User login
FDA: Gastrointestinal Drugs Advisory Committee (GIDAC) & Drug Safety and Risk Management Advisory Committee (DSaRM) Meeting
FDA advisers back vedolizumab approval to treat moderate to severe inflammatory bowel disease
SILVER SPRING, MD. – The benefits of vedolizumab, an integrin antagonist, outweigh the potential for progressive multifocal encephalopathy and other possible risks as a treatment for moderate to severely active Crohn’s disease and ulcerative colitis, and should be approved, according to two Food and Drug Administration advisory panels.
At a meeting of the FDA’s Gastrointestinal Drugs and Drug Safety and Risk Management advisory committees, the panels unanimously supported approval of vedolizumab for both indications, although they were less confident about the efficacy data of vedolizumab for induction in Crohn’s disease. They also strongly recommended a postmarketing program to monitor safety issues after approval, including progressive multifocal encephalopathy (PML), other infections, and other types of adverse events such as autoimmune hepatitis.
Vedolizumab is a monoclonal antibody that binds exclusively to the alpha 4 beta 7 integrin, "a key mediator of gastrointestinal inflammation," according to the manufacturer, Takeda Pharmaceuticals USA. It is administered intravenously at 0, 2, and 6 weeks, followed by once every 8 weeks for maintenance therapy. No cases of PML have been reported in more than 3,100 patients treated with vedolizumab worldwide, including 906 treated for 3 or more years, according to Takeda. But because it is thought to disrupt integrin function, like natalizumab (Tysabri), which is approved for multiple sclerosis and Crohn’s disease and is associated with an increased risk of PML, the potential for PML with vedolizumab therapy was the main safety issue raised by the FDA. Natalizumab is approved with a Risk Evaluation and Mitigation Strategy (REMS) that addresses the risk of PML, the usually fatal demyelinating CNS infection.
The phase III studies evaluating vedolizumab in patients with Crohn’s disease and ulcerative colitis were similarly designed, randomizing patients to vedolizumab or placebo in 6-week induction studies, and randomizing patients who responded to vedolizumab induction therapy to vedolizumab or placebo in 52-week maintenance studies.
In a Crohn’s disease induction and maintenance study of 368 patients, 14.5% of those on vedolizumab had achieved a clinical remission at week 6, vs. almost 7% of those on placebo, a statistically significant difference. At week 52, 39% of those who were treated every 8 weeks as maintenance therapy had a clinical remission, vs. almost 22% of those on placebo, also a significant difference. But in a second induction study of 315 patients, mostly treatment-refractory patients, there was not a significant difference in 6-week clinical remission rates between those on vedolizumab (15%) and those on placebo (12%).
The panelists voted 12 to 9 that these data supported efficacy for Crohn’s disease induction, and voted 20 to 0, with one abstention, that the data supported approval of the maintenance indication, agreeing the maintenance data were robust. Most (14) agreed that the benefits outweighed the risks to support approval of patients with Crohn’s who have failed treatment with steroids or immunosuppressants or tumor necrosis factor (TNF)–alpha blockers, the indication proposed by the manufacturer. Six panelists supported approval, but for the narrower use, in patients who had failed treatment with immunosuppressants or TNF-alpha antagonists, an indication that would not include patients who had failed steroids only. (The remaining panelist abstained.)
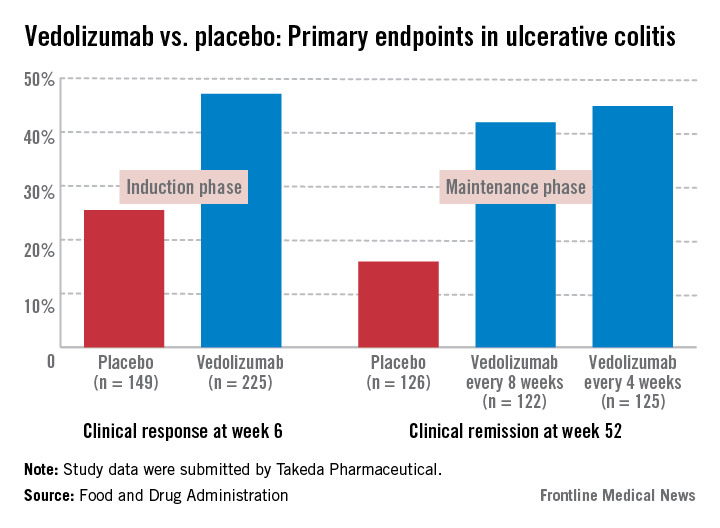
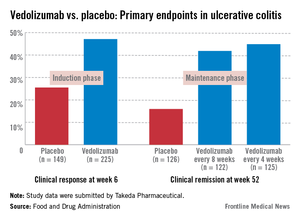
In the induction and maintenance trial of 374 patients with ulcerative colitis, the clinical response rate at 6 weeks was 47% among those on vedolizumab, vs. 25.5% among those on placebo, a statistically significant difference. In this study, almost 42% of those on vedolizumab every 8 weeks as maintenance therapy had achieved clinical remission at week 52, vs. 16% of those on placebo, also a statistically significant difference.
All panel members supported approval for the ulcerative colitis indication, based on the benefits and risks, but differed on the wording of the indication, with 13 panelists supporting approval for patients with ulcerative colitis who have failed steroids or immunosuppressants or TNF-alpha antagonists. The remaining eight panelists supported approval for patients who have failed immunosuppressants or TNF-alpha antagonists, which would not include an indication for patients who had failed treatment with steroids only.
The overall infection rate was higher among the patients treated with vedolizumab than among those on placebo in these studies (primarily upper respiratory tract infections and pharyngitis), which did not appear to be related to the number of infusions or concomitant immunosuppressive treatment. The rates of serious infections (3%-4%) were similar among those on vedolizumab and placebo. There were four patients with serious hepatic adverse events – acute hepatitis – that resolved. The 12 deaths among those on vedolizumab were not related to the drug, and there was no apparent increase in malignancies among treated patients, according to the FDA reviewers.
In a unanimous 21-0 vote, the panel agreed that Takeda had "adequately characterized the potential risk of PML" to support approval, but panelists recommended postmarketing follow-up for PML and other serious adverse events in treated patients.
The FDA is expected to make a decision on approval for the ulcerative colitis indication by Feb. 18, 2014, and for the Crohn’s disease indication by June 18, 2014.
The FDA usually follows the recommendations of its advisory panels. Members of FDA panels have usually been cleared of conflicts related to the product under review; occasionally, a panelist is given a waiver, but not at this meeting.
If approved, Takeda plans to market vedolizumab as Entyvio.
SILVER SPRING, MD. – The benefits of vedolizumab, an integrin antagonist, outweigh the potential for progressive multifocal encephalopathy and other possible risks as a treatment for moderate to severely active Crohn’s disease and ulcerative colitis, and should be approved, according to two Food and Drug Administration advisory panels.
At a meeting of the FDA’s Gastrointestinal Drugs and Drug Safety and Risk Management advisory committees, the panels unanimously supported approval of vedolizumab for both indications, although they were less confident about the efficacy data of vedolizumab for induction in Crohn’s disease. They also strongly recommended a postmarketing program to monitor safety issues after approval, including progressive multifocal encephalopathy (PML), other infections, and other types of adverse events such as autoimmune hepatitis.

Vedolizumab is a monoclonal antibody that binds exclusively to the alpha 4 beta 7 integrin, "a key mediator of gastrointestinal inflammation," according to the manufacturer, Takeda Pharmaceuticals USA. It is administered intravenously at 0, 2, and 6 weeks, followed by once every 8 weeks for maintenance therapy. No cases of PML have been reported in more than 3,100 patients treated with vedolizumab worldwide, including 906 treated for 3 or more years, according to Takeda. But because it is thought to disrupt integrin function, like natalizumab (Tysabri), which is approved for multiple sclerosis and Crohn’s disease and is associated with an increased risk of PML, the potential for PML with vedolizumab therapy was the main safety issue raised by the FDA. Natalizumab is approved with a Risk Evaluation and Mitigation Strategy (REMS) that addresses the risk of PML, the usually fatal demyelinating CNS infection.
The phase III studies evaluating vedolizumab in patients with Crohn’s disease and ulcerative colitis were similarly designed, randomizing patients to vedolizumab or placebo in 6-week induction studies, and randomizing patients who responded to vedolizumab induction therapy to vedolizumab or placebo in 52-week maintenance studies.
In a Crohn’s disease induction and maintenance study of 368 patients, 14.5% of those on vedolizumab had achieved a clinical remission at week 6, vs. almost 7% of those on placebo, a statistically significant difference. At week 52, 39% of those who were treated every 8 weeks as maintenance therapy had a clinical remission, vs. almost 22% of those on placebo, also a significant difference. But in a second induction study of 315 patients, mostly treatment-refractory patients, there was not a significant difference in 6-week clinical remission rates between those on vedolizumab (15%) and those on placebo (12%).
The panelists voted 12 to 9 that these data supported efficacy for Crohn’s disease induction, and voted 20 to 0, with one abstention, that the data supported approval of the maintenance indication, agreeing the maintenance data were robust. Most (14) agreed that the benefits outweighed the risks to support approval of patients with Crohn’s who have failed treatment with steroids or immunosuppressants or tumor necrosis factor (TNF)–alpha blockers, the indication proposed by the manufacturer. Six panelists supported approval, but for the narrower use, in patients who had failed treatment with immunosuppressants or TNF-alpha antagonists, an indication that would not include patients who had failed steroids only. (The remaining panelist abstained.)
In the induction and maintenance trial of 374 patients with ulcerative colitis, the clinical response rate at 6 weeks was 47% among those on vedolizumab, vs. 25.5% among those on placebo, a statistically significant difference. In this study, almost 42% of those on vedolizumab every 8 weeks as maintenance therapy had achieved clinical remission at week 52, vs. 16% of those on placebo, also a statistically significant difference.
All panel members supported approval for the ulcerative colitis indication, based on the benefits and risks, but differed on the wording of the indication, with 13 panelists supporting approval for patients with ulcerative colitis who have failed steroids or immunosuppressants or TNF-alpha antagonists. The remaining eight panelists supported approval for patients who have failed immunosuppressants or TNF-alpha antagonists, which would not include an indication for patients who had failed treatment with steroids only.
The overall infection rate was higher among the patients treated with vedolizumab than among those on placebo in these studies (primarily upper respiratory tract infections and pharyngitis), which did not appear to be related to the number of infusions or concomitant immunosuppressive treatment. The rates of serious infections (3%-4%) were similar among those on vedolizumab and placebo. There were four patients with serious hepatic adverse events – acute hepatitis – that resolved. The 12 deaths among those on vedolizumab were not related to the drug, and there was no apparent increase in malignancies among treated patients, according to the FDA reviewers.
In a unanimous 21-0 vote, the panel agreed that Takeda had "adequately characterized the potential risk of PML" to support approval, but panelists recommended postmarketing follow-up for PML and other serious adverse events in treated patients.
The FDA is expected to make a decision on approval for the ulcerative colitis indication by Feb. 18, 2014, and for the Crohn’s disease indication by June 18, 2014.
The FDA usually follows the recommendations of its advisory panels. Members of FDA panels have usually been cleared of conflicts related to the product under review; occasionally, a panelist is given a waiver, but not at this meeting.
If approved, Takeda plans to market vedolizumab as Entyvio.
SILVER SPRING, MD. – The benefits of vedolizumab, an integrin antagonist, outweigh the potential for progressive multifocal encephalopathy and other possible risks as a treatment for moderate to severely active Crohn’s disease and ulcerative colitis, and should be approved, according to two Food and Drug Administration advisory panels.
At a meeting of the FDA’s Gastrointestinal Drugs and Drug Safety and Risk Management advisory committees, the panels unanimously supported approval of vedolizumab for both indications, although they were less confident about the efficacy data of vedolizumab for induction in Crohn’s disease. They also strongly recommended a postmarketing program to monitor safety issues after approval, including progressive multifocal encephalopathy (PML), other infections, and other types of adverse events such as autoimmune hepatitis.

Vedolizumab is a monoclonal antibody that binds exclusively to the alpha 4 beta 7 integrin, "a key mediator of gastrointestinal inflammation," according to the manufacturer, Takeda Pharmaceuticals USA. It is administered intravenously at 0, 2, and 6 weeks, followed by once every 8 weeks for maintenance therapy. No cases of PML have been reported in more than 3,100 patients treated with vedolizumab worldwide, including 906 treated for 3 or more years, according to Takeda. But because it is thought to disrupt integrin function, like natalizumab (Tysabri), which is approved for multiple sclerosis and Crohn’s disease and is associated with an increased risk of PML, the potential for PML with vedolizumab therapy was the main safety issue raised by the FDA. Natalizumab is approved with a Risk Evaluation and Mitigation Strategy (REMS) that addresses the risk of PML, the usually fatal demyelinating CNS infection.
The phase III studies evaluating vedolizumab in patients with Crohn’s disease and ulcerative colitis were similarly designed, randomizing patients to vedolizumab or placebo in 6-week induction studies, and randomizing patients who responded to vedolizumab induction therapy to vedolizumab or placebo in 52-week maintenance studies.
In a Crohn’s disease induction and maintenance study of 368 patients, 14.5% of those on vedolizumab had achieved a clinical remission at week 6, vs. almost 7% of those on placebo, a statistically significant difference. At week 52, 39% of those who were treated every 8 weeks as maintenance therapy had a clinical remission, vs. almost 22% of those on placebo, also a significant difference. But in a second induction study of 315 patients, mostly treatment-refractory patients, there was not a significant difference in 6-week clinical remission rates between those on vedolizumab (15%) and those on placebo (12%).
The panelists voted 12 to 9 that these data supported efficacy for Crohn’s disease induction, and voted 20 to 0, with one abstention, that the data supported approval of the maintenance indication, agreeing the maintenance data were robust. Most (14) agreed that the benefits outweighed the risks to support approval of patients with Crohn’s who have failed treatment with steroids or immunosuppressants or tumor necrosis factor (TNF)–alpha blockers, the indication proposed by the manufacturer. Six panelists supported approval, but for the narrower use, in patients who had failed treatment with immunosuppressants or TNF-alpha antagonists, an indication that would not include patients who had failed steroids only. (The remaining panelist abstained.)
In the induction and maintenance trial of 374 patients with ulcerative colitis, the clinical response rate at 6 weeks was 47% among those on vedolizumab, vs. 25.5% among those on placebo, a statistically significant difference. In this study, almost 42% of those on vedolizumab every 8 weeks as maintenance therapy had achieved clinical remission at week 52, vs. 16% of those on placebo, also a statistically significant difference.
All panel members supported approval for the ulcerative colitis indication, based on the benefits and risks, but differed on the wording of the indication, with 13 panelists supporting approval for patients with ulcerative colitis who have failed steroids or immunosuppressants or TNF-alpha antagonists. The remaining eight panelists supported approval for patients who have failed immunosuppressants or TNF-alpha antagonists, which would not include an indication for patients who had failed treatment with steroids only.
The overall infection rate was higher among the patients treated with vedolizumab than among those on placebo in these studies (primarily upper respiratory tract infections and pharyngitis), which did not appear to be related to the number of infusions or concomitant immunosuppressive treatment. The rates of serious infections (3%-4%) were similar among those on vedolizumab and placebo. There were four patients with serious hepatic adverse events – acute hepatitis – that resolved. The 12 deaths among those on vedolizumab were not related to the drug, and there was no apparent increase in malignancies among treated patients, according to the FDA reviewers.
In a unanimous 21-0 vote, the panel agreed that Takeda had "adequately characterized the potential risk of PML" to support approval, but panelists recommended postmarketing follow-up for PML and other serious adverse events in treated patients.
The FDA is expected to make a decision on approval for the ulcerative colitis indication by Feb. 18, 2014, and for the Crohn’s disease indication by June 18, 2014.
The FDA usually follows the recommendations of its advisory panels. Members of FDA panels have usually been cleared of conflicts related to the product under review; occasionally, a panelist is given a waiver, but not at this meeting.
If approved, Takeda plans to market vedolizumab as Entyvio.
AT AN FDA ADVISORY COMMITTEE MEETING