User login
42-year-old woman with abnormal uterine bleeding
A) Endometrial polyp INCORRECT
Endometrial polyps on ultrasonography appear as focal echogenic (hyperechoic) masses or as nonspecific endometrial thickening.1 Color Doppler often demonstrates a vascular stalk, which is a nonspecific finding that also can be seen in submucosal fibroids and endometrial cancer.2 On sonohysterography (SHG), endometrial polyps typically appear as well-defined echogenic/hyperechoic polypoid lesions (tissue appearance similar to that of normal endometrium) protruding into the endometrial canal but still preserving the endometrial−myometrial interface.2,3
B) Submucosal fibroid CORRECT
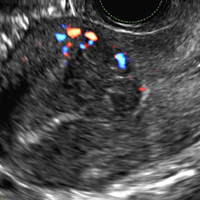
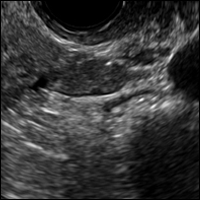
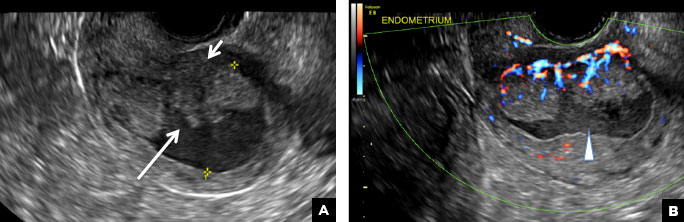
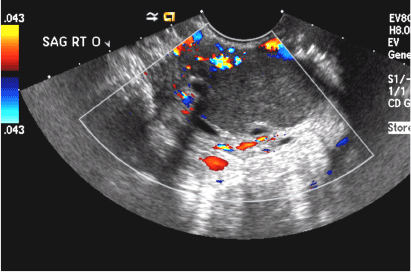
Submucosal fibroids on ultrasonography appear as heterogeneous hypoechoic lesions distorting the endometrial cavity.1 In contrast to endometrial polyps, which involve the endometrium only, submucosal (intracavitary) fibroids originate in the myometrium, as clarified in Figures 3A & B. SHG demonstrates a broad-based mixed hypoechoic/isoechoic lesion protruding into the endometrial canal but preserving the echogenic endometrium, distinguishing myometrial from endometrial lesions. Submucosal fibroids often distort the endometrial myometrial interface and demonstrate acoustic shadowing.2,3
C) Endometrial carcinoma INCORRECT
Endometrial carcinoma on SHG appears as an irregular inhomogeneous lobulated vascular mass distorting the endometrial−myometrial interface.3 Additionally, irregular frond-like projections can be seen extending from the mass into the endometrial cavity, which are distended with echogenic fluid.2
D) Endometrial hyperplasia INCORRECT
On ultrasonography, endometrial hyperplasia has a nonspecific appearance, often presenting as diffuse smooth endometrial thickening.1 SHG typically demonstrates diffuse thickening of the echogenic endometrial stripe without a focal lesion and, when a focal lesion is present, can mimic a broad-based endometrial polyp.2,3

- Nalaboff KM, Pellerito JS, Ben-Levi E. Imaging the endometrium: disease and normal variants. Radiographics. 2001;21(6):1409-1424.
- Davis PC, O'Neill MJ, Yoder IC, Lee SI, Mueller PR. Sonohysterographic findings of endometrial and subendometrial conditions. Radiographics. 2002;22(4):803- 816.
- Yang T, Pandya A, Marcal L, et al. Sonohysterography: principles, technique and role in diagnosis of endometrial pathology. World J Radiol. 2013;5(3):81-87.
A) Endometrial polyp INCORRECT
Endometrial polyps on ultrasonography appear as focal echogenic (hyperechoic) masses or as nonspecific endometrial thickening.1 Color Doppler often demonstrates a vascular stalk, which is a nonspecific finding that also can be seen in submucosal fibroids and endometrial cancer.2 On sonohysterography (SHG), endometrial polyps typically appear as well-defined echogenic/hyperechoic polypoid lesions (tissue appearance similar to that of normal endometrium) protruding into the endometrial canal but still preserving the endometrial−myometrial interface.2,3

B) Submucosal fibroid CORRECT
Submucosal fibroids on ultrasonography appear as heterogeneous hypoechoic lesions distorting the endometrial cavity.1 In contrast to endometrial polyps, which involve the endometrium only, submucosal (intracavitary) fibroids originate in the myometrium, as clarified in Figures 3A & B. SHG demonstrates a broad-based mixed hypoechoic/isoechoic lesion protruding into the endometrial canal but preserving the echogenic endometrium, distinguishing myometrial from endometrial lesions. Submucosal fibroids often distort the endometrial myometrial interface and demonstrate acoustic shadowing.2,3

C) Endometrial carcinoma INCORRECT
Endometrial carcinoma on SHG appears as an irregular inhomogeneous lobulated vascular mass distorting the endometrial−myometrial interface.3 Additionally, irregular frond-like projections can be seen extending from the mass into the endometrial cavity, which are distended with echogenic fluid.2

D) Endometrial hyperplasia INCORRECT
On ultrasonography, endometrial hyperplasia has a nonspecific appearance, often presenting as diffuse smooth endometrial thickening.1 SHG typically demonstrates diffuse thickening of the echogenic endometrial stripe without a focal lesion and, when a focal lesion is present, can mimic a broad-based endometrial polyp.2,3

A) Endometrial polyp INCORRECT
Endometrial polyps on ultrasonography appear as focal echogenic (hyperechoic) masses or as nonspecific endometrial thickening.1 Color Doppler often demonstrates a vascular stalk, which is a nonspecific finding that also can be seen in submucosal fibroids and endometrial cancer.2 On sonohysterography (SHG), endometrial polyps typically appear as well-defined echogenic/hyperechoic polypoid lesions (tissue appearance similar to that of normal endometrium) protruding into the endometrial canal but still preserving the endometrial−myometrial interface.2,3

B) Submucosal fibroid CORRECT
Submucosal fibroids on ultrasonography appear as heterogeneous hypoechoic lesions distorting the endometrial cavity.1 In contrast to endometrial polyps, which involve the endometrium only, submucosal (intracavitary) fibroids originate in the myometrium, as clarified in Figures 3A & B. SHG demonstrates a broad-based mixed hypoechoic/isoechoic lesion protruding into the endometrial canal but preserving the echogenic endometrium, distinguishing myometrial from endometrial lesions. Submucosal fibroids often distort the endometrial myometrial interface and demonstrate acoustic shadowing.2,3

C) Endometrial carcinoma INCORRECT
Endometrial carcinoma on SHG appears as an irregular inhomogeneous lobulated vascular mass distorting the endometrial−myometrial interface.3 Additionally, irregular frond-like projections can be seen extending from the mass into the endometrial cavity, which are distended with echogenic fluid.2

D) Endometrial hyperplasia INCORRECT
On ultrasonography, endometrial hyperplasia has a nonspecific appearance, often presenting as diffuse smooth endometrial thickening.1 SHG typically demonstrates diffuse thickening of the echogenic endometrial stripe without a focal lesion and, when a focal lesion is present, can mimic a broad-based endometrial polyp.2,3

- Nalaboff KM, Pellerito JS, Ben-Levi E. Imaging the endometrium: disease and normal variants. Radiographics. 2001;21(6):1409-1424.
- Davis PC, O'Neill MJ, Yoder IC, Lee SI, Mueller PR. Sonohysterographic findings of endometrial and subendometrial conditions. Radiographics. 2002;22(4):803- 816.
- Yang T, Pandya A, Marcal L, et al. Sonohysterography: principles, technique and role in diagnosis of endometrial pathology. World J Radiol. 2013;5(3):81-87.
- Nalaboff KM, Pellerito JS, Ben-Levi E. Imaging the endometrium: disease and normal variants. Radiographics. 2001;21(6):1409-1424.
- Davis PC, O'Neill MJ, Yoder IC, Lee SI, Mueller PR. Sonohysterographic findings of endometrial and subendometrial conditions. Radiographics. 2002;22(4):803- 816.
- Yang T, Pandya A, Marcal L, et al. Sonohysterography: principles, technique and role in diagnosis of endometrial pathology. World J Radiol. 2013;5(3):81-87.
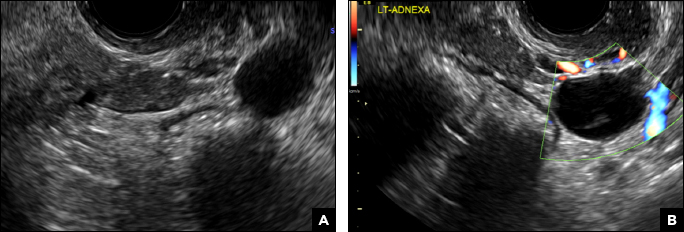
A 42-year-old woman presents to her gynecologist's office with abnormal uterine bleeding. Pelvic ultrasonography of the uterus is performed with color Doppler (A) and subsequent sonohysterogram (SHG)/saline infusion sonohysterography (SIS) (B). Figures shown above.
32-year-old woman with pelvic pain and irregular menstrual periods
(A) Paratubal cyst CORRECT
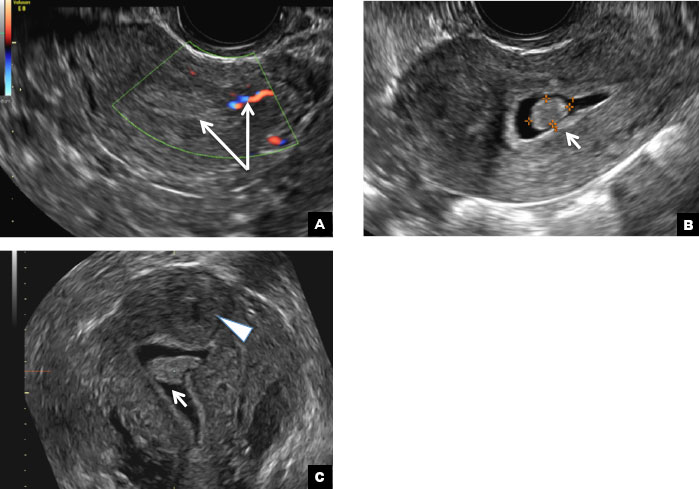
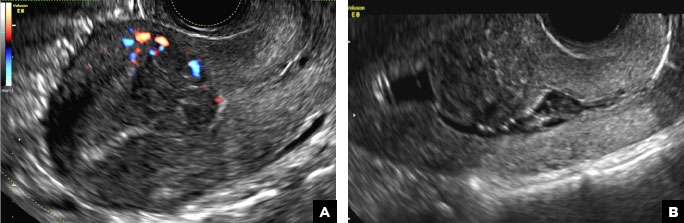
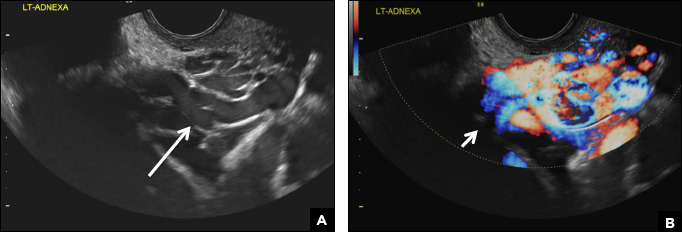
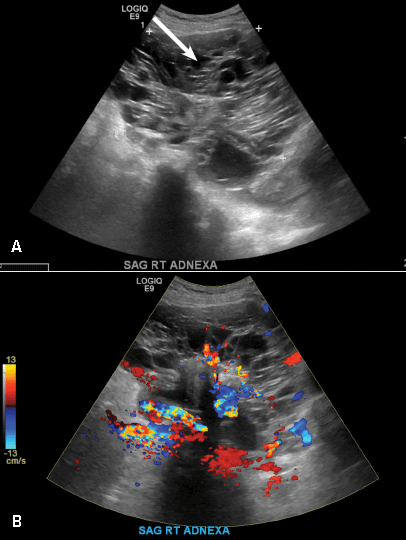
Paratubal, or paraovarian, cysts typically are round or oval avascular hypoechoic cysts (long arrow) separate from the adjacent ovary (short arrow). Since they are congenital remnants of the Wolffian duct, they arise from the mesosalpinx, specifically the broad ligament or fallopian tube.1,2 They usually are seen in close proximity to but separate from the ovary without distorting the ovary’s architecture.1,2
B) Hydrosalpinx INCORRECT
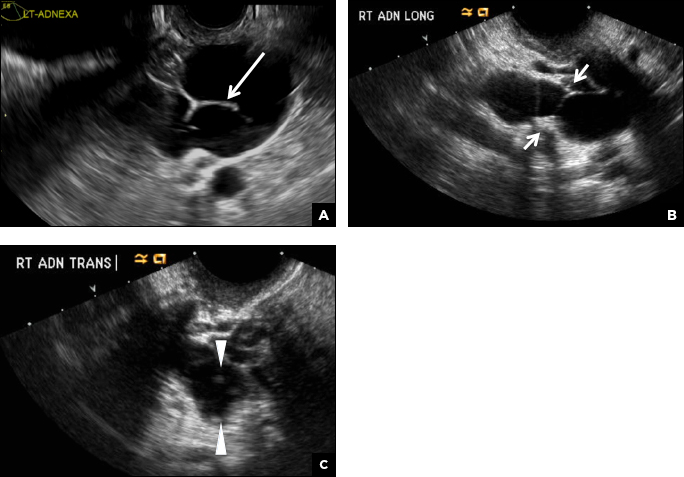
A hydrosalpinx appears as an elongated C- or S-shaped, thin-walled tubular serpiginous cystic lesion separate from the ovary. It often has incomplete septations that are infolding of the tube on itself (long arrow).3 Other findings include diametrically opposed indentations (short arrows) of the wall (Waist sign) and short linear mucosal or submucosal folds (arrowhead) that when viewed in cross section appear similar to the spokes of a cogwheel (Cogwheel sign).1–3 Prior tubal infection or gynecologic surgery represent risk factors for hydrosalpinx.
C) Peritoneal inclusion cyst INCORRECT
A peritoneal inclusion cyst appears as an anechoic cystic mass that conforms passively to the shape of the peritoneal cavity/pelvic sidewall (long arrow) and may contain entrapped ovaries (short arrow) within or along the periphery of the fluid collection.1,2 Septations within it are likely from peritoneal adhesions (arrowhead) and may show vascularity.2 Prior (often multiple) gynecologic surgeries represent a risk factor for peritoneal inclusion cysts.

D) Dilated pelvic veins INCORRECT
Dilated pelvic veins appear on sonography as a cluster of elongated and tubular cystic lesions in the adnexa along the broad ligament and demonstrate low level echoes due to sluggish flow (long arrow) and visible red blood cell rouleaux formation. This can be confirmed on color Doppler images (short arrow) and help differentiate it from hydrosalpinx.
- Laing FC, Allison SF. US of the ovary and adnexa: to worry or not to worry? Radiographics. 2012:32(6):1621−1639.
- Moyle PL, Kataoka MY, Nakai A, Takahata A, Reinhold C, Sala E. Nonovarian cystic lesions of the pelvis. Radiographics. 2010;30(4):921−938.
- Rezvani M, Shaaban AM. Fallopian tube disease in the nonpregnant patient. Radiographics. 2011;31(2):527−548.
(A) Paratubal cyst CORRECT
Paratubal, or paraovarian, cysts typically are round or oval avascular hypoechoic cysts (long arrow) separate from the adjacent ovary (short arrow). Since they are congenital remnants of the Wolffian duct, they arise from the mesosalpinx, specifically the broad ligament or fallopian tube.1,2 They usually are seen in close proximity to but separate from the ovary without distorting the ovary’s architecture.1,2

B) Hydrosalpinx INCORRECT
A hydrosalpinx appears as an elongated C- or S-shaped, thin-walled tubular serpiginous cystic lesion separate from the ovary. It often has incomplete septations that are infolding of the tube on itself (long arrow).3 Other findings include diametrically opposed indentations (short arrows) of the wall (Waist sign) and short linear mucosal or submucosal folds (arrowhead) that when viewed in cross section appear similar to the spokes of a cogwheel (Cogwheel sign).1–3 Prior tubal infection or gynecologic surgery represent risk factors for hydrosalpinx.

C) Peritoneal inclusion cyst INCORRECT
A peritoneal inclusion cyst appears as an anechoic cystic mass that conforms passively to the shape of the peritoneal cavity/pelvic sidewall (long arrow) and may contain entrapped ovaries (short arrow) within or along the periphery of the fluid collection.1,2 Septations within it are likely from peritoneal adhesions (arrowhead) and may show vascularity.2 Prior (often multiple) gynecologic surgeries represent a risk factor for peritoneal inclusion cysts.

D) Dilated pelvic veins INCORRECT
Dilated pelvic veins appear on sonography as a cluster of elongated and tubular cystic lesions in the adnexa along the broad ligament and demonstrate low level echoes due to sluggish flow (long arrow) and visible red blood cell rouleaux formation. This can be confirmed on color Doppler images (short arrow) and help differentiate it from hydrosalpinx.

(A) Paratubal cyst CORRECT
Paratubal, or paraovarian, cysts typically are round or oval avascular hypoechoic cysts (long arrow) separate from the adjacent ovary (short arrow). Since they are congenital remnants of the Wolffian duct, they arise from the mesosalpinx, specifically the broad ligament or fallopian tube.1,2 They usually are seen in close proximity to but separate from the ovary without distorting the ovary’s architecture.1,2

B) Hydrosalpinx INCORRECT
A hydrosalpinx appears as an elongated C- or S-shaped, thin-walled tubular serpiginous cystic lesion separate from the ovary. It often has incomplete septations that are infolding of the tube on itself (long arrow).3 Other findings include diametrically opposed indentations (short arrows) of the wall (Waist sign) and short linear mucosal or submucosal folds (arrowhead) that when viewed in cross section appear similar to the spokes of a cogwheel (Cogwheel sign).1–3 Prior tubal infection or gynecologic surgery represent risk factors for hydrosalpinx.

C) Peritoneal inclusion cyst INCORRECT
A peritoneal inclusion cyst appears as an anechoic cystic mass that conforms passively to the shape of the peritoneal cavity/pelvic sidewall (long arrow) and may contain entrapped ovaries (short arrow) within or along the periphery of the fluid collection.1,2 Septations within it are likely from peritoneal adhesions (arrowhead) and may show vascularity.2 Prior (often multiple) gynecologic surgeries represent a risk factor for peritoneal inclusion cysts.

D) Dilated pelvic veins INCORRECT
Dilated pelvic veins appear on sonography as a cluster of elongated and tubular cystic lesions in the adnexa along the broad ligament and demonstrate low level echoes due to sluggish flow (long arrow) and visible red blood cell rouleaux formation. This can be confirmed on color Doppler images (short arrow) and help differentiate it from hydrosalpinx.

- Laing FC, Allison SF. US of the ovary and adnexa: to worry or not to worry? Radiographics. 2012:32(6):1621−1639.
- Moyle PL, Kataoka MY, Nakai A, Takahata A, Reinhold C, Sala E. Nonovarian cystic lesions of the pelvis. Radiographics. 2010;30(4):921−938.
- Rezvani M, Shaaban AM. Fallopian tube disease in the nonpregnant patient. Radiographics. 2011;31(2):527−548.
- Laing FC, Allison SF. US of the ovary and adnexa: to worry or not to worry? Radiographics. 2012:32(6):1621−1639.
- Moyle PL, Kataoka MY, Nakai A, Takahata A, Reinhold C, Sala E. Nonovarian cystic lesions of the pelvis. Radiographics. 2010;30(4):921−938.
- Rezvani M, Shaaban AM. Fallopian tube disease in the nonpregnant patient. Radiographics. 2011;31(2):527−548.
A 32-year-old women presents to her gynecologist’s office reporting pelvic pain and irregular menstrual periods. Results of a urine pregnancy test are negative. Pelvic ultrasonography is performed, with gray scale ( A ) and color Doppler ( B ) images of the left adnexa obtained. Figures shown above.
2-week left-sided pelvic pain
(A) Simple ovarian cyst INCORRECT
Here is an example of a well circumscribed round or oval anechoic, avascular simple ovarian cyst with posterior acoustic enhancement and thin smooth walls.1 No septations or solid components are identified.
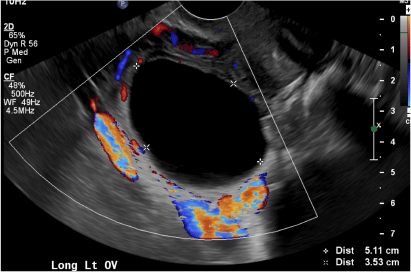
(B) Hemorrhagic cyst CORRECT
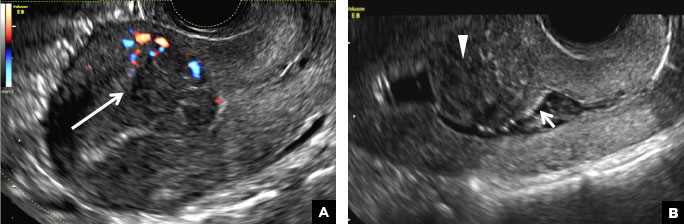
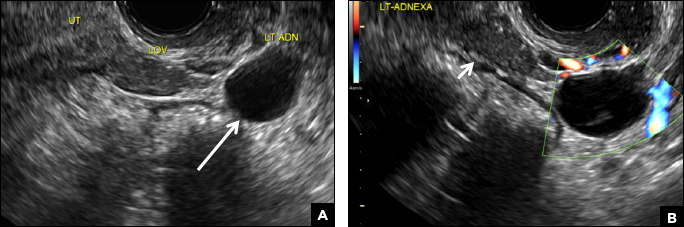
This type of cyst is well circumscribed and hypoechoic, with posterior acoustic enhancement and demonstrates a lacy reticular pattern of internal echoes due to fibrin strands (long arrow). The internal echoes also may be solid appearing with concave margins (short arrow) due to retractile hemorrhagic clot.1 The absence of internal vascular flow on color Doppler helps differentiate it from the solid components seen in ovarian neoplasm.
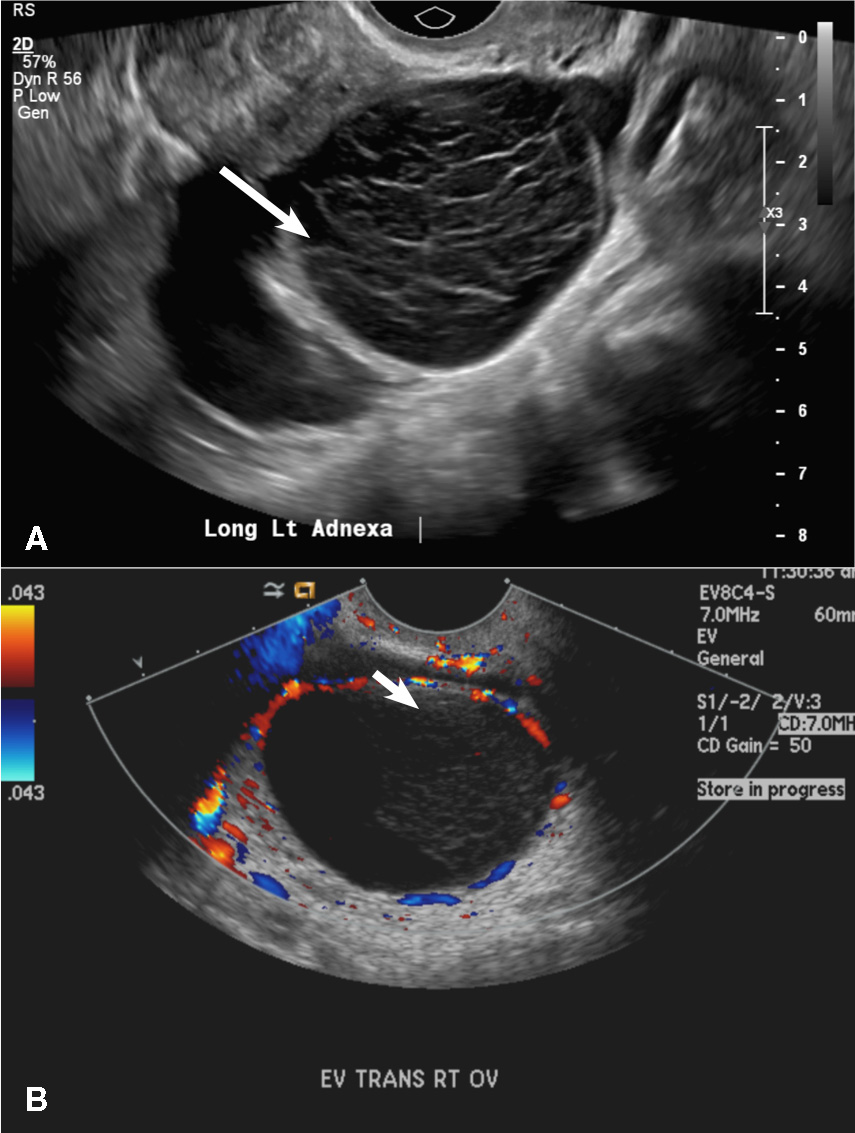
(C) Endometrioma INCORRECT
This mass is a well-circumscribed hypoechoic cyst with homogeneous ground glass or low level echoes and increased through transmission.1 It is also avascular without solid components.
(D) Dermoid INCORRECT
This common benign ovarian tumor has varying appearances. The most common appearance is a cystic lesion with a focal echogenic nodule (long arrow) protruding into the cyst (Rokitansky nodule).2 The second most common appearance is a focal or diffuse hyperechoic mass with areas of acoustic shadowing (arrowhead) from the sebaceous material and hair (tip of the iceberg sign). A third common appearance is a cystic lesion with multiple thin echogenic bands (lines and dots), which are hair floating within the cyst (short arrow). There is no internal vascular flow identified.

(E) Cystic ovarian neoplasm INCORRECT
These are large complex masses with both cystic and solid components demonstrating internal vascular flow. They usually demonstrate a thick irregular wall, multiple septations, and nodular papillary projections.3
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2010;256:(3):943−954.
- Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475−490.
- Wasnik AP, Menias CO, Platt JF, Lalchandani UR, Bedi DG, Elsayes KM. Multimodality imaging of ovarian cystic lesions: review with an imaging based algorithmic approach. World J Radiol. 2013;5(3):113−125.
(A) Simple ovarian cyst INCORRECT
Here is an example of a well circumscribed round or oval anechoic, avascular simple ovarian cyst with posterior acoustic enhancement and thin smooth walls.1 No septations or solid components are identified.

(B) Hemorrhagic cyst CORRECT
This type of cyst is well circumscribed and hypoechoic, with posterior acoustic enhancement and demonstrates a lacy reticular pattern of internal echoes due to fibrin strands (long arrow). The internal echoes also may be solid appearing with concave margins (short arrow) due to retractile hemorrhagic clot.1 The absence of internal vascular flow on color Doppler helps differentiate it from the solid components seen in ovarian neoplasm.

(C) Endometrioma INCORRECT
This mass is a well-circumscribed hypoechoic cyst with homogeneous ground glass or low level echoes and increased through transmission.1 It is also avascular without solid components.

(D) Dermoid INCORRECT
This common benign ovarian tumor has varying appearances. The most common appearance is a cystic lesion with a focal echogenic nodule (long arrow) protruding into the cyst (Rokitansky nodule).2 The second most common appearance is a focal or diffuse hyperechoic mass with areas of acoustic shadowing (arrowhead) from the sebaceous material and hair (tip of the iceberg sign). A third common appearance is a cystic lesion with multiple thin echogenic bands (lines and dots), which are hair floating within the cyst (short arrow). There is no internal vascular flow identified.

(E) Cystic ovarian neoplasm INCORRECT
These are large complex masses with both cystic and solid components demonstrating internal vascular flow. They usually demonstrate a thick irregular wall, multiple septations, and nodular papillary projections.3

(A) Simple ovarian cyst INCORRECT
Here is an example of a well circumscribed round or oval anechoic, avascular simple ovarian cyst with posterior acoustic enhancement and thin smooth walls.1 No septations or solid components are identified.

(B) Hemorrhagic cyst CORRECT
This type of cyst is well circumscribed and hypoechoic, with posterior acoustic enhancement and demonstrates a lacy reticular pattern of internal echoes due to fibrin strands (long arrow). The internal echoes also may be solid appearing with concave margins (short arrow) due to retractile hemorrhagic clot.1 The absence of internal vascular flow on color Doppler helps differentiate it from the solid components seen in ovarian neoplasm.

(C) Endometrioma INCORRECT
This mass is a well-circumscribed hypoechoic cyst with homogeneous ground glass or low level echoes and increased through transmission.1 It is also avascular without solid components.

(D) Dermoid INCORRECT
This common benign ovarian tumor has varying appearances. The most common appearance is a cystic lesion with a focal echogenic nodule (long arrow) protruding into the cyst (Rokitansky nodule).2 The second most common appearance is a focal or diffuse hyperechoic mass with areas of acoustic shadowing (arrowhead) from the sebaceous material and hair (tip of the iceberg sign). A third common appearance is a cystic lesion with multiple thin echogenic bands (lines and dots), which are hair floating within the cyst (short arrow). There is no internal vascular flow identified.

(E) Cystic ovarian neoplasm INCORRECT
These are large complex masses with both cystic and solid components demonstrating internal vascular flow. They usually demonstrate a thick irregular wall, multiple septations, and nodular papillary projections.3

- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2010;256:(3):943−954.
- Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475−490.
- Wasnik AP, Menias CO, Platt JF, Lalchandani UR, Bedi DG, Elsayes KM. Multimodality imaging of ovarian cystic lesions: review with an imaging based algorithmic approach. World J Radiol. 2013;5(3):113−125.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2010;256:(3):943−954.
- Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475−490.
- Wasnik AP, Menias CO, Platt JF, Lalchandani UR, Bedi DG, Elsayes KM. Multimodality imaging of ovarian cystic lesions: review with an imaging based algorithmic approach. World J Radiol. 2013;5(3):113−125.
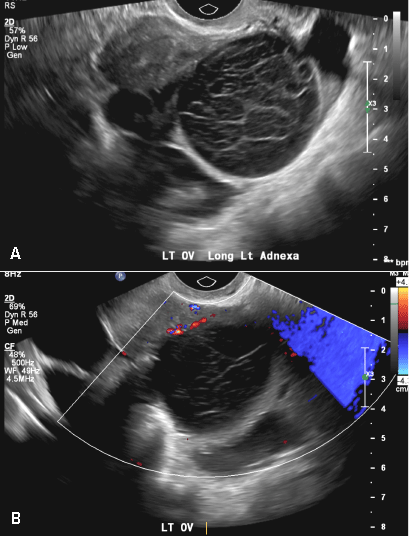
A 37-year-old woman presents to the emergency department reporting left-sided pelvic pain for 2 weeks duration. She has a negative urine pregnancy test. Pelvic ultrasonography of the left adnexa is performed with gray scale (A) and color Doppler images (B). Figures shown above.