User login
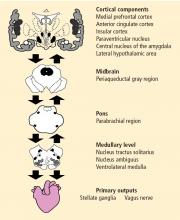
The neurovisceral integration model of cardiac vagal tone integrates autonomic, attentional, and affective systems into a functional and structural network. This neural network can be indexed by heart rate variability (HRV). High HRV is associated with greater prefrontal inhibitory tone. A lack of inhibition leads to undifferentiated threat responses to environmental challenges.
THE CENTRAL AUTONOMIC NETWORK
Activity of the heart permits us to infer activity in this set of neural structures. Excitatory and inhibitory pathways form the connections between the prefrontal cortex and the autonomic output regions in the medullary area, with further connections to heart rate (HR) and HRV.
Central, respiratory, cardiopulmonary, and arterial baroreflex influences on the brainstem signal the sinoatrial node of the heart. Autonomic inputs at the heart have a differential influence. The sympathetic inputs to the sinoatrial node of the heart are relatively slow, such that a burst of sympathetic outflow from the brain produces an effect on the heart several seconds later. In contrast, inputs to the cholinergic or vagal pathway are relatively fast, on the order of milliseconds. The interplay of sympathetic and vagal neural control of the heart produces a complex variability in heart rhythm that characterizes a healthy system.
PARASYMPATHETIC CONTROL AND THE RIGHT VAGUS NERVE
Pharmacologic blockade of prefrontal cortex
The effect of pharmacologic blockade of the prefrontal cortex on HR and HRV was investigated in patients undergoing preoperative evaluation for epilepsy surgery.2 The hypothesis was that inactivation of the prefrontal cortex (using an injection of intracarotid sodium amobarbital) would be associated with an increase in HR and a decrease in vagally mediated HRV.
During 10 minutes of inactivation, an increase in HR was observed in both the left and right hemispheres. HR peaked 3 to 4 minutes postinjection and decreased gradually, returning to preinjection baseline at about 10 minutes. The increase was larger in the right hemisphere, a finding that is consistent with the known neuroanatomy in which the right-sided neural inputs selectively signal the sinoatrial node, and the left-sided inputs signal the atrioventricular node. The pronounced effect on HR in the right hemisphere was related specifically to the vagally mediated (high-frequency) component of HRV. This experiment strongly suggests that cerebral structures tonically inhibit sympathoexcitatory circuits, and that the inhibition is mediated via vagal mechanisms.
Further analysis, in which the subjects were divided into tertiles based on age, revealed disinhibition of brainstem sympathoexcitatory circuits, resulting in an increase in HR of approximately 9 beats per minute in the youngest individuals (mean age, 20 years), but an absence of a laterality effect, which suggests that the prefrontal cortex is not fully developed in this young age group. Disinhibition of sympathoexcitatory circuits as indicated by a HR increase of 11 beats per minute and a right-sided laterality effect occurred in subjects in the second tertile (mean age, 33 years). In the oldest age group (mean age, 45 years), the disinhibition effect on HR was only 3 beats per minute, consistent with the known changes in prefrontal inhibitory tone and prefrontal activity that occur with age.3
Confirmation from neuroimaging studies
Neuroimaging studies support the predominant role of the right hemisphere in the regulation of vagal tone during emotion. Twelve healthy females underwent measurements of cerebral blood flow and the high-frequency component of HRV during two stimulus modalities (film, recall) and six stimulus conditions (happiness, sadness, disgust, and three neutral conditions), for a total of 12 conditions.4 Significant covariation (increased activity associated with increased HRV) was found for four brain areas: the right superior prefrontal cortex, the right dorsal lateral prefrontal cortex, the right parietal cortex, and the left anterior cingulate.
THE INFLAMMATORY REFLEX
The cholinergic anti-inflammatory pathway
Acetylcholine and parasympathetic tone inhibit proinflammatory cytokines such as interleukin (IL)-6. These proinflammatory cytokines are under tonic inhibitory control via the vagus nerve, and this function may have important implications for health and disease.5
The cholinergic anti-inflammatory pathway is associated with efferent activity in the vagus nerve, leading to acetylcholine release in the reticuloendothelial system that includes the liver, heart, spleen, and gastrointestinal tract. Acetylcholine interacts with the alpha-7 nicotinic receptor on tissue macrophages to inhibit the release of proinflammatory cytokines, but not anti-inflammatory cytokines such as IL-10.
Approximately 80% of the fibers of the vagus nerve are sensory; ie, they sense the presence of proinflammatory cytokines and convey the signal to the brain. Efferent vagus nerve activity leads to the release of acetylcholine, which inhibits tumor necrosis factor (TNF)-alpha on the macrophages. Cytokine regulation also involves the sympathetic nervous system and the endocrine system (the hypothalamic-pituitary axis).
The sympathetic system has both pro- and anti-inflammatory influences. The inflammatory response is a cascade of cytokines, such that it may begin with the release of TNF-alpha, leading to the production of IL-1 and IL-6. IL-6 has both pro- and anti-inflammatory properties and represents a negative feedback mechanism. Expression of IL-6 in the liver promotes the production of the acute-phase reactant C-reactive protein (CRP). Therefore, activation of the cholinergic receptor to induce acetylcholine release may be an early intervention to short-circuit this inflammatory cascade, a potential therapeutic strategy to blunt inflammatory-mediated disease.
Inverse relationship between HRV and CRP
In a study of 613 airplane factory workers in southern Germany, vagally mediated HRV was inversely related to high-sensitivity CRP in men and premenopausal women, even after controlling for urinary norepinephrine as an index of sympathetic activity.6 Most previous studies in which the relationship between HRV and CRP (or other inflammatory markers) was assessed failed to control for sympathetic nervous system activity. In the total sample and in men, the parasympathetic effect on CRP was comparable with that of smoking; in women, the effect was 4 times larger and comparable with that of high body mass index. A negative association was again found between vagally mediated HRV and white blood cell count.
Inverse relationship between HRV and fibrinogen
In a related report from the same study, vagal modulation of fibrinogen was investigated.7 Fibrinogen is a large glycoprotein that is synthesized by the liver. Plasma fibrinogen is a measure of systemic inflammation crucially involved in atherosclerosis. Meta-analyses have shown a prospective association between elevated plasma fibrinogen levels even in the normal range and an increased risk of coronary artery disease in different populations. We investigated the relationship between nighttime HRV, assessed by root mean square of successive R-R interval differences (RMSSD), and fibrinogen in 559 mostly male workers from southern Germany. Among all workers, there was a mean ± SEM increase of 0.41 ± 0.13 mg/dL fibrinogen for each ms decrease in nighttime RMSSD, even after controlling for established cardiovascular risk factors. The increase in men was 0.28 ± 0.13 mg/dL and, in women, 1.16 ± 0.41 mg/dL for each ms decrease in nighttime RMSSD. Such an autonomic mechanism might contribute to the atherosclerotic process and its thrombotic complications.
Vagal regulation of allostatic systems
Whereas the role of the autonomic nervous system, and the vagus nerve in particular, in the regulation of the cardiovascular system seems clear, the role of the vagus nerve in the regulation of other systems associated with allostasis is less evident. In addition to the regulation of inflammatory markers as discussed thus far, decreased vagal function and HRV have been associated with increased fasting glucose and glycated hemoglobin (HbA1c) levels, and with increased overnight urinary cortisol.8 These factors have been associated with increased allostatic load and poor health. Thus, vagal activity seems to have an inhibitory function in the regulation of allostatic systems. The prefrontal cortex and the amygdala are important central nervous system structures linked to the regulation of these allostatic systems, including inflammation via the vagus nerve. The next section describes evidence for the prefrontal regulation of inflammation.
Prefrontal cortical activity and immune indices
Ohira et al used neuroimaging to explore the association between the brain and immune function.9 Their study examined the neural basis of the top-down modulation accompanying cognitive appraisal during a controllable or uncontrollable acute stressor. HR and blood pressure increased significantly during a mental arithmetic task and returned to baseline soon after termination of the task. HR increased to a greater extent in the controllable versus the uncontrollable condition; blood pressure was unaffected by controllability. Endocrine and immune indices were also affected by the acute stress task: the proportions of natural killer cells increased and helper T cells decreased acutely during the stressor.
Importantly, cerebral blood flow measurements demonstrated that the areas of the prefrontal cortex that we have found to be associated with HRV, including the medial prefrontal cortex and the insula, were also associated with immune indices (medial and lateral orbitofrontal cortices and insula), suggesting prefrontal or frontal modulation of immune responses possibly via the same vagal pathways.
VAGAL ACTIVITY AND CARDIOVASCULAR RISK FACTORS
The regulation of physiologic systems that are important for health and disease has been linked to vagal function and HRV. We have recently reviewed the literature on the relationship between vagal function and the risk for cardiovascular disease and stroke. The National Heart, Lung, and Blood Institute lists eight risk factors for heart disease and stroke.10 Six are considered modifiable. Of the six modifiable factors, three are associated with what could be called biologic factors: high blood pressure (hypertension), diabetes, and abnormal cholesterol; the other three could be considered lifestyle factors: tobacco use (smoking), physical inactivity (exercise), and overweight (obesity). Two factors, age and family history of early heart disease or stroke, are considered nonmodifiable. At least some data suggest that each of these risk factors is associated with decreased vagal function as indexed by HRV.11
Interventions to modify HRV include exercise, ingestion of omega-3 fatty acids, stress reduction (eg, mediation), pharmacologic manipulations, and vagus nerve stimulation, suggesting that methods that increase vagus nerve activity might favorably modify an individual’s risk profile.
CONCLUSION
The brain and the heart are intimately connected. Both epidemiologic and experimental data suggest an association between HRV and inflammation, including similar neural mechanisms. Evidence of an association between HRV and inflammation supports the concept of a cholinergic anti-inflammatory pathway.
- Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 1993; 68:988–1001.
- Ahern GL, Sollers JJ, Lane RD, et al. Heart rate and heart rate variability changes in the intracarotid sodium amobarbital test. Epilepsia 2001; 42:912–921.
- Thayer JF, Sollers JJ, Labiner DM, et al Age-related differences in prefrontal control of heart rate in humans: a pharmacological blockade study [published online ahead of print September 19, 2008] Int J Psychophysioldoi: 10.1016/j.ijpsycho.2008.04.007.
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. Neuroimage 2009; 44:213–222.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Thayer JF, Fischer JE Heart rate variability, overnight urinary norepinephrine, and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults [published online ahead of print November 15, 2008] J Intern Meddoi: 10.1111/j.1365-2796.2008.02023.x.
- von Kanel R, Thayer JF, Fischer JE Night-time vagal cardiac control and plasma fibrinogen levels in a population of working men and women Ann Noninvasive Electrocardiol In press
- Thayer JF, Sternberg EM. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci 2006; 1088:361–372.
- Ohira H, Isowa T, Nomura M, et al. Imaging brain and immune association accompanying cognitive appraisal of an acute stressor. Neuroimage 2008; 39:500–514.
- Your guide to lowering blood pressure: risk factors. National Heart, Lung, and Blood Institute Web site. http://www.nhlbi.nih.gov/hbp/hbp/hdrf.htm. Accessed January 13, 2009.
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol 2007; 74:224–242.
The neurovisceral integration model of cardiac vagal tone integrates autonomic, attentional, and affective systems into a functional and structural network. This neural network can be indexed by heart rate variability (HRV). High HRV is associated with greater prefrontal inhibitory tone. A lack of inhibition leads to undifferentiated threat responses to environmental challenges.
THE CENTRAL AUTONOMIC NETWORK
Activity of the heart permits us to infer activity in this set of neural structures. Excitatory and inhibitory pathways form the connections between the prefrontal cortex and the autonomic output regions in the medullary area, with further connections to heart rate (HR) and HRV.
Central, respiratory, cardiopulmonary, and arterial baroreflex influences on the brainstem signal the sinoatrial node of the heart. Autonomic inputs at the heart have a differential influence. The sympathetic inputs to the sinoatrial node of the heart are relatively slow, such that a burst of sympathetic outflow from the brain produces an effect on the heart several seconds later. In contrast, inputs to the cholinergic or vagal pathway are relatively fast, on the order of milliseconds. The interplay of sympathetic and vagal neural control of the heart produces a complex variability in heart rhythm that characterizes a healthy system.
PARASYMPATHETIC CONTROL AND THE RIGHT VAGUS NERVE
Pharmacologic blockade of prefrontal cortex
The effect of pharmacologic blockade of the prefrontal cortex on HR and HRV was investigated in patients undergoing preoperative evaluation for epilepsy surgery.2 The hypothesis was that inactivation of the prefrontal cortex (using an injection of intracarotid sodium amobarbital) would be associated with an increase in HR and a decrease in vagally mediated HRV.
During 10 minutes of inactivation, an increase in HR was observed in both the left and right hemispheres. HR peaked 3 to 4 minutes postinjection and decreased gradually, returning to preinjection baseline at about 10 minutes. The increase was larger in the right hemisphere, a finding that is consistent with the known neuroanatomy in which the right-sided neural inputs selectively signal the sinoatrial node, and the left-sided inputs signal the atrioventricular node. The pronounced effect on HR in the right hemisphere was related specifically to the vagally mediated (high-frequency) component of HRV. This experiment strongly suggests that cerebral structures tonically inhibit sympathoexcitatory circuits, and that the inhibition is mediated via vagal mechanisms.
Further analysis, in which the subjects were divided into tertiles based on age, revealed disinhibition of brainstem sympathoexcitatory circuits, resulting in an increase in HR of approximately 9 beats per minute in the youngest individuals (mean age, 20 years), but an absence of a laterality effect, which suggests that the prefrontal cortex is not fully developed in this young age group. Disinhibition of sympathoexcitatory circuits as indicated by a HR increase of 11 beats per minute and a right-sided laterality effect occurred in subjects in the second tertile (mean age, 33 years). In the oldest age group (mean age, 45 years), the disinhibition effect on HR was only 3 beats per minute, consistent with the known changes in prefrontal inhibitory tone and prefrontal activity that occur with age.3
Confirmation from neuroimaging studies
Neuroimaging studies support the predominant role of the right hemisphere in the regulation of vagal tone during emotion. Twelve healthy females underwent measurements of cerebral blood flow and the high-frequency component of HRV during two stimulus modalities (film, recall) and six stimulus conditions (happiness, sadness, disgust, and three neutral conditions), for a total of 12 conditions.4 Significant covariation (increased activity associated with increased HRV) was found for four brain areas: the right superior prefrontal cortex, the right dorsal lateral prefrontal cortex, the right parietal cortex, and the left anterior cingulate.
THE INFLAMMATORY REFLEX
The cholinergic anti-inflammatory pathway
Acetylcholine and parasympathetic tone inhibit proinflammatory cytokines such as interleukin (IL)-6. These proinflammatory cytokines are under tonic inhibitory control via the vagus nerve, and this function may have important implications for health and disease.5
The cholinergic anti-inflammatory pathway is associated with efferent activity in the vagus nerve, leading to acetylcholine release in the reticuloendothelial system that includes the liver, heart, spleen, and gastrointestinal tract. Acetylcholine interacts with the alpha-7 nicotinic receptor on tissue macrophages to inhibit the release of proinflammatory cytokines, but not anti-inflammatory cytokines such as IL-10.
Approximately 80% of the fibers of the vagus nerve are sensory; ie, they sense the presence of proinflammatory cytokines and convey the signal to the brain. Efferent vagus nerve activity leads to the release of acetylcholine, which inhibits tumor necrosis factor (TNF)-alpha on the macrophages. Cytokine regulation also involves the sympathetic nervous system and the endocrine system (the hypothalamic-pituitary axis).
The sympathetic system has both pro- and anti-inflammatory influences. The inflammatory response is a cascade of cytokines, such that it may begin with the release of TNF-alpha, leading to the production of IL-1 and IL-6. IL-6 has both pro- and anti-inflammatory properties and represents a negative feedback mechanism. Expression of IL-6 in the liver promotes the production of the acute-phase reactant C-reactive protein (CRP). Therefore, activation of the cholinergic receptor to induce acetylcholine release may be an early intervention to short-circuit this inflammatory cascade, a potential therapeutic strategy to blunt inflammatory-mediated disease.
Inverse relationship between HRV and CRP
In a study of 613 airplane factory workers in southern Germany, vagally mediated HRV was inversely related to high-sensitivity CRP in men and premenopausal women, even after controlling for urinary norepinephrine as an index of sympathetic activity.6 Most previous studies in which the relationship between HRV and CRP (or other inflammatory markers) was assessed failed to control for sympathetic nervous system activity. In the total sample and in men, the parasympathetic effect on CRP was comparable with that of smoking; in women, the effect was 4 times larger and comparable with that of high body mass index. A negative association was again found between vagally mediated HRV and white blood cell count.
Inverse relationship between HRV and fibrinogen
In a related report from the same study, vagal modulation of fibrinogen was investigated.7 Fibrinogen is a large glycoprotein that is synthesized by the liver. Plasma fibrinogen is a measure of systemic inflammation crucially involved in atherosclerosis. Meta-analyses have shown a prospective association between elevated plasma fibrinogen levels even in the normal range and an increased risk of coronary artery disease in different populations. We investigated the relationship between nighttime HRV, assessed by root mean square of successive R-R interval differences (RMSSD), and fibrinogen in 559 mostly male workers from southern Germany. Among all workers, there was a mean ± SEM increase of 0.41 ± 0.13 mg/dL fibrinogen for each ms decrease in nighttime RMSSD, even after controlling for established cardiovascular risk factors. The increase in men was 0.28 ± 0.13 mg/dL and, in women, 1.16 ± 0.41 mg/dL for each ms decrease in nighttime RMSSD. Such an autonomic mechanism might contribute to the atherosclerotic process and its thrombotic complications.
Vagal regulation of allostatic systems
Whereas the role of the autonomic nervous system, and the vagus nerve in particular, in the regulation of the cardiovascular system seems clear, the role of the vagus nerve in the regulation of other systems associated with allostasis is less evident. In addition to the regulation of inflammatory markers as discussed thus far, decreased vagal function and HRV have been associated with increased fasting glucose and glycated hemoglobin (HbA1c) levels, and with increased overnight urinary cortisol.8 These factors have been associated with increased allostatic load and poor health. Thus, vagal activity seems to have an inhibitory function in the regulation of allostatic systems. The prefrontal cortex and the amygdala are important central nervous system structures linked to the regulation of these allostatic systems, including inflammation via the vagus nerve. The next section describes evidence for the prefrontal regulation of inflammation.
Prefrontal cortical activity and immune indices
Ohira et al used neuroimaging to explore the association between the brain and immune function.9 Their study examined the neural basis of the top-down modulation accompanying cognitive appraisal during a controllable or uncontrollable acute stressor. HR and blood pressure increased significantly during a mental arithmetic task and returned to baseline soon after termination of the task. HR increased to a greater extent in the controllable versus the uncontrollable condition; blood pressure was unaffected by controllability. Endocrine and immune indices were also affected by the acute stress task: the proportions of natural killer cells increased and helper T cells decreased acutely during the stressor.
Importantly, cerebral blood flow measurements demonstrated that the areas of the prefrontal cortex that we have found to be associated with HRV, including the medial prefrontal cortex and the insula, were also associated with immune indices (medial and lateral orbitofrontal cortices and insula), suggesting prefrontal or frontal modulation of immune responses possibly via the same vagal pathways.
VAGAL ACTIVITY AND CARDIOVASCULAR RISK FACTORS
The regulation of physiologic systems that are important for health and disease has been linked to vagal function and HRV. We have recently reviewed the literature on the relationship between vagal function and the risk for cardiovascular disease and stroke. The National Heart, Lung, and Blood Institute lists eight risk factors for heart disease and stroke.10 Six are considered modifiable. Of the six modifiable factors, three are associated with what could be called biologic factors: high blood pressure (hypertension), diabetes, and abnormal cholesterol; the other three could be considered lifestyle factors: tobacco use (smoking), physical inactivity (exercise), and overweight (obesity). Two factors, age and family history of early heart disease or stroke, are considered nonmodifiable. At least some data suggest that each of these risk factors is associated with decreased vagal function as indexed by HRV.11
Interventions to modify HRV include exercise, ingestion of omega-3 fatty acids, stress reduction (eg, mediation), pharmacologic manipulations, and vagus nerve stimulation, suggesting that methods that increase vagus nerve activity might favorably modify an individual’s risk profile.
CONCLUSION
The brain and the heart are intimately connected. Both epidemiologic and experimental data suggest an association between HRV and inflammation, including similar neural mechanisms. Evidence of an association between HRV and inflammation supports the concept of a cholinergic anti-inflammatory pathway.
The neurovisceral integration model of cardiac vagal tone integrates autonomic, attentional, and affective systems into a functional and structural network. This neural network can be indexed by heart rate variability (HRV). High HRV is associated with greater prefrontal inhibitory tone. A lack of inhibition leads to undifferentiated threat responses to environmental challenges.
THE CENTRAL AUTONOMIC NETWORK
Activity of the heart permits us to infer activity in this set of neural structures. Excitatory and inhibitory pathways form the connections between the prefrontal cortex and the autonomic output regions in the medullary area, with further connections to heart rate (HR) and HRV.
Central, respiratory, cardiopulmonary, and arterial baroreflex influences on the brainstem signal the sinoatrial node of the heart. Autonomic inputs at the heart have a differential influence. The sympathetic inputs to the sinoatrial node of the heart are relatively slow, such that a burst of sympathetic outflow from the brain produces an effect on the heart several seconds later. In contrast, inputs to the cholinergic or vagal pathway are relatively fast, on the order of milliseconds. The interplay of sympathetic and vagal neural control of the heart produces a complex variability in heart rhythm that characterizes a healthy system.
PARASYMPATHETIC CONTROL AND THE RIGHT VAGUS NERVE
Pharmacologic blockade of prefrontal cortex
The effect of pharmacologic blockade of the prefrontal cortex on HR and HRV was investigated in patients undergoing preoperative evaluation for epilepsy surgery.2 The hypothesis was that inactivation of the prefrontal cortex (using an injection of intracarotid sodium amobarbital) would be associated with an increase in HR and a decrease in vagally mediated HRV.
During 10 minutes of inactivation, an increase in HR was observed in both the left and right hemispheres. HR peaked 3 to 4 minutes postinjection and decreased gradually, returning to preinjection baseline at about 10 minutes. The increase was larger in the right hemisphere, a finding that is consistent with the known neuroanatomy in which the right-sided neural inputs selectively signal the sinoatrial node, and the left-sided inputs signal the atrioventricular node. The pronounced effect on HR in the right hemisphere was related specifically to the vagally mediated (high-frequency) component of HRV. This experiment strongly suggests that cerebral structures tonically inhibit sympathoexcitatory circuits, and that the inhibition is mediated via vagal mechanisms.
Further analysis, in which the subjects were divided into tertiles based on age, revealed disinhibition of brainstem sympathoexcitatory circuits, resulting in an increase in HR of approximately 9 beats per minute in the youngest individuals (mean age, 20 years), but an absence of a laterality effect, which suggests that the prefrontal cortex is not fully developed in this young age group. Disinhibition of sympathoexcitatory circuits as indicated by a HR increase of 11 beats per minute and a right-sided laterality effect occurred in subjects in the second tertile (mean age, 33 years). In the oldest age group (mean age, 45 years), the disinhibition effect on HR was only 3 beats per minute, consistent with the known changes in prefrontal inhibitory tone and prefrontal activity that occur with age.3
Confirmation from neuroimaging studies
Neuroimaging studies support the predominant role of the right hemisphere in the regulation of vagal tone during emotion. Twelve healthy females underwent measurements of cerebral blood flow and the high-frequency component of HRV during two stimulus modalities (film, recall) and six stimulus conditions (happiness, sadness, disgust, and three neutral conditions), for a total of 12 conditions.4 Significant covariation (increased activity associated with increased HRV) was found for four brain areas: the right superior prefrontal cortex, the right dorsal lateral prefrontal cortex, the right parietal cortex, and the left anterior cingulate.
THE INFLAMMATORY REFLEX
The cholinergic anti-inflammatory pathway
Acetylcholine and parasympathetic tone inhibit proinflammatory cytokines such as interleukin (IL)-6. These proinflammatory cytokines are under tonic inhibitory control via the vagus nerve, and this function may have important implications for health and disease.5
The cholinergic anti-inflammatory pathway is associated with efferent activity in the vagus nerve, leading to acetylcholine release in the reticuloendothelial system that includes the liver, heart, spleen, and gastrointestinal tract. Acetylcholine interacts with the alpha-7 nicotinic receptor on tissue macrophages to inhibit the release of proinflammatory cytokines, but not anti-inflammatory cytokines such as IL-10.
Approximately 80% of the fibers of the vagus nerve are sensory; ie, they sense the presence of proinflammatory cytokines and convey the signal to the brain. Efferent vagus nerve activity leads to the release of acetylcholine, which inhibits tumor necrosis factor (TNF)-alpha on the macrophages. Cytokine regulation also involves the sympathetic nervous system and the endocrine system (the hypothalamic-pituitary axis).
The sympathetic system has both pro- and anti-inflammatory influences. The inflammatory response is a cascade of cytokines, such that it may begin with the release of TNF-alpha, leading to the production of IL-1 and IL-6. IL-6 has both pro- and anti-inflammatory properties and represents a negative feedback mechanism. Expression of IL-6 in the liver promotes the production of the acute-phase reactant C-reactive protein (CRP). Therefore, activation of the cholinergic receptor to induce acetylcholine release may be an early intervention to short-circuit this inflammatory cascade, a potential therapeutic strategy to blunt inflammatory-mediated disease.
Inverse relationship between HRV and CRP
In a study of 613 airplane factory workers in southern Germany, vagally mediated HRV was inversely related to high-sensitivity CRP in men and premenopausal women, even after controlling for urinary norepinephrine as an index of sympathetic activity.6 Most previous studies in which the relationship between HRV and CRP (or other inflammatory markers) was assessed failed to control for sympathetic nervous system activity. In the total sample and in men, the parasympathetic effect on CRP was comparable with that of smoking; in women, the effect was 4 times larger and comparable with that of high body mass index. A negative association was again found between vagally mediated HRV and white blood cell count.
Inverse relationship between HRV and fibrinogen
In a related report from the same study, vagal modulation of fibrinogen was investigated.7 Fibrinogen is a large glycoprotein that is synthesized by the liver. Plasma fibrinogen is a measure of systemic inflammation crucially involved in atherosclerosis. Meta-analyses have shown a prospective association between elevated plasma fibrinogen levels even in the normal range and an increased risk of coronary artery disease in different populations. We investigated the relationship between nighttime HRV, assessed by root mean square of successive R-R interval differences (RMSSD), and fibrinogen in 559 mostly male workers from southern Germany. Among all workers, there was a mean ± SEM increase of 0.41 ± 0.13 mg/dL fibrinogen for each ms decrease in nighttime RMSSD, even after controlling for established cardiovascular risk factors. The increase in men was 0.28 ± 0.13 mg/dL and, in women, 1.16 ± 0.41 mg/dL for each ms decrease in nighttime RMSSD. Such an autonomic mechanism might contribute to the atherosclerotic process and its thrombotic complications.
Vagal regulation of allostatic systems
Whereas the role of the autonomic nervous system, and the vagus nerve in particular, in the regulation of the cardiovascular system seems clear, the role of the vagus nerve in the regulation of other systems associated with allostasis is less evident. In addition to the regulation of inflammatory markers as discussed thus far, decreased vagal function and HRV have been associated with increased fasting glucose and glycated hemoglobin (HbA1c) levels, and with increased overnight urinary cortisol.8 These factors have been associated with increased allostatic load and poor health. Thus, vagal activity seems to have an inhibitory function in the regulation of allostatic systems. The prefrontal cortex and the amygdala are important central nervous system structures linked to the regulation of these allostatic systems, including inflammation via the vagus nerve. The next section describes evidence for the prefrontal regulation of inflammation.
Prefrontal cortical activity and immune indices
Ohira et al used neuroimaging to explore the association between the brain and immune function.9 Their study examined the neural basis of the top-down modulation accompanying cognitive appraisal during a controllable or uncontrollable acute stressor. HR and blood pressure increased significantly during a mental arithmetic task and returned to baseline soon after termination of the task. HR increased to a greater extent in the controllable versus the uncontrollable condition; blood pressure was unaffected by controllability. Endocrine and immune indices were also affected by the acute stress task: the proportions of natural killer cells increased and helper T cells decreased acutely during the stressor.
Importantly, cerebral blood flow measurements demonstrated that the areas of the prefrontal cortex that we have found to be associated with HRV, including the medial prefrontal cortex and the insula, were also associated with immune indices (medial and lateral orbitofrontal cortices and insula), suggesting prefrontal or frontal modulation of immune responses possibly via the same vagal pathways.
VAGAL ACTIVITY AND CARDIOVASCULAR RISK FACTORS
The regulation of physiologic systems that are important for health and disease has been linked to vagal function and HRV. We have recently reviewed the literature on the relationship between vagal function and the risk for cardiovascular disease and stroke. The National Heart, Lung, and Blood Institute lists eight risk factors for heart disease and stroke.10 Six are considered modifiable. Of the six modifiable factors, three are associated with what could be called biologic factors: high blood pressure (hypertension), diabetes, and abnormal cholesterol; the other three could be considered lifestyle factors: tobacco use (smoking), physical inactivity (exercise), and overweight (obesity). Two factors, age and family history of early heart disease or stroke, are considered nonmodifiable. At least some data suggest that each of these risk factors is associated with decreased vagal function as indexed by HRV.11
Interventions to modify HRV include exercise, ingestion of omega-3 fatty acids, stress reduction (eg, mediation), pharmacologic manipulations, and vagus nerve stimulation, suggesting that methods that increase vagus nerve activity might favorably modify an individual’s risk profile.
CONCLUSION
The brain and the heart are intimately connected. Both epidemiologic and experimental data suggest an association between HRV and inflammation, including similar neural mechanisms. Evidence of an association between HRV and inflammation supports the concept of a cholinergic anti-inflammatory pathway.
- Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 1993; 68:988–1001.
- Ahern GL, Sollers JJ, Lane RD, et al. Heart rate and heart rate variability changes in the intracarotid sodium amobarbital test. Epilepsia 2001; 42:912–921.
- Thayer JF, Sollers JJ, Labiner DM, et al Age-related differences in prefrontal control of heart rate in humans: a pharmacological blockade study [published online ahead of print September 19, 2008] Int J Psychophysioldoi: 10.1016/j.ijpsycho.2008.04.007.
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. Neuroimage 2009; 44:213–222.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Thayer JF, Fischer JE Heart rate variability, overnight urinary norepinephrine, and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults [published online ahead of print November 15, 2008] J Intern Meddoi: 10.1111/j.1365-2796.2008.02023.x.
- von Kanel R, Thayer JF, Fischer JE Night-time vagal cardiac control and plasma fibrinogen levels in a population of working men and women Ann Noninvasive Electrocardiol In press
- Thayer JF, Sternberg EM. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci 2006; 1088:361–372.
- Ohira H, Isowa T, Nomura M, et al. Imaging brain and immune association accompanying cognitive appraisal of an acute stressor. Neuroimage 2008; 39:500–514.
- Your guide to lowering blood pressure: risk factors. National Heart, Lung, and Blood Institute Web site. http://www.nhlbi.nih.gov/hbp/hbp/hdrf.htm. Accessed January 13, 2009.
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol 2007; 74:224–242.
- Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 1993; 68:988–1001.
- Ahern GL, Sollers JJ, Lane RD, et al. Heart rate and heart rate variability changes in the intracarotid sodium amobarbital test. Epilepsia 2001; 42:912–921.
- Thayer JF, Sollers JJ, Labiner DM, et al Age-related differences in prefrontal control of heart rate in humans: a pharmacological blockade study [published online ahead of print September 19, 2008] Int J Psychophysioldoi: 10.1016/j.ijpsycho.2008.04.007.
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. Neuroimage 2009; 44:213–222.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Thayer JF, Fischer JE Heart rate variability, overnight urinary norepinephrine, and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults [published online ahead of print November 15, 2008] J Intern Meddoi: 10.1111/j.1365-2796.2008.02023.x.
- von Kanel R, Thayer JF, Fischer JE Night-time vagal cardiac control and plasma fibrinogen levels in a population of working men and women Ann Noninvasive Electrocardiol In press
- Thayer JF, Sternberg EM. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci 2006; 1088:361–372.
- Ohira H, Isowa T, Nomura M, et al. Imaging brain and immune association accompanying cognitive appraisal of an acute stressor. Neuroimage 2008; 39:500–514.
- Your guide to lowering blood pressure: risk factors. National Heart, Lung, and Blood Institute Web site. http://www.nhlbi.nih.gov/hbp/hbp/hdrf.htm. Accessed January 13, 2009.
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol 2007; 74:224–242.