User login
- A stepwise approach to antidiabetic therapy allows for the treatment to change in response to disease progression. This usually means beginning with oral agents and adding insulin as required (B).
- Treatment strategies must address both fasting and prandial hyperglycemia because prandial hyperglycemia has been shown to be an independent risk factor for cardiovascular events and mortality (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
More than 80% of patients with type 2 diabetes—including more than a third of patients with good metabolic control—have excessive postprandial hyperglycemia.1 That’s unwelcome news for the 20 million Americans with type 2 diabetes, especially when you consider that post-prandial hyperglycemia is a strong independent risk factor for all-cause mortality and cardiovascular events.2-5
To help our type 2 diabetes patients gain ideal control, we need to do at least 2 things better:
- Measure and act on glycosylated hemoglobin (A1c) levels.
- Take a stepped approach to glycemic control, making full use of prandial insulin.
A1clevels and the important role they play
Analysis of A1c is the “gold standard” for monitoring glycemic control in patients with diabetes because it provides an indication of mean plasma glucose levels during the preceding 120 days.6 The relative contribution of fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) to A1c levels is a dynamic function of the extent of day-long hyperglycemia; FPG has a greater influence at higher A1c levels and PPG has a predominant role at lower A1c levels.7
The relationship between hyperglycemia, as measured by A1c, and increased morbidity and mortality (including cardiovascular events) was demonstrated several years ago in the United Kingdom Prospective Diabetes Study (UKPDS).8 Interestingly, several studies have also found that fasting glucose levels alone are not a reliable predictor for hyperglycemia-related morbidity or mortality, whereas postprandial hyperglycemia, as noted in the introduction, is a strong independent risk factor for all-cause mortality and cardiovascular events.2-5
Continued management of A1c through tight control of both FPG and PPG may therefore improve patient long-term health outcomes. A1c should be evaluated every 3 to 6 months, and appropriate changes to the patients’ treatment regimens should be made accordingly.
An algorithm for the stepwise approach
We typically use oral antidiabetic drugs typically are used as initial therapy for patients with newly diagnosed type 2 diabetes, especially those with initial A1c levels of 6.0% to 8.0%.9 Three recent publications10-12 provide an excellent analysis of the rationale for combination therapy to address multiple physiologic defects, as well as the relative efficacy of agents.
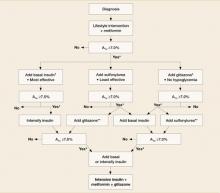
In 2006 the American Diabetes Association (ADA) and the European Association for the Study of Diabetes published a consensus statement that presented an algorithm for the initiation and adjustment of type 2 diabetes therapy (Figure).13 In this evidence-and experience-based treatment algorithm, the authors emphasize the achievement and maintenance of normal glycemic goals, initiating therapy with lifestyle intervention and metformin (Glucophage), not delaying therapy and transitioning to new regimens when glycemic targets are not achieved, and adding insulin therapy early to the regimens of patients who are not meeting glycemic targets.9,13
You may also consider newer therapeutic options not included in the ADA’s 2007 treatment guidelines.14 Incretin mimetics and dipeptidyl-peptidase IV (DPP-IV) inhibitors are 2 new classes of antidiabetic agents that are effective for patients with type 2 diabetes. (See “2 new classes of antidiabetic agents: Incretin mimetics and DPP-IV inhibitors” 15-22 ) Most patients with type 2 diabetes will, however, eventually require insulin therapy to maintain optimal glycemic control.23
Advancement to insulin therapy
Basal insulin replacement achieves glycemic control
The addition of once-daily basal insulin to oral antidiabetic drug regimens is a simple way to introduce insulin therapy and achieve glycemic control. (See “A guide to basal insulin dosing and titration”14,24-28 )
- In a randomized, parallel, multi-center study, treatment with once-daily insulin glargine (Lantus) or neutral protamine Hagedorn (NPH) insulin (Humulin, Novolin) added to preexisting oral antidiabetic drug regimens for 756 patients with inadequately controlled type 2 diabetes (A1c >7.5%) effectively achieved the goal A1c of ≤7.0% for most patients. More patients in the insulin glargine group were able to reach goal without experiencing any nocturnal hypoglycemia, compared with those in the NPH group (33.2% vs 26.7%, P<.05).24
- A similar study used a forced-titration algorithm to compare NPH insulin with insulin detemir (Levemir).26 This study demonstrated that a similar reduction in A1c could be achieved with insulin detemir and NPH insulin over 24 weeks (1.8% and 1.9%, P=ns), but weight gain and nocturnal hypoglycemia incidence were significantly lower with insulin detemir compared with NPH insulin (1.2 kg vs 2.8 kg, and 160 vs 349 events, respectively, P<.001 for both).
Prandial insulin is as effective as carbohydrate counting
If a patient doesn’t reach his or her A1c targets despite appropriate titration of the basal insulin dose, injections of a rapid-acting insulin analog at mealtime may be necessary. You can add rapid-acting insulin to the regimen, starting with 1 injection at the largest meal of the day and then adding an injection at additional meals as needed.
- A typical starting dose of rapid-acting prandial insulin (insulin aspart [NovoLog], insulin glulisine [Apidra], or insulin lispro [Humalog]) would be 5 to 10 U per meal.29
- The range of daily dose, when used at 3 meals per day, would be 0.2 to 0.5 U/kg per day (ie, 0.1 to 0.15 U/kg per meal).29 For a patient who weighs 100 kg, that would mean 20 U (6–7 units before each meal) per day.
FIGURE
An evidence-based algorithm for achieving normal glycemic goals in patients with type 2 diabetes
Note: Reinforce lifestyle intervention at every visit.
*Check A1c every 3 months until <7% and then at least every 6 months.
†At A1C >9%.
‡At A1C ≤8%.
**Although 3 oral agents can be used, initiation and intensification of insulin therapy is preferred based on effectiveness and expense.
Reprinted with permission from the American Diabetes Association.13
Incretin mimetics
Incretins, such as glucagonlike peptide-1 (GLP-1), enhance glucose-dependent insulin secretion by the pancreatic beta cells and exhibit other antihyperglycemic actions after release into circulation by the gut.
Exenatide (Byetta) is the first agent in this class to be approved by the Food and Drug Administration for use in type 2 diabetes. Exenatide may be used in combination with oral therapy (a sulfonylurea and/or metformin) by patients who have not achieved adequate glycemic control on oral therapy alone. Exenatide mimics the antihyperglycemic actions of GLP-1 but maintains a prolonged duration of action compared with endogenous GLP-1.15 This agent effectively addresses postprandial hyperglycemia by restoring a rapid postprandial insulin response; consider it when reduction of postprandial glycemic excursions is required.
When injected twice daily (before morning and evening meals), exenatide reduces hyperglycemia and promotes satiety, which in turn reduces caloric intake and body weight. In a recent study, exenatide achieved reductions in A1c (1.11%) similar to those observed with insulin glargine when it was added to sulfonylurea/metformin combination therapy for patients with type 2 diabetes.16 Exenatide reduced fasting plasma glucose (FPG) to a greater degree, whereas insulin glargine had a greater effect on FPG. This suggests that a basal insulin may be more appropriate when FPG levels are elevated (ie, patients with A1c levels >8.0%) and exenatide may be more useful for patients with A1c levels <8.0%, when elevated PPG levels are predominant.17 However, some patients with A1c levels >8.0% also may benefit from such intervention.
Liraglutide, another incretin mimetic, is in development for the treatment of type 2 diabetes. Liraglutide is a long-acting GLP-1 analog in phase 3 development for once-daily treatment of type 2 diabetes. The mechanism of action is similar to that of exenatide, but with a longer duration of action. Liraglutide may be suitable for once-daily administration. Initial data indicate that liraglutide improved glycemic control while providing modest weight loss for patients with type 2 diabetes.18 (Available online at: www.novonordisk.com/science/pipeline/rd_pipeline.asp. Accessed August 2, 2007.)
DPP-IV inhibitors
A new class of oral antidiabetic agents, DPP-IV inhibitors slow the degradation of incretin hormones, allowing these hormones to stimulate insulin secretion and decrease glucagon levels in the circulation in a glucose-dependent manner.19,20
Sitagliptin (Januvia) has been approved for use as monotherapy or in combination with metformin, pioglitazone, or rosiglitazone for the treatment of type 2 diabetes. Once-daily sitagliptin improves glycemic control, reducing A1c from 0.4% to 1.1% and decreasing fasting plasma glucose from 12 to 17 mg/dL and 2-hour postprandial glucose from 49 to 62 mg/dL in clinical trials. It is well-tolerated,21,22 but its long-term efficacy and safety are unknown.
Vildagliptin (Galvus), another DPP-IV inhibitor has completed phase 3 testing and is pending FDA approval at this time. (Available online at: www.diabeteshealth.com/read/2007/03/16/5039.html. Accessed August 2, 2007.)
Recent data indicate that a simple treatment algorithm based on preprandial glucose patterns can be as effective as carbohydrate counting for the dose titration of prandial insulin.30 In this 24-week study, insulin glulisine was added to basal-prandial insulin therapy, with insulin glargine as the basal insulin component. Glulisine was adjusted to target using either a simple algorithm of adding 1, 2, or 3 U based on premeal glucose patterns or standard carbohydrate counting. The carbohydrate counting–based dose adjustment and the algorithm-based titration treatment arms achieved similar A1c reductions. However, patients using the simple algorithm experienced significantly less symptomatic hypoglycemia (P=.02).
- Initiate basal insulin with a 10 U once-daily dose of insulin glargine, insulin detemir, or NPH insulin.24 (Note: NPH insulin and insulin detemir may require twice-daily dosing.)25
- Titrate weekly to a target fasting plasma glucose [FPG] of ≤100 mg/dL based on the average self-monitored FPG values from the preceding 2 days as follows:24
- If FPG is ≥180 mg/dL, increase insulin dosage by 8 U/d.
- If FPG is 140–180 mg/dL, increase insulin dosage by 6 U/d.
- If FPG is 120–140 mg/dL, increase insulin dosage by 4 U/d.
- If FPG is 100–120 mg/dL, increase insulin dosage by 2 U/d.
- If FPG is <72 mg/dL at any time during the week, do not increase insulin dosage.
- If FPG is <56 mg/dL, decrease insulin dosage by 2–4 U/d.
Keep in mind…
- A similar titration schedule to the one described here was effective in a study with insulin detemir and NPH insulin.26
- An alternative titration strategy to the one here would be to increase basal insulin dose by 2 U every 3 days to reach an FPG level of ≤100 mg/dL.27,28
- Less stringent A1c goals may be appropriate for patients with limited life expectancies, very young children, the elderly, and individuals with comorbid conditions.14
Rapid-acting analogs allow more flexible administration
Prior to the development of rapid-acting insulin analogs, regular human insulin (RHI) was the only available insulin suitable for prandial glycemic control. However, it had significant limitations, including the need for it to be injected 30 to 45 minutes before eating (and the poor compliance with this requirement), variability in peak levels (between patients and with the same patient), variability in absorption based on injection site, and frequent episodes of hypoglycemia.31,32
Newer rapid-acting insulin analogs such as insulin aspart, insulin glulisine, and insulin lispro demonstrate improved pharmacokinetic profiles with more rapid onset, faster time to peak activity, and shorter duration of action than RHI.32,33 These rapid-acting analogs allow administration right before or right after a meal, resulting in improved glycemic control without increased hypoglycemia or weight gain.34,35 Whereas the rapid onset of action of these analogs allows for administration 5 to 15 minutes before a meal, the patient can administer insulin glulisine within 20 minutes of the start of the meal.36 The addition of just 1 dose of prandial insulin to existing basal insulin plus oral antidiabetic drug therapy offers patients a substantial benefit.37
A new option: inhaled insulin
The US Food and Drug Administration recently approved an inhaled prandial insulin. Research has shown that it effectively addresses postprandial glucose excursions for patients with type 2 diabetes.38,39 A 12-week trial comparing A1c levels among patients switched to inhaled insulin (Exubera) before meals (n=76) or rosiglitazone (Avandia) 4 mg twice daily (n=69) found that inhaled insulin reduced A1c to a greater degree than rosiglitazone (–2.3% vs –1.4%); however, patients receiving inhaled insulin experienced a greater incidence of hypoglycemia (0.7 vs 0.05 episodes per subject-month).39
Inhaled insulin can be used as monotherapy or in conjunction with oral agents or a long-acting basal insulin. Inhaled insulin has a rapid onset of action (within 10–20 minutes, comparable with rapid-acting insulin analogs) and a duration of glucose-lowering activity of approximately 6 hours (comparable with RHI).40 This is useful for patients reluctant to begin insulin therapy because of injections; however, you will need to closely monitor hypoglycemia.
TABLE
How to use sensitivity factors to calculate 24-hour insulin need
| Characteristic | Dosage (U/kg) |
|---|---|
| Phenotype | |
| Normal weight | |
| Extremely physically active | 0.3 baseline |
| Moderately physically active | 0.4 baseline |
| Minimally active | 0.5 baseline |
| Obese | |
| Extremely physically active | 0.5 baseline |
| Moderately physically active | 0.6 baseline |
| Minimally active | 0.8 baseline |
| Renal failure | Subtract 0.2 |
| Coexisting illness raising risk of hypoglycemia | Subtract 0.2 |
| Eating habits (“big eater”) | Add 0.1 |
| New-onset type 1 diabetes, <30 years of age | 0.3 baseline |
| Reprinted with permission from Leahy, Insulin Therapy 2002.29 | |
Basal-prandial insulin in new type 2 diabetes
In certain cases, it may be more appropriate to initiate insulin therapy using a basal-prandial regimen that includes injections of prandial insulin with each meal of the day. Such cases include patients with newly diagnosed type 2 diabetes who have A1c levels >10.0%, or insulin-naive patients on oral antidiabetic drug regimens who have A1c levels >8.5%.25
You can calculate the starting total 24-hour insulin dosage for both the basal and prandial insulin components by multiplying body weight in kg by a factor based on the patient’s estimated insulin sensitivity ( TABLE ).29
Once you have this 24-hour insulin dose, you’ll then need to calculate the dose of basal insulin, which is 50% of the 24-hour total insulin dose, administered once daily. The remaining 50% of the total 24-hour dose provides prandial insulin coverage and is usually administered as follows:
- 30% to 40% at breakfast
- 30% at lunch
- 30% to 40% at dinner
Patients will need to adjust prandial insulin doses based on self-monitored blood glucose values.
Premixed insulin formulations
You should have your patients administer basal-prandial insulin as separate injections (eg, insulin glargine and insulin glulisine,30 or insulin detemir and insulin aspart25 ). The premixed (NPH based) formulations provide fixed doses of an intermediate-or long-acting insulin combined with a short-acting insulin. Although this method may be convenient to administer, it is more rigid and may not account for mealtimes and exercise. As a result, insulin levels will not match physiological insulin and thus, the risk for hypoglycemia increases. Another disadvantage is that adjustments to the dose based on self-monitored glucose levels are not possible with pre-mixed formulations.41
Separating the basal and prandial insulin components allows the insulin regimen to be adapted to an individual’s needs, thereby providing glycemic control with less propensity for hypoglycemia.
Acknowledgments
This article was supported by Sanofi-Aventis US. While the author is responsible for all content, he gratefully acknowledges the embryon scientific staff, who assisted in the preparation of a first draft of this article based on an author-approved outline, and also assisted in implementing author revisions.
Correspondence
George E. Dailey, MD, Senior Consultant, Division of Diabetes and Endocrinology, Head, Diabetes Research, Scripps Clinic, 10666 N. Torrey Pines Road, La Jolla, CA 92037; [email protected]
1. Bonora E, Corrao G, Bagnardi V, et al. Prevalence and correlates of post-prandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitus. Diabetologia 2006;49:846-854.
2. Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999;22:920-924.
3. The DECODE study group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet 1999;354:617-621.
4. Decode Study Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 2003;26:688-696.
5. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia 2001;44:2107-2114.
6. Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care 2002;25:275-278.
7. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care 2003;26:881-885.
8. Stratton IM, Adler AI, Neil HAW, et al. on behalf of the UK Prospective Diabetes Study Group. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-412.
9. ACE/AACE Diabetes Road Map Task Force. American Association of Clinical Endocrinologists Website. Road map for the prevention and treatment of type 2 diabetes. Available at: www.aace.com/meetings/consensus/odimplementation/roadmap.pdf. Accessed August 6, 2007.
10. Riddle MC, Rosenstock J. Oral monotherapy and combination therapy. In: Cefalu WT, Gerich JE, Le-Roith D, eds. The CADRE Handbook of Diabetes Management. New York, NY: Medical Information Press;2004:127-144.
11. Sheehan MT. Current therapeutic options in type 2 diabetes mellitus: a practical approach. Clin Med Res 2003;1:189-200.
12. Giorgino F, Laviola L, Leonardini A. Pathophysiology of type 2 diabetes: rationale for different oral antidiabetic treatment strategies. Diabetes Res Clin Pract 2005;68(suppl 1):S22-S29.
13. Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care 2006;29:1963-1972.
14. American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care 2007;30(suppl 1):S4-S41.
15. Lam S, See S. Exenatide: a novel incretin mimetic agent for treating type 2 diabetes mellitus. Cardiol Rev 2006;14:205-211.
16. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: A randomized trial. Ann Intern Med 2005;143:559-569.
17. Kennedy L. Exenatide versus glargine—complementary therapies rather than competing [electronic letter]. Ann Intern Med 2005; 143. Available at: www.annals.org/cgi/eletters/143/8/559#2404. Accessed August 3, 2007.
18. Feinglos MN, Saad MF, Pi-Sunyer FX, An B, Santiago O. on behalf of the Liraglutide Dose-Response Study Group. Effects of liraglutide (NN2211), a long-acting GLP-1 analogue, on glycaemic control and bodyweight in subjects with Type 2 diabetes. Diabetes Med 2005;22:1016-1023.
19. Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006;29:2632-2637.
20. Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006;49:2564-2571.
21. Miller SA, St Onge EL. Sitagliptin: a dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Ann Pharmacother 2006;40:1336-1343.
22. Herman GA, Bergman A, Liu F, et al. Pharmacokinetics and pharmacodynamic effects of the oral DPP-4 inhibitor sitagliptin in middle-aged obese subjects. J Clin Pharmacol 2006;46:876-886.
23. Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR. for the UK Prospective Diabetes Study Group. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57). Diabetes Care 2002;25:330-336.
24. Riddle MC, Rosenstock J, Gerich J. on behalf of the Insulin Glargine 4002 Study Investigators. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080-3086.
25. Raslová K, Bogoev M, Raz I, Leth G, Gall MA, Hâncu N. Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. Diabetes Res Clin Pract 2004;66:193-201.
26. Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. on behalf of the Levemir Treat-to-Target Study. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naïve people with type 2 diabetes. Diabetes Care 2006;29:1269-1274.
27. Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R, Lantus Study Group. AT.LANTUS trial investigating treatment algorithms for insulin glargine (LANTUS): results of the Type 2 Study [abstract]. Diabetes 2004;53(suppl 2):A473.-Abstract 1980 PO.
28. Ryysy L, Yki-Jarvinen H, Hänninen J. Simplifying treat to target—the LANMET study. In: Program and abstracts of the 40th European Association for the Study of Diabetes Annual Meeting. 2004;No. 749. PS 064.
29. Leahy JL. Intensive insulin therapy in type 1 diabetes mellitus. In: Leahy, JL, Cefalu WT, eds. Insulin Therapy New York, NY: Marcel Dekker;2002:87-112.
30. Bergenstal R, Johnson ML, Powers MA, Wynne AG, Vlajnic A, Hollander PA. Using a simple algorithm (ALG) to adjust mealtime glulisine (GLU) based on pre-prandial glucose patterns is a safe and effective alternative to carbohydrate counting (Carb Count). Diabetes 2006;55(suppl 1):A105.-
31. Overmann H, Heinemann L. Injection-meal interval: recommendations of diabetologists and how patients handle it. Diabetes Res Clin Pract 1999;43:137-142.
32. Wittlin SD, Woehrle HJ, Gerich JE. Insulin pharmacokinetics. In: Leahy JL, Cefalu WT, eds. Insulin Therapy New York, NY: Marcel Dekker;2002:73-85.
33. Becker RHA, Frick AD, Burger F, Potgieter JH, Scholtz H. Insulin glulisine, a new rapid-acting insulin analogue, displays a rapid time-action profile in obese non-diabetic subjects. Exp Clin Endocrinol Diabetes 2005;13:435-443.
34. Chase HP, Lockspeiser T, Peery B, et al. The impact of the diabetes control and complications trial and humalog insulin on glycohemoglobin levels and severe hypoglycemia in type 1 diabetes. Diabetes Care 2001;24:430-434.
35. Dailey G, Rosenstock J, Moses RG, Ways K. Insulin glulisine provides improved glycemic control in patients with type 2 diabetes. Diabetes Care 2004;27:2363-2368.
36. Apidra [package insert] Kansas City, Mo: Aventis Pharmaceuticals Inc;2004.
37. Lankisch M, Stahr B, Alawi H, Ferlinz K, Scherbaum W. Basal insulin and oral antihyperglycemic therapy (BOT) plus a single dose of insulin glusine at breakfast or at the predominant meal lower HbA1c in patients with type 2 diabetes. Diabetes 2006;55(suppl 1).:Abstract 514-P.
38. Rosenstock J, Zinman B, Murphy LJ, et al. Inhaled insulin improves glycemic control when substituted for or added to oral combination therapy in type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2005;143:549-558.
39. DeFronzo RA, Bergenstal RM, Cefalu WT, et al. Efficacy of inhaled insulin in patients with type 2 diabetes not controlled with diet and exercise: a 12-week, randomized, comparative trial. Diabetes Care 2005;28:1922-1928.
40. Exubera [package insert] New York, NY: Pfizer Inc;2006.
41. Hirsch IB. Insulin analogues. N Engl J Med 2005;352:174-183.
- A stepwise approach to antidiabetic therapy allows for the treatment to change in response to disease progression. This usually means beginning with oral agents and adding insulin as required (B).
- Treatment strategies must address both fasting and prandial hyperglycemia because prandial hyperglycemia has been shown to be an independent risk factor for cardiovascular events and mortality (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
More than 80% of patients with type 2 diabetes—including more than a third of patients with good metabolic control—have excessive postprandial hyperglycemia.1 That’s unwelcome news for the 20 million Americans with type 2 diabetes, especially when you consider that post-prandial hyperglycemia is a strong independent risk factor for all-cause mortality and cardiovascular events.2-5
To help our type 2 diabetes patients gain ideal control, we need to do at least 2 things better:
- Measure and act on glycosylated hemoglobin (A1c) levels.
- Take a stepped approach to glycemic control, making full use of prandial insulin.
A1clevels and the important role they play
Analysis of A1c is the “gold standard” for monitoring glycemic control in patients with diabetes because it provides an indication of mean plasma glucose levels during the preceding 120 days.6 The relative contribution of fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) to A1c levels is a dynamic function of the extent of day-long hyperglycemia; FPG has a greater influence at higher A1c levels and PPG has a predominant role at lower A1c levels.7
The relationship between hyperglycemia, as measured by A1c, and increased morbidity and mortality (including cardiovascular events) was demonstrated several years ago in the United Kingdom Prospective Diabetes Study (UKPDS).8 Interestingly, several studies have also found that fasting glucose levels alone are not a reliable predictor for hyperglycemia-related morbidity or mortality, whereas postprandial hyperglycemia, as noted in the introduction, is a strong independent risk factor for all-cause mortality and cardiovascular events.2-5
Continued management of A1c through tight control of both FPG and PPG may therefore improve patient long-term health outcomes. A1c should be evaluated every 3 to 6 months, and appropriate changes to the patients’ treatment regimens should be made accordingly.
An algorithm for the stepwise approach
We typically use oral antidiabetic drugs typically are used as initial therapy for patients with newly diagnosed type 2 diabetes, especially those with initial A1c levels of 6.0% to 8.0%.9 Three recent publications10-12 provide an excellent analysis of the rationale for combination therapy to address multiple physiologic defects, as well as the relative efficacy of agents.
In 2006 the American Diabetes Association (ADA) and the European Association for the Study of Diabetes published a consensus statement that presented an algorithm for the initiation and adjustment of type 2 diabetes therapy (Figure).13 In this evidence-and experience-based treatment algorithm, the authors emphasize the achievement and maintenance of normal glycemic goals, initiating therapy with lifestyle intervention and metformin (Glucophage), not delaying therapy and transitioning to new regimens when glycemic targets are not achieved, and adding insulin therapy early to the regimens of patients who are not meeting glycemic targets.9,13
You may also consider newer therapeutic options not included in the ADA’s 2007 treatment guidelines.14 Incretin mimetics and dipeptidyl-peptidase IV (DPP-IV) inhibitors are 2 new classes of antidiabetic agents that are effective for patients with type 2 diabetes. (See “2 new classes of antidiabetic agents: Incretin mimetics and DPP-IV inhibitors” 15-22 ) Most patients with type 2 diabetes will, however, eventually require insulin therapy to maintain optimal glycemic control.23
Advancement to insulin therapy
Basal insulin replacement achieves glycemic control
The addition of once-daily basal insulin to oral antidiabetic drug regimens is a simple way to introduce insulin therapy and achieve glycemic control. (See “A guide to basal insulin dosing and titration”14,24-28 )
- In a randomized, parallel, multi-center study, treatment with once-daily insulin glargine (Lantus) or neutral protamine Hagedorn (NPH) insulin (Humulin, Novolin) added to preexisting oral antidiabetic drug regimens for 756 patients with inadequately controlled type 2 diabetes (A1c >7.5%) effectively achieved the goal A1c of ≤7.0% for most patients. More patients in the insulin glargine group were able to reach goal without experiencing any nocturnal hypoglycemia, compared with those in the NPH group (33.2% vs 26.7%, P<.05).24
- A similar study used a forced-titration algorithm to compare NPH insulin with insulin detemir (Levemir).26 This study demonstrated that a similar reduction in A1c could be achieved with insulin detemir and NPH insulin over 24 weeks (1.8% and 1.9%, P=ns), but weight gain and nocturnal hypoglycemia incidence were significantly lower with insulin detemir compared with NPH insulin (1.2 kg vs 2.8 kg, and 160 vs 349 events, respectively, P<.001 for both).
Prandial insulin is as effective as carbohydrate counting
If a patient doesn’t reach his or her A1c targets despite appropriate titration of the basal insulin dose, injections of a rapid-acting insulin analog at mealtime may be necessary. You can add rapid-acting insulin to the regimen, starting with 1 injection at the largest meal of the day and then adding an injection at additional meals as needed.
- A typical starting dose of rapid-acting prandial insulin (insulin aspart [NovoLog], insulin glulisine [Apidra], or insulin lispro [Humalog]) would be 5 to 10 U per meal.29
- The range of daily dose, when used at 3 meals per day, would be 0.2 to 0.5 U/kg per day (ie, 0.1 to 0.15 U/kg per meal).29 For a patient who weighs 100 kg, that would mean 20 U (6–7 units before each meal) per day.
FIGURE
An evidence-based algorithm for achieving normal glycemic goals in patients with type 2 diabetes
Note: Reinforce lifestyle intervention at every visit.
*Check A1c every 3 months until <7% and then at least every 6 months.
†At A1C >9%.
‡At A1C ≤8%.
**Although 3 oral agents can be used, initiation and intensification of insulin therapy is preferred based on effectiveness and expense.
Reprinted with permission from the American Diabetes Association.13
Incretin mimetics
Incretins, such as glucagonlike peptide-1 (GLP-1), enhance glucose-dependent insulin secretion by the pancreatic beta cells and exhibit other antihyperglycemic actions after release into circulation by the gut.
Exenatide (Byetta) is the first agent in this class to be approved by the Food and Drug Administration for use in type 2 diabetes. Exenatide may be used in combination with oral therapy (a sulfonylurea and/or metformin) by patients who have not achieved adequate glycemic control on oral therapy alone. Exenatide mimics the antihyperglycemic actions of GLP-1 but maintains a prolonged duration of action compared with endogenous GLP-1.15 This agent effectively addresses postprandial hyperglycemia by restoring a rapid postprandial insulin response; consider it when reduction of postprandial glycemic excursions is required.
When injected twice daily (before morning and evening meals), exenatide reduces hyperglycemia and promotes satiety, which in turn reduces caloric intake and body weight. In a recent study, exenatide achieved reductions in A1c (1.11%) similar to those observed with insulin glargine when it was added to sulfonylurea/metformin combination therapy for patients with type 2 diabetes.16 Exenatide reduced fasting plasma glucose (FPG) to a greater degree, whereas insulin glargine had a greater effect on FPG. This suggests that a basal insulin may be more appropriate when FPG levels are elevated (ie, patients with A1c levels >8.0%) and exenatide may be more useful for patients with A1c levels <8.0%, when elevated PPG levels are predominant.17 However, some patients with A1c levels >8.0% also may benefit from such intervention.
Liraglutide, another incretin mimetic, is in development for the treatment of type 2 diabetes. Liraglutide is a long-acting GLP-1 analog in phase 3 development for once-daily treatment of type 2 diabetes. The mechanism of action is similar to that of exenatide, but with a longer duration of action. Liraglutide may be suitable for once-daily administration. Initial data indicate that liraglutide improved glycemic control while providing modest weight loss for patients with type 2 diabetes.18 (Available online at: www.novonordisk.com/science/pipeline/rd_pipeline.asp. Accessed August 2, 2007.)
DPP-IV inhibitors
A new class of oral antidiabetic agents, DPP-IV inhibitors slow the degradation of incretin hormones, allowing these hormones to stimulate insulin secretion and decrease glucagon levels in the circulation in a glucose-dependent manner.19,20
Sitagliptin (Januvia) has been approved for use as monotherapy or in combination with metformin, pioglitazone, or rosiglitazone for the treatment of type 2 diabetes. Once-daily sitagliptin improves glycemic control, reducing A1c from 0.4% to 1.1% and decreasing fasting plasma glucose from 12 to 17 mg/dL and 2-hour postprandial glucose from 49 to 62 mg/dL in clinical trials. It is well-tolerated,21,22 but its long-term efficacy and safety are unknown.
Vildagliptin (Galvus), another DPP-IV inhibitor has completed phase 3 testing and is pending FDA approval at this time. (Available online at: www.diabeteshealth.com/read/2007/03/16/5039.html. Accessed August 2, 2007.)
Recent data indicate that a simple treatment algorithm based on preprandial glucose patterns can be as effective as carbohydrate counting for the dose titration of prandial insulin.30 In this 24-week study, insulin glulisine was added to basal-prandial insulin therapy, with insulin glargine as the basal insulin component. Glulisine was adjusted to target using either a simple algorithm of adding 1, 2, or 3 U based on premeal glucose patterns or standard carbohydrate counting. The carbohydrate counting–based dose adjustment and the algorithm-based titration treatment arms achieved similar A1c reductions. However, patients using the simple algorithm experienced significantly less symptomatic hypoglycemia (P=.02).
- Initiate basal insulin with a 10 U once-daily dose of insulin glargine, insulin detemir, or NPH insulin.24 (Note: NPH insulin and insulin detemir may require twice-daily dosing.)25
- Titrate weekly to a target fasting plasma glucose [FPG] of ≤100 mg/dL based on the average self-monitored FPG values from the preceding 2 days as follows:24
- If FPG is ≥180 mg/dL, increase insulin dosage by 8 U/d.
- If FPG is 140–180 mg/dL, increase insulin dosage by 6 U/d.
- If FPG is 120–140 mg/dL, increase insulin dosage by 4 U/d.
- If FPG is 100–120 mg/dL, increase insulin dosage by 2 U/d.
- If FPG is <72 mg/dL at any time during the week, do not increase insulin dosage.
- If FPG is <56 mg/dL, decrease insulin dosage by 2–4 U/d.
Keep in mind…
- A similar titration schedule to the one described here was effective in a study with insulin detemir and NPH insulin.26
- An alternative titration strategy to the one here would be to increase basal insulin dose by 2 U every 3 days to reach an FPG level of ≤100 mg/dL.27,28
- Less stringent A1c goals may be appropriate for patients with limited life expectancies, very young children, the elderly, and individuals with comorbid conditions.14
Rapid-acting analogs allow more flexible administration
Prior to the development of rapid-acting insulin analogs, regular human insulin (RHI) was the only available insulin suitable for prandial glycemic control. However, it had significant limitations, including the need for it to be injected 30 to 45 minutes before eating (and the poor compliance with this requirement), variability in peak levels (between patients and with the same patient), variability in absorption based on injection site, and frequent episodes of hypoglycemia.31,32
Newer rapid-acting insulin analogs such as insulin aspart, insulin glulisine, and insulin lispro demonstrate improved pharmacokinetic profiles with more rapid onset, faster time to peak activity, and shorter duration of action than RHI.32,33 These rapid-acting analogs allow administration right before or right after a meal, resulting in improved glycemic control without increased hypoglycemia or weight gain.34,35 Whereas the rapid onset of action of these analogs allows for administration 5 to 15 minutes before a meal, the patient can administer insulin glulisine within 20 minutes of the start of the meal.36 The addition of just 1 dose of prandial insulin to existing basal insulin plus oral antidiabetic drug therapy offers patients a substantial benefit.37
A new option: inhaled insulin
The US Food and Drug Administration recently approved an inhaled prandial insulin. Research has shown that it effectively addresses postprandial glucose excursions for patients with type 2 diabetes.38,39 A 12-week trial comparing A1c levels among patients switched to inhaled insulin (Exubera) before meals (n=76) or rosiglitazone (Avandia) 4 mg twice daily (n=69) found that inhaled insulin reduced A1c to a greater degree than rosiglitazone (–2.3% vs –1.4%); however, patients receiving inhaled insulin experienced a greater incidence of hypoglycemia (0.7 vs 0.05 episodes per subject-month).39
Inhaled insulin can be used as monotherapy or in conjunction with oral agents or a long-acting basal insulin. Inhaled insulin has a rapid onset of action (within 10–20 minutes, comparable with rapid-acting insulin analogs) and a duration of glucose-lowering activity of approximately 6 hours (comparable with RHI).40 This is useful for patients reluctant to begin insulin therapy because of injections; however, you will need to closely monitor hypoglycemia.
TABLE
How to use sensitivity factors to calculate 24-hour insulin need
| Characteristic | Dosage (U/kg) |
|---|---|
| Phenotype | |
| Normal weight | |
| Extremely physically active | 0.3 baseline |
| Moderately physically active | 0.4 baseline |
| Minimally active | 0.5 baseline |
| Obese | |
| Extremely physically active | 0.5 baseline |
| Moderately physically active | 0.6 baseline |
| Minimally active | 0.8 baseline |
| Renal failure | Subtract 0.2 |
| Coexisting illness raising risk of hypoglycemia | Subtract 0.2 |
| Eating habits (“big eater”) | Add 0.1 |
| New-onset type 1 diabetes, <30 years of age | 0.3 baseline |
| Reprinted with permission from Leahy, Insulin Therapy 2002.29 | |
Basal-prandial insulin in new type 2 diabetes
In certain cases, it may be more appropriate to initiate insulin therapy using a basal-prandial regimen that includes injections of prandial insulin with each meal of the day. Such cases include patients with newly diagnosed type 2 diabetes who have A1c levels >10.0%, or insulin-naive patients on oral antidiabetic drug regimens who have A1c levels >8.5%.25
You can calculate the starting total 24-hour insulin dosage for both the basal and prandial insulin components by multiplying body weight in kg by a factor based on the patient’s estimated insulin sensitivity ( TABLE ).29
Once you have this 24-hour insulin dose, you’ll then need to calculate the dose of basal insulin, which is 50% of the 24-hour total insulin dose, administered once daily. The remaining 50% of the total 24-hour dose provides prandial insulin coverage and is usually administered as follows:
- 30% to 40% at breakfast
- 30% at lunch
- 30% to 40% at dinner
Patients will need to adjust prandial insulin doses based on self-monitored blood glucose values.
Premixed insulin formulations
You should have your patients administer basal-prandial insulin as separate injections (eg, insulin glargine and insulin glulisine,30 or insulin detemir and insulin aspart25 ). The premixed (NPH based) formulations provide fixed doses of an intermediate-or long-acting insulin combined with a short-acting insulin. Although this method may be convenient to administer, it is more rigid and may not account for mealtimes and exercise. As a result, insulin levels will not match physiological insulin and thus, the risk for hypoglycemia increases. Another disadvantage is that adjustments to the dose based on self-monitored glucose levels are not possible with pre-mixed formulations.41
Separating the basal and prandial insulin components allows the insulin regimen to be adapted to an individual’s needs, thereby providing glycemic control with less propensity for hypoglycemia.
Acknowledgments
This article was supported by Sanofi-Aventis US. While the author is responsible for all content, he gratefully acknowledges the embryon scientific staff, who assisted in the preparation of a first draft of this article based on an author-approved outline, and also assisted in implementing author revisions.
Correspondence
George E. Dailey, MD, Senior Consultant, Division of Diabetes and Endocrinology, Head, Diabetes Research, Scripps Clinic, 10666 N. Torrey Pines Road, La Jolla, CA 92037; [email protected]
- A stepwise approach to antidiabetic therapy allows for the treatment to change in response to disease progression. This usually means beginning with oral agents and adding insulin as required (B).
- Treatment strategies must address both fasting and prandial hyperglycemia because prandial hyperglycemia has been shown to be an independent risk factor for cardiovascular events and mortality (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
More than 80% of patients with type 2 diabetes—including more than a third of patients with good metabolic control—have excessive postprandial hyperglycemia.1 That’s unwelcome news for the 20 million Americans with type 2 diabetes, especially when you consider that post-prandial hyperglycemia is a strong independent risk factor for all-cause mortality and cardiovascular events.2-5
To help our type 2 diabetes patients gain ideal control, we need to do at least 2 things better:
- Measure and act on glycosylated hemoglobin (A1c) levels.
- Take a stepped approach to glycemic control, making full use of prandial insulin.
A1clevels and the important role they play
Analysis of A1c is the “gold standard” for monitoring glycemic control in patients with diabetes because it provides an indication of mean plasma glucose levels during the preceding 120 days.6 The relative contribution of fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) to A1c levels is a dynamic function of the extent of day-long hyperglycemia; FPG has a greater influence at higher A1c levels and PPG has a predominant role at lower A1c levels.7
The relationship between hyperglycemia, as measured by A1c, and increased morbidity and mortality (including cardiovascular events) was demonstrated several years ago in the United Kingdom Prospective Diabetes Study (UKPDS).8 Interestingly, several studies have also found that fasting glucose levels alone are not a reliable predictor for hyperglycemia-related morbidity or mortality, whereas postprandial hyperglycemia, as noted in the introduction, is a strong independent risk factor for all-cause mortality and cardiovascular events.2-5
Continued management of A1c through tight control of both FPG and PPG may therefore improve patient long-term health outcomes. A1c should be evaluated every 3 to 6 months, and appropriate changes to the patients’ treatment regimens should be made accordingly.
An algorithm for the stepwise approach
We typically use oral antidiabetic drugs typically are used as initial therapy for patients with newly diagnosed type 2 diabetes, especially those with initial A1c levels of 6.0% to 8.0%.9 Three recent publications10-12 provide an excellent analysis of the rationale for combination therapy to address multiple physiologic defects, as well as the relative efficacy of agents.
In 2006 the American Diabetes Association (ADA) and the European Association for the Study of Diabetes published a consensus statement that presented an algorithm for the initiation and adjustment of type 2 diabetes therapy (Figure).13 In this evidence-and experience-based treatment algorithm, the authors emphasize the achievement and maintenance of normal glycemic goals, initiating therapy with lifestyle intervention and metformin (Glucophage), not delaying therapy and transitioning to new regimens when glycemic targets are not achieved, and adding insulin therapy early to the regimens of patients who are not meeting glycemic targets.9,13
You may also consider newer therapeutic options not included in the ADA’s 2007 treatment guidelines.14 Incretin mimetics and dipeptidyl-peptidase IV (DPP-IV) inhibitors are 2 new classes of antidiabetic agents that are effective for patients with type 2 diabetes. (See “2 new classes of antidiabetic agents: Incretin mimetics and DPP-IV inhibitors” 15-22 ) Most patients with type 2 diabetes will, however, eventually require insulin therapy to maintain optimal glycemic control.23
Advancement to insulin therapy
Basal insulin replacement achieves glycemic control
The addition of once-daily basal insulin to oral antidiabetic drug regimens is a simple way to introduce insulin therapy and achieve glycemic control. (See “A guide to basal insulin dosing and titration”14,24-28 )
- In a randomized, parallel, multi-center study, treatment with once-daily insulin glargine (Lantus) or neutral protamine Hagedorn (NPH) insulin (Humulin, Novolin) added to preexisting oral antidiabetic drug regimens for 756 patients with inadequately controlled type 2 diabetes (A1c >7.5%) effectively achieved the goal A1c of ≤7.0% for most patients. More patients in the insulin glargine group were able to reach goal without experiencing any nocturnal hypoglycemia, compared with those in the NPH group (33.2% vs 26.7%, P<.05).24
- A similar study used a forced-titration algorithm to compare NPH insulin with insulin detemir (Levemir).26 This study demonstrated that a similar reduction in A1c could be achieved with insulin detemir and NPH insulin over 24 weeks (1.8% and 1.9%, P=ns), but weight gain and nocturnal hypoglycemia incidence were significantly lower with insulin detemir compared with NPH insulin (1.2 kg vs 2.8 kg, and 160 vs 349 events, respectively, P<.001 for both).
Prandial insulin is as effective as carbohydrate counting
If a patient doesn’t reach his or her A1c targets despite appropriate titration of the basal insulin dose, injections of a rapid-acting insulin analog at mealtime may be necessary. You can add rapid-acting insulin to the regimen, starting with 1 injection at the largest meal of the day and then adding an injection at additional meals as needed.
- A typical starting dose of rapid-acting prandial insulin (insulin aspart [NovoLog], insulin glulisine [Apidra], or insulin lispro [Humalog]) would be 5 to 10 U per meal.29
- The range of daily dose, when used at 3 meals per day, would be 0.2 to 0.5 U/kg per day (ie, 0.1 to 0.15 U/kg per meal).29 For a patient who weighs 100 kg, that would mean 20 U (6–7 units before each meal) per day.
FIGURE
An evidence-based algorithm for achieving normal glycemic goals in patients with type 2 diabetes
Note: Reinforce lifestyle intervention at every visit.
*Check A1c every 3 months until <7% and then at least every 6 months.
†At A1C >9%.
‡At A1C ≤8%.
**Although 3 oral agents can be used, initiation and intensification of insulin therapy is preferred based on effectiveness and expense.
Reprinted with permission from the American Diabetes Association.13
Incretin mimetics
Incretins, such as glucagonlike peptide-1 (GLP-1), enhance glucose-dependent insulin secretion by the pancreatic beta cells and exhibit other antihyperglycemic actions after release into circulation by the gut.
Exenatide (Byetta) is the first agent in this class to be approved by the Food and Drug Administration for use in type 2 diabetes. Exenatide may be used in combination with oral therapy (a sulfonylurea and/or metformin) by patients who have not achieved adequate glycemic control on oral therapy alone. Exenatide mimics the antihyperglycemic actions of GLP-1 but maintains a prolonged duration of action compared with endogenous GLP-1.15 This agent effectively addresses postprandial hyperglycemia by restoring a rapid postprandial insulin response; consider it when reduction of postprandial glycemic excursions is required.
When injected twice daily (before morning and evening meals), exenatide reduces hyperglycemia and promotes satiety, which in turn reduces caloric intake and body weight. In a recent study, exenatide achieved reductions in A1c (1.11%) similar to those observed with insulin glargine when it was added to sulfonylurea/metformin combination therapy for patients with type 2 diabetes.16 Exenatide reduced fasting plasma glucose (FPG) to a greater degree, whereas insulin glargine had a greater effect on FPG. This suggests that a basal insulin may be more appropriate when FPG levels are elevated (ie, patients with A1c levels >8.0%) and exenatide may be more useful for patients with A1c levels <8.0%, when elevated PPG levels are predominant.17 However, some patients with A1c levels >8.0% also may benefit from such intervention.
Liraglutide, another incretin mimetic, is in development for the treatment of type 2 diabetes. Liraglutide is a long-acting GLP-1 analog in phase 3 development for once-daily treatment of type 2 diabetes. The mechanism of action is similar to that of exenatide, but with a longer duration of action. Liraglutide may be suitable for once-daily administration. Initial data indicate that liraglutide improved glycemic control while providing modest weight loss for patients with type 2 diabetes.18 (Available online at: www.novonordisk.com/science/pipeline/rd_pipeline.asp. Accessed August 2, 2007.)
DPP-IV inhibitors
A new class of oral antidiabetic agents, DPP-IV inhibitors slow the degradation of incretin hormones, allowing these hormones to stimulate insulin secretion and decrease glucagon levels in the circulation in a glucose-dependent manner.19,20
Sitagliptin (Januvia) has been approved for use as monotherapy or in combination with metformin, pioglitazone, or rosiglitazone for the treatment of type 2 diabetes. Once-daily sitagliptin improves glycemic control, reducing A1c from 0.4% to 1.1% and decreasing fasting plasma glucose from 12 to 17 mg/dL and 2-hour postprandial glucose from 49 to 62 mg/dL in clinical trials. It is well-tolerated,21,22 but its long-term efficacy and safety are unknown.
Vildagliptin (Galvus), another DPP-IV inhibitor has completed phase 3 testing and is pending FDA approval at this time. (Available online at: www.diabeteshealth.com/read/2007/03/16/5039.html. Accessed August 2, 2007.)
Recent data indicate that a simple treatment algorithm based on preprandial glucose patterns can be as effective as carbohydrate counting for the dose titration of prandial insulin.30 In this 24-week study, insulin glulisine was added to basal-prandial insulin therapy, with insulin glargine as the basal insulin component. Glulisine was adjusted to target using either a simple algorithm of adding 1, 2, or 3 U based on premeal glucose patterns or standard carbohydrate counting. The carbohydrate counting–based dose adjustment and the algorithm-based titration treatment arms achieved similar A1c reductions. However, patients using the simple algorithm experienced significantly less symptomatic hypoglycemia (P=.02).
- Initiate basal insulin with a 10 U once-daily dose of insulin glargine, insulin detemir, or NPH insulin.24 (Note: NPH insulin and insulin detemir may require twice-daily dosing.)25
- Titrate weekly to a target fasting plasma glucose [FPG] of ≤100 mg/dL based on the average self-monitored FPG values from the preceding 2 days as follows:24
- If FPG is ≥180 mg/dL, increase insulin dosage by 8 U/d.
- If FPG is 140–180 mg/dL, increase insulin dosage by 6 U/d.
- If FPG is 120–140 mg/dL, increase insulin dosage by 4 U/d.
- If FPG is 100–120 mg/dL, increase insulin dosage by 2 U/d.
- If FPG is <72 mg/dL at any time during the week, do not increase insulin dosage.
- If FPG is <56 mg/dL, decrease insulin dosage by 2–4 U/d.
Keep in mind…
- A similar titration schedule to the one described here was effective in a study with insulin detemir and NPH insulin.26
- An alternative titration strategy to the one here would be to increase basal insulin dose by 2 U every 3 days to reach an FPG level of ≤100 mg/dL.27,28
- Less stringent A1c goals may be appropriate for patients with limited life expectancies, very young children, the elderly, and individuals with comorbid conditions.14
Rapid-acting analogs allow more flexible administration
Prior to the development of rapid-acting insulin analogs, regular human insulin (RHI) was the only available insulin suitable for prandial glycemic control. However, it had significant limitations, including the need for it to be injected 30 to 45 minutes before eating (and the poor compliance with this requirement), variability in peak levels (between patients and with the same patient), variability in absorption based on injection site, and frequent episodes of hypoglycemia.31,32
Newer rapid-acting insulin analogs such as insulin aspart, insulin glulisine, and insulin lispro demonstrate improved pharmacokinetic profiles with more rapid onset, faster time to peak activity, and shorter duration of action than RHI.32,33 These rapid-acting analogs allow administration right before or right after a meal, resulting in improved glycemic control without increased hypoglycemia or weight gain.34,35 Whereas the rapid onset of action of these analogs allows for administration 5 to 15 minutes before a meal, the patient can administer insulin glulisine within 20 minutes of the start of the meal.36 The addition of just 1 dose of prandial insulin to existing basal insulin plus oral antidiabetic drug therapy offers patients a substantial benefit.37
A new option: inhaled insulin
The US Food and Drug Administration recently approved an inhaled prandial insulin. Research has shown that it effectively addresses postprandial glucose excursions for patients with type 2 diabetes.38,39 A 12-week trial comparing A1c levels among patients switched to inhaled insulin (Exubera) before meals (n=76) or rosiglitazone (Avandia) 4 mg twice daily (n=69) found that inhaled insulin reduced A1c to a greater degree than rosiglitazone (–2.3% vs –1.4%); however, patients receiving inhaled insulin experienced a greater incidence of hypoglycemia (0.7 vs 0.05 episodes per subject-month).39
Inhaled insulin can be used as monotherapy or in conjunction with oral agents or a long-acting basal insulin. Inhaled insulin has a rapid onset of action (within 10–20 minutes, comparable with rapid-acting insulin analogs) and a duration of glucose-lowering activity of approximately 6 hours (comparable with RHI).40 This is useful for patients reluctant to begin insulin therapy because of injections; however, you will need to closely monitor hypoglycemia.
TABLE
How to use sensitivity factors to calculate 24-hour insulin need
| Characteristic | Dosage (U/kg) |
|---|---|
| Phenotype | |
| Normal weight | |
| Extremely physically active | 0.3 baseline |
| Moderately physically active | 0.4 baseline |
| Minimally active | 0.5 baseline |
| Obese | |
| Extremely physically active | 0.5 baseline |
| Moderately physically active | 0.6 baseline |
| Minimally active | 0.8 baseline |
| Renal failure | Subtract 0.2 |
| Coexisting illness raising risk of hypoglycemia | Subtract 0.2 |
| Eating habits (“big eater”) | Add 0.1 |
| New-onset type 1 diabetes, <30 years of age | 0.3 baseline |
| Reprinted with permission from Leahy, Insulin Therapy 2002.29 | |
Basal-prandial insulin in new type 2 diabetes
In certain cases, it may be more appropriate to initiate insulin therapy using a basal-prandial regimen that includes injections of prandial insulin with each meal of the day. Such cases include patients with newly diagnosed type 2 diabetes who have A1c levels >10.0%, or insulin-naive patients on oral antidiabetic drug regimens who have A1c levels >8.5%.25
You can calculate the starting total 24-hour insulin dosage for both the basal and prandial insulin components by multiplying body weight in kg by a factor based on the patient’s estimated insulin sensitivity ( TABLE ).29
Once you have this 24-hour insulin dose, you’ll then need to calculate the dose of basal insulin, which is 50% of the 24-hour total insulin dose, administered once daily. The remaining 50% of the total 24-hour dose provides prandial insulin coverage and is usually administered as follows:
- 30% to 40% at breakfast
- 30% at lunch
- 30% to 40% at dinner
Patients will need to adjust prandial insulin doses based on self-monitored blood glucose values.
Premixed insulin formulations
You should have your patients administer basal-prandial insulin as separate injections (eg, insulin glargine and insulin glulisine,30 or insulin detemir and insulin aspart25 ). The premixed (NPH based) formulations provide fixed doses of an intermediate-or long-acting insulin combined with a short-acting insulin. Although this method may be convenient to administer, it is more rigid and may not account for mealtimes and exercise. As a result, insulin levels will not match physiological insulin and thus, the risk for hypoglycemia increases. Another disadvantage is that adjustments to the dose based on self-monitored glucose levels are not possible with pre-mixed formulations.41
Separating the basal and prandial insulin components allows the insulin regimen to be adapted to an individual’s needs, thereby providing glycemic control with less propensity for hypoglycemia.
Acknowledgments
This article was supported by Sanofi-Aventis US. While the author is responsible for all content, he gratefully acknowledges the embryon scientific staff, who assisted in the preparation of a first draft of this article based on an author-approved outline, and also assisted in implementing author revisions.
Correspondence
George E. Dailey, MD, Senior Consultant, Division of Diabetes and Endocrinology, Head, Diabetes Research, Scripps Clinic, 10666 N. Torrey Pines Road, La Jolla, CA 92037; [email protected]
1. Bonora E, Corrao G, Bagnardi V, et al. Prevalence and correlates of post-prandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitus. Diabetologia 2006;49:846-854.
2. Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999;22:920-924.
3. The DECODE study group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet 1999;354:617-621.
4. Decode Study Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 2003;26:688-696.
5. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia 2001;44:2107-2114.
6. Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care 2002;25:275-278.
7. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care 2003;26:881-885.
8. Stratton IM, Adler AI, Neil HAW, et al. on behalf of the UK Prospective Diabetes Study Group. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-412.
9. ACE/AACE Diabetes Road Map Task Force. American Association of Clinical Endocrinologists Website. Road map for the prevention and treatment of type 2 diabetes. Available at: www.aace.com/meetings/consensus/odimplementation/roadmap.pdf. Accessed August 6, 2007.
10. Riddle MC, Rosenstock J. Oral monotherapy and combination therapy. In: Cefalu WT, Gerich JE, Le-Roith D, eds. The CADRE Handbook of Diabetes Management. New York, NY: Medical Information Press;2004:127-144.
11. Sheehan MT. Current therapeutic options in type 2 diabetes mellitus: a practical approach. Clin Med Res 2003;1:189-200.
12. Giorgino F, Laviola L, Leonardini A. Pathophysiology of type 2 diabetes: rationale for different oral antidiabetic treatment strategies. Diabetes Res Clin Pract 2005;68(suppl 1):S22-S29.
13. Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care 2006;29:1963-1972.
14. American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care 2007;30(suppl 1):S4-S41.
15. Lam S, See S. Exenatide: a novel incretin mimetic agent for treating type 2 diabetes mellitus. Cardiol Rev 2006;14:205-211.
16. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: A randomized trial. Ann Intern Med 2005;143:559-569.
17. Kennedy L. Exenatide versus glargine—complementary therapies rather than competing [electronic letter]. Ann Intern Med 2005; 143. Available at: www.annals.org/cgi/eletters/143/8/559#2404. Accessed August 3, 2007.
18. Feinglos MN, Saad MF, Pi-Sunyer FX, An B, Santiago O. on behalf of the Liraglutide Dose-Response Study Group. Effects of liraglutide (NN2211), a long-acting GLP-1 analogue, on glycaemic control and bodyweight in subjects with Type 2 diabetes. Diabetes Med 2005;22:1016-1023.
19. Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006;29:2632-2637.
20. Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006;49:2564-2571.
21. Miller SA, St Onge EL. Sitagliptin: a dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Ann Pharmacother 2006;40:1336-1343.
22. Herman GA, Bergman A, Liu F, et al. Pharmacokinetics and pharmacodynamic effects of the oral DPP-4 inhibitor sitagliptin in middle-aged obese subjects. J Clin Pharmacol 2006;46:876-886.
23. Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR. for the UK Prospective Diabetes Study Group. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57). Diabetes Care 2002;25:330-336.
24. Riddle MC, Rosenstock J, Gerich J. on behalf of the Insulin Glargine 4002 Study Investigators. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080-3086.
25. Raslová K, Bogoev M, Raz I, Leth G, Gall MA, Hâncu N. Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. Diabetes Res Clin Pract 2004;66:193-201.
26. Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. on behalf of the Levemir Treat-to-Target Study. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naïve people with type 2 diabetes. Diabetes Care 2006;29:1269-1274.
27. Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R, Lantus Study Group. AT.LANTUS trial investigating treatment algorithms for insulin glargine (LANTUS): results of the Type 2 Study [abstract]. Diabetes 2004;53(suppl 2):A473.-Abstract 1980 PO.
28. Ryysy L, Yki-Jarvinen H, Hänninen J. Simplifying treat to target—the LANMET study. In: Program and abstracts of the 40th European Association for the Study of Diabetes Annual Meeting. 2004;No. 749. PS 064.
29. Leahy JL. Intensive insulin therapy in type 1 diabetes mellitus. In: Leahy, JL, Cefalu WT, eds. Insulin Therapy New York, NY: Marcel Dekker;2002:87-112.
30. Bergenstal R, Johnson ML, Powers MA, Wynne AG, Vlajnic A, Hollander PA. Using a simple algorithm (ALG) to adjust mealtime glulisine (GLU) based on pre-prandial glucose patterns is a safe and effective alternative to carbohydrate counting (Carb Count). Diabetes 2006;55(suppl 1):A105.-
31. Overmann H, Heinemann L. Injection-meal interval: recommendations of diabetologists and how patients handle it. Diabetes Res Clin Pract 1999;43:137-142.
32. Wittlin SD, Woehrle HJ, Gerich JE. Insulin pharmacokinetics. In: Leahy JL, Cefalu WT, eds. Insulin Therapy New York, NY: Marcel Dekker;2002:73-85.
33. Becker RHA, Frick AD, Burger F, Potgieter JH, Scholtz H. Insulin glulisine, a new rapid-acting insulin analogue, displays a rapid time-action profile in obese non-diabetic subjects. Exp Clin Endocrinol Diabetes 2005;13:435-443.
34. Chase HP, Lockspeiser T, Peery B, et al. The impact of the diabetes control and complications trial and humalog insulin on glycohemoglobin levels and severe hypoglycemia in type 1 diabetes. Diabetes Care 2001;24:430-434.
35. Dailey G, Rosenstock J, Moses RG, Ways K. Insulin glulisine provides improved glycemic control in patients with type 2 diabetes. Diabetes Care 2004;27:2363-2368.
36. Apidra [package insert] Kansas City, Mo: Aventis Pharmaceuticals Inc;2004.
37. Lankisch M, Stahr B, Alawi H, Ferlinz K, Scherbaum W. Basal insulin and oral antihyperglycemic therapy (BOT) plus a single dose of insulin glusine at breakfast or at the predominant meal lower HbA1c in patients with type 2 diabetes. Diabetes 2006;55(suppl 1).:Abstract 514-P.
38. Rosenstock J, Zinman B, Murphy LJ, et al. Inhaled insulin improves glycemic control when substituted for or added to oral combination therapy in type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2005;143:549-558.
39. DeFronzo RA, Bergenstal RM, Cefalu WT, et al. Efficacy of inhaled insulin in patients with type 2 diabetes not controlled with diet and exercise: a 12-week, randomized, comparative trial. Diabetes Care 2005;28:1922-1928.
40. Exubera [package insert] New York, NY: Pfizer Inc;2006.
41. Hirsch IB. Insulin analogues. N Engl J Med 2005;352:174-183.
1. Bonora E, Corrao G, Bagnardi V, et al. Prevalence and correlates of post-prandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitus. Diabetologia 2006;49:846-854.
2. Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999;22:920-924.
3. The DECODE study group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet 1999;354:617-621.
4. Decode Study Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 2003;26:688-696.
5. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia 2001;44:2107-2114.
6. Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care 2002;25:275-278.
7. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care 2003;26:881-885.
8. Stratton IM, Adler AI, Neil HAW, et al. on behalf of the UK Prospective Diabetes Study Group. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-412.
9. ACE/AACE Diabetes Road Map Task Force. American Association of Clinical Endocrinologists Website. Road map for the prevention and treatment of type 2 diabetes. Available at: www.aace.com/meetings/consensus/odimplementation/roadmap.pdf. Accessed August 6, 2007.
10. Riddle MC, Rosenstock J. Oral monotherapy and combination therapy. In: Cefalu WT, Gerich JE, Le-Roith D, eds. The CADRE Handbook of Diabetes Management. New York, NY: Medical Information Press;2004:127-144.
11. Sheehan MT. Current therapeutic options in type 2 diabetes mellitus: a practical approach. Clin Med Res 2003;1:189-200.
12. Giorgino F, Laviola L, Leonardini A. Pathophysiology of type 2 diabetes: rationale for different oral antidiabetic treatment strategies. Diabetes Res Clin Pract 2005;68(suppl 1):S22-S29.
13. Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care 2006;29:1963-1972.
14. American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care 2007;30(suppl 1):S4-S41.
15. Lam S, See S. Exenatide: a novel incretin mimetic agent for treating type 2 diabetes mellitus. Cardiol Rev 2006;14:205-211.
16. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: A randomized trial. Ann Intern Med 2005;143:559-569.
17. Kennedy L. Exenatide versus glargine—complementary therapies rather than competing [electronic letter]. Ann Intern Med 2005; 143. Available at: www.annals.org/cgi/eletters/143/8/559#2404. Accessed August 3, 2007.
18. Feinglos MN, Saad MF, Pi-Sunyer FX, An B, Santiago O. on behalf of the Liraglutide Dose-Response Study Group. Effects of liraglutide (NN2211), a long-acting GLP-1 analogue, on glycaemic control and bodyweight in subjects with Type 2 diabetes. Diabetes Med 2005;22:1016-1023.
19. Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006;29:2632-2637.
20. Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006;49:2564-2571.
21. Miller SA, St Onge EL. Sitagliptin: a dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Ann Pharmacother 2006;40:1336-1343.
22. Herman GA, Bergman A, Liu F, et al. Pharmacokinetics and pharmacodynamic effects of the oral DPP-4 inhibitor sitagliptin in middle-aged obese subjects. J Clin Pharmacol 2006;46:876-886.
23. Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR. for the UK Prospective Diabetes Study Group. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57). Diabetes Care 2002;25:330-336.
24. Riddle MC, Rosenstock J, Gerich J. on behalf of the Insulin Glargine 4002 Study Investigators. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080-3086.
25. Raslová K, Bogoev M, Raz I, Leth G, Gall MA, Hâncu N. Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. Diabetes Res Clin Pract 2004;66:193-201.
26. Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. on behalf of the Levemir Treat-to-Target Study. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naïve people with type 2 diabetes. Diabetes Care 2006;29:1269-1274.
27. Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R, Lantus Study Group. AT.LANTUS trial investigating treatment algorithms for insulin glargine (LANTUS): results of the Type 2 Study [abstract]. Diabetes 2004;53(suppl 2):A473.-Abstract 1980 PO.
28. Ryysy L, Yki-Jarvinen H, Hänninen J. Simplifying treat to target—the LANMET study. In: Program and abstracts of the 40th European Association for the Study of Diabetes Annual Meeting. 2004;No. 749. PS 064.
29. Leahy JL. Intensive insulin therapy in type 1 diabetes mellitus. In: Leahy, JL, Cefalu WT, eds. Insulin Therapy New York, NY: Marcel Dekker;2002:87-112.
30. Bergenstal R, Johnson ML, Powers MA, Wynne AG, Vlajnic A, Hollander PA. Using a simple algorithm (ALG) to adjust mealtime glulisine (GLU) based on pre-prandial glucose patterns is a safe and effective alternative to carbohydrate counting (Carb Count). Diabetes 2006;55(suppl 1):A105.-
31. Overmann H, Heinemann L. Injection-meal interval: recommendations of diabetologists and how patients handle it. Diabetes Res Clin Pract 1999;43:137-142.
32. Wittlin SD, Woehrle HJ, Gerich JE. Insulin pharmacokinetics. In: Leahy JL, Cefalu WT, eds. Insulin Therapy New York, NY: Marcel Dekker;2002:73-85.
33. Becker RHA, Frick AD, Burger F, Potgieter JH, Scholtz H. Insulin glulisine, a new rapid-acting insulin analogue, displays a rapid time-action profile in obese non-diabetic subjects. Exp Clin Endocrinol Diabetes 2005;13:435-443.
34. Chase HP, Lockspeiser T, Peery B, et al. The impact of the diabetes control and complications trial and humalog insulin on glycohemoglobin levels and severe hypoglycemia in type 1 diabetes. Diabetes Care 2001;24:430-434.
35. Dailey G, Rosenstock J, Moses RG, Ways K. Insulin glulisine provides improved glycemic control in patients with type 2 diabetes. Diabetes Care 2004;27:2363-2368.
36. Apidra [package insert] Kansas City, Mo: Aventis Pharmaceuticals Inc;2004.
37. Lankisch M, Stahr B, Alawi H, Ferlinz K, Scherbaum W. Basal insulin and oral antihyperglycemic therapy (BOT) plus a single dose of insulin glusine at breakfast or at the predominant meal lower HbA1c in patients with type 2 diabetes. Diabetes 2006;55(suppl 1).:Abstract 514-P.
38. Rosenstock J, Zinman B, Murphy LJ, et al. Inhaled insulin improves glycemic control when substituted for or added to oral combination therapy in type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2005;143:549-558.
39. DeFronzo RA, Bergenstal RM, Cefalu WT, et al. Efficacy of inhaled insulin in patients with type 2 diabetes not controlled with diet and exercise: a 12-week, randomized, comparative trial. Diabetes Care 2005;28:1922-1928.
40. Exubera [package insert] New York, NY: Pfizer Inc;2006.
41. Hirsch IB. Insulin analogues. N Engl J Med 2005;352:174-183.