User login
VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
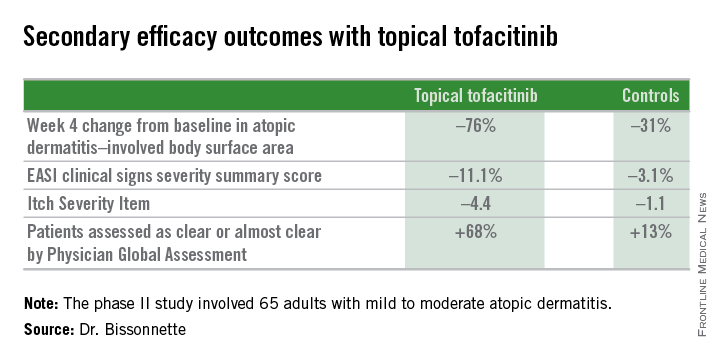
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.
VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
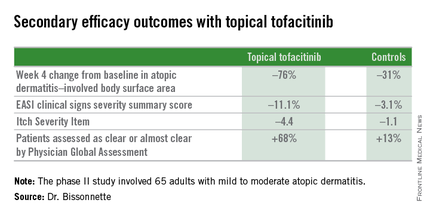
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.

VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.

AT WCD 2015