User login
Network meta-analysis ranks first-line H. pylori regimens
A network meta-analysis of current first-line dual, triple, and quadruple therapies for Helicobacter pylori infection found that vonoprazan triple therapy was most effective, while standard triple therapy of a proton pump inhibitor (PPI), amoxicillin, and clarithromycin was least effective. Levofloxacin-containing triple therapy performed best in Western countries and West Asia, while reverse hybrid therapy was most effective in East Asia.
The results “[suggest that] a new approach concerning H. pylori treatment is now needed and that the time for transitioning from trial and error to antimicrobial stewardship [of H. pylori infection] has arrived,” wrote Theodore Rokkas, PhD, MD, of the European University of Cyprus in Engomi, and colleagues. Their study was published online April 8 in Gastroenterology.
H. pylori infection is the primary cause of gastritis, peptic ulcer disease, gastric mucosa–associated lymphoid tissue lymphoma, and gastric cancer.
Since H. pylori infection was first recognized, physicians have employed a range of drugs in double, triple, and quadruple combinations to combat it.
Despite those efforts, treatment success is lower than with many other infectious diseases. A newcomer is the potassium-competing acid blocker vonoprazan, which increases efficacy of amoxicillin combination therapies and has, thereby, generated renewed interest in all combination therapies, according to the study authors. Vonoprazan is currently available in some Asian countries, but not the United States or Europe.
Current guidelines for H. pylori treatment relied on randomized controlled trials and relevant pair-wise meta-analyses, but no previous pairwise analysis has included all currently available medications, the authors noted. Network meta-analyses can help fill this evidence gap: They incorporate both direct and indirect evidence from a collection of randomized controlled trials to estimate the comparative effectiveness of three or more regimens.
The researchers conducted a network meta-analysis that included 68 randomized, controlled trials totaling 22,975 patients. The following regimens were included in the analysis: Concomitant quadruple bismuth treatment (bismuth quadruple therapy), concomitant quadruple nonbismuth treatment (nonbismuth quadruple therapy), high-dose amoxicillin double treatment (Amox-dual therapy), levofloxacin-containing treatment (Levo-therapy), reverse hybrid therapy (R-hybrid therapy), sequential quadruple treatment (sequential therapy), standard triple treatment (triple therapy), and vonoprazan-containing therapy (Vono-triple therapy).
Statistically significant results were found with Vono-triple therapy versus triple therapy (odds ratio, 3.80; 95% confidence interval, 1.62-8.94), sequential therapy versus triple therapy (OR, 1.79; 95% CI, 1.26-2.53), nonbismuth quadruple therapy versus triple therapy (OR, 2.08; 95% CI, 1.45-2.98), bismuth quadruple therapy versus triple therapy (OR, 1.47; 95% CI, 1.02-2.11), and Levo-therapy versus triple therapy (OR, 1.79; 95% CI, 1.26-2.53).
In the overall data, mean cure rates greater than 90% were seen only in Vono-triple therapy (91.4%; 95% CI, 88.5-93.5%) and R-hybrid therapy (93.6%; 95% CI, 90.4-96.8%). Cure rates were lower for Nonbismuth quadruple therapy (84.3%; 95% CI, 82.7-85.8%), Levo-therapy (83.8%; 95% CI, 82.1-85.4%), Sequential therapy (83.7%; 95% CI, 82.7-84.7%), bismuth quadruple therapy (81.3%; 95% CI, 79.5-83.1%), Amox-dual therapy (80.2%; 75.3%-84.4%), and triple therapy (75.7%; 95% CI, 74.9-76.4%). Levo-therapy performed best in Western countries (88.5%; 95% CI, 86.5-90.5%) and West Asia (88.4%; 95% CI, 84.6-91.1%). R-hybrid therapy performed best in East Asia (93.6%; 95% CI, 90.4-96.8%).
A surface under the cumulative ranking (SUCRA) value, which represents the efficacy of the intervention compared to an ideal intervention, was 92.4% for Vono-triple therapy. The second highest SUCRA value was for 68.8% for nonbismuth quadruple therapy. The SUCRA value of standard triple therapy was 4.7%.
A key limitation to the study is that Vono-triple therapy was tested only in Japan, and requires additional study in other geographic regions.
The study received support from the Department of Veteran Affairs. The authors have consulted for and received research funding from various pharmaceutical companies.
In this perspective, the network meta-analysis by Rokkas and colleagues is very important: The purpose of this study is not only to identify those regimens with the highest treatment success in comparison but also stratifies for world regions and time-shift aspects. The key value of the network approach, however, is the ability for indirect comparisons, as presented here. Using the surface under the cumulative ranking values, vonoprazan-based triple therapy may be the most promising candidate for the future, non–bismuth quadruple and R-hybrid therapies are also suitable.
In this perspective, with currently sparse vonoprazan data limited to Japan, I still prefer to go primarily for the non–bismuth quadruple therapy (56 pills to be taken in 1 week), and from my own published data, this regimen will still work if only taken for 5 days. Vice versa, in the presence of macrolide resistance, amoxicillin allergy, previous treatment failures, I go for the bismuth quadruple therapy – if I can expect good treatment compliance because proton pump inhibitor plus potassium, metronidazole, and tetracycline for 10 days can mean 140 pills. Gerhard G. Treiber, MD, AGAF, is with the department of internal medicine at Saarland University Hospital, Homburg, Germany. He has no conflicts of interest.
In this perspective, the network meta-analysis by Rokkas and colleagues is very important: The purpose of this study is not only to identify those regimens with the highest treatment success in comparison but also stratifies for world regions and time-shift aspects. The key value of the network approach, however, is the ability for indirect comparisons, as presented here. Using the surface under the cumulative ranking values, vonoprazan-based triple therapy may be the most promising candidate for the future, non–bismuth quadruple and R-hybrid therapies are also suitable.
In this perspective, with currently sparse vonoprazan data limited to Japan, I still prefer to go primarily for the non–bismuth quadruple therapy (56 pills to be taken in 1 week), and from my own published data, this regimen will still work if only taken for 5 days. Vice versa, in the presence of macrolide resistance, amoxicillin allergy, previous treatment failures, I go for the bismuth quadruple therapy – if I can expect good treatment compliance because proton pump inhibitor plus potassium, metronidazole, and tetracycline for 10 days can mean 140 pills. Gerhard G. Treiber, MD, AGAF, is with the department of internal medicine at Saarland University Hospital, Homburg, Germany. He has no conflicts of interest.
In this perspective, the network meta-analysis by Rokkas and colleagues is very important: The purpose of this study is not only to identify those regimens with the highest treatment success in comparison but also stratifies for world regions and time-shift aspects. The key value of the network approach, however, is the ability for indirect comparisons, as presented here. Using the surface under the cumulative ranking values, vonoprazan-based triple therapy may be the most promising candidate for the future, non–bismuth quadruple and R-hybrid therapies are also suitable.
In this perspective, with currently sparse vonoprazan data limited to Japan, I still prefer to go primarily for the non–bismuth quadruple therapy (56 pills to be taken in 1 week), and from my own published data, this regimen will still work if only taken for 5 days. Vice versa, in the presence of macrolide resistance, amoxicillin allergy, previous treatment failures, I go for the bismuth quadruple therapy – if I can expect good treatment compliance because proton pump inhibitor plus potassium, metronidazole, and tetracycline for 10 days can mean 140 pills. Gerhard G. Treiber, MD, AGAF, is with the department of internal medicine at Saarland University Hospital, Homburg, Germany. He has no conflicts of interest.
A network meta-analysis of current first-line dual, triple, and quadruple therapies for Helicobacter pylori infection found that vonoprazan triple therapy was most effective, while standard triple therapy of a proton pump inhibitor (PPI), amoxicillin, and clarithromycin was least effective. Levofloxacin-containing triple therapy performed best in Western countries and West Asia, while reverse hybrid therapy was most effective in East Asia.
The results “[suggest that] a new approach concerning H. pylori treatment is now needed and that the time for transitioning from trial and error to antimicrobial stewardship [of H. pylori infection] has arrived,” wrote Theodore Rokkas, PhD, MD, of the European University of Cyprus in Engomi, and colleagues. Their study was published online April 8 in Gastroenterology.
H. pylori infection is the primary cause of gastritis, peptic ulcer disease, gastric mucosa–associated lymphoid tissue lymphoma, and gastric cancer.
Since H. pylori infection was first recognized, physicians have employed a range of drugs in double, triple, and quadruple combinations to combat it.
Despite those efforts, treatment success is lower than with many other infectious diseases. A newcomer is the potassium-competing acid blocker vonoprazan, which increases efficacy of amoxicillin combination therapies and has, thereby, generated renewed interest in all combination therapies, according to the study authors. Vonoprazan is currently available in some Asian countries, but not the United States or Europe.
Current guidelines for H. pylori treatment relied on randomized controlled trials and relevant pair-wise meta-analyses, but no previous pairwise analysis has included all currently available medications, the authors noted. Network meta-analyses can help fill this evidence gap: They incorporate both direct and indirect evidence from a collection of randomized controlled trials to estimate the comparative effectiveness of three or more regimens.
The researchers conducted a network meta-analysis that included 68 randomized, controlled trials totaling 22,975 patients. The following regimens were included in the analysis: Concomitant quadruple bismuth treatment (bismuth quadruple therapy), concomitant quadruple nonbismuth treatment (nonbismuth quadruple therapy), high-dose amoxicillin double treatment (Amox-dual therapy), levofloxacin-containing treatment (Levo-therapy), reverse hybrid therapy (R-hybrid therapy), sequential quadruple treatment (sequential therapy), standard triple treatment (triple therapy), and vonoprazan-containing therapy (Vono-triple therapy).
Statistically significant results were found with Vono-triple therapy versus triple therapy (odds ratio, 3.80; 95% confidence interval, 1.62-8.94), sequential therapy versus triple therapy (OR, 1.79; 95% CI, 1.26-2.53), nonbismuth quadruple therapy versus triple therapy (OR, 2.08; 95% CI, 1.45-2.98), bismuth quadruple therapy versus triple therapy (OR, 1.47; 95% CI, 1.02-2.11), and Levo-therapy versus triple therapy (OR, 1.79; 95% CI, 1.26-2.53).
In the overall data, mean cure rates greater than 90% were seen only in Vono-triple therapy (91.4%; 95% CI, 88.5-93.5%) and R-hybrid therapy (93.6%; 95% CI, 90.4-96.8%). Cure rates were lower for Nonbismuth quadruple therapy (84.3%; 95% CI, 82.7-85.8%), Levo-therapy (83.8%; 95% CI, 82.1-85.4%), Sequential therapy (83.7%; 95% CI, 82.7-84.7%), bismuth quadruple therapy (81.3%; 95% CI, 79.5-83.1%), Amox-dual therapy (80.2%; 75.3%-84.4%), and triple therapy (75.7%; 95% CI, 74.9-76.4%). Levo-therapy performed best in Western countries (88.5%; 95% CI, 86.5-90.5%) and West Asia (88.4%; 95% CI, 84.6-91.1%). R-hybrid therapy performed best in East Asia (93.6%; 95% CI, 90.4-96.8%).
A surface under the cumulative ranking (SUCRA) value, which represents the efficacy of the intervention compared to an ideal intervention, was 92.4% for Vono-triple therapy. The second highest SUCRA value was for 68.8% for nonbismuth quadruple therapy. The SUCRA value of standard triple therapy was 4.7%.
A key limitation to the study is that Vono-triple therapy was tested only in Japan, and requires additional study in other geographic regions.
The study received support from the Department of Veteran Affairs. The authors have consulted for and received research funding from various pharmaceutical companies.
A network meta-analysis of current first-line dual, triple, and quadruple therapies for Helicobacter pylori infection found that vonoprazan triple therapy was most effective, while standard triple therapy of a proton pump inhibitor (PPI), amoxicillin, and clarithromycin was least effective. Levofloxacin-containing triple therapy performed best in Western countries and West Asia, while reverse hybrid therapy was most effective in East Asia.
The results “[suggest that] a new approach concerning H. pylori treatment is now needed and that the time for transitioning from trial and error to antimicrobial stewardship [of H. pylori infection] has arrived,” wrote Theodore Rokkas, PhD, MD, of the European University of Cyprus in Engomi, and colleagues. Their study was published online April 8 in Gastroenterology.
H. pylori infection is the primary cause of gastritis, peptic ulcer disease, gastric mucosa–associated lymphoid tissue lymphoma, and gastric cancer.
Since H. pylori infection was first recognized, physicians have employed a range of drugs in double, triple, and quadruple combinations to combat it.
Despite those efforts, treatment success is lower than with many other infectious diseases. A newcomer is the potassium-competing acid blocker vonoprazan, which increases efficacy of amoxicillin combination therapies and has, thereby, generated renewed interest in all combination therapies, according to the study authors. Vonoprazan is currently available in some Asian countries, but not the United States or Europe.
Current guidelines for H. pylori treatment relied on randomized controlled trials and relevant pair-wise meta-analyses, but no previous pairwise analysis has included all currently available medications, the authors noted. Network meta-analyses can help fill this evidence gap: They incorporate both direct and indirect evidence from a collection of randomized controlled trials to estimate the comparative effectiveness of three or more regimens.
The researchers conducted a network meta-analysis that included 68 randomized, controlled trials totaling 22,975 patients. The following regimens were included in the analysis: Concomitant quadruple bismuth treatment (bismuth quadruple therapy), concomitant quadruple nonbismuth treatment (nonbismuth quadruple therapy), high-dose amoxicillin double treatment (Amox-dual therapy), levofloxacin-containing treatment (Levo-therapy), reverse hybrid therapy (R-hybrid therapy), sequential quadruple treatment (sequential therapy), standard triple treatment (triple therapy), and vonoprazan-containing therapy (Vono-triple therapy).
Statistically significant results were found with Vono-triple therapy versus triple therapy (odds ratio, 3.80; 95% confidence interval, 1.62-8.94), sequential therapy versus triple therapy (OR, 1.79; 95% CI, 1.26-2.53), nonbismuth quadruple therapy versus triple therapy (OR, 2.08; 95% CI, 1.45-2.98), bismuth quadruple therapy versus triple therapy (OR, 1.47; 95% CI, 1.02-2.11), and Levo-therapy versus triple therapy (OR, 1.79; 95% CI, 1.26-2.53).
In the overall data, mean cure rates greater than 90% were seen only in Vono-triple therapy (91.4%; 95% CI, 88.5-93.5%) and R-hybrid therapy (93.6%; 95% CI, 90.4-96.8%). Cure rates were lower for Nonbismuth quadruple therapy (84.3%; 95% CI, 82.7-85.8%), Levo-therapy (83.8%; 95% CI, 82.1-85.4%), Sequential therapy (83.7%; 95% CI, 82.7-84.7%), bismuth quadruple therapy (81.3%; 95% CI, 79.5-83.1%), Amox-dual therapy (80.2%; 75.3%-84.4%), and triple therapy (75.7%; 95% CI, 74.9-76.4%). Levo-therapy performed best in Western countries (88.5%; 95% CI, 86.5-90.5%) and West Asia (88.4%; 95% CI, 84.6-91.1%). R-hybrid therapy performed best in East Asia (93.6%; 95% CI, 90.4-96.8%).
A surface under the cumulative ranking (SUCRA) value, which represents the efficacy of the intervention compared to an ideal intervention, was 92.4% for Vono-triple therapy. The second highest SUCRA value was for 68.8% for nonbismuth quadruple therapy. The SUCRA value of standard triple therapy was 4.7%.
A key limitation to the study is that Vono-triple therapy was tested only in Japan, and requires additional study in other geographic regions.
The study received support from the Department of Veteran Affairs. The authors have consulted for and received research funding from various pharmaceutical companies.
FROM GASTROENTEROLOGY
Who’s at risk for enterocolitis in Hirschsprung’s?
In a small study of Hirschsprung’s disease (HSCR) patients, those with a low-fiber colonic mucosal acetylcholinesterase-positive (AChE+) innervation phenotype were more likely to suffer from postoperative enterocolitis, which can be life-threatening.
The study lends insight into crosstalk between the human enteric nervous and immune systems. It suggests a role for acetylcholine-secreting (cholinergic) nerve fibers in aganglionic sections of colon in patients with HSCR, which is a congenital disorder marked by the absence of enteric neuronal cells in the distal part of the gut.
There are also potential clinical implications. “These observations suggest that HSCR patients with low-fiber phenotype might have a higher risk of developing postoperative enterocolitis and that the fiber phenotype could serve as a predictive marker for development of prophylactic therapy,” wrote Simone Keck, PhD, of the University of Basel (Switzerland) and colleagues in a study published in Cellular and Molecular Gastroenterology and Hepatology.
HSCR is a multigenetic congenital condition that includes a lack of enteric ganglia cells (aganglionosis) in the distal part of the colon, leading to intestinal obstruction and prestenotic megacolon. Treatment consists of pull-through surgery to remove the aganglionic portion of the bowel, but 20%-50% of patients develop life-threatening HSCR-associated enterocolitis before or after surgery. Although the mechanism of the complication is uncertain, immune cells, intestinal barrier function, and the microbiome may play a role.
Mouse models have shown connections between the immune and nervous system, but it has been challenging to study the effects of specific neurotransmitters in humans. There are more than 30 separate neurotransmitters in the enteric nervous system, making it difficult to tease apart individual functions. But there are comparatively few enteric nervous system neurotransmitters in patients with HSCR and the aganglionic colon in these patients contains enlarged AChE+ nerve fibers, “neuronal cholinergic function can be examined particularly well” among these patients. .
The researchers of the current study from analyzed tissue from 44 pediatric HSCR patients who underwent pull-through surgery, along with 6 non-HSCR controls who had surgery for various other reasons. Tissue samples were semiquantitatively categorized according to the extent of colonic mucosal AChE+ innervation: Low-fiber rectosigmoid tissue lacked intrinsic nerve cell bodies and mucosal ACHe+ innervation, while high-fiber tissue lacked nerve cell bodies but had mucosal AChE+ innervation. The researchers also determined tissue cytokine profile and immune cell frequencies, and used confocal immunofluorescence microscopy to determine proximity of macrophages to nerve fibers and 16S-rDNA sequencing to determine microbial populations.
They found that aganglionic low-fiber samples had higher levels of inflammatory cytokines such as interleukin-17, IL-1-beta, and IL6. Levels of these cytokines were lower in both ganglionic sections of the colon and in high-fiber samples with mucosal AChE+ nerve fibers. Low-fiber samples also had elevated Th17 T cells, compared with high-fiber, aganglionic, and ganglionic distal colon samples. Regulatory T cells were highest in cholinergic high-fiber segments.
Out of 42 patients, 9 developed enterocolitis within 1 year of surgery; 7 had a low-fiber phenotype, while 2 were high-fiber. This difference was not statistically significant, but the researchers then performed a retrospective analysis of 29 HSCR patients to validate the findings. Of these, 14 developed enterocolitis after surgery, with 12 of the cases occurring among children with the low-fiber phenotype, and 2 cases occurred among those with the high-fiber phenotype.
The findings could help guide postsurgical management of HSCR by allowing clinicians to employ preventive measures against enterocolitis, such as high-volume enemas, antibiotics, prebiotics, probiotics, or dietary changes. Th17 cells are known to migrate to nearby mesenteric lymph nodes, where they may promote enterocolitis, and this site is usually not removed during HSCR surgery. Fiber phenotype could prompt a surgeon to also remove mesenteric lymph nodes to reduce enterocolitis risk. A potential therapeutic strategy is to target IL-17 or IL-23.
The study was funded by the University of Basel. The authors have no relevant financial disclosures.
Hirschsprung’s disease is a hereditary childhood disorder in which the enteric nervous system develops abnormally in the distal bowel. As a consequence, peristalsis fails in the aganglionic segment, causing obstruction and prestenotic megacolon. Standard of care is the surgical removal of the affected part of the colon and the connection of healthy ganglionic tissue to the anus. Unfortunately, a large fraction of Hirschsprung’s patients suffer from enterocolitis, diarrhea, and abdominal distention either before or after surgery, which can progress to life-threatening sepsis and organ failure.
Further research is needed to determine the reason for different levels of cholinergic fibers in the aganglionic colon and to validate these findings in a separate patient cohort.
Klaus H. Kaestner, PhD, MS, is director of the Next Generation Sequencing Center at the University of Pennsylvania, Philadelphia. He has no conflicts of interest.
Hirschsprung’s disease is a hereditary childhood disorder in which the enteric nervous system develops abnormally in the distal bowel. As a consequence, peristalsis fails in the aganglionic segment, causing obstruction and prestenotic megacolon. Standard of care is the surgical removal of the affected part of the colon and the connection of healthy ganglionic tissue to the anus. Unfortunately, a large fraction of Hirschsprung’s patients suffer from enterocolitis, diarrhea, and abdominal distention either before or after surgery, which can progress to life-threatening sepsis and organ failure.
Further research is needed to determine the reason for different levels of cholinergic fibers in the aganglionic colon and to validate these findings in a separate patient cohort.
Klaus H. Kaestner, PhD, MS, is director of the Next Generation Sequencing Center at the University of Pennsylvania, Philadelphia. He has no conflicts of interest.
Hirschsprung’s disease is a hereditary childhood disorder in which the enteric nervous system develops abnormally in the distal bowel. As a consequence, peristalsis fails in the aganglionic segment, causing obstruction and prestenotic megacolon. Standard of care is the surgical removal of the affected part of the colon and the connection of healthy ganglionic tissue to the anus. Unfortunately, a large fraction of Hirschsprung’s patients suffer from enterocolitis, diarrhea, and abdominal distention either before or after surgery, which can progress to life-threatening sepsis and organ failure.
Further research is needed to determine the reason for different levels of cholinergic fibers in the aganglionic colon and to validate these findings in a separate patient cohort.
Klaus H. Kaestner, PhD, MS, is director of the Next Generation Sequencing Center at the University of Pennsylvania, Philadelphia. He has no conflicts of interest.
In a small study of Hirschsprung’s disease (HSCR) patients, those with a low-fiber colonic mucosal acetylcholinesterase-positive (AChE+) innervation phenotype were more likely to suffer from postoperative enterocolitis, which can be life-threatening.
The study lends insight into crosstalk between the human enteric nervous and immune systems. It suggests a role for acetylcholine-secreting (cholinergic) nerve fibers in aganglionic sections of colon in patients with HSCR, which is a congenital disorder marked by the absence of enteric neuronal cells in the distal part of the gut.
There are also potential clinical implications. “These observations suggest that HSCR patients with low-fiber phenotype might have a higher risk of developing postoperative enterocolitis and that the fiber phenotype could serve as a predictive marker for development of prophylactic therapy,” wrote Simone Keck, PhD, of the University of Basel (Switzerland) and colleagues in a study published in Cellular and Molecular Gastroenterology and Hepatology.
HSCR is a multigenetic congenital condition that includes a lack of enteric ganglia cells (aganglionosis) in the distal part of the colon, leading to intestinal obstruction and prestenotic megacolon. Treatment consists of pull-through surgery to remove the aganglionic portion of the bowel, but 20%-50% of patients develop life-threatening HSCR-associated enterocolitis before or after surgery. Although the mechanism of the complication is uncertain, immune cells, intestinal barrier function, and the microbiome may play a role.
Mouse models have shown connections between the immune and nervous system, but it has been challenging to study the effects of specific neurotransmitters in humans. There are more than 30 separate neurotransmitters in the enteric nervous system, making it difficult to tease apart individual functions. But there are comparatively few enteric nervous system neurotransmitters in patients with HSCR and the aganglionic colon in these patients contains enlarged AChE+ nerve fibers, “neuronal cholinergic function can be examined particularly well” among these patients. .
The researchers of the current study from analyzed tissue from 44 pediatric HSCR patients who underwent pull-through surgery, along with 6 non-HSCR controls who had surgery for various other reasons. Tissue samples were semiquantitatively categorized according to the extent of colonic mucosal AChE+ innervation: Low-fiber rectosigmoid tissue lacked intrinsic nerve cell bodies and mucosal ACHe+ innervation, while high-fiber tissue lacked nerve cell bodies but had mucosal AChE+ innervation. The researchers also determined tissue cytokine profile and immune cell frequencies, and used confocal immunofluorescence microscopy to determine proximity of macrophages to nerve fibers and 16S-rDNA sequencing to determine microbial populations.
They found that aganglionic low-fiber samples had higher levels of inflammatory cytokines such as interleukin-17, IL-1-beta, and IL6. Levels of these cytokines were lower in both ganglionic sections of the colon and in high-fiber samples with mucosal AChE+ nerve fibers. Low-fiber samples also had elevated Th17 T cells, compared with high-fiber, aganglionic, and ganglionic distal colon samples. Regulatory T cells were highest in cholinergic high-fiber segments.
Out of 42 patients, 9 developed enterocolitis within 1 year of surgery; 7 had a low-fiber phenotype, while 2 were high-fiber. This difference was not statistically significant, but the researchers then performed a retrospective analysis of 29 HSCR patients to validate the findings. Of these, 14 developed enterocolitis after surgery, with 12 of the cases occurring among children with the low-fiber phenotype, and 2 cases occurred among those with the high-fiber phenotype.
The findings could help guide postsurgical management of HSCR by allowing clinicians to employ preventive measures against enterocolitis, such as high-volume enemas, antibiotics, prebiotics, probiotics, or dietary changes. Th17 cells are known to migrate to nearby mesenteric lymph nodes, where they may promote enterocolitis, and this site is usually not removed during HSCR surgery. Fiber phenotype could prompt a surgeon to also remove mesenteric lymph nodes to reduce enterocolitis risk. A potential therapeutic strategy is to target IL-17 or IL-23.
The study was funded by the University of Basel. The authors have no relevant financial disclosures.
In a small study of Hirschsprung’s disease (HSCR) patients, those with a low-fiber colonic mucosal acetylcholinesterase-positive (AChE+) innervation phenotype were more likely to suffer from postoperative enterocolitis, which can be life-threatening.
The study lends insight into crosstalk between the human enteric nervous and immune systems. It suggests a role for acetylcholine-secreting (cholinergic) nerve fibers in aganglionic sections of colon in patients with HSCR, which is a congenital disorder marked by the absence of enteric neuronal cells in the distal part of the gut.
There are also potential clinical implications. “These observations suggest that HSCR patients with low-fiber phenotype might have a higher risk of developing postoperative enterocolitis and that the fiber phenotype could serve as a predictive marker for development of prophylactic therapy,” wrote Simone Keck, PhD, of the University of Basel (Switzerland) and colleagues in a study published in Cellular and Molecular Gastroenterology and Hepatology.
HSCR is a multigenetic congenital condition that includes a lack of enteric ganglia cells (aganglionosis) in the distal part of the colon, leading to intestinal obstruction and prestenotic megacolon. Treatment consists of pull-through surgery to remove the aganglionic portion of the bowel, but 20%-50% of patients develop life-threatening HSCR-associated enterocolitis before or after surgery. Although the mechanism of the complication is uncertain, immune cells, intestinal barrier function, and the microbiome may play a role.
Mouse models have shown connections between the immune and nervous system, but it has been challenging to study the effects of specific neurotransmitters in humans. There are more than 30 separate neurotransmitters in the enteric nervous system, making it difficult to tease apart individual functions. But there are comparatively few enteric nervous system neurotransmitters in patients with HSCR and the aganglionic colon in these patients contains enlarged AChE+ nerve fibers, “neuronal cholinergic function can be examined particularly well” among these patients. .
The researchers of the current study from analyzed tissue from 44 pediatric HSCR patients who underwent pull-through surgery, along with 6 non-HSCR controls who had surgery for various other reasons. Tissue samples were semiquantitatively categorized according to the extent of colonic mucosal AChE+ innervation: Low-fiber rectosigmoid tissue lacked intrinsic nerve cell bodies and mucosal ACHe+ innervation, while high-fiber tissue lacked nerve cell bodies but had mucosal AChE+ innervation. The researchers also determined tissue cytokine profile and immune cell frequencies, and used confocal immunofluorescence microscopy to determine proximity of macrophages to nerve fibers and 16S-rDNA sequencing to determine microbial populations.
They found that aganglionic low-fiber samples had higher levels of inflammatory cytokines such as interleukin-17, IL-1-beta, and IL6. Levels of these cytokines were lower in both ganglionic sections of the colon and in high-fiber samples with mucosal AChE+ nerve fibers. Low-fiber samples also had elevated Th17 T cells, compared with high-fiber, aganglionic, and ganglionic distal colon samples. Regulatory T cells were highest in cholinergic high-fiber segments.
Out of 42 patients, 9 developed enterocolitis within 1 year of surgery; 7 had a low-fiber phenotype, while 2 were high-fiber. This difference was not statistically significant, but the researchers then performed a retrospective analysis of 29 HSCR patients to validate the findings. Of these, 14 developed enterocolitis after surgery, with 12 of the cases occurring among children with the low-fiber phenotype, and 2 cases occurred among those with the high-fiber phenotype.
The findings could help guide postsurgical management of HSCR by allowing clinicians to employ preventive measures against enterocolitis, such as high-volume enemas, antibiotics, prebiotics, probiotics, or dietary changes. Th17 cells are known to migrate to nearby mesenteric lymph nodes, where they may promote enterocolitis, and this site is usually not removed during HSCR surgery. Fiber phenotype could prompt a surgeon to also remove mesenteric lymph nodes to reduce enterocolitis risk. A potential therapeutic strategy is to target IL-17 or IL-23.
The study was funded by the University of Basel. The authors have no relevant financial disclosures.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
PPIs could be bad news for oral cancer therapies
A substantial proportion of patients with cancer use proton pump inhibitors (PPIs), and up to one-third of these patients are also using oral cancer treatments that could be adversely affected by concomitant PPI use, according to a cross-sectional analysis.
Amit Patel, MD, a gastroenterologist with Duke University, Durham, N.C., was not involved in the study but commented on it in an interview. The “sobering” study findings highlight the need for “clinicians to carefully and regularly assess the indications and need for PPI, which are often overutilized, and consider ‘deprescribing’ based on clinical guidance,” he explained.
Previous research indicates the use of PPIs can lower the bioavailability and efficacy of oral cancer treatments, such as tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors. In the current study, published in JAMA Network Open, researchers sought to identify how many patients with cancer were taking treatments at risk for altered efficacy from PPI use and what factors were associated with use of PPIs.
The study findings
Jean-Luc Raoul, MD, and colleagues, analyzed physician-reported medical data of 566 women and 306 men with cancer from four comprehensive cancer centers in France, with a median age of 63 years. A total of 229 patients in the study (26.3%) were taking PPIs.
Most patients (71.1%) were using PPIs on a regular basis; reasons included epigastric pain (50.0%), retrosternal pain (14.0%), proven esophageal or gastric ulcer (8.0%), or gastroprotection (15.0%).
Factors associated with PPI use in this cohort included older age (odds ratio, 1.02; P <.001), Eastern Cooperative Oncology Group performance status (PS) (PS 1: OR, 1.92; PS 2: OR, 2.51; PS 3: OR, 2.33; P <.001), receipt of hormone therapy (OR, 0.59; P =.01), metastatic stage (P =.03), and tumor site (P =.045).
Older age and PS are particularly important characteristics, explained Dr. Patel. “Unfortunately, older patients with cancer and/or poor PS are more likely to have medical interactions that may result in their being prescribed PPI medications, often for indications that may not justify their use, and/or for indefinite durations.”
He noted that clinicians who are considering prescribing PPI medications should carefully address the indications for PPIs in the clinical scenario, the evidence supporting PPI use for the indication, ratio of benefits and risks, and potential alternatives to PPI use to mitigate potential issues with other therapies.
Approximately 29% of patients who took drugs whose efficacy might be affected by PPI use were also taking other medications, including capecitabine (n = 5), sunitinib (n = 5), cabozantinib (n = 2), pazopanib (n = 1), gefitinib (n = 1), erlotinib (n = 1), and sorafenib (n = 1). Another 39 out of 90 patients (25.6%) taking PPIs were also receiving checkpoint inhibitors. Of the 20 patients who took TKIs and PPIs, a total of 16 reported long-term PPI use. The most common reason for long-term use of PPIs was related to epigastric pain (n = 11).
Since this study was based on physician-reported data, the analysis was limited by the lack of data for all patients seen by each participating physician. In spite of this limitation, the investigators reported no sources of major bias and suggested the study’s prospective nature and relatively large-sized cohorts strengthened the analysis.
PPI use and cancer care
Although issues exist with PPIs in respect to cancer therapies, there are some strategies which may help reduce possible negative effects, Dr. Patel said. “When PPI medications are prescribed, they should be used at the lowest effective dose for the shortest necessary duration, and their use should be regularly reevaluated for dose reduction and/or potential discontinuation.”
Dr. Patel noted that, based on the indication for PPIs, alternatives to PPIs should be considered in the setting of potential drug-drug interactions that may affect the efficacy of oral cancer therapies. “For example, for intermittent typical reflux symptoms such as heartburn, over-the-counter antacids may be considered, along with reflux lifestyle medications,” he explained.
Likewise, the study authors stated in their research letter that “PPIs should be actively identified and substituted” in certain cases. The authors added that antacids are also the best option for patients taking checkpoint inhibitors.
“For those patients who absolutely must take TKI and PPI, clinicians can also consider staggering the dosing schedule, such as taking the TKI in the morning at least 2 hours before PPI and/or with an acidic beverage,” added Dr. Patel.
Although the findings from this study raise potential concerns, Dr. Patel stated further clinical investigations are needed to help the medical community better understand the specific effects of PPIs on the efficacy of various chemotherapeutic agents and to also help develop better management options for patients in these settings.
The authors reported relationships with Bayer, Merck, Transgene, and others. Dr. Patel has no relevant conflicts of interest to report.
A substantial proportion of patients with cancer use proton pump inhibitors (PPIs), and up to one-third of these patients are also using oral cancer treatments that could be adversely affected by concomitant PPI use, according to a cross-sectional analysis.
Amit Patel, MD, a gastroenterologist with Duke University, Durham, N.C., was not involved in the study but commented on it in an interview. The “sobering” study findings highlight the need for “clinicians to carefully and regularly assess the indications and need for PPI, which are often overutilized, and consider ‘deprescribing’ based on clinical guidance,” he explained.
Previous research indicates the use of PPIs can lower the bioavailability and efficacy of oral cancer treatments, such as tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors. In the current study, published in JAMA Network Open, researchers sought to identify how many patients with cancer were taking treatments at risk for altered efficacy from PPI use and what factors were associated with use of PPIs.
The study findings
Jean-Luc Raoul, MD, and colleagues, analyzed physician-reported medical data of 566 women and 306 men with cancer from four comprehensive cancer centers in France, with a median age of 63 years. A total of 229 patients in the study (26.3%) were taking PPIs.
Most patients (71.1%) were using PPIs on a regular basis; reasons included epigastric pain (50.0%), retrosternal pain (14.0%), proven esophageal or gastric ulcer (8.0%), or gastroprotection (15.0%).
Factors associated with PPI use in this cohort included older age (odds ratio, 1.02; P <.001), Eastern Cooperative Oncology Group performance status (PS) (PS 1: OR, 1.92; PS 2: OR, 2.51; PS 3: OR, 2.33; P <.001), receipt of hormone therapy (OR, 0.59; P =.01), metastatic stage (P =.03), and tumor site (P =.045).
Older age and PS are particularly important characteristics, explained Dr. Patel. “Unfortunately, older patients with cancer and/or poor PS are more likely to have medical interactions that may result in their being prescribed PPI medications, often for indications that may not justify their use, and/or for indefinite durations.”
He noted that clinicians who are considering prescribing PPI medications should carefully address the indications for PPIs in the clinical scenario, the evidence supporting PPI use for the indication, ratio of benefits and risks, and potential alternatives to PPI use to mitigate potential issues with other therapies.
Approximately 29% of patients who took drugs whose efficacy might be affected by PPI use were also taking other medications, including capecitabine (n = 5), sunitinib (n = 5), cabozantinib (n = 2), pazopanib (n = 1), gefitinib (n = 1), erlotinib (n = 1), and sorafenib (n = 1). Another 39 out of 90 patients (25.6%) taking PPIs were also receiving checkpoint inhibitors. Of the 20 patients who took TKIs and PPIs, a total of 16 reported long-term PPI use. The most common reason for long-term use of PPIs was related to epigastric pain (n = 11).
Since this study was based on physician-reported data, the analysis was limited by the lack of data for all patients seen by each participating physician. In spite of this limitation, the investigators reported no sources of major bias and suggested the study’s prospective nature and relatively large-sized cohorts strengthened the analysis.
PPI use and cancer care
Although issues exist with PPIs in respect to cancer therapies, there are some strategies which may help reduce possible negative effects, Dr. Patel said. “When PPI medications are prescribed, they should be used at the lowest effective dose for the shortest necessary duration, and their use should be regularly reevaluated for dose reduction and/or potential discontinuation.”
Dr. Patel noted that, based on the indication for PPIs, alternatives to PPIs should be considered in the setting of potential drug-drug interactions that may affect the efficacy of oral cancer therapies. “For example, for intermittent typical reflux symptoms such as heartburn, over-the-counter antacids may be considered, along with reflux lifestyle medications,” he explained.
Likewise, the study authors stated in their research letter that “PPIs should be actively identified and substituted” in certain cases. The authors added that antacids are also the best option for patients taking checkpoint inhibitors.
“For those patients who absolutely must take TKI and PPI, clinicians can also consider staggering the dosing schedule, such as taking the TKI in the morning at least 2 hours before PPI and/or with an acidic beverage,” added Dr. Patel.
Although the findings from this study raise potential concerns, Dr. Patel stated further clinical investigations are needed to help the medical community better understand the specific effects of PPIs on the efficacy of various chemotherapeutic agents and to also help develop better management options for patients in these settings.
The authors reported relationships with Bayer, Merck, Transgene, and others. Dr. Patel has no relevant conflicts of interest to report.
A substantial proportion of patients with cancer use proton pump inhibitors (PPIs), and up to one-third of these patients are also using oral cancer treatments that could be adversely affected by concomitant PPI use, according to a cross-sectional analysis.
Amit Patel, MD, a gastroenterologist with Duke University, Durham, N.C., was not involved in the study but commented on it in an interview. The “sobering” study findings highlight the need for “clinicians to carefully and regularly assess the indications and need for PPI, which are often overutilized, and consider ‘deprescribing’ based on clinical guidance,” he explained.
Previous research indicates the use of PPIs can lower the bioavailability and efficacy of oral cancer treatments, such as tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors. In the current study, published in JAMA Network Open, researchers sought to identify how many patients with cancer were taking treatments at risk for altered efficacy from PPI use and what factors were associated with use of PPIs.
The study findings
Jean-Luc Raoul, MD, and colleagues, analyzed physician-reported medical data of 566 women and 306 men with cancer from four comprehensive cancer centers in France, with a median age of 63 years. A total of 229 patients in the study (26.3%) were taking PPIs.
Most patients (71.1%) were using PPIs on a regular basis; reasons included epigastric pain (50.0%), retrosternal pain (14.0%), proven esophageal or gastric ulcer (8.0%), or gastroprotection (15.0%).
Factors associated with PPI use in this cohort included older age (odds ratio, 1.02; P <.001), Eastern Cooperative Oncology Group performance status (PS) (PS 1: OR, 1.92; PS 2: OR, 2.51; PS 3: OR, 2.33; P <.001), receipt of hormone therapy (OR, 0.59; P =.01), metastatic stage (P =.03), and tumor site (P =.045).
Older age and PS are particularly important characteristics, explained Dr. Patel. “Unfortunately, older patients with cancer and/or poor PS are more likely to have medical interactions that may result in their being prescribed PPI medications, often for indications that may not justify their use, and/or for indefinite durations.”
He noted that clinicians who are considering prescribing PPI medications should carefully address the indications for PPIs in the clinical scenario, the evidence supporting PPI use for the indication, ratio of benefits and risks, and potential alternatives to PPI use to mitigate potential issues with other therapies.
Approximately 29% of patients who took drugs whose efficacy might be affected by PPI use were also taking other medications, including capecitabine (n = 5), sunitinib (n = 5), cabozantinib (n = 2), pazopanib (n = 1), gefitinib (n = 1), erlotinib (n = 1), and sorafenib (n = 1). Another 39 out of 90 patients (25.6%) taking PPIs were also receiving checkpoint inhibitors. Of the 20 patients who took TKIs and PPIs, a total of 16 reported long-term PPI use. The most common reason for long-term use of PPIs was related to epigastric pain (n = 11).
Since this study was based on physician-reported data, the analysis was limited by the lack of data for all patients seen by each participating physician. In spite of this limitation, the investigators reported no sources of major bias and suggested the study’s prospective nature and relatively large-sized cohorts strengthened the analysis.
PPI use and cancer care
Although issues exist with PPIs in respect to cancer therapies, there are some strategies which may help reduce possible negative effects, Dr. Patel said. “When PPI medications are prescribed, they should be used at the lowest effective dose for the shortest necessary duration, and their use should be regularly reevaluated for dose reduction and/or potential discontinuation.”
Dr. Patel noted that, based on the indication for PPIs, alternatives to PPIs should be considered in the setting of potential drug-drug interactions that may affect the efficacy of oral cancer therapies. “For example, for intermittent typical reflux symptoms such as heartburn, over-the-counter antacids may be considered, along with reflux lifestyle medications,” he explained.
Likewise, the study authors stated in their research letter that “PPIs should be actively identified and substituted” in certain cases. The authors added that antacids are also the best option for patients taking checkpoint inhibitors.
“For those patients who absolutely must take TKI and PPI, clinicians can also consider staggering the dosing schedule, such as taking the TKI in the morning at least 2 hours before PPI and/or with an acidic beverage,” added Dr. Patel.
Although the findings from this study raise potential concerns, Dr. Patel stated further clinical investigations are needed to help the medical community better understand the specific effects of PPIs on the efficacy of various chemotherapeutic agents and to also help develop better management options for patients in these settings.
The authors reported relationships with Bayer, Merck, Transgene, and others. Dr. Patel has no relevant conflicts of interest to report.
FROM JAMA NETWORK OPEN
Microscopic colitis: A common, yet often overlooked, cause of chronic diarrhea
Microscopic colitis is an inflammatory disease of the colon and a frequent cause of chronic or recurrent watery diarrhea, particularly in older persons. MC consists of two subtypes, collagenous colitis (CC) and lymphocytic colitis (LC). While the primary symptom is diarrhea, other signs and symptoms such as abdominal pain, weight loss, and dehydration or electrolyte abnormalities may also be present depending on disease severity.1 In MC, the colonic mucosa usually appears normal on colonoscopy, and the diagnosis is made by histologic findings of intraepithelial lymphocytosis with (CC) or without (LC) a prominent subepithelial collagen band. The management approaches to CC and LC are similar and should be directed based on the severity of symptoms.2 We review the epidemiology, risk factors, pathophysiology, diagnosis, and clinical management for this condition, as well as novel therapeutic approaches.
Epidemiology
Although the incidence of MC increased in the late twentieth century, more recently, it has stabilized with an estimated incidence varying from 1 to 25 per 100,000 person-years.3-5 A recent meta-analysis revealed a pooled incidence of 4.85 per 100,000 persons for LC and 4.14 per 100,000 persons for CC.6 Proposed explanations for the rising incidence in the late twentieth century include improved clinical awareness of the disease, possible increased use of drugs associated with MC, and increased performance of diagnostic colonoscopies for chronic diarrhea. Since MC is now well-recognized, the recent plateau in incidence rates may reflect decreased detection bias.
The prevalence of MC ranges from 10%-20% in patients undergoing colonoscopy for chronic watery diarrhea.6,7 The prevalence of LC is approximately 63.1 cases per 100,000 person-years and, for CC, is 49.2 cases per 100,000 person-years.6-8 Recent studies have demonstrated increasing prevalence of MC likely resulting from an aging population.9,10
Risk stratification
Female gender, increasing age, concomitant autoimmune disease, and the use of certain drugs, including NSAIDs, proton pump inhibitors (PPIs), statins, and selective serotonin reuptake inhibitors (SSRIs), have been associated with an increased risk of MC.11,12 Autoimmune disorders, including celiac disease (CD), rheumatoid arthritis, hypothyroidism, and hyperthyroidism, are more common in patients with MC. The association with CD, in particular, is clinically important, as CD is associated with a 50-70 times greater risk of MC, and 2%-9% of patients with MC have CD.13,14
Several medications have been associated with MC. In a British multicenter prospective study, MC was associated with the use of NSAIDs, PPIs, and SSRIs;15 however, recent studies have questioned the association of MC with some of these medications, which might worsen diarrhea but not actually cause MC.16
An additional risk factor for MC is smoking. A recent meta-analysis demonstrated that current and former smokers had an increased risk of MC (odds ratio, 2.99; 95% confidence interval, 2.15-4.15 and OR, 1.63; 95% CI, 1.37-1.94, respectively), compared with nonsmokers.17 Smokers develop MC at a younger age, and smoking is associated with increased disease severity and decreased likelihood of attaining remission.18,19
Pathogenesis
The pathogenesis of MC remains largely unknown, although there are several hypotheses. The leading proposed mechanisms include reaction to luminal antigens, dysregulated collagen metabolism, genetic predisposition, autoimmunity, and bile acid malabsorption.
MC may be caused by abnormal epithelial barrier function, leading to increased permeability and reaction to luminal antigens, including dietary antigens, certain drugs, and bacterial products, 20,21 which themselves lead to the immune dysregulation and intestinal inflammation seen in MC. This mechanism may explain the association of several drugs with MC. Histological changes resembling LC are reported in patients with CD who consume gluten; however, large population-based studies have not found specific dietary associations with the development of MC.22
Another potential mechanism of MC is dysregulated collagen deposition. Collagen accumulation in the subepithelial layer in CC may result from increased levels of fibroblast growth factor, transforming growth factor–beta and vascular endothelial growth factor.23 Nonetheless, studies have not found an association between the severity of diarrhea in patients with CC and the thickness of the subepithelial collagen band.
Thirdly, autoimmunity and genetic predisposition have been postulated in the pathogenesis of MC. As previously discussed, MC is associated with several autoimmune diseases and predominantly occurs in women, a distinctive feature of autoimmune disorders. Several studies have demonstrated an association between MC and HLA-DQ2 and -DQ3 haplotypes,24 as well as potential polymorphisms in the serotonin transporter gene promoter.25 It is important to note, however, that only a few familial cases of MC have been reported to date.26
Lastly, bile acid malabsorption may play a role in the etiology of MC. Histologic findings of inflammation, along with villous atrophy and collagen deposition, have been reported in the ileum of patients with MC;27,28 however, because patients with MC without bile acid malabsorption may also respond to bile acid binders such as cholestyramine, these findings unlikely to be the sole mechanism explaining the development of the disease.
Despite the different proposed mechanisms for the pathogenesis of MC, no definite conclusions can be drawn because of the limited size of these studies and their often conflicting results.

Clinical features
Clinicians should suspect MC in patients with chronic or recurrent watery diarrhea, particularly in older persons. Other risk factors include female gender, use of certain culprit medications, smoking, and presence of other autoimmune diseases. The clinical manifestations of MC subtypes LC and CC are similar with no significant clinical differences.1,2 In addition to diarrhea, patients with MC may have abdominal pain, fatigue, and dehydration or electrolyte abnormalities depending on disease severity. Patients may also present with fecal urgency, incontinence, and nocturnal stools. Quality of life is often reduced in these patients, predominantly in those with severe or refractory symptoms.29,30 The natural course of MC is highly variable, with some patients achieving spontaneous resolution after one episode and others developing chronic symptoms.
Diagnosis
The differential diagnosis of chronic watery diarrhea is broad and includes malabsorption/maldigestion, inflammatory bowel disease (IBD), irritable bowel syndrome, and medication side effects. In addition, although gastrointestinal infections typically cause acute or subacute diarrhea, some can present with chronic diarrhea. Malabsorption/maldigestion may occur because of CD, lactose intolerance, and pancreatic insufficiency, among other conditions. A thorough history, regarding recent antibiotic and medication use, travel, and immunosuppression, should be obtained in patients with chronic diarrhea. Additionally, laboratory and endoscopic evaluation with random biopsies of the colon can further help differentiate these diseases from MC. A few studies suggest fecal calprotectin may be used to differentiate MC from other noninflammatory conditions such as irritable bowel syndrome, as well as to monitor disease activity. This test is not expected to distinguish MC from other inflammatory causes of diarrhea, such as IBD, and therefore, its role in clinical practice is uncertain.31
The diagnosis of MC is made by biopsy of the colonic mucosa demonstrating characteristic pathologic features.32 Unlike in diseases such as Crohn’s disease or ulcerative colitis, the colon usually appears normal in MC, although mild nonspecific changes, such as erythema or edema, may be visualized. There is no consensus on the ideal location to obtain biopsies for MC or whether biopsies from both the left and the right colon are required.2,33 The procedure of choice for the diagnosis of MC is colonoscopy with random biopsies taken throughout the colon. More limited evaluation by flexible sigmoidoscopy with biopsies may miss cases of MC as inflammation and collagen thickening are not necessarily uniform throughout the colon; however, in a patient that has undergone a recent colonoscopy for colon cancer screening without colon biopsies, a flexible sigmoidoscopy may be a reasonable next test for evaluation of MC, provided biopsies are obtained above the rectosigmoid colon.34
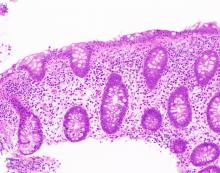
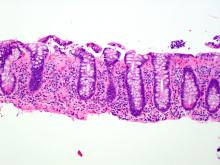
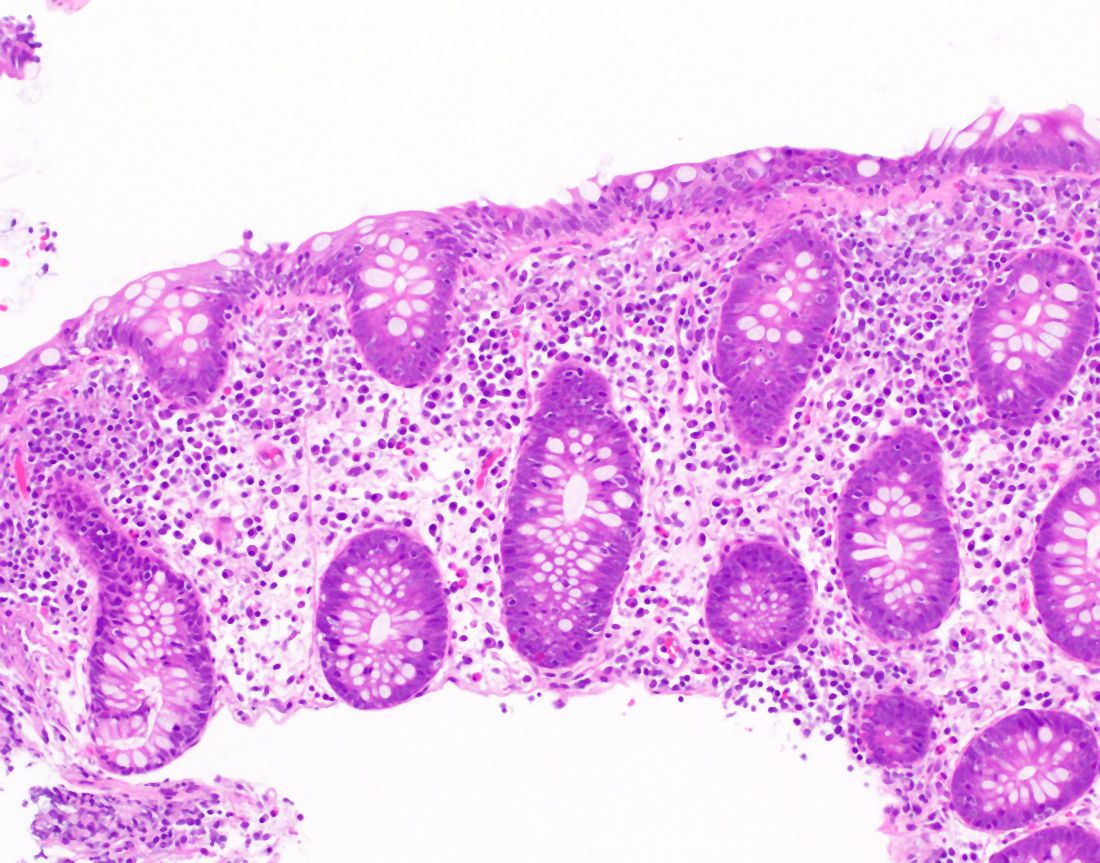
The MC subtypes are differentiated based on histology. The hallmark of LC is less than 20 intraepithelial lymphocytes per 100 surface epithelial cells (normal, less than 5) (Figure 1A). CC is characterized by a thickened subepithelial collagen band greater than 7-10 micrometers (normal, less than 5) (Figure 1B). For a subgroup of patients with milder abnormalities that do not meet these histological criteria, the terms “microscopic colitis, not otherwise specified” or “microscopic colitis, incomplete” may be used.35 These patients often respond to standard treatments for MC. There is an additional subset of patients with biopsy demonstrating features of both CC and LC simultaneously, as well as patients transitioning from one MC subtype to another over time.32,35
Management approach
The first step in management of patients with MC includes stopping culprit medications if there is a temporal relationship between the initiation of the medication and the onset of diarrhea, as well as encouraging smoking cessation. These steps alone, however, are unlikely to achieve clinical remission in most patients. A stepwise pharmacological approach is used in the management of MC based on disease severity (Figure 2). For patients with mild symptoms, antidiarrheal medications, such as loperamide, may be helpful.36 Long-term use of loperamide at therapeutic doses no greater than 16 mg daily appears to be safe if required to maintain symptom response. For those with persistent symptoms despite antidiarrheal medications, bismuth subsalicylate at three 262 mg tablets three times daily for 6-8 weeks can be considered. Long-term use of bismuth subsalicylate is not advised, especially at this dose, because of possible neurotoxicity.37
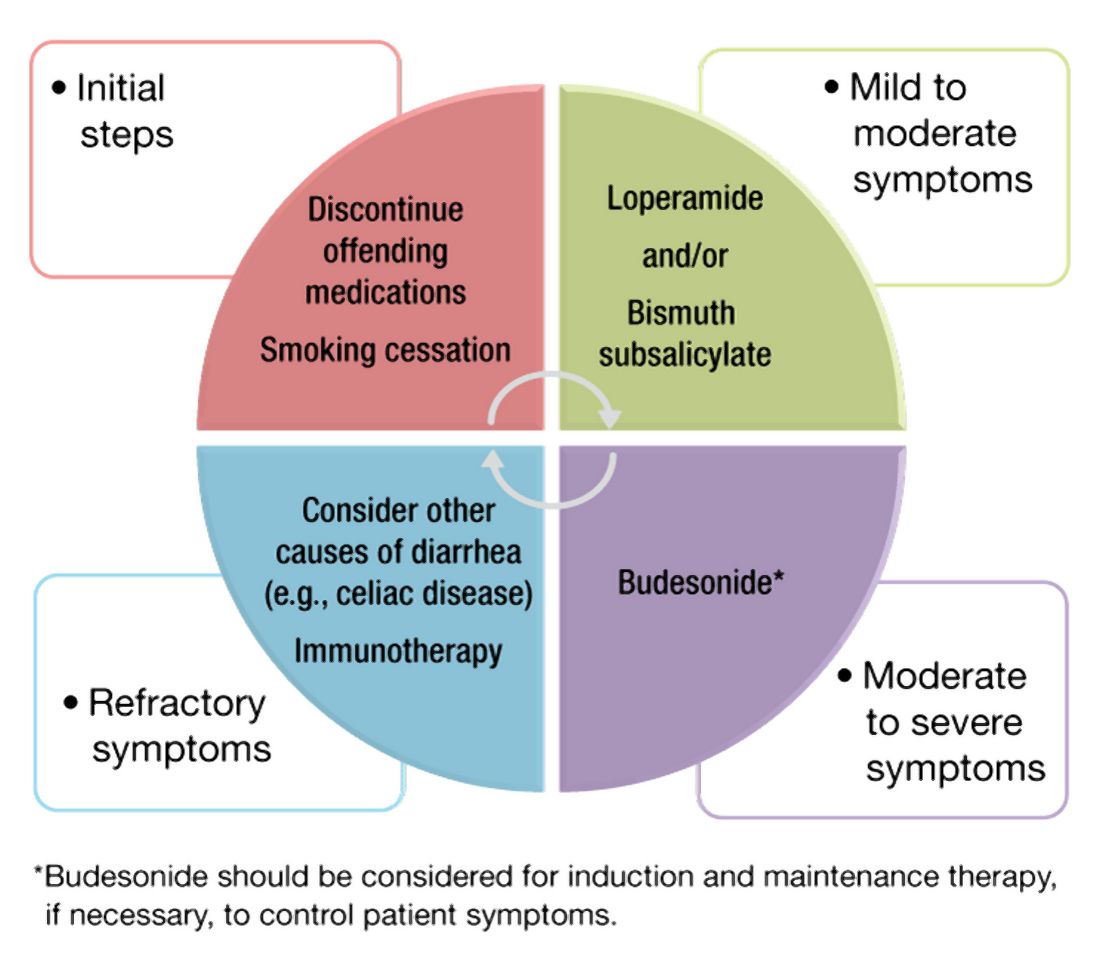
For patients refractory to the above treatments or those with moderate-to-severe symptoms, an 8-week course of budesonide at 9 mg daily is the first-line treatment.38 The dose was tapered before discontinuation in some studies but not in others. Both strategies appear effective. A recent meta-analysis of nine randomized trials demonstrated pooled ORs of 7.34 (95% CI, 4.08-13.19) and 8.35 (95% CI, 4.14-16.85) for response to budesonide induction and maintenance, respectively.39
Cholestyramine is another medication considered in the management of MC and warrants further investigation. To date, no randomized clinical trials have been conducted to evaluate bile acid sequestrants in MC, but they should be considered before placing patients on immunosuppressive medications. Some providers use mesalamine in this setting, although mesalamine is inferior to budesonide in the induction of clinical remission in MC.40
Despite high rates of response to budesonide, relapse after discontinuation is frequent (60%-80%), and time to relapse is variable41,42 The American Gastroenterological Association recommends budesonide for maintenance of remission in patients with recurrence following discontinuation of induction therapy. The lowest effective dose that maintains resolution of symptoms should be prescribed, ideally at 6 mg daily or lower.38 Although budesonide has a greater first-pass metabolism, compared with other glucocorticoids, patients should be monitored for possible side effects including hypertension, diabetes, and osteoporosis, as well as ophthalmologic disease, including cataracts and glaucoma.
For those who are intolerant to budesonide or have refractory symptoms, concomitant disorders such as CD that may be contributing to symptoms must be excluded. Immunosuppressive medications – such as thiopurines and biologic agents, including tumor necrosis factor–alpha inhibitors or vedolizumab – may be considered in refractory cases.43,44 Of note, there are limited studies evaluating the use of these medications for MC. Lastly, surgeries including ileostomy with or without colectomy have been performed in the most severe cases for resistant disease that has failed numerous pharmacological therapies.45
Patients should be counseled that, while symptoms from MC can be quite bothersome and disabling, there appears to be a normal life expectancy and no association between MC and colon cancer, unlike with other inflammatory conditions of the colon such as IBD.46,47
Conclusion and future outlook
As a common cause of chronic watery diarrhea, MC will be commonly encountered in primary care and gastroenterology practices. The diagnosis should be suspected in patients presenting with chronic or recurrent watery diarrhea, especially with female gender, autoimmune disease, and increasing age. The management of MC requires an algorithmic approach directed by symptom severity, with a subgroup of patients requiring maintenance therapy for relapsing symptoms. The care of patients with MC will continue to evolve in the future. Further work is needed to explore long-term safety outcomes with budesonide and the role of immunomodulators and newer biologic agents for patients with complex, refractory disease.
Dr. Tome is with the department of internal medicine at the Mayo Clinic, Rochester, Minn. Dr. Kamboj, and Dr. Pardi are with the division of gastroenterology and hepatology at the Mayo Clinic. Dr. Pardi has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda and has consulted for Vedanta and Otsuka. The other authors have no conflicts of interest to report.
References
1. Nyhlin N et al. Aliment Pharmacol Ther. 2014;39:963-72.
2. Miehlke S et al. United European Gastroenterol J. 2020;20-8.
3. Pardi DS et al. Gut. 2007;56:504-8.
4. Fernández-Bañares F et al. J Crohn’s Colitis.2016;10(7):805-11.
5. Gentile NM et al. Clin Gastroenterol Hepatol. 2014;12(5):838-42.
6. Tong J et al. Am J Gastroenterol. 2015;110:265-76.
7. Olesen M et al. Gut. 2004;53(3):346-50.
8. Bergman D et al. Aliment Pharmacol Ther. 2019;49(11):1395-400.
9. Guagnozzi D et al. Dig Liver Dis. 2012;44(5):384-8.
10. Münch A et al. J Crohns Colitis. 2012;6(9):932-45.
11. Macaigne G et al. Am J Gastroenterol. 2014; 09(9):1461-70.
12. Verhaegh BP et al. Aliment Pharmacol Ther. 2016;43(9):1004-13.
13. Stewart M et al. Aliment Pharmacol Ther. 2011;33(12):1340-9.
14. Green PHR et al. Clin Gastroenterol Hepatol. 2009;7(11):1210-6.
15. Masclee GM et al. Am J Gastroenterol. 2015;110:749-59.
16. Zylberberg H et al. Ailment Pharmacol Ther. 2021 Jun;53(11)1209-15.
17. Jaruvongvanich V et al. Inflamm Bowel Dis. 2019;25(4):672-8.
18. Fernández-Bañares F et al. Inflamm Bowel Dis. 2013; 19(7):1470-6.
19. Yen EF et al. Inflamm Bowel Dis. 2012;18(10):1835-41.
20. Barmeyer C et al. J Gastroenterol. 2017;52(10):1090-100.
21. Morgan DM et al. Clin Gastroenterol Hepatol. 2020;18(4):984-6.
22. Larsson JK et al. Eur J Clin Nutr. 2016;70:1309-17.
23. Madisch A et al.. Inflamm Bowel Dis. 2011;17(11):2295-8.
24. Stahl E et al. Gastroenterology. 2020;159(2):549-61.
25. Sikander A et al. Dig Dis Sci. 2015; 60:887-94.
26. Abdo AA et al. Can J Gastroenterol. 2001;15(5):341-3.
27. Fernandez-Bañares F et al. Dig Dis Sci.2001;46(10):2231-8.
28. Lyutakov I et al. Eur J Gastroenterol Hepatol. 2021;1;33(3):380-7.
29. Hjortswang H et al. Dig Liver Dis. 2011 Feb;43(2):102-9.
30. Cotter TG= et al. Gut. 2018;67(3):441-6.
31. Von Arnim U et al. Clin Exp Gastroenterol. 2016;9:97-103.
32. Langner C et al. Histopathology. 2015;66:613-26.
33. ASGE Standards of Practice Committee and Sharaf RN et al. Gastrointest Endosc. 2013;78:216-24.
34. Macaigne G et al. Clin Res Hepatol Gastroenterol. 2017;41(3):333-40.
35. Bjørnbak C et al. Aliment Pharmacol Ther. 2011;34(10):1225-34.
36. Pardi DS et al. Gastroenterology. 2016;150(1):247-74.
37. Masannat Y and Nazer E. West Virginia Med J. 2013;109(3):32-4.
38. Nguyen GC et al. Gastroenterology. 2016; 150(1):242-6.
39. Sebastian S et al. Eur J Gastroenterol Hepatol. 2019 Aug;31(8):919-27.
40. Miehlke S et al. Gastroenterology. 2014;146(5):1222-30.
41. Gentile NM et al. Am J Gastroenterol. 2013;108:256-9.
42. Münch A et al. Gut. 2016; 65(1):47-56.
43. Cotter TG et al. Aliment Pharmacol Ther. 2017; 46(2):169-74.
44. Esteve M et al. J Crohns Colitis. 2011;5(6):612-8.
45. Cottreau J et al. Clin J Gastroenterol. 2016;9:140-4.
46. Kamboj AK et al. Program No. P1876. ACG 2018 Annual Scientific Meeting Abstracts. Philadelphia, Pennsylvania: American College of Gastroenterology.
47. Yen EF et al. Dig Dis Sci. 2012;57:161-9.
Microscopic colitis is an inflammatory disease of the colon and a frequent cause of chronic or recurrent watery diarrhea, particularly in older persons. MC consists of two subtypes, collagenous colitis (CC) and lymphocytic colitis (LC). While the primary symptom is diarrhea, other signs and symptoms such as abdominal pain, weight loss, and dehydration or electrolyte abnormalities may also be present depending on disease severity.1 In MC, the colonic mucosa usually appears normal on colonoscopy, and the diagnosis is made by histologic findings of intraepithelial lymphocytosis with (CC) or without (LC) a prominent subepithelial collagen band. The management approaches to CC and LC are similar and should be directed based on the severity of symptoms.2 We review the epidemiology, risk factors, pathophysiology, diagnosis, and clinical management for this condition, as well as novel therapeutic approaches.
Epidemiology
Although the incidence of MC increased in the late twentieth century, more recently, it has stabilized with an estimated incidence varying from 1 to 25 per 100,000 person-years.3-5 A recent meta-analysis revealed a pooled incidence of 4.85 per 100,000 persons for LC and 4.14 per 100,000 persons for CC.6 Proposed explanations for the rising incidence in the late twentieth century include improved clinical awareness of the disease, possible increased use of drugs associated with MC, and increased performance of diagnostic colonoscopies for chronic diarrhea. Since MC is now well-recognized, the recent plateau in incidence rates may reflect decreased detection bias.
The prevalence of MC ranges from 10%-20% in patients undergoing colonoscopy for chronic watery diarrhea.6,7 The prevalence of LC is approximately 63.1 cases per 100,000 person-years and, for CC, is 49.2 cases per 100,000 person-years.6-8 Recent studies have demonstrated increasing prevalence of MC likely resulting from an aging population.9,10
Risk stratification
Female gender, increasing age, concomitant autoimmune disease, and the use of certain drugs, including NSAIDs, proton pump inhibitors (PPIs), statins, and selective serotonin reuptake inhibitors (SSRIs), have been associated with an increased risk of MC.11,12 Autoimmune disorders, including celiac disease (CD), rheumatoid arthritis, hypothyroidism, and hyperthyroidism, are more common in patients with MC. The association with CD, in particular, is clinically important, as CD is associated with a 50-70 times greater risk of MC, and 2%-9% of patients with MC have CD.13,14
Several medications have been associated with MC. In a British multicenter prospective study, MC was associated with the use of NSAIDs, PPIs, and SSRIs;15 however, recent studies have questioned the association of MC with some of these medications, which might worsen diarrhea but not actually cause MC.16
An additional risk factor for MC is smoking. A recent meta-analysis demonstrated that current and former smokers had an increased risk of MC (odds ratio, 2.99; 95% confidence interval, 2.15-4.15 and OR, 1.63; 95% CI, 1.37-1.94, respectively), compared with nonsmokers.17 Smokers develop MC at a younger age, and smoking is associated with increased disease severity and decreased likelihood of attaining remission.18,19
Pathogenesis
The pathogenesis of MC remains largely unknown, although there are several hypotheses. The leading proposed mechanisms include reaction to luminal antigens, dysregulated collagen metabolism, genetic predisposition, autoimmunity, and bile acid malabsorption.
MC may be caused by abnormal epithelial barrier function, leading to increased permeability and reaction to luminal antigens, including dietary antigens, certain drugs, and bacterial products, 20,21 which themselves lead to the immune dysregulation and intestinal inflammation seen in MC. This mechanism may explain the association of several drugs with MC. Histological changes resembling LC are reported in patients with CD who consume gluten; however, large population-based studies have not found specific dietary associations with the development of MC.22
Another potential mechanism of MC is dysregulated collagen deposition. Collagen accumulation in the subepithelial layer in CC may result from increased levels of fibroblast growth factor, transforming growth factor–beta and vascular endothelial growth factor.23 Nonetheless, studies have not found an association between the severity of diarrhea in patients with CC and the thickness of the subepithelial collagen band.
Thirdly, autoimmunity and genetic predisposition have been postulated in the pathogenesis of MC. As previously discussed, MC is associated with several autoimmune diseases and predominantly occurs in women, a distinctive feature of autoimmune disorders. Several studies have demonstrated an association between MC and HLA-DQ2 and -DQ3 haplotypes,24 as well as potential polymorphisms in the serotonin transporter gene promoter.25 It is important to note, however, that only a few familial cases of MC have been reported to date.26
Lastly, bile acid malabsorption may play a role in the etiology of MC. Histologic findings of inflammation, along with villous atrophy and collagen deposition, have been reported in the ileum of patients with MC;27,28 however, because patients with MC without bile acid malabsorption may also respond to bile acid binders such as cholestyramine, these findings unlikely to be the sole mechanism explaining the development of the disease.
Despite the different proposed mechanisms for the pathogenesis of MC, no definite conclusions can be drawn because of the limited size of these studies and their often conflicting results.

Clinical features
Clinicians should suspect MC in patients with chronic or recurrent watery diarrhea, particularly in older persons. Other risk factors include female gender, use of certain culprit medications, smoking, and presence of other autoimmune diseases. The clinical manifestations of MC subtypes LC and CC are similar with no significant clinical differences.1,2 In addition to diarrhea, patients with MC may have abdominal pain, fatigue, and dehydration or electrolyte abnormalities depending on disease severity. Patients may also present with fecal urgency, incontinence, and nocturnal stools. Quality of life is often reduced in these patients, predominantly in those with severe or refractory symptoms.29,30 The natural course of MC is highly variable, with some patients achieving spontaneous resolution after one episode and others developing chronic symptoms.
Diagnosis
The differential diagnosis of chronic watery diarrhea is broad and includes malabsorption/maldigestion, inflammatory bowel disease (IBD), irritable bowel syndrome, and medication side effects. In addition, although gastrointestinal infections typically cause acute or subacute diarrhea, some can present with chronic diarrhea. Malabsorption/maldigestion may occur because of CD, lactose intolerance, and pancreatic insufficiency, among other conditions. A thorough history, regarding recent antibiotic and medication use, travel, and immunosuppression, should be obtained in patients with chronic diarrhea. Additionally, laboratory and endoscopic evaluation with random biopsies of the colon can further help differentiate these diseases from MC. A few studies suggest fecal calprotectin may be used to differentiate MC from other noninflammatory conditions such as irritable bowel syndrome, as well as to monitor disease activity. This test is not expected to distinguish MC from other inflammatory causes of diarrhea, such as IBD, and therefore, its role in clinical practice is uncertain.31
The diagnosis of MC is made by biopsy of the colonic mucosa demonstrating characteristic pathologic features.32 Unlike in diseases such as Crohn’s disease or ulcerative colitis, the colon usually appears normal in MC, although mild nonspecific changes, such as erythema or edema, may be visualized. There is no consensus on the ideal location to obtain biopsies for MC or whether biopsies from both the left and the right colon are required.2,33 The procedure of choice for the diagnosis of MC is colonoscopy with random biopsies taken throughout the colon. More limited evaluation by flexible sigmoidoscopy with biopsies may miss cases of MC as inflammation and collagen thickening are not necessarily uniform throughout the colon; however, in a patient that has undergone a recent colonoscopy for colon cancer screening without colon biopsies, a flexible sigmoidoscopy may be a reasonable next test for evaluation of MC, provided biopsies are obtained above the rectosigmoid colon.34
The MC subtypes are differentiated based on histology. The hallmark of LC is less than 20 intraepithelial lymphocytes per 100 surface epithelial cells (normal, less than 5) (Figure 1A). CC is characterized by a thickened subepithelial collagen band greater than 7-10 micrometers (normal, less than 5) (Figure 1B). For a subgroup of patients with milder abnormalities that do not meet these histological criteria, the terms “microscopic colitis, not otherwise specified” or “microscopic colitis, incomplete” may be used.35 These patients often respond to standard treatments for MC. There is an additional subset of patients with biopsy demonstrating features of both CC and LC simultaneously, as well as patients transitioning from one MC subtype to another over time.32,35
Management approach
The first step in management of patients with MC includes stopping culprit medications if there is a temporal relationship between the initiation of the medication and the onset of diarrhea, as well as encouraging smoking cessation. These steps alone, however, are unlikely to achieve clinical remission in most patients. A stepwise pharmacological approach is used in the management of MC based on disease severity (Figure 2). For patients with mild symptoms, antidiarrheal medications, such as loperamide, may be helpful.36 Long-term use of loperamide at therapeutic doses no greater than 16 mg daily appears to be safe if required to maintain symptom response. For those with persistent symptoms despite antidiarrheal medications, bismuth subsalicylate at three 262 mg tablets three times daily for 6-8 weeks can be considered. Long-term use of bismuth subsalicylate is not advised, especially at this dose, because of possible neurotoxicity.37

For patients refractory to the above treatments or those with moderate-to-severe symptoms, an 8-week course of budesonide at 9 mg daily is the first-line treatment.38 The dose was tapered before discontinuation in some studies but not in others. Both strategies appear effective. A recent meta-analysis of nine randomized trials demonstrated pooled ORs of 7.34 (95% CI, 4.08-13.19) and 8.35 (95% CI, 4.14-16.85) for response to budesonide induction and maintenance, respectively.39
Cholestyramine is another medication considered in the management of MC and warrants further investigation. To date, no randomized clinical trials have been conducted to evaluate bile acid sequestrants in MC, but they should be considered before placing patients on immunosuppressive medications. Some providers use mesalamine in this setting, although mesalamine is inferior to budesonide in the induction of clinical remission in MC.40
Despite high rates of response to budesonide, relapse after discontinuation is frequent (60%-80%), and time to relapse is variable41,42 The American Gastroenterological Association recommends budesonide for maintenance of remission in patients with recurrence following discontinuation of induction therapy. The lowest effective dose that maintains resolution of symptoms should be prescribed, ideally at 6 mg daily or lower.38 Although budesonide has a greater first-pass metabolism, compared with other glucocorticoids, patients should be monitored for possible side effects including hypertension, diabetes, and osteoporosis, as well as ophthalmologic disease, including cataracts and glaucoma.
For those who are intolerant to budesonide or have refractory symptoms, concomitant disorders such as CD that may be contributing to symptoms must be excluded. Immunosuppressive medications – such as thiopurines and biologic agents, including tumor necrosis factor–alpha inhibitors or vedolizumab – may be considered in refractory cases.43,44 Of note, there are limited studies evaluating the use of these medications for MC. Lastly, surgeries including ileostomy with or without colectomy have been performed in the most severe cases for resistant disease that has failed numerous pharmacological therapies.45
Patients should be counseled that, while symptoms from MC can be quite bothersome and disabling, there appears to be a normal life expectancy and no association between MC and colon cancer, unlike with other inflammatory conditions of the colon such as IBD.46,47
Conclusion and future outlook
As a common cause of chronic watery diarrhea, MC will be commonly encountered in primary care and gastroenterology practices. The diagnosis should be suspected in patients presenting with chronic or recurrent watery diarrhea, especially with female gender, autoimmune disease, and increasing age. The management of MC requires an algorithmic approach directed by symptom severity, with a subgroup of patients requiring maintenance therapy for relapsing symptoms. The care of patients with MC will continue to evolve in the future. Further work is needed to explore long-term safety outcomes with budesonide and the role of immunomodulators and newer biologic agents for patients with complex, refractory disease.
Dr. Tome is with the department of internal medicine at the Mayo Clinic, Rochester, Minn. Dr. Kamboj, and Dr. Pardi are with the division of gastroenterology and hepatology at the Mayo Clinic. Dr. Pardi has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda and has consulted for Vedanta and Otsuka. The other authors have no conflicts of interest to report.
References
1. Nyhlin N et al. Aliment Pharmacol Ther. 2014;39:963-72.
2. Miehlke S et al. United European Gastroenterol J. 2020;20-8.
3. Pardi DS et al. Gut. 2007;56:504-8.
4. Fernández-Bañares F et al. J Crohn’s Colitis.2016;10(7):805-11.
5. Gentile NM et al. Clin Gastroenterol Hepatol. 2014;12(5):838-42.
6. Tong J et al. Am J Gastroenterol. 2015;110:265-76.
7. Olesen M et al. Gut. 2004;53(3):346-50.
8. Bergman D et al. Aliment Pharmacol Ther. 2019;49(11):1395-400.
9. Guagnozzi D et al. Dig Liver Dis. 2012;44(5):384-8.
10. Münch A et al. J Crohns Colitis. 2012;6(9):932-45.
11. Macaigne G et al. Am J Gastroenterol. 2014; 09(9):1461-70.
12. Verhaegh BP et al. Aliment Pharmacol Ther. 2016;43(9):1004-13.
13. Stewart M et al. Aliment Pharmacol Ther. 2011;33(12):1340-9.
14. Green PHR et al. Clin Gastroenterol Hepatol. 2009;7(11):1210-6.
15. Masclee GM et al. Am J Gastroenterol. 2015;110:749-59.
16. Zylberberg H et al. Ailment Pharmacol Ther. 2021 Jun;53(11)1209-15.
17. Jaruvongvanich V et al. Inflamm Bowel Dis. 2019;25(4):672-8.
18. Fernández-Bañares F et al. Inflamm Bowel Dis. 2013; 19(7):1470-6.
19. Yen EF et al. Inflamm Bowel Dis. 2012;18(10):1835-41.
20. Barmeyer C et al. J Gastroenterol. 2017;52(10):1090-100.
21. Morgan DM et al. Clin Gastroenterol Hepatol. 2020;18(4):984-6.
22. Larsson JK et al. Eur J Clin Nutr. 2016;70:1309-17.
23. Madisch A et al.. Inflamm Bowel Dis. 2011;17(11):2295-8.
24. Stahl E et al. Gastroenterology. 2020;159(2):549-61.
25. Sikander A et al. Dig Dis Sci. 2015; 60:887-94.
26. Abdo AA et al. Can J Gastroenterol. 2001;15(5):341-3.
27. Fernandez-Bañares F et al. Dig Dis Sci.2001;46(10):2231-8.
28. Lyutakov I et al. Eur J Gastroenterol Hepatol. 2021;1;33(3):380-7.
29. Hjortswang H et al. Dig Liver Dis. 2011 Feb;43(2):102-9.
30. Cotter TG= et al. Gut. 2018;67(3):441-6.
31. Von Arnim U et al. Clin Exp Gastroenterol. 2016;9:97-103.
32. Langner C et al. Histopathology. 2015;66:613-26.
33. ASGE Standards of Practice Committee and Sharaf RN et al. Gastrointest Endosc. 2013;78:216-24.
34. Macaigne G et al. Clin Res Hepatol Gastroenterol. 2017;41(3):333-40.
35. Bjørnbak C et al. Aliment Pharmacol Ther. 2011;34(10):1225-34.
36. Pardi DS et al. Gastroenterology. 2016;150(1):247-74.
37. Masannat Y and Nazer E. West Virginia Med J. 2013;109(3):32-4.
38. Nguyen GC et al. Gastroenterology. 2016; 150(1):242-6.
39. Sebastian S et al. Eur J Gastroenterol Hepatol. 2019 Aug;31(8):919-27.
40. Miehlke S et al. Gastroenterology. 2014;146(5):1222-30.
41. Gentile NM et al. Am J Gastroenterol. 2013;108:256-9.
42. Münch A et al. Gut. 2016; 65(1):47-56.
43. Cotter TG et al. Aliment Pharmacol Ther. 2017; 46(2):169-74.
44. Esteve M et al. J Crohns Colitis. 2011;5(6):612-8.
45. Cottreau J et al. Clin J Gastroenterol. 2016;9:140-4.
46. Kamboj AK et al. Program No. P1876. ACG 2018 Annual Scientific Meeting Abstracts. Philadelphia, Pennsylvania: American College of Gastroenterology.
47. Yen EF et al. Dig Dis Sci. 2012;57:161-9.
Microscopic colitis is an inflammatory disease of the colon and a frequent cause of chronic or recurrent watery diarrhea, particularly in older persons. MC consists of two subtypes, collagenous colitis (CC) and lymphocytic colitis (LC). While the primary symptom is diarrhea, other signs and symptoms such as abdominal pain, weight loss, and dehydration or electrolyte abnormalities may also be present depending on disease severity.1 In MC, the colonic mucosa usually appears normal on colonoscopy, and the diagnosis is made by histologic findings of intraepithelial lymphocytosis with (CC) or without (LC) a prominent subepithelial collagen band. The management approaches to CC and LC are similar and should be directed based on the severity of symptoms.2 We review the epidemiology, risk factors, pathophysiology, diagnosis, and clinical management for this condition, as well as novel therapeutic approaches.
Epidemiology
Although the incidence of MC increased in the late twentieth century, more recently, it has stabilized with an estimated incidence varying from 1 to 25 per 100,000 person-years.3-5 A recent meta-analysis revealed a pooled incidence of 4.85 per 100,000 persons for LC and 4.14 per 100,000 persons for CC.6 Proposed explanations for the rising incidence in the late twentieth century include improved clinical awareness of the disease, possible increased use of drugs associated with MC, and increased performance of diagnostic colonoscopies for chronic diarrhea. Since MC is now well-recognized, the recent plateau in incidence rates may reflect decreased detection bias.
The prevalence of MC ranges from 10%-20% in patients undergoing colonoscopy for chronic watery diarrhea.6,7 The prevalence of LC is approximately 63.1 cases per 100,000 person-years and, for CC, is 49.2 cases per 100,000 person-years.6-8 Recent studies have demonstrated increasing prevalence of MC likely resulting from an aging population.9,10
Risk stratification
Female gender, increasing age, concomitant autoimmune disease, and the use of certain drugs, including NSAIDs, proton pump inhibitors (PPIs), statins, and selective serotonin reuptake inhibitors (SSRIs), have been associated with an increased risk of MC.11,12 Autoimmune disorders, including celiac disease (CD), rheumatoid arthritis, hypothyroidism, and hyperthyroidism, are more common in patients with MC. The association with CD, in particular, is clinically important, as CD is associated with a 50-70 times greater risk of MC, and 2%-9% of patients with MC have CD.13,14
Several medications have been associated with MC. In a British multicenter prospective study, MC was associated with the use of NSAIDs, PPIs, and SSRIs;15 however, recent studies have questioned the association of MC with some of these medications, which might worsen diarrhea but not actually cause MC.16
An additional risk factor for MC is smoking. A recent meta-analysis demonstrated that current and former smokers had an increased risk of MC (odds ratio, 2.99; 95% confidence interval, 2.15-4.15 and OR, 1.63; 95% CI, 1.37-1.94, respectively), compared with nonsmokers.17 Smokers develop MC at a younger age, and smoking is associated with increased disease severity and decreased likelihood of attaining remission.18,19
Pathogenesis
The pathogenesis of MC remains largely unknown, although there are several hypotheses. The leading proposed mechanisms include reaction to luminal antigens, dysregulated collagen metabolism, genetic predisposition, autoimmunity, and bile acid malabsorption.
MC may be caused by abnormal epithelial barrier function, leading to increased permeability and reaction to luminal antigens, including dietary antigens, certain drugs, and bacterial products, 20,21 which themselves lead to the immune dysregulation and intestinal inflammation seen in MC. This mechanism may explain the association of several drugs with MC. Histological changes resembling LC are reported in patients with CD who consume gluten; however, large population-based studies have not found specific dietary associations with the development of MC.22
Another potential mechanism of MC is dysregulated collagen deposition. Collagen accumulation in the subepithelial layer in CC may result from increased levels of fibroblast growth factor, transforming growth factor–beta and vascular endothelial growth factor.23 Nonetheless, studies have not found an association between the severity of diarrhea in patients with CC and the thickness of the subepithelial collagen band.
Thirdly, autoimmunity and genetic predisposition have been postulated in the pathogenesis of MC. As previously discussed, MC is associated with several autoimmune diseases and predominantly occurs in women, a distinctive feature of autoimmune disorders. Several studies have demonstrated an association between MC and HLA-DQ2 and -DQ3 haplotypes,24 as well as potential polymorphisms in the serotonin transporter gene promoter.25 It is important to note, however, that only a few familial cases of MC have been reported to date.26
Lastly, bile acid malabsorption may play a role in the etiology of MC. Histologic findings of inflammation, along with villous atrophy and collagen deposition, have been reported in the ileum of patients with MC;27,28 however, because patients with MC without bile acid malabsorption may also respond to bile acid binders such as cholestyramine, these findings unlikely to be the sole mechanism explaining the development of the disease.
Despite the different proposed mechanisms for the pathogenesis of MC, no definite conclusions can be drawn because of the limited size of these studies and their often conflicting results.

Clinical features
Clinicians should suspect MC in patients with chronic or recurrent watery diarrhea, particularly in older persons. Other risk factors include female gender, use of certain culprit medications, smoking, and presence of other autoimmune diseases. The clinical manifestations of MC subtypes LC and CC are similar with no significant clinical differences.1,2 In addition to diarrhea, patients with MC may have abdominal pain, fatigue, and dehydration or electrolyte abnormalities depending on disease severity. Patients may also present with fecal urgency, incontinence, and nocturnal stools. Quality of life is often reduced in these patients, predominantly in those with severe or refractory symptoms.29,30 The natural course of MC is highly variable, with some patients achieving spontaneous resolution after one episode and others developing chronic symptoms.
Diagnosis
The differential diagnosis of chronic watery diarrhea is broad and includes malabsorption/maldigestion, inflammatory bowel disease (IBD), irritable bowel syndrome, and medication side effects. In addition, although gastrointestinal infections typically cause acute or subacute diarrhea, some can present with chronic diarrhea. Malabsorption/maldigestion may occur because of CD, lactose intolerance, and pancreatic insufficiency, among other conditions. A thorough history, regarding recent antibiotic and medication use, travel, and immunosuppression, should be obtained in patients with chronic diarrhea. Additionally, laboratory and endoscopic evaluation with random biopsies of the colon can further help differentiate these diseases from MC. A few studies suggest fecal calprotectin may be used to differentiate MC from other noninflammatory conditions such as irritable bowel syndrome, as well as to monitor disease activity. This test is not expected to distinguish MC from other inflammatory causes of diarrhea, such as IBD, and therefore, its role in clinical practice is uncertain.31
The diagnosis of MC is made by biopsy of the colonic mucosa demonstrating characteristic pathologic features.32 Unlike in diseases such as Crohn’s disease or ulcerative colitis, the colon usually appears normal in MC, although mild nonspecific changes, such as erythema or edema, may be visualized. There is no consensus on the ideal location to obtain biopsies for MC or whether biopsies from both the left and the right colon are required.2,33 The procedure of choice for the diagnosis of MC is colonoscopy with random biopsies taken throughout the colon. More limited evaluation by flexible sigmoidoscopy with biopsies may miss cases of MC as inflammation and collagen thickening are not necessarily uniform throughout the colon; however, in a patient that has undergone a recent colonoscopy for colon cancer screening without colon biopsies, a flexible sigmoidoscopy may be a reasonable next test for evaluation of MC, provided biopsies are obtained above the rectosigmoid colon.34
The MC subtypes are differentiated based on histology. The hallmark of LC is less than 20 intraepithelial lymphocytes per 100 surface epithelial cells (normal, less than 5) (Figure 1A). CC is characterized by a thickened subepithelial collagen band greater than 7-10 micrometers (normal, less than 5) (Figure 1B). For a subgroup of patients with milder abnormalities that do not meet these histological criteria, the terms “microscopic colitis, not otherwise specified” or “microscopic colitis, incomplete” may be used.35 These patients often respond to standard treatments for MC. There is an additional subset of patients with biopsy demonstrating features of both CC and LC simultaneously, as well as patients transitioning from one MC subtype to another over time.32,35
Management approach
The first step in management of patients with MC includes stopping culprit medications if there is a temporal relationship between the initiation of the medication and the onset of diarrhea, as well as encouraging smoking cessation. These steps alone, however, are unlikely to achieve clinical remission in most patients. A stepwise pharmacological approach is used in the management of MC based on disease severity (Figure 2). For patients with mild symptoms, antidiarrheal medications, such as loperamide, may be helpful.36 Long-term use of loperamide at therapeutic doses no greater than 16 mg daily appears to be safe if required to maintain symptom response. For those with persistent symptoms despite antidiarrheal medications, bismuth subsalicylate at three 262 mg tablets three times daily for 6-8 weeks can be considered. Long-term use of bismuth subsalicylate is not advised, especially at this dose, because of possible neurotoxicity.37

For patients refractory to the above treatments or those with moderate-to-severe symptoms, an 8-week course of budesonide at 9 mg daily is the first-line treatment.38 The dose was tapered before discontinuation in some studies but not in others. Both strategies appear effective. A recent meta-analysis of nine randomized trials demonstrated pooled ORs of 7.34 (95% CI, 4.08-13.19) and 8.35 (95% CI, 4.14-16.85) for response to budesonide induction and maintenance, respectively.39
Cholestyramine is another medication considered in the management of MC and warrants further investigation. To date, no randomized clinical trials have been conducted to evaluate bile acid sequestrants in MC, but they should be considered before placing patients on immunosuppressive medications. Some providers use mesalamine in this setting, although mesalamine is inferior to budesonide in the induction of clinical remission in MC.40
Despite high rates of response to budesonide, relapse after discontinuation is frequent (60%-80%), and time to relapse is variable41,42 The American Gastroenterological Association recommends budesonide for maintenance of remission in patients with recurrence following discontinuation of induction therapy. The lowest effective dose that maintains resolution of symptoms should be prescribed, ideally at 6 mg daily or lower.38 Although budesonide has a greater first-pass metabolism, compared with other glucocorticoids, patients should be monitored for possible side effects including hypertension, diabetes, and osteoporosis, as well as ophthalmologic disease, including cataracts and glaucoma.
For those who are intolerant to budesonide or have refractory symptoms, concomitant disorders such as CD that may be contributing to symptoms must be excluded. Immunosuppressive medications – such as thiopurines and biologic agents, including tumor necrosis factor–alpha inhibitors or vedolizumab – may be considered in refractory cases.43,44 Of note, there are limited studies evaluating the use of these medications for MC. Lastly, surgeries including ileostomy with or without colectomy have been performed in the most severe cases for resistant disease that has failed numerous pharmacological therapies.45
Patients should be counseled that, while symptoms from MC can be quite bothersome and disabling, there appears to be a normal life expectancy and no association between MC and colon cancer, unlike with other inflammatory conditions of the colon such as IBD.46,47
Conclusion and future outlook
As a common cause of chronic watery diarrhea, MC will be commonly encountered in primary care and gastroenterology practices. The diagnosis should be suspected in patients presenting with chronic or recurrent watery diarrhea, especially with female gender, autoimmune disease, and increasing age. The management of MC requires an algorithmic approach directed by symptom severity, with a subgroup of patients requiring maintenance therapy for relapsing symptoms. The care of patients with MC will continue to evolve in the future. Further work is needed to explore long-term safety outcomes with budesonide and the role of immunomodulators and newer biologic agents for patients with complex, refractory disease.
Dr. Tome is with the department of internal medicine at the Mayo Clinic, Rochester, Minn. Dr. Kamboj, and Dr. Pardi are with the division of gastroenterology and hepatology at the Mayo Clinic. Dr. Pardi has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda and has consulted for Vedanta and Otsuka. The other authors have no conflicts of interest to report.
References
1. Nyhlin N et al. Aliment Pharmacol Ther. 2014;39:963-72.
2. Miehlke S et al. United European Gastroenterol J. 2020;20-8.
3. Pardi DS et al. Gut. 2007;56:504-8.
4. Fernández-Bañares F et al. J Crohn’s Colitis.2016;10(7):805-11.
5. Gentile NM et al. Clin Gastroenterol Hepatol. 2014;12(5):838-42.
6. Tong J et al. Am J Gastroenterol. 2015;110:265-76.
7. Olesen M et al. Gut. 2004;53(3):346-50.
8. Bergman D et al. Aliment Pharmacol Ther. 2019;49(11):1395-400.
9. Guagnozzi D et al. Dig Liver Dis. 2012;44(5):384-8.
10. Münch A et al. J Crohns Colitis. 2012;6(9):932-45.
11. Macaigne G et al. Am J Gastroenterol. 2014; 09(9):1461-70.
12. Verhaegh BP et al. Aliment Pharmacol Ther. 2016;43(9):1004-13.
13. Stewart M et al. Aliment Pharmacol Ther. 2011;33(12):1340-9.
14. Green PHR et al. Clin Gastroenterol Hepatol. 2009;7(11):1210-6.
15. Masclee GM et al. Am J Gastroenterol. 2015;110:749-59.
16. Zylberberg H et al. Ailment Pharmacol Ther. 2021 Jun;53(11)1209-15.
17. Jaruvongvanich V et al. Inflamm Bowel Dis. 2019;25(4):672-8.
18. Fernández-Bañares F et al. Inflamm Bowel Dis. 2013; 19(7):1470-6.
19. Yen EF et al. Inflamm Bowel Dis. 2012;18(10):1835-41.
20. Barmeyer C et al. J Gastroenterol. 2017;52(10):1090-100.
21. Morgan DM et al. Clin Gastroenterol Hepatol. 2020;18(4):984-6.
22. Larsson JK et al. Eur J Clin Nutr. 2016;70:1309-17.
23. Madisch A et al.. Inflamm Bowel Dis. 2011;17(11):2295-8.
24. Stahl E et al. Gastroenterology. 2020;159(2):549-61.
25. Sikander A et al. Dig Dis Sci. 2015; 60:887-94.
26. Abdo AA et al. Can J Gastroenterol. 2001;15(5):341-3.
27. Fernandez-Bañares F et al. Dig Dis Sci.2001;46(10):2231-8.
28. Lyutakov I et al. Eur J Gastroenterol Hepatol. 2021;1;33(3):380-7.
29. Hjortswang H et al. Dig Liver Dis. 2011 Feb;43(2):102-9.
30. Cotter TG= et al. Gut. 2018;67(3):441-6.
31. Von Arnim U et al. Clin Exp Gastroenterol. 2016;9:97-103.
32. Langner C et al. Histopathology. 2015;66:613-26.
33. ASGE Standards of Practice Committee and Sharaf RN et al. Gastrointest Endosc. 2013;78:216-24.
34. Macaigne G et al. Clin Res Hepatol Gastroenterol. 2017;41(3):333-40.
35. Bjørnbak C et al. Aliment Pharmacol Ther. 2011;34(10):1225-34.
36. Pardi DS et al. Gastroenterology. 2016;150(1):247-74.
37. Masannat Y and Nazer E. West Virginia Med J. 2013;109(3):32-4.
38. Nguyen GC et al. Gastroenterology. 2016; 150(1):242-6.
39. Sebastian S et al. Eur J Gastroenterol Hepatol. 2019 Aug;31(8):919-27.
40. Miehlke S et al. Gastroenterology. 2014;146(5):1222-30.
41. Gentile NM et al. Am J Gastroenterol. 2013;108:256-9.
42. Münch A et al. Gut. 2016; 65(1):47-56.
43. Cotter TG et al. Aliment Pharmacol Ther. 2017; 46(2):169-74.
44. Esteve M et al. J Crohns Colitis. 2011;5(6):612-8.
45. Cottreau J et al. Clin J Gastroenterol. 2016;9:140-4.
46. Kamboj AK et al. Program No. P1876. ACG 2018 Annual Scientific Meeting Abstracts. Philadelphia, Pennsylvania: American College of Gastroenterology.
47. Yen EF et al. Dig Dis Sci. 2012;57:161-9.
Clostridioides difficile: Two sets of guidelines disagree
With two sets of Clostridioides difficile recommendations being published within a month of each other, clinicians may find themselves trying to reconcile some of the conflicts between the two guidelines.
The first set, published June 1 by the American College of Gastroenterology, focuses on fecal microbiota transplantation (FMT) and the antibiotic vancomycin. The second, published June 24 by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, drives a shift in treatment for initial episodes and short-term recurrence from vancomycin to fidaxomicin and, in some cases, adding on the monoclonal antibody bezlotoxumab, both made by Merck.
The updates are timely because researchers are now recognizing that C. difficile can colonize people without causing symptoms, David Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine, Norfolk, said in an interview. He was not involved in writing either set of guidelines. “C. diff infection was a hospital-type infection, but we’re now seeing it in up to approximately 35%-50% of patients coming from the community, so it’s a big concern.”
Although the guidelines agree on which treatments are effective, the recommendations give the options a different emphasis.
Infectious disease specialist Stuart Johnson, MD, professor of medicine at Loyola University Medical Center in Maywood, Ill., and a physician researcher at Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., is the first author in the IDSA/SHEA guidelines. He told this news organization that one reason the two sets of recommendations may diverge in emphasis for initial and recurrent C. difficile is that “everyone has a different way of looking at things.” Compared with infectious disease specialists like him, he said, gastroenterologists “for the most part see the world a little different and have their own bent on things.”
The differences between the two guidelines relate to the first-line therapy for people with an initial or recurrent C. difficile episode. For an initial episode, the IDSA/SHEA authors conditionally recommend fidaxomicin as first preferred choice over vancomycin, with a moderate certainty of evidence. They noted that implementing this recommendation depends on “available resources,” a reference to the higher cost and difficulty of access associated with fidaxomicin.
Gastroenterologist Monika Fischer, MD, an associate professor of medicine at Indiana University, Indianapolis, is one of the authors of the ACG guidelines. She told this news organization that the cost difference between fidaxomicin and vancomycin is considerable and finds the choice to foreground fidaxomicin puzzling. “They did not reference any new data compared to those we have published.” Their recommendation may make sense in terms of efficacy, but real-world demands require attention to cost and reimbursement. “They themselves state this in their recommendations,” she noted.
Dr. Fischer cited a ballpark of about $100 for a course of vancomycin, compared with about $3,000 for a course of fidaxomicin. The IDSA/SHEA guidelines do cite vancomycin as an acceptable alternative. According to Dr. Fischer, the ACG guidelines authors discussed fidaxomicin and concluded that there just wasn’t enough evidence to justify favoring this antibiotic over vancomycin, given the cost-benefit imbalance. The ACG guidelines call for a standard course of oral vancomycin for a first, nonsevere C. difficile episode, listing oral fidaxomicin or oral metronidazole as alternatives.
For a recurrence, the IDSA/SHEA authors also favor fidaxomicin in a conditional recommendation over a standard course of vancomycin. For multiple recurrences, a tapered and pulsed vancomycin regimen, vancomycin followed by rifaximin, or FMT are also options.
Dr. David Johnson said that these recommendations favoring fidaxomicin are “surprising,” and that lower costs of vancomycin outweigh the benefit of fidaxomicin, given more-or-less comparable data on cure rates.
In contrast, the ACG guidelines recommend that an initial recurrence be treated with a tapering dose of vancomycin, and call for FMT for patients who are eligible and who experience a second or more C. difficile recurrences after a round of pulsed vancomycin.
Dr. Stuart Johnson said that FMT carries its own special set of issues. “If you don’t have a donor program set up, you have to rely on a stool bank,” noting that one widely used stool bank “basically had to stop making the product because of the coronavirus.” Costs for FMT products have doubled in recent years, and because Food and Drug Administration approval of the therapy is lacking, insurance does not cover it.
Dr. David Johnson also said that he is not “terribly happy” about the ACG recommendation for vancomycin prophylaxis. “It may help, but it also can have off-target effects against colonic bacterial flora, so we would not agree with that recommendation.”
The IDSA/SHEA authors also conditionally recommend bezlotoxumab, on very low certainty of evidence, as a cotherapy with standard of care antibiotics for recurrence prevention in patients with an episode in the last 6 months, particularly for patients at high recurrence risk “where logistics is not an issue.” The FDA has warned that this monoclonal antibody should be used with great care in patients with heart failure and only when benefits outweigh risks.
The ACG guidelines conditionally recommend considering bezlotoxumab to prevent recurrence in patients with specific risk factors, including age over 65 years and severe presentation. The IDSA/SHEA guidelines expand this population to anyone with a recurrence within 6 months, Dr. Fischer pointed out.
The antibody treatment “does offer another 10% absolute reduction in recurrent C. diff disease,” said Dr. Stuart Johnson, which is a “helpful option and primarily for people who have had recurrent C. diff already.” In general, he said, for both drugs, “access is still something we have to work with.”
In a commentary on the ACG guidelines, Dr. David Johnson wrote that there is good evidence that bezlotoxumab prevents relapse, especially in patients with specific risk factors. The hitch is the $4,500 price tag for a 1,000-mg vial, with a recommended dose of 10 mg/kg.
Dr. Stuart Johnson agreed that the costs of the fidaxomicin and bezlotoxumab are important considerations. In addition, there are logistical issues with using the antibody because most hospitals don’t offer infusions, which pushes patients to infusion centers.
Regardless, he added, “we’re happy that we have new options.”
Dr. Fischer, Dr. Stuart Johnson, and Dr. David Johnson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With two sets of Clostridioides difficile recommendations being published within a month of each other, clinicians may find themselves trying to reconcile some of the conflicts between the two guidelines.
The first set, published June 1 by the American College of Gastroenterology, focuses on fecal microbiota transplantation (FMT) and the antibiotic vancomycin. The second, published June 24 by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, drives a shift in treatment for initial episodes and short-term recurrence from vancomycin to fidaxomicin and, in some cases, adding on the monoclonal antibody bezlotoxumab, both made by Merck.
The updates are timely because researchers are now recognizing that C. difficile can colonize people without causing symptoms, David Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine, Norfolk, said in an interview. He was not involved in writing either set of guidelines. “C. diff infection was a hospital-type infection, but we’re now seeing it in up to approximately 35%-50% of patients coming from the community, so it’s a big concern.”
Although the guidelines agree on which treatments are effective, the recommendations give the options a different emphasis.
Infectious disease specialist Stuart Johnson, MD, professor of medicine at Loyola University Medical Center in Maywood, Ill., and a physician researcher at Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., is the first author in the IDSA/SHEA guidelines. He told this news organization that one reason the two sets of recommendations may diverge in emphasis for initial and recurrent C. difficile is that “everyone has a different way of looking at things.” Compared with infectious disease specialists like him, he said, gastroenterologists “for the most part see the world a little different and have their own bent on things.”
The differences between the two guidelines relate to the first-line therapy for people with an initial or recurrent C. difficile episode. For an initial episode, the IDSA/SHEA authors conditionally recommend fidaxomicin as first preferred choice over vancomycin, with a moderate certainty of evidence. They noted that implementing this recommendation depends on “available resources,” a reference to the higher cost and difficulty of access associated with fidaxomicin.
Gastroenterologist Monika Fischer, MD, an associate professor of medicine at Indiana University, Indianapolis, is one of the authors of the ACG guidelines. She told this news organization that the cost difference between fidaxomicin and vancomycin is considerable and finds the choice to foreground fidaxomicin puzzling. “They did not reference any new data compared to those we have published.” Their recommendation may make sense in terms of efficacy, but real-world demands require attention to cost and reimbursement. “They themselves state this in their recommendations,” she noted.
Dr. Fischer cited a ballpark of about $100 for a course of vancomycin, compared with about $3,000 for a course of fidaxomicin. The IDSA/SHEA guidelines do cite vancomycin as an acceptable alternative. According to Dr. Fischer, the ACG guidelines authors discussed fidaxomicin and concluded that there just wasn’t enough evidence to justify favoring this antibiotic over vancomycin, given the cost-benefit imbalance. The ACG guidelines call for a standard course of oral vancomycin for a first, nonsevere C. difficile episode, listing oral fidaxomicin or oral metronidazole as alternatives.
For a recurrence, the IDSA/SHEA authors also favor fidaxomicin in a conditional recommendation over a standard course of vancomycin. For multiple recurrences, a tapered and pulsed vancomycin regimen, vancomycin followed by rifaximin, or FMT are also options.
Dr. David Johnson said that these recommendations favoring fidaxomicin are “surprising,” and that lower costs of vancomycin outweigh the benefit of fidaxomicin, given more-or-less comparable data on cure rates.
In contrast, the ACG guidelines recommend that an initial recurrence be treated with a tapering dose of vancomycin, and call for FMT for patients who are eligible and who experience a second or more C. difficile recurrences after a round of pulsed vancomycin.
Dr. Stuart Johnson said that FMT carries its own special set of issues. “If you don’t have a donor program set up, you have to rely on a stool bank,” noting that one widely used stool bank “basically had to stop making the product because of the coronavirus.” Costs for FMT products have doubled in recent years, and because Food and Drug Administration approval of the therapy is lacking, insurance does not cover it.
Dr. David Johnson also said that he is not “terribly happy” about the ACG recommendation for vancomycin prophylaxis. “It may help, but it also can have off-target effects against colonic bacterial flora, so we would not agree with that recommendation.”
The IDSA/SHEA authors also conditionally recommend bezlotoxumab, on very low certainty of evidence, as a cotherapy with standard of care antibiotics for recurrence prevention in patients with an episode in the last 6 months, particularly for patients at high recurrence risk “where logistics is not an issue.” The FDA has warned that this monoclonal antibody should be used with great care in patients with heart failure and only when benefits outweigh risks.
The ACG guidelines conditionally recommend considering bezlotoxumab to prevent recurrence in patients with specific risk factors, including age over 65 years and severe presentation. The IDSA/SHEA guidelines expand this population to anyone with a recurrence within 6 months, Dr. Fischer pointed out.
The antibody treatment “does offer another 10% absolute reduction in recurrent C. diff disease,” said Dr. Stuart Johnson, which is a “helpful option and primarily for people who have had recurrent C. diff already.” In general, he said, for both drugs, “access is still something we have to work with.”
In a commentary on the ACG guidelines, Dr. David Johnson wrote that there is good evidence that bezlotoxumab prevents relapse, especially in patients with specific risk factors. The hitch is the $4,500 price tag for a 1,000-mg vial, with a recommended dose of 10 mg/kg.
Dr. Stuart Johnson agreed that the costs of the fidaxomicin and bezlotoxumab are important considerations. In addition, there are logistical issues with using the antibody because most hospitals don’t offer infusions, which pushes patients to infusion centers.
Regardless, he added, “we’re happy that we have new options.”
Dr. Fischer, Dr. Stuart Johnson, and Dr. David Johnson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With two sets of Clostridioides difficile recommendations being published within a month of each other, clinicians may find themselves trying to reconcile some of the conflicts between the two guidelines.
The first set, published June 1 by the American College of Gastroenterology, focuses on fecal microbiota transplantation (FMT) and the antibiotic vancomycin. The second, published June 24 by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, drives a shift in treatment for initial episodes and short-term recurrence from vancomycin to fidaxomicin and, in some cases, adding on the monoclonal antibody bezlotoxumab, both made by Merck.
The updates are timely because researchers are now recognizing that C. difficile can colonize people without causing symptoms, David Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine, Norfolk, said in an interview. He was not involved in writing either set of guidelines. “C. diff infection was a hospital-type infection, but we’re now seeing it in up to approximately 35%-50% of patients coming from the community, so it’s a big concern.”
Although the guidelines agree on which treatments are effective, the recommendations give the options a different emphasis.
Infectious disease specialist Stuart Johnson, MD, professor of medicine at Loyola University Medical Center in Maywood, Ill., and a physician researcher at Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., is the first author in the IDSA/SHEA guidelines. He told this news organization that one reason the two sets of recommendations may diverge in emphasis for initial and recurrent C. difficile is that “everyone has a different way of looking at things.” Compared with infectious disease specialists like him, he said, gastroenterologists “for the most part see the world a little different and have their own bent on things.”
The differences between the two guidelines relate to the first-line therapy for people with an initial or recurrent C. difficile episode. For an initial episode, the IDSA/SHEA authors conditionally recommend fidaxomicin as first preferred choice over vancomycin, with a moderate certainty of evidence. They noted that implementing this recommendation depends on “available resources,” a reference to the higher cost and difficulty of access associated with fidaxomicin.
Gastroenterologist Monika Fischer, MD, an associate professor of medicine at Indiana University, Indianapolis, is one of the authors of the ACG guidelines. She told this news organization that the cost difference between fidaxomicin and vancomycin is considerable and finds the choice to foreground fidaxomicin puzzling. “They did not reference any new data compared to those we have published.” Their recommendation may make sense in terms of efficacy, but real-world demands require attention to cost and reimbursement. “They themselves state this in their recommendations,” she noted.
Dr. Fischer cited a ballpark of about $100 for a course of vancomycin, compared with about $3,000 for a course of fidaxomicin. The IDSA/SHEA guidelines do cite vancomycin as an acceptable alternative. According to Dr. Fischer, the ACG guidelines authors discussed fidaxomicin and concluded that there just wasn’t enough evidence to justify favoring this antibiotic over vancomycin, given the cost-benefit imbalance. The ACG guidelines call for a standard course of oral vancomycin for a first, nonsevere C. difficile episode, listing oral fidaxomicin or oral metronidazole as alternatives.
For a recurrence, the IDSA/SHEA authors also favor fidaxomicin in a conditional recommendation over a standard course of vancomycin. For multiple recurrences, a tapered and pulsed vancomycin regimen, vancomycin followed by rifaximin, or FMT are also options.
Dr. David Johnson said that these recommendations favoring fidaxomicin are “surprising,” and that lower costs of vancomycin outweigh the benefit of fidaxomicin, given more-or-less comparable data on cure rates.
In contrast, the ACG guidelines recommend that an initial recurrence be treated with a tapering dose of vancomycin, and call for FMT for patients who are eligible and who experience a second or more C. difficile recurrences after a round of pulsed vancomycin.
Dr. Stuart Johnson said that FMT carries its own special set of issues. “If you don’t have a donor program set up, you have to rely on a stool bank,” noting that one widely used stool bank “basically had to stop making the product because of the coronavirus.” Costs for FMT products have doubled in recent years, and because Food and Drug Administration approval of the therapy is lacking, insurance does not cover it.
Dr. David Johnson also said that he is not “terribly happy” about the ACG recommendation for vancomycin prophylaxis. “It may help, but it also can have off-target effects against colonic bacterial flora, so we would not agree with that recommendation.”
The IDSA/SHEA authors also conditionally recommend bezlotoxumab, on very low certainty of evidence, as a cotherapy with standard of care antibiotics for recurrence prevention in patients with an episode in the last 6 months, particularly for patients at high recurrence risk “where logistics is not an issue.” The FDA has warned that this monoclonal antibody should be used with great care in patients with heart failure and only when benefits outweigh risks.
The ACG guidelines conditionally recommend considering bezlotoxumab to prevent recurrence in patients with specific risk factors, including age over 65 years and severe presentation. The IDSA/SHEA guidelines expand this population to anyone with a recurrence within 6 months, Dr. Fischer pointed out.
The antibody treatment “does offer another 10% absolute reduction in recurrent C. diff disease,” said Dr. Stuart Johnson, which is a “helpful option and primarily for people who have had recurrent C. diff already.” In general, he said, for both drugs, “access is still something we have to work with.”
In a commentary on the ACG guidelines, Dr. David Johnson wrote that there is good evidence that bezlotoxumab prevents relapse, especially in patients with specific risk factors. The hitch is the $4,500 price tag for a 1,000-mg vial, with a recommended dose of 10 mg/kg.
Dr. Stuart Johnson agreed that the costs of the fidaxomicin and bezlotoxumab are important considerations. In addition, there are logistical issues with using the antibody because most hospitals don’t offer infusions, which pushes patients to infusion centers.
Regardless, he added, “we’re happy that we have new options.”
Dr. Fischer, Dr. Stuart Johnson, and Dr. David Johnson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Novel oral inhibitor may block intestinal damage in celiac disease
A novel oral inhibitor of transglutaminase 2 appears to block gluten-induced mucosal damage in patients with celiac disease at three different doses, based on proof-of-concept trial data from 132 patients.
“Currently, no drug therapy reliably prevents the effects of dietary gluten or has been approved by regulators to treat celiac disease,” which remains an unmet need in these patients, many of whom struggle with symptoms even when they adhere to a gluten-free diet, wrote Detlef Schuppan, MD, of Johannes Gutenberg University of Mainz (Germany) and colleagues.
Celiac disease is driven in part by the enzyme transglutaminase 2, and a transglutaminase 2 inhibitor known as ZED1227 has been tested safely in phase 1 trials, they reported.
“ZED1227 targets the intestinal mucosa predominantly and thereby mediates protection; thus, it is unaffected by the complexity of the food matrix and is less dependent on the timing of ingestion of gluten-containing food,” the researchers explained.
In a study published in the New England Journal of Medicine, the researchers assessed the safety and efficacy of three dose levels of ZED1227. Adults with controlled celiac disease were randomized to doses of 10 mg (41 patients), 50 mg (41 patients), and 100 mg (41 patients), and 40 patients received a placebo. Of these, 35, 39, 28, and 30 patients, respectively, had sufficient duodenal biopsy samples for analysis.
Patients underwent a daily gluten challenge of 3 g for 6 weeks. At the end of 6 weeks, the primary study endpoint of attenuation of gluten-induced mucosal damage was measured by the ratio of villus height to crypt depth.
Patients in all three treatment groups showed significant attenuation of mucosal damage. The change in the average ratio of villus height to crypt depth compared to placebo in the 10-mg, 50-mg, and 100-mg groups was 0.44, 0.49, and 0.48, respectively, with P values equal to .001 in the 10-mg group and less than .001 in the 50-mg and 100-mg groups.
Adverse events were similar across all treatment groups and the placebo group, with the exception of a rash in three patients in the 100-mg group. A total of 74 patients reported adverse events, and the most common were headache, nausea, diarrhea, vomiting, and abdominal pain. The investigators determined that from 34% to 55% of the adverse events across groups were related to the study drug or placebo.
Two patients developed serious adverse events that were deemed related to the study drug or placebo; one patient in the 50-mg group developed migraine with aura, and one placebo patient developed ventricular extrasystoles. The patients recovered after discontinuing the drug or placebo.
Secondary endpoints included intraepithelial lymphocyte density, the Celiac Symptom Index score, and the Celiac Disease Questionnaire score. Estimated changes in intraepithelial lymphocyte density, compared with placebo, were –2.7 cells per 100 epithelial cells in the 10-mg group, −4.2 cells per 100 epithelial cells in the 50-mg group, and −9.6 cells per 100 epithelial cells in the 100-mg group. Compared with those of patients taking placebo, the 6-week changes in Celiac Symptom Index scores and Celiac Disease Questionnaire scores suggested slight improvements in symptoms and quality of life for the 100-mg dose.
The study findings were limited by several factors including missing data and loss of several patients to follow-up, as well as the short trial duration and use of controlled gluten ingestion, the researchers noted. Larger studies involving real-world conditions of minor gluten ingestion are needed to support the preliminary signs of safety and efficacy, they said.
Study strengths include high levels of patient adherence to the treatment and the gluten challenge, they said. “Future studies of ZED1227 in more patients are needed to provide additional evidence of the safety and efficacy of the drug, potentially in real-life conditions with minor gluten ingestion,” they concluded.
Translating potential into practice
“An absence of mucosal damage is a critical criterion to ensure the long-term health of a patient, and this clinical trial in celiac disease meets this important endpoint,” Bana Jabri, MD, of the University of Chicago, wrote in an accompanying editorial.
The primary endpoint of no mucosal damage is “especially notable because it was achieved under a controlled gluten challenge, albeit with a relatively moderate amount of gluten (a regular diet contains 12 g of gluten daily, whereas the challenge involved 3 g daily) and for a short period of time,” Dr. Jabri said. The reduction of disease-associated symptoms and apparent improvement in quality of life with 100-mg dose added value to the findings, she said.
Future research areas include whether cross-reactive T cells, which were not analyzed in the current study, might “expand and become pathogenic after a long-term gluten challenge,” Dr. Jabri noted.
However, “ZED1227 is the first nondietary treatment that has preliminarily shown the capacity to prevent mucosal damage in persons with celiac disease,” she said.
“Although this trial is very encouraging, whether treatment with ZED1227, and more generally transglutaminase 2 inhibition, in patients with celiac disease will be efficient in real life and during long-term gluten exposure remains to be determined,” Dr. Jabri concluded.
Need for data on dosing consistency
“Celiac disease affects up to 2% of the population in many countries, and the main therapy of celiac disease is avoidance of gluten,” Kim Isaacs, MD, of the University of North Carolina, Chapel Hill, said in an interview. “This is challenging due to the ubiquitous nature of gluten in many food products,” she said. “Restrictive eating also affects social interaction which is often focused around food,” she added. “Availability of an oral therapy that is effective to treat celiac in the face of gluten exposure will have a profound impact on patients in terms of liberalization of dietary intake.”
Overall, “the changes in the villus height to crypt depth was similar between all the active treatment groups, whereas there was a dose-dependent reduction in transepithelial lymphocyte density,” Dr. Isaacs noted. “The symptom improvement was greatest in the 100-mg group, suggesting that symptoms may be related to a greater extent to the lymphocyte density than the minimal differences in villus height to crypt depth ratios seen in the active treatment groups,” she said.
Potential barriers to the use of the treatment include cost because “this will need to be a daily long-term therapy,” said Dr. Isaacs. “Compliance is a potential barrier as well,” she said. “This study looks at daily administration of the transglutaminase 2 inhibitor and shows a benefit, but it is not clear whether missing doses of the medication will have a prolonged impact on efficacy,” she emphasized. Consequently, long-term efficacy studies are needed, Dr. Isaacs said. Other research questions to answer include whether patients will become refractory to the beneficial effects over time, the effect of missing doses, and whether patients would lose all the benefits of the therapy if dosing is not consistent, she emphasized.
The study was funded by Dr. Falk Pharma. The researchers, as well as Dr. Jabri and Dr. Isaacs, had no financial conflicts to disclose. Dr. Isaacs is on the editorial advisory board of GI & Hepatology News.
A novel oral inhibitor of transglutaminase 2 appears to block gluten-induced mucosal damage in patients with celiac disease at three different doses, based on proof-of-concept trial data from 132 patients.
“Currently, no drug therapy reliably prevents the effects of dietary gluten or has been approved by regulators to treat celiac disease,” which remains an unmet need in these patients, many of whom struggle with symptoms even when they adhere to a gluten-free diet, wrote Detlef Schuppan, MD, of Johannes Gutenberg University of Mainz (Germany) and colleagues.
Celiac disease is driven in part by the enzyme transglutaminase 2, and a transglutaminase 2 inhibitor known as ZED1227 has been tested safely in phase 1 trials, they reported.
“ZED1227 targets the intestinal mucosa predominantly and thereby mediates protection; thus, it is unaffected by the complexity of the food matrix and is less dependent on the timing of ingestion of gluten-containing food,” the researchers explained.
In a study published in the New England Journal of Medicine, the researchers assessed the safety and efficacy of three dose levels of ZED1227. Adults with controlled celiac disease were randomized to doses of 10 mg (41 patients), 50 mg (41 patients), and 100 mg (41 patients), and 40 patients received a placebo. Of these, 35, 39, 28, and 30 patients, respectively, had sufficient duodenal biopsy samples for analysis.
Patients underwent a daily gluten challenge of 3 g for 6 weeks. At the end of 6 weeks, the primary study endpoint of attenuation of gluten-induced mucosal damage was measured by the ratio of villus height to crypt depth.
Patients in all three treatment groups showed significant attenuation of mucosal damage. The change in the average ratio of villus height to crypt depth compared to placebo in the 10-mg, 50-mg, and 100-mg groups was 0.44, 0.49, and 0.48, respectively, with P values equal to .001 in the 10-mg group and less than .001 in the 50-mg and 100-mg groups.
Adverse events were similar across all treatment groups and the placebo group, with the exception of a rash in three patients in the 100-mg group. A total of 74 patients reported adverse events, and the most common were headache, nausea, diarrhea, vomiting, and abdominal pain. The investigators determined that from 34% to 55% of the adverse events across groups were related to the study drug or placebo.
Two patients developed serious adverse events that were deemed related to the study drug or placebo; one patient in the 50-mg group developed migraine with aura, and one placebo patient developed ventricular extrasystoles. The patients recovered after discontinuing the drug or placebo.
Secondary endpoints included intraepithelial lymphocyte density, the Celiac Symptom Index score, and the Celiac Disease Questionnaire score. Estimated changes in intraepithelial lymphocyte density, compared with placebo, were –2.7 cells per 100 epithelial cells in the 10-mg group, −4.2 cells per 100 epithelial cells in the 50-mg group, and −9.6 cells per 100 epithelial cells in the 100-mg group. Compared with those of patients taking placebo, the 6-week changes in Celiac Symptom Index scores and Celiac Disease Questionnaire scores suggested slight improvements in symptoms and quality of life for the 100-mg dose.
The study findings were limited by several factors including missing data and loss of several patients to follow-up, as well as the short trial duration and use of controlled gluten ingestion, the researchers noted. Larger studies involving real-world conditions of minor gluten ingestion are needed to support the preliminary signs of safety and efficacy, they said.
Study strengths include high levels of patient adherence to the treatment and the gluten challenge, they said. “Future studies of ZED1227 in more patients are needed to provide additional evidence of the safety and efficacy of the drug, potentially in real-life conditions with minor gluten ingestion,” they concluded.
Translating potential into practice
“An absence of mucosal damage is a critical criterion to ensure the long-term health of a patient, and this clinical trial in celiac disease meets this important endpoint,” Bana Jabri, MD, of the University of Chicago, wrote in an accompanying editorial.
The primary endpoint of no mucosal damage is “especially notable because it was achieved under a controlled gluten challenge, albeit with a relatively moderate amount of gluten (a regular diet contains 12 g of gluten daily, whereas the challenge involved 3 g daily) and for a short period of time,” Dr. Jabri said. The reduction of disease-associated symptoms and apparent improvement in quality of life with 100-mg dose added value to the findings, she said.
Future research areas include whether cross-reactive T cells, which were not analyzed in the current study, might “expand and become pathogenic after a long-term gluten challenge,” Dr. Jabri noted.
However, “ZED1227 is the first nondietary treatment that has preliminarily shown the capacity to prevent mucosal damage in persons with celiac disease,” she said.
“Although this trial is very encouraging, whether treatment with ZED1227, and more generally transglutaminase 2 inhibition, in patients with celiac disease will be efficient in real life and during long-term gluten exposure remains to be determined,” Dr. Jabri concluded.
Need for data on dosing consistency
“Celiac disease affects up to 2% of the population in many countries, and the main therapy of celiac disease is avoidance of gluten,” Kim Isaacs, MD, of the University of North Carolina, Chapel Hill, said in an interview. “This is challenging due to the ubiquitous nature of gluten in many food products,” she said. “Restrictive eating also affects social interaction which is often focused around food,” she added. “Availability of an oral therapy that is effective to treat celiac in the face of gluten exposure will have a profound impact on patients in terms of liberalization of dietary intake.”
Overall, “the changes in the villus height to crypt depth was similar between all the active treatment groups, whereas there was a dose-dependent reduction in transepithelial lymphocyte density,” Dr. Isaacs noted. “The symptom improvement was greatest in the 100-mg group, suggesting that symptoms may be related to a greater extent to the lymphocyte density than the minimal differences in villus height to crypt depth ratios seen in the active treatment groups,” she said.
Potential barriers to the use of the treatment include cost because “this will need to be a daily long-term therapy,” said Dr. Isaacs. “Compliance is a potential barrier as well,” she said. “This study looks at daily administration of the transglutaminase 2 inhibitor and shows a benefit, but it is not clear whether missing doses of the medication will have a prolonged impact on efficacy,” she emphasized. Consequently, long-term efficacy studies are needed, Dr. Isaacs said. Other research questions to answer include whether patients will become refractory to the beneficial effects over time, the effect of missing doses, and whether patients would lose all the benefits of the therapy if dosing is not consistent, she emphasized.
The study was funded by Dr. Falk Pharma. The researchers, as well as Dr. Jabri and Dr. Isaacs, had no financial conflicts to disclose. Dr. Isaacs is on the editorial advisory board of GI & Hepatology News.
A novel oral inhibitor of transglutaminase 2 appears to block gluten-induced mucosal damage in patients with celiac disease at three different doses, based on proof-of-concept trial data from 132 patients.
“Currently, no drug therapy reliably prevents the effects of dietary gluten or has been approved by regulators to treat celiac disease,” which remains an unmet need in these patients, many of whom struggle with symptoms even when they adhere to a gluten-free diet, wrote Detlef Schuppan, MD, of Johannes Gutenberg University of Mainz (Germany) and colleagues.
Celiac disease is driven in part by the enzyme transglutaminase 2, and a transglutaminase 2 inhibitor known as ZED1227 has been tested safely in phase 1 trials, they reported.
“ZED1227 targets the intestinal mucosa predominantly and thereby mediates protection; thus, it is unaffected by the complexity of the food matrix and is less dependent on the timing of ingestion of gluten-containing food,” the researchers explained.
In a study published in the New England Journal of Medicine, the researchers assessed the safety and efficacy of three dose levels of ZED1227. Adults with controlled celiac disease were randomized to doses of 10 mg (41 patients), 50 mg (41 patients), and 100 mg (41 patients), and 40 patients received a placebo. Of these, 35, 39, 28, and 30 patients, respectively, had sufficient duodenal biopsy samples for analysis.
Patients underwent a daily gluten challenge of 3 g for 6 weeks. At the end of 6 weeks, the primary study endpoint of attenuation of gluten-induced mucosal damage was measured by the ratio of villus height to crypt depth.
Patients in all three treatment groups showed significant attenuation of mucosal damage. The change in the average ratio of villus height to crypt depth compared to placebo in the 10-mg, 50-mg, and 100-mg groups was 0.44, 0.49, and 0.48, respectively, with P values equal to .001 in the 10-mg group and less than .001 in the 50-mg and 100-mg groups.
Adverse events were similar across all treatment groups and the placebo group, with the exception of a rash in three patients in the 100-mg group. A total of 74 patients reported adverse events, and the most common were headache, nausea, diarrhea, vomiting, and abdominal pain. The investigators determined that from 34% to 55% of the adverse events across groups were related to the study drug or placebo.
Two patients developed serious adverse events that were deemed related to the study drug or placebo; one patient in the 50-mg group developed migraine with aura, and one placebo patient developed ventricular extrasystoles. The patients recovered after discontinuing the drug or placebo.
Secondary endpoints included intraepithelial lymphocyte density, the Celiac Symptom Index score, and the Celiac Disease Questionnaire score. Estimated changes in intraepithelial lymphocyte density, compared with placebo, were –2.7 cells per 100 epithelial cells in the 10-mg group, −4.2 cells per 100 epithelial cells in the 50-mg group, and −9.6 cells per 100 epithelial cells in the 100-mg group. Compared with those of patients taking placebo, the 6-week changes in Celiac Symptom Index scores and Celiac Disease Questionnaire scores suggested slight improvements in symptoms and quality of life for the 100-mg dose.
The study findings were limited by several factors including missing data and loss of several patients to follow-up, as well as the short trial duration and use of controlled gluten ingestion, the researchers noted. Larger studies involving real-world conditions of minor gluten ingestion are needed to support the preliminary signs of safety and efficacy, they said.
Study strengths include high levels of patient adherence to the treatment and the gluten challenge, they said. “Future studies of ZED1227 in more patients are needed to provide additional evidence of the safety and efficacy of the drug, potentially in real-life conditions with minor gluten ingestion,” they concluded.
Translating potential into practice
“An absence of mucosal damage is a critical criterion to ensure the long-term health of a patient, and this clinical trial in celiac disease meets this important endpoint,” Bana Jabri, MD, of the University of Chicago, wrote in an accompanying editorial.
The primary endpoint of no mucosal damage is “especially notable because it was achieved under a controlled gluten challenge, albeit with a relatively moderate amount of gluten (a regular diet contains 12 g of gluten daily, whereas the challenge involved 3 g daily) and for a short period of time,” Dr. Jabri said. The reduction of disease-associated symptoms and apparent improvement in quality of life with 100-mg dose added value to the findings, she said.
Future research areas include whether cross-reactive T cells, which were not analyzed in the current study, might “expand and become pathogenic after a long-term gluten challenge,” Dr. Jabri noted.
However, “ZED1227 is the first nondietary treatment that has preliminarily shown the capacity to prevent mucosal damage in persons with celiac disease,” she said.
“Although this trial is very encouraging, whether treatment with ZED1227, and more generally transglutaminase 2 inhibition, in patients with celiac disease will be efficient in real life and during long-term gluten exposure remains to be determined,” Dr. Jabri concluded.
Need for data on dosing consistency
“Celiac disease affects up to 2% of the population in many countries, and the main therapy of celiac disease is avoidance of gluten,” Kim Isaacs, MD, of the University of North Carolina, Chapel Hill, said in an interview. “This is challenging due to the ubiquitous nature of gluten in many food products,” she said. “Restrictive eating also affects social interaction which is often focused around food,” she added. “Availability of an oral therapy that is effective to treat celiac in the face of gluten exposure will have a profound impact on patients in terms of liberalization of dietary intake.”
Overall, “the changes in the villus height to crypt depth was similar between all the active treatment groups, whereas there was a dose-dependent reduction in transepithelial lymphocyte density,” Dr. Isaacs noted. “The symptom improvement was greatest in the 100-mg group, suggesting that symptoms may be related to a greater extent to the lymphocyte density than the minimal differences in villus height to crypt depth ratios seen in the active treatment groups,” she said.
Potential barriers to the use of the treatment include cost because “this will need to be a daily long-term therapy,” said Dr. Isaacs. “Compliance is a potential barrier as well,” she said. “This study looks at daily administration of the transglutaminase 2 inhibitor and shows a benefit, but it is not clear whether missing doses of the medication will have a prolonged impact on efficacy,” she emphasized. Consequently, long-term efficacy studies are needed, Dr. Isaacs said. Other research questions to answer include whether patients will become refractory to the beneficial effects over time, the effect of missing doses, and whether patients would lose all the benefits of the therapy if dosing is not consistent, she emphasized.
The study was funded by Dr. Falk Pharma. The researchers, as well as Dr. Jabri and Dr. Isaacs, had no financial conflicts to disclose. Dr. Isaacs is on the editorial advisory board of GI & Hepatology News.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Anti-TNF therapy increases risk for herpes zoster in patients with IBD
Key clinical point: The use of antitumor necrosis factor (anti-TNF) therapy was associated with an increased risk for herpes zoster (HZ) in patients with inflammatory bowel disease (IBD).
Major finding: Compared with nonuse, current use of anti-TNF therapy was associated with an increased risk for HZ (adjusted odds ratio [aOR], 1.5; 95% confidence interval [CI], 1.1-2.1), particularly in patients below 50 years of age (aOR, 2.0; 95% CI, 1.2-3.5) and those additionally using steroids and immunosuppressants (aOR, 4.1; 95% CI, 2.3-7.2).
Study details: This was a nested case-control study of 15,454 patients with incident IBD. Patients diagnosed with HZ (n=824) were matched with 8,240 HZ-free controls.
Disclosures: The study was supported by the Division of Gastroenterology and Hepatology McGill University health center. Some of the authors reported serving as a speaker, advisory board member, and/or receiving unrestricted research grants from various sources.
Source: Santella C et al. Inflamm Bowel Dis. 2021 May 17. doi: 10.1093/ibd/izab092.
Key clinical point: The use of antitumor necrosis factor (anti-TNF) therapy was associated with an increased risk for herpes zoster (HZ) in patients with inflammatory bowel disease (IBD).
Major finding: Compared with nonuse, current use of anti-TNF therapy was associated with an increased risk for HZ (adjusted odds ratio [aOR], 1.5; 95% confidence interval [CI], 1.1-2.1), particularly in patients below 50 years of age (aOR, 2.0; 95% CI, 1.2-3.5) and those additionally using steroids and immunosuppressants (aOR, 4.1; 95% CI, 2.3-7.2).
Study details: This was a nested case-control study of 15,454 patients with incident IBD. Patients diagnosed with HZ (n=824) were matched with 8,240 HZ-free controls.
Disclosures: The study was supported by the Division of Gastroenterology and Hepatology McGill University health center. Some of the authors reported serving as a speaker, advisory board member, and/or receiving unrestricted research grants from various sources.
Source: Santella C et al. Inflamm Bowel Dis. 2021 May 17. doi: 10.1093/ibd/izab092.
Key clinical point: The use of antitumor necrosis factor (anti-TNF) therapy was associated with an increased risk for herpes zoster (HZ) in patients with inflammatory bowel disease (IBD).
Major finding: Compared with nonuse, current use of anti-TNF therapy was associated with an increased risk for HZ (adjusted odds ratio [aOR], 1.5; 95% confidence interval [CI], 1.1-2.1), particularly in patients below 50 years of age (aOR, 2.0; 95% CI, 1.2-3.5) and those additionally using steroids and immunosuppressants (aOR, 4.1; 95% CI, 2.3-7.2).
Study details: This was a nested case-control study of 15,454 patients with incident IBD. Patients diagnosed with HZ (n=824) were matched with 8,240 HZ-free controls.
Disclosures: The study was supported by the Division of Gastroenterology and Hepatology McGill University health center. Some of the authors reported serving as a speaker, advisory board member, and/or receiving unrestricted research grants from various sources.
Source: Santella C et al. Inflamm Bowel Dis. 2021 May 17. doi: 10.1093/ibd/izab092.
Obesity tied to early readmission risk in patients hospitalized with IBD
Key clinical point: Obesity among patients with inflammatory bowel disease (IBD) was associated with significantly higher all-cause early readmission than nonobese patients with IBD.
Major finding: Independent association was observed between obesity and higher all-cause readmission rates at 30 days (adjusted odds ratio [aOR], 1.16; P = .005) and 90 days (aOR, 1.27; P less than .0001) compared with those without obesity.
Study details: Findings are from a retrospective cohort study of 143,190 patients hospitalized with IBD, of which 9.1% of patients were obese (body mass index [BMI], 30 kg/m2 or higher) and the remaining 90.9% were nonobese (BMI less than 30 kg/m2).
Disclosures: No funding information was reported. The authors had no financial disclosures and conflicts of interest relevant to this article.
Source: Weissman S et al. J Crohns Colitis. 2021 May 17. doi: 10.1093/ecco-jcc/jjab088.
Key clinical point: Obesity among patients with inflammatory bowel disease (IBD) was associated with significantly higher all-cause early readmission than nonobese patients with IBD.
Major finding: Independent association was observed between obesity and higher all-cause readmission rates at 30 days (adjusted odds ratio [aOR], 1.16; P = .005) and 90 days (aOR, 1.27; P less than .0001) compared with those without obesity.
Study details: Findings are from a retrospective cohort study of 143,190 patients hospitalized with IBD, of which 9.1% of patients were obese (body mass index [BMI], 30 kg/m2 or higher) and the remaining 90.9% were nonobese (BMI less than 30 kg/m2).
Disclosures: No funding information was reported. The authors had no financial disclosures and conflicts of interest relevant to this article.
Source: Weissman S et al. J Crohns Colitis. 2021 May 17. doi: 10.1093/ecco-jcc/jjab088.
Key clinical point: Obesity among patients with inflammatory bowel disease (IBD) was associated with significantly higher all-cause early readmission than nonobese patients with IBD.
Major finding: Independent association was observed between obesity and higher all-cause readmission rates at 30 days (adjusted odds ratio [aOR], 1.16; P = .005) and 90 days (aOR, 1.27; P less than .0001) compared with those without obesity.
Study details: Findings are from a retrospective cohort study of 143,190 patients hospitalized with IBD, of which 9.1% of patients were obese (body mass index [BMI], 30 kg/m2 or higher) and the remaining 90.9% were nonobese (BMI less than 30 kg/m2).
Disclosures: No funding information was reported. The authors had no financial disclosures and conflicts of interest relevant to this article.
Source: Weissman S et al. J Crohns Colitis. 2021 May 17. doi: 10.1093/ecco-jcc/jjab088.
Hyperbaric oxygen therapy safe and effective in various IBD phenotypes
Key clinical point: Hyperbaric oxygen therapy (HBOT) was safe and associated with substantial rates of clinical remission in patients with various inflammatory bowel disease (IBD) phenotypes refractory to conventional therapies.
Major finding: HBOT resulted in substantial rates of clinical remission across multiple IBD phenotypes including ulcerative colitis (67%; 95% confidence interval [CI], 39%-86%), luminal Crohn’s disease (CD; 87.5%; 95% CI, 46.3%-98.3%), perianal CD (55%; 95% CI, 44%-65%), inflammatory disorders of the pouch (31%; 95% CI, 16%-50%), pyoderma gangrenosum (91.7%; 95% CI, 37.8%-99.5%), and perianal sinus/metastatic CD (65.2%; 95% CI, 10%-96.9%). Overall, 15% of patients reported minor adverse events.
Study details: Data come from a systematic review and proportional meta-analysis of 19 studies.
Disclosures: No information on funding was available. Some of the authors disclosed receiving consultancy fees and/or honoraria from multiple sources. All other authors had no conflicts of interest to disclose.
Source: McCurdy J et al. Inflamm Bowel Dis. 2021 May 18. doi: 10.1093/ibd/izab098.
Key clinical point: Hyperbaric oxygen therapy (HBOT) was safe and associated with substantial rates of clinical remission in patients with various inflammatory bowel disease (IBD) phenotypes refractory to conventional therapies.
Major finding: HBOT resulted in substantial rates of clinical remission across multiple IBD phenotypes including ulcerative colitis (67%; 95% confidence interval [CI], 39%-86%), luminal Crohn’s disease (CD; 87.5%; 95% CI, 46.3%-98.3%), perianal CD (55%; 95% CI, 44%-65%), inflammatory disorders of the pouch (31%; 95% CI, 16%-50%), pyoderma gangrenosum (91.7%; 95% CI, 37.8%-99.5%), and perianal sinus/metastatic CD (65.2%; 95% CI, 10%-96.9%). Overall, 15% of patients reported minor adverse events.
Study details: Data come from a systematic review and proportional meta-analysis of 19 studies.
Disclosures: No information on funding was available. Some of the authors disclosed receiving consultancy fees and/or honoraria from multiple sources. All other authors had no conflicts of interest to disclose.
Source: McCurdy J et al. Inflamm Bowel Dis. 2021 May 18. doi: 10.1093/ibd/izab098.
Key clinical point: Hyperbaric oxygen therapy (HBOT) was safe and associated with substantial rates of clinical remission in patients with various inflammatory bowel disease (IBD) phenotypes refractory to conventional therapies.
Major finding: HBOT resulted in substantial rates of clinical remission across multiple IBD phenotypes including ulcerative colitis (67%; 95% confidence interval [CI], 39%-86%), luminal Crohn’s disease (CD; 87.5%; 95% CI, 46.3%-98.3%), perianal CD (55%; 95% CI, 44%-65%), inflammatory disorders of the pouch (31%; 95% CI, 16%-50%), pyoderma gangrenosum (91.7%; 95% CI, 37.8%-99.5%), and perianal sinus/metastatic CD (65.2%; 95% CI, 10%-96.9%). Overall, 15% of patients reported minor adverse events.
Study details: Data come from a systematic review and proportional meta-analysis of 19 studies.
Disclosures: No information on funding was available. Some of the authors disclosed receiving consultancy fees and/or honoraria from multiple sources. All other authors had no conflicts of interest to disclose.
Source: McCurdy J et al. Inflamm Bowel Dis. 2021 May 18. doi: 10.1093/ibd/izab098.
Multiple switches from infliximab to biosimilars effective and safe in IBD
Key clinical point: In patients with inflammatory bowel disease (IBD), multiple successive switching from originator infliximab (IFX) to biosimilars (CT-P13 and SB2) was effective and safe, particularly if patients were in remission during the switch.
Major finding: At 12 months after the most recent switch, 76.9%, 65.7%, and 76.9% of patients successively switching from IFX to CT-P13 and then to SB2, from CT-P13 to SB2, and from IFX to CT-P13, respectively, were in clinical remission. Rate of clinical remission (P = .375), C-reactive protein (P = .582), and fecal calprotectin remission (P = .641) was not significantly different between the 3 groups. Overall, infusion reactions occurred in 1.7% of patients.
Study details: Data come from a multicenter prospective cohort study of 176 patients with IBD who switched from originator IFX to CT-P13 and then to SB2 (n=69), from CT-P13 to SB2 (n=80), or from IFX to CT-P13 (n=27).
Disclosures: No information on funding was available. Some of the authors reported serving on advisory boards or as a speaker or consultant for and receiving speaker’s fees, consultancy fees, and/or honoraria from multiple sources.
Source: Hanzel J et al. Inflamm Bowel Dis. 2021 May 20. doi: 10.1093/ibd/izab099.
Key clinical point: In patients with inflammatory bowel disease (IBD), multiple successive switching from originator infliximab (IFX) to biosimilars (CT-P13 and SB2) was effective and safe, particularly if patients were in remission during the switch.
Major finding: At 12 months after the most recent switch, 76.9%, 65.7%, and 76.9% of patients successively switching from IFX to CT-P13 and then to SB2, from CT-P13 to SB2, and from IFX to CT-P13, respectively, were in clinical remission. Rate of clinical remission (P = .375), C-reactive protein (P = .582), and fecal calprotectin remission (P = .641) was not significantly different between the 3 groups. Overall, infusion reactions occurred in 1.7% of patients.
Study details: Data come from a multicenter prospective cohort study of 176 patients with IBD who switched from originator IFX to CT-P13 and then to SB2 (n=69), from CT-P13 to SB2 (n=80), or from IFX to CT-P13 (n=27).
Disclosures: No information on funding was available. Some of the authors reported serving on advisory boards or as a speaker or consultant for and receiving speaker’s fees, consultancy fees, and/or honoraria from multiple sources.
Source: Hanzel J et al. Inflamm Bowel Dis. 2021 May 20. doi: 10.1093/ibd/izab099.
Key clinical point: In patients with inflammatory bowel disease (IBD), multiple successive switching from originator infliximab (IFX) to biosimilars (CT-P13 and SB2) was effective and safe, particularly if patients were in remission during the switch.
Major finding: At 12 months after the most recent switch, 76.9%, 65.7%, and 76.9% of patients successively switching from IFX to CT-P13 and then to SB2, from CT-P13 to SB2, and from IFX to CT-P13, respectively, were in clinical remission. Rate of clinical remission (P = .375), C-reactive protein (P = .582), and fecal calprotectin remission (P = .641) was not significantly different between the 3 groups. Overall, infusion reactions occurred in 1.7% of patients.
Study details: Data come from a multicenter prospective cohort study of 176 patients with IBD who switched from originator IFX to CT-P13 and then to SB2 (n=69), from CT-P13 to SB2 (n=80), or from IFX to CT-P13 (n=27).
Disclosures: No information on funding was available. Some of the authors reported serving on advisory boards or as a speaker or consultant for and receiving speaker’s fees, consultancy fees, and/or honoraria from multiple sources.
Source: Hanzel J et al. Inflamm Bowel Dis. 2021 May 20. doi: 10.1093/ibd/izab099.