User login
Novel COVID-19 vaccine could fill the void for patients with blood cancers
according to study results presented at the annual meeting of the American Association for Cancer Research.
The phase 1/2 trial included 54 patients with a B-cell deficiency (mean age, 63 years; 28% female): 4 had congenital B-cell deficiency and 50 had a blood cancer (lymphocytic leukemia or lymphoma). T-cell immune responses were observed in 86% of patients 28 days after vaccination with a single CoVac-1 dose. The potency of CoVac-1–induced T-cell responses exceeded those seen typically with B cell–deficient patient responses after mRNA vaccine treatment and were comparable with those seen among nonimmunocompromised COVID-19 patients.
In the majority of individuals, currently approved SARS-CoV-2 vaccines induce a robust immune response, however, their efficacy, has been shown to be decreased among individuals who are immunocompromised. Patients treated for hematologic cancers, in particular, receive treatment regimens that damage healthy immune cells, particularly B cells, said Juliane Walz, MD, the study’s senior author and professor of medicine at University Hospital Tübingen (Germany).
“In the clinic, we see many cancer patients who do not mount sufficient humoral immune responses after vaccination with available SARS-CoV-2 vaccines,” Dr. Walz said. “These patients are at a high risk for a severe course of COVID-19.”
B-cell deficiency, she stated, can be compensated for by enhancing T-cell responses against SARS-CoV-2, which can then combat infections in the absence of neutralizing antibodies.
In a prior study of CoVac-1 among 36 adults without immune deficiency, the vaccine elicited T-cell responses that were still robust 3 months post vaccination, and that included responses against omicron and other key SARS-CoV-2 variants.
While mRNA-based or adenoviral vector-based vaccines are limited to the spike protein and are thus prone to loss of activity because of viral mutations, CoVac-1–induced T-cell immunity is far more intense and broader, Dr. Walz said.
CoVac-1 is a peptide vaccine that is injected directly rather than being encoded via mRNA and targets different viral components. It would not be given, however, to healthy, immunocompetent adults because it is important for them to have both B-cell antibody and T-cell response.
The patients with B-cell deficiency recruited for the study were given a single dose of CoVac-1 and assessed for safety and immunogenicity until day 56. Prior vaccinations with an approved SARS-CoV-2 vaccine had failed to elicit a humoral response in 87% of the subjects.
“Our vaccine does not induce antibody responses,” Dr. Walz said. “However, it could be used to induce broad T-cell responses as a complementary or additive vaccine for elderly adults. In the elderly, antibody responses decline very, very fast after vaccination.”
Dr. Walz said that CoVac-1 could find application in various syndromes associated with congenital B-cell deficiencies, in autoimmune diseases such as rheumatoid arthritis and multiple sclerosis, or diseases treated with rituximab or other B cell–depleting therapies (for example, ofatumumab, blinatumomab, or chimeric antigen receptor T cells), and in transplant patients.
A phase 3 study of CoVac-1 versus placebo is under discussion and would require about 300-500 subjects, Dr. Walz said.
“CoVac-1 is designed to induce broad and long-lasting SARS-CoV-2 T-cell immunity, even in individuals who have impaired ability to mount sufficient immunity from a currently approved vaccine, and thus protect these high-risk patients from a severe course of COVID-19,” Dr. Walz said.
“Having an option for these patients is just critical – so this is significant work,” said Ana Maria Lopez, MD, MPH, of the Sidney Kimmel Cancer Center–Jefferson Health, Philadelphia.
Limitations of this study included the small sample size with low racial and ethnic diversity, Dr. Walz stated.
Funding was provided by the Ministry of Science, Research and the Arts of the state of Baden-Württemberg; the Federal Ministry of Research and Education in Germany; the German Research Foundation under Germany’s Excellence Strategy; and the Clinical Cooperation Unit Translational Immunology at University Hospital Tübingen. Dr. Walz holds the CoVac-1 patent.
according to study results presented at the annual meeting of the American Association for Cancer Research.
The phase 1/2 trial included 54 patients with a B-cell deficiency (mean age, 63 years; 28% female): 4 had congenital B-cell deficiency and 50 had a blood cancer (lymphocytic leukemia or lymphoma). T-cell immune responses were observed in 86% of patients 28 days after vaccination with a single CoVac-1 dose. The potency of CoVac-1–induced T-cell responses exceeded those seen typically with B cell–deficient patient responses after mRNA vaccine treatment and were comparable with those seen among nonimmunocompromised COVID-19 patients.
In the majority of individuals, currently approved SARS-CoV-2 vaccines induce a robust immune response, however, their efficacy, has been shown to be decreased among individuals who are immunocompromised. Patients treated for hematologic cancers, in particular, receive treatment regimens that damage healthy immune cells, particularly B cells, said Juliane Walz, MD, the study’s senior author and professor of medicine at University Hospital Tübingen (Germany).
“In the clinic, we see many cancer patients who do not mount sufficient humoral immune responses after vaccination with available SARS-CoV-2 vaccines,” Dr. Walz said. “These patients are at a high risk for a severe course of COVID-19.”
B-cell deficiency, she stated, can be compensated for by enhancing T-cell responses against SARS-CoV-2, which can then combat infections in the absence of neutralizing antibodies.
In a prior study of CoVac-1 among 36 adults without immune deficiency, the vaccine elicited T-cell responses that were still robust 3 months post vaccination, and that included responses against omicron and other key SARS-CoV-2 variants.
While mRNA-based or adenoviral vector-based vaccines are limited to the spike protein and are thus prone to loss of activity because of viral mutations, CoVac-1–induced T-cell immunity is far more intense and broader, Dr. Walz said.
CoVac-1 is a peptide vaccine that is injected directly rather than being encoded via mRNA and targets different viral components. It would not be given, however, to healthy, immunocompetent adults because it is important for them to have both B-cell antibody and T-cell response.
The patients with B-cell deficiency recruited for the study were given a single dose of CoVac-1 and assessed for safety and immunogenicity until day 56. Prior vaccinations with an approved SARS-CoV-2 vaccine had failed to elicit a humoral response in 87% of the subjects.
“Our vaccine does not induce antibody responses,” Dr. Walz said. “However, it could be used to induce broad T-cell responses as a complementary or additive vaccine for elderly adults. In the elderly, antibody responses decline very, very fast after vaccination.”
Dr. Walz said that CoVac-1 could find application in various syndromes associated with congenital B-cell deficiencies, in autoimmune diseases such as rheumatoid arthritis and multiple sclerosis, or diseases treated with rituximab or other B cell–depleting therapies (for example, ofatumumab, blinatumomab, or chimeric antigen receptor T cells), and in transplant patients.
A phase 3 study of CoVac-1 versus placebo is under discussion and would require about 300-500 subjects, Dr. Walz said.
“CoVac-1 is designed to induce broad and long-lasting SARS-CoV-2 T-cell immunity, even in individuals who have impaired ability to mount sufficient immunity from a currently approved vaccine, and thus protect these high-risk patients from a severe course of COVID-19,” Dr. Walz said.
“Having an option for these patients is just critical – so this is significant work,” said Ana Maria Lopez, MD, MPH, of the Sidney Kimmel Cancer Center–Jefferson Health, Philadelphia.
Limitations of this study included the small sample size with low racial and ethnic diversity, Dr. Walz stated.
Funding was provided by the Ministry of Science, Research and the Arts of the state of Baden-Württemberg; the Federal Ministry of Research and Education in Germany; the German Research Foundation under Germany’s Excellence Strategy; and the Clinical Cooperation Unit Translational Immunology at University Hospital Tübingen. Dr. Walz holds the CoVac-1 patent.
according to study results presented at the annual meeting of the American Association for Cancer Research.
The phase 1/2 trial included 54 patients with a B-cell deficiency (mean age, 63 years; 28% female): 4 had congenital B-cell deficiency and 50 had a blood cancer (lymphocytic leukemia or lymphoma). T-cell immune responses were observed in 86% of patients 28 days after vaccination with a single CoVac-1 dose. The potency of CoVac-1–induced T-cell responses exceeded those seen typically with B cell–deficient patient responses after mRNA vaccine treatment and were comparable with those seen among nonimmunocompromised COVID-19 patients.
In the majority of individuals, currently approved SARS-CoV-2 vaccines induce a robust immune response, however, their efficacy, has been shown to be decreased among individuals who are immunocompromised. Patients treated for hematologic cancers, in particular, receive treatment regimens that damage healthy immune cells, particularly B cells, said Juliane Walz, MD, the study’s senior author and professor of medicine at University Hospital Tübingen (Germany).
“In the clinic, we see many cancer patients who do not mount sufficient humoral immune responses after vaccination with available SARS-CoV-2 vaccines,” Dr. Walz said. “These patients are at a high risk for a severe course of COVID-19.”
B-cell deficiency, she stated, can be compensated for by enhancing T-cell responses against SARS-CoV-2, which can then combat infections in the absence of neutralizing antibodies.
In a prior study of CoVac-1 among 36 adults without immune deficiency, the vaccine elicited T-cell responses that were still robust 3 months post vaccination, and that included responses against omicron and other key SARS-CoV-2 variants.
While mRNA-based or adenoviral vector-based vaccines are limited to the spike protein and are thus prone to loss of activity because of viral mutations, CoVac-1–induced T-cell immunity is far more intense and broader, Dr. Walz said.
CoVac-1 is a peptide vaccine that is injected directly rather than being encoded via mRNA and targets different viral components. It would not be given, however, to healthy, immunocompetent adults because it is important for them to have both B-cell antibody and T-cell response.
The patients with B-cell deficiency recruited for the study were given a single dose of CoVac-1 and assessed for safety and immunogenicity until day 56. Prior vaccinations with an approved SARS-CoV-2 vaccine had failed to elicit a humoral response in 87% of the subjects.
“Our vaccine does not induce antibody responses,” Dr. Walz said. “However, it could be used to induce broad T-cell responses as a complementary or additive vaccine for elderly adults. In the elderly, antibody responses decline very, very fast after vaccination.”
Dr. Walz said that CoVac-1 could find application in various syndromes associated with congenital B-cell deficiencies, in autoimmune diseases such as rheumatoid arthritis and multiple sclerosis, or diseases treated with rituximab or other B cell–depleting therapies (for example, ofatumumab, blinatumomab, or chimeric antigen receptor T cells), and in transplant patients.
A phase 3 study of CoVac-1 versus placebo is under discussion and would require about 300-500 subjects, Dr. Walz said.
“CoVac-1 is designed to induce broad and long-lasting SARS-CoV-2 T-cell immunity, even in individuals who have impaired ability to mount sufficient immunity from a currently approved vaccine, and thus protect these high-risk patients from a severe course of COVID-19,” Dr. Walz said.
“Having an option for these patients is just critical – so this is significant work,” said Ana Maria Lopez, MD, MPH, of the Sidney Kimmel Cancer Center–Jefferson Health, Philadelphia.
Limitations of this study included the small sample size with low racial and ethnic diversity, Dr. Walz stated.
Funding was provided by the Ministry of Science, Research and the Arts of the state of Baden-Württemberg; the Federal Ministry of Research and Education in Germany; the German Research Foundation under Germany’s Excellence Strategy; and the Clinical Cooperation Unit Translational Immunology at University Hospital Tübingen. Dr. Walz holds the CoVac-1 patent.
FROM AACR 2022
Neutropenia and Leukopenia After Cross Taper From Quetiapine to Divalproex for the Treatment of Borderline Personality Disorder
Valproic acid (VPA) and its derivative, divalproex (DVP) are prescribed for a variety of indications, commonly for seizure control in patients with epilepsy, mood stabilization in patients with bipolar disorder, and migraine prophylaxis. Gastrointestinal distress and sedation are among the most reported adverse effects (AEs) with DVP therapy.1 Although serious hepatic and hematologic AEs are rare, monitoring is still recommended. DVP can cause various hematologic dyscrasias, the most common being thrombocytopenia.1,2 Neutropenia and leukopenia have been reported in isolated cases, most occurring in pediatric patients or patients with epilepsy.3-14
Several case reports of DVP-related neutropenia (absolute neutrophil count [ANC] < 1.50 103/mcL) and leukopenia (white blood cell count [WBC] < 4.0 103/mcL) were reviewed during our literature search, some caused by DVP monotherapy; others were thought to be related to concomitant use of DVP and another drug.15-25 Quetiapine was the antipsychotic most commonly implicated in causing hematologic abnormalities when combined with DVP. We report a case of neutropenia and leukopenia that presented after a cross taper from quetiapine to DVP for the treatment of borderline personality disorder (BPD).
Although no medications have been approved by the US Food and Drug Administration (FDA) for the treatment of BPD, mood stabilizers, including DVP, have literature to support their use for the treatment of affective dysregulation and impulsive behavioral dyscontrol.26-28 A therapeutic range for DVP in the treatment of BPD has not been defined; therefore, for this case report, the generally accepted range of 50 to 100 µg/mL will be considered therapeutic.1
Case Presentation
A 34-year-old male patient presented to the mental health clinic pharmacist reporting that his current psychotropic medication regimen was not effective. His medical history included posttraumatic stress disorder (PTSD), opioid use disorder, alcohol use disorder, stimulant use disorder, cannabis use, BPD, hypertension, hyperlipidemia, prediabetes, gastroesophageal reflex disease, and a pulmonary nodule. On initial presentation, the patient was prescribed buprenorphine 24 mg/naloxone 6 mg, quetiapine 400 mg, duloxetine 120 mg, and prazosin 15 mg per day. At the time of pharmacy consultation, last reported alcohol or nonprescribed opioid use was about 6 months prior, and methamphetamine use about 1 month prior, with ongoing cannabis use. The patient had a history of participating in cognitive processing therapy, dialectical behavior therapy (DBT), and residential treatment for both PTSD and substance use. Additionally, he was actively participating in contingency management for stimulant use disorder and self-management and recovery training group.
The patient reported ongoing mood lability, hypervigilance, and oversedation with current psychotropic regimen. The prescriber of his medication for opioid use disorder also reported the patient experienced labile mood, impulsive behavior, and anger outbursts. In the setting of intolerability due to oversedation with quetiapine, cardiometabolic risk, and lack of clear indication for use, the patient and health care practitioner (HCP) agreed to taper quetiapine and initiate a trial of DVP for affective dysregulation and impulsive-behavioral dyscontrol. To prevent cholinergic rebound and insomnia with abrupt discontinuation of quetiapine, DVP and quetiapine were cross tapered. The following cross taper was prescribed: quetiapine 300 mg and DVP 500 mg per day for week 1; quetiapine 200 mg and DVP 500 mg per day for week 2; quetiapine 100 mg and DVP 1000 mg per day for week 3; quetiapine 50 mg and DVP 1000 mg per day for week 4; followed by DVP 1000 mg per day and discontinuation of quetiapine.
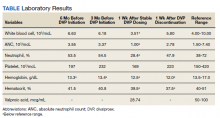
During a 4-week follow-up appointment, the patient reported appropriate completion of cross taper but stopped taking the DVP 3 days prior to the appointment due to self-reported lack of efficacy. For this reason, serum VPA level was not obtained. After discussion with his HCP, the patient restarted DVP 1000 mg per day without retitration with plans to get laboratory tests in 1 week. The next week, laboratory tests were notable for VPA level 28.74 (reference range, 50-100) µg/mL, low WBC 3.51 (reference range, 4.00-10.00) 103/mcL, platelets 169 (reference range, 150-420) 103/mcL, and low ANC 1.00 (reference range, 1.50-7.40) 103/mcL (Table). This raised clinical concern as the patient had no history of documented neutropenia or leukopenia, with most recent complete blood count (CBC) prior to DVP initiation 3 months earlier while prescribed quetiapine.
On further review, the HCP opted to cease administration of DVP and repeat CBC with differential in 1 week. Nine days later, laboratory tests were performed and compared with those collected the week before, revealing resolution of neutropenia and leukopenia. A score of 7 on the Naranjo Adverse Drug Reaction Probability Scale (NADRPS) was determined based on previous conclusive reports on the reaction (+1), appeared after suspected drug administration (+2), improved with drug discontinuation (+1), confirmed by objective evidence (+1), and no alternative causes could be found (+2).29 With a NADRPS score of 7, an AE of probable DVP-induced neutropenia was documented and medication was not resumed.
Discussion
Our case report describes isolated neutropenia and leukopenia that developed after a cross taper from quetiapine to DVP. Hematologic abnormalities resolved after discontinuation of DVP, suggesting a likely correlation. DVP has a well-established, dose-related prevalence of thrombocytopenia occurring in up to 27% of patients.1 Fewer case reports exist on neutropenia and leukopenia. DVP-induced neutropenia is thought to be a result of direct bone marrow suppression, whereas the more commonly occurring blood dyscrasia, thrombocytopenia, is thought to be caused by an antibody-mediated destruction of platelets.6
Management of DVP-induced thrombocytopenia is often dependent on the severity of the reaction. In mild-to-moderate cases, intervention may not be necessary as thrombocytopenia has been shown to resolve without adjustment to DVP therapy.1 In more severe or symptomatic cases, dose reduction or discontinuation of the offending agent is recommended, typically resulting in resolution shortly following pharmacologic intervention.
Guidance on the management of other drug-induced hematologic abnormalities, such as neutropenia and leukopenia are not as well established. A 2019 systematic review of idiosyncratic drug-induced neutropenia suggested that continuing the offending drug with strict monitoring could be considered in cases of mild neutropenia. In cases of moderate neutropenia, the author suggests temporary cessation of the drug and reinstatement once neutrophil count normalizes and definitive cessation of the drug in severe cases.30
In our case, continuing the offending agent with close monitoring was considered, similar to the well-established management of clozapine-induced neutropenia. However, due to the concern that the ANC was bordering moderate neutropenia in the absence of a therapeutic VPA level as well as a significant reduction in platelets, although not meeting criteria for thrombocytopenia, the decision was made to err on the side of caution and discontinue the most likely offending agent.
It is important to highlight that DVP was replacing quetiapine in the form of a cross taper. Quetiapine is structurally similar to clozapine. While clozapine has strict monitoring requirements related to neutropenia, blood dyscrasias with quetiapine therapy are rare. Quetiapine-induced hematologic abnormalities may be due to direct toxicity or to an immune-mediated mechanism, leading to bone marrow suppression.20 Case reports documenting blood dyscrasias with the combination of DVP and quetiapine were identified during literature review.15-19 Despite these case reports, we believe DVP was the primary offending agent in our case as the patient’s last dose of quetiapine was 2 weeks before obtaining the abnormal CBC. There was no history of blood dyscrasias with quetiapine monotherapy; however, the effect of the combination of DVP and quetiapine is unknown as no CBC was obtained during the cross-taper period.
Although there are no FDA-approved medications for the treatment of BPD, mood stabilizers, including DVP, have some research to support their use for the treatment of affective dysregulation and impulsive-behavioral dyscontrol.26-28 In our case, DVP was selected due to the evidence for use in BPD and ability to assess adherence with therapeutic monitoring. Although polypharmacy is a concern in patients with BPD, in our case we believed that the patient’s ongoing mood lability and impulsive behaviors warranted pharmacologic intervention. Additionally, DVP provided an advantage in its ability to quickly titrate to therapeutic dose when compared with lamotrigine and a lower risk of cognitive AEs when compared with topiramate.
Conclusions
To our knowledge, this case report demonstrates the first published case of neutropenia and leukopenia related to DVP therapy for the treatment of BPD. Routine CBC monitoring is recommended with DVP therapy, and our case highlights the importance of evaluating for not only thrombocytopenia, but also other blood dyscrasias during the titration phase even in the absence of a therapeutic VPA level. Further studies are warranted to determine incidence of DVP-related neutropenia and leukopenia and to evaluate the safety of continuing DVP in cases of mild-to-moderate neutropenia with close monitoring.
1. Depakote (valproic acid). Package insert. Abbott Laboratories; June 2000.
2. Conley EL, Coley KC, Pollock BG, Dapos SV, Maxwell R, Branch RA. Prevalence and risk of thrombocytopenia with valproic acid: experience at a psychiatric teaching hospital. Pharmacotherapy. 2001;21(11):1325-1330. doi:10.1592/phco.21.17.1325.34418
3. Jaeken J, van Goethem C, Casaer P, Devlieger H, Eggermont E, Pilet M. Neutropenia during sodium valproate therapy. Arch Dis Child. 1979;54(12):986-987. doi:10.1136/adc.54.12.986
4. Barr RD, Copeland SA, Stockwell MC, Morris N, Kelton JC. Valproic acid and immune thrombocytopenia. Arch Dis Child. 1982;57(9):681-684. doi:10.1136/adc.57.9.681
5. Symon DNK, Russell G. Sodium valproate and neutropenia (letter). Arch Dis Child. 1983;58:235. doi:10.1136/adc.58.3.235
6. Watts RG, Emanuel PD, Zuckerman KS, Howard TH. Valproic acid-induced cytopenias: evidence for a dose-related suppression of hematopoiesis. J Pediatr. 1990;117(3):495-499. doi:10.1016/s0022-3476(05)81105-9
7. Blackburn SC, Oliart AD, García-Rodríguez LA, Pérez Gutthann S. Antiepileptics and blood dyscrasias: a cohort study. Pharmacotherapy. 1998;18(6):1277-1283.
8. Acharya S, Bussel JB. Hematologic toxicity of sodium valproate. J Pediatr Hematol Oncol. 2000;22(1):62-65. doi:10.1097/00043426-200001000-00012
9. Vesta KS, Medina PJ. Valproic acid-induced neutropenia. Ann Pharmacother. 2003;37(6):819-821. doi:10.1345/aph.1C381
10. Kohli U, Gulati, S. Sodium valproate induced isolated neutropenia. Indian J Pediatr. 2006;73(9):844-844. doi:10.1007/BF02790401
11. Hsu HC, Tseng HK, Wang SC, Wang YY. Valproic acid-induced agranulocytosis. Int J Gerontol. 2009;3(2):137-139. doi:10.1016/S1873-9598(09)70036-5
12. Chakraborty S, Chakraborty J, Mandal S, Ghosal MK. A rare occurrence of isolated neutropenia with valproic acid: a case report. J Indian Med Assoc. 2011;109(5):345-346.
13. Stoner SC, Deal E, Lurk JT. Delayed-onset neutropenia with divalproex sodium. Ann Pharmacother. 2008;42(10):1507-1510. doi:10.1345/aph.1L239
14. Storch DD. Severe leukopenia with valproate. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208-1209. doi:10.1097/00004583-200010000-00003
15. Rahman A, Mican LM, Fischer C, Campbell AH. Evaluating the incidence of leukopenia and neutropenia with valproate, quetiapine, or the combination in children and adolescents. Ann Pharmacother. 2009;43:822-830. doi:10.1345/aph.1L617
16. Hung WC, Hsieh MH. Neutropenia associated with the comedication of quetiapine and valproate in 2 elderly patients. J Clin Psychopharmacol. 2012;32(3):416-417. doi:10.1097/JCP.0b013e3182549d2d
17. Park HJ, Kim JY. Incidence of neutropenia with valproate and quetiapine combination treatment in subjects with acquired brain injuries. Arch Phys Med Rehabil. 2016;97(2):183-188. doi:10.1016/j.apmr.2015.09.004
18. Estabrook KR, Pheister M. A case of quetiapine XR and divalproex-associated neutropenia followed by successful use of ziprasidone. J Clin Psychopharmacol. 2012;32(3):417-418. doi:10.1097/JCP.0b013e318253a071
19. Nair P, Lippmann S. Is leukopenia associated with divalproex and/or quetiapine? Psychosomatics. 2005;46(2):188-189. doi:10.1176/appi.psy.46.2.188
20. Cowan C, Oakley C. Leukopenia and neutropenia induced by quetiapine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):292-294. doi:10.1016/j.pnpbp.2006.07.003
21. Fan KY, Chen WY, Huang MC. Quetiapine-associated leucopenia and thrombocytopenia: a case report. BMC Psychiatry. 2015;15:110. doi:10.1186/s12888-015-0495-9
22. Malik S, Lally J, Ajnakina O, et al. Sodium valproate and clozapine induced neutropenia: A case control study using register data. Schizophr Res. 2018;195:267-273. doi:10.1016/j.schres.2017.08.041
23. Pantelis C, Adesanya A. Increased risk of neutropaenia and agranulocytosis with sodium valproate used adjunctively with clozapine. Aust N Z J Psychiatry. 2001;35(4):544-545. doi:10.1046/j.1440-1614.2001.0911f.x
24. Madeb R, Hirschmann S, Kurs R, Turkie A, Modai I. Combined clozapine and valproic acid treatment-induced agranulocytosis. Eur Psychiatry. 2002;17(4):238-239. doi:10.1016/s0924-9338(02)00659-4
25. Dose M, Hellweg R, Yassouridis A, Theison M, Emrich HM. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry. 1998;31(4):122-125. doi:10.1055/s-2007-979312
26. Ingenhoven T, Lafay P, Rinne T, Passchier J, Duivenvoorden H. Effectiveness of pharmacotherapy for severe personality disorders: meta-analyses of randomized controlled trials. J Clin Psychiatry. 2010;71:14. doi:10.4088/jcp.08r04526gre
27. Mercer D, Douglass AB, Links PS. Meta-analyses of mood stabilizers, antidepressants and antipsychotics in the treatment of borderline personality disorder: effectiveness for depression and anger symptoms. J Pers Disord. 2009;23(2):156-174. doi:10.1521/pedi.2009.23.2.156
28. Hollander E, Swann AC, Coccaro EF, Jiang P, Smith TB. Impact of trait impulsivity and state aggression on divalproex versus placebo response in borderline personality disorder. Am J Psychiatry. 2005;162(3):621-624. doi:10.1176/appi.ajp.162.3.621
29. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. doi:10.1038/clpt.1981.154
30. Andrès E, Villalba NL, Zulfiqar AA, Serraj K, Mourot-Cottet R, Gottenberg AJ. State of art of idiosyncratic drug-induced neutropenia or agranulocytosis, with a focus on biotherapies. J Clin Med. 2019;8(9):1351. doi:10.3390/jcm8091351
Valproic acid (VPA) and its derivative, divalproex (DVP) are prescribed for a variety of indications, commonly for seizure control in patients with epilepsy, mood stabilization in patients with bipolar disorder, and migraine prophylaxis. Gastrointestinal distress and sedation are among the most reported adverse effects (AEs) with DVP therapy.1 Although serious hepatic and hematologic AEs are rare, monitoring is still recommended. DVP can cause various hematologic dyscrasias, the most common being thrombocytopenia.1,2 Neutropenia and leukopenia have been reported in isolated cases, most occurring in pediatric patients or patients with epilepsy.3-14
Several case reports of DVP-related neutropenia (absolute neutrophil count [ANC] < 1.50 103/mcL) and leukopenia (white blood cell count [WBC] < 4.0 103/mcL) were reviewed during our literature search, some caused by DVP monotherapy; others were thought to be related to concomitant use of DVP and another drug.15-25 Quetiapine was the antipsychotic most commonly implicated in causing hematologic abnormalities when combined with DVP. We report a case of neutropenia and leukopenia that presented after a cross taper from quetiapine to DVP for the treatment of borderline personality disorder (BPD).
Although no medications have been approved by the US Food and Drug Administration (FDA) for the treatment of BPD, mood stabilizers, including DVP, have literature to support their use for the treatment of affective dysregulation and impulsive behavioral dyscontrol.26-28 A therapeutic range for DVP in the treatment of BPD has not been defined; therefore, for this case report, the generally accepted range of 50 to 100 µg/mL will be considered therapeutic.1
Case Presentation
A 34-year-old male patient presented to the mental health clinic pharmacist reporting that his current psychotropic medication regimen was not effective. His medical history included posttraumatic stress disorder (PTSD), opioid use disorder, alcohol use disorder, stimulant use disorder, cannabis use, BPD, hypertension, hyperlipidemia, prediabetes, gastroesophageal reflex disease, and a pulmonary nodule. On initial presentation, the patient was prescribed buprenorphine 24 mg/naloxone 6 mg, quetiapine 400 mg, duloxetine 120 mg, and prazosin 15 mg per day. At the time of pharmacy consultation, last reported alcohol or nonprescribed opioid use was about 6 months prior, and methamphetamine use about 1 month prior, with ongoing cannabis use. The patient had a history of participating in cognitive processing therapy, dialectical behavior therapy (DBT), and residential treatment for both PTSD and substance use. Additionally, he was actively participating in contingency management for stimulant use disorder and self-management and recovery training group.
The patient reported ongoing mood lability, hypervigilance, and oversedation with current psychotropic regimen. The prescriber of his medication for opioid use disorder also reported the patient experienced labile mood, impulsive behavior, and anger outbursts. In the setting of intolerability due to oversedation with quetiapine, cardiometabolic risk, and lack of clear indication for use, the patient and health care practitioner (HCP) agreed to taper quetiapine and initiate a trial of DVP for affective dysregulation and impulsive-behavioral dyscontrol. To prevent cholinergic rebound and insomnia with abrupt discontinuation of quetiapine, DVP and quetiapine were cross tapered. The following cross taper was prescribed: quetiapine 300 mg and DVP 500 mg per day for week 1; quetiapine 200 mg and DVP 500 mg per day for week 2; quetiapine 100 mg and DVP 1000 mg per day for week 3; quetiapine 50 mg and DVP 1000 mg per day for week 4; followed by DVP 1000 mg per day and discontinuation of quetiapine.
During a 4-week follow-up appointment, the patient reported appropriate completion of cross taper but stopped taking the DVP 3 days prior to the appointment due to self-reported lack of efficacy. For this reason, serum VPA level was not obtained. After discussion with his HCP, the patient restarted DVP 1000 mg per day without retitration with plans to get laboratory tests in 1 week. The next week, laboratory tests were notable for VPA level 28.74 (reference range, 50-100) µg/mL, low WBC 3.51 (reference range, 4.00-10.00) 103/mcL, platelets 169 (reference range, 150-420) 103/mcL, and low ANC 1.00 (reference range, 1.50-7.40) 103/mcL (Table). This raised clinical concern as the patient had no history of documented neutropenia or leukopenia, with most recent complete blood count (CBC) prior to DVP initiation 3 months earlier while prescribed quetiapine.
On further review, the HCP opted to cease administration of DVP and repeat CBC with differential in 1 week. Nine days later, laboratory tests were performed and compared with those collected the week before, revealing resolution of neutropenia and leukopenia. A score of 7 on the Naranjo Adverse Drug Reaction Probability Scale (NADRPS) was determined based on previous conclusive reports on the reaction (+1), appeared after suspected drug administration (+2), improved with drug discontinuation (+1), confirmed by objective evidence (+1), and no alternative causes could be found (+2).29 With a NADRPS score of 7, an AE of probable DVP-induced neutropenia was documented and medication was not resumed.
Discussion
Our case report describes isolated neutropenia and leukopenia that developed after a cross taper from quetiapine to DVP. Hematologic abnormalities resolved after discontinuation of DVP, suggesting a likely correlation. DVP has a well-established, dose-related prevalence of thrombocytopenia occurring in up to 27% of patients.1 Fewer case reports exist on neutropenia and leukopenia. DVP-induced neutropenia is thought to be a result of direct bone marrow suppression, whereas the more commonly occurring blood dyscrasia, thrombocytopenia, is thought to be caused by an antibody-mediated destruction of platelets.6
Management of DVP-induced thrombocytopenia is often dependent on the severity of the reaction. In mild-to-moderate cases, intervention may not be necessary as thrombocytopenia has been shown to resolve without adjustment to DVP therapy.1 In more severe or symptomatic cases, dose reduction or discontinuation of the offending agent is recommended, typically resulting in resolution shortly following pharmacologic intervention.
Guidance on the management of other drug-induced hematologic abnormalities, such as neutropenia and leukopenia are not as well established. A 2019 systematic review of idiosyncratic drug-induced neutropenia suggested that continuing the offending drug with strict monitoring could be considered in cases of mild neutropenia. In cases of moderate neutropenia, the author suggests temporary cessation of the drug and reinstatement once neutrophil count normalizes and definitive cessation of the drug in severe cases.30
In our case, continuing the offending agent with close monitoring was considered, similar to the well-established management of clozapine-induced neutropenia. However, due to the concern that the ANC was bordering moderate neutropenia in the absence of a therapeutic VPA level as well as a significant reduction in platelets, although not meeting criteria for thrombocytopenia, the decision was made to err on the side of caution and discontinue the most likely offending agent.
It is important to highlight that DVP was replacing quetiapine in the form of a cross taper. Quetiapine is structurally similar to clozapine. While clozapine has strict monitoring requirements related to neutropenia, blood dyscrasias with quetiapine therapy are rare. Quetiapine-induced hematologic abnormalities may be due to direct toxicity or to an immune-mediated mechanism, leading to bone marrow suppression.20 Case reports documenting blood dyscrasias with the combination of DVP and quetiapine were identified during literature review.15-19 Despite these case reports, we believe DVP was the primary offending agent in our case as the patient’s last dose of quetiapine was 2 weeks before obtaining the abnormal CBC. There was no history of blood dyscrasias with quetiapine monotherapy; however, the effect of the combination of DVP and quetiapine is unknown as no CBC was obtained during the cross-taper period.
Although there are no FDA-approved medications for the treatment of BPD, mood stabilizers, including DVP, have some research to support their use for the treatment of affective dysregulation and impulsive-behavioral dyscontrol.26-28 In our case, DVP was selected due to the evidence for use in BPD and ability to assess adherence with therapeutic monitoring. Although polypharmacy is a concern in patients with BPD, in our case we believed that the patient’s ongoing mood lability and impulsive behaviors warranted pharmacologic intervention. Additionally, DVP provided an advantage in its ability to quickly titrate to therapeutic dose when compared with lamotrigine and a lower risk of cognitive AEs when compared with topiramate.
Conclusions
To our knowledge, this case report demonstrates the first published case of neutropenia and leukopenia related to DVP therapy for the treatment of BPD. Routine CBC monitoring is recommended with DVP therapy, and our case highlights the importance of evaluating for not only thrombocytopenia, but also other blood dyscrasias during the titration phase even in the absence of a therapeutic VPA level. Further studies are warranted to determine incidence of DVP-related neutropenia and leukopenia and to evaluate the safety of continuing DVP in cases of mild-to-moderate neutropenia with close monitoring.
Valproic acid (VPA) and its derivative, divalproex (DVP) are prescribed for a variety of indications, commonly for seizure control in patients with epilepsy, mood stabilization in patients with bipolar disorder, and migraine prophylaxis. Gastrointestinal distress and sedation are among the most reported adverse effects (AEs) with DVP therapy.1 Although serious hepatic and hematologic AEs are rare, monitoring is still recommended. DVP can cause various hematologic dyscrasias, the most common being thrombocytopenia.1,2 Neutropenia and leukopenia have been reported in isolated cases, most occurring in pediatric patients or patients with epilepsy.3-14
Several case reports of DVP-related neutropenia (absolute neutrophil count [ANC] < 1.50 103/mcL) and leukopenia (white blood cell count [WBC] < 4.0 103/mcL) were reviewed during our literature search, some caused by DVP monotherapy; others were thought to be related to concomitant use of DVP and another drug.15-25 Quetiapine was the antipsychotic most commonly implicated in causing hematologic abnormalities when combined with DVP. We report a case of neutropenia and leukopenia that presented after a cross taper from quetiapine to DVP for the treatment of borderline personality disorder (BPD).
Although no medications have been approved by the US Food and Drug Administration (FDA) for the treatment of BPD, mood stabilizers, including DVP, have literature to support their use for the treatment of affective dysregulation and impulsive behavioral dyscontrol.26-28 A therapeutic range for DVP in the treatment of BPD has not been defined; therefore, for this case report, the generally accepted range of 50 to 100 µg/mL will be considered therapeutic.1
Case Presentation
A 34-year-old male patient presented to the mental health clinic pharmacist reporting that his current psychotropic medication regimen was not effective. His medical history included posttraumatic stress disorder (PTSD), opioid use disorder, alcohol use disorder, stimulant use disorder, cannabis use, BPD, hypertension, hyperlipidemia, prediabetes, gastroesophageal reflex disease, and a pulmonary nodule. On initial presentation, the patient was prescribed buprenorphine 24 mg/naloxone 6 mg, quetiapine 400 mg, duloxetine 120 mg, and prazosin 15 mg per day. At the time of pharmacy consultation, last reported alcohol or nonprescribed opioid use was about 6 months prior, and methamphetamine use about 1 month prior, with ongoing cannabis use. The patient had a history of participating in cognitive processing therapy, dialectical behavior therapy (DBT), and residential treatment for both PTSD and substance use. Additionally, he was actively participating in contingency management for stimulant use disorder and self-management and recovery training group.
The patient reported ongoing mood lability, hypervigilance, and oversedation with current psychotropic regimen. The prescriber of his medication for opioid use disorder also reported the patient experienced labile mood, impulsive behavior, and anger outbursts. In the setting of intolerability due to oversedation with quetiapine, cardiometabolic risk, and lack of clear indication for use, the patient and health care practitioner (HCP) agreed to taper quetiapine and initiate a trial of DVP for affective dysregulation and impulsive-behavioral dyscontrol. To prevent cholinergic rebound and insomnia with abrupt discontinuation of quetiapine, DVP and quetiapine were cross tapered. The following cross taper was prescribed: quetiapine 300 mg and DVP 500 mg per day for week 1; quetiapine 200 mg and DVP 500 mg per day for week 2; quetiapine 100 mg and DVP 1000 mg per day for week 3; quetiapine 50 mg and DVP 1000 mg per day for week 4; followed by DVP 1000 mg per day and discontinuation of quetiapine.
During a 4-week follow-up appointment, the patient reported appropriate completion of cross taper but stopped taking the DVP 3 days prior to the appointment due to self-reported lack of efficacy. For this reason, serum VPA level was not obtained. After discussion with his HCP, the patient restarted DVP 1000 mg per day without retitration with plans to get laboratory tests in 1 week. The next week, laboratory tests were notable for VPA level 28.74 (reference range, 50-100) µg/mL, low WBC 3.51 (reference range, 4.00-10.00) 103/mcL, platelets 169 (reference range, 150-420) 103/mcL, and low ANC 1.00 (reference range, 1.50-7.40) 103/mcL (Table). This raised clinical concern as the patient had no history of documented neutropenia or leukopenia, with most recent complete blood count (CBC) prior to DVP initiation 3 months earlier while prescribed quetiapine.
On further review, the HCP opted to cease administration of DVP and repeat CBC with differential in 1 week. Nine days later, laboratory tests were performed and compared with those collected the week before, revealing resolution of neutropenia and leukopenia. A score of 7 on the Naranjo Adverse Drug Reaction Probability Scale (NADRPS) was determined based on previous conclusive reports on the reaction (+1), appeared after suspected drug administration (+2), improved with drug discontinuation (+1), confirmed by objective evidence (+1), and no alternative causes could be found (+2).29 With a NADRPS score of 7, an AE of probable DVP-induced neutropenia was documented and medication was not resumed.
Discussion
Our case report describes isolated neutropenia and leukopenia that developed after a cross taper from quetiapine to DVP. Hematologic abnormalities resolved after discontinuation of DVP, suggesting a likely correlation. DVP has a well-established, dose-related prevalence of thrombocytopenia occurring in up to 27% of patients.1 Fewer case reports exist on neutropenia and leukopenia. DVP-induced neutropenia is thought to be a result of direct bone marrow suppression, whereas the more commonly occurring blood dyscrasia, thrombocytopenia, is thought to be caused by an antibody-mediated destruction of platelets.6
Management of DVP-induced thrombocytopenia is often dependent on the severity of the reaction. In mild-to-moderate cases, intervention may not be necessary as thrombocytopenia has been shown to resolve without adjustment to DVP therapy.1 In more severe or symptomatic cases, dose reduction or discontinuation of the offending agent is recommended, typically resulting in resolution shortly following pharmacologic intervention.
Guidance on the management of other drug-induced hematologic abnormalities, such as neutropenia and leukopenia are not as well established. A 2019 systematic review of idiosyncratic drug-induced neutropenia suggested that continuing the offending drug with strict monitoring could be considered in cases of mild neutropenia. In cases of moderate neutropenia, the author suggests temporary cessation of the drug and reinstatement once neutrophil count normalizes and definitive cessation of the drug in severe cases.30
In our case, continuing the offending agent with close monitoring was considered, similar to the well-established management of clozapine-induced neutropenia. However, due to the concern that the ANC was bordering moderate neutropenia in the absence of a therapeutic VPA level as well as a significant reduction in platelets, although not meeting criteria for thrombocytopenia, the decision was made to err on the side of caution and discontinue the most likely offending agent.
It is important to highlight that DVP was replacing quetiapine in the form of a cross taper. Quetiapine is structurally similar to clozapine. While clozapine has strict monitoring requirements related to neutropenia, blood dyscrasias with quetiapine therapy are rare. Quetiapine-induced hematologic abnormalities may be due to direct toxicity or to an immune-mediated mechanism, leading to bone marrow suppression.20 Case reports documenting blood dyscrasias with the combination of DVP and quetiapine were identified during literature review.15-19 Despite these case reports, we believe DVP was the primary offending agent in our case as the patient’s last dose of quetiapine was 2 weeks before obtaining the abnormal CBC. There was no history of blood dyscrasias with quetiapine monotherapy; however, the effect of the combination of DVP and quetiapine is unknown as no CBC was obtained during the cross-taper period.
Although there are no FDA-approved medications for the treatment of BPD, mood stabilizers, including DVP, have some research to support their use for the treatment of affective dysregulation and impulsive-behavioral dyscontrol.26-28 In our case, DVP was selected due to the evidence for use in BPD and ability to assess adherence with therapeutic monitoring. Although polypharmacy is a concern in patients with BPD, in our case we believed that the patient’s ongoing mood lability and impulsive behaviors warranted pharmacologic intervention. Additionally, DVP provided an advantage in its ability to quickly titrate to therapeutic dose when compared with lamotrigine and a lower risk of cognitive AEs when compared with topiramate.
Conclusions
To our knowledge, this case report demonstrates the first published case of neutropenia and leukopenia related to DVP therapy for the treatment of BPD. Routine CBC monitoring is recommended with DVP therapy, and our case highlights the importance of evaluating for not only thrombocytopenia, but also other blood dyscrasias during the titration phase even in the absence of a therapeutic VPA level. Further studies are warranted to determine incidence of DVP-related neutropenia and leukopenia and to evaluate the safety of continuing DVP in cases of mild-to-moderate neutropenia with close monitoring.
1. Depakote (valproic acid). Package insert. Abbott Laboratories; June 2000.
2. Conley EL, Coley KC, Pollock BG, Dapos SV, Maxwell R, Branch RA. Prevalence and risk of thrombocytopenia with valproic acid: experience at a psychiatric teaching hospital. Pharmacotherapy. 2001;21(11):1325-1330. doi:10.1592/phco.21.17.1325.34418
3. Jaeken J, van Goethem C, Casaer P, Devlieger H, Eggermont E, Pilet M. Neutropenia during sodium valproate therapy. Arch Dis Child. 1979;54(12):986-987. doi:10.1136/adc.54.12.986
4. Barr RD, Copeland SA, Stockwell MC, Morris N, Kelton JC. Valproic acid and immune thrombocytopenia. Arch Dis Child. 1982;57(9):681-684. doi:10.1136/adc.57.9.681
5. Symon DNK, Russell G. Sodium valproate and neutropenia (letter). Arch Dis Child. 1983;58:235. doi:10.1136/adc.58.3.235
6. Watts RG, Emanuel PD, Zuckerman KS, Howard TH. Valproic acid-induced cytopenias: evidence for a dose-related suppression of hematopoiesis. J Pediatr. 1990;117(3):495-499. doi:10.1016/s0022-3476(05)81105-9
7. Blackburn SC, Oliart AD, García-Rodríguez LA, Pérez Gutthann S. Antiepileptics and blood dyscrasias: a cohort study. Pharmacotherapy. 1998;18(6):1277-1283.
8. Acharya S, Bussel JB. Hematologic toxicity of sodium valproate. J Pediatr Hematol Oncol. 2000;22(1):62-65. doi:10.1097/00043426-200001000-00012
9. Vesta KS, Medina PJ. Valproic acid-induced neutropenia. Ann Pharmacother. 2003;37(6):819-821. doi:10.1345/aph.1C381
10. Kohli U, Gulati, S. Sodium valproate induced isolated neutropenia. Indian J Pediatr. 2006;73(9):844-844. doi:10.1007/BF02790401
11. Hsu HC, Tseng HK, Wang SC, Wang YY. Valproic acid-induced agranulocytosis. Int J Gerontol. 2009;3(2):137-139. doi:10.1016/S1873-9598(09)70036-5
12. Chakraborty S, Chakraborty J, Mandal S, Ghosal MK. A rare occurrence of isolated neutropenia with valproic acid: a case report. J Indian Med Assoc. 2011;109(5):345-346.
13. Stoner SC, Deal E, Lurk JT. Delayed-onset neutropenia with divalproex sodium. Ann Pharmacother. 2008;42(10):1507-1510. doi:10.1345/aph.1L239
14. Storch DD. Severe leukopenia with valproate. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208-1209. doi:10.1097/00004583-200010000-00003
15. Rahman A, Mican LM, Fischer C, Campbell AH. Evaluating the incidence of leukopenia and neutropenia with valproate, quetiapine, or the combination in children and adolescents. Ann Pharmacother. 2009;43:822-830. doi:10.1345/aph.1L617
16. Hung WC, Hsieh MH. Neutropenia associated with the comedication of quetiapine and valproate in 2 elderly patients. J Clin Psychopharmacol. 2012;32(3):416-417. doi:10.1097/JCP.0b013e3182549d2d
17. Park HJ, Kim JY. Incidence of neutropenia with valproate and quetiapine combination treatment in subjects with acquired brain injuries. Arch Phys Med Rehabil. 2016;97(2):183-188. doi:10.1016/j.apmr.2015.09.004
18. Estabrook KR, Pheister M. A case of quetiapine XR and divalproex-associated neutropenia followed by successful use of ziprasidone. J Clin Psychopharmacol. 2012;32(3):417-418. doi:10.1097/JCP.0b013e318253a071
19. Nair P, Lippmann S. Is leukopenia associated with divalproex and/or quetiapine? Psychosomatics. 2005;46(2):188-189. doi:10.1176/appi.psy.46.2.188
20. Cowan C, Oakley C. Leukopenia and neutropenia induced by quetiapine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):292-294. doi:10.1016/j.pnpbp.2006.07.003
21. Fan KY, Chen WY, Huang MC. Quetiapine-associated leucopenia and thrombocytopenia: a case report. BMC Psychiatry. 2015;15:110. doi:10.1186/s12888-015-0495-9
22. Malik S, Lally J, Ajnakina O, et al. Sodium valproate and clozapine induced neutropenia: A case control study using register data. Schizophr Res. 2018;195:267-273. doi:10.1016/j.schres.2017.08.041
23. Pantelis C, Adesanya A. Increased risk of neutropaenia and agranulocytosis with sodium valproate used adjunctively with clozapine. Aust N Z J Psychiatry. 2001;35(4):544-545. doi:10.1046/j.1440-1614.2001.0911f.x
24. Madeb R, Hirschmann S, Kurs R, Turkie A, Modai I. Combined clozapine and valproic acid treatment-induced agranulocytosis. Eur Psychiatry. 2002;17(4):238-239. doi:10.1016/s0924-9338(02)00659-4
25. Dose M, Hellweg R, Yassouridis A, Theison M, Emrich HM. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry. 1998;31(4):122-125. doi:10.1055/s-2007-979312
26. Ingenhoven T, Lafay P, Rinne T, Passchier J, Duivenvoorden H. Effectiveness of pharmacotherapy for severe personality disorders: meta-analyses of randomized controlled trials. J Clin Psychiatry. 2010;71:14. doi:10.4088/jcp.08r04526gre
27. Mercer D, Douglass AB, Links PS. Meta-analyses of mood stabilizers, antidepressants and antipsychotics in the treatment of borderline personality disorder: effectiveness for depression and anger symptoms. J Pers Disord. 2009;23(2):156-174. doi:10.1521/pedi.2009.23.2.156
28. Hollander E, Swann AC, Coccaro EF, Jiang P, Smith TB. Impact of trait impulsivity and state aggression on divalproex versus placebo response in borderline personality disorder. Am J Psychiatry. 2005;162(3):621-624. doi:10.1176/appi.ajp.162.3.621
29. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. doi:10.1038/clpt.1981.154
30. Andrès E, Villalba NL, Zulfiqar AA, Serraj K, Mourot-Cottet R, Gottenberg AJ. State of art of idiosyncratic drug-induced neutropenia or agranulocytosis, with a focus on biotherapies. J Clin Med. 2019;8(9):1351. doi:10.3390/jcm8091351
1. Depakote (valproic acid). Package insert. Abbott Laboratories; June 2000.
2. Conley EL, Coley KC, Pollock BG, Dapos SV, Maxwell R, Branch RA. Prevalence and risk of thrombocytopenia with valproic acid: experience at a psychiatric teaching hospital. Pharmacotherapy. 2001;21(11):1325-1330. doi:10.1592/phco.21.17.1325.34418
3. Jaeken J, van Goethem C, Casaer P, Devlieger H, Eggermont E, Pilet M. Neutropenia during sodium valproate therapy. Arch Dis Child. 1979;54(12):986-987. doi:10.1136/adc.54.12.986
4. Barr RD, Copeland SA, Stockwell MC, Morris N, Kelton JC. Valproic acid and immune thrombocytopenia. Arch Dis Child. 1982;57(9):681-684. doi:10.1136/adc.57.9.681
5. Symon DNK, Russell G. Sodium valproate and neutropenia (letter). Arch Dis Child. 1983;58:235. doi:10.1136/adc.58.3.235
6. Watts RG, Emanuel PD, Zuckerman KS, Howard TH. Valproic acid-induced cytopenias: evidence for a dose-related suppression of hematopoiesis. J Pediatr. 1990;117(3):495-499. doi:10.1016/s0022-3476(05)81105-9
7. Blackburn SC, Oliart AD, García-Rodríguez LA, Pérez Gutthann S. Antiepileptics and blood dyscrasias: a cohort study. Pharmacotherapy. 1998;18(6):1277-1283.
8. Acharya S, Bussel JB. Hematologic toxicity of sodium valproate. J Pediatr Hematol Oncol. 2000;22(1):62-65. doi:10.1097/00043426-200001000-00012
9. Vesta KS, Medina PJ. Valproic acid-induced neutropenia. Ann Pharmacother. 2003;37(6):819-821. doi:10.1345/aph.1C381
10. Kohli U, Gulati, S. Sodium valproate induced isolated neutropenia. Indian J Pediatr. 2006;73(9):844-844. doi:10.1007/BF02790401
11. Hsu HC, Tseng HK, Wang SC, Wang YY. Valproic acid-induced agranulocytosis. Int J Gerontol. 2009;3(2):137-139. doi:10.1016/S1873-9598(09)70036-5
12. Chakraborty S, Chakraborty J, Mandal S, Ghosal MK. A rare occurrence of isolated neutropenia with valproic acid: a case report. J Indian Med Assoc. 2011;109(5):345-346.
13. Stoner SC, Deal E, Lurk JT. Delayed-onset neutropenia with divalproex sodium. Ann Pharmacother. 2008;42(10):1507-1510. doi:10.1345/aph.1L239
14. Storch DD. Severe leukopenia with valproate. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208-1209. doi:10.1097/00004583-200010000-00003
15. Rahman A, Mican LM, Fischer C, Campbell AH. Evaluating the incidence of leukopenia and neutropenia with valproate, quetiapine, or the combination in children and adolescents. Ann Pharmacother. 2009;43:822-830. doi:10.1345/aph.1L617
16. Hung WC, Hsieh MH. Neutropenia associated with the comedication of quetiapine and valproate in 2 elderly patients. J Clin Psychopharmacol. 2012;32(3):416-417. doi:10.1097/JCP.0b013e3182549d2d
17. Park HJ, Kim JY. Incidence of neutropenia with valproate and quetiapine combination treatment in subjects with acquired brain injuries. Arch Phys Med Rehabil. 2016;97(2):183-188. doi:10.1016/j.apmr.2015.09.004
18. Estabrook KR, Pheister M. A case of quetiapine XR and divalproex-associated neutropenia followed by successful use of ziprasidone. J Clin Psychopharmacol. 2012;32(3):417-418. doi:10.1097/JCP.0b013e318253a071
19. Nair P, Lippmann S. Is leukopenia associated with divalproex and/or quetiapine? Psychosomatics. 2005;46(2):188-189. doi:10.1176/appi.psy.46.2.188
20. Cowan C, Oakley C. Leukopenia and neutropenia induced by quetiapine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):292-294. doi:10.1016/j.pnpbp.2006.07.003
21. Fan KY, Chen WY, Huang MC. Quetiapine-associated leucopenia and thrombocytopenia: a case report. BMC Psychiatry. 2015;15:110. doi:10.1186/s12888-015-0495-9
22. Malik S, Lally J, Ajnakina O, et al. Sodium valproate and clozapine induced neutropenia: A case control study using register data. Schizophr Res. 2018;195:267-273. doi:10.1016/j.schres.2017.08.041
23. Pantelis C, Adesanya A. Increased risk of neutropaenia and agranulocytosis with sodium valproate used adjunctively with clozapine. Aust N Z J Psychiatry. 2001;35(4):544-545. doi:10.1046/j.1440-1614.2001.0911f.x
24. Madeb R, Hirschmann S, Kurs R, Turkie A, Modai I. Combined clozapine and valproic acid treatment-induced agranulocytosis. Eur Psychiatry. 2002;17(4):238-239. doi:10.1016/s0924-9338(02)00659-4
25. Dose M, Hellweg R, Yassouridis A, Theison M, Emrich HM. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry. 1998;31(4):122-125. doi:10.1055/s-2007-979312
26. Ingenhoven T, Lafay P, Rinne T, Passchier J, Duivenvoorden H. Effectiveness of pharmacotherapy for severe personality disorders: meta-analyses of randomized controlled trials. J Clin Psychiatry. 2010;71:14. doi:10.4088/jcp.08r04526gre
27. Mercer D, Douglass AB, Links PS. Meta-analyses of mood stabilizers, antidepressants and antipsychotics in the treatment of borderline personality disorder: effectiveness for depression and anger symptoms. J Pers Disord. 2009;23(2):156-174. doi:10.1521/pedi.2009.23.2.156
28. Hollander E, Swann AC, Coccaro EF, Jiang P, Smith TB. Impact of trait impulsivity and state aggression on divalproex versus placebo response in borderline personality disorder. Am J Psychiatry. 2005;162(3):621-624. doi:10.1176/appi.ajp.162.3.621
29. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. doi:10.1038/clpt.1981.154
30. Andrès E, Villalba NL, Zulfiqar AA, Serraj K, Mourot-Cottet R, Gottenberg AJ. State of art of idiosyncratic drug-induced neutropenia or agranulocytosis, with a focus on biotherapies. J Clin Med. 2019;8(9):1351. doi:10.3390/jcm8091351
Some leukemias detectable up to 16 years before diagnosis?
Previous analyses showed that monoclonal B-cell lymphocytosis (MBL), a CLL precursor state, has been detected up to 6 years before CLL diagnosis, the investigators explained, noting that “[a]nother prognostically relevant immunogenetic feature of CLL concerns the stereotype of the B-cell receptor immunoglobulins (BcR IG).”
“Indeed, distinct stereotyped subsets can be defined by the expression of shared sequence motifs and are associated with particular presentation and outcomes,” P. Martijn Kolijn, PhD, a researcher in the department of immunology at Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues wrote in a brief report published online in Blood. In an effort to “gain insight into the composition of the BcR IG repertoire during the early stages of CLL,” the investigators utilized next-generation sequencing to analyze 124 blood samples taken from healthy individuals up to 22 years before they received a diagnosis of CLL or small lymphocytic leukemia (SLL). An additional 118 matched control samples were also analyzed.
Study subjects were participants in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.
“First, unsurprisingly, we observed a significant difference in the frequency of the dominant clonotype in CLL patients versus controls with a median frequency of 54.9%, compared to only 0.38% in controls,” they wrote.
Among 28 patients whose lymphocyte counts were measured at baseline, 10 showed evidence of lymphocytosis up to 8 years before CLL diagnosis.
This suggests undiagnosed instances of high-count MBL (cases with a cell count above 0.5x 109 cells/L, which can progress to CLL) or asymptomatic CLL, they explained.
“In contrast, next-generation sequencing results showed detectable skewing of the IGH gene repertoire in 21/28 patients up to 15 years before CLL diagnosis, often in the absence of elevated lymphocyte counts,” they wrote. “Remarkably, some patients with CLL requiring treatment and clinical transformation to an aggressive B-cell lymphoma displayed considerable skewing in the IGH gene repertoire even 16 years before CLL diagnosis.”
Patients with a prediagnostic IGHV-unmutated dominant clonotype had significantly shorter overall survival after CLL diagnosis than did those with an IGHV-mutated clonotype, they noted.
“Furthermore, at early timepoints (>10 years before diagnosis), patients with a high dominant clonotype frequency were more likely to be IGHV mutated, whereas closer to diagnosis this tendency was lost, indicating that the prediagnostic phase may be even longer than 16 years for [mutated] CLL patients,” they added.
The investigators also found that:
- Twenty-five patients carried stereotyped BcR IG up to 17 years prior to CLL diagnosis, and of these, 10 clonotypes were assigned to minor subsets and 15 to major CLL subsets. Among the latter, 14 of the 15 belonged to high-risk subsets, and most of those showed a trend for faster disease evolution.
- High frequency of the dominant clonotype was evident in samples obtained less than 6 years before diagnosis, whereas high-risk stereotyped clonotypes found longer before diagnosis (as early as 16 years) tended to have a lower dominant clonotype frequency (<20% of IGH gene repertoire)
- The stereotyped BcR IG matched the clonotype at diagnosis for both patients with diagnostic material.
- No stereotyped subsets were identified among the dominant clonotypes of the healthy controls.
“To our knowledge, the dynamics of the emergence of biclonality in an MBL patient and subsequent progression to CLL have never been captured in such a convincing manner,” they noted.
The findings “extend current knowledge on the evolution of the IGH repertoire prior to CLL diagnosis, highlighting that even high-risk CLL subtypes may display a prolonged indolent preclinical stage,” they added, speculating that “somatic genetic aberrations, (auto)stimulation, epigenetic and/or microenvironmental influences are required for the transformation into overt CLL.”
The investigators also noted that since the observed skewing in the IGH gene repertoire often occurs prior to B-cell lymphocytosis, they consider the findings “a novel extension to the characterization of MBL.”
“Further studies may prove invaluable in the clinical distinction between ‘progressing’ MBL versus ‘stable’ MBL. Notwithstanding the above, we emphasize that early detection is only warranted if it provides clear benefits to patient care,” they concluded.
In a related commentary, Gerald Marti, MD, PhD, of the National Heart, Lung, and Blood Institute, emphasized that the findings “represent the earliest detection of a clonotypic precursor cell for CLL.” .
They also raise new questions and point to new directions for research, Dr. Marti noted.
“Where do we go from here? CLL has a long evolutionary history in which early branching may start as an oligoclonal process (antigen stimulation) and include driver mutations,” he wrote. “A long-term analysis of the B-cell repertoire in familial CLL might shed light on this process. Further clarification of the mechanisms of age-related immune senescence is also of interest.”
The study authors and Dr. Marti reported having no competing financial interests.
Previous analyses showed that monoclonal B-cell lymphocytosis (MBL), a CLL precursor state, has been detected up to 6 years before CLL diagnosis, the investigators explained, noting that “[a]nother prognostically relevant immunogenetic feature of CLL concerns the stereotype of the B-cell receptor immunoglobulins (BcR IG).”
“Indeed, distinct stereotyped subsets can be defined by the expression of shared sequence motifs and are associated with particular presentation and outcomes,” P. Martijn Kolijn, PhD, a researcher in the department of immunology at Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues wrote in a brief report published online in Blood. In an effort to “gain insight into the composition of the BcR IG repertoire during the early stages of CLL,” the investigators utilized next-generation sequencing to analyze 124 blood samples taken from healthy individuals up to 22 years before they received a diagnosis of CLL or small lymphocytic leukemia (SLL). An additional 118 matched control samples were also analyzed.
Study subjects were participants in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.
“First, unsurprisingly, we observed a significant difference in the frequency of the dominant clonotype in CLL patients versus controls with a median frequency of 54.9%, compared to only 0.38% in controls,” they wrote.
Among 28 patients whose lymphocyte counts were measured at baseline, 10 showed evidence of lymphocytosis up to 8 years before CLL diagnosis.
This suggests undiagnosed instances of high-count MBL (cases with a cell count above 0.5x 109 cells/L, which can progress to CLL) or asymptomatic CLL, they explained.
“In contrast, next-generation sequencing results showed detectable skewing of the IGH gene repertoire in 21/28 patients up to 15 years before CLL diagnosis, often in the absence of elevated lymphocyte counts,” they wrote. “Remarkably, some patients with CLL requiring treatment and clinical transformation to an aggressive B-cell lymphoma displayed considerable skewing in the IGH gene repertoire even 16 years before CLL diagnosis.”
Patients with a prediagnostic IGHV-unmutated dominant clonotype had significantly shorter overall survival after CLL diagnosis than did those with an IGHV-mutated clonotype, they noted.
“Furthermore, at early timepoints (>10 years before diagnosis), patients with a high dominant clonotype frequency were more likely to be IGHV mutated, whereas closer to diagnosis this tendency was lost, indicating that the prediagnostic phase may be even longer than 16 years for [mutated] CLL patients,” they added.
The investigators also found that:
- Twenty-five patients carried stereotyped BcR IG up to 17 years prior to CLL diagnosis, and of these, 10 clonotypes were assigned to minor subsets and 15 to major CLL subsets. Among the latter, 14 of the 15 belonged to high-risk subsets, and most of those showed a trend for faster disease evolution.
- High frequency of the dominant clonotype was evident in samples obtained less than 6 years before diagnosis, whereas high-risk stereotyped clonotypes found longer before diagnosis (as early as 16 years) tended to have a lower dominant clonotype frequency (<20% of IGH gene repertoire)
- The stereotyped BcR IG matched the clonotype at diagnosis for both patients with diagnostic material.
- No stereotyped subsets were identified among the dominant clonotypes of the healthy controls.
“To our knowledge, the dynamics of the emergence of biclonality in an MBL patient and subsequent progression to CLL have never been captured in such a convincing manner,” they noted.
The findings “extend current knowledge on the evolution of the IGH repertoire prior to CLL diagnosis, highlighting that even high-risk CLL subtypes may display a prolonged indolent preclinical stage,” they added, speculating that “somatic genetic aberrations, (auto)stimulation, epigenetic and/or microenvironmental influences are required for the transformation into overt CLL.”
The investigators also noted that since the observed skewing in the IGH gene repertoire often occurs prior to B-cell lymphocytosis, they consider the findings “a novel extension to the characterization of MBL.”
“Further studies may prove invaluable in the clinical distinction between ‘progressing’ MBL versus ‘stable’ MBL. Notwithstanding the above, we emphasize that early detection is only warranted if it provides clear benefits to patient care,” they concluded.
In a related commentary, Gerald Marti, MD, PhD, of the National Heart, Lung, and Blood Institute, emphasized that the findings “represent the earliest detection of a clonotypic precursor cell for CLL.” .
They also raise new questions and point to new directions for research, Dr. Marti noted.
“Where do we go from here? CLL has a long evolutionary history in which early branching may start as an oligoclonal process (antigen stimulation) and include driver mutations,” he wrote. “A long-term analysis of the B-cell repertoire in familial CLL might shed light on this process. Further clarification of the mechanisms of age-related immune senescence is also of interest.”
The study authors and Dr. Marti reported having no competing financial interests.
Previous analyses showed that monoclonal B-cell lymphocytosis (MBL), a CLL precursor state, has been detected up to 6 years before CLL diagnosis, the investigators explained, noting that “[a]nother prognostically relevant immunogenetic feature of CLL concerns the stereotype of the B-cell receptor immunoglobulins (BcR IG).”
“Indeed, distinct stereotyped subsets can be defined by the expression of shared sequence motifs and are associated with particular presentation and outcomes,” P. Martijn Kolijn, PhD, a researcher in the department of immunology at Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues wrote in a brief report published online in Blood. In an effort to “gain insight into the composition of the BcR IG repertoire during the early stages of CLL,” the investigators utilized next-generation sequencing to analyze 124 blood samples taken from healthy individuals up to 22 years before they received a diagnosis of CLL or small lymphocytic leukemia (SLL). An additional 118 matched control samples were also analyzed.
Study subjects were participants in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.
“First, unsurprisingly, we observed a significant difference in the frequency of the dominant clonotype in CLL patients versus controls with a median frequency of 54.9%, compared to only 0.38% in controls,” they wrote.
Among 28 patients whose lymphocyte counts were measured at baseline, 10 showed evidence of lymphocytosis up to 8 years before CLL diagnosis.
This suggests undiagnosed instances of high-count MBL (cases with a cell count above 0.5x 109 cells/L, which can progress to CLL) or asymptomatic CLL, they explained.
“In contrast, next-generation sequencing results showed detectable skewing of the IGH gene repertoire in 21/28 patients up to 15 years before CLL diagnosis, often in the absence of elevated lymphocyte counts,” they wrote. “Remarkably, some patients with CLL requiring treatment and clinical transformation to an aggressive B-cell lymphoma displayed considerable skewing in the IGH gene repertoire even 16 years before CLL diagnosis.”
Patients with a prediagnostic IGHV-unmutated dominant clonotype had significantly shorter overall survival after CLL diagnosis than did those with an IGHV-mutated clonotype, they noted.
“Furthermore, at early timepoints (>10 years before diagnosis), patients with a high dominant clonotype frequency were more likely to be IGHV mutated, whereas closer to diagnosis this tendency was lost, indicating that the prediagnostic phase may be even longer than 16 years for [mutated] CLL patients,” they added.
The investigators also found that:
- Twenty-five patients carried stereotyped BcR IG up to 17 years prior to CLL diagnosis, and of these, 10 clonotypes were assigned to minor subsets and 15 to major CLL subsets. Among the latter, 14 of the 15 belonged to high-risk subsets, and most of those showed a trend for faster disease evolution.
- High frequency of the dominant clonotype was evident in samples obtained less than 6 years before diagnosis, whereas high-risk stereotyped clonotypes found longer before diagnosis (as early as 16 years) tended to have a lower dominant clonotype frequency (<20% of IGH gene repertoire)
- The stereotyped BcR IG matched the clonotype at diagnosis for both patients with diagnostic material.
- No stereotyped subsets were identified among the dominant clonotypes of the healthy controls.
“To our knowledge, the dynamics of the emergence of biclonality in an MBL patient and subsequent progression to CLL have never been captured in such a convincing manner,” they noted.
The findings “extend current knowledge on the evolution of the IGH repertoire prior to CLL diagnosis, highlighting that even high-risk CLL subtypes may display a prolonged indolent preclinical stage,” they added, speculating that “somatic genetic aberrations, (auto)stimulation, epigenetic and/or microenvironmental influences are required for the transformation into overt CLL.”
The investigators also noted that since the observed skewing in the IGH gene repertoire often occurs prior to B-cell lymphocytosis, they consider the findings “a novel extension to the characterization of MBL.”
“Further studies may prove invaluable in the clinical distinction between ‘progressing’ MBL versus ‘stable’ MBL. Notwithstanding the above, we emphasize that early detection is only warranted if it provides clear benefits to patient care,” they concluded.
In a related commentary, Gerald Marti, MD, PhD, of the National Heart, Lung, and Blood Institute, emphasized that the findings “represent the earliest detection of a clonotypic precursor cell for CLL.” .
They also raise new questions and point to new directions for research, Dr. Marti noted.
“Where do we go from here? CLL has a long evolutionary history in which early branching may start as an oligoclonal process (antigen stimulation) and include driver mutations,” he wrote. “A long-term analysis of the B-cell repertoire in familial CLL might shed light on this process. Further clarification of the mechanisms of age-related immune senescence is also of interest.”
The study authors and Dr. Marti reported having no competing financial interests.
FROM BLOOD
Cancer Data Trends 2022
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
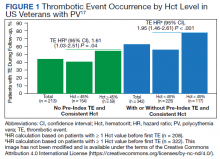
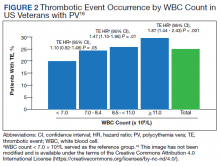
Hematocrit, White Blood Cells, and Thrombotic Events in the Veteran Population With Polycythemia Vera
Polycythemia vera (PV) is a rare myeloproliferative neoplasm affecting 44 to 57 individuals per 100,000 in the United States.1,2 It is characterized by somatic mutations in the hematopoietic stem cell, resulting in hyperproliferation of mature myeloid lineage cells.2 Sustained erythrocytosis is a hallmark of PV, although many patients also have leukocytosis and thrombocytosis.2,3 These patients have increased inherent thrombotic risk with arterial events reported to occur at rates of 7 to 21/1000 person-years and venous thrombotic events at 5 to 20/1000 person-years.4-7 Thrombotic and cardiovascular events are leading causes of morbidity and mortality, resulting in a reduced overall survival of patients with PV compared with the general population.3,8-10
Blood Cell Counts and Thrombotic Events in PV
Treatment strategies for patients with PV mainly aim to prevent or manage thrombotic and bleeding complications through normalization of blood counts.11 Hematocrit (Hct) control has been reported to be associated with reduced thrombotic risk in patients with PV. This was shown and popularized by the prospective, randomized Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial in which participants were randomized 1:1 to maintaining either a low (< 45%) or high (45%-50%) Hct for 5 years to examine the long-term effects of more- or less-intensive cytoreductive therapy.12 Patients in the low-Hct group were found to have a lower rate of death from cardiovascular events or major thrombosis (1.1/100 person-years in the low-Hct group vs 4.4 in the high-Hct group; hazard ratio [HR], 3.91; 95% confidence interval [CI], 1.45-10.53; P = .007). Likewise, cardiovascular events occurred at a lower rate in patients in the low-Hct group compared with the high-Hct group (4.4% vs 10.9% of patients, respectively; HR, 2.69; 95% CI, 1.19-6.12; P = .02).12
Leukocytosis has also been linked to elevated risk for vascular events as shown in several studies, including the real-world European Collaboration on Low-Dose Aspirin in PV (ECLAP) observational study and a post hoc subanalysis of the CYTO-PV study.13,14 In a multivariate, time-dependent analysis in ECLAP, patients with white blood cell (WBC) counts > 15 × 109/L had a significant increase in the risk of thrombosis compared with those who had lower WBC counts, with higher WBC count more strongly associated with arterial than venous thromboembolism.13 In CYTO-PV, a significant correlation between elevated WBC count (≥ 11 × 109/L vs reference level of < 7 × 109/L) and time-dependent risk of major thrombosis was shown (HR, 3.9; 95% CI, 1.24-12.3; P = .02).14 Likewise, WBC count ≥ 11 × 109/L was found to be a predictor of subsequent venous events in a separate single-center multivariate analysis of patients with PV.8
Although CYTO-PV remains one of the largest prospective landmark studies in PV demonstrating the impact of Hct control on thrombosis, it is worthwhile to note that the patients in the high-Hct group who received less frequent myelosuppressive therapy with hydroxyurea than the low-Hct group also had higher WBC counts.12,15 Work is needed to determine the relative effects of high Hct and high WBC counts on PV independent of each other.
The Veteran Population with PV
Two recently published retrospective analyses from Parasuraman and colleagues used data from the Veterans Health Administration (VHA), the largest integrated health care system in the US, with an aim to replicate findings from CYTO-PV in a real-world population.16,17 The 2 analyses focused independently on the effects of Hct control and WBC count on the risk of a thrombotic event in patients with PV.
In the first retrospective analysis, 213 patients with PV and no prior thrombosis were placed into groups based on whether Hct levels were consistently either < 45% or ≥ 45% throughout the study period.17 The mean follow-up time was 2.3 years, during which 44.1% of patients experienced a thrombotic event (Figure 1). Patients with Hct levels < 45% had a lower rate of thrombotic events compared to those with levels ≥ 45% (40.3% vs 54.2%, respectively; HR, 1.61; 95% CI, 1.03-2.51; P = .04). In a sensitivity analysis that included patients with pre-index thrombotic events (N = 342), similar results were noted (55.6% vs 76.9% between the < 45% and ≥ 45% groups, respectively; HR, 1.95; 95% CI, 1.46-2.61; P < .001).
In the second analysis, the authors investigated the relationship between WBC counts and thrombotic events.16 Evaluable patients (N = 1565) were grouped into 1 of 4 cohorts based on the last WBC measurement taken during the study period before a thrombotic event or through the end of follow-up: (1) WBC < 7.0 × 109/L, (2) 7.0 to 8.4 × 109/L, (3) 8.5 to < 11.0 × 109/L, or (4) ≥ 11.0 × 109/L. Mean follow-up time ranged from 3.6 to 4.5 years among WBC count cohorts, during which 24.9% of patients experienced a thrombotic event. Compared with the reference cohort (WBC < 7.0 × 109/L), a significant positive association between WBC counts and thrombotic event occurrence was observed among patients with WBC counts of 8.5 to < 11.0 × 109/L (HR, 1.47; 95% CI, 1.10-1.96; P < .01) and ≥ 11 × 109/L (HR, 1.87; 95% CI, 1.44-2.43; P < .001) (Figure 2).16 When including all patients in a sensitivity analysis regardless of whether they experienced thrombotic events before the index date (N = 1876), similar results were obtained (7.0-8.4 × 109/L group: HR, 1.22; 95% CI, 0.97-1.55; P = .0959; 8.5 - 11.0 × 109/L group: HR, 1.41; 95% CI, 1.10-1.81; P = .0062; ≥ 11.0 × 109/L group: HR, 1.53; 95% CI, 1.23-1.91; P < .001; compared with < 7.0 × 109/L reference group). Rates of phlebotomy and cytoreductive treatments were similar across groups.16
Some limitations to these studies are attributable to their retrospective design, reliance on health records, and the VHA population characteristics, which differ from the general population. For example, in this analysis, patients with PV in the VHA population had significantly increased risk of thrombotic events, even at a lower WBC count threshold (≥ 8.5 × 109/L) compared with those reported in CYTO-PV (≥ 11 × 109/L). Furthermore, approximately one-third of patients had elevated WBC levels, compared with 25.5% in the CYTO-PV study.14,16 This is most likely due to the unique nature of the VHA patient population, who are predominantly older adult men and generally have a higher comorbidity burden. A notable pre-index comorbidity burden was reported in the VHA population in the Hct analysis, even when compared to patients with PV in the general US population (Charlson Comorbidity Index score, 1.3 vs 0.8).6,17 Comorbid conditions such as hypertension, diabetes, and tobacco use, which are most common among the VHA population, are independently associated with higher risk of cardiovascular and thrombotic events.18,19 However, whether these higher levels of comorbidities affected the type of treatments they received was not elucidated, and the effectiveness of treatments to maintain target Hct levels was not addressed in the study.
Current PV Management and Future Implications
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology in myeloproliferative neoplasms recommend maintaining Hct levels < 45% in patients with PV.11 Patients with high-risk disease (age ≥ 60 years and/or history of thrombosis) are monitored for new thrombosis or bleeding and are managed for their cardiovascular risk factors. In addition, they receive low-dose aspirin (81-100 mg/day), undergo phlebotomy to maintain an Hct < 45%, and are managed with pharmacologic cytoreductive therapy. Cytoreductive therapy primarily consists of hydroxyurea or peginterferon alfa-2a for younger patients. Ruxolitinib, a Janus kinase (JAK1)/JAK2 inhibitor, is now approved by the US Food and Drug Administration as second-line treatment for those with PV that is intolerant or unresponsive to hydroxyurea or peginterferon alfa-2a treatments.11,20 However, the role of cytoreductive therapy is not clear for patients with low-risk disease (age < 60 years and no history of thrombosis). These patients are managed for their cardiovascular risk factors, undergo phlebotomy to maintain an Hct < 45%, are maintained on low-dose aspirin (81-100 mg/day), and are monitored for indications for cytoreductive therapy, which include any new thrombosis or disease-related major bleeding, frequent or persistent need for phlebotomy with poor tolerance for the procedure, splenomegaly, thrombocytosis, leukocytosis, and disease-related symptoms (eg, aquagenic pruritus, night sweats, fatigue).
Even though the current guidelines recommend maintaining a target Hct of < 45% in patients with high-risk PV, the role of Hct as the main determinant of thrombotic risk in patients with PV is still debated.21 In JAK2V617F-positive essential thrombocythemia, Hct levels are usually normal but risk of thrombosis is nevertheless still significant.22 The risk of thrombosis is significantly lower in primary familial and congenital polycythemia and much lower in secondary erythrocytosis such as cyanotic heart disease, long-term native dwellers of high altitude, and those with high-oxygen–affinity hemoglobins.21,23 In secondary erythrocytosis from hypoxia or upregulated hypoxic pathway such as hypoxia inducible factor-2α (HIF-2α) mutation and Chuvash erythrocytosis, the risk of thrombosis is more associated with the upregulated HIF pathway and its downstream consequences, rather than the elevated Hct level.24
However, most current literature supports the association of increased risk of thrombosis with higher Hct and high WBC count in patients with PV. In addition, the underlying mechanism of thrombogenesis still remains elusive; it is likely a complex process that involves interactions among multiple components, including elevated blood counts arising from clonal hematopoiesis, JAK2V617F allele burden, and platelet and WBC activation and their interaction with endothelial cells and inflammatory cytokines.25
Nevertheless, Hct control and aspirin use are current standard of care for patients with PV to mitigate thrombotic risk, and the results from the 2 analyses by Parasuraman and colleagues, using real-world data from the VHA, support the current practice guidelines to maintain Hct < 45% in these patients. They also provide additional support for considering WBC counts when determining patient risk and treatment plans. Although treatment response criteria from the European LeukemiaNet include achieving normal WBC levels to decrease the risk of thrombosis, current NCCN guidelines do not include WBC counts as a component for establishing patient risk or provide a target WBC count to guide patient management.11,26,27 Updates to these practice guidelines may be warranted. In addition, further study is needed to understand the mechanism of thrombogenesis in PV and other myeloproliferative disorders in order to develop novel therapeutic targets and improve patient outcomes.
Acknowledgments
Writing assistance was provided by Tania Iqbal, PhD, an employee of ICON (North Wales, PA), and was funded by Incyte Corporation (Wilmington, DE).
1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi:10.1182/blood-2016-03-643544
3. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163
4. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. doi:10.1200/JCO.2005.07.062
5. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840-846. doi:10.1182/blood-2006-12-064287
6. Goyal RK, Davis KL, Cote I, Mounedji N, Kaye JA. Increased incidence of thromboembolic event rates in patients diagnosed with polycythemia vera: results from an observational cohort study. Blood (ASH Annual Meeting Abstracts). 2014;124:4840. doi:10.1182/blood.V124.21.4840.4840
7. Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. doi:10.1182/blood-2014-07-591610 8. Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi:10.1038/s41408-017-0035-6
9. Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965-1976. doi:10.1007/s00277-014-2205-y
10. Hultcrantz M, Kristinsson SY, Andersson TM-L, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995-3001. doi:10.1200/JCO.2012.42.1925
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in myeloproliferative neoplasms (Version 1.2020). Accessed March 3, 2022. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500
13. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446-2452. doi:10.1182/blood-2006-08-042515
14. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560-561. doi:10.1182/blood-2015-04-638593
15. Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. doi:10.1056/NEJMc1301262
16. Parasuraman S, Yu J, Paranagama D, et al. Elevated white blood cell levels and thrombotic events in patients with polycythemia vera: a real-world analysis of Veterans Health Administration data. Clin Lymphoma Myeloma Leuk. 2020;20(2):63-69. doi:10.1016/j.clml.2019.11.010
17. Parasuraman S, Yu J, Paranagama D, et al. Hematocrit levels and thrombotic events in patients with polycythemia vera: an analysis of Veterans Health Administration data. Ann Hematol. 2019;98(11):2533-2539. doi:10.1007/s00277-019-03793-w
18. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345. doi:10.1016/S2214-109X(19)30318-3.
19. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi:10.1161/CIRCULATIONAHA.107.699579
20. Jakafi. Package insert. Incyte Corporation; 2020.
21. Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653-658. doi:10.3324/haematol.2018.210732
22. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857-5859. doi:10.1182/blood-2011-02-339002
23. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87(6):1954-1959. doi:10.1161/01.cir.87.6.1954
24. Gordeuk VR, Miasnikova GY, Sergueeva AI, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90. doi:10.3324/haematol.2019.216267
25. Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29(4):215-221. doi:10.1016/j.blre.2014.12.002
26. Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. doi:10.1038/s41375-018-0077-1
27. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. doi:10.1182/blood-2013-01-478891
Polycythemia vera (PV) is a rare myeloproliferative neoplasm affecting 44 to 57 individuals per 100,000 in the United States.1,2 It is characterized by somatic mutations in the hematopoietic stem cell, resulting in hyperproliferation of mature myeloid lineage cells.2 Sustained erythrocytosis is a hallmark of PV, although many patients also have leukocytosis and thrombocytosis.2,3 These patients have increased inherent thrombotic risk with arterial events reported to occur at rates of 7 to 21/1000 person-years and venous thrombotic events at 5 to 20/1000 person-years.4-7 Thrombotic and cardiovascular events are leading causes of morbidity and mortality, resulting in a reduced overall survival of patients with PV compared with the general population.3,8-10
Blood Cell Counts and Thrombotic Events in PV
Treatment strategies for patients with PV mainly aim to prevent or manage thrombotic and bleeding complications through normalization of blood counts.11 Hematocrit (Hct) control has been reported to be associated with reduced thrombotic risk in patients with PV. This was shown and popularized by the prospective, randomized Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial in which participants were randomized 1:1 to maintaining either a low (< 45%) or high (45%-50%) Hct for 5 years to examine the long-term effects of more- or less-intensive cytoreductive therapy.12 Patients in the low-Hct group were found to have a lower rate of death from cardiovascular events or major thrombosis (1.1/100 person-years in the low-Hct group vs 4.4 in the high-Hct group; hazard ratio [HR], 3.91; 95% confidence interval [CI], 1.45-10.53; P = .007). Likewise, cardiovascular events occurred at a lower rate in patients in the low-Hct group compared with the high-Hct group (4.4% vs 10.9% of patients, respectively; HR, 2.69; 95% CI, 1.19-6.12; P = .02).12
Leukocytosis has also been linked to elevated risk for vascular events as shown in several studies, including the real-world European Collaboration on Low-Dose Aspirin in PV (ECLAP) observational study and a post hoc subanalysis of the CYTO-PV study.13,14 In a multivariate, time-dependent analysis in ECLAP, patients with white blood cell (WBC) counts > 15 × 109/L had a significant increase in the risk of thrombosis compared with those who had lower WBC counts, with higher WBC count more strongly associated with arterial than venous thromboembolism.13 In CYTO-PV, a significant correlation between elevated WBC count (≥ 11 × 109/L vs reference level of < 7 × 109/L) and time-dependent risk of major thrombosis was shown (HR, 3.9; 95% CI, 1.24-12.3; P = .02).14 Likewise, WBC count ≥ 11 × 109/L was found to be a predictor of subsequent venous events in a separate single-center multivariate analysis of patients with PV.8
Although CYTO-PV remains one of the largest prospective landmark studies in PV demonstrating the impact of Hct control on thrombosis, it is worthwhile to note that the patients in the high-Hct group who received less frequent myelosuppressive therapy with hydroxyurea than the low-Hct group also had higher WBC counts.12,15 Work is needed to determine the relative effects of high Hct and high WBC counts on PV independent of each other.
The Veteran Population with PV
Two recently published retrospective analyses from Parasuraman and colleagues used data from the Veterans Health Administration (VHA), the largest integrated health care system in the US, with an aim to replicate findings from CYTO-PV in a real-world population.16,17 The 2 analyses focused independently on the effects of Hct control and WBC count on the risk of a thrombotic event in patients with PV.
In the first retrospective analysis, 213 patients with PV and no prior thrombosis were placed into groups based on whether Hct levels were consistently either < 45% or ≥ 45% throughout the study period.17 The mean follow-up time was 2.3 years, during which 44.1% of patients experienced a thrombotic event (Figure 1). Patients with Hct levels < 45% had a lower rate of thrombotic events compared to those with levels ≥ 45% (40.3% vs 54.2%, respectively; HR, 1.61; 95% CI, 1.03-2.51; P = .04). In a sensitivity analysis that included patients with pre-index thrombotic events (N = 342), similar results were noted (55.6% vs 76.9% between the < 45% and ≥ 45% groups, respectively; HR, 1.95; 95% CI, 1.46-2.61; P < .001).
In the second analysis, the authors investigated the relationship between WBC counts and thrombotic events.16 Evaluable patients (N = 1565) were grouped into 1 of 4 cohorts based on the last WBC measurement taken during the study period before a thrombotic event or through the end of follow-up: (1) WBC < 7.0 × 109/L, (2) 7.0 to 8.4 × 109/L, (3) 8.5 to < 11.0 × 109/L, or (4) ≥ 11.0 × 109/L. Mean follow-up time ranged from 3.6 to 4.5 years among WBC count cohorts, during which 24.9% of patients experienced a thrombotic event. Compared with the reference cohort (WBC < 7.0 × 109/L), a significant positive association between WBC counts and thrombotic event occurrence was observed among patients with WBC counts of 8.5 to < 11.0 × 109/L (HR, 1.47; 95% CI, 1.10-1.96; P < .01) and ≥ 11 × 109/L (HR, 1.87; 95% CI, 1.44-2.43; P < .001) (Figure 2).16 When including all patients in a sensitivity analysis regardless of whether they experienced thrombotic events before the index date (N = 1876), similar results were obtained (7.0-8.4 × 109/L group: HR, 1.22; 95% CI, 0.97-1.55; P = .0959; 8.5 - 11.0 × 109/L group: HR, 1.41; 95% CI, 1.10-1.81; P = .0062; ≥ 11.0 × 109/L group: HR, 1.53; 95% CI, 1.23-1.91; P < .001; compared with < 7.0 × 109/L reference group). Rates of phlebotomy and cytoreductive treatments were similar across groups.16
Some limitations to these studies are attributable to their retrospective design, reliance on health records, and the VHA population characteristics, which differ from the general population. For example, in this analysis, patients with PV in the VHA population had significantly increased risk of thrombotic events, even at a lower WBC count threshold (≥ 8.5 × 109/L) compared with those reported in CYTO-PV (≥ 11 × 109/L). Furthermore, approximately one-third of patients had elevated WBC levels, compared with 25.5% in the CYTO-PV study.14,16 This is most likely due to the unique nature of the VHA patient population, who are predominantly older adult men and generally have a higher comorbidity burden. A notable pre-index comorbidity burden was reported in the VHA population in the Hct analysis, even when compared to patients with PV in the general US population (Charlson Comorbidity Index score, 1.3 vs 0.8).6,17 Comorbid conditions such as hypertension, diabetes, and tobacco use, which are most common among the VHA population, are independently associated with higher risk of cardiovascular and thrombotic events.18,19 However, whether these higher levels of comorbidities affected the type of treatments they received was not elucidated, and the effectiveness of treatments to maintain target Hct levels was not addressed in the study.
Current PV Management and Future Implications
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology in myeloproliferative neoplasms recommend maintaining Hct levels < 45% in patients with PV.11 Patients with high-risk disease (age ≥ 60 years and/or history of thrombosis) are monitored for new thrombosis or bleeding and are managed for their cardiovascular risk factors. In addition, they receive low-dose aspirin (81-100 mg/day), undergo phlebotomy to maintain an Hct < 45%, and are managed with pharmacologic cytoreductive therapy. Cytoreductive therapy primarily consists of hydroxyurea or peginterferon alfa-2a for younger patients. Ruxolitinib, a Janus kinase (JAK1)/JAK2 inhibitor, is now approved by the US Food and Drug Administration as second-line treatment for those with PV that is intolerant or unresponsive to hydroxyurea or peginterferon alfa-2a treatments.11,20 However, the role of cytoreductive therapy is not clear for patients with low-risk disease (age < 60 years and no history of thrombosis). These patients are managed for their cardiovascular risk factors, undergo phlebotomy to maintain an Hct < 45%, are maintained on low-dose aspirin (81-100 mg/day), and are monitored for indications for cytoreductive therapy, which include any new thrombosis or disease-related major bleeding, frequent or persistent need for phlebotomy with poor tolerance for the procedure, splenomegaly, thrombocytosis, leukocytosis, and disease-related symptoms (eg, aquagenic pruritus, night sweats, fatigue).
Even though the current guidelines recommend maintaining a target Hct of < 45% in patients with high-risk PV, the role of Hct as the main determinant of thrombotic risk in patients with PV is still debated.21 In JAK2V617F-positive essential thrombocythemia, Hct levels are usually normal but risk of thrombosis is nevertheless still significant.22 The risk of thrombosis is significantly lower in primary familial and congenital polycythemia and much lower in secondary erythrocytosis such as cyanotic heart disease, long-term native dwellers of high altitude, and those with high-oxygen–affinity hemoglobins.21,23 In secondary erythrocytosis from hypoxia or upregulated hypoxic pathway such as hypoxia inducible factor-2α (HIF-2α) mutation and Chuvash erythrocytosis, the risk of thrombosis is more associated with the upregulated HIF pathway and its downstream consequences, rather than the elevated Hct level.24
However, most current literature supports the association of increased risk of thrombosis with higher Hct and high WBC count in patients with PV. In addition, the underlying mechanism of thrombogenesis still remains elusive; it is likely a complex process that involves interactions among multiple components, including elevated blood counts arising from clonal hematopoiesis, JAK2V617F allele burden, and platelet and WBC activation and their interaction with endothelial cells and inflammatory cytokines.25
Nevertheless, Hct control and aspirin use are current standard of care for patients with PV to mitigate thrombotic risk, and the results from the 2 analyses by Parasuraman and colleagues, using real-world data from the VHA, support the current practice guidelines to maintain Hct < 45% in these patients. They also provide additional support for considering WBC counts when determining patient risk and treatment plans. Although treatment response criteria from the European LeukemiaNet include achieving normal WBC levels to decrease the risk of thrombosis, current NCCN guidelines do not include WBC counts as a component for establishing patient risk or provide a target WBC count to guide patient management.11,26,27 Updates to these practice guidelines may be warranted. In addition, further study is needed to understand the mechanism of thrombogenesis in PV and other myeloproliferative disorders in order to develop novel therapeutic targets and improve patient outcomes.
Acknowledgments
Writing assistance was provided by Tania Iqbal, PhD, an employee of ICON (North Wales, PA), and was funded by Incyte Corporation (Wilmington, DE).
Polycythemia vera (PV) is a rare myeloproliferative neoplasm affecting 44 to 57 individuals per 100,000 in the United States.1,2 It is characterized by somatic mutations in the hematopoietic stem cell, resulting in hyperproliferation of mature myeloid lineage cells.2 Sustained erythrocytosis is a hallmark of PV, although many patients also have leukocytosis and thrombocytosis.2,3 These patients have increased inherent thrombotic risk with arterial events reported to occur at rates of 7 to 21/1000 person-years and venous thrombotic events at 5 to 20/1000 person-years.4-7 Thrombotic and cardiovascular events are leading causes of morbidity and mortality, resulting in a reduced overall survival of patients with PV compared with the general population.3,8-10
Blood Cell Counts and Thrombotic Events in PV
Treatment strategies for patients with PV mainly aim to prevent or manage thrombotic and bleeding complications through normalization of blood counts.11 Hematocrit (Hct) control has been reported to be associated with reduced thrombotic risk in patients with PV. This was shown and popularized by the prospective, randomized Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial in which participants were randomized 1:1 to maintaining either a low (< 45%) or high (45%-50%) Hct for 5 years to examine the long-term effects of more- or less-intensive cytoreductive therapy.12 Patients in the low-Hct group were found to have a lower rate of death from cardiovascular events or major thrombosis (1.1/100 person-years in the low-Hct group vs 4.4 in the high-Hct group; hazard ratio [HR], 3.91; 95% confidence interval [CI], 1.45-10.53; P = .007). Likewise, cardiovascular events occurred at a lower rate in patients in the low-Hct group compared with the high-Hct group (4.4% vs 10.9% of patients, respectively; HR, 2.69; 95% CI, 1.19-6.12; P = .02).12
Leukocytosis has also been linked to elevated risk for vascular events as shown in several studies, including the real-world European Collaboration on Low-Dose Aspirin in PV (ECLAP) observational study and a post hoc subanalysis of the CYTO-PV study.13,14 In a multivariate, time-dependent analysis in ECLAP, patients with white blood cell (WBC) counts > 15 × 109/L had a significant increase in the risk of thrombosis compared with those who had lower WBC counts, with higher WBC count more strongly associated with arterial than venous thromboembolism.13 In CYTO-PV, a significant correlation between elevated WBC count (≥ 11 × 109/L vs reference level of < 7 × 109/L) and time-dependent risk of major thrombosis was shown (HR, 3.9; 95% CI, 1.24-12.3; P = .02).14 Likewise, WBC count ≥ 11 × 109/L was found to be a predictor of subsequent venous events in a separate single-center multivariate analysis of patients with PV.8
Although CYTO-PV remains one of the largest prospective landmark studies in PV demonstrating the impact of Hct control on thrombosis, it is worthwhile to note that the patients in the high-Hct group who received less frequent myelosuppressive therapy with hydroxyurea than the low-Hct group also had higher WBC counts.12,15 Work is needed to determine the relative effects of high Hct and high WBC counts on PV independent of each other.
The Veteran Population with PV
Two recently published retrospective analyses from Parasuraman and colleagues used data from the Veterans Health Administration (VHA), the largest integrated health care system in the US, with an aim to replicate findings from CYTO-PV in a real-world population.16,17 The 2 analyses focused independently on the effects of Hct control and WBC count on the risk of a thrombotic event in patients with PV.
In the first retrospective analysis, 213 patients with PV and no prior thrombosis were placed into groups based on whether Hct levels were consistently either < 45% or ≥ 45% throughout the study period.17 The mean follow-up time was 2.3 years, during which 44.1% of patients experienced a thrombotic event (Figure 1). Patients with Hct levels < 45% had a lower rate of thrombotic events compared to those with levels ≥ 45% (40.3% vs 54.2%, respectively; HR, 1.61; 95% CI, 1.03-2.51; P = .04). In a sensitivity analysis that included patients with pre-index thrombotic events (N = 342), similar results were noted (55.6% vs 76.9% between the < 45% and ≥ 45% groups, respectively; HR, 1.95; 95% CI, 1.46-2.61; P < .001).
In the second analysis, the authors investigated the relationship between WBC counts and thrombotic events.16 Evaluable patients (N = 1565) were grouped into 1 of 4 cohorts based on the last WBC measurement taken during the study period before a thrombotic event or through the end of follow-up: (1) WBC < 7.0 × 109/L, (2) 7.0 to 8.4 × 109/L, (3) 8.5 to < 11.0 × 109/L, or (4) ≥ 11.0 × 109/L. Mean follow-up time ranged from 3.6 to 4.5 years among WBC count cohorts, during which 24.9% of patients experienced a thrombotic event. Compared with the reference cohort (WBC < 7.0 × 109/L), a significant positive association between WBC counts and thrombotic event occurrence was observed among patients with WBC counts of 8.5 to < 11.0 × 109/L (HR, 1.47; 95% CI, 1.10-1.96; P < .01) and ≥ 11 × 109/L (HR, 1.87; 95% CI, 1.44-2.43; P < .001) (Figure 2).16 When including all patients in a sensitivity analysis regardless of whether they experienced thrombotic events before the index date (N = 1876), similar results were obtained (7.0-8.4 × 109/L group: HR, 1.22; 95% CI, 0.97-1.55; P = .0959; 8.5 - 11.0 × 109/L group: HR, 1.41; 95% CI, 1.10-1.81; P = .0062; ≥ 11.0 × 109/L group: HR, 1.53; 95% CI, 1.23-1.91; P < .001; compared with < 7.0 × 109/L reference group). Rates of phlebotomy and cytoreductive treatments were similar across groups.16
Some limitations to these studies are attributable to their retrospective design, reliance on health records, and the VHA population characteristics, which differ from the general population. For example, in this analysis, patients with PV in the VHA population had significantly increased risk of thrombotic events, even at a lower WBC count threshold (≥ 8.5 × 109/L) compared with those reported in CYTO-PV (≥ 11 × 109/L). Furthermore, approximately one-third of patients had elevated WBC levels, compared with 25.5% in the CYTO-PV study.14,16 This is most likely due to the unique nature of the VHA patient population, who are predominantly older adult men and generally have a higher comorbidity burden. A notable pre-index comorbidity burden was reported in the VHA population in the Hct analysis, even when compared to patients with PV in the general US population (Charlson Comorbidity Index score, 1.3 vs 0.8).6,17 Comorbid conditions such as hypertension, diabetes, and tobacco use, which are most common among the VHA population, are independently associated with higher risk of cardiovascular and thrombotic events.18,19 However, whether these higher levels of comorbidities affected the type of treatments they received was not elucidated, and the effectiveness of treatments to maintain target Hct levels was not addressed in the study.
Current PV Management and Future Implications
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology in myeloproliferative neoplasms recommend maintaining Hct levels < 45% in patients with PV.11 Patients with high-risk disease (age ≥ 60 years and/or history of thrombosis) are monitored for new thrombosis or bleeding and are managed for their cardiovascular risk factors. In addition, they receive low-dose aspirin (81-100 mg/day), undergo phlebotomy to maintain an Hct < 45%, and are managed with pharmacologic cytoreductive therapy. Cytoreductive therapy primarily consists of hydroxyurea or peginterferon alfa-2a for younger patients. Ruxolitinib, a Janus kinase (JAK1)/JAK2 inhibitor, is now approved by the US Food and Drug Administration as second-line treatment for those with PV that is intolerant or unresponsive to hydroxyurea or peginterferon alfa-2a treatments.11,20 However, the role of cytoreductive therapy is not clear for patients with low-risk disease (age < 60 years and no history of thrombosis). These patients are managed for their cardiovascular risk factors, undergo phlebotomy to maintain an Hct < 45%, are maintained on low-dose aspirin (81-100 mg/day), and are monitored for indications for cytoreductive therapy, which include any new thrombosis or disease-related major bleeding, frequent or persistent need for phlebotomy with poor tolerance for the procedure, splenomegaly, thrombocytosis, leukocytosis, and disease-related symptoms (eg, aquagenic pruritus, night sweats, fatigue).
Even though the current guidelines recommend maintaining a target Hct of < 45% in patients with high-risk PV, the role of Hct as the main determinant of thrombotic risk in patients with PV is still debated.21 In JAK2V617F-positive essential thrombocythemia, Hct levels are usually normal but risk of thrombosis is nevertheless still significant.22 The risk of thrombosis is significantly lower in primary familial and congenital polycythemia and much lower in secondary erythrocytosis such as cyanotic heart disease, long-term native dwellers of high altitude, and those with high-oxygen–affinity hemoglobins.21,23 In secondary erythrocytosis from hypoxia or upregulated hypoxic pathway such as hypoxia inducible factor-2α (HIF-2α) mutation and Chuvash erythrocytosis, the risk of thrombosis is more associated with the upregulated HIF pathway and its downstream consequences, rather than the elevated Hct level.24
However, most current literature supports the association of increased risk of thrombosis with higher Hct and high WBC count in patients with PV. In addition, the underlying mechanism of thrombogenesis still remains elusive; it is likely a complex process that involves interactions among multiple components, including elevated blood counts arising from clonal hematopoiesis, JAK2V617F allele burden, and platelet and WBC activation and their interaction with endothelial cells and inflammatory cytokines.25
Nevertheless, Hct control and aspirin use are current standard of care for patients with PV to mitigate thrombotic risk, and the results from the 2 analyses by Parasuraman and colleagues, using real-world data from the VHA, support the current practice guidelines to maintain Hct < 45% in these patients. They also provide additional support for considering WBC counts when determining patient risk and treatment plans. Although treatment response criteria from the European LeukemiaNet include achieving normal WBC levels to decrease the risk of thrombosis, current NCCN guidelines do not include WBC counts as a component for establishing patient risk or provide a target WBC count to guide patient management.11,26,27 Updates to these practice guidelines may be warranted. In addition, further study is needed to understand the mechanism of thrombogenesis in PV and other myeloproliferative disorders in order to develop novel therapeutic targets and improve patient outcomes.
Acknowledgments
Writing assistance was provided by Tania Iqbal, PhD, an employee of ICON (North Wales, PA), and was funded by Incyte Corporation (Wilmington, DE).
1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi:10.1182/blood-2016-03-643544
3. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163
4. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. doi:10.1200/JCO.2005.07.062
5. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840-846. doi:10.1182/blood-2006-12-064287
6. Goyal RK, Davis KL, Cote I, Mounedji N, Kaye JA. Increased incidence of thromboembolic event rates in patients diagnosed with polycythemia vera: results from an observational cohort study. Blood (ASH Annual Meeting Abstracts). 2014;124:4840. doi:10.1182/blood.V124.21.4840.4840
7. Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. doi:10.1182/blood-2014-07-591610 8. Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi:10.1038/s41408-017-0035-6
9. Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965-1976. doi:10.1007/s00277-014-2205-y
10. Hultcrantz M, Kristinsson SY, Andersson TM-L, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995-3001. doi:10.1200/JCO.2012.42.1925
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in myeloproliferative neoplasms (Version 1.2020). Accessed March 3, 2022. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500
13. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446-2452. doi:10.1182/blood-2006-08-042515
14. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560-561. doi:10.1182/blood-2015-04-638593
15. Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. doi:10.1056/NEJMc1301262
16. Parasuraman S, Yu J, Paranagama D, et al. Elevated white blood cell levels and thrombotic events in patients with polycythemia vera: a real-world analysis of Veterans Health Administration data. Clin Lymphoma Myeloma Leuk. 2020;20(2):63-69. doi:10.1016/j.clml.2019.11.010
17. Parasuraman S, Yu J, Paranagama D, et al. Hematocrit levels and thrombotic events in patients with polycythemia vera: an analysis of Veterans Health Administration data. Ann Hematol. 2019;98(11):2533-2539. doi:10.1007/s00277-019-03793-w
18. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345. doi:10.1016/S2214-109X(19)30318-3.
19. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi:10.1161/CIRCULATIONAHA.107.699579
20. Jakafi. Package insert. Incyte Corporation; 2020.
21. Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653-658. doi:10.3324/haematol.2018.210732
22. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857-5859. doi:10.1182/blood-2011-02-339002
23. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87(6):1954-1959. doi:10.1161/01.cir.87.6.1954
24. Gordeuk VR, Miasnikova GY, Sergueeva AI, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90. doi:10.3324/haematol.2019.216267
25. Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29(4):215-221. doi:10.1016/j.blre.2014.12.002
26. Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. doi:10.1038/s41375-018-0077-1
27. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. doi:10.1182/blood-2013-01-478891
1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi:10.1182/blood-2016-03-643544
3. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163
4. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. doi:10.1200/JCO.2005.07.062
5. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840-846. doi:10.1182/blood-2006-12-064287
6. Goyal RK, Davis KL, Cote I, Mounedji N, Kaye JA. Increased incidence of thromboembolic event rates in patients diagnosed with polycythemia vera: results from an observational cohort study. Blood (ASH Annual Meeting Abstracts). 2014;124:4840. doi:10.1182/blood.V124.21.4840.4840
7. Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. doi:10.1182/blood-2014-07-591610 8. Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi:10.1038/s41408-017-0035-6
9. Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965-1976. doi:10.1007/s00277-014-2205-y
10. Hultcrantz M, Kristinsson SY, Andersson TM-L, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995-3001. doi:10.1200/JCO.2012.42.1925
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in myeloproliferative neoplasms (Version 1.2020). Accessed March 3, 2022. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500
13. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446-2452. doi:10.1182/blood-2006-08-042515
14. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560-561. doi:10.1182/blood-2015-04-638593
15. Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. doi:10.1056/NEJMc1301262
16. Parasuraman S, Yu J, Paranagama D, et al. Elevated white blood cell levels and thrombotic events in patients with polycythemia vera: a real-world analysis of Veterans Health Administration data. Clin Lymphoma Myeloma Leuk. 2020;20(2):63-69. doi:10.1016/j.clml.2019.11.010
17. Parasuraman S, Yu J, Paranagama D, et al. Hematocrit levels and thrombotic events in patients with polycythemia vera: an analysis of Veterans Health Administration data. Ann Hematol. 2019;98(11):2533-2539. doi:10.1007/s00277-019-03793-w
18. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345. doi:10.1016/S2214-109X(19)30318-3.
19. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi:10.1161/CIRCULATIONAHA.107.699579
20. Jakafi. Package insert. Incyte Corporation; 2020.
21. Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653-658. doi:10.3324/haematol.2018.210732
22. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857-5859. doi:10.1182/blood-2011-02-339002
23. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87(6):1954-1959. doi:10.1161/01.cir.87.6.1954
24. Gordeuk VR, Miasnikova GY, Sergueeva AI, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90. doi:10.3324/haematol.2019.216267
25. Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29(4):215-221. doi:10.1016/j.blre.2014.12.002
26. Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. doi:10.1038/s41375-018-0077-1
27. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. doi:10.1182/blood-2013-01-478891
Complex link between gut microbiome and immunotherapy response in advanced melanoma
A large-scale than previously thought.
Overall, researchers identified a panel of species, including Roseburia spp. and Akkermansia muciniphila, associated with responses to ICI therapy. However, no single species was a “fully consistent biomarker” across the studies, the authors explain.
This “machine learning analysis confirmed the link between the microbiome and overall response rates (ORRs) and progression-free survival (PFS) with ICIs but also revealed limited reproducibility of microbiome-based signatures across cohorts,” Karla A. Lee, PhD, a clinical research fellow at King’s College London, and colleagues report. The results suggest that “the microbiome is predictive of response in some, but not all, cohorts.”
The findings were published online Feb. 28 in Nature Medicine.
Despite recent advances in targeted therapies for melanoma, less than half of the those who receive a single-agent ICI respond, and those who receive combination ICI therapy often suffer from severe drug toxicity problems. That is why finding patients more likely to respond to a single-agent ICI has become a priority.
Previous studies have identified the gut microbiome as “a potential biomarker of response, as well as a therapeutic target” in melanoma and other malignancies, but “little consensus exists on which microbiome characteristics are associated with treatment responses in the human setting,” the authors explain.
To further clarify the microbiome–immunotherapy relationship, the researchers performed metagenomic sequencing of stool samples collected from 165 ICI-naive patients with unresectable stage III or IV cutaneous melanoma from 5 observational cohorts in the Netherlands, United Kingdom, and Spain. These data were integrated with 147 samples from publicly available datasets.
First, the authors highlighted the variability in findings across these observational studies. For instance, they analyzed stool samples from one UK-based observational study of patients with melanoma (PRIMM-UK) and found a small but statistically significant difference in the microbiome composition of immunotherapy responders versus nonresponders (P = .05) but did not find such an association in a parallel study in the Netherlands (PRIMM-NL, P = .61).
The investigators also explored biomarkers of response across different cohorts and found several standouts. In trials using ORR as an endpoint, two uncultivated Roseburia species (CAG:182 and CAG:471) were associated with responses to ICIs. For patients with available PFS data, Phascolarctobacterium succinatutens and Lactobacillus vaginalis were “enriched in responders” across 7 datasets and significant in 3 of the 8 meta-analysis approaches. A muciniphila and Dorea formicigenerans were also associated with ORR and PFS at 12 months in several meta-analyses.
However, “no single bacterium was a fully consistent biomarker of response across all datasets,” the authors wrote.
Still, the findings could have important implications for the more than 50% of patients with advanced melanoma who don’t respond to single-agent ICI therapy.
“Our study shows that studying the microbiome is important to improve and personalize immunotherapy treatments for melanoma,” study coauthor Nicola Segata, PhD, principal investigator in the Laboratory of Computational Metagenomics, University of Trento, Italy, said in a press release. “However, it also suggests that because of the person-to-person variability of the gut microbiome, even larger studies must be carried out to understand the specific gut microbial features that are more likely to lead to a positive response to immunotherapy.”
Coauthor Tim Spector, PhD, head of the Department of Twin Research & Genetic Epidemiology at King’s College London, added that “the ultimate goal is to identify which specific features of the microbiome are directly influencing the clinical benefits of immunotherapy to exploit these features in new personalized approaches to support cancer immunotherapy.”
In the meantime, he said, “this study highlights the potential impact of good diet and gut health on chances of survival in patients undergoing immunotherapy.”
This study was coordinated by King’s College London, CIBIO Department of the University of Trento and European Institute of Oncology in Italy, and the University of Groningen in the Netherlands, and was funded by the Seerave Foundation. Dr. Lee, Dr. Segata, and Dr. Spector have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large-scale than previously thought.
Overall, researchers identified a panel of species, including Roseburia spp. and Akkermansia muciniphila, associated with responses to ICI therapy. However, no single species was a “fully consistent biomarker” across the studies, the authors explain.
This “machine learning analysis confirmed the link between the microbiome and overall response rates (ORRs) and progression-free survival (PFS) with ICIs but also revealed limited reproducibility of microbiome-based signatures across cohorts,” Karla A. Lee, PhD, a clinical research fellow at King’s College London, and colleagues report. The results suggest that “the microbiome is predictive of response in some, but not all, cohorts.”
The findings were published online Feb. 28 in Nature Medicine.
Despite recent advances in targeted therapies for melanoma, less than half of the those who receive a single-agent ICI respond, and those who receive combination ICI therapy often suffer from severe drug toxicity problems. That is why finding patients more likely to respond to a single-agent ICI has become a priority.
Previous studies have identified the gut microbiome as “a potential biomarker of response, as well as a therapeutic target” in melanoma and other malignancies, but “little consensus exists on which microbiome characteristics are associated with treatment responses in the human setting,” the authors explain.
To further clarify the microbiome–immunotherapy relationship, the researchers performed metagenomic sequencing of stool samples collected from 165 ICI-naive patients with unresectable stage III or IV cutaneous melanoma from 5 observational cohorts in the Netherlands, United Kingdom, and Spain. These data were integrated with 147 samples from publicly available datasets.
First, the authors highlighted the variability in findings across these observational studies. For instance, they analyzed stool samples from one UK-based observational study of patients with melanoma (PRIMM-UK) and found a small but statistically significant difference in the microbiome composition of immunotherapy responders versus nonresponders (P = .05) but did not find such an association in a parallel study in the Netherlands (PRIMM-NL, P = .61).
The investigators also explored biomarkers of response across different cohorts and found several standouts. In trials using ORR as an endpoint, two uncultivated Roseburia species (CAG:182 and CAG:471) were associated with responses to ICIs. For patients with available PFS data, Phascolarctobacterium succinatutens and Lactobacillus vaginalis were “enriched in responders” across 7 datasets and significant in 3 of the 8 meta-analysis approaches. A muciniphila and Dorea formicigenerans were also associated with ORR and PFS at 12 months in several meta-analyses.
However, “no single bacterium was a fully consistent biomarker of response across all datasets,” the authors wrote.
Still, the findings could have important implications for the more than 50% of patients with advanced melanoma who don’t respond to single-agent ICI therapy.
“Our study shows that studying the microbiome is important to improve and personalize immunotherapy treatments for melanoma,” study coauthor Nicola Segata, PhD, principal investigator in the Laboratory of Computational Metagenomics, University of Trento, Italy, said in a press release. “However, it also suggests that because of the person-to-person variability of the gut microbiome, even larger studies must be carried out to understand the specific gut microbial features that are more likely to lead to a positive response to immunotherapy.”
Coauthor Tim Spector, PhD, head of the Department of Twin Research & Genetic Epidemiology at King’s College London, added that “the ultimate goal is to identify which specific features of the microbiome are directly influencing the clinical benefits of immunotherapy to exploit these features in new personalized approaches to support cancer immunotherapy.”
In the meantime, he said, “this study highlights the potential impact of good diet and gut health on chances of survival in patients undergoing immunotherapy.”
This study was coordinated by King’s College London, CIBIO Department of the University of Trento and European Institute of Oncology in Italy, and the University of Groningen in the Netherlands, and was funded by the Seerave Foundation. Dr. Lee, Dr. Segata, and Dr. Spector have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large-scale than previously thought.
Overall, researchers identified a panel of species, including Roseburia spp. and Akkermansia muciniphila, associated with responses to ICI therapy. However, no single species was a “fully consistent biomarker” across the studies, the authors explain.
This “machine learning analysis confirmed the link between the microbiome and overall response rates (ORRs) and progression-free survival (PFS) with ICIs but also revealed limited reproducibility of microbiome-based signatures across cohorts,” Karla A. Lee, PhD, a clinical research fellow at King’s College London, and colleagues report. The results suggest that “the microbiome is predictive of response in some, but not all, cohorts.”
The findings were published online Feb. 28 in Nature Medicine.
Despite recent advances in targeted therapies for melanoma, less than half of the those who receive a single-agent ICI respond, and those who receive combination ICI therapy often suffer from severe drug toxicity problems. That is why finding patients more likely to respond to a single-agent ICI has become a priority.
Previous studies have identified the gut microbiome as “a potential biomarker of response, as well as a therapeutic target” in melanoma and other malignancies, but “little consensus exists on which microbiome characteristics are associated with treatment responses in the human setting,” the authors explain.
To further clarify the microbiome–immunotherapy relationship, the researchers performed metagenomic sequencing of stool samples collected from 165 ICI-naive patients with unresectable stage III or IV cutaneous melanoma from 5 observational cohorts in the Netherlands, United Kingdom, and Spain. These data were integrated with 147 samples from publicly available datasets.
First, the authors highlighted the variability in findings across these observational studies. For instance, they analyzed stool samples from one UK-based observational study of patients with melanoma (PRIMM-UK) and found a small but statistically significant difference in the microbiome composition of immunotherapy responders versus nonresponders (P = .05) but did not find such an association in a parallel study in the Netherlands (PRIMM-NL, P = .61).
The investigators also explored biomarkers of response across different cohorts and found several standouts. In trials using ORR as an endpoint, two uncultivated Roseburia species (CAG:182 and CAG:471) were associated with responses to ICIs. For patients with available PFS data, Phascolarctobacterium succinatutens and Lactobacillus vaginalis were “enriched in responders” across 7 datasets and significant in 3 of the 8 meta-analysis approaches. A muciniphila and Dorea formicigenerans were also associated with ORR and PFS at 12 months in several meta-analyses.
However, “no single bacterium was a fully consistent biomarker of response across all datasets,” the authors wrote.
Still, the findings could have important implications for the more than 50% of patients with advanced melanoma who don’t respond to single-agent ICI therapy.
“Our study shows that studying the microbiome is important to improve and personalize immunotherapy treatments for melanoma,” study coauthor Nicola Segata, PhD, principal investigator in the Laboratory of Computational Metagenomics, University of Trento, Italy, said in a press release. “However, it also suggests that because of the person-to-person variability of the gut microbiome, even larger studies must be carried out to understand the specific gut microbial features that are more likely to lead to a positive response to immunotherapy.”
Coauthor Tim Spector, PhD, head of the Department of Twin Research & Genetic Epidemiology at King’s College London, added that “the ultimate goal is to identify which specific features of the microbiome are directly influencing the clinical benefits of immunotherapy to exploit these features in new personalized approaches to support cancer immunotherapy.”
In the meantime, he said, “this study highlights the potential impact of good diet and gut health on chances of survival in patients undergoing immunotherapy.”
This study was coordinated by King’s College London, CIBIO Department of the University of Trento and European Institute of Oncology in Italy, and the University of Groningen in the Netherlands, and was funded by the Seerave Foundation. Dr. Lee, Dr. Segata, and Dr. Spector have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves new CAR T-cell treatment for multiple myeloma
A new treatment option for patients with refractory/relapsed multiple myeloma who have already tried four or more therapies has been approved by the U.S. Food and Drug Administration.
There are already two other therapies on the market that target BCMA – another CAR T cell, idecabtagene vicleucel (Abecma), which was approved by the FDA in March 2021, and a drug conjugate, belantamab mafodotin (Blenrep), which was approved in August 2020.
The approval of cilta-cel was based on clinical data from the CARTITUDE-1 study, which were initially presented in December 2020 at the annual meeting of the American Society of Hematology, as reported at the time by this news organization.
The trial involved 97 patients with relapsed/refractory multiple myeloma who had already received a median of six previous treatments (range, three to 18), including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
“The treatment journey for the majority of patients living with multiple myeloma is a relentless cycle of remission and relapse, with fewer patients achieving a deep response as they progress through later lines of therapy,” commented Sundar Jagannath, MBBS, professor of medicine, hematology, and medical oncology at Mount Sinai, who was a principal investigator on the pivotal study.
“That is why I have been really excited about the results from the CARTITUDE-1 study, which has demonstrated that cilta-cel can provide deep and durable responses and long-term treatment-free intervals, even in this heavily pretreated multiple myeloma patient population,” he said.
“Today’s approval of Carvykti helps address a great unmet need for these patients,” he commented in a press release from the manufacturer.
Like other CAR T-cell therapies, ciltacabtagene autoleucel is a one-time treatment. It involves collecting blood from the patient, extracting T cells, genetically engineering them, then transfusing them back to the patient, who in the meantime has undergone conditioning.
The results from CARTITUDE-1 show that this one-time treatment resulted in deep and durable responses.
The overall response rate was 98%, and the majority of patients (78%) achieved a stringent complete response, in which physicians are unable to observe any signs or symptoms of disease via imaging or other tests after treatment.
At a median of 18 months’ follow-up, the median duration of response was 21.8 months.
“The responses in the CARTITUDE-1 study showed durability over time and resulted in the majority of heavily pretreated patients achieving deep responses after 18-month follow-up,” commented Mr. Jagannath.
“The approval of cilta-cel provides physicians an immunotherapy treatment option that offers patients an opportunity to be free from anti-myeloma therapies for a period of time,” he added.
As with other CAR T-cell therapies, there were serious side effects, and these products are available only through restricted programs under a risk evaluation and mitigation strategy.
The product information for Cartykti includes a boxed warning that mentions cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome, parkinsonism, Guillain-Barré syndrome, hemophagocytic lymphohistiocytosis/macrophage activation syndrome, and prolonged and/or recurrent cytopenias.
The most common adverse reactions (reported in greater than or equal to 20% of patients) are pyrexia, CRS, hypogammaglobulinemia, hypotension, musculoskeletal pain, fatigue, infections–pathogens unspecified, cough, chills, diarrhea, nausea, encephalopathy, decreased appetite, upper respiratory tract infection, headache, tachycardia, dizziness, dyspnea, edema, viral infections, coagulopathy, constipation, and vomiting.
A version of this article first appeared on Medscape.com.
A new treatment option for patients with refractory/relapsed multiple myeloma who have already tried four or more therapies has been approved by the U.S. Food and Drug Administration.
There are already two other therapies on the market that target BCMA – another CAR T cell, idecabtagene vicleucel (Abecma), which was approved by the FDA in March 2021, and a drug conjugate, belantamab mafodotin (Blenrep), which was approved in August 2020.
The approval of cilta-cel was based on clinical data from the CARTITUDE-1 study, which were initially presented in December 2020 at the annual meeting of the American Society of Hematology, as reported at the time by this news organization.
The trial involved 97 patients with relapsed/refractory multiple myeloma who had already received a median of six previous treatments (range, three to 18), including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
“The treatment journey for the majority of patients living with multiple myeloma is a relentless cycle of remission and relapse, with fewer patients achieving a deep response as they progress through later lines of therapy,” commented Sundar Jagannath, MBBS, professor of medicine, hematology, and medical oncology at Mount Sinai, who was a principal investigator on the pivotal study.
“That is why I have been really excited about the results from the CARTITUDE-1 study, which has demonstrated that cilta-cel can provide deep and durable responses and long-term treatment-free intervals, even in this heavily pretreated multiple myeloma patient population,” he said.
“Today’s approval of Carvykti helps address a great unmet need for these patients,” he commented in a press release from the manufacturer.
Like other CAR T-cell therapies, ciltacabtagene autoleucel is a one-time treatment. It involves collecting blood from the patient, extracting T cells, genetically engineering them, then transfusing them back to the patient, who in the meantime has undergone conditioning.
The results from CARTITUDE-1 show that this one-time treatment resulted in deep and durable responses.
The overall response rate was 98%, and the majority of patients (78%) achieved a stringent complete response, in which physicians are unable to observe any signs or symptoms of disease via imaging or other tests after treatment.
At a median of 18 months’ follow-up, the median duration of response was 21.8 months.
“The responses in the CARTITUDE-1 study showed durability over time and resulted in the majority of heavily pretreated patients achieving deep responses after 18-month follow-up,” commented Mr. Jagannath.
“The approval of cilta-cel provides physicians an immunotherapy treatment option that offers patients an opportunity to be free from anti-myeloma therapies for a period of time,” he added.
As with other CAR T-cell therapies, there were serious side effects, and these products are available only through restricted programs under a risk evaluation and mitigation strategy.
The product information for Cartykti includes a boxed warning that mentions cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome, parkinsonism, Guillain-Barré syndrome, hemophagocytic lymphohistiocytosis/macrophage activation syndrome, and prolonged and/or recurrent cytopenias.
The most common adverse reactions (reported in greater than or equal to 20% of patients) are pyrexia, CRS, hypogammaglobulinemia, hypotension, musculoskeletal pain, fatigue, infections–pathogens unspecified, cough, chills, diarrhea, nausea, encephalopathy, decreased appetite, upper respiratory tract infection, headache, tachycardia, dizziness, dyspnea, edema, viral infections, coagulopathy, constipation, and vomiting.
A version of this article first appeared on Medscape.com.
A new treatment option for patients with refractory/relapsed multiple myeloma who have already tried four or more therapies has been approved by the U.S. Food and Drug Administration.
There are already two other therapies on the market that target BCMA – another CAR T cell, idecabtagene vicleucel (Abecma), which was approved by the FDA in March 2021, and a drug conjugate, belantamab mafodotin (Blenrep), which was approved in August 2020.
The approval of cilta-cel was based on clinical data from the CARTITUDE-1 study, which were initially presented in December 2020 at the annual meeting of the American Society of Hematology, as reported at the time by this news organization.
The trial involved 97 patients with relapsed/refractory multiple myeloma who had already received a median of six previous treatments (range, three to 18), including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
“The treatment journey for the majority of patients living with multiple myeloma is a relentless cycle of remission and relapse, with fewer patients achieving a deep response as they progress through later lines of therapy,” commented Sundar Jagannath, MBBS, professor of medicine, hematology, and medical oncology at Mount Sinai, who was a principal investigator on the pivotal study.
“That is why I have been really excited about the results from the CARTITUDE-1 study, which has demonstrated that cilta-cel can provide deep and durable responses and long-term treatment-free intervals, even in this heavily pretreated multiple myeloma patient population,” he said.
“Today’s approval of Carvykti helps address a great unmet need for these patients,” he commented in a press release from the manufacturer.
Like other CAR T-cell therapies, ciltacabtagene autoleucel is a one-time treatment. It involves collecting blood from the patient, extracting T cells, genetically engineering them, then transfusing them back to the patient, who in the meantime has undergone conditioning.
The results from CARTITUDE-1 show that this one-time treatment resulted in deep and durable responses.
The overall response rate was 98%, and the majority of patients (78%) achieved a stringent complete response, in which physicians are unable to observe any signs or symptoms of disease via imaging or other tests after treatment.
At a median of 18 months’ follow-up, the median duration of response was 21.8 months.
“The responses in the CARTITUDE-1 study showed durability over time and resulted in the majority of heavily pretreated patients achieving deep responses after 18-month follow-up,” commented Mr. Jagannath.
“The approval of cilta-cel provides physicians an immunotherapy treatment option that offers patients an opportunity to be free from anti-myeloma therapies for a period of time,” he added.
As with other CAR T-cell therapies, there were serious side effects, and these products are available only through restricted programs under a risk evaluation and mitigation strategy.
The product information for Cartykti includes a boxed warning that mentions cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome, parkinsonism, Guillain-Barré syndrome, hemophagocytic lymphohistiocytosis/macrophage activation syndrome, and prolonged and/or recurrent cytopenias.
The most common adverse reactions (reported in greater than or equal to 20% of patients) are pyrexia, CRS, hypogammaglobulinemia, hypotension, musculoskeletal pain, fatigue, infections–pathogens unspecified, cough, chills, diarrhea, nausea, encephalopathy, decreased appetite, upper respiratory tract infection, headache, tachycardia, dizziness, dyspnea, edema, viral infections, coagulopathy, constipation, and vomiting.
A version of this article first appeared on Medscape.com.
AGILE: ‘Exciting’ survival results for IDH1-mutated AML
ATLANTA – Patients with acute myeloid leukemia (AML) bearing mutations in IDH1 who could not withstand the rigors of intensive therapy had improved event-free and overall survival when they were treated with the combination of ivosidenib (Tibsovo) and azacitidine (Onureg, Vidaza), compared with azacitidine alone.
The results come from the phase 3 AGILE trial., reported Hartmut Döhner, MD, from Ulm (Germany) University Hospital.
Median overall survival was 24 months with the combination, compared with 7.9 months for azacitidine-placebo, translating into a hazard ratio for death with the IVO-AZA of 0.44 (P = .0005).
“The IVO-AZA combination was safe and tolerable, with fewer infections reported relative to placebo plus AZA. Additionally, the clinical benefit of the combination was supported by favorable health-related quality of life,” he said.
Dr. Döhner spoke at a press briefing prior to the presentation of the trial results at the annual meeting of the American Society of Hematology.
“I’m really excited by the results from the AGILE trial,” commented Mikkael A. Sekeres, MD, chief of the division of hematology at the University of Miami’s Sylvester Comprehensive Cancer Center, who moderated the briefing.
The results show “survival that’s three times longer for a combination of ivosidenib plus azacitidine versus azacitidine alone in a very distinct population of patients who have an IDH1 mutation,” he said.
“The question that will arise is, if the standard of care is now to give azacitidine and venetoclax [Venclexta] to patients who don’t receive intensive chemotherapy in the inpatient setting, where does this trial end? And I would answer [by saying] that the combination of azacitidine is not truly nonintensive therapy, and it’s probably on a spectrum between nonintensive and intensive therapy, and probably closer to seven plus three than a lot of people recognize,” Dr. Sekeres said.
The combination of ivosidenib and azacitidine may therefore be a better choice for treatment of older patients with IDH1-mutated AML – particularly those with comorbidities – who may not be able to tolerate venetoclax plus azacitidine, he added.
AGILE results
For the analysis presented at the meeting, there was a data cutoff in March 2021, at which point 146 patients out of a planned 200 had been enrolled and randomly assigned. They received treatment with either oral ivosidenib 500 mg daily plus azacitidine 75 mg/m2, delivered subcutaneously or intravenously, or azacitidine plus placebo.
In May 2021, after an interim analysis, the independent data-monitoring committee recommended a halt to the trial because of significant improvements in outcomes among patients assigned to the combination, and those data are reported at the meeting.
The median patient age was about 76 years in each group. Approximately 75% of patients in each arm had de novo AML, and about 25% had AML secondary to treatment, myelodysplastic syndrome, or myeloproliferative neoplasms. The majority of patients in each group had intermediate cytogenetic risk disease.
The analysis was by intention to treat, with patients who did not have complete remission (CR) by week 24 considered to have had an event on day 1 of randomization.
At the time of the interim analysis, with the longest follow-up out to 29 months (the investigators did not report median follow-up time for the study), there were significantly fewer study events – defined as treatment failure by week 24, relapse from remission, or death from any cause – in the ivosidenib/azacitidine combination, with a hazard ratio of 0.33 (P = .0011).
The EFS benefit and the overall survival benefit were consistent across subgroups, the researchers noted, including in patients with de novo disease, demographics, baseline cytogenetic risk status, World Health Organization AML classification, baseline white blood cell count, and baseline percentage of bone marrow blasts.
Clinical and hematologic responses also favored the combination, with a complete response (CR) rate of 34%, compared with 11% for azacitidine alone (odds ratio, 4.8; P < .0001), and respective overall response rates of 45% versus 14% (odds ratio, 7.2; P < .0001).
Health-related quality of life measures also trended better with the combination across all subscales, and were significantly better at day 1 of cycle 5 in the diarrhea and appetite loss domains.
Treatment-emergent adverse events included grade 2 or higher differentiation syndrome, which occurred in 14.1% of patients treated with IVO-AZA versus 8.2% treated with AZA alone. Grade 3 or higher QT interval prolongation was also more frequent with the combination, at 9.9% versus 4.1%.
Any-grade infections were less common with IVO-AZA, however, at 28.2% versus 49.3% with AZA alone. At the briefing, this news organization asked coinvestigator Stephane de Botton, MD, Gustave Roussy Cancer Center, Villejuif, France, whether he could explain this seemingly paradoxical result.
He replied that the combination results in greater production of neutrophils and therefore better protection against infections, compared with azacitidine alone.
The study was funded by Agios Pharmaceuticals, now a part of Servier Pharmaceuticals. Dr. Döhner disclosed consultancy and other relationships with various companies. Dr. Sekeres disclosed consulting/advising for Novartis, Takea/Millennium, and Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
ATLANTA – Patients with acute myeloid leukemia (AML) bearing mutations in IDH1 who could not withstand the rigors of intensive therapy had improved event-free and overall survival when they were treated with the combination of ivosidenib (Tibsovo) and azacitidine (Onureg, Vidaza), compared with azacitidine alone.
The results come from the phase 3 AGILE trial., reported Hartmut Döhner, MD, from Ulm (Germany) University Hospital.
Median overall survival was 24 months with the combination, compared with 7.9 months for azacitidine-placebo, translating into a hazard ratio for death with the IVO-AZA of 0.44 (P = .0005).
“The IVO-AZA combination was safe and tolerable, with fewer infections reported relative to placebo plus AZA. Additionally, the clinical benefit of the combination was supported by favorable health-related quality of life,” he said.
Dr. Döhner spoke at a press briefing prior to the presentation of the trial results at the annual meeting of the American Society of Hematology.
“I’m really excited by the results from the AGILE trial,” commented Mikkael A. Sekeres, MD, chief of the division of hematology at the University of Miami’s Sylvester Comprehensive Cancer Center, who moderated the briefing.
The results show “survival that’s three times longer for a combination of ivosidenib plus azacitidine versus azacitidine alone in a very distinct population of patients who have an IDH1 mutation,” he said.
“The question that will arise is, if the standard of care is now to give azacitidine and venetoclax [Venclexta] to patients who don’t receive intensive chemotherapy in the inpatient setting, where does this trial end? And I would answer [by saying] that the combination of azacitidine is not truly nonintensive therapy, and it’s probably on a spectrum between nonintensive and intensive therapy, and probably closer to seven plus three than a lot of people recognize,” Dr. Sekeres said.
The combination of ivosidenib and azacitidine may therefore be a better choice for treatment of older patients with IDH1-mutated AML – particularly those with comorbidities – who may not be able to tolerate venetoclax plus azacitidine, he added.
AGILE results
For the analysis presented at the meeting, there was a data cutoff in March 2021, at which point 146 patients out of a planned 200 had been enrolled and randomly assigned. They received treatment with either oral ivosidenib 500 mg daily plus azacitidine 75 mg/m2, delivered subcutaneously or intravenously, or azacitidine plus placebo.
In May 2021, after an interim analysis, the independent data-monitoring committee recommended a halt to the trial because of significant improvements in outcomes among patients assigned to the combination, and those data are reported at the meeting.
The median patient age was about 76 years in each group. Approximately 75% of patients in each arm had de novo AML, and about 25% had AML secondary to treatment, myelodysplastic syndrome, or myeloproliferative neoplasms. The majority of patients in each group had intermediate cytogenetic risk disease.
The analysis was by intention to treat, with patients who did not have complete remission (CR) by week 24 considered to have had an event on day 1 of randomization.
At the time of the interim analysis, with the longest follow-up out to 29 months (the investigators did not report median follow-up time for the study), there were significantly fewer study events – defined as treatment failure by week 24, relapse from remission, or death from any cause – in the ivosidenib/azacitidine combination, with a hazard ratio of 0.33 (P = .0011).
The EFS benefit and the overall survival benefit were consistent across subgroups, the researchers noted, including in patients with de novo disease, demographics, baseline cytogenetic risk status, World Health Organization AML classification, baseline white blood cell count, and baseline percentage of bone marrow blasts.
Clinical and hematologic responses also favored the combination, with a complete response (CR) rate of 34%, compared with 11% for azacitidine alone (odds ratio, 4.8; P < .0001), and respective overall response rates of 45% versus 14% (odds ratio, 7.2; P < .0001).
Health-related quality of life measures also trended better with the combination across all subscales, and were significantly better at day 1 of cycle 5 in the diarrhea and appetite loss domains.
Treatment-emergent adverse events included grade 2 or higher differentiation syndrome, which occurred in 14.1% of patients treated with IVO-AZA versus 8.2% treated with AZA alone. Grade 3 or higher QT interval prolongation was also more frequent with the combination, at 9.9% versus 4.1%.
Any-grade infections were less common with IVO-AZA, however, at 28.2% versus 49.3% with AZA alone. At the briefing, this news organization asked coinvestigator Stephane de Botton, MD, Gustave Roussy Cancer Center, Villejuif, France, whether he could explain this seemingly paradoxical result.
He replied that the combination results in greater production of neutrophils and therefore better protection against infections, compared with azacitidine alone.
The study was funded by Agios Pharmaceuticals, now a part of Servier Pharmaceuticals. Dr. Döhner disclosed consultancy and other relationships with various companies. Dr. Sekeres disclosed consulting/advising for Novartis, Takea/Millennium, and Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
ATLANTA – Patients with acute myeloid leukemia (AML) bearing mutations in IDH1 who could not withstand the rigors of intensive therapy had improved event-free and overall survival when they were treated with the combination of ivosidenib (Tibsovo) and azacitidine (Onureg, Vidaza), compared with azacitidine alone.
The results come from the phase 3 AGILE trial., reported Hartmut Döhner, MD, from Ulm (Germany) University Hospital.
Median overall survival was 24 months with the combination, compared with 7.9 months for azacitidine-placebo, translating into a hazard ratio for death with the IVO-AZA of 0.44 (P = .0005).
“The IVO-AZA combination was safe and tolerable, with fewer infections reported relative to placebo plus AZA. Additionally, the clinical benefit of the combination was supported by favorable health-related quality of life,” he said.
Dr. Döhner spoke at a press briefing prior to the presentation of the trial results at the annual meeting of the American Society of Hematology.
“I’m really excited by the results from the AGILE trial,” commented Mikkael A. Sekeres, MD, chief of the division of hematology at the University of Miami’s Sylvester Comprehensive Cancer Center, who moderated the briefing.
The results show “survival that’s three times longer for a combination of ivosidenib plus azacitidine versus azacitidine alone in a very distinct population of patients who have an IDH1 mutation,” he said.
“The question that will arise is, if the standard of care is now to give azacitidine and venetoclax [Venclexta] to patients who don’t receive intensive chemotherapy in the inpatient setting, where does this trial end? And I would answer [by saying] that the combination of azacitidine is not truly nonintensive therapy, and it’s probably on a spectrum between nonintensive and intensive therapy, and probably closer to seven plus three than a lot of people recognize,” Dr. Sekeres said.
The combination of ivosidenib and azacitidine may therefore be a better choice for treatment of older patients with IDH1-mutated AML – particularly those with comorbidities – who may not be able to tolerate venetoclax plus azacitidine, he added.
AGILE results
For the analysis presented at the meeting, there was a data cutoff in March 2021, at which point 146 patients out of a planned 200 had been enrolled and randomly assigned. They received treatment with either oral ivosidenib 500 mg daily plus azacitidine 75 mg/m2, delivered subcutaneously or intravenously, or azacitidine plus placebo.
In May 2021, after an interim analysis, the independent data-monitoring committee recommended a halt to the trial because of significant improvements in outcomes among patients assigned to the combination, and those data are reported at the meeting.
The median patient age was about 76 years in each group. Approximately 75% of patients in each arm had de novo AML, and about 25% had AML secondary to treatment, myelodysplastic syndrome, or myeloproliferative neoplasms. The majority of patients in each group had intermediate cytogenetic risk disease.
The analysis was by intention to treat, with patients who did not have complete remission (CR) by week 24 considered to have had an event on day 1 of randomization.
At the time of the interim analysis, with the longest follow-up out to 29 months (the investigators did not report median follow-up time for the study), there were significantly fewer study events – defined as treatment failure by week 24, relapse from remission, or death from any cause – in the ivosidenib/azacitidine combination, with a hazard ratio of 0.33 (P = .0011).
The EFS benefit and the overall survival benefit were consistent across subgroups, the researchers noted, including in patients with de novo disease, demographics, baseline cytogenetic risk status, World Health Organization AML classification, baseline white blood cell count, and baseline percentage of bone marrow blasts.
Clinical and hematologic responses also favored the combination, with a complete response (CR) rate of 34%, compared with 11% for azacitidine alone (odds ratio, 4.8; P < .0001), and respective overall response rates of 45% versus 14% (odds ratio, 7.2; P < .0001).
Health-related quality of life measures also trended better with the combination across all subscales, and were significantly better at day 1 of cycle 5 in the diarrhea and appetite loss domains.
Treatment-emergent adverse events included grade 2 or higher differentiation syndrome, which occurred in 14.1% of patients treated with IVO-AZA versus 8.2% treated with AZA alone. Grade 3 or higher QT interval prolongation was also more frequent with the combination, at 9.9% versus 4.1%.
Any-grade infections were less common with IVO-AZA, however, at 28.2% versus 49.3% with AZA alone. At the briefing, this news organization asked coinvestigator Stephane de Botton, MD, Gustave Roussy Cancer Center, Villejuif, France, whether he could explain this seemingly paradoxical result.
He replied that the combination results in greater production of neutrophils and therefore better protection against infections, compared with azacitidine alone.
The study was funded by Agios Pharmaceuticals, now a part of Servier Pharmaceuticals. Dr. Döhner disclosed consultancy and other relationships with various companies. Dr. Sekeres disclosed consulting/advising for Novartis, Takea/Millennium, and Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
AT ASH 2021
Isatuximab added to RVd boosts response in new myeloma
ATLANTA -
The drug is isatuximab (Sarclisa, Sanofi), an anti-CD38 antibody that was approved last year for use in patients with advanced disease.
Now it has shown benefit in patients who have been newly diagnosed with the disease. When isatuximab was added onto a usual triplet therapy for myeloma, it increased the likelihood that patients would be negative for minimal residual disease (MRD) at the end of the induction phase of treatment, thereby increasing their chances for a successful autologous stem cell transplant (ASCT).
The new results come from the GMMG-HD7 trial, in which all patients were treated with the triplet combination of lenalidomide (Revlimid), bortezomib (Velcade), and dexamethasone (RVd).
Some patients, after randomization, also received isatuximab, and in this group, the MRD-negativity rate was 50.1% at the end of induction therapy compared with 35.6% for patients treated with RVd alone.
Patients who are MRD-negative at the time of ASCT have significantly better outcomes than patients who remain MRD-positive.
“Isa-RVd is the first regimen to demonstrate significant MRD-negativity benefit at the end of induction versus RVd in a phase 3 trial,” reported Hartmut Goldschmidt, MD, from University Hospital Heidelberg, Germany.
“The benefits of the addition of Isa to RVd versus RVd regarding MRD negativity after induction therapy was consistent in all subgroups,” he added.
Dr. Goldschmidt spoke at a press briefing prior to his presentation of the data here at the annual meeting of the American Society of Hematology (ASH).
“I think that these data are encouraging, but they are preliminary, and we need mature data to be absolutely certain about whether this presents a major advance in treatment,” commented Ravi Vij, MD, from the Siteman Cancer Center and Washington University School of Medicine in St. Louis. Dr. Vij was not involved in the study.
“We know that for transplant-eligible patients, for whom this trial was conducted, the field is moving toward giving four drugs for induction,” he said in an interview with this news organization.
He noted that the combination of RVd with the other currently available anti-CD38 antibody, daratumumab (Darzalex), was approved for this indication in the United States in Jan. 2021.
Dr. Vij said that isatuximab has been slow to catch on in the United States both because it was approved after clinicians had already become familiar with daratumumab and because it is given intravenously, compared with subcutaneous administration of the latest formulation of daratumumab.
“Whereas isatuximab can take an hour-and-a-half with each infusion, daratumumab takes 5 minutes for an injection and the patient is out of there, so it is convenient both for the patient and the treating institution,” he said.
MRD vs. CR?
Dr. Goldschmidt was asked during the briefing about whether MRD-negativity or complete response rates are better predictors of progression-free survival (PFS). He replied that with current standardized sequencing techniques and sensitivity down to 10-6, “it’s a big benefit to analyze MRD negativity, and there is ongoing discussion between colleagues from the myeloma group with the Food and Drug Administration about how we can merge the data and predict PFS and overall survival.”
Laurie Sehn, MD, MPH, from the BC Cancer Centre for Lymphoid Cancer, Vancouver, who moderated the briefing, commented that “we’re desperately looking for surrogate markers to speed up answers to clinical trials, and I think MRD in myeloma is quickly becoming a very important surrogate marker.”
GMMG-7 results
For their trial, Dr. Goldschmidt and colleagues enrolled 662 patients with newly diagnosed multiple myeloma who were candidates for high-dose therapy and ASCT and after stratification by revised International Staging System (r-ISS) criteria, randomly assigned them six three-week cycles of induction therapy with Isa-RVd or RVd alone.
Following ASCT, patients were again randomized to maintenance with either isatuximab plus lenalidomide or lenalidomide alone.
As noted before, MRD rates at the end of induction were 50.1% with Isa-RVd versus 35.6% with RVd alone, translating to a hazard ratio favoring the four-drug combination of 1.83 (P < .001).
Treatment with Isa-RVd was the only significant predictor for the likelihood of MRD negativity in a multivariate analysis controlling for treatment group, r-ISS status, performance status, renal impairment, age, and sex.
Although the rate of complete responses at the end of induction was similar between the treatment groups, the rate of very good partial response or better was higher with the isatuximab-containing combination (77.3% vs. 60.5%; P < .001).
The respective rates of disease progression at the end of induction in the Isa-RVd and RVd groups were 1.5% versus 4.0%.
The rates of adverse events were generally similar between the groups, except a higher proportion of patients had leukocytopenia or neutropenia in the Isa-RVd than the RVdgroup (26.4% vs. 9.1%). There were four deaths in the Isa-RVd group and eight in the RVd group. Most of the deaths were attributable to disease progression or COVID-19, said Dr. Goldschmidt.
The study was funded by Sanofi. Dr. Goldschmidt has disclosed honoraria and research grants from Sanofi and others. Dr. Vij has disclosed honoraria or advisory board activities from various companies, including Sanofi. Dr. Sehn is a consultant for and has received honoraria from various companies, not including Sanofi.
A version of this article first appeared on Medscape.com.
ATLANTA -
The drug is isatuximab (Sarclisa, Sanofi), an anti-CD38 antibody that was approved last year for use in patients with advanced disease.
Now it has shown benefit in patients who have been newly diagnosed with the disease. When isatuximab was added onto a usual triplet therapy for myeloma, it increased the likelihood that patients would be negative for minimal residual disease (MRD) at the end of the induction phase of treatment, thereby increasing their chances for a successful autologous stem cell transplant (ASCT).
The new results come from the GMMG-HD7 trial, in which all patients were treated with the triplet combination of lenalidomide (Revlimid), bortezomib (Velcade), and dexamethasone (RVd).
Some patients, after randomization, also received isatuximab, and in this group, the MRD-negativity rate was 50.1% at the end of induction therapy compared with 35.6% for patients treated with RVd alone.
Patients who are MRD-negative at the time of ASCT have significantly better outcomes than patients who remain MRD-positive.
“Isa-RVd is the first regimen to demonstrate significant MRD-negativity benefit at the end of induction versus RVd in a phase 3 trial,” reported Hartmut Goldschmidt, MD, from University Hospital Heidelberg, Germany.
“The benefits of the addition of Isa to RVd versus RVd regarding MRD negativity after induction therapy was consistent in all subgroups,” he added.
Dr. Goldschmidt spoke at a press briefing prior to his presentation of the data here at the annual meeting of the American Society of Hematology (ASH).
“I think that these data are encouraging, but they are preliminary, and we need mature data to be absolutely certain about whether this presents a major advance in treatment,” commented Ravi Vij, MD, from the Siteman Cancer Center and Washington University School of Medicine in St. Louis. Dr. Vij was not involved in the study.
“We know that for transplant-eligible patients, for whom this trial was conducted, the field is moving toward giving four drugs for induction,” he said in an interview with this news organization.
He noted that the combination of RVd with the other currently available anti-CD38 antibody, daratumumab (Darzalex), was approved for this indication in the United States in Jan. 2021.
Dr. Vij said that isatuximab has been slow to catch on in the United States both because it was approved after clinicians had already become familiar with daratumumab and because it is given intravenously, compared with subcutaneous administration of the latest formulation of daratumumab.
“Whereas isatuximab can take an hour-and-a-half with each infusion, daratumumab takes 5 minutes for an injection and the patient is out of there, so it is convenient both for the patient and the treating institution,” he said.
MRD vs. CR?
Dr. Goldschmidt was asked during the briefing about whether MRD-negativity or complete response rates are better predictors of progression-free survival (PFS). He replied that with current standardized sequencing techniques and sensitivity down to 10-6, “it’s a big benefit to analyze MRD negativity, and there is ongoing discussion between colleagues from the myeloma group with the Food and Drug Administration about how we can merge the data and predict PFS and overall survival.”
Laurie Sehn, MD, MPH, from the BC Cancer Centre for Lymphoid Cancer, Vancouver, who moderated the briefing, commented that “we’re desperately looking for surrogate markers to speed up answers to clinical trials, and I think MRD in myeloma is quickly becoming a very important surrogate marker.”
GMMG-7 results
For their trial, Dr. Goldschmidt and colleagues enrolled 662 patients with newly diagnosed multiple myeloma who were candidates for high-dose therapy and ASCT and after stratification by revised International Staging System (r-ISS) criteria, randomly assigned them six three-week cycles of induction therapy with Isa-RVd or RVd alone.
Following ASCT, patients were again randomized to maintenance with either isatuximab plus lenalidomide or lenalidomide alone.
As noted before, MRD rates at the end of induction were 50.1% with Isa-RVd versus 35.6% with RVd alone, translating to a hazard ratio favoring the four-drug combination of 1.83 (P < .001).
Treatment with Isa-RVd was the only significant predictor for the likelihood of MRD negativity in a multivariate analysis controlling for treatment group, r-ISS status, performance status, renal impairment, age, and sex.
Although the rate of complete responses at the end of induction was similar between the treatment groups, the rate of very good partial response or better was higher with the isatuximab-containing combination (77.3% vs. 60.5%; P < .001).
The respective rates of disease progression at the end of induction in the Isa-RVd and RVd groups were 1.5% versus 4.0%.
The rates of adverse events were generally similar between the groups, except a higher proportion of patients had leukocytopenia or neutropenia in the Isa-RVd than the RVdgroup (26.4% vs. 9.1%). There were four deaths in the Isa-RVd group and eight in the RVd group. Most of the deaths were attributable to disease progression or COVID-19, said Dr. Goldschmidt.
The study was funded by Sanofi. Dr. Goldschmidt has disclosed honoraria and research grants from Sanofi and others. Dr. Vij has disclosed honoraria or advisory board activities from various companies, including Sanofi. Dr. Sehn is a consultant for and has received honoraria from various companies, not including Sanofi.
A version of this article first appeared on Medscape.com.
ATLANTA -
The drug is isatuximab (Sarclisa, Sanofi), an anti-CD38 antibody that was approved last year for use in patients with advanced disease.
Now it has shown benefit in patients who have been newly diagnosed with the disease. When isatuximab was added onto a usual triplet therapy for myeloma, it increased the likelihood that patients would be negative for minimal residual disease (MRD) at the end of the induction phase of treatment, thereby increasing their chances for a successful autologous stem cell transplant (ASCT).
The new results come from the GMMG-HD7 trial, in which all patients were treated with the triplet combination of lenalidomide (Revlimid), bortezomib (Velcade), and dexamethasone (RVd).
Some patients, after randomization, also received isatuximab, and in this group, the MRD-negativity rate was 50.1% at the end of induction therapy compared with 35.6% for patients treated with RVd alone.
Patients who are MRD-negative at the time of ASCT have significantly better outcomes than patients who remain MRD-positive.
“Isa-RVd is the first regimen to demonstrate significant MRD-negativity benefit at the end of induction versus RVd in a phase 3 trial,” reported Hartmut Goldschmidt, MD, from University Hospital Heidelberg, Germany.
“The benefits of the addition of Isa to RVd versus RVd regarding MRD negativity after induction therapy was consistent in all subgroups,” he added.
Dr. Goldschmidt spoke at a press briefing prior to his presentation of the data here at the annual meeting of the American Society of Hematology (ASH).
“I think that these data are encouraging, but they are preliminary, and we need mature data to be absolutely certain about whether this presents a major advance in treatment,” commented Ravi Vij, MD, from the Siteman Cancer Center and Washington University School of Medicine in St. Louis. Dr. Vij was not involved in the study.
“We know that for transplant-eligible patients, for whom this trial was conducted, the field is moving toward giving four drugs for induction,” he said in an interview with this news organization.
He noted that the combination of RVd with the other currently available anti-CD38 antibody, daratumumab (Darzalex), was approved for this indication in the United States in Jan. 2021.
Dr. Vij said that isatuximab has been slow to catch on in the United States both because it was approved after clinicians had already become familiar with daratumumab and because it is given intravenously, compared with subcutaneous administration of the latest formulation of daratumumab.
“Whereas isatuximab can take an hour-and-a-half with each infusion, daratumumab takes 5 minutes for an injection and the patient is out of there, so it is convenient both for the patient and the treating institution,” he said.
MRD vs. CR?
Dr. Goldschmidt was asked during the briefing about whether MRD-negativity or complete response rates are better predictors of progression-free survival (PFS). He replied that with current standardized sequencing techniques and sensitivity down to 10-6, “it’s a big benefit to analyze MRD negativity, and there is ongoing discussion between colleagues from the myeloma group with the Food and Drug Administration about how we can merge the data and predict PFS and overall survival.”
Laurie Sehn, MD, MPH, from the BC Cancer Centre for Lymphoid Cancer, Vancouver, who moderated the briefing, commented that “we’re desperately looking for surrogate markers to speed up answers to clinical trials, and I think MRD in myeloma is quickly becoming a very important surrogate marker.”
GMMG-7 results
For their trial, Dr. Goldschmidt and colleagues enrolled 662 patients with newly diagnosed multiple myeloma who were candidates for high-dose therapy and ASCT and after stratification by revised International Staging System (r-ISS) criteria, randomly assigned them six three-week cycles of induction therapy with Isa-RVd or RVd alone.
Following ASCT, patients were again randomized to maintenance with either isatuximab plus lenalidomide or lenalidomide alone.
As noted before, MRD rates at the end of induction were 50.1% with Isa-RVd versus 35.6% with RVd alone, translating to a hazard ratio favoring the four-drug combination of 1.83 (P < .001).
Treatment with Isa-RVd was the only significant predictor for the likelihood of MRD negativity in a multivariate analysis controlling for treatment group, r-ISS status, performance status, renal impairment, age, and sex.
Although the rate of complete responses at the end of induction was similar between the treatment groups, the rate of very good partial response or better was higher with the isatuximab-containing combination (77.3% vs. 60.5%; P < .001).
The respective rates of disease progression at the end of induction in the Isa-RVd and RVd groups were 1.5% versus 4.0%.
The rates of adverse events were generally similar between the groups, except a higher proportion of patients had leukocytopenia or neutropenia in the Isa-RVd than the RVdgroup (26.4% vs. 9.1%). There were four deaths in the Isa-RVd group and eight in the RVd group. Most of the deaths were attributable to disease progression or COVID-19, said Dr. Goldschmidt.
The study was funded by Sanofi. Dr. Goldschmidt has disclosed honoraria and research grants from Sanofi and others. Dr. Vij has disclosed honoraria or advisory board activities from various companies, including Sanofi. Dr. Sehn is a consultant for and has received honoraria from various companies, not including Sanofi.
A version of this article first appeared on Medscape.com.
AT ASH 2021
Talk early to patients with high-risk AML about end-of-life decisions
The prognosis isn’t good for high-risk AML, defined in the study as either relapsing/recurrent disease or a diagnosis made past the age of 59 years. Almost 60% of the patients (114) died during the 7 years of the study, which started in 2014.
Therefore, it’s important to bring up end-of-life decisions when patients are still able to discuss them, so families aren’t left struggling to guess how aggressive their loved ones might have wanted their final care to be, said lead investigator Hannah Abrams, MD, an internal medicine resident at Massachusetts General. She presented these findings at the annual meeting of the American Society of Hematology.
Much of the time, however, end-of-life discussions come too late. The study team found that nearly 40% (45/114) of the patients who died during the study were not involved in their final code decisions, which most often were to administer comfort care only. Many patients were too ill to participate; the median time between the last code change and death was just 2 days.
Dr. Abrams said she’s seen how families agonize when patients haven’t addressed the issue beforehand. “Witnessing that made me think this is really important to look at. Having these conversations upfront is really important,” she said in an interview.
When asked for comment, hematologist-oncologist Toby Campbell, MD, chief of palliative care at the University of Wisconsin, Madison, agreed.
He called this issue a “missed opportunity for patient autonomy and self-determination. Patients with high-risk AML commonly experience rapid changes in their clinical condition, which catch everyone by surprise. Healthcare providers should do more to prepare patients and families, rather than allow them to be surprised,” Dr. Campbell said.
Part of the problem, Dr. Abrams said, is that end-of-life discussions can fall through the cracks amid urgent discussions about chemotherapy options and other matters.
“One of the biggest things to make this more feasible is to schedule and reimburse time in clinic for this to happen,” she said, noting a need to carve out and protect “15 minutes for patients and clinicians to talk about this.”
Another aspect is that patients are often overly optimistic about their prognoses, so end-of-life discussions don’t seem as pressing. Educational materials about the meaning of various code options and when they are appropriate could help, Dr. Abrams said.
As for the psychological impact of bringing up end-of-life decisions early on, Mikkael Sekeres, MD, chief of the division of hematology at the University of Miami, stressed the importance of telling patients, “We are having this conversation because you are doing well, not because you are doing poorly, and this is the time to have it.”
“Sometimes it does take a sentinel event like an ICU stay before some people want to engage in that conversation, and unfortunately, that is often too late,” said Dr. Sekeres, who moderated Dr. Abrams’ presentation at the meeting.
Among other findings, Dr. Abrams and her team reported that at diagnosis, 86.0% of patients were full-code, and 8.5% had restrictions on life-sustaining therapies. Overall, 57% (114/200) of patients experienced a code status transition, with a median of two transitions during their illness.
Among patients who died, older age and receipt of non-intensive chemotherapy were associated with earlier discussions about code status.
The next step in the project is to determine if palliative care consults yield earlier discussions and greater patient involvement.
There was no commercial funding for the study, and Dr. Abrams and Dr. Campbell didn’t have any relevant disclosures. Dr. Sekeres is an advisor to Novartis, Takeda, and BMS.
[email protected]
The prognosis isn’t good for high-risk AML, defined in the study as either relapsing/recurrent disease or a diagnosis made past the age of 59 years. Almost 60% of the patients (114) died during the 7 years of the study, which started in 2014.
Therefore, it’s important to bring up end-of-life decisions when patients are still able to discuss them, so families aren’t left struggling to guess how aggressive their loved ones might have wanted their final care to be, said lead investigator Hannah Abrams, MD, an internal medicine resident at Massachusetts General. She presented these findings at the annual meeting of the American Society of Hematology.
Much of the time, however, end-of-life discussions come too late. The study team found that nearly 40% (45/114) of the patients who died during the study were not involved in their final code decisions, which most often were to administer comfort care only. Many patients were too ill to participate; the median time between the last code change and death was just 2 days.
Dr. Abrams said she’s seen how families agonize when patients haven’t addressed the issue beforehand. “Witnessing that made me think this is really important to look at. Having these conversations upfront is really important,” she said in an interview.
When asked for comment, hematologist-oncologist Toby Campbell, MD, chief of palliative care at the University of Wisconsin, Madison, agreed.
He called this issue a “missed opportunity for patient autonomy and self-determination. Patients with high-risk AML commonly experience rapid changes in their clinical condition, which catch everyone by surprise. Healthcare providers should do more to prepare patients and families, rather than allow them to be surprised,” Dr. Campbell said.
Part of the problem, Dr. Abrams said, is that end-of-life discussions can fall through the cracks amid urgent discussions about chemotherapy options and other matters.
“One of the biggest things to make this more feasible is to schedule and reimburse time in clinic for this to happen,” she said, noting a need to carve out and protect “15 minutes for patients and clinicians to talk about this.”
Another aspect is that patients are often overly optimistic about their prognoses, so end-of-life discussions don’t seem as pressing. Educational materials about the meaning of various code options and when they are appropriate could help, Dr. Abrams said.
As for the psychological impact of bringing up end-of-life decisions early on, Mikkael Sekeres, MD, chief of the division of hematology at the University of Miami, stressed the importance of telling patients, “We are having this conversation because you are doing well, not because you are doing poorly, and this is the time to have it.”
“Sometimes it does take a sentinel event like an ICU stay before some people want to engage in that conversation, and unfortunately, that is often too late,” said Dr. Sekeres, who moderated Dr. Abrams’ presentation at the meeting.
Among other findings, Dr. Abrams and her team reported that at diagnosis, 86.0% of patients were full-code, and 8.5% had restrictions on life-sustaining therapies. Overall, 57% (114/200) of patients experienced a code status transition, with a median of two transitions during their illness.
Among patients who died, older age and receipt of non-intensive chemotherapy were associated with earlier discussions about code status.
The next step in the project is to determine if palliative care consults yield earlier discussions and greater patient involvement.
There was no commercial funding for the study, and Dr. Abrams and Dr. Campbell didn’t have any relevant disclosures. Dr. Sekeres is an advisor to Novartis, Takeda, and BMS.
[email protected]
The prognosis isn’t good for high-risk AML, defined in the study as either relapsing/recurrent disease or a diagnosis made past the age of 59 years. Almost 60% of the patients (114) died during the 7 years of the study, which started in 2014.
Therefore, it’s important to bring up end-of-life decisions when patients are still able to discuss them, so families aren’t left struggling to guess how aggressive their loved ones might have wanted their final care to be, said lead investigator Hannah Abrams, MD, an internal medicine resident at Massachusetts General. She presented these findings at the annual meeting of the American Society of Hematology.
Much of the time, however, end-of-life discussions come too late. The study team found that nearly 40% (45/114) of the patients who died during the study were not involved in their final code decisions, which most often were to administer comfort care only. Many patients were too ill to participate; the median time between the last code change and death was just 2 days.
Dr. Abrams said she’s seen how families agonize when patients haven’t addressed the issue beforehand. “Witnessing that made me think this is really important to look at. Having these conversations upfront is really important,” she said in an interview.
When asked for comment, hematologist-oncologist Toby Campbell, MD, chief of palliative care at the University of Wisconsin, Madison, agreed.
He called this issue a “missed opportunity for patient autonomy and self-determination. Patients with high-risk AML commonly experience rapid changes in their clinical condition, which catch everyone by surprise. Healthcare providers should do more to prepare patients and families, rather than allow them to be surprised,” Dr. Campbell said.
Part of the problem, Dr. Abrams said, is that end-of-life discussions can fall through the cracks amid urgent discussions about chemotherapy options and other matters.
“One of the biggest things to make this more feasible is to schedule and reimburse time in clinic for this to happen,” she said, noting a need to carve out and protect “15 minutes for patients and clinicians to talk about this.”
Another aspect is that patients are often overly optimistic about their prognoses, so end-of-life discussions don’t seem as pressing. Educational materials about the meaning of various code options and when they are appropriate could help, Dr. Abrams said.
As for the psychological impact of bringing up end-of-life decisions early on, Mikkael Sekeres, MD, chief of the division of hematology at the University of Miami, stressed the importance of telling patients, “We are having this conversation because you are doing well, not because you are doing poorly, and this is the time to have it.”
“Sometimes it does take a sentinel event like an ICU stay before some people want to engage in that conversation, and unfortunately, that is often too late,” said Dr. Sekeres, who moderated Dr. Abrams’ presentation at the meeting.
Among other findings, Dr. Abrams and her team reported that at diagnosis, 86.0% of patients were full-code, and 8.5% had restrictions on life-sustaining therapies. Overall, 57% (114/200) of patients experienced a code status transition, with a median of two transitions during their illness.
Among patients who died, older age and receipt of non-intensive chemotherapy were associated with earlier discussions about code status.
The next step in the project is to determine if palliative care consults yield earlier discussions and greater patient involvement.
There was no commercial funding for the study, and Dr. Abrams and Dr. Campbell didn’t have any relevant disclosures. Dr. Sekeres is an advisor to Novartis, Takeda, and BMS.
[email protected]
FROM ASH 2021