User login
Case
While placing a central line, you sustain a needlestick. You’ve washed the area thoroughly with soap and water, but you are concerned about contracting a bloodborne pathogen. What is the risk of contracting such a pathogen, and what can be done to reduce this risk?
Overview
Needlestick injuries are a common occupational hazard in the hospital setting. According to the International Health Care Worker Safety Center (IHCWSC), approximately 295,000 hospital-based healthcare workers experience occupational percutaneous injuries annually. In 1991, Mangione et al surveyed internal-medicine house staff and found an annual incidence of 674 needlestick injuries per 1,000 participants.1 Other retrospective data estimate this risk to be as high as 839 per 1,000 healthcare workers annually.2 Evidence from the Centers for Disease Control and Prevention (CDC) in 2004 suggests that because these are only self-reported injuries, the annual incidence of such injuries is in fact much higher than the current estimates suggest.2,3,4
More than 20 bloodborne pathogens (see Table 1, right) might be transmitted from contaminated needles or sharps, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). A quick and appropriate response to a needlestick injury can greatly decrease the risk of disease transmission following an occupational exposure to potentially infectious materials.
Review of the Data
After any needlestick injury, an affected healthcare worker should wash the area with soap and water immediately. There is no contraindication to using antiseptic solutions, but there is also no evidence to suggest that this reduces the rates of disease transmission.
As decisions for post-exposure prophylaxis often need to be made within hours, a healthcare worker should seek care in the facility areas responsible for managing occupational exposures. Healthcare providers should always be encouraged and supported to report all sharps-related injuries to such departments.
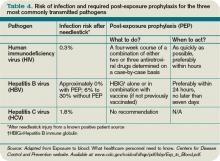
The source patient should be identified and evaluated for potentially transmissible diseases, including HIV, HBV, and HCV. If indicated, the source patient should then undergo appropriate serological testing, and any indicated antiviral prophylaxis should be initiated (see Table 2, p. 19).
Risk of Seroconversion
For all bloodborne pathogens, a needlestick injury carries a greater risk for transmission than other occupational exposures (e.g. mucous membrane exposure). If a needlestick injury occurs in the setting of an infected patient source, the risk of disease transmission varies for HIV, HBV, and HCV (see Table 3, p. 19). In general, risk for seroconversion is increased with a deep injury, an injury with a device visibly contaminated with the source patient’s blood, or an injury involving a needle placed in the source patient’s artery or vein.3,5,6
Human immunodeficiency virus. Contracting HIV after needlestick injury is rare. From 1981 to 2006, the CDC documented only 57 cases of HIV/AIDS in healthcare workers following occupational exposure and identified an additional “possible” 140 cases post-exposure.5,6 Of the 57 documented cases, 48 sustained a percutaneous injury.
Following needlestick injury involving a known HIV-positive source, the one-year risk of seroconversion has been estimated to be 0.3%.5,6 In 1997, Cardo and colleagues identified four factors associated with increased risk for seroconversion after a needlestick/sharps injury from a known positive-HIV source:
- Deep injury;
- Injury with a device visibly contaminated with the source patient’s blood;
- A procedure involving a needle placed in the source patient’s artery or vein; and
- Exposure to a source patient who died of AIDS in the two months following the occupational exposure.5
Hepatitis B virus. Wides-pread immunization of healthcare workers has led to a dramatic decline in occupationally acquired HBV. The CDC estimated that in 1985, approximately 12,500 new HBV infections occurred in healthcare workers.3 This estimate plummeted to approximately 500 new occupationally acquired HBV infections in 1997.3
Despite this, hospital-based healthcare personnel remain at risk for HBV transmission after a needlestick injury from a known positive patient source. Few studies have evaluated the occupational risk of HBV transmission after a needlestick injury. Buergler et al reported that following a needlestick injury involving a known HBV-positive source, the one-year risk of seroconversion was 0.76% to 7.35% for nonimmunized surgeons, and 0.23% to 2.28% for nonimmunized anesthesiologists.7
In the absence of post-exposure prophylaxis, an exposed healthcare worker has a 6% to 30% risk of becoming infected with HBV.3,8 The risk is greatest if the patient source is known to be hepatitis B e antigen-positive, a marker for greater disease infectivity. When given within one week of injury, post-exposure prophylaxis (PEP) with multiple doses of hepatitis B immune globulin (HBIG) provides an estimated 75% protection from transmission.
Healthcare workers who have received the hepatitis B vaccine and developed immunity have virtually no risk for infection.6,7
Hepatitis C virus. Prospective evaluation has demonstrated that the average risk of HCV transmission after percutaneous exposure to a known HCV-positive source is from 0% to 7%.3 The Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections evaluated HCV seroconversion within six months of a reported exposure with enzyme immunoassay and immunoblot assay. In this study, the authors found a seroconversion rate of 1.2%.9
Further, they suggested that HCV seroconversion only occurred from hollow-bore needles, as no seroconversions were noted in healthcare workers who sustained injuries with solid sharp objects.
Post-Exposure Management
The CDC does not recommend prophylaxis when source fluids make contact with intact skin. However, if a percutaneous occupational exposure has occurred, PEPs exist for HIV and HBV but not for HCV.3,6 If a source patient’s HIV, HBV, and HCV statuses are unknown, occupational-health personnel can interview the patient to evaluate his or her risks and initiate testing. Specific information about the time and nature of exposure should be documented.
When testing is indicated, it should be done following institutional and state-specific exposure-control policies and informed consent guidelines. In all situations, the decision to begin antiviral PEP should be carefully considered, weighing benefits of PEP versus the risks and toxicity of treatment.
Human immunodeficiency virus. If a source patient is known to be HIV-positive, has a positive rapid HIV test, or if HIV status cannot be quickly determined, PEP is indicated. Healthcare providers should be aware of rare cases in which the source patient initially tested HIV-seronegative but was subsequently found to have primary HIV infection.
Per 2004 CDC recommendations, PEP is indicated for all healthcare workers who sustain a percuanteous injury from a known HIV-positive source.3,8 For a less severe injury (e.g. solid needle or superficial injury), PEP with either a basic two-drug or three-drug regimen is indicated, depending on the source patient’s viral load.3,5,6,8
If the source patient has unknown HIV status, two-drug PEP is indicated based on the source patient’s HIV risk factors. In such patients, rapid HIV testing also is indicated to aid in determining the need for PEP. When the source HIV status is unknown, PEP is indicated in settings where exposure to HIV-infected persons is likely.
If PEP is indicated, it should be started as quickly as possible. The 2005 U.S. Public Health Service Recommendations for PEP recommend initiating two nucleosides for low-risk exposures and two nucleosides plus a boosted protease inhibitor for high-risk exposures.
Examples of commonly used dual nucleoside regimens are Zidovudine plus Lamivudine (coformulated as Combivir) or Tenofovir plus Emtricitabine (coformulated as Truvada). Current recommendations indicate that PEP should be continued for four weeks, with concurrent clinical and laboratory evaluation for drug toxicity.
Hepatitis B virus. Numerous prospective studies have evaluated the post-exposure effectiveness of HBIG. When administered within 24 hours of exposure, HBIG might offer immediate passive protection against HBV infection. Additionally, if initiated within one week of percutaneous injury with a known HBV-positive source, multiple doses of HGIB provide an estimated 75% protection from transmission.
Although the combination of HBIG and the hepatitis vaccine B series has not been evaluated as PEP in the occupational setting, evidence in the perinatal setting suggests this regimen is more effective than HBIG alone.3,6,8
Hepatitis C virus. No PEP exists for HCV, and current recommendations for post-exposure management focus on early identification and treatment of chronic disease. There are insufficient data for a treatment recommendation for patients with acute HCV infection with no evidence of disease; the appropriate dosing of such a regimen is unknown. Further, evidence suggests that treatment started early in the course of chronic infection could be just as effective and might eliminate the need to treat persons whose infection will spontaneously resolve.7
Back to the Case
Your needlestick occurred while using a hollow-bore needle to cannulate a source patient’s vein, placing you at higher risk for seroconversion. You immediately reported the exposure to the department of occupational health at your hospital. The source patient’s HIV, HBV, and HCV serological statuses were tested, and the patient was found to be HBV-positive. After appropriate counseling, you decide to receive HGIB prophylaxis to reduce your chances of becoming infected with HBV infection.
Bottom Line
Healthcare workers who suffer occupational needlestick injuries require immediate identification and attention to avoid transmission of such infectious diseases as HIV, HBV, and HCV. Source patients should undergo rapid serological testing to determine appropriate PEP. TH
Dr. Zehnder is a hospitalist in the Section of Hospital Medicine at the University of Colorado Denver.
References
- Mangione CM, Gerberding JL, Cummings, SR. Occupational exposure to HIV: Frequency and rates of underreporting of percutaneous and mucocutaneous exposures by medical housestaff. Am J Med. 1991;90(1):85-90.
- Lee JM, Botteman MF, Nicklasson L, et al. Needlestick injury in acute care nurses caring for patients with diabetes mellitus: a retrospective study. Curr Med Res Opinion. 2005;21(5):741-747.
- Workbook for designing, implementing, and evaluating a sharps injury prevention program. Centers for Disease Control and Prevention website. Available at: www.cdc.gov/sharpssafety/pdf/WorkbookComplete.pdf. Accessed Sept. 13, 2010.
- Lee JM, Botteman MF, Xanthakos N, Nicklasson L. Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. AAOHN J. 2005;53(3):117-133.
- Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485-1490.
- Exposure to blood: What healthcare personnel need to know. CDC website. Available at: www.cdc.gov/ncidod /dhqp/pdf/bbp/Exp_to_Blood.pdf. Accessed Aug. 31, 2010.
- Buergler JM, Kim R, Thisted RA, Cohn SJ, Lichtor JL, Roizen MF. Risk of human immunodeficiency virus in surgeons, anesthesiologists, and medical students. Anesth Analg. 1992;75(1):118-124.
- Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. CDC website. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm. Accessed Aug. 31, 2010.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposure in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23(5):273-277.
Case
While placing a central line, you sustain a needlestick. You’ve washed the area thoroughly with soap and water, but you are concerned about contracting a bloodborne pathogen. What is the risk of contracting such a pathogen, and what can be done to reduce this risk?
Overview
Needlestick injuries are a common occupational hazard in the hospital setting. According to the International Health Care Worker Safety Center (IHCWSC), approximately 295,000 hospital-based healthcare workers experience occupational percutaneous injuries annually. In 1991, Mangione et al surveyed internal-medicine house staff and found an annual incidence of 674 needlestick injuries per 1,000 participants.1 Other retrospective data estimate this risk to be as high as 839 per 1,000 healthcare workers annually.2 Evidence from the Centers for Disease Control and Prevention (CDC) in 2004 suggests that because these are only self-reported injuries, the annual incidence of such injuries is in fact much higher than the current estimates suggest.2,3,4
More than 20 bloodborne pathogens (see Table 1, right) might be transmitted from contaminated needles or sharps, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). A quick and appropriate response to a needlestick injury can greatly decrease the risk of disease transmission following an occupational exposure to potentially infectious materials.
Review of the Data
After any needlestick injury, an affected healthcare worker should wash the area with soap and water immediately. There is no contraindication to using antiseptic solutions, but there is also no evidence to suggest that this reduces the rates of disease transmission.
As decisions for post-exposure prophylaxis often need to be made within hours, a healthcare worker should seek care in the facility areas responsible for managing occupational exposures. Healthcare providers should always be encouraged and supported to report all sharps-related injuries to such departments.
The source patient should be identified and evaluated for potentially transmissible diseases, including HIV, HBV, and HCV. If indicated, the source patient should then undergo appropriate serological testing, and any indicated antiviral prophylaxis should be initiated (see Table 2, p. 19).
Risk of Seroconversion
For all bloodborne pathogens, a needlestick injury carries a greater risk for transmission than other occupational exposures (e.g. mucous membrane exposure). If a needlestick injury occurs in the setting of an infected patient source, the risk of disease transmission varies for HIV, HBV, and HCV (see Table 3, p. 19). In general, risk for seroconversion is increased with a deep injury, an injury with a device visibly contaminated with the source patient’s blood, or an injury involving a needle placed in the source patient’s artery or vein.3,5,6
Human immunodeficiency virus. Contracting HIV after needlestick injury is rare. From 1981 to 2006, the CDC documented only 57 cases of HIV/AIDS in healthcare workers following occupational exposure and identified an additional “possible” 140 cases post-exposure.5,6 Of the 57 documented cases, 48 sustained a percutaneous injury.
Following needlestick injury involving a known HIV-positive source, the one-year risk of seroconversion has been estimated to be 0.3%.5,6 In 1997, Cardo and colleagues identified four factors associated with increased risk for seroconversion after a needlestick/sharps injury from a known positive-HIV source:
- Deep injury;
- Injury with a device visibly contaminated with the source patient’s blood;
- A procedure involving a needle placed in the source patient’s artery or vein; and
- Exposure to a source patient who died of AIDS in the two months following the occupational exposure.5
Hepatitis B virus. Wides-pread immunization of healthcare workers has led to a dramatic decline in occupationally acquired HBV. The CDC estimated that in 1985, approximately 12,500 new HBV infections occurred in healthcare workers.3 This estimate plummeted to approximately 500 new occupationally acquired HBV infections in 1997.3
Despite this, hospital-based healthcare personnel remain at risk for HBV transmission after a needlestick injury from a known positive patient source. Few studies have evaluated the occupational risk of HBV transmission after a needlestick injury. Buergler et al reported that following a needlestick injury involving a known HBV-positive source, the one-year risk of seroconversion was 0.76% to 7.35% for nonimmunized surgeons, and 0.23% to 2.28% for nonimmunized anesthesiologists.7
In the absence of post-exposure prophylaxis, an exposed healthcare worker has a 6% to 30% risk of becoming infected with HBV.3,8 The risk is greatest if the patient source is known to be hepatitis B e antigen-positive, a marker for greater disease infectivity. When given within one week of injury, post-exposure prophylaxis (PEP) with multiple doses of hepatitis B immune globulin (HBIG) provides an estimated 75% protection from transmission.
Healthcare workers who have received the hepatitis B vaccine and developed immunity have virtually no risk for infection.6,7
Hepatitis C virus. Prospective evaluation has demonstrated that the average risk of HCV transmission after percutaneous exposure to a known HCV-positive source is from 0% to 7%.3 The Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections evaluated HCV seroconversion within six months of a reported exposure with enzyme immunoassay and immunoblot assay. In this study, the authors found a seroconversion rate of 1.2%.9
Further, they suggested that HCV seroconversion only occurred from hollow-bore needles, as no seroconversions were noted in healthcare workers who sustained injuries with solid sharp objects.
Post-Exposure Management
The CDC does not recommend prophylaxis when source fluids make contact with intact skin. However, if a percutaneous occupational exposure has occurred, PEPs exist for HIV and HBV but not for HCV.3,6 If a source patient’s HIV, HBV, and HCV statuses are unknown, occupational-health personnel can interview the patient to evaluate his or her risks and initiate testing. Specific information about the time and nature of exposure should be documented.
When testing is indicated, it should be done following institutional and state-specific exposure-control policies and informed consent guidelines. In all situations, the decision to begin antiviral PEP should be carefully considered, weighing benefits of PEP versus the risks and toxicity of treatment.
Human immunodeficiency virus. If a source patient is known to be HIV-positive, has a positive rapid HIV test, or if HIV status cannot be quickly determined, PEP is indicated. Healthcare providers should be aware of rare cases in which the source patient initially tested HIV-seronegative but was subsequently found to have primary HIV infection.
Per 2004 CDC recommendations, PEP is indicated for all healthcare workers who sustain a percuanteous injury from a known HIV-positive source.3,8 For a less severe injury (e.g. solid needle or superficial injury), PEP with either a basic two-drug or three-drug regimen is indicated, depending on the source patient’s viral load.3,5,6,8
If the source patient has unknown HIV status, two-drug PEP is indicated based on the source patient’s HIV risk factors. In such patients, rapid HIV testing also is indicated to aid in determining the need for PEP. When the source HIV status is unknown, PEP is indicated in settings where exposure to HIV-infected persons is likely.
If PEP is indicated, it should be started as quickly as possible. The 2005 U.S. Public Health Service Recommendations for PEP recommend initiating two nucleosides for low-risk exposures and two nucleosides plus a boosted protease inhibitor for high-risk exposures.
Examples of commonly used dual nucleoside regimens are Zidovudine plus Lamivudine (coformulated as Combivir) or Tenofovir plus Emtricitabine (coformulated as Truvada). Current recommendations indicate that PEP should be continued for four weeks, with concurrent clinical and laboratory evaluation for drug toxicity.
Hepatitis B virus. Numerous prospective studies have evaluated the post-exposure effectiveness of HBIG. When administered within 24 hours of exposure, HBIG might offer immediate passive protection against HBV infection. Additionally, if initiated within one week of percutaneous injury with a known HBV-positive source, multiple doses of HGIB provide an estimated 75% protection from transmission.
Although the combination of HBIG and the hepatitis vaccine B series has not been evaluated as PEP in the occupational setting, evidence in the perinatal setting suggests this regimen is more effective than HBIG alone.3,6,8
Hepatitis C virus. No PEP exists for HCV, and current recommendations for post-exposure management focus on early identification and treatment of chronic disease. There are insufficient data for a treatment recommendation for patients with acute HCV infection with no evidence of disease; the appropriate dosing of such a regimen is unknown. Further, evidence suggests that treatment started early in the course of chronic infection could be just as effective and might eliminate the need to treat persons whose infection will spontaneously resolve.7
Back to the Case
Your needlestick occurred while using a hollow-bore needle to cannulate a source patient’s vein, placing you at higher risk for seroconversion. You immediately reported the exposure to the department of occupational health at your hospital. The source patient’s HIV, HBV, and HCV serological statuses were tested, and the patient was found to be HBV-positive. After appropriate counseling, you decide to receive HGIB prophylaxis to reduce your chances of becoming infected with HBV infection.
Bottom Line
Healthcare workers who suffer occupational needlestick injuries require immediate identification and attention to avoid transmission of such infectious diseases as HIV, HBV, and HCV. Source patients should undergo rapid serological testing to determine appropriate PEP. TH
Dr. Zehnder is a hospitalist in the Section of Hospital Medicine at the University of Colorado Denver.
References
- Mangione CM, Gerberding JL, Cummings, SR. Occupational exposure to HIV: Frequency and rates of underreporting of percutaneous and mucocutaneous exposures by medical housestaff. Am J Med. 1991;90(1):85-90.
- Lee JM, Botteman MF, Nicklasson L, et al. Needlestick injury in acute care nurses caring for patients with diabetes mellitus: a retrospective study. Curr Med Res Opinion. 2005;21(5):741-747.
- Workbook for designing, implementing, and evaluating a sharps injury prevention program. Centers for Disease Control and Prevention website. Available at: www.cdc.gov/sharpssafety/pdf/WorkbookComplete.pdf. Accessed Sept. 13, 2010.
- Lee JM, Botteman MF, Xanthakos N, Nicklasson L. Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. AAOHN J. 2005;53(3):117-133.
- Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485-1490.
- Exposure to blood: What healthcare personnel need to know. CDC website. Available at: www.cdc.gov/ncidod /dhqp/pdf/bbp/Exp_to_Blood.pdf. Accessed Aug. 31, 2010.
- Buergler JM, Kim R, Thisted RA, Cohn SJ, Lichtor JL, Roizen MF. Risk of human immunodeficiency virus in surgeons, anesthesiologists, and medical students. Anesth Analg. 1992;75(1):118-124.
- Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. CDC website. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm. Accessed Aug. 31, 2010.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposure in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23(5):273-277.
Case
While placing a central line, you sustain a needlestick. You’ve washed the area thoroughly with soap and water, but you are concerned about contracting a bloodborne pathogen. What is the risk of contracting such a pathogen, and what can be done to reduce this risk?
Overview
Needlestick injuries are a common occupational hazard in the hospital setting. According to the International Health Care Worker Safety Center (IHCWSC), approximately 295,000 hospital-based healthcare workers experience occupational percutaneous injuries annually. In 1991, Mangione et al surveyed internal-medicine house staff and found an annual incidence of 674 needlestick injuries per 1,000 participants.1 Other retrospective data estimate this risk to be as high as 839 per 1,000 healthcare workers annually.2 Evidence from the Centers for Disease Control and Prevention (CDC) in 2004 suggests that because these are only self-reported injuries, the annual incidence of such injuries is in fact much higher than the current estimates suggest.2,3,4
More than 20 bloodborne pathogens (see Table 1, right) might be transmitted from contaminated needles or sharps, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). A quick and appropriate response to a needlestick injury can greatly decrease the risk of disease transmission following an occupational exposure to potentially infectious materials.
Review of the Data
After any needlestick injury, an affected healthcare worker should wash the area with soap and water immediately. There is no contraindication to using antiseptic solutions, but there is also no evidence to suggest that this reduces the rates of disease transmission.
As decisions for post-exposure prophylaxis often need to be made within hours, a healthcare worker should seek care in the facility areas responsible for managing occupational exposures. Healthcare providers should always be encouraged and supported to report all sharps-related injuries to such departments.
The source patient should be identified and evaluated for potentially transmissible diseases, including HIV, HBV, and HCV. If indicated, the source patient should then undergo appropriate serological testing, and any indicated antiviral prophylaxis should be initiated (see Table 2, p. 19).
Risk of Seroconversion
For all bloodborne pathogens, a needlestick injury carries a greater risk for transmission than other occupational exposures (e.g. mucous membrane exposure). If a needlestick injury occurs in the setting of an infected patient source, the risk of disease transmission varies for HIV, HBV, and HCV (see Table 3, p. 19). In general, risk for seroconversion is increased with a deep injury, an injury with a device visibly contaminated with the source patient’s blood, or an injury involving a needle placed in the source patient’s artery or vein.3,5,6
Human immunodeficiency virus. Contracting HIV after needlestick injury is rare. From 1981 to 2006, the CDC documented only 57 cases of HIV/AIDS in healthcare workers following occupational exposure and identified an additional “possible” 140 cases post-exposure.5,6 Of the 57 documented cases, 48 sustained a percutaneous injury.
Following needlestick injury involving a known HIV-positive source, the one-year risk of seroconversion has been estimated to be 0.3%.5,6 In 1997, Cardo and colleagues identified four factors associated with increased risk for seroconversion after a needlestick/sharps injury from a known positive-HIV source:
- Deep injury;
- Injury with a device visibly contaminated with the source patient’s blood;
- A procedure involving a needle placed in the source patient’s artery or vein; and
- Exposure to a source patient who died of AIDS in the two months following the occupational exposure.5
Hepatitis B virus. Wides-pread immunization of healthcare workers has led to a dramatic decline in occupationally acquired HBV. The CDC estimated that in 1985, approximately 12,500 new HBV infections occurred in healthcare workers.3 This estimate plummeted to approximately 500 new occupationally acquired HBV infections in 1997.3
Despite this, hospital-based healthcare personnel remain at risk for HBV transmission after a needlestick injury from a known positive patient source. Few studies have evaluated the occupational risk of HBV transmission after a needlestick injury. Buergler et al reported that following a needlestick injury involving a known HBV-positive source, the one-year risk of seroconversion was 0.76% to 7.35% for nonimmunized surgeons, and 0.23% to 2.28% for nonimmunized anesthesiologists.7
In the absence of post-exposure prophylaxis, an exposed healthcare worker has a 6% to 30% risk of becoming infected with HBV.3,8 The risk is greatest if the patient source is known to be hepatitis B e antigen-positive, a marker for greater disease infectivity. When given within one week of injury, post-exposure prophylaxis (PEP) with multiple doses of hepatitis B immune globulin (HBIG) provides an estimated 75% protection from transmission.
Healthcare workers who have received the hepatitis B vaccine and developed immunity have virtually no risk for infection.6,7
Hepatitis C virus. Prospective evaluation has demonstrated that the average risk of HCV transmission after percutaneous exposure to a known HCV-positive source is from 0% to 7%.3 The Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections evaluated HCV seroconversion within six months of a reported exposure with enzyme immunoassay and immunoblot assay. In this study, the authors found a seroconversion rate of 1.2%.9
Further, they suggested that HCV seroconversion only occurred from hollow-bore needles, as no seroconversions were noted in healthcare workers who sustained injuries with solid sharp objects.
Post-Exposure Management
The CDC does not recommend prophylaxis when source fluids make contact with intact skin. However, if a percutaneous occupational exposure has occurred, PEPs exist for HIV and HBV but not for HCV.3,6 If a source patient’s HIV, HBV, and HCV statuses are unknown, occupational-health personnel can interview the patient to evaluate his or her risks and initiate testing. Specific information about the time and nature of exposure should be documented.
When testing is indicated, it should be done following institutional and state-specific exposure-control policies and informed consent guidelines. In all situations, the decision to begin antiviral PEP should be carefully considered, weighing benefits of PEP versus the risks and toxicity of treatment.
Human immunodeficiency virus. If a source patient is known to be HIV-positive, has a positive rapid HIV test, or if HIV status cannot be quickly determined, PEP is indicated. Healthcare providers should be aware of rare cases in which the source patient initially tested HIV-seronegative but was subsequently found to have primary HIV infection.
Per 2004 CDC recommendations, PEP is indicated for all healthcare workers who sustain a percuanteous injury from a known HIV-positive source.3,8 For a less severe injury (e.g. solid needle or superficial injury), PEP with either a basic two-drug or three-drug regimen is indicated, depending on the source patient’s viral load.3,5,6,8
If the source patient has unknown HIV status, two-drug PEP is indicated based on the source patient’s HIV risk factors. In such patients, rapid HIV testing also is indicated to aid in determining the need for PEP. When the source HIV status is unknown, PEP is indicated in settings where exposure to HIV-infected persons is likely.
If PEP is indicated, it should be started as quickly as possible. The 2005 U.S. Public Health Service Recommendations for PEP recommend initiating two nucleosides for low-risk exposures and two nucleosides plus a boosted protease inhibitor for high-risk exposures.
Examples of commonly used dual nucleoside regimens are Zidovudine plus Lamivudine (coformulated as Combivir) or Tenofovir plus Emtricitabine (coformulated as Truvada). Current recommendations indicate that PEP should be continued for four weeks, with concurrent clinical and laboratory evaluation for drug toxicity.
Hepatitis B virus. Numerous prospective studies have evaluated the post-exposure effectiveness of HBIG. When administered within 24 hours of exposure, HBIG might offer immediate passive protection against HBV infection. Additionally, if initiated within one week of percutaneous injury with a known HBV-positive source, multiple doses of HGIB provide an estimated 75% protection from transmission.
Although the combination of HBIG and the hepatitis vaccine B series has not been evaluated as PEP in the occupational setting, evidence in the perinatal setting suggests this regimen is more effective than HBIG alone.3,6,8
Hepatitis C virus. No PEP exists for HCV, and current recommendations for post-exposure management focus on early identification and treatment of chronic disease. There are insufficient data for a treatment recommendation for patients with acute HCV infection with no evidence of disease; the appropriate dosing of such a regimen is unknown. Further, evidence suggests that treatment started early in the course of chronic infection could be just as effective and might eliminate the need to treat persons whose infection will spontaneously resolve.7
Back to the Case
Your needlestick occurred while using a hollow-bore needle to cannulate a source patient’s vein, placing you at higher risk for seroconversion. You immediately reported the exposure to the department of occupational health at your hospital. The source patient’s HIV, HBV, and HCV serological statuses were tested, and the patient was found to be HBV-positive. After appropriate counseling, you decide to receive HGIB prophylaxis to reduce your chances of becoming infected with HBV infection.
Bottom Line
Healthcare workers who suffer occupational needlestick injuries require immediate identification and attention to avoid transmission of such infectious diseases as HIV, HBV, and HCV. Source patients should undergo rapid serological testing to determine appropriate PEP. TH
Dr. Zehnder is a hospitalist in the Section of Hospital Medicine at the University of Colorado Denver.
References
- Mangione CM, Gerberding JL, Cummings, SR. Occupational exposure to HIV: Frequency and rates of underreporting of percutaneous and mucocutaneous exposures by medical housestaff. Am J Med. 1991;90(1):85-90.
- Lee JM, Botteman MF, Nicklasson L, et al. Needlestick injury in acute care nurses caring for patients with diabetes mellitus: a retrospective study. Curr Med Res Opinion. 2005;21(5):741-747.
- Workbook for designing, implementing, and evaluating a sharps injury prevention program. Centers for Disease Control and Prevention website. Available at: www.cdc.gov/sharpssafety/pdf/WorkbookComplete.pdf. Accessed Sept. 13, 2010.
- Lee JM, Botteman MF, Xanthakos N, Nicklasson L. Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. AAOHN J. 2005;53(3):117-133.
- Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485-1490.
- Exposure to blood: What healthcare personnel need to know. CDC website. Available at: www.cdc.gov/ncidod /dhqp/pdf/bbp/Exp_to_Blood.pdf. Accessed Aug. 31, 2010.
- Buergler JM, Kim R, Thisted RA, Cohn SJ, Lichtor JL, Roizen MF. Risk of human immunodeficiency virus in surgeons, anesthesiologists, and medical students. Anesth Analg. 1992;75(1):118-124.
- Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. CDC website. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm. Accessed Aug. 31, 2010.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposure in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23(5):273-277.