User login
- Update on ovarian cancer screening
David G. Mutch, MD; Nora Kizer, MD (July 2010)
A majority of ovarian cancers are diagnosed at an advanced stage, requiring extensive surgical cytoreductive procedures.1 Because the presence of residual macroscopic disease correlates highly with decreased survival,2 these procedures can be lengthy, complicated, and risky for the patient. Many patients who undergo cytoreduction will be left with a suboptimal result despite surgery.
Better identification and improved treatment of patients who are at high risk of a suboptimal result are clearly needed. One treatment option is neoadjuvant chemotherapy, the administration of chemotherapy prior to the main treatment. Although early data suggested that it was associated with worse outcomes, recent studies have yielded new information:
- Neoadjuvant chemotherapy followed by interval debulking surgery is not inferior to primary debulking surgery followed by chemotherapy for patients who have bulky stage III or IV ovarian cancer
- In patients who have advanced ovarian cancer, neoadjuvant chemotherapy followed by surgical cytoreduction is associated with improved perioperative outcomes
- Postoperative intraperitoneal chemotherapy after neoadjuvant chemotherapy has not yet proved to be associated with improved survival.
Several questions prompted by these findings include:
- Will neoadjuvant chemotherapy improve surgical outcomes in patients who have advanced ovarian cancer and, thus, improve survival?
- Is neoadjuvant chemotherapy a better strategy for all patients?
- Will neoadjuvant chemotherapy reduce the surgical effort necessary to achieve an optimal result?
- What is the role of intraperitoneal chemotherapy in patients who undergo neoadjuvant chemotherapy?
Further national (or international) data are needed to confirm a survival advantage for patients who receive neoadjuvant chemotherapy, compared with those who undergo primary surgery before the administration of chemotherapy.
Neoadjuvant chemotherapy is an acceptable alternative to primary surgical cytoreduction
Vergote I, Tropé CG, Amant F, et al; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
Historically, the standard of care in ovarian cancer treatment has been surgical cytoreduction followed by chemotherapy.3-6 However, data from prospective randomized trials to support this practice are limited. Neoadjuvant chemotherapy is an alternative strategy that has been explored as a way to improve outcomes from interval surgical debulking in patients who have ovarian cancer in whom suboptimal cytoreduction is otherwise expected. Vergote and coworkers attempted to determine which strategy is better through a randomized trial of 632 patients.
Participants had to have biopsy-proven stage IIIc or IV ovarian, fallopian tube, or primary peritoneal cancer. The two treatment arms were:
- primary debulking surgery followed by at least 6 cycles of platinum-based chemotherapy
- 3 cycles of platinum-based neoadjuvant chemotherapy followed by interval debulking surgery in responders and those who had stable disease. These patients then received an additional 3 cycles of platinum-based chemotherapy post-operatively.
All surgical procedures were completed by qualified gynecologic oncologists, and all patients were evaluated for eligibility before randomization, with no additional selection criteria.
Postoperative death occurred in 2.5% of patients in the primary-surgery group, compared with 0.7% of patients in the neoadjuvant-chemotherapy group. Grade 3 or 4 hemorrhage occurred in 7.4% of patients after primary debulking, compared with 4.1% of patients after interval debulking. Patients who received neoadjuvant chemotherapy experienced a lower rate of infection (1.7% versus 8.1%) and venous complications (0% versus 2.6%).
Overall and progression-free survival rates were similar between the two groups. After multivariate analysis, the strongest predictors of survival were absence of residual disease after surgery (P<.001), small tumor size before randomization (P=.001), and endometrioid histology (P=.001)
Neoadjuvant chemotherapy is a preferred treatment strategy for patients who are expected to have a suboptimal result after surgery. Because neoadjuvant chemotherapy has a survival outcome similar to that of primary surgery followed by chemotherapy, it may be considered for all patients who have bulky stage IIIc or IV disease.
Although neoadjuvant chemotherapy improves the rate of optimal surgical cytoreduction, data are lacking to demonstrate that this improvement boosts survival.
Administration of neoadjuvant chemotherapy in these patients may improve perioperative morbidity and mortality, although no formal analysis was conducted in this study.
Neoadjuvant chemotherapy improves perioperative outcomes
Milam MR, Tao X, Coleman RL, et al. Neoadjuvant chemotherapy is associated with prolonged primary treatment intervals in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2011;21(1):66–71.
Milam and coworkers investigated chemotherapy-associated morbidity and timing in two groups of patients who had advanced epithelial ovarian cancer:
- those undergoing neoadjuvant chemotherapy followed by maximal cytoreductive surgery
- those undergoing primary surgery followed by chemotherapy.
Their retrospective study involved 263 consecutive patients who were treated at MD Anderson Cancer Center from 1993 to 2005. In this cohort, 47 women (18%) received neoadjuvant chemotherapy. These patients experienced less blood loss (400 mL versus 750 mL) and a shorter hospital stay (6 versus 8 days). Time to the initiation of chemotherapy from the date of diagnosis did not differ between groups, and the amount of residual disease and rate of survival were also similar between arms. However, patients who received neoadjuvant chemotherapy underwent more cycles of chemotherapy over a longer treatment period.
Although neoadjuvant chemotherapy does not appear to offer a survival advantage, it is equivalent to primary surgery followed by adjuvant chemotherapy and may be associated with improved perioperative outcomes.
The results of the trial by Vergote and colleagues (page 25), should discourage oncologists from prescribing more than 6 cycles of chemotherapy in the neoadjuvant setting; patients from their study in the neoadjuvant group received a total of 6 cycles and had survival outcomes equivalent to those of women in the primary surgery group.
In the pipeline: Data on intraperitoneal chemotherapy after neoadjuvant chemotherapy
Le T, Latifah H, Jolicoeur L, et al. Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol. 2011;121(3):451–454.
Although several studies have demonstrated that intraperitoneal (IP) chemotherapy provides a survival advantage, compared with intravenous (IV) chemotherapy, after primary surgical debulking, it remains unclear whether IP chemotherapy would provide a similar superior survival outcome following neoadjuvant chemotherapy (FIGURE).
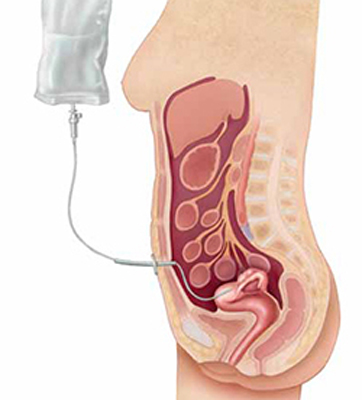
Intraperitoneal chemotherapy: How efficacious?
The jury is still out on whether intraperitoneal chemotherapy improves survival after neoadjuvant chemotherapy and interval debulking in stages III and IV ovarian cancer.The authors of this paper attempted to answer this question through a retrospective review of 71 patients. All patients were treated with neoadjuvant chemotherapy followed by interval debulking and either IP or IV chemotherapy. Overall, 17 patients (24%) received IP chemotherapy, and 54 patients (76%) received IV chemotherapy. The median number of cycles given prior to and after surgery was the same for both groups (3 for both neoadjuvant chemotherapy and chemotherapy following surgery).
Although patients who received IP chemotherapy had a higher overall response rate (82% versus 67%), there were no differences between groups in terms of progression-free (P=.42) and overall survival (P=.72).
One important limitation of this study was its small sample size and lack of statistical power. In addition, more patients in the IP group had macroscopic residual disease than in the IV group (71% versus 52%; P=.17).
A phase II/III study is under way to evaluate the use of IP chemotherapy following neoadjuvant chemotherapy in ovarian cancer patients.7 The two-stage randomized trial will compare IV chemotherapy with platinum-based IP chemotherapy in women who have undergone optimal surgical debulking (>1 cm) after 3 to 4 cycles of platinum-based neoadjuvant chemotherapy. This study is led by the US National Cancer Institute in collaboration with the Society of Gynecologic Oncologists of Canada, the UK National Cancer Research Institute, the Spanish Ovarian Cancer Research Group, and the US Southwest Oncology Group.
Data are limited on the use of intraperitoneal (IP) chemotherapy following neoadjuvant chemotherapy and interval surgical cytoreduction. We await the results of larger prospective studies to definitively determine whether there is a role for IP chemotherapy in this setting. For now, patients who receive neoadjuvant chemotherapy are limited to IV chemotherapy following surgery.
We want to hear from you! Tell us what you think.
1. Howlader N, Noone AM, Krapcho M, et all. eds. SEER Cancer Statistics Review 1975-2008. National Cancer Institute. http://seer.cancer.gov/csr/1975_2008. Published April 15, 2011. Accessed June 10, 2011.
2. du Bois A, Ruess A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials; by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115(6):1234-1244.
3. Meigs JV. Tumors of the pelvic organs. New York: Macmillan: 1934.
4. Aure JC, Hoeg K, Kolstad P. Clinical and histologic studies of ovarian carcinoma. Long-term follow-up of 990 cases. Obstet Gynecol. 1971;37(1):1-9.
5. Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58(1):131-142.
6. du Bois A, Quinn M, Thigpen T, et al. 2004 Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16(suppl 8):viii7-viii12.
7. Mackay HJ, Provencheur D, Heywood M, et al. Phase II/III study of intraperitoneal chemotherapy after neoadjuvant chemotherapy for ovarian cancer: ncic ctg ov.21. Curr Oncol. 2011;18(2):84-90.
- Update on ovarian cancer screening
David G. Mutch, MD; Nora Kizer, MD (July 2010)
A majority of ovarian cancers are diagnosed at an advanced stage, requiring extensive surgical cytoreductive procedures.1 Because the presence of residual macroscopic disease correlates highly with decreased survival,2 these procedures can be lengthy, complicated, and risky for the patient. Many patients who undergo cytoreduction will be left with a suboptimal result despite surgery.
Better identification and improved treatment of patients who are at high risk of a suboptimal result are clearly needed. One treatment option is neoadjuvant chemotherapy, the administration of chemotherapy prior to the main treatment. Although early data suggested that it was associated with worse outcomes, recent studies have yielded new information:
- Neoadjuvant chemotherapy followed by interval debulking surgery is not inferior to primary debulking surgery followed by chemotherapy for patients who have bulky stage III or IV ovarian cancer
- In patients who have advanced ovarian cancer, neoadjuvant chemotherapy followed by surgical cytoreduction is associated with improved perioperative outcomes
- Postoperative intraperitoneal chemotherapy after neoadjuvant chemotherapy has not yet proved to be associated with improved survival.
Several questions prompted by these findings include:
- Will neoadjuvant chemotherapy improve surgical outcomes in patients who have advanced ovarian cancer and, thus, improve survival?
- Is neoadjuvant chemotherapy a better strategy for all patients?
- Will neoadjuvant chemotherapy reduce the surgical effort necessary to achieve an optimal result?
- What is the role of intraperitoneal chemotherapy in patients who undergo neoadjuvant chemotherapy?
Further national (or international) data are needed to confirm a survival advantage for patients who receive neoadjuvant chemotherapy, compared with those who undergo primary surgery before the administration of chemotherapy.
Neoadjuvant chemotherapy is an acceptable alternative to primary surgical cytoreduction
Vergote I, Tropé CG, Amant F, et al; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
Historically, the standard of care in ovarian cancer treatment has been surgical cytoreduction followed by chemotherapy.3-6 However, data from prospective randomized trials to support this practice are limited. Neoadjuvant chemotherapy is an alternative strategy that has been explored as a way to improve outcomes from interval surgical debulking in patients who have ovarian cancer in whom suboptimal cytoreduction is otherwise expected. Vergote and coworkers attempted to determine which strategy is better through a randomized trial of 632 patients.
Participants had to have biopsy-proven stage IIIc or IV ovarian, fallopian tube, or primary peritoneal cancer. The two treatment arms were:
- primary debulking surgery followed by at least 6 cycles of platinum-based chemotherapy
- 3 cycles of platinum-based neoadjuvant chemotherapy followed by interval debulking surgery in responders and those who had stable disease. These patients then received an additional 3 cycles of platinum-based chemotherapy post-operatively.
All surgical procedures were completed by qualified gynecologic oncologists, and all patients were evaluated for eligibility before randomization, with no additional selection criteria.
Postoperative death occurred in 2.5% of patients in the primary-surgery group, compared with 0.7% of patients in the neoadjuvant-chemotherapy group. Grade 3 or 4 hemorrhage occurred in 7.4% of patients after primary debulking, compared with 4.1% of patients after interval debulking. Patients who received neoadjuvant chemotherapy experienced a lower rate of infection (1.7% versus 8.1%) and venous complications (0% versus 2.6%).
Overall and progression-free survival rates were similar between the two groups. After multivariate analysis, the strongest predictors of survival were absence of residual disease after surgery (P<.001), small tumor size before randomization (P=.001), and endometrioid histology (P=.001)
Neoadjuvant chemotherapy is a preferred treatment strategy for patients who are expected to have a suboptimal result after surgery. Because neoadjuvant chemotherapy has a survival outcome similar to that of primary surgery followed by chemotherapy, it may be considered for all patients who have bulky stage IIIc or IV disease.
Although neoadjuvant chemotherapy improves the rate of optimal surgical cytoreduction, data are lacking to demonstrate that this improvement boosts survival.
Administration of neoadjuvant chemotherapy in these patients may improve perioperative morbidity and mortality, although no formal analysis was conducted in this study.
Neoadjuvant chemotherapy improves perioperative outcomes
Milam MR, Tao X, Coleman RL, et al. Neoadjuvant chemotherapy is associated with prolonged primary treatment intervals in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2011;21(1):66–71.
Milam and coworkers investigated chemotherapy-associated morbidity and timing in two groups of patients who had advanced epithelial ovarian cancer:
- those undergoing neoadjuvant chemotherapy followed by maximal cytoreductive surgery
- those undergoing primary surgery followed by chemotherapy.
Their retrospective study involved 263 consecutive patients who were treated at MD Anderson Cancer Center from 1993 to 2005. In this cohort, 47 women (18%) received neoadjuvant chemotherapy. These patients experienced less blood loss (400 mL versus 750 mL) and a shorter hospital stay (6 versus 8 days). Time to the initiation of chemotherapy from the date of diagnosis did not differ between groups, and the amount of residual disease and rate of survival were also similar between arms. However, patients who received neoadjuvant chemotherapy underwent more cycles of chemotherapy over a longer treatment period.
Although neoadjuvant chemotherapy does not appear to offer a survival advantage, it is equivalent to primary surgery followed by adjuvant chemotherapy and may be associated with improved perioperative outcomes.
The results of the trial by Vergote and colleagues (page 25), should discourage oncologists from prescribing more than 6 cycles of chemotherapy in the neoadjuvant setting; patients from their study in the neoadjuvant group received a total of 6 cycles and had survival outcomes equivalent to those of women in the primary surgery group.
In the pipeline: Data on intraperitoneal chemotherapy after neoadjuvant chemotherapy
Le T, Latifah H, Jolicoeur L, et al. Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol. 2011;121(3):451–454.
Although several studies have demonstrated that intraperitoneal (IP) chemotherapy provides a survival advantage, compared with intravenous (IV) chemotherapy, after primary surgical debulking, it remains unclear whether IP chemotherapy would provide a similar superior survival outcome following neoadjuvant chemotherapy (FIGURE).

Intraperitoneal chemotherapy: How efficacious?
The jury is still out on whether intraperitoneal chemotherapy improves survival after neoadjuvant chemotherapy and interval debulking in stages III and IV ovarian cancer.The authors of this paper attempted to answer this question through a retrospective review of 71 patients. All patients were treated with neoadjuvant chemotherapy followed by interval debulking and either IP or IV chemotherapy. Overall, 17 patients (24%) received IP chemotherapy, and 54 patients (76%) received IV chemotherapy. The median number of cycles given prior to and after surgery was the same for both groups (3 for both neoadjuvant chemotherapy and chemotherapy following surgery).
Although patients who received IP chemotherapy had a higher overall response rate (82% versus 67%), there were no differences between groups in terms of progression-free (P=.42) and overall survival (P=.72).
One important limitation of this study was its small sample size and lack of statistical power. In addition, more patients in the IP group had macroscopic residual disease than in the IV group (71% versus 52%; P=.17).
A phase II/III study is under way to evaluate the use of IP chemotherapy following neoadjuvant chemotherapy in ovarian cancer patients.7 The two-stage randomized trial will compare IV chemotherapy with platinum-based IP chemotherapy in women who have undergone optimal surgical debulking (>1 cm) after 3 to 4 cycles of platinum-based neoadjuvant chemotherapy. This study is led by the US National Cancer Institute in collaboration with the Society of Gynecologic Oncologists of Canada, the UK National Cancer Research Institute, the Spanish Ovarian Cancer Research Group, and the US Southwest Oncology Group.
Data are limited on the use of intraperitoneal (IP) chemotherapy following neoadjuvant chemotherapy and interval surgical cytoreduction. We await the results of larger prospective studies to definitively determine whether there is a role for IP chemotherapy in this setting. For now, patients who receive neoadjuvant chemotherapy are limited to IV chemotherapy following surgery.
We want to hear from you! Tell us what you think.
- Update on ovarian cancer screening
David G. Mutch, MD; Nora Kizer, MD (July 2010)
A majority of ovarian cancers are diagnosed at an advanced stage, requiring extensive surgical cytoreductive procedures.1 Because the presence of residual macroscopic disease correlates highly with decreased survival,2 these procedures can be lengthy, complicated, and risky for the patient. Many patients who undergo cytoreduction will be left with a suboptimal result despite surgery.
Better identification and improved treatment of patients who are at high risk of a suboptimal result are clearly needed. One treatment option is neoadjuvant chemotherapy, the administration of chemotherapy prior to the main treatment. Although early data suggested that it was associated with worse outcomes, recent studies have yielded new information:
- Neoadjuvant chemotherapy followed by interval debulking surgery is not inferior to primary debulking surgery followed by chemotherapy for patients who have bulky stage III or IV ovarian cancer
- In patients who have advanced ovarian cancer, neoadjuvant chemotherapy followed by surgical cytoreduction is associated with improved perioperative outcomes
- Postoperative intraperitoneal chemotherapy after neoadjuvant chemotherapy has not yet proved to be associated with improved survival.
Several questions prompted by these findings include:
- Will neoadjuvant chemotherapy improve surgical outcomes in patients who have advanced ovarian cancer and, thus, improve survival?
- Is neoadjuvant chemotherapy a better strategy for all patients?
- Will neoadjuvant chemotherapy reduce the surgical effort necessary to achieve an optimal result?
- What is the role of intraperitoneal chemotherapy in patients who undergo neoadjuvant chemotherapy?
Further national (or international) data are needed to confirm a survival advantage for patients who receive neoadjuvant chemotherapy, compared with those who undergo primary surgery before the administration of chemotherapy.
Neoadjuvant chemotherapy is an acceptable alternative to primary surgical cytoreduction
Vergote I, Tropé CG, Amant F, et al; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
Historically, the standard of care in ovarian cancer treatment has been surgical cytoreduction followed by chemotherapy.3-6 However, data from prospective randomized trials to support this practice are limited. Neoadjuvant chemotherapy is an alternative strategy that has been explored as a way to improve outcomes from interval surgical debulking in patients who have ovarian cancer in whom suboptimal cytoreduction is otherwise expected. Vergote and coworkers attempted to determine which strategy is better through a randomized trial of 632 patients.
Participants had to have biopsy-proven stage IIIc or IV ovarian, fallopian tube, or primary peritoneal cancer. The two treatment arms were:
- primary debulking surgery followed by at least 6 cycles of platinum-based chemotherapy
- 3 cycles of platinum-based neoadjuvant chemotherapy followed by interval debulking surgery in responders and those who had stable disease. These patients then received an additional 3 cycles of platinum-based chemotherapy post-operatively.
All surgical procedures were completed by qualified gynecologic oncologists, and all patients were evaluated for eligibility before randomization, with no additional selection criteria.
Postoperative death occurred in 2.5% of patients in the primary-surgery group, compared with 0.7% of patients in the neoadjuvant-chemotherapy group. Grade 3 or 4 hemorrhage occurred in 7.4% of patients after primary debulking, compared with 4.1% of patients after interval debulking. Patients who received neoadjuvant chemotherapy experienced a lower rate of infection (1.7% versus 8.1%) and venous complications (0% versus 2.6%).
Overall and progression-free survival rates were similar between the two groups. After multivariate analysis, the strongest predictors of survival were absence of residual disease after surgery (P<.001), small tumor size before randomization (P=.001), and endometrioid histology (P=.001)
Neoadjuvant chemotherapy is a preferred treatment strategy for patients who are expected to have a suboptimal result after surgery. Because neoadjuvant chemotherapy has a survival outcome similar to that of primary surgery followed by chemotherapy, it may be considered for all patients who have bulky stage IIIc or IV disease.
Although neoadjuvant chemotherapy improves the rate of optimal surgical cytoreduction, data are lacking to demonstrate that this improvement boosts survival.
Administration of neoadjuvant chemotherapy in these patients may improve perioperative morbidity and mortality, although no formal analysis was conducted in this study.
Neoadjuvant chemotherapy improves perioperative outcomes
Milam MR, Tao X, Coleman RL, et al. Neoadjuvant chemotherapy is associated with prolonged primary treatment intervals in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2011;21(1):66–71.
Milam and coworkers investigated chemotherapy-associated morbidity and timing in two groups of patients who had advanced epithelial ovarian cancer:
- those undergoing neoadjuvant chemotherapy followed by maximal cytoreductive surgery
- those undergoing primary surgery followed by chemotherapy.
Their retrospective study involved 263 consecutive patients who were treated at MD Anderson Cancer Center from 1993 to 2005. In this cohort, 47 women (18%) received neoadjuvant chemotherapy. These patients experienced less blood loss (400 mL versus 750 mL) and a shorter hospital stay (6 versus 8 days). Time to the initiation of chemotherapy from the date of diagnosis did not differ between groups, and the amount of residual disease and rate of survival were also similar between arms. However, patients who received neoadjuvant chemotherapy underwent more cycles of chemotherapy over a longer treatment period.
Although neoadjuvant chemotherapy does not appear to offer a survival advantage, it is equivalent to primary surgery followed by adjuvant chemotherapy and may be associated with improved perioperative outcomes.
The results of the trial by Vergote and colleagues (page 25), should discourage oncologists from prescribing more than 6 cycles of chemotherapy in the neoadjuvant setting; patients from their study in the neoadjuvant group received a total of 6 cycles and had survival outcomes equivalent to those of women in the primary surgery group.
In the pipeline: Data on intraperitoneal chemotherapy after neoadjuvant chemotherapy
Le T, Latifah H, Jolicoeur L, et al. Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol. 2011;121(3):451–454.
Although several studies have demonstrated that intraperitoneal (IP) chemotherapy provides a survival advantage, compared with intravenous (IV) chemotherapy, after primary surgical debulking, it remains unclear whether IP chemotherapy would provide a similar superior survival outcome following neoadjuvant chemotherapy (FIGURE).

Intraperitoneal chemotherapy: How efficacious?
The jury is still out on whether intraperitoneal chemotherapy improves survival after neoadjuvant chemotherapy and interval debulking in stages III and IV ovarian cancer.The authors of this paper attempted to answer this question through a retrospective review of 71 patients. All patients were treated with neoadjuvant chemotherapy followed by interval debulking and either IP or IV chemotherapy. Overall, 17 patients (24%) received IP chemotherapy, and 54 patients (76%) received IV chemotherapy. The median number of cycles given prior to and after surgery was the same for both groups (3 for both neoadjuvant chemotherapy and chemotherapy following surgery).
Although patients who received IP chemotherapy had a higher overall response rate (82% versus 67%), there were no differences between groups in terms of progression-free (P=.42) and overall survival (P=.72).
One important limitation of this study was its small sample size and lack of statistical power. In addition, more patients in the IP group had macroscopic residual disease than in the IV group (71% versus 52%; P=.17).
A phase II/III study is under way to evaluate the use of IP chemotherapy following neoadjuvant chemotherapy in ovarian cancer patients.7 The two-stage randomized trial will compare IV chemotherapy with platinum-based IP chemotherapy in women who have undergone optimal surgical debulking (>1 cm) after 3 to 4 cycles of platinum-based neoadjuvant chemotherapy. This study is led by the US National Cancer Institute in collaboration with the Society of Gynecologic Oncologists of Canada, the UK National Cancer Research Institute, the Spanish Ovarian Cancer Research Group, and the US Southwest Oncology Group.
Data are limited on the use of intraperitoneal (IP) chemotherapy following neoadjuvant chemotherapy and interval surgical cytoreduction. We await the results of larger prospective studies to definitively determine whether there is a role for IP chemotherapy in this setting. For now, patients who receive neoadjuvant chemotherapy are limited to IV chemotherapy following surgery.
We want to hear from you! Tell us what you think.
1. Howlader N, Noone AM, Krapcho M, et all. eds. SEER Cancer Statistics Review 1975-2008. National Cancer Institute. http://seer.cancer.gov/csr/1975_2008. Published April 15, 2011. Accessed June 10, 2011.
2. du Bois A, Ruess A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials; by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115(6):1234-1244.
3. Meigs JV. Tumors of the pelvic organs. New York: Macmillan: 1934.
4. Aure JC, Hoeg K, Kolstad P. Clinical and histologic studies of ovarian carcinoma. Long-term follow-up of 990 cases. Obstet Gynecol. 1971;37(1):1-9.
5. Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58(1):131-142.
6. du Bois A, Quinn M, Thigpen T, et al. 2004 Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16(suppl 8):viii7-viii12.
7. Mackay HJ, Provencheur D, Heywood M, et al. Phase II/III study of intraperitoneal chemotherapy after neoadjuvant chemotherapy for ovarian cancer: ncic ctg ov.21. Curr Oncol. 2011;18(2):84-90.
1. Howlader N, Noone AM, Krapcho M, et all. eds. SEER Cancer Statistics Review 1975-2008. National Cancer Institute. http://seer.cancer.gov/csr/1975_2008. Published April 15, 2011. Accessed June 10, 2011.
2. du Bois A, Ruess A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials; by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115(6):1234-1244.
3. Meigs JV. Tumors of the pelvic organs. New York: Macmillan: 1934.
4. Aure JC, Hoeg K, Kolstad P. Clinical and histologic studies of ovarian carcinoma. Long-term follow-up of 990 cases. Obstet Gynecol. 1971;37(1):1-9.
5. Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58(1):131-142.
6. du Bois A, Quinn M, Thigpen T, et al. 2004 Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16(suppl 8):viii7-viii12.
7. Mackay HJ, Provencheur D, Heywood M, et al. Phase II/III study of intraperitoneal chemotherapy after neoadjuvant chemotherapy for ovarian cancer: ncic ctg ov.21. Curr Oncol. 2011;18(2):84-90.

