User login
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
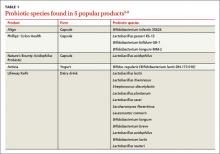
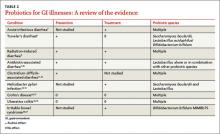
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
REFERENCES
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
REFERENCES
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
REFERENCES
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.