User login
Although measles is generally considered a disease of children, it affects people of all ages. While the incidence of measles in the United States is significantly lower than in 1963, when an effective measles vaccine was first introduced, recent increases in the number of sporadic cases and community outbreaks in adults show that measles is still a significant health problem.
PATHOGENESIS OF MEASLES
Measles is a highly contagious viral infection, whose manifestations have been recognized since the 7th century. The measles virus is an RNA virus of the Paramyxoviridae family. It is very difficult to isolate from clinical specimens, requiring special cell lines for in vitro propagation.
After acquisition, the measles virus establishes localized infection of the respiratory epithelium and then spreads to the regional lymphatics. A primary viremia then occurs, in which the virus replicates at the site of inoculation and in the reticuloendothelial tissues. A secondary viremia follows, in which the virus infects and replicates in the skin, conjunctiva, respiratory tract, and other distant organs.
The measles rash is thought to be due to a hypersensitivity reaction.1 Cell-mediated responses are the main line of defense against measles, as evidenced by the fact that people with cell-mediated deficiencies develop severe measles infection.2 Immunity to wild-type measles is believed to be lifelong.3,4
MEASLES IS HIGHLY CONTAGIOUS
Measles is one of the most contagious infectious diseases, with a secondary attack rate of at least 90% in susceptible household contacts. 4 The fact that emergency departments and physicians’ offices have become sites of measles transmission in recent years underscores the transmissibility of the virus.5–7
Although the virus is very labile, it can remain infective in respiratory droplets from the air for many hours. Thus, measles virus spreads from person to person by direct contact with droplets from respiratory secretions of infected persons.
The period of maximal contagion is the late prodrome, ie, 2 to 4 days before the onset of the rash. People who are generally in good health are contagious through 4 days after the onset of the rash, whereas people with compromised immunity can continue to shed the virus for the entire duration of the illness.
Airborne transmission precautions are required for 4 days after the onset of the rash in hospitalized, non-immunocompromised patients with measles, and for the duration of the illness for immunocompromised patients.
In the absence of widespread measles vaccination, measles infection peaks in late winter and early spring.
EPIDEMIOLOGIC TRENDS: CAUSE FOR CONCERN
Since an effective vaccine became available in 1963, the annual incidence of measles cases in the United States has decreased by more than 99%. A significant resurgence from 1989 to 1991 affected mainly unvaccinated preschoolers and resulted in more than 55,000 cases and 130 deaths.8 This resurgence prompted widespread, intensive immunization efforts and the recommendation that a second dose of measles vaccine be given to school-aged children. This led to the effective elimination of endemic transmission of measles in the United States.9
Although 90% of these cases either were directly imported or were associated with importation from other countries,10 the reason for the large number of cases was clearly the greater transmission after importation of the virus into the United States. This transmission was the direct result of the fact that 91% of the cases occurred in unvaccinated people or people whose vaccination status was not known or was not documented. A high proportion— at least 61 (47%)—of the 131 measles cases in 2008 were in school-aged children whose parents chose not to have them vaccinated. Although no deaths were reported in these 131 patients, 15 required hospitalization.
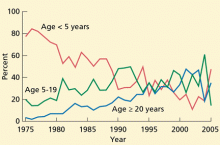
Although most reported measles cases are still in young and school-aged children, recent cases and outbreaks have also occurred in isolated communities of adults. Approximately 25% of the cases reported in 2008 were in people age 20 and older. Most adults who contracted measles had unknown or undocumented vaccination status. Similarly, a small measles outbreak occurred in Indiana in 2005, when an adolescent US citizen traveling in Europe became infected in Romania and exposed 500 people at a church gathering upon her return. Thirty-four cases of measles were reported from this exposure, and many were in adults.11
The recent increase in the number of cases reported and the continued reports of outbreaks highlight the fact that measles outbreaks can occur in communities with a high number of unvaccinated people, and underscore the need for high overall measles vaccination coverage to limit the spread of this infection.12
CLINICAL FEATURES OF MEASLES
The first sign of measles is a distinct prodrome, which occurs after an incubation period of 10 to 12 days. The prodrome is characterized by fever, malaise, anorexia, conjunctivitis, coryza, and cough and may resemble an upper respiratory tract infection; it lasts 2 to 4 days.
Towards the end of the prodrome, the body temperature can rise to as high as 40°C, and Koplik spots, pathognomonic for measles, appear. Koplik spots, bluish-gray specks on an erythematous base, usually appear on the buccal mucosa opposite the second molars 1 to 2 days before the onset of the rash, and last for 1 to 2 days after the onset of the rash. Thus, it is not unusual for Koplik spots to have disappeared at the time the diagnosis of measles is entertained.
The classic measles rash is an erythematous maculopapular eruption that begins on the head and face and spreads to involve the entire body. It usually persists for 4 to 5 days and is most confluent on the face and upper body. The rash fades in order of appearance, and may desquamate. People with measles appear ill, especially 1 to 2 days after the rash appears.
The entire course of measles usually lasts 7 to 10 days in patients with a healthy immune system. The cough, a manifestation of tracheobronchitis, is usually the last symptom to resolve. Patients are contagious 2 to 4 days before the onset of the rash, and remain so through 4 days after the onset of the rash.
COMPLICATIONS
Respiratory complications
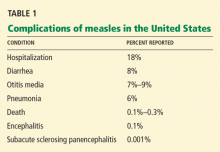
Pneumonia is responsible for 60% of deaths associated with measles.13 Although radiographic evidence of pneumonia is found in measles patients with no complications, symptomatic pneumonia occurs in 1% to 6% of patients. It is the result of either direct invasion by the virus or secondary bacterial infection,17 most often with Staphylococcus aureus and Streptococcus pneumoniae. Other respiratory complications include otitis media, sinusitis, and laryngotracheobronchitis.
Neurologic complications
Acute measles encephalitis is more common in adults than in children. Occuring in 1 in 1,000 to 2,000 patients,18 it is characterized by the resurgence of fever during the convalescent phase of the illness, along with headaches, seizures, and altered consciousness. These manifestations may be mild or severe, but they lead to permanent neurologic sequelae in a substantial proportion of affected patients. It is not clear whether acute measles encephalitis represents direct invasion of the virus or a postinfectious process from a hypersensitivity to the virus.19
Subacute sclerosing panencephalitis is a rare, chronic, degenerative central nervous system disease that occurs secondary to persistent infection with a defective measles virus.20 The prevalence is estimated at 1 per 100,000 cases. Signs and symptoms appear an average of 7 years after the initial infection and include personality changes, myoclonic seizures, and motor disturbances. Often, coma and death follow.
This condition occurs particularly in those who had measles at a very young age, ie, before the age of 2 years, and it occurs despite a vigorous host-immune response to the virus. Patients have high titers of measles-specific antibody in the sera and cerebrospinal fluid.
Other complications
Diarrhea and stomatitis account for much of the sickness and death from measles in developing countries.
Subclinical hepatitis occurs in at least 30% of adult measles patients.
Less common complications include thrombocytopenia, appendicitis, ileocolitis, pericarditis, myocarditis, and hypocalcemia.
MEASLES DURING PREGNANCY
Measles during pregnancy may be severe, mainly due to primary measles pneumonia.21 Measles is associated with a risk of miscarriage and prematurity, but congenital anomalies of the fetus have not been described, as they have for rubella infection.22
MEASLES IN COMPROMISED IMMUNITY
Measles patients with deficiencies of cellmediated immunity have a prolonged, severe, and often fatal course.2,23,24 This includes patients with:
- Human immunodeficiency virus (HIV) infection
- Congenital immunodeficiencies
- Disorders requiring chemotherapeutic and immunosuppressive therapy.
These patients are particularly susceptible to acute progressive encephalitis and measles pneumonitis. Case-fatality rates of 70% in cancer patients and 40% in HIV-infected patients have been reported.24
The diagnosis of measles may be difficult in patients without cell-mediated immunity, as 25% to 40% of them do not develop the characteristic rash.2,23 The absence of rash supports the theory that the rash is a hypersensitivity reaction to the virus.
MODIFIED AND ATYPICAL MEASLES
Modified measles
A modified form of measles can occur in people with some degree of passive immunity to the virus, including those previously vaccinated. It occurs mostly in patients who recently received immunoglobulin products, or in young infants who have residual maternal antibody. A modified measles illness can also follow vaccination with live-virus vaccine (see later discussion).
The clinical manifestations vary, and the illness may not have the classic features of prodrome, rash, and Koplik spots.
Atypical measles
Atypical measles is an unusual form that can occur when a person previously vaccinated with a killed-virus measles vaccine (used from 1963 to 1967) is exposed to wild-type measles.25 Features include a shorter prodrome (1 to 2 days), followed by appearance of a rash that begins on the distal extremities and spreads centripetally, usually sparing the neck, face, and head. The rash may be petechial, maculopapular, urticarial, vesicular, or a combination. The rash is accompanied by high fever and edema of the extremities. Complications such as pneumonia and hepatitis may occur.
The course of atypical measles is more prolonged than with classic measles, but because these patients are thought to have partial protection against the virus, they do not transmit it and are not considered contagious.26
DIAGNOSIS OF MEASLES
The classic clinical features are usually enough to distinguish measles from other febrile illnesses with similar clinical manifestions, such as rubella, dengue, parvovirus B19 infection, erythema multiforme, Stevens-Johnson syndrome, and streptococcal scarlet fever. The distinctive measles prodrome, Koplik spots, the progression of the rash from the head and neck to the trunk and the extremities, and the severity of disease are distinctive features of measles.
Laboratory tests to confirm the diagnosis are often used in areas where measles is rare, and laboratory confirmation is currently recommended in the United States. Because viral isolation is technically difficult and is not widely available, serologic testing is the method most commonly used. The measles-specific immunoglobulin M (IgM) antibody assay, the test used most often, is almost 100% sensitive when done 2 to 3 days after the onset of the rash.27,28 Measles IgM antibody peaks at 4 weeks after the infection and disappears by 6 to 8 weeks.
It is important to remember that false-positive measles IgM antibody may occur with other viral infections, such as parvovirus B19 and rubella. Because measles-specific IgG antibody is produced with the onset of infection and peaks at 4 weeks, a fourfold rise in the IgG titer is useful in confirming the diagnosis. Measles IgG antibody after infection is sustained for life.
Reverse transcription-polymerase chain reaction testing can also detect measles virus in the blood and urine when direct evidence of the virus is necessary, such as in immunocompromised patients.29
TREATMENT IS SUPPORTIVE
Treatment of measles mainly involves supportive measures, such as fluids and antipyretics. Antiviral agents such as ribavirin and interferon have in vitro activity against the measles virus and have been used to treat severe measles infection in immunocompromised patients. However, their clinical efficacy is unproven.30
Routine use of antibacterial agents to prevent secondary bacterial infection is not recommended.
CURRENT RECOMMENDATIONS FOR ACTIVE IMMUNIZATION
Active immunization for measles has been available since 1963. Between 1963 and 1967, both killed-virus and live-virus vaccines were available. As atypical measles cases became recognized, the killed-virus vaccine was withdrawn.
The vaccine currently available in the United States is a live-attenuated strain prepared in chicken embryo cell culture and combined with mumps and rubella vaccine (MMR) or mumps, rubella, and varicella vaccine (MMRV).
Two doses of live-virus measles vaccine are recommended for all healthy children before they begin school, with the first dose given at 12 to 15 months of age. A second dose is needed because the failure rate with one dose is 5%. More than 99% of people who receive two doses separated by 4 weeks develop serologic evidence of measles.
Waning immunity after vaccination occurs very rarely, with approximately 5% of children developing secondary vaccine failure 10 to 15 years after vaccination.3,31
Although rates of vaccination in the United States are high, cases of measles continue to occur in unvaccinated infants and in children who are either too young to be vaccinated or whose parents claimed exemption because of religious or personal beliefs.
Because of the occurrence of measles cases in adolescents, young adults, and adults, potentially susceptible people should be identified and vaccinated according to current guidelines. People should be considered susceptible unless they have documentation of at least two doses of measles vaccine given at least 28 days apart, physician-diagnosed measles, laboratory evidence of immunity to measles, or were born before 1957. All adults who are susceptible should receive at least one dose of measles vaccine.10 Adults at higher risk of contracting measles include:
- Students in high school and college
- International travelers
- Health care personnel.
For these adults, two doses of measles vaccine, at least 28 days apart, are recommended.32
Postexposure prophylaxis
Measles vaccination given to susceptible contacts within 72 hours of exposure as postexposure prophylaxis may protect against infection and induces protection against subsequent exposures to measles.33,34 Vaccination is the intervention of choice for susceptible individuals older than 12 months of age who are exposed to measles and who do not have a contraindication to measles vaccination.35 Active rather than passive immunization is also the strategy of choice for controlling measles outbreaks.
Passive immunization with intramuscular immune globulin within 6 days of exposure can be used in selected circumstances to prevent transmission or to modify the clinical course of the infection.36 Immune globulin therapy is recommended for susceptible individuals who are exposed to measles and who are at high risk of developing severe or fatal measles. This includes individuals who are being treated with immunosuppressive agents, those with HIV infection, pregnant women, and infants less than 1 year of age. Immune globulin should not be used to control measles outbreaks.
ADVERSE EFFECTS OF MEASLES VACCINE
Live-virus measles vaccine has an excellent safety record. A transient fever, which may be accompanied by a measles-like rash, occurs in 5% to 15% of people 5 to 12 days after vaccination. The rash may be discrete or confluent and is self-limited.
Although measles vaccine is a live-attenuated vaccine, vaccinated people do not transmit the virus to susceptible contacts and are not considered contagious, even if they develop a vaccine-associated rash. Thus, the vaccine can be safely given to close contacts of immunocompromised and other susceptible people. Encephalitis is exceedingly rare following vaccination.
There is no scientific evidence that the risk of autism is higher in children who receive measles or MMR vaccine than in unvaccinated children.37 An Institute of Medicine report in 2001 rejected a causal relationship between MMR vaccine and autism spectrum disorders.38
CONTRAINDICATIONS TO MEASLES VACCINATION
Measles vaccine is contraindicated for:
- People who have cell-mediated immune deficiencies (except patients wtih HIV infection—see discussion just below)
- Pregnant women
- Those who had a severe allergic reaction to a vaccine component after a previous dose
- Those with moderate or severe acute illness
- Those who have recently received immune globulin products.
HIV-infected patients with severe immunosuppression should not receive the liveattenuated measles vaccine. However, because patients with HIV are at risk of severe measles, and because the vaccine has been shown to be safe in HIV patients who do not have severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression. 39
After receiving immune globulin
Anyone who has recently received immune globulin should not receive measles vaccine until sufficient time has passed, since passively acquired antibodies interfere with the immune response to live-virus vaccines. How long to wait depends on the type of immune globulin, the indication, the amount, and the route of administration. In general, the waiting period is:
- At least 3 months after intramuscular immune globulin or tetanus, hepatitis A, or hepatitis B prophylaxis
- At least 4 months after intramuscular immune globulin for rabies, or 6 months after intravenous immune globulin for cytomegalovirus (dose, 150 mg/kg)
- At least 8 months after intravenous immune globulin as replacement or therapy for immune deficiencies (dose, 400 mg/kg), or after intravenous immune globulin for immune thrombocytopenic purpura (400 mg/kg)
- At least 10 months after intravenous immune globulin for immune thrombocytopenic pupura at a dose of 1 g/kg.39
Egg allergy is not a contraindication
Although measles vaccine is produced in chick embryo cell culture, the vaccine has been shown to be safe in people with egg allergy, so they may be vaccinated without first being tested for egg allergy.39,40
- Lachmann PJ. Immunopathology of measles. Proc R Soc Med 1974; 67:1120–1122.
- Enders JF, McCarthy K, Mitus A, Cheatham WJ. Isolation of measles virus at autopsy in case of giant cell pneumonia without rash. N Engl J Med 1959; 261:875–881.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stokes J, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Farizo KM, Stehr-Green PA, Simpson DM, Markowitz LE. Pediatric emergency room visits: a risk factor for acquiring measles. Pediatrics 1991; 87:74–79.
- Bloch AB, Orenstein W, Ewing WM, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics 1985; 75:676–683.
- Remington PL, Hall WN, Davis IH, et al. Airborne transmission of measles in a physician’s office. JAMA 1985; 253:1574–1577.
- US Centers for Disease Control and Prevention. Reported vaccine-preventable diseases—United States, 1993, and the Childhood Immunization Initiative. MMWR 1994; 43:57–60.
- Orenstein WA, Papania MJ, Wharton ME. Measles elimination in the United States. J Infect Dis 2004; 189 (suppl 1):S1–S3.
- US Centers for Disease Control and Prevention. Update: Measles—United States, January–July 2008. MMWR 2008; 57:893–896.
- Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med 2006; 355:447–455.
- Mulholland EK. Measles in the United States, 2006. N Engl J Med 2006; 355:440–443.
- Barkin RM. Measles mortality. Analysis of the primary cause of death. Am J Dis Child 1975; 129:307–309.
- Barkin RM. Measles mortality: a retrospective look at the vaccine era. Am J Epidemiol 1975; 102:341–349.
- US Centers for Disease Control and Prevention. Public-sector vaccination efforts in response to the resurgence of measles among preschool-aged children—United States, 1989–1991. MMWR 1992; 41:522–525.
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. an analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189( suppl 1):S4–S16.
- Johnson RT, Griffin DE, Hirsch RL, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Gershon AA. Chickenpox, measles, and mumps. In:Remington J, Klein J, eds. Infectious Diseases of the Fetus and Newborn Infant. Elsevier Saunders: Philadelphia, 2006:693–738.
- Mitus A, Enders JF, Craig JM, Holloway A. Persistence of measles virus and depression of antibody formation in patients with giant-cell pneumonia after measles. N Engl J Med 1959; 261:882–889.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Mayo DR, Brennan T, Cormier DP, Hadler J, Lamb P. Evaluation of a commercial measles virus immunoglobulin M enzyme immunoassay. J Clin Microbiol 1991; 29:2865–2867.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187( suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Forni Al, Schluger NW, Roberts RB. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis 1994; 19:454–462.
- Anders JF, Jacobsen RM, Poland GA, Jacobsen SJ, Wollan PC. Secondary failure rates of measles vaccines: a metaanalysis of published studes. Pediar Infect Dis J 1996; 15:62–66.
- US Centers for Disease Control and Prevention. Measles—United States, January 1–April 25, 2008. MMWR 2008; 57:494–498.
- Berkovitz S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
- Strebel PM, Papania MJ, Halsey NA. Measles vaccine. In:Plotkin SA, Orenstein WA, eds. Vaccines. Saunders: New York, 2004:389–440.
- US Centers for Disease Control and Prevention. Measles, mumps and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998; 47( RR-8):1–57. http://www.cdc.gov/mmwr/PDF/rr/rr4708.pdf. Accessed December 30, 2009.
- Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med 2002; 347:1477–1482.
- Straton K, Gable A, Shetty P, McCormick M; for the Institute of Medicine. Immunization Safety Review: Measles-Mumps-Rubella Vaccine and Autism. National Academy Press: Washington, DC, 2001.
- Pickerington LK, Baker CJ, Long SS, McMillan JA, editors. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:444–455.
- James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med 1995; 332:1262–1266.
Although measles is generally considered a disease of children, it affects people of all ages. While the incidence of measles in the United States is significantly lower than in 1963, when an effective measles vaccine was first introduced, recent increases in the number of sporadic cases and community outbreaks in adults show that measles is still a significant health problem.
PATHOGENESIS OF MEASLES
Measles is a highly contagious viral infection, whose manifestations have been recognized since the 7th century. The measles virus is an RNA virus of the Paramyxoviridae family. It is very difficult to isolate from clinical specimens, requiring special cell lines for in vitro propagation.
After acquisition, the measles virus establishes localized infection of the respiratory epithelium and then spreads to the regional lymphatics. A primary viremia then occurs, in which the virus replicates at the site of inoculation and in the reticuloendothelial tissues. A secondary viremia follows, in which the virus infects and replicates in the skin, conjunctiva, respiratory tract, and other distant organs.
The measles rash is thought to be due to a hypersensitivity reaction.1 Cell-mediated responses are the main line of defense against measles, as evidenced by the fact that people with cell-mediated deficiencies develop severe measles infection.2 Immunity to wild-type measles is believed to be lifelong.3,4
MEASLES IS HIGHLY CONTAGIOUS
Measles is one of the most contagious infectious diseases, with a secondary attack rate of at least 90% in susceptible household contacts. 4 The fact that emergency departments and physicians’ offices have become sites of measles transmission in recent years underscores the transmissibility of the virus.5–7
Although the virus is very labile, it can remain infective in respiratory droplets from the air for many hours. Thus, measles virus spreads from person to person by direct contact with droplets from respiratory secretions of infected persons.
The period of maximal contagion is the late prodrome, ie, 2 to 4 days before the onset of the rash. People who are generally in good health are contagious through 4 days after the onset of the rash, whereas people with compromised immunity can continue to shed the virus for the entire duration of the illness.
Airborne transmission precautions are required for 4 days after the onset of the rash in hospitalized, non-immunocompromised patients with measles, and for the duration of the illness for immunocompromised patients.
In the absence of widespread measles vaccination, measles infection peaks in late winter and early spring.
EPIDEMIOLOGIC TRENDS: CAUSE FOR CONCERN
Since an effective vaccine became available in 1963, the annual incidence of measles cases in the United States has decreased by more than 99%. A significant resurgence from 1989 to 1991 affected mainly unvaccinated preschoolers and resulted in more than 55,000 cases and 130 deaths.8 This resurgence prompted widespread, intensive immunization efforts and the recommendation that a second dose of measles vaccine be given to school-aged children. This led to the effective elimination of endemic transmission of measles in the United States.9
Although 90% of these cases either were directly imported or were associated with importation from other countries,10 the reason for the large number of cases was clearly the greater transmission after importation of the virus into the United States. This transmission was the direct result of the fact that 91% of the cases occurred in unvaccinated people or people whose vaccination status was not known or was not documented. A high proportion— at least 61 (47%)—of the 131 measles cases in 2008 were in school-aged children whose parents chose not to have them vaccinated. Although no deaths were reported in these 131 patients, 15 required hospitalization.
Although most reported measles cases are still in young and school-aged children, recent cases and outbreaks have also occurred in isolated communities of adults. Approximately 25% of the cases reported in 2008 were in people age 20 and older. Most adults who contracted measles had unknown or undocumented vaccination status. Similarly, a small measles outbreak occurred in Indiana in 2005, when an adolescent US citizen traveling in Europe became infected in Romania and exposed 500 people at a church gathering upon her return. Thirty-four cases of measles were reported from this exposure, and many were in adults.11
The recent increase in the number of cases reported and the continued reports of outbreaks highlight the fact that measles outbreaks can occur in communities with a high number of unvaccinated people, and underscore the need for high overall measles vaccination coverage to limit the spread of this infection.12
CLINICAL FEATURES OF MEASLES
The first sign of measles is a distinct prodrome, which occurs after an incubation period of 10 to 12 days. The prodrome is characterized by fever, malaise, anorexia, conjunctivitis, coryza, and cough and may resemble an upper respiratory tract infection; it lasts 2 to 4 days.
Towards the end of the prodrome, the body temperature can rise to as high as 40°C, and Koplik spots, pathognomonic for measles, appear. Koplik spots, bluish-gray specks on an erythematous base, usually appear on the buccal mucosa opposite the second molars 1 to 2 days before the onset of the rash, and last for 1 to 2 days after the onset of the rash. Thus, it is not unusual for Koplik spots to have disappeared at the time the diagnosis of measles is entertained.
The classic measles rash is an erythematous maculopapular eruption that begins on the head and face and spreads to involve the entire body. It usually persists for 4 to 5 days and is most confluent on the face and upper body. The rash fades in order of appearance, and may desquamate. People with measles appear ill, especially 1 to 2 days after the rash appears.
The entire course of measles usually lasts 7 to 10 days in patients with a healthy immune system. The cough, a manifestation of tracheobronchitis, is usually the last symptom to resolve. Patients are contagious 2 to 4 days before the onset of the rash, and remain so through 4 days after the onset of the rash.
COMPLICATIONS
Respiratory complications
Pneumonia is responsible for 60% of deaths associated with measles.13 Although radiographic evidence of pneumonia is found in measles patients with no complications, symptomatic pneumonia occurs in 1% to 6% of patients. It is the result of either direct invasion by the virus or secondary bacterial infection,17 most often with Staphylococcus aureus and Streptococcus pneumoniae. Other respiratory complications include otitis media, sinusitis, and laryngotracheobronchitis.
Neurologic complications
Acute measles encephalitis is more common in adults than in children. Occuring in 1 in 1,000 to 2,000 patients,18 it is characterized by the resurgence of fever during the convalescent phase of the illness, along with headaches, seizures, and altered consciousness. These manifestations may be mild or severe, but they lead to permanent neurologic sequelae in a substantial proportion of affected patients. It is not clear whether acute measles encephalitis represents direct invasion of the virus or a postinfectious process from a hypersensitivity to the virus.19
Subacute sclerosing panencephalitis is a rare, chronic, degenerative central nervous system disease that occurs secondary to persistent infection with a defective measles virus.20 The prevalence is estimated at 1 per 100,000 cases. Signs and symptoms appear an average of 7 years after the initial infection and include personality changes, myoclonic seizures, and motor disturbances. Often, coma and death follow.
This condition occurs particularly in those who had measles at a very young age, ie, before the age of 2 years, and it occurs despite a vigorous host-immune response to the virus. Patients have high titers of measles-specific antibody in the sera and cerebrospinal fluid.
Other complications
Diarrhea and stomatitis account for much of the sickness and death from measles in developing countries.
Subclinical hepatitis occurs in at least 30% of adult measles patients.
Less common complications include thrombocytopenia, appendicitis, ileocolitis, pericarditis, myocarditis, and hypocalcemia.
MEASLES DURING PREGNANCY
Measles during pregnancy may be severe, mainly due to primary measles pneumonia.21 Measles is associated with a risk of miscarriage and prematurity, but congenital anomalies of the fetus have not been described, as they have for rubella infection.22
MEASLES IN COMPROMISED IMMUNITY
Measles patients with deficiencies of cellmediated immunity have a prolonged, severe, and often fatal course.2,23,24 This includes patients with:
- Human immunodeficiency virus (HIV) infection
- Congenital immunodeficiencies
- Disorders requiring chemotherapeutic and immunosuppressive therapy.
These patients are particularly susceptible to acute progressive encephalitis and measles pneumonitis. Case-fatality rates of 70% in cancer patients and 40% in HIV-infected patients have been reported.24
The diagnosis of measles may be difficult in patients without cell-mediated immunity, as 25% to 40% of them do not develop the characteristic rash.2,23 The absence of rash supports the theory that the rash is a hypersensitivity reaction to the virus.
MODIFIED AND ATYPICAL MEASLES
Modified measles
A modified form of measles can occur in people with some degree of passive immunity to the virus, including those previously vaccinated. It occurs mostly in patients who recently received immunoglobulin products, or in young infants who have residual maternal antibody. A modified measles illness can also follow vaccination with live-virus vaccine (see later discussion).
The clinical manifestations vary, and the illness may not have the classic features of prodrome, rash, and Koplik spots.
Atypical measles
Atypical measles is an unusual form that can occur when a person previously vaccinated with a killed-virus measles vaccine (used from 1963 to 1967) is exposed to wild-type measles.25 Features include a shorter prodrome (1 to 2 days), followed by appearance of a rash that begins on the distal extremities and spreads centripetally, usually sparing the neck, face, and head. The rash may be petechial, maculopapular, urticarial, vesicular, or a combination. The rash is accompanied by high fever and edema of the extremities. Complications such as pneumonia and hepatitis may occur.
The course of atypical measles is more prolonged than with classic measles, but because these patients are thought to have partial protection against the virus, they do not transmit it and are not considered contagious.26
DIAGNOSIS OF MEASLES
The classic clinical features are usually enough to distinguish measles from other febrile illnesses with similar clinical manifestions, such as rubella, dengue, parvovirus B19 infection, erythema multiforme, Stevens-Johnson syndrome, and streptococcal scarlet fever. The distinctive measles prodrome, Koplik spots, the progression of the rash from the head and neck to the trunk and the extremities, and the severity of disease are distinctive features of measles.
Laboratory tests to confirm the diagnosis are often used in areas where measles is rare, and laboratory confirmation is currently recommended in the United States. Because viral isolation is technically difficult and is not widely available, serologic testing is the method most commonly used. The measles-specific immunoglobulin M (IgM) antibody assay, the test used most often, is almost 100% sensitive when done 2 to 3 days after the onset of the rash.27,28 Measles IgM antibody peaks at 4 weeks after the infection and disappears by 6 to 8 weeks.
It is important to remember that false-positive measles IgM antibody may occur with other viral infections, such as parvovirus B19 and rubella. Because measles-specific IgG antibody is produced with the onset of infection and peaks at 4 weeks, a fourfold rise in the IgG titer is useful in confirming the diagnosis. Measles IgG antibody after infection is sustained for life.
Reverse transcription-polymerase chain reaction testing can also detect measles virus in the blood and urine when direct evidence of the virus is necessary, such as in immunocompromised patients.29
TREATMENT IS SUPPORTIVE
Treatment of measles mainly involves supportive measures, such as fluids and antipyretics. Antiviral agents such as ribavirin and interferon have in vitro activity against the measles virus and have been used to treat severe measles infection in immunocompromised patients. However, their clinical efficacy is unproven.30
Routine use of antibacterial agents to prevent secondary bacterial infection is not recommended.
CURRENT RECOMMENDATIONS FOR ACTIVE IMMUNIZATION
Active immunization for measles has been available since 1963. Between 1963 and 1967, both killed-virus and live-virus vaccines were available. As atypical measles cases became recognized, the killed-virus vaccine was withdrawn.
The vaccine currently available in the United States is a live-attenuated strain prepared in chicken embryo cell culture and combined with mumps and rubella vaccine (MMR) or mumps, rubella, and varicella vaccine (MMRV).
Two doses of live-virus measles vaccine are recommended for all healthy children before they begin school, with the first dose given at 12 to 15 months of age. A second dose is needed because the failure rate with one dose is 5%. More than 99% of people who receive two doses separated by 4 weeks develop serologic evidence of measles.
Waning immunity after vaccination occurs very rarely, with approximately 5% of children developing secondary vaccine failure 10 to 15 years after vaccination.3,31
Although rates of vaccination in the United States are high, cases of measles continue to occur in unvaccinated infants and in children who are either too young to be vaccinated or whose parents claimed exemption because of religious or personal beliefs.
Because of the occurrence of measles cases in adolescents, young adults, and adults, potentially susceptible people should be identified and vaccinated according to current guidelines. People should be considered susceptible unless they have documentation of at least two doses of measles vaccine given at least 28 days apart, physician-diagnosed measles, laboratory evidence of immunity to measles, or were born before 1957. All adults who are susceptible should receive at least one dose of measles vaccine.10 Adults at higher risk of contracting measles include:
- Students in high school and college
- International travelers
- Health care personnel.
For these adults, two doses of measles vaccine, at least 28 days apart, are recommended.32
Postexposure prophylaxis
Measles vaccination given to susceptible contacts within 72 hours of exposure as postexposure prophylaxis may protect against infection and induces protection against subsequent exposures to measles.33,34 Vaccination is the intervention of choice for susceptible individuals older than 12 months of age who are exposed to measles and who do not have a contraindication to measles vaccination.35 Active rather than passive immunization is also the strategy of choice for controlling measles outbreaks.
Passive immunization with intramuscular immune globulin within 6 days of exposure can be used in selected circumstances to prevent transmission or to modify the clinical course of the infection.36 Immune globulin therapy is recommended for susceptible individuals who are exposed to measles and who are at high risk of developing severe or fatal measles. This includes individuals who are being treated with immunosuppressive agents, those with HIV infection, pregnant women, and infants less than 1 year of age. Immune globulin should not be used to control measles outbreaks.
ADVERSE EFFECTS OF MEASLES VACCINE
Live-virus measles vaccine has an excellent safety record. A transient fever, which may be accompanied by a measles-like rash, occurs in 5% to 15% of people 5 to 12 days after vaccination. The rash may be discrete or confluent and is self-limited.
Although measles vaccine is a live-attenuated vaccine, vaccinated people do not transmit the virus to susceptible contacts and are not considered contagious, even if they develop a vaccine-associated rash. Thus, the vaccine can be safely given to close contacts of immunocompromised and other susceptible people. Encephalitis is exceedingly rare following vaccination.
There is no scientific evidence that the risk of autism is higher in children who receive measles or MMR vaccine than in unvaccinated children.37 An Institute of Medicine report in 2001 rejected a causal relationship between MMR vaccine and autism spectrum disorders.38
CONTRAINDICATIONS TO MEASLES VACCINATION
Measles vaccine is contraindicated for:
- People who have cell-mediated immune deficiencies (except patients wtih HIV infection—see discussion just below)
- Pregnant women
- Those who had a severe allergic reaction to a vaccine component after a previous dose
- Those with moderate or severe acute illness
- Those who have recently received immune globulin products.
HIV-infected patients with severe immunosuppression should not receive the liveattenuated measles vaccine. However, because patients with HIV are at risk of severe measles, and because the vaccine has been shown to be safe in HIV patients who do not have severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression. 39
After receiving immune globulin
Anyone who has recently received immune globulin should not receive measles vaccine until sufficient time has passed, since passively acquired antibodies interfere with the immune response to live-virus vaccines. How long to wait depends on the type of immune globulin, the indication, the amount, and the route of administration. In general, the waiting period is:
- At least 3 months after intramuscular immune globulin or tetanus, hepatitis A, or hepatitis B prophylaxis
- At least 4 months after intramuscular immune globulin for rabies, or 6 months after intravenous immune globulin for cytomegalovirus (dose, 150 mg/kg)
- At least 8 months after intravenous immune globulin as replacement or therapy for immune deficiencies (dose, 400 mg/kg), or after intravenous immune globulin for immune thrombocytopenic purpura (400 mg/kg)
- At least 10 months after intravenous immune globulin for immune thrombocytopenic pupura at a dose of 1 g/kg.39
Egg allergy is not a contraindication
Although measles vaccine is produced in chick embryo cell culture, the vaccine has been shown to be safe in people with egg allergy, so they may be vaccinated without first being tested for egg allergy.39,40
Although measles is generally considered a disease of children, it affects people of all ages. While the incidence of measles in the United States is significantly lower than in 1963, when an effective measles vaccine was first introduced, recent increases in the number of sporadic cases and community outbreaks in adults show that measles is still a significant health problem.
PATHOGENESIS OF MEASLES
Measles is a highly contagious viral infection, whose manifestations have been recognized since the 7th century. The measles virus is an RNA virus of the Paramyxoviridae family. It is very difficult to isolate from clinical specimens, requiring special cell lines for in vitro propagation.
After acquisition, the measles virus establishes localized infection of the respiratory epithelium and then spreads to the regional lymphatics. A primary viremia then occurs, in which the virus replicates at the site of inoculation and in the reticuloendothelial tissues. A secondary viremia follows, in which the virus infects and replicates in the skin, conjunctiva, respiratory tract, and other distant organs.
The measles rash is thought to be due to a hypersensitivity reaction.1 Cell-mediated responses are the main line of defense against measles, as evidenced by the fact that people with cell-mediated deficiencies develop severe measles infection.2 Immunity to wild-type measles is believed to be lifelong.3,4
MEASLES IS HIGHLY CONTAGIOUS
Measles is one of the most contagious infectious diseases, with a secondary attack rate of at least 90% in susceptible household contacts. 4 The fact that emergency departments and physicians’ offices have become sites of measles transmission in recent years underscores the transmissibility of the virus.5–7
Although the virus is very labile, it can remain infective in respiratory droplets from the air for many hours. Thus, measles virus spreads from person to person by direct contact with droplets from respiratory secretions of infected persons.
The period of maximal contagion is the late prodrome, ie, 2 to 4 days before the onset of the rash. People who are generally in good health are contagious through 4 days after the onset of the rash, whereas people with compromised immunity can continue to shed the virus for the entire duration of the illness.
Airborne transmission precautions are required for 4 days after the onset of the rash in hospitalized, non-immunocompromised patients with measles, and for the duration of the illness for immunocompromised patients.
In the absence of widespread measles vaccination, measles infection peaks in late winter and early spring.
EPIDEMIOLOGIC TRENDS: CAUSE FOR CONCERN
Since an effective vaccine became available in 1963, the annual incidence of measles cases in the United States has decreased by more than 99%. A significant resurgence from 1989 to 1991 affected mainly unvaccinated preschoolers and resulted in more than 55,000 cases and 130 deaths.8 This resurgence prompted widespread, intensive immunization efforts and the recommendation that a second dose of measles vaccine be given to school-aged children. This led to the effective elimination of endemic transmission of measles in the United States.9
Although 90% of these cases either were directly imported or were associated with importation from other countries,10 the reason for the large number of cases was clearly the greater transmission after importation of the virus into the United States. This transmission was the direct result of the fact that 91% of the cases occurred in unvaccinated people or people whose vaccination status was not known or was not documented. A high proportion— at least 61 (47%)—of the 131 measles cases in 2008 were in school-aged children whose parents chose not to have them vaccinated. Although no deaths were reported in these 131 patients, 15 required hospitalization.
Although most reported measles cases are still in young and school-aged children, recent cases and outbreaks have also occurred in isolated communities of adults. Approximately 25% of the cases reported in 2008 were in people age 20 and older. Most adults who contracted measles had unknown or undocumented vaccination status. Similarly, a small measles outbreak occurred in Indiana in 2005, when an adolescent US citizen traveling in Europe became infected in Romania and exposed 500 people at a church gathering upon her return. Thirty-four cases of measles were reported from this exposure, and many were in adults.11
The recent increase in the number of cases reported and the continued reports of outbreaks highlight the fact that measles outbreaks can occur in communities with a high number of unvaccinated people, and underscore the need for high overall measles vaccination coverage to limit the spread of this infection.12
CLINICAL FEATURES OF MEASLES
The first sign of measles is a distinct prodrome, which occurs after an incubation period of 10 to 12 days. The prodrome is characterized by fever, malaise, anorexia, conjunctivitis, coryza, and cough and may resemble an upper respiratory tract infection; it lasts 2 to 4 days.
Towards the end of the prodrome, the body temperature can rise to as high as 40°C, and Koplik spots, pathognomonic for measles, appear. Koplik spots, bluish-gray specks on an erythematous base, usually appear on the buccal mucosa opposite the second molars 1 to 2 days before the onset of the rash, and last for 1 to 2 days after the onset of the rash. Thus, it is not unusual for Koplik spots to have disappeared at the time the diagnosis of measles is entertained.
The classic measles rash is an erythematous maculopapular eruption that begins on the head and face and spreads to involve the entire body. It usually persists for 4 to 5 days and is most confluent on the face and upper body. The rash fades in order of appearance, and may desquamate. People with measles appear ill, especially 1 to 2 days after the rash appears.
The entire course of measles usually lasts 7 to 10 days in patients with a healthy immune system. The cough, a manifestation of tracheobronchitis, is usually the last symptom to resolve. Patients are contagious 2 to 4 days before the onset of the rash, and remain so through 4 days after the onset of the rash.
COMPLICATIONS
Respiratory complications
Pneumonia is responsible for 60% of deaths associated with measles.13 Although radiographic evidence of pneumonia is found in measles patients with no complications, symptomatic pneumonia occurs in 1% to 6% of patients. It is the result of either direct invasion by the virus or secondary bacterial infection,17 most often with Staphylococcus aureus and Streptococcus pneumoniae. Other respiratory complications include otitis media, sinusitis, and laryngotracheobronchitis.
Neurologic complications
Acute measles encephalitis is more common in adults than in children. Occuring in 1 in 1,000 to 2,000 patients,18 it is characterized by the resurgence of fever during the convalescent phase of the illness, along with headaches, seizures, and altered consciousness. These manifestations may be mild or severe, but they lead to permanent neurologic sequelae in a substantial proportion of affected patients. It is not clear whether acute measles encephalitis represents direct invasion of the virus or a postinfectious process from a hypersensitivity to the virus.19
Subacute sclerosing panencephalitis is a rare, chronic, degenerative central nervous system disease that occurs secondary to persistent infection with a defective measles virus.20 The prevalence is estimated at 1 per 100,000 cases. Signs and symptoms appear an average of 7 years after the initial infection and include personality changes, myoclonic seizures, and motor disturbances. Often, coma and death follow.
This condition occurs particularly in those who had measles at a very young age, ie, before the age of 2 years, and it occurs despite a vigorous host-immune response to the virus. Patients have high titers of measles-specific antibody in the sera and cerebrospinal fluid.
Other complications
Diarrhea and stomatitis account for much of the sickness and death from measles in developing countries.
Subclinical hepatitis occurs in at least 30% of adult measles patients.
Less common complications include thrombocytopenia, appendicitis, ileocolitis, pericarditis, myocarditis, and hypocalcemia.
MEASLES DURING PREGNANCY
Measles during pregnancy may be severe, mainly due to primary measles pneumonia.21 Measles is associated with a risk of miscarriage and prematurity, but congenital anomalies of the fetus have not been described, as they have for rubella infection.22
MEASLES IN COMPROMISED IMMUNITY
Measles patients with deficiencies of cellmediated immunity have a prolonged, severe, and often fatal course.2,23,24 This includes patients with:
- Human immunodeficiency virus (HIV) infection
- Congenital immunodeficiencies
- Disorders requiring chemotherapeutic and immunosuppressive therapy.
These patients are particularly susceptible to acute progressive encephalitis and measles pneumonitis. Case-fatality rates of 70% in cancer patients and 40% in HIV-infected patients have been reported.24
The diagnosis of measles may be difficult in patients without cell-mediated immunity, as 25% to 40% of them do not develop the characteristic rash.2,23 The absence of rash supports the theory that the rash is a hypersensitivity reaction to the virus.
MODIFIED AND ATYPICAL MEASLES
Modified measles
A modified form of measles can occur in people with some degree of passive immunity to the virus, including those previously vaccinated. It occurs mostly in patients who recently received immunoglobulin products, or in young infants who have residual maternal antibody. A modified measles illness can also follow vaccination with live-virus vaccine (see later discussion).
The clinical manifestations vary, and the illness may not have the classic features of prodrome, rash, and Koplik spots.
Atypical measles
Atypical measles is an unusual form that can occur when a person previously vaccinated with a killed-virus measles vaccine (used from 1963 to 1967) is exposed to wild-type measles.25 Features include a shorter prodrome (1 to 2 days), followed by appearance of a rash that begins on the distal extremities and spreads centripetally, usually sparing the neck, face, and head. The rash may be petechial, maculopapular, urticarial, vesicular, or a combination. The rash is accompanied by high fever and edema of the extremities. Complications such as pneumonia and hepatitis may occur.
The course of atypical measles is more prolonged than with classic measles, but because these patients are thought to have partial protection against the virus, they do not transmit it and are not considered contagious.26
DIAGNOSIS OF MEASLES
The classic clinical features are usually enough to distinguish measles from other febrile illnesses with similar clinical manifestions, such as rubella, dengue, parvovirus B19 infection, erythema multiforme, Stevens-Johnson syndrome, and streptococcal scarlet fever. The distinctive measles prodrome, Koplik spots, the progression of the rash from the head and neck to the trunk and the extremities, and the severity of disease are distinctive features of measles.
Laboratory tests to confirm the diagnosis are often used in areas where measles is rare, and laboratory confirmation is currently recommended in the United States. Because viral isolation is technically difficult and is not widely available, serologic testing is the method most commonly used. The measles-specific immunoglobulin M (IgM) antibody assay, the test used most often, is almost 100% sensitive when done 2 to 3 days after the onset of the rash.27,28 Measles IgM antibody peaks at 4 weeks after the infection and disappears by 6 to 8 weeks.
It is important to remember that false-positive measles IgM antibody may occur with other viral infections, such as parvovirus B19 and rubella. Because measles-specific IgG antibody is produced with the onset of infection and peaks at 4 weeks, a fourfold rise in the IgG titer is useful in confirming the diagnosis. Measles IgG antibody after infection is sustained for life.
Reverse transcription-polymerase chain reaction testing can also detect measles virus in the blood and urine when direct evidence of the virus is necessary, such as in immunocompromised patients.29
TREATMENT IS SUPPORTIVE
Treatment of measles mainly involves supportive measures, such as fluids and antipyretics. Antiviral agents such as ribavirin and interferon have in vitro activity against the measles virus and have been used to treat severe measles infection in immunocompromised patients. However, their clinical efficacy is unproven.30
Routine use of antibacterial agents to prevent secondary bacterial infection is not recommended.
CURRENT RECOMMENDATIONS FOR ACTIVE IMMUNIZATION
Active immunization for measles has been available since 1963. Between 1963 and 1967, both killed-virus and live-virus vaccines were available. As atypical measles cases became recognized, the killed-virus vaccine was withdrawn.
The vaccine currently available in the United States is a live-attenuated strain prepared in chicken embryo cell culture and combined with mumps and rubella vaccine (MMR) or mumps, rubella, and varicella vaccine (MMRV).
Two doses of live-virus measles vaccine are recommended for all healthy children before they begin school, with the first dose given at 12 to 15 months of age. A second dose is needed because the failure rate with one dose is 5%. More than 99% of people who receive two doses separated by 4 weeks develop serologic evidence of measles.
Waning immunity after vaccination occurs very rarely, with approximately 5% of children developing secondary vaccine failure 10 to 15 years after vaccination.3,31
Although rates of vaccination in the United States are high, cases of measles continue to occur in unvaccinated infants and in children who are either too young to be vaccinated or whose parents claimed exemption because of religious or personal beliefs.
Because of the occurrence of measles cases in adolescents, young adults, and adults, potentially susceptible people should be identified and vaccinated according to current guidelines. People should be considered susceptible unless they have documentation of at least two doses of measles vaccine given at least 28 days apart, physician-diagnosed measles, laboratory evidence of immunity to measles, or were born before 1957. All adults who are susceptible should receive at least one dose of measles vaccine.10 Adults at higher risk of contracting measles include:
- Students in high school and college
- International travelers
- Health care personnel.
For these adults, two doses of measles vaccine, at least 28 days apart, are recommended.32
Postexposure prophylaxis
Measles vaccination given to susceptible contacts within 72 hours of exposure as postexposure prophylaxis may protect against infection and induces protection against subsequent exposures to measles.33,34 Vaccination is the intervention of choice for susceptible individuals older than 12 months of age who are exposed to measles and who do not have a contraindication to measles vaccination.35 Active rather than passive immunization is also the strategy of choice for controlling measles outbreaks.
Passive immunization with intramuscular immune globulin within 6 days of exposure can be used in selected circumstances to prevent transmission or to modify the clinical course of the infection.36 Immune globulin therapy is recommended for susceptible individuals who are exposed to measles and who are at high risk of developing severe or fatal measles. This includes individuals who are being treated with immunosuppressive agents, those with HIV infection, pregnant women, and infants less than 1 year of age. Immune globulin should not be used to control measles outbreaks.
ADVERSE EFFECTS OF MEASLES VACCINE
Live-virus measles vaccine has an excellent safety record. A transient fever, which may be accompanied by a measles-like rash, occurs in 5% to 15% of people 5 to 12 days after vaccination. The rash may be discrete or confluent and is self-limited.
Although measles vaccine is a live-attenuated vaccine, vaccinated people do not transmit the virus to susceptible contacts and are not considered contagious, even if they develop a vaccine-associated rash. Thus, the vaccine can be safely given to close contacts of immunocompromised and other susceptible people. Encephalitis is exceedingly rare following vaccination.
There is no scientific evidence that the risk of autism is higher in children who receive measles or MMR vaccine than in unvaccinated children.37 An Institute of Medicine report in 2001 rejected a causal relationship between MMR vaccine and autism spectrum disorders.38
CONTRAINDICATIONS TO MEASLES VACCINATION
Measles vaccine is contraindicated for:
- People who have cell-mediated immune deficiencies (except patients wtih HIV infection—see discussion just below)
- Pregnant women
- Those who had a severe allergic reaction to a vaccine component after a previous dose
- Those with moderate or severe acute illness
- Those who have recently received immune globulin products.
HIV-infected patients with severe immunosuppression should not receive the liveattenuated measles vaccine. However, because patients with HIV are at risk of severe measles, and because the vaccine has been shown to be safe in HIV patients who do not have severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression. 39
After receiving immune globulin
Anyone who has recently received immune globulin should not receive measles vaccine until sufficient time has passed, since passively acquired antibodies interfere with the immune response to live-virus vaccines. How long to wait depends on the type of immune globulin, the indication, the amount, and the route of administration. In general, the waiting period is:
- At least 3 months after intramuscular immune globulin or tetanus, hepatitis A, or hepatitis B prophylaxis
- At least 4 months after intramuscular immune globulin for rabies, or 6 months after intravenous immune globulin for cytomegalovirus (dose, 150 mg/kg)
- At least 8 months after intravenous immune globulin as replacement or therapy for immune deficiencies (dose, 400 mg/kg), or after intravenous immune globulin for immune thrombocytopenic purpura (400 mg/kg)
- At least 10 months after intravenous immune globulin for immune thrombocytopenic pupura at a dose of 1 g/kg.39
Egg allergy is not a contraindication
Although measles vaccine is produced in chick embryo cell culture, the vaccine has been shown to be safe in people with egg allergy, so they may be vaccinated without first being tested for egg allergy.39,40
- Lachmann PJ. Immunopathology of measles. Proc R Soc Med 1974; 67:1120–1122.
- Enders JF, McCarthy K, Mitus A, Cheatham WJ. Isolation of measles virus at autopsy in case of giant cell pneumonia without rash. N Engl J Med 1959; 261:875–881.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stokes J, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Farizo KM, Stehr-Green PA, Simpson DM, Markowitz LE. Pediatric emergency room visits: a risk factor for acquiring measles. Pediatrics 1991; 87:74–79.
- Bloch AB, Orenstein W, Ewing WM, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics 1985; 75:676–683.
- Remington PL, Hall WN, Davis IH, et al. Airborne transmission of measles in a physician’s office. JAMA 1985; 253:1574–1577.
- US Centers for Disease Control and Prevention. Reported vaccine-preventable diseases—United States, 1993, and the Childhood Immunization Initiative. MMWR 1994; 43:57–60.
- Orenstein WA, Papania MJ, Wharton ME. Measles elimination in the United States. J Infect Dis 2004; 189 (suppl 1):S1–S3.
- US Centers for Disease Control and Prevention. Update: Measles—United States, January–July 2008. MMWR 2008; 57:893–896.
- Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med 2006; 355:447–455.
- Mulholland EK. Measles in the United States, 2006. N Engl J Med 2006; 355:440–443.
- Barkin RM. Measles mortality. Analysis of the primary cause of death. Am J Dis Child 1975; 129:307–309.
- Barkin RM. Measles mortality: a retrospective look at the vaccine era. Am J Epidemiol 1975; 102:341–349.
- US Centers for Disease Control and Prevention. Public-sector vaccination efforts in response to the resurgence of measles among preschool-aged children—United States, 1989–1991. MMWR 1992; 41:522–525.
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. an analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189( suppl 1):S4–S16.
- Johnson RT, Griffin DE, Hirsch RL, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Gershon AA. Chickenpox, measles, and mumps. In:Remington J, Klein J, eds. Infectious Diseases of the Fetus and Newborn Infant. Elsevier Saunders: Philadelphia, 2006:693–738.
- Mitus A, Enders JF, Craig JM, Holloway A. Persistence of measles virus and depression of antibody formation in patients with giant-cell pneumonia after measles. N Engl J Med 1959; 261:882–889.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Mayo DR, Brennan T, Cormier DP, Hadler J, Lamb P. Evaluation of a commercial measles virus immunoglobulin M enzyme immunoassay. J Clin Microbiol 1991; 29:2865–2867.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187( suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Forni Al, Schluger NW, Roberts RB. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis 1994; 19:454–462.
- Anders JF, Jacobsen RM, Poland GA, Jacobsen SJ, Wollan PC. Secondary failure rates of measles vaccines: a metaanalysis of published studes. Pediar Infect Dis J 1996; 15:62–66.
- US Centers for Disease Control and Prevention. Measles—United States, January 1–April 25, 2008. MMWR 2008; 57:494–498.
- Berkovitz S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
- Strebel PM, Papania MJ, Halsey NA. Measles vaccine. In:Plotkin SA, Orenstein WA, eds. Vaccines. Saunders: New York, 2004:389–440.
- US Centers for Disease Control and Prevention. Measles, mumps and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998; 47( RR-8):1–57. http://www.cdc.gov/mmwr/PDF/rr/rr4708.pdf. Accessed December 30, 2009.
- Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med 2002; 347:1477–1482.
- Straton K, Gable A, Shetty P, McCormick M; for the Institute of Medicine. Immunization Safety Review: Measles-Mumps-Rubella Vaccine and Autism. National Academy Press: Washington, DC, 2001.
- Pickerington LK, Baker CJ, Long SS, McMillan JA, editors. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:444–455.
- James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med 1995; 332:1262–1266.
- Lachmann PJ. Immunopathology of measles. Proc R Soc Med 1974; 67:1120–1122.
- Enders JF, McCarthy K, Mitus A, Cheatham WJ. Isolation of measles virus at autopsy in case of giant cell pneumonia without rash. N Engl J Med 1959; 261:875–881.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stokes J, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Farizo KM, Stehr-Green PA, Simpson DM, Markowitz LE. Pediatric emergency room visits: a risk factor for acquiring measles. Pediatrics 1991; 87:74–79.
- Bloch AB, Orenstein W, Ewing WM, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics 1985; 75:676–683.
- Remington PL, Hall WN, Davis IH, et al. Airborne transmission of measles in a physician’s office. JAMA 1985; 253:1574–1577.
- US Centers for Disease Control and Prevention. Reported vaccine-preventable diseases—United States, 1993, and the Childhood Immunization Initiative. MMWR 1994; 43:57–60.
- Orenstein WA, Papania MJ, Wharton ME. Measles elimination in the United States. J Infect Dis 2004; 189 (suppl 1):S1–S3.
- US Centers for Disease Control and Prevention. Update: Measles—United States, January–July 2008. MMWR 2008; 57:893–896.
- Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med 2006; 355:447–455.
- Mulholland EK. Measles in the United States, 2006. N Engl J Med 2006; 355:440–443.
- Barkin RM. Measles mortality. Analysis of the primary cause of death. Am J Dis Child 1975; 129:307–309.
- Barkin RM. Measles mortality: a retrospective look at the vaccine era. Am J Epidemiol 1975; 102:341–349.
- US Centers for Disease Control and Prevention. Public-sector vaccination efforts in response to the resurgence of measles among preschool-aged children—United States, 1989–1991. MMWR 1992; 41:522–525.
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. an analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189( suppl 1):S4–S16.
- Johnson RT, Griffin DE, Hirsch RL, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Gershon AA. Chickenpox, measles, and mumps. In:Remington J, Klein J, eds. Infectious Diseases of the Fetus and Newborn Infant. Elsevier Saunders: Philadelphia, 2006:693–738.
- Mitus A, Enders JF, Craig JM, Holloway A. Persistence of measles virus and depression of antibody formation in patients with giant-cell pneumonia after measles. N Engl J Med 1959; 261:882–889.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Mayo DR, Brennan T, Cormier DP, Hadler J, Lamb P. Evaluation of a commercial measles virus immunoglobulin M enzyme immunoassay. J Clin Microbiol 1991; 29:2865–2867.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187( suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Forni Al, Schluger NW, Roberts RB. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis 1994; 19:454–462.
- Anders JF, Jacobsen RM, Poland GA, Jacobsen SJ, Wollan PC. Secondary failure rates of measles vaccines: a metaanalysis of published studes. Pediar Infect Dis J 1996; 15:62–66.
- US Centers for Disease Control and Prevention. Measles—United States, January 1–April 25, 2008. MMWR 2008; 57:494–498.
- Berkovitz S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
- Strebel PM, Papania MJ, Halsey NA. Measles vaccine. In:Plotkin SA, Orenstein WA, eds. Vaccines. Saunders: New York, 2004:389–440.
- US Centers for Disease Control and Prevention. Measles, mumps and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998; 47( RR-8):1–57. http://www.cdc.gov/mmwr/PDF/rr/rr4708.pdf. Accessed December 30, 2009.
- Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med 2002; 347:1477–1482.
- Straton K, Gable A, Shetty P, McCormick M; for the Institute of Medicine. Immunization Safety Review: Measles-Mumps-Rubella Vaccine and Autism. National Academy Press: Washington, DC, 2001.
- Pickerington LK, Baker CJ, Long SS, McMillan JA, editors. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:444–455.
- James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med 1995; 332:1262–1266.
KEY POINTS
- Measles is one of the most contagious infectious diseases, with a secondary attack rate of at least 90% in susceptible household contacts.
- Since 1993, most reported cases of measles have been directly or indirectly linked to international travel, and many have occurred in adults.
- Acute measles encephalitis, a neurologic complication of measles, is more common in adults than in children and is characterized by the resurgence of fever during the convalescent phase, along with headaches, seizures, and altered consciousness.