User login
MILWAUKEE – Despite extensive testing, seemingly innocuous wet wipes have been linked to allergic contact dermatitis in children.
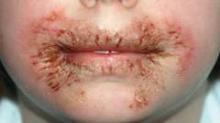
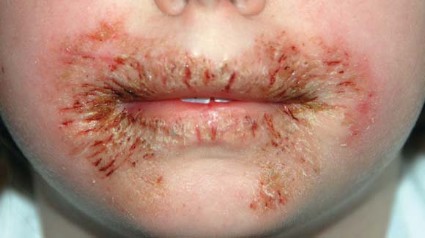
The first reported case occurred in a healthy 8-year-old girl who presented with chronic, recalcitrant erythematous, eczematous patches and plaques with crusting and fissuring around the mouth and perianal area. She had been using wipes containing methylisothiazolinone (MI), without methylchloroisothiazolinone (MCI).
Within 22 months, an additional five children had been diagnosed with allergic contact dermatitis to MI in wet wipes, Dr. Mary Wu Chang and Ms. Radhika Nakrani reported at the annual meeting of the Society for Pediatric Dermatology.
All cases were confirmed on patch testing, and all patients had rapid, complete resolution within about 2 days after discontinuing use of the wipes. Ms. Nakrani also interviewed the parents to determine the type of wipes used, identified as Cottonelle and Huggies brands (both manufactured by Kimberly-Clark). The preservative MI was identified in these brands.
"This is going to explode," Dr. Chang, a pediatric dermatologist at the University of Connecticut, West Hartford, said in an interview. "There’s more marketing [of wipes] now for non–diaper wearing children, and older people use wipes out of convenience."
The case series was described as the first report of pediatric allergic contact dermatitis to MI in wet wipes in the United States.
Although the MCI/MI patch test (T.R.U.E. Test) detected contact allergy to MI in the six children, identifying MI contact sensitization may require specialized MI patch tests with higher concentrations of MI or the use of small squares of the wipes themselves, Dr. Chang observed. Patch testing containing 100 ppm of MCI/MI mixture consists of only 25 ppm of MI and may be inadequate to detect MI alone, missing 33%-60% of cases.
Acute contact dermatitis was suspected in the index case, when the 8-year-old girl presented to pediatric dermatology after suffering for 11 months. She was initially misdiagnosed with impetiginized eczema and had received numerous oral and topical corticosteroids and antibiotics, said Dr. Chang. Questioning revealed that the patient used wet wipes for toileting and facial cleansing, and the rash resolved after the wipes were discontinued. MI sensitivity was confirmed on patch testing. Ms. Nakrani also interviewed the parents to determine the type of wipes used, and the preservative MI was identified in these brands.
"Remember to ask about wet wipe use, even in individuals who do not wear diapers and even if the rash in on the face," Dr. Chang said.
In the five other cases, allergic reactions to MI in the wet wipes were misdiagnosed as impetigo, psoriasis, diaper dermatitis, or atopic dermatitis, and unsuccessfully treated with a multitude of medications including steroids, antifungals, and topical tacrolimus (Protopic). The children, aged 3-8 years, had experienced symptoms for 1-12 months, according to the authors, who earned top honors for their poster presentation at the meeting.
The index patient also suffered from chronic retroauricular dermatitis, which resolved after discontinuing a shampoo containing MI.
MI was named the 2013 contact allergen of the year by the American Contact Dermatitis Society. In another recent study, Australian researchers reported 23 reactions to MI from a variety of personal care products, including 7 cases of hand dermatitis in parents of young children that was caused by an allergic reaction to MI in baby wipes (Australas. J. Dermatol. 2013 May 29 [doi: 10.1111/ajd.12062]).
The preservative MI was previously used only in a 3:1 MCI/MI combination (Kathon CG) that is widely known to cause allergic contact dermatitis. In an attempt to minimize such reactions, MI has been used alone, explained Ms. Nakrani, a medical student at the University of Connecticut.
However, its permitted concentration has increased by more than 25 times – from 3.7 ppm to 100 ppm – because it was thought to be a weaker sensitizer than MCI, she said.
"Once we found out the culprit was MI in the wet wipes and instructed parents to read labels and avoid MI, parents became more vigilant about the other products they were using like shampoos and soaps, and it really turned things right around once they stopped using these products," Ms. Nakrani said. "One mom, very grateful that the rashes finally cleared, said her child was going to school and everyone thought it was contagious."
The investigators have not contacted Kimberly-Clark regarding their findings. Bob Brand, director of external communications for Kimberly-Clark, would not specifically say whether they had received complaints of allergic contact dermatitis to MI in their wet wipes, but said all of their products undergo a thorough safety review prior to commercialization.
"While we understand there might be concern regarding a potential reaction to one of our products, consumers can use our wipes with confidence and know that the concentration levels of MI in our products are considered safe and well within the recommended levels as established by scientific and regulatory bodies such as the Cosmetic Ingredient Review Expert Panel in the U.S.A. and the European Commission Scientific Committee on Consumer Safety," he told Skin & Allergy News.
The formulation of the wipes appears to vary by product. Ms. Dianna Kenneally, principal scientist, baby care scientific communication, for Procter & Gamble, said in a separate interview that Procter & Gamble baby wipe brands do not contain MI.
Dr. Chang and Ms. Nakrani reported no relevant disclosures.
MILWAUKEE – Despite extensive testing, seemingly innocuous wet wipes have been linked to allergic contact dermatitis in children.
The first reported case occurred in a healthy 8-year-old girl who presented with chronic, recalcitrant erythematous, eczematous patches and plaques with crusting and fissuring around the mouth and perianal area. She had been using wipes containing methylisothiazolinone (MI), without methylchloroisothiazolinone (MCI).
Within 22 months, an additional five children had been diagnosed with allergic contact dermatitis to MI in wet wipes, Dr. Mary Wu Chang and Ms. Radhika Nakrani reported at the annual meeting of the Society for Pediatric Dermatology.
All cases were confirmed on patch testing, and all patients had rapid, complete resolution within about 2 days after discontinuing use of the wipes. Ms. Nakrani also interviewed the parents to determine the type of wipes used, identified as Cottonelle and Huggies brands (both manufactured by Kimberly-Clark). The preservative MI was identified in these brands.
"This is going to explode," Dr. Chang, a pediatric dermatologist at the University of Connecticut, West Hartford, said in an interview. "There’s more marketing [of wipes] now for non–diaper wearing children, and older people use wipes out of convenience."
The case series was described as the first report of pediatric allergic contact dermatitis to MI in wet wipes in the United States.
Although the MCI/MI patch test (T.R.U.E. Test) detected contact allergy to MI in the six children, identifying MI contact sensitization may require specialized MI patch tests with higher concentrations of MI or the use of small squares of the wipes themselves, Dr. Chang observed. Patch testing containing 100 ppm of MCI/MI mixture consists of only 25 ppm of MI and may be inadequate to detect MI alone, missing 33%-60% of cases.
Acute contact dermatitis was suspected in the index case, when the 8-year-old girl presented to pediatric dermatology after suffering for 11 months. She was initially misdiagnosed with impetiginized eczema and had received numerous oral and topical corticosteroids and antibiotics, said Dr. Chang. Questioning revealed that the patient used wet wipes for toileting and facial cleansing, and the rash resolved after the wipes were discontinued. MI sensitivity was confirmed on patch testing. Ms. Nakrani also interviewed the parents to determine the type of wipes used, and the preservative MI was identified in these brands.
"Remember to ask about wet wipe use, even in individuals who do not wear diapers and even if the rash in on the face," Dr. Chang said.
In the five other cases, allergic reactions to MI in the wet wipes were misdiagnosed as impetigo, psoriasis, diaper dermatitis, or atopic dermatitis, and unsuccessfully treated with a multitude of medications including steroids, antifungals, and topical tacrolimus (Protopic). The children, aged 3-8 years, had experienced symptoms for 1-12 months, according to the authors, who earned top honors for their poster presentation at the meeting.
The index patient also suffered from chronic retroauricular dermatitis, which resolved after discontinuing a shampoo containing MI.
MI was named the 2013 contact allergen of the year by the American Contact Dermatitis Society. In another recent study, Australian researchers reported 23 reactions to MI from a variety of personal care products, including 7 cases of hand dermatitis in parents of young children that was caused by an allergic reaction to MI in baby wipes (Australas. J. Dermatol. 2013 May 29 [doi: 10.1111/ajd.12062]).
The preservative MI was previously used only in a 3:1 MCI/MI combination (Kathon CG) that is widely known to cause allergic contact dermatitis. In an attempt to minimize such reactions, MI has been used alone, explained Ms. Nakrani, a medical student at the University of Connecticut.
However, its permitted concentration has increased by more than 25 times – from 3.7 ppm to 100 ppm – because it was thought to be a weaker sensitizer than MCI, she said.
"Once we found out the culprit was MI in the wet wipes and instructed parents to read labels and avoid MI, parents became more vigilant about the other products they were using like shampoos and soaps, and it really turned things right around once they stopped using these products," Ms. Nakrani said. "One mom, very grateful that the rashes finally cleared, said her child was going to school and everyone thought it was contagious."
The investigators have not contacted Kimberly-Clark regarding their findings. Bob Brand, director of external communications for Kimberly-Clark, would not specifically say whether they had received complaints of allergic contact dermatitis to MI in their wet wipes, but said all of their products undergo a thorough safety review prior to commercialization.
"While we understand there might be concern regarding a potential reaction to one of our products, consumers can use our wipes with confidence and know that the concentration levels of MI in our products are considered safe and well within the recommended levels as established by scientific and regulatory bodies such as the Cosmetic Ingredient Review Expert Panel in the U.S.A. and the European Commission Scientific Committee on Consumer Safety," he told Skin & Allergy News.
The formulation of the wipes appears to vary by product. Ms. Dianna Kenneally, principal scientist, baby care scientific communication, for Procter & Gamble, said in a separate interview that Procter & Gamble baby wipe brands do not contain MI.
Dr. Chang and Ms. Nakrani reported no relevant disclosures.
MILWAUKEE – Despite extensive testing, seemingly innocuous wet wipes have been linked to allergic contact dermatitis in children.
The first reported case occurred in a healthy 8-year-old girl who presented with chronic, recalcitrant erythematous, eczematous patches and plaques with crusting and fissuring around the mouth and perianal area. She had been using wipes containing methylisothiazolinone (MI), without methylchloroisothiazolinone (MCI).
Within 22 months, an additional five children had been diagnosed with allergic contact dermatitis to MI in wet wipes, Dr. Mary Wu Chang and Ms. Radhika Nakrani reported at the annual meeting of the Society for Pediatric Dermatology.
All cases were confirmed on patch testing, and all patients had rapid, complete resolution within about 2 days after discontinuing use of the wipes. Ms. Nakrani also interviewed the parents to determine the type of wipes used, identified as Cottonelle and Huggies brands (both manufactured by Kimberly-Clark). The preservative MI was identified in these brands.
"This is going to explode," Dr. Chang, a pediatric dermatologist at the University of Connecticut, West Hartford, said in an interview. "There’s more marketing [of wipes] now for non–diaper wearing children, and older people use wipes out of convenience."
The case series was described as the first report of pediatric allergic contact dermatitis to MI in wet wipes in the United States.
Although the MCI/MI patch test (T.R.U.E. Test) detected contact allergy to MI in the six children, identifying MI contact sensitization may require specialized MI patch tests with higher concentrations of MI or the use of small squares of the wipes themselves, Dr. Chang observed. Patch testing containing 100 ppm of MCI/MI mixture consists of only 25 ppm of MI and may be inadequate to detect MI alone, missing 33%-60% of cases.
Acute contact dermatitis was suspected in the index case, when the 8-year-old girl presented to pediatric dermatology after suffering for 11 months. She was initially misdiagnosed with impetiginized eczema and had received numerous oral and topical corticosteroids and antibiotics, said Dr. Chang. Questioning revealed that the patient used wet wipes for toileting and facial cleansing, and the rash resolved after the wipes were discontinued. MI sensitivity was confirmed on patch testing. Ms. Nakrani also interviewed the parents to determine the type of wipes used, and the preservative MI was identified in these brands.
"Remember to ask about wet wipe use, even in individuals who do not wear diapers and even if the rash in on the face," Dr. Chang said.
In the five other cases, allergic reactions to MI in the wet wipes were misdiagnosed as impetigo, psoriasis, diaper dermatitis, or atopic dermatitis, and unsuccessfully treated with a multitude of medications including steroids, antifungals, and topical tacrolimus (Protopic). The children, aged 3-8 years, had experienced symptoms for 1-12 months, according to the authors, who earned top honors for their poster presentation at the meeting.
The index patient also suffered from chronic retroauricular dermatitis, which resolved after discontinuing a shampoo containing MI.
MI was named the 2013 contact allergen of the year by the American Contact Dermatitis Society. In another recent study, Australian researchers reported 23 reactions to MI from a variety of personal care products, including 7 cases of hand dermatitis in parents of young children that was caused by an allergic reaction to MI in baby wipes (Australas. J. Dermatol. 2013 May 29 [doi: 10.1111/ajd.12062]).
The preservative MI was previously used only in a 3:1 MCI/MI combination (Kathon CG) that is widely known to cause allergic contact dermatitis. In an attempt to minimize such reactions, MI has been used alone, explained Ms. Nakrani, a medical student at the University of Connecticut.
However, its permitted concentration has increased by more than 25 times – from 3.7 ppm to 100 ppm – because it was thought to be a weaker sensitizer than MCI, she said.
"Once we found out the culprit was MI in the wet wipes and instructed parents to read labels and avoid MI, parents became more vigilant about the other products they were using like shampoos and soaps, and it really turned things right around once they stopped using these products," Ms. Nakrani said. "One mom, very grateful that the rashes finally cleared, said her child was going to school and everyone thought it was contagious."
The investigators have not contacted Kimberly-Clark regarding their findings. Bob Brand, director of external communications for Kimberly-Clark, would not specifically say whether they had received complaints of allergic contact dermatitis to MI in their wet wipes, but said all of their products undergo a thorough safety review prior to commercialization.
"While we understand there might be concern regarding a potential reaction to one of our products, consumers can use our wipes with confidence and know that the concentration levels of MI in our products are considered safe and well within the recommended levels as established by scientific and regulatory bodies such as the Cosmetic Ingredient Review Expert Panel in the U.S.A. and the European Commission Scientific Committee on Consumer Safety," he told Skin & Allergy News.
The formulation of the wipes appears to vary by product. Ms. Dianna Kenneally, principal scientist, baby care scientific communication, for Procter & Gamble, said in a separate interview that Procter & Gamble baby wipe brands do not contain MI.
Dr. Chang and Ms. Nakrani reported no relevant disclosures.
AT THE SPD ANNUAL MEETING
Major finding: Six cases of pediatric allergic contact dermatitis to methylisothiazolinone in wet wipes were confirmed on patch testing, and resolved within 1-2 days after discontinuing the wipes.
Data source: A case series of six children with allergic contact dermatitis to methylisothiazolinone.
Disclosures: Dr. Chang and Ms. Nakrani reported no relevant disclosures.