User login
Managing Hyponatremia Patients With SIADH
Why is SIADH Important to Hospitalists?
Disorders of body fluids, and particularly hyponatremia, are among the most commonly encountered problems in clinical medicine, affecting up to 30% of hospitalized patients. In a study of 303,577 laboratory samples collected from 120,137 patients, the prevalence of hyponatremia (serum [Na+] 135 mmol/L) on initial presentation to a healthcare provider was 28.2% among those treated in an acute hospital care setting, 21% among those treated in an ambulatory hospital care setting, and 7.2% in community care centers.1 Numerous other studies have corroborated a high prevalence of hyponatremia in hospitalized patients,2 which reflects the increased vulnerability of this patient population to disruptions of body fluid homeostasis. Recognizing the many possible causes of hyponatremia in hospitalized patients and implementing appropriate treatment strategies therefore are critical steps toward optimizing care and improving outcomes in hospitalized patients with hyponatremia.
In addition to its frequency, hyponatremia is also important because it has been associated with worse clinical outcomes across the entire range of inpatient care, from the general hospital population to those treated in the intensive care unit (ICU). In a study of 4123 patients age 65 years or older who were admitted to a community hospital, 3.5% had clinically significant hyponatremia (serum [Na+] 130 mmol/L) at admission. Compared with nonhyponatremic patients, those with hyponatremia were twice as likely to die during their hospital stay (relative risk [RR], 1.95; P 0.05).3 In another study of 2188 patients admitted to a medical ICU over a 5‐year period, 13.7% had hyponatremia. The overall rate of in‐hospital mortality among all ICU patients was high at 37.7%. However, severe hyponatremia (serum [Na+] 125 mmol/L) more than doubled the risk of in‐hospital mortality (RR, 2.10; P 0.001).4 In addition to the general hospital population, in virtually every disease ever studied, the presence of hyponatremia has been found to be an independent risk factor for increased mortality, from congestive heart failure to tuberculosis to liver failure.2
What Causes Hyponatremia in Patients with SIADH?
Hyponatremia can be caused by 1 of 2 potential disruptions in fluid balance: dilution from retained water, or depletion from electrolyte losses in excess of water. Dilutional hyponatremias are associated with either a normal (euvolemic) or an increased (hypervolemic) extracellular fluid (ECF) volume, whereas depletional hyponatremias generally are associated with a decreased ECF volume (hypovolemic). Dilutional hyponatremia can arise from a primary defect in osmoregulation, such as in SIADH, or as a result of ECF volume expansion, as seen in conditions associated with concomitant secondary hyperaldosteronism such as heart failure, hepatic cirrhosis, or nephrotic syndrome. Among some hospitalized patient groups, euvolemic hyponatremia is the most common presentation of abnormally low serum [Na+]. In a study of patients who developed clinically significant postoperative hyponatremia (defined as a serum [Na+] 130 mmol/L) in a large teaching hospital, only 8% were hypovolemic, whereas 42% were euvolemic and 21% were hypervolemic.5
Euvolemic hyponatremia results from an increase in total body water, but with normal or near‐normal total body sodium. As a result, there is an absence of clinical manifestations of ECF volume expansion, such as subcutaneous edema or ascites. It is important to recognize that although SIADH clearly represents a state of volume expansion due to water retention, it rarely causes clinically recognizable hypervolemia since the retained water is distributed across the intracellular fluid (ICF) as well as the ECF, and because volume regulatory processes act to decrease the actual degree of ECF volume expansion.6 Euvolemic hyponatremia can accompany a wide variety of pathological processes, but the most common cause by far is SIADH. Normally, increased plasma osmolality activates osmoreceptors located in the anterior hypothalamus and stimulates the secretion of arginine vasopressin (AVP), also called antidiuretic hormone (ADH), a key neurohormone that regulates fluid homeostasis. In patients with euvolemic hyponatremia due to SIADH, plasma AVP levels are not suppressed despite normal or decreased plasma osmolality.7 This can be a result of ectopic production of AVP by tumors, or stimulation of endogenous pituitary AVP secretion as a result of nonosmotic stimuli that also stimulate vasopressinergic neurons, which include hypovolemia, hypotension, angiotensin II, nausea, hypoxia, hypercarbia, hypoglycemia, stress, and physical activity. Nonsuppressed AVP levels have been documented in the majority of hyponatremic patients, including those with SIADH8 and heart failure.9
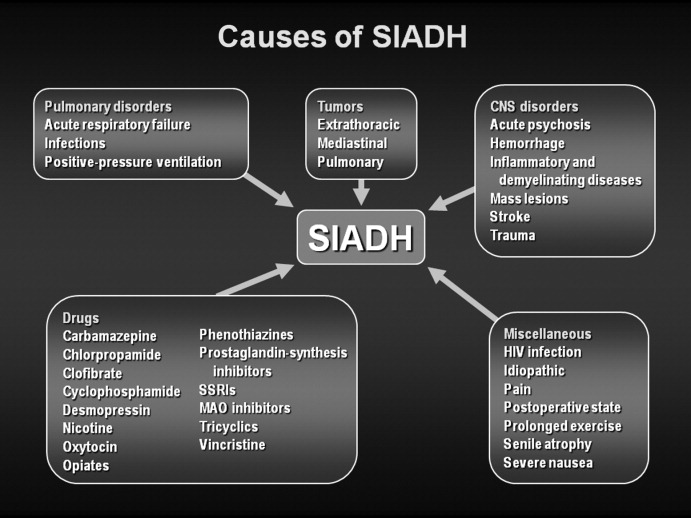
SIADH can develop as the result of many different disease processes that disrupt the normal mechanisms that regulate AVP secretion, including pneumonias and other lung infections, thoracic and extrathoracic tumors, a variety of different central nervous system disorders, the postoperative state, human immunodeficiency virus (HIV), and many different drugs (Figure 1). Given the multiplicity of disorders and drugs that can cause disrupted AVP secretion, it is not surprising that hyponatremia is the most common electrolyte abnormality seen in clinical practice.
What Symptoms are Associated With SIADH?
Symptoms of hyponatremia correlate both with the degree of decrease in the serum [Na+] and with the chronicity of the hyponatremia. Acute hyponatremia, defined as 48 hours in duration, is often associated with life‐threatening clinical features such as obtundation, seizures, coma, and respiratory arrest. These symptoms can occur abruptly, sometimes with little warning.10 In the most severe cases, death can occur as a result of cerebral edema with tentorial herniation. Hypoxia secondary to neurogenic pulmonary edema can increase the severity of brain swelling.11
In contrast, chronic hyponatremia is much less symptomatic, and the reason for the profound differences between the symptoms of acute and chronic hyponatremia is now well understood to be due to the process of brain volume regulation.12 It is essential that this process be understood in order to understand the full spectrum of hyponatremic symptoms. As the ECF [Na+] decreases, regardless of whether due to a loss of sodium or a gain of water, there is an obligate movement of water into the brain along osmotic gradients. That water shift causes swelling of the brain, or cerebral edema. If the increased brain water reaches approximately 8% in adults, it exceeds the capacity of the skull to accommodate brain expansion, leading to tentorial herniation and death from respiratory arrest and/or ischemic brain damage. However, if the patient survives the initial hyponatremia, a very strong volume regulatory process follows, consisting of loss of electrolytes and small organic molecules called osmolytes from brain cells into brain ECF, and eventually the peripheral ECF.12, 13 As the solute content of the brain decreases, the water content is allowed to normalize, eventually reaching a state in which brain edema is virtually absent, and as a result symptoms are markedly less than with acute hyponatremia. Although the time required for the brain to acieve a volume‐regulated state varies across patients, this process is completed within 48 hours in experinmental animal studies, and probably follows a similar time course in humans.
Despite this powerful adaptation process, chronic hyponatremia is frequently associated with neurological symptomatology, albeit milder and more subtle in nature. A recent report found a fairly high incidence of symptoms in 223 patients with chronic hyponatremia as a result of thiazide administration: 49% had malaise/lethargy, 47% had dizzy spells, 35% had vomiting, 17% had confusion/obtundation, 17% experienced falls, 6% had headaches, and 0.9% had seizures.14 Although dizziness can potentially be attributed to a diuretic‐induced hypovolemia, symptoms such as confusion, obtundation and seizures are more consistent with hyponatremic symptomatology. Because thiazide‐induced hyponatremia can be readily corrected by stopping the thiazide and/or administering sodium, this represents an ideal situation in which to assess improvement in hyponatremia symptomatology with normalization of the serum [Na+]; in this study, all of these symptoms improved with correction of the hyponatremia. This represents one of the best examples demonstrating reversal of the symptoms associated with chronic hyponatremia by correction of the hyponatremia, because the patients in this study did not in general have severe underlying comorbidities that might complicate interpretation of their symptoms, as is often the case in patients with SIADH.
What Is Required for Making a Diagnosis of SIADH in Hospitalized Patients?
In patients with hypotonic hypoosmolality, ascertainment of their ECF volume status (ie, hypovolemic, euvolemic, or hypervolemic) is an essential first step, as this will segregate patients into different treatment paradigms. For example, in patients who are truly clinically hypovolemic with a decreased ECF volume by clinical parameters, treatment would generally consist of solute repletion with sodium, generally isotonic saline infusion with or without potassium, until the sodium levels normalize. In patients who are hypervolemic, treatment should focus first on the underlying disease rather than addressing the serum [Na+] directly. In patients with clinical euvolemia, the standard diagnostic pathway should be followed to confirm a diagnosis of SIADH as described below.
Assessing ECF volume status can be difficult, even for the most experienced clinicians. Physical signs such as orthostatic decreases in blood pressure and increases in pulse rate, dry mucus membranes, and skin tenting indicate hypovolemic hyponatremia, while signs such as subcutaneous edema, ascites, or anasarca indicate hypervolemic hyponatremia. Patients without any of these findings are generally considered to be euvolemic. However, in any situation these signs are only applicable if there are no other reasons to suspect an altered ECF volume. Along with a complete history and physical examination that includes a careful neurological evaluation, several laboratory tests can help to assess the etiology of the hyponatremia, once serum sodium concentrations have been shown to be below normal ([Na+] 135 mmol/L):
-
Urine osmolality. A urine osmolality (Uosm) less than 100 mOsm/kg H2O can indicate low dietary solute intake, primary polydipsia, or a reset osmostat after suppression of AVP release by a decrease in plasma osmolality below the osmotic threshold for AVP secretion, usually as a result of increased water loading.
-
Urine sodium concentration. Excretion of sodium, as measured by a spot urine [Na+] (UNa), can indicate depletional hyponatremia if the concentration is less than 30 mmol/L.15 A low UNa reflects a volume depleted state unless the patient has secondary hyperaldosteronism from heart failure or cirrhosis. Patients with a low UNa are more likely to respond to isotonic saline. Euvolemic patients who have a normal dietary sodium intake will generally have spot UNa 30 mmol/L and will not benefit from isotonic saline administration.15 In fact, in SIADH, these patients may respond to isotonic saline with a worsening of hyponatremia, since the sodium from the isotonic saline will be excreted in a concentrated urine while the free water is reabsorbed in the kidney collecting ducts. If the patient is on diuretic therapy, urine sodium values cannot always be accurately interpreted, since a UNa 30 mmol/L may reflect the natriuretic effect of the diuretic and not a volume replete state.
-
Blood tests. Additional indicators of volume status include serum blood nitrogen (BUN) and uric acid levels. A BUN 10 mg/dL and uric acid 4 mg/dL are generally consistent with a euvolemic state, particularly when there is glomerular hyperfiltration, which is often present in SIADH. Elevated serum BUN and uric acid levels (BUN >20 mg/dL and uric acid >6 mg/dL), especially if prior values are available for comparison, can also help to establish whether ineffective vascular volume status may be contributing to the pathophysiology of the hyponatremia. In certain clinical scenarios, the B‐type natriuretic protein (BNP) can be helpful to support a clinical impression of congestive heart failure.
The criteria necessary for a diagnosis of SIADH remain essentially as defined by Bartter and Schwartz16 in 1967 (Table 1), but several points deserve emphasis.17 First, true ECF hypoosmolality must be present and hyponatremia secondary to pseudohyponatremia or hyperglycemia excluded. Second, urinary osmolality must be inappropriate for plasma hypoosmolality (Posm). This does not require a Uosm>Posm, but simply that the urine osmolality is greater than maximally dilute (ie, Uosm>100 mOsm/kg H2O in adults). Furthermore, urine osmolality need not be inappropriately elevated at all levels of Posm but simply at some level under 275 mOsm/kg H2O, since in patients with a reset osmostat, AVP secretion can be suppressed at some level of osmolality resulting in maximal urinary dilution and free water excretion at plasma osmolalities below this level.18 Although some consider a reset osmostat to be a separate disorder rather than a variant of SIADH, such cases nonetheless illustrate that some hypoosmolar patients can exhibit an appropriately dilute urine at some, though not all, plasma osmolalities. Third, clinical euvolemia must be present to diagnose SIADH, and this diagnosis cannot be made in a hypovolemic or edematous patient. Importantly, this does not mean that patients with SIADH cannot become hypovolemic for other reasons, but in such cases it is impossible to diagnose the underlying SIADH until the patient is rendered euvolemic. The fourth criterion, renal salt wasting, has probably caused the most confusion in the diagnosis of SIADH. As noted above, the importance of this criterion lies in its usefulness in differentiating hypoosmolality caused by a decreased effective intravascular volume with high aldosterone levels in which case renal Na+ conservation occurs, from dilutional disorders in which urine Na+ excretion is normal or increased due to ECF volume expansion and a suppressed renin‐angiotensin‐aldosterone system. However, UNa can also be high in renal causes of solute depletion such as diuretic use or Addison's disease, and conversely patients with SIADH can have a low UNa if they subsequently become hypovolemic or solute depleted, conditions sometimes produced by imposed salt and water restriction. Consequently, although high urinary Na+ excretion is generally the rule in most patients with SIADH, its presence does not necessarily confirm this diagnosis, nor does its absence rule out the diagnosis. The final criterion emphasizes that SIADH remains a diagnosis of exclusion, and the absence of other potential causes of hypoosmolality must always be verified. Glucocorticoid deficiency and SIADH can be especially difficult to distinguish, since both primary and secondary hypocortisolism can cause elevated plasma AVP levels in addition to direct renal effects that prevent maximal urinary dilution.19 Therefore, no patient with chronic hyponatremia should be diagnosed as having SIADH without a thorough evaluation of adrenal function, preferably via a rapid adrenocorticotropic hormone (ACTH) stimulation test. Acute hyponatremia of obvious etiology, such as postoperatively or in association with pneumonitis, may be treated without adrenal testing as long as there are no other clinical signs or symptoms suggestive of adrenal dysfunction.20
|
| Essential |
| Decreased effective osmolality of the extracellular fluid (Posm 275 mOsm/kg H2O). |
| Inappropriate urinary concentration (Uosm >100 mOsm/kg H2O with normal renal function) at some level of hypoosmolality. |
| Clinical euvolemia, as defined by the absence of signs of hypovolemia (orthostasis, tachycardia, decreased skin turgor, dry mucous membranes) or hypervolemia (subcutaneous edema, ascites). |
| Elevated urinary sodium excretion while on a normal salt and water intake. |
| Absence of other potential causes of euvolemic hypoosmolality: hypothyroidism, hypocortisolism (Addison's disease or secondary adrenal insufficiency) and diuretic use. |
| Supplemental |
| Abnormal water load test (inability to excrete at least 90% of a 20 mL/kg water load in 4 hours and/or failure to dilute Uosm to 100 mOsm/kg H2O). |
| Plasma AVP level inappropriately elevated relative to plasma osmolality. |
| No significant correction of serum [Na+] with volume expansion but improvement after fluid restriction. |
Hyponatremia is a particularly common complication in elderly hospitalized patients, increasing in prevalence from approximately 7% in the general older population to 18% to 22% among elderly patients in chronic care facilities.21 Despite the many known causes of SIADH (Figure 1), hyponatremia is often associated with idiopathic SIADH in the elderly population. In a study of 119 nursing home residents aged 60 to 103 years, 53% had at least 1 episode of hyponatremia during the previous 12 months.22 Of these patients, 26% were diagnosed with idiopathic SIADH. In another study of elderly patients with hyponatremia and SIADH, 60% were diagnosed with idiopathic SIADH. Among remaining patients, the 2 main causes identified were pneumonia (9 cases/18%) and medications (6 cases/12%).23 Therefore, more than half of elderly patients who present with hyponatremia due to SIADH may have an idiopathic form, with no detectable underlying treatable disease.
Which Hospital Patients With SIADH are Candidates for Treatment of Hyponatremia?
Correction of hyponatremia is associated with markedly improved neurological outcomes in patients with severely symptomatic hyponatremia. In a retrospective review of patients who presented with severe neurological symptoms and serum [Na+] 125 mmol/L, prompt therapy with isotonic or hypertonic saline resulted in a correction in the range of 20 mmol/L over several days and neurological recovery in almost all cases. In contrast, in patients who were treated with fluid restriction alone, there was very little correction over the study period (5 mmol/L over 72 hours), and the neurological outcomes were much worse, with most of these patients either dying or entering a persistently vegetative state.24 Consequently, prompt therapy to rapidly increase the serum [Na+] represents the standard‐of‐care for treatment of patients presenting with severe life‐threatening symptoms of hyponatremia.
As discussed earlier, chronic hyponatremia is much less symptomatic as a result of the process of brain volume regulation. Because of this adaptation process, chronic hyponatremia is arguably a condition that clinicians feel they may not need to be as concerned about, and in some publications this has been called asymptomatic hyponatremia. However, such patients often do have neurological symptoms, even if milder and more subtle in nature, including headaches, nausea, mood disturbances, depression, difficulty concentrating, slowed reaction times, unstable gait, increased falls, confusion, and disorientation. Consequently, any patient with hyponatremia secondary to SIADH who manifests any neurological symptoms that could be related to the hyponatremia should be considered as appropriate candidates for treatment of the hyponatremia, regardless of the chronicity of the hyponatremia or the level of serum [Na+].
What Therapies are Currently Available to Manage SIADH in Hospitalized Patients?
Conventional management strategies for euvolemic hyponatremia range from saline infusion and fluid restriction to pharmacologic adjustment of fluid balance. Consideration of treatment options should include an evaluation of the benefits as well as the potential toxicities of any therapy (Table 2). Sometimes, simply stopping treatment with an agent that is associated with hyponatremia is sufficient to reverse a low serum [Na+].
| Therapy | Targets Underlying Pathophysiology | Limitations |
|---|---|---|
| ||
| Isotonic saline | Ineffective in dilutional hyponatremias; exacerbates the volume overload if used in edema‐forming disorders; no controlled safety database. | |
| Hypertonic saline | No consensus regarding appropriate infusion rates; overcorrection can cause osmotic demyelination; exacerbates the volume overload if used in edema‐forming disorders; no controlled safety database. | |
| Fluid restriction | Slow to correct over many days; poorly tolerated due to thirst; can not be used effectively in patients with high AVP levels and urine osmolalities. | |
| Demeclocycline | ✓ | Not FDA approved for hyponatremia; slow to correct; nephrotoxic in cirrhosis and heart failure. |
| Mineralocorticoids | Only one report in elderly patients with SIADH; no safety database; exacerbates the volume overload if used in edema‐forming disorders. | |
| Urea | Not FDA‐approved for hyponatremia; poor palatability. | |
| AVP receptor antagonists (vaptans) | ✓ | Conivaptan approved only for in‐hospital use secondary to CYP3A4 inhibition; infusion‐site reactions with intravenous use. Tolvaptan must be initiated and reinitiated in the hospital, as serum sodium needs to be monitored closely to avoid overly rapid correction of hyponatremia. |
Isotonic Saline
The treatment of choice for depletional hyponatremia (ie, hypovolemic hyponatremia) is isotonic saline ([Na+] = 154 mmol/L) to restore ECF volume and ensure adequate organ perfusion. This initial therapy is appropriate for patients who either have clinical signs of hypovolemia, or in whom a spot UNa+ is 30 mmol/L. However, this therapy is ineffective for dilutional hyponatremias such as SIADH,25 and continued inappropriate administration of isotonic saline to a euvolemic patient may worsen their hyponatremia,26 and/or cause fluid overload. Although isotonic saline may improve the serum [Na+] in patients with hypervolemic hyponatremia, their volume status will generally worsen with this therapy, so unless the hyponatremia is profound isotonic saline should be avoided.
Hypertonic Saline
Acute hyponatremia presenting with severe neurological symptoms is life‐threatening, and should be treated promptly with hypertonic solutions, typically 3% NaCl ([Na+] = 513 mmol/L), as this represents the most reliable method to quickly raise the serum [Na+]. A continuous infusion of hypertonic NaCl is usually utilized in inpatient settings. Various formulae have been suggested for calculating the initial rate of infusion of hypertonic solutions,27 but perhaps the simplest utilizes the following relationship:
- .Age and gender as risk factors for hyponatremia and hypernatremia.Clin Chim Acta.2003;337(1‐2):169–172.
- ,,.Incidence and prevalence of hyponatremia.Am J Med.2006;119(7 Suppl 1):S30–S35.
- ,,.Admission hyponatremia in the elderly: factors influencing prognosis.J Gen Intern Med1994;9:89–91.
- ,,, et al.[Incidence, causes and prognostic factors of hyponatremia in intensive care].Rev Med Interne.2003;24(4):224–229.
- ,,,.Postoperative hyponatremia. A prospective study.Arch Int Med.1986;146:333–336.
- .Whole‐body volume regulation and escape from antidiuresis.Am J Med.2006;119(7 Suppl 1):S21–S29.
- ,,.Neurogenic disorders of osmoregulation.Am J Med.1982;72:339–353.
- ,,.Vasopressin function in the syndrome of inappropriate antidiuresis.Annu Rev Med.1980;31:315–327.
- ,,,,,.Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure.N Eng J Med.1981;305:263–266.
- ,.Epidemiology, pathophysiology, and management of hyponatremic encephalopathy.Am J Med.1997;102:67–77.
- ,.Pulmonary complications of hyponatremic encephalopathy. noncardiogenic pulmonary edema and hypercapnic respiratory failure [see comments].Chest.1995;107(2):517–521.
- ,.Control of brain volume during hyperosmolar and hypoosmolar conditions.Annu Rev Med.1993;44:289–301.
- .Control of brain volume during hypoosmolality and hyperosmolality.Adv Exp Med Biol.2006;576:113–129.
- ,,.Clinical studies of thiazide‐induced hyponatremia.J Natl Med Assoc.2004;96(10):1305–1308.
- ,,,.Clinical assessment of extracellular fluid volume in hyponatremia.Am J Med.1987;83:905–908.
- ,.The syndrome of inappropriate secretion of antidiuretic hormone.Am J Med.1967;42:790–806.
- .Hyponatremia and Hypo‐osmolar Disorders. In: Greenberg A, Cheung AK, Coffman TM, Falk RJ, Jennette JC, eds.Primer on Kidney Diseases.Philadelphia:Saunders Elsevier,2009:52–59.
- ,,,.Reset of osmoreceptors in association with normovolemic hyponatremia.Am J Med Sci.1974;267:267–273.
- .Hyponatremia and inappropriate secretion of vasopressin (antidiuretic hormone) in patients with hypopituitarism.N Eng J Med.1989;321:492–496.
- .The Syndrome of Inappropriate Antidiuretic Hormone Secretion and Other Hypoosmolar Disorders. In: Schrier RW, ed.Diseases of the Kidney and Urinary Tract.Philadelphia:Lippincott Williams 27:156–161.
- ,,.Hyponatremia in a nursing home population.J Am Geriatr Soc.1995;43(12):1410–1413.
- ,.The syndrome of inappropriate antidiuretic hormone secretion in the elderly.Am J Med.1997;103(4):270–273.
- .Diuretic‐induced hyponatremia [editorial].Arch Intern Med.1986;146(7):1295–1296.
- ,,,.A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone.Am J Med.1957;23:529–542.
- ,,,,,.Postoperative hyponatremia despite near‐isotonic saline infusion: a phenomenon of desalination [see comments].Ann Intern Med.1997;126(1):20–25.
- ,.Hyponatremia.N Engl J Med.2000;342(21):1581–1589.
- ,,, et al.Statement of the Second International Exercise‐Associated Hyponatremia Consensus Development Conference, New Zealand, 2007.Clin J Sport Med.2008;18(2):111–121.
- ,,,.Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective.J Am Soc Nephrol.1994;4:1522–1530.
- ,,,.Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury.Crit Care Med.2005;33(1):196–202.
- ,,.Osmotic demyelination syndrome following correction of hyponatremia.N Engl J Med.1986;314:1535–1542.
- .Regulation of arginine vasopressin in the syndrome of inappropriate antidiuresis.Am J Med.2006;119(7 Suppl 1):S36–S42.
- .The syndrome of inappropriate secretion of antidiuretic hormone (SIADH).Semin Nephrol.2009;29(3):239–256.
- .Impact of solute intake on urine flow and water excretion.J Am Soc Nephrol.2008;19(6):1076–1078.
- ,.Demeclocycline‐induced nephrogenic diabetes insipidus. In‐vivo and in‐ vitro studies.Ann Intern Med.1973;79(5):679–683.
- ,,, et al.Involvement of arginine vasopressin and renal sodium handling in pathogenesis of hyponatremia in elderly patients.Endocr J.1996;43(1):101–108.
- ,.Urea for long‐term treatment of syndrome of inappropriate secretion of antidiuretic hormone.Br Med J (Clin Res Ed).1981;283:1081–1083.
- ,.Vasopressin receptor antagonists.Kidney Int.2006;69(12):2124–2130.
- ,,, et al.Potent aquaretic agent. A novel nonpeptide selective vasopressin 2 antagonist (OPC‐31260) in men.J Clin Invest.1993;92(6):2653–2659.
- ,,,,.Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia.Am J Nephrol.2007;27(5):447–457.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355(20):2099–2112.
- Vaprisol (conivaptan hydrochloride injection) prescribing information.Deerfield, IL:Astellas Pharma US, Inc.,2006.
- ,,,,.Hyponatremia treatment guidelines 2007: expert panel recommendations.Am J Med.2007;120(11 Suppl 1):S1–S21.
- Otsuka Pharmaceutical Co L, Tokyo J. Samsca (tolvaptan) prescribing information.2009.
- ,.Clinical practice. The syndrome of inappropriate antidiuresis.N Engl J Med.2007;356(20):2064–2072.
- ,,.The treatment of hyponatremia.Semin Nephrol.2009;29(3):282–299.
- .Hyponatremia and Hypo‐osmolar Disorders. In: Greenberg A, Cheung AK, Coffman TM, Falk RJ, Jennette JC, eds.Primer on Kidney Diseases.Philadelphia. PA:Saunders Elsevier;2009:52–59.
- ,,,.Risk factors for symptomatic hyponatraemia: the role of pre‐existing asymptomatic hyponatraemia.Intern Med J.2007;37(3):149–155.
- ,,,,.Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits.Am J Med.2006;119(1):71.
- ,,,,.Mild hyponatremia and risk of fracture in the ambulatory elderly.QJM.2008;101(7):583–588.
- ,,,,.Hyponatremia associated with large‐bone fracture in elderly patients.Int Urol Nephrol.2009;41(3):733–737.
- ,,, et al.Hyponatremia‐induced osteoporosis.J Bone Miner Res.2010;25(3):554–563.
Why is SIADH Important to Hospitalists?
Disorders of body fluids, and particularly hyponatremia, are among the most commonly encountered problems in clinical medicine, affecting up to 30% of hospitalized patients. In a study of 303,577 laboratory samples collected from 120,137 patients, the prevalence of hyponatremia (serum [Na+] 135 mmol/L) on initial presentation to a healthcare provider was 28.2% among those treated in an acute hospital care setting, 21% among those treated in an ambulatory hospital care setting, and 7.2% in community care centers.1 Numerous other studies have corroborated a high prevalence of hyponatremia in hospitalized patients,2 which reflects the increased vulnerability of this patient population to disruptions of body fluid homeostasis. Recognizing the many possible causes of hyponatremia in hospitalized patients and implementing appropriate treatment strategies therefore are critical steps toward optimizing care and improving outcomes in hospitalized patients with hyponatremia.
In addition to its frequency, hyponatremia is also important because it has been associated with worse clinical outcomes across the entire range of inpatient care, from the general hospital population to those treated in the intensive care unit (ICU). In a study of 4123 patients age 65 years or older who were admitted to a community hospital, 3.5% had clinically significant hyponatremia (serum [Na+] 130 mmol/L) at admission. Compared with nonhyponatremic patients, those with hyponatremia were twice as likely to die during their hospital stay (relative risk [RR], 1.95; P 0.05).3 In another study of 2188 patients admitted to a medical ICU over a 5‐year period, 13.7% had hyponatremia. The overall rate of in‐hospital mortality among all ICU patients was high at 37.7%. However, severe hyponatremia (serum [Na+] 125 mmol/L) more than doubled the risk of in‐hospital mortality (RR, 2.10; P 0.001).4 In addition to the general hospital population, in virtually every disease ever studied, the presence of hyponatremia has been found to be an independent risk factor for increased mortality, from congestive heart failure to tuberculosis to liver failure.2
What Causes Hyponatremia in Patients with SIADH?
Hyponatremia can be caused by 1 of 2 potential disruptions in fluid balance: dilution from retained water, or depletion from electrolyte losses in excess of water. Dilutional hyponatremias are associated with either a normal (euvolemic) or an increased (hypervolemic) extracellular fluid (ECF) volume, whereas depletional hyponatremias generally are associated with a decreased ECF volume (hypovolemic). Dilutional hyponatremia can arise from a primary defect in osmoregulation, such as in SIADH, or as a result of ECF volume expansion, as seen in conditions associated with concomitant secondary hyperaldosteronism such as heart failure, hepatic cirrhosis, or nephrotic syndrome. Among some hospitalized patient groups, euvolemic hyponatremia is the most common presentation of abnormally low serum [Na+]. In a study of patients who developed clinically significant postoperative hyponatremia (defined as a serum [Na+] 130 mmol/L) in a large teaching hospital, only 8% were hypovolemic, whereas 42% were euvolemic and 21% were hypervolemic.5
Euvolemic hyponatremia results from an increase in total body water, but with normal or near‐normal total body sodium. As a result, there is an absence of clinical manifestations of ECF volume expansion, such as subcutaneous edema or ascites. It is important to recognize that although SIADH clearly represents a state of volume expansion due to water retention, it rarely causes clinically recognizable hypervolemia since the retained water is distributed across the intracellular fluid (ICF) as well as the ECF, and because volume regulatory processes act to decrease the actual degree of ECF volume expansion.6 Euvolemic hyponatremia can accompany a wide variety of pathological processes, but the most common cause by far is SIADH. Normally, increased plasma osmolality activates osmoreceptors located in the anterior hypothalamus and stimulates the secretion of arginine vasopressin (AVP), also called antidiuretic hormone (ADH), a key neurohormone that regulates fluid homeostasis. In patients with euvolemic hyponatremia due to SIADH, plasma AVP levels are not suppressed despite normal or decreased plasma osmolality.7 This can be a result of ectopic production of AVP by tumors, or stimulation of endogenous pituitary AVP secretion as a result of nonosmotic stimuli that also stimulate vasopressinergic neurons, which include hypovolemia, hypotension, angiotensin II, nausea, hypoxia, hypercarbia, hypoglycemia, stress, and physical activity. Nonsuppressed AVP levels have been documented in the majority of hyponatremic patients, including those with SIADH8 and heart failure.9
SIADH can develop as the result of many different disease processes that disrupt the normal mechanisms that regulate AVP secretion, including pneumonias and other lung infections, thoracic and extrathoracic tumors, a variety of different central nervous system disorders, the postoperative state, human immunodeficiency virus (HIV), and many different drugs (Figure 1). Given the multiplicity of disorders and drugs that can cause disrupted AVP secretion, it is not surprising that hyponatremia is the most common electrolyte abnormality seen in clinical practice.

What Symptoms are Associated With SIADH?
Symptoms of hyponatremia correlate both with the degree of decrease in the serum [Na+] and with the chronicity of the hyponatremia. Acute hyponatremia, defined as 48 hours in duration, is often associated with life‐threatening clinical features such as obtundation, seizures, coma, and respiratory arrest. These symptoms can occur abruptly, sometimes with little warning.10 In the most severe cases, death can occur as a result of cerebral edema with tentorial herniation. Hypoxia secondary to neurogenic pulmonary edema can increase the severity of brain swelling.11
In contrast, chronic hyponatremia is much less symptomatic, and the reason for the profound differences between the symptoms of acute and chronic hyponatremia is now well understood to be due to the process of brain volume regulation.12 It is essential that this process be understood in order to understand the full spectrum of hyponatremic symptoms. As the ECF [Na+] decreases, regardless of whether due to a loss of sodium or a gain of water, there is an obligate movement of water into the brain along osmotic gradients. That water shift causes swelling of the brain, or cerebral edema. If the increased brain water reaches approximately 8% in adults, it exceeds the capacity of the skull to accommodate brain expansion, leading to tentorial herniation and death from respiratory arrest and/or ischemic brain damage. However, if the patient survives the initial hyponatremia, a very strong volume regulatory process follows, consisting of loss of electrolytes and small organic molecules called osmolytes from brain cells into brain ECF, and eventually the peripheral ECF.12, 13 As the solute content of the brain decreases, the water content is allowed to normalize, eventually reaching a state in which brain edema is virtually absent, and as a result symptoms are markedly less than with acute hyponatremia. Although the time required for the brain to acieve a volume‐regulated state varies across patients, this process is completed within 48 hours in experinmental animal studies, and probably follows a similar time course in humans.
Despite this powerful adaptation process, chronic hyponatremia is frequently associated with neurological symptomatology, albeit milder and more subtle in nature. A recent report found a fairly high incidence of symptoms in 223 patients with chronic hyponatremia as a result of thiazide administration: 49% had malaise/lethargy, 47% had dizzy spells, 35% had vomiting, 17% had confusion/obtundation, 17% experienced falls, 6% had headaches, and 0.9% had seizures.14 Although dizziness can potentially be attributed to a diuretic‐induced hypovolemia, symptoms such as confusion, obtundation and seizures are more consistent with hyponatremic symptomatology. Because thiazide‐induced hyponatremia can be readily corrected by stopping the thiazide and/or administering sodium, this represents an ideal situation in which to assess improvement in hyponatremia symptomatology with normalization of the serum [Na+]; in this study, all of these symptoms improved with correction of the hyponatremia. This represents one of the best examples demonstrating reversal of the symptoms associated with chronic hyponatremia by correction of the hyponatremia, because the patients in this study did not in general have severe underlying comorbidities that might complicate interpretation of their symptoms, as is often the case in patients with SIADH.
What Is Required for Making a Diagnosis of SIADH in Hospitalized Patients?
In patients with hypotonic hypoosmolality, ascertainment of their ECF volume status (ie, hypovolemic, euvolemic, or hypervolemic) is an essential first step, as this will segregate patients into different treatment paradigms. For example, in patients who are truly clinically hypovolemic with a decreased ECF volume by clinical parameters, treatment would generally consist of solute repletion with sodium, generally isotonic saline infusion with or without potassium, until the sodium levels normalize. In patients who are hypervolemic, treatment should focus first on the underlying disease rather than addressing the serum [Na+] directly. In patients with clinical euvolemia, the standard diagnostic pathway should be followed to confirm a diagnosis of SIADH as described below.
Assessing ECF volume status can be difficult, even for the most experienced clinicians. Physical signs such as orthostatic decreases in blood pressure and increases in pulse rate, dry mucus membranes, and skin tenting indicate hypovolemic hyponatremia, while signs such as subcutaneous edema, ascites, or anasarca indicate hypervolemic hyponatremia. Patients without any of these findings are generally considered to be euvolemic. However, in any situation these signs are only applicable if there are no other reasons to suspect an altered ECF volume. Along with a complete history and physical examination that includes a careful neurological evaluation, several laboratory tests can help to assess the etiology of the hyponatremia, once serum sodium concentrations have been shown to be below normal ([Na+] 135 mmol/L):
-
Urine osmolality. A urine osmolality (Uosm) less than 100 mOsm/kg H2O can indicate low dietary solute intake, primary polydipsia, or a reset osmostat after suppression of AVP release by a decrease in plasma osmolality below the osmotic threshold for AVP secretion, usually as a result of increased water loading.
-
Urine sodium concentration. Excretion of sodium, as measured by a spot urine [Na+] (UNa), can indicate depletional hyponatremia if the concentration is less than 30 mmol/L.15 A low UNa reflects a volume depleted state unless the patient has secondary hyperaldosteronism from heart failure or cirrhosis. Patients with a low UNa are more likely to respond to isotonic saline. Euvolemic patients who have a normal dietary sodium intake will generally have spot UNa 30 mmol/L and will not benefit from isotonic saline administration.15 In fact, in SIADH, these patients may respond to isotonic saline with a worsening of hyponatremia, since the sodium from the isotonic saline will be excreted in a concentrated urine while the free water is reabsorbed in the kidney collecting ducts. If the patient is on diuretic therapy, urine sodium values cannot always be accurately interpreted, since a UNa 30 mmol/L may reflect the natriuretic effect of the diuretic and not a volume replete state.
-
Blood tests. Additional indicators of volume status include serum blood nitrogen (BUN) and uric acid levels. A BUN 10 mg/dL and uric acid 4 mg/dL are generally consistent with a euvolemic state, particularly when there is glomerular hyperfiltration, which is often present in SIADH. Elevated serum BUN and uric acid levels (BUN >20 mg/dL and uric acid >6 mg/dL), especially if prior values are available for comparison, can also help to establish whether ineffective vascular volume status may be contributing to the pathophysiology of the hyponatremia. In certain clinical scenarios, the B‐type natriuretic protein (BNP) can be helpful to support a clinical impression of congestive heart failure.
The criteria necessary for a diagnosis of SIADH remain essentially as defined by Bartter and Schwartz16 in 1967 (Table 1), but several points deserve emphasis.17 First, true ECF hypoosmolality must be present and hyponatremia secondary to pseudohyponatremia or hyperglycemia excluded. Second, urinary osmolality must be inappropriate for plasma hypoosmolality (Posm). This does not require a Uosm>Posm, but simply that the urine osmolality is greater than maximally dilute (ie, Uosm>100 mOsm/kg H2O in adults). Furthermore, urine osmolality need not be inappropriately elevated at all levels of Posm but simply at some level under 275 mOsm/kg H2O, since in patients with a reset osmostat, AVP secretion can be suppressed at some level of osmolality resulting in maximal urinary dilution and free water excretion at plasma osmolalities below this level.18 Although some consider a reset osmostat to be a separate disorder rather than a variant of SIADH, such cases nonetheless illustrate that some hypoosmolar patients can exhibit an appropriately dilute urine at some, though not all, plasma osmolalities. Third, clinical euvolemia must be present to diagnose SIADH, and this diagnosis cannot be made in a hypovolemic or edematous patient. Importantly, this does not mean that patients with SIADH cannot become hypovolemic for other reasons, but in such cases it is impossible to diagnose the underlying SIADH until the patient is rendered euvolemic. The fourth criterion, renal salt wasting, has probably caused the most confusion in the diagnosis of SIADH. As noted above, the importance of this criterion lies in its usefulness in differentiating hypoosmolality caused by a decreased effective intravascular volume with high aldosterone levels in which case renal Na+ conservation occurs, from dilutional disorders in which urine Na+ excretion is normal or increased due to ECF volume expansion and a suppressed renin‐angiotensin‐aldosterone system. However, UNa can also be high in renal causes of solute depletion such as diuretic use or Addison's disease, and conversely patients with SIADH can have a low UNa if they subsequently become hypovolemic or solute depleted, conditions sometimes produced by imposed salt and water restriction. Consequently, although high urinary Na+ excretion is generally the rule in most patients with SIADH, its presence does not necessarily confirm this diagnosis, nor does its absence rule out the diagnosis. The final criterion emphasizes that SIADH remains a diagnosis of exclusion, and the absence of other potential causes of hypoosmolality must always be verified. Glucocorticoid deficiency and SIADH can be especially difficult to distinguish, since both primary and secondary hypocortisolism can cause elevated plasma AVP levels in addition to direct renal effects that prevent maximal urinary dilution.19 Therefore, no patient with chronic hyponatremia should be diagnosed as having SIADH without a thorough evaluation of adrenal function, preferably via a rapid adrenocorticotropic hormone (ACTH) stimulation test. Acute hyponatremia of obvious etiology, such as postoperatively or in association with pneumonitis, may be treated without adrenal testing as long as there are no other clinical signs or symptoms suggestive of adrenal dysfunction.20
|
| Essential |
| Decreased effective osmolality of the extracellular fluid (Posm 275 mOsm/kg H2O). |
| Inappropriate urinary concentration (Uosm >100 mOsm/kg H2O with normal renal function) at some level of hypoosmolality. |
| Clinical euvolemia, as defined by the absence of signs of hypovolemia (orthostasis, tachycardia, decreased skin turgor, dry mucous membranes) or hypervolemia (subcutaneous edema, ascites). |
| Elevated urinary sodium excretion while on a normal salt and water intake. |
| Absence of other potential causes of euvolemic hypoosmolality: hypothyroidism, hypocortisolism (Addison's disease or secondary adrenal insufficiency) and diuretic use. |
| Supplemental |
| Abnormal water load test (inability to excrete at least 90% of a 20 mL/kg water load in 4 hours and/or failure to dilute Uosm to 100 mOsm/kg H2O). |
| Plasma AVP level inappropriately elevated relative to plasma osmolality. |
| No significant correction of serum [Na+] with volume expansion but improvement after fluid restriction. |
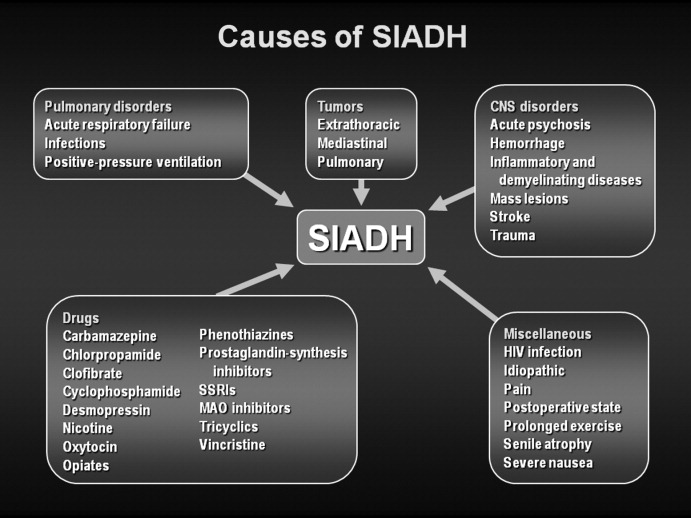
Hyponatremia is a particularly common complication in elderly hospitalized patients, increasing in prevalence from approximately 7% in the general older population to 18% to 22% among elderly patients in chronic care facilities.21 Despite the many known causes of SIADH (Figure 1), hyponatremia is often associated with idiopathic SIADH in the elderly population. In a study of 119 nursing home residents aged 60 to 103 years, 53% had at least 1 episode of hyponatremia during the previous 12 months.22 Of these patients, 26% were diagnosed with idiopathic SIADH. In another study of elderly patients with hyponatremia and SIADH, 60% were diagnosed with idiopathic SIADH. Among remaining patients, the 2 main causes identified were pneumonia (9 cases/18%) and medications (6 cases/12%).23 Therefore, more than half of elderly patients who present with hyponatremia due to SIADH may have an idiopathic form, with no detectable underlying treatable disease.
Which Hospital Patients With SIADH are Candidates for Treatment of Hyponatremia?
Correction of hyponatremia is associated with markedly improved neurological outcomes in patients with severely symptomatic hyponatremia. In a retrospective review of patients who presented with severe neurological symptoms and serum [Na+] 125 mmol/L, prompt therapy with isotonic or hypertonic saline resulted in a correction in the range of 20 mmol/L over several days and neurological recovery in almost all cases. In contrast, in patients who were treated with fluid restriction alone, there was very little correction over the study period (5 mmol/L over 72 hours), and the neurological outcomes were much worse, with most of these patients either dying or entering a persistently vegetative state.24 Consequently, prompt therapy to rapidly increase the serum [Na+] represents the standard‐of‐care for treatment of patients presenting with severe life‐threatening symptoms of hyponatremia.
As discussed earlier, chronic hyponatremia is much less symptomatic as a result of the process of brain volume regulation. Because of this adaptation process, chronic hyponatremia is arguably a condition that clinicians feel they may not need to be as concerned about, and in some publications this has been called asymptomatic hyponatremia. However, such patients often do have neurological symptoms, even if milder and more subtle in nature, including headaches, nausea, mood disturbances, depression, difficulty concentrating, slowed reaction times, unstable gait, increased falls, confusion, and disorientation. Consequently, any patient with hyponatremia secondary to SIADH who manifests any neurological symptoms that could be related to the hyponatremia should be considered as appropriate candidates for treatment of the hyponatremia, regardless of the chronicity of the hyponatremia or the level of serum [Na+].
What Therapies are Currently Available to Manage SIADH in Hospitalized Patients?
Conventional management strategies for euvolemic hyponatremia range from saline infusion and fluid restriction to pharmacologic adjustment of fluid balance. Consideration of treatment options should include an evaluation of the benefits as well as the potential toxicities of any therapy (Table 2). Sometimes, simply stopping treatment with an agent that is associated with hyponatremia is sufficient to reverse a low serum [Na+].
| Therapy | Targets Underlying Pathophysiology | Limitations |
|---|---|---|
| ||
| Isotonic saline | Ineffective in dilutional hyponatremias; exacerbates the volume overload if used in edema‐forming disorders; no controlled safety database. | |
| Hypertonic saline | No consensus regarding appropriate infusion rates; overcorrection can cause osmotic demyelination; exacerbates the volume overload if used in edema‐forming disorders; no controlled safety database. | |
| Fluid restriction | Slow to correct over many days; poorly tolerated due to thirst; can not be used effectively in patients with high AVP levels and urine osmolalities. | |
| Demeclocycline | ✓ | Not FDA approved for hyponatremia; slow to correct; nephrotoxic in cirrhosis and heart failure. |
| Mineralocorticoids | Only one report in elderly patients with SIADH; no safety database; exacerbates the volume overload if used in edema‐forming disorders. | |
| Urea | Not FDA‐approved for hyponatremia; poor palatability. | |
| AVP receptor antagonists (vaptans) | ✓ | Conivaptan approved only for in‐hospital use secondary to CYP3A4 inhibition; infusion‐site reactions with intravenous use. Tolvaptan must be initiated and reinitiated in the hospital, as serum sodium needs to be monitored closely to avoid overly rapid correction of hyponatremia. |
Isotonic Saline
The treatment of choice for depletional hyponatremia (ie, hypovolemic hyponatremia) is isotonic saline ([Na+] = 154 mmol/L) to restore ECF volume and ensure adequate organ perfusion. This initial therapy is appropriate for patients who either have clinical signs of hypovolemia, or in whom a spot UNa+ is 30 mmol/L. However, this therapy is ineffective for dilutional hyponatremias such as SIADH,25 and continued inappropriate administration of isotonic saline to a euvolemic patient may worsen their hyponatremia,26 and/or cause fluid overload. Although isotonic saline may improve the serum [Na+] in patients with hypervolemic hyponatremia, their volume status will generally worsen with this therapy, so unless the hyponatremia is profound isotonic saline should be avoided.
Hypertonic Saline
Acute hyponatremia presenting with severe neurological symptoms is life‐threatening, and should be treated promptly with hypertonic solutions, typically 3% NaCl ([Na+] = 513 mmol/L), as this represents the most reliable method to quickly raise the serum [Na+]. A continuous infusion of hypertonic NaCl is usually utilized in inpatient settings. Various formulae have been suggested for calculating the initial rate of infusion of hypertonic solutions,27 but perhaps the simplest utilizes the following relationship:
Why is SIADH Important to Hospitalists?
Disorders of body fluids, and particularly hyponatremia, are among the most commonly encountered problems in clinical medicine, affecting up to 30% of hospitalized patients. In a study of 303,577 laboratory samples collected from 120,137 patients, the prevalence of hyponatremia (serum [Na+] 135 mmol/L) on initial presentation to a healthcare provider was 28.2% among those treated in an acute hospital care setting, 21% among those treated in an ambulatory hospital care setting, and 7.2% in community care centers.1 Numerous other studies have corroborated a high prevalence of hyponatremia in hospitalized patients,2 which reflects the increased vulnerability of this patient population to disruptions of body fluid homeostasis. Recognizing the many possible causes of hyponatremia in hospitalized patients and implementing appropriate treatment strategies therefore are critical steps toward optimizing care and improving outcomes in hospitalized patients with hyponatremia.
In addition to its frequency, hyponatremia is also important because it has been associated with worse clinical outcomes across the entire range of inpatient care, from the general hospital population to those treated in the intensive care unit (ICU). In a study of 4123 patients age 65 years or older who were admitted to a community hospital, 3.5% had clinically significant hyponatremia (serum [Na+] 130 mmol/L) at admission. Compared with nonhyponatremic patients, those with hyponatremia were twice as likely to die during their hospital stay (relative risk [RR], 1.95; P 0.05).3 In another study of 2188 patients admitted to a medical ICU over a 5‐year period, 13.7% had hyponatremia. The overall rate of in‐hospital mortality among all ICU patients was high at 37.7%. However, severe hyponatremia (serum [Na+] 125 mmol/L) more than doubled the risk of in‐hospital mortality (RR, 2.10; P 0.001).4 In addition to the general hospital population, in virtually every disease ever studied, the presence of hyponatremia has been found to be an independent risk factor for increased mortality, from congestive heart failure to tuberculosis to liver failure.2
What Causes Hyponatremia in Patients with SIADH?
Hyponatremia can be caused by 1 of 2 potential disruptions in fluid balance: dilution from retained water, or depletion from electrolyte losses in excess of water. Dilutional hyponatremias are associated with either a normal (euvolemic) or an increased (hypervolemic) extracellular fluid (ECF) volume, whereas depletional hyponatremias generally are associated with a decreased ECF volume (hypovolemic). Dilutional hyponatremia can arise from a primary defect in osmoregulation, such as in SIADH, or as a result of ECF volume expansion, as seen in conditions associated with concomitant secondary hyperaldosteronism such as heart failure, hepatic cirrhosis, or nephrotic syndrome. Among some hospitalized patient groups, euvolemic hyponatremia is the most common presentation of abnormally low serum [Na+]. In a study of patients who developed clinically significant postoperative hyponatremia (defined as a serum [Na+] 130 mmol/L) in a large teaching hospital, only 8% were hypovolemic, whereas 42% were euvolemic and 21% were hypervolemic.5
Euvolemic hyponatremia results from an increase in total body water, but with normal or near‐normal total body sodium. As a result, there is an absence of clinical manifestations of ECF volume expansion, such as subcutaneous edema or ascites. It is important to recognize that although SIADH clearly represents a state of volume expansion due to water retention, it rarely causes clinically recognizable hypervolemia since the retained water is distributed across the intracellular fluid (ICF) as well as the ECF, and because volume regulatory processes act to decrease the actual degree of ECF volume expansion.6 Euvolemic hyponatremia can accompany a wide variety of pathological processes, but the most common cause by far is SIADH. Normally, increased plasma osmolality activates osmoreceptors located in the anterior hypothalamus and stimulates the secretion of arginine vasopressin (AVP), also called antidiuretic hormone (ADH), a key neurohormone that regulates fluid homeostasis. In patients with euvolemic hyponatremia due to SIADH, plasma AVP levels are not suppressed despite normal or decreased plasma osmolality.7 This can be a result of ectopic production of AVP by tumors, or stimulation of endogenous pituitary AVP secretion as a result of nonosmotic stimuli that also stimulate vasopressinergic neurons, which include hypovolemia, hypotension, angiotensin II, nausea, hypoxia, hypercarbia, hypoglycemia, stress, and physical activity. Nonsuppressed AVP levels have been documented in the majority of hyponatremic patients, including those with SIADH8 and heart failure.9
SIADH can develop as the result of many different disease processes that disrupt the normal mechanisms that regulate AVP secretion, including pneumonias and other lung infections, thoracic and extrathoracic tumors, a variety of different central nervous system disorders, the postoperative state, human immunodeficiency virus (HIV), and many different drugs (Figure 1). Given the multiplicity of disorders and drugs that can cause disrupted AVP secretion, it is not surprising that hyponatremia is the most common electrolyte abnormality seen in clinical practice.

What Symptoms are Associated With SIADH?
Symptoms of hyponatremia correlate both with the degree of decrease in the serum [Na+] and with the chronicity of the hyponatremia. Acute hyponatremia, defined as 48 hours in duration, is often associated with life‐threatening clinical features such as obtundation, seizures, coma, and respiratory arrest. These symptoms can occur abruptly, sometimes with little warning.10 In the most severe cases, death can occur as a result of cerebral edema with tentorial herniation. Hypoxia secondary to neurogenic pulmonary edema can increase the severity of brain swelling.11
In contrast, chronic hyponatremia is much less symptomatic, and the reason for the profound differences between the symptoms of acute and chronic hyponatremia is now well understood to be due to the process of brain volume regulation.12 It is essential that this process be understood in order to understand the full spectrum of hyponatremic symptoms. As the ECF [Na+] decreases, regardless of whether due to a loss of sodium or a gain of water, there is an obligate movement of water into the brain along osmotic gradients. That water shift causes swelling of the brain, or cerebral edema. If the increased brain water reaches approximately 8% in adults, it exceeds the capacity of the skull to accommodate brain expansion, leading to tentorial herniation and death from respiratory arrest and/or ischemic brain damage. However, if the patient survives the initial hyponatremia, a very strong volume regulatory process follows, consisting of loss of electrolytes and small organic molecules called osmolytes from brain cells into brain ECF, and eventually the peripheral ECF.12, 13 As the solute content of the brain decreases, the water content is allowed to normalize, eventually reaching a state in which brain edema is virtually absent, and as a result symptoms are markedly less than with acute hyponatremia. Although the time required for the brain to acieve a volume‐regulated state varies across patients, this process is completed within 48 hours in experinmental animal studies, and probably follows a similar time course in humans.
Despite this powerful adaptation process, chronic hyponatremia is frequently associated with neurological symptomatology, albeit milder and more subtle in nature. A recent report found a fairly high incidence of symptoms in 223 patients with chronic hyponatremia as a result of thiazide administration: 49% had malaise/lethargy, 47% had dizzy spells, 35% had vomiting, 17% had confusion/obtundation, 17% experienced falls, 6% had headaches, and 0.9% had seizures.14 Although dizziness can potentially be attributed to a diuretic‐induced hypovolemia, symptoms such as confusion, obtundation and seizures are more consistent with hyponatremic symptomatology. Because thiazide‐induced hyponatremia can be readily corrected by stopping the thiazide and/or administering sodium, this represents an ideal situation in which to assess improvement in hyponatremia symptomatology with normalization of the serum [Na+]; in this study, all of these symptoms improved with correction of the hyponatremia. This represents one of the best examples demonstrating reversal of the symptoms associated with chronic hyponatremia by correction of the hyponatremia, because the patients in this study did not in general have severe underlying comorbidities that might complicate interpretation of their symptoms, as is often the case in patients with SIADH.
What Is Required for Making a Diagnosis of SIADH in Hospitalized Patients?
In patients with hypotonic hypoosmolality, ascertainment of their ECF volume status (ie, hypovolemic, euvolemic, or hypervolemic) is an essential first step, as this will segregate patients into different treatment paradigms. For example, in patients who are truly clinically hypovolemic with a decreased ECF volume by clinical parameters, treatment would generally consist of solute repletion with sodium, generally isotonic saline infusion with or without potassium, until the sodium levels normalize. In patients who are hypervolemic, treatment should focus first on the underlying disease rather than addressing the serum [Na+] directly. In patients with clinical euvolemia, the standard diagnostic pathway should be followed to confirm a diagnosis of SIADH as described below.
Assessing ECF volume status can be difficult, even for the most experienced clinicians. Physical signs such as orthostatic decreases in blood pressure and increases in pulse rate, dry mucus membranes, and skin tenting indicate hypovolemic hyponatremia, while signs such as subcutaneous edema, ascites, or anasarca indicate hypervolemic hyponatremia. Patients without any of these findings are generally considered to be euvolemic. However, in any situation these signs are only applicable if there are no other reasons to suspect an altered ECF volume. Along with a complete history and physical examination that includes a careful neurological evaluation, several laboratory tests can help to assess the etiology of the hyponatremia, once serum sodium concentrations have been shown to be below normal ([Na+] 135 mmol/L):
-
Urine osmolality. A urine osmolality (Uosm) less than 100 mOsm/kg H2O can indicate low dietary solute intake, primary polydipsia, or a reset osmostat after suppression of AVP release by a decrease in plasma osmolality below the osmotic threshold for AVP secretion, usually as a result of increased water loading.
-
Urine sodium concentration. Excretion of sodium, as measured by a spot urine [Na+] (UNa), can indicate depletional hyponatremia if the concentration is less than 30 mmol/L.15 A low UNa reflects a volume depleted state unless the patient has secondary hyperaldosteronism from heart failure or cirrhosis. Patients with a low UNa are more likely to respond to isotonic saline. Euvolemic patients who have a normal dietary sodium intake will generally have spot UNa 30 mmol/L and will not benefit from isotonic saline administration.15 In fact, in SIADH, these patients may respond to isotonic saline with a worsening of hyponatremia, since the sodium from the isotonic saline will be excreted in a concentrated urine while the free water is reabsorbed in the kidney collecting ducts. If the patient is on diuretic therapy, urine sodium values cannot always be accurately interpreted, since a UNa 30 mmol/L may reflect the natriuretic effect of the diuretic and not a volume replete state.
-
Blood tests. Additional indicators of volume status include serum blood nitrogen (BUN) and uric acid levels. A BUN 10 mg/dL and uric acid 4 mg/dL are generally consistent with a euvolemic state, particularly when there is glomerular hyperfiltration, which is often present in SIADH. Elevated serum BUN and uric acid levels (BUN >20 mg/dL and uric acid >6 mg/dL), especially if prior values are available for comparison, can also help to establish whether ineffective vascular volume status may be contributing to the pathophysiology of the hyponatremia. In certain clinical scenarios, the B‐type natriuretic protein (BNP) can be helpful to support a clinical impression of congestive heart failure.
The criteria necessary for a diagnosis of SIADH remain essentially as defined by Bartter and Schwartz16 in 1967 (Table 1), but several points deserve emphasis.17 First, true ECF hypoosmolality must be present and hyponatremia secondary to pseudohyponatremia or hyperglycemia excluded. Second, urinary osmolality must be inappropriate for plasma hypoosmolality (Posm). This does not require a Uosm>Posm, but simply that the urine osmolality is greater than maximally dilute (ie, Uosm>100 mOsm/kg H2O in adults). Furthermore, urine osmolality need not be inappropriately elevated at all levels of Posm but simply at some level under 275 mOsm/kg H2O, since in patients with a reset osmostat, AVP secretion can be suppressed at some level of osmolality resulting in maximal urinary dilution and free water excretion at plasma osmolalities below this level.18 Although some consider a reset osmostat to be a separate disorder rather than a variant of SIADH, such cases nonetheless illustrate that some hypoosmolar patients can exhibit an appropriately dilute urine at some, though not all, plasma osmolalities. Third, clinical euvolemia must be present to diagnose SIADH, and this diagnosis cannot be made in a hypovolemic or edematous patient. Importantly, this does not mean that patients with SIADH cannot become hypovolemic for other reasons, but in such cases it is impossible to diagnose the underlying SIADH until the patient is rendered euvolemic. The fourth criterion, renal salt wasting, has probably caused the most confusion in the diagnosis of SIADH. As noted above, the importance of this criterion lies in its usefulness in differentiating hypoosmolality caused by a decreased effective intravascular volume with high aldosterone levels in which case renal Na+ conservation occurs, from dilutional disorders in which urine Na+ excretion is normal or increased due to ECF volume expansion and a suppressed renin‐angiotensin‐aldosterone system. However, UNa can also be high in renal causes of solute depletion such as diuretic use or Addison's disease, and conversely patients with SIADH can have a low UNa if they subsequently become hypovolemic or solute depleted, conditions sometimes produced by imposed salt and water restriction. Consequently, although high urinary Na+ excretion is generally the rule in most patients with SIADH, its presence does not necessarily confirm this diagnosis, nor does its absence rule out the diagnosis. The final criterion emphasizes that SIADH remains a diagnosis of exclusion, and the absence of other potential causes of hypoosmolality must always be verified. Glucocorticoid deficiency and SIADH can be especially difficult to distinguish, since both primary and secondary hypocortisolism can cause elevated plasma AVP levels in addition to direct renal effects that prevent maximal urinary dilution.19 Therefore, no patient with chronic hyponatremia should be diagnosed as having SIADH without a thorough evaluation of adrenal function, preferably via a rapid adrenocorticotropic hormone (ACTH) stimulation test. Acute hyponatremia of obvious etiology, such as postoperatively or in association with pneumonitis, may be treated without adrenal testing as long as there are no other clinical signs or symptoms suggestive of adrenal dysfunction.20
|
| Essential |
| Decreased effective osmolality of the extracellular fluid (Posm 275 mOsm/kg H2O). |
| Inappropriate urinary concentration (Uosm >100 mOsm/kg H2O with normal renal function) at some level of hypoosmolality. |
| Clinical euvolemia, as defined by the absence of signs of hypovolemia (orthostasis, tachycardia, decreased skin turgor, dry mucous membranes) or hypervolemia (subcutaneous edema, ascites). |
| Elevated urinary sodium excretion while on a normal salt and water intake. |
| Absence of other potential causes of euvolemic hypoosmolality: hypothyroidism, hypocortisolism (Addison's disease or secondary adrenal insufficiency) and diuretic use. |
| Supplemental |
| Abnormal water load test (inability to excrete at least 90% of a 20 mL/kg water load in 4 hours and/or failure to dilute Uosm to 100 mOsm/kg H2O). |
| Plasma AVP level inappropriately elevated relative to plasma osmolality. |
| No significant correction of serum [Na+] with volume expansion but improvement after fluid restriction. |
Hyponatremia is a particularly common complication in elderly hospitalized patients, increasing in prevalence from approximately 7% in the general older population to 18% to 22% among elderly patients in chronic care facilities.21 Despite the many known causes of SIADH (Figure 1), hyponatremia is often associated with idiopathic SIADH in the elderly population. In a study of 119 nursing home residents aged 60 to 103 years, 53% had at least 1 episode of hyponatremia during the previous 12 months.22 Of these patients, 26% were diagnosed with idiopathic SIADH. In another study of elderly patients with hyponatremia and SIADH, 60% were diagnosed with idiopathic SIADH. Among remaining patients, the 2 main causes identified were pneumonia (9 cases/18%) and medications (6 cases/12%).23 Therefore, more than half of elderly patients who present with hyponatremia due to SIADH may have an idiopathic form, with no detectable underlying treatable disease.
Which Hospital Patients With SIADH are Candidates for Treatment of Hyponatremia?
Correction of hyponatremia is associated with markedly improved neurological outcomes in patients with severely symptomatic hyponatremia. In a retrospective review of patients who presented with severe neurological symptoms and serum [Na+] 125 mmol/L, prompt therapy with isotonic or hypertonic saline resulted in a correction in the range of 20 mmol/L over several days and neurological recovery in almost all cases. In contrast, in patients who were treated with fluid restriction alone, there was very little correction over the study period (5 mmol/L over 72 hours), and the neurological outcomes were much worse, with most of these patients either dying or entering a persistently vegetative state.24 Consequently, prompt therapy to rapidly increase the serum [Na+] represents the standard‐of‐care for treatment of patients presenting with severe life‐threatening symptoms of hyponatremia.
As discussed earlier, chronic hyponatremia is much less symptomatic as a result of the process of brain volume regulation. Because of this adaptation process, chronic hyponatremia is arguably a condition that clinicians feel they may not need to be as concerned about, and in some publications this has been called asymptomatic hyponatremia. However, such patients often do have neurological symptoms, even if milder and more subtle in nature, including headaches, nausea, mood disturbances, depression, difficulty concentrating, slowed reaction times, unstable gait, increased falls, confusion, and disorientation. Consequently, any patient with hyponatremia secondary to SIADH who manifests any neurological symptoms that could be related to the hyponatremia should be considered as appropriate candidates for treatment of the hyponatremia, regardless of the chronicity of the hyponatremia or the level of serum [Na+].
What Therapies are Currently Available to Manage SIADH in Hospitalized Patients?
Conventional management strategies for euvolemic hyponatremia range from saline infusion and fluid restriction to pharmacologic adjustment of fluid balance. Consideration of treatment options should include an evaluation of the benefits as well as the potential toxicities of any therapy (Table 2). Sometimes, simply stopping treatment with an agent that is associated with hyponatremia is sufficient to reverse a low serum [Na+].
| Therapy | Targets Underlying Pathophysiology | Limitations |
|---|---|---|
| ||
| Isotonic saline | Ineffective in dilutional hyponatremias; exacerbates the volume overload if used in edema‐forming disorders; no controlled safety database. | |
| Hypertonic saline | No consensus regarding appropriate infusion rates; overcorrection can cause osmotic demyelination; exacerbates the volume overload if used in edema‐forming disorders; no controlled safety database. | |
| Fluid restriction | Slow to correct over many days; poorly tolerated due to thirst; can not be used effectively in patients with high AVP levels and urine osmolalities. | |
| Demeclocycline | ✓ | Not FDA approved for hyponatremia; slow to correct; nephrotoxic in cirrhosis and heart failure. |
| Mineralocorticoids | Only one report in elderly patients with SIADH; no safety database; exacerbates the volume overload if used in edema‐forming disorders. | |
| Urea | Not FDA‐approved for hyponatremia; poor palatability. | |
| AVP receptor antagonists (vaptans) | ✓ | Conivaptan approved only for in‐hospital use secondary to CYP3A4 inhibition; infusion‐site reactions with intravenous use. Tolvaptan must be initiated and reinitiated in the hospital, as serum sodium needs to be monitored closely to avoid overly rapid correction of hyponatremia. |
Isotonic Saline
The treatment of choice for depletional hyponatremia (ie, hypovolemic hyponatremia) is isotonic saline ([Na+] = 154 mmol/L) to restore ECF volume and ensure adequate organ perfusion. This initial therapy is appropriate for patients who either have clinical signs of hypovolemia, or in whom a spot UNa+ is 30 mmol/L. However, this therapy is ineffective for dilutional hyponatremias such as SIADH,25 and continued inappropriate administration of isotonic saline to a euvolemic patient may worsen their hyponatremia,26 and/or cause fluid overload. Although isotonic saline may improve the serum [Na+] in patients with hypervolemic hyponatremia, their volume status will generally worsen with this therapy, so unless the hyponatremia is profound isotonic saline should be avoided.
Hypertonic Saline
Acute hyponatremia presenting with severe neurological symptoms is life‐threatening, and should be treated promptly with hypertonic solutions, typically 3% NaCl ([Na+] = 513 mmol/L), as this represents the most reliable method to quickly raise the serum [Na+]. A continuous infusion of hypertonic NaCl is usually utilized in inpatient settings. Various formulae have been suggested for calculating the initial rate of infusion of hypertonic solutions,27 but perhaps the simplest utilizes the following relationship:
- .Age and gender as risk factors for hyponatremia and hypernatremia.Clin Chim Acta.2003;337(1‐2):169–172.
- ,,.Incidence and prevalence of hyponatremia.Am J Med.2006;119(7 Suppl 1):S30–S35.
- ,,.Admission hyponatremia in the elderly: factors influencing prognosis.J Gen Intern Med1994;9:89–91.
- ,,, et al.[Incidence, causes and prognostic factors of hyponatremia in intensive care].Rev Med Interne.2003;24(4):224–229.
- ,,,.Postoperative hyponatremia. A prospective study.Arch Int Med.1986;146:333–336.
- .Whole‐body volume regulation and escape from antidiuresis.Am J Med.2006;119(7 Suppl 1):S21–S29.
- ,,.Neurogenic disorders of osmoregulation.Am J Med.1982;72:339–353.
- ,,.Vasopressin function in the syndrome of inappropriate antidiuresis.Annu Rev Med.1980;31:315–327.
- ,,,,,.Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure.N Eng J Med.1981;305:263–266.
- ,.Epidemiology, pathophysiology, and management of hyponatremic encephalopathy.Am J Med.1997;102:67–77.
- ,.Pulmonary complications of hyponatremic encephalopathy. noncardiogenic pulmonary edema and hypercapnic respiratory failure [see comments].Chest.1995;107(2):517–521.
- ,.Control of brain volume during hyperosmolar and hypoosmolar conditions.Annu Rev Med.1993;44:289–301.
- .Control of brain volume during hypoosmolality and hyperosmolality.Adv Exp Med Biol.2006;576:113–129.
- ,,.Clinical studies of thiazide‐induced hyponatremia.J Natl Med Assoc.2004;96(10):1305–1308.
- ,,,.Clinical assessment of extracellular fluid volume in hyponatremia.Am J Med.1987;83:905–908.
- ,.The syndrome of inappropriate secretion of antidiuretic hormone.Am J Med.1967;42:790–806.
- .Hyponatremia and Hypo‐osmolar Disorders. In: Greenberg A, Cheung AK, Coffman TM, Falk RJ, Jennette JC, eds.Primer on Kidney Diseases.Philadelphia:Saunders Elsevier,2009:52–59.
- ,,,.Reset of osmoreceptors in association with normovolemic hyponatremia.Am J Med Sci.1974;267:267–273.
- .Hyponatremia and inappropriate secretion of vasopressin (antidiuretic hormone) in patients with hypopituitarism.N Eng J Med.1989;321:492–496.
- .The Syndrome of Inappropriate Antidiuretic Hormone Secretion and Other Hypoosmolar Disorders. In: Schrier RW, ed.Diseases of the Kidney and Urinary Tract.Philadelphia:Lippincott Williams 27:156–161.
- ,,.Hyponatremia in a nursing home population.J Am Geriatr Soc.1995;43(12):1410–1413.
- ,.The syndrome of inappropriate antidiuretic hormone secretion in the elderly.Am J Med.1997;103(4):270–273.
- .Diuretic‐induced hyponatremia [editorial].Arch Intern Med.1986;146(7):1295–1296.
- ,,,.A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone.Am J Med.1957;23:529–542.
- ,,,,,.Postoperative hyponatremia despite near‐isotonic saline infusion: a phenomenon of desalination [see comments].Ann Intern Med.1997;126(1):20–25.
- ,.Hyponatremia.N Engl J Med.2000;342(21):1581–1589.
- ,,, et al.Statement of the Second International Exercise‐Associated Hyponatremia Consensus Development Conference, New Zealand, 2007.Clin J Sport Med.2008;18(2):111–121.
- ,,,.Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective.J Am Soc Nephrol.1994;4:1522–1530.
- ,,,.Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury.Crit Care Med.2005;33(1):196–202.
- ,,.Osmotic demyelination syndrome following correction of hyponatremia.N Engl J Med.1986;314:1535–1542.
- .Regulation of arginine vasopressin in the syndrome of inappropriate antidiuresis.Am J Med.2006;119(7 Suppl 1):S36–S42.
- .The syndrome of inappropriate secretion of antidiuretic hormone (SIADH).Semin Nephrol.2009;29(3):239–256.
- .Impact of solute intake on urine flow and water excretion.J Am Soc Nephrol.2008;19(6):1076–1078.
- ,.Demeclocycline‐induced nephrogenic diabetes insipidus. In‐vivo and in‐ vitro studies.Ann Intern Med.1973;79(5):679–683.
- ,,, et al.Involvement of arginine vasopressin and renal sodium handling in pathogenesis of hyponatremia in elderly patients.Endocr J.1996;43(1):101–108.
- ,.Urea for long‐term treatment of syndrome of inappropriate secretion of antidiuretic hormone.Br Med J (Clin Res Ed).1981;283:1081–1083.
- ,.Vasopressin receptor antagonists.Kidney Int.2006;69(12):2124–2130.
- ,,, et al.Potent aquaretic agent. A novel nonpeptide selective vasopressin 2 antagonist (OPC‐31260) in men.J Clin Invest.1993;92(6):2653–2659.
- ,,,,.Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia.Am J Nephrol.2007;27(5):447–457.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355(20):2099–2112.
- Vaprisol (conivaptan hydrochloride injection) prescribing information.Deerfield, IL:Astellas Pharma US, Inc.,2006.
- ,,,,.Hyponatremia treatment guidelines 2007: expert panel recommendations.Am J Med.2007;120(11 Suppl 1):S1–S21.
- Otsuka Pharmaceutical Co L, Tokyo J. Samsca (tolvaptan) prescribing information.2009.
- ,.Clinical practice. The syndrome of inappropriate antidiuresis.N Engl J Med.2007;356(20):2064–2072.
- ,,.The treatment of hyponatremia.Semin Nephrol.2009;29(3):282–299.
- .Hyponatremia and Hypo‐osmolar Disorders. In: Greenberg A, Cheung AK, Coffman TM, Falk RJ, Jennette JC, eds.Primer on Kidney Diseases.Philadelphia. PA:Saunders Elsevier;2009:52–59.
- ,,,.Risk factors for symptomatic hyponatraemia: the role of pre‐existing asymptomatic hyponatraemia.Intern Med J.2007;37(3):149–155.
- ,,,,.Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits.Am J Med.2006;119(1):71.
- ,,,,.Mild hyponatremia and risk of fracture in the ambulatory elderly.QJM.2008;101(7):583–588.
- ,,,,.Hyponatremia associated with large‐bone fracture in elderly patients.Int Urol Nephrol.2009;41(3):733–737.
- ,,, et al.Hyponatremia‐induced osteoporosis.J Bone Miner Res.2010;25(3):554–563.
- .Age and gender as risk factors for hyponatremia and hypernatremia.Clin Chim Acta.2003;337(1‐2):169–172.
- ,,.Incidence and prevalence of hyponatremia.Am J Med.2006;119(7 Suppl 1):S30–S35.
- ,,.Admission hyponatremia in the elderly: factors influencing prognosis.J Gen Intern Med1994;9:89–91.
- ,,, et al.[Incidence, causes and prognostic factors of hyponatremia in intensive care].Rev Med Interne.2003;24(4):224–229.
- ,,,.Postoperative hyponatremia. A prospective study.Arch Int Med.1986;146:333–336.
- .Whole‐body volume regulation and escape from antidiuresis.Am J Med.2006;119(7 Suppl 1):S21–S29.
- ,,.Neurogenic disorders of osmoregulation.Am J Med.1982;72:339–353.
- ,,.Vasopressin function in the syndrome of inappropriate antidiuresis.Annu Rev Med.1980;31:315–327.
- ,,,,,.Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure.N Eng J Med.1981;305:263–266.
- ,.Epidemiology, pathophysiology, and management of hyponatremic encephalopathy.Am J Med.1997;102:67–77.
- ,.Pulmonary complications of hyponatremic encephalopathy. noncardiogenic pulmonary edema and hypercapnic respiratory failure [see comments].Chest.1995;107(2):517–521.
- ,.Control of brain volume during hyperosmolar and hypoosmolar conditions.Annu Rev Med.1993;44:289–301.
- .Control of brain volume during hypoosmolality and hyperosmolality.Adv Exp Med Biol.2006;576:113–129.
- ,,.Clinical studies of thiazide‐induced hyponatremia.J Natl Med Assoc.2004;96(10):1305–1308.
- ,,,.Clinical assessment of extracellular fluid volume in hyponatremia.Am J Med.1987;83:905–908.
- ,.The syndrome of inappropriate secretion of antidiuretic hormone.Am J Med.1967;42:790–806.
- .Hyponatremia and Hypo‐osmolar Disorders. In: Greenberg A, Cheung AK, Coffman TM, Falk RJ, Jennette JC, eds.Primer on Kidney Diseases.Philadelphia:Saunders Elsevier,2009:52–59.
- ,,,.Reset of osmoreceptors in association with normovolemic hyponatremia.Am J Med Sci.1974;267:267–273.
- .Hyponatremia and inappropriate secretion of vasopressin (antidiuretic hormone) in patients with hypopituitarism.N Eng J Med.1989;321:492–496.
- .The Syndrome of Inappropriate Antidiuretic Hormone Secretion and Other Hypoosmolar Disorders. In: Schrier RW, ed.Diseases of the Kidney and Urinary Tract.Philadelphia:Lippincott Williams 27:156–161.
- ,,.Hyponatremia in a nursing home population.J Am Geriatr Soc.1995;43(12):1410–1413.
- ,.The syndrome of inappropriate antidiuretic hormone secretion in the elderly.Am J Med.1997;103(4):270–273.
- .Diuretic‐induced hyponatremia [editorial].Arch Intern Med.1986;146(7):1295–1296.
- ,,,.A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone.Am J Med.1957;23:529–542.
- ,,,,,.Postoperative hyponatremia despite near‐isotonic saline infusion: a phenomenon of desalination [see comments].Ann Intern Med.1997;126(1):20–25.
- ,.Hyponatremia.N Engl J Med.2000;342(21):1581–1589.
- ,,, et al.Statement of the Second International Exercise‐Associated Hyponatremia Consensus Development Conference, New Zealand, 2007.Clin J Sport Med.2008;18(2):111–121.
- ,,,.Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective.J Am Soc Nephrol.1994;4:1522–1530.
- ,,,.Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury.Crit Care Med.2005;33(1):196–202.
- ,,.Osmotic demyelination syndrome following correction of hyponatremia.N Engl J Med.1986;314:1535–1542.
- .Regulation of arginine vasopressin in the syndrome of inappropriate antidiuresis.Am J Med.2006;119(7 Suppl 1):S36–S42.
- .The syndrome of inappropriate secretion of antidiuretic hormone (SIADH).Semin Nephrol.2009;29(3):239–256.
- .Impact of solute intake on urine flow and water excretion.J Am Soc Nephrol.2008;19(6):1076–1078.
- ,.Demeclocycline‐induced nephrogenic diabetes insipidus. In‐vivo and in‐ vitro studies.Ann Intern Med.1973;79(5):679–683.
- ,,, et al.Involvement of arginine vasopressin and renal sodium handling in pathogenesis of hyponatremia in elderly patients.Endocr J.1996;43(1):101–108.
- ,.Urea for long‐term treatment of syndrome of inappropriate secretion of antidiuretic hormone.Br Med J (Clin Res Ed).1981;283:1081–1083.
- ,.Vasopressin receptor antagonists.Kidney Int.2006;69(12):2124–2130.
- ,,, et al.Potent aquaretic agent. A novel nonpeptide selective vasopressin 2 antagonist (OPC‐31260) in men.J Clin Invest.1993;92(6):2653–2659.
- ,,,,.Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia.Am J Nephrol.2007;27(5):447–457.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355(20):2099–2112.
- Vaprisol (conivaptan hydrochloride injection) prescribing information.Deerfield, IL:Astellas Pharma US, Inc.,2006.
- ,,,,.Hyponatremia treatment guidelines 2007: expert panel recommendations.Am J Med.2007;120(11 Suppl 1):S1–S21.
- Otsuka Pharmaceutical Co L, Tokyo J. Samsca (tolvaptan) prescribing information.2009.
- ,.Clinical practice. The syndrome of inappropriate antidiuresis.N Engl J Med.2007;356(20):2064–2072.
- ,,.The treatment of hyponatremia.Semin Nephrol.2009;29(3):282–299.
- .Hyponatremia and Hypo‐osmolar Disorders. In: Greenberg A, Cheung AK, Coffman TM, Falk RJ, Jennette JC, eds.Primer on Kidney Diseases.Philadelphia. PA:Saunders Elsevier;2009:52–59.
- ,,,.Risk factors for symptomatic hyponatraemia: the role of pre‐existing asymptomatic hyponatraemia.Intern Med J.2007;37(3):149–155.
- ,,,,.Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits.Am J Med.2006;119(1):71.
- ,,,,.Mild hyponatremia and risk of fracture in the ambulatory elderly.QJM.2008;101(7):583–588.
- ,,,,.Hyponatremia associated with large‐bone fracture in elderly patients.Int Urol Nephrol.2009;41(3):733–737.
- ,,, et al.Hyponatremia‐induced osteoporosis.J Bone Miner Res.2010;25(3):554–563.