User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Medical Roundtable: Hodgkin Lymphoma - Discussing Recent Evidence & Practice Options
Moderated by: Stephen Ansell, MD, PhD1
Discussants: Craig Moskowitz, MD2; Catherine Diefenbach, MD3; Andrew M. Evens, DO, MSc4
From Mayo Clinic, Rochester, MN1; Memorial Sloan Kettering Cancer Center and Weill Medical College of Cornell University, New York, NY2; NYU School of Medicine and NYU Perlmutter Cancer Center, New York, NY3; Tufts University School of Medicine and Tufts Medical Center, Boston, MA4
Address for correspondence: Stephen Ansell, MD, PhD, Mayo Clinic, 200 First Street SW #W10, Rochester, MN 55905
E-mail: [email protected]
Biographical sketch:
From Weill Medical College of Cornell University:
Dr. Moskowitz serves as principal investigator and co-investigator for a number of clinical trials aimed at improving the care of patients with lymphoma. His research has focused on improving the outcome of patients with poor-risk diffuse large B-cell lymphoma (DLBCL) and Hodgkin lymphoma (HL). This effort has been conducted along two tracks. One effort is focused on improving therapy for patients with disease that has returned or is not responding to standard therapy (refractory disease), through the use of high-dose therapy and autologous stem cell transplantation as well as new agents that can be incorporated into such "salvage" therapy. The second is aimed at developing risk-adapted strategies to optimize the treatment of newly diagnosed DLBCL by using what we have learned in the relapsed and refractory setting.
Dr. Moskowitz has been recognized for his research on a national level through multiple awards. He has lectured worldwide on lymphoma and stem cell transplantation. In addition, he is a member of the research council at MSKCC, and on the steering committees for the bi-annual international lymphoma conference in Lugano and international Hodgkin lymphoma conference in Cologne.
From NYU School of Medicine and NYU Perlmutter Cancer Center:
An alumna of the University of Pennsylvania School of Medicine, Dr. Diefenbach completed her internship and residency at the Johns Hopkins Hospital and her oncology fellowship at Memorial Sloan-Kettering Cancer Center, where she spent an additional year focusing on translational immunology.
Her scientific research focuses on the relationship between lymphoma and immunity; on developing novel and immune based treatment strategies for patients with relapsed lymphoma; and on biomarker discovery. She is currently leading a national clinical trial for relapsed Hodgkin lymphoma investigating the combination of the antibody drug conjugate brentuximab with the immune activating agents ipilimumab and nivolumab.
Dr. Diefenbach directs the lymphoma clinical research within the Hematology/ Oncology Division at the Perlmutter Cancer Center. She is a member of the ECOG Lymphoma Committee, the NCI Lymphoma Steering Committee Clinical Trials Planning Meeting, and the Editorial Board of Clinical Cancer Research. Her research is supported by the Lymphoma Research Foundation, the American Cancer Society and the National Cancer Institute (NCI).
From Tufts University School of Medicine and Tufts Medical Center:
DR. ANSELL: My name is Stephen Ansell, from Mayo Clinic, Rochester, Minnesota. I’m joined by Drs. Craig Moskowitz, Attending Physician at Memorial Sloan Kettering Cancer Center and Professor of Medicine at Weill Medical College of Cornell University, Catherine Diefenbach, Assistant Professor of Medicine at the NYU School of Medicine and the NYU Perlmutter Cancer Center, and Andy Evens, Professor of Medicine at Tufts University School of Medicine, and Faculty Member at Tufts Medical Center in Boston.
Welcome to all of you and thank you for participating in this medical roundtable discussion. The focus today is going to be on practical management of Hodgkin lymphoma. This is a very experienced group of roundtable participants whom I hope will give us valuable insights into some of the questions relating to managing Hodgkin lymphoma.
Let’s talk about patients with early stage Hodgkin lymphoma and discuss what we feel the standard management might be and how we would use positron emission tomographic (PET) scans to direct therapy. Craig, I’m going to ask you to start. Please discuss what your standard management of early stage Hodgkin lymphoma is and how you use PET scans to direct your treatment.
DR. MOSKOWITZ: I divide Hodgkin lymphoma, early stage, into three groups; favorable early stage, unfavorable early stage without tumor bulk, and stage two disease with tumor bulk. The standard management is well borne out these days from the German Hodgkin Lymphoma Study Group.1,2 For early stage favorable Hodgkin lymphoma I treat men and women quite differently.
For men with stage IA or IIA, non-bulky disease, I tend to use standard treatment, which is short course combined modality therapy with two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and involved-field radiation. I do not use PET imaging in that setting. For women, however, as you know, the median age is young in this patient population. I’ve adopted a RAPID approach for these folks. I give 3 months of chemotherapy and then repeat the PET scan and if the PET scan is negative I stop treatment. If the PET scan is positive, I treat the patient as per that study, which was one more cycle of chemotherapy and radiation. But unfavorable early-stage disease without tumor bulk, once again, the issue is, should patients get chemotherapy alone or should they get combined modality therapy?
If I’m giving combined modality therapy, I do not use PET imaging. If I’m using chemotherapy alone and if this is a patient eligible for a RAPID approach I treat as above. If the patient was not eligible for RAPID because they had stage IIB disease, I usually give full course chemotherapy and I do not use PET imaging.
For patients with bulky stage II disease, I treat based upon the randomized cooperative group study, which is four to six cycles of chemotherapy followed by radiation. I do not use PET imaging in that setting.
DR. ANSELL: Craig, thanks. Andy, do you actually escalate therapy at any time based on PET results, or deescalate therapy? We heard from Craig that he follows some of the RAPID guidelines. Give us your perspective.
DR. EVENS: I think they’re all good questions. In a way, it’s not one size fits all. I think it has to be a very individualized patient-by-patient treatment decision. In other words, I might approach a 19-year-old woman with a bulky mediastinal mass differently than a 45-year-old man with a right cervical lymph node. This is one important point to convey. In terms of PET-adapted, I think it is evolving. The question is, is there any actionable evidence to go on?
In terms of where there are more data, which is in early negativity in terms of PET-2 negativity, there have been a couple publications alluded to—RAPID and the European Organisation for Research and Treatment of Cancer (EORTC)3—and I’m not sure those data changed the opinion if we didn’t have an early negative PET scan. We know, without an early negative PET scan, that patients who do not receive consolidative radiation have a small improvement in progression-free survival (PFS) and no difference in overall survival (OS).
What both of those studies showed is that basically persists. That margin of difference might be a little less—instead of 6%–8% difference in PFS, it might now be 4%–6%, but both studies proved to be not noninferior, so I’m not quite sure that has changed how I treat someone. In other words, an early negative PET scan. For PET positivity, the data are really evolving. I think, before the Lugano meeting this past June I would have said no.
I just saw a very recent second opinion on a younger patient in her mid-20s who had a bulky mediastinal mass, and then after two cycles was definitely better, but still PET positive, defined as it was a smidge greater than the liver, and the data that emanated from Lugano on early PET positive showed not only a PFS advantage, but a borderline OS, and that, of course, would be a game changer if PET-adapted therapy is pointing toward an OS advantage.
DR. ANSELL: Catherine, what’s your sense of the role of radiation therapy in early stage Hodgkin lymphoma?
DR. DIEFENBACH: As Andy said, the role of PET to stratify early stage patients is evolving. Another study that was reported in Lugano was the Response-adjusted Therapy for Hodgkin Lymphoma (RATHL) study4 where it appeared that for patients who had an early PET negative, the bleomycin could be omitted and the patients ended up with a PFS that was equivalent to patients who did not have bleomycin omitted. The decision of combined modality therapy vs standard therapy—I think Andy and Craig put it really well—will have to be individualized to the patient’s situation: specifically, to their age, sex, bulk of disease, and response after early disease assessment.
DR. MOSKOWITZ: That’s why it’s so hard to study this patient population. You have three folks on the phone and we’re all treating patients differently with an individualized approach. It’s almost impossible to come up with a clinical trial that everybody would be comfortable with in this patient population, which is why we have difficulty in writing one, which is disappointing.
DR. ANSELL: Craig, I wanted to circle back to you. The German Hodgkin Lymphoma Study Group has shown that if you omit bleomycin there is a slight decrease in outcome;5 however, the RATHL trial would suggest maybe not.6 Has that impacted your practice at all? Do you omit bleomycin in your regimens or do you use it standardly?
DR. MOSKOWITZ: Well, RATHL is for advanced stage disease and I’ve already stopped giving bleomycin to patients who are PET negative after 2 months of ABVD based on that approach; but I do not omit bleomycin for an early stage disease based upon the results from the German Hodgkin Lymphoma Study Group. We’re not giving full course chemotherapy in the early stage setting, so I’m willing to give the appropriate number of cycles, at least for now.
DR. ANSELL: Thanks for your perspectives. I think as it was pointed out, there’s a lot of individualization here and we still have an evolving role of PET scanning. Also, there are multiple new agents we’ll begin to talk about later in the program that may impact things further. So, I think this remains an area where it’s quite challenging to determine the optimal approach.
I want to turn our attention to talk about patients with advanced stage disease—predominantly stage III and IV disease. Catherine, how do you treat patients with advanced stage disease? What’s your approach, and as new agents are coming along, agents like brentuximab vedotin, where do you think they fit into your approach?
DR. DIEFENBACH: Just as Craig said, there isn’t really a one-size-fits-all approach to advanced-stage patients either. Just as early-stage patients are divided into at least three groups, we look at advanced-stage patients as a very heterogeneous population. One of the ways we stratify advanced-stage patients is based on what we call their risk score.
Traditionally, we’ve used the Hasenclever risk score—or the International Prognostic Score (IPS)7—which looks at seven clinical factors to get a sense of how these advanced-stage patients are going to do in terms of their PFS and OS.7 More recently, we have a manuscript where we’re looking in the modern era at using a streamlined score with only three of these factors instead of seven factors8 and the group from British Columbia has showed that, while this Hasenclever risk score is still relevant,9 it looks in the modern era with modern chemotherapy that it may be less helpful than it previously was, because the patients who did much worse are doing better, so the curves are narrowed.
Nonetheless, biologically and clinically, there’s a big difference between advanced-stage patients who have zero to two risk factors and advanced-stage patients who have five, six, or seven risk factors. The standard of care for treatment for advanced-stage patients both in the US and internationally has been either the chemotherapy regimen of ABVD or the chemotherapy regimen of escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP).10,11 There has been a controversy between those who prefer escalated BEACOPP and those who prefer ABVD. Escalated BEACOPP has a superior PFS to ABVD, which didn’t necessarily translate into an OS benefit, and patients who fail ABVD may be salvaged with stem cell transplant and second line chemotherapy and stem cell transplant.
Patients with escalated BEACOPP also have substantial therapy-related toxicity that patients with ABVD do not have, such as infertility and myelosuppression, higher rates of neutropenic fever, and higher rates of secondary leukemia, so there’s a cost associated with being treated with escalated BEACOPP that’s not associated with ABVD. With both regimens, the cure rate is approximately 75%–80%, so those who favor ABVD—I would consider myself one of them—would argue that you spare a certain number of patients who are salvaged with ABVD and who are cured unnecessary toxicity with the escalated BEACOPP. Those who are in favor of escalated BEACOPP for all patients would probably argue that an increased PFS should translate into an increased OS, and there have in fact been meta-analyses that show improved OS in some older trials with BEACOPP compared to ABVD, but this is a meta-analysis across many different trials.
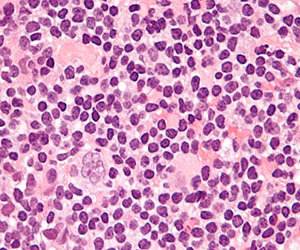
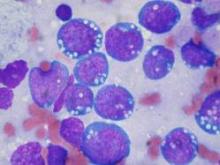
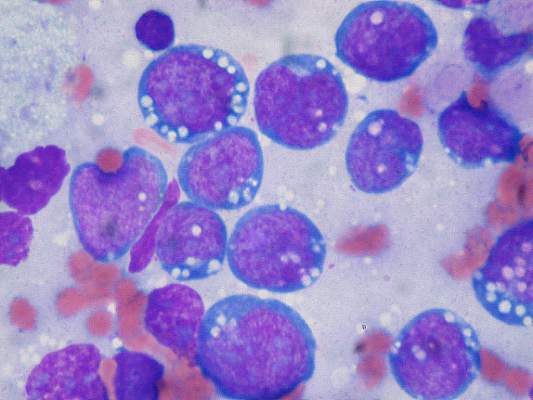
More recently, brentuximab vedotin—an antibody drug conjugate, against CD30, which is expressed on the Hodgkin Reed-Sternberg (HRS) cell—combined with auristatin—a taxane-like cytotoxic chemotherapy—which acts like a Trojan horse, is delivered to the HRS cell, taken up by the HRS cell and then blows up the HRS cell while sparing, to a large extent, the microenvironment, has been incorporated into upfront therapy with ABVD.
Brentuximab was approved by the Food and Drug Administration (FDA) to treat relapsed Hodgkin lymphoma based on the pivotal study, which had an overall response rate of approximately 75% for relapsed patients.12 Based on this it was incorporated into upfront therapy. There was a phase I study which I think, Steve, you actually led, which combined ABVD with brentuximab vedotin.13 This, however, resulted in excessive pulmonary toxicity secondary to bleomycin, so bleomycin was then omitted. The data were reported at the American Society of Hematology (ASH) meeting and showed a 3-year failure free survival of 96% and OS of 100%, which is extremely impressive in this population. The Phase 3 Frontline Therapy Trial in Patients With Advanced Classical Hodgkin Lymphoma (ECHELON) [NCT01712490] is currently underway and should complete accrual this year.
I think that these data will certainly help to answer the question of whether incorporating brentuximab into the upfront regimen of ABVD can improve both PFS and OS for these patients and perhaps put BEACOPP, finally, to rest.
DR. ANSELL: Andy, do you have a place in your practice where you think escalated BEACOPP has a role?
DR. EVENS: The quick answer is yes, but that sliver of practice is extremely small, and as time is going on, it’s getting smaller. I think in the pre-response adapted era—5 to 10 years ago when BEACOPP was first published—it did show an OS advantage, but many people would argue the comparator or COPP/ABVD arm isn’t equivalent, so to speak, to contemporary ABVD.
I was using it mainly in patients with high IPS—patients who had a five, six, or seven. That, frankly, is not a large percent of the patient population. Now, in the PET-adapted, we don’t quite have that actionable evidence, as much has accumulated quite yet, as early stage disease. Even in the patients now with high IPS, I have to admit, I’m really starting with ABVD in everyone and only considering escalating in patients who have a positive PET scan, which I guess in my practice is somewhat lower than the clinical trial data.
The clinical trial data in advanced-stage disease would say that’s 15%–20% of the patients.14 I tend to find it’s closer in the 5% range. I think part of that is viewing the PET-adapted part we keep talking about—it can’t be viewed in a vacuum, meaning you shouldn’t review a report. You should sit side-by-side with your radiologist and try to review that scan, and while doing that, have a clear meaning of what defines PET positive and PET negative, because almost every study we talked about has it slightly different. Where’s the cut point? Is it at the liver—meaning a Deauville score of four or five—or is it less in the liver, etc? So, I think those types of things are very important to understand and appreciate when treating the patients.
DR. DIEFENBACH: Andy, I completely agree with you and I also would have a role for BEACOPP in a patient who was not responding to ABVD with interim scans. I also, at that time, if the patient was by PET/computed tomography unequivocally refractory, I would consider clinical trial probably in the same vein as the escalated BEACOPP. So, I agree with Andy that I have a sliver of patients for whom I do consider escalated BEACOPP, but that is a very small sliver.
DR. ANSELL: Craig, what’s your comment about modifications that are happening, even to escalated BEACOPP? The BrECADD regimen is incorporating new agents like brentuximab vedotin.15 What’s your thinking about that regimen?
DR. MOSKOWITZ: I don’t treat patients off protocol with protocol based treatment, so my familiarity with it is low. Just to digress for one second, I think that I have definitely changed my practice and I would say that I used to give BEACOPP quite a bit for patients with four to seven risk factors, which is about 20% of the patient population. I think, based upon two fairly large studies that will have about 1700 patients on, the RATHL study and the intergroup study in the United States—it’s fairly clear—to me at least—that one can start with ABVD chemotherapy.
Since 80% of the patients have a negative interim PET scan, despite the number of pre-treatment risk factors they have, you’re really limiting the number of patients who get crossed over to a more complicated program, so I’ve been using that approach. I’m very comfortable with it.
The issue of giving brentuximab vedotin with AVD as part of primary therapy is, once again, investigational, and I have many concerns about the cost of that treatment program and it’s applicability worldwide even if the study is positive.
DR. ANSELL: I think that’s a good point. One of the things I find very interesting as we talk about the escalated BEACOPP vs. ABVD comparison is that as brentuximab vedotin has been mixed into those two regimens, it’s almost as if those two regimens are coming toward each other. ABVD is being somewhat escalated in intensity and escalated BEACOPP being modified down. I think at the end of the day, we’re going to come to a combined approach that suits people on both sides of the intensity debate. Cost, however, is going to be an issue to make this an internationally usable regimen, so I think that’s a real challenge.
I want to pick up, Craig, on the point you made about risks of relapse and so on. When and if patients relapse, what do you use in your practice as the optimal salvage regimen? Also, I know you’re a big advocate for your patients to attain a complete remission before going to transplant, would you like to explain why?
DR. MOSKOWITZ: Once again my opinion about this has evolved over the years, but I think that I’m fairly comfortable right now that almost all these salvage regimens are quite similar. They have variable toxicity profiles, but if the treatment is given correctly and you look at the published literature, somewhere between 60% and 80% of the patients will likely be in remission after salvage chemotherapy. As defined as a negative PET, based upon standard criteria, those patients have a marked survival advantage, looking at published literature thus far.
As I’m maturing, I’ve been trying to treat patients with less aggressive salvage therapy to try to get them into remission. For example, in patients with early stage disease who relapse with non-bulky and non-widespread disease, I’d be more inclined to give an outpatient chemotherapy regimen. Patients with widespread, extranodal involvement, I’d be more inclined to give inpatient treatment off study. On clinical trials, that’s a different story, but we’ve published now, multiple times, that overwhelmingly a complete response (CR) prior to a stem cell transplant abrogates almost all the other prognostic factors.
DR. ANSELL: Right. Andy, if a patient had a response, but not a CR, or maybe only a modest improvement, would you say that’s a deal breaker for moving onto something like an autologous transplant?
DR. EVENS: I would say for the majority of patients, yes. There might be that patient who, depending on what they’ve received in the past—particularly if they have already received brentuximab vedotin—might be someone we still take to an autologous transplant.
There’s a significant minority you can still cure who are chemo-resistant, but I think I completely agree with Craig. In the era of novel therapeutics, that’s becoming less and less. As more options come on the table, I think if you really have somebody who’s chemotherapy resistant—that can be defined, I guess, as a positive PET, although I do think there are some gray zones in defining positive PET—I think it would be someone I would really look toward a novel therapeutic.
DR. ANSELL: Catherine, there have been some recent data about maintenance therapy after an autologous stem cell transplant. What’s your take on that?
DR. DIEFENBACH: I think the most recent data on maintenance therapy post autologous stem cell transplant comes from the AETHERA study,16 which Craig was the lead author on, and this investigated the question of whether using brentuximab vedotin as a consolidative therapy after transplant—as a maintenance therapy—improved PFS in this patient population.
The patients in this study who had relapsed disease were randomized between receiving brentuximab vedotin or being observed. Patients were treated for 16 cycles, which is approximately one year with this therapy, given every 3 weeks. Data from this study were in favor of brentuximab vedotin. The group that received brentuximab vedotin as opposed to being observed had a PFS of 42.9 months vs a PFS of approximately 24 months in the group that wasn’t treated with brentuximab vedotin.
This says a few interesting things. This demonstrates that most patients who are going into transplant are not doing so with optimal disease control. If all patients were going into transplant in a CR with only micrometastatic disease, there would not be such a stark difference between these arms. I think this study very nicely demonstrates that patients who benefit most strongly from the maintenance therapy are the patients who are considered to be the highest risk.
These are the patients with refractory disease who go into transplant with a high degree of tumor bulk, or patients who relapse within a short time—less than 12 months after their initial therapy—suggesting they didn’t really obtain optimal disease control with their therapy, or they had extranodal disease or disease outside of the lymph nodes affecting vital organs or bone marrow. These high-risk patients, based on these criteria, appear to be the ones who benefit the most from brentuximab maintenance. I think it gets a little tricky, however, because none of these patients receive brentuximab in their upfront therapy. So, going forward, it’s going to be an even harder question to ask—how are high-risk patients who receive brentuximab initially going to benefit from receiving a therapy that they relapsed from after receiving?
Getting brentuximab was not a free ride. There was a significantly higher amount of both sensory neuropathy, and about 20% of patients had motor neuropathy in the brentuximab group vs the non-brentuximab treated patients.
Finally, there was no difference yet—and this will still mature—in OS between the groups because the patients who didn’t get brentuximab were able to cross over and get it. This goes back to the old question with rituximab when rituximab maintenance was looked at. Is it better to receive brentuximab vedotin maintenance after transplant or is it better to receive it if you relapse after transplant? I think we won’t know the answer to that question for a few more years when we really see if the PFS translates into durable survival in the brentuximab treated group vs the non-brentuximab treated group. As Craig has alluded to with regard to the cost with respect to upfront therapy, I think this adds substantial costs subsequent to stem cell transplant. If we are going to use this therapy, I think we’ll have to be very clever in how we risk assess the patients who proceed to transplant, both prior to transplant and in terms of deciding whether they receive therapy.
There are actually efforts underway right now—internationally—to use better risk discrimination criteria for relapsed Hodgkin lymphoma to define a higher population of patients who are truly high risk, you might have the highest likelihood of benefiting from this sort of consolidative strategy.
DR. ANSELL: Craig, please comment. Catherine mentioned that the addition of brentuximab wasn’t without financial cost, and that there is also a toxicity cost. You led the study, what are your comments on the side effects of a year’s worth of brentuximab vedotin?
DR. MOSKOWITZ: I think, shockingly, the median number of doses that patients received on the study was 15, so almost a year or full course. In general, neuropathy is similar to every other single study that’s been looked at with single agent brentuximab. Peripheral neuropathy is real. For me, if a patient gets a dose reduction and the neuropathy does not improve, I would just stop the treatment. That’s how I practice.
I think the question Catherine raises is an extremely good one about patients who had a brentuximab vedotin pretransplant. Should they get brentuximab vedotin post-transplant? That’s something that’s clearly going to evolve, and the number of doses that a patient will receive post-transplant is going to evolve, but I think for the audience listening, this is here to stay for the next couple of years.
The FDA approved this August 17, so transplant physicians are going to initiate brentuximab vedotin therapy prior to the patient returning to their medical oncologist. It’s the medical oncologist who’s going to decide how many more cycles of brentuximab should be administered, because these folks would already have received three cycles by then.
Transplant physicians have to report 90-day efficacy data. In general, therefore, transplanters will see the patients up to around day 90. Brentuximab is administered between days 30 and 45 post-transplant, so by definition, the Hodgkin lymphoma patients who have met the criteria of the study are going to receive it. Medical oncologists need to decide what to do after that time point.
DR. DIEFENBACH: Craig, my question to you is given what the study showed—that the patients with the highest risk receive the most benefit—would you recommend giving this to everyone who undergoes a transplant, or really to the patients per the study who are considered to have at least one of these three risk factors?
DR. MOSKOWITZ: I only treat patients with maintenance who were potentially eligible for the AETHERA study. That means remission duration of less than one year, primary refractory disease, or extranodal involvement. If they did not meet those criteria, I do not recommend maintenance.
DR. ANSELL: Most of what we’ve discussed to far, with all of our therapeutic options, have been most likely to be utilized in younger patients—patients who can tolerate the intensive regimens, patients who can receive salvage therapy and can get a transplant. Elderly patients are a real challenge to treat. Andy, you’re an expert in this area—how do you manage a 70-year-old who presents with Hodgkin lymphoma? Are there some new options we should bear in mind as we think about these patients?
DR. EVENS: Yes, it’s definitely a challenging patient population. A recurring theme is there’s not a one-size-fits-all. Age 70 might fall right in the middle, but the definition of elderly, for better or for worse, has been greater than age 60 in most clinical studies and other analyses, but again, that 60-year-old vs the 85-year-old in a wheelchair will be approached differently.
Let’s say we take that sweet spot of someone in their early 70s who is still performing all their activities of daily living and most of their instrumental activities of daily living with a performance status of one—the quick answer off of a clinical study is I’d probably use AVD (AVBD without the bleomycin). We, and others, have reported the incidence of bleomycin lung toxicity and the number one risk factor is age. That’s part and parcel related to the renal clearance of bleomycin and knowing that that is a risk factor.
Are there other risk factors, such as preexisting lung disease, etc? Yes. We recently looked at what we called the contemporary era, meaning post 2000, when I was at Northwestern in Chicago—we collected close to 100 patients.17 It was more of a real-world population, it was whether or not you were on a clinical study, and a third of patients in that analysis had developed bleomycin lung toxicity. The mortality rate, if you developed bleomycin lung toxicity, was 30%.
If anyone’s ever had a patient die from that, they know it’s quite significant. We corroborated those data when we took a subset analyses out from E2406, the phase III randomized study of Stanford V vs ABVD. The rate of bleomycin lung was not quite as high. This again was a clinical study—probably a healthier population—but it was just under 30% with a mortality rate of just under 20%. To me it’s just too slippery of a slope. It’s hard, besides age, to predict who’s going to develop it. I think we’ve already mentioned some of the data. If we had to say which is the weakest link or the least potent of the ABVD, it would be the B.
That’s all for the clinical trial. Thankfully there are, now, clinical trials specifically carved out for this patient population of untreated older patients with Hodgkin lymphoma. We’re participating in a clinical trial with Paul Hamlin at Memorial Sloan Kettering and other sites where we’re utilizing a sequential approach integrating brentuximab vedotin. We rationalized that concurrent therapy would likely be too tough for these patients, so it’s designed in more of a window study where we start with two cycles of brentuximab vedotin given every 3 weeks, followed by chemotherapy, AVD, and then followed by consolidation therapy.
The study is not done yet. We have reported interim results at the recent Lugano meeting that was alluded to,18 and we showed yes, there’s still some toxicity, including to the brentuximab vedotin. But the disease related outcomes were phenomenal in the early report, upwards of 95%, which again is another theme in elderly Hodgkin that I didn’t talk about. One is the tolerability of therapy, and second is a strong sentiment—if not scientific hypothesis—that it’s a different disease biology, in other words, more aggressive. You see more mixalarity, Epstein Barr Virus related. Those are a couple of considerations. There are also studies out there that are looking—and especially the frail patients, maybe that 85 year old or so—what about single-agent novel therapeutics such as brentuximab vedotin or I’ve even heard through the grapevine now PD-1 inhibitors being tested as single agents in this patient population.
DR. ANSELL: Right, I think these are exciting times and there are data to be watched for in elderly patients. I want to talk about patients that have failed an autologous transplant. In the past, the typical next modality of therapy was an allogeneic (ALLO) transplant. Seeing as Craig is a guy that’s done a lot of transplants in the past—what do you see as the role of ALLO stem cell transplants? There are a lot of data now for new drugs in post-autologous failure patients, including brentuximab vedotin and PD-1 blockade. So, I guess the question is, has ALLO stem cell transplant for Hodgkin lymphoma gone away?
DR. MOSKOWITZ: Well, I see it as a slow, painful death to be perfectly honest.
DR. EVENS: No pun intended.
DR. MOSKOWITZ: No pun intended. We’ve been studying the checkpoint inhibitors as have you, Steve, for about 2 and a half years, and I will say that during this time, I have sent one patient to an ALLO stem cell transplant and that was a patient who really had a fairly poor response to nivolumab.
I find it very hard to pull the trigger, so to speak, to send a patient for an ALLO transplant now when the patients are receiving modern checkpoint inhibition and tolerating it so well with stable Hodgkin lymphoma that is not affecting their day-to-day life.
This, to me, is the most difficult question you’ve addressed so far on this teleconference. For someone who’s been doing this for a long time, I’m not sure at the present time who should get an ALLO transplant for Hodgkin lymphoma. I think it’s a difficult area. It’s unclear to me how to study it.
DR. ANSELL: Catherine, you’ve also done a lot of work with immune checkpoint inhibitors and combination studies. Give us your perspective on when to use brentuximab vedotin, when to use PD-1 inhibition, and when to use combinations.
DR. DIEFENBACH: I think most of these, Steve, are still research questions to a certain extent. Of these novel agents, the only one that is FDA approved for use in a relapse patient is brentuximab vedotin, which is approved for patients who have failed two or more chemotherapy regimens.
In practice, brentuximab is used much more commonly. If I have a patient who doesn’t have a huge amount of bulky relapse and is for some reason not a trial candidate I might well consider second line therapy with brentuximab as opposed to ifosfamide, carboplatin, and etoposide (ICE) chemotherapy. There are data both out of City of Hope and Sloan Kettering showing that the efficacy for brentuximab vedotin in second line is at least equivalent to the data we saw in later line with respect both to the CR rate and the overall response rate, if not better.19,20
I think there’s an established role for brentuximab vedotin. There are other agents which are being used in the community that are not approved but are certainly looked at in combination or being used off-label, such as bendamustine, which also has published phase II data showing that it is also effective in relapsed Hodgkin lymphoma.21 I think really, with regard to second line, the goal is to get to a CR, and anything that can get you to a good CR to make autologous transplantation more effective is probably a good way to go. But I think the more interesting question is really how are we, in the future, going to design therapeutic strategies and therapeutic platforms that are really biologically based and relevant to Hodgkin lymphoma biology, rather than just taking something from column A and something from column B off the shelf?
I think the checkpoint inhibitors particularly speak to Hodgkin lymphoma biology, because what they’re doing—you have the PDL-1, which is expressed on the Hodgkin tumor cell (the HRS cell), and the PD-1 is actually on the T cells of the Hodgkin lymphoma microenvironment, and by blocking the ability of the T cells to interact with the Hodgkin lymphoma cells, you’re not actually directly killing anything.
What you’re doing is actually taking the activated T cells, which are switched off, and turning them back on the way you’d turn a light on and saying go do your job, go kill the HRS cells. We actually have a trial that’s open right now in which we’re combining brentuximab vedotin with a checkpoint inhibitor, ipilimumab, trying to do just that. We are trying to use the brentuximab to kill the HRS cells in bulk and release antigen and stimulate the T cells and we’re going to combine this with the PD-1 inhibitor nivolumab as well, and we’re planning to look at the triplet combination of dual checkpoint inhibition with ipilimumab and nivolumab with brentuximab and that study is open [Ipilimumab, Nivolumab, and Brentuximab Vedotin in Treating Patients With Relapsed or Refractory Hodgkin Lymphoma; NCT01896999].
There are other studies ongoing, looking at brentuximab and nivolumab in combination as well. I think there’s a study planned, as Andy alluded to, in the elderly population as well as another study that is planned by pharmaceutical companies.
I think with regard to immune agents, we’ve only really scratched the surface, and everyone is very excited right now about these checkpoint inhibitors, but the immune microenvironment is composed of more than just some CD-4 cells that are sitting around in a switched off state. We have macrophages and dendritic cells and natural killer cells and I think there a lot of other exciting immunologic agents that are both being used right now in solid tumors and are being used pre-clinically that may have very exciting applicability for Hodgkin lymphoma.
Finally, there are the signaling agents—not to ignore them—like, the JAK/STAT inhibitors which is a pathway that’s highly upregulated in Hodgkin lymphoma cells and other agents, such as epigenetic agents, and I think going forward, the rational platforms are really going to combine these agents in intelligent ways and try to target the Hodgkin lymphoma in a way that can really obviate the need for ALLO transplant. I, as Craig, have the same issue with my patients who are on PD-1 inhibitor therapy. It’s very hard to see them doing so well and to really pull the trigger on referring them for an ALLO transplant.
DR. ANSELL: I’d like to summarize our roundtable discussion by saying These are exciting times. There are lots of changes in Hodgkin lymphoma that are developing as we watch, and the exciting thing is to see how we can optimize early stage therapy to minimize toxicity and maintain benefit., optimize advanced stage initial treatment to get the best results, again with the least toxicity, and then how to integrate these new agents with great promise into frontline therapies and salvage therapies so that, hopefully, down the line, we see more and more patients that are cured of their disease right away with initial treatment.
I want to thank Craig Moskowitz, Catherine Diefenbach, and Andy Evens for their participation.
References
1 Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363(7):640–652.
2 Eich HT, Diehl V, Görgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28(27):4199–4206.
3 Raemaekers JM, André MP, Federico M, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32(12):1188–1194.
4 Barrington SF, O’Doherty MJ, Roberts TH, et al. PET-CT for staging and early response—results from the Response Adapted Therapy in Advanced Hodgkin Lymphoma (RATHL) study. Hematol Oncol. 2013;31(Suppl. I):96–150. Abstract 18.
5 Behringer K, Goergen H, Hitz F, et al. for the German Hodgkin Study Group; Swiss Group for Clinical Cancer Research; Arbeitsgemeinschaft Medikamentöse Tumortherapie. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin's lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet. 2015;385(9976):1418–1427.
6 Johnson PMW, et al. Response-adapted therapy based on interim FDG-PET scans in advanced Hodgkin lymphoma: 1st analysis of the safety of de-escalation & efficacy of escalation in the international RATHL study CRUK/07/033. Plenary Session: 13th International Conference on Malignant Lymphoma; June 17–20, 2015; Lugano, Switzerland.
7 Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339(21):1506–1514.
8 Diefenbach CS, Li H, Hong F, et al. Evaluation of the International Prognostic Score (IPS-7) and a Simpler Prognostic Score (IPS-3) for advanced Hodgkin lymphoma in the modern era. Br J Haematol. 2015. doi: 10.1111/bjh.13634. [Epub ahead of print]
9 Moccia AA, Donaldson J, Chhanabhai M, et al. International Prognostic Score in advanced-stage Hodgkin's lymphoma: altered utility in the modern era. J Clin Oncol. 2012;30(27):3383–3388.
10 Diehl V, Franklin J, Pfreundschuh M, et al. for the German Hodgkin's Lymphoma Study Group. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med. 2003;348(24):2386–2395.
11 Viviani S, Zinzani PL, Rambaldi A, et al for the Michelangelo Foundtaion; Gruppo Italiano di Terapie Innovative nei Linfomi; Intergruppo Italiano Linfomi. ABVD versus BEACOPP for Hodgkin's lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365(3):203–212.
12 Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183–2189.
13 Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin's lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14(13):1348–1356.
14 Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31(6):684–691.
15 Borchmann P, Eichenauer DA, Plütschow A, et al. Targeted BEACOPP variants in patients with newly diagnosed advanced stage classical Hodgkin lymphoma: interim results of a randomized phase II study. Blood. 2013;122(21)4344.
16 Moskowitz CH, Nadamanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2015;385(9980):1853–1862.
17 Evens AM, Helenowski I, Ramsdale E, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood. 2012;119(3):692–695.
18 Evens AM, et al. Sequential brentuximab vedotin and AVD for older Hodgkin lymphoma patients: initial results from a phase 2 multicentre study. In: Hematological Oncology – Special Issue: 13-ICML, 13th International Conference on Malignant Lymphoma, Palazzo dei Congressi, Lugano (Switzerland), 17–20 June, 2015. Abstract 89.
19 Chen RW, Palmer J, Siddiqi T, et al. Brentuximab vedotin as first line salvage therapy in relapsed/refractory HL. Poster presented at: 54th ASH Annual Meeting and Exposition; December 8–11, 2012; Atlanta, GA.
20 Moskowitz AJ, Schoder H, Yahalom J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin's lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16(3):284–292.
21 Moskowitz AJ, Hamlin PA, Jr, Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31(4):456–460.
Moderated by: Stephen Ansell, MD, PhD1
Discussants: Craig Moskowitz, MD2; Catherine Diefenbach, MD3; Andrew M. Evens, DO, MSc4
From Mayo Clinic, Rochester, MN1; Memorial Sloan Kettering Cancer Center and Weill Medical College of Cornell University, New York, NY2; NYU School of Medicine and NYU Perlmutter Cancer Center, New York, NY3; Tufts University School of Medicine and Tufts Medical Center, Boston, MA4
Address for correspondence: Stephen Ansell, MD, PhD, Mayo Clinic, 200 First Street SW #W10, Rochester, MN 55905
E-mail: [email protected]
Biographical sketch:
From Weill Medical College of Cornell University:
Dr. Moskowitz serves as principal investigator and co-investigator for a number of clinical trials aimed at improving the care of patients with lymphoma. His research has focused on improving the outcome of patients with poor-risk diffuse large B-cell lymphoma (DLBCL) and Hodgkin lymphoma (HL). This effort has been conducted along two tracks. One effort is focused on improving therapy for patients with disease that has returned or is not responding to standard therapy (refractory disease), through the use of high-dose therapy and autologous stem cell transplantation as well as new agents that can be incorporated into such "salvage" therapy. The second is aimed at developing risk-adapted strategies to optimize the treatment of newly diagnosed DLBCL by using what we have learned in the relapsed and refractory setting.
Dr. Moskowitz has been recognized for his research on a national level through multiple awards. He has lectured worldwide on lymphoma and stem cell transplantation. In addition, he is a member of the research council at MSKCC, and on the steering committees for the bi-annual international lymphoma conference in Lugano and international Hodgkin lymphoma conference in Cologne.
From NYU School of Medicine and NYU Perlmutter Cancer Center:
An alumna of the University of Pennsylvania School of Medicine, Dr. Diefenbach completed her internship and residency at the Johns Hopkins Hospital and her oncology fellowship at Memorial Sloan-Kettering Cancer Center, where she spent an additional year focusing on translational immunology.
Her scientific research focuses on the relationship between lymphoma and immunity; on developing novel and immune based treatment strategies for patients with relapsed lymphoma; and on biomarker discovery. She is currently leading a national clinical trial for relapsed Hodgkin lymphoma investigating the combination of the antibody drug conjugate brentuximab with the immune activating agents ipilimumab and nivolumab.
Dr. Diefenbach directs the lymphoma clinical research within the Hematology/ Oncology Division at the Perlmutter Cancer Center. She is a member of the ECOG Lymphoma Committee, the NCI Lymphoma Steering Committee Clinical Trials Planning Meeting, and the Editorial Board of Clinical Cancer Research. Her research is supported by the Lymphoma Research Foundation, the American Cancer Society and the National Cancer Institute (NCI).
From Tufts University School of Medicine and Tufts Medical Center:
DR. ANSELL: My name is Stephen Ansell, from Mayo Clinic, Rochester, Minnesota. I’m joined by Drs. Craig Moskowitz, Attending Physician at Memorial Sloan Kettering Cancer Center and Professor of Medicine at Weill Medical College of Cornell University, Catherine Diefenbach, Assistant Professor of Medicine at the NYU School of Medicine and the NYU Perlmutter Cancer Center, and Andy Evens, Professor of Medicine at Tufts University School of Medicine, and Faculty Member at Tufts Medical Center in Boston.
Welcome to all of you and thank you for participating in this medical roundtable discussion. The focus today is going to be on practical management of Hodgkin lymphoma. This is a very experienced group of roundtable participants whom I hope will give us valuable insights into some of the questions relating to managing Hodgkin lymphoma.
Let’s talk about patients with early stage Hodgkin lymphoma and discuss what we feel the standard management might be and how we would use positron emission tomographic (PET) scans to direct therapy. Craig, I’m going to ask you to start. Please discuss what your standard management of early stage Hodgkin lymphoma is and how you use PET scans to direct your treatment.
DR. MOSKOWITZ: I divide Hodgkin lymphoma, early stage, into three groups; favorable early stage, unfavorable early stage without tumor bulk, and stage two disease with tumor bulk. The standard management is well borne out these days from the German Hodgkin Lymphoma Study Group.1,2 For early stage favorable Hodgkin lymphoma I treat men and women quite differently.
For men with stage IA or IIA, non-bulky disease, I tend to use standard treatment, which is short course combined modality therapy with two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and involved-field radiation. I do not use PET imaging in that setting. For women, however, as you know, the median age is young in this patient population. I’ve adopted a RAPID approach for these folks. I give 3 months of chemotherapy and then repeat the PET scan and if the PET scan is negative I stop treatment. If the PET scan is positive, I treat the patient as per that study, which was one more cycle of chemotherapy and radiation. But unfavorable early-stage disease without tumor bulk, once again, the issue is, should patients get chemotherapy alone or should they get combined modality therapy?
If I’m giving combined modality therapy, I do not use PET imaging. If I’m using chemotherapy alone and if this is a patient eligible for a RAPID approach I treat as above. If the patient was not eligible for RAPID because they had stage IIB disease, I usually give full course chemotherapy and I do not use PET imaging.
For patients with bulky stage II disease, I treat based upon the randomized cooperative group study, which is four to six cycles of chemotherapy followed by radiation. I do not use PET imaging in that setting.
DR. ANSELL: Craig, thanks. Andy, do you actually escalate therapy at any time based on PET results, or deescalate therapy? We heard from Craig that he follows some of the RAPID guidelines. Give us your perspective.
DR. EVENS: I think they’re all good questions. In a way, it’s not one size fits all. I think it has to be a very individualized patient-by-patient treatment decision. In other words, I might approach a 19-year-old woman with a bulky mediastinal mass differently than a 45-year-old man with a right cervical lymph node. This is one important point to convey. In terms of PET-adapted, I think it is evolving. The question is, is there any actionable evidence to go on?
In terms of where there are more data, which is in early negativity in terms of PET-2 negativity, there have been a couple publications alluded to—RAPID and the European Organisation for Research and Treatment of Cancer (EORTC)3—and I’m not sure those data changed the opinion if we didn’t have an early negative PET scan. We know, without an early negative PET scan, that patients who do not receive consolidative radiation have a small improvement in progression-free survival (PFS) and no difference in overall survival (OS).
What both of those studies showed is that basically persists. That margin of difference might be a little less—instead of 6%–8% difference in PFS, it might now be 4%–6%, but both studies proved to be not noninferior, so I’m not quite sure that has changed how I treat someone. In other words, an early negative PET scan. For PET positivity, the data are really evolving. I think, before the Lugano meeting this past June I would have said no.
I just saw a very recent second opinion on a younger patient in her mid-20s who had a bulky mediastinal mass, and then after two cycles was definitely better, but still PET positive, defined as it was a smidge greater than the liver, and the data that emanated from Lugano on early PET positive showed not only a PFS advantage, but a borderline OS, and that, of course, would be a game changer if PET-adapted therapy is pointing toward an OS advantage.
DR. ANSELL: Catherine, what’s your sense of the role of radiation therapy in early stage Hodgkin lymphoma?
DR. DIEFENBACH: As Andy said, the role of PET to stratify early stage patients is evolving. Another study that was reported in Lugano was the Response-adjusted Therapy for Hodgkin Lymphoma (RATHL) study4 where it appeared that for patients who had an early PET negative, the bleomycin could be omitted and the patients ended up with a PFS that was equivalent to patients who did not have bleomycin omitted. The decision of combined modality therapy vs standard therapy—I think Andy and Craig put it really well—will have to be individualized to the patient’s situation: specifically, to their age, sex, bulk of disease, and response after early disease assessment.
DR. MOSKOWITZ: That’s why it’s so hard to study this patient population. You have three folks on the phone and we’re all treating patients differently with an individualized approach. It’s almost impossible to come up with a clinical trial that everybody would be comfortable with in this patient population, which is why we have difficulty in writing one, which is disappointing.
DR. ANSELL: Craig, I wanted to circle back to you. The German Hodgkin Lymphoma Study Group has shown that if you omit bleomycin there is a slight decrease in outcome;5 however, the RATHL trial would suggest maybe not.6 Has that impacted your practice at all? Do you omit bleomycin in your regimens or do you use it standardly?
DR. MOSKOWITZ: Well, RATHL is for advanced stage disease and I’ve already stopped giving bleomycin to patients who are PET negative after 2 months of ABVD based on that approach; but I do not omit bleomycin for an early stage disease based upon the results from the German Hodgkin Lymphoma Study Group. We’re not giving full course chemotherapy in the early stage setting, so I’m willing to give the appropriate number of cycles, at least for now.
DR. ANSELL: Thanks for your perspectives. I think as it was pointed out, there’s a lot of individualization here and we still have an evolving role of PET scanning. Also, there are multiple new agents we’ll begin to talk about later in the program that may impact things further. So, I think this remains an area where it’s quite challenging to determine the optimal approach.
I want to turn our attention to talk about patients with advanced stage disease—predominantly stage III and IV disease. Catherine, how do you treat patients with advanced stage disease? What’s your approach, and as new agents are coming along, agents like brentuximab vedotin, where do you think they fit into your approach?
DR. DIEFENBACH: Just as Craig said, there isn’t really a one-size-fits-all approach to advanced-stage patients either. Just as early-stage patients are divided into at least three groups, we look at advanced-stage patients as a very heterogeneous population. One of the ways we stratify advanced-stage patients is based on what we call their risk score.
Traditionally, we’ve used the Hasenclever risk score—or the International Prognostic Score (IPS)7—which looks at seven clinical factors to get a sense of how these advanced-stage patients are going to do in terms of their PFS and OS.7 More recently, we have a manuscript where we’re looking in the modern era at using a streamlined score with only three of these factors instead of seven factors8 and the group from British Columbia has showed that, while this Hasenclever risk score is still relevant,9 it looks in the modern era with modern chemotherapy that it may be less helpful than it previously was, because the patients who did much worse are doing better, so the curves are narrowed.
Nonetheless, biologically and clinically, there’s a big difference between advanced-stage patients who have zero to two risk factors and advanced-stage patients who have five, six, or seven risk factors. The standard of care for treatment for advanced-stage patients both in the US and internationally has been either the chemotherapy regimen of ABVD or the chemotherapy regimen of escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP).10,11 There has been a controversy between those who prefer escalated BEACOPP and those who prefer ABVD. Escalated BEACOPP has a superior PFS to ABVD, which didn’t necessarily translate into an OS benefit, and patients who fail ABVD may be salvaged with stem cell transplant and second line chemotherapy and stem cell transplant.
Patients with escalated BEACOPP also have substantial therapy-related toxicity that patients with ABVD do not have, such as infertility and myelosuppression, higher rates of neutropenic fever, and higher rates of secondary leukemia, so there’s a cost associated with being treated with escalated BEACOPP that’s not associated with ABVD. With both regimens, the cure rate is approximately 75%–80%, so those who favor ABVD—I would consider myself one of them—would argue that you spare a certain number of patients who are salvaged with ABVD and who are cured unnecessary toxicity with the escalated BEACOPP. Those who are in favor of escalated BEACOPP for all patients would probably argue that an increased PFS should translate into an increased OS, and there have in fact been meta-analyses that show improved OS in some older trials with BEACOPP compared to ABVD, but this is a meta-analysis across many different trials.
More recently, brentuximab vedotin—an antibody drug conjugate, against CD30, which is expressed on the Hodgkin Reed-Sternberg (HRS) cell—combined with auristatin—a taxane-like cytotoxic chemotherapy—which acts like a Trojan horse, is delivered to the HRS cell, taken up by the HRS cell and then blows up the HRS cell while sparing, to a large extent, the microenvironment, has been incorporated into upfront therapy with ABVD.
Brentuximab was approved by the Food and Drug Administration (FDA) to treat relapsed Hodgkin lymphoma based on the pivotal study, which had an overall response rate of approximately 75% for relapsed patients.12 Based on this it was incorporated into upfront therapy. There was a phase I study which I think, Steve, you actually led, which combined ABVD with brentuximab vedotin.13 This, however, resulted in excessive pulmonary toxicity secondary to bleomycin, so bleomycin was then omitted. The data were reported at the American Society of Hematology (ASH) meeting and showed a 3-year failure free survival of 96% and OS of 100%, which is extremely impressive in this population. The Phase 3 Frontline Therapy Trial in Patients With Advanced Classical Hodgkin Lymphoma (ECHELON) [NCT01712490] is currently underway and should complete accrual this year.
I think that these data will certainly help to answer the question of whether incorporating brentuximab into the upfront regimen of ABVD can improve both PFS and OS for these patients and perhaps put BEACOPP, finally, to rest.
DR. ANSELL: Andy, do you have a place in your practice where you think escalated BEACOPP has a role?
DR. EVENS: The quick answer is yes, but that sliver of practice is extremely small, and as time is going on, it’s getting smaller. I think in the pre-response adapted era—5 to 10 years ago when BEACOPP was first published—it did show an OS advantage, but many people would argue the comparator or COPP/ABVD arm isn’t equivalent, so to speak, to contemporary ABVD.
I was using it mainly in patients with high IPS—patients who had a five, six, or seven. That, frankly, is not a large percent of the patient population. Now, in the PET-adapted, we don’t quite have that actionable evidence, as much has accumulated quite yet, as early stage disease. Even in the patients now with high IPS, I have to admit, I’m really starting with ABVD in everyone and only considering escalating in patients who have a positive PET scan, which I guess in my practice is somewhat lower than the clinical trial data.
The clinical trial data in advanced-stage disease would say that’s 15%–20% of the patients.14 I tend to find it’s closer in the 5% range. I think part of that is viewing the PET-adapted part we keep talking about—it can’t be viewed in a vacuum, meaning you shouldn’t review a report. You should sit side-by-side with your radiologist and try to review that scan, and while doing that, have a clear meaning of what defines PET positive and PET negative, because almost every study we talked about has it slightly different. Where’s the cut point? Is it at the liver—meaning a Deauville score of four or five—or is it less in the liver, etc? So, I think those types of things are very important to understand and appreciate when treating the patients.
DR. DIEFENBACH: Andy, I completely agree with you and I also would have a role for BEACOPP in a patient who was not responding to ABVD with interim scans. I also, at that time, if the patient was by PET/computed tomography unequivocally refractory, I would consider clinical trial probably in the same vein as the escalated BEACOPP. So, I agree with Andy that I have a sliver of patients for whom I do consider escalated BEACOPP, but that is a very small sliver.
DR. ANSELL: Craig, what’s your comment about modifications that are happening, even to escalated BEACOPP? The BrECADD regimen is incorporating new agents like brentuximab vedotin.15 What’s your thinking about that regimen?
DR. MOSKOWITZ: I don’t treat patients off protocol with protocol based treatment, so my familiarity with it is low. Just to digress for one second, I think that I have definitely changed my practice and I would say that I used to give BEACOPP quite a bit for patients with four to seven risk factors, which is about 20% of the patient population. I think, based upon two fairly large studies that will have about 1700 patients on, the RATHL study and the intergroup study in the United States—it’s fairly clear—to me at least—that one can start with ABVD chemotherapy.
Since 80% of the patients have a negative interim PET scan, despite the number of pre-treatment risk factors they have, you’re really limiting the number of patients who get crossed over to a more complicated program, so I’ve been using that approach. I’m very comfortable with it.
The issue of giving brentuximab vedotin with AVD as part of primary therapy is, once again, investigational, and I have many concerns about the cost of that treatment program and it’s applicability worldwide even if the study is positive.
DR. ANSELL: I think that’s a good point. One of the things I find very interesting as we talk about the escalated BEACOPP vs. ABVD comparison is that as brentuximab vedotin has been mixed into those two regimens, it’s almost as if those two regimens are coming toward each other. ABVD is being somewhat escalated in intensity and escalated BEACOPP being modified down. I think at the end of the day, we’re going to come to a combined approach that suits people on both sides of the intensity debate. Cost, however, is going to be an issue to make this an internationally usable regimen, so I think that’s a real challenge.
I want to pick up, Craig, on the point you made about risks of relapse and so on. When and if patients relapse, what do you use in your practice as the optimal salvage regimen? Also, I know you’re a big advocate for your patients to attain a complete remission before going to transplant, would you like to explain why?
DR. MOSKOWITZ: Once again my opinion about this has evolved over the years, but I think that I’m fairly comfortable right now that almost all these salvage regimens are quite similar. They have variable toxicity profiles, but if the treatment is given correctly and you look at the published literature, somewhere between 60% and 80% of the patients will likely be in remission after salvage chemotherapy. As defined as a negative PET, based upon standard criteria, those patients have a marked survival advantage, looking at published literature thus far.
As I’m maturing, I’ve been trying to treat patients with less aggressive salvage therapy to try to get them into remission. For example, in patients with early stage disease who relapse with non-bulky and non-widespread disease, I’d be more inclined to give an outpatient chemotherapy regimen. Patients with widespread, extranodal involvement, I’d be more inclined to give inpatient treatment off study. On clinical trials, that’s a different story, but we’ve published now, multiple times, that overwhelmingly a complete response (CR) prior to a stem cell transplant abrogates almost all the other prognostic factors.
DR. ANSELL: Right. Andy, if a patient had a response, but not a CR, or maybe only a modest improvement, would you say that’s a deal breaker for moving onto something like an autologous transplant?
DR. EVENS: I would say for the majority of patients, yes. There might be that patient who, depending on what they’ve received in the past—particularly if they have already received brentuximab vedotin—might be someone we still take to an autologous transplant.
There’s a significant minority you can still cure who are chemo-resistant, but I think I completely agree with Craig. In the era of novel therapeutics, that’s becoming less and less. As more options come on the table, I think if you really have somebody who’s chemotherapy resistant—that can be defined, I guess, as a positive PET, although I do think there are some gray zones in defining positive PET—I think it would be someone I would really look toward a novel therapeutic.
DR. ANSELL: Catherine, there have been some recent data about maintenance therapy after an autologous stem cell transplant. What’s your take on that?
DR. DIEFENBACH: I think the most recent data on maintenance therapy post autologous stem cell transplant comes from the AETHERA study,16 which Craig was the lead author on, and this investigated the question of whether using brentuximab vedotin as a consolidative therapy after transplant—as a maintenance therapy—improved PFS in this patient population.
The patients in this study who had relapsed disease were randomized between receiving brentuximab vedotin or being observed. Patients were treated for 16 cycles, which is approximately one year with this therapy, given every 3 weeks. Data from this study were in favor of brentuximab vedotin. The group that received brentuximab vedotin as opposed to being observed had a PFS of 42.9 months vs a PFS of approximately 24 months in the group that wasn’t treated with brentuximab vedotin.
This says a few interesting things. This demonstrates that most patients who are going into transplant are not doing so with optimal disease control. If all patients were going into transplant in a CR with only micrometastatic disease, there would not be such a stark difference between these arms. I think this study very nicely demonstrates that patients who benefit most strongly from the maintenance therapy are the patients who are considered to be the highest risk.
These are the patients with refractory disease who go into transplant with a high degree of tumor bulk, or patients who relapse within a short time—less than 12 months after their initial therapy—suggesting they didn’t really obtain optimal disease control with their therapy, or they had extranodal disease or disease outside of the lymph nodes affecting vital organs or bone marrow. These high-risk patients, based on these criteria, appear to be the ones who benefit the most from brentuximab maintenance. I think it gets a little tricky, however, because none of these patients receive brentuximab in their upfront therapy. So, going forward, it’s going to be an even harder question to ask—how are high-risk patients who receive brentuximab initially going to benefit from receiving a therapy that they relapsed from after receiving?
Getting brentuximab was not a free ride. There was a significantly higher amount of both sensory neuropathy, and about 20% of patients had motor neuropathy in the brentuximab group vs the non-brentuximab treated patients.
Finally, there was no difference yet—and this will still mature—in OS between the groups because the patients who didn’t get brentuximab were able to cross over and get it. This goes back to the old question with rituximab when rituximab maintenance was looked at. Is it better to receive brentuximab vedotin maintenance after transplant or is it better to receive it if you relapse after transplant? I think we won’t know the answer to that question for a few more years when we really see if the PFS translates into durable survival in the brentuximab treated group vs the non-brentuximab treated group. As Craig has alluded to with regard to the cost with respect to upfront therapy, I think this adds substantial costs subsequent to stem cell transplant. If we are going to use this therapy, I think we’ll have to be very clever in how we risk assess the patients who proceed to transplant, both prior to transplant and in terms of deciding whether they receive therapy.
There are actually efforts underway right now—internationally—to use better risk discrimination criteria for relapsed Hodgkin lymphoma to define a higher population of patients who are truly high risk, you might have the highest likelihood of benefiting from this sort of consolidative strategy.
DR. ANSELL: Craig, please comment. Catherine mentioned that the addition of brentuximab wasn’t without financial cost, and that there is also a toxicity cost. You led the study, what are your comments on the side effects of a year’s worth of brentuximab vedotin?
DR. MOSKOWITZ: I think, shockingly, the median number of doses that patients received on the study was 15, so almost a year or full course. In general, neuropathy is similar to every other single study that’s been looked at with single agent brentuximab. Peripheral neuropathy is real. For me, if a patient gets a dose reduction and the neuropathy does not improve, I would just stop the treatment. That’s how I practice.
I think the question Catherine raises is an extremely good one about patients who had a brentuximab vedotin pretransplant. Should they get brentuximab vedotin post-transplant? That’s something that’s clearly going to evolve, and the number of doses that a patient will receive post-transplant is going to evolve, but I think for the audience listening, this is here to stay for the next couple of years.
The FDA approved this August 17, so transplant physicians are going to initiate brentuximab vedotin therapy prior to the patient returning to their medical oncologist. It’s the medical oncologist who’s going to decide how many more cycles of brentuximab should be administered, because these folks would already have received three cycles by then.
Transplant physicians have to report 90-day efficacy data. In general, therefore, transplanters will see the patients up to around day 90. Brentuximab is administered between days 30 and 45 post-transplant, so by definition, the Hodgkin lymphoma patients who have met the criteria of the study are going to receive it. Medical oncologists need to decide what to do after that time point.
DR. DIEFENBACH: Craig, my question to you is given what the study showed—that the patients with the highest risk receive the most benefit—would you recommend giving this to everyone who undergoes a transplant, or really to the patients per the study who are considered to have at least one of these three risk factors?
DR. MOSKOWITZ: I only treat patients with maintenance who were potentially eligible for the AETHERA study. That means remission duration of less than one year, primary refractory disease, or extranodal involvement. If they did not meet those criteria, I do not recommend maintenance.
DR. ANSELL: Most of what we’ve discussed to far, with all of our therapeutic options, have been most likely to be utilized in younger patients—patients who can tolerate the intensive regimens, patients who can receive salvage therapy and can get a transplant. Elderly patients are a real challenge to treat. Andy, you’re an expert in this area—how do you manage a 70-year-old who presents with Hodgkin lymphoma? Are there some new options we should bear in mind as we think about these patients?
DR. EVENS: Yes, it’s definitely a challenging patient population. A recurring theme is there’s not a one-size-fits-all. Age 70 might fall right in the middle, but the definition of elderly, for better or for worse, has been greater than age 60 in most clinical studies and other analyses, but again, that 60-year-old vs the 85-year-old in a wheelchair will be approached differently.
Let’s say we take that sweet spot of someone in their early 70s who is still performing all their activities of daily living and most of their instrumental activities of daily living with a performance status of one—the quick answer off of a clinical study is I’d probably use AVD (AVBD without the bleomycin). We, and others, have reported the incidence of bleomycin lung toxicity and the number one risk factor is age. That’s part and parcel related to the renal clearance of bleomycin and knowing that that is a risk factor.
Are there other risk factors, such as preexisting lung disease, etc? Yes. We recently looked at what we called the contemporary era, meaning post 2000, when I was at Northwestern in Chicago—we collected close to 100 patients.17 It was more of a real-world population, it was whether or not you were on a clinical study, and a third of patients in that analysis had developed bleomycin lung toxicity. The mortality rate, if you developed bleomycin lung toxicity, was 30%.
If anyone’s ever had a patient die from that, they know it’s quite significant. We corroborated those data when we took a subset analyses out from E2406, the phase III randomized study of Stanford V vs ABVD. The rate of bleomycin lung was not quite as high. This again was a clinical study—probably a healthier population—but it was just under 30% with a mortality rate of just under 20%. To me it’s just too slippery of a slope. It’s hard, besides age, to predict who’s going to develop it. I think we’ve already mentioned some of the data. If we had to say which is the weakest link or the least potent of the ABVD, it would be the B.
That’s all for the clinical trial. Thankfully there are, now, clinical trials specifically carved out for this patient population of untreated older patients with Hodgkin lymphoma. We’re participating in a clinical trial with Paul Hamlin at Memorial Sloan Kettering and other sites where we’re utilizing a sequential approach integrating brentuximab vedotin. We rationalized that concurrent therapy would likely be too tough for these patients, so it’s designed in more of a window study where we start with two cycles of brentuximab vedotin given every 3 weeks, followed by chemotherapy, AVD, and then followed by consolidation therapy.
The study is not done yet. We have reported interim results at the recent Lugano meeting that was alluded to,18 and we showed yes, there’s still some toxicity, including to the brentuximab vedotin. But the disease related outcomes were phenomenal in the early report, upwards of 95%, which again is another theme in elderly Hodgkin that I didn’t talk about. One is the tolerability of therapy, and second is a strong sentiment—if not scientific hypothesis—that it’s a different disease biology, in other words, more aggressive. You see more mixalarity, Epstein Barr Virus related. Those are a couple of considerations. There are also studies out there that are looking—and especially the frail patients, maybe that 85 year old or so—what about single-agent novel therapeutics such as brentuximab vedotin or I’ve even heard through the grapevine now PD-1 inhibitors being tested as single agents in this patient population.
DR. ANSELL: Right, I think these are exciting times and there are data to be watched for in elderly patients. I want to talk about patients that have failed an autologous transplant. In the past, the typical next modality of therapy was an allogeneic (ALLO) transplant. Seeing as Craig is a guy that’s done a lot of transplants in the past—what do you see as the role of ALLO stem cell transplants? There are a lot of data now for new drugs in post-autologous failure patients, including brentuximab vedotin and PD-1 blockade. So, I guess the question is, has ALLO stem cell transplant for Hodgkin lymphoma gone away?
DR. MOSKOWITZ: Well, I see it as a slow, painful death to be perfectly honest.
DR. EVENS: No pun intended.
DR. MOSKOWITZ: No pun intended. We’ve been studying the checkpoint inhibitors as have you, Steve, for about 2 and a half years, and I will say that during this time, I have sent one patient to an ALLO stem cell transplant and that was a patient who really had a fairly poor response to nivolumab.
I find it very hard to pull the trigger, so to speak, to send a patient for an ALLO transplant now when the patients are receiving modern checkpoint inhibition and tolerating it so well with stable Hodgkin lymphoma that is not affecting their day-to-day life.
This, to me, is the most difficult question you’ve addressed so far on this teleconference. For someone who’s been doing this for a long time, I’m not sure at the present time who should get an ALLO transplant for Hodgkin lymphoma. I think it’s a difficult area. It’s unclear to me how to study it.
DR. ANSELL: Catherine, you’ve also done a lot of work with immune checkpoint inhibitors and combination studies. Give us your perspective on when to use brentuximab vedotin, when to use PD-1 inhibition, and when to use combinations.
DR. DIEFENBACH: I think most of these, Steve, are still research questions to a certain extent. Of these novel agents, the only one that is FDA approved for use in a relapse patient is brentuximab vedotin, which is approved for patients who have failed two or more chemotherapy regimens.
In practice, brentuximab is used much more commonly. If I have a patient who doesn’t have a huge amount of bulky relapse and is for some reason not a trial candidate I might well consider second line therapy with brentuximab as opposed to ifosfamide, carboplatin, and etoposide (ICE) chemotherapy. There are data both out of City of Hope and Sloan Kettering showing that the efficacy for brentuximab vedotin in second line is at least equivalent to the data we saw in later line with respect both to the CR rate and the overall response rate, if not better.19,20
I think there’s an established role for brentuximab vedotin. There are other agents which are being used in the community that are not approved but are certainly looked at in combination or being used off-label, such as bendamustine, which also has published phase II data showing that it is also effective in relapsed Hodgkin lymphoma.21 I think really, with regard to second line, the goal is to get to a CR, and anything that can get you to a good CR to make autologous transplantation more effective is probably a good way to go. But I think the more interesting question is really how are we, in the future, going to design therapeutic strategies and therapeutic platforms that are really biologically based and relevant to Hodgkin lymphoma biology, rather than just taking something from column A and something from column B off the shelf?
I think the checkpoint inhibitors particularly speak to Hodgkin lymphoma biology, because what they’re doing—you have the PDL-1, which is expressed on the Hodgkin tumor cell (the HRS cell), and the PD-1 is actually on the T cells of the Hodgkin lymphoma microenvironment, and by blocking the ability of the T cells to interact with the Hodgkin lymphoma cells, you’re not actually directly killing anything.
What you’re doing is actually taking the activated T cells, which are switched off, and turning them back on the way you’d turn a light on and saying go do your job, go kill the HRS cells. We actually have a trial that’s open right now in which we’re combining brentuximab vedotin with a checkpoint inhibitor, ipilimumab, trying to do just that. We are trying to use the brentuximab to kill the HRS cells in bulk and release antigen and stimulate the T cells and we’re going to combine this with the PD-1 inhibitor nivolumab as well, and we’re planning to look at the triplet combination of dual checkpoint inhibition with ipilimumab and nivolumab with brentuximab and that study is open [Ipilimumab, Nivolumab, and Brentuximab Vedotin in Treating Patients With Relapsed or Refractory Hodgkin Lymphoma; NCT01896999].
There are other studies ongoing, looking at brentuximab and nivolumab in combination as well. I think there’s a study planned, as Andy alluded to, in the elderly population as well as another study that is planned by pharmaceutical companies.
I think with regard to immune agents, we’ve only really scratched the surface, and everyone is very excited right now about these checkpoint inhibitors, but the immune microenvironment is composed of more than just some CD-4 cells that are sitting around in a switched off state. We have macrophages and dendritic cells and natural killer cells and I think there a lot of other exciting immunologic agents that are both being used right now in solid tumors and are being used pre-clinically that may have very exciting applicability for Hodgkin lymphoma.
Finally, there are the signaling agents—not to ignore them—like, the JAK/STAT inhibitors which is a pathway that’s highly upregulated in Hodgkin lymphoma cells and other agents, such as epigenetic agents, and I think going forward, the rational platforms are really going to combine these agents in intelligent ways and try to target the Hodgkin lymphoma in a way that can really obviate the need for ALLO transplant. I, as Craig, have the same issue with my patients who are on PD-1 inhibitor therapy. It’s very hard to see them doing so well and to really pull the trigger on referring them for an ALLO transplant.
DR. ANSELL: I’d like to summarize our roundtable discussion by saying These are exciting times. There are lots of changes in Hodgkin lymphoma that are developing as we watch, and the exciting thing is to see how we can optimize early stage therapy to minimize toxicity and maintain benefit., optimize advanced stage initial treatment to get the best results, again with the least toxicity, and then how to integrate these new agents with great promise into frontline therapies and salvage therapies so that, hopefully, down the line, we see more and more patients that are cured of their disease right away with initial treatment.
I want to thank Craig Moskowitz, Catherine Diefenbach, and Andy Evens for their participation.
References
1 Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363(7):640–652.
2 Eich HT, Diehl V, Görgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28(27):4199–4206.
3 Raemaekers JM, André MP, Federico M, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32(12):1188–1194.
4 Barrington SF, O’Doherty MJ, Roberts TH, et al. PET-CT for staging and early response—results from the Response Adapted Therapy in Advanced Hodgkin Lymphoma (RATHL) study. Hematol Oncol. 2013;31(Suppl. I):96–150. Abstract 18.
5 Behringer K, Goergen H, Hitz F, et al. for the German Hodgkin Study Group; Swiss Group for Clinical Cancer Research; Arbeitsgemeinschaft Medikamentöse Tumortherapie. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin's lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet. 2015;385(9976):1418–1427.
6 Johnson PMW, et al. Response-adapted therapy based on interim FDG-PET scans in advanced Hodgkin lymphoma: 1st analysis of the safety of de-escalation & efficacy of escalation in the international RATHL study CRUK/07/033. Plenary Session: 13th International Conference on Malignant Lymphoma; June 17–20, 2015; Lugano, Switzerland.
7 Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339(21):1506–1514.
8 Diefenbach CS, Li H, Hong F, et al. Evaluation of the International Prognostic Score (IPS-7) and a Simpler Prognostic Score (IPS-3) for advanced Hodgkin lymphoma in the modern era. Br J Haematol. 2015. doi: 10.1111/bjh.13634. [Epub ahead of print]
9 Moccia AA, Donaldson J, Chhanabhai M, et al. International Prognostic Score in advanced-stage Hodgkin's lymphoma: altered utility in the modern era. J Clin Oncol. 2012;30(27):3383–3388.
10 Diehl V, Franklin J, Pfreundschuh M, et al. for the German Hodgkin's Lymphoma Study Group. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med. 2003;348(24):2386–2395.
11 Viviani S, Zinzani PL, Rambaldi A, et al for the Michelangelo Foundtaion; Gruppo Italiano di Terapie Innovative nei Linfomi; Intergruppo Italiano Linfomi. ABVD versus BEACOPP for Hodgkin's lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365(3):203–212.
12 Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183–2189.
13 Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin's lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14(13):1348–1356.
14 Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31(6):684–691.
15 Borchmann P, Eichenauer DA, Plütschow A, et al. Targeted BEACOPP variants in patients with newly diagnosed advanced stage classical Hodgkin lymphoma: interim results of a randomized phase II study. Blood. 2013;122(21)4344.
16 Moskowitz CH, Nadamanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2015;385(9980):1853–1862.
17 Evens AM, Helenowski I, Ramsdale E, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood. 2012;119(3):692–695.
18 Evens AM, et al. Sequential brentuximab vedotin and AVD for older Hodgkin lymphoma patients: initial results from a phase 2 multicentre study. In: Hematological Oncology – Special Issue: 13-ICML, 13th International Conference on Malignant Lymphoma, Palazzo dei Congressi, Lugano (Switzerland), 17–20 June, 2015. Abstract 89.
19 Chen RW, Palmer J, Siddiqi T, et al. Brentuximab vedotin as first line salvage therapy in relapsed/refractory HL. Poster presented at: 54th ASH Annual Meeting and Exposition; December 8–11, 2012; Atlanta, GA.
20 Moskowitz AJ, Schoder H, Yahalom J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin's lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16(3):284–292.
21 Moskowitz AJ, Hamlin PA, Jr, Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31(4):456–460.
Moderated by: Stephen Ansell, MD, PhD1
Discussants: Craig Moskowitz, MD2; Catherine Diefenbach, MD3; Andrew M. Evens, DO, MSc4
From Mayo Clinic, Rochester, MN1; Memorial Sloan Kettering Cancer Center and Weill Medical College of Cornell University, New York, NY2; NYU School of Medicine and NYU Perlmutter Cancer Center, New York, NY3; Tufts University School of Medicine and Tufts Medical Center, Boston, MA4
Address for correspondence: Stephen Ansell, MD, PhD, Mayo Clinic, 200 First Street SW #W10, Rochester, MN 55905
E-mail: [email protected]
Biographical sketch:
From Weill Medical College of Cornell University:
Dr. Moskowitz serves as principal investigator and co-investigator for a number of clinical trials aimed at improving the care of patients with lymphoma. His research has focused on improving the outcome of patients with poor-risk diffuse large B-cell lymphoma (DLBCL) and Hodgkin lymphoma (HL). This effort has been conducted along two tracks. One effort is focused on improving therapy for patients with disease that has returned or is not responding to standard therapy (refractory disease), through the use of high-dose therapy and autologous stem cell transplantation as well as new agents that can be incorporated into such "salvage" therapy. The second is aimed at developing risk-adapted strategies to optimize the treatment of newly diagnosed DLBCL by using what we have learned in the relapsed and refractory setting.
Dr. Moskowitz has been recognized for his research on a national level through multiple awards. He has lectured worldwide on lymphoma and stem cell transplantation. In addition, he is a member of the research council at MSKCC, and on the steering committees for the bi-annual international lymphoma conference in Lugano and international Hodgkin lymphoma conference in Cologne.
From NYU School of Medicine and NYU Perlmutter Cancer Center:
An alumna of the University of Pennsylvania School of Medicine, Dr. Diefenbach completed her internship and residency at the Johns Hopkins Hospital and her oncology fellowship at Memorial Sloan-Kettering Cancer Center, where she spent an additional year focusing on translational immunology.
Her scientific research focuses on the relationship between lymphoma and immunity; on developing novel and immune based treatment strategies for patients with relapsed lymphoma; and on biomarker discovery. She is currently leading a national clinical trial for relapsed Hodgkin lymphoma investigating the combination of the antibody drug conjugate brentuximab with the immune activating agents ipilimumab and nivolumab.
Dr. Diefenbach directs the lymphoma clinical research within the Hematology/ Oncology Division at the Perlmutter Cancer Center. She is a member of the ECOG Lymphoma Committee, the NCI Lymphoma Steering Committee Clinical Trials Planning Meeting, and the Editorial Board of Clinical Cancer Research. Her research is supported by the Lymphoma Research Foundation, the American Cancer Society and the National Cancer Institute (NCI).
From Tufts University School of Medicine and Tufts Medical Center:
DR. ANSELL: My name is Stephen Ansell, from Mayo Clinic, Rochester, Minnesota. I’m joined by Drs. Craig Moskowitz, Attending Physician at Memorial Sloan Kettering Cancer Center and Professor of Medicine at Weill Medical College of Cornell University, Catherine Diefenbach, Assistant Professor of Medicine at the NYU School of Medicine and the NYU Perlmutter Cancer Center, and Andy Evens, Professor of Medicine at Tufts University School of Medicine, and Faculty Member at Tufts Medical Center in Boston.
Welcome to all of you and thank you for participating in this medical roundtable discussion. The focus today is going to be on practical management of Hodgkin lymphoma. This is a very experienced group of roundtable participants whom I hope will give us valuable insights into some of the questions relating to managing Hodgkin lymphoma.
Let’s talk about patients with early stage Hodgkin lymphoma and discuss what we feel the standard management might be and how we would use positron emission tomographic (PET) scans to direct therapy. Craig, I’m going to ask you to start. Please discuss what your standard management of early stage Hodgkin lymphoma is and how you use PET scans to direct your treatment.
DR. MOSKOWITZ: I divide Hodgkin lymphoma, early stage, into three groups; favorable early stage, unfavorable early stage without tumor bulk, and stage two disease with tumor bulk. The standard management is well borne out these days from the German Hodgkin Lymphoma Study Group.1,2 For early stage favorable Hodgkin lymphoma I treat men and women quite differently.
For men with stage IA or IIA, non-bulky disease, I tend to use standard treatment, which is short course combined modality therapy with two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and involved-field radiation. I do not use PET imaging in that setting. For women, however, as you know, the median age is young in this patient population. I’ve adopted a RAPID approach for these folks. I give 3 months of chemotherapy and then repeat the PET scan and if the PET scan is negative I stop treatment. If the PET scan is positive, I treat the patient as per that study, which was one more cycle of chemotherapy and radiation. But unfavorable early-stage disease without tumor bulk, once again, the issue is, should patients get chemotherapy alone or should they get combined modality therapy?
If I’m giving combined modality therapy, I do not use PET imaging. If I’m using chemotherapy alone and if this is a patient eligible for a RAPID approach I treat as above. If the patient was not eligible for RAPID because they had stage IIB disease, I usually give full course chemotherapy and I do not use PET imaging.
For patients with bulky stage II disease, I treat based upon the randomized cooperative group study, which is four to six cycles of chemotherapy followed by radiation. I do not use PET imaging in that setting.
DR. ANSELL: Craig, thanks. Andy, do you actually escalate therapy at any time based on PET results, or deescalate therapy? We heard from Craig that he follows some of the RAPID guidelines. Give us your perspective.
DR. EVENS: I think they’re all good questions. In a way, it’s not one size fits all. I think it has to be a very individualized patient-by-patient treatment decision. In other words, I might approach a 19-year-old woman with a bulky mediastinal mass differently than a 45-year-old man with a right cervical lymph node. This is one important point to convey. In terms of PET-adapted, I think it is evolving. The question is, is there any actionable evidence to go on?
In terms of where there are more data, which is in early negativity in terms of PET-2 negativity, there have been a couple publications alluded to—RAPID and the European Organisation for Research and Treatment of Cancer (EORTC)3—and I’m not sure those data changed the opinion if we didn’t have an early negative PET scan. We know, without an early negative PET scan, that patients who do not receive consolidative radiation have a small improvement in progression-free survival (PFS) and no difference in overall survival (OS).
What both of those studies showed is that basically persists. That margin of difference might be a little less—instead of 6%–8% difference in PFS, it might now be 4%–6%, but both studies proved to be not noninferior, so I’m not quite sure that has changed how I treat someone. In other words, an early negative PET scan. For PET positivity, the data are really evolving. I think, before the Lugano meeting this past June I would have said no.
I just saw a very recent second opinion on a younger patient in her mid-20s who had a bulky mediastinal mass, and then after two cycles was definitely better, but still PET positive, defined as it was a smidge greater than the liver, and the data that emanated from Lugano on early PET positive showed not only a PFS advantage, but a borderline OS, and that, of course, would be a game changer if PET-adapted therapy is pointing toward an OS advantage.
DR. ANSELL: Catherine, what’s your sense of the role of radiation therapy in early stage Hodgkin lymphoma?
DR. DIEFENBACH: As Andy said, the role of PET to stratify early stage patients is evolving. Another study that was reported in Lugano was the Response-adjusted Therapy for Hodgkin Lymphoma (RATHL) study4 where it appeared that for patients who had an early PET negative, the bleomycin could be omitted and the patients ended up with a PFS that was equivalent to patients who did not have bleomycin omitted. The decision of combined modality therapy vs standard therapy—I think Andy and Craig put it really well—will have to be individualized to the patient’s situation: specifically, to their age, sex, bulk of disease, and response after early disease assessment.
DR. MOSKOWITZ: That’s why it’s so hard to study this patient population. You have three folks on the phone and we’re all treating patients differently with an individualized approach. It’s almost impossible to come up with a clinical trial that everybody would be comfortable with in this patient population, which is why we have difficulty in writing one, which is disappointing.
DR. ANSELL: Craig, I wanted to circle back to you. The German Hodgkin Lymphoma Study Group has shown that if you omit bleomycin there is a slight decrease in outcome;5 however, the RATHL trial would suggest maybe not.6 Has that impacted your practice at all? Do you omit bleomycin in your regimens or do you use it standardly?
DR. MOSKOWITZ: Well, RATHL is for advanced stage disease and I’ve already stopped giving bleomycin to patients who are PET negative after 2 months of ABVD based on that approach; but I do not omit bleomycin for an early stage disease based upon the results from the German Hodgkin Lymphoma Study Group. We’re not giving full course chemotherapy in the early stage setting, so I’m willing to give the appropriate number of cycles, at least for now.
DR. ANSELL: Thanks for your perspectives. I think as it was pointed out, there’s a lot of individualization here and we still have an evolving role of PET scanning. Also, there are multiple new agents we’ll begin to talk about later in the program that may impact things further. So, I think this remains an area where it’s quite challenging to determine the optimal approach.
I want to turn our attention to talk about patients with advanced stage disease—predominantly stage III and IV disease. Catherine, how do you treat patients with advanced stage disease? What’s your approach, and as new agents are coming along, agents like brentuximab vedotin, where do you think they fit into your approach?
DR. DIEFENBACH: Just as Craig said, there isn’t really a one-size-fits-all approach to advanced-stage patients either. Just as early-stage patients are divided into at least three groups, we look at advanced-stage patients as a very heterogeneous population. One of the ways we stratify advanced-stage patients is based on what we call their risk score.
Traditionally, we’ve used the Hasenclever risk score—or the International Prognostic Score (IPS)7—which looks at seven clinical factors to get a sense of how these advanced-stage patients are going to do in terms of their PFS and OS.7 More recently, we have a manuscript where we’re looking in the modern era at using a streamlined score with only three of these factors instead of seven factors8 and the group from British Columbia has showed that, while this Hasenclever risk score is still relevant,9 it looks in the modern era with modern chemotherapy that it may be less helpful than it previously was, because the patients who did much worse are doing better, so the curves are narrowed.
Nonetheless, biologically and clinically, there’s a big difference between advanced-stage patients who have zero to two risk factors and advanced-stage patients who have five, six, or seven risk factors. The standard of care for treatment for advanced-stage patients both in the US and internationally has been either the chemotherapy regimen of ABVD or the chemotherapy regimen of escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP).10,11 There has been a controversy between those who prefer escalated BEACOPP and those who prefer ABVD. Escalated BEACOPP has a superior PFS to ABVD, which didn’t necessarily translate into an OS benefit, and patients who fail ABVD may be salvaged with stem cell transplant and second line chemotherapy and stem cell transplant.
Patients with escalated BEACOPP also have substantial therapy-related toxicity that patients with ABVD do not have, such as infertility and myelosuppression, higher rates of neutropenic fever, and higher rates of secondary leukemia, so there’s a cost associated with being treated with escalated BEACOPP that’s not associated with ABVD. With both regimens, the cure rate is approximately 75%–80%, so those who favor ABVD—I would consider myself one of them—would argue that you spare a certain number of patients who are salvaged with ABVD and who are cured unnecessary toxicity with the escalated BEACOPP. Those who are in favor of escalated BEACOPP for all patients would probably argue that an increased PFS should translate into an increased OS, and there have in fact been meta-analyses that show improved OS in some older trials with BEACOPP compared to ABVD, but this is a meta-analysis across many different trials.
More recently, brentuximab vedotin—an antibody drug conjugate, against CD30, which is expressed on the Hodgkin Reed-Sternberg (HRS) cell—combined with auristatin—a taxane-like cytotoxic chemotherapy—which acts like a Trojan horse, is delivered to the HRS cell, taken up by the HRS cell and then blows up the HRS cell while sparing, to a large extent, the microenvironment, has been incorporated into upfront therapy with ABVD.
Brentuximab was approved by the Food and Drug Administration (FDA) to treat relapsed Hodgkin lymphoma based on the pivotal study, which had an overall response rate of approximately 75% for relapsed patients.12 Based on this it was incorporated into upfront therapy. There was a phase I study which I think, Steve, you actually led, which combined ABVD with brentuximab vedotin.13 This, however, resulted in excessive pulmonary toxicity secondary to bleomycin, so bleomycin was then omitted. The data were reported at the American Society of Hematology (ASH) meeting and showed a 3-year failure free survival of 96% and OS of 100%, which is extremely impressive in this population. The Phase 3 Frontline Therapy Trial in Patients With Advanced Classical Hodgkin Lymphoma (ECHELON) [NCT01712490] is currently underway and should complete accrual this year.
I think that these data will certainly help to answer the question of whether incorporating brentuximab into the upfront regimen of ABVD can improve both PFS and OS for these patients and perhaps put BEACOPP, finally, to rest.
DR. ANSELL: Andy, do you have a place in your practice where you think escalated BEACOPP has a role?
DR. EVENS: The quick answer is yes, but that sliver of practice is extremely small, and as time is going on, it’s getting smaller. I think in the pre-response adapted era—5 to 10 years ago when BEACOPP was first published—it did show an OS advantage, but many people would argue the comparator or COPP/ABVD arm isn’t equivalent, so to speak, to contemporary ABVD.
I was using it mainly in patients with high IPS—patients who had a five, six, or seven. That, frankly, is not a large percent of the patient population. Now, in the PET-adapted, we don’t quite have that actionable evidence, as much has accumulated quite yet, as early stage disease. Even in the patients now with high IPS, I have to admit, I’m really starting with ABVD in everyone and only considering escalating in patients who have a positive PET scan, which I guess in my practice is somewhat lower than the clinical trial data.
The clinical trial data in advanced-stage disease would say that’s 15%–20% of the patients.14 I tend to find it’s closer in the 5% range. I think part of that is viewing the PET-adapted part we keep talking about—it can’t be viewed in a vacuum, meaning you shouldn’t review a report. You should sit side-by-side with your radiologist and try to review that scan, and while doing that, have a clear meaning of what defines PET positive and PET negative, because almost every study we talked about has it slightly different. Where’s the cut point? Is it at the liver—meaning a Deauville score of four or five—or is it less in the liver, etc? So, I think those types of things are very important to understand and appreciate when treating the patients.
DR. DIEFENBACH: Andy, I completely agree with you and I also would have a role for BEACOPP in a patient who was not responding to ABVD with interim scans. I also, at that time, if the patient was by PET/computed tomography unequivocally refractory, I would consider clinical trial probably in the same vein as the escalated BEACOPP. So, I agree with Andy that I have a sliver of patients for whom I do consider escalated BEACOPP, but that is a very small sliver.
DR. ANSELL: Craig, what’s your comment about modifications that are happening, even to escalated BEACOPP? The BrECADD regimen is incorporating new agents like brentuximab vedotin.15 What’s your thinking about that regimen?
DR. MOSKOWITZ: I don’t treat patients off protocol with protocol based treatment, so my familiarity with it is low. Just to digress for one second, I think that I have definitely changed my practice and I would say that I used to give BEACOPP quite a bit for patients with four to seven risk factors, which is about 20% of the patient population. I think, based upon two fairly large studies that will have about 1700 patients on, the RATHL study and the intergroup study in the United States—it’s fairly clear—to me at least—that one can start with ABVD chemotherapy.
Since 80% of the patients have a negative interim PET scan, despite the number of pre-treatment risk factors they have, you’re really limiting the number of patients who get crossed over to a more complicated program, so I’ve been using that approach. I’m very comfortable with it.
The issue of giving brentuximab vedotin with AVD as part of primary therapy is, once again, investigational, and I have many concerns about the cost of that treatment program and it’s applicability worldwide even if the study is positive.
DR. ANSELL: I think that’s a good point. One of the things I find very interesting as we talk about the escalated BEACOPP vs. ABVD comparison is that as brentuximab vedotin has been mixed into those two regimens, it’s almost as if those two regimens are coming toward each other. ABVD is being somewhat escalated in intensity and escalated BEACOPP being modified down. I think at the end of the day, we’re going to come to a combined approach that suits people on both sides of the intensity debate. Cost, however, is going to be an issue to make this an internationally usable regimen, so I think that’s a real challenge.
I want to pick up, Craig, on the point you made about risks of relapse and so on. When and if patients relapse, what do you use in your practice as the optimal salvage regimen? Also, I know you’re a big advocate for your patients to attain a complete remission before going to transplant, would you like to explain why?
DR. MOSKOWITZ: Once again my opinion about this has evolved over the years, but I think that I’m fairly comfortable right now that almost all these salvage regimens are quite similar. They have variable toxicity profiles, but if the treatment is given correctly and you look at the published literature, somewhere between 60% and 80% of the patients will likely be in remission after salvage chemotherapy. As defined as a negative PET, based upon standard criteria, those patients have a marked survival advantage, looking at published literature thus far.
As I’m maturing, I’ve been trying to treat patients with less aggressive salvage therapy to try to get them into remission. For example, in patients with early stage disease who relapse with non-bulky and non-widespread disease, I’d be more inclined to give an outpatient chemotherapy regimen. Patients with widespread, extranodal involvement, I’d be more inclined to give inpatient treatment off study. On clinical trials, that’s a different story, but we’ve published now, multiple times, that overwhelmingly a complete response (CR) prior to a stem cell transplant abrogates almost all the other prognostic factors.
DR. ANSELL: Right. Andy, if a patient had a response, but not a CR, or maybe only a modest improvement, would you say that’s a deal breaker for moving onto something like an autologous transplant?
DR. EVENS: I would say for the majority of patients, yes. There might be that patient who, depending on what they’ve received in the past—particularly if they have already received brentuximab vedotin—might be someone we still take to an autologous transplant.
There’s a significant minority you can still cure who are chemo-resistant, but I think I completely agree with Craig. In the era of novel therapeutics, that’s becoming less and less. As more options come on the table, I think if you really have somebody who’s chemotherapy resistant—that can be defined, I guess, as a positive PET, although I do think there are some gray zones in defining positive PET—I think it would be someone I would really look toward a novel therapeutic.
DR. ANSELL: Catherine, there have been some recent data about maintenance therapy after an autologous stem cell transplant. What’s your take on that?
DR. DIEFENBACH: I think the most recent data on maintenance therapy post autologous stem cell transplant comes from the AETHERA study,16 which Craig was the lead author on, and this investigated the question of whether using brentuximab vedotin as a consolidative therapy after transplant—as a maintenance therapy—improved PFS in this patient population.
The patients in this study who had relapsed disease were randomized between receiving brentuximab vedotin or being observed. Patients were treated for 16 cycles, which is approximately one year with this therapy, given every 3 weeks. Data from this study were in favor of brentuximab vedotin. The group that received brentuximab vedotin as opposed to being observed had a PFS of 42.9 months vs a PFS of approximately 24 months in the group that wasn’t treated with brentuximab vedotin.
This says a few interesting things. This demonstrates that most patients who are going into transplant are not doing so with optimal disease control. If all patients were going into transplant in a CR with only micrometastatic disease, there would not be such a stark difference between these arms. I think this study very nicely demonstrates that patients who benefit most strongly from the maintenance therapy are the patients who are considered to be the highest risk.
These are the patients with refractory disease who go into transplant with a high degree of tumor bulk, or patients who relapse within a short time—less than 12 months after their initial therapy—suggesting they didn’t really obtain optimal disease control with their therapy, or they had extranodal disease or disease outside of the lymph nodes affecting vital organs or bone marrow. These high-risk patients, based on these criteria, appear to be the ones who benefit the most from brentuximab maintenance. I think it gets a little tricky, however, because none of these patients receive brentuximab in their upfront therapy. So, going forward, it’s going to be an even harder question to ask—how are high-risk patients who receive brentuximab initially going to benefit from receiving a therapy that they relapsed from after receiving?
Getting brentuximab was not a free ride. There was a significantly higher amount of both sensory neuropathy, and about 20% of patients had motor neuropathy in the brentuximab group vs the non-brentuximab treated patients.
Finally, there was no difference yet—and this will still mature—in OS between the groups because the patients who didn’t get brentuximab were able to cross over and get it. This goes back to the old question with rituximab when rituximab maintenance was looked at. Is it better to receive brentuximab vedotin maintenance after transplant or is it better to receive it if you relapse after transplant? I think we won’t know the answer to that question for a few more years when we really see if the PFS translates into durable survival in the brentuximab treated group vs the non-brentuximab treated group. As Craig has alluded to with regard to the cost with respect to upfront therapy, I think this adds substantial costs subsequent to stem cell transplant. If we are going to use this therapy, I think we’ll have to be very clever in how we risk assess the patients who proceed to transplant, both prior to transplant and in terms of deciding whether they receive therapy.
There are actually efforts underway right now—internationally—to use better risk discrimination criteria for relapsed Hodgkin lymphoma to define a higher population of patients who are truly high risk, you might have the highest likelihood of benefiting from this sort of consolidative strategy.
DR. ANSELL: Craig, please comment. Catherine mentioned that the addition of brentuximab wasn’t without financial cost, and that there is also a toxicity cost. You led the study, what are your comments on the side effects of a year’s worth of brentuximab vedotin?
DR. MOSKOWITZ: I think, shockingly, the median number of doses that patients received on the study was 15, so almost a year or full course. In general, neuropathy is similar to every other single study that’s been looked at with single agent brentuximab. Peripheral neuropathy is real. For me, if a patient gets a dose reduction and the neuropathy does not improve, I would just stop the treatment. That’s how I practice.
I think the question Catherine raises is an extremely good one about patients who had a brentuximab vedotin pretransplant. Should they get brentuximab vedotin post-transplant? That’s something that’s clearly going to evolve, and the number of doses that a patient will receive post-transplant is going to evolve, but I think for the audience listening, this is here to stay for the next couple of years.
The FDA approved this August 17, so transplant physicians are going to initiate brentuximab vedotin therapy prior to the patient returning to their medical oncologist. It’s the medical oncologist who’s going to decide how many more cycles of brentuximab should be administered, because these folks would already have received three cycles by then.
Transplant physicians have to report 90-day efficacy data. In general, therefore, transplanters will see the patients up to around day 90. Brentuximab is administered between days 30 and 45 post-transplant, so by definition, the Hodgkin lymphoma patients who have met the criteria of the study are going to receive it. Medical oncologists need to decide what to do after that time point.
DR. DIEFENBACH: Craig, my question to you is given what the study showed—that the patients with the highest risk receive the most benefit—would you recommend giving this to everyone who undergoes a transplant, or really to the patients per the study who are considered to have at least one of these three risk factors?
DR. MOSKOWITZ: I only treat patients with maintenance who were potentially eligible for the AETHERA study. That means remission duration of less than one year, primary refractory disease, or extranodal involvement. If they did not meet those criteria, I do not recommend maintenance.
DR. ANSELL: Most of what we’ve discussed to far, with all of our therapeutic options, have been most likely to be utilized in younger patients—patients who can tolerate the intensive regimens, patients who can receive salvage therapy and can get a transplant. Elderly patients are a real challenge to treat. Andy, you’re an expert in this area—how do you manage a 70-year-old who presents with Hodgkin lymphoma? Are there some new options we should bear in mind as we think about these patients?
DR. EVENS: Yes, it’s definitely a challenging patient population. A recurring theme is there’s not a one-size-fits-all. Age 70 might fall right in the middle, but the definition of elderly, for better or for worse, has been greater than age 60 in most clinical studies and other analyses, but again, that 60-year-old vs the 85-year-old in a wheelchair will be approached differently.
Let’s say we take that sweet spot of someone in their early 70s who is still performing all their activities of daily living and most of their instrumental activities of daily living with a performance status of one—the quick answer off of a clinical study is I’d probably use AVD (AVBD without the bleomycin). We, and others, have reported the incidence of bleomycin lung toxicity and the number one risk factor is age. That’s part and parcel related to the renal clearance of bleomycin and knowing that that is a risk factor.
Are there other risk factors, such as preexisting lung disease, etc? Yes. We recently looked at what we called the contemporary era, meaning post 2000, when I was at Northwestern in Chicago—we collected close to 100 patients.17 It was more of a real-world population, it was whether or not you were on a clinical study, and a third of patients in that analysis had developed bleomycin lung toxicity. The mortality rate, if you developed bleomycin lung toxicity, was 30%.
If anyone’s ever had a patient die from that, they know it’s quite significant. We corroborated those data when we took a subset analyses out from E2406, the phase III randomized study of Stanford V vs ABVD. The rate of bleomycin lung was not quite as high. This again was a clinical study—probably a healthier population—but it was just under 30% with a mortality rate of just under 20%. To me it’s just too slippery of a slope. It’s hard, besides age, to predict who’s going to develop it. I think we’ve already mentioned some of the data. If we had to say which is the weakest link or the least potent of the ABVD, it would be the B.
That’s all for the clinical trial. Thankfully there are, now, clinical trials specifically carved out for this patient population of untreated older patients with Hodgkin lymphoma. We’re participating in a clinical trial with Paul Hamlin at Memorial Sloan Kettering and other sites where we’re utilizing a sequential approach integrating brentuximab vedotin. We rationalized that concurrent therapy would likely be too tough for these patients, so it’s designed in more of a window study where we start with two cycles of brentuximab vedotin given every 3 weeks, followed by chemotherapy, AVD, and then followed by consolidation therapy.
The study is not done yet. We have reported interim results at the recent Lugano meeting that was alluded to,18 and we showed yes, there’s still some toxicity, including to the brentuximab vedotin. But the disease related outcomes were phenomenal in the early report, upwards of 95%, which again is another theme in elderly Hodgkin that I didn’t talk about. One is the tolerability of therapy, and second is a strong sentiment—if not scientific hypothesis—that it’s a different disease biology, in other words, more aggressive. You see more mixalarity, Epstein Barr Virus related. Those are a couple of considerations. There are also studies out there that are looking—and especially the frail patients, maybe that 85 year old or so—what about single-agent novel therapeutics such as brentuximab vedotin or I’ve even heard through the grapevine now PD-1 inhibitors being tested as single agents in this patient population.
DR. ANSELL: Right, I think these are exciting times and there are data to be watched for in elderly patients. I want to talk about patients that have failed an autologous transplant. In the past, the typical next modality of therapy was an allogeneic (ALLO) transplant. Seeing as Craig is a guy that’s done a lot of transplants in the past—what do you see as the role of ALLO stem cell transplants? There are a lot of data now for new drugs in post-autologous failure patients, including brentuximab vedotin and PD-1 blockade. So, I guess the question is, has ALLO stem cell transplant for Hodgkin lymphoma gone away?
DR. MOSKOWITZ: Well, I see it as a slow, painful death to be perfectly honest.
DR. EVENS: No pun intended.
DR. MOSKOWITZ: No pun intended. We’ve been studying the checkpoint inhibitors as have you, Steve, for about 2 and a half years, and I will say that during this time, I have sent one patient to an ALLO stem cell transplant and that was a patient who really had a fairly poor response to nivolumab.
I find it very hard to pull the trigger, so to speak, to send a patient for an ALLO transplant now when the patients are receiving modern checkpoint inhibition and tolerating it so well with stable Hodgkin lymphoma that is not affecting their day-to-day life.
This, to me, is the most difficult question you’ve addressed so far on this teleconference. For someone who’s been doing this for a long time, I’m not sure at the present time who should get an ALLO transplant for Hodgkin lymphoma. I think it’s a difficult area. It’s unclear to me how to study it.
DR. ANSELL: Catherine, you’ve also done a lot of work with immune checkpoint inhibitors and combination studies. Give us your perspective on when to use brentuximab vedotin, when to use PD-1 inhibition, and when to use combinations.
DR. DIEFENBACH: I think most of these, Steve, are still research questions to a certain extent. Of these novel agents, the only one that is FDA approved for use in a relapse patient is brentuximab vedotin, which is approved for patients who have failed two or more chemotherapy regimens.
In practice, brentuximab is used much more commonly. If I have a patient who doesn’t have a huge amount of bulky relapse and is for some reason not a trial candidate I might well consider second line therapy with brentuximab as opposed to ifosfamide, carboplatin, and etoposide (ICE) chemotherapy. There are data both out of City of Hope and Sloan Kettering showing that the efficacy for brentuximab vedotin in second line is at least equivalent to the data we saw in later line with respect both to the CR rate and the overall response rate, if not better.19,20
I think there’s an established role for brentuximab vedotin. There are other agents which are being used in the community that are not approved but are certainly looked at in combination or being used off-label, such as bendamustine, which also has published phase II data showing that it is also effective in relapsed Hodgkin lymphoma.21 I think really, with regard to second line, the goal is to get to a CR, and anything that can get you to a good CR to make autologous transplantation more effective is probably a good way to go. But I think the more interesting question is really how are we, in the future, going to design therapeutic strategies and therapeutic platforms that are really biologically based and relevant to Hodgkin lymphoma biology, rather than just taking something from column A and something from column B off the shelf?
I think the checkpoint inhibitors particularly speak to Hodgkin lymphoma biology, because what they’re doing—you have the PDL-1, which is expressed on the Hodgkin tumor cell (the HRS cell), and the PD-1 is actually on the T cells of the Hodgkin lymphoma microenvironment, and by blocking the ability of the T cells to interact with the Hodgkin lymphoma cells, you’re not actually directly killing anything.
What you’re doing is actually taking the activated T cells, which are switched off, and turning them back on the way you’d turn a light on and saying go do your job, go kill the HRS cells. We actually have a trial that’s open right now in which we’re combining brentuximab vedotin with a checkpoint inhibitor, ipilimumab, trying to do just that. We are trying to use the brentuximab to kill the HRS cells in bulk and release antigen and stimulate the T cells and we’re going to combine this with the PD-1 inhibitor nivolumab as well, and we’re planning to look at the triplet combination of dual checkpoint inhibition with ipilimumab and nivolumab with brentuximab and that study is open [Ipilimumab, Nivolumab, and Brentuximab Vedotin in Treating Patients With Relapsed or Refractory Hodgkin Lymphoma; NCT01896999].
There are other studies ongoing, looking at brentuximab and nivolumab in combination as well. I think there’s a study planned, as Andy alluded to, in the elderly population as well as another study that is planned by pharmaceutical companies.
I think with regard to immune agents, we’ve only really scratched the surface, and everyone is very excited right now about these checkpoint inhibitors, but the immune microenvironment is composed of more than just some CD-4 cells that are sitting around in a switched off state. We have macrophages and dendritic cells and natural killer cells and I think there a lot of other exciting immunologic agents that are both being used right now in solid tumors and are being used pre-clinically that may have very exciting applicability for Hodgkin lymphoma.
Finally, there are the signaling agents—not to ignore them—like, the JAK/STAT inhibitors which is a pathway that’s highly upregulated in Hodgkin lymphoma cells and other agents, such as epigenetic agents, and I think going forward, the rational platforms are really going to combine these agents in intelligent ways and try to target the Hodgkin lymphoma in a way that can really obviate the need for ALLO transplant. I, as Craig, have the same issue with my patients who are on PD-1 inhibitor therapy. It’s very hard to see them doing so well and to really pull the trigger on referring them for an ALLO transplant.
DR. ANSELL: I’d like to summarize our roundtable discussion by saying These are exciting times. There are lots of changes in Hodgkin lymphoma that are developing as we watch, and the exciting thing is to see how we can optimize early stage therapy to minimize toxicity and maintain benefit., optimize advanced stage initial treatment to get the best results, again with the least toxicity, and then how to integrate these new agents with great promise into frontline therapies and salvage therapies so that, hopefully, down the line, we see more and more patients that are cured of their disease right away with initial treatment.
I want to thank Craig Moskowitz, Catherine Diefenbach, and Andy Evens for their participation.
References
1 Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363(7):640–652.
2 Eich HT, Diehl V, Görgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28(27):4199–4206.
3 Raemaekers JM, André MP, Federico M, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32(12):1188–1194.
4 Barrington SF, O’Doherty MJ, Roberts TH, et al. PET-CT for staging and early response—results from the Response Adapted Therapy in Advanced Hodgkin Lymphoma (RATHL) study. Hematol Oncol. 2013;31(Suppl. I):96–150. Abstract 18.
5 Behringer K, Goergen H, Hitz F, et al. for the German Hodgkin Study Group; Swiss Group for Clinical Cancer Research; Arbeitsgemeinschaft Medikamentöse Tumortherapie. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin's lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet. 2015;385(9976):1418–1427.
6 Johnson PMW, et al. Response-adapted therapy based on interim FDG-PET scans in advanced Hodgkin lymphoma: 1st analysis of the safety of de-escalation & efficacy of escalation in the international RATHL study CRUK/07/033. Plenary Session: 13th International Conference on Malignant Lymphoma; June 17–20, 2015; Lugano, Switzerland.
7 Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339(21):1506–1514.
8 Diefenbach CS, Li H, Hong F, et al. Evaluation of the International Prognostic Score (IPS-7) and a Simpler Prognostic Score (IPS-3) for advanced Hodgkin lymphoma in the modern era. Br J Haematol. 2015. doi: 10.1111/bjh.13634. [Epub ahead of print]
9 Moccia AA, Donaldson J, Chhanabhai M, et al. International Prognostic Score in advanced-stage Hodgkin's lymphoma: altered utility in the modern era. J Clin Oncol. 2012;30(27):3383–3388.
10 Diehl V, Franklin J, Pfreundschuh M, et al. for the German Hodgkin's Lymphoma Study Group. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med. 2003;348(24):2386–2395.
11 Viviani S, Zinzani PL, Rambaldi A, et al for the Michelangelo Foundtaion; Gruppo Italiano di Terapie Innovative nei Linfomi; Intergruppo Italiano Linfomi. ABVD versus BEACOPP for Hodgkin's lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365(3):203–212.
12 Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183–2189.
13 Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin's lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14(13):1348–1356.
14 Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31(6):684–691.
15 Borchmann P, Eichenauer DA, Plütschow A, et al. Targeted BEACOPP variants in patients with newly diagnosed advanced stage classical Hodgkin lymphoma: interim results of a randomized phase II study. Blood. 2013;122(21)4344.
16 Moskowitz CH, Nadamanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2015;385(9980):1853–1862.
17 Evens AM, Helenowski I, Ramsdale E, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood. 2012;119(3):692–695.
18 Evens AM, et al. Sequential brentuximab vedotin and AVD for older Hodgkin lymphoma patients: initial results from a phase 2 multicentre study. In: Hematological Oncology – Special Issue: 13-ICML, 13th International Conference on Malignant Lymphoma, Palazzo dei Congressi, Lugano (Switzerland), 17–20 June, 2015. Abstract 89.
19 Chen RW, Palmer J, Siddiqi T, et al. Brentuximab vedotin as first line salvage therapy in relapsed/refractory HL. Poster presented at: 54th ASH Annual Meeting and Exposition; December 8–11, 2012; Atlanta, GA.
20 Moskowitz AJ, Schoder H, Yahalom J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin's lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16(3):284–292.
21 Moskowitz AJ, Hamlin PA, Jr, Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31(4):456–460.
Routine imaging for diffuse large B-cell lymphoma offers no survival benefit
A population-based comparison of patients with diffuse large B-cell lymphoma (DLBCL) in first complete remission indicated that routine imaging surveillance did not improve outcomes, researchers reported.
Overall survival was similar for Danish and Swedish populations who received similar follow-up care, except that routine imaging surveillance is the standard of care in Denmark, but not in Sweden. The 3-year overall survival for Danish and Swedish patients was 92% and 91%, respectively.
Outcomes grouped by international prognostic index (IPI) also showed no significant differences between populations (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.62.0229.).
“An imaging-based follow-up strategy does not improve postremission [overall survival] for DLBCL,” wrote Dr. Tarec Christoffer El-Galaly, of Aalborg University Hospital, Denmark, and colleagues.
They observed that aside from using IPI as risk stratification, the study “also points to baseline [lactate dehyrogenase] as a single discriminator of patients with high versus low risk of progression,” (Hazard ratio, 3.12; 95% CI, 1.78-5.48; P less than .01).
The retrospective study examined records of patients with DLBCL from Sweden (n=696) and Denmark (n=525) who achieved first complete remission after first-line therapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP-like regimens from 2007 to 2012. The proportion of patients with IPI greater than two were similar for both groups, though more Danish patients received radiotherapy compared with their Swedish counterparts (35% v. 9%).
Standard follow-up care after first complete remission is similar in Denmark and Sweden and typically includes symptom assessment, clinical examination, and blood tests at 3- to 4-month intervals for 2 years, and 6-month intervals in the third year. After 3 years, Swedish patients are seen annually for 2 years and then follow-up is ended for most patients. In Denmark, 6-month checks are continued until 5 years and then follow-up is usually ended. However, in Denmark guidelines support routine computerized tomography (CT) scans of the neck, abdomen, and thorax every 6 months for 2 years, which is not encouraged by guidelines in Sweden.
Early relapse detection aims to improve survival, and although low disease burden is associated with durable survival in patients treated for relapsed DLBCL, most studies show similar outcomes for imaging versus non-imaging detection. Additionally, previous retrospective studies that have reported survival differences based on relapse detection method are prone to lead-time bias, according to the researchers.
Given that a majority of patients with recurrent DLBCL experience symptoms before relapse, that elevated lactate dehyrogenase or abnormal physical examination may raise suspicion, and that exposure to ionizing radiation from medical imaging can lead to radiation-induced cancers, “routine imaging for DLBCL in first [complete remission] is not recommended,” the authors wrote.
The research was supported in part by the North Denmark Region. Dr. El-Galaly and coauthors reported having no financial disclosures.
The best way to determine the effectiveness of surveillance imaging would be a randomized trial including patients with diffuse large B-cell lymphoma (DLBCL) after first complete remission, but it is unlikely that such a study will be done. The study by El-Galaly et al may be the next best approach. Taking advantage of the fact that neighboring countries Denmark and Sweden have opposite policies for surveillance imaging but otherwise similar follow-up visit schedules and testing, the authors identified factors that predicted relapse (e.g., age greater than 60 years and elevated LDH), and they found that routine surveillance imaging had no impact on outcome. The study presents the strongest argument yet published against routine surveillance imaging.
The two other outstanding issues of routine surveillance are long-term safety and cost benefit. The study by El-Galaly et al, in combination with several other reports, suggests that routine surveillance imaging, in the absence of new or suspicious symptoms, physical findings, or change in laboratory results, is unlikely to benefit patients, may add to the patient’s stress, may cause long-term health problems, and incurs substantial economic cost.
Dr. James O. Armitage and Dr. Julie M. Vose are both at the University of Nebraska, Omaha. Dr. Armitage disclosed a leadership role with Tesaro and consulting or advisory roles with GlaxoSmithKline, Roche, Spectrum Pharmaceuticals, ZIOPHARM Oncology, Conatus, and Celgene. Dr. Vose reported honoraria from Sanofi-Aventis and Seattle Genetics; consulting or advisory roles with Bioconnections; and institutional research funding from Spectrum Pharmaceuticals, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte, Janssen Biotech, Pharmacyclics, Acerta, and Kite Pharma. These remarks were adapted from their accompanying editorial (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.63.5946).
The best way to determine the effectiveness of surveillance imaging would be a randomized trial including patients with diffuse large B-cell lymphoma (DLBCL) after first complete remission, but it is unlikely that such a study will be done. The study by El-Galaly et al may be the next best approach. Taking advantage of the fact that neighboring countries Denmark and Sweden have opposite policies for surveillance imaging but otherwise similar follow-up visit schedules and testing, the authors identified factors that predicted relapse (e.g., age greater than 60 years and elevated LDH), and they found that routine surveillance imaging had no impact on outcome. The study presents the strongest argument yet published against routine surveillance imaging.
The two other outstanding issues of routine surveillance are long-term safety and cost benefit. The study by El-Galaly et al, in combination with several other reports, suggests that routine surveillance imaging, in the absence of new or suspicious symptoms, physical findings, or change in laboratory results, is unlikely to benefit patients, may add to the patient’s stress, may cause long-term health problems, and incurs substantial economic cost.
Dr. James O. Armitage and Dr. Julie M. Vose are both at the University of Nebraska, Omaha. Dr. Armitage disclosed a leadership role with Tesaro and consulting or advisory roles with GlaxoSmithKline, Roche, Spectrum Pharmaceuticals, ZIOPHARM Oncology, Conatus, and Celgene. Dr. Vose reported honoraria from Sanofi-Aventis and Seattle Genetics; consulting or advisory roles with Bioconnections; and institutional research funding from Spectrum Pharmaceuticals, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte, Janssen Biotech, Pharmacyclics, Acerta, and Kite Pharma. These remarks were adapted from their accompanying editorial (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.63.5946).
The best way to determine the effectiveness of surveillance imaging would be a randomized trial including patients with diffuse large B-cell lymphoma (DLBCL) after first complete remission, but it is unlikely that such a study will be done. The study by El-Galaly et al may be the next best approach. Taking advantage of the fact that neighboring countries Denmark and Sweden have opposite policies for surveillance imaging but otherwise similar follow-up visit schedules and testing, the authors identified factors that predicted relapse (e.g., age greater than 60 years and elevated LDH), and they found that routine surveillance imaging had no impact on outcome. The study presents the strongest argument yet published against routine surveillance imaging.
The two other outstanding issues of routine surveillance are long-term safety and cost benefit. The study by El-Galaly et al, in combination with several other reports, suggests that routine surveillance imaging, in the absence of new or suspicious symptoms, physical findings, or change in laboratory results, is unlikely to benefit patients, may add to the patient’s stress, may cause long-term health problems, and incurs substantial economic cost.
Dr. James O. Armitage and Dr. Julie M. Vose are both at the University of Nebraska, Omaha. Dr. Armitage disclosed a leadership role with Tesaro and consulting or advisory roles with GlaxoSmithKline, Roche, Spectrum Pharmaceuticals, ZIOPHARM Oncology, Conatus, and Celgene. Dr. Vose reported honoraria from Sanofi-Aventis and Seattle Genetics; consulting or advisory roles with Bioconnections; and institutional research funding from Spectrum Pharmaceuticals, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte, Janssen Biotech, Pharmacyclics, Acerta, and Kite Pharma. These remarks were adapted from their accompanying editorial (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.63.5946).
A population-based comparison of patients with diffuse large B-cell lymphoma (DLBCL) in first complete remission indicated that routine imaging surveillance did not improve outcomes, researchers reported.
Overall survival was similar for Danish and Swedish populations who received similar follow-up care, except that routine imaging surveillance is the standard of care in Denmark, but not in Sweden. The 3-year overall survival for Danish and Swedish patients was 92% and 91%, respectively.
Outcomes grouped by international prognostic index (IPI) also showed no significant differences between populations (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.62.0229.).
“An imaging-based follow-up strategy does not improve postremission [overall survival] for DLBCL,” wrote Dr. Tarec Christoffer El-Galaly, of Aalborg University Hospital, Denmark, and colleagues.
They observed that aside from using IPI as risk stratification, the study “also points to baseline [lactate dehyrogenase] as a single discriminator of patients with high versus low risk of progression,” (Hazard ratio, 3.12; 95% CI, 1.78-5.48; P less than .01).
The retrospective study examined records of patients with DLBCL from Sweden (n=696) and Denmark (n=525) who achieved first complete remission after first-line therapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP-like regimens from 2007 to 2012. The proportion of patients with IPI greater than two were similar for both groups, though more Danish patients received radiotherapy compared with their Swedish counterparts (35% v. 9%).
Standard follow-up care after first complete remission is similar in Denmark and Sweden and typically includes symptom assessment, clinical examination, and blood tests at 3- to 4-month intervals for 2 years, and 6-month intervals in the third year. After 3 years, Swedish patients are seen annually for 2 years and then follow-up is ended for most patients. In Denmark, 6-month checks are continued until 5 years and then follow-up is usually ended. However, in Denmark guidelines support routine computerized tomography (CT) scans of the neck, abdomen, and thorax every 6 months for 2 years, which is not encouraged by guidelines in Sweden.
Early relapse detection aims to improve survival, and although low disease burden is associated with durable survival in patients treated for relapsed DLBCL, most studies show similar outcomes for imaging versus non-imaging detection. Additionally, previous retrospective studies that have reported survival differences based on relapse detection method are prone to lead-time bias, according to the researchers.
Given that a majority of patients with recurrent DLBCL experience symptoms before relapse, that elevated lactate dehyrogenase or abnormal physical examination may raise suspicion, and that exposure to ionizing radiation from medical imaging can lead to radiation-induced cancers, “routine imaging for DLBCL in first [complete remission] is not recommended,” the authors wrote.
The research was supported in part by the North Denmark Region. Dr. El-Galaly and coauthors reported having no financial disclosures.
A population-based comparison of patients with diffuse large B-cell lymphoma (DLBCL) in first complete remission indicated that routine imaging surveillance did not improve outcomes, researchers reported.
Overall survival was similar for Danish and Swedish populations who received similar follow-up care, except that routine imaging surveillance is the standard of care in Denmark, but not in Sweden. The 3-year overall survival for Danish and Swedish patients was 92% and 91%, respectively.
Outcomes grouped by international prognostic index (IPI) also showed no significant differences between populations (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.62.0229.).
“An imaging-based follow-up strategy does not improve postremission [overall survival] for DLBCL,” wrote Dr. Tarec Christoffer El-Galaly, of Aalborg University Hospital, Denmark, and colleagues.
They observed that aside from using IPI as risk stratification, the study “also points to baseline [lactate dehyrogenase] as a single discriminator of patients with high versus low risk of progression,” (Hazard ratio, 3.12; 95% CI, 1.78-5.48; P less than .01).
The retrospective study examined records of patients with DLBCL from Sweden (n=696) and Denmark (n=525) who achieved first complete remission after first-line therapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP-like regimens from 2007 to 2012. The proportion of patients with IPI greater than two were similar for both groups, though more Danish patients received radiotherapy compared with their Swedish counterparts (35% v. 9%).
Standard follow-up care after first complete remission is similar in Denmark and Sweden and typically includes symptom assessment, clinical examination, and blood tests at 3- to 4-month intervals for 2 years, and 6-month intervals in the third year. After 3 years, Swedish patients are seen annually for 2 years and then follow-up is ended for most patients. In Denmark, 6-month checks are continued until 5 years and then follow-up is usually ended. However, in Denmark guidelines support routine computerized tomography (CT) scans of the neck, abdomen, and thorax every 6 months for 2 years, which is not encouraged by guidelines in Sweden.
Early relapse detection aims to improve survival, and although low disease burden is associated with durable survival in patients treated for relapsed DLBCL, most studies show similar outcomes for imaging versus non-imaging detection. Additionally, previous retrospective studies that have reported survival differences based on relapse detection method are prone to lead-time bias, according to the researchers.
Given that a majority of patients with recurrent DLBCL experience symptoms before relapse, that elevated lactate dehyrogenase or abnormal physical examination may raise suspicion, and that exposure to ionizing radiation from medical imaging can lead to radiation-induced cancers, “routine imaging for DLBCL in first [complete remission] is not recommended,” the authors wrote.
The research was supported in part by the North Denmark Region. Dr. El-Galaly and coauthors reported having no financial disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:Danish patients with diffuse large B-cell lymphoma (DLBCL) who received routine imaging during follow up had similar survival to Swedish patients who did not undergo routine imaging surveillance.
Major finding: After first complete remission, the 3-year overall survival for Danish and Swedish patients was 92% and 91%, respectively.
Data source: Population-based study of 525 Danish patients and 696 Swedish patients with DLBCL who achieved first complete remission after R-CHOP/CHOP-like first-line therapies from 2007 to 2012.
Disclosures: The research was supported in part by the North Denmark Region. Dr. El-Galaly and coauthors reported having no financial disclosures.
Obinutuzumab trends better than rituxumab in relapsed indolent lymphoma
Patients with relapsed follicular lymphoma who were treated with obinutuzumab experienced higher response rates than did patients given rituximab with an acceptable safety profile, according to new findings.
However, the difference did not translate into an improvement in progression-free survival, so the clinical value of obinutuzumab in this patient population is still unclear.
The quality of remissions was better with obinutuzumab, with an almost twofold higher complete response/unconfirmed complete response rate (41.9% vs. 22.7%; P = .006),” wrote Dr. Laurie Sehn from the Centre for Lymphoid Cancer, British Columbia Cancer Agency and the University of British Columbia, Vancouver, and her colleagues (J Clin Oncol. 2015 Aug 17. doi:10.1200/JCO.2014.59.2139).
On the basis of an independent review, the best overall response was better in the obinutuzumab arm (P = .04), but the complete response/unconfirmed response rate was not different for the two groups.
The study was published online Aug. 17 in the Journal of Clinical Oncology.
A total of 175 patients with relapsed CD20+ indolent lymphoma were randomized 1:1 to four once-per-week infusions of either obinutuzumab (1,000 mg) or rituximab (375 mg/m2). Those without any evidence of disease progression after completing induction therapy received obinutuzumab or rituximab maintenance therapy every 2 months for up to 2 years.
At the end of induction, the investigator assessed overall response rate was 44.6% in the obinutuzumab arm and 33.3% in the rituximab arm (P = .08); nine patients receiving obinutuzumab (12.2%) and four given rituximab (5.3%) achieved complete response or unconfirmed complete response, but the difference was not significant (P = .07).
Independent review also found the overall response rate to be higher with obinutuzumab vs. rituximab (44.6% vs. 26.7%; P = .01), but with no difference in complete response/unconfirmed complete response rate (5.4 vs. 4.0; P = .34).
Adverse events were similar in each group, and most episodes were grade 1 to 2. Higher rates of infusion-related reactions (74% vs. 51%) and cough (24% vs. 9%) were observed in the obinutuzumab vs. the rituximab arm.
Dr. Sehn receives research funding and honoraria from, and serves in a consulting or advisory role to, Roche/Genentech, the maker of obinutuzumab (Gyzyva) and rituximab (Rituxan). She also receives honoraria from and serves in a consulting or advisory role to Amgen, Janssen, Seattle Genetics, Lundbeck, and Celgene.
Patients with relapsed follicular lymphoma who were treated with obinutuzumab experienced higher response rates than did patients given rituximab with an acceptable safety profile, according to new findings.
However, the difference did not translate into an improvement in progression-free survival, so the clinical value of obinutuzumab in this patient population is still unclear.
The quality of remissions was better with obinutuzumab, with an almost twofold higher complete response/unconfirmed complete response rate (41.9% vs. 22.7%; P = .006),” wrote Dr. Laurie Sehn from the Centre for Lymphoid Cancer, British Columbia Cancer Agency and the University of British Columbia, Vancouver, and her colleagues (J Clin Oncol. 2015 Aug 17. doi:10.1200/JCO.2014.59.2139).
On the basis of an independent review, the best overall response was better in the obinutuzumab arm (P = .04), but the complete response/unconfirmed response rate was not different for the two groups.
The study was published online Aug. 17 in the Journal of Clinical Oncology.
A total of 175 patients with relapsed CD20+ indolent lymphoma were randomized 1:1 to four once-per-week infusions of either obinutuzumab (1,000 mg) or rituximab (375 mg/m2). Those without any evidence of disease progression after completing induction therapy received obinutuzumab or rituximab maintenance therapy every 2 months for up to 2 years.
At the end of induction, the investigator assessed overall response rate was 44.6% in the obinutuzumab arm and 33.3% in the rituximab arm (P = .08); nine patients receiving obinutuzumab (12.2%) and four given rituximab (5.3%) achieved complete response or unconfirmed complete response, but the difference was not significant (P = .07).
Independent review also found the overall response rate to be higher with obinutuzumab vs. rituximab (44.6% vs. 26.7%; P = .01), but with no difference in complete response/unconfirmed complete response rate (5.4 vs. 4.0; P = .34).
Adverse events were similar in each group, and most episodes were grade 1 to 2. Higher rates of infusion-related reactions (74% vs. 51%) and cough (24% vs. 9%) were observed in the obinutuzumab vs. the rituximab arm.
Dr. Sehn receives research funding and honoraria from, and serves in a consulting or advisory role to, Roche/Genentech, the maker of obinutuzumab (Gyzyva) and rituximab (Rituxan). She also receives honoraria from and serves in a consulting or advisory role to Amgen, Janssen, Seattle Genetics, Lundbeck, and Celgene.
Patients with relapsed follicular lymphoma who were treated with obinutuzumab experienced higher response rates than did patients given rituximab with an acceptable safety profile, according to new findings.
However, the difference did not translate into an improvement in progression-free survival, so the clinical value of obinutuzumab in this patient population is still unclear.
The quality of remissions was better with obinutuzumab, with an almost twofold higher complete response/unconfirmed complete response rate (41.9% vs. 22.7%; P = .006),” wrote Dr. Laurie Sehn from the Centre for Lymphoid Cancer, British Columbia Cancer Agency and the University of British Columbia, Vancouver, and her colleagues (J Clin Oncol. 2015 Aug 17. doi:10.1200/JCO.2014.59.2139).
On the basis of an independent review, the best overall response was better in the obinutuzumab arm (P = .04), but the complete response/unconfirmed response rate was not different for the two groups.
The study was published online Aug. 17 in the Journal of Clinical Oncology.
A total of 175 patients with relapsed CD20+ indolent lymphoma were randomized 1:1 to four once-per-week infusions of either obinutuzumab (1,000 mg) or rituximab (375 mg/m2). Those without any evidence of disease progression after completing induction therapy received obinutuzumab or rituximab maintenance therapy every 2 months for up to 2 years.
At the end of induction, the investigator assessed overall response rate was 44.6% in the obinutuzumab arm and 33.3% in the rituximab arm (P = .08); nine patients receiving obinutuzumab (12.2%) and four given rituximab (5.3%) achieved complete response or unconfirmed complete response, but the difference was not significant (P = .07).
Independent review also found the overall response rate to be higher with obinutuzumab vs. rituximab (44.6% vs. 26.7%; P = .01), but with no difference in complete response/unconfirmed complete response rate (5.4 vs. 4.0; P = .34).
Adverse events were similar in each group, and most episodes were grade 1 to 2. Higher rates of infusion-related reactions (74% vs. 51%) and cough (24% vs. 9%) were observed in the obinutuzumab vs. the rituximab arm.
Dr. Sehn receives research funding and honoraria from, and serves in a consulting or advisory role to, Roche/Genentech, the maker of obinutuzumab (Gyzyva) and rituximab (Rituxan). She also receives honoraria from and serves in a consulting or advisory role to Amgen, Janssen, Seattle Genetics, Lundbeck, and Celgene.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Obinutuzumab was associated with a higher overall response rate as compared with rituximab, but obinutuzumab’s clinical benefit in non–Hodgkin lymphoma is still unclear.
Major finding: Among patients with follicular lymphoma (n = 149), overall response rate trended higher for obinutuzumab, compared with rituximab (44.6% vs. 33.3%; P = .08).
Data source: An open-label, multicenter, randomized, phase II study of 175 patients with relapsed CD20+ indolent lymphoma that compared induction with obinutuzumab vs. rituximab.
Disclosures: Dr. Sehn receives research funding and honoraria from, and serves in a consulting or advisory role to Roche/Genentech, the maker of obinutuzumab (Gyzyva) and rituximab (Rituxan). She also receives honoraria from and serves in a consulting or advisory role to Amgen, Janssen, Seattle Genetics, Lundbeck, and Celgene.
Lenalidomide + rituximab combo effective in recurrent follicular lymphoma
The combination of lenalidomide and rituximab was more active in patients with recurrent follicular lymphoma, compared with lenalidomide alone, and significantly increased the overall response rate, according to new data published online Aug. 24 in the Journal of Clinical Oncology.
Although both lenalidomide and rituximab are active agents in follicular lymphoma, their combined use in recurrent follicular lymphoma has not been previously evaluated in randomized clinical trials, said Dr. John P. Leonard of Cornell University, New York, and his colleagues.
The overall response rate of patients receiving the combination regimen was significantly higher than that of patients who received lenalidomide alone (P = .029). In the cohort receiving lenalidomide alone, 24 patients (53%) achieved an objective response (9 complete responses [20%]), while 35 patients (76%) in the lenalidomide/rituximab group were responders (18 complete responses [39%]).
At a median follow-up of 2.5 years (range, 0.1-4.8 years), the addition of rituximab to lenalidomide in this population also significantly increased the median time to progression: 1.1 year for lenalidomide alone versus 2 years for the combined therapy (P = .002).
Overall survival was 4.5 years for lenalidomide alone and has not yet been reached for the combination arm (P = .149.
This trial helps to establish the safety profile of single-agent lenalidomide in follicular lymphoma, while its “randomized nature also allows a direct assessment of potential toxicity resulting from the addition of rituximab to lenalidomide,” wrote Dr. Leonard and his associates (J Clin Oncol. 2015 Aug 24. doi: 10.1200/JCO.2014.59.9258).
“There was no evidence of increased toxicity from the lenalidomide/rituximab combination compared with lenalidomide alone,” they pointed out.
Both lenalidomide alone and lenalidomide/rituximab were well tolerated, with grade 3-4 adverse events occurring in 58% and 53% of patients, respectively, with 9% and 11% of patients experiencing grade 4 toxicity, respectively. The most common grade 3-4 adverse events included neutropenia (16% vs. 20%), fatigue (9% vs. 13%), and rash (4% vs. 4%).
The study was supported in part by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology. Dr. Leonard reported financial relationships with Celgene and Genentech, and several coauthors also reported relationships with industry.
The combination of lenalidomide and rituximab was more active in patients with recurrent follicular lymphoma, compared with lenalidomide alone, and significantly increased the overall response rate, according to new data published online Aug. 24 in the Journal of Clinical Oncology.
Although both lenalidomide and rituximab are active agents in follicular lymphoma, their combined use in recurrent follicular lymphoma has not been previously evaluated in randomized clinical trials, said Dr. John P. Leonard of Cornell University, New York, and his colleagues.
The overall response rate of patients receiving the combination regimen was significantly higher than that of patients who received lenalidomide alone (P = .029). In the cohort receiving lenalidomide alone, 24 patients (53%) achieved an objective response (9 complete responses [20%]), while 35 patients (76%) in the lenalidomide/rituximab group were responders (18 complete responses [39%]).
At a median follow-up of 2.5 years (range, 0.1-4.8 years), the addition of rituximab to lenalidomide in this population also significantly increased the median time to progression: 1.1 year for lenalidomide alone versus 2 years for the combined therapy (P = .002).
Overall survival was 4.5 years for lenalidomide alone and has not yet been reached for the combination arm (P = .149.
This trial helps to establish the safety profile of single-agent lenalidomide in follicular lymphoma, while its “randomized nature also allows a direct assessment of potential toxicity resulting from the addition of rituximab to lenalidomide,” wrote Dr. Leonard and his associates (J Clin Oncol. 2015 Aug 24. doi: 10.1200/JCO.2014.59.9258).
“There was no evidence of increased toxicity from the lenalidomide/rituximab combination compared with lenalidomide alone,” they pointed out.
Both lenalidomide alone and lenalidomide/rituximab were well tolerated, with grade 3-4 adverse events occurring in 58% and 53% of patients, respectively, with 9% and 11% of patients experiencing grade 4 toxicity, respectively. The most common grade 3-4 adverse events included neutropenia (16% vs. 20%), fatigue (9% vs. 13%), and rash (4% vs. 4%).
The study was supported in part by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology. Dr. Leonard reported financial relationships with Celgene and Genentech, and several coauthors also reported relationships with industry.
The combination of lenalidomide and rituximab was more active in patients with recurrent follicular lymphoma, compared with lenalidomide alone, and significantly increased the overall response rate, according to new data published online Aug. 24 in the Journal of Clinical Oncology.
Although both lenalidomide and rituximab are active agents in follicular lymphoma, their combined use in recurrent follicular lymphoma has not been previously evaluated in randomized clinical trials, said Dr. John P. Leonard of Cornell University, New York, and his colleagues.
The overall response rate of patients receiving the combination regimen was significantly higher than that of patients who received lenalidomide alone (P = .029). In the cohort receiving lenalidomide alone, 24 patients (53%) achieved an objective response (9 complete responses [20%]), while 35 patients (76%) in the lenalidomide/rituximab group were responders (18 complete responses [39%]).
At a median follow-up of 2.5 years (range, 0.1-4.8 years), the addition of rituximab to lenalidomide in this population also significantly increased the median time to progression: 1.1 year for lenalidomide alone versus 2 years for the combined therapy (P = .002).
Overall survival was 4.5 years for lenalidomide alone and has not yet been reached for the combination arm (P = .149.
This trial helps to establish the safety profile of single-agent lenalidomide in follicular lymphoma, while its “randomized nature also allows a direct assessment of potential toxicity resulting from the addition of rituximab to lenalidomide,” wrote Dr. Leonard and his associates (J Clin Oncol. 2015 Aug 24. doi: 10.1200/JCO.2014.59.9258).
“There was no evidence of increased toxicity from the lenalidomide/rituximab combination compared with lenalidomide alone,” they pointed out.
Both lenalidomide alone and lenalidomide/rituximab were well tolerated, with grade 3-4 adverse events occurring in 58% and 53% of patients, respectively, with 9% and 11% of patients experiencing grade 4 toxicity, respectively. The most common grade 3-4 adverse events included neutropenia (16% vs. 20%), fatigue (9% vs. 13%), and rash (4% vs. 4%).
The study was supported in part by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology. Dr. Leonard reported financial relationships with Celgene and Genentech, and several coauthors also reported relationships with industry.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Lenalidomide combined with rituximab is more active in recurrent follicular lymphoma, compared with lenalidomide monotherapy.
Major finding: Overall response rate was 53% (20% complete response) for lenalidomide alone versus 76% (39% complete response) for lenalidomide and rituximab (P = .029).
Data source: Randomized phase II clinical trial of 91 patients with follicular lymphoma who were assigned to receive lenalidomide alone or lenalidomide combined with rituximab.
Disclosures: The study was supported in part by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology. Dr. Leonard reported financial relationships with Celgene and Genentech, and several coauthors also reported relationships with industry.
Secondary CNS lymphoma regimen linked to 41% survival at 5 years
Treatment with high doses of antimetabolites followed by rituximab plus high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation was feasible and effective in a multicenter phase II study of 38 patients with aggressive B-cell lymphoma and secondary central nervous system involvement.
The patients, aged 18 to 70 years with Eastern Cooperative Oncology Group performance status of 3 or less at enrollment, were treated with high doses of methotrexate and cytarabine, followed by rituximab plus high-dose sequential chemoimmunotherapy (R-HDS) consisting of cylcophosphamide, cytarabine, and etoposide supported by autologous stem-cell transplantation in eligible patients.
Toxicity was typically manageable, but 30 treatment courses were complicated by grade 3 or 4 febrile neutropenia and/or infections; 4 patients died because of toxicity, Dr. Andres J. M. Ferreri of San Raffaele Scientific Institute, Milan, Italy and colleagues reported online Aug. 17 in the Journal of Clinical Oncology.
The complete response rate was 63% (24 patients), and 17 patients remained relapse free at a median follow-up of 48 months, with a 2-year event-free survival rate of 50%, and a 5-year survival rate of 41%, the investigators said (J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.61.1236).
This novel radiotherapy-free regimen, developed based on encouraging outcomes with antimetabolites in patients with primary CNS lymphoma and with R-HDS in relapsed aggressive lymphoma, appears safe and effective in the setting of secondary CNS lymphoma (SCNSL).
“We propose this strategy as the standard of care for patients with SCNSL and as a comparison control regimen for future trials,” they concluded.
Dr. Ferreri reported having no disclosures. One co-author, Dr. Federico Caligaris-Cappio, reported serving in a consulting or advisory role for Janssen and Pharmacyclics.
The findings of Ferreri et al highlight the significant progress that has been made toward finding a cure for aggressive B-cell lymphoma with concomitant or subsequent CNS involvement.
Just a few years ago, this condition was fatal in nearly all cases, but in light of these and other recent findings, needed improvements in treatment seem possible.
Among other strategies, complete elimination of non-[blood-brain barrier]-crossing agents and administration of more than two cycles of induction chemotherapy might prove to be of value. In addition, oral targeted therapies with small molecules, most of which easily cross the BBB, hold promise.
Dr. Norbert Schmitz and Huei-Shan Wuthey are with Asklepios Hospital St. Georg, Hamburg, Germany. They made their remarks in an editorial(J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.63.1143) that accompanied the study. Dr. Schmitz reported serving in a consulting or advisory role for Roche and receiving research funding from Roche. Huei-Shan Wu reported having no disclosures.
The findings of Ferreri et al highlight the significant progress that has been made toward finding a cure for aggressive B-cell lymphoma with concomitant or subsequent CNS involvement.
Just a few years ago, this condition was fatal in nearly all cases, but in light of these and other recent findings, needed improvements in treatment seem possible.
Among other strategies, complete elimination of non-[blood-brain barrier]-crossing agents and administration of more than two cycles of induction chemotherapy might prove to be of value. In addition, oral targeted therapies with small molecules, most of which easily cross the BBB, hold promise.
Dr. Norbert Schmitz and Huei-Shan Wuthey are with Asklepios Hospital St. Georg, Hamburg, Germany. They made their remarks in an editorial(J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.63.1143) that accompanied the study. Dr. Schmitz reported serving in a consulting or advisory role for Roche and receiving research funding from Roche. Huei-Shan Wu reported having no disclosures.
The findings of Ferreri et al highlight the significant progress that has been made toward finding a cure for aggressive B-cell lymphoma with concomitant or subsequent CNS involvement.
Just a few years ago, this condition was fatal in nearly all cases, but in light of these and other recent findings, needed improvements in treatment seem possible.
Among other strategies, complete elimination of non-[blood-brain barrier]-crossing agents and administration of more than two cycles of induction chemotherapy might prove to be of value. In addition, oral targeted therapies with small molecules, most of which easily cross the BBB, hold promise.
Dr. Norbert Schmitz and Huei-Shan Wuthey are with Asklepios Hospital St. Georg, Hamburg, Germany. They made their remarks in an editorial(J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.63.1143) that accompanied the study. Dr. Schmitz reported serving in a consulting or advisory role for Roche and receiving research funding from Roche. Huei-Shan Wu reported having no disclosures.
Treatment with high doses of antimetabolites followed by rituximab plus high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation was feasible and effective in a multicenter phase II study of 38 patients with aggressive B-cell lymphoma and secondary central nervous system involvement.
The patients, aged 18 to 70 years with Eastern Cooperative Oncology Group performance status of 3 or less at enrollment, were treated with high doses of methotrexate and cytarabine, followed by rituximab plus high-dose sequential chemoimmunotherapy (R-HDS) consisting of cylcophosphamide, cytarabine, and etoposide supported by autologous stem-cell transplantation in eligible patients.
Toxicity was typically manageable, but 30 treatment courses were complicated by grade 3 or 4 febrile neutropenia and/or infections; 4 patients died because of toxicity, Dr. Andres J. M. Ferreri of San Raffaele Scientific Institute, Milan, Italy and colleagues reported online Aug. 17 in the Journal of Clinical Oncology.
The complete response rate was 63% (24 patients), and 17 patients remained relapse free at a median follow-up of 48 months, with a 2-year event-free survival rate of 50%, and a 5-year survival rate of 41%, the investigators said (J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.61.1236).
This novel radiotherapy-free regimen, developed based on encouraging outcomes with antimetabolites in patients with primary CNS lymphoma and with R-HDS in relapsed aggressive lymphoma, appears safe and effective in the setting of secondary CNS lymphoma (SCNSL).
“We propose this strategy as the standard of care for patients with SCNSL and as a comparison control regimen for future trials,” they concluded.
Dr. Ferreri reported having no disclosures. One co-author, Dr. Federico Caligaris-Cappio, reported serving in a consulting or advisory role for Janssen and Pharmacyclics.
Treatment with high doses of antimetabolites followed by rituximab plus high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation was feasible and effective in a multicenter phase II study of 38 patients with aggressive B-cell lymphoma and secondary central nervous system involvement.
The patients, aged 18 to 70 years with Eastern Cooperative Oncology Group performance status of 3 or less at enrollment, were treated with high doses of methotrexate and cytarabine, followed by rituximab plus high-dose sequential chemoimmunotherapy (R-HDS) consisting of cylcophosphamide, cytarabine, and etoposide supported by autologous stem-cell transplantation in eligible patients.
Toxicity was typically manageable, but 30 treatment courses were complicated by grade 3 or 4 febrile neutropenia and/or infections; 4 patients died because of toxicity, Dr. Andres J. M. Ferreri of San Raffaele Scientific Institute, Milan, Italy and colleagues reported online Aug. 17 in the Journal of Clinical Oncology.
The complete response rate was 63% (24 patients), and 17 patients remained relapse free at a median follow-up of 48 months, with a 2-year event-free survival rate of 50%, and a 5-year survival rate of 41%, the investigators said (J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.61.1236).
This novel radiotherapy-free regimen, developed based on encouraging outcomes with antimetabolites in patients with primary CNS lymphoma and with R-HDS in relapsed aggressive lymphoma, appears safe and effective in the setting of secondary CNS lymphoma (SCNSL).
“We propose this strategy as the standard of care for patients with SCNSL and as a comparison control regimen for future trials,” they concluded.
Dr. Ferreri reported having no disclosures. One co-author, Dr. Federico Caligaris-Cappio, reported serving in a consulting or advisory role for Janssen and Pharmacyclics.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: High doses of antimetabolites followed by R-HDS and autologous stem-cell transplantation was feasible and effective in 38 patients with aggressive B-cell lymphoma and secondary central nervous system involvement.
Major finding: The 2-year event-free survival rate was 50%, and the 5-year survival rate was 41%
Data source: A multicenter phase II study of 38 adults.
Disclosures: Dr. Ferreri reported having no disclosures. One co-author, Dr. Federico Caligaris-Cappio, reported serving in a consulting or advisory role for Janssen and Pharmacyclics.
Lymph2Cx assay identifies outcomes in DLBCL
In patients with diffuse large B-cell lymphoma (DLBCL) treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), the Lymph2Cx assay was able to separate the cohort into groups with significantly different outcomes based on cell of origin (COO), investigators reported online in the Journal of Clinical Oncology.
In pairwise multivariable analyses, COO was associated with outcomes that were independent of International Prognostic Index score (IPI) and MYC/BCL2 immunohistochemistry (IHC).
Gene expression profiling of DLBCL has provided classification into two distinct subtypes: germinal center B-cell–like (GCB) and activated B-cell–like (ABC) subtypes. Using the cell of origin classification can define subgroups with distinct biology ad pathogenesis, as well as identify patient groups with different outcomes after treatment. Improvements in technology have allowed for the use of formalin-fixed paraffin-embedded tissue (FFPET) biopsies for more reliable gene expression profiling.
“The size of the study cohort allowed exploration of the prognostic value of COO in comparison with other prognostic tools,” wrote Dr. David W. Scott of the British Columbia Cancer Agency, Vancouver, and his colleagues (J Clin Oncol. 2015 Aug 3. doi: 10.1200/JCO.2014.60.2383).“Although the IPI remains the most powerful tool for risk stratification, COO assignment provides additional prognostic information, particularly evident in the intermediate IPI score group,” the authors noted.
The consistency and reproducibility of COO assignment using the Lymph2Cx assay was evaluated in a large patient cohort treated with R-CHOP therapy, and the relationship between COO, MYC/BCL2 dual expression, and IPI score with respect to defining prognosis in patients with DLBCL was also investigated.
Reproducibility of COO assignment using the Lymph2Cx assay was tested using repeated sampling within tumor biopsies and changes in reagent lots, and concordance of COO calls across the two reagent lots was 100%.
The COO was then determined in 344 patients with de novo DLBCL who were treated with R-CHOP at a single center. MYC and BCL2 protein expression was assessed using immunohistochemistry on tissue microarrays, and the median follow-up of living patients was 6.5 years (range, 0.75-13.2 years).
COO was a prognostic biomarker in the patient cohort. Those with activated B-cell–like DLBCL had significantly inferior outcomes as compared to patients with germinal center B-cell–like DLBCL (log-rank P less than .001 for time to progression, progression-free survival, disease-specific survival, and overall survival).
When reviewing the relationship between COO, IPI score, and MYC/BCL2 IHC, pairwise multivariable analyses demonstrated that the prognostic impact of COO is independent of IPI score. The prognostic value added by COO was most notable when patients with intermediate IPI scores were evaluated (ABC vs. GCB: 5-year time to progression, 53% v 74%; log-rank P = .003).
Both COO and MYC/BCL2 IHC identified high-risk groups that were similar in size (32% vs. 31%) with comparable outcomes (5-year time to progression, 51% vs. 51%). In pairwise multivariable analyses that included COO and MYC/BCL2 IHC as variables, COO remained significant, thus showing that COO had prognostic power “beyond that conferred merely by enrichment of MYC-positive/BCL2-positive patient cases of the ABC subtype,” wrote the authors.
When COO, IPI, and MYC/BCL2 IHC were all included in multivariable analyses, COO remained significantly associated with time to progression and progression-free survival.
“We anticipate that over the next few years, with the emergence of agents with selective activity in ABC or GCB DLBCL, the determination of COO will become part of the foundation for optimal patient care,” the authors concluded.
In patients with diffuse large B-cell lymphoma (DLBCL) treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), the Lymph2Cx assay was able to separate the cohort into groups with significantly different outcomes based on cell of origin (COO), investigators reported online in the Journal of Clinical Oncology.
In pairwise multivariable analyses, COO was associated with outcomes that were independent of International Prognostic Index score (IPI) and MYC/BCL2 immunohistochemistry (IHC).
Gene expression profiling of DLBCL has provided classification into two distinct subtypes: germinal center B-cell–like (GCB) and activated B-cell–like (ABC) subtypes. Using the cell of origin classification can define subgroups with distinct biology ad pathogenesis, as well as identify patient groups with different outcomes after treatment. Improvements in technology have allowed for the use of formalin-fixed paraffin-embedded tissue (FFPET) biopsies for more reliable gene expression profiling.
“The size of the study cohort allowed exploration of the prognostic value of COO in comparison with other prognostic tools,” wrote Dr. David W. Scott of the British Columbia Cancer Agency, Vancouver, and his colleagues (J Clin Oncol. 2015 Aug 3. doi: 10.1200/JCO.2014.60.2383).“Although the IPI remains the most powerful tool for risk stratification, COO assignment provides additional prognostic information, particularly evident in the intermediate IPI score group,” the authors noted.
The consistency and reproducibility of COO assignment using the Lymph2Cx assay was evaluated in a large patient cohort treated with R-CHOP therapy, and the relationship between COO, MYC/BCL2 dual expression, and IPI score with respect to defining prognosis in patients with DLBCL was also investigated.
Reproducibility of COO assignment using the Lymph2Cx assay was tested using repeated sampling within tumor biopsies and changes in reagent lots, and concordance of COO calls across the two reagent lots was 100%.
The COO was then determined in 344 patients with de novo DLBCL who were treated with R-CHOP at a single center. MYC and BCL2 protein expression was assessed using immunohistochemistry on tissue microarrays, and the median follow-up of living patients was 6.5 years (range, 0.75-13.2 years).
COO was a prognostic biomarker in the patient cohort. Those with activated B-cell–like DLBCL had significantly inferior outcomes as compared to patients with germinal center B-cell–like DLBCL (log-rank P less than .001 for time to progression, progression-free survival, disease-specific survival, and overall survival).
When reviewing the relationship between COO, IPI score, and MYC/BCL2 IHC, pairwise multivariable analyses demonstrated that the prognostic impact of COO is independent of IPI score. The prognostic value added by COO was most notable when patients with intermediate IPI scores were evaluated (ABC vs. GCB: 5-year time to progression, 53% v 74%; log-rank P = .003).
Both COO and MYC/BCL2 IHC identified high-risk groups that were similar in size (32% vs. 31%) with comparable outcomes (5-year time to progression, 51% vs. 51%). In pairwise multivariable analyses that included COO and MYC/BCL2 IHC as variables, COO remained significant, thus showing that COO had prognostic power “beyond that conferred merely by enrichment of MYC-positive/BCL2-positive patient cases of the ABC subtype,” wrote the authors.
When COO, IPI, and MYC/BCL2 IHC were all included in multivariable analyses, COO remained significantly associated with time to progression and progression-free survival.
“We anticipate that over the next few years, with the emergence of agents with selective activity in ABC or GCB DLBCL, the determination of COO will become part of the foundation for optimal patient care,” the authors concluded.
In patients with diffuse large B-cell lymphoma (DLBCL) treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), the Lymph2Cx assay was able to separate the cohort into groups with significantly different outcomes based on cell of origin (COO), investigators reported online in the Journal of Clinical Oncology.
In pairwise multivariable analyses, COO was associated with outcomes that were independent of International Prognostic Index score (IPI) and MYC/BCL2 immunohistochemistry (IHC).
Gene expression profiling of DLBCL has provided classification into two distinct subtypes: germinal center B-cell–like (GCB) and activated B-cell–like (ABC) subtypes. Using the cell of origin classification can define subgroups with distinct biology ad pathogenesis, as well as identify patient groups with different outcomes after treatment. Improvements in technology have allowed for the use of formalin-fixed paraffin-embedded tissue (FFPET) biopsies for more reliable gene expression profiling.
“The size of the study cohort allowed exploration of the prognostic value of COO in comparison with other prognostic tools,” wrote Dr. David W. Scott of the British Columbia Cancer Agency, Vancouver, and his colleagues (J Clin Oncol. 2015 Aug 3. doi: 10.1200/JCO.2014.60.2383).“Although the IPI remains the most powerful tool for risk stratification, COO assignment provides additional prognostic information, particularly evident in the intermediate IPI score group,” the authors noted.
The consistency and reproducibility of COO assignment using the Lymph2Cx assay was evaluated in a large patient cohort treated with R-CHOP therapy, and the relationship between COO, MYC/BCL2 dual expression, and IPI score with respect to defining prognosis in patients with DLBCL was also investigated.
Reproducibility of COO assignment using the Lymph2Cx assay was tested using repeated sampling within tumor biopsies and changes in reagent lots, and concordance of COO calls across the two reagent lots was 100%.
The COO was then determined in 344 patients with de novo DLBCL who were treated with R-CHOP at a single center. MYC and BCL2 protein expression was assessed using immunohistochemistry on tissue microarrays, and the median follow-up of living patients was 6.5 years (range, 0.75-13.2 years).
COO was a prognostic biomarker in the patient cohort. Those with activated B-cell–like DLBCL had significantly inferior outcomes as compared to patients with germinal center B-cell–like DLBCL (log-rank P less than .001 for time to progression, progression-free survival, disease-specific survival, and overall survival).
When reviewing the relationship between COO, IPI score, and MYC/BCL2 IHC, pairwise multivariable analyses demonstrated that the prognostic impact of COO is independent of IPI score. The prognostic value added by COO was most notable when patients with intermediate IPI scores were evaluated (ABC vs. GCB: 5-year time to progression, 53% v 74%; log-rank P = .003).
Both COO and MYC/BCL2 IHC identified high-risk groups that were similar in size (32% vs. 31%) with comparable outcomes (5-year time to progression, 51% vs. 51%). In pairwise multivariable analyses that included COO and MYC/BCL2 IHC as variables, COO remained significant, thus showing that COO had prognostic power “beyond that conferred merely by enrichment of MYC-positive/BCL2-positive patient cases of the ABC subtype,” wrote the authors.
When COO, IPI, and MYC/BCL2 IHC were all included in multivariable analyses, COO remained significantly associated with time to progression and progression-free survival.
“We anticipate that over the next few years, with the emergence of agents with selective activity in ABC or GCB DLBCL, the determination of COO will become part of the foundation for optimal patient care,” the authors concluded.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The Lymph2Cx is an accurate assay for DLBCL cell of origin assignment, and identified groups with significantly different outcomes after treatment with R-CHOP.
Major finding: The Lymph2Cx assay provided concordant COO calls in 100% of 83 formalin-fixed paraffin-embedded tissue biopsies, and COO was associated with significantly different outcomes independent of IPI score and MYC/BCL2 immunohistochemistry
Data source: The Lymph2Cx assay was compared with the IPI) score and MYC/BCL2 coexpression status in FFPET biopsies from 344 patients with DLBCL uniformly treated with R-CHOP.
Disclosures: The study was supported by the Terry Fox Foundation and by the British Columbia Cancer Foundation. Dr. Scott is potentially named inventor on pending patent on use of gene expression profiling to assign COO in DLBCL. Dr. Scott and several coauthors also report financial relationships with industry.
Treatment of choice for NLPHL? Involved-field RT alone
Involved-field radiotherapy alone should be the treatment of choice for stage 1A nodular lymphocyte-predominant Hodgkin lymphoma because it yields similar survival outcomes but fewer toxic effects than other therapies, investigators reported online Aug. 3 in Journal of Clinical Oncology.
Few studies have examined the long-term outcomes of patients who have this generally indolent malignancy, even though late treatment-associated effects are their major cause of death, said Dr. Dennis A. Eichenauer and his associates at University Hospital Cologne and University Hospital Münster, both in Germany.
To assess long-term outcomes in nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), the investigators analyzed data for 256 patients who participated in seven prospective randomized clinical trials comparing various treatments during a 21-year period, who were followed for a median of 91 months. The study participants’ median age at diagnosis was 39 years (range, 16-75 years), and all achieved remission with therapy. They were categorized by treatment type: 108 received involved-field radiotherapy (IF-RT) alone; 49 received extended-field radiotherapy (EF-RT) alone; 72 received combined modality treatment involving various combinations of doxorubicin, bleomycin, vinblastine, dacarbazine, IF-RT, EF-RT, and/or rituximab; and 27 received rituximab alone.
At 8 years, progression-free survival was 91.9% with IF-RT, 84.3% with EF-RT, and 88.5% with combined modalities, which are nonsignificant differences. Overall survival was 99.0%, 95.7%, and 98.6%, respectively, which also are nonsignificant differences. Rituximab alone was markedly less effective than the other treatments, with a 4-year progression-free survival of only 81.0%. “Compared with patients who received IF-RT, patients treated with rituximab had a hazard ratio of 4.99 for relapse,” Dr. Eichenauer and his associates wrote (J Clin Oncol. 2015 Aug 3 [doi: 10.1200/JCO. 2014.60.4363]).
Only 3.7% of patients treated with IF-RT developed a second malignancy during follow-up, compared with 11.1% of those treated with combined modalities, 6.1% of those treated with EF-RT, and 7.4% of those treated with rituximab. The rate of relapse was markedly higher with rituximab than with the other treatments.
The main study findings are twofold. First, IF-RT was at least as effective as other treatments in controlling NLPHL, was less toxic acutely, and carried similar or reduced risks of late adverse effects such as relapse and second malignancies. It should be considered the first-line treatment of choice. Second, rituximab alone should not be used routinely in these patients because it yields poorer survival outcomes and a higher relapse rate. “However, it might represent an option for individual patients, such as young women with abdominal disease, to avoid gonadotoxic effects of radiotherapy,” the investigators said.
Involved-field radiotherapy alone should be the treatment of choice for stage 1A nodular lymphocyte-predominant Hodgkin lymphoma because it yields similar survival outcomes but fewer toxic effects than other therapies, investigators reported online Aug. 3 in Journal of Clinical Oncology.
Few studies have examined the long-term outcomes of patients who have this generally indolent malignancy, even though late treatment-associated effects are their major cause of death, said Dr. Dennis A. Eichenauer and his associates at University Hospital Cologne and University Hospital Münster, both in Germany.
To assess long-term outcomes in nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), the investigators analyzed data for 256 patients who participated in seven prospective randomized clinical trials comparing various treatments during a 21-year period, who were followed for a median of 91 months. The study participants’ median age at diagnosis was 39 years (range, 16-75 years), and all achieved remission with therapy. They were categorized by treatment type: 108 received involved-field radiotherapy (IF-RT) alone; 49 received extended-field radiotherapy (EF-RT) alone; 72 received combined modality treatment involving various combinations of doxorubicin, bleomycin, vinblastine, dacarbazine, IF-RT, EF-RT, and/or rituximab; and 27 received rituximab alone.
At 8 years, progression-free survival was 91.9% with IF-RT, 84.3% with EF-RT, and 88.5% with combined modalities, which are nonsignificant differences. Overall survival was 99.0%, 95.7%, and 98.6%, respectively, which also are nonsignificant differences. Rituximab alone was markedly less effective than the other treatments, with a 4-year progression-free survival of only 81.0%. “Compared with patients who received IF-RT, patients treated with rituximab had a hazard ratio of 4.99 for relapse,” Dr. Eichenauer and his associates wrote (J Clin Oncol. 2015 Aug 3 [doi: 10.1200/JCO. 2014.60.4363]).
Only 3.7% of patients treated with IF-RT developed a second malignancy during follow-up, compared with 11.1% of those treated with combined modalities, 6.1% of those treated with EF-RT, and 7.4% of those treated with rituximab. The rate of relapse was markedly higher with rituximab than with the other treatments.
The main study findings are twofold. First, IF-RT was at least as effective as other treatments in controlling NLPHL, was less toxic acutely, and carried similar or reduced risks of late adverse effects such as relapse and second malignancies. It should be considered the first-line treatment of choice. Second, rituximab alone should not be used routinely in these patients because it yields poorer survival outcomes and a higher relapse rate. “However, it might represent an option for individual patients, such as young women with abdominal disease, to avoid gonadotoxic effects of radiotherapy,” the investigators said.
Involved-field radiotherapy alone should be the treatment of choice for stage 1A nodular lymphocyte-predominant Hodgkin lymphoma because it yields similar survival outcomes but fewer toxic effects than other therapies, investigators reported online Aug. 3 in Journal of Clinical Oncology.
Few studies have examined the long-term outcomes of patients who have this generally indolent malignancy, even though late treatment-associated effects are their major cause of death, said Dr. Dennis A. Eichenauer and his associates at University Hospital Cologne and University Hospital Münster, both in Germany.
To assess long-term outcomes in nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), the investigators analyzed data for 256 patients who participated in seven prospective randomized clinical trials comparing various treatments during a 21-year period, who were followed for a median of 91 months. The study participants’ median age at diagnosis was 39 years (range, 16-75 years), and all achieved remission with therapy. They were categorized by treatment type: 108 received involved-field radiotherapy (IF-RT) alone; 49 received extended-field radiotherapy (EF-RT) alone; 72 received combined modality treatment involving various combinations of doxorubicin, bleomycin, vinblastine, dacarbazine, IF-RT, EF-RT, and/or rituximab; and 27 received rituximab alone.
At 8 years, progression-free survival was 91.9% with IF-RT, 84.3% with EF-RT, and 88.5% with combined modalities, which are nonsignificant differences. Overall survival was 99.0%, 95.7%, and 98.6%, respectively, which also are nonsignificant differences. Rituximab alone was markedly less effective than the other treatments, with a 4-year progression-free survival of only 81.0%. “Compared with patients who received IF-RT, patients treated with rituximab had a hazard ratio of 4.99 for relapse,” Dr. Eichenauer and his associates wrote (J Clin Oncol. 2015 Aug 3 [doi: 10.1200/JCO. 2014.60.4363]).
Only 3.7% of patients treated with IF-RT developed a second malignancy during follow-up, compared with 11.1% of those treated with combined modalities, 6.1% of those treated with EF-RT, and 7.4% of those treated with rituximab. The rate of relapse was markedly higher with rituximab than with the other treatments.
The main study findings are twofold. First, IF-RT was at least as effective as other treatments in controlling NLPHL, was less toxic acutely, and carried similar or reduced risks of late adverse effects such as relapse and second malignancies. It should be considered the first-line treatment of choice. Second, rituximab alone should not be used routinely in these patients because it yields poorer survival outcomes and a higher relapse rate. “However, it might represent an option for individual patients, such as young women with abdominal disease, to avoid gonadotoxic effects of radiotherapy,” the investigators said.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Involved-field radiotherapy alone yields similar survival but fewer toxic effects than do other treatments for stage 1A nodular lymphocyte–predominant Hodgkin lymphoma.
Major finding: Progression-free survival was 91.9% with IF-RT, 84.3% with EF-RT, and 88.5% with combined modalities, and overall survival was 99.0%, 95.7%, and 98.6%, respectively.
Data source: A retrospective analysis of outcomes of seven German prospective clinical trials involving 256 patients followed for a median of 91 months.
Disclosures: This study was supported by University Hospital Cologne and University Hospital Münster. Dr. Eichenauer reported having no relevant financial disclosures; his associates reported ties to Amgen, Takeda, Novartis, Gilead, and Takeda/Millennium.
Less toxic chemo for HIV-positive Burkitt lymphoma
For HIV-positive patients with Burkitt lymphoma, a modified intensive chemotherapy regimen produced overall and progression-free survival rates comparable with those seen in HIV-free patients with Burkitt, with manageable toxicities, reported researchers in a multicenter clinical trial.
The AIDS Malignancy Consortium (AMC) 048 study looked at the use of a modified version of the dose intensive CODOX-M/IVAC regimen, consisting of cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine. Compared with the standard regimen, the investigators added rituximab, reduced and/or rescheduled cyclophosphamide and methotrexate, limited the use of vincristine, and used combination intrathecal chemotherapy to prevent central nervous system involvement.
The study included 34 HIV-positive patients (30 men and 4 women) with Burkitt, 26 of whom were also receiving highly active antiretroviral therapy (HAART). The patients ranged in age from 19-55 (median 42) years. Of the 34 patients, 25 had Ann Arbor stage IV disease, 2 had stage III, 1 had stage IIE, 2 had stage II, and 4 had stage I. Median age was 42 years (range, 19-55 years).
The median CD4 count was 195 cells/mL; five patients had fewer than 100 cells/mL
Progression-free survival at 1 year was 69%, and 1- and 2-year overall survival were 72% and 69%, respectively.
The modified CODOX-M/IVAC regimen was associated with a grade 3 to 4 toxicity rate of 79%, with no grade 3 or 4 mucositis reported. In contrast, virtually all patients who receive the unmodified regimen develop at least one grade 3 or greater toxicity.
In total, there were 20 hematologic, 14 infectious, and 6 metabolic toxicities. Five patients did not complete treatment because of adverse events.
There were 11 deaths, including 1 treatment-related death of a patient with encephalopathy, hepatic failure, hepatitis B, and pneumonia cited as contributing causes. Of the remaining 10 patients, 8 died from systemic disease progression, and 2 died during follow-up, 1 during remission from a fungal infection and 1 from nonmalignant complications of HIV.
The investigators say that the addition of rituximab may have contributed to the favorable outcomes, and that rescheduling and limiting the amount of high-dose methotrexate delivered likely contributed to lower incidences of both severe mucositis and neutropenic fever.
Although a separate trial is evaluating a different regimen (EPOCH-R; etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in Burkitt lymphoma, “the modified AMC 048 version of CODOX-M/IVAC-R may better serve patients who present with CNS disease or are at high risk for CNS relapse (e.g., patients with bone marrow, testicular, or multiple extranodal sites), because it contains high-dose cytarabine and methotrexate, drugs that cross the blood-brain barrier. Consequently, AMC048 represents a reasonable treatment option in the appropriate setting, possibly irrespective of HIV status.”
The study by Dr. Ariela Noy from the Memorial Sloan Kettering Cancer Center in New York and her colleagues, is published in Blood.
Although the results of AMC 048 are encouraging and demonstrate that intensive regimens for AIDS-related Burkitt lymphoma are both tolerable and efficacious, the question of whether multiagent dose intense regimens are needed remains unanswered. Using a short course of EPOCH (infusional etoposide, oral prednisone, infusional vincristine, bolus cyclophosphamide, and infusional doxorubicin) with a double dose of rituximab (SC-EPOCH-RR) to treat 11 patients with AIDS-related Burkitt lymphoma, researchers at the National Cancer Institute have observed progression-free survival of 100% and overall survival of 90% at a median follow-up of 73 months. This regimen omits systemic ifosfamide and high-dose methotrexate. Although both agents are thought to be important for disease control in Burkitt lymphoma, especially to treat and/or prevent lymphomatous CNS involvement, they also have substantial toxicities. In the NCI study, only 1 patient had CNS involvement at baseline and was successfully treated with intrathecal methotrexate alone. No patient relapsed in the CNS. However, given the small number of patients enrolled in this single institution study, there remains significant concern that omission of these agents will jeopardize disease control, specifically in high-risk patients. It will be interesting to see whether the results of the NCI study will be maintained in the ongoing larger cooperative group trial that currently evaluates dose-adjusted EPOCH-R.
Dr. Stefan K. Barta is with the Fox Chase Cancer Center/Temple University Health System in Philadelphia. He made his remarks in an editorial that accompanied the study.
Although the results of AMC 048 are encouraging and demonstrate that intensive regimens for AIDS-related Burkitt lymphoma are both tolerable and efficacious, the question of whether multiagent dose intense regimens are needed remains unanswered. Using a short course of EPOCH (infusional etoposide, oral prednisone, infusional vincristine, bolus cyclophosphamide, and infusional doxorubicin) with a double dose of rituximab (SC-EPOCH-RR) to treat 11 patients with AIDS-related Burkitt lymphoma, researchers at the National Cancer Institute have observed progression-free survival of 100% and overall survival of 90% at a median follow-up of 73 months. This regimen omits systemic ifosfamide and high-dose methotrexate. Although both agents are thought to be important for disease control in Burkitt lymphoma, especially to treat and/or prevent lymphomatous CNS involvement, they also have substantial toxicities. In the NCI study, only 1 patient had CNS involvement at baseline and was successfully treated with intrathecal methotrexate alone. No patient relapsed in the CNS. However, given the small number of patients enrolled in this single institution study, there remains significant concern that omission of these agents will jeopardize disease control, specifically in high-risk patients. It will be interesting to see whether the results of the NCI study will be maintained in the ongoing larger cooperative group trial that currently evaluates dose-adjusted EPOCH-R.
Dr. Stefan K. Barta is with the Fox Chase Cancer Center/Temple University Health System in Philadelphia. He made his remarks in an editorial that accompanied the study.
Although the results of AMC 048 are encouraging and demonstrate that intensive regimens for AIDS-related Burkitt lymphoma are both tolerable and efficacious, the question of whether multiagent dose intense regimens are needed remains unanswered. Using a short course of EPOCH (infusional etoposide, oral prednisone, infusional vincristine, bolus cyclophosphamide, and infusional doxorubicin) with a double dose of rituximab (SC-EPOCH-RR) to treat 11 patients with AIDS-related Burkitt lymphoma, researchers at the National Cancer Institute have observed progression-free survival of 100% and overall survival of 90% at a median follow-up of 73 months. This regimen omits systemic ifosfamide and high-dose methotrexate. Although both agents are thought to be important for disease control in Burkitt lymphoma, especially to treat and/or prevent lymphomatous CNS involvement, they also have substantial toxicities. In the NCI study, only 1 patient had CNS involvement at baseline and was successfully treated with intrathecal methotrexate alone. No patient relapsed in the CNS. However, given the small number of patients enrolled in this single institution study, there remains significant concern that omission of these agents will jeopardize disease control, specifically in high-risk patients. It will be interesting to see whether the results of the NCI study will be maintained in the ongoing larger cooperative group trial that currently evaluates dose-adjusted EPOCH-R.
Dr. Stefan K. Barta is with the Fox Chase Cancer Center/Temple University Health System in Philadelphia. He made his remarks in an editorial that accompanied the study.
For HIV-positive patients with Burkitt lymphoma, a modified intensive chemotherapy regimen produced overall and progression-free survival rates comparable with those seen in HIV-free patients with Burkitt, with manageable toxicities, reported researchers in a multicenter clinical trial.
The AIDS Malignancy Consortium (AMC) 048 study looked at the use of a modified version of the dose intensive CODOX-M/IVAC regimen, consisting of cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine. Compared with the standard regimen, the investigators added rituximab, reduced and/or rescheduled cyclophosphamide and methotrexate, limited the use of vincristine, and used combination intrathecal chemotherapy to prevent central nervous system involvement.
The study included 34 HIV-positive patients (30 men and 4 women) with Burkitt, 26 of whom were also receiving highly active antiretroviral therapy (HAART). The patients ranged in age from 19-55 (median 42) years. Of the 34 patients, 25 had Ann Arbor stage IV disease, 2 had stage III, 1 had stage IIE, 2 had stage II, and 4 had stage I. Median age was 42 years (range, 19-55 years).
The median CD4 count was 195 cells/mL; five patients had fewer than 100 cells/mL
Progression-free survival at 1 year was 69%, and 1- and 2-year overall survival were 72% and 69%, respectively.
The modified CODOX-M/IVAC regimen was associated with a grade 3 to 4 toxicity rate of 79%, with no grade 3 or 4 mucositis reported. In contrast, virtually all patients who receive the unmodified regimen develop at least one grade 3 or greater toxicity.
In total, there were 20 hematologic, 14 infectious, and 6 metabolic toxicities. Five patients did not complete treatment because of adverse events.
There were 11 deaths, including 1 treatment-related death of a patient with encephalopathy, hepatic failure, hepatitis B, and pneumonia cited as contributing causes. Of the remaining 10 patients, 8 died from systemic disease progression, and 2 died during follow-up, 1 during remission from a fungal infection and 1 from nonmalignant complications of HIV.
The investigators say that the addition of rituximab may have contributed to the favorable outcomes, and that rescheduling and limiting the amount of high-dose methotrexate delivered likely contributed to lower incidences of both severe mucositis and neutropenic fever.
Although a separate trial is evaluating a different regimen (EPOCH-R; etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in Burkitt lymphoma, “the modified AMC 048 version of CODOX-M/IVAC-R may better serve patients who present with CNS disease or are at high risk for CNS relapse (e.g., patients with bone marrow, testicular, or multiple extranodal sites), because it contains high-dose cytarabine and methotrexate, drugs that cross the blood-brain barrier. Consequently, AMC048 represents a reasonable treatment option in the appropriate setting, possibly irrespective of HIV status.”
The study by Dr. Ariela Noy from the Memorial Sloan Kettering Cancer Center in New York and her colleagues, is published in Blood.
For HIV-positive patients with Burkitt lymphoma, a modified intensive chemotherapy regimen produced overall and progression-free survival rates comparable with those seen in HIV-free patients with Burkitt, with manageable toxicities, reported researchers in a multicenter clinical trial.
The AIDS Malignancy Consortium (AMC) 048 study looked at the use of a modified version of the dose intensive CODOX-M/IVAC regimen, consisting of cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine. Compared with the standard regimen, the investigators added rituximab, reduced and/or rescheduled cyclophosphamide and methotrexate, limited the use of vincristine, and used combination intrathecal chemotherapy to prevent central nervous system involvement.
The study included 34 HIV-positive patients (30 men and 4 women) with Burkitt, 26 of whom were also receiving highly active antiretroviral therapy (HAART). The patients ranged in age from 19-55 (median 42) years. Of the 34 patients, 25 had Ann Arbor stage IV disease, 2 had stage III, 1 had stage IIE, 2 had stage II, and 4 had stage I. Median age was 42 years (range, 19-55 years).
The median CD4 count was 195 cells/mL; five patients had fewer than 100 cells/mL
Progression-free survival at 1 year was 69%, and 1- and 2-year overall survival were 72% and 69%, respectively.
The modified CODOX-M/IVAC regimen was associated with a grade 3 to 4 toxicity rate of 79%, with no grade 3 or 4 mucositis reported. In contrast, virtually all patients who receive the unmodified regimen develop at least one grade 3 or greater toxicity.
In total, there were 20 hematologic, 14 infectious, and 6 metabolic toxicities. Five patients did not complete treatment because of adverse events.
There were 11 deaths, including 1 treatment-related death of a patient with encephalopathy, hepatic failure, hepatitis B, and pneumonia cited as contributing causes. Of the remaining 10 patients, 8 died from systemic disease progression, and 2 died during follow-up, 1 during remission from a fungal infection and 1 from nonmalignant complications of HIV.
The investigators say that the addition of rituximab may have contributed to the favorable outcomes, and that rescheduling and limiting the amount of high-dose methotrexate delivered likely contributed to lower incidences of both severe mucositis and neutropenic fever.
Although a separate trial is evaluating a different regimen (EPOCH-R; etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in Burkitt lymphoma, “the modified AMC 048 version of CODOX-M/IVAC-R may better serve patients who present with CNS disease or are at high risk for CNS relapse (e.g., patients with bone marrow, testicular, or multiple extranodal sites), because it contains high-dose cytarabine and methotrexate, drugs that cross the blood-brain barrier. Consequently, AMC048 represents a reasonable treatment option in the appropriate setting, possibly irrespective of HIV status.”
The study by Dr. Ariela Noy from the Memorial Sloan Kettering Cancer Center in New York and her colleagues, is published in Blood.
FROM BLOOD
Key clinical point: A modified form of a standard chemotherapy regimen for Burkitt lymphoma is effective in HIV-positive patients, with lower rates of adverse events.
Major finding: 1-year overall survival was 72%, and 2-year OS was 69%.
Data source: Open-label study of a modified chemotherapy regimen in 34 HIV-positive patients with Burkitt lymphoma.
Disclosures: The trial was supported by a grant from the National Cancer Institute, The authors and Dr. Barta declare no conflicts of interest.
Early follicular lymphoma progression signals poor outcomes
For patients with follicular lymphoma treated with a rituximab-based combination chemotherapy regimen, early disease progression is associated with significantly worse overall survival, suggesting the need for additional interventions, according to results of a multicenter study.
Among 588 patients with stage 2-4 follicular lymphoma treated with first-line R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) and followed for a median of 7 years in the National LymphoCare Study, overall survival (OS) at 2 years was 68% for those who had disease progression within 2 years, compared with 97% for patients with no disease progression during that time.
Similarly, 5-year overall survival was 50% for patients with early progression of disease, compared with 90% for patients with no early progression, write Dr. Carla Casulo of the University of Rochester (N.Y.) Medical Center and colleagues. The study is in anearly online publication in the Journal of Clinical Oncology.
“Given our findings, early relapse after diagnosis in patients treated with first-line chemoimmunotherapy is a powerful prognostic indicator of outcome and should be used to stratify the risk of patients in studies of relapsed follicular lymphoma,” the authors wrote.
The findings were validated in an independent cohort of patients with follicular lymphoma treated with R-CHOP from the University of Iowa and Mayo Clinical Molecular Epidemiology Resource, and are consistent with findings from other studies of patients treated with different rituximab-based regimens, the investigators reported.
In unadjusted analysis, early disease progression was associated with a hazard ratio (HR) of 7.17 (95% confidence interval [CI] 4.83-10.65); the effect remained after adjustment for the Follicular Lymphoma International Prognostic Index (FLIPI) score (HR 6.44, 95% CI, 4.33-9.58).
Factors associated with early progression included age, Eastern Cooperative Oncology Group performance score, nodal sites, and disease stage.
Early use of aggressive salvage therapies or autologous stem-cell transplantation could improve outcomes in patients with early disease progression, the authors wrote. However, only 8 patients among the 110 with early progression went on to transplant, not a large enough sample for meaningful analysis, they added.
“This newly defined high-risk group of patients represents a distinct population in whom further study is warranted in both directed prospective clinical trials of follicular lymphoma biology and treatment. Moreover, we propose that 2-year progression-free survival may be a practical and meaningful clinical end point for trials involving a chemoimmunotherapy backbone,” they concluded.
If, in studying the immunologic and inflammatory host response to, and the genetic landscape of, these lymphomas, we are able to define this high-risk subgroup of patients with follicular lymphoma, the question becomes whether we could use this information to effectively treat these patients differently. Although high-dose chemotherapy and autologous stem-cell transplantation (HDC-ASCT) in first remission seems to have no effect on OS in all comers, results might be different for this cohort of high-risk patients. To study this would require an ability to identify these patients at diagnosis. Given that the efficacy of HDC-ASCT is maintained in the case of chemosensitive relapse, reserving HDC-ASCT for patients who relapse within the first 2 years of their initial therapy may be a more prudent strategy.
However, it may be that this is a particularly chemoresistant population and that, instead, attention should be paid to targeting the biologic and genetic factors that contribute to the poor prognosis of this group. Given the negative differential outcomes in patients with decreased tumor-infiltrating lymphocytes and increased monocyte/macrophage activation, immunologic approaches in the salvage setting, including immune checkpoint blockade drugs, chimeric antigen receptor T cells, and allogeneic transplantation may be biologically relevant.
Dr. Caron A. Jacobson and Dr. Arnold S. Freedman, of the Dana-Farber Cancer Institute and Harvard Medical School, Boston, made their remarks in an editorial accompanying the study.
If, in studying the immunologic and inflammatory host response to, and the genetic landscape of, these lymphomas, we are able to define this high-risk subgroup of patients with follicular lymphoma, the question becomes whether we could use this information to effectively treat these patients differently. Although high-dose chemotherapy and autologous stem-cell transplantation (HDC-ASCT) in first remission seems to have no effect on OS in all comers, results might be different for this cohort of high-risk patients. To study this would require an ability to identify these patients at diagnosis. Given that the efficacy of HDC-ASCT is maintained in the case of chemosensitive relapse, reserving HDC-ASCT for patients who relapse within the first 2 years of their initial therapy may be a more prudent strategy.
However, it may be that this is a particularly chemoresistant population and that, instead, attention should be paid to targeting the biologic and genetic factors that contribute to the poor prognosis of this group. Given the negative differential outcomes in patients with decreased tumor-infiltrating lymphocytes and increased monocyte/macrophage activation, immunologic approaches in the salvage setting, including immune checkpoint blockade drugs, chimeric antigen receptor T cells, and allogeneic transplantation may be biologically relevant.
Dr. Caron A. Jacobson and Dr. Arnold S. Freedman, of the Dana-Farber Cancer Institute and Harvard Medical School, Boston, made their remarks in an editorial accompanying the study.
If, in studying the immunologic and inflammatory host response to, and the genetic landscape of, these lymphomas, we are able to define this high-risk subgroup of patients with follicular lymphoma, the question becomes whether we could use this information to effectively treat these patients differently. Although high-dose chemotherapy and autologous stem-cell transplantation (HDC-ASCT) in first remission seems to have no effect on OS in all comers, results might be different for this cohort of high-risk patients. To study this would require an ability to identify these patients at diagnosis. Given that the efficacy of HDC-ASCT is maintained in the case of chemosensitive relapse, reserving HDC-ASCT for patients who relapse within the first 2 years of their initial therapy may be a more prudent strategy.
However, it may be that this is a particularly chemoresistant population and that, instead, attention should be paid to targeting the biologic and genetic factors that contribute to the poor prognosis of this group. Given the negative differential outcomes in patients with decreased tumor-infiltrating lymphocytes and increased monocyte/macrophage activation, immunologic approaches in the salvage setting, including immune checkpoint blockade drugs, chimeric antigen receptor T cells, and allogeneic transplantation may be biologically relevant.
Dr. Caron A. Jacobson and Dr. Arnold S. Freedman, of the Dana-Farber Cancer Institute and Harvard Medical School, Boston, made their remarks in an editorial accompanying the study.
For patients with follicular lymphoma treated with a rituximab-based combination chemotherapy regimen, early disease progression is associated with significantly worse overall survival, suggesting the need for additional interventions, according to results of a multicenter study.
Among 588 patients with stage 2-4 follicular lymphoma treated with first-line R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) and followed for a median of 7 years in the National LymphoCare Study, overall survival (OS) at 2 years was 68% for those who had disease progression within 2 years, compared with 97% for patients with no disease progression during that time.
Similarly, 5-year overall survival was 50% for patients with early progression of disease, compared with 90% for patients with no early progression, write Dr. Carla Casulo of the University of Rochester (N.Y.) Medical Center and colleagues. The study is in anearly online publication in the Journal of Clinical Oncology.
“Given our findings, early relapse after diagnosis in patients treated with first-line chemoimmunotherapy is a powerful prognostic indicator of outcome and should be used to stratify the risk of patients in studies of relapsed follicular lymphoma,” the authors wrote.
The findings were validated in an independent cohort of patients with follicular lymphoma treated with R-CHOP from the University of Iowa and Mayo Clinical Molecular Epidemiology Resource, and are consistent with findings from other studies of patients treated with different rituximab-based regimens, the investigators reported.
In unadjusted analysis, early disease progression was associated with a hazard ratio (HR) of 7.17 (95% confidence interval [CI] 4.83-10.65); the effect remained after adjustment for the Follicular Lymphoma International Prognostic Index (FLIPI) score (HR 6.44, 95% CI, 4.33-9.58).
Factors associated with early progression included age, Eastern Cooperative Oncology Group performance score, nodal sites, and disease stage.
Early use of aggressive salvage therapies or autologous stem-cell transplantation could improve outcomes in patients with early disease progression, the authors wrote. However, only 8 patients among the 110 with early progression went on to transplant, not a large enough sample for meaningful analysis, they added.
“This newly defined high-risk group of patients represents a distinct population in whom further study is warranted in both directed prospective clinical trials of follicular lymphoma biology and treatment. Moreover, we propose that 2-year progression-free survival may be a practical and meaningful clinical end point for trials involving a chemoimmunotherapy backbone,” they concluded.
For patients with follicular lymphoma treated with a rituximab-based combination chemotherapy regimen, early disease progression is associated with significantly worse overall survival, suggesting the need for additional interventions, according to results of a multicenter study.
Among 588 patients with stage 2-4 follicular lymphoma treated with first-line R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) and followed for a median of 7 years in the National LymphoCare Study, overall survival (OS) at 2 years was 68% for those who had disease progression within 2 years, compared with 97% for patients with no disease progression during that time.
Similarly, 5-year overall survival was 50% for patients with early progression of disease, compared with 90% for patients with no early progression, write Dr. Carla Casulo of the University of Rochester (N.Y.) Medical Center and colleagues. The study is in anearly online publication in the Journal of Clinical Oncology.
“Given our findings, early relapse after diagnosis in patients treated with first-line chemoimmunotherapy is a powerful prognostic indicator of outcome and should be used to stratify the risk of patients in studies of relapsed follicular lymphoma,” the authors wrote.
The findings were validated in an independent cohort of patients with follicular lymphoma treated with R-CHOP from the University of Iowa and Mayo Clinical Molecular Epidemiology Resource, and are consistent with findings from other studies of patients treated with different rituximab-based regimens, the investigators reported.
In unadjusted analysis, early disease progression was associated with a hazard ratio (HR) of 7.17 (95% confidence interval [CI] 4.83-10.65); the effect remained after adjustment for the Follicular Lymphoma International Prognostic Index (FLIPI) score (HR 6.44, 95% CI, 4.33-9.58).
Factors associated with early progression included age, Eastern Cooperative Oncology Group performance score, nodal sites, and disease stage.
Early use of aggressive salvage therapies or autologous stem-cell transplantation could improve outcomes in patients with early disease progression, the authors wrote. However, only 8 patients among the 110 with early progression went on to transplant, not a large enough sample for meaningful analysis, they added.
“This newly defined high-risk group of patients represents a distinct population in whom further study is warranted in both directed prospective clinical trials of follicular lymphoma biology and treatment. Moreover, we propose that 2-year progression-free survival may be a practical and meaningful clinical end point for trials involving a chemoimmunotherapy backbone,” they concluded.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Disease progression within 2 years of chemotherapy for follicular lymphoma is associated with poor outcomes.
Major finding: Five-year overall survival was 50% for patients with follicular lymphoma with disease progression within 2-years of R-CHOP, vs. 90% for patients with no early progression.
Data source: Retrospective review involving 588 patients in the longitudinal National LymphoCare Study.
Disclosures: Genentech and F. Hoffmann-La Roche supported the study. Dr. Casulo and Dr. Jacobson reported no relevant disclosures. Dr. Freedman reported ties with UpToDate, Axio, and Immunogen.
Guideline updated on hematopoietic colony-stimulating factors
Hematopoietic colony-stimulating factors should now be considered for patients who are over age 64 years, have diffuse aggressive lymphoma, and are receiving curative chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab), particularly those who have comorbidities.
This is one of several recommendations noted in the American Society of Clinical Oncology’s updated practice guidelines, published online in the Journal of Clinical Oncology, on the use of hematopoietic colony-stimulating factors (CSFs) to prevent or treat neutropenia and its complications in adults and children receiving chemotherapy.
This “moderately strong” recommendation is based on a single randomized clinical trial that found pegfilgrastim significantly reduced the risk of febrile neutropenia in this patient population, according to the guidelines (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.62.3488]).
The updated guideline incorporates new evidence from 66 randomized controlled trials and meta-analyses published since its last update in 2006, said cochair Dr. Thomas J. Smith of the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, and his associates on the update committee.
In addition to pegfilgrastim and filgrastim, the guideline now addresses the use of tbo-filgrastim, filgrastim-sndz, and other biosimilars as they become available. These new agents are effective at preventing chemotherapy-related febrile neutropenia, so the choice of agent depends on convenience, cost, and clinical factors, and in some cases may be dictated by the patient’s treatment schedule. Certain off-label uses of pegfilgrastim can now be considered, such as giving it on the same day as chemotherapy if that is the only feasible timing for some patients.
CSFs should only be used to enable dose-dense chemotherapy regimens “if supported by convincing efficacy data or within an appropriately designed clinical trial” – for example, to support treatment of urothelial cancer or high-risk breast cancer targeted with high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin.
In contrast, the use of CSFs to enable dose-dense chemotherapy for Hodgkin lymphoma is not recommended at this time because the current data supporting such use are limited and conflicting. Similarly, the current evidence strongly argues against giving CSFs to enable dose-dense chemotherapy for other lymphomas, lung cancer, ovarian cancer, osteosarcoma, or sarcoma.
The guideline update was supported by the American Society of Clinical Oncology. Dr. Smith reported stock or other ownership in United Healthcare; his associates reported ties to numerous industry sources.
The full guideline and supplementary material, including slide sets and clinical tools, are available at www.asco.org/guidelines/wbcgf.
Hematopoietic colony-stimulating factors should now be considered for patients who are over age 64 years, have diffuse aggressive lymphoma, and are receiving curative chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab), particularly those who have comorbidities.
This is one of several recommendations noted in the American Society of Clinical Oncology’s updated practice guidelines, published online in the Journal of Clinical Oncology, on the use of hematopoietic colony-stimulating factors (CSFs) to prevent or treat neutropenia and its complications in adults and children receiving chemotherapy.
This “moderately strong” recommendation is based on a single randomized clinical trial that found pegfilgrastim significantly reduced the risk of febrile neutropenia in this patient population, according to the guidelines (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.62.3488]).
The updated guideline incorporates new evidence from 66 randomized controlled trials and meta-analyses published since its last update in 2006, said cochair Dr. Thomas J. Smith of the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, and his associates on the update committee.
In addition to pegfilgrastim and filgrastim, the guideline now addresses the use of tbo-filgrastim, filgrastim-sndz, and other biosimilars as they become available. These new agents are effective at preventing chemotherapy-related febrile neutropenia, so the choice of agent depends on convenience, cost, and clinical factors, and in some cases may be dictated by the patient’s treatment schedule. Certain off-label uses of pegfilgrastim can now be considered, such as giving it on the same day as chemotherapy if that is the only feasible timing for some patients.
CSFs should only be used to enable dose-dense chemotherapy regimens “if supported by convincing efficacy data or within an appropriately designed clinical trial” – for example, to support treatment of urothelial cancer or high-risk breast cancer targeted with high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin.
In contrast, the use of CSFs to enable dose-dense chemotherapy for Hodgkin lymphoma is not recommended at this time because the current data supporting such use are limited and conflicting. Similarly, the current evidence strongly argues against giving CSFs to enable dose-dense chemotherapy for other lymphomas, lung cancer, ovarian cancer, osteosarcoma, or sarcoma.
The guideline update was supported by the American Society of Clinical Oncology. Dr. Smith reported stock or other ownership in United Healthcare; his associates reported ties to numerous industry sources.
The full guideline and supplementary material, including slide sets and clinical tools, are available at www.asco.org/guidelines/wbcgf.
Hematopoietic colony-stimulating factors should now be considered for patients who are over age 64 years, have diffuse aggressive lymphoma, and are receiving curative chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab), particularly those who have comorbidities.
This is one of several recommendations noted in the American Society of Clinical Oncology’s updated practice guidelines, published online in the Journal of Clinical Oncology, on the use of hematopoietic colony-stimulating factors (CSFs) to prevent or treat neutropenia and its complications in adults and children receiving chemotherapy.
This “moderately strong” recommendation is based on a single randomized clinical trial that found pegfilgrastim significantly reduced the risk of febrile neutropenia in this patient population, according to the guidelines (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.62.3488]).
The updated guideline incorporates new evidence from 66 randomized controlled trials and meta-analyses published since its last update in 2006, said cochair Dr. Thomas J. Smith of the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, and his associates on the update committee.
In addition to pegfilgrastim and filgrastim, the guideline now addresses the use of tbo-filgrastim, filgrastim-sndz, and other biosimilars as they become available. These new agents are effective at preventing chemotherapy-related febrile neutropenia, so the choice of agent depends on convenience, cost, and clinical factors, and in some cases may be dictated by the patient’s treatment schedule. Certain off-label uses of pegfilgrastim can now be considered, such as giving it on the same day as chemotherapy if that is the only feasible timing for some patients.
CSFs should only be used to enable dose-dense chemotherapy regimens “if supported by convincing efficacy data or within an appropriately designed clinical trial” – for example, to support treatment of urothelial cancer or high-risk breast cancer targeted with high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin.
In contrast, the use of CSFs to enable dose-dense chemotherapy for Hodgkin lymphoma is not recommended at this time because the current data supporting such use are limited and conflicting. Similarly, the current evidence strongly argues against giving CSFs to enable dose-dense chemotherapy for other lymphomas, lung cancer, ovarian cancer, osteosarcoma, or sarcoma.
The guideline update was supported by the American Society of Clinical Oncology. Dr. Smith reported stock or other ownership in United Healthcare; his associates reported ties to numerous industry sources.
The full guideline and supplementary material, including slide sets and clinical tools, are available at www.asco.org/guidelines/wbcgf.
FROM THE JOURNAL OF CLINICAL ONCOLOGY