User login
Dr. Mutch reports that he has received grant or research support from Lilly and Genentech. He serves as a speaker for GSK, Lilly, and Merck. Dr. Rimel reports no financial relationships relevant to this article.
Endometrial cancer is a great concern in industrialized nations, where it is the most common gynecologic cancer—with incidence increasing every year. Survival is generally very good for women who have low-grade disease confined to the uterus. However, for patients who have high-grade disease, an aggressive histologic type, or other features that suggest a poor prognosis, the cure rate approaches 75%.1
Primary surgery is the mainstay of initial treatment and basis of FIGO staging ( TABLE ), which requires:
- total hysterectomy
- bilateral salpingo-oophorectomy
- complete examination of the abdomen
- pelvic washings
- lymph-adenectomy (anatomic boundaries and node counts aren’t specified).
Controversy clouds our understanding of the optimal type of surgery, utility of pelvic lymphadenectomy, and possible benefit of adjuvant radiation therapy. During the past year, fuel has been added to this debate:
- Two randomized, controlled trials of surgery with and without pelvic lymphadenectomy in early-stage patients demonstrated no survival benefit. Earlier studies investigating the benefits of lymphadenectomy in endometrial cancer have been largely retrospective, and results have varied.
- A concurrent randomized, controlled trial of external-beam radiotherapy for women who have intermediate- or high-risk disease showed no improvement in overall survival, although local control increased by 3%.
TABLE
FIGO surgical staging for endometrial cancer
| Stage | Description |
|---|---|
| I | Tumor is confined to uterine fundus |
| IA | Tumor is limited to endometrium |
| IB | Tumor invades less than half of the myometrial thickness |
| IC | Tumor invades more than half of the myometrial thickness |
| II | Tumor extends to cervix |
| IIA | Cervical extension is limited to endocervical glands |
| IIB | Tumor invades cervical stroma |
| III | There is regional tumor spread |
| IIIA | Tumor invades uterine serosa or adnexa, or cells in the peritoneum show signs of cancer |
| IIIB | Vaginal metastases are present |
| IIIC | Tumor has spread to lymph nodes near the uterus |
| IV | There is bulky pelvic disease or distant spread |
| IVA | Tumor has spread to bladder or rectum |
| IVB | Distant metastases are present |
No survival advantage to pelvic lymphadenectomy—but it has other benefits
ASTEC study group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136.
Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716.
Among the arguments for lymphadenectomy in endometrial cancer staging are:
- It aids in the selection of women for radiation or other adjuvant treatment
- It may have a direct survival benefit, as suggested by retrospective studies.
But lymphadenectomy is time-consuming, requires a specialized gynecologic surgeon, and is associated with some increase in the risk of morbidity—namely, lymphedema, lymphocyst formation, deep-vein thrombosis (DVT), and blood loss.
The much-anticipated report of the 85-center, multinational ASTEC trial [ A S urgical T rial of E ndometrial C ancer], published earlier this year, offers further insight into the practice of lymphadenectomy. ASTEC involved two randomizations: The first, to pelvic lymphadenectomy; the second, to radiation therapy.
The ASTEC trial enrolled 1,408 women who had histologically confirmed endometrial carcinoma that was believed to be confined to the uterus. How this determination was made was not specified. Patients who had enlarged lymph nodes corroborated by computed tomography or magnetic resonance imaging were not excluded.
Participants were randomized to either of the following treatment groups:
- traditional surgery with total hysterectomy and bilateral salpingo-oophorectomy, pelvic washings, and palpation of para-aortic nodes
- the same surgery plus systematic lymphadenectomy of the iliac and obturator nodes.
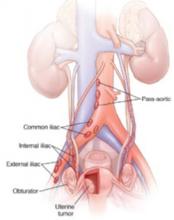
If any para-aortic nodes were suspicious, biopsy or lymphadenectomy was performed at the discretion of the surgeon ( FIGURE ).
FIGURE Nodes reveal when cancer has spread
Women in the ASTEC trial were randomized to traditional surgery (total hysterectomy and bilateral salpingo-oophorectomy), pelvic washings, and palpation of para-aortic nodes or to the same surgery plus lymphadenectomy of the iliac and obturator nodes.
Operative findings determined a patient’s level of risk
After surgery, patients were categorized as having one of the following:
- low-risk, early-stage disease. This group included patients who had disease classified as stage IA or IB, grade 1 or 2. They were deemed to have a suitably low risk of recurrence to be offered further treatment according to their physician’s standard practice.
- intermediate- or high-risk, early-stage disease. These patients were randomized to the ASTEC radiation-therapy trial, which compared external-beam radiotherapy with no external-beam radiotherapy. The authors assert that this second randomization was necessary to prevent over- or undertreatment of patients who had unknown node status, which might alter survival outcomes.
- advanced disease. These patients were referred to their physician for further treatment.
In both surgical groups (with and without lymphadenectomy), approximately 80% of patients had disease confined to the uterus. Nodes were harvested in 91% of the patients in the lymphadenectomy group, compared with 5% of patients in the traditional-surgery group. Nine percent of women in the lymphadenectomy group had positive nodes.
The authors observe that more women had deeply invasive disease and adverse histologic types in the group that underwent lymphadenectomy. There were no differences between the two groups in overall survival; disease-specific survival; recurrence-free survival; or recurrence-free, disease-specific survival, after adjustment for baseline differences. Subgroup analysis for low-risk, high-risk, and advanced disease also failed to demonstrate differences in overall survival and recurrence-free survival.
Study from Italy produces similar findings
An independent randomized, controlled trial examining survival outcomes for endometrial cancer patients with and without lymphadenectomy was released by the Italian group in late 2008. In this study, 537 patients who had histologically confirmed endometrial carcinoma believed to be confined to the uterus were randomized to total abdominal hysterectomy and bilateral salpingo-oophorectomy with or without pelvic lymphadenectomy.
Anatomic boundaries of the pelvic lymph-node dissection were clearly defined, and a minimum lymph-node count of 20 was specified for inclusion. Intraoperative frozen section was utilized to exclude patients who had grade-1 disease that was less than 50% invasive. The option of para-aortic lymph-node dissection or sampling was left to the discretion of the surgeon. If pelvic nodes were larger than 1 cm, they were removed or sampled regardless of randomization.
Unlike the ASTEC trial, this study did not attempt to control adjuvant treatment. Patients were treated according to the discretion of the physician. Most patients received no further therapy; only 20% underwent radiation therapy, and 7% received chemotherapy.
Given the findings of these two, large, multi-institutional trials with strikingly similar results but major problems, what is a gynecologist to do? Can lymphadenectomy be avoided in patients whose disease is believed to be confined to the uterus?
For now, the answer is a tentative “No.”
There appears to be no survival advantage to removal of lymph nodes when disease is confined to the uterus, but that is not to say there is no benefit to systematic lymphadenectomy—just that there is no survival benefit afforded by the procedure. Benefits of lymphadenectomy, which include more precise definition of the extent of disease, minimization of over- or undertreatment, and a reduction in overall treatment and cost, still remain. The concept of surgical debulking put forward by Bristow and coworkers still has merit, and any gross disease should be removed, if feasible.2
Lymphadenectomy in endometrial cancer remains controversial and complex, especially as we lack a precise method for determining which patients will have nodal disease. Our practice remains to remove the lymph nodes whenever possible to better tailor any adjuvant treatment.—DAVID G. MUTCH, MD; B. J. RIMEL, MD
Women in the lymphadenectomy group were more likely to have stage-IIIC disease, which is directly attributable to histologic evaluation of the lymph nodes in this group. The authors point out that these patients had more accurate assessment of their prognosis, allowing for the tailoring of adjuvant treatment.
The overall survival and disease-free survival curves for the two experimental groups were similar, consistent with data from the ASTEC trial. This proved to be true for both the intention-to-treat and according-to-protocol groups. The authors note that their results are similar to those of the ASTEC trial, despite the significant difference in the number of nodes removed in each trial.
Some aspects of the trials hamper interpretation and comparison
Outcomes are improved when surgery is performed by a trained gynecologic oncologist. In the ASTEC trial, each lymphadenectomy was performed by a specialized gynecologic surgeon who was “skilled in the procedure.” In the Italian study, the type of surgeon was not specified, but the specific anatomic boundaries of the dissection and the minimum node count were. More specific data are needed before any conclusions can be drawn about the effect of surgical skill on outcome in these trials.
In the ASTEC trial, 9% of patients in the lymphadenectomy group had no nodes removed, and more than 60% of patients would not have met criteria for inclusion in the lymphadenectomy arm of the Italian study—suggesting that the majority of women in the ASTEC trial had inadequate lymphadenectomy. Para-aortic lymphadenectomy was left to the discretion of the attending surgeon, and some patients did have resection of these nodes. The data do not include information about whether these patients were treated in the para-aortic region based on the histology of these nodes.
Randomization in a prospective study is supposed to equalize the risks between groups. In the ASTEC trial, despite randomization, there were 10% more patients who had deeply invasive disease in the lymphadenectomy group, along with 3% more adverse histologies and high-grade (grade-3) tumors. Given the higher incidence of positive nodes and poorer outcome in these cases, this difference may have had a significant impact on the evaluation of the groups for overall or disease-specific survival.
External-beam radiotherapy reduces local recurrence of endometrial Ca but does not improve survival
ASTEC/EN.5 Study Group, Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–146.
Radiation therapy has been a standard treatment for endometrial cancer when there is high risk of recurrence. This report combines two independent randomized, controlled trials investigating the benefit of postoperative adjuvant pelvic radiation in women who had early-stage disease and who met histologic criteria for high risk of recurrence and death. The trials are the EN.5 trial from Canada, and the radiation-therapy randomization of the ASTEC trial). Neither found a benefit in terms of overall survival, disease-specific survival, or recurrence-free survival, although local recurrence was reduced by 2.9% The authors also provide a review of the literature and a meta-analysis of other randomized, controlled trials on this subject.
Details of the EN.5 and ASTEC radiation-therapy trials
Criteria for enrollment were similar for the two trials, which focused on women who had histologically confirmed endometrial cancer and an intermediate or high risk of recurrence. This included women who had FIGO stage IA or stage IB (grade 3), stage IC (all grades), or papillary serous or clear-cell histology (all stages).
Survival is the primary goal of cancer treatment. External-beam radiotherapy does not improve survival, but does provide a small but real increase in local control. Regrettably, this improvement in local control comes at a cost: 3% of patients experience acute severe or life-threatening toxicity from treatment. The absolute difference in local recurrence between women who received external-beam radiotherapy and those who did not was only 2.9%. Local recurrences are largely salvageable in women who have not been irradiated.
Therefore, external-beam radiotherapy, as delivered in this trial, regardless of node status, should not be the standard of care. Improvement in technology with intensity-modulated radiotherapy, and the further evaluation of vaginal brachytherapy alone, may provide new ways to apply this kind of treatment in endometrial cancer.
This aspect of endometrial cancer treatment clearly needs further investigation. Trials are under way that may determine the role of radiation therapy in women who have endometrial cancer.—DAVID G. MUTCH, MD; B. J. RIMEL, MD
Lymphadenectomy was not required for patients enrolled in EN.5, but was part of the surgical randomization for ASTEC. This distinction could confound the results of the combined trials, as the investigators were trying to answer two questions within one patient population.
In both the EN.5 and ASTEC trials, women were randomized to observation or external-beam radiotherapy, with these parameters:
- Radiation therapy was to begin no later than 12 weeks after surgery (most patients began radiation therapy 6 to 8 weeks after surgery)
- For ASTEC, the target dosage was 40–46 Gy in 20–26 daily fractions to the pelvis, with treatment five times each week. For EN.5, the dosage and timing were very similar: 45 Gy, 25 daily fractions, five times weekly
- In both trials, vaginal brachytherapy was allowed if it was the local practice or the center’s policy
- Women were classified as being at intermediate risk or high risk, based on the likelihood of distant recurrence, as defined by GOG99 and PORTEC1 studies. Intermediate risk included all patients who had stage-IA or -IB (grade-3) or stage-IC or -IIA (grade-1 or -2) disease. Women who had papillary serous or clear-cell histology, stage-IC or -IIA (grade-3) disease, or any stage-IIB disease were considered at high risk.
The primary outcome evaluated for both trials was overall survival. Secondary endpoints were:
- disease-specific survival
- recurrence-free survival
- locoregional recurrence
- treatment toxicity.
A total of 905 women were enrolled in the ASTEC and EN.5 trials, with most patients having endometrial histology (83%) and being categorized as at intermediate risk (75%). Approximately half the patients in both trials received brachytherapy, which was allowed according to local practice. Only 47% of the observation group actually received no treatment.
Findings were remarkably similar in EN.5 and ASTEC
Here are the main findings:
- no difference between groups in overall survival, disease-specific survival, and recurrence-free survival
- significantly fewer isolated vaginal or pelvic initial recurrences in the external-beam radiotherapy group, with an absolute difference of 2.9%. (Only 35% of all recurrences were isolated recurrences)
- no significant difference between groups in distant or local and distant recurrences
- as expected, higher toxicity in the group receiving external-beam radiotherapy, including life-threatening toxicity (acute toxicity, 3% vs <1%; late toxicity, 1% vs 0%).
Subgroup analysis comparing overall survival in intermediate- and high-risk patients demonstrated no improvement with external-beam radiotherapy. Nor was overall survival altered by lymphadenectomy. The authors performed a meta-analysis using data from GOG99, PORTEC1, and this combined trial, and found no significant difference in overall survival or disease-specific survival, regardless of histologic risk group.
Trial has notable strengths and weaknesses
This large prospective trial has significant strengths: its size and its multi-institutional nature. The authors also evaluated their data in combination with other randomized, controlled trials to further investigate the effect of external-beam radiotherapy on survival. However, allowing brachytherapy somewhat confounds the true effect of external-beam radiotherapy on local recurrence. (There were few local recurrences, and the authors did not evaluate whether women who had an isolated vaginal recurrence received vaginal brachytherapy.) Moreover, 15% of women who were randomized to external-beam radiotherapy did not complete it.
In addition, secondary randomization of patients in the intermediate-risk and high-risk categories to external-beam radiotherapy versus no treatment may have significantly confounded the results of the entire ASTEC trial. Because women were, or were not, randomized to treatment regardless of node status, some patients who had positive nodes failed to receive adjuvant treatment. This may have had a significant effect on overall survival, as positive lymph nodes are a negative prognostic factor.
1. Keys HM, Roberts JA, Brunetto VL, et al. Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744-751.
2. Bristow RE, Zahurak ML, Alexander CJ, Zellars RC, Montz FJ. FIGO stage IIIC endometrial carcinoma: resection of macroscopic nodal disease and other determinants of survival. Int J Gynecol Cancer. 2003;13:664-672.
Dr. Mutch reports that he has received grant or research support from Lilly and Genentech. He serves as a speaker for GSK, Lilly, and Merck. Dr. Rimel reports no financial relationships relevant to this article.
Endometrial cancer is a great concern in industrialized nations, where it is the most common gynecologic cancer—with incidence increasing every year. Survival is generally very good for women who have low-grade disease confined to the uterus. However, for patients who have high-grade disease, an aggressive histologic type, or other features that suggest a poor prognosis, the cure rate approaches 75%.1
Primary surgery is the mainstay of initial treatment and basis of FIGO staging ( TABLE ), which requires:
- total hysterectomy
- bilateral salpingo-oophorectomy
- complete examination of the abdomen
- pelvic washings
- lymph-adenectomy (anatomic boundaries and node counts aren’t specified).
Controversy clouds our understanding of the optimal type of surgery, utility of pelvic lymphadenectomy, and possible benefit of adjuvant radiation therapy. During the past year, fuel has been added to this debate:
- Two randomized, controlled trials of surgery with and without pelvic lymphadenectomy in early-stage patients demonstrated no survival benefit. Earlier studies investigating the benefits of lymphadenectomy in endometrial cancer have been largely retrospective, and results have varied.
- A concurrent randomized, controlled trial of external-beam radiotherapy for women who have intermediate- or high-risk disease showed no improvement in overall survival, although local control increased by 3%.
TABLE
FIGO surgical staging for endometrial cancer
| Stage | Description |
|---|---|
| I | Tumor is confined to uterine fundus |
| IA | Tumor is limited to endometrium |
| IB | Tumor invades less than half of the myometrial thickness |
| IC | Tumor invades more than half of the myometrial thickness |
| II | Tumor extends to cervix |
| IIA | Cervical extension is limited to endocervical glands |
| IIB | Tumor invades cervical stroma |
| III | There is regional tumor spread |
| IIIA | Tumor invades uterine serosa or adnexa, or cells in the peritoneum show signs of cancer |
| IIIB | Vaginal metastases are present |
| IIIC | Tumor has spread to lymph nodes near the uterus |
| IV | There is bulky pelvic disease or distant spread |
| IVA | Tumor has spread to bladder or rectum |
| IVB | Distant metastases are present |
No survival advantage to pelvic lymphadenectomy—but it has other benefits
ASTEC study group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136.
Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716.
Among the arguments for lymphadenectomy in endometrial cancer staging are:
- It aids in the selection of women for radiation or other adjuvant treatment
- It may have a direct survival benefit, as suggested by retrospective studies.
But lymphadenectomy is time-consuming, requires a specialized gynecologic surgeon, and is associated with some increase in the risk of morbidity—namely, lymphedema, lymphocyst formation, deep-vein thrombosis (DVT), and blood loss.
The much-anticipated report of the 85-center, multinational ASTEC trial [ A S urgical T rial of E ndometrial C ancer], published earlier this year, offers further insight into the practice of lymphadenectomy. ASTEC involved two randomizations: The first, to pelvic lymphadenectomy; the second, to radiation therapy.
The ASTEC trial enrolled 1,408 women who had histologically confirmed endometrial carcinoma that was believed to be confined to the uterus. How this determination was made was not specified. Patients who had enlarged lymph nodes corroborated by computed tomography or magnetic resonance imaging were not excluded.
Participants were randomized to either of the following treatment groups:
- traditional surgery with total hysterectomy and bilateral salpingo-oophorectomy, pelvic washings, and palpation of para-aortic nodes
- the same surgery plus systematic lymphadenectomy of the iliac and obturator nodes.
If any para-aortic nodes were suspicious, biopsy or lymphadenectomy was performed at the discretion of the surgeon ( FIGURE ).
FIGURE Nodes reveal when cancer has spread
Women in the ASTEC trial were randomized to traditional surgery (total hysterectomy and bilateral salpingo-oophorectomy), pelvic washings, and palpation of para-aortic nodes or to the same surgery plus lymphadenectomy of the iliac and obturator nodes.
Operative findings determined a patient’s level of risk
After surgery, patients were categorized as having one of the following:
- low-risk, early-stage disease. This group included patients who had disease classified as stage IA or IB, grade 1 or 2. They were deemed to have a suitably low risk of recurrence to be offered further treatment according to their physician’s standard practice.
- intermediate- or high-risk, early-stage disease. These patients were randomized to the ASTEC radiation-therapy trial, which compared external-beam radiotherapy with no external-beam radiotherapy. The authors assert that this second randomization was necessary to prevent over- or undertreatment of patients who had unknown node status, which might alter survival outcomes.
- advanced disease. These patients were referred to their physician for further treatment.
In both surgical groups (with and without lymphadenectomy), approximately 80% of patients had disease confined to the uterus. Nodes were harvested in 91% of the patients in the lymphadenectomy group, compared with 5% of patients in the traditional-surgery group. Nine percent of women in the lymphadenectomy group had positive nodes.
The authors observe that more women had deeply invasive disease and adverse histologic types in the group that underwent lymphadenectomy. There were no differences between the two groups in overall survival; disease-specific survival; recurrence-free survival; or recurrence-free, disease-specific survival, after adjustment for baseline differences. Subgroup analysis for low-risk, high-risk, and advanced disease also failed to demonstrate differences in overall survival and recurrence-free survival.
Study from Italy produces similar findings
An independent randomized, controlled trial examining survival outcomes for endometrial cancer patients with and without lymphadenectomy was released by the Italian group in late 2008. In this study, 537 patients who had histologically confirmed endometrial carcinoma believed to be confined to the uterus were randomized to total abdominal hysterectomy and bilateral salpingo-oophorectomy with or without pelvic lymphadenectomy.
Anatomic boundaries of the pelvic lymph-node dissection were clearly defined, and a minimum lymph-node count of 20 was specified for inclusion. Intraoperative frozen section was utilized to exclude patients who had grade-1 disease that was less than 50% invasive. The option of para-aortic lymph-node dissection or sampling was left to the discretion of the surgeon. If pelvic nodes were larger than 1 cm, they were removed or sampled regardless of randomization.
Unlike the ASTEC trial, this study did not attempt to control adjuvant treatment. Patients were treated according to the discretion of the physician. Most patients received no further therapy; only 20% underwent radiation therapy, and 7% received chemotherapy.
Given the findings of these two, large, multi-institutional trials with strikingly similar results but major problems, what is a gynecologist to do? Can lymphadenectomy be avoided in patients whose disease is believed to be confined to the uterus?
For now, the answer is a tentative “No.”
There appears to be no survival advantage to removal of lymph nodes when disease is confined to the uterus, but that is not to say there is no benefit to systematic lymphadenectomy—just that there is no survival benefit afforded by the procedure. Benefits of lymphadenectomy, which include more precise definition of the extent of disease, minimization of over- or undertreatment, and a reduction in overall treatment and cost, still remain. The concept of surgical debulking put forward by Bristow and coworkers still has merit, and any gross disease should be removed, if feasible.2
Lymphadenectomy in endometrial cancer remains controversial and complex, especially as we lack a precise method for determining which patients will have nodal disease. Our practice remains to remove the lymph nodes whenever possible to better tailor any adjuvant treatment.—DAVID G. MUTCH, MD; B. J. RIMEL, MD
Women in the lymphadenectomy group were more likely to have stage-IIIC disease, which is directly attributable to histologic evaluation of the lymph nodes in this group. The authors point out that these patients had more accurate assessment of their prognosis, allowing for the tailoring of adjuvant treatment.
The overall survival and disease-free survival curves for the two experimental groups were similar, consistent with data from the ASTEC trial. This proved to be true for both the intention-to-treat and according-to-protocol groups. The authors note that their results are similar to those of the ASTEC trial, despite the significant difference in the number of nodes removed in each trial.
Some aspects of the trials hamper interpretation and comparison
Outcomes are improved when surgery is performed by a trained gynecologic oncologist. In the ASTEC trial, each lymphadenectomy was performed by a specialized gynecologic surgeon who was “skilled in the procedure.” In the Italian study, the type of surgeon was not specified, but the specific anatomic boundaries of the dissection and the minimum node count were. More specific data are needed before any conclusions can be drawn about the effect of surgical skill on outcome in these trials.
In the ASTEC trial, 9% of patients in the lymphadenectomy group had no nodes removed, and more than 60% of patients would not have met criteria for inclusion in the lymphadenectomy arm of the Italian study—suggesting that the majority of women in the ASTEC trial had inadequate lymphadenectomy. Para-aortic lymphadenectomy was left to the discretion of the attending surgeon, and some patients did have resection of these nodes. The data do not include information about whether these patients were treated in the para-aortic region based on the histology of these nodes.
Randomization in a prospective study is supposed to equalize the risks between groups. In the ASTEC trial, despite randomization, there were 10% more patients who had deeply invasive disease in the lymphadenectomy group, along with 3% more adverse histologies and high-grade (grade-3) tumors. Given the higher incidence of positive nodes and poorer outcome in these cases, this difference may have had a significant impact on the evaluation of the groups for overall or disease-specific survival.
External-beam radiotherapy reduces local recurrence of endometrial Ca but does not improve survival
ASTEC/EN.5 Study Group, Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–146.
Radiation therapy has been a standard treatment for endometrial cancer when there is high risk of recurrence. This report combines two independent randomized, controlled trials investigating the benefit of postoperative adjuvant pelvic radiation in women who had early-stage disease and who met histologic criteria for high risk of recurrence and death. The trials are the EN.5 trial from Canada, and the radiation-therapy randomization of the ASTEC trial). Neither found a benefit in terms of overall survival, disease-specific survival, or recurrence-free survival, although local recurrence was reduced by 2.9% The authors also provide a review of the literature and a meta-analysis of other randomized, controlled trials on this subject.
Details of the EN.5 and ASTEC radiation-therapy trials
Criteria for enrollment were similar for the two trials, which focused on women who had histologically confirmed endometrial cancer and an intermediate or high risk of recurrence. This included women who had FIGO stage IA or stage IB (grade 3), stage IC (all grades), or papillary serous or clear-cell histology (all stages).
Survival is the primary goal of cancer treatment. External-beam radiotherapy does not improve survival, but does provide a small but real increase in local control. Regrettably, this improvement in local control comes at a cost: 3% of patients experience acute severe or life-threatening toxicity from treatment. The absolute difference in local recurrence between women who received external-beam radiotherapy and those who did not was only 2.9%. Local recurrences are largely salvageable in women who have not been irradiated.
Therefore, external-beam radiotherapy, as delivered in this trial, regardless of node status, should not be the standard of care. Improvement in technology with intensity-modulated radiotherapy, and the further evaluation of vaginal brachytherapy alone, may provide new ways to apply this kind of treatment in endometrial cancer.
This aspect of endometrial cancer treatment clearly needs further investigation. Trials are under way that may determine the role of radiation therapy in women who have endometrial cancer.—DAVID G. MUTCH, MD; B. J. RIMEL, MD
Lymphadenectomy was not required for patients enrolled in EN.5, but was part of the surgical randomization for ASTEC. This distinction could confound the results of the combined trials, as the investigators were trying to answer two questions within one patient population.
In both the EN.5 and ASTEC trials, women were randomized to observation or external-beam radiotherapy, with these parameters:
- Radiation therapy was to begin no later than 12 weeks after surgery (most patients began radiation therapy 6 to 8 weeks after surgery)
- For ASTEC, the target dosage was 40–46 Gy in 20–26 daily fractions to the pelvis, with treatment five times each week. For EN.5, the dosage and timing were very similar: 45 Gy, 25 daily fractions, five times weekly
- In both trials, vaginal brachytherapy was allowed if it was the local practice or the center’s policy
- Women were classified as being at intermediate risk or high risk, based on the likelihood of distant recurrence, as defined by GOG99 and PORTEC1 studies. Intermediate risk included all patients who had stage-IA or -IB (grade-3) or stage-IC or -IIA (grade-1 or -2) disease. Women who had papillary serous or clear-cell histology, stage-IC or -IIA (grade-3) disease, or any stage-IIB disease were considered at high risk.
The primary outcome evaluated for both trials was overall survival. Secondary endpoints were:
- disease-specific survival
- recurrence-free survival
- locoregional recurrence
- treatment toxicity.
A total of 905 women were enrolled in the ASTEC and EN.5 trials, with most patients having endometrial histology (83%) and being categorized as at intermediate risk (75%). Approximately half the patients in both trials received brachytherapy, which was allowed according to local practice. Only 47% of the observation group actually received no treatment.
Findings were remarkably similar in EN.5 and ASTEC
Here are the main findings:
- no difference between groups in overall survival, disease-specific survival, and recurrence-free survival
- significantly fewer isolated vaginal or pelvic initial recurrences in the external-beam radiotherapy group, with an absolute difference of 2.9%. (Only 35% of all recurrences were isolated recurrences)
- no significant difference between groups in distant or local and distant recurrences
- as expected, higher toxicity in the group receiving external-beam radiotherapy, including life-threatening toxicity (acute toxicity, 3% vs <1%; late toxicity, 1% vs 0%).
Subgroup analysis comparing overall survival in intermediate- and high-risk patients demonstrated no improvement with external-beam radiotherapy. Nor was overall survival altered by lymphadenectomy. The authors performed a meta-analysis using data from GOG99, PORTEC1, and this combined trial, and found no significant difference in overall survival or disease-specific survival, regardless of histologic risk group.
Trial has notable strengths and weaknesses
This large prospective trial has significant strengths: its size and its multi-institutional nature. The authors also evaluated their data in combination with other randomized, controlled trials to further investigate the effect of external-beam radiotherapy on survival. However, allowing brachytherapy somewhat confounds the true effect of external-beam radiotherapy on local recurrence. (There were few local recurrences, and the authors did not evaluate whether women who had an isolated vaginal recurrence received vaginal brachytherapy.) Moreover, 15% of women who were randomized to external-beam radiotherapy did not complete it.
In addition, secondary randomization of patients in the intermediate-risk and high-risk categories to external-beam radiotherapy versus no treatment may have significantly confounded the results of the entire ASTEC trial. Because women were, or were not, randomized to treatment regardless of node status, some patients who had positive nodes failed to receive adjuvant treatment. This may have had a significant effect on overall survival, as positive lymph nodes are a negative prognostic factor.
Dr. Mutch reports that he has received grant or research support from Lilly and Genentech. He serves as a speaker for GSK, Lilly, and Merck. Dr. Rimel reports no financial relationships relevant to this article.
Endometrial cancer is a great concern in industrialized nations, where it is the most common gynecologic cancer—with incidence increasing every year. Survival is generally very good for women who have low-grade disease confined to the uterus. However, for patients who have high-grade disease, an aggressive histologic type, or other features that suggest a poor prognosis, the cure rate approaches 75%.1
Primary surgery is the mainstay of initial treatment and basis of FIGO staging ( TABLE ), which requires:
- total hysterectomy
- bilateral salpingo-oophorectomy
- complete examination of the abdomen
- pelvic washings
- lymph-adenectomy (anatomic boundaries and node counts aren’t specified).
Controversy clouds our understanding of the optimal type of surgery, utility of pelvic lymphadenectomy, and possible benefit of adjuvant radiation therapy. During the past year, fuel has been added to this debate:
- Two randomized, controlled trials of surgery with and without pelvic lymphadenectomy in early-stage patients demonstrated no survival benefit. Earlier studies investigating the benefits of lymphadenectomy in endometrial cancer have been largely retrospective, and results have varied.
- A concurrent randomized, controlled trial of external-beam radiotherapy for women who have intermediate- or high-risk disease showed no improvement in overall survival, although local control increased by 3%.
TABLE
FIGO surgical staging for endometrial cancer
| Stage | Description |
|---|---|
| I | Tumor is confined to uterine fundus |
| IA | Tumor is limited to endometrium |
| IB | Tumor invades less than half of the myometrial thickness |
| IC | Tumor invades more than half of the myometrial thickness |
| II | Tumor extends to cervix |
| IIA | Cervical extension is limited to endocervical glands |
| IIB | Tumor invades cervical stroma |
| III | There is regional tumor spread |
| IIIA | Tumor invades uterine serosa or adnexa, or cells in the peritoneum show signs of cancer |
| IIIB | Vaginal metastases are present |
| IIIC | Tumor has spread to lymph nodes near the uterus |
| IV | There is bulky pelvic disease or distant spread |
| IVA | Tumor has spread to bladder or rectum |
| IVB | Distant metastases are present |
No survival advantage to pelvic lymphadenectomy—but it has other benefits
ASTEC study group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136.
Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716.
Among the arguments for lymphadenectomy in endometrial cancer staging are:
- It aids in the selection of women for radiation or other adjuvant treatment
- It may have a direct survival benefit, as suggested by retrospective studies.
But lymphadenectomy is time-consuming, requires a specialized gynecologic surgeon, and is associated with some increase in the risk of morbidity—namely, lymphedema, lymphocyst formation, deep-vein thrombosis (DVT), and blood loss.
The much-anticipated report of the 85-center, multinational ASTEC trial [ A S urgical T rial of E ndometrial C ancer], published earlier this year, offers further insight into the practice of lymphadenectomy. ASTEC involved two randomizations: The first, to pelvic lymphadenectomy; the second, to radiation therapy.
The ASTEC trial enrolled 1,408 women who had histologically confirmed endometrial carcinoma that was believed to be confined to the uterus. How this determination was made was not specified. Patients who had enlarged lymph nodes corroborated by computed tomography or magnetic resonance imaging were not excluded.
Participants were randomized to either of the following treatment groups:
- traditional surgery with total hysterectomy and bilateral salpingo-oophorectomy, pelvic washings, and palpation of para-aortic nodes
- the same surgery plus systematic lymphadenectomy of the iliac and obturator nodes.
If any para-aortic nodes were suspicious, biopsy or lymphadenectomy was performed at the discretion of the surgeon ( FIGURE ).
FIGURE Nodes reveal when cancer has spread
Women in the ASTEC trial were randomized to traditional surgery (total hysterectomy and bilateral salpingo-oophorectomy), pelvic washings, and palpation of para-aortic nodes or to the same surgery plus lymphadenectomy of the iliac and obturator nodes.
Operative findings determined a patient’s level of risk
After surgery, patients were categorized as having one of the following:
- low-risk, early-stage disease. This group included patients who had disease classified as stage IA or IB, grade 1 or 2. They were deemed to have a suitably low risk of recurrence to be offered further treatment according to their physician’s standard practice.
- intermediate- or high-risk, early-stage disease. These patients were randomized to the ASTEC radiation-therapy trial, which compared external-beam radiotherapy with no external-beam radiotherapy. The authors assert that this second randomization was necessary to prevent over- or undertreatment of patients who had unknown node status, which might alter survival outcomes.
- advanced disease. These patients were referred to their physician for further treatment.
In both surgical groups (with and without lymphadenectomy), approximately 80% of patients had disease confined to the uterus. Nodes were harvested in 91% of the patients in the lymphadenectomy group, compared with 5% of patients in the traditional-surgery group. Nine percent of women in the lymphadenectomy group had positive nodes.
The authors observe that more women had deeply invasive disease and adverse histologic types in the group that underwent lymphadenectomy. There were no differences between the two groups in overall survival; disease-specific survival; recurrence-free survival; or recurrence-free, disease-specific survival, after adjustment for baseline differences. Subgroup analysis for low-risk, high-risk, and advanced disease also failed to demonstrate differences in overall survival and recurrence-free survival.
Study from Italy produces similar findings
An independent randomized, controlled trial examining survival outcomes for endometrial cancer patients with and without lymphadenectomy was released by the Italian group in late 2008. In this study, 537 patients who had histologically confirmed endometrial carcinoma believed to be confined to the uterus were randomized to total abdominal hysterectomy and bilateral salpingo-oophorectomy with or without pelvic lymphadenectomy.
Anatomic boundaries of the pelvic lymph-node dissection were clearly defined, and a minimum lymph-node count of 20 was specified for inclusion. Intraoperative frozen section was utilized to exclude patients who had grade-1 disease that was less than 50% invasive. The option of para-aortic lymph-node dissection or sampling was left to the discretion of the surgeon. If pelvic nodes were larger than 1 cm, they were removed or sampled regardless of randomization.
Unlike the ASTEC trial, this study did not attempt to control adjuvant treatment. Patients were treated according to the discretion of the physician. Most patients received no further therapy; only 20% underwent radiation therapy, and 7% received chemotherapy.
Given the findings of these two, large, multi-institutional trials with strikingly similar results but major problems, what is a gynecologist to do? Can lymphadenectomy be avoided in patients whose disease is believed to be confined to the uterus?
For now, the answer is a tentative “No.”
There appears to be no survival advantage to removal of lymph nodes when disease is confined to the uterus, but that is not to say there is no benefit to systematic lymphadenectomy—just that there is no survival benefit afforded by the procedure. Benefits of lymphadenectomy, which include more precise definition of the extent of disease, minimization of over- or undertreatment, and a reduction in overall treatment and cost, still remain. The concept of surgical debulking put forward by Bristow and coworkers still has merit, and any gross disease should be removed, if feasible.2
Lymphadenectomy in endometrial cancer remains controversial and complex, especially as we lack a precise method for determining which patients will have nodal disease. Our practice remains to remove the lymph nodes whenever possible to better tailor any adjuvant treatment.—DAVID G. MUTCH, MD; B. J. RIMEL, MD
Women in the lymphadenectomy group were more likely to have stage-IIIC disease, which is directly attributable to histologic evaluation of the lymph nodes in this group. The authors point out that these patients had more accurate assessment of their prognosis, allowing for the tailoring of adjuvant treatment.
The overall survival and disease-free survival curves for the two experimental groups were similar, consistent with data from the ASTEC trial. This proved to be true for both the intention-to-treat and according-to-protocol groups. The authors note that their results are similar to those of the ASTEC trial, despite the significant difference in the number of nodes removed in each trial.
Some aspects of the trials hamper interpretation and comparison
Outcomes are improved when surgery is performed by a trained gynecologic oncologist. In the ASTEC trial, each lymphadenectomy was performed by a specialized gynecologic surgeon who was “skilled in the procedure.” In the Italian study, the type of surgeon was not specified, but the specific anatomic boundaries of the dissection and the minimum node count were. More specific data are needed before any conclusions can be drawn about the effect of surgical skill on outcome in these trials.
In the ASTEC trial, 9% of patients in the lymphadenectomy group had no nodes removed, and more than 60% of patients would not have met criteria for inclusion in the lymphadenectomy arm of the Italian study—suggesting that the majority of women in the ASTEC trial had inadequate lymphadenectomy. Para-aortic lymphadenectomy was left to the discretion of the attending surgeon, and some patients did have resection of these nodes. The data do not include information about whether these patients were treated in the para-aortic region based on the histology of these nodes.
Randomization in a prospective study is supposed to equalize the risks between groups. In the ASTEC trial, despite randomization, there were 10% more patients who had deeply invasive disease in the lymphadenectomy group, along with 3% more adverse histologies and high-grade (grade-3) tumors. Given the higher incidence of positive nodes and poorer outcome in these cases, this difference may have had a significant impact on the evaluation of the groups for overall or disease-specific survival.
External-beam radiotherapy reduces local recurrence of endometrial Ca but does not improve survival
ASTEC/EN.5 Study Group, Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–146.
Radiation therapy has been a standard treatment for endometrial cancer when there is high risk of recurrence. This report combines two independent randomized, controlled trials investigating the benefit of postoperative adjuvant pelvic radiation in women who had early-stage disease and who met histologic criteria for high risk of recurrence and death. The trials are the EN.5 trial from Canada, and the radiation-therapy randomization of the ASTEC trial). Neither found a benefit in terms of overall survival, disease-specific survival, or recurrence-free survival, although local recurrence was reduced by 2.9% The authors also provide a review of the literature and a meta-analysis of other randomized, controlled trials on this subject.
Details of the EN.5 and ASTEC radiation-therapy trials
Criteria for enrollment were similar for the two trials, which focused on women who had histologically confirmed endometrial cancer and an intermediate or high risk of recurrence. This included women who had FIGO stage IA or stage IB (grade 3), stage IC (all grades), or papillary serous or clear-cell histology (all stages).
Survival is the primary goal of cancer treatment. External-beam radiotherapy does not improve survival, but does provide a small but real increase in local control. Regrettably, this improvement in local control comes at a cost: 3% of patients experience acute severe or life-threatening toxicity from treatment. The absolute difference in local recurrence between women who received external-beam radiotherapy and those who did not was only 2.9%. Local recurrences are largely salvageable in women who have not been irradiated.
Therefore, external-beam radiotherapy, as delivered in this trial, regardless of node status, should not be the standard of care. Improvement in technology with intensity-modulated radiotherapy, and the further evaluation of vaginal brachytherapy alone, may provide new ways to apply this kind of treatment in endometrial cancer.
This aspect of endometrial cancer treatment clearly needs further investigation. Trials are under way that may determine the role of radiation therapy in women who have endometrial cancer.—DAVID G. MUTCH, MD; B. J. RIMEL, MD
Lymphadenectomy was not required for patients enrolled in EN.5, but was part of the surgical randomization for ASTEC. This distinction could confound the results of the combined trials, as the investigators were trying to answer two questions within one patient population.
In both the EN.5 and ASTEC trials, women were randomized to observation or external-beam radiotherapy, with these parameters:
- Radiation therapy was to begin no later than 12 weeks after surgery (most patients began radiation therapy 6 to 8 weeks after surgery)
- For ASTEC, the target dosage was 40–46 Gy in 20–26 daily fractions to the pelvis, with treatment five times each week. For EN.5, the dosage and timing were very similar: 45 Gy, 25 daily fractions, five times weekly
- In both trials, vaginal brachytherapy was allowed if it was the local practice or the center’s policy
- Women were classified as being at intermediate risk or high risk, based on the likelihood of distant recurrence, as defined by GOG99 and PORTEC1 studies. Intermediate risk included all patients who had stage-IA or -IB (grade-3) or stage-IC or -IIA (grade-1 or -2) disease. Women who had papillary serous or clear-cell histology, stage-IC or -IIA (grade-3) disease, or any stage-IIB disease were considered at high risk.
The primary outcome evaluated for both trials was overall survival. Secondary endpoints were:
- disease-specific survival
- recurrence-free survival
- locoregional recurrence
- treatment toxicity.
A total of 905 women were enrolled in the ASTEC and EN.5 trials, with most patients having endometrial histology (83%) and being categorized as at intermediate risk (75%). Approximately half the patients in both trials received brachytherapy, which was allowed according to local practice. Only 47% of the observation group actually received no treatment.
Findings were remarkably similar in EN.5 and ASTEC
Here are the main findings:
- no difference between groups in overall survival, disease-specific survival, and recurrence-free survival
- significantly fewer isolated vaginal or pelvic initial recurrences in the external-beam radiotherapy group, with an absolute difference of 2.9%. (Only 35% of all recurrences were isolated recurrences)
- no significant difference between groups in distant or local and distant recurrences
- as expected, higher toxicity in the group receiving external-beam radiotherapy, including life-threatening toxicity (acute toxicity, 3% vs <1%; late toxicity, 1% vs 0%).
Subgroup analysis comparing overall survival in intermediate- and high-risk patients demonstrated no improvement with external-beam radiotherapy. Nor was overall survival altered by lymphadenectomy. The authors performed a meta-analysis using data from GOG99, PORTEC1, and this combined trial, and found no significant difference in overall survival or disease-specific survival, regardless of histologic risk group.
Trial has notable strengths and weaknesses
This large prospective trial has significant strengths: its size and its multi-institutional nature. The authors also evaluated their data in combination with other randomized, controlled trials to further investigate the effect of external-beam radiotherapy on survival. However, allowing brachytherapy somewhat confounds the true effect of external-beam radiotherapy on local recurrence. (There were few local recurrences, and the authors did not evaluate whether women who had an isolated vaginal recurrence received vaginal brachytherapy.) Moreover, 15% of women who were randomized to external-beam radiotherapy did not complete it.
In addition, secondary randomization of patients in the intermediate-risk and high-risk categories to external-beam radiotherapy versus no treatment may have significantly confounded the results of the entire ASTEC trial. Because women were, or were not, randomized to treatment regardless of node status, some patients who had positive nodes failed to receive adjuvant treatment. This may have had a significant effect on overall survival, as positive lymph nodes are a negative prognostic factor.
1. Keys HM, Roberts JA, Brunetto VL, et al. Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744-751.
2. Bristow RE, Zahurak ML, Alexander CJ, Zellars RC, Montz FJ. FIGO stage IIIC endometrial carcinoma: resection of macroscopic nodal disease and other determinants of survival. Int J Gynecol Cancer. 2003;13:664-672.
1. Keys HM, Roberts JA, Brunetto VL, et al. Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744-751.
2. Bristow RE, Zahurak ML, Alexander CJ, Zellars RC, Montz FJ. FIGO stage IIIC endometrial carcinoma: resection of macroscopic nodal disease and other determinants of survival. Int J Gynecol Cancer. 2003;13:664-672.