User login
BEST PRACTICES IN: Extended-Regimen Oral Contraception:Modifying the Hormone-Free Interval
A supplement to Ob.Gyn. News. This supplement was sponsored by TEVA Women's Health.
•Topics
•Faculty/Faculty Disclosure

To view the supplement, click the image above.
Topics
• History of the Hormone-Free Interval
• Physiologic Effects of a Modified Hormone-Free Interval
• Safety and Efficacy of Extended-Regimen Oral Contraception
Faculty/Faculty Disclosure
David J. Portman, MD
Director and Principal Investigator
Columbus Center for Women's
Health Research
Columbus, Ohio
Dr. Portman is a consultant to Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim GmbH, GlaxoSmithKline plc, and TEVA Women's Health. He has received funding for clinical grants from Bayer, Boehringer Ingelheim, Depomed, Inc., Pfizer Inc., TEVA Women's Health, and Warner Chilcott.
A supplement to Ob.Gyn. News. This supplement was sponsored by TEVA Women's Health.
•Topics
•Faculty/Faculty Disclosure

To view the supplement, click the image above.
Topics
• History of the Hormone-Free Interval
• Physiologic Effects of a Modified Hormone-Free Interval
• Safety and Efficacy of Extended-Regimen Oral Contraception
Faculty/Faculty Disclosure
David J. Portman, MD
Director and Principal Investigator
Columbus Center for Women's
Health Research
Columbus, Ohio
Dr. Portman is a consultant to Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim GmbH, GlaxoSmithKline plc, and TEVA Women's Health. He has received funding for clinical grants from Bayer, Boehringer Ingelheim, Depomed, Inc., Pfizer Inc., TEVA Women's Health, and Warner Chilcott.
A supplement to Ob.Gyn. News. This supplement was sponsored by TEVA Women's Health.
•Topics
•Faculty/Faculty Disclosure

To view the supplement, click the image above.
Topics
• History of the Hormone-Free Interval
• Physiologic Effects of a Modified Hormone-Free Interval
• Safety and Efficacy of Extended-Regimen Oral Contraception
Faculty/Faculty Disclosure
David J. Portman, MD
Director and Principal Investigator
Columbus Center for Women's
Health Research
Columbus, Ohio
Dr. Portman is a consultant to Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim GmbH, GlaxoSmithKline plc, and TEVA Women's Health. He has received funding for clinical grants from Bayer, Boehringer Ingelheim, Depomed, Inc., Pfizer Inc., TEVA Women's Health, and Warner Chilcott.
Clinical Perspectives on the Role of Hormone Therapy in Menopausal Management
A supplement to Ob.Gyn. News.
This supplement is based on physician interviews. It is supported by Kenwood Therapeutics, a division of Bradley Pharmaceuticals, Inc.

To view the supplement, click the image above.
Topic Highlights
• Menopause and Hormone Therapy
• Current Recommendations for Postmenopausal Hormone Therapy
• Clinical Trial of Transdermal Estrogen
• Other Considerations of Transdermal Hormone Therapy
Faculty/Faculty Disclosures
Copyright © 2007 by Elsevier Inc.
A supplement to Ob.Gyn. News.
This supplement is based on physician interviews. It is supported by Kenwood Therapeutics, a division of Bradley Pharmaceuticals, Inc.

To view the supplement, click the image above.
Topic Highlights
• Menopause and Hormone Therapy
• Current Recommendations for Postmenopausal Hormone Therapy
• Clinical Trial of Transdermal Estrogen
• Other Considerations of Transdermal Hormone Therapy
Faculty/Faculty Disclosures
Copyright © 2007 by Elsevier Inc.
A supplement to Ob.Gyn. News.
This supplement is based on physician interviews. It is supported by Kenwood Therapeutics, a division of Bradley Pharmaceuticals, Inc.

To view the supplement, click the image above.
Topic Highlights
• Menopause and Hormone Therapy
• Current Recommendations for Postmenopausal Hormone Therapy
• Clinical Trial of Transdermal Estrogen
• Other Considerations of Transdermal Hormone Therapy
Faculty/Faculty Disclosures
Copyright © 2007 by Elsevier Inc.
BEST PRACTICES IN: Prenatal Screening & Cord Blood Banking
A supplement to Ob.Gyn. News. This supplement was sponsored by PerkinElmer.
•Topics
•Faculty/Faculty Disclosure

To view the supplement, click the image above.
Topics
• Introduction
• Screening for Fetal Chromosomal Abnormalities
• UCB Banking
• Conclusions
Faculty/Faculty Disclosure
Joanne Stone, MD
Director, Perinatal Ultrasound
Division Director, Maternal Fetal Medicine
Department of Obstetrics, Gynecology, and Reproductive Medicine
Mt. Sinai School of Medicine
New York, NY
University of California, San Diego
La Jolla, California
Dr. Stone has nothing to disclose.
Copyright © 2009 by Elsevier Inc.
A supplement to Ob.Gyn. News. This supplement was sponsored by PerkinElmer.
•Topics
•Faculty/Faculty Disclosure

To view the supplement, click the image above.
Topics
• Introduction
• Screening for Fetal Chromosomal Abnormalities
• UCB Banking
• Conclusions
Faculty/Faculty Disclosure
Joanne Stone, MD
Director, Perinatal Ultrasound
Division Director, Maternal Fetal Medicine
Department of Obstetrics, Gynecology, and Reproductive Medicine
Mt. Sinai School of Medicine
New York, NY
University of California, San Diego
La Jolla, California
Dr. Stone has nothing to disclose.
Copyright © 2009 by Elsevier Inc.
A supplement to Ob.Gyn. News. This supplement was sponsored by PerkinElmer.
•Topics
•Faculty/Faculty Disclosure

To view the supplement, click the image above.
Topics
• Introduction
• Screening for Fetal Chromosomal Abnormalities
• UCB Banking
• Conclusions
Faculty/Faculty Disclosure
Joanne Stone, MD
Director, Perinatal Ultrasound
Division Director, Maternal Fetal Medicine
Department of Obstetrics, Gynecology, and Reproductive Medicine
Mt. Sinai School of Medicine
New York, NY
University of California, San Diego
La Jolla, California
Dr. Stone has nothing to disclose.
Copyright © 2009 by Elsevier Inc.
Cervical Cancer Screening in the Era of Improved Technology and HPV Vaccines
A supplement to Ob.Gyn. News.
This supplement was supported by CYTYC Corporation.
The articles are based on interviews with the faculty.

To view the supplement, click the image above.
Topic Highlights/Faculty
Introduction
Mark H. Einstein, MD, MS, Chair
Director, Clinical Research
Division of Gynecologic Oncology
Department of Obstetrics and Gynecology and Women's Health
Montefiore Medical Center
Bronx, N.Y.
Dr Einstein has received clinical grants from GlaxoSmithKline, Merck & Co., Inc., and Tigris Pharmaceuticals, Inc.
Milestones in Cervical Cancer Detection and Prevention: Significance in Clinical Practice
Mark H. Einstein, MD, MS, Chair
Improving HSIL and Glandular Disease Detection: What the Recent Data Show
Richard Lozano, MD
Director of Cytology
Pathology and Cytology Laboratories, Inc.
Lexington, Ky.
Dr Lozano has nothing to disclose.
and
Harold J. Sauer, MD, FACOG
Associate Professor and Acting Chair
Department of Obstetrics and Gynecology and Reproductive Biology
Michigan State University
Lansing
Dr Sauer has nothing to disclose.
Weighing the Costs and Benefits: Technologic Advances in Cervical Cancer Screening
Warner K. Huh, MD, FACOG, FACS
Assistant Professor
Division of Gynecologic Oncology
University of Alabama at Birmingham
Dr Huh has received clinical grants from 3M Pharmaceuticals, Cytyc Corporation, GlaxoSmithKline, MGI PHARMA, Merck, Roche Molecular Systems, and Tigris Pharmaceuticals. He is a consultant to GlaxoSmithKline, MGI PHARMA, Roche Molecular Systems, and mtm laboratories AG.
Efficacy of HPV Screening Versus Liquid-Based Cervical Cytology and Imaging: What the Data Really Show
Michael Karram, MD, FACOG
President and Medical Director
Seven Hills Women's Health Centers
Cincinnati, Ohio
Dr Karram has nothing to disclose.
and
Michael L. Krychman, MD
Associate Clinical Attending
Sexual Medicine Program
Memorial Sloan-Kettering Cancer Center
New York, N.Y.
Dr Krychman is a consultant to Cytyc.
Copyright © 2007 by Elsevier Inc.
A supplement to Ob.Gyn. News.
This supplement was supported by CYTYC Corporation.
The articles are based on interviews with the faculty.

To view the supplement, click the image above.
Topic Highlights/Faculty
Introduction
Mark H. Einstein, MD, MS, Chair
Director, Clinical Research
Division of Gynecologic Oncology
Department of Obstetrics and Gynecology and Women's Health
Montefiore Medical Center
Bronx, N.Y.
Dr Einstein has received clinical grants from GlaxoSmithKline, Merck & Co., Inc., and Tigris Pharmaceuticals, Inc.
Milestones in Cervical Cancer Detection and Prevention: Significance in Clinical Practice
Mark H. Einstein, MD, MS, Chair
Improving HSIL and Glandular Disease Detection: What the Recent Data Show
Richard Lozano, MD
Director of Cytology
Pathology and Cytology Laboratories, Inc.
Lexington, Ky.
Dr Lozano has nothing to disclose.
and
Harold J. Sauer, MD, FACOG
Associate Professor and Acting Chair
Department of Obstetrics and Gynecology and Reproductive Biology
Michigan State University
Lansing
Dr Sauer has nothing to disclose.
Weighing the Costs and Benefits: Technologic Advances in Cervical Cancer Screening
Warner K. Huh, MD, FACOG, FACS
Assistant Professor
Division of Gynecologic Oncology
University of Alabama at Birmingham
Dr Huh has received clinical grants from 3M Pharmaceuticals, Cytyc Corporation, GlaxoSmithKline, MGI PHARMA, Merck, Roche Molecular Systems, and Tigris Pharmaceuticals. He is a consultant to GlaxoSmithKline, MGI PHARMA, Roche Molecular Systems, and mtm laboratories AG.
Efficacy of HPV Screening Versus Liquid-Based Cervical Cytology and Imaging: What the Data Really Show
Michael Karram, MD, FACOG
President and Medical Director
Seven Hills Women's Health Centers
Cincinnati, Ohio
Dr Karram has nothing to disclose.
and
Michael L. Krychman, MD
Associate Clinical Attending
Sexual Medicine Program
Memorial Sloan-Kettering Cancer Center
New York, N.Y.
Dr Krychman is a consultant to Cytyc.
Copyright © 2007 by Elsevier Inc.
A supplement to Ob.Gyn. News.
This supplement was supported by CYTYC Corporation.
The articles are based on interviews with the faculty.

To view the supplement, click the image above.
Topic Highlights/Faculty
Introduction
Mark H. Einstein, MD, MS, Chair
Director, Clinical Research
Division of Gynecologic Oncology
Department of Obstetrics and Gynecology and Women's Health
Montefiore Medical Center
Bronx, N.Y.
Dr Einstein has received clinical grants from GlaxoSmithKline, Merck & Co., Inc., and Tigris Pharmaceuticals, Inc.
Milestones in Cervical Cancer Detection and Prevention: Significance in Clinical Practice
Mark H. Einstein, MD, MS, Chair
Improving HSIL and Glandular Disease Detection: What the Recent Data Show
Richard Lozano, MD
Director of Cytology
Pathology and Cytology Laboratories, Inc.
Lexington, Ky.
Dr Lozano has nothing to disclose.
and
Harold J. Sauer, MD, FACOG
Associate Professor and Acting Chair
Department of Obstetrics and Gynecology and Reproductive Biology
Michigan State University
Lansing
Dr Sauer has nothing to disclose.
Weighing the Costs and Benefits: Technologic Advances in Cervical Cancer Screening
Warner K. Huh, MD, FACOG, FACS
Assistant Professor
Division of Gynecologic Oncology
University of Alabama at Birmingham
Dr Huh has received clinical grants from 3M Pharmaceuticals, Cytyc Corporation, GlaxoSmithKline, MGI PHARMA, Merck, Roche Molecular Systems, and Tigris Pharmaceuticals. He is a consultant to GlaxoSmithKline, MGI PHARMA, Roche Molecular Systems, and mtm laboratories AG.
Efficacy of HPV Screening Versus Liquid-Based Cervical Cytology and Imaging: What the Data Really Show
Michael Karram, MD, FACOG
President and Medical Director
Seven Hills Women's Health Centers
Cincinnati, Ohio
Dr Karram has nothing to disclose.
and
Michael L. Krychman, MD
Associate Clinical Attending
Sexual Medicine Program
Memorial Sloan-Kettering Cancer Center
New York, N.Y.
Dr Krychman is a consultant to Cytyc.
Copyright © 2007 by Elsevier Inc.
CLINICAL UPDATE: Women's Health and Nutrition: Demographic Challenges
A supplement to Ob.Gyn. News.
This supplement is supported by Xanodyne Pharmaceuticals, Inc.
•Topic Highlights
•Faculty/Faculty Disclosures

To view the supplement, click the image above.
Topic Highlights
• Nutritional Gaps for Women in the United States
• Role of Obstetricians and Gynecologists in Women's Health Care
Faculty/Faculty Disclosures
Linda D. Bradley MD
Vice Chair, Obstetrics
Gynecology & Women's Health Institute
Cleveland Clinic
Cleveland, OH
Dr. Bradley is a consultant for Xanodyne Pharmaceuticals, Inc.
Beth Reardon, MS, RD, LDN
Integrative Nutritionist
Duke Integrative Nutrition
Durham, NC
Dr. Reardon has nothing to disclose.
John M. Thorp, Jr., MD
McAllister Distinguished Professor
Department of Obstetrics and Gynecology
University of North Carolina at Chapel Hill
Chapel Hill, NC
Dr. Thorp has nothing to disclose.
Barbara A. Underwood, PhD
Adjunct Professor of Nutrition
Columbia University
Institute of Human Nutrition
New York, NY
Dr. Underwood has nothing to disclose.
Fernando E. Viteri, MD, ScD
Professor (Emeritus)
Department of Nutritional Sciences and Toxicology
University of California
Berkeley, CA
and
Scientist
Children's Hospital
Oakland Research Institute
Oakland, CA
Dr. Viteri has received clinical grant funding from the University of California Institute for Mexico and the United States.
Copyright © 2009 by Elsevier Inc.
A supplement to Ob.Gyn. News.
This supplement is supported by Xanodyne Pharmaceuticals, Inc.
•Topic Highlights
•Faculty/Faculty Disclosures

To view the supplement, click the image above.
Topic Highlights
• Nutritional Gaps for Women in the United States
• Role of Obstetricians and Gynecologists in Women's Health Care
Faculty/Faculty Disclosures
Linda D. Bradley MD
Vice Chair, Obstetrics
Gynecology & Women's Health Institute
Cleveland Clinic
Cleveland, OH
Dr. Bradley is a consultant for Xanodyne Pharmaceuticals, Inc.
Beth Reardon, MS, RD, LDN
Integrative Nutritionist
Duke Integrative Nutrition
Durham, NC
Dr. Reardon has nothing to disclose.
John M. Thorp, Jr., MD
McAllister Distinguished Professor
Department of Obstetrics and Gynecology
University of North Carolina at Chapel Hill
Chapel Hill, NC
Dr. Thorp has nothing to disclose.
Barbara A. Underwood, PhD
Adjunct Professor of Nutrition
Columbia University
Institute of Human Nutrition
New York, NY
Dr. Underwood has nothing to disclose.
Fernando E. Viteri, MD, ScD
Professor (Emeritus)
Department of Nutritional Sciences and Toxicology
University of California
Berkeley, CA
and
Scientist
Children's Hospital
Oakland Research Institute
Oakland, CA
Dr. Viteri has received clinical grant funding from the University of California Institute for Mexico and the United States.
Copyright © 2009 by Elsevier Inc.
A supplement to Ob.Gyn. News.
This supplement is supported by Xanodyne Pharmaceuticals, Inc.
•Topic Highlights
•Faculty/Faculty Disclosures

To view the supplement, click the image above.
Topic Highlights
• Nutritional Gaps for Women in the United States
• Role of Obstetricians and Gynecologists in Women's Health Care
Faculty/Faculty Disclosures
Linda D. Bradley MD
Vice Chair, Obstetrics
Gynecology & Women's Health Institute
Cleveland Clinic
Cleveland, OH
Dr. Bradley is a consultant for Xanodyne Pharmaceuticals, Inc.
Beth Reardon, MS, RD, LDN
Integrative Nutritionist
Duke Integrative Nutrition
Durham, NC
Dr. Reardon has nothing to disclose.
John M. Thorp, Jr., MD
McAllister Distinguished Professor
Department of Obstetrics and Gynecology
University of North Carolina at Chapel Hill
Chapel Hill, NC
Dr. Thorp has nothing to disclose.
Barbara A. Underwood, PhD
Adjunct Professor of Nutrition
Columbia University
Institute of Human Nutrition
New York, NY
Dr. Underwood has nothing to disclose.
Fernando E. Viteri, MD, ScD
Professor (Emeritus)
Department of Nutritional Sciences and Toxicology
University of California
Berkeley, CA
and
Scientist
Children's Hospital
Oakland Research Institute
Oakland, CA
Dr. Viteri has received clinical grant funding from the University of California Institute for Mexico and the United States.
Copyright © 2009 by Elsevier Inc.
The Changing Landscape of Cervical Cancer Screening and Implications for the Clinician
A supplement to Ob.Gyn. News.
This educational supplement was supported by an educational grant from CYTYC Corporation.
The articles are based on clinical dialogues with the faculty.

To view the supplement, click the image above.
Topic Highlights/Faculty
Implications of Computer-Assisted Cervical Screening for the Ob.Gyn. Clinician
Co-Chairs:
Randall K. Gibb, MD
Assistant Professor, Division of Gynecologic Oncology
Washington University School of Medicine
St. Louis, Mo.
Thomas J. Herzog, MD
Director, Division of Gynecologic Oncology
Columbia University College of Physicians and Surgeons
New York, N.Y.
Comparison of Manual and Image-Directed Screening of Liquid-Based Cervical Cytology in a Large Metropolitan Cytology Practice
James R. Lingle, MD
Lingle, Gore, and Harding, P.C.
Englewood, Colo.
Fern S. Miller, MSN, CT(ASCP)
Cytology Manager, Cytology Department
Metropolitan Pathologists
Denver, Colo.
Performance of a Computer-Assisted Imaging System in Detecting High-Grade Squamous Intraepithelial Lesions
Bruce R. Dziura, MD
Chief of Pathology
New England Pathology Associates
Mercy Medical Center
Springfield, Mass
Timothy Kelly Fitzpatrick, MD
Attending Physician
Mercy Medical Center
Springfield, Mass.
Evaluation of a Computer-Assisted Imaging System in Diagnosing Uncommon Malignancies
Andrea E. Dawson, MD
Staff Pathologist
Cleveland Clinic Foundation
Cleveland, Ohio
Holly L. Thacker, MD
Director, Women's Health Center
Cleveland Clinic Foundation
Cleveland, Ohio
The faculty report they have nothing to disclose.
Copyright © 2005 by International Medical News Group
A supplement to Ob.Gyn. News.
This educational supplement was supported by an educational grant from CYTYC Corporation.
The articles are based on clinical dialogues with the faculty.

To view the supplement, click the image above.
Topic Highlights/Faculty
Implications of Computer-Assisted Cervical Screening for the Ob.Gyn. Clinician
Co-Chairs:
Randall K. Gibb, MD
Assistant Professor, Division of Gynecologic Oncology
Washington University School of Medicine
St. Louis, Mo.
Thomas J. Herzog, MD
Director, Division of Gynecologic Oncology
Columbia University College of Physicians and Surgeons
New York, N.Y.
Comparison of Manual and Image-Directed Screening of Liquid-Based Cervical Cytology in a Large Metropolitan Cytology Practice
James R. Lingle, MD
Lingle, Gore, and Harding, P.C.
Englewood, Colo.
Fern S. Miller, MSN, CT(ASCP)
Cytology Manager, Cytology Department
Metropolitan Pathologists
Denver, Colo.
Performance of a Computer-Assisted Imaging System in Detecting High-Grade Squamous Intraepithelial Lesions
Bruce R. Dziura, MD
Chief of Pathology
New England Pathology Associates
Mercy Medical Center
Springfield, Mass
Timothy Kelly Fitzpatrick, MD
Attending Physician
Mercy Medical Center
Springfield, Mass.
Evaluation of a Computer-Assisted Imaging System in Diagnosing Uncommon Malignancies
Andrea E. Dawson, MD
Staff Pathologist
Cleveland Clinic Foundation
Cleveland, Ohio
Holly L. Thacker, MD
Director, Women's Health Center
Cleveland Clinic Foundation
Cleveland, Ohio
The faculty report they have nothing to disclose.
Copyright © 2005 by International Medical News Group
A supplement to Ob.Gyn. News.
This educational supplement was supported by an educational grant from CYTYC Corporation.
The articles are based on clinical dialogues with the faculty.

To view the supplement, click the image above.
Topic Highlights/Faculty
Implications of Computer-Assisted Cervical Screening for the Ob.Gyn. Clinician
Co-Chairs:
Randall K. Gibb, MD
Assistant Professor, Division of Gynecologic Oncology
Washington University School of Medicine
St. Louis, Mo.
Thomas J. Herzog, MD
Director, Division of Gynecologic Oncology
Columbia University College of Physicians and Surgeons
New York, N.Y.
Comparison of Manual and Image-Directed Screening of Liquid-Based Cervical Cytology in a Large Metropolitan Cytology Practice
James R. Lingle, MD
Lingle, Gore, and Harding, P.C.
Englewood, Colo.
Fern S. Miller, MSN, CT(ASCP)
Cytology Manager, Cytology Department
Metropolitan Pathologists
Denver, Colo.
Performance of a Computer-Assisted Imaging System in Detecting High-Grade Squamous Intraepithelial Lesions
Bruce R. Dziura, MD
Chief of Pathology
New England Pathology Associates
Mercy Medical Center
Springfield, Mass
Timothy Kelly Fitzpatrick, MD
Attending Physician
Mercy Medical Center
Springfield, Mass.
Evaluation of a Computer-Assisted Imaging System in Diagnosing Uncommon Malignancies
Andrea E. Dawson, MD
Staff Pathologist
Cleveland Clinic Foundation
Cleveland, Ohio
Holly L. Thacker, MD
Director, Women's Health Center
Cleveland Clinic Foundation
Cleveland, Ohio
The faculty report they have nothing to disclose.
Copyright © 2005 by International Medical News Group
Chronic Dysfunctional Uterine Bleeding: Identifying Patients and Helping Them Understand Their Treatment Options
A supplement to Ob.Gyn. News.
Supported by an educational grant from Gynecare Worldwide, a division of Ethicon, Inc., a Johnson & Johnson Company.
The articles in this supplement are based on clinical dialogues with the faculty.
•Contents
•Faculty/Faculty Disclosure Statement

To view the supplement, click the image above.
Contents
Introduction
Consequences of Heavy Menstrual Bleeding
Types, Patterns, and Causes of Abnormal Uterine Bleeding
• Evaluating the Endometrial Cavity
Treatment Options: Entering the Dialogue
• Medical Therapy
• Surgical Interventions
• Endometrial Ablation Procedures
Considering Cases:
• An Overweight Patient
• A Patient Who Prefers to Avoid Hysterectomy
• A Patient With Postsurgical HMB
Helping Patients Choose
Conclusion
Faculty/Faculty Disclosure Statement
Mary Jane Minkin, MD, FACOG, Chair
Clinical Professor
Department of Obstetrics and Gynecology
Yale University School of Medicine
New Haven, Conn.
Developed a Web site for Gynecare; Speaker's Bureau: Berlex, Inc.
Charles E. Miller, MD, FACOG
Clinical Associate Professor
Department of Obstetrics and Gynecology
University of Illinois at Chicago
Clinical Associate
Department of Obstetrics and Gynecology
University of Chicago
Consultant: Gynecare Worldwide.
Malcolm G. Munro, MD, FRCS(c), FACOG
Professor
Department of Obstetrics and Gynecology
The David Geffen School of Medicine at UCLA
Los Angeles
Attending Staff
Department of Obstetrics and Gynecology
Kaiser Permanente Los Angeles Medical Center
Received Funding for Clinical Grants: Kaiser Research Foundation and Karl Storz Endoscopy-America, Inc.M
Consultant: Boston Scientific Corporation, Gynecare, and Karl Storz Endoscopy.
Robert K. Zurawin, MD, FACOG
Associate Professor
Department of Obstetrics and Gynecology
Baylor College of Medicine
Houston
Consultant/Speaker: Gynecare Worldwide.
Copyright © 2004 by International Medical News Group
A supplement to Ob.Gyn. News.
Supported by an educational grant from Gynecare Worldwide, a division of Ethicon, Inc., a Johnson & Johnson Company.
The articles in this supplement are based on clinical dialogues with the faculty.
•Contents
•Faculty/Faculty Disclosure Statement

To view the supplement, click the image above.
Contents
Introduction
Consequences of Heavy Menstrual Bleeding
Types, Patterns, and Causes of Abnormal Uterine Bleeding
• Evaluating the Endometrial Cavity
Treatment Options: Entering the Dialogue
• Medical Therapy
• Surgical Interventions
• Endometrial Ablation Procedures
Considering Cases:
• An Overweight Patient
• A Patient Who Prefers to Avoid Hysterectomy
• A Patient With Postsurgical HMB
Helping Patients Choose
Conclusion
Faculty/Faculty Disclosure Statement
Mary Jane Minkin, MD, FACOG, Chair
Clinical Professor
Department of Obstetrics and Gynecology
Yale University School of Medicine
New Haven, Conn.
Developed a Web site for Gynecare; Speaker's Bureau: Berlex, Inc.
Charles E. Miller, MD, FACOG
Clinical Associate Professor
Department of Obstetrics and Gynecology
University of Illinois at Chicago
Clinical Associate
Department of Obstetrics and Gynecology
University of Chicago
Consultant: Gynecare Worldwide.
Malcolm G. Munro, MD, FRCS(c), FACOG
Professor
Department of Obstetrics and Gynecology
The David Geffen School of Medicine at UCLA
Los Angeles
Attending Staff
Department of Obstetrics and Gynecology
Kaiser Permanente Los Angeles Medical Center
Received Funding for Clinical Grants: Kaiser Research Foundation and Karl Storz Endoscopy-America, Inc.M
Consultant: Boston Scientific Corporation, Gynecare, and Karl Storz Endoscopy.
Robert K. Zurawin, MD, FACOG
Associate Professor
Department of Obstetrics and Gynecology
Baylor College of Medicine
Houston
Consultant/Speaker: Gynecare Worldwide.
Copyright © 2004 by International Medical News Group
A supplement to Ob.Gyn. News.
Supported by an educational grant from Gynecare Worldwide, a division of Ethicon, Inc., a Johnson & Johnson Company.
The articles in this supplement are based on clinical dialogues with the faculty.
•Contents
•Faculty/Faculty Disclosure Statement

To view the supplement, click the image above.
Contents
Introduction
Consequences of Heavy Menstrual Bleeding
Types, Patterns, and Causes of Abnormal Uterine Bleeding
• Evaluating the Endometrial Cavity
Treatment Options: Entering the Dialogue
• Medical Therapy
• Surgical Interventions
• Endometrial Ablation Procedures
Considering Cases:
• An Overweight Patient
• A Patient Who Prefers to Avoid Hysterectomy
• A Patient With Postsurgical HMB
Helping Patients Choose
Conclusion
Faculty/Faculty Disclosure Statement
Mary Jane Minkin, MD, FACOG, Chair
Clinical Professor
Department of Obstetrics and Gynecology
Yale University School of Medicine
New Haven, Conn.
Developed a Web site for Gynecare; Speaker's Bureau: Berlex, Inc.
Charles E. Miller, MD, FACOG
Clinical Associate Professor
Department of Obstetrics and Gynecology
University of Illinois at Chicago
Clinical Associate
Department of Obstetrics and Gynecology
University of Chicago
Consultant: Gynecare Worldwide.
Malcolm G. Munro, MD, FRCS(c), FACOG
Professor
Department of Obstetrics and Gynecology
The David Geffen School of Medicine at UCLA
Los Angeles
Attending Staff
Department of Obstetrics and Gynecology
Kaiser Permanente Los Angeles Medical Center
Received Funding for Clinical Grants: Kaiser Research Foundation and Karl Storz Endoscopy-America, Inc.M
Consultant: Boston Scientific Corporation, Gynecare, and Karl Storz Endoscopy.
Robert K. Zurawin, MD, FACOG
Associate Professor
Department of Obstetrics and Gynecology
Baylor College of Medicine
Houston
Consultant/Speaker: Gynecare Worldwide.
Copyright © 2004 by International Medical News Group
A Practical Update on Sexually Transmitted Infections: Advances in Diagnosis and Treatment
A supplement to Ob. Gyn. News.
Supported by a restricted educational grant from 3M Pharmaceuticals.
Highlights of presentations that took place at a continuing medical education conference held April 5-6, 2003, Washington, DC.

To view the supplement, click the image above.
Contents/Faculty Disclosure
Introduction
Jack D. Sobel, MD
Professor, Chief
Division of Infectious Disease
Wayne State University School of Medicine
Harper Hospital
Detroit, MI
Clinical Grants: 3M Pharmaceuticals, Pfizer, and Ortho-McNeil. Discusses the investigational use of fluconazole and 17.4% topical flucytosine cream for treating Candida glabrata, and 10% hydrocortisone for treating erosive lichen planus.
Phillip G. Stubblefield, MD
Professor and Chairman, Obstetrics and Gynecology
Boston University School of Medicine
Director, Obstetrics and Gynecology
Boston Medical Center
Boston, MA
Nothing to disclose.
Jonathan M. Zenilman, MD
Professor, Infectious Diseases Division
Johns Hopkins School of Medicine
Baltimore, MD
Clinical Grants/Research: Osmetech PLC; Consultant: Merck and Company; Speaker's Bureau: GlaxoSmithKline, Pfizer, Inc.
Normal Vaginal Flora
Jeanne M. Marrazzo, MD, MPH
Assistant Professor, Department of Medicine
Division of Allergy and Infectious Diseases
University of Washington, Harborview Medical Center
Seattle, WA
Discusses the use of intravaginal Lactobacillus crispatus capsules for the treatment of bacterial vaginosis.
Diagnosis and Treatment of Routine and Resistant Trichomoniasis
Anne M. Rompalo, MD, ScM
Associate Professor, Infectious Diseases Division
Johns Hopkins School of Medicine
Baltimore, MD
Clinical Grants/Research: GlaxoSmithKline; Speaker's Bureau: GlaxoSmithKline, Pfizer, Inc.
New Findings in Routine and Recurrent Vulvovaginal Candidiasis Treatment
Jack D. Sobel, MD
Overview of Bacterial Vaginosis and Its Role in Upper Genital Tract Infection
David A. Eschenbach, MD
Professor and Director, Women's Health
Chair, Department of Obstetrics and Gynecology
University of Washington
Seattle, WA
Consultant: 3M Pharmaceuticals.
Noninfectious Vulvovaginal Symptoms as Manifestations of Systemic Diseases
Jack D. Sobel, MD
Copyright © 2003 by International Medical News Group
A supplement to Ob. Gyn. News.
Supported by a restricted educational grant from 3M Pharmaceuticals.
Highlights of presentations that took place at a continuing medical education conference held April 5-6, 2003, Washington, DC.

To view the supplement, click the image above.
Contents/Faculty Disclosure
Introduction
Jack D. Sobel, MD
Professor, Chief
Division of Infectious Disease
Wayne State University School of Medicine
Harper Hospital
Detroit, MI
Clinical Grants: 3M Pharmaceuticals, Pfizer, and Ortho-McNeil. Discusses the investigational use of fluconazole and 17.4% topical flucytosine cream for treating Candida glabrata, and 10% hydrocortisone for treating erosive lichen planus.
Phillip G. Stubblefield, MD
Professor and Chairman, Obstetrics and Gynecology
Boston University School of Medicine
Director, Obstetrics and Gynecology
Boston Medical Center
Boston, MA
Nothing to disclose.
Jonathan M. Zenilman, MD
Professor, Infectious Diseases Division
Johns Hopkins School of Medicine
Baltimore, MD
Clinical Grants/Research: Osmetech PLC; Consultant: Merck and Company; Speaker's Bureau: GlaxoSmithKline, Pfizer, Inc.
Normal Vaginal Flora
Jeanne M. Marrazzo, MD, MPH
Assistant Professor, Department of Medicine
Division of Allergy and Infectious Diseases
University of Washington, Harborview Medical Center
Seattle, WA
Discusses the use of intravaginal Lactobacillus crispatus capsules for the treatment of bacterial vaginosis.
Diagnosis and Treatment of Routine and Resistant Trichomoniasis
Anne M. Rompalo, MD, ScM
Associate Professor, Infectious Diseases Division
Johns Hopkins School of Medicine
Baltimore, MD
Clinical Grants/Research: GlaxoSmithKline; Speaker's Bureau: GlaxoSmithKline, Pfizer, Inc.
New Findings in Routine and Recurrent Vulvovaginal Candidiasis Treatment
Jack D. Sobel, MD
Overview of Bacterial Vaginosis and Its Role in Upper Genital Tract Infection
David A. Eschenbach, MD
Professor and Director, Women's Health
Chair, Department of Obstetrics and Gynecology
University of Washington
Seattle, WA
Consultant: 3M Pharmaceuticals.
Noninfectious Vulvovaginal Symptoms as Manifestations of Systemic Diseases
Jack D. Sobel, MD
Copyright © 2003 by International Medical News Group
A supplement to Ob. Gyn. News.
Supported by a restricted educational grant from 3M Pharmaceuticals.
Highlights of presentations that took place at a continuing medical education conference held April 5-6, 2003, Washington, DC.

To view the supplement, click the image above.
Contents/Faculty Disclosure
Introduction
Jack D. Sobel, MD
Professor, Chief
Division of Infectious Disease
Wayne State University School of Medicine
Harper Hospital
Detroit, MI
Clinical Grants: 3M Pharmaceuticals, Pfizer, and Ortho-McNeil. Discusses the investigational use of fluconazole and 17.4% topical flucytosine cream for treating Candida glabrata, and 10% hydrocortisone for treating erosive lichen planus.
Phillip G. Stubblefield, MD
Professor and Chairman, Obstetrics and Gynecology
Boston University School of Medicine
Director, Obstetrics and Gynecology
Boston Medical Center
Boston, MA
Nothing to disclose.
Jonathan M. Zenilman, MD
Professor, Infectious Diseases Division
Johns Hopkins School of Medicine
Baltimore, MD
Clinical Grants/Research: Osmetech PLC; Consultant: Merck and Company; Speaker's Bureau: GlaxoSmithKline, Pfizer, Inc.
Normal Vaginal Flora
Jeanne M. Marrazzo, MD, MPH
Assistant Professor, Department of Medicine
Division of Allergy and Infectious Diseases
University of Washington, Harborview Medical Center
Seattle, WA
Discusses the use of intravaginal Lactobacillus crispatus capsules for the treatment of bacterial vaginosis.
Diagnosis and Treatment of Routine and Resistant Trichomoniasis
Anne M. Rompalo, MD, ScM
Associate Professor, Infectious Diseases Division
Johns Hopkins School of Medicine
Baltimore, MD
Clinical Grants/Research: GlaxoSmithKline; Speaker's Bureau: GlaxoSmithKline, Pfizer, Inc.
New Findings in Routine and Recurrent Vulvovaginal Candidiasis Treatment
Jack D. Sobel, MD
Overview of Bacterial Vaginosis and Its Role in Upper Genital Tract Infection
David A. Eschenbach, MD
Professor and Director, Women's Health
Chair, Department of Obstetrics and Gynecology
University of Washington
Seattle, WA
Consultant: 3M Pharmaceuticals.
Noninfectious Vulvovaginal Symptoms as Manifestations of Systemic Diseases
Jack D. Sobel, MD
Copyright © 2003 by International Medical News Group
Clinical Update: Managing Women's Digestive Health
A supplement to Ob. Gyn. News.
This Clinical Update is supported by Braintree Laboratories, Inc.
The articles are based on interviews with the faculty.

To view the supplement, click the image above.
Topic Highlights
Rectal Anatomy Is a Key Factor in Constipation
Lawrence R. Schiller, MD, FACP, FACG
Program Director, Gastroenterology Fellowship
Department of Gastroenterology
Baylor University Medical Center
Dallas, TX
Consultant: Braintree Laboratories, Inc. and Novartis Pharmaceuticals.
Focus Evaluation of Constipation on Two Causes: Medication and Function
Jack A. DiPalma, MD, FACP, FACG
Professor and Director
Division of Gastroenterology
University of South Alabama College of Medicine
Mobile, AL
Clinical Grants: Braintree Laboratories, Inc. He discusses the unlabeled use of Polyethylene Glycol 3350 for chronic constipation.
Ob/Gyn: The Year in Review
GI Disorders Often Present to Gynecologists First
Christine L. Frissora, MD, FACG, FACP
Faculty Member, Division of Gastroenterology and Hepatology
The Weill Medical College of Cornell University
The New York-Presbyterian Hospital
New York, NY
Consultant: Novartis Pharmaceuticals and Vela Pharmaceuticals; Research Grants: Forest Laboratories.
Treat Constipation Before Gynecologic Surgery
Marie Fidela R. Paraiso, MD, FACOG
Director of Residency Education
Associate Fellowship Director, Urogynecology and Reconstructive Pelvic Surgery
Staff, Department of Obstetrics and Gynecology and the Urological Institute
The Cleveland Clinic Foundation
Cleveland, OH
Member of the Braintree Advisory Board.
Scientific Posters: Selected Summaries
Copyright © 2003 by International Medical News Group
A supplement to Ob. Gyn. News.
This Clinical Update is supported by Braintree Laboratories, Inc.
The articles are based on interviews with the faculty.

To view the supplement, click the image above.
Topic Highlights
Rectal Anatomy Is a Key Factor in Constipation
Lawrence R. Schiller, MD, FACP, FACG
Program Director, Gastroenterology Fellowship
Department of Gastroenterology
Baylor University Medical Center
Dallas, TX
Consultant: Braintree Laboratories, Inc. and Novartis Pharmaceuticals.
Focus Evaluation of Constipation on Two Causes: Medication and Function
Jack A. DiPalma, MD, FACP, FACG
Professor and Director
Division of Gastroenterology
University of South Alabama College of Medicine
Mobile, AL
Clinical Grants: Braintree Laboratories, Inc. He discusses the unlabeled use of Polyethylene Glycol 3350 for chronic constipation.
Ob/Gyn: The Year in Review
GI Disorders Often Present to Gynecologists First
Christine L. Frissora, MD, FACG, FACP
Faculty Member, Division of Gastroenterology and Hepatology
The Weill Medical College of Cornell University
The New York-Presbyterian Hospital
New York, NY
Consultant: Novartis Pharmaceuticals and Vela Pharmaceuticals; Research Grants: Forest Laboratories.
Treat Constipation Before Gynecologic Surgery
Marie Fidela R. Paraiso, MD, FACOG
Director of Residency Education
Associate Fellowship Director, Urogynecology and Reconstructive Pelvic Surgery
Staff, Department of Obstetrics and Gynecology and the Urological Institute
The Cleveland Clinic Foundation
Cleveland, OH
Member of the Braintree Advisory Board.
Scientific Posters: Selected Summaries
Copyright © 2003 by International Medical News Group
A supplement to Ob. Gyn. News.
This Clinical Update is supported by Braintree Laboratories, Inc.
The articles are based on interviews with the faculty.

To view the supplement, click the image above.
Topic Highlights
Rectal Anatomy Is a Key Factor in Constipation
Lawrence R. Schiller, MD, FACP, FACG
Program Director, Gastroenterology Fellowship
Department of Gastroenterology
Baylor University Medical Center
Dallas, TX
Consultant: Braintree Laboratories, Inc. and Novartis Pharmaceuticals.
Focus Evaluation of Constipation on Two Causes: Medication and Function
Jack A. DiPalma, MD, FACP, FACG
Professor and Director
Division of Gastroenterology
University of South Alabama College of Medicine
Mobile, AL
Clinical Grants: Braintree Laboratories, Inc. He discusses the unlabeled use of Polyethylene Glycol 3350 for chronic constipation.
Ob/Gyn: The Year in Review
GI Disorders Often Present to Gynecologists First
Christine L. Frissora, MD, FACG, FACP
Faculty Member, Division of Gastroenterology and Hepatology
The Weill Medical College of Cornell University
The New York-Presbyterian Hospital
New York, NY
Consultant: Novartis Pharmaceuticals and Vela Pharmaceuticals; Research Grants: Forest Laboratories.
Treat Constipation Before Gynecologic Surgery
Marie Fidela R. Paraiso, MD, FACOG
Director of Residency Education
Associate Fellowship Director, Urogynecology and Reconstructive Pelvic Surgery
Staff, Department of Obstetrics and Gynecology and the Urological Institute
The Cleveland Clinic Foundation
Cleveland, OH
Member of the Braintree Advisory Board.
Scientific Posters: Selected Summaries
Copyright © 2003 by International Medical News Group
10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery
Cesarean delivery is not risk-free, despite its high prevalence (30% overall, but almost 100% in women who have more than two prior cesareans). It increases the risks of adhesions, severe blood loss, and injury to the bowel, bladder and ureters, particularly among women undergoing the procedure for the second or third time.
Morbidly obese women (i.e., those who have a body mass index [BMI] of 40 or above) are in a particular bind: They have an elevated risk of cesarean delivery, and when they undergo the procedure, they have a significantly heightened risk of cardiopulmonary complications, anesthetic complications, wound complications, thromboembolism, and prolonged skin incision-to-delivery time.
A number of studies have described the technical aspects of cesarean delivery, but debate continues about a number of issues:
- the risks and benefits of types of skin incision
- whether the rectus muscle should be separated bluntly or sharply
- whether or not to close the peritoneum
- the best method of closing the skin (i.e., subcuticular sutures or staples).
In this review, I offer 10 practical, evidence-based recommendations that help clarify these issues, including several that focus on the morbidl obese population.
1. Anticipate anesthetic complications
In morbidly obese pregnant women, plan for potential complications
Vricella LK, Lois JM, Mercer BM, Bolden N. Anesthesia complications during scheduled cesarean delivery for morbidly obese women. Am J Obstet Gynecol. 2010;203(3):276.e1–5.
Knight M, Kurinczuk JJ, Spark P, Brocklehurst P; UK Obstetric Surveillance System. Extreme obesity in pregnancy in the United Kingdom. Obstet Gynecol. 2010;115(5):989–997.
In a national cohort study of 665 women who had a BMI of 50 or above, 11% experienced problems with epidural anesthesia, including failure; 6% required general anesthesia; and 3% required admission to intensive care. A similar, but retrospective, study of 142 morbidly obese women found an anesthesia complication rate of 8.5%.
These studies suggest that planning and antenatal consultation with anesthesiologists are important to help avert anesthetic complications during cesarean delivery. Requirements include detailed evaluation at admission, early placement of an epidural catheter, preparation for general anesthesia in case of failure of regional anesthesia, and ensuring the availability of an anesthesiologist who has expertise in this population.
2. Reduce the interval from decision to delivery
Plan, implement, and rehearse a protocol to move from decision to incision and delivery in 30 minutes in morbidly obese women
Lucas DN. The 30-minute decision to delivery time is unrealistic in morbidly obese women. Int J Obstet Anesth. 2010;19(4):431–435. Comment by: Dresner M. Int J Obstet Anesth. 2010;19(4):435–437.
Although several national bodies recommend a decision-to-incision or delivery interval of 30 minutes or less, this approach is not backed by definitive data. Moreover, the 30-minute goal poses major challenges to the nursing, anesthesia, and surgical teams that provide care to morbidly obese women who require emergent cesarean delivery. This is especially true in cases that involve catastrophic events, such as abruptio placentae, cord prolapse, uterine rupture, or vasa previa—where minutes matter.
Nevertheless, efforts to reduce this interval are vital. Consider four phases:
- how long it takes to transfer the patient to the operating room
- the time it takes to position and prepare the patient for surgery
- the time required to administer anesthesia
- how long it takes to move from skin incision to delivery of the fetus.
Because all four phases will be prolonged in morbidly obese patients, it is prudent for obstetric units to develop protocols to identify and flag women who are at risk, and to have policies and procedures in place to reduce these times. This will necessitate drills for rehearsal and testing of response times and skills of the various providers. In addition, whenever emergent cesarean is performed, the actual response time and effectiveness of interventions should be evaluated.
3. Consider a transverse skin incision
In morbidly obese women who undergo emergent cesarean delivery, a transverse skin incision may provide benefit
Wylie BJ, Gilbert S, Landon MB, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Comparison of transverse and vertical skin incision for emergency cesarean delivery. Obstet Gynecol. 2010;115(6):1134–1140.
Bell J, Bell S, Vahratian A, Awonuga AO. Abdominal surgical incisions and perioperative morbidity among morbidly obese women undergoing cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;154(1):16–19.
No randomized trials have compared the benefits and risks of vertical and transverse skin incisions during cesarean delivery. In general, a vertical incision is believed to shorten the time to delivery, but is associated with a greater need for transfusion, greater postoperative pain, and higher rates of wound dehiscence and infection, compared with a transverse incision.
A prospective cohort study of all emergent cesarean deliveries performed at 13 medical centers compared maternal and neonatal outcomes between 2,498 women who had a vertical incision and 1,027 who had a transverse incision. The use of a vertical incision shortened the median incision-to-delivery interval by 1 minute (3 vs 4 minutes; P < .001) for primary cesarean and by 2 minutes (3 vs 5 minutes; P < .001) for repeat cesarean. However, a vertical incision was associated with higher rates of endometritis (15% vs 11%; P = .006) and postpartum transfusion (7% vs 5%; P = .01) for primary cesarean, as well as a higher rate of postpartum transfusion (15% vs 8%; P = .02) for repeat cesarean. No differences in the rates of wound hematoma and infection were noted.
A retrospective cohort study in 424 morbidly obese women compared maternal morbidity between 41 women who had a vertical skin incision and 383 women who had a transverse incision for cesarean delivery. A vertical incision was associated with a dramatic increase in the risk of a classical uterine incision (65.9% vs 7.3%; P < .001), but there were no differences in the rates of blood transfusion or wound breakdown or infection between the two groups. However, these findings should be interpreted with caution because women who received a vertical incision were older (31.0 ± 6.2 years vs 27.1 ± 6.7 years; P <.001), and there was no mention of the type of vertical skin incision in relation to the umbilicus or the use of drains. A randomized trial is needed to determine the optimal skin incision in morbidly obese women.
4. Use blunt, not sharp, expansion of the uterine incision
Blunt expansion is associated with less blood loss
Sekhavat L, Firouzabadi RD, Mojiri P. Effect of expansion technique of uterine incision on maternal blood loss in cesarean section. Arch Gynecol Obstet. 2010;282(5):475–479.
A prospective, randomized trial explored the rate of lateral extension of the uterine incision and estimated blood loss in 200 full-term primiparas undergoing cesarean delivery. Women were assigned to blunt expansion (n = 100) or sharp expansion (n = 100). Blunt expansion was associated with lower estimated blood loss (375 ± 95 mL vs 443 ± 86 mL; P <.05) but no differences in the rate of lateral extension (5% vs 6%). These findings reveal that blunt expansion of the uterine incision in primiparas is safer and easier than sharp expansion.
Nonclosure after cesarean delivery is associated with a higher rate of adhesion formation
Cheong YC, Premkumar G, Metwally M, Peacock JL, Li TC. To close or not to close? A systematic review and a meta-analysis of peritoneal non-closure and adhesion formation after caesarean section. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):3–8.
Shi Z, Ma L, Yang Y, Wang H, et al. Adhesion formation after previous caesarean section—a meta-analysis and systematic review. BJOG. 2011;118(4):410–422. doi: 10.1111/j.1471-0528.2010.02808.x.
A systematic review and meta-analysis that included two randomized trials and one prospective study compared the rate of adhesions after cesarean delivery between women who had peritoneal closure (n = 110) and those who did not (n = 139). Nonclosure was associated with a substantial increase in the rate of subsequent adhesion formation (adjusted odds ratio, 4.23; 95% confidence interval [CI], 2.06–8.69). However, this review did not consider risk factors such as creation of a bladder flap or type of uterine incision.
A subsequent systematic review (n = 4,423) compared the rate of adhesions associated with closure and nonclosure of the peritoneum according to cesarean technique (Stark’s, modified Stark’s, or classic lower-segment). The classic lower-segment technique involves dissecting the bladder off the uterus and closure of both peritoneal layers (visceral and peritoneal). Neither Stark’s technique nor the modified Stark’s technique dissects the bladder from the uterus; both techniques use single-layer closure of the uterine incision. Stark’s technique leaves the peritoneal layer open, whereas the modified Stark’s technique closes the peritoneal layer. This review revealed that closing the peritoneum in modified Stark’s cesarean delivery was associated with a lower rate of subsequent adhesions—both in terms of total adhesions and individual grades of adhesions.
6. Use double-layer uterine closure
Despite its lack of effect on maternal morbidity, double-layer closure reduces risk of rupture during VBAC
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
This large multicenter, randomized trial evaluated maternal infectious morbidity in women undergoing single- (n = 1,483) and double-layer (n = 1,496) closure of the uterine incision. The total rates of maternal infectious morbidity (16.1% vs 16.9%), wound infection (12.8% vs 12.7%), severe morbidity (0.5% vs 0.7%), and readmission within 6 weeks (2.6% vs 2.7%) were similar between groups for single- and double-layer closure, respectively. However, retrospective and case-control studies reveal that double-layer closure is associated with lower rates of uterine dehiscence and rupture during vaginal birth after cesarean (VBAC).
7. Keep the risk of adhesions in mind
Closing the peritoneum may reduce long-term adhesion formation
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
According to the findings of this large, multicenter, randomized trial, the rate of maternal infectious morbidity did not change whether or not a drain was used or the peritoneum was closed. The study evaluated only maternal infectious morbidity related to peritoneal closure—not long-term adhesion formation. Nevertheless, it appears that closure of the peritoneum at the time of cesarean delivery is associated with a lower rate of long-term adhesion formation.
8. Forget adhesion barriers
Their use to prevent intra-abdominal adhesions is ill-advised
Albright CM, Rouse DJ. Adhesion barriers at cesarean delivery: advertising compared with the evidence. Obstet Gynecol. 2011;118(1):157–160.
After cesarean delivery, there is a potential for intra-abdominal adhesions to form, which can lead to pain, small bowel obstruction, and injury during repeat surgery. A review of the literature suggests that the use of adhesion barriers at cesarean delivery is not cost-effective. Randomized trials are needed.
9. Be vigilant for bladder and ureteral injuries
Know the risk factors and preventive strategies for these injuries
Gungorduk K, Asicioglu O, Celikkol O, et al. Iatrogenic bladder injuries during caesarean delivery: a case control study. J Obstet Gynaecol. 2010;30(7):667–670.
Karram M. Avoiding and managing lower urinary tract injury during vaginal and abdominal deliveries. In: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011:179–187.
The reported incidence of bladder injury at the time of cesarean delivery ranges from 0.13% to 0.31% during primary cesarean and reaches 0.6% during repeat cesarean. During primary cesarean, bladder injury usually occurs during entry into the peritoneal cavity and involves the high extraperitoneal aspect of the bladder. In repeat cesarean, it usually occurs during dissection of the bladder flap and involves the intraperitoneal aspect of the bladder (dependent portion).
Bladder and ureteral injuries are more likely to occur in the presence of one or more of the following risk factors:
- emergency or crash cesarean delivery
- cesarean delivery after prolonged pushing
- history of uterine or abdominal surgery
- central placenta previa or accreta
- lateral or downward extension of the uterine incision
- uterine rupture
- need for hysterectomy.
Inadvertent bladder injury during entrance into the peritoneum should be managed with layered closure of the cystotomy. Because such injury occurs high in the bladder, it requires only a short period of postoperative drainage. In contrast, injury to the base of the bladder requires appropriate mobilization of the bladder off any adherent structures to allow tension-free closure of the injury. Since this type of injury lies in the dependent portion of the bladder, it requires postoperative drainage for 10 to 14 days. Close the injury in two layers, using fine, delayed, absorbable suture in interrupted or running fashion, with the first layer approximating the mucosa and the second layer imbricating the muscularis.
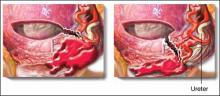
Ureteral injury is rare during cesarean delivery. When it does occur, it usually occurs during repair of lacerations from the uterine incision or control of excessive bleeding from the lower segment or broad ligament (FIGURE). The most common site of injury from uterine lacerations is at the level of the uterine vessels, whereas the most common site of injury at cesarean hysterectomy is the lower portion of the ureter near the uterosacral ligaments.
If you suspect injury, confirm ureteral patency by making a cystotomy in the bladder dome to visualize the orifices, and attempt to pass a ureteral catheter or pediatric feeding tube through the orifice into the ureter until you reach a point above the area of concern. If there is an obstruction, kinking, or transection, consult a urogynecologist or urologist.
Risks of lateral and downward extension
Avoid lateral and downward extension of the uterine incision, which may injure the blood vessels or ureter.
Source: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011.
10. Close the skin with subcuticular suture
This approach is associated with lower risk of wound separation and infection than is closure with staples
Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682–690.
Wound complications following cesarean can include hematoma, seroma, complete separation, or infection (superficial or deep). Wound complications may be more likely with staple closure of a transverse skin incision than with subcuticular suture. Other issues to consider when choosing a type of skin closure include postoperative pain, cosmesis, and how long it takes to deliver the infant.
This systematic review and meta-analysis of five randomized trials and one prospective study compared outcomes after skin staple closure (n = 803) with those after subcuticular suture closure (n = 684) in women who had a transverse incision. Staple closure was associated with a higher rate of wound infection or separation (13.4% vs 6.6%; pooled odds ratio, 2.06; 95% CI, 1.43–2.98). Staple closure was associated with a shorter operating time (range, 3.3–9.3 minutes). Both techniques were similar in terms of postoperative pain, cosmetic appearance, and patient satisfaction.
We want to hear from you! Tell us what you think.
- 10 practical recommendations to improve maternal and perinatal outcomes in patients with eclampsia
Baha M. Sibai, MD (November 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai, MD (June 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011) - 10 practical, evidence-based suggestions to improve your gyn practice now
Mark D. Walters, MD (January 2011)
Cesarean delivery is not risk-free, despite its high prevalence (30% overall, but almost 100% in women who have more than two prior cesareans). It increases the risks of adhesions, severe blood loss, and injury to the bowel, bladder and ureters, particularly among women undergoing the procedure for the second or third time.
Morbidly obese women (i.e., those who have a body mass index [BMI] of 40 or above) are in a particular bind: They have an elevated risk of cesarean delivery, and when they undergo the procedure, they have a significantly heightened risk of cardiopulmonary complications, anesthetic complications, wound complications, thromboembolism, and prolonged skin incision-to-delivery time.
A number of studies have described the technical aspects of cesarean delivery, but debate continues about a number of issues:
- the risks and benefits of types of skin incision
- whether the rectus muscle should be separated bluntly or sharply
- whether or not to close the peritoneum
- the best method of closing the skin (i.e., subcuticular sutures or staples).
In this review, I offer 10 practical, evidence-based recommendations that help clarify these issues, including several that focus on the morbidl obese population.
1. Anticipate anesthetic complications
In morbidly obese pregnant women, plan for potential complications
Vricella LK, Lois JM, Mercer BM, Bolden N. Anesthesia complications during scheduled cesarean delivery for morbidly obese women. Am J Obstet Gynecol. 2010;203(3):276.e1–5.
Knight M, Kurinczuk JJ, Spark P, Brocklehurst P; UK Obstetric Surveillance System. Extreme obesity in pregnancy in the United Kingdom. Obstet Gynecol. 2010;115(5):989–997.
In a national cohort study of 665 women who had a BMI of 50 or above, 11% experienced problems with epidural anesthesia, including failure; 6% required general anesthesia; and 3% required admission to intensive care. A similar, but retrospective, study of 142 morbidly obese women found an anesthesia complication rate of 8.5%.
These studies suggest that planning and antenatal consultation with anesthesiologists are important to help avert anesthetic complications during cesarean delivery. Requirements include detailed evaluation at admission, early placement of an epidural catheter, preparation for general anesthesia in case of failure of regional anesthesia, and ensuring the availability of an anesthesiologist who has expertise in this population.
2. Reduce the interval from decision to delivery
Plan, implement, and rehearse a protocol to move from decision to incision and delivery in 30 minutes in morbidly obese women
Lucas DN. The 30-minute decision to delivery time is unrealistic in morbidly obese women. Int J Obstet Anesth. 2010;19(4):431–435. Comment by: Dresner M. Int J Obstet Anesth. 2010;19(4):435–437.
Although several national bodies recommend a decision-to-incision or delivery interval of 30 minutes or less, this approach is not backed by definitive data. Moreover, the 30-minute goal poses major challenges to the nursing, anesthesia, and surgical teams that provide care to morbidly obese women who require emergent cesarean delivery. This is especially true in cases that involve catastrophic events, such as abruptio placentae, cord prolapse, uterine rupture, or vasa previa—where minutes matter.
Nevertheless, efforts to reduce this interval are vital. Consider four phases:
- how long it takes to transfer the patient to the operating room
- the time it takes to position and prepare the patient for surgery
- the time required to administer anesthesia
- how long it takes to move from skin incision to delivery of the fetus.
Because all four phases will be prolonged in morbidly obese patients, it is prudent for obstetric units to develop protocols to identify and flag women who are at risk, and to have policies and procedures in place to reduce these times. This will necessitate drills for rehearsal and testing of response times and skills of the various providers. In addition, whenever emergent cesarean is performed, the actual response time and effectiveness of interventions should be evaluated.
3. Consider a transverse skin incision
In morbidly obese women who undergo emergent cesarean delivery, a transverse skin incision may provide benefit
Wylie BJ, Gilbert S, Landon MB, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Comparison of transverse and vertical skin incision for emergency cesarean delivery. Obstet Gynecol. 2010;115(6):1134–1140.
Bell J, Bell S, Vahratian A, Awonuga AO. Abdominal surgical incisions and perioperative morbidity among morbidly obese women undergoing cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;154(1):16–19.
No randomized trials have compared the benefits and risks of vertical and transverse skin incisions during cesarean delivery. In general, a vertical incision is believed to shorten the time to delivery, but is associated with a greater need for transfusion, greater postoperative pain, and higher rates of wound dehiscence and infection, compared with a transverse incision.
A prospective cohort study of all emergent cesarean deliveries performed at 13 medical centers compared maternal and neonatal outcomes between 2,498 women who had a vertical incision and 1,027 who had a transverse incision. The use of a vertical incision shortened the median incision-to-delivery interval by 1 minute (3 vs 4 minutes; P < .001) for primary cesarean and by 2 minutes (3 vs 5 minutes; P < .001) for repeat cesarean. However, a vertical incision was associated with higher rates of endometritis (15% vs 11%; P = .006) and postpartum transfusion (7% vs 5%; P = .01) for primary cesarean, as well as a higher rate of postpartum transfusion (15% vs 8%; P = .02) for repeat cesarean. No differences in the rates of wound hematoma and infection were noted.
A retrospective cohort study in 424 morbidly obese women compared maternal morbidity between 41 women who had a vertical skin incision and 383 women who had a transverse incision for cesarean delivery. A vertical incision was associated with a dramatic increase in the risk of a classical uterine incision (65.9% vs 7.3%; P < .001), but there were no differences in the rates of blood transfusion or wound breakdown or infection between the two groups. However, these findings should be interpreted with caution because women who received a vertical incision were older (31.0 ± 6.2 years vs 27.1 ± 6.7 years; P <.001), and there was no mention of the type of vertical skin incision in relation to the umbilicus or the use of drains. A randomized trial is needed to determine the optimal skin incision in morbidly obese women.
4. Use blunt, not sharp, expansion of the uterine incision
Blunt expansion is associated with less blood loss
Sekhavat L, Firouzabadi RD, Mojiri P. Effect of expansion technique of uterine incision on maternal blood loss in cesarean section. Arch Gynecol Obstet. 2010;282(5):475–479.
A prospective, randomized trial explored the rate of lateral extension of the uterine incision and estimated blood loss in 200 full-term primiparas undergoing cesarean delivery. Women were assigned to blunt expansion (n = 100) or sharp expansion (n = 100). Blunt expansion was associated with lower estimated blood loss (375 ± 95 mL vs 443 ± 86 mL; P <.05) but no differences in the rate of lateral extension (5% vs 6%). These findings reveal that blunt expansion of the uterine incision in primiparas is safer and easier than sharp expansion.
Nonclosure after cesarean delivery is associated with a higher rate of adhesion formation
Cheong YC, Premkumar G, Metwally M, Peacock JL, Li TC. To close or not to close? A systematic review and a meta-analysis of peritoneal non-closure and adhesion formation after caesarean section. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):3–8.
Shi Z, Ma L, Yang Y, Wang H, et al. Adhesion formation after previous caesarean section—a meta-analysis and systematic review. BJOG. 2011;118(4):410–422. doi: 10.1111/j.1471-0528.2010.02808.x.
A systematic review and meta-analysis that included two randomized trials and one prospective study compared the rate of adhesions after cesarean delivery between women who had peritoneal closure (n = 110) and those who did not (n = 139). Nonclosure was associated with a substantial increase in the rate of subsequent adhesion formation (adjusted odds ratio, 4.23; 95% confidence interval [CI], 2.06–8.69). However, this review did not consider risk factors such as creation of a bladder flap or type of uterine incision.
A subsequent systematic review (n = 4,423) compared the rate of adhesions associated with closure and nonclosure of the peritoneum according to cesarean technique (Stark’s, modified Stark’s, or classic lower-segment). The classic lower-segment technique involves dissecting the bladder off the uterus and closure of both peritoneal layers (visceral and peritoneal). Neither Stark’s technique nor the modified Stark’s technique dissects the bladder from the uterus; both techniques use single-layer closure of the uterine incision. Stark’s technique leaves the peritoneal layer open, whereas the modified Stark’s technique closes the peritoneal layer. This review revealed that closing the peritoneum in modified Stark’s cesarean delivery was associated with a lower rate of subsequent adhesions—both in terms of total adhesions and individual grades of adhesions.
6. Use double-layer uterine closure
Despite its lack of effect on maternal morbidity, double-layer closure reduces risk of rupture during VBAC
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
This large multicenter, randomized trial evaluated maternal infectious morbidity in women undergoing single- (n = 1,483) and double-layer (n = 1,496) closure of the uterine incision. The total rates of maternal infectious morbidity (16.1% vs 16.9%), wound infection (12.8% vs 12.7%), severe morbidity (0.5% vs 0.7%), and readmission within 6 weeks (2.6% vs 2.7%) were similar between groups for single- and double-layer closure, respectively. However, retrospective and case-control studies reveal that double-layer closure is associated with lower rates of uterine dehiscence and rupture during vaginal birth after cesarean (VBAC).
7. Keep the risk of adhesions in mind
Closing the peritoneum may reduce long-term adhesion formation
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
According to the findings of this large, multicenter, randomized trial, the rate of maternal infectious morbidity did not change whether or not a drain was used or the peritoneum was closed. The study evaluated only maternal infectious morbidity related to peritoneal closure—not long-term adhesion formation. Nevertheless, it appears that closure of the peritoneum at the time of cesarean delivery is associated with a lower rate of long-term adhesion formation.
8. Forget adhesion barriers
Their use to prevent intra-abdominal adhesions is ill-advised
Albright CM, Rouse DJ. Adhesion barriers at cesarean delivery: advertising compared with the evidence. Obstet Gynecol. 2011;118(1):157–160.
After cesarean delivery, there is a potential for intra-abdominal adhesions to form, which can lead to pain, small bowel obstruction, and injury during repeat surgery. A review of the literature suggests that the use of adhesion barriers at cesarean delivery is not cost-effective. Randomized trials are needed.
9. Be vigilant for bladder and ureteral injuries
Know the risk factors and preventive strategies for these injuries
Gungorduk K, Asicioglu O, Celikkol O, et al. Iatrogenic bladder injuries during caesarean delivery: a case control study. J Obstet Gynaecol. 2010;30(7):667–670.
Karram M. Avoiding and managing lower urinary tract injury during vaginal and abdominal deliveries. In: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011:179–187.
The reported incidence of bladder injury at the time of cesarean delivery ranges from 0.13% to 0.31% during primary cesarean and reaches 0.6% during repeat cesarean. During primary cesarean, bladder injury usually occurs during entry into the peritoneal cavity and involves the high extraperitoneal aspect of the bladder. In repeat cesarean, it usually occurs during dissection of the bladder flap and involves the intraperitoneal aspect of the bladder (dependent portion).
Bladder and ureteral injuries are more likely to occur in the presence of one or more of the following risk factors:
- emergency or crash cesarean delivery
- cesarean delivery after prolonged pushing
- history of uterine or abdominal surgery
- central placenta previa or accreta
- lateral or downward extension of the uterine incision
- uterine rupture
- need for hysterectomy.
Inadvertent bladder injury during entrance into the peritoneum should be managed with layered closure of the cystotomy. Because such injury occurs high in the bladder, it requires only a short period of postoperative drainage. In contrast, injury to the base of the bladder requires appropriate mobilization of the bladder off any adherent structures to allow tension-free closure of the injury. Since this type of injury lies in the dependent portion of the bladder, it requires postoperative drainage for 10 to 14 days. Close the injury in two layers, using fine, delayed, absorbable suture in interrupted or running fashion, with the first layer approximating the mucosa and the second layer imbricating the muscularis.
Ureteral injury is rare during cesarean delivery. When it does occur, it usually occurs during repair of lacerations from the uterine incision or control of excessive bleeding from the lower segment or broad ligament (FIGURE). The most common site of injury from uterine lacerations is at the level of the uterine vessels, whereas the most common site of injury at cesarean hysterectomy is the lower portion of the ureter near the uterosacral ligaments.
If you suspect injury, confirm ureteral patency by making a cystotomy in the bladder dome to visualize the orifices, and attempt to pass a ureteral catheter or pediatric feeding tube through the orifice into the ureter until you reach a point above the area of concern. If there is an obstruction, kinking, or transection, consult a urogynecologist or urologist.
Risks of lateral and downward extension
Avoid lateral and downward extension of the uterine incision, which may injure the blood vessels or ureter.
Source: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011.
10. Close the skin with subcuticular suture
This approach is associated with lower risk of wound separation and infection than is closure with staples
Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682–690.
Wound complications following cesarean can include hematoma, seroma, complete separation, or infection (superficial or deep). Wound complications may be more likely with staple closure of a transverse skin incision than with subcuticular suture. Other issues to consider when choosing a type of skin closure include postoperative pain, cosmesis, and how long it takes to deliver the infant.
This systematic review and meta-analysis of five randomized trials and one prospective study compared outcomes after skin staple closure (n = 803) with those after subcuticular suture closure (n = 684) in women who had a transverse incision. Staple closure was associated with a higher rate of wound infection or separation (13.4% vs 6.6%; pooled odds ratio, 2.06; 95% CI, 1.43–2.98). Staple closure was associated with a shorter operating time (range, 3.3–9.3 minutes). Both techniques were similar in terms of postoperative pain, cosmetic appearance, and patient satisfaction.
We want to hear from you! Tell us what you think.
- 10 practical recommendations to improve maternal and perinatal outcomes in patients with eclampsia
Baha M. Sibai, MD (November 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai, MD (June 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011) - 10 practical, evidence-based suggestions to improve your gyn practice now
Mark D. Walters, MD (January 2011)
Cesarean delivery is not risk-free, despite its high prevalence (30% overall, but almost 100% in women who have more than two prior cesareans). It increases the risks of adhesions, severe blood loss, and injury to the bowel, bladder and ureters, particularly among women undergoing the procedure for the second or third time.
Morbidly obese women (i.e., those who have a body mass index [BMI] of 40 or above) are in a particular bind: They have an elevated risk of cesarean delivery, and when they undergo the procedure, they have a significantly heightened risk of cardiopulmonary complications, anesthetic complications, wound complications, thromboembolism, and prolonged skin incision-to-delivery time.
A number of studies have described the technical aspects of cesarean delivery, but debate continues about a number of issues:
- the risks and benefits of types of skin incision
- whether the rectus muscle should be separated bluntly or sharply
- whether or not to close the peritoneum
- the best method of closing the skin (i.e., subcuticular sutures or staples).
In this review, I offer 10 practical, evidence-based recommendations that help clarify these issues, including several that focus on the morbidl obese population.
1. Anticipate anesthetic complications
In morbidly obese pregnant women, plan for potential complications
Vricella LK, Lois JM, Mercer BM, Bolden N. Anesthesia complications during scheduled cesarean delivery for morbidly obese women. Am J Obstet Gynecol. 2010;203(3):276.e1–5.
Knight M, Kurinczuk JJ, Spark P, Brocklehurst P; UK Obstetric Surveillance System. Extreme obesity in pregnancy in the United Kingdom. Obstet Gynecol. 2010;115(5):989–997.
In a national cohort study of 665 women who had a BMI of 50 or above, 11% experienced problems with epidural anesthesia, including failure; 6% required general anesthesia; and 3% required admission to intensive care. A similar, but retrospective, study of 142 morbidly obese women found an anesthesia complication rate of 8.5%.
These studies suggest that planning and antenatal consultation with anesthesiologists are important to help avert anesthetic complications during cesarean delivery. Requirements include detailed evaluation at admission, early placement of an epidural catheter, preparation for general anesthesia in case of failure of regional anesthesia, and ensuring the availability of an anesthesiologist who has expertise in this population.
2. Reduce the interval from decision to delivery
Plan, implement, and rehearse a protocol to move from decision to incision and delivery in 30 minutes in morbidly obese women
Lucas DN. The 30-minute decision to delivery time is unrealistic in morbidly obese women. Int J Obstet Anesth. 2010;19(4):431–435. Comment by: Dresner M. Int J Obstet Anesth. 2010;19(4):435–437.
Although several national bodies recommend a decision-to-incision or delivery interval of 30 minutes or less, this approach is not backed by definitive data. Moreover, the 30-minute goal poses major challenges to the nursing, anesthesia, and surgical teams that provide care to morbidly obese women who require emergent cesarean delivery. This is especially true in cases that involve catastrophic events, such as abruptio placentae, cord prolapse, uterine rupture, or vasa previa—where minutes matter.
Nevertheless, efforts to reduce this interval are vital. Consider four phases:
- how long it takes to transfer the patient to the operating room
- the time it takes to position and prepare the patient for surgery
- the time required to administer anesthesia
- how long it takes to move from skin incision to delivery of the fetus.
Because all four phases will be prolonged in morbidly obese patients, it is prudent for obstetric units to develop protocols to identify and flag women who are at risk, and to have policies and procedures in place to reduce these times. This will necessitate drills for rehearsal and testing of response times and skills of the various providers. In addition, whenever emergent cesarean is performed, the actual response time and effectiveness of interventions should be evaluated.
3. Consider a transverse skin incision
In morbidly obese women who undergo emergent cesarean delivery, a transverse skin incision may provide benefit
Wylie BJ, Gilbert S, Landon MB, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Comparison of transverse and vertical skin incision for emergency cesarean delivery. Obstet Gynecol. 2010;115(6):1134–1140.
Bell J, Bell S, Vahratian A, Awonuga AO. Abdominal surgical incisions and perioperative morbidity among morbidly obese women undergoing cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;154(1):16–19.
No randomized trials have compared the benefits and risks of vertical and transverse skin incisions during cesarean delivery. In general, a vertical incision is believed to shorten the time to delivery, but is associated with a greater need for transfusion, greater postoperative pain, and higher rates of wound dehiscence and infection, compared with a transverse incision.
A prospective cohort study of all emergent cesarean deliveries performed at 13 medical centers compared maternal and neonatal outcomes between 2,498 women who had a vertical incision and 1,027 who had a transverse incision. The use of a vertical incision shortened the median incision-to-delivery interval by 1 minute (3 vs 4 minutes; P < .001) for primary cesarean and by 2 minutes (3 vs 5 minutes; P < .001) for repeat cesarean. However, a vertical incision was associated with higher rates of endometritis (15% vs 11%; P = .006) and postpartum transfusion (7% vs 5%; P = .01) for primary cesarean, as well as a higher rate of postpartum transfusion (15% vs 8%; P = .02) for repeat cesarean. No differences in the rates of wound hematoma and infection were noted.
A retrospective cohort study in 424 morbidly obese women compared maternal morbidity between 41 women who had a vertical skin incision and 383 women who had a transverse incision for cesarean delivery. A vertical incision was associated with a dramatic increase in the risk of a classical uterine incision (65.9% vs 7.3%; P < .001), but there were no differences in the rates of blood transfusion or wound breakdown or infection between the two groups. However, these findings should be interpreted with caution because women who received a vertical incision were older (31.0 ± 6.2 years vs 27.1 ± 6.7 years; P <.001), and there was no mention of the type of vertical skin incision in relation to the umbilicus or the use of drains. A randomized trial is needed to determine the optimal skin incision in morbidly obese women.
4. Use blunt, not sharp, expansion of the uterine incision
Blunt expansion is associated with less blood loss
Sekhavat L, Firouzabadi RD, Mojiri P. Effect of expansion technique of uterine incision on maternal blood loss in cesarean section. Arch Gynecol Obstet. 2010;282(5):475–479.
A prospective, randomized trial explored the rate of lateral extension of the uterine incision and estimated blood loss in 200 full-term primiparas undergoing cesarean delivery. Women were assigned to blunt expansion (n = 100) or sharp expansion (n = 100). Blunt expansion was associated with lower estimated blood loss (375 ± 95 mL vs 443 ± 86 mL; P <.05) but no differences in the rate of lateral extension (5% vs 6%). These findings reveal that blunt expansion of the uterine incision in primiparas is safer and easier than sharp expansion.
Nonclosure after cesarean delivery is associated with a higher rate of adhesion formation
Cheong YC, Premkumar G, Metwally M, Peacock JL, Li TC. To close or not to close? A systematic review and a meta-analysis of peritoneal non-closure and adhesion formation after caesarean section. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):3–8.
Shi Z, Ma L, Yang Y, Wang H, et al. Adhesion formation after previous caesarean section—a meta-analysis and systematic review. BJOG. 2011;118(4):410–422. doi: 10.1111/j.1471-0528.2010.02808.x.
A systematic review and meta-analysis that included two randomized trials and one prospective study compared the rate of adhesions after cesarean delivery between women who had peritoneal closure (n = 110) and those who did not (n = 139). Nonclosure was associated with a substantial increase in the rate of subsequent adhesion formation (adjusted odds ratio, 4.23; 95% confidence interval [CI], 2.06–8.69). However, this review did not consider risk factors such as creation of a bladder flap or type of uterine incision.
A subsequent systematic review (n = 4,423) compared the rate of adhesions associated with closure and nonclosure of the peritoneum according to cesarean technique (Stark’s, modified Stark’s, or classic lower-segment). The classic lower-segment technique involves dissecting the bladder off the uterus and closure of both peritoneal layers (visceral and peritoneal). Neither Stark’s technique nor the modified Stark’s technique dissects the bladder from the uterus; both techniques use single-layer closure of the uterine incision. Stark’s technique leaves the peritoneal layer open, whereas the modified Stark’s technique closes the peritoneal layer. This review revealed that closing the peritoneum in modified Stark’s cesarean delivery was associated with a lower rate of subsequent adhesions—both in terms of total adhesions and individual grades of adhesions.
6. Use double-layer uterine closure
Despite its lack of effect on maternal morbidity, double-layer closure reduces risk of rupture during VBAC
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
This large multicenter, randomized trial evaluated maternal infectious morbidity in women undergoing single- (n = 1,483) and double-layer (n = 1,496) closure of the uterine incision. The total rates of maternal infectious morbidity (16.1% vs 16.9%), wound infection (12.8% vs 12.7%), severe morbidity (0.5% vs 0.7%), and readmission within 6 weeks (2.6% vs 2.7%) were similar between groups for single- and double-layer closure, respectively. However, retrospective and case-control studies reveal that double-layer closure is associated with lower rates of uterine dehiscence and rupture during vaginal birth after cesarean (VBAC).
7. Keep the risk of adhesions in mind
Closing the peritoneum may reduce long-term adhesion formation
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
According to the findings of this large, multicenter, randomized trial, the rate of maternal infectious morbidity did not change whether or not a drain was used or the peritoneum was closed. The study evaluated only maternal infectious morbidity related to peritoneal closure—not long-term adhesion formation. Nevertheless, it appears that closure of the peritoneum at the time of cesarean delivery is associated with a lower rate of long-term adhesion formation.
8. Forget adhesion barriers
Their use to prevent intra-abdominal adhesions is ill-advised
Albright CM, Rouse DJ. Adhesion barriers at cesarean delivery: advertising compared with the evidence. Obstet Gynecol. 2011;118(1):157–160.
After cesarean delivery, there is a potential for intra-abdominal adhesions to form, which can lead to pain, small bowel obstruction, and injury during repeat surgery. A review of the literature suggests that the use of adhesion barriers at cesarean delivery is not cost-effective. Randomized trials are needed.
9. Be vigilant for bladder and ureteral injuries
Know the risk factors and preventive strategies for these injuries
Gungorduk K, Asicioglu O, Celikkol O, et al. Iatrogenic bladder injuries during caesarean delivery: a case control study. J Obstet Gynaecol. 2010;30(7):667–670.
Karram M. Avoiding and managing lower urinary tract injury during vaginal and abdominal deliveries. In: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011:179–187.
The reported incidence of bladder injury at the time of cesarean delivery ranges from 0.13% to 0.31% during primary cesarean and reaches 0.6% during repeat cesarean. During primary cesarean, bladder injury usually occurs during entry into the peritoneal cavity and involves the high extraperitoneal aspect of the bladder. In repeat cesarean, it usually occurs during dissection of the bladder flap and involves the intraperitoneal aspect of the bladder (dependent portion).
Bladder and ureteral injuries are more likely to occur in the presence of one or more of the following risk factors:
- emergency or crash cesarean delivery
- cesarean delivery after prolonged pushing
- history of uterine or abdominal surgery
- central placenta previa or accreta
- lateral or downward extension of the uterine incision
- uterine rupture
- need for hysterectomy.
Inadvertent bladder injury during entrance into the peritoneum should be managed with layered closure of the cystotomy. Because such injury occurs high in the bladder, it requires only a short period of postoperative drainage. In contrast, injury to the base of the bladder requires appropriate mobilization of the bladder off any adherent structures to allow tension-free closure of the injury. Since this type of injury lies in the dependent portion of the bladder, it requires postoperative drainage for 10 to 14 days. Close the injury in two layers, using fine, delayed, absorbable suture in interrupted or running fashion, with the first layer approximating the mucosa and the second layer imbricating the muscularis.
Ureteral injury is rare during cesarean delivery. When it does occur, it usually occurs during repair of lacerations from the uterine incision or control of excessive bleeding from the lower segment or broad ligament (FIGURE). The most common site of injury from uterine lacerations is at the level of the uterine vessels, whereas the most common site of injury at cesarean hysterectomy is the lower portion of the ureter near the uterosacral ligaments.
If you suspect injury, confirm ureteral patency by making a cystotomy in the bladder dome to visualize the orifices, and attempt to pass a ureteral catheter or pediatric feeding tube through the orifice into the ureter until you reach a point above the area of concern. If there is an obstruction, kinking, or transection, consult a urogynecologist or urologist.
Risks of lateral and downward extension
Avoid lateral and downward extension of the uterine incision, which may injure the blood vessels or ureter.
Source: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011.
10. Close the skin with subcuticular suture
This approach is associated with lower risk of wound separation and infection than is closure with staples
Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682–690.
Wound complications following cesarean can include hematoma, seroma, complete separation, or infection (superficial or deep). Wound complications may be more likely with staple closure of a transverse skin incision than with subcuticular suture. Other issues to consider when choosing a type of skin closure include postoperative pain, cosmesis, and how long it takes to deliver the infant.
This systematic review and meta-analysis of five randomized trials and one prospective study compared outcomes after skin staple closure (n = 803) with those after subcuticular suture closure (n = 684) in women who had a transverse incision. Staple closure was associated with a higher rate of wound infection or separation (13.4% vs 6.6%; pooled odds ratio, 2.06; 95% CI, 1.43–2.98). Staple closure was associated with a shorter operating time (range, 3.3–9.3 minutes). Both techniques were similar in terms of postoperative pain, cosmetic appearance, and patient satisfaction.
We want to hear from you! Tell us what you think.
- 10 practical recommendations to improve maternal and perinatal outcomes in patients with eclampsia
Baha M. Sibai, MD (November 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai, MD (June 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011) - 10 practical, evidence-based suggestions to improve your gyn practice now
Mark D. Walters, MD (January 2011)