User login
- Consider recommending HPV vaccine for 11- and 12-year-old girls in your practice, before sexual activity puts them at risk of viral infection (A). The FDA has also approved the HPV vaccine for women up to 26 years of age.
- If women older than 26 years ask to be vaccinated, make sure they understand it is an off-label use for them (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Presexual adolescent girls and sexually active women can now lower their lifetime risk of cervical cancer, thanks to a newly available quadrivalent vaccine (Gardasil) directed at human papillomavirus (HPV). This gives us the opportunity to educate parents and adolescents (the primary target group for the vaccine), many of whom remain uninformed about the direct link between HPV infection and cervical cancer.
Ethical, cultural, social, and religious issues that will require attention1 are beyond the scope of this article.
Who should receive the HPV vaccine?
Pre-adolescent and adolescent girls
Girls ages 11 to 12 years—most of whom have not started sexual activity—are the primary targets of immunization. However, the US Food and Drug Administration also approved the use of Gardasil for girls as young as 9. Girls this age may require other vaccines, such as meningococcal conjugate and tetanus-diphtheria-acellular pertussis, and experience thus far indicates no negative immune effects with co-administration of vaccines.1,2
According to one study, vaccination of the entire US population of 12-year-old girls would prevent more than 200,000 HPV infections, 100,000 abnormal Pap tests, and 3300 cases of cervical cancer.3 Parental as well as health care provider acceptance of HPV vaccines for adolescents will be critical to the success of the vaccination effort (see “What makes FPs recommend the HPV vaccine” ).4
Practical issues. As with any new vaccine added to the childhood/adolescent vaccination schedule, a host of issues will need to be resolved to ensure adequate coverage. Factors likely to influence use of HPV vaccine among adolescents are cost and reimbursement, and adherence to the 3-dose regimen that spans 6 months.
The American Academy of Pediatrics’ Committee on Infectious Diseases and the Advisory Committee on Immunization Practices (ACIP) recommends universal use of the HPV vaccine for girls, with a focus on 11- to 12-year-olds. The vaccine is also recommended for 13- to 26-year-old girls and women who have received or completed the 3-dose vaccine series.
Why not vaccinate boys? HPV infection is highly prevalent in sexually active men.5 The efficacy of vaccinating boys against HPV infection is currently being explored.6 However, one model has suggested that vaccinating adolescent males with a bivalent HPV vaccine would only slightly reduce the incidence of cervical cancer cases beyond that achieved by vaccination of adolescent girls, and with an extremely high cost-effectiveness ratio compared with female-only vaccination.5
Women ≤26 years
Indications under FDA approval also include women up to 26 years. Even adults who have been sexually active for years may not have been exposed to all high-risk HPV covered by the vaccine.
Are women older than 26 years eligible?
Though FDA approval of the vaccine is for females aged 9 to 26 years, a recent working group on HPV prevention concluded that any sexually active person may benefit from vaccination and should have the opportunity to receive the vaccine.1 Importantly, women older than 26 years who request the vaccine should be made fully aware of its off-label application in their case.
The rationale behind the recommendations
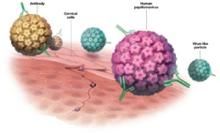
HPV transmission occurs easily with skin-to-skin contact.8-11 HPV can infect the external genitalia during non-intercourse sexual activities, including manual and oral genital contact. Sexual intercourse is the most frequent mode of infection of the cervix. Condoms may help protect against transmission of HPV but are not fully effective.8,12
Adolescents are particularly vulnerable to HPV, but respond best to vaccine. The cervix is especially susceptible to HPV infection in adolescence because the squamous columnar cell junction transformation zone is more exposed. The adult cervix is less susceptible to HPV than the adolescent cervix because of the smaller area of cervical ectopy comprised of columnar epithelial cells.13 However, in adolescents, the immune response to HPV exposure is greater than in than adults.
Risk for acquiring HPV infection. Risk factors for acquiring HPV infection are listed in the TABLE .8,14,15 According to the Centers for Disease Control and Prevention, sexually active men and women have a 50% lifetime risk of acquiring HPV infection.16 An estimated 6.2 million people in the US become infected with HPV each year,16 and approximately 20 million currently harbor HPV infections.17 This estimate includes more than 9 million sexually active adolescents and young adults 15 to 24 years of age, the group in which nearly 75% of new HPV infections occur.18 Among women <25 years of age, between 28% and 46% are infected with HPV.19,20
Infection cannot always be cleared. Most HPV infections (whether high-risk or low-risk type) are asymptomatic and are efficiently cleared (ie, no detection of DNA for a specific HPV type) by the immune system.21,22 However, if the infection cannot be cleared or controlled by the immune system, it may become a persistent infection.
Persistent infection with HPV increases the probability of progression to high-grade cervical intraepithelial neoplasia (CIN) and invasive carcinoma ( FIGURE ).18-19 Evidence also increasingly shows that high-risk HPV types likely cause anal, penile, scrotal, vulvar, vaginal, and some head and neck cancers.25
After HPV vaccination, neutralizing antibodies are secreted from memory B cells, and bind to their target HPV type, preventing infection before it occurs, thereby blocking the initial step toward development of cervical cancer.
15 high-risk oncogenic types. Papillomaviruses such as HPV are nonenveloped, double-stranded, DNA viruses. They infect cutaneous and mucosal epithelial tissues. More than 100 HPV types have been identified,3 about 30 to 40 of which are spread by sexual contact.4 Of the many known HPVs, only 15 are high-risk oncogenic types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 73) that can cause cervical cancer.5.6 Of these high-risk oncogenic types, HPV 16 and 18 account for about 70% of all cervical cancers.7
The new HPV vaccines, Gardasil and Cervarix, (see Web table) both contain virosomal antigens to vaccinate against HPV types 16 and 18. Persistent infection with these high-risk HPV types is necessary for the development of cervical cancer. Chronic infection with low-risk HPV types (eg, HPV 6 or 11) may lead to the development of anogenital warts and other low-grade genital abnormalities, as well as laryngeal cancer or recurrent respiratory papillomatosis. Gardasil also contains virosome antigens for these 2 HPV types. Warts on the hands are usually attributable to HPV 7.8
Viral integration is a necessary step in the malignant transformation of HPV infection; infection may progress from residential to episomal, and, finally, to an integrated form. Residential infection typically occurs a minimum of 6 weeks from exposure, can persist without detection for decades, and can be low risk or high risk. In the episomal state, virally active HPV is located in the cell nucleus, separate from the human DNA. In the integrated form of infection, the HPV DNA circle has opened and joined the human DNA. Integrated HPV—always high risk—produces an abnormal Papanicolaou (Pap) test. If recognized on colposcopy, it must be treated to prevent progression to cervical cancer.
FIGURE
How HPV infection progresses to cervical cancer
Adapted with permission from Pinto and Crum 200023 and Schlecht et al 2001.24
TABLE
Factors that put women at risk for HPV infection
| Young age (peak age group: 20–24 years) |
| Lifetime number of sexual partners |
| First sexual intercourse at early age |
| Male partner sexual behavior |
| Smoking |
| Oral contraceptive use |
| Uncircumcised male partners |
| Sources: Winer et al 2003;8 Schiffman and castle 2003;14 Insinga et al 2003.15 |
Why screening alone isn’t enough
New technologies for Pap testing, HPV DNA testing, and revisions in the Bethesda system for reporting cervical cytology have led to better treatment recommendations for patients with abnormal cytology results.26 But despite these advances, cervical screening is underused or not used at all for many women at risk.
For example, some women with abnormal cervical cytology—especially those of lower socioeconomic status, who often are medically underserved or lack insurance—may not receive adequate follow-up care.27 Though widespread cervical screening in the future may significantly decrease morbidity and mortality associated with cervical cancer, HPV vaccination can also help achieve this goal.
The case for vaccination plus screening
It will likely take at least a decade to assess the impact of HPV vaccination on invasive cervical cancer, and perhaps 20 to 30 years to achieve the maximum benefit from such a program. A computer-based model of the natural history of HPV and cervical cancer developed by the Harvard School of Public Health considered different cancer prevention policies, including vaccination against HPV types 16 and 18 (initiated at the age of 12 years), cytologic screening (initiated at 18, 21, 25, 30, or 35 years,) and combined vaccination and screening strategies. The model showed the combination strategy to be most effective.28
Dramatic reductions expected. The model predicts that with current screening and vaccination against HPV, low-grade cervical abnormalities associated with HPV-16 and HPV-18 infections would be reduced by 15% and high-grade lesions by 49%. Vaccination would decrease the number of cases of cervical cancer by about 66% in conjunction with screening. The vaccine, however, would not prevent cancers caused by other high-risk HPV types.
According to the model, HPV vaccination would produce health gains that are well worth the cost. Specifically, the cost per additional quality-adjusted life-year gained with vaccinating only females was estimated to be $21,000. This ratio compares favorably with many adult and pediatric vaccines currently used in the US.
A recent survey of attitudes about HPV vaccination among members of the American Academy of Family Physicians (AAFP) found that survey respondents would be more likely to administer an HPV vaccine to girls than to boys and to older rather than younger adolescents.4 Female gender, knowledge about HPV, and attitudes about vaccination were independently associated with family physicians’ intentions to recommend HPV vaccination.
It will take decades to see cervical cancer rates drop, but we will soon see fewer CIN 2/3 lesions once HPV 16/18 vaccination is routine.
HPV types 6 and 11 cause 90% of genital warts
Looking forward
The long-term efficacy of HPV vaccines remains to be determined. Sustained efficacy up to 4.5 years has been documented29 but it could be that boosters will be needed.
Research has shown that adolescents and parents, and even some providers of adolescent health care, may have a significant misunderstanding about HPV infection and its possible sequelae,30 suggesting the need for educational programs about the disease and its prevention. Education and vaccine advocacy from professional organizations such as the AAFP, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists will be essential to foster acceptance of HPV vaccination.
CORRESPONDENCE
Michael E. Pichichero, MD, Elmwood Pediatric Group, 601 Elmwood Avenue, Box 672, Rochester, NY 14642. [email protected]
1. Frazer IH, Cox JT, Mayeaux Jr EJ, et al. advances in prevention of cervical cancer and other human papillomavirus-related diseases. Pediatr Infect Dis J 2006;25:S65-S81.
2. Bonnez W. Immunization against genital human papillomaviruses. J Infect Dis 2005;24:1005-1006.
3. Sanders GD, Taira AV. cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003;9:37-48.
4. Riedesel JM, Rosenthal SL, Zimet GD, et al. Attitudes about HPV vaccine among family physicians. J Pediatr Adolesc Gynecol 2005;18:391-398.
5. Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis 2006;194:1044-1057.
6. Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in mail and female adolescents and young adult women. Pediatrics 2006;118:2135-2145.
7. Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004;19:1915-1923.
8. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003;157:218-226.
8. Kjaer SK, Chackerian B, van den Brule AJC, et al. High-risk human papillomavirus is sexually transmitted: Evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev 2001;10:101-106.
10. Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003;95:1772-1783.
11. Smith EM, Ritchie JM, Yankowitz J, et al. Human papillomavirus prevalence and types in newborns and parents: concordance and modes of transmission. Sex Transm Dis 2004;31:57-62.
12. Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med 2006;354:2645-2654.
13. Kahn JA, Hillard PA. Human papillomavirus and cervical cytology in adolescents. Adolesc Med Clin 2004;15:301-321.
14. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.
15. Insinga RP, Dasbach EF, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003;36:1397-1403.
16. Centers for Disease Control and Prevention. Genital HPV Infection Fact Sheet. Rockville, Md: CDC National Prevention Information Network; 2004.
17. Cates W, Jr. and the American Social Health Association Panel. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex Transm Dis 1999;26(suppl):S2-S7.
18. Weinstock H, Berman S, Cates W, Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 2004;36:6-10.
19. Tarkowski TA, Koumans EH, Sawyer M, et al. Epidemiology of human papillomavirus infection and abnormal cytologic test results in an urban adolescent population. J Infect Dis 2004;189:46-50.
20. Revzina NV, Diclemente RJ. Prevalence and incidence of human papillomavirus infection in women in the USA: a systematic review. Int J STD AIDS 2005;16:528-537.
21. Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis 2005;191:182-192.
22. Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev 2003;12:485-490.
23. Pinto AP, Crum CP. Natural history of cervical neoplasia: defining progression and its consequence. Clin Obstet Gynecol 2000;43:352-362.
24. Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of CIN. JAMA 2001;286:3106-3114.
25. Hernandez BY, McDuffie K, Zhu X, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol Biomarkers Prev 2005;14:2550-2556.
26. Holcomb K, Runowicz CD. Cervical cancer screening. Surg Oncol Clin N Am 2005;14:777-797.
27. Benard VB, Lawson HW, Eheman CR, Anderson C, Helsel W. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol 2005;105:1323-1328.
28. Goldie SJ, Kohli M, Grimm D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004;96:604-615.
29. Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet 2006;367:1247-1255.
30. Dell DL, Chen H, Ahmad F, Stewart DE. Knowledge about human papillomavirus among adolescents. Obstet Gynecol 2000;96:653-656.
31. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004;324:17-27.
32. Howley PM. Papillomavirinae: The viruses and their replication. In: Fields BN, knipe DM, Howley PM, eds. Fields Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996:2045-2076.
33. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.
34. Wiley DJ, Douglas J, Beutner K, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis 2002;35:S210-S224.
35. Clifford GM, Smith JS, Aguadp T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer 2003;89:101-105.
36. de Villiers EM, Neumann C, Oltersdorf T, Fierlbeck G, zur Hausen H. Butcher’s wart virus (HPV 7) infections in non-butchers. J Invest Dermatol 1986;87:236-238.
- Consider recommending HPV vaccine for 11- and 12-year-old girls in your practice, before sexual activity puts them at risk of viral infection (A). The FDA has also approved the HPV vaccine for women up to 26 years of age.
- If women older than 26 years ask to be vaccinated, make sure they understand it is an off-label use for them (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Presexual adolescent girls and sexually active women can now lower their lifetime risk of cervical cancer, thanks to a newly available quadrivalent vaccine (Gardasil) directed at human papillomavirus (HPV). This gives us the opportunity to educate parents and adolescents (the primary target group for the vaccine), many of whom remain uninformed about the direct link between HPV infection and cervical cancer.
Ethical, cultural, social, and religious issues that will require attention1 are beyond the scope of this article.
Who should receive the HPV vaccine?
Pre-adolescent and adolescent girls
Girls ages 11 to 12 years—most of whom have not started sexual activity—are the primary targets of immunization. However, the US Food and Drug Administration also approved the use of Gardasil for girls as young as 9. Girls this age may require other vaccines, such as meningococcal conjugate and tetanus-diphtheria-acellular pertussis, and experience thus far indicates no negative immune effects with co-administration of vaccines.1,2
According to one study, vaccination of the entire US population of 12-year-old girls would prevent more than 200,000 HPV infections, 100,000 abnormal Pap tests, and 3300 cases of cervical cancer.3 Parental as well as health care provider acceptance of HPV vaccines for adolescents will be critical to the success of the vaccination effort (see “What makes FPs recommend the HPV vaccine” ).4
Practical issues. As with any new vaccine added to the childhood/adolescent vaccination schedule, a host of issues will need to be resolved to ensure adequate coverage. Factors likely to influence use of HPV vaccine among adolescents are cost and reimbursement, and adherence to the 3-dose regimen that spans 6 months.
The American Academy of Pediatrics’ Committee on Infectious Diseases and the Advisory Committee on Immunization Practices (ACIP) recommends universal use of the HPV vaccine for girls, with a focus on 11- to 12-year-olds. The vaccine is also recommended for 13- to 26-year-old girls and women who have received or completed the 3-dose vaccine series.
Why not vaccinate boys? HPV infection is highly prevalent in sexually active men.5 The efficacy of vaccinating boys against HPV infection is currently being explored.6 However, one model has suggested that vaccinating adolescent males with a bivalent HPV vaccine would only slightly reduce the incidence of cervical cancer cases beyond that achieved by vaccination of adolescent girls, and with an extremely high cost-effectiveness ratio compared with female-only vaccination.5
Women ≤26 years
Indications under FDA approval also include women up to 26 years. Even adults who have been sexually active for years may not have been exposed to all high-risk HPV covered by the vaccine.
Are women older than 26 years eligible?
Though FDA approval of the vaccine is for females aged 9 to 26 years, a recent working group on HPV prevention concluded that any sexually active person may benefit from vaccination and should have the opportunity to receive the vaccine.1 Importantly, women older than 26 years who request the vaccine should be made fully aware of its off-label application in their case.
The rationale behind the recommendations
HPV transmission occurs easily with skin-to-skin contact.8-11 HPV can infect the external genitalia during non-intercourse sexual activities, including manual and oral genital contact. Sexual intercourse is the most frequent mode of infection of the cervix. Condoms may help protect against transmission of HPV but are not fully effective.8,12
Adolescents are particularly vulnerable to HPV, but respond best to vaccine. The cervix is especially susceptible to HPV infection in adolescence because the squamous columnar cell junction transformation zone is more exposed. The adult cervix is less susceptible to HPV than the adolescent cervix because of the smaller area of cervical ectopy comprised of columnar epithelial cells.13 However, in adolescents, the immune response to HPV exposure is greater than in than adults.
Risk for acquiring HPV infection. Risk factors for acquiring HPV infection are listed in the TABLE .8,14,15 According to the Centers for Disease Control and Prevention, sexually active men and women have a 50% lifetime risk of acquiring HPV infection.16 An estimated 6.2 million people in the US become infected with HPV each year,16 and approximately 20 million currently harbor HPV infections.17 This estimate includes more than 9 million sexually active adolescents and young adults 15 to 24 years of age, the group in which nearly 75% of new HPV infections occur.18 Among women <25 years of age, between 28% and 46% are infected with HPV.19,20
Infection cannot always be cleared. Most HPV infections (whether high-risk or low-risk type) are asymptomatic and are efficiently cleared (ie, no detection of DNA for a specific HPV type) by the immune system.21,22 However, if the infection cannot be cleared or controlled by the immune system, it may become a persistent infection.
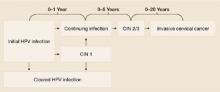
Persistent infection with HPV increases the probability of progression to high-grade cervical intraepithelial neoplasia (CIN) and invasive carcinoma ( FIGURE ).18-19 Evidence also increasingly shows that high-risk HPV types likely cause anal, penile, scrotal, vulvar, vaginal, and some head and neck cancers.25
After HPV vaccination, neutralizing antibodies are secreted from memory B cells, and bind to their target HPV type, preventing infection before it occurs, thereby blocking the initial step toward development of cervical cancer.
15 high-risk oncogenic types. Papillomaviruses such as HPV are nonenveloped, double-stranded, DNA viruses. They infect cutaneous and mucosal epithelial tissues. More than 100 HPV types have been identified,3 about 30 to 40 of which are spread by sexual contact.4 Of the many known HPVs, only 15 are high-risk oncogenic types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 73) that can cause cervical cancer.5.6 Of these high-risk oncogenic types, HPV 16 and 18 account for about 70% of all cervical cancers.7
The new HPV vaccines, Gardasil and Cervarix, (see Web table) both contain virosomal antigens to vaccinate against HPV types 16 and 18. Persistent infection with these high-risk HPV types is necessary for the development of cervical cancer. Chronic infection with low-risk HPV types (eg, HPV 6 or 11) may lead to the development of anogenital warts and other low-grade genital abnormalities, as well as laryngeal cancer or recurrent respiratory papillomatosis. Gardasil also contains virosome antigens for these 2 HPV types. Warts on the hands are usually attributable to HPV 7.8
Viral integration is a necessary step in the malignant transformation of HPV infection; infection may progress from residential to episomal, and, finally, to an integrated form. Residential infection typically occurs a minimum of 6 weeks from exposure, can persist without detection for decades, and can be low risk or high risk. In the episomal state, virally active HPV is located in the cell nucleus, separate from the human DNA. In the integrated form of infection, the HPV DNA circle has opened and joined the human DNA. Integrated HPV—always high risk—produces an abnormal Papanicolaou (Pap) test. If recognized on colposcopy, it must be treated to prevent progression to cervical cancer.
FIGURE
How HPV infection progresses to cervical cancer
Adapted with permission from Pinto and Crum 200023 and Schlecht et al 2001.24
TABLE
Factors that put women at risk for HPV infection
| Young age (peak age group: 20–24 years) |
| Lifetime number of sexual partners |
| First sexual intercourse at early age |
| Male partner sexual behavior |
| Smoking |
| Oral contraceptive use |
| Uncircumcised male partners |
| Sources: Winer et al 2003;8 Schiffman and castle 2003;14 Insinga et al 2003.15 |
Why screening alone isn’t enough
New technologies for Pap testing, HPV DNA testing, and revisions in the Bethesda system for reporting cervical cytology have led to better treatment recommendations for patients with abnormal cytology results.26 But despite these advances, cervical screening is underused or not used at all for many women at risk.
For example, some women with abnormal cervical cytology—especially those of lower socioeconomic status, who often are medically underserved or lack insurance—may not receive adequate follow-up care.27 Though widespread cervical screening in the future may significantly decrease morbidity and mortality associated with cervical cancer, HPV vaccination can also help achieve this goal.
The case for vaccination plus screening
It will likely take at least a decade to assess the impact of HPV vaccination on invasive cervical cancer, and perhaps 20 to 30 years to achieve the maximum benefit from such a program. A computer-based model of the natural history of HPV and cervical cancer developed by the Harvard School of Public Health considered different cancer prevention policies, including vaccination against HPV types 16 and 18 (initiated at the age of 12 years), cytologic screening (initiated at 18, 21, 25, 30, or 35 years,) and combined vaccination and screening strategies. The model showed the combination strategy to be most effective.28
Dramatic reductions expected. The model predicts that with current screening and vaccination against HPV, low-grade cervical abnormalities associated with HPV-16 and HPV-18 infections would be reduced by 15% and high-grade lesions by 49%. Vaccination would decrease the number of cases of cervical cancer by about 66% in conjunction with screening. The vaccine, however, would not prevent cancers caused by other high-risk HPV types.
According to the model, HPV vaccination would produce health gains that are well worth the cost. Specifically, the cost per additional quality-adjusted life-year gained with vaccinating only females was estimated to be $21,000. This ratio compares favorably with many adult and pediatric vaccines currently used in the US.
A recent survey of attitudes about HPV vaccination among members of the American Academy of Family Physicians (AAFP) found that survey respondents would be more likely to administer an HPV vaccine to girls than to boys and to older rather than younger adolescents.4 Female gender, knowledge about HPV, and attitudes about vaccination were independently associated with family physicians’ intentions to recommend HPV vaccination.
It will take decades to see cervical cancer rates drop, but we will soon see fewer CIN 2/3 lesions once HPV 16/18 vaccination is routine.
HPV types 6 and 11 cause 90% of genital warts
Looking forward
The long-term efficacy of HPV vaccines remains to be determined. Sustained efficacy up to 4.5 years has been documented29 but it could be that boosters will be needed.
Research has shown that adolescents and parents, and even some providers of adolescent health care, may have a significant misunderstanding about HPV infection and its possible sequelae,30 suggesting the need for educational programs about the disease and its prevention. Education and vaccine advocacy from professional organizations such as the AAFP, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists will be essential to foster acceptance of HPV vaccination.
CORRESPONDENCE
Michael E. Pichichero, MD, Elmwood Pediatric Group, 601 Elmwood Avenue, Box 672, Rochester, NY 14642. [email protected]
- Consider recommending HPV vaccine for 11- and 12-year-old girls in your practice, before sexual activity puts them at risk of viral infection (A). The FDA has also approved the HPV vaccine for women up to 26 years of age.
- If women older than 26 years ask to be vaccinated, make sure they understand it is an off-label use for them (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Presexual adolescent girls and sexually active women can now lower their lifetime risk of cervical cancer, thanks to a newly available quadrivalent vaccine (Gardasil) directed at human papillomavirus (HPV). This gives us the opportunity to educate parents and adolescents (the primary target group for the vaccine), many of whom remain uninformed about the direct link between HPV infection and cervical cancer.
Ethical, cultural, social, and religious issues that will require attention1 are beyond the scope of this article.
Who should receive the HPV vaccine?
Pre-adolescent and adolescent girls
Girls ages 11 to 12 years—most of whom have not started sexual activity—are the primary targets of immunization. However, the US Food and Drug Administration also approved the use of Gardasil for girls as young as 9. Girls this age may require other vaccines, such as meningococcal conjugate and tetanus-diphtheria-acellular pertussis, and experience thus far indicates no negative immune effects with co-administration of vaccines.1,2
According to one study, vaccination of the entire US population of 12-year-old girls would prevent more than 200,000 HPV infections, 100,000 abnormal Pap tests, and 3300 cases of cervical cancer.3 Parental as well as health care provider acceptance of HPV vaccines for adolescents will be critical to the success of the vaccination effort (see “What makes FPs recommend the HPV vaccine” ).4
Practical issues. As with any new vaccine added to the childhood/adolescent vaccination schedule, a host of issues will need to be resolved to ensure adequate coverage. Factors likely to influence use of HPV vaccine among adolescents are cost and reimbursement, and adherence to the 3-dose regimen that spans 6 months.
The American Academy of Pediatrics’ Committee on Infectious Diseases and the Advisory Committee on Immunization Practices (ACIP) recommends universal use of the HPV vaccine for girls, with a focus on 11- to 12-year-olds. The vaccine is also recommended for 13- to 26-year-old girls and women who have received or completed the 3-dose vaccine series.
Why not vaccinate boys? HPV infection is highly prevalent in sexually active men.5 The efficacy of vaccinating boys against HPV infection is currently being explored.6 However, one model has suggested that vaccinating adolescent males with a bivalent HPV vaccine would only slightly reduce the incidence of cervical cancer cases beyond that achieved by vaccination of adolescent girls, and with an extremely high cost-effectiveness ratio compared with female-only vaccination.5
Women ≤26 years
Indications under FDA approval also include women up to 26 years. Even adults who have been sexually active for years may not have been exposed to all high-risk HPV covered by the vaccine.
Are women older than 26 years eligible?
Though FDA approval of the vaccine is for females aged 9 to 26 years, a recent working group on HPV prevention concluded that any sexually active person may benefit from vaccination and should have the opportunity to receive the vaccine.1 Importantly, women older than 26 years who request the vaccine should be made fully aware of its off-label application in their case.
The rationale behind the recommendations
HPV transmission occurs easily with skin-to-skin contact.8-11 HPV can infect the external genitalia during non-intercourse sexual activities, including manual and oral genital contact. Sexual intercourse is the most frequent mode of infection of the cervix. Condoms may help protect against transmission of HPV but are not fully effective.8,12
Adolescents are particularly vulnerable to HPV, but respond best to vaccine. The cervix is especially susceptible to HPV infection in adolescence because the squamous columnar cell junction transformation zone is more exposed. The adult cervix is less susceptible to HPV than the adolescent cervix because of the smaller area of cervical ectopy comprised of columnar epithelial cells.13 However, in adolescents, the immune response to HPV exposure is greater than in than adults.
Risk for acquiring HPV infection. Risk factors for acquiring HPV infection are listed in the TABLE .8,14,15 According to the Centers for Disease Control and Prevention, sexually active men and women have a 50% lifetime risk of acquiring HPV infection.16 An estimated 6.2 million people in the US become infected with HPV each year,16 and approximately 20 million currently harbor HPV infections.17 This estimate includes more than 9 million sexually active adolescents and young adults 15 to 24 years of age, the group in which nearly 75% of new HPV infections occur.18 Among women <25 years of age, between 28% and 46% are infected with HPV.19,20
Infection cannot always be cleared. Most HPV infections (whether high-risk or low-risk type) are asymptomatic and are efficiently cleared (ie, no detection of DNA for a specific HPV type) by the immune system.21,22 However, if the infection cannot be cleared or controlled by the immune system, it may become a persistent infection.
Persistent infection with HPV increases the probability of progression to high-grade cervical intraepithelial neoplasia (CIN) and invasive carcinoma ( FIGURE ).18-19 Evidence also increasingly shows that high-risk HPV types likely cause anal, penile, scrotal, vulvar, vaginal, and some head and neck cancers.25
After HPV vaccination, neutralizing antibodies are secreted from memory B cells, and bind to their target HPV type, preventing infection before it occurs, thereby blocking the initial step toward development of cervical cancer.
15 high-risk oncogenic types. Papillomaviruses such as HPV are nonenveloped, double-stranded, DNA viruses. They infect cutaneous and mucosal epithelial tissues. More than 100 HPV types have been identified,3 about 30 to 40 of which are spread by sexual contact.4 Of the many known HPVs, only 15 are high-risk oncogenic types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 73) that can cause cervical cancer.5.6 Of these high-risk oncogenic types, HPV 16 and 18 account for about 70% of all cervical cancers.7
The new HPV vaccines, Gardasil and Cervarix, (see Web table) both contain virosomal antigens to vaccinate against HPV types 16 and 18. Persistent infection with these high-risk HPV types is necessary for the development of cervical cancer. Chronic infection with low-risk HPV types (eg, HPV 6 or 11) may lead to the development of anogenital warts and other low-grade genital abnormalities, as well as laryngeal cancer or recurrent respiratory papillomatosis. Gardasil also contains virosome antigens for these 2 HPV types. Warts on the hands are usually attributable to HPV 7.8
Viral integration is a necessary step in the malignant transformation of HPV infection; infection may progress from residential to episomal, and, finally, to an integrated form. Residential infection typically occurs a minimum of 6 weeks from exposure, can persist without detection for decades, and can be low risk or high risk. In the episomal state, virally active HPV is located in the cell nucleus, separate from the human DNA. In the integrated form of infection, the HPV DNA circle has opened and joined the human DNA. Integrated HPV—always high risk—produces an abnormal Papanicolaou (Pap) test. If recognized on colposcopy, it must be treated to prevent progression to cervical cancer.
FIGURE
How HPV infection progresses to cervical cancer
Adapted with permission from Pinto and Crum 200023 and Schlecht et al 2001.24
TABLE
Factors that put women at risk for HPV infection
| Young age (peak age group: 20–24 years) |
| Lifetime number of sexual partners |
| First sexual intercourse at early age |
| Male partner sexual behavior |
| Smoking |
| Oral contraceptive use |
| Uncircumcised male partners |
| Sources: Winer et al 2003;8 Schiffman and castle 2003;14 Insinga et al 2003.15 |
Why screening alone isn’t enough
New technologies for Pap testing, HPV DNA testing, and revisions in the Bethesda system for reporting cervical cytology have led to better treatment recommendations for patients with abnormal cytology results.26 But despite these advances, cervical screening is underused or not used at all for many women at risk.
For example, some women with abnormal cervical cytology—especially those of lower socioeconomic status, who often are medically underserved or lack insurance—may not receive adequate follow-up care.27 Though widespread cervical screening in the future may significantly decrease morbidity and mortality associated with cervical cancer, HPV vaccination can also help achieve this goal.
The case for vaccination plus screening
It will likely take at least a decade to assess the impact of HPV vaccination on invasive cervical cancer, and perhaps 20 to 30 years to achieve the maximum benefit from such a program. A computer-based model of the natural history of HPV and cervical cancer developed by the Harvard School of Public Health considered different cancer prevention policies, including vaccination against HPV types 16 and 18 (initiated at the age of 12 years), cytologic screening (initiated at 18, 21, 25, 30, or 35 years,) and combined vaccination and screening strategies. The model showed the combination strategy to be most effective.28
Dramatic reductions expected. The model predicts that with current screening and vaccination against HPV, low-grade cervical abnormalities associated with HPV-16 and HPV-18 infections would be reduced by 15% and high-grade lesions by 49%. Vaccination would decrease the number of cases of cervical cancer by about 66% in conjunction with screening. The vaccine, however, would not prevent cancers caused by other high-risk HPV types.
According to the model, HPV vaccination would produce health gains that are well worth the cost. Specifically, the cost per additional quality-adjusted life-year gained with vaccinating only females was estimated to be $21,000. This ratio compares favorably with many adult and pediatric vaccines currently used in the US.
A recent survey of attitudes about HPV vaccination among members of the American Academy of Family Physicians (AAFP) found that survey respondents would be more likely to administer an HPV vaccine to girls than to boys and to older rather than younger adolescents.4 Female gender, knowledge about HPV, and attitudes about vaccination were independently associated with family physicians’ intentions to recommend HPV vaccination.
It will take decades to see cervical cancer rates drop, but we will soon see fewer CIN 2/3 lesions once HPV 16/18 vaccination is routine.
HPV types 6 and 11 cause 90% of genital warts
Looking forward
The long-term efficacy of HPV vaccines remains to be determined. Sustained efficacy up to 4.5 years has been documented29 but it could be that boosters will be needed.
Research has shown that adolescents and parents, and even some providers of adolescent health care, may have a significant misunderstanding about HPV infection and its possible sequelae,30 suggesting the need for educational programs about the disease and its prevention. Education and vaccine advocacy from professional organizations such as the AAFP, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists will be essential to foster acceptance of HPV vaccination.
CORRESPONDENCE
Michael E. Pichichero, MD, Elmwood Pediatric Group, 601 Elmwood Avenue, Box 672, Rochester, NY 14642. [email protected]
1. Frazer IH, Cox JT, Mayeaux Jr EJ, et al. advances in prevention of cervical cancer and other human papillomavirus-related diseases. Pediatr Infect Dis J 2006;25:S65-S81.
2. Bonnez W. Immunization against genital human papillomaviruses. J Infect Dis 2005;24:1005-1006.
3. Sanders GD, Taira AV. cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003;9:37-48.
4. Riedesel JM, Rosenthal SL, Zimet GD, et al. Attitudes about HPV vaccine among family physicians. J Pediatr Adolesc Gynecol 2005;18:391-398.
5. Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis 2006;194:1044-1057.
6. Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in mail and female adolescents and young adult women. Pediatrics 2006;118:2135-2145.
7. Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004;19:1915-1923.
8. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003;157:218-226.
8. Kjaer SK, Chackerian B, van den Brule AJC, et al. High-risk human papillomavirus is sexually transmitted: Evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev 2001;10:101-106.
10. Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003;95:1772-1783.
11. Smith EM, Ritchie JM, Yankowitz J, et al. Human papillomavirus prevalence and types in newborns and parents: concordance and modes of transmission. Sex Transm Dis 2004;31:57-62.
12. Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med 2006;354:2645-2654.
13. Kahn JA, Hillard PA. Human papillomavirus and cervical cytology in adolescents. Adolesc Med Clin 2004;15:301-321.
14. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.
15. Insinga RP, Dasbach EF, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003;36:1397-1403.
16. Centers for Disease Control and Prevention. Genital HPV Infection Fact Sheet. Rockville, Md: CDC National Prevention Information Network; 2004.
17. Cates W, Jr. and the American Social Health Association Panel. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex Transm Dis 1999;26(suppl):S2-S7.
18. Weinstock H, Berman S, Cates W, Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 2004;36:6-10.
19. Tarkowski TA, Koumans EH, Sawyer M, et al. Epidemiology of human papillomavirus infection and abnormal cytologic test results in an urban adolescent population. J Infect Dis 2004;189:46-50.
20. Revzina NV, Diclemente RJ. Prevalence and incidence of human papillomavirus infection in women in the USA: a systematic review. Int J STD AIDS 2005;16:528-537.
21. Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis 2005;191:182-192.
22. Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev 2003;12:485-490.
23. Pinto AP, Crum CP. Natural history of cervical neoplasia: defining progression and its consequence. Clin Obstet Gynecol 2000;43:352-362.
24. Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of CIN. JAMA 2001;286:3106-3114.
25. Hernandez BY, McDuffie K, Zhu X, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol Biomarkers Prev 2005;14:2550-2556.
26. Holcomb K, Runowicz CD. Cervical cancer screening. Surg Oncol Clin N Am 2005;14:777-797.
27. Benard VB, Lawson HW, Eheman CR, Anderson C, Helsel W. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol 2005;105:1323-1328.
28. Goldie SJ, Kohli M, Grimm D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004;96:604-615.
29. Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet 2006;367:1247-1255.
30. Dell DL, Chen H, Ahmad F, Stewart DE. Knowledge about human papillomavirus among adolescents. Obstet Gynecol 2000;96:653-656.
31. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004;324:17-27.
32. Howley PM. Papillomavirinae: The viruses and their replication. In: Fields BN, knipe DM, Howley PM, eds. Fields Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996:2045-2076.
33. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.
34. Wiley DJ, Douglas J, Beutner K, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis 2002;35:S210-S224.
35. Clifford GM, Smith JS, Aguadp T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer 2003;89:101-105.
36. de Villiers EM, Neumann C, Oltersdorf T, Fierlbeck G, zur Hausen H. Butcher’s wart virus (HPV 7) infections in non-butchers. J Invest Dermatol 1986;87:236-238.
1. Frazer IH, Cox JT, Mayeaux Jr EJ, et al. advances in prevention of cervical cancer and other human papillomavirus-related diseases. Pediatr Infect Dis J 2006;25:S65-S81.
2. Bonnez W. Immunization against genital human papillomaviruses. J Infect Dis 2005;24:1005-1006.
3. Sanders GD, Taira AV. cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003;9:37-48.
4. Riedesel JM, Rosenthal SL, Zimet GD, et al. Attitudes about HPV vaccine among family physicians. J Pediatr Adolesc Gynecol 2005;18:391-398.
5. Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis 2006;194:1044-1057.
6. Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in mail and female adolescents and young adult women. Pediatrics 2006;118:2135-2145.
7. Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004;19:1915-1923.
8. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003;157:218-226.
8. Kjaer SK, Chackerian B, van den Brule AJC, et al. High-risk human papillomavirus is sexually transmitted: Evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev 2001;10:101-106.
10. Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003;95:1772-1783.
11. Smith EM, Ritchie JM, Yankowitz J, et al. Human papillomavirus prevalence and types in newborns and parents: concordance and modes of transmission. Sex Transm Dis 2004;31:57-62.
12. Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med 2006;354:2645-2654.
13. Kahn JA, Hillard PA. Human papillomavirus and cervical cytology in adolescents. Adolesc Med Clin 2004;15:301-321.
14. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.
15. Insinga RP, Dasbach EF, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003;36:1397-1403.
16. Centers for Disease Control and Prevention. Genital HPV Infection Fact Sheet. Rockville, Md: CDC National Prevention Information Network; 2004.
17. Cates W, Jr. and the American Social Health Association Panel. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex Transm Dis 1999;26(suppl):S2-S7.
18. Weinstock H, Berman S, Cates W, Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 2004;36:6-10.
19. Tarkowski TA, Koumans EH, Sawyer M, et al. Epidemiology of human papillomavirus infection and abnormal cytologic test results in an urban adolescent population. J Infect Dis 2004;189:46-50.
20. Revzina NV, Diclemente RJ. Prevalence and incidence of human papillomavirus infection in women in the USA: a systematic review. Int J STD AIDS 2005;16:528-537.
21. Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis 2005;191:182-192.
22. Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev 2003;12:485-490.
23. Pinto AP, Crum CP. Natural history of cervical neoplasia: defining progression and its consequence. Clin Obstet Gynecol 2000;43:352-362.
24. Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of CIN. JAMA 2001;286:3106-3114.
25. Hernandez BY, McDuffie K, Zhu X, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol Biomarkers Prev 2005;14:2550-2556.
26. Holcomb K, Runowicz CD. Cervical cancer screening. Surg Oncol Clin N Am 2005;14:777-797.
27. Benard VB, Lawson HW, Eheman CR, Anderson C, Helsel W. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol 2005;105:1323-1328.
28. Goldie SJ, Kohli M, Grimm D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004;96:604-615.
29. Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet 2006;367:1247-1255.
30. Dell DL, Chen H, Ahmad F, Stewart DE. Knowledge about human papillomavirus among adolescents. Obstet Gynecol 2000;96:653-656.
31. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004;324:17-27.
32. Howley PM. Papillomavirinae: The viruses and their replication. In: Fields BN, knipe DM, Howley PM, eds. Fields Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996:2045-2076.
33. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.
34. Wiley DJ, Douglas J, Beutner K, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis 2002;35:S210-S224.
35. Clifford GM, Smith JS, Aguadp T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer 2003;89:101-105.
36. de Villiers EM, Neumann C, Oltersdorf T, Fierlbeck G, zur Hausen H. Butcher’s wart virus (HPV 7) infections in non-butchers. J Invest Dermatol 1986;87:236-238.