User login
The year 2007 was rather calm, compared to the 3 previous years in regards to new vaccines and vaccine recommendations. Although no breakthrough vaccine products came onto the market in 2007, there were new recommendations and licensure for new age groups for existing vaccines and a recall of some lots of Hib vaccines.
Meningococcal vaccine
Recommendations on the use of the quadrivalent meningococcal conjugate vaccine (MCV4) have evolved since its licensure in 2005 for use in persons 11 to 55 years of age. The first set of recommendations focused on universal vaccination of preteens, aged 11 to 12, those entering high school who had not received the vaccine previously, and others at risk for meningococcal disease including college freshmen living in dormitories.1 The MCV4 was preferred to the older polysaccharide vaccine (MPSV4) which was recommended only for children aged 2 to 10 and adults over age 55 at increased risk.
In 2007, the CDC changed 2 of the 2005 recommendations:
- The first, in August, simplified the recommendations for teens, making MCV4 universally recommended for all those aged 11 to 18 at the earliest opportunity.2
- The second, in December, followed FDA approval for use of MCV4 in children aged 2 to 10 years. The CDC now recommends MCV4 as the preferred vaccine in this age group for those at risk (TABLE 1).3
TABLE 1
Populations at increased risk for meningococcal disease who should receive quadrivalent meningococcal conjugate vaccine
|
If someone at ongoing risk for meningococcal disease has been previously vaccinated with MPSV4, they should be revaccinated 3 years later with MCV4. It is not known if repeat doses of MCV4 will be needed, and if so, after what amount of time.
The MCV4 has been linked to Guillain-Barré syndrome (GBS), and a history of GBS is a precaution for its use. For those with a history of GBS who need protection against meningococcal infection, MPSV4 is an alternative.
Hepatitis A vaccine
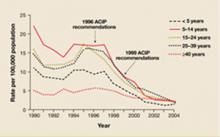
Widespread use of inactivated hepatitis A vaccine (HAV), first licensed in 1995, has markedly reduced the incidence of hepatitis A infection (FIGURE). Recommendations for its use have been periodically revised; current recommendations include universal vaccination of all children at age 12 to 23 months, catch-up vaccination in older children in areas of high prevalence, and vaccination of those at increased risk for hepatitis A including travelers to endemic areas, users of illicit drugs and men who have sex with men.4
FIGURE
Reduction in incidence of hepatitis A infection
Source: Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2006; 55(RR-07).
For those unvaccinated who are acutely exposed to hepatitis A virus and those traveling to areas of high prevalence who do not have time to complete the 2 doses of HAV, the only prevention available until recently has been IG. This has now changed and HAV can be used in both groups. The new recommendation for postexposure prophylaxis is that either a single dose of HAV or use of IG is acceptable.5 At ages 12 months to 40 years, vaccine is preferred. For those over age 40, IG is preferred but vaccine is acceptable. For children less than 12 months, the immune suppressed, and those with chronic liver disease, IG should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either HAV or IG. A single dose of HAV is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those under age 12 months, those who choose not to receive the vaccine, and those who are allergic to the vaccine should be offered IG. Both IG and HAV should be considered for individuals who plan to travel within 2 weeks of the first HAV dose; those over age 40, the immune compromised, and those with chronic liver disease or other chronic medical conditions.
Live attenuated influenza vaccine
FluMist, the live attenuated influenza vaccine (LAIV), which is administered as an intranasal spray, is now approved for use among those 2 to 4 years of age.6 Previously, the LAIV was approved only for healthy, non pregnant persons, 5 to 49 years of age. The LAIV may actually be the preferred product in children as it has been shown to prevent more influenza illness than the trivalent inactivated vaccine (TIV). The LAIV should not be used in anyone with a condition listed in TABLE 2 and should not be administered to children under age 5 who have recurrent wheezing.
FluMist has also been modified in several advantageous ways:
- The dose in the sprayer is now 0.2 mL (previously 0.5 mL). One half of the dose should be administered in each nostril.
- The product no longer has to be stored frozen; it should be kept at 35° to 46°F.
- When 2 doses are needed in children under age 9 being vaccinated for the first time, the interval between doses is now 4 weeks (previously 6 weeks).
TABLE 2
LAIV (FluMist) should not be used in these groups
|
Children under age 9 years who receive only 1 dose of vaccine (either TIV or LAIV) the first year they are vaccinated should receive 2 doses the next year.6 If they fail to receive 2 doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only 1 dose was recommended in this situation.7
Alternative schedule for combined hepatitis A and B vaccine
The FDA approved an alternate, 4-dose schedule for the combined hepatitis A and hepatitis B vaccine (Twinrix): at 0, 7, 21 days, and 12 months.8 It was previously approved only for a 3-dose schedule: at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart in less than a month’s time.
Merck recalls some lots of Hib vaccine
On December 11, 2007, Merck announced a voluntary recall of specific lots of Haemophilus influenza type b (Hib) conjugate vaccine products: 10 lots of a monovalent Hib vaccine, PedvaxHIB, and 2 lots of a combined hepatitis B/Hib vaccine, Comvax.
Consult Merck’s Web site for the lots involved and for instructions on returning vaccine (www.merckvaccines. com/PCHRecall.pdf). The recall was prompted by concern about equipment sterility, although no vaccine has been shown to be contaminated. Children vaccinated with Merck products do not need to be revaccinated or obtain any special follow-up.
Shortage expected. It is unknown when Merck will resume production, but it is not anticipated until at least late in 2008. Other Hib-containing products are produced by Sanofi Pasteur but the supply of these products will not make up for the expected shortage.
Interim recommendations. The recall resulted in interim recommendations from the CDC.9 These recommendations are complicated because the dosing schedule for Hib vaccine differs by the product and the age of receipt of first vaccine when children are not on schedule. TABLE 3 lists the Hib-containing products, the recommended primary series schedule, and booster dose.
TABLE 3
Hib products
| PRIMARY SERIES | BOOSTER | |||
|---|---|---|---|---|
| Merck Products | ||||
| PedvaxHIB | Monovalent Hib vaccine | 2, 4 months | 12–15 months* | |
| Comvax | Combined Hib/hepatitis B vaccine | 2, 4 months | 12–15 months* | |
| Sanofi Pasteur products | ||||
| ActHIB | Monovalent hib vaccine | 2, 4, 6 months | 12–15 months* | |
| TriHIBit | DTaP/Hib vaccine | Not licensed for this age group | 15–18 months* | |
| * Can follow a primary series of any product or serve as the only dose for a child up to 59 months, not previously immunized. | ||||
The main points are:
- Defer the booster dose at age 12 to 15 months until the shortage is resolved, except for high-risk children.
- High-risk children, who should continue to receive the booster at ages 12 to 15 months, include those with asplenia, sickle cell disease, HIV infection, and certain other immune deficiencies and cancers, and American Indian/Alaskan Native children.
- Physicians should keep track of children who have the booster deferred so they can be vaccinated when the supply improves.
- Non-recalled lots of PedvaxHIB and Comvax in the CDC stockpile will be prioritized to providers who care for predominantly American Indian/Alaskan Native children, who are at markedly in creased risk of Hib infection.
- If a child has received only 1 dose of PedvaxHIB or Comvax, their primary series can be completed with ActHIB, but 3 total doses are needed.
Children through age 59 months who are behind schedule should complete a primary series according to published recommendations.10 Physicians should call their local health department if they have any questions about what to do in a specific case.
1. CDC. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54(RR-7):1-21.
2. CDC. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007;56:794-795.
3. CDC. Recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MCV4) in children aged 2-10 years at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep 2007;56:1265-1266.
4. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the ACIP. MMWR Morb Mortal Wkly Rep 2007;56:1080-1084.
5. Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-07):1-23.
6. CDC. Expansion of use of live attenuated influenza vaccine to children aged 2-4 years and other Flu-Mist changes for the 2007-2008 influenza season. MMWR Morb Mortal Wkly Rep 2007;56:1217-1219.
7. Fiore AE, Shay DK, Haber P, et al. Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007;56(RR-6):1-54.
8. CDC. FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). MMWR Morb Mortal Wkly Rep 2007;56:1057.-
9. CDC. Interim recommendations for the use of Haemophilus influenza Type b (Hib) conjugate vaccines related to the recall of certain lots of Hib-containing vaccines (PedvaxHIB and Comvax). MMWR Morb Mortal Wkly Rep 2007;56:1318-1320.
10. CDC. Catch-up immunization schedule for persons aged 4 months-18 years who start late or are more than one month behind. Available at www.cdc.gov/vaccines/recs/schedules/downloads/child/2007/child-schedule-color-print.pdf. Accessed February 11, 2008.
The year 2007 was rather calm, compared to the 3 previous years in regards to new vaccines and vaccine recommendations. Although no breakthrough vaccine products came onto the market in 2007, there were new recommendations and licensure for new age groups for existing vaccines and a recall of some lots of Hib vaccines.
Meningococcal vaccine
Recommendations on the use of the quadrivalent meningococcal conjugate vaccine (MCV4) have evolved since its licensure in 2005 for use in persons 11 to 55 years of age. The first set of recommendations focused on universal vaccination of preteens, aged 11 to 12, those entering high school who had not received the vaccine previously, and others at risk for meningococcal disease including college freshmen living in dormitories.1 The MCV4 was preferred to the older polysaccharide vaccine (MPSV4) which was recommended only for children aged 2 to 10 and adults over age 55 at increased risk.
In 2007, the CDC changed 2 of the 2005 recommendations:
- The first, in August, simplified the recommendations for teens, making MCV4 universally recommended for all those aged 11 to 18 at the earliest opportunity.2
- The second, in December, followed FDA approval for use of MCV4 in children aged 2 to 10 years. The CDC now recommends MCV4 as the preferred vaccine in this age group for those at risk (TABLE 1).3
TABLE 1
Populations at increased risk for meningococcal disease who should receive quadrivalent meningococcal conjugate vaccine
|
If someone at ongoing risk for meningococcal disease has been previously vaccinated with MPSV4, they should be revaccinated 3 years later with MCV4. It is not known if repeat doses of MCV4 will be needed, and if so, after what amount of time.
The MCV4 has been linked to Guillain-Barré syndrome (GBS), and a history of GBS is a precaution for its use. For those with a history of GBS who need protection against meningococcal infection, MPSV4 is an alternative.
Hepatitis A vaccine
Widespread use of inactivated hepatitis A vaccine (HAV), first licensed in 1995, has markedly reduced the incidence of hepatitis A infection (FIGURE). Recommendations for its use have been periodically revised; current recommendations include universal vaccination of all children at age 12 to 23 months, catch-up vaccination in older children in areas of high prevalence, and vaccination of those at increased risk for hepatitis A including travelers to endemic areas, users of illicit drugs and men who have sex with men.4
FIGURE
Reduction in incidence of hepatitis A infection
Source: Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2006; 55(RR-07).
For those unvaccinated who are acutely exposed to hepatitis A virus and those traveling to areas of high prevalence who do not have time to complete the 2 doses of HAV, the only prevention available until recently has been IG. This has now changed and HAV can be used in both groups. The new recommendation for postexposure prophylaxis is that either a single dose of HAV or use of IG is acceptable.5 At ages 12 months to 40 years, vaccine is preferred. For those over age 40, IG is preferred but vaccine is acceptable. For children less than 12 months, the immune suppressed, and those with chronic liver disease, IG should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either HAV or IG. A single dose of HAV is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those under age 12 months, those who choose not to receive the vaccine, and those who are allergic to the vaccine should be offered IG. Both IG and HAV should be considered for individuals who plan to travel within 2 weeks of the first HAV dose; those over age 40, the immune compromised, and those with chronic liver disease or other chronic medical conditions.
Live attenuated influenza vaccine
FluMist, the live attenuated influenza vaccine (LAIV), which is administered as an intranasal spray, is now approved for use among those 2 to 4 years of age.6 Previously, the LAIV was approved only for healthy, non pregnant persons, 5 to 49 years of age. The LAIV may actually be the preferred product in children as it has been shown to prevent more influenza illness than the trivalent inactivated vaccine (TIV). The LAIV should not be used in anyone with a condition listed in TABLE 2 and should not be administered to children under age 5 who have recurrent wheezing.
FluMist has also been modified in several advantageous ways:
- The dose in the sprayer is now 0.2 mL (previously 0.5 mL). One half of the dose should be administered in each nostril.
- The product no longer has to be stored frozen; it should be kept at 35° to 46°F.
- When 2 doses are needed in children under age 9 being vaccinated for the first time, the interval between doses is now 4 weeks (previously 6 weeks).
TABLE 2
LAIV (FluMist) should not be used in these groups
|
Children under age 9 years who receive only 1 dose of vaccine (either TIV or LAIV) the first year they are vaccinated should receive 2 doses the next year.6 If they fail to receive 2 doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only 1 dose was recommended in this situation.7
Alternative schedule for combined hepatitis A and B vaccine
The FDA approved an alternate, 4-dose schedule for the combined hepatitis A and hepatitis B vaccine (Twinrix): at 0, 7, 21 days, and 12 months.8 It was previously approved only for a 3-dose schedule: at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart in less than a month’s time.
Merck recalls some lots of Hib vaccine
On December 11, 2007, Merck announced a voluntary recall of specific lots of Haemophilus influenza type b (Hib) conjugate vaccine products: 10 lots of a monovalent Hib vaccine, PedvaxHIB, and 2 lots of a combined hepatitis B/Hib vaccine, Comvax.
Consult Merck’s Web site for the lots involved and for instructions on returning vaccine (www.merckvaccines. com/PCHRecall.pdf). The recall was prompted by concern about equipment sterility, although no vaccine has been shown to be contaminated. Children vaccinated with Merck products do not need to be revaccinated or obtain any special follow-up.
Shortage expected. It is unknown when Merck will resume production, but it is not anticipated until at least late in 2008. Other Hib-containing products are produced by Sanofi Pasteur but the supply of these products will not make up for the expected shortage.
Interim recommendations. The recall resulted in interim recommendations from the CDC.9 These recommendations are complicated because the dosing schedule for Hib vaccine differs by the product and the age of receipt of first vaccine when children are not on schedule. TABLE 3 lists the Hib-containing products, the recommended primary series schedule, and booster dose.
TABLE 3
Hib products
| PRIMARY SERIES | BOOSTER | |||
|---|---|---|---|---|
| Merck Products | ||||
| PedvaxHIB | Monovalent Hib vaccine | 2, 4 months | 12–15 months* | |
| Comvax | Combined Hib/hepatitis B vaccine | 2, 4 months | 12–15 months* | |
| Sanofi Pasteur products | ||||
| ActHIB | Monovalent hib vaccine | 2, 4, 6 months | 12–15 months* | |
| TriHIBit | DTaP/Hib vaccine | Not licensed for this age group | 15–18 months* | |
| * Can follow a primary series of any product or serve as the only dose for a child up to 59 months, not previously immunized. | ||||
The main points are:
- Defer the booster dose at age 12 to 15 months until the shortage is resolved, except for high-risk children.
- High-risk children, who should continue to receive the booster at ages 12 to 15 months, include those with asplenia, sickle cell disease, HIV infection, and certain other immune deficiencies and cancers, and American Indian/Alaskan Native children.
- Physicians should keep track of children who have the booster deferred so they can be vaccinated when the supply improves.
- Non-recalled lots of PedvaxHIB and Comvax in the CDC stockpile will be prioritized to providers who care for predominantly American Indian/Alaskan Native children, who are at markedly in creased risk of Hib infection.
- If a child has received only 1 dose of PedvaxHIB or Comvax, their primary series can be completed with ActHIB, but 3 total doses are needed.
Children through age 59 months who are behind schedule should complete a primary series according to published recommendations.10 Physicians should call their local health department if they have any questions about what to do in a specific case.
The year 2007 was rather calm, compared to the 3 previous years in regards to new vaccines and vaccine recommendations. Although no breakthrough vaccine products came onto the market in 2007, there were new recommendations and licensure for new age groups for existing vaccines and a recall of some lots of Hib vaccines.
Meningococcal vaccine
Recommendations on the use of the quadrivalent meningococcal conjugate vaccine (MCV4) have evolved since its licensure in 2005 for use in persons 11 to 55 years of age. The first set of recommendations focused on universal vaccination of preteens, aged 11 to 12, those entering high school who had not received the vaccine previously, and others at risk for meningococcal disease including college freshmen living in dormitories.1 The MCV4 was preferred to the older polysaccharide vaccine (MPSV4) which was recommended only for children aged 2 to 10 and adults over age 55 at increased risk.
In 2007, the CDC changed 2 of the 2005 recommendations:
- The first, in August, simplified the recommendations for teens, making MCV4 universally recommended for all those aged 11 to 18 at the earliest opportunity.2
- The second, in December, followed FDA approval for use of MCV4 in children aged 2 to 10 years. The CDC now recommends MCV4 as the preferred vaccine in this age group for those at risk (TABLE 1).3
TABLE 1
Populations at increased risk for meningococcal disease who should receive quadrivalent meningococcal conjugate vaccine
|
If someone at ongoing risk for meningococcal disease has been previously vaccinated with MPSV4, they should be revaccinated 3 years later with MCV4. It is not known if repeat doses of MCV4 will be needed, and if so, after what amount of time.
The MCV4 has been linked to Guillain-Barré syndrome (GBS), and a history of GBS is a precaution for its use. For those with a history of GBS who need protection against meningococcal infection, MPSV4 is an alternative.
Hepatitis A vaccine
Widespread use of inactivated hepatitis A vaccine (HAV), first licensed in 1995, has markedly reduced the incidence of hepatitis A infection (FIGURE). Recommendations for its use have been periodically revised; current recommendations include universal vaccination of all children at age 12 to 23 months, catch-up vaccination in older children in areas of high prevalence, and vaccination of those at increased risk for hepatitis A including travelers to endemic areas, users of illicit drugs and men who have sex with men.4
FIGURE
Reduction in incidence of hepatitis A infection
Source: Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2006; 55(RR-07).
For those unvaccinated who are acutely exposed to hepatitis A virus and those traveling to areas of high prevalence who do not have time to complete the 2 doses of HAV, the only prevention available until recently has been IG. This has now changed and HAV can be used in both groups. The new recommendation for postexposure prophylaxis is that either a single dose of HAV or use of IG is acceptable.5 At ages 12 months to 40 years, vaccine is preferred. For those over age 40, IG is preferred but vaccine is acceptable. For children less than 12 months, the immune suppressed, and those with chronic liver disease, IG should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either HAV or IG. A single dose of HAV is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those under age 12 months, those who choose not to receive the vaccine, and those who are allergic to the vaccine should be offered IG. Both IG and HAV should be considered for individuals who plan to travel within 2 weeks of the first HAV dose; those over age 40, the immune compromised, and those with chronic liver disease or other chronic medical conditions.
Live attenuated influenza vaccine
FluMist, the live attenuated influenza vaccine (LAIV), which is administered as an intranasal spray, is now approved for use among those 2 to 4 years of age.6 Previously, the LAIV was approved only for healthy, non pregnant persons, 5 to 49 years of age. The LAIV may actually be the preferred product in children as it has been shown to prevent more influenza illness than the trivalent inactivated vaccine (TIV). The LAIV should not be used in anyone with a condition listed in TABLE 2 and should not be administered to children under age 5 who have recurrent wheezing.
FluMist has also been modified in several advantageous ways:
- The dose in the sprayer is now 0.2 mL (previously 0.5 mL). One half of the dose should be administered in each nostril.
- The product no longer has to be stored frozen; it should be kept at 35° to 46°F.
- When 2 doses are needed in children under age 9 being vaccinated for the first time, the interval between doses is now 4 weeks (previously 6 weeks).
TABLE 2
LAIV (FluMist) should not be used in these groups
|
Children under age 9 years who receive only 1 dose of vaccine (either TIV or LAIV) the first year they are vaccinated should receive 2 doses the next year.6 If they fail to receive 2 doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only 1 dose was recommended in this situation.7
Alternative schedule for combined hepatitis A and B vaccine
The FDA approved an alternate, 4-dose schedule for the combined hepatitis A and hepatitis B vaccine (Twinrix): at 0, 7, 21 days, and 12 months.8 It was previously approved only for a 3-dose schedule: at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart in less than a month’s time.
Merck recalls some lots of Hib vaccine
On December 11, 2007, Merck announced a voluntary recall of specific lots of Haemophilus influenza type b (Hib) conjugate vaccine products: 10 lots of a monovalent Hib vaccine, PedvaxHIB, and 2 lots of a combined hepatitis B/Hib vaccine, Comvax.
Consult Merck’s Web site for the lots involved and for instructions on returning vaccine (www.merckvaccines. com/PCHRecall.pdf). The recall was prompted by concern about equipment sterility, although no vaccine has been shown to be contaminated. Children vaccinated with Merck products do not need to be revaccinated or obtain any special follow-up.
Shortage expected. It is unknown when Merck will resume production, but it is not anticipated until at least late in 2008. Other Hib-containing products are produced by Sanofi Pasteur but the supply of these products will not make up for the expected shortage.
Interim recommendations. The recall resulted in interim recommendations from the CDC.9 These recommendations are complicated because the dosing schedule for Hib vaccine differs by the product and the age of receipt of first vaccine when children are not on schedule. TABLE 3 lists the Hib-containing products, the recommended primary series schedule, and booster dose.
TABLE 3
Hib products
| PRIMARY SERIES | BOOSTER | |||
|---|---|---|---|---|
| Merck Products | ||||
| PedvaxHIB | Monovalent Hib vaccine | 2, 4 months | 12–15 months* | |
| Comvax | Combined Hib/hepatitis B vaccine | 2, 4 months | 12–15 months* | |
| Sanofi Pasteur products | ||||
| ActHIB | Monovalent hib vaccine | 2, 4, 6 months | 12–15 months* | |
| TriHIBit | DTaP/Hib vaccine | Not licensed for this age group | 15–18 months* | |
| * Can follow a primary series of any product or serve as the only dose for a child up to 59 months, not previously immunized. | ||||
The main points are:
- Defer the booster dose at age 12 to 15 months until the shortage is resolved, except for high-risk children.
- High-risk children, who should continue to receive the booster at ages 12 to 15 months, include those with asplenia, sickle cell disease, HIV infection, and certain other immune deficiencies and cancers, and American Indian/Alaskan Native children.
- Physicians should keep track of children who have the booster deferred so they can be vaccinated when the supply improves.
- Non-recalled lots of PedvaxHIB and Comvax in the CDC stockpile will be prioritized to providers who care for predominantly American Indian/Alaskan Native children, who are at markedly in creased risk of Hib infection.
- If a child has received only 1 dose of PedvaxHIB or Comvax, their primary series can be completed with ActHIB, but 3 total doses are needed.
Children through age 59 months who are behind schedule should complete a primary series according to published recommendations.10 Physicians should call their local health department if they have any questions about what to do in a specific case.
1. CDC. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54(RR-7):1-21.
2. CDC. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007;56:794-795.
3. CDC. Recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MCV4) in children aged 2-10 years at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep 2007;56:1265-1266.
4. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the ACIP. MMWR Morb Mortal Wkly Rep 2007;56:1080-1084.
5. Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-07):1-23.
6. CDC. Expansion of use of live attenuated influenza vaccine to children aged 2-4 years and other Flu-Mist changes for the 2007-2008 influenza season. MMWR Morb Mortal Wkly Rep 2007;56:1217-1219.
7. Fiore AE, Shay DK, Haber P, et al. Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007;56(RR-6):1-54.
8. CDC. FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). MMWR Morb Mortal Wkly Rep 2007;56:1057.-
9. CDC. Interim recommendations for the use of Haemophilus influenza Type b (Hib) conjugate vaccines related to the recall of certain lots of Hib-containing vaccines (PedvaxHIB and Comvax). MMWR Morb Mortal Wkly Rep 2007;56:1318-1320.
10. CDC. Catch-up immunization schedule for persons aged 4 months-18 years who start late or are more than one month behind. Available at www.cdc.gov/vaccines/recs/schedules/downloads/child/2007/child-schedule-color-print.pdf. Accessed February 11, 2008.
1. CDC. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54(RR-7):1-21.
2. CDC. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007;56:794-795.
3. CDC. Recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MCV4) in children aged 2-10 years at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep 2007;56:1265-1266.
4. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the ACIP. MMWR Morb Mortal Wkly Rep 2007;56:1080-1084.
5. Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-07):1-23.
6. CDC. Expansion of use of live attenuated influenza vaccine to children aged 2-4 years and other Flu-Mist changes for the 2007-2008 influenza season. MMWR Morb Mortal Wkly Rep 2007;56:1217-1219.
7. Fiore AE, Shay DK, Haber P, et al. Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007;56(RR-6):1-54.
8. CDC. FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). MMWR Morb Mortal Wkly Rep 2007;56:1057.-
9. CDC. Interim recommendations for the use of Haemophilus influenza Type b (Hib) conjugate vaccines related to the recall of certain lots of Hib-containing vaccines (PedvaxHIB and Comvax). MMWR Morb Mortal Wkly Rep 2007;56:1318-1320.
10. CDC. Catch-up immunization schedule for persons aged 4 months-18 years who start late or are more than one month behind. Available at www.cdc.gov/vaccines/recs/schedules/downloads/child/2007/child-schedule-color-print.pdf. Accessed February 11, 2008.