User login
Intranasal Postop Steroids Same as Saline in Select Patients
CHICAGO – Intranasal steroids were as good as saline alone as postoperative care in a randomized, double-blinded study of patients with chronic rhinosinusitis and Samter’s triad.
There was no difference in disease recurrence rate, complications, or quality of life at 6 months and 1-year postoperative using intranasal saline, saline plus budesonide, or saline and budesonide combined.
The surprising finding runs contrary to general practice and has several implications including how best to counsel patients for postoperative care, lead author Dr. Brian Rotenberg said at the Combined Otolaryngology Spring Meetings.
"If nasal steroids as done in this population don’t confer any additional benefit postoperatively, should we still prescribe them?" he asked. "Should we be prescribing something different or perhaps nothing at all? Is there a potential plus side here in terms of health-care cost savings?"
During a discussion of the study, an attendee expressed concern that insurers would interpret the results too broadly and deny coverage of postoperative nasal steroids for all patients with rhinosinusitis and polyposis, and not just those with Samter’s triad, a condition consisting of asthma, aspirin sensitivity, and nasal polyposis.
Another attendee agreed that nasal steroids are not potent enough in this population and said a pulse course of oral steroids 60 mg for 4 days can knock down symptoms in 80% of those with recurrence and be maintained with topical steroids. In the absence of a federally approved product for nasal use, he also suggested that dexamethasone eye drops can be effective.
Dr. Rotenberg replied that all patients received 3 weeks of postoperative oral prednisone, but that pulse-dosing of steroids was limited to one patient with early recurrence.
The 60 patients in the analysis had failed medical management for chronic rhinosinusitis with nasal polyposis and had a minimum preoperative Lund-Kennedy score of 8 out of 12. Nineteen patients were randomized to saline, 21 to saline plus budesonide, and 20 to saline/budesonide combination. Their mean Lund-Mackay scores were 20.6, 19.9, and 20.5, respectively.
Exclusion criteria included revision functional endoscopic sinus surgery, use of corticosteroids for other medical conditions, smoking, and concurrent disease with steroid contraindication.
At 6 months postoperatively, there were no significant differences between the saline, saline plus budesonide, and saline/budesonide combination groups with regard to Lund-Kennedy scores (1.5, 0.9, and 1.2, respectively), adrenocorticotropic hormone (ACTH) ranges (all normal), and intraocular pressure (12.4, 12.9, and 13.9 mm Hg, respectively), said Dr. Rotenberg of the University of Western Ontario in London, Ontario. Quality of life as assessed using the 21-item Sino-Nasal Outcome Test (SNOT-21) was also similar at a mean of 29.7, 27.4 and 28.2, respectively.
At 1 year of follow-up, once again there were no significant differences between the saline, saline plus budesonide, and saline/budesonide groups in Lund-Kennedy scores (3.7, 4.4, and 4.1, respectively), Lund-Mackay scores (11.8, 12.7, and 13.4, respectively), SNOT-21 scores (42.5, 47.9, and 42.2, respectively), intraocular pressure (13.1, 13.4,and 12.9 mm Hg, respectively). ACTH ranges were all normal.
A within-group analysis showed a significant improvement in all outcomes from baseline to 6 months, and a general worsening of outcomes at 1 year compared with the first 6 months, although they were still improved over baseline, Dr. Rotenberg said.
He pointed out that the literature is lacking in evidence guiding the postoperative management of patients with chronic rhinosinusitis with nasal polyps undergoing surgery. One study reported that steroid nasal spray did not influence polyp recurrence rate after surgery (Clin. Exp. Allergy. 2004;34:1395-400), while another showed that normal and buffered hypertonic saline nasal sprays had no beneficial effect on postoperative symptoms compared with no treatment (Am. J. Rhinol. 2006;20:191-6).
Dr. Rotenberg reported no relevant financial disclosures.
Session moderator Dr. Brent A. Senior said in an interview that the study was well designed and sufficiently powered, but would have been strengthened by the inclusion of a nontreatment group and more information on oral medications, as they can have a significant impact on disease. Follow-up to 18 months also would be useful, as the endoscopic appearance of the nasal cavity at 18 months has been shown to be predictive of how patients will do years after surgery.
"This is a good study, but not a game changer," he said, adding that additional work is needed to confirm the findings.
Dr. Senior is chief of rhinology, allergy and sinus surgery at the University of North Carolina at Chapel Hill.
rhinosinusitis and polyposis, dexamethasone eye drops,
Session moderator Dr. Brent A. Senior said in an interview that the study was well designed and sufficiently powered, but would have been strengthened by the inclusion of a nontreatment group and more information on oral medications, as they can have a significant impact on disease. Follow-up to 18 months also would be useful, as the endoscopic appearance of the nasal cavity at 18 months has been shown to be predictive of how patients will do years after surgery.
"This is a good study, but not a game changer," he said, adding that additional work is needed to confirm the findings.
Dr. Senior is chief of rhinology, allergy and sinus surgery at the University of North Carolina at Chapel Hill.
Session moderator Dr. Brent A. Senior said in an interview that the study was well designed and sufficiently powered, but would have been strengthened by the inclusion of a nontreatment group and more information on oral medications, as they can have a significant impact on disease. Follow-up to 18 months also would be useful, as the endoscopic appearance of the nasal cavity at 18 months has been shown to be predictive of how patients will do years after surgery.
"This is a good study, but not a game changer," he said, adding that additional work is needed to confirm the findings.
Dr. Senior is chief of rhinology, allergy and sinus surgery at the University of North Carolina at Chapel Hill.
CHICAGO – Intranasal steroids were as good as saline alone as postoperative care in a randomized, double-blinded study of patients with chronic rhinosinusitis and Samter’s triad.
There was no difference in disease recurrence rate, complications, or quality of life at 6 months and 1-year postoperative using intranasal saline, saline plus budesonide, or saline and budesonide combined.
The surprising finding runs contrary to general practice and has several implications including how best to counsel patients for postoperative care, lead author Dr. Brian Rotenberg said at the Combined Otolaryngology Spring Meetings.
"If nasal steroids as done in this population don’t confer any additional benefit postoperatively, should we still prescribe them?" he asked. "Should we be prescribing something different or perhaps nothing at all? Is there a potential plus side here in terms of health-care cost savings?"
During a discussion of the study, an attendee expressed concern that insurers would interpret the results too broadly and deny coverage of postoperative nasal steroids for all patients with rhinosinusitis and polyposis, and not just those with Samter’s triad, a condition consisting of asthma, aspirin sensitivity, and nasal polyposis.
Another attendee agreed that nasal steroids are not potent enough in this population and said a pulse course of oral steroids 60 mg for 4 days can knock down symptoms in 80% of those with recurrence and be maintained with topical steroids. In the absence of a federally approved product for nasal use, he also suggested that dexamethasone eye drops can be effective.
Dr. Rotenberg replied that all patients received 3 weeks of postoperative oral prednisone, but that pulse-dosing of steroids was limited to one patient with early recurrence.
The 60 patients in the analysis had failed medical management for chronic rhinosinusitis with nasal polyposis and had a minimum preoperative Lund-Kennedy score of 8 out of 12. Nineteen patients were randomized to saline, 21 to saline plus budesonide, and 20 to saline/budesonide combination. Their mean Lund-Mackay scores were 20.6, 19.9, and 20.5, respectively.
Exclusion criteria included revision functional endoscopic sinus surgery, use of corticosteroids for other medical conditions, smoking, and concurrent disease with steroid contraindication.
At 6 months postoperatively, there were no significant differences between the saline, saline plus budesonide, and saline/budesonide combination groups with regard to Lund-Kennedy scores (1.5, 0.9, and 1.2, respectively), adrenocorticotropic hormone (ACTH) ranges (all normal), and intraocular pressure (12.4, 12.9, and 13.9 mm Hg, respectively), said Dr. Rotenberg of the University of Western Ontario in London, Ontario. Quality of life as assessed using the 21-item Sino-Nasal Outcome Test (SNOT-21) was also similar at a mean of 29.7, 27.4 and 28.2, respectively.
At 1 year of follow-up, once again there were no significant differences between the saline, saline plus budesonide, and saline/budesonide groups in Lund-Kennedy scores (3.7, 4.4, and 4.1, respectively), Lund-Mackay scores (11.8, 12.7, and 13.4, respectively), SNOT-21 scores (42.5, 47.9, and 42.2, respectively), intraocular pressure (13.1, 13.4,and 12.9 mm Hg, respectively). ACTH ranges were all normal.
A within-group analysis showed a significant improvement in all outcomes from baseline to 6 months, and a general worsening of outcomes at 1 year compared with the first 6 months, although they were still improved over baseline, Dr. Rotenberg said.
He pointed out that the literature is lacking in evidence guiding the postoperative management of patients with chronic rhinosinusitis with nasal polyps undergoing surgery. One study reported that steroid nasal spray did not influence polyp recurrence rate after surgery (Clin. Exp. Allergy. 2004;34:1395-400), while another showed that normal and buffered hypertonic saline nasal sprays had no beneficial effect on postoperative symptoms compared with no treatment (Am. J. Rhinol. 2006;20:191-6).
Dr. Rotenberg reported no relevant financial disclosures.
CHICAGO – Intranasal steroids were as good as saline alone as postoperative care in a randomized, double-blinded study of patients with chronic rhinosinusitis and Samter’s triad.
There was no difference in disease recurrence rate, complications, or quality of life at 6 months and 1-year postoperative using intranasal saline, saline plus budesonide, or saline and budesonide combined.
The surprising finding runs contrary to general practice and has several implications including how best to counsel patients for postoperative care, lead author Dr. Brian Rotenberg said at the Combined Otolaryngology Spring Meetings.
"If nasal steroids as done in this population don’t confer any additional benefit postoperatively, should we still prescribe them?" he asked. "Should we be prescribing something different or perhaps nothing at all? Is there a potential plus side here in terms of health-care cost savings?"
During a discussion of the study, an attendee expressed concern that insurers would interpret the results too broadly and deny coverage of postoperative nasal steroids for all patients with rhinosinusitis and polyposis, and not just those with Samter’s triad, a condition consisting of asthma, aspirin sensitivity, and nasal polyposis.
Another attendee agreed that nasal steroids are not potent enough in this population and said a pulse course of oral steroids 60 mg for 4 days can knock down symptoms in 80% of those with recurrence and be maintained with topical steroids. In the absence of a federally approved product for nasal use, he also suggested that dexamethasone eye drops can be effective.
Dr. Rotenberg replied that all patients received 3 weeks of postoperative oral prednisone, but that pulse-dosing of steroids was limited to one patient with early recurrence.
The 60 patients in the analysis had failed medical management for chronic rhinosinusitis with nasal polyposis and had a minimum preoperative Lund-Kennedy score of 8 out of 12. Nineteen patients were randomized to saline, 21 to saline plus budesonide, and 20 to saline/budesonide combination. Their mean Lund-Mackay scores were 20.6, 19.9, and 20.5, respectively.
Exclusion criteria included revision functional endoscopic sinus surgery, use of corticosteroids for other medical conditions, smoking, and concurrent disease with steroid contraindication.
At 6 months postoperatively, there were no significant differences between the saline, saline plus budesonide, and saline/budesonide combination groups with regard to Lund-Kennedy scores (1.5, 0.9, and 1.2, respectively), adrenocorticotropic hormone (ACTH) ranges (all normal), and intraocular pressure (12.4, 12.9, and 13.9 mm Hg, respectively), said Dr. Rotenberg of the University of Western Ontario in London, Ontario. Quality of life as assessed using the 21-item Sino-Nasal Outcome Test (SNOT-21) was also similar at a mean of 29.7, 27.4 and 28.2, respectively.
At 1 year of follow-up, once again there were no significant differences between the saline, saline plus budesonide, and saline/budesonide groups in Lund-Kennedy scores (3.7, 4.4, and 4.1, respectively), Lund-Mackay scores (11.8, 12.7, and 13.4, respectively), SNOT-21 scores (42.5, 47.9, and 42.2, respectively), intraocular pressure (13.1, 13.4,and 12.9 mm Hg, respectively). ACTH ranges were all normal.
A within-group analysis showed a significant improvement in all outcomes from baseline to 6 months, and a general worsening of outcomes at 1 year compared with the first 6 months, although they were still improved over baseline, Dr. Rotenberg said.
He pointed out that the literature is lacking in evidence guiding the postoperative management of patients with chronic rhinosinusitis with nasal polyps undergoing surgery. One study reported that steroid nasal spray did not influence polyp recurrence rate after surgery (Clin. Exp. Allergy. 2004;34:1395-400), while another showed that normal and buffered hypertonic saline nasal sprays had no beneficial effect on postoperative symptoms compared with no treatment (Am. J. Rhinol. 2006;20:191-6).
Dr. Rotenberg reported no relevant financial disclosures.
rhinosinusitis and polyposis, dexamethasone eye drops,
rhinosinusitis and polyposis, dexamethasone eye drops,
FROM THE COMBINED OTOLARYNGOLOGY SPRING MEETINGS
Major Finding: At 6 months postoperatively, there were no significant differences between the saline, saline plus budesonide, and saline/budesonide combination groups with regard to Lund-Kennedy scores (1.5, 0.9, and 1.2, respectively), adrenocorticotropic hormone (ACTH) ranges (all normal), and intraocular pressure (12.4, 12.9, and 13.9 mm Hg, respectively).
Data Source: Prospective, double-blind study in 60 patients with chronic rhinosinusitis and Samter’s triad who underwent functional endoscopic sinus surgery.
Disclosures: Dr. Rotenberg reported no relevant financial disclosures. Dr. Senior reported receiving honorarium from and serving as a consultant for BrainLAB, ENTrigue, and Gyrus ACMI, a subsidiary of Olympus.
Intranasal Postop Steroids Same as Saline in Select Patients
CHICAGO – Intranasal steroids were as good as saline alone as postoperative care in a randomized, double-blinded study of patients with chronic rhinosinusitis and Samter’s triad.
There was no difference in disease recurrence rate, complications, or quality of life at 6 months and 1-year postoperative using intranasal saline, saline plus budesonide, or saline and budesonide combined.
The surprising finding runs contrary to general practice and has several implications including how best to counsel patients for postoperative care, lead author Dr. Brian Rotenberg said at the Combined Otolaryngology Spring Meetings.
"If nasal steroids as done in this population don’t confer any additional benefit postoperatively, should we still prescribe them?" he asked. "Should we be prescribing something different or perhaps nothing at all? Is there a potential plus side here in terms of health-care cost savings?"
During a discussion of the study, an attendee expressed concern that insurers would interpret the results too broadly and deny coverage of postoperative nasal steroids for all patients with rhinosinusitis and polyposis, and not just those with Samter’s triad, a condition consisting of asthma, aspirin sensitivity, and nasal polyposis.
Another attendee agreed that nasal steroids are not potent enough in this population and said a pulse course of oral steroids 60 mg for 4 days can knock down symptoms in 80% of those with recurrence and be maintained with topical steroids. In the absence of a federally approved product for nasal use, he also suggested that dexamethasone eye drops can be effective.
Dr. Rotenberg replied that all patients received 3 weeks of postoperative oral prednisone, but that pulse-dosing of steroids was limited to one patient with early recurrence.
The 60 patients in the analysis had failed medical management for chronic rhinosinusitis with nasal polyposis and had a minimum preoperative Lund-Kennedy score of 8 out of 12. Nineteen patients were randomized to saline, 21 to saline plus budesonide, and 20 to saline/budesonide combination. Their mean Lund-Mackay scores were 20.6, 19.9, and 20.5, respectively.
Exclusion criteria included revision functional endoscopic sinus surgery, use of corticosteroids for other medical conditions, smoking, and concurrent disease with steroid contraindication.
At 6 months postoperatively, there were no significant differences between the saline, saline plus budesonide, and saline/budesonide combination groups with regard to Lund-Kennedy scores (1.5, 0.9, and 1.2, respectively), adrenocorticotropic hormone (ACTH) ranges (all normal), and intraocular pressure (12.4, 12.9, and 13.9 mm Hg, respectively), said Dr. Rotenberg of the University of Western Ontario in London, Ontario. Quality of life as assessed using the 21-item Sino-Nasal Outcome Test (SNOT-21) was also similar at a mean of 29.7, 27.4 and 28.2, respectively.
At 1 year of follow-up, once again there were no significant differences between the saline, saline plus budesonide, and saline/budesonide groups in Lund-Kennedy scores (3.7, 4.4, and 4.1, respectively), Lund-Mackay scores (11.8, 12.7, and 13.4, respectively), SNOT-21 scores (42.5, 47.9, and 42.2, respectively), intraocular pressure (13.1, 13.4,and 12.9 mm Hg, respectively). ACTH ranges were all normal.
A within-group analysis showed a significant improvement in all outcomes from baseline to 6 months, and a general worsening of outcomes at 1 year compared with the first 6 months, although they were still improved over baseline, Dr. Rotenberg said.
He pointed out that the literature is lacking in evidence guiding the postoperative management of patients with chronic rhinosinusitis with nasal polyps undergoing surgery. One study reported that steroid nasal spray did not influence polyp recurrence rate after surgery (Clin. Exp. Allergy. 2004;34:1395-400), while another showed that normal and buffered hypertonic saline nasal sprays had no beneficial effect on postoperative symptoms compared with no treatment (Am. J. Rhinol. 2006;20:191-6).
Dr. Rotenberg reported no relevant financial disclosures.
Session moderator Dr. Brent A. Senior said in an interview that the study was well designed and sufficiently powered, but would have been strengthened by the inclusion of a nontreatment group and more information on oral medications, as they can have a significant impact on disease. Follow-up to 18 months also would be useful, as the endoscopic appearance of the nasal cavity at 18 months has been shown to be predictive of how patients will do years after surgery.
"This is a good study, but not a game changer," he said, adding that additional work is needed to confirm the findings.
Dr. Senior is chief of rhinology, allergy and sinus surgery at the University of North Carolina at Chapel Hill.
rhinosinusitis and polyposis, dexamethasone eye drops,
Session moderator Dr. Brent A. Senior said in an interview that the study was well designed and sufficiently powered, but would have been strengthened by the inclusion of a nontreatment group and more information on oral medications, as they can have a significant impact on disease. Follow-up to 18 months also would be useful, as the endoscopic appearance of the nasal cavity at 18 months has been shown to be predictive of how patients will do years after surgery.
"This is a good study, but not a game changer," he said, adding that additional work is needed to confirm the findings.
Dr. Senior is chief of rhinology, allergy and sinus surgery at the University of North Carolina at Chapel Hill.
Session moderator Dr. Brent A. Senior said in an interview that the study was well designed and sufficiently powered, but would have been strengthened by the inclusion of a nontreatment group and more information on oral medications, as they can have a significant impact on disease. Follow-up to 18 months also would be useful, as the endoscopic appearance of the nasal cavity at 18 months has been shown to be predictive of how patients will do years after surgery.
"This is a good study, but not a game changer," he said, adding that additional work is needed to confirm the findings.
Dr. Senior is chief of rhinology, allergy and sinus surgery at the University of North Carolina at Chapel Hill.
CHICAGO – Intranasal steroids were as good as saline alone as postoperative care in a randomized, double-blinded study of patients with chronic rhinosinusitis and Samter’s triad.
There was no difference in disease recurrence rate, complications, or quality of life at 6 months and 1-year postoperative using intranasal saline, saline plus budesonide, or saline and budesonide combined.
The surprising finding runs contrary to general practice and has several implications including how best to counsel patients for postoperative care, lead author Dr. Brian Rotenberg said at the Combined Otolaryngology Spring Meetings.
"If nasal steroids as done in this population don’t confer any additional benefit postoperatively, should we still prescribe them?" he asked. "Should we be prescribing something different or perhaps nothing at all? Is there a potential plus side here in terms of health-care cost savings?"
During a discussion of the study, an attendee expressed concern that insurers would interpret the results too broadly and deny coverage of postoperative nasal steroids for all patients with rhinosinusitis and polyposis, and not just those with Samter’s triad, a condition consisting of asthma, aspirin sensitivity, and nasal polyposis.
Another attendee agreed that nasal steroids are not potent enough in this population and said a pulse course of oral steroids 60 mg for 4 days can knock down symptoms in 80% of those with recurrence and be maintained with topical steroids. In the absence of a federally approved product for nasal use, he also suggested that dexamethasone eye drops can be effective.
Dr. Rotenberg replied that all patients received 3 weeks of postoperative oral prednisone, but that pulse-dosing of steroids was limited to one patient with early recurrence.
The 60 patients in the analysis had failed medical management for chronic rhinosinusitis with nasal polyposis and had a minimum preoperative Lund-Kennedy score of 8 out of 12. Nineteen patients were randomized to saline, 21 to saline plus budesonide, and 20 to saline/budesonide combination. Their mean Lund-Mackay scores were 20.6, 19.9, and 20.5, respectively.
Exclusion criteria included revision functional endoscopic sinus surgery, use of corticosteroids for other medical conditions, smoking, and concurrent disease with steroid contraindication.
At 6 months postoperatively, there were no significant differences between the saline, saline plus budesonide, and saline/budesonide combination groups with regard to Lund-Kennedy scores (1.5, 0.9, and 1.2, respectively), adrenocorticotropic hormone (ACTH) ranges (all normal), and intraocular pressure (12.4, 12.9, and 13.9 mm Hg, respectively), said Dr. Rotenberg of the University of Western Ontario in London, Ontario. Quality of life as assessed using the 21-item Sino-Nasal Outcome Test (SNOT-21) was also similar at a mean of 29.7, 27.4 and 28.2, respectively.
At 1 year of follow-up, once again there were no significant differences between the saline, saline plus budesonide, and saline/budesonide groups in Lund-Kennedy scores (3.7, 4.4, and 4.1, respectively), Lund-Mackay scores (11.8, 12.7, and 13.4, respectively), SNOT-21 scores (42.5, 47.9, and 42.2, respectively), intraocular pressure (13.1, 13.4,and 12.9 mm Hg, respectively). ACTH ranges were all normal.
A within-group analysis showed a significant improvement in all outcomes from baseline to 6 months, and a general worsening of outcomes at 1 year compared with the first 6 months, although they were still improved over baseline, Dr. Rotenberg said.
He pointed out that the literature is lacking in evidence guiding the postoperative management of patients with chronic rhinosinusitis with nasal polyps undergoing surgery. One study reported that steroid nasal spray did not influence polyp recurrence rate after surgery (Clin. Exp. Allergy. 2004;34:1395-400), while another showed that normal and buffered hypertonic saline nasal sprays had no beneficial effect on postoperative symptoms compared with no treatment (Am. J. Rhinol. 2006;20:191-6).
Dr. Rotenberg reported no relevant financial disclosures.
CHICAGO – Intranasal steroids were as good as saline alone as postoperative care in a randomized, double-blinded study of patients with chronic rhinosinusitis and Samter’s triad.
There was no difference in disease recurrence rate, complications, or quality of life at 6 months and 1-year postoperative using intranasal saline, saline plus budesonide, or saline and budesonide combined.
The surprising finding runs contrary to general practice and has several implications including how best to counsel patients for postoperative care, lead author Dr. Brian Rotenberg said at the Combined Otolaryngology Spring Meetings.
"If nasal steroids as done in this population don’t confer any additional benefit postoperatively, should we still prescribe them?" he asked. "Should we be prescribing something different or perhaps nothing at all? Is there a potential plus side here in terms of health-care cost savings?"
During a discussion of the study, an attendee expressed concern that insurers would interpret the results too broadly and deny coverage of postoperative nasal steroids for all patients with rhinosinusitis and polyposis, and not just those with Samter’s triad, a condition consisting of asthma, aspirin sensitivity, and nasal polyposis.
Another attendee agreed that nasal steroids are not potent enough in this population and said a pulse course of oral steroids 60 mg for 4 days can knock down symptoms in 80% of those with recurrence and be maintained with topical steroids. In the absence of a federally approved product for nasal use, he also suggested that dexamethasone eye drops can be effective.
Dr. Rotenberg replied that all patients received 3 weeks of postoperative oral prednisone, but that pulse-dosing of steroids was limited to one patient with early recurrence.
The 60 patients in the analysis had failed medical management for chronic rhinosinusitis with nasal polyposis and had a minimum preoperative Lund-Kennedy score of 8 out of 12. Nineteen patients were randomized to saline, 21 to saline plus budesonide, and 20 to saline/budesonide combination. Their mean Lund-Mackay scores were 20.6, 19.9, and 20.5, respectively.
Exclusion criteria included revision functional endoscopic sinus surgery, use of corticosteroids for other medical conditions, smoking, and concurrent disease with steroid contraindication.
At 6 months postoperatively, there were no significant differences between the saline, saline plus budesonide, and saline/budesonide combination groups with regard to Lund-Kennedy scores (1.5, 0.9, and 1.2, respectively), adrenocorticotropic hormone (ACTH) ranges (all normal), and intraocular pressure (12.4, 12.9, and 13.9 mm Hg, respectively), said Dr. Rotenberg of the University of Western Ontario in London, Ontario. Quality of life as assessed using the 21-item Sino-Nasal Outcome Test (SNOT-21) was also similar at a mean of 29.7, 27.4 and 28.2, respectively.
At 1 year of follow-up, once again there were no significant differences between the saline, saline plus budesonide, and saline/budesonide groups in Lund-Kennedy scores (3.7, 4.4, and 4.1, respectively), Lund-Mackay scores (11.8, 12.7, and 13.4, respectively), SNOT-21 scores (42.5, 47.9, and 42.2, respectively), intraocular pressure (13.1, 13.4,and 12.9 mm Hg, respectively). ACTH ranges were all normal.
A within-group analysis showed a significant improvement in all outcomes from baseline to 6 months, and a general worsening of outcomes at 1 year compared with the first 6 months, although they were still improved over baseline, Dr. Rotenberg said.
He pointed out that the literature is lacking in evidence guiding the postoperative management of patients with chronic rhinosinusitis with nasal polyps undergoing surgery. One study reported that steroid nasal spray did not influence polyp recurrence rate after surgery (Clin. Exp. Allergy. 2004;34:1395-400), while another showed that normal and buffered hypertonic saline nasal sprays had no beneficial effect on postoperative symptoms compared with no treatment (Am. J. Rhinol. 2006;20:191-6).
Dr. Rotenberg reported no relevant financial disclosures.
rhinosinusitis and polyposis, dexamethasone eye drops,
rhinosinusitis and polyposis, dexamethasone eye drops,
FROM THE COMBINED OTOLARYNGOLOGY SPRING MEETINGS
Intranasal Postop Steroids Same as Saline in Select Patients
CHICAGO – Intranasal steroids were as good as saline alone as postoperative care in a randomized, double-blinded study of patients with chronic rhinosinusitis and Samter’s triad.
There was no difference in disease recurrence rate, complications, or quality of life at 6 months and 1-year postoperative using intranasal saline, saline plus budesonide, or saline and budesonide combined.
The surprising finding runs contrary to general practice and has several implications including how best to counsel patients for postoperative care, lead author Dr. Brian Rotenberg said at the Combined Otolaryngology Spring Meetings.
"If nasal steroids as done in this population don’t confer any additional benefit postoperatively, should we still prescribe them?" he asked. "Should we be prescribing something different or perhaps nothing at all? Is there a potential plus side here in terms of health-care cost savings?"
During a discussion of the study, an attendee expressed concern that insurers would interpret the results too broadly and deny coverage of postoperative nasal steroids for all patients with rhinosinusitis and polyposis, and not just those with Samter’s triad, a condition consisting of asthma, aspirin sensitivity, and nasal polyposis.
Another attendee agreed that nasal steroids are not potent enough in this population and said a pulse course of oral steroids 60 mg for 4 days can knock down symptoms in 80% of those with recurrence and be maintained with topical steroids. In the absence of a federally approved product for nasal use, he also suggested that dexamethasone eye drops can be effective.
Dr. Rotenberg replied that all patients received 3 weeks of postoperative oral prednisone, but that pulse-dosing of steroids was limited to one patient with early recurrence.
The 60 patients in the analysis had failed medical management for chronic rhinosinusitis with nasal polyposis and had a minimum preoperative Lund-Kennedy score of 8 out of 12. Nineteen patients were randomized to saline, 21 to saline plus budesonide, and 20 to saline/budesonide combination. Their mean Lund-Mackay scores were 20.6, 19.9, and 20.5, respectively.
Exclusion criteria included revision functional endoscopic sinus surgery, use of corticosteroids for other medical conditions, smoking, and concurrent disease with steroid contraindication.
At 6 months postoperatively, there were no significant differences between the saline, saline plus budesonide, and saline/budesonide combination groups with regard to Lund-Kennedy scores (1.5, 0.9, and 1.2, respectively), adrenocorticotropic hormone (ACTH) ranges (all normal), and intraocular pressure (12.4, 12.9, and 13.9 mm Hg, respectively), said Dr. Rotenberg of the University of Western Ontario in London, Ontario. Quality of life as assessed using the 21-item Sino-Nasal Outcome Test (SNOT-21) was also similar at a mean of 29.7, 27.4 and 28.2, respectively.
At 1 year of follow-up, once again there were no significant differences between the saline, saline plus budesonide, and saline/budesonide groups in Lund-Kennedy scores (3.7, 4.4, and 4.1, respectively), Lund-Mackay scores (11.8, 12.7, and 13.4, respectively), SNOT-21 scores (42.5, 47.9, and 42.2, respectively), intraocular pressure (13.1, 13.4,and 12.9 mm Hg, respectively). ACTH ranges were all normal.
A within-group analysis showed a significant improvement in all outcomes from baseline to 6 months, and a general worsening of outcomes at 1 year compared with the first 6 months, although they were still improved over baseline, Dr. Rotenberg said.
He pointed out that the literature is lacking in evidence guiding the postoperative management of patients with chronic rhinosinusitis with nasal polyps undergoing surgery. One study reported that steroid nasal spray did not influence polyp recurrence rate after surgery (Clin. Exp. Allergy. 2004;34:1395-400), while another showed that normal and buffered hypertonic saline nasal sprays had no beneficial effect on postoperative symptoms compared with no treatment (Am. J. Rhinol. 2006;20:191-6).
Dr. Rotenberg reported no relevant financial disclosures.
Session moderator Dr. Brent A. Senior said in an interview that the study was well designed and sufficiently powered, but would have been strengthened by the inclusion of a nontreatment group and more information on oral medications, as they can have a significant impact on disease. Follow-up to 18 months also would be useful, as the endoscopic appearance of the nasal cavity at 18 months has been shown to be predictive of how patients will do years after surgery.
"This is a good study, but not a game changer," he said, adding that additional work is needed to confirm the findings.
Dr. Senior is chief of rhinology, allergy and sinus surgery at the University of North Carolina at Chapel Hill.
rhinosinusitis and polyposis, dexamethasone eye drops,
Session moderator Dr. Brent A. Senior said in an interview that the study was well designed and sufficiently powered, but would have been strengthened by the inclusion of a nontreatment group and more information on oral medications, as they can have a significant impact on disease. Follow-up to 18 months also would be useful, as the endoscopic appearance of the nasal cavity at 18 months has been shown to be predictive of how patients will do years after surgery.
"This is a good study, but not a game changer," he said, adding that additional work is needed to confirm the findings.
Dr. Senior is chief of rhinology, allergy and sinus surgery at the University of North Carolina at Chapel Hill.
Session moderator Dr. Brent A. Senior said in an interview that the study was well designed and sufficiently powered, but would have been strengthened by the inclusion of a nontreatment group and more information on oral medications, as they can have a significant impact on disease. Follow-up to 18 months also would be useful, as the endoscopic appearance of the nasal cavity at 18 months has been shown to be predictive of how patients will do years after surgery.
"This is a good study, but not a game changer," he said, adding that additional work is needed to confirm the findings.
Dr. Senior is chief of rhinology, allergy and sinus surgery at the University of North Carolina at Chapel Hill.
CHICAGO – Intranasal steroids were as good as saline alone as postoperative care in a randomized, double-blinded study of patients with chronic rhinosinusitis and Samter’s triad.
There was no difference in disease recurrence rate, complications, or quality of life at 6 months and 1-year postoperative using intranasal saline, saline plus budesonide, or saline and budesonide combined.
The surprising finding runs contrary to general practice and has several implications including how best to counsel patients for postoperative care, lead author Dr. Brian Rotenberg said at the Combined Otolaryngology Spring Meetings.
"If nasal steroids as done in this population don’t confer any additional benefit postoperatively, should we still prescribe them?" he asked. "Should we be prescribing something different or perhaps nothing at all? Is there a potential plus side here in terms of health-care cost savings?"
During a discussion of the study, an attendee expressed concern that insurers would interpret the results too broadly and deny coverage of postoperative nasal steroids for all patients with rhinosinusitis and polyposis, and not just those with Samter’s triad, a condition consisting of asthma, aspirin sensitivity, and nasal polyposis.
Another attendee agreed that nasal steroids are not potent enough in this population and said a pulse course of oral steroids 60 mg for 4 days can knock down symptoms in 80% of those with recurrence and be maintained with topical steroids. In the absence of a federally approved product for nasal use, he also suggested that dexamethasone eye drops can be effective.
Dr. Rotenberg replied that all patients received 3 weeks of postoperative oral prednisone, but that pulse-dosing of steroids was limited to one patient with early recurrence.
The 60 patients in the analysis had failed medical management for chronic rhinosinusitis with nasal polyposis and had a minimum preoperative Lund-Kennedy score of 8 out of 12. Nineteen patients were randomized to saline, 21 to saline plus budesonide, and 20 to saline/budesonide combination. Their mean Lund-Mackay scores were 20.6, 19.9, and 20.5, respectively.
Exclusion criteria included revision functional endoscopic sinus surgery, use of corticosteroids for other medical conditions, smoking, and concurrent disease with steroid contraindication.
At 6 months postoperatively, there were no significant differences between the saline, saline plus budesonide, and saline/budesonide combination groups with regard to Lund-Kennedy scores (1.5, 0.9, and 1.2, respectively), adrenocorticotropic hormone (ACTH) ranges (all normal), and intraocular pressure (12.4, 12.9, and 13.9 mm Hg, respectively), said Dr. Rotenberg of the University of Western Ontario in London, Ontario. Quality of life as assessed using the 21-item Sino-Nasal Outcome Test (SNOT-21) was also similar at a mean of 29.7, 27.4 and 28.2, respectively.
At 1 year of follow-up, once again there were no significant differences between the saline, saline plus budesonide, and saline/budesonide groups in Lund-Kennedy scores (3.7, 4.4, and 4.1, respectively), Lund-Mackay scores (11.8, 12.7, and 13.4, respectively), SNOT-21 scores (42.5, 47.9, and 42.2, respectively), intraocular pressure (13.1, 13.4,and 12.9 mm Hg, respectively). ACTH ranges were all normal.
A within-group analysis showed a significant improvement in all outcomes from baseline to 6 months, and a general worsening of outcomes at 1 year compared with the first 6 months, although they were still improved over baseline, Dr. Rotenberg said.
He pointed out that the literature is lacking in evidence guiding the postoperative management of patients with chronic rhinosinusitis with nasal polyps undergoing surgery. One study reported that steroid nasal spray did not influence polyp recurrence rate after surgery (Clin. Exp. Allergy. 2004;34:1395-400), while another showed that normal and buffered hypertonic saline nasal sprays had no beneficial effect on postoperative symptoms compared with no treatment (Am. J. Rhinol. 2006;20:191-6).
Dr. Rotenberg reported no relevant financial disclosures.
CHICAGO – Intranasal steroids were as good as saline alone as postoperative care in a randomized, double-blinded study of patients with chronic rhinosinusitis and Samter’s triad.
There was no difference in disease recurrence rate, complications, or quality of life at 6 months and 1-year postoperative using intranasal saline, saline plus budesonide, or saline and budesonide combined.
The surprising finding runs contrary to general practice and has several implications including how best to counsel patients for postoperative care, lead author Dr. Brian Rotenberg said at the Combined Otolaryngology Spring Meetings.
"If nasal steroids as done in this population don’t confer any additional benefit postoperatively, should we still prescribe them?" he asked. "Should we be prescribing something different or perhaps nothing at all? Is there a potential plus side here in terms of health-care cost savings?"
During a discussion of the study, an attendee expressed concern that insurers would interpret the results too broadly and deny coverage of postoperative nasal steroids for all patients with rhinosinusitis and polyposis, and not just those with Samter’s triad, a condition consisting of asthma, aspirin sensitivity, and nasal polyposis.
Another attendee agreed that nasal steroids are not potent enough in this population and said a pulse course of oral steroids 60 mg for 4 days can knock down symptoms in 80% of those with recurrence and be maintained with topical steroids. In the absence of a federally approved product for nasal use, he also suggested that dexamethasone eye drops can be effective.
Dr. Rotenberg replied that all patients received 3 weeks of postoperative oral prednisone, but that pulse-dosing of steroids was limited to one patient with early recurrence.
The 60 patients in the analysis had failed medical management for chronic rhinosinusitis with nasal polyposis and had a minimum preoperative Lund-Kennedy score of 8 out of 12. Nineteen patients were randomized to saline, 21 to saline plus budesonide, and 20 to saline/budesonide combination. Their mean Lund-Mackay scores were 20.6, 19.9, and 20.5, respectively.
Exclusion criteria included revision functional endoscopic sinus surgery, use of corticosteroids for other medical conditions, smoking, and concurrent disease with steroid contraindication.
At 6 months postoperatively, there were no significant differences between the saline, saline plus budesonide, and saline/budesonide combination groups with regard to Lund-Kennedy scores (1.5, 0.9, and 1.2, respectively), adrenocorticotropic hormone (ACTH) ranges (all normal), and intraocular pressure (12.4, 12.9, and 13.9 mm Hg, respectively), said Dr. Rotenberg of the University of Western Ontario in London, Ontario. Quality of life as assessed using the 21-item Sino-Nasal Outcome Test (SNOT-21) was also similar at a mean of 29.7, 27.4 and 28.2, respectively.
At 1 year of follow-up, once again there were no significant differences between the saline, saline plus budesonide, and saline/budesonide groups in Lund-Kennedy scores (3.7, 4.4, and 4.1, respectively), Lund-Mackay scores (11.8, 12.7, and 13.4, respectively), SNOT-21 scores (42.5, 47.9, and 42.2, respectively), intraocular pressure (13.1, 13.4,and 12.9 mm Hg, respectively). ACTH ranges were all normal.
A within-group analysis showed a significant improvement in all outcomes from baseline to 6 months, and a general worsening of outcomes at 1 year compared with the first 6 months, although they were still improved over baseline, Dr. Rotenberg said.
He pointed out that the literature is lacking in evidence guiding the postoperative management of patients with chronic rhinosinusitis with nasal polyps undergoing surgery. One study reported that steroid nasal spray did not influence polyp recurrence rate after surgery (Clin. Exp. Allergy. 2004;34:1395-400), while another showed that normal and buffered hypertonic saline nasal sprays had no beneficial effect on postoperative symptoms compared with no treatment (Am. J. Rhinol. 2006;20:191-6).
Dr. Rotenberg reported no relevant financial disclosures.
rhinosinusitis and polyposis, dexamethasone eye drops,
rhinosinusitis and polyposis, dexamethasone eye drops,
FROM THE COMBINED OTOLARYNGOLOGY SPRING MEETINGS
Major Finding: At 6 months postoperatively, there were no significant differences between the saline, saline plus budesonide, and saline/budesonide combination groups with regard to Lund-Kennedy scores (1.5, 0.9, and 1.2, respectively), adrenocorticotropic hormone (ACTH) ranges (all normal), and intraocular pressure (12.4, 12.9, and 13.9 mm Hg, respectively).
Data Source: Prospective, double-blind study in 60 patients with chronic rhinosinusitis and Samter’s triad who underwent functional endoscopic sinus surgery.
Disclosures: Dr. Rotenberg reported no relevant financial disclosures. Dr. Senior reported receiving honorarium from and serving as a consultant for BrainLAB, ENTrigue, and Gyrus ACMI, a subsidiary of Olympus.
Steroid-Eluting Sinus Stent May Minimize Postop Events
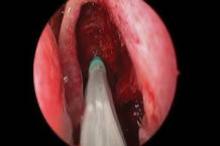
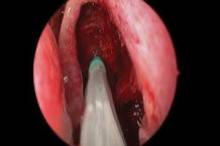
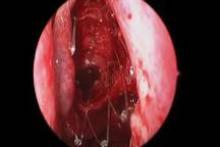
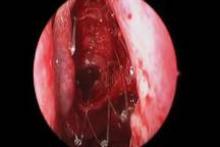
CHICAGO – A bioabsorbable steroid-eluting stent preserved sinus patency following endoscopic sinus surgery in 50 patients with chronic rhinosinusitis in a prospective, multicenter study.
The stent was associated with low rates of polyp formation, inflammation, and adhesions, despite a challenging patient population and withholding of postoperative oral and topical steroids for 30 days, lead author Dr. Keith D. Forwith said at the Combined Otolaryngology Spring Meetings.
The results were consistent across different patient populations and consistent with the pilot study in 43 patients (Int. Forum Allergy Rhinol. 2011;1:23-32).
"I think with any kind of new technique or device, the ultimate thing is how the surgeon feels about it and his or her results," he said. "What I noticed most of all when we stopped doing the study is that I missed using this device. Patients I had used it in during the study had very clean results, very easy debridements. ... We’re very pleased with the results and think it’s a promising technology."
Study sponsor Intersect ENT Inc. has submitted the investigational stent for federal review and hopes to market the device within 6-9 months.
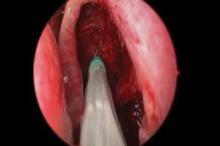
The springlike stent maintains patency by propping open the sinus cavity and releasing mometasone furoate into the sinus lining over 30 days before being resorbed. If approved, it could provide patients with an alternative to space-filling packing materials and silicone stents, and reduce the use of systemic steroids.
The current study involved 50 adults from seven centers with chronic rhinosinusitis for at least 8 consecutive weeks that was confirmed by CT scan (mean CT stage, 11.2). Antibiotics could be prescribed per physician standard of care, and saline irrigation was permitted postoperatively as needed. Polyps were present in 66% of patients, and 28% had undergone a prior sinus procedure. Their mean age was 44 years, and 52% were male.
Ethmoidectomy and maxillary antrostomy were performed in all 50 patients, with 28 also undergoing frontal sinusotomy and 31 sphenoidotomy. Stents were placed unilaterally in 10 patients and bilaterally in 40 patients. Device placement was successful in 100% of sinuses treated, and the implants were resorbed as predicted, Dr. Forwith said. At postoperative day 30, 15% of the material remained and 0.2% remained at day 60.
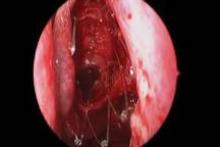
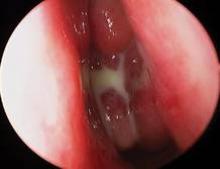
Endoscopic follow-up at 1 month revealed polypoid edema in 10% of patients, significant adhesion formation in 1%, and middle turbinate lateralization in only 4.4%, said Dr. Forwith, who is in private practice in Louisville, Ky.
When patients were asked about the procedure, their mean score on the 22-question Sino-Nasal Outcome Test improved significantly from baseline through 6 months. The same was true using the Rhinosinusitis Disability Index. "We were pretty pleased that our patients liked the results of the intervention," he said.
No clinically significant changes from baseline occurred in lens opacities or intraocular pressure, which was a theoretical concern given the close proximity of the device. The patients’ mean intraocular pressure was 15 mm Hg at baseline and 14.3 mm Hg at day 30.
One patient experienced headache with sinus pressure/irritation at day 21 that was determined to be related primarily to the surgery and was exacerbated in intensity by the presence of crust on the device. The device was removed and the event resolved without sequelae by day 28.
When asked during a discussion of the study whether the drug-eluting stent resulted in any systemic complications or adrenal corticol suppression, Dr. Forwith replied that they did not specifically look at adrenal suppression, but added that the 370-mcg dose of mometasone furoate is lower than the dose patients receive with most b.i.d. nasal spray administration.
Stent maker Intersect ENT provided funding, administrative support, and materials for the study. Dr. Forwith reported no conflicts of interest.
CHICAGO – A bioabsorbable steroid-eluting stent preserved sinus patency following endoscopic sinus surgery in 50 patients with chronic rhinosinusitis in a prospective, multicenter study.
The stent was associated with low rates of polyp formation, inflammation, and adhesions, despite a challenging patient population and withholding of postoperative oral and topical steroids for 30 days, lead author Dr. Keith D. Forwith said at the Combined Otolaryngology Spring Meetings.
The results were consistent across different patient populations and consistent with the pilot study in 43 patients (Int. Forum Allergy Rhinol. 2011;1:23-32).
"I think with any kind of new technique or device, the ultimate thing is how the surgeon feels about it and his or her results," he said. "What I noticed most of all when we stopped doing the study is that I missed using this device. Patients I had used it in during the study had very clean results, very easy debridements. ... We’re very pleased with the results and think it’s a promising technology."
Study sponsor Intersect ENT Inc. has submitted the investigational stent for federal review and hopes to market the device within 6-9 months.
The springlike stent maintains patency by propping open the sinus cavity and releasing mometasone furoate into the sinus lining over 30 days before being resorbed. If approved, it could provide patients with an alternative to space-filling packing materials and silicone stents, and reduce the use of systemic steroids.
The current study involved 50 adults from seven centers with chronic rhinosinusitis for at least 8 consecutive weeks that was confirmed by CT scan (mean CT stage, 11.2). Antibiotics could be prescribed per physician standard of care, and saline irrigation was permitted postoperatively as needed. Polyps were present in 66% of patients, and 28% had undergone a prior sinus procedure. Their mean age was 44 years, and 52% were male.
Ethmoidectomy and maxillary antrostomy were performed in all 50 patients, with 28 also undergoing frontal sinusotomy and 31 sphenoidotomy. Stents were placed unilaterally in 10 patients and bilaterally in 40 patients. Device placement was successful in 100% of sinuses treated, and the implants were resorbed as predicted, Dr. Forwith said. At postoperative day 30, 15% of the material remained and 0.2% remained at day 60.
Endoscopic follow-up at 1 month revealed polypoid edema in 10% of patients, significant adhesion formation in 1%, and middle turbinate lateralization in only 4.4%, said Dr. Forwith, who is in private practice in Louisville, Ky.
When patients were asked about the procedure, their mean score on the 22-question Sino-Nasal Outcome Test improved significantly from baseline through 6 months. The same was true using the Rhinosinusitis Disability Index. "We were pretty pleased that our patients liked the results of the intervention," he said.
No clinically significant changes from baseline occurred in lens opacities or intraocular pressure, which was a theoretical concern given the close proximity of the device. The patients’ mean intraocular pressure was 15 mm Hg at baseline and 14.3 mm Hg at day 30.
One patient experienced headache with sinus pressure/irritation at day 21 that was determined to be related primarily to the surgery and was exacerbated in intensity by the presence of crust on the device. The device was removed and the event resolved without sequelae by day 28.
When asked during a discussion of the study whether the drug-eluting stent resulted in any systemic complications or adrenal corticol suppression, Dr. Forwith replied that they did not specifically look at adrenal suppression, but added that the 370-mcg dose of mometasone furoate is lower than the dose patients receive with most b.i.d. nasal spray administration.
Stent maker Intersect ENT provided funding, administrative support, and materials for the study. Dr. Forwith reported no conflicts of interest.
CHICAGO – A bioabsorbable steroid-eluting stent preserved sinus patency following endoscopic sinus surgery in 50 patients with chronic rhinosinusitis in a prospective, multicenter study.
The stent was associated with low rates of polyp formation, inflammation, and adhesions, despite a challenging patient population and withholding of postoperative oral and topical steroids for 30 days, lead author Dr. Keith D. Forwith said at the Combined Otolaryngology Spring Meetings.
The results were consistent across different patient populations and consistent with the pilot study in 43 patients (Int. Forum Allergy Rhinol. 2011;1:23-32).
"I think with any kind of new technique or device, the ultimate thing is how the surgeon feels about it and his or her results," he said. "What I noticed most of all when we stopped doing the study is that I missed using this device. Patients I had used it in during the study had very clean results, very easy debridements. ... We’re very pleased with the results and think it’s a promising technology."
Study sponsor Intersect ENT Inc. has submitted the investigational stent for federal review and hopes to market the device within 6-9 months.
The springlike stent maintains patency by propping open the sinus cavity and releasing mometasone furoate into the sinus lining over 30 days before being resorbed. If approved, it could provide patients with an alternative to space-filling packing materials and silicone stents, and reduce the use of systemic steroids.
The current study involved 50 adults from seven centers with chronic rhinosinusitis for at least 8 consecutive weeks that was confirmed by CT scan (mean CT stage, 11.2). Antibiotics could be prescribed per physician standard of care, and saline irrigation was permitted postoperatively as needed. Polyps were present in 66% of patients, and 28% had undergone a prior sinus procedure. Their mean age was 44 years, and 52% were male.
Ethmoidectomy and maxillary antrostomy were performed in all 50 patients, with 28 also undergoing frontal sinusotomy and 31 sphenoidotomy. Stents were placed unilaterally in 10 patients and bilaterally in 40 patients. Device placement was successful in 100% of sinuses treated, and the implants were resorbed as predicted, Dr. Forwith said. At postoperative day 30, 15% of the material remained and 0.2% remained at day 60.
Endoscopic follow-up at 1 month revealed polypoid edema in 10% of patients, significant adhesion formation in 1%, and middle turbinate lateralization in only 4.4%, said Dr. Forwith, who is in private practice in Louisville, Ky.
When patients were asked about the procedure, their mean score on the 22-question Sino-Nasal Outcome Test improved significantly from baseline through 6 months. The same was true using the Rhinosinusitis Disability Index. "We were pretty pleased that our patients liked the results of the intervention," he said.
No clinically significant changes from baseline occurred in lens opacities or intraocular pressure, which was a theoretical concern given the close proximity of the device. The patients’ mean intraocular pressure was 15 mm Hg at baseline and 14.3 mm Hg at day 30.
One patient experienced headache with sinus pressure/irritation at day 21 that was determined to be related primarily to the surgery and was exacerbated in intensity by the presence of crust on the device. The device was removed and the event resolved without sequelae by day 28.
When asked during a discussion of the study whether the drug-eluting stent resulted in any systemic complications or adrenal corticol suppression, Dr. Forwith replied that they did not specifically look at adrenal suppression, but added that the 370-mcg dose of mometasone furoate is lower than the dose patients receive with most b.i.d. nasal spray administration.
Stent maker Intersect ENT provided funding, administrative support, and materials for the study. Dr. Forwith reported no conflicts of interest.
FROM THE COMBINED OTOLARYNGOLOGY SPRING MEETINGS
Major Finding: At 1 month follow-up, the occurrence of polypoid edema was 10%, significant adhesion formation 1%, and middle turbinate lateralization 4.4%.
Data Source: Prospective, multicenter study in 50 patients with chronic rhinosinusitis undergoing endoscopic sinus surgery.
Disclosures: Stent maker Intersect ENT provided funding, administrative support, and materials for the study. Dr. Forwith reported no conflicts of interest.
Steroid-Eluting Sinus Stent May Minimize Postop Events
CHICAGO – A bioabsorbable steroid-eluting stent preserved sinus patency following endoscopic sinus surgery in 50 patients with chronic rhinosinusitis in a prospective, multicenter study.
The stent was associated with low rates of polyp formation, inflammation, and adhesions, despite a challenging patient population and withholding of postoperative oral and topical steroids for 30 days, lead author Dr. Keith D. Forwith said at the Combined Otolaryngology Spring Meetings.
The results were consistent across different patient populations and consistent with the pilot study in 43 patients (Int. Forum Allergy Rhinol. 2011;1:23-32).
"I think with any kind of new technique or device, the ultimate thing is how the surgeon feels about it and his or her results," he said. "What I noticed most of all when we stopped doing the study is that I missed using this device. Patients I had used it in during the study had very clean results, very easy debridements. ... We’re very pleased with the results and think it’s a promising technology."
Study sponsor Intersect ENT Inc. has submitted the investigational stent for federal review and hopes to market the device within 6-9 months.
The springlike stent maintains patency by propping open the sinus cavity and releasing mometasone furoate into the sinus lining over 30 days before being resorbed. If approved, it could provide patients with an alternative to space-filling packing materials and silicone stents, and reduce the use of systemic steroids.
The current study involved 50 adults from seven centers with chronic rhinosinusitis for at least 8 consecutive weeks that was confirmed by CT scan (mean CT stage, 11.2). Antibiotics could be prescribed per physician standard of care, and saline irrigation was permitted postoperatively as needed. Polyps were present in 66% of patients, and 28% had undergone a prior sinus procedure. Their mean age was 44 years, and 52% were male.
Ethmoidectomy and maxillary antrostomy were performed in all 50 patients, with 28 also undergoing frontal sinusotomy and 31 sphenoidotomy. Stents were placed unilaterally in 10 patients and bilaterally in 40 patients. Device placement was successful in 100% of sinuses treated, and the implants were resorbed as predicted, Dr. Forwith said. At postoperative day 30, 15% of the material remained and 0.2% remained at day 60.
Endoscopic follow-up at 1 month revealed polypoid edema in 10% of patients, significant adhesion formation in 1%, and middle turbinate lateralization in only 4.4%, said Dr. Forwith, who is in private practice in Louisville, Ky.
When patients were asked about the procedure, their mean score on the 22-question Sino-Nasal Outcome Test improved significantly from baseline through 6 months. The same was true using the Rhinosinusitis Disability Index. "We were pretty pleased that our patients liked the results of the intervention," he said.
No clinically significant changes from baseline occurred in lens opacities or intraocular pressure, which was a theoretical concern given the close proximity of the device. The patients’ mean intraocular pressure was 15 mm Hg at baseline and 14.3 mm Hg at day 30.
One patient experienced headache with sinus pressure/irritation at day 21 that was determined to be related primarily to the surgery and was exacerbated in intensity by the presence of crust on the device. The device was removed and the event resolved without sequelae by day 28.
When asked during a discussion of the study whether the drug-eluting stent resulted in any systemic complications or adrenal corticol suppression, Dr. Forwith replied that they did not specifically look at adrenal suppression, but added that the 370-mcg dose of mometasone furoate is lower than the dose patients receive with most b.i.d. nasal spray administration.
Stent maker Intersect ENT provided funding, administrative support, and materials for the study. Dr. Forwith reported no conflicts of interest.
CHICAGO – A bioabsorbable steroid-eluting stent preserved sinus patency following endoscopic sinus surgery in 50 patients with chronic rhinosinusitis in a prospective, multicenter study.
The stent was associated with low rates of polyp formation, inflammation, and adhesions, despite a challenging patient population and withholding of postoperative oral and topical steroids for 30 days, lead author Dr. Keith D. Forwith said at the Combined Otolaryngology Spring Meetings.
The results were consistent across different patient populations and consistent with the pilot study in 43 patients (Int. Forum Allergy Rhinol. 2011;1:23-32).
"I think with any kind of new technique or device, the ultimate thing is how the surgeon feels about it and his or her results," he said. "What I noticed most of all when we stopped doing the study is that I missed using this device. Patients I had used it in during the study had very clean results, very easy debridements. ... We’re very pleased with the results and think it’s a promising technology."
Study sponsor Intersect ENT Inc. has submitted the investigational stent for federal review and hopes to market the device within 6-9 months.
The springlike stent maintains patency by propping open the sinus cavity and releasing mometasone furoate into the sinus lining over 30 days before being resorbed. If approved, it could provide patients with an alternative to space-filling packing materials and silicone stents, and reduce the use of systemic steroids.
The current study involved 50 adults from seven centers with chronic rhinosinusitis for at least 8 consecutive weeks that was confirmed by CT scan (mean CT stage, 11.2). Antibiotics could be prescribed per physician standard of care, and saline irrigation was permitted postoperatively as needed. Polyps were present in 66% of patients, and 28% had undergone a prior sinus procedure. Their mean age was 44 years, and 52% were male.
Ethmoidectomy and maxillary antrostomy were performed in all 50 patients, with 28 also undergoing frontal sinusotomy and 31 sphenoidotomy. Stents were placed unilaterally in 10 patients and bilaterally in 40 patients. Device placement was successful in 100% of sinuses treated, and the implants were resorbed as predicted, Dr. Forwith said. At postoperative day 30, 15% of the material remained and 0.2% remained at day 60.
Endoscopic follow-up at 1 month revealed polypoid edema in 10% of patients, significant adhesion formation in 1%, and middle turbinate lateralization in only 4.4%, said Dr. Forwith, who is in private practice in Louisville, Ky.
When patients were asked about the procedure, their mean score on the 22-question Sino-Nasal Outcome Test improved significantly from baseline through 6 months. The same was true using the Rhinosinusitis Disability Index. "We were pretty pleased that our patients liked the results of the intervention," he said.
No clinically significant changes from baseline occurred in lens opacities or intraocular pressure, which was a theoretical concern given the close proximity of the device. The patients’ mean intraocular pressure was 15 mm Hg at baseline and 14.3 mm Hg at day 30.
One patient experienced headache with sinus pressure/irritation at day 21 that was determined to be related primarily to the surgery and was exacerbated in intensity by the presence of crust on the device. The device was removed and the event resolved without sequelae by day 28.
When asked during a discussion of the study whether the drug-eluting stent resulted in any systemic complications or adrenal corticol suppression, Dr. Forwith replied that they did not specifically look at adrenal suppression, but added that the 370-mcg dose of mometasone furoate is lower than the dose patients receive with most b.i.d. nasal spray administration.
Stent maker Intersect ENT provided funding, administrative support, and materials for the study. Dr. Forwith reported no conflicts of interest.
CHICAGO – A bioabsorbable steroid-eluting stent preserved sinus patency following endoscopic sinus surgery in 50 patients with chronic rhinosinusitis in a prospective, multicenter study.
The stent was associated with low rates of polyp formation, inflammation, and adhesions, despite a challenging patient population and withholding of postoperative oral and topical steroids for 30 days, lead author Dr. Keith D. Forwith said at the Combined Otolaryngology Spring Meetings.
The results were consistent across different patient populations and consistent with the pilot study in 43 patients (Int. Forum Allergy Rhinol. 2011;1:23-32).
"I think with any kind of new technique or device, the ultimate thing is how the surgeon feels about it and his or her results," he said. "What I noticed most of all when we stopped doing the study is that I missed using this device. Patients I had used it in during the study had very clean results, very easy debridements. ... We’re very pleased with the results and think it’s a promising technology."
Study sponsor Intersect ENT Inc. has submitted the investigational stent for federal review and hopes to market the device within 6-9 months.
The springlike stent maintains patency by propping open the sinus cavity and releasing mometasone furoate into the sinus lining over 30 days before being resorbed. If approved, it could provide patients with an alternative to space-filling packing materials and silicone stents, and reduce the use of systemic steroids.
The current study involved 50 adults from seven centers with chronic rhinosinusitis for at least 8 consecutive weeks that was confirmed by CT scan (mean CT stage, 11.2). Antibiotics could be prescribed per physician standard of care, and saline irrigation was permitted postoperatively as needed. Polyps were present in 66% of patients, and 28% had undergone a prior sinus procedure. Their mean age was 44 years, and 52% were male.
Ethmoidectomy and maxillary antrostomy were performed in all 50 patients, with 28 also undergoing frontal sinusotomy and 31 sphenoidotomy. Stents were placed unilaterally in 10 patients and bilaterally in 40 patients. Device placement was successful in 100% of sinuses treated, and the implants were resorbed as predicted, Dr. Forwith said. At postoperative day 30, 15% of the material remained and 0.2% remained at day 60.
Endoscopic follow-up at 1 month revealed polypoid edema in 10% of patients, significant adhesion formation in 1%, and middle turbinate lateralization in only 4.4%, said Dr. Forwith, who is in private practice in Louisville, Ky.
When patients were asked about the procedure, their mean score on the 22-question Sino-Nasal Outcome Test improved significantly from baseline through 6 months. The same was true using the Rhinosinusitis Disability Index. "We were pretty pleased that our patients liked the results of the intervention," he said.
No clinically significant changes from baseline occurred in lens opacities or intraocular pressure, which was a theoretical concern given the close proximity of the device. The patients’ mean intraocular pressure was 15 mm Hg at baseline and 14.3 mm Hg at day 30.
One patient experienced headache with sinus pressure/irritation at day 21 that was determined to be related primarily to the surgery and was exacerbated in intensity by the presence of crust on the device. The device was removed and the event resolved without sequelae by day 28.
When asked during a discussion of the study whether the drug-eluting stent resulted in any systemic complications or adrenal corticol suppression, Dr. Forwith replied that they did not specifically look at adrenal suppression, but added that the 370-mcg dose of mometasone furoate is lower than the dose patients receive with most b.i.d. nasal spray administration.
Stent maker Intersect ENT provided funding, administrative support, and materials for the study. Dr. Forwith reported no conflicts of interest.
FROM THE COMBINED OTOLARYNGOLOGY SPRING MEETINGS
Steroid-Eluting Sinus Stent May Minimize Postop Events
CHICAGO – A bioabsorbable steroid-eluting stent preserved sinus patency following endoscopic sinus surgery in 50 patients with chronic rhinosinusitis in a prospective, multicenter study.
The stent was associated with low rates of polyp formation, inflammation, and adhesions, despite a challenging patient population and withholding of postoperative oral and topical steroids for 30 days, lead author Dr. Keith D. Forwith said at the Combined Otolaryngology Spring Meetings.
The results were consistent across different patient populations and consistent with the pilot study in 43 patients (Int. Forum Allergy Rhinol. 2011;1:23-32).
"I think with any kind of new technique or device, the ultimate thing is how the surgeon feels about it and his or her results," he said. "What I noticed most of all when we stopped doing the study is that I missed using this device. Patients I had used it in during the study had very clean results, very easy debridements. ... We’re very pleased with the results and think it’s a promising technology."
Study sponsor Intersect ENT Inc. has submitted the investigational stent for federal review and hopes to market the device within 6-9 months.
The springlike stent maintains patency by propping open the sinus cavity and releasing mometasone furoate into the sinus lining over 30 days before being resorbed. If approved, it could provide patients with an alternative to space-filling packing materials and silicone stents, and reduce the use of systemic steroids.
The current study involved 50 adults from seven centers with chronic rhinosinusitis for at least 8 consecutive weeks that was confirmed by CT scan (mean CT stage, 11.2). Antibiotics could be prescribed per physician standard of care, and saline irrigation was permitted postoperatively as needed. Polyps were present in 66% of patients, and 28% had undergone a prior sinus procedure. Their mean age was 44 years, and 52% were male.
Ethmoidectomy and maxillary antrostomy were performed in all 50 patients, with 28 also undergoing frontal sinusotomy and 31 sphenoidotomy. Stents were placed unilaterally in 10 patients and bilaterally in 40 patients. Device placement was successful in 100% of sinuses treated, and the implants were resorbed as predicted, Dr. Forwith said. At postoperative day 30, 15% of the material remained and 0.2% remained at day 60.
Endoscopic follow-up at 1 month revealed polypoid edema in 10% of patients, significant adhesion formation in 1%, and middle turbinate lateralization in only 4.4%, said Dr. Forwith, who is in private practice in Louisville, Ky.
When patients were asked about the procedure, their mean score on the 22-question Sino-Nasal Outcome Test improved significantly from baseline through 6 months. The same was true using the Rhinosinusitis Disability Index. "We were pretty pleased that our patients liked the results of the intervention," he said.
No clinically significant changes from baseline occurred in lens opacities or intraocular pressure, which was a theoretical concern given the close proximity of the device. The patients’ mean intraocular pressure was 15 mm Hg at baseline and 14.3 mm Hg at day 30.
One patient experienced headache with sinus pressure/irritation at day 21 that was determined to be related primarily to the surgery and was exacerbated in intensity by the presence of crust on the device. The device was removed and the event resolved without sequelae by day 28.
When asked during a discussion of the study whether the drug-eluting stent resulted in any systemic complications or adrenal corticol suppression, Dr. Forwith replied that they did not specifically look at adrenal suppression, but added that the 370-mcg dose of mometasone furoate is lower than the dose patients receive with most b.i.d. nasal spray administration.
Stent maker Intersect ENT provided funding, administrative support, and materials for the study. Dr. Forwith reported no conflicts of interest.
CHICAGO – A bioabsorbable steroid-eluting stent preserved sinus patency following endoscopic sinus surgery in 50 patients with chronic rhinosinusitis in a prospective, multicenter study.
The stent was associated with low rates of polyp formation, inflammation, and adhesions, despite a challenging patient population and withholding of postoperative oral and topical steroids for 30 days, lead author Dr. Keith D. Forwith said at the Combined Otolaryngology Spring Meetings.
The results were consistent across different patient populations and consistent with the pilot study in 43 patients (Int. Forum Allergy Rhinol. 2011;1:23-32).
"I think with any kind of new technique or device, the ultimate thing is how the surgeon feels about it and his or her results," he said. "What I noticed most of all when we stopped doing the study is that I missed using this device. Patients I had used it in during the study had very clean results, very easy debridements. ... We’re very pleased with the results and think it’s a promising technology."
Study sponsor Intersect ENT Inc. has submitted the investigational stent for federal review and hopes to market the device within 6-9 months.
The springlike stent maintains patency by propping open the sinus cavity and releasing mometasone furoate into the sinus lining over 30 days before being resorbed. If approved, it could provide patients with an alternative to space-filling packing materials and silicone stents, and reduce the use of systemic steroids.
The current study involved 50 adults from seven centers with chronic rhinosinusitis for at least 8 consecutive weeks that was confirmed by CT scan (mean CT stage, 11.2). Antibiotics could be prescribed per physician standard of care, and saline irrigation was permitted postoperatively as needed. Polyps were present in 66% of patients, and 28% had undergone a prior sinus procedure. Their mean age was 44 years, and 52% were male.
Ethmoidectomy and maxillary antrostomy were performed in all 50 patients, with 28 also undergoing frontal sinusotomy and 31 sphenoidotomy. Stents were placed unilaterally in 10 patients and bilaterally in 40 patients. Device placement was successful in 100% of sinuses treated, and the implants were resorbed as predicted, Dr. Forwith said. At postoperative day 30, 15% of the material remained and 0.2% remained at day 60.
Endoscopic follow-up at 1 month revealed polypoid edema in 10% of patients, significant adhesion formation in 1%, and middle turbinate lateralization in only 4.4%, said Dr. Forwith, who is in private practice in Louisville, Ky.
When patients were asked about the procedure, their mean score on the 22-question Sino-Nasal Outcome Test improved significantly from baseline through 6 months. The same was true using the Rhinosinusitis Disability Index. "We were pretty pleased that our patients liked the results of the intervention," he said.
No clinically significant changes from baseline occurred in lens opacities or intraocular pressure, which was a theoretical concern given the close proximity of the device. The patients’ mean intraocular pressure was 15 mm Hg at baseline and 14.3 mm Hg at day 30.
One patient experienced headache with sinus pressure/irritation at day 21 that was determined to be related primarily to the surgery and was exacerbated in intensity by the presence of crust on the device. The device was removed and the event resolved without sequelae by day 28.
When asked during a discussion of the study whether the drug-eluting stent resulted in any systemic complications or adrenal corticol suppression, Dr. Forwith replied that they did not specifically look at adrenal suppression, but added that the 370-mcg dose of mometasone furoate is lower than the dose patients receive with most b.i.d. nasal spray administration.
Stent maker Intersect ENT provided funding, administrative support, and materials for the study. Dr. Forwith reported no conflicts of interest.
CHICAGO – A bioabsorbable steroid-eluting stent preserved sinus patency following endoscopic sinus surgery in 50 patients with chronic rhinosinusitis in a prospective, multicenter study.
The stent was associated with low rates of polyp formation, inflammation, and adhesions, despite a challenging patient population and withholding of postoperative oral and topical steroids for 30 days, lead author Dr. Keith D. Forwith said at the Combined Otolaryngology Spring Meetings.
The results were consistent across different patient populations and consistent with the pilot study in 43 patients (Int. Forum Allergy Rhinol. 2011;1:23-32).
"I think with any kind of new technique or device, the ultimate thing is how the surgeon feels about it and his or her results," he said. "What I noticed most of all when we stopped doing the study is that I missed using this device. Patients I had used it in during the study had very clean results, very easy debridements. ... We’re very pleased with the results and think it’s a promising technology."
Study sponsor Intersect ENT Inc. has submitted the investigational stent for federal review and hopes to market the device within 6-9 months.
The springlike stent maintains patency by propping open the sinus cavity and releasing mometasone furoate into the sinus lining over 30 days before being resorbed. If approved, it could provide patients with an alternative to space-filling packing materials and silicone stents, and reduce the use of systemic steroids.
The current study involved 50 adults from seven centers with chronic rhinosinusitis for at least 8 consecutive weeks that was confirmed by CT scan (mean CT stage, 11.2). Antibiotics could be prescribed per physician standard of care, and saline irrigation was permitted postoperatively as needed. Polyps were present in 66% of patients, and 28% had undergone a prior sinus procedure. Their mean age was 44 years, and 52% were male.
Ethmoidectomy and maxillary antrostomy were performed in all 50 patients, with 28 also undergoing frontal sinusotomy and 31 sphenoidotomy. Stents were placed unilaterally in 10 patients and bilaterally in 40 patients. Device placement was successful in 100% of sinuses treated, and the implants were resorbed as predicted, Dr. Forwith said. At postoperative day 30, 15% of the material remained and 0.2% remained at day 60.
Endoscopic follow-up at 1 month revealed polypoid edema in 10% of patients, significant adhesion formation in 1%, and middle turbinate lateralization in only 4.4%, said Dr. Forwith, who is in private practice in Louisville, Ky.
When patients were asked about the procedure, their mean score on the 22-question Sino-Nasal Outcome Test improved significantly from baseline through 6 months. The same was true using the Rhinosinusitis Disability Index. "We were pretty pleased that our patients liked the results of the intervention," he said.
No clinically significant changes from baseline occurred in lens opacities or intraocular pressure, which was a theoretical concern given the close proximity of the device. The patients’ mean intraocular pressure was 15 mm Hg at baseline and 14.3 mm Hg at day 30.
One patient experienced headache with sinus pressure/irritation at day 21 that was determined to be related primarily to the surgery and was exacerbated in intensity by the presence of crust on the device. The device was removed and the event resolved without sequelae by day 28.
When asked during a discussion of the study whether the drug-eluting stent resulted in any systemic complications or adrenal corticol suppression, Dr. Forwith replied that they did not specifically look at adrenal suppression, but added that the 370-mcg dose of mometasone furoate is lower than the dose patients receive with most b.i.d. nasal spray administration.
Stent maker Intersect ENT provided funding, administrative support, and materials for the study. Dr. Forwith reported no conflicts of interest.
FROM THE COMBINED OTOLARYNGOLOGY SPRING MEETINGS
Major Finding: At 1 month follow-up, the occurrence of polypoid edema was 10%, significant adhesion formation 1%, and middle turbinate lateralization 4.4%.
Data Source: Prospective, multicenter study in 50 patients with chronic rhinosinusitis undergoing endoscopic sinus surgery.
Disclosures: Stent maker Intersect ENT provided funding, administrative support, and materials for the study. Dr. Forwith reported no conflicts of interest.