User login
Thoracic Oncology & Chest Procedures Network
Interventional Procedures Section
Mind the gap: Improving adherence to lung cancer screening follow-up
The gap in adherence rates between a disciplined clinical trial and the heterogenous patchwork of U.S. health care is hardly unusual, but as lung cancer remains the number one cancer killer both worldwide and in the United States, one such disparity bears closer scrutiny.
In 2011, the National Lung Screening Trial (NLST) demonstrated a 20% reduction in lung cancer mortality with the implementation of low dose CT scan screening with 95% adherence to CT scan follow-up within 15 months of initial screening imaging (Aberle, et al. N Engl J Med. 2011;365[5]:395-409). Unfortunately, estimates of real-world adherence to lung cancer screening (LCS) follow-up fall to 51% even within an extended 18-month window (Hirsch, et al. Ann Am Thorac Soc. 2019;16[10]:1329-32).
Recent studies compared adherence to LCS follow-up between centralized and decentralized screening programs. Centralized programs used dedicated program coordinators and a tracking system, while decentralized programs relied on primary care providers.(Sakoda, et al. JAMA Network Open. 2021;4[4]:e218559). A subsequent study demonstrated adherence of 70% vs 41% among patients in centralized vs decentralized programs, respectively (Smith, et al. Chest. 2022;161[3]:818-25).
This gap is even more pronounced in majority-Black populations. Kunitomo and colleagues showed 33% lower odds of adherence to LCS follow-up compared with White patients (Kunitomo, et al. Chest. 2022;161[1]:266-75). Another study in a diverse, majority-Black patient population showed only 31% adherence to LCS follow-up at 1 year (Erkmen, et al. Cancer Causes Control. 2021;32[3]:291-8).
How could we close this gap? Centralized LCS programs show promise of increasing adherence to LCS follow-up. Heightened awareness of and targeted investment to mitigate racial inequities in LCS is imperative.
Jose De Cardenas MD
John Howe, MD
Members-at-Large
Interventional Procedures Section
Mind the gap: Improving adherence to lung cancer screening follow-up
The gap in adherence rates between a disciplined clinical trial and the heterogenous patchwork of U.S. health care is hardly unusual, but as lung cancer remains the number one cancer killer both worldwide and in the United States, one such disparity bears closer scrutiny.
In 2011, the National Lung Screening Trial (NLST) demonstrated a 20% reduction in lung cancer mortality with the implementation of low dose CT scan screening with 95% adherence to CT scan follow-up within 15 months of initial screening imaging (Aberle, et al. N Engl J Med. 2011;365[5]:395-409). Unfortunately, estimates of real-world adherence to lung cancer screening (LCS) follow-up fall to 51% even within an extended 18-month window (Hirsch, et al. Ann Am Thorac Soc. 2019;16[10]:1329-32).
Recent studies compared adherence to LCS follow-up between centralized and decentralized screening programs. Centralized programs used dedicated program coordinators and a tracking system, while decentralized programs relied on primary care providers.(Sakoda, et al. JAMA Network Open. 2021;4[4]:e218559). A subsequent study demonstrated adherence of 70% vs 41% among patients in centralized vs decentralized programs, respectively (Smith, et al. Chest. 2022;161[3]:818-25).
This gap is even more pronounced in majority-Black populations. Kunitomo and colleagues showed 33% lower odds of adherence to LCS follow-up compared with White patients (Kunitomo, et al. Chest. 2022;161[1]:266-75). Another study in a diverse, majority-Black patient population showed only 31% adherence to LCS follow-up at 1 year (Erkmen, et al. Cancer Causes Control. 2021;32[3]:291-8).
How could we close this gap? Centralized LCS programs show promise of increasing adherence to LCS follow-up. Heightened awareness of and targeted investment to mitigate racial inequities in LCS is imperative.
Jose De Cardenas MD
John Howe, MD
Members-at-Large
Interventional Procedures Section
Mind the gap: Improving adherence to lung cancer screening follow-up
The gap in adherence rates between a disciplined clinical trial and the heterogenous patchwork of U.S. health care is hardly unusual, but as lung cancer remains the number one cancer killer both worldwide and in the United States, one such disparity bears closer scrutiny.
In 2011, the National Lung Screening Trial (NLST) demonstrated a 20% reduction in lung cancer mortality with the implementation of low dose CT scan screening with 95% adherence to CT scan follow-up within 15 months of initial screening imaging (Aberle, et al. N Engl J Med. 2011;365[5]:395-409). Unfortunately, estimates of real-world adherence to lung cancer screening (LCS) follow-up fall to 51% even within an extended 18-month window (Hirsch, et al. Ann Am Thorac Soc. 2019;16[10]:1329-32).
Recent studies compared adherence to LCS follow-up between centralized and decentralized screening programs. Centralized programs used dedicated program coordinators and a tracking system, while decentralized programs relied on primary care providers.(Sakoda, et al. JAMA Network Open. 2021;4[4]:e218559). A subsequent study demonstrated adherence of 70% vs 41% among patients in centralized vs decentralized programs, respectively (Smith, et al. Chest. 2022;161[3]:818-25).
This gap is even more pronounced in majority-Black populations. Kunitomo and colleagues showed 33% lower odds of adherence to LCS follow-up compared with White patients (Kunitomo, et al. Chest. 2022;161[1]:266-75). Another study in a diverse, majority-Black patient population showed only 31% adherence to LCS follow-up at 1 year (Erkmen, et al. Cancer Causes Control. 2021;32[3]:291-8).
How could we close this gap? Centralized LCS programs show promise of increasing adherence to LCS follow-up. Heightened awareness of and targeted investment to mitigate racial inequities in LCS is imperative.
Jose De Cardenas MD
John Howe, MD
Members-at-Large
Sleep Medicine Network
Home-based Mechanical Ventilation and Neuromuscular Disease Section
Navigating the latest device supply chain challenge: Mechanical airway clearance
(distal airways). Cough augmentation techniques provide lung volume recruitment on the insufflation phase, in addition to mobilization of secretions with augmentation of the peak expiratory flow rate to >160 L/min on the exhalation phase.
A mechanical insufflation-exsufflation (MI-E) device (T70 Cough Assist - Phillips) is now on indefinite backorder. This creates a dangerous situation for our patients requiring cough augmentation for survival. Alternative options that provide both MI-E and high frequency oscillation include two systems (Synclara Cough System – Hill-rom and the Biwaze Cough System-ABM Respiratory Care).
The Synclara can only be obtained in a direct-to-patient model, contracting with individual respiratory therapists, outside of the standard durable medical equipment model. The final MI-E model option is the VOCSYN multifunctional ventilator (ventilator, cough assist, nebulizer, oxygen concentrator, suction). This multifunction ventilator has had variable acceptance with HCPCS code E0467. If the VOCSYN is chosen, the patient cannot have been issued any component devices or have reached the 36-month cap for oxygen equipment (CR 10854 special payment rule, 42 CFR414.222).
As the supply of devices is exhausted, we will need to shift to evidence-based manual options. Manual cough augmentation can be done effectively with a bag-valve mask, using breath stacking to achieve maximal lung insufflation, optimizing the length tension relationship of elastic recoil on exhalation to increase peak cough flow (PCF).
This can be done alone but is more effective when combined with manually assisted cough (Bach JR. Chest. 1993;104[5]:1553-62). These interventions require training of the caregivers, using resources such as those found at www.canventottawa.ca.
With continued supply chain instability, manual airway clearance techniques should be considered in patients with less advanced cough impairment (PCF 160-270 L/min), to save the remaining devices for those with PCF of <160 L/min.
Jeanette Brown, MD, PhD
Karin Provost, DO, PhD
Members-at-Large
Home-based Mechanical Ventilation and Neuromuscular Disease Section
Navigating the latest device supply chain challenge: Mechanical airway clearance
(distal airways). Cough augmentation techniques provide lung volume recruitment on the insufflation phase, in addition to mobilization of secretions with augmentation of the peak expiratory flow rate to >160 L/min on the exhalation phase.
A mechanical insufflation-exsufflation (MI-E) device (T70 Cough Assist - Phillips) is now on indefinite backorder. This creates a dangerous situation for our patients requiring cough augmentation for survival. Alternative options that provide both MI-E and high frequency oscillation include two systems (Synclara Cough System – Hill-rom and the Biwaze Cough System-ABM Respiratory Care).
The Synclara can only be obtained in a direct-to-patient model, contracting with individual respiratory therapists, outside of the standard durable medical equipment model. The final MI-E model option is the VOCSYN multifunctional ventilator (ventilator, cough assist, nebulizer, oxygen concentrator, suction). This multifunction ventilator has had variable acceptance with HCPCS code E0467. If the VOCSYN is chosen, the patient cannot have been issued any component devices or have reached the 36-month cap for oxygen equipment (CR 10854 special payment rule, 42 CFR414.222).
As the supply of devices is exhausted, we will need to shift to evidence-based manual options. Manual cough augmentation can be done effectively with a bag-valve mask, using breath stacking to achieve maximal lung insufflation, optimizing the length tension relationship of elastic recoil on exhalation to increase peak cough flow (PCF).
This can be done alone but is more effective when combined with manually assisted cough (Bach JR. Chest. 1993;104[5]:1553-62). These interventions require training of the caregivers, using resources such as those found at www.canventottawa.ca.
With continued supply chain instability, manual airway clearance techniques should be considered in patients with less advanced cough impairment (PCF 160-270 L/min), to save the remaining devices for those with PCF of <160 L/min.
Jeanette Brown, MD, PhD
Karin Provost, DO, PhD
Members-at-Large
Home-based Mechanical Ventilation and Neuromuscular Disease Section
Navigating the latest device supply chain challenge: Mechanical airway clearance
(distal airways). Cough augmentation techniques provide lung volume recruitment on the insufflation phase, in addition to mobilization of secretions with augmentation of the peak expiratory flow rate to >160 L/min on the exhalation phase.
A mechanical insufflation-exsufflation (MI-E) device (T70 Cough Assist - Phillips) is now on indefinite backorder. This creates a dangerous situation for our patients requiring cough augmentation for survival. Alternative options that provide both MI-E and high frequency oscillation include two systems (Synclara Cough System – Hill-rom and the Biwaze Cough System-ABM Respiratory Care).
The Synclara can only be obtained in a direct-to-patient model, contracting with individual respiratory therapists, outside of the standard durable medical equipment model. The final MI-E model option is the VOCSYN multifunctional ventilator (ventilator, cough assist, nebulizer, oxygen concentrator, suction). This multifunction ventilator has had variable acceptance with HCPCS code E0467. If the VOCSYN is chosen, the patient cannot have been issued any component devices or have reached the 36-month cap for oxygen equipment (CR 10854 special payment rule, 42 CFR414.222).
As the supply of devices is exhausted, we will need to shift to evidence-based manual options. Manual cough augmentation can be done effectively with a bag-valve mask, using breath stacking to achieve maximal lung insufflation, optimizing the length tension relationship of elastic recoil on exhalation to increase peak cough flow (PCF).
This can be done alone but is more effective when combined with manually assisted cough (Bach JR. Chest. 1993;104[5]:1553-62). These interventions require training of the caregivers, using resources such as those found at www.canventottawa.ca.
With continued supply chain instability, manual airway clearance techniques should be considered in patients with less advanced cough impairment (PCF 160-270 L/min), to save the remaining devices for those with PCF of <160 L/min.
Jeanette Brown, MD, PhD
Karin Provost, DO, PhD
Members-at-Large
Airways Disorders Network
Asthma and COPD Section
Go TEAM! Shared decision-making tool for patient-clinician collaboration in severe asthma
Shared decision-making is associated with improved medication adherence in adults (Wilson, et al. Am J Respir Crit Care Med. 2010;181[6]:566-77) and quality of life and asthma control in children (Taylor, et al. J Asthma. 2018;55[6]:675-83). The Global Initiative for Asthma committee recommends a patient-clinician partnership. Activated and engaged patients play a major role in their asthma management (https://ginasthma.org/gina-reports). Shared decision-making discussions should include potential benefits and harms of the therapeutic options, patient’s values and lifestyle preferences, and addressing concerns.
The CHEST Foundation, the Allergy and Asthma Network, and the American College of Allergy, Asthma, and Immunology developed an online shared decision- making tool for severe asthma (https://asthma.chestnet.org/sdm-tool).
This tool utilizes patient’s values, specifics about triggers, asthma control, medication side effects, and lifestyle preferences to identify personalized management options. The tool provides information about recommended therapeutic options in simple terms, including potential benefits, possible side effects, expected treatment frequency and duration, and financial aid information. The treatment options currently explained in this tool include anti-immunoglobulin E, anti-interleukin-5, anti-interleukin-4/13, bronchial thermoplasty, long-acting muscarinic antagonist, macrolides, oral corticosteroids, and standard of care.
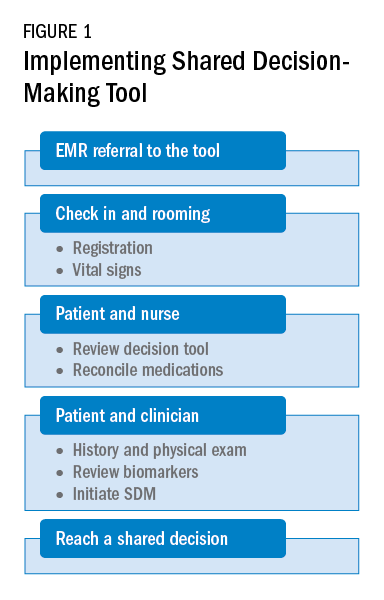
As a team, the patient and the health care professional can use this tool during office visits to help guide management. Figure 1 shows a suggested workflow to utilize the tool in clinical practice.
Potential barriers include excess time and increased human resources. Barrier mitigation may include reviewing the tool and reconciling the medications before the clinician enters the room. With these interventions, many clinician encounters may be completed in 10 to 15 minutes.
Farrukh Abbas, MBBS
Fellow-in-Training
Asthma and COPD Section
Go TEAM! Shared decision-making tool for patient-clinician collaboration in severe asthma
Shared decision-making is associated with improved medication adherence in adults (Wilson, et al. Am J Respir Crit Care Med. 2010;181[6]:566-77) and quality of life and asthma control in children (Taylor, et al. J Asthma. 2018;55[6]:675-83). The Global Initiative for Asthma committee recommends a patient-clinician partnership. Activated and engaged patients play a major role in their asthma management (https://ginasthma.org/gina-reports). Shared decision-making discussions should include potential benefits and harms of the therapeutic options, patient’s values and lifestyle preferences, and addressing concerns.
The CHEST Foundation, the Allergy and Asthma Network, and the American College of Allergy, Asthma, and Immunology developed an online shared decision- making tool for severe asthma (https://asthma.chestnet.org/sdm-tool).
This tool utilizes patient’s values, specifics about triggers, asthma control, medication side effects, and lifestyle preferences to identify personalized management options. The tool provides information about recommended therapeutic options in simple terms, including potential benefits, possible side effects, expected treatment frequency and duration, and financial aid information. The treatment options currently explained in this tool include anti-immunoglobulin E, anti-interleukin-5, anti-interleukin-4/13, bronchial thermoplasty, long-acting muscarinic antagonist, macrolides, oral corticosteroids, and standard of care.
As a team, the patient and the health care professional can use this tool during office visits to help guide management. Figure 1 shows a suggested workflow to utilize the tool in clinical practice.
Potential barriers include excess time and increased human resources. Barrier mitigation may include reviewing the tool and reconciling the medications before the clinician enters the room. With these interventions, many clinician encounters may be completed in 10 to 15 minutes.

Farrukh Abbas, MBBS
Fellow-in-Training
Asthma and COPD Section
Go TEAM! Shared decision-making tool for patient-clinician collaboration in severe asthma
Shared decision-making is associated with improved medication adherence in adults (Wilson, et al. Am J Respir Crit Care Med. 2010;181[6]:566-77) and quality of life and asthma control in children (Taylor, et al. J Asthma. 2018;55[6]:675-83). The Global Initiative for Asthma committee recommends a patient-clinician partnership. Activated and engaged patients play a major role in their asthma management (https://ginasthma.org/gina-reports). Shared decision-making discussions should include potential benefits and harms of the therapeutic options, patient’s values and lifestyle preferences, and addressing concerns.
The CHEST Foundation, the Allergy and Asthma Network, and the American College of Allergy, Asthma, and Immunology developed an online shared decision- making tool for severe asthma (https://asthma.chestnet.org/sdm-tool).
This tool utilizes patient’s values, specifics about triggers, asthma control, medication side effects, and lifestyle preferences to identify personalized management options. The tool provides information about recommended therapeutic options in simple terms, including potential benefits, possible side effects, expected treatment frequency and duration, and financial aid information. The treatment options currently explained in this tool include anti-immunoglobulin E, anti-interleukin-5, anti-interleukin-4/13, bronchial thermoplasty, long-acting muscarinic antagonist, macrolides, oral corticosteroids, and standard of care.
As a team, the patient and the health care professional can use this tool during office visits to help guide management. Figure 1 shows a suggested workflow to utilize the tool in clinical practice.
Potential barriers include excess time and increased human resources. Barrier mitigation may include reviewing the tool and reconciling the medications before the clinician enters the room. With these interventions, many clinician encounters may be completed in 10 to 15 minutes.

Farrukh Abbas, MBBS
Fellow-in-Training
Robotic bronchoscopy 2022
Over the last several years, hundreds of millions of dollars have been spent on robotic bronchoscopy systems in the United States. The release of robotic scopes was made to great fanfare, translating into the market being infiltrated with these systems. With base costs in the hundreds of thousands of dollars, robotic bronchoscope systems are easily the most expensive singular capital investment in the bronchoscopy suite. I frequently get asked questions from those who have not yet made that purchase: “Should I buy a robot?” “How could I justify a new robot purchase to my hospital?” “Is the hype real?” These are complex questions to answer. Before one can answer, I think it’s best to look back on the last 2 decades of bronchoscopy for peripheral lung nodules to get a better understanding of the value proposition robotic bronchoscopes may offer.
Guided bronchoscopy for lung nodules has significantly evolved over the past 2 decades, shifting diagnostic procedures from interventional radiologist to the pulmonologist. Some of these advances were based in redesigns of the bronchoscope (ultrathin bronchoscopy) or application of technology to the bronchoscope (radial EBUS, virtual bronchoscopy); but, these were not broadly applicable to the pulmonology community at large. It was not until the development of electromagnetic navigational bronchoscopy (ENB) that widespread adoption of bronchoscopy for lung nodules occurred. By and large, ENB fueled a rapid expansion of nodule bronchoscopy, mainly due to its ease of use and novel approach. Initial studies of ENB had impressive results; however, studies were criticized for having small numbers, inadequate follow-up, spurious definitions of yield, and that they were being done at highly specialized centers. The NAVIGATE trial was launched to address these criticisms among “real world” conditions. Sponsored by Medtronic, it studied ENB (superDimension platform, v6.0 or higher) across 29 academic and medical centers in the United States, enrolling over 1,000 patients (Folch EE, et al. J Thorac Oncol. 2019;14[3]:445-58. Epub 2018 Nov 23), and reported a diagnostic yield of 73%.
This led to a drive to improve upon yield, resulting in development of new technologies specifically designed to address some of the factors thought associated with diminished yield, and, out of this, robotic bronchoscopy was born. These factors included CT scan-body registration divergence, deflection of the extended working channel (EWC) by rigid biopsy tools, and inability to accurately “aim” the EWC-biopsy tool at the nodule; these were especially problematic in nodules not associated with airways. Robotic scopes were specifically designed to reach into the peripheral lung airways similar to an EWC, but with better structural integrity and steerability. This tip integrity would resist tool-related displacement, and steerability would allow for improved targeting of nodules during the biopsy.
There are two robots approved by the FDA at the time of this writing (Auris Monarch, Intuitive Ion), with a third awaiting FDA clearance (Noah Galaxy). In general, though the engineering of the robotic scopes to improve structural tip integrity are similar, the approach to navigation and targeting vary significantly. The Monarch platform uses electromagnetic guidance, similar to other traditional ENB platforms. The Ion platform does not use ENB; instead, it uses fiberoptic shape sensing technology, which analyzes the shape and orientation of the scope to provide location information. There are potential advantages to shape sensing, the most notable being the absence of electromagnetics; this allows for use of fluoroscopy during the procedure, which otherwise would have interfered with ENB-based navigation. There are other subtle differences between the two robots. The Monarch uses a scope-in-scope design, with a robotic scope contained within a robotic sheath; the Ion uses a single robotic scope. The Ion scope diameter is 3.5 mm, whereas the Monarch diameter is 4.4 mm; this may be a potential advantage when having to navigate through smaller airways.
So, which robot is better suited to reach peripheral nodules more consistently and accurately? I get asked this question a lot, since I have both platforms at my institution. But, answering with my own opinion based on my institution’s anecdotal experience would be irresponsible. I’m more of a “what does the data show?” person. Luckily, we do have clinical trials in both robot technologies. It should be noted here that there will likely never be a head-to-head randomized trial, so evaluating published studies with each platform is going to be the best method we have for comparison going forward, albeit an imperfect one. It should also be noted that many of the early robotic bronchoscopy trials have to be looked at with caution, as yield definitions tended not to be conservative and/or the follow-up of non-malignant was not robust. With that in mind, let’s review representative high-quality studies for each platform.
The best study to date using the Ion platform came out of Memorial Sloan Kettering Cancer Center (Kalchiem-Dekel O, et al. Chest. 2022;161[2];572-82). This single-site study reported on 159 nodule biopsies, with the primary outcome being diagnostic yield. The patients had 1 year of follow-up, and the definition of yield was conservative. The average lesion size was 18 mm, and nodule locations and characteristics were representative of real-world conditions. Overall diagnostic yield was 81.7%; however, it dropped to under 70% for nodules under 20 mm in size.
The largest study to date using the Monarch platform was also a single center study, this from the University of Chicago (Agrawal, et al. Ann Thorac Surg. 2022 Jan 17;S0003-4975(22)00042-X. Online ahead of print). This study included 124 nodules with at least 12 months of follow-up; diagnostic yield definition was conservative. Median nodule size was 20.5 mm, with distribution and characteristics representative of real-world conditions. Overall accuracy was 77%, and, similar to the Ion study, dropped to under 70% when nodule size was smaller than 20 mm.
Overall, both robot studies seemed to show a modest improvement in diagnostic yield when compared with ENB, and their outcomes were overall similar. It is important to remember that these were studies of each center’s first experiences with early versions of each technology; over time, the technology will continue to improve, as will operator skill and experience, and with that, perhaps improvements in yield will be seen, as well.
Interestingly, both studies evaluated target localization using radial EBUS (rEBUS), which also allowed for airway-nodule relationships to be reported. In Kalchiem-Dekel’s study, 85% of cases used rEBUS to determine localization, and, of these, 91.2% of cases showed accurate localization. In Agrawal’s study, rEBUS was used in all cases with a reported localization of 94%. In both, yield did not seem to be affected by airway-nodule relationships, perhaps explained by more robust tip control of the robotic scope. However, localization did not equate to yield in all cases, which brings up a very important question: Can the yield of robotic bronchoscopy be further improved with better real-time on-board imaging, such as CBCT scanning or C-arm based tomography? Currently, there is a study using 3D technology (Cios 3D Mobile Spin) in conjunction with the Ion platform to evaluate this.
So, let’s circle back to where we started. I think if you look at the totality of the data, it is clear that the robotic platforms currently offer a modest improvement in diagnostic yield over traditional ENB, with individual performances that are somewhat equivalent despite differences in design and operation. But does this improvement in yield justify the cost? Individual hospitals will have to make that decision. The capital cost and per-use price of the scope is significant, which has to be balanced against each center’s current performance with non-robotic bronchoscopy.
To date, there have been over 25,000 robotic procedures performed in the United States, so enthusiasm across diverse centers is being maintained. Whether this enthusiasm is driven by yield or novelty, or both, I’m not sure. With other nonrobotic platforms having reached, or soon to reach, the market, this is a good time to be in the business of bronchoscopy.
Dr. Cicenia is in the Section of Bronchoscopy at Cleveland Clinic’s Respiratory Institute, Cleveland, Ohio.
Over the last several years, hundreds of millions of dollars have been spent on robotic bronchoscopy systems in the United States. The release of robotic scopes was made to great fanfare, translating into the market being infiltrated with these systems. With base costs in the hundreds of thousands of dollars, robotic bronchoscope systems are easily the most expensive singular capital investment in the bronchoscopy suite. I frequently get asked questions from those who have not yet made that purchase: “Should I buy a robot?” “How could I justify a new robot purchase to my hospital?” “Is the hype real?” These are complex questions to answer. Before one can answer, I think it’s best to look back on the last 2 decades of bronchoscopy for peripheral lung nodules to get a better understanding of the value proposition robotic bronchoscopes may offer.
Guided bronchoscopy for lung nodules has significantly evolved over the past 2 decades, shifting diagnostic procedures from interventional radiologist to the pulmonologist. Some of these advances were based in redesigns of the bronchoscope (ultrathin bronchoscopy) or application of technology to the bronchoscope (radial EBUS, virtual bronchoscopy); but, these were not broadly applicable to the pulmonology community at large. It was not until the development of electromagnetic navigational bronchoscopy (ENB) that widespread adoption of bronchoscopy for lung nodules occurred. By and large, ENB fueled a rapid expansion of nodule bronchoscopy, mainly due to its ease of use and novel approach. Initial studies of ENB had impressive results; however, studies were criticized for having small numbers, inadequate follow-up, spurious definitions of yield, and that they were being done at highly specialized centers. The NAVIGATE trial was launched to address these criticisms among “real world” conditions. Sponsored by Medtronic, it studied ENB (superDimension platform, v6.0 or higher) across 29 academic and medical centers in the United States, enrolling over 1,000 patients (Folch EE, et al. J Thorac Oncol. 2019;14[3]:445-58. Epub 2018 Nov 23), and reported a diagnostic yield of 73%.
This led to a drive to improve upon yield, resulting in development of new technologies specifically designed to address some of the factors thought associated with diminished yield, and, out of this, robotic bronchoscopy was born. These factors included CT scan-body registration divergence, deflection of the extended working channel (EWC) by rigid biopsy tools, and inability to accurately “aim” the EWC-biopsy tool at the nodule; these were especially problematic in nodules not associated with airways. Robotic scopes were specifically designed to reach into the peripheral lung airways similar to an EWC, but with better structural integrity and steerability. This tip integrity would resist tool-related displacement, and steerability would allow for improved targeting of nodules during the biopsy.
There are two robots approved by the FDA at the time of this writing (Auris Monarch, Intuitive Ion), with a third awaiting FDA clearance (Noah Galaxy). In general, though the engineering of the robotic scopes to improve structural tip integrity are similar, the approach to navigation and targeting vary significantly. The Monarch platform uses electromagnetic guidance, similar to other traditional ENB platforms. The Ion platform does not use ENB; instead, it uses fiberoptic shape sensing technology, which analyzes the shape and orientation of the scope to provide location information. There are potential advantages to shape sensing, the most notable being the absence of electromagnetics; this allows for use of fluoroscopy during the procedure, which otherwise would have interfered with ENB-based navigation. There are other subtle differences between the two robots. The Monarch uses a scope-in-scope design, with a robotic scope contained within a robotic sheath; the Ion uses a single robotic scope. The Ion scope diameter is 3.5 mm, whereas the Monarch diameter is 4.4 mm; this may be a potential advantage when having to navigate through smaller airways.
So, which robot is better suited to reach peripheral nodules more consistently and accurately? I get asked this question a lot, since I have both platforms at my institution. But, answering with my own opinion based on my institution’s anecdotal experience would be irresponsible. I’m more of a “what does the data show?” person. Luckily, we do have clinical trials in both robot technologies. It should be noted here that there will likely never be a head-to-head randomized trial, so evaluating published studies with each platform is going to be the best method we have for comparison going forward, albeit an imperfect one. It should also be noted that many of the early robotic bronchoscopy trials have to be looked at with caution, as yield definitions tended not to be conservative and/or the follow-up of non-malignant was not robust. With that in mind, let’s review representative high-quality studies for each platform.
The best study to date using the Ion platform came out of Memorial Sloan Kettering Cancer Center (Kalchiem-Dekel O, et al. Chest. 2022;161[2];572-82). This single-site study reported on 159 nodule biopsies, with the primary outcome being diagnostic yield. The patients had 1 year of follow-up, and the definition of yield was conservative. The average lesion size was 18 mm, and nodule locations and characteristics were representative of real-world conditions. Overall diagnostic yield was 81.7%; however, it dropped to under 70% for nodules under 20 mm in size.
The largest study to date using the Monarch platform was also a single center study, this from the University of Chicago (Agrawal, et al. Ann Thorac Surg. 2022 Jan 17;S0003-4975(22)00042-X. Online ahead of print). This study included 124 nodules with at least 12 months of follow-up; diagnostic yield definition was conservative. Median nodule size was 20.5 mm, with distribution and characteristics representative of real-world conditions. Overall accuracy was 77%, and, similar to the Ion study, dropped to under 70% when nodule size was smaller than 20 mm.
Overall, both robot studies seemed to show a modest improvement in diagnostic yield when compared with ENB, and their outcomes were overall similar. It is important to remember that these were studies of each center’s first experiences with early versions of each technology; over time, the technology will continue to improve, as will operator skill and experience, and with that, perhaps improvements in yield will be seen, as well.
Interestingly, both studies evaluated target localization using radial EBUS (rEBUS), which also allowed for airway-nodule relationships to be reported. In Kalchiem-Dekel’s study, 85% of cases used rEBUS to determine localization, and, of these, 91.2% of cases showed accurate localization. In Agrawal’s study, rEBUS was used in all cases with a reported localization of 94%. In both, yield did not seem to be affected by airway-nodule relationships, perhaps explained by more robust tip control of the robotic scope. However, localization did not equate to yield in all cases, which brings up a very important question: Can the yield of robotic bronchoscopy be further improved with better real-time on-board imaging, such as CBCT scanning or C-arm based tomography? Currently, there is a study using 3D technology (Cios 3D Mobile Spin) in conjunction with the Ion platform to evaluate this.
So, let’s circle back to where we started. I think if you look at the totality of the data, it is clear that the robotic platforms currently offer a modest improvement in diagnostic yield over traditional ENB, with individual performances that are somewhat equivalent despite differences in design and operation. But does this improvement in yield justify the cost? Individual hospitals will have to make that decision. The capital cost and per-use price of the scope is significant, which has to be balanced against each center’s current performance with non-robotic bronchoscopy.
To date, there have been over 25,000 robotic procedures performed in the United States, so enthusiasm across diverse centers is being maintained. Whether this enthusiasm is driven by yield or novelty, or both, I’m not sure. With other nonrobotic platforms having reached, or soon to reach, the market, this is a good time to be in the business of bronchoscopy.
Dr. Cicenia is in the Section of Bronchoscopy at Cleveland Clinic’s Respiratory Institute, Cleveland, Ohio.
Over the last several years, hundreds of millions of dollars have been spent on robotic bronchoscopy systems in the United States. The release of robotic scopes was made to great fanfare, translating into the market being infiltrated with these systems. With base costs in the hundreds of thousands of dollars, robotic bronchoscope systems are easily the most expensive singular capital investment in the bronchoscopy suite. I frequently get asked questions from those who have not yet made that purchase: “Should I buy a robot?” “How could I justify a new robot purchase to my hospital?” “Is the hype real?” These are complex questions to answer. Before one can answer, I think it’s best to look back on the last 2 decades of bronchoscopy for peripheral lung nodules to get a better understanding of the value proposition robotic bronchoscopes may offer.
Guided bronchoscopy for lung nodules has significantly evolved over the past 2 decades, shifting diagnostic procedures from interventional radiologist to the pulmonologist. Some of these advances were based in redesigns of the bronchoscope (ultrathin bronchoscopy) or application of technology to the bronchoscope (radial EBUS, virtual bronchoscopy); but, these were not broadly applicable to the pulmonology community at large. It was not until the development of electromagnetic navigational bronchoscopy (ENB) that widespread adoption of bronchoscopy for lung nodules occurred. By and large, ENB fueled a rapid expansion of nodule bronchoscopy, mainly due to its ease of use and novel approach. Initial studies of ENB had impressive results; however, studies were criticized for having small numbers, inadequate follow-up, spurious definitions of yield, and that they were being done at highly specialized centers. The NAVIGATE trial was launched to address these criticisms among “real world” conditions. Sponsored by Medtronic, it studied ENB (superDimension platform, v6.0 or higher) across 29 academic and medical centers in the United States, enrolling over 1,000 patients (Folch EE, et al. J Thorac Oncol. 2019;14[3]:445-58. Epub 2018 Nov 23), and reported a diagnostic yield of 73%.
This led to a drive to improve upon yield, resulting in development of new technologies specifically designed to address some of the factors thought associated with diminished yield, and, out of this, robotic bronchoscopy was born. These factors included CT scan-body registration divergence, deflection of the extended working channel (EWC) by rigid biopsy tools, and inability to accurately “aim” the EWC-biopsy tool at the nodule; these were especially problematic in nodules not associated with airways. Robotic scopes were specifically designed to reach into the peripheral lung airways similar to an EWC, but with better structural integrity and steerability. This tip integrity would resist tool-related displacement, and steerability would allow for improved targeting of nodules during the biopsy.
There are two robots approved by the FDA at the time of this writing (Auris Monarch, Intuitive Ion), with a third awaiting FDA clearance (Noah Galaxy). In general, though the engineering of the robotic scopes to improve structural tip integrity are similar, the approach to navigation and targeting vary significantly. The Monarch platform uses electromagnetic guidance, similar to other traditional ENB platforms. The Ion platform does not use ENB; instead, it uses fiberoptic shape sensing technology, which analyzes the shape and orientation of the scope to provide location information. There are potential advantages to shape sensing, the most notable being the absence of electromagnetics; this allows for use of fluoroscopy during the procedure, which otherwise would have interfered with ENB-based navigation. There are other subtle differences between the two robots. The Monarch uses a scope-in-scope design, with a robotic scope contained within a robotic sheath; the Ion uses a single robotic scope. The Ion scope diameter is 3.5 mm, whereas the Monarch diameter is 4.4 mm; this may be a potential advantage when having to navigate through smaller airways.
So, which robot is better suited to reach peripheral nodules more consistently and accurately? I get asked this question a lot, since I have both platforms at my institution. But, answering with my own opinion based on my institution’s anecdotal experience would be irresponsible. I’m more of a “what does the data show?” person. Luckily, we do have clinical trials in both robot technologies. It should be noted here that there will likely never be a head-to-head randomized trial, so evaluating published studies with each platform is going to be the best method we have for comparison going forward, albeit an imperfect one. It should also be noted that many of the early robotic bronchoscopy trials have to be looked at with caution, as yield definitions tended not to be conservative and/or the follow-up of non-malignant was not robust. With that in mind, let’s review representative high-quality studies for each platform.
The best study to date using the Ion platform came out of Memorial Sloan Kettering Cancer Center (Kalchiem-Dekel O, et al. Chest. 2022;161[2];572-82). This single-site study reported on 159 nodule biopsies, with the primary outcome being diagnostic yield. The patients had 1 year of follow-up, and the definition of yield was conservative. The average lesion size was 18 mm, and nodule locations and characteristics were representative of real-world conditions. Overall diagnostic yield was 81.7%; however, it dropped to under 70% for nodules under 20 mm in size.
The largest study to date using the Monarch platform was also a single center study, this from the University of Chicago (Agrawal, et al. Ann Thorac Surg. 2022 Jan 17;S0003-4975(22)00042-X. Online ahead of print). This study included 124 nodules with at least 12 months of follow-up; diagnostic yield definition was conservative. Median nodule size was 20.5 mm, with distribution and characteristics representative of real-world conditions. Overall accuracy was 77%, and, similar to the Ion study, dropped to under 70% when nodule size was smaller than 20 mm.
Overall, both robot studies seemed to show a modest improvement in diagnostic yield when compared with ENB, and their outcomes were overall similar. It is important to remember that these were studies of each center’s first experiences with early versions of each technology; over time, the technology will continue to improve, as will operator skill and experience, and with that, perhaps improvements in yield will be seen, as well.
Interestingly, both studies evaluated target localization using radial EBUS (rEBUS), which also allowed for airway-nodule relationships to be reported. In Kalchiem-Dekel’s study, 85% of cases used rEBUS to determine localization, and, of these, 91.2% of cases showed accurate localization. In Agrawal’s study, rEBUS was used in all cases with a reported localization of 94%. In both, yield did not seem to be affected by airway-nodule relationships, perhaps explained by more robust tip control of the robotic scope. However, localization did not equate to yield in all cases, which brings up a very important question: Can the yield of robotic bronchoscopy be further improved with better real-time on-board imaging, such as CBCT scanning or C-arm based tomography? Currently, there is a study using 3D technology (Cios 3D Mobile Spin) in conjunction with the Ion platform to evaluate this.
So, let’s circle back to where we started. I think if you look at the totality of the data, it is clear that the robotic platforms currently offer a modest improvement in diagnostic yield over traditional ENB, with individual performances that are somewhat equivalent despite differences in design and operation. But does this improvement in yield justify the cost? Individual hospitals will have to make that decision. The capital cost and per-use price of the scope is significant, which has to be balanced against each center’s current performance with non-robotic bronchoscopy.
To date, there have been over 25,000 robotic procedures performed in the United States, so enthusiasm across diverse centers is being maintained. Whether this enthusiasm is driven by yield or novelty, or both, I’m not sure. With other nonrobotic platforms having reached, or soon to reach, the market, this is a good time to be in the business of bronchoscopy.
Dr. Cicenia is in the Section of Bronchoscopy at Cleveland Clinic’s Respiratory Institute, Cleveland, Ohio.
This month in the journal CHEST®
Dupilumab Reduces Oral Corticosteroid Use in Patients With Corticosteroid-Dependent Severe Asthma. By Lawrence D. Sher, MD, et al.
Carriage and Transmission of Macrolide Resistance Genes in Patients With Chronic Respiratory Conditions and Their Close Contacts. By Yiming Wang, MSc, et al.
An Evaluation of Factors That Influence Referral to Pulmonary Rehabilitation Programs Among People With COPD. By Sarah Hug, BSc, et al.
Prevalence and Outcomes of Previously Healthy Adults Among Patients Hospitalized With Community-Onset Sepsis. By Mohammad Alrawashdeh, PhD, MSN, et al.
Screening Strategies for Pulmonary Hypertension in Patients With Interstitial Lung Disease: A Multidisciplinary Delphi Study. By Franck F. Rahaghi, MD, et al.
Race- and Ethnicity-Based Spirometry Reference Equations: Are They Accurate for Genetically Admixed Children? By Jonathan Witonsky, MD, et al.
No VTE Recurrence After 1-Year Follow Up of Hospitalized Patients With COVID-19 and a VTE Event: A Prospective Study. By Maxime Delrue, MD, PhD, et al.
Patient Perspectives on Longitudinal Adherence to Lung Cancer Screening. By Anna Holman, BS, et al.
Dupilumab Reduces Oral Corticosteroid Use in Patients With Corticosteroid-Dependent Severe Asthma. By Lawrence D. Sher, MD, et al.
Carriage and Transmission of Macrolide Resistance Genes in Patients With Chronic Respiratory Conditions and Their Close Contacts. By Yiming Wang, MSc, et al.
An Evaluation of Factors That Influence Referral to Pulmonary Rehabilitation Programs Among People With COPD. By Sarah Hug, BSc, et al.
Prevalence and Outcomes of Previously Healthy Adults Among Patients Hospitalized With Community-Onset Sepsis. By Mohammad Alrawashdeh, PhD, MSN, et al.
Screening Strategies for Pulmonary Hypertension in Patients With Interstitial Lung Disease: A Multidisciplinary Delphi Study. By Franck F. Rahaghi, MD, et al.
Race- and Ethnicity-Based Spirometry Reference Equations: Are They Accurate for Genetically Admixed Children? By Jonathan Witonsky, MD, et al.
No VTE Recurrence After 1-Year Follow Up of Hospitalized Patients With COVID-19 and a VTE Event: A Prospective Study. By Maxime Delrue, MD, PhD, et al.
Patient Perspectives on Longitudinal Adherence to Lung Cancer Screening. By Anna Holman, BS, et al.
Dupilumab Reduces Oral Corticosteroid Use in Patients With Corticosteroid-Dependent Severe Asthma. By Lawrence D. Sher, MD, et al.
Carriage and Transmission of Macrolide Resistance Genes in Patients With Chronic Respiratory Conditions and Their Close Contacts. By Yiming Wang, MSc, et al.
An Evaluation of Factors That Influence Referral to Pulmonary Rehabilitation Programs Among People With COPD. By Sarah Hug, BSc, et al.
Prevalence and Outcomes of Previously Healthy Adults Among Patients Hospitalized With Community-Onset Sepsis. By Mohammad Alrawashdeh, PhD, MSN, et al.
Screening Strategies for Pulmonary Hypertension in Patients With Interstitial Lung Disease: A Multidisciplinary Delphi Study. By Franck F. Rahaghi, MD, et al.
Race- and Ethnicity-Based Spirometry Reference Equations: Are They Accurate for Genetically Admixed Children? By Jonathan Witonsky, MD, et al.
No VTE Recurrence After 1-Year Follow Up of Hospitalized Patients With COVID-19 and a VTE Event: A Prospective Study. By Maxime Delrue, MD, PhD, et al.
Patient Perspectives on Longitudinal Adherence to Lung Cancer Screening. By Anna Holman, BS, et al.
Starting CHEST 2022 off with a step kick
, with a bang—or, more accurately, with a step kick, swivel, and stomp—at the Wildhorse Saloon.
The Wildhorse is famous for hosting daily line dancing lessons on the largest dance floor in the downtown area and for having a menu full of Nashville favorites, including Nashville hot chicken and a hearty selection of entrees (as well as a decadent bananas foster) with a “Jack Daniels” single barrel whiskey glaze.
The opening reception offers attendees the opportunity to relax and reconnect with their peers from across the fields of pulmonary, critical care, and sleep medicine before the jam-packed schedule of more than 300 educational sessions starts the following day.
But the fun doesn’t stop there. Attendees interested in exploring the city after hours have a host of options, from world-class music venues to iconic distilleries and restaurants. The Music City Center, where CHEST 2022 will be held, is located in the SoBro neighborhood of Nashville, not far from the Arts District, Downtown, and Music Row.
According to Nashville local and CHEST member Meredith Pugh, MD, MSCI, “it goes without saying that we have the best music scene in the country, but it’s a great place for outdoor activities and food.”
For those who don’t get their fill at the Wildhorse, Dr. Pugh recommends attendees check out the Assembly Food Hall (.3 miles from the convention center) to try the city’s famous Nashville Hot Chicken and a variety of other local options. And, don’t miss the many excellent options for BBQ. Fellow Nashville transplant and CHEST member Todd Rice, MD, FCCP, suggests Martin’s Bar-B-Que Joint and Jack’s Bar-B-Que—both within walking distance of the Music City Center—as well as other local options.
To learn more about everything Nashville has to offer, and get more recommendations from Drs. Rice and Pugh, check out the latest CHEST 2022 blog on chestnet.org
, with a bang—or, more accurately, with a step kick, swivel, and stomp—at the Wildhorse Saloon.
The Wildhorse is famous for hosting daily line dancing lessons on the largest dance floor in the downtown area and for having a menu full of Nashville favorites, including Nashville hot chicken and a hearty selection of entrees (as well as a decadent bananas foster) with a “Jack Daniels” single barrel whiskey glaze.
The opening reception offers attendees the opportunity to relax and reconnect with their peers from across the fields of pulmonary, critical care, and sleep medicine before the jam-packed schedule of more than 300 educational sessions starts the following day.
But the fun doesn’t stop there. Attendees interested in exploring the city after hours have a host of options, from world-class music venues to iconic distilleries and restaurants. The Music City Center, where CHEST 2022 will be held, is located in the SoBro neighborhood of Nashville, not far from the Arts District, Downtown, and Music Row.
According to Nashville local and CHEST member Meredith Pugh, MD, MSCI, “it goes without saying that we have the best music scene in the country, but it’s a great place for outdoor activities and food.”
For those who don’t get their fill at the Wildhorse, Dr. Pugh recommends attendees check out the Assembly Food Hall (.3 miles from the convention center) to try the city’s famous Nashville Hot Chicken and a variety of other local options. And, don’t miss the many excellent options for BBQ. Fellow Nashville transplant and CHEST member Todd Rice, MD, FCCP, suggests Martin’s Bar-B-Que Joint and Jack’s Bar-B-Que—both within walking distance of the Music City Center—as well as other local options.
To learn more about everything Nashville has to offer, and get more recommendations from Drs. Rice and Pugh, check out the latest CHEST 2022 blog on chestnet.org
, with a bang—or, more accurately, with a step kick, swivel, and stomp—at the Wildhorse Saloon.
The Wildhorse is famous for hosting daily line dancing lessons on the largest dance floor in the downtown area and for having a menu full of Nashville favorites, including Nashville hot chicken and a hearty selection of entrees (as well as a decadent bananas foster) with a “Jack Daniels” single barrel whiskey glaze.
The opening reception offers attendees the opportunity to relax and reconnect with their peers from across the fields of pulmonary, critical care, and sleep medicine before the jam-packed schedule of more than 300 educational sessions starts the following day.
But the fun doesn’t stop there. Attendees interested in exploring the city after hours have a host of options, from world-class music venues to iconic distilleries and restaurants. The Music City Center, where CHEST 2022 will be held, is located in the SoBro neighborhood of Nashville, not far from the Arts District, Downtown, and Music Row.
According to Nashville local and CHEST member Meredith Pugh, MD, MSCI, “it goes without saying that we have the best music scene in the country, but it’s a great place for outdoor activities and food.”
For those who don’t get their fill at the Wildhorse, Dr. Pugh recommends attendees check out the Assembly Food Hall (.3 miles from the convention center) to try the city’s famous Nashville Hot Chicken and a variety of other local options. And, don’t miss the many excellent options for BBQ. Fellow Nashville transplant and CHEST member Todd Rice, MD, FCCP, suggests Martin’s Bar-B-Que Joint and Jack’s Bar-B-Que—both within walking distance of the Music City Center—as well as other local options.
To learn more about everything Nashville has to offer, and get more recommendations from Drs. Rice and Pugh, check out the latest CHEST 2022 blog on chestnet.org
Beyond CPAP: Looking to alternative treatments for obstructive sleep apnea
Overview of the problem
Obstructive sleep apnea (OSA) is an extraordinarily common condition impacting nearly 1 billion individuals globally (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687). For the past 40 years, the mainstay of treatment has been continuous positive airway pressure (CPAP). However, CPAP usage is highly variable, and not all sleep apnea is created the same with respect to underlying mechanism or patient symptoms. Currently, there is a global CPAP shortage, which has expedited the need for alternative therapies in OSA (Owens RL, et al. Am J Respir Crit Care Med. 2021;204[8]:887).
Characterizing OSA
First, it is important to understand that sleep apnea emerges for multiple reasons. Some examples include: an excessively collapsible airway, insufficient upper airway reflexes, low arousal threshold (awakening easily to ventilatory disturbance), as well as an unstable chemoreflex system. This list is not comprehensive. However, we believe that the future of OSA management will be targeted therapy for individual OSA traits.
Notably, the patient experience of OSA is also highly variable. Some individuals are excessively sleepy. Some individuals experience OSA as insomnia. Other patients are asymptomatic, but present to the sleep clinic at the behest of a disgruntled bed partner. These individual factors should all be kept in mind when deciding when and how to treat sleep apnea.
OSA scoring – past, present, and future
The traditional method for scoring sleep apnea severity is the apnea-hypopnea index (AHI), with mild, moderate, and severe OSA being stratified by the number of events per hour. This metric has shaped many of the modern sleep practices and consensus recommendations but is simply not sophisticated enough to capture the nuance of how or why an individual’s sleep is disrupted from flow-limited breathing. As such, there has been a push in recent times to tailor treatment for OSA to an individual’s physiology. Examples of alternative metrics which quantify sleep apnea traits include the apnea-hypopnea event duration, the sleep apnea-specific hypoxic burden (area under the SpO2 curve for flow-limited events), as well as the arousal intensity from sleep in the setting of flow-limited breathing. There are numerous other metrics that have been proposed but are beyond the scope of this review (Malhotra A, et al. Sleep. 2021;44[7]:zsab030).
What therapies are available and how can we individualize them to our patients?
As noted, CPAP has been the gold-standard for OSA treatment for 40 years but is not always accepted or tolerated (Malhotra A, et al. Chest. 2018;153[4]:843). Broad categories of OSA management are presented as follows.
Surgery for OSA
Upper airway surgery is effective for pediatric OSA treatment, where enlarged tonsils are often the culprit for flow-limited breathing in sleep. For adults, however, there is no one best surgery or surgical candidate. For instance, surgery can be used to improve CPAP tolerance or as a primary OSA treatment. Many individuals with sinus disease may require sinus surgery or septoplasty to improve CPAP tolerability by creating more space for airflow through the nasopharynx. Retrognathic individuals, on the other hand, may benefit from maxillomandibular advancement. Others may benefit from genioglossus advancement or hyoid suspension. The characteristics of the soft palate can be predictive of surgical success with respect to uvulopalatopharyngoplasty (UPPP), with longer uvulas and redundant soft palate tissue being attractive surgical targets. Obviously, this list is far from comprehensive, but Friedman tongue position, tonsil size, and body mass index also appear to be important in predicting surgical success (MacKay S, et al. JAMA. 2020;324[12]:1168).
Hypoglossal nerve stimulation is one surgical treatment option for patients with moderate-severe OSA who are unable or unwilling to use CPAP therapy, have a BMI <32-35 kg/m2 (center-dependent), no concentric velopharyngeal collapse on drug-induced sleep endoscopy, and fewer than 25% central/mixed apneas on their sleep study. Areas for further study are whether unilateral or bilateral stimulation are most effective, as well as which of the sleep apnea traits are most predictive of a treatment response (Strohl MM, et al. Curr Sleep Med Rep. 2017;3[3]:133).
Notably, surgical techniques are highly variable, and there are individual patient characteristics, such as lower loop gain (more stable ventilatory control), which may have a greater likelihood of successful upper airway surgery. This is likely because making the upper airway more patent allows for ventilatory overshoots and thereby airway collapse and cyclic, unstable breathing in those with an unstable ventilatory control system. Trials with prespecified surgical techniques based on individual traits are welcome. Additionally, the metrics of a successful surgical treatment for OSA, much like the AHI, are in need of evolution. The Sher criteria, for instance (50% AHI reduction to an AHI < 20/h), are arbitrary, and their clinical utility is unclear.
Oral appliances
Oral appliances fall into two broad categories – tongue-retaining devices and mandibular advancement splints (MAS). Of the two, MAS are much more commonly prescribed. Of the MAS devices, custom made devices by an American Academy of Dental Sleep Medicine (AADSM)–trained dentist are recommended over noncustom MAS in the treatment of primary snoring or OSA for those unwilling or unable to wear CPAP. Notably, the 2015 American Academy of Sleep Medicine (AASM) and AADSM shared guidelines were unable to make OSA treatment recommendations based on severity of disease as stratified by the AHI due to the limited quality of evidence. These devices are broadly thought to work by protruding the mandible/tongue and, in-turn, advancing multiple soft tissue components of the velopharynx. Relatively recent work suggests that the following OSA traits are associated with MAS efficacy: lower loop gain, higher arousal threshold, lower ventilatory response to arousal, moderate pharyngeal collapsibility, and weaker upper airway dilator muscle compensation. However, in order for these devices to be successful, close follow-up for titration with a AADSM-certified dentist, as well as a follow-up efficacy sleep study, are recommended. Adherence for custom device use appears to be about 70% use greater than 4 hours per night, with 35% to 40% of those prescribed a device achieving an AHI less than 5/h. Over the counter devices are not routinely recommended, though some practices do use these devices as a trial to see if patients may tolerate custom made devices (Ramar K, et al. J Clin Sleep Med. 2015;11[7]:773).
Upper airway training
Upper airway training has been shown possibly to be effective in treating OSA, though the ideal endotype is still being established. Upper airway training has taken many forms, from woodwind instrument playing, to nocturnal electrical stimulation of the tongue, and, more recently, daytime awake transoral neuromuscular stimulation. These interventions appear to be effective for mild sleep apnea and snoring, but the best training regimen has yet to be established. Equally, as with other routine exercise, there appears to be a “use it or lose it” component, and the ideal maintenance regimen for each of these therapies is yet to be determined.
Weight loss and bariatric surgery
Obesity is a common, reversible risk factor for OSA. However, not all obese individuals develop OSA (typically those with robust upper airway reflexes). Improvements in weight appear to correlate with reductions in tongue fat, which correlate to AHI reduction. Weight loss also creates lower CPAP requirements for many individuals, conceivably improving tolerability. Ongoing work is seeking to understand whether there are changes in upper airway muscle recruitability as well as other change in endotype traits following weight loss surgery.
Pharmacotherapy for OSA
There is a great deal of promise in tailoring pharmacotherapy to individual sleep traits. Acetazolamide, for instance, results in improvements an AHI for both obstructive and central sleep apnea through changes in chemosensitivity and is generally well-tolerated (Schmickl CN, et al. Physiol Rep. 2021;9[20]:e15071). Eszopiclone has been used to raise the arousal threshold for those who awaken from breathing events too easily. With added time, individuals with a low arousal threshold can more effectively recruit upper airway dilator muscles without waking up. Pharmacotherapy to improve upper airway recruitability with combination noradrenergic stimulation and antimuscarinic activity has limited data thus far but may be a useful part of the sleep armamentarium moving forward.
Summary
OSA is a public health priority, and the current global CPAP shortage emphasizes the need for alternative OSA therapies. The ideal therapy for a given patient requires a careful consideration of their individual traits and will be much more refined when endotyping is available in a routine clinical setting. Individualized sleep apnea treatment is the future of sleep medicine and a one-size fits all approach no longer meets the needs of our patients given the current state of sleep medicine knowledge.
Dr. Nokes, Dr. Schmickl, and Dr. Malhotra are with the University of California, San Diego, Division of Pulmonary, Critical Care, and Sleep Medicine, La, Jolla, CA. Dr. Nokes also is with the Veterans Affairs San Diego Healthcare System, sleep section, San Diego, CA. Dr. Vahabzadeh-Hagh is with the University of California, San Diego, Department of Otolaryngology, San Diego, CA.
Overview of the problem
Obstructive sleep apnea (OSA) is an extraordinarily common condition impacting nearly 1 billion individuals globally (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687). For the past 40 years, the mainstay of treatment has been continuous positive airway pressure (CPAP). However, CPAP usage is highly variable, and not all sleep apnea is created the same with respect to underlying mechanism or patient symptoms. Currently, there is a global CPAP shortage, which has expedited the need for alternative therapies in OSA (Owens RL, et al. Am J Respir Crit Care Med. 2021;204[8]:887).
Characterizing OSA
First, it is important to understand that sleep apnea emerges for multiple reasons. Some examples include: an excessively collapsible airway, insufficient upper airway reflexes, low arousal threshold (awakening easily to ventilatory disturbance), as well as an unstable chemoreflex system. This list is not comprehensive. However, we believe that the future of OSA management will be targeted therapy for individual OSA traits.
Notably, the patient experience of OSA is also highly variable. Some individuals are excessively sleepy. Some individuals experience OSA as insomnia. Other patients are asymptomatic, but present to the sleep clinic at the behest of a disgruntled bed partner. These individual factors should all be kept in mind when deciding when and how to treat sleep apnea.
OSA scoring – past, present, and future
The traditional method for scoring sleep apnea severity is the apnea-hypopnea index (AHI), with mild, moderate, and severe OSA being stratified by the number of events per hour. This metric has shaped many of the modern sleep practices and consensus recommendations but is simply not sophisticated enough to capture the nuance of how or why an individual’s sleep is disrupted from flow-limited breathing. As such, there has been a push in recent times to tailor treatment for OSA to an individual’s physiology. Examples of alternative metrics which quantify sleep apnea traits include the apnea-hypopnea event duration, the sleep apnea-specific hypoxic burden (area under the SpO2 curve for flow-limited events), as well as the arousal intensity from sleep in the setting of flow-limited breathing. There are numerous other metrics that have been proposed but are beyond the scope of this review (Malhotra A, et al. Sleep. 2021;44[7]:zsab030).
What therapies are available and how can we individualize them to our patients?
As noted, CPAP has been the gold-standard for OSA treatment for 40 years but is not always accepted or tolerated (Malhotra A, et al. Chest. 2018;153[4]:843). Broad categories of OSA management are presented as follows.
Surgery for OSA
Upper airway surgery is effective for pediatric OSA treatment, where enlarged tonsils are often the culprit for flow-limited breathing in sleep. For adults, however, there is no one best surgery or surgical candidate. For instance, surgery can be used to improve CPAP tolerance or as a primary OSA treatment. Many individuals with sinus disease may require sinus surgery or septoplasty to improve CPAP tolerability by creating more space for airflow through the nasopharynx. Retrognathic individuals, on the other hand, may benefit from maxillomandibular advancement. Others may benefit from genioglossus advancement or hyoid suspension. The characteristics of the soft palate can be predictive of surgical success with respect to uvulopalatopharyngoplasty (UPPP), with longer uvulas and redundant soft palate tissue being attractive surgical targets. Obviously, this list is far from comprehensive, but Friedman tongue position, tonsil size, and body mass index also appear to be important in predicting surgical success (MacKay S, et al. JAMA. 2020;324[12]:1168).
Hypoglossal nerve stimulation is one surgical treatment option for patients with moderate-severe OSA who are unable or unwilling to use CPAP therapy, have a BMI <32-35 kg/m2 (center-dependent), no concentric velopharyngeal collapse on drug-induced sleep endoscopy, and fewer than 25% central/mixed apneas on their sleep study. Areas for further study are whether unilateral or bilateral stimulation are most effective, as well as which of the sleep apnea traits are most predictive of a treatment response (Strohl MM, et al. Curr Sleep Med Rep. 2017;3[3]:133).
Notably, surgical techniques are highly variable, and there are individual patient characteristics, such as lower loop gain (more stable ventilatory control), which may have a greater likelihood of successful upper airway surgery. This is likely because making the upper airway more patent allows for ventilatory overshoots and thereby airway collapse and cyclic, unstable breathing in those with an unstable ventilatory control system. Trials with prespecified surgical techniques based on individual traits are welcome. Additionally, the metrics of a successful surgical treatment for OSA, much like the AHI, are in need of evolution. The Sher criteria, for instance (50% AHI reduction to an AHI < 20/h), are arbitrary, and their clinical utility is unclear.
Oral appliances
Oral appliances fall into two broad categories – tongue-retaining devices and mandibular advancement splints (MAS). Of the two, MAS are much more commonly prescribed. Of the MAS devices, custom made devices by an American Academy of Dental Sleep Medicine (AADSM)–trained dentist are recommended over noncustom MAS in the treatment of primary snoring or OSA for those unwilling or unable to wear CPAP. Notably, the 2015 American Academy of Sleep Medicine (AASM) and AADSM shared guidelines were unable to make OSA treatment recommendations based on severity of disease as stratified by the AHI due to the limited quality of evidence. These devices are broadly thought to work by protruding the mandible/tongue and, in-turn, advancing multiple soft tissue components of the velopharynx. Relatively recent work suggests that the following OSA traits are associated with MAS efficacy: lower loop gain, higher arousal threshold, lower ventilatory response to arousal, moderate pharyngeal collapsibility, and weaker upper airway dilator muscle compensation. However, in order for these devices to be successful, close follow-up for titration with a AADSM-certified dentist, as well as a follow-up efficacy sleep study, are recommended. Adherence for custom device use appears to be about 70% use greater than 4 hours per night, with 35% to 40% of those prescribed a device achieving an AHI less than 5/h. Over the counter devices are not routinely recommended, though some practices do use these devices as a trial to see if patients may tolerate custom made devices (Ramar K, et al. J Clin Sleep Med. 2015;11[7]:773).
Upper airway training
Upper airway training has been shown possibly to be effective in treating OSA, though the ideal endotype is still being established. Upper airway training has taken many forms, from woodwind instrument playing, to nocturnal electrical stimulation of the tongue, and, more recently, daytime awake transoral neuromuscular stimulation. These interventions appear to be effective for mild sleep apnea and snoring, but the best training regimen has yet to be established. Equally, as with other routine exercise, there appears to be a “use it or lose it” component, and the ideal maintenance regimen for each of these therapies is yet to be determined.
Weight loss and bariatric surgery
Obesity is a common, reversible risk factor for OSA. However, not all obese individuals develop OSA (typically those with robust upper airway reflexes). Improvements in weight appear to correlate with reductions in tongue fat, which correlate to AHI reduction. Weight loss also creates lower CPAP requirements for many individuals, conceivably improving tolerability. Ongoing work is seeking to understand whether there are changes in upper airway muscle recruitability as well as other change in endotype traits following weight loss surgery.
Pharmacotherapy for OSA
There is a great deal of promise in tailoring pharmacotherapy to individual sleep traits. Acetazolamide, for instance, results in improvements an AHI for both obstructive and central sleep apnea through changes in chemosensitivity and is generally well-tolerated (Schmickl CN, et al. Physiol Rep. 2021;9[20]:e15071). Eszopiclone has been used to raise the arousal threshold for those who awaken from breathing events too easily. With added time, individuals with a low arousal threshold can more effectively recruit upper airway dilator muscles without waking up. Pharmacotherapy to improve upper airway recruitability with combination noradrenergic stimulation and antimuscarinic activity has limited data thus far but may be a useful part of the sleep armamentarium moving forward.
Summary
OSA is a public health priority, and the current global CPAP shortage emphasizes the need for alternative OSA therapies. The ideal therapy for a given patient requires a careful consideration of their individual traits and will be much more refined when endotyping is available in a routine clinical setting. Individualized sleep apnea treatment is the future of sleep medicine and a one-size fits all approach no longer meets the needs of our patients given the current state of sleep medicine knowledge.
Dr. Nokes, Dr. Schmickl, and Dr. Malhotra are with the University of California, San Diego, Division of Pulmonary, Critical Care, and Sleep Medicine, La, Jolla, CA. Dr. Nokes also is with the Veterans Affairs San Diego Healthcare System, sleep section, San Diego, CA. Dr. Vahabzadeh-Hagh is with the University of California, San Diego, Department of Otolaryngology, San Diego, CA.
Overview of the problem
Obstructive sleep apnea (OSA) is an extraordinarily common condition impacting nearly 1 billion individuals globally (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687). For the past 40 years, the mainstay of treatment has been continuous positive airway pressure (CPAP). However, CPAP usage is highly variable, and not all sleep apnea is created the same with respect to underlying mechanism or patient symptoms. Currently, there is a global CPAP shortage, which has expedited the need for alternative therapies in OSA (Owens RL, et al. Am J Respir Crit Care Med. 2021;204[8]:887).
Characterizing OSA
First, it is important to understand that sleep apnea emerges for multiple reasons. Some examples include: an excessively collapsible airway, insufficient upper airway reflexes, low arousal threshold (awakening easily to ventilatory disturbance), as well as an unstable chemoreflex system. This list is not comprehensive. However, we believe that the future of OSA management will be targeted therapy for individual OSA traits.
Notably, the patient experience of OSA is also highly variable. Some individuals are excessively sleepy. Some individuals experience OSA as insomnia. Other patients are asymptomatic, but present to the sleep clinic at the behest of a disgruntled bed partner. These individual factors should all be kept in mind when deciding when and how to treat sleep apnea.
OSA scoring – past, present, and future
The traditional method for scoring sleep apnea severity is the apnea-hypopnea index (AHI), with mild, moderate, and severe OSA being stratified by the number of events per hour. This metric has shaped many of the modern sleep practices and consensus recommendations but is simply not sophisticated enough to capture the nuance of how or why an individual’s sleep is disrupted from flow-limited breathing. As such, there has been a push in recent times to tailor treatment for OSA to an individual’s physiology. Examples of alternative metrics which quantify sleep apnea traits include the apnea-hypopnea event duration, the sleep apnea-specific hypoxic burden (area under the SpO2 curve for flow-limited events), as well as the arousal intensity from sleep in the setting of flow-limited breathing. There are numerous other metrics that have been proposed but are beyond the scope of this review (Malhotra A, et al. Sleep. 2021;44[7]:zsab030).
What therapies are available and how can we individualize them to our patients?
As noted, CPAP has been the gold-standard for OSA treatment for 40 years but is not always accepted or tolerated (Malhotra A, et al. Chest. 2018;153[4]:843). Broad categories of OSA management are presented as follows.
Surgery for OSA
Upper airway surgery is effective for pediatric OSA treatment, where enlarged tonsils are often the culprit for flow-limited breathing in sleep. For adults, however, there is no one best surgery or surgical candidate. For instance, surgery can be used to improve CPAP tolerance or as a primary OSA treatment. Many individuals with sinus disease may require sinus surgery or septoplasty to improve CPAP tolerability by creating more space for airflow through the nasopharynx. Retrognathic individuals, on the other hand, may benefit from maxillomandibular advancement. Others may benefit from genioglossus advancement or hyoid suspension. The characteristics of the soft palate can be predictive of surgical success with respect to uvulopalatopharyngoplasty (UPPP), with longer uvulas and redundant soft palate tissue being attractive surgical targets. Obviously, this list is far from comprehensive, but Friedman tongue position, tonsil size, and body mass index also appear to be important in predicting surgical success (MacKay S, et al. JAMA. 2020;324[12]:1168).
Hypoglossal nerve stimulation is one surgical treatment option for patients with moderate-severe OSA who are unable or unwilling to use CPAP therapy, have a BMI <32-35 kg/m2 (center-dependent), no concentric velopharyngeal collapse on drug-induced sleep endoscopy, and fewer than 25% central/mixed apneas on their sleep study. Areas for further study are whether unilateral or bilateral stimulation are most effective, as well as which of the sleep apnea traits are most predictive of a treatment response (Strohl MM, et al. Curr Sleep Med Rep. 2017;3[3]:133).
Notably, surgical techniques are highly variable, and there are individual patient characteristics, such as lower loop gain (more stable ventilatory control), which may have a greater likelihood of successful upper airway surgery. This is likely because making the upper airway more patent allows for ventilatory overshoots and thereby airway collapse and cyclic, unstable breathing in those with an unstable ventilatory control system. Trials with prespecified surgical techniques based on individual traits are welcome. Additionally, the metrics of a successful surgical treatment for OSA, much like the AHI, are in need of evolution. The Sher criteria, for instance (50% AHI reduction to an AHI < 20/h), are arbitrary, and their clinical utility is unclear.
Oral appliances
Oral appliances fall into two broad categories – tongue-retaining devices and mandibular advancement splints (MAS). Of the two, MAS are much more commonly prescribed. Of the MAS devices, custom made devices by an American Academy of Dental Sleep Medicine (AADSM)–trained dentist are recommended over noncustom MAS in the treatment of primary snoring or OSA for those unwilling or unable to wear CPAP. Notably, the 2015 American Academy of Sleep Medicine (AASM) and AADSM shared guidelines were unable to make OSA treatment recommendations based on severity of disease as stratified by the AHI due to the limited quality of evidence. These devices are broadly thought to work by protruding the mandible/tongue and, in-turn, advancing multiple soft tissue components of the velopharynx. Relatively recent work suggests that the following OSA traits are associated with MAS efficacy: lower loop gain, higher arousal threshold, lower ventilatory response to arousal, moderate pharyngeal collapsibility, and weaker upper airway dilator muscle compensation. However, in order for these devices to be successful, close follow-up for titration with a AADSM-certified dentist, as well as a follow-up efficacy sleep study, are recommended. Adherence for custom device use appears to be about 70% use greater than 4 hours per night, with 35% to 40% of those prescribed a device achieving an AHI less than 5/h. Over the counter devices are not routinely recommended, though some practices do use these devices as a trial to see if patients may tolerate custom made devices (Ramar K, et al. J Clin Sleep Med. 2015;11[7]:773).
Upper airway training
Upper airway training has been shown possibly to be effective in treating OSA, though the ideal endotype is still being established. Upper airway training has taken many forms, from woodwind instrument playing, to nocturnal electrical stimulation of the tongue, and, more recently, daytime awake transoral neuromuscular stimulation. These interventions appear to be effective for mild sleep apnea and snoring, but the best training regimen has yet to be established. Equally, as with other routine exercise, there appears to be a “use it or lose it” component, and the ideal maintenance regimen for each of these therapies is yet to be determined.
Weight loss and bariatric surgery
Obesity is a common, reversible risk factor for OSA. However, not all obese individuals develop OSA (typically those with robust upper airway reflexes). Improvements in weight appear to correlate with reductions in tongue fat, which correlate to AHI reduction. Weight loss also creates lower CPAP requirements for many individuals, conceivably improving tolerability. Ongoing work is seeking to understand whether there are changes in upper airway muscle recruitability as well as other change in endotype traits following weight loss surgery.
Pharmacotherapy for OSA
There is a great deal of promise in tailoring pharmacotherapy to individual sleep traits. Acetazolamide, for instance, results in improvements an AHI for both obstructive and central sleep apnea through changes in chemosensitivity and is generally well-tolerated (Schmickl CN, et al. Physiol Rep. 2021;9[20]:e15071). Eszopiclone has been used to raise the arousal threshold for those who awaken from breathing events too easily. With added time, individuals with a low arousal threshold can more effectively recruit upper airway dilator muscles without waking up. Pharmacotherapy to improve upper airway recruitability with combination noradrenergic stimulation and antimuscarinic activity has limited data thus far but may be a useful part of the sleep armamentarium moving forward.
Summary
OSA is a public health priority, and the current global CPAP shortage emphasizes the need for alternative OSA therapies. The ideal therapy for a given patient requires a careful consideration of their individual traits and will be much more refined when endotyping is available in a routine clinical setting. Individualized sleep apnea treatment is the future of sleep medicine and a one-size fits all approach no longer meets the needs of our patients given the current state of sleep medicine knowledge.
Dr. Nokes, Dr. Schmickl, and Dr. Malhotra are with the University of California, San Diego, Division of Pulmonary, Critical Care, and Sleep Medicine, La, Jolla, CA. Dr. Nokes also is with the Veterans Affairs San Diego Healthcare System, sleep section, San Diego, CA. Dr. Vahabzadeh-Hagh is with the University of California, San Diego, Department of Otolaryngology, San Diego, CA.
From the President: A day in the life
For those of you in the Northern Hemisphere, like me, spring has transitioned into summer, allowing us all to spend more time outdoors, gathering again with family, friends, and colleagues. And, while people are always happy to hear about what’s going on with the family, or how things are going at Emory, the most common question I get is “So what’s it like to be President of CHEST?” Now that I’ve been on the job for 6 months, I thought it was well enough time to pull back the curtain on the role for all of you out in CHEST-land who might be interested, as well. For the purposes of this column, I’ll be incorporating things that occurred over the past week.
As I’ve previously reported, the most important decisions that relate to CHEST strategy are made by the Board of Regents. While I do have the privilege of organizing and running Board meetings, most presidential duties between these meetings focus on communication: with our members, our leaders, and other organizations. One of the best parts of the job is the opportunity to interact with our members; between the [email protected] account and my own, I receive a couple of emails each day with questions about navigating CHEST, or ideas about ways that things might be better accomplished. With our recent Network and section reorganization, many of those questions have focused on leadership opportunities, inquiring about whether the writer should apply, or asking for information about the qualities that might increase the odds of earning a position. My answer is almost always the same: go ahead and throw your name in the hat; for most members, the sections are the first place to start the journey in CHEST leadership. And I’m pleased to say that I’ve had the chance to see some of the members who’ve reached out to me in the past selected for the positions to which they’d applied (in full disclosure, I have little role in selecting leadership positions; Network and section positions are chosen by current members of those Networks and sections). I look forward to watching their progress in our organizational leadership.
While CHEST CEO Robert Musacchio and I communicate almost every day, Wednesday is our weekly meeting during which we review progress on our organizational goals, the status of ongoing projects, and concerns from our membership and leadership. I also have the pleasure of meeting with my co-Presidents every other week; though Jack, Doreen, and Steve have always been happy to offer their counsel on very short notice, this semimonthly meeting helps to provide continuity in leadership, as well as a more formal opportunity for me to meet with trusted advisors to get a sounding board on active issues that affect CHEST. And, this gets to the other main job of CHEST President, which is to facilitate the making of important decisions on behalf of the College. I receive sporadic emails from CHEST staff as we are approached by other organizations or international partners for input on or approval for statements that they wish to make. In the case where the topic is clearly in the CHEST wheelhouse and the statement is consistent with our organizational goals, I can unilaterally sign off; a common example of something that fits in this category is content related to tobacco cessation. In the more frequent situation where the statement for approval is a bit more complex, I will usually refer the request to one of our committee, Network, or section chairs for consideration. Since the turnaround time on these requests is usually pretty short, I may ask them to advise me on their own, although they sometimes opt to run things by their own membership for further input or to achieve consensus.
The CHEST President also serves as ambassador to other organizations; this week, I had the pleasure of participating in a meeting with the American Board of Internal Medicine and a number of medical specialty leaders focused on how professional societies can help to mitigate the spread of medical misinformation. I also interfaced with several of our international partners in the pulmonary space, as they plan their own international meetings, to see how CHEST can contribute to the success of those endeavors by contributing content, speakers, or both. At the time of this writing, the CHEST Congress in Bologna, Italy, is right around the corner, and so I also spent time working with our Italian partners and program co-chair William Kelly, MD, FCCP, to finalize the meeting’s opening session. Though our own international meeting is still months away, work continues with the annual meeting innovations group, and I’ve been working with my own small team on some special surprises that you’ll hear more about in the coming months! The other CHEST meeting-related item on the front burner is the selection of the keynote speaker. The way this works is that I outline in broad strokes a sense of the flavor I’m targeting, and the CHEST staff work with a consulting group to propose some options. They provide me with a brief biography in clips, and we narrow the list down. As I write, we are finalizing our invitation, and I look forward to formally announcing the CHEST 2022 keynote speaker shortly!
After I explain the breadth of duties involved in my role, the most common follow-up question asked of me is whether I am enjoying the position. I’ll concede that it’s not for everyone. There’s a lot less independent decision-making than people assume. But, if you like getting to meet and interact with people from around the globe, helping them to see how CHEST can help them in their pursuits or career goals (and how they can help CHEST in our mission), it’s a super fun job. And I’ll most definitely miss it when I’m done.
So that, in brief, is an overview of what the CHEST President does. But each week is different. And, I get better at the job each day, as I learn something new about the position, the organization, and our outstanding members, leaders, and staff. I look forward to continuing to represent each of you in making decisions and communicating on behalf of CHEST. As always, I remain interested in your input as to how things are going; please consider reaching out to me at [email protected] at your convenience. ... I expect to have a few minutes to write back sometime next Thursday.
Until next time,
David
For those of you in the Northern Hemisphere, like me, spring has transitioned into summer, allowing us all to spend more time outdoors, gathering again with family, friends, and colleagues. And, while people are always happy to hear about what’s going on with the family, or how things are going at Emory, the most common question I get is “So what’s it like to be President of CHEST?” Now that I’ve been on the job for 6 months, I thought it was well enough time to pull back the curtain on the role for all of you out in CHEST-land who might be interested, as well. For the purposes of this column, I’ll be incorporating things that occurred over the past week.
As I’ve previously reported, the most important decisions that relate to CHEST strategy are made by the Board of Regents. While I do have the privilege of organizing and running Board meetings, most presidential duties between these meetings focus on communication: with our members, our leaders, and other organizations. One of the best parts of the job is the opportunity to interact with our members; between the [email protected] account and my own, I receive a couple of emails each day with questions about navigating CHEST, or ideas about ways that things might be better accomplished. With our recent Network and section reorganization, many of those questions have focused on leadership opportunities, inquiring about whether the writer should apply, or asking for information about the qualities that might increase the odds of earning a position. My answer is almost always the same: go ahead and throw your name in the hat; for most members, the sections are the first place to start the journey in CHEST leadership. And I’m pleased to say that I’ve had the chance to see some of the members who’ve reached out to me in the past selected for the positions to which they’d applied (in full disclosure, I have little role in selecting leadership positions; Network and section positions are chosen by current members of those Networks and sections). I look forward to watching their progress in our organizational leadership.
While CHEST CEO Robert Musacchio and I communicate almost every day, Wednesday is our weekly meeting during which we review progress on our organizational goals, the status of ongoing projects, and concerns from our membership and leadership. I also have the pleasure of meeting with my co-Presidents every other week; though Jack, Doreen, and Steve have always been happy to offer their counsel on very short notice, this semimonthly meeting helps to provide continuity in leadership, as well as a more formal opportunity for me to meet with trusted advisors to get a sounding board on active issues that affect CHEST. And, this gets to the other main job of CHEST President, which is to facilitate the making of important decisions on behalf of the College. I receive sporadic emails from CHEST staff as we are approached by other organizations or international partners for input on or approval for statements that they wish to make. In the case where the topic is clearly in the CHEST wheelhouse and the statement is consistent with our organizational goals, I can unilaterally sign off; a common example of something that fits in this category is content related to tobacco cessation. In the more frequent situation where the statement for approval is a bit more complex, I will usually refer the request to one of our committee, Network, or section chairs for consideration. Since the turnaround time on these requests is usually pretty short, I may ask them to advise me on their own, although they sometimes opt to run things by their own membership for further input or to achieve consensus.
The CHEST President also serves as ambassador to other organizations; this week, I had the pleasure of participating in a meeting with the American Board of Internal Medicine and a number of medical specialty leaders focused on how professional societies can help to mitigate the spread of medical misinformation. I also interfaced with several of our international partners in the pulmonary space, as they plan their own international meetings, to see how CHEST can contribute to the success of those endeavors by contributing content, speakers, or both. At the time of this writing, the CHEST Congress in Bologna, Italy, is right around the corner, and so I also spent time working with our Italian partners and program co-chair William Kelly, MD, FCCP, to finalize the meeting’s opening session. Though our own international meeting is still months away, work continues with the annual meeting innovations group, and I’ve been working with my own small team on some special surprises that you’ll hear more about in the coming months! The other CHEST meeting-related item on the front burner is the selection of the keynote speaker. The way this works is that I outline in broad strokes a sense of the flavor I’m targeting, and the CHEST staff work with a consulting group to propose some options. They provide me with a brief biography in clips, and we narrow the list down. As I write, we are finalizing our invitation, and I look forward to formally announcing the CHEST 2022 keynote speaker shortly!
After I explain the breadth of duties involved in my role, the most common follow-up question asked of me is whether I am enjoying the position. I’ll concede that it’s not for everyone. There’s a lot less independent decision-making than people assume. But, if you like getting to meet and interact with people from around the globe, helping them to see how CHEST can help them in their pursuits or career goals (and how they can help CHEST in our mission), it’s a super fun job. And I’ll most definitely miss it when I’m done.
So that, in brief, is an overview of what the CHEST President does. But each week is different. And, I get better at the job each day, as I learn something new about the position, the organization, and our outstanding members, leaders, and staff. I look forward to continuing to represent each of you in making decisions and communicating on behalf of CHEST. As always, I remain interested in your input as to how things are going; please consider reaching out to me at [email protected] at your convenience. ... I expect to have a few minutes to write back sometime next Thursday.
Until next time,
David
For those of you in the Northern Hemisphere, like me, spring has transitioned into summer, allowing us all to spend more time outdoors, gathering again with family, friends, and colleagues. And, while people are always happy to hear about what’s going on with the family, or how things are going at Emory, the most common question I get is “So what’s it like to be President of CHEST?” Now that I’ve been on the job for 6 months, I thought it was well enough time to pull back the curtain on the role for all of you out in CHEST-land who might be interested, as well. For the purposes of this column, I’ll be incorporating things that occurred over the past week.
As I’ve previously reported, the most important decisions that relate to CHEST strategy are made by the Board of Regents. While I do have the privilege of organizing and running Board meetings, most presidential duties between these meetings focus on communication: with our members, our leaders, and other organizations. One of the best parts of the job is the opportunity to interact with our members; between the [email protected] account and my own, I receive a couple of emails each day with questions about navigating CHEST, or ideas about ways that things might be better accomplished. With our recent Network and section reorganization, many of those questions have focused on leadership opportunities, inquiring about whether the writer should apply, or asking for information about the qualities that might increase the odds of earning a position. My answer is almost always the same: go ahead and throw your name in the hat; for most members, the sections are the first place to start the journey in CHEST leadership. And I’m pleased to say that I’ve had the chance to see some of the members who’ve reached out to me in the past selected for the positions to which they’d applied (in full disclosure, I have little role in selecting leadership positions; Network and section positions are chosen by current members of those Networks and sections). I look forward to watching their progress in our organizational leadership.
While CHEST CEO Robert Musacchio and I communicate almost every day, Wednesday is our weekly meeting during which we review progress on our organizational goals, the status of ongoing projects, and concerns from our membership and leadership. I also have the pleasure of meeting with my co-Presidents every other week; though Jack, Doreen, and Steve have always been happy to offer their counsel on very short notice, this semimonthly meeting helps to provide continuity in leadership, as well as a more formal opportunity for me to meet with trusted advisors to get a sounding board on active issues that affect CHEST. And, this gets to the other main job of CHEST President, which is to facilitate the making of important decisions on behalf of the College. I receive sporadic emails from CHEST staff as we are approached by other organizations or international partners for input on or approval for statements that they wish to make. In the case where the topic is clearly in the CHEST wheelhouse and the statement is consistent with our organizational goals, I can unilaterally sign off; a common example of something that fits in this category is content related to tobacco cessation. In the more frequent situation where the statement for approval is a bit more complex, I will usually refer the request to one of our committee, Network, or section chairs for consideration. Since the turnaround time on these requests is usually pretty short, I may ask them to advise me on their own, although they sometimes opt to run things by their own membership for further input or to achieve consensus.
The CHEST President also serves as ambassador to other organizations; this week, I had the pleasure of participating in a meeting with the American Board of Internal Medicine and a number of medical specialty leaders focused on how professional societies can help to mitigate the spread of medical misinformation. I also interfaced with several of our international partners in the pulmonary space, as they plan their own international meetings, to see how CHEST can contribute to the success of those endeavors by contributing content, speakers, or both. At the time of this writing, the CHEST Congress in Bologna, Italy, is right around the corner, and so I also spent time working with our Italian partners and program co-chair William Kelly, MD, FCCP, to finalize the meeting’s opening session. Though our own international meeting is still months away, work continues with the annual meeting innovations group, and I’ve been working with my own small team on some special surprises that you’ll hear more about in the coming months! The other CHEST meeting-related item on the front burner is the selection of the keynote speaker. The way this works is that I outline in broad strokes a sense of the flavor I’m targeting, and the CHEST staff work with a consulting group to propose some options. They provide me with a brief biography in clips, and we narrow the list down. As I write, we are finalizing our invitation, and I look forward to formally announcing the CHEST 2022 keynote speaker shortly!
After I explain the breadth of duties involved in my role, the most common follow-up question asked of me is whether I am enjoying the position. I’ll concede that it’s not for everyone. There’s a lot less independent decision-making than people assume. But, if you like getting to meet and interact with people from around the globe, helping them to see how CHEST can help them in their pursuits or career goals (and how they can help CHEST in our mission), it’s a super fun job. And I’ll most definitely miss it when I’m done.
So that, in brief, is an overview of what the CHEST President does. But each week is different. And, I get better at the job each day, as I learn something new about the position, the organization, and our outstanding members, leaders, and staff. I look forward to continuing to represent each of you in making decisions and communicating on behalf of CHEST. As always, I remain interested in your input as to how things are going; please consider reaching out to me at [email protected] at your convenience. ... I expect to have a few minutes to write back sometime next Thursday.
Until next time,
David
And, they’re off! Belmont Stakes Dinner and Auction fundraises for patient education
For a night of fun and philanthropy, CHEST leadership and supporters of the CHEST Foundation came together in New York City to watch the Belmont Stakes race and raise money to support patient education.
What started 8 years ago as a brunch in the living room of Doreen Addrizzo-Harris, MD, FCCP, to support the initiatives of the CHEST Foundation.
Spearheaded by Dr. Addrizzo- Harris, President-Elect of the American College of Chest Physicians, this year’s event was focused entirely on patient education and advocacy. The attendees heard the moving stories of Betsy Glaeser and Fred Schick who are both patients living with lung disease and advocates for others living with like afflictions. Betsy is living with nontuberculous mycobacteria (NTM) disease and Fred with idiopathic pulmonary fibrosis (IPF). Betsy and Fred have used their experiences to serve as support for others in similar positions.
Betsy Glaeser, a longtime patient of Dr. Addrizzo-Harris, shared a story about the struggle of being one of the first cases of NTM bronchiectasis and helping to define the course of action. She shared that her original doctors gave her 5 years to live. The room erupted in applause when she shared that with the exceptional treatment she’s received, over 20 years later, she is standing in front of the supporters of the CHEST Foundation to share her story.
Because of the rarity of her disease, she was hospitalized multiple times with pneumonia before finally reaching her diagnosis of NTM disease. She channeled the accompanying frustrations into helping others who were recently diagnosed with the NTM disorder by sharing her experiences. “I would give them guidance on treatment options because in my years of living with the disease, I’d been there and tried almost everything,” said Betsy. “I would get calls from my doctor all of the time to speak with someone who just received an NTM disease diagnosis. I was happy to do so – at the time, the Internet didn’t exist, and firsthand experiences were all we had. Since forming our physical support group, the most memorable experience I can recall is when a woman, newly diagnosed with NTM, walked into the room and immediately burst into tears. She shared that she expected to see all of us on oxygen and wheelchair-bound, but that wasn’t the case at all. That day, we were able to give her hope. That’s why I do what I do, and I’m proud to do it.”
Fred Schick shared with the attendees his story of struggling to find his IPF diagnosis and how incredibly frustrating it was to be so short of breath that he needed to be rescued from the water while on vacation. With a history of cardiac complications, Fred’s doctors were looking at his heart.
For Fred to get to his IPF diagnosis, it took the careful ear of a primary care doctor Fred started to see when his previous doctor retired.
“It was almost like she was listening with different ears and was hearing what others didn’t. Once she recommended speaking with a pulmonologist, everything fell into place,” said Fred. “From my experience, IPF is best treated by a lot of pieces coming together and working together. It takes the dedication of a care team in the hospital, proper diet and exercise and, just as importantly, it takes a support group to guide you through the process. I’m grateful to my care team, but I’m equally thankful for the work I get to do as an advocate for others living with IPF.”
When she spoke to the attendees, Lisa Moores, MD, FCCP, reflected on what the patients shared. “We saw great examples of why we’re here tonight,” said Dr. Moores. “One of the things CHEST and the Foundation are focusing on is earlier diagnosis for interstitial lung diseases like pulmonary fibrosis and, with voices like Fred Schick, we’ll get there. The patients remind us why we’re here. We’re here for our patients; we’re here for Fred; and we’re here for Betsy.”
Laurence Feldman, Vice President of the Feldman Family Foundation that partners with the CHEST Foundation for their annual casino fundraiser benefiting pulmonary fibrosis, was able to participate in the dinner and theauction.
He shared, “Tonight, I was so impressed with the generosity of the attendees and the organization of this event. It reminded me that if you ask your supporters to give, they’ll be there for you. Almost like the ‘Field of Dreams’ quote of ‘if you build it, they will come.’ Being at the Belmont Stakes Dinner and Auction makes me that much more excited for our upcoming Irv Feldman Casino Night and Texas Hold ‘Em Tournament coming up in late August. Thanks to our corporate partners and the support of the CHEST Foundation, we’re able to produce an excellent event like the Belmont Stakes fundraiser that helps bring in donations that can make a difference in the lives of patients.”
At the end of the day, medicine is all about the patients and, by dedicating the night to patient education and patient advocates, the Belmont Stakes event brought the focus to where it should always be – improving care and helping patients.
To learn more and to support the various initiatives of the CHEST Foundation, visit foundation.chestnet.org/donate.
For a night of fun and philanthropy, CHEST leadership and supporters of the CHEST Foundation came together in New York City to watch the Belmont Stakes race and raise money to support patient education.
What started 8 years ago as a brunch in the living room of Doreen Addrizzo-Harris, MD, FCCP, to support the initiatives of the CHEST Foundation.
Spearheaded by Dr. Addrizzo- Harris, President-Elect of the American College of Chest Physicians, this year’s event was focused entirely on patient education and advocacy. The attendees heard the moving stories of Betsy Glaeser and Fred Schick who are both patients living with lung disease and advocates for others living with like afflictions. Betsy is living with nontuberculous mycobacteria (NTM) disease and Fred with idiopathic pulmonary fibrosis (IPF). Betsy and Fred have used their experiences to serve as support for others in similar positions.
Betsy Glaeser, a longtime patient of Dr. Addrizzo-Harris, shared a story about the struggle of being one of the first cases of NTM bronchiectasis and helping to define the course of action. She shared that her original doctors gave her 5 years to live. The room erupted in applause when she shared that with the exceptional treatment she’s received, over 20 years later, she is standing in front of the supporters of the CHEST Foundation to share her story.
Because of the rarity of her disease, she was hospitalized multiple times with pneumonia before finally reaching her diagnosis of NTM disease. She channeled the accompanying frustrations into helping others who were recently diagnosed with the NTM disorder by sharing her experiences. “I would give them guidance on treatment options because in my years of living with the disease, I’d been there and tried almost everything,” said Betsy. “I would get calls from my doctor all of the time to speak with someone who just received an NTM disease diagnosis. I was happy to do so – at the time, the Internet didn’t exist, and firsthand experiences were all we had. Since forming our physical support group, the most memorable experience I can recall is when a woman, newly diagnosed with NTM, walked into the room and immediately burst into tears. She shared that she expected to see all of us on oxygen and wheelchair-bound, but that wasn’t the case at all. That day, we were able to give her hope. That’s why I do what I do, and I’m proud to do it.”
Fred Schick shared with the attendees his story of struggling to find his IPF diagnosis and how incredibly frustrating it was to be so short of breath that he needed to be rescued from the water while on vacation. With a history of cardiac complications, Fred’s doctors were looking at his heart.
For Fred to get to his IPF diagnosis, it took the careful ear of a primary care doctor Fred started to see when his previous doctor retired.
“It was almost like she was listening with different ears and was hearing what others didn’t. Once she recommended speaking with a pulmonologist, everything fell into place,” said Fred. “From my experience, IPF is best treated by a lot of pieces coming together and working together. It takes the dedication of a care team in the hospital, proper diet and exercise and, just as importantly, it takes a support group to guide you through the process. I’m grateful to my care team, but I’m equally thankful for the work I get to do as an advocate for others living with IPF.”
When she spoke to the attendees, Lisa Moores, MD, FCCP, reflected on what the patients shared. “We saw great examples of why we’re here tonight,” said Dr. Moores. “One of the things CHEST and the Foundation are focusing on is earlier diagnosis for interstitial lung diseases like pulmonary fibrosis and, with voices like Fred Schick, we’ll get there. The patients remind us why we’re here. We’re here for our patients; we’re here for Fred; and we’re here for Betsy.”
Laurence Feldman, Vice President of the Feldman Family Foundation that partners with the CHEST Foundation for their annual casino fundraiser benefiting pulmonary fibrosis, was able to participate in the dinner and theauction.
He shared, “Tonight, I was so impressed with the generosity of the attendees and the organization of this event. It reminded me that if you ask your supporters to give, they’ll be there for you. Almost like the ‘Field of Dreams’ quote of ‘if you build it, they will come.’ Being at the Belmont Stakes Dinner and Auction makes me that much more excited for our upcoming Irv Feldman Casino Night and Texas Hold ‘Em Tournament coming up in late August. Thanks to our corporate partners and the support of the CHEST Foundation, we’re able to produce an excellent event like the Belmont Stakes fundraiser that helps bring in donations that can make a difference in the lives of patients.”
At the end of the day, medicine is all about the patients and, by dedicating the night to patient education and patient advocates, the Belmont Stakes event brought the focus to where it should always be – improving care and helping patients.
To learn more and to support the various initiatives of the CHEST Foundation, visit foundation.chestnet.org/donate.
For a night of fun and philanthropy, CHEST leadership and supporters of the CHEST Foundation came together in New York City to watch the Belmont Stakes race and raise money to support patient education.
What started 8 years ago as a brunch in the living room of Doreen Addrizzo-Harris, MD, FCCP, to support the initiatives of the CHEST Foundation.
Spearheaded by Dr. Addrizzo- Harris, President-Elect of the American College of Chest Physicians, this year’s event was focused entirely on patient education and advocacy. The attendees heard the moving stories of Betsy Glaeser and Fred Schick who are both patients living with lung disease and advocates for others living with like afflictions. Betsy is living with nontuberculous mycobacteria (NTM) disease and Fred with idiopathic pulmonary fibrosis (IPF). Betsy and Fred have used their experiences to serve as support for others in similar positions.
Betsy Glaeser, a longtime patient of Dr. Addrizzo-Harris, shared a story about the struggle of being one of the first cases of NTM bronchiectasis and helping to define the course of action. She shared that her original doctors gave her 5 years to live. The room erupted in applause when she shared that with the exceptional treatment she’s received, over 20 years later, she is standing in front of the supporters of the CHEST Foundation to share her story.
Because of the rarity of her disease, she was hospitalized multiple times with pneumonia before finally reaching her diagnosis of NTM disease. She channeled the accompanying frustrations into helping others who were recently diagnosed with the NTM disorder by sharing her experiences. “I would give them guidance on treatment options because in my years of living with the disease, I’d been there and tried almost everything,” said Betsy. “I would get calls from my doctor all of the time to speak with someone who just received an NTM disease diagnosis. I was happy to do so – at the time, the Internet didn’t exist, and firsthand experiences were all we had. Since forming our physical support group, the most memorable experience I can recall is when a woman, newly diagnosed with NTM, walked into the room and immediately burst into tears. She shared that she expected to see all of us on oxygen and wheelchair-bound, but that wasn’t the case at all. That day, we were able to give her hope. That’s why I do what I do, and I’m proud to do it.”
Fred Schick shared with the attendees his story of struggling to find his IPF diagnosis and how incredibly frustrating it was to be so short of breath that he needed to be rescued from the water while on vacation. With a history of cardiac complications, Fred’s doctors were looking at his heart.
For Fred to get to his IPF diagnosis, it took the careful ear of a primary care doctor Fred started to see when his previous doctor retired.
“It was almost like she was listening with different ears and was hearing what others didn’t. Once she recommended speaking with a pulmonologist, everything fell into place,” said Fred. “From my experience, IPF is best treated by a lot of pieces coming together and working together. It takes the dedication of a care team in the hospital, proper diet and exercise and, just as importantly, it takes a support group to guide you through the process. I’m grateful to my care team, but I’m equally thankful for the work I get to do as an advocate for others living with IPF.”
When she spoke to the attendees, Lisa Moores, MD, FCCP, reflected on what the patients shared. “We saw great examples of why we’re here tonight,” said Dr. Moores. “One of the things CHEST and the Foundation are focusing on is earlier diagnosis for interstitial lung diseases like pulmonary fibrosis and, with voices like Fred Schick, we’ll get there. The patients remind us why we’re here. We’re here for our patients; we’re here for Fred; and we’re here for Betsy.”
Laurence Feldman, Vice President of the Feldman Family Foundation that partners with the CHEST Foundation for their annual casino fundraiser benefiting pulmonary fibrosis, was able to participate in the dinner and theauction.
He shared, “Tonight, I was so impressed with the generosity of the attendees and the organization of this event. It reminded me that if you ask your supporters to give, they’ll be there for you. Almost like the ‘Field of Dreams’ quote of ‘if you build it, they will come.’ Being at the Belmont Stakes Dinner and Auction makes me that much more excited for our upcoming Irv Feldman Casino Night and Texas Hold ‘Em Tournament coming up in late August. Thanks to our corporate partners and the support of the CHEST Foundation, we’re able to produce an excellent event like the Belmont Stakes fundraiser that helps bring in donations that can make a difference in the lives of patients.”
At the end of the day, medicine is all about the patients and, by dedicating the night to patient education and patient advocates, the Belmont Stakes event brought the focus to where it should always be – improving care and helping patients.
To learn more and to support the various initiatives of the CHEST Foundation, visit foundation.chestnet.org/donate.
Diffuse Lung Disease & Transplant Network
Pulmonary Physiology & Rehabilitation Section
Interpretive strategies for routine lung function tests
In December 2021, the European Respiratory Journal published the, ERS/ATS technical standard on interpretive strategies for routine lung function tests (Stanojevic S, et al. Eur Respir J. 2021 Dec 23;2101499). Briefly, a few of the updated recommendations are discussed here.
First, the task force recommends the use of Global Lung Initiative (GLI) reference values for spirometry, lung volumes, and diffusing capacity of carbon monoxide. GLI equations were derived from the largest sample of healthy individuals to date and provide an internal consistency across all ages.
Second, it is now recommended that z-scores are used as opposed to percent predicted in grading severity of impairment. Z-scores, which refer to the number of standard deviations a measurement is positioned from the predicted value, centered at zero, account better for age, sex, and height biases compared with percent predicted, and is simplified into mild (z-score -1.65 to -2.5), moderate (-2.51 to -4), and severe (< -4) categories.
A bronchodilator response is now defined as a > 10% change from the predicted value in FEV1 or FVC while the concept of a conditional change score in children and FEV1Q in adults has been introduced to describe lung function change.
The recommendations reflect and reiterate a shift in reporting a range of values, rather than using absolute threshold values, with an emphasis on the classification of physiologic impairments. The uncertainty present as lung function approaches the lower limit of normal is acknowledged, emphasizing the importance of pretest probability in making a clinical diagnosis and/or clinical decision. We encourage all pulmonary clinicians to review this important paper for more detailed information on these changes.
Tom DeCato, MD, Vice-Chair
Gina Lee, MD, Member-at-Large
Pulmonary Physiology & Rehabilitation Section
Interpretive strategies for routine lung function tests
In December 2021, the European Respiratory Journal published the, ERS/ATS technical standard on interpretive strategies for routine lung function tests (Stanojevic S, et al. Eur Respir J. 2021 Dec 23;2101499). Briefly, a few of the updated recommendations are discussed here.
First, the task force recommends the use of Global Lung Initiative (GLI) reference values for spirometry, lung volumes, and diffusing capacity of carbon monoxide. GLI equations were derived from the largest sample of healthy individuals to date and provide an internal consistency across all ages.
Second, it is now recommended that z-scores are used as opposed to percent predicted in grading severity of impairment. Z-scores, which refer to the number of standard deviations a measurement is positioned from the predicted value, centered at zero, account better for age, sex, and height biases compared with percent predicted, and is simplified into mild (z-score -1.65 to -2.5), moderate (-2.51 to -4), and severe (< -4) categories.
A bronchodilator response is now defined as a > 10% change from the predicted value in FEV1 or FVC while the concept of a conditional change score in children and FEV1Q in adults has been introduced to describe lung function change.
The recommendations reflect and reiterate a shift in reporting a range of values, rather than using absolute threshold values, with an emphasis on the classification of physiologic impairments. The uncertainty present as lung function approaches the lower limit of normal is acknowledged, emphasizing the importance of pretest probability in making a clinical diagnosis and/or clinical decision. We encourage all pulmonary clinicians to review this important paper for more detailed information on these changes.
Tom DeCato, MD, Vice-Chair
Gina Lee, MD, Member-at-Large
Pulmonary Physiology & Rehabilitation Section
Interpretive strategies for routine lung function tests
In December 2021, the European Respiratory Journal published the, ERS/ATS technical standard on interpretive strategies for routine lung function tests (Stanojevic S, et al. Eur Respir J. 2021 Dec 23;2101499). Briefly, a few of the updated recommendations are discussed here.
First, the task force recommends the use of Global Lung Initiative (GLI) reference values for spirometry, lung volumes, and diffusing capacity of carbon monoxide. GLI equations were derived from the largest sample of healthy individuals to date and provide an internal consistency across all ages.
Second, it is now recommended that z-scores are used as opposed to percent predicted in grading severity of impairment. Z-scores, which refer to the number of standard deviations a measurement is positioned from the predicted value, centered at zero, account better for age, sex, and height biases compared with percent predicted, and is simplified into mild (z-score -1.65 to -2.5), moderate (-2.51 to -4), and severe (< -4) categories.
A bronchodilator response is now defined as a > 10% change from the predicted value in FEV1 or FVC while the concept of a conditional change score in children and FEV1Q in adults has been introduced to describe lung function change.
The recommendations reflect and reiterate a shift in reporting a range of values, rather than using absolute threshold values, with an emphasis on the classification of physiologic impairments. The uncertainty present as lung function approaches the lower limit of normal is acknowledged, emphasizing the importance of pretest probability in making a clinical diagnosis and/or clinical decision. We encourage all pulmonary clinicians to review this important paper for more detailed information on these changes.
Tom DeCato, MD, Vice-Chair
Gina Lee, MD, Member-at-Large














