User login
Variation in COVID-19 Mortality Across 117 US Hospitals in High- and Low-Burden Settings
It is clear that certain patient-level factors, such as age, sex, and comorbidities, predict outcomes of SARS-CoV-2 infection.1,2 Less is known about whether hospital-level factors, including surges of patients with COVID-19, are associated with patient outcomes.
In a multicenter cohort study of 2,215 patients with COVID-19 in 65 intensive care units (ICU) across the United States, mortality rates varied widely (6.6%-80.8%), with improved survival for patients admitted to a hospital with more (>100) rather than fewer (<50) ICU beds.3 A different study found that at the state level, COVID-19 mortality increased with increasing COVID-19 admissions.4 Together, these studies suggest that surges in COVID-19 patient volume may be associated with excess mortality. However, the first study was restricted to the ICU population, limiting generalizability, and did not consider admission volume, only ICU bed count. Meanwhile, the second study considered both hospital capacity and patient volume, but it describes a relatively small sample, did not adjust for patient-level predictors of mortality, and does not report outcomes at the hospital level.
Here, we used a large dataset to compare in-hospital mortality rates for patients with COVID-19 across US hospitals, hypothesizing that mortality would be higher in hospitals with the highest burden of COVID-19 admissions. By adjusting for patient-level predictors of mortality and normalizing admission volume for hospital size, we are able to describe residual variability in mortality that may be attributable to differences in COVID-19 patient volume.
METHODS
We included patients with an International Statistical Classification of Diseases, Tenth Revision (ICD)-10 diagnosis of COVID-19 (U07.1) who were admitted to a US hospital that contracts with CarePort Health.5 CarePort is a platform for discharge planning and care coordination that contracts with hospitals in all US regions and auto-extracts data using interface feeds.
We restricted the population to patients admitted between April 1 and April 30, 2020, after a new ICD-10 code for confirmed COVID-19 infection became available, and to hospitals that provided real-time ICD-10 data and pertinent demographic information and could be linked to Centers for Medicare & Medicaid Services (CMS) data by National Provider Identifier. We assumed that the 145 patients (1.0%) who remained hospitalized at 5 weeks all survived. For the 5.9% of patients with multiple admissions during the study period, we included only the first admission with a diagnosis code for COVID-19.
We adjusted for patient age, sex, and the 31 comorbidities in the Elixhauser index, defined by ICD-10 codes. This set of comorbidities includes those previously associated with COVID-19 survival.1,2,6 Unfortunately, inconsistent reporting of vital signs and laboratory data precluded adjusting for acute illness severity. For those patients whose residence zip code was known, we report the racial breakdown (White vs non-White) and adjusted gross income (AGI), based on linked information from the 2018 American Community Survey.7
We defined COVID-19 burden as the quotient of COVID-19 admissions in April 2020 and each hospital’s certified bed count, as reported to the CMS.8 This allowed us to normalize COVID-19 patient volume for variation in hospital size, acknowledging that admitting 10 patients with COVID-19 to a 1,000-bed hospital is different from admitting 10 patients with COVID-19 to a 20-bed hospital. Certified bed count seemed the ideal denominator because it excludes beds not readily deployable to care for patients with COVID-19 (eg, radiology suites, labor and delivery rooms).
We computed hospital-specific adjusted mortality proportions and 95% confidence intervals based on hierarchical multivariable logistic regression, adjusting for age, sex, and comorbidities, and a random effect for each hospital.9,10 Hypothesizing that there may be a threshold of burden beyond which mortality begins to rise, we compared the in-hospital mortality rate at hospitals in the highest quintile of COVID-19 burden to all other hospitals.
We conducted eight post-hoc sensitivity analyses: (1) restricting the study population to patients aged 75 years and older; (2) restricting study hospitals to those with at least 100 beds and 20 COVID-19 admissions; (3) assuming that all patients who remained hospitalized at 5 weeks had died; (4) using each patient’s last admission during the month of April rather than the first; sequentially incorporating (5) zip code–level information on race (limited to White, non-White) and (6) AGI (treated as a continuous variable) into our model; (7) computing two burdens for each hospital (one for each half of April) and using whichever was higher; and (8) treating COVID-19 burden as a continuous predictor. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc) using the GLIMMIX procedure. This study was deemed exempt by the University of California, San Francisco Institutional Review Board.
RESULTS
The study population included 14,226 patients with COVID-19 (median age, 66 years [range, 0-110 years]; 45.2% women) at 117 US hospitals. Based on patients’ zip code of residence, we estimate that 47.0% of patients were White and 29.1% Black, and that the mean household AGI was $61,956. Most hospitals were nonprofit (56%) or private (39%), with approximately one quarter coming from each US census region (range, 25 hospitals [21%] in Midwest to 33 hospitals [28%] in Northeast). Nine hospitals (8%) had more than 700 beds, 40 (34%) had 300 to 700 beds, and 68 (58%) had fewer than 300 beds. Thirty-six hospitals (30.8%) admitted fewer than 20 patients with COVID-19, while six hospitals (5.1%) admitted more than 500 such patients. COVID burden ranged from 0.004 to 2.03 admissions per bed.
As of June 5, 2020, 78.1% of patients had been discharged alive, 20.9% had died, and 1.0% remained hospitalized. At the hospital level, the observed mortality ranged from 0% to 44.4%, was 17.1% among hospitals in COVID-19 burden quintiles one through four, and was 22.7% in the highest burden quintile (Table). 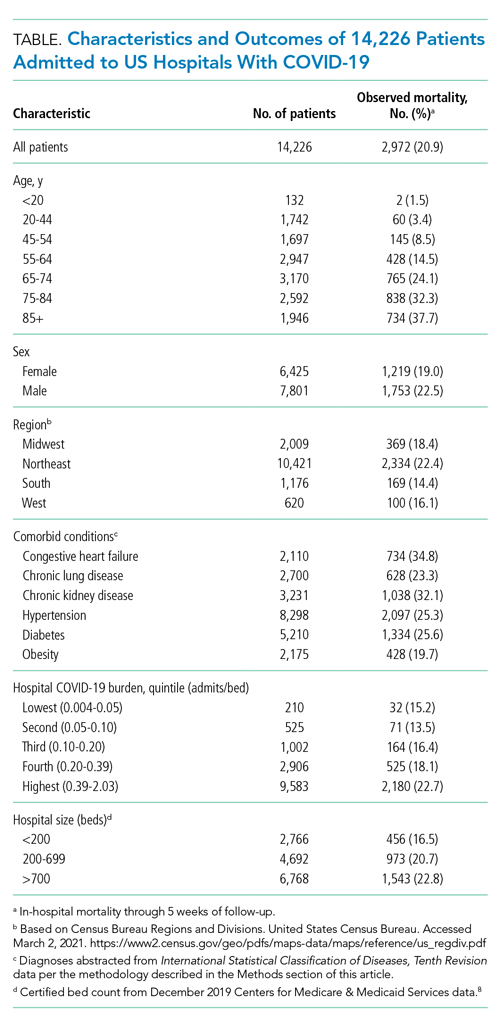

Results were similar across multiple sensitivity analyses (see Appendix Table), although the relationship between COVID-19 burden and in-hospital mortality was attenuated and not significant when the sample was restricted to hospitals with at least 100 beds and 20 COVID-19 admissions, or in analyses adjusted for race and AGI.
DISCUSSION
In this study of 14,226 patients with COVID-19 across 117 US hospitals, those patients admitted to the most burdened hospitals had a higher odds of death. This relationship, which persisted after adjusting for age, sex, and comorbid conditions, suggests that a threshold exists at which patient surges may cause excess mortality.
Notably, in sensitivity analyses adjusting for race and AGI, COVID-19 burden was no longer associated with in-hospital mortality and the point estimate was attenuated. This raises the possibility that our primary results are confounded by these factors. However, prior studies of hospitalized patients have not found race to be predictive of mortality, after adjusting for other factors.11,12
We also note that the relationship between COVID-19 burden and mortality was not significant (P = .07) when the sample was restricted to larger hospitals with more than 20 COVID-19 admissions; again, the point estimate was attenuated. This suggests that larger hospitals may be more resilient in the face of patient surges. Whether this is due to increased availability of staff who can be redeployed to patient care (as with researchers at academic centers), increased experience managing severe respiratory failure, or other factors is uncertain.
Interestingly, in-hospital mortality varied widely across study hospitals, even among the most-burdened hospitals. The reasons for this residual variability—after adjusting for age, sex, and comorbidities and stratifying by COVID-19 burden—are uncertain. To the extent that this variability reflects differences in patient management, hospital staffing, or use of investigational or advanced therapies, it will be critical to identify and disseminate any replicable best practices from high-burden hospitals with low mortality rates.
Whereas other reports have often described single-center or regional experiences,13-15 leaving open the possibility that their results were highly influenced by the local nature of the pandemic in their respective settings, our report from a large sample of hospitals across the United States in high- and low-burden settings provides a more generalizable description of mortality rates for hospitalized patients. Additional study strengths include our adjustment for comorbidities known to be associated with COVID-19 survival, the reporting of definitive outcomes for 99% of patients, and the inclusion of multiple sensitivity analyses to assess the stability of findings.
Our principal limitation is the inability to adjust for severity of acute illness due to inconsistent reporting of laboratory and vital signs data from study hospitals and missing information on interhospital transfers. While our adjusted analyses clearly suggest an association between COVID-19 burden and patient outcomes, our results may still be confounded by differences in illness severity at study hospitals. Thus, our findings should be considered hypothesis-generating and will require confirmation in future studies that include adjustment for acute illness severity.
Other limitations of our study include overrepresentation of large urban hospitals in the Northeast, although this represents the geography of the US pandemic during the study period. Our adjustment for race/ethnicity and socioeconomic status was limited in that we only had zip code-of-residence level information, did not know the zip code of residence for one quarter of study patients, and had to bifurcate the population into White/non-White categories. Finally, our definition of burden does not account for hospital resources, including staffing, ICU capacity, and the availability of advanced or investigational therapies.
CONCLUSION
In this study of 14,226 patients with COVID-19 admitted to 1 of 117 US hospitals, we found that the odds of in-hospital mortality were higher in hospitals that had the highest burden of COVID-19 admissions. This relationship, which persisted after adjustment for age, sex, and comorbid conditions, suggests that patient surges may be an independent risk factor for in-hospital death among patients with COVID-19.
ACKNOWLEGMENTS
The authors thank Bocheng Jing, MS, Senior Statistician at the UCSF Pepper Center, for providing code to identify Elixhauser conditions from ICD-10 data; and Scott Kerber, BS, and Scott Magnoni, MS, both of CarePort Health, for assistance with data extraction. They were not compensated for this work beyond their regular salaries.
1. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Centers for Disease Control and Prevention. Updated November 2, 2020. Accessed December 29, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html
2. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/S0140-6736(20)31189-2
3. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1-12. https://doi.org/10.1001/jamainternmed.2020.4568
4. Karaca-Mandic P, Sen S, Georgiou A, Zhu Y, Basu A. Association of COVID-19-related hospital use and overall covid-19 mortality in the USA. J Gen Intern Med. 2020:1-3. https://doi.org/10.1007/s11606-020-06084-7
5. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. Centers for Disease Control and Prevention. Accessed June 2, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf
6. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
7. About the American Community Survey. United States Census Bureau. Updated January 4, 2021. Accessed March 2, 2021. https://www.census.gov/programs-surveys/acs/about.html
8. Provider of service files. Centers for Medicare & Medicaid Services. Revised January 15, 2020. Accessed March 2, 2021. https://www.cms.gov/research-statistics-data-systems/provider-services-current-files/2019-pos-file
9. Ash AS, Fienberg SE, Louis TA, et al. Statistical issues in assessing hospital performance. Committee of Presidents of Statistical Societies white paper. January 2012. Accessed March 1, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Statistical-Issues-in-Assessing-Hospital-Performance.pdf
10. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLoS One. 2011;12;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401
11. Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33-41. https://doi.org/10.7326/M20-3905
12. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/NEJMsa2011686
13. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012-2022. https://doi.org/10.1056/NEJMoa2004500
14. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. https://doi.org/10.1016/S2213-2600(20)30079-5
15. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
It is clear that certain patient-level factors, such as age, sex, and comorbidities, predict outcomes of SARS-CoV-2 infection.1,2 Less is known about whether hospital-level factors, including surges of patients with COVID-19, are associated with patient outcomes.
In a multicenter cohort study of 2,215 patients with COVID-19 in 65 intensive care units (ICU) across the United States, mortality rates varied widely (6.6%-80.8%), with improved survival for patients admitted to a hospital with more (>100) rather than fewer (<50) ICU beds.3 A different study found that at the state level, COVID-19 mortality increased with increasing COVID-19 admissions.4 Together, these studies suggest that surges in COVID-19 patient volume may be associated with excess mortality. However, the first study was restricted to the ICU population, limiting generalizability, and did not consider admission volume, only ICU bed count. Meanwhile, the second study considered both hospital capacity and patient volume, but it describes a relatively small sample, did not adjust for patient-level predictors of mortality, and does not report outcomes at the hospital level.
Here, we used a large dataset to compare in-hospital mortality rates for patients with COVID-19 across US hospitals, hypothesizing that mortality would be higher in hospitals with the highest burden of COVID-19 admissions. By adjusting for patient-level predictors of mortality and normalizing admission volume for hospital size, we are able to describe residual variability in mortality that may be attributable to differences in COVID-19 patient volume.
METHODS
We included patients with an International Statistical Classification of Diseases, Tenth Revision (ICD)-10 diagnosis of COVID-19 (U07.1) who were admitted to a US hospital that contracts with CarePort Health.5 CarePort is a platform for discharge planning and care coordination that contracts with hospitals in all US regions and auto-extracts data using interface feeds.
We restricted the population to patients admitted between April 1 and April 30, 2020, after a new ICD-10 code for confirmed COVID-19 infection became available, and to hospitals that provided real-time ICD-10 data and pertinent demographic information and could be linked to Centers for Medicare & Medicaid Services (CMS) data by National Provider Identifier. We assumed that the 145 patients (1.0%) who remained hospitalized at 5 weeks all survived. For the 5.9% of patients with multiple admissions during the study period, we included only the first admission with a diagnosis code for COVID-19.
We adjusted for patient age, sex, and the 31 comorbidities in the Elixhauser index, defined by ICD-10 codes. This set of comorbidities includes those previously associated with COVID-19 survival.1,2,6 Unfortunately, inconsistent reporting of vital signs and laboratory data precluded adjusting for acute illness severity. For those patients whose residence zip code was known, we report the racial breakdown (White vs non-White) and adjusted gross income (AGI), based on linked information from the 2018 American Community Survey.7
We defined COVID-19 burden as the quotient of COVID-19 admissions in April 2020 and each hospital’s certified bed count, as reported to the CMS.8 This allowed us to normalize COVID-19 patient volume for variation in hospital size, acknowledging that admitting 10 patients with COVID-19 to a 1,000-bed hospital is different from admitting 10 patients with COVID-19 to a 20-bed hospital. Certified bed count seemed the ideal denominator because it excludes beds not readily deployable to care for patients with COVID-19 (eg, radiology suites, labor and delivery rooms).
We computed hospital-specific adjusted mortality proportions and 95% confidence intervals based on hierarchical multivariable logistic regression, adjusting for age, sex, and comorbidities, and a random effect for each hospital.9,10 Hypothesizing that there may be a threshold of burden beyond which mortality begins to rise, we compared the in-hospital mortality rate at hospitals in the highest quintile of COVID-19 burden to all other hospitals.
We conducted eight post-hoc sensitivity analyses: (1) restricting the study population to patients aged 75 years and older; (2) restricting study hospitals to those with at least 100 beds and 20 COVID-19 admissions; (3) assuming that all patients who remained hospitalized at 5 weeks had died; (4) using each patient’s last admission during the month of April rather than the first; sequentially incorporating (5) zip code–level information on race (limited to White, non-White) and (6) AGI (treated as a continuous variable) into our model; (7) computing two burdens for each hospital (one for each half of April) and using whichever was higher; and (8) treating COVID-19 burden as a continuous predictor. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc) using the GLIMMIX procedure. This study was deemed exempt by the University of California, San Francisco Institutional Review Board.
RESULTS
The study population included 14,226 patients with COVID-19 (median age, 66 years [range, 0-110 years]; 45.2% women) at 117 US hospitals. Based on patients’ zip code of residence, we estimate that 47.0% of patients were White and 29.1% Black, and that the mean household AGI was $61,956. Most hospitals were nonprofit (56%) or private (39%), with approximately one quarter coming from each US census region (range, 25 hospitals [21%] in Midwest to 33 hospitals [28%] in Northeast). Nine hospitals (8%) had more than 700 beds, 40 (34%) had 300 to 700 beds, and 68 (58%) had fewer than 300 beds. Thirty-six hospitals (30.8%) admitted fewer than 20 patients with COVID-19, while six hospitals (5.1%) admitted more than 500 such patients. COVID burden ranged from 0.004 to 2.03 admissions per bed.
As of June 5, 2020, 78.1% of patients had been discharged alive, 20.9% had died, and 1.0% remained hospitalized. At the hospital level, the observed mortality ranged from 0% to 44.4%, was 17.1% among hospitals in COVID-19 burden quintiles one through four, and was 22.7% in the highest burden quintile (Table). 

Results were similar across multiple sensitivity analyses (see Appendix Table), although the relationship between COVID-19 burden and in-hospital mortality was attenuated and not significant when the sample was restricted to hospitals with at least 100 beds and 20 COVID-19 admissions, or in analyses adjusted for race and AGI.
DISCUSSION
In this study of 14,226 patients with COVID-19 across 117 US hospitals, those patients admitted to the most burdened hospitals had a higher odds of death. This relationship, which persisted after adjusting for age, sex, and comorbid conditions, suggests that a threshold exists at which patient surges may cause excess mortality.
Notably, in sensitivity analyses adjusting for race and AGI, COVID-19 burden was no longer associated with in-hospital mortality and the point estimate was attenuated. This raises the possibility that our primary results are confounded by these factors. However, prior studies of hospitalized patients have not found race to be predictive of mortality, after adjusting for other factors.11,12
We also note that the relationship between COVID-19 burden and mortality was not significant (P = .07) when the sample was restricted to larger hospitals with more than 20 COVID-19 admissions; again, the point estimate was attenuated. This suggests that larger hospitals may be more resilient in the face of patient surges. Whether this is due to increased availability of staff who can be redeployed to patient care (as with researchers at academic centers), increased experience managing severe respiratory failure, or other factors is uncertain.
Interestingly, in-hospital mortality varied widely across study hospitals, even among the most-burdened hospitals. The reasons for this residual variability—after adjusting for age, sex, and comorbidities and stratifying by COVID-19 burden—are uncertain. To the extent that this variability reflects differences in patient management, hospital staffing, or use of investigational or advanced therapies, it will be critical to identify and disseminate any replicable best practices from high-burden hospitals with low mortality rates.
Whereas other reports have often described single-center or regional experiences,13-15 leaving open the possibility that their results were highly influenced by the local nature of the pandemic in their respective settings, our report from a large sample of hospitals across the United States in high- and low-burden settings provides a more generalizable description of mortality rates for hospitalized patients. Additional study strengths include our adjustment for comorbidities known to be associated with COVID-19 survival, the reporting of definitive outcomes for 99% of patients, and the inclusion of multiple sensitivity analyses to assess the stability of findings.
Our principal limitation is the inability to adjust for severity of acute illness due to inconsistent reporting of laboratory and vital signs data from study hospitals and missing information on interhospital transfers. While our adjusted analyses clearly suggest an association between COVID-19 burden and patient outcomes, our results may still be confounded by differences in illness severity at study hospitals. Thus, our findings should be considered hypothesis-generating and will require confirmation in future studies that include adjustment for acute illness severity.
Other limitations of our study include overrepresentation of large urban hospitals in the Northeast, although this represents the geography of the US pandemic during the study period. Our adjustment for race/ethnicity and socioeconomic status was limited in that we only had zip code-of-residence level information, did not know the zip code of residence for one quarter of study patients, and had to bifurcate the population into White/non-White categories. Finally, our definition of burden does not account for hospital resources, including staffing, ICU capacity, and the availability of advanced or investigational therapies.
CONCLUSION
In this study of 14,226 patients with COVID-19 admitted to 1 of 117 US hospitals, we found that the odds of in-hospital mortality were higher in hospitals that had the highest burden of COVID-19 admissions. This relationship, which persisted after adjustment for age, sex, and comorbid conditions, suggests that patient surges may be an independent risk factor for in-hospital death among patients with COVID-19.
ACKNOWLEGMENTS
The authors thank Bocheng Jing, MS, Senior Statistician at the UCSF Pepper Center, for providing code to identify Elixhauser conditions from ICD-10 data; and Scott Kerber, BS, and Scott Magnoni, MS, both of CarePort Health, for assistance with data extraction. They were not compensated for this work beyond their regular salaries.
It is clear that certain patient-level factors, such as age, sex, and comorbidities, predict outcomes of SARS-CoV-2 infection.1,2 Less is known about whether hospital-level factors, including surges of patients with COVID-19, are associated with patient outcomes.
In a multicenter cohort study of 2,215 patients with COVID-19 in 65 intensive care units (ICU) across the United States, mortality rates varied widely (6.6%-80.8%), with improved survival for patients admitted to a hospital with more (>100) rather than fewer (<50) ICU beds.3 A different study found that at the state level, COVID-19 mortality increased with increasing COVID-19 admissions.4 Together, these studies suggest that surges in COVID-19 patient volume may be associated with excess mortality. However, the first study was restricted to the ICU population, limiting generalizability, and did not consider admission volume, only ICU bed count. Meanwhile, the second study considered both hospital capacity and patient volume, but it describes a relatively small sample, did not adjust for patient-level predictors of mortality, and does not report outcomes at the hospital level.
Here, we used a large dataset to compare in-hospital mortality rates for patients with COVID-19 across US hospitals, hypothesizing that mortality would be higher in hospitals with the highest burden of COVID-19 admissions. By adjusting for patient-level predictors of mortality and normalizing admission volume for hospital size, we are able to describe residual variability in mortality that may be attributable to differences in COVID-19 patient volume.
METHODS
We included patients with an International Statistical Classification of Diseases, Tenth Revision (ICD)-10 diagnosis of COVID-19 (U07.1) who were admitted to a US hospital that contracts with CarePort Health.5 CarePort is a platform for discharge planning and care coordination that contracts with hospitals in all US regions and auto-extracts data using interface feeds.
We restricted the population to patients admitted between April 1 and April 30, 2020, after a new ICD-10 code for confirmed COVID-19 infection became available, and to hospitals that provided real-time ICD-10 data and pertinent demographic information and could be linked to Centers for Medicare & Medicaid Services (CMS) data by National Provider Identifier. We assumed that the 145 patients (1.0%) who remained hospitalized at 5 weeks all survived. For the 5.9% of patients with multiple admissions during the study period, we included only the first admission with a diagnosis code for COVID-19.
We adjusted for patient age, sex, and the 31 comorbidities in the Elixhauser index, defined by ICD-10 codes. This set of comorbidities includes those previously associated with COVID-19 survival.1,2,6 Unfortunately, inconsistent reporting of vital signs and laboratory data precluded adjusting for acute illness severity. For those patients whose residence zip code was known, we report the racial breakdown (White vs non-White) and adjusted gross income (AGI), based on linked information from the 2018 American Community Survey.7
We defined COVID-19 burden as the quotient of COVID-19 admissions in April 2020 and each hospital’s certified bed count, as reported to the CMS.8 This allowed us to normalize COVID-19 patient volume for variation in hospital size, acknowledging that admitting 10 patients with COVID-19 to a 1,000-bed hospital is different from admitting 10 patients with COVID-19 to a 20-bed hospital. Certified bed count seemed the ideal denominator because it excludes beds not readily deployable to care for patients with COVID-19 (eg, radiology suites, labor and delivery rooms).
We computed hospital-specific adjusted mortality proportions and 95% confidence intervals based on hierarchical multivariable logistic regression, adjusting for age, sex, and comorbidities, and a random effect for each hospital.9,10 Hypothesizing that there may be a threshold of burden beyond which mortality begins to rise, we compared the in-hospital mortality rate at hospitals in the highest quintile of COVID-19 burden to all other hospitals.
We conducted eight post-hoc sensitivity analyses: (1) restricting the study population to patients aged 75 years and older; (2) restricting study hospitals to those with at least 100 beds and 20 COVID-19 admissions; (3) assuming that all patients who remained hospitalized at 5 weeks had died; (4) using each patient’s last admission during the month of April rather than the first; sequentially incorporating (5) zip code–level information on race (limited to White, non-White) and (6) AGI (treated as a continuous variable) into our model; (7) computing two burdens for each hospital (one for each half of April) and using whichever was higher; and (8) treating COVID-19 burden as a continuous predictor. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc) using the GLIMMIX procedure. This study was deemed exempt by the University of California, San Francisco Institutional Review Board.
RESULTS
The study population included 14,226 patients with COVID-19 (median age, 66 years [range, 0-110 years]; 45.2% women) at 117 US hospitals. Based on patients’ zip code of residence, we estimate that 47.0% of patients were White and 29.1% Black, and that the mean household AGI was $61,956. Most hospitals were nonprofit (56%) or private (39%), with approximately one quarter coming from each US census region (range, 25 hospitals [21%] in Midwest to 33 hospitals [28%] in Northeast). Nine hospitals (8%) had more than 700 beds, 40 (34%) had 300 to 700 beds, and 68 (58%) had fewer than 300 beds. Thirty-six hospitals (30.8%) admitted fewer than 20 patients with COVID-19, while six hospitals (5.1%) admitted more than 500 such patients. COVID burden ranged from 0.004 to 2.03 admissions per bed.
As of June 5, 2020, 78.1% of patients had been discharged alive, 20.9% had died, and 1.0% remained hospitalized. At the hospital level, the observed mortality ranged from 0% to 44.4%, was 17.1% among hospitals in COVID-19 burden quintiles one through four, and was 22.7% in the highest burden quintile (Table). 

Results were similar across multiple sensitivity analyses (see Appendix Table), although the relationship between COVID-19 burden and in-hospital mortality was attenuated and not significant when the sample was restricted to hospitals with at least 100 beds and 20 COVID-19 admissions, or in analyses adjusted for race and AGI.
DISCUSSION
In this study of 14,226 patients with COVID-19 across 117 US hospitals, those patients admitted to the most burdened hospitals had a higher odds of death. This relationship, which persisted after adjusting for age, sex, and comorbid conditions, suggests that a threshold exists at which patient surges may cause excess mortality.
Notably, in sensitivity analyses adjusting for race and AGI, COVID-19 burden was no longer associated with in-hospital mortality and the point estimate was attenuated. This raises the possibility that our primary results are confounded by these factors. However, prior studies of hospitalized patients have not found race to be predictive of mortality, after adjusting for other factors.11,12
We also note that the relationship between COVID-19 burden and mortality was not significant (P = .07) when the sample was restricted to larger hospitals with more than 20 COVID-19 admissions; again, the point estimate was attenuated. This suggests that larger hospitals may be more resilient in the face of patient surges. Whether this is due to increased availability of staff who can be redeployed to patient care (as with researchers at academic centers), increased experience managing severe respiratory failure, or other factors is uncertain.
Interestingly, in-hospital mortality varied widely across study hospitals, even among the most-burdened hospitals. The reasons for this residual variability—after adjusting for age, sex, and comorbidities and stratifying by COVID-19 burden—are uncertain. To the extent that this variability reflects differences in patient management, hospital staffing, or use of investigational or advanced therapies, it will be critical to identify and disseminate any replicable best practices from high-burden hospitals with low mortality rates.
Whereas other reports have often described single-center or regional experiences,13-15 leaving open the possibility that their results were highly influenced by the local nature of the pandemic in their respective settings, our report from a large sample of hospitals across the United States in high- and low-burden settings provides a more generalizable description of mortality rates for hospitalized patients. Additional study strengths include our adjustment for comorbidities known to be associated with COVID-19 survival, the reporting of definitive outcomes for 99% of patients, and the inclusion of multiple sensitivity analyses to assess the stability of findings.
Our principal limitation is the inability to adjust for severity of acute illness due to inconsistent reporting of laboratory and vital signs data from study hospitals and missing information on interhospital transfers. While our adjusted analyses clearly suggest an association between COVID-19 burden and patient outcomes, our results may still be confounded by differences in illness severity at study hospitals. Thus, our findings should be considered hypothesis-generating and will require confirmation in future studies that include adjustment for acute illness severity.
Other limitations of our study include overrepresentation of large urban hospitals in the Northeast, although this represents the geography of the US pandemic during the study period. Our adjustment for race/ethnicity and socioeconomic status was limited in that we only had zip code-of-residence level information, did not know the zip code of residence for one quarter of study patients, and had to bifurcate the population into White/non-White categories. Finally, our definition of burden does not account for hospital resources, including staffing, ICU capacity, and the availability of advanced or investigational therapies.
CONCLUSION
In this study of 14,226 patients with COVID-19 admitted to 1 of 117 US hospitals, we found that the odds of in-hospital mortality were higher in hospitals that had the highest burden of COVID-19 admissions. This relationship, which persisted after adjustment for age, sex, and comorbid conditions, suggests that patient surges may be an independent risk factor for in-hospital death among patients with COVID-19.
ACKNOWLEGMENTS
The authors thank Bocheng Jing, MS, Senior Statistician at the UCSF Pepper Center, for providing code to identify Elixhauser conditions from ICD-10 data; and Scott Kerber, BS, and Scott Magnoni, MS, both of CarePort Health, for assistance with data extraction. They were not compensated for this work beyond their regular salaries.
1. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Centers for Disease Control and Prevention. Updated November 2, 2020. Accessed December 29, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html
2. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/S0140-6736(20)31189-2
3. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1-12. https://doi.org/10.1001/jamainternmed.2020.4568
4. Karaca-Mandic P, Sen S, Georgiou A, Zhu Y, Basu A. Association of COVID-19-related hospital use and overall covid-19 mortality in the USA. J Gen Intern Med. 2020:1-3. https://doi.org/10.1007/s11606-020-06084-7
5. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. Centers for Disease Control and Prevention. Accessed June 2, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf
6. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
7. About the American Community Survey. United States Census Bureau. Updated January 4, 2021. Accessed March 2, 2021. https://www.census.gov/programs-surveys/acs/about.html
8. Provider of service files. Centers for Medicare & Medicaid Services. Revised January 15, 2020. Accessed March 2, 2021. https://www.cms.gov/research-statistics-data-systems/provider-services-current-files/2019-pos-file
9. Ash AS, Fienberg SE, Louis TA, et al. Statistical issues in assessing hospital performance. Committee of Presidents of Statistical Societies white paper. January 2012. Accessed March 1, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Statistical-Issues-in-Assessing-Hospital-Performance.pdf
10. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLoS One. 2011;12;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401
11. Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33-41. https://doi.org/10.7326/M20-3905
12. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/NEJMsa2011686
13. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012-2022. https://doi.org/10.1056/NEJMoa2004500
14. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. https://doi.org/10.1016/S2213-2600(20)30079-5
15. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
1. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Centers for Disease Control and Prevention. Updated November 2, 2020. Accessed December 29, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html
2. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/S0140-6736(20)31189-2
3. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1-12. https://doi.org/10.1001/jamainternmed.2020.4568
4. Karaca-Mandic P, Sen S, Georgiou A, Zhu Y, Basu A. Association of COVID-19-related hospital use and overall covid-19 mortality in the USA. J Gen Intern Med. 2020:1-3. https://doi.org/10.1007/s11606-020-06084-7
5. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. Centers for Disease Control and Prevention. Accessed June 2, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf
6. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
7. About the American Community Survey. United States Census Bureau. Updated January 4, 2021. Accessed March 2, 2021. https://www.census.gov/programs-surveys/acs/about.html
8. Provider of service files. Centers for Medicare & Medicaid Services. Revised January 15, 2020. Accessed March 2, 2021. https://www.cms.gov/research-statistics-data-systems/provider-services-current-files/2019-pos-file
9. Ash AS, Fienberg SE, Louis TA, et al. Statistical issues in assessing hospital performance. Committee of Presidents of Statistical Societies white paper. January 2012. Accessed March 1, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Statistical-Issues-in-Assessing-Hospital-Performance.pdf
10. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLoS One. 2011;12;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401
11. Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33-41. https://doi.org/10.7326/M20-3905
12. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/NEJMsa2011686
13. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012-2022. https://doi.org/10.1056/NEJMoa2004500
14. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. https://doi.org/10.1016/S2213-2600(20)30079-5
15. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
© 2021 Society of Hospital Medicine
Supine-Related Pseudoanemia in Hospitalized Patients
The World Health Organization (WHO) defines anemia as a hemoglobin value less than 12 g/dL in women and less than 13 g/dL in men.1 Hospital-acquired anemia is loosely defined as normal hemoglobin levels on admission that, at their nadir during hospitalization or on discharge, are less than WHO sex-defined cutoffs. Hospital-acquired anemia or significant decreases in hemoglobin are often identified during hospitalization.2-6 Potential causes include blood loss from phlebotomy, occult gastrointestinal bleeding, hemolysis, anemia of inflammation, and hemodilution due to fluid resuscitation. Of these causes, some are dangerous to patients, some are iatrogenic, and some are due to laboratory error.7 Physicians often evaluate decreases in hemoglobin, which could otherwise be explained by laboratory error, hemodilution, or expected decrease in hemoglobin due to hospitalization, to identify causes that may lead to potential harm.
Jacob et al8 demonstrated the effect of posture on hemoglobin concentrations in healthy volunteers, showing an average 11% relative increase in hemoglobin when going from lying to standing. This increase was attributed to shifts in plasma volume to the vascular space with recumbence. They hypothesized that the initial hemoglobin on admission is measured when patients are upright or recently upright, whereas after admission, patients are more likely to be supine, resulting in lower hemoglobin concentrations. Others have also demonstrated similar effects of patient posture on hemoglobin concentration.9-13 However, these prior results are not readily generalizable to hospitalized patients. These prior studies enrolled healthy volunteers, and most examined postural changes from the supine and standing positions; blood is rarely obtained from hospitalized patients when they are standing.
The aim of this study was to investigate whether postural changes in hemoglobin can be demonstrated in positions that patients routinely encountered during in-hospital phlebotomy: upright in a chair or recumbent in a bed. Patient position, which is not standardized during blood draws, may contribute to lower measured hemoglobin concentrations in some patients, especially sicker individuals who are recumbent more frequently. We hypothesized that going from supine to upright in a chair would result in a relative increase in hemoglobin concentration of 5% to 6%, approximately half the value of going from supine to standing.8 To investigate this, we conducted a quasi-experimental study exploring the effect of position (supine or sitting in chair) on hemoglobin concentrations in medical inpatients.
METHODS
Participants
Patients were enrolled in this single-center study between October 2017 and August 2018. Patients aged 18 years or older who were hospitalized on the general internal medicine wards were screened to determine if they met the following inclusion criteria: hospitalized for <5 days, had blood work scheduled as part of routine care (in order to decrease phlebotomy required by this study), had baseline hemoglobin >8 g/dL, and were able to remain supine without interruption overnight and able to sit in a chair for at least 1 hour the following morning. Patients were excluded from the study if they had a hematologic malignancy, were at risk of >100 mL of blood loss (eg, admitted for gastrointestinal bleeding, planned surgery), had a transfusion requirement, or received intravascular modifiers such as fluid (>100 cc/h) or intravenous diuretics. The Johns Hopkins Institutional Review Board approved this study, and all patients provided written informed consent.
Study Design
Patients enrolled in this quasi-experimental study were asked to remain supine for at least 6 hours overnight. Adherence to the recumbent position was tracked by patient self-report and by corroboration with the patient’s nurse overnight. Any interruptions to supine positioning resulted in exclusion from the study. The following morning, a member of the study team performed phlebotomy while the patient remained supine. Patients were then asked to sit comfortably in a chair for at least 1 hour with their feet on the ground; the blood draw was then repeated. All blood samples were acquired by venipuncture. Prior to each blood draw, a tourniquet was placed over the upper arm below the axilla. An antecubital vein on either arm was visualized under ultrasound guidance, and a 23-G × 3/4” butterfly needle was used for venipuncture. The vials of blood were immediately inverted after blood collection. Hemoglobin assays were processed and analyzed using Sysmex XN-10 analyzer (Sysmex Corporation). The reference range for hemoglobin in our facility was 12.0 to 15.0 g/dL for women and 13.9 to 16.3 g/dL for men. Laboratory technicians were blinded to and uninvolved in the study.
We determined, a priori, that 33 enrolled patients would provide 80% power (alpha 0.05) to detect an average hemoglobin change of 4.1%, assuming that the standard deviation of the hemoglobin change was twice the mean (ie, SD = 8.2%). The Wilcoxon signed-rank test was used to test the significance of postural hemoglobin changes. Analyses were conducted using JMP Pro 13.0 (SAS) and GraphPad Prism 8 (GraphPad Software). Significance was defined at P < .05 for all analyses.
RESULTS
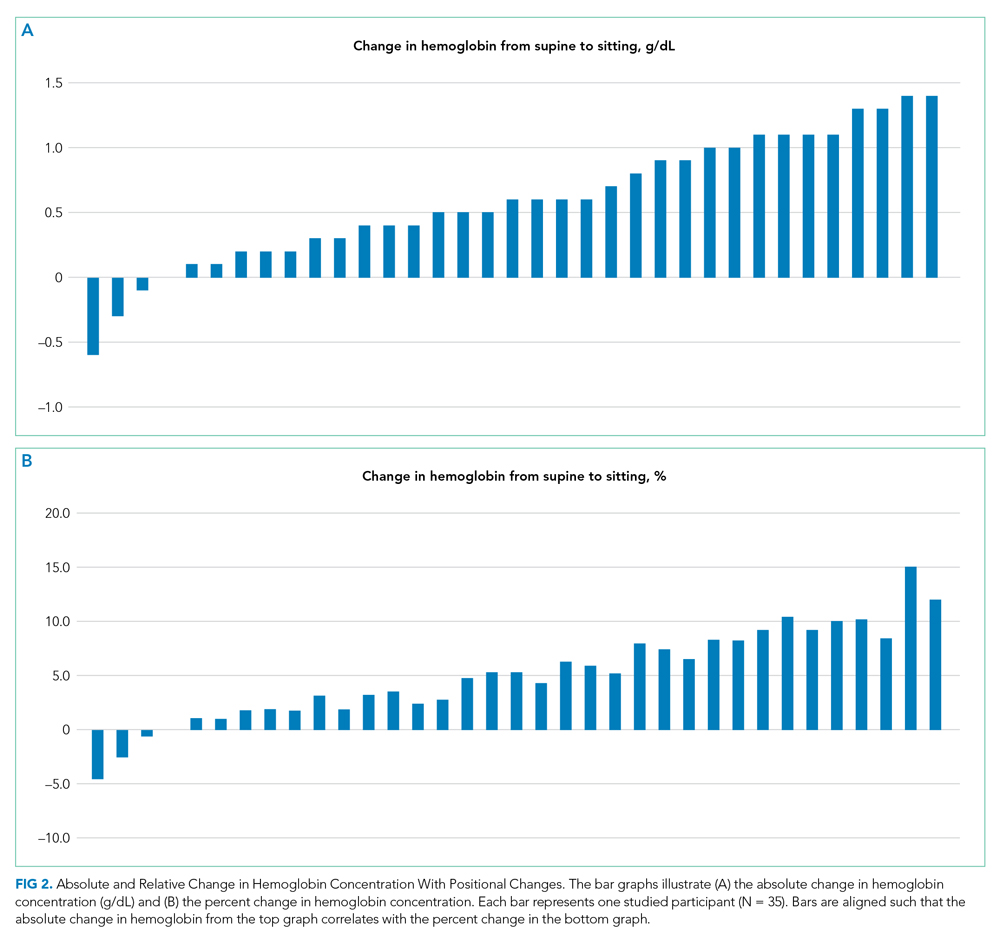
Thirty-nine patients were consented and enrolled in the study; four patients were excluded prior to blood draw (two patients because of interruption of supine time, two patients because of refusal in the morning). Of the 35 patients who completed the study, 13 were women (37%); median age was 49 years (range, 25-83 years). Median supine hemoglobin concentration in our sample was 11.7 g/dL (range, 9.3-18.1 g/dL), and median baseline creatinine level was 0.70 mg/dL (range, 0.5-2.5 mg/dL). Median supine hemoglobin levels were 11.7 g/dL (range, 9.6-13.2 g/dL) in women and 11.8 g/dL (range, 9.3-18.1 g/dL) in men. In aggregate, patients had a median increase in hemoglobin concentration of 0.60 g/dL (range, –0.6 to 1.4 g/dL) with sitting, a 5.2% (range, –4.5% to 15.1%) relative change (P < .001) (Figure 1). 
DISCUSSION
International blood collection guidelines acknowledge postural changes in laboratory values and recommend standardization of patient position to either sitting in a chair or lying flat in a bed, without changes in position for 15 minutes prior to blood draw.14 When these positional accommodations cannot be met, documenting positional disruptions is recommended so that laboratory values can be interpreted accordingly. To the best of our knowledge, no hospital in the United States has standardized patient position as part of phlebotomy procedure such that patient position is documented and can be made available to interpreting providers.
Relative increases in hemoglobin or hematocrit range from 7% to 12% when patients go from supine to standing.8,9,11 The reverse relationship has also been shown, where upright-to-supine position results in decreases in hemoglobin concentrations.10,13 We found that going from supine to a seated position resulted in significant increases in hemoglobin of 0.6 g/dL and in a more than 1 g/dL increase in 29% of the patients. Although four of the 35 patients experienced either no change or a slight decrease in their hemoglobin concentration when going from supine to upright and not all patients saw a uniform effect, providers should be aware that the patient’s position can contribute to changes in hemoglobin concentration in the hospitalized setting. Providers may be able to use this information to avoid an extensive diagnostic workup when anemia is identified in hospitalized patients, although more research is needed to identify patient subsets who are at higher risk for this effect.
Until hospitals implement protocols that require phlebotomists to report patient position during phlebotomy in a standardized fashion, providers should be alert to the fact that supine positioning may result in a hemoglobin level that is significantly lower than that when drawn in a sitting position, and in almost one-third of patients, this difference may be 1.0 g/dL or greater.
Given our study criteria requiring supine positions of at least 6 hours and a baseline hemoglobin concentration >8 g/dL, our sample of patients may have been younger and healthier than the average hospitalized patient on general internal medicine wards. Since greater relative changes in plasma volume shifts and hemoglobin might be seen in patients with lower baseline hemoglobin and lower baseline plasma protein, this selection bias may underestimate the effects of position on hemoglobin changes for the average inpatient population. Additionally, we intentionally sought to obtain sitting hemoglobin levels after the supine samples to avoid the possibility of incorrectly attributing dropping hemoglobin levels to progressive hospital-acquired anemia from phlebotomy or illness. Any concomitant trend of falling hemoglobin levels in our patients would be expected to lead to a systematic underestimation of the positional change in hemoglobin we observed. We did not objectively observe adherence to supine and upright position and instead relied on patient self-reporting, which is one possible contributor to the variable effects of position on hemoglobin concentration, with some patients having no change or decreases in hemoglobin concentrations.
CONCLUSION
Posture can significantly influence hemoglobin levels in hospitalized patients on general medicine wards. Further research can determine whether it would be cost and time effective to standardize patient positions prior to phlebotomy, or at least to report patient positioning with the laboratory testing results.
1. DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38(3):302-316.
2. Martin ND, Scantling D. Hospital-acquired anemia. J Infus Nurs. 2015;38(5):330-338. https://doi.org/10.1097/NAN.0000000000000121
3. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
4. Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646-1653. https://doi.org/10.1001/archinternmed.2011.361
5. Languasco A, Cazap N, Marciano S, et al. Hemoglobin concentration variations over time in general medical inpatients. J Hosp Med. 2010;5(5):283-288. https://doi.org/10.1002/jhm.650
6. van der Bom JG, Cannegieter SC. Hospital-acquired anemia: the contribution of diagnostic blood loss. J Thromb Haemost. 2015;13(6):1157-1159. https://doi.org/10.1111/jth.12886
7. Berkow L. Factors affecting hemoglobin measurement. J Clin Monit Comput. 2013;27(5):499-508. https://doi.org/10.1007/s10877-013-9456-3
8. Jacob G, Raj SR, Ketch T, et al. Postural pseudoanemia: posture-dependent change in hematocrit. Mayo Clin Proc. 2005;80(5):611-614. https://doi.org/10.4065/80.5.611
9. Fawcett JK, Wynn V. Effects of posture on plasma volume and some blood constituents. J Clin Pathol. 1960;13(4):304-310. https://doi.org/10.1136/jcp.13.4.304
10. Tombridge TL. Effect of posture on hematology results. Am J ClinPathol. 1968;49(4):491-493. https://doi.org/10.1093/ajcp/49.4.491
11. Hagan RD, Diaz FJ, Horvath SM. Plasma volume changes with movement to supine and standing positions. J Appl Physiol. 1978;45(3):414-417. https://doi.org/10.1152/jappl.1978.45.3.414
12. Maw GJ, Mackenzie IL, Taylor NA. Redistribution of body fluids during postural manipulations. Acta Physiol Scand. 1995;155(2):157-163. https://doi.org/10.1111/j.1748-1716.1995.tb09960.x
13. Lima-Oliveira G, Guidi GC, Salvagno GL, Danese E, Montagnana M, Lippi G. Patient posture for blood collection by venipuncture: recall for standardization after 28 years. Rev Bras Hematol Hemoter. 2017;39(2):127-132. https://doi.org/10.1016/j.bjhh.2017.01.004
14. Simundic AM, Bölenius K, Cadamuro J, et al. Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI). Joint EFLM-COLABIOCLI recommendation for venous blood sampling. Clin Chem Lab Med. 2018;56(12):2015-2038. https://doi.org/10.1515/cclm-2018-0602
The World Health Organization (WHO) defines anemia as a hemoglobin value less than 12 g/dL in women and less than 13 g/dL in men.1 Hospital-acquired anemia is loosely defined as normal hemoglobin levels on admission that, at their nadir during hospitalization or on discharge, are less than WHO sex-defined cutoffs. Hospital-acquired anemia or significant decreases in hemoglobin are often identified during hospitalization.2-6 Potential causes include blood loss from phlebotomy, occult gastrointestinal bleeding, hemolysis, anemia of inflammation, and hemodilution due to fluid resuscitation. Of these causes, some are dangerous to patients, some are iatrogenic, and some are due to laboratory error.7 Physicians often evaluate decreases in hemoglobin, which could otherwise be explained by laboratory error, hemodilution, or expected decrease in hemoglobin due to hospitalization, to identify causes that may lead to potential harm.
Jacob et al8 demonstrated the effect of posture on hemoglobin concentrations in healthy volunteers, showing an average 11% relative increase in hemoglobin when going from lying to standing. This increase was attributed to shifts in plasma volume to the vascular space with recumbence. They hypothesized that the initial hemoglobin on admission is measured when patients are upright or recently upright, whereas after admission, patients are more likely to be supine, resulting in lower hemoglobin concentrations. Others have also demonstrated similar effects of patient posture on hemoglobin concentration.9-13 However, these prior results are not readily generalizable to hospitalized patients. These prior studies enrolled healthy volunteers, and most examined postural changes from the supine and standing positions; blood is rarely obtained from hospitalized patients when they are standing.
The aim of this study was to investigate whether postural changes in hemoglobin can be demonstrated in positions that patients routinely encountered during in-hospital phlebotomy: upright in a chair or recumbent in a bed. Patient position, which is not standardized during blood draws, may contribute to lower measured hemoglobin concentrations in some patients, especially sicker individuals who are recumbent more frequently. We hypothesized that going from supine to upright in a chair would result in a relative increase in hemoglobin concentration of 5% to 6%, approximately half the value of going from supine to standing.8 To investigate this, we conducted a quasi-experimental study exploring the effect of position (supine or sitting in chair) on hemoglobin concentrations in medical inpatients.
METHODS
Participants
Patients were enrolled in this single-center study between October 2017 and August 2018. Patients aged 18 years or older who were hospitalized on the general internal medicine wards were screened to determine if they met the following inclusion criteria: hospitalized for <5 days, had blood work scheduled as part of routine care (in order to decrease phlebotomy required by this study), had baseline hemoglobin >8 g/dL, and were able to remain supine without interruption overnight and able to sit in a chair for at least 1 hour the following morning. Patients were excluded from the study if they had a hematologic malignancy, were at risk of >100 mL of blood loss (eg, admitted for gastrointestinal bleeding, planned surgery), had a transfusion requirement, or received intravascular modifiers such as fluid (>100 cc/h) or intravenous diuretics. The Johns Hopkins Institutional Review Board approved this study, and all patients provided written informed consent.
Study Design
Patients enrolled in this quasi-experimental study were asked to remain supine for at least 6 hours overnight. Adherence to the recumbent position was tracked by patient self-report and by corroboration with the patient’s nurse overnight. Any interruptions to supine positioning resulted in exclusion from the study. The following morning, a member of the study team performed phlebotomy while the patient remained supine. Patients were then asked to sit comfortably in a chair for at least 1 hour with their feet on the ground; the blood draw was then repeated. All blood samples were acquired by venipuncture. Prior to each blood draw, a tourniquet was placed over the upper arm below the axilla. An antecubital vein on either arm was visualized under ultrasound guidance, and a 23-G × 3/4” butterfly needle was used for venipuncture. The vials of blood were immediately inverted after blood collection. Hemoglobin assays were processed and analyzed using Sysmex XN-10 analyzer (Sysmex Corporation). The reference range for hemoglobin in our facility was 12.0 to 15.0 g/dL for women and 13.9 to 16.3 g/dL for men. Laboratory technicians were blinded to and uninvolved in the study.
We determined, a priori, that 33 enrolled patients would provide 80% power (alpha 0.05) to detect an average hemoglobin change of 4.1%, assuming that the standard deviation of the hemoglobin change was twice the mean (ie, SD = 8.2%). The Wilcoxon signed-rank test was used to test the significance of postural hemoglobin changes. Analyses were conducted using JMP Pro 13.0 (SAS) and GraphPad Prism 8 (GraphPad Software). Significance was defined at P < .05 for all analyses.
RESULTS
Thirty-nine patients were consented and enrolled in the study; four patients were excluded prior to blood draw (two patients because of interruption of supine time, two patients because of refusal in the morning). Of the 35 patients who completed the study, 13 were women (37%); median age was 49 years (range, 25-83 years). Median supine hemoglobin concentration in our sample was 11.7 g/dL (range, 9.3-18.1 g/dL), and median baseline creatinine level was 0.70 mg/dL (range, 0.5-2.5 mg/dL). Median supine hemoglobin levels were 11.7 g/dL (range, 9.6-13.2 g/dL) in women and 11.8 g/dL (range, 9.3-18.1 g/dL) in men. In aggregate, patients had a median increase in hemoglobin concentration of 0.60 g/dL (range, –0.6 to 1.4 g/dL) with sitting, a 5.2% (range, –4.5% to 15.1%) relative change (P < .001) (Figure 1). 

DISCUSSION
International blood collection guidelines acknowledge postural changes in laboratory values and recommend standardization of patient position to either sitting in a chair or lying flat in a bed, without changes in position for 15 minutes prior to blood draw.14 When these positional accommodations cannot be met, documenting positional disruptions is recommended so that laboratory values can be interpreted accordingly. To the best of our knowledge, no hospital in the United States has standardized patient position as part of phlebotomy procedure such that patient position is documented and can be made available to interpreting providers.
Relative increases in hemoglobin or hematocrit range from 7% to 12% when patients go from supine to standing.8,9,11 The reverse relationship has also been shown, where upright-to-supine position results in decreases in hemoglobin concentrations.10,13 We found that going from supine to a seated position resulted in significant increases in hemoglobin of 0.6 g/dL and in a more than 1 g/dL increase in 29% of the patients. Although four of the 35 patients experienced either no change or a slight decrease in their hemoglobin concentration when going from supine to upright and not all patients saw a uniform effect, providers should be aware that the patient’s position can contribute to changes in hemoglobin concentration in the hospitalized setting. Providers may be able to use this information to avoid an extensive diagnostic workup when anemia is identified in hospitalized patients, although more research is needed to identify patient subsets who are at higher risk for this effect.
Until hospitals implement protocols that require phlebotomists to report patient position during phlebotomy in a standardized fashion, providers should be alert to the fact that supine positioning may result in a hemoglobin level that is significantly lower than that when drawn in a sitting position, and in almost one-third of patients, this difference may be 1.0 g/dL or greater.
Given our study criteria requiring supine positions of at least 6 hours and a baseline hemoglobin concentration >8 g/dL, our sample of patients may have been younger and healthier than the average hospitalized patient on general internal medicine wards. Since greater relative changes in plasma volume shifts and hemoglobin might be seen in patients with lower baseline hemoglobin and lower baseline plasma protein, this selection bias may underestimate the effects of position on hemoglobin changes for the average inpatient population. Additionally, we intentionally sought to obtain sitting hemoglobin levels after the supine samples to avoid the possibility of incorrectly attributing dropping hemoglobin levels to progressive hospital-acquired anemia from phlebotomy or illness. Any concomitant trend of falling hemoglobin levels in our patients would be expected to lead to a systematic underestimation of the positional change in hemoglobin we observed. We did not objectively observe adherence to supine and upright position and instead relied on patient self-reporting, which is one possible contributor to the variable effects of position on hemoglobin concentration, with some patients having no change or decreases in hemoglobin concentrations.
CONCLUSION
Posture can significantly influence hemoglobin levels in hospitalized patients on general medicine wards. Further research can determine whether it would be cost and time effective to standardize patient positions prior to phlebotomy, or at least to report patient positioning with the laboratory testing results.
The World Health Organization (WHO) defines anemia as a hemoglobin value less than 12 g/dL in women and less than 13 g/dL in men.1 Hospital-acquired anemia is loosely defined as normal hemoglobin levels on admission that, at their nadir during hospitalization or on discharge, are less than WHO sex-defined cutoffs. Hospital-acquired anemia or significant decreases in hemoglobin are often identified during hospitalization.2-6 Potential causes include blood loss from phlebotomy, occult gastrointestinal bleeding, hemolysis, anemia of inflammation, and hemodilution due to fluid resuscitation. Of these causes, some are dangerous to patients, some are iatrogenic, and some are due to laboratory error.7 Physicians often evaluate decreases in hemoglobin, which could otherwise be explained by laboratory error, hemodilution, or expected decrease in hemoglobin due to hospitalization, to identify causes that may lead to potential harm.
Jacob et al8 demonstrated the effect of posture on hemoglobin concentrations in healthy volunteers, showing an average 11% relative increase in hemoglobin when going from lying to standing. This increase was attributed to shifts in plasma volume to the vascular space with recumbence. They hypothesized that the initial hemoglobin on admission is measured when patients are upright or recently upright, whereas after admission, patients are more likely to be supine, resulting in lower hemoglobin concentrations. Others have also demonstrated similar effects of patient posture on hemoglobin concentration.9-13 However, these prior results are not readily generalizable to hospitalized patients. These prior studies enrolled healthy volunteers, and most examined postural changes from the supine and standing positions; blood is rarely obtained from hospitalized patients when they are standing.
The aim of this study was to investigate whether postural changes in hemoglobin can be demonstrated in positions that patients routinely encountered during in-hospital phlebotomy: upright in a chair or recumbent in a bed. Patient position, which is not standardized during blood draws, may contribute to lower measured hemoglobin concentrations in some patients, especially sicker individuals who are recumbent more frequently. We hypothesized that going from supine to upright in a chair would result in a relative increase in hemoglobin concentration of 5% to 6%, approximately half the value of going from supine to standing.8 To investigate this, we conducted a quasi-experimental study exploring the effect of position (supine or sitting in chair) on hemoglobin concentrations in medical inpatients.
METHODS
Participants
Patients were enrolled in this single-center study between October 2017 and August 2018. Patients aged 18 years or older who were hospitalized on the general internal medicine wards were screened to determine if they met the following inclusion criteria: hospitalized for <5 days, had blood work scheduled as part of routine care (in order to decrease phlebotomy required by this study), had baseline hemoglobin >8 g/dL, and were able to remain supine without interruption overnight and able to sit in a chair for at least 1 hour the following morning. Patients were excluded from the study if they had a hematologic malignancy, were at risk of >100 mL of blood loss (eg, admitted for gastrointestinal bleeding, planned surgery), had a transfusion requirement, or received intravascular modifiers such as fluid (>100 cc/h) or intravenous diuretics. The Johns Hopkins Institutional Review Board approved this study, and all patients provided written informed consent.
Study Design
Patients enrolled in this quasi-experimental study were asked to remain supine for at least 6 hours overnight. Adherence to the recumbent position was tracked by patient self-report and by corroboration with the patient’s nurse overnight. Any interruptions to supine positioning resulted in exclusion from the study. The following morning, a member of the study team performed phlebotomy while the patient remained supine. Patients were then asked to sit comfortably in a chair for at least 1 hour with their feet on the ground; the blood draw was then repeated. All blood samples were acquired by venipuncture. Prior to each blood draw, a tourniquet was placed over the upper arm below the axilla. An antecubital vein on either arm was visualized under ultrasound guidance, and a 23-G × 3/4” butterfly needle was used for venipuncture. The vials of blood were immediately inverted after blood collection. Hemoglobin assays were processed and analyzed using Sysmex XN-10 analyzer (Sysmex Corporation). The reference range for hemoglobin in our facility was 12.0 to 15.0 g/dL for women and 13.9 to 16.3 g/dL for men. Laboratory technicians were blinded to and uninvolved in the study.
We determined, a priori, that 33 enrolled patients would provide 80% power (alpha 0.05) to detect an average hemoglobin change of 4.1%, assuming that the standard deviation of the hemoglobin change was twice the mean (ie, SD = 8.2%). The Wilcoxon signed-rank test was used to test the significance of postural hemoglobin changes. Analyses were conducted using JMP Pro 13.0 (SAS) and GraphPad Prism 8 (GraphPad Software). Significance was defined at P < .05 for all analyses.
RESULTS
Thirty-nine patients were consented and enrolled in the study; four patients were excluded prior to blood draw (two patients because of interruption of supine time, two patients because of refusal in the morning). Of the 35 patients who completed the study, 13 were women (37%); median age was 49 years (range, 25-83 years). Median supine hemoglobin concentration in our sample was 11.7 g/dL (range, 9.3-18.1 g/dL), and median baseline creatinine level was 0.70 mg/dL (range, 0.5-2.5 mg/dL). Median supine hemoglobin levels were 11.7 g/dL (range, 9.6-13.2 g/dL) in women and 11.8 g/dL (range, 9.3-18.1 g/dL) in men. In aggregate, patients had a median increase in hemoglobin concentration of 0.60 g/dL (range, –0.6 to 1.4 g/dL) with sitting, a 5.2% (range, –4.5% to 15.1%) relative change (P < .001) (Figure 1). 

DISCUSSION
International blood collection guidelines acknowledge postural changes in laboratory values and recommend standardization of patient position to either sitting in a chair or lying flat in a bed, without changes in position for 15 minutes prior to blood draw.14 When these positional accommodations cannot be met, documenting positional disruptions is recommended so that laboratory values can be interpreted accordingly. To the best of our knowledge, no hospital in the United States has standardized patient position as part of phlebotomy procedure such that patient position is documented and can be made available to interpreting providers.
Relative increases in hemoglobin or hematocrit range from 7% to 12% when patients go from supine to standing.8,9,11 The reverse relationship has also been shown, where upright-to-supine position results in decreases in hemoglobin concentrations.10,13 We found that going from supine to a seated position resulted in significant increases in hemoglobin of 0.6 g/dL and in a more than 1 g/dL increase in 29% of the patients. Although four of the 35 patients experienced either no change or a slight decrease in their hemoglobin concentration when going from supine to upright and not all patients saw a uniform effect, providers should be aware that the patient’s position can contribute to changes in hemoglobin concentration in the hospitalized setting. Providers may be able to use this information to avoid an extensive diagnostic workup when anemia is identified in hospitalized patients, although more research is needed to identify patient subsets who are at higher risk for this effect.
Until hospitals implement protocols that require phlebotomists to report patient position during phlebotomy in a standardized fashion, providers should be alert to the fact that supine positioning may result in a hemoglobin level that is significantly lower than that when drawn in a sitting position, and in almost one-third of patients, this difference may be 1.0 g/dL or greater.
Given our study criteria requiring supine positions of at least 6 hours and a baseline hemoglobin concentration >8 g/dL, our sample of patients may have been younger and healthier than the average hospitalized patient on general internal medicine wards. Since greater relative changes in plasma volume shifts and hemoglobin might be seen in patients with lower baseline hemoglobin and lower baseline plasma protein, this selection bias may underestimate the effects of position on hemoglobin changes for the average inpatient population. Additionally, we intentionally sought to obtain sitting hemoglobin levels after the supine samples to avoid the possibility of incorrectly attributing dropping hemoglobin levels to progressive hospital-acquired anemia from phlebotomy or illness. Any concomitant trend of falling hemoglobin levels in our patients would be expected to lead to a systematic underestimation of the positional change in hemoglobin we observed. We did not objectively observe adherence to supine and upright position and instead relied on patient self-reporting, which is one possible contributor to the variable effects of position on hemoglobin concentration, with some patients having no change or decreases in hemoglobin concentrations.
CONCLUSION
Posture can significantly influence hemoglobin levels in hospitalized patients on general medicine wards. Further research can determine whether it would be cost and time effective to standardize patient positions prior to phlebotomy, or at least to report patient positioning with the laboratory testing results.
1. DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38(3):302-316.
2. Martin ND, Scantling D. Hospital-acquired anemia. J Infus Nurs. 2015;38(5):330-338. https://doi.org/10.1097/NAN.0000000000000121
3. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
4. Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646-1653. https://doi.org/10.1001/archinternmed.2011.361
5. Languasco A, Cazap N, Marciano S, et al. Hemoglobin concentration variations over time in general medical inpatients. J Hosp Med. 2010;5(5):283-288. https://doi.org/10.1002/jhm.650
6. van der Bom JG, Cannegieter SC. Hospital-acquired anemia: the contribution of diagnostic blood loss. J Thromb Haemost. 2015;13(6):1157-1159. https://doi.org/10.1111/jth.12886
7. Berkow L. Factors affecting hemoglobin measurement. J Clin Monit Comput. 2013;27(5):499-508. https://doi.org/10.1007/s10877-013-9456-3
8. Jacob G, Raj SR, Ketch T, et al. Postural pseudoanemia: posture-dependent change in hematocrit. Mayo Clin Proc. 2005;80(5):611-614. https://doi.org/10.4065/80.5.611
9. Fawcett JK, Wynn V. Effects of posture on plasma volume and some blood constituents. J Clin Pathol. 1960;13(4):304-310. https://doi.org/10.1136/jcp.13.4.304
10. Tombridge TL. Effect of posture on hematology results. Am J ClinPathol. 1968;49(4):491-493. https://doi.org/10.1093/ajcp/49.4.491
11. Hagan RD, Diaz FJ, Horvath SM. Plasma volume changes with movement to supine and standing positions. J Appl Physiol. 1978;45(3):414-417. https://doi.org/10.1152/jappl.1978.45.3.414
12. Maw GJ, Mackenzie IL, Taylor NA. Redistribution of body fluids during postural manipulations. Acta Physiol Scand. 1995;155(2):157-163. https://doi.org/10.1111/j.1748-1716.1995.tb09960.x
13. Lima-Oliveira G, Guidi GC, Salvagno GL, Danese E, Montagnana M, Lippi G. Patient posture for blood collection by venipuncture: recall for standardization after 28 years. Rev Bras Hematol Hemoter. 2017;39(2):127-132. https://doi.org/10.1016/j.bjhh.2017.01.004
14. Simundic AM, Bölenius K, Cadamuro J, et al. Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI). Joint EFLM-COLABIOCLI recommendation for venous blood sampling. Clin Chem Lab Med. 2018;56(12):2015-2038. https://doi.org/10.1515/cclm-2018-0602
1. DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38(3):302-316.
2. Martin ND, Scantling D. Hospital-acquired anemia. J Infus Nurs. 2015;38(5):330-338. https://doi.org/10.1097/NAN.0000000000000121
3. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
4. Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646-1653. https://doi.org/10.1001/archinternmed.2011.361
5. Languasco A, Cazap N, Marciano S, et al. Hemoglobin concentration variations over time in general medical inpatients. J Hosp Med. 2010;5(5):283-288. https://doi.org/10.1002/jhm.650
6. van der Bom JG, Cannegieter SC. Hospital-acquired anemia: the contribution of diagnostic blood loss. J Thromb Haemost. 2015;13(6):1157-1159. https://doi.org/10.1111/jth.12886
7. Berkow L. Factors affecting hemoglobin measurement. J Clin Monit Comput. 2013;27(5):499-508. https://doi.org/10.1007/s10877-013-9456-3
8. Jacob G, Raj SR, Ketch T, et al. Postural pseudoanemia: posture-dependent change in hematocrit. Mayo Clin Proc. 2005;80(5):611-614. https://doi.org/10.4065/80.5.611
9. Fawcett JK, Wynn V. Effects of posture on plasma volume and some blood constituents. J Clin Pathol. 1960;13(4):304-310. https://doi.org/10.1136/jcp.13.4.304
10. Tombridge TL. Effect of posture on hematology results. Am J ClinPathol. 1968;49(4):491-493. https://doi.org/10.1093/ajcp/49.4.491
11. Hagan RD, Diaz FJ, Horvath SM. Plasma volume changes with movement to supine and standing positions. J Appl Physiol. 1978;45(3):414-417. https://doi.org/10.1152/jappl.1978.45.3.414
12. Maw GJ, Mackenzie IL, Taylor NA. Redistribution of body fluids during postural manipulations. Acta Physiol Scand. 1995;155(2):157-163. https://doi.org/10.1111/j.1748-1716.1995.tb09960.x
13. Lima-Oliveira G, Guidi GC, Salvagno GL, Danese E, Montagnana M, Lippi G. Patient posture for blood collection by venipuncture: recall for standardization after 28 years. Rev Bras Hematol Hemoter. 2017;39(2):127-132. https://doi.org/10.1016/j.bjhh.2017.01.004
14. Simundic AM, Bölenius K, Cadamuro J, et al. Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI). Joint EFLM-COLABIOCLI recommendation for venous blood sampling. Clin Chem Lab Med. 2018;56(12):2015-2038. https://doi.org/10.1515/cclm-2018-0602
© 2021 Society of Hospital Medicine
The COVID-19 Pandemic and Changes in Healthcare Utilization for Pediatric Respiratory and Nonrespiratory Illnesses in the United States
In the United States, respiratory illnesses are the most common cause of emergency department (ED) visits and hospitalizations in children.1 In response to the ongoing COVID-19 pandemic, several public health interventions, including school and business closures, stay-at-home orders, and mask mandates, were implemented to limit transmission of SARS-CoV-2.2,3 Studies have shown that children can contribute to the spread of SARS-CoV-2 infections, especially within households.4-6 Recent data suggest that COVID-19, and the associated public health measures enacted to slow its spread, may have affected the transmission of other respiratory pathogens.7 Similarly, the pandemic has likely affected healthcare utilization for nonrespiratory illnesses through adoption of social distancing recommendations, suspension and delays in nonemergent elective care, avoidance of healthcare settings, and the effect of decreased respiratory disease on exacerbation of chronic illness.8 The objective of this study was to examine associations between the COVID-19 pandemic and healthcare utilization for pediatric respiratory and nonrespiratory illnesses at US pediatric hospitals.
METHODS
Study Design
This is a multicenter, cross-sectional study of encounters at 44 pediatric hospitals that reported data to the Pediatric Health Information System (PHIS) database maintained by the Children’s Hospital Association (Lenexa, Kansas).
Study Population
Children 2 months to 18 years of age discharged from ED or inpatient settings with a nonsurgical diagnosis from January 1 to September 30 over a 4-year period (2017-2020) were included.
Exposure
The primary exposure was the 2020 COVID-19 pandemic time, divided into three periods: pre-COVID-19 (January-February 2020, the period prior to the pandemic in the United States), early COVID-19 (March-April 2020, coinciding with the first reported US pediatric case of COVID-19 on March 2, 2020), and COVID-19 (May-September 2020, marked by the implementation of at least two of the following containment measures in every US state: stay-at-home/shelter orders, school closures, nonessential business closures, restaurant closures, or prohibition of gatherings of more than 10 people).2
Outcomes
Statistical Analysis
Categorical variables were summarized using frequencies and percentages and compared using chi-square tests. Continuous variables were summarized as median and interquartile range (IQR) and compared using Wilcoxon rank sum tests. Weekly observed-to-expected (O:E) ratios were calculated for each hospital by dividing the number of observed respiratory illness and nonrespiratory illness encounters in a given week in 2020 (observed) by the average number of encounters for that same week during 2017-2019 (expected). O:E ratios were then aggregated over the three COVID-19 study periods, and 95% confidence intervals were established around mean O:E ratios across individual hospitals. Outcomes were then stratified by respiratory illness subgroups, geographic region, and age. Additional details can be found in the Supplemental Methods in the Appendix.
RESULTS
Study Population
A total of 9,051,980 encounters were included in the study, 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. Median age was 5 years (IQR, 1-11 years), and 52.7% of the population was male (Appendix Table 2 and Appendix Table 3).
Respiratory vs Nonrespiratory Illness During the COVID-19 Pandemic
Over the study period, fewer respiratory and nonrespiratory illness encounters were observed than expected, with a larger decrease in respiratory illness encounters (Table, Appendix Table 4). 

Respiratory Subgroup Analyses
The O:E ratio decreased for all respiratory subgroups over the study period (Table, Appendix Table 4). There were significant differences in specific respiratory subgroups, including asthma, bronchiolitis, croup, influenza, and pneumonia (Appendix Figure 1A). Temporal trends in respiratory encounters were consistent across hospital settings, ages, and geographic regions (Appendix Figure 1B-D). When comparing the with and without COVID-19 subgroups in the “other respiratory illnesses” cohort, other respiratory illness without COVID-19 decreased and remained lower than expected over the rest of the study period, while other respiratory illness with COVID-19 increased markedly during the summer months and declined thereafter (Appendix Figure 2).
All age groups had reductions in respiratory illness encounters during the early COVID-19 and COVID-19 periods, although the decline was less pronounced in the 12- to 17-year-old group (Appendix Figure 1B). Similarly, while all age groups experienced increases in encounters for respiratory illnesses during the summer months, only children in the 12- to 17-year-old group experienced increases beyond pre-COVID-19 levels. Importantly, this increase in respiratory encounters was largely driven by COVID-19 diagnoses (Appendix Figure 3). The trend in nonrespiratory illness encounters stratified by age is shown in Appendix Figure 4.
When patients were stratified by hospital setting, there were no differences between those hospitalized and those discharged from the ED (Appendix Figure 1C). Patterns in respiratory illnesses by geographic location were qualitatively similar until the beginning of the summer 2020, after which geographical variation became more evident (Appendix Figure 1D).
DISCUSSION
In this large, multicenter study evaluating ED visits and hospitalizations for respiratory and nonrespiratory illnesses at US pediatric hospitals during the 2020 COVID-19 pandemic, we found a significant and substantial decrease in healthcare encounters for respiratory illnesses. A rapid and marked decline in encounters for respiratory illness in a relatively short period of time (March 12-April 2) was observed across all hospitals and US regions. Declines were consistent across common respiratory illnesses. More modest, yet still substantial, declines were also observed for nonrespiratory illnesses.
There are likely multiple underlying reasons for the observed reductions. Social distancing measures almost certainly played an important role in interrupting respiratory infection transmission. Rapid reduction in influenza transmission during the early COVID-19 period has been attributed to social distancing measures,3 and influenza transmission in children decreases with school closures.9 It is also possible that some families delayed seeking care at hospitals due to COVID-19, leading to less frequent encounters but more severe illness. The similar decrease in O:E ratio for ED visits and hospitalizations, however, is inconsistent with this explanation.
We also found relative differences in changes in encounters for respiratory illness by age. Adolescents’ levels of respiratory healthcare use declined less and recovered at a faster rate than those of younger children, returning to pre-COVID-19 levels by the end of the study period. The reason for this age differential is likely multifaceted. Infections, such as bronchiolitis and pneumonia, are more likely to be a source of respiratory illness in younger than in older children. It is also likely that disproportionate relaxation of social distancing measures among adolescents, who are known to have a stronger pattern of social interaction, contributed to the faster rise in respiratory illness–related encounters in this age group.11 Adolescents have been reported to be more susceptible to, and more likely to transmit, SARS-CoV-2 compared to younger age groups.12 More modest, albeit similar, age-based changes were observed in encounters for nonrespiratory illnesses
Emerging evidence suggests that school-age children may play an important role in SARS-CoV-2 transmission in the community.4,14 Our finding that, compared to younger children, adolescents had significantly fewer reductions in respiratory illness encounters is concerning. These findings suggest that community-based efforts to help prevent respiratory illnesses, especially COVID-19, should focus on adolescents, who are most likely to maintain social interactions and transmit respiratory infections in the school setting and their households.
This study is limited by the inclusion of only tertiary care children’s hospitals, which may not be nationally representative, and the inability to assess the precise timing of when specific public health interventions were introduced. Moreover, previous studies suggest that social distancing behaviors may have changed even before formal recommendations were enacted.15 Future studies should investigate the local impact of state- and municipality-specific mandates on the burden of COVID-19 and other respiratory illnesses.
The COVID-19 pandemic was associated with substantial reductions in encounters for respiratory diseases, and also with more modest but still sizable reductions in encounters for nonrespiratory diseases. These reductions varied by age. Encounters among adolescents declined less and returned to previous levels faster compared with those of younger children.
ACKNOWLEDGMENT
This publication is dedicated to the memory of our coauthor, Dr. Michael Bendel-Stenzel. Dr. Bendel-Stenzel was dedicated to bettering the lives of children and advancing our knowledge of pediatrics through his research.
1. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
2. Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324(9):859-870. https://doi.org/10.1001/jama.2020.14348
3. Wiese AD, Everson J, Grijalva CG. Social distancing measures: evidence of interruption of seasonal influenza activity and early lessons of the SARS-CoV-2 pandemic. Clin Infect Dis. Published online June 20, 2020. https://doi.org/10.1093/cid/ciaa834
4. Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 infections in households - Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1631-1634. https://doi.org/10.15585/mmwr.mm6944e1
5. Worby CJ, Chaves SS, Wallinga J, Lipsitch M, Finelli L, Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10-16. https://doi.org/10.1016/j.epidem.2015.04.003
6. Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. Published online January 8, 2021. https://doi.org/10.1542/peds.2020-048090
7. Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146(4):e2020006460. https://doi.org/10.1542/peds.2020-006460
8. Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F. Trends in pediatric emergency department utilization after institution of coronavirus disease-19 mandatory social distancing. J Pediatr. 2020;226:274-277.e1. https://doi.org/10.1016/j.jpeds.2020.07.048
9. Luca G, Kerckhove KV, Coletti P, et al. The impact of regular school closure on seasonal influenza epidemics: a data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. https://doi.org/10.1186/s12879-017-2934-3
10. Taquechel K, Diwadkar AR, Sayed S, et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(10):3378-3387.e11. https://doi.org/10.1016/j.jaip.2020.07.057
11. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465-2468. https://doi.org/10.3201/eid2610.201315
12. Davies NG, Klepac P, Liu Y, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-1211. https://doi.org/10.1038/s41591-020-0962-9
13. Hill RM, Rufino K, Kurian S, Saxena J, Saxena K, Williams L. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. Published online December 16, 2020. https://doi.org/10.1542/peds.2020-029280
14. Flasche S, Edmunds WJ. The role of schools and school-aged children in SARS-CoV-2 transmission. Lancet Infect Dis. Published online December 8, 2020. https://doi.org/10.1016/S1473-3099(20)30927-0
15. Sehra ST, George M, Wiebe DJ, Fundin S, Baker JF. Cell phone activity in categories of places and associations with growth in cases of COVID-19 in the US. JAMA Intern Med. Published online August 31, 2020. https://doi.org/10.1001/jamainternmed.2020.4288
In the United States, respiratory illnesses are the most common cause of emergency department (ED) visits and hospitalizations in children.1 In response to the ongoing COVID-19 pandemic, several public health interventions, including school and business closures, stay-at-home orders, and mask mandates, were implemented to limit transmission of SARS-CoV-2.2,3 Studies have shown that children can contribute to the spread of SARS-CoV-2 infections, especially within households.4-6 Recent data suggest that COVID-19, and the associated public health measures enacted to slow its spread, may have affected the transmission of other respiratory pathogens.7 Similarly, the pandemic has likely affected healthcare utilization for nonrespiratory illnesses through adoption of social distancing recommendations, suspension and delays in nonemergent elective care, avoidance of healthcare settings, and the effect of decreased respiratory disease on exacerbation of chronic illness.8 The objective of this study was to examine associations between the COVID-19 pandemic and healthcare utilization for pediatric respiratory and nonrespiratory illnesses at US pediatric hospitals.
METHODS
Study Design
This is a multicenter, cross-sectional study of encounters at 44 pediatric hospitals that reported data to the Pediatric Health Information System (PHIS) database maintained by the Children’s Hospital Association (Lenexa, Kansas).
Study Population
Children 2 months to 18 years of age discharged from ED or inpatient settings with a nonsurgical diagnosis from January 1 to September 30 over a 4-year period (2017-2020) were included.
Exposure
The primary exposure was the 2020 COVID-19 pandemic time, divided into three periods: pre-COVID-19 (January-February 2020, the period prior to the pandemic in the United States), early COVID-19 (March-April 2020, coinciding with the first reported US pediatric case of COVID-19 on March 2, 2020), and COVID-19 (May-September 2020, marked by the implementation of at least two of the following containment measures in every US state: stay-at-home/shelter orders, school closures, nonessential business closures, restaurant closures, or prohibition of gatherings of more than 10 people).2
Outcomes
Statistical Analysis
Categorical variables were summarized using frequencies and percentages and compared using chi-square tests. Continuous variables were summarized as median and interquartile range (IQR) and compared using Wilcoxon rank sum tests. Weekly observed-to-expected (O:E) ratios were calculated for each hospital by dividing the number of observed respiratory illness and nonrespiratory illness encounters in a given week in 2020 (observed) by the average number of encounters for that same week during 2017-2019 (expected). O:E ratios were then aggregated over the three COVID-19 study periods, and 95% confidence intervals were established around mean O:E ratios across individual hospitals. Outcomes were then stratified by respiratory illness subgroups, geographic region, and age. Additional details can be found in the Supplemental Methods in the Appendix.
RESULTS
Study Population
A total of 9,051,980 encounters were included in the study, 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. Median age was 5 years (IQR, 1-11 years), and 52.7% of the population was male (Appendix Table 2 and Appendix Table 3).
Respiratory vs Nonrespiratory Illness During the COVID-19 Pandemic
Over the study period, fewer respiratory and nonrespiratory illness encounters were observed than expected, with a larger decrease in respiratory illness encounters (Table, Appendix Table 4). 

Respiratory Subgroup Analyses
The O:E ratio decreased for all respiratory subgroups over the study period (Table, Appendix Table 4). There were significant differences in specific respiratory subgroups, including asthma, bronchiolitis, croup, influenza, and pneumonia (Appendix Figure 1A). Temporal trends in respiratory encounters were consistent across hospital settings, ages, and geographic regions (Appendix Figure 1B-D). When comparing the with and without COVID-19 subgroups in the “other respiratory illnesses” cohort, other respiratory illness without COVID-19 decreased and remained lower than expected over the rest of the study period, while other respiratory illness with COVID-19 increased markedly during the summer months and declined thereafter (Appendix Figure 2).
All age groups had reductions in respiratory illness encounters during the early COVID-19 and COVID-19 periods, although the decline was less pronounced in the 12- to 17-year-old group (Appendix Figure 1B). Similarly, while all age groups experienced increases in encounters for respiratory illnesses during the summer months, only children in the 12- to 17-year-old group experienced increases beyond pre-COVID-19 levels. Importantly, this increase in respiratory encounters was largely driven by COVID-19 diagnoses (Appendix Figure 3). The trend in nonrespiratory illness encounters stratified by age is shown in Appendix Figure 4.
When patients were stratified by hospital setting, there were no differences between those hospitalized and those discharged from the ED (Appendix Figure 1C). Patterns in respiratory illnesses by geographic location were qualitatively similar until the beginning of the summer 2020, after which geographical variation became more evident (Appendix Figure 1D).
DISCUSSION
In this large, multicenter study evaluating ED visits and hospitalizations for respiratory and nonrespiratory illnesses at US pediatric hospitals during the 2020 COVID-19 pandemic, we found a significant and substantial decrease in healthcare encounters for respiratory illnesses. A rapid and marked decline in encounters for respiratory illness in a relatively short period of time (March 12-April 2) was observed across all hospitals and US regions. Declines were consistent across common respiratory illnesses. More modest, yet still substantial, declines were also observed for nonrespiratory illnesses.
There are likely multiple underlying reasons for the observed reductions. Social distancing measures almost certainly played an important role in interrupting respiratory infection transmission. Rapid reduction in influenza transmission during the early COVID-19 period has been attributed to social distancing measures,3 and influenza transmission in children decreases with school closures.9 It is also possible that some families delayed seeking care at hospitals due to COVID-19, leading to less frequent encounters but more severe illness. The similar decrease in O:E ratio for ED visits and hospitalizations, however, is inconsistent with this explanation.
We also found relative differences in changes in encounters for respiratory illness by age. Adolescents’ levels of respiratory healthcare use declined less and recovered at a faster rate than those of younger children, returning to pre-COVID-19 levels by the end of the study period. The reason for this age differential is likely multifaceted. Infections, such as bronchiolitis and pneumonia, are more likely to be a source of respiratory illness in younger than in older children. It is also likely that disproportionate relaxation of social distancing measures among adolescents, who are known to have a stronger pattern of social interaction, contributed to the faster rise in respiratory illness–related encounters in this age group.11 Adolescents have been reported to be more susceptible to, and more likely to transmit, SARS-CoV-2 compared to younger age groups.12 More modest, albeit similar, age-based changes were observed in encounters for nonrespiratory illnesses
Emerging evidence suggests that school-age children may play an important role in SARS-CoV-2 transmission in the community.4,14 Our finding that, compared to younger children, adolescents had significantly fewer reductions in respiratory illness encounters is concerning. These findings suggest that community-based efforts to help prevent respiratory illnesses, especially COVID-19, should focus on adolescents, who are most likely to maintain social interactions and transmit respiratory infections in the school setting and their households.
This study is limited by the inclusion of only tertiary care children’s hospitals, which may not be nationally representative, and the inability to assess the precise timing of when specific public health interventions were introduced. Moreover, previous studies suggest that social distancing behaviors may have changed even before formal recommendations were enacted.15 Future studies should investigate the local impact of state- and municipality-specific mandates on the burden of COVID-19 and other respiratory illnesses.
The COVID-19 pandemic was associated with substantial reductions in encounters for respiratory diseases, and also with more modest but still sizable reductions in encounters for nonrespiratory diseases. These reductions varied by age. Encounters among adolescents declined less and returned to previous levels faster compared with those of younger children.
ACKNOWLEDGMENT
This publication is dedicated to the memory of our coauthor, Dr. Michael Bendel-Stenzel. Dr. Bendel-Stenzel was dedicated to bettering the lives of children and advancing our knowledge of pediatrics through his research.
In the United States, respiratory illnesses are the most common cause of emergency department (ED) visits and hospitalizations in children.1 In response to the ongoing COVID-19 pandemic, several public health interventions, including school and business closures, stay-at-home orders, and mask mandates, were implemented to limit transmission of SARS-CoV-2.2,3 Studies have shown that children can contribute to the spread of SARS-CoV-2 infections, especially within households.4-6 Recent data suggest that COVID-19, and the associated public health measures enacted to slow its spread, may have affected the transmission of other respiratory pathogens.7 Similarly, the pandemic has likely affected healthcare utilization for nonrespiratory illnesses through adoption of social distancing recommendations, suspension and delays in nonemergent elective care, avoidance of healthcare settings, and the effect of decreased respiratory disease on exacerbation of chronic illness.8 The objective of this study was to examine associations between the COVID-19 pandemic and healthcare utilization for pediatric respiratory and nonrespiratory illnesses at US pediatric hospitals.
METHODS
Study Design
This is a multicenter, cross-sectional study of encounters at 44 pediatric hospitals that reported data to the Pediatric Health Information System (PHIS) database maintained by the Children’s Hospital Association (Lenexa, Kansas).
Study Population
Children 2 months to 18 years of age discharged from ED or inpatient settings with a nonsurgical diagnosis from January 1 to September 30 over a 4-year period (2017-2020) were included.
Exposure
The primary exposure was the 2020 COVID-19 pandemic time, divided into three periods: pre-COVID-19 (January-February 2020, the period prior to the pandemic in the United States), early COVID-19 (March-April 2020, coinciding with the first reported US pediatric case of COVID-19 on March 2, 2020), and COVID-19 (May-September 2020, marked by the implementation of at least two of the following containment measures in every US state: stay-at-home/shelter orders, school closures, nonessential business closures, restaurant closures, or prohibition of gatherings of more than 10 people).2
Outcomes
Statistical Analysis
Categorical variables were summarized using frequencies and percentages and compared using chi-square tests. Continuous variables were summarized as median and interquartile range (IQR) and compared using Wilcoxon rank sum tests. Weekly observed-to-expected (O:E) ratios were calculated for each hospital by dividing the number of observed respiratory illness and nonrespiratory illness encounters in a given week in 2020 (observed) by the average number of encounters for that same week during 2017-2019 (expected). O:E ratios were then aggregated over the three COVID-19 study periods, and 95% confidence intervals were established around mean O:E ratios across individual hospitals. Outcomes were then stratified by respiratory illness subgroups, geographic region, and age. Additional details can be found in the Supplemental Methods in the Appendix.
RESULTS
Study Population
A total of 9,051,980 encounters were included in the study, 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. Median age was 5 years (IQR, 1-11 years), and 52.7% of the population was male (Appendix Table 2 and Appendix Table 3).
Respiratory vs Nonrespiratory Illness During the COVID-19 Pandemic
Over the study period, fewer respiratory and nonrespiratory illness encounters were observed than expected, with a larger decrease in respiratory illness encounters (Table, Appendix Table 4). 

Respiratory Subgroup Analyses
The O:E ratio decreased for all respiratory subgroups over the study period (Table, Appendix Table 4). There were significant differences in specific respiratory subgroups, including asthma, bronchiolitis, croup, influenza, and pneumonia (Appendix Figure 1A). Temporal trends in respiratory encounters were consistent across hospital settings, ages, and geographic regions (Appendix Figure 1B-D). When comparing the with and without COVID-19 subgroups in the “other respiratory illnesses” cohort, other respiratory illness without COVID-19 decreased and remained lower than expected over the rest of the study period, while other respiratory illness with COVID-19 increased markedly during the summer months and declined thereafter (Appendix Figure 2).
All age groups had reductions in respiratory illness encounters during the early COVID-19 and COVID-19 periods, although the decline was less pronounced in the 12- to 17-year-old group (Appendix Figure 1B). Similarly, while all age groups experienced increases in encounters for respiratory illnesses during the summer months, only children in the 12- to 17-year-old group experienced increases beyond pre-COVID-19 levels. Importantly, this increase in respiratory encounters was largely driven by COVID-19 diagnoses (Appendix Figure 3). The trend in nonrespiratory illness encounters stratified by age is shown in Appendix Figure 4.
When patients were stratified by hospital setting, there were no differences between those hospitalized and those discharged from the ED (Appendix Figure 1C). Patterns in respiratory illnesses by geographic location were qualitatively similar until the beginning of the summer 2020, after which geographical variation became more evident (Appendix Figure 1D).
DISCUSSION
In this large, multicenter study evaluating ED visits and hospitalizations for respiratory and nonrespiratory illnesses at US pediatric hospitals during the 2020 COVID-19 pandemic, we found a significant and substantial decrease in healthcare encounters for respiratory illnesses. A rapid and marked decline in encounters for respiratory illness in a relatively short period of time (March 12-April 2) was observed across all hospitals and US regions. Declines were consistent across common respiratory illnesses. More modest, yet still substantial, declines were also observed for nonrespiratory illnesses.
There are likely multiple underlying reasons for the observed reductions. Social distancing measures almost certainly played an important role in interrupting respiratory infection transmission. Rapid reduction in influenza transmission during the early COVID-19 period has been attributed to social distancing measures,3 and influenza transmission in children decreases with school closures.9 It is also possible that some families delayed seeking care at hospitals due to COVID-19, leading to less frequent encounters but more severe illness. The similar decrease in O:E ratio for ED visits and hospitalizations, however, is inconsistent with this explanation.
We also found relative differences in changes in encounters for respiratory illness by age. Adolescents’ levels of respiratory healthcare use declined less and recovered at a faster rate than those of younger children, returning to pre-COVID-19 levels by the end of the study period. The reason for this age differential is likely multifaceted. Infections, such as bronchiolitis and pneumonia, are more likely to be a source of respiratory illness in younger than in older children. It is also likely that disproportionate relaxation of social distancing measures among adolescents, who are known to have a stronger pattern of social interaction, contributed to the faster rise in respiratory illness–related encounters in this age group.11 Adolescents have been reported to be more susceptible to, and more likely to transmit, SARS-CoV-2 compared to younger age groups.12 More modest, albeit similar, age-based changes were observed in encounters for nonrespiratory illnesses
Emerging evidence suggests that school-age children may play an important role in SARS-CoV-2 transmission in the community.4,14 Our finding that, compared to younger children, adolescents had significantly fewer reductions in respiratory illness encounters is concerning. These findings suggest that community-based efforts to help prevent respiratory illnesses, especially COVID-19, should focus on adolescents, who are most likely to maintain social interactions and transmit respiratory infections in the school setting and their households.
This study is limited by the inclusion of only tertiary care children’s hospitals, which may not be nationally representative, and the inability to assess the precise timing of when specific public health interventions were introduced. Moreover, previous studies suggest that social distancing behaviors may have changed even before formal recommendations were enacted.15 Future studies should investigate the local impact of state- and municipality-specific mandates on the burden of COVID-19 and other respiratory illnesses.
The COVID-19 pandemic was associated with substantial reductions in encounters for respiratory diseases, and also with more modest but still sizable reductions in encounters for nonrespiratory diseases. These reductions varied by age. Encounters among adolescents declined less and returned to previous levels faster compared with those of younger children.
ACKNOWLEDGMENT
This publication is dedicated to the memory of our coauthor, Dr. Michael Bendel-Stenzel. Dr. Bendel-Stenzel was dedicated to bettering the lives of children and advancing our knowledge of pediatrics through his research.
1. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
2. Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324(9):859-870. https://doi.org/10.1001/jama.2020.14348
3. Wiese AD, Everson J, Grijalva CG. Social distancing measures: evidence of interruption of seasonal influenza activity and early lessons of the SARS-CoV-2 pandemic. Clin Infect Dis. Published online June 20, 2020. https://doi.org/10.1093/cid/ciaa834
4. Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 infections in households - Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1631-1634. https://doi.org/10.15585/mmwr.mm6944e1
5. Worby CJ, Chaves SS, Wallinga J, Lipsitch M, Finelli L, Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10-16. https://doi.org/10.1016/j.epidem.2015.04.003
6. Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. Published online January 8, 2021. https://doi.org/10.1542/peds.2020-048090
7. Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146(4):e2020006460. https://doi.org/10.1542/peds.2020-006460
8. Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F. Trends in pediatric emergency department utilization after institution of coronavirus disease-19 mandatory social distancing. J Pediatr. 2020;226:274-277.e1. https://doi.org/10.1016/j.jpeds.2020.07.048
9. Luca G, Kerckhove KV, Coletti P, et al. The impact of regular school closure on seasonal influenza epidemics: a data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. https://doi.org/10.1186/s12879-017-2934-3
10. Taquechel K, Diwadkar AR, Sayed S, et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(10):3378-3387.e11. https://doi.org/10.1016/j.jaip.2020.07.057
11. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465-2468. https://doi.org/10.3201/eid2610.201315
12. Davies NG, Klepac P, Liu Y, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-1211. https://doi.org/10.1038/s41591-020-0962-9
13. Hill RM, Rufino K, Kurian S, Saxena J, Saxena K, Williams L. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. Published online December 16, 2020. https://doi.org/10.1542/peds.2020-029280
14. Flasche S, Edmunds WJ. The role of schools and school-aged children in SARS-CoV-2 transmission. Lancet Infect Dis. Published online December 8, 2020. https://doi.org/10.1016/S1473-3099(20)30927-0
15. Sehra ST, George M, Wiebe DJ, Fundin S, Baker JF. Cell phone activity in categories of places and associations with growth in cases of COVID-19 in the US. JAMA Intern Med. Published online August 31, 2020. https://doi.org/10.1001/jamainternmed.2020.4288
1. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
2. Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324(9):859-870. https://doi.org/10.1001/jama.2020.14348
3. Wiese AD, Everson J, Grijalva CG. Social distancing measures: evidence of interruption of seasonal influenza activity and early lessons of the SARS-CoV-2 pandemic. Clin Infect Dis. Published online June 20, 2020. https://doi.org/10.1093/cid/ciaa834
4. Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 infections in households - Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1631-1634. https://doi.org/10.15585/mmwr.mm6944e1
5. Worby CJ, Chaves SS, Wallinga J, Lipsitch M, Finelli L, Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10-16. https://doi.org/10.1016/j.epidem.2015.04.003
6. Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. Published online January 8, 2021. https://doi.org/10.1542/peds.2020-048090
7. Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146(4):e2020006460. https://doi.org/10.1542/peds.2020-006460
8. Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F. Trends in pediatric emergency department utilization after institution of coronavirus disease-19 mandatory social distancing. J Pediatr. 2020;226:274-277.e1. https://doi.org/10.1016/j.jpeds.2020.07.048
9. Luca G, Kerckhove KV, Coletti P, et al. The impact of regular school closure on seasonal influenza epidemics: a data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. https://doi.org/10.1186/s12879-017-2934-3
10. Taquechel K, Diwadkar AR, Sayed S, et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(10):3378-3387.e11. https://doi.org/10.1016/j.jaip.2020.07.057
11. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465-2468. https://doi.org/10.3201/eid2610.201315
12. Davies NG, Klepac P, Liu Y, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-1211. https://doi.org/10.1038/s41591-020-0962-9
13. Hill RM, Rufino K, Kurian S, Saxena J, Saxena K, Williams L. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. Published online December 16, 2020. https://doi.org/10.1542/peds.2020-029280
14. Flasche S, Edmunds WJ. The role of schools and school-aged children in SARS-CoV-2 transmission. Lancet Infect Dis. Published online December 8, 2020. https://doi.org/10.1016/S1473-3099(20)30927-0
15. Sehra ST, George M, Wiebe DJ, Fundin S, Baker JF. Cell phone activity in categories of places and associations with growth in cases of COVID-19 in the US. JAMA Intern Med. Published online August 31, 2020. https://doi.org/10.1001/jamainternmed.2020.4288
© 2021 Society of Hospital Medicine
Trends in Risk-Adjusted 28-Day Mortality Rates for Patients Hospitalized with COVID-19 in England
The early phase of the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK) was characterized by uncertainty as clinicians grappled to understand and manage an unfamiliar disease that affected very high numbers of patients amid radically evolving working environments, with little evidence to support their efforts. Early reports indicated high mortality in patients hospitalized with COVID-19.
As the disease became better understood, treatment evolved and the mortality appears to have decreased. For example, two recent papers, a national study of critical care patients in the UK and a single-site study from New York, have demonstrated a significant reduction in adjusted mortality between the pre- and post-peak periods.1,2 However, the UK study was restricted to patients receiving critical care, potentially introducing bias due to varying critical care admission thresholds over time, while the single-site US study may not be generalizable. Moreover, both studies measured only in-hospital mortality. It remains uncertain therefore whether overall mortality has decreased on a broad scale after accounting for changes in patient characteristics.
The aim of this study was to use a national dataset to assess the
METHODS
We conducted a retrospective, secondary analysis of English National Health Services (NHS) hospitals’ admissions of patients at least 18 years of age between March 1 and July 31, 2020. Data were obtained from the Hospital Episode Statistics (HES) admitted patient care dataset.3 This is an administrative dataset that contains data on diagnoses and procedures as well as organizational characteristics and patient demographics for all NHS activity in England. We included all patients with an International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis of U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified).
The primary outcome of death within 28 days of admission was obtained by linking to the Civil Registrations (Deaths) - Secondary Care Cut - Information dataset, which includes the date, place, and cause of death from the Office for National Statistics4 and which was complete through September 31, 2020. The time horizon of 28 days from admission was chosen to approximate the Public Health England definition of a death from COVID-19 as being within 28 days of testing positive.5 We restricted our analysis to emergency admissions of persons age >18 years. If a patient had multiple emergency admissions, we restricted our analysis to the first admission to ensure comparability across hospitalizations and to best represent outcomes from the earliest onset of COVID-19.
We estimated a modified Poisson regression6 to predict death at 28 days, with month of admission, region, source of admission, age, deprivation, gender, ethnic group, and the 29 comorbidities in the Elixhauser comorbidity measure as variables in the regression.7 The derivation of each of these variables from the HES dataset is shown in Appendix Table 1.
Deprivation was measured by the Index of Multiple Deprivation, a methodology used widely within the UK to classify relative deprivation.8 To control for clustering, hospital system (known as Trust) was added as a random effect. Robust errors were estimated using the sandwich package.9 Modified Poisson regression was chosen in preference to the more common logistic regression because the coefficients can be interpreted as relative risks and not odds ratios. The model was fitted using R, version 4.0.3, geepack library.10 We carried out three sensitivity analyses, restricting to laboratory-confirmed COVID-19, length of stay ≥3 days, and primary respiratory disease.
For each month, we obtained a standardized mortality ratio (SMR) by fixing the month to the reference month of March 2020 and repredicting the outcome using the existing model. We calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month, comparing observed deaths to the number we would have expected had those patients been hospitalized in March. We then multiplied each period’s SMR by the March crude mortality to generate monthly adjusted mortality rates. We calculated Poisson confidence intervals around the SMR and used these to obtain confidence intervals for the adjusted rate. The binomial exact method was used to obtain confidence intervals for the crude rate. Multicollinearity was assessed using both the variance inflation factor (VIF) and the condition number test.7 All analyses used two-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The study was exempt from UK National Research Ethics Committee approval because it involved secondary analysis of anonymized data.
RESULTS
The dataset included 115,643 emergency admissions from 179 healthcare systems, of which 103,202 were first admissions eligible for inclusion. A total of 592 patients were excluded due to missing demographic data (0.5%), resulting in 102,610 admissions included in the analysis. Peak hospitalizations occurred in late March to mid April, accounting for 44% of the hospitalizations (Table). Median length of stay for patients who died was 7 days (interquartile range, 3-12). The median age and number of Elixhauser comorbidities decreased in July. The proportion of men decreased between May and July. 
The modified Poisson regression had a C statistic of 0.743 (95% CI, 0.740-0.746) (Appendix Table 4). The VIF and condition number test found no evidence of multicollinearity.11
Adjusted mortality decreased each month, from 33.4% in March to 17.4% in July (Figure). The relative risk of death declined progressively to a minimum of 0.52 (95% CI, 0.34-0.80) in July, compared to March.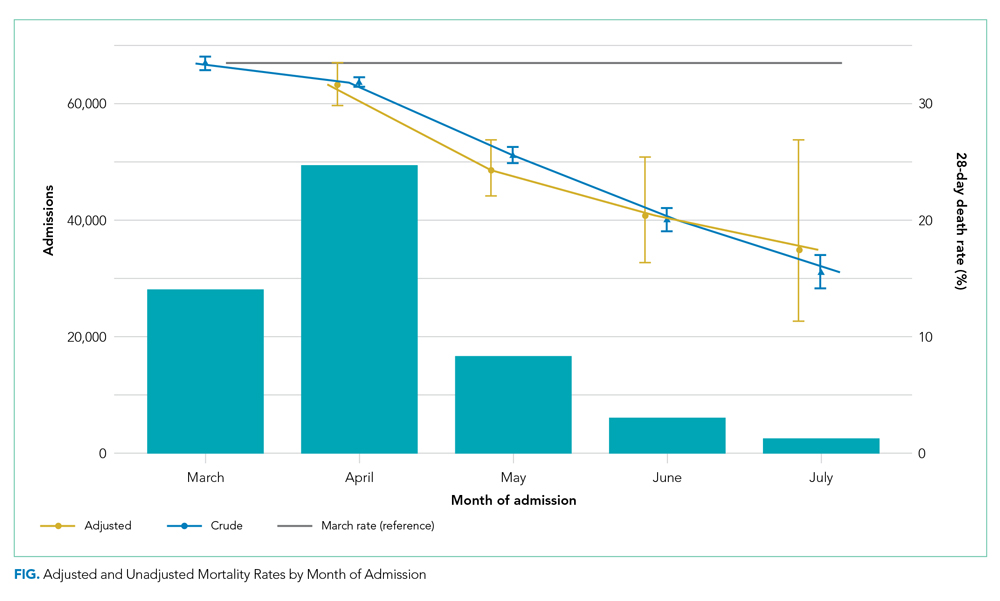
Admission from another hospital and being female were associated with reduced risk of death. Admission from a skilled nursing facility and being >75 years were associated with increased risk of death. Ten of the 29 Elixhauser comorbidities were associated with increased risk of mortality (cardiac arrhythmia, peripheral vascular disease, other neurologic disorders, renal failure, lymphoma, metastatic cancer, solid tumor without metastasis, coagulopathy, fluid and electrolyte disorders, and anemia). Deprivation and ethnic group were not associated with death among hospitalized patients.
DISCUSSION
Our study of all emergency hospital admissions in England during the first wave of the COVID-19 pandemic demonstrated that, even after adjusting for patient comorbidity and risk factors, the mortality rate decreased by approximately half over the first 5 months. Although the demographics of hospitalized patients changed over that period (with both the median age and the number of comorbidities decreasing), this does not fully explain the decrease in mortality. It is therefore likely that the decrease is due, at least in part, to an improvement in treatment and/or a reduction in hospital strain.
For example, initially the use of corticosteroids was controversial, in part due to previous experience with severe acute respiratory syndrome and Middle East respiratory syndrome (in which a Cochrane review demonstrated no benefit but potential harm). However, this changed as a result of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12 which showed a significant survival benefit.One of the positive defining characteristics of the COVID-19 pandemic has been the intensive collaborative research effort combined with the rapid dissemination and discussion of new management protocols. The RECOVERY trial randomly assigned >11,000 participants in just 3 months, amounting to approximately 15% of all patients hospitalized with COVID-19 in the UK. Its results were widely publicized via professional networks and rapidly adopted into widespread clinical practice.
Examples of other changes include a higher threshold for mechanical ventilation (and a lower threshold for noninvasive ventilation), increased clinician experience, and, potentially, a reduced viral load arising from increased social distancing and mask wearing. Finally, the hospitals and staff themselves were under enormous physical and mental strain in the early months from multiple factors, including unfamiliar working environments, the large-scale redeployment of inexperienced staff, and very high numbers of patients with an unfamiliar disease. These factors all lessened as the initial peak passed. It is therefore likely that the reduction in adjusted mortality we observed arises from a combination of all these factors, as well as other incremental benefits.
The factors associated with increased mortality risk in our study (increasing age, male gender, certain comorbidities, and frailty [with care home residency acting as a proxy in our study]) are consistent with multiple previous reports. Although not the focus of our analysis, we found no effect of ethnicity or deprivation on mortality. This is consistent with many US studies that demonstrate that the widely reported effect of these factors is likely due to differences in exposure to the disease. Once patients are hospitalized, adjusted mortality risks are similar across ethnic groups and deprivation levels.
The strengths of this study include complete capture of hospitalizations across all hospitals and areas in England. Likewise, linking the hospital data to death data from the Office for National Statistics allows complete capture of outcomes, irrespective of where the patient died. This is a significant strength compared to prior studies, which only included in-hospital mortality. Our results are therefore likely robust and a true observation of the mortality trend.
Limitations include the lack of physiologic and laboratory data; having these would have allowed us to adjust for disease severity on admission and strengthened the risk stratification. Likewise, although the complete national coverage is overall a significant strength, aggregating data from numerous areas that might be at different stages of local outbreaks, have different management strategies, and have differing data quality introduces its own biases.
Furthermore, these results predate the second wave in the UK, so we cannot distinguish whether the reduced mortality is due to improved treatment, a seasonal effect, evolution of the virus itself, or a reduction in the strain on hospitals.
CONCLUSION
This nationwide study indicates that, even after accounting for changing patient characteristics, the mortality of patients hospitalized with COVID-19 in England decreased significantly as the outbreak progressed. This is likely due to a combination of incremental treatment improvements.
1. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020;16(2):90-92. https://doi.org/10.12788/jhm.3552
2. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. https://doi.org/10.1097/CCM.0000000000004747
3. NHS Digital. Hospital Episode Statistics Data Dictionary. Published March 2018. Accessed October 15, 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary
4. NHS Digital. HES-ONS Linked Mortality Data Dictionary. Accessed October 15, 2020. https://digital.nhs.uk/binaries/content/assets/legacy/word/i/p/hes-ons_linked_mortality_data_dictionary_-_mar_20181.docx
5. Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. Accessed November 11, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/916035/RA_Technical_Summary_-_PHE_Data_Series_COVID_19_Deaths_20200812.pdf
6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. https://doi.org/10.1093/aje/kwh090
7. van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org /10.1097/MLR.0b013e31819432e5
8. Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). Published September 26, 2020. Accessed January 15, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
9. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. 2006;16:1-16. https://doi.org/10.18637/jss.v016.i09
10. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Software. 2006;15:1-11. https://doi.org/10.18637/jss.v015.i02
11. Belsley DA, Kuh E, Welsch RE. Diagnostics: Identifying Influential Data and Sources of Collinearity. John Wiley & Sons; 1980.
12. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
The early phase of the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK) was characterized by uncertainty as clinicians grappled to understand and manage an unfamiliar disease that affected very high numbers of patients amid radically evolving working environments, with little evidence to support their efforts. Early reports indicated high mortality in patients hospitalized with COVID-19.
As the disease became better understood, treatment evolved and the mortality appears to have decreased. For example, two recent papers, a national study of critical care patients in the UK and a single-site study from New York, have demonstrated a significant reduction in adjusted mortality between the pre- and post-peak periods.1,2 However, the UK study was restricted to patients receiving critical care, potentially introducing bias due to varying critical care admission thresholds over time, while the single-site US study may not be generalizable. Moreover, both studies measured only in-hospital mortality. It remains uncertain therefore whether overall mortality has decreased on a broad scale after accounting for changes in patient characteristics.
The aim of this study was to use a national dataset to assess the
METHODS
We conducted a retrospective, secondary analysis of English National Health Services (NHS) hospitals’ admissions of patients at least 18 years of age between March 1 and July 31, 2020. Data were obtained from the Hospital Episode Statistics (HES) admitted patient care dataset.3 This is an administrative dataset that contains data on diagnoses and procedures as well as organizational characteristics and patient demographics for all NHS activity in England. We included all patients with an International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis of U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified).
The primary outcome of death within 28 days of admission was obtained by linking to the Civil Registrations (Deaths) - Secondary Care Cut - Information dataset, which includes the date, place, and cause of death from the Office for National Statistics4 and which was complete through September 31, 2020. The time horizon of 28 days from admission was chosen to approximate the Public Health England definition of a death from COVID-19 as being within 28 days of testing positive.5 We restricted our analysis to emergency admissions of persons age >18 years. If a patient had multiple emergency admissions, we restricted our analysis to the first admission to ensure comparability across hospitalizations and to best represent outcomes from the earliest onset of COVID-19.
We estimated a modified Poisson regression6 to predict death at 28 days, with month of admission, region, source of admission, age, deprivation, gender, ethnic group, and the 29 comorbidities in the Elixhauser comorbidity measure as variables in the regression.7 The derivation of each of these variables from the HES dataset is shown in Appendix Table 1.
Deprivation was measured by the Index of Multiple Deprivation, a methodology used widely within the UK to classify relative deprivation.8 To control for clustering, hospital system (known as Trust) was added as a random effect. Robust errors were estimated using the sandwich package.9 Modified Poisson regression was chosen in preference to the more common logistic regression because the coefficients can be interpreted as relative risks and not odds ratios. The model was fitted using R, version 4.0.3, geepack library.10 We carried out three sensitivity analyses, restricting to laboratory-confirmed COVID-19, length of stay ≥3 days, and primary respiratory disease.
For each month, we obtained a standardized mortality ratio (SMR) by fixing the month to the reference month of March 2020 and repredicting the outcome using the existing model. We calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month, comparing observed deaths to the number we would have expected had those patients been hospitalized in March. We then multiplied each period’s SMR by the March crude mortality to generate monthly adjusted mortality rates. We calculated Poisson confidence intervals around the SMR and used these to obtain confidence intervals for the adjusted rate. The binomial exact method was used to obtain confidence intervals for the crude rate. Multicollinearity was assessed using both the variance inflation factor (VIF) and the condition number test.7 All analyses used two-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The study was exempt from UK National Research Ethics Committee approval because it involved secondary analysis of anonymized data.
RESULTS
The dataset included 115,643 emergency admissions from 179 healthcare systems, of which 103,202 were first admissions eligible for inclusion. A total of 592 patients were excluded due to missing demographic data (0.5%), resulting in 102,610 admissions included in the analysis. Peak hospitalizations occurred in late March to mid April, accounting for 44% of the hospitalizations (Table). Median length of stay for patients who died was 7 days (interquartile range, 3-12). The median age and number of Elixhauser comorbidities decreased in July. The proportion of men decreased between May and July. 
The modified Poisson regression had a C statistic of 0.743 (95% CI, 0.740-0.746) (Appendix Table 4). The VIF and condition number test found no evidence of multicollinearity.11
Adjusted mortality decreased each month, from 33.4% in March to 17.4% in July (Figure). The relative risk of death declined progressively to a minimum of 0.52 (95% CI, 0.34-0.80) in July, compared to March.
Admission from another hospital and being female were associated with reduced risk of death. Admission from a skilled nursing facility and being >75 years were associated with increased risk of death. Ten of the 29 Elixhauser comorbidities were associated with increased risk of mortality (cardiac arrhythmia, peripheral vascular disease, other neurologic disorders, renal failure, lymphoma, metastatic cancer, solid tumor without metastasis, coagulopathy, fluid and electrolyte disorders, and anemia). Deprivation and ethnic group were not associated with death among hospitalized patients.
DISCUSSION
Our study of all emergency hospital admissions in England during the first wave of the COVID-19 pandemic demonstrated that, even after adjusting for patient comorbidity and risk factors, the mortality rate decreased by approximately half over the first 5 months. Although the demographics of hospitalized patients changed over that period (with both the median age and the number of comorbidities decreasing), this does not fully explain the decrease in mortality. It is therefore likely that the decrease is due, at least in part, to an improvement in treatment and/or a reduction in hospital strain.
For example, initially the use of corticosteroids was controversial, in part due to previous experience with severe acute respiratory syndrome and Middle East respiratory syndrome (in which a Cochrane review demonstrated no benefit but potential harm). However, this changed as a result of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12 which showed a significant survival benefit.One of the positive defining characteristics of the COVID-19 pandemic has been the intensive collaborative research effort combined with the rapid dissemination and discussion of new management protocols. The RECOVERY trial randomly assigned >11,000 participants in just 3 months, amounting to approximately 15% of all patients hospitalized with COVID-19 in the UK. Its results were widely publicized via professional networks and rapidly adopted into widespread clinical practice.
Examples of other changes include a higher threshold for mechanical ventilation (and a lower threshold for noninvasive ventilation), increased clinician experience, and, potentially, a reduced viral load arising from increased social distancing and mask wearing. Finally, the hospitals and staff themselves were under enormous physical and mental strain in the early months from multiple factors, including unfamiliar working environments, the large-scale redeployment of inexperienced staff, and very high numbers of patients with an unfamiliar disease. These factors all lessened as the initial peak passed. It is therefore likely that the reduction in adjusted mortality we observed arises from a combination of all these factors, as well as other incremental benefits.
The factors associated with increased mortality risk in our study (increasing age, male gender, certain comorbidities, and frailty [with care home residency acting as a proxy in our study]) are consistent with multiple previous reports. Although not the focus of our analysis, we found no effect of ethnicity or deprivation on mortality. This is consistent with many US studies that demonstrate that the widely reported effect of these factors is likely due to differences in exposure to the disease. Once patients are hospitalized, adjusted mortality risks are similar across ethnic groups and deprivation levels.
The strengths of this study include complete capture of hospitalizations across all hospitals and areas in England. Likewise, linking the hospital data to death data from the Office for National Statistics allows complete capture of outcomes, irrespective of where the patient died. This is a significant strength compared to prior studies, which only included in-hospital mortality. Our results are therefore likely robust and a true observation of the mortality trend.
Limitations include the lack of physiologic and laboratory data; having these would have allowed us to adjust for disease severity on admission and strengthened the risk stratification. Likewise, although the complete national coverage is overall a significant strength, aggregating data from numerous areas that might be at different stages of local outbreaks, have different management strategies, and have differing data quality introduces its own biases.
Furthermore, these results predate the second wave in the UK, so we cannot distinguish whether the reduced mortality is due to improved treatment, a seasonal effect, evolution of the virus itself, or a reduction in the strain on hospitals.
CONCLUSION
This nationwide study indicates that, even after accounting for changing patient characteristics, the mortality of patients hospitalized with COVID-19 in England decreased significantly as the outbreak progressed. This is likely due to a combination of incremental treatment improvements.
The early phase of the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK) was characterized by uncertainty as clinicians grappled to understand and manage an unfamiliar disease that affected very high numbers of patients amid radically evolving working environments, with little evidence to support their efforts. Early reports indicated high mortality in patients hospitalized with COVID-19.
As the disease became better understood, treatment evolved and the mortality appears to have decreased. For example, two recent papers, a national study of critical care patients in the UK and a single-site study from New York, have demonstrated a significant reduction in adjusted mortality between the pre- and post-peak periods.1,2 However, the UK study was restricted to patients receiving critical care, potentially introducing bias due to varying critical care admission thresholds over time, while the single-site US study may not be generalizable. Moreover, both studies measured only in-hospital mortality. It remains uncertain therefore whether overall mortality has decreased on a broad scale after accounting for changes in patient characteristics.
The aim of this study was to use a national dataset to assess the
METHODS
We conducted a retrospective, secondary analysis of English National Health Services (NHS) hospitals’ admissions of patients at least 18 years of age between March 1 and July 31, 2020. Data were obtained from the Hospital Episode Statistics (HES) admitted patient care dataset.3 This is an administrative dataset that contains data on diagnoses and procedures as well as organizational characteristics and patient demographics for all NHS activity in England. We included all patients with an International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis of U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified).
The primary outcome of death within 28 days of admission was obtained by linking to the Civil Registrations (Deaths) - Secondary Care Cut - Information dataset, which includes the date, place, and cause of death from the Office for National Statistics4 and which was complete through September 31, 2020. The time horizon of 28 days from admission was chosen to approximate the Public Health England definition of a death from COVID-19 as being within 28 days of testing positive.5 We restricted our analysis to emergency admissions of persons age >18 years. If a patient had multiple emergency admissions, we restricted our analysis to the first admission to ensure comparability across hospitalizations and to best represent outcomes from the earliest onset of COVID-19.
We estimated a modified Poisson regression6 to predict death at 28 days, with month of admission, region, source of admission, age, deprivation, gender, ethnic group, and the 29 comorbidities in the Elixhauser comorbidity measure as variables in the regression.7 The derivation of each of these variables from the HES dataset is shown in Appendix Table 1.
Deprivation was measured by the Index of Multiple Deprivation, a methodology used widely within the UK to classify relative deprivation.8 To control for clustering, hospital system (known as Trust) was added as a random effect. Robust errors were estimated using the sandwich package.9 Modified Poisson regression was chosen in preference to the more common logistic regression because the coefficients can be interpreted as relative risks and not odds ratios. The model was fitted using R, version 4.0.3, geepack library.10 We carried out three sensitivity analyses, restricting to laboratory-confirmed COVID-19, length of stay ≥3 days, and primary respiratory disease.
For each month, we obtained a standardized mortality ratio (SMR) by fixing the month to the reference month of March 2020 and repredicting the outcome using the existing model. We calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month, comparing observed deaths to the number we would have expected had those patients been hospitalized in March. We then multiplied each period’s SMR by the March crude mortality to generate monthly adjusted mortality rates. We calculated Poisson confidence intervals around the SMR and used these to obtain confidence intervals for the adjusted rate. The binomial exact method was used to obtain confidence intervals for the crude rate. Multicollinearity was assessed using both the variance inflation factor (VIF) and the condition number test.7 All analyses used two-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The study was exempt from UK National Research Ethics Committee approval because it involved secondary analysis of anonymized data.
RESULTS
The dataset included 115,643 emergency admissions from 179 healthcare systems, of which 103,202 were first admissions eligible for inclusion. A total of 592 patients were excluded due to missing demographic data (0.5%), resulting in 102,610 admissions included in the analysis. Peak hospitalizations occurred in late March to mid April, accounting for 44% of the hospitalizations (Table). Median length of stay for patients who died was 7 days (interquartile range, 3-12). The median age and number of Elixhauser comorbidities decreased in July. The proportion of men decreased between May and July. 
The modified Poisson regression had a C statistic of 0.743 (95% CI, 0.740-0.746) (Appendix Table 4). The VIF and condition number test found no evidence of multicollinearity.11
Adjusted mortality decreased each month, from 33.4% in March to 17.4% in July (Figure). The relative risk of death declined progressively to a minimum of 0.52 (95% CI, 0.34-0.80) in July, compared to March.
Admission from another hospital and being female were associated with reduced risk of death. Admission from a skilled nursing facility and being >75 years were associated with increased risk of death. Ten of the 29 Elixhauser comorbidities were associated with increased risk of mortality (cardiac arrhythmia, peripheral vascular disease, other neurologic disorders, renal failure, lymphoma, metastatic cancer, solid tumor without metastasis, coagulopathy, fluid and electrolyte disorders, and anemia). Deprivation and ethnic group were not associated with death among hospitalized patients.
DISCUSSION
Our study of all emergency hospital admissions in England during the first wave of the COVID-19 pandemic demonstrated that, even after adjusting for patient comorbidity and risk factors, the mortality rate decreased by approximately half over the first 5 months. Although the demographics of hospitalized patients changed over that period (with both the median age and the number of comorbidities decreasing), this does not fully explain the decrease in mortality. It is therefore likely that the decrease is due, at least in part, to an improvement in treatment and/or a reduction in hospital strain.
For example, initially the use of corticosteroids was controversial, in part due to previous experience with severe acute respiratory syndrome and Middle East respiratory syndrome (in which a Cochrane review demonstrated no benefit but potential harm). However, this changed as a result of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12 which showed a significant survival benefit.One of the positive defining characteristics of the COVID-19 pandemic has been the intensive collaborative research effort combined with the rapid dissemination and discussion of new management protocols. The RECOVERY trial randomly assigned >11,000 participants in just 3 months, amounting to approximately 15% of all patients hospitalized with COVID-19 in the UK. Its results were widely publicized via professional networks and rapidly adopted into widespread clinical practice.
Examples of other changes include a higher threshold for mechanical ventilation (and a lower threshold for noninvasive ventilation), increased clinician experience, and, potentially, a reduced viral load arising from increased social distancing and mask wearing. Finally, the hospitals and staff themselves were under enormous physical and mental strain in the early months from multiple factors, including unfamiliar working environments, the large-scale redeployment of inexperienced staff, and very high numbers of patients with an unfamiliar disease. These factors all lessened as the initial peak passed. It is therefore likely that the reduction in adjusted mortality we observed arises from a combination of all these factors, as well as other incremental benefits.
The factors associated with increased mortality risk in our study (increasing age, male gender, certain comorbidities, and frailty [with care home residency acting as a proxy in our study]) are consistent with multiple previous reports. Although not the focus of our analysis, we found no effect of ethnicity or deprivation on mortality. This is consistent with many US studies that demonstrate that the widely reported effect of these factors is likely due to differences in exposure to the disease. Once patients are hospitalized, adjusted mortality risks are similar across ethnic groups and deprivation levels.
The strengths of this study include complete capture of hospitalizations across all hospitals and areas in England. Likewise, linking the hospital data to death data from the Office for National Statistics allows complete capture of outcomes, irrespective of where the patient died. This is a significant strength compared to prior studies, which only included in-hospital mortality. Our results are therefore likely robust and a true observation of the mortality trend.
Limitations include the lack of physiologic and laboratory data; having these would have allowed us to adjust for disease severity on admission and strengthened the risk stratification. Likewise, although the complete national coverage is overall a significant strength, aggregating data from numerous areas that might be at different stages of local outbreaks, have different management strategies, and have differing data quality introduces its own biases.
Furthermore, these results predate the second wave in the UK, so we cannot distinguish whether the reduced mortality is due to improved treatment, a seasonal effect, evolution of the virus itself, or a reduction in the strain on hospitals.
CONCLUSION
This nationwide study indicates that, even after accounting for changing patient characteristics, the mortality of patients hospitalized with COVID-19 in England decreased significantly as the outbreak progressed. This is likely due to a combination of incremental treatment improvements.
1. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020;16(2):90-92. https://doi.org/10.12788/jhm.3552
2. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. https://doi.org/10.1097/CCM.0000000000004747
3. NHS Digital. Hospital Episode Statistics Data Dictionary. Published March 2018. Accessed October 15, 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary
4. NHS Digital. HES-ONS Linked Mortality Data Dictionary. Accessed October 15, 2020. https://digital.nhs.uk/binaries/content/assets/legacy/word/i/p/hes-ons_linked_mortality_data_dictionary_-_mar_20181.docx
5. Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. Accessed November 11, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/916035/RA_Technical_Summary_-_PHE_Data_Series_COVID_19_Deaths_20200812.pdf
6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. https://doi.org/10.1093/aje/kwh090
7. van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org /10.1097/MLR.0b013e31819432e5
8. Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). Published September 26, 2020. Accessed January 15, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
9. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. 2006;16:1-16. https://doi.org/10.18637/jss.v016.i09
10. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Software. 2006;15:1-11. https://doi.org/10.18637/jss.v015.i02
11. Belsley DA, Kuh E, Welsch RE. Diagnostics: Identifying Influential Data and Sources of Collinearity. John Wiley & Sons; 1980.
12. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
1. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020;16(2):90-92. https://doi.org/10.12788/jhm.3552
2. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. https://doi.org/10.1097/CCM.0000000000004747
3. NHS Digital. Hospital Episode Statistics Data Dictionary. Published March 2018. Accessed October 15, 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary
4. NHS Digital. HES-ONS Linked Mortality Data Dictionary. Accessed October 15, 2020. https://digital.nhs.uk/binaries/content/assets/legacy/word/i/p/hes-ons_linked_mortality_data_dictionary_-_mar_20181.docx
5. Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. Accessed November 11, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/916035/RA_Technical_Summary_-_PHE_Data_Series_COVID_19_Deaths_20200812.pdf
6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. https://doi.org/10.1093/aje/kwh090
7. van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org /10.1097/MLR.0b013e31819432e5
8. Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). Published September 26, 2020. Accessed January 15, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
9. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. 2006;16:1-16. https://doi.org/10.18637/jss.v016.i09
10. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Software. 2006;15:1-11. https://doi.org/10.18637/jss.v015.i02
11. Belsley DA, Kuh E, Welsch RE. Diagnostics: Identifying Influential Data and Sources of Collinearity. John Wiley & Sons; 1980.
12. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
© 2021 Society of Hospital Medicine
ECHO-CT: An Interdisciplinary Videoconference Model for Identifying Potential Postdischarge Transition-of-Care Events
As the population of the United States continues to age, hospitals are seeing an increasing number of older patients with significant medical and social complexity. Medicare data have shown that an increasing number require post–acute care after a hospitalization.1 Discharges to post–acute care settings are often longer and more costly compared with discharges to other settings, which suggests that targeting quality improvement efforts at this transition period may improve the value of care.2
The transition from the hospital setting to a post–acute care facility can be dangerous and complicated due to lapses in communication, medication errors, and the complexity of medical treatment plans. Suboptimal transitions in care can result in adverse events for the patient, as well as confusion in medication regimens or incomplete plans for follow-up care.3
The Project ECHO (Extension for Community Healthcare Outcomes) model was first developed and launched by Sanjeev Arora, MD, in New Mexico in 2003 to expand access to subspecialist care using videoconferencing.4 We first applied this model in 2013 to evaluate the impact of this interdisciplinary videoconferencing tool on the care of patients discharged to post–acute settings.5 We found that patients participating in the Extension for Community Healthcare Outcomes–Care Transitions (ECHO-CT) model experienced decreased risk of rehospitalization, decreased skilled nursing facility (SNF) length of stay, and reduced 30-day healthcare costs, compared with those patients not enrolled in this program; these outcomes were likely due to identification and correction of medication-related errors, improved care coordination, improved disease management, and clarification of goals of care.6 Though these investigations did identify some issues arising during the care transition process, they did not fully describe the types of problems uncovered. We sought to better characterize the clinical and operational issues identified through the ECHO-CT conference, hereafter known as transition-of-care events (TCEs). These issues may include new or evolving medical concerns, an adverse event, or a “near miss.” Identification and classification of TCEs that may contribute to unsafe or fractured care transitions are critical in developing systematic solutions to improve transitions of care, which can ultimately improve patient safety and potentially avoid preventable errors.
METHODS
ECHO-CT Multidisciplinary Video Conference
We conducted ECHO-CT at a large, tertiary care academic medical center. The project design for the ECHO-CT program has been previously described.5 In brief, the program is a weekly, multidisciplinary videoconference between a hospital-based team and post–acute care providers to discuss patients discharged from inpatient services to post–acute care sites, including SNFs and long-term acute care hospitals (LTACHs), during the preceding week. All patients discharged from the tertiary care inpatient site to one of the eight participating SNFs or LTACHs, from either a medical or surgical service, are eligible to be discussed at this weekly interdisciplinary conference. Long-term care facilities were not included in this study. The ECHO-CT program used HIPAA (Health Insurance Portability and Accountability Act)-compliant videoconferencing technology to connect hospital and post–acute care providers.
During the videoconferences, each patient’s hospital course and discharge documentation are reviewed by a hospitalist, and a pharmacist performs a medication reconciliation of each patient’s admission, discharge, and post–acute care medication list. The discharging attending, primary care providers, residents, other trainees, and subspecialist providers are invited to attend. Typically, the interdisciplinary team at the post–acute care sites includes physicians, nurse practitioners, physical therapists, social workers, and case managers. Between 10 and 20 patients are discussed in a case-based format, which includes a summary of the patient’s hospital course, an update from the post–acute care team on the patient’s care, and an opportunity for a discussion regarding any concerns or questions raised by the post–acute care or inpatient care teams. The content and duration of discussion typically lasts approximately 3 to 10 minutes, depending on the needs of the patient and the care team. Each of the eight post–acute care sites participating in the project are assigned a 10- to 15-minute block. A copy of the ECHO-CT session process document is included in the Appendix.
Data Collection
At each interdisciplinary patient review, TCEs were identified and recorded. These events were categorized in real time by the ECHO-CT data collection team into the following categories: medication related, medical, discharge communication/coordination, or other, and recorded in a secured, deidentified database. For individuals whose TCEs could represent more than one category, authors reviewed the available information about the TCEs and determined the most appropriate category; if more than one category was felt to be applicable to a patient’s situation, the events were reclassified into all applicable categories. Data about individual patients, including gender, age at the time of discharge, and other demographic information, were obtained from hospital databases. Number of diagnoses included any diagnosis billed during the patient’s hospital stay, and these data were obtained from a hospital billing database. Average number of medications at discharge was obtained from a hospital pharmacy database.
RESULTS
A total of 675 patients (experiencing 743 hospitalizations) were discharged from a medical or surgical service to one of the participating post–acute care sites from January 2016 to October 2018, and were discussed at the interdisciplinary conference. During that time, 139 TCEs were recorded for review, involving 132 patients (Table 1). Patients who experienced TCEs were noted to have a slightly higher average number of diagnoses than did those in the non-TCE group (21 vs 18, respectively) and number of medications (18 vs 15).
Representative examples of TCEs are provided in Table 2. Fifty-eight issues were identified as discharge communication or coordination issues (eg, discharge summary was late or missing at time of discharge to facility, transitional issues were unclear, follow-up appointments were not appropriately scheduled or documented). An additional 52 TCEs were identified as pharmacy or medication issues (eg, medications were inadvertently omitted from discharge medication list, prehospital medication list was incorrect). Medical issues accounted for an additional 27 concerns (eg, patient was hypoglycemic on arrival, inadequate pain control, discovery of new acute medical issues or medical diagnoses that were not clearly documented or communicated by the inpatient team). “Other” issues (two) included unaddressed social concerns, such as insurance issues.

DISCUSSION
The ECHO-CT model unites hospital and post–acute care providers to improve transitions of care and is unique in its focus on the transition from hospital to post–acute care rather than to home care. In 2 years of data collection, we identified several TCEs encompassing a range of concerns. Of the 675 patients discussed, 132 (20%) were noted to have a TCE. When these percentages are applied to the 140 million Medicare hospital discharges that took place during 2000 to 2015, we would estimate nearly 5.5 million TCEs, or 375,000 TCEs per year, that may have affected this population.
The majority of TCEs were communication and coordination errors. Missing or incomplete discharge paperwork, inadequate documentation of inpatient care, and confusion about medical devices or postoperative needs (eg, slings, braces, wound care, drains) were commonly reported. Follow-up appointments with specialists were often not appropriately scheduled or communicated. This may have resulted from unstandardized discharge documentation and a lower priority given to documentation in the setting of multiple clinical demands (eg, direct patient care, complex care coordination, and clinical paperwork and charting). Studies have demonstrated that fewer than one-third of discharge summaries are received by outpatient providers before postdischarge follow-up, and additionally that nearly 40% of patients did not undergo recommended workups for medical issues identified during their hospital stay.7,8 All of this is problematic because appropriate documentation in discharge summaries is associated with a decreased risk of hospital readmission.9
Pharmacy issues were the second most common TCE identified. One member of the post–acute care team noted that “omissions, additions, and replacements” relating to medications were common occurrences. Additionally, it was noted that medications were inadvertently continued for longer than planned or not adjusted appropriately with changing clinical parameters, such as renal function. The results of our analysis are consistent with current literature, which suggests that up to 60% of all medication errors occur during the period surrounding transitions of care.10
There were several limitations to this investigation. Though recording of identified TCEs occurred in real time, analysis of these identified events occurred retrospectively; therefore, investigators had limited ability to retroactively review or recategorize recorded issues, which potentially could have resulted in misclassification or misinterpretation. Additionally, the data were intended to be descriptive; therefore, outcomes such as hospital readmission and patient harm could not be linked to specific TCEs. Furthermore, it is possible that events were not detected by either the postdischarge team or the hospital-based team and, therefore, not captured in this analysis. Further work would be helpful to determine the root causes underlying the identified issues in care transitions, with the goal of improving patient safety and avoiding preventable errors during transitions of care. Although there is comprehensive literature related to errors and medication-related adverse events,11 there is not a consensus of how to classify and report, in a standardized fashion, events arising during the transition period. A validated structure for systematically identifying, monitoring, recording, and reporting issues arising during care transitions will be critical in preventing errors and ensuring patient safety during this high-risk period.
CONCLUSION
Our model is a unique intervention that uses the expertise and engagement of an interdisciplinary team and seeks to identify and remedy issues arising during transitions of care—in real time—to prevent direct harm to vulnerable patients. We have already implemented interventions to improve care based on our experiences with this videoconference-based program. For example, direct feedback was given to discharging teams to improve the discharge summary and associated documentation, and changes to the medication-ordering system were implemented to address specific medication errors discovered. The TCEs identified in this investigation highlight specific areas for improvement with the goal of providing high-quality care for patients and seamless transitions to post–acute care. As health systems and hospitals face new challenges in communication and care coordination, especially due to the recent COVID-19 pandemic, the technology and communication methods used in the ECHO-CT model may become even more relevant for promoting clear communication and patient safety during transitions of care.
Acknowledgment
The ECHO CT team thanks Sabrina Carretie for her contributions in data collection and analysis.
1. Werner RM, Konetzka RT. Trends in post-acute care use among medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616–1617. https://doi.org/10.1001/jama.2018.2408
2. Tian W. An All-Payer View of Hospital Discharge to Postacute Care, 2013. Statistical Brief #205. Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality; May 2016. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.pdf
3. Kessler C, Williams MC, Moustoukas JN, Pappas C. Transitions of care for the geriatric patient in the emergency department. Clin Geriatr Med. 2013;29(1):49-69. https://doi.org/10.1016/j.cger.2012.10.005
4. Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122(Suppl 2):74-77. https://doi.org/10.1177/00333549071220s214
5. Farris G, Sircar M, Bortinger J, et al. Extension for community healthcare outcomes–care transitions: enhancing geriatric care transitions through a multidisciplinary videoconference. J Am Geriatr Soc. 2017;65(3):598-602. https://doi.org/10.1111/jgs.14690
6. Moore AB, Krupp JE, Dufour AB, et al. Improving transitions to postacute care for elderly patients using a novel video-conferencing program: ECHO-Care transitions. Am J Med. 2017;130(10):1199-1204. https://doi.org/10.1016/j.amjmed.2017.04.041
7. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841. https://doi.org/10.1001/jama.297.8.831
8. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305-1311. https://doi.org/10.1001/archinte.167.12.1305
9. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186-192. https://doi.org/10.1046/j.1525-1497.2002.10741.x
10. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
11. Claeys C, Nève J, Tulkens PM, Spinewine A. Content validity and inter-rater reliability of an instrument to characterize unintentional medication discrepancies. Drugs Aging. 2012;29(7):577-591. https://doi.org/10.1007/bf03262275
As the population of the United States continues to age, hospitals are seeing an increasing number of older patients with significant medical and social complexity. Medicare data have shown that an increasing number require post–acute care after a hospitalization.1 Discharges to post–acute care settings are often longer and more costly compared with discharges to other settings, which suggests that targeting quality improvement efforts at this transition period may improve the value of care.2
The transition from the hospital setting to a post–acute care facility can be dangerous and complicated due to lapses in communication, medication errors, and the complexity of medical treatment plans. Suboptimal transitions in care can result in adverse events for the patient, as well as confusion in medication regimens or incomplete plans for follow-up care.3
The Project ECHO (Extension for Community Healthcare Outcomes) model was first developed and launched by Sanjeev Arora, MD, in New Mexico in 2003 to expand access to subspecialist care using videoconferencing.4 We first applied this model in 2013 to evaluate the impact of this interdisciplinary videoconferencing tool on the care of patients discharged to post–acute settings.5 We found that patients participating in the Extension for Community Healthcare Outcomes–Care Transitions (ECHO-CT) model experienced decreased risk of rehospitalization, decreased skilled nursing facility (SNF) length of stay, and reduced 30-day healthcare costs, compared with those patients not enrolled in this program; these outcomes were likely due to identification and correction of medication-related errors, improved care coordination, improved disease management, and clarification of goals of care.6 Though these investigations did identify some issues arising during the care transition process, they did not fully describe the types of problems uncovered. We sought to better characterize the clinical and operational issues identified through the ECHO-CT conference, hereafter known as transition-of-care events (TCEs). These issues may include new or evolving medical concerns, an adverse event, or a “near miss.” Identification and classification of TCEs that may contribute to unsafe or fractured care transitions are critical in developing systematic solutions to improve transitions of care, which can ultimately improve patient safety and potentially avoid preventable errors.
METHODS
ECHO-CT Multidisciplinary Video Conference
We conducted ECHO-CT at a large, tertiary care academic medical center. The project design for the ECHO-CT program has been previously described.5 In brief, the program is a weekly, multidisciplinary videoconference between a hospital-based team and post–acute care providers to discuss patients discharged from inpatient services to post–acute care sites, including SNFs and long-term acute care hospitals (LTACHs), during the preceding week. All patients discharged from the tertiary care inpatient site to one of the eight participating SNFs or LTACHs, from either a medical or surgical service, are eligible to be discussed at this weekly interdisciplinary conference. Long-term care facilities were not included in this study. The ECHO-CT program used HIPAA (Health Insurance Portability and Accountability Act)-compliant videoconferencing technology to connect hospital and post–acute care providers.
During the videoconferences, each patient’s hospital course and discharge documentation are reviewed by a hospitalist, and a pharmacist performs a medication reconciliation of each patient’s admission, discharge, and post–acute care medication list. The discharging attending, primary care providers, residents, other trainees, and subspecialist providers are invited to attend. Typically, the interdisciplinary team at the post–acute care sites includes physicians, nurse practitioners, physical therapists, social workers, and case managers. Between 10 and 20 patients are discussed in a case-based format, which includes a summary of the patient’s hospital course, an update from the post–acute care team on the patient’s care, and an opportunity for a discussion regarding any concerns or questions raised by the post–acute care or inpatient care teams. The content and duration of discussion typically lasts approximately 3 to 10 minutes, depending on the needs of the patient and the care team. Each of the eight post–acute care sites participating in the project are assigned a 10- to 15-minute block. A copy of the ECHO-CT session process document is included in the Appendix.
Data Collection
At each interdisciplinary patient review, TCEs were identified and recorded. These events were categorized in real time by the ECHO-CT data collection team into the following categories: medication related, medical, discharge communication/coordination, or other, and recorded in a secured, deidentified database. For individuals whose TCEs could represent more than one category, authors reviewed the available information about the TCEs and determined the most appropriate category; if more than one category was felt to be applicable to a patient’s situation, the events were reclassified into all applicable categories. Data about individual patients, including gender, age at the time of discharge, and other demographic information, were obtained from hospital databases. Number of diagnoses included any diagnosis billed during the patient’s hospital stay, and these data were obtained from a hospital billing database. Average number of medications at discharge was obtained from a hospital pharmacy database.
RESULTS
A total of 675 patients (experiencing 743 hospitalizations) were discharged from a medical or surgical service to one of the participating post–acute care sites from January 2016 to October 2018, and were discussed at the interdisciplinary conference. During that time, 139 TCEs were recorded for review, involving 132 patients (Table 1). Patients who experienced TCEs were noted to have a slightly higher average number of diagnoses than did those in the non-TCE group (21 vs 18, respectively) and number of medications (18 vs 15).

Representative examples of TCEs are provided in Table 2. Fifty-eight issues were identified as discharge communication or coordination issues (eg, discharge summary was late or missing at time of discharge to facility, transitional issues were unclear, follow-up appointments were not appropriately scheduled or documented). An additional 52 TCEs were identified as pharmacy or medication issues (eg, medications were inadvertently omitted from discharge medication list, prehospital medication list was incorrect). Medical issues accounted for an additional 27 concerns (eg, patient was hypoglycemic on arrival, inadequate pain control, discovery of new acute medical issues or medical diagnoses that were not clearly documented or communicated by the inpatient team). “Other” issues (two) included unaddressed social concerns, such as insurance issues.

DISCUSSION
The ECHO-CT model unites hospital and post–acute care providers to improve transitions of care and is unique in its focus on the transition from hospital to post–acute care rather than to home care. In 2 years of data collection, we identified several TCEs encompassing a range of concerns. Of the 675 patients discussed, 132 (20%) were noted to have a TCE. When these percentages are applied to the 140 million Medicare hospital discharges that took place during 2000 to 2015, we would estimate nearly 5.5 million TCEs, or 375,000 TCEs per year, that may have affected this population.
The majority of TCEs were communication and coordination errors. Missing or incomplete discharge paperwork, inadequate documentation of inpatient care, and confusion about medical devices or postoperative needs (eg, slings, braces, wound care, drains) were commonly reported. Follow-up appointments with specialists were often not appropriately scheduled or communicated. This may have resulted from unstandardized discharge documentation and a lower priority given to documentation in the setting of multiple clinical demands (eg, direct patient care, complex care coordination, and clinical paperwork and charting). Studies have demonstrated that fewer than one-third of discharge summaries are received by outpatient providers before postdischarge follow-up, and additionally that nearly 40% of patients did not undergo recommended workups for medical issues identified during their hospital stay.7,8 All of this is problematic because appropriate documentation in discharge summaries is associated with a decreased risk of hospital readmission.9
Pharmacy issues were the second most common TCE identified. One member of the post–acute care team noted that “omissions, additions, and replacements” relating to medications were common occurrences. Additionally, it was noted that medications were inadvertently continued for longer than planned or not adjusted appropriately with changing clinical parameters, such as renal function. The results of our analysis are consistent with current literature, which suggests that up to 60% of all medication errors occur during the period surrounding transitions of care.10
There were several limitations to this investigation. Though recording of identified TCEs occurred in real time, analysis of these identified events occurred retrospectively; therefore, investigators had limited ability to retroactively review or recategorize recorded issues, which potentially could have resulted in misclassification or misinterpretation. Additionally, the data were intended to be descriptive; therefore, outcomes such as hospital readmission and patient harm could not be linked to specific TCEs. Furthermore, it is possible that events were not detected by either the postdischarge team or the hospital-based team and, therefore, not captured in this analysis. Further work would be helpful to determine the root causes underlying the identified issues in care transitions, with the goal of improving patient safety and avoiding preventable errors during transitions of care. Although there is comprehensive literature related to errors and medication-related adverse events,11 there is not a consensus of how to classify and report, in a standardized fashion, events arising during the transition period. A validated structure for systematically identifying, monitoring, recording, and reporting issues arising during care transitions will be critical in preventing errors and ensuring patient safety during this high-risk period.
CONCLUSION
Our model is a unique intervention that uses the expertise and engagement of an interdisciplinary team and seeks to identify and remedy issues arising during transitions of care—in real time—to prevent direct harm to vulnerable patients. We have already implemented interventions to improve care based on our experiences with this videoconference-based program. For example, direct feedback was given to discharging teams to improve the discharge summary and associated documentation, and changes to the medication-ordering system were implemented to address specific medication errors discovered. The TCEs identified in this investigation highlight specific areas for improvement with the goal of providing high-quality care for patients and seamless transitions to post–acute care. As health systems and hospitals face new challenges in communication and care coordination, especially due to the recent COVID-19 pandemic, the technology and communication methods used in the ECHO-CT model may become even more relevant for promoting clear communication and patient safety during transitions of care.
Acknowledgment
The ECHO CT team thanks Sabrina Carretie for her contributions in data collection and analysis.
As the population of the United States continues to age, hospitals are seeing an increasing number of older patients with significant medical and social complexity. Medicare data have shown that an increasing number require post–acute care after a hospitalization.1 Discharges to post–acute care settings are often longer and more costly compared with discharges to other settings, which suggests that targeting quality improvement efforts at this transition period may improve the value of care.2
The transition from the hospital setting to a post–acute care facility can be dangerous and complicated due to lapses in communication, medication errors, and the complexity of medical treatment plans. Suboptimal transitions in care can result in adverse events for the patient, as well as confusion in medication regimens or incomplete plans for follow-up care.3
The Project ECHO (Extension for Community Healthcare Outcomes) model was first developed and launched by Sanjeev Arora, MD, in New Mexico in 2003 to expand access to subspecialist care using videoconferencing.4 We first applied this model in 2013 to evaluate the impact of this interdisciplinary videoconferencing tool on the care of patients discharged to post–acute settings.5 We found that patients participating in the Extension for Community Healthcare Outcomes–Care Transitions (ECHO-CT) model experienced decreased risk of rehospitalization, decreased skilled nursing facility (SNF) length of stay, and reduced 30-day healthcare costs, compared with those patients not enrolled in this program; these outcomes were likely due to identification and correction of medication-related errors, improved care coordination, improved disease management, and clarification of goals of care.6 Though these investigations did identify some issues arising during the care transition process, they did not fully describe the types of problems uncovered. We sought to better characterize the clinical and operational issues identified through the ECHO-CT conference, hereafter known as transition-of-care events (TCEs). These issues may include new or evolving medical concerns, an adverse event, or a “near miss.” Identification and classification of TCEs that may contribute to unsafe or fractured care transitions are critical in developing systematic solutions to improve transitions of care, which can ultimately improve patient safety and potentially avoid preventable errors.
METHODS
ECHO-CT Multidisciplinary Video Conference
We conducted ECHO-CT at a large, tertiary care academic medical center. The project design for the ECHO-CT program has been previously described.5 In brief, the program is a weekly, multidisciplinary videoconference between a hospital-based team and post–acute care providers to discuss patients discharged from inpatient services to post–acute care sites, including SNFs and long-term acute care hospitals (LTACHs), during the preceding week. All patients discharged from the tertiary care inpatient site to one of the eight participating SNFs or LTACHs, from either a medical or surgical service, are eligible to be discussed at this weekly interdisciplinary conference. Long-term care facilities were not included in this study. The ECHO-CT program used HIPAA (Health Insurance Portability and Accountability Act)-compliant videoconferencing technology to connect hospital and post–acute care providers.
During the videoconferences, each patient’s hospital course and discharge documentation are reviewed by a hospitalist, and a pharmacist performs a medication reconciliation of each patient’s admission, discharge, and post–acute care medication list. The discharging attending, primary care providers, residents, other trainees, and subspecialist providers are invited to attend. Typically, the interdisciplinary team at the post–acute care sites includes physicians, nurse practitioners, physical therapists, social workers, and case managers. Between 10 and 20 patients are discussed in a case-based format, which includes a summary of the patient’s hospital course, an update from the post–acute care team on the patient’s care, and an opportunity for a discussion regarding any concerns or questions raised by the post–acute care or inpatient care teams. The content and duration of discussion typically lasts approximately 3 to 10 minutes, depending on the needs of the patient and the care team. Each of the eight post–acute care sites participating in the project are assigned a 10- to 15-minute block. A copy of the ECHO-CT session process document is included in the Appendix.
Data Collection
At each interdisciplinary patient review, TCEs were identified and recorded. These events were categorized in real time by the ECHO-CT data collection team into the following categories: medication related, medical, discharge communication/coordination, or other, and recorded in a secured, deidentified database. For individuals whose TCEs could represent more than one category, authors reviewed the available information about the TCEs and determined the most appropriate category; if more than one category was felt to be applicable to a patient’s situation, the events were reclassified into all applicable categories. Data about individual patients, including gender, age at the time of discharge, and other demographic information, were obtained from hospital databases. Number of diagnoses included any diagnosis billed during the patient’s hospital stay, and these data were obtained from a hospital billing database. Average number of medications at discharge was obtained from a hospital pharmacy database.
RESULTS
A total of 675 patients (experiencing 743 hospitalizations) were discharged from a medical or surgical service to one of the participating post–acute care sites from January 2016 to October 2018, and were discussed at the interdisciplinary conference. During that time, 139 TCEs were recorded for review, involving 132 patients (Table 1). Patients who experienced TCEs were noted to have a slightly higher average number of diagnoses than did those in the non-TCE group (21 vs 18, respectively) and number of medications (18 vs 15).

Representative examples of TCEs are provided in Table 2. Fifty-eight issues were identified as discharge communication or coordination issues (eg, discharge summary was late or missing at time of discharge to facility, transitional issues were unclear, follow-up appointments were not appropriately scheduled or documented). An additional 52 TCEs were identified as pharmacy or medication issues (eg, medications were inadvertently omitted from discharge medication list, prehospital medication list was incorrect). Medical issues accounted for an additional 27 concerns (eg, patient was hypoglycemic on arrival, inadequate pain control, discovery of new acute medical issues or medical diagnoses that were not clearly documented or communicated by the inpatient team). “Other” issues (two) included unaddressed social concerns, such as insurance issues.

DISCUSSION
The ECHO-CT model unites hospital and post–acute care providers to improve transitions of care and is unique in its focus on the transition from hospital to post–acute care rather than to home care. In 2 years of data collection, we identified several TCEs encompassing a range of concerns. Of the 675 patients discussed, 132 (20%) were noted to have a TCE. When these percentages are applied to the 140 million Medicare hospital discharges that took place during 2000 to 2015, we would estimate nearly 5.5 million TCEs, or 375,000 TCEs per year, that may have affected this population.
The majority of TCEs were communication and coordination errors. Missing or incomplete discharge paperwork, inadequate documentation of inpatient care, and confusion about medical devices or postoperative needs (eg, slings, braces, wound care, drains) were commonly reported. Follow-up appointments with specialists were often not appropriately scheduled or communicated. This may have resulted from unstandardized discharge documentation and a lower priority given to documentation in the setting of multiple clinical demands (eg, direct patient care, complex care coordination, and clinical paperwork and charting). Studies have demonstrated that fewer than one-third of discharge summaries are received by outpatient providers before postdischarge follow-up, and additionally that nearly 40% of patients did not undergo recommended workups for medical issues identified during their hospital stay.7,8 All of this is problematic because appropriate documentation in discharge summaries is associated with a decreased risk of hospital readmission.9
Pharmacy issues were the second most common TCE identified. One member of the post–acute care team noted that “omissions, additions, and replacements” relating to medications were common occurrences. Additionally, it was noted that medications were inadvertently continued for longer than planned or not adjusted appropriately with changing clinical parameters, such as renal function. The results of our analysis are consistent with current literature, which suggests that up to 60% of all medication errors occur during the period surrounding transitions of care.10
There were several limitations to this investigation. Though recording of identified TCEs occurred in real time, analysis of these identified events occurred retrospectively; therefore, investigators had limited ability to retroactively review or recategorize recorded issues, which potentially could have resulted in misclassification or misinterpretation. Additionally, the data were intended to be descriptive; therefore, outcomes such as hospital readmission and patient harm could not be linked to specific TCEs. Furthermore, it is possible that events were not detected by either the postdischarge team or the hospital-based team and, therefore, not captured in this analysis. Further work would be helpful to determine the root causes underlying the identified issues in care transitions, with the goal of improving patient safety and avoiding preventable errors during transitions of care. Although there is comprehensive literature related to errors and medication-related adverse events,11 there is not a consensus of how to classify and report, in a standardized fashion, events arising during the transition period. A validated structure for systematically identifying, monitoring, recording, and reporting issues arising during care transitions will be critical in preventing errors and ensuring patient safety during this high-risk period.
CONCLUSION
Our model is a unique intervention that uses the expertise and engagement of an interdisciplinary team and seeks to identify and remedy issues arising during transitions of care—in real time—to prevent direct harm to vulnerable patients. We have already implemented interventions to improve care based on our experiences with this videoconference-based program. For example, direct feedback was given to discharging teams to improve the discharge summary and associated documentation, and changes to the medication-ordering system were implemented to address specific medication errors discovered. The TCEs identified in this investigation highlight specific areas for improvement with the goal of providing high-quality care for patients and seamless transitions to post–acute care. As health systems and hospitals face new challenges in communication and care coordination, especially due to the recent COVID-19 pandemic, the technology and communication methods used in the ECHO-CT model may become even more relevant for promoting clear communication and patient safety during transitions of care.
Acknowledgment
The ECHO CT team thanks Sabrina Carretie for her contributions in data collection and analysis.
1. Werner RM, Konetzka RT. Trends in post-acute care use among medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616–1617. https://doi.org/10.1001/jama.2018.2408
2. Tian W. An All-Payer View of Hospital Discharge to Postacute Care, 2013. Statistical Brief #205. Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality; May 2016. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.pdf
3. Kessler C, Williams MC, Moustoukas JN, Pappas C. Transitions of care for the geriatric patient in the emergency department. Clin Geriatr Med. 2013;29(1):49-69. https://doi.org/10.1016/j.cger.2012.10.005
4. Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122(Suppl 2):74-77. https://doi.org/10.1177/00333549071220s214
5. Farris G, Sircar M, Bortinger J, et al. Extension for community healthcare outcomes–care transitions: enhancing geriatric care transitions through a multidisciplinary videoconference. J Am Geriatr Soc. 2017;65(3):598-602. https://doi.org/10.1111/jgs.14690
6. Moore AB, Krupp JE, Dufour AB, et al. Improving transitions to postacute care for elderly patients using a novel video-conferencing program: ECHO-Care transitions. Am J Med. 2017;130(10):1199-1204. https://doi.org/10.1016/j.amjmed.2017.04.041
7. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841. https://doi.org/10.1001/jama.297.8.831
8. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305-1311. https://doi.org/10.1001/archinte.167.12.1305
9. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186-192. https://doi.org/10.1046/j.1525-1497.2002.10741.x
10. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
11. Claeys C, Nève J, Tulkens PM, Spinewine A. Content validity and inter-rater reliability of an instrument to characterize unintentional medication discrepancies. Drugs Aging. 2012;29(7):577-591. https://doi.org/10.1007/bf03262275
1. Werner RM, Konetzka RT. Trends in post-acute care use among medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616–1617. https://doi.org/10.1001/jama.2018.2408
2. Tian W. An All-Payer View of Hospital Discharge to Postacute Care, 2013. Statistical Brief #205. Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality; May 2016. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.pdf
3. Kessler C, Williams MC, Moustoukas JN, Pappas C. Transitions of care for the geriatric patient in the emergency department. Clin Geriatr Med. 2013;29(1):49-69. https://doi.org/10.1016/j.cger.2012.10.005
4. Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122(Suppl 2):74-77. https://doi.org/10.1177/00333549071220s214
5. Farris G, Sircar M, Bortinger J, et al. Extension for community healthcare outcomes–care transitions: enhancing geriatric care transitions through a multidisciplinary videoconference. J Am Geriatr Soc. 2017;65(3):598-602. https://doi.org/10.1111/jgs.14690
6. Moore AB, Krupp JE, Dufour AB, et al. Improving transitions to postacute care for elderly patients using a novel video-conferencing program: ECHO-Care transitions. Am J Med. 2017;130(10):1199-1204. https://doi.org/10.1016/j.amjmed.2017.04.041
7. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841. https://doi.org/10.1001/jama.297.8.831
8. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305-1311. https://doi.org/10.1001/archinte.167.12.1305
9. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186-192. https://doi.org/10.1046/j.1525-1497.2002.10741.x
10. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
11. Claeys C, Nève J, Tulkens PM, Spinewine A. Content validity and inter-rater reliability of an instrument to characterize unintentional medication discrepancies. Drugs Aging. 2012;29(7):577-591. https://doi.org/10.1007/bf03262275
© 2021 Society of Hospital Medicine
Analysis of Hospital Resource Availability and COVID-19 Mortality Across the United States
The COVID-19 pandemic is a crisis of mismatch between resources and infection burden. There is extraordinary heterogeneity across time and geography in the pandemic impact, with hospitals in New York City initially inundated while hospitals in major urban areas of California were comparatively quiet. Efforts to “flatten the curve” are intended to improve outcomes by reducing health system overload.1 In the case of hospital-based care, health systems’ primary resources include emergency and critical care bed and staff capacity.
Prior work has documented wide variability in intensive care capacity across the United States and hypothesized that even moderate disease outbreaks could overwhelm hospital referral regions (HRRs).2,3 Various simulations of outbreaks suggested that thousands of deaths are potentially preventable depending on the health system’s response,4 although the degree to which resource limitations have contributed to mortality during this COVID-19 pandemic has yet to be explored. The objective of this analysis was to examine the association between hospital resources and COVID-19 deaths amongst HRRs in the United States in the period from March 1 to July 26, 2020.
METHODS
Data
This was an analysis of the American Hospital Association Annual Survey Database from 2017 and 2018, including hospital resource variables such as total hospital beds, hospitalists, intensive care beds, intensivists, emergency physicians, and nurses.5 The analysis was limited to general medical and surgical hospitals capable of providing acute care services, defined as those reporting at least 500 emergency department visits in 2018. Where data were missing on analysis variables (26.0% missing overall), the data were drawn from the 2017 survey results (reduced to 23.8% missing) from the same site as available, and the remainder were imputed with multivariate imputation by chained equations. An identical analysis without imputation was performed as a sensitivity analysis that showed a similar pattern of results. Total resources were tabulated amongst HRRs, and the hospital resources per COVID-19 case calculated. HRRs are a geographic category devised to represent regional health care markets, and each includes hospital sites performing major procedures.3 These were the focus of the analysis because they may represent a meaningful geographic division of hospital-based resources. COVID-19 case and death counts (as of July 26, 2020) were drawn from publicly available county-level data curated by the New York Times from state and local governments as well as health departments nationwide,6 separated by month (ie, March, April, May, June, and July). Data on New York City were available in aggregate (rather than separated by borough). Cases and deaths were therefore apportioned to the three HRRs involving New York City in proportion to that area’s population. To adjust for the lag between COVID-19 cases and deaths,7,8 we offset deaths 2 weeks into the future so that the April COVID-19 death count for a given HRR included deaths that occurred for 1 month beginning 2 weeks after the start of April, and so on.
Analysis
We estimated Poisson distribution regressions for the outcome of COVID-19 death count in each HRR and month with one model for each of our six hospital-based resource variables. The offset (exposure) variable was COVID-19 case count. To adjust for the possibility of varying effects of hospital resources on deaths by month (ie, in anticipation that health systems might evolve in response to the pandemic over time), each model includes terms for the interaction between hospital-based resource and an indicator variable for month, as well as a fifth term for month. Standard errors were adjusted for clustering within HRR. We report resultant incident rate ratios (IRRs) with 95% CIs, and we report these as statistically significant at the 5% level only after adjustment for multiple comparisons across our six hospital-resource variables using the conservative Bonferroni adjustment. The pseudo-R2 for each of these six models is also reported to summarize the amount of variation in deaths explained. For our model with ICU beds per COVID-19 case, we perform postestimation prediction of number of deaths by HRR, assuming the counterfactual in which HRRs with fewer than average ICU beds per COVID-19 case instead had the average observed number of ICU beds per COVID-19 case by HRR in April, which functioned as a measure of early excess deaths potentially related to resource limitations. The study was classified as exempt by the Institutional Review Board at the Yale School of Medicine, New Haven, Connecticut. Analyses were conducted in Stata 15 (StataCorp LLC) and R.
RESULTS
A total of 4,453 hospitals across 306 HRRs were included and linked to 2,827 county-level COVID-19 case and death counts in each of 5 months (March through July 2020). The median HRR in our analysis included 14 hospitals, with a maximum of 76 hospitals (Los Angeles, California) and a minimum of 1 (Longview, Texas). Among HRRs, 206 (67.3%) had experienced caseloads exceeding 20 per 10,000 population, while 85 (27.8%) had experienced greater than 100 per 10,000 population in the peak month during the study period. The Table depicts results of each of six Poisson distribution regression models, with the finding that greater number of ICU beds (IRR, 0.194; 95% CI, 0.076-0.491), general medical/surgical beds (IRR, 0.800; 95% CI, 0.696-0.920), and nurses (IRR, 0.927; 95% CI, 0.888-0.967) per COVID-19 case in April were statistically significantly associated with reduced deaths.
The model including ICU beds per COVID-19 case had the largest pseudo-R2 at 0.6018, which suggests that ICU bed availability explains the most variation in death count among hospital resource variables analyzed. The incident rate ratio in this model implies that, for an entire additional ICU bed for each COVID-19 case (a one-unit increase in that variable), there is an associated one-fifth decrease in incidence rate (IRR, 0.194) of death in April. The mean value among HRRs in April was 0.25 ICU beds per case (one ICU bed for every four COVID-19 cases), but it was as low as 0.01 to 0.005 in hard-hit areas (one ICU bed for every 100 to 200 COVID-19 cases). The early excess deaths observed in April were not observed in later months. The magnitude of this effect can be summarized as follows: If the 152 HRRs in April with fewer than the mean number of ICU beds per COVID-19 case were to instead have the mean number (one ICU bed for every four COVID-19 cases), our model estimates that there would have been 15,571 fewer deaths that month. The HRRs with the largest number of early excess deaths were Manhattan in New York City (1,466), Bronx in New York City (1,315), Boston, Massachusetts (1,293), Philadelphia, Pennsylvania (955), Hartford, Connecticut (682), Detroit, Michigan (499), and Camden, New Jersey (484). The Figure depicts HRRs in the United States with early excess deaths by this measure in April.
DISCUSSION
We found significant associations between availability of hospital-based resources and COVID-19 deaths in the month of April 2020. This observation was consistent across measures of both hospital bed and staff capacity but not statistically significant in all cases. This provides empiric evidence in support of a preprint simulation publication by Branas et al showing the potential for thousands of excess deaths related to lack of available resources.4 Interestingly, the relationship between hospital-based resources per COVID-19 case and death count is not seen in May, June, or July. This may be because hospitals and health systems were rapidly adapting to pandemic demands9 by shifting resources or reorganizing existing infrastructure to free up beds and personnel.
Our findings underscore the importance of analyses that address heterogeneity in health system response over time and across different geographic areas. That the relationship is not seen after the early pandemic period, when hospitals and health systems were most overwhelmed, suggests that health systems and communities were able to adapt. Importantly, this work does not address the likely complex relationships among hospital resources and outcomes (for example, the benefit of ICU bed availability is likely limited when there are insufficient intensivists and nurses). These complexities should be a focus of future work. Furthermore, hospital resource flexibility, community efforts to slow transmission, and improvements in testing availability and the management of COVID-19 among hospitalized patients may all play a role in attenuating the relationship between baseline resource limitations and outcomes for patients with COVID-19.
These results merit further granular studies to examine specific hospital resources and observed variation in outcomes. Prior literature has linked inpatient capacity—variously defined as high census, acuity, turnover, or delayed admission—to outcomes including mortality among patients with stroke, among those with acute coronary syndrome, and among those requiring intensive care.10 Literature from Italy’s experience shows there was large variation in the case fatality rate among regions of Northern Italy and argues this was partially due to hospital resource limitations.11 Future work can also address whether just-in-time resource mobilization, such as temporary ICU expansion, physician cross-staffing, telemedicine, and dedicated units for COVID-19 patients, attenuated the impact of potential hospital resource scarcity on outcomes.
The present analysis is limited by the quality of the data. There is likely variation of available COVID-19 testing by HRR. It may be that areas with larger outbreaks early on generally tested a smaller, sicker proportion of population-level cases than did those with smaller outbreaks. This effect may be reversed if larger HRRs in urban areas have health systems and public health departments more inclined toward or capable of doing more testing. Furthermore, deaths related to COVID-19 are likely related to community-based factors, including nonhealthcare resources and underlying population characteristics, that likely correlate with the availability of hospital-based resources within HRRs. Some have called into question whether, a priori, we should expect hospital-based capacity to be an important driver of mortality at all,12 arguing that, when critical care capacity is exceeded, resources may be efficiently reallocated away from patients who are least likely to benefit. Because we used the American Hospital Association data, this snapshot of hospital resources is not limited to critical care capacity because there could be alternative explanations for situations in which mortality for both COVID-19 and non–COVID-19 patients may be lower and hospital resources are better matched with demand. For example, patients may seek care earlier in their disease course (whether COVID-19 or otherwise)13 if their local emergency department is not thought to be overwhelmed with case volume.
CONCLUSION
We find that COVID-19 deaths vary among HRRs. The availability of several hospital-based resources is associated with death rates and supports early efforts across the United States to “flatten the curve” to prevent hospital overload. Continued surveillance of this relationship is essential to guide policymakers and hospitals seeking to balance the still limited supply of resources with the demands of caring for both infectious and noninfectious patients in the coming months of this outbreak and in future pandemics.
Acknowledgment
The authors gratefully acknowledge the help of Carolyn Lusch, AICP, in generating depictions of results in Geographic Information Systems.
1. Phua J, Weng L, Ling L, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506-517. https://doi.org/10.1016/s2213-2600(20)30161-2
2. Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371-1372. https://doi.org/10.1001/jama.2010.394
3. General FAQ. Dartmouth Atlas Project. 2020. Accessed July 8, 2020. https://www.dartmouthatlas.org/faq/
4. Branas CC, Rundle A, Pei S, et al. Flattening the curve before it flattens us: hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties. medRxiv. Preprint posted online April 6, 2020. https://doi.org/10.1101/2020.04.01.20049759
5. American Hospital Association Annual Survey Database. American Hospital Association. 2018. Accessed July 8, 2020. https://www.ahadata.com/aha-annual-survey-database
6. An Ongoing Repository of Data on Coronavirus Cases and Deaths in the U.S. New York Times. 2020. Accessed July 8, 2020. https://github.com/nytimes/covid-19-data
7. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. https://doi.org/10.1016/s1473-3099(20)30195-x
8. Rosakis P, Marketou ME. Rethinking case fatality ratios for COVID-19 from a data-driven viewpoint. J Infect. 2020;81(2);e162-e164. https://doi.org/10.1016/j.jinf.2020.06.010
9. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
10. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guide JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
11. Volpato S, Landi F, Incalzi RA. A frail health care system for an old population: lesson form [sic] the COVID-19 outbreak in Italy. J Gerontol Series A. 2020;75(9):e126-e127. https://doi.org/10.1093/gerona/glaa087
12. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447-455. https://doi.org/10.7326/0003-4819-159-7-201310010-00004
13. Moroni F, Gramegna M, Agello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(10):1620-1624. https://doi.org/10.1016/j.jaccas.2020.04.010
The COVID-19 pandemic is a crisis of mismatch between resources and infection burden. There is extraordinary heterogeneity across time and geography in the pandemic impact, with hospitals in New York City initially inundated while hospitals in major urban areas of California were comparatively quiet. Efforts to “flatten the curve” are intended to improve outcomes by reducing health system overload.1 In the case of hospital-based care, health systems’ primary resources include emergency and critical care bed and staff capacity.
Prior work has documented wide variability in intensive care capacity across the United States and hypothesized that even moderate disease outbreaks could overwhelm hospital referral regions (HRRs).2,3 Various simulations of outbreaks suggested that thousands of deaths are potentially preventable depending on the health system’s response,4 although the degree to which resource limitations have contributed to mortality during this COVID-19 pandemic has yet to be explored. The objective of this analysis was to examine the association between hospital resources and COVID-19 deaths amongst HRRs in the United States in the period from March 1 to July 26, 2020.
METHODS
Data
This was an analysis of the American Hospital Association Annual Survey Database from 2017 and 2018, including hospital resource variables such as total hospital beds, hospitalists, intensive care beds, intensivists, emergency physicians, and nurses.5 The analysis was limited to general medical and surgical hospitals capable of providing acute care services, defined as those reporting at least 500 emergency department visits in 2018. Where data were missing on analysis variables (26.0% missing overall), the data were drawn from the 2017 survey results (reduced to 23.8% missing) from the same site as available, and the remainder were imputed with multivariate imputation by chained equations. An identical analysis without imputation was performed as a sensitivity analysis that showed a similar pattern of results. Total resources were tabulated amongst HRRs, and the hospital resources per COVID-19 case calculated. HRRs are a geographic category devised to represent regional health care markets, and each includes hospital sites performing major procedures.3 These were the focus of the analysis because they may represent a meaningful geographic division of hospital-based resources. COVID-19 case and death counts (as of July 26, 2020) were drawn from publicly available county-level data curated by the New York Times from state and local governments as well as health departments nationwide,6 separated by month (ie, March, April, May, June, and July). Data on New York City were available in aggregate (rather than separated by borough). Cases and deaths were therefore apportioned to the three HRRs involving New York City in proportion to that area’s population. To adjust for the lag between COVID-19 cases and deaths,7,8 we offset deaths 2 weeks into the future so that the April COVID-19 death count for a given HRR included deaths that occurred for 1 month beginning 2 weeks after the start of April, and so on.
Analysis
We estimated Poisson distribution regressions for the outcome of COVID-19 death count in each HRR and month with one model for each of our six hospital-based resource variables. The offset (exposure) variable was COVID-19 case count. To adjust for the possibility of varying effects of hospital resources on deaths by month (ie, in anticipation that health systems might evolve in response to the pandemic over time), each model includes terms for the interaction between hospital-based resource and an indicator variable for month, as well as a fifth term for month. Standard errors were adjusted for clustering within HRR. We report resultant incident rate ratios (IRRs) with 95% CIs, and we report these as statistically significant at the 5% level only after adjustment for multiple comparisons across our six hospital-resource variables using the conservative Bonferroni adjustment. The pseudo-R2 for each of these six models is also reported to summarize the amount of variation in deaths explained. For our model with ICU beds per COVID-19 case, we perform postestimation prediction of number of deaths by HRR, assuming the counterfactual in which HRRs with fewer than average ICU beds per COVID-19 case instead had the average observed number of ICU beds per COVID-19 case by HRR in April, which functioned as a measure of early excess deaths potentially related to resource limitations. The study was classified as exempt by the Institutional Review Board at the Yale School of Medicine, New Haven, Connecticut. Analyses were conducted in Stata 15 (StataCorp LLC) and R.
RESULTS
A total of 4,453 hospitals across 306 HRRs were included and linked to 2,827 county-level COVID-19 case and death counts in each of 5 months (March through July 2020). The median HRR in our analysis included 14 hospitals, with a maximum of 76 hospitals (Los Angeles, California) and a minimum of 1 (Longview, Texas). Among HRRs, 206 (67.3%) had experienced caseloads exceeding 20 per 10,000 population, while 85 (27.8%) had experienced greater than 100 per 10,000 population in the peak month during the study period. The Table depicts results of each of six Poisson distribution regression models, with the finding that greater number of ICU beds (IRR, 0.194; 95% CI, 0.076-0.491), general medical/surgical beds (IRR, 0.800; 95% CI, 0.696-0.920), and nurses (IRR, 0.927; 95% CI, 0.888-0.967) per COVID-19 case in April were statistically significantly associated with reduced deaths.

The model including ICU beds per COVID-19 case had the largest pseudo-R2 at 0.6018, which suggests that ICU bed availability explains the most variation in death count among hospital resource variables analyzed. The incident rate ratio in this model implies that, for an entire additional ICU bed for each COVID-19 case (a one-unit increase in that variable), there is an associated one-fifth decrease in incidence rate (IRR, 0.194) of death in April. The mean value among HRRs in April was 0.25 ICU beds per case (one ICU bed for every four COVID-19 cases), but it was as low as 0.01 to 0.005 in hard-hit areas (one ICU bed for every 100 to 200 COVID-19 cases). The early excess deaths observed in April were not observed in later months. The magnitude of this effect can be summarized as follows: If the 152 HRRs in April with fewer than the mean number of ICU beds per COVID-19 case were to instead have the mean number (one ICU bed for every four COVID-19 cases), our model estimates that there would have been 15,571 fewer deaths that month. The HRRs with the largest number of early excess deaths were Manhattan in New York City (1,466), Bronx in New York City (1,315), Boston, Massachusetts (1,293), Philadelphia, Pennsylvania (955), Hartford, Connecticut (682), Detroit, Michigan (499), and Camden, New Jersey (484). The Figure depicts HRRs in the United States with early excess deaths by this measure in April.

DISCUSSION
We found significant associations between availability of hospital-based resources and COVID-19 deaths in the month of April 2020. This observation was consistent across measures of both hospital bed and staff capacity but not statistically significant in all cases. This provides empiric evidence in support of a preprint simulation publication by Branas et al showing the potential for thousands of excess deaths related to lack of available resources.4 Interestingly, the relationship between hospital-based resources per COVID-19 case and death count is not seen in May, June, or July. This may be because hospitals and health systems were rapidly adapting to pandemic demands9 by shifting resources or reorganizing existing infrastructure to free up beds and personnel.
Our findings underscore the importance of analyses that address heterogeneity in health system response over time and across different geographic areas. That the relationship is not seen after the early pandemic period, when hospitals and health systems were most overwhelmed, suggests that health systems and communities were able to adapt. Importantly, this work does not address the likely complex relationships among hospital resources and outcomes (for example, the benefit of ICU bed availability is likely limited when there are insufficient intensivists and nurses). These complexities should be a focus of future work. Furthermore, hospital resource flexibility, community efforts to slow transmission, and improvements in testing availability and the management of COVID-19 among hospitalized patients may all play a role in attenuating the relationship between baseline resource limitations and outcomes for patients with COVID-19.
These results merit further granular studies to examine specific hospital resources and observed variation in outcomes. Prior literature has linked inpatient capacity—variously defined as high census, acuity, turnover, or delayed admission—to outcomes including mortality among patients with stroke, among those with acute coronary syndrome, and among those requiring intensive care.10 Literature from Italy’s experience shows there was large variation in the case fatality rate among regions of Northern Italy and argues this was partially due to hospital resource limitations.11 Future work can also address whether just-in-time resource mobilization, such as temporary ICU expansion, physician cross-staffing, telemedicine, and dedicated units for COVID-19 patients, attenuated the impact of potential hospital resource scarcity on outcomes.
The present analysis is limited by the quality of the data. There is likely variation of available COVID-19 testing by HRR. It may be that areas with larger outbreaks early on generally tested a smaller, sicker proportion of population-level cases than did those with smaller outbreaks. This effect may be reversed if larger HRRs in urban areas have health systems and public health departments more inclined toward or capable of doing more testing. Furthermore, deaths related to COVID-19 are likely related to community-based factors, including nonhealthcare resources and underlying population characteristics, that likely correlate with the availability of hospital-based resources within HRRs. Some have called into question whether, a priori, we should expect hospital-based capacity to be an important driver of mortality at all,12 arguing that, when critical care capacity is exceeded, resources may be efficiently reallocated away from patients who are least likely to benefit. Because we used the American Hospital Association data, this snapshot of hospital resources is not limited to critical care capacity because there could be alternative explanations for situations in which mortality for both COVID-19 and non–COVID-19 patients may be lower and hospital resources are better matched with demand. For example, patients may seek care earlier in their disease course (whether COVID-19 or otherwise)13 if their local emergency department is not thought to be overwhelmed with case volume.
CONCLUSION
We find that COVID-19 deaths vary among HRRs. The availability of several hospital-based resources is associated with death rates and supports early efforts across the United States to “flatten the curve” to prevent hospital overload. Continued surveillance of this relationship is essential to guide policymakers and hospitals seeking to balance the still limited supply of resources with the demands of caring for both infectious and noninfectious patients in the coming months of this outbreak and in future pandemics.
Acknowledgment
The authors gratefully acknowledge the help of Carolyn Lusch, AICP, in generating depictions of results in Geographic Information Systems.
The COVID-19 pandemic is a crisis of mismatch between resources and infection burden. There is extraordinary heterogeneity across time and geography in the pandemic impact, with hospitals in New York City initially inundated while hospitals in major urban areas of California were comparatively quiet. Efforts to “flatten the curve” are intended to improve outcomes by reducing health system overload.1 In the case of hospital-based care, health systems’ primary resources include emergency and critical care bed and staff capacity.
Prior work has documented wide variability in intensive care capacity across the United States and hypothesized that even moderate disease outbreaks could overwhelm hospital referral regions (HRRs).2,3 Various simulations of outbreaks suggested that thousands of deaths are potentially preventable depending on the health system’s response,4 although the degree to which resource limitations have contributed to mortality during this COVID-19 pandemic has yet to be explored. The objective of this analysis was to examine the association between hospital resources and COVID-19 deaths amongst HRRs in the United States in the period from March 1 to July 26, 2020.
METHODS
Data
This was an analysis of the American Hospital Association Annual Survey Database from 2017 and 2018, including hospital resource variables such as total hospital beds, hospitalists, intensive care beds, intensivists, emergency physicians, and nurses.5 The analysis was limited to general medical and surgical hospitals capable of providing acute care services, defined as those reporting at least 500 emergency department visits in 2018. Where data were missing on analysis variables (26.0% missing overall), the data were drawn from the 2017 survey results (reduced to 23.8% missing) from the same site as available, and the remainder were imputed with multivariate imputation by chained equations. An identical analysis without imputation was performed as a sensitivity analysis that showed a similar pattern of results. Total resources were tabulated amongst HRRs, and the hospital resources per COVID-19 case calculated. HRRs are a geographic category devised to represent regional health care markets, and each includes hospital sites performing major procedures.3 These were the focus of the analysis because they may represent a meaningful geographic division of hospital-based resources. COVID-19 case and death counts (as of July 26, 2020) were drawn from publicly available county-level data curated by the New York Times from state and local governments as well as health departments nationwide,6 separated by month (ie, March, April, May, June, and July). Data on New York City were available in aggregate (rather than separated by borough). Cases and deaths were therefore apportioned to the three HRRs involving New York City in proportion to that area’s population. To adjust for the lag between COVID-19 cases and deaths,7,8 we offset deaths 2 weeks into the future so that the April COVID-19 death count for a given HRR included deaths that occurred for 1 month beginning 2 weeks after the start of April, and so on.
Analysis
We estimated Poisson distribution regressions for the outcome of COVID-19 death count in each HRR and month with one model for each of our six hospital-based resource variables. The offset (exposure) variable was COVID-19 case count. To adjust for the possibility of varying effects of hospital resources on deaths by month (ie, in anticipation that health systems might evolve in response to the pandemic over time), each model includes terms for the interaction between hospital-based resource and an indicator variable for month, as well as a fifth term for month. Standard errors were adjusted for clustering within HRR. We report resultant incident rate ratios (IRRs) with 95% CIs, and we report these as statistically significant at the 5% level only after adjustment for multiple comparisons across our six hospital-resource variables using the conservative Bonferroni adjustment. The pseudo-R2 for each of these six models is also reported to summarize the amount of variation in deaths explained. For our model with ICU beds per COVID-19 case, we perform postestimation prediction of number of deaths by HRR, assuming the counterfactual in which HRRs with fewer than average ICU beds per COVID-19 case instead had the average observed number of ICU beds per COVID-19 case by HRR in April, which functioned as a measure of early excess deaths potentially related to resource limitations. The study was classified as exempt by the Institutional Review Board at the Yale School of Medicine, New Haven, Connecticut. Analyses were conducted in Stata 15 (StataCorp LLC) and R.
RESULTS
A total of 4,453 hospitals across 306 HRRs were included and linked to 2,827 county-level COVID-19 case and death counts in each of 5 months (March through July 2020). The median HRR in our analysis included 14 hospitals, with a maximum of 76 hospitals (Los Angeles, California) and a minimum of 1 (Longview, Texas). Among HRRs, 206 (67.3%) had experienced caseloads exceeding 20 per 10,000 population, while 85 (27.8%) had experienced greater than 100 per 10,000 population in the peak month during the study period. The Table depicts results of each of six Poisson distribution regression models, with the finding that greater number of ICU beds (IRR, 0.194; 95% CI, 0.076-0.491), general medical/surgical beds (IRR, 0.800; 95% CI, 0.696-0.920), and nurses (IRR, 0.927; 95% CI, 0.888-0.967) per COVID-19 case in April were statistically significantly associated with reduced deaths.

The model including ICU beds per COVID-19 case had the largest pseudo-R2 at 0.6018, which suggests that ICU bed availability explains the most variation in death count among hospital resource variables analyzed. The incident rate ratio in this model implies that, for an entire additional ICU bed for each COVID-19 case (a one-unit increase in that variable), there is an associated one-fifth decrease in incidence rate (IRR, 0.194) of death in April. The mean value among HRRs in April was 0.25 ICU beds per case (one ICU bed for every four COVID-19 cases), but it was as low as 0.01 to 0.005 in hard-hit areas (one ICU bed for every 100 to 200 COVID-19 cases). The early excess deaths observed in April were not observed in later months. The magnitude of this effect can be summarized as follows: If the 152 HRRs in April with fewer than the mean number of ICU beds per COVID-19 case were to instead have the mean number (one ICU bed for every four COVID-19 cases), our model estimates that there would have been 15,571 fewer deaths that month. The HRRs with the largest number of early excess deaths were Manhattan in New York City (1,466), Bronx in New York City (1,315), Boston, Massachusetts (1,293), Philadelphia, Pennsylvania (955), Hartford, Connecticut (682), Detroit, Michigan (499), and Camden, New Jersey (484). The Figure depicts HRRs in the United States with early excess deaths by this measure in April.

DISCUSSION
We found significant associations between availability of hospital-based resources and COVID-19 deaths in the month of April 2020. This observation was consistent across measures of both hospital bed and staff capacity but not statistically significant in all cases. This provides empiric evidence in support of a preprint simulation publication by Branas et al showing the potential for thousands of excess deaths related to lack of available resources.4 Interestingly, the relationship between hospital-based resources per COVID-19 case and death count is not seen in May, June, or July. This may be because hospitals and health systems were rapidly adapting to pandemic demands9 by shifting resources or reorganizing existing infrastructure to free up beds and personnel.
Our findings underscore the importance of analyses that address heterogeneity in health system response over time and across different geographic areas. That the relationship is not seen after the early pandemic period, when hospitals and health systems were most overwhelmed, suggests that health systems and communities were able to adapt. Importantly, this work does not address the likely complex relationships among hospital resources and outcomes (for example, the benefit of ICU bed availability is likely limited when there are insufficient intensivists and nurses). These complexities should be a focus of future work. Furthermore, hospital resource flexibility, community efforts to slow transmission, and improvements in testing availability and the management of COVID-19 among hospitalized patients may all play a role in attenuating the relationship between baseline resource limitations and outcomes for patients with COVID-19.
These results merit further granular studies to examine specific hospital resources and observed variation in outcomes. Prior literature has linked inpatient capacity—variously defined as high census, acuity, turnover, or delayed admission—to outcomes including mortality among patients with stroke, among those with acute coronary syndrome, and among those requiring intensive care.10 Literature from Italy’s experience shows there was large variation in the case fatality rate among regions of Northern Italy and argues this was partially due to hospital resource limitations.11 Future work can also address whether just-in-time resource mobilization, such as temporary ICU expansion, physician cross-staffing, telemedicine, and dedicated units for COVID-19 patients, attenuated the impact of potential hospital resource scarcity on outcomes.
The present analysis is limited by the quality of the data. There is likely variation of available COVID-19 testing by HRR. It may be that areas with larger outbreaks early on generally tested a smaller, sicker proportion of population-level cases than did those with smaller outbreaks. This effect may be reversed if larger HRRs in urban areas have health systems and public health departments more inclined toward or capable of doing more testing. Furthermore, deaths related to COVID-19 are likely related to community-based factors, including nonhealthcare resources and underlying population characteristics, that likely correlate with the availability of hospital-based resources within HRRs. Some have called into question whether, a priori, we should expect hospital-based capacity to be an important driver of mortality at all,12 arguing that, when critical care capacity is exceeded, resources may be efficiently reallocated away from patients who are least likely to benefit. Because we used the American Hospital Association data, this snapshot of hospital resources is not limited to critical care capacity because there could be alternative explanations for situations in which mortality for both COVID-19 and non–COVID-19 patients may be lower and hospital resources are better matched with demand. For example, patients may seek care earlier in their disease course (whether COVID-19 or otherwise)13 if their local emergency department is not thought to be overwhelmed with case volume.
CONCLUSION
We find that COVID-19 deaths vary among HRRs. The availability of several hospital-based resources is associated with death rates and supports early efforts across the United States to “flatten the curve” to prevent hospital overload. Continued surveillance of this relationship is essential to guide policymakers and hospitals seeking to balance the still limited supply of resources with the demands of caring for both infectious and noninfectious patients in the coming months of this outbreak and in future pandemics.
Acknowledgment
The authors gratefully acknowledge the help of Carolyn Lusch, AICP, in generating depictions of results in Geographic Information Systems.
1. Phua J, Weng L, Ling L, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506-517. https://doi.org/10.1016/s2213-2600(20)30161-2
2. Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371-1372. https://doi.org/10.1001/jama.2010.394
3. General FAQ. Dartmouth Atlas Project. 2020. Accessed July 8, 2020. https://www.dartmouthatlas.org/faq/
4. Branas CC, Rundle A, Pei S, et al. Flattening the curve before it flattens us: hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties. medRxiv. Preprint posted online April 6, 2020. https://doi.org/10.1101/2020.04.01.20049759
5. American Hospital Association Annual Survey Database. American Hospital Association. 2018. Accessed July 8, 2020. https://www.ahadata.com/aha-annual-survey-database
6. An Ongoing Repository of Data on Coronavirus Cases and Deaths in the U.S. New York Times. 2020. Accessed July 8, 2020. https://github.com/nytimes/covid-19-data
7. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. https://doi.org/10.1016/s1473-3099(20)30195-x
8. Rosakis P, Marketou ME. Rethinking case fatality ratios for COVID-19 from a data-driven viewpoint. J Infect. 2020;81(2);e162-e164. https://doi.org/10.1016/j.jinf.2020.06.010
9. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
10. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guide JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
11. Volpato S, Landi F, Incalzi RA. A frail health care system for an old population: lesson form [sic] the COVID-19 outbreak in Italy. J Gerontol Series A. 2020;75(9):e126-e127. https://doi.org/10.1093/gerona/glaa087
12. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447-455. https://doi.org/10.7326/0003-4819-159-7-201310010-00004
13. Moroni F, Gramegna M, Agello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(10):1620-1624. https://doi.org/10.1016/j.jaccas.2020.04.010
1. Phua J, Weng L, Ling L, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506-517. https://doi.org/10.1016/s2213-2600(20)30161-2
2. Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371-1372. https://doi.org/10.1001/jama.2010.394
3. General FAQ. Dartmouth Atlas Project. 2020. Accessed July 8, 2020. https://www.dartmouthatlas.org/faq/
4. Branas CC, Rundle A, Pei S, et al. Flattening the curve before it flattens us: hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties. medRxiv. Preprint posted online April 6, 2020. https://doi.org/10.1101/2020.04.01.20049759
5. American Hospital Association Annual Survey Database. American Hospital Association. 2018. Accessed July 8, 2020. https://www.ahadata.com/aha-annual-survey-database
6. An Ongoing Repository of Data on Coronavirus Cases and Deaths in the U.S. New York Times. 2020. Accessed July 8, 2020. https://github.com/nytimes/covid-19-data
7. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. https://doi.org/10.1016/s1473-3099(20)30195-x
8. Rosakis P, Marketou ME. Rethinking case fatality ratios for COVID-19 from a data-driven viewpoint. J Infect. 2020;81(2);e162-e164. https://doi.org/10.1016/j.jinf.2020.06.010
9. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
10. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guide JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
11. Volpato S, Landi F, Incalzi RA. A frail health care system for an old population: lesson form [sic] the COVID-19 outbreak in Italy. J Gerontol Series A. 2020;75(9):e126-e127. https://doi.org/10.1093/gerona/glaa087
12. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447-455. https://doi.org/10.7326/0003-4819-159-7-201310010-00004
13. Moroni F, Gramegna M, Agello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(10):1620-1624. https://doi.org/10.1016/j.jaccas.2020.04.010
© 2021 Society of Hospital Medicine