User login
Symptoms of psychosis and OCD in a patient with postpartum depression
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
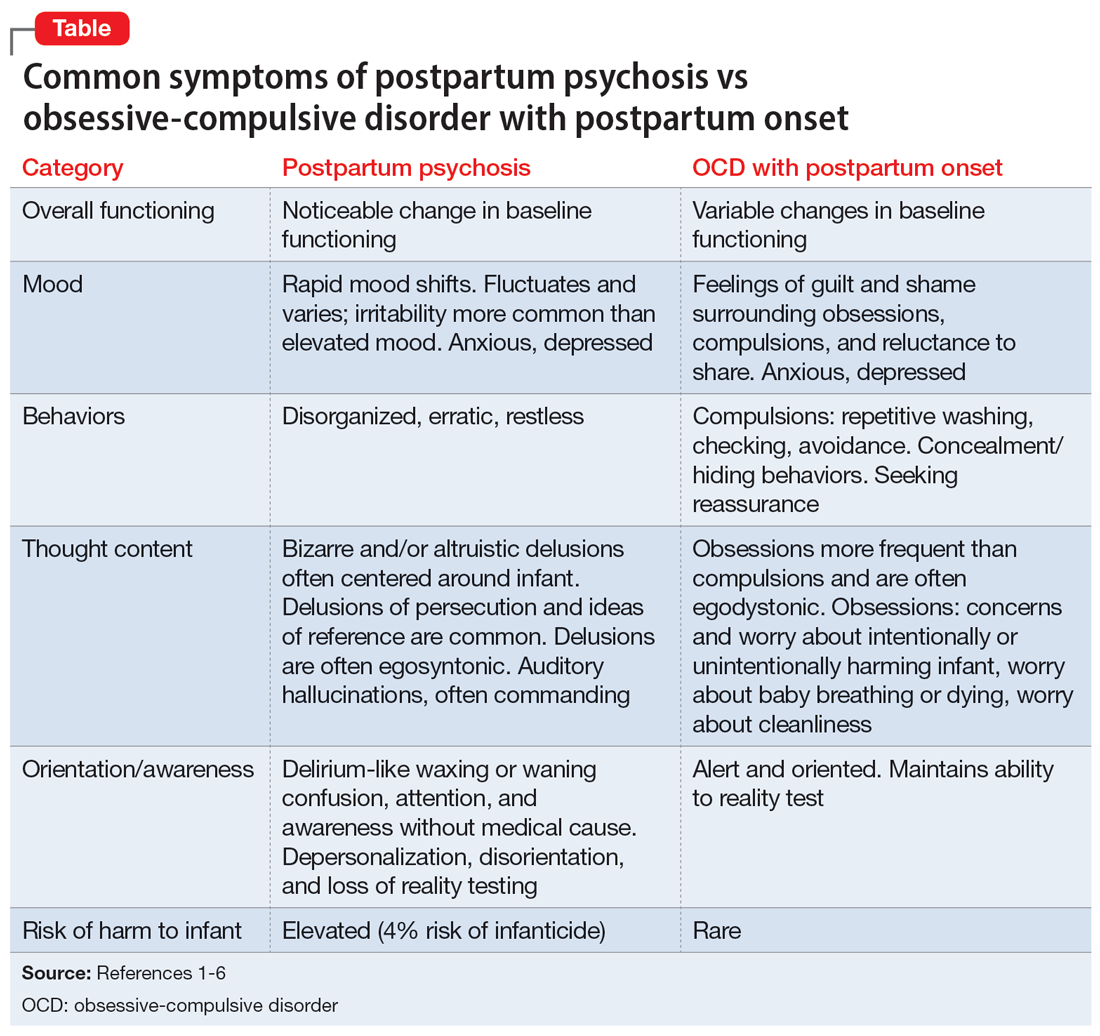
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.
Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.
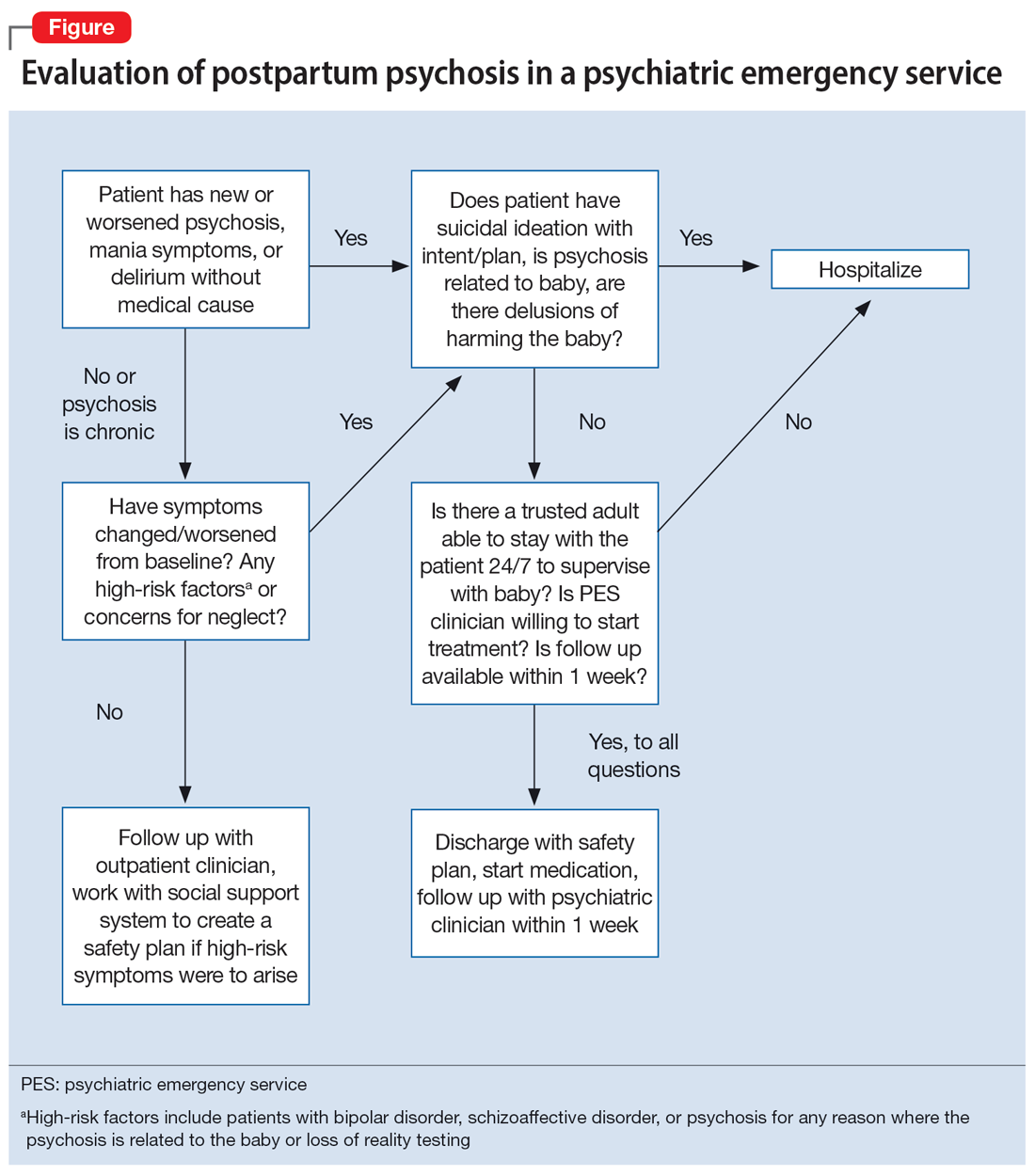
If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.

Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.

If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.

Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.

If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
Auditory hallucinations in a patient who is hearing impaired
CASE New-onset auditory hallucinations
Ms. L, age 78, presents to our hospital with worsening anxiety due to auditory hallucinations. She has been hearing music, which she reports is worse at night and consists of songs, usually the song Jingle Bells, sometimes just melodies and other times with lyrics. Ms. L denies paranoia, visual hallucinations, or worsening mood.
Two weeks ago, Ms. L had visited another hospital, describing 5 days of right-side hearing loss accompanied by pain and burning in her ear and face, along with vesicular lesions in a dermatomal pattern extending into her auditory canal. During this visit, Ms. L’s complete blood count, urine culture, urine drug screen, electrolytes, liver panel, thyroid studies, and vitamin levels were unremarkable. A CT scan of her head showed no abnormalities.
Ms. L was diagnosed with Ramsay Hunt syndrome (herpes zoster oticus), which affects cranial nerves, because of physical examination findings with a dermatomal pattern of lesion distribution and associated pain. Ramsay Hunt syndrome can cause facial paralysis and hearing loss in the affected ear. She was discharged with prescriptions for prednisone 60 mg/d for 7 days and valacyclovir 1 g/d for 7 days and told to follow up with her primary care physician. During the present visit to our hospital, Ms. L’s home health nurse reports that she still has her entire bottles of valacyclovir and prednisone left. Ms. L also has left-side hearing loss that began 5 years ago and a history of recurrent major depressive disorder (MDD) and generalized anxiety disorder. Due to the recent onset of right-side hearing loss, her hearing impairment requires her to communicate via writing or via a voice-to-text app.
HISTORY Depressed and living alone
Ms. L was diagnosed with MDD more than 4 decades ago and has been receiving medication since then. She reports no prior psychiatric hospitalizations, suicide attempts, manic symptoms, or psychotic symptoms. For more than 20 years, she has seen a nurse practitioner, who had prescribed mirtazapine 30 mg/d for MDD, poor appetite, and sleep. Within the last 5 years, her nurse practitioner added risperidone 0.5 mg/d at night to augment the mirtazapine for tearfulness, irritability, and mood swings.
Ms. L’s medical history also includes hypertension and chronic obstructive pulmonary disease. She is a retired teacher and lives alone. She has a chore worker who visits her home for 1 hour 5 days a week to help with cleaning and lifting, and support from her son. Ms. L no longer drives and relies on others for transportation, but is able to manage her finances, activities of daily living, cooking, and walking without any assistance.
[polldaddy:12807642]
EVALUATION Identifying the cause of the music
Ms. L is alert and oriented to time and situation, her concentration is appropriate, and her recent and remote memories are preserved. A full cognitive screen is not performed, but she is able to spell WORLD forwards and backwards and adequately perform a serial 7s test. An examination of her ear does not reveal any open vesicular lesions or swelling, but she continues to report pain and tingling in the C7 dermatomal pattern. Her urine drug screen and infectious and autoimmune laboratory testing are unremarkable. She does not have electrolyte, renal function, or blood count abnormalities. An MRI of her brain that is performed to rule out intracranial pathology due to acute hearing loss shows no acute intracranial abnormalities, with some artifact effect due to motion. Because temporal lobe epilepsy can present with hallucinations,1 an EEG is performed to rule out seizure activity; it shows a normal wake pattern.
Psychiatry is consulted for management of the auditory hallucinations because Ms. L is distressed by hearing music. Ms. L is evaluated by Neurology and Otolaryngology. Neurology recommends a repeat brain MRI in the outpatient setting after seeing an artifact in the inpatient imaging, as well as follow-up with her primary care physician. Otolaryngology believes her symptoms are secondary to Ramsay Hunt syndrome with incomplete treatment, which is consistent with the initial diagnosis from her previous hospital visit, and recommends another course of oral corticosteroids, along with Audiology and Otolaryngology follow-up.
Continue to: The authors' observations
The authors’ observations
This is the first case we have seen detailing musical hallucinations (MH) secondary to Ramsay Hunt syndrome, although musical hallucinations have been associated with other etiologies of hearing loss. MH is a “release phenomenon” believed to be caused by deprivation of stimulation of the auditory cortex.2 They are categorized as complex auditory hallucinations made up of melodies and rhythms and may be present in up to 2.5% of patients with hearing impairment.1 The condition is mostly seen in older adults because this population is more likely to experience hearing loss. MH is more common among women (70% to 80% of cases) and is highly comorbid with psychiatric disorders such as schizophrenia, obsessive-compulsive disorder, or (as was the case for Ms. L) MDD.3 Hallucinations secondary to hearing loss may be more common in left-side hearing loss.4 In a 2005 study, Warner et al5 found religious music such as hymns or Christmas carols was most commonly heard, possibly due to repetitive past exposure.
There is no consensus on treatment for MH. Current treatment guidance comes from case reports and case series. Treatment is generally most successful when the etiology of the hallucination is both apparent and treatable, such as an infectious eitiology.3 In the case of MH due to hearing loss, hallucinations may improve following treatment with hearing aids or cochlear implants,1,3,6,7 which is what was advised for Ms. L. Table 17-9 outlines other possible measures for addressing musical hallucinations.
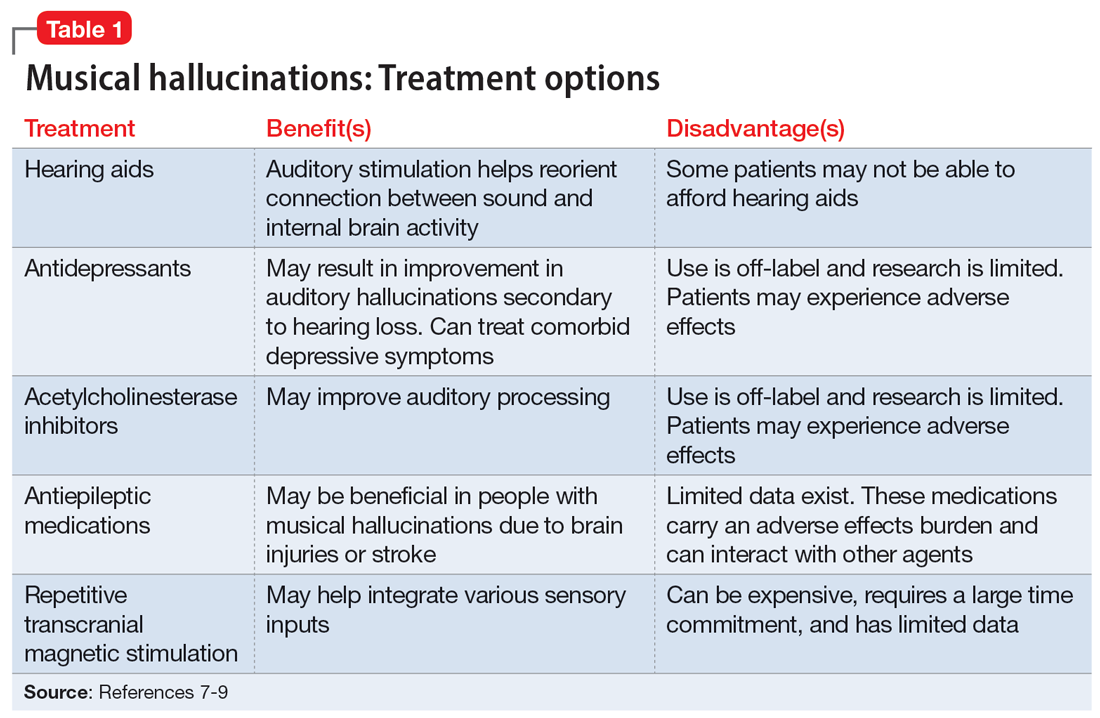
Anticholinesterases, antidepressants, and antiepileptics may provide some benefit.8 However, pharmacotherapy is generally less efficacious and can cause adverse effects, so environmental support and hearing aids may be a safer approach. No medications have been shown to completely cure MH.
TREATMENT Hearing loss management and follow-up
When speaking with the consulting psychiatry team, Ms. L reports her outpatient psychotropic regimen has been helpful. The team decides to continue mirtazapine 30 mg/d and risperidone 0.5 mg/d at night. We recommend that Ms. L discuss tapering off risperidone with her outpatient clinician if they feel it may be indicated to reduce the risk of adverse effects. The treatment team decides not to start corticosteroids due to the risk of steroid-induced psychotic symptoms. The team discusses hallucinations related to hearing loss with Ms. L and advise her to follow up with Audiology and Otolaryngology in the outpatient setting.
The authors’ observations
Approximately 40% of people age >60 struggle with hearing impairment4,9; this impacts their general quality of life and how clinicians communicate with such patients.10 People with hearing loss are more likely to develop feelings of social isolation, depression, and delirium (Table 28,10,11).11
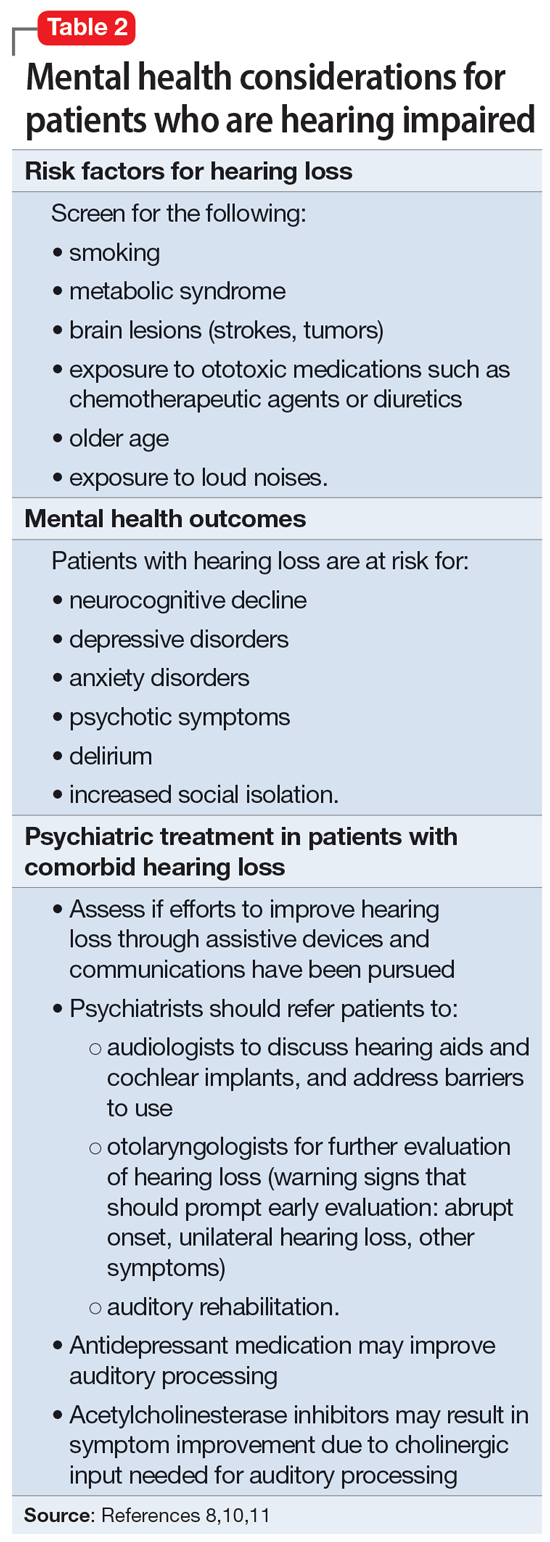
Risk factors for hearing loss include tobacco use, metabolic syndrome, exposure to loud noises, and exposure to certain ototoxic medications such as chemotherapeutic agents.11 As psychiatrists, it is important to identify patients who may be at risk for hearing loss and refer them to the appropriate medical professional. If hearing loss is new onset, refer the patient to an otolaryngologist for a full evaluation. Unilateral hearing loss should warrant further workup because this could be due to an acoustic neuroma.11
When providing care for a patient who uses a hearing aid, discuss adherence, barriers to adherence, and difficulties with adjusting the hearing aid. A referral to an audiologist may help patients address these barriers. Patients with hearing impairment or loss may benefit from auditory rehabilitation programs that provide communication strategies, ways to adapt to hearing loss, and information about different assistive options.11 Such programs are often run by audiologists or speech language pathologists and contain both counseling and group components.
Continue to: Is is critical for psychiatrists...
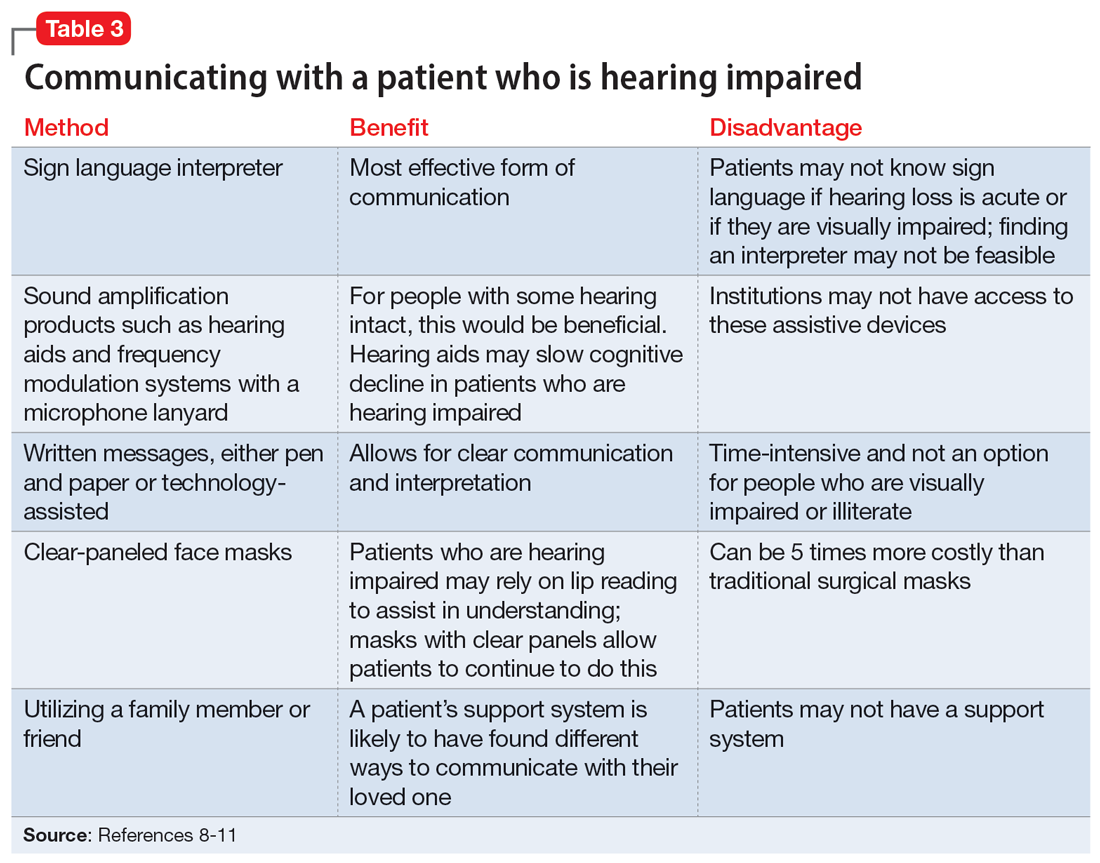
It is critical for psychiatrists to ensure appropriate communication with patients who are hearing impaired (Table 38-11). The use of assistive devices such as sound amplifiers, written messages, or family members to assist in communication is needed to prevent miscommunication.9-11
OUTCOME Lack of follow-up
A home health worker visits Ms. L, communicating with her using voice-to-text. Ms. L has not yet gone to her primary care physician, audiologist, or outpatient psychiatrist for follow-up because she needs to arrange transportation. Ms. L remains distressed by the music she is hearing, which is worse at night, along with her acute hearing loss.
Bottom Line
Hearing loss can predispose a person to psychiatric disorders and symptoms, including depression, delirium, and auditory hallucinations. Psychiatrists should strive to ensure clear communication with patients who are hearing impaired and should refer such patients to appropriate resources to improve outcomes.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- Sosland MD, Pinninti N. 5 ways to quiet auditory hallucinations. Current Psychiatry. 2005;4(4):110.
- Convery E, Keidser G, McLelland M, et al. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed E-Health. 2020;26(6):798-804. doi:10.1089/ tmj.2019.0109
Drug Brand Names
Mirtazapine • Remeron
Prednisone • Rayos
Risperidone • Risperdal
Valacyclovir • Valtrex
1. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. doi:10.1002/gps.618
2. Alvarez Perez P, Garcia-Antelo MJ, Rubio-Nazabal E. “Doctor, I hear music”: a brief review about musical hallucinations. Open Neurol J. 2017;11:11-14. doi:10.2174/1874205X01711010011
3. Sanchez TG, Rocha SCM, Knobel KAB, et al. Musical hallucination associated with hearing loss. Arq Neuropsiquiatr. 2011;69(2B):395-400. doi:10.1590/S0004-282X2011000300024
4. Teunisse RJ, Olde Rikkert MGM. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20(12):1075-1077. doi:10.1097/JGP.0b013e31823e31c4
5. Warner N, Aziz V. Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry. 2005;20(7):658-660. doi:10.1002/gps.1338
6. Low WK, Tham CA, D’Souza VD, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854-858. doi:10.1017/S0022215113001758
7. Brunner JP, Amedee RG. Musical hallucinations in a patient with presbycusis: a case report. Ochsner J. 2015;15(1):89-91.
8. Coebergh JAF, Lauw RF, Bots R, et al. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi:10.3389/fpsyg.2015.00814
9. Ten Hulzen RD, Fabry DA. Impact of hearing loss and universal face masking in the COVID-19 era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
10. Shukla A, Nieman CL, Price C, et al. Impact of hearing loss on patient-provider communication among hospitalized patients: a systematic review. Am J Med Qual. 2019;34(3):284-292. doi:10.1177/1062860618798926
11. Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi:10.1017/S0033291718003409
CASE New-onset auditory hallucinations
Ms. L, age 78, presents to our hospital with worsening anxiety due to auditory hallucinations. She has been hearing music, which she reports is worse at night and consists of songs, usually the song Jingle Bells, sometimes just melodies and other times with lyrics. Ms. L denies paranoia, visual hallucinations, or worsening mood.
Two weeks ago, Ms. L had visited another hospital, describing 5 days of right-side hearing loss accompanied by pain and burning in her ear and face, along with vesicular lesions in a dermatomal pattern extending into her auditory canal. During this visit, Ms. L’s complete blood count, urine culture, urine drug screen, electrolytes, liver panel, thyroid studies, and vitamin levels were unremarkable. A CT scan of her head showed no abnormalities.
Ms. L was diagnosed with Ramsay Hunt syndrome (herpes zoster oticus), which affects cranial nerves, because of physical examination findings with a dermatomal pattern of lesion distribution and associated pain. Ramsay Hunt syndrome can cause facial paralysis and hearing loss in the affected ear. She was discharged with prescriptions for prednisone 60 mg/d for 7 days and valacyclovir 1 g/d for 7 days and told to follow up with her primary care physician. During the present visit to our hospital, Ms. L’s home health nurse reports that she still has her entire bottles of valacyclovir and prednisone left. Ms. L also has left-side hearing loss that began 5 years ago and a history of recurrent major depressive disorder (MDD) and generalized anxiety disorder. Due to the recent onset of right-side hearing loss, her hearing impairment requires her to communicate via writing or via a voice-to-text app.
HISTORY Depressed and living alone
Ms. L was diagnosed with MDD more than 4 decades ago and has been receiving medication since then. She reports no prior psychiatric hospitalizations, suicide attempts, manic symptoms, or psychotic symptoms. For more than 20 years, she has seen a nurse practitioner, who had prescribed mirtazapine 30 mg/d for MDD, poor appetite, and sleep. Within the last 5 years, her nurse practitioner added risperidone 0.5 mg/d at night to augment the mirtazapine for tearfulness, irritability, and mood swings.
Ms. L’s medical history also includes hypertension and chronic obstructive pulmonary disease. She is a retired teacher and lives alone. She has a chore worker who visits her home for 1 hour 5 days a week to help with cleaning and lifting, and support from her son. Ms. L no longer drives and relies on others for transportation, but is able to manage her finances, activities of daily living, cooking, and walking without any assistance.
[polldaddy:12807642]
EVALUATION Identifying the cause of the music
Ms. L is alert and oriented to time and situation, her concentration is appropriate, and her recent and remote memories are preserved. A full cognitive screen is not performed, but she is able to spell WORLD forwards and backwards and adequately perform a serial 7s test. An examination of her ear does not reveal any open vesicular lesions or swelling, but she continues to report pain and tingling in the C7 dermatomal pattern. Her urine drug screen and infectious and autoimmune laboratory testing are unremarkable. She does not have electrolyte, renal function, or blood count abnormalities. An MRI of her brain that is performed to rule out intracranial pathology due to acute hearing loss shows no acute intracranial abnormalities, with some artifact effect due to motion. Because temporal lobe epilepsy can present with hallucinations,1 an EEG is performed to rule out seizure activity; it shows a normal wake pattern.
Psychiatry is consulted for management of the auditory hallucinations because Ms. L is distressed by hearing music. Ms. L is evaluated by Neurology and Otolaryngology. Neurology recommends a repeat brain MRI in the outpatient setting after seeing an artifact in the inpatient imaging, as well as follow-up with her primary care physician. Otolaryngology believes her symptoms are secondary to Ramsay Hunt syndrome with incomplete treatment, which is consistent with the initial diagnosis from her previous hospital visit, and recommends another course of oral corticosteroids, along with Audiology and Otolaryngology follow-up.
Continue to: The authors' observations
The authors’ observations
This is the first case we have seen detailing musical hallucinations (MH) secondary to Ramsay Hunt syndrome, although musical hallucinations have been associated with other etiologies of hearing loss. MH is a “release phenomenon” believed to be caused by deprivation of stimulation of the auditory cortex.2 They are categorized as complex auditory hallucinations made up of melodies and rhythms and may be present in up to 2.5% of patients with hearing impairment.1 The condition is mostly seen in older adults because this population is more likely to experience hearing loss. MH is more common among women (70% to 80% of cases) and is highly comorbid with psychiatric disorders such as schizophrenia, obsessive-compulsive disorder, or (as was the case for Ms. L) MDD.3 Hallucinations secondary to hearing loss may be more common in left-side hearing loss.4 In a 2005 study, Warner et al5 found religious music such as hymns or Christmas carols was most commonly heard, possibly due to repetitive past exposure.
There is no consensus on treatment for MH. Current treatment guidance comes from case reports and case series. Treatment is generally most successful when the etiology of the hallucination is both apparent and treatable, such as an infectious eitiology.3 In the case of MH due to hearing loss, hallucinations may improve following treatment with hearing aids or cochlear implants,1,3,6,7 which is what was advised for Ms. L. Table 17-9 outlines other possible measures for addressing musical hallucinations.

Anticholinesterases, antidepressants, and antiepileptics may provide some benefit.8 However, pharmacotherapy is generally less efficacious and can cause adverse effects, so environmental support and hearing aids may be a safer approach. No medications have been shown to completely cure MH.
TREATMENT Hearing loss management and follow-up
When speaking with the consulting psychiatry team, Ms. L reports her outpatient psychotropic regimen has been helpful. The team decides to continue mirtazapine 30 mg/d and risperidone 0.5 mg/d at night. We recommend that Ms. L discuss tapering off risperidone with her outpatient clinician if they feel it may be indicated to reduce the risk of adverse effects. The treatment team decides not to start corticosteroids due to the risk of steroid-induced psychotic symptoms. The team discusses hallucinations related to hearing loss with Ms. L and advise her to follow up with Audiology and Otolaryngology in the outpatient setting.
The authors’ observations
Approximately 40% of people age >60 struggle with hearing impairment4,9; this impacts their general quality of life and how clinicians communicate with such patients.10 People with hearing loss are more likely to develop feelings of social isolation, depression, and delirium (Table 28,10,11).11

Risk factors for hearing loss include tobacco use, metabolic syndrome, exposure to loud noises, and exposure to certain ototoxic medications such as chemotherapeutic agents.11 As psychiatrists, it is important to identify patients who may be at risk for hearing loss and refer them to the appropriate medical professional. If hearing loss is new onset, refer the patient to an otolaryngologist for a full evaluation. Unilateral hearing loss should warrant further workup because this could be due to an acoustic neuroma.11
When providing care for a patient who uses a hearing aid, discuss adherence, barriers to adherence, and difficulties with adjusting the hearing aid. A referral to an audiologist may help patients address these barriers. Patients with hearing impairment or loss may benefit from auditory rehabilitation programs that provide communication strategies, ways to adapt to hearing loss, and information about different assistive options.11 Such programs are often run by audiologists or speech language pathologists and contain both counseling and group components.
Continue to: Is is critical for psychiatrists...
It is critical for psychiatrists to ensure appropriate communication with patients who are hearing impaired (Table 38-11). The use of assistive devices such as sound amplifiers, written messages, or family members to assist in communication is needed to prevent miscommunication.9-11

OUTCOME Lack of follow-up
A home health worker visits Ms. L, communicating with her using voice-to-text. Ms. L has not yet gone to her primary care physician, audiologist, or outpatient psychiatrist for follow-up because she needs to arrange transportation. Ms. L remains distressed by the music she is hearing, which is worse at night, along with her acute hearing loss.
Bottom Line
Hearing loss can predispose a person to psychiatric disorders and symptoms, including depression, delirium, and auditory hallucinations. Psychiatrists should strive to ensure clear communication with patients who are hearing impaired and should refer such patients to appropriate resources to improve outcomes.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- Sosland MD, Pinninti N. 5 ways to quiet auditory hallucinations. Current Psychiatry. 2005;4(4):110.
- Convery E, Keidser G, McLelland M, et al. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed E-Health. 2020;26(6):798-804. doi:10.1089/ tmj.2019.0109
Drug Brand Names
Mirtazapine • Remeron
Prednisone • Rayos
Risperidone • Risperdal
Valacyclovir • Valtrex
CASE New-onset auditory hallucinations
Ms. L, age 78, presents to our hospital with worsening anxiety due to auditory hallucinations. She has been hearing music, which she reports is worse at night and consists of songs, usually the song Jingle Bells, sometimes just melodies and other times with lyrics. Ms. L denies paranoia, visual hallucinations, or worsening mood.
Two weeks ago, Ms. L had visited another hospital, describing 5 days of right-side hearing loss accompanied by pain and burning in her ear and face, along with vesicular lesions in a dermatomal pattern extending into her auditory canal. During this visit, Ms. L’s complete blood count, urine culture, urine drug screen, electrolytes, liver panel, thyroid studies, and vitamin levels were unremarkable. A CT scan of her head showed no abnormalities.
Ms. L was diagnosed with Ramsay Hunt syndrome (herpes zoster oticus), which affects cranial nerves, because of physical examination findings with a dermatomal pattern of lesion distribution and associated pain. Ramsay Hunt syndrome can cause facial paralysis and hearing loss in the affected ear. She was discharged with prescriptions for prednisone 60 mg/d for 7 days and valacyclovir 1 g/d for 7 days and told to follow up with her primary care physician. During the present visit to our hospital, Ms. L’s home health nurse reports that she still has her entire bottles of valacyclovir and prednisone left. Ms. L also has left-side hearing loss that began 5 years ago and a history of recurrent major depressive disorder (MDD) and generalized anxiety disorder. Due to the recent onset of right-side hearing loss, her hearing impairment requires her to communicate via writing or via a voice-to-text app.
HISTORY Depressed and living alone
Ms. L was diagnosed with MDD more than 4 decades ago and has been receiving medication since then. She reports no prior psychiatric hospitalizations, suicide attempts, manic symptoms, or psychotic symptoms. For more than 20 years, she has seen a nurse practitioner, who had prescribed mirtazapine 30 mg/d for MDD, poor appetite, and sleep. Within the last 5 years, her nurse practitioner added risperidone 0.5 mg/d at night to augment the mirtazapine for tearfulness, irritability, and mood swings.
Ms. L’s medical history also includes hypertension and chronic obstructive pulmonary disease. She is a retired teacher and lives alone. She has a chore worker who visits her home for 1 hour 5 days a week to help with cleaning and lifting, and support from her son. Ms. L no longer drives and relies on others for transportation, but is able to manage her finances, activities of daily living, cooking, and walking without any assistance.
[polldaddy:12807642]
EVALUATION Identifying the cause of the music
Ms. L is alert and oriented to time and situation, her concentration is appropriate, and her recent and remote memories are preserved. A full cognitive screen is not performed, but she is able to spell WORLD forwards and backwards and adequately perform a serial 7s test. An examination of her ear does not reveal any open vesicular lesions or swelling, but she continues to report pain and tingling in the C7 dermatomal pattern. Her urine drug screen and infectious and autoimmune laboratory testing are unremarkable. She does not have electrolyte, renal function, or blood count abnormalities. An MRI of her brain that is performed to rule out intracranial pathology due to acute hearing loss shows no acute intracranial abnormalities, with some artifact effect due to motion. Because temporal lobe epilepsy can present with hallucinations,1 an EEG is performed to rule out seizure activity; it shows a normal wake pattern.
Psychiatry is consulted for management of the auditory hallucinations because Ms. L is distressed by hearing music. Ms. L is evaluated by Neurology and Otolaryngology. Neurology recommends a repeat brain MRI in the outpatient setting after seeing an artifact in the inpatient imaging, as well as follow-up with her primary care physician. Otolaryngology believes her symptoms are secondary to Ramsay Hunt syndrome with incomplete treatment, which is consistent with the initial diagnosis from her previous hospital visit, and recommends another course of oral corticosteroids, along with Audiology and Otolaryngology follow-up.
Continue to: The authors' observations
The authors’ observations
This is the first case we have seen detailing musical hallucinations (MH) secondary to Ramsay Hunt syndrome, although musical hallucinations have been associated with other etiologies of hearing loss. MH is a “release phenomenon” believed to be caused by deprivation of stimulation of the auditory cortex.2 They are categorized as complex auditory hallucinations made up of melodies and rhythms and may be present in up to 2.5% of patients with hearing impairment.1 The condition is mostly seen in older adults because this population is more likely to experience hearing loss. MH is more common among women (70% to 80% of cases) and is highly comorbid with psychiatric disorders such as schizophrenia, obsessive-compulsive disorder, or (as was the case for Ms. L) MDD.3 Hallucinations secondary to hearing loss may be more common in left-side hearing loss.4 In a 2005 study, Warner et al5 found religious music such as hymns or Christmas carols was most commonly heard, possibly due to repetitive past exposure.
There is no consensus on treatment for MH. Current treatment guidance comes from case reports and case series. Treatment is generally most successful when the etiology of the hallucination is both apparent and treatable, such as an infectious eitiology.3 In the case of MH due to hearing loss, hallucinations may improve following treatment with hearing aids or cochlear implants,1,3,6,7 which is what was advised for Ms. L. Table 17-9 outlines other possible measures for addressing musical hallucinations.

Anticholinesterases, antidepressants, and antiepileptics may provide some benefit.8 However, pharmacotherapy is generally less efficacious and can cause adverse effects, so environmental support and hearing aids may be a safer approach. No medications have been shown to completely cure MH.
TREATMENT Hearing loss management and follow-up
When speaking with the consulting psychiatry team, Ms. L reports her outpatient psychotropic regimen has been helpful. The team decides to continue mirtazapine 30 mg/d and risperidone 0.5 mg/d at night. We recommend that Ms. L discuss tapering off risperidone with her outpatient clinician if they feel it may be indicated to reduce the risk of adverse effects. The treatment team decides not to start corticosteroids due to the risk of steroid-induced psychotic symptoms. The team discusses hallucinations related to hearing loss with Ms. L and advise her to follow up with Audiology and Otolaryngology in the outpatient setting.
The authors’ observations
Approximately 40% of people age >60 struggle with hearing impairment4,9; this impacts their general quality of life and how clinicians communicate with such patients.10 People with hearing loss are more likely to develop feelings of social isolation, depression, and delirium (Table 28,10,11).11

Risk factors for hearing loss include tobacco use, metabolic syndrome, exposure to loud noises, and exposure to certain ototoxic medications such as chemotherapeutic agents.11 As psychiatrists, it is important to identify patients who may be at risk for hearing loss and refer them to the appropriate medical professional. If hearing loss is new onset, refer the patient to an otolaryngologist for a full evaluation. Unilateral hearing loss should warrant further workup because this could be due to an acoustic neuroma.11
When providing care for a patient who uses a hearing aid, discuss adherence, barriers to adherence, and difficulties with adjusting the hearing aid. A referral to an audiologist may help patients address these barriers. Patients with hearing impairment or loss may benefit from auditory rehabilitation programs that provide communication strategies, ways to adapt to hearing loss, and information about different assistive options.11 Such programs are often run by audiologists or speech language pathologists and contain both counseling and group components.
Continue to: Is is critical for psychiatrists...
It is critical for psychiatrists to ensure appropriate communication with patients who are hearing impaired (Table 38-11). The use of assistive devices such as sound amplifiers, written messages, or family members to assist in communication is needed to prevent miscommunication.9-11

OUTCOME Lack of follow-up
A home health worker visits Ms. L, communicating with her using voice-to-text. Ms. L has not yet gone to her primary care physician, audiologist, or outpatient psychiatrist for follow-up because she needs to arrange transportation. Ms. L remains distressed by the music she is hearing, which is worse at night, along with her acute hearing loss.
Bottom Line
Hearing loss can predispose a person to psychiatric disorders and symptoms, including depression, delirium, and auditory hallucinations. Psychiatrists should strive to ensure clear communication with patients who are hearing impaired and should refer such patients to appropriate resources to improve outcomes.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- Sosland MD, Pinninti N. 5 ways to quiet auditory hallucinations. Current Psychiatry. 2005;4(4):110.
- Convery E, Keidser G, McLelland M, et al. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed E-Health. 2020;26(6):798-804. doi:10.1089/ tmj.2019.0109
Drug Brand Names
Mirtazapine • Remeron
Prednisone • Rayos
Risperidone • Risperdal
Valacyclovir • Valtrex
1. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. doi:10.1002/gps.618
2. Alvarez Perez P, Garcia-Antelo MJ, Rubio-Nazabal E. “Doctor, I hear music”: a brief review about musical hallucinations. Open Neurol J. 2017;11:11-14. doi:10.2174/1874205X01711010011
3. Sanchez TG, Rocha SCM, Knobel KAB, et al. Musical hallucination associated with hearing loss. Arq Neuropsiquiatr. 2011;69(2B):395-400. doi:10.1590/S0004-282X2011000300024
4. Teunisse RJ, Olde Rikkert MGM. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20(12):1075-1077. doi:10.1097/JGP.0b013e31823e31c4
5. Warner N, Aziz V. Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry. 2005;20(7):658-660. doi:10.1002/gps.1338
6. Low WK, Tham CA, D’Souza VD, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854-858. doi:10.1017/S0022215113001758
7. Brunner JP, Amedee RG. Musical hallucinations in a patient with presbycusis: a case report. Ochsner J. 2015;15(1):89-91.
8. Coebergh JAF, Lauw RF, Bots R, et al. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi:10.3389/fpsyg.2015.00814
9. Ten Hulzen RD, Fabry DA. Impact of hearing loss and universal face masking in the COVID-19 era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
10. Shukla A, Nieman CL, Price C, et al. Impact of hearing loss on patient-provider communication among hospitalized patients: a systematic review. Am J Med Qual. 2019;34(3):284-292. doi:10.1177/1062860618798926
11. Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi:10.1017/S0033291718003409
1. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. doi:10.1002/gps.618
2. Alvarez Perez P, Garcia-Antelo MJ, Rubio-Nazabal E. “Doctor, I hear music”: a brief review about musical hallucinations. Open Neurol J. 2017;11:11-14. doi:10.2174/1874205X01711010011
3. Sanchez TG, Rocha SCM, Knobel KAB, et al. Musical hallucination associated with hearing loss. Arq Neuropsiquiatr. 2011;69(2B):395-400. doi:10.1590/S0004-282X2011000300024
4. Teunisse RJ, Olde Rikkert MGM. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20(12):1075-1077. doi:10.1097/JGP.0b013e31823e31c4
5. Warner N, Aziz V. Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry. 2005;20(7):658-660. doi:10.1002/gps.1338
6. Low WK, Tham CA, D’Souza VD, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854-858. doi:10.1017/S0022215113001758
7. Brunner JP, Amedee RG. Musical hallucinations in a patient with presbycusis: a case report. Ochsner J. 2015;15(1):89-91.
8. Coebergh JAF, Lauw RF, Bots R, et al. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi:10.3389/fpsyg.2015.00814
9. Ten Hulzen RD, Fabry DA. Impact of hearing loss and universal face masking in the COVID-19 era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
10. Shukla A, Nieman CL, Price C, et al. Impact of hearing loss on patient-provider communication among hospitalized patients: a systematic review. Am J Med Qual. 2019;34(3):284-292. doi:10.1177/1062860618798926
11. Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi:10.1017/S0033291718003409
Agitated and depressed with a traumatic brain injury
CASE TBI as a result of self-harm
Mr. N, age 46, presents to the emergency department (ED) after his neighbors report hearing “loud banging sounds” coming from his apartment for approximately 3 days. Emergency medical services found him repeatedly beating his head into a table. Upon admission to the ED, his injuries include a right temporal lobe contusion, right temporal subdural hematoma, facial fractures, bilateral foot fractures, and prevertebral swelling at the C4 vertebrate.
Mr. N is admitted to the surgical intensive care unit for hourly neurology checks. Neurosurgery recommends nonoperative management and for Mr. N to wear a cervical collar for 1 month. He is sedated after he experiences auditory hallucinations and becomes agitated toward the staff, which is later determined to be delirium. The Psychiatry team recommends inpatient psychiatric hospitalization because Mr. N’s self-harming behavior resulted in severe and dangerous injuries.
HISTORY Alcohol use disorder, insomnia, anxiety, and depression
As Mr. N becomes alert and oriented, he reports a history of alcohol use disorder (AUD), insomnia, anxiety, and major depressive disorder (MDD), but no personal or family history of bipolar disorder (BD). He says he has had insomnia and anxiety since age 18, for which he received diazepam and zolpidem for 16 years. He stopped diazepam soon after a recent change in psychiatrists and subsequently had difficulty sleeping. Mr. N started taking mirtazapine, but found minimal relief and stopped it several months ago.
[polldaddy:12704471]
The authors’ observations
The term “agitated depression” refers to a mixed state that includes symptoms of depression plus marked anxiety, restlessness, and delusions. Agitated depression is not a distinct diagnosis in DSM-5, but is classified as depression with mixed features.1 To meet the criteria for the mixed features specifier, a patient who meets the criteria for a major depressive episode needs to have ≥3 of the following manic/hypomanic symptoms1:
- Elevated, expansive mood
- Inflated self-esteem or grandiosity
- More talkative than usual
- Flight of ideas or racing thoughts
- Increase in energy or goal-directed activity
- Increased involvement in activities that have a high potential for painful consequences
- Decreased need for sleep.
The diagnosis for individuals who meet the full criteria for mania or hypomania would be BD I or BD II.1 Additionally, mixed features associated with a major depressive episode are a significant risk factor for BD.1
EVALUATION Agitation and hallucinations
Mr. N recalls multiple falls at home in the weeks prior to hospitalization, but says he does not remember repeatedly hitting his head against a table. He reports sleeping for approximately 2 hours per night since his father’s death 2 months ago, an acute stressor that likely precipitated this depressive episode. Mr. N says he had been experiencing visual hallucinations of his father and a younger version of himself for weeks before presenting to the ED. It is not clear if Mr. N does not recall beating his head on the table due to his traumatic brain injury (TBI) or because it occurred during an acute manic or psychotic episode with hallucinations.
The treatment team assigns Mr. N a working diagnosis of agitated depression with a risk for BD, mixed episode. He meets the criteria for agitated depression (major depressive episode, motor agitation, and psychic agitation), but also has many features of BD; a manic episode may have led to hospitalization. The treating clinicians continue to monitor the progression of Mr. N’s symptoms to clarify his diagnoses. During the course of his hospitalization, Mr. N’s psychiatric diagnoses include delirium (resolved), alcohol withdrawal, catatonia, substance-induced mood disorder, and agitated depression. Mixed episode BD is ruled out.
Continue to: The authors' observations
The authors’ observations
There is significant symptomatic overlap between agitated depression and BD. It can be difficult to differentiate the diagnoses, as psychomotor agitation can be seen in MDD and agitated depression can be seen in BD. Serra et al2 investigated the prevalence of agitated depression in patients with BD and found that agitation accompanied bipolar depression in at least one-third of cases and was associated with concurrent somatic depressive symptoms, which are common features of mixed manic states. Psychomotor agitation was also associated with lifetime experience of mixed mania, comorbid panic disorder, and increased suicidal behavior.2
Though antidepressants are considered a first-line treatment for depression, they should not be used to treat agitated depression because they may increase insomnia, agitation, and suicide risk, and may trigger the onset of psychotic symptoms. In a similar vein, antidepressant monotherapy is contraindicated in BD because it may induce mania or hypomania states.2
TREATMENT Neuroprotective psychotropics
Due to Mr. N’s medical complexity (particularly cervical collar and physical therapy needs), he is not transferred to a psychiatric facility. Instead, the consultation-liaison psychiatry team follows him and provides psychiatric care in the hospital.
Due to concerns for continued self-harm, Mr. N is observed by continuous video monitoring. After initial stabilization, the care team starts valproic acid 250 mg twice daily and titrates it to 500 mg/d in the morning and 1,000 mg/d in the evening for mood stabilization, gabapentin 300 mg 3 times daily, melatonin 3 mg/d at bedtime for insomnia, and lorazepam 1 mg/d at bedtime to rule out catatonia and 1 mg/d as needed for agitation. After starting valproic acid, the care team routinely checks Mr. N’s ammonia levels throughout his hospitalization.
[polldaddy:12704473]
The authors’ observations
Treatment of agitated depression both in isolation and in the context of BD presents a clinical challenge because antidepressants are contraindicated for both agitated depression and BD. In the context of TBI, treatment of agitated depression becomes more complicated because neuroprotection is the priority. Neuroprotection refers to a medication’s ability to prevent neuronal cell death or further injury or damage through neurochemical modulation.
Continue to: To treat agitation associated with MDD...
To treat agitation associated with MDD, second-generation antipsychotics and valproic acid have shown significant neuroprotective effects. The proposed mechanisms for neuroprotection include not only antioxidant effects but 5HT1A agonist properties, with the latter thought to protect against excitotoxic injury that may exacerbate agitation due to brain trauma.3
There is no consensus on which antipsychotics are most efficacious for treating agitation in the setting of an acute TBI. Williamson et al4 reviewed various medications that may treat agitation in the setting of acute TBI with fewer adverse effects.
Though haloperidol is often prescribed to treat agitation in patients with TBI, animal studies have shown it is inferior to second-generation antipsychotics in protecting against excitotoxic/oxidative injury, and haloperidol has been associated with neuronal loss. Haloperidol has been linked to adverse clinical outcomes for patients with aggression after TBI, including prolonged amnesia, which is thought to be linked to haloperidol’s strong and selective dopamine-2 receptor antagonism and the mesocortical and nigrostriatal pathways involved.4
Carbamazepine, phenytoin, and methylphenidate cause oxidative stress and/or apoptosis, and therefore offer no neuroprotection. Data on gabapentin are mixed; a few studies suggest it may block synapse formation or decrease quantities of antioxidant enzymes in the brain, though it’s known to protect against glutamate-induced neuronal injury.3
Additional research is needed to assess which second-generation antipsychotics offer the most neuroprotection. However, based on existing literature, olanzapine and aripiprazole may offer the most benefit because they have the greatest antioxidant—and thus, neuroprotective—activity. Cognitive enhancers such as memantine and donepezil exhibit neuroprotection, particularly in Alzheimer disease. Anticonvulsants such as levetiracetam, lacosamide, and lamotrigine offer neuroprotection and may be considered for seizure prevention.3 The Table3-6 lists psychotropic medications used to treat TBI.
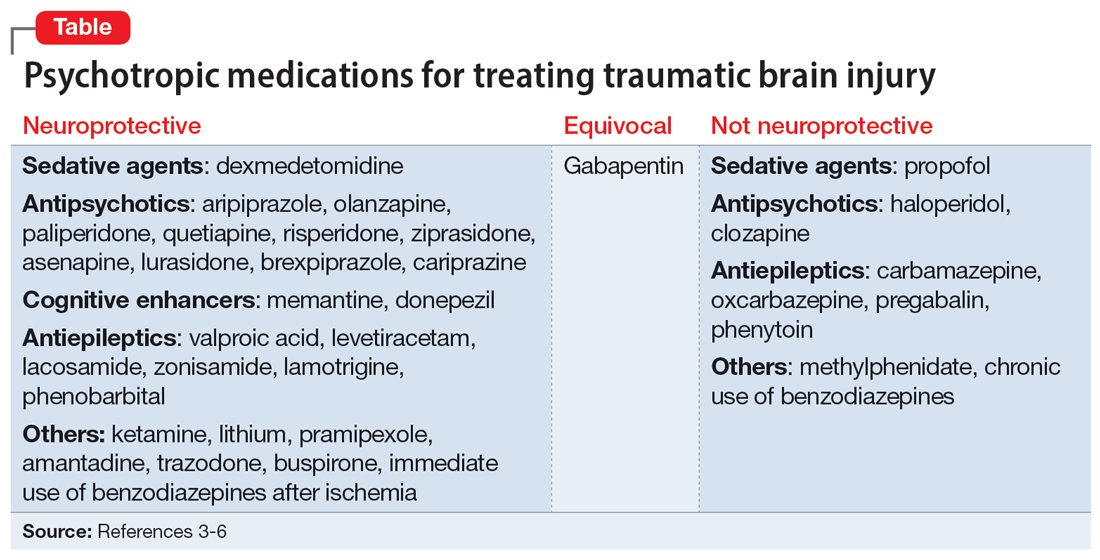
Continue to: Valproic acid stands out among...
Valproic acid stands out among anticonvulsants because its superior antioxidant effects, in combination with its antiepileptic effect in patients with TBI, offer more neuroprotection than other medications.5 It is important to regularly monitor ammonia levels in patients receiving valproic acid because elevated levels can cause hyperammonemic encephalopathy.
A 2005 study by DeBattista et al5 investigated the impact of valproic acid on agitation in 12 adults with MDD who were being treated with antidepressants. Participants were given a low dose of valproic acid for 4 weeks and their agitation, anxiety, and depressed mood were independently assessed by separate rating scales. There was a modest decrease in scores for mood symptoms but a particularly sharp decrease in agitation scores.5
Valproic acid has been shown to be a potentially safe and efficacious treatment for alcohol withdrawal. A clinical trial examining patients with moderate alcohol withdrawal found a faster and more consistent resolution of symptoms in patients given valproic acid detoxification compared to a control group that received the standard benzodiazepine detoxification.6 Additionally, patients who continued maintenance valproic acid following detoxification were completely abstinent at 6-week follow-up compared to patients who did not receive this maintenance therapy.6
Valproic acid was a particularly optimal medication choice for Mr. N due to its neuroprotective properties in the context of TBI, its ability to treat delirium,7 its lack of abuse potential compared with benzodiazepines, and its potential efficacy for managing alcohol withdrawal and AUD.
OUTCOME Improvement and discharge
Mr. N is medically cleared for discharge. Although the psychiatry team initially was concerned about his willingness to attend follow-up appointments and adhere to proper cervical collar use, Mr. N becomes more cooperative with psychiatric care as his stay continues, and he is psychiatrically cleared for discharge 1 month after admission. Discharge plans include attending an intensive outpatient program, continuing the inpatient psychiatric medication regimen, participating in regular outpatient psychiatric follow-up, as well as following up with orthopedic surgery, neurosurgery, podiatry, and ear, nose, and throat for medical conditions.
Bottom Line
Agitated depression is a mixed state that includes features of depression and manic/hypomanic symptoms. Diagnosis and treatment can be challenging because symptoms of agitated depression overlap with bipolar disorder and antidepressants are contraindicated. In a patient with a traumatic brain injury, pharmacotherapy that provides neuroprotection is a priority.
Related Resources
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
- Sampogna G, Del Vecchio V, Giallonardo V, et al. Diagnosis, clinical features, and therapeutic implications of agitated depression. Psychiatr Clin North Am. 2020;43(1):47-57. doi: 10.1016/j.psc.2019.10.011
Drug Brand Names
Amantadine • Gocovri
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Buspirone • BuSpar
Carbamazepine • Tegretol
Cariprazine • Vraylar
Clozapine • Clozaril
Dexmedetomidine • Igalmi
Diazepam • Valium
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lacosamide • Vimpat
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lithium • Lithobid
Lorazepam • Ativan
Lurasidone • Latuda
Memantine • Namenda
Methylphenidate • Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Paliperidone • Invega
Phenytoin • Dilantin
Pramipexole • Mirapex
Pregabalin • Lyrica
Quetiapine • Seroquel
Risperidone • Risperdal
Trazodone • Oleptro
Valproic acid • Depakene
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Serra F, Gordon‐Smith K, Perry A, et al. Agitated depression in bipolar disorder. Bipolar Disord. 2019;21(6):547-555. doi:10.1111/bdi.12778
3. Meresh E, Daniels D, Owens JH, et al. Psychotropics and neuroprotection: literature review and case series report. OBM Neurobiol. 2020;4(1). doi:10.21926/obm.neurobiol.2001048
4. Williamson DR, Frenette AJ, Burry L, et al. Pharmacological interventions for agitation in patients with traumatic brain injury: protocol for a systematic review and meta-analysis. Syst Rev. 2016;5(1):193. doi:10.1186/s13643-016-0374-6
5. DeBattista C, Solomon A, Arnow B, et al. The efficacy of divalproex sodium in the treatment of agitation associated with major depression. J Clin Psychopharmacol. 2005;25(5):476-479. doi:10.1097/01.jcp.0000177552.21338.b0
6. Longo LP, Campbell T, Hubatch, S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis. 2002;21(2):55-64. doi:10.1300/J069v21n02_05
7. Sher Y, Cramer ACM, Ament A, et al. Valproic acid for treatment of hyperactive or mixed delirium: rationale and literature review. Psychosomatics. 2015;56(6):615-625. doi:10.1016/j.psym.2015.09.008
CASE TBI as a result of self-harm
Mr. N, age 46, presents to the emergency department (ED) after his neighbors report hearing “loud banging sounds” coming from his apartment for approximately 3 days. Emergency medical services found him repeatedly beating his head into a table. Upon admission to the ED, his injuries include a right temporal lobe contusion, right temporal subdural hematoma, facial fractures, bilateral foot fractures, and prevertebral swelling at the C4 vertebrate.
Mr. N is admitted to the surgical intensive care unit for hourly neurology checks. Neurosurgery recommends nonoperative management and for Mr. N to wear a cervical collar for 1 month. He is sedated after he experiences auditory hallucinations and becomes agitated toward the staff, which is later determined to be delirium. The Psychiatry team recommends inpatient psychiatric hospitalization because Mr. N’s self-harming behavior resulted in severe and dangerous injuries.
HISTORY Alcohol use disorder, insomnia, anxiety, and depression
As Mr. N becomes alert and oriented, he reports a history of alcohol use disorder (AUD), insomnia, anxiety, and major depressive disorder (MDD), but no personal or family history of bipolar disorder (BD). He says he has had insomnia and anxiety since age 18, for which he received diazepam and zolpidem for 16 years. He stopped diazepam soon after a recent change in psychiatrists and subsequently had difficulty sleeping. Mr. N started taking mirtazapine, but found minimal relief and stopped it several months ago.
[polldaddy:12704471]
The authors’ observations
The term “agitated depression” refers to a mixed state that includes symptoms of depression plus marked anxiety, restlessness, and delusions. Agitated depression is not a distinct diagnosis in DSM-5, but is classified as depression with mixed features.1 To meet the criteria for the mixed features specifier, a patient who meets the criteria for a major depressive episode needs to have ≥3 of the following manic/hypomanic symptoms1:
- Elevated, expansive mood
- Inflated self-esteem or grandiosity
- More talkative than usual
- Flight of ideas or racing thoughts
- Increase in energy or goal-directed activity
- Increased involvement in activities that have a high potential for painful consequences
- Decreased need for sleep.
The diagnosis for individuals who meet the full criteria for mania or hypomania would be BD I or BD II.1 Additionally, mixed features associated with a major depressive episode are a significant risk factor for BD.1
EVALUATION Agitation and hallucinations
Mr. N recalls multiple falls at home in the weeks prior to hospitalization, but says he does not remember repeatedly hitting his head against a table. He reports sleeping for approximately 2 hours per night since his father’s death 2 months ago, an acute stressor that likely precipitated this depressive episode. Mr. N says he had been experiencing visual hallucinations of his father and a younger version of himself for weeks before presenting to the ED. It is not clear if Mr. N does not recall beating his head on the table due to his traumatic brain injury (TBI) or because it occurred during an acute manic or psychotic episode with hallucinations.
The treatment team assigns Mr. N a working diagnosis of agitated depression with a risk for BD, mixed episode. He meets the criteria for agitated depression (major depressive episode, motor agitation, and psychic agitation), but also has many features of BD; a manic episode may have led to hospitalization. The treating clinicians continue to monitor the progression of Mr. N’s symptoms to clarify his diagnoses. During the course of his hospitalization, Mr. N’s psychiatric diagnoses include delirium (resolved), alcohol withdrawal, catatonia, substance-induced mood disorder, and agitated depression. Mixed episode BD is ruled out.
Continue to: The authors' observations
The authors’ observations
There is significant symptomatic overlap between agitated depression and BD. It can be difficult to differentiate the diagnoses, as psychomotor agitation can be seen in MDD and agitated depression can be seen in BD. Serra et al2 investigated the prevalence of agitated depression in patients with BD and found that agitation accompanied bipolar depression in at least one-third of cases and was associated with concurrent somatic depressive symptoms, which are common features of mixed manic states. Psychomotor agitation was also associated with lifetime experience of mixed mania, comorbid panic disorder, and increased suicidal behavior.2
Though antidepressants are considered a first-line treatment for depression, they should not be used to treat agitated depression because they may increase insomnia, agitation, and suicide risk, and may trigger the onset of psychotic symptoms. In a similar vein, antidepressant monotherapy is contraindicated in BD because it may induce mania or hypomania states.2
TREATMENT Neuroprotective psychotropics
Due to Mr. N’s medical complexity (particularly cervical collar and physical therapy needs), he is not transferred to a psychiatric facility. Instead, the consultation-liaison psychiatry team follows him and provides psychiatric care in the hospital.
Due to concerns for continued self-harm, Mr. N is observed by continuous video monitoring. After initial stabilization, the care team starts valproic acid 250 mg twice daily and titrates it to 500 mg/d in the morning and 1,000 mg/d in the evening for mood stabilization, gabapentin 300 mg 3 times daily, melatonin 3 mg/d at bedtime for insomnia, and lorazepam 1 mg/d at bedtime to rule out catatonia and 1 mg/d as needed for agitation. After starting valproic acid, the care team routinely checks Mr. N’s ammonia levels throughout his hospitalization.
[polldaddy:12704473]
The authors’ observations
Treatment of agitated depression both in isolation and in the context of BD presents a clinical challenge because antidepressants are contraindicated for both agitated depression and BD. In the context of TBI, treatment of agitated depression becomes more complicated because neuroprotection is the priority. Neuroprotection refers to a medication’s ability to prevent neuronal cell death or further injury or damage through neurochemical modulation.
Continue to: To treat agitation associated with MDD...
To treat agitation associated with MDD, second-generation antipsychotics and valproic acid have shown significant neuroprotective effects. The proposed mechanisms for neuroprotection include not only antioxidant effects but 5HT1A agonist properties, with the latter thought to protect against excitotoxic injury that may exacerbate agitation due to brain trauma.3
There is no consensus on which antipsychotics are most efficacious for treating agitation in the setting of an acute TBI. Williamson et al4 reviewed various medications that may treat agitation in the setting of acute TBI with fewer adverse effects.
Though haloperidol is often prescribed to treat agitation in patients with TBI, animal studies have shown it is inferior to second-generation antipsychotics in protecting against excitotoxic/oxidative injury, and haloperidol has been associated with neuronal loss. Haloperidol has been linked to adverse clinical outcomes for patients with aggression after TBI, including prolonged amnesia, which is thought to be linked to haloperidol’s strong and selective dopamine-2 receptor antagonism and the mesocortical and nigrostriatal pathways involved.4
Carbamazepine, phenytoin, and methylphenidate cause oxidative stress and/or apoptosis, and therefore offer no neuroprotection. Data on gabapentin are mixed; a few studies suggest it may block synapse formation or decrease quantities of antioxidant enzymes in the brain, though it’s known to protect against glutamate-induced neuronal injury.3
Additional research is needed to assess which second-generation antipsychotics offer the most neuroprotection. However, based on existing literature, olanzapine and aripiprazole may offer the most benefit because they have the greatest antioxidant—and thus, neuroprotective—activity. Cognitive enhancers such as memantine and donepezil exhibit neuroprotection, particularly in Alzheimer disease. Anticonvulsants such as levetiracetam, lacosamide, and lamotrigine offer neuroprotection and may be considered for seizure prevention.3 The Table3-6 lists psychotropic medications used to treat TBI.

Continue to: Valproic acid stands out among...
Valproic acid stands out among anticonvulsants because its superior antioxidant effects, in combination with its antiepileptic effect in patients with TBI, offer more neuroprotection than other medications.5 It is important to regularly monitor ammonia levels in patients receiving valproic acid because elevated levels can cause hyperammonemic encephalopathy.
A 2005 study by DeBattista et al5 investigated the impact of valproic acid on agitation in 12 adults with MDD who were being treated with antidepressants. Participants were given a low dose of valproic acid for 4 weeks and their agitation, anxiety, and depressed mood were independently assessed by separate rating scales. There was a modest decrease in scores for mood symptoms but a particularly sharp decrease in agitation scores.5
Valproic acid has been shown to be a potentially safe and efficacious treatment for alcohol withdrawal. A clinical trial examining patients with moderate alcohol withdrawal found a faster and more consistent resolution of symptoms in patients given valproic acid detoxification compared to a control group that received the standard benzodiazepine detoxification.6 Additionally, patients who continued maintenance valproic acid following detoxification were completely abstinent at 6-week follow-up compared to patients who did not receive this maintenance therapy.6
Valproic acid was a particularly optimal medication choice for Mr. N due to its neuroprotective properties in the context of TBI, its ability to treat delirium,7 its lack of abuse potential compared with benzodiazepines, and its potential efficacy for managing alcohol withdrawal and AUD.
OUTCOME Improvement and discharge
Mr. N is medically cleared for discharge. Although the psychiatry team initially was concerned about his willingness to attend follow-up appointments and adhere to proper cervical collar use, Mr. N becomes more cooperative with psychiatric care as his stay continues, and he is psychiatrically cleared for discharge 1 month after admission. Discharge plans include attending an intensive outpatient program, continuing the inpatient psychiatric medication regimen, participating in regular outpatient psychiatric follow-up, as well as following up with orthopedic surgery, neurosurgery, podiatry, and ear, nose, and throat for medical conditions.
Bottom Line
Agitated depression is a mixed state that includes features of depression and manic/hypomanic symptoms. Diagnosis and treatment can be challenging because symptoms of agitated depression overlap with bipolar disorder and antidepressants are contraindicated. In a patient with a traumatic brain injury, pharmacotherapy that provides neuroprotection is a priority.
Related Resources
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
- Sampogna G, Del Vecchio V, Giallonardo V, et al. Diagnosis, clinical features, and therapeutic implications of agitated depression. Psychiatr Clin North Am. 2020;43(1):47-57. doi: 10.1016/j.psc.2019.10.011
Drug Brand Names
Amantadine • Gocovri
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Buspirone • BuSpar
Carbamazepine • Tegretol
Cariprazine • Vraylar
Clozapine • Clozaril
Dexmedetomidine • Igalmi
Diazepam • Valium
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lacosamide • Vimpat
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lithium • Lithobid
Lorazepam • Ativan
Lurasidone • Latuda
Memantine • Namenda
Methylphenidate • Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Paliperidone • Invega
Phenytoin • Dilantin
Pramipexole • Mirapex
Pregabalin • Lyrica
Quetiapine • Seroquel
Risperidone • Risperdal
Trazodone • Oleptro
Valproic acid • Depakene
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
CASE TBI as a result of self-harm
Mr. N, age 46, presents to the emergency department (ED) after his neighbors report hearing “loud banging sounds” coming from his apartment for approximately 3 days. Emergency medical services found him repeatedly beating his head into a table. Upon admission to the ED, his injuries include a right temporal lobe contusion, right temporal subdural hematoma, facial fractures, bilateral foot fractures, and prevertebral swelling at the C4 vertebrate.
Mr. N is admitted to the surgical intensive care unit for hourly neurology checks. Neurosurgery recommends nonoperative management and for Mr. N to wear a cervical collar for 1 month. He is sedated after he experiences auditory hallucinations and becomes agitated toward the staff, which is later determined to be delirium. The Psychiatry team recommends inpatient psychiatric hospitalization because Mr. N’s self-harming behavior resulted in severe and dangerous injuries.
HISTORY Alcohol use disorder, insomnia, anxiety, and depression
As Mr. N becomes alert and oriented, he reports a history of alcohol use disorder (AUD), insomnia, anxiety, and major depressive disorder (MDD), but no personal or family history of bipolar disorder (BD). He says he has had insomnia and anxiety since age 18, for which he received diazepam and zolpidem for 16 years. He stopped diazepam soon after a recent change in psychiatrists and subsequently had difficulty sleeping. Mr. N started taking mirtazapine, but found minimal relief and stopped it several months ago.
[polldaddy:12704471]
The authors’ observations
The term “agitated depression” refers to a mixed state that includes symptoms of depression plus marked anxiety, restlessness, and delusions. Agitated depression is not a distinct diagnosis in DSM-5, but is classified as depression with mixed features.1 To meet the criteria for the mixed features specifier, a patient who meets the criteria for a major depressive episode needs to have ≥3 of the following manic/hypomanic symptoms1:
- Elevated, expansive mood
- Inflated self-esteem or grandiosity
- More talkative than usual
- Flight of ideas or racing thoughts
- Increase in energy or goal-directed activity
- Increased involvement in activities that have a high potential for painful consequences
- Decreased need for sleep.
The diagnosis for individuals who meet the full criteria for mania or hypomania would be BD I or BD II.1 Additionally, mixed features associated with a major depressive episode are a significant risk factor for BD.1
EVALUATION Agitation and hallucinations
Mr. N recalls multiple falls at home in the weeks prior to hospitalization, but says he does not remember repeatedly hitting his head against a table. He reports sleeping for approximately 2 hours per night since his father’s death 2 months ago, an acute stressor that likely precipitated this depressive episode. Mr. N says he had been experiencing visual hallucinations of his father and a younger version of himself for weeks before presenting to the ED. It is not clear if Mr. N does not recall beating his head on the table due to his traumatic brain injury (TBI) or because it occurred during an acute manic or psychotic episode with hallucinations.
The treatment team assigns Mr. N a working diagnosis of agitated depression with a risk for BD, mixed episode. He meets the criteria for agitated depression (major depressive episode, motor agitation, and psychic agitation), but also has many features of BD; a manic episode may have led to hospitalization. The treating clinicians continue to monitor the progression of Mr. N’s symptoms to clarify his diagnoses. During the course of his hospitalization, Mr. N’s psychiatric diagnoses include delirium (resolved), alcohol withdrawal, catatonia, substance-induced mood disorder, and agitated depression. Mixed episode BD is ruled out.
Continue to: The authors' observations
The authors’ observations
There is significant symptomatic overlap between agitated depression and BD. It can be difficult to differentiate the diagnoses, as psychomotor agitation can be seen in MDD and agitated depression can be seen in BD. Serra et al2 investigated the prevalence of agitated depression in patients with BD and found that agitation accompanied bipolar depression in at least one-third of cases and was associated with concurrent somatic depressive symptoms, which are common features of mixed manic states. Psychomotor agitation was also associated with lifetime experience of mixed mania, comorbid panic disorder, and increased suicidal behavior.2
Though antidepressants are considered a first-line treatment for depression, they should not be used to treat agitated depression because they may increase insomnia, agitation, and suicide risk, and may trigger the onset of psychotic symptoms. In a similar vein, antidepressant monotherapy is contraindicated in BD because it may induce mania or hypomania states.2
TREATMENT Neuroprotective psychotropics
Due to Mr. N’s medical complexity (particularly cervical collar and physical therapy needs), he is not transferred to a psychiatric facility. Instead, the consultation-liaison psychiatry team follows him and provides psychiatric care in the hospital.
Due to concerns for continued self-harm, Mr. N is observed by continuous video monitoring. After initial stabilization, the care team starts valproic acid 250 mg twice daily and titrates it to 500 mg/d in the morning and 1,000 mg/d in the evening for mood stabilization, gabapentin 300 mg 3 times daily, melatonin 3 mg/d at bedtime for insomnia, and lorazepam 1 mg/d at bedtime to rule out catatonia and 1 mg/d as needed for agitation. After starting valproic acid, the care team routinely checks Mr. N’s ammonia levels throughout his hospitalization.
[polldaddy:12704473]
The authors’ observations
Treatment of agitated depression both in isolation and in the context of BD presents a clinical challenge because antidepressants are contraindicated for both agitated depression and BD. In the context of TBI, treatment of agitated depression becomes more complicated because neuroprotection is the priority. Neuroprotection refers to a medication’s ability to prevent neuronal cell death or further injury or damage through neurochemical modulation.
Continue to: To treat agitation associated with MDD...
To treat agitation associated with MDD, second-generation antipsychotics and valproic acid have shown significant neuroprotective effects. The proposed mechanisms for neuroprotection include not only antioxidant effects but 5HT1A agonist properties, with the latter thought to protect against excitotoxic injury that may exacerbate agitation due to brain trauma.3
There is no consensus on which antipsychotics are most efficacious for treating agitation in the setting of an acute TBI. Williamson et al4 reviewed various medications that may treat agitation in the setting of acute TBI with fewer adverse effects.
Though haloperidol is often prescribed to treat agitation in patients with TBI, animal studies have shown it is inferior to second-generation antipsychotics in protecting against excitotoxic/oxidative injury, and haloperidol has been associated with neuronal loss. Haloperidol has been linked to adverse clinical outcomes for patients with aggression after TBI, including prolonged amnesia, which is thought to be linked to haloperidol’s strong and selective dopamine-2 receptor antagonism and the mesocortical and nigrostriatal pathways involved.4
Carbamazepine, phenytoin, and methylphenidate cause oxidative stress and/or apoptosis, and therefore offer no neuroprotection. Data on gabapentin are mixed; a few studies suggest it may block synapse formation or decrease quantities of antioxidant enzymes in the brain, though it’s known to protect against glutamate-induced neuronal injury.3
Additional research is needed to assess which second-generation antipsychotics offer the most neuroprotection. However, based on existing literature, olanzapine and aripiprazole may offer the most benefit because they have the greatest antioxidant—and thus, neuroprotective—activity. Cognitive enhancers such as memantine and donepezil exhibit neuroprotection, particularly in Alzheimer disease. Anticonvulsants such as levetiracetam, lacosamide, and lamotrigine offer neuroprotection and may be considered for seizure prevention.3 The Table3-6 lists psychotropic medications used to treat TBI.

Continue to: Valproic acid stands out among...
Valproic acid stands out among anticonvulsants because its superior antioxidant effects, in combination with its antiepileptic effect in patients with TBI, offer more neuroprotection than other medications.5 It is important to regularly monitor ammonia levels in patients receiving valproic acid because elevated levels can cause hyperammonemic encephalopathy.
A 2005 study by DeBattista et al5 investigated the impact of valproic acid on agitation in 12 adults with MDD who were being treated with antidepressants. Participants were given a low dose of valproic acid for 4 weeks and their agitation, anxiety, and depressed mood were independently assessed by separate rating scales. There was a modest decrease in scores for mood symptoms but a particularly sharp decrease in agitation scores.5
Valproic acid has been shown to be a potentially safe and efficacious treatment for alcohol withdrawal. A clinical trial examining patients with moderate alcohol withdrawal found a faster and more consistent resolution of symptoms in patients given valproic acid detoxification compared to a control group that received the standard benzodiazepine detoxification.6 Additionally, patients who continued maintenance valproic acid following detoxification were completely abstinent at 6-week follow-up compared to patients who did not receive this maintenance therapy.6
Valproic acid was a particularly optimal medication choice for Mr. N due to its neuroprotective properties in the context of TBI, its ability to treat delirium,7 its lack of abuse potential compared with benzodiazepines, and its potential efficacy for managing alcohol withdrawal and AUD.
OUTCOME Improvement and discharge
Mr. N is medically cleared for discharge. Although the psychiatry team initially was concerned about his willingness to attend follow-up appointments and adhere to proper cervical collar use, Mr. N becomes more cooperative with psychiatric care as his stay continues, and he is psychiatrically cleared for discharge 1 month after admission. Discharge plans include attending an intensive outpatient program, continuing the inpatient psychiatric medication regimen, participating in regular outpatient psychiatric follow-up, as well as following up with orthopedic surgery, neurosurgery, podiatry, and ear, nose, and throat for medical conditions.
Bottom Line
Agitated depression is a mixed state that includes features of depression and manic/hypomanic symptoms. Diagnosis and treatment can be challenging because symptoms of agitated depression overlap with bipolar disorder and antidepressants are contraindicated. In a patient with a traumatic brain injury, pharmacotherapy that provides neuroprotection is a priority.
Related Resources
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
- Sampogna G, Del Vecchio V, Giallonardo V, et al. Diagnosis, clinical features, and therapeutic implications of agitated depression. Psychiatr Clin North Am. 2020;43(1):47-57. doi: 10.1016/j.psc.2019.10.011
Drug Brand Names
Amantadine • Gocovri
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Buspirone • BuSpar
Carbamazepine • Tegretol
Cariprazine • Vraylar
Clozapine • Clozaril
Dexmedetomidine • Igalmi
Diazepam • Valium
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lacosamide • Vimpat
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lithium • Lithobid
Lorazepam • Ativan
Lurasidone • Latuda
Memantine • Namenda
Methylphenidate • Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Paliperidone • Invega
Phenytoin • Dilantin
Pramipexole • Mirapex
Pregabalin • Lyrica
Quetiapine • Seroquel
Risperidone • Risperdal
Trazodone • Oleptro
Valproic acid • Depakene
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Serra F, Gordon‐Smith K, Perry A, et al. Agitated depression in bipolar disorder. Bipolar Disord. 2019;21(6):547-555. doi:10.1111/bdi.12778
3. Meresh E, Daniels D, Owens JH, et al. Psychotropics and neuroprotection: literature review and case series report. OBM Neurobiol. 2020;4(1). doi:10.21926/obm.neurobiol.2001048
4. Williamson DR, Frenette AJ, Burry L, et al. Pharmacological interventions for agitation in patients with traumatic brain injury: protocol for a systematic review and meta-analysis. Syst Rev. 2016;5(1):193. doi:10.1186/s13643-016-0374-6
5. DeBattista C, Solomon A, Arnow B, et al. The efficacy of divalproex sodium in the treatment of agitation associated with major depression. J Clin Psychopharmacol. 2005;25(5):476-479. doi:10.1097/01.jcp.0000177552.21338.b0
6. Longo LP, Campbell T, Hubatch, S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis. 2002;21(2):55-64. doi:10.1300/J069v21n02_05
7. Sher Y, Cramer ACM, Ament A, et al. Valproic acid for treatment of hyperactive or mixed delirium: rationale and literature review. Psychosomatics. 2015;56(6):615-625. doi:10.1016/j.psym.2015.09.008
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Serra F, Gordon‐Smith K, Perry A, et al. Agitated depression in bipolar disorder. Bipolar Disord. 2019;21(6):547-555. doi:10.1111/bdi.12778
3. Meresh E, Daniels D, Owens JH, et al. Psychotropics and neuroprotection: literature review and case series report. OBM Neurobiol. 2020;4(1). doi:10.21926/obm.neurobiol.2001048
4. Williamson DR, Frenette AJ, Burry L, et al. Pharmacological interventions for agitation in patients with traumatic brain injury: protocol for a systematic review and meta-analysis. Syst Rev. 2016;5(1):193. doi:10.1186/s13643-016-0374-6
5. DeBattista C, Solomon A, Arnow B, et al. The efficacy of divalproex sodium in the treatment of agitation associated with major depression. J Clin Psychopharmacol. 2005;25(5):476-479. doi:10.1097/01.jcp.0000177552.21338.b0
6. Longo LP, Campbell T, Hubatch, S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis. 2002;21(2):55-64. doi:10.1300/J069v21n02_05
7. Sher Y, Cramer ACM, Ament A, et al. Valproic acid for treatment of hyperactive or mixed delirium: rationale and literature review. Psychosomatics. 2015;56(6):615-625. doi:10.1016/j.psym.2015.09.008
Infested with worms, but are they really there?
CASE Detoxification and preoccupation with parasites
Mr. H, age 51, has an extensive history of alcohol and methamphetamine use. He presents to the emergency department (ED) requesting inpatient detoxification. He says he had been drinking alcohol but is unable to say how much. His blood ethanol level is 61 mg/dL (unintoxicated level: <50 mg/dL), and a urine drug screen is positive for methamphetamine; Mr. H also admits to using fentanyl. The ED team treats Mr. H’s electrolyte abnormalities, initiates thiamine supplementation, and transfers him to a unit for inpatient withdrawal management.
On the detoxification unit, Mr. H receives a total of 1,950 mg of phenobarbital for alcohol withdrawal and stabilizes on a buprenorphine/naloxone maintenance dose of 8 mg/2 mg twice daily for methamphetamine and fentanyl use. Though he was not taking any psychiatric medications prior to his arrival at the ED, Mr. H agrees to restart quetiapine
During Mr. H’s 3-day detoxification, the psychiatry team evaluates him. Mr. H says he believes he is infested with worms. He describes a prior sensation of “meth mites,” or the feeling of bugs crawling under his skin, while using methamphetamines. However, Mr. H says his current infestation feels distinctively different, and he had continued to experience these
The psychiatry team expresses concern over his preoccupation with infestations, disheveled appearance, poor hygiene, and healed scars from excoriation. Mr. H also reports poor sleep and appetite and was observed writing an incomprehensible “experiment” on a paper towel. Due to his bizarre behavior, delusional thoughts, and concerns about his inability to care for himself, the team admits Mr. H to the acute inpatient psychiatric unit on a voluntary commitment.
HISTORY Long-standing drug use and repeated hospital visits
Mr. H reports a history of drug use. His first documented ED visit was >5 years before his current admission. He has a family history of substance abuse and reports previously using methamphetamine, heroin, and alcohol. Mr. H was never diagnosed with a psychiatric illness, but when he was younger, there were suspicions of bipolar depression, with no contributing family psychiatric history. Though he took quetiapine at an unspecified younger age, Mr. H did not follow through with any outpatient mental health services or medications.
Mr. H first reported infestation
In the 6 months prior to his current admission, Mr. H came to the hospital >20 times for various reasons, including methamphetamine abuse, alcohol withdrawal, opiate overdose, cellulitis, wound checks, and 3 visits for hallucinations for which he requested physical evaluation and medical care. His substance use was the suspected cause of his tactile and visual hallucinations of infestation because formication
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis (DP), also known as delusional infestation or Ekbom Syndrome, is a condition characterized by the fixed, false belief of an infestation without any objective evidence. This condition was previously defined in DSM-IV, but was removed from DSM-5-TR. In DSM-5-TR, DP is most closely associated with delusional disorder
DP is rare, affecting approximately 1.9 per 100,000 people. There has not been consistent data supporting differences in prevalence between sexes, but there is evidence for increasing incidence with age, with a mean age of diagnosis of 61.4.2,3 DP can be divided into 2 types based on the history and etiology of the symptoms: primary DP and secondary DP. Primary DP occurs when there is a failure to identify an organic cause for the occurrence of the symptoms. Therefore, primary DP requires an extensive investigation by a multidisciplinary team that commonly includes medical specialists for a nonpsychiatric workup. Secondary DP occurs when the patient has delusional symptoms associated with a primary diagnosis of schizophrenia, depression, stroke, diabetes, vitamin B12 deficiency, or substance use.4
Though Mr. H initially presented to the ED, patients with DP commonly present to a primary care physician or dermatologist with the complaint of itching or feelings of insects, worms, or unclear organisms inside them. Patients with DP may often develop poor working relationships with physicians while obtaining multiple negative results. They may seek opinions from multiple specialists; however, patients typically do not consider psychiatrists as a source of help. When patients seek psychiatric care, often after a recommendation from a primary care physician or dermatologist, mental health clinicians should listen to and evaluate the patient holistically, continuing to rule out other possible etiologies.
[polldaddy:12570072]
TREATMENT Finding the right antipsychotic
In the psychiatric unit, Mr. H says he believes worms are exiting his ears, mouth, toenail, and self-inflicted scratch wounds. He believes he has been dealing with the parasites for >1 year and they are slowly draining his energy. Mr. H insists he contracted the “infection” from his home carpet, which was wet due to a flood in his house, and after he had fallen asleep following drug use. He also believes he acquired the parasites while walking barefoot along the beach and collecting rocks, and that there are multiple species living inside him, all intelligent enough to hide, making it difficult to prove their existence. He notes they vary in size, and some have red eyes.
During admission, Mr. H voices his frustration that clinicians had not found the worms he has been seeing. He continuously requests to review imaging performed during his visit and wants a multidisciplinary team to evaluate his case. He demands to test a cup with spit-up “samples,” believing the parasites would be visible under a microscope. Throughout his admission, Mr. H continues to take buprenorphine/naloxone and does not experience withdrawal symptoms. The treatment team titrates his quetiapine to 400 mg/d. Due to the lack of improvement, the team initiates olanzapine 5 mg/d at bedtime. However, Mr. H reports significant tinnitus and requests a medication change. He is started on haloperidol 5 mg twice daily.
Continue to: Mr. H begins to see improvements...
Mr. H begins to see improvements on Day 7 of taking haloperidol. He no longer brings up infestation but still acknowledges having worms inside him when directly asked. He says the worms cause him less distress than before and he is hopeful to live without discomfort. He also demonstrates an ability to conduct activities of daily living. Because Mr. H is being monitored on an acute inpatient psychiatric basis, he is deemed appropriate for discharge even though his symptoms have not yet fully resolved. After a 19-day hospital stay, Mr. H is discharged on haloperidol 15 mg/d and quetiapine 200 mg/d.
[polldaddy:12570074]
The authors’ observations
Mr. H asked to have his sputum examined. The “specimen sign,” also called “matchbox sign” or “Ziploc bag sign,” in which patients collect what they believe to be infected tissue or organisms in a container, is a well-studied part of DP.5 Such samples should be considered during initial encounters and can be examined for formal evaluation, but cautiously. Overtesting may incur a financial burden or reinforce deleterious beliefs and behaviors.
It can be difficult to identify triggers of DP. Research shows DP may arise from nonorganic and stressful life events, home floods, or contact with people infected with parasites.6,7 Organic causes have also been found, such as patients taking multiple medications for Parkinson disease who developed delusional symptoms.8 Buscarino et al9 reported the case of a woman who started to develop symptoms of delusions and hallucinations after being on high-dose amphetamines for attention-deficit/hyperactivity disorder. Research shows that stopping the suspected medication commonly improves such symptoms.9,10 Although methamphetamine can remain detectable in urine for up to 4 days after use and potentially a few days longer for chronic users due to circulating levels,11 Mr. H’s symptoms continued for weeks after all substances of abuse should have been cleared from his system. This suggests he was experiencing a psychiatric illness and was accurate in distinguishing methamphetamine-induced from psychiatric-induced sensations. Regardless, polysubstance use has been shown to potentially increase the risk and play a role in the onset and progression of delusional illness, as seen in prior cases as well as in this case.9
It has been hypothesized that the pathophysiology of DP is associated with the deterioration of the striatal dopaminergic pathway, leading to an increase in extracellular dopamine levels. The striatum is responsible for most dopamine reuptake in the brain; therefore, certain drugs such as cocaine, methamphetamine, and methylphenidate may precipitate symptoms of DP due to their blockade of presynaptic dopamine reuptake.12 Additionally, conditions that decrease the functioning of striatal dopamine transporters, such as schizophrenia or depression, may be underlying causes of DP.13
Treatment of DP remains a topic of debate. Most current recommendations appear to be based on a small, nonrandomized placebo-controlled trial.14 The first-generation antipsychotic pimozide had been a first-line treatment for DP, but its adverse effect profile, which includes QTc prolongation and extrapyramidal symptoms, led to the exploration of second-generation antipsychotics such as olanzapine and risperidone.15,16 There is a dearth of literature about the use of haloperidol, quetiapine, or a combination of both as treatment options for DP, though the combination of these 2 medications proved effective for Mr. H. Further research is necessary to justify changes to current treatment standards, but this finding highlights a successful symptom reduction achieved with this combination.
Continue to: Patients may experience genuine symptoms...
Patients may experience genuine symptoms despite the delusional nature of DP, and it is important for clinicians to recognize the potential burden and anxiety these individuals face. Patients may present with self-inflicted bruises, cuts, and erosions to gain access to infected areas, which may be confused with skin picking disorder. Excessive cleansing or use of irritant products can also cause skin damage, leading to other dermatological conditions that reinforce the patient’s belief that something is medically wrong. During treatment, consider medications for relief of pruritus or pain. Focus on offering patients the opportunity to express their concerns, treat them with empathy, avoid stigmatizing language such as “delusions” or “psychosis,” and refrain from contradicting them until a strong rapport has been established (Table 217).
Symptoms of DP can persist for months to years. Patients who fully recovered experienced a median duration of 0.5 years until symptom resolution, compared to incompletely recovered patients, who took approximately 1 year.18 Primary DP has slower improvement rates compared to secondary DP, with the median onset of effects occurring at Week 1.5 and peak improvements occurring at Week 6.16
OUTCOME Continued ED visits
Unfortunately, Mr. H does not follow through with his outpatient psychiatry appointments. In the 7 months following discharge, he visits the ED 8 times for alcohol intoxication, alcohol withdrawal, and methamphetamine abuse, in addition to 2 admissions for inpatient detoxification, during which he was still receiving the same scheduled medications (haloperidol 15 mg/d and quetiapine 200 mg/d). At each of his ED visits, there was no documentation of DP symptoms, which suggests his symptoms may have resolved.
Bottom Line
Because delusional parasitosis symptoms feel real to patients, it is crucial to build rapport to recommend and successfully initiate treatment. After ruling out nonpsychiatric etiologies, consider traditional treatment with antipsychotics, and consider medications for relief of pruritus or pain.
Related Resources
- Sellman D, Phan SV, Inyang M. Bugs on her skin—but nobody else sees them. Current Psychiatry. 2018;17(8):48,50-53.
- Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434. doi:10.1016/j.jaad.2018.12.012
Drug Brand Names
Buprenorphine/naloxone • Suboxone
Haloperidol • Haldol
Hydroxyzine • Vistaril
Lithium • Eskalith, Lithobid
Methylphenidate • Concerta
Olanzapine • Zyprexa
Permethrin • Elimite
Phenobarbital • Solfoton, Tedral, Luminal
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakote
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2013.
2. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976-2010. Br J Dermatol. 2014;170(5):1130-1135. doi:10.1111/bjd.12848
3. Kohorst JJ, Bailey CH, Andersen LK, et al. Prevalence of delusional infestation-a population-based study. JAMA Dermatol. 2018;154(5):615-617. doi:10.1001/jamadermatol.2018.0004
4. Freinhar JP. Delusions of parasitosis. Psychosomatics. 1984;25(1):47-53. doi:10.1016/S0033-3182(84)73096-9
5. Reich A, Kwiatkowska D, Pacan P. Delusions of parasitosis: an update. Dermatol Ther (Heidelb). 2019;9(4):631-638. doi:10.1007/s13555-019-00324-3
6. Berrios GE. Delusional parasitosis and physical disease. Compr Psychiatry. 1985;26(5):395-403. doi:10.1016/0010-440x(85)90077-x
7. Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry. 1991;159:716-717. doi:10.1192/bjp.159.5.716
8. Flann S, Shotbolt J, Kessel B, et al. Three cases of delusional parasitosis caused by dopamine agonists. Clin Exp Dermatol. 2010;35(7):740-742. doi:10.1111/j.1365-2230.2010.03810.x
9. Buscarino M, Saal J, Young JL. Delusional parasitosis in a female treated with mixed amphetamine salts: a case report and literature review. Case Rep Psychiatry. 2012;2012:624235. doi:10.1155/2012/624235
10. Elpern DJ. Cocaine abuse and delusions of parasitosis. Cutis. 1988;42(4):273-274.
11. Richards JR, Laurin EG. Methamphetamine toxicity. StatPearls Publishing; 2023. Updated January 8, 2023. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430895/
12. Huber M, Kirchler E, Karner M, et al. Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med Hypotheses. 2007;68(6):1351-1358. doi:10.1016/j.mehy.2006.07.061
13. Lipman ZM, Yosipovitch G. Substance use disorders and chronic itch. J Am Acad Dermatol. 2021;84(1):148-155. doi:10.1016/j.jaad.2020.08.117
14. Kenchaiah BK, Kumar S, Tharyan P. Atypical anti-psychotics in delusional parasitosis: a retrospective case series of 20 patients. Int J Dermatol. 2010;49(1):95-100. doi:10.1111/j.1365-4632.2009.04312.x
15. Laidler N. Delusions of parasitosis: a brief review of the literature and pathway for diagnosis and treatment. Dermatol Online J. 2018;24(1):13030/qt1fh739nx.
16. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508. doi:10.1097/JCP.0b013e318185e774
17. Mumcuoglu KY, Leibovici V, Reuveni I, et al. Delusional parasitosis: diagnosis and treatment. Isr Med Assoc J. 2018;20(7):456-460.
18. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246. doi:10.1159/000284934
CASE Detoxification and preoccupation with parasites
Mr. H, age 51, has an extensive history of alcohol and methamphetamine use. He presents to the emergency department (ED) requesting inpatient detoxification. He says he had been drinking alcohol but is unable to say how much. His blood ethanol level is 61 mg/dL (unintoxicated level: <50 mg/dL), and a urine drug screen is positive for methamphetamine; Mr. H also admits to using fentanyl. The ED team treats Mr. H’s electrolyte abnormalities, initiates thiamine supplementation, and transfers him to a unit for inpatient withdrawal management.
On the detoxification unit, Mr. H receives a total of 1,950 mg of phenobarbital for alcohol withdrawal and stabilizes on a buprenorphine/naloxone maintenance dose of 8 mg/2 mg twice daily for methamphetamine and fentanyl use. Though he was not taking any psychiatric medications prior to his arrival at the ED, Mr. H agrees to restart quetiapine
During Mr. H’s 3-day detoxification, the psychiatry team evaluates him. Mr. H says he believes he is infested with worms. He describes a prior sensation of “meth mites,” or the feeling of bugs crawling under his skin, while using methamphetamines. However, Mr. H says his current infestation feels distinctively different, and he had continued to experience these
The psychiatry team expresses concern over his preoccupation with infestations, disheveled appearance, poor hygiene, and healed scars from excoriation. Mr. H also reports poor sleep and appetite and was observed writing an incomprehensible “experiment” on a paper towel. Due to his bizarre behavior, delusional thoughts, and concerns about his inability to care for himself, the team admits Mr. H to the acute inpatient psychiatric unit on a voluntary commitment.
HISTORY Long-standing drug use and repeated hospital visits
Mr. H reports a history of drug use. His first documented ED visit was >5 years before his current admission. He has a family history of substance abuse and reports previously using methamphetamine, heroin, and alcohol. Mr. H was never diagnosed with a psychiatric illness, but when he was younger, there were suspicions of bipolar depression, with no contributing family psychiatric history. Though he took quetiapine at an unspecified younger age, Mr. H did not follow through with any outpatient mental health services or medications.
Mr. H first reported infestation
In the 6 months prior to his current admission, Mr. H came to the hospital >20 times for various reasons, including methamphetamine abuse, alcohol withdrawal, opiate overdose, cellulitis, wound checks, and 3 visits for hallucinations for which he requested physical evaluation and medical care. His substance use was the suspected cause of his tactile and visual hallucinations of infestation because formication
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis (DP), also known as delusional infestation or Ekbom Syndrome, is a condition characterized by the fixed, false belief of an infestation without any objective evidence. This condition was previously defined in DSM-IV, but was removed from DSM-5-TR. In DSM-5-TR, DP is most closely associated with delusional disorder
DP is rare, affecting approximately 1.9 per 100,000 people. There has not been consistent data supporting differences in prevalence between sexes, but there is evidence for increasing incidence with age, with a mean age of diagnosis of 61.4.2,3 DP can be divided into 2 types based on the history and etiology of the symptoms: primary DP and secondary DP. Primary DP occurs when there is a failure to identify an organic cause for the occurrence of the symptoms. Therefore, primary DP requires an extensive investigation by a multidisciplinary team that commonly includes medical specialists for a nonpsychiatric workup. Secondary DP occurs when the patient has delusional symptoms associated with a primary diagnosis of schizophrenia, depression, stroke, diabetes, vitamin B12 deficiency, or substance use.4
Though Mr. H initially presented to the ED, patients with DP commonly present to a primary care physician or dermatologist with the complaint of itching or feelings of insects, worms, or unclear organisms inside them. Patients with DP may often develop poor working relationships with physicians while obtaining multiple negative results. They may seek opinions from multiple specialists; however, patients typically do not consider psychiatrists as a source of help. When patients seek psychiatric care, often after a recommendation from a primary care physician or dermatologist, mental health clinicians should listen to and evaluate the patient holistically, continuing to rule out other possible etiologies.
[polldaddy:12570072]
TREATMENT Finding the right antipsychotic
In the psychiatric unit, Mr. H says he believes worms are exiting his ears, mouth, toenail, and self-inflicted scratch wounds. He believes he has been dealing with the parasites for >1 year and they are slowly draining his energy. Mr. H insists he contracted the “infection” from his home carpet, which was wet due to a flood in his house, and after he had fallen asleep following drug use. He also believes he acquired the parasites while walking barefoot along the beach and collecting rocks, and that there are multiple species living inside him, all intelligent enough to hide, making it difficult to prove their existence. He notes they vary in size, and some have red eyes.
During admission, Mr. H voices his frustration that clinicians had not found the worms he has been seeing. He continuously requests to review imaging performed during his visit and wants a multidisciplinary team to evaluate his case. He demands to test a cup with spit-up “samples,” believing the parasites would be visible under a microscope. Throughout his admission, Mr. H continues to take buprenorphine/naloxone and does not experience withdrawal symptoms. The treatment team titrates his quetiapine to 400 mg/d. Due to the lack of improvement, the team initiates olanzapine 5 mg/d at bedtime. However, Mr. H reports significant tinnitus and requests a medication change. He is started on haloperidol 5 mg twice daily.
Continue to: Mr. H begins to see improvements...
Mr. H begins to see improvements on Day 7 of taking haloperidol. He no longer brings up infestation but still acknowledges having worms inside him when directly asked. He says the worms cause him less distress than before and he is hopeful to live without discomfort. He also demonstrates an ability to conduct activities of daily living. Because Mr. H is being monitored on an acute inpatient psychiatric basis, he is deemed appropriate for discharge even though his symptoms have not yet fully resolved. After a 19-day hospital stay, Mr. H is discharged on haloperidol 15 mg/d and quetiapine 200 mg/d.
[polldaddy:12570074]
The authors’ observations
Mr. H asked to have his sputum examined. The “specimen sign,” also called “matchbox sign” or “Ziploc bag sign,” in which patients collect what they believe to be infected tissue or organisms in a container, is a well-studied part of DP.5 Such samples should be considered during initial encounters and can be examined for formal evaluation, but cautiously. Overtesting may incur a financial burden or reinforce deleterious beliefs and behaviors.
It can be difficult to identify triggers of DP. Research shows DP may arise from nonorganic and stressful life events, home floods, or contact with people infected with parasites.6,7 Organic causes have also been found, such as patients taking multiple medications for Parkinson disease who developed delusional symptoms.8 Buscarino et al9 reported the case of a woman who started to develop symptoms of delusions and hallucinations after being on high-dose amphetamines for attention-deficit/hyperactivity disorder. Research shows that stopping the suspected medication commonly improves such symptoms.9,10 Although methamphetamine can remain detectable in urine for up to 4 days after use and potentially a few days longer for chronic users due to circulating levels,11 Mr. H’s symptoms continued for weeks after all substances of abuse should have been cleared from his system. This suggests he was experiencing a psychiatric illness and was accurate in distinguishing methamphetamine-induced from psychiatric-induced sensations. Regardless, polysubstance use has been shown to potentially increase the risk and play a role in the onset and progression of delusional illness, as seen in prior cases as well as in this case.9
It has been hypothesized that the pathophysiology of DP is associated with the deterioration of the striatal dopaminergic pathway, leading to an increase in extracellular dopamine levels. The striatum is responsible for most dopamine reuptake in the brain; therefore, certain drugs such as cocaine, methamphetamine, and methylphenidate may precipitate symptoms of DP due to their blockade of presynaptic dopamine reuptake.12 Additionally, conditions that decrease the functioning of striatal dopamine transporters, such as schizophrenia or depression, may be underlying causes of DP.13
Treatment of DP remains a topic of debate. Most current recommendations appear to be based on a small, nonrandomized placebo-controlled trial.14 The first-generation antipsychotic pimozide had been a first-line treatment for DP, but its adverse effect profile, which includes QTc prolongation and extrapyramidal symptoms, led to the exploration of second-generation antipsychotics such as olanzapine and risperidone.15,16 There is a dearth of literature about the use of haloperidol, quetiapine, or a combination of both as treatment options for DP, though the combination of these 2 medications proved effective for Mr. H. Further research is necessary to justify changes to current treatment standards, but this finding highlights a successful symptom reduction achieved with this combination.
Continue to: Patients may experience genuine symptoms...
Patients may experience genuine symptoms despite the delusional nature of DP, and it is important for clinicians to recognize the potential burden and anxiety these individuals face. Patients may present with self-inflicted bruises, cuts, and erosions to gain access to infected areas, which may be confused with skin picking disorder. Excessive cleansing or use of irritant products can also cause skin damage, leading to other dermatological conditions that reinforce the patient’s belief that something is medically wrong. During treatment, consider medications for relief of pruritus or pain. Focus on offering patients the opportunity to express their concerns, treat them with empathy, avoid stigmatizing language such as “delusions” or “psychosis,” and refrain from contradicting them until a strong rapport has been established (Table 217).
Symptoms of DP can persist for months to years. Patients who fully recovered experienced a median duration of 0.5 years until symptom resolution, compared to incompletely recovered patients, who took approximately 1 year.18 Primary DP has slower improvement rates compared to secondary DP, with the median onset of effects occurring at Week 1.5 and peak improvements occurring at Week 6.16
OUTCOME Continued ED visits
Unfortunately, Mr. H does not follow through with his outpatient psychiatry appointments. In the 7 months following discharge, he visits the ED 8 times for alcohol intoxication, alcohol withdrawal, and methamphetamine abuse, in addition to 2 admissions for inpatient detoxification, during which he was still receiving the same scheduled medications (haloperidol 15 mg/d and quetiapine 200 mg/d). At each of his ED visits, there was no documentation of DP symptoms, which suggests his symptoms may have resolved.
Bottom Line
Because delusional parasitosis symptoms feel real to patients, it is crucial to build rapport to recommend and successfully initiate treatment. After ruling out nonpsychiatric etiologies, consider traditional treatment with antipsychotics, and consider medications for relief of pruritus or pain.
Related Resources
- Sellman D, Phan SV, Inyang M. Bugs on her skin—but nobody else sees them. Current Psychiatry. 2018;17(8):48,50-53.
- Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434. doi:10.1016/j.jaad.2018.12.012
Drug Brand Names
Buprenorphine/naloxone • Suboxone
Haloperidol • Haldol
Hydroxyzine • Vistaril
Lithium • Eskalith, Lithobid
Methylphenidate • Concerta
Olanzapine • Zyprexa
Permethrin • Elimite
Phenobarbital • Solfoton, Tedral, Luminal
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakote
CASE Detoxification and preoccupation with parasites
Mr. H, age 51, has an extensive history of alcohol and methamphetamine use. He presents to the emergency department (ED) requesting inpatient detoxification. He says he had been drinking alcohol but is unable to say how much. His blood ethanol level is 61 mg/dL (unintoxicated level: <50 mg/dL), and a urine drug screen is positive for methamphetamine; Mr. H also admits to using fentanyl. The ED team treats Mr. H’s electrolyte abnormalities, initiates thiamine supplementation, and transfers him to a unit for inpatient withdrawal management.
On the detoxification unit, Mr. H receives a total of 1,950 mg of phenobarbital for alcohol withdrawal and stabilizes on a buprenorphine/naloxone maintenance dose of 8 mg/2 mg twice daily for methamphetamine and fentanyl use. Though he was not taking any psychiatric medications prior to his arrival at the ED, Mr. H agrees to restart quetiapine
During Mr. H’s 3-day detoxification, the psychiatry team evaluates him. Mr. H says he believes he is infested with worms. He describes a prior sensation of “meth mites,” or the feeling of bugs crawling under his skin, while using methamphetamines. However, Mr. H says his current infestation feels distinctively different, and he had continued to experience these
The psychiatry team expresses concern over his preoccupation with infestations, disheveled appearance, poor hygiene, and healed scars from excoriation. Mr. H also reports poor sleep and appetite and was observed writing an incomprehensible “experiment” on a paper towel. Due to his bizarre behavior, delusional thoughts, and concerns about his inability to care for himself, the team admits Mr. H to the acute inpatient psychiatric unit on a voluntary commitment.
HISTORY Long-standing drug use and repeated hospital visits
Mr. H reports a history of drug use. His first documented ED visit was >5 years before his current admission. He has a family history of substance abuse and reports previously using methamphetamine, heroin, and alcohol. Mr. H was never diagnosed with a psychiatric illness, but when he was younger, there were suspicions of bipolar depression, with no contributing family psychiatric history. Though he took quetiapine at an unspecified younger age, Mr. H did not follow through with any outpatient mental health services or medications.
Mr. H first reported infestation
In the 6 months prior to his current admission, Mr. H came to the hospital >20 times for various reasons, including methamphetamine abuse, alcohol withdrawal, opiate overdose, cellulitis, wound checks, and 3 visits for hallucinations for which he requested physical evaluation and medical care. His substance use was the suspected cause of his tactile and visual hallucinations of infestation because formication
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis (DP), also known as delusional infestation or Ekbom Syndrome, is a condition characterized by the fixed, false belief of an infestation without any objective evidence. This condition was previously defined in DSM-IV, but was removed from DSM-5-TR. In DSM-5-TR, DP is most closely associated with delusional disorder
DP is rare, affecting approximately 1.9 per 100,000 people. There has not been consistent data supporting differences in prevalence between sexes, but there is evidence for increasing incidence with age, with a mean age of diagnosis of 61.4.2,3 DP can be divided into 2 types based on the history and etiology of the symptoms: primary DP and secondary DP. Primary DP occurs when there is a failure to identify an organic cause for the occurrence of the symptoms. Therefore, primary DP requires an extensive investigation by a multidisciplinary team that commonly includes medical specialists for a nonpsychiatric workup. Secondary DP occurs when the patient has delusional symptoms associated with a primary diagnosis of schizophrenia, depression, stroke, diabetes, vitamin B12 deficiency, or substance use.4
Though Mr. H initially presented to the ED, patients with DP commonly present to a primary care physician or dermatologist with the complaint of itching or feelings of insects, worms, or unclear organisms inside them. Patients with DP may often develop poor working relationships with physicians while obtaining multiple negative results. They may seek opinions from multiple specialists; however, patients typically do not consider psychiatrists as a source of help. When patients seek psychiatric care, often after a recommendation from a primary care physician or dermatologist, mental health clinicians should listen to and evaluate the patient holistically, continuing to rule out other possible etiologies.
[polldaddy:12570072]
TREATMENT Finding the right antipsychotic
In the psychiatric unit, Mr. H says he believes worms are exiting his ears, mouth, toenail, and self-inflicted scratch wounds. He believes he has been dealing with the parasites for >1 year and they are slowly draining his energy. Mr. H insists he contracted the “infection” from his home carpet, which was wet due to a flood in his house, and after he had fallen asleep following drug use. He also believes he acquired the parasites while walking barefoot along the beach and collecting rocks, and that there are multiple species living inside him, all intelligent enough to hide, making it difficult to prove their existence. He notes they vary in size, and some have red eyes.
During admission, Mr. H voices his frustration that clinicians had not found the worms he has been seeing. He continuously requests to review imaging performed during his visit and wants a multidisciplinary team to evaluate his case. He demands to test a cup with spit-up “samples,” believing the parasites would be visible under a microscope. Throughout his admission, Mr. H continues to take buprenorphine/naloxone and does not experience withdrawal symptoms. The treatment team titrates his quetiapine to 400 mg/d. Due to the lack of improvement, the team initiates olanzapine 5 mg/d at bedtime. However, Mr. H reports significant tinnitus and requests a medication change. He is started on haloperidol 5 mg twice daily.
Continue to: Mr. H begins to see improvements...
Mr. H begins to see improvements on Day 7 of taking haloperidol. He no longer brings up infestation but still acknowledges having worms inside him when directly asked. He says the worms cause him less distress than before and he is hopeful to live without discomfort. He also demonstrates an ability to conduct activities of daily living. Because Mr. H is being monitored on an acute inpatient psychiatric basis, he is deemed appropriate for discharge even though his symptoms have not yet fully resolved. After a 19-day hospital stay, Mr. H is discharged on haloperidol 15 mg/d and quetiapine 200 mg/d.
[polldaddy:12570074]
The authors’ observations
Mr. H asked to have his sputum examined. The “specimen sign,” also called “matchbox sign” or “Ziploc bag sign,” in which patients collect what they believe to be infected tissue or organisms in a container, is a well-studied part of DP.5 Such samples should be considered during initial encounters and can be examined for formal evaluation, but cautiously. Overtesting may incur a financial burden or reinforce deleterious beliefs and behaviors.
It can be difficult to identify triggers of DP. Research shows DP may arise from nonorganic and stressful life events, home floods, or contact with people infected with parasites.6,7 Organic causes have also been found, such as patients taking multiple medications for Parkinson disease who developed delusional symptoms.8 Buscarino et al9 reported the case of a woman who started to develop symptoms of delusions and hallucinations after being on high-dose amphetamines for attention-deficit/hyperactivity disorder. Research shows that stopping the suspected medication commonly improves such symptoms.9,10 Although methamphetamine can remain detectable in urine for up to 4 days after use and potentially a few days longer for chronic users due to circulating levels,11 Mr. H’s symptoms continued for weeks after all substances of abuse should have been cleared from his system. This suggests he was experiencing a psychiatric illness and was accurate in distinguishing methamphetamine-induced from psychiatric-induced sensations. Regardless, polysubstance use has been shown to potentially increase the risk and play a role in the onset and progression of delusional illness, as seen in prior cases as well as in this case.9
It has been hypothesized that the pathophysiology of DP is associated with the deterioration of the striatal dopaminergic pathway, leading to an increase in extracellular dopamine levels. The striatum is responsible for most dopamine reuptake in the brain; therefore, certain drugs such as cocaine, methamphetamine, and methylphenidate may precipitate symptoms of DP due to their blockade of presynaptic dopamine reuptake.12 Additionally, conditions that decrease the functioning of striatal dopamine transporters, such as schizophrenia or depression, may be underlying causes of DP.13
Treatment of DP remains a topic of debate. Most current recommendations appear to be based on a small, nonrandomized placebo-controlled trial.14 The first-generation antipsychotic pimozide had been a first-line treatment for DP, but its adverse effect profile, which includes QTc prolongation and extrapyramidal symptoms, led to the exploration of second-generation antipsychotics such as olanzapine and risperidone.15,16 There is a dearth of literature about the use of haloperidol, quetiapine, or a combination of both as treatment options for DP, though the combination of these 2 medications proved effective for Mr. H. Further research is necessary to justify changes to current treatment standards, but this finding highlights a successful symptom reduction achieved with this combination.
Continue to: Patients may experience genuine symptoms...
Patients may experience genuine symptoms despite the delusional nature of DP, and it is important for clinicians to recognize the potential burden and anxiety these individuals face. Patients may present with self-inflicted bruises, cuts, and erosions to gain access to infected areas, which may be confused with skin picking disorder. Excessive cleansing or use of irritant products can also cause skin damage, leading to other dermatological conditions that reinforce the patient’s belief that something is medically wrong. During treatment, consider medications for relief of pruritus or pain. Focus on offering patients the opportunity to express their concerns, treat them with empathy, avoid stigmatizing language such as “delusions” or “psychosis,” and refrain from contradicting them until a strong rapport has been established (Table 217).
Symptoms of DP can persist for months to years. Patients who fully recovered experienced a median duration of 0.5 years until symptom resolution, compared to incompletely recovered patients, who took approximately 1 year.18 Primary DP has slower improvement rates compared to secondary DP, with the median onset of effects occurring at Week 1.5 and peak improvements occurring at Week 6.16
OUTCOME Continued ED visits
Unfortunately, Mr. H does not follow through with his outpatient psychiatry appointments. In the 7 months following discharge, he visits the ED 8 times for alcohol intoxication, alcohol withdrawal, and methamphetamine abuse, in addition to 2 admissions for inpatient detoxification, during which he was still receiving the same scheduled medications (haloperidol 15 mg/d and quetiapine 200 mg/d). At each of his ED visits, there was no documentation of DP symptoms, which suggests his symptoms may have resolved.
Bottom Line
Because delusional parasitosis symptoms feel real to patients, it is crucial to build rapport to recommend and successfully initiate treatment. After ruling out nonpsychiatric etiologies, consider traditional treatment with antipsychotics, and consider medications for relief of pruritus or pain.
Related Resources
- Sellman D, Phan SV, Inyang M. Bugs on her skin—but nobody else sees them. Current Psychiatry. 2018;17(8):48,50-53.
- Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434. doi:10.1016/j.jaad.2018.12.012
Drug Brand Names
Buprenorphine/naloxone • Suboxone
Haloperidol • Haldol
Hydroxyzine • Vistaril
Lithium • Eskalith, Lithobid
Methylphenidate • Concerta
Olanzapine • Zyprexa
Permethrin • Elimite
Phenobarbital • Solfoton, Tedral, Luminal
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakote
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2013.
2. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976-2010. Br J Dermatol. 2014;170(5):1130-1135. doi:10.1111/bjd.12848
3. Kohorst JJ, Bailey CH, Andersen LK, et al. Prevalence of delusional infestation-a population-based study. JAMA Dermatol. 2018;154(5):615-617. doi:10.1001/jamadermatol.2018.0004
4. Freinhar JP. Delusions of parasitosis. Psychosomatics. 1984;25(1):47-53. doi:10.1016/S0033-3182(84)73096-9
5. Reich A, Kwiatkowska D, Pacan P. Delusions of parasitosis: an update. Dermatol Ther (Heidelb). 2019;9(4):631-638. doi:10.1007/s13555-019-00324-3
6. Berrios GE. Delusional parasitosis and physical disease. Compr Psychiatry. 1985;26(5):395-403. doi:10.1016/0010-440x(85)90077-x
7. Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry. 1991;159:716-717. doi:10.1192/bjp.159.5.716
8. Flann S, Shotbolt J, Kessel B, et al. Three cases of delusional parasitosis caused by dopamine agonists. Clin Exp Dermatol. 2010;35(7):740-742. doi:10.1111/j.1365-2230.2010.03810.x
9. Buscarino M, Saal J, Young JL. Delusional parasitosis in a female treated with mixed amphetamine salts: a case report and literature review. Case Rep Psychiatry. 2012;2012:624235. doi:10.1155/2012/624235
10. Elpern DJ. Cocaine abuse and delusions of parasitosis. Cutis. 1988;42(4):273-274.
11. Richards JR, Laurin EG. Methamphetamine toxicity. StatPearls Publishing; 2023. Updated January 8, 2023. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430895/
12. Huber M, Kirchler E, Karner M, et al. Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med Hypotheses. 2007;68(6):1351-1358. doi:10.1016/j.mehy.2006.07.061
13. Lipman ZM, Yosipovitch G. Substance use disorders and chronic itch. J Am Acad Dermatol. 2021;84(1):148-155. doi:10.1016/j.jaad.2020.08.117
14. Kenchaiah BK, Kumar S, Tharyan P. Atypical anti-psychotics in delusional parasitosis: a retrospective case series of 20 patients. Int J Dermatol. 2010;49(1):95-100. doi:10.1111/j.1365-4632.2009.04312.x
15. Laidler N. Delusions of parasitosis: a brief review of the literature and pathway for diagnosis and treatment. Dermatol Online J. 2018;24(1):13030/qt1fh739nx.
16. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508. doi:10.1097/JCP.0b013e318185e774
17. Mumcuoglu KY, Leibovici V, Reuveni I, et al. Delusional parasitosis: diagnosis and treatment. Isr Med Assoc J. 2018;20(7):456-460.
18. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246. doi:10.1159/000284934
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2013.
2. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976-2010. Br J Dermatol. 2014;170(5):1130-1135. doi:10.1111/bjd.12848
3. Kohorst JJ, Bailey CH, Andersen LK, et al. Prevalence of delusional infestation-a population-based study. JAMA Dermatol. 2018;154(5):615-617. doi:10.1001/jamadermatol.2018.0004
4. Freinhar JP. Delusions of parasitosis. Psychosomatics. 1984;25(1):47-53. doi:10.1016/S0033-3182(84)73096-9
5. Reich A, Kwiatkowska D, Pacan P. Delusions of parasitosis: an update. Dermatol Ther (Heidelb). 2019;9(4):631-638. doi:10.1007/s13555-019-00324-3
6. Berrios GE. Delusional parasitosis and physical disease. Compr Psychiatry. 1985;26(5):395-403. doi:10.1016/0010-440x(85)90077-x
7. Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry. 1991;159:716-717. doi:10.1192/bjp.159.5.716
8. Flann S, Shotbolt J, Kessel B, et al. Three cases of delusional parasitosis caused by dopamine agonists. Clin Exp Dermatol. 2010;35(7):740-742. doi:10.1111/j.1365-2230.2010.03810.x
9. Buscarino M, Saal J, Young JL. Delusional parasitosis in a female treated with mixed amphetamine salts: a case report and literature review. Case Rep Psychiatry. 2012;2012:624235. doi:10.1155/2012/624235
10. Elpern DJ. Cocaine abuse and delusions of parasitosis. Cutis. 1988;42(4):273-274.
11. Richards JR, Laurin EG. Methamphetamine toxicity. StatPearls Publishing; 2023. Updated January 8, 2023. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430895/
12. Huber M, Kirchler E, Karner M, et al. Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med Hypotheses. 2007;68(6):1351-1358. doi:10.1016/j.mehy.2006.07.061
13. Lipman ZM, Yosipovitch G. Substance use disorders and chronic itch. J Am Acad Dermatol. 2021;84(1):148-155. doi:10.1016/j.jaad.2020.08.117
14. Kenchaiah BK, Kumar S, Tharyan P. Atypical anti-psychotics in delusional parasitosis: a retrospective case series of 20 patients. Int J Dermatol. 2010;49(1):95-100. doi:10.1111/j.1365-4632.2009.04312.x
15. Laidler N. Delusions of parasitosis: a brief review of the literature and pathway for diagnosis and treatment. Dermatol Online J. 2018;24(1):13030/qt1fh739nx.
16. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508. doi:10.1097/JCP.0b013e318185e774
17. Mumcuoglu KY, Leibovici V, Reuveni I, et al. Delusional parasitosis: diagnosis and treatment. Isr Med Assoc J. 2018;20(7):456-460.
18. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246. doi:10.1159/000284934
Serious complications due to ‘huffing’
CASE A relapse and crisis
Ms. G, age 32, is brought to the emergency department (ED) by police after being found in a stupor-like state in a public restroom. The consultation-liaison (CL) psychiatry team assesses her for concerns of self-harm and suicide behavior. Ms. G discloses that she “huffs” an average of 4 canisters of air dusters daily to cope with psychosocial stressors and achieve a euphoric state. She recently lost her job, which led to homelessness, financial difficulties, a relapse to aerosol use after 2 years of abstinence, and stealing aerosol cans. The latest incident follows 2 prior arrests, which led officers to bring her to the ED for medical evaluation. Ms. G has a history of bipolar disorder (BD), generalized anxiety disorder (GAD), insomnia, and inhalant use disorder.
HISTORY Inhalant abuse and suicide attempt
Ms. G reports a longstanding history of severe inhalant abuse, primarily with air dusters due to their accessibility and low cost. She previously underwent inpatient rehab for inhalant abuse, and received inpatient psychiatry treatment 5 years ago for a suicide attempt by overdose linked to psychosocial stressors. In addition to BD, GAD, insomnia, and inhalant use disorder, Ms. G has a history of neuropathy, seizures, and recurrent hypokalemia. She is single and does not have insurance.
[polldaddy:12318871]
The authors’ observations
Inhalant abuse is the intentional inhalation of volatile substances to achieve an altered mental state. Inhalants are commercially available products that can produce intoxication if inhaled, such as glue, toluene, spray paint, gasoline, and lighter fluid (Table 11).
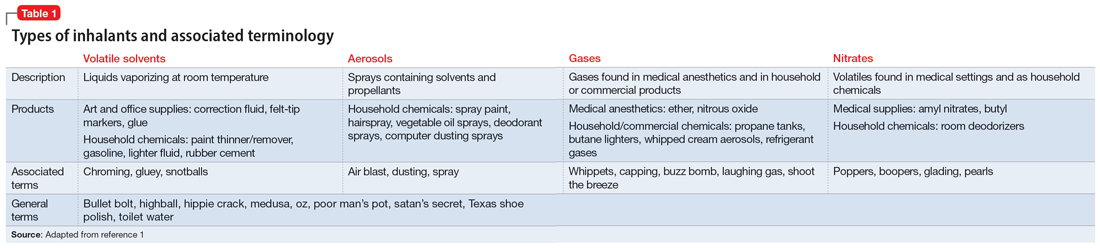
The epidemiology of inhalant abuse is difficult to accurately report due to a lack of recognition and social stigma. Due to inhalants’ ease of access and low cost, this form of substance abuse is popular among adolescents, adults of low socioeconomic status, individuals who live in rural areas, and those living in institutions. Inhalants act as reinforcers, producing a euphoric state. Rapid pulmonary absorption and lipid solubility of the substance rapidly alters the brain. Inhalant abuse can result in chemical and thermal burns, withdrawal symptoms, persistent mental illness, and catastrophic medical emergencies such as ventricular arrhythmias leading to disruptive myocardial electrical propagation. Chronic abuse can cause irreversible neurological and neuropsychological effects, cardiomyopathy, rapid airway compromise, pulmonary debilitations, renal tubular acidosis, bone marrow toxicity, reduced immunity, and peripheral neuropathy.2 Ms. G’s diagnosis of inhalant use disorder was based on her mental state and history of severe inhalant misuse, specifically with air dusters. Several additional factors further support this diagnosis, including the fact she survived a suicide attempt by overdose 5 years ago, had an inpatient rehabilitation placement for inhalant abuse, experiences insomnia, and was attempting to self-treat a depressive episode relapse with inhalants.
EVALUATION Depressed but cooperative
After being monitored in the ED for several hours, Ms. G is no longer in a stupor-like state. She has poor body habitus, appears older than her stated age, and is unkempt in appearance/attire. She is mildly distressed but relatively cooperative and engaged during the interview. Ms. G has a depressed mood and is anxious, with mood-congruent affect, and is tearful at times, especially when discussing recent stressors. She denies suicidality, homicidality, paranoia, delusions, and hallucinations. Her thought process is linear, goal-directed, and logical. She has fair insight, but relatively poor and impulsive judgment. The nursing staff expresses concerns that Ms. G was possibly responding to internal stimuli and behaving bizarrely during her initial presentation; this was not evident upon examination.
Ms. G reports having acute-on-chronic headaches, intermittent myalgias and weakness in her lower extremities (acute), and polyneuropathy (chronic). She denies a history of manic episodes or psychosis but reports previous relative hypomanic episodes that vacillated with periods of recurrent depressive episodes. Ms. G denies using illicit substances other than tobacco and inhalants. She says she had adhered to her outpatient psychiatric management services and medication regimen (duloxetine 60 mg/d at bedtime for mood/migraines, trazodone 150 mg/d at bedtime for insomnia, ziprasidone 40 mg/d at bedtime for BD, carbamazepine 200 mg twice daily for neuropathy/migraines, gabapentin 400 mg 3 times daily for neuropathy migraines/anxiety, and propranolol 10 mg 3 times daily for anxiety/tremors/migraine prophylaxis) until 4 days before her current presentation to the ED, when she used inhalants and was arrested.
Ms. G’s vitals are mostly unremarkable, but her heart rate is 116 beats per minute. There are no acute findings on physical examination. She is not pregnant, and her creatinine, glomerular filtration rate, complete blood count, and thyroid-stimulating hormone are all within normal limits. Her blood sugar is high (120 mg/dL; reference range 70 to 100 mg/dL). She has slight transaminitis with high aspartate aminotransferase (93 U/L; reference range 17 to 59 U/L) and high alanine aminotransferase (69 U/L; reference range 20 to 35 U/L); chronic hypokalemia (2.4 mmol/L; reference range 3.5 to 5.2 mmol/L), which leads the primary team to initiate a potassium replacement protocol; lactic acidosis (2.2 mmol/L; normal levels <2 mmol/L); and creatine kinase (CK) 5,930 U/L.
[polldaddy:12318873]
Continue to: The authors' observations
The authors’ observations
Efforts to improve the laboratory diagnosis of inhalant abuse are ongoing, but they have not yet been widely implemented. Systemic screening and assessment of inhalant use can help prevent and treat complications. For Ms. G, we considered several possible complications, including hypoglycemia. Although the classic triad of myalgia, weakness, and myoglobinuria (tea-colored urine) was not present, elevated CK levels in the context of Ms. G’s intermittent myalgia and lower extremity weakness led us to suspect she was experiencing moderate rhabdomyolysis (Table 23).
Rhabdomyolysis can be caused by several factors, including drug abuse, trauma, neuromuscular syndrome, and immobility. Treatment is mainly supportive, with a focus on preserving the ABCs (airway, breathing, circulation) and renal function through vigorous rehydration.4 We postulated Ms. G’s rhabdomyolysis was caused by muscle damage directly resulting from inhalant abuse and compounded by her remaining in prolonged fixed position on the ground after overdosing on inhalants.
TREATMENT Rehydration and psychotropics
The treatment team initiates IV fluid hydration of chloride 0.9% 150 mL/h and monitors Ms. G until she is stable and the trajectory of her CK levels begins to decline. On hospital Day 2, Ms. G’s CK decreases to 2,475 U/L and her lactic acid levels normalize. Ms. G restarts her regimen of duloxetine 60 mg/d, trazodone 150 mg/d, ziprasidone 40 mg/d, carbamazepine 200 mg twice daily, gabapentin 400 mg 3 times daily, and propranolol 10 mg 3 times daily. The team adds quetiapine 25 mg as needed for hallucinations, paranoia, and/or anxiety. Ms. G is closely monitored due to the potential risk of toxicity-induced or withdrawal-induced psychotic symptoms.
[polldaddy:12318869]
The authors’ observations
Presently, there are no effective treatments for acute inhalant intoxication or withdrawal, which makes supportive care and vigilant monitoring the only options.5 Although clinical research has not led to any FDA-approved treatments for chronic inhalant use disorder, a multipronged biopsychosocial treatment approach is critical in light of the negative consequences of inhalant abuse, including poor academic performance, criminal behavior, abuse of other substances, social maladjustment, low self-esteem, and suicidality.6
Ms. G had a moderate form of rhabdomyolysis, which was managed with IV fluid rehydration. Education and counseling were crucial to help Ms. G understand the unintended complications and potentially life-threatening consequences of inhalant abuse, with rehabilitation services to encourage abstinence. Ms. G had previously undergone successful inpatient rehabilitation and was willing to start such services again. She reported success with gabapentin for her polyneuropathy and migraines, which may be long-term consequences of prolonged inhalant abuse with neurological lesions. Ziprasidone may have mitigated some of the impulsivity and hypomanic symptoms of her BD that could make her more likely to engage in risky self-harm behaviors.
Continue to: After extensive discussion...
After extensive discussion on the long-term complications of inhalant abuse, Ms. G was motivated, cooperative, and sought care to return to rehabilitation services. The CL psychiatry team collaborated with the social work team to address the psychosocial components of Ms. G’s homelessness and facilitated an application for a local resource to obtain rehabilitation placement and living assistance. Her years of abstinence from inhalant use and success with rehabilitation demonstrate the need for a multimodal approach to manage and treat inhalant use disorder. Outpatient follow-up arrangements were made with local mental health resources.
OUTCOME Improved outlook and discharge
Ms. G reports improved mood and willingness to change her substance use habits. The treatment team counsels her on the acute risk of fatal arrhythmias and end-organ complications of inhalant abuse. They warn her about the potential long-term effects of mood alterations, neurological lesions, and polyneuropathy that could possibly worsen with substance abuse. Ms. G expresses appreciation for this counseling, the help associated with her aftercare, and the referral to restart the 30-day inpatient rehabilitation services. The team arranges follow-up with outpatient psychiatry and outpatient therapy services to enhance Ms. G’s coping skills and mitigate her reliance on inhalants to regulate her mood.
Bottom Line
Inhalant use is a poorly understood form of substance abuse that disproportionately affects vulnerable populations. It can lead to life-threatening medical emergencies such as rhabdomyolysis. Clinicians need to be able to identify and manage inhalant abuse and associated complications, as well as provide appropriate education and counseling to prevent further misuse.
Related Resources
- Gude J, Bisen V, Fujii K. Medication-induced rhabdomyolysis. Current Psychiatry. 2023;22(2):39-40. doi:10.12788/cp.0332
- Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity--a Euro-DEN study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal. pone.0246297
Drug Brand Names
Carbamazepine • Tegretol
Duloxetine • Cymbalta
Gabapentin • Neurontin
Propranolol • Inderal
Quetiapine • Seroquel
Trazodone • Oleptro
Ziprasidone • Geodon
1. Ahern NR, Falsafi N. Inhalant abuse: youth at risk. J Psychosoc Nurs Ment Health Serv. 2013;51(8):19-24. doi:10.3928/02793695-20130612-02
2. Howard MO, Bowen SE, Garland EL, et al. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Prac. 2011;6(1):18-31.
3. Farkas J. Rhabdomyolysis. Internet Book of Critical Care. June 25, 2021. Accessed February 24, 2023. https://emcrit.org/ibcc/rhabdo/
4. Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
5. Muller AA, Muller GF. Inhalant abuse. J Emerg Nurs. 2006;32(5):447-448. doi:10.1016/j.jen.2006.05.018
6. Kozel N, Sloboda Z, De La Rosa M, eds. Epidemiology of Inhalant Abuse: An International Perspective; Nida Research Monograph 148. National Institute on Drug Abuse Research, US Dept of Health and Human Services; 1995. Accessed April 20, 2023. https://archives.nida.nih.gov/sites/default/files/monograph148.pdf
CASE A relapse and crisis
Ms. G, age 32, is brought to the emergency department (ED) by police after being found in a stupor-like state in a public restroom. The consultation-liaison (CL) psychiatry team assesses her for concerns of self-harm and suicide behavior. Ms. G discloses that she “huffs” an average of 4 canisters of air dusters daily to cope with psychosocial stressors and achieve a euphoric state. She recently lost her job, which led to homelessness, financial difficulties, a relapse to aerosol use after 2 years of abstinence, and stealing aerosol cans. The latest incident follows 2 prior arrests, which led officers to bring her to the ED for medical evaluation. Ms. G has a history of bipolar disorder (BD), generalized anxiety disorder (GAD), insomnia, and inhalant use disorder.
HISTORY Inhalant abuse and suicide attempt
Ms. G reports a longstanding history of severe inhalant abuse, primarily with air dusters due to their accessibility and low cost. She previously underwent inpatient rehab for inhalant abuse, and received inpatient psychiatry treatment 5 years ago for a suicide attempt by overdose linked to psychosocial stressors. In addition to BD, GAD, insomnia, and inhalant use disorder, Ms. G has a history of neuropathy, seizures, and recurrent hypokalemia. She is single and does not have insurance.
[polldaddy:12318871]
The authors’ observations
Inhalant abuse is the intentional inhalation of volatile substances to achieve an altered mental state. Inhalants are commercially available products that can produce intoxication if inhaled, such as glue, toluene, spray paint, gasoline, and lighter fluid (Table 11).

The epidemiology of inhalant abuse is difficult to accurately report due to a lack of recognition and social stigma. Due to inhalants’ ease of access and low cost, this form of substance abuse is popular among adolescents, adults of low socioeconomic status, individuals who live in rural areas, and those living in institutions. Inhalants act as reinforcers, producing a euphoric state. Rapid pulmonary absorption and lipid solubility of the substance rapidly alters the brain. Inhalant abuse can result in chemical and thermal burns, withdrawal symptoms, persistent mental illness, and catastrophic medical emergencies such as ventricular arrhythmias leading to disruptive myocardial electrical propagation. Chronic abuse can cause irreversible neurological and neuropsychological effects, cardiomyopathy, rapid airway compromise, pulmonary debilitations, renal tubular acidosis, bone marrow toxicity, reduced immunity, and peripheral neuropathy.2 Ms. G’s diagnosis of inhalant use disorder was based on her mental state and history of severe inhalant misuse, specifically with air dusters. Several additional factors further support this diagnosis, including the fact she survived a suicide attempt by overdose 5 years ago, had an inpatient rehabilitation placement for inhalant abuse, experiences insomnia, and was attempting to self-treat a depressive episode relapse with inhalants.
EVALUATION Depressed but cooperative
After being monitored in the ED for several hours, Ms. G is no longer in a stupor-like state. She has poor body habitus, appears older than her stated age, and is unkempt in appearance/attire. She is mildly distressed but relatively cooperative and engaged during the interview. Ms. G has a depressed mood and is anxious, with mood-congruent affect, and is tearful at times, especially when discussing recent stressors. She denies suicidality, homicidality, paranoia, delusions, and hallucinations. Her thought process is linear, goal-directed, and logical. She has fair insight, but relatively poor and impulsive judgment. The nursing staff expresses concerns that Ms. G was possibly responding to internal stimuli and behaving bizarrely during her initial presentation; this was not evident upon examination.
Ms. G reports having acute-on-chronic headaches, intermittent myalgias and weakness in her lower extremities (acute), and polyneuropathy (chronic). She denies a history of manic episodes or psychosis but reports previous relative hypomanic episodes that vacillated with periods of recurrent depressive episodes. Ms. G denies using illicit substances other than tobacco and inhalants. She says she had adhered to her outpatient psychiatric management services and medication regimen (duloxetine 60 mg/d at bedtime for mood/migraines, trazodone 150 mg/d at bedtime for insomnia, ziprasidone 40 mg/d at bedtime for BD, carbamazepine 200 mg twice daily for neuropathy/migraines, gabapentin 400 mg 3 times daily for neuropathy migraines/anxiety, and propranolol 10 mg 3 times daily for anxiety/tremors/migraine prophylaxis) until 4 days before her current presentation to the ED, when she used inhalants and was arrested.
Ms. G’s vitals are mostly unremarkable, but her heart rate is 116 beats per minute. There are no acute findings on physical examination. She is not pregnant, and her creatinine, glomerular filtration rate, complete blood count, and thyroid-stimulating hormone are all within normal limits. Her blood sugar is high (120 mg/dL; reference range 70 to 100 mg/dL). She has slight transaminitis with high aspartate aminotransferase (93 U/L; reference range 17 to 59 U/L) and high alanine aminotransferase (69 U/L; reference range 20 to 35 U/L); chronic hypokalemia (2.4 mmol/L; reference range 3.5 to 5.2 mmol/L), which leads the primary team to initiate a potassium replacement protocol; lactic acidosis (2.2 mmol/L; normal levels <2 mmol/L); and creatine kinase (CK) 5,930 U/L.
[polldaddy:12318873]
Continue to: The authors' observations
The authors’ observations
Efforts to improve the laboratory diagnosis of inhalant abuse are ongoing, but they have not yet been widely implemented. Systemic screening and assessment of inhalant use can help prevent and treat complications. For Ms. G, we considered several possible complications, including hypoglycemia. Although the classic triad of myalgia, weakness, and myoglobinuria (tea-colored urine) was not present, elevated CK levels in the context of Ms. G’s intermittent myalgia and lower extremity weakness led us to suspect she was experiencing moderate rhabdomyolysis (Table 23).

Rhabdomyolysis can be caused by several factors, including drug abuse, trauma, neuromuscular syndrome, and immobility. Treatment is mainly supportive, with a focus on preserving the ABCs (airway, breathing, circulation) and renal function through vigorous rehydration.4 We postulated Ms. G’s rhabdomyolysis was caused by muscle damage directly resulting from inhalant abuse and compounded by her remaining in prolonged fixed position on the ground after overdosing on inhalants.
TREATMENT Rehydration and psychotropics
The treatment team initiates IV fluid hydration of chloride 0.9% 150 mL/h and monitors Ms. G until she is stable and the trajectory of her CK levels begins to decline. On hospital Day 2, Ms. G’s CK decreases to 2,475 U/L and her lactic acid levels normalize. Ms. G restarts her regimen of duloxetine 60 mg/d, trazodone 150 mg/d, ziprasidone 40 mg/d, carbamazepine 200 mg twice daily, gabapentin 400 mg 3 times daily, and propranolol 10 mg 3 times daily. The team adds quetiapine 25 mg as needed for hallucinations, paranoia, and/or anxiety. Ms. G is closely monitored due to the potential risk of toxicity-induced or withdrawal-induced psychotic symptoms.
[polldaddy:12318869]
The authors’ observations
Presently, there are no effective treatments for acute inhalant intoxication or withdrawal, which makes supportive care and vigilant monitoring the only options.5 Although clinical research has not led to any FDA-approved treatments for chronic inhalant use disorder, a multipronged biopsychosocial treatment approach is critical in light of the negative consequences of inhalant abuse, including poor academic performance, criminal behavior, abuse of other substances, social maladjustment, low self-esteem, and suicidality.6
Ms. G had a moderate form of rhabdomyolysis, which was managed with IV fluid rehydration. Education and counseling were crucial to help Ms. G understand the unintended complications and potentially life-threatening consequences of inhalant abuse, with rehabilitation services to encourage abstinence. Ms. G had previously undergone successful inpatient rehabilitation and was willing to start such services again. She reported success with gabapentin for her polyneuropathy and migraines, which may be long-term consequences of prolonged inhalant abuse with neurological lesions. Ziprasidone may have mitigated some of the impulsivity and hypomanic symptoms of her BD that could make her more likely to engage in risky self-harm behaviors.
Continue to: After extensive discussion...
After extensive discussion on the long-term complications of inhalant abuse, Ms. G was motivated, cooperative, and sought care to return to rehabilitation services. The CL psychiatry team collaborated with the social work team to address the psychosocial components of Ms. G’s homelessness and facilitated an application for a local resource to obtain rehabilitation placement and living assistance. Her years of abstinence from inhalant use and success with rehabilitation demonstrate the need for a multimodal approach to manage and treat inhalant use disorder. Outpatient follow-up arrangements were made with local mental health resources.
OUTCOME Improved outlook and discharge
Ms. G reports improved mood and willingness to change her substance use habits. The treatment team counsels her on the acute risk of fatal arrhythmias and end-organ complications of inhalant abuse. They warn her about the potential long-term effects of mood alterations, neurological lesions, and polyneuropathy that could possibly worsen with substance abuse. Ms. G expresses appreciation for this counseling, the help associated with her aftercare, and the referral to restart the 30-day inpatient rehabilitation services. The team arranges follow-up with outpatient psychiatry and outpatient therapy services to enhance Ms. G’s coping skills and mitigate her reliance on inhalants to regulate her mood.
Bottom Line
Inhalant use is a poorly understood form of substance abuse that disproportionately affects vulnerable populations. It can lead to life-threatening medical emergencies such as rhabdomyolysis. Clinicians need to be able to identify and manage inhalant abuse and associated complications, as well as provide appropriate education and counseling to prevent further misuse.
Related Resources
- Gude J, Bisen V, Fujii K. Medication-induced rhabdomyolysis. Current Psychiatry. 2023;22(2):39-40. doi:10.12788/cp.0332
- Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity--a Euro-DEN study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal. pone.0246297
Drug Brand Names
Carbamazepine • Tegretol
Duloxetine • Cymbalta
Gabapentin • Neurontin
Propranolol • Inderal
Quetiapine • Seroquel
Trazodone • Oleptro
Ziprasidone • Geodon
CASE A relapse and crisis
Ms. G, age 32, is brought to the emergency department (ED) by police after being found in a stupor-like state in a public restroom. The consultation-liaison (CL) psychiatry team assesses her for concerns of self-harm and suicide behavior. Ms. G discloses that she “huffs” an average of 4 canisters of air dusters daily to cope with psychosocial stressors and achieve a euphoric state. She recently lost her job, which led to homelessness, financial difficulties, a relapse to aerosol use after 2 years of abstinence, and stealing aerosol cans. The latest incident follows 2 prior arrests, which led officers to bring her to the ED for medical evaluation. Ms. G has a history of bipolar disorder (BD), generalized anxiety disorder (GAD), insomnia, and inhalant use disorder.
HISTORY Inhalant abuse and suicide attempt
Ms. G reports a longstanding history of severe inhalant abuse, primarily with air dusters due to their accessibility and low cost. She previously underwent inpatient rehab for inhalant abuse, and received inpatient psychiatry treatment 5 years ago for a suicide attempt by overdose linked to psychosocial stressors. In addition to BD, GAD, insomnia, and inhalant use disorder, Ms. G has a history of neuropathy, seizures, and recurrent hypokalemia. She is single and does not have insurance.
[polldaddy:12318871]
The authors’ observations
Inhalant abuse is the intentional inhalation of volatile substances to achieve an altered mental state. Inhalants are commercially available products that can produce intoxication if inhaled, such as glue, toluene, spray paint, gasoline, and lighter fluid (Table 11).

The epidemiology of inhalant abuse is difficult to accurately report due to a lack of recognition and social stigma. Due to inhalants’ ease of access and low cost, this form of substance abuse is popular among adolescents, adults of low socioeconomic status, individuals who live in rural areas, and those living in institutions. Inhalants act as reinforcers, producing a euphoric state. Rapid pulmonary absorption and lipid solubility of the substance rapidly alters the brain. Inhalant abuse can result in chemical and thermal burns, withdrawal symptoms, persistent mental illness, and catastrophic medical emergencies such as ventricular arrhythmias leading to disruptive myocardial electrical propagation. Chronic abuse can cause irreversible neurological and neuropsychological effects, cardiomyopathy, rapid airway compromise, pulmonary debilitations, renal tubular acidosis, bone marrow toxicity, reduced immunity, and peripheral neuropathy.2 Ms. G’s diagnosis of inhalant use disorder was based on her mental state and history of severe inhalant misuse, specifically with air dusters. Several additional factors further support this diagnosis, including the fact she survived a suicide attempt by overdose 5 years ago, had an inpatient rehabilitation placement for inhalant abuse, experiences insomnia, and was attempting to self-treat a depressive episode relapse with inhalants.
EVALUATION Depressed but cooperative
After being monitored in the ED for several hours, Ms. G is no longer in a stupor-like state. She has poor body habitus, appears older than her stated age, and is unkempt in appearance/attire. She is mildly distressed but relatively cooperative and engaged during the interview. Ms. G has a depressed mood and is anxious, with mood-congruent affect, and is tearful at times, especially when discussing recent stressors. She denies suicidality, homicidality, paranoia, delusions, and hallucinations. Her thought process is linear, goal-directed, and logical. She has fair insight, but relatively poor and impulsive judgment. The nursing staff expresses concerns that Ms. G was possibly responding to internal stimuli and behaving bizarrely during her initial presentation; this was not evident upon examination.
Ms. G reports having acute-on-chronic headaches, intermittent myalgias and weakness in her lower extremities (acute), and polyneuropathy (chronic). She denies a history of manic episodes or psychosis but reports previous relative hypomanic episodes that vacillated with periods of recurrent depressive episodes. Ms. G denies using illicit substances other than tobacco and inhalants. She says she had adhered to her outpatient psychiatric management services and medication regimen (duloxetine 60 mg/d at bedtime for mood/migraines, trazodone 150 mg/d at bedtime for insomnia, ziprasidone 40 mg/d at bedtime for BD, carbamazepine 200 mg twice daily for neuropathy/migraines, gabapentin 400 mg 3 times daily for neuropathy migraines/anxiety, and propranolol 10 mg 3 times daily for anxiety/tremors/migraine prophylaxis) until 4 days before her current presentation to the ED, when she used inhalants and was arrested.
Ms. G’s vitals are mostly unremarkable, but her heart rate is 116 beats per minute. There are no acute findings on physical examination. She is not pregnant, and her creatinine, glomerular filtration rate, complete blood count, and thyroid-stimulating hormone are all within normal limits. Her blood sugar is high (120 mg/dL; reference range 70 to 100 mg/dL). She has slight transaminitis with high aspartate aminotransferase (93 U/L; reference range 17 to 59 U/L) and high alanine aminotransferase (69 U/L; reference range 20 to 35 U/L); chronic hypokalemia (2.4 mmol/L; reference range 3.5 to 5.2 mmol/L), which leads the primary team to initiate a potassium replacement protocol; lactic acidosis (2.2 mmol/L; normal levels <2 mmol/L); and creatine kinase (CK) 5,930 U/L.
[polldaddy:12318873]
Continue to: The authors' observations
The authors’ observations
Efforts to improve the laboratory diagnosis of inhalant abuse are ongoing, but they have not yet been widely implemented. Systemic screening and assessment of inhalant use can help prevent and treat complications. For Ms. G, we considered several possible complications, including hypoglycemia. Although the classic triad of myalgia, weakness, and myoglobinuria (tea-colored urine) was not present, elevated CK levels in the context of Ms. G’s intermittent myalgia and lower extremity weakness led us to suspect she was experiencing moderate rhabdomyolysis (Table 23).

Rhabdomyolysis can be caused by several factors, including drug abuse, trauma, neuromuscular syndrome, and immobility. Treatment is mainly supportive, with a focus on preserving the ABCs (airway, breathing, circulation) and renal function through vigorous rehydration.4 We postulated Ms. G’s rhabdomyolysis was caused by muscle damage directly resulting from inhalant abuse and compounded by her remaining in prolonged fixed position on the ground after overdosing on inhalants.
TREATMENT Rehydration and psychotropics
The treatment team initiates IV fluid hydration of chloride 0.9% 150 mL/h and monitors Ms. G until she is stable and the trajectory of her CK levels begins to decline. On hospital Day 2, Ms. G’s CK decreases to 2,475 U/L and her lactic acid levels normalize. Ms. G restarts her regimen of duloxetine 60 mg/d, trazodone 150 mg/d, ziprasidone 40 mg/d, carbamazepine 200 mg twice daily, gabapentin 400 mg 3 times daily, and propranolol 10 mg 3 times daily. The team adds quetiapine 25 mg as needed for hallucinations, paranoia, and/or anxiety. Ms. G is closely monitored due to the potential risk of toxicity-induced or withdrawal-induced psychotic symptoms.
[polldaddy:12318869]
The authors’ observations
Presently, there are no effective treatments for acute inhalant intoxication or withdrawal, which makes supportive care and vigilant monitoring the only options.5 Although clinical research has not led to any FDA-approved treatments for chronic inhalant use disorder, a multipronged biopsychosocial treatment approach is critical in light of the negative consequences of inhalant abuse, including poor academic performance, criminal behavior, abuse of other substances, social maladjustment, low self-esteem, and suicidality.6
Ms. G had a moderate form of rhabdomyolysis, which was managed with IV fluid rehydration. Education and counseling were crucial to help Ms. G understand the unintended complications and potentially life-threatening consequences of inhalant abuse, with rehabilitation services to encourage abstinence. Ms. G had previously undergone successful inpatient rehabilitation and was willing to start such services again. She reported success with gabapentin for her polyneuropathy and migraines, which may be long-term consequences of prolonged inhalant abuse with neurological lesions. Ziprasidone may have mitigated some of the impulsivity and hypomanic symptoms of her BD that could make her more likely to engage in risky self-harm behaviors.
Continue to: After extensive discussion...
After extensive discussion on the long-term complications of inhalant abuse, Ms. G was motivated, cooperative, and sought care to return to rehabilitation services. The CL psychiatry team collaborated with the social work team to address the psychosocial components of Ms. G’s homelessness and facilitated an application for a local resource to obtain rehabilitation placement and living assistance. Her years of abstinence from inhalant use and success with rehabilitation demonstrate the need for a multimodal approach to manage and treat inhalant use disorder. Outpatient follow-up arrangements were made with local mental health resources.
OUTCOME Improved outlook and discharge
Ms. G reports improved mood and willingness to change her substance use habits. The treatment team counsels her on the acute risk of fatal arrhythmias and end-organ complications of inhalant abuse. They warn her about the potential long-term effects of mood alterations, neurological lesions, and polyneuropathy that could possibly worsen with substance abuse. Ms. G expresses appreciation for this counseling, the help associated with her aftercare, and the referral to restart the 30-day inpatient rehabilitation services. The team arranges follow-up with outpatient psychiatry and outpatient therapy services to enhance Ms. G’s coping skills and mitigate her reliance on inhalants to regulate her mood.
Bottom Line
Inhalant use is a poorly understood form of substance abuse that disproportionately affects vulnerable populations. It can lead to life-threatening medical emergencies such as rhabdomyolysis. Clinicians need to be able to identify and manage inhalant abuse and associated complications, as well as provide appropriate education and counseling to prevent further misuse.
Related Resources
- Gude J, Bisen V, Fujii K. Medication-induced rhabdomyolysis. Current Psychiatry. 2023;22(2):39-40. doi:10.12788/cp.0332
- Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity--a Euro-DEN study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal. pone.0246297
Drug Brand Names
Carbamazepine • Tegretol
Duloxetine • Cymbalta
Gabapentin • Neurontin
Propranolol • Inderal
Quetiapine • Seroquel
Trazodone • Oleptro
Ziprasidone • Geodon
1. Ahern NR, Falsafi N. Inhalant abuse: youth at risk. J Psychosoc Nurs Ment Health Serv. 2013;51(8):19-24. doi:10.3928/02793695-20130612-02
2. Howard MO, Bowen SE, Garland EL, et al. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Prac. 2011;6(1):18-31.
3. Farkas J. Rhabdomyolysis. Internet Book of Critical Care. June 25, 2021. Accessed February 24, 2023. https://emcrit.org/ibcc/rhabdo/
4. Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
5. Muller AA, Muller GF. Inhalant abuse. J Emerg Nurs. 2006;32(5):447-448. doi:10.1016/j.jen.2006.05.018
6. Kozel N, Sloboda Z, De La Rosa M, eds. Epidemiology of Inhalant Abuse: An International Perspective; Nida Research Monograph 148. National Institute on Drug Abuse Research, US Dept of Health and Human Services; 1995. Accessed April 20, 2023. https://archives.nida.nih.gov/sites/default/files/monograph148.pdf
1. Ahern NR, Falsafi N. Inhalant abuse: youth at risk. J Psychosoc Nurs Ment Health Serv. 2013;51(8):19-24. doi:10.3928/02793695-20130612-02
2. Howard MO, Bowen SE, Garland EL, et al. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Prac. 2011;6(1):18-31.
3. Farkas J. Rhabdomyolysis. Internet Book of Critical Care. June 25, 2021. Accessed February 24, 2023. https://emcrit.org/ibcc/rhabdo/
4. Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
5. Muller AA, Muller GF. Inhalant abuse. J Emerg Nurs. 2006;32(5):447-448. doi:10.1016/j.jen.2006.05.018
6. Kozel N, Sloboda Z, De La Rosa M, eds. Epidemiology of Inhalant Abuse: An International Perspective; Nida Research Monograph 148. National Institute on Drug Abuse Research, US Dept of Health and Human Services; 1995. Accessed April 20, 2023. https://archives.nida.nih.gov/sites/default/files/monograph148.pdf