User login
Talc pleurodesis, ICS, cardiopulmonary exercise testing
Interventional Chest/Diagnostic Procedures
Review of The AMPLE Trial: is talc making a comeback?
A proposed advantage of indwelling pleural catheters (IPC) is their purported ability to reduce hospitalization time when compared with the more traditional talc pleurodesis procedure. The recently published AMPLE trial was a multicenter randomized trial comparing the impact of IPCs vs talc pleurodesis on hospitalization days in patients with malignant pleural effusions. One-hundred forty-six patients were randomized for pleurodesis to either IPC vs pleurodesis via talc slurry in nine centers in Australia, New Zealand, Singapore, and Hong Kong. Patients were followed for up to 12 months. Secondary outcomes included need for further pleural intervention, breathlessness, quality of life, and adverse events.
Patients randomized to IPC spent on average 2 days less in the hospital (10 vs 12 days), a difference that was statistically significant, though of questionable clinical relevance, and somewhat disappointing in light of a prior prospective study from the same group suggesting a benefit of 6 to 7 days (Fysh. Chest. 2012;142[2]:394. As in previous studies, additional pleural procedures were more common in the talc group, adverse events occurred more frequently with IPC, but breathlessness and quality of life were identical in both groups.
This study raises interesting questions. Clearly, IPCs have been favored over talc pleurodesis in the US in the last decade, primarily because of a perceived benefit in terms of hospitalization time. In the absence of clear advantage of IPC on time spent in the hospital, impact on breathlessness and quality of life, and considering the inconvenience of frequent drainage, co-pay incurred by patients, and increased adverse events with IPC, the pendulum may swing again toward talc pleurodesis.
Christine Argento, MD, FCCP
Fabien Maldonado, MD, FCCP
Steering Committee Members
Pediatric Chest Medicine
Early escalation of inhaled corticosteroids: does it help prevent asthma exacerbations?
Asthma is one of the most common chronic conditions in children. The importance of effective control of asthma to prevent exacerbations is well accepted. Inhaled corticosteroids (ICS) are a preferred component of treatment to improve asthma control in children with persistent asthma; however, exacerbations can still occur and result in significant morbidity. Most patients receive systemic corticosteroids during acute asthma exacerbations. The most recent Global Initiative for Asthma (GINA) guidelines recommend increasing ICS at the first signs of an asthma exacerbation in an effort to lessen the need for systemic corticosteroids (GINA. Global strategy for asthma management and prevention. 2017. http://www.ginasthma.org/).
In a recent issue of the New England Journal of Medicine, Jackson and colleagues at the National Heart, Lung, and Blood Institute AsthmaNet published the results of a randomized, double-blind 48-week trial, which included 254 children between ages 5 and 11 years with mild-moderate asthma. Their objectives were to compare exacerbation rates, time to first exacerbation, acute care visits, and bronchodilator use in children randomized to treatment with either high (5 x baseline ICS dose x 7 days) or low dose inhaled corticosteroids early in a drop to the “yellow zone” (Jackson, et al. N Engl J Med. 2018;378[10]:891).
Time to asthma exacerbations and exacerbations that required treatment with corticosteroids did not significantly differ between the low dose and high dose groups. Unexpectedly, the rate of exacerbations was higher with the high dose compared with the low dose group (0.48 vs 0.37). The children who were in the high dose group received 16% more ICS compared with the low dose group. Although not significant, there was a lower linear growth rate, ~0.23 cm per year seen in this high-dose group than in the low-dose group. Additionally, the use of bronchodilator, symptoms, and the rates of evaluation by a physician (ie, emergency department or urgent care visits) did not significantly differ between the two groups.
This study was specific to school-age children with mild-moderate persistent asthma treated with low dose ICS with a history of good adherence. Overall, this well-designed study helps address a question that many clinicians have regarding escalating ICS in the “yellow zone.” Escalating ICS did not reduce exacerbations at the cost of a lower linear growth rate. When it comes to escalating ICS for asthma exacerbation, more is not better.
In conclusion, in children with mild-to-moderate persistent asthma treated with daily inhaled glucocorticoids, quintupling the dose at the early signs of loss of asthma control did not reduce the rate of severe asthma exacerbations or improve other asthma outcomes and may be associated with diminished linear growth. (Funded by the National Heart, Lung, and Blood Institute; STICS ClinicalTrials.gov number, NCT02066129).
John Bishara, DO
Fellow-in-Training Member
Pulmonary Physiology, Function, and Rehabilitation
Understanding cardiopulmonary exercise testing
The cardiopulmonary exercise test (CPET) is an underutilized tool for evaluating patients with dyspnea of uncertain etiology. This is often due to the daunting task of trying to make sense of seemingly large amounts of interacting data, along with clinicians not having been taught a systematic approach for interpreting the results. Unlike other typical tests we order that point to a specific laboratory or anatomic radiographic abnormality, narrowing our differential to a few possibilities, one needs a different mindset when interpreting a CPET. This is a study to demonstrate the body’s normal or abnormal physiologic responses to increasing levels of physical stress. Because different conditions can give similar findings, the physiologic abnormalities must be interpreted in the context of the clinical presentation. If the results do not entirely fit the suspected diagnosis, they should be reported in a manner that may help guide the ordering physician down an alternate pathway. This CHEST NetWork has sought ways to reach out to members to promote a better understanding of the utilization of the basics of pulmonary physiology in the management of patients. We created an online two-part video demonstrating a basic systematic approach toward understanding the combinations of findings one often sees when performing a CPET. A comprehensive understanding cannot be shown in a 40-minute video series, but, hopefully, this will give a starting point to make this task easier and more enjoyable.
Zachary Morris, MD, FCCP
Steering Committee Member
Pulmonary Vascular Disease
BMPR2 mutation regulates singular millimetric fibrovascular lesions in bronchial circulation in PAH
Patients with PAH with BMPR2 mutation are younger with worse hemodynamics, ie, higher mean PAP with higher PVR and a lower cardiac index in comparison to the noncarriers. A systematic analysis of pulmonary imaging using CT angiography or magnetic resonance imaging in patients with PAH demonstrated increased bronchial arterial hypertrophy in BMPR2 mutation carriers compared with those without the mutation. Moreover, hemoptysis is more frequently encountered in patients with PAH with BMPR2 mutation and presumably related to bronchial artery remodeling and angiogenesis. French investigators described, in histopathology findings of explanted lungs of 44 patients with PAH (23 carriers of BMPR2 and 21 noncarriers), unusual singular millimetric fibrovascular lesions (SiMFi) in patients with BMPR2 mutations. The SiMFi is a structure of millimetric dimension with fibrovascular characteristics that are extremely rich in collagen and displayed more than one vascular channel. SiMFi did not show a classic glomeruloid pattern with predominant endothelial cell proliferation as seen in plexiform lesions but rather a large conglomerate of hypertrophic vessels. Performing an ink injection experiment in a freshly explanted lung highlighted a patent connection between bronchial/systemic vessels and pulmonary septal veins. SiMFis had an increased amount of bronchial microvessels and showed increased hypertrophy of larger bronchial arteries. SiMFi is directly related to hypertrophy and/or angiogenesis of vasa vasorum/bronchial arteries in the vicinity of the diseased artery. In patients with PAH with BMPR2 mutations, bronchial angiogenesis is more prevalent compared with patients with PAH lacking these mutations. This highlights the role of bronchial arteries in the spectrum of PAH.
Hector Cajigas, MD, FCCP
Sandeep Sahay, MD, FCCP
Steering Committee Members
References
1.Ghigna MR, et al. BMPR2 mutation status influences bronchial vascular changes in pulmonary arterial hypertension. Eur Respir J. 2016;48[6]:1668. Epub 2016 Nov 3.
2. Tio D, et al. Risk factors for hemoptysis in idiopathic and hereditary pulmonary arterial hypertension. PLoS One. 2013;8:e78132.
3. Elliott CG, et al. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation. 2006;113[21]:2509.
Thoracic Oncology
We have a lung cancer screening test but we could use it better
The American Lung Association recently demonstrated the majority of current and former smokers don’t know about lung cancer screening (LCS) with low-dose CT scanning.1 Researchers estimate less than 5% of eligible persons received LCS.2 Awareness campaigns targeting patients and health care providers at the local level can improve LCS uptake.3,4 While any new clinical practice has an expected implementation delay, LCS has another implementation barrier: complex eligibility criteria (age 55 – 80 years PLUS 30+ pack-year smoking history PLUS quit time less than 15 years). Electronic health record (EHR) tools might accelerate the adoption curve to identify eligible persons.5 Moreover, assessing and recording a qualitative smoking history is challenging, at best. One center showed 96.2% discordance between EHR smoking history and that obtained during shared decision-making visit for LCS.6 Mostly, the EHR underreported quantitative pack-year history; meaning LCS-eligible patients might fail to be identified by EHR review alone. Another small pilot showed that some patients age 55 – 79 years will update their EHR smoking history using patient portal, but this will not be effective for all patients.7 For current smokers, age alone may be an effective identification strategy, given the average start time for most smokers.8
Even though current LCS guidelines leave out some individuals at high risk for lung cancer, we must continue efforts to offer this potentially life-saving service to patients now eligible. Using EHR tools may help proactively identify those who are eligible for lung cancer screening.
A bbie Begnaud, MD
NetWork Member
References
1. New Study from American Lung Association’s LUNG FORCE Reveals Low Awareness of Lifesaving Lung Cancer Screening Among Those at Greatest Risk. (2017). http://www.lung.org/about-us/media/press-releases/new-study-lung-cancer-screening.html. Accessed April 19, 2018.
2. Soneji S, et al. Underuse of Chest Radiography Versus Computed Tomography for Lung Cancer Screening. Am J Public Health. 2017;107(8):1248.
3. Cardarelli R, et al. Terminate lung cancer (TLC) study-A mixed-methods population approach to increase lung cancer screening awareness and low-dose computed tomography in Eastern Kentucky. Cancer Epidemiol. 2017;46:1.
4. Jessup, DL, et al. Implementation of digital awareness strategies to engage patients and providers in a lung cancer screening program: retrospective study. J Med Internet Res. 2018;20(2):e52.
5. Comparison of the Electronic Medical Record versus a Shared Decision Making Conversation. Ann Am Thorac Soc. 2018. In press.
6. Modin HE, et al. Pack-year cigarette smoking history for determination of lung cancer screening eligibility. Ann Am Thorac Soc. 2017 Aug;14(8):1320-1325.
7. Begnaud AL, et al. Randomized electronic promotion of lung cancer screening: a pilot. JCO Clinical Cancer Informatics(1), 1-6. doi:10.1200/cci.17.00033
8. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. (2014). Atlanta, GA.
Interventional Chest/Diagnostic Procedures
Review of The AMPLE Trial: is talc making a comeback?
A proposed advantage of indwelling pleural catheters (IPC) is their purported ability to reduce hospitalization time when compared with the more traditional talc pleurodesis procedure. The recently published AMPLE trial was a multicenter randomized trial comparing the impact of IPCs vs talc pleurodesis on hospitalization days in patients with malignant pleural effusions. One-hundred forty-six patients were randomized for pleurodesis to either IPC vs pleurodesis via talc slurry in nine centers in Australia, New Zealand, Singapore, and Hong Kong. Patients were followed for up to 12 months. Secondary outcomes included need for further pleural intervention, breathlessness, quality of life, and adverse events.
Patients randomized to IPC spent on average 2 days less in the hospital (10 vs 12 days), a difference that was statistically significant, though of questionable clinical relevance, and somewhat disappointing in light of a prior prospective study from the same group suggesting a benefit of 6 to 7 days (Fysh. Chest. 2012;142[2]:394. As in previous studies, additional pleural procedures were more common in the talc group, adverse events occurred more frequently with IPC, but breathlessness and quality of life were identical in both groups.
This study raises interesting questions. Clearly, IPCs have been favored over talc pleurodesis in the US in the last decade, primarily because of a perceived benefit in terms of hospitalization time. In the absence of clear advantage of IPC on time spent in the hospital, impact on breathlessness and quality of life, and considering the inconvenience of frequent drainage, co-pay incurred by patients, and increased adverse events with IPC, the pendulum may swing again toward talc pleurodesis.
Christine Argento, MD, FCCP
Fabien Maldonado, MD, FCCP
Steering Committee Members
Pediatric Chest Medicine
Early escalation of inhaled corticosteroids: does it help prevent asthma exacerbations?
Asthma is one of the most common chronic conditions in children. The importance of effective control of asthma to prevent exacerbations is well accepted. Inhaled corticosteroids (ICS) are a preferred component of treatment to improve asthma control in children with persistent asthma; however, exacerbations can still occur and result in significant morbidity. Most patients receive systemic corticosteroids during acute asthma exacerbations. The most recent Global Initiative for Asthma (GINA) guidelines recommend increasing ICS at the first signs of an asthma exacerbation in an effort to lessen the need for systemic corticosteroids (GINA. Global strategy for asthma management and prevention. 2017. http://www.ginasthma.org/).
In a recent issue of the New England Journal of Medicine, Jackson and colleagues at the National Heart, Lung, and Blood Institute AsthmaNet published the results of a randomized, double-blind 48-week trial, which included 254 children between ages 5 and 11 years with mild-moderate asthma. Their objectives were to compare exacerbation rates, time to first exacerbation, acute care visits, and bronchodilator use in children randomized to treatment with either high (5 x baseline ICS dose x 7 days) or low dose inhaled corticosteroids early in a drop to the “yellow zone” (Jackson, et al. N Engl J Med. 2018;378[10]:891).
Time to asthma exacerbations and exacerbations that required treatment with corticosteroids did not significantly differ between the low dose and high dose groups. Unexpectedly, the rate of exacerbations was higher with the high dose compared with the low dose group (0.48 vs 0.37). The children who were in the high dose group received 16% more ICS compared with the low dose group. Although not significant, there was a lower linear growth rate, ~0.23 cm per year seen in this high-dose group than in the low-dose group. Additionally, the use of bronchodilator, symptoms, and the rates of evaluation by a physician (ie, emergency department or urgent care visits) did not significantly differ between the two groups.
This study was specific to school-age children with mild-moderate persistent asthma treated with low dose ICS with a history of good adherence. Overall, this well-designed study helps address a question that many clinicians have regarding escalating ICS in the “yellow zone.” Escalating ICS did not reduce exacerbations at the cost of a lower linear growth rate. When it comes to escalating ICS for asthma exacerbation, more is not better.
In conclusion, in children with mild-to-moderate persistent asthma treated with daily inhaled glucocorticoids, quintupling the dose at the early signs of loss of asthma control did not reduce the rate of severe asthma exacerbations or improve other asthma outcomes and may be associated with diminished linear growth. (Funded by the National Heart, Lung, and Blood Institute; STICS ClinicalTrials.gov number, NCT02066129).
John Bishara, DO
Fellow-in-Training Member
Pulmonary Physiology, Function, and Rehabilitation
Understanding cardiopulmonary exercise testing
The cardiopulmonary exercise test (CPET) is an underutilized tool for evaluating patients with dyspnea of uncertain etiology. This is often due to the daunting task of trying to make sense of seemingly large amounts of interacting data, along with clinicians not having been taught a systematic approach for interpreting the results. Unlike other typical tests we order that point to a specific laboratory or anatomic radiographic abnormality, narrowing our differential to a few possibilities, one needs a different mindset when interpreting a CPET. This is a study to demonstrate the body’s normal or abnormal physiologic responses to increasing levels of physical stress. Because different conditions can give similar findings, the physiologic abnormalities must be interpreted in the context of the clinical presentation. If the results do not entirely fit the suspected diagnosis, they should be reported in a manner that may help guide the ordering physician down an alternate pathway. This CHEST NetWork has sought ways to reach out to members to promote a better understanding of the utilization of the basics of pulmonary physiology in the management of patients. We created an online two-part video demonstrating a basic systematic approach toward understanding the combinations of findings one often sees when performing a CPET. A comprehensive understanding cannot be shown in a 40-minute video series, but, hopefully, this will give a starting point to make this task easier and more enjoyable.
Zachary Morris, MD, FCCP
Steering Committee Member
Pulmonary Vascular Disease
BMPR2 mutation regulates singular millimetric fibrovascular lesions in bronchial circulation in PAH
Patients with PAH with BMPR2 mutation are younger with worse hemodynamics, ie, higher mean PAP with higher PVR and a lower cardiac index in comparison to the noncarriers. A systematic analysis of pulmonary imaging using CT angiography or magnetic resonance imaging in patients with PAH demonstrated increased bronchial arterial hypertrophy in BMPR2 mutation carriers compared with those without the mutation. Moreover, hemoptysis is more frequently encountered in patients with PAH with BMPR2 mutation and presumably related to bronchial artery remodeling and angiogenesis. French investigators described, in histopathology findings of explanted lungs of 44 patients with PAH (23 carriers of BMPR2 and 21 noncarriers), unusual singular millimetric fibrovascular lesions (SiMFi) in patients with BMPR2 mutations. The SiMFi is a structure of millimetric dimension with fibrovascular characteristics that are extremely rich in collagen and displayed more than one vascular channel. SiMFi did not show a classic glomeruloid pattern with predominant endothelial cell proliferation as seen in plexiform lesions but rather a large conglomerate of hypertrophic vessels. Performing an ink injection experiment in a freshly explanted lung highlighted a patent connection between bronchial/systemic vessels and pulmonary septal veins. SiMFis had an increased amount of bronchial microvessels and showed increased hypertrophy of larger bronchial arteries. SiMFi is directly related to hypertrophy and/or angiogenesis of vasa vasorum/bronchial arteries in the vicinity of the diseased artery. In patients with PAH with BMPR2 mutations, bronchial angiogenesis is more prevalent compared with patients with PAH lacking these mutations. This highlights the role of bronchial arteries in the spectrum of PAH.
Hector Cajigas, MD, FCCP
Sandeep Sahay, MD, FCCP
Steering Committee Members
References
1.Ghigna MR, et al. BMPR2 mutation status influences bronchial vascular changes in pulmonary arterial hypertension. Eur Respir J. 2016;48[6]:1668. Epub 2016 Nov 3.
2. Tio D, et al. Risk factors for hemoptysis in idiopathic and hereditary pulmonary arterial hypertension. PLoS One. 2013;8:e78132.
3. Elliott CG, et al. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation. 2006;113[21]:2509.
Thoracic Oncology
We have a lung cancer screening test but we could use it better
The American Lung Association recently demonstrated the majority of current and former smokers don’t know about lung cancer screening (LCS) with low-dose CT scanning.1 Researchers estimate less than 5% of eligible persons received LCS.2 Awareness campaigns targeting patients and health care providers at the local level can improve LCS uptake.3,4 While any new clinical practice has an expected implementation delay, LCS has another implementation barrier: complex eligibility criteria (age 55 – 80 years PLUS 30+ pack-year smoking history PLUS quit time less than 15 years). Electronic health record (EHR) tools might accelerate the adoption curve to identify eligible persons.5 Moreover, assessing and recording a qualitative smoking history is challenging, at best. One center showed 96.2% discordance between EHR smoking history and that obtained during shared decision-making visit for LCS.6 Mostly, the EHR underreported quantitative pack-year history; meaning LCS-eligible patients might fail to be identified by EHR review alone. Another small pilot showed that some patients age 55 – 79 years will update their EHR smoking history using patient portal, but this will not be effective for all patients.7 For current smokers, age alone may be an effective identification strategy, given the average start time for most smokers.8
Even though current LCS guidelines leave out some individuals at high risk for lung cancer, we must continue efforts to offer this potentially life-saving service to patients now eligible. Using EHR tools may help proactively identify those who are eligible for lung cancer screening.
A bbie Begnaud, MD
NetWork Member
References
1. New Study from American Lung Association’s LUNG FORCE Reveals Low Awareness of Lifesaving Lung Cancer Screening Among Those at Greatest Risk. (2017). http://www.lung.org/about-us/media/press-releases/new-study-lung-cancer-screening.html. Accessed April 19, 2018.
2. Soneji S, et al. Underuse of Chest Radiography Versus Computed Tomography for Lung Cancer Screening. Am J Public Health. 2017;107(8):1248.
3. Cardarelli R, et al. Terminate lung cancer (TLC) study-A mixed-methods population approach to increase lung cancer screening awareness and low-dose computed tomography in Eastern Kentucky. Cancer Epidemiol. 2017;46:1.
4. Jessup, DL, et al. Implementation of digital awareness strategies to engage patients and providers in a lung cancer screening program: retrospective study. J Med Internet Res. 2018;20(2):e52.
5. Comparison of the Electronic Medical Record versus a Shared Decision Making Conversation. Ann Am Thorac Soc. 2018. In press.
6. Modin HE, et al. Pack-year cigarette smoking history for determination of lung cancer screening eligibility. Ann Am Thorac Soc. 2017 Aug;14(8):1320-1325.
7. Begnaud AL, et al. Randomized electronic promotion of lung cancer screening: a pilot. JCO Clinical Cancer Informatics(1), 1-6. doi:10.1200/cci.17.00033
8. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. (2014). Atlanta, GA.
Interventional Chest/Diagnostic Procedures
Review of The AMPLE Trial: is talc making a comeback?
A proposed advantage of indwelling pleural catheters (IPC) is their purported ability to reduce hospitalization time when compared with the more traditional talc pleurodesis procedure. The recently published AMPLE trial was a multicenter randomized trial comparing the impact of IPCs vs talc pleurodesis on hospitalization days in patients with malignant pleural effusions. One-hundred forty-six patients were randomized for pleurodesis to either IPC vs pleurodesis via talc slurry in nine centers in Australia, New Zealand, Singapore, and Hong Kong. Patients were followed for up to 12 months. Secondary outcomes included need for further pleural intervention, breathlessness, quality of life, and adverse events.
Patients randomized to IPC spent on average 2 days less in the hospital (10 vs 12 days), a difference that was statistically significant, though of questionable clinical relevance, and somewhat disappointing in light of a prior prospective study from the same group suggesting a benefit of 6 to 7 days (Fysh. Chest. 2012;142[2]:394. As in previous studies, additional pleural procedures were more common in the talc group, adverse events occurred more frequently with IPC, but breathlessness and quality of life were identical in both groups.
This study raises interesting questions. Clearly, IPCs have been favored over talc pleurodesis in the US in the last decade, primarily because of a perceived benefit in terms of hospitalization time. In the absence of clear advantage of IPC on time spent in the hospital, impact on breathlessness and quality of life, and considering the inconvenience of frequent drainage, co-pay incurred by patients, and increased adverse events with IPC, the pendulum may swing again toward talc pleurodesis.
Christine Argento, MD, FCCP
Fabien Maldonado, MD, FCCP
Steering Committee Members
Pediatric Chest Medicine
Early escalation of inhaled corticosteroids: does it help prevent asthma exacerbations?
Asthma is one of the most common chronic conditions in children. The importance of effective control of asthma to prevent exacerbations is well accepted. Inhaled corticosteroids (ICS) are a preferred component of treatment to improve asthma control in children with persistent asthma; however, exacerbations can still occur and result in significant morbidity. Most patients receive systemic corticosteroids during acute asthma exacerbations. The most recent Global Initiative for Asthma (GINA) guidelines recommend increasing ICS at the first signs of an asthma exacerbation in an effort to lessen the need for systemic corticosteroids (GINA. Global strategy for asthma management and prevention. 2017. http://www.ginasthma.org/).
In a recent issue of the New England Journal of Medicine, Jackson and colleagues at the National Heart, Lung, and Blood Institute AsthmaNet published the results of a randomized, double-blind 48-week trial, which included 254 children between ages 5 and 11 years with mild-moderate asthma. Their objectives were to compare exacerbation rates, time to first exacerbation, acute care visits, and bronchodilator use in children randomized to treatment with either high (5 x baseline ICS dose x 7 days) or low dose inhaled corticosteroids early in a drop to the “yellow zone” (Jackson, et al. N Engl J Med. 2018;378[10]:891).
Time to asthma exacerbations and exacerbations that required treatment with corticosteroids did not significantly differ between the low dose and high dose groups. Unexpectedly, the rate of exacerbations was higher with the high dose compared with the low dose group (0.48 vs 0.37). The children who were in the high dose group received 16% more ICS compared with the low dose group. Although not significant, there was a lower linear growth rate, ~0.23 cm per year seen in this high-dose group than in the low-dose group. Additionally, the use of bronchodilator, symptoms, and the rates of evaluation by a physician (ie, emergency department or urgent care visits) did not significantly differ between the two groups.
This study was specific to school-age children with mild-moderate persistent asthma treated with low dose ICS with a history of good adherence. Overall, this well-designed study helps address a question that many clinicians have regarding escalating ICS in the “yellow zone.” Escalating ICS did not reduce exacerbations at the cost of a lower linear growth rate. When it comes to escalating ICS for asthma exacerbation, more is not better.
In conclusion, in children with mild-to-moderate persistent asthma treated with daily inhaled glucocorticoids, quintupling the dose at the early signs of loss of asthma control did not reduce the rate of severe asthma exacerbations or improve other asthma outcomes and may be associated with diminished linear growth. (Funded by the National Heart, Lung, and Blood Institute; STICS ClinicalTrials.gov number, NCT02066129).
John Bishara, DO
Fellow-in-Training Member
Pulmonary Physiology, Function, and Rehabilitation
Understanding cardiopulmonary exercise testing
The cardiopulmonary exercise test (CPET) is an underutilized tool for evaluating patients with dyspnea of uncertain etiology. This is often due to the daunting task of trying to make sense of seemingly large amounts of interacting data, along with clinicians not having been taught a systematic approach for interpreting the results. Unlike other typical tests we order that point to a specific laboratory or anatomic radiographic abnormality, narrowing our differential to a few possibilities, one needs a different mindset when interpreting a CPET. This is a study to demonstrate the body’s normal or abnormal physiologic responses to increasing levels of physical stress. Because different conditions can give similar findings, the physiologic abnormalities must be interpreted in the context of the clinical presentation. If the results do not entirely fit the suspected diagnosis, they should be reported in a manner that may help guide the ordering physician down an alternate pathway. This CHEST NetWork has sought ways to reach out to members to promote a better understanding of the utilization of the basics of pulmonary physiology in the management of patients. We created an online two-part video demonstrating a basic systematic approach toward understanding the combinations of findings one often sees when performing a CPET. A comprehensive understanding cannot be shown in a 40-minute video series, but, hopefully, this will give a starting point to make this task easier and more enjoyable.
Zachary Morris, MD, FCCP
Steering Committee Member
Pulmonary Vascular Disease
BMPR2 mutation regulates singular millimetric fibrovascular lesions in bronchial circulation in PAH
Patients with PAH with BMPR2 mutation are younger with worse hemodynamics, ie, higher mean PAP with higher PVR and a lower cardiac index in comparison to the noncarriers. A systematic analysis of pulmonary imaging using CT angiography or magnetic resonance imaging in patients with PAH demonstrated increased bronchial arterial hypertrophy in BMPR2 mutation carriers compared with those without the mutation. Moreover, hemoptysis is more frequently encountered in patients with PAH with BMPR2 mutation and presumably related to bronchial artery remodeling and angiogenesis. French investigators described, in histopathology findings of explanted lungs of 44 patients with PAH (23 carriers of BMPR2 and 21 noncarriers), unusual singular millimetric fibrovascular lesions (SiMFi) in patients with BMPR2 mutations. The SiMFi is a structure of millimetric dimension with fibrovascular characteristics that are extremely rich in collagen and displayed more than one vascular channel. SiMFi did not show a classic glomeruloid pattern with predominant endothelial cell proliferation as seen in plexiform lesions but rather a large conglomerate of hypertrophic vessels. Performing an ink injection experiment in a freshly explanted lung highlighted a patent connection between bronchial/systemic vessels and pulmonary septal veins. SiMFis had an increased amount of bronchial microvessels and showed increased hypertrophy of larger bronchial arteries. SiMFi is directly related to hypertrophy and/or angiogenesis of vasa vasorum/bronchial arteries in the vicinity of the diseased artery. In patients with PAH with BMPR2 mutations, bronchial angiogenesis is more prevalent compared with patients with PAH lacking these mutations. This highlights the role of bronchial arteries in the spectrum of PAH.
Hector Cajigas, MD, FCCP
Sandeep Sahay, MD, FCCP
Steering Committee Members
References
1.Ghigna MR, et al. BMPR2 mutation status influences bronchial vascular changes in pulmonary arterial hypertension. Eur Respir J. 2016;48[6]:1668. Epub 2016 Nov 3.
2. Tio D, et al. Risk factors for hemoptysis in idiopathic and hereditary pulmonary arterial hypertension. PLoS One. 2013;8:e78132.
3. Elliott CG, et al. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation. 2006;113[21]:2509.
Thoracic Oncology
We have a lung cancer screening test but we could use it better
The American Lung Association recently demonstrated the majority of current and former smokers don’t know about lung cancer screening (LCS) with low-dose CT scanning.1 Researchers estimate less than 5% of eligible persons received LCS.2 Awareness campaigns targeting patients and health care providers at the local level can improve LCS uptake.3,4 While any new clinical practice has an expected implementation delay, LCS has another implementation barrier: complex eligibility criteria (age 55 – 80 years PLUS 30+ pack-year smoking history PLUS quit time less than 15 years). Electronic health record (EHR) tools might accelerate the adoption curve to identify eligible persons.5 Moreover, assessing and recording a qualitative smoking history is challenging, at best. One center showed 96.2% discordance between EHR smoking history and that obtained during shared decision-making visit for LCS.6 Mostly, the EHR underreported quantitative pack-year history; meaning LCS-eligible patients might fail to be identified by EHR review alone. Another small pilot showed that some patients age 55 – 79 years will update their EHR smoking history using patient portal, but this will not be effective for all patients.7 For current smokers, age alone may be an effective identification strategy, given the average start time for most smokers.8
Even though current LCS guidelines leave out some individuals at high risk for lung cancer, we must continue efforts to offer this potentially life-saving service to patients now eligible. Using EHR tools may help proactively identify those who are eligible for lung cancer screening.
A bbie Begnaud, MD
NetWork Member
References
1. New Study from American Lung Association’s LUNG FORCE Reveals Low Awareness of Lifesaving Lung Cancer Screening Among Those at Greatest Risk. (2017). http://www.lung.org/about-us/media/press-releases/new-study-lung-cancer-screening.html. Accessed April 19, 2018.
2. Soneji S, et al. Underuse of Chest Radiography Versus Computed Tomography for Lung Cancer Screening. Am J Public Health. 2017;107(8):1248.
3. Cardarelli R, et al. Terminate lung cancer (TLC) study-A mixed-methods population approach to increase lung cancer screening awareness and low-dose computed tomography in Eastern Kentucky. Cancer Epidemiol. 2017;46:1.
4. Jessup, DL, et al. Implementation of digital awareness strategies to engage patients and providers in a lung cancer screening program: retrospective study. J Med Internet Res. 2018;20(2):e52.
5. Comparison of the Electronic Medical Record versus a Shared Decision Making Conversation. Ann Am Thorac Soc. 2018. In press.
6. Modin HE, et al. Pack-year cigarette smoking history for determination of lung cancer screening eligibility. Ann Am Thorac Soc. 2017 Aug;14(8):1320-1325.
7. Begnaud AL, et al. Randomized electronic promotion of lung cancer screening: a pilot. JCO Clinical Cancer Informatics(1), 1-6. doi:10.1200/cci.17.00033
8. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. (2014). Atlanta, GA.
Palliative care screening, sleep devices, novel biologics
Palliative and end-of-life care
Nurse-driven palliative care screening
Palliative care (PC) aims to improve quality of life for patients with a life-threatening illness, providing holistic patient-centered support along the continuum of the disease process. Although frequently implemented in critical care settings, integrating PC in the neuro ICU has been difficult to adopt in practice due to the uncertainty in prognostication of definitive outcomes and practice culture beliefs such as the self-fulfilling prophecy (Frontera, et al. Crit Care Med. 2015;43[9]:1964; Rubin, et al. Curr Opin Crit Care. 2017;23[2]:134; Knies, et al. Semin Neurol. 2016;36[6]:631).
At our institution, a nursing education project was conducted to pilot nurse-driven PC screenings on admission to the neuro ICU. The project evaluated nurse comfort and knowledge with identifying and recommending PC consults. Pre- and post-intervention surveys revealed that education and introduction of a PC screening tool significantly increased nurse comfort and knowledge of PC eligibility.
PC in the neuro ICU can exist to contribute to successful outcomes in patient and family care. Within neurocritical care, incorporating PC is essential to provide extra support to patients and families (Frontera, et al. 2015).
For these reasons and data from the project, nurse-driven screening may encourage appropriate early PC consults. Patient-centered care is the ultimate goal in the management of our patients. Nurse-driven PC screening can help bring various unmet PC needs to the health-care team for opportunities that might not have been met or otherwise assessed. Consider implementing nurse-driven PC screening protocols at your institution to aid in collaborative and proactive interdisciplinary care.
Danielle McCamey, ACNP
Steering Committee Member
Sleep medicine
Diagnostics, devices, and sleep
The past several months have been busy for the Sleep Medicine NetWork. We have been working to represent the interests of our membership and our patients in many arenas.
Devices coded as E0464, defined as life support mechanical ventilators used with mask-based ventilation in the home are being more frequently used. According to the Office of the Inspector General (OIG), there has been an 89-fold increase in billing for E0464 ventilators for Medicare and its beneficiaries between 2009 and 2015, increasing from $3.8M to $340M. In response, the Agency for Healthcare Research and Quality (AHRQ) requested a response to specific questions related to these devices.
In 2018, the CHEST Sleep Medicine NetWork will be participating in a Federal Drug Association-sponsored workshop entitled “Study Design Considerations for Devices including Digital Health Technologies for Sleep-Disordered Breathing (SDB) in Adults,” along with other national organizations and leaders in our field. This workshop will address available technologies for the diagnosis, monitoring, and treatment of SDB, as well as trends for digital health technologies and clinical trial design considerations.
Finally, the Sleep Medicine NetWork has wasted no time after a successful CHEST 2017 in Toronto in planning for the next annual meeting in San Antonio. We are excited to present an exciting curriculum in Sleep Medicine at CHEST 2018, so stay tuned.
Aneesa M. Das, MD, FCCP
NetWork Chair
Occupational and environmental health
Post-deployment lung disease
Since the early 1990s, ongoing military deployments to Southwest Asia remain a unique challenge from a pulmonary symptomology and diagnostic perspective.
Various airborne hazards in the deployment environment include geologic dusts, burn pit smoke, vehicle emissions, and industrial air pollution. Exposures can give rise to both acute respiratory symptoms and, in some instances, chronic lung disease. Currently, data are limited on whether inhalation of airborne particulate matter by military personnel is linked to increases in pulmonary diseases (Morris MJ, et al. US Army Med Dep J. 2016:173).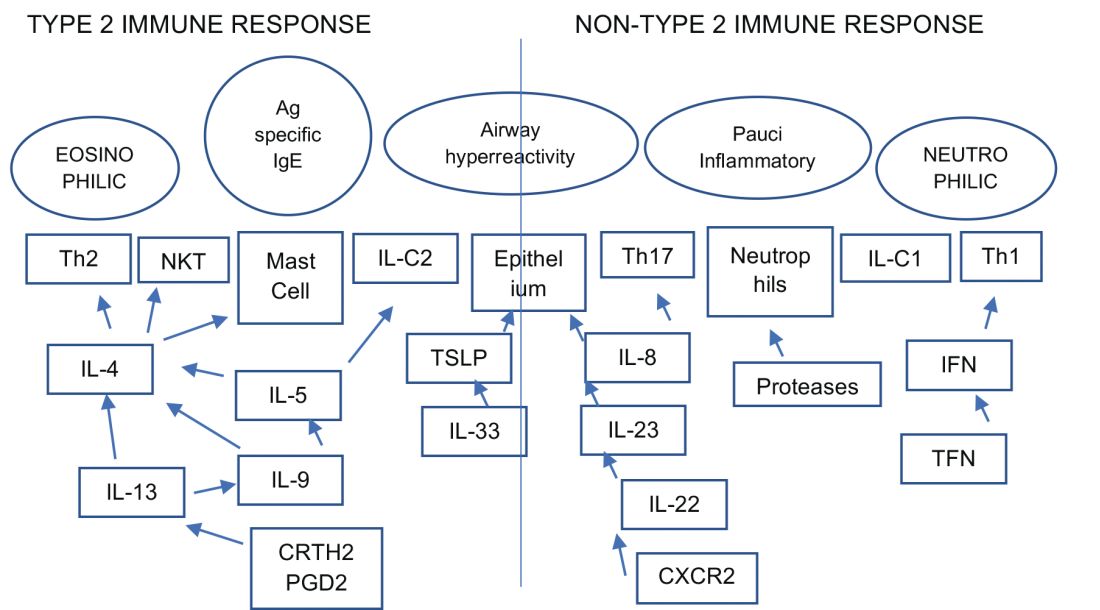
Ongoing research by the Veterans Affairs continues to enroll post-deployed personnel in an Airborne Hazard and Burn Pit Registry. Past approaches in evaluation of deployed individuals ranged from common tests such as spirometry, HRCT scanning, full PFTs, bronchoprovocation challenges, and, in some instances, lung biopsies (Krefft SD, et al. Fed Pract. 2015;32[6]:32). More novel evaluations of postdeployment dyspnea include impulse oscillometry, exhaled nitric oxide, bronchoscopy, and cardiopulmonary exercise testing (Huprikar, et al. Chest. 2016;150[4]:S934A).
Members of the CHEST Occupational and Environmental Health NetWork are currently updating comprehensive approaches to evaluate military personnel with chronic respiratory symptoms from deployments. Continued emphasis, however, should be placed on diagnosing and treating common diseases such as asthma, exercise-induced bronchospasm, GERD, and upper airway disorders.
Pedro F. Lucero, MD, FCCP
Steering Committee Member
Clinical pulmonary medicine
Biologics – Birth of a new era of precision management in asthma
An estimated 10% to 20% of patients with severe uncontrolled asthma do not respond to maximal best standard treatments, leading to substantial health-care costs. A paradigm shift is now underway in our approach to the care of these patients with the emergence of novel biologics targeting the complex and interconnected inflammatory pathways in asthma that result in a diverse profile of asthma endotypes and phenotypes (Fig 1).
Current FDA-approved biologics primarily target patients with a T2 high phenotype (Table1).
Dupilumab binds to the alpha unit of the IL-4 receptor and blocks both IL-4 and IL-13. It shows potential efficacy in patients with T2 high asthma with or without eosinophilia but has not yet received FDA approval.
Multiple newer biologics are currently in development (Table 2).
Pulmonologists need to get familiar with the logistics of administration of these novel agents. The two common methods of administering biologics are (1) buy and bill – where the provider buys the drug directly from the distributor; and (2) assignment of benefits (typically administered by a Pharmacy Benefit Manager) - specific dose of the medication is shipped to the physician’s office and physician only bills for the administration. CPT and J codes are shown in Table 1.
Shyamsunder Subramanian, MD, FCCP
Steering Committee Member
Palliative and end-of-life care
Nurse-driven palliative care screening
Palliative care (PC) aims to improve quality of life for patients with a life-threatening illness, providing holistic patient-centered support along the continuum of the disease process. Although frequently implemented in critical care settings, integrating PC in the neuro ICU has been difficult to adopt in practice due to the uncertainty in prognostication of definitive outcomes and practice culture beliefs such as the self-fulfilling prophecy (Frontera, et al. Crit Care Med. 2015;43[9]:1964; Rubin, et al. Curr Opin Crit Care. 2017;23[2]:134; Knies, et al. Semin Neurol. 2016;36[6]:631).
At our institution, a nursing education project was conducted to pilot nurse-driven PC screenings on admission to the neuro ICU. The project evaluated nurse comfort and knowledge with identifying and recommending PC consults. Pre- and post-intervention surveys revealed that education and introduction of a PC screening tool significantly increased nurse comfort and knowledge of PC eligibility.
PC in the neuro ICU can exist to contribute to successful outcomes in patient and family care. Within neurocritical care, incorporating PC is essential to provide extra support to patients and families (Frontera, et al. 2015).
For these reasons and data from the project, nurse-driven screening may encourage appropriate early PC consults. Patient-centered care is the ultimate goal in the management of our patients. Nurse-driven PC screening can help bring various unmet PC needs to the health-care team for opportunities that might not have been met or otherwise assessed. Consider implementing nurse-driven PC screening protocols at your institution to aid in collaborative and proactive interdisciplinary care.
Danielle McCamey, ACNP
Steering Committee Member
Sleep medicine
Diagnostics, devices, and sleep
The past several months have been busy for the Sleep Medicine NetWork. We have been working to represent the interests of our membership and our patients in many arenas.
Devices coded as E0464, defined as life support mechanical ventilators used with mask-based ventilation in the home are being more frequently used. According to the Office of the Inspector General (OIG), there has been an 89-fold increase in billing for E0464 ventilators for Medicare and its beneficiaries between 2009 and 2015, increasing from $3.8M to $340M. In response, the Agency for Healthcare Research and Quality (AHRQ) requested a response to specific questions related to these devices.
In 2018, the CHEST Sleep Medicine NetWork will be participating in a Federal Drug Association-sponsored workshop entitled “Study Design Considerations for Devices including Digital Health Technologies for Sleep-Disordered Breathing (SDB) in Adults,” along with other national organizations and leaders in our field. This workshop will address available technologies for the diagnosis, monitoring, and treatment of SDB, as well as trends for digital health technologies and clinical trial design considerations.
Finally, the Sleep Medicine NetWork has wasted no time after a successful CHEST 2017 in Toronto in planning for the next annual meeting in San Antonio. We are excited to present an exciting curriculum in Sleep Medicine at CHEST 2018, so stay tuned.
Aneesa M. Das, MD, FCCP
NetWork Chair
Occupational and environmental health
Post-deployment lung disease
Since the early 1990s, ongoing military deployments to Southwest Asia remain a unique challenge from a pulmonary symptomology and diagnostic perspective.
Various airborne hazards in the deployment environment include geologic dusts, burn pit smoke, vehicle emissions, and industrial air pollution. Exposures can give rise to both acute respiratory symptoms and, in some instances, chronic lung disease. Currently, data are limited on whether inhalation of airborne particulate matter by military personnel is linked to increases in pulmonary diseases (Morris MJ, et al. US Army Med Dep J. 2016:173).
Ongoing research by the Veterans Affairs continues to enroll post-deployed personnel in an Airborne Hazard and Burn Pit Registry. Past approaches in evaluation of deployed individuals ranged from common tests such as spirometry, HRCT scanning, full PFTs, bronchoprovocation challenges, and, in some instances, lung biopsies (Krefft SD, et al. Fed Pract. 2015;32[6]:32). More novel evaluations of postdeployment dyspnea include impulse oscillometry, exhaled nitric oxide, bronchoscopy, and cardiopulmonary exercise testing (Huprikar, et al. Chest. 2016;150[4]:S934A).
Members of the CHEST Occupational and Environmental Health NetWork are currently updating comprehensive approaches to evaluate military personnel with chronic respiratory symptoms from deployments. Continued emphasis, however, should be placed on diagnosing and treating common diseases such as asthma, exercise-induced bronchospasm, GERD, and upper airway disorders.
Pedro F. Lucero, MD, FCCP
Steering Committee Member
Clinical pulmonary medicine
Biologics – Birth of a new era of precision management in asthma
An estimated 10% to 20% of patients with severe uncontrolled asthma do not respond to maximal best standard treatments, leading to substantial health-care costs. A paradigm shift is now underway in our approach to the care of these patients with the emergence of novel biologics targeting the complex and interconnected inflammatory pathways in asthma that result in a diverse profile of asthma endotypes and phenotypes (Fig 1).
Current FDA-approved biologics primarily target patients with a T2 high phenotype (Table1).
Dupilumab binds to the alpha unit of the IL-4 receptor and blocks both IL-4 and IL-13. It shows potential efficacy in patients with T2 high asthma with or without eosinophilia but has not yet received FDA approval.
Multiple newer biologics are currently in development (Table 2).
Pulmonologists need to get familiar with the logistics of administration of these novel agents. The two common methods of administering biologics are (1) buy and bill – where the provider buys the drug directly from the distributor; and (2) assignment of benefits (typically administered by a Pharmacy Benefit Manager) - specific dose of the medication is shipped to the physician’s office and physician only bills for the administration. CPT and J codes are shown in Table 1.
Shyamsunder Subramanian, MD, FCCP
Steering Committee Member
Palliative and end-of-life care
Nurse-driven palliative care screening
Palliative care (PC) aims to improve quality of life for patients with a life-threatening illness, providing holistic patient-centered support along the continuum of the disease process. Although frequently implemented in critical care settings, integrating PC in the neuro ICU has been difficult to adopt in practice due to the uncertainty in prognostication of definitive outcomes and practice culture beliefs such as the self-fulfilling prophecy (Frontera, et al. Crit Care Med. 2015;43[9]:1964; Rubin, et al. Curr Opin Crit Care. 2017;23[2]:134; Knies, et al. Semin Neurol. 2016;36[6]:631).
At our institution, a nursing education project was conducted to pilot nurse-driven PC screenings on admission to the neuro ICU. The project evaluated nurse comfort and knowledge with identifying and recommending PC consults. Pre- and post-intervention surveys revealed that education and introduction of a PC screening tool significantly increased nurse comfort and knowledge of PC eligibility.
PC in the neuro ICU can exist to contribute to successful outcomes in patient and family care. Within neurocritical care, incorporating PC is essential to provide extra support to patients and families (Frontera, et al. 2015).
For these reasons and data from the project, nurse-driven screening may encourage appropriate early PC consults. Patient-centered care is the ultimate goal in the management of our patients. Nurse-driven PC screening can help bring various unmet PC needs to the health-care team for opportunities that might not have been met or otherwise assessed. Consider implementing nurse-driven PC screening protocols at your institution to aid in collaborative and proactive interdisciplinary care.
Danielle McCamey, ACNP
Steering Committee Member
Sleep medicine
Diagnostics, devices, and sleep
The past several months have been busy for the Sleep Medicine NetWork. We have been working to represent the interests of our membership and our patients in many arenas.
Devices coded as E0464, defined as life support mechanical ventilators used with mask-based ventilation in the home are being more frequently used. According to the Office of the Inspector General (OIG), there has been an 89-fold increase in billing for E0464 ventilators for Medicare and its beneficiaries between 2009 and 2015, increasing from $3.8M to $340M. In response, the Agency for Healthcare Research and Quality (AHRQ) requested a response to specific questions related to these devices.
In 2018, the CHEST Sleep Medicine NetWork will be participating in a Federal Drug Association-sponsored workshop entitled “Study Design Considerations for Devices including Digital Health Technologies for Sleep-Disordered Breathing (SDB) in Adults,” along with other national organizations and leaders in our field. This workshop will address available technologies for the diagnosis, monitoring, and treatment of SDB, as well as trends for digital health technologies and clinical trial design considerations.
Finally, the Sleep Medicine NetWork has wasted no time after a successful CHEST 2017 in Toronto in planning for the next annual meeting in San Antonio. We are excited to present an exciting curriculum in Sleep Medicine at CHEST 2018, so stay tuned.
Aneesa M. Das, MD, FCCP
NetWork Chair
Occupational and environmental health
Post-deployment lung disease
Since the early 1990s, ongoing military deployments to Southwest Asia remain a unique challenge from a pulmonary symptomology and diagnostic perspective.
Various airborne hazards in the deployment environment include geologic dusts, burn pit smoke, vehicle emissions, and industrial air pollution. Exposures can give rise to both acute respiratory symptoms and, in some instances, chronic lung disease. Currently, data are limited on whether inhalation of airborne particulate matter by military personnel is linked to increases in pulmonary diseases (Morris MJ, et al. US Army Med Dep J. 2016:173).
Ongoing research by the Veterans Affairs continues to enroll post-deployed personnel in an Airborne Hazard and Burn Pit Registry. Past approaches in evaluation of deployed individuals ranged from common tests such as spirometry, HRCT scanning, full PFTs, bronchoprovocation challenges, and, in some instances, lung biopsies (Krefft SD, et al. Fed Pract. 2015;32[6]:32). More novel evaluations of postdeployment dyspnea include impulse oscillometry, exhaled nitric oxide, bronchoscopy, and cardiopulmonary exercise testing (Huprikar, et al. Chest. 2016;150[4]:S934A).
Members of the CHEST Occupational and Environmental Health NetWork are currently updating comprehensive approaches to evaluate military personnel with chronic respiratory symptoms from deployments. Continued emphasis, however, should be placed on diagnosing and treating common diseases such as asthma, exercise-induced bronchospasm, GERD, and upper airway disorders.
Pedro F. Lucero, MD, FCCP
Steering Committee Member
Clinical pulmonary medicine
Biologics – Birth of a new era of precision management in asthma
An estimated 10% to 20% of patients with severe uncontrolled asthma do not respond to maximal best standard treatments, leading to substantial health-care costs. A paradigm shift is now underway in our approach to the care of these patients with the emergence of novel biologics targeting the complex and interconnected inflammatory pathways in asthma that result in a diverse profile of asthma endotypes and phenotypes (Fig 1).
Current FDA-approved biologics primarily target patients with a T2 high phenotype (Table1).
Dupilumab binds to the alpha unit of the IL-4 receptor and blocks both IL-4 and IL-13. It shows potential efficacy in patients with T2 high asthma with or without eosinophilia but has not yet received FDA approval.
Multiple newer biologics are currently in development (Table 2).
Pulmonologists need to get familiar with the logistics of administration of these novel agents. The two common methods of administering biologics are (1) buy and bill – where the provider buys the drug directly from the distributor; and (2) assignment of benefits (typically administered by a Pharmacy Benefit Manager) - specific dose of the medication is shipped to the physician’s office and physician only bills for the administration. CPT and J codes are shown in Table 1.
Shyamsunder Subramanian, MD, FCCP
Steering Committee Member
Hurricane Maria, Bloodstream Infections, Lung Cancer in Women
Disaster Response
A Natural Disaster Creates Nationwide Threat
Hurricane Maria devastated Puerto Rico in late September 2017, and the lessons learned endure as the storm exposed the vulnerability of an increasingly interconnected and fragile medical community across the continental United States. According to the US Food and Drug Administration (FDA), Puerto Rico manufactures more drug products than any US state and just under 10% of all drugs consumed by Americans, some of which do not have therapeutic alternatives. In addition, certain medical devices are only produced in Puerto Rico. The humanitarian crisis caused by Hurricane Maria consequently created critical medication and medical device shortages across the United States (FDA. https://www.fda.gov/NewsEvents/. Accessed Feb 01, 2018).
The disruption and disorganization caused by Hurricane Maria was perhaps best exemplified by the resultant shortage of small-volume 0.9% saline injection bags, which coincided with a particularly bad flu season. The FDA temporarily allowed import of saline bags from outside the United States while concurrently expediting the approval of IV solutions from new manufacturers. The American Society for Health-System Pharmacists (ASHP), meanwhile, contributed guidance on managing fluid shortages (ASHP. https://www.ashp.org/Drug-Shortages/. Accessed Feb 01, 2018).
Hurricane Maria was a wake-up call for medical professionals across the United States to modernize institutional procedures and to develop contingency plans to deal with medication shortages, particularly IV fluids, since this is a recurring problem across the United States since 2014. Ultimately, the goal of health-care providers across the United States is to manage natural catastrophes, however distant, by effectively planning for and adapting to medical product shortages to ensure patient care is not interrupted and that critical shortages remain invisible to patients themselves.
Cristian Madar, MD
Steering Committee Member
Practice Operations
Are All Regulations Well Thought Out? Point of View!
Current medicine is complex, with patients presenting in the ICU with multiorgan dysfunction. The art and science of medicine is being replaced by protocolized medicine. To help streamline the care, societies and colleges are coming up with guidelines. The guideline, though, changes from year to year what has been practiced in the past has been obsolete, and what is current may not hold true in the future. With increasing health-care costs affecting physician and hospital practices, innovations are being undertaken on a daily basis. The payers, on the other hand, are trying to come up with regulations, whether one likes it or not, that have become the beacon for penalty and reward. Sometimes those regulations conflict with what is sound judgment and prudent care, cornering the providers in the box with unnecessary penalties.
Approximately 250,000 bloodstream infections occur in the United States yearly, mostly attributed to the presence of intravascular devices. The rate of central line-associated blood stream infection (CLABSI) in the United States is 0.8 per 1,000 central line days. The desirable rate is zero rate of CLABSI. The hospitals are being pushed to be prudent with the use of central lines and removal if not needed. The technique and sterile field along with appropriate innovation in dressing technique have been effective in reducing the CLABSI by 46% from 2008 to 2013. The hospitals and ICUs are being very vigilant in trying to avoid CLABSI and are striving to achieve the goal of a zero percentage CLABSI rate, leading to almost a state of paranoia. The efforts are being undertaken in many institutions to get all the cultures on admission to identify the organism on admission so as to be designated as a bloodstream infection (BSI) due to other causes and to avoid the CLABSI attribution. The CLABSI attribution follows a complex algorithm with no waiver for the exception outside the strict definition that is changing (The 2015 definition change resulted in an 83% increase in CLABSI rate.).
We hereby present a simple scenario for point of view, where there is very clear-cut evidence of the bloodstream infection due to abdominal sources but that BSI would be designated as CLABSI as defined by National Healthcare Safety Network (NHSN). The patient postoperatively presents with fever, nausea, and abdominal discomfort. The CT scan showed fluid collection suggestive of infection. Culture from the abscess grew Escherichia coli and the blood culture grew Bacteroides fargilis. This patient was labeled as BSI due to intra-abdominal cause. On the other hand, patient has pus pockets in the abdominal wall with swelling and tenderness. Cultures from the pustule grew Streptococcus Group B and the blood culture grew Staphylococcus aureus. This would be classified as soft tissue infection and primary BSI and if the patient has the central line for 2 days, it would be classified as CLABSI, even though there was a clear cut source from where the infection originated. On the other hand, if a patient has a CT scan of the abdomen or any imaging study done, which showed the pus pocket, and even if there is no abscess culture done, and if there is BSI, it would be labelled as BSI due to intra-abdominal cause rather than CLABSI.
This is one of the many examples where there is unnecessary imaging needed to avoid the designation of CLABSI, or, in other instances, unnecessary cultures on admission to avoid the CLABSI or catheter-related urinary tract infection (CAUTI) when patient is coming in from other institutions or nursing facilities to avoid the attribution of CLABSI and CAUTI, rather than what is good for the patient. We are in the time of protocol-driven medicine, which has helped in improving the patient care in certain aspects, but where are the days when the physical examination meant something rather than having to prove it by imaging and laboratory studies? Are the guidelines and regulations a solution to health-care cost and waste, or are they part of problem? You be the judge.
Adel Bassily-Marcus, MD, FCCP
Chair
Salim Surani, MD, FCCP
Vice-Chair
References
Grimes L, McMullen KM, Leone C, et al. Impact of 2015 National Healthcare Safety Network (NHSN) Definition Changes on Intensive Care Unit (ICU) Central Line-Associated Bloodstream Infection (CLABSI) at a Large Healthcare System. Open Forum Infectious Diseases. 2016;3(suppl1):946-946.
Central line related blood infection. Center for Disease Control and Prevention. https://www.cdc.gov/hai/bsi/bsi.html. Accessed on 1/28/2018
Preventing central line related blood infections. A Global challenge and Global perspective. https://www.jointcommission.org/assets/1/18/CLABSI_Monograph.pdf. Accessed on 1/28/2018
Transplant
Radius of Change: Will Expanding Organ Sharing Beyond Donor Service Area Enhance Access in Lung Transplantation?
In November 2017, the US Department of Health and Human Services (HHS) prompted the United Network for Organ Sharing Organ Procurement and Transplant Network (UNOS/OPTN) to reconsider geographical boundaries of donor allocation. The impetus for change was driven by a recent litigation and data that challenged the current organ allocation algorithm on the premise that it overlooked potential high acuity candidates listed at centers outside the primary DSA (donor service area) of the donor hospital, in favor of less sick local recipients. In response, the UNOS/OPTN Executive Committee recommended the adoption of a 250-nautical mile radius from the donor hospital in lieu of the DSA as the first circle or zone A of distribution for lungs. The putative merits of this change, due to last an experimental year, is intended to provide sicker candidates with access to a broader geographic range of donors. Its impact will then be evaluated by the Thoracic Organ Transplantation Committee to make further recommendations, including possibly extending zone A to 500 miles.
The extended geographical limits have organ-specific implications. In contrast to other organs, constraints of cold ischemia limit the duration within which lungs and hearts must be transplanted. Indeed, this latter point is the basis for using a radius from the donor hospital, rather than the region, as the first circle of distribution. Furthermore, DSAs vary substantially in both size and population and performance, leading to considerable variation in access to organs for candidates based on their region of residence. Currently, more than 50% of the lung allocation in the United States occurs locally to recipients with lung allocation scores (LAS) less than 50 (Iribarne et al. Chest. 2009;135[4]:923). In addition, waiting time mortality remains high and actuarial survival remains low for those with higher LAS (Russo et al. Chest. 2010;137[3]:651). The new recommendations broaden the concentric circle approach and potentially provide enhanced access for the sickest candidates on the waiting list. However, this may increase duration of waitlist time for those with lower LAS, certain disease groups such as COPD and those listed in more conservative centers. It may conversely, however, drive transplantation in the sickest patients and increase the use of bridging strategies in high volume centers and those with ECMO capabilities, as there will now be a greater reassurance of donor offers with the wider catchment area. The implications are unclear at this time, and over the next year, the efficacy and the potential unintended consequences of this newly implemented directive should become more apparent.
Anupam Kumar, MD
Fellow-in-Training Member
J. W. Awori Hayanga, MD, MPH
Steering Committee Member
Women’s Health
Lung Cancer and Steroid Hormones: An Evolving Paradigm
Lung cancer remains to be the second most common cancer and the leading cause of cancer-related mortality in women. The risk for developing lung cancer in women is 1/17 and increases with age and smoking history. Women with stage I NSLC have better prognosis after surgical treatment compared with men (Graham et al. South Med J. 2013;106[10]:582); however. they are less likely to have undergone a low dose screening CT scan, even after meeting high risk criteria (Lamb et al. Chest. 2017;152[suppl]A623). The prognosis in advanced stage lung cancer at diagnosis does not differ among the genders or age groups (Santoro et al. J Bras Pneumol. 2017;43[6]:431).
There is increasing interest in the role of steroid hormones in lung biology in health and disease with estrogen and progesterone receptors identified in both healthy and malignant tissue. The role of hormone receptors as a prognostication tool and a therapeutic target is being actively investigated.
Estrogen receptor Beta (ER-Beta) is the predominantly expressed estrogen receptor in lung cancer cells (Raso et al. Clin Cancer Res. 2009;15[17]:5359). Increased cytoplasmic ER-alpha and ER-beta is associated with tobacco smoking and likely indicates a hormonal-smoking interaction (Siegfried. Mol Cancer Res. 2014;12[1]:24). A higher nuclear expression ER-beta in women may be protective against hormone-related lung cancer (Schwartz et al. J Clin Oncol. 2007; 25[36]:5785), whereas higher cytoplasmic expression of ER-alpha and ER-beta was associated with worse lung cancer survival (Cheng. J Natl Cancer Inst. 2018; Jan 13). Therapies targeting ER-beta1 and its downregulation resulted in sensitizing the cells to epidermal growth factor receptor-tyrosine kinase inhibitors and may result in reversing EGFR-TK resistance (Fu et al. Oncol Rep. 2018;39[3]:1313).
The presence of progesterone receptors is associated with longer survival in NSCLC, and treatment with progesterone has been shown to induce apoptosis and inhibit migration and invasion of lung cancer cell lines (Ishibashi et al. Cancer Res. 2005;65[14]:6450). Women over the age of 60 were found to have significant survival benefit when compared with both men and younger women (Wakelee et al. J Thoracic Oncol. 2007b; 2:S570), whereas a worse survival and earlier age of occurrence of lung cancer was associated with the exposure to HRT (Ganti et al. J Clin Oncol. 2006;24[1]:59).
The future of hormone receptor targets in lung cancer may provide new therapeutic options for patients with lung adenocarcinoma, especially those with acquired resistance to the EGFR antagonists (Hsu et al. Int J Mol Sci. 2017; 18[8] pii: E1713. doi: 10.3390/ijms18081713).
Fidaa Shaib, MD, FCCP
Steering Committee Member
Ali Jiwani, MD
NetWork Member
Disaster Response
A Natural Disaster Creates Nationwide Threat
Hurricane Maria devastated Puerto Rico in late September 2017, and the lessons learned endure as the storm exposed the vulnerability of an increasingly interconnected and fragile medical community across the continental United States. According to the US Food and Drug Administration (FDA), Puerto Rico manufactures more drug products than any US state and just under 10% of all drugs consumed by Americans, some of which do not have therapeutic alternatives. In addition, certain medical devices are only produced in Puerto Rico. The humanitarian crisis caused by Hurricane Maria consequently created critical medication and medical device shortages across the United States (FDA. https://www.fda.gov/NewsEvents/. Accessed Feb 01, 2018).
The disruption and disorganization caused by Hurricane Maria was perhaps best exemplified by the resultant shortage of small-volume 0.9% saline injection bags, which coincided with a particularly bad flu season. The FDA temporarily allowed import of saline bags from outside the United States while concurrently expediting the approval of IV solutions from new manufacturers. The American Society for Health-System Pharmacists (ASHP), meanwhile, contributed guidance on managing fluid shortages (ASHP. https://www.ashp.org/Drug-Shortages/. Accessed Feb 01, 2018).
Hurricane Maria was a wake-up call for medical professionals across the United States to modernize institutional procedures and to develop contingency plans to deal with medication shortages, particularly IV fluids, since this is a recurring problem across the United States since 2014. Ultimately, the goal of health-care providers across the United States is to manage natural catastrophes, however distant, by effectively planning for and adapting to medical product shortages to ensure patient care is not interrupted and that critical shortages remain invisible to patients themselves.
Cristian Madar, MD
Steering Committee Member
Practice Operations
Are All Regulations Well Thought Out? Point of View!
Current medicine is complex, with patients presenting in the ICU with multiorgan dysfunction. The art and science of medicine is being replaced by protocolized medicine. To help streamline the care, societies and colleges are coming up with guidelines. The guideline, though, changes from year to year what has been practiced in the past has been obsolete, and what is current may not hold true in the future. With increasing health-care costs affecting physician and hospital practices, innovations are being undertaken on a daily basis. The payers, on the other hand, are trying to come up with regulations, whether one likes it or not, that have become the beacon for penalty and reward. Sometimes those regulations conflict with what is sound judgment and prudent care, cornering the providers in the box with unnecessary penalties.
Approximately 250,000 bloodstream infections occur in the United States yearly, mostly attributed to the presence of intravascular devices. The rate of central line-associated blood stream infection (CLABSI) in the United States is 0.8 per 1,000 central line days. The desirable rate is zero rate of CLABSI. The hospitals are being pushed to be prudent with the use of central lines and removal if not needed. The technique and sterile field along with appropriate innovation in dressing technique have been effective in reducing the CLABSI by 46% from 2008 to 2013. The hospitals and ICUs are being very vigilant in trying to avoid CLABSI and are striving to achieve the goal of a zero percentage CLABSI rate, leading to almost a state of paranoia. The efforts are being undertaken in many institutions to get all the cultures on admission to identify the organism on admission so as to be designated as a bloodstream infection (BSI) due to other causes and to avoid the CLABSI attribution. The CLABSI attribution follows a complex algorithm with no waiver for the exception outside the strict definition that is changing (The 2015 definition change resulted in an 83% increase in CLABSI rate.).
We hereby present a simple scenario for point of view, where there is very clear-cut evidence of the bloodstream infection due to abdominal sources but that BSI would be designated as CLABSI as defined by National Healthcare Safety Network (NHSN). The patient postoperatively presents with fever, nausea, and abdominal discomfort. The CT scan showed fluid collection suggestive of infection. Culture from the abscess grew Escherichia coli and the blood culture grew Bacteroides fargilis. This patient was labeled as BSI due to intra-abdominal cause. On the other hand, patient has pus pockets in the abdominal wall with swelling and tenderness. Cultures from the pustule grew Streptococcus Group B and the blood culture grew Staphylococcus aureus. This would be classified as soft tissue infection and primary BSI and if the patient has the central line for 2 days, it would be classified as CLABSI, even though there was a clear cut source from where the infection originated. On the other hand, if a patient has a CT scan of the abdomen or any imaging study done, which showed the pus pocket, and even if there is no abscess culture done, and if there is BSI, it would be labelled as BSI due to intra-abdominal cause rather than CLABSI.
This is one of the many examples where there is unnecessary imaging needed to avoid the designation of CLABSI, or, in other instances, unnecessary cultures on admission to avoid the CLABSI or catheter-related urinary tract infection (CAUTI) when patient is coming in from other institutions or nursing facilities to avoid the attribution of CLABSI and CAUTI, rather than what is good for the patient. We are in the time of protocol-driven medicine, which has helped in improving the patient care in certain aspects, but where are the days when the physical examination meant something rather than having to prove it by imaging and laboratory studies? Are the guidelines and regulations a solution to health-care cost and waste, or are they part of problem? You be the judge.
Adel Bassily-Marcus, MD, FCCP
Chair
Salim Surani, MD, FCCP
Vice-Chair
References
Grimes L, McMullen KM, Leone C, et al. Impact of 2015 National Healthcare Safety Network (NHSN) Definition Changes on Intensive Care Unit (ICU) Central Line-Associated Bloodstream Infection (CLABSI) at a Large Healthcare System. Open Forum Infectious Diseases. 2016;3(suppl1):946-946.
Central line related blood infection. Center for Disease Control and Prevention. https://www.cdc.gov/hai/bsi/bsi.html. Accessed on 1/28/2018
Preventing central line related blood infections. A Global challenge and Global perspective. https://www.jointcommission.org/assets/1/18/CLABSI_Monograph.pdf. Accessed on 1/28/2018
Transplant
Radius of Change: Will Expanding Organ Sharing Beyond Donor Service Area Enhance Access in Lung Transplantation?
In November 2017, the US Department of Health and Human Services (HHS) prompted the United Network for Organ Sharing Organ Procurement and Transplant Network (UNOS/OPTN) to reconsider geographical boundaries of donor allocation. The impetus for change was driven by a recent litigation and data that challenged the current organ allocation algorithm on the premise that it overlooked potential high acuity candidates listed at centers outside the primary DSA (donor service area) of the donor hospital, in favor of less sick local recipients. In response, the UNOS/OPTN Executive Committee recommended the adoption of a 250-nautical mile radius from the donor hospital in lieu of the DSA as the first circle or zone A of distribution for lungs. The putative merits of this change, due to last an experimental year, is intended to provide sicker candidates with access to a broader geographic range of donors. Its impact will then be evaluated by the Thoracic Organ Transplantation Committee to make further recommendations, including possibly extending zone A to 500 miles.
The extended geographical limits have organ-specific implications. In contrast to other organs, constraints of cold ischemia limit the duration within which lungs and hearts must be transplanted. Indeed, this latter point is the basis for using a radius from the donor hospital, rather than the region, as the first circle of distribution. Furthermore, DSAs vary substantially in both size and population and performance, leading to considerable variation in access to organs for candidates based on their region of residence. Currently, more than 50% of the lung allocation in the United States occurs locally to recipients with lung allocation scores (LAS) less than 50 (Iribarne et al. Chest. 2009;135[4]:923). In addition, waiting time mortality remains high and actuarial survival remains low for those with higher LAS (Russo et al. Chest. 2010;137[3]:651). The new recommendations broaden the concentric circle approach and potentially provide enhanced access for the sickest candidates on the waiting list. However, this may increase duration of waitlist time for those with lower LAS, certain disease groups such as COPD and those listed in more conservative centers. It may conversely, however, drive transplantation in the sickest patients and increase the use of bridging strategies in high volume centers and those with ECMO capabilities, as there will now be a greater reassurance of donor offers with the wider catchment area. The implications are unclear at this time, and over the next year, the efficacy and the potential unintended consequences of this newly implemented directive should become more apparent.
Anupam Kumar, MD
Fellow-in-Training Member
J. W. Awori Hayanga, MD, MPH
Steering Committee Member
Women’s Health
Lung Cancer and Steroid Hormones: An Evolving Paradigm
Lung cancer remains to be the second most common cancer and the leading cause of cancer-related mortality in women. The risk for developing lung cancer in women is 1/17 and increases with age and smoking history. Women with stage I NSLC have better prognosis after surgical treatment compared with men (Graham et al. South Med J. 2013;106[10]:582); however. they are less likely to have undergone a low dose screening CT scan, even after meeting high risk criteria (Lamb et al. Chest. 2017;152[suppl]A623). The prognosis in advanced stage lung cancer at diagnosis does not differ among the genders or age groups (Santoro et al. J Bras Pneumol. 2017;43[6]:431).
There is increasing interest in the role of steroid hormones in lung biology in health and disease with estrogen and progesterone receptors identified in both healthy and malignant tissue. The role of hormone receptors as a prognostication tool and a therapeutic target is being actively investigated.
Estrogen receptor Beta (ER-Beta) is the predominantly expressed estrogen receptor in lung cancer cells (Raso et al. Clin Cancer Res. 2009;15[17]:5359). Increased cytoplasmic ER-alpha and ER-beta is associated with tobacco smoking and likely indicates a hormonal-smoking interaction (Siegfried. Mol Cancer Res. 2014;12[1]:24). A higher nuclear expression ER-beta in women may be protective against hormone-related lung cancer (Schwartz et al. J Clin Oncol. 2007; 25[36]:5785), whereas higher cytoplasmic expression of ER-alpha and ER-beta was associated with worse lung cancer survival (Cheng. J Natl Cancer Inst. 2018; Jan 13). Therapies targeting ER-beta1 and its downregulation resulted in sensitizing the cells to epidermal growth factor receptor-tyrosine kinase inhibitors and may result in reversing EGFR-TK resistance (Fu et al. Oncol Rep. 2018;39[3]:1313).
The presence of progesterone receptors is associated with longer survival in NSCLC, and treatment with progesterone has been shown to induce apoptosis and inhibit migration and invasion of lung cancer cell lines (Ishibashi et al. Cancer Res. 2005;65[14]:6450). Women over the age of 60 were found to have significant survival benefit when compared with both men and younger women (Wakelee et al. J Thoracic Oncol. 2007b; 2:S570), whereas a worse survival and earlier age of occurrence of lung cancer was associated with the exposure to HRT (Ganti et al. J Clin Oncol. 2006;24[1]:59).
The future of hormone receptor targets in lung cancer may provide new therapeutic options for patients with lung adenocarcinoma, especially those with acquired resistance to the EGFR antagonists (Hsu et al. Int J Mol Sci. 2017; 18[8] pii: E1713. doi: 10.3390/ijms18081713).
Fidaa Shaib, MD, FCCP
Steering Committee Member
Ali Jiwani, MD
NetWork Member
Disaster Response
A Natural Disaster Creates Nationwide Threat
Hurricane Maria devastated Puerto Rico in late September 2017, and the lessons learned endure as the storm exposed the vulnerability of an increasingly interconnected and fragile medical community across the continental United States. According to the US Food and Drug Administration (FDA), Puerto Rico manufactures more drug products than any US state and just under 10% of all drugs consumed by Americans, some of which do not have therapeutic alternatives. In addition, certain medical devices are only produced in Puerto Rico. The humanitarian crisis caused by Hurricane Maria consequently created critical medication and medical device shortages across the United States (FDA. https://www.fda.gov/NewsEvents/. Accessed Feb 01, 2018).
The disruption and disorganization caused by Hurricane Maria was perhaps best exemplified by the resultant shortage of small-volume 0.9% saline injection bags, which coincided with a particularly bad flu season. The FDA temporarily allowed import of saline bags from outside the United States while concurrently expediting the approval of IV solutions from new manufacturers. The American Society for Health-System Pharmacists (ASHP), meanwhile, contributed guidance on managing fluid shortages (ASHP. https://www.ashp.org/Drug-Shortages/. Accessed Feb 01, 2018).
Hurricane Maria was a wake-up call for medical professionals across the United States to modernize institutional procedures and to develop contingency plans to deal with medication shortages, particularly IV fluids, since this is a recurring problem across the United States since 2014. Ultimately, the goal of health-care providers across the United States is to manage natural catastrophes, however distant, by effectively planning for and adapting to medical product shortages to ensure patient care is not interrupted and that critical shortages remain invisible to patients themselves.
Cristian Madar, MD
Steering Committee Member
Practice Operations
Are All Regulations Well Thought Out? Point of View!
Current medicine is complex, with patients presenting in the ICU with multiorgan dysfunction. The art and science of medicine is being replaced by protocolized medicine. To help streamline the care, societies and colleges are coming up with guidelines. The guideline, though, changes from year to year what has been practiced in the past has been obsolete, and what is current may not hold true in the future. With increasing health-care costs affecting physician and hospital practices, innovations are being undertaken on a daily basis. The payers, on the other hand, are trying to come up with regulations, whether one likes it or not, that have become the beacon for penalty and reward. Sometimes those regulations conflict with what is sound judgment and prudent care, cornering the providers in the box with unnecessary penalties.
Approximately 250,000 bloodstream infections occur in the United States yearly, mostly attributed to the presence of intravascular devices. The rate of central line-associated blood stream infection (CLABSI) in the United States is 0.8 per 1,000 central line days. The desirable rate is zero rate of CLABSI. The hospitals are being pushed to be prudent with the use of central lines and removal if not needed. The technique and sterile field along with appropriate innovation in dressing technique have been effective in reducing the CLABSI by 46% from 2008 to 2013. The hospitals and ICUs are being very vigilant in trying to avoid CLABSI and are striving to achieve the goal of a zero percentage CLABSI rate, leading to almost a state of paranoia. The efforts are being undertaken in many institutions to get all the cultures on admission to identify the organism on admission so as to be designated as a bloodstream infection (BSI) due to other causes and to avoid the CLABSI attribution. The CLABSI attribution follows a complex algorithm with no waiver for the exception outside the strict definition that is changing (The 2015 definition change resulted in an 83% increase in CLABSI rate.).
We hereby present a simple scenario for point of view, where there is very clear-cut evidence of the bloodstream infection due to abdominal sources but that BSI would be designated as CLABSI as defined by National Healthcare Safety Network (NHSN). The patient postoperatively presents with fever, nausea, and abdominal discomfort. The CT scan showed fluid collection suggestive of infection. Culture from the abscess grew Escherichia coli and the blood culture grew Bacteroides fargilis. This patient was labeled as BSI due to intra-abdominal cause. On the other hand, patient has pus pockets in the abdominal wall with swelling and tenderness. Cultures from the pustule grew Streptococcus Group B and the blood culture grew Staphylococcus aureus. This would be classified as soft tissue infection and primary BSI and if the patient has the central line for 2 days, it would be classified as CLABSI, even though there was a clear cut source from where the infection originated. On the other hand, if a patient has a CT scan of the abdomen or any imaging study done, which showed the pus pocket, and even if there is no abscess culture done, and if there is BSI, it would be labelled as BSI due to intra-abdominal cause rather than CLABSI.
This is one of the many examples where there is unnecessary imaging needed to avoid the designation of CLABSI, or, in other instances, unnecessary cultures on admission to avoid the CLABSI or catheter-related urinary tract infection (CAUTI) when patient is coming in from other institutions or nursing facilities to avoid the attribution of CLABSI and CAUTI, rather than what is good for the patient. We are in the time of protocol-driven medicine, which has helped in improving the patient care in certain aspects, but where are the days when the physical examination meant something rather than having to prove it by imaging and laboratory studies? Are the guidelines and regulations a solution to health-care cost and waste, or are they part of problem? You be the judge.
Adel Bassily-Marcus, MD, FCCP
Chair
Salim Surani, MD, FCCP
Vice-Chair
References
Grimes L, McMullen KM, Leone C, et al. Impact of 2015 National Healthcare Safety Network (NHSN) Definition Changes on Intensive Care Unit (ICU) Central Line-Associated Bloodstream Infection (CLABSI) at a Large Healthcare System. Open Forum Infectious Diseases. 2016;3(suppl1):946-946.
Central line related blood infection. Center for Disease Control and Prevention. https://www.cdc.gov/hai/bsi/bsi.html. Accessed on 1/28/2018
Preventing central line related blood infections. A Global challenge and Global perspective. https://www.jointcommission.org/assets/1/18/CLABSI_Monograph.pdf. Accessed on 1/28/2018
Transplant
Radius of Change: Will Expanding Organ Sharing Beyond Donor Service Area Enhance Access in Lung Transplantation?
In November 2017, the US Department of Health and Human Services (HHS) prompted the United Network for Organ Sharing Organ Procurement and Transplant Network (UNOS/OPTN) to reconsider geographical boundaries of donor allocation. The impetus for change was driven by a recent litigation and data that challenged the current organ allocation algorithm on the premise that it overlooked potential high acuity candidates listed at centers outside the primary DSA (donor service area) of the donor hospital, in favor of less sick local recipients. In response, the UNOS/OPTN Executive Committee recommended the adoption of a 250-nautical mile radius from the donor hospital in lieu of the DSA as the first circle or zone A of distribution for lungs. The putative merits of this change, due to last an experimental year, is intended to provide sicker candidates with access to a broader geographic range of donors. Its impact will then be evaluated by the Thoracic Organ Transplantation Committee to make further recommendations, including possibly extending zone A to 500 miles.
The extended geographical limits have organ-specific implications. In contrast to other organs, constraints of cold ischemia limit the duration within which lungs and hearts must be transplanted. Indeed, this latter point is the basis for using a radius from the donor hospital, rather than the region, as the first circle of distribution. Furthermore, DSAs vary substantially in both size and population and performance, leading to considerable variation in access to organs for candidates based on their region of residence. Currently, more than 50% of the lung allocation in the United States occurs locally to recipients with lung allocation scores (LAS) less than 50 (Iribarne et al. Chest. 2009;135[4]:923). In addition, waiting time mortality remains high and actuarial survival remains low for those with higher LAS (Russo et al. Chest. 2010;137[3]:651). The new recommendations broaden the concentric circle approach and potentially provide enhanced access for the sickest candidates on the waiting list. However, this may increase duration of waitlist time for those with lower LAS, certain disease groups such as COPD and those listed in more conservative centers. It may conversely, however, drive transplantation in the sickest patients and increase the use of bridging strategies in high volume centers and those with ECMO capabilities, as there will now be a greater reassurance of donor offers with the wider catchment area. The implications are unclear at this time, and over the next year, the efficacy and the potential unintended consequences of this newly implemented directive should become more apparent.
Anupam Kumar, MD
Fellow-in-Training Member
J. W. Awori Hayanga, MD, MPH
Steering Committee Member
Women’s Health
Lung Cancer and Steroid Hormones: An Evolving Paradigm
Lung cancer remains to be the second most common cancer and the leading cause of cancer-related mortality in women. The risk for developing lung cancer in women is 1/17 and increases with age and smoking history. Women with stage I NSLC have better prognosis after surgical treatment compared with men (Graham et al. South Med J. 2013;106[10]:582); however. they are less likely to have undergone a low dose screening CT scan, even after meeting high risk criteria (Lamb et al. Chest. 2017;152[suppl]A623). The prognosis in advanced stage lung cancer at diagnosis does not differ among the genders or age groups (Santoro et al. J Bras Pneumol. 2017;43[6]:431).
There is increasing interest in the role of steroid hormones in lung biology in health and disease with estrogen and progesterone receptors identified in both healthy and malignant tissue. The role of hormone receptors as a prognostication tool and a therapeutic target is being actively investigated.
Estrogen receptor Beta (ER-Beta) is the predominantly expressed estrogen receptor in lung cancer cells (Raso et al. Clin Cancer Res. 2009;15[17]:5359). Increased cytoplasmic ER-alpha and ER-beta is associated with tobacco smoking and likely indicates a hormonal-smoking interaction (Siegfried. Mol Cancer Res. 2014;12[1]:24). A higher nuclear expression ER-beta in women may be protective against hormone-related lung cancer (Schwartz et al. J Clin Oncol. 2007; 25[36]:5785), whereas higher cytoplasmic expression of ER-alpha and ER-beta was associated with worse lung cancer survival (Cheng. J Natl Cancer Inst. 2018; Jan 13). Therapies targeting ER-beta1 and its downregulation resulted in sensitizing the cells to epidermal growth factor receptor-tyrosine kinase inhibitors and may result in reversing EGFR-TK resistance (Fu et al. Oncol Rep. 2018;39[3]:1313).
The presence of progesterone receptors is associated with longer survival in NSCLC, and treatment with progesterone has been shown to induce apoptosis and inhibit migration and invasion of lung cancer cell lines (Ishibashi et al. Cancer Res. 2005;65[14]:6450). Women over the age of 60 were found to have significant survival benefit when compared with both men and younger women (Wakelee et al. J Thoracic Oncol. 2007b; 2:S570), whereas a worse survival and earlier age of occurrence of lung cancer was associated with the exposure to HRT (Ganti et al. J Clin Oncol. 2006;24[1]:59).
The future of hormone receptor targets in lung cancer may provide new therapeutic options for patients with lung adenocarcinoma, especially those with acquired resistance to the EGFR antagonists (Hsu et al. Int J Mol Sci. 2017; 18[8] pii: E1713. doi: 10.3390/ijms18081713).
Fidaa Shaib, MD, FCCP
Steering Committee Member
Ali Jiwani, MD
NetWork Member
Adult bronchiectasis, asthma therapy, frailty in ILD
Clinical Research
New guidelines for adult bronchiectasis
Clinically significant bronchiectasis is a combination of radiologic bronchial dilatation with clinical symptoms. Guidelines on management of adult bronchiectasis were recently published (Eur Respir J. 2017; Sep 10;50[3]).
For all adult patients with clinically significant bronchiectasis, the guidelines suggest standardized minimum testing with differential blood count, serum immunoglobulins, and testing for allergic bronchopulmonary aspergillosis with any further workup on an individual basis. Annual sputum surveillance is suggested for clinically stable adult patients; however, the evidence for this recommendation came from studies done on patients with cystic fibrosis.
Inhaled bronchodilators are suggested as the first-line treatment in symptomatic patients. Long-term antibiotics (greater than 3 months) are recommended in patients with greater than 3 exacerbations/year after optimizing airway clearance and disease-specific treatment. Pseudomonas aeruginosa infections are to be treated with inhaled antibiotics (colistin or gentamicin) (Charles SH, et al. Am J Respir Crit Care Med. 2014;189[8]975; Murray P, et al. Am J Respir Crit Care Med. 2011;183[4]:491; and nonpseudomonal infections are to be treated with macrolides (Conroy W, et al. Lancet. 2012;380[9842]:660; Altenburg J, et al. JAMA. 2013;309[12];1251), although interchangeable for intolerance. Sputum cultured early will guide therapy among poor responders. Long-term mucolytic agents are suggested in appropriate tolerating patients. Pulmonary rehabilitation for 6-8 weeks is strongly recommended in adult bronchiectasis with impaired exercise capacity. Surgical interventions for bronchiectasis are reserved for a small group of patients who have localized disease and high exacerbation rates despite maximal medical therapy. Inhaled corticosteroids are suggested not to be used in adult bronchiectasis. Guidelines recommend against the use of statins and recombinant human DNase as it increases exacerbations (Chest. 1998;113[5]:1329. The task force acknowledged the low quality of evidence for their recommendations requiring more research in the field of adult bronchiectasis.
Bharat Bajantri, MD
Fellow-in-Training Member
Airways Disorders
ICS/LABA combo therapy: black box warning removed
Publication of the Salmeterol Multicenter Asthma Research Trial (SMART) in 2006 caused panic among asthmatics and the physicians who treat them (Nelson et al. Chest. 2006;129[1]:15). The study suggested that the long-acting beta-2-agonist (LABA) salmeterol leads to an increased risk of asthma/respiratory-related deaths compared with placebo. This finding was more pronounced in the African American subpopulation. The study left many questions unanswered, including whether or not this risk is present when LABA therapy is combined with inhaled corticosteroids (ICS) (O’Byrne. Chest. 2006;129[1]:3). Subsequent meta-analyses confirmed the increased risk with LABA monotherapy but not with LABA/ICS (Salpeter et al. Ann Inter Med. 2006;144[12]: 904; Jaeschke et al. Am J Respir Crit Care Med. 2008;178[10]:1009). Still, a black box warning relating LABA to asthma-related death was applied to LABA/ICS products.
In 2011, the Food and Drug Administration (FDA) mandated large, randomized controlled trials be performed for LABA/ICS products to assess safety. These trials were recently completed, showing no difference in asthma-related deaths between LABA/ICS and ICS alone. There were 41, 297 patients across four trials, three included teenagers and adults (age ≥ 12) and one enrolled children (ages 4-11). These studies prompted the FDA to remove the black box warning from salmeterol/fluticasone, formoterol/budesonide, and formoterol/mometasone (https://wwwfdagov/downloads/Drugs/DrugSafety/UCM589997pdf).
Conclusion: Because LABA/ICS therapy is effective for asthma, most pulmonologists continue to prescribe it despite the SMART study results and FDA warning. In a practical sense, we don’t expect the FDA findings to radically change asthma care. Still, it seems we can finally put this question to rest – LABA/ICS is indeed safe for asthmatics. Most physicians will continue to avoid LABA monotherapy, and now that tiotropium is included in the 2017 GINA guidelines, it’s only matter of time before we’re debating whether LABA/long-acting muscarinic antagonist (LAMA) is safe for asthmatics.
Aaron Holley, MD, FCCP
Steering Committee Member
Navitha Ramesh, MD, MBBS
Fellow-in-Training Member
Critical Care
Standardized handoffs in the ICU: room for improvement?
Transitions in patient care are commonplace in the ICU. But handoffs are particularly susceptible to error given the complexity of the patient population. Impacts of less-than-ideal handoffs likely include adverse events, delays in medical diagnosis and treatment, redundant communications, redundant activities such as additional procedures and tests, lower provider and patient satisfaction, higher costs, longer hospital stays, more hospital admissions, and less effective training for health -care providers. Yet, there is great heterogeneity in handoff practiced, and the impact of standardized handoffs in the ICU is unclear (Cochran A. JAMA Surg. 2018 Jan 3. doi: 10.1001/jamasurg.2017.5468. [Epub ahead of print]).
In a survey of over 600 academic intensivists, 55% of the participants stated that attending handoffs in the ICU should be standardized, yet, only 13% of those participating in handoffs reported using a standardized process (Lane-Fall M. Crit Care Med. 2016;44[4]690). Clinician miscommunication contributes to an estimated 250,000 deaths in US hospitals per year (Makary M. BMJ. 2016 May 3;353:i2139. doi: 10.1136/bmj.i2139). Standardized handoffs may improve outcomes in the ICU.
In many ICUs that do use standardized sign-out templates, higher clinician satisfaction and fewer unexpected patient events have been reported (Bavare AC. J Healthc Qual. 2015;37[5]:267; Nanchal R. BMJ Qual Saf. 2017;26[12]:987). In a recent randomized controlled trial, use of a standardized handoff curriculum in the ICU resulted in a significant 3% decrease in communication errors, without any change in the duration of the handoff. There also was a clinician-reported improvement in team communication and patient safety; but no changes in ICU length of stay, duration of mechanical ventilation, or number of re-intubations were noted (JAMA Surg. 2018 Jan 3. doi: 10.1001/jamasurg.2017.5440. [Epub ahead of print]).
Unfortunately, despite interest in improving patient handoffs, there are few tools to evaluate the effectiveness of different handoff strategies. Most studies report clinician perceptions rather than patient-centered outcomes. Further research is required to examine the optimal approach to handover communication. However, based on the available evidence, a standardized approach to handoffs is likely better than a nonstandardized format.
Shruti Gadre, MD
Fellow-in-Training Member
Christopher Carroll, MD, FCCP
Vice-Chair
Home-Based Mechanical Ventilation and Neuromuscular Disease
Update on two recent FDA-approved therapies for ALS and SMA
Amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA) are neuromuscular diseases often deteriorating to progressive respiratory failure. Two medications received recent FDA approval and are now available in clinical practice – edaravone for ALS and nusinersen for SMA. We present a balanced overview of the favorable data along with realistic challenges.
Edaravone (Radicava) is the second FDA-approved medication for management of ALS (Riluzole was approved over 20 years ago). Edaravone is a free radical scavenger that reduces oxidative stress, resulting in a protective effect on neuronal cells. It originally showed promise in acute ischemic stroke in Japan and was subsequently studied for ALS. A phase 3 randomized, double-blind placebo-controlled study performed in Japan (Lancet Neurol. 2017;16[7]:505) compared ALSFRS-R scores of a specific subset of ALS patients receiving edaravone vs placebo. This study revealed that patients with early ALS (2 years duration or less) with rapid progression (ALSFRS-R score of 7.5 in 6 months) had a 33% decrease in their degree of progression (reducing their ALSFRS-R score to 5) in the edaravone group. Of note, there was also slowing in the decline of FVC, though not clinically significant. Although the drug was rapidly approved by the FDA, there are obvious challenges that must be recognized. First, it is unclear if patients will discern such a mild degree of slowing of disease progression. In addition, the annual cost may be prohibitive, and lifelong IV administration of the medication for 10 days every month may pose logistical barriers.
Nusinersen (Spinraza) is the first FDA-approved therapeutic medication for spinal muscular atrophy (SMA). SMA is a hereditary neuromuscular disorder leading to degeneration of motor neuron cells and ultimately diffuse muscle weakness and often respiratory failure. Nusinersen is an antisense oligonucleotide that modifies splicing of the SMN-2 gene to increase production of normal, full-length SMN protein, which is deficient in SMA. The ENDEAR trial (Finkel RS, et al. N Engl J Med. 2017;377[18]:1723) was a phase 3, multicenter, double-blind study that enrolled SMA infants to receive nusinersen vs sham. Infants who received treatment had improvements in motor milestones (41% vs 0%) and less permanent-assisted ventilation or death in the nusinersen group (39% vs 68%), a 47% reduction in risk of death. The therapy is safe and tolerable, although there is reported risk of bleeding abnormalities, renal toxicity, and constipation. Administered intrathecally, there is a series of four loading doses, followed by maintenance doses every 4 months – presumably lifelong. Although FDA approved all three SMA subtypes, the eventual impact is uncertain, especially in cases of advanced muscle weakness. There are realistic challenges: the high cost ($125,000/dose), limited longitudinal evidence, technical administration, and limited access.
Pulmonologists should be aware of both medications as new therapeutic options for ALS and SMA; however, the long-term impact is yet to be determined.
Ashraf Elsayegh, MD, FCCP
Steering Committee Member
Won Y. Lee, MD
Steering Committee Member
Interstitial and Diffuse Lung Disease
Frailty as a measure of disease activity in ILD
Frailty is a systemic geriatric syndrome characterized by age-related accumulation of physiologic deficits across several systems with an attenuated response to biological stress. Considering that interstitial lung disease (ILD), particularly, idiopathic pulmonary fibrosis (IPF), is a disease of the aging population, frailty is an emerging area of clinical interest. The biological pathways driving the association of frailty with worse prognosis are complex but hinge on cellular senescence, systemic inflammation, and sarcopenia.
There is a high prevalence of frailty in adults with chronic lung diseases and is associated with worse prognosis. The current literature, though, is mostly derived from patients with COPD. Frailty measured using the 42-item patient-reported frailty index is associated with dyspnea severity in patients with fibrotic ILD (Milne et al. Respirology. 2017;22[4]:728) and systemic sclerosis-associated ILD (Guler et al. Respir Med. 2017 Aug;129:1-7. doi: 10.1016/j.rmed.2017.05.012. Epub 2017 May 25.). The SHARE-Frailty and the Edmonton Frail Scale instruments utilized to measure frailty in the University of Alabama at Birmingham IPF cohort detected a high-percentage of frail and pre-frail patients (Luckhardt et al. Am J Respir Crit Care Med. 2017;195:A7012). However, there are differences in targeted domains between the various frailty instruments, and this could affect the identification of the frailty syndrome in patients.
Frailty as a measure of disease activity and progression is not currently employed in clinical trials for ILD, primarily due to lack of standardized tools for this patient population. Future studies designed to utilize the frailty syndrome as outcome measures may further our understanding of the clinical manifestations and underlying mechanisms, as well as identify potential therapeutic interventions for patients with ILD.
Tejaswini Kulkarni MD, MPH
Fellow-in-Training Member
Clinical Research
New guidelines for adult bronchiectasis
Clinically significant bronchiectasis is a combination of radiologic bronchial dilatation with clinical symptoms. Guidelines on management of adult bronchiectasis were recently published (Eur Respir J. 2017; Sep 10;50[3]).
For all adult patients with clinically significant bronchiectasis, the guidelines suggest standardized minimum testing with differential blood count, serum immunoglobulins, and testing for allergic bronchopulmonary aspergillosis with any further workup on an individual basis. Annual sputum surveillance is suggested for clinically stable adult patients; however, the evidence for this recommendation came from studies done on patients with cystic fibrosis.
Inhaled bronchodilators are suggested as the first-line treatment in symptomatic patients. Long-term antibiotics (greater than 3 months) are recommended in patients with greater than 3 exacerbations/year after optimizing airway clearance and disease-specific treatment. Pseudomonas aeruginosa infections are to be treated with inhaled antibiotics (colistin or gentamicin) (Charles SH, et al. Am J Respir Crit Care Med. 2014;189[8]975; Murray P, et al. Am J Respir Crit Care Med. 2011;183[4]:491; and nonpseudomonal infections are to be treated with macrolides (Conroy W, et al. Lancet. 2012;380[9842]:660; Altenburg J, et al. JAMA. 2013;309[12];1251), although interchangeable for intolerance. Sputum cultured early will guide therapy among poor responders. Long-term mucolytic agents are suggested in appropriate tolerating patients. Pulmonary rehabilitation for 6-8 weeks is strongly recommended in adult bronchiectasis with impaired exercise capacity. Surgical interventions for bronchiectasis are reserved for a small group of patients who have localized disease and high exacerbation rates despite maximal medical therapy. Inhaled corticosteroids are suggested not to be used in adult bronchiectasis. Guidelines recommend against the use of statins and recombinant human DNase as it increases exacerbations (Chest. 1998;113[5]:1329. The task force acknowledged the low quality of evidence for their recommendations requiring more research in the field of adult bronchiectasis.
Bharat Bajantri, MD
Fellow-in-Training Member
Airways Disorders
ICS/LABA combo therapy: black box warning removed
Publication of the Salmeterol Multicenter Asthma Research Trial (SMART) in 2006 caused panic among asthmatics and the physicians who treat them (Nelson et al. Chest. 2006;129[1]:15). The study suggested that the long-acting beta-2-agonist (LABA) salmeterol leads to an increased risk of asthma/respiratory-related deaths compared with placebo. This finding was more pronounced in the African American subpopulation. The study left many questions unanswered, including whether or not this risk is present when LABA therapy is combined with inhaled corticosteroids (ICS) (O’Byrne. Chest. 2006;129[1]:3). Subsequent meta-analyses confirmed the increased risk with LABA monotherapy but not with LABA/ICS (Salpeter et al. Ann Inter Med. 2006;144[12]: 904; Jaeschke et al. Am J Respir Crit Care Med. 2008;178[10]:1009). Still, a black box warning relating LABA to asthma-related death was applied to LABA/ICS products.
In 2011, the Food and Drug Administration (FDA) mandated large, randomized controlled trials be performed for LABA/ICS products to assess safety. These trials were recently completed, showing no difference in asthma-related deaths between LABA/ICS and ICS alone. There were 41, 297 patients across four trials, three included teenagers and adults (age ≥ 12) and one enrolled children (ages 4-11). These studies prompted the FDA to remove the black box warning from salmeterol/fluticasone, formoterol/budesonide, and formoterol/mometasone (https://wwwfdagov/downloads/Drugs/DrugSafety/UCM589997pdf).
Conclusion: Because LABA/ICS therapy is effective for asthma, most pulmonologists continue to prescribe it despite the SMART study results and FDA warning. In a practical sense, we don’t expect the FDA findings to radically change asthma care. Still, it seems we can finally put this question to rest – LABA/ICS is indeed safe for asthmatics. Most physicians will continue to avoid LABA monotherapy, and now that tiotropium is included in the 2017 GINA guidelines, it’s only matter of time before we’re debating whether LABA/long-acting muscarinic antagonist (LAMA) is safe for asthmatics.
Aaron Holley, MD, FCCP
Steering Committee Member
Navitha Ramesh, MD, MBBS
Fellow-in-Training Member
Critical Care
Standardized handoffs in the ICU: room for improvement?
Transitions in patient care are commonplace in the ICU. But handoffs are particularly susceptible to error given the complexity of the patient population. Impacts of less-than-ideal handoffs likely include adverse events, delays in medical diagnosis and treatment, redundant communications, redundant activities such as additional procedures and tests, lower provider and patient satisfaction, higher costs, longer hospital stays, more hospital admissions, and less effective training for health -care providers. Yet, there is great heterogeneity in handoff practiced, and the impact of standardized handoffs in the ICU is unclear (Cochran A. JAMA Surg. 2018 Jan 3. doi: 10.1001/jamasurg.2017.5468. [Epub ahead of print]).
In a survey of over 600 academic intensivists, 55% of the participants stated that attending handoffs in the ICU should be standardized, yet, only 13% of those participating in handoffs reported using a standardized process (Lane-Fall M. Crit Care Med. 2016;44[4]690). Clinician miscommunication contributes to an estimated 250,000 deaths in US hospitals per year (Makary M. BMJ. 2016 May 3;353:i2139. doi: 10.1136/bmj.i2139). Standardized handoffs may improve outcomes in the ICU.
In many ICUs that do use standardized sign-out templates, higher clinician satisfaction and fewer unexpected patient events have been reported (Bavare AC. J Healthc Qual. 2015;37[5]:267; Nanchal R. BMJ Qual Saf. 2017;26[12]:987). In a recent randomized controlled trial, use of a standardized handoff curriculum in the ICU resulted in a significant 3% decrease in communication errors, without any change in the duration of the handoff. There also was a clinician-reported improvement in team communication and patient safety; but no changes in ICU length of stay, duration of mechanical ventilation, or number of re-intubations were noted (JAMA Surg. 2018 Jan 3. doi: 10.1001/jamasurg.2017.5440. [Epub ahead of print]).
Unfortunately, despite interest in improving patient handoffs, there are few tools to evaluate the effectiveness of different handoff strategies. Most studies report clinician perceptions rather than patient-centered outcomes. Further research is required to examine the optimal approach to handover communication. However, based on the available evidence, a standardized approach to handoffs is likely better than a nonstandardized format.
Shruti Gadre, MD
Fellow-in-Training Member
Christopher Carroll, MD, FCCP
Vice-Chair
Home-Based Mechanical Ventilation and Neuromuscular Disease
Update on two recent FDA-approved therapies for ALS and SMA
Amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA) are neuromuscular diseases often deteriorating to progressive respiratory failure. Two medications received recent FDA approval and are now available in clinical practice – edaravone for ALS and nusinersen for SMA. We present a balanced overview of the favorable data along with realistic challenges.
Edaravone (Radicava) is the second FDA-approved medication for management of ALS (Riluzole was approved over 20 years ago). Edaravone is a free radical scavenger that reduces oxidative stress, resulting in a protective effect on neuronal cells. It originally showed promise in acute ischemic stroke in Japan and was subsequently studied for ALS. A phase 3 randomized, double-blind placebo-controlled study performed in Japan (Lancet Neurol. 2017;16[7]:505) compared ALSFRS-R scores of a specific subset of ALS patients receiving edaravone vs placebo. This study revealed that patients with early ALS (2 years duration or less) with rapid progression (ALSFRS-R score of 7.5 in 6 months) had a 33% decrease in their degree of progression (reducing their ALSFRS-R score to 5) in the edaravone group. Of note, there was also slowing in the decline of FVC, though not clinically significant. Although the drug was rapidly approved by the FDA, there are obvious challenges that must be recognized. First, it is unclear if patients will discern such a mild degree of slowing of disease progression. In addition, the annual cost may be prohibitive, and lifelong IV administration of the medication for 10 days every month may pose logistical barriers.
Nusinersen (Spinraza) is the first FDA-approved therapeutic medication for spinal muscular atrophy (SMA). SMA is a hereditary neuromuscular disorder leading to degeneration of motor neuron cells and ultimately diffuse muscle weakness and often respiratory failure. Nusinersen is an antisense oligonucleotide that modifies splicing of the SMN-2 gene to increase production of normal, full-length SMN protein, which is deficient in SMA. The ENDEAR trial (Finkel RS, et al. N Engl J Med. 2017;377[18]:1723) was a phase 3, multicenter, double-blind study that enrolled SMA infants to receive nusinersen vs sham. Infants who received treatment had improvements in motor milestones (41% vs 0%) and less permanent-assisted ventilation or death in the nusinersen group (39% vs 68%), a 47% reduction in risk of death. The therapy is safe and tolerable, although there is reported risk of bleeding abnormalities, renal toxicity, and constipation. Administered intrathecally, there is a series of four loading doses, followed by maintenance doses every 4 months – presumably lifelong. Although FDA approved all three SMA subtypes, the eventual impact is uncertain, especially in cases of advanced muscle weakness. There are realistic challenges: the high cost ($125,000/dose), limited longitudinal evidence, technical administration, and limited access.
Pulmonologists should be aware of both medications as new therapeutic options for ALS and SMA; however, the long-term impact is yet to be determined.
Ashraf Elsayegh, MD, FCCP
Steering Committee Member
Won Y. Lee, MD
Steering Committee Member
Interstitial and Diffuse Lung Disease
Frailty as a measure of disease activity in ILD
Frailty is a systemic geriatric syndrome characterized by age-related accumulation of physiologic deficits across several systems with an attenuated response to biological stress. Considering that interstitial lung disease (ILD), particularly, idiopathic pulmonary fibrosis (IPF), is a disease of the aging population, frailty is an emerging area of clinical interest. The biological pathways driving the association of frailty with worse prognosis are complex but hinge on cellular senescence, systemic inflammation, and sarcopenia.
There is a high prevalence of frailty in adults with chronic lung diseases and is associated with worse prognosis. The current literature, though, is mostly derived from patients with COPD. Frailty measured using the 42-item patient-reported frailty index is associated with dyspnea severity in patients with fibrotic ILD (Milne et al. Respirology. 2017;22[4]:728) and systemic sclerosis-associated ILD (Guler et al. Respir Med. 2017 Aug;129:1-7. doi: 10.1016/j.rmed.2017.05.012. Epub 2017 May 25.). The SHARE-Frailty and the Edmonton Frail Scale instruments utilized to measure frailty in the University of Alabama at Birmingham IPF cohort detected a high-percentage of frail and pre-frail patients (Luckhardt et al. Am J Respir Crit Care Med. 2017;195:A7012). However, there are differences in targeted domains between the various frailty instruments, and this could affect the identification of the frailty syndrome in patients.
Frailty as a measure of disease activity and progression is not currently employed in clinical trials for ILD, primarily due to lack of standardized tools for this patient population. Future studies designed to utilize the frailty syndrome as outcome measures may further our understanding of the clinical manifestations and underlying mechanisms, as well as identify potential therapeutic interventions for patients with ILD.
Tejaswini Kulkarni MD, MPH
Fellow-in-Training Member
Clinical Research
New guidelines for adult bronchiectasis
Clinically significant bronchiectasis is a combination of radiologic bronchial dilatation with clinical symptoms. Guidelines on management of adult bronchiectasis were recently published (Eur Respir J. 2017; Sep 10;50[3]).
For all adult patients with clinically significant bronchiectasis, the guidelines suggest standardized minimum testing with differential blood count, serum immunoglobulins, and testing for allergic bronchopulmonary aspergillosis with any further workup on an individual basis. Annual sputum surveillance is suggested for clinically stable adult patients; however, the evidence for this recommendation came from studies done on patients with cystic fibrosis.
Inhaled bronchodilators are suggested as the first-line treatment in symptomatic patients. Long-term antibiotics (greater than 3 months) are recommended in patients with greater than 3 exacerbations/year after optimizing airway clearance and disease-specific treatment. Pseudomonas aeruginosa infections are to be treated with inhaled antibiotics (colistin or gentamicin) (Charles SH, et al. Am J Respir Crit Care Med. 2014;189[8]975; Murray P, et al. Am J Respir Crit Care Med. 2011;183[4]:491; and nonpseudomonal infections are to be treated with macrolides (Conroy W, et al. Lancet. 2012;380[9842]:660; Altenburg J, et al. JAMA. 2013;309[12];1251), although interchangeable for intolerance. Sputum cultured early will guide therapy among poor responders. Long-term mucolytic agents are suggested in appropriate tolerating patients. Pulmonary rehabilitation for 6-8 weeks is strongly recommended in adult bronchiectasis with impaired exercise capacity. Surgical interventions for bronchiectasis are reserved for a small group of patients who have localized disease and high exacerbation rates despite maximal medical therapy. Inhaled corticosteroids are suggested not to be used in adult bronchiectasis. Guidelines recommend against the use of statins and recombinant human DNase as it increases exacerbations (Chest. 1998;113[5]:1329. The task force acknowledged the low quality of evidence for their recommendations requiring more research in the field of adult bronchiectasis.
Bharat Bajantri, MD
Fellow-in-Training Member
Airways Disorders
ICS/LABA combo therapy: black box warning removed
Publication of the Salmeterol Multicenter Asthma Research Trial (SMART) in 2006 caused panic among asthmatics and the physicians who treat them (Nelson et al. Chest. 2006;129[1]:15). The study suggested that the long-acting beta-2-agonist (LABA) salmeterol leads to an increased risk of asthma/respiratory-related deaths compared with placebo. This finding was more pronounced in the African American subpopulation. The study left many questions unanswered, including whether or not this risk is present when LABA therapy is combined with inhaled corticosteroids (ICS) (O’Byrne. Chest. 2006;129[1]:3). Subsequent meta-analyses confirmed the increased risk with LABA monotherapy but not with LABA/ICS (Salpeter et al. Ann Inter Med. 2006;144[12]: 904; Jaeschke et al. Am J Respir Crit Care Med. 2008;178[10]:1009). Still, a black box warning relating LABA to asthma-related death was applied to LABA/ICS products.
In 2011, the Food and Drug Administration (FDA) mandated large, randomized controlled trials be performed for LABA/ICS products to assess safety. These trials were recently completed, showing no difference in asthma-related deaths between LABA/ICS and ICS alone. There were 41, 297 patients across four trials, three included teenagers and adults (age ≥ 12) and one enrolled children (ages 4-11). These studies prompted the FDA to remove the black box warning from salmeterol/fluticasone, formoterol/budesonide, and formoterol/mometasone (https://wwwfdagov/downloads/Drugs/DrugSafety/UCM589997pdf).
Conclusion: Because LABA/ICS therapy is effective for asthma, most pulmonologists continue to prescribe it despite the SMART study results and FDA warning. In a practical sense, we don’t expect the FDA findings to radically change asthma care. Still, it seems we can finally put this question to rest – LABA/ICS is indeed safe for asthmatics. Most physicians will continue to avoid LABA monotherapy, and now that tiotropium is included in the 2017 GINA guidelines, it’s only matter of time before we’re debating whether LABA/long-acting muscarinic antagonist (LAMA) is safe for asthmatics.
Aaron Holley, MD, FCCP
Steering Committee Member
Navitha Ramesh, MD, MBBS
Fellow-in-Training Member
Critical Care
Standardized handoffs in the ICU: room for improvement?
Transitions in patient care are commonplace in the ICU. But handoffs are particularly susceptible to error given the complexity of the patient population. Impacts of less-than-ideal handoffs likely include adverse events, delays in medical diagnosis and treatment, redundant communications, redundant activities such as additional procedures and tests, lower provider and patient satisfaction, higher costs, longer hospital stays, more hospital admissions, and less effective training for health -care providers. Yet, there is great heterogeneity in handoff practiced, and the impact of standardized handoffs in the ICU is unclear (Cochran A. JAMA Surg. 2018 Jan 3. doi: 10.1001/jamasurg.2017.5468. [Epub ahead of print]).
In a survey of over 600 academic intensivists, 55% of the participants stated that attending handoffs in the ICU should be standardized, yet, only 13% of those participating in handoffs reported using a standardized process (Lane-Fall M. Crit Care Med. 2016;44[4]690). Clinician miscommunication contributes to an estimated 250,000 deaths in US hospitals per year (Makary M. BMJ. 2016 May 3;353:i2139. doi: 10.1136/bmj.i2139). Standardized handoffs may improve outcomes in the ICU.
In many ICUs that do use standardized sign-out templates, higher clinician satisfaction and fewer unexpected patient events have been reported (Bavare AC. J Healthc Qual. 2015;37[5]:267; Nanchal R. BMJ Qual Saf. 2017;26[12]:987). In a recent randomized controlled trial, use of a standardized handoff curriculum in the ICU resulted in a significant 3% decrease in communication errors, without any change in the duration of the handoff. There also was a clinician-reported improvement in team communication and patient safety; but no changes in ICU length of stay, duration of mechanical ventilation, or number of re-intubations were noted (JAMA Surg. 2018 Jan 3. doi: 10.1001/jamasurg.2017.5440. [Epub ahead of print]).
Unfortunately, despite interest in improving patient handoffs, there are few tools to evaluate the effectiveness of different handoff strategies. Most studies report clinician perceptions rather than patient-centered outcomes. Further research is required to examine the optimal approach to handover communication. However, based on the available evidence, a standardized approach to handoffs is likely better than a nonstandardized format.
Shruti Gadre, MD
Fellow-in-Training Member
Christopher Carroll, MD, FCCP
Vice-Chair
Home-Based Mechanical Ventilation and Neuromuscular Disease
Update on two recent FDA-approved therapies for ALS and SMA
Amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA) are neuromuscular diseases often deteriorating to progressive respiratory failure. Two medications received recent FDA approval and are now available in clinical practice – edaravone for ALS and nusinersen for SMA. We present a balanced overview of the favorable data along with realistic challenges.
Edaravone (Radicava) is the second FDA-approved medication for management of ALS (Riluzole was approved over 20 years ago). Edaravone is a free radical scavenger that reduces oxidative stress, resulting in a protective effect on neuronal cells. It originally showed promise in acute ischemic stroke in Japan and was subsequently studied for ALS. A phase 3 randomized, double-blind placebo-controlled study performed in Japan (Lancet Neurol. 2017;16[7]:505) compared ALSFRS-R scores of a specific subset of ALS patients receiving edaravone vs placebo. This study revealed that patients with early ALS (2 years duration or less) with rapid progression (ALSFRS-R score of 7.5 in 6 months) had a 33% decrease in their degree of progression (reducing their ALSFRS-R score to 5) in the edaravone group. Of note, there was also slowing in the decline of FVC, though not clinically significant. Although the drug was rapidly approved by the FDA, there are obvious challenges that must be recognized. First, it is unclear if patients will discern such a mild degree of slowing of disease progression. In addition, the annual cost may be prohibitive, and lifelong IV administration of the medication for 10 days every month may pose logistical barriers.
Nusinersen (Spinraza) is the first FDA-approved therapeutic medication for spinal muscular atrophy (SMA). SMA is a hereditary neuromuscular disorder leading to degeneration of motor neuron cells and ultimately diffuse muscle weakness and often respiratory failure. Nusinersen is an antisense oligonucleotide that modifies splicing of the SMN-2 gene to increase production of normal, full-length SMN protein, which is deficient in SMA. The ENDEAR trial (Finkel RS, et al. N Engl J Med. 2017;377[18]:1723) was a phase 3, multicenter, double-blind study that enrolled SMA infants to receive nusinersen vs sham. Infants who received treatment had improvements in motor milestones (41% vs 0%) and less permanent-assisted ventilation or death in the nusinersen group (39% vs 68%), a 47% reduction in risk of death. The therapy is safe and tolerable, although there is reported risk of bleeding abnormalities, renal toxicity, and constipation. Administered intrathecally, there is a series of four loading doses, followed by maintenance doses every 4 months – presumably lifelong. Although FDA approved all three SMA subtypes, the eventual impact is uncertain, especially in cases of advanced muscle weakness. There are realistic challenges: the high cost ($125,000/dose), limited longitudinal evidence, technical administration, and limited access.
Pulmonologists should be aware of both medications as new therapeutic options for ALS and SMA; however, the long-term impact is yet to be determined.
Ashraf Elsayegh, MD, FCCP
Steering Committee Member
Won Y. Lee, MD
Steering Committee Member
Interstitial and Diffuse Lung Disease
Frailty as a measure of disease activity in ILD
Frailty is a systemic geriatric syndrome characterized by age-related accumulation of physiologic deficits across several systems with an attenuated response to biological stress. Considering that interstitial lung disease (ILD), particularly, idiopathic pulmonary fibrosis (IPF), is a disease of the aging population, frailty is an emerging area of clinical interest. The biological pathways driving the association of frailty with worse prognosis are complex but hinge on cellular senescence, systemic inflammation, and sarcopenia.
There is a high prevalence of frailty in adults with chronic lung diseases and is associated with worse prognosis. The current literature, though, is mostly derived from patients with COPD. Frailty measured using the 42-item patient-reported frailty index is associated with dyspnea severity in patients with fibrotic ILD (Milne et al. Respirology. 2017;22[4]:728) and systemic sclerosis-associated ILD (Guler et al. Respir Med. 2017 Aug;129:1-7. doi: 10.1016/j.rmed.2017.05.012. Epub 2017 May 25.). The SHARE-Frailty and the Edmonton Frail Scale instruments utilized to measure frailty in the University of Alabama at Birmingham IPF cohort detected a high-percentage of frail and pre-frail patients (Luckhardt et al. Am J Respir Crit Care Med. 2017;195:A7012). However, there are differences in targeted domains between the various frailty instruments, and this could affect the identification of the frailty syndrome in patients.
Frailty as a measure of disease activity and progression is not currently employed in clinical trials for ILD, primarily due to lack of standardized tools for this patient population. Future studies designed to utilize the frailty syndrome as outcome measures may further our understanding of the clinical manifestations and underlying mechanisms, as well as identify potential therapeutic interventions for patients with ILD.
Tejaswini Kulkarni MD, MPH
Fellow-in-Training Member
BP targets questioned, Candida auris infections
Cardiovascular Medicine and Surgery
The Holy Grail of Blood Pressure Management?
Blood pressure treatment recommendations have been confusing over the past few years. The Joint National Committee (JNC) 8 stirred up controversy in 2014 because they raised the recommended tolerating systolic blood pressures, in certain people aged 60 and above, up to 150 mm Hg [James, et al. JAMA. 2014;311(5):507-520]. The new AHA/ACC hypertension guidelines cosponsored by 11 societies generated controversy because they changed the definition of hypertension (normal <120/80 mm Hg, elevated 120-129/80-89, stage 1 130-139/80-89, or stage 2 >140/90) [Whelton et al. J Am Coll Cardiol. 2017 pii:S0735-1097(17)41519-1]. The SPRINT trial [Wright, et al. N Engl J Med. 2015;373:2103-2116] largely influenced these recommendations. SPRINT demonstrated a 25% relative risk reduction of heart attack, stroke, cardiovascular death, or decompensated heart failure with more aggressive blood pressure management (BP goal <120/90 vs <140/90).
This new classification would label 46% of Americans, or 103.3 million people, as hypertensive. However, there is uncertainty in how broadly applicable the SPRINT results are, particularly in those under the age of 45. The majority of large clinical trials, including SPRINT, have limited numbers of patients who were less than 50 years old and, therefore, it is unknown if younger patients benefit to the same degree. The absolute improvement is also questionable because as an editorial points out [Welch, “Don’t Let New Blood Pressure Guidelines Raise Yours” NY Times. Nov. 15, 2017], the primary endpoint in SPRINT only occurred in less than or equal to 8% of patients.
These guidelines reinforce the need to measure ambulatory blood pressures, perform proper in-office blood pressure measurements, and emphasize lifestyle modifications. Whether aggressive blood pressure management is worth the potential risks and the degree to which ideal blood pressure measurement can be applied to real world practices, remains uncertain.
David J. Nagel, MD, PhD Steering Committee Member
Chest Infections
Candida auris
Invasive fungal infections are frequently managed by ICU physicians and are a leading cause of mortality among critically ill patients. Invasive candidiasis is associated with an attributable mortality rate of up to 49%. Historically, the majority of these infections has been caused by Candida albicans, but this may be changing.
The first outbreak of Candida auris in the Americas (18 patients) occurred in the ICU of a hospital in Venezuela. Resistance to common azoles was documented, and half of the isolates showed decreased susceptibility to amphotericin B. As of August 2017, a total 153 clinical cases of C auris infection have been reported to CDC from 10 US states; most have occurred in New York and New Jersey.
What has been learned from these cases is that close contacts can be colonized, colonization can be persistent (approximately 9 months), the yeast can survive in the hospital environment, bleach or sporicide is needed for elimination, isolation precautions are recommended as for MDRO bacteria, and serial resistance to echinocandins has been observed.
Principal takeaways:
1Candida auris isolates are often MDR, with some strains having elevated MICs to drugs in all the three major classes of antifungal medications.
2The isolates are difficult to identify and require specialized methods, such as MALDI-TOF or molecular identification based on sequencing.
3Misidentification may lead to inappropriate treatment.
4C auris has the propensity to cause outbreaks in health-care settings, as has been reported in several countries, and resistance may result in treatment failure.
Richard Winn, MD, MS, FCCPImmediate Past Chair
References
1. Sarma S. Current perspective on emergence, diagnosis and drug resistance in Candida auris. Infect Drug Resistance. 2017;10:155–165.
2. Pan American Health Organization/World Health Organization. Epidemiological Alert: Candida auris outbreaks in health care services. October 3, Washington, DC: PAHO/WHO; 2016.
3. Centers for Disease Control and Prevention. Global emergence of invasive infections caused by the multidrug-resistant yeast Candida auris. CDC; 2016 [updated June 24, 2016]
Cardiovascular Medicine and Surgery
The Holy Grail of Blood Pressure Management?
Blood pressure treatment recommendations have been confusing over the past few years. The Joint National Committee (JNC) 8 stirred up controversy in 2014 because they raised the recommended tolerating systolic blood pressures, in certain people aged 60 and above, up to 150 mm Hg [James, et al. JAMA. 2014;311(5):507-520]. The new AHA/ACC hypertension guidelines cosponsored by 11 societies generated controversy because they changed the definition of hypertension (normal <120/80 mm Hg, elevated 120-129/80-89, stage 1 130-139/80-89, or stage 2 >140/90) [Whelton et al. J Am Coll Cardiol. 2017 pii:S0735-1097(17)41519-1]. The SPRINT trial [Wright, et al. N Engl J Med. 2015;373:2103-2116] largely influenced these recommendations. SPRINT demonstrated a 25% relative risk reduction of heart attack, stroke, cardiovascular death, or decompensated heart failure with more aggressive blood pressure management (BP goal <120/90 vs <140/90).
This new classification would label 46% of Americans, or 103.3 million people, as hypertensive. However, there is uncertainty in how broadly applicable the SPRINT results are, particularly in those under the age of 45. The majority of large clinical trials, including SPRINT, have limited numbers of patients who were less than 50 years old and, therefore, it is unknown if younger patients benefit to the same degree. The absolute improvement is also questionable because as an editorial points out [Welch, “Don’t Let New Blood Pressure Guidelines Raise Yours” NY Times. Nov. 15, 2017], the primary endpoint in SPRINT only occurred in less than or equal to 8% of patients.
These guidelines reinforce the need to measure ambulatory blood pressures, perform proper in-office blood pressure measurements, and emphasize lifestyle modifications. Whether aggressive blood pressure management is worth the potential risks and the degree to which ideal blood pressure measurement can be applied to real world practices, remains uncertain.
David J. Nagel, MD, PhD Steering Committee Member
Chest Infections
Candida auris
Invasive fungal infections are frequently managed by ICU physicians and are a leading cause of mortality among critically ill patients. Invasive candidiasis is associated with an attributable mortality rate of up to 49%. Historically, the majority of these infections has been caused by Candida albicans, but this may be changing.
The first outbreak of Candida auris in the Americas (18 patients) occurred in the ICU of a hospital in Venezuela. Resistance to common azoles was documented, and half of the isolates showed decreased susceptibility to amphotericin B. As of August 2017, a total 153 clinical cases of C auris infection have been reported to CDC from 10 US states; most have occurred in New York and New Jersey.
What has been learned from these cases is that close contacts can be colonized, colonization can be persistent (approximately 9 months), the yeast can survive in the hospital environment, bleach or sporicide is needed for elimination, isolation precautions are recommended as for MDRO bacteria, and serial resistance to echinocandins has been observed.
Principal takeaways:
1Candida auris isolates are often MDR, with some strains having elevated MICs to drugs in all the three major classes of antifungal medications.
2The isolates are difficult to identify and require specialized methods, such as MALDI-TOF or molecular identification based on sequencing.
3Misidentification may lead to inappropriate treatment.
4C auris has the propensity to cause outbreaks in health-care settings, as has been reported in several countries, and resistance may result in treatment failure.
Richard Winn, MD, MS, FCCPImmediate Past Chair
References
1. Sarma S. Current perspective on emergence, diagnosis and drug resistance in Candida auris. Infect Drug Resistance. 2017;10:155–165.
2. Pan American Health Organization/World Health Organization. Epidemiological Alert: Candida auris outbreaks in health care services. October 3, Washington, DC: PAHO/WHO; 2016.
3. Centers for Disease Control and Prevention. Global emergence of invasive infections caused by the multidrug-resistant yeast Candida auris. CDC; 2016 [updated June 24, 2016]
Cardiovascular Medicine and Surgery
The Holy Grail of Blood Pressure Management?
Blood pressure treatment recommendations have been confusing over the past few years. The Joint National Committee (JNC) 8 stirred up controversy in 2014 because they raised the recommended tolerating systolic blood pressures, in certain people aged 60 and above, up to 150 mm Hg [James, et al. JAMA. 2014;311(5):507-520]. The new AHA/ACC hypertension guidelines cosponsored by 11 societies generated controversy because they changed the definition of hypertension (normal <120/80 mm Hg, elevated 120-129/80-89, stage 1 130-139/80-89, or stage 2 >140/90) [Whelton et al. J Am Coll Cardiol. 2017 pii:S0735-1097(17)41519-1]. The SPRINT trial [Wright, et al. N Engl J Med. 2015;373:2103-2116] largely influenced these recommendations. SPRINT demonstrated a 25% relative risk reduction of heart attack, stroke, cardiovascular death, or decompensated heart failure with more aggressive blood pressure management (BP goal <120/90 vs <140/90).
This new classification would label 46% of Americans, or 103.3 million people, as hypertensive. However, there is uncertainty in how broadly applicable the SPRINT results are, particularly in those under the age of 45. The majority of large clinical trials, including SPRINT, have limited numbers of patients who were less than 50 years old and, therefore, it is unknown if younger patients benefit to the same degree. The absolute improvement is also questionable because as an editorial points out [Welch, “Don’t Let New Blood Pressure Guidelines Raise Yours” NY Times. Nov. 15, 2017], the primary endpoint in SPRINT only occurred in less than or equal to 8% of patients.
These guidelines reinforce the need to measure ambulatory blood pressures, perform proper in-office blood pressure measurements, and emphasize lifestyle modifications. Whether aggressive blood pressure management is worth the potential risks and the degree to which ideal blood pressure measurement can be applied to real world practices, remains uncertain.
David J. Nagel, MD, PhD Steering Committee Member
Chest Infections
Candida auris
Invasive fungal infections are frequently managed by ICU physicians and are a leading cause of mortality among critically ill patients. Invasive candidiasis is associated with an attributable mortality rate of up to 49%. Historically, the majority of these infections has been caused by Candida albicans, but this may be changing.
The first outbreak of Candida auris in the Americas (18 patients) occurred in the ICU of a hospital in Venezuela. Resistance to common azoles was documented, and half of the isolates showed decreased susceptibility to amphotericin B. As of August 2017, a total 153 clinical cases of C auris infection have been reported to CDC from 10 US states; most have occurred in New York and New Jersey.
What has been learned from these cases is that close contacts can be colonized, colonization can be persistent (approximately 9 months), the yeast can survive in the hospital environment, bleach or sporicide is needed for elimination, isolation precautions are recommended as for MDRO bacteria, and serial resistance to echinocandins has been observed.
Principal takeaways:
1Candida auris isolates are often MDR, with some strains having elevated MICs to drugs in all the three major classes of antifungal medications.
2The isolates are difficult to identify and require specialized methods, such as MALDI-TOF or molecular identification based on sequencing.
3Misidentification may lead to inappropriate treatment.
4C auris has the propensity to cause outbreaks in health-care settings, as has been reported in several countries, and resistance may result in treatment failure.
Richard Winn, MD, MS, FCCPImmediate Past Chair
References
1. Sarma S. Current perspective on emergence, diagnosis and drug resistance in Candida auris. Infect Drug Resistance. 2017;10:155–165.
2. Pan American Health Organization/World Health Organization. Epidemiological Alert: Candida auris outbreaks in health care services. October 3, Washington, DC: PAHO/WHO; 2016.
3. Centers for Disease Control and Prevention. Global emergence of invasive infections caused by the multidrug-resistant yeast Candida auris. CDC; 2016 [updated June 24, 2016]
Transbronchial cryobiopsy, updated guidelines for chronic cough in children, PD-1 inhibition
Interventional Chest/Diagnostic Procedures
Cryobiopsy for ILD: Careful stewardship needed
Interest in transbronchial cryobiopsy has accelerated rapidly in recent years. This procedure is performed by advancing a cryoprobe into the peripheral lung via flexible bronchoscopy, where lung tissue freezes and adheres to the probe and is subsequently extracted as a cryobiopsy. The number of cryobiopsy-related publications has increased exponentially since it was described in 2009 (Babiak A, et al. Respiration. 2009;78[2]:203). This interest stems from reports of high diagnostic yields in patients with interstitial lung disease (ILD) while maintaining complication rates similar to that of conventional bronchoscopic biopsy.
Traditional bronchoscopic biopsies are notoriously insensitive; a specific diagnosis can be established in fewer than a third of cases (Sheth JS, et al. Chest. 2017;151[2]:389). As such, surgical lung biopsy continues to be recommended but is associated with significant mortality (2%) and morbidity (30%) in patients with ILD (Hutchinson JP, et al. ARJCCM. 2016;193[10]:1161). Cryobiopsy, which appears to rival surgical lung biopsy in terms of ability to contribute to a specific diagnosis, is, therefore, a highly promising alternative (Tomassetti S, et al. AJRCCM. 2016;193[7]:745).
As cryobiopsy is increasingly adopted around the world, however, troubling reports of serious complications have surfaced. Most notable is the recently reported experience of the initial 25 cases performed at the University of Pennsylvania, in which almost one in four patients suffered serious complications (DiBardino DM, et al. Ann Am Thorac Soc. 2017;14[6]:851). The authors pointed to lack of a predefined procedural protocol, as well as several choices relating to the specific technique used, including inconsistent use of fluoroscopy, lack of prophylactic bronchial blocker placement, and predominant use of laryngeal mask airways as potential contributing factors. Indeed, many variations of the basic cryobiopsy procedure have been described (Lentz RJ, et al. J Thoracic Dis. 2017;9[7]:2186), with no formal guidance or training available to inform advanced bronchoscopists interested in this procedure.
It is incumbent on the interventional pulmonology and ILD specialist communities to be responsible stewards of this promising procedure. Implementation of three parallel efforts to standardize and rigorously study this procedure should be considered as soon as possible: creation of expert consensus guidelines establishing best-practices for safe and effective biopsy technique; a training requirement before independent performance of the procedure; and creation of an international cryobiopsy registry to facilitate higher-quality research into optimal technique and outcomes. We owe this to our patients.
Robert J. Lentz, MD
NetWork Member
Fabien Maldonado, MD, FCCP
NetWork Member
Pediatric Chest Medicine
Chronic cough in children: New guidelines
A chronic cough is a common complaint among children whose parents seek medical evaluation. Chronic wet cough can indicate an underlying illness; therefore, an early diagnosis can lead to prevention of complications of the disease and improvement in quality of life.
CHEST is a leading resource in evidence and consensus-based guidelines on important topics affecting children. The most recent guidelines entitled Management of Children with Chronic Wet Cough and Protracted Bacterial Bronchitis (Chest. 2017;151(4):884-890) and Use of Management Pathways or Algorithms in Children with Chronic Cough (Chest. 2017;151(4):875-873) are updates from the 2006 CHEST guidelines on chronic cough in children.
The present updates utilized the CHEST methodological guidelines with chronic wet or productive cough and Grading of Recommendations Assessment, Development, and Evaluation framework and also performed a systematic review addressing key questions concerning the management of childhood disease for children 14 years and younger.
Guidance provided by the expert panel focused on recommendations to answer six key questions concerning the management of children 14 years and younger with a chronic wet cough unrelated to established chronic lung disease. The recommendations are:
1. Chronic cough is defined as the presence of a cough 4 weeks or longer in duration.
2. Assessment of the effect of the cough on the child and the family be undertaken as part of clinical consultation.
3. Evaluation of a chronic cough should be done with a systematic approach with pediatric-specific cough management protocols or algorithms.
4. Chest radiograph and, when age appropriate, spirometry with bronchodilator be undertaken as evaluation; tests for pertussis infection only to be performed if clinically suspected.
5. Chronic wet cough with no specific clinical features should receive antibiotics for 2 weeks targeted for common respiratory bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis).
6. When cough persists despite 2 weeks of appropriate antibiotics, it is recommended to continue for an additional 2 weeks.
7. Additional tests (eg skin prick test, Mantoux, bronchoscopy, chest CT scan) should be individualized in accordance with the clinical setting and child’s clinical symptoms and signs.
The panel recognizes the need for prospective studies to assess current algorithms outcomes of children with chronic cough. Both articles can be found on the guidelines section of the CHEST site.
John Bishara, DO
Fellow-in-Training Member
Pulmonary Physiology, Function, and Rehabilitation
Functional imaging of the lung
Quantifying heterogeneity of ventilation and gas exchange in lung diseases remains a clinical challenge. Conventional pulmonary function test is insensitive to regional changes. The multiple inert gas elimination technique can quantify ventilation-perfusion distribution, but it requires invasive instrumentation (eg, pulmonary artery catheterization) and is not practical for clinical use. Computed tomography (CT) scans delineate spatial changes in lung structures but do not directly measure changes in ventilation and gas exchange. With its radiation, it is difficult to apply CT scanning repeatedly in patients. More recently, MR imaging techniques have been developed to directly “visualize” and quantify regional lung function (Kruger SJ, et al. J Magn Reson Imaging. 2016;43(2):295; Roos JE, et al. Magn Reson Imaging Clin N Am. 2015;23(2):217). These techniques employ inhalation of gases, such as oxygen, perfluorinated gases, and hyperpolarized 3He and 129Xe. Hyperpolarized 3He has been studied the most; however, the dwindling supply of 3He gas and its rising cost have prevented its further development. 129Xe has abundant supply and has emerged to be the inert gas of choice for MR imaging. Hyperpolarized 129Xe can measure ventilation, like hyperpolarized 3He. In addition, Xe diffuses into alveolar barrier (interstitium and plasma) and red blood cells, where it exhibits distinct resonant frequency shifts that can be captured by MR. Therefore, in one test, information on pulmonary ventilation and gas transfer can be obtained. To date, the results from MR imaging studies have provided new insights into the pathophysiology of obstructive and restrictive lung diseases. With continuous development, MR imaging of the lung could become a clinically useful tool in the near future.
Yuh-Chin T. Huang, MD, MHS, FCCP
Steering Committee Member
Thoracic Oncology
Immune-mediated pneumonitis and PD-1 inhibition
Inhibitors of the programmed cell death 1 receptor (PD-1) have shown significant promise in the treatment of advanced stage malignancy. With the recent expansion of indications for use of these agents, the number of patients treated will continue to grow. Clinicians must be aware of their potential for serious adverse side effects, including dermatitis, colitis, and potentially life-threatening pneumonitis.
The development of pneumonitis secondary to PD-1 inhibitions is reported to occur in 2% to 5% of patients and can present at any time during therapy, with 1% of patients developing grade 3 or higher pneumonitis.1,2 The most common symptoms are dyspnea and cough, though one-third of patients are asymptomatic at presentation.2 Radiographic and pathologic features vary greatly and include organizing pneumonia, interstitial pneumonitis, hypersensitivity pneumonitis, or diffuse alveolar damage.3 While pneumonitis due to PD-1 inhibition is reportedly uncommon, the increasing number of patients expected to receive these medications will predictably result in increasing overall frequency of pneumonitis cases. In addition, the lack of large prospective randomized trials and reliance on radiographic rather than pathologic data in diagnosing immune-mediated pneumonitis gives one pause. Given the variability of presentation, lack of routine pathologic data, and increasing use of dual agents (eg, PD-1 and CTLA-4), chest physicians and medical oncologists should have a high index of suspicion yet practice equipoise in patients receiving immunotherapy who develop unexplained pulmonary symptoms or infiltrates. More research is needed to help improve the multidisciplinary diagnosis and treatment of this potentially serious complication.
David Maurice Chambers, MD
Fellow-in-Training Member
Jason Atticus Akulian, MD, MPH
Steering Committee Member
References
1. Nishino M, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncology. 2016;2(12):1607.
2. Naidoo J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709.
3. Nishino M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051.
Pulmonary Vascular Disease
Pulmonary Arterial Hypertension Associated With SLE
While pulmonary arterial hypertension (PAH) commonly complicates scleroderma (SSc), it is a rare complication of other connective tissue diseases (CTD), such as systemic lupus erythematosus (SLE). In the few prospective studies that utilize right-sided heart catheterization (RHC), the estimated prevalence of PAH in SLE is about 4%. However, since the prevalence of SLE is 10 to 15 times greater than SSc in the United States, the true prevalence of SLE-PAH may be higher than previously thought, and, thus, clinically relevant. Despite this, little is known about SLE-PAH.
A recent retrospective study from the French Pulmonary Hypertension Registry has added significantly to our understanding of this complication of SLE. Hachulla and colleagues studied 51 patients with RHC-proven SLE-PAH compared with 101 SLE control subjects without PAH. While the authors did not find any relevant differences in the demographics between groups, they did find a significantly higher prevalence of SSA and SSB antibodies in SLE-PAH. Interestingly, the presence of anti-U1 RNP antibody appeared to be less common in SLE-PAH patients; this lack of association is in contrast to prior studies in mixed CTD patients with anti-U1 RNP antibodies in which the prevalence of PAH can be as high as 60%. Further, none of the SLE-PAH patients demonstrated an acute response to vasodilator challenge during RHC, emphasizing that this maneuver does not need to be performed in SLE patients at risk of PAH. Trends toward improved survival in SLE-PAH patients treated with hydroxychloroquine are preliminary and hypothesis-generating but require confirmation in larger clinical studies.
Stephen Mathai, MD, FCCP
Chair
Leena Palwar, MD
Fellow-in-Training Member
References
Hachulla E, Jais X, Cinquetti G, et al. Pulmonary arterial hypertension associated with SLE: Results from the French pulmonary hypertension registry. Chest. 2017 Aug 26. pii: S0012-3692(17)31430-7. doi: 10.1016/j.chest.2017.08.014. [Epub ahead of print]
Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138:1383-1394.
Shirai Y, Yasuoka H, Okano Y, Takeuchi T, Satoh T, Kuwana M. Clinical characteristics and survival of Japanese patients with connective tissue disease and pulmonary arterial hypertension: a singlecentre cohort. Rheumatology. 2012;51:1846-1854.
Hao YJ, Jiang X, Zhou W, et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J. 2014;44: 963-972.
Huang C, Li M, Liu Y, et al. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Medicine. 2016;95:e2761.
Pérez-Peñate GM, Rúa-Figueroa I, Juliá- Serdá G, et al. Pulmonary arterial hypertension in systemic lupus erythematosus: prevalence and predictors. J Rheumatol. 2016;43:323-329.
Alpert MA, Goldberg SH, Sindem BH, et al. Cardiovascular manifestations of mixed connective tissue disease in adults. Circulation. 1983;63:1182-1193.
Interventional Chest/Diagnostic Procedures
Cryobiopsy for ILD: Careful stewardship needed
Interest in transbronchial cryobiopsy has accelerated rapidly in recent years. This procedure is performed by advancing a cryoprobe into the peripheral lung via flexible bronchoscopy, where lung tissue freezes and adheres to the probe and is subsequently extracted as a cryobiopsy. The number of cryobiopsy-related publications has increased exponentially since it was described in 2009 (Babiak A, et al. Respiration. 2009;78[2]:203). This interest stems from reports of high diagnostic yields in patients with interstitial lung disease (ILD) while maintaining complication rates similar to that of conventional bronchoscopic biopsy.
Traditional bronchoscopic biopsies are notoriously insensitive; a specific diagnosis can be established in fewer than a third of cases (Sheth JS, et al. Chest. 2017;151[2]:389). As such, surgical lung biopsy continues to be recommended but is associated with significant mortality (2%) and morbidity (30%) in patients with ILD (Hutchinson JP, et al. ARJCCM. 2016;193[10]:1161). Cryobiopsy, which appears to rival surgical lung biopsy in terms of ability to contribute to a specific diagnosis, is, therefore, a highly promising alternative (Tomassetti S, et al. AJRCCM. 2016;193[7]:745).
As cryobiopsy is increasingly adopted around the world, however, troubling reports of serious complications have surfaced. Most notable is the recently reported experience of the initial 25 cases performed at the University of Pennsylvania, in which almost one in four patients suffered serious complications (DiBardino DM, et al. Ann Am Thorac Soc. 2017;14[6]:851). The authors pointed to lack of a predefined procedural protocol, as well as several choices relating to the specific technique used, including inconsistent use of fluoroscopy, lack of prophylactic bronchial blocker placement, and predominant use of laryngeal mask airways as potential contributing factors. Indeed, many variations of the basic cryobiopsy procedure have been described (Lentz RJ, et al. J Thoracic Dis. 2017;9[7]:2186), with no formal guidance or training available to inform advanced bronchoscopists interested in this procedure.
It is incumbent on the interventional pulmonology and ILD specialist communities to be responsible stewards of this promising procedure. Implementation of three parallel efforts to standardize and rigorously study this procedure should be considered as soon as possible: creation of expert consensus guidelines establishing best-practices for safe and effective biopsy technique; a training requirement before independent performance of the procedure; and creation of an international cryobiopsy registry to facilitate higher-quality research into optimal technique and outcomes. We owe this to our patients.
Robert J. Lentz, MD
NetWork Member
Fabien Maldonado, MD, FCCP
NetWork Member
Pediatric Chest Medicine
Chronic cough in children: New guidelines
A chronic cough is a common complaint among children whose parents seek medical evaluation. Chronic wet cough can indicate an underlying illness; therefore, an early diagnosis can lead to prevention of complications of the disease and improvement in quality of life.
CHEST is a leading resource in evidence and consensus-based guidelines on important topics affecting children. The most recent guidelines entitled Management of Children with Chronic Wet Cough and Protracted Bacterial Bronchitis (Chest. 2017;151(4):884-890) and Use of Management Pathways or Algorithms in Children with Chronic Cough (Chest. 2017;151(4):875-873) are updates from the 2006 CHEST guidelines on chronic cough in children.
The present updates utilized the CHEST methodological guidelines with chronic wet or productive cough and Grading of Recommendations Assessment, Development, and Evaluation framework and also performed a systematic review addressing key questions concerning the management of childhood disease for children 14 years and younger.
Guidance provided by the expert panel focused on recommendations to answer six key questions concerning the management of children 14 years and younger with a chronic wet cough unrelated to established chronic lung disease. The recommendations are:
1. Chronic cough is defined as the presence of a cough 4 weeks or longer in duration.
2. Assessment of the effect of the cough on the child and the family be undertaken as part of clinical consultation.
3. Evaluation of a chronic cough should be done with a systematic approach with pediatric-specific cough management protocols or algorithms.
4. Chest radiograph and, when age appropriate, spirometry with bronchodilator be undertaken as evaluation; tests for pertussis infection only to be performed if clinically suspected.
5. Chronic wet cough with no specific clinical features should receive antibiotics for 2 weeks targeted for common respiratory bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis).
6. When cough persists despite 2 weeks of appropriate antibiotics, it is recommended to continue for an additional 2 weeks.
7. Additional tests (eg skin prick test, Mantoux, bronchoscopy, chest CT scan) should be individualized in accordance with the clinical setting and child’s clinical symptoms and signs.
The panel recognizes the need for prospective studies to assess current algorithms outcomes of children with chronic cough. Both articles can be found on the guidelines section of the CHEST site.
John Bishara, DO
Fellow-in-Training Member
Pulmonary Physiology, Function, and Rehabilitation
Functional imaging of the lung
Quantifying heterogeneity of ventilation and gas exchange in lung diseases remains a clinical challenge. Conventional pulmonary function test is insensitive to regional changes. The multiple inert gas elimination technique can quantify ventilation-perfusion distribution, but it requires invasive instrumentation (eg, pulmonary artery catheterization) and is not practical for clinical use. Computed tomography (CT) scans delineate spatial changes in lung structures but do not directly measure changes in ventilation and gas exchange. With its radiation, it is difficult to apply CT scanning repeatedly in patients. More recently, MR imaging techniques have been developed to directly “visualize” and quantify regional lung function (Kruger SJ, et al. J Magn Reson Imaging. 2016;43(2):295; Roos JE, et al. Magn Reson Imaging Clin N Am. 2015;23(2):217). These techniques employ inhalation of gases, such as oxygen, perfluorinated gases, and hyperpolarized 3He and 129Xe. Hyperpolarized 3He has been studied the most; however, the dwindling supply of 3He gas and its rising cost have prevented its further development. 129Xe has abundant supply and has emerged to be the inert gas of choice for MR imaging. Hyperpolarized 129Xe can measure ventilation, like hyperpolarized 3He. In addition, Xe diffuses into alveolar barrier (interstitium and plasma) and red blood cells, where it exhibits distinct resonant frequency shifts that can be captured by MR. Therefore, in one test, information on pulmonary ventilation and gas transfer can be obtained. To date, the results from MR imaging studies have provided new insights into the pathophysiology of obstructive and restrictive lung diseases. With continuous development, MR imaging of the lung could become a clinically useful tool in the near future.
Yuh-Chin T. Huang, MD, MHS, FCCP
Steering Committee Member
Thoracic Oncology
Immune-mediated pneumonitis and PD-1 inhibition
Inhibitors of the programmed cell death 1 receptor (PD-1) have shown significant promise in the treatment of advanced stage malignancy. With the recent expansion of indications for use of these agents, the number of patients treated will continue to grow. Clinicians must be aware of their potential for serious adverse side effects, including dermatitis, colitis, and potentially life-threatening pneumonitis.
The development of pneumonitis secondary to PD-1 inhibitions is reported to occur in 2% to 5% of patients and can present at any time during therapy, with 1% of patients developing grade 3 or higher pneumonitis.1,2 The most common symptoms are dyspnea and cough, though one-third of patients are asymptomatic at presentation.2 Radiographic and pathologic features vary greatly and include organizing pneumonia, interstitial pneumonitis, hypersensitivity pneumonitis, or diffuse alveolar damage.3 While pneumonitis due to PD-1 inhibition is reportedly uncommon, the increasing number of patients expected to receive these medications will predictably result in increasing overall frequency of pneumonitis cases. In addition, the lack of large prospective randomized trials and reliance on radiographic rather than pathologic data in diagnosing immune-mediated pneumonitis gives one pause. Given the variability of presentation, lack of routine pathologic data, and increasing use of dual agents (eg, PD-1 and CTLA-4), chest physicians and medical oncologists should have a high index of suspicion yet practice equipoise in patients receiving immunotherapy who develop unexplained pulmonary symptoms or infiltrates. More research is needed to help improve the multidisciplinary diagnosis and treatment of this potentially serious complication.
David Maurice Chambers, MD
Fellow-in-Training Member
Jason Atticus Akulian, MD, MPH
Steering Committee Member
References
1. Nishino M, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncology. 2016;2(12):1607.
2. Naidoo J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709.
3. Nishino M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051.
Pulmonary Vascular Disease
Pulmonary Arterial Hypertension Associated With SLE
While pulmonary arterial hypertension (PAH) commonly complicates scleroderma (SSc), it is a rare complication of other connective tissue diseases (CTD), such as systemic lupus erythematosus (SLE). In the few prospective studies that utilize right-sided heart catheterization (RHC), the estimated prevalence of PAH in SLE is about 4%. However, since the prevalence of SLE is 10 to 15 times greater than SSc in the United States, the true prevalence of SLE-PAH may be higher than previously thought, and, thus, clinically relevant. Despite this, little is known about SLE-PAH.
A recent retrospective study from the French Pulmonary Hypertension Registry has added significantly to our understanding of this complication of SLE. Hachulla and colleagues studied 51 patients with RHC-proven SLE-PAH compared with 101 SLE control subjects without PAH. While the authors did not find any relevant differences in the demographics between groups, they did find a significantly higher prevalence of SSA and SSB antibodies in SLE-PAH. Interestingly, the presence of anti-U1 RNP antibody appeared to be less common in SLE-PAH patients; this lack of association is in contrast to prior studies in mixed CTD patients with anti-U1 RNP antibodies in which the prevalence of PAH can be as high as 60%. Further, none of the SLE-PAH patients demonstrated an acute response to vasodilator challenge during RHC, emphasizing that this maneuver does not need to be performed in SLE patients at risk of PAH. Trends toward improved survival in SLE-PAH patients treated with hydroxychloroquine are preliminary and hypothesis-generating but require confirmation in larger clinical studies.
Stephen Mathai, MD, FCCP
Chair
Leena Palwar, MD
Fellow-in-Training Member
References
Hachulla E, Jais X, Cinquetti G, et al. Pulmonary arterial hypertension associated with SLE: Results from the French pulmonary hypertension registry. Chest. 2017 Aug 26. pii: S0012-3692(17)31430-7. doi: 10.1016/j.chest.2017.08.014. [Epub ahead of print]
Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138:1383-1394.
Shirai Y, Yasuoka H, Okano Y, Takeuchi T, Satoh T, Kuwana M. Clinical characteristics and survival of Japanese patients with connective tissue disease and pulmonary arterial hypertension: a singlecentre cohort. Rheumatology. 2012;51:1846-1854.
Hao YJ, Jiang X, Zhou W, et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J. 2014;44: 963-972.
Huang C, Li M, Liu Y, et al. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Medicine. 2016;95:e2761.
Pérez-Peñate GM, Rúa-Figueroa I, Juliá- Serdá G, et al. Pulmonary arterial hypertension in systemic lupus erythematosus: prevalence and predictors. J Rheumatol. 2016;43:323-329.
Alpert MA, Goldberg SH, Sindem BH, et al. Cardiovascular manifestations of mixed connective tissue disease in adults. Circulation. 1983;63:1182-1193.
Interventional Chest/Diagnostic Procedures
Cryobiopsy for ILD: Careful stewardship needed
Interest in transbronchial cryobiopsy has accelerated rapidly in recent years. This procedure is performed by advancing a cryoprobe into the peripheral lung via flexible bronchoscopy, where lung tissue freezes and adheres to the probe and is subsequently extracted as a cryobiopsy. The number of cryobiopsy-related publications has increased exponentially since it was described in 2009 (Babiak A, et al. Respiration. 2009;78[2]:203). This interest stems from reports of high diagnostic yields in patients with interstitial lung disease (ILD) while maintaining complication rates similar to that of conventional bronchoscopic biopsy.
Traditional bronchoscopic biopsies are notoriously insensitive; a specific diagnosis can be established in fewer than a third of cases (Sheth JS, et al. Chest. 2017;151[2]:389). As such, surgical lung biopsy continues to be recommended but is associated with significant mortality (2%) and morbidity (30%) in patients with ILD (Hutchinson JP, et al. ARJCCM. 2016;193[10]:1161). Cryobiopsy, which appears to rival surgical lung biopsy in terms of ability to contribute to a specific diagnosis, is, therefore, a highly promising alternative (Tomassetti S, et al. AJRCCM. 2016;193[7]:745).
As cryobiopsy is increasingly adopted around the world, however, troubling reports of serious complications have surfaced. Most notable is the recently reported experience of the initial 25 cases performed at the University of Pennsylvania, in which almost one in four patients suffered serious complications (DiBardino DM, et al. Ann Am Thorac Soc. 2017;14[6]:851). The authors pointed to lack of a predefined procedural protocol, as well as several choices relating to the specific technique used, including inconsistent use of fluoroscopy, lack of prophylactic bronchial blocker placement, and predominant use of laryngeal mask airways as potential contributing factors. Indeed, many variations of the basic cryobiopsy procedure have been described (Lentz RJ, et al. J Thoracic Dis. 2017;9[7]:2186), with no formal guidance or training available to inform advanced bronchoscopists interested in this procedure.
It is incumbent on the interventional pulmonology and ILD specialist communities to be responsible stewards of this promising procedure. Implementation of three parallel efforts to standardize and rigorously study this procedure should be considered as soon as possible: creation of expert consensus guidelines establishing best-practices for safe and effective biopsy technique; a training requirement before independent performance of the procedure; and creation of an international cryobiopsy registry to facilitate higher-quality research into optimal technique and outcomes. We owe this to our patients.
Robert J. Lentz, MD
NetWork Member
Fabien Maldonado, MD, FCCP
NetWork Member
Pediatric Chest Medicine
Chronic cough in children: New guidelines
A chronic cough is a common complaint among children whose parents seek medical evaluation. Chronic wet cough can indicate an underlying illness; therefore, an early diagnosis can lead to prevention of complications of the disease and improvement in quality of life.
CHEST is a leading resource in evidence and consensus-based guidelines on important topics affecting children. The most recent guidelines entitled Management of Children with Chronic Wet Cough and Protracted Bacterial Bronchitis (Chest. 2017;151(4):884-890) and Use of Management Pathways or Algorithms in Children with Chronic Cough (Chest. 2017;151(4):875-873) are updates from the 2006 CHEST guidelines on chronic cough in children.
The present updates utilized the CHEST methodological guidelines with chronic wet or productive cough and Grading of Recommendations Assessment, Development, and Evaluation framework and also performed a systematic review addressing key questions concerning the management of childhood disease for children 14 years and younger.
Guidance provided by the expert panel focused on recommendations to answer six key questions concerning the management of children 14 years and younger with a chronic wet cough unrelated to established chronic lung disease. The recommendations are:
1. Chronic cough is defined as the presence of a cough 4 weeks or longer in duration.
2. Assessment of the effect of the cough on the child and the family be undertaken as part of clinical consultation.
3. Evaluation of a chronic cough should be done with a systematic approach with pediatric-specific cough management protocols or algorithms.
4. Chest radiograph and, when age appropriate, spirometry with bronchodilator be undertaken as evaluation; tests for pertussis infection only to be performed if clinically suspected.
5. Chronic wet cough with no specific clinical features should receive antibiotics for 2 weeks targeted for common respiratory bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis).
6. When cough persists despite 2 weeks of appropriate antibiotics, it is recommended to continue for an additional 2 weeks.
7. Additional tests (eg skin prick test, Mantoux, bronchoscopy, chest CT scan) should be individualized in accordance with the clinical setting and child’s clinical symptoms and signs.
The panel recognizes the need for prospective studies to assess current algorithms outcomes of children with chronic cough. Both articles can be found on the guidelines section of the CHEST site.
John Bishara, DO
Fellow-in-Training Member
Pulmonary Physiology, Function, and Rehabilitation
Functional imaging of the lung
Quantifying heterogeneity of ventilation and gas exchange in lung diseases remains a clinical challenge. Conventional pulmonary function test is insensitive to regional changes. The multiple inert gas elimination technique can quantify ventilation-perfusion distribution, but it requires invasive instrumentation (eg, pulmonary artery catheterization) and is not practical for clinical use. Computed tomography (CT) scans delineate spatial changes in lung structures but do not directly measure changes in ventilation and gas exchange. With its radiation, it is difficult to apply CT scanning repeatedly in patients. More recently, MR imaging techniques have been developed to directly “visualize” and quantify regional lung function (Kruger SJ, et al. J Magn Reson Imaging. 2016;43(2):295; Roos JE, et al. Magn Reson Imaging Clin N Am. 2015;23(2):217). These techniques employ inhalation of gases, such as oxygen, perfluorinated gases, and hyperpolarized 3He and 129Xe. Hyperpolarized 3He has been studied the most; however, the dwindling supply of 3He gas and its rising cost have prevented its further development. 129Xe has abundant supply and has emerged to be the inert gas of choice for MR imaging. Hyperpolarized 129Xe can measure ventilation, like hyperpolarized 3He. In addition, Xe diffuses into alveolar barrier (interstitium and plasma) and red blood cells, where it exhibits distinct resonant frequency shifts that can be captured by MR. Therefore, in one test, information on pulmonary ventilation and gas transfer can be obtained. To date, the results from MR imaging studies have provided new insights into the pathophysiology of obstructive and restrictive lung diseases. With continuous development, MR imaging of the lung could become a clinically useful tool in the near future.
Yuh-Chin T. Huang, MD, MHS, FCCP
Steering Committee Member
Thoracic Oncology
Immune-mediated pneumonitis and PD-1 inhibition
Inhibitors of the programmed cell death 1 receptor (PD-1) have shown significant promise in the treatment of advanced stage malignancy. With the recent expansion of indications for use of these agents, the number of patients treated will continue to grow. Clinicians must be aware of their potential for serious adverse side effects, including dermatitis, colitis, and potentially life-threatening pneumonitis.
The development of pneumonitis secondary to PD-1 inhibitions is reported to occur in 2% to 5% of patients and can present at any time during therapy, with 1% of patients developing grade 3 or higher pneumonitis.1,2 The most common symptoms are dyspnea and cough, though one-third of patients are asymptomatic at presentation.2 Radiographic and pathologic features vary greatly and include organizing pneumonia, interstitial pneumonitis, hypersensitivity pneumonitis, or diffuse alveolar damage.3 While pneumonitis due to PD-1 inhibition is reportedly uncommon, the increasing number of patients expected to receive these medications will predictably result in increasing overall frequency of pneumonitis cases. In addition, the lack of large prospective randomized trials and reliance on radiographic rather than pathologic data in diagnosing immune-mediated pneumonitis gives one pause. Given the variability of presentation, lack of routine pathologic data, and increasing use of dual agents (eg, PD-1 and CTLA-4), chest physicians and medical oncologists should have a high index of suspicion yet practice equipoise in patients receiving immunotherapy who develop unexplained pulmonary symptoms or infiltrates. More research is needed to help improve the multidisciplinary diagnosis and treatment of this potentially serious complication.
David Maurice Chambers, MD
Fellow-in-Training Member
Jason Atticus Akulian, MD, MPH
Steering Committee Member
References
1. Nishino M, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncology. 2016;2(12):1607.
2. Naidoo J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709.
3. Nishino M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051.
Pulmonary Vascular Disease
Pulmonary Arterial Hypertension Associated With SLE
While pulmonary arterial hypertension (PAH) commonly complicates scleroderma (SSc), it is a rare complication of other connective tissue diseases (CTD), such as systemic lupus erythematosus (SLE). In the few prospective studies that utilize right-sided heart catheterization (RHC), the estimated prevalence of PAH in SLE is about 4%. However, since the prevalence of SLE is 10 to 15 times greater than SSc in the United States, the true prevalence of SLE-PAH may be higher than previously thought, and, thus, clinically relevant. Despite this, little is known about SLE-PAH.
A recent retrospective study from the French Pulmonary Hypertension Registry has added significantly to our understanding of this complication of SLE. Hachulla and colleagues studied 51 patients with RHC-proven SLE-PAH compared with 101 SLE control subjects without PAH. While the authors did not find any relevant differences in the demographics between groups, they did find a significantly higher prevalence of SSA and SSB antibodies in SLE-PAH. Interestingly, the presence of anti-U1 RNP antibody appeared to be less common in SLE-PAH patients; this lack of association is in contrast to prior studies in mixed CTD patients with anti-U1 RNP antibodies in which the prevalence of PAH can be as high as 60%. Further, none of the SLE-PAH patients demonstrated an acute response to vasodilator challenge during RHC, emphasizing that this maneuver does not need to be performed in SLE patients at risk of PAH. Trends toward improved survival in SLE-PAH patients treated with hydroxychloroquine are preliminary and hypothesis-generating but require confirmation in larger clinical studies.
Stephen Mathai, MD, FCCP
Chair
Leena Palwar, MD
Fellow-in-Training Member
References
Hachulla E, Jais X, Cinquetti G, et al. Pulmonary arterial hypertension associated with SLE: Results from the French pulmonary hypertension registry. Chest. 2017 Aug 26. pii: S0012-3692(17)31430-7. doi: 10.1016/j.chest.2017.08.014. [Epub ahead of print]
Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138:1383-1394.
Shirai Y, Yasuoka H, Okano Y, Takeuchi T, Satoh T, Kuwana M. Clinical characteristics and survival of Japanese patients with connective tissue disease and pulmonary arterial hypertension: a singlecentre cohort. Rheumatology. 2012;51:1846-1854.
Hao YJ, Jiang X, Zhou W, et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J. 2014;44: 963-972.
Huang C, Li M, Liu Y, et al. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Medicine. 2016;95:e2761.
Pérez-Peñate GM, Rúa-Figueroa I, Juliá- Serdá G, et al. Pulmonary arterial hypertension in systemic lupus erythematosus: prevalence and predictors. J Rheumatol. 2016;43:323-329.
Alpert MA, Goldberg SH, Sindem BH, et al. Cardiovascular manifestations of mixed connective tissue disease in adults. Circulation. 1983;63:1182-1193.
Transbronchial cryobiopsy, updated guidelines for chronic cough in children, PD-1 inhibition
Interventional Chest/Diagnostic Procedures
Cryobiopsy for ILD: Careful stewardship needed
Interest in transbronchial cryobiopsy has accelerated rapidly in recent years. This procedure is performed by advancing a cryoprobe into the peripheral lung via flexible bronchoscopy, where lung tissue freezes and adheres to the probe and is subsequently extracted as a cryobiopsy. The number of cryobiopsy-related publications has increased exponentially since it was described in 2009 (Babiak A, et al. Respiration. 2009;78[2]:203). This interest stems from reports of high diagnostic yields in patients with interstitial lung disease (ILD) while maintaining complication rates similar to that of conventional bronchoscopic biopsy.
Traditional bronchoscopic biopsies are notoriously insensitive; a specific diagnosis can be established in fewer than a third of cases (Sheth JS, et al. Chest. 2017;151[2]:389). As such, surgical lung biopsy continues to be recommended but is associated with significant mortality (2%) and morbidity (30%) in patients with ILD (Hutchinson JP, et al. ARJCCM. 2016;193[10]:1161). Cryobiopsy, which appears to rival surgical lung biopsy in terms of ability to contribute to a specific diagnosis, is, therefore, a highly promising alternative (Tomassetti S, et al. AJRCCM. 2016;193[7]:745).
As cryobiopsy is increasingly adopted around the world, however, troubling reports of serious complications have surfaced. Most notable is the recently reported experience of the initial 25 cases performed at the University of Pennsylvania, in which almost one in four patients suffered serious complications (DiBardino DM, et al. Ann Am Thorac Soc. 2017;14[6]:851). The authors pointed to lack of a predefined procedural protocol, as well as several choices relating to the specific technique used, including inconsistent use of fluoroscopy, lack of prophylactic bronchial blocker placement, and predominant use of laryngeal mask airways as potential contributing factors. Indeed, many variations of the basic cryobiopsy procedure have been described (Lentz RJ, et al. J Thoracic Dis. 2017;9[7]:2186), with no formal guidance or training available to inform advanced bronchoscopists interested in this procedure.
It is incumbent on the interventional pulmonology and ILD specialist communities to be responsible stewards of this promising procedure. Implementation of three parallel efforts to standardize and rigorously study this procedure should be considered as soon as possible: creation of expert consensus guidelines establishing best-practices for safe and effective biopsy technique; a training requirement before independent performance of the procedure; and creation of an international cryobiopsy registry to facilitate higher-quality research into optimal technique and outcomes. We owe this to our patients.
Robert J. Lentz, MD
NetWork Member
Fabien Maldonado, MD, FCCP
NetWork Member
Pediatric Chest Medicine
Chronic cough in children: New guidelines
A chronic cough is a common complaint among children whose parents seek medical evaluation. Chronic wet cough can indicate an underlying illness; therefore, an early diagnosis can lead to prevention of complications of the disease and improvement in quality of life.
CHEST is a leading resource in evidence and consensus-based guidelines on important topics affecting children. The most recent guidelines entitled Management of Children with Chronic Wet Cough and Protracted Bacterial Bronchitis (Chest. 2017;151(4):884-890) and Use of Management Pathways or Algorithms in Children with Chronic Cough (Chest. 2017;151(4):875-873) are updates from the 2006 CHEST guidelines on chronic cough in children.
The present updates utilized the CHEST methodological guidelines with chronic wet or productive cough and Grading of Recommendations Assessment, Development, and Evaluation framework and also performed a systematic review addressing key questions concerning the management of childhood disease for children 14 years and younger.
Guidance provided by the expert panel focused on recommendations to answer six key questions concerning the management of children 14 years and younger with a chronic wet cough unrelated to established chronic lung disease. The recommendations are:
1. Chronic cough is defined as the presence of a cough 4 weeks or longer in duration.
2. Assessment of the effect of the cough on the child and the family be undertaken as part of clinical consultation.
3. Evaluation of a chronic cough should be done with a systematic approach with pediatric-specific cough management protocols or algorithms.
4. Chest radiograph and, when age appropriate, spirometry with bronchodilator be undertaken as evaluation; tests for pertussis infection only to be performed if clinically suspected.
5. Chronic wet cough with no specific clinical features should receive antibiotics for 2 weeks targeted for common respiratory bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis).
6. When cough persists despite 2 weeks of appropriate antibiotics, it is recommended to continue for an additional 2 weeks.
7. Additional tests (eg skin prick test, Mantoux, bronchoscopy, chest CT scan) should be individualized in accordance with the clinical setting and child’s clinical symptoms and signs.
The panel recognizes the need for prospective studies to assess current algorithms outcomes of children with chronic cough. Both articles can be found on the guidelines section of the CHEST site.
John Bishara, DO
Fellow-in-Training Member
Pulmonary Physiology, Function, and Rehabilitation
Functional imaging of the lung
Quantifying heterogeneity of ventilation and gas exchange in lung diseases remains a clinical challenge. Conventional pulmonary function test is insensitive to regional changes. The multiple inert gas elimination technique can quantify ventilation-perfusion distribution, but it requires invasive instrumentation (eg, pulmonary artery catheterization) and is not practical for clinical use. Computed tomography (CT) scans delineate spatial changes in lung structures but do not directly measure changes in ventilation and gas exchange. With its radiation, it is difficult to apply CT scanning repeatedly in patients. More recently, MR imaging techniques have been developed to directly “visualize” and quantify regional lung function (Kruger SJ, et al. J Magn Reson Imaging. 2016;43(2):295; Roos JE, et al. Magn Reson Imaging Clin N Am. 2015;23(2):217). These techniques employ inhalation of gases, such as oxygen, perfluorinated gases, and hyperpolarized 3He and 129Xe. Hyperpolarized 3He has been studied the most; however, the dwindling supply of 3He gas and its rising cost have prevented its further development. 129Xe has abundant supply and has emerged to be the inert gas of choice for MR imaging. Hyperpolarized 129Xe can measure ventilation, like hyperpolarized 3He. In addition, Xe diffuses into alveolar barrier (interstitium and plasma) and red blood cells, where it exhibits distinct resonant frequency shifts that can be captured by MR. Therefore, in one test, information on pulmonary ventilation and gas transfer can be obtained. To date, the results from MR imaging studies have provided new insights into the pathophysiology of obstructive and restrictive lung diseases. With continuous development, MR imaging of the lung could become a clinically useful tool in the near future.
Yuh-Chin T. Huang, MD, MHS, FCCP
Steering Committee Member
Thoracic Oncology
Immune-mediated pneumonitis and PD-1 inhibition
Inhibitors of the programmed cell death 1 receptor (PD-1) have shown significant promise in the treatment of advanced stage malignancy. With the recent expansion of indications for use of these agents, the number of patients treated will continue to grow. Clinicians must be aware of their potential for serious adverse side effects, including dermatitis, colitis, and potentially life-threatening pneumonitis.
The development of pneumonitis secondary to PD-1 inhibitions is reported to occur in 2% to 5% of patients and can present at any time during therapy, with 1% of patients developing grade 3 or higher pneumonitis.1,2 The most common symptoms are dyspnea and cough, though one-third of patients are asymptomatic at presentation.2 Radiographic and pathologic features vary greatly and include organizing pneumonia, interstitial pneumonitis, hypersensitivity pneumonitis, or diffuse alveolar damage.3 While pneumonitis due to PD-1 inhibition is reportedly uncommon, the increasing number of patients expected to receive these medications will predictably result in increasing overall frequency of pneumonitis cases. In addition, the lack of large prospective randomized trials and reliance on radiographic rather than pathologic data in diagnosing immune-mediated pneumonitis gives one pause. Given the variability of presentation, lack of routine pathologic data, and increasing use of dual agents (eg, PD-1 and CTLA-4), chest physicians and medical oncologists should have a high index of suspicion yet practice equipoise in patients receiving immunotherapy who develop unexplained pulmonary symptoms or infiltrates. More research is needed to help improve the multidisciplinary diagnosis and treatment of this potentially serious complication.
David Maurice Chambers, MD
Fellow-in-Training Member
Jason Atticus Akulian, MD, MPH
Steering Committee Member
References
1. Nishino M, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncology. 2016;2(12):1607.
2. Naidoo J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709.
3. Nishino M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051.
Pulmonary Vascular Disease
Pulmonary Arterial Hypertension Associated With SLE
While pulmonary arterial hypertension (PAH) commonly complicates scleroderma (SSc), it is a rare complication of other connective tissue diseases (CTD), such as systemic lupus erythematosus (SLE). In the few prospective studies that utilize right-sided heart catheterization (RHC), the estimated prevalence of PAH in SLE is about 4%. However, since the prevalence of SLE is 10 to 15 times greater than SSc in the United States, the true prevalence of SLE-PAH may be higher than previously thought, and, thus, clinically relevant. Despite this, little is known about SLE-PAH.
A recent retrospective study from the French Pulmonary Hypertension Registry has added significantly to our understanding of this complication of SLE. Hachulla and colleagues studied 51 patients with RHC-proven SLE-PAH compared with 101 SLE control subjects without PAH. While the authors did not find any relevant differences in the demographics between groups, they did find a significantly higher prevalence of SSA and SSB antibodies in SLE-PAH. Interestingly, the presence of anti-U1 RNP antibody appeared to be less common in SLE-PAH patients; this lack of association is in contrast to prior studies in mixed CTD patients with anti-U1 RNP antibodies in which the prevalence of PAH can be as high as 60%. Further, none of the SLE-PAH patients demonstrated an acute response to vasodilator challenge during RHC, emphasizing that this maneuver does not need to be performed in SLE patients at risk of PAH. Trends toward improved survival in SLE-PAH patients treated with hydroxychloroquine are preliminary and hypothesis-generating but require confirmation in larger clinical studies.
Stephen Mathai, MD, FCCP
Chair
Leena Palwar, MD
Fellow-in-Training Member
References
Hachulla E, Jais X, Cinquetti G, et al. Pulmonary arterial hypertension associated with SLE: Results from the French pulmonary hypertension registry. Chest. 2017 Aug 26. pii: S0012-3692(17)31430-7. doi: 10.1016/j.chest.2017.08.014. [Epub ahead of print]
Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138:1383-1394.
Shirai Y, Yasuoka H, Okano Y, Takeuchi T, Satoh T, Kuwana M. Clinical characteristics and survival of Japanese patients with connective tissue disease and pulmonary arterial hypertension: a singlecentre cohort. Rheumatology. 2012;51:1846-1854.
Hao YJ, Jiang X, Zhou W, et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J. 2014;44: 963-972.
Huang C, Li M, Liu Y, et al. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Medicine. 2016;95:e2761.
Pérez-Peñate GM, Rúa-Figueroa I, Juliá- Serdá G, et al. Pulmonary arterial hypertension in systemic lupus erythematosus: prevalence and predictors. J Rheumatol. 2016;43:323-329.
Alpert MA, Goldberg SH, Sindem BH, et al. Cardiovascular manifestations of mixed connective tissue disease in adults. Circulation. 1983;63:1182-1193.
Interventional Chest/Diagnostic Procedures
Cryobiopsy for ILD: Careful stewardship needed
Interest in transbronchial cryobiopsy has accelerated rapidly in recent years. This procedure is performed by advancing a cryoprobe into the peripheral lung via flexible bronchoscopy, where lung tissue freezes and adheres to the probe and is subsequently extracted as a cryobiopsy. The number of cryobiopsy-related publications has increased exponentially since it was described in 2009 (Babiak A, et al. Respiration. 2009;78[2]:203). This interest stems from reports of high diagnostic yields in patients with interstitial lung disease (ILD) while maintaining complication rates similar to that of conventional bronchoscopic biopsy.
Traditional bronchoscopic biopsies are notoriously insensitive; a specific diagnosis can be established in fewer than a third of cases (Sheth JS, et al. Chest. 2017;151[2]:389). As such, surgical lung biopsy continues to be recommended but is associated with significant mortality (2%) and morbidity (30%) in patients with ILD (Hutchinson JP, et al. ARJCCM. 2016;193[10]:1161). Cryobiopsy, which appears to rival surgical lung biopsy in terms of ability to contribute to a specific diagnosis, is, therefore, a highly promising alternative (Tomassetti S, et al. AJRCCM. 2016;193[7]:745).
As cryobiopsy is increasingly adopted around the world, however, troubling reports of serious complications have surfaced. Most notable is the recently reported experience of the initial 25 cases performed at the University of Pennsylvania, in which almost one in four patients suffered serious complications (DiBardino DM, et al. Ann Am Thorac Soc. 2017;14[6]:851). The authors pointed to lack of a predefined procedural protocol, as well as several choices relating to the specific technique used, including inconsistent use of fluoroscopy, lack of prophylactic bronchial blocker placement, and predominant use of laryngeal mask airways as potential contributing factors. Indeed, many variations of the basic cryobiopsy procedure have been described (Lentz RJ, et al. J Thoracic Dis. 2017;9[7]:2186), with no formal guidance or training available to inform advanced bronchoscopists interested in this procedure.
It is incumbent on the interventional pulmonology and ILD specialist communities to be responsible stewards of this promising procedure. Implementation of three parallel efforts to standardize and rigorously study this procedure should be considered as soon as possible: creation of expert consensus guidelines establishing best-practices for safe and effective biopsy technique; a training requirement before independent performance of the procedure; and creation of an international cryobiopsy registry to facilitate higher-quality research into optimal technique and outcomes. We owe this to our patients.
Robert J. Lentz, MD
NetWork Member
Fabien Maldonado, MD, FCCP
NetWork Member
Pediatric Chest Medicine
Chronic cough in children: New guidelines
A chronic cough is a common complaint among children whose parents seek medical evaluation. Chronic wet cough can indicate an underlying illness; therefore, an early diagnosis can lead to prevention of complications of the disease and improvement in quality of life.
CHEST is a leading resource in evidence and consensus-based guidelines on important topics affecting children. The most recent guidelines entitled Management of Children with Chronic Wet Cough and Protracted Bacterial Bronchitis (Chest. 2017;151(4):884-890) and Use of Management Pathways or Algorithms in Children with Chronic Cough (Chest. 2017;151(4):875-873) are updates from the 2006 CHEST guidelines on chronic cough in children.
The present updates utilized the CHEST methodological guidelines with chronic wet or productive cough and Grading of Recommendations Assessment, Development, and Evaluation framework and also performed a systematic review addressing key questions concerning the management of childhood disease for children 14 years and younger.
Guidance provided by the expert panel focused on recommendations to answer six key questions concerning the management of children 14 years and younger with a chronic wet cough unrelated to established chronic lung disease. The recommendations are:
1. Chronic cough is defined as the presence of a cough 4 weeks or longer in duration.
2. Assessment of the effect of the cough on the child and the family be undertaken as part of clinical consultation.
3. Evaluation of a chronic cough should be done with a systematic approach with pediatric-specific cough management protocols or algorithms.
4. Chest radiograph and, when age appropriate, spirometry with bronchodilator be undertaken as evaluation; tests for pertussis infection only to be performed if clinically suspected.
5. Chronic wet cough with no specific clinical features should receive antibiotics for 2 weeks targeted for common respiratory bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis).
6. When cough persists despite 2 weeks of appropriate antibiotics, it is recommended to continue for an additional 2 weeks.
7. Additional tests (eg skin prick test, Mantoux, bronchoscopy, chest CT scan) should be individualized in accordance with the clinical setting and child’s clinical symptoms and signs.
The panel recognizes the need for prospective studies to assess current algorithms outcomes of children with chronic cough. Both articles can be found on the guidelines section of the CHEST site.
John Bishara, DO
Fellow-in-Training Member
Pulmonary Physiology, Function, and Rehabilitation
Functional imaging of the lung
Quantifying heterogeneity of ventilation and gas exchange in lung diseases remains a clinical challenge. Conventional pulmonary function test is insensitive to regional changes. The multiple inert gas elimination technique can quantify ventilation-perfusion distribution, but it requires invasive instrumentation (eg, pulmonary artery catheterization) and is not practical for clinical use. Computed tomography (CT) scans delineate spatial changes in lung structures but do not directly measure changes in ventilation and gas exchange. With its radiation, it is difficult to apply CT scanning repeatedly in patients. More recently, MR imaging techniques have been developed to directly “visualize” and quantify regional lung function (Kruger SJ, et al. J Magn Reson Imaging. 2016;43(2):295; Roos JE, et al. Magn Reson Imaging Clin N Am. 2015;23(2):217). These techniques employ inhalation of gases, such as oxygen, perfluorinated gases, and hyperpolarized 3He and 129Xe. Hyperpolarized 3He has been studied the most; however, the dwindling supply of 3He gas and its rising cost have prevented its further development. 129Xe has abundant supply and has emerged to be the inert gas of choice for MR imaging. Hyperpolarized 129Xe can measure ventilation, like hyperpolarized 3He. In addition, Xe diffuses into alveolar barrier (interstitium and plasma) and red blood cells, where it exhibits distinct resonant frequency shifts that can be captured by MR. Therefore, in one test, information on pulmonary ventilation and gas transfer can be obtained. To date, the results from MR imaging studies have provided new insights into the pathophysiology of obstructive and restrictive lung diseases. With continuous development, MR imaging of the lung could become a clinically useful tool in the near future.
Yuh-Chin T. Huang, MD, MHS, FCCP
Steering Committee Member
Thoracic Oncology
Immune-mediated pneumonitis and PD-1 inhibition
Inhibitors of the programmed cell death 1 receptor (PD-1) have shown significant promise in the treatment of advanced stage malignancy. With the recent expansion of indications for use of these agents, the number of patients treated will continue to grow. Clinicians must be aware of their potential for serious adverse side effects, including dermatitis, colitis, and potentially life-threatening pneumonitis.
The development of pneumonitis secondary to PD-1 inhibitions is reported to occur in 2% to 5% of patients and can present at any time during therapy, with 1% of patients developing grade 3 or higher pneumonitis.1,2 The most common symptoms are dyspnea and cough, though one-third of patients are asymptomatic at presentation.2 Radiographic and pathologic features vary greatly and include organizing pneumonia, interstitial pneumonitis, hypersensitivity pneumonitis, or diffuse alveolar damage.3 While pneumonitis due to PD-1 inhibition is reportedly uncommon, the increasing number of patients expected to receive these medications will predictably result in increasing overall frequency of pneumonitis cases. In addition, the lack of large prospective randomized trials and reliance on radiographic rather than pathologic data in diagnosing immune-mediated pneumonitis gives one pause. Given the variability of presentation, lack of routine pathologic data, and increasing use of dual agents (eg, PD-1 and CTLA-4), chest physicians and medical oncologists should have a high index of suspicion yet practice equipoise in patients receiving immunotherapy who develop unexplained pulmonary symptoms or infiltrates. More research is needed to help improve the multidisciplinary diagnosis and treatment of this potentially serious complication.
David Maurice Chambers, MD
Fellow-in-Training Member
Jason Atticus Akulian, MD, MPH
Steering Committee Member
References
1. Nishino M, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncology. 2016;2(12):1607.
2. Naidoo J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709.
3. Nishino M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051.
Pulmonary Vascular Disease
Pulmonary Arterial Hypertension Associated With SLE
While pulmonary arterial hypertension (PAH) commonly complicates scleroderma (SSc), it is a rare complication of other connective tissue diseases (CTD), such as systemic lupus erythematosus (SLE). In the few prospective studies that utilize right-sided heart catheterization (RHC), the estimated prevalence of PAH in SLE is about 4%. However, since the prevalence of SLE is 10 to 15 times greater than SSc in the United States, the true prevalence of SLE-PAH may be higher than previously thought, and, thus, clinically relevant. Despite this, little is known about SLE-PAH.
A recent retrospective study from the French Pulmonary Hypertension Registry has added significantly to our understanding of this complication of SLE. Hachulla and colleagues studied 51 patients with RHC-proven SLE-PAH compared with 101 SLE control subjects without PAH. While the authors did not find any relevant differences in the demographics between groups, they did find a significantly higher prevalence of SSA and SSB antibodies in SLE-PAH. Interestingly, the presence of anti-U1 RNP antibody appeared to be less common in SLE-PAH patients; this lack of association is in contrast to prior studies in mixed CTD patients with anti-U1 RNP antibodies in which the prevalence of PAH can be as high as 60%. Further, none of the SLE-PAH patients demonstrated an acute response to vasodilator challenge during RHC, emphasizing that this maneuver does not need to be performed in SLE patients at risk of PAH. Trends toward improved survival in SLE-PAH patients treated with hydroxychloroquine are preliminary and hypothesis-generating but require confirmation in larger clinical studies.
Stephen Mathai, MD, FCCP
Chair
Leena Palwar, MD
Fellow-in-Training Member
References
Hachulla E, Jais X, Cinquetti G, et al. Pulmonary arterial hypertension associated with SLE: Results from the French pulmonary hypertension registry. Chest. 2017 Aug 26. pii: S0012-3692(17)31430-7. doi: 10.1016/j.chest.2017.08.014. [Epub ahead of print]
Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138:1383-1394.
Shirai Y, Yasuoka H, Okano Y, Takeuchi T, Satoh T, Kuwana M. Clinical characteristics and survival of Japanese patients with connective tissue disease and pulmonary arterial hypertension: a singlecentre cohort. Rheumatology. 2012;51:1846-1854.
Hao YJ, Jiang X, Zhou W, et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J. 2014;44: 963-972.
Huang C, Li M, Liu Y, et al. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Medicine. 2016;95:e2761.
Pérez-Peñate GM, Rúa-Figueroa I, Juliá- Serdá G, et al. Pulmonary arterial hypertension in systemic lupus erythematosus: prevalence and predictors. J Rheumatol. 2016;43:323-329.
Alpert MA, Goldberg SH, Sindem BH, et al. Cardiovascular manifestations of mixed connective tissue disease in adults. Circulation. 1983;63:1182-1193.
Interventional Chest/Diagnostic Procedures
Cryobiopsy for ILD: Careful stewardship needed
Interest in transbronchial cryobiopsy has accelerated rapidly in recent years. This procedure is performed by advancing a cryoprobe into the peripheral lung via flexible bronchoscopy, where lung tissue freezes and adheres to the probe and is subsequently extracted as a cryobiopsy. The number of cryobiopsy-related publications has increased exponentially since it was described in 2009 (Babiak A, et al. Respiration. 2009;78[2]:203). This interest stems from reports of high diagnostic yields in patients with interstitial lung disease (ILD) while maintaining complication rates similar to that of conventional bronchoscopic biopsy.
Traditional bronchoscopic biopsies are notoriously insensitive; a specific diagnosis can be established in fewer than a third of cases (Sheth JS, et al. Chest. 2017;151[2]:389). As such, surgical lung biopsy continues to be recommended but is associated with significant mortality (2%) and morbidity (30%) in patients with ILD (Hutchinson JP, et al. ARJCCM. 2016;193[10]:1161). Cryobiopsy, which appears to rival surgical lung biopsy in terms of ability to contribute to a specific diagnosis, is, therefore, a highly promising alternative (Tomassetti S, et al. AJRCCM. 2016;193[7]:745).
As cryobiopsy is increasingly adopted around the world, however, troubling reports of serious complications have surfaced. Most notable is the recently reported experience of the initial 25 cases performed at the University of Pennsylvania, in which almost one in four patients suffered serious complications (DiBardino DM, et al. Ann Am Thorac Soc. 2017;14[6]:851). The authors pointed to lack of a predefined procedural protocol, as well as several choices relating to the specific technique used, including inconsistent use of fluoroscopy, lack of prophylactic bronchial blocker placement, and predominant use of laryngeal mask airways as potential contributing factors. Indeed, many variations of the basic cryobiopsy procedure have been described (Lentz RJ, et al. J Thoracic Dis. 2017;9[7]:2186), with no formal guidance or training available to inform advanced bronchoscopists interested in this procedure.
It is incumbent on the interventional pulmonology and ILD specialist communities to be responsible stewards of this promising procedure. Implementation of three parallel efforts to standardize and rigorously study this procedure should be considered as soon as possible: creation of expert consensus guidelines establishing best-practices for safe and effective biopsy technique; a training requirement before independent performance of the procedure; and creation of an international cryobiopsy registry to facilitate higher-quality research into optimal technique and outcomes. We owe this to our patients.
Robert J. Lentz, MD
NetWork Member
Fabien Maldonado, MD, FCCP
NetWork Member
Pediatric Chest Medicine
Chronic cough in children: New guidelines
A chronic cough is a common complaint among children whose parents seek medical evaluation. Chronic wet cough can indicate an underlying illness; therefore, an early diagnosis can lead to prevention of complications of the disease and improvement in quality of life.
CHEST is a leading resource in evidence and consensus-based guidelines on important topics affecting children. The most recent guidelines entitled Management of Children with Chronic Wet Cough and Protracted Bacterial Bronchitis (Chest. 2017;151(4):884-890) and Use of Management Pathways or Algorithms in Children with Chronic Cough (Chest. 2017;151(4):875-873) are updates from the 2006 CHEST guidelines on chronic cough in children.
The present updates utilized the CHEST methodological guidelines with chronic wet or productive cough and Grading of Recommendations Assessment, Development, and Evaluation framework and also performed a systematic review addressing key questions concerning the management of childhood disease for children 14 years and younger.
Guidance provided by the expert panel focused on recommendations to answer six key questions concerning the management of children 14 years and younger with a chronic wet cough unrelated to established chronic lung disease. The recommendations are:
1. Chronic cough is defined as the presence of a cough 4 weeks or longer in duration.
2. Assessment of the effect of the cough on the child and the family be undertaken as part of clinical consultation.
3. Evaluation of a chronic cough should be done with a systematic approach with pediatric-specific cough management protocols or algorithms.
4. Chest radiograph and, when age appropriate, spirometry with bronchodilator be undertaken as evaluation; tests for pertussis infection only to be performed if clinically suspected.
5. Chronic wet cough with no specific clinical features should receive antibiotics for 2 weeks targeted for common respiratory bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis).
6. When cough persists despite 2 weeks of appropriate antibiotics, it is recommended to continue for an additional 2 weeks.
7. Additional tests (eg skin prick test, Mantoux, bronchoscopy, chest CT scan) should be individualized in accordance with the clinical setting and child’s clinical symptoms and signs.
The panel recognizes the need for prospective studies to assess current algorithms outcomes of children with chronic cough. Both articles can be found on the guidelines section of the CHEST site.
John Bishara, DO
Fellow-in-Training Member
Pulmonary Physiology, Function, and Rehabilitation
Functional imaging of the lung
Quantifying heterogeneity of ventilation and gas exchange in lung diseases remains a clinical challenge. Conventional pulmonary function test is insensitive to regional changes. The multiple inert gas elimination technique can quantify ventilation-perfusion distribution, but it requires invasive instrumentation (eg, pulmonary artery catheterization) and is not practical for clinical use. Computed tomography (CT) scans delineate spatial changes in lung structures but do not directly measure changes in ventilation and gas exchange. With its radiation, it is difficult to apply CT scanning repeatedly in patients. More recently, MR imaging techniques have been developed to directly “visualize” and quantify regional lung function (Kruger SJ, et al. J Magn Reson Imaging. 2016;43(2):295; Roos JE, et al. Magn Reson Imaging Clin N Am. 2015;23(2):217). These techniques employ inhalation of gases, such as oxygen, perfluorinated gases, and hyperpolarized 3He and 129Xe. Hyperpolarized 3He has been studied the most; however, the dwindling supply of 3He gas and its rising cost have prevented its further development. 129Xe has abundant supply and has emerged to be the inert gas of choice for MR imaging. Hyperpolarized 129Xe can measure ventilation, like hyperpolarized 3He. In addition, Xe diffuses into alveolar barrier (interstitium and plasma) and red blood cells, where it exhibits distinct resonant frequency shifts that can be captured by MR. Therefore, in one test, information on pulmonary ventilation and gas transfer can be obtained. To date, the results from MR imaging studies have provided new insights into the pathophysiology of obstructive and restrictive lung diseases. With continuous development, MR imaging of the lung could become a clinically useful tool in the near future.
Yuh-Chin T. Huang, MD, MHS, FCCP
Steering Committee Member
Thoracic Oncology
Immune-mediated pneumonitis and PD-1 inhibition
Inhibitors of the programmed cell death 1 receptor (PD-1) have shown significant promise in the treatment of advanced stage malignancy. With the recent expansion of indications for use of these agents, the number of patients treated will continue to grow. Clinicians must be aware of their potential for serious adverse side effects, including dermatitis, colitis, and potentially life-threatening pneumonitis.
The development of pneumonitis secondary to PD-1 inhibitions is reported to occur in 2% to 5% of patients and can present at any time during therapy, with 1% of patients developing grade 3 or higher pneumonitis.1,2 The most common symptoms are dyspnea and cough, though one-third of patients are asymptomatic at presentation.2 Radiographic and pathologic features vary greatly and include organizing pneumonia, interstitial pneumonitis, hypersensitivity pneumonitis, or diffuse alveolar damage.3 While pneumonitis due to PD-1 inhibition is reportedly uncommon, the increasing number of patients expected to receive these medications will predictably result in increasing overall frequency of pneumonitis cases. In addition, the lack of large prospective randomized trials and reliance on radiographic rather than pathologic data in diagnosing immune-mediated pneumonitis gives one pause. Given the variability of presentation, lack of routine pathologic data, and increasing use of dual agents (eg, PD-1 and CTLA-4), chest physicians and medical oncologists should have a high index of suspicion yet practice equipoise in patients receiving immunotherapy who develop unexplained pulmonary symptoms or infiltrates. More research is needed to help improve the multidisciplinary diagnosis and treatment of this potentially serious complication.
David Maurice Chambers, MD
Fellow-in-Training Member
Jason Atticus Akulian, MD, MPH
Steering Committee Member
References
1. Nishino M, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncology. 2016;2(12):1607.
2. Naidoo J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709.
3. Nishino M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051.
Pulmonary Vascular Disease
Pulmonary Arterial Hypertension Associated With SLE
While pulmonary arterial hypertension (PAH) commonly complicates scleroderma (SSc), it is a rare complication of other connective tissue diseases (CTD), such as systemic lupus erythematosus (SLE). In the few prospective studies that utilize right-sided heart catheterization (RHC), the estimated prevalence of PAH in SLE is about 4%. However, since the prevalence of SLE is 10 to 15 times greater than SSc in the United States, the true prevalence of SLE-PAH may be higher than previously thought, and, thus, clinically relevant. Despite this, little is known about SLE-PAH.
A recent retrospective study from the French Pulmonary Hypertension Registry has added significantly to our understanding of this complication of SLE. Hachulla and colleagues studied 51 patients with RHC-proven SLE-PAH compared with 101 SLE control subjects without PAH. While the authors did not find any relevant differences in the demographics between groups, they did find a significantly higher prevalence of SSA and SSB antibodies in SLE-PAH. Interestingly, the presence of anti-U1 RNP antibody appeared to be less common in SLE-PAH patients; this lack of association is in contrast to prior studies in mixed CTD patients with anti-U1 RNP antibodies in which the prevalence of PAH can be as high as 60%. Further, none of the SLE-PAH patients demonstrated an acute response to vasodilator challenge during RHC, emphasizing that this maneuver does not need to be performed in SLE patients at risk of PAH. Trends toward improved survival in SLE-PAH patients treated with hydroxychloroquine are preliminary and hypothesis-generating but require confirmation in larger clinical studies.
Stephen Mathai, MD, FCCP
Chair
Leena Palwar, MD
Fellow-in-Training Member
References
Hachulla E, Jais X, Cinquetti G, et al. Pulmonary arterial hypertension associated with SLE: Results from the French pulmonary hypertension registry. Chest. 2017 Aug 26. pii: S0012-3692(17)31430-7. doi: 10.1016/j.chest.2017.08.014. [Epub ahead of print]
Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138:1383-1394.
Shirai Y, Yasuoka H, Okano Y, Takeuchi T, Satoh T, Kuwana M. Clinical characteristics and survival of Japanese patients with connective tissue disease and pulmonary arterial hypertension: a singlecentre cohort. Rheumatology. 2012;51:1846-1854.
Hao YJ, Jiang X, Zhou W, et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J. 2014;44: 963-972.
Huang C, Li M, Liu Y, et al. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Medicine. 2016;95:e2761.
Pérez-Peñate GM, Rúa-Figueroa I, Juliá- Serdá G, et al. Pulmonary arterial hypertension in systemic lupus erythematosus: prevalence and predictors. J Rheumatol. 2016;43:323-329.
Alpert MA, Goldberg SH, Sindem BH, et al. Cardiovascular manifestations of mixed connective tissue disease in adults. Circulation. 1983;63:1182-1193.
NetWorks: Oxygen therapy, electronic consent, diagnosing ILD
Oxygen therapy in patients with COPD with moderate desaturation
Two landmark trials from the early 1980s demonstrated survival benefit with long-term oxygen therapy (LTOT) in patients with COPD with severe resting hypoxemia [Sao2 less than 89%; (Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93[3]:391) or Pao2 40-60 mm Hg and cor pulmonale (Report of the Medical Research Council Working Party. Lancet. 1981;1[8222]:681).
The potential benefits of LTOT in COPD with mild-moderate hypoxemia have not been clearly established. The LOTT trial (The Long-Term Oxygen Treatment Trial Research Group. N Engl J Med. 2016;375[17]:1617), a recent multicenter randomized study, attempted to answer this question. They studied 738 stable patients with COPD with mild to moderate resting desaturation (Spo2 89%-93%) or exercise-induced moderate desaturation (Spo2 greater than or equal to 80% for greater than or equal to 5 minutes and Spo2 less than 90% for greater than or equal to 10 seconds during 6- minute walk test). After a median follow-up of 18.4 months, LTOT did not demonstrate a decrease in the time to death or first hospitalization and did not show improvement in quality of life or functional status. Notable adverse events from oxygen included 23 instances of tripping over equipment, with two patients requiring hospitalization and six fires with one patient hospitalized for burns.
A Cochrane meta-analysis, which did not include LOTT data, revealed that oxygen relieved breathlessness during acute exercise in mildly-moderately hypoxemic patients with COPD, but there was insufficient evidence of benefit in daily life or in health-related quality of life (Cochrane Database Syst Rev. 2016;11:CD006429).
Whether or not to continue prescribing oxygen to patients with moderate desaturation remains a controversial issue. A trial of oxygen may be warranted in those who are already on maximal evidence-based therapy for COPD and still complain of severe breathlessness (Ekstrom M; N Engl J Med. 2016;375[17]:1683). Conversely, a patient with COPD and moderate desaturation who resists being placed on supplemental oxygen, can be reassured that this is a reasonable course based on current evidence (Baliksoon R. COPD. 2017;4:71).
Navitha Ramesh, MD, MBBS
Fellow-in-Training Steering Committee Member
Allen Blaivas, DO, FCCP
Steering Committee Member
Informed consent: Do we need to change our practice?
Informed consent is the keystone of clinical research and helps respect and protect the rights of the participants/subjects. While the informed consent process has been standardized, some challenges still remain, such as pieces of information that should be disclosed, how to disclose information and document understanding of participants, and how detailed that disclosure should be (Grady C. N Engl J Med. 2015;372[9]:855). Digital technology can and has been used to improve the process of obtaining informed consent. Smartphones now comprise 75% of all mobile phones sold worldwide. They are being used to reach a larger and diverse population to conduct trials.
Substituting long and complex written forms with electronic consent (e-consent), however, has issues. Few people read through online agreements before clicking “agree,” which may lead to participants consenting without a clear understanding of what they are consenting to. On the other hand, it is also possible to use e-consent to improve comprehension by including videos and graphics. Interactive quizzes can assess the understanding of the participants, and embedded links to audios or videos can further enhance the grasp of information. With e-consents, queries from participants can be answered via phone call or email, etc. When e-consent is obtained remotely, the identity can be confirmed by electronic signatures, username, password, or biometrics.
E-consent has advantages, can be done remotely, no paper is needed, etc. It has potential disadvantages like being costly, videos can add time to the process, and multicenter international trials can be difficult (Grady C, et al. N Engl J Med. 2017;376[20]:e43). Studying e-consents to identify gaps in communication between the researcher and the participant in the digitalized world may help improve the process and allow research to proceed with better understanding of the risks and benefits of involvement in clinical research.
Moshsin Ijaz, MD, FCCP
Steering Committee Member
Early ID and treatment in sepsis
PRISM, the latest meta-analysis of three multicenter trials (ProCESS, ARISE, and ProMISe) found no difference in mortality with early goal-directed therapy vs usual care (N Engl J Med. 2017; 376[23]:2223). These clinical trials promoted early recognition of sepsis and prompt delivery of IV fluids and antimicrobial agents before randomization. It seems that early identification and treatment of sepsis and the rapid administration of antibiotics (following the timing recommended for sepsis bundle protocols) are the most effective interventions in sepsis (Seymour WS, et al. N Engl J Med. 2017;376[23]:2235). Other interventions over the past decade designed to reduce mortality associated with sepsis have been unsuccessful.
Maximiliano Tamae Kakazu, MD, FCCP
Steering Committee Member
The changing landscape of home mechanical ventilation
The greatest advances in home mechanical ventilation for conditions associated with chronic respiratory failure have been associated with the implementation of noninvasivepositive pressure ventilation (NIPPV) via mask interface. This dynamic growth is accredited to NIPPV efficacy and technologic improvements in ventilator and mask. For neuromuscular and restrictive thoracic diseases, NIPPV has been shown to increase survival, decrease hospital admissions, and improve quality of life (Chatwin A, et al. Plos One. 2015;10[5]:e0125839; Annane D, et al. Cochrane Database Syst Rev. 2014;13[12]:CD001941). From this success, NIPPV has been extended to conditions associated with respiratory impairment (eg, COPD, obesity hypoventilation, sleep-disordered breathing). A recent randomized study comparing home oxygen therapy (HOT) plus NIPPV vs HOT alone in post-hospitalized patients with COPD with persistent hypercapnia showed that addition of NIPPV significantly prolonged time to readmission or death from 1.4 to 4.3 months (Murphy P, et al. JAMA. 2017;317[21]:2177). Overall, however, evidence to support NIPPV in these groups is less compelling.
Current CMS guidelines defer to the provider’s clinical judgment regarding the severity of patient’s respiratory condition and if a ventilator or RAD would be most appropriate. It is important to recognize the proper patient (and setting) who would benefit from advanced respiratory support. The choice of ventilator should be reserved for severe or progressive respiratory impairment, specifically for patients who would benefit from daytime use, and for whom interruption of respiratory support would lead to serious consequences.
Michelle Cao, DO, FCCP
Steering Committee Member
Improving diagnostic capabilities in diffuse parenchymal lung disease
With the approval of two antifibrotic drugs for the treatment of idiopathic pulmonary fibrosis, there has been renewed focus in the NetWork in improving diagnosis in interstitial lung disease. There is considerable interest in exploring novel techniques and paradigms in the classification and diagnosis of diffuse parenchymal lung diseases (DPLDs). Given the invasive nature of surgical lung biopsy and its associated morbidity in elderly patients, there is a need for safer techniques to obtain lung tissue for histopathologic analysis. Transbronchial cryobiopsy may be a safe and accurate alternative for obtaining lung tissue, and we hope to better understand the role of this procedure in disease diagnosis. It is also possible that in the future, we may be able to classify these diseases without having to obtain lung tissue. More studies are being done in novel imaging techniques, such as molecular imaging, optical coherence tomography, and confocal laser endomicroscopy, that may negate the need for lung tissue in the future. Biomarker discovery and identification of biomarker signatures that can help differentiate DPLDs and provide prognostic information are also a particular focus and of importance for our NetWork. With this increased focus on better diagnostic techniques for classification of DPLD, the NetWork is featuring a lecture at CHEST 2017 on “Molecular Endotyping of Pulmonary Fibrosis,” and two sessions that will explore the current diagnostic difficulties that confront clinicians. As we move forward in our understanding of how to classify and diagnose interstitial lung disease, there is potential for more targeted interventions in individual patients.
Tracy Luckhardt, MD
Steering Committee Member
Oxygen therapy in patients with COPD with moderate desaturation
Two landmark trials from the early 1980s demonstrated survival benefit with long-term oxygen therapy (LTOT) in patients with COPD with severe resting hypoxemia [Sao2 less than 89%; (Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93[3]:391) or Pao2 40-60 mm Hg and cor pulmonale (Report of the Medical Research Council Working Party. Lancet. 1981;1[8222]:681).
The potential benefits of LTOT in COPD with mild-moderate hypoxemia have not been clearly established. The LOTT trial (The Long-Term Oxygen Treatment Trial Research Group. N Engl J Med. 2016;375[17]:1617), a recent multicenter randomized study, attempted to answer this question. They studied 738 stable patients with COPD with mild to moderate resting desaturation (Spo2 89%-93%) or exercise-induced moderate desaturation (Spo2 greater than or equal to 80% for greater than or equal to 5 minutes and Spo2 less than 90% for greater than or equal to 10 seconds during 6- minute walk test). After a median follow-up of 18.4 months, LTOT did not demonstrate a decrease in the time to death or first hospitalization and did not show improvement in quality of life or functional status. Notable adverse events from oxygen included 23 instances of tripping over equipment, with two patients requiring hospitalization and six fires with one patient hospitalized for burns.
A Cochrane meta-analysis, which did not include LOTT data, revealed that oxygen relieved breathlessness during acute exercise in mildly-moderately hypoxemic patients with COPD, but there was insufficient evidence of benefit in daily life or in health-related quality of life (Cochrane Database Syst Rev. 2016;11:CD006429).
Whether or not to continue prescribing oxygen to patients with moderate desaturation remains a controversial issue. A trial of oxygen may be warranted in those who are already on maximal evidence-based therapy for COPD and still complain of severe breathlessness (Ekstrom M; N Engl J Med. 2016;375[17]:1683). Conversely, a patient with COPD and moderate desaturation who resists being placed on supplemental oxygen, can be reassured that this is a reasonable course based on current evidence (Baliksoon R. COPD. 2017;4:71).
Navitha Ramesh, MD, MBBS
Fellow-in-Training Steering Committee Member
Allen Blaivas, DO, FCCP
Steering Committee Member
Informed consent: Do we need to change our practice?
Informed consent is the keystone of clinical research and helps respect and protect the rights of the participants/subjects. While the informed consent process has been standardized, some challenges still remain, such as pieces of information that should be disclosed, how to disclose information and document understanding of participants, and how detailed that disclosure should be (Grady C. N Engl J Med. 2015;372[9]:855). Digital technology can and has been used to improve the process of obtaining informed consent. Smartphones now comprise 75% of all mobile phones sold worldwide. They are being used to reach a larger and diverse population to conduct trials.
Substituting long and complex written forms with electronic consent (e-consent), however, has issues. Few people read through online agreements before clicking “agree,” which may lead to participants consenting without a clear understanding of what they are consenting to. On the other hand, it is also possible to use e-consent to improve comprehension by including videos and graphics. Interactive quizzes can assess the understanding of the participants, and embedded links to audios or videos can further enhance the grasp of information. With e-consents, queries from participants can be answered via phone call or email, etc. When e-consent is obtained remotely, the identity can be confirmed by electronic signatures, username, password, or biometrics.
E-consent has advantages, can be done remotely, no paper is needed, etc. It has potential disadvantages like being costly, videos can add time to the process, and multicenter international trials can be difficult (Grady C, et al. N Engl J Med. 2017;376[20]:e43). Studying e-consents to identify gaps in communication between the researcher and the participant in the digitalized world may help improve the process and allow research to proceed with better understanding of the risks and benefits of involvement in clinical research.
Moshsin Ijaz, MD, FCCP
Steering Committee Member
Early ID and treatment in sepsis
PRISM, the latest meta-analysis of three multicenter trials (ProCESS, ARISE, and ProMISe) found no difference in mortality with early goal-directed therapy vs usual care (N Engl J Med. 2017; 376[23]:2223). These clinical trials promoted early recognition of sepsis and prompt delivery of IV fluids and antimicrobial agents before randomization. It seems that early identification and treatment of sepsis and the rapid administration of antibiotics (following the timing recommended for sepsis bundle protocols) are the most effective interventions in sepsis (Seymour WS, et al. N Engl J Med. 2017;376[23]:2235). Other interventions over the past decade designed to reduce mortality associated with sepsis have been unsuccessful.
Maximiliano Tamae Kakazu, MD, FCCP
Steering Committee Member
The changing landscape of home mechanical ventilation
The greatest advances in home mechanical ventilation for conditions associated with chronic respiratory failure have been associated with the implementation of noninvasivepositive pressure ventilation (NIPPV) via mask interface. This dynamic growth is accredited to NIPPV efficacy and technologic improvements in ventilator and mask. For neuromuscular and restrictive thoracic diseases, NIPPV has been shown to increase survival, decrease hospital admissions, and improve quality of life (Chatwin A, et al. Plos One. 2015;10[5]:e0125839; Annane D, et al. Cochrane Database Syst Rev. 2014;13[12]:CD001941). From this success, NIPPV has been extended to conditions associated with respiratory impairment (eg, COPD, obesity hypoventilation, sleep-disordered breathing). A recent randomized study comparing home oxygen therapy (HOT) plus NIPPV vs HOT alone in post-hospitalized patients with COPD with persistent hypercapnia showed that addition of NIPPV significantly prolonged time to readmission or death from 1.4 to 4.3 months (Murphy P, et al. JAMA. 2017;317[21]:2177). Overall, however, evidence to support NIPPV in these groups is less compelling.
Current CMS guidelines defer to the provider’s clinical judgment regarding the severity of patient’s respiratory condition and if a ventilator or RAD would be most appropriate. It is important to recognize the proper patient (and setting) who would benefit from advanced respiratory support. The choice of ventilator should be reserved for severe or progressive respiratory impairment, specifically for patients who would benefit from daytime use, and for whom interruption of respiratory support would lead to serious consequences.
Michelle Cao, DO, FCCP
Steering Committee Member
Improving diagnostic capabilities in diffuse parenchymal lung disease
With the approval of two antifibrotic drugs for the treatment of idiopathic pulmonary fibrosis, there has been renewed focus in the NetWork in improving diagnosis in interstitial lung disease. There is considerable interest in exploring novel techniques and paradigms in the classification and diagnosis of diffuse parenchymal lung diseases (DPLDs). Given the invasive nature of surgical lung biopsy and its associated morbidity in elderly patients, there is a need for safer techniques to obtain lung tissue for histopathologic analysis. Transbronchial cryobiopsy may be a safe and accurate alternative for obtaining lung tissue, and we hope to better understand the role of this procedure in disease diagnosis. It is also possible that in the future, we may be able to classify these diseases without having to obtain lung tissue. More studies are being done in novel imaging techniques, such as molecular imaging, optical coherence tomography, and confocal laser endomicroscopy, that may negate the need for lung tissue in the future. Biomarker discovery and identification of biomarker signatures that can help differentiate DPLDs and provide prognostic information are also a particular focus and of importance for our NetWork. With this increased focus on better diagnostic techniques for classification of DPLD, the NetWork is featuring a lecture at CHEST 2017 on “Molecular Endotyping of Pulmonary Fibrosis,” and two sessions that will explore the current diagnostic difficulties that confront clinicians. As we move forward in our understanding of how to classify and diagnose interstitial lung disease, there is potential for more targeted interventions in individual patients.
Tracy Luckhardt, MD
Steering Committee Member
Oxygen therapy in patients with COPD with moderate desaturation
Two landmark trials from the early 1980s demonstrated survival benefit with long-term oxygen therapy (LTOT) in patients with COPD with severe resting hypoxemia [Sao2 less than 89%; (Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93[3]:391) or Pao2 40-60 mm Hg and cor pulmonale (Report of the Medical Research Council Working Party. Lancet. 1981;1[8222]:681).
The potential benefits of LTOT in COPD with mild-moderate hypoxemia have not been clearly established. The LOTT trial (The Long-Term Oxygen Treatment Trial Research Group. N Engl J Med. 2016;375[17]:1617), a recent multicenter randomized study, attempted to answer this question. They studied 738 stable patients with COPD with mild to moderate resting desaturation (Spo2 89%-93%) or exercise-induced moderate desaturation (Spo2 greater than or equal to 80% for greater than or equal to 5 minutes and Spo2 less than 90% for greater than or equal to 10 seconds during 6- minute walk test). After a median follow-up of 18.4 months, LTOT did not demonstrate a decrease in the time to death or first hospitalization and did not show improvement in quality of life or functional status. Notable adverse events from oxygen included 23 instances of tripping over equipment, with two patients requiring hospitalization and six fires with one patient hospitalized for burns.
A Cochrane meta-analysis, which did not include LOTT data, revealed that oxygen relieved breathlessness during acute exercise in mildly-moderately hypoxemic patients with COPD, but there was insufficient evidence of benefit in daily life or in health-related quality of life (Cochrane Database Syst Rev. 2016;11:CD006429).
Whether or not to continue prescribing oxygen to patients with moderate desaturation remains a controversial issue. A trial of oxygen may be warranted in those who are already on maximal evidence-based therapy for COPD and still complain of severe breathlessness (Ekstrom M; N Engl J Med. 2016;375[17]:1683). Conversely, a patient with COPD and moderate desaturation who resists being placed on supplemental oxygen, can be reassured that this is a reasonable course based on current evidence (Baliksoon R. COPD. 2017;4:71).
Navitha Ramesh, MD, MBBS
Fellow-in-Training Steering Committee Member
Allen Blaivas, DO, FCCP
Steering Committee Member
Informed consent: Do we need to change our practice?
Informed consent is the keystone of clinical research and helps respect and protect the rights of the participants/subjects. While the informed consent process has been standardized, some challenges still remain, such as pieces of information that should be disclosed, how to disclose information and document understanding of participants, and how detailed that disclosure should be (Grady C. N Engl J Med. 2015;372[9]:855). Digital technology can and has been used to improve the process of obtaining informed consent. Smartphones now comprise 75% of all mobile phones sold worldwide. They are being used to reach a larger and diverse population to conduct trials.
Substituting long and complex written forms with electronic consent (e-consent), however, has issues. Few people read through online agreements before clicking “agree,” which may lead to participants consenting without a clear understanding of what they are consenting to. On the other hand, it is also possible to use e-consent to improve comprehension by including videos and graphics. Interactive quizzes can assess the understanding of the participants, and embedded links to audios or videos can further enhance the grasp of information. With e-consents, queries from participants can be answered via phone call or email, etc. When e-consent is obtained remotely, the identity can be confirmed by electronic signatures, username, password, or biometrics.
E-consent has advantages, can be done remotely, no paper is needed, etc. It has potential disadvantages like being costly, videos can add time to the process, and multicenter international trials can be difficult (Grady C, et al. N Engl J Med. 2017;376[20]:e43). Studying e-consents to identify gaps in communication between the researcher and the participant in the digitalized world may help improve the process and allow research to proceed with better understanding of the risks and benefits of involvement in clinical research.
Moshsin Ijaz, MD, FCCP
Steering Committee Member
Early ID and treatment in sepsis
PRISM, the latest meta-analysis of three multicenter trials (ProCESS, ARISE, and ProMISe) found no difference in mortality with early goal-directed therapy vs usual care (N Engl J Med. 2017; 376[23]:2223). These clinical trials promoted early recognition of sepsis and prompt delivery of IV fluids and antimicrobial agents before randomization. It seems that early identification and treatment of sepsis and the rapid administration of antibiotics (following the timing recommended for sepsis bundle protocols) are the most effective interventions in sepsis (Seymour WS, et al. N Engl J Med. 2017;376[23]:2235). Other interventions over the past decade designed to reduce mortality associated with sepsis have been unsuccessful.
Maximiliano Tamae Kakazu, MD, FCCP
Steering Committee Member
The changing landscape of home mechanical ventilation
The greatest advances in home mechanical ventilation for conditions associated with chronic respiratory failure have been associated with the implementation of noninvasivepositive pressure ventilation (NIPPV) via mask interface. This dynamic growth is accredited to NIPPV efficacy and technologic improvements in ventilator and mask. For neuromuscular and restrictive thoracic diseases, NIPPV has been shown to increase survival, decrease hospital admissions, and improve quality of life (Chatwin A, et al. Plos One. 2015;10[5]:e0125839; Annane D, et al. Cochrane Database Syst Rev. 2014;13[12]:CD001941). From this success, NIPPV has been extended to conditions associated with respiratory impairment (eg, COPD, obesity hypoventilation, sleep-disordered breathing). A recent randomized study comparing home oxygen therapy (HOT) plus NIPPV vs HOT alone in post-hospitalized patients with COPD with persistent hypercapnia showed that addition of NIPPV significantly prolonged time to readmission or death from 1.4 to 4.3 months (Murphy P, et al. JAMA. 2017;317[21]:2177). Overall, however, evidence to support NIPPV in these groups is less compelling.
Current CMS guidelines defer to the provider’s clinical judgment regarding the severity of patient’s respiratory condition and if a ventilator or RAD would be most appropriate. It is important to recognize the proper patient (and setting) who would benefit from advanced respiratory support. The choice of ventilator should be reserved for severe or progressive respiratory impairment, specifically for patients who would benefit from daytime use, and for whom interruption of respiratory support would lead to serious consequences.
Michelle Cao, DO, FCCP
Steering Committee Member
Improving diagnostic capabilities in diffuse parenchymal lung disease
With the approval of two antifibrotic drugs for the treatment of idiopathic pulmonary fibrosis, there has been renewed focus in the NetWork in improving diagnosis in interstitial lung disease. There is considerable interest in exploring novel techniques and paradigms in the classification and diagnosis of diffuse parenchymal lung diseases (DPLDs). Given the invasive nature of surgical lung biopsy and its associated morbidity in elderly patients, there is a need for safer techniques to obtain lung tissue for histopathologic analysis. Transbronchial cryobiopsy may be a safe and accurate alternative for obtaining lung tissue, and we hope to better understand the role of this procedure in disease diagnosis. It is also possible that in the future, we may be able to classify these diseases without having to obtain lung tissue. More studies are being done in novel imaging techniques, such as molecular imaging, optical coherence tomography, and confocal laser endomicroscopy, that may negate the need for lung tissue in the future. Biomarker discovery and identification of biomarker signatures that can help differentiate DPLDs and provide prognostic information are also a particular focus and of importance for our NetWork. With this increased focus on better diagnostic techniques for classification of DPLD, the NetWork is featuring a lecture at CHEST 2017 on “Molecular Endotyping of Pulmonary Fibrosis,” and two sessions that will explore the current diagnostic difficulties that confront clinicians. As we move forward in our understanding of how to classify and diagnose interstitial lung disease, there is potential for more targeted interventions in individual patients.
Tracy Luckhardt, MD
Steering Committee Member
CHEST NetWorks Submassive PE, antibiotic resistance, advanced practice providers
Cardiovascular Medicine and Surgery
Catch 22 of Submassive Pulmonary Emboli
Venous thromboembolism (including deep vein thrombosis (DVT) and pulmonary embolism [PE]) occurs in approximately 1 per 1,000 patients (Piran S, Schulman S. Thromb J. 2016;14[S1]:23) and can be fatal. Pulmonary embolus severity is classified as low risk, intermediate-risk/submassive PE, and massive PE. There is significant controversy about the management of submassive PE, which is defined as PE with right-sided heart strain (elevated troponin or B-type natriuretic peptide, right-axis deviation on ECG, or e
David J. Nagel, MD
Steering Committee Member
Olivier Axler, MD, FCCP
Vice-Chair
Chest Infections
Antibiotic Resistance
One-hundred years ago, infectious diseases caused 5 of the 10 most common causes of deaths in the United States. In 2016, only one infection remained on this list (influenza/pneumonia) (MMWR Morb Mortal Wkly Rep. 2017;66:413).
How medicine has improved with antibiotics. An unfortunate and unintended consequence of widespread antibiotic use has been the progressive resistance to these drugs. It is estimated that, if current trends continue, 10 million lives a year will be at risk from resistant organisms by 2050 (O’Neill, J. (2016). https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf).
Pathogens acquire antibiotic resistance by passing genetic material to one another through plasmids, bacteriophages, or naked DNA. Once acquired, resistance manifests via a number of mechanisms under the stress imposed by antibiotics (Levy SB, et al. Nat Med. 2004;10:S122).
Among the best studied is enzymatic degradation of the antibiotic. This occurs when beta-lactamases degrade penicillin. A second mechanism alters cell transport, thereby blocking cell entry or actively ejecting the antibiotic from the cell. Finally, overexpression or alteration of the antibiotic target may render a drug ineffective at inhibiting any vital cell function.
At the pace with which resistance now develops, the medical community faces a crisis, whereby infections caused by evolving superbugs are no longer effectively controlled by the available menu of antimicrobial agents.
This challenge must be met collectively by the more prudent prescribing of antibiotics, potentially with the help of rapid diagnostics; isolation of patients potentially infected with resistant organisms; and a focus on developing newer drugs that defy known resistant mechanisms.
Marc Feinstein, MD, FCCP
Steering Committee Member
Clinical Pulmonary Medicine
COPD and sleep-disordered breathing; A missing comorbid condition
Subjective, as well as objective, sleep complaints are common in patients with COPD (Krachman S, et al. Proc Am Thorac Soc. 2008;5[4]:536), and sleeping difficulties are ranked the third most frequent complaint (behind dyspnea and fatigue) in patients with COPD (Kinsman RA, et al. Chest. 1983;83[5]:755). Also, sleep quality is poor, and patients with moderate to severe COPD may have higher-than-expected incidence of OSA (Soler X, et al. Ann Am Thorac Soc. 2015;12[8]:1219).
Unfortunately, sleep is usually not assessed during a COPD evaluation. Up to 27% of patients with COPD without hypoxia during wakefulness can experience important desaturation during sleep, so called nocturnal oxygen desaturation (NOD) (Fletcher EC, et al. Chest. 1987;92[4]:604), that may lead to pulmonary hypertension (Chaouat A, et a
Although identification and effective treatment of COPD comorbidities are becoming the cornerstone of COPD management, sleep-disordered breathing has not been identified in current guidelines yet as a true potential contributor in poor outcomes despite emergent clinical evidence. Multidisciplinary programs, such as pulmonary rehabilitation, that improve dyspnea, exercise capacity, and quality of life may also positively impact sleep (Soler X, et al. COPD. 2013;10[2]:156). Because of the background of the staff involved, the comprehensive approach to patient assessment, and access to number of COPD subjects, pulmonary rehabilitation may be an optimal opportunity to assess sleep and identify an important comorbid condition often overlooked in patients with more advanced COPD.
Xavier Soler, MD, PhD
Steering Committee Member
Interprofessional Team
Finding Home
Outside our internal medicine curriculum, there is no formal pulmonary training or post-masters fellowship in pulmonary medicine for Advanced Practice Providers (APPs). Because of this, APPs are left to their own devices to fill educational gaps. To perform at the level expected by the physicians I work for, journal reviews and memorizing guidelines were not going to be enough. Since there is no formal pulmonary APP society, there were no peers to reach out to either. Off to conferences I went.
At first, I found CHEST daunting. After all, it’s run by the American College of Chest “Physicians,” not Nurse Practitioners. I spent most of the first day with my nametag turned around worried I’d be found out as a nonphysician attendee who snuck in. And then the unthinkable happened, I ran into another unicorn—another APP seeking the same information, only her nametag was turned the right way. The best advice she gave was to attend the Interprofessional NetWork meeting. This was ground zero of the conference as far as I was concerned. There I found myself surrounded by RTs, RNs, NPs, PAs, and yes, even physicians.
Over the years, as I’ve gotten further involved with CHEST NetWorks, I have found from top to bottom CHEST striving to incorporate APPs and advance our education. From including us in the FCCP program, reducing conference pricing for APPs, and focusing this year’s conference theme around being team focused, CHEST is creating a home for APPs.
Corinne Preston Young, FNP, FCCP
Steering Committee Member
Cardiovascular Medicine and Surgery
Catch 22 of Submassive Pulmonary Emboli
Venous thromboembolism (including deep vein thrombosis (DVT) and pulmonary embolism [PE]) occurs in approximately 1 per 1,000 patients (Piran S, Schulman S. Thromb J. 2016;14[S1]:23) and can be fatal. Pulmonary embolus severity is classified as low risk, intermediate-risk/submassive PE, and massive PE. There is significant controversy about the management of submassive PE, which is defined as PE with right-sided heart strain (elevated troponin or B-type natriuretic peptide, right-axis deviation on ECG, or e
David J. Nagel, MD
Steering Committee Member
Olivier Axler, MD, FCCP
Vice-Chair
Chest Infections
Antibiotic Resistance
One-hundred years ago, infectious diseases caused 5 of the 10 most common causes of deaths in the United States. In 2016, only one infection remained on this list (influenza/pneumonia) (MMWR Morb Mortal Wkly Rep. 2017;66:413).
How medicine has improved with antibiotics. An unfortunate and unintended consequence of widespread antibiotic use has been the progressive resistance to these drugs. It is estimated that, if current trends continue, 10 million lives a year will be at risk from resistant organisms by 2050 (O’Neill, J. (2016). https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf).
Pathogens acquire antibiotic resistance by passing genetic material to one another through plasmids, bacteriophages, or naked DNA. Once acquired, resistance manifests via a number of mechanisms under the stress imposed by antibiotics (Levy SB, et al. Nat Med. 2004;10:S122).
Among the best studied is enzymatic degradation of the antibiotic. This occurs when beta-lactamases degrade penicillin. A second mechanism alters cell transport, thereby blocking cell entry or actively ejecting the antibiotic from the cell. Finally, overexpression or alteration of the antibiotic target may render a drug ineffective at inhibiting any vital cell function.
At the pace with which resistance now develops, the medical community faces a crisis, whereby infections caused by evolving superbugs are no longer effectively controlled by the available menu of antimicrobial agents.
This challenge must be met collectively by the more prudent prescribing of antibiotics, potentially with the help of rapid diagnostics; isolation of patients potentially infected with resistant organisms; and a focus on developing newer drugs that defy known resistant mechanisms.
Marc Feinstein, MD, FCCP
Steering Committee Member
Clinical Pulmonary Medicine
COPD and sleep-disordered breathing; A missing comorbid condition
Subjective, as well as objective, sleep complaints are common in patients with COPD (Krachman S, et al. Proc Am Thorac Soc. 2008;5[4]:536), and sleeping difficulties are ranked the third most frequent complaint (behind dyspnea and fatigue) in patients with COPD (Kinsman RA, et al. Chest. 1983;83[5]:755). Also, sleep quality is poor, and patients with moderate to severe COPD may have higher-than-expected incidence of OSA (Soler X, et al. Ann Am Thorac Soc. 2015;12[8]:1219).
Unfortunately, sleep is usually not assessed during a COPD evaluation. Up to 27% of patients with COPD without hypoxia during wakefulness can experience important desaturation during sleep, so called nocturnal oxygen desaturation (NOD) (Fletcher EC, et al. Chest. 1987;92[4]:604), that may lead to pulmonary hypertension (Chaouat A, et a
Although identification and effective treatment of COPD comorbidities are becoming the cornerstone of COPD management, sleep-disordered breathing has not been identified in current guidelines yet as a true potential contributor in poor outcomes despite emergent clinical evidence. Multidisciplinary programs, such as pulmonary rehabilitation, that improve dyspnea, exercise capacity, and quality of life may also positively impact sleep (Soler X, et al. COPD. 2013;10[2]:156). Because of the background of the staff involved, the comprehensive approach to patient assessment, and access to number of COPD subjects, pulmonary rehabilitation may be an optimal opportunity to assess sleep and identify an important comorbid condition often overlooked in patients with more advanced COPD.
Xavier Soler, MD, PhD
Steering Committee Member
Interprofessional Team
Finding Home
Outside our internal medicine curriculum, there is no formal pulmonary training or post-masters fellowship in pulmonary medicine for Advanced Practice Providers (APPs). Because of this, APPs are left to their own devices to fill educational gaps. To perform at the level expected by the physicians I work for, journal reviews and memorizing guidelines were not going to be enough. Since there is no formal pulmonary APP society, there were no peers to reach out to either. Off to conferences I went.
At first, I found CHEST daunting. After all, it’s run by the American College of Chest “Physicians,” not Nurse Practitioners. I spent most of the first day with my nametag turned around worried I’d be found out as a nonphysician attendee who snuck in. And then the unthinkable happened, I ran into another unicorn—another APP seeking the same information, only her nametag was turned the right way. The best advice she gave was to attend the Interprofessional NetWork meeting. This was ground zero of the conference as far as I was concerned. There I found myself surrounded by RTs, RNs, NPs, PAs, and yes, even physicians.
Over the years, as I’ve gotten further involved with CHEST NetWorks, I have found from top to bottom CHEST striving to incorporate APPs and advance our education. From including us in the FCCP program, reducing conference pricing for APPs, and focusing this year’s conference theme around being team focused, CHEST is creating a home for APPs.
Corinne Preston Young, FNP, FCCP
Steering Committee Member
Cardiovascular Medicine and Surgery
Catch 22 of Submassive Pulmonary Emboli
Venous thromboembolism (including deep vein thrombosis (DVT) and pulmonary embolism [PE]) occurs in approximately 1 per 1,000 patients (Piran S, Schulman S. Thromb J. 2016;14[S1]:23) and can be fatal. Pulmonary embolus severity is classified as low risk, intermediate-risk/submassive PE, and massive PE. There is significant controversy about the management of submassive PE, which is defined as PE with right-sided heart strain (elevated troponin or B-type natriuretic peptide, right-axis deviation on ECG, or e
David J. Nagel, MD
Steering Committee Member
Olivier Axler, MD, FCCP
Vice-Chair
Chest Infections
Antibiotic Resistance
One-hundred years ago, infectious diseases caused 5 of the 10 most common causes of deaths in the United States. In 2016, only one infection remained on this list (influenza/pneumonia) (MMWR Morb Mortal Wkly Rep. 2017;66:413).
How medicine has improved with antibiotics. An unfortunate and unintended consequence of widespread antibiotic use has been the progressive resistance to these drugs. It is estimated that, if current trends continue, 10 million lives a year will be at risk from resistant organisms by 2050 (O’Neill, J. (2016). https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf).
Pathogens acquire antibiotic resistance by passing genetic material to one another through plasmids, bacteriophages, or naked DNA. Once acquired, resistance manifests via a number of mechanisms under the stress imposed by antibiotics (Levy SB, et al. Nat Med. 2004;10:S122).
Among the best studied is enzymatic degradation of the antibiotic. This occurs when beta-lactamases degrade penicillin. A second mechanism alters cell transport, thereby blocking cell entry or actively ejecting the antibiotic from the cell. Finally, overexpression or alteration of the antibiotic target may render a drug ineffective at inhibiting any vital cell function.
At the pace with which resistance now develops, the medical community faces a crisis, whereby infections caused by evolving superbugs are no longer effectively controlled by the available menu of antimicrobial agents.
This challenge must be met collectively by the more prudent prescribing of antibiotics, potentially with the help of rapid diagnostics; isolation of patients potentially infected with resistant organisms; and a focus on developing newer drugs that defy known resistant mechanisms.
Marc Feinstein, MD, FCCP
Steering Committee Member
Clinical Pulmonary Medicine
COPD and sleep-disordered breathing; A missing comorbid condition
Subjective, as well as objective, sleep complaints are common in patients with COPD (Krachman S, et al. Proc Am Thorac Soc. 2008;5[4]:536), and sleeping difficulties are ranked the third most frequent complaint (behind dyspnea and fatigue) in patients with COPD (Kinsman RA, et al. Chest. 1983;83[5]:755). Also, sleep quality is poor, and patients with moderate to severe COPD may have higher-than-expected incidence of OSA (Soler X, et al. Ann Am Thorac Soc. 2015;12[8]:1219).
Unfortunately, sleep is usually not assessed during a COPD evaluation. Up to 27% of patients with COPD without hypoxia during wakefulness can experience important desaturation during sleep, so called nocturnal oxygen desaturation (NOD) (Fletcher EC, et al. Chest. 1987;92[4]:604), that may lead to pulmonary hypertension (Chaouat A, et a
Although identification and effective treatment of COPD comorbidities are becoming the cornerstone of COPD management, sleep-disordered breathing has not been identified in current guidelines yet as a true potential contributor in poor outcomes despite emergent clinical evidence. Multidisciplinary programs, such as pulmonary rehabilitation, that improve dyspnea, exercise capacity, and quality of life may also positively impact sleep (Soler X, et al. COPD. 2013;10[2]:156). Because of the background of the staff involved, the comprehensive approach to patient assessment, and access to number of COPD subjects, pulmonary rehabilitation may be an optimal opportunity to assess sleep and identify an important comorbid condition often overlooked in patients with more advanced COPD.
Xavier Soler, MD, PhD
Steering Committee Member
Interprofessional Team
Finding Home
Outside our internal medicine curriculum, there is no formal pulmonary training or post-masters fellowship in pulmonary medicine for Advanced Practice Providers (APPs). Because of this, APPs are left to their own devices to fill educational gaps. To perform at the level expected by the physicians I work for, journal reviews and memorizing guidelines were not going to be enough. Since there is no formal pulmonary APP society, there were no peers to reach out to either. Off to conferences I went.
At first, I found CHEST daunting. After all, it’s run by the American College of Chest “Physicians,” not Nurse Practitioners. I spent most of the first day with my nametag turned around worried I’d be found out as a nonphysician attendee who snuck in. And then the unthinkable happened, I ran into another unicorn—another APP seeking the same information, only her nametag was turned the right way. The best advice she gave was to attend the Interprofessional NetWork meeting. This was ground zero of the conference as far as I was concerned. There I found myself surrounded by RTs, RNs, NPs, PAs, and yes, even physicians.
Over the years, as I’ve gotten further involved with CHEST NetWorks, I have found from top to bottom CHEST striving to incorporate APPs and advance our education. From including us in the FCCP program, reducing conference pricing for APPs, and focusing this year’s conference theme around being team focused, CHEST is creating a home for APPs.
Corinne Preston Young, FNP, FCCP
Steering Committee Member























