User login
Although we are not able to cover all of the important developments in fertility medicine over the past year, there were 3 important articles published in the past 12 months that we highlight here. First, we discuss an American College of Obstetricians and Gynecologists (ACOG) committee opinion on genetic carrier screening that was reaffirmed in 2019. Second, we explore an interesting retrospective analysis of time-lapse videos and clinical outcomes of more than 10,000 embryos from 8 IVF clinics, across 4 countries. The authors assessed whether a deep learning model could predict the probability of pregnancy with fetal heart from time-lapse videos in the hopes that their research can improve prioritization of the most viable embryo for single embryo transfer. Last, we consider a review of the data on obstetric and reproductive health effects of preconception and prenatal exposure to several environmental toxicants, including heavy metals, endocrine-disrupting chemicals, pesticides, and air pollution.
Preconception genetic carrier screening: Standardize your counseling approach
American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
With the rapid development of advanced and high throughput platforms for DNA sequencing in the past several years, the cost of genetic testing has decreased dramatically. Women's health care providers in general, and fertility specialists in particular, are uniquely positioned to take advantage of these novel and yet affordable technologies by counseling prospective parents during the preconception counseling, or early prenatal period, about the availability of genetic carrier screening and its potential to provide actionable information in a timely manner. The ultimate objective of genetic carrier screening is to enable individuals to make an informed decision regarding their reproductive choices based on their personal values. In a study by Larsen and colleagues, the uptake of genetic carrier screening was significantly higher when offered in the preconception period (68.7%), compared with during pregnancy (35.1%), which highlights the significance of early counseling.1
Based on the Centers for Disease Control and Prevention's Birth/Infant Death Data set, birth defects affect 1 in every 33 (about 3%) of all babies born in the United States each year and account for 20% of infant mortality.2 About 20% of birth defects are caused by single-gene (monogenic) disorders, and although some of these are due to dominant conditions or de novo mutations, a significant proportion are due to autosomal recessive, or X-chromosome linked conditions that are commonly assessed by genetic carrier screening.
ACOG published a committee opinion on "Carrier Screening in the Age of Genomic Medicine" in March 2017, which was reaffirmed in 2019.3
Residual risk. Several points discussed in this document are of paramount importance, including the need for pretest and posttest counseling and consent, as well as a discussion of "residual risk." Newer platforms employ sequencing techniques that potentially can detect most, if not all, of the disease-causing variants in the tested genes, such as the gene for cystic fibrosis and, therefore, have a higher detection rate compared with the older PCR-based techniques for a limited number of specific mutations included in the panel. Due to a variety of technical and biological limitations, however, such as allelic dropouts and the occurrence of de novo mutations, the detection rate is not 100%; there is always a residual risk that needs to be estimated and provided to individuals based on the existing knowledge on frequency of gene, penetrance of phenotype, and prevalence of condition in the general and specific ethnic populations.
Continue to: Expanded vs panethnic screening...
Expanded vs panethnic screening. Furthermore, although sequencing technology has made "expanded carrier screening" for several hundred conditions, simultaneous to and independent of ethnicity and family history, more easily available and affordable, ethnic-specific and panethnic screening for a more limited number of conditions are still acceptable approaches. Having said this, when the first partner screened is identified to be a carrier, his/her reproductive partners must be offered next-generation sequencing to identify less common disease-causing variants.4
A cautionary point to consider when expanded carrier screening panels are requested is the significant variability among commercial laboratories with regard to the conditions included in their panels. In addition, consider the absence of a well-defined or predictable phenotype for some of the included conditions.
Perhaps the most important matter when it comes to genetic carrier screening is to have a standard counseling approach that is persistently followed and offers the opportunity for individuals to know about their genetic testing options and available reproductive choices, including the use of donor gametes, preimplantation genetic testing for monogenic disease (PGT-M, formerly known as preimplantation genetic diagnosis, or PGD), prenatal testing, and pregnancy management options. For couples and/or individuals who decide to proceed with an affected pregnancy, earlier diagnosis can assist with postnatal management.
Medicolegal responsibility. Genetic carrier screening also is of specific relevance to the field of fertility medicine and assisted reproductive technology (ART) as a potential liability issue. Couples and individuals who are undergoing fertility treatment with in vitro fertilization (IVF) for a variety of medical or personal reasons are a specific group that certainly should be offered genetic carrier screening, as they have the option of "adding on" PGT-M (PGD) to their existing treatment plan at a fraction of the cost and treatment burden that would have otherwise been needed if they were not undergoing IVF. After counseling, some individuals and couples may ultimately opt out of genetic carrier screening. The counseling discussion needs to be clearly documented in the medical chart.
The preconception period is the perfect time to have a discussion about genetic carrier screening; it offers the opportunity for timely interventions if desired by the couples or individuals.
Continue to: Artificial intelligence and embryo selection...
Artificial intelligence and embryo selection
With continued improvements in embryo culture conditions and cryopreservation technology, there has been a tremendous amount of interest in developing better methods for embryo selection. These efforts are aimed at encouraging elective single embryo transfer (eSET) for women of all ages, thereby lowering the risk of multiple pregnancy and its associated adverse neonatal and obstetric outcomes—without compromising the pregnancy rates per transfer or lengthening the time to pregnancy.
One of the most extensively studied methods for this purpose is preimplantation genetic testing for aneuploidy (PGT-A, formerly known as PGS), but emerging data from large multicenter randomized clinical trials (RCTs) have again cast significant doubt on PGT-A's efficacy and utility.5 Meanwhile, alternative methods for embryo selection are currently under investigation, including noninvasive PGT-A and morphokinetic assessment of embryo development via analysis of images obtained by time-lapse imaging.
The potential of time-lapse imaging
Despite the initial promising results from time-lapse imaging, subsequent RCTs have not shown a significant clinical benefit.6 However, these early methods of morphokinetic assessment are mainly dependent on the embryologists' subjective assessment of individual static frames and "annotation" of observed spatial and temporal features of embryo development. In addition to being a very time-consuming task, this process is subject to significant interobserver and intraobserver variability.
Considering these limitations, even machine-based algorithms that incorporate these annotations along with such other clinical variables as parental age and prior obstetric history, have a low predictive power for the outcome of embryo transfer, with an area under the curve (AUC) of the ROC curve of 0.65 to 0.74. (An AUC of 0.5 represents completely random prediction and an AUC of 1.0 suggests perfect prediction.)7
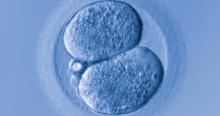
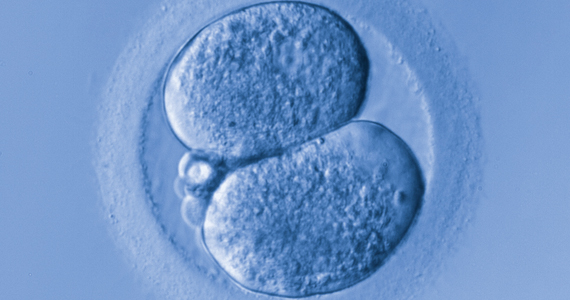
A recent study by Tran and colleagues has employed a deep learning (neural network) model to analyze the entire raw time-lapse videos in an automated manner without prior annotation by embryologists. After analysis of 10,638 embryos from 8 different IVF clinics in 4 different countries, they have reported an AUC of 0.93 (95% confidence interval, 0.92-0.94) for prediction of fetal heart rate activity detected at 7 weeks of gestation or beyond. Although these data are very preliminary and have not yet been validated prospectively in larger datasets for live birth, it may herald the beginning of a new era for the automation and standardization of embryo assessment with artificial intelligence—similar to the rapidly increasing role of facial recognition technology for various applications.
Improved standardization of noninvasive embryo selection with growing use of artificial intelligence is a promising new tool to improve the safety and efficacy of ART.
Continue to: Environmental toxicants: The hidden danger...
Environmental toxicants: The hidden danger
Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112:613-621.
We receive news daily about the existential risk to humans of climate change. However, a risk that is likely as serious goes almost unseen by the public and most health care providers. That risk is environmental toxicants.8
More than 80,000 chemicals are registered in the United States, most in the last 75 years. These chemicals are ubiquitous. All of us are continuously exposed to and suffused with these toxicants and their metabolites. Air pollution adds insult to injury. Since this exposure has especially significant implications for fertility, infertility, pregnancy, perinatal health, childhood development, adult diseases, and later generational reproduction, it is imperative that reproductive health professionals take responsibility for helping mitigate this environmental crisis.
The problem is exceptionally complicated
The risks posed by environmental toxicants are much less visible than those for climate change, so the public, policymakers, and providers are largely unaware or may even seem uncaring. Few health professionals have sufficient knowledge to deliver care in this area, know which questions to ask, or have adequate information/medical record tools to assist them in care—and what are the possible interventions?
Addressing risk posed by individual toxicants
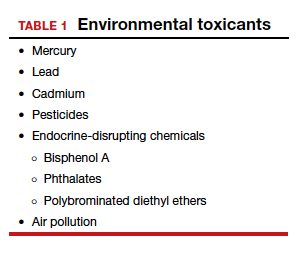
Addressing the problem clinically requires asking patients questions about exposure and recommending interventions. Toxicant chemicals include the neurotoxin mercury, which can be addressed by limiting intake of fish, especially certain types.
Lead was used before 1978 in paint, it also was used in gas and in water pipes. People living in older homes may be exposed, as well as those in occupations exposed to lead. Others with lead exposure risk include immigrants from areas without lead regulations and people using pica- or lead-glazed pottery. Lead exposure has been associated with multiple pregnancy complications and permanently impaired intellectual development in children. If lead testing reveals high levels, chelation therapy can help.
Cadmium is a heavy metal used in rechargeable batteries, paint pigment, and plastic production. Exposure results from food intake, smoking, and second-hand smoke. Cadmium accumulates in the liver, kidneys, testes, ovaries, and placenta. Exposure causes itai-itai disease, which is characterized by osteomalacia and renal tubular dysfunction as well as epigenetic changes in placental DNA and damage to the reproductive system. Eating organic food and reducing industrial exposure to cadmium are preventive strategies.
Pesticides are ubiquitous, with 90% of the US population having detectable levels. Exposure during the preconception period can lead to intrauterine growth restriction, low birth weight, subsequent cancers, and other problems. Eating organic food can reduce risk, as can frequent hand washing when exposed to pesticides, using protective gear, and removing shoes in the home.
Endocrine-disrupting chemicals (EDCs) are chemicals that can mimic or block endogenous hormones, which leads to adverse health outcomes. In addition to heavy metals, 3 important EDCs are bisphenol A (BPA), phthalates, and polybrominated diethyl ethers (PBDEs). Exposure is ubiquitous from industrial food processing, personal care products, cosmetics, and dust. Phthalates and BPA have short half-lives of hours to days, while PBDEs can persist in adipose tissue for months. Abnormal urogenital and neurologic development and thyroid disruption can result. Eating organic food, eating at home, and decreasing processed food intake can reduce exposure.
BPA is used in plastics, canned food liners, cash register receipts, and epoxy resins. Exposure is through inhalation, ingestion, and dermal absorption and affects semen quality, fertilization, placentation, and early reproduction. Limiting the use of plastic containers, not microwaving food in plastic, and avoiding thermal paper cash register receipts can reduce exposure.
Phthalates are synthetically derived and used as plasticizers in personal and medical products. The major source of phthalate exposure is food; exposure causes sperm, egg, and DNA damage. Phthalate avoidance involves replacing plastic bottles with glass or stainless steel, avoiding reheating food in plastic containers, and choosing "fragrance free" products.
PBDEs are used in flame retardants on upholstery, textiles, carpeting, and some electronics. Most PBDEs have been replaced by alternatives; however, their half-life is up to 12 years. Complications caused by PBDEs include thyroid disruption, resulting in abnormal fetal brain development. Avoiding dust and furniture that contain PBDEs, as well as hand washing, reduces exposure risk.
Air pollutants are associated with adverse obstetric outcomes and lower cognitive function in children. Avoiding areas with heavy traffic, staying indoors when air is heavily polluted, and using a HEPA filter in the home can reduce chemicals from air pollution.
Recommendations
The magnitude of the problem that environmental toxicant exposure creates requires health care providers to take action. The table in the publication by Segal and Giudice can be used as a tool that patients can answer first themselves before review by their provider.2 It can be added to your electronic health record and/or patient portal. Even making general comments to raise awareness, asking questions regarding exposure, and making recommendations can be helpful (TABLES 1 and 2). When possible, we also should advocate for public awareness and policy changes that address this significant health issue.

Environmental toxicants are a significant health problem that can be effectively mitigated through patient questions and recommended interventions.
- Larsen D, Ma J, Strassberg M, et al. The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn. 2019;39:319-323.
- Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1-30.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
- Gregg AR, Edwards JG. Prenatal genetic carrier screening in the genomic age. Semin Perinatol. 2018;42:303-306.
- Munné S, Kaplan B, Frattarelli JL, et al; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071-1079. e7.
- Goodman LR, Goldberg J, Falcone T, et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-285.e10.
- Blank C, Wildeboer RR, DeCroo I, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111:318- 326.
- The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society for Reproductive Medicine Practice Committee; The University of California, San Francisco Program on Reproductive Health and the Environment. ACOG Committee Opinion No. 575. Exposure to environmental toxic agents. Fertil Steril. 2013;100:931-934.
Although we are not able to cover all of the important developments in fertility medicine over the past year, there were 3 important articles published in the past 12 months that we highlight here. First, we discuss an American College of Obstetricians and Gynecologists (ACOG) committee opinion on genetic carrier screening that was reaffirmed in 2019. Second, we explore an interesting retrospective analysis of time-lapse videos and clinical outcomes of more than 10,000 embryos from 8 IVF clinics, across 4 countries. The authors assessed whether a deep learning model could predict the probability of pregnancy with fetal heart from time-lapse videos in the hopes that their research can improve prioritization of the most viable embryo for single embryo transfer. Last, we consider a review of the data on obstetric and reproductive health effects of preconception and prenatal exposure to several environmental toxicants, including heavy metals, endocrine-disrupting chemicals, pesticides, and air pollution.
Preconception genetic carrier screening: Standardize your counseling approach
American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
With the rapid development of advanced and high throughput platforms for DNA sequencing in the past several years, the cost of genetic testing has decreased dramatically. Women's health care providers in general, and fertility specialists in particular, are uniquely positioned to take advantage of these novel and yet affordable technologies by counseling prospective parents during the preconception counseling, or early prenatal period, about the availability of genetic carrier screening and its potential to provide actionable information in a timely manner. The ultimate objective of genetic carrier screening is to enable individuals to make an informed decision regarding their reproductive choices based on their personal values. In a study by Larsen and colleagues, the uptake of genetic carrier screening was significantly higher when offered in the preconception period (68.7%), compared with during pregnancy (35.1%), which highlights the significance of early counseling.1
Based on the Centers for Disease Control and Prevention's Birth/Infant Death Data set, birth defects affect 1 in every 33 (about 3%) of all babies born in the United States each year and account for 20% of infant mortality.2 About 20% of birth defects are caused by single-gene (monogenic) disorders, and although some of these are due to dominant conditions or de novo mutations, a significant proportion are due to autosomal recessive, or X-chromosome linked conditions that are commonly assessed by genetic carrier screening.
ACOG published a committee opinion on "Carrier Screening in the Age of Genomic Medicine" in March 2017, which was reaffirmed in 2019.3
Residual risk. Several points discussed in this document are of paramount importance, including the need for pretest and posttest counseling and consent, as well as a discussion of "residual risk." Newer platforms employ sequencing techniques that potentially can detect most, if not all, of the disease-causing variants in the tested genes, such as the gene for cystic fibrosis and, therefore, have a higher detection rate compared with the older PCR-based techniques for a limited number of specific mutations included in the panel. Due to a variety of technical and biological limitations, however, such as allelic dropouts and the occurrence of de novo mutations, the detection rate is not 100%; there is always a residual risk that needs to be estimated and provided to individuals based on the existing knowledge on frequency of gene, penetrance of phenotype, and prevalence of condition in the general and specific ethnic populations.
Continue to: Expanded vs panethnic screening...
Expanded vs panethnic screening. Furthermore, although sequencing technology has made "expanded carrier screening" for several hundred conditions, simultaneous to and independent of ethnicity and family history, more easily available and affordable, ethnic-specific and panethnic screening for a more limited number of conditions are still acceptable approaches. Having said this, when the first partner screened is identified to be a carrier, his/her reproductive partners must be offered next-generation sequencing to identify less common disease-causing variants.4
A cautionary point to consider when expanded carrier screening panels are requested is the significant variability among commercial laboratories with regard to the conditions included in their panels. In addition, consider the absence of a well-defined or predictable phenotype for some of the included conditions.
Perhaps the most important matter when it comes to genetic carrier screening is to have a standard counseling approach that is persistently followed and offers the opportunity for individuals to know about their genetic testing options and available reproductive choices, including the use of donor gametes, preimplantation genetic testing for monogenic disease (PGT-M, formerly known as preimplantation genetic diagnosis, or PGD), prenatal testing, and pregnancy management options. For couples and/or individuals who decide to proceed with an affected pregnancy, earlier diagnosis can assist with postnatal management.
Medicolegal responsibility. Genetic carrier screening also is of specific relevance to the field of fertility medicine and assisted reproductive technology (ART) as a potential liability issue. Couples and individuals who are undergoing fertility treatment with in vitro fertilization (IVF) for a variety of medical or personal reasons are a specific group that certainly should be offered genetic carrier screening, as they have the option of "adding on" PGT-M (PGD) to their existing treatment plan at a fraction of the cost and treatment burden that would have otherwise been needed if they were not undergoing IVF. After counseling, some individuals and couples may ultimately opt out of genetic carrier screening. The counseling discussion needs to be clearly documented in the medical chart.
The preconception period is the perfect time to have a discussion about genetic carrier screening; it offers the opportunity for timely interventions if desired by the couples or individuals.
Continue to: Artificial intelligence and embryo selection...
Artificial intelligence and embryo selection
With continued improvements in embryo culture conditions and cryopreservation technology, there has been a tremendous amount of interest in developing better methods for embryo selection. These efforts are aimed at encouraging elective single embryo transfer (eSET) for women of all ages, thereby lowering the risk of multiple pregnancy and its associated adverse neonatal and obstetric outcomes—without compromising the pregnancy rates per transfer or lengthening the time to pregnancy.
One of the most extensively studied methods for this purpose is preimplantation genetic testing for aneuploidy (PGT-A, formerly known as PGS), but emerging data from large multicenter randomized clinical trials (RCTs) have again cast significant doubt on PGT-A's efficacy and utility.5 Meanwhile, alternative methods for embryo selection are currently under investigation, including noninvasive PGT-A and morphokinetic assessment of embryo development via analysis of images obtained by time-lapse imaging.
The potential of time-lapse imaging
Despite the initial promising results from time-lapse imaging, subsequent RCTs have not shown a significant clinical benefit.6 However, these early methods of morphokinetic assessment are mainly dependent on the embryologists' subjective assessment of individual static frames and "annotation" of observed spatial and temporal features of embryo development. In addition to being a very time-consuming task, this process is subject to significant interobserver and intraobserver variability.
Considering these limitations, even machine-based algorithms that incorporate these annotations along with such other clinical variables as parental age and prior obstetric history, have a low predictive power for the outcome of embryo transfer, with an area under the curve (AUC) of the ROC curve of 0.65 to 0.74. (An AUC of 0.5 represents completely random prediction and an AUC of 1.0 suggests perfect prediction.)7
A recent study by Tran and colleagues has employed a deep learning (neural network) model to analyze the entire raw time-lapse videos in an automated manner without prior annotation by embryologists. After analysis of 10,638 embryos from 8 different IVF clinics in 4 different countries, they have reported an AUC of 0.93 (95% confidence interval, 0.92-0.94) for prediction of fetal heart rate activity detected at 7 weeks of gestation or beyond. Although these data are very preliminary and have not yet been validated prospectively in larger datasets for live birth, it may herald the beginning of a new era for the automation and standardization of embryo assessment with artificial intelligence—similar to the rapidly increasing role of facial recognition technology for various applications.
Improved standardization of noninvasive embryo selection with growing use of artificial intelligence is a promising new tool to improve the safety and efficacy of ART.
Continue to: Environmental toxicants: The hidden danger...
Environmental toxicants: The hidden danger
Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112:613-621.
We receive news daily about the existential risk to humans of climate change. However, a risk that is likely as serious goes almost unseen by the public and most health care providers. That risk is environmental toxicants.8
More than 80,000 chemicals are registered in the United States, most in the last 75 years. These chemicals are ubiquitous. All of us are continuously exposed to and suffused with these toxicants and their metabolites. Air pollution adds insult to injury. Since this exposure has especially significant implications for fertility, infertility, pregnancy, perinatal health, childhood development, adult diseases, and later generational reproduction, it is imperative that reproductive health professionals take responsibility for helping mitigate this environmental crisis.
The problem is exceptionally complicated
The risks posed by environmental toxicants are much less visible than those for climate change, so the public, policymakers, and providers are largely unaware or may even seem uncaring. Few health professionals have sufficient knowledge to deliver care in this area, know which questions to ask, or have adequate information/medical record tools to assist them in care—and what are the possible interventions?
Addressing risk posed by individual toxicants
Addressing the problem clinically requires asking patients questions about exposure and recommending interventions. Toxicant chemicals include the neurotoxin mercury, which can be addressed by limiting intake of fish, especially certain types.
Lead was used before 1978 in paint, it also was used in gas and in water pipes. People living in older homes may be exposed, as well as those in occupations exposed to lead. Others with lead exposure risk include immigrants from areas without lead regulations and people using pica- or lead-glazed pottery. Lead exposure has been associated with multiple pregnancy complications and permanently impaired intellectual development in children. If lead testing reveals high levels, chelation therapy can help.
Cadmium is a heavy metal used in rechargeable batteries, paint pigment, and plastic production. Exposure results from food intake, smoking, and second-hand smoke. Cadmium accumulates in the liver, kidneys, testes, ovaries, and placenta. Exposure causes itai-itai disease, which is characterized by osteomalacia and renal tubular dysfunction as well as epigenetic changes in placental DNA and damage to the reproductive system. Eating organic food and reducing industrial exposure to cadmium are preventive strategies.
Pesticides are ubiquitous, with 90% of the US population having detectable levels. Exposure during the preconception period can lead to intrauterine growth restriction, low birth weight, subsequent cancers, and other problems. Eating organic food can reduce risk, as can frequent hand washing when exposed to pesticides, using protective gear, and removing shoes in the home.
Endocrine-disrupting chemicals (EDCs) are chemicals that can mimic or block endogenous hormones, which leads to adverse health outcomes. In addition to heavy metals, 3 important EDCs are bisphenol A (BPA), phthalates, and polybrominated diethyl ethers (PBDEs). Exposure is ubiquitous from industrial food processing, personal care products, cosmetics, and dust. Phthalates and BPA have short half-lives of hours to days, while PBDEs can persist in adipose tissue for months. Abnormal urogenital and neurologic development and thyroid disruption can result. Eating organic food, eating at home, and decreasing processed food intake can reduce exposure.
BPA is used in plastics, canned food liners, cash register receipts, and epoxy resins. Exposure is through inhalation, ingestion, and dermal absorption and affects semen quality, fertilization, placentation, and early reproduction. Limiting the use of plastic containers, not microwaving food in plastic, and avoiding thermal paper cash register receipts can reduce exposure.
Phthalates are synthetically derived and used as plasticizers in personal and medical products. The major source of phthalate exposure is food; exposure causes sperm, egg, and DNA damage. Phthalate avoidance involves replacing plastic bottles with glass or stainless steel, avoiding reheating food in plastic containers, and choosing "fragrance free" products.
PBDEs are used in flame retardants on upholstery, textiles, carpeting, and some electronics. Most PBDEs have been replaced by alternatives; however, their half-life is up to 12 years. Complications caused by PBDEs include thyroid disruption, resulting in abnormal fetal brain development. Avoiding dust and furniture that contain PBDEs, as well as hand washing, reduces exposure risk.
Air pollutants are associated with adverse obstetric outcomes and lower cognitive function in children. Avoiding areas with heavy traffic, staying indoors when air is heavily polluted, and using a HEPA filter in the home can reduce chemicals from air pollution.
Recommendations
The magnitude of the problem that environmental toxicant exposure creates requires health care providers to take action. The table in the publication by Segal and Giudice can be used as a tool that patients can answer first themselves before review by their provider.2 It can be added to your electronic health record and/or patient portal. Even making general comments to raise awareness, asking questions regarding exposure, and making recommendations can be helpful (TABLES 1 and 2). When possible, we also should advocate for public awareness and policy changes that address this significant health issue.


Environmental toxicants are a significant health problem that can be effectively mitigated through patient questions and recommended interventions.
Although we are not able to cover all of the important developments in fertility medicine over the past year, there were 3 important articles published in the past 12 months that we highlight here. First, we discuss an American College of Obstetricians and Gynecologists (ACOG) committee opinion on genetic carrier screening that was reaffirmed in 2019. Second, we explore an interesting retrospective analysis of time-lapse videos and clinical outcomes of more than 10,000 embryos from 8 IVF clinics, across 4 countries. The authors assessed whether a deep learning model could predict the probability of pregnancy with fetal heart from time-lapse videos in the hopes that their research can improve prioritization of the most viable embryo for single embryo transfer. Last, we consider a review of the data on obstetric and reproductive health effects of preconception and prenatal exposure to several environmental toxicants, including heavy metals, endocrine-disrupting chemicals, pesticides, and air pollution.
Preconception genetic carrier screening: Standardize your counseling approach
American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
With the rapid development of advanced and high throughput platforms for DNA sequencing in the past several years, the cost of genetic testing has decreased dramatically. Women's health care providers in general, and fertility specialists in particular, are uniquely positioned to take advantage of these novel and yet affordable technologies by counseling prospective parents during the preconception counseling, or early prenatal period, about the availability of genetic carrier screening and its potential to provide actionable information in a timely manner. The ultimate objective of genetic carrier screening is to enable individuals to make an informed decision regarding their reproductive choices based on their personal values. In a study by Larsen and colleagues, the uptake of genetic carrier screening was significantly higher when offered in the preconception period (68.7%), compared with during pregnancy (35.1%), which highlights the significance of early counseling.1
Based on the Centers for Disease Control and Prevention's Birth/Infant Death Data set, birth defects affect 1 in every 33 (about 3%) of all babies born in the United States each year and account for 20% of infant mortality.2 About 20% of birth defects are caused by single-gene (monogenic) disorders, and although some of these are due to dominant conditions or de novo mutations, a significant proportion are due to autosomal recessive, or X-chromosome linked conditions that are commonly assessed by genetic carrier screening.
ACOG published a committee opinion on "Carrier Screening in the Age of Genomic Medicine" in March 2017, which was reaffirmed in 2019.3
Residual risk. Several points discussed in this document are of paramount importance, including the need for pretest and posttest counseling and consent, as well as a discussion of "residual risk." Newer platforms employ sequencing techniques that potentially can detect most, if not all, of the disease-causing variants in the tested genes, such as the gene for cystic fibrosis and, therefore, have a higher detection rate compared with the older PCR-based techniques for a limited number of specific mutations included in the panel. Due to a variety of technical and biological limitations, however, such as allelic dropouts and the occurrence of de novo mutations, the detection rate is not 100%; there is always a residual risk that needs to be estimated and provided to individuals based on the existing knowledge on frequency of gene, penetrance of phenotype, and prevalence of condition in the general and specific ethnic populations.
Continue to: Expanded vs panethnic screening...
Expanded vs panethnic screening. Furthermore, although sequencing technology has made "expanded carrier screening" for several hundred conditions, simultaneous to and independent of ethnicity and family history, more easily available and affordable, ethnic-specific and panethnic screening for a more limited number of conditions are still acceptable approaches. Having said this, when the first partner screened is identified to be a carrier, his/her reproductive partners must be offered next-generation sequencing to identify less common disease-causing variants.4
A cautionary point to consider when expanded carrier screening panels are requested is the significant variability among commercial laboratories with regard to the conditions included in their panels. In addition, consider the absence of a well-defined or predictable phenotype for some of the included conditions.
Perhaps the most important matter when it comes to genetic carrier screening is to have a standard counseling approach that is persistently followed and offers the opportunity for individuals to know about their genetic testing options and available reproductive choices, including the use of donor gametes, preimplantation genetic testing for monogenic disease (PGT-M, formerly known as preimplantation genetic diagnosis, or PGD), prenatal testing, and pregnancy management options. For couples and/or individuals who decide to proceed with an affected pregnancy, earlier diagnosis can assist with postnatal management.
Medicolegal responsibility. Genetic carrier screening also is of specific relevance to the field of fertility medicine and assisted reproductive technology (ART) as a potential liability issue. Couples and individuals who are undergoing fertility treatment with in vitro fertilization (IVF) for a variety of medical or personal reasons are a specific group that certainly should be offered genetic carrier screening, as they have the option of "adding on" PGT-M (PGD) to their existing treatment plan at a fraction of the cost and treatment burden that would have otherwise been needed if they were not undergoing IVF. After counseling, some individuals and couples may ultimately opt out of genetic carrier screening. The counseling discussion needs to be clearly documented in the medical chart.
The preconception period is the perfect time to have a discussion about genetic carrier screening; it offers the opportunity for timely interventions if desired by the couples or individuals.
Continue to: Artificial intelligence and embryo selection...
Artificial intelligence and embryo selection
With continued improvements in embryo culture conditions and cryopreservation technology, there has been a tremendous amount of interest in developing better methods for embryo selection. These efforts are aimed at encouraging elective single embryo transfer (eSET) for women of all ages, thereby lowering the risk of multiple pregnancy and its associated adverse neonatal and obstetric outcomes—without compromising the pregnancy rates per transfer or lengthening the time to pregnancy.
One of the most extensively studied methods for this purpose is preimplantation genetic testing for aneuploidy (PGT-A, formerly known as PGS), but emerging data from large multicenter randomized clinical trials (RCTs) have again cast significant doubt on PGT-A's efficacy and utility.5 Meanwhile, alternative methods for embryo selection are currently under investigation, including noninvasive PGT-A and morphokinetic assessment of embryo development via analysis of images obtained by time-lapse imaging.
The potential of time-lapse imaging
Despite the initial promising results from time-lapse imaging, subsequent RCTs have not shown a significant clinical benefit.6 However, these early methods of morphokinetic assessment are mainly dependent on the embryologists' subjective assessment of individual static frames and "annotation" of observed spatial and temporal features of embryo development. In addition to being a very time-consuming task, this process is subject to significant interobserver and intraobserver variability.
Considering these limitations, even machine-based algorithms that incorporate these annotations along with such other clinical variables as parental age and prior obstetric history, have a low predictive power for the outcome of embryo transfer, with an area under the curve (AUC) of the ROC curve of 0.65 to 0.74. (An AUC of 0.5 represents completely random prediction and an AUC of 1.0 suggests perfect prediction.)7
A recent study by Tran and colleagues has employed a deep learning (neural network) model to analyze the entire raw time-lapse videos in an automated manner without prior annotation by embryologists. After analysis of 10,638 embryos from 8 different IVF clinics in 4 different countries, they have reported an AUC of 0.93 (95% confidence interval, 0.92-0.94) for prediction of fetal heart rate activity detected at 7 weeks of gestation or beyond. Although these data are very preliminary and have not yet been validated prospectively in larger datasets for live birth, it may herald the beginning of a new era for the automation and standardization of embryo assessment with artificial intelligence—similar to the rapidly increasing role of facial recognition technology for various applications.
Improved standardization of noninvasive embryo selection with growing use of artificial intelligence is a promising new tool to improve the safety and efficacy of ART.
Continue to: Environmental toxicants: The hidden danger...
Environmental toxicants: The hidden danger
Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112:613-621.
We receive news daily about the existential risk to humans of climate change. However, a risk that is likely as serious goes almost unseen by the public and most health care providers. That risk is environmental toxicants.8
More than 80,000 chemicals are registered in the United States, most in the last 75 years. These chemicals are ubiquitous. All of us are continuously exposed to and suffused with these toxicants and their metabolites. Air pollution adds insult to injury. Since this exposure has especially significant implications for fertility, infertility, pregnancy, perinatal health, childhood development, adult diseases, and later generational reproduction, it is imperative that reproductive health professionals take responsibility for helping mitigate this environmental crisis.
The problem is exceptionally complicated
The risks posed by environmental toxicants are much less visible than those for climate change, so the public, policymakers, and providers are largely unaware or may even seem uncaring. Few health professionals have sufficient knowledge to deliver care in this area, know which questions to ask, or have adequate information/medical record tools to assist them in care—and what are the possible interventions?
Addressing risk posed by individual toxicants
Addressing the problem clinically requires asking patients questions about exposure and recommending interventions. Toxicant chemicals include the neurotoxin mercury, which can be addressed by limiting intake of fish, especially certain types.
Lead was used before 1978 in paint, it also was used in gas and in water pipes. People living in older homes may be exposed, as well as those in occupations exposed to lead. Others with lead exposure risk include immigrants from areas without lead regulations and people using pica- or lead-glazed pottery. Lead exposure has been associated with multiple pregnancy complications and permanently impaired intellectual development in children. If lead testing reveals high levels, chelation therapy can help.
Cadmium is a heavy metal used in rechargeable batteries, paint pigment, and plastic production. Exposure results from food intake, smoking, and second-hand smoke. Cadmium accumulates in the liver, kidneys, testes, ovaries, and placenta. Exposure causes itai-itai disease, which is characterized by osteomalacia and renal tubular dysfunction as well as epigenetic changes in placental DNA and damage to the reproductive system. Eating organic food and reducing industrial exposure to cadmium are preventive strategies.
Pesticides are ubiquitous, with 90% of the US population having detectable levels. Exposure during the preconception period can lead to intrauterine growth restriction, low birth weight, subsequent cancers, and other problems. Eating organic food can reduce risk, as can frequent hand washing when exposed to pesticides, using protective gear, and removing shoes in the home.
Endocrine-disrupting chemicals (EDCs) are chemicals that can mimic or block endogenous hormones, which leads to adverse health outcomes. In addition to heavy metals, 3 important EDCs are bisphenol A (BPA), phthalates, and polybrominated diethyl ethers (PBDEs). Exposure is ubiquitous from industrial food processing, personal care products, cosmetics, and dust. Phthalates and BPA have short half-lives of hours to days, while PBDEs can persist in adipose tissue for months. Abnormal urogenital and neurologic development and thyroid disruption can result. Eating organic food, eating at home, and decreasing processed food intake can reduce exposure.
BPA is used in plastics, canned food liners, cash register receipts, and epoxy resins. Exposure is through inhalation, ingestion, and dermal absorption and affects semen quality, fertilization, placentation, and early reproduction. Limiting the use of plastic containers, not microwaving food in plastic, and avoiding thermal paper cash register receipts can reduce exposure.
Phthalates are synthetically derived and used as plasticizers in personal and medical products. The major source of phthalate exposure is food; exposure causes sperm, egg, and DNA damage. Phthalate avoidance involves replacing plastic bottles with glass or stainless steel, avoiding reheating food in plastic containers, and choosing "fragrance free" products.
PBDEs are used in flame retardants on upholstery, textiles, carpeting, and some electronics. Most PBDEs have been replaced by alternatives; however, their half-life is up to 12 years. Complications caused by PBDEs include thyroid disruption, resulting in abnormal fetal brain development. Avoiding dust and furniture that contain PBDEs, as well as hand washing, reduces exposure risk.
Air pollutants are associated with adverse obstetric outcomes and lower cognitive function in children. Avoiding areas with heavy traffic, staying indoors when air is heavily polluted, and using a HEPA filter in the home can reduce chemicals from air pollution.
Recommendations
The magnitude of the problem that environmental toxicant exposure creates requires health care providers to take action. The table in the publication by Segal and Giudice can be used as a tool that patients can answer first themselves before review by their provider.2 It can be added to your electronic health record and/or patient portal. Even making general comments to raise awareness, asking questions regarding exposure, and making recommendations can be helpful (TABLES 1 and 2). When possible, we also should advocate for public awareness and policy changes that address this significant health issue.


Environmental toxicants are a significant health problem that can be effectively mitigated through patient questions and recommended interventions.
- Larsen D, Ma J, Strassberg M, et al. The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn. 2019;39:319-323.
- Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1-30.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
- Gregg AR, Edwards JG. Prenatal genetic carrier screening in the genomic age. Semin Perinatol. 2018;42:303-306.
- Munné S, Kaplan B, Frattarelli JL, et al; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071-1079. e7.
- Goodman LR, Goldberg J, Falcone T, et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-285.e10.
- Blank C, Wildeboer RR, DeCroo I, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111:318- 326.
- The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society for Reproductive Medicine Practice Committee; The University of California, San Francisco Program on Reproductive Health and the Environment. ACOG Committee Opinion No. 575. Exposure to environmental toxic agents. Fertil Steril. 2013;100:931-934.
- Larsen D, Ma J, Strassberg M, et al. The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn. 2019;39:319-323.
- Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1-30.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
- Gregg AR, Edwards JG. Prenatal genetic carrier screening in the genomic age. Semin Perinatol. 2018;42:303-306.
- Munné S, Kaplan B, Frattarelli JL, et al; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071-1079. e7.
- Goodman LR, Goldberg J, Falcone T, et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-285.e10.
- Blank C, Wildeboer RR, DeCroo I, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111:318- 326.
- The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society for Reproductive Medicine Practice Committee; The University of California, San Francisco Program on Reproductive Health and the Environment. ACOG Committee Opinion No. 575. Exposure to environmental toxic agents. Fertil Steril. 2013;100:931-934.