User login
October 2016 Quiz 2
Q2: Answer: C
Critique
Isolated gastric varices can be a complication of splenic vein thrombosis. The splenic vein courses superior to the pancreas from the splenic hilum to the main portal vein. Recurrent (or severe acute) inflammation of the pancreas can lead to splenic vein thrombosis, which can cause gastric varices due to backup of blood flow into the short gastric veins. The incidence of gastric varices in those with splenic vein thrombosis ranges from 15% to 55%.
Diagnosis can be made with a CT of the abdomen with contrast, and definitive treatment is splenectomy. Bleeding from a splenic artery pseudoaneurysm can also occur after pancreatitis (hemosuccus pancreaticus), and causes hemodynanically significant bleeding into the peritoneum or the bowel lumen via the pancreatic duct. However, it does not result in gastric varices.
Reference
- Henry Z., Uppal D., Caldwell S., et al. Gastric and ectopic varices. Clin Liver Dis. 2014;18:371-88.
Q2: Answer: C
Critique
Isolated gastric varices can be a complication of splenic vein thrombosis. The splenic vein courses superior to the pancreas from the splenic hilum to the main portal vein. Recurrent (or severe acute) inflammation of the pancreas can lead to splenic vein thrombosis, which can cause gastric varices due to backup of blood flow into the short gastric veins. The incidence of gastric varices in those with splenic vein thrombosis ranges from 15% to 55%.
Diagnosis can be made with a CT of the abdomen with contrast, and definitive treatment is splenectomy. Bleeding from a splenic artery pseudoaneurysm can also occur after pancreatitis (hemosuccus pancreaticus), and causes hemodynanically significant bleeding into the peritoneum or the bowel lumen via the pancreatic duct. However, it does not result in gastric varices.
Q2: Answer: C
Critique
Isolated gastric varices can be a complication of splenic vein thrombosis. The splenic vein courses superior to the pancreas from the splenic hilum to the main portal vein. Recurrent (or severe acute) inflammation of the pancreas can lead to splenic vein thrombosis, which can cause gastric varices due to backup of blood flow into the short gastric veins. The incidence of gastric varices in those with splenic vein thrombosis ranges from 15% to 55%.
Diagnosis can be made with a CT of the abdomen with contrast, and definitive treatment is splenectomy. Bleeding from a splenic artery pseudoaneurysm can also occur after pancreatitis (hemosuccus pancreaticus), and causes hemodynanically significant bleeding into the peritoneum or the bowel lumen via the pancreatic duct. However, it does not result in gastric varices.
Reference
- Henry Z., Uppal D., Caldwell S., et al. Gastric and ectopic varices. Clin Liver Dis. 2014;18:371-88.
Reference
- Henry Z., Uppal D., Caldwell S., et al. Gastric and ectopic varices. Clin Liver Dis. 2014;18:371-88.
A 52-year-old woman with a history of both alcohol and IV drug abuse presents to the emergency department with hematemesis and melena. She was recently discharged from the hospital after an admission for severe acute pancreatitis and has been hospitalized multiple times with alcoholic pancreatitis. After resuscitation, she undergoes urgent endoscopy and is found to have a large gastric varix with a red wale sign, but no active bleeding. There are no esophageal varices.
October 2016 Quiz 1
Q1: Answer: A
Rationale
Vibrio cholerae is a cause of secretory diarrhea. Fecal osmotic gap calculation can be useful in the evaluation of a patient with chronic diarrhea to differentiate between osmotic and secretory diarrhea. It is calculated by subtracting measured fecal electrolytes from normal lumen osmolality (290 – 2(fecal Na+ fecal K)). Secretory diarrhea is characterized by a fecal osmotic gap of less than 50 mOsm/kg, therefore answer A is the correct answer. Osmotic diarrhea is characterized by a fecal osmotic gap greater than 50 mOsm/kg or more specifically, more than 100 mOsm/kg, therefore answer B is incorrect. Accurate fecal osmotic gap calculation depends on normal stool osmolality, which is 290 mOsm/kg. Hypertonic (answer D) or dilute (answer C) stool samples are not appropriate for the calculation of the fecal osmotic gap, therefore answer C and D are incorrect.
Reference
- Eherer A.J., Fordtran J.S. Fecal osmotic gap and pH in experimental diarrhea of various causes. Gastroenterology. 1992;103:545-51.
Q1: Answer: A
Rationale
Vibrio cholerae is a cause of secretory diarrhea. Fecal osmotic gap calculation can be useful in the evaluation of a patient with chronic diarrhea to differentiate between osmotic and secretory diarrhea. It is calculated by subtracting measured fecal electrolytes from normal lumen osmolality (290 – 2(fecal Na+ fecal K)). Secretory diarrhea is characterized by a fecal osmotic gap of less than 50 mOsm/kg, therefore answer A is the correct answer. Osmotic diarrhea is characterized by a fecal osmotic gap greater than 50 mOsm/kg or more specifically, more than 100 mOsm/kg, therefore answer B is incorrect. Accurate fecal osmotic gap calculation depends on normal stool osmolality, which is 290 mOsm/kg. Hypertonic (answer D) or dilute (answer C) stool samples are not appropriate for the calculation of the fecal osmotic gap, therefore answer C and D are incorrect.
Q1: Answer: A
Rationale
Vibrio cholerae is a cause of secretory diarrhea. Fecal osmotic gap calculation can be useful in the evaluation of a patient with chronic diarrhea to differentiate between osmotic and secretory diarrhea. It is calculated by subtracting measured fecal electrolytes from normal lumen osmolality (290 – 2(fecal Na+ fecal K)). Secretory diarrhea is characterized by a fecal osmotic gap of less than 50 mOsm/kg, therefore answer A is the correct answer. Osmotic diarrhea is characterized by a fecal osmotic gap greater than 50 mOsm/kg or more specifically, more than 100 mOsm/kg, therefore answer B is incorrect. Accurate fecal osmotic gap calculation depends on normal stool osmolality, which is 290 mOsm/kg. Hypertonic (answer D) or dilute (answer C) stool samples are not appropriate for the calculation of the fecal osmotic gap, therefore answer C and D are incorrect.
Reference
- Eherer A.J., Fordtran J.S. Fecal osmotic gap and pH in experimental diarrhea of various causes. Gastroenterology. 1992;103:545-51.
Reference
- Eherer A.J., Fordtran J.S. Fecal osmotic gap and pH in experimental diarrhea of various causes. Gastroenterology. 1992;103:545-51.
A 37-year-old man presents with acute nausea, vomiting, and diarrhea consistent with an acute Vibrio cholerae infection.
May 2016 Quiz 2
Q2: Answer: A
Campylobacter species are a major cause of diarrheal illness in the world. The organism inhabits the intestinal tracts of a wide range of animal hosts, notably poultry; contamination from these sources can lead to food-borne disease. Given the self-limited nature of most Campylobacter infections and the limited efficacy of routine antimicrobial therapy, treatment is warranted only for patients with features of severe disease or risk for severe disease.
Patients with severe disease include individuals with bloody stools, high fever, extraintestinal infection, worsening or relapsing symptoms, or symptoms lasting longer than 1 week. Those at risk for severe disease include patients who are elderly, pregnant, or immunocompromised. First-line agents for treatment of Campylobacter infection include fluoroquinolones (if sensitive) or azithromycin. Campylobacter is inherently resistant to trimethoprim and beta-lactam antibiotics, including penicillin and most cephalosporins.
In the United States, the rate of resistance to fluoroquinolones also is increasing. The rate of ciprofloxacin resistance among Campylobacter isolated in the United States increased from 0% to 19% between 1989 and 2001. Inappropriate and overprescription of fluoroquinolones in humans, combined with increased fluoroquinolone use in the poultry industry in particular, have contributed to the increased prevalence of fluoroquinolone resistance.
The rate of macrolide resistance among Campylobacter has remained stable at less than 5% in most parts of the world.
References
- Dasti J.I., Tareen A.M., Lugert R., et al. Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol. 2010;300:205-11.
- Gupta A., Nelson J.M., Barrett T.J., et al. Antimicrobial resistance among Campylobacter strains, United States, 1997-2001. Emerging infectious diseases. Jun 2004;10:1102-9.
Q2: Answer: A
Campylobacter species are a major cause of diarrheal illness in the world. The organism inhabits the intestinal tracts of a wide range of animal hosts, notably poultry; contamination from these sources can lead to food-borne disease. Given the self-limited nature of most Campylobacter infections and the limited efficacy of routine antimicrobial therapy, treatment is warranted only for patients with features of severe disease or risk for severe disease.
Patients with severe disease include individuals with bloody stools, high fever, extraintestinal infection, worsening or relapsing symptoms, or symptoms lasting longer than 1 week. Those at risk for severe disease include patients who are elderly, pregnant, or immunocompromised. First-line agents for treatment of Campylobacter infection include fluoroquinolones (if sensitive) or azithromycin. Campylobacter is inherently resistant to trimethoprim and beta-lactam antibiotics, including penicillin and most cephalosporins.
In the United States, the rate of resistance to fluoroquinolones also is increasing. The rate of ciprofloxacin resistance among Campylobacter isolated in the United States increased from 0% to 19% between 1989 and 2001. Inappropriate and overprescription of fluoroquinolones in humans, combined with increased fluoroquinolone use in the poultry industry in particular, have contributed to the increased prevalence of fluoroquinolone resistance.
The rate of macrolide resistance among Campylobacter has remained stable at less than 5% in most parts of the world.
References
- Dasti J.I., Tareen A.M., Lugert R., et al. Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol. 2010;300:205-11.
- Gupta A., Nelson J.M., Barrett T.J., et al. Antimicrobial resistance among Campylobacter strains, United States, 1997-2001. Emerging infectious diseases. Jun 2004;10:1102-9.
Q2: Answer: A
Campylobacter species are a major cause of diarrheal illness in the world. The organism inhabits the intestinal tracts of a wide range of animal hosts, notably poultry; contamination from these sources can lead to food-borne disease. Given the self-limited nature of most Campylobacter infections and the limited efficacy of routine antimicrobial therapy, treatment is warranted only for patients with features of severe disease or risk for severe disease.
Patients with severe disease include individuals with bloody stools, high fever, extraintestinal infection, worsening or relapsing symptoms, or symptoms lasting longer than 1 week. Those at risk for severe disease include patients who are elderly, pregnant, or immunocompromised. First-line agents for treatment of Campylobacter infection include fluoroquinolones (if sensitive) or azithromycin. Campylobacter is inherently resistant to trimethoprim and beta-lactam antibiotics, including penicillin and most cephalosporins.
In the United States, the rate of resistance to fluoroquinolones also is increasing. The rate of ciprofloxacin resistance among Campylobacter isolated in the United States increased from 0% to 19% between 1989 and 2001. Inappropriate and overprescription of fluoroquinolones in humans, combined with increased fluoroquinolone use in the poultry industry in particular, have contributed to the increased prevalence of fluoroquinolone resistance.
The rate of macrolide resistance among Campylobacter has remained stable at less than 5% in most parts of the world.
References
- Dasti J.I., Tareen A.M., Lugert R., et al. Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol. 2010;300:205-11.
- Gupta A., Nelson J.M., Barrett T.J., et al. Antimicrobial resistance among Campylobacter strains, United States, 1997-2001. Emerging infectious diseases. Jun 2004;10:1102-9.
A 37-year-old man presents to the clinic with a 1-week history of diarrhea. He is a poultry farmer. His symptoms started with nausea and abdominal cramps. Subsequently, he developed diarrhea, reported as 10-12 loose stools with passage of blood. He also reported high fever. Abdominal examination revealed right lower quadrant abdominal tenderness. Stool cultures were ordered and came back positive for Campylobacter infection.
May 2016 Quiz 1
Q1: Answer: D
Rationale: The most frequent mechanism for gastroesophageal reflux is transient lower esophageal sphincter relaxation (TLESR). In the setting of a compliant esophagogastric junction, TLESRs allow movement of content from stomach to esophagus. Gastric distension following meals is a prime trigger for TLESRs, and this is why reflux is most frequent after a meal.
Further, there can be an unbuffered layer of acid floating above the ingested meal (acid pocket) in close proximity to the esophagogastric junction, which is immediately available for reflux during TLESRs. All the other mechanisms listed contribute to the pathophysiology of reflux disease. Increased intra-abdominal pressure contributes to the pressure gradient promoting reflux.
Weak lower esophageal sphincter tone and hiatus hernia are structural deficiencies at the esophagogastric junction that add to the compliance of the esophagogastric junction, making it easier for reflux to occur during a TLESR. Ineffective esophageal body motility contributes to prolonged exposure of the esophageal mucosa to refluxed content, as this can impact clearance of the refluxate, particularly in the recumbent position.
References
- Wu J.C., Mui L.M., Cheung C.M., et al. Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology 2007;132:883-9.
- Mittal R.K., Lange R.C., McCallum R.W. Identification and mechanism of delayed esophageal acid clearance in subjects with hiatus hernia. Gastroenterology 1987;92:130-5.
Q1: Answer: D
Rationale: The most frequent mechanism for gastroesophageal reflux is transient lower esophageal sphincter relaxation (TLESR). In the setting of a compliant esophagogastric junction, TLESRs allow movement of content from stomach to esophagus. Gastric distension following meals is a prime trigger for TLESRs, and this is why reflux is most frequent after a meal.
Further, there can be an unbuffered layer of acid floating above the ingested meal (acid pocket) in close proximity to the esophagogastric junction, which is immediately available for reflux during TLESRs. All the other mechanisms listed contribute to the pathophysiology of reflux disease. Increased intra-abdominal pressure contributes to the pressure gradient promoting reflux.
Weak lower esophageal sphincter tone and hiatus hernia are structural deficiencies at the esophagogastric junction that add to the compliance of the esophagogastric junction, making it easier for reflux to occur during a TLESR. Ineffective esophageal body motility contributes to prolonged exposure of the esophageal mucosa to refluxed content, as this can impact clearance of the refluxate, particularly in the recumbent position.
References
- Wu J.C., Mui L.M., Cheung C.M., et al. Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology 2007;132:883-9.
- Mittal R.K., Lange R.C., McCallum R.W. Identification and mechanism of delayed esophageal acid clearance in subjects with hiatus hernia. Gastroenterology 1987;92:130-5.
Q1: Answer: D
Rationale: The most frequent mechanism for gastroesophageal reflux is transient lower esophageal sphincter relaxation (TLESR). In the setting of a compliant esophagogastric junction, TLESRs allow movement of content from stomach to esophagus. Gastric distension following meals is a prime trigger for TLESRs, and this is why reflux is most frequent after a meal.
Further, there can be an unbuffered layer of acid floating above the ingested meal (acid pocket) in close proximity to the esophagogastric junction, which is immediately available for reflux during TLESRs. All the other mechanisms listed contribute to the pathophysiology of reflux disease. Increased intra-abdominal pressure contributes to the pressure gradient promoting reflux.
Weak lower esophageal sphincter tone and hiatus hernia are structural deficiencies at the esophagogastric junction that add to the compliance of the esophagogastric junction, making it easier for reflux to occur during a TLESR. Ineffective esophageal body motility contributes to prolonged exposure of the esophageal mucosa to refluxed content, as this can impact clearance of the refluxate, particularly in the recumbent position.
References
- Wu J.C., Mui L.M., Cheung C.M., et al. Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology 2007;132:883-9.
- Mittal R.K., Lange R.C., McCallum R.W. Identification and mechanism of delayed esophageal acid clearance in subjects with hiatus hernia. Gastroenterology 1987;92:130-5.
A 24-year-old woman presents for continuing management of gastroesophageal reflux disease. Although her heartburn has resolved on twice-a-day omeprazole therapy, she continues to have postprandial regurgitation. She has no nocturnal symptoms. Prior evaluation has demonstrated a 3-cm sliding hiatus hernia, but no erosive esophagitis. She is overweight with a body mass index of 32 kg/m2. Physical examination is normal.
May 2016 Quiz 1
Q1: Answer: D
Rationale: The most frequent mechanism for gastroesophageal reflux is transient lower esophageal sphincter relaxation (TLESR). In the setting of a compliant esophagogastric junction, TLESRs allow movement of content from stomach to esophagus. Gastric distension following meals is a prime trigger for TLESRs, and this is why reflux is most frequent after a meal.
Further, there can be an unbuffered layer of acid floating above the ingested meal (acid pocket) in close proximity to the esophagogastric junction, which is immediately available for reflux during TLESRs. All the other mechanisms listed contribute to the pathophysiology of reflux disease. Increased intra-abdominal pressure contributes to the pressure gradient promoting reflux.
Weak lower esophageal sphincter tone and hiatus hernia are structural deficiencies at the esophagogastric junction that add to the compliance of the esophagogastric junction, making it easier for reflux to occur during a TLESR. Ineffective esophageal body motility contributes to prolonged exposure of the esophageal mucosa to refluxed content, as this can impact clearance of the refluxate, particularly in the recumbent position.
References
- Wu J.C., Mui L.M., Cheung C.M., et al. Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology 2007;132:883-9.
- Mittal R.K., Lange R.C., McCallum R.W. Identification and mechanism of delayed esophageal acid clearance in subjects with hiatus hernia. Gastroenterology 1987;92:130-5.
Q1: Answer: D
Rationale: The most frequent mechanism for gastroesophageal reflux is transient lower esophageal sphincter relaxation (TLESR). In the setting of a compliant esophagogastric junction, TLESRs allow movement of content from stomach to esophagus. Gastric distension following meals is a prime trigger for TLESRs, and this is why reflux is most frequent after a meal.
Further, there can be an unbuffered layer of acid floating above the ingested meal (acid pocket) in close proximity to the esophagogastric junction, which is immediately available for reflux during TLESRs. All the other mechanisms listed contribute to the pathophysiology of reflux disease. Increased intra-abdominal pressure contributes to the pressure gradient promoting reflux.
Weak lower esophageal sphincter tone and hiatus hernia are structural deficiencies at the esophagogastric junction that add to the compliance of the esophagogastric junction, making it easier for reflux to occur during a TLESR. Ineffective esophageal body motility contributes to prolonged exposure of the esophageal mucosa to refluxed content, as this can impact clearance of the refluxate, particularly in the recumbent position.
References
- Wu J.C., Mui L.M., Cheung C.M., et al. Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology 2007;132:883-9.
- Mittal R.K., Lange R.C., McCallum R.W. Identification and mechanism of delayed esophageal acid clearance in subjects with hiatus hernia. Gastroenterology 1987;92:130-5.
Q1: Answer: D
Rationale: The most frequent mechanism for gastroesophageal reflux is transient lower esophageal sphincter relaxation (TLESR). In the setting of a compliant esophagogastric junction, TLESRs allow movement of content from stomach to esophagus. Gastric distension following meals is a prime trigger for TLESRs, and this is why reflux is most frequent after a meal.
Further, there can be an unbuffered layer of acid floating above the ingested meal (acid pocket) in close proximity to the esophagogastric junction, which is immediately available for reflux during TLESRs. All the other mechanisms listed contribute to the pathophysiology of reflux disease. Increased intra-abdominal pressure contributes to the pressure gradient promoting reflux.
Weak lower esophageal sphincter tone and hiatus hernia are structural deficiencies at the esophagogastric junction that add to the compliance of the esophagogastric junction, making it easier for reflux to occur during a TLESR. Ineffective esophageal body motility contributes to prolonged exposure of the esophageal mucosa to refluxed content, as this can impact clearance of the refluxate, particularly in the recumbent position.
References
- Wu J.C., Mui L.M., Cheung C.M., et al. Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology 2007;132:883-9.
- Mittal R.K., Lange R.C., McCallum R.W. Identification and mechanism of delayed esophageal acid clearance in subjects with hiatus hernia. Gastroenterology 1987;92:130-5.
April 2016 Quiz 2
Q2: ANSWER: D
Critique
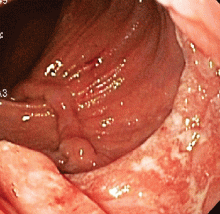
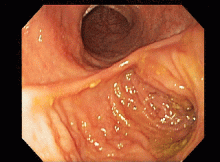
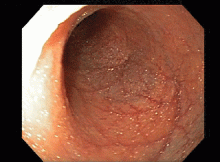
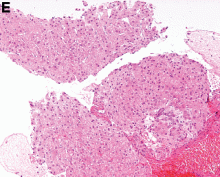
This series of endoscopic findings shows changes consistent with inflammation of the cuff.
Up to 20%-30% of patients with ulcerative colitis ultimately require colectomy due to medically refractory colitis or to the development of dysplasia. A total abdominal protocolectomy with ileal pouch anal anastomosis has become the surgical procedure of choice for most ulcerative colitis patients who require surgery.
There are both early and late complications of this type of surgery. Late complications include anastomotic stricture, pouchitis, abscesses, inflammation of the cuff or cuffitis, and functional difficulties such as irritable pouch syndrome, anal pain, and pouch stasis. Patients may also present with inflammation of the pouch and the prepouch ileum that is consistent with Crohn’s disease. Endoscopy can help to narrow down the differential diagnosis of these pouch complications.
In this series of photos the prepouch ileum has no inflammation. The pouch also has no ulcerations or evidence of pouchitis or Crohn’s disease. The cuff is inflamed consistent with a diagnosis of cuffitis. If the inflammation becomes difficult to manage, immunosuppressive therapy may be required but at this point it is reasonable to start with topical therapy to the inflamed area.
Reference
- Li, Y. Shen, B. Evaluating pouch problems. Gastroenterology Clinics North Am. 2012;41:355-78.
- Shen, G. Diagnosis and management of post-operative ileal pouch disorders. Clin Colon Rectal Surg. 2010;23:259-68.
Q2: ANSWER: D
Critique
This series of endoscopic findings shows changes consistent with inflammation of the cuff.
Up to 20%-30% of patients with ulcerative colitis ultimately require colectomy due to medically refractory colitis or to the development of dysplasia. A total abdominal protocolectomy with ileal pouch anal anastomosis has become the surgical procedure of choice for most ulcerative colitis patients who require surgery.
There are both early and late complications of this type of surgery. Late complications include anastomotic stricture, pouchitis, abscesses, inflammation of the cuff or cuffitis, and functional difficulties such as irritable pouch syndrome, anal pain, and pouch stasis. Patients may also present with inflammation of the pouch and the prepouch ileum that is consistent with Crohn’s disease. Endoscopy can help to narrow down the differential diagnosis of these pouch complications.
In this series of photos the prepouch ileum has no inflammation. The pouch also has no ulcerations or evidence of pouchitis or Crohn’s disease. The cuff is inflamed consistent with a diagnosis of cuffitis. If the inflammation becomes difficult to manage, immunosuppressive therapy may be required but at this point it is reasonable to start with topical therapy to the inflamed area.
Reference
- Li, Y. Shen, B. Evaluating pouch problems. Gastroenterology Clinics North Am. 2012;41:355-78.
- Shen, G. Diagnosis and management of post-operative ileal pouch disorders. Clin Colon Rectal Surg. 2010;23:259-68.
Q2: ANSWER: D
Critique
This series of endoscopic findings shows changes consistent with inflammation of the cuff.
Up to 20%-30% of patients with ulcerative colitis ultimately require colectomy due to medically refractory colitis or to the development of dysplasia. A total abdominal protocolectomy with ileal pouch anal anastomosis has become the surgical procedure of choice for most ulcerative colitis patients who require surgery.
There are both early and late complications of this type of surgery. Late complications include anastomotic stricture, pouchitis, abscesses, inflammation of the cuff or cuffitis, and functional difficulties such as irritable pouch syndrome, anal pain, and pouch stasis. Patients may also present with inflammation of the pouch and the prepouch ileum that is consistent with Crohn’s disease. Endoscopy can help to narrow down the differential diagnosis of these pouch complications.
In this series of photos the prepouch ileum has no inflammation. The pouch also has no ulcerations or evidence of pouchitis or Crohn’s disease. The cuff is inflamed consistent with a diagnosis of cuffitis. If the inflammation becomes difficult to manage, immunosuppressive therapy may be required but at this point it is reasonable to start with topical therapy to the inflamed area.
Reference
- Li, Y. Shen, B. Evaluating pouch problems. Gastroenterology Clinics North Am. 2012;41:355-78.
- Shen, G. Diagnosis and management of post-operative ileal pouch disorders. Clin Colon Rectal Surg. 2010;23:259-68.
April 2016 Quiz 1
Q1: ANSWER: E
Critique
Different types of meat have different risks of resulting in meat impaction in patients with predisposing esophageal factors. The ranking order is as follows: Beef more than pork more than turkey more than chicken more than fish.
Reference
1. Gasiorowska, A. et al. Gasteroenterol Hepatol. 2009;5:269-79.
Q1: ANSWER: E
Critique
Different types of meat have different risks of resulting in meat impaction in patients with predisposing esophageal factors. The ranking order is as follows: Beef more than pork more than turkey more than chicken more than fish.
Reference
1. Gasiorowska, A. et al. Gasteroenterol Hepatol. 2009;5:269-79.
Q1: ANSWER: E
Critique
Different types of meat have different risks of resulting in meat impaction in patients with predisposing esophageal factors. The ranking order is as follows: Beef more than pork more than turkey more than chicken more than fish.
Reference
1. Gasiorowska, A. et al. Gasteroenterol Hepatol. 2009;5:269-79.
A 45-year-old male, who has experienced two episodes of meat impaction in the last 10 years, was found to have a lower esophageal ring as the underlying cause. The patient underwent dilations but was still concerned about another future episode of meat impaction. The patient would like to know which type of meat is the least likely to result in food impaction.
March 2016 Quiz 2
Q2: ANSWER: E
Critique
These results could be due to Lynch syndrome as a result of an EPCAM mutation. With hereditary nonpolyposis colorectal cancer (HNPCC), also known as Lynch syndrome, patients are at an increased risk for colorectal and several other cancers owing to inactivating germline mutations in mismatch repair genes (MMR), including MLH1, MSH2, MSH6, and PMS2. Germline EPCAM deletions in the 3’ region can cause HNPCC. The EPCAM deletions lead to methylation of the MSH2 promoter and ultimately silencing of MSH2 gene. Silencing of the MSH2 gene results in a pattern of MSH2 and MSH6 loss on immunohistochemistry. Since immunohistochemistry shows no loss of expression of MLH1 or PMS2 proteins, this indicates the absence of a mutation in MLH1 and PMS2 genes. The presence of high microsatellite instability and loss of expression of two mismatch repair proteins indicates the presence of Lynch syndrome and not a sporadic colorectal cancer. Therefore MLH1 hypermethylation and BRAF testing is not indicated.
Reference
1. Umar A., Boland C., Terdiman J.P., et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261-8.
Q2: ANSWER: E
Critique
These results could be due to Lynch syndrome as a result of an EPCAM mutation. With hereditary nonpolyposis colorectal cancer (HNPCC), also known as Lynch syndrome, patients are at an increased risk for colorectal and several other cancers owing to inactivating germline mutations in mismatch repair genes (MMR), including MLH1, MSH2, MSH6, and PMS2. Germline EPCAM deletions in the 3’ region can cause HNPCC. The EPCAM deletions lead to methylation of the MSH2 promoter and ultimately silencing of MSH2 gene. Silencing of the MSH2 gene results in a pattern of MSH2 and MSH6 loss on immunohistochemistry. Since immunohistochemistry shows no loss of expression of MLH1 or PMS2 proteins, this indicates the absence of a mutation in MLH1 and PMS2 genes. The presence of high microsatellite instability and loss of expression of two mismatch repair proteins indicates the presence of Lynch syndrome and not a sporadic colorectal cancer. Therefore MLH1 hypermethylation and BRAF testing is not indicated.
Reference
1. Umar A., Boland C., Terdiman J.P., et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261-8.
Q2: ANSWER: E
Critique
These results could be due to Lynch syndrome as a result of an EPCAM mutation. With hereditary nonpolyposis colorectal cancer (HNPCC), also known as Lynch syndrome, patients are at an increased risk for colorectal and several other cancers owing to inactivating germline mutations in mismatch repair genes (MMR), including MLH1, MSH2, MSH6, and PMS2. Germline EPCAM deletions in the 3’ region can cause HNPCC. The EPCAM deletions lead to methylation of the MSH2 promoter and ultimately silencing of MSH2 gene. Silencing of the MSH2 gene results in a pattern of MSH2 and MSH6 loss on immunohistochemistry. Since immunohistochemistry shows no loss of expression of MLH1 or PMS2 proteins, this indicates the absence of a mutation in MLH1 and PMS2 genes. The presence of high microsatellite instability and loss of expression of two mismatch repair proteins indicates the presence of Lynch syndrome and not a sporadic colorectal cancer. Therefore MLH1 hypermethylation and BRAF testing is not indicated.
Reference
1. Umar A., Boland C., Terdiman J.P., et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261-8.
March 2016 Quiz 1
Q1: ANSWER: C
Critique
The patient is morbidly obese, with persisting esophagitis despite twice-a-day PPI, and structural disruption of the gastroesophageal junction with a moderate-sized hiatus hernia and a patulous gastroesophageal junction. This is a setting in which a surgical approach is worthwhile. Since the patient is morbidly obese with hypertension and diabetes, the option of Roux-en-Y gastric bypass surgery is worth considering. This is a viable option for the surgical management of persisting reflux in the morbidly obese. Sucralfate has limited additive gain in persistent esophagitis despite PPI therapy. There is no net gain in switching the route of PPI administration to intravenous, and this is mostly indicated in the setting of gastrointestinal bleeding. Metoclopramide does not result in worthwhile augmentation of esophageal motility, and may not necessarily provide further symptom improvement or healing of esophagitis. Lifestyle measures complement pharmacologic management of reflux disease, but have not been shown to heal esophagitis.
Reference
1. De Groot N.L., Burgerhart J.S., Van De Meeberg P.C., et al. Systematic review: the effects of conservative and surgical treatment for obesity on gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2009;30:1091-102.
Q1: ANSWER: C
Critique
The patient is morbidly obese, with persisting esophagitis despite twice-a-day PPI, and structural disruption of the gastroesophageal junction with a moderate-sized hiatus hernia and a patulous gastroesophageal junction. This is a setting in which a surgical approach is worthwhile. Since the patient is morbidly obese with hypertension and diabetes, the option of Roux-en-Y gastric bypass surgery is worth considering. This is a viable option for the surgical management of persisting reflux in the morbidly obese. Sucralfate has limited additive gain in persistent esophagitis despite PPI therapy. There is no net gain in switching the route of PPI administration to intravenous, and this is mostly indicated in the setting of gastrointestinal bleeding. Metoclopramide does not result in worthwhile augmentation of esophageal motility, and may not necessarily provide further symptom improvement or healing of esophagitis. Lifestyle measures complement pharmacologic management of reflux disease, but have not been shown to heal esophagitis.
Reference
1. De Groot N.L., Burgerhart J.S., Van De Meeberg P.C., et al. Systematic review: the effects of conservative and surgical treatment for obesity on gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2009;30:1091-102.
Q1: ANSWER: C
Critique
The patient is morbidly obese, with persisting esophagitis despite twice-a-day PPI, and structural disruption of the gastroesophageal junction with a moderate-sized hiatus hernia and a patulous gastroesophageal junction. This is a setting in which a surgical approach is worthwhile. Since the patient is morbidly obese with hypertension and diabetes, the option of Roux-en-Y gastric bypass surgery is worth considering. This is a viable option for the surgical management of persisting reflux in the morbidly obese. Sucralfate has limited additive gain in persistent esophagitis despite PPI therapy. There is no net gain in switching the route of PPI administration to intravenous, and this is mostly indicated in the setting of gastrointestinal bleeding. Metoclopramide does not result in worthwhile augmentation of esophageal motility, and may not necessarily provide further symptom improvement or healing of esophagitis. Lifestyle measures complement pharmacologic management of reflux disease, but have not been shown to heal esophagitis.
Reference
1. De Groot N.L., Burgerhart J.S., Van De Meeberg P.C., et al. Systematic review: the effects of conservative and surgical treatment for obesity on gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2009;30:1091-102.
February 2016 Quiz 2
Q2: ANSWER: D
Critique
This is a patient with severe alcoholic hepatitis complicated by sepsis with a Maddrey’s discriminant function greater than 32. Pentoxifylline 400 mg t.i.d. would be the most appropriate choice for treatment. Pentoxifylline is a nonselective phosphodiesterase inhibitor that decreases tumor necrosis factor gene transcription. In one study of severe alcoholic hepatitis, it appeared to reduce both mortality and renal failure. It may have a beneficial effect in preventing HRS. Prednisone would not be optimal in the setting of active infection and sepsis. Anti-TNF treatment has been associated with increased risk of severe infections and this and propylthiouracil have not shown benefit in the treatment of alcoholic hepatitis. For this patient with severe alcoholic hepatitis, treatment will improve survival and observation would not be adequate.
References
- O’Shea R.S., Dasarathy S., McCullough A.J.. Alcoholic liver disease. Hepatology 2010;51:307-28.
- Mathurin P., Mendenhall C.L., et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:480-7.
Q2: ANSWER: D
Critique
This is a patient with severe alcoholic hepatitis complicated by sepsis with a Maddrey’s discriminant function greater than 32. Pentoxifylline 400 mg t.i.d. would be the most appropriate choice for treatment. Pentoxifylline is a nonselective phosphodiesterase inhibitor that decreases tumor necrosis factor gene transcription. In one study of severe alcoholic hepatitis, it appeared to reduce both mortality and renal failure. It may have a beneficial effect in preventing HRS. Prednisone would not be optimal in the setting of active infection and sepsis. Anti-TNF treatment has been associated with increased risk of severe infections and this and propylthiouracil have not shown benefit in the treatment of alcoholic hepatitis. For this patient with severe alcoholic hepatitis, treatment will improve survival and observation would not be adequate.
References
- O’Shea R.S., Dasarathy S., McCullough A.J.. Alcoholic liver disease. Hepatology 2010;51:307-28.
- Mathurin P., Mendenhall C.L., et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:480-7.
Q2: ANSWER: D
Critique
This is a patient with severe alcoholic hepatitis complicated by sepsis with a Maddrey’s discriminant function greater than 32. Pentoxifylline 400 mg t.i.d. would be the most appropriate choice for treatment. Pentoxifylline is a nonselective phosphodiesterase inhibitor that decreases tumor necrosis factor gene transcription. In one study of severe alcoholic hepatitis, it appeared to reduce both mortality and renal failure. It may have a beneficial effect in preventing HRS. Prednisone would not be optimal in the setting of active infection and sepsis. Anti-TNF treatment has been associated with increased risk of severe infections and this and propylthiouracil have not shown benefit in the treatment of alcoholic hepatitis. For this patient with severe alcoholic hepatitis, treatment will improve survival and observation would not be adequate.
References
- O’Shea R.S., Dasarathy S., McCullough A.J.. Alcoholic liver disease. Hepatology 2010;51:307-28.
- Mathurin P., Mendenhall C.L., et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:480-7.