User login
From the Gastroenterology and Hepatology Section, Baylor College of Medicine, Houston, TX.
Abstract
- Objective: To provide an update on management of Barrett’s esophagus.
- Methods: Review of the literature.
- Results: Management of Barrett’s esophagus depends on the degree of dysplasia. Surveillance by endoscopy every 3–5 years is recommended in patients with Barrett’s esophagus without dysplasia. Patients with Barrett’s esophagus and low-grade dysplasia should undergo surveillance by endoscopy in 3 months for confirmation of the diagnosis; if the diagnosis is confirmed then surveillance by endoscopy or eradication of Barrett’s epithelium by ablation or endoscopic resection are recommended. There is a sufficient evidence to recommend radiofrequency ablation of high-grade dysplasia within Barrett’s esophagus or to perform endoscopic mucosal resection of nodular Barrett’s esophagus with any degree of dysplasia. Early esophageal cancers that are limited to the mucosa can be treated by endoscopic resection, while cancer invading into the deep submucosa or muscularis propria may need esophagectomy with or without chemoradiation.
- Conclusion: The management of Barrett’s esophagus depends on the degree of dysplasia. Radiofrequency ablation and endoscopic mucosal resection are the most commonly used treatment for Barrett’s esophagus with dysplasia.
Keywords: Barrett’s esophagus; radiofrequency ablation; endoscopic submucosal dissection; endoscopic mucosal resection; early esophageal cancer.
Barrett’s esophagus is a common complication of chronic reflux disease [1]. Metaplastic changes that occur at the distal esophageal epithelium are usually asymptomatic [2,3] and occur as reparative adaptations to the insult of the gastric acid [4]. The management of Barrett’s esophagus after diagnosis is currently debated amongst experts without a clear consensus [5,6]. This review is generally consistent with the 2016 guidelines from the American College of Gastroenterology [1], a 2012 guideline from the American Society of Gastrointestinal Endoscopy [7], a 2011 guideline, and a 2016 expert review from the American Gastroenterological Association [8,9].
D efinition
Barrett’s esophagus is a metaplasia of the stratified squamous epithelium to a specialized columnar intestinal epithelium of mucus cells and goblet cells at the distal esophagus secondary to gastroesophageal reflux disease (GERD) [7,10]. Barrett’s esophagus is unstable tissue which can progress to esophageal adenocarcinoma. When unmanaged, the risk of cancer in dysplastic mucosa is at least thirty-fold greater than that for the general population [11–14], with recent studies suggesting a 0.4–0.7 occurrence rate per year [11,15]. With no dysplasia, the risk is low [16].
Epidemiology
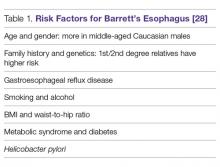
The prevalence of Barrett’s esophagus is ~10% in patients with GERD [11–13,17], an estimate tied to the prevalence of GERD. However, due to the lack of symptoms of Barrett’s esophagus, no solid data supports this assumption [18–20]. A European study estimated the prevalence of Barrett’s esophagus to be 1.6% among the general population [21,22]. Barrett’s esophagus is usually diagnosed during endoscopic examinations of middle-aged and older adults, with the mean age being 55 years of age. It is most commonly found in Caucasian males and associated with the use of smoking tobacco. The male-to-female ratio is approximately 2:1 [1] and it appears to be uncommon in African Americans [23,24]. Abdominal obesity as measured by an increased waist-to-hip ratio is associated with an increased risk of Barrett’s esophagus [25,26]. Germline mutations in the MSR1, ASCC1, and CTHRC1 genes have been associated with the presence of Barrett’s esophagus and esophageal adenocarcinoma [27]. Risk factors are listed in Table 1.
Clinical Symptoms
Columnar metaplasia itself does not cause any symptoms but is merely the adaptation of the cells to the repeated effect of the acid. The main clinical symptoms of the disease would initially be symptoms associated with GERD, such as heartburn, water brash, and dysphagia [1]. Severe presentations of GERD, such as esophageal ulceration, stricture, and hemorrhage, usually occur with long-segment Barrett’s esophagus [29,30]. However, 40% of patients presenting with adenocarcinoma had no history of GERD or symptoms of heartburn [13]. Furthermore, as few as 5% of those presenting with adenocarcinoma were known to have Barrett’s esophagus [31].
D iagnosis
Barrett’s esophagus generally requires an endoscopic examination with biopsy confirmation from the distal esophagus showing specialized intestinal columnar epithelium [32]. The biopsy specimen is acquired from the cellular lining proximal to gastroesophageal junction [5,33]. Barrett’s esophagus is classified into long- and short-segment based on the length of salmon-colored mucosa in the distal esophagus. A distance longer than 3 cm is
The Prague classification was presented by an international research group in 2006 and is regarded as the standard for measuring the length of Barrett’s esophagus. The lower measurement boundary is formed by the proximal cardial notch, and the 2 upper measurement boundaries are marked by the proximal limit of the circumferential Barrett’s segment and the longest tongue of Barrett’s [37].Confirmation of the diagnosis of dysplastic
Once the initial diagnosis of Barrett’s esophagus is made, we recommend referring the patient to a Barrett’s esophagus specialized center in order to offer the patient a second opinion from a team of experts in Barrett’s esophagus. This would avoid possible false-positive results, which can be as high as 40% [1,8]. It would also offer the patient a comprehensive multidisciplinary approach and adequate long-term management. It is further preferred if an advanced intervention is offered to the patient such as endoscopic mucosal resection or endoscopic submucosal dissection. Expert endoscopists
The availability of advanced endoscopic tools improves diagnostic yield. Adopting advanced techniques can reduce errors during biopsy sampling [40–42]. Some of the newer advanced imaging endoscopic tools include chromoendoscopy, optical coherence tomography, confocal microendoscopy, autofluorescence endoscopy, narrow band imaging (NBI), and Fujinon intelligent chromoendoscopy (FICE) [43,44]. In a meta-analysis examining whether advanced techniques improved diagnostic yield, it was found that advanced imaging increased the diagnostic yield by 34%. Advanced technology mentioned here is not mandated in current guidelines [5,45].
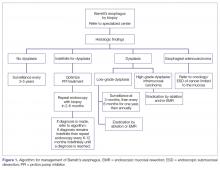
Histopathology categories and TNM staging system for Barrett’s esophagus are shown in Table 2 and Table 3. A management algorithm based on histologic findings is presented in Figure 1.
S creening
Logically, Barrett’s esophagus screening should be offered to every patient with GERD; however, this is against current recommendations because it is cost-prohibitive [46,47]. It is a given that the rationale behind the screening is to decrease morbidity and mortality from esophageal adenocarcinoma by offering early and definitive intervention [14,48,49]. The gold standard for screening is upper endoscopy, although nonendoscopic methods are being studied [1]. For example, a capsule attached to a string can be swallowed by the patient, the capsule is then deployed and pulled through the esophagus to obtains a brush sample of the cells. It is a promising technology due to high sensitivity and specificity [50]; however, it is not regularly utilized in practice.
Approaches to screening for Barrett’s esophagus has been addressed by multiple societies with several guidelines currently available [51]. None of these approaches have been proven to be superior in clinical studies. The American Gastroenterological Association (AGA) recommends screening patients with multiple risk factors associated with esophageal adenocarcinoma for Barrett’s esophagus. Risk factors listed by the AGA include white patients, male patients, patients above the age of 50 with a history of chronic GERD, hiatal hernia, elevated body mass index, and certain body fat distribution. The AGA recommends against screening the general population with GERD [9]. The American College of Gastroenterology (ACG) recommends upper endoscopy only in the presence of alarm symptoms (eg, dysphagia, weight loss, gastrointestinal bleeding) and for screening of patients at high risk for complications [52]. The American College of Physicians recommends upper endoscopy for screening for Barrett’s esophagus in men older than 50 years with GERD symptoms for more than 5 years with any risk factors like nocturnal reflux symptoms, hiatus hernia, elevated body mass index, tobacco use, and intra-abdominal distribution of fat [1].
Overall, the sensitivity of endoscopy to diagnose Barrett’s esophagus, as seen in a Veterans Affairs (VA) cohort study for the detection of Barrett’s, is about 80%. The concluded 80% sensitivity rate was based on the performance of 2 endoscopies, 6 weeks apart for each patient, with subsequent labeling of the diagnosis if intestinal metaplasia was found in either of the 2 biopsy samples taken from the 2 procedures [53].
Promising molecular biomarkers have been associated with Barrett’s esophagus including p53 and cyclin D1 expression. However, additional studies are needed before they are incorporated as part of screening practices [5].
General Management
Medical Management of GERD
All patients with Barrett’s esophagus should be treated with a proton pump inhibitor (PPI) indefinitely based on multiple studies [54–56]. Effective control of GERD was associated with a decreased risk of dysplasia and adenocarcinoma (adjusted odds ratio 0.29, 95% CI 0.12–0.79) [57].
Effective control of GERD decreases chronic esophageal inflammation, which could progress to Barrett’s esophagus and has a risk of possible progression to adenocarcinoma. In one study, patients with proven Barrett’s esophagus showed partial regression of the intestinal metaplasia with aggressive PPI therapy [57–59]. Despite this, it is not clear whether the regression decreased risk of malignant progression [58,60,61]. In a study of 68 patients, aggressive acid reduction with omeprazole 40 mg twice a day lead to partial regression of Barrett’s esophagus when compared to mild suppression with ranitidine 150 mg twice a day. However, there was no reduction in the risk of cancer [59].
Surveillance
The reasoning behind surveillance is to detect dysplasia or adenocarcinoma in patients known to have Barrett’s esophagus early enough to provide early and efficient treatment to improve the outcome. Surveillance is done by endoscopy with biopsies in addition to sampling any irregularity [1,62,63]; however, the evidence to back up the benefit of surveillance is not clear [5,64]. It should be noted that surveillance carries risks, and morbidity associated with repeated procedures may affect patients psychologically and financially. Patients who have Barrett’s esophagus are more likely to die from other more common diseases, such as coronary heart disease, prior to developing adenocarcinoma [65]. In a recent meta-analysis, the mortality rate due to esophageal adenocarcinoma was 3.0 per 1000 person-years, whereas the mortality rate due to other causes was 37.1 per 1000 person-years [65]. An ongoing randomized, multicenter trial which assesses scheduled endoscopy every 2 years will shed more light on the overall survival after applying surveillance recommendations for each grade of dysplasia [66].
The following are ACG recommendations for surveillance of Barrett’s esophagus based on the histopathology report. The histopathology report delineates 1 of 3 types of columnar epithelium [68]: cardiac epithelium of mucus-secreting cells, atrophic gastric fundic type epithelium, or specialized columnar cells with goblet cells. The latter type is the most common with high potential for cancer [32]. Degrees of dysplasia and possible adenocarcinoma is usually described in the report as well. The degree of dysplasia if found helps the endoscopist plan the next step in management. It is worth mentioning that sampling errors can lead to missing a diagnosis. In a meta-analysis, 13% of patients diagnosed with high-grade dysplasia who underwent resection were found to have invasive cancer [69].
Surveillance for Patients with No Dysplasia
We suggest surveillance every 3 to 5 years since the rate of neoplasia is low [14]. For management of select patients with no dysplasia and with additional risk factors, radiofrequency ablation (RFA) may be an option, although it remains a controversial approach. For example, in a patient under 50 years of age, family history would be an argument for proceeding with RFA instead of prolonged surveillance. In a prospective cohort study of 139 patients with 10-year follow-up after ablation, recurrent Barrett’s occurred in less than 5% of the patients. [70]
Surveillance for Patients with Biopsy Showing “Indefinite for Dysplasia”
Aggressive treatment with PPI twice daily is recommended to avoid the misinterpretation of reactive esophageal changes secondary to reflux as dysplasia on the following endoscopy with biopsy. These patients will require a repeat endoscopy with biopsies after 3 months of aggressive treatment with PPI. Biopsies should be taken every 1 centimeter within Barrett’s epithelium [1].
If it remains indefinite, biopsies should be examined by a second pathologist with expertise in Barrett’s esophagus. If the second pathologist agrees on the indefinite diagnosis for dysplasia, then endoscopy every 12 months is recommended [1]. Treatment versus surveillance after repeat endoscopy and biopsy should be tailored to the new histopathology results on the most recent exam.
Surveillance for Patients with Biopsy Results showing Low-Grade Dysplasia (LGD), High-Grade Dysplasia (HGD), or Intramucosal Carcinoma
Surveillance recommendations are discussed under Management of Dysplasia or Intramucosal Carcinoma, below.
Efficacy of Surveillance
Asymptomatic adenocarcinoma could be discovered during surveillance, and neoplasia detected during surveillance is usually less advanced than those found after development of symptoms such as dysphagia, bleeding or weight loss [2,3,71–75]. These studies obviously had lead–time bias and did not document terminal cancer in patients adherent to surveillance protocol.
Management of Dysplasia or Intramucosal Carcinoma
Overview
Historically, dysplasia was managed with esophagectomy, which was associated with high morbidity and mortality. With advancement in the field of endoscopy, dysplasia is managed quite differently today, with endoscopic eradication therapy, which includes the use of endoscopic ablation techniques and endoscopic resection. The advantage of endoscopic resection is preservation of resected tissue for further examination, thus providing valuable information regarding the stage of the tumor (depth). Histological examination is not possible with photo or thermal ablation techniques as destroyed mucosa cannot be submitted for tissue analysis.
Low-Grade Dysplasia
If low-grade dysplasia is found, it is followed by a repeat endoscopy 8 weeks after aggressive PPI therapy. The repeat endoscopy should be performed with high definition/high-resolution endoscopy. The rationale of a second endoscopy is to ensure that the metaplastic mucosa was adequately inspected and biopsied prior to further intervention [1,9]. If the diagnosis is confirmed as low-grade dysplasia, and the patient prefers to go with the path of intervention instead of conservative management (endoscopic surveillance every 6 months for 1 year then annually with biopsies), then multiple options are available for the patient, including RFA or cryotherapy [8] [76]. In a randomized clinical trial in patients with Barrett’s esophagus and LGD, RFA was shown to reduce risk of neoplastic progression over a 3-year follow-up. The study included 136 patients randomized to receive ablation and 68 patients who underwent endoscopic surveillance. In the ablation group, the risk of progression to HGD or esophageal adenocarcinoma was reduced by 25% and the risk of progression to adenocarcinoma was reduced by 7.4%. In the ablation group, complete eradication of dysplasia and intestinal metaplasia occurred in 92.6% and 88.2% of patients respectively. In the endoscopic surveillance group, complete eradication of dysplasia and intestinal metaplasia was seen in 27.9% and 0.0% of patients respectively. [76]. Treatment-related adverse events occurred in 19.1% of patients receiving ablation (P < 0.001). The most common adverse event was stricture, occurring in 8 patients receiving ablation (11.8%), all resolved by endoscopic dilation [76,78].
Surveillance after ablation of LGD is still an ongoing debate, and further evidence is needed to establish guidelines [8]. Due to lack of evidence, we would lean towards surveillance for those patients with an annual esophagogastroduodenoscopy with biopsy examination (author’s opinion, no associated level of evidence).
High-Grade Dysplasia or Intramucosal Carcinoma
For patients with HGD or intramucosal carcinoma without submucosal invasion, eradication is the treatment of choice. Current guidelines advocate for endoscopic eradication therapy for most if not all patients with HGD or intramucosal carcinoma with a goal of removing all metaplastic and dysplastic tissue [1,5,62,64]. It should be noted that the biopsy specimen should be extensive to decrease the error margin. If the diagnosis were made on endoscopy without procuring extensive biopsies, then repeat endoscopy with extensive biopsies is needed prior to deciding the treatment path. The rationale behind extensive biopsies is to confirm the diagnosis and to determine the extent of dysplasia. Other factors potentially influencing the treatment path include the patient’s age, comorbid conditions, quality of life, and available access to an advanced endoscopist or specialized surgeon. Patient’s preferences and adherence to follow-up visits should also be a consideration.
The long-term benefits of endoscopic intervention versus surgical intervention are not well established. Esophagectomy is no longer a preferred method of treatment due to high morbidity and mortality associated with the procedure when compared to endoscopic interventions. However, it is still a preferred choice amongst a select group of patients unwilling to follow-up. A cost-effective analysis found that endoscopic ablation provided the longest quality adjusted life expectancy for Barrett’s esophagus with HGD [79,80].
Endoscopic Therapy in Barrett’s Esophagus
Endoscopic Ablative Therapies
With the advancement of endoscopic intervention, we now have multiple tools to ablate abnormal epithelium in Barrett’s esophagus. Examples of ablation techniques include thermal, photochemical, and mechanical techniques [81,82]. RFA is the treatment of choice for ablation [83]. However, non-contact ablative therapy, such as cryoablation, may be prefered if topography of the esophagus doesn’t allow contact ablation.
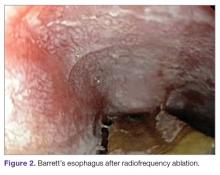
Radiofrequency Ablation (Figure 2). RFA is a procedure in which heat is generated from medium frequency alternating current and leads to thermal injury [84]. In Barrett’s esophagus, RFA uses radiofrequency energy delivered by a balloon that has a series of closely spaced electrodes in a
Most patients will require multiple sessions of RFA to achieve eradication. It is very rarely a one-time procedure. In a meta-analysis of 18 studies including 3082 patients, the most common adverse effects of RFA were stricture in 5% [76,98], bleeding in 1%, and pain in 3% of patients [99].
It is crucial for successful RFA to continue medical treatment for acid suppression, in order to allow healthy regeneration of the squamous cell lining. It is suggested to use PPI twice a day with sucralfate and ranitidine after the intervention [100,101]. Adhering to a liquid diet for 24 hours is needed, followed by a soft diet to allow faster regeneration of the epithelium.
The caveat with RFA is that new evidence shows a higher rate of recurrence than previously thought. In one study of 246 patients, recurrence of dysplasia occurred in 25% of patients at 48 months after eradication in 80% of the patients, and metaplasia occurred in 50% at 60 months [102]. The other risk is buried Barrett’s, a condition occurring after incomplete ablation, in which squamous cell epithelium covers patches of incompletely destroyed intestinal lining, leading to possible progression of the disease to adenocarcinoma under the surface [103].
It has been reported that patients who underwent RFA had remarkable improvement in quality of life even if RFA did not achieve eradication. Patients reported less depression, less stress and better quality of life [104].
Based on a survey of experts, follow-up at 3 months, 6 months, and then annually is recommended after ablation [1,105]. Biopsies should be taken distal to neosquamous epithelium and from suspicious areas [97,106].
Endoscopic Spray Cryotherapy. This technique involves application of liquid nitrogen or carbon dioxide gas by endoscope on the tissue to freeze it off. Although it has been shown to eliminate HGD in over 95% of the cases and all dysplasia in over 85% of the cases, it was effective in eradicating intestinal metaplasia in only 55% of patients [103,108,109]. Thus, RFA as ablation therapy is still superior to cryotherapy and is still the first-line treatment for dysplastic Barrett’s esophagus. In comparison to cryotherapy, RFA efficacy has been studied extensively with well documented outcomes. However, there is a role for cryotherapy over RFA in certain clinical situations (such as severe chest pain from RFA or lack of efficacy in eradicating intestinal metaplasia or dysplasia by RFA).
Similar to RFA, on occasions of partial ablation, the remaining metaplastic tissue may get buried beneath a layer of squamous epithelium and can possibly progress to adenocarcinoma [110].
Photodynamic Therapy (PDT). This technique works by producing cytotoxicity at the cellular level by exposure to light at a specific wavelength in the presence of a chemical agent known as photosensitize [107]. Although superior to omeprazole, PDT has a significant rate of complications, mainly stricture, and a high occurrence of esophageal cancer during follow-up. For this reason, it is less favorable compared to RFA [107] and mentioned here as a historical therapy.
Endoscopic Resection Techniques: Endoscopic Mucosal Resection (EMR) and Endoscopic Submucosal Dissection (ESD)
Unlike flat mucosa in Barrett’s esophagus, which respond to ablative techniques such as RFA or cryotherapy, nodular Barrett’s esophagus is hard to treat and requires endoscopic resection prior to ablation. Endoscopic mucosal resection (EMR) is the most widely used technique and it is available in most tertiary referral centers. Another technique named endoscopic submucosal dissection (ESD) allows the removal of large nodular areas of Barrett’s esophagus in one piece to ensure complete removal of nodular dysplasia. ESD is technically challenging and it is only available in a handful of centers in the US. Endoscopic resection techniques are the preferred interventions for nodular dysplasia due to their ability to provide valuable information for staging the lesion [111–113]. Endoscopic resection techniques are safer and more effective with similar or better results when compared with other approaches [114].
EMR is completed by the excision of esophageal mucosa down to the submucosa and submitting a large tissue specimen to the pathologist. It additionally serves as a therapeutic measure in cases of no submucosal extension. Another advantage of EMR is the ability to predict lymph node metastasis. The rationale is based on the fact that the most important predictor of lymph node metastasis is the depth of the tumor; hence, invasive tumors would likely be associated with lymph node metastasis [115,116].
In a systematic review of 11 studies, complete EMR was as equally effective in the short-term treatment of dysplastic Barrett’s esophagus when compared to RFA, but adverse event rates were greater with complete EMR (mainly strictures). Strictures are more likely to occur in patients undergoing extensive EMR. In another meta-analysis of 22 studies comparing the efficacy of EMR to RFA, both techniques were effective in eradicating dysplasia (95% in EMR group and 92% in RFA group). However, extensive EMR was associated with higher complication rates suggesting that a combined endoscopic approach of focal EMR followed by RFA is preferred over extensive EMR alone [86].
It should be noted that EMR and ESD information were derived from highly specialized center and these results may not be duplicated in community settings [113,117].
Efficacy of Endoscopic Resection. Endoscopic resection has a success rate comparable to surgical esophagectomy with fewer complications [113,114,118–121] in patients with HGD and early stages of esophageal cancer [122]. Complete remission can be as high as 89%. Recurrence occurred in 6% to 30% of patients [114,118,119], which was attributed to incomplete removal, large lesions, failure to use adjunct therapy, or lack of follow-up [123]. Even when recurrence occurred, it was successfully managed by endoscopic intervention [124].
In a large cohort study of 1000 patients with early mucosal adenocarcinoma who were treated with endoscopic resection, long-term complete remission occurred in 94% of patients. There was no mortality and less than 2% of patients had major complications. Infrequent complications include bleeding, perforations, and strictures [123,125,126]. The rate of complications is lower in highly specialized centers [127–129].
Surgery was necessary in 12 patients (3.7%) after endoscopic therapy failed [123]. Post-resection care and follow-up is similar to the post-RFA care discussed above.
Management of Invasive Esophageal Adenocarcinoma
Patients diagnosed with an invasive adenocarcinoma need to be referred to an oncologist for staging and to discuss treatment options. A select number of patients may be referred by oncology for endoscopic resection, yet the need for a multidisciplinary approach in these situations is absolutely necessary [1].
Esophagectomy
Esophagectomy offers the complete removal of the HGD along with any adenocarcinoma in the regional lymph nodes. However, mortality rates are as high as 12% immediately after the procedure [130]. The multitude of short- and long-term morbidity has significant effects on quality of life. Short-term morbidity is as high as 30%. Patients may develop serious postoperative complications such as myocardial infarction, hospital associated pneumonia, or anatomic leak [131].
Examples of long-term morbidity include dysphagia, transection of vagal nerve, and dumping syndrome. Recent development in minimally invasive surgeries for esophagectomy has not reduced postoperative morbidity rates [132].
Advocates of esophagectomy illustrate the advantage of eradication of occult lymph node metastasis. The counter argument has been established by a systemic review in which occult lymph node metastasis occurred in less than 2% of patients with HGD and intramucosal carcinoma; whereas the mortality rate after esophagectomy is substantially higher with no guarantee of curing metastatic disease [133].
Prevention of Barrett’s Esophagus
Since Barrett’s esophagus precedes most of the cases of EAC if not all [1,134], methods that aim at decreasing the incidence of Barrett’s esophagus could help in prevention. The modifiable risk factors listed by the AGA include BMI, GERD, and hiatal hernia management. Along with diet and exercise, the advent of new therapies to help patients manage their weight could in return help in avoiding a plethora of medical conditions including Barrett’s esophagus. Hiatal hernia management could lower the risk of Barrett’s by restoring normal anatomy. Lastly, proper management of GERD would lower the risk of developing Barrett’s esophagus as discussed in this article [1,9].
It is worth noting that a large trial on the efficacy and safety of aspirin for prevention of adenocarcinoma progression in Barrett’s esophagus is ongoing in the UK (AspECT trial). The AspECT trial examines the efficacy of low dose vs. high dose PPI with or without aspirin for the chemoprevention of esophageal adenocarcinoma. The theory behind the study is the inhibition of COX 2 receptors in Barrett’s cells can decrease tissue progression to cancer. This chempreventive effect of nonsteroidal anti-inflammatory drugs was shown to be augmented when combined with statin intake [56,135–138].
Conclusion
Barrett’s esophagus is usually diagnosed during routine endoscopic examination. The initial symptoms are those associated with GERD, like heartburn, dyspepsia, and regurgitation. Specialized columnar epithelium is the hallmark of histopathological diagnosis. Recommendations of the ACG and AGA suggest treatment based on biopsy results. The intervention would vary on a wide spectrum starting from acid suppression, radiofrequency ablation, endoscopic resection therapy, and rarely, esophagectomy.
Corresponding author: Mohamed O. Othman, MD, Gastroenterology and Hepatology Section, Baylor College of Medicine, 7200 Cambridge St., Suite 8C, Houston, TX 77030, [email protected].
Financial disclosures: Dr. Othman has received grant support from Abbvie and has served as a consultant for Olympus.
1. Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016;111:30–50; quiz 51.
2. Peters JH, Clark GW, Ireland AP. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg 1994;108:813–21; discussion 821–2.
3. Streitz JM Jr, Andrews CW Jr, Ellis FH Jr. Endoscopic surveillance of Barrett’s esophagus. Does it help? J Thorac Cardiovasc Surg 1993;105:383–7; discussion 387–8.
4. Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med 2014;371:836–45.
5. Spechler SJ, Sharma P, Souza RF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084–91.
6. Sharma P, McQuaid K, Dent J, et al; AGA Chicago Workshop. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology 2004;127:310–30.
7. ASGE Standards of Practice Committee, Evans JA, Early DS; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc 2012;76(6):1087–94.
8. Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and management of low–grade dysplasia in Barrett’s esophagus: Expert review from the clinical practice updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151:822–835.
9. American Gastroenterological, A., et al., American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology, 2011. 140(3): p. 1084–91. [Same as #5]
10. Van Eyken P. Definition of Barrett’s oesophagus. Acta Gastroenterol Belg 2000;63:10–2.
11. Hirota WK, Loughney TM, Lazas DJ, et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology 1999;116(2):277–85.
12. Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar–lined (Barrett’s) esophagus. Comparison of population–based clinical and autopsy findings. Gastroenterology 1990;99:918–22.
13. Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology 2002;123:461–7.
14. Van der Veen AH, Dees J, Blankensteijn JD, Van Blankenstein M. Adenocarcinoma in Barrett’s oesophagus: an overrated risk. Gut 1989;30:14–8.
15. Winters C Jr, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987;92:118–24.
16. Wani S, Falk G, Hall M, et al. Patients with nondysplastic Barrett’s esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2011;9:220–7;quiz e26.
17. Ward EM, Wolfsen HC, Achem SR, et al. Barrett’s esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol 2006;101:12–7.
18. Rex DK, Cummings OW, Shaw M. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology 2003;125:1670–7.
19. Rubenstein JH, Taylor JB. Meta–analysis: the association of oesophageal adenocarcinoma with symptoms of gastro–oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
20. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
21. Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology, 2005;129:1825–31.
22. Sampliner RE. A population prevalence of Barrett’s esophagus––finally. Gastroenterology 2005; 129:2101–3.
23. Abrams JA, Fields S, Lightdale CJ, Neugut AI. Racial and ethnic disparities in the prevalence of Barrett’s esophagus among patients who undergo upper endoscopy. Clin Gastroenterol Hepatol 2008;6:30–4.
24. Corley DA, Kubo A, Levin TR, et al. Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community–based study, 1994–2006. Gut 2009;58:182–8.
25. Kamat P, Wen S, Morris J, Anandasabapathy S. Exploring the association between elevated body mass index and Barrett’s esophagus: a systematic review and meta–analysis. Ann Thorac Surg 2009;87:655–62.
26. Jacobson BC, Chan AT, Giovannucci EL, Fuchs CS. Body mass index and Barrett’s oesophagus in women. Gut 2009;58:1460–6.
27. Orloff M, Peterson C, He X, et al. Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma. JAMA 2011;306:410–9.
28. Sharma N, Ho KY. Risk Factors for Barrett’s oesophagus. Gastrointest Tumors 2016;3:103–8.
29. Spechler SJ. Barrett’s esophagus. Semin Gastrointest Dis 1996;7:51–60.
30. Ronkainen J, Talley NJ, Storskrubb T. Erosive esophagitis is a risk factor for Barrett’s esophagus: a community–based endoscopic follow–up study. Am J Gastroenterol 2011;106:1946–52.
31. Kim SL, Wo JM, Hunter JG. The prevalence of intestinal metaplasia in patients with and without peptic strictures. Am J Gastroenterol 1998;93:53–5.
32. Spechler SJ. Clinical practice. Barrett’s esophagus. N Engl J Med 2002;346:836–42.
33. Riddell RH, Odze RD. Definition of Barrett’s esophagus: time for a rethink––is intestinal metaplasia dead? Am J Gastroenterol 2009;104:2588–94.
34. Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus––the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol 1998;93:1033–6.
35. Eloubeidi MA, Provenzale D. Does this patient have Barrett’s esophagus? The utility of predicting Barrett’s esophagus at the index endoscopy. Am J Gastroenterol 1999;94:937–43.
36. Rudolph RE, Vaughan TL, Storer BE, et al. Effect of segment length on risk for neoplastic progression in patients with Barrett esophagus. Ann Intern Med 2000;132:612–20.
37. Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392–9.
38. Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population–based study. J Natl Cancer Inst 2011;103:1049–57.
39. Shaheen NJ, Dulai GS, Ascher B, et al. Effect of a new diagnosis of Barrett’s esophagus on insurance status. Am J Gastroenterol 2005;100:577–80.
40. Canto MI, Setrakian S, Willis J, et al. Methylene blue–directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest Endosc 2000;51:560–8.
41. Scotiniotis IA, Kochman ML, Lewis JD. Accuracy of EUS in the evaluation of Barrett’s esophagus and high–grade dysplasia or intramucosal carcinoma. Gastrointest Endosc 2001;54:689–96.
42. Kobayashi K, Izatt JA, Kulkarni MD, et al. High–resolution cross–sectional imaging of the gastrointestinal tract using optical coherence tomography: preliminary results. Gastrointest Endosc 1998;47:515–23.
43. Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance, and light–scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology 2001. 120: 1620–9.
44. Wallace MB, Sharma P, Lightdale C, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe–based confocal laser endomicroscopy. Gastrointest Endosc 2010;72:19–24.
45. Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta–analysis and systematic review. Clin Gastroenterol Hepatol 2013;11:1562–70.e1–2.–
46. DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1999;94:1434–42.
47. Inadomi JM, Sampliner R, Lagergren J. Screening and surveillance for Barrett esophagus in high–risk groups: a cost–utility analysis. Ann Intern Med 2003;138:176–86.
48. Conio M, Blanchi S, Lapertosa G, et al. Long–term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol 2003;98:1931–9.
49. Rastogi A, Puli S, El–Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high–grade dysplasia: a meta–analysis. Gastrointest Endosc 2008;67:394–8.
50. Kadri SR, Lao–Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non–endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ 2010;341:c4372.
51. Sharma, P., et al., A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology, 2004. 127(1): p. 310–30. [Same as #6]
52. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–28; quiz 329.
53. Kim SL, Waring JP, Spechler SJ, et al. Diagnostic inconsistencies in Barrett’s esophagus. Department of Veterans Affairs Gastroesophageal Reflux Study Group. Gastroenterology 1994;107:945–9.
54. Kastelein F, Spaander MC, Steyerberg EW, et al; ProBar Study Group. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2013;11:382–8.
55. El–Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol 2004;99:1877–83.
56. Nguyen DM, El–Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
57. Singh S, Garg SK, Singh PP, et al. Acid–suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta–analysis. Gut 2014;63:1229–37.
58. Spechler SJ, Lee E, Ahnen D, et al. Long–term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow–up of a randomized controlled trial. JAMA 2001;285:2331–8.
59. Peters FT, Ganesh S, Kuipers EJ, et al. Endoscopic regression of Barrett’s oesophagus during omeprazole treatment; a randomised double blind study. Gut 1999;45:489–94.
60. Ouatu–Lascar R, Triadafilopoulos G. –Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal acid reflux in patients with Barrett’s esophagus. Am J Gastroenterol 1998;93:711–6.
61. Maret–Ouda J, Konings P, Lagergren J, Brusselaers N. Antireflux surgery and risk of esophageal adenocarcinoma: A systematic review and meta–analysis. Ann Surg 2016;263:251–7.
62. Evans, J.A., et al., The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc, 2012. 76(6): p. 1087–94. [Same as #7]
63. ASGE Standards of Practice Committee, Evans JA, Early DS,et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
64. Bennett C, Moayyedi P, Corley DA, et al; BOB CAT Consortium. BOB CAT: A large–scale review and delphi consensus for ,anagement of Barrett’s esophagus with no dysplasia, indefinite for, or low–grade dysplasia. Am J Gastroenterol 2015;110:662–82; quiz 683.
65. Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta–analysis. Clin Gastroenterol Hepatol 2010;8:235–44; quiz e32.
66. Old O, Moayyedi P, Love S, et al; BOSS Trial Team. Barrett’s Oesophagus Surveillance versus endoscopy at need Study (BOSS): protocol and analysis plan for a multicentre randomized controlled trial. J Med Screen 2015;22:158–64.
67. Weston AP, Sharma P, Topalovski M, et al. Long–term follow–up of Barrett’s high–grade dysplasia. Am J Gastroenterol 2000;95:1888–93.
68. Paull A, Trier JS, Dalton MD, et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med 1976;295:476–80.
69. Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high–grade dysplasia in Barrett’s esophagus overestimated? Clin Gastroenterol Hepatol 2008;6:159–64.
70. Allison H, Banchs MA, Bonis PA, Guelrud M. Long–term remission of nondysplastic Barrett’s esophagus after multipolar electrocoagulation ablation: report of 139 patients with 10 years of follow–up. Gastrointest Endosc 2011;73:651–8.
71. Corley DA, Levin TR, Habel LA, Weiss NS, et al. Surveillance and survival in Barrett’s adenocarcinomas: a population–based study. Gastroenterology 2002;122:633–40.
72. Wong T, Tian J, Nagar AB. Barrett’s surveillance identifies patients with early esophageal adenocarcinoma. Am J Med 2010;123:462–7.
73. Fountoulakis A, Zafirellis KD, Dolan K, et al. Effect of surveillance of Barrett’s oesophagus on the clinical outcome of oesophageal cancer. Br J Surg 2004;91:997–1003.
74. Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population–based cohort study. Am J Gastroenterol 2014;109:1215–22.
75. Kastelein F, van Olphen SH, Steyerberg EW, et al. Impact of surveillance for Barrett’s oesophagus on tumour stage and survival of patients with neoplastic progression. Gut 2016;65:548–54.
76. Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low–grade dysplasia: a randomized clinical trial. JAMA 2014;311: 1209–17.
77. Wang WL, Chang IW, Chen CC, et al. Radiofrequency ablation versus endoscopic submucosal dissection in treating large early esophageal squamous cell neoplasia. Medicine (Baltimore) 2015;94:e2240.
78. Lim CH, Treanor D, Dixon MF, Axon AT. Low–grade dysplasia in Barrett’s esophagus has a high risk of progression. Endoscopy 2007;39:581–7.
79. Shaheen NJ, Inadomi JM, Overholt BF, Sharma P. What is the best management strategy for high grade dysplasia in Barrett’s oesophagus? A cost effectiveness analysis. Gut 2004;53:1736–44.
80. Vij R, Triadafilopoulos G, Owens DK, et al. Cost–effectiveness of photodynamic therapy for high–grade dysplasia in Barrett’s esophagus. Gastrointest Endosc 2004;60:739–56.
81. van den Boogert J, v an Hillegersberg R, Siersema PD, et al. Endoscopic ablation therapy for Barrett’s esophagus with high–grade dysplasia: a review. Am J Gastroenterol 1999;94:1153–60.
82. Sampliner RE. Endoscopic ablative therapy for Barrett’s esophagus: current status. Gastrointest Endosc 2004;59:66–9.
83. Sharma VK, Wang KK, Overholt BF, et al. Balloon–based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1–year follow–up of 100 patients. Gastrointest Endosc 2007;65:185–95.
84. Hanlon CR. Textbook of surgery: The biological basis of modern surgical practice, 14th edition. Ann Surg 1992;216:94.
85. Bright T, Watson DI, Tam W, et al. Randomized trial of argon plasma coagulation versus endoscopic surveillance for barrett esophagus after antireflux surgery: late results. Ann Surg 2007;246:1016–20.
86. Chadwick G, Groene O, Markar SR, et al. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett’s esophagus: a critical assessment of histologic outcomes and adverse events. Gastrointest Endosc 2014;79:718–31.e3.
87. Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett’s neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy 2008;40:370–9.
88. Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol 2010;8:23–9.
89. Kim HP, Bulsiewicz WJ, Cotton CC, et al. Focal endoscopic mucosal resection before radiofrequency ablation is equally effective and safe compared with radiofrequency ablation alone for the eradication of Barrett’s esophagus with advanced neoplasia. Gastrointest Endosc 2012;76:733–9.
90. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic ablation of Barrett’s esophagus: a multicenter study with 2.5–year follow–up. Gastrointest Endosc 2008;68:867–76.
91. Sharma VK, Jae Kim H, Das A, et al. Circumferential and focal ablation of Barrett’s esophagus containing dysplasia. Am J Gastroenterol 2009;104:310–7.
92. Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett’s esophagus that contains high–grade dysplasia: a U.S. multicenter registry. Gastrointest Endosc 2008;68:35–40.
93. Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology 2013;145:79–86.e1.
94. Lyday WD, Corbett FS, Kuperman DA, et al. Radiofrequency ablation of Barrett’s esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy 2010;42:272–8.
95. Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12:1840–7.e1.
96. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5–year outcomes from a prospective multicenter trial. Endoscopy 2010;42:781–9.
97. Weston AP, Sharma P, Banerjee S, et al. Visible endoscopic and histologic changes in the cardia, before and after complete Barrett’s esophagus ablation. Gastrointest Endosc 2005;61:515–21.
98. Beaumont H, Gondrie JJ, McMahon BP, et al. Stepwise radiofrequency ablation of Barrett’s esophagus preserves esophageal inner diameter, compliance, and motility. Endoscopy 2009;41:2–8.
99. Orman ES, Li N, Shaheen NJ.Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta–analysis. Clin Gastroenterol Hepatol 2013;11:1245–55.
100. Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 2012;143:576–81.
101. Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra–esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett’s esophagus. Dig Dis Sci 2012;57:2625–32.
102. Small AJ, Sutherland SE, Hightower JS, et al. Comparative risk of recurrence of dysplasia and carcinoma after endoluminal eradication therapy of high–grade dysplasia versus intramucosal carcinoma in Barrett’s esophagus. Gastrointest Endosc 2015;81:1158–66.e1–4.
103. Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high–grade dysplasia. Gastrointest Endosc 2010;71:680–5.
104. Shaheen NJ, Peery AF, Hawes RH, et al. Quality of life following radiofrequency ablation of dysplastic Barrett’s esophagus. Endoscopy 2010;42:790–9.
105. Bedi AO, Kwon RS, Rubenstein JH, et al. A survey of expert follow–up practices after successful endoscopic eradication therapy for Barrett’s esophagus with high–grade dysplasia and intramucosal adenocarcinoma. Gastrointest Endosc 2013;78:696–701.
106. Sampliner RE, Camargo E, Prasad AR. Prasad, Association of ablation of Barrett’s esophagus with high grade dysplasia and adenocarcinoma of the gastric cardia. Dis Esophagus 2006;19:277–9.
107. Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: long–term results. Gastrointest Endosc 2003;58:183–8.
108. Gosain S, Mercer K, Twaddell WS, et al. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high–grade dysplasia: long–term results. Gastrointest Endosc 2013;78:260–5.
109. Canto MI, Shin EJ, Khashab MA, et al. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett’s esophagus. Endoscopy 2015;47:582–91.
110. Van Laethem JL, Peny MO, Salmon I, et al. Intramucosal adenocarcinoma arising under squamous re–epithelialisation of Barrett’s oesophagus. Gut 2000;46:574–7.
111. Pech O, May A, Gossner L, et al. Management of pre–malignant and malignant lesions by endoscopic resection. Best Pract Res Clin Gastroenterol 2004;18:61–76.
112. Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003;57:567–79.
113. Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high–grade dysplasia in Barrett’s esophagus. Gastroenterology 2000;118:670–7.
114. Pech O, Behrens A, May A, et al. Long–term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high–grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008;57:1200–6.
115. Vieth M, Ell C, Gossner L, et al. Histological analysis of endoscopic resection specimens from 326 patients with Barrett’s esophagus and early neoplasia. Endoscopy 2004;36:776–81.
116. Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high–grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004;60:703–10.
117. Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high–grade dysplasia within Barrett’s esophagus. Gastrointest Endosc 2000;52:328–32.
118. Esaki M, Matsumoto T, Hirakawa K, et al. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy 2007;39:41–5.
119. Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007;65:3–10.
120. Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high–grade dysplasia and intramucosal carcinoma––an American single–center experience. Am J Gastroenterol 2009;104:2684–92.
121. Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high–volume centers. Ann Surg 2011;254:67–72.
122. Wu J, Pan YM, Wang TT, et al. Endotherapy versus surgery for early neoplasia in Barrett’s esophagus: a meta–analysis. Gastrointest Endosc 2014;79:233–241.e2.
123. Pech O, May A, Manner H, et al. Long–term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652–660.e1.
124. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
125. May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high–grade neoplasia and early adenocarcinoma in Barrett’s oesophagus: acute–phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002;14(10):1085–91.
126. Gerke H, Siddiqui J, Nasr I, et al. Efficacy and safety of EMR to completely remove Barrett’s esophagus: experience in 41 patients. Gastrointest Endosc 2011;74:761–71.
127. Lewis JJ, Rubenstein JH, Singal AG, et al. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett’s esophagus. Gastrointest Endosc 2011;74:753–60.
128. Choi IJ, Kim CG, Chang HJ, et al. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc 2005;62:860–5.
129. Deprez PH, Bergman JJ, Meisner S, et al. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy 2010;42:853–8.
130. van Lanschot JJ, Hulscher JB, Buskens CJ, et al. Hospital volume and hospital mortality for esophagectomy. Cancer 2001;91:1574–8.
131. Karl RC, Schreiber R, Boulware D, et al. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg 2000;231:635–43.
132. Young MM, Deschamps C, Trastek VF, et al. Esophageal reconstruction for benign disease: early morbidity, mortality, and functional results. Ann Thorac Surg 2000;70:1651–5.
133. Dunbar KB, Spechler SJ. The risk of lymph–node metastases in patients with high–grade dysplasia or intramucosal carcinoma in Barrett’s esophagus: a systematic review. Am J Gastroenterol 2012;107:850–62; quiz 863.
134. Morales CP, Souza RF, Spechler SJ. Hallmarks of cancer progression in Barrett’s oesophagus. Lancet 2002;360:1587–9.
135. Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012;10:722–7.
136. Abnet CC, Freedman ND, Kamangar F, et al. Non–steroidal anti–inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta–analysis. Br J Cancer 2009;100:551–7.
137. Kastelein F, Spaander MC, Biermann K, et al. Nonsteroidal anti–inflammatory drugs and statins have chemopreventative effects in patients with Barrett’s esophagus. Gastroenterology 2011;141:2000–8; quiz e13–4.
138. Zhang S, Zhang XQ, Ding XW, et al. Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a meta–analysis. Br J Cancer 2014;110: 2378–88.
From the Gastroenterology and Hepatology Section, Baylor College of Medicine, Houston, TX.
Abstract
- Objective: To provide an update on management of Barrett’s esophagus.
- Methods: Review of the literature.
- Results: Management of Barrett’s esophagus depends on the degree of dysplasia. Surveillance by endoscopy every 3–5 years is recommended in patients with Barrett’s esophagus without dysplasia. Patients with Barrett’s esophagus and low-grade dysplasia should undergo surveillance by endoscopy in 3 months for confirmation of the diagnosis; if the diagnosis is confirmed then surveillance by endoscopy or eradication of Barrett’s epithelium by ablation or endoscopic resection are recommended. There is a sufficient evidence to recommend radiofrequency ablation of high-grade dysplasia within Barrett’s esophagus or to perform endoscopic mucosal resection of nodular Barrett’s esophagus with any degree of dysplasia. Early esophageal cancers that are limited to the mucosa can be treated by endoscopic resection, while cancer invading into the deep submucosa or muscularis propria may need esophagectomy with or without chemoradiation.
- Conclusion: The management of Barrett’s esophagus depends on the degree of dysplasia. Radiofrequency ablation and endoscopic mucosal resection are the most commonly used treatment for Barrett’s esophagus with dysplasia.
Keywords: Barrett’s esophagus; radiofrequency ablation; endoscopic submucosal dissection; endoscopic mucosal resection; early esophageal cancer.
Barrett’s esophagus is a common complication of chronic reflux disease [1]. Metaplastic changes that occur at the distal esophageal epithelium are usually asymptomatic [2,3] and occur as reparative adaptations to the insult of the gastric acid [4]. The management of Barrett’s esophagus after diagnosis is currently debated amongst experts without a clear consensus [5,6]. This review is generally consistent with the 2016 guidelines from the American College of Gastroenterology [1], a 2012 guideline from the American Society of Gastrointestinal Endoscopy [7], a 2011 guideline, and a 2016 expert review from the American Gastroenterological Association [8,9].
D efinition
Barrett’s esophagus is a metaplasia of the stratified squamous epithelium to a specialized columnar intestinal epithelium of mucus cells and goblet cells at the distal esophagus secondary to gastroesophageal reflux disease (GERD) [7,10]. Barrett’s esophagus is unstable tissue which can progress to esophageal adenocarcinoma. When unmanaged, the risk of cancer in dysplastic mucosa is at least thirty-fold greater than that for the general population [11–14], with recent studies suggesting a 0.4–0.7 occurrence rate per year [11,15]. With no dysplasia, the risk is low [16].
Epidemiology
The prevalence of Barrett’s esophagus is ~10% in patients with GERD [11–13,17], an estimate tied to the prevalence of GERD. However, due to the lack of symptoms of Barrett’s esophagus, no solid data supports this assumption [18–20]. A European study estimated the prevalence of Barrett’s esophagus to be 1.6% among the general population [21,22]. Barrett’s esophagus is usually diagnosed during endoscopic examinations of middle-aged and older adults, with the mean age being 55 years of age. It is most commonly found in Caucasian males and associated with the use of smoking tobacco. The male-to-female ratio is approximately 2:1 [1] and it appears to be uncommon in African Americans [23,24]. Abdominal obesity as measured by an increased waist-to-hip ratio is associated with an increased risk of Barrett’s esophagus [25,26]. Germline mutations in the MSR1, ASCC1, and CTHRC1 genes have been associated with the presence of Barrett’s esophagus and esophageal adenocarcinoma [27]. Risk factors are listed in Table 1.
Clinical Symptoms
Columnar metaplasia itself does not cause any symptoms but is merely the adaptation of the cells to the repeated effect of the acid. The main clinical symptoms of the disease would initially be symptoms associated with GERD, such as heartburn, water brash, and dysphagia [1]. Severe presentations of GERD, such as esophageal ulceration, stricture, and hemorrhage, usually occur with long-segment Barrett’s esophagus [29,30]. However, 40% of patients presenting with adenocarcinoma had no history of GERD or symptoms of heartburn [13]. Furthermore, as few as 5% of those presenting with adenocarcinoma were known to have Barrett’s esophagus [31].
D iagnosis
Barrett’s esophagus generally requires an endoscopic examination with biopsy confirmation from the distal esophagus showing specialized intestinal columnar epithelium [32]. The biopsy specimen is acquired from the cellular lining proximal to gastroesophageal junction [5,33]. Barrett’s esophagus is classified into long- and short-segment based on the length of salmon-colored mucosa in the distal esophagus. A distance longer than 3 cm is
The Prague classification was presented by an international research group in 2006 and is regarded as the standard for measuring the length of Barrett’s esophagus. The lower measurement boundary is formed by the proximal cardial notch, and the 2 upper measurement boundaries are marked by the proximal limit of the circumferential Barrett’s segment and the longest tongue of Barrett’s [37].Confirmation of the diagnosis of dysplastic
Once the initial diagnosis of Barrett’s esophagus is made, we recommend referring the patient to a Barrett’s esophagus specialized center in order to offer the patient a second opinion from a team of experts in Barrett’s esophagus. This would avoid possible false-positive results, which can be as high as 40% [1,8]. It would also offer the patient a comprehensive multidisciplinary approach and adequate long-term management. It is further preferred if an advanced intervention is offered to the patient such as endoscopic mucosal resection or endoscopic submucosal dissection. Expert endoscopists
The availability of advanced endoscopic tools improves diagnostic yield. Adopting advanced techniques can reduce errors during biopsy sampling [40–42]. Some of the newer advanced imaging endoscopic tools include chromoendoscopy, optical coherence tomography, confocal microendoscopy, autofluorescence endoscopy, narrow band imaging (NBI), and Fujinon intelligent chromoendoscopy (FICE) [43,44]. In a meta-analysis examining whether advanced techniques improved diagnostic yield, it was found that advanced imaging increased the diagnostic yield by 34%. Advanced technology mentioned here is not mandated in current guidelines [5,45].
Histopathology categories and TNM staging system for Barrett’s esophagus are shown in Table 2 and Table 3. A management algorithm based on histologic findings is presented in Figure 1.
S creening
Logically, Barrett’s esophagus screening should be offered to every patient with GERD; however, this is against current recommendations because it is cost-prohibitive [46,47]. It is a given that the rationale behind the screening is to decrease morbidity and mortality from esophageal adenocarcinoma by offering early and definitive intervention [14,48,49]. The gold standard for screening is upper endoscopy, although nonendoscopic methods are being studied [1]. For example, a capsule attached to a string can be swallowed by the patient, the capsule is then deployed and pulled through the esophagus to obtains a brush sample of the cells. It is a promising technology due to high sensitivity and specificity [50]; however, it is not regularly utilized in practice.
Approaches to screening for Barrett’s esophagus has been addressed by multiple societies with several guidelines currently available [51]. None of these approaches have been proven to be superior in clinical studies. The American Gastroenterological Association (AGA) recommends screening patients with multiple risk factors associated with esophageal adenocarcinoma for Barrett’s esophagus. Risk factors listed by the AGA include white patients, male patients, patients above the age of 50 with a history of chronic GERD, hiatal hernia, elevated body mass index, and certain body fat distribution. The AGA recommends against screening the general population with GERD [9]. The American College of Gastroenterology (ACG) recommends upper endoscopy only in the presence of alarm symptoms (eg, dysphagia, weight loss, gastrointestinal bleeding) and for screening of patients at high risk for complications [52]. The American College of Physicians recommends upper endoscopy for screening for Barrett’s esophagus in men older than 50 years with GERD symptoms for more than 5 years with any risk factors like nocturnal reflux symptoms, hiatus hernia, elevated body mass index, tobacco use, and intra-abdominal distribution of fat [1].
Overall, the sensitivity of endoscopy to diagnose Barrett’s esophagus, as seen in a Veterans Affairs (VA) cohort study for the detection of Barrett’s, is about 80%. The concluded 80% sensitivity rate was based on the performance of 2 endoscopies, 6 weeks apart for each patient, with subsequent labeling of the diagnosis if intestinal metaplasia was found in either of the 2 biopsy samples taken from the 2 procedures [53].
Promising molecular biomarkers have been associated with Barrett’s esophagus including p53 and cyclin D1 expression. However, additional studies are needed before they are incorporated as part of screening practices [5].
General Management
Medical Management of GERD
All patients with Barrett’s esophagus should be treated with a proton pump inhibitor (PPI) indefinitely based on multiple studies [54–56]. Effective control of GERD was associated with a decreased risk of dysplasia and adenocarcinoma (adjusted odds ratio 0.29, 95% CI 0.12–0.79) [57].
Effective control of GERD decreases chronic esophageal inflammation, which could progress to Barrett’s esophagus and has a risk of possible progression to adenocarcinoma. In one study, patients with proven Barrett’s esophagus showed partial regression of the intestinal metaplasia with aggressive PPI therapy [57–59]. Despite this, it is not clear whether the regression decreased risk of malignant progression [58,60,61]. In a study of 68 patients, aggressive acid reduction with omeprazole 40 mg twice a day lead to partial regression of Barrett’s esophagus when compared to mild suppression with ranitidine 150 mg twice a day. However, there was no reduction in the risk of cancer [59].
Surveillance
The reasoning behind surveillance is to detect dysplasia or adenocarcinoma in patients known to have Barrett’s esophagus early enough to provide early and efficient treatment to improve the outcome. Surveillance is done by endoscopy with biopsies in addition to sampling any irregularity [1,62,63]; however, the evidence to back up the benefit of surveillance is not clear [5,64]. It should be noted that surveillance carries risks, and morbidity associated with repeated procedures may affect patients psychologically and financially. Patients who have Barrett’s esophagus are more likely to die from other more common diseases, such as coronary heart disease, prior to developing adenocarcinoma [65]. In a recent meta-analysis, the mortality rate due to esophageal adenocarcinoma was 3.0 per 1000 person-years, whereas the mortality rate due to other causes was 37.1 per 1000 person-years [65]. An ongoing randomized, multicenter trial which assesses scheduled endoscopy every 2 years will shed more light on the overall survival after applying surveillance recommendations for each grade of dysplasia [66].
The following are ACG recommendations for surveillance of Barrett’s esophagus based on the histopathology report. The histopathology report delineates 1 of 3 types of columnar epithelium [68]: cardiac epithelium of mucus-secreting cells, atrophic gastric fundic type epithelium, or specialized columnar cells with goblet cells. The latter type is the most common with high potential for cancer [32]. Degrees of dysplasia and possible adenocarcinoma is usually described in the report as well. The degree of dysplasia if found helps the endoscopist plan the next step in management. It is worth mentioning that sampling errors can lead to missing a diagnosis. In a meta-analysis, 13% of patients diagnosed with high-grade dysplasia who underwent resection were found to have invasive cancer [69].
Surveillance for Patients with No Dysplasia
We suggest surveillance every 3 to 5 years since the rate of neoplasia is low [14]. For management of select patients with no dysplasia and with additional risk factors, radiofrequency ablation (RFA) may be an option, although it remains a controversial approach. For example, in a patient under 50 years of age, family history would be an argument for proceeding with RFA instead of prolonged surveillance. In a prospective cohort study of 139 patients with 10-year follow-up after ablation, recurrent Barrett’s occurred in less than 5% of the patients. [70]
Surveillance for Patients with Biopsy Showing “Indefinite for Dysplasia”
Aggressive treatment with PPI twice daily is recommended to avoid the misinterpretation of reactive esophageal changes secondary to reflux as dysplasia on the following endoscopy with biopsy. These patients will require a repeat endoscopy with biopsies after 3 months of aggressive treatment with PPI. Biopsies should be taken every 1 centimeter within Barrett’s epithelium [1].
If it remains indefinite, biopsies should be examined by a second pathologist with expertise in Barrett’s esophagus. If the second pathologist agrees on the indefinite diagnosis for dysplasia, then endoscopy every 12 months is recommended [1]. Treatment versus surveillance after repeat endoscopy and biopsy should be tailored to the new histopathology results on the most recent exam.
Surveillance for Patients with Biopsy Results showing Low-Grade Dysplasia (LGD), High-Grade Dysplasia (HGD), or Intramucosal Carcinoma
Surveillance recommendations are discussed under Management of Dysplasia or Intramucosal Carcinoma, below.
Efficacy of Surveillance
Asymptomatic adenocarcinoma could be discovered during surveillance, and neoplasia detected during surveillance is usually less advanced than those found after development of symptoms such as dysphagia, bleeding or weight loss [2,3,71–75]. These studies obviously had lead–time bias and did not document terminal cancer in patients adherent to surveillance protocol.
Management of Dysplasia or Intramucosal Carcinoma
Overview
Historically, dysplasia was managed with esophagectomy, which was associated with high morbidity and mortality. With advancement in the field of endoscopy, dysplasia is managed quite differently today, with endoscopic eradication therapy, which includes the use of endoscopic ablation techniques and endoscopic resection. The advantage of endoscopic resection is preservation of resected tissue for further examination, thus providing valuable information regarding the stage of the tumor (depth). Histological examination is not possible with photo or thermal ablation techniques as destroyed mucosa cannot be submitted for tissue analysis.
Low-Grade Dysplasia
If low-grade dysplasia is found, it is followed by a repeat endoscopy 8 weeks after aggressive PPI therapy. The repeat endoscopy should be performed with high definition/high-resolution endoscopy. The rationale of a second endoscopy is to ensure that the metaplastic mucosa was adequately inspected and biopsied prior to further intervention [1,9]. If the diagnosis is confirmed as low-grade dysplasia, and the patient prefers to go with the path of intervention instead of conservative management (endoscopic surveillance every 6 months for 1 year then annually with biopsies), then multiple options are available for the patient, including RFA or cryotherapy [8] [76]. In a randomized clinical trial in patients with Barrett’s esophagus and LGD, RFA was shown to reduce risk of neoplastic progression over a 3-year follow-up. The study included 136 patients randomized to receive ablation and 68 patients who underwent endoscopic surveillance. In the ablation group, the risk of progression to HGD or esophageal adenocarcinoma was reduced by 25% and the risk of progression to adenocarcinoma was reduced by 7.4%. In the ablation group, complete eradication of dysplasia and intestinal metaplasia occurred in 92.6% and 88.2% of patients respectively. In the endoscopic surveillance group, complete eradication of dysplasia and intestinal metaplasia was seen in 27.9% and 0.0% of patients respectively. [76]. Treatment-related adverse events occurred in 19.1% of patients receiving ablation (P < 0.001). The most common adverse event was stricture, occurring in 8 patients receiving ablation (11.8%), all resolved by endoscopic dilation [76,78].
Surveillance after ablation of LGD is still an ongoing debate, and further evidence is needed to establish guidelines [8]. Due to lack of evidence, we would lean towards surveillance for those patients with an annual esophagogastroduodenoscopy with biopsy examination (author’s opinion, no associated level of evidence).
High-Grade Dysplasia or Intramucosal Carcinoma
For patients with HGD or intramucosal carcinoma without submucosal invasion, eradication is the treatment of choice. Current guidelines advocate for endoscopic eradication therapy for most if not all patients with HGD or intramucosal carcinoma with a goal of removing all metaplastic and dysplastic tissue [1,5,62,64]. It should be noted that the biopsy specimen should be extensive to decrease the error margin. If the diagnosis were made on endoscopy without procuring extensive biopsies, then repeat endoscopy with extensive biopsies is needed prior to deciding the treatment path. The rationale behind extensive biopsies is to confirm the diagnosis and to determine the extent of dysplasia. Other factors potentially influencing the treatment path include the patient’s age, comorbid conditions, quality of life, and available access to an advanced endoscopist or specialized surgeon. Patient’s preferences and adherence to follow-up visits should also be a consideration.
The long-term benefits of endoscopic intervention versus surgical intervention are not well established. Esophagectomy is no longer a preferred method of treatment due to high morbidity and mortality associated with the procedure when compared to endoscopic interventions. However, it is still a preferred choice amongst a select group of patients unwilling to follow-up. A cost-effective analysis found that endoscopic ablation provided the longest quality adjusted life expectancy for Barrett’s esophagus with HGD [79,80].
Endoscopic Therapy in Barrett’s Esophagus
Endoscopic Ablative Therapies
With the advancement of endoscopic intervention, we now have multiple tools to ablate abnormal epithelium in Barrett’s esophagus. Examples of ablation techniques include thermal, photochemical, and mechanical techniques [81,82]. RFA is the treatment of choice for ablation [83]. However, non-contact ablative therapy, such as cryoablation, may be prefered if topography of the esophagus doesn’t allow contact ablation.
Radiofrequency Ablation (Figure 2). RFA is a procedure in which heat is generated from medium frequency alternating current and leads to thermal injury [84]. In Barrett’s esophagus, RFA uses radiofrequency energy delivered by a balloon that has a series of closely spaced electrodes in a
Most patients will require multiple sessions of RFA to achieve eradication. It is very rarely a one-time procedure. In a meta-analysis of 18 studies including 3082 patients, the most common adverse effects of RFA were stricture in 5% [76,98], bleeding in 1%, and pain in 3% of patients [99].
It is crucial for successful RFA to continue medical treatment for acid suppression, in order to allow healthy regeneration of the squamous cell lining. It is suggested to use PPI twice a day with sucralfate and ranitidine after the intervention [100,101]. Adhering to a liquid diet for 24 hours is needed, followed by a soft diet to allow faster regeneration of the epithelium.
The caveat with RFA is that new evidence shows a higher rate of recurrence than previously thought. In one study of 246 patients, recurrence of dysplasia occurred in 25% of patients at 48 months after eradication in 80% of the patients, and metaplasia occurred in 50% at 60 months [102]. The other risk is buried Barrett’s, a condition occurring after incomplete ablation, in which squamous cell epithelium covers patches of incompletely destroyed intestinal lining, leading to possible progression of the disease to adenocarcinoma under the surface [103].
It has been reported that patients who underwent RFA had remarkable improvement in quality of life even if RFA did not achieve eradication. Patients reported less depression, less stress and better quality of life [104].
Based on a survey of experts, follow-up at 3 months, 6 months, and then annually is recommended after ablation [1,105]. Biopsies should be taken distal to neosquamous epithelium and from suspicious areas [97,106].
Endoscopic Spray Cryotherapy. This technique involves application of liquid nitrogen or carbon dioxide gas by endoscope on the tissue to freeze it off. Although it has been shown to eliminate HGD in over 95% of the cases and all dysplasia in over 85% of the cases, it was effective in eradicating intestinal metaplasia in only 55% of patients [103,108,109]. Thus, RFA as ablation therapy is still superior to cryotherapy and is still the first-line treatment for dysplastic Barrett’s esophagus. In comparison to cryotherapy, RFA efficacy has been studied extensively with well documented outcomes. However, there is a role for cryotherapy over RFA in certain clinical situations (such as severe chest pain from RFA or lack of efficacy in eradicating intestinal metaplasia or dysplasia by RFA).
Similar to RFA, on occasions of partial ablation, the remaining metaplastic tissue may get buried beneath a layer of squamous epithelium and can possibly progress to adenocarcinoma [110].
Photodynamic Therapy (PDT). This technique works by producing cytotoxicity at the cellular level by exposure to light at a specific wavelength in the presence of a chemical agent known as photosensitize [107]. Although superior to omeprazole, PDT has a significant rate of complications, mainly stricture, and a high occurrence of esophageal cancer during follow-up. For this reason, it is less favorable compared to RFA [107] and mentioned here as a historical therapy.
Endoscopic Resection Techniques: Endoscopic Mucosal Resection (EMR) and Endoscopic Submucosal Dissection (ESD)
Unlike flat mucosa in Barrett’s esophagus, which respond to ablative techniques such as RFA or cryotherapy, nodular Barrett’s esophagus is hard to treat and requires endoscopic resection prior to ablation. Endoscopic mucosal resection (EMR) is the most widely used technique and it is available in most tertiary referral centers. Another technique named endoscopic submucosal dissection (ESD) allows the removal of large nodular areas of Barrett’s esophagus in one piece to ensure complete removal of nodular dysplasia. ESD is technically challenging and it is only available in a handful of centers in the US. Endoscopic resection techniques are the preferred interventions for nodular dysplasia due to their ability to provide valuable information for staging the lesion [111–113]. Endoscopic resection techniques are safer and more effective with similar or better results when compared with other approaches [114].
EMR is completed by the excision of esophageal mucosa down to the submucosa and submitting a large tissue specimen to the pathologist. It additionally serves as a therapeutic measure in cases of no submucosal extension. Another advantage of EMR is the ability to predict lymph node metastasis. The rationale is based on the fact that the most important predictor of lymph node metastasis is the depth of the tumor; hence, invasive tumors would likely be associated with lymph node metastasis [115,116].
In a systematic review of 11 studies, complete EMR was as equally effective in the short-term treatment of dysplastic Barrett’s esophagus when compared to RFA, but adverse event rates were greater with complete EMR (mainly strictures). Strictures are more likely to occur in patients undergoing extensive EMR. In another meta-analysis of 22 studies comparing the efficacy of EMR to RFA, both techniques were effective in eradicating dysplasia (95% in EMR group and 92% in RFA group). However, extensive EMR was associated with higher complication rates suggesting that a combined endoscopic approach of focal EMR followed by RFA is preferred over extensive EMR alone [86].
It should be noted that EMR and ESD information were derived from highly specialized center and these results may not be duplicated in community settings [113,117].
Efficacy of Endoscopic Resection. Endoscopic resection has a success rate comparable to surgical esophagectomy with fewer complications [113,114,118–121] in patients with HGD and early stages of esophageal cancer [122]. Complete remission can be as high as 89%. Recurrence occurred in 6% to 30% of patients [114,118,119], which was attributed to incomplete removal, large lesions, failure to use adjunct therapy, or lack of follow-up [123]. Even when recurrence occurred, it was successfully managed by endoscopic intervention [124].
In a large cohort study of 1000 patients with early mucosal adenocarcinoma who were treated with endoscopic resection, long-term complete remission occurred in 94% of patients. There was no mortality and less than 2% of patients had major complications. Infrequent complications include bleeding, perforations, and strictures [123,125,126]. The rate of complications is lower in highly specialized centers [127–129].
Surgery was necessary in 12 patients (3.7%) after endoscopic therapy failed [123]. Post-resection care and follow-up is similar to the post-RFA care discussed above.
Management of Invasive Esophageal Adenocarcinoma
Patients diagnosed with an invasive adenocarcinoma need to be referred to an oncologist for staging and to discuss treatment options. A select number of patients may be referred by oncology for endoscopic resection, yet the need for a multidisciplinary approach in these situations is absolutely necessary [1].
Esophagectomy
Esophagectomy offers the complete removal of the HGD along with any adenocarcinoma in the regional lymph nodes. However, mortality rates are as high as 12% immediately after the procedure [130]. The multitude of short- and long-term morbidity has significant effects on quality of life. Short-term morbidity is as high as 30%. Patients may develop serious postoperative complications such as myocardial infarction, hospital associated pneumonia, or anatomic leak [131].
Examples of long-term morbidity include dysphagia, transection of vagal nerve, and dumping syndrome. Recent development in minimally invasive surgeries for esophagectomy has not reduced postoperative morbidity rates [132].
Advocates of esophagectomy illustrate the advantage of eradication of occult lymph node metastasis. The counter argument has been established by a systemic review in which occult lymph node metastasis occurred in less than 2% of patients with HGD and intramucosal carcinoma; whereas the mortality rate after esophagectomy is substantially higher with no guarantee of curing metastatic disease [133].
Prevention of Barrett’s Esophagus
Since Barrett’s esophagus precedes most of the cases of EAC if not all [1,134], methods that aim at decreasing the incidence of Barrett’s esophagus could help in prevention. The modifiable risk factors listed by the AGA include BMI, GERD, and hiatal hernia management. Along with diet and exercise, the advent of new therapies to help patients manage their weight could in return help in avoiding a plethora of medical conditions including Barrett’s esophagus. Hiatal hernia management could lower the risk of Barrett’s by restoring normal anatomy. Lastly, proper management of GERD would lower the risk of developing Barrett’s esophagus as discussed in this article [1,9].
It is worth noting that a large trial on the efficacy and safety of aspirin for prevention of adenocarcinoma progression in Barrett’s esophagus is ongoing in the UK (AspECT trial). The AspECT trial examines the efficacy of low dose vs. high dose PPI with or without aspirin for the chemoprevention of esophageal adenocarcinoma. The theory behind the study is the inhibition of COX 2 receptors in Barrett’s cells can decrease tissue progression to cancer. This chempreventive effect of nonsteroidal anti-inflammatory drugs was shown to be augmented when combined with statin intake [56,135–138].
Conclusion
Barrett’s esophagus is usually diagnosed during routine endoscopic examination. The initial symptoms are those associated with GERD, like heartburn, dyspepsia, and regurgitation. Specialized columnar epithelium is the hallmark of histopathological diagnosis. Recommendations of the ACG and AGA suggest treatment based on biopsy results. The intervention would vary on a wide spectrum starting from acid suppression, radiofrequency ablation, endoscopic resection therapy, and rarely, esophagectomy.
Corresponding author: Mohamed O. Othman, MD, Gastroenterology and Hepatology Section, Baylor College of Medicine, 7200 Cambridge St., Suite 8C, Houston, TX 77030, [email protected].
Financial disclosures: Dr. Othman has received grant support from Abbvie and has served as a consultant for Olympus.
From the Gastroenterology and Hepatology Section, Baylor College of Medicine, Houston, TX.
Abstract
- Objective: To provide an update on management of Barrett’s esophagus.
- Methods: Review of the literature.
- Results: Management of Barrett’s esophagus depends on the degree of dysplasia. Surveillance by endoscopy every 3–5 years is recommended in patients with Barrett’s esophagus without dysplasia. Patients with Barrett’s esophagus and low-grade dysplasia should undergo surveillance by endoscopy in 3 months for confirmation of the diagnosis; if the diagnosis is confirmed then surveillance by endoscopy or eradication of Barrett’s epithelium by ablation or endoscopic resection are recommended. There is a sufficient evidence to recommend radiofrequency ablation of high-grade dysplasia within Barrett’s esophagus or to perform endoscopic mucosal resection of nodular Barrett’s esophagus with any degree of dysplasia. Early esophageal cancers that are limited to the mucosa can be treated by endoscopic resection, while cancer invading into the deep submucosa or muscularis propria may need esophagectomy with or without chemoradiation.
- Conclusion: The management of Barrett’s esophagus depends on the degree of dysplasia. Radiofrequency ablation and endoscopic mucosal resection are the most commonly used treatment for Barrett’s esophagus with dysplasia.
Keywords: Barrett’s esophagus; radiofrequency ablation; endoscopic submucosal dissection; endoscopic mucosal resection; early esophageal cancer.
Barrett’s esophagus is a common complication of chronic reflux disease [1]. Metaplastic changes that occur at the distal esophageal epithelium are usually asymptomatic [2,3] and occur as reparative adaptations to the insult of the gastric acid [4]. The management of Barrett’s esophagus after diagnosis is currently debated amongst experts without a clear consensus [5,6]. This review is generally consistent with the 2016 guidelines from the American College of Gastroenterology [1], a 2012 guideline from the American Society of Gastrointestinal Endoscopy [7], a 2011 guideline, and a 2016 expert review from the American Gastroenterological Association [8,9].
D efinition
Barrett’s esophagus is a metaplasia of the stratified squamous epithelium to a specialized columnar intestinal epithelium of mucus cells and goblet cells at the distal esophagus secondary to gastroesophageal reflux disease (GERD) [7,10]. Barrett’s esophagus is unstable tissue which can progress to esophageal adenocarcinoma. When unmanaged, the risk of cancer in dysplastic mucosa is at least thirty-fold greater than that for the general population [11–14], with recent studies suggesting a 0.4–0.7 occurrence rate per year [11,15]. With no dysplasia, the risk is low [16].
Epidemiology
The prevalence of Barrett’s esophagus is ~10% in patients with GERD [11–13,17], an estimate tied to the prevalence of GERD. However, due to the lack of symptoms of Barrett’s esophagus, no solid data supports this assumption [18–20]. A European study estimated the prevalence of Barrett’s esophagus to be 1.6% among the general population [21,22]. Barrett’s esophagus is usually diagnosed during endoscopic examinations of middle-aged and older adults, with the mean age being 55 years of age. It is most commonly found in Caucasian males and associated with the use of smoking tobacco. The male-to-female ratio is approximately 2:1 [1] and it appears to be uncommon in African Americans [23,24]. Abdominal obesity as measured by an increased waist-to-hip ratio is associated with an increased risk of Barrett’s esophagus [25,26]. Germline mutations in the MSR1, ASCC1, and CTHRC1 genes have been associated with the presence of Barrett’s esophagus and esophageal adenocarcinoma [27]. Risk factors are listed in Table 1.
Clinical Symptoms
Columnar metaplasia itself does not cause any symptoms but is merely the adaptation of the cells to the repeated effect of the acid. The main clinical symptoms of the disease would initially be symptoms associated with GERD, such as heartburn, water brash, and dysphagia [1]. Severe presentations of GERD, such as esophageal ulceration, stricture, and hemorrhage, usually occur with long-segment Barrett’s esophagus [29,30]. However, 40% of patients presenting with adenocarcinoma had no history of GERD or symptoms of heartburn [13]. Furthermore, as few as 5% of those presenting with adenocarcinoma were known to have Barrett’s esophagus [31].
D iagnosis
Barrett’s esophagus generally requires an endoscopic examination with biopsy confirmation from the distal esophagus showing specialized intestinal columnar epithelium [32]. The biopsy specimen is acquired from the cellular lining proximal to gastroesophageal junction [5,33]. Barrett’s esophagus is classified into long- and short-segment based on the length of salmon-colored mucosa in the distal esophagus. A distance longer than 3 cm is
The Prague classification was presented by an international research group in 2006 and is regarded as the standard for measuring the length of Barrett’s esophagus. The lower measurement boundary is formed by the proximal cardial notch, and the 2 upper measurement boundaries are marked by the proximal limit of the circumferential Barrett’s segment and the longest tongue of Barrett’s [37].Confirmation of the diagnosis of dysplastic
Once the initial diagnosis of Barrett’s esophagus is made, we recommend referring the patient to a Barrett’s esophagus specialized center in order to offer the patient a second opinion from a team of experts in Barrett’s esophagus. This would avoid possible false-positive results, which can be as high as 40% [1,8]. It would also offer the patient a comprehensive multidisciplinary approach and adequate long-term management. It is further preferred if an advanced intervention is offered to the patient such as endoscopic mucosal resection or endoscopic submucosal dissection. Expert endoscopists
The availability of advanced endoscopic tools improves diagnostic yield. Adopting advanced techniques can reduce errors during biopsy sampling [40–42]. Some of the newer advanced imaging endoscopic tools include chromoendoscopy, optical coherence tomography, confocal microendoscopy, autofluorescence endoscopy, narrow band imaging (NBI), and Fujinon intelligent chromoendoscopy (FICE) [43,44]. In a meta-analysis examining whether advanced techniques improved diagnostic yield, it was found that advanced imaging increased the diagnostic yield by 34%. Advanced technology mentioned here is not mandated in current guidelines [5,45].
Histopathology categories and TNM staging system for Barrett’s esophagus are shown in Table 2 and Table 3. A management algorithm based on histologic findings is presented in Figure 1.
S creening
Logically, Barrett’s esophagus screening should be offered to every patient with GERD; however, this is against current recommendations because it is cost-prohibitive [46,47]. It is a given that the rationale behind the screening is to decrease morbidity and mortality from esophageal adenocarcinoma by offering early and definitive intervention [14,48,49]. The gold standard for screening is upper endoscopy, although nonendoscopic methods are being studied [1]. For example, a capsule attached to a string can be swallowed by the patient, the capsule is then deployed and pulled through the esophagus to obtains a brush sample of the cells. It is a promising technology due to high sensitivity and specificity [50]; however, it is not regularly utilized in practice.
Approaches to screening for Barrett’s esophagus has been addressed by multiple societies with several guidelines currently available [51]. None of these approaches have been proven to be superior in clinical studies. The American Gastroenterological Association (AGA) recommends screening patients with multiple risk factors associated with esophageal adenocarcinoma for Barrett’s esophagus. Risk factors listed by the AGA include white patients, male patients, patients above the age of 50 with a history of chronic GERD, hiatal hernia, elevated body mass index, and certain body fat distribution. The AGA recommends against screening the general population with GERD [9]. The American College of Gastroenterology (ACG) recommends upper endoscopy only in the presence of alarm symptoms (eg, dysphagia, weight loss, gastrointestinal bleeding) and for screening of patients at high risk for complications [52]. The American College of Physicians recommends upper endoscopy for screening for Barrett’s esophagus in men older than 50 years with GERD symptoms for more than 5 years with any risk factors like nocturnal reflux symptoms, hiatus hernia, elevated body mass index, tobacco use, and intra-abdominal distribution of fat [1].
Overall, the sensitivity of endoscopy to diagnose Barrett’s esophagus, as seen in a Veterans Affairs (VA) cohort study for the detection of Barrett’s, is about 80%. The concluded 80% sensitivity rate was based on the performance of 2 endoscopies, 6 weeks apart for each patient, with subsequent labeling of the diagnosis if intestinal metaplasia was found in either of the 2 biopsy samples taken from the 2 procedures [53].
Promising molecular biomarkers have been associated with Barrett’s esophagus including p53 and cyclin D1 expression. However, additional studies are needed before they are incorporated as part of screening practices [5].
General Management
Medical Management of GERD
All patients with Barrett’s esophagus should be treated with a proton pump inhibitor (PPI) indefinitely based on multiple studies [54–56]. Effective control of GERD was associated with a decreased risk of dysplasia and adenocarcinoma (adjusted odds ratio 0.29, 95% CI 0.12–0.79) [57].
Effective control of GERD decreases chronic esophageal inflammation, which could progress to Barrett’s esophagus and has a risk of possible progression to adenocarcinoma. In one study, patients with proven Barrett’s esophagus showed partial regression of the intestinal metaplasia with aggressive PPI therapy [57–59]. Despite this, it is not clear whether the regression decreased risk of malignant progression [58,60,61]. In a study of 68 patients, aggressive acid reduction with omeprazole 40 mg twice a day lead to partial regression of Barrett’s esophagus when compared to mild suppression with ranitidine 150 mg twice a day. However, there was no reduction in the risk of cancer [59].
Surveillance
The reasoning behind surveillance is to detect dysplasia or adenocarcinoma in patients known to have Barrett’s esophagus early enough to provide early and efficient treatment to improve the outcome. Surveillance is done by endoscopy with biopsies in addition to sampling any irregularity [1,62,63]; however, the evidence to back up the benefit of surveillance is not clear [5,64]. It should be noted that surveillance carries risks, and morbidity associated with repeated procedures may affect patients psychologically and financially. Patients who have Barrett’s esophagus are more likely to die from other more common diseases, such as coronary heart disease, prior to developing adenocarcinoma [65]. In a recent meta-analysis, the mortality rate due to esophageal adenocarcinoma was 3.0 per 1000 person-years, whereas the mortality rate due to other causes was 37.1 per 1000 person-years [65]. An ongoing randomized, multicenter trial which assesses scheduled endoscopy every 2 years will shed more light on the overall survival after applying surveillance recommendations for each grade of dysplasia [66].
The following are ACG recommendations for surveillance of Barrett’s esophagus based on the histopathology report. The histopathology report delineates 1 of 3 types of columnar epithelium [68]: cardiac epithelium of mucus-secreting cells, atrophic gastric fundic type epithelium, or specialized columnar cells with goblet cells. The latter type is the most common with high potential for cancer [32]. Degrees of dysplasia and possible adenocarcinoma is usually described in the report as well. The degree of dysplasia if found helps the endoscopist plan the next step in management. It is worth mentioning that sampling errors can lead to missing a diagnosis. In a meta-analysis, 13% of patients diagnosed with high-grade dysplasia who underwent resection were found to have invasive cancer [69].
Surveillance for Patients with No Dysplasia
We suggest surveillance every 3 to 5 years since the rate of neoplasia is low [14]. For management of select patients with no dysplasia and with additional risk factors, radiofrequency ablation (RFA) may be an option, although it remains a controversial approach. For example, in a patient under 50 years of age, family history would be an argument for proceeding with RFA instead of prolonged surveillance. In a prospective cohort study of 139 patients with 10-year follow-up after ablation, recurrent Barrett’s occurred in less than 5% of the patients. [70]
Surveillance for Patients with Biopsy Showing “Indefinite for Dysplasia”
Aggressive treatment with PPI twice daily is recommended to avoid the misinterpretation of reactive esophageal changes secondary to reflux as dysplasia on the following endoscopy with biopsy. These patients will require a repeat endoscopy with biopsies after 3 months of aggressive treatment with PPI. Biopsies should be taken every 1 centimeter within Barrett’s epithelium [1].
If it remains indefinite, biopsies should be examined by a second pathologist with expertise in Barrett’s esophagus. If the second pathologist agrees on the indefinite diagnosis for dysplasia, then endoscopy every 12 months is recommended [1]. Treatment versus surveillance after repeat endoscopy and biopsy should be tailored to the new histopathology results on the most recent exam.
Surveillance for Patients with Biopsy Results showing Low-Grade Dysplasia (LGD), High-Grade Dysplasia (HGD), or Intramucosal Carcinoma
Surveillance recommendations are discussed under Management of Dysplasia or Intramucosal Carcinoma, below.
Efficacy of Surveillance
Asymptomatic adenocarcinoma could be discovered during surveillance, and neoplasia detected during surveillance is usually less advanced than those found after development of symptoms such as dysphagia, bleeding or weight loss [2,3,71–75]. These studies obviously had lead–time bias and did not document terminal cancer in patients adherent to surveillance protocol.
Management of Dysplasia or Intramucosal Carcinoma
Overview
Historically, dysplasia was managed with esophagectomy, which was associated with high morbidity and mortality. With advancement in the field of endoscopy, dysplasia is managed quite differently today, with endoscopic eradication therapy, which includes the use of endoscopic ablation techniques and endoscopic resection. The advantage of endoscopic resection is preservation of resected tissue for further examination, thus providing valuable information regarding the stage of the tumor (depth). Histological examination is not possible with photo or thermal ablation techniques as destroyed mucosa cannot be submitted for tissue analysis.
Low-Grade Dysplasia
If low-grade dysplasia is found, it is followed by a repeat endoscopy 8 weeks after aggressive PPI therapy. The repeat endoscopy should be performed with high definition/high-resolution endoscopy. The rationale of a second endoscopy is to ensure that the metaplastic mucosa was adequately inspected and biopsied prior to further intervention [1,9]. If the diagnosis is confirmed as low-grade dysplasia, and the patient prefers to go with the path of intervention instead of conservative management (endoscopic surveillance every 6 months for 1 year then annually with biopsies), then multiple options are available for the patient, including RFA or cryotherapy [8] [76]. In a randomized clinical trial in patients with Barrett’s esophagus and LGD, RFA was shown to reduce risk of neoplastic progression over a 3-year follow-up. The study included 136 patients randomized to receive ablation and 68 patients who underwent endoscopic surveillance. In the ablation group, the risk of progression to HGD or esophageal adenocarcinoma was reduced by 25% and the risk of progression to adenocarcinoma was reduced by 7.4%. In the ablation group, complete eradication of dysplasia and intestinal metaplasia occurred in 92.6% and 88.2% of patients respectively. In the endoscopic surveillance group, complete eradication of dysplasia and intestinal metaplasia was seen in 27.9% and 0.0% of patients respectively. [76]. Treatment-related adverse events occurred in 19.1% of patients receiving ablation (P < 0.001). The most common adverse event was stricture, occurring in 8 patients receiving ablation (11.8%), all resolved by endoscopic dilation [76,78].
Surveillance after ablation of LGD is still an ongoing debate, and further evidence is needed to establish guidelines [8]. Due to lack of evidence, we would lean towards surveillance for those patients with an annual esophagogastroduodenoscopy with biopsy examination (author’s opinion, no associated level of evidence).
High-Grade Dysplasia or Intramucosal Carcinoma
For patients with HGD or intramucosal carcinoma without submucosal invasion, eradication is the treatment of choice. Current guidelines advocate for endoscopic eradication therapy for most if not all patients with HGD or intramucosal carcinoma with a goal of removing all metaplastic and dysplastic tissue [1,5,62,64]. It should be noted that the biopsy specimen should be extensive to decrease the error margin. If the diagnosis were made on endoscopy without procuring extensive biopsies, then repeat endoscopy with extensive biopsies is needed prior to deciding the treatment path. The rationale behind extensive biopsies is to confirm the diagnosis and to determine the extent of dysplasia. Other factors potentially influencing the treatment path include the patient’s age, comorbid conditions, quality of life, and available access to an advanced endoscopist or specialized surgeon. Patient’s preferences and adherence to follow-up visits should also be a consideration.
The long-term benefits of endoscopic intervention versus surgical intervention are not well established. Esophagectomy is no longer a preferred method of treatment due to high morbidity and mortality associated with the procedure when compared to endoscopic interventions. However, it is still a preferred choice amongst a select group of patients unwilling to follow-up. A cost-effective analysis found that endoscopic ablation provided the longest quality adjusted life expectancy for Barrett’s esophagus with HGD [79,80].
Endoscopic Therapy in Barrett’s Esophagus
Endoscopic Ablative Therapies
With the advancement of endoscopic intervention, we now have multiple tools to ablate abnormal epithelium in Barrett’s esophagus. Examples of ablation techniques include thermal, photochemical, and mechanical techniques [81,82]. RFA is the treatment of choice for ablation [83]. However, non-contact ablative therapy, such as cryoablation, may be prefered if topography of the esophagus doesn’t allow contact ablation.
Radiofrequency Ablation (Figure 2). RFA is a procedure in which heat is generated from medium frequency alternating current and leads to thermal injury [84]. In Barrett’s esophagus, RFA uses radiofrequency energy delivered by a balloon that has a series of closely spaced electrodes in a
Most patients will require multiple sessions of RFA to achieve eradication. It is very rarely a one-time procedure. In a meta-analysis of 18 studies including 3082 patients, the most common adverse effects of RFA were stricture in 5% [76,98], bleeding in 1%, and pain in 3% of patients [99].
It is crucial for successful RFA to continue medical treatment for acid suppression, in order to allow healthy regeneration of the squamous cell lining. It is suggested to use PPI twice a day with sucralfate and ranitidine after the intervention [100,101]. Adhering to a liquid diet for 24 hours is needed, followed by a soft diet to allow faster regeneration of the epithelium.
The caveat with RFA is that new evidence shows a higher rate of recurrence than previously thought. In one study of 246 patients, recurrence of dysplasia occurred in 25% of patients at 48 months after eradication in 80% of the patients, and metaplasia occurred in 50% at 60 months [102]. The other risk is buried Barrett’s, a condition occurring after incomplete ablation, in which squamous cell epithelium covers patches of incompletely destroyed intestinal lining, leading to possible progression of the disease to adenocarcinoma under the surface [103].
It has been reported that patients who underwent RFA had remarkable improvement in quality of life even if RFA did not achieve eradication. Patients reported less depression, less stress and better quality of life [104].
Based on a survey of experts, follow-up at 3 months, 6 months, and then annually is recommended after ablation [1,105]. Biopsies should be taken distal to neosquamous epithelium and from suspicious areas [97,106].
Endoscopic Spray Cryotherapy. This technique involves application of liquid nitrogen or carbon dioxide gas by endoscope on the tissue to freeze it off. Although it has been shown to eliminate HGD in over 95% of the cases and all dysplasia in over 85% of the cases, it was effective in eradicating intestinal metaplasia in only 55% of patients [103,108,109]. Thus, RFA as ablation therapy is still superior to cryotherapy and is still the first-line treatment for dysplastic Barrett’s esophagus. In comparison to cryotherapy, RFA efficacy has been studied extensively with well documented outcomes. However, there is a role for cryotherapy over RFA in certain clinical situations (such as severe chest pain from RFA or lack of efficacy in eradicating intestinal metaplasia or dysplasia by RFA).
Similar to RFA, on occasions of partial ablation, the remaining metaplastic tissue may get buried beneath a layer of squamous epithelium and can possibly progress to adenocarcinoma [110].
Photodynamic Therapy (PDT). This technique works by producing cytotoxicity at the cellular level by exposure to light at a specific wavelength in the presence of a chemical agent known as photosensitize [107]. Although superior to omeprazole, PDT has a significant rate of complications, mainly stricture, and a high occurrence of esophageal cancer during follow-up. For this reason, it is less favorable compared to RFA [107] and mentioned here as a historical therapy.
Endoscopic Resection Techniques: Endoscopic Mucosal Resection (EMR) and Endoscopic Submucosal Dissection (ESD)
Unlike flat mucosa in Barrett’s esophagus, which respond to ablative techniques such as RFA or cryotherapy, nodular Barrett’s esophagus is hard to treat and requires endoscopic resection prior to ablation. Endoscopic mucosal resection (EMR) is the most widely used technique and it is available in most tertiary referral centers. Another technique named endoscopic submucosal dissection (ESD) allows the removal of large nodular areas of Barrett’s esophagus in one piece to ensure complete removal of nodular dysplasia. ESD is technically challenging and it is only available in a handful of centers in the US. Endoscopic resection techniques are the preferred interventions for nodular dysplasia due to their ability to provide valuable information for staging the lesion [111–113]. Endoscopic resection techniques are safer and more effective with similar or better results when compared with other approaches [114].
EMR is completed by the excision of esophageal mucosa down to the submucosa and submitting a large tissue specimen to the pathologist. It additionally serves as a therapeutic measure in cases of no submucosal extension. Another advantage of EMR is the ability to predict lymph node metastasis. The rationale is based on the fact that the most important predictor of lymph node metastasis is the depth of the tumor; hence, invasive tumors would likely be associated with lymph node metastasis [115,116].
In a systematic review of 11 studies, complete EMR was as equally effective in the short-term treatment of dysplastic Barrett’s esophagus when compared to RFA, but adverse event rates were greater with complete EMR (mainly strictures). Strictures are more likely to occur in patients undergoing extensive EMR. In another meta-analysis of 22 studies comparing the efficacy of EMR to RFA, both techniques were effective in eradicating dysplasia (95% in EMR group and 92% in RFA group). However, extensive EMR was associated with higher complication rates suggesting that a combined endoscopic approach of focal EMR followed by RFA is preferred over extensive EMR alone [86].
It should be noted that EMR and ESD information were derived from highly specialized center and these results may not be duplicated in community settings [113,117].
Efficacy of Endoscopic Resection. Endoscopic resection has a success rate comparable to surgical esophagectomy with fewer complications [113,114,118–121] in patients with HGD and early stages of esophageal cancer [122]. Complete remission can be as high as 89%. Recurrence occurred in 6% to 30% of patients [114,118,119], which was attributed to incomplete removal, large lesions, failure to use adjunct therapy, or lack of follow-up [123]. Even when recurrence occurred, it was successfully managed by endoscopic intervention [124].
In a large cohort study of 1000 patients with early mucosal adenocarcinoma who were treated with endoscopic resection, long-term complete remission occurred in 94% of patients. There was no mortality and less than 2% of patients had major complications. Infrequent complications include bleeding, perforations, and strictures [123,125,126]. The rate of complications is lower in highly specialized centers [127–129].
Surgery was necessary in 12 patients (3.7%) after endoscopic therapy failed [123]. Post-resection care and follow-up is similar to the post-RFA care discussed above.
Management of Invasive Esophageal Adenocarcinoma
Patients diagnosed with an invasive adenocarcinoma need to be referred to an oncologist for staging and to discuss treatment options. A select number of patients may be referred by oncology for endoscopic resection, yet the need for a multidisciplinary approach in these situations is absolutely necessary [1].
Esophagectomy
Esophagectomy offers the complete removal of the HGD along with any adenocarcinoma in the regional lymph nodes. However, mortality rates are as high as 12% immediately after the procedure [130]. The multitude of short- and long-term morbidity has significant effects on quality of life. Short-term morbidity is as high as 30%. Patients may develop serious postoperative complications such as myocardial infarction, hospital associated pneumonia, or anatomic leak [131].
Examples of long-term morbidity include dysphagia, transection of vagal nerve, and dumping syndrome. Recent development in minimally invasive surgeries for esophagectomy has not reduced postoperative morbidity rates [132].
Advocates of esophagectomy illustrate the advantage of eradication of occult lymph node metastasis. The counter argument has been established by a systemic review in which occult lymph node metastasis occurred in less than 2% of patients with HGD and intramucosal carcinoma; whereas the mortality rate after esophagectomy is substantially higher with no guarantee of curing metastatic disease [133].
Prevention of Barrett’s Esophagus
Since Barrett’s esophagus precedes most of the cases of EAC if not all [1,134], methods that aim at decreasing the incidence of Barrett’s esophagus could help in prevention. The modifiable risk factors listed by the AGA include BMI, GERD, and hiatal hernia management. Along with diet and exercise, the advent of new therapies to help patients manage their weight could in return help in avoiding a plethora of medical conditions including Barrett’s esophagus. Hiatal hernia management could lower the risk of Barrett’s by restoring normal anatomy. Lastly, proper management of GERD would lower the risk of developing Barrett’s esophagus as discussed in this article [1,9].
It is worth noting that a large trial on the efficacy and safety of aspirin for prevention of adenocarcinoma progression in Barrett’s esophagus is ongoing in the UK (AspECT trial). The AspECT trial examines the efficacy of low dose vs. high dose PPI with or without aspirin for the chemoprevention of esophageal adenocarcinoma. The theory behind the study is the inhibition of COX 2 receptors in Barrett’s cells can decrease tissue progression to cancer. This chempreventive effect of nonsteroidal anti-inflammatory drugs was shown to be augmented when combined with statin intake [56,135–138].
Conclusion
Barrett’s esophagus is usually diagnosed during routine endoscopic examination. The initial symptoms are those associated with GERD, like heartburn, dyspepsia, and regurgitation. Specialized columnar epithelium is the hallmark of histopathological diagnosis. Recommendations of the ACG and AGA suggest treatment based on biopsy results. The intervention would vary on a wide spectrum starting from acid suppression, radiofrequency ablation, endoscopic resection therapy, and rarely, esophagectomy.
Corresponding author: Mohamed O. Othman, MD, Gastroenterology and Hepatology Section, Baylor College of Medicine, 7200 Cambridge St., Suite 8C, Houston, TX 77030, [email protected].
Financial disclosures: Dr. Othman has received grant support from Abbvie and has served as a consultant for Olympus.
1. Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016;111:30–50; quiz 51.
2. Peters JH, Clark GW, Ireland AP. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg 1994;108:813–21; discussion 821–2.
3. Streitz JM Jr, Andrews CW Jr, Ellis FH Jr. Endoscopic surveillance of Barrett’s esophagus. Does it help? J Thorac Cardiovasc Surg 1993;105:383–7; discussion 387–8.
4. Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med 2014;371:836–45.
5. Spechler SJ, Sharma P, Souza RF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084–91.
6. Sharma P, McQuaid K, Dent J, et al; AGA Chicago Workshop. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology 2004;127:310–30.
7. ASGE Standards of Practice Committee, Evans JA, Early DS; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc 2012;76(6):1087–94.
8. Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and management of low–grade dysplasia in Barrett’s esophagus: Expert review from the clinical practice updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151:822–835.
9. American Gastroenterological, A., et al., American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology, 2011. 140(3): p. 1084–91. [Same as #5]
10. Van Eyken P. Definition of Barrett’s oesophagus. Acta Gastroenterol Belg 2000;63:10–2.
11. Hirota WK, Loughney TM, Lazas DJ, et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology 1999;116(2):277–85.
12. Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar–lined (Barrett’s) esophagus. Comparison of population–based clinical and autopsy findings. Gastroenterology 1990;99:918–22.
13. Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology 2002;123:461–7.
14. Van der Veen AH, Dees J, Blankensteijn JD, Van Blankenstein M. Adenocarcinoma in Barrett’s oesophagus: an overrated risk. Gut 1989;30:14–8.
15. Winters C Jr, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987;92:118–24.
16. Wani S, Falk G, Hall M, et al. Patients with nondysplastic Barrett’s esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2011;9:220–7;quiz e26.
17. Ward EM, Wolfsen HC, Achem SR, et al. Barrett’s esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol 2006;101:12–7.
18. Rex DK, Cummings OW, Shaw M. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology 2003;125:1670–7.
19. Rubenstein JH, Taylor JB. Meta–analysis: the association of oesophageal adenocarcinoma with symptoms of gastro–oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
20. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
21. Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology, 2005;129:1825–31.
22. Sampliner RE. A population prevalence of Barrett’s esophagus––finally. Gastroenterology 2005; 129:2101–3.
23. Abrams JA, Fields S, Lightdale CJ, Neugut AI. Racial and ethnic disparities in the prevalence of Barrett’s esophagus among patients who undergo upper endoscopy. Clin Gastroenterol Hepatol 2008;6:30–4.
24. Corley DA, Kubo A, Levin TR, et al. Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community–based study, 1994–2006. Gut 2009;58:182–8.
25. Kamat P, Wen S, Morris J, Anandasabapathy S. Exploring the association between elevated body mass index and Barrett’s esophagus: a systematic review and meta–analysis. Ann Thorac Surg 2009;87:655–62.
26. Jacobson BC, Chan AT, Giovannucci EL, Fuchs CS. Body mass index and Barrett’s oesophagus in women. Gut 2009;58:1460–6.
27. Orloff M, Peterson C, He X, et al. Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma. JAMA 2011;306:410–9.
28. Sharma N, Ho KY. Risk Factors for Barrett’s oesophagus. Gastrointest Tumors 2016;3:103–8.
29. Spechler SJ. Barrett’s esophagus. Semin Gastrointest Dis 1996;7:51–60.
30. Ronkainen J, Talley NJ, Storskrubb T. Erosive esophagitis is a risk factor for Barrett’s esophagus: a community–based endoscopic follow–up study. Am J Gastroenterol 2011;106:1946–52.
31. Kim SL, Wo JM, Hunter JG. The prevalence of intestinal metaplasia in patients with and without peptic strictures. Am J Gastroenterol 1998;93:53–5.
32. Spechler SJ. Clinical practice. Barrett’s esophagus. N Engl J Med 2002;346:836–42.
33. Riddell RH, Odze RD. Definition of Barrett’s esophagus: time for a rethink––is intestinal metaplasia dead? Am J Gastroenterol 2009;104:2588–94.
34. Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus––the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol 1998;93:1033–6.
35. Eloubeidi MA, Provenzale D. Does this patient have Barrett’s esophagus? The utility of predicting Barrett’s esophagus at the index endoscopy. Am J Gastroenterol 1999;94:937–43.
36. Rudolph RE, Vaughan TL, Storer BE, et al. Effect of segment length on risk for neoplastic progression in patients with Barrett esophagus. Ann Intern Med 2000;132:612–20.
37. Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392–9.
38. Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population–based study. J Natl Cancer Inst 2011;103:1049–57.
39. Shaheen NJ, Dulai GS, Ascher B, et al. Effect of a new diagnosis of Barrett’s esophagus on insurance status. Am J Gastroenterol 2005;100:577–80.
40. Canto MI, Setrakian S, Willis J, et al. Methylene blue–directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest Endosc 2000;51:560–8.
41. Scotiniotis IA, Kochman ML, Lewis JD. Accuracy of EUS in the evaluation of Barrett’s esophagus and high–grade dysplasia or intramucosal carcinoma. Gastrointest Endosc 2001;54:689–96.
42. Kobayashi K, Izatt JA, Kulkarni MD, et al. High–resolution cross–sectional imaging of the gastrointestinal tract using optical coherence tomography: preliminary results. Gastrointest Endosc 1998;47:515–23.
43. Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance, and light–scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology 2001. 120: 1620–9.
44. Wallace MB, Sharma P, Lightdale C, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe–based confocal laser endomicroscopy. Gastrointest Endosc 2010;72:19–24.
45. Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta–analysis and systematic review. Clin Gastroenterol Hepatol 2013;11:1562–70.e1–2.–
46. DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1999;94:1434–42.
47. Inadomi JM, Sampliner R, Lagergren J. Screening and surveillance for Barrett esophagus in high–risk groups: a cost–utility analysis. Ann Intern Med 2003;138:176–86.
48. Conio M, Blanchi S, Lapertosa G, et al. Long–term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol 2003;98:1931–9.
49. Rastogi A, Puli S, El–Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high–grade dysplasia: a meta–analysis. Gastrointest Endosc 2008;67:394–8.
50. Kadri SR, Lao–Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non–endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ 2010;341:c4372.
51. Sharma, P., et al., A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology, 2004. 127(1): p. 310–30. [Same as #6]
52. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–28; quiz 329.
53. Kim SL, Waring JP, Spechler SJ, et al. Diagnostic inconsistencies in Barrett’s esophagus. Department of Veterans Affairs Gastroesophageal Reflux Study Group. Gastroenterology 1994;107:945–9.
54. Kastelein F, Spaander MC, Steyerberg EW, et al; ProBar Study Group. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2013;11:382–8.
55. El–Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol 2004;99:1877–83.
56. Nguyen DM, El–Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
57. Singh S, Garg SK, Singh PP, et al. Acid–suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta–analysis. Gut 2014;63:1229–37.
58. Spechler SJ, Lee E, Ahnen D, et al. Long–term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow–up of a randomized controlled trial. JAMA 2001;285:2331–8.
59. Peters FT, Ganesh S, Kuipers EJ, et al. Endoscopic regression of Barrett’s oesophagus during omeprazole treatment; a randomised double blind study. Gut 1999;45:489–94.
60. Ouatu–Lascar R, Triadafilopoulos G. –Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal acid reflux in patients with Barrett’s esophagus. Am J Gastroenterol 1998;93:711–6.
61. Maret–Ouda J, Konings P, Lagergren J, Brusselaers N. Antireflux surgery and risk of esophageal adenocarcinoma: A systematic review and meta–analysis. Ann Surg 2016;263:251–7.
62. Evans, J.A., et al., The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc, 2012. 76(6): p. 1087–94. [Same as #7]
63. ASGE Standards of Practice Committee, Evans JA, Early DS,et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
64. Bennett C, Moayyedi P, Corley DA, et al; BOB CAT Consortium. BOB CAT: A large–scale review and delphi consensus for ,anagement of Barrett’s esophagus with no dysplasia, indefinite for, or low–grade dysplasia. Am J Gastroenterol 2015;110:662–82; quiz 683.
65. Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta–analysis. Clin Gastroenterol Hepatol 2010;8:235–44; quiz e32.
66. Old O, Moayyedi P, Love S, et al; BOSS Trial Team. Barrett’s Oesophagus Surveillance versus endoscopy at need Study (BOSS): protocol and analysis plan for a multicentre randomized controlled trial. J Med Screen 2015;22:158–64.
67. Weston AP, Sharma P, Topalovski M, et al. Long–term follow–up of Barrett’s high–grade dysplasia. Am J Gastroenterol 2000;95:1888–93.
68. Paull A, Trier JS, Dalton MD, et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med 1976;295:476–80.
69. Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high–grade dysplasia in Barrett’s esophagus overestimated? Clin Gastroenterol Hepatol 2008;6:159–64.
70. Allison H, Banchs MA, Bonis PA, Guelrud M. Long–term remission of nondysplastic Barrett’s esophagus after multipolar electrocoagulation ablation: report of 139 patients with 10 years of follow–up. Gastrointest Endosc 2011;73:651–8.
71. Corley DA, Levin TR, Habel LA, Weiss NS, et al. Surveillance and survival in Barrett’s adenocarcinomas: a population–based study. Gastroenterology 2002;122:633–40.
72. Wong T, Tian J, Nagar AB. Barrett’s surveillance identifies patients with early esophageal adenocarcinoma. Am J Med 2010;123:462–7.
73. Fountoulakis A, Zafirellis KD, Dolan K, et al. Effect of surveillance of Barrett’s oesophagus on the clinical outcome of oesophageal cancer. Br J Surg 2004;91:997–1003.
74. Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population–based cohort study. Am J Gastroenterol 2014;109:1215–22.
75. Kastelein F, van Olphen SH, Steyerberg EW, et al. Impact of surveillance for Barrett’s oesophagus on tumour stage and survival of patients with neoplastic progression. Gut 2016;65:548–54.
76. Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low–grade dysplasia: a randomized clinical trial. JAMA 2014;311: 1209–17.
77. Wang WL, Chang IW, Chen CC, et al. Radiofrequency ablation versus endoscopic submucosal dissection in treating large early esophageal squamous cell neoplasia. Medicine (Baltimore) 2015;94:e2240.
78. Lim CH, Treanor D, Dixon MF, Axon AT. Low–grade dysplasia in Barrett’s esophagus has a high risk of progression. Endoscopy 2007;39:581–7.
79. Shaheen NJ, Inadomi JM, Overholt BF, Sharma P. What is the best management strategy for high grade dysplasia in Barrett’s oesophagus? A cost effectiveness analysis. Gut 2004;53:1736–44.
80. Vij R, Triadafilopoulos G, Owens DK, et al. Cost–effectiveness of photodynamic therapy for high–grade dysplasia in Barrett’s esophagus. Gastrointest Endosc 2004;60:739–56.
81. van den Boogert J, v an Hillegersberg R, Siersema PD, et al. Endoscopic ablation therapy for Barrett’s esophagus with high–grade dysplasia: a review. Am J Gastroenterol 1999;94:1153–60.
82. Sampliner RE. Endoscopic ablative therapy for Barrett’s esophagus: current status. Gastrointest Endosc 2004;59:66–9.
83. Sharma VK, Wang KK, Overholt BF, et al. Balloon–based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1–year follow–up of 100 patients. Gastrointest Endosc 2007;65:185–95.
84. Hanlon CR. Textbook of surgery: The biological basis of modern surgical practice, 14th edition. Ann Surg 1992;216:94.
85. Bright T, Watson DI, Tam W, et al. Randomized trial of argon plasma coagulation versus endoscopic surveillance for barrett esophagus after antireflux surgery: late results. Ann Surg 2007;246:1016–20.
86. Chadwick G, Groene O, Markar SR, et al. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett’s esophagus: a critical assessment of histologic outcomes and adverse events. Gastrointest Endosc 2014;79:718–31.e3.
87. Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett’s neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy 2008;40:370–9.
88. Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol 2010;8:23–9.
89. Kim HP, Bulsiewicz WJ, Cotton CC, et al. Focal endoscopic mucosal resection before radiofrequency ablation is equally effective and safe compared with radiofrequency ablation alone for the eradication of Barrett’s esophagus with advanced neoplasia. Gastrointest Endosc 2012;76:733–9.
90. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic ablation of Barrett’s esophagus: a multicenter study with 2.5–year follow–up. Gastrointest Endosc 2008;68:867–76.
91. Sharma VK, Jae Kim H, Das A, et al. Circumferential and focal ablation of Barrett’s esophagus containing dysplasia. Am J Gastroenterol 2009;104:310–7.
92. Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett’s esophagus that contains high–grade dysplasia: a U.S. multicenter registry. Gastrointest Endosc 2008;68:35–40.
93. Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology 2013;145:79–86.e1.
94. Lyday WD, Corbett FS, Kuperman DA, et al. Radiofrequency ablation of Barrett’s esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy 2010;42:272–8.
95. Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12:1840–7.e1.
96. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5–year outcomes from a prospective multicenter trial. Endoscopy 2010;42:781–9.
97. Weston AP, Sharma P, Banerjee S, et al. Visible endoscopic and histologic changes in the cardia, before and after complete Barrett’s esophagus ablation. Gastrointest Endosc 2005;61:515–21.
98. Beaumont H, Gondrie JJ, McMahon BP, et al. Stepwise radiofrequency ablation of Barrett’s esophagus preserves esophageal inner diameter, compliance, and motility. Endoscopy 2009;41:2–8.
99. Orman ES, Li N, Shaheen NJ.Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta–analysis. Clin Gastroenterol Hepatol 2013;11:1245–55.
100. Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 2012;143:576–81.
101. Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra–esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett’s esophagus. Dig Dis Sci 2012;57:2625–32.
102. Small AJ, Sutherland SE, Hightower JS, et al. Comparative risk of recurrence of dysplasia and carcinoma after endoluminal eradication therapy of high–grade dysplasia versus intramucosal carcinoma in Barrett’s esophagus. Gastrointest Endosc 2015;81:1158–66.e1–4.
103. Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high–grade dysplasia. Gastrointest Endosc 2010;71:680–5.
104. Shaheen NJ, Peery AF, Hawes RH, et al. Quality of life following radiofrequency ablation of dysplastic Barrett’s esophagus. Endoscopy 2010;42:790–9.
105. Bedi AO, Kwon RS, Rubenstein JH, et al. A survey of expert follow–up practices after successful endoscopic eradication therapy for Barrett’s esophagus with high–grade dysplasia and intramucosal adenocarcinoma. Gastrointest Endosc 2013;78:696–701.
106. Sampliner RE, Camargo E, Prasad AR. Prasad, Association of ablation of Barrett’s esophagus with high grade dysplasia and adenocarcinoma of the gastric cardia. Dis Esophagus 2006;19:277–9.
107. Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: long–term results. Gastrointest Endosc 2003;58:183–8.
108. Gosain S, Mercer K, Twaddell WS, et al. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high–grade dysplasia: long–term results. Gastrointest Endosc 2013;78:260–5.
109. Canto MI, Shin EJ, Khashab MA, et al. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett’s esophagus. Endoscopy 2015;47:582–91.
110. Van Laethem JL, Peny MO, Salmon I, et al. Intramucosal adenocarcinoma arising under squamous re–epithelialisation of Barrett’s oesophagus. Gut 2000;46:574–7.
111. Pech O, May A, Gossner L, et al. Management of pre–malignant and malignant lesions by endoscopic resection. Best Pract Res Clin Gastroenterol 2004;18:61–76.
112. Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003;57:567–79.
113. Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high–grade dysplasia in Barrett’s esophagus. Gastroenterology 2000;118:670–7.
114. Pech O, Behrens A, May A, et al. Long–term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high–grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008;57:1200–6.
115. Vieth M, Ell C, Gossner L, et al. Histological analysis of endoscopic resection specimens from 326 patients with Barrett’s esophagus and early neoplasia. Endoscopy 2004;36:776–81.
116. Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high–grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004;60:703–10.
117. Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high–grade dysplasia within Barrett’s esophagus. Gastrointest Endosc 2000;52:328–32.
118. Esaki M, Matsumoto T, Hirakawa K, et al. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy 2007;39:41–5.
119. Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007;65:3–10.
120. Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high–grade dysplasia and intramucosal carcinoma––an American single–center experience. Am J Gastroenterol 2009;104:2684–92.
121. Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high–volume centers. Ann Surg 2011;254:67–72.
122. Wu J, Pan YM, Wang TT, et al. Endotherapy versus surgery for early neoplasia in Barrett’s esophagus: a meta–analysis. Gastrointest Endosc 2014;79:233–241.e2.
123. Pech O, May A, Manner H, et al. Long–term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652–660.e1.
124. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
125. May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high–grade neoplasia and early adenocarcinoma in Barrett’s oesophagus: acute–phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002;14(10):1085–91.
126. Gerke H, Siddiqui J, Nasr I, et al. Efficacy and safety of EMR to completely remove Barrett’s esophagus: experience in 41 patients. Gastrointest Endosc 2011;74:761–71.
127. Lewis JJ, Rubenstein JH, Singal AG, et al. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett’s esophagus. Gastrointest Endosc 2011;74:753–60.
128. Choi IJ, Kim CG, Chang HJ, et al. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc 2005;62:860–5.
129. Deprez PH, Bergman JJ, Meisner S, et al. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy 2010;42:853–8.
130. van Lanschot JJ, Hulscher JB, Buskens CJ, et al. Hospital volume and hospital mortality for esophagectomy. Cancer 2001;91:1574–8.
131. Karl RC, Schreiber R, Boulware D, et al. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg 2000;231:635–43.
132. Young MM, Deschamps C, Trastek VF, et al. Esophageal reconstruction for benign disease: early morbidity, mortality, and functional results. Ann Thorac Surg 2000;70:1651–5.
133. Dunbar KB, Spechler SJ. The risk of lymph–node metastases in patients with high–grade dysplasia or intramucosal carcinoma in Barrett’s esophagus: a systematic review. Am J Gastroenterol 2012;107:850–62; quiz 863.
134. Morales CP, Souza RF, Spechler SJ. Hallmarks of cancer progression in Barrett’s oesophagus. Lancet 2002;360:1587–9.
135. Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012;10:722–7.
136. Abnet CC, Freedman ND, Kamangar F, et al. Non–steroidal anti–inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta–analysis. Br J Cancer 2009;100:551–7.
137. Kastelein F, Spaander MC, Biermann K, et al. Nonsteroidal anti–inflammatory drugs and statins have chemopreventative effects in patients with Barrett’s esophagus. Gastroenterology 2011;141:2000–8; quiz e13–4.
138. Zhang S, Zhang XQ, Ding XW, et al. Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a meta–analysis. Br J Cancer 2014;110: 2378–88.
1. Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016;111:30–50; quiz 51.
2. Peters JH, Clark GW, Ireland AP. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg 1994;108:813–21; discussion 821–2.
3. Streitz JM Jr, Andrews CW Jr, Ellis FH Jr. Endoscopic surveillance of Barrett’s esophagus. Does it help? J Thorac Cardiovasc Surg 1993;105:383–7; discussion 387–8.
4. Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med 2014;371:836–45.
5. Spechler SJ, Sharma P, Souza RF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084–91.
6. Sharma P, McQuaid K, Dent J, et al; AGA Chicago Workshop. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology 2004;127:310–30.
7. ASGE Standards of Practice Committee, Evans JA, Early DS; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc 2012;76(6):1087–94.
8. Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and management of low–grade dysplasia in Barrett’s esophagus: Expert review from the clinical practice updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151:822–835.
9. American Gastroenterological, A., et al., American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology, 2011. 140(3): p. 1084–91. [Same as #5]
10. Van Eyken P. Definition of Barrett’s oesophagus. Acta Gastroenterol Belg 2000;63:10–2.
11. Hirota WK, Loughney TM, Lazas DJ, et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology 1999;116(2):277–85.
12. Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar–lined (Barrett’s) esophagus. Comparison of population–based clinical and autopsy findings. Gastroenterology 1990;99:918–22.
13. Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology 2002;123:461–7.
14. Van der Veen AH, Dees J, Blankensteijn JD, Van Blankenstein M. Adenocarcinoma in Barrett’s oesophagus: an overrated risk. Gut 1989;30:14–8.
15. Winters C Jr, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987;92:118–24.
16. Wani S, Falk G, Hall M, et al. Patients with nondysplastic Barrett’s esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2011;9:220–7;quiz e26.
17. Ward EM, Wolfsen HC, Achem SR, et al. Barrett’s esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol 2006;101:12–7.
18. Rex DK, Cummings OW, Shaw M. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology 2003;125:1670–7.
19. Rubenstein JH, Taylor JB. Meta–analysis: the association of oesophageal adenocarcinoma with symptoms of gastro–oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
20. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
21. Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology, 2005;129:1825–31.
22. Sampliner RE. A population prevalence of Barrett’s esophagus––finally. Gastroenterology 2005; 129:2101–3.
23. Abrams JA, Fields S, Lightdale CJ, Neugut AI. Racial and ethnic disparities in the prevalence of Barrett’s esophagus among patients who undergo upper endoscopy. Clin Gastroenterol Hepatol 2008;6:30–4.
24. Corley DA, Kubo A, Levin TR, et al. Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community–based study, 1994–2006. Gut 2009;58:182–8.
25. Kamat P, Wen S, Morris J, Anandasabapathy S. Exploring the association between elevated body mass index and Barrett’s esophagus: a systematic review and meta–analysis. Ann Thorac Surg 2009;87:655–62.
26. Jacobson BC, Chan AT, Giovannucci EL, Fuchs CS. Body mass index and Barrett’s oesophagus in women. Gut 2009;58:1460–6.
27. Orloff M, Peterson C, He X, et al. Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma. JAMA 2011;306:410–9.
28. Sharma N, Ho KY. Risk Factors for Barrett’s oesophagus. Gastrointest Tumors 2016;3:103–8.
29. Spechler SJ. Barrett’s esophagus. Semin Gastrointest Dis 1996;7:51–60.
30. Ronkainen J, Talley NJ, Storskrubb T. Erosive esophagitis is a risk factor for Barrett’s esophagus: a community–based endoscopic follow–up study. Am J Gastroenterol 2011;106:1946–52.
31. Kim SL, Wo JM, Hunter JG. The prevalence of intestinal metaplasia in patients with and without peptic strictures. Am J Gastroenterol 1998;93:53–5.
32. Spechler SJ. Clinical practice. Barrett’s esophagus. N Engl J Med 2002;346:836–42.
33. Riddell RH, Odze RD. Definition of Barrett’s esophagus: time for a rethink––is intestinal metaplasia dead? Am J Gastroenterol 2009;104:2588–94.
34. Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus––the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol 1998;93:1033–6.
35. Eloubeidi MA, Provenzale D. Does this patient have Barrett’s esophagus? The utility of predicting Barrett’s esophagus at the index endoscopy. Am J Gastroenterol 1999;94:937–43.
36. Rudolph RE, Vaughan TL, Storer BE, et al. Effect of segment length on risk for neoplastic progression in patients with Barrett esophagus. Ann Intern Med 2000;132:612–20.
37. Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392–9.
38. Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population–based study. J Natl Cancer Inst 2011;103:1049–57.
39. Shaheen NJ, Dulai GS, Ascher B, et al. Effect of a new diagnosis of Barrett’s esophagus on insurance status. Am J Gastroenterol 2005;100:577–80.
40. Canto MI, Setrakian S, Willis J, et al. Methylene blue–directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest Endosc 2000;51:560–8.
41. Scotiniotis IA, Kochman ML, Lewis JD. Accuracy of EUS in the evaluation of Barrett’s esophagus and high–grade dysplasia or intramucosal carcinoma. Gastrointest Endosc 2001;54:689–96.
42. Kobayashi K, Izatt JA, Kulkarni MD, et al. High–resolution cross–sectional imaging of the gastrointestinal tract using optical coherence tomography: preliminary results. Gastrointest Endosc 1998;47:515–23.
43. Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance, and light–scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology 2001. 120: 1620–9.
44. Wallace MB, Sharma P, Lightdale C, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe–based confocal laser endomicroscopy. Gastrointest Endosc 2010;72:19–24.
45. Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta–analysis and systematic review. Clin Gastroenterol Hepatol 2013;11:1562–70.e1–2.–
46. DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1999;94:1434–42.
47. Inadomi JM, Sampliner R, Lagergren J. Screening and surveillance for Barrett esophagus in high–risk groups: a cost–utility analysis. Ann Intern Med 2003;138:176–86.
48. Conio M, Blanchi S, Lapertosa G, et al. Long–term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol 2003;98:1931–9.
49. Rastogi A, Puli S, El–Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high–grade dysplasia: a meta–analysis. Gastrointest Endosc 2008;67:394–8.
50. Kadri SR, Lao–Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non–endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ 2010;341:c4372.
51. Sharma, P., et al., A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology, 2004. 127(1): p. 310–30. [Same as #6]
52. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–28; quiz 329.
53. Kim SL, Waring JP, Spechler SJ, et al. Diagnostic inconsistencies in Barrett’s esophagus. Department of Veterans Affairs Gastroesophageal Reflux Study Group. Gastroenterology 1994;107:945–9.
54. Kastelein F, Spaander MC, Steyerberg EW, et al; ProBar Study Group. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2013;11:382–8.
55. El–Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol 2004;99:1877–83.
56. Nguyen DM, El–Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
57. Singh S, Garg SK, Singh PP, et al. Acid–suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta–analysis. Gut 2014;63:1229–37.
58. Spechler SJ, Lee E, Ahnen D, et al. Long–term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow–up of a randomized controlled trial. JAMA 2001;285:2331–8.
59. Peters FT, Ganesh S, Kuipers EJ, et al. Endoscopic regression of Barrett’s oesophagus during omeprazole treatment; a randomised double blind study. Gut 1999;45:489–94.
60. Ouatu–Lascar R, Triadafilopoulos G. –Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal acid reflux in patients with Barrett’s esophagus. Am J Gastroenterol 1998;93:711–6.
61. Maret–Ouda J, Konings P, Lagergren J, Brusselaers N. Antireflux surgery and risk of esophageal adenocarcinoma: A systematic review and meta–analysis. Ann Surg 2016;263:251–7.
62. Evans, J.A., et al., The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc, 2012. 76(6): p. 1087–94. [Same as #7]
63. ASGE Standards of Practice Committee, Evans JA, Early DS,et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
64. Bennett C, Moayyedi P, Corley DA, et al; BOB CAT Consortium. BOB CAT: A large–scale review and delphi consensus for ,anagement of Barrett’s esophagus with no dysplasia, indefinite for, or low–grade dysplasia. Am J Gastroenterol 2015;110:662–82; quiz 683.
65. Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta–analysis. Clin Gastroenterol Hepatol 2010;8:235–44; quiz e32.
66. Old O, Moayyedi P, Love S, et al; BOSS Trial Team. Barrett’s Oesophagus Surveillance versus endoscopy at need Study (BOSS): protocol and analysis plan for a multicentre randomized controlled trial. J Med Screen 2015;22:158–64.
67. Weston AP, Sharma P, Topalovski M, et al. Long–term follow–up of Barrett’s high–grade dysplasia. Am J Gastroenterol 2000;95:1888–93.
68. Paull A, Trier JS, Dalton MD, et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med 1976;295:476–80.
69. Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high–grade dysplasia in Barrett’s esophagus overestimated? Clin Gastroenterol Hepatol 2008;6:159–64.
70. Allison H, Banchs MA, Bonis PA, Guelrud M. Long–term remission of nondysplastic Barrett’s esophagus after multipolar electrocoagulation ablation: report of 139 patients with 10 years of follow–up. Gastrointest Endosc 2011;73:651–8.
71. Corley DA, Levin TR, Habel LA, Weiss NS, et al. Surveillance and survival in Barrett’s adenocarcinomas: a population–based study. Gastroenterology 2002;122:633–40.
72. Wong T, Tian J, Nagar AB. Barrett’s surveillance identifies patients with early esophageal adenocarcinoma. Am J Med 2010;123:462–7.
73. Fountoulakis A, Zafirellis KD, Dolan K, et al. Effect of surveillance of Barrett’s oesophagus on the clinical outcome of oesophageal cancer. Br J Surg 2004;91:997–1003.
74. Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population–based cohort study. Am J Gastroenterol 2014;109:1215–22.
75. Kastelein F, van Olphen SH, Steyerberg EW, et al. Impact of surveillance for Barrett’s oesophagus on tumour stage and survival of patients with neoplastic progression. Gut 2016;65:548–54.
76. Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low–grade dysplasia: a randomized clinical trial. JAMA 2014;311: 1209–17.
77. Wang WL, Chang IW, Chen CC, et al. Radiofrequency ablation versus endoscopic submucosal dissection in treating large early esophageal squamous cell neoplasia. Medicine (Baltimore) 2015;94:e2240.
78. Lim CH, Treanor D, Dixon MF, Axon AT. Low–grade dysplasia in Barrett’s esophagus has a high risk of progression. Endoscopy 2007;39:581–7.
79. Shaheen NJ, Inadomi JM, Overholt BF, Sharma P. What is the best management strategy for high grade dysplasia in Barrett’s oesophagus? A cost effectiveness analysis. Gut 2004;53:1736–44.
80. Vij R, Triadafilopoulos G, Owens DK, et al. Cost–effectiveness of photodynamic therapy for high–grade dysplasia in Barrett’s esophagus. Gastrointest Endosc 2004;60:739–56.
81. van den Boogert J, v an Hillegersberg R, Siersema PD, et al. Endoscopic ablation therapy for Barrett’s esophagus with high–grade dysplasia: a review. Am J Gastroenterol 1999;94:1153–60.
82. Sampliner RE. Endoscopic ablative therapy for Barrett’s esophagus: current status. Gastrointest Endosc 2004;59:66–9.
83. Sharma VK, Wang KK, Overholt BF, et al. Balloon–based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1–year follow–up of 100 patients. Gastrointest Endosc 2007;65:185–95.
84. Hanlon CR. Textbook of surgery: The biological basis of modern surgical practice, 14th edition. Ann Surg 1992;216:94.
85. Bright T, Watson DI, Tam W, et al. Randomized trial of argon plasma coagulation versus endoscopic surveillance for barrett esophagus after antireflux surgery: late results. Ann Surg 2007;246:1016–20.
86. Chadwick G, Groene O, Markar SR, et al. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett’s esophagus: a critical assessment of histologic outcomes and adverse events. Gastrointest Endosc 2014;79:718–31.e3.
87. Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett’s neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy 2008;40:370–9.
88. Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol 2010;8:23–9.
89. Kim HP, Bulsiewicz WJ, Cotton CC, et al. Focal endoscopic mucosal resection before radiofrequency ablation is equally effective and safe compared with radiofrequency ablation alone for the eradication of Barrett’s esophagus with advanced neoplasia. Gastrointest Endosc 2012;76:733–9.
90. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic ablation of Barrett’s esophagus: a multicenter study with 2.5–year follow–up. Gastrointest Endosc 2008;68:867–76.
91. Sharma VK, Jae Kim H, Das A, et al. Circumferential and focal ablation of Barrett’s esophagus containing dysplasia. Am J Gastroenterol 2009;104:310–7.
92. Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett’s esophagus that contains high–grade dysplasia: a U.S. multicenter registry. Gastrointest Endosc 2008;68:35–40.
93. Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology 2013;145:79–86.e1.
94. Lyday WD, Corbett FS, Kuperman DA, et al. Radiofrequency ablation of Barrett’s esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy 2010;42:272–8.
95. Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12:1840–7.e1.
96. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5–year outcomes from a prospective multicenter trial. Endoscopy 2010;42:781–9.
97. Weston AP, Sharma P, Banerjee S, et al. Visible endoscopic and histologic changes in the cardia, before and after complete Barrett’s esophagus ablation. Gastrointest Endosc 2005;61:515–21.
98. Beaumont H, Gondrie JJ, McMahon BP, et al. Stepwise radiofrequency ablation of Barrett’s esophagus preserves esophageal inner diameter, compliance, and motility. Endoscopy 2009;41:2–8.
99. Orman ES, Li N, Shaheen NJ.Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta–analysis. Clin Gastroenterol Hepatol 2013;11:1245–55.
100. Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 2012;143:576–81.
101. Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra–esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett’s esophagus. Dig Dis Sci 2012;57:2625–32.
102. Small AJ, Sutherland SE, Hightower JS, et al. Comparative risk of recurrence of dysplasia and carcinoma after endoluminal eradication therapy of high–grade dysplasia versus intramucosal carcinoma in Barrett’s esophagus. Gastrointest Endosc 2015;81:1158–66.e1–4.
103. Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high–grade dysplasia. Gastrointest Endosc 2010;71:680–5.
104. Shaheen NJ, Peery AF, Hawes RH, et al. Quality of life following radiofrequency ablation of dysplastic Barrett’s esophagus. Endoscopy 2010;42:790–9.
105. Bedi AO, Kwon RS, Rubenstein JH, et al. A survey of expert follow–up practices after successful endoscopic eradication therapy for Barrett’s esophagus with high–grade dysplasia and intramucosal adenocarcinoma. Gastrointest Endosc 2013;78:696–701.
106. Sampliner RE, Camargo E, Prasad AR. Prasad, Association of ablation of Barrett’s esophagus with high grade dysplasia and adenocarcinoma of the gastric cardia. Dis Esophagus 2006;19:277–9.
107. Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: long–term results. Gastrointest Endosc 2003;58:183–8.
108. Gosain S, Mercer K, Twaddell WS, et al. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high–grade dysplasia: long–term results. Gastrointest Endosc 2013;78:260–5.
109. Canto MI, Shin EJ, Khashab MA, et al. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett’s esophagus. Endoscopy 2015;47:582–91.
110. Van Laethem JL, Peny MO, Salmon I, et al. Intramucosal adenocarcinoma arising under squamous re–epithelialisation of Barrett’s oesophagus. Gut 2000;46:574–7.
111. Pech O, May A, Gossner L, et al. Management of pre–malignant and malignant lesions by endoscopic resection. Best Pract Res Clin Gastroenterol 2004;18:61–76.
112. Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003;57:567–79.
113. Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high–grade dysplasia in Barrett’s esophagus. Gastroenterology 2000;118:670–7.
114. Pech O, Behrens A, May A, et al. Long–term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high–grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008;57:1200–6.
115. Vieth M, Ell C, Gossner L, et al. Histological analysis of endoscopic resection specimens from 326 patients with Barrett’s esophagus and early neoplasia. Endoscopy 2004;36:776–81.
116. Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high–grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004;60:703–10.
117. Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high–grade dysplasia within Barrett’s esophagus. Gastrointest Endosc 2000;52:328–32.
118. Esaki M, Matsumoto T, Hirakawa K, et al. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy 2007;39:41–5.
119. Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007;65:3–10.
120. Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high–grade dysplasia and intramucosal carcinoma––an American single–center experience. Am J Gastroenterol 2009;104:2684–92.
121. Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high–volume centers. Ann Surg 2011;254:67–72.
122. Wu J, Pan YM, Wang TT, et al. Endotherapy versus surgery for early neoplasia in Barrett’s esophagus: a meta–analysis. Gastrointest Endosc 2014;79:233–241.e2.
123. Pech O, May A, Manner H, et al. Long–term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652–660.e1.
124. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
125. May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high–grade neoplasia and early adenocarcinoma in Barrett’s oesophagus: acute–phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002;14(10):1085–91.
126. Gerke H, Siddiqui J, Nasr I, et al. Efficacy and safety of EMR to completely remove Barrett’s esophagus: experience in 41 patients. Gastrointest Endosc 2011;74:761–71.
127. Lewis JJ, Rubenstein JH, Singal AG, et al. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett’s esophagus. Gastrointest Endosc 2011;74:753–60.
128. Choi IJ, Kim CG, Chang HJ, et al. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc 2005;62:860–5.
129. Deprez PH, Bergman JJ, Meisner S, et al. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy 2010;42:853–8.
130. van Lanschot JJ, Hulscher JB, Buskens CJ, et al. Hospital volume and hospital mortality for esophagectomy. Cancer 2001;91:1574–8.
131. Karl RC, Schreiber R, Boulware D, et al. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg 2000;231:635–43.
132. Young MM, Deschamps C, Trastek VF, et al. Esophageal reconstruction for benign disease: early morbidity, mortality, and functional results. Ann Thorac Surg 2000;70:1651–5.
133. Dunbar KB, Spechler SJ. The risk of lymph–node metastases in patients with high–grade dysplasia or intramucosal carcinoma in Barrett’s esophagus: a systematic review. Am J Gastroenterol 2012;107:850–62; quiz 863.
134. Morales CP, Souza RF, Spechler SJ. Hallmarks of cancer progression in Barrett’s oesophagus. Lancet 2002;360:1587–9.
135. Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012;10:722–7.
136. Abnet CC, Freedman ND, Kamangar F, et al. Non–steroidal anti–inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta–analysis. Br J Cancer 2009;100:551–7.
137. Kastelein F, Spaander MC, Biermann K, et al. Nonsteroidal anti–inflammatory drugs and statins have chemopreventative effects in patients with Barrett’s esophagus. Gastroenterology 2011;141:2000–8; quiz e13–4.
138. Zhang S, Zhang XQ, Ding XW, et al. Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a meta–analysis. Br J Cancer 2014;110: 2378–88.