User login
CASE: OBESE PATIENT REQUESTS TOTAL LAPAROSCOPIC HYSTERECTOMY
A 45-year-old woman (G2P2), who delivered both children by cesarean section, schedules an office visit for a complaint of abnormal uterine bleeding. She is obese, with a body mass index (BMI) of 35 kg/m2, and has an enlarged uterus of approximately 14 weeks’ size with minimal descensus. An earlier trial of hormone therapy failed to provide relief. After you counsel her extensively about her treatment options, she elects to undergo total laparoscopic hysterectomy.
What anatomy would you review to help ensure the procedure’s success?
Although the vaginal route is preferred for hysterectomy, total laparoscopic hysterectomy is another minimally invasive option that offers lower morbidity and a shorter hospital stay than the abdominal approach.1 Perhaps more than any other variable, the key to safe, efficient, and effective laparoscopic surgery is a comprehensive knowledge of anatomy. For example, a thorough understanding of the anatomy of the anterior abdominal wall is critical to laparoscopic entry.2,3 Also, pelvic anatomy visualized two-dimensionally under magnification during traditional laparoscopy can look very different than it does during conventional surgery, due to the effects of the pneumoperitoneum, steep Trendelenburg position, and/or the use of uterine manipulators.3
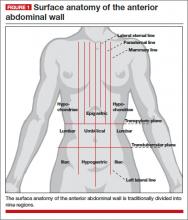
The abdominal cavity is traditionally divided into nine regions. Regardless of the quadrants chosen for laparoscopic access, thorough knowledge of the relevant surface anatomy increases patient safety during surgery (FIGURE 1).
PRIMARY PORT PLACEMENT
Primary port placement, including insertion of the Veress needle, accounts for approximately 40% of laparoscopic complications.4 To help minimize complications, surgeons should ensure that the operating table remains level during placement. As the patient is moved into the Trendelenburg position, the great vessels are more in line with the 45-degree angle that most surgeons use when placing their Veress needle and primary trocar, which can lead to an increased risk of injury. Thus, proper positioning in relationship to anatomy is critical to successful laparoscopic surgery.
Veress or closed technique
Most gynecologists employ the closed method or Veress needle approach to establish pneumoperitoneum.5,6 an initial intraperitoneal pressure below 10 mm Hg, regardless of a woman’s body habitus, height, or age, indicates correct placement of the Veress needle.7,8 Vilos and colleagues demonstrated that Veress intraperitoneal pressure correlates positively with a woman’s weight and BMI and correlates negatively with her parity.8
Hasson or open technique
During the Hasson or open technique, many surgeons use the umbilical ring to gain entry into the abdominal cavity.9 Many view the umbilical ring as a window into the anterior abdominal wall, through which access to the peritoneal cavity can be achieved, but it can also be a site of hernia development.10 The shape of the umbilical ring can vary, appearing round or oval, but it also can be obliterated, slitted, or covered completely by a connecting band, which can result in more difficult laparoscopic entry.10
Palmer’s point
In the 1940s, the French gynecologist Raoul Palmer advocated placing the laparoscope at a point in the left midclavicular line, approximately 3 cm caudal to the costal margin, because visceral-parietal adhesions rarely were found there. Gynecologists still favor this entry site when intra-abdominal adhesions are likely, especially in patients with a history of significant adhesions or multiple previous pelvic surgeries.11 In a study published by Agarwala and colleagues, which included 504 patients with intra-abdominal adhesions, left upper quadrant entry was found to be safe with a complication rate as low as 0.39%.12
If supraumbilical or left upper quadrant port sites are used, the surface anatomy of the spleen and stomach become relevant. The portion of the stomach that is in contact with the abdominal wall is represented roughly by a triangular area extending between the tip of the 10th left costal cartilage, the tip of the ninth right cartilage, and the end of the eighth left costal cartilage.13 The size and shape of the stomach differs by position. Some authors recommend emptying the stomach using a nasogastric or oral gastric tube prior to port insertion to avoid injury.12
The spleen can be mapped using the 10th rib as representing its long axis; vertically, the spleen is situated between the upper border of the ninth and lower border of the 11th ribs.13 In patients without splenic enlargement, the spleen should not be found below the rib cage.
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (Surgical Technique, August 2013)
CASE CONTINUED
On the day of surgery, your patient is brought to the operating room. you use the Veress needle for insufflation. Your opening pressure is 5 mm Hg. You know that an opening pressure of less than 10 mm Hg indicates proper placement, so you continue on to place a 10-mm port. After inserting the primary umbilical port through the umbilicus, you decide to insert secondary ports through lower quadrants. Upon insertion, you note active bleeding at one of the secondary port sites.
How do you proceed?
VASCULAR ANATOMY OF THE ANTERIOR ABDOMINAL WALL
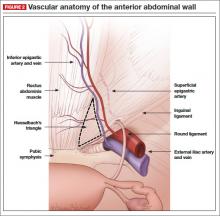
Understanding anterior abdominal wall anatomy and the course of the deep inferior and superficial epigastric vessels is essential to the safe placement of secondary laparoscopic ports. epigastric vessels are the most commonly injured vessels during laparoscopic surgery.14,15 The inferior epigastric vessels originate at the external iliac, immediately above the inguinal ligament. They course medially to the round ligament and travel beneath the lateral third of the rectus abdominis muscle. Using anterior abdominal wall landmarks, the inferior epigastric artery can be identified midway between the anterior superior iliac spine and the pubic symphysis as it travels toward the umbilicus. The inferior epigastric artery also serves as the lateral boundary of Hesselbach’s triangle; the other two boundaries are the lateral edge of the rectus abdominis and the medial aspect of the inguinal ligament (FIGURE 2).13
As the inferior epigastric vessels course cranially, the distance from the midline
decreases. the average distance from the midline at the pubis is approximately
7.5 cm. At the umbilicus, it is approximately 4.6 cm.16,17 The most efficient way to identify laparoscopically the inferior epigastric vessel is to first identify the round ligament. This can be done using a uterine manipulator to deviate the uterus to the contralateral side. Then observe the course of the inferior epigastric vessel just medial to the entry of the round ligament into the inguinal canal. The laparoscopic surgeon can then follow the course of the inferior epigastric vessels to determine the safest location for placement of secondary ports. Transillumination can identify the superficial epigastric vessels, which course within the subcutaneous tissue of the anterior abdominal wall, although it doesn’t identify the deep inferior epigastric vessels that are beneath the lateral third of the rectus muscle. The superficial epigastric vessels follow a course similar to that of the deep inferior epigastric vessels, however, and can serve as a surrogate to guide safe placement of accessory ports.17
Landmarks of the anterior abdominal wall during laparoscopic visualization can also guide placement of secondary ports. The median umbilical fold, which is the peritoneal covering of the umbilical ligament/urachus, travels between the bladder and umbilicus in the midline anterior abdominal wall. Immediately lateral is the medial umbilical fold, which is the peritoneal covering of the obliterated umbilical artery, a branch of the superior vesical artery that comes off the anterior trunk of the internal iliac artery.2 The lateral umbilical folds are lateral to the medial umbilical fold and are the peritoneal covering of the deep inferior epigastric vessels. Identification of these anterior abdominal wall landmarks can assist the surgeon in placing lateral ports so as to avoid injury to these vessels.
Related article: How to avoid major vessel injury during gynecologic laparoscopy Michael Baggish, MD (Surgical Technique, August 2012)
MAJOR RETROPERITONEAL VESSELS
Although major retroperitoneal vessel injury is uncommon, occurring in only 0.3% to 1.0% of laparoscopic surgeries, it has the potential to be catastrophic.18 Therefore, an understanding of the surface anatomy of the major vessels is essential for midline port placement.
The abdominal aorta begins about 4 cm above the transpyloric line and extends to
2 cm inferior and to the left of the umbilicus, or, more accurately, to a point 2 cm left of the middle line on a line that passes through the highest points of the iliac crests. The point of termination of the abdominal aorta corresponds to the level of the fourth lumbar vertebra; a line drawn from it to a point midway between the anterior superior iliac spine and the symphysis pubis indicates the common and external iliac arteries. The common iliac is represented by the upper third of this line and the external iliac, by the remaining two-thirds.13
Related article: How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy Michael Baggish, MD (Surgical Technique, October 2012)
OBESITY AND LAPAROSCOPIC SURGERY
Over two-thirds of the US adult population is now classified as overweight or obese.19 Research has shown that, compared with abdominal hysterectomy, laparoscopic surgery entails a shorter hospital stay, less blood loss, and fewer abdominal wall and wound infections, which are important advantages for this particular population.20
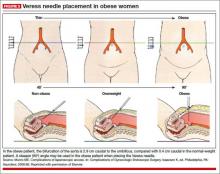
Laparoscopic entry can be particularly challenging in the obese patient. A study by Hurd and colleagues showed that the mean umbilical location was 2.4 cm caudal to the bifurcation of the aorta in the overweight population and 2.9 cm caudal in the obese population.21 Because the bifurcation of the aorta is more cephalad to the umbilicus in overweight and obese patients, the laparoscopic surgeon can introduce the Veress needle at a steeper angle and more perpendicular to the abdominal wall than for a thinner patient (FIGURE 3).
RELEVANT NERVES OF THE ANTERIOR ABDOMINAL WALL
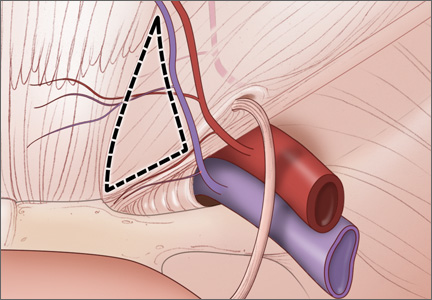
The iliohypogastric and ilioinguinal nerves are also at risk for injury with laterally placed trocars through direct trauma or nerve entrapment. These nerves emerge from the T12 to L1 and L1 to L2 regions, respectively, and course through the muscles of the anterior abdominal wall. Specifically, the iliohypogastric nerve penetrates the fascia of the internal oblique muscle, and the ilioinguinal nerve penetrates the fascia of the transverse abdominus muscle.22 Fascial closure at lateral port sites can also increase the risk of injury to those nerves (FIGURE 4).23
CASE CONTINUED
As you continue your case, you have had to replace your right lower quadrant port several times. During the last insertion, you notice that you have an enlarging abdominal wall hematoma. You suspect that you have injured the inferior epigastric vessel.
How should it be repaired?
HOW TO PREVENT AND REPAIR INJURED DEEP INFERIOR EPIGASTRIC VESSELS
A thorough knowledge of anatomy is the most effective way to prevent these types of injuries. The use of bladeless radially expanding trocars and smooth conical-tip trocars that push the vessels away may result in fewer port-site bleeding complications and injuries than large pyramidal or cutting trocars.24–26 It is important to inspect all ports sites at the end of any laparoscopic procedure because the port itself can tamponade an injured anterior abdominal wall vessel and obscure an injury.
If an injury occurs, leave the trocars in place until a plan for repair is devised. First, start by compressing the bleeding point by moving the cannula against it. Because there are two bleeding ends, the vessels must be sutured cephalad and caudad to the site of injury. The use of electrosurgical desiccation is usually less successful.25 In obese patients we prefer to suture-ligate the bleeder intracorporeally or use a laparoscopic port closure device. In thin and pediatric patients, percutaneous suture ligation can be done easily.
Another option to control bleeding at the cannula site is placement of a Foley catheter to tamponade the vessel using a large balloon placed on tension.27 If abdominal loop sutures are used to control bleeding, the sutures typically are left intact for 8 hours prior to removal.25 If identification of the bleeding point is difficult, percutaneous placement of a suture ligature over a roll of gauze or using a Foley catheter to tamponade the bleeder can be helpful.
CASE RESOLVED
A laparoscopic port closure device is used to suture ligate the bleeding vessel. Hemostasis is achieved and the laparoscopic hysterectomy is completed without further complications.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Share your thoughts by sending a letter to [email protected]. Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- AAGL position statement: Route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1–3.
- Tokar B, Yucel F. Anatomical variations of medial umbilical ligament: clinical significance in laparoscopic exploration of children. Pediatr Surg Int. 2009;25(12):1077–1080.
- Nezhat CH, Nezhat F, Brill AI, Nezhat C. Normal variations of abdominal and pelvic anatomy evaluated at laparoscopy. Obstet Gynecol. 1999;94(2):238–242.
- Fuller J, Ashar BS, Carey-Corrado J. Trocar-associated injuries and fatalities: an analysis of 1,399 reports to the FDA. J Minim Invasive Gynecol. 2005;12(4):302–307.
- Jansen FW, Kolkman W, Bakkum EA, de Kroon CD, Trimbos-Kemper TC, Trimbos JB. Complications of laparoscopy: An inquiry about closed- versus open-entry technique. Am J Obstet Gynecol. 2004;190(3):634–638.
- Yuzpe AA. Pneumoperitoneum needle and trocar injuries in laparoscopy. A survey on possible contributing factors and prevention. J Reprod Med. 1990;35(5):485–490.
- Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10(3):415–420.
- Vilos AG, Vilos GA, Abu-Rafea B, Hollett-Caines J, Al-Omran M. Effect of body habitus and parity on the initial Veress intraperitoneal CO2 insufflation pressure during laparoscopic access in women. J Minim Invasive Gynecol. 2006;13(2):108–113.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–887.
- Oh CS, Won HS, Kwon CH, Chung IH. Morphologic variations of the umbilical ring, umbilical ligaments and ligamentum teres hepatis. Yonsei Med J. 2008;49(6):1004–1007.
- Chandler JG, Corson SL, Way LW. Three spectra of laparoscopic entry access injuries. J Am Coll Surg. 2001;192(4):478–491.
- Agarwala N, Liu CY. Safe entry techniques during laparoscopy: left upper quadrant entry using the ninth intercostal space—a review of 918 procedures. J Minim Invasive Gynecol. 2005;12(1):55–61.
- Williams PL, Warwick R, eds. Gray’s anatomy. 36th ed. Philadelphia, PA: Churchill Livingstone; 1980.
- Hurd WW, Pearl ML, DeLancey JO, Quint EH, Garnett B, Bude RO. Laparoscopic injury of abdominal wall blood vessels: A report of three cases. Obstet Gynecol. 1993;82(4 Pt 2 Suppl):673–676.
- Lin P, Grow DR. Complications of laparoscopy. Strategies for prevention and cure. Obstet Gynecol Clin North Am. 1999;26(1):23–38, v.
- Saber AA, Meslemani AM, Davis R, Pimentel R. Safety zones for anterior abdominal wall entry during laparoscopy: A CT scan mapping of epigastric vessels. Ann Surg. 2004;239(2):182–185.
- Hurd WW, Bude RO, DeLancey JO, Newman JS. The location of abdominal wall blood vessels in relationship to abdominal landmarks apparent at laparoscopy. Am J Obstet Gynecol. 1994;171(3):642–646.
- Sandadi S, Johannigman JA, Wong VL, Blebea J, Altose MD, Hurd WW. Recognition and management of major vessel injury during laparoscopy. J minim invasive gynecol. 2010;17(6):692–702.
- Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol rev. 2007;29:6–28.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane database syst rev. 2009;(3):CD003677.
- Hurd WW, Bude RO, DeLancey JO, Pearl ML. The relationship of the umbilicus to the aortic bifurcation: implications for laparoscopic technique. Obstet gynecol. 1992;80(1):48–51.
- Clarke-Pearson DL, Geller EJ. Complications of hysterectomy. Obstet gynecol. 2013;121(3):654–673.
- Shin JH, Howard FM. Abdominal wall nerve injury during laparoscopic gynecologic surgery: incidence, risk factors, and treatment outcomes. J minim invasive gynecol. 2012;19(4):448–453.
- Bhoyrul S, Payne J, Steffes B, Swanstrom L, Way LW. A randomized prospective study of radially expanding trocars in laparoscopic surgery. J gastrointest surg. 2000;4(4):392–397.
- Shirk GJ, Johns A, Redwine DB. Complications of laparoscopic surgery: How to avoid them and how to repair them. J minim invasive gynecol. 2006;13(4):352–361.
- Tews G, Arzt W, Bohaumilitzky T, Füreder R, Frölich H. Significant reduction of operational risk in laparoscopy through the use of a new blunt trocar. Surg gynecol obstet. 1991;173(1):67–68.
- Najmaldin A, Guillou P. A guide to laparoscopic surgery. 1st ed. Wiley-Blackwell; 1998.
CASE: OBESE PATIENT REQUESTS TOTAL LAPAROSCOPIC HYSTERECTOMY
A 45-year-old woman (G2P2), who delivered both children by cesarean section, schedules an office visit for a complaint of abnormal uterine bleeding. She is obese, with a body mass index (BMI) of 35 kg/m2, and has an enlarged uterus of approximately 14 weeks’ size with minimal descensus. An earlier trial of hormone therapy failed to provide relief. After you counsel her extensively about her treatment options, she elects to undergo total laparoscopic hysterectomy.
What anatomy would you review to help ensure the procedure’s success?
Although the vaginal route is preferred for hysterectomy, total laparoscopic hysterectomy is another minimally invasive option that offers lower morbidity and a shorter hospital stay than the abdominal approach.1 Perhaps more than any other variable, the key to safe, efficient, and effective laparoscopic surgery is a comprehensive knowledge of anatomy. For example, a thorough understanding of the anatomy of the anterior abdominal wall is critical to laparoscopic entry.2,3 Also, pelvic anatomy visualized two-dimensionally under magnification during traditional laparoscopy can look very different than it does during conventional surgery, due to the effects of the pneumoperitoneum, steep Trendelenburg position, and/or the use of uterine manipulators.3
The abdominal cavity is traditionally divided into nine regions. Regardless of the quadrants chosen for laparoscopic access, thorough knowledge of the relevant surface anatomy increases patient safety during surgery (FIGURE 1).
PRIMARY PORT PLACEMENT
Primary port placement, including insertion of the Veress needle, accounts for approximately 40% of laparoscopic complications.4 To help minimize complications, surgeons should ensure that the operating table remains level during placement. As the patient is moved into the Trendelenburg position, the great vessels are more in line with the 45-degree angle that most surgeons use when placing their Veress needle and primary trocar, which can lead to an increased risk of injury. Thus, proper positioning in relationship to anatomy is critical to successful laparoscopic surgery.
Veress or closed technique
Most gynecologists employ the closed method or Veress needle approach to establish pneumoperitoneum.5,6 an initial intraperitoneal pressure below 10 mm Hg, regardless of a woman’s body habitus, height, or age, indicates correct placement of the Veress needle.7,8 Vilos and colleagues demonstrated that Veress intraperitoneal pressure correlates positively with a woman’s weight and BMI and correlates negatively with her parity.8
Hasson or open technique
During the Hasson or open technique, many surgeons use the umbilical ring to gain entry into the abdominal cavity.9 Many view the umbilical ring as a window into the anterior abdominal wall, through which access to the peritoneal cavity can be achieved, but it can also be a site of hernia development.10 The shape of the umbilical ring can vary, appearing round or oval, but it also can be obliterated, slitted, or covered completely by a connecting band, which can result in more difficult laparoscopic entry.10
Palmer’s point
In the 1940s, the French gynecologist Raoul Palmer advocated placing the laparoscope at a point in the left midclavicular line, approximately 3 cm caudal to the costal margin, because visceral-parietal adhesions rarely were found there. Gynecologists still favor this entry site when intra-abdominal adhesions are likely, especially in patients with a history of significant adhesions or multiple previous pelvic surgeries.11 In a study published by Agarwala and colleagues, which included 504 patients with intra-abdominal adhesions, left upper quadrant entry was found to be safe with a complication rate as low as 0.39%.12
If supraumbilical or left upper quadrant port sites are used, the surface anatomy of the spleen and stomach become relevant. The portion of the stomach that is in contact with the abdominal wall is represented roughly by a triangular area extending between the tip of the 10th left costal cartilage, the tip of the ninth right cartilage, and the end of the eighth left costal cartilage.13 The size and shape of the stomach differs by position. Some authors recommend emptying the stomach using a nasogastric or oral gastric tube prior to port insertion to avoid injury.12
The spleen can be mapped using the 10th rib as representing its long axis; vertically, the spleen is situated between the upper border of the ninth and lower border of the 11th ribs.13 In patients without splenic enlargement, the spleen should not be found below the rib cage.
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (Surgical Technique, August 2013)
CASE CONTINUED
On the day of surgery, your patient is brought to the operating room. you use the Veress needle for insufflation. Your opening pressure is 5 mm Hg. You know that an opening pressure of less than 10 mm Hg indicates proper placement, so you continue on to place a 10-mm port. After inserting the primary umbilical port through the umbilicus, you decide to insert secondary ports through lower quadrants. Upon insertion, you note active bleeding at one of the secondary port sites.
How do you proceed?
VASCULAR ANATOMY OF THE ANTERIOR ABDOMINAL WALL
Understanding anterior abdominal wall anatomy and the course of the deep inferior and superficial epigastric vessels is essential to the safe placement of secondary laparoscopic ports. epigastric vessels are the most commonly injured vessels during laparoscopic surgery.14,15 The inferior epigastric vessels originate at the external iliac, immediately above the inguinal ligament. They course medially to the round ligament and travel beneath the lateral third of the rectus abdominis muscle. Using anterior abdominal wall landmarks, the inferior epigastric artery can be identified midway between the anterior superior iliac spine and the pubic symphysis as it travels toward the umbilicus. The inferior epigastric artery also serves as the lateral boundary of Hesselbach’s triangle; the other two boundaries are the lateral edge of the rectus abdominis and the medial aspect of the inguinal ligament (FIGURE 2).13
As the inferior epigastric vessels course cranially, the distance from the midline
decreases. the average distance from the midline at the pubis is approximately
7.5 cm. At the umbilicus, it is approximately 4.6 cm.16,17 The most efficient way to identify laparoscopically the inferior epigastric vessel is to first identify the round ligament. This can be done using a uterine manipulator to deviate the uterus to the contralateral side. Then observe the course of the inferior epigastric vessel just medial to the entry of the round ligament into the inguinal canal. The laparoscopic surgeon can then follow the course of the inferior epigastric vessels to determine the safest location for placement of secondary ports. Transillumination can identify the superficial epigastric vessels, which course within the subcutaneous tissue of the anterior abdominal wall, although it doesn’t identify the deep inferior epigastric vessels that are beneath the lateral third of the rectus muscle. The superficial epigastric vessels follow a course similar to that of the deep inferior epigastric vessels, however, and can serve as a surrogate to guide safe placement of accessory ports.17
Landmarks of the anterior abdominal wall during laparoscopic visualization can also guide placement of secondary ports. The median umbilical fold, which is the peritoneal covering of the umbilical ligament/urachus, travels between the bladder and umbilicus in the midline anterior abdominal wall. Immediately lateral is the medial umbilical fold, which is the peritoneal covering of the obliterated umbilical artery, a branch of the superior vesical artery that comes off the anterior trunk of the internal iliac artery.2 The lateral umbilical folds are lateral to the medial umbilical fold and are the peritoneal covering of the deep inferior epigastric vessels. Identification of these anterior abdominal wall landmarks can assist the surgeon in placing lateral ports so as to avoid injury to these vessels.
Related article: How to avoid major vessel injury during gynecologic laparoscopy Michael Baggish, MD (Surgical Technique, August 2012)
MAJOR RETROPERITONEAL VESSELS
Although major retroperitoneal vessel injury is uncommon, occurring in only 0.3% to 1.0% of laparoscopic surgeries, it has the potential to be catastrophic.18 Therefore, an understanding of the surface anatomy of the major vessels is essential for midline port placement.
The abdominal aorta begins about 4 cm above the transpyloric line and extends to
2 cm inferior and to the left of the umbilicus, or, more accurately, to a point 2 cm left of the middle line on a line that passes through the highest points of the iliac crests. The point of termination of the abdominal aorta corresponds to the level of the fourth lumbar vertebra; a line drawn from it to a point midway between the anterior superior iliac spine and the symphysis pubis indicates the common and external iliac arteries. The common iliac is represented by the upper third of this line and the external iliac, by the remaining two-thirds.13
Related article: How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy Michael Baggish, MD (Surgical Technique, October 2012)
OBESITY AND LAPAROSCOPIC SURGERY
Over two-thirds of the US adult population is now classified as overweight or obese.19 Research has shown that, compared with abdominal hysterectomy, laparoscopic surgery entails a shorter hospital stay, less blood loss, and fewer abdominal wall and wound infections, which are important advantages for this particular population.20
Laparoscopic entry can be particularly challenging in the obese patient. A study by Hurd and colleagues showed that the mean umbilical location was 2.4 cm caudal to the bifurcation of the aorta in the overweight population and 2.9 cm caudal in the obese population.21 Because the bifurcation of the aorta is more cephalad to the umbilicus in overweight and obese patients, the laparoscopic surgeon can introduce the Veress needle at a steeper angle and more perpendicular to the abdominal wall than for a thinner patient (FIGURE 3).
RELEVANT NERVES OF THE ANTERIOR ABDOMINAL WALL
The iliohypogastric and ilioinguinal nerves are also at risk for injury with laterally placed trocars through direct trauma or nerve entrapment. These nerves emerge from the T12 to L1 and L1 to L2 regions, respectively, and course through the muscles of the anterior abdominal wall. Specifically, the iliohypogastric nerve penetrates the fascia of the internal oblique muscle, and the ilioinguinal nerve penetrates the fascia of the transverse abdominus muscle.22 Fascial closure at lateral port sites can also increase the risk of injury to those nerves (FIGURE 4).23
CASE CONTINUED
As you continue your case, you have had to replace your right lower quadrant port several times. During the last insertion, you notice that you have an enlarging abdominal wall hematoma. You suspect that you have injured the inferior epigastric vessel.
How should it be repaired?
HOW TO PREVENT AND REPAIR INJURED DEEP INFERIOR EPIGASTRIC VESSELS
A thorough knowledge of anatomy is the most effective way to prevent these types of injuries. The use of bladeless radially expanding trocars and smooth conical-tip trocars that push the vessels away may result in fewer port-site bleeding complications and injuries than large pyramidal or cutting trocars.24–26 It is important to inspect all ports sites at the end of any laparoscopic procedure because the port itself can tamponade an injured anterior abdominal wall vessel and obscure an injury.
If an injury occurs, leave the trocars in place until a plan for repair is devised. First, start by compressing the bleeding point by moving the cannula against it. Because there are two bleeding ends, the vessels must be sutured cephalad and caudad to the site of injury. The use of electrosurgical desiccation is usually less successful.25 In obese patients we prefer to suture-ligate the bleeder intracorporeally or use a laparoscopic port closure device. In thin and pediatric patients, percutaneous suture ligation can be done easily.
Another option to control bleeding at the cannula site is placement of a Foley catheter to tamponade the vessel using a large balloon placed on tension.27 If abdominal loop sutures are used to control bleeding, the sutures typically are left intact for 8 hours prior to removal.25 If identification of the bleeding point is difficult, percutaneous placement of a suture ligature over a roll of gauze or using a Foley catheter to tamponade the bleeder can be helpful.
CASE RESOLVED
A laparoscopic port closure device is used to suture ligate the bleeding vessel. Hemostasis is achieved and the laparoscopic hysterectomy is completed without further complications.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Share your thoughts by sending a letter to [email protected]. Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
CASE: OBESE PATIENT REQUESTS TOTAL LAPAROSCOPIC HYSTERECTOMY
A 45-year-old woman (G2P2), who delivered both children by cesarean section, schedules an office visit for a complaint of abnormal uterine bleeding. She is obese, with a body mass index (BMI) of 35 kg/m2, and has an enlarged uterus of approximately 14 weeks’ size with minimal descensus. An earlier trial of hormone therapy failed to provide relief. After you counsel her extensively about her treatment options, she elects to undergo total laparoscopic hysterectomy.
What anatomy would you review to help ensure the procedure’s success?
Although the vaginal route is preferred for hysterectomy, total laparoscopic hysterectomy is another minimally invasive option that offers lower morbidity and a shorter hospital stay than the abdominal approach.1 Perhaps more than any other variable, the key to safe, efficient, and effective laparoscopic surgery is a comprehensive knowledge of anatomy. For example, a thorough understanding of the anatomy of the anterior abdominal wall is critical to laparoscopic entry.2,3 Also, pelvic anatomy visualized two-dimensionally under magnification during traditional laparoscopy can look very different than it does during conventional surgery, due to the effects of the pneumoperitoneum, steep Trendelenburg position, and/or the use of uterine manipulators.3
The abdominal cavity is traditionally divided into nine regions. Regardless of the quadrants chosen for laparoscopic access, thorough knowledge of the relevant surface anatomy increases patient safety during surgery (FIGURE 1).
PRIMARY PORT PLACEMENT
Primary port placement, including insertion of the Veress needle, accounts for approximately 40% of laparoscopic complications.4 To help minimize complications, surgeons should ensure that the operating table remains level during placement. As the patient is moved into the Trendelenburg position, the great vessels are more in line with the 45-degree angle that most surgeons use when placing their Veress needle and primary trocar, which can lead to an increased risk of injury. Thus, proper positioning in relationship to anatomy is critical to successful laparoscopic surgery.
Veress or closed technique
Most gynecologists employ the closed method or Veress needle approach to establish pneumoperitoneum.5,6 an initial intraperitoneal pressure below 10 mm Hg, regardless of a woman’s body habitus, height, or age, indicates correct placement of the Veress needle.7,8 Vilos and colleagues demonstrated that Veress intraperitoneal pressure correlates positively with a woman’s weight and BMI and correlates negatively with her parity.8
Hasson or open technique
During the Hasson or open technique, many surgeons use the umbilical ring to gain entry into the abdominal cavity.9 Many view the umbilical ring as a window into the anterior abdominal wall, through which access to the peritoneal cavity can be achieved, but it can also be a site of hernia development.10 The shape of the umbilical ring can vary, appearing round or oval, but it also can be obliterated, slitted, or covered completely by a connecting band, which can result in more difficult laparoscopic entry.10
Palmer’s point
In the 1940s, the French gynecologist Raoul Palmer advocated placing the laparoscope at a point in the left midclavicular line, approximately 3 cm caudal to the costal margin, because visceral-parietal adhesions rarely were found there. Gynecologists still favor this entry site when intra-abdominal adhesions are likely, especially in patients with a history of significant adhesions or multiple previous pelvic surgeries.11 In a study published by Agarwala and colleagues, which included 504 patients with intra-abdominal adhesions, left upper quadrant entry was found to be safe with a complication rate as low as 0.39%.12
If supraumbilical or left upper quadrant port sites are used, the surface anatomy of the spleen and stomach become relevant. The portion of the stomach that is in contact with the abdominal wall is represented roughly by a triangular area extending between the tip of the 10th left costal cartilage, the tip of the ninth right cartilage, and the end of the eighth left costal cartilage.13 The size and shape of the stomach differs by position. Some authors recommend emptying the stomach using a nasogastric or oral gastric tube prior to port insertion to avoid injury.12
The spleen can be mapped using the 10th rib as representing its long axis; vertically, the spleen is situated between the upper border of the ninth and lower border of the 11th ribs.13 In patients without splenic enlargement, the spleen should not be found below the rib cage.
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (Surgical Technique, August 2013)
CASE CONTINUED
On the day of surgery, your patient is brought to the operating room. you use the Veress needle for insufflation. Your opening pressure is 5 mm Hg. You know that an opening pressure of less than 10 mm Hg indicates proper placement, so you continue on to place a 10-mm port. After inserting the primary umbilical port through the umbilicus, you decide to insert secondary ports through lower quadrants. Upon insertion, you note active bleeding at one of the secondary port sites.
How do you proceed?
VASCULAR ANATOMY OF THE ANTERIOR ABDOMINAL WALL
Understanding anterior abdominal wall anatomy and the course of the deep inferior and superficial epigastric vessels is essential to the safe placement of secondary laparoscopic ports. epigastric vessels are the most commonly injured vessels during laparoscopic surgery.14,15 The inferior epigastric vessels originate at the external iliac, immediately above the inguinal ligament. They course medially to the round ligament and travel beneath the lateral third of the rectus abdominis muscle. Using anterior abdominal wall landmarks, the inferior epigastric artery can be identified midway between the anterior superior iliac spine and the pubic symphysis as it travels toward the umbilicus. The inferior epigastric artery also serves as the lateral boundary of Hesselbach’s triangle; the other two boundaries are the lateral edge of the rectus abdominis and the medial aspect of the inguinal ligament (FIGURE 2).13
As the inferior epigastric vessels course cranially, the distance from the midline
decreases. the average distance from the midline at the pubis is approximately
7.5 cm. At the umbilicus, it is approximately 4.6 cm.16,17 The most efficient way to identify laparoscopically the inferior epigastric vessel is to first identify the round ligament. This can be done using a uterine manipulator to deviate the uterus to the contralateral side. Then observe the course of the inferior epigastric vessel just medial to the entry of the round ligament into the inguinal canal. The laparoscopic surgeon can then follow the course of the inferior epigastric vessels to determine the safest location for placement of secondary ports. Transillumination can identify the superficial epigastric vessels, which course within the subcutaneous tissue of the anterior abdominal wall, although it doesn’t identify the deep inferior epigastric vessels that are beneath the lateral third of the rectus muscle. The superficial epigastric vessels follow a course similar to that of the deep inferior epigastric vessels, however, and can serve as a surrogate to guide safe placement of accessory ports.17
Landmarks of the anterior abdominal wall during laparoscopic visualization can also guide placement of secondary ports. The median umbilical fold, which is the peritoneal covering of the umbilical ligament/urachus, travels between the bladder and umbilicus in the midline anterior abdominal wall. Immediately lateral is the medial umbilical fold, which is the peritoneal covering of the obliterated umbilical artery, a branch of the superior vesical artery that comes off the anterior trunk of the internal iliac artery.2 The lateral umbilical folds are lateral to the medial umbilical fold and are the peritoneal covering of the deep inferior epigastric vessels. Identification of these anterior abdominal wall landmarks can assist the surgeon in placing lateral ports so as to avoid injury to these vessels.
Related article: How to avoid major vessel injury during gynecologic laparoscopy Michael Baggish, MD (Surgical Technique, August 2012)
MAJOR RETROPERITONEAL VESSELS
Although major retroperitoneal vessel injury is uncommon, occurring in only 0.3% to 1.0% of laparoscopic surgeries, it has the potential to be catastrophic.18 Therefore, an understanding of the surface anatomy of the major vessels is essential for midline port placement.
The abdominal aorta begins about 4 cm above the transpyloric line and extends to
2 cm inferior and to the left of the umbilicus, or, more accurately, to a point 2 cm left of the middle line on a line that passes through the highest points of the iliac crests. The point of termination of the abdominal aorta corresponds to the level of the fourth lumbar vertebra; a line drawn from it to a point midway between the anterior superior iliac spine and the symphysis pubis indicates the common and external iliac arteries. The common iliac is represented by the upper third of this line and the external iliac, by the remaining two-thirds.13
Related article: How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy Michael Baggish, MD (Surgical Technique, October 2012)
OBESITY AND LAPAROSCOPIC SURGERY
Over two-thirds of the US adult population is now classified as overweight or obese.19 Research has shown that, compared with abdominal hysterectomy, laparoscopic surgery entails a shorter hospital stay, less blood loss, and fewer abdominal wall and wound infections, which are important advantages for this particular population.20
Laparoscopic entry can be particularly challenging in the obese patient. A study by Hurd and colleagues showed that the mean umbilical location was 2.4 cm caudal to the bifurcation of the aorta in the overweight population and 2.9 cm caudal in the obese population.21 Because the bifurcation of the aorta is more cephalad to the umbilicus in overweight and obese patients, the laparoscopic surgeon can introduce the Veress needle at a steeper angle and more perpendicular to the abdominal wall than for a thinner patient (FIGURE 3).
RELEVANT NERVES OF THE ANTERIOR ABDOMINAL WALL
The iliohypogastric and ilioinguinal nerves are also at risk for injury with laterally placed trocars through direct trauma or nerve entrapment. These nerves emerge from the T12 to L1 and L1 to L2 regions, respectively, and course through the muscles of the anterior abdominal wall. Specifically, the iliohypogastric nerve penetrates the fascia of the internal oblique muscle, and the ilioinguinal nerve penetrates the fascia of the transverse abdominus muscle.22 Fascial closure at lateral port sites can also increase the risk of injury to those nerves (FIGURE 4).23
CASE CONTINUED
As you continue your case, you have had to replace your right lower quadrant port several times. During the last insertion, you notice that you have an enlarging abdominal wall hematoma. You suspect that you have injured the inferior epigastric vessel.
How should it be repaired?
HOW TO PREVENT AND REPAIR INJURED DEEP INFERIOR EPIGASTRIC VESSELS
A thorough knowledge of anatomy is the most effective way to prevent these types of injuries. The use of bladeless radially expanding trocars and smooth conical-tip trocars that push the vessels away may result in fewer port-site bleeding complications and injuries than large pyramidal or cutting trocars.24–26 It is important to inspect all ports sites at the end of any laparoscopic procedure because the port itself can tamponade an injured anterior abdominal wall vessel and obscure an injury.
If an injury occurs, leave the trocars in place until a plan for repair is devised. First, start by compressing the bleeding point by moving the cannula against it. Because there are two bleeding ends, the vessels must be sutured cephalad and caudad to the site of injury. The use of electrosurgical desiccation is usually less successful.25 In obese patients we prefer to suture-ligate the bleeder intracorporeally or use a laparoscopic port closure device. In thin and pediatric patients, percutaneous suture ligation can be done easily.
Another option to control bleeding at the cannula site is placement of a Foley catheter to tamponade the vessel using a large balloon placed on tension.27 If abdominal loop sutures are used to control bleeding, the sutures typically are left intact for 8 hours prior to removal.25 If identification of the bleeding point is difficult, percutaneous placement of a suture ligature over a roll of gauze or using a Foley catheter to tamponade the bleeder can be helpful.
CASE RESOLVED
A laparoscopic port closure device is used to suture ligate the bleeding vessel. Hemostasis is achieved and the laparoscopic hysterectomy is completed without further complications.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Share your thoughts by sending a letter to [email protected]. Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- AAGL position statement: Route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1–3.
- Tokar B, Yucel F. Anatomical variations of medial umbilical ligament: clinical significance in laparoscopic exploration of children. Pediatr Surg Int. 2009;25(12):1077–1080.
- Nezhat CH, Nezhat F, Brill AI, Nezhat C. Normal variations of abdominal and pelvic anatomy evaluated at laparoscopy. Obstet Gynecol. 1999;94(2):238–242.
- Fuller J, Ashar BS, Carey-Corrado J. Trocar-associated injuries and fatalities: an analysis of 1,399 reports to the FDA. J Minim Invasive Gynecol. 2005;12(4):302–307.
- Jansen FW, Kolkman W, Bakkum EA, de Kroon CD, Trimbos-Kemper TC, Trimbos JB. Complications of laparoscopy: An inquiry about closed- versus open-entry technique. Am J Obstet Gynecol. 2004;190(3):634–638.
- Yuzpe AA. Pneumoperitoneum needle and trocar injuries in laparoscopy. A survey on possible contributing factors and prevention. J Reprod Med. 1990;35(5):485–490.
- Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10(3):415–420.
- Vilos AG, Vilos GA, Abu-Rafea B, Hollett-Caines J, Al-Omran M. Effect of body habitus and parity on the initial Veress intraperitoneal CO2 insufflation pressure during laparoscopic access in women. J Minim Invasive Gynecol. 2006;13(2):108–113.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–887.
- Oh CS, Won HS, Kwon CH, Chung IH. Morphologic variations of the umbilical ring, umbilical ligaments and ligamentum teres hepatis. Yonsei Med J. 2008;49(6):1004–1007.
- Chandler JG, Corson SL, Way LW. Three spectra of laparoscopic entry access injuries. J Am Coll Surg. 2001;192(4):478–491.
- Agarwala N, Liu CY. Safe entry techniques during laparoscopy: left upper quadrant entry using the ninth intercostal space—a review of 918 procedures. J Minim Invasive Gynecol. 2005;12(1):55–61.
- Williams PL, Warwick R, eds. Gray’s anatomy. 36th ed. Philadelphia, PA: Churchill Livingstone; 1980.
- Hurd WW, Pearl ML, DeLancey JO, Quint EH, Garnett B, Bude RO. Laparoscopic injury of abdominal wall blood vessels: A report of three cases. Obstet Gynecol. 1993;82(4 Pt 2 Suppl):673–676.
- Lin P, Grow DR. Complications of laparoscopy. Strategies for prevention and cure. Obstet Gynecol Clin North Am. 1999;26(1):23–38, v.
- Saber AA, Meslemani AM, Davis R, Pimentel R. Safety zones for anterior abdominal wall entry during laparoscopy: A CT scan mapping of epigastric vessels. Ann Surg. 2004;239(2):182–185.
- Hurd WW, Bude RO, DeLancey JO, Newman JS. The location of abdominal wall blood vessels in relationship to abdominal landmarks apparent at laparoscopy. Am J Obstet Gynecol. 1994;171(3):642–646.
- Sandadi S, Johannigman JA, Wong VL, Blebea J, Altose MD, Hurd WW. Recognition and management of major vessel injury during laparoscopy. J minim invasive gynecol. 2010;17(6):692–702.
- Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol rev. 2007;29:6–28.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane database syst rev. 2009;(3):CD003677.
- Hurd WW, Bude RO, DeLancey JO, Pearl ML. The relationship of the umbilicus to the aortic bifurcation: implications for laparoscopic technique. Obstet gynecol. 1992;80(1):48–51.
- Clarke-Pearson DL, Geller EJ. Complications of hysterectomy. Obstet gynecol. 2013;121(3):654–673.
- Shin JH, Howard FM. Abdominal wall nerve injury during laparoscopic gynecologic surgery: incidence, risk factors, and treatment outcomes. J minim invasive gynecol. 2012;19(4):448–453.
- Bhoyrul S, Payne J, Steffes B, Swanstrom L, Way LW. A randomized prospective study of radially expanding trocars in laparoscopic surgery. J gastrointest surg. 2000;4(4):392–397.
- Shirk GJ, Johns A, Redwine DB. Complications of laparoscopic surgery: How to avoid them and how to repair them. J minim invasive gynecol. 2006;13(4):352–361.
- Tews G, Arzt W, Bohaumilitzky T, Füreder R, Frölich H. Significant reduction of operational risk in laparoscopy through the use of a new blunt trocar. Surg gynecol obstet. 1991;173(1):67–68.
- Najmaldin A, Guillou P. A guide to laparoscopic surgery. 1st ed. Wiley-Blackwell; 1998.
- AAGL position statement: Route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1–3.
- Tokar B, Yucel F. Anatomical variations of medial umbilical ligament: clinical significance in laparoscopic exploration of children. Pediatr Surg Int. 2009;25(12):1077–1080.
- Nezhat CH, Nezhat F, Brill AI, Nezhat C. Normal variations of abdominal and pelvic anatomy evaluated at laparoscopy. Obstet Gynecol. 1999;94(2):238–242.
- Fuller J, Ashar BS, Carey-Corrado J. Trocar-associated injuries and fatalities: an analysis of 1,399 reports to the FDA. J Minim Invasive Gynecol. 2005;12(4):302–307.
- Jansen FW, Kolkman W, Bakkum EA, de Kroon CD, Trimbos-Kemper TC, Trimbos JB. Complications of laparoscopy: An inquiry about closed- versus open-entry technique. Am J Obstet Gynecol. 2004;190(3):634–638.
- Yuzpe AA. Pneumoperitoneum needle and trocar injuries in laparoscopy. A survey on possible contributing factors and prevention. J Reprod Med. 1990;35(5):485–490.
- Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10(3):415–420.
- Vilos AG, Vilos GA, Abu-Rafea B, Hollett-Caines J, Al-Omran M. Effect of body habitus and parity on the initial Veress intraperitoneal CO2 insufflation pressure during laparoscopic access in women. J Minim Invasive Gynecol. 2006;13(2):108–113.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–887.
- Oh CS, Won HS, Kwon CH, Chung IH. Morphologic variations of the umbilical ring, umbilical ligaments and ligamentum teres hepatis. Yonsei Med J. 2008;49(6):1004–1007.
- Chandler JG, Corson SL, Way LW. Three spectra of laparoscopic entry access injuries. J Am Coll Surg. 2001;192(4):478–491.
- Agarwala N, Liu CY. Safe entry techniques during laparoscopy: left upper quadrant entry using the ninth intercostal space—a review of 918 procedures. J Minim Invasive Gynecol. 2005;12(1):55–61.
- Williams PL, Warwick R, eds. Gray’s anatomy. 36th ed. Philadelphia, PA: Churchill Livingstone; 1980.
- Hurd WW, Pearl ML, DeLancey JO, Quint EH, Garnett B, Bude RO. Laparoscopic injury of abdominal wall blood vessels: A report of three cases. Obstet Gynecol. 1993;82(4 Pt 2 Suppl):673–676.
- Lin P, Grow DR. Complications of laparoscopy. Strategies for prevention and cure. Obstet Gynecol Clin North Am. 1999;26(1):23–38, v.
- Saber AA, Meslemani AM, Davis R, Pimentel R. Safety zones for anterior abdominal wall entry during laparoscopy: A CT scan mapping of epigastric vessels. Ann Surg. 2004;239(2):182–185.
- Hurd WW, Bude RO, DeLancey JO, Newman JS. The location of abdominal wall blood vessels in relationship to abdominal landmarks apparent at laparoscopy. Am J Obstet Gynecol. 1994;171(3):642–646.
- Sandadi S, Johannigman JA, Wong VL, Blebea J, Altose MD, Hurd WW. Recognition and management of major vessel injury during laparoscopy. J minim invasive gynecol. 2010;17(6):692–702.
- Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol rev. 2007;29:6–28.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane database syst rev. 2009;(3):CD003677.
- Hurd WW, Bude RO, DeLancey JO, Pearl ML. The relationship of the umbilicus to the aortic bifurcation: implications for laparoscopic technique. Obstet gynecol. 1992;80(1):48–51.
- Clarke-Pearson DL, Geller EJ. Complications of hysterectomy. Obstet gynecol. 2013;121(3):654–673.
- Shin JH, Howard FM. Abdominal wall nerve injury during laparoscopic gynecologic surgery: incidence, risk factors, and treatment outcomes. J minim invasive gynecol. 2012;19(4):448–453.
- Bhoyrul S, Payne J, Steffes B, Swanstrom L, Way LW. A randomized prospective study of radially expanding trocars in laparoscopic surgery. J gastrointest surg. 2000;4(4):392–397.
- Shirk GJ, Johns A, Redwine DB. Complications of laparoscopic surgery: How to avoid them and how to repair them. J minim invasive gynecol. 2006;13(4):352–361.
- Tews G, Arzt W, Bohaumilitzky T, Füreder R, Frölich H. Significant reduction of operational risk in laparoscopy through the use of a new blunt trocar. Surg gynecol obstet. 1991;173(1):67–68.
- Najmaldin A, Guillou P. A guide to laparoscopic surgery. 1st ed. Wiley-Blackwell; 1998.