User login
SPARTAN (SPondyloArthritis Research & Treatment Network): Annual Meeting
‘Troubling’ trend in narcotic prescribing for ankylosing spondylitis
DENVER – Even as prescribing of tumor necrosis factor inhibitors for patients with ankylosing spondylitis climbed in the last half-dozen years, patients’ use of prescription narcotics increased as well.
This was the unexpected finding of a comprehensive analysis of trends in medication use over a 12-year period among 1,018 ankylosing spondylitis patients participating in the ongoing multicenter Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS), Dr. Mary C. Gibson reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“Even with treatment with TNF inhibitors, patients are often requiring additional pain control with narcotic medications. This could reflect inadequate pain control from directed therapy or a trend toward change in pain control use in general practice,” observed Dr. Gibson of the University of Texas, Houston.
The patients enrolled in PSOAS during 2003-2014 are being followed longitudinally. They were recruited from community practice settings as well as academic centers. Dr. Gibson analyzed their medication use at the time of their baseline enrollment visit on a year-by-year basis, then stepped back to look at broader trends, comparing baseline medication use for patients enrolled in 2003-2008 to those enrolled during 2009-2014.
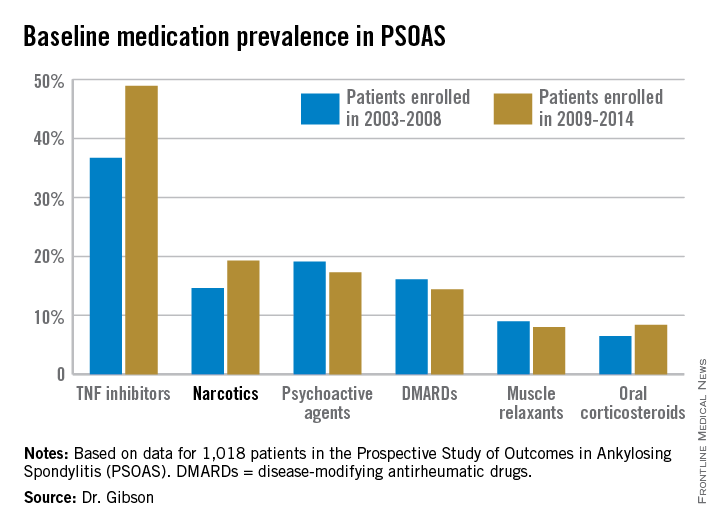
Not surprisingly, significantly more patients entered the study on a TNF inhibitor during the enrollment years 2009-2014 than during the first half of the study period: 49% compared with 37% during 2003-2008. What came as a surprise, however, was the time trend in narcotic use: 14.6% of ankylosing spondylitis patients in the 2003-2008 cohort were on narcotics, compared with 19.3% who enrolled since then.
“That’s a little bit troubling,” said Dr. John D. Reveille, professor and vice chair of rheumatology at the University of Texas, Houston, and head of PSOAS.
Oxycodone use nearly tripled between the first and second half of the study period: 1.1% were on the drug at enrollment in 2003-2008 compared with 3.1% in 2009-2014.
Of note, baseline use of oxycodone peaked in the enrollment class of 2012, 5.6% of whom were on the medication when they joined PSOAS. For narcotics overall, the peak year was 2008: over 27% of patients who enrolled in the study that year were on narcotic therapy at the time.
The use of NSAIDs – a guideline-recommended first-line therapy in ankylosing spondylitis – trended lower over time, albeit not significantly so. Among patients who enrolled in PSOAS during 2003-2008, over 70% were on NSAID therapy, compared with 66% of those who enrolled later.
There was no significant change over time in the use of DMARDs, psychoactive medications, muscle relaxants, or corticosteroids.
PSOAS is sponsored by the National Institutes of Health. Dr. Gibson reported having no financial conflicts.
DENVER – Even as prescribing of tumor necrosis factor inhibitors for patients with ankylosing spondylitis climbed in the last half-dozen years, patients’ use of prescription narcotics increased as well.
This was the unexpected finding of a comprehensive analysis of trends in medication use over a 12-year period among 1,018 ankylosing spondylitis patients participating in the ongoing multicenter Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS), Dr. Mary C. Gibson reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“Even with treatment with TNF inhibitors, patients are often requiring additional pain control with narcotic medications. This could reflect inadequate pain control from directed therapy or a trend toward change in pain control use in general practice,” observed Dr. Gibson of the University of Texas, Houston.

The patients enrolled in PSOAS during 2003-2014 are being followed longitudinally. They were recruited from community practice settings as well as academic centers. Dr. Gibson analyzed their medication use at the time of their baseline enrollment visit on a year-by-year basis, then stepped back to look at broader trends, comparing baseline medication use for patients enrolled in 2003-2008 to those enrolled during 2009-2014.
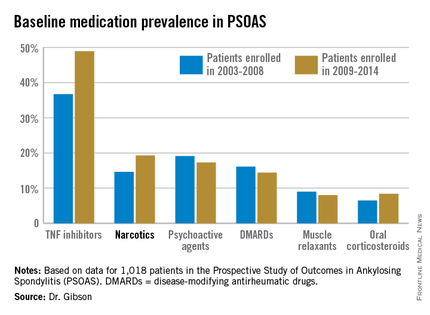
Not surprisingly, significantly more patients entered the study on a TNF inhibitor during the enrollment years 2009-2014 than during the first half of the study period: 49% compared with 37% during 2003-2008. What came as a surprise, however, was the time trend in narcotic use: 14.6% of ankylosing spondylitis patients in the 2003-2008 cohort were on narcotics, compared with 19.3% who enrolled since then.
“That’s a little bit troubling,” said Dr. John D. Reveille, professor and vice chair of rheumatology at the University of Texas, Houston, and head of PSOAS.
Oxycodone use nearly tripled between the first and second half of the study period: 1.1% were on the drug at enrollment in 2003-2008 compared with 3.1% in 2009-2014.
Of note, baseline use of oxycodone peaked in the enrollment class of 2012, 5.6% of whom were on the medication when they joined PSOAS. For narcotics overall, the peak year was 2008: over 27% of patients who enrolled in the study that year were on narcotic therapy at the time.
The use of NSAIDs – a guideline-recommended first-line therapy in ankylosing spondylitis – trended lower over time, albeit not significantly so. Among patients who enrolled in PSOAS during 2003-2008, over 70% were on NSAID therapy, compared with 66% of those who enrolled later.
There was no significant change over time in the use of DMARDs, psychoactive medications, muscle relaxants, or corticosteroids.
PSOAS is sponsored by the National Institutes of Health. Dr. Gibson reported having no financial conflicts.
DENVER – Even as prescribing of tumor necrosis factor inhibitors for patients with ankylosing spondylitis climbed in the last half-dozen years, patients’ use of prescription narcotics increased as well.
This was the unexpected finding of a comprehensive analysis of trends in medication use over a 12-year period among 1,018 ankylosing spondylitis patients participating in the ongoing multicenter Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS), Dr. Mary C. Gibson reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“Even with treatment with TNF inhibitors, patients are often requiring additional pain control with narcotic medications. This could reflect inadequate pain control from directed therapy or a trend toward change in pain control use in general practice,” observed Dr. Gibson of the University of Texas, Houston.

The patients enrolled in PSOAS during 2003-2014 are being followed longitudinally. They were recruited from community practice settings as well as academic centers. Dr. Gibson analyzed their medication use at the time of their baseline enrollment visit on a year-by-year basis, then stepped back to look at broader trends, comparing baseline medication use for patients enrolled in 2003-2008 to those enrolled during 2009-2014.
Not surprisingly, significantly more patients entered the study on a TNF inhibitor during the enrollment years 2009-2014 than during the first half of the study period: 49% compared with 37% during 2003-2008. What came as a surprise, however, was the time trend in narcotic use: 14.6% of ankylosing spondylitis patients in the 2003-2008 cohort were on narcotics, compared with 19.3% who enrolled since then.
“That’s a little bit troubling,” said Dr. John D. Reveille, professor and vice chair of rheumatology at the University of Texas, Houston, and head of PSOAS.
Oxycodone use nearly tripled between the first and second half of the study period: 1.1% were on the drug at enrollment in 2003-2008 compared with 3.1% in 2009-2014.
Of note, baseline use of oxycodone peaked in the enrollment class of 2012, 5.6% of whom were on the medication when they joined PSOAS. For narcotics overall, the peak year was 2008: over 27% of patients who enrolled in the study that year were on narcotic therapy at the time.
The use of NSAIDs – a guideline-recommended first-line therapy in ankylosing spondylitis – trended lower over time, albeit not significantly so. Among patients who enrolled in PSOAS during 2003-2008, over 70% were on NSAID therapy, compared with 66% of those who enrolled later.
There was no significant change over time in the use of DMARDs, psychoactive medications, muscle relaxants, or corticosteroids.
PSOAS is sponsored by the National Institutes of Health. Dr. Gibson reported having no financial conflicts.
AT THE 2015 SPARTAN ANNUAL MEETING
Key clinical point: The use of prescription narcotics by patients with ankylosing spondylitis has gone up during the past 12 years, even as their use of tumor necrosis factor inhibitor therapy has grown.
Major finding: Among a large cohort of patients with ankylosing spondylitis, 14.6% were on prescription narcotic therapy during 2003-2008, compared with 19.3% in 2009-2014.
Data source: Analysis of 1,018 patients with ankylosing spondylitis, from PSOAS – a prospective, multicenter, longitudinal cohort study.
Disclosures: The ongoing PSOAS study is funded by the National Institutes of Health. The presenter reported having no conflicts of interest.
Exercise boosts medication’s benefits in ankylosing spondylitis
DENVER – Recreational exercise and tumor necrosis factor–inhibitor therapy provide synergistic improvement in the functional status of patients with ankylosing spondylitis, according to an analysis from the multicenter Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS).
Among 683 PSOAS participants prospectively followed for up to 12 years, those who consistently engaged in moderate recreational exercise, defined as at least 120 minutes of exercise per week, had significantly lower, more favorable Bath Ankylosing Spondylitis Functional Index (BASFI) scores during years 1-5 than did those who exercised less, even after controlling for baseline function and other potential confounders in a multivariate analysis, Dr. Lianne S. Gensler said at the annual meeting of the Spondyloarthritis Research and Treatment Network.
Additionally, a significant interaction was observed between exercise time and anti-TNF therapy. BASFI scores improved through 5 years of follow-up in TNF inhibitor users regardless of their exercise status, but the impact on functional status was “strikingly” greater in those patients on a TNF inhibitor who engaged in at least 120 minutes of exercise per week, according to Dr. Gensler of the University of California, San Francisco.
“This suggests that it’s not simply taking a TNF inhibitor that’s going to promote long-term function. These patients should also be exercising to synergize and get the best out of their therapy,” she emphasized.
The PSOAS cohort included averaged 43 years of age at baseline, with a mean 19-year disease duration and an average of 2.1 comorbid conditions. In a multivariate longitudinal analysis, several factors emerged as significant predictors of more pronounced functional impairment over time: being age 40 or older at baseline was associated with a 25% increased risk, opiate use was associated with a 12% increase in risk, a baseline Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score of at least 40 conferred a 19% increase in risk, as did a modified Stoke Ankylosing Spondylitis Spinal Score of 4 or greater.
Dr. Gensler drew a sharp distinction between the effects of moderate recreational exercise and occupational physical activity in ankylosing spondylitis patients. In an earlier landmark study involving 397 members of the PSOAS cohort with at least a 20-year history of ankylosing spondylitis, investigators mapped the patients’ work histories, the physical activities their work entailed, and the time they spent at each activity. Jobs that involved repeated bending, stretching, twisting, and vibration exposure were associated with worse BASFI scores and greater radiographic disease severity (Arthritis Rheum. 2008 Jun 15;59[6]:822-32).
Taking a step back to summarize what’s known today about what affects outcomes in ankylosing spondylitis patients, Dr. Gensler listed in the ‘bad’ column smoking, ongoing inflammation, baseline damage to the spine, certain occupational activities involving biomechanical stress, adiposity, and low socioeconomic status.
What’s good for patients with ankylosing spondylitis is physical therapy and back exercises – both will receive favorable marks in soon-to-be-published American College of Rheumatology guidelines on the management of ankylosing spondylitis that Dr. Gensler worked on – as well as TNF-inhibitor therapy, NSAIDs, and recreational exercise, which wasn’t addressed in the former guidelines.
Dr. Gensler reported receiving research funds from or serving as a consultant to AbbVie, Amgen, Celgene, Janssen, and UCB.
DENVER – Recreational exercise and tumor necrosis factor–inhibitor therapy provide synergistic improvement in the functional status of patients with ankylosing spondylitis, according to an analysis from the multicenter Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS).
Among 683 PSOAS participants prospectively followed for up to 12 years, those who consistently engaged in moderate recreational exercise, defined as at least 120 minutes of exercise per week, had significantly lower, more favorable Bath Ankylosing Spondylitis Functional Index (BASFI) scores during years 1-5 than did those who exercised less, even after controlling for baseline function and other potential confounders in a multivariate analysis, Dr. Lianne S. Gensler said at the annual meeting of the Spondyloarthritis Research and Treatment Network.
Additionally, a significant interaction was observed between exercise time and anti-TNF therapy. BASFI scores improved through 5 years of follow-up in TNF inhibitor users regardless of their exercise status, but the impact on functional status was “strikingly” greater in those patients on a TNF inhibitor who engaged in at least 120 minutes of exercise per week, according to Dr. Gensler of the University of California, San Francisco.
“This suggests that it’s not simply taking a TNF inhibitor that’s going to promote long-term function. These patients should also be exercising to synergize and get the best out of their therapy,” she emphasized.
The PSOAS cohort included averaged 43 years of age at baseline, with a mean 19-year disease duration and an average of 2.1 comorbid conditions. In a multivariate longitudinal analysis, several factors emerged as significant predictors of more pronounced functional impairment over time: being age 40 or older at baseline was associated with a 25% increased risk, opiate use was associated with a 12% increase in risk, a baseline Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score of at least 40 conferred a 19% increase in risk, as did a modified Stoke Ankylosing Spondylitis Spinal Score of 4 or greater.
Dr. Gensler drew a sharp distinction between the effects of moderate recreational exercise and occupational physical activity in ankylosing spondylitis patients. In an earlier landmark study involving 397 members of the PSOAS cohort with at least a 20-year history of ankylosing spondylitis, investigators mapped the patients’ work histories, the physical activities their work entailed, and the time they spent at each activity. Jobs that involved repeated bending, stretching, twisting, and vibration exposure were associated with worse BASFI scores and greater radiographic disease severity (Arthritis Rheum. 2008 Jun 15;59[6]:822-32).
Taking a step back to summarize what’s known today about what affects outcomes in ankylosing spondylitis patients, Dr. Gensler listed in the ‘bad’ column smoking, ongoing inflammation, baseline damage to the spine, certain occupational activities involving biomechanical stress, adiposity, and low socioeconomic status.
What’s good for patients with ankylosing spondylitis is physical therapy and back exercises – both will receive favorable marks in soon-to-be-published American College of Rheumatology guidelines on the management of ankylosing spondylitis that Dr. Gensler worked on – as well as TNF-inhibitor therapy, NSAIDs, and recreational exercise, which wasn’t addressed in the former guidelines.
Dr. Gensler reported receiving research funds from or serving as a consultant to AbbVie, Amgen, Celgene, Janssen, and UCB.
DENVER – Recreational exercise and tumor necrosis factor–inhibitor therapy provide synergistic improvement in the functional status of patients with ankylosing spondylitis, according to an analysis from the multicenter Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS).
Among 683 PSOAS participants prospectively followed for up to 12 years, those who consistently engaged in moderate recreational exercise, defined as at least 120 minutes of exercise per week, had significantly lower, more favorable Bath Ankylosing Spondylitis Functional Index (BASFI) scores during years 1-5 than did those who exercised less, even after controlling for baseline function and other potential confounders in a multivariate analysis, Dr. Lianne S. Gensler said at the annual meeting of the Spondyloarthritis Research and Treatment Network.
Additionally, a significant interaction was observed between exercise time and anti-TNF therapy. BASFI scores improved through 5 years of follow-up in TNF inhibitor users regardless of their exercise status, but the impact on functional status was “strikingly” greater in those patients on a TNF inhibitor who engaged in at least 120 minutes of exercise per week, according to Dr. Gensler of the University of California, San Francisco.
“This suggests that it’s not simply taking a TNF inhibitor that’s going to promote long-term function. These patients should also be exercising to synergize and get the best out of their therapy,” she emphasized.
The PSOAS cohort included averaged 43 years of age at baseline, with a mean 19-year disease duration and an average of 2.1 comorbid conditions. In a multivariate longitudinal analysis, several factors emerged as significant predictors of more pronounced functional impairment over time: being age 40 or older at baseline was associated with a 25% increased risk, opiate use was associated with a 12% increase in risk, a baseline Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score of at least 40 conferred a 19% increase in risk, as did a modified Stoke Ankylosing Spondylitis Spinal Score of 4 or greater.
Dr. Gensler drew a sharp distinction between the effects of moderate recreational exercise and occupational physical activity in ankylosing spondylitis patients. In an earlier landmark study involving 397 members of the PSOAS cohort with at least a 20-year history of ankylosing spondylitis, investigators mapped the patients’ work histories, the physical activities their work entailed, and the time they spent at each activity. Jobs that involved repeated bending, stretching, twisting, and vibration exposure were associated with worse BASFI scores and greater radiographic disease severity (Arthritis Rheum. 2008 Jun 15;59[6]:822-32).
Taking a step back to summarize what’s known today about what affects outcomes in ankylosing spondylitis patients, Dr. Gensler listed in the ‘bad’ column smoking, ongoing inflammation, baseline damage to the spine, certain occupational activities involving biomechanical stress, adiposity, and low socioeconomic status.
What’s good for patients with ankylosing spondylitis is physical therapy and back exercises – both will receive favorable marks in soon-to-be-published American College of Rheumatology guidelines on the management of ankylosing spondylitis that Dr. Gensler worked on – as well as TNF-inhibitor therapy, NSAIDs, and recreational exercise, which wasn’t addressed in the former guidelines.
Dr. Gensler reported receiving research funds from or serving as a consultant to AbbVie, Amgen, Celgene, Janssen, and UCB.
EXPERT ANALYSIS FROM THE 2015 SPARTAN ANNUAL MEETING
New era ahead in spondyloarthritis therapy
DENVER – The emerging new era in the treatment of spondyloarthritis is combined blockade of tumor necrosis factor–alpha (TNF-alpha) and interleukin-17 using a bispecific antibody, Dr. Siba P. Raychaudhuri predicted at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“This is the next generation of antibody therapies for spondyloarthritis,” said Dr. Raychaudhuri, a rheumatologist at the University of California, Davis.
This novel approach has a sound theoretical basis. Moreover, studies in mouse models, including models of rheumatoid arthritis (Arthritis Rheumatol. 2015 Jan;67[1]:51-62), have been positive. And proprietary bispecific anti-TNF-alpha/IL-17 antibodies are now in phase I and phase II clinical trials for various forms of spondyloarthritis, he noted.
The impetus for development of this new therapy is widespread agreement that while TNF inhibition has been a step forward in the treatment of axial spondyloarthritis, the efficacy is less than hoped for with these potent and costly agents.
“Things have been sort of gray for almost the last 10 years. All the evidence suggests we’re not getting full efficacy with anti-TNF therapy. We’re getting stuck at about a 60% ASAS 20 response,” he observed.
The explanation for this merely middling success rate appears to be that TNF inhibition induces a counterregulatory uptick in the IL-17 pathway. And in animal models, IL-17 is a key mediator of joint inflammation and destruction.
Dr. Raychaudhuri noted that brodalumab, an investigational human monoclonal antibody directed against IL-17 receptor A, has been shown “reasonably effective” in a phase II study in patients with psoriatic arthritis, which is part of the spondyloarthritis family of autoimmune diseases, achieving an ACR 20 response rate of 39% at week 12 in the high-dose arm (N Engl J Med. 2014 Jun 12;370[24]:2295-306).
The expectation is that by inhibiting both TNF-alpha and IL-17 using a bispecific protein composed of fragments of two different monoclonal antibodies, the result will be reduced growth and differentiation of proinflammatory T cells, decreased T cell migration, and synergistic clinical efficacy, compared with what’s attainable by addressing either target alone.
Dr. Raychaudhuri reported receiving research grants from or serving as a consultant to Abbott, Amgen, Celgene, UCB, and Janssen Biotech.
DENVER – The emerging new era in the treatment of spondyloarthritis is combined blockade of tumor necrosis factor–alpha (TNF-alpha) and interleukin-17 using a bispecific antibody, Dr. Siba P. Raychaudhuri predicted at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“This is the next generation of antibody therapies for spondyloarthritis,” said Dr. Raychaudhuri, a rheumatologist at the University of California, Davis.
This novel approach has a sound theoretical basis. Moreover, studies in mouse models, including models of rheumatoid arthritis (Arthritis Rheumatol. 2015 Jan;67[1]:51-62), have been positive. And proprietary bispecific anti-TNF-alpha/IL-17 antibodies are now in phase I and phase II clinical trials for various forms of spondyloarthritis, he noted.
The impetus for development of this new therapy is widespread agreement that while TNF inhibition has been a step forward in the treatment of axial spondyloarthritis, the efficacy is less than hoped for with these potent and costly agents.
“Things have been sort of gray for almost the last 10 years. All the evidence suggests we’re not getting full efficacy with anti-TNF therapy. We’re getting stuck at about a 60% ASAS 20 response,” he observed.
The explanation for this merely middling success rate appears to be that TNF inhibition induces a counterregulatory uptick in the IL-17 pathway. And in animal models, IL-17 is a key mediator of joint inflammation and destruction.
Dr. Raychaudhuri noted that brodalumab, an investigational human monoclonal antibody directed against IL-17 receptor A, has been shown “reasonably effective” in a phase II study in patients with psoriatic arthritis, which is part of the spondyloarthritis family of autoimmune diseases, achieving an ACR 20 response rate of 39% at week 12 in the high-dose arm (N Engl J Med. 2014 Jun 12;370[24]:2295-306).
The expectation is that by inhibiting both TNF-alpha and IL-17 using a bispecific protein composed of fragments of two different monoclonal antibodies, the result will be reduced growth and differentiation of proinflammatory T cells, decreased T cell migration, and synergistic clinical efficacy, compared with what’s attainable by addressing either target alone.
Dr. Raychaudhuri reported receiving research grants from or serving as a consultant to Abbott, Amgen, Celgene, UCB, and Janssen Biotech.
DENVER – The emerging new era in the treatment of spondyloarthritis is combined blockade of tumor necrosis factor–alpha (TNF-alpha) and interleukin-17 using a bispecific antibody, Dr. Siba P. Raychaudhuri predicted at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“This is the next generation of antibody therapies for spondyloarthritis,” said Dr. Raychaudhuri, a rheumatologist at the University of California, Davis.
This novel approach has a sound theoretical basis. Moreover, studies in mouse models, including models of rheumatoid arthritis (Arthritis Rheumatol. 2015 Jan;67[1]:51-62), have been positive. And proprietary bispecific anti-TNF-alpha/IL-17 antibodies are now in phase I and phase II clinical trials for various forms of spondyloarthritis, he noted.
The impetus for development of this new therapy is widespread agreement that while TNF inhibition has been a step forward in the treatment of axial spondyloarthritis, the efficacy is less than hoped for with these potent and costly agents.
“Things have been sort of gray for almost the last 10 years. All the evidence suggests we’re not getting full efficacy with anti-TNF therapy. We’re getting stuck at about a 60% ASAS 20 response,” he observed.
The explanation for this merely middling success rate appears to be that TNF inhibition induces a counterregulatory uptick in the IL-17 pathway. And in animal models, IL-17 is a key mediator of joint inflammation and destruction.
Dr. Raychaudhuri noted that brodalumab, an investigational human monoclonal antibody directed against IL-17 receptor A, has been shown “reasonably effective” in a phase II study in patients with psoriatic arthritis, which is part of the spondyloarthritis family of autoimmune diseases, achieving an ACR 20 response rate of 39% at week 12 in the high-dose arm (N Engl J Med. 2014 Jun 12;370[24]:2295-306).
The expectation is that by inhibiting both TNF-alpha and IL-17 using a bispecific protein composed of fragments of two different monoclonal antibodies, the result will be reduced growth and differentiation of proinflammatory T cells, decreased T cell migration, and synergistic clinical efficacy, compared with what’s attainable by addressing either target alone.
Dr. Raychaudhuri reported receiving research grants from or serving as a consultant to Abbott, Amgen, Celgene, UCB, and Janssen Biotech.
EXPERT ANALYSIS FROM THE 2015 SPARTAN ANNUAL MEETING
New spondyloarthritis criteria in the works
DENVER – The Spondyloarthritis Research and Treatment Network has voted decisively to create new classification criteria for axial spondyloarthritis, including both ankylosing spondylitis and nonradiographic axial spondyloarthritis.
Following the vote, in which 64% of SPARTAN members attending the group’s annual meeting voted that “this is the right time to proceed,” comoderator Dr. Liron Caplan declared, “This is a very strong push. We’ll need to begin work on our proposal immediately.”
The detailed proposal will contain the justification for new classification criteria as well as outline how SPARTAN will go about creating the criteria in an evidence-based manner with input and financing from industry and patient support groups. The proposal needs to be filed by next March in order to be considered for roughly $135,000 in joint funding from the American College of Rheumatology and the European League Against Rheumatism.
A major impetus for the ambitious project was a frank conversation SPARTAN officers had in the Spring of 2014 with officials at the Food and Drug Administration. The rheumatologists sought an explanation for the FDA’s seemingly counterintuitive rejection of adalimumab (Humira) and certolizumab pegol (Cimzia) for treatment of nonradiographic axial spondyloarthritis (nr-axSpA) on the basis of the very same randomized, controlled trials that earlier resulted in approval for that indication by the European Medicines Agency.
The FDA representatives deemed the Assessment of SpondyloArthritis Society (ASAS) classification criteria (Ann Rheum Dis. 2009;68:777-83) used in those and other SpA trials to be overly broad. They felt the ASAS criteria lack sufficient sensitivity and specificity to ensure that large numbers of patients with fibromyalgia – a far more common disorder – wouldn’t be misclassified as having SpA and treated inappropriately with biologics, which aren’t effective in fibromyalgia, explained Dr. John D. Reveille, professor and vice-chair of rheumatology at the University of Texas, Houston, who attended the meeting.
“The only physicians who can prescribe an approved anti-TNF drug or other biologic in Europe are rheumatologists. The reason the FDA was nervous about approving drugs using the ASAS criteria is because any physician in the U.S. can prescribe those drugs once they’re approved. So the authorities made a regulatory decision about who the people are who are educated or not educated in the diagnosis and management of patients with SpA,” according to Dr. Michael H. Weisman, chair and director of rheumatology at Cedars-Sinai Medical Center in Los Angeles.
Dr. Caplan of the University of Colorado, Denver, observed that developing new and improved criteria for SpA will be a multiyear endeavor. A top goal is to shed light on the natural history of nr-axSpA, about which almost nothing is known. This will probably entail developing a sizable cohort of patients with nr-axSpA, following them for 5 years or so to see who develops ankylosing spondylitis, then looking back to identify reliable predictors.
The SPARTAN vote was preceded by a formal debate on the merits of replacing the ASAS criteria. Dr. Muhammad Asim Khan, professor of medicine at Case Western Reserve University, Cleveland, who 30 years ago was a pioneer in the concept of spondylitic disease without x-ray evidence of sacroiliitis, argued that the ASAS criteria aren’t adequately doing what disease classification criteria are supposed to do: provide a simple, clear means of identifying a homogeneous population for clinical trials. By letting in substantial numbers of patients with fibromyalgia, the criteria water down the clinical outcomes of investigational therapies for SpA.
The opposing argument was provided by ASAS representative Dr. Filip Van den Bosch. He conceded the ASAS classification criteria “aren’t perfect,” but over time they have become familiar to rheumatologists worldwide.
“Modifying the criteria will lead to years of uncertainty,” argued Dr. Van den Bosch, professor of rheumatology at Ghent (Belgium) University.
If, as some have proposed, SPARTAN were to tighten the ASAS criteria by insisting that a patient lacking x-ray evidence of sacroiliitis must possess three or four SpA features instead of the two now required, the result would be a drastic reduction in the sensitivity of the criteria, he continued.
“The challenge of making a correct diagnosis of axSpA cannot be solved by modifying the ASAS criteria. I cannot think of a new SpA feature that’s been identified in the last 5 years that could be added on top of what’s already in there,” the rheumatologist said.
What’s needed, he added, is a concerted effort to better educate physicians about axSpA, including interpretation of spinal x-rays and MRIs, the disease’s signs and symptoms, and how to differentiate SpA from other disorders that cause back pain.
While nearly two-thirds of the SPARTAN membership voted in favor of developing new classification criteria, some prominent voices were raised in dissent.
“I agree with Filip,” said Dr. Weisman. “The problem is not with the criteria, it’s who’s using them.”
Dr. Robert D. Inman, professor of medicine at the University of Toronto, predicted “it’s an exercise in futility” to try to come up with a single set of classification criteria that will provide the high degree of sensitivity that patients and clinicians want so that people who present with early axSpA are not missed while at the same time providing the maximum specificity that payers and regulatory officials seek.
Dr. Sindhu Johnson, who as cochair of the ACR Classification Criteria Subcommittee has shepherded the development of new criteria for a number of rheumatologic diseases, offered the SPARTAN group some practical tips. Because European studies tend to be comprised of monoethnic cohorts, the ACR will look favorably upon a successful SPARTAN effort to incorporate African Americans, Asians, Native Americans, and other minorities in their study cohorts. And SPARTAN should be open and receptive to critical feedback from stakeholders during the criteria development process.
“Get buy in. There’s no point in developing criteria that are extremely rigorous scientifically but that the global community feels are useless, that use tests they don’t have access to. This is one of the criticisms around the Sjögren’s criteria: they require ophthalmologic testing and salivary gland biopsy, and for rheumatologists – even academic rheumatologists at recruiting centers for clinical trials – they don’t have access to this, or it takes too much time. You need to come up with something pragmatic to get buy in,” advised Dr. Johnson of the University of Toronto.
By the way, she added, if the project wins ACR and EULAR funding it can only be named an ACR/EULAR product. “No other organization can be named in the headline,” according to Dr. Johnson.
DENVER – The Spondyloarthritis Research and Treatment Network has voted decisively to create new classification criteria for axial spondyloarthritis, including both ankylosing spondylitis and nonradiographic axial spondyloarthritis.
Following the vote, in which 64% of SPARTAN members attending the group’s annual meeting voted that “this is the right time to proceed,” comoderator Dr. Liron Caplan declared, “This is a very strong push. We’ll need to begin work on our proposal immediately.”
The detailed proposal will contain the justification for new classification criteria as well as outline how SPARTAN will go about creating the criteria in an evidence-based manner with input and financing from industry and patient support groups. The proposal needs to be filed by next March in order to be considered for roughly $135,000 in joint funding from the American College of Rheumatology and the European League Against Rheumatism.
A major impetus for the ambitious project was a frank conversation SPARTAN officers had in the Spring of 2014 with officials at the Food and Drug Administration. The rheumatologists sought an explanation for the FDA’s seemingly counterintuitive rejection of adalimumab (Humira) and certolizumab pegol (Cimzia) for treatment of nonradiographic axial spondyloarthritis (nr-axSpA) on the basis of the very same randomized, controlled trials that earlier resulted in approval for that indication by the European Medicines Agency.
The FDA representatives deemed the Assessment of SpondyloArthritis Society (ASAS) classification criteria (Ann Rheum Dis. 2009;68:777-83) used in those and other SpA trials to be overly broad. They felt the ASAS criteria lack sufficient sensitivity and specificity to ensure that large numbers of patients with fibromyalgia – a far more common disorder – wouldn’t be misclassified as having SpA and treated inappropriately with biologics, which aren’t effective in fibromyalgia, explained Dr. John D. Reveille, professor and vice-chair of rheumatology at the University of Texas, Houston, who attended the meeting.
“The only physicians who can prescribe an approved anti-TNF drug or other biologic in Europe are rheumatologists. The reason the FDA was nervous about approving drugs using the ASAS criteria is because any physician in the U.S. can prescribe those drugs once they’re approved. So the authorities made a regulatory decision about who the people are who are educated or not educated in the diagnosis and management of patients with SpA,” according to Dr. Michael H. Weisman, chair and director of rheumatology at Cedars-Sinai Medical Center in Los Angeles.
Dr. Caplan of the University of Colorado, Denver, observed that developing new and improved criteria for SpA will be a multiyear endeavor. A top goal is to shed light on the natural history of nr-axSpA, about which almost nothing is known. This will probably entail developing a sizable cohort of patients with nr-axSpA, following them for 5 years or so to see who develops ankylosing spondylitis, then looking back to identify reliable predictors.
The SPARTAN vote was preceded by a formal debate on the merits of replacing the ASAS criteria. Dr. Muhammad Asim Khan, professor of medicine at Case Western Reserve University, Cleveland, who 30 years ago was a pioneer in the concept of spondylitic disease without x-ray evidence of sacroiliitis, argued that the ASAS criteria aren’t adequately doing what disease classification criteria are supposed to do: provide a simple, clear means of identifying a homogeneous population for clinical trials. By letting in substantial numbers of patients with fibromyalgia, the criteria water down the clinical outcomes of investigational therapies for SpA.
The opposing argument was provided by ASAS representative Dr. Filip Van den Bosch. He conceded the ASAS classification criteria “aren’t perfect,” but over time they have become familiar to rheumatologists worldwide.
“Modifying the criteria will lead to years of uncertainty,” argued Dr. Van den Bosch, professor of rheumatology at Ghent (Belgium) University.
If, as some have proposed, SPARTAN were to tighten the ASAS criteria by insisting that a patient lacking x-ray evidence of sacroiliitis must possess three or four SpA features instead of the two now required, the result would be a drastic reduction in the sensitivity of the criteria, he continued.
“The challenge of making a correct diagnosis of axSpA cannot be solved by modifying the ASAS criteria. I cannot think of a new SpA feature that’s been identified in the last 5 years that could be added on top of what’s already in there,” the rheumatologist said.
What’s needed, he added, is a concerted effort to better educate physicians about axSpA, including interpretation of spinal x-rays and MRIs, the disease’s signs and symptoms, and how to differentiate SpA from other disorders that cause back pain.
While nearly two-thirds of the SPARTAN membership voted in favor of developing new classification criteria, some prominent voices were raised in dissent.
“I agree with Filip,” said Dr. Weisman. “The problem is not with the criteria, it’s who’s using them.”
Dr. Robert D. Inman, professor of medicine at the University of Toronto, predicted “it’s an exercise in futility” to try to come up with a single set of classification criteria that will provide the high degree of sensitivity that patients and clinicians want so that people who present with early axSpA are not missed while at the same time providing the maximum specificity that payers and regulatory officials seek.
Dr. Sindhu Johnson, who as cochair of the ACR Classification Criteria Subcommittee has shepherded the development of new criteria for a number of rheumatologic diseases, offered the SPARTAN group some practical tips. Because European studies tend to be comprised of monoethnic cohorts, the ACR will look favorably upon a successful SPARTAN effort to incorporate African Americans, Asians, Native Americans, and other minorities in their study cohorts. And SPARTAN should be open and receptive to critical feedback from stakeholders during the criteria development process.
“Get buy in. There’s no point in developing criteria that are extremely rigorous scientifically but that the global community feels are useless, that use tests they don’t have access to. This is one of the criticisms around the Sjögren’s criteria: they require ophthalmologic testing and salivary gland biopsy, and for rheumatologists – even academic rheumatologists at recruiting centers for clinical trials – they don’t have access to this, or it takes too much time. You need to come up with something pragmatic to get buy in,” advised Dr. Johnson of the University of Toronto.
By the way, she added, if the project wins ACR and EULAR funding it can only be named an ACR/EULAR product. “No other organization can be named in the headline,” according to Dr. Johnson.
DENVER – The Spondyloarthritis Research and Treatment Network has voted decisively to create new classification criteria for axial spondyloarthritis, including both ankylosing spondylitis and nonradiographic axial spondyloarthritis.
Following the vote, in which 64% of SPARTAN members attending the group’s annual meeting voted that “this is the right time to proceed,” comoderator Dr. Liron Caplan declared, “This is a very strong push. We’ll need to begin work on our proposal immediately.”
The detailed proposal will contain the justification for new classification criteria as well as outline how SPARTAN will go about creating the criteria in an evidence-based manner with input and financing from industry and patient support groups. The proposal needs to be filed by next March in order to be considered for roughly $135,000 in joint funding from the American College of Rheumatology and the European League Against Rheumatism.
A major impetus for the ambitious project was a frank conversation SPARTAN officers had in the Spring of 2014 with officials at the Food and Drug Administration. The rheumatologists sought an explanation for the FDA’s seemingly counterintuitive rejection of adalimumab (Humira) and certolizumab pegol (Cimzia) for treatment of nonradiographic axial spondyloarthritis (nr-axSpA) on the basis of the very same randomized, controlled trials that earlier resulted in approval for that indication by the European Medicines Agency.
The FDA representatives deemed the Assessment of SpondyloArthritis Society (ASAS) classification criteria (Ann Rheum Dis. 2009;68:777-83) used in those and other SpA trials to be overly broad. They felt the ASAS criteria lack sufficient sensitivity and specificity to ensure that large numbers of patients with fibromyalgia – a far more common disorder – wouldn’t be misclassified as having SpA and treated inappropriately with biologics, which aren’t effective in fibromyalgia, explained Dr. John D. Reveille, professor and vice-chair of rheumatology at the University of Texas, Houston, who attended the meeting.
“The only physicians who can prescribe an approved anti-TNF drug or other biologic in Europe are rheumatologists. The reason the FDA was nervous about approving drugs using the ASAS criteria is because any physician in the U.S. can prescribe those drugs once they’re approved. So the authorities made a regulatory decision about who the people are who are educated or not educated in the diagnosis and management of patients with SpA,” according to Dr. Michael H. Weisman, chair and director of rheumatology at Cedars-Sinai Medical Center in Los Angeles.
Dr. Caplan of the University of Colorado, Denver, observed that developing new and improved criteria for SpA will be a multiyear endeavor. A top goal is to shed light on the natural history of nr-axSpA, about which almost nothing is known. This will probably entail developing a sizable cohort of patients with nr-axSpA, following them for 5 years or so to see who develops ankylosing spondylitis, then looking back to identify reliable predictors.
The SPARTAN vote was preceded by a formal debate on the merits of replacing the ASAS criteria. Dr. Muhammad Asim Khan, professor of medicine at Case Western Reserve University, Cleveland, who 30 years ago was a pioneer in the concept of spondylitic disease without x-ray evidence of sacroiliitis, argued that the ASAS criteria aren’t adequately doing what disease classification criteria are supposed to do: provide a simple, clear means of identifying a homogeneous population for clinical trials. By letting in substantial numbers of patients with fibromyalgia, the criteria water down the clinical outcomes of investigational therapies for SpA.
The opposing argument was provided by ASAS representative Dr. Filip Van den Bosch. He conceded the ASAS classification criteria “aren’t perfect,” but over time they have become familiar to rheumatologists worldwide.
“Modifying the criteria will lead to years of uncertainty,” argued Dr. Van den Bosch, professor of rheumatology at Ghent (Belgium) University.
If, as some have proposed, SPARTAN were to tighten the ASAS criteria by insisting that a patient lacking x-ray evidence of sacroiliitis must possess three or four SpA features instead of the two now required, the result would be a drastic reduction in the sensitivity of the criteria, he continued.
“The challenge of making a correct diagnosis of axSpA cannot be solved by modifying the ASAS criteria. I cannot think of a new SpA feature that’s been identified in the last 5 years that could be added on top of what’s already in there,” the rheumatologist said.
What’s needed, he added, is a concerted effort to better educate physicians about axSpA, including interpretation of spinal x-rays and MRIs, the disease’s signs and symptoms, and how to differentiate SpA from other disorders that cause back pain.
While nearly two-thirds of the SPARTAN membership voted in favor of developing new classification criteria, some prominent voices were raised in dissent.
“I agree with Filip,” said Dr. Weisman. “The problem is not with the criteria, it’s who’s using them.”
Dr. Robert D. Inman, professor of medicine at the University of Toronto, predicted “it’s an exercise in futility” to try to come up with a single set of classification criteria that will provide the high degree of sensitivity that patients and clinicians want so that people who present with early axSpA are not missed while at the same time providing the maximum specificity that payers and regulatory officials seek.
Dr. Sindhu Johnson, who as cochair of the ACR Classification Criteria Subcommittee has shepherded the development of new criteria for a number of rheumatologic diseases, offered the SPARTAN group some practical tips. Because European studies tend to be comprised of monoethnic cohorts, the ACR will look favorably upon a successful SPARTAN effort to incorporate African Americans, Asians, Native Americans, and other minorities in their study cohorts. And SPARTAN should be open and receptive to critical feedback from stakeholders during the criteria development process.
“Get buy in. There’s no point in developing criteria that are extremely rigorous scientifically but that the global community feels are useless, that use tests they don’t have access to. This is one of the criticisms around the Sjögren’s criteria: they require ophthalmologic testing and salivary gland biopsy, and for rheumatologists – even academic rheumatologists at recruiting centers for clinical trials – they don’t have access to this, or it takes too much time. You need to come up with something pragmatic to get buy in,” advised Dr. Johnson of the University of Toronto.
By the way, she added, if the project wins ACR and EULAR funding it can only be named an ACR/EULAR product. “No other organization can be named in the headline,” according to Dr. Johnson.
AT THE 2015 SPARTAN ANNUAL MEETING
Effective primary care screen identified for axial spondyloarthritis
DENVER – Spondyloarthritis experts have zeroed in on a simple and effective screening and referral strategy for nonrheumatologists to use in deciding which patients with chronic low back pain to send to rheumatologists for definitive diagnosis and treatment of axial spondyloarthritis.
The goal is to reduce the unacceptably long delay to diagnosis of axial spondyloarthritis – on average, 7 years – without flooding rheumatologists with enormous numbers of patients who have mechanical low back pain, a vastly more common yet generally self-limited condition, Dr. Abhijeet Danve explained at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
He presented a systematic review of seven studies that examined various screening and referral strategies in the diagnosis of axial spondyloarthritis (axSpA). The studies totaled more than 3,300 patients with chronic low back pain.
Two key conclusions jumped out from this structured review: One, simple referral strategies and considerably more complex approaches work equally well, resulting in 35%-45% of referred patients ultimately being diagnosed by a rheumatologist as having axSpA.
And two, one strategy emerged on balance as the clear winner: “In our opinion, referring patients with chronic low back pain for more than 3 months, age of onset less than 45 years, and who meet one of three criteria – inflammatory back pain, HLA- [human leukocyte antigen] B27-positivity, or sacroiliitis on imaging – to a rheumatologist for assessment of possible axSpA would be convenient, practical, and widely acceptable,” according to Dr. Danve of Yale University, New Haven, Conn.
The more of these three criteria a patient possessed, the greater the rate of rheumatologist-diagnosed axSpa. If, for example, a patient under age 45 years with a history of low back pain for more than 3 months had classic inflammatory back pain but was HLA-B27-negative and didn’t show sacroiliitis on imaging, the rate of a confirmed diagnosis of axSpa was 25%. The axSpa diagnosis rate rose to 45% with two positive criteria and jumped to 75% in those who met all three criteria.
With appropriate application of any of the strategies evaluated in the systematic review, rheumatologists can expect to see two to three people with chronic low back pain in order to identify one patient with axSpA, he added.
SPARTAN is gearing up for a major educational initiative aimed at primary care physicians, orthopedists, physical therapists, and chiropractors. The goal is to improve early recognition of possible axSpA and bring affected patients to rheumatologists for definitive diagnosis and specialized care. Of note, Dr. Danve’s senior coinvestigator in the referral strategy study was Dr. Atul Deodhar, SPARTAN chair and professor of medicine and medical director of rheumatology clinics at Oregon Health and Science University, Portland.
DENVER – Spondyloarthritis experts have zeroed in on a simple and effective screening and referral strategy for nonrheumatologists to use in deciding which patients with chronic low back pain to send to rheumatologists for definitive diagnosis and treatment of axial spondyloarthritis.
The goal is to reduce the unacceptably long delay to diagnosis of axial spondyloarthritis – on average, 7 years – without flooding rheumatologists with enormous numbers of patients who have mechanical low back pain, a vastly more common yet generally self-limited condition, Dr. Abhijeet Danve explained at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
He presented a systematic review of seven studies that examined various screening and referral strategies in the diagnosis of axial spondyloarthritis (axSpA). The studies totaled more than 3,300 patients with chronic low back pain.
Two key conclusions jumped out from this structured review: One, simple referral strategies and considerably more complex approaches work equally well, resulting in 35%-45% of referred patients ultimately being diagnosed by a rheumatologist as having axSpA.
And two, one strategy emerged on balance as the clear winner: “In our opinion, referring patients with chronic low back pain for more than 3 months, age of onset less than 45 years, and who meet one of three criteria – inflammatory back pain, HLA- [human leukocyte antigen] B27-positivity, or sacroiliitis on imaging – to a rheumatologist for assessment of possible axSpA would be convenient, practical, and widely acceptable,” according to Dr. Danve of Yale University, New Haven, Conn.
The more of these three criteria a patient possessed, the greater the rate of rheumatologist-diagnosed axSpa. If, for example, a patient under age 45 years with a history of low back pain for more than 3 months had classic inflammatory back pain but was HLA-B27-negative and didn’t show sacroiliitis on imaging, the rate of a confirmed diagnosis of axSpa was 25%. The axSpa diagnosis rate rose to 45% with two positive criteria and jumped to 75% in those who met all three criteria.
With appropriate application of any of the strategies evaluated in the systematic review, rheumatologists can expect to see two to three people with chronic low back pain in order to identify one patient with axSpA, he added.
SPARTAN is gearing up for a major educational initiative aimed at primary care physicians, orthopedists, physical therapists, and chiropractors. The goal is to improve early recognition of possible axSpA and bring affected patients to rheumatologists for definitive diagnosis and specialized care. Of note, Dr. Danve’s senior coinvestigator in the referral strategy study was Dr. Atul Deodhar, SPARTAN chair and professor of medicine and medical director of rheumatology clinics at Oregon Health and Science University, Portland.
DENVER – Spondyloarthritis experts have zeroed in on a simple and effective screening and referral strategy for nonrheumatologists to use in deciding which patients with chronic low back pain to send to rheumatologists for definitive diagnosis and treatment of axial spondyloarthritis.
The goal is to reduce the unacceptably long delay to diagnosis of axial spondyloarthritis – on average, 7 years – without flooding rheumatologists with enormous numbers of patients who have mechanical low back pain, a vastly more common yet generally self-limited condition, Dr. Abhijeet Danve explained at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
He presented a systematic review of seven studies that examined various screening and referral strategies in the diagnosis of axial spondyloarthritis (axSpA). The studies totaled more than 3,300 patients with chronic low back pain.
Two key conclusions jumped out from this structured review: One, simple referral strategies and considerably more complex approaches work equally well, resulting in 35%-45% of referred patients ultimately being diagnosed by a rheumatologist as having axSpA.
And two, one strategy emerged on balance as the clear winner: “In our opinion, referring patients with chronic low back pain for more than 3 months, age of onset less than 45 years, and who meet one of three criteria – inflammatory back pain, HLA- [human leukocyte antigen] B27-positivity, or sacroiliitis on imaging – to a rheumatologist for assessment of possible axSpA would be convenient, practical, and widely acceptable,” according to Dr. Danve of Yale University, New Haven, Conn.
The more of these three criteria a patient possessed, the greater the rate of rheumatologist-diagnosed axSpa. If, for example, a patient under age 45 years with a history of low back pain for more than 3 months had classic inflammatory back pain but was HLA-B27-negative and didn’t show sacroiliitis on imaging, the rate of a confirmed diagnosis of axSpa was 25%. The axSpa diagnosis rate rose to 45% with two positive criteria and jumped to 75% in those who met all three criteria.
With appropriate application of any of the strategies evaluated in the systematic review, rheumatologists can expect to see two to three people with chronic low back pain in order to identify one patient with axSpA, he added.
SPARTAN is gearing up for a major educational initiative aimed at primary care physicians, orthopedists, physical therapists, and chiropractors. The goal is to improve early recognition of possible axSpA and bring affected patients to rheumatologists for definitive diagnosis and specialized care. Of note, Dr. Danve’s senior coinvestigator in the referral strategy study was Dr. Atul Deodhar, SPARTAN chair and professor of medicine and medical director of rheumatology clinics at Oregon Health and Science University, Portland.
EXPERT ANALYSIS FROM THE 2015 SPARTAN ANNUAL MEETING
Uveitis prevalence, risk eyeballed in spondyloarthritis patients
DENVER – Anterior uveitis occurred in 13% of military veterans with spondyloarthritis followed longitudinally in the PULSAR registry, Dr. Maureen Dubreuil reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
One factor proved to be independently associated with the risk of developing uveitis in a multivariate logistic regression analysis: being positive for HLA-B27. This conferred an 11.3-fold increased risk of developing the serious ophthalmologic complication, according to Dr. Dubreuil of Boston University.
PULSAR (the Program to Understand Long-Term Outcomes in Spondyloarthritis) is an eight-site longitudinal registry funded by the Department of Veterans Affairs since 2007.
Thirty-five of 268 men (13.1%) with spondyloarthritis (SpA) were diagnosed with anterior uveitis a mean of 10.3 years following diagnosis of SpA. The diagnosis of uveitis was made by a participating PULSAR rheumatologist and confirmed by an ophthalmologist.
Sixty-two percent of subjects with uveitis experienced a mean of 5.7 recurrences, while the remainder had encountered a solitary episode at the time of the study. Two-thirds of the time, uveitis episodes occurred concurrently with periods of SpA activity. The uveitis occurred in an alternating unilateral pattern in nearly half of cases.
One-quarter of subjects with uveitis experienced complications, including cataracts, glaucoma, and blindness in two patients.
Uveitis is recognized as one of the most common extra-articular manifestations of radiographic axial SpA; however, the picture in patients with non-axial SpA has been far less clear. In the PULSAR analysis, the prevalence of uveitis was 24% among the 95 subjects with axial SpA. In a univariate analysis, this translated to a 2.45-fold increased likelihood of uveitis in individuals with axial SpA, compared with patients with non-axial SpA. Nonetheless, in a multivariate logistic regression analysis, axial SpA wasn’t associated with a significantly increased risk of uveitis. Neither were demographic variables, smoking status, SpA duration, or inflammatory markers, Dr. Dubreuil said.
She reported having no financial conflicts regarding the study, which was funded by the Department of Veterans Affairs.
DENVER – Anterior uveitis occurred in 13% of military veterans with spondyloarthritis followed longitudinally in the PULSAR registry, Dr. Maureen Dubreuil reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
One factor proved to be independently associated with the risk of developing uveitis in a multivariate logistic regression analysis: being positive for HLA-B27. This conferred an 11.3-fold increased risk of developing the serious ophthalmologic complication, according to Dr. Dubreuil of Boston University.
PULSAR (the Program to Understand Long-Term Outcomes in Spondyloarthritis) is an eight-site longitudinal registry funded by the Department of Veterans Affairs since 2007.
Thirty-five of 268 men (13.1%) with spondyloarthritis (SpA) were diagnosed with anterior uveitis a mean of 10.3 years following diagnosis of SpA. The diagnosis of uveitis was made by a participating PULSAR rheumatologist and confirmed by an ophthalmologist.
Sixty-two percent of subjects with uveitis experienced a mean of 5.7 recurrences, while the remainder had encountered a solitary episode at the time of the study. Two-thirds of the time, uveitis episodes occurred concurrently with periods of SpA activity. The uveitis occurred in an alternating unilateral pattern in nearly half of cases.
One-quarter of subjects with uveitis experienced complications, including cataracts, glaucoma, and blindness in two patients.
Uveitis is recognized as one of the most common extra-articular manifestations of radiographic axial SpA; however, the picture in patients with non-axial SpA has been far less clear. In the PULSAR analysis, the prevalence of uveitis was 24% among the 95 subjects with axial SpA. In a univariate analysis, this translated to a 2.45-fold increased likelihood of uveitis in individuals with axial SpA, compared with patients with non-axial SpA. Nonetheless, in a multivariate logistic regression analysis, axial SpA wasn’t associated with a significantly increased risk of uveitis. Neither were demographic variables, smoking status, SpA duration, or inflammatory markers, Dr. Dubreuil said.
She reported having no financial conflicts regarding the study, which was funded by the Department of Veterans Affairs.
DENVER – Anterior uveitis occurred in 13% of military veterans with spondyloarthritis followed longitudinally in the PULSAR registry, Dr. Maureen Dubreuil reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
One factor proved to be independently associated with the risk of developing uveitis in a multivariate logistic regression analysis: being positive for HLA-B27. This conferred an 11.3-fold increased risk of developing the serious ophthalmologic complication, according to Dr. Dubreuil of Boston University.
PULSAR (the Program to Understand Long-Term Outcomes in Spondyloarthritis) is an eight-site longitudinal registry funded by the Department of Veterans Affairs since 2007.
Thirty-five of 268 men (13.1%) with spondyloarthritis (SpA) were diagnosed with anterior uveitis a mean of 10.3 years following diagnosis of SpA. The diagnosis of uveitis was made by a participating PULSAR rheumatologist and confirmed by an ophthalmologist.
Sixty-two percent of subjects with uveitis experienced a mean of 5.7 recurrences, while the remainder had encountered a solitary episode at the time of the study. Two-thirds of the time, uveitis episodes occurred concurrently with periods of SpA activity. The uveitis occurred in an alternating unilateral pattern in nearly half of cases.
One-quarter of subjects with uveitis experienced complications, including cataracts, glaucoma, and blindness in two patients.
Uveitis is recognized as one of the most common extra-articular manifestations of radiographic axial SpA; however, the picture in patients with non-axial SpA has been far less clear. In the PULSAR analysis, the prevalence of uveitis was 24% among the 95 subjects with axial SpA. In a univariate analysis, this translated to a 2.45-fold increased likelihood of uveitis in individuals with axial SpA, compared with patients with non-axial SpA. Nonetheless, in a multivariate logistic regression analysis, axial SpA wasn’t associated with a significantly increased risk of uveitis. Neither were demographic variables, smoking status, SpA duration, or inflammatory markers, Dr. Dubreuil said.
She reported having no financial conflicts regarding the study, which was funded by the Department of Veterans Affairs.
AT THE 2015 SPARTAN ANNUAL MEETING
Key clinical point: The risk of anterior uveitis in patients with spondyloarthritis was 11.3-fold greater among those who were HLA-B27-positive.
Major finding: Thirteen percent of men with spondyloarthritis developed anterior uveitis a mean of 10.3 years following diagnosis of their rheumatologic disease.
Data source: An analysis of 268 male military veterans with spondyloarthritis in the Program to Understand Long-Term Outcomes in Spondyloarthritis (PULSAR) registry.
Disclosures: PULSAR is funded by the Department of Veterans Affairs. The presenter reported having no financial conflicts of interest.
Study yields insight into nonradiographic axial spondyloarthritis progression
DENVER – Only one-quarter of patients newly identified as having nonradiographic axial spondyloarthritis will progress to ankylosing spondylitis within 15 years, according to findings from the Rochester (Minn.) Epidemiology Project.
Disease progression occurred far more frequently and rapidly in patients who qualified for nonradiographic axial spondyloarthritis (nr-AxSpA) on the basis of positive pelvic MRI findings than in those who met only the clinical criteria for nr-AxSpA, Dr. Runsheng Wang reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
The Rochester Epidemiology Project is a unique health care research resource. Essentially all residents in Olmsted County, Minn., the home of the Mayo Clinic, have signed off on participation in the project. Dr. Weng’s study focused on 16- to 45-year-old county residents who reported new-onset chronic back pain during 1985-2010, none of whom showed the radiographic evidence of sacroiliitis required for a diagnosis of ankylosing spondylitis.
The study began with 1,142 patients who for a variety of reasons underwent a pelvic MRI. Eighteen of them ultimately met ASAS (Assessment of Spondyloarthritis International Society) imaging criteria for nr-AxSpA. They formed the imaging arm of the study.
Another 1,009 patients in the target age group presented with chronic back pain but no MRI findings. Sixty-five of them were entered into the clinical nr-AxSpA arm of the study on the basis of being positive for HLA-B27, explained Dr. Wang, a clinical scholar in rheumatology at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Subjects were followed for a mean of 10.7 years. The overall rate of progression from nr-AxSpA to ankylosing spondylitis was 6.4% at 5 years, 17.3% at 10 years, and 26.4% at 15 years.
These data help fill an unmet need regarding understanding of the prognosis of nr-AxSpA. The two previous studies by other investigators have limitations: one German study showed an 11.2% rate of conversion to ankylosing spondylitis but was limited to 2 years of follow-up (Ann Rheum Dis. 2011 Aug;70[8]:1369-1374), while a Norwegian study comprising 20 patients showed a 20% progression rate over 8 years, Dr. Wang noted.
In her Minnesota study, patients who met criteria for nr-AxSpA based upon MRI criteria were 3.5-fold more likely to progress to ankylosing spondylitis with evidence of sacroiliitis on X-ray, compared with those in the clinical arm. There was a hint of a gender difference: Men were 1.58 times more likely to progress to ankylosing spondylitis than were women. However, this difference didn’t reach statistical significance.
Audience members were particularly interested in whether the Minnesota data demonstrated that treatment with NSAIDs and other agents had an impact upon the progression rate. Dr. Wang replied that the study size was simply too small to allow for a reasonable multivariate analysis examining this key question.
The National Institutes of Health funded the study. Dr. Wang reported having no financial conflicts of interest.
DENVER – Only one-quarter of patients newly identified as having nonradiographic axial spondyloarthritis will progress to ankylosing spondylitis within 15 years, according to findings from the Rochester (Minn.) Epidemiology Project.
Disease progression occurred far more frequently and rapidly in patients who qualified for nonradiographic axial spondyloarthritis (nr-AxSpA) on the basis of positive pelvic MRI findings than in those who met only the clinical criteria for nr-AxSpA, Dr. Runsheng Wang reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
The Rochester Epidemiology Project is a unique health care research resource. Essentially all residents in Olmsted County, Minn., the home of the Mayo Clinic, have signed off on participation in the project. Dr. Weng’s study focused on 16- to 45-year-old county residents who reported new-onset chronic back pain during 1985-2010, none of whom showed the radiographic evidence of sacroiliitis required for a diagnosis of ankylosing spondylitis.
The study began with 1,142 patients who for a variety of reasons underwent a pelvic MRI. Eighteen of them ultimately met ASAS (Assessment of Spondyloarthritis International Society) imaging criteria for nr-AxSpA. They formed the imaging arm of the study.
Another 1,009 patients in the target age group presented with chronic back pain but no MRI findings. Sixty-five of them were entered into the clinical nr-AxSpA arm of the study on the basis of being positive for HLA-B27, explained Dr. Wang, a clinical scholar in rheumatology at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Subjects were followed for a mean of 10.7 years. The overall rate of progression from nr-AxSpA to ankylosing spondylitis was 6.4% at 5 years, 17.3% at 10 years, and 26.4% at 15 years.
These data help fill an unmet need regarding understanding of the prognosis of nr-AxSpA. The two previous studies by other investigators have limitations: one German study showed an 11.2% rate of conversion to ankylosing spondylitis but was limited to 2 years of follow-up (Ann Rheum Dis. 2011 Aug;70[8]:1369-1374), while a Norwegian study comprising 20 patients showed a 20% progression rate over 8 years, Dr. Wang noted.
In her Minnesota study, patients who met criteria for nr-AxSpA based upon MRI criteria were 3.5-fold more likely to progress to ankylosing spondylitis with evidence of sacroiliitis on X-ray, compared with those in the clinical arm. There was a hint of a gender difference: Men were 1.58 times more likely to progress to ankylosing spondylitis than were women. However, this difference didn’t reach statistical significance.
Audience members were particularly interested in whether the Minnesota data demonstrated that treatment with NSAIDs and other agents had an impact upon the progression rate. Dr. Wang replied that the study size was simply too small to allow for a reasonable multivariate analysis examining this key question.
The National Institutes of Health funded the study. Dr. Wang reported having no financial conflicts of interest.
DENVER – Only one-quarter of patients newly identified as having nonradiographic axial spondyloarthritis will progress to ankylosing spondylitis within 15 years, according to findings from the Rochester (Minn.) Epidemiology Project.
Disease progression occurred far more frequently and rapidly in patients who qualified for nonradiographic axial spondyloarthritis (nr-AxSpA) on the basis of positive pelvic MRI findings than in those who met only the clinical criteria for nr-AxSpA, Dr. Runsheng Wang reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
The Rochester Epidemiology Project is a unique health care research resource. Essentially all residents in Olmsted County, Minn., the home of the Mayo Clinic, have signed off on participation in the project. Dr. Weng’s study focused on 16- to 45-year-old county residents who reported new-onset chronic back pain during 1985-2010, none of whom showed the radiographic evidence of sacroiliitis required for a diagnosis of ankylosing spondylitis.
The study began with 1,142 patients who for a variety of reasons underwent a pelvic MRI. Eighteen of them ultimately met ASAS (Assessment of Spondyloarthritis International Society) imaging criteria for nr-AxSpA. They formed the imaging arm of the study.
Another 1,009 patients in the target age group presented with chronic back pain but no MRI findings. Sixty-five of them were entered into the clinical nr-AxSpA arm of the study on the basis of being positive for HLA-B27, explained Dr. Wang, a clinical scholar in rheumatology at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Subjects were followed for a mean of 10.7 years. The overall rate of progression from nr-AxSpA to ankylosing spondylitis was 6.4% at 5 years, 17.3% at 10 years, and 26.4% at 15 years.
These data help fill an unmet need regarding understanding of the prognosis of nr-AxSpA. The two previous studies by other investigators have limitations: one German study showed an 11.2% rate of conversion to ankylosing spondylitis but was limited to 2 years of follow-up (Ann Rheum Dis. 2011 Aug;70[8]:1369-1374), while a Norwegian study comprising 20 patients showed a 20% progression rate over 8 years, Dr. Wang noted.
In her Minnesota study, patients who met criteria for nr-AxSpA based upon MRI criteria were 3.5-fold more likely to progress to ankylosing spondylitis with evidence of sacroiliitis on X-ray, compared with those in the clinical arm. There was a hint of a gender difference: Men were 1.58 times more likely to progress to ankylosing spondylitis than were women. However, this difference didn’t reach statistical significance.
Audience members were particularly interested in whether the Minnesota data demonstrated that treatment with NSAIDs and other agents had an impact upon the progression rate. Dr. Wang replied that the study size was simply too small to allow for a reasonable multivariate analysis examining this key question.
The National Institutes of Health funded the study. Dr. Wang reported having no financial conflicts of interest.
AT THE 2015 SPARTAN ANNUAL MEETING
Key clinical point: Radiographic evidence of sacroiliitis is s-l-o-w to develop in patients with nonradiographic axial spondyloarthritis.
Major finding: Fifteen years after patients were identified as having nonradiographic axial spondyloarthritis based upon MRI or clinical findings, 26% of them had progressed to ankylosing spondylitis as defined by sacroiliitis on x-ray.
Data source: The Rochester (Minn.) Epidemiology Project, a unique medical records database incorporating all residents of Olmsted County, Minn.
Disclosures: The National Institutes of Health funded the study. The presenter reported having no relevant financial disclosures.