User login
Post-COVID Cough Linked to Neurological Dysfunction
Chronic cough remains a common reason for consultation in pulmonology post–COVID-19. But what do we really know about this condition, now 5 years after the pandemic’s onset? This topic was discussed at the recent French-Speaking Pneumology Congress held in Marseille, France, from January 24-26, 2025.
Before discussing post-COVID cough, it is crucial to differentiate between an acute cough, often viral in origin (including those associated with SARS-CoV-2), a subacute cough (lasting 3-8 weeks), and a chronic cough (persisting over 8 weeks).
“This distinction allows us to tailor treatment and prescribe the appropriate investigations, according to the duration and the probability of symptom resolution,” explained Laurent Guilleminault, MD, PhD, pulmonologist at Toulouse University Hospital Centre, Toulouse, France.
In the case of an acute cough, for instance, after a viral infection, the probability of spontaneous resolution is very high. It is often unnecessary to carry out additional examinations or initiate specific treatments because none has proven its effectiveness in shortening this type of cough. On the other hand, when a cough persists beyond 8 weeks, the chance of spontaneous resolution decreases considerably. “This is when an assessment is necessary to identify a possible underlying cause,” Guilleminault noted.
“The absence of coughing during the consultation should not lead to ruling out a diagnosis,” he added.
Neurological Link
A large-scale French study of 70,000 patients examined the demographic profiles of patients with COVID-19. It revealed a lower frequency of coughing among children and older individuals, with a notable prevalence among adults aged 30-60 years.
Furthermore, during the acute phase of COVID, coughing did not appear to indicate severity. A comparison between survivors and nonsurvivors revealed no significant differences in the frequency and severity of coughing. Another study concluded that, contrary to expectations, COVID-related pneumonia, although potentially severe, does not necessarily involve severe cough.
These findings highlight the absence of a direct link between coughing and pulmonary involvement in patients with COVID-19.
“Coughing appears to be more closely linked to neurological dysfunction than to classic respiratory involvement. A distinction that is essential for better understanding the pathophysiology of the disease and guiding therapeutic strategies,” Guilleminault noted.
Cough Mechanism
“The analysis of cough in the context of phylogenetic evolution is fascinating,” explained Guilleminault. “It illustrates how this reflex has provided an advantage to the virus for its propagation.” Studies on the transmission of SARS-CoV-2 have confirmed that coughing plays a key role in the spread of viral particles. However, this mechanism does not involve severe pulmonary damage. The primary goal of the virus is to induce neurological dysfunction in the host by triggering a cough reflex. This neurological activation enables the virus to trigger a cough reflex for dissemination even without significant pulmonary damage. This mechanism provides an evolutionary advantage by enhancing the ability of the virus to spread and colonize new hosts.
The cough mechanism remains partially understood and involves cough hypersensitivity, characterized by increased neural responsivity to a range of stimuli that affect the airways, lungs, and other tissues innervated by common nerve supplies. The cough reflex begins with the activation of sensitive peripheral receptors located mainly in the respiratory tract that detect irritants or abnormalities.
These receptors, such as P2X2, P2X3, and others, transmit information to the brainstem, which coordinates the reflex response. This process is modulated by cortical controls that normally inhibit spontaneous coughing, explaining why we do not cough constantly even in the presence of moderate stimuli.
However, when there is an imbalance in this inhibition mechanism, coughing can be triggered either excessively or uncontrollably. SARS-CoV-2 appears to interact directly with these peripheral receptors, stimulating the cough reflex. The widespread presence and density of these receptors make this mechanism highly effective for the virus’s transmission.
Additionally, the vagus nerve likely plays a central role in triggering cough, particularly in viral infections. Studies of influenza have shown the involvement of sensory cells associated with the vagus nerve.
The virus stimulates the vagus nerve, which activates the cough reflex. Research suggests that neurotropism, neuroinflammation, and neuroimmunomodulation via the vagal sensory nerves, which are involved in SARS-CoV-2 infection, lead to cough hypersensitivity.
One question remains: Could vagus nerve involvement prolong coughing beyond the active phase of viral infection? The data indicate that viral infection significantly increases the sensitivity of the cough reflex, regardless of the level of irritation. The brain areas involved in inhibiting this reflex appear less effective during viral infection, resulting in reduced inhibitory control and easier triggering of cough. This phenomenon reflects temporary dysfunction of the neurological modulation system, which gradually recovers after recovery.
Long-Term Effects
The epidemiology of post-COVID cough and its integration into the framework of the long COVID framework remain subjects of ongoing debate. Early studies have revealed that cough could be either an isolated symptom or associated with other manifestations of long COVID. These studies were often conducted over relatively short periods (14-110 days) and estimated that approximately 19% of patients with long COVID experienced persistent cough. Another study found that 14% of patients reported cough between 3 weeks and 3 months after hospital discharge for COVID-19.
Longer follow-up periods showed a significant decrease in the prevalence of cough over time. For instance, a 1-year study reported that only 2.5% of patients had episodes of chronic cough.
However, a 2023 study published in JAMA found that the prevalence of post-COVID chronic cough exceeded 30% in some groups of patients.
“It is not relevant to wait so long before acting,” Guilleminault said. A reasonable threshold for evaluation and treatment is 8-12 weeks postinfection to begin investigations and consider appropriate treatment. What should be done when a patient presents with “Doctor, I had COVID, I have a cough, and it hasn’t stopped?” These situations are common in clinical practice. In terms of severity, quality of life, and overall impact, patients with chronic post-COVID cough are not significantly different from those with other chronic coughs. Moreover, both conditions involve a real neurological dysfunction.
Same Diagnostic Steps
Management should follow existing guidelines, including the recent French recommendations for chronic cough.
A visual analog scale can be used, and possible complications should be assessed. A chest x-ray is recommended to identify any warning signs, such as cough, although linked to COVID — may coincide with other conditions, such as bronchial cancer. In smokers, chest CT should be considered to rule out neoplastic pathology. The presence of interstitial lesions, particularly fibrosing lesions, suggests that fibrosing interstitial pneumonia requires specialized management.
Smoking, which is an aggravating factor, should be discontinued. Discontinuing angiotensin-converting enzyme inhibitors for 4 weeks can help determine if they contribute to cough.
The three most common causes of chronic cough — rhinosinusitis, asthma, and gastroesophageal reflux disease — should be ruled out. Diagnosis is based on history, physical examination, and specific tests: Nasofibroscopy for rhinosinusitis, spirometry, fractional exhaled nitric oxide for asthma and clinical history of gastroesophageal reflux disease. Studies have indicated that asthma may develop after a COVID infection.
Laryngeal abnormalities are also common in chronic post-COVID cough. One study found that a quarter of patients had increased laryngeal sensitivity or voice changes. “The larynx, a highly cough-producing organ, causes more coughing than the lungs,” Guilleminault explained.
Laryngeal abnormalities are frequently observed. A study found that 63% of patients experienced dysphonia, 56% had a sensation of a foreign body in the larynx, and 10% experienced laryngospasms.
These issues are common in patients with post-COVID cough and are often associated with neurological dysfunction. Innervation of the larynx is complex and can be affected by viruses, leading to hypersensitivity, paresthesia, and other sensory disturbances, which may explain the laryngeal symptoms observed in these patients.
Next Steps
If common causes such as asthma, abnormal imaging findings, or laryngeal pathology are ruled out, the condition may be classified as a chronic refractory or unexplained cough. In these cases, the neurological origin is likely due to nervous system dysfunction. Neuromodulatory treatments including amitriptyline, pregabalin, and gabapentin may be considered in some cases. Corticosteroids are generally ineffective against chronic coughs.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Chronic cough remains a common reason for consultation in pulmonology post–COVID-19. But what do we really know about this condition, now 5 years after the pandemic’s onset? This topic was discussed at the recent French-Speaking Pneumology Congress held in Marseille, France, from January 24-26, 2025.
Before discussing post-COVID cough, it is crucial to differentiate between an acute cough, often viral in origin (including those associated with SARS-CoV-2), a subacute cough (lasting 3-8 weeks), and a chronic cough (persisting over 8 weeks).
“This distinction allows us to tailor treatment and prescribe the appropriate investigations, according to the duration and the probability of symptom resolution,” explained Laurent Guilleminault, MD, PhD, pulmonologist at Toulouse University Hospital Centre, Toulouse, France.
In the case of an acute cough, for instance, after a viral infection, the probability of spontaneous resolution is very high. It is often unnecessary to carry out additional examinations or initiate specific treatments because none has proven its effectiveness in shortening this type of cough. On the other hand, when a cough persists beyond 8 weeks, the chance of spontaneous resolution decreases considerably. “This is when an assessment is necessary to identify a possible underlying cause,” Guilleminault noted.
“The absence of coughing during the consultation should not lead to ruling out a diagnosis,” he added.
Neurological Link
A large-scale French study of 70,000 patients examined the demographic profiles of patients with COVID-19. It revealed a lower frequency of coughing among children and older individuals, with a notable prevalence among adults aged 30-60 years.
Furthermore, during the acute phase of COVID, coughing did not appear to indicate severity. A comparison between survivors and nonsurvivors revealed no significant differences in the frequency and severity of coughing. Another study concluded that, contrary to expectations, COVID-related pneumonia, although potentially severe, does not necessarily involve severe cough.
These findings highlight the absence of a direct link between coughing and pulmonary involvement in patients with COVID-19.
“Coughing appears to be more closely linked to neurological dysfunction than to classic respiratory involvement. A distinction that is essential for better understanding the pathophysiology of the disease and guiding therapeutic strategies,” Guilleminault noted.
Cough Mechanism
“The analysis of cough in the context of phylogenetic evolution is fascinating,” explained Guilleminault. “It illustrates how this reflex has provided an advantage to the virus for its propagation.” Studies on the transmission of SARS-CoV-2 have confirmed that coughing plays a key role in the spread of viral particles. However, this mechanism does not involve severe pulmonary damage. The primary goal of the virus is to induce neurological dysfunction in the host by triggering a cough reflex. This neurological activation enables the virus to trigger a cough reflex for dissemination even without significant pulmonary damage. This mechanism provides an evolutionary advantage by enhancing the ability of the virus to spread and colonize new hosts.
The cough mechanism remains partially understood and involves cough hypersensitivity, characterized by increased neural responsivity to a range of stimuli that affect the airways, lungs, and other tissues innervated by common nerve supplies. The cough reflex begins with the activation of sensitive peripheral receptors located mainly in the respiratory tract that detect irritants or abnormalities.
These receptors, such as P2X2, P2X3, and others, transmit information to the brainstem, which coordinates the reflex response. This process is modulated by cortical controls that normally inhibit spontaneous coughing, explaining why we do not cough constantly even in the presence of moderate stimuli.
However, when there is an imbalance in this inhibition mechanism, coughing can be triggered either excessively or uncontrollably. SARS-CoV-2 appears to interact directly with these peripheral receptors, stimulating the cough reflex. The widespread presence and density of these receptors make this mechanism highly effective for the virus’s transmission.
Additionally, the vagus nerve likely plays a central role in triggering cough, particularly in viral infections. Studies of influenza have shown the involvement of sensory cells associated with the vagus nerve.
The virus stimulates the vagus nerve, which activates the cough reflex. Research suggests that neurotropism, neuroinflammation, and neuroimmunomodulation via the vagal sensory nerves, which are involved in SARS-CoV-2 infection, lead to cough hypersensitivity.
One question remains: Could vagus nerve involvement prolong coughing beyond the active phase of viral infection? The data indicate that viral infection significantly increases the sensitivity of the cough reflex, regardless of the level of irritation. The brain areas involved in inhibiting this reflex appear less effective during viral infection, resulting in reduced inhibitory control and easier triggering of cough. This phenomenon reflects temporary dysfunction of the neurological modulation system, which gradually recovers after recovery.
Long-Term Effects
The epidemiology of post-COVID cough and its integration into the framework of the long COVID framework remain subjects of ongoing debate. Early studies have revealed that cough could be either an isolated symptom or associated with other manifestations of long COVID. These studies were often conducted over relatively short periods (14-110 days) and estimated that approximately 19% of patients with long COVID experienced persistent cough. Another study found that 14% of patients reported cough between 3 weeks and 3 months after hospital discharge for COVID-19.
Longer follow-up periods showed a significant decrease in the prevalence of cough over time. For instance, a 1-year study reported that only 2.5% of patients had episodes of chronic cough.
However, a 2023 study published in JAMA found that the prevalence of post-COVID chronic cough exceeded 30% in some groups of patients.
“It is not relevant to wait so long before acting,” Guilleminault said. A reasonable threshold for evaluation and treatment is 8-12 weeks postinfection to begin investigations and consider appropriate treatment. What should be done when a patient presents with “Doctor, I had COVID, I have a cough, and it hasn’t stopped?” These situations are common in clinical practice. In terms of severity, quality of life, and overall impact, patients with chronic post-COVID cough are not significantly different from those with other chronic coughs. Moreover, both conditions involve a real neurological dysfunction.
Same Diagnostic Steps
Management should follow existing guidelines, including the recent French recommendations for chronic cough.
A visual analog scale can be used, and possible complications should be assessed. A chest x-ray is recommended to identify any warning signs, such as cough, although linked to COVID — may coincide with other conditions, such as bronchial cancer. In smokers, chest CT should be considered to rule out neoplastic pathology. The presence of interstitial lesions, particularly fibrosing lesions, suggests that fibrosing interstitial pneumonia requires specialized management.
Smoking, which is an aggravating factor, should be discontinued. Discontinuing angiotensin-converting enzyme inhibitors for 4 weeks can help determine if they contribute to cough.
The three most common causes of chronic cough — rhinosinusitis, asthma, and gastroesophageal reflux disease — should be ruled out. Diagnosis is based on history, physical examination, and specific tests: Nasofibroscopy for rhinosinusitis, spirometry, fractional exhaled nitric oxide for asthma and clinical history of gastroesophageal reflux disease. Studies have indicated that asthma may develop after a COVID infection.
Laryngeal abnormalities are also common in chronic post-COVID cough. One study found that a quarter of patients had increased laryngeal sensitivity or voice changes. “The larynx, a highly cough-producing organ, causes more coughing than the lungs,” Guilleminault explained.
Laryngeal abnormalities are frequently observed. A study found that 63% of patients experienced dysphonia, 56% had a sensation of a foreign body in the larynx, and 10% experienced laryngospasms.
These issues are common in patients with post-COVID cough and are often associated with neurological dysfunction. Innervation of the larynx is complex and can be affected by viruses, leading to hypersensitivity, paresthesia, and other sensory disturbances, which may explain the laryngeal symptoms observed in these patients.
Next Steps
If common causes such as asthma, abnormal imaging findings, or laryngeal pathology are ruled out, the condition may be classified as a chronic refractory or unexplained cough. In these cases, the neurological origin is likely due to nervous system dysfunction. Neuromodulatory treatments including amitriptyline, pregabalin, and gabapentin may be considered in some cases. Corticosteroids are generally ineffective against chronic coughs.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Chronic cough remains a common reason for consultation in pulmonology post–COVID-19. But what do we really know about this condition, now 5 years after the pandemic’s onset? This topic was discussed at the recent French-Speaking Pneumology Congress held in Marseille, France, from January 24-26, 2025.
Before discussing post-COVID cough, it is crucial to differentiate between an acute cough, often viral in origin (including those associated with SARS-CoV-2), a subacute cough (lasting 3-8 weeks), and a chronic cough (persisting over 8 weeks).
“This distinction allows us to tailor treatment and prescribe the appropriate investigations, according to the duration and the probability of symptom resolution,” explained Laurent Guilleminault, MD, PhD, pulmonologist at Toulouse University Hospital Centre, Toulouse, France.
In the case of an acute cough, for instance, after a viral infection, the probability of spontaneous resolution is very high. It is often unnecessary to carry out additional examinations or initiate specific treatments because none has proven its effectiveness in shortening this type of cough. On the other hand, when a cough persists beyond 8 weeks, the chance of spontaneous resolution decreases considerably. “This is when an assessment is necessary to identify a possible underlying cause,” Guilleminault noted.
“The absence of coughing during the consultation should not lead to ruling out a diagnosis,” he added.
Neurological Link
A large-scale French study of 70,000 patients examined the demographic profiles of patients with COVID-19. It revealed a lower frequency of coughing among children and older individuals, with a notable prevalence among adults aged 30-60 years.
Furthermore, during the acute phase of COVID, coughing did not appear to indicate severity. A comparison between survivors and nonsurvivors revealed no significant differences in the frequency and severity of coughing. Another study concluded that, contrary to expectations, COVID-related pneumonia, although potentially severe, does not necessarily involve severe cough.
These findings highlight the absence of a direct link between coughing and pulmonary involvement in patients with COVID-19.
“Coughing appears to be more closely linked to neurological dysfunction than to classic respiratory involvement. A distinction that is essential for better understanding the pathophysiology of the disease and guiding therapeutic strategies,” Guilleminault noted.
Cough Mechanism
“The analysis of cough in the context of phylogenetic evolution is fascinating,” explained Guilleminault. “It illustrates how this reflex has provided an advantage to the virus for its propagation.” Studies on the transmission of SARS-CoV-2 have confirmed that coughing plays a key role in the spread of viral particles. However, this mechanism does not involve severe pulmonary damage. The primary goal of the virus is to induce neurological dysfunction in the host by triggering a cough reflex. This neurological activation enables the virus to trigger a cough reflex for dissemination even without significant pulmonary damage. This mechanism provides an evolutionary advantage by enhancing the ability of the virus to spread and colonize new hosts.
The cough mechanism remains partially understood and involves cough hypersensitivity, characterized by increased neural responsivity to a range of stimuli that affect the airways, lungs, and other tissues innervated by common nerve supplies. The cough reflex begins with the activation of sensitive peripheral receptors located mainly in the respiratory tract that detect irritants or abnormalities.
These receptors, such as P2X2, P2X3, and others, transmit information to the brainstem, which coordinates the reflex response. This process is modulated by cortical controls that normally inhibit spontaneous coughing, explaining why we do not cough constantly even in the presence of moderate stimuli.
However, when there is an imbalance in this inhibition mechanism, coughing can be triggered either excessively or uncontrollably. SARS-CoV-2 appears to interact directly with these peripheral receptors, stimulating the cough reflex. The widespread presence and density of these receptors make this mechanism highly effective for the virus’s transmission.
Additionally, the vagus nerve likely plays a central role in triggering cough, particularly in viral infections. Studies of influenza have shown the involvement of sensory cells associated with the vagus nerve.
The virus stimulates the vagus nerve, which activates the cough reflex. Research suggests that neurotropism, neuroinflammation, and neuroimmunomodulation via the vagal sensory nerves, which are involved in SARS-CoV-2 infection, lead to cough hypersensitivity.
One question remains: Could vagus nerve involvement prolong coughing beyond the active phase of viral infection? The data indicate that viral infection significantly increases the sensitivity of the cough reflex, regardless of the level of irritation. The brain areas involved in inhibiting this reflex appear less effective during viral infection, resulting in reduced inhibitory control and easier triggering of cough. This phenomenon reflects temporary dysfunction of the neurological modulation system, which gradually recovers after recovery.
Long-Term Effects
The epidemiology of post-COVID cough and its integration into the framework of the long COVID framework remain subjects of ongoing debate. Early studies have revealed that cough could be either an isolated symptom or associated with other manifestations of long COVID. These studies were often conducted over relatively short periods (14-110 days) and estimated that approximately 19% of patients with long COVID experienced persistent cough. Another study found that 14% of patients reported cough between 3 weeks and 3 months after hospital discharge for COVID-19.
Longer follow-up periods showed a significant decrease in the prevalence of cough over time. For instance, a 1-year study reported that only 2.5% of patients had episodes of chronic cough.
However, a 2023 study published in JAMA found that the prevalence of post-COVID chronic cough exceeded 30% in some groups of patients.
“It is not relevant to wait so long before acting,” Guilleminault said. A reasonable threshold for evaluation and treatment is 8-12 weeks postinfection to begin investigations and consider appropriate treatment. What should be done when a patient presents with “Doctor, I had COVID, I have a cough, and it hasn’t stopped?” These situations are common in clinical practice. In terms of severity, quality of life, and overall impact, patients with chronic post-COVID cough are not significantly different from those with other chronic coughs. Moreover, both conditions involve a real neurological dysfunction.
Same Diagnostic Steps
Management should follow existing guidelines, including the recent French recommendations for chronic cough.
A visual analog scale can be used, and possible complications should be assessed. A chest x-ray is recommended to identify any warning signs, such as cough, although linked to COVID — may coincide with other conditions, such as bronchial cancer. In smokers, chest CT should be considered to rule out neoplastic pathology. The presence of interstitial lesions, particularly fibrosing lesions, suggests that fibrosing interstitial pneumonia requires specialized management.
Smoking, which is an aggravating factor, should be discontinued. Discontinuing angiotensin-converting enzyme inhibitors for 4 weeks can help determine if they contribute to cough.
The three most common causes of chronic cough — rhinosinusitis, asthma, and gastroesophageal reflux disease — should be ruled out. Diagnosis is based on history, physical examination, and specific tests: Nasofibroscopy for rhinosinusitis, spirometry, fractional exhaled nitric oxide for asthma and clinical history of gastroesophageal reflux disease. Studies have indicated that asthma may develop after a COVID infection.
Laryngeal abnormalities are also common in chronic post-COVID cough. One study found that a quarter of patients had increased laryngeal sensitivity or voice changes. “The larynx, a highly cough-producing organ, causes more coughing than the lungs,” Guilleminault explained.
Laryngeal abnormalities are frequently observed. A study found that 63% of patients experienced dysphonia, 56% had a sensation of a foreign body in the larynx, and 10% experienced laryngospasms.
These issues are common in patients with post-COVID cough and are often associated with neurological dysfunction. Innervation of the larynx is complex and can be affected by viruses, leading to hypersensitivity, paresthesia, and other sensory disturbances, which may explain the laryngeal symptoms observed in these patients.
Next Steps
If common causes such as asthma, abnormal imaging findings, or laryngeal pathology are ruled out, the condition may be classified as a chronic refractory or unexplained cough. In these cases, the neurological origin is likely due to nervous system dysfunction. Neuromodulatory treatments including amitriptyline, pregabalin, and gabapentin may be considered in some cases. Corticosteroids are generally ineffective against chronic coughs.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Updated Alzheimer’s Guidelines Chart the Full Diagnostic Journey
This is the first update since 2001 for specialists and the first guideline for primary care physicians. Executive summaries of the guidelines were published in three articles online on December 23 in a special issue of Alzheimer’s & Dementia.
What’s New?
“With this guideline, we expand the scope of prior guidelines by providing recommendations for practicing clinicians on the process from start to finish,” coauthor Brad Dickerson, MD, director of the Massachusetts General Hospital Frontotemporal Disorders Unit and professor of neurology at Harvard Medical School, Boston, said in a statement.
“If clinicians adopt these recommendations and healthcare systems provide adequate resources, outcomes should improve in most patients in most practice settings,” Dickerson added in an interview.
Through a modified-Delphi approach and guideline-development process, an expert workgroup representing primary and specialty care reviewed 7374 publications, of which 133 met inclusion criteria.
Based on the information, the workgroup outlined a three-step patient-centered evaluation process, which includes assessing cognitive functional status, identifying the cognitive-behavioral syndrome based on specific symptoms, and determining the likely brain diseases or conditions causing the symptoms.
What Are the Key Recommendations?
The guidelines include 19 “practical” recommendations that are applicable to any practice setting. They capture the core elements of a high-quality evaluation and disclosure process, the author said. Here is a brief summary of the recommendations:
Initial evaluation: Perform a multitiered evaluation for patients who self-report or whose care partner or clinician reports cognitive, behavioral, or functional changes.
Patient-centered communication: Partner with the patient and/or care partner to establish shared goals for the evaluation process; assess the patient’s capacity to engage in goal setting.
Diagnostic formulation: Use a tiered approach to assessments and tests based on individual presentation, risk factors, and profile, aiming to determine the level of impairment, cognitive-behavioral syndrome, and likely causes and contributing factors.
History taking: Gather reliable information from informants about changes in cognition, activities of daily living, mood, neuropsychiatric symptoms, and sensory/motor functions. Document individualized risk factors for cognitive decline.
Examination: Conduct a comprehensive examination of cognition, mood, behavior, and a dementia-focused neurologic evaluation using validated tools.
Laboratory tests: Perform tiered, individualized laboratory evaluations, starting with routine tests for all patients.
Structural imaging: Obtain structural brain imaging (MRI preferred, CT as an alternative) to help establish a cause.
Ongoing communication: Engage in ongoing dialogue with patient/care partner to guide them throughout the diagnostic process.
Diagnostic disclosure: Share findings honestly and compassionately, explaining the syndrome, its severity, probable cause, prognosis, treatment options and support resources.
Specialist referral: Refer patients with atypical, uncertain, early-onset, or rapidly progressing symptoms to a dementia subspecialist.
Neuropsychological testing: Use in instances of diagnostic uncertainty or patients with complex clinical profiles. At a minimum, the neuropsychological evaluation should include normed neuropsychological testing of the domains of learning and memory (in particular delayed free and cued recall/recognition), attention, executive function, visuospatial function, and language.
Advanced diagnostic testing: When diagnostic uncertainty remains, obtain additional laboratory tests tailored to individual patient profiles.
Molecular imaging: In a patient with an established cognitive-behavioral syndrome in whom there is continued diagnostic uncertainty regarding cause(s) after structural imaging, a dementia specialist can obtain molecular imaging with fluorodeoxyglucose PET to improve diagnostic accuracy.
Cerebrospinal fluid (CSF) analysis: Utilize CSF biomarkers to evaluate amyloid beta and tau profiles in cases with unresolved diagnostic uncertainty.
Amyloid PET imaging: Perform amyloid PET scans for patients with persistent diagnostic uncertainty after other assessments.
Genetic counseling and testing: Consider genetic testing for patients with strong autosomal dominant family histories and involve a genetic counselor.
Future Directions?
Maria C. Carrillo, PhD, chief science officer and medical affairs lead for the Alzheimer’s Association, encourages clinicians to incorporate these guidelines into their practice.
“These guidelines are important because they guide clinicians in the evaluation of memory complaints, which could have many underlying causes. That is the necessary start for an early and accurate Alzheimer’s diagnosis,” Carrillo said in a statement.
Dickerson said the new guidelines do not address blood-based biomarkers “because nobody really feels that they are ready for prime time yet, even though they’re getting rolled out as clinical products.”
However, the recommendations will be revised as needed. “That’s one of the values of setting this up as a process; whenever any new development occurs, it will be easy to update the guidelines to show where that new test or new biomarker fits in the overall process,” he said.
New Appropriate Use Guidance
A separate workgroup, jointly convened by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging, has revised appropriate use criteria (AUC) for amyloid PET imaging and developed AUC for tau PET imaging.
They were simultaneously published online in Alzheimer’s & Dementia and The Journal of Nuclear Medicine. They are the first revision since the initial AUC for amyloid PET was introduced in 2013.
“The updated amyloid/tau appropriate use criteria will help ensure these tracers are used in a cost-effective manner and the scan results will be used appropriately to add value to the diagnosis and management of dementia,” said workgroup members Kevin Donohoe, MD, with Beth Israel Deaconess Medical Center, Boston, and Phillip Kuo, MD, with City of Hope National Medical Center, Duarte, California.
The AUC include 17 real-world scenarios in which amyloid or tau PET may be considered, with the two tests considered separately and given their own rating for each scenario.
Overall, the strongest evidence for their use includes assessment and prognosis for people with mild cognitive impairment; assessment of people with dementia when the cause is not clearly known; and determining eligibility for treatment with new disease-modifying therapies, and monitoring response to these treatments, the workgroup said.
“Whereas the prior AUC was written at a time when only the deposition of amyloid could be documented, the new therapeutic agents allow us to demonstrate the actual clearance of amyloid during therapy,” Donohoe and Kuo explained.
“These new therapeutic agents are expensive and, as with most medications, may cause unwanted side effects. The most recent version of the AUC includes information about the appropriate use of amyloid imaging for both documenting the presence of amyloid deposits in the brain, making anti-amyloid therapy an option, as well as documenting the effectiveness of the therapeutic agents as amyloid is (or is not) cleared from the brain,” Donahoe and Kuo noted.
The revised AUC also state that, in most cases, amyloid and tau PET tests should not be used for people who do not have cognitive impairment, even if they carry the APOE4 risk-related gene for Alzheimer’s disease; nonmedical use such as for legal concerns, insurance coverage, or employment screening; and in place of genetic testing in patients suspected of carrying a disease-causing genetic mutation.
In a statement, lead author Gil D. Rabinovici, MD, with University of California, San Francisco, emphasized that the AUC “should be considered guidelines for clinicians, not a substitute for careful clinical judgment that considers the full clinical context for each patient with cognitive complaints.”
This research was funded by the Alzheimer’s Association. Disclosures for guideline authors are available with the original articles.
A version of this article first appeared on Medscape.com.
This is the first update since 2001 for specialists and the first guideline for primary care physicians. Executive summaries of the guidelines were published in three articles online on December 23 in a special issue of Alzheimer’s & Dementia.
What’s New?
“With this guideline, we expand the scope of prior guidelines by providing recommendations for practicing clinicians on the process from start to finish,” coauthor Brad Dickerson, MD, director of the Massachusetts General Hospital Frontotemporal Disorders Unit and professor of neurology at Harvard Medical School, Boston, said in a statement.
“If clinicians adopt these recommendations and healthcare systems provide adequate resources, outcomes should improve in most patients in most practice settings,” Dickerson added in an interview.
Through a modified-Delphi approach and guideline-development process, an expert workgroup representing primary and specialty care reviewed 7374 publications, of which 133 met inclusion criteria.
Based on the information, the workgroup outlined a three-step patient-centered evaluation process, which includes assessing cognitive functional status, identifying the cognitive-behavioral syndrome based on specific symptoms, and determining the likely brain diseases or conditions causing the symptoms.
What Are the Key Recommendations?
The guidelines include 19 “practical” recommendations that are applicable to any practice setting. They capture the core elements of a high-quality evaluation and disclosure process, the author said. Here is a brief summary of the recommendations:
Initial evaluation: Perform a multitiered evaluation for patients who self-report or whose care partner or clinician reports cognitive, behavioral, or functional changes.
Patient-centered communication: Partner with the patient and/or care partner to establish shared goals for the evaluation process; assess the patient’s capacity to engage in goal setting.
Diagnostic formulation: Use a tiered approach to assessments and tests based on individual presentation, risk factors, and profile, aiming to determine the level of impairment, cognitive-behavioral syndrome, and likely causes and contributing factors.
History taking: Gather reliable information from informants about changes in cognition, activities of daily living, mood, neuropsychiatric symptoms, and sensory/motor functions. Document individualized risk factors for cognitive decline.
Examination: Conduct a comprehensive examination of cognition, mood, behavior, and a dementia-focused neurologic evaluation using validated tools.
Laboratory tests: Perform tiered, individualized laboratory evaluations, starting with routine tests for all patients.
Structural imaging: Obtain structural brain imaging (MRI preferred, CT as an alternative) to help establish a cause.
Ongoing communication: Engage in ongoing dialogue with patient/care partner to guide them throughout the diagnostic process.
Diagnostic disclosure: Share findings honestly and compassionately, explaining the syndrome, its severity, probable cause, prognosis, treatment options and support resources.
Specialist referral: Refer patients with atypical, uncertain, early-onset, or rapidly progressing symptoms to a dementia subspecialist.
Neuropsychological testing: Use in instances of diagnostic uncertainty or patients with complex clinical profiles. At a minimum, the neuropsychological evaluation should include normed neuropsychological testing of the domains of learning and memory (in particular delayed free and cued recall/recognition), attention, executive function, visuospatial function, and language.
Advanced diagnostic testing: When diagnostic uncertainty remains, obtain additional laboratory tests tailored to individual patient profiles.
Molecular imaging: In a patient with an established cognitive-behavioral syndrome in whom there is continued diagnostic uncertainty regarding cause(s) after structural imaging, a dementia specialist can obtain molecular imaging with fluorodeoxyglucose PET to improve diagnostic accuracy.
Cerebrospinal fluid (CSF) analysis: Utilize CSF biomarkers to evaluate amyloid beta and tau profiles in cases with unresolved diagnostic uncertainty.
Amyloid PET imaging: Perform amyloid PET scans for patients with persistent diagnostic uncertainty after other assessments.
Genetic counseling and testing: Consider genetic testing for patients with strong autosomal dominant family histories and involve a genetic counselor.
Future Directions?
Maria C. Carrillo, PhD, chief science officer and medical affairs lead for the Alzheimer’s Association, encourages clinicians to incorporate these guidelines into their practice.
“These guidelines are important because they guide clinicians in the evaluation of memory complaints, which could have many underlying causes. That is the necessary start for an early and accurate Alzheimer’s diagnosis,” Carrillo said in a statement.
Dickerson said the new guidelines do not address blood-based biomarkers “because nobody really feels that they are ready for prime time yet, even though they’re getting rolled out as clinical products.”
However, the recommendations will be revised as needed. “That’s one of the values of setting this up as a process; whenever any new development occurs, it will be easy to update the guidelines to show where that new test or new biomarker fits in the overall process,” he said.
New Appropriate Use Guidance
A separate workgroup, jointly convened by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging, has revised appropriate use criteria (AUC) for amyloid PET imaging and developed AUC for tau PET imaging.
They were simultaneously published online in Alzheimer’s & Dementia and The Journal of Nuclear Medicine. They are the first revision since the initial AUC for amyloid PET was introduced in 2013.
“The updated amyloid/tau appropriate use criteria will help ensure these tracers are used in a cost-effective manner and the scan results will be used appropriately to add value to the diagnosis and management of dementia,” said workgroup members Kevin Donohoe, MD, with Beth Israel Deaconess Medical Center, Boston, and Phillip Kuo, MD, with City of Hope National Medical Center, Duarte, California.
The AUC include 17 real-world scenarios in which amyloid or tau PET may be considered, with the two tests considered separately and given their own rating for each scenario.
Overall, the strongest evidence for their use includes assessment and prognosis for people with mild cognitive impairment; assessment of people with dementia when the cause is not clearly known; and determining eligibility for treatment with new disease-modifying therapies, and monitoring response to these treatments, the workgroup said.
“Whereas the prior AUC was written at a time when only the deposition of amyloid could be documented, the new therapeutic agents allow us to demonstrate the actual clearance of amyloid during therapy,” Donohoe and Kuo explained.
“These new therapeutic agents are expensive and, as with most medications, may cause unwanted side effects. The most recent version of the AUC includes information about the appropriate use of amyloid imaging for both documenting the presence of amyloid deposits in the brain, making anti-amyloid therapy an option, as well as documenting the effectiveness of the therapeutic agents as amyloid is (or is not) cleared from the brain,” Donahoe and Kuo noted.
The revised AUC also state that, in most cases, amyloid and tau PET tests should not be used for people who do not have cognitive impairment, even if they carry the APOE4 risk-related gene for Alzheimer’s disease; nonmedical use such as for legal concerns, insurance coverage, or employment screening; and in place of genetic testing in patients suspected of carrying a disease-causing genetic mutation.
In a statement, lead author Gil D. Rabinovici, MD, with University of California, San Francisco, emphasized that the AUC “should be considered guidelines for clinicians, not a substitute for careful clinical judgment that considers the full clinical context for each patient with cognitive complaints.”
This research was funded by the Alzheimer’s Association. Disclosures for guideline authors are available with the original articles.
A version of this article first appeared on Medscape.com.
This is the first update since 2001 for specialists and the first guideline for primary care physicians. Executive summaries of the guidelines were published in three articles online on December 23 in a special issue of Alzheimer’s & Dementia.
What’s New?
“With this guideline, we expand the scope of prior guidelines by providing recommendations for practicing clinicians on the process from start to finish,” coauthor Brad Dickerson, MD, director of the Massachusetts General Hospital Frontotemporal Disorders Unit and professor of neurology at Harvard Medical School, Boston, said in a statement.
“If clinicians adopt these recommendations and healthcare systems provide adequate resources, outcomes should improve in most patients in most practice settings,” Dickerson added in an interview.
Through a modified-Delphi approach and guideline-development process, an expert workgroup representing primary and specialty care reviewed 7374 publications, of which 133 met inclusion criteria.
Based on the information, the workgroup outlined a three-step patient-centered evaluation process, which includes assessing cognitive functional status, identifying the cognitive-behavioral syndrome based on specific symptoms, and determining the likely brain diseases or conditions causing the symptoms.
What Are the Key Recommendations?
The guidelines include 19 “practical” recommendations that are applicable to any practice setting. They capture the core elements of a high-quality evaluation and disclosure process, the author said. Here is a brief summary of the recommendations:
Initial evaluation: Perform a multitiered evaluation for patients who self-report or whose care partner or clinician reports cognitive, behavioral, or functional changes.
Patient-centered communication: Partner with the patient and/or care partner to establish shared goals for the evaluation process; assess the patient’s capacity to engage in goal setting.
Diagnostic formulation: Use a tiered approach to assessments and tests based on individual presentation, risk factors, and profile, aiming to determine the level of impairment, cognitive-behavioral syndrome, and likely causes and contributing factors.
History taking: Gather reliable information from informants about changes in cognition, activities of daily living, mood, neuropsychiatric symptoms, and sensory/motor functions. Document individualized risk factors for cognitive decline.
Examination: Conduct a comprehensive examination of cognition, mood, behavior, and a dementia-focused neurologic evaluation using validated tools.
Laboratory tests: Perform tiered, individualized laboratory evaluations, starting with routine tests for all patients.
Structural imaging: Obtain structural brain imaging (MRI preferred, CT as an alternative) to help establish a cause.
Ongoing communication: Engage in ongoing dialogue with patient/care partner to guide them throughout the diagnostic process.
Diagnostic disclosure: Share findings honestly and compassionately, explaining the syndrome, its severity, probable cause, prognosis, treatment options and support resources.
Specialist referral: Refer patients with atypical, uncertain, early-onset, or rapidly progressing symptoms to a dementia subspecialist.
Neuropsychological testing: Use in instances of diagnostic uncertainty or patients with complex clinical profiles. At a minimum, the neuropsychological evaluation should include normed neuropsychological testing of the domains of learning and memory (in particular delayed free and cued recall/recognition), attention, executive function, visuospatial function, and language.
Advanced diagnostic testing: When diagnostic uncertainty remains, obtain additional laboratory tests tailored to individual patient profiles.
Molecular imaging: In a patient with an established cognitive-behavioral syndrome in whom there is continued diagnostic uncertainty regarding cause(s) after structural imaging, a dementia specialist can obtain molecular imaging with fluorodeoxyglucose PET to improve diagnostic accuracy.
Cerebrospinal fluid (CSF) analysis: Utilize CSF biomarkers to evaluate amyloid beta and tau profiles in cases with unresolved diagnostic uncertainty.
Amyloid PET imaging: Perform amyloid PET scans for patients with persistent diagnostic uncertainty after other assessments.
Genetic counseling and testing: Consider genetic testing for patients with strong autosomal dominant family histories and involve a genetic counselor.
Future Directions?
Maria C. Carrillo, PhD, chief science officer and medical affairs lead for the Alzheimer’s Association, encourages clinicians to incorporate these guidelines into their practice.
“These guidelines are important because they guide clinicians in the evaluation of memory complaints, which could have many underlying causes. That is the necessary start for an early and accurate Alzheimer’s diagnosis,” Carrillo said in a statement.
Dickerson said the new guidelines do not address blood-based biomarkers “because nobody really feels that they are ready for prime time yet, even though they’re getting rolled out as clinical products.”
However, the recommendations will be revised as needed. “That’s one of the values of setting this up as a process; whenever any new development occurs, it will be easy to update the guidelines to show where that new test or new biomarker fits in the overall process,” he said.
New Appropriate Use Guidance
A separate workgroup, jointly convened by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging, has revised appropriate use criteria (AUC) for amyloid PET imaging and developed AUC for tau PET imaging.
They were simultaneously published online in Alzheimer’s & Dementia and The Journal of Nuclear Medicine. They are the first revision since the initial AUC for amyloid PET was introduced in 2013.
“The updated amyloid/tau appropriate use criteria will help ensure these tracers are used in a cost-effective manner and the scan results will be used appropriately to add value to the diagnosis and management of dementia,” said workgroup members Kevin Donohoe, MD, with Beth Israel Deaconess Medical Center, Boston, and Phillip Kuo, MD, with City of Hope National Medical Center, Duarte, California.
The AUC include 17 real-world scenarios in which amyloid or tau PET may be considered, with the two tests considered separately and given their own rating for each scenario.
Overall, the strongest evidence for their use includes assessment and prognosis for people with mild cognitive impairment; assessment of people with dementia when the cause is not clearly known; and determining eligibility for treatment with new disease-modifying therapies, and monitoring response to these treatments, the workgroup said.
“Whereas the prior AUC was written at a time when only the deposition of amyloid could be documented, the new therapeutic agents allow us to demonstrate the actual clearance of amyloid during therapy,” Donohoe and Kuo explained.
“These new therapeutic agents are expensive and, as with most medications, may cause unwanted side effects. The most recent version of the AUC includes information about the appropriate use of amyloid imaging for both documenting the presence of amyloid deposits in the brain, making anti-amyloid therapy an option, as well as documenting the effectiveness of the therapeutic agents as amyloid is (or is not) cleared from the brain,” Donahoe and Kuo noted.
The revised AUC also state that, in most cases, amyloid and tau PET tests should not be used for people who do not have cognitive impairment, even if they carry the APOE4 risk-related gene for Alzheimer’s disease; nonmedical use such as for legal concerns, insurance coverage, or employment screening; and in place of genetic testing in patients suspected of carrying a disease-causing genetic mutation.
In a statement, lead author Gil D. Rabinovici, MD, with University of California, San Francisco, emphasized that the AUC “should be considered guidelines for clinicians, not a substitute for careful clinical judgment that considers the full clinical context for each patient with cognitive complaints.”
This research was funded by the Alzheimer’s Association. Disclosures for guideline authors are available with the original articles.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S & DEMENTIA
Losing Your Mind Trying to Understand the BP-Dementia Link
You could be forgiven if you are confused about how blood pressure (BP) affects dementia. First, you read an article extolling the benefits of BP lowering, then a study about how stopping antihypertensives slows cognitive decline in nursing home residents. It’s enough to make you lose your mind.
The Brain Benefits of BP Lowering
It should be stated unequivocally that you should absolutely treat high BP. It may have once been acceptable to state, “The greatest danger to a man with high blood pressure lies in its discovery, because then some fool is certain to try and reduce it.” But those dark days are long behind us.
In these divided times, at least we can agree that we should treat high BP. The cardiovascular (CV) benefits, in and of themselves, justify the decision. But BP’s relationship with dementia is more complex. There are different types of dementia even though we tend to lump them all into one category. Vascular dementia is driven by the same pathophysiology and risk factors as cardiac disease. It’s intuitive that treating hypertension, diabetes, hypercholesterolemia, and smoking will decrease the risk for stroke and limit the damage to the brain that we see with repeated vascular insults. For Alzheimer’s disease, high BP and other CV risk factors seem to increase the risk even if the mechanism is not fully elucidated.
Estimates suggest that if we could lower the prevalence of hypertension by 25%, there would be 160,000 fewer cases of Alzheimer’s disease. But the data are not as robust as one might hope. A 2021 Cochrane review found that hypertension treatment slowed cognitive decline, but the quality of the evidence was low. Short duration of follow-up, dropouts, crossovers, and other problems with the data precluded any certainty. What’s more, hypertension in midlife is associated with cognitive decline and dementia, but its impact in those over age 70 is less clear. Later in life, or once cognitive impairment has already developed, it may be too late for BP lowering to have any impact.
Potential Harms of Lowering BP
All this needs to be weighed against the potential harms of treating hypertension. I will reiterate that hypertension should be treated and treated aggressively for the prevention of CV events. But overtreatment, especially in older patients, is associated with hypotension, falls, and syncope. Older patients are also at risk for polypharmacy and drug-drug interactions.
A Korean nationwide survey showed a U-shaped association between BP and Alzheimer’s disease risk in adults (mean age, 67 years), with both high and low BPs associated with a higher risk for Alzheimer’s disease. Though not all studies agree. A post hoc analysis of SPRINT MIND did not find any negative impact of intensive BP lowering on cognitive outcomes or cerebral perfusion in older adults (mean age, 68 years). But it didn’t do much good either. Given the heterogeneity of the data, doubts remain on whether aggressive BP lowering might be detrimental in older patients with comorbidities and preexisting dementia. The obvious corollary then is whether deprescribing hypertensive medications could be beneficial.
A recent publication in JAMA Internal Medicine attempted to address this very question. The cohort study used data from Veterans Affairs nursing home residents (mean age, 78 years) to emulate a randomized trial on deprescribing antihypertensives and cognitive decline. Many of the residents’ cognitive scores worsened over the course of follow-up; however, the decline was less pronounced in the deprescribing group (10% vs 12%). The same group did a similar analysis looking at CV outcomes and found no increased risk for heart attack or stroke with deprescribing BP medications. Taken together, these nursing home data suggest that deprescribing may help slow cognitive decline without the expected trade-off of increased CV events.
Deprescribing, Yes or No?
However, randomized data would obviously be preferable, and these are in short supply. One such trial, the DANTE study, found no benefit to deprescribing in terms of cognition in adults aged 75 years or older with mild cognitive impairment. The study follow-up was only 16 weeks, however, which is hardly enough time to demonstrate any effect, positive or negative. The most that can be said is that it didn’t cause many short-term adverse events.
Perhaps the best conclusion to draw from this somewhat underwhelming collection of data is that lowering high BP is important, but less important the closer we get to the end of life. Hypotension is obviously bad, and overly aggressive BP lowering is going to lead to negative outcomes in older adults because gravity is an unforgiving mistress.
Deprescribing antihypertensives in older adults is probably not going to cause major negative outcomes, but whether it will do much good in nonhypotensive patients is debatable. The bigger problem is the millions of people with undiagnosed or undertreated hypertension. We would probably have less dementia if we treated hypertension when it does the most good: as a primary-prevention strategy in midlife.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, Quebec, Canada. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
You could be forgiven if you are confused about how blood pressure (BP) affects dementia. First, you read an article extolling the benefits of BP lowering, then a study about how stopping antihypertensives slows cognitive decline in nursing home residents. It’s enough to make you lose your mind.
The Brain Benefits of BP Lowering
It should be stated unequivocally that you should absolutely treat high BP. It may have once been acceptable to state, “The greatest danger to a man with high blood pressure lies in its discovery, because then some fool is certain to try and reduce it.” But those dark days are long behind us.
In these divided times, at least we can agree that we should treat high BP. The cardiovascular (CV) benefits, in and of themselves, justify the decision. But BP’s relationship with dementia is more complex. There are different types of dementia even though we tend to lump them all into one category. Vascular dementia is driven by the same pathophysiology and risk factors as cardiac disease. It’s intuitive that treating hypertension, diabetes, hypercholesterolemia, and smoking will decrease the risk for stroke and limit the damage to the brain that we see with repeated vascular insults. For Alzheimer’s disease, high BP and other CV risk factors seem to increase the risk even if the mechanism is not fully elucidated.
Estimates suggest that if we could lower the prevalence of hypertension by 25%, there would be 160,000 fewer cases of Alzheimer’s disease. But the data are not as robust as one might hope. A 2021 Cochrane review found that hypertension treatment slowed cognitive decline, but the quality of the evidence was low. Short duration of follow-up, dropouts, crossovers, and other problems with the data precluded any certainty. What’s more, hypertension in midlife is associated with cognitive decline and dementia, but its impact in those over age 70 is less clear. Later in life, or once cognitive impairment has already developed, it may be too late for BP lowering to have any impact.
Potential Harms of Lowering BP
All this needs to be weighed against the potential harms of treating hypertension. I will reiterate that hypertension should be treated and treated aggressively for the prevention of CV events. But overtreatment, especially in older patients, is associated with hypotension, falls, and syncope. Older patients are also at risk for polypharmacy and drug-drug interactions.
A Korean nationwide survey showed a U-shaped association between BP and Alzheimer’s disease risk in adults (mean age, 67 years), with both high and low BPs associated with a higher risk for Alzheimer’s disease. Though not all studies agree. A post hoc analysis of SPRINT MIND did not find any negative impact of intensive BP lowering on cognitive outcomes or cerebral perfusion in older adults (mean age, 68 years). But it didn’t do much good either. Given the heterogeneity of the data, doubts remain on whether aggressive BP lowering might be detrimental in older patients with comorbidities and preexisting dementia. The obvious corollary then is whether deprescribing hypertensive medications could be beneficial.
A recent publication in JAMA Internal Medicine attempted to address this very question. The cohort study used data from Veterans Affairs nursing home residents (mean age, 78 years) to emulate a randomized trial on deprescribing antihypertensives and cognitive decline. Many of the residents’ cognitive scores worsened over the course of follow-up; however, the decline was less pronounced in the deprescribing group (10% vs 12%). The same group did a similar analysis looking at CV outcomes and found no increased risk for heart attack or stroke with deprescribing BP medications. Taken together, these nursing home data suggest that deprescribing may help slow cognitive decline without the expected trade-off of increased CV events.
Deprescribing, Yes or No?
However, randomized data would obviously be preferable, and these are in short supply. One such trial, the DANTE study, found no benefit to deprescribing in terms of cognition in adults aged 75 years or older with mild cognitive impairment. The study follow-up was only 16 weeks, however, which is hardly enough time to demonstrate any effect, positive or negative. The most that can be said is that it didn’t cause many short-term adverse events.
Perhaps the best conclusion to draw from this somewhat underwhelming collection of data is that lowering high BP is important, but less important the closer we get to the end of life. Hypotension is obviously bad, and overly aggressive BP lowering is going to lead to negative outcomes in older adults because gravity is an unforgiving mistress.
Deprescribing antihypertensives in older adults is probably not going to cause major negative outcomes, but whether it will do much good in nonhypotensive patients is debatable. The bigger problem is the millions of people with undiagnosed or undertreated hypertension. We would probably have less dementia if we treated hypertension when it does the most good: as a primary-prevention strategy in midlife.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, Quebec, Canada. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
You could be forgiven if you are confused about how blood pressure (BP) affects dementia. First, you read an article extolling the benefits of BP lowering, then a study about how stopping antihypertensives slows cognitive decline in nursing home residents. It’s enough to make you lose your mind.
The Brain Benefits of BP Lowering
It should be stated unequivocally that you should absolutely treat high BP. It may have once been acceptable to state, “The greatest danger to a man with high blood pressure lies in its discovery, because then some fool is certain to try and reduce it.” But those dark days are long behind us.
In these divided times, at least we can agree that we should treat high BP. The cardiovascular (CV) benefits, in and of themselves, justify the decision. But BP’s relationship with dementia is more complex. There are different types of dementia even though we tend to lump them all into one category. Vascular dementia is driven by the same pathophysiology and risk factors as cardiac disease. It’s intuitive that treating hypertension, diabetes, hypercholesterolemia, and smoking will decrease the risk for stroke and limit the damage to the brain that we see with repeated vascular insults. For Alzheimer’s disease, high BP and other CV risk factors seem to increase the risk even if the mechanism is not fully elucidated.
Estimates suggest that if we could lower the prevalence of hypertension by 25%, there would be 160,000 fewer cases of Alzheimer’s disease. But the data are not as robust as one might hope. A 2021 Cochrane review found that hypertension treatment slowed cognitive decline, but the quality of the evidence was low. Short duration of follow-up, dropouts, crossovers, and other problems with the data precluded any certainty. What’s more, hypertension in midlife is associated with cognitive decline and dementia, but its impact in those over age 70 is less clear. Later in life, or once cognitive impairment has already developed, it may be too late for BP lowering to have any impact.
Potential Harms of Lowering BP
All this needs to be weighed against the potential harms of treating hypertension. I will reiterate that hypertension should be treated and treated aggressively for the prevention of CV events. But overtreatment, especially in older patients, is associated with hypotension, falls, and syncope. Older patients are also at risk for polypharmacy and drug-drug interactions.
A Korean nationwide survey showed a U-shaped association between BP and Alzheimer’s disease risk in adults (mean age, 67 years), with both high and low BPs associated with a higher risk for Alzheimer’s disease. Though not all studies agree. A post hoc analysis of SPRINT MIND did not find any negative impact of intensive BP lowering on cognitive outcomes or cerebral perfusion in older adults (mean age, 68 years). But it didn’t do much good either. Given the heterogeneity of the data, doubts remain on whether aggressive BP lowering might be detrimental in older patients with comorbidities and preexisting dementia. The obvious corollary then is whether deprescribing hypertensive medications could be beneficial.
A recent publication in JAMA Internal Medicine attempted to address this very question. The cohort study used data from Veterans Affairs nursing home residents (mean age, 78 years) to emulate a randomized trial on deprescribing antihypertensives and cognitive decline. Many of the residents’ cognitive scores worsened over the course of follow-up; however, the decline was less pronounced in the deprescribing group (10% vs 12%). The same group did a similar analysis looking at CV outcomes and found no increased risk for heart attack or stroke with deprescribing BP medications. Taken together, these nursing home data suggest that deprescribing may help slow cognitive decline without the expected trade-off of increased CV events.
Deprescribing, Yes or No?
However, randomized data would obviously be preferable, and these are in short supply. One such trial, the DANTE study, found no benefit to deprescribing in terms of cognition in adults aged 75 years or older with mild cognitive impairment. The study follow-up was only 16 weeks, however, which is hardly enough time to demonstrate any effect, positive or negative. The most that can be said is that it didn’t cause many short-term adverse events.
Perhaps the best conclusion to draw from this somewhat underwhelming collection of data is that lowering high BP is important, but less important the closer we get to the end of life. Hypotension is obviously bad, and overly aggressive BP lowering is going to lead to negative outcomes in older adults because gravity is an unforgiving mistress.
Deprescribing antihypertensives in older adults is probably not going to cause major negative outcomes, but whether it will do much good in nonhypotensive patients is debatable. The bigger problem is the millions of people with undiagnosed or undertreated hypertension. We would probably have less dementia if we treated hypertension when it does the most good: as a primary-prevention strategy in midlife.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, Quebec, Canada. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Around 5% of US Population Diagnosed With Autoimmune Disease
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Early-Onset Asthma May Slow Memory Development
Children with asthma scored significantly lower than those without asthma on measures of episodic memory, based on longitudinal data from nearly 500 individuals.
Animal models have shown associations between asthma and memory problems, but data for children are lacking, wrote Nicholas J. Christopher-Hayes, MA, of the University of California, Davis, and colleagues.
“Asthma is very frequent among children, and there is mounting evidence from rodent models that asthma may result in neural injury in the hippocampus, which in turn may cause memory loss,” Christopher-Hayes said in an interview. “Although there is also a good amount of research with older adults, very little research has been done with children, the period that is most frequently linked to asthma onset,” he said. Therefore, the researchers leveraged a large national study on child development to examine development of memory as a function of asthma exposure.
In this study published in JAMA Network Open, the researchers conducted both a longitudinal and cross-sectional analysis of data from the Adolescent Brain Cognitive Development Study, which began in 2015. Children were enrolled at ages 9-10 years with a follow-up assessment 1-2 years later.
The participants were categorized as early childhood-onset asthma (asthma at baseline and follow-up), later childhood-onset asthma (asthma at follow-up only), or no asthma history. The primary outcome of the longitudinal analysis was episodic memory. Approximately half of the participants were boys, and slightly more than half were White.
Overall, those with early-onset asthma showed significantly lower rates of longitudinal memory improvements at follow-up compared with the comparison group (P < .01).
Developmental memory improvement in children with later-onset asthma was not significantly different from the control individuals.
Secondary outcomes included processing speed and inhibition, and attention. In a cross-sectional analysis with a larger sample of 2062 children from the same database (1031 with any asthma), those with asthma scored significantly lower on measures not only of episodic memory but also processing speed and inhibition/attention than children with no asthma, with P values of .04, .01, and .02, respectively.
The results were limited by several factors, including the reliance on parent reports for indicators of asthma and the lack of data on the potential effect of prescription corticosteroid use on neurocognitive development, the researchers noted.
The mechanism behind the association remains unclear; the inflammation associated with asthma may disrupt neural processing and manifest as cognitive dysfunction, as has been seen in rodent models of asthma, the researchers wrote. “It is possible that associations between asthma and developmental trajectories emerge earlier for memory, perhaps due to its sensitivity to subtle hippocampal injury,” they noted.
Longer follow-up studies are needed to fully understand how childhood asthma predicts memory declines or difficulties in childhood and beyond, said Christopher-Hayes. “We also need additional studies to understand why children who were diagnosed earlier and had asthma for longer seem to be particularly affected,” he said.
The results of this study were consistent with previous findings and therefore not surprising, senior author Simona Ghetti, PhD, a professor of psychology at the University of California, Davis, said in an interview. However, the finding that the extent of exposure to asthma was associated with slower memory improvement in childhood was striking, she said. That children with an earlier asthma onset who had disease indicators for a longer period showed a slower development of memory over time, suggests that asthma exposure may affect the developmental trajectory of memory, Ghetti noted.
“Recommendations to clinicians are premature because we need a better understanding of the boundary conditions, such as the minimal level of asthma exposure that might generate memory difficulties,” said Ghetti.
“Nevertheless, our results underscore the importance of looking at asthma as a potential source of cognitive difficulty in children,” she said.
Asthma’s Extensive Effect
Evidence is mounting that a diagnosis of asthma may have implications outside the pulmonary system, Diego J. Maselli, MD, professor and chief of the Division of Pulmonary Diseases & Critical Care at UT Health, San Antonio, said in an interview.
“Asthmatics may be at risk of nasal polyps, allergic rhinitis, and other allergic conditions, but there is emerging of evidence inflammation associated with asthma may affect other organ systems,” said Maselli, who was not involved in the study.
“For example, chronic inflammation in asthmatics may increase the risk of cardiovascular disease,” he said.
Although less is known about the effects of asthma on the nervous system, animal models suggest that inflammation associated with asthma may result in neuronal injury and potential effects on memory, said Maselli.
The findings of this study provide evidence of potential detrimental effects on the memory of children with asthma but should be interpreted with caution, Maselli said. “Children with chronic medical conditions may have an inherent disadvantage compared with their peers due to the burden of their disease, medication utilization and side effects, absenteeism from school, physical limitations, and other disease-specific circumstances,” he noted.
“Uncontrolled asthma, in particular, has strong links to low socioeconomic factors that are closely tied to access to adequate medical care, nutrition, educational institutions, and other relevant contributors to normal cognitive development,” Maselli said. Although the authors account for some of these socioeconomic factors by evaluating income and race, other variables may have influenced the results, he added.
Overall, this study’s findings suggested that the diagnosis of asthma in children may be associated with memory deficits and may influence neurodevelopment; however, more research is needed to determine whether the findings are replicated in other cohorts, said Maselli. “In particular, evaluating the effects of the severity of asthma and different asthma endotypes would be crucial to identify children with a higher risk of memory or cognitive deficits and confirm these associations,” he said.
This study was funded by the Memory and Plasticity Program at the University of California, Davis, and by a Learning, Memory, and Plasticity Training Program Fellowship grant from the National Institutes of Health. The researchers and Maselli had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Children with asthma scored significantly lower than those without asthma on measures of episodic memory, based on longitudinal data from nearly 500 individuals.
Animal models have shown associations between asthma and memory problems, but data for children are lacking, wrote Nicholas J. Christopher-Hayes, MA, of the University of California, Davis, and colleagues.
“Asthma is very frequent among children, and there is mounting evidence from rodent models that asthma may result in neural injury in the hippocampus, which in turn may cause memory loss,” Christopher-Hayes said in an interview. “Although there is also a good amount of research with older adults, very little research has been done with children, the period that is most frequently linked to asthma onset,” he said. Therefore, the researchers leveraged a large national study on child development to examine development of memory as a function of asthma exposure.
In this study published in JAMA Network Open, the researchers conducted both a longitudinal and cross-sectional analysis of data from the Adolescent Brain Cognitive Development Study, which began in 2015. Children were enrolled at ages 9-10 years with a follow-up assessment 1-2 years later.
The participants were categorized as early childhood-onset asthma (asthma at baseline and follow-up), later childhood-onset asthma (asthma at follow-up only), or no asthma history. The primary outcome of the longitudinal analysis was episodic memory. Approximately half of the participants were boys, and slightly more than half were White.
Overall, those with early-onset asthma showed significantly lower rates of longitudinal memory improvements at follow-up compared with the comparison group (P < .01).
Developmental memory improvement in children with later-onset asthma was not significantly different from the control individuals.
Secondary outcomes included processing speed and inhibition, and attention. In a cross-sectional analysis with a larger sample of 2062 children from the same database (1031 with any asthma), those with asthma scored significantly lower on measures not only of episodic memory but also processing speed and inhibition/attention than children with no asthma, with P values of .04, .01, and .02, respectively.
The results were limited by several factors, including the reliance on parent reports for indicators of asthma and the lack of data on the potential effect of prescription corticosteroid use on neurocognitive development, the researchers noted.
The mechanism behind the association remains unclear; the inflammation associated with asthma may disrupt neural processing and manifest as cognitive dysfunction, as has been seen in rodent models of asthma, the researchers wrote. “It is possible that associations between asthma and developmental trajectories emerge earlier for memory, perhaps due to its sensitivity to subtle hippocampal injury,” they noted.
Longer follow-up studies are needed to fully understand how childhood asthma predicts memory declines or difficulties in childhood and beyond, said Christopher-Hayes. “We also need additional studies to understand why children who were diagnosed earlier and had asthma for longer seem to be particularly affected,” he said.
The results of this study were consistent with previous findings and therefore not surprising, senior author Simona Ghetti, PhD, a professor of psychology at the University of California, Davis, said in an interview. However, the finding that the extent of exposure to asthma was associated with slower memory improvement in childhood was striking, she said. That children with an earlier asthma onset who had disease indicators for a longer period showed a slower development of memory over time, suggests that asthma exposure may affect the developmental trajectory of memory, Ghetti noted.
“Recommendations to clinicians are premature because we need a better understanding of the boundary conditions, such as the minimal level of asthma exposure that might generate memory difficulties,” said Ghetti.
“Nevertheless, our results underscore the importance of looking at asthma as a potential source of cognitive difficulty in children,” she said.
Asthma’s Extensive Effect
Evidence is mounting that a diagnosis of asthma may have implications outside the pulmonary system, Diego J. Maselli, MD, professor and chief of the Division of Pulmonary Diseases & Critical Care at UT Health, San Antonio, said in an interview.
“Asthmatics may be at risk of nasal polyps, allergic rhinitis, and other allergic conditions, but there is emerging of evidence inflammation associated with asthma may affect other organ systems,” said Maselli, who was not involved in the study.
“For example, chronic inflammation in asthmatics may increase the risk of cardiovascular disease,” he said.
Although less is known about the effects of asthma on the nervous system, animal models suggest that inflammation associated with asthma may result in neuronal injury and potential effects on memory, said Maselli.
The findings of this study provide evidence of potential detrimental effects on the memory of children with asthma but should be interpreted with caution, Maselli said. “Children with chronic medical conditions may have an inherent disadvantage compared with their peers due to the burden of their disease, medication utilization and side effects, absenteeism from school, physical limitations, and other disease-specific circumstances,” he noted.
“Uncontrolled asthma, in particular, has strong links to low socioeconomic factors that are closely tied to access to adequate medical care, nutrition, educational institutions, and other relevant contributors to normal cognitive development,” Maselli said. Although the authors account for some of these socioeconomic factors by evaluating income and race, other variables may have influenced the results, he added.
Overall, this study’s findings suggested that the diagnosis of asthma in children may be associated with memory deficits and may influence neurodevelopment; however, more research is needed to determine whether the findings are replicated in other cohorts, said Maselli. “In particular, evaluating the effects of the severity of asthma and different asthma endotypes would be crucial to identify children with a higher risk of memory or cognitive deficits and confirm these associations,” he said.
This study was funded by the Memory and Plasticity Program at the University of California, Davis, and by a Learning, Memory, and Plasticity Training Program Fellowship grant from the National Institutes of Health. The researchers and Maselli had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Children with asthma scored significantly lower than those without asthma on measures of episodic memory, based on longitudinal data from nearly 500 individuals.
Animal models have shown associations between asthma and memory problems, but data for children are lacking, wrote Nicholas J. Christopher-Hayes, MA, of the University of California, Davis, and colleagues.
“Asthma is very frequent among children, and there is mounting evidence from rodent models that asthma may result in neural injury in the hippocampus, which in turn may cause memory loss,” Christopher-Hayes said in an interview. “Although there is also a good amount of research with older adults, very little research has been done with children, the period that is most frequently linked to asthma onset,” he said. Therefore, the researchers leveraged a large national study on child development to examine development of memory as a function of asthma exposure.
In this study published in JAMA Network Open, the researchers conducted both a longitudinal and cross-sectional analysis of data from the Adolescent Brain Cognitive Development Study, which began in 2015. Children were enrolled at ages 9-10 years with a follow-up assessment 1-2 years later.
The participants were categorized as early childhood-onset asthma (asthma at baseline and follow-up), later childhood-onset asthma (asthma at follow-up only), or no asthma history. The primary outcome of the longitudinal analysis was episodic memory. Approximately half of the participants were boys, and slightly more than half were White.
Overall, those with early-onset asthma showed significantly lower rates of longitudinal memory improvements at follow-up compared with the comparison group (P < .01).
Developmental memory improvement in children with later-onset asthma was not significantly different from the control individuals.
Secondary outcomes included processing speed and inhibition, and attention. In a cross-sectional analysis with a larger sample of 2062 children from the same database (1031 with any asthma), those with asthma scored significantly lower on measures not only of episodic memory but also processing speed and inhibition/attention than children with no asthma, with P values of .04, .01, and .02, respectively.
The results were limited by several factors, including the reliance on parent reports for indicators of asthma and the lack of data on the potential effect of prescription corticosteroid use on neurocognitive development, the researchers noted.
The mechanism behind the association remains unclear; the inflammation associated with asthma may disrupt neural processing and manifest as cognitive dysfunction, as has been seen in rodent models of asthma, the researchers wrote. “It is possible that associations between asthma and developmental trajectories emerge earlier for memory, perhaps due to its sensitivity to subtle hippocampal injury,” they noted.
Longer follow-up studies are needed to fully understand how childhood asthma predicts memory declines or difficulties in childhood and beyond, said Christopher-Hayes. “We also need additional studies to understand why children who were diagnosed earlier and had asthma for longer seem to be particularly affected,” he said.
The results of this study were consistent with previous findings and therefore not surprising, senior author Simona Ghetti, PhD, a professor of psychology at the University of California, Davis, said in an interview. However, the finding that the extent of exposure to asthma was associated with slower memory improvement in childhood was striking, she said. That children with an earlier asthma onset who had disease indicators for a longer period showed a slower development of memory over time, suggests that asthma exposure may affect the developmental trajectory of memory, Ghetti noted.
“Recommendations to clinicians are premature because we need a better understanding of the boundary conditions, such as the minimal level of asthma exposure that might generate memory difficulties,” said Ghetti.
“Nevertheless, our results underscore the importance of looking at asthma as a potential source of cognitive difficulty in children,” she said.
Asthma’s Extensive Effect
Evidence is mounting that a diagnosis of asthma may have implications outside the pulmonary system, Diego J. Maselli, MD, professor and chief of the Division of Pulmonary Diseases & Critical Care at UT Health, San Antonio, said in an interview.
“Asthmatics may be at risk of nasal polyps, allergic rhinitis, and other allergic conditions, but there is emerging of evidence inflammation associated with asthma may affect other organ systems,” said Maselli, who was not involved in the study.
“For example, chronic inflammation in asthmatics may increase the risk of cardiovascular disease,” he said.
Although less is known about the effects of asthma on the nervous system, animal models suggest that inflammation associated with asthma may result in neuronal injury and potential effects on memory, said Maselli.
The findings of this study provide evidence of potential detrimental effects on the memory of children with asthma but should be interpreted with caution, Maselli said. “Children with chronic medical conditions may have an inherent disadvantage compared with their peers due to the burden of their disease, medication utilization and side effects, absenteeism from school, physical limitations, and other disease-specific circumstances,” he noted.
“Uncontrolled asthma, in particular, has strong links to low socioeconomic factors that are closely tied to access to adequate medical care, nutrition, educational institutions, and other relevant contributors to normal cognitive development,” Maselli said. Although the authors account for some of these socioeconomic factors by evaluating income and race, other variables may have influenced the results, he added.
Overall, this study’s findings suggested that the diagnosis of asthma in children may be associated with memory deficits and may influence neurodevelopment; however, more research is needed to determine whether the findings are replicated in other cohorts, said Maselli. “In particular, evaluating the effects of the severity of asthma and different asthma endotypes would be crucial to identify children with a higher risk of memory or cognitive deficits and confirm these associations,” he said.
This study was funded by the Memory and Plasticity Program at the University of California, Davis, and by a Learning, Memory, and Plasticity Training Program Fellowship grant from the National Institutes of Health. The researchers and Maselli had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Dementia Risk Higher for Stroke Survivors
TOPLINE:
Risk for dementia is nearly 80% higher in stroke survivors than in those without stroke, a new study reveals. The data suggest risk declines within 1 year after stroke but remains elevated for up to 20 years.
METHODOLOGY:
- Researchers conducted a population-wide analysis of over 15 million people in Canada between 2002 and 2022. The study focused on adults hospitalized for ischemic stroke, intracerebral hemorrhage, or acute myocardial infarction (AMI).
- Of 175,980 stroke survivors, 99% were matched 1:1 to residents without stroke on the basis of age, sex, rural residence, neighborhood deprivation, and vascular comorbidities. In addition, 90% of patients were matched to those with AMI.
- Incident dementia diagnoses were tracked starting 90 days after stroke until death, emigration, or the end of the study, using a validated algorithm based on hospitalization for dementia, prescriptions for cholinesterase inhibitors, or physician claims within 2 years.
- The mean follow-up duration was 5.6 years.
TAKEAWAY:
- Among stroke survivors, 19% were diagnosed with dementia vs 12.5% in the reference population. The dementia rate per 100 person-years was higher among stroke survivors than in the reference population over the entire follow-up period (3.34 vs 1.89).
- Over the entire study period, dementia was 76% more likely among stroke patients (hazard ratio [HR], 1.76; 95% CI, 1.73-1.79) and 82% more likely in the AMI cohort (HR, 1.82; 95% CI, 1.79-1.85) than in the reference population.
- Time-varying analysis revealed that dementia risk was highest within the first year after stroke, with a > 2.5-fold increase at 6 months (HR, 2.51; 95% CI, 2.42-2.59), which decreased to a 1.5-fold increase at 5 years (HR, 1.51; 95% CI, 1.48-1.56) but remained elevated compared with the reference population even 20 years after the index stroke.
- Recurrent stroke was associated with an approximately threefold increased risk for dementia (single recurrent stroke adjusted HR, 2.64; 95% CI, 2.54-2.74; multiple recurrent strokes adjusted HR, 3.05; 95% CI, 2.81-3.33).
IN PRACTICE:
“While much research has been focused on reducing the risk of a second stroke, our findings make it clear that more research also is needed on developing interventions to help prevent dementia after stroke,” lead author Raed A. Joundi, MD, DPhil, McMaster University, Hamilton, Ontario, Canada, said in a press release.
“There is a need to accelerate the implementation of promising interventions or multipronged approaches into large randomized controlled trials to lower the risk of dementia,” the investigators wrote.
SOURCE:
The study was published online on December 4 in Neurology.
LIMITATIONS:
The study’s limitations included reliance on administrative coding without imaging data, potential underestimation of mild dementia, and lack of granular information on stroke severity, disability, and prestroke cognitive decline. While adjustments were made for healthcare contact and secondary prevention medications, residual biases may have persisted.
DISCLOSURES:
This study received funding from the Canada Brain Research Fund, Heart & Stroke Foundation of Canada, and Canadian Stroke Consortium. Two authors hold awards and positions from national organizations and academic institutions in Canada. Additional details are provided in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Risk for dementia is nearly 80% higher in stroke survivors than in those without stroke, a new study reveals. The data suggest risk declines within 1 year after stroke but remains elevated for up to 20 years.
METHODOLOGY:
- Researchers conducted a population-wide analysis of over 15 million people in Canada between 2002 and 2022. The study focused on adults hospitalized for ischemic stroke, intracerebral hemorrhage, or acute myocardial infarction (AMI).
- Of 175,980 stroke survivors, 99% were matched 1:1 to residents without stroke on the basis of age, sex, rural residence, neighborhood deprivation, and vascular comorbidities. In addition, 90% of patients were matched to those with AMI.
- Incident dementia diagnoses were tracked starting 90 days after stroke until death, emigration, or the end of the study, using a validated algorithm based on hospitalization for dementia, prescriptions for cholinesterase inhibitors, or physician claims within 2 years.
- The mean follow-up duration was 5.6 years.
TAKEAWAY:
- Among stroke survivors, 19% were diagnosed with dementia vs 12.5% in the reference population. The dementia rate per 100 person-years was higher among stroke survivors than in the reference population over the entire follow-up period (3.34 vs 1.89).
- Over the entire study period, dementia was 76% more likely among stroke patients (hazard ratio [HR], 1.76; 95% CI, 1.73-1.79) and 82% more likely in the AMI cohort (HR, 1.82; 95% CI, 1.79-1.85) than in the reference population.
- Time-varying analysis revealed that dementia risk was highest within the first year after stroke, with a > 2.5-fold increase at 6 months (HR, 2.51; 95% CI, 2.42-2.59), which decreased to a 1.5-fold increase at 5 years (HR, 1.51; 95% CI, 1.48-1.56) but remained elevated compared with the reference population even 20 years after the index stroke.
- Recurrent stroke was associated with an approximately threefold increased risk for dementia (single recurrent stroke adjusted HR, 2.64; 95% CI, 2.54-2.74; multiple recurrent strokes adjusted HR, 3.05; 95% CI, 2.81-3.33).
IN PRACTICE:
“While much research has been focused on reducing the risk of a second stroke, our findings make it clear that more research also is needed on developing interventions to help prevent dementia after stroke,” lead author Raed A. Joundi, MD, DPhil, McMaster University, Hamilton, Ontario, Canada, said in a press release.
“There is a need to accelerate the implementation of promising interventions or multipronged approaches into large randomized controlled trials to lower the risk of dementia,” the investigators wrote.
SOURCE:
The study was published online on December 4 in Neurology.
LIMITATIONS:
The study’s limitations included reliance on administrative coding without imaging data, potential underestimation of mild dementia, and lack of granular information on stroke severity, disability, and prestroke cognitive decline. While adjustments were made for healthcare contact and secondary prevention medications, residual biases may have persisted.
DISCLOSURES:
This study received funding from the Canada Brain Research Fund, Heart & Stroke Foundation of Canada, and Canadian Stroke Consortium. Two authors hold awards and positions from national organizations and academic institutions in Canada. Additional details are provided in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Risk for dementia is nearly 80% higher in stroke survivors than in those without stroke, a new study reveals. The data suggest risk declines within 1 year after stroke but remains elevated for up to 20 years.
METHODOLOGY:
- Researchers conducted a population-wide analysis of over 15 million people in Canada between 2002 and 2022. The study focused on adults hospitalized for ischemic stroke, intracerebral hemorrhage, or acute myocardial infarction (AMI).
- Of 175,980 stroke survivors, 99% were matched 1:1 to residents without stroke on the basis of age, sex, rural residence, neighborhood deprivation, and vascular comorbidities. In addition, 90% of patients were matched to those with AMI.
- Incident dementia diagnoses were tracked starting 90 days after stroke until death, emigration, or the end of the study, using a validated algorithm based on hospitalization for dementia, prescriptions for cholinesterase inhibitors, or physician claims within 2 years.
- The mean follow-up duration was 5.6 years.
TAKEAWAY:
- Among stroke survivors, 19% were diagnosed with dementia vs 12.5% in the reference population. The dementia rate per 100 person-years was higher among stroke survivors than in the reference population over the entire follow-up period (3.34 vs 1.89).
- Over the entire study period, dementia was 76% more likely among stroke patients (hazard ratio [HR], 1.76; 95% CI, 1.73-1.79) and 82% more likely in the AMI cohort (HR, 1.82; 95% CI, 1.79-1.85) than in the reference population.
- Time-varying analysis revealed that dementia risk was highest within the first year after stroke, with a > 2.5-fold increase at 6 months (HR, 2.51; 95% CI, 2.42-2.59), which decreased to a 1.5-fold increase at 5 years (HR, 1.51; 95% CI, 1.48-1.56) but remained elevated compared with the reference population even 20 years after the index stroke.
- Recurrent stroke was associated with an approximately threefold increased risk for dementia (single recurrent stroke adjusted HR, 2.64; 95% CI, 2.54-2.74; multiple recurrent strokes adjusted HR, 3.05; 95% CI, 2.81-3.33).
IN PRACTICE:
“While much research has been focused on reducing the risk of a second stroke, our findings make it clear that more research also is needed on developing interventions to help prevent dementia after stroke,” lead author Raed A. Joundi, MD, DPhil, McMaster University, Hamilton, Ontario, Canada, said in a press release.
“There is a need to accelerate the implementation of promising interventions or multipronged approaches into large randomized controlled trials to lower the risk of dementia,” the investigators wrote.
SOURCE:
The study was published online on December 4 in Neurology.
LIMITATIONS:
The study’s limitations included reliance on administrative coding without imaging data, potential underestimation of mild dementia, and lack of granular information on stroke severity, disability, and prestroke cognitive decline. While adjustments were made for healthcare contact and secondary prevention medications, residual biases may have persisted.
DISCLOSURES:
This study received funding from the Canada Brain Research Fund, Heart & Stroke Foundation of Canada, and Canadian Stroke Consortium. Two authors hold awards and positions from national organizations and academic institutions in Canada. Additional details are provided in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Can GLP-1s Reduce Alzheimer’s Disease Risk?
Tina is a lovely 67-year-old woman who was recently found to be an APOE gene carrier (a gene associated with increased risk of developing Alzheimer’s disease as well as an earlier age of disease onset), with diffused amyloid protein deposition her brain.
Her neuropsychiatric testing was consistent with mild cognitive impairment. Although Tina is not a doctor herself, her entire family consists of doctors, and she came to me under their advisement to consider semaglutide (Ozempic) for early Alzheimer’s disease prevention.
This would usually be simple, but in Tina’s case, there was a complicating factor: At 5’ and 90 pounds, she was already considerably underweight and was at risk of becoming severely undernourished.
To understand the potential role for glucagon-like peptide-1 (GLP-1) receptor agonists such as Ozempic in prevention, a quick primer on Alzheimer’s Disease is necessary.
The exact cause of Alzheimer’s disease remains elusive, but it is probably due to a combination of factors, including:
- Buildup of abnormal amyloid and tau proteins around brain cells
- Brain shrinkage, with subsequent damage to blood vessels and mitochondria, and inflammation
- Genetic predisposition
- Lifestyle factors, including obesity, high blood pressure, high cholesterol, and diabetes.
Once in the brain, they can reduce inflammation and improve functioning of the neurons. In early rodent trials, GLP-1 receptor agonists led to reduced amyloid and tau aggregation, downregulation of inflammation, and improved memory.
In 2021, multiple studies showed that liraglutide, an early GLP-1 receptor agonist, improved cognitive function and MRI volume in patients with Alzheimer’s disease.
A study recently published in Alzheimer’s & Dementia analyzed data from 1 million people with type 2 diabetes and no prior Alzheimer’s disease diagnosis. The authors compared Alzheimer’s disease occurrence in patients taking various diabetes medications, including insulin, metformin, and GLP-1 receptor agonists. The study found that participants taking semaglutide had up to a 70% reduction in Alzheimer’s risk. The results were consistent across gender, age, and weight.
Given the reassuring safety profile of GLP-1 receptor agonists and lack of other effective treatment or prophylaxis for Alzheimer’s disease, I agreed to start her on dulaglutide (Trulicity). My rationale was twofold:
1. In studies, dulaglutide has the highest uptake in the brain tissue at 68%. By contrast, there is virtually zero uptake in brain tissue for semaglutide (Ozempic/Wegovy) and tirzepatide (Mounjaro/Zepbound). Because this class of drugs exert their effects in the brain tissue, I wanted to give her a GLP-1 receptor agonist with a high percent uptake.
2. Trulicity has a minimal effect on weight loss compared with the newer-generation GLP-1 receptor agonists. Even so, I connected Tina to my dietitian to ensure that she would receive a high-protein, high-calorie diet.
Tina has now been taking Trulicity for 6 months. Although it is certainly too early to draw firm conclusions about the efficacy of her treatment, she is not experiencing any weight loss and is cognitively stable, according to her neurologist.
The EVOKE and EVOKE+ phase 3 trials are currently underway to evaluate the efficacy of semaglutide to treat mild cognitive impairment and early Alzheimer’s in amyloid-positive patients. Results are expected in 2025, but in the meantime, I feel comforted knowing that Tina is receiving a potentially beneficial and definitively low-risk treatment.
Dr Messer, Clinical Assistant Professor, Mount Sinai School of Medicine; Associate Professor, Hofstra School of Medicine, New York, NY, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Tina is a lovely 67-year-old woman who was recently found to be an APOE gene carrier (a gene associated with increased risk of developing Alzheimer’s disease as well as an earlier age of disease onset), with diffused amyloid protein deposition her brain.
Her neuropsychiatric testing was consistent with mild cognitive impairment. Although Tina is not a doctor herself, her entire family consists of doctors, and she came to me under their advisement to consider semaglutide (Ozempic) for early Alzheimer’s disease prevention.
This would usually be simple, but in Tina’s case, there was a complicating factor: At 5’ and 90 pounds, she was already considerably underweight and was at risk of becoming severely undernourished.
To understand the potential role for glucagon-like peptide-1 (GLP-1) receptor agonists such as Ozempic in prevention, a quick primer on Alzheimer’s Disease is necessary.
The exact cause of Alzheimer’s disease remains elusive, but it is probably due to a combination of factors, including:
- Buildup of abnormal amyloid and tau proteins around brain cells
- Brain shrinkage, with subsequent damage to blood vessels and mitochondria, and inflammation
- Genetic predisposition
- Lifestyle factors, including obesity, high blood pressure, high cholesterol, and diabetes.
Once in the brain, they can reduce inflammation and improve functioning of the neurons. In early rodent trials, GLP-1 receptor agonists led to reduced amyloid and tau aggregation, downregulation of inflammation, and improved memory.
In 2021, multiple studies showed that liraglutide, an early GLP-1 receptor agonist, improved cognitive function and MRI volume in patients with Alzheimer’s disease.
A study recently published in Alzheimer’s & Dementia analyzed data from 1 million people with type 2 diabetes and no prior Alzheimer’s disease diagnosis. The authors compared Alzheimer’s disease occurrence in patients taking various diabetes medications, including insulin, metformin, and GLP-1 receptor agonists. The study found that participants taking semaglutide had up to a 70% reduction in Alzheimer’s risk. The results were consistent across gender, age, and weight.
Given the reassuring safety profile of GLP-1 receptor agonists and lack of other effective treatment or prophylaxis for Alzheimer’s disease, I agreed to start her on dulaglutide (Trulicity). My rationale was twofold:
1. In studies, dulaglutide has the highest uptake in the brain tissue at 68%. By contrast, there is virtually zero uptake in brain tissue for semaglutide (Ozempic/Wegovy) and tirzepatide (Mounjaro/Zepbound). Because this class of drugs exert their effects in the brain tissue, I wanted to give her a GLP-1 receptor agonist with a high percent uptake.
2. Trulicity has a minimal effect on weight loss compared with the newer-generation GLP-1 receptor agonists. Even so, I connected Tina to my dietitian to ensure that she would receive a high-protein, high-calorie diet.
Tina has now been taking Trulicity for 6 months. Although it is certainly too early to draw firm conclusions about the efficacy of her treatment, she is not experiencing any weight loss and is cognitively stable, according to her neurologist.
The EVOKE and EVOKE+ phase 3 trials are currently underway to evaluate the efficacy of semaglutide to treat mild cognitive impairment and early Alzheimer’s in amyloid-positive patients. Results are expected in 2025, but in the meantime, I feel comforted knowing that Tina is receiving a potentially beneficial and definitively low-risk treatment.
Dr Messer, Clinical Assistant Professor, Mount Sinai School of Medicine; Associate Professor, Hofstra School of Medicine, New York, NY, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Tina is a lovely 67-year-old woman who was recently found to be an APOE gene carrier (a gene associated with increased risk of developing Alzheimer’s disease as well as an earlier age of disease onset), with diffused amyloid protein deposition her brain.
Her neuropsychiatric testing was consistent with mild cognitive impairment. Although Tina is not a doctor herself, her entire family consists of doctors, and she came to me under their advisement to consider semaglutide (Ozempic) for early Alzheimer’s disease prevention.
This would usually be simple, but in Tina’s case, there was a complicating factor: At 5’ and 90 pounds, she was already considerably underweight and was at risk of becoming severely undernourished.
To understand the potential role for glucagon-like peptide-1 (GLP-1) receptor agonists such as Ozempic in prevention, a quick primer on Alzheimer’s Disease is necessary.
The exact cause of Alzheimer’s disease remains elusive, but it is probably due to a combination of factors, including:
- Buildup of abnormal amyloid and tau proteins around brain cells
- Brain shrinkage, with subsequent damage to blood vessels and mitochondria, and inflammation
- Genetic predisposition
- Lifestyle factors, including obesity, high blood pressure, high cholesterol, and diabetes.
Once in the brain, they can reduce inflammation and improve functioning of the neurons. In early rodent trials, GLP-1 receptor agonists led to reduced amyloid and tau aggregation, downregulation of inflammation, and improved memory.
In 2021, multiple studies showed that liraglutide, an early GLP-1 receptor agonist, improved cognitive function and MRI volume in patients with Alzheimer’s disease.
A study recently published in Alzheimer’s & Dementia analyzed data from 1 million people with type 2 diabetes and no prior Alzheimer’s disease diagnosis. The authors compared Alzheimer’s disease occurrence in patients taking various diabetes medications, including insulin, metformin, and GLP-1 receptor agonists. The study found that participants taking semaglutide had up to a 70% reduction in Alzheimer’s risk. The results were consistent across gender, age, and weight.
Given the reassuring safety profile of GLP-1 receptor agonists and lack of other effective treatment or prophylaxis for Alzheimer’s disease, I agreed to start her on dulaglutide (Trulicity). My rationale was twofold:
1. In studies, dulaglutide has the highest uptake in the brain tissue at 68%. By contrast, there is virtually zero uptake in brain tissue for semaglutide (Ozempic/Wegovy) and tirzepatide (Mounjaro/Zepbound). Because this class of drugs exert their effects in the brain tissue, I wanted to give her a GLP-1 receptor agonist with a high percent uptake.
2. Trulicity has a minimal effect on weight loss compared with the newer-generation GLP-1 receptor agonists. Even so, I connected Tina to my dietitian to ensure that she would receive a high-protein, high-calorie diet.
Tina has now been taking Trulicity for 6 months. Although it is certainly too early to draw firm conclusions about the efficacy of her treatment, she is not experiencing any weight loss and is cognitively stable, according to her neurologist.
The EVOKE and EVOKE+ phase 3 trials are currently underway to evaluate the efficacy of semaglutide to treat mild cognitive impairment and early Alzheimer’s in amyloid-positive patients. Results are expected in 2025, but in the meantime, I feel comforted knowing that Tina is receiving a potentially beneficial and definitively low-risk treatment.
Dr Messer, Clinical Assistant Professor, Mount Sinai School of Medicine; Associate Professor, Hofstra School of Medicine, New York, NY, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Traumatic Brain Injury May Reactivate Herpes Virus Leading to Neurodegeneration
a new study suggested.
Using a three-dimensional (3D) human brain tissue model, researchers observed that quiescent HSV-1 can be reactivated by a mechanical jolt mimicking concussion, leading to signature markers of Alzheimer’s disease, including neuroinflammation and production of amyloid beta and phosphorylated tau (p-tau) and gliosis — a phenotype made worse by repeated head injury.
“This opens the question as to whether antiviral drugs or anti-inflammatory agents might be useful as early preventive treatments after head trauma to stop HSV-1 activation in its tracks and lower the risk of Alzheimer’s disease,” lead investigator Dana Cairns, PhD, with the Department of Biomedical Engineering at Tufts University, Medford, Massachusetts, said in a statement.
But outside experts urged caution in drawing any firm conclusions, pending further study.
The study was published online in the journal Science Signaling.
HSV-1: A Major Alzheimer’s Disease Risk Factor?
TBI is a major risk factor for Alzheimer’s disease and dementia, but the pathways in the brain leading from TBI to dementia are unknown.
HSV-1 is found in over 80% of people; varicella zoster virus (VZV) is found in about 95%. Both viruses are known to enter the brain and lay dormant in neurons and glial cells. Prior evidence indicates that HSV-1 in the brain of APOE-ε4 carriers confers a strong risk for Alzheimer’s disease.
A number of years ago, the team created a 3D model of human brain tissue to study the link between TBI, the viruses, and dementia. The model is 6 mm wide, shaped like a donut, and made of a spongy material of silk protein and collagen saturated with neural stem cells. The cells mature into neurons, communicate with each other, and form a network that mimics the brain environment.
In an earlier study using the model quiescently infected with HSV-1, Cairns and colleagues found that subsequent exposure to VZV created the inflammatory conditions that led to reactivation of HSV-1.
This led them to wonder what would happen if they subjected the brain tissue model to a physical disruption akin to a concussion. Would HSV-1 wake up and start the process of neurodegeneration?
To investigate, they examined the effects of one or more controlled blows to the 3D human brain tissue model in the absence or presence of quiescent HSV-1 infection.
After repeated, mild controlled blows, researchers found that the latently infected 3D brain tissue displayed reactivated HSV-1 and the production and accumulation of amyloid beta and p-tau — which promotes neurodegeneration. The blows also activated gliosis, which is associated with destructive neuroinflammation.
These effects are collectively associated with Alzheimer’s disease, dementia, and chronic traumatic encephalopathy, they pointed out, and were increased with additional injury but were absent in tissue not infected with HSV-1.
“These data suggest that HSV-1 in the brain is pivotal in increasing the risk of Alzheimer’s disease, as other recent studies using cerebral organoids have suggested,” the researchers wrote.
They propose that following brain injury, “whether by infection or mechanical damage, the resulting inflammation induces HSV-1 reactivation in the brain leading to the development of Alzheimer’s disease/dementia and that HSV-1 is a major cause of the disease, especially in APOE4 carriers.”
Future studies should investigate “possible ways of mitigating or stopping the damage caused by head injury, thereby reducing subsequent development of Alzheimer’s disease by implementing efforts to prevent the reactivation of virus in brain such as anti-inflammatory and/or antiviral treatment post-injury,” researchers suggested.
Outside Experts Weigh in
Several outside experts offered perspective on the study in a statement from the UK nonprofit Science Media Centre.
Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, London, England, said that, while the study is interesting, there are limitations.
“The increase in Alzheimer’s-like brain changes in these latent virus-containing cells subjected to injury does not resemble the pathology that is found in the brain of people with Alzheimer’s disease,” Spires-Jones noted.
“These experiments were also in cells grown in artificial conditions without important Alzheimer’s-related factors such as age and blood vessel changes. Finally, these experiments were repeated in a small number of experimental replicates (three times per experiment), so these results will need to be confirmed in more relevant biological systems with larger studies to be sure there is a biological link between latent herpes simplex virus type 1, brain injury, and Alzheimer’s pathology,” Spires-Jones cautioned.
Robert Howard, MD, MRCPsych, University College London (UCL) Division of Psychiatry, said the study suggests a possible mechanism for the association between HSV-1, brain injury, and Alzheimer’s disease.
“However, as so often in science, it is very important to bear in mind that association does not mean causation. Much more research will be needed before this can be seriously considered a plausible mechanism for the development of dementia,” Howard cautioned.
“Avoidance of brain injuries, such as those encountered in some contact sports, is already known to be an important way to prevent dementia, and I’m unconvinced that this reflects anything more complicated than mechanical damage causing death of brain cells,” he added.
Jennifer Pocock, PhD, with UCL Queen Square Institute of Neurology, noted the role of microglia, which are activated by mild and repetitive TBI, isn’t addressed in the study.
“This paper seems to suggest that only astrocytes contribute to the reported neuroinflammation in brain tissue. Also, the inclusion of APOE3/4 is not clearly defined. Because of this, the findings are likely to represent an over interpretation for the ‘real world’ as the inclusion of microglia may negate or accentuate them, depending on the severity of the TBI,” Pocock said.
The study was funded by the US Army Research Office and Department of Defense. The authors have declared no relevant conflicts of interest. Spires-Jones and Howard had no relevant disclosures related to this study. Pocock has received research funding from AstraZeneca and Daiichi Sankyo.
A version of this article appeared on Medscape.com.
a new study suggested.
Using a three-dimensional (3D) human brain tissue model, researchers observed that quiescent HSV-1 can be reactivated by a mechanical jolt mimicking concussion, leading to signature markers of Alzheimer’s disease, including neuroinflammation and production of amyloid beta and phosphorylated tau (p-tau) and gliosis — a phenotype made worse by repeated head injury.
“This opens the question as to whether antiviral drugs or anti-inflammatory agents might be useful as early preventive treatments after head trauma to stop HSV-1 activation in its tracks and lower the risk of Alzheimer’s disease,” lead investigator Dana Cairns, PhD, with the Department of Biomedical Engineering at Tufts University, Medford, Massachusetts, said in a statement.
But outside experts urged caution in drawing any firm conclusions, pending further study.
The study was published online in the journal Science Signaling.
HSV-1: A Major Alzheimer’s Disease Risk Factor?
TBI is a major risk factor for Alzheimer’s disease and dementia, but the pathways in the brain leading from TBI to dementia are unknown.
HSV-1 is found in over 80% of people; varicella zoster virus (VZV) is found in about 95%. Both viruses are known to enter the brain and lay dormant in neurons and glial cells. Prior evidence indicates that HSV-1 in the brain of APOE-ε4 carriers confers a strong risk for Alzheimer’s disease.
A number of years ago, the team created a 3D model of human brain tissue to study the link between TBI, the viruses, and dementia. The model is 6 mm wide, shaped like a donut, and made of a spongy material of silk protein and collagen saturated with neural stem cells. The cells mature into neurons, communicate with each other, and form a network that mimics the brain environment.
In an earlier study using the model quiescently infected with HSV-1, Cairns and colleagues found that subsequent exposure to VZV created the inflammatory conditions that led to reactivation of HSV-1.
This led them to wonder what would happen if they subjected the brain tissue model to a physical disruption akin to a concussion. Would HSV-1 wake up and start the process of neurodegeneration?
To investigate, they examined the effects of one or more controlled blows to the 3D human brain tissue model in the absence or presence of quiescent HSV-1 infection.
After repeated, mild controlled blows, researchers found that the latently infected 3D brain tissue displayed reactivated HSV-1 and the production and accumulation of amyloid beta and p-tau — which promotes neurodegeneration. The blows also activated gliosis, which is associated with destructive neuroinflammation.
These effects are collectively associated with Alzheimer’s disease, dementia, and chronic traumatic encephalopathy, they pointed out, and were increased with additional injury but were absent in tissue not infected with HSV-1.
“These data suggest that HSV-1 in the brain is pivotal in increasing the risk of Alzheimer’s disease, as other recent studies using cerebral organoids have suggested,” the researchers wrote.
They propose that following brain injury, “whether by infection or mechanical damage, the resulting inflammation induces HSV-1 reactivation in the brain leading to the development of Alzheimer’s disease/dementia and that HSV-1 is a major cause of the disease, especially in APOE4 carriers.”
Future studies should investigate “possible ways of mitigating or stopping the damage caused by head injury, thereby reducing subsequent development of Alzheimer’s disease by implementing efforts to prevent the reactivation of virus in brain such as anti-inflammatory and/or antiviral treatment post-injury,” researchers suggested.
Outside Experts Weigh in
Several outside experts offered perspective on the study in a statement from the UK nonprofit Science Media Centre.
Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, London, England, said that, while the study is interesting, there are limitations.
“The increase in Alzheimer’s-like brain changes in these latent virus-containing cells subjected to injury does not resemble the pathology that is found in the brain of people with Alzheimer’s disease,” Spires-Jones noted.
“These experiments were also in cells grown in artificial conditions without important Alzheimer’s-related factors such as age and blood vessel changes. Finally, these experiments were repeated in a small number of experimental replicates (three times per experiment), so these results will need to be confirmed in more relevant biological systems with larger studies to be sure there is a biological link between latent herpes simplex virus type 1, brain injury, and Alzheimer’s pathology,” Spires-Jones cautioned.
Robert Howard, MD, MRCPsych, University College London (UCL) Division of Psychiatry, said the study suggests a possible mechanism for the association between HSV-1, brain injury, and Alzheimer’s disease.
“However, as so often in science, it is very important to bear in mind that association does not mean causation. Much more research will be needed before this can be seriously considered a plausible mechanism for the development of dementia,” Howard cautioned.
“Avoidance of brain injuries, such as those encountered in some contact sports, is already known to be an important way to prevent dementia, and I’m unconvinced that this reflects anything more complicated than mechanical damage causing death of brain cells,” he added.
Jennifer Pocock, PhD, with UCL Queen Square Institute of Neurology, noted the role of microglia, which are activated by mild and repetitive TBI, isn’t addressed in the study.
“This paper seems to suggest that only astrocytes contribute to the reported neuroinflammation in brain tissue. Also, the inclusion of APOE3/4 is not clearly defined. Because of this, the findings are likely to represent an over interpretation for the ‘real world’ as the inclusion of microglia may negate or accentuate them, depending on the severity of the TBI,” Pocock said.
The study was funded by the US Army Research Office and Department of Defense. The authors have declared no relevant conflicts of interest. Spires-Jones and Howard had no relevant disclosures related to this study. Pocock has received research funding from AstraZeneca and Daiichi Sankyo.
A version of this article appeared on Medscape.com.
a new study suggested.
Using a three-dimensional (3D) human brain tissue model, researchers observed that quiescent HSV-1 can be reactivated by a mechanical jolt mimicking concussion, leading to signature markers of Alzheimer’s disease, including neuroinflammation and production of amyloid beta and phosphorylated tau (p-tau) and gliosis — a phenotype made worse by repeated head injury.
“This opens the question as to whether antiviral drugs or anti-inflammatory agents might be useful as early preventive treatments after head trauma to stop HSV-1 activation in its tracks and lower the risk of Alzheimer’s disease,” lead investigator Dana Cairns, PhD, with the Department of Biomedical Engineering at Tufts University, Medford, Massachusetts, said in a statement.
But outside experts urged caution in drawing any firm conclusions, pending further study.
The study was published online in the journal Science Signaling.
HSV-1: A Major Alzheimer’s Disease Risk Factor?
TBI is a major risk factor for Alzheimer’s disease and dementia, but the pathways in the brain leading from TBI to dementia are unknown.
HSV-1 is found in over 80% of people; varicella zoster virus (VZV) is found in about 95%. Both viruses are known to enter the brain and lay dormant in neurons and glial cells. Prior evidence indicates that HSV-1 in the brain of APOE-ε4 carriers confers a strong risk for Alzheimer’s disease.
A number of years ago, the team created a 3D model of human brain tissue to study the link between TBI, the viruses, and dementia. The model is 6 mm wide, shaped like a donut, and made of a spongy material of silk protein and collagen saturated with neural stem cells. The cells mature into neurons, communicate with each other, and form a network that mimics the brain environment.
In an earlier study using the model quiescently infected with HSV-1, Cairns and colleagues found that subsequent exposure to VZV created the inflammatory conditions that led to reactivation of HSV-1.
This led them to wonder what would happen if they subjected the brain tissue model to a physical disruption akin to a concussion. Would HSV-1 wake up and start the process of neurodegeneration?
To investigate, they examined the effects of one or more controlled blows to the 3D human brain tissue model in the absence or presence of quiescent HSV-1 infection.
After repeated, mild controlled blows, researchers found that the latently infected 3D brain tissue displayed reactivated HSV-1 and the production and accumulation of amyloid beta and p-tau — which promotes neurodegeneration. The blows also activated gliosis, which is associated with destructive neuroinflammation.
These effects are collectively associated with Alzheimer’s disease, dementia, and chronic traumatic encephalopathy, they pointed out, and were increased with additional injury but were absent in tissue not infected with HSV-1.
“These data suggest that HSV-1 in the brain is pivotal in increasing the risk of Alzheimer’s disease, as other recent studies using cerebral organoids have suggested,” the researchers wrote.
They propose that following brain injury, “whether by infection or mechanical damage, the resulting inflammation induces HSV-1 reactivation in the brain leading to the development of Alzheimer’s disease/dementia and that HSV-1 is a major cause of the disease, especially in APOE4 carriers.”
Future studies should investigate “possible ways of mitigating or stopping the damage caused by head injury, thereby reducing subsequent development of Alzheimer’s disease by implementing efforts to prevent the reactivation of virus in brain such as anti-inflammatory and/or antiviral treatment post-injury,” researchers suggested.
Outside Experts Weigh in
Several outside experts offered perspective on the study in a statement from the UK nonprofit Science Media Centre.
Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, London, England, said that, while the study is interesting, there are limitations.
“The increase in Alzheimer’s-like brain changes in these latent virus-containing cells subjected to injury does not resemble the pathology that is found in the brain of people with Alzheimer’s disease,” Spires-Jones noted.
“These experiments were also in cells grown in artificial conditions without important Alzheimer’s-related factors such as age and blood vessel changes. Finally, these experiments were repeated in a small number of experimental replicates (three times per experiment), so these results will need to be confirmed in more relevant biological systems with larger studies to be sure there is a biological link between latent herpes simplex virus type 1, brain injury, and Alzheimer’s pathology,” Spires-Jones cautioned.
Robert Howard, MD, MRCPsych, University College London (UCL) Division of Psychiatry, said the study suggests a possible mechanism for the association between HSV-1, brain injury, and Alzheimer’s disease.
“However, as so often in science, it is very important to bear in mind that association does not mean causation. Much more research will be needed before this can be seriously considered a plausible mechanism for the development of dementia,” Howard cautioned.
“Avoidance of brain injuries, such as those encountered in some contact sports, is already known to be an important way to prevent dementia, and I’m unconvinced that this reflects anything more complicated than mechanical damage causing death of brain cells,” he added.
Jennifer Pocock, PhD, with UCL Queen Square Institute of Neurology, noted the role of microglia, which are activated by mild and repetitive TBI, isn’t addressed in the study.
“This paper seems to suggest that only astrocytes contribute to the reported neuroinflammation in brain tissue. Also, the inclusion of APOE3/4 is not clearly defined. Because of this, the findings are likely to represent an over interpretation for the ‘real world’ as the inclusion of microglia may negate or accentuate them, depending on the severity of the TBI,” Pocock said.
The study was funded by the US Army Research Office and Department of Defense. The authors have declared no relevant conflicts of interest. Spires-Jones and Howard had no relevant disclosures related to this study. Pocock has received research funding from AstraZeneca and Daiichi Sankyo.
A version of this article appeared on Medscape.com.
FROM SCIENCE SIGNALING
Improving Interprofessional Neurology Training Using Tele-Education
Improving Interprofessional Neurology Training Using Tele-Education
Neurologic disorders are major causes of death and disability. Globally, the burden of neurologic disorders continues to increase. The prevalence of disabling neurologic disorders significantly increases with age. As people live longer, health care systems will face increasing demands for treatment, rehabilitation, and support services for neurologic disorders. The scarcity of established modifiable risks for most of the neurologic burden demonstrates how new knowledge is required to develop effective prevention and treatment strategies.1
A single-center study for chronic headache at a rural institution found that, when combined with public education, clinician education not only can increase access to care but also reduce specialist overuse, hospitalizations, polypharmacy, and emergency department visits.2 A predicted shortage of neurologists has sparked increased interest in the field and individual neurology educators are helping fuel its popularity.3-5
TELE-EDUCATION
Educating the next generation of health professionals is 1 of 4 statutory missions of the US Department of Veterans Affairs (VA).6 Tele-education (also known as telelearning and distance learning) deviates from traditional in-person classroom settings, in which the lecture has been a core pedagogic method.7 Audio, video, and online technologies provide health education and can overcome geographic barriers for rural and remote clinicians.8 Recent technological improvements have allowed for inexpensive and efficient dissemination of educational materials, including video lectures, podcasts, online modules, assessment materials, and even entire curricula.9
There has been an increase in the awareness of the parallel curriculum involving self-directed and asynchronous learning opportunities. 10 Several studies report knowledge gained via tele-education is comparable to conventional classroom learning.11-13 A systematic review of e-learning perceptions among health care students suggested benefits (eg, learning flexibility, pedagogical design, online interactions, basic computer skills, and access to technology) and drawbacks (eg, limited acquisition of clinical skills, internet connection problems, and issues with using educational platforms).1
The COVID-19 pandemic forced an abrupt cessation of traditional in-person education, forcing educational institutions and medical organizations to transition to telelearning. Solutions in the education field appeared during the pandemic, such as videoconferencing, social media, and telemedicine, that effectively addressed the sudden cessation of in-person medical education.15
Graduate medical education in neurology residency programs served as an experimental set up for tele-education during the pandemic. Residents from neurology training programs outlined the benefits of a volunteer lecturer-based online didactic program that was established to meet this need, which included exposure to subspeciality topics, access to subspecialist experts not available within the department, exposure to different pedagogic methods, interaction with members of other educational institutions and training programs, career development opportunities, and the potential for forming a community of learning.16
Not all recent educational developments are technology-based. For example, instruction focused on specific patient experiences, and learning processes that emphasize problem solving and personal responsibility over specific knowledge have been successful in neurology.17,18 Departments and institutions must be creative in finding ways to fund continuing education, especially when budgets are limited.19
ANNUAL NEUROLOGY SEMINAR
An annual Veterans Health Administration (VHA) neurology seminar began in 2019 as a 1-day in-person event. Neurologists at the Michael E. DeBakey VA Medical Center in Houston presented in 50-minute sessions. Nonspecialist clinical personnel and neurology clinicians attended the event. Attendees requested making the presentations widely available and regularly repeating the seminar.
The second neurology seminar took place during the COVID-19 pandemic. It was conducted online and advertised across the Veterans Integrated Services Network (VISN) 16. The 1-day program had 204 participants who were primarily nurses (59%) and physicians (21%); 94% agreed with the program objectives (Table 1). Participants could earn CME credits for the 7 presentations primarily by VHA experts.
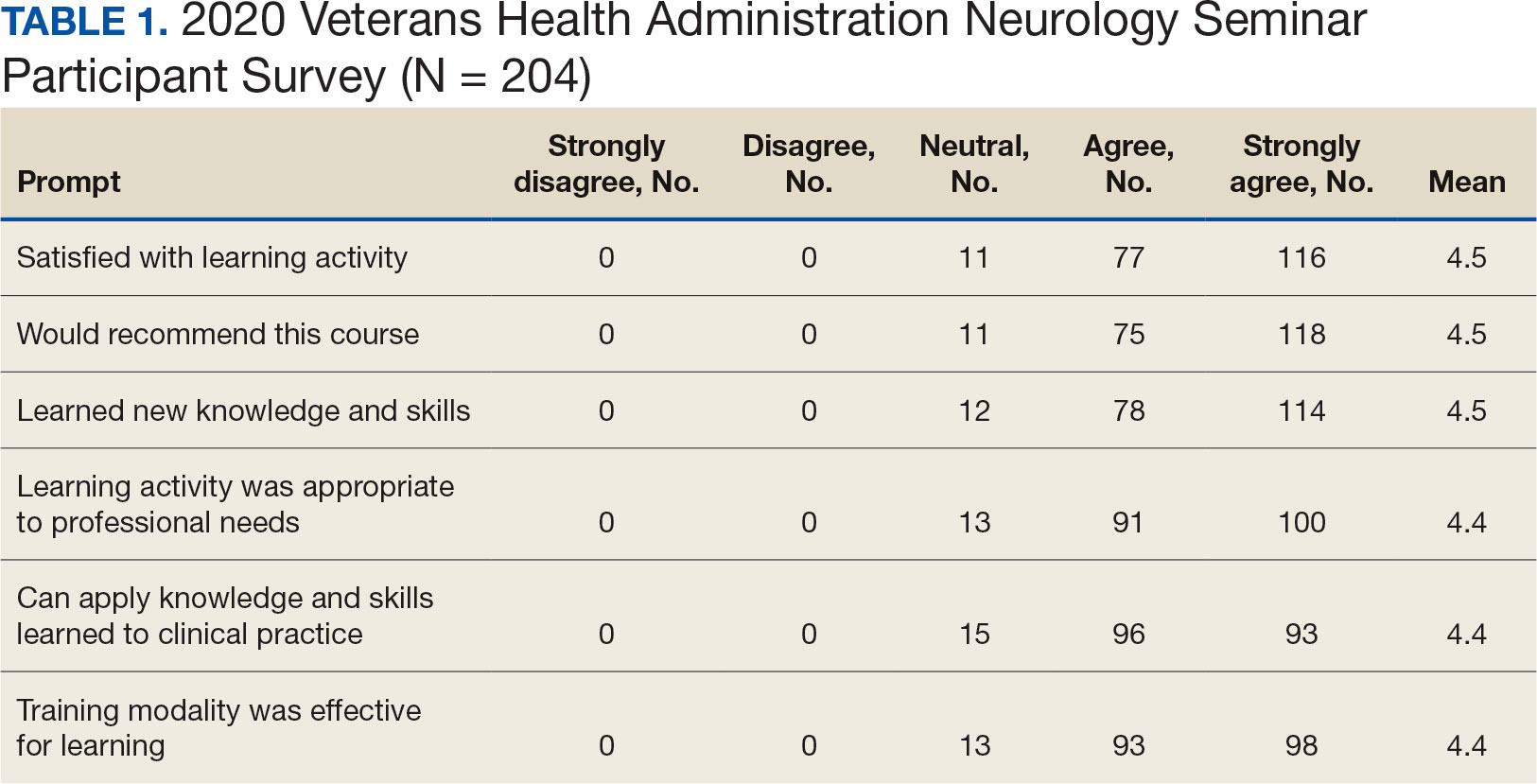
Based on feedback and a needs assessment, the program expanded in 2021 and 2022. With support from the national VHA neurology office and VHA Employee Education System (EES), the Institute for Learning, Education, and Development (ILEAD), the feedback identified topics that resonate with VHA clinicians. Neurological disorders in the fields of stroke, dementia, and headache were included since veterans with these disorders regularly visit primary care, geriatrics, mental health, and other clinical offices. Updates provided in the diagnosis and treatment of common neurological disorders were well received. Almost all speakers were VHA clinicians, which allowed them to focus on topics relevant to clinical practice at the VHA.
Attendance has increased annually. In 2021, 550 clinicians registered (52% nurses) and 433 completed the postseminar survey (Table 2). In 2022, 635 participants registered and 342 completed evaluations, including attendees from other federal agencies who were invited to participate via EES TRAIN (Training Finder Real-time Affiliate Integrated Network). Forty-seven participants from other federal agencies, including the US Department of Defense, National Institute of Health, and Centers for Disease Control and Prevention, completed the feedback evaluation via TRAIN (Table 3). Participants report high levels of satisfaction each year (mean of 4.5 on a 5-point scale). Respondents preferred conventional lecture presentation and case-based discussions for the teaching format and dementia was the most requested topic for future seminars (Table 4).
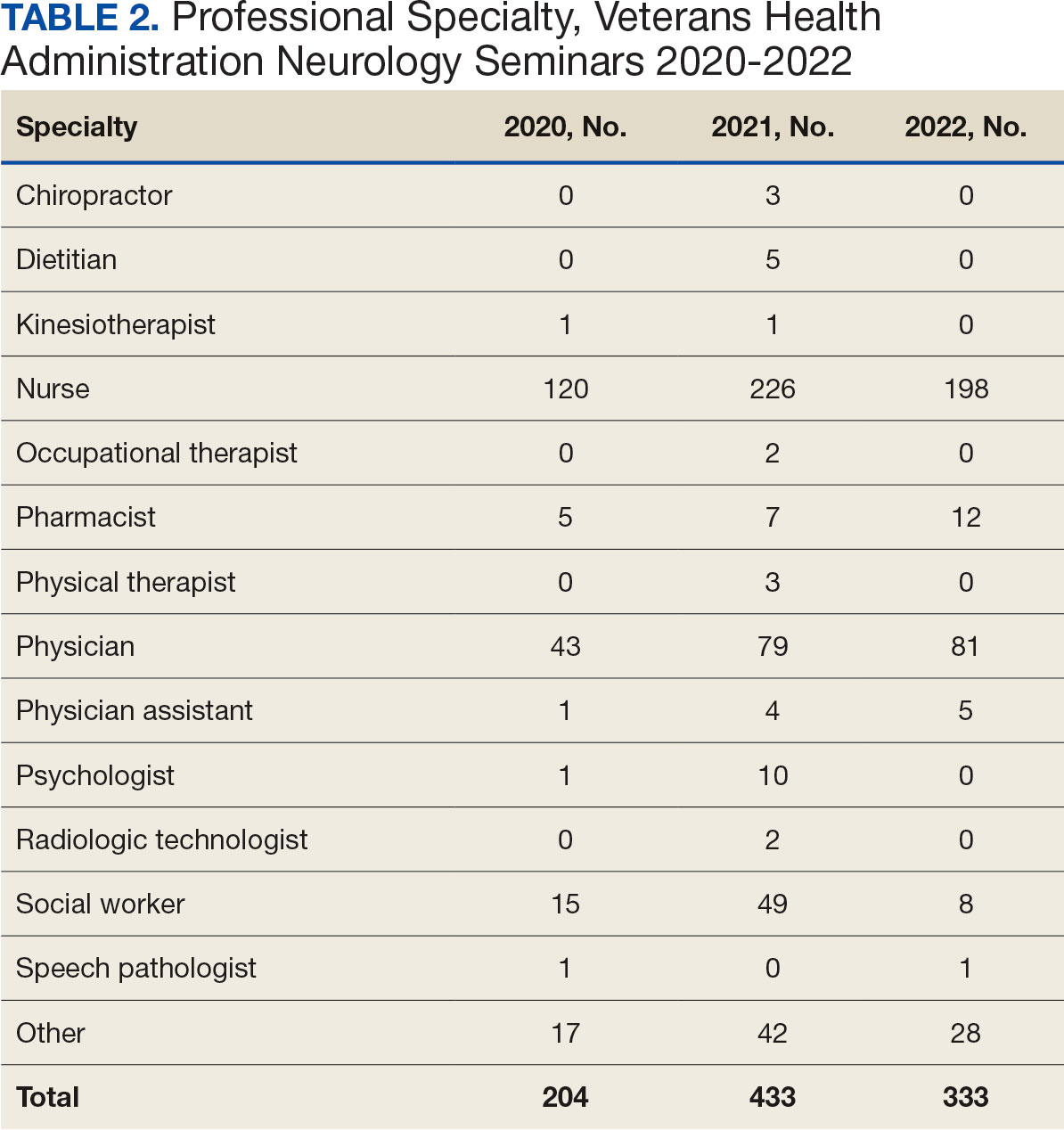

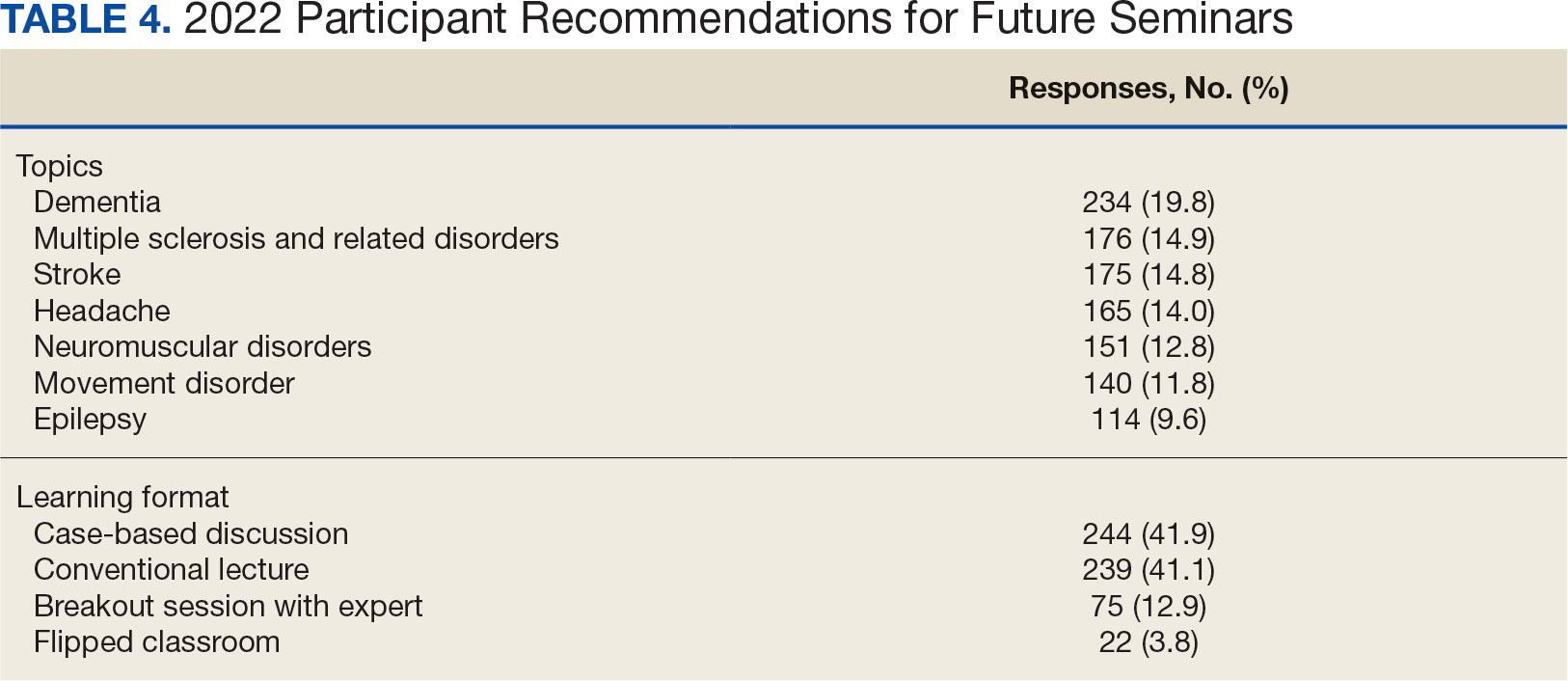
The content of each seminar was designed to include . 1 topic relevant to current clinical practice. The 2020 seminar covered topics of cerebrovascular complications of COVID- 19 and living well with neurodegenerative disease in the COVID-19 era. In 2021, the seminar included COVID-19 and neurologic manifestations. In 2022, topics included trends in stroke rehabilitation. In addition, ≥ 1 session addressed neurologic issues within the VHA. In 2020, the VA Deputy National Director of Neurology presented on the VHA stroke systems of care. In 2021, there was a presentation on traumatic brain injury (TBI) in the military. In 2022, sessions covered long term neurologic consequences of TBI and use of telemedicine for neurologic disorders. Feedback on the sessions were positive (eAppendix, available at doi:10.12788/fp.0545).

At the request of the participants, individual presentations were shared via email by the course director and speakers. In collaboration with the EES, each session was recorded and the 2022 seminar was made available to registrants in TMS and EES TRAIN and via the VHA Neurology SharePoint.
DISCUSSION
The annual VHA neurology seminar is a 1-day neurology conference that provides education to general neurologists and other clinicians caring for patients with neurologic disorders. It is the first of its kind neurology education program in the VHA covering most subspecialties in neurology and aims at improving neurologic patient care and access through education. Sessions have covered stroke, epilepsy, sleep, amyotrophic lateral sclerosis, neuropathy, dementia, movement disorders and Parkinson disease, headaches, multiple sclerosis, neurorehabilitation, and telehealth.
The seminar has transitioned from an inperson meeting to a virtual format, making neurology education more convenient and accessible. The virtual format provides the means to increase educational collaborations and share lecture platforms with other federal agencies. The program offers CME credits at no cost to government employees. Recorded lectures can also be asynchronously viewed from the Neurology SharePoint without the ability to earn CME credits. These recordings may be used to educate trainees as well.
The seminar aims to educate all health care professionals caring for patients with neurologic disorders. It aims to eliminate neurophobia, the fear of neural sciences and clinical neurology, and help general practitioners, especially in rural areas, take care of patients with neurologic disorders. The seminars introduce general practitioners to VHA neurology experts; the epilepsy, headache multiple sclerosis, and Parkinson disease centers of excellence; and the national programs for telestroke and teleneurology.
Education Support in the VHA
The EES/ILEAD provides a wide variety of learning opportunities to VHA employees on a broad range of topics, making it one of the largest medical education programs in the country. Pharmacists, social workers, psychologists, therapists, nurses, physician assistants, and physicians have access to certified training opportunities to gain knowledge and skills needed to provide high-quality, veteran-centered care.
A review of geriatrics learning activities through the EES found > 15,000 lectures from 1999 to 2009 for > 300,000 attendees.20 To our knowledge, a review of neurology-related learning activities offered by the EES/ILEAD has not been completed, but the study on geriatrics shows that a similar review would be feasible, given the integrated education system, and helpful in identifying what topics are covered, formats are used, and participants are engaged in neurology education at the VHA. This is a future project planned by the neurology education workgroup.
The EES/ILEAD arranged CME credit for the VHA Neurology Seminar and assisted in organizing an online event with > 500 attendees. Technology support and tools provided by EES during the virtual seminar, such as polling and chat features, kept the audience engaged. Other specialties may similarly value a virtual, all-day seminar format that is efficient and can encourage increased participation from practitioners, nurses, and clinicians.
Future Growth
We plan to increase future participation in the annual neurology seminar with primary care, geriatrics, neurology, and other specialties by instituting an improved and earlier marketing strategy. This includes working with the VHA neurology office to inform neurology practitioners as well as other program offices in the VHA. We intend to host the seminar the same day every year to make it easy for attendees to plan accordingly. In the future we may consider hybrid in-person and virtual modalities if feasible. We plan to focus on reaching out to other government agencies through platforms like TRAIN and the American Academy of Neurology government sections. Securing funding, administrative staff, and protected time in the future may help expand the program further.
Limitations
While a virtual format offers several advantages, using it removes the feel of an in-person meeting, which could be viewed by some attendees as a limitation. The other challenges and drawbacks of transitioning to the virtual platform for a national meeting are similar to those reported in the literature: time zone differences, internet issues, and participants having difficulty using certain online platforms. Attendance could also be limited by scheduling conflicts.16 Despite a large audience attending the seminar, many clinicians do not get protected time from their institutions. Institutional and leadership support at national and local levels will likely improve participation and help participants earn CME credits. While we are still doing a preliminary needs assessment, a formal needs assessment across federal governmental organizations will be helpful.
CONCLUSIONS
The annual VHA neurology seminar promotes interprofessional education, introduces neurology subspecialty centers of excellence, improves access to renowned neurology experts, and provides neurology-related updates through a VHA lens. The program not only provides educational updates to neurology clinicians, but also increases the confidence of non-neurology clinicians called to care for veterans with neurological disorders in their respective clinics.
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990- 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. doi:10.1016/S1474-4422(18)30499-X
- Baker V, Hack N. Improving access to care for patients with migraine in a remote Pacific population. Neurol Clin Pract. 2020;10(5):444-448. doi:10.1212/CPJ.0000000000000774
- Gutmann L, Cahill C, Jordan JT, et al. Characteristics of graduating US allopathic medical students pursuing a career in neurology. Neurology. 2019;92(17):e2051-e2063. doi:10.1212/WNL.0000000000007369
- Jordan JT, Cahill C, Ostendorf T, et al. Attracting neurology’s next generation: a qualitative study of specialty choice and perceptions. Neurology. 2020;95(8):e1080- e1090. doi:10.1212/WNL.0000000000009461
- Minen MT, Kaplan K, Akter S, et al. Understanding how to strengthen the neurology pipeline with insights from undergraduate neuroscience students. Neurology 2022;98(8):314-323. doi:10.1212/WNL.0000000000013259
- US Department of Veterans Affairs, Office of Academic Affiliations. To Educate for VA and the Nation. Updated August 1, 2024. Accessed August 15, 2024. https://www.va.gov/oaa/
- Schaefer SM, Dominguez M, Moeller JJ. The future of the lecture in neurology education. Semin Neurol. 2018;38(4):418-427. doi:10.1055/s-0038-1667042
- Curran VR. Tele-education. J Telemed Telecare. 2006;12(2):57-63. doi:10.1258/135763306776084400
- Lau KHV, Lakhan SE, Achike F. New media, technology and neurology education. Semin Neurol. 2018;38(4):457- 464. doi:10.1055/s-0038-1666985
- Quirk M, Chumley H. The adaptive medical curriculum: a model for continuous improvement. Med Teach. 2018;40(8):786-790. doi:10.1080/0142159X.2018.1484896
- Brockfeld T, Müller B, de Laffolie J. Video versus live lecture courses: a comparative evaluation of lecture types and results. Med Educ Online. 2018;23(1):1555434. doi:10.1080/10872981.2018.1555434
- Davis J, Crabb S, Rogers E, Zamora J, Khan K. Computer-based teaching is as good as face to face lecture-based teaching of evidence based medicine: a randomized controlled trial. Med Teach. 2008;30(3):302-307. doi:10.1080/01421590701784349
- Markova T, Roth LM, Monsur J. Synchronous distance learning as an effective and feasible method for delivering residency didactics. Fam Med. 2005;37(8):570-575.
- Naciri A, Radid M, Kharbach A, Chemsi G. E-learning in health professions education during the COVID-19 pandemic: a systematic review. J Educ Eval Health Prof. 2021;18:27. doi:10.3352/jeehp.2021.18.27
- Dedeilia A, Sotiropoulos MG, Hanrahan JG, Janga D, Dedeilias P, Sideris M. Medical and surgical education challenges and innovations in the COVID-19 era: a systematic review. In Vivo. 2020;34(3 Suppl):1603-1611. doi:10.21873/invivo.11950
- Weber DJ, Albert DVF, Aravamuthan BR, Bernson-Leung ME, Bhatti D, Milligan TA. Training in neurology: rapid implementation of cross-institutional neurology resident education in the time of COVID-19. Neurology. 2020;95(19):883-886. doi:10.1212/WNL.0000000000010753
- Frey J, Neeley B, Umer A, et al. Training in neurology: neuro day: an innovative curriculum connecting medical students with patients. Neurology. 2021;96(10):e1482- e1486. doi:10.1212/WNL.0000000000010859
- Schwartzstein RM, Dienstag JL, King RW, et al. The Harvard Medical School Pathways Curriculum: reimagining developmentally appropriate medical education for contemporary learners. Acad Med. 2020;95(11):1687-1695. doi:10.1097/ACM.0000000000003270
- Greer DM, Moeller J, Torres DR, et al. Funding the educational mission in neurology. Neurology. 2021;96(12):574- 582. doi:10.1212/WNL.0000000000011635
- Thielke S, Tumosa N, Lindenfeld R, Shay K. Geriatric focused educational offerings in the Department of Veterans Affairs from 1999 to 2009. Gerontol Geriatr Educ. 2011;32(1):38-53. doi:10.1080/02701960.2011.550214
Neurologic disorders are major causes of death and disability. Globally, the burden of neurologic disorders continues to increase. The prevalence of disabling neurologic disorders significantly increases with age. As people live longer, health care systems will face increasing demands for treatment, rehabilitation, and support services for neurologic disorders. The scarcity of established modifiable risks for most of the neurologic burden demonstrates how new knowledge is required to develop effective prevention and treatment strategies.1
A single-center study for chronic headache at a rural institution found that, when combined with public education, clinician education not only can increase access to care but also reduce specialist overuse, hospitalizations, polypharmacy, and emergency department visits.2 A predicted shortage of neurologists has sparked increased interest in the field and individual neurology educators are helping fuel its popularity.3-5
TELE-EDUCATION
Educating the next generation of health professionals is 1 of 4 statutory missions of the US Department of Veterans Affairs (VA).6 Tele-education (also known as telelearning and distance learning) deviates from traditional in-person classroom settings, in which the lecture has been a core pedagogic method.7 Audio, video, and online technologies provide health education and can overcome geographic barriers for rural and remote clinicians.8 Recent technological improvements have allowed for inexpensive and efficient dissemination of educational materials, including video lectures, podcasts, online modules, assessment materials, and even entire curricula.9
There has been an increase in the awareness of the parallel curriculum involving self-directed and asynchronous learning opportunities. 10 Several studies report knowledge gained via tele-education is comparable to conventional classroom learning.11-13 A systematic review of e-learning perceptions among health care students suggested benefits (eg, learning flexibility, pedagogical design, online interactions, basic computer skills, and access to technology) and drawbacks (eg, limited acquisition of clinical skills, internet connection problems, and issues with using educational platforms).1
The COVID-19 pandemic forced an abrupt cessation of traditional in-person education, forcing educational institutions and medical organizations to transition to telelearning. Solutions in the education field appeared during the pandemic, such as videoconferencing, social media, and telemedicine, that effectively addressed the sudden cessation of in-person medical education.15
Graduate medical education in neurology residency programs served as an experimental set up for tele-education during the pandemic. Residents from neurology training programs outlined the benefits of a volunteer lecturer-based online didactic program that was established to meet this need, which included exposure to subspeciality topics, access to subspecialist experts not available within the department, exposure to different pedagogic methods, interaction with members of other educational institutions and training programs, career development opportunities, and the potential for forming a community of learning.16
Not all recent educational developments are technology-based. For example, instruction focused on specific patient experiences, and learning processes that emphasize problem solving and personal responsibility over specific knowledge have been successful in neurology.17,18 Departments and institutions must be creative in finding ways to fund continuing education, especially when budgets are limited.19
ANNUAL NEUROLOGY SEMINAR
An annual Veterans Health Administration (VHA) neurology seminar began in 2019 as a 1-day in-person event. Neurologists at the Michael E. DeBakey VA Medical Center in Houston presented in 50-minute sessions. Nonspecialist clinical personnel and neurology clinicians attended the event. Attendees requested making the presentations widely available and regularly repeating the seminar.
The second neurology seminar took place during the COVID-19 pandemic. It was conducted online and advertised across the Veterans Integrated Services Network (VISN) 16. The 1-day program had 204 participants who were primarily nurses (59%) and physicians (21%); 94% agreed with the program objectives (Table 1). Participants could earn CME credits for the 7 presentations primarily by VHA experts.

Based on feedback and a needs assessment, the program expanded in 2021 and 2022. With support from the national VHA neurology office and VHA Employee Education System (EES), the Institute for Learning, Education, and Development (ILEAD), the feedback identified topics that resonate with VHA clinicians. Neurological disorders in the fields of stroke, dementia, and headache were included since veterans with these disorders regularly visit primary care, geriatrics, mental health, and other clinical offices. Updates provided in the diagnosis and treatment of common neurological disorders were well received. Almost all speakers were VHA clinicians, which allowed them to focus on topics relevant to clinical practice at the VHA.
Attendance has increased annually. In 2021, 550 clinicians registered (52% nurses) and 433 completed the postseminar survey (Table 2). In 2022, 635 participants registered and 342 completed evaluations, including attendees from other federal agencies who were invited to participate via EES TRAIN (Training Finder Real-time Affiliate Integrated Network). Forty-seven participants from other federal agencies, including the US Department of Defense, National Institute of Health, and Centers for Disease Control and Prevention, completed the feedback evaluation via TRAIN (Table 3). Participants report high levels of satisfaction each year (mean of 4.5 on a 5-point scale). Respondents preferred conventional lecture presentation and case-based discussions for the teaching format and dementia was the most requested topic for future seminars (Table 4).



The content of each seminar was designed to include . 1 topic relevant to current clinical practice. The 2020 seminar covered topics of cerebrovascular complications of COVID- 19 and living well with neurodegenerative disease in the COVID-19 era. In 2021, the seminar included COVID-19 and neurologic manifestations. In 2022, topics included trends in stroke rehabilitation. In addition, ≥ 1 session addressed neurologic issues within the VHA. In 2020, the VA Deputy National Director of Neurology presented on the VHA stroke systems of care. In 2021, there was a presentation on traumatic brain injury (TBI) in the military. In 2022, sessions covered long term neurologic consequences of TBI and use of telemedicine for neurologic disorders. Feedback on the sessions were positive (eAppendix, available at doi:10.12788/fp.0545).

At the request of the participants, individual presentations were shared via email by the course director and speakers. In collaboration with the EES, each session was recorded and the 2022 seminar was made available to registrants in TMS and EES TRAIN and via the VHA Neurology SharePoint.
DISCUSSION
The annual VHA neurology seminar is a 1-day neurology conference that provides education to general neurologists and other clinicians caring for patients with neurologic disorders. It is the first of its kind neurology education program in the VHA covering most subspecialties in neurology and aims at improving neurologic patient care and access through education. Sessions have covered stroke, epilepsy, sleep, amyotrophic lateral sclerosis, neuropathy, dementia, movement disorders and Parkinson disease, headaches, multiple sclerosis, neurorehabilitation, and telehealth.
The seminar has transitioned from an inperson meeting to a virtual format, making neurology education more convenient and accessible. The virtual format provides the means to increase educational collaborations and share lecture platforms with other federal agencies. The program offers CME credits at no cost to government employees. Recorded lectures can also be asynchronously viewed from the Neurology SharePoint without the ability to earn CME credits. These recordings may be used to educate trainees as well.
The seminar aims to educate all health care professionals caring for patients with neurologic disorders. It aims to eliminate neurophobia, the fear of neural sciences and clinical neurology, and help general practitioners, especially in rural areas, take care of patients with neurologic disorders. The seminars introduce general practitioners to VHA neurology experts; the epilepsy, headache multiple sclerosis, and Parkinson disease centers of excellence; and the national programs for telestroke and teleneurology.
Education Support in the VHA
The EES/ILEAD provides a wide variety of learning opportunities to VHA employees on a broad range of topics, making it one of the largest medical education programs in the country. Pharmacists, social workers, psychologists, therapists, nurses, physician assistants, and physicians have access to certified training opportunities to gain knowledge and skills needed to provide high-quality, veteran-centered care.
A review of geriatrics learning activities through the EES found > 15,000 lectures from 1999 to 2009 for > 300,000 attendees.20 To our knowledge, a review of neurology-related learning activities offered by the EES/ILEAD has not been completed, but the study on geriatrics shows that a similar review would be feasible, given the integrated education system, and helpful in identifying what topics are covered, formats are used, and participants are engaged in neurology education at the VHA. This is a future project planned by the neurology education workgroup.
The EES/ILEAD arranged CME credit for the VHA Neurology Seminar and assisted in organizing an online event with > 500 attendees. Technology support and tools provided by EES during the virtual seminar, such as polling and chat features, kept the audience engaged. Other specialties may similarly value a virtual, all-day seminar format that is efficient and can encourage increased participation from practitioners, nurses, and clinicians.
Future Growth
We plan to increase future participation in the annual neurology seminar with primary care, geriatrics, neurology, and other specialties by instituting an improved and earlier marketing strategy. This includes working with the VHA neurology office to inform neurology practitioners as well as other program offices in the VHA. We intend to host the seminar the same day every year to make it easy for attendees to plan accordingly. In the future we may consider hybrid in-person and virtual modalities if feasible. We plan to focus on reaching out to other government agencies through platforms like TRAIN and the American Academy of Neurology government sections. Securing funding, administrative staff, and protected time in the future may help expand the program further.
Limitations
While a virtual format offers several advantages, using it removes the feel of an in-person meeting, which could be viewed by some attendees as a limitation. The other challenges and drawbacks of transitioning to the virtual platform for a national meeting are similar to those reported in the literature: time zone differences, internet issues, and participants having difficulty using certain online platforms. Attendance could also be limited by scheduling conflicts.16 Despite a large audience attending the seminar, many clinicians do not get protected time from their institutions. Institutional and leadership support at national and local levels will likely improve participation and help participants earn CME credits. While we are still doing a preliminary needs assessment, a formal needs assessment across federal governmental organizations will be helpful.
CONCLUSIONS
The annual VHA neurology seminar promotes interprofessional education, introduces neurology subspecialty centers of excellence, improves access to renowned neurology experts, and provides neurology-related updates through a VHA lens. The program not only provides educational updates to neurology clinicians, but also increases the confidence of non-neurology clinicians called to care for veterans with neurological disorders in their respective clinics.
Neurologic disorders are major causes of death and disability. Globally, the burden of neurologic disorders continues to increase. The prevalence of disabling neurologic disorders significantly increases with age. As people live longer, health care systems will face increasing demands for treatment, rehabilitation, and support services for neurologic disorders. The scarcity of established modifiable risks for most of the neurologic burden demonstrates how new knowledge is required to develop effective prevention and treatment strategies.1
A single-center study for chronic headache at a rural institution found that, when combined with public education, clinician education not only can increase access to care but also reduce specialist overuse, hospitalizations, polypharmacy, and emergency department visits.2 A predicted shortage of neurologists has sparked increased interest in the field and individual neurology educators are helping fuel its popularity.3-5
TELE-EDUCATION
Educating the next generation of health professionals is 1 of 4 statutory missions of the US Department of Veterans Affairs (VA).6 Tele-education (also known as telelearning and distance learning) deviates from traditional in-person classroom settings, in which the lecture has been a core pedagogic method.7 Audio, video, and online technologies provide health education and can overcome geographic barriers for rural and remote clinicians.8 Recent technological improvements have allowed for inexpensive and efficient dissemination of educational materials, including video lectures, podcasts, online modules, assessment materials, and even entire curricula.9
There has been an increase in the awareness of the parallel curriculum involving self-directed and asynchronous learning opportunities. 10 Several studies report knowledge gained via tele-education is comparable to conventional classroom learning.11-13 A systematic review of e-learning perceptions among health care students suggested benefits (eg, learning flexibility, pedagogical design, online interactions, basic computer skills, and access to technology) and drawbacks (eg, limited acquisition of clinical skills, internet connection problems, and issues with using educational platforms).1
The COVID-19 pandemic forced an abrupt cessation of traditional in-person education, forcing educational institutions and medical organizations to transition to telelearning. Solutions in the education field appeared during the pandemic, such as videoconferencing, social media, and telemedicine, that effectively addressed the sudden cessation of in-person medical education.15
Graduate medical education in neurology residency programs served as an experimental set up for tele-education during the pandemic. Residents from neurology training programs outlined the benefits of a volunteer lecturer-based online didactic program that was established to meet this need, which included exposure to subspeciality topics, access to subspecialist experts not available within the department, exposure to different pedagogic methods, interaction with members of other educational institutions and training programs, career development opportunities, and the potential for forming a community of learning.16
Not all recent educational developments are technology-based. For example, instruction focused on specific patient experiences, and learning processes that emphasize problem solving and personal responsibility over specific knowledge have been successful in neurology.17,18 Departments and institutions must be creative in finding ways to fund continuing education, especially when budgets are limited.19
ANNUAL NEUROLOGY SEMINAR
An annual Veterans Health Administration (VHA) neurology seminar began in 2019 as a 1-day in-person event. Neurologists at the Michael E. DeBakey VA Medical Center in Houston presented in 50-minute sessions. Nonspecialist clinical personnel and neurology clinicians attended the event. Attendees requested making the presentations widely available and regularly repeating the seminar.
The second neurology seminar took place during the COVID-19 pandemic. It was conducted online and advertised across the Veterans Integrated Services Network (VISN) 16. The 1-day program had 204 participants who were primarily nurses (59%) and physicians (21%); 94% agreed with the program objectives (Table 1). Participants could earn CME credits for the 7 presentations primarily by VHA experts.

Based on feedback and a needs assessment, the program expanded in 2021 and 2022. With support from the national VHA neurology office and VHA Employee Education System (EES), the Institute for Learning, Education, and Development (ILEAD), the feedback identified topics that resonate with VHA clinicians. Neurological disorders in the fields of stroke, dementia, and headache were included since veterans with these disorders regularly visit primary care, geriatrics, mental health, and other clinical offices. Updates provided in the diagnosis and treatment of common neurological disorders were well received. Almost all speakers were VHA clinicians, which allowed them to focus on topics relevant to clinical practice at the VHA.
Attendance has increased annually. In 2021, 550 clinicians registered (52% nurses) and 433 completed the postseminar survey (Table 2). In 2022, 635 participants registered and 342 completed evaluations, including attendees from other federal agencies who were invited to participate via EES TRAIN (Training Finder Real-time Affiliate Integrated Network). Forty-seven participants from other federal agencies, including the US Department of Defense, National Institute of Health, and Centers for Disease Control and Prevention, completed the feedback evaluation via TRAIN (Table 3). Participants report high levels of satisfaction each year (mean of 4.5 on a 5-point scale). Respondents preferred conventional lecture presentation and case-based discussions for the teaching format and dementia was the most requested topic for future seminars (Table 4).



The content of each seminar was designed to include . 1 topic relevant to current clinical practice. The 2020 seminar covered topics of cerebrovascular complications of COVID- 19 and living well with neurodegenerative disease in the COVID-19 era. In 2021, the seminar included COVID-19 and neurologic manifestations. In 2022, topics included trends in stroke rehabilitation. In addition, ≥ 1 session addressed neurologic issues within the VHA. In 2020, the VA Deputy National Director of Neurology presented on the VHA stroke systems of care. In 2021, there was a presentation on traumatic brain injury (TBI) in the military. In 2022, sessions covered long term neurologic consequences of TBI and use of telemedicine for neurologic disorders. Feedback on the sessions were positive (eAppendix, available at doi:10.12788/fp.0545).

At the request of the participants, individual presentations were shared via email by the course director and speakers. In collaboration with the EES, each session was recorded and the 2022 seminar was made available to registrants in TMS and EES TRAIN and via the VHA Neurology SharePoint.
DISCUSSION
The annual VHA neurology seminar is a 1-day neurology conference that provides education to general neurologists and other clinicians caring for patients with neurologic disorders. It is the first of its kind neurology education program in the VHA covering most subspecialties in neurology and aims at improving neurologic patient care and access through education. Sessions have covered stroke, epilepsy, sleep, amyotrophic lateral sclerosis, neuropathy, dementia, movement disorders and Parkinson disease, headaches, multiple sclerosis, neurorehabilitation, and telehealth.
The seminar has transitioned from an inperson meeting to a virtual format, making neurology education more convenient and accessible. The virtual format provides the means to increase educational collaborations and share lecture platforms with other federal agencies. The program offers CME credits at no cost to government employees. Recorded lectures can also be asynchronously viewed from the Neurology SharePoint without the ability to earn CME credits. These recordings may be used to educate trainees as well.
The seminar aims to educate all health care professionals caring for patients with neurologic disorders. It aims to eliminate neurophobia, the fear of neural sciences and clinical neurology, and help general practitioners, especially in rural areas, take care of patients with neurologic disorders. The seminars introduce general practitioners to VHA neurology experts; the epilepsy, headache multiple sclerosis, and Parkinson disease centers of excellence; and the national programs for telestroke and teleneurology.
Education Support in the VHA
The EES/ILEAD provides a wide variety of learning opportunities to VHA employees on a broad range of topics, making it one of the largest medical education programs in the country. Pharmacists, social workers, psychologists, therapists, nurses, physician assistants, and physicians have access to certified training opportunities to gain knowledge and skills needed to provide high-quality, veteran-centered care.
A review of geriatrics learning activities through the EES found > 15,000 lectures from 1999 to 2009 for > 300,000 attendees.20 To our knowledge, a review of neurology-related learning activities offered by the EES/ILEAD has not been completed, but the study on geriatrics shows that a similar review would be feasible, given the integrated education system, and helpful in identifying what topics are covered, formats are used, and participants are engaged in neurology education at the VHA. This is a future project planned by the neurology education workgroup.
The EES/ILEAD arranged CME credit for the VHA Neurology Seminar and assisted in organizing an online event with > 500 attendees. Technology support and tools provided by EES during the virtual seminar, such as polling and chat features, kept the audience engaged. Other specialties may similarly value a virtual, all-day seminar format that is efficient and can encourage increased participation from practitioners, nurses, and clinicians.
Future Growth
We plan to increase future participation in the annual neurology seminar with primary care, geriatrics, neurology, and other specialties by instituting an improved and earlier marketing strategy. This includes working with the VHA neurology office to inform neurology practitioners as well as other program offices in the VHA. We intend to host the seminar the same day every year to make it easy for attendees to plan accordingly. In the future we may consider hybrid in-person and virtual modalities if feasible. We plan to focus on reaching out to other government agencies through platforms like TRAIN and the American Academy of Neurology government sections. Securing funding, administrative staff, and protected time in the future may help expand the program further.
Limitations
While a virtual format offers several advantages, using it removes the feel of an in-person meeting, which could be viewed by some attendees as a limitation. The other challenges and drawbacks of transitioning to the virtual platform for a national meeting are similar to those reported in the literature: time zone differences, internet issues, and participants having difficulty using certain online platforms. Attendance could also be limited by scheduling conflicts.16 Despite a large audience attending the seminar, many clinicians do not get protected time from their institutions. Institutional and leadership support at national and local levels will likely improve participation and help participants earn CME credits. While we are still doing a preliminary needs assessment, a formal needs assessment across federal governmental organizations will be helpful.
CONCLUSIONS
The annual VHA neurology seminar promotes interprofessional education, introduces neurology subspecialty centers of excellence, improves access to renowned neurology experts, and provides neurology-related updates through a VHA lens. The program not only provides educational updates to neurology clinicians, but also increases the confidence of non-neurology clinicians called to care for veterans with neurological disorders in their respective clinics.
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990- 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. doi:10.1016/S1474-4422(18)30499-X
- Baker V, Hack N. Improving access to care for patients with migraine in a remote Pacific population. Neurol Clin Pract. 2020;10(5):444-448. doi:10.1212/CPJ.0000000000000774
- Gutmann L, Cahill C, Jordan JT, et al. Characteristics of graduating US allopathic medical students pursuing a career in neurology. Neurology. 2019;92(17):e2051-e2063. doi:10.1212/WNL.0000000000007369
- Jordan JT, Cahill C, Ostendorf T, et al. Attracting neurology’s next generation: a qualitative study of specialty choice and perceptions. Neurology. 2020;95(8):e1080- e1090. doi:10.1212/WNL.0000000000009461
- Minen MT, Kaplan K, Akter S, et al. Understanding how to strengthen the neurology pipeline with insights from undergraduate neuroscience students. Neurology 2022;98(8):314-323. doi:10.1212/WNL.0000000000013259
- US Department of Veterans Affairs, Office of Academic Affiliations. To Educate for VA and the Nation. Updated August 1, 2024. Accessed August 15, 2024. https://www.va.gov/oaa/
- Schaefer SM, Dominguez M, Moeller JJ. The future of the lecture in neurology education. Semin Neurol. 2018;38(4):418-427. doi:10.1055/s-0038-1667042
- Curran VR. Tele-education. J Telemed Telecare. 2006;12(2):57-63. doi:10.1258/135763306776084400
- Lau KHV, Lakhan SE, Achike F. New media, technology and neurology education. Semin Neurol. 2018;38(4):457- 464. doi:10.1055/s-0038-1666985
- Quirk M, Chumley H. The adaptive medical curriculum: a model for continuous improvement. Med Teach. 2018;40(8):786-790. doi:10.1080/0142159X.2018.1484896
- Brockfeld T, Müller B, de Laffolie J. Video versus live lecture courses: a comparative evaluation of lecture types and results. Med Educ Online. 2018;23(1):1555434. doi:10.1080/10872981.2018.1555434
- Davis J, Crabb S, Rogers E, Zamora J, Khan K. Computer-based teaching is as good as face to face lecture-based teaching of evidence based medicine: a randomized controlled trial. Med Teach. 2008;30(3):302-307. doi:10.1080/01421590701784349
- Markova T, Roth LM, Monsur J. Synchronous distance learning as an effective and feasible method for delivering residency didactics. Fam Med. 2005;37(8):570-575.
- Naciri A, Radid M, Kharbach A, Chemsi G. E-learning in health professions education during the COVID-19 pandemic: a systematic review. J Educ Eval Health Prof. 2021;18:27. doi:10.3352/jeehp.2021.18.27
- Dedeilia A, Sotiropoulos MG, Hanrahan JG, Janga D, Dedeilias P, Sideris M. Medical and surgical education challenges and innovations in the COVID-19 era: a systematic review. In Vivo. 2020;34(3 Suppl):1603-1611. doi:10.21873/invivo.11950
- Weber DJ, Albert DVF, Aravamuthan BR, Bernson-Leung ME, Bhatti D, Milligan TA. Training in neurology: rapid implementation of cross-institutional neurology resident education in the time of COVID-19. Neurology. 2020;95(19):883-886. doi:10.1212/WNL.0000000000010753
- Frey J, Neeley B, Umer A, et al. Training in neurology: neuro day: an innovative curriculum connecting medical students with patients. Neurology. 2021;96(10):e1482- e1486. doi:10.1212/WNL.0000000000010859
- Schwartzstein RM, Dienstag JL, King RW, et al. The Harvard Medical School Pathways Curriculum: reimagining developmentally appropriate medical education for contemporary learners. Acad Med. 2020;95(11):1687-1695. doi:10.1097/ACM.0000000000003270
- Greer DM, Moeller J, Torres DR, et al. Funding the educational mission in neurology. Neurology. 2021;96(12):574- 582. doi:10.1212/WNL.0000000000011635
- Thielke S, Tumosa N, Lindenfeld R, Shay K. Geriatric focused educational offerings in the Department of Veterans Affairs from 1999 to 2009. Gerontol Geriatr Educ. 2011;32(1):38-53. doi:10.1080/02701960.2011.550214
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990- 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. doi:10.1016/S1474-4422(18)30499-X
- Baker V, Hack N. Improving access to care for patients with migraine in a remote Pacific population. Neurol Clin Pract. 2020;10(5):444-448. doi:10.1212/CPJ.0000000000000774
- Gutmann L, Cahill C, Jordan JT, et al. Characteristics of graduating US allopathic medical students pursuing a career in neurology. Neurology. 2019;92(17):e2051-e2063. doi:10.1212/WNL.0000000000007369
- Jordan JT, Cahill C, Ostendorf T, et al. Attracting neurology’s next generation: a qualitative study of specialty choice and perceptions. Neurology. 2020;95(8):e1080- e1090. doi:10.1212/WNL.0000000000009461
- Minen MT, Kaplan K, Akter S, et al. Understanding how to strengthen the neurology pipeline with insights from undergraduate neuroscience students. Neurology 2022;98(8):314-323. doi:10.1212/WNL.0000000000013259
- US Department of Veterans Affairs, Office of Academic Affiliations. To Educate for VA and the Nation. Updated August 1, 2024. Accessed August 15, 2024. https://www.va.gov/oaa/
- Schaefer SM, Dominguez M, Moeller JJ. The future of the lecture in neurology education. Semin Neurol. 2018;38(4):418-427. doi:10.1055/s-0038-1667042
- Curran VR. Tele-education. J Telemed Telecare. 2006;12(2):57-63. doi:10.1258/135763306776084400
- Lau KHV, Lakhan SE, Achike F. New media, technology and neurology education. Semin Neurol. 2018;38(4):457- 464. doi:10.1055/s-0038-1666985
- Quirk M, Chumley H. The adaptive medical curriculum: a model for continuous improvement. Med Teach. 2018;40(8):786-790. doi:10.1080/0142159X.2018.1484896
- Brockfeld T, Müller B, de Laffolie J. Video versus live lecture courses: a comparative evaluation of lecture types and results. Med Educ Online. 2018;23(1):1555434. doi:10.1080/10872981.2018.1555434
- Davis J, Crabb S, Rogers E, Zamora J, Khan K. Computer-based teaching is as good as face to face lecture-based teaching of evidence based medicine: a randomized controlled trial. Med Teach. 2008;30(3):302-307. doi:10.1080/01421590701784349
- Markova T, Roth LM, Monsur J. Synchronous distance learning as an effective and feasible method for delivering residency didactics. Fam Med. 2005;37(8):570-575.
- Naciri A, Radid M, Kharbach A, Chemsi G. E-learning in health professions education during the COVID-19 pandemic: a systematic review. J Educ Eval Health Prof. 2021;18:27. doi:10.3352/jeehp.2021.18.27
- Dedeilia A, Sotiropoulos MG, Hanrahan JG, Janga D, Dedeilias P, Sideris M. Medical and surgical education challenges and innovations in the COVID-19 era: a systematic review. In Vivo. 2020;34(3 Suppl):1603-1611. doi:10.21873/invivo.11950
- Weber DJ, Albert DVF, Aravamuthan BR, Bernson-Leung ME, Bhatti D, Milligan TA. Training in neurology: rapid implementation of cross-institutional neurology resident education in the time of COVID-19. Neurology. 2020;95(19):883-886. doi:10.1212/WNL.0000000000010753
- Frey J, Neeley B, Umer A, et al. Training in neurology: neuro day: an innovative curriculum connecting medical students with patients. Neurology. 2021;96(10):e1482- e1486. doi:10.1212/WNL.0000000000010859
- Schwartzstein RM, Dienstag JL, King RW, et al. The Harvard Medical School Pathways Curriculum: reimagining developmentally appropriate medical education for contemporary learners. Acad Med. 2020;95(11):1687-1695. doi:10.1097/ACM.0000000000003270
- Greer DM, Moeller J, Torres DR, et al. Funding the educational mission in neurology. Neurology. 2021;96(12):574- 582. doi:10.1212/WNL.0000000000011635
- Thielke S, Tumosa N, Lindenfeld R, Shay K. Geriatric focused educational offerings in the Department of Veterans Affairs from 1999 to 2009. Gerontol Geriatr Educ. 2011;32(1):38-53. doi:10.1080/02701960.2011.550214
Improving Interprofessional Neurology Training Using Tele-Education
Improving Interprofessional Neurology Training Using Tele-Education
Gestational Eclampsia Linked to Fivefold Epilepsy Risk
Gestational hypertension, preeclampsia, or eclampsia is associated with a significantly higher risk for neurologic disorders such as migraine or epilepsy in the years following a first pregnancy, new research suggests.
The risk was highest in those with gestational eclampsia, who had a 70% increased chance of developing a neurologic disorder, including a fivefold increased risk for epilepsy, investigators found.
“When consulting women with new-onset neurological disorders, it’s important to inquire about their pregnancy history, as pregnancy complications such as gestational hypertension, preeclampsia, and eclampsia have been associated with an increased risk of neurological disorders later in life.” Therese Friis, MD, PhD student, Department of Women’s and Children’s Health, Uppsala University in Sweden, said in an interview.
The findings were published online in JAMA Neurology.
Most studies of maternal outcomes after gestational hypertension, preeclampsia, or eclampsia have focused on long-term risks for cardiovascular disease or neurologic complications such as stroke, dementia, and cognitive impairment. And many of these studies were relatively small and based on interviews or questionnaires.
“We wanted to investigate whether women with a hypertensive disorder of pregnancy also had a risk of other neurological complications, closer in time to the pregnancy,” said Friis.
The new study included 648,385 women (mean age, 28.5 years) whose first pregnancy occurred between 2005 and 2018. Of these, 94% had a normotensive pregnancy, 2% had gestational hypertension, 4% had preeclampsia without eclampsia, and 0.1% had eclampsia.
Gestational hypertension was defined as new-onset systolic blood pressure of ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg; preeclampsia was defined as gestational hypertension accompanied by proteinuria; and eclampsia was defined as tonic-clonic seizures without other etiology accompanied by preeclampsia.
Researchers used linked Swedish national registries that collect data on pregnancies, births, and infant and maternal characteristics. Among other things, they controlled for maternal age, early pregnancy body mass index, education level, and pregestational and gestational diabetes.
The primary outcome was a composite of five new-onset neurologic diagnoses: Migraine, headache, epilepsy, sleep disorder, and mental fatigue (neurasthenia), although one diagnosis was sufficient, from 42 days to 15 years after childbirth. Mean follow-up was 7.7 years.
Fivefold Epilepsy Risk
Compared with normotensive pregnancies, the risk for the primary outcome was 70% greater in those with eclampsia (adjusted hazard ratio [aHR], 1.70; 95% CI, 1.16-2.50), 27% higher for gestational hypertension (aHR, 1.27; 95% CI, 1.12-1.45), and 32% for preeclampsia (aHR, 1.32; 95% CI, 1.22-1.42).
Researchers also looked at the risk for individual neurologic disorders. There were too few neurasthenia events to generate meaningful results, so this diagnosis was omitted from the analysis.
Here, the study found women with eclampsia had five times the risk for epilepsy (aHR, 5.31; 95% CI, 2.85-9.89) compared with women with normotensive pregnancies.
The underlying mechanism for this association is unclear. However, said Friis, eclampsia and epilepsy share common pathways, such as neuroinflammation, and women with epilepsy before pregnancy run an increased risk for eclampsia.
“So common underlying pathways might increase the risk both for eclampsia in cases of a seizure disorder and a future seizure disorder after eclampsia,” she said.
In addition, preeclampsia and, in particular, eclampsia can cause irreversible subclinical cerebral infarcts found in areas of cerebral edema, she added. “These infarcts or scarring of brain tissue could potentially serve as foci for later epileptic activity.”
Researchers separated women with preeclampsia (with or without eclampsia) into those with preterm (less than 37 weeks; 21%) and term (79%) deliveries. As Friis explained, women with preterm preeclampsia have a higher risk for acute complications and long-term cardiovascular outcomes than those with term preeclampsia.
Compared with those with normotensive pregnancies, investigators found an increased risk for the composite neurologic outcome among women with preterm preeclampsia (aHR, 1.54; 95% CI, 1.34-1.79), but also for those with term preeclampsia (aHR, 1.27; 95% CI, 1.17-1.38).
Common Vascular Component
The study also showed gestational hypertension and preeclampsia were associated with a later diagnosis of migraine, suggesting a possible common underlying vascular component.
“The increased risk of migraine following preeclampsia could be linked to endothelial damage at the blood-brain barrier level and alterations in cerebral blood flow and arterial vasospasm found in eclampsia, but this is only speculation,” said Friis.
The analysis also found an association between preeclampsia and a later diagnosis of headache. But this result likely encompasses several headache diagnoses, including migraine, so “it’s challenging to draw conclusions about the underlying mechanisms,” Friis added.
The study didn’t consider diagnoses from primary healthcare, which resulted in relatively few outcomes. The authors explained they could only identify the most severe cases; for example, women referred to specialized care.
Another potential study limitation is that Swedish registers don’t include information on race or ethnicity. Evidence shows there are racial differences in the risk for cardiovascular outcomes after preeclampsia.
This area of research is important as most women will experience at least one pregnancy in their lifetime, and preeclampsia affects 3%-5% of pregnancies. Further research is needed to understand the underlying pathophysiological mechanisms and the long-term consequences of this disorder, said Friis.
She added she hopes more therapeutic options will be available in the future for neuroprotective treatment for women with gestational hypertensive disorders.
Asked to comment, Thomas Vidic, MD, clinical professor of neurology, Indiana University School of Medicine, South Bend, said in an interview that this is an important study that includes robust data.
In his opinion, the most significant study finding is the marked increase in epilepsy risk after gestational eclampsia.
“In women who have new-onset epilepsy of unknown cause, asking about having eclampsia or preeclampsia during pregnancy is definitely a worthwhile question,” he said.
Confirming an etiology paints “a better picture” for patients wondering why they’re experiencing seizures, he added.
As with any registry-based study, this one had some acknowledged limitations, “but at the same time, the authors were able to have such a large database that I think this study is very worthwhile,” said Vidic.
The study received support from the Swedish Research Council. Neither Friis nor Vidic had relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Gestational hypertension, preeclampsia, or eclampsia is associated with a significantly higher risk for neurologic disorders such as migraine or epilepsy in the years following a first pregnancy, new research suggests.
The risk was highest in those with gestational eclampsia, who had a 70% increased chance of developing a neurologic disorder, including a fivefold increased risk for epilepsy, investigators found.
“When consulting women with new-onset neurological disorders, it’s important to inquire about their pregnancy history, as pregnancy complications such as gestational hypertension, preeclampsia, and eclampsia have been associated with an increased risk of neurological disorders later in life.” Therese Friis, MD, PhD student, Department of Women’s and Children’s Health, Uppsala University in Sweden, said in an interview.
The findings were published online in JAMA Neurology.
Most studies of maternal outcomes after gestational hypertension, preeclampsia, or eclampsia have focused on long-term risks for cardiovascular disease or neurologic complications such as stroke, dementia, and cognitive impairment. And many of these studies were relatively small and based on interviews or questionnaires.
“We wanted to investigate whether women with a hypertensive disorder of pregnancy also had a risk of other neurological complications, closer in time to the pregnancy,” said Friis.
The new study included 648,385 women (mean age, 28.5 years) whose first pregnancy occurred between 2005 and 2018. Of these, 94% had a normotensive pregnancy, 2% had gestational hypertension, 4% had preeclampsia without eclampsia, and 0.1% had eclampsia.
Gestational hypertension was defined as new-onset systolic blood pressure of ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg; preeclampsia was defined as gestational hypertension accompanied by proteinuria; and eclampsia was defined as tonic-clonic seizures without other etiology accompanied by preeclampsia.
Researchers used linked Swedish national registries that collect data on pregnancies, births, and infant and maternal characteristics. Among other things, they controlled for maternal age, early pregnancy body mass index, education level, and pregestational and gestational diabetes.
The primary outcome was a composite of five new-onset neurologic diagnoses: Migraine, headache, epilepsy, sleep disorder, and mental fatigue (neurasthenia), although one diagnosis was sufficient, from 42 days to 15 years after childbirth. Mean follow-up was 7.7 years.
Fivefold Epilepsy Risk
Compared with normotensive pregnancies, the risk for the primary outcome was 70% greater in those with eclampsia (adjusted hazard ratio [aHR], 1.70; 95% CI, 1.16-2.50), 27% higher for gestational hypertension (aHR, 1.27; 95% CI, 1.12-1.45), and 32% for preeclampsia (aHR, 1.32; 95% CI, 1.22-1.42).
Researchers also looked at the risk for individual neurologic disorders. There were too few neurasthenia events to generate meaningful results, so this diagnosis was omitted from the analysis.
Here, the study found women with eclampsia had five times the risk for epilepsy (aHR, 5.31; 95% CI, 2.85-9.89) compared with women with normotensive pregnancies.
The underlying mechanism for this association is unclear. However, said Friis, eclampsia and epilepsy share common pathways, such as neuroinflammation, and women with epilepsy before pregnancy run an increased risk for eclampsia.
“So common underlying pathways might increase the risk both for eclampsia in cases of a seizure disorder and a future seizure disorder after eclampsia,” she said.
In addition, preeclampsia and, in particular, eclampsia can cause irreversible subclinical cerebral infarcts found in areas of cerebral edema, she added. “These infarcts or scarring of brain tissue could potentially serve as foci for later epileptic activity.”
Researchers separated women with preeclampsia (with or without eclampsia) into those with preterm (less than 37 weeks; 21%) and term (79%) deliveries. As Friis explained, women with preterm preeclampsia have a higher risk for acute complications and long-term cardiovascular outcomes than those with term preeclampsia.
Compared with those with normotensive pregnancies, investigators found an increased risk for the composite neurologic outcome among women with preterm preeclampsia (aHR, 1.54; 95% CI, 1.34-1.79), but also for those with term preeclampsia (aHR, 1.27; 95% CI, 1.17-1.38).
Common Vascular Component
The study also showed gestational hypertension and preeclampsia were associated with a later diagnosis of migraine, suggesting a possible common underlying vascular component.
“The increased risk of migraine following preeclampsia could be linked to endothelial damage at the blood-brain barrier level and alterations in cerebral blood flow and arterial vasospasm found in eclampsia, but this is only speculation,” said Friis.
The analysis also found an association between preeclampsia and a later diagnosis of headache. But this result likely encompasses several headache diagnoses, including migraine, so “it’s challenging to draw conclusions about the underlying mechanisms,” Friis added.
The study didn’t consider diagnoses from primary healthcare, which resulted in relatively few outcomes. The authors explained they could only identify the most severe cases; for example, women referred to specialized care.
Another potential study limitation is that Swedish registers don’t include information on race or ethnicity. Evidence shows there are racial differences in the risk for cardiovascular outcomes after preeclampsia.
This area of research is important as most women will experience at least one pregnancy in their lifetime, and preeclampsia affects 3%-5% of pregnancies. Further research is needed to understand the underlying pathophysiological mechanisms and the long-term consequences of this disorder, said Friis.
She added she hopes more therapeutic options will be available in the future for neuroprotective treatment for women with gestational hypertensive disorders.
Asked to comment, Thomas Vidic, MD, clinical professor of neurology, Indiana University School of Medicine, South Bend, said in an interview that this is an important study that includes robust data.
In his opinion, the most significant study finding is the marked increase in epilepsy risk after gestational eclampsia.
“In women who have new-onset epilepsy of unknown cause, asking about having eclampsia or preeclampsia during pregnancy is definitely a worthwhile question,” he said.
Confirming an etiology paints “a better picture” for patients wondering why they’re experiencing seizures, he added.
As with any registry-based study, this one had some acknowledged limitations, “but at the same time, the authors were able to have such a large database that I think this study is very worthwhile,” said Vidic.
The study received support from the Swedish Research Council. Neither Friis nor Vidic had relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Gestational hypertension, preeclampsia, or eclampsia is associated with a significantly higher risk for neurologic disorders such as migraine or epilepsy in the years following a first pregnancy, new research suggests.
The risk was highest in those with gestational eclampsia, who had a 70% increased chance of developing a neurologic disorder, including a fivefold increased risk for epilepsy, investigators found.
“When consulting women with new-onset neurological disorders, it’s important to inquire about their pregnancy history, as pregnancy complications such as gestational hypertension, preeclampsia, and eclampsia have been associated with an increased risk of neurological disorders later in life.” Therese Friis, MD, PhD student, Department of Women’s and Children’s Health, Uppsala University in Sweden, said in an interview.
The findings were published online in JAMA Neurology.
Most studies of maternal outcomes after gestational hypertension, preeclampsia, or eclampsia have focused on long-term risks for cardiovascular disease or neurologic complications such as stroke, dementia, and cognitive impairment. And many of these studies were relatively small and based on interviews or questionnaires.
“We wanted to investigate whether women with a hypertensive disorder of pregnancy also had a risk of other neurological complications, closer in time to the pregnancy,” said Friis.
The new study included 648,385 women (mean age, 28.5 years) whose first pregnancy occurred between 2005 and 2018. Of these, 94% had a normotensive pregnancy, 2% had gestational hypertension, 4% had preeclampsia without eclampsia, and 0.1% had eclampsia.
Gestational hypertension was defined as new-onset systolic blood pressure of ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg; preeclampsia was defined as gestational hypertension accompanied by proteinuria; and eclampsia was defined as tonic-clonic seizures without other etiology accompanied by preeclampsia.
Researchers used linked Swedish national registries that collect data on pregnancies, births, and infant and maternal characteristics. Among other things, they controlled for maternal age, early pregnancy body mass index, education level, and pregestational and gestational diabetes.
The primary outcome was a composite of five new-onset neurologic diagnoses: Migraine, headache, epilepsy, sleep disorder, and mental fatigue (neurasthenia), although one diagnosis was sufficient, from 42 days to 15 years after childbirth. Mean follow-up was 7.7 years.
Fivefold Epilepsy Risk
Compared with normotensive pregnancies, the risk for the primary outcome was 70% greater in those with eclampsia (adjusted hazard ratio [aHR], 1.70; 95% CI, 1.16-2.50), 27% higher for gestational hypertension (aHR, 1.27; 95% CI, 1.12-1.45), and 32% for preeclampsia (aHR, 1.32; 95% CI, 1.22-1.42).
Researchers also looked at the risk for individual neurologic disorders. There were too few neurasthenia events to generate meaningful results, so this diagnosis was omitted from the analysis.
Here, the study found women with eclampsia had five times the risk for epilepsy (aHR, 5.31; 95% CI, 2.85-9.89) compared with women with normotensive pregnancies.
The underlying mechanism for this association is unclear. However, said Friis, eclampsia and epilepsy share common pathways, such as neuroinflammation, and women with epilepsy before pregnancy run an increased risk for eclampsia.
“So common underlying pathways might increase the risk both for eclampsia in cases of a seizure disorder and a future seizure disorder after eclampsia,” she said.
In addition, preeclampsia and, in particular, eclampsia can cause irreversible subclinical cerebral infarcts found in areas of cerebral edema, she added. “These infarcts or scarring of brain tissue could potentially serve as foci for later epileptic activity.”
Researchers separated women with preeclampsia (with or without eclampsia) into those with preterm (less than 37 weeks; 21%) and term (79%) deliveries. As Friis explained, women with preterm preeclampsia have a higher risk for acute complications and long-term cardiovascular outcomes than those with term preeclampsia.
Compared with those with normotensive pregnancies, investigators found an increased risk for the composite neurologic outcome among women with preterm preeclampsia (aHR, 1.54; 95% CI, 1.34-1.79), but also for those with term preeclampsia (aHR, 1.27; 95% CI, 1.17-1.38).
Common Vascular Component
The study also showed gestational hypertension and preeclampsia were associated with a later diagnosis of migraine, suggesting a possible common underlying vascular component.
“The increased risk of migraine following preeclampsia could be linked to endothelial damage at the blood-brain barrier level and alterations in cerebral blood flow and arterial vasospasm found in eclampsia, but this is only speculation,” said Friis.
The analysis also found an association between preeclampsia and a later diagnosis of headache. But this result likely encompasses several headache diagnoses, including migraine, so “it’s challenging to draw conclusions about the underlying mechanisms,” Friis added.
The study didn’t consider diagnoses from primary healthcare, which resulted in relatively few outcomes. The authors explained they could only identify the most severe cases; for example, women referred to specialized care.
Another potential study limitation is that Swedish registers don’t include information on race or ethnicity. Evidence shows there are racial differences in the risk for cardiovascular outcomes after preeclampsia.
This area of research is important as most women will experience at least one pregnancy in their lifetime, and preeclampsia affects 3%-5% of pregnancies. Further research is needed to understand the underlying pathophysiological mechanisms and the long-term consequences of this disorder, said Friis.
She added she hopes more therapeutic options will be available in the future for neuroprotective treatment for women with gestational hypertensive disorders.
Asked to comment, Thomas Vidic, MD, clinical professor of neurology, Indiana University School of Medicine, South Bend, said in an interview that this is an important study that includes robust data.
In his opinion, the most significant study finding is the marked increase in epilepsy risk after gestational eclampsia.
“In women who have new-onset epilepsy of unknown cause, asking about having eclampsia or preeclampsia during pregnancy is definitely a worthwhile question,” he said.
Confirming an etiology paints “a better picture” for patients wondering why they’re experiencing seizures, he added.
As with any registry-based study, this one had some acknowledged limitations, “but at the same time, the authors were able to have such a large database that I think this study is very worthwhile,” said Vidic.
The study received support from the Swedish Research Council. Neither Friis nor Vidic had relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JAMA NEUROLOGY