User login
An automated breast ultrasound system is on track to become the first such device with an indication for use as an adjunct to mammography when screening asymptomatic women with dense breasts for breast cancer.
The Food and Drug Administration’s Radiological Devices Panel voted 13-0 that the benefits of U-Systems Inc.’s somo-v automated breast ultrasound system outweigh the risks for breast cancer screening.
During their meeting, the advisory group also voted unanimously that the noninvasive device is safe and effective, despite FDA concerns over whether the firm’s pivotal study data could be generalized to a broader population.
Approval is not guaranteed, but the agency usually follows the advice of its advisory panels.
U-Systems is seeking premarket approval to increase breast cancer detection in asymptomatic women with dense breasts who have not had a previous clinical breast intervention following a negative mammogram screening. Somo-v was 510(k) cleared in 2005 for diagnostic use as an adjunct to x-ray mammography.
A key question the FDA asked the panel was whether the proposed expanded indication – and the way it would be applied in practice – was appropriately reflected in the firm’s pivotal study design. The panel agreed that it was.
The proposed indication for use "is fine the way it’s written," said panel member Dr. Carl D’Orsi, professor of radiology and hematology/oncology at Emory University in Atlanta. "It should not be changed," Dr. D’Orsi said, reflecting the panel’s consensus.
Premarket approval would make somo-v the first ultrasound device available in the United States with a breast cancer screening indication, according to the company. The system is already the only ultrasound device approved for breast cancer screening in Canada and Europe.
Supporting the premarket approval application is a retrospective, multireader study that enrolled 200 subjects at 13 U.S. sites – culled from the firm’s larger SOMO-INSIGHT clinical trial – to evaluate whether digital mammography in combination with the somo-v automated breast ultrasound system (ABUS) is more sensitive in detecting breast cancer in women with dense breast tissue than x-ray mammography screening alone.
The primary end point of the study was to determine radiologist readers’ performance with and without ABUS using a statistical methodology called "receiver operator characteristic" curve analysis. The mean area under the curve (AUC) values were 0.604 for x-ray mammography alone and 0.747 for x-ray mammography plus ABUS.
"Results from our pivotal clinical retrospective reader study demonstrate that use of screening mammography with ABUS provides a substantial, statistically significant clinical improvement in a reader’s ability to detect mammographically negative breast cancers," said CEO Ronald Ho during the meeting.
"Our primary end point was met," added principal investigator Maryellen Giger, Ph.D., professor of radiology at the University of Chicago.
The difference of 0.143 in the AUC values "was statistically significant, given that the lower bound of an estimated 95% confidence interval for the difference in AUC is 0.074," the FDA agreed.
According to the company, mammography’s sensitivity for the detection of breast cancer is 85% in women overall, but the rate is reduced to 65% in women with dense breast tissue. "Dense breast tissue has been proven to conceal malignant lesions, limiting the effectiveness of mammography," Mr. Ho said. Dense breast tissue also increases a woman’s risk of breast cancer by up to four to six times, according to U-Systems.
"What differentiates our device is that we can detect lesions that are missed by mammography ... and we do it in a very fast work flow that’s reproducible and operator independent," the firm stated. The technology is designed to follow the same work flow as a traditional mammography procedure and provide an exam in 15 minutes.
Real-World Management Questioned
The FDA questioned whether the pivotal study’s results could be extrapolated to a real-world population of patients. One hundred sixty-four subjects were used in the study’s primary statistical analysis (133 normal cases chosen at random and 31 cancer cases), with 12 noncancer cases being evaluated by mammography alone.
Patients with prior clinical breast interventions, such as those who had a breast augmentation or breast cancer treatments including biopsy, radiation, lumpectomy, or mastectomy, were excluded from the study. U-Systems said it excluded these patients to eliminate potential bias and/or confounding effects.
But while the firm is seeking approval of somo-v as a screening tool for women with dense breasts "who have not had a previous clinical breast intervention," the FDA argued that in the real world, "patient management that includes an ABUS scan may be considered for patients who have had prior clinical breast intervention."
The panel discussed whether the exclusion of patients with a prior clinical breast intervention should be specified in the indication, and agreed that it should, given the pivotal study design. Additional studies would be needed to remove the restriction, the panel said.
Some panelists expressed concern over whether somo-v use on biopsy patients, for instance, might increase the device’s false-positive rate, given surgical scars’ similarity to malignancies on ultrasound.
Nevertheless, a majority did not seem overly concerned about potential off-label use on patients with prior interventions. "It seems to me that that’s not a big problem," said panel member Dr. Daniel Kopans, professor of radiology at Harvard Medical School, Boston. "If FDA approves the technology even with these limitations, those of us in breast imaging will use it as we see fit. ... I don’t think that [the indication] is a huge limitation."
Operator Training Concerns
The panel also discussed the sponsor’s proposed physician training program, concluding that it is adequate. However, after the panel voted on safety and effectiveness, some members debated whether potential use of the system by nonimaging specialists could adversely impact outcomes and whether controls were needed to restrict use to accredited radiologists.
According to the company, somo-v’s automation is intended to "reduce operator dependence," and the firm claims that "any medical staff member can be trained to operate the ABUS device consistently and reproducibly." But some panel members cautioned that the simplicity could lead to harm in clinical practice.
"Potentially, you could be in a situation where the mammogram is done by breast-imaging specialists, but the ultrasound is done by nonimaging specialists, which could significantly affect results," said panelist Dr. Robert Faulk, a diagnostic radiologist in private practice in Omaha, Neb.
Dr. Kopans agreed, citing the Mammography Quality Standards Act’s radiologist accreditation training and urging that "similar constraints" are needed for somo-v.
But in response, Janine Morris, the FDA’s acting radiologic devices division director, reminded the panelists that the FDA "doesn’t regulate [the] practice of medicine."
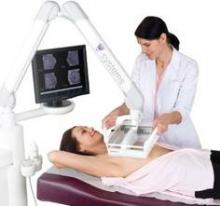
Somo-v relies on proprietary hardware and software, and incorporates a scan head that automatically acquires wide field-of-view breast images while the patient lies supine on a standard exam table.
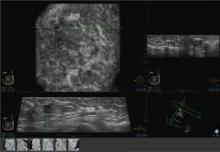
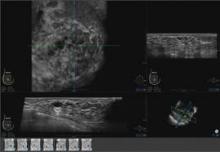
The images are transferred to the system’s somo-VIEWer 3D workstation, which displays the images in a 3D reconstructed coronal view, as well as standard ultrasound formats, including transverse, radial, multislice, sagittal and antiradial views.
Elsevier Global Medical News and "The Gray Sheet" are owned by Elsevier.
An automated breast ultrasound system is on track to become the first such device with an indication for use as an adjunct to mammography when screening asymptomatic women with dense breasts for breast cancer.
The Food and Drug Administration’s Radiological Devices Panel voted 13-0 that the benefits of U-Systems Inc.’s somo-v automated breast ultrasound system outweigh the risks for breast cancer screening.
During their meeting, the advisory group also voted unanimously that the noninvasive device is safe and effective, despite FDA concerns over whether the firm’s pivotal study data could be generalized to a broader population.
Approval is not guaranteed, but the agency usually follows the advice of its advisory panels.
U-Systems is seeking premarket approval to increase breast cancer detection in asymptomatic women with dense breasts who have not had a previous clinical breast intervention following a negative mammogram screening. Somo-v was 510(k) cleared in 2005 for diagnostic use as an adjunct to x-ray mammography.
A key question the FDA asked the panel was whether the proposed expanded indication – and the way it would be applied in practice – was appropriately reflected in the firm’s pivotal study design. The panel agreed that it was.
The proposed indication for use "is fine the way it’s written," said panel member Dr. Carl D’Orsi, professor of radiology and hematology/oncology at Emory University in Atlanta. "It should not be changed," Dr. D’Orsi said, reflecting the panel’s consensus.
Premarket approval would make somo-v the first ultrasound device available in the United States with a breast cancer screening indication, according to the company. The system is already the only ultrasound device approved for breast cancer screening in Canada and Europe.
Supporting the premarket approval application is a retrospective, multireader study that enrolled 200 subjects at 13 U.S. sites – culled from the firm’s larger SOMO-INSIGHT clinical trial – to evaluate whether digital mammography in combination with the somo-v automated breast ultrasound system (ABUS) is more sensitive in detecting breast cancer in women with dense breast tissue than x-ray mammography screening alone.
The primary end point of the study was to determine radiologist readers’ performance with and without ABUS using a statistical methodology called "receiver operator characteristic" curve analysis. The mean area under the curve (AUC) values were 0.604 for x-ray mammography alone and 0.747 for x-ray mammography plus ABUS.
"Results from our pivotal clinical retrospective reader study demonstrate that use of screening mammography with ABUS provides a substantial, statistically significant clinical improvement in a reader’s ability to detect mammographically negative breast cancers," said CEO Ronald Ho during the meeting.
"Our primary end point was met," added principal investigator Maryellen Giger, Ph.D., professor of radiology at the University of Chicago.
The difference of 0.143 in the AUC values "was statistically significant, given that the lower bound of an estimated 95% confidence interval for the difference in AUC is 0.074," the FDA agreed.
According to the company, mammography’s sensitivity for the detection of breast cancer is 85% in women overall, but the rate is reduced to 65% in women with dense breast tissue. "Dense breast tissue has been proven to conceal malignant lesions, limiting the effectiveness of mammography," Mr. Ho said. Dense breast tissue also increases a woman’s risk of breast cancer by up to four to six times, according to U-Systems.
"What differentiates our device is that we can detect lesions that are missed by mammography ... and we do it in a very fast work flow that’s reproducible and operator independent," the firm stated. The technology is designed to follow the same work flow as a traditional mammography procedure and provide an exam in 15 minutes.
Real-World Management Questioned
The FDA questioned whether the pivotal study’s results could be extrapolated to a real-world population of patients. One hundred sixty-four subjects were used in the study’s primary statistical analysis (133 normal cases chosen at random and 31 cancer cases), with 12 noncancer cases being evaluated by mammography alone.
Patients with prior clinical breast interventions, such as those who had a breast augmentation or breast cancer treatments including biopsy, radiation, lumpectomy, or mastectomy, were excluded from the study. U-Systems said it excluded these patients to eliminate potential bias and/or confounding effects.
But while the firm is seeking approval of somo-v as a screening tool for women with dense breasts "who have not had a previous clinical breast intervention," the FDA argued that in the real world, "patient management that includes an ABUS scan may be considered for patients who have had prior clinical breast intervention."
The panel discussed whether the exclusion of patients with a prior clinical breast intervention should be specified in the indication, and agreed that it should, given the pivotal study design. Additional studies would be needed to remove the restriction, the panel said.
Some panelists expressed concern over whether somo-v use on biopsy patients, for instance, might increase the device’s false-positive rate, given surgical scars’ similarity to malignancies on ultrasound.
Nevertheless, a majority did not seem overly concerned about potential off-label use on patients with prior interventions. "It seems to me that that’s not a big problem," said panel member Dr. Daniel Kopans, professor of radiology at Harvard Medical School, Boston. "If FDA approves the technology even with these limitations, those of us in breast imaging will use it as we see fit. ... I don’t think that [the indication] is a huge limitation."
Operator Training Concerns
The panel also discussed the sponsor’s proposed physician training program, concluding that it is adequate. However, after the panel voted on safety and effectiveness, some members debated whether potential use of the system by nonimaging specialists could adversely impact outcomes and whether controls were needed to restrict use to accredited radiologists.
According to the company, somo-v’s automation is intended to "reduce operator dependence," and the firm claims that "any medical staff member can be trained to operate the ABUS device consistently and reproducibly." But some panel members cautioned that the simplicity could lead to harm in clinical practice.
"Potentially, you could be in a situation where the mammogram is done by breast-imaging specialists, but the ultrasound is done by nonimaging specialists, which could significantly affect results," said panelist Dr. Robert Faulk, a diagnostic radiologist in private practice in Omaha, Neb.
Dr. Kopans agreed, citing the Mammography Quality Standards Act’s radiologist accreditation training and urging that "similar constraints" are needed for somo-v.
But in response, Janine Morris, the FDA’s acting radiologic devices division director, reminded the panelists that the FDA "doesn’t regulate [the] practice of medicine."
Somo-v relies on proprietary hardware and software, and incorporates a scan head that automatically acquires wide field-of-view breast images while the patient lies supine on a standard exam table.
The images are transferred to the system’s somo-VIEWer 3D workstation, which displays the images in a 3D reconstructed coronal view, as well as standard ultrasound formats, including transverse, radial, multislice, sagittal and antiradial views.
Elsevier Global Medical News and "The Gray Sheet" are owned by Elsevier.
An automated breast ultrasound system is on track to become the first such device with an indication for use as an adjunct to mammography when screening asymptomatic women with dense breasts for breast cancer.
The Food and Drug Administration’s Radiological Devices Panel voted 13-0 that the benefits of U-Systems Inc.’s somo-v automated breast ultrasound system outweigh the risks for breast cancer screening.
During their meeting, the advisory group also voted unanimously that the noninvasive device is safe and effective, despite FDA concerns over whether the firm’s pivotal study data could be generalized to a broader population.
Approval is not guaranteed, but the agency usually follows the advice of its advisory panels.
U-Systems is seeking premarket approval to increase breast cancer detection in asymptomatic women with dense breasts who have not had a previous clinical breast intervention following a negative mammogram screening. Somo-v was 510(k) cleared in 2005 for diagnostic use as an adjunct to x-ray mammography.
A key question the FDA asked the panel was whether the proposed expanded indication – and the way it would be applied in practice – was appropriately reflected in the firm’s pivotal study design. The panel agreed that it was.
The proposed indication for use "is fine the way it’s written," said panel member Dr. Carl D’Orsi, professor of radiology and hematology/oncology at Emory University in Atlanta. "It should not be changed," Dr. D’Orsi said, reflecting the panel’s consensus.
Premarket approval would make somo-v the first ultrasound device available in the United States with a breast cancer screening indication, according to the company. The system is already the only ultrasound device approved for breast cancer screening in Canada and Europe.
Supporting the premarket approval application is a retrospective, multireader study that enrolled 200 subjects at 13 U.S. sites – culled from the firm’s larger SOMO-INSIGHT clinical trial – to evaluate whether digital mammography in combination with the somo-v automated breast ultrasound system (ABUS) is more sensitive in detecting breast cancer in women with dense breast tissue than x-ray mammography screening alone.
The primary end point of the study was to determine radiologist readers’ performance with and without ABUS using a statistical methodology called "receiver operator characteristic" curve analysis. The mean area under the curve (AUC) values were 0.604 for x-ray mammography alone and 0.747 for x-ray mammography plus ABUS.
"Results from our pivotal clinical retrospective reader study demonstrate that use of screening mammography with ABUS provides a substantial, statistically significant clinical improvement in a reader’s ability to detect mammographically negative breast cancers," said CEO Ronald Ho during the meeting.
"Our primary end point was met," added principal investigator Maryellen Giger, Ph.D., professor of radiology at the University of Chicago.
The difference of 0.143 in the AUC values "was statistically significant, given that the lower bound of an estimated 95% confidence interval for the difference in AUC is 0.074," the FDA agreed.
According to the company, mammography’s sensitivity for the detection of breast cancer is 85% in women overall, but the rate is reduced to 65% in women with dense breast tissue. "Dense breast tissue has been proven to conceal malignant lesions, limiting the effectiveness of mammography," Mr. Ho said. Dense breast tissue also increases a woman’s risk of breast cancer by up to four to six times, according to U-Systems.
"What differentiates our device is that we can detect lesions that are missed by mammography ... and we do it in a very fast work flow that’s reproducible and operator independent," the firm stated. The technology is designed to follow the same work flow as a traditional mammography procedure and provide an exam in 15 minutes.
Real-World Management Questioned
The FDA questioned whether the pivotal study’s results could be extrapolated to a real-world population of patients. One hundred sixty-four subjects were used in the study’s primary statistical analysis (133 normal cases chosen at random and 31 cancer cases), with 12 noncancer cases being evaluated by mammography alone.
Patients with prior clinical breast interventions, such as those who had a breast augmentation or breast cancer treatments including biopsy, radiation, lumpectomy, or mastectomy, were excluded from the study. U-Systems said it excluded these patients to eliminate potential bias and/or confounding effects.
But while the firm is seeking approval of somo-v as a screening tool for women with dense breasts "who have not had a previous clinical breast intervention," the FDA argued that in the real world, "patient management that includes an ABUS scan may be considered for patients who have had prior clinical breast intervention."
The panel discussed whether the exclusion of patients with a prior clinical breast intervention should be specified in the indication, and agreed that it should, given the pivotal study design. Additional studies would be needed to remove the restriction, the panel said.
Some panelists expressed concern over whether somo-v use on biopsy patients, for instance, might increase the device’s false-positive rate, given surgical scars’ similarity to malignancies on ultrasound.
Nevertheless, a majority did not seem overly concerned about potential off-label use on patients with prior interventions. "It seems to me that that’s not a big problem," said panel member Dr. Daniel Kopans, professor of radiology at Harvard Medical School, Boston. "If FDA approves the technology even with these limitations, those of us in breast imaging will use it as we see fit. ... I don’t think that [the indication] is a huge limitation."
Operator Training Concerns
The panel also discussed the sponsor’s proposed physician training program, concluding that it is adequate. However, after the panel voted on safety and effectiveness, some members debated whether potential use of the system by nonimaging specialists could adversely impact outcomes and whether controls were needed to restrict use to accredited radiologists.
According to the company, somo-v’s automation is intended to "reduce operator dependence," and the firm claims that "any medical staff member can be trained to operate the ABUS device consistently and reproducibly." But some panel members cautioned that the simplicity could lead to harm in clinical practice.
"Potentially, you could be in a situation where the mammogram is done by breast-imaging specialists, but the ultrasound is done by nonimaging specialists, which could significantly affect results," said panelist Dr. Robert Faulk, a diagnostic radiologist in private practice in Omaha, Neb.
Dr. Kopans agreed, citing the Mammography Quality Standards Act’s radiologist accreditation training and urging that "similar constraints" are needed for somo-v.
But in response, Janine Morris, the FDA’s acting radiologic devices division director, reminded the panelists that the FDA "doesn’t regulate [the] practice of medicine."
Somo-v relies on proprietary hardware and software, and incorporates a scan head that automatically acquires wide field-of-view breast images while the patient lies supine on a standard exam table.
The images are transferred to the system’s somo-VIEWer 3D workstation, which displays the images in a 3D reconstructed coronal view, as well as standard ultrasound formats, including transverse, radial, multislice, sagittal and antiradial views.
Elsevier Global Medical News and "The Gray Sheet" are owned by Elsevier.