User login
Ultrasound’s value for arthralgia may be to rule out IA
Ultrasound evaluations to look for subclinical inflammation in joints of patients with arthralgia appear best at ruling out inflammatory arthritis (IA) 1 year in the future rather than ruling it in, according to findings from a multicenter cohort study published online in Arthritis Research and Therapy.
The imaging modality’s ability to identify those who will not go on to develop IA complemented the serologic and clinical factors that help to discriminate the individuals with arthralgia who are most at risk of the condition.
The ultimate goal of using imaging such as ultrasound in patients with arthralgia is to identify those who would benefit from starting treatment with disease-modifying antirheumatic drugs as early as possible to potentially improve outcomes, but it also could help to discriminate between the anti-citrullinated protein antibody (ACPA)-positive and seronegative individuals without clinical signs of inflammation at baseline who may progress from arthralgia to IA.
“Although ACPA positivity is a very good predictor for those patients who will develop IA within 1 year, it is still difficult to identify the exact individuals who will develop IA, because any ACPA-positive individual has an a priori chance of 50% of developing IA. In seronegative patients, the prediction of IA is even more difficult, because only 5% develop IA within the subsequent year. Imaging techniques have been shown to be able to detect synovitis before its clinical appearance and could be of help in identifying those at risk of IA,” the investigators wrote (Arthritis Res Ther. 2017;19:202. doi: 10.1186/s13075-017-1405-y).
Dr. van der Ven and her associates found that 31 (16%) of 196 patients who had arthralgia for less than 1 year in the hands, feet, or shoulders went on to develop IA after 1 year of follow-up. In this group of 196 patients at baseline, 72 (37%) had synovitis on ultrasound – defined as a greyscale grade of 2 or 3 and/or the presence of power Doppler signal (grade 1, 2, or 3) – including 32 with a positive power Doppler. A total of 18 patients were lost to follow-up during the first 6 months and another 19 were lost during months 6-12.
Rheumatologists who were unaware of ultrasound findings had to confirm soft-tissue swelling as arthritis at 1 year to classify it as incident IA. The positive predictive value of ultrasound for IA was only 26% when at least 1 joint out of 26 assessed was positive, but the negative predictive value when no joints were positive on ultrasound was 89%.
Overall, at 1 year, 15 of the 31 patients with IA had started therapy with a disease-modifying antirheumatic drug and 22 did not have a definite diagnosis; 12 had monoarthritis and 10 had polyarthritis. The remaining nine patients included four with rheumatoid arthritis, four with psoriatic arthritis, and one with spondyloarthritis.
At baseline, individuals with IA were more often older (mean age 50 vs. 44 years; P = .005), had synovitis on ultrasound (59% vs. 32%; P = .007), and had a positive power Doppler signal (31% vs. 12%; P = .012). A multivariate analysis revealed that IA at 1 year of follow-up could be independently predicted according to age (odds ratio, 1.06), morning stiffness lasting more than 30 minutes (OR, 2.80), ACPA positivity (OR, 2.35), and synovitis on ultrasound (OR, 2.65).
The investigators noted that the study’s limitations relate to requirements for patients to have at least two painful joints in hands, feet, or shoulders at baseline and two criteria related to inflammation. The possible inflammation-related criteria required for entry included morning stiffness for more than 1 hour, inability to clench a fist in the morning, pain when shaking someone’s hand, pins and needles in the fingers, difficulties wearing rings or shoes, family history of rheumatoid arthritis, and/or unexplained fatigue for less than 1 year.
Rheumatologists who enrolled patients into the cohort also may have “recruited clinically suspected patients with possibly more severe symptoms,” the investigators noted. Another potential source of bias related to the group of 38 patients who chose not to participate: It’s possible these patients had less severe symptoms than those who participated in the study.
The study was funded by an investigator-initiated grant from Pfizer. The authors declared that they have no competing interests.
Ultrasound evaluations to look for subclinical inflammation in joints of patients with arthralgia appear best at ruling out inflammatory arthritis (IA) 1 year in the future rather than ruling it in, according to findings from a multicenter cohort study published online in Arthritis Research and Therapy.
The imaging modality’s ability to identify those who will not go on to develop IA complemented the serologic and clinical factors that help to discriminate the individuals with arthralgia who are most at risk of the condition.
The ultimate goal of using imaging such as ultrasound in patients with arthralgia is to identify those who would benefit from starting treatment with disease-modifying antirheumatic drugs as early as possible to potentially improve outcomes, but it also could help to discriminate between the anti-citrullinated protein antibody (ACPA)-positive and seronegative individuals without clinical signs of inflammation at baseline who may progress from arthralgia to IA.
“Although ACPA positivity is a very good predictor for those patients who will develop IA within 1 year, it is still difficult to identify the exact individuals who will develop IA, because any ACPA-positive individual has an a priori chance of 50% of developing IA. In seronegative patients, the prediction of IA is even more difficult, because only 5% develop IA within the subsequent year. Imaging techniques have been shown to be able to detect synovitis before its clinical appearance and could be of help in identifying those at risk of IA,” the investigators wrote (Arthritis Res Ther. 2017;19:202. doi: 10.1186/s13075-017-1405-y).
Dr. van der Ven and her associates found that 31 (16%) of 196 patients who had arthralgia for less than 1 year in the hands, feet, or shoulders went on to develop IA after 1 year of follow-up. In this group of 196 patients at baseline, 72 (37%) had synovitis on ultrasound – defined as a greyscale grade of 2 or 3 and/or the presence of power Doppler signal (grade 1, 2, or 3) – including 32 with a positive power Doppler. A total of 18 patients were lost to follow-up during the first 6 months and another 19 were lost during months 6-12.
Rheumatologists who were unaware of ultrasound findings had to confirm soft-tissue swelling as arthritis at 1 year to classify it as incident IA. The positive predictive value of ultrasound for IA was only 26% when at least 1 joint out of 26 assessed was positive, but the negative predictive value when no joints were positive on ultrasound was 89%.
Overall, at 1 year, 15 of the 31 patients with IA had started therapy with a disease-modifying antirheumatic drug and 22 did not have a definite diagnosis; 12 had monoarthritis and 10 had polyarthritis. The remaining nine patients included four with rheumatoid arthritis, four with psoriatic arthritis, and one with spondyloarthritis.
At baseline, individuals with IA were more often older (mean age 50 vs. 44 years; P = .005), had synovitis on ultrasound (59% vs. 32%; P = .007), and had a positive power Doppler signal (31% vs. 12%; P = .012). A multivariate analysis revealed that IA at 1 year of follow-up could be independently predicted according to age (odds ratio, 1.06), morning stiffness lasting more than 30 minutes (OR, 2.80), ACPA positivity (OR, 2.35), and synovitis on ultrasound (OR, 2.65).
The investigators noted that the study’s limitations relate to requirements for patients to have at least two painful joints in hands, feet, or shoulders at baseline and two criteria related to inflammation. The possible inflammation-related criteria required for entry included morning stiffness for more than 1 hour, inability to clench a fist in the morning, pain when shaking someone’s hand, pins and needles in the fingers, difficulties wearing rings or shoes, family history of rheumatoid arthritis, and/or unexplained fatigue for less than 1 year.
Rheumatologists who enrolled patients into the cohort also may have “recruited clinically suspected patients with possibly more severe symptoms,” the investigators noted. Another potential source of bias related to the group of 38 patients who chose not to participate: It’s possible these patients had less severe symptoms than those who participated in the study.
The study was funded by an investigator-initiated grant from Pfizer. The authors declared that they have no competing interests.
Ultrasound evaluations to look for subclinical inflammation in joints of patients with arthralgia appear best at ruling out inflammatory arthritis (IA) 1 year in the future rather than ruling it in, according to findings from a multicenter cohort study published online in Arthritis Research and Therapy.
The imaging modality’s ability to identify those who will not go on to develop IA complemented the serologic and clinical factors that help to discriminate the individuals with arthralgia who are most at risk of the condition.
The ultimate goal of using imaging such as ultrasound in patients with arthralgia is to identify those who would benefit from starting treatment with disease-modifying antirheumatic drugs as early as possible to potentially improve outcomes, but it also could help to discriminate between the anti-citrullinated protein antibody (ACPA)-positive and seronegative individuals without clinical signs of inflammation at baseline who may progress from arthralgia to IA.
“Although ACPA positivity is a very good predictor for those patients who will develop IA within 1 year, it is still difficult to identify the exact individuals who will develop IA, because any ACPA-positive individual has an a priori chance of 50% of developing IA. In seronegative patients, the prediction of IA is even more difficult, because only 5% develop IA within the subsequent year. Imaging techniques have been shown to be able to detect synovitis before its clinical appearance and could be of help in identifying those at risk of IA,” the investigators wrote (Arthritis Res Ther. 2017;19:202. doi: 10.1186/s13075-017-1405-y).
Dr. van der Ven and her associates found that 31 (16%) of 196 patients who had arthralgia for less than 1 year in the hands, feet, or shoulders went on to develop IA after 1 year of follow-up. In this group of 196 patients at baseline, 72 (37%) had synovitis on ultrasound – defined as a greyscale grade of 2 or 3 and/or the presence of power Doppler signal (grade 1, 2, or 3) – including 32 with a positive power Doppler. A total of 18 patients were lost to follow-up during the first 6 months and another 19 were lost during months 6-12.
Rheumatologists who were unaware of ultrasound findings had to confirm soft-tissue swelling as arthritis at 1 year to classify it as incident IA. The positive predictive value of ultrasound for IA was only 26% when at least 1 joint out of 26 assessed was positive, but the negative predictive value when no joints were positive on ultrasound was 89%.
Overall, at 1 year, 15 of the 31 patients with IA had started therapy with a disease-modifying antirheumatic drug and 22 did not have a definite diagnosis; 12 had monoarthritis and 10 had polyarthritis. The remaining nine patients included four with rheumatoid arthritis, four with psoriatic arthritis, and one with spondyloarthritis.
At baseline, individuals with IA were more often older (mean age 50 vs. 44 years; P = .005), had synovitis on ultrasound (59% vs. 32%; P = .007), and had a positive power Doppler signal (31% vs. 12%; P = .012). A multivariate analysis revealed that IA at 1 year of follow-up could be independently predicted according to age (odds ratio, 1.06), morning stiffness lasting more than 30 minutes (OR, 2.80), ACPA positivity (OR, 2.35), and synovitis on ultrasound (OR, 2.65).
The investigators noted that the study’s limitations relate to requirements for patients to have at least two painful joints in hands, feet, or shoulders at baseline and two criteria related to inflammation. The possible inflammation-related criteria required for entry included morning stiffness for more than 1 hour, inability to clench a fist in the morning, pain when shaking someone’s hand, pins and needles in the fingers, difficulties wearing rings or shoes, family history of rheumatoid arthritis, and/or unexplained fatigue for less than 1 year.
Rheumatologists who enrolled patients into the cohort also may have “recruited clinically suspected patients with possibly more severe symptoms,” the investigators noted. Another potential source of bias related to the group of 38 patients who chose not to participate: It’s possible these patients had less severe symptoms than those who participated in the study.
The study was funded by an investigator-initiated grant from Pfizer. The authors declared that they have no competing interests.
FROM ARTHRITIS RESEARCH AND THERAPY
Key clinical point:
Major finding: The positive predictive value of ultrasound for IA was only 26% when at least 1 joint out of 26 assessed was positive, but the negative predictive value when no joints were positive on ultrasound was 89%.
Data source: A multicenter cohort study of 196 patients with arthralgia in at least two joints for less than 1 year.
Disclosures: The study was funded by an investigator-initiated grant from Pfizer. The authors declared that they have no competing interests.
New recommendations tout biosimilars for rheumatologic diseases
The available evidence is sufficient to support switching appropriate patients with rheumatologic diseases from a bio-originator agent to an approved biosimilar agent, according to new consensus-based recommendations from an international multidisciplinary task force.
“Treatment with biological agents has dramatically improved the outcome for patients with inflammatory diseases. However, the high cost of these medications has limited access for many patients,” Jonathan Kay, MD, of UMass Memorial Medical Center and the University of Massachusetts, Worcester, and his colleagues wrote on behalf of the Task Force on the Use of Biosimilars to Treat Rheumatological Diseases. Biosimilars of agents no longer protected by patent allow for increased availability at lower costs, they noted. In the European Union, the United States, Japan, and other countries, biosimilars of adalimumab, etanercept, infliximab, and rituximab have been approved, and those for which the bio-originator is no longer protected by patent have been marketed.
The task force, convened in 2016 to address the matter at an international level, included 25 experts from Europe, Japan, and the United States, including 17 rheumatologists, a rheumatologist/regulator, a dermatologist, a gastroenterologist, 2 pharmacologists, 2 patients, and a research fellow. The task force identified five overarching principles and made eight specific recommendations, based on expert opinion and an extensive literature review that yielded 29 relevant full-text papers and 20 relevant abstracts from the 2015 and 2016 American College of Rheumatology and European League Against Rheumatism annual meetings (Ann Rheum Dis. 2017 Sep 2. doi: 10.1136/annrheumdis-2017-211937).
“This statement was intended both to guide clinicians and to serve as a framework for future educational efforts,” they wrote.
The experts based all five overarching principles for the use of biosimilars on level 5, grade D evidence, indicating that they were derived mainly from expert opinion. They determined that:
- Treatment of rheumatic diseases is based on a shared decision-making process between patients and their rheumatologists.
- The contextual aspects of the health care system should be taken into consideration when treatment decisions are made.
- A biosimilar, as approved by authorities in a highly regulated area, is neither better nor worse in efficacy and is not inferior in safety to its bio-originator.
- Patients and health care providers should be informed about the nature of biosimilars, their approval process, and their safety and efficacy.
- Harmonized methods should be established to obtain reliable pharmacovigilance data, including traceability, about both biosimilars and bio-originators.
These principles represent the key issues regarding biosimilars as identified by the task force. As for the specific recommendations, the task force agreed that:
1. The availability of biosimilars must significantly lower the cost of treating an individual patient and increase access to optimal therapy for all patients with rheumatic diseases (level 5, grade D evidence).
2. Approved biosimilars can be used to treat appropriate patients in the same way as their bio-originators (level 1b, grade A evidence, indicating that the recommendation is based on an individual randomized, controlled trial and that the level 1 evidence is consistent).
3. Antidrug antibodies to biosimilars need not be measured in clinical practice as no significant differences have been detected between biosimilars and their bio-originators (level 2b, grade B evidence, indicating that the recommendation is based on an individual cohort study/low-quality randomized, controlled trial and consistent level 2 or 3 evidence).
4. Relevant preclinical and phase 1 data on a biosimilar should be available when phase 3 data are published (level 5, grade D evidence).
5. Confirmation of efficacy and safety in a single indication is sufficient for extrapolation to other diseases for which the bio-originator has been approved because biosimilars are equivalent in physiochemical, functional, and pharmacokinetic properties to the bio-originator (level 5, grade D evidence).
6. Available evidence suggests that a single switch from a bio-originator to one of its biosimilars is safe and effective; there is no reason to expect a different clinical outcome. However, patient perspectives must be considered (level 1b, grade A evidence).
7. Multiple switching between biosimilars and their bio-originators or other biosimilars should be assessed in registries (level 5, grade D evidence).
8. No switch to or among biosimilars should be initiated without the prior awareness of the patient and the treating health care provider (level 5, grade D evidence).
Differing opinions about the use of biosimilars as published by various subspecialty organizations highlight a lack of confidence among many clinicians with respect to appropriate use of the products, but that is changing amid a rapidly growing body of evidence, the task force said. The group achieved a high level of agreement about both the evaluation of biosimilars and their use to treat rheumatologic diseases, reaching 100% consensus for six of the recommendations and 91% and 96% for the other two.
“Data available as of December 2016 support the use of biosimilars by rheumatologists to encourage a fair and competitive market for biologics. Biosimilars now provide an opportunity to expand access to effective but expensive medications, increasing the number of available treatment choices and helping to control rapidly increasing drug expenditures,” they concluded.
The task force’s work was funded by an unrestricted educational grant from Amgen. Dr. Kay and his coauthors reported financial relationships with multiple pharmaceutical companies, many of which are developing biosimilars.
The available evidence is sufficient to support switching appropriate patients with rheumatologic diseases from a bio-originator agent to an approved biosimilar agent, according to new consensus-based recommendations from an international multidisciplinary task force.
“Treatment with biological agents has dramatically improved the outcome for patients with inflammatory diseases. However, the high cost of these medications has limited access for many patients,” Jonathan Kay, MD, of UMass Memorial Medical Center and the University of Massachusetts, Worcester, and his colleagues wrote on behalf of the Task Force on the Use of Biosimilars to Treat Rheumatological Diseases. Biosimilars of agents no longer protected by patent allow for increased availability at lower costs, they noted. In the European Union, the United States, Japan, and other countries, biosimilars of adalimumab, etanercept, infliximab, and rituximab have been approved, and those for which the bio-originator is no longer protected by patent have been marketed.
The task force, convened in 2016 to address the matter at an international level, included 25 experts from Europe, Japan, and the United States, including 17 rheumatologists, a rheumatologist/regulator, a dermatologist, a gastroenterologist, 2 pharmacologists, 2 patients, and a research fellow. The task force identified five overarching principles and made eight specific recommendations, based on expert opinion and an extensive literature review that yielded 29 relevant full-text papers and 20 relevant abstracts from the 2015 and 2016 American College of Rheumatology and European League Against Rheumatism annual meetings (Ann Rheum Dis. 2017 Sep 2. doi: 10.1136/annrheumdis-2017-211937).
“This statement was intended both to guide clinicians and to serve as a framework for future educational efforts,” they wrote.
The experts based all five overarching principles for the use of biosimilars on level 5, grade D evidence, indicating that they were derived mainly from expert opinion. They determined that:
- Treatment of rheumatic diseases is based on a shared decision-making process between patients and their rheumatologists.
- The contextual aspects of the health care system should be taken into consideration when treatment decisions are made.
- A biosimilar, as approved by authorities in a highly regulated area, is neither better nor worse in efficacy and is not inferior in safety to its bio-originator.
- Patients and health care providers should be informed about the nature of biosimilars, their approval process, and their safety and efficacy.
- Harmonized methods should be established to obtain reliable pharmacovigilance data, including traceability, about both biosimilars and bio-originators.
These principles represent the key issues regarding biosimilars as identified by the task force. As for the specific recommendations, the task force agreed that:
1. The availability of biosimilars must significantly lower the cost of treating an individual patient and increase access to optimal therapy for all patients with rheumatic diseases (level 5, grade D evidence).
2. Approved biosimilars can be used to treat appropriate patients in the same way as their bio-originators (level 1b, grade A evidence, indicating that the recommendation is based on an individual randomized, controlled trial and that the level 1 evidence is consistent).
3. Antidrug antibodies to biosimilars need not be measured in clinical practice as no significant differences have been detected between biosimilars and their bio-originators (level 2b, grade B evidence, indicating that the recommendation is based on an individual cohort study/low-quality randomized, controlled trial and consistent level 2 or 3 evidence).
4. Relevant preclinical and phase 1 data on a biosimilar should be available when phase 3 data are published (level 5, grade D evidence).
5. Confirmation of efficacy and safety in a single indication is sufficient for extrapolation to other diseases for which the bio-originator has been approved because biosimilars are equivalent in physiochemical, functional, and pharmacokinetic properties to the bio-originator (level 5, grade D evidence).
6. Available evidence suggests that a single switch from a bio-originator to one of its biosimilars is safe and effective; there is no reason to expect a different clinical outcome. However, patient perspectives must be considered (level 1b, grade A evidence).
7. Multiple switching between biosimilars and their bio-originators or other biosimilars should be assessed in registries (level 5, grade D evidence).
8. No switch to or among biosimilars should be initiated without the prior awareness of the patient and the treating health care provider (level 5, grade D evidence).
Differing opinions about the use of biosimilars as published by various subspecialty organizations highlight a lack of confidence among many clinicians with respect to appropriate use of the products, but that is changing amid a rapidly growing body of evidence, the task force said. The group achieved a high level of agreement about both the evaluation of biosimilars and their use to treat rheumatologic diseases, reaching 100% consensus for six of the recommendations and 91% and 96% for the other two.
“Data available as of December 2016 support the use of biosimilars by rheumatologists to encourage a fair and competitive market for biologics. Biosimilars now provide an opportunity to expand access to effective but expensive medications, increasing the number of available treatment choices and helping to control rapidly increasing drug expenditures,” they concluded.
The task force’s work was funded by an unrestricted educational grant from Amgen. Dr. Kay and his coauthors reported financial relationships with multiple pharmaceutical companies, many of which are developing biosimilars.
The available evidence is sufficient to support switching appropriate patients with rheumatologic diseases from a bio-originator agent to an approved biosimilar agent, according to new consensus-based recommendations from an international multidisciplinary task force.
“Treatment with biological agents has dramatically improved the outcome for patients with inflammatory diseases. However, the high cost of these medications has limited access for many patients,” Jonathan Kay, MD, of UMass Memorial Medical Center and the University of Massachusetts, Worcester, and his colleagues wrote on behalf of the Task Force on the Use of Biosimilars to Treat Rheumatological Diseases. Biosimilars of agents no longer protected by patent allow for increased availability at lower costs, they noted. In the European Union, the United States, Japan, and other countries, biosimilars of adalimumab, etanercept, infliximab, and rituximab have been approved, and those for which the bio-originator is no longer protected by patent have been marketed.
The task force, convened in 2016 to address the matter at an international level, included 25 experts from Europe, Japan, and the United States, including 17 rheumatologists, a rheumatologist/regulator, a dermatologist, a gastroenterologist, 2 pharmacologists, 2 patients, and a research fellow. The task force identified five overarching principles and made eight specific recommendations, based on expert opinion and an extensive literature review that yielded 29 relevant full-text papers and 20 relevant abstracts from the 2015 and 2016 American College of Rheumatology and European League Against Rheumatism annual meetings (Ann Rheum Dis. 2017 Sep 2. doi: 10.1136/annrheumdis-2017-211937).
“This statement was intended both to guide clinicians and to serve as a framework for future educational efforts,” they wrote.
The experts based all five overarching principles for the use of biosimilars on level 5, grade D evidence, indicating that they were derived mainly from expert opinion. They determined that:
- Treatment of rheumatic diseases is based on a shared decision-making process between patients and their rheumatologists.
- The contextual aspects of the health care system should be taken into consideration when treatment decisions are made.
- A biosimilar, as approved by authorities in a highly regulated area, is neither better nor worse in efficacy and is not inferior in safety to its bio-originator.
- Patients and health care providers should be informed about the nature of biosimilars, their approval process, and their safety and efficacy.
- Harmonized methods should be established to obtain reliable pharmacovigilance data, including traceability, about both biosimilars and bio-originators.
These principles represent the key issues regarding biosimilars as identified by the task force. As for the specific recommendations, the task force agreed that:
1. The availability of biosimilars must significantly lower the cost of treating an individual patient and increase access to optimal therapy for all patients with rheumatic diseases (level 5, grade D evidence).
2. Approved biosimilars can be used to treat appropriate patients in the same way as their bio-originators (level 1b, grade A evidence, indicating that the recommendation is based on an individual randomized, controlled trial and that the level 1 evidence is consistent).
3. Antidrug antibodies to biosimilars need not be measured in clinical practice as no significant differences have been detected between biosimilars and their bio-originators (level 2b, grade B evidence, indicating that the recommendation is based on an individual cohort study/low-quality randomized, controlled trial and consistent level 2 or 3 evidence).
4. Relevant preclinical and phase 1 data on a biosimilar should be available when phase 3 data are published (level 5, grade D evidence).
5. Confirmation of efficacy and safety in a single indication is sufficient for extrapolation to other diseases for which the bio-originator has been approved because biosimilars are equivalent in physiochemical, functional, and pharmacokinetic properties to the bio-originator (level 5, grade D evidence).
6. Available evidence suggests that a single switch from a bio-originator to one of its biosimilars is safe and effective; there is no reason to expect a different clinical outcome. However, patient perspectives must be considered (level 1b, grade A evidence).
7. Multiple switching between biosimilars and their bio-originators or other biosimilars should be assessed in registries (level 5, grade D evidence).
8. No switch to or among biosimilars should be initiated without the prior awareness of the patient and the treating health care provider (level 5, grade D evidence).
Differing opinions about the use of biosimilars as published by various subspecialty organizations highlight a lack of confidence among many clinicians with respect to appropriate use of the products, but that is changing amid a rapidly growing body of evidence, the task force said. The group achieved a high level of agreement about both the evaluation of biosimilars and their use to treat rheumatologic diseases, reaching 100% consensus for six of the recommendations and 91% and 96% for the other two.
“Data available as of December 2016 support the use of biosimilars by rheumatologists to encourage a fair and competitive market for biologics. Biosimilars now provide an opportunity to expand access to effective but expensive medications, increasing the number of available treatment choices and helping to control rapidly increasing drug expenditures,” they concluded.
The task force’s work was funded by an unrestricted educational grant from Amgen. Dr. Kay and his coauthors reported financial relationships with multiple pharmaceutical companies, many of which are developing biosimilars.
FROM ANNALS OF THE RHEUMATIC DISEASES
FDA approves second adalimumab biosimilar for multiple conditions
The Food and Drug Administration has approved Cyltezo (adalimumab-adbm) for multiple conditions.
Cyltezo is an injectable tumor necrosis factor blocker, and is a biosimilar to adalimumab (Humira). The drug is indicated to treat moderate to severe active rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, moderate to severe active Crohn’s disease, moderate to severe active ulcerative colitis, moderately to severely active polyarticular juvenile idiopathic arthritis in patients 4 years of age and older, and moderate to severe plaque psoriasis.
Find the Cyltezo labeling information here.
The Food and Drug Administration has approved Cyltezo (adalimumab-adbm) for multiple conditions.
Cyltezo is an injectable tumor necrosis factor blocker, and is a biosimilar to adalimumab (Humira). The drug is indicated to treat moderate to severe active rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, moderate to severe active Crohn’s disease, moderate to severe active ulcerative colitis, moderately to severely active polyarticular juvenile idiopathic arthritis in patients 4 years of age and older, and moderate to severe plaque psoriasis.
Find the Cyltezo labeling information here.
The Food and Drug Administration has approved Cyltezo (adalimumab-adbm) for multiple conditions.
Cyltezo is an injectable tumor necrosis factor blocker, and is a biosimilar to adalimumab (Humira). The drug is indicated to treat moderate to severe active rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, moderate to severe active Crohn’s disease, moderate to severe active ulcerative colitis, moderately to severely active polyarticular juvenile idiopathic arthritis in patients 4 years of age and older, and moderate to severe plaque psoriasis.
Find the Cyltezo labeling information here.
Axial SpA features don’t guarantee its diagnosis in chronic back pain
The manifestation of multiple features of spondyloarthritis (SpA) in patients with chronic back pain is not sufficient for a diagnosis of axial spondyloarthritis, according to a report from Zineb Ez-Zaitouni and associates.
In a group of 250 people with chronic back pain who were not diagnosed with axial SpA, the most common alternative diagnosis was nonspecific back pain, followed by mechanical back pain, degenerative disc disease, and myalgia/fibromyalgia. Sacroiliitis on either radiographs or MRI and HLA-B27 was uncommon, and HLA-B27 positivity was also infrequent.
A total of 18 patients within the study group had at least four features of SpA but did not have axial SpA. Within this group, the most common SpA features were inflammatory back pain, a positive family history of SpA, a good response to nonsteroidal anti-inflammatory drugs, elevated C-reactive protein or erythrocyte sedimentation rate, and enthesitis. No patients had positive imaging, and only four were positive for HLA-B27.
“These findings show that rheumatologists in clinical practice rightly dispute a diagnosis of axSpA even when there is a high number of SpA features, especially when imaging is normal and patients are negative for HLA-B27,” the investigators concluded.
Find the full report in Annals of the Rheumatic Diseases (doi: 10.1136/annrheumdis-2017-212175)
The manifestation of multiple features of spondyloarthritis (SpA) in patients with chronic back pain is not sufficient for a diagnosis of axial spondyloarthritis, according to a report from Zineb Ez-Zaitouni and associates.
In a group of 250 people with chronic back pain who were not diagnosed with axial SpA, the most common alternative diagnosis was nonspecific back pain, followed by mechanical back pain, degenerative disc disease, and myalgia/fibromyalgia. Sacroiliitis on either radiographs or MRI and HLA-B27 was uncommon, and HLA-B27 positivity was also infrequent.
A total of 18 patients within the study group had at least four features of SpA but did not have axial SpA. Within this group, the most common SpA features were inflammatory back pain, a positive family history of SpA, a good response to nonsteroidal anti-inflammatory drugs, elevated C-reactive protein or erythrocyte sedimentation rate, and enthesitis. No patients had positive imaging, and only four were positive for HLA-B27.
“These findings show that rheumatologists in clinical practice rightly dispute a diagnosis of axSpA even when there is a high number of SpA features, especially when imaging is normal and patients are negative for HLA-B27,” the investigators concluded.
Find the full report in Annals of the Rheumatic Diseases (doi: 10.1136/annrheumdis-2017-212175)
The manifestation of multiple features of spondyloarthritis (SpA) in patients with chronic back pain is not sufficient for a diagnosis of axial spondyloarthritis, according to a report from Zineb Ez-Zaitouni and associates.
In a group of 250 people with chronic back pain who were not diagnosed with axial SpA, the most common alternative diagnosis was nonspecific back pain, followed by mechanical back pain, degenerative disc disease, and myalgia/fibromyalgia. Sacroiliitis on either radiographs or MRI and HLA-B27 was uncommon, and HLA-B27 positivity was also infrequent.
A total of 18 patients within the study group had at least four features of SpA but did not have axial SpA. Within this group, the most common SpA features were inflammatory back pain, a positive family history of SpA, a good response to nonsteroidal anti-inflammatory drugs, elevated C-reactive protein or erythrocyte sedimentation rate, and enthesitis. No patients had positive imaging, and only four were positive for HLA-B27.
“These findings show that rheumatologists in clinical practice rightly dispute a diagnosis of axSpA even when there is a high number of SpA features, especially when imaging is normal and patients are negative for HLA-B27,” the investigators concluded.
Find the full report in Annals of the Rheumatic Diseases (doi: 10.1136/annrheumdis-2017-212175)
FROM ANNALS OF THE RHEUMATIC DISEASES
Radiographic progression in axial spondyloarthritis moves slowly in first 5 years
Sacroiliac joint radiographic progression during the first 5 years of the onset of axial spondyloarthritis occurs to an extent related to the degree of inflammation seen on MRI at baseline, according to new findings from 416 French patients in the DESIR cohort.
Maxime Dougados, MD, of Paris Descartes University, and his colleagues found that 15% of patients at baseline met modified New York (mNY) criteria – and therefore had radiographic axial spondyloarthritis (r-axSpA) – and this increased to 20% at 5 years. During the 5-year follow-up, the net percentage of patients who progressed was 5% (those who went from nonradiographic axial spondyloarthritis [nr-axSpA] to r-axSpA minus those who regressed from r-axSpA to nr-axSpA). Overall, 13% changed at least one grade on mNY criteria, and if an mNY criteria grade change from zero to one was not considered, only 10% experienced a change in at least one grade. These patients overall had a mean age of 34 years and had inflammatory back pain that had lasted at least 3 months but less than 3 years.
“The association between baseline MRI inflammation and 5-year SIJ damage was consistently found, regardless of the analytical method and the definition of SIJ progression,” the investigators wrote.
The estimated risk for progression by at least one mNY criteria grade varied from as high as 18% in HLA-B27–positive individuals with baseline SIJ inflammation on MRI and elevated C-reactive protein to just 1% in those who were negative for those three variables.
Read the full report online (Ann Rheum Dis. 2017 Jul 6. doi: 10.1136/annrheumdis-2017-211596).
Sacroiliac joint radiographic progression during the first 5 years of the onset of axial spondyloarthritis occurs to an extent related to the degree of inflammation seen on MRI at baseline, according to new findings from 416 French patients in the DESIR cohort.
Maxime Dougados, MD, of Paris Descartes University, and his colleagues found that 15% of patients at baseline met modified New York (mNY) criteria – and therefore had radiographic axial spondyloarthritis (r-axSpA) – and this increased to 20% at 5 years. During the 5-year follow-up, the net percentage of patients who progressed was 5% (those who went from nonradiographic axial spondyloarthritis [nr-axSpA] to r-axSpA minus those who regressed from r-axSpA to nr-axSpA). Overall, 13% changed at least one grade on mNY criteria, and if an mNY criteria grade change from zero to one was not considered, only 10% experienced a change in at least one grade. These patients overall had a mean age of 34 years and had inflammatory back pain that had lasted at least 3 months but less than 3 years.
“The association between baseline MRI inflammation and 5-year SIJ damage was consistently found, regardless of the analytical method and the definition of SIJ progression,” the investigators wrote.
The estimated risk for progression by at least one mNY criteria grade varied from as high as 18% in HLA-B27–positive individuals with baseline SIJ inflammation on MRI and elevated C-reactive protein to just 1% in those who were negative for those three variables.
Read the full report online (Ann Rheum Dis. 2017 Jul 6. doi: 10.1136/annrheumdis-2017-211596).
Sacroiliac joint radiographic progression during the first 5 years of the onset of axial spondyloarthritis occurs to an extent related to the degree of inflammation seen on MRI at baseline, according to new findings from 416 French patients in the DESIR cohort.
Maxime Dougados, MD, of Paris Descartes University, and his colleagues found that 15% of patients at baseline met modified New York (mNY) criteria – and therefore had radiographic axial spondyloarthritis (r-axSpA) – and this increased to 20% at 5 years. During the 5-year follow-up, the net percentage of patients who progressed was 5% (those who went from nonradiographic axial spondyloarthritis [nr-axSpA] to r-axSpA minus those who regressed from r-axSpA to nr-axSpA). Overall, 13% changed at least one grade on mNY criteria, and if an mNY criteria grade change from zero to one was not considered, only 10% experienced a change in at least one grade. These patients overall had a mean age of 34 years and had inflammatory back pain that had lasted at least 3 months but less than 3 years.
“The association between baseline MRI inflammation and 5-year SIJ damage was consistently found, regardless of the analytical method and the definition of SIJ progression,” the investigators wrote.
The estimated risk for progression by at least one mNY criteria grade varied from as high as 18% in HLA-B27–positive individuals with baseline SIJ inflammation on MRI and elevated C-reactive protein to just 1% in those who were negative for those three variables.
Read the full report online (Ann Rheum Dis. 2017 Jul 6. doi: 10.1136/annrheumdis-2017-211596).
FROM ANNALS OF THE RHEUMATIC DISEASES
First interchangeability study for an adalimumab biosimilar has begun
The VOLTAIRE-X study of a biosimilar candidate for adalimumab (Humira) for chronic plaque psoriasis has enrolled its first patient, announced Boehringer Ingelheim, the biosimilar’s developer, on July 27.
This is the first study in the United States to investigate whether a biosimilar candidate should be granted an interchangeability designation with adalimumab. The candidate, BI 695501, is up against adalimumab’s 40-mg injection.
In VOLTAIRE-X, some patients will alternate between adalimumab and BI 695501, and others will take adalimumab continuously. The study will compare the pharmacokinetics, clinical outcomes, safety, immunogenicity, and efficacy between the two groups of patients. The estimated enrollment of adult patients with moderate to severe chronic plaque psoriasis is 240, and the study is expected to conclude in July 2019.
A phase 3 study of BI 695501’s performance for rheumatoid arthritis patients, completed in 2016, demonstrated similar efficacy, safety, and immunogenicity.
The VOLTAIRE-X study of a biosimilar candidate for adalimumab (Humira) for chronic plaque psoriasis has enrolled its first patient, announced Boehringer Ingelheim, the biosimilar’s developer, on July 27.
This is the first study in the United States to investigate whether a biosimilar candidate should be granted an interchangeability designation with adalimumab. The candidate, BI 695501, is up against adalimumab’s 40-mg injection.
In VOLTAIRE-X, some patients will alternate between adalimumab and BI 695501, and others will take adalimumab continuously. The study will compare the pharmacokinetics, clinical outcomes, safety, immunogenicity, and efficacy between the two groups of patients. The estimated enrollment of adult patients with moderate to severe chronic plaque psoriasis is 240, and the study is expected to conclude in July 2019.
A phase 3 study of BI 695501’s performance for rheumatoid arthritis patients, completed in 2016, demonstrated similar efficacy, safety, and immunogenicity.
The VOLTAIRE-X study of a biosimilar candidate for adalimumab (Humira) for chronic plaque psoriasis has enrolled its first patient, announced Boehringer Ingelheim, the biosimilar’s developer, on July 27.
This is the first study in the United States to investigate whether a biosimilar candidate should be granted an interchangeability designation with adalimumab. The candidate, BI 695501, is up against adalimumab’s 40-mg injection.
In VOLTAIRE-X, some patients will alternate between adalimumab and BI 695501, and others will take adalimumab continuously. The study will compare the pharmacokinetics, clinical outcomes, safety, immunogenicity, and efficacy between the two groups of patients. The estimated enrollment of adult patients with moderate to severe chronic plaque psoriasis is 240, and the study is expected to conclude in July 2019.
A phase 3 study of BI 695501’s performance for rheumatoid arthritis patients, completed in 2016, demonstrated similar efficacy, safety, and immunogenicity.
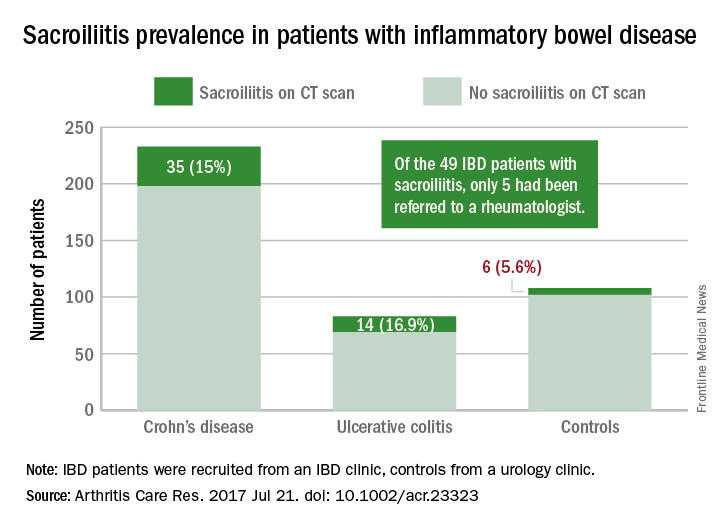
CT scoring system may improve sacroiliitis treatment in IBD
A standardized scoring system to identify sacroiliitis could enable patients with inflammatory bowel disease to get earlier rheumatology referrals and improve treatment, according to an analysis of IBD patients with pre-existing abdominal CT scans.
Of the 316 patients recruited from an IBD clinic in Toronto, the validated CT scan scoring system identified 49 with sacroiliitis, of whom only 5 had been referred to an outpatient rheumatology clinic in the city. Rates of sacroiliitis were similar between the 233 patients with Crohn’s disease (15%) and the 83 with ulcerative colitis (16.9%). The scoring system indicated sacroiliitis in 6 (5.6%) of the 108 control subjects, who were recruited from a urology clinic and had no prior history of chronic back pain, reported Jonathan Chan, MD, of the University of Toronto and his associates (Arthritis Care Res. 2017 Jul 21. doi: 10.1002/acr.23323).“Previous studies using CT scan to detect sacroiliitis have relied upon an adaptation of the [modified New York] criteria or a radiologist’s gestalt. Such an adaptation may not be appropriate since changes suggestive of sacroiliitis can be found in the healthy population due to the increased sensitivity of CT scans,” the investigators said. They developed a CT-scan scoring system in which the sacroiliac joints are divided into left/right and iliac/sacral segments. The slice with the greatest number of erosions in each of the four segments contributes that value to the total erosion score, with a score of 3 or greater identifying the presence of sacroiliitis.
By demonstrating that sacroiliitis is three times more prevalent among IBD patients and can be reliably detected in CT scans performed in the clinical care of those patients, this study suggests that “more timely referral for rheumatology assessment” could avoid unnecessary treatment with biologics, Dr. Chan and his associates wrote.
The study was supported in part by a fellowship grant from the Assessment of Spondyloarthritis International Society and in part by Janssen. The investigators reported having no conflicts of interest for the study.
A standardized scoring system to identify sacroiliitis could enable patients with inflammatory bowel disease to get earlier rheumatology referrals and improve treatment, according to an analysis of IBD patients with pre-existing abdominal CT scans.
Of the 316 patients recruited from an IBD clinic in Toronto, the validated CT scan scoring system identified 49 with sacroiliitis, of whom only 5 had been referred to an outpatient rheumatology clinic in the city. Rates of sacroiliitis were similar between the 233 patients with Crohn’s disease (15%) and the 83 with ulcerative colitis (16.9%). The scoring system indicated sacroiliitis in 6 (5.6%) of the 108 control subjects, who were recruited from a urology clinic and had no prior history of chronic back pain, reported Jonathan Chan, MD, of the University of Toronto and his associates (Arthritis Care Res. 2017 Jul 21. doi: 10.1002/acr.23323).“Previous studies using CT scan to detect sacroiliitis have relied upon an adaptation of the [modified New York] criteria or a radiologist’s gestalt. Such an adaptation may not be appropriate since changes suggestive of sacroiliitis can be found in the healthy population due to the increased sensitivity of CT scans,” the investigators said. They developed a CT-scan scoring system in which the sacroiliac joints are divided into left/right and iliac/sacral segments. The slice with the greatest number of erosions in each of the four segments contributes that value to the total erosion score, with a score of 3 or greater identifying the presence of sacroiliitis.
By demonstrating that sacroiliitis is three times more prevalent among IBD patients and can be reliably detected in CT scans performed in the clinical care of those patients, this study suggests that “more timely referral for rheumatology assessment” could avoid unnecessary treatment with biologics, Dr. Chan and his associates wrote.
The study was supported in part by a fellowship grant from the Assessment of Spondyloarthritis International Society and in part by Janssen. The investigators reported having no conflicts of interest for the study.
A standardized scoring system to identify sacroiliitis could enable patients with inflammatory bowel disease to get earlier rheumatology referrals and improve treatment, according to an analysis of IBD patients with pre-existing abdominal CT scans.
Of the 316 patients recruited from an IBD clinic in Toronto, the validated CT scan scoring system identified 49 with sacroiliitis, of whom only 5 had been referred to an outpatient rheumatology clinic in the city. Rates of sacroiliitis were similar between the 233 patients with Crohn’s disease (15%) and the 83 with ulcerative colitis (16.9%). The scoring system indicated sacroiliitis in 6 (5.6%) of the 108 control subjects, who were recruited from a urology clinic and had no prior history of chronic back pain, reported Jonathan Chan, MD, of the University of Toronto and his associates (Arthritis Care Res. 2017 Jul 21. doi: 10.1002/acr.23323).“Previous studies using CT scan to detect sacroiliitis have relied upon an adaptation of the [modified New York] criteria or a radiologist’s gestalt. Such an adaptation may not be appropriate since changes suggestive of sacroiliitis can be found in the healthy population due to the increased sensitivity of CT scans,” the investigators said. They developed a CT-scan scoring system in which the sacroiliac joints are divided into left/right and iliac/sacral segments. The slice with the greatest number of erosions in each of the four segments contributes that value to the total erosion score, with a score of 3 or greater identifying the presence of sacroiliitis.
By demonstrating that sacroiliitis is three times more prevalent among IBD patients and can be reliably detected in CT scans performed in the clinical care of those patients, this study suggests that “more timely referral for rheumatology assessment” could avoid unnecessary treatment with biologics, Dr. Chan and his associates wrote.
The study was supported in part by a fellowship grant from the Assessment of Spondyloarthritis International Society and in part by Janssen. The investigators reported having no conflicts of interest for the study.
FROM ARTHRITIS CARE & RESEARCH
TNFi treatment halves ankylosing spondylitis progression
MADRID – At least 2 years of tumor necrosis factor–inhibitor treatment of patients with ankylosing spondylitis nearly halved the rate of spinal radiographic progression in a study involving 432 Swiss patients.
In addition, patients on a tumor necrosis factor inhibitor (TNFi) who achieved low disease activity, reflected in an Ankylosing Spondylitis (AS) Disease Activity Score of 1.3 or less, showed virtually no spinal radiographic progression during a 2-year follow-up, Adrian Ciurea, MD, reported at the European Congress of Rheumatology.
He cautioned, however, that the evidence only shows correlation and can’t prove a causal relationship between TNFi treatment and slowed spinal radiographic progression because of potential residual confounding.
Dr. Ciurea and his associates analyzed records for AS patients enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases cohort who underwent at least two spinal radiographs separated by a 2-year gap. They assessed the radiographs using the modified Stoke AS Spinal Score (mSASSS), and they defined progression as a gain of at least two units on the mSASSS during a 2-year period between radiographs.
The 432 AS patients in the study averaged 40 years old, two-thirds were men, and they had AS symptoms for an average of nearly 14 years. Their average AS Disease Activity Score (ASDAS) at entry was 2.8.
A multivariate analysis that controlled for several variables, including sex, smoking history, baseline mSASSS, and exercise, identified three parameters that had significant correlations with radiographic progression: Men had more than double the rate of progression, compared with women; higher baseline mSASSS was linked with a higher rate of progression; and a greater-than-2-year history of treatment with a TNFi was linked with a 48% reduced rate of progression, reported Dr. Ciurea, a rheumatologist at the Zürich University Hospital.
The duration of treatment also mattered. Patients who received at least 4 years of TNFi treatment had a statistically significant 68% reduced rate of radiographic spinal progression. In contrast, patients who received a TNFi for fewer than 4 years but more than 2 years had a 42% lower rate of progression that was of borderline statistical significance. TNFi treatment that started during the 2 years immediately preceding the radiograph failed to show a significant link with reduced progression.
Further analysis also showed a tight correlation between patients’ disease activity while on TNFi treatment and radiographic progression. Patients who maintained an average ASDAS of 2.1 or less during the 2 years prior to radiographic assessment showed an average mSASSS gain of 0.31 units over that 2-year period, compared with an average 1.45-unit mSASSS gain among patients whose average ASDAS remained above 2.1, a statistically significant difference between these two groups. Patients with even more inactive disease on TNFi treatment – those who maintained an average ASDAS of 1.3 or less – had an average 0.01-unit rise in their mSASSS after 2 years of treatment, compared with an average 0.52-unit mSASSS rise after 2 years in patients with an ASDAS of more than 1.3 but less than 2.1, he said.
The cohort study received partial support from Merck Sharpe & Dohme. Dr. Ciurea has been a consultant to or speaker for Abbvie, Celgene, Eli Lilly, Janssen-Cilag, Merck Sharp & Dohme, Novartis, Pfizer, and UCB.
[email protected]
On Twitter @mitchelzoler
MADRID – At least 2 years of tumor necrosis factor–inhibitor treatment of patients with ankylosing spondylitis nearly halved the rate of spinal radiographic progression in a study involving 432 Swiss patients.
In addition, patients on a tumor necrosis factor inhibitor (TNFi) who achieved low disease activity, reflected in an Ankylosing Spondylitis (AS) Disease Activity Score of 1.3 or less, showed virtually no spinal radiographic progression during a 2-year follow-up, Adrian Ciurea, MD, reported at the European Congress of Rheumatology.
He cautioned, however, that the evidence only shows correlation and can’t prove a causal relationship between TNFi treatment and slowed spinal radiographic progression because of potential residual confounding.
Dr. Ciurea and his associates analyzed records for AS patients enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases cohort who underwent at least two spinal radiographs separated by a 2-year gap. They assessed the radiographs using the modified Stoke AS Spinal Score (mSASSS), and they defined progression as a gain of at least two units on the mSASSS during a 2-year period between radiographs.
The 432 AS patients in the study averaged 40 years old, two-thirds were men, and they had AS symptoms for an average of nearly 14 years. Their average AS Disease Activity Score (ASDAS) at entry was 2.8.
A multivariate analysis that controlled for several variables, including sex, smoking history, baseline mSASSS, and exercise, identified three parameters that had significant correlations with radiographic progression: Men had more than double the rate of progression, compared with women; higher baseline mSASSS was linked with a higher rate of progression; and a greater-than-2-year history of treatment with a TNFi was linked with a 48% reduced rate of progression, reported Dr. Ciurea, a rheumatologist at the Zürich University Hospital.
The duration of treatment also mattered. Patients who received at least 4 years of TNFi treatment had a statistically significant 68% reduced rate of radiographic spinal progression. In contrast, patients who received a TNFi for fewer than 4 years but more than 2 years had a 42% lower rate of progression that was of borderline statistical significance. TNFi treatment that started during the 2 years immediately preceding the radiograph failed to show a significant link with reduced progression.
Further analysis also showed a tight correlation between patients’ disease activity while on TNFi treatment and radiographic progression. Patients who maintained an average ASDAS of 2.1 or less during the 2 years prior to radiographic assessment showed an average mSASSS gain of 0.31 units over that 2-year period, compared with an average 1.45-unit mSASSS gain among patients whose average ASDAS remained above 2.1, a statistically significant difference between these two groups. Patients with even more inactive disease on TNFi treatment – those who maintained an average ASDAS of 1.3 or less – had an average 0.01-unit rise in their mSASSS after 2 years of treatment, compared with an average 0.52-unit mSASSS rise after 2 years in patients with an ASDAS of more than 1.3 but less than 2.1, he said.
The cohort study received partial support from Merck Sharpe & Dohme. Dr. Ciurea has been a consultant to or speaker for Abbvie, Celgene, Eli Lilly, Janssen-Cilag, Merck Sharp & Dohme, Novartis, Pfizer, and UCB.
[email protected]
On Twitter @mitchelzoler
MADRID – At least 2 years of tumor necrosis factor–inhibitor treatment of patients with ankylosing spondylitis nearly halved the rate of spinal radiographic progression in a study involving 432 Swiss patients.
In addition, patients on a tumor necrosis factor inhibitor (TNFi) who achieved low disease activity, reflected in an Ankylosing Spondylitis (AS) Disease Activity Score of 1.3 or less, showed virtually no spinal radiographic progression during a 2-year follow-up, Adrian Ciurea, MD, reported at the European Congress of Rheumatology.
He cautioned, however, that the evidence only shows correlation and can’t prove a causal relationship between TNFi treatment and slowed spinal radiographic progression because of potential residual confounding.
Dr. Ciurea and his associates analyzed records for AS patients enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases cohort who underwent at least two spinal radiographs separated by a 2-year gap. They assessed the radiographs using the modified Stoke AS Spinal Score (mSASSS), and they defined progression as a gain of at least two units on the mSASSS during a 2-year period between radiographs.
The 432 AS patients in the study averaged 40 years old, two-thirds were men, and they had AS symptoms for an average of nearly 14 years. Their average AS Disease Activity Score (ASDAS) at entry was 2.8.
A multivariate analysis that controlled for several variables, including sex, smoking history, baseline mSASSS, and exercise, identified three parameters that had significant correlations with radiographic progression: Men had more than double the rate of progression, compared with women; higher baseline mSASSS was linked with a higher rate of progression; and a greater-than-2-year history of treatment with a TNFi was linked with a 48% reduced rate of progression, reported Dr. Ciurea, a rheumatologist at the Zürich University Hospital.
The duration of treatment also mattered. Patients who received at least 4 years of TNFi treatment had a statistically significant 68% reduced rate of radiographic spinal progression. In contrast, patients who received a TNFi for fewer than 4 years but more than 2 years had a 42% lower rate of progression that was of borderline statistical significance. TNFi treatment that started during the 2 years immediately preceding the radiograph failed to show a significant link with reduced progression.
Further analysis also showed a tight correlation between patients’ disease activity while on TNFi treatment and radiographic progression. Patients who maintained an average ASDAS of 2.1 or less during the 2 years prior to radiographic assessment showed an average mSASSS gain of 0.31 units over that 2-year period, compared with an average 1.45-unit mSASSS gain among patients whose average ASDAS remained above 2.1, a statistically significant difference between these two groups. Patients with even more inactive disease on TNFi treatment – those who maintained an average ASDAS of 1.3 or less – had an average 0.01-unit rise in their mSASSS after 2 years of treatment, compared with an average 0.52-unit mSASSS rise after 2 years in patients with an ASDAS of more than 1.3 but less than 2.1, he said.
The cohort study received partial support from Merck Sharpe & Dohme. Dr. Ciurea has been a consultant to or speaker for Abbvie, Celgene, Eli Lilly, Janssen-Cilag, Merck Sharp & Dohme, Novartis, Pfizer, and UCB.
[email protected]
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Prolonged TNFi treatment was linked with a 48% lower rate of spinal radiographic progression, compared with shorter treatment.
Data source: Review of 432 patients in the Swiss Clinical Quality Management in Rheumatic Diseases cohort.
Disclosures: The cohort study received partial support from Merck Sharpe & Dohme. Dr. Ciurea has been a consultant to or speaker for Abbvie, Celgene, Eli Lilly, Janssen-Cilag, Merck Sharp & Dohme, Novartis, Pfizer, and UCB.
Obesity blunts TNFi response in axial spondyloarthritis
MADRID – Obese patients with axial spondyloarthritis were substantially less responsive to treatment with a tumor necrosis factor inhibitor than were healthy-weight patients in a multicenter Swiss study with 531 patients.
In a multivariate analysis that controlled for several demographic and clinical factors, including baseline disease severity, obese patients with axial spondyloarthritis (SpA) were 70% less likely to achieve a 40% or better improvement in their Assessment in SpondyloArthritis International Society improvement criteria (ASAS 40) when compared with patients with a healthy body mass index (BMI), Raphael Micheroli, MD, reported in a poster at the European Congress of Rheumatology.
The finding supplies a third reason why patients with newly diagnosed axial SpA should try to lose weight if they are obese (or overweight) – to potentially improve their responsiveness to a TNFi. The other two reasons are to reduce cardiovascular disease risk in patients who are already at risk for these complications because of their disease, and to also help improve their ability to perform physical activities, he explained in an interview.
Dr. Micheroli proposed three possible reasons why obese patients with axial SpA might be less responsive to a TNFi than healthy-weight patients: They receive an inadequate TNFi dosage, their increased adipose tissue produces excess proinflammatory cytokines that exacerbate their axial SpA, or it is possible that obese patients are more likely to be misdiagnosed with axial SpA and because they don’t really have this disease their symptoms cannot improve with TNFi treatment. They may instead have, for example, degenerative back pain, a condition that can be challenging to distinguish from axial SpA, he said.
A role for obesity in blunting the beneficial effects of TNFi treatment has been well described for psoriatic arthritis, for example, in an Italian study with 138 patients (Ann Rheum Dis. 2014 June;73[6]:1157-62), and in a Danish study with more than 1,200 patients (Rheumatology [Oxford]. 2016 Dec;55[12]:2191-9).
Dr. Micheroli’s study included 624 patients with axial SpA enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases axial spondyloarthritis cohort who met the ASAS classification criteria for axial SpA and started treatment with their first TNFi after they entered the cohort. Follow-up data after 1 year on treatment were available for 531 of these patients. The entry group included 332 patients (53%) with a healthy BMI, 204 (33%) with an overweight BMI (25-30 kg/m2), and 88 (14%) obese patients (BMI more than 30 kg/m2). The patients averaged about 40 years old and had been symptomatic for an average of about 13 years. About one-third of patients started on adalimumab (Humira) treatment, about one-quarter started etanercept (Enbrel), more than one-fifth began infliximab (Remicade), and some patients started treatment with either golimumab (Simponi) or certolizumab pegol (Cimzia).
After 1 year on TNFi treatment, ASAS 40 improvement occurred in 44% of 282 healthy-BMI patients, 34% of 178 overweight patients, and in 29% of 71 obese patients, Dr. Micheroli reported. In a baseline-adjusted multivariate model, this difference translated into an odds ratio of 0.30 for obese patients achieving an ASAS 40 response, compared with the healthy-BMI patients after 1 year, a statistically significant difference. Further analysis showed no statistically significant differences in TNFi discontinuation rates among the three BMI subgroups.
Dr. Micheroli had no disclosures.
[email protected]
On Twitter @mitchelzoler
MADRID – Obese patients with axial spondyloarthritis were substantially less responsive to treatment with a tumor necrosis factor inhibitor than were healthy-weight patients in a multicenter Swiss study with 531 patients.
In a multivariate analysis that controlled for several demographic and clinical factors, including baseline disease severity, obese patients with axial spondyloarthritis (SpA) were 70% less likely to achieve a 40% or better improvement in their Assessment in SpondyloArthritis International Society improvement criteria (ASAS 40) when compared with patients with a healthy body mass index (BMI), Raphael Micheroli, MD, reported in a poster at the European Congress of Rheumatology.
The finding supplies a third reason why patients with newly diagnosed axial SpA should try to lose weight if they are obese (or overweight) – to potentially improve their responsiveness to a TNFi. The other two reasons are to reduce cardiovascular disease risk in patients who are already at risk for these complications because of their disease, and to also help improve their ability to perform physical activities, he explained in an interview.
Dr. Micheroli proposed three possible reasons why obese patients with axial SpA might be less responsive to a TNFi than healthy-weight patients: They receive an inadequate TNFi dosage, their increased adipose tissue produces excess proinflammatory cytokines that exacerbate their axial SpA, or it is possible that obese patients are more likely to be misdiagnosed with axial SpA and because they don’t really have this disease their symptoms cannot improve with TNFi treatment. They may instead have, for example, degenerative back pain, a condition that can be challenging to distinguish from axial SpA, he said.
A role for obesity in blunting the beneficial effects of TNFi treatment has been well described for psoriatic arthritis, for example, in an Italian study with 138 patients (Ann Rheum Dis. 2014 June;73[6]:1157-62), and in a Danish study with more than 1,200 patients (Rheumatology [Oxford]. 2016 Dec;55[12]:2191-9).
Dr. Micheroli’s study included 624 patients with axial SpA enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases axial spondyloarthritis cohort who met the ASAS classification criteria for axial SpA and started treatment with their first TNFi after they entered the cohort. Follow-up data after 1 year on treatment were available for 531 of these patients. The entry group included 332 patients (53%) with a healthy BMI, 204 (33%) with an overweight BMI (25-30 kg/m2), and 88 (14%) obese patients (BMI more than 30 kg/m2). The patients averaged about 40 years old and had been symptomatic for an average of about 13 years. About one-third of patients started on adalimumab (Humira) treatment, about one-quarter started etanercept (Enbrel), more than one-fifth began infliximab (Remicade), and some patients started treatment with either golimumab (Simponi) or certolizumab pegol (Cimzia).
After 1 year on TNFi treatment, ASAS 40 improvement occurred in 44% of 282 healthy-BMI patients, 34% of 178 overweight patients, and in 29% of 71 obese patients, Dr. Micheroli reported. In a baseline-adjusted multivariate model, this difference translated into an odds ratio of 0.30 for obese patients achieving an ASAS 40 response, compared with the healthy-BMI patients after 1 year, a statistically significant difference. Further analysis showed no statistically significant differences in TNFi discontinuation rates among the three BMI subgroups.
Dr. Micheroli had no disclosures.
[email protected]
On Twitter @mitchelzoler
MADRID – Obese patients with axial spondyloarthritis were substantially less responsive to treatment with a tumor necrosis factor inhibitor than were healthy-weight patients in a multicenter Swiss study with 531 patients.
In a multivariate analysis that controlled for several demographic and clinical factors, including baseline disease severity, obese patients with axial spondyloarthritis (SpA) were 70% less likely to achieve a 40% or better improvement in their Assessment in SpondyloArthritis International Society improvement criteria (ASAS 40) when compared with patients with a healthy body mass index (BMI), Raphael Micheroli, MD, reported in a poster at the European Congress of Rheumatology.
The finding supplies a third reason why patients with newly diagnosed axial SpA should try to lose weight if they are obese (or overweight) – to potentially improve their responsiveness to a TNFi. The other two reasons are to reduce cardiovascular disease risk in patients who are already at risk for these complications because of their disease, and to also help improve their ability to perform physical activities, he explained in an interview.
Dr. Micheroli proposed three possible reasons why obese patients with axial SpA might be less responsive to a TNFi than healthy-weight patients: They receive an inadequate TNFi dosage, their increased adipose tissue produces excess proinflammatory cytokines that exacerbate their axial SpA, or it is possible that obese patients are more likely to be misdiagnosed with axial SpA and because they don’t really have this disease their symptoms cannot improve with TNFi treatment. They may instead have, for example, degenerative back pain, a condition that can be challenging to distinguish from axial SpA, he said.
A role for obesity in blunting the beneficial effects of TNFi treatment has been well described for psoriatic arthritis, for example, in an Italian study with 138 patients (Ann Rheum Dis. 2014 June;73[6]:1157-62), and in a Danish study with more than 1,200 patients (Rheumatology [Oxford]. 2016 Dec;55[12]:2191-9).
Dr. Micheroli’s study included 624 patients with axial SpA enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases axial spondyloarthritis cohort who met the ASAS classification criteria for axial SpA and started treatment with their first TNFi after they entered the cohort. Follow-up data after 1 year on treatment were available for 531 of these patients. The entry group included 332 patients (53%) with a healthy BMI, 204 (33%) with an overweight BMI (25-30 kg/m2), and 88 (14%) obese patients (BMI more than 30 kg/m2). The patients averaged about 40 years old and had been symptomatic for an average of about 13 years. About one-third of patients started on adalimumab (Humira) treatment, about one-quarter started etanercept (Enbrel), more than one-fifth began infliximab (Remicade), and some patients started treatment with either golimumab (Simponi) or certolizumab pegol (Cimzia).
After 1 year on TNFi treatment, ASAS 40 improvement occurred in 44% of 282 healthy-BMI patients, 34% of 178 overweight patients, and in 29% of 71 obese patients, Dr. Micheroli reported. In a baseline-adjusted multivariate model, this difference translated into an odds ratio of 0.30 for obese patients achieving an ASAS 40 response, compared with the healthy-BMI patients after 1 year, a statistically significant difference. Further analysis showed no statistically significant differences in TNFi discontinuation rates among the three BMI subgroups.
Dr. Micheroli had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Obese patients had a 70% lower response rate to a tumor necrosis factor inhibitor, compared with healthy-weight patients.
Data source: A cohort of 531 axial spondyloarthritis patients enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases program.
Disclosures: Dr. Micheroli had no disclosures.
VIDEO: Rheumatology biosimilars gain U.S. momentum
MADRID – With biosimilar infliximab on the U.S. market since November 2016 and producing an immediate, albeit modest, price drop for this tumor necrosis factor inhibitor (TNFi) and a second biosimilar infliximab now approved by the Food and Drug Administration and awaiting market entry, biosimilars are in a new phase of integration into U.S. practice.
“Physicians are willing to prescribe Inflectra,” the first biosimilar infliximab and the first TNFi to be sold in the United States last November, Jonathan Kay, MD, said in a video interview during the European Congress of Rheumatology. “Rheumatologists who were initially skeptical are now on the bandwagon and willing to prescribe biosimilars,” said Dr. Kay, a rheumatologist who has often consulted on biosimilar issues and has recently spoken to rheumatologists at various state society meetings to explain the U.S. biosimilar regulatory concepts and spread the message of the societal value of these agents.
“This is not a quick and casual drug evaluation” that produces “knockoff drugs,” but a “careful and extensive” FDA review that results in drugs that are equivalent in efficacy, safety, and immunogenicity to the reference drug and only compete on price, he explained.
When Pfizer began marketing Inflectra last Fall, it set the drug’s list price 15% lower than the list price at the time for Remicade, the reference-product infliximab. However, complex pricing and rebate strategies actually led to Remicade selling for a lower price than Inflectra, at least for some U.S. hospitals, including the University of Massachusetts in Worcester, where Dr. Kay is a professor of medicine.
“The effect of biosimilars is to reduce the cost to patients of an effective treatment. Whether that cost is for the reference drug or for the biosimilar drug doesn’t matter [from society’s perspective] as long as patients are able to receive an effective therapy at a [more] affordable cost, making the effective therapy available to more patients,” he said.
While Inflectra’s price impact my have been modest so far, the biosimilar effect on infliximab’s cost may soon intensify now that a second biosimilar of this TNFi, Renflexis – made by Samsung Bioepis and with U.S. marketing by Merck, received FDA approval on April 21, 2017. Until recently, U.S. pharmaceutical regulations had been understood to require a 180-day hiatus between FDA marketing approval for a biosimilar and the start of U.S. sales. But, on June 12, 2017, the U.S. Supreme Court, in a 9-0 decision, ruled that this 180-day wait was not required, making it possible for U.S. marketing of Renflexis to begin soon. (In mid-June, a statement on the Merck U.S. website for Renflexis says that the product is not currently available.)
Availability of a second biosimilar infliximab “is likely to drive the price down rapidly,” predicted Dr. Kay, citing what happened when multiple biosimilars for a reference drug came onto the European market.
Two other biosimilar TNFi have also received FDA marketing approvals but remain on hold as patent issues and litigation barriers play out. Erelzi – biosimilar etanercept – received FDA approval in August 2016, and Amjevita, biosimilar adalimumab, received FDA approval last September.
The efficacy and safety of Inflectra specifically, and by extension all biosimilars, received a recent boost with publication of findings from a randomized study with 482 patients that provided a real-world test of the core principle of biosimilar equivalence. After Inflectra came onto the Norwegian market, during July 2014 to August 2015, Norwegian researchers ran the NOR-SWTICH trial, which randomized patients who were on stable treatment with Remicade for a variety of indications (including 41% with a rheumatologic disease) to either stay on Remicade or to abruptly switch to treatment with Inflectra. During 1-year follow-up, the incidence of adverse effects and of episodes of disease worsening were virtually identical in the two treatment arms (Lancet. 2017 June 10;389[10086]:2304-16).
Dr. Kay has been a consultant to several companies that develop or market biosimilars, including Samsung Bioepis, Amgen, Pfizer, and Sandoz (Novartis), and to AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Janssen, Roche, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
MADRID – With biosimilar infliximab on the U.S. market since November 2016 and producing an immediate, albeit modest, price drop for this tumor necrosis factor inhibitor (TNFi) and a second biosimilar infliximab now approved by the Food and Drug Administration and awaiting market entry, biosimilars are in a new phase of integration into U.S. practice.
“Physicians are willing to prescribe Inflectra,” the first biosimilar infliximab and the first TNFi to be sold in the United States last November, Jonathan Kay, MD, said in a video interview during the European Congress of Rheumatology. “Rheumatologists who were initially skeptical are now on the bandwagon and willing to prescribe biosimilars,” said Dr. Kay, a rheumatologist who has often consulted on biosimilar issues and has recently spoken to rheumatologists at various state society meetings to explain the U.S. biosimilar regulatory concepts and spread the message of the societal value of these agents.
“This is not a quick and casual drug evaluation” that produces “knockoff drugs,” but a “careful and extensive” FDA review that results in drugs that are equivalent in efficacy, safety, and immunogenicity to the reference drug and only compete on price, he explained.
When Pfizer began marketing Inflectra last Fall, it set the drug’s list price 15% lower than the list price at the time for Remicade, the reference-product infliximab. However, complex pricing and rebate strategies actually led to Remicade selling for a lower price than Inflectra, at least for some U.S. hospitals, including the University of Massachusetts in Worcester, where Dr. Kay is a professor of medicine.
“The effect of biosimilars is to reduce the cost to patients of an effective treatment. Whether that cost is for the reference drug or for the biosimilar drug doesn’t matter [from society’s perspective] as long as patients are able to receive an effective therapy at a [more] affordable cost, making the effective therapy available to more patients,” he said.
While Inflectra’s price impact my have been modest so far, the biosimilar effect on infliximab’s cost may soon intensify now that a second biosimilar of this TNFi, Renflexis – made by Samsung Bioepis and with U.S. marketing by Merck, received FDA approval on April 21, 2017. Until recently, U.S. pharmaceutical regulations had been understood to require a 180-day hiatus between FDA marketing approval for a biosimilar and the start of U.S. sales. But, on June 12, 2017, the U.S. Supreme Court, in a 9-0 decision, ruled that this 180-day wait was not required, making it possible for U.S. marketing of Renflexis to begin soon. (In mid-June, a statement on the Merck U.S. website for Renflexis says that the product is not currently available.)
Availability of a second biosimilar infliximab “is likely to drive the price down rapidly,” predicted Dr. Kay, citing what happened when multiple biosimilars for a reference drug came onto the European market.
Two other biosimilar TNFi have also received FDA marketing approvals but remain on hold as patent issues and litigation barriers play out. Erelzi – biosimilar etanercept – received FDA approval in August 2016, and Amjevita, biosimilar adalimumab, received FDA approval last September.
The efficacy and safety of Inflectra specifically, and by extension all biosimilars, received a recent boost with publication of findings from a randomized study with 482 patients that provided a real-world test of the core principle of biosimilar equivalence. After Inflectra came onto the Norwegian market, during July 2014 to August 2015, Norwegian researchers ran the NOR-SWTICH trial, which randomized patients who were on stable treatment with Remicade for a variety of indications (including 41% with a rheumatologic disease) to either stay on Remicade or to abruptly switch to treatment with Inflectra. During 1-year follow-up, the incidence of adverse effects and of episodes of disease worsening were virtually identical in the two treatment arms (Lancet. 2017 June 10;389[10086]:2304-16).
Dr. Kay has been a consultant to several companies that develop or market biosimilars, including Samsung Bioepis, Amgen, Pfizer, and Sandoz (Novartis), and to AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Janssen, Roche, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
MADRID – With biosimilar infliximab on the U.S. market since November 2016 and producing an immediate, albeit modest, price drop for this tumor necrosis factor inhibitor (TNFi) and a second biosimilar infliximab now approved by the Food and Drug Administration and awaiting market entry, biosimilars are in a new phase of integration into U.S. practice.
“Physicians are willing to prescribe Inflectra,” the first biosimilar infliximab and the first TNFi to be sold in the United States last November, Jonathan Kay, MD, said in a video interview during the European Congress of Rheumatology. “Rheumatologists who were initially skeptical are now on the bandwagon and willing to prescribe biosimilars,” said Dr. Kay, a rheumatologist who has often consulted on biosimilar issues and has recently spoken to rheumatologists at various state society meetings to explain the U.S. biosimilar regulatory concepts and spread the message of the societal value of these agents.
“This is not a quick and casual drug evaluation” that produces “knockoff drugs,” but a “careful and extensive” FDA review that results in drugs that are equivalent in efficacy, safety, and immunogenicity to the reference drug and only compete on price, he explained.
When Pfizer began marketing Inflectra last Fall, it set the drug’s list price 15% lower than the list price at the time for Remicade, the reference-product infliximab. However, complex pricing and rebate strategies actually led to Remicade selling for a lower price than Inflectra, at least for some U.S. hospitals, including the University of Massachusetts in Worcester, where Dr. Kay is a professor of medicine.
“The effect of biosimilars is to reduce the cost to patients of an effective treatment. Whether that cost is for the reference drug or for the biosimilar drug doesn’t matter [from society’s perspective] as long as patients are able to receive an effective therapy at a [more] affordable cost, making the effective therapy available to more patients,” he said.
While Inflectra’s price impact my have been modest so far, the biosimilar effect on infliximab’s cost may soon intensify now that a second biosimilar of this TNFi, Renflexis – made by Samsung Bioepis and with U.S. marketing by Merck, received FDA approval on April 21, 2017. Until recently, U.S. pharmaceutical regulations had been understood to require a 180-day hiatus between FDA marketing approval for a biosimilar and the start of U.S. sales. But, on June 12, 2017, the U.S. Supreme Court, in a 9-0 decision, ruled that this 180-day wait was not required, making it possible for U.S. marketing of Renflexis to begin soon. (In mid-June, a statement on the Merck U.S. website for Renflexis says that the product is not currently available.)
Availability of a second biosimilar infliximab “is likely to drive the price down rapidly,” predicted Dr. Kay, citing what happened when multiple biosimilars for a reference drug came onto the European market.
Two other biosimilar TNFi have also received FDA marketing approvals but remain on hold as patent issues and litigation barriers play out. Erelzi – biosimilar etanercept – received FDA approval in August 2016, and Amjevita, biosimilar adalimumab, received FDA approval last September.
The efficacy and safety of Inflectra specifically, and by extension all biosimilars, received a recent boost with publication of findings from a randomized study with 482 patients that provided a real-world test of the core principle of biosimilar equivalence. After Inflectra came onto the Norwegian market, during July 2014 to August 2015, Norwegian researchers ran the NOR-SWTICH trial, which randomized patients who were on stable treatment with Remicade for a variety of indications (including 41% with a rheumatologic disease) to either stay on Remicade or to abruptly switch to treatment with Inflectra. During 1-year follow-up, the incidence of adverse effects and of episodes of disease worsening were virtually identical in the two treatment arms (Lancet. 2017 June 10;389[10086]:2304-16).
Dr. Kay has been a consultant to several companies that develop or market biosimilars, including Samsung Bioepis, Amgen, Pfizer, and Sandoz (Novartis), and to AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Janssen, Roche, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE EULAR 2017 CONGRESS