User login
The head and neck are the most common sites of involvement at initial presentation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]). Head and neck manifestations occur initially in 73% of patients, and eventually, up to 92% of patients with GPA are affected.1 Many of these compromise the upper airway. Although treatment is multidisciplinary, the effects on the airway make it important to understand upper airway presentations and treatments. This article examines upper airway disease presentations, their assessment, and their advocated interventions.
DISEASE COURSE
Because head and neck involvement may be associated with a less aggressive form of GPA, outcomes for patients with predominantly head and neck involvement may be better compared with those who have involvement of other systems.2
The natural course of GPA may be indolent or rapidly progressive. Regardless, left untreated, it progresses to a generalized systemic disease that often leads to significant morbidity and likely mortality. Most patients (96%) achieve remission with immunosuppressive therapy, but nearly half (49%) have at least one relapse.1 For this reason, systemic immunosuppressive medications play a dominant role in systemic and localized head and neck disease control. Patients often require maintenance medications along with additional therapies during disease exacerbation.3 Therefore, key partnerships between internists, rheumatologists, and otolaryngologists are paramount in the treatment and follow-up of these patients.
DIAGNOSIS: MAINSTAY IS SEROLOGIC EVALUATION
The differential diagnosis of GPA includes infection, lymphoproliferative disease (T-cell lymphoma), systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and other granulomatous diseases such as eosinophilic GPA (Churg-Strauss syndrome), polyarteritis nodosa, and microscopic polyangiitis. Appropriate diagnosis is critical because treatment of these entities varies drastically.
The mainstay of GPA diagnosis is serologic evaluation for a cytoplasmic pattern of antineutrophil cytoplasmic antibodies (cANCA), which are reactive toward proteinase-3 (PR3) or myeloperoxidase (MPO). Testing for cANCA yields a pooled sensitivity of 91% and specificity of 99%. Sensitivity falls significantly (63%) when the disease is in nonacute stages, while the specificity remains high.4 These cANCA test characteristics allow a high positive predictive value for this rare disease.
Biopsy is typically reserved for cases in which serologic ANCA testing is nondiagnostic. Biopsy tissue may be readily accessible from the head and neck, but these biopsies may bear significant false-negative rates.4–6 Diagnosis requires demonstration of palisading granulomas as vascular or extravascular lesions within the upper respiratory tract tissues. The specific site biopsied from within the head and neck has been shown to influence diagnostic yield, with sinonasal biopsies producing the highest yield.
SINONASAL MANIFESTATIONS
The nose and paranasal sinuses are the most frequently affected sites in the head and neck, noted in 64% to 80% of patients. Additionally, the nose is the only site of involvement in 30% of patients.7 Given the high frequency of sinonasal manifestations, GPA should be considered as a potential diagnosis among patients with persistent sinonasal disease.
Pathophysiology and disease course
The pathophysiologic mechanisms leading to the changes in the sinonasal tract in GPA have not been established. GPA is believed to be an immunologic disease that manifests as a vasculitis of small- and medium-sized vessels. Multiple potential causative factors have been identified, including fibrinoid necrosis of small blood vessels, epithelial granulomas, chronic inflammation, and prior surgical intervention.8,9 The acute and chronic inflammation, coupled with the epithelioid granuloma formation, damages adjacent small- to medium-sized vessels. The vasculitis leads to diminished blood flow and subsequent avascular necrosis, which may promote tissue necrosis and bone destruction. This destructive process typically starts in the midseptum supplied by Kiesselbach plexus and in the turbinates. The process then eventually spreads to the paranasal sinuses.8
Patient evaluation
Examination of the nasal cavities is typically performed by rigid or flexible nasal endoscopy and often reveals nasal crusting, friable erythematous mucosa, granulation, and even signs of sinusitis. All or part of the cartilaginous septum may be involved, leading to significant septal defects. As the degree of cartilage destruction increases, nasal dorsal support decreases, leading to a visible depression of the external nose known as a “saddle-nose” deformity, which is present in 23% of patients with GPA.7,10
Imaging assessment by computed tomography (CT) is needed to establish disease extent and involvement. Atypical findings may include bony erosion and destruction of the septum and turbinates; erosion of bony partitions within the ethmoid sinuses; neo-osteogenesis of the maxillary, frontal, and sphenoid sinuses; and complete bony obliteration of the maxillary, frontal, and sphenoid sinuses.9,11
Clinical presentation
Sinonasal disease indicates the degree of disease activity.12 Clinical findings may vary, but they have a significant impact on quality of life in these patients.13 Most patients with active disease present with nasal crusting (69%), chronic rhinosinusitis (CRS) symptoms (61%), nasal obstruction (58%), and serosanguinous nasal discharge (52%).10 Patients may also complain of foul-smelling rhinorrhea, recurrent epistaxis, hyposmia, anosmia, and epiphora (from granulomatous compression or obstruction of the lacrimal system). In a series of 120 patients with GPA, Cannady et al found that four (3.3%) patients had mucoceles and three (2.5%) had orbital pseudotumor.10
Any structure in the sinonasal cavity, including mucosa, septum, turbinates, and sinuses proper, may be affected because of the vasculitic involvement of mucosal blood vessels that causes diminished blood flow and subsequent necrosis. The area of the anterior septum supplied by Kiesselbach plexus is the most common site of active nasal disease, which can eventually lead to the common presentation of an anterior nasal septal perforation.
Otologic disease secondary to sinonasal GPA
Otologic involvement is observed in 19% to 38% of patients with GPA.14,15 Most patients with GPA who exhibit otologic symptoms have middle ear or mastoid disease. It typically appears as chronic otitis media (COM) with conductive hearing loss.16 In most cases, the otologic involvement is secondary to Eustachian tube dysfunction caused by the presence of extensive disease in the nasopharynx.
Additionally, chronic mastoiditis can result from direct mastoid involvement with GPA. Facial nerve palsy secondary to infective bony destruction is a rare but repeatedly reported complication of GPA.14,15
Inner ear involvement is a relatively common otologic presentation of GPA. Patients may experience sensorineural hearing loss (SNHL) as well as vertigo, which may mimic Cogan syndrome. Importantly, patients may exhibit inner ear involvement with or without middle ear and mastoid disease. The SNHL observed in patients with GPA may be responsive to steroid or immunosuppressive therapy.
Treatment
Refractory CRS in GPA is a complex problem for which aggressive surgical intervention is often counterproductive. Unfortunately, traditional medical therapies are also often inadequate to treat progressive sinonasal symptomatology. As the nasal tissue becomes devascularized, loss of normal mucociliary function aggravates the sinus pathology, and clinical symptoms may worsen. Simple antibiotic regimens used to manage uncomplicated sinusitis are often inadequate in these patients. The subsequent progression to frank necrosis in localized regions creates an intranasal foreign body, allowing bacterial colonization, which is often refractory to antibiotics because of the inability of drug tissue penetration into these devascularized nasal structures.12,17
Medical management must be tailored to be effective in this complex intranasal milieu. Successful treatment requires a multifaceted and often prolonged treatment course. A high index of suspicion should be maintained for Staphylococcus aureus. As a rule, endoscopically obtained cultures should be used to guide antibiotic selection. Several weeks of culture-directed antibiotics followed by topical antibiotic irrigations (eg, mupirocin irrigations) can be useful to reduce the frequency of sinonasal exacerbations.
Frequent saline irrigations using high-volume, high-flow irrigation devices (as opposed to low-volume, low-flow applicators such as nasal spray bottles) can be an excellent adjunct to maintenance therapy and are effective in clearing debris and augmenting mucociliary clearance in affected nasal cavities and those with septal perforations. Occasional in-office endoscopic debridement of large crusts adherent to intranasal structures or the edges of a septal perforation can also help to improve obstructive symptoms.
Surgery for refractory cases. Surgery should be reserved for refractory cases unresponsive to maximal medical efforts or those cases with impending complications (ie, mucoceles). Overall, only 16% of patients with sinonasal GPA required surgical intervention in a large series of 120 patients at our institution. In this series, one-third of all patients had undergone previous functional nasal surgery at an outside institution without resolution of symptoms. Anecdotal evidence suggests that surgery for GPA can contribute to additional scarring and lead to protracted sinonasal symptoms.10,18
The decision to perform surgery is individualized and based on severity of the disease process, patient expectations, and surgeon expertise. In our experience at Cleveland Clinic, functional endoscopic sinus surgery in the setting of GPA is a surgical challenge, given extensive alteration of the sinonasal anatomy from previous surgery, prior and ongoing inflammation, chronic crusting, and scarring. Consequently, it has been our practice to employ conservative efforts prior to consideration of surgery. A complete surgical cure is exceedingly rare, and the patient should be counseled about the possible need for revision surgery and ongoing nonsurgical therapies. Meticulous postoperative care with weekly postoperative debridement, saline or antibiotic irrigations, and culture-directed antibiotics, is essential during the early postoperative recovery phase.
Management of epiphora. The most common ophthalmologic findings in patients with GPA include chronic epiphora and orbital pseudotumor. With the advent of advanced endoscopic techniques, the otolaryngologist plays a greater role in the surgical management of these ophthalmologic disease entities. In a series reported by Cannady et al,10 endoscopic dacrocystorhinostomy was performed successfully in seven patients, including one revision.
Nasal reconstruction for saddle-nose deformity: effective in selected patients. The progressive loss of septal support that occurs with the enlarging anterior septal perforation often results in significant collapse of the cartilaginous midvault of the nose. The tip cartilages in turn also begin to lose projection, resulting in a shortened nose with the characteristic saddle-nose deformity. The psychologic impact of this disfiguring facial abnormality is significant. The loss of midvault support also results in worsening nasal obstruction and increases the incidence of anosmia as the superior nasal vault becomes obstructed. For these reasons, patients often seek referral for potential reconstruction.
Despite the potential benefits, the general consensus in the medical community has been that surgical procedures on the nose should be avoided in GPA patients.17 Most nasal destruction in these patients is the consequence of poor tissue perfusion from the active vasculitis. Poor wound healing, reconstructive graft resorption, and worsening necrosis have been observed in patients who have undergone ill-advised surgical procedures.
These poor outcomes do not, however, preclude the potential for safe and effective surgical intervention. In three small published series, good surgical outcomes were achieved but the procedures were done in very highly selected patients and were modified to address the specific clinical issues seen in GPA patients.19–21 The critical step in achieving a good outcome is working closely with the patient’s rheumatologist to identify an appropriate clinical window during which the patient’s disease process is in a period of relative remission. The second major factor is to modify the surgical techniques to take into account the very poor vascular framework of the recipient nasal bed.
Management of COM. Because the COM in patients with GPA is frequently secondary to nasopharyngeal disease, systemic control of GPA is the first priority. Systemic control is also the first-line treatment for patients with mixed or sensorineural hearing loss, or with vertigo. For continued or symptomatic middle ear effusions that do not resolve with systemic therapy, placement of a ventilation tube may be considered. In patients with significant hearing loss, hearing amplification devices may be warranted.15,22 Cochlear implant devices in GPA patients are experimental and may pose undue risks of meningitis.
SUBGLOTTIC STENOSIS AND TRACHEAL MANIFESTATIONS
Subglottic stenosis affects 10% to 20% of patients with GPA.1,23,24 Because of its potential life-threatening airway complications, patients should be carefully assessed for this disease manifestation. It may be the only manifestation of GPA or may be part of a spectrum of other disease manifestations. Therefore, the work-up for subglottic stenosis of unknown etiology should always include an evaluation for GPA.
Pathophysiology and disease course
The etiology of subglottic stenosis in GPA is not well understood. Theories primarily involve the vulnerability of the subglottic tissues to damage, chronic inflammation, and scarring.25 The combination of vasculitis in the setting of active inflammation may synergistically produce a hyperactive reparative mechanism in GPA patients that leads to cartilaginous fibrotic scarring and stenosis. Wound healing can be divided into the phases of inflammation, proliferation, and remodeling. An imbalance or exaggerated response of any of these levels (and likely all) produces an abnormal healing response.26 Similarly, each of these phases may be targeted to improve the healing process.
Patient evaluation
The presence of subglottic stenosis must be considered in a GPA patient with respiratory symptoms. As part of the routine initial evaluation, an office-based nasopharyngeal/laryngeal endoscopy using a flexible laryngoscope should be performed to assess the presence and severity of luminal airway narrowing. Flexible laryngoscopy reveals a circumferential narrowing of the subglottis. The stenotic tissue may vary from friable with erythematous and inflamed mucosa to a rigid mature fibrotic band, depending on the inflammatory state of the stenosis.18,27
Subclinical stenosis may be identified with routine endoscopy. An appropriate baseline is needed to follow the progression of disease and to adjust the timing of any potential intervention. The ability to digitally record a patient’s examination allows further tracking of disease and is commonly used in our practices.
Although flexible fiberoptic examination is critical in diagnosis and follow-up, intraoperative direct laryngoscopy using rigid laryngoscopes and telescopes provides the optimum view of the subglottis. In particular, this view provides greater information on the length and degree of the stenosis and allows evaluation of potential stenotic segments in the inferior trachea.
Spiral CT with 3-dimensional reconstruction of the laryngotracheal lumen and virtual bronchoscopy may provide information that complements laryngoscopy. CT may permit assessment of the entire tracheobronchial pathway. Because 15% to 55% of GPA patients have additional bronchial stenotic segments, assessment of the entire airway is important.28,29
Clinical presentation
Diagnosis of GPA in patients younger than 20 years is associated with the development of subglottic stenosis.23,30 The GPA patient with subglottic stenosis may or may not have other active systemic symptoms. The efficacy of systemic therapy often does not correlate with the degree of subglottic stenosis. Importantly, when systemic disease enters remission, the subglottic stenosis may remain due to residual scarring of the subglottis.31
Patients with subglottic stenosis may present with hoarseness, cough, wheeze, stridor, or dyspnea on exertion.27,32 The stridor and wheeze may be confused with the wheeze of asthma, often leading to misdiagnosis.17
Subglottic stenosis likely begins at a small degree and increases gradually, allowing the patient to adjust his or her breathing pattern until a critical stenotic airway area is reached. Typically, and dependent on their pulmonary health, patients are asymptomatic until about 75% airway stenosis (60% in children).33,34 At this point, symptoms may become evident and correlate with the degree of stenosis, ranging from cough and mild shortness of breath to life-threatening stridor and obstruction. Importantly, as the airway caliber narrows, mucous plugging becomes a greater concern, as it can cause acute stridulous exacerbations and airway obstruction.
A significant proportion of patients with GPA who have subclinical asymptomatic stenosis may not receive laryngeal examination. Patients who have suspicious clinical histories should be referred for evaluation of subglottic stenosis prior to symptom worsening.
Patients with significant (approximately 80%) stenosis can present with respiratory symptoms that may be life-threatening. Because airway management in this setting is substantially more difficult, the goal should be to obtain a diagnosis and perform intervention before this advanced presentation develops.
Pauzner et al described a possible association between GPA tracheal stenosis and pregnancy.35 Women of childbearing age who have GPA should be counseled about this possible association and the need for close follow-up during the partum and postpartum periods.
Treatment is controversial
The treatment of subglottic stenosis of GPA requires multidisciplinary management by the rheumatologist, otolaryngologist, and pulmonologist. Systemic manifestations of disease are managed by immunosuppressive therapy, but up to 80% of patients may require surgical management of subglottic stenosis, and the remaining 20% will respond to systemic medical therapy.22,23,36,37 Overall, the treatment of this disease is controversial and varies by center. The therapeutic arsenal consists of conventional immunosuppressive therapy, endoscopic dilation, endoscopic or laser excision, and surgical resection of the stenotic segment followed by reconstruction.
Tracheotomy. Historically, tracheotomies were performed in approximately one-half of patients with airway manifestations of GPA when the patient had active disease or when airway patency could not be adequately maintained. Most of these patients were eventually decannulated.23,25 At present, tracheotomy is performed infrequently and is reserved for patients who have either a severely tenuous airway (with tracheotomy the only safe option available to obtain a secure airway) or who express a preference for tracheotomy. In a recent study by Hoffman et al,38 tracheotomy was avoided in 21 patients through the use of stenosis dilation procedures.
Dilation. Endoscopic subglottic dilation is the currently advocated method of treatment, and has shown promising results. In two studies with a total of 41 GPA patients who were able to avoid tracheotomy and open surgical procedures, 24% underwent decannulation of previously placed tracheotomies and 24% required only one procedure at an average follow-up of 3.4 and 5 years per study. In these studies, the technique of intralesional corticosteroid with mechanical dilation (ILCD) was performed.31,36,38
Preferred: Dilation plus medical therapy
Because of the inflammatory etiology of this condition, surgical intervention has the risk of potentially worsening the stenosis. However, combining dilation of the stenosis with aggressive local medical treatment to prevent scar formation and cellular proliferation has been shown to be effective and safe. This treatment modality was recently recommended as the preferred therapy based on a number of relatively small clinical trials for subglottic stenosis, without the benefit of large controlled trials.
Our patient population consists of two subsets: (1) those who respond well to ILCD and systemic medical therapy, requiring a minimal number of dilations before no longer needing procedures because of a possible “burn out” of the subglottic disease, and (2) those who continue to have recurrence of stenosis, requiring repeat ILCD. The latter group requires close long-term observation.
To counter the effects of the exaggerated healing reaction of inflammation (early) and proliferation (late) following injury, two medications are applied to the area of repaired stenosis. The stenotic lesion is first injected submucosally with a long-acting corticosteroid suspension such as methylprednisolone. The solution is injected along the submucosal-perichondrial plane. Incisions are made in a star-like fashion, employing sharp metal microlaryngeal blades or, less commonly, the carbon dioxide laser. These incisions release the constricting stenotic ring and break it up, widening the diameter of the airway and simultaneously preserving islands of intact mucous membrane between the incisions. This epithelium is intended to regenerate and resurface the expanded lumen. Progressive serial dilations are performed using semirigid, flexible, smooth dilators or high-pressure balloon dilation. The next stage involves repeated topical applications of mitomycin-C to further inhibit fibrosis and restenosis by inhibiting cellular proliferation of the vigorous injury cycles of these lesions. Application of mitomycin-C to the dilated area of a laryngotracheal stenosis has been associated with a decreased rate of stenosis relapse.39
Our group at Cleveland Clinic has never used laser surgery alone without dilation on the subglottic stenosis caused by GPA. Incidentally, patients treated with laser surgery in other institutions prior to their referral to the Cleveland Clinic have developed complicating secondary stenoses that required more extensive surgical intervention to overcome the severe secondary superimposed damage. In theory, use of the laser may create unnecessary thermal injury that likely worsens local damage. These patients required laryngotracheal reconstructive procedures or had to undergo establishment of permanent tracheotomies.
CONCLUSION
Granulomatosis with polyangiitis is a rare disease that may manifest in multiple areas of the head and neck. Careful attention to diagnosis and management is critical, as these patients tend to have progressive disease with debilitating sequelae. The rheumatologist, otolaryngologist, and internist should identify patients with any constellation of symptoms that may be typical of GPA. A collaborative effort to diagnose, treat, and follow these patients is paramount to successful disease management.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Mahr A, Girard T, Agher R, Guillevin L. Analysis of factors predictive of survival based on 49 patients with systemic Wegener’s granulomatosis and prospective follow-up. Rheumatology (Oxford) 2001; 40:492–498.
- Wung PK, Stone JH. Therapeutics of Wegener’s granulomatosis. Nat Clin Pract Rheumatol 2006; 2:192–200.
- Rao JK, Weinberger M, Oddone EZ, Allen NB, Landsman P, Feussner JR. The role of antineutrophil cytoplasmic antibody (c-ANCA) testing in the diagnosis of Wegener granulomatosis: a literature review and meta-analysis. Ann Intern Med 1995; 123:925–932.
- Devaney KO, Travis WD, Hoffman G, Leavitt R, Lebovics R, Fauci AS. Interpretation of head and neck biopsies in Wegener’s granulomatosis: a pathologic study of 126 biopsies in 70 patients. Am J Surg Pathol 1990; 14:555–564.
- Jennings CR, Jones NS, Dugar J, Powell RJ, Lowe J. Wegener’s granulomatosis—a review of diagnosis and treatment in 53 subjects. Rhinology 1998; 36:188–191.
- McDonald TJ, DeRemee RA. Wegener’s granulomatosis. Laryngoscope 1983; 93:220–231.
- Lloyd G, Lund VJ, Beale T, Howard D. Rhinologic changes in Wegener’s granulomatosis. J Laryngol Otol 2002; 116:565–569.
- Yang C, Talbot JM, Hwang PH. Bony abnormalities of the paranasal sinuses in patients with Wegener’s granulomatosis. Am J Rhinol 2001; 15:121–125.
- Cannady SB, Batra PS, Koening C, et al. Sinonasal Wegener granulomatosis: a single-institution experience with 120 cases. Laryngoscope 2009; 119:757–761.
- Grindler D, Cannady S, Batra PS. Computed tomography findings in sinonasal Wegener’s granulomatosis. Am J Rhinol Allergy 2009; 23:497–501.
- Hughes RG, Drake-Lee A. Nasal manifestations of granulomatous disease. Hosp Med 2001; 62:417–421.
- Srouji IA, Andrews P, Edwards C, Lund VJ. General and rhinosinusitis-related quality of life in patients with Wegener’s granulomatosis. Laryngoscope 2006; 116:1621–1625.
- Kornblut AD, Wolff SM, deFries HO, Fauci AS. Wegener’s granulomatosis. Laryngoscope 1980; 90:1453–1465.
- McCaffrey TV, McDonald TJ, Facer GW, DeRemee RA. Otologic manifestations of Wegener’s granulomatosis. Otolaryngol Head Neck Surg 1980; 88:586–593.
- Bradley PJ. Wegener’s granulomatosis of the ear. J Laryngol Otol 1983; 97:623–626.
- Rasmussen N. Management of the ear, nose, and throat manifestations of Wegener granulomatosis: an otorhinolaryngologist’s perspective. Curr Opin Rheumatol 2001; 13:3–11.
- Erickson VR, Hwang PH. Wegener’s granulomatosis: current trends in diagnosis and management. Curr Opin Otolaryngol Head Neck Surg 2007; 15:170–176.
- Congdon D, Sherris DA, Specks U, McDonald T. Long-term follow-up of repair of external nasal deformities in patients with Wegener’s granulomatosis. Laryngoscope 2002; 112:731–737.
- Duffy FJ, Rossi RM, Pribaz JJ. Reconstruction of Wegener’s nasal deformity using bilateral facial artery musculomucosal flaps. Plast Reconstr Surg 1998; 101:1330–1333.
- Shipchandler TZ, Chung BJ, Alam DS. Saddle nose deformity reconstruction with a split calvarial bone L-shaped strut. Arch Facial Plast Surg 2008; 10:305–311.
- Hernández-Rodríguez J, Hoffman GS, Koening CL. Surgical interventions and local therapy for Wegener’s granulomatosis. Curr Opin Rheumatol 2010; 22:29–36.
- Lebovics RS, Hoffman GS, Leavitt RY, et al. The management of subglottic stenosis in patients with Wegener’s granulomatosis. Laryngoscope 1992; 102:1341–1345.
- Waxman J, Bose WJ. Laryngeal manifestations of Wegener’s granulomatosis: case reports and review of the literature. J Rheumatol 1986; 13:408–411.
- Maronian NC, Azadeh H, Waugh P, Hillel A. Association of laryngopharyngeal reflux disease and subglottic stenosis. Ann Otol Rhinol Laryngol 2001; 110:606–612.
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004; 9:283–289.
- Gubbels SP, Barkhuizen A, Hwang PH. Head and neck manifestations of Wegener’s granulomatosis. Otolaryngol Clin North Am 2003; 36:685–705.
- Polychronopoulos VS, Prakash UB, Golbin JM, Edell ES, Specks U. Airway involvement in Wegener’s granulomatosis. Rheum Dis Clin North Am 2007; 33:755–775.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
- Rottem M, Fauci AS, Hallahan CW, et al Wegener granulomatosis in children and adolescents: clinical presentation and outcome. J Pediatr 1993; 122:26–31.
- Eliachar I, Chan J, Akst L. New approaches to the management of subglottic stenosis in Wegener’s granulomatosis. Cleve Clin J Med 2002; 69( suppl 2):SII149–SII151.
- Solans-Laqué R, Bosch-Gil J, Canela M, Lorente J, Pallisa E, Vilardell-Tarrés M. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Lupus 2008; 17:832–836.
- Sandu K, Monnier P. Cricotracheal resection. Otolaryngol Clin North Am 2008; 41:981–998.
- Brouns M, Jayaraju ST, Lacor C, De Mey J, et al. Tracheal stenosis: a flow dynamics study [published online ahead of print November 30, 2006]. J Appl Physiol 2007; 102:1178–1184. doi: 10.1152/japplphysiol.01063.2006
- Pauzner R, Mayan H, Hershko E, Alcalay M, Farfel Z. Exacerbation of Wegener’s granulomatosis during pregnancy: report of a case with tracheal stenosis and literature review. J Rheumatol 1994; 21:1153–1156.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Schokkenbroek AA, Franssen CF, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis [published online ahead of print November 14, 2007]. Eur Arch Otorhinolaryngol 2008; 265:549–555. doi: 10.1007/s00405-007-0518-3
- Hoffman GS, Thomas-Golbanov CK, Chan J, Akst LM, Eliachar I. Treatment of subglottic stenosis, due to Wegener’s granulomatosis, with intralesional corticosteroids and dilation. J Rheumatol 2003; 30:1017–1021.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
The head and neck are the most common sites of involvement at initial presentation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]). Head and neck manifestations occur initially in 73% of patients, and eventually, up to 92% of patients with GPA are affected.1 Many of these compromise the upper airway. Although treatment is multidisciplinary, the effects on the airway make it important to understand upper airway presentations and treatments. This article examines upper airway disease presentations, their assessment, and their advocated interventions.
DISEASE COURSE
Because head and neck involvement may be associated with a less aggressive form of GPA, outcomes for patients with predominantly head and neck involvement may be better compared with those who have involvement of other systems.2
The natural course of GPA may be indolent or rapidly progressive. Regardless, left untreated, it progresses to a generalized systemic disease that often leads to significant morbidity and likely mortality. Most patients (96%) achieve remission with immunosuppressive therapy, but nearly half (49%) have at least one relapse.1 For this reason, systemic immunosuppressive medications play a dominant role in systemic and localized head and neck disease control. Patients often require maintenance medications along with additional therapies during disease exacerbation.3 Therefore, key partnerships between internists, rheumatologists, and otolaryngologists are paramount in the treatment and follow-up of these patients.
DIAGNOSIS: MAINSTAY IS SEROLOGIC EVALUATION
The differential diagnosis of GPA includes infection, lymphoproliferative disease (T-cell lymphoma), systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and other granulomatous diseases such as eosinophilic GPA (Churg-Strauss syndrome), polyarteritis nodosa, and microscopic polyangiitis. Appropriate diagnosis is critical because treatment of these entities varies drastically.
The mainstay of GPA diagnosis is serologic evaluation for a cytoplasmic pattern of antineutrophil cytoplasmic antibodies (cANCA), which are reactive toward proteinase-3 (PR3) or myeloperoxidase (MPO). Testing for cANCA yields a pooled sensitivity of 91% and specificity of 99%. Sensitivity falls significantly (63%) when the disease is in nonacute stages, while the specificity remains high.4 These cANCA test characteristics allow a high positive predictive value for this rare disease.
Biopsy is typically reserved for cases in which serologic ANCA testing is nondiagnostic. Biopsy tissue may be readily accessible from the head and neck, but these biopsies may bear significant false-negative rates.4–6 Diagnosis requires demonstration of palisading granulomas as vascular or extravascular lesions within the upper respiratory tract tissues. The specific site biopsied from within the head and neck has been shown to influence diagnostic yield, with sinonasal biopsies producing the highest yield.
SINONASAL MANIFESTATIONS
The nose and paranasal sinuses are the most frequently affected sites in the head and neck, noted in 64% to 80% of patients. Additionally, the nose is the only site of involvement in 30% of patients.7 Given the high frequency of sinonasal manifestations, GPA should be considered as a potential diagnosis among patients with persistent sinonasal disease.
Pathophysiology and disease course
The pathophysiologic mechanisms leading to the changes in the sinonasal tract in GPA have not been established. GPA is believed to be an immunologic disease that manifests as a vasculitis of small- and medium-sized vessels. Multiple potential causative factors have been identified, including fibrinoid necrosis of small blood vessels, epithelial granulomas, chronic inflammation, and prior surgical intervention.8,9 The acute and chronic inflammation, coupled with the epithelioid granuloma formation, damages adjacent small- to medium-sized vessels. The vasculitis leads to diminished blood flow and subsequent avascular necrosis, which may promote tissue necrosis and bone destruction. This destructive process typically starts in the midseptum supplied by Kiesselbach plexus and in the turbinates. The process then eventually spreads to the paranasal sinuses.8
Patient evaluation
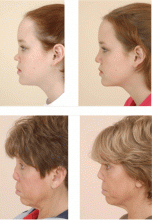
Examination of the nasal cavities is typically performed by rigid or flexible nasal endoscopy and often reveals nasal crusting, friable erythematous mucosa, granulation, and even signs of sinusitis. All or part of the cartilaginous septum may be involved, leading to significant septal defects. As the degree of cartilage destruction increases, nasal dorsal support decreases, leading to a visible depression of the external nose known as a “saddle-nose” deformity, which is present in 23% of patients with GPA.7,10
Imaging assessment by computed tomography (CT) is needed to establish disease extent and involvement. Atypical findings may include bony erosion and destruction of the septum and turbinates; erosion of bony partitions within the ethmoid sinuses; neo-osteogenesis of the maxillary, frontal, and sphenoid sinuses; and complete bony obliteration of the maxillary, frontal, and sphenoid sinuses.9,11
Clinical presentation
Sinonasal disease indicates the degree of disease activity.12 Clinical findings may vary, but they have a significant impact on quality of life in these patients.13 Most patients with active disease present with nasal crusting (69%), chronic rhinosinusitis (CRS) symptoms (61%), nasal obstruction (58%), and serosanguinous nasal discharge (52%).10 Patients may also complain of foul-smelling rhinorrhea, recurrent epistaxis, hyposmia, anosmia, and epiphora (from granulomatous compression or obstruction of the lacrimal system). In a series of 120 patients with GPA, Cannady et al found that four (3.3%) patients had mucoceles and three (2.5%) had orbital pseudotumor.10
Any structure in the sinonasal cavity, including mucosa, septum, turbinates, and sinuses proper, may be affected because of the vasculitic involvement of mucosal blood vessels that causes diminished blood flow and subsequent necrosis. The area of the anterior septum supplied by Kiesselbach plexus is the most common site of active nasal disease, which can eventually lead to the common presentation of an anterior nasal septal perforation.
Otologic disease secondary to sinonasal GPA
Otologic involvement is observed in 19% to 38% of patients with GPA.14,15 Most patients with GPA who exhibit otologic symptoms have middle ear or mastoid disease. It typically appears as chronic otitis media (COM) with conductive hearing loss.16 In most cases, the otologic involvement is secondary to Eustachian tube dysfunction caused by the presence of extensive disease in the nasopharynx.
Additionally, chronic mastoiditis can result from direct mastoid involvement with GPA. Facial nerve palsy secondary to infective bony destruction is a rare but repeatedly reported complication of GPA.14,15
Inner ear involvement is a relatively common otologic presentation of GPA. Patients may experience sensorineural hearing loss (SNHL) as well as vertigo, which may mimic Cogan syndrome. Importantly, patients may exhibit inner ear involvement with or without middle ear and mastoid disease. The SNHL observed in patients with GPA may be responsive to steroid or immunosuppressive therapy.
Treatment
Refractory CRS in GPA is a complex problem for which aggressive surgical intervention is often counterproductive. Unfortunately, traditional medical therapies are also often inadequate to treat progressive sinonasal symptomatology. As the nasal tissue becomes devascularized, loss of normal mucociliary function aggravates the sinus pathology, and clinical symptoms may worsen. Simple antibiotic regimens used to manage uncomplicated sinusitis are often inadequate in these patients. The subsequent progression to frank necrosis in localized regions creates an intranasal foreign body, allowing bacterial colonization, which is often refractory to antibiotics because of the inability of drug tissue penetration into these devascularized nasal structures.12,17
Medical management must be tailored to be effective in this complex intranasal milieu. Successful treatment requires a multifaceted and often prolonged treatment course. A high index of suspicion should be maintained for Staphylococcus aureus. As a rule, endoscopically obtained cultures should be used to guide antibiotic selection. Several weeks of culture-directed antibiotics followed by topical antibiotic irrigations (eg, mupirocin irrigations) can be useful to reduce the frequency of sinonasal exacerbations.
Frequent saline irrigations using high-volume, high-flow irrigation devices (as opposed to low-volume, low-flow applicators such as nasal spray bottles) can be an excellent adjunct to maintenance therapy and are effective in clearing debris and augmenting mucociliary clearance in affected nasal cavities and those with septal perforations. Occasional in-office endoscopic debridement of large crusts adherent to intranasal structures or the edges of a septal perforation can also help to improve obstructive symptoms.
Surgery for refractory cases. Surgery should be reserved for refractory cases unresponsive to maximal medical efforts or those cases with impending complications (ie, mucoceles). Overall, only 16% of patients with sinonasal GPA required surgical intervention in a large series of 120 patients at our institution. In this series, one-third of all patients had undergone previous functional nasal surgery at an outside institution without resolution of symptoms. Anecdotal evidence suggests that surgery for GPA can contribute to additional scarring and lead to protracted sinonasal symptoms.10,18
The decision to perform surgery is individualized and based on severity of the disease process, patient expectations, and surgeon expertise. In our experience at Cleveland Clinic, functional endoscopic sinus surgery in the setting of GPA is a surgical challenge, given extensive alteration of the sinonasal anatomy from previous surgery, prior and ongoing inflammation, chronic crusting, and scarring. Consequently, it has been our practice to employ conservative efforts prior to consideration of surgery. A complete surgical cure is exceedingly rare, and the patient should be counseled about the possible need for revision surgery and ongoing nonsurgical therapies. Meticulous postoperative care with weekly postoperative debridement, saline or antibiotic irrigations, and culture-directed antibiotics, is essential during the early postoperative recovery phase.
Management of epiphora. The most common ophthalmologic findings in patients with GPA include chronic epiphora and orbital pseudotumor. With the advent of advanced endoscopic techniques, the otolaryngologist plays a greater role in the surgical management of these ophthalmologic disease entities. In a series reported by Cannady et al,10 endoscopic dacrocystorhinostomy was performed successfully in seven patients, including one revision.
Nasal reconstruction for saddle-nose deformity: effective in selected patients. The progressive loss of septal support that occurs with the enlarging anterior septal perforation often results in significant collapse of the cartilaginous midvault of the nose. The tip cartilages in turn also begin to lose projection, resulting in a shortened nose with the characteristic saddle-nose deformity. The psychologic impact of this disfiguring facial abnormality is significant. The loss of midvault support also results in worsening nasal obstruction and increases the incidence of anosmia as the superior nasal vault becomes obstructed. For these reasons, patients often seek referral for potential reconstruction.
Despite the potential benefits, the general consensus in the medical community has been that surgical procedures on the nose should be avoided in GPA patients.17 Most nasal destruction in these patients is the consequence of poor tissue perfusion from the active vasculitis. Poor wound healing, reconstructive graft resorption, and worsening necrosis have been observed in patients who have undergone ill-advised surgical procedures.
These poor outcomes do not, however, preclude the potential for safe and effective surgical intervention. In three small published series, good surgical outcomes were achieved but the procedures were done in very highly selected patients and were modified to address the specific clinical issues seen in GPA patients.19–21 The critical step in achieving a good outcome is working closely with the patient’s rheumatologist to identify an appropriate clinical window during which the patient’s disease process is in a period of relative remission. The second major factor is to modify the surgical techniques to take into account the very poor vascular framework of the recipient nasal bed.
Management of COM. Because the COM in patients with GPA is frequently secondary to nasopharyngeal disease, systemic control of GPA is the first priority. Systemic control is also the first-line treatment for patients with mixed or sensorineural hearing loss, or with vertigo. For continued or symptomatic middle ear effusions that do not resolve with systemic therapy, placement of a ventilation tube may be considered. In patients with significant hearing loss, hearing amplification devices may be warranted.15,22 Cochlear implant devices in GPA patients are experimental and may pose undue risks of meningitis.
SUBGLOTTIC STENOSIS AND TRACHEAL MANIFESTATIONS
Subglottic stenosis affects 10% to 20% of patients with GPA.1,23,24 Because of its potential life-threatening airway complications, patients should be carefully assessed for this disease manifestation. It may be the only manifestation of GPA or may be part of a spectrum of other disease manifestations. Therefore, the work-up for subglottic stenosis of unknown etiology should always include an evaluation for GPA.
Pathophysiology and disease course
The etiology of subglottic stenosis in GPA is not well understood. Theories primarily involve the vulnerability of the subglottic tissues to damage, chronic inflammation, and scarring.25 The combination of vasculitis in the setting of active inflammation may synergistically produce a hyperactive reparative mechanism in GPA patients that leads to cartilaginous fibrotic scarring and stenosis. Wound healing can be divided into the phases of inflammation, proliferation, and remodeling. An imbalance or exaggerated response of any of these levels (and likely all) produces an abnormal healing response.26 Similarly, each of these phases may be targeted to improve the healing process.
Patient evaluation
The presence of subglottic stenosis must be considered in a GPA patient with respiratory symptoms. As part of the routine initial evaluation, an office-based nasopharyngeal/laryngeal endoscopy using a flexible laryngoscope should be performed to assess the presence and severity of luminal airway narrowing. Flexible laryngoscopy reveals a circumferential narrowing of the subglottis. The stenotic tissue may vary from friable with erythematous and inflamed mucosa to a rigid mature fibrotic band, depending on the inflammatory state of the stenosis.18,27
Subclinical stenosis may be identified with routine endoscopy. An appropriate baseline is needed to follow the progression of disease and to adjust the timing of any potential intervention. The ability to digitally record a patient’s examination allows further tracking of disease and is commonly used in our practices.
Although flexible fiberoptic examination is critical in diagnosis and follow-up, intraoperative direct laryngoscopy using rigid laryngoscopes and telescopes provides the optimum view of the subglottis. In particular, this view provides greater information on the length and degree of the stenosis and allows evaluation of potential stenotic segments in the inferior trachea.
Spiral CT with 3-dimensional reconstruction of the laryngotracheal lumen and virtual bronchoscopy may provide information that complements laryngoscopy. CT may permit assessment of the entire tracheobronchial pathway. Because 15% to 55% of GPA patients have additional bronchial stenotic segments, assessment of the entire airway is important.28,29
Clinical presentation
Diagnosis of GPA in patients younger than 20 years is associated with the development of subglottic stenosis.23,30 The GPA patient with subglottic stenosis may or may not have other active systemic symptoms. The efficacy of systemic therapy often does not correlate with the degree of subglottic stenosis. Importantly, when systemic disease enters remission, the subglottic stenosis may remain due to residual scarring of the subglottis.31
Patients with subglottic stenosis may present with hoarseness, cough, wheeze, stridor, or dyspnea on exertion.27,32 The stridor and wheeze may be confused with the wheeze of asthma, often leading to misdiagnosis.17
Subglottic stenosis likely begins at a small degree and increases gradually, allowing the patient to adjust his or her breathing pattern until a critical stenotic airway area is reached. Typically, and dependent on their pulmonary health, patients are asymptomatic until about 75% airway stenosis (60% in children).33,34 At this point, symptoms may become evident and correlate with the degree of stenosis, ranging from cough and mild shortness of breath to life-threatening stridor and obstruction. Importantly, as the airway caliber narrows, mucous plugging becomes a greater concern, as it can cause acute stridulous exacerbations and airway obstruction.
A significant proportion of patients with GPA who have subclinical asymptomatic stenosis may not receive laryngeal examination. Patients who have suspicious clinical histories should be referred for evaluation of subglottic stenosis prior to symptom worsening.
Patients with significant (approximately 80%) stenosis can present with respiratory symptoms that may be life-threatening. Because airway management in this setting is substantially more difficult, the goal should be to obtain a diagnosis and perform intervention before this advanced presentation develops.
Pauzner et al described a possible association between GPA tracheal stenosis and pregnancy.35 Women of childbearing age who have GPA should be counseled about this possible association and the need for close follow-up during the partum and postpartum periods.
Treatment is controversial
The treatment of subglottic stenosis of GPA requires multidisciplinary management by the rheumatologist, otolaryngologist, and pulmonologist. Systemic manifestations of disease are managed by immunosuppressive therapy, but up to 80% of patients may require surgical management of subglottic stenosis, and the remaining 20% will respond to systemic medical therapy.22,23,36,37 Overall, the treatment of this disease is controversial and varies by center. The therapeutic arsenal consists of conventional immunosuppressive therapy, endoscopic dilation, endoscopic or laser excision, and surgical resection of the stenotic segment followed by reconstruction.
Tracheotomy. Historically, tracheotomies were performed in approximately one-half of patients with airway manifestations of GPA when the patient had active disease or when airway patency could not be adequately maintained. Most of these patients were eventually decannulated.23,25 At present, tracheotomy is performed infrequently and is reserved for patients who have either a severely tenuous airway (with tracheotomy the only safe option available to obtain a secure airway) or who express a preference for tracheotomy. In a recent study by Hoffman et al,38 tracheotomy was avoided in 21 patients through the use of stenosis dilation procedures.
Dilation. Endoscopic subglottic dilation is the currently advocated method of treatment, and has shown promising results. In two studies with a total of 41 GPA patients who were able to avoid tracheotomy and open surgical procedures, 24% underwent decannulation of previously placed tracheotomies and 24% required only one procedure at an average follow-up of 3.4 and 5 years per study. In these studies, the technique of intralesional corticosteroid with mechanical dilation (ILCD) was performed.31,36,38
Preferred: Dilation plus medical therapy
Because of the inflammatory etiology of this condition, surgical intervention has the risk of potentially worsening the stenosis. However, combining dilation of the stenosis with aggressive local medical treatment to prevent scar formation and cellular proliferation has been shown to be effective and safe. This treatment modality was recently recommended as the preferred therapy based on a number of relatively small clinical trials for subglottic stenosis, without the benefit of large controlled trials.
Our patient population consists of two subsets: (1) those who respond well to ILCD and systemic medical therapy, requiring a minimal number of dilations before no longer needing procedures because of a possible “burn out” of the subglottic disease, and (2) those who continue to have recurrence of stenosis, requiring repeat ILCD. The latter group requires close long-term observation.
To counter the effects of the exaggerated healing reaction of inflammation (early) and proliferation (late) following injury, two medications are applied to the area of repaired stenosis. The stenotic lesion is first injected submucosally with a long-acting corticosteroid suspension such as methylprednisolone. The solution is injected along the submucosal-perichondrial plane. Incisions are made in a star-like fashion, employing sharp metal microlaryngeal blades or, less commonly, the carbon dioxide laser. These incisions release the constricting stenotic ring and break it up, widening the diameter of the airway and simultaneously preserving islands of intact mucous membrane between the incisions. This epithelium is intended to regenerate and resurface the expanded lumen. Progressive serial dilations are performed using semirigid, flexible, smooth dilators or high-pressure balloon dilation. The next stage involves repeated topical applications of mitomycin-C to further inhibit fibrosis and restenosis by inhibiting cellular proliferation of the vigorous injury cycles of these lesions. Application of mitomycin-C to the dilated area of a laryngotracheal stenosis has been associated with a decreased rate of stenosis relapse.39
Our group at Cleveland Clinic has never used laser surgery alone without dilation on the subglottic stenosis caused by GPA. Incidentally, patients treated with laser surgery in other institutions prior to their referral to the Cleveland Clinic have developed complicating secondary stenoses that required more extensive surgical intervention to overcome the severe secondary superimposed damage. In theory, use of the laser may create unnecessary thermal injury that likely worsens local damage. These patients required laryngotracheal reconstructive procedures or had to undergo establishment of permanent tracheotomies.
CONCLUSION
Granulomatosis with polyangiitis is a rare disease that may manifest in multiple areas of the head and neck. Careful attention to diagnosis and management is critical, as these patients tend to have progressive disease with debilitating sequelae. The rheumatologist, otolaryngologist, and internist should identify patients with any constellation of symptoms that may be typical of GPA. A collaborative effort to diagnose, treat, and follow these patients is paramount to successful disease management.
The head and neck are the most common sites of involvement at initial presentation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]). Head and neck manifestations occur initially in 73% of patients, and eventually, up to 92% of patients with GPA are affected.1 Many of these compromise the upper airway. Although treatment is multidisciplinary, the effects on the airway make it important to understand upper airway presentations and treatments. This article examines upper airway disease presentations, their assessment, and their advocated interventions.
DISEASE COURSE
Because head and neck involvement may be associated with a less aggressive form of GPA, outcomes for patients with predominantly head and neck involvement may be better compared with those who have involvement of other systems.2
The natural course of GPA may be indolent or rapidly progressive. Regardless, left untreated, it progresses to a generalized systemic disease that often leads to significant morbidity and likely mortality. Most patients (96%) achieve remission with immunosuppressive therapy, but nearly half (49%) have at least one relapse.1 For this reason, systemic immunosuppressive medications play a dominant role in systemic and localized head and neck disease control. Patients often require maintenance medications along with additional therapies during disease exacerbation.3 Therefore, key partnerships between internists, rheumatologists, and otolaryngologists are paramount in the treatment and follow-up of these patients.
DIAGNOSIS: MAINSTAY IS SEROLOGIC EVALUATION
The differential diagnosis of GPA includes infection, lymphoproliferative disease (T-cell lymphoma), systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and other granulomatous diseases such as eosinophilic GPA (Churg-Strauss syndrome), polyarteritis nodosa, and microscopic polyangiitis. Appropriate diagnosis is critical because treatment of these entities varies drastically.
The mainstay of GPA diagnosis is serologic evaluation for a cytoplasmic pattern of antineutrophil cytoplasmic antibodies (cANCA), which are reactive toward proteinase-3 (PR3) or myeloperoxidase (MPO). Testing for cANCA yields a pooled sensitivity of 91% and specificity of 99%. Sensitivity falls significantly (63%) when the disease is in nonacute stages, while the specificity remains high.4 These cANCA test characteristics allow a high positive predictive value for this rare disease.
Biopsy is typically reserved for cases in which serologic ANCA testing is nondiagnostic. Biopsy tissue may be readily accessible from the head and neck, but these biopsies may bear significant false-negative rates.4–6 Diagnosis requires demonstration of palisading granulomas as vascular or extravascular lesions within the upper respiratory tract tissues. The specific site biopsied from within the head and neck has been shown to influence diagnostic yield, with sinonasal biopsies producing the highest yield.
SINONASAL MANIFESTATIONS
The nose and paranasal sinuses are the most frequently affected sites in the head and neck, noted in 64% to 80% of patients. Additionally, the nose is the only site of involvement in 30% of patients.7 Given the high frequency of sinonasal manifestations, GPA should be considered as a potential diagnosis among patients with persistent sinonasal disease.
Pathophysiology and disease course
The pathophysiologic mechanisms leading to the changes in the sinonasal tract in GPA have not been established. GPA is believed to be an immunologic disease that manifests as a vasculitis of small- and medium-sized vessels. Multiple potential causative factors have been identified, including fibrinoid necrosis of small blood vessels, epithelial granulomas, chronic inflammation, and prior surgical intervention.8,9 The acute and chronic inflammation, coupled with the epithelioid granuloma formation, damages adjacent small- to medium-sized vessels. The vasculitis leads to diminished blood flow and subsequent avascular necrosis, which may promote tissue necrosis and bone destruction. This destructive process typically starts in the midseptum supplied by Kiesselbach plexus and in the turbinates. The process then eventually spreads to the paranasal sinuses.8
Patient evaluation
Examination of the nasal cavities is typically performed by rigid or flexible nasal endoscopy and often reveals nasal crusting, friable erythematous mucosa, granulation, and even signs of sinusitis. All or part of the cartilaginous septum may be involved, leading to significant septal defects. As the degree of cartilage destruction increases, nasal dorsal support decreases, leading to a visible depression of the external nose known as a “saddle-nose” deformity, which is present in 23% of patients with GPA.7,10
Imaging assessment by computed tomography (CT) is needed to establish disease extent and involvement. Atypical findings may include bony erosion and destruction of the septum and turbinates; erosion of bony partitions within the ethmoid sinuses; neo-osteogenesis of the maxillary, frontal, and sphenoid sinuses; and complete bony obliteration of the maxillary, frontal, and sphenoid sinuses.9,11
Clinical presentation
Sinonasal disease indicates the degree of disease activity.12 Clinical findings may vary, but they have a significant impact on quality of life in these patients.13 Most patients with active disease present with nasal crusting (69%), chronic rhinosinusitis (CRS) symptoms (61%), nasal obstruction (58%), and serosanguinous nasal discharge (52%).10 Patients may also complain of foul-smelling rhinorrhea, recurrent epistaxis, hyposmia, anosmia, and epiphora (from granulomatous compression or obstruction of the lacrimal system). In a series of 120 patients with GPA, Cannady et al found that four (3.3%) patients had mucoceles and three (2.5%) had orbital pseudotumor.10
Any structure in the sinonasal cavity, including mucosa, septum, turbinates, and sinuses proper, may be affected because of the vasculitic involvement of mucosal blood vessels that causes diminished blood flow and subsequent necrosis. The area of the anterior septum supplied by Kiesselbach plexus is the most common site of active nasal disease, which can eventually lead to the common presentation of an anterior nasal septal perforation.
Otologic disease secondary to sinonasal GPA
Otologic involvement is observed in 19% to 38% of patients with GPA.14,15 Most patients with GPA who exhibit otologic symptoms have middle ear or mastoid disease. It typically appears as chronic otitis media (COM) with conductive hearing loss.16 In most cases, the otologic involvement is secondary to Eustachian tube dysfunction caused by the presence of extensive disease in the nasopharynx.
Additionally, chronic mastoiditis can result from direct mastoid involvement with GPA. Facial nerve palsy secondary to infective bony destruction is a rare but repeatedly reported complication of GPA.14,15
Inner ear involvement is a relatively common otologic presentation of GPA. Patients may experience sensorineural hearing loss (SNHL) as well as vertigo, which may mimic Cogan syndrome. Importantly, patients may exhibit inner ear involvement with or without middle ear and mastoid disease. The SNHL observed in patients with GPA may be responsive to steroid or immunosuppressive therapy.
Treatment
Refractory CRS in GPA is a complex problem for which aggressive surgical intervention is often counterproductive. Unfortunately, traditional medical therapies are also often inadequate to treat progressive sinonasal symptomatology. As the nasal tissue becomes devascularized, loss of normal mucociliary function aggravates the sinus pathology, and clinical symptoms may worsen. Simple antibiotic regimens used to manage uncomplicated sinusitis are often inadequate in these patients. The subsequent progression to frank necrosis in localized regions creates an intranasal foreign body, allowing bacterial colonization, which is often refractory to antibiotics because of the inability of drug tissue penetration into these devascularized nasal structures.12,17
Medical management must be tailored to be effective in this complex intranasal milieu. Successful treatment requires a multifaceted and often prolonged treatment course. A high index of suspicion should be maintained for Staphylococcus aureus. As a rule, endoscopically obtained cultures should be used to guide antibiotic selection. Several weeks of culture-directed antibiotics followed by topical antibiotic irrigations (eg, mupirocin irrigations) can be useful to reduce the frequency of sinonasal exacerbations.
Frequent saline irrigations using high-volume, high-flow irrigation devices (as opposed to low-volume, low-flow applicators such as nasal spray bottles) can be an excellent adjunct to maintenance therapy and are effective in clearing debris and augmenting mucociliary clearance in affected nasal cavities and those with septal perforations. Occasional in-office endoscopic debridement of large crusts adherent to intranasal structures or the edges of a septal perforation can also help to improve obstructive symptoms.
Surgery for refractory cases. Surgery should be reserved for refractory cases unresponsive to maximal medical efforts or those cases with impending complications (ie, mucoceles). Overall, only 16% of patients with sinonasal GPA required surgical intervention in a large series of 120 patients at our institution. In this series, one-third of all patients had undergone previous functional nasal surgery at an outside institution without resolution of symptoms. Anecdotal evidence suggests that surgery for GPA can contribute to additional scarring and lead to protracted sinonasal symptoms.10,18
The decision to perform surgery is individualized and based on severity of the disease process, patient expectations, and surgeon expertise. In our experience at Cleveland Clinic, functional endoscopic sinus surgery in the setting of GPA is a surgical challenge, given extensive alteration of the sinonasal anatomy from previous surgery, prior and ongoing inflammation, chronic crusting, and scarring. Consequently, it has been our practice to employ conservative efforts prior to consideration of surgery. A complete surgical cure is exceedingly rare, and the patient should be counseled about the possible need for revision surgery and ongoing nonsurgical therapies. Meticulous postoperative care with weekly postoperative debridement, saline or antibiotic irrigations, and culture-directed antibiotics, is essential during the early postoperative recovery phase.
Management of epiphora. The most common ophthalmologic findings in patients with GPA include chronic epiphora and orbital pseudotumor. With the advent of advanced endoscopic techniques, the otolaryngologist plays a greater role in the surgical management of these ophthalmologic disease entities. In a series reported by Cannady et al,10 endoscopic dacrocystorhinostomy was performed successfully in seven patients, including one revision.
Nasal reconstruction for saddle-nose deformity: effective in selected patients. The progressive loss of septal support that occurs with the enlarging anterior septal perforation often results in significant collapse of the cartilaginous midvault of the nose. The tip cartilages in turn also begin to lose projection, resulting in a shortened nose with the characteristic saddle-nose deformity. The psychologic impact of this disfiguring facial abnormality is significant. The loss of midvault support also results in worsening nasal obstruction and increases the incidence of anosmia as the superior nasal vault becomes obstructed. For these reasons, patients often seek referral for potential reconstruction.
Despite the potential benefits, the general consensus in the medical community has been that surgical procedures on the nose should be avoided in GPA patients.17 Most nasal destruction in these patients is the consequence of poor tissue perfusion from the active vasculitis. Poor wound healing, reconstructive graft resorption, and worsening necrosis have been observed in patients who have undergone ill-advised surgical procedures.
These poor outcomes do not, however, preclude the potential for safe and effective surgical intervention. In three small published series, good surgical outcomes were achieved but the procedures were done in very highly selected patients and were modified to address the specific clinical issues seen in GPA patients.19–21 The critical step in achieving a good outcome is working closely with the patient’s rheumatologist to identify an appropriate clinical window during which the patient’s disease process is in a period of relative remission. The second major factor is to modify the surgical techniques to take into account the very poor vascular framework of the recipient nasal bed.
Management of COM. Because the COM in patients with GPA is frequently secondary to nasopharyngeal disease, systemic control of GPA is the first priority. Systemic control is also the first-line treatment for patients with mixed or sensorineural hearing loss, or with vertigo. For continued or symptomatic middle ear effusions that do not resolve with systemic therapy, placement of a ventilation tube may be considered. In patients with significant hearing loss, hearing amplification devices may be warranted.15,22 Cochlear implant devices in GPA patients are experimental and may pose undue risks of meningitis.
SUBGLOTTIC STENOSIS AND TRACHEAL MANIFESTATIONS
Subglottic stenosis affects 10% to 20% of patients with GPA.1,23,24 Because of its potential life-threatening airway complications, patients should be carefully assessed for this disease manifestation. It may be the only manifestation of GPA or may be part of a spectrum of other disease manifestations. Therefore, the work-up for subglottic stenosis of unknown etiology should always include an evaluation for GPA.
Pathophysiology and disease course
The etiology of subglottic stenosis in GPA is not well understood. Theories primarily involve the vulnerability of the subglottic tissues to damage, chronic inflammation, and scarring.25 The combination of vasculitis in the setting of active inflammation may synergistically produce a hyperactive reparative mechanism in GPA patients that leads to cartilaginous fibrotic scarring and stenosis. Wound healing can be divided into the phases of inflammation, proliferation, and remodeling. An imbalance or exaggerated response of any of these levels (and likely all) produces an abnormal healing response.26 Similarly, each of these phases may be targeted to improve the healing process.
Patient evaluation
The presence of subglottic stenosis must be considered in a GPA patient with respiratory symptoms. As part of the routine initial evaluation, an office-based nasopharyngeal/laryngeal endoscopy using a flexible laryngoscope should be performed to assess the presence and severity of luminal airway narrowing. Flexible laryngoscopy reveals a circumferential narrowing of the subglottis. The stenotic tissue may vary from friable with erythematous and inflamed mucosa to a rigid mature fibrotic band, depending on the inflammatory state of the stenosis.18,27
Subclinical stenosis may be identified with routine endoscopy. An appropriate baseline is needed to follow the progression of disease and to adjust the timing of any potential intervention. The ability to digitally record a patient’s examination allows further tracking of disease and is commonly used in our practices.
Although flexible fiberoptic examination is critical in diagnosis and follow-up, intraoperative direct laryngoscopy using rigid laryngoscopes and telescopes provides the optimum view of the subglottis. In particular, this view provides greater information on the length and degree of the stenosis and allows evaluation of potential stenotic segments in the inferior trachea.
Spiral CT with 3-dimensional reconstruction of the laryngotracheal lumen and virtual bronchoscopy may provide information that complements laryngoscopy. CT may permit assessment of the entire tracheobronchial pathway. Because 15% to 55% of GPA patients have additional bronchial stenotic segments, assessment of the entire airway is important.28,29
Clinical presentation
Diagnosis of GPA in patients younger than 20 years is associated with the development of subglottic stenosis.23,30 The GPA patient with subglottic stenosis may or may not have other active systemic symptoms. The efficacy of systemic therapy often does not correlate with the degree of subglottic stenosis. Importantly, when systemic disease enters remission, the subglottic stenosis may remain due to residual scarring of the subglottis.31
Patients with subglottic stenosis may present with hoarseness, cough, wheeze, stridor, or dyspnea on exertion.27,32 The stridor and wheeze may be confused with the wheeze of asthma, often leading to misdiagnosis.17
Subglottic stenosis likely begins at a small degree and increases gradually, allowing the patient to adjust his or her breathing pattern until a critical stenotic airway area is reached. Typically, and dependent on their pulmonary health, patients are asymptomatic until about 75% airway stenosis (60% in children).33,34 At this point, symptoms may become evident and correlate with the degree of stenosis, ranging from cough and mild shortness of breath to life-threatening stridor and obstruction. Importantly, as the airway caliber narrows, mucous plugging becomes a greater concern, as it can cause acute stridulous exacerbations and airway obstruction.
A significant proportion of patients with GPA who have subclinical asymptomatic stenosis may not receive laryngeal examination. Patients who have suspicious clinical histories should be referred for evaluation of subglottic stenosis prior to symptom worsening.
Patients with significant (approximately 80%) stenosis can present with respiratory symptoms that may be life-threatening. Because airway management in this setting is substantially more difficult, the goal should be to obtain a diagnosis and perform intervention before this advanced presentation develops.
Pauzner et al described a possible association between GPA tracheal stenosis and pregnancy.35 Women of childbearing age who have GPA should be counseled about this possible association and the need for close follow-up during the partum and postpartum periods.
Treatment is controversial
The treatment of subglottic stenosis of GPA requires multidisciplinary management by the rheumatologist, otolaryngologist, and pulmonologist. Systemic manifestations of disease are managed by immunosuppressive therapy, but up to 80% of patients may require surgical management of subglottic stenosis, and the remaining 20% will respond to systemic medical therapy.22,23,36,37 Overall, the treatment of this disease is controversial and varies by center. The therapeutic arsenal consists of conventional immunosuppressive therapy, endoscopic dilation, endoscopic or laser excision, and surgical resection of the stenotic segment followed by reconstruction.
Tracheotomy. Historically, tracheotomies were performed in approximately one-half of patients with airway manifestations of GPA when the patient had active disease or when airway patency could not be adequately maintained. Most of these patients were eventually decannulated.23,25 At present, tracheotomy is performed infrequently and is reserved for patients who have either a severely tenuous airway (with tracheotomy the only safe option available to obtain a secure airway) or who express a preference for tracheotomy. In a recent study by Hoffman et al,38 tracheotomy was avoided in 21 patients through the use of stenosis dilation procedures.
Dilation. Endoscopic subglottic dilation is the currently advocated method of treatment, and has shown promising results. In two studies with a total of 41 GPA patients who were able to avoid tracheotomy and open surgical procedures, 24% underwent decannulation of previously placed tracheotomies and 24% required only one procedure at an average follow-up of 3.4 and 5 years per study. In these studies, the technique of intralesional corticosteroid with mechanical dilation (ILCD) was performed.31,36,38
Preferred: Dilation plus medical therapy
Because of the inflammatory etiology of this condition, surgical intervention has the risk of potentially worsening the stenosis. However, combining dilation of the stenosis with aggressive local medical treatment to prevent scar formation and cellular proliferation has been shown to be effective and safe. This treatment modality was recently recommended as the preferred therapy based on a number of relatively small clinical trials for subglottic stenosis, without the benefit of large controlled trials.
Our patient population consists of two subsets: (1) those who respond well to ILCD and systemic medical therapy, requiring a minimal number of dilations before no longer needing procedures because of a possible “burn out” of the subglottic disease, and (2) those who continue to have recurrence of stenosis, requiring repeat ILCD. The latter group requires close long-term observation.
To counter the effects of the exaggerated healing reaction of inflammation (early) and proliferation (late) following injury, two medications are applied to the area of repaired stenosis. The stenotic lesion is first injected submucosally with a long-acting corticosteroid suspension such as methylprednisolone. The solution is injected along the submucosal-perichondrial plane. Incisions are made in a star-like fashion, employing sharp metal microlaryngeal blades or, less commonly, the carbon dioxide laser. These incisions release the constricting stenotic ring and break it up, widening the diameter of the airway and simultaneously preserving islands of intact mucous membrane between the incisions. This epithelium is intended to regenerate and resurface the expanded lumen. Progressive serial dilations are performed using semirigid, flexible, smooth dilators or high-pressure balloon dilation. The next stage involves repeated topical applications of mitomycin-C to further inhibit fibrosis and restenosis by inhibiting cellular proliferation of the vigorous injury cycles of these lesions. Application of mitomycin-C to the dilated area of a laryngotracheal stenosis has been associated with a decreased rate of stenosis relapse.39
Our group at Cleveland Clinic has never used laser surgery alone without dilation on the subglottic stenosis caused by GPA. Incidentally, patients treated with laser surgery in other institutions prior to their referral to the Cleveland Clinic have developed complicating secondary stenoses that required more extensive surgical intervention to overcome the severe secondary superimposed damage. In theory, use of the laser may create unnecessary thermal injury that likely worsens local damage. These patients required laryngotracheal reconstructive procedures or had to undergo establishment of permanent tracheotomies.
CONCLUSION
Granulomatosis with polyangiitis is a rare disease that may manifest in multiple areas of the head and neck. Careful attention to diagnosis and management is critical, as these patients tend to have progressive disease with debilitating sequelae. The rheumatologist, otolaryngologist, and internist should identify patients with any constellation of symptoms that may be typical of GPA. A collaborative effort to diagnose, treat, and follow these patients is paramount to successful disease management.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Mahr A, Girard T, Agher R, Guillevin L. Analysis of factors predictive of survival based on 49 patients with systemic Wegener’s granulomatosis and prospective follow-up. Rheumatology (Oxford) 2001; 40:492–498.
- Wung PK, Stone JH. Therapeutics of Wegener’s granulomatosis. Nat Clin Pract Rheumatol 2006; 2:192–200.
- Rao JK, Weinberger M, Oddone EZ, Allen NB, Landsman P, Feussner JR. The role of antineutrophil cytoplasmic antibody (c-ANCA) testing in the diagnosis of Wegener granulomatosis: a literature review and meta-analysis. Ann Intern Med 1995; 123:925–932.
- Devaney KO, Travis WD, Hoffman G, Leavitt R, Lebovics R, Fauci AS. Interpretation of head and neck biopsies in Wegener’s granulomatosis: a pathologic study of 126 biopsies in 70 patients. Am J Surg Pathol 1990; 14:555–564.
- Jennings CR, Jones NS, Dugar J, Powell RJ, Lowe J. Wegener’s granulomatosis—a review of diagnosis and treatment in 53 subjects. Rhinology 1998; 36:188–191.
- McDonald TJ, DeRemee RA. Wegener’s granulomatosis. Laryngoscope 1983; 93:220–231.
- Lloyd G, Lund VJ, Beale T, Howard D. Rhinologic changes in Wegener’s granulomatosis. J Laryngol Otol 2002; 116:565–569.
- Yang C, Talbot JM, Hwang PH. Bony abnormalities of the paranasal sinuses in patients with Wegener’s granulomatosis. Am J Rhinol 2001; 15:121–125.
- Cannady SB, Batra PS, Koening C, et al. Sinonasal Wegener granulomatosis: a single-institution experience with 120 cases. Laryngoscope 2009; 119:757–761.
- Grindler D, Cannady S, Batra PS. Computed tomography findings in sinonasal Wegener’s granulomatosis. Am J Rhinol Allergy 2009; 23:497–501.
- Hughes RG, Drake-Lee A. Nasal manifestations of granulomatous disease. Hosp Med 2001; 62:417–421.
- Srouji IA, Andrews P, Edwards C, Lund VJ. General and rhinosinusitis-related quality of life in patients with Wegener’s granulomatosis. Laryngoscope 2006; 116:1621–1625.
- Kornblut AD, Wolff SM, deFries HO, Fauci AS. Wegener’s granulomatosis. Laryngoscope 1980; 90:1453–1465.
- McCaffrey TV, McDonald TJ, Facer GW, DeRemee RA. Otologic manifestations of Wegener’s granulomatosis. Otolaryngol Head Neck Surg 1980; 88:586–593.
- Bradley PJ. Wegener’s granulomatosis of the ear. J Laryngol Otol 1983; 97:623–626.
- Rasmussen N. Management of the ear, nose, and throat manifestations of Wegener granulomatosis: an otorhinolaryngologist’s perspective. Curr Opin Rheumatol 2001; 13:3–11.
- Erickson VR, Hwang PH. Wegener’s granulomatosis: current trends in diagnosis and management. Curr Opin Otolaryngol Head Neck Surg 2007; 15:170–176.
- Congdon D, Sherris DA, Specks U, McDonald T. Long-term follow-up of repair of external nasal deformities in patients with Wegener’s granulomatosis. Laryngoscope 2002; 112:731–737.
- Duffy FJ, Rossi RM, Pribaz JJ. Reconstruction of Wegener’s nasal deformity using bilateral facial artery musculomucosal flaps. Plast Reconstr Surg 1998; 101:1330–1333.
- Shipchandler TZ, Chung BJ, Alam DS. Saddle nose deformity reconstruction with a split calvarial bone L-shaped strut. Arch Facial Plast Surg 2008; 10:305–311.
- Hernández-Rodríguez J, Hoffman GS, Koening CL. Surgical interventions and local therapy for Wegener’s granulomatosis. Curr Opin Rheumatol 2010; 22:29–36.
- Lebovics RS, Hoffman GS, Leavitt RY, et al. The management of subglottic stenosis in patients with Wegener’s granulomatosis. Laryngoscope 1992; 102:1341–1345.
- Waxman J, Bose WJ. Laryngeal manifestations of Wegener’s granulomatosis: case reports and review of the literature. J Rheumatol 1986; 13:408–411.
- Maronian NC, Azadeh H, Waugh P, Hillel A. Association of laryngopharyngeal reflux disease and subglottic stenosis. Ann Otol Rhinol Laryngol 2001; 110:606–612.
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004; 9:283–289.
- Gubbels SP, Barkhuizen A, Hwang PH. Head and neck manifestations of Wegener’s granulomatosis. Otolaryngol Clin North Am 2003; 36:685–705.
- Polychronopoulos VS, Prakash UB, Golbin JM, Edell ES, Specks U. Airway involvement in Wegener’s granulomatosis. Rheum Dis Clin North Am 2007; 33:755–775.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
- Rottem M, Fauci AS, Hallahan CW, et al Wegener granulomatosis in children and adolescents: clinical presentation and outcome. J Pediatr 1993; 122:26–31.
- Eliachar I, Chan J, Akst L. New approaches to the management of subglottic stenosis in Wegener’s granulomatosis. Cleve Clin J Med 2002; 69( suppl 2):SII149–SII151.
- Solans-Laqué R, Bosch-Gil J, Canela M, Lorente J, Pallisa E, Vilardell-Tarrés M. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Lupus 2008; 17:832–836.
- Sandu K, Monnier P. Cricotracheal resection. Otolaryngol Clin North Am 2008; 41:981–998.
- Brouns M, Jayaraju ST, Lacor C, De Mey J, et al. Tracheal stenosis: a flow dynamics study [published online ahead of print November 30, 2006]. J Appl Physiol 2007; 102:1178–1184. doi: 10.1152/japplphysiol.01063.2006
- Pauzner R, Mayan H, Hershko E, Alcalay M, Farfel Z. Exacerbation of Wegener’s granulomatosis during pregnancy: report of a case with tracheal stenosis and literature review. J Rheumatol 1994; 21:1153–1156.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Schokkenbroek AA, Franssen CF, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis [published online ahead of print November 14, 2007]. Eur Arch Otorhinolaryngol 2008; 265:549–555. doi: 10.1007/s00405-007-0518-3
- Hoffman GS, Thomas-Golbanov CK, Chan J, Akst LM, Eliachar I. Treatment of subglottic stenosis, due to Wegener’s granulomatosis, with intralesional corticosteroids and dilation. J Rheumatol 2003; 30:1017–1021.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Mahr A, Girard T, Agher R, Guillevin L. Analysis of factors predictive of survival based on 49 patients with systemic Wegener’s granulomatosis and prospective follow-up. Rheumatology (Oxford) 2001; 40:492–498.
- Wung PK, Stone JH. Therapeutics of Wegener’s granulomatosis. Nat Clin Pract Rheumatol 2006; 2:192–200.
- Rao JK, Weinberger M, Oddone EZ, Allen NB, Landsman P, Feussner JR. The role of antineutrophil cytoplasmic antibody (c-ANCA) testing in the diagnosis of Wegener granulomatosis: a literature review and meta-analysis. Ann Intern Med 1995; 123:925–932.
- Devaney KO, Travis WD, Hoffman G, Leavitt R, Lebovics R, Fauci AS. Interpretation of head and neck biopsies in Wegener’s granulomatosis: a pathologic study of 126 biopsies in 70 patients. Am J Surg Pathol 1990; 14:555–564.
- Jennings CR, Jones NS, Dugar J, Powell RJ, Lowe J. Wegener’s granulomatosis—a review of diagnosis and treatment in 53 subjects. Rhinology 1998; 36:188–191.
- McDonald TJ, DeRemee RA. Wegener’s granulomatosis. Laryngoscope 1983; 93:220–231.
- Lloyd G, Lund VJ, Beale T, Howard D. Rhinologic changes in Wegener’s granulomatosis. J Laryngol Otol 2002; 116:565–569.
- Yang C, Talbot JM, Hwang PH. Bony abnormalities of the paranasal sinuses in patients with Wegener’s granulomatosis. Am J Rhinol 2001; 15:121–125.
- Cannady SB, Batra PS, Koening C, et al. Sinonasal Wegener granulomatosis: a single-institution experience with 120 cases. Laryngoscope 2009; 119:757–761.
- Grindler D, Cannady S, Batra PS. Computed tomography findings in sinonasal Wegener’s granulomatosis. Am J Rhinol Allergy 2009; 23:497–501.
- Hughes RG, Drake-Lee A. Nasal manifestations of granulomatous disease. Hosp Med 2001; 62:417–421.
- Srouji IA, Andrews P, Edwards C, Lund VJ. General and rhinosinusitis-related quality of life in patients with Wegener’s granulomatosis. Laryngoscope 2006; 116:1621–1625.
- Kornblut AD, Wolff SM, deFries HO, Fauci AS. Wegener’s granulomatosis. Laryngoscope 1980; 90:1453–1465.
- McCaffrey TV, McDonald TJ, Facer GW, DeRemee RA. Otologic manifestations of Wegener’s granulomatosis. Otolaryngol Head Neck Surg 1980; 88:586–593.
- Bradley PJ. Wegener’s granulomatosis of the ear. J Laryngol Otol 1983; 97:623–626.
- Rasmussen N. Management of the ear, nose, and throat manifestations of Wegener granulomatosis: an otorhinolaryngologist’s perspective. Curr Opin Rheumatol 2001; 13:3–11.
- Erickson VR, Hwang PH. Wegener’s granulomatosis: current trends in diagnosis and management. Curr Opin Otolaryngol Head Neck Surg 2007; 15:170–176.
- Congdon D, Sherris DA, Specks U, McDonald T. Long-term follow-up of repair of external nasal deformities in patients with Wegener’s granulomatosis. Laryngoscope 2002; 112:731–737.
- Duffy FJ, Rossi RM, Pribaz JJ. Reconstruction of Wegener’s nasal deformity using bilateral facial artery musculomucosal flaps. Plast Reconstr Surg 1998; 101:1330–1333.
- Shipchandler TZ, Chung BJ, Alam DS. Saddle nose deformity reconstruction with a split calvarial bone L-shaped strut. Arch Facial Plast Surg 2008; 10:305–311.
- Hernández-Rodríguez J, Hoffman GS, Koening CL. Surgical interventions and local therapy for Wegener’s granulomatosis. Curr Opin Rheumatol 2010; 22:29–36.
- Lebovics RS, Hoffman GS, Leavitt RY, et al. The management of subglottic stenosis in patients with Wegener’s granulomatosis. Laryngoscope 1992; 102:1341–1345.
- Waxman J, Bose WJ. Laryngeal manifestations of Wegener’s granulomatosis: case reports and review of the literature. J Rheumatol 1986; 13:408–411.
- Maronian NC, Azadeh H, Waugh P, Hillel A. Association of laryngopharyngeal reflux disease and subglottic stenosis. Ann Otol Rhinol Laryngol 2001; 110:606–612.
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004; 9:283–289.
- Gubbels SP, Barkhuizen A, Hwang PH. Head and neck manifestations of Wegener’s granulomatosis. Otolaryngol Clin North Am 2003; 36:685–705.
- Polychronopoulos VS, Prakash UB, Golbin JM, Edell ES, Specks U. Airway involvement in Wegener’s granulomatosis. Rheum Dis Clin North Am 2007; 33:755–775.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
- Rottem M, Fauci AS, Hallahan CW, et al Wegener granulomatosis in children and adolescents: clinical presentation and outcome. J Pediatr 1993; 122:26–31.
- Eliachar I, Chan J, Akst L. New approaches to the management of subglottic stenosis in Wegener’s granulomatosis. Cleve Clin J Med 2002; 69( suppl 2):SII149–SII151.
- Solans-Laqué R, Bosch-Gil J, Canela M, Lorente J, Pallisa E, Vilardell-Tarrés M. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Lupus 2008; 17:832–836.
- Sandu K, Monnier P. Cricotracheal resection. Otolaryngol Clin North Am 2008; 41:981–998.
- Brouns M, Jayaraju ST, Lacor C, De Mey J, et al. Tracheal stenosis: a flow dynamics study [published online ahead of print November 30, 2006]. J Appl Physiol 2007; 102:1178–1184. doi: 10.1152/japplphysiol.01063.2006
- Pauzner R, Mayan H, Hershko E, Alcalay M, Farfel Z. Exacerbation of Wegener’s granulomatosis during pregnancy: report of a case with tracheal stenosis and literature review. J Rheumatol 1994; 21:1153–1156.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Schokkenbroek AA, Franssen CF, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis [published online ahead of print November 14, 2007]. Eur Arch Otorhinolaryngol 2008; 265:549–555. doi: 10.1007/s00405-007-0518-3
- Hoffman GS, Thomas-Golbanov CK, Chan J, Akst LM, Eliachar I. Treatment of subglottic stenosis, due to Wegener’s granulomatosis, with intralesional corticosteroids and dilation. J Rheumatol 2003; 30:1017–1021.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.