User login
Time to rethink endometrial ablation: A gyn oncology perspective on the sequelae of an overused procedure
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
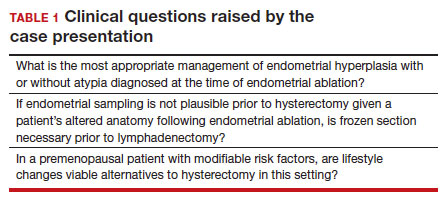
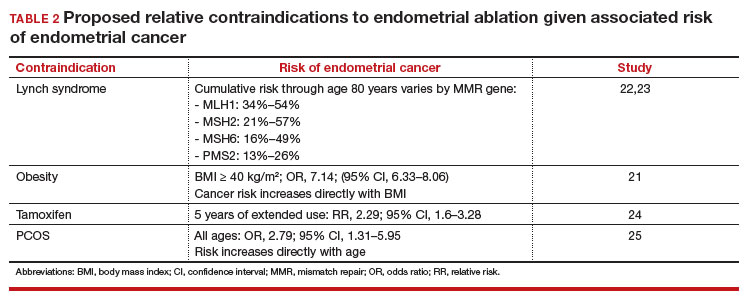
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.
Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
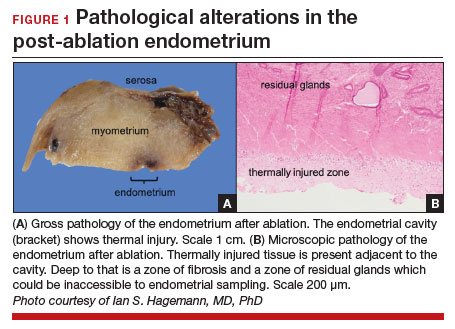
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.
Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.
Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
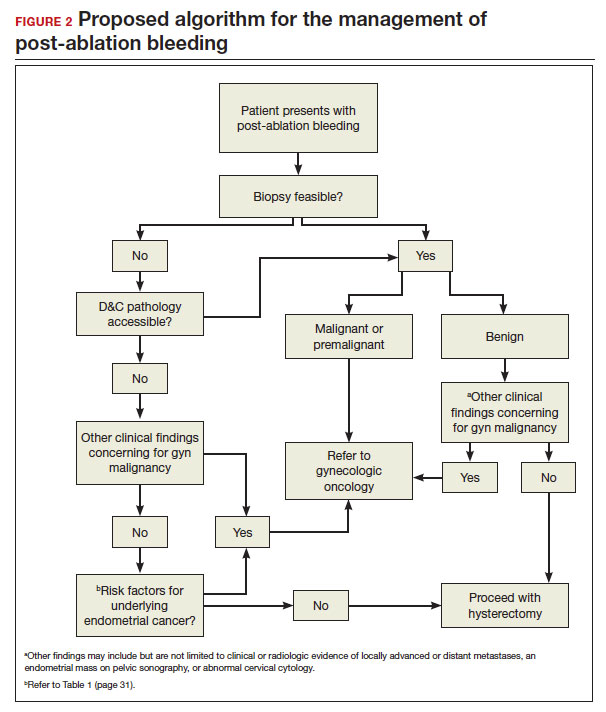
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.
CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.

Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.

Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.

Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.

CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.

Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.

Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.

Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.

CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
Multi-cancer early detection liquid biopsy testing: A predictive genetic test not quite ready for prime time
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?
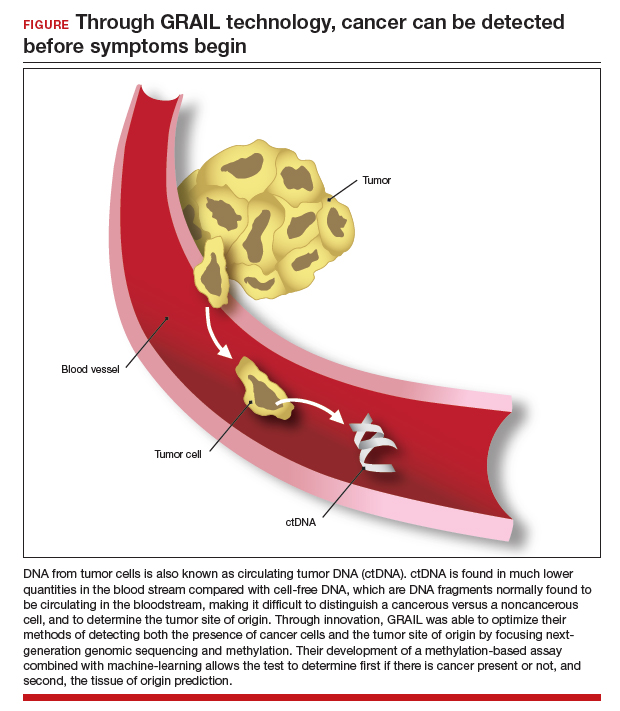
The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?

The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?

The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
Defending access to reproductive health care
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
Cervical cancer: A path to eradication
David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.

David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●

David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.
Should secondary cytoreduction be performed for platinum-sensitive recurrent ovarian cancer?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
Morcellation use in gynecologic surgery: Current clinical recommendations and cautions
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7
Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7

Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7

Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
Should we abandon minimally invasive surgery for cervical cancer?
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
Does low-dose aspirin decrease a woman’s risk of ovarian cancer?
EXPERT COMMENTARY
Epidemiologic studies conducted in ovarian cancer suggest an association between chronic inflammation and incidence of disease.1 Nonsteroidal anti-inflammatory drugs (NSAIDs) work to decrease inflammation through the inhibition of cyclo-oxygenase (COX). Therefore, anti-inflammatory agents such as NSAIDs have been proposed to play a role in the pathophysiology of ovarian cancer.
Previous studies of this association show conflicting data. The majority of these studies are retrospective, and those that are prospective do not include detailed data regarding dosing and frequency of ASA use.2-6
_
Details of the study
This study by Barnard and colleagues is a prospective cohort study evaluating a total of 205,498 women from 1980–2015 from 2 separate cohorts (the Nurses’ Health Study and the Nurses’ Health Study II). The primary outcome was “to evaluate whether regular aspirin or nonaspirin NSAID use and patterns of use are associated with lower ovarian cancer risk.” Analgesic use and data regarding covariates were obtained via self-reported questionnaires. Ovarian cancer diagnosis was confirmed via medical records.
Results demonstrated that current low-dose aspirin use was associated with a decreased risk of ovarian cancer (hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.61–0.96). This significance was not maintained upon further controlling for inflammatory factors (hypertension, autoimmune disease, inflammatory diet scores, smoking, etc) (HR, 0.94; 95% CI, 0.69–1.26). Other significant findings included an increased risk of developing ovarian cancer with standard-dose ASA use of ≥5 years or standard-dose use at 6 to 9 tablets per week (HR, 1.77; 95% CI, 1.13–2.77 and HR, 2.00; 95% CI, 1.27–3.15, respectively). An increased risk of developing ovarian cancer also was found for >10-year use or use of >10 tablets per week of nonaspirin NSAIDs (HR, 2.00; 95% CI, 1.27–3.15 and HR, 1.35; 95% CI, 1.02–1.79, respectively).
The authors concluded that there was a slight inverse association for low-dose aspirin and ovarian cancer risk and that standard aspirin or NSAID use actually may be associated with an increased risk of ovarian cancer.
Study strengths and weaknesses
This study has many strengths. It was a large prospective cohort investigation with adequate power to detect clinically significant differences. The authors collected detailed exposure data, which was novel. They also considered a latency period prior to the diagnosis of ovarian cancer during which a patient may increase their analgesic use in order to treat pain caused by the impending cancer.
However, the conclusions of the authors seem to be overstated in the setting of the data. Specifically, the deduction regarding a decreased risk of ovarian cancer with low-dose aspirin use given the loss of the statistical significance when controlling for pertinent cofounders. Further, the study authors did not evaluate adverse effects associated with low-dose aspirin use, which would be clinically applicable when determining whether the results from this study should become formal recommendations. Lastly, other important clinical factors, such as the presence of genetic mutations or endometriosis, were not considered, and these considerations would greatly affect results.
In the setting of previous large prospective studies that suggest no association between ASA use and ovarian cancer risk,4-6 data from this study are not compelling enough to recommend regular low-dose aspirin use to all women.
Based on these current data, there is insufficient evidence to suggest the use of low-dose aspirin for chemoprophylaxis of ovarian cancer. In order to suggest the use of a drug for prophylaxis the benefits must outweigh the risks, and in the case of NSAIDs, this has yet to be confirmed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Poole EM, Lee IM, Ridker PM, et al. A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor alpha receptor 2 levels and risk of ovarian cancer. Am J Epidemiol. 2013;178:1256-1264.
- Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
- Peres LC, Camacho F, Abbott SE, et al. Analgesic medication use and risk of epithelial ovarian cancer in African American women. Br J Cancer. 2016;114(7):819-825.
- Murphy MA, Trabert B, Yang HP, et al. Non-steroidal antiinflammatory drug use and ovarian cancer risk: findings from the NIH-AARP Diet and Health Study and systematic review. Cancer Causes Control. 2012;23:1839-1852.
- Brasky TM, Liu J, White E, et al. Non-steroidal antiinflammatory drugs and cancer risk in women: results from the Women’s Health Initiative. Int J Cancer. 2014;135:1869-1883.
- Lacey JV Jr, Sherman ME, Hartge P, et al. Medication use and risk of ovarian carcinoma: a prospective study. Int J Cancer. 2004;108:281-286.
EXPERT COMMENTARY
Epidemiologic studies conducted in ovarian cancer suggest an association between chronic inflammation and incidence of disease.1 Nonsteroidal anti-inflammatory drugs (NSAIDs) work to decrease inflammation through the inhibition of cyclo-oxygenase (COX). Therefore, anti-inflammatory agents such as NSAIDs have been proposed to play a role in the pathophysiology of ovarian cancer.
Previous studies of this association show conflicting data. The majority of these studies are retrospective, and those that are prospective do not include detailed data regarding dosing and frequency of ASA use.2-6
_
Details of the study
This study by Barnard and colleagues is a prospective cohort study evaluating a total of 205,498 women from 1980–2015 from 2 separate cohorts (the Nurses’ Health Study and the Nurses’ Health Study II). The primary outcome was “to evaluate whether regular aspirin or nonaspirin NSAID use and patterns of use are associated with lower ovarian cancer risk.” Analgesic use and data regarding covariates were obtained via self-reported questionnaires. Ovarian cancer diagnosis was confirmed via medical records.
Results demonstrated that current low-dose aspirin use was associated with a decreased risk of ovarian cancer (hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.61–0.96). This significance was not maintained upon further controlling for inflammatory factors (hypertension, autoimmune disease, inflammatory diet scores, smoking, etc) (HR, 0.94; 95% CI, 0.69–1.26). Other significant findings included an increased risk of developing ovarian cancer with standard-dose ASA use of ≥5 years or standard-dose use at 6 to 9 tablets per week (HR, 1.77; 95% CI, 1.13–2.77 and HR, 2.00; 95% CI, 1.27–3.15, respectively). An increased risk of developing ovarian cancer also was found for >10-year use or use of >10 tablets per week of nonaspirin NSAIDs (HR, 2.00; 95% CI, 1.27–3.15 and HR, 1.35; 95% CI, 1.02–1.79, respectively).
The authors concluded that there was a slight inverse association for low-dose aspirin and ovarian cancer risk and that standard aspirin or NSAID use actually may be associated with an increased risk of ovarian cancer.
Study strengths and weaknesses
This study has many strengths. It was a large prospective cohort investigation with adequate power to detect clinically significant differences. The authors collected detailed exposure data, which was novel. They also considered a latency period prior to the diagnosis of ovarian cancer during which a patient may increase their analgesic use in order to treat pain caused by the impending cancer.
However, the conclusions of the authors seem to be overstated in the setting of the data. Specifically, the deduction regarding a decreased risk of ovarian cancer with low-dose aspirin use given the loss of the statistical significance when controlling for pertinent cofounders. Further, the study authors did not evaluate adverse effects associated with low-dose aspirin use, which would be clinically applicable when determining whether the results from this study should become formal recommendations. Lastly, other important clinical factors, such as the presence of genetic mutations or endometriosis, were not considered, and these considerations would greatly affect results.
In the setting of previous large prospective studies that suggest no association between ASA use and ovarian cancer risk,4-6 data from this study are not compelling enough to recommend regular low-dose aspirin use to all women.
Based on these current data, there is insufficient evidence to suggest the use of low-dose aspirin for chemoprophylaxis of ovarian cancer. In order to suggest the use of a drug for prophylaxis the benefits must outweigh the risks, and in the case of NSAIDs, this has yet to be confirmed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Epidemiologic studies conducted in ovarian cancer suggest an association between chronic inflammation and incidence of disease.1 Nonsteroidal anti-inflammatory drugs (NSAIDs) work to decrease inflammation through the inhibition of cyclo-oxygenase (COX). Therefore, anti-inflammatory agents such as NSAIDs have been proposed to play a role in the pathophysiology of ovarian cancer.
Previous studies of this association show conflicting data. The majority of these studies are retrospective, and those that are prospective do not include detailed data regarding dosing and frequency of ASA use.2-6
_
Details of the study
This study by Barnard and colleagues is a prospective cohort study evaluating a total of 205,498 women from 1980–2015 from 2 separate cohorts (the Nurses’ Health Study and the Nurses’ Health Study II). The primary outcome was “to evaluate whether regular aspirin or nonaspirin NSAID use and patterns of use are associated with lower ovarian cancer risk.” Analgesic use and data regarding covariates were obtained via self-reported questionnaires. Ovarian cancer diagnosis was confirmed via medical records.
Results demonstrated that current low-dose aspirin use was associated with a decreased risk of ovarian cancer (hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.61–0.96). This significance was not maintained upon further controlling for inflammatory factors (hypertension, autoimmune disease, inflammatory diet scores, smoking, etc) (HR, 0.94; 95% CI, 0.69–1.26). Other significant findings included an increased risk of developing ovarian cancer with standard-dose ASA use of ≥5 years or standard-dose use at 6 to 9 tablets per week (HR, 1.77; 95% CI, 1.13–2.77 and HR, 2.00; 95% CI, 1.27–3.15, respectively). An increased risk of developing ovarian cancer also was found for >10-year use or use of >10 tablets per week of nonaspirin NSAIDs (HR, 2.00; 95% CI, 1.27–3.15 and HR, 1.35; 95% CI, 1.02–1.79, respectively).
The authors concluded that there was a slight inverse association for low-dose aspirin and ovarian cancer risk and that standard aspirin or NSAID use actually may be associated with an increased risk of ovarian cancer.
Study strengths and weaknesses
This study has many strengths. It was a large prospective cohort investigation with adequate power to detect clinically significant differences. The authors collected detailed exposure data, which was novel. They also considered a latency period prior to the diagnosis of ovarian cancer during which a patient may increase their analgesic use in order to treat pain caused by the impending cancer.
However, the conclusions of the authors seem to be overstated in the setting of the data. Specifically, the deduction regarding a decreased risk of ovarian cancer with low-dose aspirin use given the loss of the statistical significance when controlling for pertinent cofounders. Further, the study authors did not evaluate adverse effects associated with low-dose aspirin use, which would be clinically applicable when determining whether the results from this study should become formal recommendations. Lastly, other important clinical factors, such as the presence of genetic mutations or endometriosis, were not considered, and these considerations would greatly affect results.
In the setting of previous large prospective studies that suggest no association between ASA use and ovarian cancer risk,4-6 data from this study are not compelling enough to recommend regular low-dose aspirin use to all women.
Based on these current data, there is insufficient evidence to suggest the use of low-dose aspirin for chemoprophylaxis of ovarian cancer. In order to suggest the use of a drug for prophylaxis the benefits must outweigh the risks, and in the case of NSAIDs, this has yet to be confirmed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Poole EM, Lee IM, Ridker PM, et al. A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor alpha receptor 2 levels and risk of ovarian cancer. Am J Epidemiol. 2013;178:1256-1264.
- Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
- Peres LC, Camacho F, Abbott SE, et al. Analgesic medication use and risk of epithelial ovarian cancer in African American women. Br J Cancer. 2016;114(7):819-825.
- Murphy MA, Trabert B, Yang HP, et al. Non-steroidal antiinflammatory drug use and ovarian cancer risk: findings from the NIH-AARP Diet and Health Study and systematic review. Cancer Causes Control. 2012;23:1839-1852.
- Brasky TM, Liu J, White E, et al. Non-steroidal antiinflammatory drugs and cancer risk in women: results from the Women’s Health Initiative. Int J Cancer. 2014;135:1869-1883.
- Lacey JV Jr, Sherman ME, Hartge P, et al. Medication use and risk of ovarian carcinoma: a prospective study. Int J Cancer. 2004;108:281-286.
- Poole EM, Lee IM, Ridker PM, et al. A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor alpha receptor 2 levels and risk of ovarian cancer. Am J Epidemiol. 2013;178:1256-1264.
- Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
- Peres LC, Camacho F, Abbott SE, et al. Analgesic medication use and risk of epithelial ovarian cancer in African American women. Br J Cancer. 2016;114(7):819-825.
- Murphy MA, Trabert B, Yang HP, et al. Non-steroidal antiinflammatory drug use and ovarian cancer risk: findings from the NIH-AARP Diet and Health Study and systematic review. Cancer Causes Control. 2012;23:1839-1852.
- Brasky TM, Liu J, White E, et al. Non-steroidal antiinflammatory drugs and cancer risk in women: results from the Women’s Health Initiative. Int J Cancer. 2014;135:1869-1883.
- Lacey JV Jr, Sherman ME, Hartge P, et al. Medication use and risk of ovarian carcinoma: a prospective study. Int J Cancer. 2004;108:281-286.
Does laparoscopic versus open abdominal surgery for stage I endometrial cancer affect oncologic outcomes?
EXPERT COMMENTARY
The objective of the study by Janda and colleagues (known as the “LACE” trial) was to evaluate the equivalency of total laparoscopic hysterectomy (TLH) with staging versus the standard procedure, which is total abdominal hysterectomy (TAH) with staging, for surgical management of women with presumed low-risk, early-stage endometrial cancer.
Related Article:
2016 Update on cancer
Details of the study
This nonblinded, randomized controlled multicenter equivalency trial included 760 women from Australia, New Zealand, and Hong Kong undergoing surgical management of presumed stage I uterine endometrioid adenocarcinoma. All surgeries were performed or supervised by trained gynecologic oncologists. Pelvic lymph node sampling was required but omission was permitted for: morbid obesity, low risk of metastasis based on frozen section results, medically unfit status, or institutional guidelines prohibiting the procedure. Patients were excluded for preoperative nonendometrioid histology, suspected ultimate FIGO stage II–IV based on preoperative imaging, or uterine size greater than 10 weeks’ gestation.
The primary outcome was disease-free survival, defined as the time from surgery to the date of first recurrence, which included disease progression, development of a new primary malignancy, or death. Secondary outcomes included disease recurrence, patterns of recurrence, and overall survival. A 7% difference in disease-free survival at 4.5 years postoperatively was prespecified and determined based on previously published literature.1–4
By Kaplan-Meier estimates, disease-free survival at 4.5 years was 81.3% in the TAH group and 81.6% in the TLH group, a 0.3% difference. In addition, there were no differences noted in secondary outcomes, further supporting equivalency of the surgical modalities. The only significantly different surgical findings included decreased operative time in the TAH group and decreased lymph node dissection completion in the TLH group.
Related Article:
Can we reduce the use of abdominal hysterectomy and increase the use of vaginal and laparoscopic approaches?
Study strengths and weaknesses
The largest previous trial of more than 2,000 patients examining the method of surgical management was the Gynecologic Oncology Group’s (GOG) noninferiority LAP2 trial.3 This trial has been used widely to promote a minimally invasive approach, but did not actually reach the prespecified statistical goals. The LACE trial, however, successfully reached its statistical targets and is now the largest randomized trial supporting an equivalence in oncologic outcomes.
It is important to recognize the limitations of the LACE trial in the current medical environment. The study population was a very specific group of low-risk women without high-risk histologic subtypes or even moderately enlarged uteri; many institutions would consider offering a minimally invasive approach to these women. In addition, this study did not include robotic minimally invasive surgery, which in many regions of the country is rapidly becoming accepted as the first choice procedure over traditional laparoscopy.5 Furthermore, the FIRES trial and others6–8 have demonstrated that utilizing a minimally invasive approach that includes sentinel lymph node identification and removal may be as diagnostic as a full dissection, adding considerations to surgical modality selection.
--Kathryn A. Mills, MD, and David G. Mutch, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346–1355.
- Stevenson AR, Solomon MJ, Lumley JW, et al; ALaCaRT Investigators. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–1363.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30(7):695–700.
- Creutzberg CL, van Putten WL, Koper PC, et al; Post Operative Radiation Therapy in Endometrial Carcinoma. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Lancet. 2000;355(9213):1404–1411.
- Wright JD, Burke WM, Tergas AI, et al. Comparative effectiveness of minimally invasive hysterectomy for endometrial cancer. J Clin Oncol. 2016;34(10):1087–1096.
- Rossi EC, Kowalski LD, Scalici JS, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384–392.
- Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;12(3):531–535.
- Darai E, Dubernard G, Bats AS, et al. Sentinel node biopsy for the management of early stage endometrial cancer: long-term results of the SENTI-ENDO study. Gynecol Oncol. 2015;136(1):54-59.
EXPERT COMMENTARY
The objective of the study by Janda and colleagues (known as the “LACE” trial) was to evaluate the equivalency of total laparoscopic hysterectomy (TLH) with staging versus the standard procedure, which is total abdominal hysterectomy (TAH) with staging, for surgical management of women with presumed low-risk, early-stage endometrial cancer.
Related Article:
2016 Update on cancer
Details of the study
This nonblinded, randomized controlled multicenter equivalency trial included 760 women from Australia, New Zealand, and Hong Kong undergoing surgical management of presumed stage I uterine endometrioid adenocarcinoma. All surgeries were performed or supervised by trained gynecologic oncologists. Pelvic lymph node sampling was required but omission was permitted for: morbid obesity, low risk of metastasis based on frozen section results, medically unfit status, or institutional guidelines prohibiting the procedure. Patients were excluded for preoperative nonendometrioid histology, suspected ultimate FIGO stage II–IV based on preoperative imaging, or uterine size greater than 10 weeks’ gestation.
The primary outcome was disease-free survival, defined as the time from surgery to the date of first recurrence, which included disease progression, development of a new primary malignancy, or death. Secondary outcomes included disease recurrence, patterns of recurrence, and overall survival. A 7% difference in disease-free survival at 4.5 years postoperatively was prespecified and determined based on previously published literature.1–4
By Kaplan-Meier estimates, disease-free survival at 4.5 years was 81.3% in the TAH group and 81.6% in the TLH group, a 0.3% difference. In addition, there were no differences noted in secondary outcomes, further supporting equivalency of the surgical modalities. The only significantly different surgical findings included decreased operative time in the TAH group and decreased lymph node dissection completion in the TLH group.
Related Article:
Can we reduce the use of abdominal hysterectomy and increase the use of vaginal and laparoscopic approaches?
Study strengths and weaknesses
The largest previous trial of more than 2,000 patients examining the method of surgical management was the Gynecologic Oncology Group’s (GOG) noninferiority LAP2 trial.3 This trial has been used widely to promote a minimally invasive approach, but did not actually reach the prespecified statistical goals. The LACE trial, however, successfully reached its statistical targets and is now the largest randomized trial supporting an equivalence in oncologic outcomes.
It is important to recognize the limitations of the LACE trial in the current medical environment. The study population was a very specific group of low-risk women without high-risk histologic subtypes or even moderately enlarged uteri; many institutions would consider offering a minimally invasive approach to these women. In addition, this study did not include robotic minimally invasive surgery, which in many regions of the country is rapidly becoming accepted as the first choice procedure over traditional laparoscopy.5 Furthermore, the FIRES trial and others6–8 have demonstrated that utilizing a minimally invasive approach that includes sentinel lymph node identification and removal may be as diagnostic as a full dissection, adding considerations to surgical modality selection.
--Kathryn A. Mills, MD, and David G. Mutch, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
The objective of the study by Janda and colleagues (known as the “LACE” trial) was to evaluate the equivalency of total laparoscopic hysterectomy (TLH) with staging versus the standard procedure, which is total abdominal hysterectomy (TAH) with staging, for surgical management of women with presumed low-risk, early-stage endometrial cancer.
Related Article:
2016 Update on cancer
Details of the study
This nonblinded, randomized controlled multicenter equivalency trial included 760 women from Australia, New Zealand, and Hong Kong undergoing surgical management of presumed stage I uterine endometrioid adenocarcinoma. All surgeries were performed or supervised by trained gynecologic oncologists. Pelvic lymph node sampling was required but omission was permitted for: morbid obesity, low risk of metastasis based on frozen section results, medically unfit status, or institutional guidelines prohibiting the procedure. Patients were excluded for preoperative nonendometrioid histology, suspected ultimate FIGO stage II–IV based on preoperative imaging, or uterine size greater than 10 weeks’ gestation.
The primary outcome was disease-free survival, defined as the time from surgery to the date of first recurrence, which included disease progression, development of a new primary malignancy, or death. Secondary outcomes included disease recurrence, patterns of recurrence, and overall survival. A 7% difference in disease-free survival at 4.5 years postoperatively was prespecified and determined based on previously published literature.1–4
By Kaplan-Meier estimates, disease-free survival at 4.5 years was 81.3% in the TAH group and 81.6% in the TLH group, a 0.3% difference. In addition, there were no differences noted in secondary outcomes, further supporting equivalency of the surgical modalities. The only significantly different surgical findings included decreased operative time in the TAH group and decreased lymph node dissection completion in the TLH group.
Related Article:
Can we reduce the use of abdominal hysterectomy and increase the use of vaginal and laparoscopic approaches?
Study strengths and weaknesses
The largest previous trial of more than 2,000 patients examining the method of surgical management was the Gynecologic Oncology Group’s (GOG) noninferiority LAP2 trial.3 This trial has been used widely to promote a minimally invasive approach, but did not actually reach the prespecified statistical goals. The LACE trial, however, successfully reached its statistical targets and is now the largest randomized trial supporting an equivalence in oncologic outcomes.
It is important to recognize the limitations of the LACE trial in the current medical environment. The study population was a very specific group of low-risk women without high-risk histologic subtypes or even moderately enlarged uteri; many institutions would consider offering a minimally invasive approach to these women. In addition, this study did not include robotic minimally invasive surgery, which in many regions of the country is rapidly becoming accepted as the first choice procedure over traditional laparoscopy.5 Furthermore, the FIRES trial and others6–8 have demonstrated that utilizing a minimally invasive approach that includes sentinel lymph node identification and removal may be as diagnostic as a full dissection, adding considerations to surgical modality selection.
--Kathryn A. Mills, MD, and David G. Mutch, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346–1355.
- Stevenson AR, Solomon MJ, Lumley JW, et al; ALaCaRT Investigators. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–1363.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30(7):695–700.
- Creutzberg CL, van Putten WL, Koper PC, et al; Post Operative Radiation Therapy in Endometrial Carcinoma. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Lancet. 2000;355(9213):1404–1411.
- Wright JD, Burke WM, Tergas AI, et al. Comparative effectiveness of minimally invasive hysterectomy for endometrial cancer. J Clin Oncol. 2016;34(10):1087–1096.
- Rossi EC, Kowalski LD, Scalici JS, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384–392.
- Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;12(3):531–535.
- Darai E, Dubernard G, Bats AS, et al. Sentinel node biopsy for the management of early stage endometrial cancer: long-term results of the SENTI-ENDO study. Gynecol Oncol. 2015;136(1):54-59.
- Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346–1355.
- Stevenson AR, Solomon MJ, Lumley JW, et al; ALaCaRT Investigators. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–1363.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30(7):695–700.
- Creutzberg CL, van Putten WL, Koper PC, et al; Post Operative Radiation Therapy in Endometrial Carcinoma. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Lancet. 2000;355(9213):1404–1411.
- Wright JD, Burke WM, Tergas AI, et al. Comparative effectiveness of minimally invasive hysterectomy for endometrial cancer. J Clin Oncol. 2016;34(10):1087–1096.
- Rossi EC, Kowalski LD, Scalici JS, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384–392.
- Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;12(3):531–535.
- Darai E, Dubernard G, Bats AS, et al. Sentinel node biopsy for the management of early stage endometrial cancer: long-term results of the SENTI-ENDO study. Gynecol Oncol. 2015;136(1):54-59.
2016 Update on cancer
Each year approximately 60,000 women are diagnosed with endometrial cancer. The majority of the identified tumors will be low grade—cancer found at an early stage that may be treated with surgery alone. Unfortunately, however, too many of the 60,000 patients will have poor prognostic features, such as serous or clear cell histology (high-grade cancer), lymphovascular space invasion, or positive lymph node status.
Advances in technology and the state of science have come a long way since the dichotomy of Type I (endometrioid) and Type II (serous and clear cell) tumors were
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations high (serous tumors)
- somatic copy number alterations low (endometrioid cancer).
In 2016, we are now understanding the molecular basis of disease and how it affects survival; these 4 categories have different survival. But why? Perhaps the answer lies within the endogenous immune system. Tumor-infiltrating lymphocytes are associated with improved survival in multiple types of cancer, including endometrial. Whether these lymphocytes are regulatory or cytotoxic T-cells convolutes the matter further.4 To understand these intricacies we need to further categorize how a tumor’s genetic mutations affect antigen exposure to the immune system, quantitate the clinical impact of the findings, and selectively target patients with novel therapeutics.
In this Update, we look at data on POLE mutations, exploring 2 studies that help us to better understand why these types of mutations have uniquely positive prognostic implications (when they logically should not have good survival rates). In addition, we discuss 2 studies that examined mismatch repair defects, in endometrial cancer specifically, and the programmed death (PD)-1 pathway in both endometrial and other cancer types. Are these molecular entities of tumors associated with better or worse prognosis, and why?
Molecular profiling: Prognostic implications of POLE mutations
Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2014;107(1):402.
van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE Proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347 - 3355.
The TCGA identified a subgroup of endometrial carcinomas with mutations of the DNA polymerase POLE. These mutants have a high rate of proofreading error and frequent base pair substitutions. This POLE subgroup (6% to 12% of endometrial tumors) is associated with endometrioid histology and high-grade tumors. Patients with these tumors would be expected to have an aggressive course with poor survival, but often these patients survive without a recurrence. We need more understanding of why.
POLE mutations and prognosis
In a secondary analysis by Church and colleagues of the PORTEC-1 and -2 studies (2 large, randomized controlled trials evaluating postoperative external beam radiation therapy [EBRT] or vaginal brachytherapy), tumors were tested for mutations in POLE (POLE-mutant and POLE wild-type). POLE mutations were detected in 6.1% of tumors overall. Despite their high grade, POLE-mutant tumors resulted in fewer recurrences (6.2% vs 14.1%) and fewer deaths (2.3% vs 9.7%) than POLE wild-type tumors. In grade 3 tumors, 0 of 15 POLE-mutant tumors recurred.
These results indicate that, even with having poor prognostic features, endometrial cancers with mutations in POLE have an excellent prognosis.5
POLE mutations and the immune response
To explain the discrepancy in the results by Church and colleagues, van Gool and colleagues analyzed endometrial cancer specimens from PORTEC-1, -2, and the TCGA studies. Endometrial cancers were categorized as POLE-mutants, POLE wild-type, or microsatellite stable (MSS) tumors. They found that POLE-mutant endometrial cancers have an increased lymphocytic infiltrate (present in 22 of 47 POLE-mutant specimens) as compared with POLE wild-type or MSS tumors.
Also, POLE-mutants had an increased density of cytotoxic T-cells (CD8+) at the tumor center and margin that significantly exceeded that of POLE wild-type or MSS tumors. The proportion of tumors with CD8+ cells exceeding the median were also higher in POLE-mutant (60%) compared with POLE wild-type (31.3%) and MSS (7.2%) tumors. Markers LAG3, TIM-3, TIGI, as well as T-cell inhibitors PD1 and CTLA-4, confirmed evidence of T-cell exhaustion--all of which correlated with CD8 expression.
These findings suggest that POLE mutations lead to hundreds of thousands of DNA fragments stimulating the immune system through prolonged antigenic exposure.6 This immune response is so powerful that even these tumors with poor prognostic features will have excellent clinical outcomes.
POLE-mutant endometrial cancers have mutations that stimulate the immune system with tremendous amounts of antigenic neopeptides. This robust immune response is demonstrated by tumor infiltrating lymphocytes that enhance antitumor effects and host killing in spite of traditional poor prognostic features.
Mismatch repair and immunology: Targeted therapy for targeted patients
McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol. 2016;34(25):3062-3068.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
The most frequent genetic mutation in endometrial cancer is mismatch repair (MMR) deficiency. Loss of this pathway leads to a failure of repairing replication errors and gives rise to small repeated sequences of DNA, known as MSI. Germline mutations in MMR (Lynch syndrome) occur in only 3% to 5% of endometrial cancers. Somatic mutations in MMR give rise to 10% to 20% of colorectal cancers and upwards of 20% to 40% of endometrial cancers.
Given this high frequency, universal screening utilizing immunohistochemistry of proteins MLH1, MSH2, MSH6, and PMS2 has become the standard of care in tumors to identify MMR deficiency. MMR-deficient endometrial tumors are associated with higher grade and lymphovascular space invasion. The actual clinical prognosis of these tumors, however, has not been well described.7 McMeekin and colleagues set out to examine prognosis.
Details of the study by McMeekin and colleagues
In the collaborative study, researchers assessed 1,024 tumors for MMR and categorized them into 1 of 4 groups: normal(62.4%), epigenetic MMR-defective (25.78%),MMR-probable mutation (9.67%), or MSI-low (2.15%). The researchers found that the pathologic features were associatedwith MMR status. For instance, MMR-defective tumors were more likely thanMMR-normal tumors to be Grade 2 (50% vs 40.7%, respectively). Lymphovascular space invasion also occurred more frequently in MMR-defective than in MMR-normal tumors (32.7% vs 17.13%, respectively). Approximately 22% of patients with MMR-defective tumors had stage III or IV disease, while only 13% to 14% of the other groups presented with such advanced stage.
On univariate analysis, an MMR-defective tumor was associated with worsened progression-free survival (hazard ratio [HR], 1.37). On subsequent multivariate analysis, no difference in survival in MMR-defective vs MMR-normal tumors was found. The authors concluded that MMR status is predictive of response to adjuvant therapy.
An intriguing biologic explanation of how MMR status affects response to adjuvant therapy is that MMR-defective tumors contain lymphocytic infiltrates, consistent with an increased immunologic response.8 Similar to the previously discussed POLE mutations, MMR-defective tumors have a tremendous increase in somatic mutations that are on the order of 10 to 100 times that of MMR-proficient tumors. These MMR-defective tumors likely give rise to increased antigen exposure to the immune system.
These immune infiltrates will show signs of exhaustion and upregulate negative feedback systems, which is the point at which the PD-1 pathway becomes critically important. The PD-1 receptor is expressed predominately on T-cells and its ligands regulate the immune system by inhibition of self-reactive T-cells.9
MMR deficiency and anti-programmed death receptor 1
The study by McMeekin and colleagues shows MMR-defective tumors have poor prognostic features but the same survival as those with MMR proficiency or good prognostic features. Why is this the case? A recent study by Le and colleagues analyzed this question.
Details of the study by Le and colleagues
The investigators performed a phase 2 trial evaluating pembrolizumab (10 mg/kg IV every 14 days), an anti-PD 1 immune checkpoint inhibitor in patients with tumors demonstrating MMR-deficiency. The 3 cohorts included: MMR-defective colorectal cancer (n = 10), MMR-proficient colorectal cancer (n = 18), and MMR-defective noncolorectal cancer (n = 7, including 2 endometrial cancers). Objective response rates were 40%, 0%, and 71% for each group, respectively.
MMR-defective tumors had a striking HR of disease progression or death of 0.04 (95% confidence interval, 0.01-0.21; P<.001). Genomic analysis was performed and identified 578 potential mutation- associated neoantigens in the MMR-defective groups (compared with only 21 in the MMR-proficient tumors). These findings promote the concept of a mutation-associated antigen component to the endogenous immune response.10
These studies support the growing evidence that molecular events have a powerful clinical impact that has the potential to supplant traditional histopathologic staging.
Conclusion
The above-stated mutations of mismatch repair and POLE are changing our perspective of endometrial cancer and shedding light on the complexities of tumor biology. As future research increasingly incorporates genomic profiling, we anticipate clinical trials may build evidence that adjuvant therapy will be directed by molecular staging, as opposed to traditional surgical or even histologic staging, as these mutations are the root cause of the tumor phenotype.
Key for readers to take away from this Update is that genomic profiling and enrollment in clinical trials is critical to understanding the implications of these mutations and how to best treat our patients. In addition, we should encourage our patients with endometrial cancer to see genetic counselors and have appropriate screening of MMR-deficiency. This will continue to advance our understanding as well as to provide patients with valuable information regarding their diagnosis.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-17.
- Kuroki LM, Mutch DG. Endometrial cancer update: the move toward personalized cancer care. OBG Manag. 2013;25(10):25-32.
- Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
- De Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105-110.
- Church DN, Steloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107(1):402.
- Van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347-3355.
- Lancaster JM, Powell CB, Chen L-M, Richardson DL; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3-7. Erratum in Gynecol. Oncol. 2015;138(3):765.
- McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34(25):3062-3068.
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145-152.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
Each year approximately 60,000 women are diagnosed with endometrial cancer. The majority of the identified tumors will be low grade—cancer found at an early stage that may be treated with surgery alone. Unfortunately, however, too many of the 60,000 patients will have poor prognostic features, such as serous or clear cell histology (high-grade cancer), lymphovascular space invasion, or positive lymph node status.
Advances in technology and the state of science have come a long way since the dichotomy of Type I (endometrioid) and Type II (serous and clear cell) tumors were
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations high (serous tumors)
- somatic copy number alterations low (endometrioid cancer).
In 2016, we are now understanding the molecular basis of disease and how it affects survival; these 4 categories have different survival. But why? Perhaps the answer lies within the endogenous immune system. Tumor-infiltrating lymphocytes are associated with improved survival in multiple types of cancer, including endometrial. Whether these lymphocytes are regulatory or cytotoxic T-cells convolutes the matter further.4 To understand these intricacies we need to further categorize how a tumor’s genetic mutations affect antigen exposure to the immune system, quantitate the clinical impact of the findings, and selectively target patients with novel therapeutics.
In this Update, we look at data on POLE mutations, exploring 2 studies that help us to better understand why these types of mutations have uniquely positive prognostic implications (when they logically should not have good survival rates). In addition, we discuss 2 studies that examined mismatch repair defects, in endometrial cancer specifically, and the programmed death (PD)-1 pathway in both endometrial and other cancer types. Are these molecular entities of tumors associated with better or worse prognosis, and why?
Molecular profiling: Prognostic implications of POLE mutations
Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2014;107(1):402.
van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE Proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347 - 3355.
The TCGA identified a subgroup of endometrial carcinomas with mutations of the DNA polymerase POLE. These mutants have a high rate of proofreading error and frequent base pair substitutions. This POLE subgroup (6% to 12% of endometrial tumors) is associated with endometrioid histology and high-grade tumors. Patients with these tumors would be expected to have an aggressive course with poor survival, but often these patients survive without a recurrence. We need more understanding of why.
POLE mutations and prognosis
In a secondary analysis by Church and colleagues of the PORTEC-1 and -2 studies (2 large, randomized controlled trials evaluating postoperative external beam radiation therapy [EBRT] or vaginal brachytherapy), tumors were tested for mutations in POLE (POLE-mutant and POLE wild-type). POLE mutations were detected in 6.1% of tumors overall. Despite their high grade, POLE-mutant tumors resulted in fewer recurrences (6.2% vs 14.1%) and fewer deaths (2.3% vs 9.7%) than POLE wild-type tumors. In grade 3 tumors, 0 of 15 POLE-mutant tumors recurred.
These results indicate that, even with having poor prognostic features, endometrial cancers with mutations in POLE have an excellent prognosis.5
POLE mutations and the immune response
To explain the discrepancy in the results by Church and colleagues, van Gool and colleagues analyzed endometrial cancer specimens from PORTEC-1, -2, and the TCGA studies. Endometrial cancers were categorized as POLE-mutants, POLE wild-type, or microsatellite stable (MSS) tumors. They found that POLE-mutant endometrial cancers have an increased lymphocytic infiltrate (present in 22 of 47 POLE-mutant specimens) as compared with POLE wild-type or MSS tumors.
Also, POLE-mutants had an increased density of cytotoxic T-cells (CD8+) at the tumor center and margin that significantly exceeded that of POLE wild-type or MSS tumors. The proportion of tumors with CD8+ cells exceeding the median were also higher in POLE-mutant (60%) compared with POLE wild-type (31.3%) and MSS (7.2%) tumors. Markers LAG3, TIM-3, TIGI, as well as T-cell inhibitors PD1 and CTLA-4, confirmed evidence of T-cell exhaustion--all of which correlated with CD8 expression.
These findings suggest that POLE mutations lead to hundreds of thousands of DNA fragments stimulating the immune system through prolonged antigenic exposure.6 This immune response is so powerful that even these tumors with poor prognostic features will have excellent clinical outcomes.
POLE-mutant endometrial cancers have mutations that stimulate the immune system with tremendous amounts of antigenic neopeptides. This robust immune response is demonstrated by tumor infiltrating lymphocytes that enhance antitumor effects and host killing in spite of traditional poor prognostic features.
Mismatch repair and immunology: Targeted therapy for targeted patients
McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol. 2016;34(25):3062-3068.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
The most frequent genetic mutation in endometrial cancer is mismatch repair (MMR) deficiency. Loss of this pathway leads to a failure of repairing replication errors and gives rise to small repeated sequences of DNA, known as MSI. Germline mutations in MMR (Lynch syndrome) occur in only 3% to 5% of endometrial cancers. Somatic mutations in MMR give rise to 10% to 20% of colorectal cancers and upwards of 20% to 40% of endometrial cancers.
Given this high frequency, universal screening utilizing immunohistochemistry of proteins MLH1, MSH2, MSH6, and PMS2 has become the standard of care in tumors to identify MMR deficiency. MMR-deficient endometrial tumors are associated with higher grade and lymphovascular space invasion. The actual clinical prognosis of these tumors, however, has not been well described.7 McMeekin and colleagues set out to examine prognosis.
Details of the study by McMeekin and colleagues
In the collaborative study, researchers assessed 1,024 tumors for MMR and categorized them into 1 of 4 groups: normal(62.4%), epigenetic MMR-defective (25.78%),MMR-probable mutation (9.67%), or MSI-low (2.15%). The researchers found that the pathologic features were associatedwith MMR status. For instance, MMR-defective tumors were more likely thanMMR-normal tumors to be Grade 2 (50% vs 40.7%, respectively). Lymphovascular space invasion also occurred more frequently in MMR-defective than in MMR-normal tumors (32.7% vs 17.13%, respectively). Approximately 22% of patients with MMR-defective tumors had stage III or IV disease, while only 13% to 14% of the other groups presented with such advanced stage.
On univariate analysis, an MMR-defective tumor was associated with worsened progression-free survival (hazard ratio [HR], 1.37). On subsequent multivariate analysis, no difference in survival in MMR-defective vs MMR-normal tumors was found. The authors concluded that MMR status is predictive of response to adjuvant therapy.
An intriguing biologic explanation of how MMR status affects response to adjuvant therapy is that MMR-defective tumors contain lymphocytic infiltrates, consistent with an increased immunologic response.8 Similar to the previously discussed POLE mutations, MMR-defective tumors have a tremendous increase in somatic mutations that are on the order of 10 to 100 times that of MMR-proficient tumors. These MMR-defective tumors likely give rise to increased antigen exposure to the immune system.
These immune infiltrates will show signs of exhaustion and upregulate negative feedback systems, which is the point at which the PD-1 pathway becomes critically important. The PD-1 receptor is expressed predominately on T-cells and its ligands regulate the immune system by inhibition of self-reactive T-cells.9
MMR deficiency and anti-programmed death receptor 1
The study by McMeekin and colleagues shows MMR-defective tumors have poor prognostic features but the same survival as those with MMR proficiency or good prognostic features. Why is this the case? A recent study by Le and colleagues analyzed this question.
Details of the study by Le and colleagues
The investigators performed a phase 2 trial evaluating pembrolizumab (10 mg/kg IV every 14 days), an anti-PD 1 immune checkpoint inhibitor in patients with tumors demonstrating MMR-deficiency. The 3 cohorts included: MMR-defective colorectal cancer (n = 10), MMR-proficient colorectal cancer (n = 18), and MMR-defective noncolorectal cancer (n = 7, including 2 endometrial cancers). Objective response rates were 40%, 0%, and 71% for each group, respectively.
MMR-defective tumors had a striking HR of disease progression or death of 0.04 (95% confidence interval, 0.01-0.21; P<.001). Genomic analysis was performed and identified 578 potential mutation- associated neoantigens in the MMR-defective groups (compared with only 21 in the MMR-proficient tumors). These findings promote the concept of a mutation-associated antigen component to the endogenous immune response.10
These studies support the growing evidence that molecular events have a powerful clinical impact that has the potential to supplant traditional histopathologic staging.
Conclusion
The above-stated mutations of mismatch repair and POLE are changing our perspective of endometrial cancer and shedding light on the complexities of tumor biology. As future research increasingly incorporates genomic profiling, we anticipate clinical trials may build evidence that adjuvant therapy will be directed by molecular staging, as opposed to traditional surgical or even histologic staging, as these mutations are the root cause of the tumor phenotype.
Key for readers to take away from this Update is that genomic profiling and enrollment in clinical trials is critical to understanding the implications of these mutations and how to best treat our patients. In addition, we should encourage our patients with endometrial cancer to see genetic counselors and have appropriate screening of MMR-deficiency. This will continue to advance our understanding as well as to provide patients with valuable information regarding their diagnosis.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Each year approximately 60,000 women are diagnosed with endometrial cancer. The majority of the identified tumors will be low grade—cancer found at an early stage that may be treated with surgery alone. Unfortunately, however, too many of the 60,000 patients will have poor prognostic features, such as serous or clear cell histology (high-grade cancer), lymphovascular space invasion, or positive lymph node status.
Advances in technology and the state of science have come a long way since the dichotomy of Type I (endometrioid) and Type II (serous and clear cell) tumors were
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations high (serous tumors)
- somatic copy number alterations low (endometrioid cancer).
In 2016, we are now understanding the molecular basis of disease and how it affects survival; these 4 categories have different survival. But why? Perhaps the answer lies within the endogenous immune system. Tumor-infiltrating lymphocytes are associated with improved survival in multiple types of cancer, including endometrial. Whether these lymphocytes are regulatory or cytotoxic T-cells convolutes the matter further.4 To understand these intricacies we need to further categorize how a tumor’s genetic mutations affect antigen exposure to the immune system, quantitate the clinical impact of the findings, and selectively target patients with novel therapeutics.
In this Update, we look at data on POLE mutations, exploring 2 studies that help us to better understand why these types of mutations have uniquely positive prognostic implications (when they logically should not have good survival rates). In addition, we discuss 2 studies that examined mismatch repair defects, in endometrial cancer specifically, and the programmed death (PD)-1 pathway in both endometrial and other cancer types. Are these molecular entities of tumors associated with better or worse prognosis, and why?
Molecular profiling: Prognostic implications of POLE mutations
Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2014;107(1):402.
van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE Proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347 - 3355.
The TCGA identified a subgroup of endometrial carcinomas with mutations of the DNA polymerase POLE. These mutants have a high rate of proofreading error and frequent base pair substitutions. This POLE subgroup (6% to 12% of endometrial tumors) is associated with endometrioid histology and high-grade tumors. Patients with these tumors would be expected to have an aggressive course with poor survival, but often these patients survive without a recurrence. We need more understanding of why.
POLE mutations and prognosis
In a secondary analysis by Church and colleagues of the PORTEC-1 and -2 studies (2 large, randomized controlled trials evaluating postoperative external beam radiation therapy [EBRT] or vaginal brachytherapy), tumors were tested for mutations in POLE (POLE-mutant and POLE wild-type). POLE mutations were detected in 6.1% of tumors overall. Despite their high grade, POLE-mutant tumors resulted in fewer recurrences (6.2% vs 14.1%) and fewer deaths (2.3% vs 9.7%) than POLE wild-type tumors. In grade 3 tumors, 0 of 15 POLE-mutant tumors recurred.
These results indicate that, even with having poor prognostic features, endometrial cancers with mutations in POLE have an excellent prognosis.5
POLE mutations and the immune response
To explain the discrepancy in the results by Church and colleagues, van Gool and colleagues analyzed endometrial cancer specimens from PORTEC-1, -2, and the TCGA studies. Endometrial cancers were categorized as POLE-mutants, POLE wild-type, or microsatellite stable (MSS) tumors. They found that POLE-mutant endometrial cancers have an increased lymphocytic infiltrate (present in 22 of 47 POLE-mutant specimens) as compared with POLE wild-type or MSS tumors.
Also, POLE-mutants had an increased density of cytotoxic T-cells (CD8+) at the tumor center and margin that significantly exceeded that of POLE wild-type or MSS tumors. The proportion of tumors with CD8+ cells exceeding the median were also higher in POLE-mutant (60%) compared with POLE wild-type (31.3%) and MSS (7.2%) tumors. Markers LAG3, TIM-3, TIGI, as well as T-cell inhibitors PD1 and CTLA-4, confirmed evidence of T-cell exhaustion--all of which correlated with CD8 expression.
These findings suggest that POLE mutations lead to hundreds of thousands of DNA fragments stimulating the immune system through prolonged antigenic exposure.6 This immune response is so powerful that even these tumors with poor prognostic features will have excellent clinical outcomes.
POLE-mutant endometrial cancers have mutations that stimulate the immune system with tremendous amounts of antigenic neopeptides. This robust immune response is demonstrated by tumor infiltrating lymphocytes that enhance antitumor effects and host killing in spite of traditional poor prognostic features.
Mismatch repair and immunology: Targeted therapy for targeted patients
McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol. 2016;34(25):3062-3068.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
The most frequent genetic mutation in endometrial cancer is mismatch repair (MMR) deficiency. Loss of this pathway leads to a failure of repairing replication errors and gives rise to small repeated sequences of DNA, known as MSI. Germline mutations in MMR (Lynch syndrome) occur in only 3% to 5% of endometrial cancers. Somatic mutations in MMR give rise to 10% to 20% of colorectal cancers and upwards of 20% to 40% of endometrial cancers.
Given this high frequency, universal screening utilizing immunohistochemistry of proteins MLH1, MSH2, MSH6, and PMS2 has become the standard of care in tumors to identify MMR deficiency. MMR-deficient endometrial tumors are associated with higher grade and lymphovascular space invasion. The actual clinical prognosis of these tumors, however, has not been well described.7 McMeekin and colleagues set out to examine prognosis.
Details of the study by McMeekin and colleagues
In the collaborative study, researchers assessed 1,024 tumors for MMR and categorized them into 1 of 4 groups: normal(62.4%), epigenetic MMR-defective (25.78%),MMR-probable mutation (9.67%), or MSI-low (2.15%). The researchers found that the pathologic features were associatedwith MMR status. For instance, MMR-defective tumors were more likely thanMMR-normal tumors to be Grade 2 (50% vs 40.7%, respectively). Lymphovascular space invasion also occurred more frequently in MMR-defective than in MMR-normal tumors (32.7% vs 17.13%, respectively). Approximately 22% of patients with MMR-defective tumors had stage III or IV disease, while only 13% to 14% of the other groups presented with such advanced stage.
On univariate analysis, an MMR-defective tumor was associated with worsened progression-free survival (hazard ratio [HR], 1.37). On subsequent multivariate analysis, no difference in survival in MMR-defective vs MMR-normal tumors was found. The authors concluded that MMR status is predictive of response to adjuvant therapy.
An intriguing biologic explanation of how MMR status affects response to adjuvant therapy is that MMR-defective tumors contain lymphocytic infiltrates, consistent with an increased immunologic response.8 Similar to the previously discussed POLE mutations, MMR-defective tumors have a tremendous increase in somatic mutations that are on the order of 10 to 100 times that of MMR-proficient tumors. These MMR-defective tumors likely give rise to increased antigen exposure to the immune system.
These immune infiltrates will show signs of exhaustion and upregulate negative feedback systems, which is the point at which the PD-1 pathway becomes critically important. The PD-1 receptor is expressed predominately on T-cells and its ligands regulate the immune system by inhibition of self-reactive T-cells.9
MMR deficiency and anti-programmed death receptor 1
The study by McMeekin and colleagues shows MMR-defective tumors have poor prognostic features but the same survival as those with MMR proficiency or good prognostic features. Why is this the case? A recent study by Le and colleagues analyzed this question.
Details of the study by Le and colleagues
The investigators performed a phase 2 trial evaluating pembrolizumab (10 mg/kg IV every 14 days), an anti-PD 1 immune checkpoint inhibitor in patients with tumors demonstrating MMR-deficiency. The 3 cohorts included: MMR-defective colorectal cancer (n = 10), MMR-proficient colorectal cancer (n = 18), and MMR-defective noncolorectal cancer (n = 7, including 2 endometrial cancers). Objective response rates were 40%, 0%, and 71% for each group, respectively.
MMR-defective tumors had a striking HR of disease progression or death of 0.04 (95% confidence interval, 0.01-0.21; P<.001). Genomic analysis was performed and identified 578 potential mutation- associated neoantigens in the MMR-defective groups (compared with only 21 in the MMR-proficient tumors). These findings promote the concept of a mutation-associated antigen component to the endogenous immune response.10
These studies support the growing evidence that molecular events have a powerful clinical impact that has the potential to supplant traditional histopathologic staging.
Conclusion
The above-stated mutations of mismatch repair and POLE are changing our perspective of endometrial cancer and shedding light on the complexities of tumor biology. As future research increasingly incorporates genomic profiling, we anticipate clinical trials may build evidence that adjuvant therapy will be directed by molecular staging, as opposed to traditional surgical or even histologic staging, as these mutations are the root cause of the tumor phenotype.
Key for readers to take away from this Update is that genomic profiling and enrollment in clinical trials is critical to understanding the implications of these mutations and how to best treat our patients. In addition, we should encourage our patients with endometrial cancer to see genetic counselors and have appropriate screening of MMR-deficiency. This will continue to advance our understanding as well as to provide patients with valuable information regarding their diagnosis.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-17.
- Kuroki LM, Mutch DG. Endometrial cancer update: the move toward personalized cancer care. OBG Manag. 2013;25(10):25-32.
- Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
- De Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105-110.
- Church DN, Steloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107(1):402.
- Van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347-3355.
- Lancaster JM, Powell CB, Chen L-M, Richardson DL; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3-7. Erratum in Gynecol. Oncol. 2015;138(3):765.
- McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34(25):3062-3068.
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145-152.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-17.
- Kuroki LM, Mutch DG. Endometrial cancer update: the move toward personalized cancer care. OBG Manag. 2013;25(10):25-32.
- Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
- De Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105-110.
- Church DN, Steloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107(1):402.
- Van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347-3355.
- Lancaster JM, Powell CB, Chen L-M, Richardson DL; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3-7. Erratum in Gynecol. Oncol. 2015;138(3):765.
- McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34(25):3062-3068.
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145-152.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
In this Article
- The prognostic significance of tumors with POLE mutations
- Can the immune system kill MMR-deficient endometrial cancer?