User login
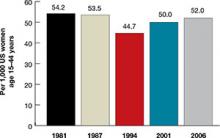
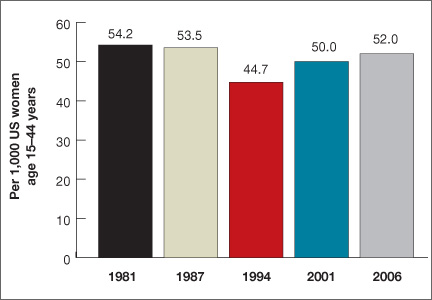
Women spend about 5 years of their reproductive lives trying to get pregnant and the other three decades trying to avoid it.1 Nearly half of all pregnancies are unintended, and 40% of these end in abortion.2 In the past 15 years, new contraceptive options have been developed to address this staggering statistic (FIGURE 1). Despite these innovations, the unintended pregnancy rate has increased continually since 1994 (FIGURE 2).23
What are we doing wrong? In this article, we will review how recent innovations are disseminated through the medical community in the context of three specific contraceptive technologies:
-
hysteroscopic sterilization (Essure)
-
ulipristal acetate emergency contraception (Ella)
-
the 13.5-mg levonorgestrel-releasing intrauterine system (Skyla).
In the process, we assess the available data on the intended and potential impacts of these technologies and describe how ObGyns can best translate these data when considering how to incorporate these new technologies into practice.
| FIGURE 2: Changes in the unintended pregnancy rate, 1981–2006 |
How contraceptive technologies spread in the medical community
Innovations spread through communication channels between individuals of a social network, who are then given time to adopt them. As opinion leaders of a social network become early adopters of a technology, dissemination of the innovation through the social network accelerates.4 This phenomenon is best described by the “diffusion of innovations theory” popularized in 1962 by sociologist Everett Rogers for agricultural applications; he also applied the model to public health.5 The variables he determined to be involved in the acceptance of an innovation are:
-
its relative advantage compared with existing technologies
-
compatibility with current practice
-
low complexity
-
high “trialability” (a potential adopter can easily attempt to use the innovation in his or her practice)
-
high “observability” (the results are easily observed and described to colleagues).
In contrast to new technology itself, medical evidence does not spread rapidly. Data generally spread far more slowly than new technology, typically taking longer than 10 years to influence medical practice.6,7 Opinion leaders can impair the dissemination of data by relying on anecdotal evidence to justify their recommendations.8 Negative findings that challenge these intuitive beliefs can take even longer to disseminate, allowing certain innovations to diffuse through the medical community faster than reports of any associated problems.9
Related article Let’s increase our use of IUDs and improve contraceptive effectiveness in this country Robert L. Barbieri, MD (Editorial, August 2012)
How hysteroscopic sterilization gained widespread adoption
Gariepy AM, Creinin MD, Schwarz EB, Smith KJ. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2):273–279.
Since its introduction into the market in 2002, more than 650,000 Essure hysteroscopic sterilization procedures have been performed worldwide.10 This procedure has diffused quickly through the medical community because of the characteristics we mentioned earlier, which ease acceptance in any network:
-
Relative advantage compared with existing technologies. Compared with existing laparoscopic sterilization methods, hysteroscopic sterilization was seen as a less invasive office procedure that could be performed more cost-effectively under local anesthesia, with very high efficacy, if successful.
-
Compatibility with current practice. Because many clinicians were providing in-office hysteroscopy, adding sterilization was a simple step.
-
Low complexity. Hysteroscopic sterilization builds on operative hysteroscopic skills with which gynecologists are familiar.
-
High trialability. The manufacturer’s representatives were willing to bring the instruments to any office for clinicians to try in their practice. The company worked with hysteroscopic equipment companies to create significant discounts for providers who would perform the procedure regularly.
-
High observability. Successful deployment of the devices, and the appearance of the confirmation test, were visualized and described easily as clinicians spoke to other clinicians, helping with dissemination.
Despite these features, however, new data suggest that hysteroscopic sterilization is less effective than laparoscopic sterilization. A successful Essure procedure requires:
-
visualization of both tubal ostia on hysteroscopy
-
successful deployment of the microinserts at the appropriate position
-
hysterosalpingography at least 3 months later (with use of an alternate form of contraception in the interim)
-
demonstrated tubal occlusion by the Essure devices (not by tubal spasm) on hysterosalpingogram.
Although 5-year data collected by the makers of Essure (and posted on their Web site) show a very high rate of efficacy and a failure rate of 0.17%, these data come from women who completed all of the required steps for successful sterilization and study follow-up.
How hysteroscopic sterilization compares with the laparoscopic approach
Gariepy and colleagues created an evidence-based clinical decision analysis to estimate the probability of successful sterilization after a hysteroscopic procedure in the operating room (OR) or office versus laparoscopic sterilization. A decision analysis, which includes the range of data available to assess different outcomes, is the best methodology to provide population-level information about likelihoods, including rare events (eg, pregnancy after sterilization), in the absence of a randomized trial.
A decision analysis assigns women to outcomes based on their intended method of sterilization, mimicking real-life situations created by the multiple steps required for successful completion of the procedure and confirmation of sterilization. When the probabilities of failing these steps are taken into account, 94% and 95% of women choosing hysteroscopic sterilization in the office and OR, respectively, would be successfully sterilized within 1 year, compared with a success rate of 99% in those who opt for laparoscopic sterilization. The estimates of hysteroscopic success include 6% of women who would attempt hysteroscopy but ultimately be sterilized via laparoscopy, and 5% of women who would decline further sterilization attempts after hysteroscopic sterilization fails.
|
WHAT THIS EVIDENCE MEANS FOR PRACTICE Hysteroscopic sterilization has its advantages, including a very high efficacy rate among women who meet all the criteria for successful occlusion. Among these criteria is confirmation, by hysterosalpingography, of occlusion 3 months after deployment of the microinserts.10 However, the efficacy of hysteroscopic sterilization is inferior at a population level; therefore, it should not be used indiscriminately. Rather, hysteroscopic sterilization may be a better option for women for whom laparoscopy itself carries a high risk, such as women with complicated diabetes or severe cardiopulmonary disease. While we await similar studies or further trials that evaluate population-based estimates of pregnancy rates, women considering sterilization should be counseled accordingly. |
How limits on access can prevent widespread use of effective contraception
American College of Obstetricians and Gynecologists. Access to emergency contraception. Committee Opinion No. 542. Obstet Gynecol. 2012;120(5):1250–1253.
Ulipristal acetate as emergency contraception (EC) was introduced to the market in 2010. As was noted in this Update in 2011, ulipristal acetate is more effective than progestin-only emergency contraception and maintains this efficacy for a longer period of time.11 Despite these clear advantages, ulipristal acetate is unlikely to realize its full potential.
Data related to EC as a public health benefit have been largely disappointing. Increased access and availability have not yet reduced the unintended pregnancy rate in the United States. Although use of EC increased from 4.2% in 2002 to 11% in 2008,12 even women with a knowledge of EC do not always use it when needed.13,14
Use of ulipristal acetate, in particular, remains limited because it lacks one important requirement for rapid diffusion—access. Although it is clinically superior to the progestin-only method of EC, is compatible with current practice, and has both high trialability and high observability, access to the drug remains too complex for easy dissemination due to its prescription-only status. Because women can now obtain progestin-only EC over the counter, the use of ulipristal acetate is likely to remain low unless the access barrier to this effective oral EC regimen is reduced.
|
WHAT THIS EVIDENCE MEANS FOR PRACTICE When counseling women of reproductive age about contraception, offer them an advance prescription for ulipristal acetate and advise them of its greater efficacy, compared with progestin-only emergency contraception. |
Skyla versus other IUDs: What the data reveal
Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril. 2012;97(3):616–622.e1–e3.
The 13.5-mg levonorgestrel-releasing intrauterine system (Skyla) boasts a smaller frame and a narrower inserter than the two intrauterine devices (IUDs) already on the market (ParaGard and Mirena), a lower amount of levonorgestrel than the other levonorgestrel-releasing IUD (Mirena), and 3 years of continuous contraception. Both of the IUDs that predated Skyla are backed by data supporting their efficacy and safety in nulliparous women,15-18 but a number of clinicians and opinion leaders have stated that Skyla’s smaller frame and inserter make it an ideal IUD for the narrower cervical canal and smaller endometrial cavity of nulliparous women,19 including Gemzell-Danielsson and colleagues.
Skyla meets the prerequisites for rapid diffusion; it is highly compatible with current practice and easy to place and use. Of all these characteristics, the relative advantage granted by its size is most likely to promote its diffusion through the medical community.
Ease of placement versus Mirena
Clinical information about Skyla is currently available from two sources. The first is the product package insert, which includes selected data from the product’s Phase 3 study. This study included 1,432 participants, of whom 556 (38.8%) were nulliparous and 540 (37.7%) were treated in the United States.20
The second source is a published, peer-reviewed Phase 2 trial comparing Mirena with two smaller, lower-dose levonorgestrel-releasing devices, with the lowest-dose product corresponding to the marketed Skyla product.21 In the Phase 2 trial, all 738 women given Mirena or the smaller devices experienced successful placement, with 98.5% of placements achieved on the first attempt. Investigators rated placement for the smaller devices “easy” in 455 of 484 (94.0%) women, compared with 219 of 254 (86.2%) women given Mirena (P <.001). Most of the women given the smaller devices rated their pain with insertion as “mild pain” or “no pain,” compared with those given Mirena (72.3% vs 57.9%; P <.001). Adverse events were similar between users of the different products, except that significantly more women were classified as having an ovarian cyst among Mirena users than among users of the smaller, low-dose devices (22% vs 6%; P <.0001).
Little difference in "clinically relevant" effects
The claim that Skyla has an advantage over Mirena or ParaGard falls short on closer inspection. Although a clinician may prefer easy insertion and a patient with no pain, only very difficult or severely painful placements have clinical relevance.
Investigators rated only 4 of 254 (1.6%) Mirena insertions as “very difficult,” compared with 4 of 484 (0.8%) for the smaller devices (P=.46). Further, women found Mirena insertion to cause severe pain in only 17 of 254 (6.7%) insertions, compared with 21 of 484 (4.3%) placements of the smaller devices (P=.22). The smaller device and inserter, therefore, may have no clinical advantage.
Adverse events were similar
The data on adverse events are similarly misleading. Investigators in the Phase 2 study found that the lower-dose levonorgestrel-releasing IUDs had an 8.6% rate of ovarian cysts and the Mirena had a 22% rate (P <.0001). However, the Phase 2 study included a pelvic ultrasound examination at every visit, and ovarian cysts were included as an adverse event if the size was 3 cm or greater, regardless of symptoms.
Complaints of abdominal or low abdominal pain were as common among Mirena users as among users of the smaller devices, so this finding likely represents asymptomatic, clinically irrelevant cysts.
Most ovarian cysts found in users of the levonorgestrel-releasing intrauterine system are asymptomatic.22
Fewer Skyla users developed amenorrhea
Bleeding patterns differed between the products. Users of the smaller, low-dose device reported slightly more spotting and bleeding over the course of a month. In the Phase 2 trial, at the end of 3 years, only 12.7% of Skyla users achieved amenorrhea, compared with 23.6% of Mirena users. The amenorrhea rate for Mirena was very similar to the 20% rate reported in earlier studies,23,24 but the rate for Skyla was even lower (6%) in the larger Phase 3 study.
What about efficacy?
If there are no real advantages to be gained from the size of the device and inserter in terms of pain, and no real improvement in adverse effects or bleeding patterns, what about efficacy?
No direct comparisons are available, but if the devices are evaluated in terms of their first-year Pearl index rating from Phase 3 studies for approval in the United States, then among a cohort of 100,000 users, about 190 Mirena users would become pregnant in the first year, compared with 410 Skyla users.
All IUDs are considered highly effective contraceptives, but small relative differences can have a large impact on a population level if the methods are not used correctly or patients are not counseled appropriately. Although it is more effective than user-dependent contraceptives such as the pill, Skyla is the least effective of the highly effective methods available. If the device has any real benefits in comparison with the other IUDs, they must be better demonstrated with additional data.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
ObGyns have done much to increase the use of long-acting reversible contraceptives such as the IUD (Mirena, ParaGard), the etonogestrel implant (Implanon, Nexplanon), and the injectable contraceptive depot medroxyprogesterone acetate (Depo-Provera). We applaud this success and urge ObGyns to continue prescribing these options.
In addition, if we want to have a positive impact on the unintended pregnancy rate, we need to increase awareness of, access to, and use of the most effective contraceptive options in our community of providers and among our patients. We also need to eliminate barriers to use of the most effective methods—eg, discussing ulipristal acetate with our patients and providing advance prescriptions. We also need to be cautious about adopting some innovations, as the data for Skyla and Essure illustrate. They may be terrific options for very specific populations of women, but indiscriminate use may, paradoxically, increase the rate of unintended pregnancy.
Women spend about 5 years of their reproductive lives trying to get pregnant and the other three decades trying to avoid it.1 Nearly half of all pregnancies are unintended, and 40% of these end in abortion.2 In the past 15 years, new contraceptive options have been developed to address this staggering statistic (FIGURE 1). Despite these innovations, the unintended pregnancy rate has increased continually since 1994 (FIGURE 2).23
What are we doing wrong? In this article, we will review how recent innovations are disseminated through the medical community in the context of three specific contraceptive technologies:
-
hysteroscopic sterilization (Essure)
-
ulipristal acetate emergency contraception (Ella)
-
the 13.5-mg levonorgestrel-releasing intrauterine system (Skyla).
In the process, we assess the available data on the intended and potential impacts of these technologies and describe how ObGyns can best translate these data when considering how to incorporate these new technologies into practice.
| FIGURE 2: Changes in the unintended pregnancy rate, 1981–2006 |
How contraceptive technologies spread in the medical community
Innovations spread through communication channels between individuals of a social network, who are then given time to adopt them. As opinion leaders of a social network become early adopters of a technology, dissemination of the innovation through the social network accelerates.4 This phenomenon is best described by the “diffusion of innovations theory” popularized in 1962 by sociologist Everett Rogers for agricultural applications; he also applied the model to public health.5 The variables he determined to be involved in the acceptance of an innovation are:
-
its relative advantage compared with existing technologies
-
compatibility with current practice
-
low complexity
-
high “trialability” (a potential adopter can easily attempt to use the innovation in his or her practice)
-
high “observability” (the results are easily observed and described to colleagues).
In contrast to new technology itself, medical evidence does not spread rapidly. Data generally spread far more slowly than new technology, typically taking longer than 10 years to influence medical practice.6,7 Opinion leaders can impair the dissemination of data by relying on anecdotal evidence to justify their recommendations.8 Negative findings that challenge these intuitive beliefs can take even longer to disseminate, allowing certain innovations to diffuse through the medical community faster than reports of any associated problems.9
Related article Let’s increase our use of IUDs and improve contraceptive effectiveness in this country Robert L. Barbieri, MD (Editorial, August 2012)
How hysteroscopic sterilization gained widespread adoption
Gariepy AM, Creinin MD, Schwarz EB, Smith KJ. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2):273–279.
Since its introduction into the market in 2002, more than 650,000 Essure hysteroscopic sterilization procedures have been performed worldwide.10 This procedure has diffused quickly through the medical community because of the characteristics we mentioned earlier, which ease acceptance in any network:
-
Relative advantage compared with existing technologies. Compared with existing laparoscopic sterilization methods, hysteroscopic sterilization was seen as a less invasive office procedure that could be performed more cost-effectively under local anesthesia, with very high efficacy, if successful.
-
Compatibility with current practice. Because many clinicians were providing in-office hysteroscopy, adding sterilization was a simple step.
-
Low complexity. Hysteroscopic sterilization builds on operative hysteroscopic skills with which gynecologists are familiar.
-
High trialability. The manufacturer’s representatives were willing to bring the instruments to any office for clinicians to try in their practice. The company worked with hysteroscopic equipment companies to create significant discounts for providers who would perform the procedure regularly.
-
High observability. Successful deployment of the devices, and the appearance of the confirmation test, were visualized and described easily as clinicians spoke to other clinicians, helping with dissemination.
Despite these features, however, new data suggest that hysteroscopic sterilization is less effective than laparoscopic sterilization. A successful Essure procedure requires:
-
visualization of both tubal ostia on hysteroscopy
-
successful deployment of the microinserts at the appropriate position
-
hysterosalpingography at least 3 months later (with use of an alternate form of contraception in the interim)
-
demonstrated tubal occlusion by the Essure devices (not by tubal spasm) on hysterosalpingogram.
Although 5-year data collected by the makers of Essure (and posted on their Web site) show a very high rate of efficacy and a failure rate of 0.17%, these data come from women who completed all of the required steps for successful sterilization and study follow-up.
How hysteroscopic sterilization compares with the laparoscopic approach
Gariepy and colleagues created an evidence-based clinical decision analysis to estimate the probability of successful sterilization after a hysteroscopic procedure in the operating room (OR) or office versus laparoscopic sterilization. A decision analysis, which includes the range of data available to assess different outcomes, is the best methodology to provide population-level information about likelihoods, including rare events (eg, pregnancy after sterilization), in the absence of a randomized trial.
A decision analysis assigns women to outcomes based on their intended method of sterilization, mimicking real-life situations created by the multiple steps required for successful completion of the procedure and confirmation of sterilization. When the probabilities of failing these steps are taken into account, 94% and 95% of women choosing hysteroscopic sterilization in the office and OR, respectively, would be successfully sterilized within 1 year, compared with a success rate of 99% in those who opt for laparoscopic sterilization. The estimates of hysteroscopic success include 6% of women who would attempt hysteroscopy but ultimately be sterilized via laparoscopy, and 5% of women who would decline further sterilization attempts after hysteroscopic sterilization fails.
|
WHAT THIS EVIDENCE MEANS FOR PRACTICE Hysteroscopic sterilization has its advantages, including a very high efficacy rate among women who meet all the criteria for successful occlusion. Among these criteria is confirmation, by hysterosalpingography, of occlusion 3 months after deployment of the microinserts.10 However, the efficacy of hysteroscopic sterilization is inferior at a population level; therefore, it should not be used indiscriminately. Rather, hysteroscopic sterilization may be a better option for women for whom laparoscopy itself carries a high risk, such as women with complicated diabetes or severe cardiopulmonary disease. While we await similar studies or further trials that evaluate population-based estimates of pregnancy rates, women considering sterilization should be counseled accordingly. |
How limits on access can prevent widespread use of effective contraception
American College of Obstetricians and Gynecologists. Access to emergency contraception. Committee Opinion No. 542. Obstet Gynecol. 2012;120(5):1250–1253.
Ulipristal acetate as emergency contraception (EC) was introduced to the market in 2010. As was noted in this Update in 2011, ulipristal acetate is more effective than progestin-only emergency contraception and maintains this efficacy for a longer period of time.11 Despite these clear advantages, ulipristal acetate is unlikely to realize its full potential.
Data related to EC as a public health benefit have been largely disappointing. Increased access and availability have not yet reduced the unintended pregnancy rate in the United States. Although use of EC increased from 4.2% in 2002 to 11% in 2008,12 even women with a knowledge of EC do not always use it when needed.13,14
Use of ulipristal acetate, in particular, remains limited because it lacks one important requirement for rapid diffusion—access. Although it is clinically superior to the progestin-only method of EC, is compatible with current practice, and has both high trialability and high observability, access to the drug remains too complex for easy dissemination due to its prescription-only status. Because women can now obtain progestin-only EC over the counter, the use of ulipristal acetate is likely to remain low unless the access barrier to this effective oral EC regimen is reduced.
|
WHAT THIS EVIDENCE MEANS FOR PRACTICE When counseling women of reproductive age about contraception, offer them an advance prescription for ulipristal acetate and advise them of its greater efficacy, compared with progestin-only emergency contraception. |
Skyla versus other IUDs: What the data reveal
Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril. 2012;97(3):616–622.e1–e3.
The 13.5-mg levonorgestrel-releasing intrauterine system (Skyla) boasts a smaller frame and a narrower inserter than the two intrauterine devices (IUDs) already on the market (ParaGard and Mirena), a lower amount of levonorgestrel than the other levonorgestrel-releasing IUD (Mirena), and 3 years of continuous contraception. Both of the IUDs that predated Skyla are backed by data supporting their efficacy and safety in nulliparous women,15-18 but a number of clinicians and opinion leaders have stated that Skyla’s smaller frame and inserter make it an ideal IUD for the narrower cervical canal and smaller endometrial cavity of nulliparous women,19 including Gemzell-Danielsson and colleagues.
Skyla meets the prerequisites for rapid diffusion; it is highly compatible with current practice and easy to place and use. Of all these characteristics, the relative advantage granted by its size is most likely to promote its diffusion through the medical community.
Ease of placement versus Mirena
Clinical information about Skyla is currently available from two sources. The first is the product package insert, which includes selected data from the product’s Phase 3 study. This study included 1,432 participants, of whom 556 (38.8%) were nulliparous and 540 (37.7%) were treated in the United States.20
The second source is a published, peer-reviewed Phase 2 trial comparing Mirena with two smaller, lower-dose levonorgestrel-releasing devices, with the lowest-dose product corresponding to the marketed Skyla product.21 In the Phase 2 trial, all 738 women given Mirena or the smaller devices experienced successful placement, with 98.5% of placements achieved on the first attempt. Investigators rated placement for the smaller devices “easy” in 455 of 484 (94.0%) women, compared with 219 of 254 (86.2%) women given Mirena (P <.001). Most of the women given the smaller devices rated their pain with insertion as “mild pain” or “no pain,” compared with those given Mirena (72.3% vs 57.9%; P <.001). Adverse events were similar between users of the different products, except that significantly more women were classified as having an ovarian cyst among Mirena users than among users of the smaller, low-dose devices (22% vs 6%; P <.0001).
Little difference in "clinically relevant" effects
The claim that Skyla has an advantage over Mirena or ParaGard falls short on closer inspection. Although a clinician may prefer easy insertion and a patient with no pain, only very difficult or severely painful placements have clinical relevance.
Investigators rated only 4 of 254 (1.6%) Mirena insertions as “very difficult,” compared with 4 of 484 (0.8%) for the smaller devices (P=.46). Further, women found Mirena insertion to cause severe pain in only 17 of 254 (6.7%) insertions, compared with 21 of 484 (4.3%) placements of the smaller devices (P=.22). The smaller device and inserter, therefore, may have no clinical advantage.
Adverse events were similar
The data on adverse events are similarly misleading. Investigators in the Phase 2 study found that the lower-dose levonorgestrel-releasing IUDs had an 8.6% rate of ovarian cysts and the Mirena had a 22% rate (P <.0001). However, the Phase 2 study included a pelvic ultrasound examination at every visit, and ovarian cysts were included as an adverse event if the size was 3 cm or greater, regardless of symptoms.
Complaints of abdominal or low abdominal pain were as common among Mirena users as among users of the smaller devices, so this finding likely represents asymptomatic, clinically irrelevant cysts.
Most ovarian cysts found in users of the levonorgestrel-releasing intrauterine system are asymptomatic.22
Fewer Skyla users developed amenorrhea
Bleeding patterns differed between the products. Users of the smaller, low-dose device reported slightly more spotting and bleeding over the course of a month. In the Phase 2 trial, at the end of 3 years, only 12.7% of Skyla users achieved amenorrhea, compared with 23.6% of Mirena users. The amenorrhea rate for Mirena was very similar to the 20% rate reported in earlier studies,23,24 but the rate for Skyla was even lower (6%) in the larger Phase 3 study.
What about efficacy?
If there are no real advantages to be gained from the size of the device and inserter in terms of pain, and no real improvement in adverse effects or bleeding patterns, what about efficacy?
No direct comparisons are available, but if the devices are evaluated in terms of their first-year Pearl index rating from Phase 3 studies for approval in the United States, then among a cohort of 100,000 users, about 190 Mirena users would become pregnant in the first year, compared with 410 Skyla users.
All IUDs are considered highly effective contraceptives, but small relative differences can have a large impact on a population level if the methods are not used correctly or patients are not counseled appropriately. Although it is more effective than user-dependent contraceptives such as the pill, Skyla is the least effective of the highly effective methods available. If the device has any real benefits in comparison with the other IUDs, they must be better demonstrated with additional data.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
ObGyns have done much to increase the use of long-acting reversible contraceptives such as the IUD (Mirena, ParaGard), the etonogestrel implant (Implanon, Nexplanon), and the injectable contraceptive depot medroxyprogesterone acetate (Depo-Provera). We applaud this success and urge ObGyns to continue prescribing these options.
In addition, if we want to have a positive impact on the unintended pregnancy rate, we need to increase awareness of, access to, and use of the most effective contraceptive options in our community of providers and among our patients. We also need to eliminate barriers to use of the most effective methods—eg, discussing ulipristal acetate with our patients and providing advance prescriptions. We also need to be cautious about adopting some innovations, as the data for Skyla and Essure illustrate. They may be terrific options for very specific populations of women, but indiscriminate use may, paradoxically, increase the rate of unintended pregnancy.
Women spend about 5 years of their reproductive lives trying to get pregnant and the other three decades trying to avoid it.1 Nearly half of all pregnancies are unintended, and 40% of these end in abortion.2 In the past 15 years, new contraceptive options have been developed to address this staggering statistic (FIGURE 1). Despite these innovations, the unintended pregnancy rate has increased continually since 1994 (FIGURE 2).23
What are we doing wrong? In this article, we will review how recent innovations are disseminated through the medical community in the context of three specific contraceptive technologies:
-
hysteroscopic sterilization (Essure)
-
ulipristal acetate emergency contraception (Ella)
-
the 13.5-mg levonorgestrel-releasing intrauterine system (Skyla).
In the process, we assess the available data on the intended and potential impacts of these technologies and describe how ObGyns can best translate these data when considering how to incorporate these new technologies into practice.
| FIGURE 2: Changes in the unintended pregnancy rate, 1981–2006 |
How contraceptive technologies spread in the medical community
Innovations spread through communication channels between individuals of a social network, who are then given time to adopt them. As opinion leaders of a social network become early adopters of a technology, dissemination of the innovation through the social network accelerates.4 This phenomenon is best described by the “diffusion of innovations theory” popularized in 1962 by sociologist Everett Rogers for agricultural applications; he also applied the model to public health.5 The variables he determined to be involved in the acceptance of an innovation are:
-
its relative advantage compared with existing technologies
-
compatibility with current practice
-
low complexity
-
high “trialability” (a potential adopter can easily attempt to use the innovation in his or her practice)
-
high “observability” (the results are easily observed and described to colleagues).
In contrast to new technology itself, medical evidence does not spread rapidly. Data generally spread far more slowly than new technology, typically taking longer than 10 years to influence medical practice.6,7 Opinion leaders can impair the dissemination of data by relying on anecdotal evidence to justify their recommendations.8 Negative findings that challenge these intuitive beliefs can take even longer to disseminate, allowing certain innovations to diffuse through the medical community faster than reports of any associated problems.9
Related article Let’s increase our use of IUDs and improve contraceptive effectiveness in this country Robert L. Barbieri, MD (Editorial, August 2012)
How hysteroscopic sterilization gained widespread adoption
Gariepy AM, Creinin MD, Schwarz EB, Smith KJ. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2):273–279.
Since its introduction into the market in 2002, more than 650,000 Essure hysteroscopic sterilization procedures have been performed worldwide.10 This procedure has diffused quickly through the medical community because of the characteristics we mentioned earlier, which ease acceptance in any network:
-
Relative advantage compared with existing technologies. Compared with existing laparoscopic sterilization methods, hysteroscopic sterilization was seen as a less invasive office procedure that could be performed more cost-effectively under local anesthesia, with very high efficacy, if successful.
-
Compatibility with current practice. Because many clinicians were providing in-office hysteroscopy, adding sterilization was a simple step.
-
Low complexity. Hysteroscopic sterilization builds on operative hysteroscopic skills with which gynecologists are familiar.
-
High trialability. The manufacturer’s representatives were willing to bring the instruments to any office for clinicians to try in their practice. The company worked with hysteroscopic equipment companies to create significant discounts for providers who would perform the procedure regularly.
-
High observability. Successful deployment of the devices, and the appearance of the confirmation test, were visualized and described easily as clinicians spoke to other clinicians, helping with dissemination.
Despite these features, however, new data suggest that hysteroscopic sterilization is less effective than laparoscopic sterilization. A successful Essure procedure requires:
-
visualization of both tubal ostia on hysteroscopy
-
successful deployment of the microinserts at the appropriate position
-
hysterosalpingography at least 3 months later (with use of an alternate form of contraception in the interim)
-
demonstrated tubal occlusion by the Essure devices (not by tubal spasm) on hysterosalpingogram.
Although 5-year data collected by the makers of Essure (and posted on their Web site) show a very high rate of efficacy and a failure rate of 0.17%, these data come from women who completed all of the required steps for successful sterilization and study follow-up.
How hysteroscopic sterilization compares with the laparoscopic approach
Gariepy and colleagues created an evidence-based clinical decision analysis to estimate the probability of successful sterilization after a hysteroscopic procedure in the operating room (OR) or office versus laparoscopic sterilization. A decision analysis, which includes the range of data available to assess different outcomes, is the best methodology to provide population-level information about likelihoods, including rare events (eg, pregnancy after sterilization), in the absence of a randomized trial.
A decision analysis assigns women to outcomes based on their intended method of sterilization, mimicking real-life situations created by the multiple steps required for successful completion of the procedure and confirmation of sterilization. When the probabilities of failing these steps are taken into account, 94% and 95% of women choosing hysteroscopic sterilization in the office and OR, respectively, would be successfully sterilized within 1 year, compared with a success rate of 99% in those who opt for laparoscopic sterilization. The estimates of hysteroscopic success include 6% of women who would attempt hysteroscopy but ultimately be sterilized via laparoscopy, and 5% of women who would decline further sterilization attempts after hysteroscopic sterilization fails.
|
WHAT THIS EVIDENCE MEANS FOR PRACTICE Hysteroscopic sterilization has its advantages, including a very high efficacy rate among women who meet all the criteria for successful occlusion. Among these criteria is confirmation, by hysterosalpingography, of occlusion 3 months after deployment of the microinserts.10 However, the efficacy of hysteroscopic sterilization is inferior at a population level; therefore, it should not be used indiscriminately. Rather, hysteroscopic sterilization may be a better option for women for whom laparoscopy itself carries a high risk, such as women with complicated diabetes or severe cardiopulmonary disease. While we await similar studies or further trials that evaluate population-based estimates of pregnancy rates, women considering sterilization should be counseled accordingly. |
How limits on access can prevent widespread use of effective contraception
American College of Obstetricians and Gynecologists. Access to emergency contraception. Committee Opinion No. 542. Obstet Gynecol. 2012;120(5):1250–1253.
Ulipristal acetate as emergency contraception (EC) was introduced to the market in 2010. As was noted in this Update in 2011, ulipristal acetate is more effective than progestin-only emergency contraception and maintains this efficacy for a longer period of time.11 Despite these clear advantages, ulipristal acetate is unlikely to realize its full potential.
Data related to EC as a public health benefit have been largely disappointing. Increased access and availability have not yet reduced the unintended pregnancy rate in the United States. Although use of EC increased from 4.2% in 2002 to 11% in 2008,12 even women with a knowledge of EC do not always use it when needed.13,14
Use of ulipristal acetate, in particular, remains limited because it lacks one important requirement for rapid diffusion—access. Although it is clinically superior to the progestin-only method of EC, is compatible with current practice, and has both high trialability and high observability, access to the drug remains too complex for easy dissemination due to its prescription-only status. Because women can now obtain progestin-only EC over the counter, the use of ulipristal acetate is likely to remain low unless the access barrier to this effective oral EC regimen is reduced.
|
WHAT THIS EVIDENCE MEANS FOR PRACTICE When counseling women of reproductive age about contraception, offer them an advance prescription for ulipristal acetate and advise them of its greater efficacy, compared with progestin-only emergency contraception. |
Skyla versus other IUDs: What the data reveal
Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril. 2012;97(3):616–622.e1–e3.
The 13.5-mg levonorgestrel-releasing intrauterine system (Skyla) boasts a smaller frame and a narrower inserter than the two intrauterine devices (IUDs) already on the market (ParaGard and Mirena), a lower amount of levonorgestrel than the other levonorgestrel-releasing IUD (Mirena), and 3 years of continuous contraception. Both of the IUDs that predated Skyla are backed by data supporting their efficacy and safety in nulliparous women,15-18 but a number of clinicians and opinion leaders have stated that Skyla’s smaller frame and inserter make it an ideal IUD for the narrower cervical canal and smaller endometrial cavity of nulliparous women,19 including Gemzell-Danielsson and colleagues.
Skyla meets the prerequisites for rapid diffusion; it is highly compatible with current practice and easy to place and use. Of all these characteristics, the relative advantage granted by its size is most likely to promote its diffusion through the medical community.
Ease of placement versus Mirena
Clinical information about Skyla is currently available from two sources. The first is the product package insert, which includes selected data from the product’s Phase 3 study. This study included 1,432 participants, of whom 556 (38.8%) were nulliparous and 540 (37.7%) were treated in the United States.20
The second source is a published, peer-reviewed Phase 2 trial comparing Mirena with two smaller, lower-dose levonorgestrel-releasing devices, with the lowest-dose product corresponding to the marketed Skyla product.21 In the Phase 2 trial, all 738 women given Mirena or the smaller devices experienced successful placement, with 98.5% of placements achieved on the first attempt. Investigators rated placement for the smaller devices “easy” in 455 of 484 (94.0%) women, compared with 219 of 254 (86.2%) women given Mirena (P <.001). Most of the women given the smaller devices rated their pain with insertion as “mild pain” or “no pain,” compared with those given Mirena (72.3% vs 57.9%; P <.001). Adverse events were similar between users of the different products, except that significantly more women were classified as having an ovarian cyst among Mirena users than among users of the smaller, low-dose devices (22% vs 6%; P <.0001).
Little difference in "clinically relevant" effects
The claim that Skyla has an advantage over Mirena or ParaGard falls short on closer inspection. Although a clinician may prefer easy insertion and a patient with no pain, only very difficult or severely painful placements have clinical relevance.
Investigators rated only 4 of 254 (1.6%) Mirena insertions as “very difficult,” compared with 4 of 484 (0.8%) for the smaller devices (P=.46). Further, women found Mirena insertion to cause severe pain in only 17 of 254 (6.7%) insertions, compared with 21 of 484 (4.3%) placements of the smaller devices (P=.22). The smaller device and inserter, therefore, may have no clinical advantage.
Adverse events were similar
The data on adverse events are similarly misleading. Investigators in the Phase 2 study found that the lower-dose levonorgestrel-releasing IUDs had an 8.6% rate of ovarian cysts and the Mirena had a 22% rate (P <.0001). However, the Phase 2 study included a pelvic ultrasound examination at every visit, and ovarian cysts were included as an adverse event if the size was 3 cm or greater, regardless of symptoms.
Complaints of abdominal or low abdominal pain were as common among Mirena users as among users of the smaller devices, so this finding likely represents asymptomatic, clinically irrelevant cysts.
Most ovarian cysts found in users of the levonorgestrel-releasing intrauterine system are asymptomatic.22
Fewer Skyla users developed amenorrhea
Bleeding patterns differed between the products. Users of the smaller, low-dose device reported slightly more spotting and bleeding over the course of a month. In the Phase 2 trial, at the end of 3 years, only 12.7% of Skyla users achieved amenorrhea, compared with 23.6% of Mirena users. The amenorrhea rate for Mirena was very similar to the 20% rate reported in earlier studies,23,24 but the rate for Skyla was even lower (6%) in the larger Phase 3 study.
What about efficacy?
If there are no real advantages to be gained from the size of the device and inserter in terms of pain, and no real improvement in adverse effects or bleeding patterns, what about efficacy?
No direct comparisons are available, but if the devices are evaluated in terms of their first-year Pearl index rating from Phase 3 studies for approval in the United States, then among a cohort of 100,000 users, about 190 Mirena users would become pregnant in the first year, compared with 410 Skyla users.
All IUDs are considered highly effective contraceptives, but small relative differences can have a large impact on a population level if the methods are not used correctly or patients are not counseled appropriately. Although it is more effective than user-dependent contraceptives such as the pill, Skyla is the least effective of the highly effective methods available. If the device has any real benefits in comparison with the other IUDs, they must be better demonstrated with additional data.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
ObGyns have done much to increase the use of long-acting reversible contraceptives such as the IUD (Mirena, ParaGard), the etonogestrel implant (Implanon, Nexplanon), and the injectable contraceptive depot medroxyprogesterone acetate (Depo-Provera). We applaud this success and urge ObGyns to continue prescribing these options.
In addition, if we want to have a positive impact on the unintended pregnancy rate, we need to increase awareness of, access to, and use of the most effective contraceptive options in our community of providers and among our patients. We also need to eliminate barriers to use of the most effective methods—eg, discussing ulipristal acetate with our patients and providing advance prescriptions. We also need to be cautious about adopting some innovations, as the data for Skyla and Essure illustrate. They may be terrific options for very specific populations of women, but indiscriminate use may, paradoxically, increase the rate of unintended pregnancy.