User login
Small Changes Yield Big Bang for Chlamydia Screening Buck
MINNEAPOLIS – A few simple administrative changes made some big differences at clinics that screen for chlamydia, increasing the number of screenings and saving more than $40,000 in just 1 year.
Chlamydia is the most common nationally reportable infectious disease, with 1.4 million cases diagnosed in the U.S. in 2011. Yet it’s also one of the most underdiagnosed, Elizabeth Torrone, Ph.D. said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Despite its ability to hide in plain sight, chlamydia can have serious health consequences for young women, said Dr. Torrone, an epidemiologist at the CDC.
"It’s most common in young women and it’s usually asymptomatic, but 1 in 10 infections results in pelvic inflammatory disease, which can have adverse effects on reproduction. CDC recommends annual screening for all sexually active women aged 25 or younger, and for older women who are at high risk for infection," such as those with new or multiple sexual partners.
Chlamydia rates seem to have increased significantly in the past decade, but that’s most likely the result of increased screening. "The more we look for it, the more we find." But screening still doesn’t match up to the CDC guidelines, she said. "Since 2001, screening among females aged 16-24 years has increased to 60% of those targeted compared to 30% before 2001. But older women still have higher screening rates than necessary, because only about 1% of the positives occur in women aged 25-39 years."
She described how three health departments have tackled the problem, and the benefits they reaped from a few low-cost changes in their screening routine.
San Francisco Enforces the Guidelines
In San Francisco, health department family planning clinics were charged with screening any woman younger than 25 years, and all women who were pregnant or getting an intrauterine birth control device inserted. But clinicians still could screen other groups: The lab requisition slip had an "other" indication, where clinicians could write in any other reason they thought a screen was necessary.
In 2008, Dr. Torrone said, 64% of the chlamydia screening in these clinics was done on women older than 26 years. "This led us to conclude they were over-screening the older population," she said.
To address the problem, the health department simply removed the "other" box on the lab slip, leaving only three choices: younger than 26 years, pregnant, or IUD insertion. The labs rejected any specimen without one of these valid reasons for testing marked.
"The only way you could screen a woman older than 26 was if she was pregnant or having an IUD inserted. So this really forced our clinicians to adhere to the screening guidelines," she said.
When they compared screening in 2008 with 2009 – after the new slips came out – there was a 25% decrease in the number of tests on women 26 and older, and a 4% increase in positive tests – indicating that more young women were being screened.
The change also dramatically decreased costs, Dr. Torrone said. "For every screen, the cost decreased by 3.5%, resulting in a savings of almost $40,000."
Kentucky’s Experience
In 2009, to assure that clinics were screening appropriately and to make screening financially feasible, CDC implemented a program designed to produce at least a 3% positivity rate at any clinic that screened for chlamydia.
"In Kentucky, 75 sites participating in [the program] had a positivity rate of less than 3% in women younger than 26, so CDC offered some assistance in getting that increased," she said.
The plan included site-specific feedback to those clinics not meeting the 3% rule. Feedback included the clinic’s positivity rate, implementing risk-based screening criteria, and individual technical assistance to help out in some sites.
By 2010, 54 sites were reporting positivity rates of less than 3%. * "Obviously Kentucky still has some work to do, but they plan to target high-volume sites and intensify communication and teamwork with providers."
Specimen Pooling in Idaho
Specimen pooling proved effective in Idaho, a state with a relatively low number of positive chlamydia tests. Specimen pooling is a two-step lab procedure that batches samples. Each batch has a single test. If the result is negative then every sample in that batch is negative, eliminating individual sample testing. If the batch tests positive, then every sample in that group must be tested.
The technique saves money on every specimen that goes into a negative batch, Dr. Torrone said.
It’s most cost-effective in areas with low chlamydia prevalence.
In 2009, the Idaho Bureau of Laboratories instituted the pooling technique. In July of that year, 1,300 samples were submitted. Using the pooling technique, the lab ran 727 tests to identify all the positive samples.
"Assuming a cost of $14.47 per test, this saved 47% on the positive tests," Dr. Torrone said. "The lab estimated that they were able to test 15,201 samples for the price of 8,866 samples."
Dr. Torrone reported having no financial disclosures.
*Correction, 4/13/12: An earlier version of this story misreported the percentage of the reduction in the number of clinics in Kentucky reporting positivity rates of less than 3%.
MINNEAPOLIS – A few simple administrative changes made some big differences at clinics that screen for chlamydia, increasing the number of screenings and saving more than $40,000 in just 1 year.
Chlamydia is the most common nationally reportable infectious disease, with 1.4 million cases diagnosed in the U.S. in 2011. Yet it’s also one of the most underdiagnosed, Elizabeth Torrone, Ph.D. said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Despite its ability to hide in plain sight, chlamydia can have serious health consequences for young women, said Dr. Torrone, an epidemiologist at the CDC.
"It’s most common in young women and it’s usually asymptomatic, but 1 in 10 infections results in pelvic inflammatory disease, which can have adverse effects on reproduction. CDC recommends annual screening for all sexually active women aged 25 or younger, and for older women who are at high risk for infection," such as those with new or multiple sexual partners.
Chlamydia rates seem to have increased significantly in the past decade, but that’s most likely the result of increased screening. "The more we look for it, the more we find." But screening still doesn’t match up to the CDC guidelines, she said. "Since 2001, screening among females aged 16-24 years has increased to 60% of those targeted compared to 30% before 2001. But older women still have higher screening rates than necessary, because only about 1% of the positives occur in women aged 25-39 years."
She described how three health departments have tackled the problem, and the benefits they reaped from a few low-cost changes in their screening routine.
San Francisco Enforces the Guidelines
In San Francisco, health department family planning clinics were charged with screening any woman younger than 25 years, and all women who were pregnant or getting an intrauterine birth control device inserted. But clinicians still could screen other groups: The lab requisition slip had an "other" indication, where clinicians could write in any other reason they thought a screen was necessary.
In 2008, Dr. Torrone said, 64% of the chlamydia screening in these clinics was done on women older than 26 years. "This led us to conclude they were over-screening the older population," she said.
To address the problem, the health department simply removed the "other" box on the lab slip, leaving only three choices: younger than 26 years, pregnant, or IUD insertion. The labs rejected any specimen without one of these valid reasons for testing marked.
"The only way you could screen a woman older than 26 was if she was pregnant or having an IUD inserted. So this really forced our clinicians to adhere to the screening guidelines," she said.
When they compared screening in 2008 with 2009 – after the new slips came out – there was a 25% decrease in the number of tests on women 26 and older, and a 4% increase in positive tests – indicating that more young women were being screened.
The change also dramatically decreased costs, Dr. Torrone said. "For every screen, the cost decreased by 3.5%, resulting in a savings of almost $40,000."
Kentucky’s Experience
In 2009, to assure that clinics were screening appropriately and to make screening financially feasible, CDC implemented a program designed to produce at least a 3% positivity rate at any clinic that screened for chlamydia.
"In Kentucky, 75 sites participating in [the program] had a positivity rate of less than 3% in women younger than 26, so CDC offered some assistance in getting that increased," she said.
The plan included site-specific feedback to those clinics not meeting the 3% rule. Feedback included the clinic’s positivity rate, implementing risk-based screening criteria, and individual technical assistance to help out in some sites.
By 2010, 54 sites were reporting positivity rates of less than 3%. * "Obviously Kentucky still has some work to do, but they plan to target high-volume sites and intensify communication and teamwork with providers."
Specimen Pooling in Idaho
Specimen pooling proved effective in Idaho, a state with a relatively low number of positive chlamydia tests. Specimen pooling is a two-step lab procedure that batches samples. Each batch has a single test. If the result is negative then every sample in that batch is negative, eliminating individual sample testing. If the batch tests positive, then every sample in that group must be tested.
The technique saves money on every specimen that goes into a negative batch, Dr. Torrone said.
It’s most cost-effective in areas with low chlamydia prevalence.
In 2009, the Idaho Bureau of Laboratories instituted the pooling technique. In July of that year, 1,300 samples were submitted. Using the pooling technique, the lab ran 727 tests to identify all the positive samples.
"Assuming a cost of $14.47 per test, this saved 47% on the positive tests," Dr. Torrone said. "The lab estimated that they were able to test 15,201 samples for the price of 8,866 samples."
Dr. Torrone reported having no financial disclosures.
*Correction, 4/13/12: An earlier version of this story misreported the percentage of the reduction in the number of clinics in Kentucky reporting positivity rates of less than 3%.
MINNEAPOLIS – A few simple administrative changes made some big differences at clinics that screen for chlamydia, increasing the number of screenings and saving more than $40,000 in just 1 year.
Chlamydia is the most common nationally reportable infectious disease, with 1.4 million cases diagnosed in the U.S. in 2011. Yet it’s also one of the most underdiagnosed, Elizabeth Torrone, Ph.D. said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Despite its ability to hide in plain sight, chlamydia can have serious health consequences for young women, said Dr. Torrone, an epidemiologist at the CDC.
"It’s most common in young women and it’s usually asymptomatic, but 1 in 10 infections results in pelvic inflammatory disease, which can have adverse effects on reproduction. CDC recommends annual screening for all sexually active women aged 25 or younger, and for older women who are at high risk for infection," such as those with new or multiple sexual partners.
Chlamydia rates seem to have increased significantly in the past decade, but that’s most likely the result of increased screening. "The more we look for it, the more we find." But screening still doesn’t match up to the CDC guidelines, she said. "Since 2001, screening among females aged 16-24 years has increased to 60% of those targeted compared to 30% before 2001. But older women still have higher screening rates than necessary, because only about 1% of the positives occur in women aged 25-39 years."
She described how three health departments have tackled the problem, and the benefits they reaped from a few low-cost changes in their screening routine.
San Francisco Enforces the Guidelines
In San Francisco, health department family planning clinics were charged with screening any woman younger than 25 years, and all women who were pregnant or getting an intrauterine birth control device inserted. But clinicians still could screen other groups: The lab requisition slip had an "other" indication, where clinicians could write in any other reason they thought a screen was necessary.
In 2008, Dr. Torrone said, 64% of the chlamydia screening in these clinics was done on women older than 26 years. "This led us to conclude they were over-screening the older population," she said.
To address the problem, the health department simply removed the "other" box on the lab slip, leaving only three choices: younger than 26 years, pregnant, or IUD insertion. The labs rejected any specimen without one of these valid reasons for testing marked.
"The only way you could screen a woman older than 26 was if she was pregnant or having an IUD inserted. So this really forced our clinicians to adhere to the screening guidelines," she said.
When they compared screening in 2008 with 2009 – after the new slips came out – there was a 25% decrease in the number of tests on women 26 and older, and a 4% increase in positive tests – indicating that more young women were being screened.
The change also dramatically decreased costs, Dr. Torrone said. "For every screen, the cost decreased by 3.5%, resulting in a savings of almost $40,000."
Kentucky’s Experience
In 2009, to assure that clinics were screening appropriately and to make screening financially feasible, CDC implemented a program designed to produce at least a 3% positivity rate at any clinic that screened for chlamydia.
"In Kentucky, 75 sites participating in [the program] had a positivity rate of less than 3% in women younger than 26, so CDC offered some assistance in getting that increased," she said.
The plan included site-specific feedback to those clinics not meeting the 3% rule. Feedback included the clinic’s positivity rate, implementing risk-based screening criteria, and individual technical assistance to help out in some sites.
By 2010, 54 sites were reporting positivity rates of less than 3%. * "Obviously Kentucky still has some work to do, but they plan to target high-volume sites and intensify communication and teamwork with providers."
Specimen Pooling in Idaho
Specimen pooling proved effective in Idaho, a state with a relatively low number of positive chlamydia tests. Specimen pooling is a two-step lab procedure that batches samples. Each batch has a single test. If the result is negative then every sample in that batch is negative, eliminating individual sample testing. If the batch tests positive, then every sample in that group must be tested.
The technique saves money on every specimen that goes into a negative batch, Dr. Torrone said.
It’s most cost-effective in areas with low chlamydia prevalence.
In 2009, the Idaho Bureau of Laboratories instituted the pooling technique. In July of that year, 1,300 samples were submitted. Using the pooling technique, the lab ran 727 tests to identify all the positive samples.
"Assuming a cost of $14.47 per test, this saved 47% on the positive tests," Dr. Torrone said. "The lab estimated that they were able to test 15,201 samples for the price of 8,866 samples."
Dr. Torrone reported having no financial disclosures.
*Correction, 4/13/12: An earlier version of this story misreported the percentage of the reduction in the number of clinics in Kentucky reporting positivity rates of less than 3%.
FROM A CONFERENCE ON STD PREVENTION SPONSORED BY THE CENTERS FOR DISEASE CONTROL AND PREVENTION
Gonorrhea Continues to Outsmart Antibiotics
MINNEAPOLIS – Neisseria gonorrhoeae is probing the final frontier of antimicrobial treatment.
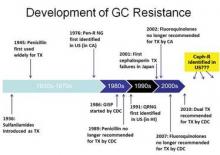
The persistent and versatile bacteria has proved its mettle against every antibiotic humans have thrown at it over the past 70 years, and now shows signs of figuring out the cephalosporins – the last bastion of effective antimicrobial treatment.
The Centers for Disease Control and Prevention have not seen any case of cephalosporin treatment failures in the United States yet. But researchers at the National STD Conference said it’s only a matter of time.
"We have already seen oral cephalosporin treatment failures in Asia and, in the last few years, in Europe," said Mark Pandori, Ph.D, of the San Francisco Department of Public Health. "None have yet been recorded in the U.S. but it’s wise to assume that it is on our doorstep, if not already here."
The bacteria developed fluoroquinolone resistance in the early 2000s, prompting CDC to drop that drug from its treatment recommendation in 2010. Now the agency recommends dual therapy: a 250-mg injection of ceftriaxone or a 400-mg dose of cefixeme, plus a 1-g dose of azithromycin or a 7-day course of doxycycline.
But now azithromycin resistance is becoming more common. Last July, the agency issued an alert about a cluster of five azithromycin-resistant isolates from a group of men who have sex with men (MSM) in San Diego.
In fact, one case of azithromycin treatment failure has occurred in the U.S., Olusegun Soge, Ph.D. reported at the meeting. After 12 days of treatment with the drug, the bacteria had already developed an increased resistance level, said Dr. Soge, an epidemiologist at the University of Washington, Seattle.
The patient was a 26-year-old MSM who received 2 grams of azithromycin. A culture showed a high level of azithromycin resistance. After 12 days of treatment, the patient was still experiencing symptoms consistent with a gonorrhea infection. He denied any sexual relations during treatment.
"We needed to know if this was a possible treatment failure, or a re-exposure," Dr. Soge said. Nucleic acid amplification testing (NAAT) determined that both isolates showed the same genetic blueprint, but the new specimen had an even higher resistance level: a minimum inhibitory concentration (MIC) of 8 mcg/mL compared to 0.5 mcg/mL in the first sample.
"The emergence of a resistant variant in an individual patient during treatment is alarming and may predict rapid emergence of gonococcal resistance," he said. He recommended that any patient who receives azithromycin as a single treatment should return for a test of cure.
At this point, cephalosporins are really the only treatment with certain effectiveness, said Dr. Sarah Guerry, medical director, of the sexually transmitted disease program at the Los Angeles County Public Health. "Not only have we had to increase the recommended dosage of ceftriaxone, but now this is our therapy of last resort," she said at the meeting.
Many STD clinics, however, are only able to distribute oral medications. Dr. Guerry and her colleagues examined whether complying with the new treatment recommendations of intramuscular ceftriaxone was delaying treatment.
She reviewed all urogenital and anorectal gonorrhea cases diagnosed in Los Angeles County from April-August 2010 – before the new treatment guidelines – and from the same period in 2011. The review focused on clinics that treated less than 10% of cases with any dose of ceftriaxone injection, and compared the overall and median time to treatment, as well as compliance with the new recommendations.
There were 508 gonorrhea cases in 2010 and 401 in 2011. Overall, 11% of the clinics surveyed were considered "low injectors."
Almost three-fourths of the clinics (72%) dispensed some form of the treatment recommended for that period. This did not change after the new guidelines came out, indicating that most clinics adopted the new guideline. But in 2010, only 13% of the facilities were giving the injected ceftriaxone. In 2011, there was a dramatic change, with 90% of the facilities giving the injection.
Time to treatment, however, was not significantly altered, indicating that switching to the injected form did not delay treatment. Overall, the time from diagnosis to treatment increased from 5 to 7 days.
Still, there is plenty of room for improvement in delivering the recommended treatment, according to Roxanne Kerani, Ph.D., an epidemiologist at the Seattle and King County Public Health Department, Wash. She collaborated with CDC researchers to estimate how frequently clinicians across the country are delivering treatment according to the new CDC guidelines. They polled clinics included in the national STD Surveillance Network: Washington, California, Colorado, Baltimore, and San Francisco.
Overall, 80% of patients with gonorrhea received ceftriaxone as part of their treatment. But only 45% received the recommended dual therapy. About a third got ceftriaxone alone.
The incidence of dual treatment varied significantly according to the type of site, she said. STD clinics were most likely to administer dual therapy (88%), which was significantly more than hospitals (79%), private providers (71%) or family planning clinics. Men, particularly MSM, were significantly more likely to receive the recommended therapy than were women, regardless of their sexual preference.
"We believe that continued surveillance of treatment practices is important, given the changing antibiotic susceptibility patterns we’re seeing, and the limited alternative treatment options for gonorrhea," she said.
Despite consternation over the creeping threat of resistance, gonorrhea is not nearly as common as it once was, said Mark Stenger, an epidemiologist at the CDC.
"We are in a good epidemiologic moment with gonorrhea rates in the U.S. right now. There have been incredible declines in rates overall."
According to the CDC, the national gonorrhea rate declined by 74% from 1975 to 1997 – largely due to a national gonorrhea control program instituted in the mid-70s, Dr. Stenger said. In 2010, the overall rate was 101 per 100,000 persons, with a total of 309,341 cases diagnosed that year.
"This is the lowest rate since we began recording it," he said.
Healthy People 2020 sets what Mr. Stenger called "realistic goals" for gonorrhea incidence: 257 per 100,000 persons for women and 198 per 100,000 persons for men by the end of that year.
"When we look at incidence among males in the 2011 unpublished data, there are a number of counties that are not meeting that goal right now ... and a number of counties that are. We need to work in those areas that don’t meet it and continue to do whatever we are doing well to keep cases low in the areas that have already met this target."
None of the presenters said they have any financial disclosures.
MINNEAPOLIS – Neisseria gonorrhoeae is probing the final frontier of antimicrobial treatment.
The persistent and versatile bacteria has proved its mettle against every antibiotic humans have thrown at it over the past 70 years, and now shows signs of figuring out the cephalosporins – the last bastion of effective antimicrobial treatment.
The Centers for Disease Control and Prevention have not seen any case of cephalosporin treatment failures in the United States yet. But researchers at the National STD Conference said it’s only a matter of time.
"We have already seen oral cephalosporin treatment failures in Asia and, in the last few years, in Europe," said Mark Pandori, Ph.D, of the San Francisco Department of Public Health. "None have yet been recorded in the U.S. but it’s wise to assume that it is on our doorstep, if not already here."
The bacteria developed fluoroquinolone resistance in the early 2000s, prompting CDC to drop that drug from its treatment recommendation in 2010. Now the agency recommends dual therapy: a 250-mg injection of ceftriaxone or a 400-mg dose of cefixeme, plus a 1-g dose of azithromycin or a 7-day course of doxycycline.
But now azithromycin resistance is becoming more common. Last July, the agency issued an alert about a cluster of five azithromycin-resistant isolates from a group of men who have sex with men (MSM) in San Diego.
In fact, one case of azithromycin treatment failure has occurred in the U.S., Olusegun Soge, Ph.D. reported at the meeting. After 12 days of treatment with the drug, the bacteria had already developed an increased resistance level, said Dr. Soge, an epidemiologist at the University of Washington, Seattle.
The patient was a 26-year-old MSM who received 2 grams of azithromycin. A culture showed a high level of azithromycin resistance. After 12 days of treatment, the patient was still experiencing symptoms consistent with a gonorrhea infection. He denied any sexual relations during treatment.
"We needed to know if this was a possible treatment failure, or a re-exposure," Dr. Soge said. Nucleic acid amplification testing (NAAT) determined that both isolates showed the same genetic blueprint, but the new specimen had an even higher resistance level: a minimum inhibitory concentration (MIC) of 8 mcg/mL compared to 0.5 mcg/mL in the first sample.
"The emergence of a resistant variant in an individual patient during treatment is alarming and may predict rapid emergence of gonococcal resistance," he said. He recommended that any patient who receives azithromycin as a single treatment should return for a test of cure.
At this point, cephalosporins are really the only treatment with certain effectiveness, said Dr. Sarah Guerry, medical director, of the sexually transmitted disease program at the Los Angeles County Public Health. "Not only have we had to increase the recommended dosage of ceftriaxone, but now this is our therapy of last resort," she said at the meeting.
Many STD clinics, however, are only able to distribute oral medications. Dr. Guerry and her colleagues examined whether complying with the new treatment recommendations of intramuscular ceftriaxone was delaying treatment.
She reviewed all urogenital and anorectal gonorrhea cases diagnosed in Los Angeles County from April-August 2010 – before the new treatment guidelines – and from the same period in 2011. The review focused on clinics that treated less than 10% of cases with any dose of ceftriaxone injection, and compared the overall and median time to treatment, as well as compliance with the new recommendations.
There were 508 gonorrhea cases in 2010 and 401 in 2011. Overall, 11% of the clinics surveyed were considered "low injectors."
Almost three-fourths of the clinics (72%) dispensed some form of the treatment recommended for that period. This did not change after the new guidelines came out, indicating that most clinics adopted the new guideline. But in 2010, only 13% of the facilities were giving the injected ceftriaxone. In 2011, there was a dramatic change, with 90% of the facilities giving the injection.
Time to treatment, however, was not significantly altered, indicating that switching to the injected form did not delay treatment. Overall, the time from diagnosis to treatment increased from 5 to 7 days.
Still, there is plenty of room for improvement in delivering the recommended treatment, according to Roxanne Kerani, Ph.D., an epidemiologist at the Seattle and King County Public Health Department, Wash. She collaborated with CDC researchers to estimate how frequently clinicians across the country are delivering treatment according to the new CDC guidelines. They polled clinics included in the national STD Surveillance Network: Washington, California, Colorado, Baltimore, and San Francisco.
Overall, 80% of patients with gonorrhea received ceftriaxone as part of their treatment. But only 45% received the recommended dual therapy. About a third got ceftriaxone alone.
The incidence of dual treatment varied significantly according to the type of site, she said. STD clinics were most likely to administer dual therapy (88%), which was significantly more than hospitals (79%), private providers (71%) or family planning clinics. Men, particularly MSM, were significantly more likely to receive the recommended therapy than were women, regardless of their sexual preference.
"We believe that continued surveillance of treatment practices is important, given the changing antibiotic susceptibility patterns we’re seeing, and the limited alternative treatment options for gonorrhea," she said.
Despite consternation over the creeping threat of resistance, gonorrhea is not nearly as common as it once was, said Mark Stenger, an epidemiologist at the CDC.
"We are in a good epidemiologic moment with gonorrhea rates in the U.S. right now. There have been incredible declines in rates overall."
According to the CDC, the national gonorrhea rate declined by 74% from 1975 to 1997 – largely due to a national gonorrhea control program instituted in the mid-70s, Dr. Stenger said. In 2010, the overall rate was 101 per 100,000 persons, with a total of 309,341 cases diagnosed that year.
"This is the lowest rate since we began recording it," he said.
Healthy People 2020 sets what Mr. Stenger called "realistic goals" for gonorrhea incidence: 257 per 100,000 persons for women and 198 per 100,000 persons for men by the end of that year.
"When we look at incidence among males in the 2011 unpublished data, there are a number of counties that are not meeting that goal right now ... and a number of counties that are. We need to work in those areas that don’t meet it and continue to do whatever we are doing well to keep cases low in the areas that have already met this target."
None of the presenters said they have any financial disclosures.
MINNEAPOLIS – Neisseria gonorrhoeae is probing the final frontier of antimicrobial treatment.
The persistent and versatile bacteria has proved its mettle against every antibiotic humans have thrown at it over the past 70 years, and now shows signs of figuring out the cephalosporins – the last bastion of effective antimicrobial treatment.
The Centers for Disease Control and Prevention have not seen any case of cephalosporin treatment failures in the United States yet. But researchers at the National STD Conference said it’s only a matter of time.
"We have already seen oral cephalosporin treatment failures in Asia and, in the last few years, in Europe," said Mark Pandori, Ph.D, of the San Francisco Department of Public Health. "None have yet been recorded in the U.S. but it’s wise to assume that it is on our doorstep, if not already here."
The bacteria developed fluoroquinolone resistance in the early 2000s, prompting CDC to drop that drug from its treatment recommendation in 2010. Now the agency recommends dual therapy: a 250-mg injection of ceftriaxone or a 400-mg dose of cefixeme, plus a 1-g dose of azithromycin or a 7-day course of doxycycline.
But now azithromycin resistance is becoming more common. Last July, the agency issued an alert about a cluster of five azithromycin-resistant isolates from a group of men who have sex with men (MSM) in San Diego.
In fact, one case of azithromycin treatment failure has occurred in the U.S., Olusegun Soge, Ph.D. reported at the meeting. After 12 days of treatment with the drug, the bacteria had already developed an increased resistance level, said Dr. Soge, an epidemiologist at the University of Washington, Seattle.
The patient was a 26-year-old MSM who received 2 grams of azithromycin. A culture showed a high level of azithromycin resistance. After 12 days of treatment, the patient was still experiencing symptoms consistent with a gonorrhea infection. He denied any sexual relations during treatment.
"We needed to know if this was a possible treatment failure, or a re-exposure," Dr. Soge said. Nucleic acid amplification testing (NAAT) determined that both isolates showed the same genetic blueprint, but the new specimen had an even higher resistance level: a minimum inhibitory concentration (MIC) of 8 mcg/mL compared to 0.5 mcg/mL in the first sample.
"The emergence of a resistant variant in an individual patient during treatment is alarming and may predict rapid emergence of gonococcal resistance," he said. He recommended that any patient who receives azithromycin as a single treatment should return for a test of cure.
At this point, cephalosporins are really the only treatment with certain effectiveness, said Dr. Sarah Guerry, medical director, of the sexually transmitted disease program at the Los Angeles County Public Health. "Not only have we had to increase the recommended dosage of ceftriaxone, but now this is our therapy of last resort," she said at the meeting.
Many STD clinics, however, are only able to distribute oral medications. Dr. Guerry and her colleagues examined whether complying with the new treatment recommendations of intramuscular ceftriaxone was delaying treatment.
She reviewed all urogenital and anorectal gonorrhea cases diagnosed in Los Angeles County from April-August 2010 – before the new treatment guidelines – and from the same period in 2011. The review focused on clinics that treated less than 10% of cases with any dose of ceftriaxone injection, and compared the overall and median time to treatment, as well as compliance with the new recommendations.
There were 508 gonorrhea cases in 2010 and 401 in 2011. Overall, 11% of the clinics surveyed were considered "low injectors."
Almost three-fourths of the clinics (72%) dispensed some form of the treatment recommended for that period. This did not change after the new guidelines came out, indicating that most clinics adopted the new guideline. But in 2010, only 13% of the facilities were giving the injected ceftriaxone. In 2011, there was a dramatic change, with 90% of the facilities giving the injection.
Time to treatment, however, was not significantly altered, indicating that switching to the injected form did not delay treatment. Overall, the time from diagnosis to treatment increased from 5 to 7 days.
Still, there is plenty of room for improvement in delivering the recommended treatment, according to Roxanne Kerani, Ph.D., an epidemiologist at the Seattle and King County Public Health Department, Wash. She collaborated with CDC researchers to estimate how frequently clinicians across the country are delivering treatment according to the new CDC guidelines. They polled clinics included in the national STD Surveillance Network: Washington, California, Colorado, Baltimore, and San Francisco.
Overall, 80% of patients with gonorrhea received ceftriaxone as part of their treatment. But only 45% received the recommended dual therapy. About a third got ceftriaxone alone.
The incidence of dual treatment varied significantly according to the type of site, she said. STD clinics were most likely to administer dual therapy (88%), which was significantly more than hospitals (79%), private providers (71%) or family planning clinics. Men, particularly MSM, were significantly more likely to receive the recommended therapy than were women, regardless of their sexual preference.
"We believe that continued surveillance of treatment practices is important, given the changing antibiotic susceptibility patterns we’re seeing, and the limited alternative treatment options for gonorrhea," she said.
Despite consternation over the creeping threat of resistance, gonorrhea is not nearly as common as it once was, said Mark Stenger, an epidemiologist at the CDC.
"We are in a good epidemiologic moment with gonorrhea rates in the U.S. right now. There have been incredible declines in rates overall."
According to the CDC, the national gonorrhea rate declined by 74% from 1975 to 1997 – largely due to a national gonorrhea control program instituted in the mid-70s, Dr. Stenger said. In 2010, the overall rate was 101 per 100,000 persons, with a total of 309,341 cases diagnosed that year.
"This is the lowest rate since we began recording it," he said.
Healthy People 2020 sets what Mr. Stenger called "realistic goals" for gonorrhea incidence: 257 per 100,000 persons for women and 198 per 100,000 persons for men by the end of that year.
"When we look at incidence among males in the 2011 unpublished data, there are a number of counties that are not meeting that goal right now ... and a number of counties that are. We need to work in those areas that don’t meet it and continue to do whatever we are doing well to keep cases low in the areas that have already met this target."
None of the presenters said they have any financial disclosures.
EXPERT ANALYSIS FROM THE NATIONAL STD CONFERENCE
Azithromycin, Doxycycline Quell Nongonococcal Urethritis in Men
MINNEAPOLIS – Azithromycin and doxycycline appear to be equally effective for men with nongonococcal urethritis.
In a randomized placebo-controlled trial, both drugs had good overall effectiveness, with an 84% clinical cure rate for azithromycin and a 78% rate for doxycycline. But some pathogens were more resistant to the drugs than others, Lisa Manhart, Ph.D., said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
The Mycoplasma Genitalium Antibiotic Susceptibility and Treatment (MEGA) trial enrolled 606 men with nongonococcal urethritis from 2007 to 2011. The main end points of the trial were clinical and microbiological cure of urethritis associated with M. genitalium infection, when treated with the standard therapy recommended by the Centers for Disease Control and Prevention: a single dose of 1 g of azithromycin, or a 7-day regimen of 100 mg of doxycycline taken twice a day.
Several pathogens cause nongonococcal urethritis, said Dr. Manhart, an epidemiologist at the University of Washington, Seattle. Among them are Chlamydia trachomatis, M. genitalium, the recently differentiated Ureaplasma urealyticum, and Trichomonas vaginalis. In some infections, no pathogen can be identified. In addition to presenting the end points for M. genitalium infections, Dr. Manhart broke the results out by other pathogens.
The mean age of the men in the trial was 34 years. Most (67%) were heterosexual, 28% were homosexual, and about 5% were bisexual. They reported a mean of six sexual partners in the past 12 months.
The most common infectious pathogen was chlamydia (26%), followed by U. urealyticum (24%). M. genitalium was present in 14% of the study group, and T. vaginalis in 2%. The remainder of the group had an idiopathic urethritis.
Treatment consisted of either a placebo azithromycin and active doxycycline or active azithromycin and placebo doxycycline. If men still showed symptoms or microbiological failure after treatment, they were offered reverse therapy (i.e., a treatment pack with the opposite active and placebo drugs), and returned for a follow-up visit in 3 weeks. If they were still not clinically or microbiologically cured, they received a course of moxifloxacin.
Overall, there was no statistically significant difference in cure rates between the two drugs. Nor were there significant differences among men with chlamydia infections, with a clinical cure rate of 87% for azithromycin and 78% for doxycycline. For microbiological cure, the rates were 86% and 90%, respectively.
Both clinical and microbiological cures rates were much lower in men infected with M. genitalium, but the rates were not significantly different between the two drugs. Clinical cure rates were 68% for azithromycin and 48% for doxycycline. For microbiological cure, the rates were 39% and 30%, respectively.
Men with U. urealyticum infections fared a little better, but again, the cure rates were not significantly different between the drugs. A clinical cure occurred in 85% of the azithromycin group and 75% of the doxycycline group. Microbiological cure rates were 75% and 69%, respectively.
For those with idiopathic urethritis, the cure rate for both drugs was identical: 87%.
Reverse therapy was still not effective in some men with U. urealyticum or M. genitalium infections. Three weeks after that treatment, 51% of those with M. genitalium and 57% of those with U. urealyticum were still positive for the pathogen. These men received a course of moxifloxacin.
"Moxifloxacin was quite effective, but at the end of the trial, we still had two men with M. genitalium and four with U. urealyticum who did not clear their infections," Dr. Manhart said.
Moxifloxacin, although highly effective, is too expensive to be used as first-time therapy in a public health department setting, she noted. "Its preinsurance cost runs up to $150, so we are still faced with a conundrum when treating these infections."
Dr. Manhart had no financial declarations.
MINNEAPOLIS – Azithromycin and doxycycline appear to be equally effective for men with nongonococcal urethritis.
In a randomized placebo-controlled trial, both drugs had good overall effectiveness, with an 84% clinical cure rate for azithromycin and a 78% rate for doxycycline. But some pathogens were more resistant to the drugs than others, Lisa Manhart, Ph.D., said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
The Mycoplasma Genitalium Antibiotic Susceptibility and Treatment (MEGA) trial enrolled 606 men with nongonococcal urethritis from 2007 to 2011. The main end points of the trial were clinical and microbiological cure of urethritis associated with M. genitalium infection, when treated with the standard therapy recommended by the Centers for Disease Control and Prevention: a single dose of 1 g of azithromycin, or a 7-day regimen of 100 mg of doxycycline taken twice a day.
Several pathogens cause nongonococcal urethritis, said Dr. Manhart, an epidemiologist at the University of Washington, Seattle. Among them are Chlamydia trachomatis, M. genitalium, the recently differentiated Ureaplasma urealyticum, and Trichomonas vaginalis. In some infections, no pathogen can be identified. In addition to presenting the end points for M. genitalium infections, Dr. Manhart broke the results out by other pathogens.
The mean age of the men in the trial was 34 years. Most (67%) were heterosexual, 28% were homosexual, and about 5% were bisexual. They reported a mean of six sexual partners in the past 12 months.
The most common infectious pathogen was chlamydia (26%), followed by U. urealyticum (24%). M. genitalium was present in 14% of the study group, and T. vaginalis in 2%. The remainder of the group had an idiopathic urethritis.
Treatment consisted of either a placebo azithromycin and active doxycycline or active azithromycin and placebo doxycycline. If men still showed symptoms or microbiological failure after treatment, they were offered reverse therapy (i.e., a treatment pack with the opposite active and placebo drugs), and returned for a follow-up visit in 3 weeks. If they were still not clinically or microbiologically cured, they received a course of moxifloxacin.
Overall, there was no statistically significant difference in cure rates between the two drugs. Nor were there significant differences among men with chlamydia infections, with a clinical cure rate of 87% for azithromycin and 78% for doxycycline. For microbiological cure, the rates were 86% and 90%, respectively.
Both clinical and microbiological cures rates were much lower in men infected with M. genitalium, but the rates were not significantly different between the two drugs. Clinical cure rates were 68% for azithromycin and 48% for doxycycline. For microbiological cure, the rates were 39% and 30%, respectively.
Men with U. urealyticum infections fared a little better, but again, the cure rates were not significantly different between the drugs. A clinical cure occurred in 85% of the azithromycin group and 75% of the doxycycline group. Microbiological cure rates were 75% and 69%, respectively.
For those with idiopathic urethritis, the cure rate for both drugs was identical: 87%.
Reverse therapy was still not effective in some men with U. urealyticum or M. genitalium infections. Three weeks after that treatment, 51% of those with M. genitalium and 57% of those with U. urealyticum were still positive for the pathogen. These men received a course of moxifloxacin.
"Moxifloxacin was quite effective, but at the end of the trial, we still had two men with M. genitalium and four with U. urealyticum who did not clear their infections," Dr. Manhart said.
Moxifloxacin, although highly effective, is too expensive to be used as first-time therapy in a public health department setting, she noted. "Its preinsurance cost runs up to $150, so we are still faced with a conundrum when treating these infections."
Dr. Manhart had no financial declarations.
MINNEAPOLIS – Azithromycin and doxycycline appear to be equally effective for men with nongonococcal urethritis.
In a randomized placebo-controlled trial, both drugs had good overall effectiveness, with an 84% clinical cure rate for azithromycin and a 78% rate for doxycycline. But some pathogens were more resistant to the drugs than others, Lisa Manhart, Ph.D., said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
The Mycoplasma Genitalium Antibiotic Susceptibility and Treatment (MEGA) trial enrolled 606 men with nongonococcal urethritis from 2007 to 2011. The main end points of the trial were clinical and microbiological cure of urethritis associated with M. genitalium infection, when treated with the standard therapy recommended by the Centers for Disease Control and Prevention: a single dose of 1 g of azithromycin, or a 7-day regimen of 100 mg of doxycycline taken twice a day.
Several pathogens cause nongonococcal urethritis, said Dr. Manhart, an epidemiologist at the University of Washington, Seattle. Among them are Chlamydia trachomatis, M. genitalium, the recently differentiated Ureaplasma urealyticum, and Trichomonas vaginalis. In some infections, no pathogen can be identified. In addition to presenting the end points for M. genitalium infections, Dr. Manhart broke the results out by other pathogens.
The mean age of the men in the trial was 34 years. Most (67%) were heterosexual, 28% were homosexual, and about 5% were bisexual. They reported a mean of six sexual partners in the past 12 months.
The most common infectious pathogen was chlamydia (26%), followed by U. urealyticum (24%). M. genitalium was present in 14% of the study group, and T. vaginalis in 2%. The remainder of the group had an idiopathic urethritis.
Treatment consisted of either a placebo azithromycin and active doxycycline or active azithromycin and placebo doxycycline. If men still showed symptoms or microbiological failure after treatment, they were offered reverse therapy (i.e., a treatment pack with the opposite active and placebo drugs), and returned for a follow-up visit in 3 weeks. If they were still not clinically or microbiologically cured, they received a course of moxifloxacin.
Overall, there was no statistically significant difference in cure rates between the two drugs. Nor were there significant differences among men with chlamydia infections, with a clinical cure rate of 87% for azithromycin and 78% for doxycycline. For microbiological cure, the rates were 86% and 90%, respectively.
Both clinical and microbiological cures rates were much lower in men infected with M. genitalium, but the rates were not significantly different between the two drugs. Clinical cure rates were 68% for azithromycin and 48% for doxycycline. For microbiological cure, the rates were 39% and 30%, respectively.
Men with U. urealyticum infections fared a little better, but again, the cure rates were not significantly different between the drugs. A clinical cure occurred in 85% of the azithromycin group and 75% of the doxycycline group. Microbiological cure rates were 75% and 69%, respectively.
For those with idiopathic urethritis, the cure rate for both drugs was identical: 87%.
Reverse therapy was still not effective in some men with U. urealyticum or M. genitalium infections. Three weeks after that treatment, 51% of those with M. genitalium and 57% of those with U. urealyticum were still positive for the pathogen. These men received a course of moxifloxacin.
"Moxifloxacin was quite effective, but at the end of the trial, we still had two men with M. genitalium and four with U. urealyticum who did not clear their infections," Dr. Manhart said.
Moxifloxacin, although highly effective, is too expensive to be used as first-time therapy in a public health department setting, she noted. "Its preinsurance cost runs up to $150, so we are still faced with a conundrum when treating these infections."
Dr. Manhart had no financial declarations.
FROM A CONFERENCE ON STD PREVENTION SPONSORED BY THE CENTERS FOR DISEASE CONTROL AND PREVENTION
When Diagnosed, Women Help Treat Partners for Chlamydia
MINNEAPOLIS – Researchers at the National STD Prevention Conference agreed: Not enough women are getting tested for chlamydia, and health care providers are missing valuable opportunities to test them.
Among those lost opportunities is the ability to stem the spread of the disease, as new findings show that women who are diagnosed with chlamydia are likely to provide treatment to their partners.
Women with chlamydia infection are usually asymptomatic, and often consult a clinician for some other sexual health concern. Most of these visits provide a perfect opportunity to add a chlamydia screening test, said Dr. Joan Chow, chief of epidemiology in the STD control branch of the California Department of Public Health.
"Annual chlamydia screening has increased during the past 10 years, but still, about 50% of young women who should be screened aren’t being screened," she said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
She and her colleagues examined data from California’s Family PACT program, which provides comprehensive family planning services to low-income women.
More than 1.5 million women are enrolled in the program, which includes more than 2,000 medical providers and uses the services of about 200 labs in the state. Chlamydia testing is part of the PACT program benefits, and is recommended for all sexually active women aged 25 years or younger.
The study period spanned 2008-2010, focusing on women aged 25 or younger who were seen at by Family PACT providers in 2009. Dr. Chow compared the chlamydia testing these women received with any testing they had in the prior 12 months, or in the following 12 months.
During 2009, 219,319 young women accessed PACT services; 74% of those had a chlamydia test in the past 12 months, leaving 25% (55,915) untested at the 2009 visit.
Overall, 89% had urine collected for either a pregnancy test or to evaluate for a urinary tract infection. Of those patients, 43% had other services that included an easy opportunity to test, including a pelvic exam.
Most of these young women (75%) visited a PACT office only once during the study period. Only half (52%) returned to an office in the following 12 months, and of those, only 50% were tested for chlamydia, despite not being tested at the previous visit.
In all, only a quarter of the untested young women were tested in the following 12 months, Dr. Chow said. "Since most had only one visit, opportunities to test were not abundant."
But most patients who are diagnosed do help treat their sexual partners if they have access to the expedited treatment packs some clinics provide.
Typically, the packs contain 1 g of azithromycin, condoms, educational material about chlamydia and other STDs, and contact information for all local STD clinics, said Meighan Rogers of the New York City Department of Health’s STD branch.
She and her colleagues conducted a follow-up study of 671 patients diagnosed with chlamydia during a 12-month period from February 2011 through February 2012. Patients could receive up to three partner packs at the visit, with three additional packs available by prescription. The analysis included only patients from heterosexual couples.
A health department representative called each patient 5 days after treatment to determine if the pack had been given to partners, and if the partners had taken the medication. The survey also asked about any adverse effects of the antibiotic and ascertained the written materials’ clarity.
Most of the patients (74%) were women. Of all those eligible for the patient packs, 52% accepted them. Surveyors were able to contact 174 of these patients; 122 of those reported having a total of 150 partners who were locatable. About 44% of these (66) were successfully contacted.
Most (74%) of the partners reported getting the packs; 94% of those said they took the medication. Two partners reported having medical problems from the medication, although the analysis did not specify what those were.
About a quarter of the partners (23%) said they visited a physician afterward. Of those who did not see a doctor, 100% said they planned to. Women partners were significantly more likely to plan a doctor’s visit than men (46% vs. 13%).
That’s good news, said Ms. Rogers. "It’s very hopeful that almost half of the women were planning to see a physician, since this might translate into a decreased risk of pelvic inflammatory disease and its reproductive sequelae."
As state and federal employees, the presenters had no financial disclosures.
MINNEAPOLIS – Researchers at the National STD Prevention Conference agreed: Not enough women are getting tested for chlamydia, and health care providers are missing valuable opportunities to test them.
Among those lost opportunities is the ability to stem the spread of the disease, as new findings show that women who are diagnosed with chlamydia are likely to provide treatment to their partners.
Women with chlamydia infection are usually asymptomatic, and often consult a clinician for some other sexual health concern. Most of these visits provide a perfect opportunity to add a chlamydia screening test, said Dr. Joan Chow, chief of epidemiology in the STD control branch of the California Department of Public Health.
"Annual chlamydia screening has increased during the past 10 years, but still, about 50% of young women who should be screened aren’t being screened," she said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
She and her colleagues examined data from California’s Family PACT program, which provides comprehensive family planning services to low-income women.
More than 1.5 million women are enrolled in the program, which includes more than 2,000 medical providers and uses the services of about 200 labs in the state. Chlamydia testing is part of the PACT program benefits, and is recommended for all sexually active women aged 25 years or younger.
The study period spanned 2008-2010, focusing on women aged 25 or younger who were seen at by Family PACT providers in 2009. Dr. Chow compared the chlamydia testing these women received with any testing they had in the prior 12 months, or in the following 12 months.
During 2009, 219,319 young women accessed PACT services; 74% of those had a chlamydia test in the past 12 months, leaving 25% (55,915) untested at the 2009 visit.
Overall, 89% had urine collected for either a pregnancy test or to evaluate for a urinary tract infection. Of those patients, 43% had other services that included an easy opportunity to test, including a pelvic exam.
Most of these young women (75%) visited a PACT office only once during the study period. Only half (52%) returned to an office in the following 12 months, and of those, only 50% were tested for chlamydia, despite not being tested at the previous visit.
In all, only a quarter of the untested young women were tested in the following 12 months, Dr. Chow said. "Since most had only one visit, opportunities to test were not abundant."
But most patients who are diagnosed do help treat their sexual partners if they have access to the expedited treatment packs some clinics provide.
Typically, the packs contain 1 g of azithromycin, condoms, educational material about chlamydia and other STDs, and contact information for all local STD clinics, said Meighan Rogers of the New York City Department of Health’s STD branch.
She and her colleagues conducted a follow-up study of 671 patients diagnosed with chlamydia during a 12-month period from February 2011 through February 2012. Patients could receive up to three partner packs at the visit, with three additional packs available by prescription. The analysis included only patients from heterosexual couples.
A health department representative called each patient 5 days after treatment to determine if the pack had been given to partners, and if the partners had taken the medication. The survey also asked about any adverse effects of the antibiotic and ascertained the written materials’ clarity.
Most of the patients (74%) were women. Of all those eligible for the patient packs, 52% accepted them. Surveyors were able to contact 174 of these patients; 122 of those reported having a total of 150 partners who were locatable. About 44% of these (66) were successfully contacted.
Most (74%) of the partners reported getting the packs; 94% of those said they took the medication. Two partners reported having medical problems from the medication, although the analysis did not specify what those were.
About a quarter of the partners (23%) said they visited a physician afterward. Of those who did not see a doctor, 100% said they planned to. Women partners were significantly more likely to plan a doctor’s visit than men (46% vs. 13%).
That’s good news, said Ms. Rogers. "It’s very hopeful that almost half of the women were planning to see a physician, since this might translate into a decreased risk of pelvic inflammatory disease and its reproductive sequelae."
As state and federal employees, the presenters had no financial disclosures.
MINNEAPOLIS – Researchers at the National STD Prevention Conference agreed: Not enough women are getting tested for chlamydia, and health care providers are missing valuable opportunities to test them.
Among those lost opportunities is the ability to stem the spread of the disease, as new findings show that women who are diagnosed with chlamydia are likely to provide treatment to their partners.
Women with chlamydia infection are usually asymptomatic, and often consult a clinician for some other sexual health concern. Most of these visits provide a perfect opportunity to add a chlamydia screening test, said Dr. Joan Chow, chief of epidemiology in the STD control branch of the California Department of Public Health.
"Annual chlamydia screening has increased during the past 10 years, but still, about 50% of young women who should be screened aren’t being screened," she said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
She and her colleagues examined data from California’s Family PACT program, which provides comprehensive family planning services to low-income women.
More than 1.5 million women are enrolled in the program, which includes more than 2,000 medical providers and uses the services of about 200 labs in the state. Chlamydia testing is part of the PACT program benefits, and is recommended for all sexually active women aged 25 years or younger.
The study period spanned 2008-2010, focusing on women aged 25 or younger who were seen at by Family PACT providers in 2009. Dr. Chow compared the chlamydia testing these women received with any testing they had in the prior 12 months, or in the following 12 months.
During 2009, 219,319 young women accessed PACT services; 74% of those had a chlamydia test in the past 12 months, leaving 25% (55,915) untested at the 2009 visit.
Overall, 89% had urine collected for either a pregnancy test or to evaluate for a urinary tract infection. Of those patients, 43% had other services that included an easy opportunity to test, including a pelvic exam.
Most of these young women (75%) visited a PACT office only once during the study period. Only half (52%) returned to an office in the following 12 months, and of those, only 50% were tested for chlamydia, despite not being tested at the previous visit.
In all, only a quarter of the untested young women were tested in the following 12 months, Dr. Chow said. "Since most had only one visit, opportunities to test were not abundant."
But most patients who are diagnosed do help treat their sexual partners if they have access to the expedited treatment packs some clinics provide.
Typically, the packs contain 1 g of azithromycin, condoms, educational material about chlamydia and other STDs, and contact information for all local STD clinics, said Meighan Rogers of the New York City Department of Health’s STD branch.
She and her colleagues conducted a follow-up study of 671 patients diagnosed with chlamydia during a 12-month period from February 2011 through February 2012. Patients could receive up to three partner packs at the visit, with three additional packs available by prescription. The analysis included only patients from heterosexual couples.
A health department representative called each patient 5 days after treatment to determine if the pack had been given to partners, and if the partners had taken the medication. The survey also asked about any adverse effects of the antibiotic and ascertained the written materials’ clarity.
Most of the patients (74%) were women. Of all those eligible for the patient packs, 52% accepted them. Surveyors were able to contact 174 of these patients; 122 of those reported having a total of 150 partners who were locatable. About 44% of these (66) were successfully contacted.
Most (74%) of the partners reported getting the packs; 94% of those said they took the medication. Two partners reported having medical problems from the medication, although the analysis did not specify what those were.
About a quarter of the partners (23%) said they visited a physician afterward. Of those who did not see a doctor, 100% said they planned to. Women partners were significantly more likely to plan a doctor’s visit than men (46% vs. 13%).
That’s good news, said Ms. Rogers. "It’s very hopeful that almost half of the women were planning to see a physician, since this might translate into a decreased risk of pelvic inflammatory disease and its reproductive sequelae."
As state and federal employees, the presenters had no financial disclosures.
FROM A CONFERENCE ON STD PREVENTION SPONSORED BY THE CENTERS FOR DISEASE CONTROL AND PREVENTION