User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Depression: More than a ‘chemical imbalance’
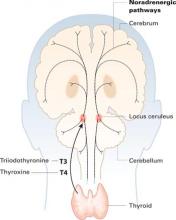
The 1960s’ catecholamine hypothesis—that depression is caused by deficiencies in neurotransmitters such as serotonin and norepinephrine—has greatly influenced how doctors, patients, and the public regard depression. On the positive side, this “chemical imbalance” concept helped reduce the stigma of depression; the illness could be seen as something other than the patient’s fault.
More subtly, though, the imbalance idea is overly simplistic: Antidepressants work in depression the way insulin does in diabetes. When patients don’t have enough of a chemical, just replace it and you have managed the disease. Consequently, some health insurers cover medication management of depression but not psychotherapy, and patients come into the office saying, “I don’t want to talk about my feelings; just give me a pill.”
A recent study suggests that depression and its treatment are more complicated than a simple chemical imbalance. In “Neuroscience News”, Dr. Edmund Higgins reviews research suggesting that scarred DNA strands cause depression.
Thus, science is catching up with what psychiatrists have learned first-hand from clinical practice:
- depression is caused by a complex interaction among neurotransmitters, genetics, and environment
- treating depression successfully is usually complex, too.
The 1960s’ catecholamine hypothesis—that depression is caused by deficiencies in neurotransmitters such as serotonin and norepinephrine—has greatly influenced how doctors, patients, and the public regard depression. On the positive side, this “chemical imbalance” concept helped reduce the stigma of depression; the illness could be seen as something other than the patient’s fault.
More subtly, though, the imbalance idea is overly simplistic: Antidepressants work in depression the way insulin does in diabetes. When patients don’t have enough of a chemical, just replace it and you have managed the disease. Consequently, some health insurers cover medication management of depression but not psychotherapy, and patients come into the office saying, “I don’t want to talk about my feelings; just give me a pill.”
A recent study suggests that depression and its treatment are more complicated than a simple chemical imbalance. In “Neuroscience News”, Dr. Edmund Higgins reviews research suggesting that scarred DNA strands cause depression.
Thus, science is catching up with what psychiatrists have learned first-hand from clinical practice:
- depression is caused by a complex interaction among neurotransmitters, genetics, and environment
- treating depression successfully is usually complex, too.
The 1960s’ catecholamine hypothesis—that depression is caused by deficiencies in neurotransmitters such as serotonin and norepinephrine—has greatly influenced how doctors, patients, and the public regard depression. On the positive side, this “chemical imbalance” concept helped reduce the stigma of depression; the illness could be seen as something other than the patient’s fault.
More subtly, though, the imbalance idea is overly simplistic: Antidepressants work in depression the way insulin does in diabetes. When patients don’t have enough of a chemical, just replace it and you have managed the disease. Consequently, some health insurers cover medication management of depression but not psychotherapy, and patients come into the office saying, “I don’t want to talk about my feelings; just give me a pill.”
A recent study suggests that depression and its treatment are more complicated than a simple chemical imbalance. In “Neuroscience News”, Dr. Edmund Higgins reviews research suggesting that scarred DNA strands cause depression.
Thus, science is catching up with what psychiatrists have learned first-hand from clinical practice:
- depression is caused by a complex interaction among neurotransmitters, genetics, and environment
- treating depression successfully is usually complex, too.
Depressed, delusional, and ‘dead’
History: Suddenly Speechless
Mr. P, age 52, is transferred to our behavioral health unit after 1 month of unsuccessful treatment at a psychiatric hospital. He is mute and disheveled with blunted affect.
Before his hospitalization, Mr. P—who is mildly retarded and has an IQ of 67—lived independently, managed his finances, held two part-time jobs, volunteered as an usher at church, and had a girlfriend. He has been medically stable with diagnoses of indolent stage-zero chronic lymphocytic leukemia (for which he took no medication), moderate obesity, and essential hypertension. For 2 years he has been taking reserpine, 0.25 mg/d, for hypertension, and weighs 200 lb at presentation (body mass index: 29 kg/m2). He has no history of mental illness.
Seven months ago, Mr. P began having trouble dressing and bathing. He also began eating considerably less—about one-third of his normal food intake—and lost 20 lbs over 6 months.
Mr. P also began standing in the street for hours at a time—calling out to passers-by that people were dying and he was causing their deaths—until family members persuaded him to return home. He was not hallucinating, but his brother—who is Mr. P’s legal guardian—said symptoms worsened after a family friend died. After Mr. P became mute, resistant to direction, and immobile, his brother got him admitted to the psychiatric hospital.
The attending physician stopped reserpine—which might cause depression—and started hydrochlorothiazide, 25 mg/d, to maintain normal blood pressure. A psychiatrist diagnosed major depressive disorder and psychosis not otherwise specified, and prescribed mirtazapine, 30 mg nightly, and quetiapine, 25 mg bid. The psychiatrist ruled out lethal catatonia, as vital signs remained stable. When Mr. P’s symptoms did not improve after 1 month, the psychiatrist recommended electroconvulsive therapy (ECT) and transferred him to our facility.
Physical examination and laboratory findings are normal except for lymphocytosis secondary to leukemia:
poll here
The authors’ observations
Mr. P. has major depression with psychotic features. His staring, catalepsy, negativism, selective mutism, and posturing indicate catatonia, and his nihilistic delusions signal Cotard’s syndrome, a delusional depressive disorder.
Catatonia consists of changes in muscle tone and activity and is accompanied by echopraxia and echolalia. Many medical conditions or medications can cause catatonia (Table 1).1 Resultant immobility and stupor can lead to contractures, pressure ulcers, venous thrombosis, and pulmonary emboli. Refusal to eat or drink can cause malnutrition, dehydration, weight loss, and muscle wasting. Approximately 9% of psychiatric inpatients develop catatonia at some point.2
DSM-IV-TR3 describes catatonia criteria as specifiers in affective illness and requires two or more of the following features for diagnosis:
- catalepsy or stupor
- purposeless, excessive motor activity
- negativism or mutism
- peculiar voluntary behaviors, such as posturing, stereotypy, or mannerisms
- echolalia or echopraxia (Table 2).
Catatonia can occur during an excited or retarded state:
- Excited catatonia—also called delirious mania or an oneiroid state—is marked by a dreamlike sensorium, rapid onset, confabulation, derealization, depersonalization, disorientation, and a mixture of catatonic features.4
- Retarded catatonia can be diagnosed using DSM-IV-TR criteria for catatonia. In mild cases or early in presentation, symptoms resemble anergy and psychomotor slowing typical of depression.
Table 1
Recognized causes of catatonia
|
Catatonia: Defining clinical characteristics
| Term | Definition |
|---|---|
| Ambitendency | Indecision, hesitance, becoming stuck regarding stimuli |
| Analgesia to painful stimuli | Failure to feel or withdraw from pain |
| Catalepsy | Posturing, including facial expressions such as exaggerated lip puckering, with waxy flexibility and automatic obedience |
| Echolalia | Repeating words and phrases |
| Echopraxia | Repeating another person’s movements |
| Excitement | Loquacious confabulation and autonomic instability |
| Mannerisms | Purposeful eccentric movements, such as saluting |
| Negativism | Rigidity and resistance to commands |
| Perseveration | Continuing a response long after it is appropriate |
| Prosectic speech | Decreased production and volume of speech |
| Selective mutism | Absence of speech |
| Stereotypy | Persistently repeating gestures that do not appear goal-directed, such as head-banging, rocking, and twirling objects |
| Verbigeration | Repeating a word, phrase, or sentence |
Cotard’s syndrome, first described in the late 1800s by French neurologist Jules Cotard, can accompany folie à deux7 or lycanthropy, the delusional belief that one has been transformed into a werewolf.8 In rare cases, patients believe that their bodies are abnormally enlarged.7 Cotard’s syndrome can exist alone or as part of a psychiatric illness with nihilistic delusions.7
poll hereTable 3
Characteristics of Cotard’s syndrome
|
The authors’ observations
Mr. P.’s episode appears to have been idiopathic.
Reserpine could have caused his decompensation, though precisely how is unclear. The medication is alleged to cause depression by depleting serotonin, dopamine, and norepinephrine, but some researchers believe it exacerbates pre-existing depression.9,10
When treating any patient with a history of depression, find out if he or she is taking reserpine. Advise the primary care physician to discontinue the drug if the patient is self-deprecating or despondent, or reports early morning insomnia, loss of appetite, or impotence.11
Treatment: False Start
To address Mr. P’s catatonia, we stop quetiapine and mirtazapine and start IM lorazepam, 2 mg qid. After 4 days his condition is stable, but he still believes that he and everyone else is dead.
poll here
The authors’ observations
Parenteral benzodiazepines typically are used to treat patients with catatonia and Cotard’s syndrome while the clinician searches for a toxic or medical cause. Most patients with nonemergent catatonia respond to a benzodiazepine.12
Although opinion differs on starting dosages of IM lorazepam in retarded catatonia, we recommend 2 mg IM and repeat doses every 3 hours if the patient does not respond.4,13 Lack of response after 20 mg (10 doses) warrants ECT.4
Consider ECT—which has shown effectiveness for treating both catatonia and Cotard’s syndrome in case reports6-8,14,15—as first-line treatment in emergent catatonia. Do not try a first- or second-generation neuroleptic, which can worsen clinical outcome.
Treatment: a Three-Week Trial
We receive informed consent from Mr. P’s brother to try 10 ECT treatments over 3 weeks. We choose left anterior right temporal electrode placement to minimize cognitive interference,16 and give Mr. P glycopyrrolate, 0.2 mg before each treatment, to manage bradycardia resulting from enhanced vagal tone after electrical stimulation. According to ECT protocol, we administer the anesthetic methohexital, 0.75 to 1.0 mg/kg, and the muscle relaxant succinylcholine, 0.5 to 1 mg/kg, to shorten seizure duration during ECT.
Mr. P also receives forced ventilation at each treatment to counteract brief succinylcholine-induced paralysis of the diaphragm and other muscle tissue. Stimulus intensity begins at 35% and is increased to 50% as the patient’s seizure threshold increases. Each morning, Mr. P also receives extended-release venlafaxine, 225 mg, for depressive symptoms, and hydrochlorothiazide, 25 mg.
After the first ECT treatment, Mr. P’s affect starts to brighten. He speaks a few words after the third treatment and begins eating larger portions by the fifth treatment. After the last treatment, he is performing activities of daily living, talking readily and coherently, and playing basketball with peers. He shows no adverse cognitive effects or other complications from ECT.
The authors’ observations
Although little evidence guides treatment of catatonia in the developmentally disabled,17 we support early use of ECT in those with serious refractory mental illness.18 Some clinicians hesitate to administer ECT to patients with mental retardation because they might be particularly vulnerable to adverse medication effects.19 ECT, however, has been found to cause minimal side effects in this population20 and does not cause or exacerbate brain damage.21
If the patient is mentally incapable of consenting to ECT, obtain informed consent from his or her legal guardian.
Conclusion: Leaving the Hospital
We discharge Mr. P after 25 days. He shows no evidence of psychosis, suicidality, or intent to harm others. He continues hydrochlorothiazide and venlafaxine at the same dosages. He returns home with his brother, and 6 months later is functioning well.
Related resources
- National Mental Health Association. Electroconvulsive therapy. www.nmha.org/infoctr/factsheets/ect.cfm.
- Bupropion • Wellbutrin
- Disulfiram • Antabuse
- Glycopyrrolate • Robinul
- Hydrochlorothiazide • Various
- Lorazepam • Ativan
- Methohexital • Brevital
- Mirtazapine • Remeron
- Quetiapine • Seroquel
- Reserpine • Serpasil
- Succinylcholine • Anectine
- Venlafaxine XR • Effexor XR
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. McCall WV, Mann SC, Shelp FE, et al. Fatal pulmonary embolism in the catatonic syndrome: two case reports and a literature review. J Clin Psychiatry 1995;56:21-5.
2. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and responses to lorazepam. J Clin Psychiatry 1990;51:357-62.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
4. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
5. Mann SC, Caroff SN, Bleier HR, et al. Electroconvulsive therapy of the lethal catatonia syndrome. Convuls Ther 1990;6:239-47.
6. Yamada K, Katsuragi S, Fujii I. A case study of Cotard’s syndrome: stages and diagnosis. Acta Psychiatr Scand 1999;100:369-99.
7. Enoch MD, Ball H. Uncommon psychiatric syndromes, 4th ed. London: Arnold Publishers; 2001.
8. Nejad AG, Toofani K. Co-existence of lycanthropy and Cotard’s syndrome in a single case. Acta Psychiat Scand 2005;111:250-2.
9. Beers MH, Passman LJ. Antihypertensive medications and depression. Drugs 1990;40:792-9.
10. Baumeister AA, Hawkins MF, Uzelac SM. The myth of reserpine-induced depression: role in the historical development of the monoamine hypothesis. J Hist Neurosci 2003;12:207-20.
11. Drug facts and comparisons. St. Louis: Wolters Kluwer; 2006.
12. Fink M. Treating neuroleptic malignant syndrome as catatonia. J Clin Psychopharmacol 2001;21:121.-
13. Caroff SN, Mann SC, Francis A, Fricchionne GL. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing; 2004.
14. Mahgoub NA, Hossain A. Cotard’s syndrome and electroconvulsive therapy. Psychiatr Serv 2004;51:1319-20.
15. Kearns A. Cotard’s syndrome in a mentally handicapped man. Brit J Psychiatry 1987;150:112-14.
16. Schwartz CM, Nelson AL. Rational electroconvulsive therapy electrode placement. Psychiatry 2005;2:37-43.
17. Gaind GS, Rosebush PI, Mazurek MF. Lorazepam treatment of acute and chronic catatonia in two mentally retarded brothers. J Clin Psychiatry 1994;55:20-3.
18. Little JD, McFarlane J, Ducharme HM. ECT use delayed in the presence of comorbid mental retardation: a review of clinical and ethical issues. J ECT 2002;18:38-42.
19. Aziz M, Maixner DF, DeQuardo J, et al. ECT and mental retardation: a review of case reports. J ECT 2001;17:149-52.
20. Friedlander RI, Solomons K. ECT: use in individuals with mental retardation. J ECT 2002;18:38-42.
21. Devanand DP, Dwark AJ, Hutchinson ER, et al. Does ECT alter brain structure? Am J Psychiatry 1994;151:951-70.
History: Suddenly Speechless
Mr. P, age 52, is transferred to our behavioral health unit after 1 month of unsuccessful treatment at a psychiatric hospital. He is mute and disheveled with blunted affect.
Before his hospitalization, Mr. P—who is mildly retarded and has an IQ of 67—lived independently, managed his finances, held two part-time jobs, volunteered as an usher at church, and had a girlfriend. He has been medically stable with diagnoses of indolent stage-zero chronic lymphocytic leukemia (for which he took no medication), moderate obesity, and essential hypertension. For 2 years he has been taking reserpine, 0.25 mg/d, for hypertension, and weighs 200 lb at presentation (body mass index: 29 kg/m2). He has no history of mental illness.
Seven months ago, Mr. P began having trouble dressing and bathing. He also began eating considerably less—about one-third of his normal food intake—and lost 20 lbs over 6 months.
Mr. P also began standing in the street for hours at a time—calling out to passers-by that people were dying and he was causing their deaths—until family members persuaded him to return home. He was not hallucinating, but his brother—who is Mr. P’s legal guardian—said symptoms worsened after a family friend died. After Mr. P became mute, resistant to direction, and immobile, his brother got him admitted to the psychiatric hospital.
The attending physician stopped reserpine—which might cause depression—and started hydrochlorothiazide, 25 mg/d, to maintain normal blood pressure. A psychiatrist diagnosed major depressive disorder and psychosis not otherwise specified, and prescribed mirtazapine, 30 mg nightly, and quetiapine, 25 mg bid. The psychiatrist ruled out lethal catatonia, as vital signs remained stable. When Mr. P’s symptoms did not improve after 1 month, the psychiatrist recommended electroconvulsive therapy (ECT) and transferred him to our facility.
Physical examination and laboratory findings are normal except for lymphocytosis secondary to leukemia:
poll here
The authors’ observations
Mr. P. has major depression with psychotic features. His staring, catalepsy, negativism, selective mutism, and posturing indicate catatonia, and his nihilistic delusions signal Cotard’s syndrome, a delusional depressive disorder.
Catatonia consists of changes in muscle tone and activity and is accompanied by echopraxia and echolalia. Many medical conditions or medications can cause catatonia (Table 1).1 Resultant immobility and stupor can lead to contractures, pressure ulcers, venous thrombosis, and pulmonary emboli. Refusal to eat or drink can cause malnutrition, dehydration, weight loss, and muscle wasting. Approximately 9% of psychiatric inpatients develop catatonia at some point.2
DSM-IV-TR3 describes catatonia criteria as specifiers in affective illness and requires two or more of the following features for diagnosis:
- catalepsy or stupor
- purposeless, excessive motor activity
- negativism or mutism
- peculiar voluntary behaviors, such as posturing, stereotypy, or mannerisms
- echolalia or echopraxia (Table 2).
Catatonia can occur during an excited or retarded state:
- Excited catatonia—also called delirious mania or an oneiroid state—is marked by a dreamlike sensorium, rapid onset, confabulation, derealization, depersonalization, disorientation, and a mixture of catatonic features.4
- Retarded catatonia can be diagnosed using DSM-IV-TR criteria for catatonia. In mild cases or early in presentation, symptoms resemble anergy and psychomotor slowing typical of depression.
Table 1
Recognized causes of catatonia
|
Catatonia: Defining clinical characteristics
| Term | Definition |
|---|---|
| Ambitendency | Indecision, hesitance, becoming stuck regarding stimuli |
| Analgesia to painful stimuli | Failure to feel or withdraw from pain |
| Catalepsy | Posturing, including facial expressions such as exaggerated lip puckering, with waxy flexibility and automatic obedience |
| Echolalia | Repeating words and phrases |
| Echopraxia | Repeating another person’s movements |
| Excitement | Loquacious confabulation and autonomic instability |
| Mannerisms | Purposeful eccentric movements, such as saluting |
| Negativism | Rigidity and resistance to commands |
| Perseveration | Continuing a response long after it is appropriate |
| Prosectic speech | Decreased production and volume of speech |
| Selective mutism | Absence of speech |
| Stereotypy | Persistently repeating gestures that do not appear goal-directed, such as head-banging, rocking, and twirling objects |
| Verbigeration | Repeating a word, phrase, or sentence |
Cotard’s syndrome, first described in the late 1800s by French neurologist Jules Cotard, can accompany folie à deux7 or lycanthropy, the delusional belief that one has been transformed into a werewolf.8 In rare cases, patients believe that their bodies are abnormally enlarged.7 Cotard’s syndrome can exist alone or as part of a psychiatric illness with nihilistic delusions.7
poll hereTable 3
Characteristics of Cotard’s syndrome
|
The authors’ observations
Mr. P.’s episode appears to have been idiopathic.
Reserpine could have caused his decompensation, though precisely how is unclear. The medication is alleged to cause depression by depleting serotonin, dopamine, and norepinephrine, but some researchers believe it exacerbates pre-existing depression.9,10
When treating any patient with a history of depression, find out if he or she is taking reserpine. Advise the primary care physician to discontinue the drug if the patient is self-deprecating or despondent, or reports early morning insomnia, loss of appetite, or impotence.11
Treatment: False Start
To address Mr. P’s catatonia, we stop quetiapine and mirtazapine and start IM lorazepam, 2 mg qid. After 4 days his condition is stable, but he still believes that he and everyone else is dead.
poll here
The authors’ observations
Parenteral benzodiazepines typically are used to treat patients with catatonia and Cotard’s syndrome while the clinician searches for a toxic or medical cause. Most patients with nonemergent catatonia respond to a benzodiazepine.12
Although opinion differs on starting dosages of IM lorazepam in retarded catatonia, we recommend 2 mg IM and repeat doses every 3 hours if the patient does not respond.4,13 Lack of response after 20 mg (10 doses) warrants ECT.4
Consider ECT—which has shown effectiveness for treating both catatonia and Cotard’s syndrome in case reports6-8,14,15—as first-line treatment in emergent catatonia. Do not try a first- or second-generation neuroleptic, which can worsen clinical outcome.
Treatment: a Three-Week Trial
We receive informed consent from Mr. P’s brother to try 10 ECT treatments over 3 weeks. We choose left anterior right temporal electrode placement to minimize cognitive interference,16 and give Mr. P glycopyrrolate, 0.2 mg before each treatment, to manage bradycardia resulting from enhanced vagal tone after electrical stimulation. According to ECT protocol, we administer the anesthetic methohexital, 0.75 to 1.0 mg/kg, and the muscle relaxant succinylcholine, 0.5 to 1 mg/kg, to shorten seizure duration during ECT.
Mr. P also receives forced ventilation at each treatment to counteract brief succinylcholine-induced paralysis of the diaphragm and other muscle tissue. Stimulus intensity begins at 35% and is increased to 50% as the patient’s seizure threshold increases. Each morning, Mr. P also receives extended-release venlafaxine, 225 mg, for depressive symptoms, and hydrochlorothiazide, 25 mg.
After the first ECT treatment, Mr. P’s affect starts to brighten. He speaks a few words after the third treatment and begins eating larger portions by the fifth treatment. After the last treatment, he is performing activities of daily living, talking readily and coherently, and playing basketball with peers. He shows no adverse cognitive effects or other complications from ECT.
The authors’ observations
Although little evidence guides treatment of catatonia in the developmentally disabled,17 we support early use of ECT in those with serious refractory mental illness.18 Some clinicians hesitate to administer ECT to patients with mental retardation because they might be particularly vulnerable to adverse medication effects.19 ECT, however, has been found to cause minimal side effects in this population20 and does not cause or exacerbate brain damage.21
If the patient is mentally incapable of consenting to ECT, obtain informed consent from his or her legal guardian.
Conclusion: Leaving the Hospital
We discharge Mr. P after 25 days. He shows no evidence of psychosis, suicidality, or intent to harm others. He continues hydrochlorothiazide and venlafaxine at the same dosages. He returns home with his brother, and 6 months later is functioning well.
Related resources
- National Mental Health Association. Electroconvulsive therapy. www.nmha.org/infoctr/factsheets/ect.cfm.
- Bupropion • Wellbutrin
- Disulfiram • Antabuse
- Glycopyrrolate • Robinul
- Hydrochlorothiazide • Various
- Lorazepam • Ativan
- Methohexital • Brevital
- Mirtazapine • Remeron
- Quetiapine • Seroquel
- Reserpine • Serpasil
- Succinylcholine • Anectine
- Venlafaxine XR • Effexor XR
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
History: Suddenly Speechless
Mr. P, age 52, is transferred to our behavioral health unit after 1 month of unsuccessful treatment at a psychiatric hospital. He is mute and disheveled with blunted affect.
Before his hospitalization, Mr. P—who is mildly retarded and has an IQ of 67—lived independently, managed his finances, held two part-time jobs, volunteered as an usher at church, and had a girlfriend. He has been medically stable with diagnoses of indolent stage-zero chronic lymphocytic leukemia (for which he took no medication), moderate obesity, and essential hypertension. For 2 years he has been taking reserpine, 0.25 mg/d, for hypertension, and weighs 200 lb at presentation (body mass index: 29 kg/m2). He has no history of mental illness.
Seven months ago, Mr. P began having trouble dressing and bathing. He also began eating considerably less—about one-third of his normal food intake—and lost 20 lbs over 6 months.
Mr. P also began standing in the street for hours at a time—calling out to passers-by that people were dying and he was causing their deaths—until family members persuaded him to return home. He was not hallucinating, but his brother—who is Mr. P’s legal guardian—said symptoms worsened after a family friend died. After Mr. P became mute, resistant to direction, and immobile, his brother got him admitted to the psychiatric hospital.
The attending physician stopped reserpine—which might cause depression—and started hydrochlorothiazide, 25 mg/d, to maintain normal blood pressure. A psychiatrist diagnosed major depressive disorder and psychosis not otherwise specified, and prescribed mirtazapine, 30 mg nightly, and quetiapine, 25 mg bid. The psychiatrist ruled out lethal catatonia, as vital signs remained stable. When Mr. P’s symptoms did not improve after 1 month, the psychiatrist recommended electroconvulsive therapy (ECT) and transferred him to our facility.
Physical examination and laboratory findings are normal except for lymphocytosis secondary to leukemia:
poll here
The authors’ observations
Mr. P. has major depression with psychotic features. His staring, catalepsy, negativism, selective mutism, and posturing indicate catatonia, and his nihilistic delusions signal Cotard’s syndrome, a delusional depressive disorder.
Catatonia consists of changes in muscle tone and activity and is accompanied by echopraxia and echolalia. Many medical conditions or medications can cause catatonia (Table 1).1 Resultant immobility and stupor can lead to contractures, pressure ulcers, venous thrombosis, and pulmonary emboli. Refusal to eat or drink can cause malnutrition, dehydration, weight loss, and muscle wasting. Approximately 9% of psychiatric inpatients develop catatonia at some point.2
DSM-IV-TR3 describes catatonia criteria as specifiers in affective illness and requires two or more of the following features for diagnosis:
- catalepsy or stupor
- purposeless, excessive motor activity
- negativism or mutism
- peculiar voluntary behaviors, such as posturing, stereotypy, or mannerisms
- echolalia or echopraxia (Table 2).
Catatonia can occur during an excited or retarded state:
- Excited catatonia—also called delirious mania or an oneiroid state—is marked by a dreamlike sensorium, rapid onset, confabulation, derealization, depersonalization, disorientation, and a mixture of catatonic features.4
- Retarded catatonia can be diagnosed using DSM-IV-TR criteria for catatonia. In mild cases or early in presentation, symptoms resemble anergy and psychomotor slowing typical of depression.
Table 1
Recognized causes of catatonia
|
Catatonia: Defining clinical characteristics
| Term | Definition |
|---|---|
| Ambitendency | Indecision, hesitance, becoming stuck regarding stimuli |
| Analgesia to painful stimuli | Failure to feel or withdraw from pain |
| Catalepsy | Posturing, including facial expressions such as exaggerated lip puckering, with waxy flexibility and automatic obedience |
| Echolalia | Repeating words and phrases |
| Echopraxia | Repeating another person’s movements |
| Excitement | Loquacious confabulation and autonomic instability |
| Mannerisms | Purposeful eccentric movements, such as saluting |
| Negativism | Rigidity and resistance to commands |
| Perseveration | Continuing a response long after it is appropriate |
| Prosectic speech | Decreased production and volume of speech |
| Selective mutism | Absence of speech |
| Stereotypy | Persistently repeating gestures that do not appear goal-directed, such as head-banging, rocking, and twirling objects |
| Verbigeration | Repeating a word, phrase, or sentence |
Cotard’s syndrome, first described in the late 1800s by French neurologist Jules Cotard, can accompany folie à deux7 or lycanthropy, the delusional belief that one has been transformed into a werewolf.8 In rare cases, patients believe that their bodies are abnormally enlarged.7 Cotard’s syndrome can exist alone or as part of a psychiatric illness with nihilistic delusions.7
poll hereTable 3
Characteristics of Cotard’s syndrome
|
The authors’ observations
Mr. P.’s episode appears to have been idiopathic.
Reserpine could have caused his decompensation, though precisely how is unclear. The medication is alleged to cause depression by depleting serotonin, dopamine, and norepinephrine, but some researchers believe it exacerbates pre-existing depression.9,10
When treating any patient with a history of depression, find out if he or she is taking reserpine. Advise the primary care physician to discontinue the drug if the patient is self-deprecating or despondent, or reports early morning insomnia, loss of appetite, or impotence.11
Treatment: False Start
To address Mr. P’s catatonia, we stop quetiapine and mirtazapine and start IM lorazepam, 2 mg qid. After 4 days his condition is stable, but he still believes that he and everyone else is dead.
poll here
The authors’ observations
Parenteral benzodiazepines typically are used to treat patients with catatonia and Cotard’s syndrome while the clinician searches for a toxic or medical cause. Most patients with nonemergent catatonia respond to a benzodiazepine.12
Although opinion differs on starting dosages of IM lorazepam in retarded catatonia, we recommend 2 mg IM and repeat doses every 3 hours if the patient does not respond.4,13 Lack of response after 20 mg (10 doses) warrants ECT.4
Consider ECT—which has shown effectiveness for treating both catatonia and Cotard’s syndrome in case reports6-8,14,15—as first-line treatment in emergent catatonia. Do not try a first- or second-generation neuroleptic, which can worsen clinical outcome.
Treatment: a Three-Week Trial
We receive informed consent from Mr. P’s brother to try 10 ECT treatments over 3 weeks. We choose left anterior right temporal electrode placement to minimize cognitive interference,16 and give Mr. P glycopyrrolate, 0.2 mg before each treatment, to manage bradycardia resulting from enhanced vagal tone after electrical stimulation. According to ECT protocol, we administer the anesthetic methohexital, 0.75 to 1.0 mg/kg, and the muscle relaxant succinylcholine, 0.5 to 1 mg/kg, to shorten seizure duration during ECT.
Mr. P also receives forced ventilation at each treatment to counteract brief succinylcholine-induced paralysis of the diaphragm and other muscle tissue. Stimulus intensity begins at 35% and is increased to 50% as the patient’s seizure threshold increases. Each morning, Mr. P also receives extended-release venlafaxine, 225 mg, for depressive symptoms, and hydrochlorothiazide, 25 mg.
After the first ECT treatment, Mr. P’s affect starts to brighten. He speaks a few words after the third treatment and begins eating larger portions by the fifth treatment. After the last treatment, he is performing activities of daily living, talking readily and coherently, and playing basketball with peers. He shows no adverse cognitive effects or other complications from ECT.
The authors’ observations
Although little evidence guides treatment of catatonia in the developmentally disabled,17 we support early use of ECT in those with serious refractory mental illness.18 Some clinicians hesitate to administer ECT to patients with mental retardation because they might be particularly vulnerable to adverse medication effects.19 ECT, however, has been found to cause minimal side effects in this population20 and does not cause or exacerbate brain damage.21
If the patient is mentally incapable of consenting to ECT, obtain informed consent from his or her legal guardian.
Conclusion: Leaving the Hospital
We discharge Mr. P after 25 days. He shows no evidence of psychosis, suicidality, or intent to harm others. He continues hydrochlorothiazide and venlafaxine at the same dosages. He returns home with his brother, and 6 months later is functioning well.
Related resources
- National Mental Health Association. Electroconvulsive therapy. www.nmha.org/infoctr/factsheets/ect.cfm.
- Bupropion • Wellbutrin
- Disulfiram • Antabuse
- Glycopyrrolate • Robinul
- Hydrochlorothiazide • Various
- Lorazepam • Ativan
- Methohexital • Brevital
- Mirtazapine • Remeron
- Quetiapine • Seroquel
- Reserpine • Serpasil
- Succinylcholine • Anectine
- Venlafaxine XR • Effexor XR
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. McCall WV, Mann SC, Shelp FE, et al. Fatal pulmonary embolism in the catatonic syndrome: two case reports and a literature review. J Clin Psychiatry 1995;56:21-5.
2. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and responses to lorazepam. J Clin Psychiatry 1990;51:357-62.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
4. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
5. Mann SC, Caroff SN, Bleier HR, et al. Electroconvulsive therapy of the lethal catatonia syndrome. Convuls Ther 1990;6:239-47.
6. Yamada K, Katsuragi S, Fujii I. A case study of Cotard’s syndrome: stages and diagnosis. Acta Psychiatr Scand 1999;100:369-99.
7. Enoch MD, Ball H. Uncommon psychiatric syndromes, 4th ed. London: Arnold Publishers; 2001.
8. Nejad AG, Toofani K. Co-existence of lycanthropy and Cotard’s syndrome in a single case. Acta Psychiat Scand 2005;111:250-2.
9. Beers MH, Passman LJ. Antihypertensive medications and depression. Drugs 1990;40:792-9.
10. Baumeister AA, Hawkins MF, Uzelac SM. The myth of reserpine-induced depression: role in the historical development of the monoamine hypothesis. J Hist Neurosci 2003;12:207-20.
11. Drug facts and comparisons. St. Louis: Wolters Kluwer; 2006.
12. Fink M. Treating neuroleptic malignant syndrome as catatonia. J Clin Psychopharmacol 2001;21:121.-
13. Caroff SN, Mann SC, Francis A, Fricchionne GL. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing; 2004.
14. Mahgoub NA, Hossain A. Cotard’s syndrome and electroconvulsive therapy. Psychiatr Serv 2004;51:1319-20.
15. Kearns A. Cotard’s syndrome in a mentally handicapped man. Brit J Psychiatry 1987;150:112-14.
16. Schwartz CM, Nelson AL. Rational electroconvulsive therapy electrode placement. Psychiatry 2005;2:37-43.
17. Gaind GS, Rosebush PI, Mazurek MF. Lorazepam treatment of acute and chronic catatonia in two mentally retarded brothers. J Clin Psychiatry 1994;55:20-3.
18. Little JD, McFarlane J, Ducharme HM. ECT use delayed in the presence of comorbid mental retardation: a review of clinical and ethical issues. J ECT 2002;18:38-42.
19. Aziz M, Maixner DF, DeQuardo J, et al. ECT and mental retardation: a review of case reports. J ECT 2001;17:149-52.
20. Friedlander RI, Solomons K. ECT: use in individuals with mental retardation. J ECT 2002;18:38-42.
21. Devanand DP, Dwark AJ, Hutchinson ER, et al. Does ECT alter brain structure? Am J Psychiatry 1994;151:951-70.
1. McCall WV, Mann SC, Shelp FE, et al. Fatal pulmonary embolism in the catatonic syndrome: two case reports and a literature review. J Clin Psychiatry 1995;56:21-5.
2. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and responses to lorazepam. J Clin Psychiatry 1990;51:357-62.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
4. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
5. Mann SC, Caroff SN, Bleier HR, et al. Electroconvulsive therapy of the lethal catatonia syndrome. Convuls Ther 1990;6:239-47.
6. Yamada K, Katsuragi S, Fujii I. A case study of Cotard’s syndrome: stages and diagnosis. Acta Psychiatr Scand 1999;100:369-99.
7. Enoch MD, Ball H. Uncommon psychiatric syndromes, 4th ed. London: Arnold Publishers; 2001.
8. Nejad AG, Toofani K. Co-existence of lycanthropy and Cotard’s syndrome in a single case. Acta Psychiat Scand 2005;111:250-2.
9. Beers MH, Passman LJ. Antihypertensive medications and depression. Drugs 1990;40:792-9.
10. Baumeister AA, Hawkins MF, Uzelac SM. The myth of reserpine-induced depression: role in the historical development of the monoamine hypothesis. J Hist Neurosci 2003;12:207-20.
11. Drug facts and comparisons. St. Louis: Wolters Kluwer; 2006.
12. Fink M. Treating neuroleptic malignant syndrome as catatonia. J Clin Psychopharmacol 2001;21:121.-
13. Caroff SN, Mann SC, Francis A, Fricchionne GL. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing; 2004.
14. Mahgoub NA, Hossain A. Cotard’s syndrome and electroconvulsive therapy. Psychiatr Serv 2004;51:1319-20.
15. Kearns A. Cotard’s syndrome in a mentally handicapped man. Brit J Psychiatry 1987;150:112-14.
16. Schwartz CM, Nelson AL. Rational electroconvulsive therapy electrode placement. Psychiatry 2005;2:37-43.
17. Gaind GS, Rosebush PI, Mazurek MF. Lorazepam treatment of acute and chronic catatonia in two mentally retarded brothers. J Clin Psychiatry 1994;55:20-3.
18. Little JD, McFarlane J, Ducharme HM. ECT use delayed in the presence of comorbid mental retardation: a review of clinical and ethical issues. J ECT 2002;18:38-42.
19. Aziz M, Maixner DF, DeQuardo J, et al. ECT and mental retardation: a review of case reports. J ECT 2001;17:149-52.
20. Friedlander RI, Solomons K. ECT: use in individuals with mental retardation. J ECT 2002;18:38-42.
21. Devanand DP, Dwark AJ, Hutchinson ER, et al. Does ECT alter brain structure? Am J Psychiatry 1994;151:951-70.
Beware ictal activity that mimics psychiatric illness
Nonconvulsive status epilepticus (NCSE) is marked by neurobehavioral disturbances that resemble primary psychiatric disorders. Mistaken diagnosis and delayed treatment increase the risk of neurologic damage, so recognizing NCSE symptoms early is important.
To help you make a timely diagnosis, this article describes:
- neuropsychiatric manifestations of NCSE
- how to narrow the differential diagnosis by reviewing clinical symptoms and using electroencephalography (EEG)
- techniques used to rapidly halt ictal activity.
Status epilepticus (SE) is an acute medical emergency. Both forms—convulsive (CSE) and nonconvulsive (NCSE)—require early recognition and treatment. In the United States, 60 SE cases occur per 100,000 population/year, with mortality rates of 20% in adults and 38% in the elderly.1,2
Mortality risk. Data suggest patients with NCSE are unlikely to die unless NCSE co-occurs with CSE or severe medical illness such as delirium or acute complications. Mortality risk does not appear linked with a type of EEG discharge.3
Neurologic injury risk. Prolonged NCSE may cause permanent neurologic damage.4 Transient memory impairment has been reported after cessation of complex partial status epilepticus (CPSE).5 CPSE also has resulted in prolonged neurologic deficits, although concomitant medical illnesses might have contributed to the deficits.6 In one study, some patients gradually returned to baseline cognitive function after CPSE stopped, but they were not tested with standardized neuropsychological tools.7
No significant postictal memory impairment was observed on neuropsychological testing in patients with NCSE of frontal origin.8 A >5-year follow-up study of absence status epilepticus (ASE) found no evidence of long-term cognitive or behavioral decline, even though most patients had recurrent ASE.9 Similarly, no long-term sequelae were seen in patients with ASE.10,11
Triggers, neurologic symptoms
NCSE is an acute but treatable medical emergency that calls for assessing and supporting cardiac and respiratory function, monitoring vital signs, temperature reduction, and fluid replacement. Prognosis is usually good unless NCSE is associated with a serious medical illness (Box).1-11
Many metabolic, neurologic, pharmacologic, and medical abnormalities can precipitate NCSE (Table 1). The most common causes are hypoxia/anoxia, stroke, infection, subtherapeutic antiepileptic levels, alcohol and benzodiazepine intoxication/withdrawal, and metabolic abnormalities.4,7,10,12
NCSE manifests as absence status epilepticus (ASE) or complex partial status epilepticus (CPSE). A generally accepted diagnostic definition is ≥30 minutes of behavioral change from baseline, with diagnostic EEG findings.4,13 EEG is indispensable because the clinical manifestations of NCSE are predominantly behavioral, with minimal or no motor activity.
Table 1
Clinical factors that may precipitate NCSE
| Medical | Recent infection, hyperventilation, trauma, menstruation, pregnancy, renal dialysis, postoperative period, sleep deprivation |
| Metabolic | Hypoparathyroidism, renal failure, hyper/hyponatremia, hyper/hypoglycemia, hypocalcemia |
| Neurologic | Mental retardation, dementia, stroke |
| Pharmacologic | Low serum levels or abrupt discontinuation of anticonvulsants, alcohol intoxication/withdrawal, benzodiazepine withdrawal lithium and neuroleptic use, psychotropic overdose |
| Source : References 9,10,12,16 | |
ASE is reported primarily in children, although de novo cases have been described in elderly patients with no history of epilepsy.10,14
CPSE is usually associated with a history of focal epilepsy and vascular disease. CPSE has a focal onset, with subsequent secondary generalization. Onset is usually temporal in origin but also can be extratemporal.
Patients with CPSE often cycle between an “epileptic twilight state” with confusion and complete unresponsiveness with stereotyped automatisms. It can present with marked behavioral fluctuation or a change in mental status and is generally followed by a prolonged postictal state.4,7,13-15 Several NCSE cases have occurred in patients with no history of seizures.9,10,16
Historically, CPSE was reported to be less common than ASE, but this misconception was most likely caused by failure to recognize CPSE’s clinical presentation and rapid generalization on EEG.7,15
Neuropsychiatric features
Patients with NCSE may be referred for evaluation of an array of behavioral changes commonly seen in psychiatric practice. The differential diagnosis is extensive (Table 2) and includes neurologic and medical conditions often associated with catatonic syndrome.17,18
In a retrospective study, Kaplan12 assessed clinical presentations and reasons for diagnostic delay in 23 adults eventually diagnosed with NCSE. Presenting symptoms included:
- confusion, agitation, aggressive behavior
- lethargy, mutism, verbal perseveration, echolalia
- delirium, blinking, staring, chewing or picking behaviors
- tremulousness or myoclonus
- bizarre behavior (inappropriate laughing, crying, or singing)
- rigidity with waxy flexibility
- delusions, hallucinations.
A prospective study of 22 patients with NCSE found that 7 had a history of psychotic depression, schizophrenia, self-mutilation, bipolar disorder, or episodic severe aggression; 12 of 18 with ASE had a history of epilepsy, and 3 of 4 with CPSE had experienced seizures associated with cerebrovascular accident, right cerebral embolus, and thiazide-induced hyponatremia, respectively.16
Table 2
Differential diagnosis of NCSE
| Metabolic disorders | Hypo/hyperglycemia, hypercalcemia, Addison’s disease, Cushing’s disease, uremia |
| Neurologic disorders | Stroke, CNS tumors, closed head trauma, transient global amnesia, seizures, inflammatory and infectious encephalopathies |
| Psychiatric disorders | Schizophrenia, mood disorders, catatonia, malignant catatonia, somatoform disorders, conversion disorder, Asperger’s syndrome, malingering |
| Toxic disorders | Toxic encephalopathy, neuroleptic malignant syndrome, serotonin syndrome, alcohol and sedative-hypnotic withdrawal, drugs (lithium toxicity, tricyclics, baclofen, tiagabine, overdose) |
| Source: Reference 17,18 | |
Cerebrovascular disease, tumors, and trauma are the most common causes of late-life NCSE.4,19 De novo NCSE occasionally presents:
- after benzodiazepine withdrawal
- with neuroleptic, tricyclic antidepressant, or lithium treatment10,16
- with metabolic abnormalities and nonpsychotropic medications.10
Clinical symptoms
Clinical features of NCSE include cognitive changes, speech abnormalities, affective disturbances, psychosis, poor impulse control, and bizarre behaviors (Table 3). Some patients develop ictal phenomena resembling catatonia or clinical and EEG changes that mimic neuroleptic malignant syndrome (NMS).20-23
Table 3
Clinical features that raise suspicion of NCSE
| Domain | Features |
|---|---|
| Cognitive changes | Prolonged confusion, executive dysfunction, obtundation, attention/memory difficulties, lack of initiative, perseveration, stupor |
| Speech | Poverty of speech with monosyllabic answers, verbal perseveration, echolalia, palilalia, aphasia, paraphasic errors, confabulation, mutism |
| Affective | Prolonged fear, affective indifferent state with blank facial expression, hypomania, psychotic depression, inappropriate laughing and crying, anxiety states |
| Psychosis | Visual, auditory and cenesthetic hallucinations, delusions |
| Impulse control | Hostility, agitation, violence, groping, genital manipulation, picking, posturing |
| Others | Catatonic signs, autonomic disturbances |
| Source: References 5,7-9,12,15-17,20-23 | |
Among 29 patients with acute catatonic syndromes, epileptic activity was identified in 4. One patient with absence status was diagnosed with NMS during the catatonic period.26 Conversely, the commonality of clinical features has led to misdiagnosis of psychogenic catatonia as NCSE. EEG is necessary to exclude NCSE in these cases.
NMS. Yoshino et al27 described two patients taking neuroleptics who met criteria for NMS and had EEG changes consistent with NCSE. They later reported another patient with NCSE complicating NMS; the point at which NCSE developed was unknown, however, because EEG activity was not recorded at NMS onset.28 Based on NMS diagnostic criteria proposed by Caroff et al,29 these patients could have developed NCSE mimicking NMS.
EEG for diagnosis
Candidates. Because differentiating NCSE from similar conditions can be difficult, use EEG to confirm your clinical observations. No guidelines exist, but consider EEG when the patient’s history suggests NCSE. Ask the patient or family about:
- changes in mental status from baseline, especially new-onset catatonia or unexplained altered consciousness
- duration of events
- presence or absence of motor activity
- behavioral fluctuations
- presence or absence of automatisms or blinking.
EEG patterns. Table 4 summarized NCSE diagnostic criteria. NCSE shows characteristic patterns in ASE and CPSE,9,10,16,23 and EEG changes can be continuous or nearly continuous in both.
Table 4
EEG findings that support a clinical diagnosis of NCSE
| Clear-cut criteria |
|---|
| Frequent or continuous focal seizures, with ictal patterns that wax and wane with change in amplitude, frequency, and/or spatial distribution |
| Frequent or continuous generalized spike wave discharges: |
|
| Periodic lateralized epileptiform discharges (“PLEDs”) or bilateral periodic epileptiform discharges (“biPEDs") occurring in patients with coma from generalized tonic-clonic status epilepticus (subtle SE) |
| Probable (equivocal) criteria |
| Patients with acute cerebral damage who also show frequent or continuous EEG abnormalities without previous similar findings |
| Patients with epilepsy who show frequent or continuous generalized EEG abnormalities and similar interictal EEG patterns but whose clinical symptoms suggest NCSE |
| Source: References 4,12-14,17 |
31,32
In CPSE, less-synchronous epileptiform activity has been described, including rhythmical slow, rhythmic spikes, or rhythmic spike and slow waves. Two types of CPSE of frontal origin have been described:
- Type 1 presents clinically with mood disturbance and minimal confusion. EEG shows a frontal focus with a normal background.
- Type 2 presents clinically with confusion. EEG shows bilateral asymmetric frontal discharges.8
- generalized in 69%
- diffuse with focal predominance in 18%
- focal in 13%.
Distinguish between ictal and interictal EEG findings with epileptiform activity, because only the former is diagnostic for NCSE. Intravenous benzodiazepines might be necessary during EEG to verify the diagnosis.33
NCSE has developed after electroconvulsive therapy (ECT), but a cause-effect relationship is debatable. Interictal and abnormal EEG findings after ECT may be misdiagnosed as NCSE.34
Neuroimaging has limited clinical value because of the need for patient cooperation and specialized equipment.4 Head CT or MRI can exclude structural abnormalities. PET and SPECT show increased metabolism and blood flow, respectively, in NCSE. MR spectroscopy shows elevated lactate and decreased N-acetyl aspartate.
Halting ictal activity
To rapidly stop ictal activity—the main goal of treatment—recognizing and correcting precipitant factors is vital:
- Consider discontinuing medications that could lower the seizure threshold.
- Order a complete blood count, serum electrolytes, calcium, arterial-blood gas, liver and renal function tests, urine toxicology screen, and serum antiepileptic drug concentrations.
- When possible, obtain neuroimaging and EEG in the emergency room for accurate diagnosis and prompt treatment.12
Response to benzodiazepines might be transient, lasting only hours or days. For instance, diazepam’s anticonvulsant effect may last
Newer antiepileptics—such as lamotrigine, levetiracetam, or topiramate—have been used with varying results, and their role in first-line treatment of NCSE is evolving. Rarely, the antiepileptic tiagabine precipitates or worsens NCSE.4,13,14
Related resources
- Epilepsy Foundation. www.epilepsyfoundation.org
- Neuroleptic Malignant Syndrome Information Service. Hotline for health professionals (888) 667-8367. www.nmsis.org
- Carbamazepine • Tegretol, Carbatrol
- Clonazepam • Klonopin
- Diazepam • Valium
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Lithium carbonate • Lithobid, Eskalith CR
- Lorazepam • Ativan
- Phenobarbital • Luminal
- Phenytoin • Dilantin
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproic acid • Depakote
The authors report no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
Acknowledgment
Dr. Goveas was a geriatric psychiatry fellow, University of Pennsylvania, when he wrote this article in collaboration with his mentors, Drs. Caroff and Riggio.
1. DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology 1996;46(4):1029-35.
2. Shorvon S. Status epilepticus: Its clinical features and treatment in children and adults Cambridge, UK: Cambridge University Press, 1994.
3. Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003;61:1066-73.
4. Walker M, Cross H, Smith S, et al. Nonconvulsive status epilepticus: Epilepsy research foundation workshop reports. Epileptic Disord 2005;7(3):53-296.
5. Engel J, Ludwig BI, Fetell M. Prolonged partial complex status epilepticus: EEG and behavioral observations. Neurology 1978;28:863-9.
6. Krumholz A, Sung GY, Fisher RS, et al. Complex partial status epilepticus accompanied by serious morbidity and mortality. Neurology 1995;45:1499-1504.
7. Ballenger CE, King DW, Gallagher BB. Partial complex status epilepticus. Neurology 1983;33:1545-52.
8. Thomas P, Zifkin B, Migneco O, et al. Nonconvulsive status epilepticus of frontal origin. Neurology 1999;52:1174-83.
9. Guberman A, Cantu-Reyna G, Stuss D, Broughton R. Nonconvulsive generalized status epilepticus: Clinical features, neuropsychological testing, and long-term follow-up. Neurology 1986;36:1284-91.
10. Thomas P, Beaumanoir A, Genton P, et al. ‘De novo’ absence status of late onset: Report of 11 cases. Neurology 1992;42:104-10.
11. Andermann F, Robb J. Absence status: a reappraisal following review of thirty-eight patients. Epilepsia 1972;13:177-87.
12. Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996;37(7):643-50.
13. Riggio S. Nonconvulsive status epilepticus: Clinical features and diagnostic challenges. Psychiatr Clin N Am 2005;28(3):653-64.
14. Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav 2000;1(5):301-14.
15. Tomson T, Lindbom U, Nilsson BY. Nonconvulsive status epilepticus in adults: Thirty-two consecutive patients from a general hospital population. Epilepsia 1992;3(5):829-35.
16. Dunne JW, Summers QA, Stewart-Wynne EG. Non-convulsive status epilepticus: A prospective study in an adult general hospital. Q J Med 1987;62(238):117-26.
17. Kaplan PW. Behavioral manifestations of nonconvulsive status epilepticus. Epilepsy Behav 2002;3(2):122-39.
18. Mann SC. Malignant catatonia. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A, eds. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing Inc, 2003:121-43.
19. Sung CY, Chu NS. Status epilepticus in elderly: etiology, seizure type and outcome. Acta Neurol Scand 1989;80:51-6.
20. McLachlan RS, Blume WT. Isolated fear in complex partial status epilepticus. Ann Neurol 1980;8:639-41.
21. Walls MJ, Bowers TC, Dilsaver SC, Swann AC. Catatonia associated with depression secondary to complex partial epilepsy. J Clin Psychiatry 1993;54(2):73.-
22. Wells CE. Transient ictal psychosis. Arch Gen Psychiatry 1975;32:1201-3.
23. Agathonikou A, Panayiotopoulos CP, Giannakodimos S, Koutroumanidis M. Typical absence status in adults: Diagnostic and syndromic considerations. Epilepsia 1998;39(12):1265-76.
24. Lim J, Yagnik P, Schraeder P, Wheeler S. Ictal catatonia as a manifestation of nonconvulsive status epilepticus. J Neurol Neurosurg Psychiatry 1986;49:833-6.
25. Drury I, Klass DW, Westmoreland BF, Sharbrough FW. An acute syndrome with psychiatric symptoms and EEG abnormalities. Neurology 1985;35(6):911-14.
26. Primavera A, Fonti A, Novello P, et al. Epileptic seizures in patients with acute catatonic syndrome. J Neurol Neurosurg Psychiatry 1994;57(11):1419-22.
27. Yoshino A, Yoshimasu H, Tatsuzawa Y, et al. Nonconvulsive status epilepticus in two patients with neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18(4):347-9.
28. Yoshino A, Yoshimasu H. Nonconvulsive status epilepticus complicating neuroleptic malignant syndrome improved by intravenous diazepam. J Clin Psychopharmacol 2000;20(3):389-90.
29. Caroff SN. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A, eds. Neuroleptic malignant syndrome and related conditions, 2nd ed. Washington, DC: American Psychiatric Publishing; 2003:1-44.
30. Lob H, Roger J, Soulayrol R. Les etats de mal generalizes a expression confusionelle. In: Gastaut H, Roger J, Lob H, eds. Les etats de mal epileptiques. Paris: Masson; 1967:91-109.
31. Granner MA, Lee SI. Nonconvulsive status epilepticus: EEG analysis in a large series. Epilepsia 1994;35(1):42-7.
32. Niedermeyer E, Fineyre F, Riley T, Uematsu S. Absence status (petit mal status) with focal characteristics. Arch Neurol 1979;36:417-21.
33. Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res 1994;18:155-66.
34. Povlsen UJ, Wildschiodtz G, Hogenhaven H, Bolwig TG. Nonconvulsive status epilepticus after electroconvulsive therapy. J ECT 2003;19(3):164-9.
Nonconvulsive status epilepticus (NCSE) is marked by neurobehavioral disturbances that resemble primary psychiatric disorders. Mistaken diagnosis and delayed treatment increase the risk of neurologic damage, so recognizing NCSE symptoms early is important.
To help you make a timely diagnosis, this article describes:
- neuropsychiatric manifestations of NCSE
- how to narrow the differential diagnosis by reviewing clinical symptoms and using electroencephalography (EEG)
- techniques used to rapidly halt ictal activity.
Status epilepticus (SE) is an acute medical emergency. Both forms—convulsive (CSE) and nonconvulsive (NCSE)—require early recognition and treatment. In the United States, 60 SE cases occur per 100,000 population/year, with mortality rates of 20% in adults and 38% in the elderly.1,2
Mortality risk. Data suggest patients with NCSE are unlikely to die unless NCSE co-occurs with CSE or severe medical illness such as delirium or acute complications. Mortality risk does not appear linked with a type of EEG discharge.3
Neurologic injury risk. Prolonged NCSE may cause permanent neurologic damage.4 Transient memory impairment has been reported after cessation of complex partial status epilepticus (CPSE).5 CPSE also has resulted in prolonged neurologic deficits, although concomitant medical illnesses might have contributed to the deficits.6 In one study, some patients gradually returned to baseline cognitive function after CPSE stopped, but they were not tested with standardized neuropsychological tools.7
No significant postictal memory impairment was observed on neuropsychological testing in patients with NCSE of frontal origin.8 A >5-year follow-up study of absence status epilepticus (ASE) found no evidence of long-term cognitive or behavioral decline, even though most patients had recurrent ASE.9 Similarly, no long-term sequelae were seen in patients with ASE.10,11
Triggers, neurologic symptoms
NCSE is an acute but treatable medical emergency that calls for assessing and supporting cardiac and respiratory function, monitoring vital signs, temperature reduction, and fluid replacement. Prognosis is usually good unless NCSE is associated with a serious medical illness (Box).1-11
Many metabolic, neurologic, pharmacologic, and medical abnormalities can precipitate NCSE (Table 1). The most common causes are hypoxia/anoxia, stroke, infection, subtherapeutic antiepileptic levels, alcohol and benzodiazepine intoxication/withdrawal, and metabolic abnormalities.4,7,10,12
NCSE manifests as absence status epilepticus (ASE) or complex partial status epilepticus (CPSE). A generally accepted diagnostic definition is ≥30 minutes of behavioral change from baseline, with diagnostic EEG findings.4,13 EEG is indispensable because the clinical manifestations of NCSE are predominantly behavioral, with minimal or no motor activity.
Table 1
Clinical factors that may precipitate NCSE
| Medical | Recent infection, hyperventilation, trauma, menstruation, pregnancy, renal dialysis, postoperative period, sleep deprivation |
| Metabolic | Hypoparathyroidism, renal failure, hyper/hyponatremia, hyper/hypoglycemia, hypocalcemia |
| Neurologic | Mental retardation, dementia, stroke |
| Pharmacologic | Low serum levels or abrupt discontinuation of anticonvulsants, alcohol intoxication/withdrawal, benzodiazepine withdrawal lithium and neuroleptic use, psychotropic overdose |
| Source : References 9,10,12,16 | |
ASE is reported primarily in children, although de novo cases have been described in elderly patients with no history of epilepsy.10,14
CPSE is usually associated with a history of focal epilepsy and vascular disease. CPSE has a focal onset, with subsequent secondary generalization. Onset is usually temporal in origin but also can be extratemporal.
Patients with CPSE often cycle between an “epileptic twilight state” with confusion and complete unresponsiveness with stereotyped automatisms. It can present with marked behavioral fluctuation or a change in mental status and is generally followed by a prolonged postictal state.4,7,13-15 Several NCSE cases have occurred in patients with no history of seizures.9,10,16
Historically, CPSE was reported to be less common than ASE, but this misconception was most likely caused by failure to recognize CPSE’s clinical presentation and rapid generalization on EEG.7,15
Neuropsychiatric features
Patients with NCSE may be referred for evaluation of an array of behavioral changes commonly seen in psychiatric practice. The differential diagnosis is extensive (Table 2) and includes neurologic and medical conditions often associated with catatonic syndrome.17,18
In a retrospective study, Kaplan12 assessed clinical presentations and reasons for diagnostic delay in 23 adults eventually diagnosed with NCSE. Presenting symptoms included:
- confusion, agitation, aggressive behavior
- lethargy, mutism, verbal perseveration, echolalia
- delirium, blinking, staring, chewing or picking behaviors
- tremulousness or myoclonus
- bizarre behavior (inappropriate laughing, crying, or singing)
- rigidity with waxy flexibility
- delusions, hallucinations.
A prospective study of 22 patients with NCSE found that 7 had a history of psychotic depression, schizophrenia, self-mutilation, bipolar disorder, or episodic severe aggression; 12 of 18 with ASE had a history of epilepsy, and 3 of 4 with CPSE had experienced seizures associated with cerebrovascular accident, right cerebral embolus, and thiazide-induced hyponatremia, respectively.16
Table 2
Differential diagnosis of NCSE
| Metabolic disorders | Hypo/hyperglycemia, hypercalcemia, Addison’s disease, Cushing’s disease, uremia |
| Neurologic disorders | Stroke, CNS tumors, closed head trauma, transient global amnesia, seizures, inflammatory and infectious encephalopathies |
| Psychiatric disorders | Schizophrenia, mood disorders, catatonia, malignant catatonia, somatoform disorders, conversion disorder, Asperger’s syndrome, malingering |
| Toxic disorders | Toxic encephalopathy, neuroleptic malignant syndrome, serotonin syndrome, alcohol and sedative-hypnotic withdrawal, drugs (lithium toxicity, tricyclics, baclofen, tiagabine, overdose) |
| Source: Reference 17,18 | |
Cerebrovascular disease, tumors, and trauma are the most common causes of late-life NCSE.4,19 De novo NCSE occasionally presents:
- after benzodiazepine withdrawal
- with neuroleptic, tricyclic antidepressant, or lithium treatment10,16
- with metabolic abnormalities and nonpsychotropic medications.10
Clinical symptoms
Clinical features of NCSE include cognitive changes, speech abnormalities, affective disturbances, psychosis, poor impulse control, and bizarre behaviors (Table 3). Some patients develop ictal phenomena resembling catatonia or clinical and EEG changes that mimic neuroleptic malignant syndrome (NMS).20-23
Table 3
Clinical features that raise suspicion of NCSE
| Domain | Features |
|---|---|
| Cognitive changes | Prolonged confusion, executive dysfunction, obtundation, attention/memory difficulties, lack of initiative, perseveration, stupor |
| Speech | Poverty of speech with monosyllabic answers, verbal perseveration, echolalia, palilalia, aphasia, paraphasic errors, confabulation, mutism |
| Affective | Prolonged fear, affective indifferent state with blank facial expression, hypomania, psychotic depression, inappropriate laughing and crying, anxiety states |
| Psychosis | Visual, auditory and cenesthetic hallucinations, delusions |
| Impulse control | Hostility, agitation, violence, groping, genital manipulation, picking, posturing |
| Others | Catatonic signs, autonomic disturbances |
| Source: References 5,7-9,12,15-17,20-23 | |
Among 29 patients with acute catatonic syndromes, epileptic activity was identified in 4. One patient with absence status was diagnosed with NMS during the catatonic period.26 Conversely, the commonality of clinical features has led to misdiagnosis of psychogenic catatonia as NCSE. EEG is necessary to exclude NCSE in these cases.
NMS. Yoshino et al27 described two patients taking neuroleptics who met criteria for NMS and had EEG changes consistent with NCSE. They later reported another patient with NCSE complicating NMS; the point at which NCSE developed was unknown, however, because EEG activity was not recorded at NMS onset.28 Based on NMS diagnostic criteria proposed by Caroff et al,29 these patients could have developed NCSE mimicking NMS.
EEG for diagnosis
Candidates. Because differentiating NCSE from similar conditions can be difficult, use EEG to confirm your clinical observations. No guidelines exist, but consider EEG when the patient’s history suggests NCSE. Ask the patient or family about:
- changes in mental status from baseline, especially new-onset catatonia or unexplained altered consciousness
- duration of events
- presence or absence of motor activity
- behavioral fluctuations
- presence or absence of automatisms or blinking.
EEG patterns. Table 4 summarized NCSE diagnostic criteria. NCSE shows characteristic patterns in ASE and CPSE,9,10,16,23 and EEG changes can be continuous or nearly continuous in both.
Table 4
EEG findings that support a clinical diagnosis of NCSE
| Clear-cut criteria |
|---|
| Frequent or continuous focal seizures, with ictal patterns that wax and wane with change in amplitude, frequency, and/or spatial distribution |
| Frequent or continuous generalized spike wave discharges: |
|
| Periodic lateralized epileptiform discharges (“PLEDs”) or bilateral periodic epileptiform discharges (“biPEDs") occurring in patients with coma from generalized tonic-clonic status epilepticus (subtle SE) |
| Probable (equivocal) criteria |
| Patients with acute cerebral damage who also show frequent or continuous EEG abnormalities without previous similar findings |
| Patients with epilepsy who show frequent or continuous generalized EEG abnormalities and similar interictal EEG patterns but whose clinical symptoms suggest NCSE |
| Source: References 4,12-14,17 |
31,32
In CPSE, less-synchronous epileptiform activity has been described, including rhythmical slow, rhythmic spikes, or rhythmic spike and slow waves. Two types of CPSE of frontal origin have been described:
- Type 1 presents clinically with mood disturbance and minimal confusion. EEG shows a frontal focus with a normal background.
- Type 2 presents clinically with confusion. EEG shows bilateral asymmetric frontal discharges.8
- generalized in 69%
- diffuse with focal predominance in 18%
- focal in 13%.
Distinguish between ictal and interictal EEG findings with epileptiform activity, because only the former is diagnostic for NCSE. Intravenous benzodiazepines might be necessary during EEG to verify the diagnosis.33
NCSE has developed after electroconvulsive therapy (ECT), but a cause-effect relationship is debatable. Interictal and abnormal EEG findings after ECT may be misdiagnosed as NCSE.34
Neuroimaging has limited clinical value because of the need for patient cooperation and specialized equipment.4 Head CT or MRI can exclude structural abnormalities. PET and SPECT show increased metabolism and blood flow, respectively, in NCSE. MR spectroscopy shows elevated lactate and decreased N-acetyl aspartate.
Halting ictal activity
To rapidly stop ictal activity—the main goal of treatment—recognizing and correcting precipitant factors is vital:
- Consider discontinuing medications that could lower the seizure threshold.
- Order a complete blood count, serum electrolytes, calcium, arterial-blood gas, liver and renal function tests, urine toxicology screen, and serum antiepileptic drug concentrations.
- When possible, obtain neuroimaging and EEG in the emergency room for accurate diagnosis and prompt treatment.12
Response to benzodiazepines might be transient, lasting only hours or days. For instance, diazepam’s anticonvulsant effect may last
Newer antiepileptics—such as lamotrigine, levetiracetam, or topiramate—have been used with varying results, and their role in first-line treatment of NCSE is evolving. Rarely, the antiepileptic tiagabine precipitates or worsens NCSE.4,13,14
Related resources
- Epilepsy Foundation. www.epilepsyfoundation.org
- Neuroleptic Malignant Syndrome Information Service. Hotline for health professionals (888) 667-8367. www.nmsis.org
- Carbamazepine • Tegretol, Carbatrol
- Clonazepam • Klonopin
- Diazepam • Valium
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Lithium carbonate • Lithobid, Eskalith CR
- Lorazepam • Ativan
- Phenobarbital • Luminal
- Phenytoin • Dilantin
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproic acid • Depakote
The authors report no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
Acknowledgment
Dr. Goveas was a geriatric psychiatry fellow, University of Pennsylvania, when he wrote this article in collaboration with his mentors, Drs. Caroff and Riggio.
Nonconvulsive status epilepticus (NCSE) is marked by neurobehavioral disturbances that resemble primary psychiatric disorders. Mistaken diagnosis and delayed treatment increase the risk of neurologic damage, so recognizing NCSE symptoms early is important.
To help you make a timely diagnosis, this article describes:
- neuropsychiatric manifestations of NCSE
- how to narrow the differential diagnosis by reviewing clinical symptoms and using electroencephalography (EEG)
- techniques used to rapidly halt ictal activity.
Status epilepticus (SE) is an acute medical emergency. Both forms—convulsive (CSE) and nonconvulsive (NCSE)—require early recognition and treatment. In the United States, 60 SE cases occur per 100,000 population/year, with mortality rates of 20% in adults and 38% in the elderly.1,2
Mortality risk. Data suggest patients with NCSE are unlikely to die unless NCSE co-occurs with CSE or severe medical illness such as delirium or acute complications. Mortality risk does not appear linked with a type of EEG discharge.3
Neurologic injury risk. Prolonged NCSE may cause permanent neurologic damage.4 Transient memory impairment has been reported after cessation of complex partial status epilepticus (CPSE).5 CPSE also has resulted in prolonged neurologic deficits, although concomitant medical illnesses might have contributed to the deficits.6 In one study, some patients gradually returned to baseline cognitive function after CPSE stopped, but they were not tested with standardized neuropsychological tools.7
No significant postictal memory impairment was observed on neuropsychological testing in patients with NCSE of frontal origin.8 A >5-year follow-up study of absence status epilepticus (ASE) found no evidence of long-term cognitive or behavioral decline, even though most patients had recurrent ASE.9 Similarly, no long-term sequelae were seen in patients with ASE.10,11
Triggers, neurologic symptoms
NCSE is an acute but treatable medical emergency that calls for assessing and supporting cardiac and respiratory function, monitoring vital signs, temperature reduction, and fluid replacement. Prognosis is usually good unless NCSE is associated with a serious medical illness (Box).1-11
Many metabolic, neurologic, pharmacologic, and medical abnormalities can precipitate NCSE (Table 1). The most common causes are hypoxia/anoxia, stroke, infection, subtherapeutic antiepileptic levels, alcohol and benzodiazepine intoxication/withdrawal, and metabolic abnormalities.4,7,10,12
NCSE manifests as absence status epilepticus (ASE) or complex partial status epilepticus (CPSE). A generally accepted diagnostic definition is ≥30 minutes of behavioral change from baseline, with diagnostic EEG findings.4,13 EEG is indispensable because the clinical manifestations of NCSE are predominantly behavioral, with minimal or no motor activity.
Table 1
Clinical factors that may precipitate NCSE
| Medical | Recent infection, hyperventilation, trauma, menstruation, pregnancy, renal dialysis, postoperative period, sleep deprivation |
| Metabolic | Hypoparathyroidism, renal failure, hyper/hyponatremia, hyper/hypoglycemia, hypocalcemia |
| Neurologic | Mental retardation, dementia, stroke |
| Pharmacologic | Low serum levels or abrupt discontinuation of anticonvulsants, alcohol intoxication/withdrawal, benzodiazepine withdrawal lithium and neuroleptic use, psychotropic overdose |
| Source : References 9,10,12,16 | |
ASE is reported primarily in children, although de novo cases have been described in elderly patients with no history of epilepsy.10,14
CPSE is usually associated with a history of focal epilepsy and vascular disease. CPSE has a focal onset, with subsequent secondary generalization. Onset is usually temporal in origin but also can be extratemporal.
Patients with CPSE often cycle between an “epileptic twilight state” with confusion and complete unresponsiveness with stereotyped automatisms. It can present with marked behavioral fluctuation or a change in mental status and is generally followed by a prolonged postictal state.4,7,13-15 Several NCSE cases have occurred in patients with no history of seizures.9,10,16
Historically, CPSE was reported to be less common than ASE, but this misconception was most likely caused by failure to recognize CPSE’s clinical presentation and rapid generalization on EEG.7,15
Neuropsychiatric features
Patients with NCSE may be referred for evaluation of an array of behavioral changes commonly seen in psychiatric practice. The differential diagnosis is extensive (Table 2) and includes neurologic and medical conditions often associated with catatonic syndrome.17,18
In a retrospective study, Kaplan12 assessed clinical presentations and reasons for diagnostic delay in 23 adults eventually diagnosed with NCSE. Presenting symptoms included:
- confusion, agitation, aggressive behavior
- lethargy, mutism, verbal perseveration, echolalia
- delirium, blinking, staring, chewing or picking behaviors
- tremulousness or myoclonus
- bizarre behavior (inappropriate laughing, crying, or singing)
- rigidity with waxy flexibility
- delusions, hallucinations.
A prospective study of 22 patients with NCSE found that 7 had a history of psychotic depression, schizophrenia, self-mutilation, bipolar disorder, or episodic severe aggression; 12 of 18 with ASE had a history of epilepsy, and 3 of 4 with CPSE had experienced seizures associated with cerebrovascular accident, right cerebral embolus, and thiazide-induced hyponatremia, respectively.16
Table 2
Differential diagnosis of NCSE
| Metabolic disorders | Hypo/hyperglycemia, hypercalcemia, Addison’s disease, Cushing’s disease, uremia |
| Neurologic disorders | Stroke, CNS tumors, closed head trauma, transient global amnesia, seizures, inflammatory and infectious encephalopathies |
| Psychiatric disorders | Schizophrenia, mood disorders, catatonia, malignant catatonia, somatoform disorders, conversion disorder, Asperger’s syndrome, malingering |
| Toxic disorders | Toxic encephalopathy, neuroleptic malignant syndrome, serotonin syndrome, alcohol and sedative-hypnotic withdrawal, drugs (lithium toxicity, tricyclics, baclofen, tiagabine, overdose) |
| Source: Reference 17,18 | |
Cerebrovascular disease, tumors, and trauma are the most common causes of late-life NCSE.4,19 De novo NCSE occasionally presents:
- after benzodiazepine withdrawal
- with neuroleptic, tricyclic antidepressant, or lithium treatment10,16
- with metabolic abnormalities and nonpsychotropic medications.10
Clinical symptoms
Clinical features of NCSE include cognitive changes, speech abnormalities, affective disturbances, psychosis, poor impulse control, and bizarre behaviors (Table 3). Some patients develop ictal phenomena resembling catatonia or clinical and EEG changes that mimic neuroleptic malignant syndrome (NMS).20-23
Table 3
Clinical features that raise suspicion of NCSE
| Domain | Features |
|---|---|
| Cognitive changes | Prolonged confusion, executive dysfunction, obtundation, attention/memory difficulties, lack of initiative, perseveration, stupor |
| Speech | Poverty of speech with monosyllabic answers, verbal perseveration, echolalia, palilalia, aphasia, paraphasic errors, confabulation, mutism |
| Affective | Prolonged fear, affective indifferent state with blank facial expression, hypomania, psychotic depression, inappropriate laughing and crying, anxiety states |
| Psychosis | Visual, auditory and cenesthetic hallucinations, delusions |
| Impulse control | Hostility, agitation, violence, groping, genital manipulation, picking, posturing |
| Others | Catatonic signs, autonomic disturbances |
| Source: References 5,7-9,12,15-17,20-23 | |
Among 29 patients with acute catatonic syndromes, epileptic activity was identified in 4. One patient with absence status was diagnosed with NMS during the catatonic period.26 Conversely, the commonality of clinical features has led to misdiagnosis of psychogenic catatonia as NCSE. EEG is necessary to exclude NCSE in these cases.
NMS. Yoshino et al27 described two patients taking neuroleptics who met criteria for NMS and had EEG changes consistent with NCSE. They later reported another patient with NCSE complicating NMS; the point at which NCSE developed was unknown, however, because EEG activity was not recorded at NMS onset.28 Based on NMS diagnostic criteria proposed by Caroff et al,29 these patients could have developed NCSE mimicking NMS.
EEG for diagnosis
Candidates. Because differentiating NCSE from similar conditions can be difficult, use EEG to confirm your clinical observations. No guidelines exist, but consider EEG when the patient’s history suggests NCSE. Ask the patient or family about:
- changes in mental status from baseline, especially new-onset catatonia or unexplained altered consciousness
- duration of events
- presence or absence of motor activity
- behavioral fluctuations
- presence or absence of automatisms or blinking.
EEG patterns. Table 4 summarized NCSE diagnostic criteria. NCSE shows characteristic patterns in ASE and CPSE,9,10,16,23 and EEG changes can be continuous or nearly continuous in both.
Table 4
EEG findings that support a clinical diagnosis of NCSE
| Clear-cut criteria |
|---|
| Frequent or continuous focal seizures, with ictal patterns that wax and wane with change in amplitude, frequency, and/or spatial distribution |
| Frequent or continuous generalized spike wave discharges: |
|
| Periodic lateralized epileptiform discharges (“PLEDs”) or bilateral periodic epileptiform discharges (“biPEDs") occurring in patients with coma from generalized tonic-clonic status epilepticus (subtle SE) |
| Probable (equivocal) criteria |
| Patients with acute cerebral damage who also show frequent or continuous EEG abnormalities without previous similar findings |
| Patients with epilepsy who show frequent or continuous generalized EEG abnormalities and similar interictal EEG patterns but whose clinical symptoms suggest NCSE |
| Source: References 4,12-14,17 |
31,32
In CPSE, less-synchronous epileptiform activity has been described, including rhythmical slow, rhythmic spikes, or rhythmic spike and slow waves. Two types of CPSE of frontal origin have been described:
- Type 1 presents clinically with mood disturbance and minimal confusion. EEG shows a frontal focus with a normal background.
- Type 2 presents clinically with confusion. EEG shows bilateral asymmetric frontal discharges.8
- generalized in 69%
- diffuse with focal predominance in 18%
- focal in 13%.
Distinguish between ictal and interictal EEG findings with epileptiform activity, because only the former is diagnostic for NCSE. Intravenous benzodiazepines might be necessary during EEG to verify the diagnosis.33
NCSE has developed after electroconvulsive therapy (ECT), but a cause-effect relationship is debatable. Interictal and abnormal EEG findings after ECT may be misdiagnosed as NCSE.34
Neuroimaging has limited clinical value because of the need for patient cooperation and specialized equipment.4 Head CT or MRI can exclude structural abnormalities. PET and SPECT show increased metabolism and blood flow, respectively, in NCSE. MR spectroscopy shows elevated lactate and decreased N-acetyl aspartate.
Halting ictal activity
To rapidly stop ictal activity—the main goal of treatment—recognizing and correcting precipitant factors is vital:
- Consider discontinuing medications that could lower the seizure threshold.
- Order a complete blood count, serum electrolytes, calcium, arterial-blood gas, liver and renal function tests, urine toxicology screen, and serum antiepileptic drug concentrations.
- When possible, obtain neuroimaging and EEG in the emergency room for accurate diagnosis and prompt treatment.12
Response to benzodiazepines might be transient, lasting only hours or days. For instance, diazepam’s anticonvulsant effect may last
Newer antiepileptics—such as lamotrigine, levetiracetam, or topiramate—have been used with varying results, and their role in first-line treatment of NCSE is evolving. Rarely, the antiepileptic tiagabine precipitates or worsens NCSE.4,13,14
Related resources
- Epilepsy Foundation. www.epilepsyfoundation.org
- Neuroleptic Malignant Syndrome Information Service. Hotline for health professionals (888) 667-8367. www.nmsis.org
- Carbamazepine • Tegretol, Carbatrol
- Clonazepam • Klonopin
- Diazepam • Valium
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Lithium carbonate • Lithobid, Eskalith CR
- Lorazepam • Ativan
- Phenobarbital • Luminal
- Phenytoin • Dilantin
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproic acid • Depakote
The authors report no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
Acknowledgment
Dr. Goveas was a geriatric psychiatry fellow, University of Pennsylvania, when he wrote this article in collaboration with his mentors, Drs. Caroff and Riggio.
1. DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology 1996;46(4):1029-35.
2. Shorvon S. Status epilepticus: Its clinical features and treatment in children and adults Cambridge, UK: Cambridge University Press, 1994.
3. Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003;61:1066-73.
4. Walker M, Cross H, Smith S, et al. Nonconvulsive status epilepticus: Epilepsy research foundation workshop reports. Epileptic Disord 2005;7(3):53-296.
5. Engel J, Ludwig BI, Fetell M. Prolonged partial complex status epilepticus: EEG and behavioral observations. Neurology 1978;28:863-9.
6. Krumholz A, Sung GY, Fisher RS, et al. Complex partial status epilepticus accompanied by serious morbidity and mortality. Neurology 1995;45:1499-1504.
7. Ballenger CE, King DW, Gallagher BB. Partial complex status epilepticus. Neurology 1983;33:1545-52.
8. Thomas P, Zifkin B, Migneco O, et al. Nonconvulsive status epilepticus of frontal origin. Neurology 1999;52:1174-83.
9. Guberman A, Cantu-Reyna G, Stuss D, Broughton R. Nonconvulsive generalized status epilepticus: Clinical features, neuropsychological testing, and long-term follow-up. Neurology 1986;36:1284-91.
10. Thomas P, Beaumanoir A, Genton P, et al. ‘De novo’ absence status of late onset: Report of 11 cases. Neurology 1992;42:104-10.
11. Andermann F, Robb J. Absence status: a reappraisal following review of thirty-eight patients. Epilepsia 1972;13:177-87.
12. Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996;37(7):643-50.
13. Riggio S. Nonconvulsive status epilepticus: Clinical features and diagnostic challenges. Psychiatr Clin N Am 2005;28(3):653-64.
14. Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav 2000;1(5):301-14.
15. Tomson T, Lindbom U, Nilsson BY. Nonconvulsive status epilepticus in adults: Thirty-two consecutive patients from a general hospital population. Epilepsia 1992;3(5):829-35.
16. Dunne JW, Summers QA, Stewart-Wynne EG. Non-convulsive status epilepticus: A prospective study in an adult general hospital. Q J Med 1987;62(238):117-26.
17. Kaplan PW. Behavioral manifestations of nonconvulsive status epilepticus. Epilepsy Behav 2002;3(2):122-39.
18. Mann SC. Malignant catatonia. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A, eds. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing Inc, 2003:121-43.
19. Sung CY, Chu NS. Status epilepticus in elderly: etiology, seizure type and outcome. Acta Neurol Scand 1989;80:51-6.
20. McLachlan RS, Blume WT. Isolated fear in complex partial status epilepticus. Ann Neurol 1980;8:639-41.
21. Walls MJ, Bowers TC, Dilsaver SC, Swann AC. Catatonia associated with depression secondary to complex partial epilepsy. J Clin Psychiatry 1993;54(2):73.-
22. Wells CE. Transient ictal psychosis. Arch Gen Psychiatry 1975;32:1201-3.
23. Agathonikou A, Panayiotopoulos CP, Giannakodimos S, Koutroumanidis M. Typical absence status in adults: Diagnostic and syndromic considerations. Epilepsia 1998;39(12):1265-76.
24. Lim J, Yagnik P, Schraeder P, Wheeler S. Ictal catatonia as a manifestation of nonconvulsive status epilepticus. J Neurol Neurosurg Psychiatry 1986;49:833-6.
25. Drury I, Klass DW, Westmoreland BF, Sharbrough FW. An acute syndrome with psychiatric symptoms and EEG abnormalities. Neurology 1985;35(6):911-14.
26. Primavera A, Fonti A, Novello P, et al. Epileptic seizures in patients with acute catatonic syndrome. J Neurol Neurosurg Psychiatry 1994;57(11):1419-22.
27. Yoshino A, Yoshimasu H, Tatsuzawa Y, et al. Nonconvulsive status epilepticus in two patients with neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18(4):347-9.
28. Yoshino A, Yoshimasu H. Nonconvulsive status epilepticus complicating neuroleptic malignant syndrome improved by intravenous diazepam. J Clin Psychopharmacol 2000;20(3):389-90.
29. Caroff SN. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A, eds. Neuroleptic malignant syndrome and related conditions, 2nd ed. Washington, DC: American Psychiatric Publishing; 2003:1-44.
30. Lob H, Roger J, Soulayrol R. Les etats de mal generalizes a expression confusionelle. In: Gastaut H, Roger J, Lob H, eds. Les etats de mal epileptiques. Paris: Masson; 1967:91-109.
31. Granner MA, Lee SI. Nonconvulsive status epilepticus: EEG analysis in a large series. Epilepsia 1994;35(1):42-7.
32. Niedermeyer E, Fineyre F, Riley T, Uematsu S. Absence status (petit mal status) with focal characteristics. Arch Neurol 1979;36:417-21.
33. Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res 1994;18:155-66.
34. Povlsen UJ, Wildschiodtz G, Hogenhaven H, Bolwig TG. Nonconvulsive status epilepticus after electroconvulsive therapy. J ECT 2003;19(3):164-9.
1. DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology 1996;46(4):1029-35.
2. Shorvon S. Status epilepticus: Its clinical features and treatment in children and adults Cambridge, UK: Cambridge University Press, 1994.
3. Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003;61:1066-73.
4. Walker M, Cross H, Smith S, et al. Nonconvulsive status epilepticus: Epilepsy research foundation workshop reports. Epileptic Disord 2005;7(3):53-296.
5. Engel J, Ludwig BI, Fetell M. Prolonged partial complex status epilepticus: EEG and behavioral observations. Neurology 1978;28:863-9.
6. Krumholz A, Sung GY, Fisher RS, et al. Complex partial status epilepticus accompanied by serious morbidity and mortality. Neurology 1995;45:1499-1504.
7. Ballenger CE, King DW, Gallagher BB. Partial complex status epilepticus. Neurology 1983;33:1545-52.
8. Thomas P, Zifkin B, Migneco O, et al. Nonconvulsive status epilepticus of frontal origin. Neurology 1999;52:1174-83.
9. Guberman A, Cantu-Reyna G, Stuss D, Broughton R. Nonconvulsive generalized status epilepticus: Clinical features, neuropsychological testing, and long-term follow-up. Neurology 1986;36:1284-91.
10. Thomas P, Beaumanoir A, Genton P, et al. ‘De novo’ absence status of late onset: Report of 11 cases. Neurology 1992;42:104-10.
11. Andermann F, Robb J. Absence status: a reappraisal following review of thirty-eight patients. Epilepsia 1972;13:177-87.
12. Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996;37(7):643-50.
13. Riggio S. Nonconvulsive status epilepticus: Clinical features and diagnostic challenges. Psychiatr Clin N Am 2005;28(3):653-64.
14. Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav 2000;1(5):301-14.
15. Tomson T, Lindbom U, Nilsson BY. Nonconvulsive status epilepticus in adults: Thirty-two consecutive patients from a general hospital population. Epilepsia 1992;3(5):829-35.
16. Dunne JW, Summers QA, Stewart-Wynne EG. Non-convulsive status epilepticus: A prospective study in an adult general hospital. Q J Med 1987;62(238):117-26.
17. Kaplan PW. Behavioral manifestations of nonconvulsive status epilepticus. Epilepsy Behav 2002;3(2):122-39.
18. Mann SC. Malignant catatonia. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A, eds. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing Inc, 2003:121-43.
19. Sung CY, Chu NS. Status epilepticus in elderly: etiology, seizure type and outcome. Acta Neurol Scand 1989;80:51-6.
20. McLachlan RS, Blume WT. Isolated fear in complex partial status epilepticus. Ann Neurol 1980;8:639-41.
21. Walls MJ, Bowers TC, Dilsaver SC, Swann AC. Catatonia associated with depression secondary to complex partial epilepsy. J Clin Psychiatry 1993;54(2):73.-
22. Wells CE. Transient ictal psychosis. Arch Gen Psychiatry 1975;32:1201-3.
23. Agathonikou A, Panayiotopoulos CP, Giannakodimos S, Koutroumanidis M. Typical absence status in adults: Diagnostic and syndromic considerations. Epilepsia 1998;39(12):1265-76.
24. Lim J, Yagnik P, Schraeder P, Wheeler S. Ictal catatonia as a manifestation of nonconvulsive status epilepticus. J Neurol Neurosurg Psychiatry 1986;49:833-6.
25. Drury I, Klass DW, Westmoreland BF, Sharbrough FW. An acute syndrome with psychiatric symptoms and EEG abnormalities. Neurology 1985;35(6):911-14.
26. Primavera A, Fonti A, Novello P, et al. Epileptic seizures in patients with acute catatonic syndrome. J Neurol Neurosurg Psychiatry 1994;57(11):1419-22.
27. Yoshino A, Yoshimasu H, Tatsuzawa Y, et al. Nonconvulsive status epilepticus in two patients with neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18(4):347-9.
28. Yoshino A, Yoshimasu H. Nonconvulsive status epilepticus complicating neuroleptic malignant syndrome improved by intravenous diazepam. J Clin Psychopharmacol 2000;20(3):389-90.
29. Caroff SN. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A, eds. Neuroleptic malignant syndrome and related conditions, 2nd ed. Washington, DC: American Psychiatric Publishing; 2003:1-44.
30. Lob H, Roger J, Soulayrol R. Les etats de mal generalizes a expression confusionelle. In: Gastaut H, Roger J, Lob H, eds. Les etats de mal epileptiques. Paris: Masson; 1967:91-109.
31. Granner MA, Lee SI. Nonconvulsive status epilepticus: EEG analysis in a large series. Epilepsia 1994;35(1):42-7.
32. Niedermeyer E, Fineyre F, Riley T, Uematsu S. Absence status (petit mal status) with focal characteristics. Arch Neurol 1979;36:417-21.
33. Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res 1994;18:155-66.
34. Povlsen UJ, Wildschiodtz G, Hogenhaven H, Bolwig TG. Nonconvulsive status epilepticus after electroconvulsive therapy. J ECT 2003;19(3):164-9.
Sexual dysfunction: What’s love got to do with it?
Concepts of love and sexual desire lurk around clinical discussions of sexual dysfunction. Love is frequently dismissed as hopelessly unscientific, whereas desire is simplified as if it were a thing called libido. Decreased libido per se tells us little about a patient’s sexual complaints; the key is to differentiate between:
- those with sexual drive but no motivation for their partners
- those with no driveFemale sexual dysfunction”).
Psychiatrists avoid talking about love; it has too many meanings and nuances, too many avenues of defeat, and is too abstract. All you have to say to a patient is, “Tell me about your marriage,” and listen closely as he or she comes to grip with love’s complexity.
This article’s aim is to help you counsel patients more effectively about relationship and sexual problems by exploring two questions: “What is love?” and “What is sexual desire?”
What is love?
Mrs. C, age 41, is being treated for depression and wonders why she has lost desire for her husband. The antidepressant she is taking improves her mood and diminishes her considerable anxiety but makes her feel sexually dead. “My husband doesn’t mind how I feel, as long as he can have sex,” she says.
After adjusting her medication, you explore other problems that might be contributing to her sexual dysfunction. She expresses uncertainty about what love is. Though faithful and committed to her husband, she has stopped enjoying the way he interacts with her, their two grade-school children, her family, and friends.
Love is the usual context within which sexual activities are viewed. Among adults, unhappiness in love predisposes to sexual concerns, and sexual concerns interfere with loving and being loved.
Our patients’ expectations for feeling and receiving love and experiencing satisfying sex are disappointed through a myriad of avenues. Clinicians may overlook it, but demoralization about love can precede the onset of anxiety, panic attacks, and lingering depression.2 Sexual love is expected to begin with connecting with a partner and to evolve for 65 or more years. Most individuals harbor the secret that they are not certain what love is (Table 1) or are surprised by their lack of words to explain it.
1. Love as transient emotion. The assumption that love is a feeling leads too many people waiting to experience the pure feeling. But unlike sadness, fear, anger, or shame, love does not indicate a discrete feeling. Saying, “I love you,” connotes at least two feelings: pleasure and interest.
- Pleasure begins with pleasantness and moves up through delight to exhilaration.
- Interest ranges from mild curiosity to preoccupying fascination.
Most events simultaneously provoke more than one feeling. Discovering that your beloved wants to marry you usually brings about at least happiness, pride, gratitude, and awe. Even if only one feeling is produced, our attitude towards that feeling complicates the experience. When a child is taught that feeling envy is wrong, for example, his experience of it evokes anxiety (from the guilt) and shame (if someone is watching).
After the family, culture, and the person have worked on a simple feeling, it becomes a layered complexity called an emotion. Love, the emotion, is quickly layered with attitudes (which are the product of feelings and defenses against them) based on the person’s sense of safety stemming from earlier attachments.3 When someone says “I love you,” he or she knows the motive for saying it and hopes for a particular response from the listener.
Sexual desire is an ingredient of love’s emotional complexity. Because “I love you” can create sexual arousal in the listener, the speaker can use the phrase when his or her primary pleasure and interest in the person is the anticipation of sex.
Meanings and motives for expressing love change all the time. When someone tells us “I love you,” we have to discern both meaning and motive. Love’s emotions and their expression to another person are always complicated by past, present, and future considerations.
2. Love as an ambition. Love is so intensely celebrated in every culture that few people grow up without longing to realize it. Table 2 shows one version of the ambition to love and be loved.4 Many clinical declarations of love for a partner signify that the person has not yet given up on this ambition.
3. Love as an arrangement. All adult sexual relationships are quid pro quo exchanges of hopes, expectations, and assets. During courtship, both people are preoccupied with answering the question: “What will this person bring to my life?”
The question has many dimensions: social, economic, aesthetic, recreational, sexual, medical, time-to-death, and more. In their first romantic relationships, people generally prefer not to think in these terms. Their embarrassment dissipates with experience.
This ordinary process can be more clearly perceived after a relationship ends by breakup, divorce, or death and the person begins anew with someone. The person then can deliberately weigh the factors that will determine his or her involvement. When an arrangement is worked out, each person perceives what has been offered by the partner. Of course, perceptions vary in accuracy.
Anticipating making a deal can be very exciting, and once the deal is formally accepted, people often feel a celebratory degree of pleasure, interest, and sexual desire. They think that life is good. In cultures where parents make the deal, the couple courts in the hope that they will fall in love by early marriage.
4. Love is an attachment. Love also means the presence of a bond or attachment. People weave their psyches together and begin to feel a hunger to be with the other person. They think of themselves as belonging with and to the other.
Sexual activities—particularly those that lead to orgasm—facilitate attachment, but the bonds within each partner’s mind do not necessarily develop at the same time or solidify at the same rate. Thus, some people are unable to answer, “I love you, too,” when the partner reveals feelings that are summarized as love.
5. Love as a moral commitment. The rituals that sanctify marriage emphasize clearly that love is a commitment for couples to try to realize the grand idealized ambition (see “Love as an ambition”). The rituals are public promises to honor and cherish each other through all of life’s vicissitudes.
This love as moral commitment instantly restructures life by generating a new set of obligations. Many hostile, disappointed, and seemingly asexual spouses who have not felt pleasure and interest in a partner for a long time will tell their doctors they love the partner. They mean they remain bound by their moral commitment.
6. Love as a mental struggle. Love’s original emotions are stimulated by an idealized version of the partner. This image is internalized early in the relationship. As time passes, discovering our partner’s limitations gradually attenuates our idealization. We think of our earlier appraisals as naïve. Even so, disappointment does not quickly cancel our commitment because of our:
- ambition to love
- obligation to live through bad moments
- ability to love the idealized version of the partner
- moral commitments to raising our children.
The moral commitment to love can sustain people for a lifetime, despite grave disappointments. It also explains the persistent guilt many feel as they contemplate extramarital affairs, divorce, and the agonizing dilemma between their commitment to live with their children and their wish to be free of unhappiness with their partner.
“I love my partner, but I am not in love with him/her,” means, “although I’m still committed, I have lost my ability to idealize my partner.”
7. Love as a force of nature. Love is a force in nature that creates a unity from two individuals. It casts our fates together, organizes reproduction, and remains vital to adult growth and development and to the maturation of children. This love is a backbone that supports the sexual and non-sexual processes of our lives.5
Among older couples, “I love my partner but I am no longer in love with her/him” may mean, “We have shared so much of our lives that my partner is an inextricable part of me. I could never be free of my partner, even though most of the pleasure is gone.”
8. Love as an illusion. We create love for our partner by internal private processes, maintain it by prudent diplomatic dishonesties, and can lose it without the partner’s knowing. To remain in an intimate relationship, the processes of love require defensive distortions of a person’s feelings, thoughts, and perceptions.
As individuals gain experience, many look back and see that their assumptions about love were self-serving illusions. When entire relationships are dismissed with “what was I thinking?” the person usually means that now I can perceive that I created illusions so as not to admit to the depth of my disappointment with my partner.
9. Love as a stop sign. When a person says, “I love you,” the listener is challenged to discern its meaning. The emotions and motives behind the sentence can be very difficult to accurately perceive. Some love relationships, after all, are deceptions.
At any particular moment, we may know what we mean by “I love you” and why we are saying it. We may not be willing, however, to have our motives, meanings, and emotions fully known by the listener. In fact, the motive for saying “I love you” is often to obscure the view:
- Lover A: I love you.
- Lover B: Why do you love me?
- Lover A: I don’t know, I just do.
- Doctor: Why do you put up with this behavior from your spouse?
- Patient: Because I love him.
- Doctor: What does that mean?
- Patient: I don’t know.
What is the meaning of ‘I love you’? Love is…
| A transient emotion |
| An ambition |
| An arrangement |
| An attachment |
| A moral commitment |
| A mental struggle |
| A force of nature |
| An illusion |
| A stop sign |
Love as ambition: 7 ideals for loving relationships
| Mutual respect |
| Behavioral reliability |
| Enjoyment of one another |
| Sexual fidelity |
| Psychological intimacy |
| Sexual pleasure |
| A comfortable balance of individuality and couplehood |
| Source: Reference 4 |
What is sexual desire?
Sexual desire—at any given moment—is the sum of biological, psychological, interpersonal, and cultural forces that incline us toward and away from sexual behavior.6 Understanding desire can help you:
- ask patients insightful questions about their relationship concerns
- formulate a hypothesis to explain how drive, motivation, and values contribute to a patient’s sexual dysfunction.
Motivation is the degree of willingness an individual has to enter into sexual behavior with a particular partner at a moment in time. Sexual motivation is a psychological force that is influenced by:
- affective states, such as joy or sorrow
- interpersonal states, such as mutual affection, disagreement, or disrespect
- relationship stage, such as short or long duration
- cognitive states, such as moral disapproval.7
Values serve an evaluative function as our minds screen personal sexual behaviors with two questions:
- Is the behavior normal or abnormal?
- Is it morally acceptable or unacceptable?
In talking with Mrs. C, for example, you learn that her family reinforced the religious prohibition against extramarital sexual expression. “When I was a teenager, my father told me not to come home if I got pregnant before I was married,” she relates.
Values augment or diminish desire by affecting our willingness to engage in sexual behaviors. Values are camouflaged as motivation; Mrs. C may not realize that values she acquired at home early in life continue to influence her and may contribute to her lack of desire for nonreproductive sex.
Related resources
- Regan PC: Love relationships. In: Muscarella F (ed). Psychological perspectives on human sexuality. New York: John Wiley & Sons; 2000:232-82.
- Aron A, Fisher H, Mashek DJ. Reward, motivation, and emotion systems associated with early stage intense romantic love. J Neurophysiology 2005;94:327-37.
Singer Tina Turner recorded “What’s Love Got To Do With It?” in 1984.
1. Basson R. Sexual desire and arousal disorders in women. N Engl J Med 2006;354(14):1497-1506.
2. Levine SB. What is love anyway? J Sex Marital Ther 2005;31(2):143-51.
3. Bowlby J. The making and breaking of affectional bonds. London: Routledge; 1989.
4. Levine SB. Sexuality in mid-life. New York: Plenum; 1998.
5. Lear J. Love and its place in nature: a philosophical interpretation of Freudian psychoanalysis. New York: Farrar, Straus & Giroux; 1990.
6. Levine SB. The nature of sexual desire: a clinician’s perspective. Arch Sex Behav 2003;32(3):279-85.
7. Clement U. Sex in long-term relationships: a systemic approach to sexual desire problems. Arch Sex Behav 2002;31(3):241-6.
Concepts of love and sexual desire lurk around clinical discussions of sexual dysfunction. Love is frequently dismissed as hopelessly unscientific, whereas desire is simplified as if it were a thing called libido. Decreased libido per se tells us little about a patient’s sexual complaints; the key is to differentiate between:
- those with sexual drive but no motivation for their partners
- those with no driveFemale sexual dysfunction”).
Psychiatrists avoid talking about love; it has too many meanings and nuances, too many avenues of defeat, and is too abstract. All you have to say to a patient is, “Tell me about your marriage,” and listen closely as he or she comes to grip with love’s complexity.
This article’s aim is to help you counsel patients more effectively about relationship and sexual problems by exploring two questions: “What is love?” and “What is sexual desire?”
What is love?
Mrs. C, age 41, is being treated for depression and wonders why she has lost desire for her husband. The antidepressant she is taking improves her mood and diminishes her considerable anxiety but makes her feel sexually dead. “My husband doesn’t mind how I feel, as long as he can have sex,” she says.
After adjusting her medication, you explore other problems that might be contributing to her sexual dysfunction. She expresses uncertainty about what love is. Though faithful and committed to her husband, she has stopped enjoying the way he interacts with her, their two grade-school children, her family, and friends.
Love is the usual context within which sexual activities are viewed. Among adults, unhappiness in love predisposes to sexual concerns, and sexual concerns interfere with loving and being loved.
Our patients’ expectations for feeling and receiving love and experiencing satisfying sex are disappointed through a myriad of avenues. Clinicians may overlook it, but demoralization about love can precede the onset of anxiety, panic attacks, and lingering depression.2 Sexual love is expected to begin with connecting with a partner and to evolve for 65 or more years. Most individuals harbor the secret that they are not certain what love is (Table 1) or are surprised by their lack of words to explain it.
1. Love as transient emotion. The assumption that love is a feeling leads too many people waiting to experience the pure feeling. But unlike sadness, fear, anger, or shame, love does not indicate a discrete feeling. Saying, “I love you,” connotes at least two feelings: pleasure and interest.
- Pleasure begins with pleasantness and moves up through delight to exhilaration.
- Interest ranges from mild curiosity to preoccupying fascination.
Most events simultaneously provoke more than one feeling. Discovering that your beloved wants to marry you usually brings about at least happiness, pride, gratitude, and awe. Even if only one feeling is produced, our attitude towards that feeling complicates the experience. When a child is taught that feeling envy is wrong, for example, his experience of it evokes anxiety (from the guilt) and shame (if someone is watching).
After the family, culture, and the person have worked on a simple feeling, it becomes a layered complexity called an emotion. Love, the emotion, is quickly layered with attitudes (which are the product of feelings and defenses against them) based on the person’s sense of safety stemming from earlier attachments.3 When someone says “I love you,” he or she knows the motive for saying it and hopes for a particular response from the listener.
Sexual desire is an ingredient of love’s emotional complexity. Because “I love you” can create sexual arousal in the listener, the speaker can use the phrase when his or her primary pleasure and interest in the person is the anticipation of sex.
Meanings and motives for expressing love change all the time. When someone tells us “I love you,” we have to discern both meaning and motive. Love’s emotions and their expression to another person are always complicated by past, present, and future considerations.
2. Love as an ambition. Love is so intensely celebrated in every culture that few people grow up without longing to realize it. Table 2 shows one version of the ambition to love and be loved.4 Many clinical declarations of love for a partner signify that the person has not yet given up on this ambition.
3. Love as an arrangement. All adult sexual relationships are quid pro quo exchanges of hopes, expectations, and assets. During courtship, both people are preoccupied with answering the question: “What will this person bring to my life?”
The question has many dimensions: social, economic, aesthetic, recreational, sexual, medical, time-to-death, and more. In their first romantic relationships, people generally prefer not to think in these terms. Their embarrassment dissipates with experience.
This ordinary process can be more clearly perceived after a relationship ends by breakup, divorce, or death and the person begins anew with someone. The person then can deliberately weigh the factors that will determine his or her involvement. When an arrangement is worked out, each person perceives what has been offered by the partner. Of course, perceptions vary in accuracy.
Anticipating making a deal can be very exciting, and once the deal is formally accepted, people often feel a celebratory degree of pleasure, interest, and sexual desire. They think that life is good. In cultures where parents make the deal, the couple courts in the hope that they will fall in love by early marriage.
4. Love is an attachment. Love also means the presence of a bond or attachment. People weave their psyches together and begin to feel a hunger to be with the other person. They think of themselves as belonging with and to the other.
Sexual activities—particularly those that lead to orgasm—facilitate attachment, but the bonds within each partner’s mind do not necessarily develop at the same time or solidify at the same rate. Thus, some people are unable to answer, “I love you, too,” when the partner reveals feelings that are summarized as love.
5. Love as a moral commitment. The rituals that sanctify marriage emphasize clearly that love is a commitment for couples to try to realize the grand idealized ambition (see “Love as an ambition”). The rituals are public promises to honor and cherish each other through all of life’s vicissitudes.
This love as moral commitment instantly restructures life by generating a new set of obligations. Many hostile, disappointed, and seemingly asexual spouses who have not felt pleasure and interest in a partner for a long time will tell their doctors they love the partner. They mean they remain bound by their moral commitment.
6. Love as a mental struggle. Love’s original emotions are stimulated by an idealized version of the partner. This image is internalized early in the relationship. As time passes, discovering our partner’s limitations gradually attenuates our idealization. We think of our earlier appraisals as naïve. Even so, disappointment does not quickly cancel our commitment because of our:
- ambition to love
- obligation to live through bad moments
- ability to love the idealized version of the partner
- moral commitments to raising our children.
The moral commitment to love can sustain people for a lifetime, despite grave disappointments. It also explains the persistent guilt many feel as they contemplate extramarital affairs, divorce, and the agonizing dilemma between their commitment to live with their children and their wish to be free of unhappiness with their partner.
“I love my partner, but I am not in love with him/her,” means, “although I’m still committed, I have lost my ability to idealize my partner.”
7. Love as a force of nature. Love is a force in nature that creates a unity from two individuals. It casts our fates together, organizes reproduction, and remains vital to adult growth and development and to the maturation of children. This love is a backbone that supports the sexual and non-sexual processes of our lives.5
Among older couples, “I love my partner but I am no longer in love with her/him” may mean, “We have shared so much of our lives that my partner is an inextricable part of me. I could never be free of my partner, even though most of the pleasure is gone.”
8. Love as an illusion. We create love for our partner by internal private processes, maintain it by prudent diplomatic dishonesties, and can lose it without the partner’s knowing. To remain in an intimate relationship, the processes of love require defensive distortions of a person’s feelings, thoughts, and perceptions.
As individuals gain experience, many look back and see that their assumptions about love were self-serving illusions. When entire relationships are dismissed with “what was I thinking?” the person usually means that now I can perceive that I created illusions so as not to admit to the depth of my disappointment with my partner.
9. Love as a stop sign. When a person says, “I love you,” the listener is challenged to discern its meaning. The emotions and motives behind the sentence can be very difficult to accurately perceive. Some love relationships, after all, are deceptions.
At any particular moment, we may know what we mean by “I love you” and why we are saying it. We may not be willing, however, to have our motives, meanings, and emotions fully known by the listener. In fact, the motive for saying “I love you” is often to obscure the view:
- Lover A: I love you.
- Lover B: Why do you love me?
- Lover A: I don’t know, I just do.
- Doctor: Why do you put up with this behavior from your spouse?
- Patient: Because I love him.
- Doctor: What does that mean?
- Patient: I don’t know.
What is the meaning of ‘I love you’? Love is…
| A transient emotion |
| An ambition |
| An arrangement |
| An attachment |
| A moral commitment |
| A mental struggle |
| A force of nature |
| An illusion |
| A stop sign |
Love as ambition: 7 ideals for loving relationships
| Mutual respect |
| Behavioral reliability |
| Enjoyment of one another |
| Sexual fidelity |
| Psychological intimacy |
| Sexual pleasure |
| A comfortable balance of individuality and couplehood |
| Source: Reference 4 |
What is sexual desire?
Sexual desire—at any given moment—is the sum of biological, psychological, interpersonal, and cultural forces that incline us toward and away from sexual behavior.6 Understanding desire can help you:
- ask patients insightful questions about their relationship concerns
- formulate a hypothesis to explain how drive, motivation, and values contribute to a patient’s sexual dysfunction.
Motivation is the degree of willingness an individual has to enter into sexual behavior with a particular partner at a moment in time. Sexual motivation is a psychological force that is influenced by:
- affective states, such as joy or sorrow
- interpersonal states, such as mutual affection, disagreement, or disrespect
- relationship stage, such as short or long duration
- cognitive states, such as moral disapproval.7
Values serve an evaluative function as our minds screen personal sexual behaviors with two questions:
- Is the behavior normal or abnormal?
- Is it morally acceptable or unacceptable?
In talking with Mrs. C, for example, you learn that her family reinforced the religious prohibition against extramarital sexual expression. “When I was a teenager, my father told me not to come home if I got pregnant before I was married,” she relates.
Values augment or diminish desire by affecting our willingness to engage in sexual behaviors. Values are camouflaged as motivation; Mrs. C may not realize that values she acquired at home early in life continue to influence her and may contribute to her lack of desire for nonreproductive sex.
Related resources
- Regan PC: Love relationships. In: Muscarella F (ed). Psychological perspectives on human sexuality. New York: John Wiley & Sons; 2000:232-82.
- Aron A, Fisher H, Mashek DJ. Reward, motivation, and emotion systems associated with early stage intense romantic love. J Neurophysiology 2005;94:327-37.
Singer Tina Turner recorded “What’s Love Got To Do With It?” in 1984.
Concepts of love and sexual desire lurk around clinical discussions of sexual dysfunction. Love is frequently dismissed as hopelessly unscientific, whereas desire is simplified as if it were a thing called libido. Decreased libido per se tells us little about a patient’s sexual complaints; the key is to differentiate between:
- those with sexual drive but no motivation for their partners
- those with no driveFemale sexual dysfunction”).
Psychiatrists avoid talking about love; it has too many meanings and nuances, too many avenues of defeat, and is too abstract. All you have to say to a patient is, “Tell me about your marriage,” and listen closely as he or she comes to grip with love’s complexity.
This article’s aim is to help you counsel patients more effectively about relationship and sexual problems by exploring two questions: “What is love?” and “What is sexual desire?”
What is love?
Mrs. C, age 41, is being treated for depression and wonders why she has lost desire for her husband. The antidepressant she is taking improves her mood and diminishes her considerable anxiety but makes her feel sexually dead. “My husband doesn’t mind how I feel, as long as he can have sex,” she says.
After adjusting her medication, you explore other problems that might be contributing to her sexual dysfunction. She expresses uncertainty about what love is. Though faithful and committed to her husband, she has stopped enjoying the way he interacts with her, their two grade-school children, her family, and friends.
Love is the usual context within which sexual activities are viewed. Among adults, unhappiness in love predisposes to sexual concerns, and sexual concerns interfere with loving and being loved.
Our patients’ expectations for feeling and receiving love and experiencing satisfying sex are disappointed through a myriad of avenues. Clinicians may overlook it, but demoralization about love can precede the onset of anxiety, panic attacks, and lingering depression.2 Sexual love is expected to begin with connecting with a partner and to evolve for 65 or more years. Most individuals harbor the secret that they are not certain what love is (Table 1) or are surprised by their lack of words to explain it.
1. Love as transient emotion. The assumption that love is a feeling leads too many people waiting to experience the pure feeling. But unlike sadness, fear, anger, or shame, love does not indicate a discrete feeling. Saying, “I love you,” connotes at least two feelings: pleasure and interest.
- Pleasure begins with pleasantness and moves up through delight to exhilaration.
- Interest ranges from mild curiosity to preoccupying fascination.
Most events simultaneously provoke more than one feeling. Discovering that your beloved wants to marry you usually brings about at least happiness, pride, gratitude, and awe. Even if only one feeling is produced, our attitude towards that feeling complicates the experience. When a child is taught that feeling envy is wrong, for example, his experience of it evokes anxiety (from the guilt) and shame (if someone is watching).
After the family, culture, and the person have worked on a simple feeling, it becomes a layered complexity called an emotion. Love, the emotion, is quickly layered with attitudes (which are the product of feelings and defenses against them) based on the person’s sense of safety stemming from earlier attachments.3 When someone says “I love you,” he or she knows the motive for saying it and hopes for a particular response from the listener.
Sexual desire is an ingredient of love’s emotional complexity. Because “I love you” can create sexual arousal in the listener, the speaker can use the phrase when his or her primary pleasure and interest in the person is the anticipation of sex.
Meanings and motives for expressing love change all the time. When someone tells us “I love you,” we have to discern both meaning and motive. Love’s emotions and their expression to another person are always complicated by past, present, and future considerations.
2. Love as an ambition. Love is so intensely celebrated in every culture that few people grow up without longing to realize it. Table 2 shows one version of the ambition to love and be loved.4 Many clinical declarations of love for a partner signify that the person has not yet given up on this ambition.
3. Love as an arrangement. All adult sexual relationships are quid pro quo exchanges of hopes, expectations, and assets. During courtship, both people are preoccupied with answering the question: “What will this person bring to my life?”
The question has many dimensions: social, economic, aesthetic, recreational, sexual, medical, time-to-death, and more. In their first romantic relationships, people generally prefer not to think in these terms. Their embarrassment dissipates with experience.
This ordinary process can be more clearly perceived after a relationship ends by breakup, divorce, or death and the person begins anew with someone. The person then can deliberately weigh the factors that will determine his or her involvement. When an arrangement is worked out, each person perceives what has been offered by the partner. Of course, perceptions vary in accuracy.
Anticipating making a deal can be very exciting, and once the deal is formally accepted, people often feel a celebratory degree of pleasure, interest, and sexual desire. They think that life is good. In cultures where parents make the deal, the couple courts in the hope that they will fall in love by early marriage.
4. Love is an attachment. Love also means the presence of a bond or attachment. People weave their psyches together and begin to feel a hunger to be with the other person. They think of themselves as belonging with and to the other.
Sexual activities—particularly those that lead to orgasm—facilitate attachment, but the bonds within each partner’s mind do not necessarily develop at the same time or solidify at the same rate. Thus, some people are unable to answer, “I love you, too,” when the partner reveals feelings that are summarized as love.
5. Love as a moral commitment. The rituals that sanctify marriage emphasize clearly that love is a commitment for couples to try to realize the grand idealized ambition (see “Love as an ambition”). The rituals are public promises to honor and cherish each other through all of life’s vicissitudes.
This love as moral commitment instantly restructures life by generating a new set of obligations. Many hostile, disappointed, and seemingly asexual spouses who have not felt pleasure and interest in a partner for a long time will tell their doctors they love the partner. They mean they remain bound by their moral commitment.
6. Love as a mental struggle. Love’s original emotions are stimulated by an idealized version of the partner. This image is internalized early in the relationship. As time passes, discovering our partner’s limitations gradually attenuates our idealization. We think of our earlier appraisals as naïve. Even so, disappointment does not quickly cancel our commitment because of our:
- ambition to love
- obligation to live through bad moments
- ability to love the idealized version of the partner
- moral commitments to raising our children.
The moral commitment to love can sustain people for a lifetime, despite grave disappointments. It also explains the persistent guilt many feel as they contemplate extramarital affairs, divorce, and the agonizing dilemma between their commitment to live with their children and their wish to be free of unhappiness with their partner.
“I love my partner, but I am not in love with him/her,” means, “although I’m still committed, I have lost my ability to idealize my partner.”
7. Love as a force of nature. Love is a force in nature that creates a unity from two individuals. It casts our fates together, organizes reproduction, and remains vital to adult growth and development and to the maturation of children. This love is a backbone that supports the sexual and non-sexual processes of our lives.5
Among older couples, “I love my partner but I am no longer in love with her/him” may mean, “We have shared so much of our lives that my partner is an inextricable part of me. I could never be free of my partner, even though most of the pleasure is gone.”
8. Love as an illusion. We create love for our partner by internal private processes, maintain it by prudent diplomatic dishonesties, and can lose it without the partner’s knowing. To remain in an intimate relationship, the processes of love require defensive distortions of a person’s feelings, thoughts, and perceptions.
As individuals gain experience, many look back and see that their assumptions about love were self-serving illusions. When entire relationships are dismissed with “what was I thinking?” the person usually means that now I can perceive that I created illusions so as not to admit to the depth of my disappointment with my partner.
9. Love as a stop sign. When a person says, “I love you,” the listener is challenged to discern its meaning. The emotions and motives behind the sentence can be very difficult to accurately perceive. Some love relationships, after all, are deceptions.
At any particular moment, we may know what we mean by “I love you” and why we are saying it. We may not be willing, however, to have our motives, meanings, and emotions fully known by the listener. In fact, the motive for saying “I love you” is often to obscure the view:
- Lover A: I love you.
- Lover B: Why do you love me?
- Lover A: I don’t know, I just do.
- Doctor: Why do you put up with this behavior from your spouse?
- Patient: Because I love him.
- Doctor: What does that mean?
- Patient: I don’t know.
What is the meaning of ‘I love you’? Love is…
| A transient emotion |
| An ambition |
| An arrangement |
| An attachment |
| A moral commitment |
| A mental struggle |
| A force of nature |
| An illusion |
| A stop sign |
Love as ambition: 7 ideals for loving relationships
| Mutual respect |
| Behavioral reliability |
| Enjoyment of one another |
| Sexual fidelity |
| Psychological intimacy |
| Sexual pleasure |
| A comfortable balance of individuality and couplehood |
| Source: Reference 4 |
What is sexual desire?
Sexual desire—at any given moment—is the sum of biological, psychological, interpersonal, and cultural forces that incline us toward and away from sexual behavior.6 Understanding desire can help you:
- ask patients insightful questions about their relationship concerns
- formulate a hypothesis to explain how drive, motivation, and values contribute to a patient’s sexual dysfunction.
Motivation is the degree of willingness an individual has to enter into sexual behavior with a particular partner at a moment in time. Sexual motivation is a psychological force that is influenced by:
- affective states, such as joy or sorrow
- interpersonal states, such as mutual affection, disagreement, or disrespect
- relationship stage, such as short or long duration
- cognitive states, such as moral disapproval.7
Values serve an evaluative function as our minds screen personal sexual behaviors with two questions:
- Is the behavior normal or abnormal?
- Is it morally acceptable or unacceptable?
In talking with Mrs. C, for example, you learn that her family reinforced the religious prohibition against extramarital sexual expression. “When I was a teenager, my father told me not to come home if I got pregnant before I was married,” she relates.
Values augment or diminish desire by affecting our willingness to engage in sexual behaviors. Values are camouflaged as motivation; Mrs. C may not realize that values she acquired at home early in life continue to influence her and may contribute to her lack of desire for nonreproductive sex.
Related resources
- Regan PC: Love relationships. In: Muscarella F (ed). Psychological perspectives on human sexuality. New York: John Wiley & Sons; 2000:232-82.
- Aron A, Fisher H, Mashek DJ. Reward, motivation, and emotion systems associated with early stage intense romantic love. J Neurophysiology 2005;94:327-37.
Singer Tina Turner recorded “What’s Love Got To Do With It?” in 1984.
1. Basson R. Sexual desire and arousal disorders in women. N Engl J Med 2006;354(14):1497-1506.
2. Levine SB. What is love anyway? J Sex Marital Ther 2005;31(2):143-51.
3. Bowlby J. The making and breaking of affectional bonds. London: Routledge; 1989.
4. Levine SB. Sexuality in mid-life. New York: Plenum; 1998.
5. Lear J. Love and its place in nature: a philosophical interpretation of Freudian psychoanalysis. New York: Farrar, Straus & Giroux; 1990.
6. Levine SB. The nature of sexual desire: a clinician’s perspective. Arch Sex Behav 2003;32(3):279-85.
7. Clement U. Sex in long-term relationships: a systemic approach to sexual desire problems. Arch Sex Behav 2002;31(3):241-6.
1. Basson R. Sexual desire and arousal disorders in women. N Engl J Med 2006;354(14):1497-1506.
2. Levine SB. What is love anyway? J Sex Marital Ther 2005;31(2):143-51.
3. Bowlby J. The making and breaking of affectional bonds. London: Routledge; 1989.
4. Levine SB. Sexuality in mid-life. New York: Plenum; 1998.
5. Lear J. Love and its place in nature: a philosophical interpretation of Freudian psychoanalysis. New York: Farrar, Straus & Giroux; 1990.
6. Levine SB. The nature of sexual desire: a clinician’s perspective. Arch Sex Behav 2003;32(3):279-85.
7. Clement U. Sex in long-term relationships: a systemic approach to sexual desire problems. Arch Sex Behav 2002;31(3):241-6.
‘Supercharge’ antidepressants by adding thyroid hormones
Prescribing thyroid hormones with antidepressants—whether to augment the antidepressant effect or accelerate patient response—is a well-researched strategy for treatment-resistant major depressive disorder (MDD). Thyroid hormones are known to boost response to tricyclics, and preliminary evidence shows they may be useful adjuvants to selective serotonin reuptake inhibitors (SSRIs) as well.
Thyroid hormones enter the brain slowly across the blood-brain barrier and choroid plexus. They accumulate in the locus ceruleus and other structures and are distributed widely along noradrenergic pathways.
Effective treatments are available for MDD, although 30% to 40% of patients do not respond to one or more antidepressant trials (Box).1-4 This article offers:
- new information about why triiodothyronine (T3) and thyroxine (T4) can “super-charge” antidepressant response
- tips on how to use thyroid hormones in patients with MDD, including effective dosages, patient monitoring, and treatment durations.
- 30% to 40% of patients with major depressive disorder (MDD) do not respond sufficiently to usual antidepressant treatment1
- Even under optimal treatment conditions, only one-third of patients achieve remission2
- Among patients who fail to respond to two pharmacologic interventions, remission rates with the next antidepressant are as low as 12%3
- A patient becomes less likely to respond clinically with each additional nonresponse to antidepressant treatment4
Why thyroid hormones?
Thyroid hormones enter the brain slowly across the blood-brain barrier and the choroid plexus—cerebrospinal fluid barriers. T4 is the main source of brain T3—after attack by 5’deiodinase—but circulating T3 also crosses the blood-brain barrier through active transport.
Thyroid hormones accumulate in the locus ceruleus and other central noradrenergic structures and are distributed widely in the brain along noradrenergic pathways.5-7 The mechanism of their therapeutic effect for MDD is not well understood, and various hypotheses have been proposed.
Subclinical hypothyroidism. Early studies such as by Howland8 of treatment-refractory MDD suggested that thyroid hormone augmentation might correct a hypothyroid state. However, blood thyroid hormone levels are not associated with resistance to antidepressant treatment, according to studies of MDD populations.9-10 Also, thyroid hormones’ therapeutic action in MDD appears unlikely to be related to treating subclinical hypothyroidism because patients’ euthyroid status was verified in all adjuvant studies since 1980.
Joffe et al11 proposed that MDD is characterized by a relative excess of T4 versus T3—probably related to a deficit in converting T4 to T3 in the periphery—and administering T3 would therefore correct this imbalance. They offered no strong evidence for an increased T4 level, however, and later studies failed to detect the postulated blood T3 abnormalities in MDD.9-10 Also—as suggested by studies with high-dose T4 augmentation12,13—the adjuvant antidepressant effect of thyroid hormones is not restricted to T3, although T3 may be more efficacious than T4.14
Neurotransmitter effects. Thyroid hormones’ role in increasing serotonin (5-HT) release could partially explain the benefit of adjuvant thyroid hormone therapy in MDD. Researchers found:
- T3 increased cortical 5-HT levels, probably by reducing the autoinhibitory effect of the presynaptic 5-HT1A receptor15
- adding T3 to clomipramine therapy increased 5-HT levels to a greater extent than T3 or clomipramine used alone16
- Low 5-HT activity, shown in hypothyroid patients, increased after T4 replacement.17
This 5-HT release theory cannot explain why thyroid hormones have a rapid clinical effect in MDD. Most studies report the hormones have clinical efficacy in MDD within 4 weeks.18 Similarly, selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels within hours of treatment onset, but the clinical effect occurs 4 to 6 weeks later.19 Therefore, increased 5-HT levels cannot fully explain thyroid hormones’ early effect.
Close interaction between thyroid hormones and the noradrenergic system also has been examined. Brain T3 is primarily localized in the central noradrenergic systems, with axonal anterograde transport of T3 from the locus ceruleus. T3 is processed and accumulated in the noradrenergic system, carried via axonal transport, then delivered from nerve cell bodies to its neuronal targets.5,7 T3 thus functions as a coneurotransmitter with norepinephrine.
Cellular energy metabolism. We recently reported that thyroid hormones’ antidepressant effect may be related to brain cellular energy metabolism. Thyroid hormones increase cellular levels of adenosine triphosphate (ATP) and phosphocreatine (PCr) in the hypothyroid brain.20 Brain imaging—phosphorus-31 nuclear magnetic resonance spectroscopy (31P-MRS)—of subjects with MDD shows decreased brain levels of ATP and increased PCr.21
Our group showed that the antidepressant effect of T3 augmentation of SSRIs is correlated with significant increases in ATP levels and decreases in PCr. This effect—which appears to represent re-normalization of brain bioenergetics in treatment responders—did not occur in nonresponders (Iosifescu et al, presented at APA, 2004).
The effect of thyroid hormones on bioenergetic metabolism is compatible with the hypothesized effects on noradrenergic and serotonergic systems.5,7 These mechanisms may represent different links in the same chain of events.
Antidepressant boosters
Thyroid hormones have been used extensively to treat MDD since the 1950s, when researchers reported that T3 monotherapy was efficacious for treating depression. These early studies had important methodologic limitations, including open designs and poorly defined diagnostic criteria and response.
For MDD, the most extensively researched uses of thyroid hormones are to augment therapy for antidepressant nonresponders and to accelerate partial response to antidepressants.
Tricyclics. Open studies primarily among outpatients in the 1970s and ‘80s suggested that thyroid hormones are a valid augmentation strategy for nonresponders to tricyclic antidepressants. Most—but not all—reported response rates >50% with T3 dosages of 20 to 50 mcg/d.22,23
Compared with outpatient studies, however, an open trial of T3 augmentation by Birkenhager et al24 found no evidence of efficacy in 14 severely depressed inpatients who had not responded to 6 weeks of tricyclics. These patients—mainly with melancholic and/or psychotic depression—showed greater response to a monoamine oxidase inhibitor (MAOI) or electroconvulsive therapy than to thyroid hormone.
Three of five double-blind controlled studies of thyroid hormone augmentation of tricyclics reported response rates of 50 to 65% (Table).14,18,25 Earlier controlled studies, including two negative studies26,27 had important methodologic limitations: all included few subjects (≤16), and two studies included both unipolar and bipolar patients. Joffe et al partially addressed these problems with two randomized, double-blind studies that were controlled with placebo or T4:
- In a 3-week trial, T3 was more effective than T4 when added to imipramine or desipramine.14
- In a 2-week trial, T3 was significantly more effective than placebo when added to imipramine or desipramine.25
In the latter study, T3 and lithium augmentation appeared equally effective. Conversely, T4 was more effective than lithium as the first augmentation strategy in a double-blind, crossover study by Spoov and Lahdelma.28
In conclusion, depressed patients given T3 with tricyclic antidepressants may be twice as likely as controls to respond to treatment, according to a meta-analysis of eight studies totaling 292 patients. This analysis by Aronson et al29 supports T3 augmentation while addressing the surveyed studies’ limitations.
Table
Treatment-resistant MDD: Controlled studies of thyroid hormone augmentation
| Study authors/design | Initial therapy | Augmentation | Results |
|---|---|---|---|
| Steiner et al, 1978 Randomized double-blind; 8 patients* | Several TCAs (6 weeks) | T3, 25 mcg/d, or placebo (35 days) | T3: 75% responders (3/4) Placebo: 75% (3/4) |
| Goodwin et al, 1982 Double-blind, mirror design; 12 patients* | Desipramine or imipramine (4 weeks) | T3, 25 to 50 mcg/d, or placebo (21 days) | T3: 33% responders (4/12) Placebo: 0% (0/6) |
| Gitlin et al, 1987 Double-blind with crossover; 16 patients | Imipramine (4 weeks) | T3, 25 mcg/d, or placebo (2 weeks); crossover (2 weeks) | No difference between T3 and placebo |
| Joffe and Singer, 1990 Randomized double-blind; 38 patients | Desipramine or imipramine (4 weeks) | T3, 37.5 mcg/d, or T4, 150 mcg/d (21 days) | T3: 53% responders (9/17) T4: 19% (4/21) |
| Joffe et al, 1993 Randomized double-blind; 33 patients | Desipramine or imipramine (5 weeks) | T3, 37.5 mcg/d, or placebo (14 days) | T3: 59% responders (10/17) Placebo: 19% (3/16) |
| Sopov and Lahdelma, 1998 Randomized double-blind with crossover; 22 patients* | TCA, MAOI antidepressants | T4, 200 mcg/d, or lithium, 500 mg/d, (4 weeks); then crossover (4 weeks) | T4: 64% responders (7/11) Lithium: 18% responders (2/11) |
| *Included patients with unipolar or bipolar depression | |||
| MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; T3: triiodothyronine; T4: thyroxine; TCA: tricyclic antidepressant. | |||
MAO inhibitors. One small report has addressed the efficacy of thyroid hormones as adjuvants to MAOIs.30 In two patients, adding T3 to phenelzine enhanced the antidepressant response.
High-dose T4. Two open studies of patients with treatment-resistant bipolar or unipolar depression12,13 have examined the efficacy of high-dose T4 augmentation. These patients were taking a variety of antidepressants, including TCAs and SSRIs.
- In the study by Baurer et al,12 clinical remission (Hamilton Depression Scale [HAM-D] score ≤10) occurred in 4 of 5 patients with severe treatment-resistant unipolar depression who received adjunctive T4, mean 482±72 mcg/d, with antidepressants.
- In the study by Rudas et al,13 clinical remission (HAM-D ≤9) occurred in 7 of 9 patients with treatment-resistant MDD after T4, 150 to 300 mcg/d, was added to their antidepressant therapy. Side effects may limit this high-dose strategy, however, because 2 of the patients dropped out with thyrotoxicosis symptoms.
SSRIs. Three open trials to date have investigated using thyroid hormones to augment SSRIs in treatment-resistant MDD. In a prospective study by Agid and Lerer,31 10 of 25 (40%) patients who did not respond to SSRI treatment did so after T3 was added. No men improved, however, which led the authors to suggest that men and women might respond differently to T3 augmentation of SSRIs.
In our study, 7 of 20 patients (35%) with MDD who did not respond to 8 weeks of SSRI therapy did so when we added T3, 50 mcg/d, for 4 weeks (Figure). Response rates were high (5/5, 100%) in patients with atypical features by DSMIV criteria and low (1/8, 12.5%) in those with melancholic features.32
Abraham et al33 added T3, 50 mcg/d, to the regimens of 12 patients with MDD who did not respond to SSRIs alone. One patient dropped out with side effects. After 4 weeks of T3 augmentation, 5 patients (42%) showed 50% or greater improvement in HAM-D scores from baseline.
Figure T3 augmentation of SSRIs in 20 patients with resistant major depressive disorder
Open T3 augmentation, 50 mcg/d, given to 20 nonresponders to 8 weeks of selective serotonin reuptake inhibitors (SSRIs) improved baseline CGI-S scores significantly (P=0.006) at 4 weeks in those with atypical depression and modestly (P>0.05) in those with melancholic depression.
Source: Reference 32Antidepressant accelerators. Five of seven early double-blind, controlled studies indicated that adding small doses of thyroid hormones at the beginning of antidepressant treatment accelerated treatment response. All were limited by small sample sizes and other methodologic problems. A more-recent meta-analysis of six studies totalling 125 patients by Altshuler et al34 found:
- T3 was significantly more effective than placebo in accelerating clinical response to tricyclics
- the acceleration effect was more pronounced for women than for men.
Clinical recommendations
Thyroid hormones can be useful to augment and accelerate treatment of MDD. Evidence strongly supports their use with tricyclic antidepressants and suggests they also can be effective adjuvants for patients who do not respond to SSRIs.
Either T3 (up to 50 mcg/d) or T4 (up to 150 mcg/d) can be used as augmentation. T3’s antidepressant properties are considered more effective than those of T4, but the only head-to-head study supporting this conclusion was small (38 patients).14 Some T4 augmentation studies used very high dosages (300 to 600 mcg/d),12 which increase the risk of acute overdose.
Start T3 augmentation at 25 mcg/d and increase, if tolerated, to 50 mcg/d after 1 week. Measure baseline serum thyroid-stimulating hormone (TSH), and do not treat patients with TSH <0.5 mIU/L). Baseline TSH, T4, or T3 levels do not predict response to T3 augmentation in euthyroid MDD patients.32
Common side effects. Adjuvant T3, 25 to 50 mcg/d, was well-tolerated in our study of 20 patients also taking SSRIs:
- 2 (10%) experienced fatigue and diaphoresis
- 1 each (5%) had tremor, dry mouth, headaches, muscle aches, and vivid dreams.32
We saw no significant changes in blood pressure, but heart rates increased significantly in our 4-week study—from 76±12 bpm (range 60 to 96) to 82±9 bpm (range 66 to 96). Thus, T3 augmentation may not be indicated for patients with coronary artery disease or chronic heart failure. Patients’ weight decreased an average 2.5±6.6 lbs (range –20 to +7).
Thyroid hormones may cause hypoglycemia and change insulin requirements in patients with diabetes. High doses of T3 or T4 may be associated with hyperthyroidism, weight loss, nervousness, sweating, tachycardia, insomnia, heat intolerance, menstrual irregularities, palpitations, psychosis, or fever. Discontinue treatment if these symptoms develop.
Onset of antidepressant effect. Assess patient response in 4 to 6 weeks, whether using augmentation to address antidepressant nonresponse14,25 or to accelerate response.33 If you detect only partial improvement, studies support continuing treatment up to 8 weeks.
Treatment duration. No guidelines exist on how long to continue thyroid hormones after the initial antidepressant response. TSH levels become suppressed (TSH <0.1 mIU/L) after 4 weeks of T3, 50 mcg/d, in patients with normal baseline thyroid function.32 This suggests thyroid hormone’s booster effect is self limited, and augmentation may not need to continue after 2 to 3 months—even in responders.
T3 augmentation at 25 mcg/d can be discontinued immediately. For 50 to 75 mcg/d, taper across 1 to 2 weeks. The hypothalamic-pituitary-thyroid axis returns to normal function 6 to 8 weeks after T3 augmentation is stopped.
In this model, thyroid hormone augmentation can be used to boost antidepressant efficacy several months at a time. If effective, the same strategy could be tried again for subsequent MDD episodes.
Related resources
- DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol 2006;20(3):11-18.
- Massachusetts General Hospital Psychiatric Academy (including web-casts on treatment-resistant depression. www.mghcme.com
Drug brand names
- Desipramine • Norpramin
- Imipramine • Tofranil
- Levothyroxine (T4) • Levoxyl, Levothroid, Synthroid, others
- Liothyronine (synthetic T3) • Cytomel
Disclosures
The author receives research support from Aspect Medical Systems, Forest Laboratories, and Janssen Pharmaceutica; is a consultant to Pfizer, Inc., and Forest Laboratories; and a speaker for Eli Lilly and Co., Pfizer, Inc., and Cephalon.
1. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 1996;19:179-200.
2. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163(1):28-40.
3. Nierenberg AA, McLean NE, Alpert JE, et al. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry 1995;152:1500-3.
4. Nierenberg AA, Papakostas GI, Petersen T, et al. Nortriptyline for treatment-resistant depression. J Clin Psychiatry 2003;64(1):35-9.
5. Rozanov CB, Dratman MB. Immunohistochemical mapping of brain triiodothyronine reveals prominent localization in central noradrenergic systems. Neuroscience 1996;74:897-915.
6. Cheng LY, Outterbridge LV, Covatta ND, et al. Film autoradiography identifies unique features of [125I]3,3’5’-(reverse) triiodothyronine transport from blood to brain. J Neurophysiol 1994;72:380-91.
7. Gordon JT, Kaminski DM, Rozanov CB, Dratman MB. Evidence that 3,3’,5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience 1999;93:943-54.
8. Howland RH. Thyroid dysfunction in refractory depression: implications for pathophysiology and treatment. J Clin Psychiatry 1993;54:47-54.
9. Joffe RT. Peripheral thyroid hormone levels in treatment resistant depression. Biol Psychiatry 1999;45:1053-5.
10. Iosifescu DV, Howarth S, Alpert JE, et al. T3 blood levels and treatment outcome in depression. Int J Psychiatry Med 2001;31:367-73.
11. Joffe RT, Roy-Byrne PP, Udhe TW, Post RM. Thyroid function and affective illness: a reappraisal. Biol Psychiatry 1984;19:1685-91.
12. Bauer M, Hellweg R, Graf KJ, Baumgartner A. Treatment of refractory depression with high-dose thyroxine. Neuropsychopharmacology 1998;18:444-55.
13. Rudas S, Schmitz M, Pichler P, Baumgartner A. Treatment of refractory chronic depression and dysthymia with high-dose thyroxine. Biol Psychiatry 1999;45:229-33.
14. Joffe RT, Singer W. A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res 1990;32:241-51.
15. Sandrini M, Vitale G, Vergoni AV, et al. Effect of acute and chronic treatment with triiodothyronine on serotonin levels and serotonergic receptor subtypes in the rat brain. Life Sci 1996;58:1551-9.
16. Gur E, Lerer B, Newman ME. Chronic clomipramine and triiodothyronine increase serotonin levels in rat frontal cortex in vivo: relationship to serotonin autoreceptor activity. J Pharmacol Exp Ther 1999;288:81-7.
17. Cleare AJ, McGregor A, Chambers SM, et al. Thyroxine replacement increases central 5-hydroxytryptamine activity and reduces depressive symptoms in hypothyroidism. Neuroendocrinology 1996;64:65-9.
18. Goodwin FK, Prange AJ, Post RM, et al. Potentiation of antidepressant effects by l-triiodothyronine in tricyclic nonresponders. Am J Psychiatry 1982;139:34-8.
19. Blier P. Pharmacology of rapid-onset antidepressant treatment strategies. J Clin Psychiatry 2001;62(suppl 15):12-7.
20. Smith CD, Ain KB. Brain metabolism in hypothyroidism studied with 31P magnetic-resonance spectroscopy. Lancet 1995;345:619-20.
21. Kato T, Murashita J, Shioiri T, et al. Effect of photic stimulation on energy metabolism in the human brain measured by 31P-MR spectroscopy. J Neuropsychiatry Clin Neurosci 1996;8:417-22.
22. Earle BV. Thyroid hormone and tricyclic antidepressants in resistant depressions. Am J Psychiatry 1970;126:1667-9.
23. Thase ME, Kupfer DJ, Jarrett DB. Treatment of imipramine-resistant recurrent depression: I. An open clinical trial of adjunctive l-triiodothyronine. J Clin Psychiatry 1989;50:385-8.
24. Birkenhager TK, Vegt M, Nolen WA. An open study of triiodothyronine augmentation of tricyclic antidepressants in inpatients with refractory depression. Pharmacopsychiatry 1997;30:23-6.
25. Joffe RT, Singer W, Levitt AJ, MacDonald C. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry 1993;50:387-93.
26. Steiner M, Radwan M, Elizur A, et al. Failure of l-triiodothyronine to potentiate tricyclic antidepressant response. Curr Ther Res 1978;23:655-9.
27. Gitlin MJ, Weiner H, Fairbanks L, et al. Failure of T3 to potentiate tricyclic antidepressant response. J Affective Disord 1987;13:267-72.
28. Spoov J, Lahdelma L. Should thyroid augmentation precede lithium augmentation—a pilot study. J Affect Disord 1998;49:235-9.
29. Aronson R, Offman HJ, Joffe RT, Naylor CD. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry 1996;53:842-8.
30. Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry 1988;49:409-10.
31. Agid O, Lerer B. Algorithm-based treatment of major depression in an outpatient clinic: clinical correlates of response to a specific serotonin reuptake inhibitor and to triiodothyronine augmentation. Int J Neuropsychopharmacol 2003;6(1):41-9.
32. Iosifescu DV, Nierenberg AA, Mischoulon D, et al. An open study of triiodothyronine augmentation of selective serotonin reuptake inhibitors in treatment-resistant major depressive disorder. J Clin Psychiatry 2005;66:1038-42.
33. Abraham G, Milev R, Stuart Lawson J. T3 augmentation of SSRI resistant depression. J Affect Disord 2006;91(2-3):211-15.
34. Altshuler LL, Bauer M, Frye MA, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 2001;158:1617-22.
Prescribing thyroid hormones with antidepressants—whether to augment the antidepressant effect or accelerate patient response—is a well-researched strategy for treatment-resistant major depressive disorder (MDD). Thyroid hormones are known to boost response to tricyclics, and preliminary evidence shows they may be useful adjuvants to selective serotonin reuptake inhibitors (SSRIs) as well.
Thyroid hormones enter the brain slowly across the blood-brain barrier and choroid plexus. They accumulate in the locus ceruleus and other structures and are distributed widely along noradrenergic pathways.
Effective treatments are available for MDD, although 30% to 40% of patients do not respond to one or more antidepressant trials (Box).1-4 This article offers:
- new information about why triiodothyronine (T3) and thyroxine (T4) can “super-charge” antidepressant response
- tips on how to use thyroid hormones in patients with MDD, including effective dosages, patient monitoring, and treatment durations.
- 30% to 40% of patients with major depressive disorder (MDD) do not respond sufficiently to usual antidepressant treatment1
- Even under optimal treatment conditions, only one-third of patients achieve remission2
- Among patients who fail to respond to two pharmacologic interventions, remission rates with the next antidepressant are as low as 12%3
- A patient becomes less likely to respond clinically with each additional nonresponse to antidepressant treatment4
Why thyroid hormones?
Thyroid hormones enter the brain slowly across the blood-brain barrier and the choroid plexus—cerebrospinal fluid barriers. T4 is the main source of brain T3—after attack by 5’deiodinase—but circulating T3 also crosses the blood-brain barrier through active transport.
Thyroid hormones accumulate in the locus ceruleus and other central noradrenergic structures and are distributed widely in the brain along noradrenergic pathways.5-7 The mechanism of their therapeutic effect for MDD is not well understood, and various hypotheses have been proposed.
Subclinical hypothyroidism. Early studies such as by Howland8 of treatment-refractory MDD suggested that thyroid hormone augmentation might correct a hypothyroid state. However, blood thyroid hormone levels are not associated with resistance to antidepressant treatment, according to studies of MDD populations.9-10 Also, thyroid hormones’ therapeutic action in MDD appears unlikely to be related to treating subclinical hypothyroidism because patients’ euthyroid status was verified in all adjuvant studies since 1980.
Joffe et al11 proposed that MDD is characterized by a relative excess of T4 versus T3—probably related to a deficit in converting T4 to T3 in the periphery—and administering T3 would therefore correct this imbalance. They offered no strong evidence for an increased T4 level, however, and later studies failed to detect the postulated blood T3 abnormalities in MDD.9-10 Also—as suggested by studies with high-dose T4 augmentation12,13—the adjuvant antidepressant effect of thyroid hormones is not restricted to T3, although T3 may be more efficacious than T4.14
Neurotransmitter effects. Thyroid hormones’ role in increasing serotonin (5-HT) release could partially explain the benefit of adjuvant thyroid hormone therapy in MDD. Researchers found:
- T3 increased cortical 5-HT levels, probably by reducing the autoinhibitory effect of the presynaptic 5-HT1A receptor15
- adding T3 to clomipramine therapy increased 5-HT levels to a greater extent than T3 or clomipramine used alone16
- Low 5-HT activity, shown in hypothyroid patients, increased after T4 replacement.17
This 5-HT release theory cannot explain why thyroid hormones have a rapid clinical effect in MDD. Most studies report the hormones have clinical efficacy in MDD within 4 weeks.18 Similarly, selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels within hours of treatment onset, but the clinical effect occurs 4 to 6 weeks later.19 Therefore, increased 5-HT levels cannot fully explain thyroid hormones’ early effect.
Close interaction between thyroid hormones and the noradrenergic system also has been examined. Brain T3 is primarily localized in the central noradrenergic systems, with axonal anterograde transport of T3 from the locus ceruleus. T3 is processed and accumulated in the noradrenergic system, carried via axonal transport, then delivered from nerve cell bodies to its neuronal targets.5,7 T3 thus functions as a coneurotransmitter with norepinephrine.
Cellular energy metabolism. We recently reported that thyroid hormones’ antidepressant effect may be related to brain cellular energy metabolism. Thyroid hormones increase cellular levels of adenosine triphosphate (ATP) and phosphocreatine (PCr) in the hypothyroid brain.20 Brain imaging—phosphorus-31 nuclear magnetic resonance spectroscopy (31P-MRS)—of subjects with MDD shows decreased brain levels of ATP and increased PCr.21
Our group showed that the antidepressant effect of T3 augmentation of SSRIs is correlated with significant increases in ATP levels and decreases in PCr. This effect—which appears to represent re-normalization of brain bioenergetics in treatment responders—did not occur in nonresponders (Iosifescu et al, presented at APA, 2004).
The effect of thyroid hormones on bioenergetic metabolism is compatible with the hypothesized effects on noradrenergic and serotonergic systems.5,7 These mechanisms may represent different links in the same chain of events.
Antidepressant boosters
Thyroid hormones have been used extensively to treat MDD since the 1950s, when researchers reported that T3 monotherapy was efficacious for treating depression. These early studies had important methodologic limitations, including open designs and poorly defined diagnostic criteria and response.
For MDD, the most extensively researched uses of thyroid hormones are to augment therapy for antidepressant nonresponders and to accelerate partial response to antidepressants.
Tricyclics. Open studies primarily among outpatients in the 1970s and ‘80s suggested that thyroid hormones are a valid augmentation strategy for nonresponders to tricyclic antidepressants. Most—but not all—reported response rates >50% with T3 dosages of 20 to 50 mcg/d.22,23
Compared with outpatient studies, however, an open trial of T3 augmentation by Birkenhager et al24 found no evidence of efficacy in 14 severely depressed inpatients who had not responded to 6 weeks of tricyclics. These patients—mainly with melancholic and/or psychotic depression—showed greater response to a monoamine oxidase inhibitor (MAOI) or electroconvulsive therapy than to thyroid hormone.
Three of five double-blind controlled studies of thyroid hormone augmentation of tricyclics reported response rates of 50 to 65% (Table).14,18,25 Earlier controlled studies, including two negative studies26,27 had important methodologic limitations: all included few subjects (≤16), and two studies included both unipolar and bipolar patients. Joffe et al partially addressed these problems with two randomized, double-blind studies that were controlled with placebo or T4:
- In a 3-week trial, T3 was more effective than T4 when added to imipramine or desipramine.14
- In a 2-week trial, T3 was significantly more effective than placebo when added to imipramine or desipramine.25
In the latter study, T3 and lithium augmentation appeared equally effective. Conversely, T4 was more effective than lithium as the first augmentation strategy in a double-blind, crossover study by Spoov and Lahdelma.28
In conclusion, depressed patients given T3 with tricyclic antidepressants may be twice as likely as controls to respond to treatment, according to a meta-analysis of eight studies totaling 292 patients. This analysis by Aronson et al29 supports T3 augmentation while addressing the surveyed studies’ limitations.
Table
Treatment-resistant MDD: Controlled studies of thyroid hormone augmentation
| Study authors/design | Initial therapy | Augmentation | Results |
|---|---|---|---|
| Steiner et al, 1978 Randomized double-blind; 8 patients* | Several TCAs (6 weeks) | T3, 25 mcg/d, or placebo (35 days) | T3: 75% responders (3/4) Placebo: 75% (3/4) |
| Goodwin et al, 1982 Double-blind, mirror design; 12 patients* | Desipramine or imipramine (4 weeks) | T3, 25 to 50 mcg/d, or placebo (21 days) | T3: 33% responders (4/12) Placebo: 0% (0/6) |
| Gitlin et al, 1987 Double-blind with crossover; 16 patients | Imipramine (4 weeks) | T3, 25 mcg/d, or placebo (2 weeks); crossover (2 weeks) | No difference between T3 and placebo |
| Joffe and Singer, 1990 Randomized double-blind; 38 patients | Desipramine or imipramine (4 weeks) | T3, 37.5 mcg/d, or T4, 150 mcg/d (21 days) | T3: 53% responders (9/17) T4: 19% (4/21) |
| Joffe et al, 1993 Randomized double-blind; 33 patients | Desipramine or imipramine (5 weeks) | T3, 37.5 mcg/d, or placebo (14 days) | T3: 59% responders (10/17) Placebo: 19% (3/16) |
| Sopov and Lahdelma, 1998 Randomized double-blind with crossover; 22 patients* | TCA, MAOI antidepressants | T4, 200 mcg/d, or lithium, 500 mg/d, (4 weeks); then crossover (4 weeks) | T4: 64% responders (7/11) Lithium: 18% responders (2/11) |
| *Included patients with unipolar or bipolar depression | |||
| MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; T3: triiodothyronine; T4: thyroxine; TCA: tricyclic antidepressant. | |||
MAO inhibitors. One small report has addressed the efficacy of thyroid hormones as adjuvants to MAOIs.30 In two patients, adding T3 to phenelzine enhanced the antidepressant response.
High-dose T4. Two open studies of patients with treatment-resistant bipolar or unipolar depression12,13 have examined the efficacy of high-dose T4 augmentation. These patients were taking a variety of antidepressants, including TCAs and SSRIs.
- In the study by Baurer et al,12 clinical remission (Hamilton Depression Scale [HAM-D] score ≤10) occurred in 4 of 5 patients with severe treatment-resistant unipolar depression who received adjunctive T4, mean 482±72 mcg/d, with antidepressants.
- In the study by Rudas et al,13 clinical remission (HAM-D ≤9) occurred in 7 of 9 patients with treatment-resistant MDD after T4, 150 to 300 mcg/d, was added to their antidepressant therapy. Side effects may limit this high-dose strategy, however, because 2 of the patients dropped out with thyrotoxicosis symptoms.
SSRIs. Three open trials to date have investigated using thyroid hormones to augment SSRIs in treatment-resistant MDD. In a prospective study by Agid and Lerer,31 10 of 25 (40%) patients who did not respond to SSRI treatment did so after T3 was added. No men improved, however, which led the authors to suggest that men and women might respond differently to T3 augmentation of SSRIs.
In our study, 7 of 20 patients (35%) with MDD who did not respond to 8 weeks of SSRI therapy did so when we added T3, 50 mcg/d, for 4 weeks (Figure). Response rates were high (5/5, 100%) in patients with atypical features by DSMIV criteria and low (1/8, 12.5%) in those with melancholic features.32
Abraham et al33 added T3, 50 mcg/d, to the regimens of 12 patients with MDD who did not respond to SSRIs alone. One patient dropped out with side effects. After 4 weeks of T3 augmentation, 5 patients (42%) showed 50% or greater improvement in HAM-D scores from baseline.
Figure T3 augmentation of SSRIs in 20 patients with resistant major depressive disorder
Open T3 augmentation, 50 mcg/d, given to 20 nonresponders to 8 weeks of selective serotonin reuptake inhibitors (SSRIs) improved baseline CGI-S scores significantly (P=0.006) at 4 weeks in those with atypical depression and modestly (P>0.05) in those with melancholic depression.
Source: Reference 32Antidepressant accelerators. Five of seven early double-blind, controlled studies indicated that adding small doses of thyroid hormones at the beginning of antidepressant treatment accelerated treatment response. All were limited by small sample sizes and other methodologic problems. A more-recent meta-analysis of six studies totalling 125 patients by Altshuler et al34 found:
- T3 was significantly more effective than placebo in accelerating clinical response to tricyclics
- the acceleration effect was more pronounced for women than for men.
Clinical recommendations
Thyroid hormones can be useful to augment and accelerate treatment of MDD. Evidence strongly supports their use with tricyclic antidepressants and suggests they also can be effective adjuvants for patients who do not respond to SSRIs.
Either T3 (up to 50 mcg/d) or T4 (up to 150 mcg/d) can be used as augmentation. T3’s antidepressant properties are considered more effective than those of T4, but the only head-to-head study supporting this conclusion was small (38 patients).14 Some T4 augmentation studies used very high dosages (300 to 600 mcg/d),12 which increase the risk of acute overdose.
Start T3 augmentation at 25 mcg/d and increase, if tolerated, to 50 mcg/d after 1 week. Measure baseline serum thyroid-stimulating hormone (TSH), and do not treat patients with TSH <0.5 mIU/L). Baseline TSH, T4, or T3 levels do not predict response to T3 augmentation in euthyroid MDD patients.32
Common side effects. Adjuvant T3, 25 to 50 mcg/d, was well-tolerated in our study of 20 patients also taking SSRIs:
- 2 (10%) experienced fatigue and diaphoresis
- 1 each (5%) had tremor, dry mouth, headaches, muscle aches, and vivid dreams.32
We saw no significant changes in blood pressure, but heart rates increased significantly in our 4-week study—from 76±12 bpm (range 60 to 96) to 82±9 bpm (range 66 to 96). Thus, T3 augmentation may not be indicated for patients with coronary artery disease or chronic heart failure. Patients’ weight decreased an average 2.5±6.6 lbs (range –20 to +7).
Thyroid hormones may cause hypoglycemia and change insulin requirements in patients with diabetes. High doses of T3 or T4 may be associated with hyperthyroidism, weight loss, nervousness, sweating, tachycardia, insomnia, heat intolerance, menstrual irregularities, palpitations, psychosis, or fever. Discontinue treatment if these symptoms develop.
Onset of antidepressant effect. Assess patient response in 4 to 6 weeks, whether using augmentation to address antidepressant nonresponse14,25 or to accelerate response.33 If you detect only partial improvement, studies support continuing treatment up to 8 weeks.
Treatment duration. No guidelines exist on how long to continue thyroid hormones after the initial antidepressant response. TSH levels become suppressed (TSH <0.1 mIU/L) after 4 weeks of T3, 50 mcg/d, in patients with normal baseline thyroid function.32 This suggests thyroid hormone’s booster effect is self limited, and augmentation may not need to continue after 2 to 3 months—even in responders.
T3 augmentation at 25 mcg/d can be discontinued immediately. For 50 to 75 mcg/d, taper across 1 to 2 weeks. The hypothalamic-pituitary-thyroid axis returns to normal function 6 to 8 weeks after T3 augmentation is stopped.
In this model, thyroid hormone augmentation can be used to boost antidepressant efficacy several months at a time. If effective, the same strategy could be tried again for subsequent MDD episodes.
Related resources
- DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol 2006;20(3):11-18.
- Massachusetts General Hospital Psychiatric Academy (including web-casts on treatment-resistant depression. www.mghcme.com
Drug brand names
- Desipramine • Norpramin
- Imipramine • Tofranil
- Levothyroxine (T4) • Levoxyl, Levothroid, Synthroid, others
- Liothyronine (synthetic T3) • Cytomel
Disclosures
The author receives research support from Aspect Medical Systems, Forest Laboratories, and Janssen Pharmaceutica; is a consultant to Pfizer, Inc., and Forest Laboratories; and a speaker for Eli Lilly and Co., Pfizer, Inc., and Cephalon.
Prescribing thyroid hormones with antidepressants—whether to augment the antidepressant effect or accelerate patient response—is a well-researched strategy for treatment-resistant major depressive disorder (MDD). Thyroid hormones are known to boost response to tricyclics, and preliminary evidence shows they may be useful adjuvants to selective serotonin reuptake inhibitors (SSRIs) as well.
Thyroid hormones enter the brain slowly across the blood-brain barrier and choroid plexus. They accumulate in the locus ceruleus and other structures and are distributed widely along noradrenergic pathways.
Effective treatments are available for MDD, although 30% to 40% of patients do not respond to one or more antidepressant trials (Box).1-4 This article offers:
- new information about why triiodothyronine (T3) and thyroxine (T4) can “super-charge” antidepressant response
- tips on how to use thyroid hormones in patients with MDD, including effective dosages, patient monitoring, and treatment durations.
- 30% to 40% of patients with major depressive disorder (MDD) do not respond sufficiently to usual antidepressant treatment1
- Even under optimal treatment conditions, only one-third of patients achieve remission2
- Among patients who fail to respond to two pharmacologic interventions, remission rates with the next antidepressant are as low as 12%3
- A patient becomes less likely to respond clinically with each additional nonresponse to antidepressant treatment4
Why thyroid hormones?
Thyroid hormones enter the brain slowly across the blood-brain barrier and the choroid plexus—cerebrospinal fluid barriers. T4 is the main source of brain T3—after attack by 5’deiodinase—but circulating T3 also crosses the blood-brain barrier through active transport.
Thyroid hormones accumulate in the locus ceruleus and other central noradrenergic structures and are distributed widely in the brain along noradrenergic pathways.5-7 The mechanism of their therapeutic effect for MDD is not well understood, and various hypotheses have been proposed.
Subclinical hypothyroidism. Early studies such as by Howland8 of treatment-refractory MDD suggested that thyroid hormone augmentation might correct a hypothyroid state. However, blood thyroid hormone levels are not associated with resistance to antidepressant treatment, according to studies of MDD populations.9-10 Also, thyroid hormones’ therapeutic action in MDD appears unlikely to be related to treating subclinical hypothyroidism because patients’ euthyroid status was verified in all adjuvant studies since 1980.
Joffe et al11 proposed that MDD is characterized by a relative excess of T4 versus T3—probably related to a deficit in converting T4 to T3 in the periphery—and administering T3 would therefore correct this imbalance. They offered no strong evidence for an increased T4 level, however, and later studies failed to detect the postulated blood T3 abnormalities in MDD.9-10 Also—as suggested by studies with high-dose T4 augmentation12,13—the adjuvant antidepressant effect of thyroid hormones is not restricted to T3, although T3 may be more efficacious than T4.14
Neurotransmitter effects. Thyroid hormones’ role in increasing serotonin (5-HT) release could partially explain the benefit of adjuvant thyroid hormone therapy in MDD. Researchers found:
- T3 increased cortical 5-HT levels, probably by reducing the autoinhibitory effect of the presynaptic 5-HT1A receptor15
- adding T3 to clomipramine therapy increased 5-HT levels to a greater extent than T3 or clomipramine used alone16
- Low 5-HT activity, shown in hypothyroid patients, increased after T4 replacement.17
This 5-HT release theory cannot explain why thyroid hormones have a rapid clinical effect in MDD. Most studies report the hormones have clinical efficacy in MDD within 4 weeks.18 Similarly, selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels within hours of treatment onset, but the clinical effect occurs 4 to 6 weeks later.19 Therefore, increased 5-HT levels cannot fully explain thyroid hormones’ early effect.
Close interaction between thyroid hormones and the noradrenergic system also has been examined. Brain T3 is primarily localized in the central noradrenergic systems, with axonal anterograde transport of T3 from the locus ceruleus. T3 is processed and accumulated in the noradrenergic system, carried via axonal transport, then delivered from nerve cell bodies to its neuronal targets.5,7 T3 thus functions as a coneurotransmitter with norepinephrine.
Cellular energy metabolism. We recently reported that thyroid hormones’ antidepressant effect may be related to brain cellular energy metabolism. Thyroid hormones increase cellular levels of adenosine triphosphate (ATP) and phosphocreatine (PCr) in the hypothyroid brain.20 Brain imaging—phosphorus-31 nuclear magnetic resonance spectroscopy (31P-MRS)—of subjects with MDD shows decreased brain levels of ATP and increased PCr.21
Our group showed that the antidepressant effect of T3 augmentation of SSRIs is correlated with significant increases in ATP levels and decreases in PCr. This effect—which appears to represent re-normalization of brain bioenergetics in treatment responders—did not occur in nonresponders (Iosifescu et al, presented at APA, 2004).
The effect of thyroid hormones on bioenergetic metabolism is compatible with the hypothesized effects on noradrenergic and serotonergic systems.5,7 These mechanisms may represent different links in the same chain of events.
Antidepressant boosters
Thyroid hormones have been used extensively to treat MDD since the 1950s, when researchers reported that T3 monotherapy was efficacious for treating depression. These early studies had important methodologic limitations, including open designs and poorly defined diagnostic criteria and response.
For MDD, the most extensively researched uses of thyroid hormones are to augment therapy for antidepressant nonresponders and to accelerate partial response to antidepressants.
Tricyclics. Open studies primarily among outpatients in the 1970s and ‘80s suggested that thyroid hormones are a valid augmentation strategy for nonresponders to tricyclic antidepressants. Most—but not all—reported response rates >50% with T3 dosages of 20 to 50 mcg/d.22,23
Compared with outpatient studies, however, an open trial of T3 augmentation by Birkenhager et al24 found no evidence of efficacy in 14 severely depressed inpatients who had not responded to 6 weeks of tricyclics. These patients—mainly with melancholic and/or psychotic depression—showed greater response to a monoamine oxidase inhibitor (MAOI) or electroconvulsive therapy than to thyroid hormone.
Three of five double-blind controlled studies of thyroid hormone augmentation of tricyclics reported response rates of 50 to 65% (Table).14,18,25 Earlier controlled studies, including two negative studies26,27 had important methodologic limitations: all included few subjects (≤16), and two studies included both unipolar and bipolar patients. Joffe et al partially addressed these problems with two randomized, double-blind studies that were controlled with placebo or T4:
- In a 3-week trial, T3 was more effective than T4 when added to imipramine or desipramine.14
- In a 2-week trial, T3 was significantly more effective than placebo when added to imipramine or desipramine.25
In the latter study, T3 and lithium augmentation appeared equally effective. Conversely, T4 was more effective than lithium as the first augmentation strategy in a double-blind, crossover study by Spoov and Lahdelma.28
In conclusion, depressed patients given T3 with tricyclic antidepressants may be twice as likely as controls to respond to treatment, according to a meta-analysis of eight studies totaling 292 patients. This analysis by Aronson et al29 supports T3 augmentation while addressing the surveyed studies’ limitations.
Table
Treatment-resistant MDD: Controlled studies of thyroid hormone augmentation
| Study authors/design | Initial therapy | Augmentation | Results |
|---|---|---|---|
| Steiner et al, 1978 Randomized double-blind; 8 patients* | Several TCAs (6 weeks) | T3, 25 mcg/d, or placebo (35 days) | T3: 75% responders (3/4) Placebo: 75% (3/4) |
| Goodwin et al, 1982 Double-blind, mirror design; 12 patients* | Desipramine or imipramine (4 weeks) | T3, 25 to 50 mcg/d, or placebo (21 days) | T3: 33% responders (4/12) Placebo: 0% (0/6) |
| Gitlin et al, 1987 Double-blind with crossover; 16 patients | Imipramine (4 weeks) | T3, 25 mcg/d, or placebo (2 weeks); crossover (2 weeks) | No difference between T3 and placebo |
| Joffe and Singer, 1990 Randomized double-blind; 38 patients | Desipramine or imipramine (4 weeks) | T3, 37.5 mcg/d, or T4, 150 mcg/d (21 days) | T3: 53% responders (9/17) T4: 19% (4/21) |
| Joffe et al, 1993 Randomized double-blind; 33 patients | Desipramine or imipramine (5 weeks) | T3, 37.5 mcg/d, or placebo (14 days) | T3: 59% responders (10/17) Placebo: 19% (3/16) |
| Sopov and Lahdelma, 1998 Randomized double-blind with crossover; 22 patients* | TCA, MAOI antidepressants | T4, 200 mcg/d, or lithium, 500 mg/d, (4 weeks); then crossover (4 weeks) | T4: 64% responders (7/11) Lithium: 18% responders (2/11) |
| *Included patients with unipolar or bipolar depression | |||
| MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; T3: triiodothyronine; T4: thyroxine; TCA: tricyclic antidepressant. | |||
MAO inhibitors. One small report has addressed the efficacy of thyroid hormones as adjuvants to MAOIs.30 In two patients, adding T3 to phenelzine enhanced the antidepressant response.
High-dose T4. Two open studies of patients with treatment-resistant bipolar or unipolar depression12,13 have examined the efficacy of high-dose T4 augmentation. These patients were taking a variety of antidepressants, including TCAs and SSRIs.
- In the study by Baurer et al,12 clinical remission (Hamilton Depression Scale [HAM-D] score ≤10) occurred in 4 of 5 patients with severe treatment-resistant unipolar depression who received adjunctive T4, mean 482±72 mcg/d, with antidepressants.
- In the study by Rudas et al,13 clinical remission (HAM-D ≤9) occurred in 7 of 9 patients with treatment-resistant MDD after T4, 150 to 300 mcg/d, was added to their antidepressant therapy. Side effects may limit this high-dose strategy, however, because 2 of the patients dropped out with thyrotoxicosis symptoms.
SSRIs. Three open trials to date have investigated using thyroid hormones to augment SSRIs in treatment-resistant MDD. In a prospective study by Agid and Lerer,31 10 of 25 (40%) patients who did not respond to SSRI treatment did so after T3 was added. No men improved, however, which led the authors to suggest that men and women might respond differently to T3 augmentation of SSRIs.
In our study, 7 of 20 patients (35%) with MDD who did not respond to 8 weeks of SSRI therapy did so when we added T3, 50 mcg/d, for 4 weeks (Figure). Response rates were high (5/5, 100%) in patients with atypical features by DSMIV criteria and low (1/8, 12.5%) in those with melancholic features.32
Abraham et al33 added T3, 50 mcg/d, to the regimens of 12 patients with MDD who did not respond to SSRIs alone. One patient dropped out with side effects. After 4 weeks of T3 augmentation, 5 patients (42%) showed 50% or greater improvement in HAM-D scores from baseline.
Figure T3 augmentation of SSRIs in 20 patients with resistant major depressive disorder
Open T3 augmentation, 50 mcg/d, given to 20 nonresponders to 8 weeks of selective serotonin reuptake inhibitors (SSRIs) improved baseline CGI-S scores significantly (P=0.006) at 4 weeks in those with atypical depression and modestly (P>0.05) in those with melancholic depression.
Source: Reference 32Antidepressant accelerators. Five of seven early double-blind, controlled studies indicated that adding small doses of thyroid hormones at the beginning of antidepressant treatment accelerated treatment response. All were limited by small sample sizes and other methodologic problems. A more-recent meta-analysis of six studies totalling 125 patients by Altshuler et al34 found:
- T3 was significantly more effective than placebo in accelerating clinical response to tricyclics
- the acceleration effect was more pronounced for women than for men.
Clinical recommendations
Thyroid hormones can be useful to augment and accelerate treatment of MDD. Evidence strongly supports their use with tricyclic antidepressants and suggests they also can be effective adjuvants for patients who do not respond to SSRIs.
Either T3 (up to 50 mcg/d) or T4 (up to 150 mcg/d) can be used as augmentation. T3’s antidepressant properties are considered more effective than those of T4, but the only head-to-head study supporting this conclusion was small (38 patients).14 Some T4 augmentation studies used very high dosages (300 to 600 mcg/d),12 which increase the risk of acute overdose.
Start T3 augmentation at 25 mcg/d and increase, if tolerated, to 50 mcg/d after 1 week. Measure baseline serum thyroid-stimulating hormone (TSH), and do not treat patients with TSH <0.5 mIU/L). Baseline TSH, T4, or T3 levels do not predict response to T3 augmentation in euthyroid MDD patients.32
Common side effects. Adjuvant T3, 25 to 50 mcg/d, was well-tolerated in our study of 20 patients also taking SSRIs:
- 2 (10%) experienced fatigue and diaphoresis
- 1 each (5%) had tremor, dry mouth, headaches, muscle aches, and vivid dreams.32
We saw no significant changes in blood pressure, but heart rates increased significantly in our 4-week study—from 76±12 bpm (range 60 to 96) to 82±9 bpm (range 66 to 96). Thus, T3 augmentation may not be indicated for patients with coronary artery disease or chronic heart failure. Patients’ weight decreased an average 2.5±6.6 lbs (range –20 to +7).
Thyroid hormones may cause hypoglycemia and change insulin requirements in patients with diabetes. High doses of T3 or T4 may be associated with hyperthyroidism, weight loss, nervousness, sweating, tachycardia, insomnia, heat intolerance, menstrual irregularities, palpitations, psychosis, or fever. Discontinue treatment if these symptoms develop.
Onset of antidepressant effect. Assess patient response in 4 to 6 weeks, whether using augmentation to address antidepressant nonresponse14,25 or to accelerate response.33 If you detect only partial improvement, studies support continuing treatment up to 8 weeks.
Treatment duration. No guidelines exist on how long to continue thyroid hormones after the initial antidepressant response. TSH levels become suppressed (TSH <0.1 mIU/L) after 4 weeks of T3, 50 mcg/d, in patients with normal baseline thyroid function.32 This suggests thyroid hormone’s booster effect is self limited, and augmentation may not need to continue after 2 to 3 months—even in responders.
T3 augmentation at 25 mcg/d can be discontinued immediately. For 50 to 75 mcg/d, taper across 1 to 2 weeks. The hypothalamic-pituitary-thyroid axis returns to normal function 6 to 8 weeks after T3 augmentation is stopped.
In this model, thyroid hormone augmentation can be used to boost antidepressant efficacy several months at a time. If effective, the same strategy could be tried again for subsequent MDD episodes.
Related resources
- DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol 2006;20(3):11-18.
- Massachusetts General Hospital Psychiatric Academy (including web-casts on treatment-resistant depression. www.mghcme.com
Drug brand names
- Desipramine • Norpramin
- Imipramine • Tofranil
- Levothyroxine (T4) • Levoxyl, Levothroid, Synthroid, others
- Liothyronine (synthetic T3) • Cytomel
Disclosures
The author receives research support from Aspect Medical Systems, Forest Laboratories, and Janssen Pharmaceutica; is a consultant to Pfizer, Inc., and Forest Laboratories; and a speaker for Eli Lilly and Co., Pfizer, Inc., and Cephalon.
1. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 1996;19:179-200.
2. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163(1):28-40.
3. Nierenberg AA, McLean NE, Alpert JE, et al. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry 1995;152:1500-3.
4. Nierenberg AA, Papakostas GI, Petersen T, et al. Nortriptyline for treatment-resistant depression. J Clin Psychiatry 2003;64(1):35-9.
5. Rozanov CB, Dratman MB. Immunohistochemical mapping of brain triiodothyronine reveals prominent localization in central noradrenergic systems. Neuroscience 1996;74:897-915.
6. Cheng LY, Outterbridge LV, Covatta ND, et al. Film autoradiography identifies unique features of [125I]3,3’5’-(reverse) triiodothyronine transport from blood to brain. J Neurophysiol 1994;72:380-91.
7. Gordon JT, Kaminski DM, Rozanov CB, Dratman MB. Evidence that 3,3’,5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience 1999;93:943-54.
8. Howland RH. Thyroid dysfunction in refractory depression: implications for pathophysiology and treatment. J Clin Psychiatry 1993;54:47-54.
9. Joffe RT. Peripheral thyroid hormone levels in treatment resistant depression. Biol Psychiatry 1999;45:1053-5.
10. Iosifescu DV, Howarth S, Alpert JE, et al. T3 blood levels and treatment outcome in depression. Int J Psychiatry Med 2001;31:367-73.
11. Joffe RT, Roy-Byrne PP, Udhe TW, Post RM. Thyroid function and affective illness: a reappraisal. Biol Psychiatry 1984;19:1685-91.
12. Bauer M, Hellweg R, Graf KJ, Baumgartner A. Treatment of refractory depression with high-dose thyroxine. Neuropsychopharmacology 1998;18:444-55.
13. Rudas S, Schmitz M, Pichler P, Baumgartner A. Treatment of refractory chronic depression and dysthymia with high-dose thyroxine. Biol Psychiatry 1999;45:229-33.
14. Joffe RT, Singer W. A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res 1990;32:241-51.
15. Sandrini M, Vitale G, Vergoni AV, et al. Effect of acute and chronic treatment with triiodothyronine on serotonin levels and serotonergic receptor subtypes in the rat brain. Life Sci 1996;58:1551-9.
16. Gur E, Lerer B, Newman ME. Chronic clomipramine and triiodothyronine increase serotonin levels in rat frontal cortex in vivo: relationship to serotonin autoreceptor activity. J Pharmacol Exp Ther 1999;288:81-7.
17. Cleare AJ, McGregor A, Chambers SM, et al. Thyroxine replacement increases central 5-hydroxytryptamine activity and reduces depressive symptoms in hypothyroidism. Neuroendocrinology 1996;64:65-9.
18. Goodwin FK, Prange AJ, Post RM, et al. Potentiation of antidepressant effects by l-triiodothyronine in tricyclic nonresponders. Am J Psychiatry 1982;139:34-8.
19. Blier P. Pharmacology of rapid-onset antidepressant treatment strategies. J Clin Psychiatry 2001;62(suppl 15):12-7.
20. Smith CD, Ain KB. Brain metabolism in hypothyroidism studied with 31P magnetic-resonance spectroscopy. Lancet 1995;345:619-20.
21. Kato T, Murashita J, Shioiri T, et al. Effect of photic stimulation on energy metabolism in the human brain measured by 31P-MR spectroscopy. J Neuropsychiatry Clin Neurosci 1996;8:417-22.
22. Earle BV. Thyroid hormone and tricyclic antidepressants in resistant depressions. Am J Psychiatry 1970;126:1667-9.
23. Thase ME, Kupfer DJ, Jarrett DB. Treatment of imipramine-resistant recurrent depression: I. An open clinical trial of adjunctive l-triiodothyronine. J Clin Psychiatry 1989;50:385-8.
24. Birkenhager TK, Vegt M, Nolen WA. An open study of triiodothyronine augmentation of tricyclic antidepressants in inpatients with refractory depression. Pharmacopsychiatry 1997;30:23-6.
25. Joffe RT, Singer W, Levitt AJ, MacDonald C. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry 1993;50:387-93.
26. Steiner M, Radwan M, Elizur A, et al. Failure of l-triiodothyronine to potentiate tricyclic antidepressant response. Curr Ther Res 1978;23:655-9.
27. Gitlin MJ, Weiner H, Fairbanks L, et al. Failure of T3 to potentiate tricyclic antidepressant response. J Affective Disord 1987;13:267-72.
28. Spoov J, Lahdelma L. Should thyroid augmentation precede lithium augmentation—a pilot study. J Affect Disord 1998;49:235-9.
29. Aronson R, Offman HJ, Joffe RT, Naylor CD. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry 1996;53:842-8.
30. Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry 1988;49:409-10.
31. Agid O, Lerer B. Algorithm-based treatment of major depression in an outpatient clinic: clinical correlates of response to a specific serotonin reuptake inhibitor and to triiodothyronine augmentation. Int J Neuropsychopharmacol 2003;6(1):41-9.
32. Iosifescu DV, Nierenberg AA, Mischoulon D, et al. An open study of triiodothyronine augmentation of selective serotonin reuptake inhibitors in treatment-resistant major depressive disorder. J Clin Psychiatry 2005;66:1038-42.
33. Abraham G, Milev R, Stuart Lawson J. T3 augmentation of SSRI resistant depression. J Affect Disord 2006;91(2-3):211-15.
34. Altshuler LL, Bauer M, Frye MA, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 2001;158:1617-22.
1. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 1996;19:179-200.
2. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163(1):28-40.
3. Nierenberg AA, McLean NE, Alpert JE, et al. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry 1995;152:1500-3.
4. Nierenberg AA, Papakostas GI, Petersen T, et al. Nortriptyline for treatment-resistant depression. J Clin Psychiatry 2003;64(1):35-9.
5. Rozanov CB, Dratman MB. Immunohistochemical mapping of brain triiodothyronine reveals prominent localization in central noradrenergic systems. Neuroscience 1996;74:897-915.
6. Cheng LY, Outterbridge LV, Covatta ND, et al. Film autoradiography identifies unique features of [125I]3,3’5’-(reverse) triiodothyronine transport from blood to brain. J Neurophysiol 1994;72:380-91.
7. Gordon JT, Kaminski DM, Rozanov CB, Dratman MB. Evidence that 3,3’,5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience 1999;93:943-54.
8. Howland RH. Thyroid dysfunction in refractory depression: implications for pathophysiology and treatment. J Clin Psychiatry 1993;54:47-54.
9. Joffe RT. Peripheral thyroid hormone levels in treatment resistant depression. Biol Psychiatry 1999;45:1053-5.
10. Iosifescu DV, Howarth S, Alpert JE, et al. T3 blood levels and treatment outcome in depression. Int J Psychiatry Med 2001;31:367-73.
11. Joffe RT, Roy-Byrne PP, Udhe TW, Post RM. Thyroid function and affective illness: a reappraisal. Biol Psychiatry 1984;19:1685-91.
12. Bauer M, Hellweg R, Graf KJ, Baumgartner A. Treatment of refractory depression with high-dose thyroxine. Neuropsychopharmacology 1998;18:444-55.
13. Rudas S, Schmitz M, Pichler P, Baumgartner A. Treatment of refractory chronic depression and dysthymia with high-dose thyroxine. Biol Psychiatry 1999;45:229-33.
14. Joffe RT, Singer W. A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res 1990;32:241-51.
15. Sandrini M, Vitale G, Vergoni AV, et al. Effect of acute and chronic treatment with triiodothyronine on serotonin levels and serotonergic receptor subtypes in the rat brain. Life Sci 1996;58:1551-9.
16. Gur E, Lerer B, Newman ME. Chronic clomipramine and triiodothyronine increase serotonin levels in rat frontal cortex in vivo: relationship to serotonin autoreceptor activity. J Pharmacol Exp Ther 1999;288:81-7.
17. Cleare AJ, McGregor A, Chambers SM, et al. Thyroxine replacement increases central 5-hydroxytryptamine activity and reduces depressive symptoms in hypothyroidism. Neuroendocrinology 1996;64:65-9.
18. Goodwin FK, Prange AJ, Post RM, et al. Potentiation of antidepressant effects by l-triiodothyronine in tricyclic nonresponders. Am J Psychiatry 1982;139:34-8.
19. Blier P. Pharmacology of rapid-onset antidepressant treatment strategies. J Clin Psychiatry 2001;62(suppl 15):12-7.
20. Smith CD, Ain KB. Brain metabolism in hypothyroidism studied with 31P magnetic-resonance spectroscopy. Lancet 1995;345:619-20.
21. Kato T, Murashita J, Shioiri T, et al. Effect of photic stimulation on energy metabolism in the human brain measured by 31P-MR spectroscopy. J Neuropsychiatry Clin Neurosci 1996;8:417-22.
22. Earle BV. Thyroid hormone and tricyclic antidepressants in resistant depressions. Am J Psychiatry 1970;126:1667-9.
23. Thase ME, Kupfer DJ, Jarrett DB. Treatment of imipramine-resistant recurrent depression: I. An open clinical trial of adjunctive l-triiodothyronine. J Clin Psychiatry 1989;50:385-8.
24. Birkenhager TK, Vegt M, Nolen WA. An open study of triiodothyronine augmentation of tricyclic antidepressants in inpatients with refractory depression. Pharmacopsychiatry 1997;30:23-6.
25. Joffe RT, Singer W, Levitt AJ, MacDonald C. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry 1993;50:387-93.
26. Steiner M, Radwan M, Elizur A, et al. Failure of l-triiodothyronine to potentiate tricyclic antidepressant response. Curr Ther Res 1978;23:655-9.
27. Gitlin MJ, Weiner H, Fairbanks L, et al. Failure of T3 to potentiate tricyclic antidepressant response. J Affective Disord 1987;13:267-72.
28. Spoov J, Lahdelma L. Should thyroid augmentation precede lithium augmentation—a pilot study. J Affect Disord 1998;49:235-9.
29. Aronson R, Offman HJ, Joffe RT, Naylor CD. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry 1996;53:842-8.
30. Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry 1988;49:409-10.
31. Agid O, Lerer B. Algorithm-based treatment of major depression in an outpatient clinic: clinical correlates of response to a specific serotonin reuptake inhibitor and to triiodothyronine augmentation. Int J Neuropsychopharmacol 2003;6(1):41-9.
32. Iosifescu DV, Nierenberg AA, Mischoulon D, et al. An open study of triiodothyronine augmentation of selective serotonin reuptake inhibitors in treatment-resistant major depressive disorder. J Clin Psychiatry 2005;66:1038-42.
33. Abraham G, Milev R, Stuart Lawson J. T3 augmentation of SSRI resistant depression. J Affect Disord 2006;91(2-3):211-15.
34. Altshuler LL, Bauer M, Frye MA, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 2001;158:1617-22.
Compulsive shopping: When spending begins to consume the consumer
Ms. A has been compulsively shopping and spending since age 19 when she first obtained credit cards. After years of intense urges to shop and remorse over the financial consequences, she seeks psychiatric help. Now age 37 and divorced, she has controlled her spending only for two 1- to 2-year periods that coincided with bankruptcy proceedings.
With easy access to credit, many persons such as Ms. A develop what is variously called compulsive buying, compulsive shopping, addictive shopping, or shopaholism. Although “medicalizing” excessive shopping may seem to obscure its broader cultural and social causes,1 increasing evidence points to a discrete shopping disorder.
Our group has contributed to compulsive buying research and continues to evaluate potential treatments. We offer evidence and practical advice to help you:
- identify compulsive shopping disorder using the patient’s history and three screening questions
- differentiate compulsive shopping from manic or hypomanic shopping sprees
- educate patients about four steps to control compulsive shopping.
Table 1
Compulsive shopping disorder’s clinical signs
| Onset in late adolescence to early adulthood |
| Female-to-male ratio may be 9:1 |
| Behaviors include shopping frequently, spending inappropriately, and fantasizing about future purchases |
| Psychiatric comorbidity—mood disorders, substance abuse, eating disorders—is common among patients and first-degree relatives |
| Chronic symptoms wax and wane, with widely varying severity |
| Irresistible urges prompt spending by some patients |
| Shopping is intensely exciting, with transitory feelings of happiness and power |
| Feelings of distress and guilt develop after shopping; patients often hide purchases |
| Patients may be in denial or feel embarrassed to disclose symptoms |
An Evolving Picture
Ms. A says shopping is her primary social activity and entertainment. Though she works full time, she shops three or more times a week, cruising expensive department stores and discount outlets on evenings and weekends. She buys clothing, shoes, makeup, jewelry, antiques, household electronics, and other items.
She says her shopping is spontaneous and impulsive. Shopping gives her an emotional “rush” that is frequently followed by periods of guilt, and she often returns or gives away purchased items. She is disappointed at her inability to control her shopping behavior and ashamed of the financial crises she has caused.
Compulsive buying is characterized by persistent or poorly controlled preoccupations, urges, or behaviors regarding shopping or spending, leading to adverse consequences.2 Onset in late adolescence to early adulthood is the usual pattern, and the disorder is thought to be chronic or recurrent. It is not listed in DSM-IV-TR but is considered an example of an impulse control disorder not otherwise specified. For this paper, we use the terms compulsive shopping and compulsive buying interchangeably.
The disorder’s tentative classification reflects debate about its conceptualization. Some clinicians and researchers consider compulsive buying an addiction similar to drug or alcohol misuse; others have linked it to depression or anxiety. Hollander3 and others have commented on its similarities with obsessive-compulsive disorder (OCD), and a recent study noted that compulsive buying is more common in patients with OCD than in matched controls.4 Still others—drawing on Kraepelin’s and Bleuler’s early work—consider compulsive buying an impulse control disorder, having features in common with pathological gambling and kleptomania.5
Prevalence. One survey estimates 2% to 8% of U.S. adults meet criteria for a compulsive shopping disorder, and community-based and clinical surveys suggest that 86% to 95% of them are women.5 The reported gender difference may be artifactual; women readily acknowledge that they enjoy shopping, whereas men are more likely to report that they “collect.”
Behavior patterns. No careful, longitudinal studies have examined compulsive buying disorder, but case reports suggest the condition is chronic, with a waxing and waning course and wide variance in symptom severity. In 20 consecutive patients with compulsive buying symptoms, one-half reported that irresistible urges prompted spending and three-quarters preferred to shop alone.6
Compulsive shoppers tend to shop frequently and spend inappropriately:
- at department and discount stores, specialty shops, and boutiques
- from mail order, television, and online merchants.
While shopping, compulsive shoppers may report feeling intensely excited, happy, and powerful. These emotions are frequently followed by distress or guilt. They may return purchases or hide them in closets or attics, never to be used.
Low-income persons who shop compulsively may do so at consignment shops or garage sales. In one of our studies, the most severe compulsive buyers had the lowest incomes,6 suggesting that:
- lack of money does not prevent compulsive shopping disorder from developing
- severe compulsive shoppers lack the ability to delay their shopping.
Psychiatric comorbidity
Compulsive buyers differ from matched controls when dimensional scales are used to measure psychopathology. One study found that compulsive buyers had elevated scores on the Beck Depression Inventory, the Spielberger Trait Anxiety Scale, and the Maudsley Obsessive Compulsive Inventory.2
Compulsive buyers and their first-degree relatives often have comorbid psychiatric disorders, particularly mood, anxiety, substance use, and eating disorders.5 Axis II disorders are also common; no particular type predominates, but the obsessive-compulsive, borderline, and avoidant personality types are seen most frequently.
McElroy et al7 defined compulsive buying disorder as:
- uncontrollable
- markedly distressing, time-consuming, and/or resulting in family, social, vocational, and/or financial difficulties
- not occurring only in the context of hypomanic or manic symptoms.
In a larger controlled study, our group8 compared 33 individuals who met the McElroy et al criteria for compulsive buying disorder and 22 control patients. The 137 first-degree relatives of the compulsive shoppers were significantly more likely than the controls’ relatives to have histories of depression, alcoholism, substance use, or multiple psychiatric diagnoses (as measured by the Family History Research Diagnostic Criteria).
Identifying a patient’s psychiatric comorbidities can help you develop:
- a biopsychosocial counseling plan—such as for a patient with borderline personality disorder who shops to relieve tension from relationship stress
- pharmacologic treatment strategies—such as prescribing a selective serotonin reuptake inhibitor (SSRI) for patients with comorbid major depression.
Manic versus compulsive behavior
Manic and hypomanic symptoms may be associated with impulsive and reckless spending. Thus, when evaluating excessive spending, always carefully evaluate patients for bipolar disorder.
Bipolar mania and excessive spending related to a compulsive buying disorder are relatively easy to differentiate:
- The manic patient’s unrestrained spending sprees correspond to manic episodes and are accompanied by euphoric mood, grandiosity, unrealistic plans, and often a giddy, overly bright affect.
- The compulsive shopper’s spending occurs year-round in a pattern suggesting ongoing preoccupation.
Not so for the manic, who may boast of his or her spending, display the evidence, and try to convince family and friends that the purchase is necessary or fits into some grandiose scheme. “Who doesn’t need two BMWs?” a manic patient said to one of the authors [DWB].
Screening and diagnosis
As with any psychiatric disorder, gathering an accurate history through a careful interview is important. This can be challenging with compulsive shopping disorder, however, because the patient may minimize symptoms out of embarrassment or denial. Your goal is to identify the shopping problem through nonjudgmental inquiries.
Diagnostic instruments. Researchers use assessment tools such as Faber and O’Guinn’s 7-item Compulsive Buying Scale9 to help diagnose this disorder. Our group developed a shopping version of the Yale-Brown Obsessive Compulsive Scale (YBOCS-SV) to help rate severity and change during clinical trials.10
Formal instruments may help in the clinical setting, but you can often elicit compulsive buying symptoms with a few screening questions (Table 2). If screening indicates a positive response, move to more detailed questions about:
- frequency of excessive shopping
- time spent shopping
- factors that trigger or worsen the shopping behavior
- amount of money spent.
Table 2
Is your patient a compulsive shopper? Ask these screening questions
| Do you feel preoccupied with shopping and spending? |
| Do you ever feel that your shopping behavior is excessive, inappropriate, or uncontrolled? |
Have your shopping desires, urges, fantasies, or behaviors ever:
|
| Source: Black DW. Assessment of compulsive buying. In: Benson AL, ed. I shop, therefore I am: Compulsive buying and the search for self. Northvale, NJ: Jason Aronson; 2000:191-216. |
Stopping uncontrolled shopping
Compulsive shopping has no standard treatment, but evidence shows benefit from some SSRIs and psychotherapies.
Fluvoxamine. An early case series suggested antidepressants could curb compulsive buying,5 but later research has yielded mixed results.
Ms. A entered an experimental drug trial. She was randomly assigned to receive fluvoxamine and—despite difficulties with oversedation—tolerated a sustained dosage of 100 mg/d. After the 9-week trial, Ms. A said she thought less frequently about shopping, felt less compulsion to shop, and was spending less money and time shopping.
This open-label trial we conducted indicated that fluvoxamine, up to 300 mg/d, could be an effective treatment for compulsive buying.11 Two subsequent randomized controlled trials, however, found fluvoxamine did no better than placebo when treating compulsive shoppers.12,13
Citalopram. In an open-label trial,14 23 women and 1 man who met diagnostic criteria for compulsive shopping disorder (YBOCS-SV scores ≥17) received citalopram for 7 weeks. Dosages started at 20 mg/d and were increased as tolerated to 60 mg/d. Fifteen patients (63%) met response criteria—“much improved” or “very much improved” as measured by the Clinical Global Impressions-Improvement scale and a ≥50% decrease in YBOCS-SV score. Three patients (13%) discontinued treatment because of adverse effects (headache, rash, insomnia).
The 15 responders were then enrolled in a 9-week double-blind, placebo-controlled trial. Compulsive shopping symptoms recurred in 5 of 8 patients (63%) assigned to placebo, compared with none of the 7 who continued taking citalopram.
By comparison, escitalopram, 10 to 20 mg/d, showed little effect for compulsive shopping symptoms in an identically designed discontinuation trial by the same investigators. During the 7-week, open-label trial, 19 of 26 patients met response criteria. In the 9-week double-blind, controlled phase, however, 63% of initial responders relapsed while taking escitalopram, compared with 67% of those randomized to placebo.15
A naturalistic follow-up study of 24 patients treated with citalopram, 20 to 60 mg/d, noted that patients who responded at 3 months were more likely to be symptom-free after 1 year than those who did not respond to acute treatment.16 Responders’ mean 2-week compulsive spending declined from $773 before treatment to $351 at 12 months, and their mean total debt declined from $17,833 to $16,752.
Because remission was not significantly associated with taking citalopram, however, the authors concluded that the mechanisms responsible for maintaining remission were unclear.
Psychotherapy. Cognitive-behavioral therapy (CBT) may help, but few therapists are familiar with this disorder. CBT challenges the patient’s cognitive distortions and faulty schemas about shopping, such as:
- “Having the latest fashions will make me more popular.”
- “Having 5 pair of new shoes will make me a happier and better person.”
Our recommendations. Medication—such as an antidepressant for major depression or a mood stabilizer for bipolar disorder—may improve compulsive shopping in patients with a comorbid psychiatric disorder. For other compulsive shoppers, however, medication trials provide little guidance for treatment.
We inform patients such as Ms. A that they cannot rely on medication to control their behavior. Instead, we recommend a four-step approach to break the compulsive shopping habit (Table 3).
Financial counseling, provided free of charge by many banks, benefits some patients. Self-help books describe strategies to overcome compulsive spending (Related resources). Debtors Anonymous, a 12-step program patterned after Alcoholics Anonymous, also can help by offering acceptance, belonging, forgiveness, and understanding.
In the most severe cases we recommend appointing a financial conservator to control the patient’s finances. We rarely advise this strategy but have encountered cases in which there seemed to be no other option. Having a conservator controls the patient’s spending but does not reverse the preoccupation with shopping.
Table 3
Patient education: 4 steps to control compulsive spending
|
For clinicians
- Black DW. Assessment of compulsive buying. In: Benson AL (ed). I shop, therefore I am: Compulsive buying and the search for self. Northvale, NJ: Jason Aronson; 2000:191-216.
- Arenson G. Born to spend: how to overcome compulsive spending. Blue Ridge Summit, PA: Tab Books, 1991.
- Benson AL. Stopping Overshopping. A site for shopaholics and the people who love them. www.stoppingovershopping.com.
- Mellan O. Money harmony: resolving money conflicts in your life and relationships. New York: Walker, 2005.
Drug brand names
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluvoxamine • Luvox
Dr. Kuzma reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Black receives grant/research support or is a consultant or speaker for Forest Laboratories and Shire Pharmaceuticals
1. Lee S, Mysyk A. The medicalization of compulsive buying. Soc Sci Med 2004;58(9):1709-18.
2. Black DW. Compulsive buying disorder: definition, assessment, epidemiology and clinical management. CNS Drugs 2001;15(1):17-27.
3. Hollander E. Obsessive compulsive related disorders. Washington, DC: American Psychiatric Press; 1993.
4. Lejoyeux M, Bailly F, Moula H, Loi S, Ades J. Study of compulsive buying in patients presenting with obsessive-compulsive disorder. Compr Psychiatry 2005;46:105-10.
5. Black DW. Compulsive shopping. In: Hollander E, Stein D (eds). Clinical manual of impulse control disorders. Washington, DC: American Psychiatric Publishing; 2003;203–27.
6. Black DW, Monahan P, Schlosser S, Repertinger S. Compulsive buying severity: an analysis of Compulsive Buying Scale results in 44 subjects. J Nerv Ment Dis 2001;189:123-7.
7. McElroy SL, Keck PE, Jr, Pope HG, Jr, et al. Compulsive buying: a report of 20 cases. J Clin Psychiatry 1994;55:242-8.
8. Black DW, Repertinger S, Gaffney GR, Gabel J. Family history and psychiatric comorbidity in persons with compulsive buying: preliminary findings. Am J Psychiatry 1998;155:960-3.
9. Faber RJ, O’Guinn TC. A clinical screener for compulsive buying. Consum Res 1992;19:459-69.
10. Monahan P, Black DW, Gabel J. Reliability and validity of a scale to measure change in persons with compulsive buying. Psychiatr Res 1996;64:59-67.
11. Black DW, Monahan P, Gabel J. Fluvoxamine in the treatment of compulsive buying. J Clin Psychiatry 1997;58:159-63.
12. Ninan PT, McElroy SL, Kane CP, et al. Placebo-controlled study of fluvoxamine in the treatment of patients with compulsive buying. J Clin Psychopharmacol 2000;20:362-6.
13. Black DW, Gabel J, Hansen J, Schlosser S. A double-blind comparison of fluvoxamine versus placebo in the treatment of compulsive buying disorder. Ann Clin Psychiatry 2000;12:205-11.
14. Koran LM, Chuong HW, Bullock KD, Smith SC. Citalopram for compulsive shopping disorder: an open-label study followed by double-blind discontinuation. J Clin Psychiatry 2003;64:793-8.
15. Koran LM. Escitalopram treatment evaluated in patients with compulsive shopping disorder. Primary Psychiatry 2005;12(12):13.-
16. Aboujaoude E, Gamel N, Koran LM. A 1-year naturalistic follow-up of patients with compulsive shopping disorder. J Clin Psychiatry 2003;64:946-50.
Ms. A has been compulsively shopping and spending since age 19 when she first obtained credit cards. After years of intense urges to shop and remorse over the financial consequences, she seeks psychiatric help. Now age 37 and divorced, she has controlled her spending only for two 1- to 2-year periods that coincided with bankruptcy proceedings.
With easy access to credit, many persons such as Ms. A develop what is variously called compulsive buying, compulsive shopping, addictive shopping, or shopaholism. Although “medicalizing” excessive shopping may seem to obscure its broader cultural and social causes,1 increasing evidence points to a discrete shopping disorder.
Our group has contributed to compulsive buying research and continues to evaluate potential treatments. We offer evidence and practical advice to help you:
- identify compulsive shopping disorder using the patient’s history and three screening questions
- differentiate compulsive shopping from manic or hypomanic shopping sprees
- educate patients about four steps to control compulsive shopping.
Table 1
Compulsive shopping disorder’s clinical signs
| Onset in late adolescence to early adulthood |
| Female-to-male ratio may be 9:1 |
| Behaviors include shopping frequently, spending inappropriately, and fantasizing about future purchases |
| Psychiatric comorbidity—mood disorders, substance abuse, eating disorders—is common among patients and first-degree relatives |
| Chronic symptoms wax and wane, with widely varying severity |
| Irresistible urges prompt spending by some patients |
| Shopping is intensely exciting, with transitory feelings of happiness and power |
| Feelings of distress and guilt develop after shopping; patients often hide purchases |
| Patients may be in denial or feel embarrassed to disclose symptoms |
An Evolving Picture
Ms. A says shopping is her primary social activity and entertainment. Though she works full time, she shops three or more times a week, cruising expensive department stores and discount outlets on evenings and weekends. She buys clothing, shoes, makeup, jewelry, antiques, household electronics, and other items.
She says her shopping is spontaneous and impulsive. Shopping gives her an emotional “rush” that is frequently followed by periods of guilt, and she often returns or gives away purchased items. She is disappointed at her inability to control her shopping behavior and ashamed of the financial crises she has caused.
Compulsive buying is characterized by persistent or poorly controlled preoccupations, urges, or behaviors regarding shopping or spending, leading to adverse consequences.2 Onset in late adolescence to early adulthood is the usual pattern, and the disorder is thought to be chronic or recurrent. It is not listed in DSM-IV-TR but is considered an example of an impulse control disorder not otherwise specified. For this paper, we use the terms compulsive shopping and compulsive buying interchangeably.
The disorder’s tentative classification reflects debate about its conceptualization. Some clinicians and researchers consider compulsive buying an addiction similar to drug or alcohol misuse; others have linked it to depression or anxiety. Hollander3 and others have commented on its similarities with obsessive-compulsive disorder (OCD), and a recent study noted that compulsive buying is more common in patients with OCD than in matched controls.4 Still others—drawing on Kraepelin’s and Bleuler’s early work—consider compulsive buying an impulse control disorder, having features in common with pathological gambling and kleptomania.5
Prevalence. One survey estimates 2% to 8% of U.S. adults meet criteria for a compulsive shopping disorder, and community-based and clinical surveys suggest that 86% to 95% of them are women.5 The reported gender difference may be artifactual; women readily acknowledge that they enjoy shopping, whereas men are more likely to report that they “collect.”
Behavior patterns. No careful, longitudinal studies have examined compulsive buying disorder, but case reports suggest the condition is chronic, with a waxing and waning course and wide variance in symptom severity. In 20 consecutive patients with compulsive buying symptoms, one-half reported that irresistible urges prompted spending and three-quarters preferred to shop alone.6
Compulsive shoppers tend to shop frequently and spend inappropriately:
- at department and discount stores, specialty shops, and boutiques
- from mail order, television, and online merchants.
While shopping, compulsive shoppers may report feeling intensely excited, happy, and powerful. These emotions are frequently followed by distress or guilt. They may return purchases or hide them in closets or attics, never to be used.
Low-income persons who shop compulsively may do so at consignment shops or garage sales. In one of our studies, the most severe compulsive buyers had the lowest incomes,6 suggesting that:
- lack of money does not prevent compulsive shopping disorder from developing
- severe compulsive shoppers lack the ability to delay their shopping.
Psychiatric comorbidity
Compulsive buyers differ from matched controls when dimensional scales are used to measure psychopathology. One study found that compulsive buyers had elevated scores on the Beck Depression Inventory, the Spielberger Trait Anxiety Scale, and the Maudsley Obsessive Compulsive Inventory.2
Compulsive buyers and their first-degree relatives often have comorbid psychiatric disorders, particularly mood, anxiety, substance use, and eating disorders.5 Axis II disorders are also common; no particular type predominates, but the obsessive-compulsive, borderline, and avoidant personality types are seen most frequently.
McElroy et al7 defined compulsive buying disorder as:
- uncontrollable
- markedly distressing, time-consuming, and/or resulting in family, social, vocational, and/or financial difficulties
- not occurring only in the context of hypomanic or manic symptoms.
In a larger controlled study, our group8 compared 33 individuals who met the McElroy et al criteria for compulsive buying disorder and 22 control patients. The 137 first-degree relatives of the compulsive shoppers were significantly more likely than the controls’ relatives to have histories of depression, alcoholism, substance use, or multiple psychiatric diagnoses (as measured by the Family History Research Diagnostic Criteria).
Identifying a patient’s psychiatric comorbidities can help you develop:
- a biopsychosocial counseling plan—such as for a patient with borderline personality disorder who shops to relieve tension from relationship stress
- pharmacologic treatment strategies—such as prescribing a selective serotonin reuptake inhibitor (SSRI) for patients with comorbid major depression.
Manic versus compulsive behavior
Manic and hypomanic symptoms may be associated with impulsive and reckless spending. Thus, when evaluating excessive spending, always carefully evaluate patients for bipolar disorder.
Bipolar mania and excessive spending related to a compulsive buying disorder are relatively easy to differentiate:
- The manic patient’s unrestrained spending sprees correspond to manic episodes and are accompanied by euphoric mood, grandiosity, unrealistic plans, and often a giddy, overly bright affect.
- The compulsive shopper’s spending occurs year-round in a pattern suggesting ongoing preoccupation.
Not so for the manic, who may boast of his or her spending, display the evidence, and try to convince family and friends that the purchase is necessary or fits into some grandiose scheme. “Who doesn’t need two BMWs?” a manic patient said to one of the authors [DWB].
Screening and diagnosis
As with any psychiatric disorder, gathering an accurate history through a careful interview is important. This can be challenging with compulsive shopping disorder, however, because the patient may minimize symptoms out of embarrassment or denial. Your goal is to identify the shopping problem through nonjudgmental inquiries.
Diagnostic instruments. Researchers use assessment tools such as Faber and O’Guinn’s 7-item Compulsive Buying Scale9 to help diagnose this disorder. Our group developed a shopping version of the Yale-Brown Obsessive Compulsive Scale (YBOCS-SV) to help rate severity and change during clinical trials.10
Formal instruments may help in the clinical setting, but you can often elicit compulsive buying symptoms with a few screening questions (Table 2). If screening indicates a positive response, move to more detailed questions about:
- frequency of excessive shopping
- time spent shopping
- factors that trigger or worsen the shopping behavior
- amount of money spent.
Table 2
Is your patient a compulsive shopper? Ask these screening questions
| Do you feel preoccupied with shopping and spending? |
| Do you ever feel that your shopping behavior is excessive, inappropriate, or uncontrolled? |
Have your shopping desires, urges, fantasies, or behaviors ever:
|
| Source: Black DW. Assessment of compulsive buying. In: Benson AL, ed. I shop, therefore I am: Compulsive buying and the search for self. Northvale, NJ: Jason Aronson; 2000:191-216. |
Stopping uncontrolled shopping
Compulsive shopping has no standard treatment, but evidence shows benefit from some SSRIs and psychotherapies.
Fluvoxamine. An early case series suggested antidepressants could curb compulsive buying,5 but later research has yielded mixed results.
Ms. A entered an experimental drug trial. She was randomly assigned to receive fluvoxamine and—despite difficulties with oversedation—tolerated a sustained dosage of 100 mg/d. After the 9-week trial, Ms. A said she thought less frequently about shopping, felt less compulsion to shop, and was spending less money and time shopping.
This open-label trial we conducted indicated that fluvoxamine, up to 300 mg/d, could be an effective treatment for compulsive buying.11 Two subsequent randomized controlled trials, however, found fluvoxamine did no better than placebo when treating compulsive shoppers.12,13
Citalopram. In an open-label trial,14 23 women and 1 man who met diagnostic criteria for compulsive shopping disorder (YBOCS-SV scores ≥17) received citalopram for 7 weeks. Dosages started at 20 mg/d and were increased as tolerated to 60 mg/d. Fifteen patients (63%) met response criteria—“much improved” or “very much improved” as measured by the Clinical Global Impressions-Improvement scale and a ≥50% decrease in YBOCS-SV score. Three patients (13%) discontinued treatment because of adverse effects (headache, rash, insomnia).
The 15 responders were then enrolled in a 9-week double-blind, placebo-controlled trial. Compulsive shopping symptoms recurred in 5 of 8 patients (63%) assigned to placebo, compared with none of the 7 who continued taking citalopram.
By comparison, escitalopram, 10 to 20 mg/d, showed little effect for compulsive shopping symptoms in an identically designed discontinuation trial by the same investigators. During the 7-week, open-label trial, 19 of 26 patients met response criteria. In the 9-week double-blind, controlled phase, however, 63% of initial responders relapsed while taking escitalopram, compared with 67% of those randomized to placebo.15
A naturalistic follow-up study of 24 patients treated with citalopram, 20 to 60 mg/d, noted that patients who responded at 3 months were more likely to be symptom-free after 1 year than those who did not respond to acute treatment.16 Responders’ mean 2-week compulsive spending declined from $773 before treatment to $351 at 12 months, and their mean total debt declined from $17,833 to $16,752.
Because remission was not significantly associated with taking citalopram, however, the authors concluded that the mechanisms responsible for maintaining remission were unclear.
Psychotherapy. Cognitive-behavioral therapy (CBT) may help, but few therapists are familiar with this disorder. CBT challenges the patient’s cognitive distortions and faulty schemas about shopping, such as:
- “Having the latest fashions will make me more popular.”
- “Having 5 pair of new shoes will make me a happier and better person.”
Our recommendations. Medication—such as an antidepressant for major depression or a mood stabilizer for bipolar disorder—may improve compulsive shopping in patients with a comorbid psychiatric disorder. For other compulsive shoppers, however, medication trials provide little guidance for treatment.
We inform patients such as Ms. A that they cannot rely on medication to control their behavior. Instead, we recommend a four-step approach to break the compulsive shopping habit (Table 3).
Financial counseling, provided free of charge by many banks, benefits some patients. Self-help books describe strategies to overcome compulsive spending (Related resources). Debtors Anonymous, a 12-step program patterned after Alcoholics Anonymous, also can help by offering acceptance, belonging, forgiveness, and understanding.
In the most severe cases we recommend appointing a financial conservator to control the patient’s finances. We rarely advise this strategy but have encountered cases in which there seemed to be no other option. Having a conservator controls the patient’s spending but does not reverse the preoccupation with shopping.
Table 3
Patient education: 4 steps to control compulsive spending
|
For clinicians
- Black DW. Assessment of compulsive buying. In: Benson AL (ed). I shop, therefore I am: Compulsive buying and the search for self. Northvale, NJ: Jason Aronson; 2000:191-216.
- Arenson G. Born to spend: how to overcome compulsive spending. Blue Ridge Summit, PA: Tab Books, 1991.
- Benson AL. Stopping Overshopping. A site for shopaholics and the people who love them. www.stoppingovershopping.com.
- Mellan O. Money harmony: resolving money conflicts in your life and relationships. New York: Walker, 2005.
Drug brand names
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluvoxamine • Luvox
Dr. Kuzma reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Black receives grant/research support or is a consultant or speaker for Forest Laboratories and Shire Pharmaceuticals
Ms. A has been compulsively shopping and spending since age 19 when she first obtained credit cards. After years of intense urges to shop and remorse over the financial consequences, she seeks psychiatric help. Now age 37 and divorced, she has controlled her spending only for two 1- to 2-year periods that coincided with bankruptcy proceedings.
With easy access to credit, many persons such as Ms. A develop what is variously called compulsive buying, compulsive shopping, addictive shopping, or shopaholism. Although “medicalizing” excessive shopping may seem to obscure its broader cultural and social causes,1 increasing evidence points to a discrete shopping disorder.
Our group has contributed to compulsive buying research and continues to evaluate potential treatments. We offer evidence and practical advice to help you:
- identify compulsive shopping disorder using the patient’s history and three screening questions
- differentiate compulsive shopping from manic or hypomanic shopping sprees
- educate patients about four steps to control compulsive shopping.
Table 1
Compulsive shopping disorder’s clinical signs
| Onset in late adolescence to early adulthood |
| Female-to-male ratio may be 9:1 |
| Behaviors include shopping frequently, spending inappropriately, and fantasizing about future purchases |
| Psychiatric comorbidity—mood disorders, substance abuse, eating disorders—is common among patients and first-degree relatives |
| Chronic symptoms wax and wane, with widely varying severity |
| Irresistible urges prompt spending by some patients |
| Shopping is intensely exciting, with transitory feelings of happiness and power |
| Feelings of distress and guilt develop after shopping; patients often hide purchases |
| Patients may be in denial or feel embarrassed to disclose symptoms |
An Evolving Picture
Ms. A says shopping is her primary social activity and entertainment. Though she works full time, she shops three or more times a week, cruising expensive department stores and discount outlets on evenings and weekends. She buys clothing, shoes, makeup, jewelry, antiques, household electronics, and other items.
She says her shopping is spontaneous and impulsive. Shopping gives her an emotional “rush” that is frequently followed by periods of guilt, and she often returns or gives away purchased items. She is disappointed at her inability to control her shopping behavior and ashamed of the financial crises she has caused.
Compulsive buying is characterized by persistent or poorly controlled preoccupations, urges, or behaviors regarding shopping or spending, leading to adverse consequences.2 Onset in late adolescence to early adulthood is the usual pattern, and the disorder is thought to be chronic or recurrent. It is not listed in DSM-IV-TR but is considered an example of an impulse control disorder not otherwise specified. For this paper, we use the terms compulsive shopping and compulsive buying interchangeably.
The disorder’s tentative classification reflects debate about its conceptualization. Some clinicians and researchers consider compulsive buying an addiction similar to drug or alcohol misuse; others have linked it to depression or anxiety. Hollander3 and others have commented on its similarities with obsessive-compulsive disorder (OCD), and a recent study noted that compulsive buying is more common in patients with OCD than in matched controls.4 Still others—drawing on Kraepelin’s and Bleuler’s early work—consider compulsive buying an impulse control disorder, having features in common with pathological gambling and kleptomania.5
Prevalence. One survey estimates 2% to 8% of U.S. adults meet criteria for a compulsive shopping disorder, and community-based and clinical surveys suggest that 86% to 95% of them are women.5 The reported gender difference may be artifactual; women readily acknowledge that they enjoy shopping, whereas men are more likely to report that they “collect.”
Behavior patterns. No careful, longitudinal studies have examined compulsive buying disorder, but case reports suggest the condition is chronic, with a waxing and waning course and wide variance in symptom severity. In 20 consecutive patients with compulsive buying symptoms, one-half reported that irresistible urges prompted spending and three-quarters preferred to shop alone.6
Compulsive shoppers tend to shop frequently and spend inappropriately:
- at department and discount stores, specialty shops, and boutiques
- from mail order, television, and online merchants.
While shopping, compulsive shoppers may report feeling intensely excited, happy, and powerful. These emotions are frequently followed by distress or guilt. They may return purchases or hide them in closets or attics, never to be used.
Low-income persons who shop compulsively may do so at consignment shops or garage sales. In one of our studies, the most severe compulsive buyers had the lowest incomes,6 suggesting that:
- lack of money does not prevent compulsive shopping disorder from developing
- severe compulsive shoppers lack the ability to delay their shopping.
Psychiatric comorbidity
Compulsive buyers differ from matched controls when dimensional scales are used to measure psychopathology. One study found that compulsive buyers had elevated scores on the Beck Depression Inventory, the Spielberger Trait Anxiety Scale, and the Maudsley Obsessive Compulsive Inventory.2
Compulsive buyers and their first-degree relatives often have comorbid psychiatric disorders, particularly mood, anxiety, substance use, and eating disorders.5 Axis II disorders are also common; no particular type predominates, but the obsessive-compulsive, borderline, and avoidant personality types are seen most frequently.
McElroy et al7 defined compulsive buying disorder as:
- uncontrollable
- markedly distressing, time-consuming, and/or resulting in family, social, vocational, and/or financial difficulties
- not occurring only in the context of hypomanic or manic symptoms.
In a larger controlled study, our group8 compared 33 individuals who met the McElroy et al criteria for compulsive buying disorder and 22 control patients. The 137 first-degree relatives of the compulsive shoppers were significantly more likely than the controls’ relatives to have histories of depression, alcoholism, substance use, or multiple psychiatric diagnoses (as measured by the Family History Research Diagnostic Criteria).
Identifying a patient’s psychiatric comorbidities can help you develop:
- a biopsychosocial counseling plan—such as for a patient with borderline personality disorder who shops to relieve tension from relationship stress
- pharmacologic treatment strategies—such as prescribing a selective serotonin reuptake inhibitor (SSRI) for patients with comorbid major depression.
Manic versus compulsive behavior
Manic and hypomanic symptoms may be associated with impulsive and reckless spending. Thus, when evaluating excessive spending, always carefully evaluate patients for bipolar disorder.
Bipolar mania and excessive spending related to a compulsive buying disorder are relatively easy to differentiate:
- The manic patient’s unrestrained spending sprees correspond to manic episodes and are accompanied by euphoric mood, grandiosity, unrealistic plans, and often a giddy, overly bright affect.
- The compulsive shopper’s spending occurs year-round in a pattern suggesting ongoing preoccupation.
Not so for the manic, who may boast of his or her spending, display the evidence, and try to convince family and friends that the purchase is necessary or fits into some grandiose scheme. “Who doesn’t need two BMWs?” a manic patient said to one of the authors [DWB].
Screening and diagnosis
As with any psychiatric disorder, gathering an accurate history through a careful interview is important. This can be challenging with compulsive shopping disorder, however, because the patient may minimize symptoms out of embarrassment or denial. Your goal is to identify the shopping problem through nonjudgmental inquiries.
Diagnostic instruments. Researchers use assessment tools such as Faber and O’Guinn’s 7-item Compulsive Buying Scale9 to help diagnose this disorder. Our group developed a shopping version of the Yale-Brown Obsessive Compulsive Scale (YBOCS-SV) to help rate severity and change during clinical trials.10
Formal instruments may help in the clinical setting, but you can often elicit compulsive buying symptoms with a few screening questions (Table 2). If screening indicates a positive response, move to more detailed questions about:
- frequency of excessive shopping
- time spent shopping
- factors that trigger or worsen the shopping behavior
- amount of money spent.
Table 2
Is your patient a compulsive shopper? Ask these screening questions
| Do you feel preoccupied with shopping and spending? |
| Do you ever feel that your shopping behavior is excessive, inappropriate, or uncontrolled? |
Have your shopping desires, urges, fantasies, or behaviors ever:
|
| Source: Black DW. Assessment of compulsive buying. In: Benson AL, ed. I shop, therefore I am: Compulsive buying and the search for self. Northvale, NJ: Jason Aronson; 2000:191-216. |
Stopping uncontrolled shopping
Compulsive shopping has no standard treatment, but evidence shows benefit from some SSRIs and psychotherapies.
Fluvoxamine. An early case series suggested antidepressants could curb compulsive buying,5 but later research has yielded mixed results.
Ms. A entered an experimental drug trial. She was randomly assigned to receive fluvoxamine and—despite difficulties with oversedation—tolerated a sustained dosage of 100 mg/d. After the 9-week trial, Ms. A said she thought less frequently about shopping, felt less compulsion to shop, and was spending less money and time shopping.
This open-label trial we conducted indicated that fluvoxamine, up to 300 mg/d, could be an effective treatment for compulsive buying.11 Two subsequent randomized controlled trials, however, found fluvoxamine did no better than placebo when treating compulsive shoppers.12,13
Citalopram. In an open-label trial,14 23 women and 1 man who met diagnostic criteria for compulsive shopping disorder (YBOCS-SV scores ≥17) received citalopram for 7 weeks. Dosages started at 20 mg/d and were increased as tolerated to 60 mg/d. Fifteen patients (63%) met response criteria—“much improved” or “very much improved” as measured by the Clinical Global Impressions-Improvement scale and a ≥50% decrease in YBOCS-SV score. Three patients (13%) discontinued treatment because of adverse effects (headache, rash, insomnia).
The 15 responders were then enrolled in a 9-week double-blind, placebo-controlled trial. Compulsive shopping symptoms recurred in 5 of 8 patients (63%) assigned to placebo, compared with none of the 7 who continued taking citalopram.
By comparison, escitalopram, 10 to 20 mg/d, showed little effect for compulsive shopping symptoms in an identically designed discontinuation trial by the same investigators. During the 7-week, open-label trial, 19 of 26 patients met response criteria. In the 9-week double-blind, controlled phase, however, 63% of initial responders relapsed while taking escitalopram, compared with 67% of those randomized to placebo.15
A naturalistic follow-up study of 24 patients treated with citalopram, 20 to 60 mg/d, noted that patients who responded at 3 months were more likely to be symptom-free after 1 year than those who did not respond to acute treatment.16 Responders’ mean 2-week compulsive spending declined from $773 before treatment to $351 at 12 months, and their mean total debt declined from $17,833 to $16,752.
Because remission was not significantly associated with taking citalopram, however, the authors concluded that the mechanisms responsible for maintaining remission were unclear.
Psychotherapy. Cognitive-behavioral therapy (CBT) may help, but few therapists are familiar with this disorder. CBT challenges the patient’s cognitive distortions and faulty schemas about shopping, such as:
- “Having the latest fashions will make me more popular.”
- “Having 5 pair of new shoes will make me a happier and better person.”
Our recommendations. Medication—such as an antidepressant for major depression or a mood stabilizer for bipolar disorder—may improve compulsive shopping in patients with a comorbid psychiatric disorder. For other compulsive shoppers, however, medication trials provide little guidance for treatment.
We inform patients such as Ms. A that they cannot rely on medication to control their behavior. Instead, we recommend a four-step approach to break the compulsive shopping habit (Table 3).
Financial counseling, provided free of charge by many banks, benefits some patients. Self-help books describe strategies to overcome compulsive spending (Related resources). Debtors Anonymous, a 12-step program patterned after Alcoholics Anonymous, also can help by offering acceptance, belonging, forgiveness, and understanding.
In the most severe cases we recommend appointing a financial conservator to control the patient’s finances. We rarely advise this strategy but have encountered cases in which there seemed to be no other option. Having a conservator controls the patient’s spending but does not reverse the preoccupation with shopping.
Table 3
Patient education: 4 steps to control compulsive spending
|
For clinicians
- Black DW. Assessment of compulsive buying. In: Benson AL (ed). I shop, therefore I am: Compulsive buying and the search for self. Northvale, NJ: Jason Aronson; 2000:191-216.
- Arenson G. Born to spend: how to overcome compulsive spending. Blue Ridge Summit, PA: Tab Books, 1991.
- Benson AL. Stopping Overshopping. A site for shopaholics and the people who love them. www.stoppingovershopping.com.
- Mellan O. Money harmony: resolving money conflicts in your life and relationships. New York: Walker, 2005.
Drug brand names
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluvoxamine • Luvox
Dr. Kuzma reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Black receives grant/research support or is a consultant or speaker for Forest Laboratories and Shire Pharmaceuticals
1. Lee S, Mysyk A. The medicalization of compulsive buying. Soc Sci Med 2004;58(9):1709-18.
2. Black DW. Compulsive buying disorder: definition, assessment, epidemiology and clinical management. CNS Drugs 2001;15(1):17-27.
3. Hollander E. Obsessive compulsive related disorders. Washington, DC: American Psychiatric Press; 1993.
4. Lejoyeux M, Bailly F, Moula H, Loi S, Ades J. Study of compulsive buying in patients presenting with obsessive-compulsive disorder. Compr Psychiatry 2005;46:105-10.
5. Black DW. Compulsive shopping. In: Hollander E, Stein D (eds). Clinical manual of impulse control disorders. Washington, DC: American Psychiatric Publishing; 2003;203–27.
6. Black DW, Monahan P, Schlosser S, Repertinger S. Compulsive buying severity: an analysis of Compulsive Buying Scale results in 44 subjects. J Nerv Ment Dis 2001;189:123-7.
7. McElroy SL, Keck PE, Jr, Pope HG, Jr, et al. Compulsive buying: a report of 20 cases. J Clin Psychiatry 1994;55:242-8.
8. Black DW, Repertinger S, Gaffney GR, Gabel J. Family history and psychiatric comorbidity in persons with compulsive buying: preliminary findings. Am J Psychiatry 1998;155:960-3.
9. Faber RJ, O’Guinn TC. A clinical screener for compulsive buying. Consum Res 1992;19:459-69.
10. Monahan P, Black DW, Gabel J. Reliability and validity of a scale to measure change in persons with compulsive buying. Psychiatr Res 1996;64:59-67.
11. Black DW, Monahan P, Gabel J. Fluvoxamine in the treatment of compulsive buying. J Clin Psychiatry 1997;58:159-63.
12. Ninan PT, McElroy SL, Kane CP, et al. Placebo-controlled study of fluvoxamine in the treatment of patients with compulsive buying. J Clin Psychopharmacol 2000;20:362-6.
13. Black DW, Gabel J, Hansen J, Schlosser S. A double-blind comparison of fluvoxamine versus placebo in the treatment of compulsive buying disorder. Ann Clin Psychiatry 2000;12:205-11.
14. Koran LM, Chuong HW, Bullock KD, Smith SC. Citalopram for compulsive shopping disorder: an open-label study followed by double-blind discontinuation. J Clin Psychiatry 2003;64:793-8.
15. Koran LM. Escitalopram treatment evaluated in patients with compulsive shopping disorder. Primary Psychiatry 2005;12(12):13.-
16. Aboujaoude E, Gamel N, Koran LM. A 1-year naturalistic follow-up of patients with compulsive shopping disorder. J Clin Psychiatry 2003;64:946-50.
1. Lee S, Mysyk A. The medicalization of compulsive buying. Soc Sci Med 2004;58(9):1709-18.
2. Black DW. Compulsive buying disorder: definition, assessment, epidemiology and clinical management. CNS Drugs 2001;15(1):17-27.
3. Hollander E. Obsessive compulsive related disorders. Washington, DC: American Psychiatric Press; 1993.
4. Lejoyeux M, Bailly F, Moula H, Loi S, Ades J. Study of compulsive buying in patients presenting with obsessive-compulsive disorder. Compr Psychiatry 2005;46:105-10.
5. Black DW. Compulsive shopping. In: Hollander E, Stein D (eds). Clinical manual of impulse control disorders. Washington, DC: American Psychiatric Publishing; 2003;203–27.
6. Black DW, Monahan P, Schlosser S, Repertinger S. Compulsive buying severity: an analysis of Compulsive Buying Scale results in 44 subjects. J Nerv Ment Dis 2001;189:123-7.
7. McElroy SL, Keck PE, Jr, Pope HG, Jr, et al. Compulsive buying: a report of 20 cases. J Clin Psychiatry 1994;55:242-8.
8. Black DW, Repertinger S, Gaffney GR, Gabel J. Family history and psychiatric comorbidity in persons with compulsive buying: preliminary findings. Am J Psychiatry 1998;155:960-3.
9. Faber RJ, O’Guinn TC. A clinical screener for compulsive buying. Consum Res 1992;19:459-69.
10. Monahan P, Black DW, Gabel J. Reliability and validity of a scale to measure change in persons with compulsive buying. Psychiatr Res 1996;64:59-67.
11. Black DW, Monahan P, Gabel J. Fluvoxamine in the treatment of compulsive buying. J Clin Psychiatry 1997;58:159-63.
12. Ninan PT, McElroy SL, Kane CP, et al. Placebo-controlled study of fluvoxamine in the treatment of patients with compulsive buying. J Clin Psychopharmacol 2000;20:362-6.
13. Black DW, Gabel J, Hansen J, Schlosser S. A double-blind comparison of fluvoxamine versus placebo in the treatment of compulsive buying disorder. Ann Clin Psychiatry 2000;12:205-11.
14. Koran LM, Chuong HW, Bullock KD, Smith SC. Citalopram for compulsive shopping disorder: an open-label study followed by double-blind discontinuation. J Clin Psychiatry 2003;64:793-8.
15. Koran LM. Escitalopram treatment evaluated in patients with compulsive shopping disorder. Primary Psychiatry 2005;12(12):13.-
16. Aboujaoude E, Gamel N, Koran LM. A 1-year naturalistic follow-up of patients with compulsive shopping disorder. J Clin Psychiatry 2003;64:946-50.
Transdermal methylphenidate
When prescribing methylphenidate to children with attention-deficit/hyperactivity disorder (ADHD), psychiatrists have had two options:
- immediate-release oral methylphenidate, which works for 3 to 5 hours, necessitating multiple daily doses
- extended-release oral methylphenidate, which can prevent irritability and other rebound symptoms caused by multiple daily dosing.1 Because its effects last 12 hours, however, once-daily dosing with this formulation is inflexible.
A new option—a transdermal methylphenidate patch FDA-approved for treating ADHD in children ages 6 to 12 (Table 1)—offers flexible methylphenidate coverage based on response to or need for the medication.
Table 1
Transdermal methylphenidate: Fast facts
| Brand name: Daytrana |
| Class: CNS stimulant |
| FDA-approved indication: ADHD in children ages 6 to 12 |
| Manufacturer: Noven Pharmaceuticals (marketed by Shire PLC) |
| Dosing forms: 10-, 15-, 20-, and 30-mg patches |
| Recommended dosage: One 10- to 30-mg patch daily, worn on the hip for 9 hours. Patient can remove patch sooner if side effects become problematic. |
Clinical implications
The transdermal patch allows dosing to be tailored—or changed day to day as needed—to maximize effectiveness and reduce side-effect risk. The manufacturer recommends that the patch be worn for 9 hours daily, but it can be removed sooner if children experience appetite loss, insomnia, or other adverse effects with 9 hours of exposure to methylphenidate.
Minimizing daily exposure to methylphenidate can also reduce the risk of long-term effects. Findings from one large, randomized clinical trial2 suggest that chronic exposure to high-dose stimulant medications might suppress growth in height and weight, although other data indicate that initial height reductions found in children receiving methylphenidate for ADHD were no longer significant in adulthood.3
The patch also could benefit youths who have trouble following dosing schedules and young children who are unable to swallow pills.
How it works
The patch contains methylphenidate dispersed in an acrylic multipolymeric adhesive that is further dispersed in a silicone adhesive.4 The methylphenidate within the acrylic adhesive flows into the skin, then into the bloodstream. The patch is worn on the hip—where it is covered by clothing and unlikely to be dislodged—and changed daily.
The patch comes in four sizes—12.5, 18.75, 25, and 37.5 cm2—which, respectively, deliver 10, 15, 20, and 30 mg of methylphenidate over 9 hours.4 Methylphenidate concentration is the same for all four sizes, so patch size and duration of use determine dose delivery.
In clinical trials, therapeutic effect was seen 2 hours after patch placement and continued through 12 hours.5 Methylphenidate is delivered continuously while the patch is in place and for as long as 2 hours after it is removed.
Pharmacokinetics
Methylphenidate, a known CNS stimulant, blocks norepinephrine and dopamine reuptake in the presynaptic neuron, thereby releasing more of these neuro-transmitters into the extraneuronal space.4 Methylphenidate’s precise therapeutic action in ADHD is not known.
Methylphenidate is a racemic mixture of d- and l-enantiomers, the first of which is believed to be more active. Whereas the liver removes the l-enantiomer from oral methylphenidate, the transdermal formulation bypasses the liver and preserves the l-enantiomer, thus increasing exposure to racemic methylphenidate. This means that optimal dosages of transdermal methylphenidate (10 to 30 mg/d) may be lower compared with the oral formulation5-7 (Table 2).
Methylphenidate’s d-enantiomer has a mean 3- to 4-hour elimination half-life, approximately twice that of the l-enantiomer. This is why transdermal methylphenidate continues to exert therapeutic effect several hours after the patch is removed.5
Table 2
Transdermal methylphenidate dosage delivery in children ages 6 to 12
| Dose delivered over 9 hours (mg) | Patch size (cm2) | Dosage rate (mg/hr) | Methylphenidate content per patch (mg) |
|---|---|---|---|
| 10 | 12.5 | 1.1 | 27.5 |
| 15 | 18.75 | 1.6 | 41.3 |
| 20 | 25 | 2.2 | 55.0 |
| 30 | 37.5 | 3.3 | 82.5 |
| Source: Reference 4 | |||
Efficacy
Results from randomized, double-blind, placebo-controlled trials support short-term use of transdermal methylphenidate in ADHD. The following studies recruited children ages 6 to 12 with the disorder.
Dose-ranging study. Thirty-three children participating in a summer treatment program received transdermal methylphenidate, 6.25, 12.5, or 25 cm2 12 hours daily for 8 days.6 All three patch sizes were associated with improved academic, social, and behavioral functioning based on a range of measures.
Dose response rate diminished with higher dosages, and significant further improvements were difficult to detect as dosages increased. The children also received intensive behavioral treatment during the study, which might have accounted for some therapeutic gains and diminished the researchers’ ability to detect subtle improvements with increased dosages.
Children also had fewer negative behaviors during the first hour when the patch was applied at 6 AM instead of 7 AM. This suggests that the patch might produce optimal effect when placed first thing in the morning.
Randomized crossover trial.7 Across 6 weeks, 27 children were given placebo or transdermal methylphenidate, 12.5, 25, or 37.5 cm2/d. The children also received behavior modification treatment on alternating weeks. Medication was randomly assigned and varied daily for 4 days per week over 6 weeks, and behavioral treatment was varied weekly for 4 weeks. Each subject took each dosage for 2 days without behavioral treatment and for 4 days with behavioral treatment.
Academic productivity, interactions with peers and adults, and compliance during class improved with all dosages compared with placebo. Although most children removed the patch by 3:30 PM after 9 hours of use, parents reported that positive behavioral effects lasted into the evening.
As in the dose-ranging study, dose response diminished with higher dosages. Optimal effects were achieved on some measures with 12.5 cm2 of transdermal methylphenidate, which produces the same plasma drug level as 10 mg of oral methylphenidate.7
Multisite crossover study.5 Eighty children received transdermal methylphenidate—12.5, 18.75, 25, or 37.5 cm2 based on response to medication—over 5 weeks for 9 hours daily. Dosages were titrated by changing patch sizes until each child reached his or her optimal dosage. Children then received their optimal dosage or placebo for 1 week, then received the opposite treatment for another week. Results were measured in a simulated classroom.
Overall, children showed statistically significant improvement in Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) Teacher Rating Scale scores while receiving their optimal dosage. Improvement was seen 2 hours after patches were applied and continued for 12 hours. Children also were more able to solve math problems during optimal dosage periods than while using placebo.
Nearly 80% of children were rated as significantly improved while receiving transdermal methylphenidate based on Clinical Global Impressions of Improvement scores. Roughly 12% of children showed significant improvement with placebo. Parents reported that their children were markedly less hyperactive and impulsive and more attentive during optimal dosage periods.
Tolerability
No serious side effects were reported during clinical trials of transdermal methylphenidate in children ages 6 to 12.5-7 Side effects commonly associated with oral methylphenidate—anorexia, decreased appetite, headache, insomnia, and abdominal pain—were most frequently reported with the patch.5
In one study,6 61% of children who wore the patch for 12 hours/day reported appetite loss and 47% reported insomnia. Insomnia prevalence diminished substantially when daily wear was limited to 9 hours.2,5 Loss of appetite was reported less often with lower-dose patches (12.5, 18.75 cm2) than with higher-dose patches (25, 37.5 cm2).
Although many children complained of erythema at the patch site,5-7 most reported minimal irritation or discomfort.5 Redness usually dissipated about 8 hours after the patch was removed.6
Despite concerns that youths with impulsive behaviors might remove the patches prematurely, very few children did so during clinical trials.5-7 Those who did had comorbid symptomatic conduct disturbances. Compliance with patch placement and maintenance was very high during dose optimization (98%) and analog classroom analysis (97%).5-7
Dosing
Start transdermal methylphenidate at 12.5 cm2 (10 mg) for children who have never taken methylphenidate or were previously stabilized on the drug. If the child does not respond after 1 week, switch to the 18.75 cm2 (15-mg) patch; keep switching to the next largest patch each week until optimal response is achieved. In clinical trials,5-7 18.75 or 25 cm2 (10 mg) of transdermal methylphenidate produced optimal response for most children.
Advise the child and parents to place the patch on the right and left hip on alternate days to minimize irritation. Counsel children to inform parents if the patch causes itching, burning, or irritation; tell parents to call you if they notice or the child complains of irritation. Children who experience intolerable skin sensitivity with the patch can resume taking oral methylphenidate the day after the patch is removed.
As with oral methylphenidate, the transdermal formulation may be discontinued without a taper and another formulation or medication may be started the next day. Ask the child and parents about side effects at each visit. See the child every 2 to 4 weeks during the titration period and monthly after symptoms are stabilized.
Consider trying a “drug holiday” for at least 2 weeks during the summer to see how the child behaves without methylphenidate, then evaluate the need for medication and determine the optimal dosage close to when the school year begins.
Related resources
- Transdermal methylphenidate Web site. www.daytrana.com.
- Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41:26S-49S.
Drug brand names
- Methylphenidate (oral) • Concerta, Ritalin, Metadate
- Methylphenidate (transdermal) • Daytrana
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Spencer TJ. ADHD treatment across the life cycle. J Clin Psychiatry 2004;65S3:22-6.
2. MTA Cooperative Group. National Institute of Mental Health multimodal treatment study of ADHD follow-up: changes in effectiveness and growth at the end of treatment. Pediatrics 2004;113:762-9.
3. Klein RG, Mannuzza S. Hyperactive boys almost grown up. III. Methylphenidate effects on ultimate height. Arch Gen Psychiatry 1988;45:1131-4.
4. Transdermal methylphenidate Web site. Available at: http://www.daytrana.com. Accessed April 11, 2006.
5. McGough JJ, Wigal SB, Abikoff H, et al. A randomized, double-blind, placebo controlled laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. J Atten Disord 2006;9:476-85.
6. Pelham WE, Jr, Manos MJ, Ezzell CE, et al. A dose-ranging study of a methylphenidate transdermal system in children with ADHD. J Am Acad Child Adolesc Psychiatry 2005;44:522-9.
7. Pelham WE, Burrows-Maclean L, Gnagy EM, et al. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Exp Clin Pharmacol 2005;13:111-26.
When prescribing methylphenidate to children with attention-deficit/hyperactivity disorder (ADHD), psychiatrists have had two options:
- immediate-release oral methylphenidate, which works for 3 to 5 hours, necessitating multiple daily doses
- extended-release oral methylphenidate, which can prevent irritability and other rebound symptoms caused by multiple daily dosing.1 Because its effects last 12 hours, however, once-daily dosing with this formulation is inflexible.
A new option—a transdermal methylphenidate patch FDA-approved for treating ADHD in children ages 6 to 12 (Table 1)—offers flexible methylphenidate coverage based on response to or need for the medication.
Table 1
Transdermal methylphenidate: Fast facts
| Brand name: Daytrana |
| Class: CNS stimulant |
| FDA-approved indication: ADHD in children ages 6 to 12 |
| Manufacturer: Noven Pharmaceuticals (marketed by Shire PLC) |
| Dosing forms: 10-, 15-, 20-, and 30-mg patches |
| Recommended dosage: One 10- to 30-mg patch daily, worn on the hip for 9 hours. Patient can remove patch sooner if side effects become problematic. |
Clinical implications
The transdermal patch allows dosing to be tailored—or changed day to day as needed—to maximize effectiveness and reduce side-effect risk. The manufacturer recommends that the patch be worn for 9 hours daily, but it can be removed sooner if children experience appetite loss, insomnia, or other adverse effects with 9 hours of exposure to methylphenidate.
Minimizing daily exposure to methylphenidate can also reduce the risk of long-term effects. Findings from one large, randomized clinical trial2 suggest that chronic exposure to high-dose stimulant medications might suppress growth in height and weight, although other data indicate that initial height reductions found in children receiving methylphenidate for ADHD were no longer significant in adulthood.3
The patch also could benefit youths who have trouble following dosing schedules and young children who are unable to swallow pills.
How it works
The patch contains methylphenidate dispersed in an acrylic multipolymeric adhesive that is further dispersed in a silicone adhesive.4 The methylphenidate within the acrylic adhesive flows into the skin, then into the bloodstream. The patch is worn on the hip—where it is covered by clothing and unlikely to be dislodged—and changed daily.
The patch comes in four sizes—12.5, 18.75, 25, and 37.5 cm2—which, respectively, deliver 10, 15, 20, and 30 mg of methylphenidate over 9 hours.4 Methylphenidate concentration is the same for all four sizes, so patch size and duration of use determine dose delivery.
In clinical trials, therapeutic effect was seen 2 hours after patch placement and continued through 12 hours.5 Methylphenidate is delivered continuously while the patch is in place and for as long as 2 hours after it is removed.
Pharmacokinetics
Methylphenidate, a known CNS stimulant, blocks norepinephrine and dopamine reuptake in the presynaptic neuron, thereby releasing more of these neuro-transmitters into the extraneuronal space.4 Methylphenidate’s precise therapeutic action in ADHD is not known.
Methylphenidate is a racemic mixture of d- and l-enantiomers, the first of which is believed to be more active. Whereas the liver removes the l-enantiomer from oral methylphenidate, the transdermal formulation bypasses the liver and preserves the l-enantiomer, thus increasing exposure to racemic methylphenidate. This means that optimal dosages of transdermal methylphenidate (10 to 30 mg/d) may be lower compared with the oral formulation5-7 (Table 2).
Methylphenidate’s d-enantiomer has a mean 3- to 4-hour elimination half-life, approximately twice that of the l-enantiomer. This is why transdermal methylphenidate continues to exert therapeutic effect several hours after the patch is removed.5
Table 2
Transdermal methylphenidate dosage delivery in children ages 6 to 12
| Dose delivered over 9 hours (mg) | Patch size (cm2) | Dosage rate (mg/hr) | Methylphenidate content per patch (mg) |
|---|---|---|---|
| 10 | 12.5 | 1.1 | 27.5 |
| 15 | 18.75 | 1.6 | 41.3 |
| 20 | 25 | 2.2 | 55.0 |
| 30 | 37.5 | 3.3 | 82.5 |
| Source: Reference 4 | |||
Efficacy
Results from randomized, double-blind, placebo-controlled trials support short-term use of transdermal methylphenidate in ADHD. The following studies recruited children ages 6 to 12 with the disorder.
Dose-ranging study. Thirty-three children participating in a summer treatment program received transdermal methylphenidate, 6.25, 12.5, or 25 cm2 12 hours daily for 8 days.6 All three patch sizes were associated with improved academic, social, and behavioral functioning based on a range of measures.
Dose response rate diminished with higher dosages, and significant further improvements were difficult to detect as dosages increased. The children also received intensive behavioral treatment during the study, which might have accounted for some therapeutic gains and diminished the researchers’ ability to detect subtle improvements with increased dosages.
Children also had fewer negative behaviors during the first hour when the patch was applied at 6 AM instead of 7 AM. This suggests that the patch might produce optimal effect when placed first thing in the morning.
Randomized crossover trial.7 Across 6 weeks, 27 children were given placebo or transdermal methylphenidate, 12.5, 25, or 37.5 cm2/d. The children also received behavior modification treatment on alternating weeks. Medication was randomly assigned and varied daily for 4 days per week over 6 weeks, and behavioral treatment was varied weekly for 4 weeks. Each subject took each dosage for 2 days without behavioral treatment and for 4 days with behavioral treatment.
Academic productivity, interactions with peers and adults, and compliance during class improved with all dosages compared with placebo. Although most children removed the patch by 3:30 PM after 9 hours of use, parents reported that positive behavioral effects lasted into the evening.
As in the dose-ranging study, dose response diminished with higher dosages. Optimal effects were achieved on some measures with 12.5 cm2 of transdermal methylphenidate, which produces the same plasma drug level as 10 mg of oral methylphenidate.7
Multisite crossover study.5 Eighty children received transdermal methylphenidate—12.5, 18.75, 25, or 37.5 cm2 based on response to medication—over 5 weeks for 9 hours daily. Dosages were titrated by changing patch sizes until each child reached his or her optimal dosage. Children then received their optimal dosage or placebo for 1 week, then received the opposite treatment for another week. Results were measured in a simulated classroom.
Overall, children showed statistically significant improvement in Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) Teacher Rating Scale scores while receiving their optimal dosage. Improvement was seen 2 hours after patches were applied and continued for 12 hours. Children also were more able to solve math problems during optimal dosage periods than while using placebo.
Nearly 80% of children were rated as significantly improved while receiving transdermal methylphenidate based on Clinical Global Impressions of Improvement scores. Roughly 12% of children showed significant improvement with placebo. Parents reported that their children were markedly less hyperactive and impulsive and more attentive during optimal dosage periods.
Tolerability
No serious side effects were reported during clinical trials of transdermal methylphenidate in children ages 6 to 12.5-7 Side effects commonly associated with oral methylphenidate—anorexia, decreased appetite, headache, insomnia, and abdominal pain—were most frequently reported with the patch.5
In one study,6 61% of children who wore the patch for 12 hours/day reported appetite loss and 47% reported insomnia. Insomnia prevalence diminished substantially when daily wear was limited to 9 hours.2,5 Loss of appetite was reported less often with lower-dose patches (12.5, 18.75 cm2) than with higher-dose patches (25, 37.5 cm2).
Although many children complained of erythema at the patch site,5-7 most reported minimal irritation or discomfort.5 Redness usually dissipated about 8 hours after the patch was removed.6
Despite concerns that youths with impulsive behaviors might remove the patches prematurely, very few children did so during clinical trials.5-7 Those who did had comorbid symptomatic conduct disturbances. Compliance with patch placement and maintenance was very high during dose optimization (98%) and analog classroom analysis (97%).5-7
Dosing
Start transdermal methylphenidate at 12.5 cm2 (10 mg) for children who have never taken methylphenidate or were previously stabilized on the drug. If the child does not respond after 1 week, switch to the 18.75 cm2 (15-mg) patch; keep switching to the next largest patch each week until optimal response is achieved. In clinical trials,5-7 18.75 or 25 cm2 (10 mg) of transdermal methylphenidate produced optimal response for most children.
Advise the child and parents to place the patch on the right and left hip on alternate days to minimize irritation. Counsel children to inform parents if the patch causes itching, burning, or irritation; tell parents to call you if they notice or the child complains of irritation. Children who experience intolerable skin sensitivity with the patch can resume taking oral methylphenidate the day after the patch is removed.
As with oral methylphenidate, the transdermal formulation may be discontinued without a taper and another formulation or medication may be started the next day. Ask the child and parents about side effects at each visit. See the child every 2 to 4 weeks during the titration period and monthly after symptoms are stabilized.
Consider trying a “drug holiday” for at least 2 weeks during the summer to see how the child behaves without methylphenidate, then evaluate the need for medication and determine the optimal dosage close to when the school year begins.
Related resources
- Transdermal methylphenidate Web site. www.daytrana.com.
- Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41:26S-49S.
Drug brand names
- Methylphenidate (oral) • Concerta, Ritalin, Metadate
- Methylphenidate (transdermal) • Daytrana
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
When prescribing methylphenidate to children with attention-deficit/hyperactivity disorder (ADHD), psychiatrists have had two options:
- immediate-release oral methylphenidate, which works for 3 to 5 hours, necessitating multiple daily doses
- extended-release oral methylphenidate, which can prevent irritability and other rebound symptoms caused by multiple daily dosing.1 Because its effects last 12 hours, however, once-daily dosing with this formulation is inflexible.
A new option—a transdermal methylphenidate patch FDA-approved for treating ADHD in children ages 6 to 12 (Table 1)—offers flexible methylphenidate coverage based on response to or need for the medication.
Table 1
Transdermal methylphenidate: Fast facts
| Brand name: Daytrana |
| Class: CNS stimulant |
| FDA-approved indication: ADHD in children ages 6 to 12 |
| Manufacturer: Noven Pharmaceuticals (marketed by Shire PLC) |
| Dosing forms: 10-, 15-, 20-, and 30-mg patches |
| Recommended dosage: One 10- to 30-mg patch daily, worn on the hip for 9 hours. Patient can remove patch sooner if side effects become problematic. |
Clinical implications
The transdermal patch allows dosing to be tailored—or changed day to day as needed—to maximize effectiveness and reduce side-effect risk. The manufacturer recommends that the patch be worn for 9 hours daily, but it can be removed sooner if children experience appetite loss, insomnia, or other adverse effects with 9 hours of exposure to methylphenidate.
Minimizing daily exposure to methylphenidate can also reduce the risk of long-term effects. Findings from one large, randomized clinical trial2 suggest that chronic exposure to high-dose stimulant medications might suppress growth in height and weight, although other data indicate that initial height reductions found in children receiving methylphenidate for ADHD were no longer significant in adulthood.3
The patch also could benefit youths who have trouble following dosing schedules and young children who are unable to swallow pills.
How it works
The patch contains methylphenidate dispersed in an acrylic multipolymeric adhesive that is further dispersed in a silicone adhesive.4 The methylphenidate within the acrylic adhesive flows into the skin, then into the bloodstream. The patch is worn on the hip—where it is covered by clothing and unlikely to be dislodged—and changed daily.
The patch comes in four sizes—12.5, 18.75, 25, and 37.5 cm2—which, respectively, deliver 10, 15, 20, and 30 mg of methylphenidate over 9 hours.4 Methylphenidate concentration is the same for all four sizes, so patch size and duration of use determine dose delivery.
In clinical trials, therapeutic effect was seen 2 hours after patch placement and continued through 12 hours.5 Methylphenidate is delivered continuously while the patch is in place and for as long as 2 hours after it is removed.
Pharmacokinetics
Methylphenidate, a known CNS stimulant, blocks norepinephrine and dopamine reuptake in the presynaptic neuron, thereby releasing more of these neuro-transmitters into the extraneuronal space.4 Methylphenidate’s precise therapeutic action in ADHD is not known.
Methylphenidate is a racemic mixture of d- and l-enantiomers, the first of which is believed to be more active. Whereas the liver removes the l-enantiomer from oral methylphenidate, the transdermal formulation bypasses the liver and preserves the l-enantiomer, thus increasing exposure to racemic methylphenidate. This means that optimal dosages of transdermal methylphenidate (10 to 30 mg/d) may be lower compared with the oral formulation5-7 (Table 2).
Methylphenidate’s d-enantiomer has a mean 3- to 4-hour elimination half-life, approximately twice that of the l-enantiomer. This is why transdermal methylphenidate continues to exert therapeutic effect several hours after the patch is removed.5
Table 2
Transdermal methylphenidate dosage delivery in children ages 6 to 12
| Dose delivered over 9 hours (mg) | Patch size (cm2) | Dosage rate (mg/hr) | Methylphenidate content per patch (mg) |
|---|---|---|---|
| 10 | 12.5 | 1.1 | 27.5 |
| 15 | 18.75 | 1.6 | 41.3 |
| 20 | 25 | 2.2 | 55.0 |
| 30 | 37.5 | 3.3 | 82.5 |
| Source: Reference 4 | |||
Efficacy
Results from randomized, double-blind, placebo-controlled trials support short-term use of transdermal methylphenidate in ADHD. The following studies recruited children ages 6 to 12 with the disorder.
Dose-ranging study. Thirty-three children participating in a summer treatment program received transdermal methylphenidate, 6.25, 12.5, or 25 cm2 12 hours daily for 8 days.6 All three patch sizes were associated with improved academic, social, and behavioral functioning based on a range of measures.
Dose response rate diminished with higher dosages, and significant further improvements were difficult to detect as dosages increased. The children also received intensive behavioral treatment during the study, which might have accounted for some therapeutic gains and diminished the researchers’ ability to detect subtle improvements with increased dosages.
Children also had fewer negative behaviors during the first hour when the patch was applied at 6 AM instead of 7 AM. This suggests that the patch might produce optimal effect when placed first thing in the morning.
Randomized crossover trial.7 Across 6 weeks, 27 children were given placebo or transdermal methylphenidate, 12.5, 25, or 37.5 cm2/d. The children also received behavior modification treatment on alternating weeks. Medication was randomly assigned and varied daily for 4 days per week over 6 weeks, and behavioral treatment was varied weekly for 4 weeks. Each subject took each dosage for 2 days without behavioral treatment and for 4 days with behavioral treatment.
Academic productivity, interactions with peers and adults, and compliance during class improved with all dosages compared with placebo. Although most children removed the patch by 3:30 PM after 9 hours of use, parents reported that positive behavioral effects lasted into the evening.
As in the dose-ranging study, dose response diminished with higher dosages. Optimal effects were achieved on some measures with 12.5 cm2 of transdermal methylphenidate, which produces the same plasma drug level as 10 mg of oral methylphenidate.7
Multisite crossover study.5 Eighty children received transdermal methylphenidate—12.5, 18.75, 25, or 37.5 cm2 based on response to medication—over 5 weeks for 9 hours daily. Dosages were titrated by changing patch sizes until each child reached his or her optimal dosage. Children then received their optimal dosage or placebo for 1 week, then received the opposite treatment for another week. Results were measured in a simulated classroom.
Overall, children showed statistically significant improvement in Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) Teacher Rating Scale scores while receiving their optimal dosage. Improvement was seen 2 hours after patches were applied and continued for 12 hours. Children also were more able to solve math problems during optimal dosage periods than while using placebo.
Nearly 80% of children were rated as significantly improved while receiving transdermal methylphenidate based on Clinical Global Impressions of Improvement scores. Roughly 12% of children showed significant improvement with placebo. Parents reported that their children were markedly less hyperactive and impulsive and more attentive during optimal dosage periods.
Tolerability
No serious side effects were reported during clinical trials of transdermal methylphenidate in children ages 6 to 12.5-7 Side effects commonly associated with oral methylphenidate—anorexia, decreased appetite, headache, insomnia, and abdominal pain—were most frequently reported with the patch.5
In one study,6 61% of children who wore the patch for 12 hours/day reported appetite loss and 47% reported insomnia. Insomnia prevalence diminished substantially when daily wear was limited to 9 hours.2,5 Loss of appetite was reported less often with lower-dose patches (12.5, 18.75 cm2) than with higher-dose patches (25, 37.5 cm2).
Although many children complained of erythema at the patch site,5-7 most reported minimal irritation or discomfort.5 Redness usually dissipated about 8 hours after the patch was removed.6
Despite concerns that youths with impulsive behaviors might remove the patches prematurely, very few children did so during clinical trials.5-7 Those who did had comorbid symptomatic conduct disturbances. Compliance with patch placement and maintenance was very high during dose optimization (98%) and analog classroom analysis (97%).5-7
Dosing
Start transdermal methylphenidate at 12.5 cm2 (10 mg) for children who have never taken methylphenidate or were previously stabilized on the drug. If the child does not respond after 1 week, switch to the 18.75 cm2 (15-mg) patch; keep switching to the next largest patch each week until optimal response is achieved. In clinical trials,5-7 18.75 or 25 cm2 (10 mg) of transdermal methylphenidate produced optimal response for most children.
Advise the child and parents to place the patch on the right and left hip on alternate days to minimize irritation. Counsel children to inform parents if the patch causes itching, burning, or irritation; tell parents to call you if they notice or the child complains of irritation. Children who experience intolerable skin sensitivity with the patch can resume taking oral methylphenidate the day after the patch is removed.
As with oral methylphenidate, the transdermal formulation may be discontinued without a taper and another formulation or medication may be started the next day. Ask the child and parents about side effects at each visit. See the child every 2 to 4 weeks during the titration period and monthly after symptoms are stabilized.
Consider trying a “drug holiday” for at least 2 weeks during the summer to see how the child behaves without methylphenidate, then evaluate the need for medication and determine the optimal dosage close to when the school year begins.
Related resources
- Transdermal methylphenidate Web site. www.daytrana.com.
- Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41:26S-49S.
Drug brand names
- Methylphenidate (oral) • Concerta, Ritalin, Metadate
- Methylphenidate (transdermal) • Daytrana
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Spencer TJ. ADHD treatment across the life cycle. J Clin Psychiatry 2004;65S3:22-6.
2. MTA Cooperative Group. National Institute of Mental Health multimodal treatment study of ADHD follow-up: changes in effectiveness and growth at the end of treatment. Pediatrics 2004;113:762-9.
3. Klein RG, Mannuzza S. Hyperactive boys almost grown up. III. Methylphenidate effects on ultimate height. Arch Gen Psychiatry 1988;45:1131-4.
4. Transdermal methylphenidate Web site. Available at: http://www.daytrana.com. Accessed April 11, 2006.
5. McGough JJ, Wigal SB, Abikoff H, et al. A randomized, double-blind, placebo controlled laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. J Atten Disord 2006;9:476-85.
6. Pelham WE, Jr, Manos MJ, Ezzell CE, et al. A dose-ranging study of a methylphenidate transdermal system in children with ADHD. J Am Acad Child Adolesc Psychiatry 2005;44:522-9.
7. Pelham WE, Burrows-Maclean L, Gnagy EM, et al. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Exp Clin Pharmacol 2005;13:111-26.
1. Spencer TJ. ADHD treatment across the life cycle. J Clin Psychiatry 2004;65S3:22-6.
2. MTA Cooperative Group. National Institute of Mental Health multimodal treatment study of ADHD follow-up: changes in effectiveness and growth at the end of treatment. Pediatrics 2004;113:762-9.
3. Klein RG, Mannuzza S. Hyperactive boys almost grown up. III. Methylphenidate effects on ultimate height. Arch Gen Psychiatry 1988;45:1131-4.
4. Transdermal methylphenidate Web site. Available at: http://www.daytrana.com. Accessed April 11, 2006.
5. McGough JJ, Wigal SB, Abikoff H, et al. A randomized, double-blind, placebo controlled laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. J Atten Disord 2006;9:476-85.
6. Pelham WE, Jr, Manos MJ, Ezzell CE, et al. A dose-ranging study of a methylphenidate transdermal system in children with ADHD. J Am Acad Child Adolesc Psychiatry 2005;44:522-9.
7. Pelham WE, Burrows-Maclean L, Gnagy EM, et al. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Exp Clin Pharmacol 2005;13:111-26.
Set 4 ground rules at the first office visit
Setting ground rules with patients at the first visit can prevent conflicts and strengthen your therapeutic alliance. Remember four “P’s”: punctuality, appointment policies, paperwork, and payment.
Punctuality
Starting and ending sessions on time gives patients a sense that they—and their time—matter. Patients can interpret late starts to mean that the previous patient was more important. For consistently late patients, starting on time lets you explain the importance of punctuality without hypocrisy. Effective time management also makes appointments predictable, which can reduce patients’ anxiety.
Appointment policies
Be realistic about what can be accomplished at each visit. Tell patients before each session how much time is available. Allow time to write prescriptions, schedule follow-up appointments, sign consent forms, and document the visit.
Complicated patients—such as those with multiple psychiatric or medical comorbidities or an extensive medication regimen—may need additional sessions for psychoeducation and treatment planning after the initial evaluation. These extra sessions can cement the therapeutic relationship and reinforce treatment adherence.
Provide a written copy of your policy on missed appointments and discuss it with patients at the first visit. Post the policy prominently in the waiting area.
Paperwork
Schedule an additional appointment to write letters or complete paperwork that patients may require. Allowing patients to assist with the writing process improves accuracy and patient control over disclosure of sensitive information.
Payment
Address fee nonpayment early and often, as non-payment can have multiple dynamic meanings that can be explored during therapy. For instance, some patients may withhold payment to “punish” the therapist for what they perceive as ineffective treatment. Discussing nonpayment can increase patients’ insight into their behavior and help you decide whether to stop treatment.
Discussing financial matters in therapy can be awkward,1 but a comfortable discussion of payment policies can help some patients open up about sensitive matters later. It also subtly affirms your confidence in the treatment you provide.
Acknowledgment
Dr. Newman thanks the Clinical Scholars and Resident Research Track residents and faculty for their help with this article.
1. Geistwhite R. Inadequacy and indebtedness. J Psychother Pract Res 2000;9:142-8.
Dr. Newman is chief resident, child and adolescent psychiatry fellowship, University of Michigan, Ann Arbor.
Setting ground rules with patients at the first visit can prevent conflicts and strengthen your therapeutic alliance. Remember four “P’s”: punctuality, appointment policies, paperwork, and payment.
Punctuality
Starting and ending sessions on time gives patients a sense that they—and their time—matter. Patients can interpret late starts to mean that the previous patient was more important. For consistently late patients, starting on time lets you explain the importance of punctuality without hypocrisy. Effective time management also makes appointments predictable, which can reduce patients’ anxiety.
Appointment policies
Be realistic about what can be accomplished at each visit. Tell patients before each session how much time is available. Allow time to write prescriptions, schedule follow-up appointments, sign consent forms, and document the visit.
Complicated patients—such as those with multiple psychiatric or medical comorbidities or an extensive medication regimen—may need additional sessions for psychoeducation and treatment planning after the initial evaluation. These extra sessions can cement the therapeutic relationship and reinforce treatment adherence.
Provide a written copy of your policy on missed appointments and discuss it with patients at the first visit. Post the policy prominently in the waiting area.
Paperwork
Schedule an additional appointment to write letters or complete paperwork that patients may require. Allowing patients to assist with the writing process improves accuracy and patient control over disclosure of sensitive information.
Payment
Address fee nonpayment early and often, as non-payment can have multiple dynamic meanings that can be explored during therapy. For instance, some patients may withhold payment to “punish” the therapist for what they perceive as ineffective treatment. Discussing nonpayment can increase patients’ insight into their behavior and help you decide whether to stop treatment.
Discussing financial matters in therapy can be awkward,1 but a comfortable discussion of payment policies can help some patients open up about sensitive matters later. It also subtly affirms your confidence in the treatment you provide.
Acknowledgment
Dr. Newman thanks the Clinical Scholars and Resident Research Track residents and faculty for their help with this article.
Setting ground rules with patients at the first visit can prevent conflicts and strengthen your therapeutic alliance. Remember four “P’s”: punctuality, appointment policies, paperwork, and payment.
Punctuality
Starting and ending sessions on time gives patients a sense that they—and their time—matter. Patients can interpret late starts to mean that the previous patient was more important. For consistently late patients, starting on time lets you explain the importance of punctuality without hypocrisy. Effective time management also makes appointments predictable, which can reduce patients’ anxiety.
Appointment policies
Be realistic about what can be accomplished at each visit. Tell patients before each session how much time is available. Allow time to write prescriptions, schedule follow-up appointments, sign consent forms, and document the visit.
Complicated patients—such as those with multiple psychiatric or medical comorbidities or an extensive medication regimen—may need additional sessions for psychoeducation and treatment planning after the initial evaluation. These extra sessions can cement the therapeutic relationship and reinforce treatment adherence.
Provide a written copy of your policy on missed appointments and discuss it with patients at the first visit. Post the policy prominently in the waiting area.
Paperwork
Schedule an additional appointment to write letters or complete paperwork that patients may require. Allowing patients to assist with the writing process improves accuracy and patient control over disclosure of sensitive information.
Payment
Address fee nonpayment early and often, as non-payment can have multiple dynamic meanings that can be explored during therapy. For instance, some patients may withhold payment to “punish” the therapist for what they perceive as ineffective treatment. Discussing nonpayment can increase patients’ insight into their behavior and help you decide whether to stop treatment.
Discussing financial matters in therapy can be awkward,1 but a comfortable discussion of payment policies can help some patients open up about sensitive matters later. It also subtly affirms your confidence in the treatment you provide.
Acknowledgment
Dr. Newman thanks the Clinical Scholars and Resident Research Track residents and faculty for their help with this article.
1. Geistwhite R. Inadequacy and indebtedness. J Psychother Pract Res 2000;9:142-8.
Dr. Newman is chief resident, child and adolescent psychiatry fellowship, University of Michigan, Ann Arbor.
1. Geistwhite R. Inadequacy and indebtedness. J Psychother Pract Res 2000;9:142-8.
Dr. Newman is chief resident, child and adolescent psychiatry fellowship, University of Michigan, Ann Arbor.
Defuse patient demands and other difficult behaviors
Mishandling patients’ suicidal thoughts, delusions, medication demands, and other difficult behaviors can damage the therapeutic alliance, cause you unnecessary consternation, and even endanger patients’ lives.
The following strategies can help you overcome six of psychiatry’s clinical conundrums (Box).
Assessing suicidality
Dealing with insistent delusions
Defusing intimidation
Weighing medication demands
Protecting patient confidentiality
Documenting patient complaints
1. Assessing suicidality
Not having a suicide plan is not necessarily protective; a patient with unremitting depression can deteriorate rapidly from “no plan” to high risk.
Besides probing for plans, ask what is stopping a patient with suicidal thoughts from completing suicide. Suspect increased risk in patients who:
- say they have not tried suicide because they fear the attempt will fail
- cannot express a reason to live.
2. Dealing with insistent delusions
If a delusional patient complains that previous physicians thought he was “lying” or “crazy” and asks if you believe his delusional statements:
- reassure him that you feel he sincerely believes what he says is true.
- affirm that you believe he is accurately and truthfully reporting his feelings.
3. Defusing intimidation
When an intimidating patient demands that you prescribe a controlled substance, be calm, patient, and firm. If the patient stands up and leans toward you or shows other threatening postures, calmly ask him to “please sit down.”
Refuse the patient’s request for the controlled substance by gently informing him that:
- the substance is not medically indicated
- the substance could be “detrimental to your health”
- prescribing the substance would not be good medical care
- you are prescribing a safer substitute.
4. Weighing medication demands
Patients who demand specific medications—controlled or not—might in fact be asking for a reasonable choice. Weigh the request against the patient’s symptoms and history. Don’t be put off by obnoxious, demanding patients who complain about providers who deny their requests for medication.
Barring contraindications or side effects, respect a competent patient’s desire to take an older medication he prefers.
Judge medication requests from incompetent or psychotic patients on a case-by-case basis. In many cases they can remember what worked best in the past.
5. Protecting patient confidentiality
Information about your patients from collateral sources can be valuable. Remember that you are not breaching the patient’s confidentiality when you:
- listen to someone who offers unsolicited information
- do not disclose that you are treating the patient to someone who calls you about him or her.
6. Documenting patient complaints
View with skepticism any history that patients tell you about collateral sources until you confirm the information. All persons—delusional or competent—filter their experiences through their own beliefs.
Be cautious about documenting a patient’s report of abusive treatment as factual. Preface documentation of derogatory or accusatory statements with comments such as, “The patient claims…” or “The patient feels….”
Dr. Roth is attending psychiatrist, Department of Veterans Affairs Medical Center, North Chicago, IL
Mishandling patients’ suicidal thoughts, delusions, medication demands, and other difficult behaviors can damage the therapeutic alliance, cause you unnecessary consternation, and even endanger patients’ lives.
The following strategies can help you overcome six of psychiatry’s clinical conundrums (Box).
Assessing suicidality
Dealing with insistent delusions
Defusing intimidation
Weighing medication demands
Protecting patient confidentiality
Documenting patient complaints
1. Assessing suicidality
Not having a suicide plan is not necessarily protective; a patient with unremitting depression can deteriorate rapidly from “no plan” to high risk.
Besides probing for plans, ask what is stopping a patient with suicidal thoughts from completing suicide. Suspect increased risk in patients who:
- say they have not tried suicide because they fear the attempt will fail
- cannot express a reason to live.
2. Dealing with insistent delusions
If a delusional patient complains that previous physicians thought he was “lying” or “crazy” and asks if you believe his delusional statements:
- reassure him that you feel he sincerely believes what he says is true.
- affirm that you believe he is accurately and truthfully reporting his feelings.
3. Defusing intimidation
When an intimidating patient demands that you prescribe a controlled substance, be calm, patient, and firm. If the patient stands up and leans toward you or shows other threatening postures, calmly ask him to “please sit down.”
Refuse the patient’s request for the controlled substance by gently informing him that:
- the substance is not medically indicated
- the substance could be “detrimental to your health”
- prescribing the substance would not be good medical care
- you are prescribing a safer substitute.
4. Weighing medication demands
Patients who demand specific medications—controlled or not—might in fact be asking for a reasonable choice. Weigh the request against the patient’s symptoms and history. Don’t be put off by obnoxious, demanding patients who complain about providers who deny their requests for medication.
Barring contraindications or side effects, respect a competent patient’s desire to take an older medication he prefers.
Judge medication requests from incompetent or psychotic patients on a case-by-case basis. In many cases they can remember what worked best in the past.
5. Protecting patient confidentiality
Information about your patients from collateral sources can be valuable. Remember that you are not breaching the patient’s confidentiality when you:
- listen to someone who offers unsolicited information
- do not disclose that you are treating the patient to someone who calls you about him or her.
6. Documenting patient complaints
View with skepticism any history that patients tell you about collateral sources until you confirm the information. All persons—delusional or competent—filter their experiences through their own beliefs.
Be cautious about documenting a patient’s report of abusive treatment as factual. Preface documentation of derogatory or accusatory statements with comments such as, “The patient claims…” or “The patient feels….”
Mishandling patients’ suicidal thoughts, delusions, medication demands, and other difficult behaviors can damage the therapeutic alliance, cause you unnecessary consternation, and even endanger patients’ lives.
The following strategies can help you overcome six of psychiatry’s clinical conundrums (Box).
Assessing suicidality
Dealing with insistent delusions
Defusing intimidation
Weighing medication demands
Protecting patient confidentiality
Documenting patient complaints
1. Assessing suicidality
Not having a suicide plan is not necessarily protective; a patient with unremitting depression can deteriorate rapidly from “no plan” to high risk.
Besides probing for plans, ask what is stopping a patient with suicidal thoughts from completing suicide. Suspect increased risk in patients who:
- say they have not tried suicide because they fear the attempt will fail
- cannot express a reason to live.
2. Dealing with insistent delusions
If a delusional patient complains that previous physicians thought he was “lying” or “crazy” and asks if you believe his delusional statements:
- reassure him that you feel he sincerely believes what he says is true.
- affirm that you believe he is accurately and truthfully reporting his feelings.
3. Defusing intimidation
When an intimidating patient demands that you prescribe a controlled substance, be calm, patient, and firm. If the patient stands up and leans toward you or shows other threatening postures, calmly ask him to “please sit down.”
Refuse the patient’s request for the controlled substance by gently informing him that:
- the substance is not medically indicated
- the substance could be “detrimental to your health”
- prescribing the substance would not be good medical care
- you are prescribing a safer substitute.
4. Weighing medication demands
Patients who demand specific medications—controlled or not—might in fact be asking for a reasonable choice. Weigh the request against the patient’s symptoms and history. Don’t be put off by obnoxious, demanding patients who complain about providers who deny their requests for medication.
Barring contraindications or side effects, respect a competent patient’s desire to take an older medication he prefers.
Judge medication requests from incompetent or psychotic patients on a case-by-case basis. In many cases they can remember what worked best in the past.
5. Protecting patient confidentiality
Information about your patients from collateral sources can be valuable. Remember that you are not breaching the patient’s confidentiality when you:
- listen to someone who offers unsolicited information
- do not disclose that you are treating the patient to someone who calls you about him or her.
6. Documenting patient complaints
View with skepticism any history that patients tell you about collateral sources until you confirm the information. All persons—delusional or competent—filter their experiences through their own beliefs.
Be cautious about documenting a patient’s report of abusive treatment as factual. Preface documentation of derogatory or accusatory statements with comments such as, “The patient claims…” or “The patient feels….”
Dr. Roth is attending psychiatrist, Department of Veterans Affairs Medical Center, North Chicago, IL
Dr. Roth is attending psychiatrist, Department of Veterans Affairs Medical Center, North Chicago, IL
Phone calls: Protect yourself when you can’t see the patient
Man attempts suicide after telephone consultations
Kitsap County (WA) Superior Court
A 38-year-old man was hospitalized after a suicide attempt. He was diagnosed as having bipolar affective disorder and treated with lithium and olanzapine. Over the next 3 months a psychiatrist treated him, discontinued olanzapine and lithium, and started valproic acid.
Four months after the suicide attempt, the patient’s wife called the psychiatrist. The patient claims his wife told the psychiatrist he was having paranoid delusions similar to those he had experienced before the suicide attempt. The psychiatrist says the wife reported only that the patient was confused. The psychiatrist told her that her husband should resume taking olanzapine and report the results in 1 to 2 days.
Two days later, the psychiatrist received a voice mail from the patient’s wife, who reported that her husband had improved. The psychiatrist testified that he returned the call and was told that the patient was doing well. The patient denied that this call was made.
The next day, the patient concealed a knife in his briefcase, drove to a wooded area, and stabbed himself three times, lacerating his heart, lung, and diaphragm. He underwent surgery and survived.
In court, the patient argued that if the psychiatrist had evaluated him in person instead of over the telephone, the psychiatrist would have recommended hospitalization. He also alleged that the psychiatrist did not obtain informed consent before stopping olanzapine.
The psychiatrist argued that the patient gave informed consent to withdraw olanzapine and that the second suicide attempt was sudden, unpredictable, and impulsive.
- The jury decided for the defense.
Called-in prescription fails to prevent suicide
Unknown Massachusetts venue
A woman with a history of depression, anxiety, and difficulty following prescriptions attempted suicide and was hospitalized after she and her husband separated.
After discharge and under the care of a psychiatrist, the patient became dependent on lorazepam. When she tried to renew her lorazepam prescription but could not reach the psychiatrist, she called the pharmacy and attempted to impersonate the psychiatrist. The pharmacy did not fill the prescription and notified the psychiatrist.
The psychiatrist called the patient that evening and spoke with the patient and her minister, who was with her. The psychiatrist informed the minister that the medication would be delivered to the house if the minister paid for it, administered it to the patient, and saw her to bed. The minister agreed and followed the psychiatrist’s instructions when the medication arrived.
Later that night, the woman broke into the minister’s church and was apprehended by police. She was released after the minister assured police that the break-in was not a criminal matter.
At home, the patient called the psychiatrist again and left a voice mail. Phone records indicate that she stayed on the line for 5 minutes. The psychiatrist reported that he did not receive the message until the next day. By that time, the patient had hanged herself with a leather strap.
The patient’s family claimed that the church break-in was a new, risky behavior that warranted an in-person evaluation. The psychiatrist argued that the patient often called his office, that the tone of her message did not suggest an imminent suicide attempt, and that neither the minister nor police feared she would harm herself. The psychiatrist’s records showed numerous office visits and telephone calls regarding the patient’s medication.
The family also claimed that the patient was extremely frustrated by her lack of progress. The psychiatrist countered that the patient refused his recommendations for further treatment.
- The case was settled for $600,000.
Dr. Grant’s observations
There are obvious benefits to dealing with patients over the telephone. First, phone consultations can prevent unnecessary office visits or a trip to the emergency room,1 especially when a patient needs reassurance rather than an assessment.
Second, telephone contact can help you cost-effectively track an acute or chronic illness.2 A short telephone conversation can spare some patients the expense of an office visit.
Recent data3 suggest that care management and psychotherapy via telephone may improve clinical outcomes for patients taking antidepressants for depression. Physician-patient telephone calls average 4.3 minutes and very few are considered urgent, so most calls will not result in a legal problem.4
The above cases reflect what many psychiatrists do routinely: assess a patient and change medication without seeing the patient. Roughly 25% of physician-patient interactions occur over the telephone.4 In one-third of these interactions, however, the physician and patient disagree on the reason for the call.5 Given this rate of miscommunication, beware of potential legal trouble when communicating with patients by telephone.
Phone management pitfalls
Improper diagnosis and treatment. The American Psychiatric Association (APA) considers starting a patient relationship without a face-to-face evaluation unethical, but office evaluations are not required when changing an established treatment plan.6 APA’s ethics committee suggests that face-to-face evaluations of established patients are required only if “clinically necessary,” so use your knowledge of the patient and the call to determine clinical necessity.
The above cases appear to stem from the psychiatrists’ failure to detect the severity of the patients’ problems and to offer more intensive interventions. Two limitations of telephone conversation can increase the risk of missed diagnosis and delayed or inappropriate treatment:
- Telephone assessments tend to be rushed and not as systematic as an office evaluation.
- Making a thorough assessment is difficult without seeing the patient’s nonverbal cues.
Breach of confidentiality occurs when a physician provides confidential medical information to someone other than the patient without the patient’s consent.7 In one study assessing physician telephone calls, the physician spoke to the patient in only 79% of cases.8
Disclosing information without consent could violate the patient’s privacy. When a caller identifies himself as your patient, make sure you know who’s on the phone. If the caller requests confidential information (such as HIV test results) and you’re not sure that the caller is your patient, tell him you’ll call back or ask the patient to come to your office for the test results. If the caller is giving but not requesting information, you are not violating the patient’s confidentiality.
In the above cases, the psychiatrists discussed symptoms and treatment with someone other than the patient. In the first case, the psychiatrist violated the patient’s confidentiality by discussing the patient’s medication needs not with him but with his wife. In the bargain, the doctor did not get informed consent. The psychiatrist should have spoken directly to the patient or asked him for permission to discuss care with his wife. The patient might have been too confused to talk with the psychiatrist, leading the psychiatrist to offer different treatment recommendations.
Changing medication or dosages requires a thorough discussion of the drug’s side effects, benefits, and alternatives with the patient.
Telephone protocol for your practice
Talk to the patient directly. As stated, discussing the patient’s treatment with a spouse or someone else without the patient’s permission violates the patient’s privacy. Also, be cautious when interpreting information provided by someone else.
Speaking with the patient directly is crucial to accurate assessment. Without visual cues, the patient’s words become crucial.
During the phone call, have the patient repeat any instructions you give.9 This will minimize the risk for error.5
Document the call. In one study of psychiatrists receiving or making calls, only 45% documented the calls in the patient’s chart.2
Your defense against a malpractice suit could hinge on the thoroughness of documentation. Make sure you record:
- the date and time of the call
- the patient’s name
- the chief complaint and his or her disposition
- your assessment and any advice given
- necessary follow-up action
- requests for prescription refills
- and any symptoms that indicate that the patient should call back.7
How long you wait to call the patient depends on his or her condition. If he or she is fairly stable, you might call after 1 week; if the condition is more serious, you might call the next day.
Avoid managing high-risk patients over the phone. In the above cases, an urgent office visit or a recommendation to report to the nearest emergency room might have been prudent.
Discuss your phone policy during the initial visit. Ask the patient if you can leave a personal message and if his or her message service is private.
Also discuss whether you will charge for phone consultation. Insurance companies often consider telephone conversations “incidental” and usually do not reimburse them separately. From an ethical standpoint, you can charge the patient for such calls if you discuss payment during the initial treatment contact.6
Telephone calls to patients can be time-consuming. Although 86% of psychiatrists feel they should receive compensation for these calls, less than 1% do.2
1. Unwin BK, Jerant AF. The home visit. Am Fam Physician 1999;60:1481-8.
2. Sorum PC, Mallick R. Physicians’ opinions on compensation for telephone calls. Pediatrics 1997;99(4):E3.-
3. Simon GE, Ludman EJ, Tutty S, et al. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment. JAMA 2004;292:935-42.
4. Radecki SE, Neville RE, Girard RA. Telephone patient management by primary care physicians. Med Care 1989;27:817-22.
5. Reisman AB, Brown KE. Preventing communication errors in telephone medicine: a case-based approach. J Gen Intern Med 2005;20:959-63.
6. American Psychiatric Association. Opinions of the Ethics Committee on the Principles of Medical Ethics. Available at: www.psych.org/psych_pract/ethics/ethics_opinions52201. Accessed April 22, 2006.
7. Phelan JP. Ambulatory obstetrical care: strategies to reduce telephone liability. Clin Obstet Gynecol 1998;41:640-6.
8. Johnson BE, Johnson CA. Telephone medicine: a general internal medicine experience. J Gen Intern Med 1990;5:234-9.
9. Bartlett EE. Managing your telephone liability risks. J Healthc Risk Manag 1995;15:30-6.
Man attempts suicide after telephone consultations
Kitsap County (WA) Superior Court
A 38-year-old man was hospitalized after a suicide attempt. He was diagnosed as having bipolar affective disorder and treated with lithium and olanzapine. Over the next 3 months a psychiatrist treated him, discontinued olanzapine and lithium, and started valproic acid.
Four months after the suicide attempt, the patient’s wife called the psychiatrist. The patient claims his wife told the psychiatrist he was having paranoid delusions similar to those he had experienced before the suicide attempt. The psychiatrist says the wife reported only that the patient was confused. The psychiatrist told her that her husband should resume taking olanzapine and report the results in 1 to 2 days.
Two days later, the psychiatrist received a voice mail from the patient’s wife, who reported that her husband had improved. The psychiatrist testified that he returned the call and was told that the patient was doing well. The patient denied that this call was made.
The next day, the patient concealed a knife in his briefcase, drove to a wooded area, and stabbed himself three times, lacerating his heart, lung, and diaphragm. He underwent surgery and survived.
In court, the patient argued that if the psychiatrist had evaluated him in person instead of over the telephone, the psychiatrist would have recommended hospitalization. He also alleged that the psychiatrist did not obtain informed consent before stopping olanzapine.
The psychiatrist argued that the patient gave informed consent to withdraw olanzapine and that the second suicide attempt was sudden, unpredictable, and impulsive.
- The jury decided for the defense.
Called-in prescription fails to prevent suicide
Unknown Massachusetts venue
A woman with a history of depression, anxiety, and difficulty following prescriptions attempted suicide and was hospitalized after she and her husband separated.
After discharge and under the care of a psychiatrist, the patient became dependent on lorazepam. When she tried to renew her lorazepam prescription but could not reach the psychiatrist, she called the pharmacy and attempted to impersonate the psychiatrist. The pharmacy did not fill the prescription and notified the psychiatrist.
The psychiatrist called the patient that evening and spoke with the patient and her minister, who was with her. The psychiatrist informed the minister that the medication would be delivered to the house if the minister paid for it, administered it to the patient, and saw her to bed. The minister agreed and followed the psychiatrist’s instructions when the medication arrived.
Later that night, the woman broke into the minister’s church and was apprehended by police. She was released after the minister assured police that the break-in was not a criminal matter.
At home, the patient called the psychiatrist again and left a voice mail. Phone records indicate that she stayed on the line for 5 minutes. The psychiatrist reported that he did not receive the message until the next day. By that time, the patient had hanged herself with a leather strap.
The patient’s family claimed that the church break-in was a new, risky behavior that warranted an in-person evaluation. The psychiatrist argued that the patient often called his office, that the tone of her message did not suggest an imminent suicide attempt, and that neither the minister nor police feared she would harm herself. The psychiatrist’s records showed numerous office visits and telephone calls regarding the patient’s medication.
The family also claimed that the patient was extremely frustrated by her lack of progress. The psychiatrist countered that the patient refused his recommendations for further treatment.
- The case was settled for $600,000.
Dr. Grant’s observations
There are obvious benefits to dealing with patients over the telephone. First, phone consultations can prevent unnecessary office visits or a trip to the emergency room,1 especially when a patient needs reassurance rather than an assessment.
Second, telephone contact can help you cost-effectively track an acute or chronic illness.2 A short telephone conversation can spare some patients the expense of an office visit.
Recent data3 suggest that care management and psychotherapy via telephone may improve clinical outcomes for patients taking antidepressants for depression. Physician-patient telephone calls average 4.3 minutes and very few are considered urgent, so most calls will not result in a legal problem.4
The above cases reflect what many psychiatrists do routinely: assess a patient and change medication without seeing the patient. Roughly 25% of physician-patient interactions occur over the telephone.4 In one-third of these interactions, however, the physician and patient disagree on the reason for the call.5 Given this rate of miscommunication, beware of potential legal trouble when communicating with patients by telephone.
Phone management pitfalls
Improper diagnosis and treatment. The American Psychiatric Association (APA) considers starting a patient relationship without a face-to-face evaluation unethical, but office evaluations are not required when changing an established treatment plan.6 APA’s ethics committee suggests that face-to-face evaluations of established patients are required only if “clinically necessary,” so use your knowledge of the patient and the call to determine clinical necessity.
The above cases appear to stem from the psychiatrists’ failure to detect the severity of the patients’ problems and to offer more intensive interventions. Two limitations of telephone conversation can increase the risk of missed diagnosis and delayed or inappropriate treatment:
- Telephone assessments tend to be rushed and not as systematic as an office evaluation.
- Making a thorough assessment is difficult without seeing the patient’s nonverbal cues.
Breach of confidentiality occurs when a physician provides confidential medical information to someone other than the patient without the patient’s consent.7 In one study assessing physician telephone calls, the physician spoke to the patient in only 79% of cases.8
Disclosing information without consent could violate the patient’s privacy. When a caller identifies himself as your patient, make sure you know who’s on the phone. If the caller requests confidential information (such as HIV test results) and you’re not sure that the caller is your patient, tell him you’ll call back or ask the patient to come to your office for the test results. If the caller is giving but not requesting information, you are not violating the patient’s confidentiality.
In the above cases, the psychiatrists discussed symptoms and treatment with someone other than the patient. In the first case, the psychiatrist violated the patient’s confidentiality by discussing the patient’s medication needs not with him but with his wife. In the bargain, the doctor did not get informed consent. The psychiatrist should have spoken directly to the patient or asked him for permission to discuss care with his wife. The patient might have been too confused to talk with the psychiatrist, leading the psychiatrist to offer different treatment recommendations.
Changing medication or dosages requires a thorough discussion of the drug’s side effects, benefits, and alternatives with the patient.
Telephone protocol for your practice
Talk to the patient directly. As stated, discussing the patient’s treatment with a spouse or someone else without the patient’s permission violates the patient’s privacy. Also, be cautious when interpreting information provided by someone else.
Speaking with the patient directly is crucial to accurate assessment. Without visual cues, the patient’s words become crucial.
During the phone call, have the patient repeat any instructions you give.9 This will minimize the risk for error.5
Document the call. In one study of psychiatrists receiving or making calls, only 45% documented the calls in the patient’s chart.2
Your defense against a malpractice suit could hinge on the thoroughness of documentation. Make sure you record:
- the date and time of the call
- the patient’s name
- the chief complaint and his or her disposition
- your assessment and any advice given
- necessary follow-up action
- requests for prescription refills
- and any symptoms that indicate that the patient should call back.7
How long you wait to call the patient depends on his or her condition. If he or she is fairly stable, you might call after 1 week; if the condition is more serious, you might call the next day.
Avoid managing high-risk patients over the phone. In the above cases, an urgent office visit or a recommendation to report to the nearest emergency room might have been prudent.
Discuss your phone policy during the initial visit. Ask the patient if you can leave a personal message and if his or her message service is private.
Also discuss whether you will charge for phone consultation. Insurance companies often consider telephone conversations “incidental” and usually do not reimburse them separately. From an ethical standpoint, you can charge the patient for such calls if you discuss payment during the initial treatment contact.6
Telephone calls to patients can be time-consuming. Although 86% of psychiatrists feel they should receive compensation for these calls, less than 1% do.2
Man attempts suicide after telephone consultations
Kitsap County (WA) Superior Court
A 38-year-old man was hospitalized after a suicide attempt. He was diagnosed as having bipolar affective disorder and treated with lithium and olanzapine. Over the next 3 months a psychiatrist treated him, discontinued olanzapine and lithium, and started valproic acid.
Four months after the suicide attempt, the patient’s wife called the psychiatrist. The patient claims his wife told the psychiatrist he was having paranoid delusions similar to those he had experienced before the suicide attempt. The psychiatrist says the wife reported only that the patient was confused. The psychiatrist told her that her husband should resume taking olanzapine and report the results in 1 to 2 days.
Two days later, the psychiatrist received a voice mail from the patient’s wife, who reported that her husband had improved. The psychiatrist testified that he returned the call and was told that the patient was doing well. The patient denied that this call was made.
The next day, the patient concealed a knife in his briefcase, drove to a wooded area, and stabbed himself three times, lacerating his heart, lung, and diaphragm. He underwent surgery and survived.
In court, the patient argued that if the psychiatrist had evaluated him in person instead of over the telephone, the psychiatrist would have recommended hospitalization. He also alleged that the psychiatrist did not obtain informed consent before stopping olanzapine.
The psychiatrist argued that the patient gave informed consent to withdraw olanzapine and that the second suicide attempt was sudden, unpredictable, and impulsive.
- The jury decided for the defense.
Called-in prescription fails to prevent suicide
Unknown Massachusetts venue
A woman with a history of depression, anxiety, and difficulty following prescriptions attempted suicide and was hospitalized after she and her husband separated.
After discharge and under the care of a psychiatrist, the patient became dependent on lorazepam. When she tried to renew her lorazepam prescription but could not reach the psychiatrist, she called the pharmacy and attempted to impersonate the psychiatrist. The pharmacy did not fill the prescription and notified the psychiatrist.
The psychiatrist called the patient that evening and spoke with the patient and her minister, who was with her. The psychiatrist informed the minister that the medication would be delivered to the house if the minister paid for it, administered it to the patient, and saw her to bed. The minister agreed and followed the psychiatrist’s instructions when the medication arrived.
Later that night, the woman broke into the minister’s church and was apprehended by police. She was released after the minister assured police that the break-in was not a criminal matter.
At home, the patient called the psychiatrist again and left a voice mail. Phone records indicate that she stayed on the line for 5 minutes. The psychiatrist reported that he did not receive the message until the next day. By that time, the patient had hanged herself with a leather strap.
The patient’s family claimed that the church break-in was a new, risky behavior that warranted an in-person evaluation. The psychiatrist argued that the patient often called his office, that the tone of her message did not suggest an imminent suicide attempt, and that neither the minister nor police feared she would harm herself. The psychiatrist’s records showed numerous office visits and telephone calls regarding the patient’s medication.
The family also claimed that the patient was extremely frustrated by her lack of progress. The psychiatrist countered that the patient refused his recommendations for further treatment.
- The case was settled for $600,000.
Dr. Grant’s observations
There are obvious benefits to dealing with patients over the telephone. First, phone consultations can prevent unnecessary office visits or a trip to the emergency room,1 especially when a patient needs reassurance rather than an assessment.
Second, telephone contact can help you cost-effectively track an acute or chronic illness.2 A short telephone conversation can spare some patients the expense of an office visit.
Recent data3 suggest that care management and psychotherapy via telephone may improve clinical outcomes for patients taking antidepressants for depression. Physician-patient telephone calls average 4.3 minutes and very few are considered urgent, so most calls will not result in a legal problem.4
The above cases reflect what many psychiatrists do routinely: assess a patient and change medication without seeing the patient. Roughly 25% of physician-patient interactions occur over the telephone.4 In one-third of these interactions, however, the physician and patient disagree on the reason for the call.5 Given this rate of miscommunication, beware of potential legal trouble when communicating with patients by telephone.
Phone management pitfalls
Improper diagnosis and treatment. The American Psychiatric Association (APA) considers starting a patient relationship without a face-to-face evaluation unethical, but office evaluations are not required when changing an established treatment plan.6 APA’s ethics committee suggests that face-to-face evaluations of established patients are required only if “clinically necessary,” so use your knowledge of the patient and the call to determine clinical necessity.
The above cases appear to stem from the psychiatrists’ failure to detect the severity of the patients’ problems and to offer more intensive interventions. Two limitations of telephone conversation can increase the risk of missed diagnosis and delayed or inappropriate treatment:
- Telephone assessments tend to be rushed and not as systematic as an office evaluation.
- Making a thorough assessment is difficult without seeing the patient’s nonverbal cues.
Breach of confidentiality occurs when a physician provides confidential medical information to someone other than the patient without the patient’s consent.7 In one study assessing physician telephone calls, the physician spoke to the patient in only 79% of cases.8
Disclosing information without consent could violate the patient’s privacy. When a caller identifies himself as your patient, make sure you know who’s on the phone. If the caller requests confidential information (such as HIV test results) and you’re not sure that the caller is your patient, tell him you’ll call back or ask the patient to come to your office for the test results. If the caller is giving but not requesting information, you are not violating the patient’s confidentiality.
In the above cases, the psychiatrists discussed symptoms and treatment with someone other than the patient. In the first case, the psychiatrist violated the patient’s confidentiality by discussing the patient’s medication needs not with him but with his wife. In the bargain, the doctor did not get informed consent. The psychiatrist should have spoken directly to the patient or asked him for permission to discuss care with his wife. The patient might have been too confused to talk with the psychiatrist, leading the psychiatrist to offer different treatment recommendations.
Changing medication or dosages requires a thorough discussion of the drug’s side effects, benefits, and alternatives with the patient.
Telephone protocol for your practice
Talk to the patient directly. As stated, discussing the patient’s treatment with a spouse or someone else without the patient’s permission violates the patient’s privacy. Also, be cautious when interpreting information provided by someone else.
Speaking with the patient directly is crucial to accurate assessment. Without visual cues, the patient’s words become crucial.
During the phone call, have the patient repeat any instructions you give.9 This will minimize the risk for error.5
Document the call. In one study of psychiatrists receiving or making calls, only 45% documented the calls in the patient’s chart.2
Your defense against a malpractice suit could hinge on the thoroughness of documentation. Make sure you record:
- the date and time of the call
- the patient’s name
- the chief complaint and his or her disposition
- your assessment and any advice given
- necessary follow-up action
- requests for prescription refills
- and any symptoms that indicate that the patient should call back.7
How long you wait to call the patient depends on his or her condition. If he or she is fairly stable, you might call after 1 week; if the condition is more serious, you might call the next day.
Avoid managing high-risk patients over the phone. In the above cases, an urgent office visit or a recommendation to report to the nearest emergency room might have been prudent.
Discuss your phone policy during the initial visit. Ask the patient if you can leave a personal message and if his or her message service is private.
Also discuss whether you will charge for phone consultation. Insurance companies often consider telephone conversations “incidental” and usually do not reimburse them separately. From an ethical standpoint, you can charge the patient for such calls if you discuss payment during the initial treatment contact.6
Telephone calls to patients can be time-consuming. Although 86% of psychiatrists feel they should receive compensation for these calls, less than 1% do.2
1. Unwin BK, Jerant AF. The home visit. Am Fam Physician 1999;60:1481-8.
2. Sorum PC, Mallick R. Physicians’ opinions on compensation for telephone calls. Pediatrics 1997;99(4):E3.-
3. Simon GE, Ludman EJ, Tutty S, et al. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment. JAMA 2004;292:935-42.
4. Radecki SE, Neville RE, Girard RA. Telephone patient management by primary care physicians. Med Care 1989;27:817-22.
5. Reisman AB, Brown KE. Preventing communication errors in telephone medicine: a case-based approach. J Gen Intern Med 2005;20:959-63.
6. American Psychiatric Association. Opinions of the Ethics Committee on the Principles of Medical Ethics. Available at: www.psych.org/psych_pract/ethics/ethics_opinions52201. Accessed April 22, 2006.
7. Phelan JP. Ambulatory obstetrical care: strategies to reduce telephone liability. Clin Obstet Gynecol 1998;41:640-6.
8. Johnson BE, Johnson CA. Telephone medicine: a general internal medicine experience. J Gen Intern Med 1990;5:234-9.
9. Bartlett EE. Managing your telephone liability risks. J Healthc Risk Manag 1995;15:30-6.
1. Unwin BK, Jerant AF. The home visit. Am Fam Physician 1999;60:1481-8.
2. Sorum PC, Mallick R. Physicians’ opinions on compensation for telephone calls. Pediatrics 1997;99(4):E3.-
3. Simon GE, Ludman EJ, Tutty S, et al. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment. JAMA 2004;292:935-42.
4. Radecki SE, Neville RE, Girard RA. Telephone patient management by primary care physicians. Med Care 1989;27:817-22.
5. Reisman AB, Brown KE. Preventing communication errors in telephone medicine: a case-based approach. J Gen Intern Med 2005;20:959-63.
6. American Psychiatric Association. Opinions of the Ethics Committee on the Principles of Medical Ethics. Available at: www.psych.org/psych_pract/ethics/ethics_opinions52201. Accessed April 22, 2006.
7. Phelan JP. Ambulatory obstetrical care: strategies to reduce telephone liability. Clin Obstet Gynecol 1998;41:640-6.
8. Johnson BE, Johnson CA. Telephone medicine: a general internal medicine experience. J Gen Intern Med 1990;5:234-9.
9. Bartlett EE. Managing your telephone liability risks. J Healthc Risk Manag 1995;15:30-6.