User login
Drugs approved in 2013
In 2013, the Food and Drug Administration approved 27 new molecular entities (i.e., drugs) for human use. Because of their indications, it is unlikely that four will be used in pregnancy or lactation, so they are not discussed here. The four agents are ospemifene (Osphena), an estrogen agonist/antagonist used for severe dyspareunia; [223Ra]radium dichloride (Xofigo), for late-stage metastatic prostate cancer; conjugated estrogens/bazedoxifene (Duavee) for hot flashes associated with menopause and to prevent osteoporosis; and flutemetamol F-18 injection (Vizamyl), a radioactive diagnostic agent to aid in the evaluation of Alzheimer’s disease and dementia.
There are two other drugs that are unlikely to be used in pregnancy: macitentan (Opsumit) and riociguat (Adempas). These drugs are oral vasodilators indicated for the treatment of pulmonary hypertension. Both are teratogenic in rats and rabbits, but there are no reports of their use in human pregnancy. For female patients of reproductive potential, they are only available through restricted programs. Pregnancy must be excluded before starting therapy, monthly during treatment, and for 1 month after treatment is stopped.
The remaining 21 agents can be classified into the following categories: anticonvulsant (1), antidepressant (1), antidiabetics (2), antineoplastics (7), antihyperlipidemic (1), anti-infectives (4), diagnostics (2), immunologic (1), and respiratory (2). It is important to note that, except for two drugs (fluticasone in a combination product and dimethyl fumarate), there is no reported human pregnancy experience for these agents. Moreover, all probably cross the placenta to the embryo and/or the fetus, at least in some part of pregnancy.
Eslicarbazepine (Aptiom) is indicated as adjunctive treatment of partial-onset seizures. Developmental toxicity was observed in three animals: teratogenicity (mice), embryolethality (rats), and fetal growth restriction (rabbits). The no-effect dose was not found in two species, and was less than the human dose based on body surface area in the third. If a pregnant woman is taking this drug, she should be encouraged to enroll in the North American Antiepileptic Drug Pregnancy Registry by calling 888-233-2334.
Vortioxetine (Brintellix) is indicated for the treatment of major depressive disorder. The drug was not teratogenic in animals but did cause developmental delays in one species. Although the antidepressant mechanism is not fully understood, it appears to be related to the inhibition of the reuptake of serotonin (5-hydroxytryptamine). If so, vortioxetine would be closely related to the drugs in the selective serotonin reuptake inhibitor (SSRI) class: citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac), fluvoxamine (Luvox), paroxetine (Paxil), sertraline (Zoloft), and vilazodone (Viibryd). The relationship could be important because the use of SSRIs or serotonin norepinephrine reuptake inhibitors (SNRIs) close to birth is related to significant toxicity in the newborn.
There are two new antidiabetic agents for the treatment of type 2 diabetes. Alogliptin (Nesina), a dipeptidyl peptidase–4 inhibitor, is in the same pharmacologic class as linagliptin (Tradjenta), saxagliptin (Onglyza), and sitagliptin (Januvia). Canagliflozin (Invokana) is a sodium-glucose cotransporter 2 inhibitor, the first drug in this class to be approved. The animal data for alogliptin suggest low risk, whereas canagliflozin caused renal toxicity in rats at exposures corresponding to the late second and third trimester in humans. Insulin remains the treatment of choice for pregnant diabetics because tight control of glucose levels is beneficial for the mother, embryo-fetus, and newborn.
The seven new antineoplastic agents are ado-trastuzumab emtansine (Kadcyla) for HER2-positive breast cancer; afatinib (Gilotrif) for non–small cell lung cancer; dabrafenib (Tafinlar) for unresectable or metastatic melanoma; ibrutinib (Imbruvica) for mantle cell lymphoma or chronic lymphocytic leukemia; obinutuzumab (Gazyva) for chronic lymphocytic leukemia; pomalidomide (Pomalyst) for multiple myeloma; and trametinib (Mekinist) for unresectable or metastatic melanoma. Only pomalidomide is contraindicated in pregnancy. Although obinutuzumab did not cause teratogenicity in monkeys, its use in the latter portion of pregnancy resulted in newborn depletion of B cells that took up to 6 months after birth to restore. Moreover, it is used in combination with chlorambucil, a known teratogen. The animal data suggest risk in the other five agents. Nevertheless, the maternal condition should determine whether any of these antineoplastics are used in a pregnant woman.
Mipomersen sodium (Kynamro) is given subcutaneously once a week as an adjunct to lipid-lowering medications. The drug caused embryo toxicity in one of three animal species.
Among the four anti-infectives are two oral agents for the treatment of chronic hepatitis C virus infection: simeprevir (Olysio) and sofosbuvir (Sovaldi). Because both agents are recommended to be combined with peginterferon alfa and ribavirin, they are classified as contraindicated in pregnancy. However, when used alone, the animal data suggest that sofosbuvir was low risk, whereas simeprevir might have higher risk.
Luliconazole (Luzu), an azole antifungal, is a cream used for the treatment of tinea pedis, tinea cruris, and tinea corporis. Systemic absorption is minimal. The animal data suggest low risk, but there are no human pregnancy reports. Nevertheless, topical use is probably compatible in pregnancy, as are the other topical azole antifungals in this pharmacologic class: clotrimazole (Lotrimin), econazole (Spectazole), ketoconazole (Kuric), miconazole (Micatin), oxiconazole (Oxistat), sertaconazole (Ertaczo), and sulconazole (Exelderm).
Dolutegravir (Tivicay) is an HIV-1 integrase strand transfer inhibitor given in combination with other antiretroviral drugs. The animal data suggest low risk. If indicated, the drug should not be withheld because of pregnancy.
Gadoterate meglumine (Dotarem), a gadolinium-based contrast agent, is indicated to detect and visualize areas with disruption of the blood brain barrier and/or abnormal vascularity. No developmental toxicity was observed in pregnant animals. Closely related diagnostic agents are gadobenate dimeglumine (MultiHance), gadodiamide (Omniscan), gadofosveset (Ablavar), gadopentetate dimeglumine (Magnevist), gadoteridol (Prohance), and gadoversetamide (OptiMARK). Although the animal data for these agents show risk, no harm has been reported in human pregnancies. However, the available human data are very limited, and the risk magnitude for embryo-fetal harm remains unknown.
Technetium (99mTc) tilmanocept (Lymphoseek) is a radioactive diagnostic agent used in patients with breast cancer or melanoma. The active ingredient is technetium (99mTc). Animal reproduction studies have not been conducted. 99mTc is probably compatible in pregnancy (see Drugs in Pregnancy and Lactation, 10th ed.; Philadelphia: Lippincott, Williams and Wilkins, 2014:1317-8; to be released in August), but the risk of the tilmanocept moiety is unknown.
The immunologic agent dimethyl fumarate (Tecfidera) is indicated for the treatment of patients with relapsing forms of multiple sclerosis. The drug caused developmental toxicity (embryolethality, impaired growth, and birth defects) in animals during all portions of pregnancy. In clinical trials, there were 38 exposed pregnancies with the following outcomes: 22 live births, 3 spontaneous abortions, 9 elective abortions, 3 ongoing pregnancies, and 1 lost to follow-up (CNS Drugs 2014;28:89-94). A pregnancy registry has been established, and patients should be encouraged to enroll by calling 800-456-2255.
Two new respiratory combination products were approved in 2013, both for chronic obstructive pulmonary disease: fluticasone/vilanterol (Breo Ellipta) and umeclidinium/vilanterol (Anoro Ellipta). Inhaled fluticasone, a corticosteroid, is compatible in pregnancy (see Drugs in Pregnancy and Lactation, 9th ed.; Philadelphia: Lippincott, Williams and Wilkins; 2011:599-601). Vilanterol is a long-acting beta2-adrenergic agonist that is probably compatible in pregnancy. The absolute bioavailability of inhaled fluticasone and vilanterol in nonpregnant adults was about 15% and 27%, respectively. The animal data for the combination or when given individually suggest low risk in pregnancy. Umeclidinium is a long-acting anticholinergic. It also is absorbed from the lung, but the amount was not specified by the manufacturer. The animal data for umeclidinium suggest low risk.
There are no reports of the above drugs being used during breastfeeding, but excretion into breast milk should be expected. The effect of these exposures on a nursing infant is unknown. However, if a mother is taking one of these drugs and breastfeeding, her infant should be monitored for adverse effects, especially those that are the most common (typically listed on the first page of the package insert) in patients taking the drug. Close monitoring is particularly important during the first 2 postpartum months. A 2003 study found that most adverse reactions in nursing infants occurred within that time period (Clin. Pediatr. 2003;42:325-40).
Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of "Drugs in Pregnancy and Lactation," and coeditor of "Diseases, Complications, and Drug Therapy in Obstetrics." He had no other relevant financial disclosures. Contact him at [email protected].
In 2013, the Food and Drug Administration approved 27 new molecular entities (i.e., drugs) for human use. Because of their indications, it is unlikely that four will be used in pregnancy or lactation, so they are not discussed here. The four agents are ospemifene (Osphena), an estrogen agonist/antagonist used for severe dyspareunia; [223Ra]radium dichloride (Xofigo), for late-stage metastatic prostate cancer; conjugated estrogens/bazedoxifene (Duavee) for hot flashes associated with menopause and to prevent osteoporosis; and flutemetamol F-18 injection (Vizamyl), a radioactive diagnostic agent to aid in the evaluation of Alzheimer’s disease and dementia.
There are two other drugs that are unlikely to be used in pregnancy: macitentan (Opsumit) and riociguat (Adempas). These drugs are oral vasodilators indicated for the treatment of pulmonary hypertension. Both are teratogenic in rats and rabbits, but there are no reports of their use in human pregnancy. For female patients of reproductive potential, they are only available through restricted programs. Pregnancy must be excluded before starting therapy, monthly during treatment, and for 1 month after treatment is stopped.
The remaining 21 agents can be classified into the following categories: anticonvulsant (1), antidepressant (1), antidiabetics (2), antineoplastics (7), antihyperlipidemic (1), anti-infectives (4), diagnostics (2), immunologic (1), and respiratory (2). It is important to note that, except for two drugs (fluticasone in a combination product and dimethyl fumarate), there is no reported human pregnancy experience for these agents. Moreover, all probably cross the placenta to the embryo and/or the fetus, at least in some part of pregnancy.
Eslicarbazepine (Aptiom) is indicated as adjunctive treatment of partial-onset seizures. Developmental toxicity was observed in three animals: teratogenicity (mice), embryolethality (rats), and fetal growth restriction (rabbits). The no-effect dose was not found in two species, and was less than the human dose based on body surface area in the third. If a pregnant woman is taking this drug, she should be encouraged to enroll in the North American Antiepileptic Drug Pregnancy Registry by calling 888-233-2334.
Vortioxetine (Brintellix) is indicated for the treatment of major depressive disorder. The drug was not teratogenic in animals but did cause developmental delays in one species. Although the antidepressant mechanism is not fully understood, it appears to be related to the inhibition of the reuptake of serotonin (5-hydroxytryptamine). If so, vortioxetine would be closely related to the drugs in the selective serotonin reuptake inhibitor (SSRI) class: citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac), fluvoxamine (Luvox), paroxetine (Paxil), sertraline (Zoloft), and vilazodone (Viibryd). The relationship could be important because the use of SSRIs or serotonin norepinephrine reuptake inhibitors (SNRIs) close to birth is related to significant toxicity in the newborn.
There are two new antidiabetic agents for the treatment of type 2 diabetes. Alogliptin (Nesina), a dipeptidyl peptidase–4 inhibitor, is in the same pharmacologic class as linagliptin (Tradjenta), saxagliptin (Onglyza), and sitagliptin (Januvia). Canagliflozin (Invokana) is a sodium-glucose cotransporter 2 inhibitor, the first drug in this class to be approved. The animal data for alogliptin suggest low risk, whereas canagliflozin caused renal toxicity in rats at exposures corresponding to the late second and third trimester in humans. Insulin remains the treatment of choice for pregnant diabetics because tight control of glucose levels is beneficial for the mother, embryo-fetus, and newborn.
The seven new antineoplastic agents are ado-trastuzumab emtansine (Kadcyla) for HER2-positive breast cancer; afatinib (Gilotrif) for non–small cell lung cancer; dabrafenib (Tafinlar) for unresectable or metastatic melanoma; ibrutinib (Imbruvica) for mantle cell lymphoma or chronic lymphocytic leukemia; obinutuzumab (Gazyva) for chronic lymphocytic leukemia; pomalidomide (Pomalyst) for multiple myeloma; and trametinib (Mekinist) for unresectable or metastatic melanoma. Only pomalidomide is contraindicated in pregnancy. Although obinutuzumab did not cause teratogenicity in monkeys, its use in the latter portion of pregnancy resulted in newborn depletion of B cells that took up to 6 months after birth to restore. Moreover, it is used in combination with chlorambucil, a known teratogen. The animal data suggest risk in the other five agents. Nevertheless, the maternal condition should determine whether any of these antineoplastics are used in a pregnant woman.
Mipomersen sodium (Kynamro) is given subcutaneously once a week as an adjunct to lipid-lowering medications. The drug caused embryo toxicity in one of three animal species.
Among the four anti-infectives are two oral agents for the treatment of chronic hepatitis C virus infection: simeprevir (Olysio) and sofosbuvir (Sovaldi). Because both agents are recommended to be combined with peginterferon alfa and ribavirin, they are classified as contraindicated in pregnancy. However, when used alone, the animal data suggest that sofosbuvir was low risk, whereas simeprevir might have higher risk.
Luliconazole (Luzu), an azole antifungal, is a cream used for the treatment of tinea pedis, tinea cruris, and tinea corporis. Systemic absorption is minimal. The animal data suggest low risk, but there are no human pregnancy reports. Nevertheless, topical use is probably compatible in pregnancy, as are the other topical azole antifungals in this pharmacologic class: clotrimazole (Lotrimin), econazole (Spectazole), ketoconazole (Kuric), miconazole (Micatin), oxiconazole (Oxistat), sertaconazole (Ertaczo), and sulconazole (Exelderm).
Dolutegravir (Tivicay) is an HIV-1 integrase strand transfer inhibitor given in combination with other antiretroviral drugs. The animal data suggest low risk. If indicated, the drug should not be withheld because of pregnancy.
Gadoterate meglumine (Dotarem), a gadolinium-based contrast agent, is indicated to detect and visualize areas with disruption of the blood brain barrier and/or abnormal vascularity. No developmental toxicity was observed in pregnant animals. Closely related diagnostic agents are gadobenate dimeglumine (MultiHance), gadodiamide (Omniscan), gadofosveset (Ablavar), gadopentetate dimeglumine (Magnevist), gadoteridol (Prohance), and gadoversetamide (OptiMARK). Although the animal data for these agents show risk, no harm has been reported in human pregnancies. However, the available human data are very limited, and the risk magnitude for embryo-fetal harm remains unknown.
Technetium (99mTc) tilmanocept (Lymphoseek) is a radioactive diagnostic agent used in patients with breast cancer or melanoma. The active ingredient is technetium (99mTc). Animal reproduction studies have not been conducted. 99mTc is probably compatible in pregnancy (see Drugs in Pregnancy and Lactation, 10th ed.; Philadelphia: Lippincott, Williams and Wilkins, 2014:1317-8; to be released in August), but the risk of the tilmanocept moiety is unknown.
The immunologic agent dimethyl fumarate (Tecfidera) is indicated for the treatment of patients with relapsing forms of multiple sclerosis. The drug caused developmental toxicity (embryolethality, impaired growth, and birth defects) in animals during all portions of pregnancy. In clinical trials, there were 38 exposed pregnancies with the following outcomes: 22 live births, 3 spontaneous abortions, 9 elective abortions, 3 ongoing pregnancies, and 1 lost to follow-up (CNS Drugs 2014;28:89-94). A pregnancy registry has been established, and patients should be encouraged to enroll by calling 800-456-2255.
Two new respiratory combination products were approved in 2013, both for chronic obstructive pulmonary disease: fluticasone/vilanterol (Breo Ellipta) and umeclidinium/vilanterol (Anoro Ellipta). Inhaled fluticasone, a corticosteroid, is compatible in pregnancy (see Drugs in Pregnancy and Lactation, 9th ed.; Philadelphia: Lippincott, Williams and Wilkins; 2011:599-601). Vilanterol is a long-acting beta2-adrenergic agonist that is probably compatible in pregnancy. The absolute bioavailability of inhaled fluticasone and vilanterol in nonpregnant adults was about 15% and 27%, respectively. The animal data for the combination or when given individually suggest low risk in pregnancy. Umeclidinium is a long-acting anticholinergic. It also is absorbed from the lung, but the amount was not specified by the manufacturer. The animal data for umeclidinium suggest low risk.
There are no reports of the above drugs being used during breastfeeding, but excretion into breast milk should be expected. The effect of these exposures on a nursing infant is unknown. However, if a mother is taking one of these drugs and breastfeeding, her infant should be monitored for adverse effects, especially those that are the most common (typically listed on the first page of the package insert) in patients taking the drug. Close monitoring is particularly important during the first 2 postpartum months. A 2003 study found that most adverse reactions in nursing infants occurred within that time period (Clin. Pediatr. 2003;42:325-40).
Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of "Drugs in Pregnancy and Lactation," and coeditor of "Diseases, Complications, and Drug Therapy in Obstetrics." He had no other relevant financial disclosures. Contact him at [email protected].
In 2013, the Food and Drug Administration approved 27 new molecular entities (i.e., drugs) for human use. Because of their indications, it is unlikely that four will be used in pregnancy or lactation, so they are not discussed here. The four agents are ospemifene (Osphena), an estrogen agonist/antagonist used for severe dyspareunia; [223Ra]radium dichloride (Xofigo), for late-stage metastatic prostate cancer; conjugated estrogens/bazedoxifene (Duavee) for hot flashes associated with menopause and to prevent osteoporosis; and flutemetamol F-18 injection (Vizamyl), a radioactive diagnostic agent to aid in the evaluation of Alzheimer’s disease and dementia.
There are two other drugs that are unlikely to be used in pregnancy: macitentan (Opsumit) and riociguat (Adempas). These drugs are oral vasodilators indicated for the treatment of pulmonary hypertension. Both are teratogenic in rats and rabbits, but there are no reports of their use in human pregnancy. For female patients of reproductive potential, they are only available through restricted programs. Pregnancy must be excluded before starting therapy, monthly during treatment, and for 1 month after treatment is stopped.
The remaining 21 agents can be classified into the following categories: anticonvulsant (1), antidepressant (1), antidiabetics (2), antineoplastics (7), antihyperlipidemic (1), anti-infectives (4), diagnostics (2), immunologic (1), and respiratory (2). It is important to note that, except for two drugs (fluticasone in a combination product and dimethyl fumarate), there is no reported human pregnancy experience for these agents. Moreover, all probably cross the placenta to the embryo and/or the fetus, at least in some part of pregnancy.
Eslicarbazepine (Aptiom) is indicated as adjunctive treatment of partial-onset seizures. Developmental toxicity was observed in three animals: teratogenicity (mice), embryolethality (rats), and fetal growth restriction (rabbits). The no-effect dose was not found in two species, and was less than the human dose based on body surface area in the third. If a pregnant woman is taking this drug, she should be encouraged to enroll in the North American Antiepileptic Drug Pregnancy Registry by calling 888-233-2334.
Vortioxetine (Brintellix) is indicated for the treatment of major depressive disorder. The drug was not teratogenic in animals but did cause developmental delays in one species. Although the antidepressant mechanism is not fully understood, it appears to be related to the inhibition of the reuptake of serotonin (5-hydroxytryptamine). If so, vortioxetine would be closely related to the drugs in the selective serotonin reuptake inhibitor (SSRI) class: citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac), fluvoxamine (Luvox), paroxetine (Paxil), sertraline (Zoloft), and vilazodone (Viibryd). The relationship could be important because the use of SSRIs or serotonin norepinephrine reuptake inhibitors (SNRIs) close to birth is related to significant toxicity in the newborn.
There are two new antidiabetic agents for the treatment of type 2 diabetes. Alogliptin (Nesina), a dipeptidyl peptidase–4 inhibitor, is in the same pharmacologic class as linagliptin (Tradjenta), saxagliptin (Onglyza), and sitagliptin (Januvia). Canagliflozin (Invokana) is a sodium-glucose cotransporter 2 inhibitor, the first drug in this class to be approved. The animal data for alogliptin suggest low risk, whereas canagliflozin caused renal toxicity in rats at exposures corresponding to the late second and third trimester in humans. Insulin remains the treatment of choice for pregnant diabetics because tight control of glucose levels is beneficial for the mother, embryo-fetus, and newborn.
The seven new antineoplastic agents are ado-trastuzumab emtansine (Kadcyla) for HER2-positive breast cancer; afatinib (Gilotrif) for non–small cell lung cancer; dabrafenib (Tafinlar) for unresectable or metastatic melanoma; ibrutinib (Imbruvica) for mantle cell lymphoma or chronic lymphocytic leukemia; obinutuzumab (Gazyva) for chronic lymphocytic leukemia; pomalidomide (Pomalyst) for multiple myeloma; and trametinib (Mekinist) for unresectable or metastatic melanoma. Only pomalidomide is contraindicated in pregnancy. Although obinutuzumab did not cause teratogenicity in monkeys, its use in the latter portion of pregnancy resulted in newborn depletion of B cells that took up to 6 months after birth to restore. Moreover, it is used in combination with chlorambucil, a known teratogen. The animal data suggest risk in the other five agents. Nevertheless, the maternal condition should determine whether any of these antineoplastics are used in a pregnant woman.
Mipomersen sodium (Kynamro) is given subcutaneously once a week as an adjunct to lipid-lowering medications. The drug caused embryo toxicity in one of three animal species.
Among the four anti-infectives are two oral agents for the treatment of chronic hepatitis C virus infection: simeprevir (Olysio) and sofosbuvir (Sovaldi). Because both agents are recommended to be combined with peginterferon alfa and ribavirin, they are classified as contraindicated in pregnancy. However, when used alone, the animal data suggest that sofosbuvir was low risk, whereas simeprevir might have higher risk.
Luliconazole (Luzu), an azole antifungal, is a cream used for the treatment of tinea pedis, tinea cruris, and tinea corporis. Systemic absorption is minimal. The animal data suggest low risk, but there are no human pregnancy reports. Nevertheless, topical use is probably compatible in pregnancy, as are the other topical azole antifungals in this pharmacologic class: clotrimazole (Lotrimin), econazole (Spectazole), ketoconazole (Kuric), miconazole (Micatin), oxiconazole (Oxistat), sertaconazole (Ertaczo), and sulconazole (Exelderm).
Dolutegravir (Tivicay) is an HIV-1 integrase strand transfer inhibitor given in combination with other antiretroviral drugs. The animal data suggest low risk. If indicated, the drug should not be withheld because of pregnancy.
Gadoterate meglumine (Dotarem), a gadolinium-based contrast agent, is indicated to detect and visualize areas with disruption of the blood brain barrier and/or abnormal vascularity. No developmental toxicity was observed in pregnant animals. Closely related diagnostic agents are gadobenate dimeglumine (MultiHance), gadodiamide (Omniscan), gadofosveset (Ablavar), gadopentetate dimeglumine (Magnevist), gadoteridol (Prohance), and gadoversetamide (OptiMARK). Although the animal data for these agents show risk, no harm has been reported in human pregnancies. However, the available human data are very limited, and the risk magnitude for embryo-fetal harm remains unknown.
Technetium (99mTc) tilmanocept (Lymphoseek) is a radioactive diagnostic agent used in patients with breast cancer or melanoma. The active ingredient is technetium (99mTc). Animal reproduction studies have not been conducted. 99mTc is probably compatible in pregnancy (see Drugs in Pregnancy and Lactation, 10th ed.; Philadelphia: Lippincott, Williams and Wilkins, 2014:1317-8; to be released in August), but the risk of the tilmanocept moiety is unknown.
The immunologic agent dimethyl fumarate (Tecfidera) is indicated for the treatment of patients with relapsing forms of multiple sclerosis. The drug caused developmental toxicity (embryolethality, impaired growth, and birth defects) in animals during all portions of pregnancy. In clinical trials, there were 38 exposed pregnancies with the following outcomes: 22 live births, 3 spontaneous abortions, 9 elective abortions, 3 ongoing pregnancies, and 1 lost to follow-up (CNS Drugs 2014;28:89-94). A pregnancy registry has been established, and patients should be encouraged to enroll by calling 800-456-2255.
Two new respiratory combination products were approved in 2013, both for chronic obstructive pulmonary disease: fluticasone/vilanterol (Breo Ellipta) and umeclidinium/vilanterol (Anoro Ellipta). Inhaled fluticasone, a corticosteroid, is compatible in pregnancy (see Drugs in Pregnancy and Lactation, 9th ed.; Philadelphia: Lippincott, Williams and Wilkins; 2011:599-601). Vilanterol is a long-acting beta2-adrenergic agonist that is probably compatible in pregnancy. The absolute bioavailability of inhaled fluticasone and vilanterol in nonpregnant adults was about 15% and 27%, respectively. The animal data for the combination or when given individually suggest low risk in pregnancy. Umeclidinium is a long-acting anticholinergic. It also is absorbed from the lung, but the amount was not specified by the manufacturer. The animal data for umeclidinium suggest low risk.
There are no reports of the above drugs being used during breastfeeding, but excretion into breast milk should be expected. The effect of these exposures on a nursing infant is unknown. However, if a mother is taking one of these drugs and breastfeeding, her infant should be monitored for adverse effects, especially those that are the most common (typically listed on the first page of the package insert) in patients taking the drug. Close monitoring is particularly important during the first 2 postpartum months. A 2003 study found that most adverse reactions in nursing infants occurred within that time period (Clin. Pediatr. 2003;42:325-40).
Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of "Drugs in Pregnancy and Lactation," and coeditor of "Diseases, Complications, and Drug Therapy in Obstetrics." He had no other relevant financial disclosures. Contact him at [email protected].
ONO-4059 makes waves in heavily pretreated CLL
NEW ORLEANS – Early data suggest that the second-generation oral BTK inhibitor ONO-4059 may give ibrutinib a run for its money in chronic lymphocytic leukemia.
The response rate to ONO-4059 monotherapy was 89% overall and 71% in those with the deleterious 17p deletion among 18 heavily pretreated patients with relapsed/refractory or high-risk CLL in a phase I, dose-escalation study.
Patients had already received a median of three prior therapies, including rituximab (84%) and fludarabine (95%), and had no higher priority therapy available to them, said Dr. Gilles Salles of Hospices Civils de Lyon (France), Universite Claude Bernard Lyon.
All patients had improved hemoglobin and platelet counts after 3 months on treatment and rapid reductions in lymph node size within the first 28-day cycle. Tumor burden was reduced by 50% for most patients, and all but one patient experienced a response that was detectable on a CT scan.
"This was true whatever their FISH status or 17p or 11q deletion status," Dr. Salles said at the annual meeting of the American Society of Hematology.
ONO-4059 is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor with antitumor activity in several preclinical models.
No patients had received prior treatment with a P13 kinase or a BTK inhibitor, including ibrutinib (Imbruvica), which recently gained accelerated approval for previously treated mantle cell lymphoma.
ONO-4059 was given at daily doses ranging from 20 mg to 320 mg for up to 6 months, with the option of additional dosing up to 2 years. Sustained BTK inhibition was established at doses of 40 mg and higher.
Overall, the best response was a partial response in 14 patients, as well as two partial responses with lymphocytosis and one stable disease, he said. No complete responses occurred.
One patient progressed roughly 1 month after showing an initial response and complete disappearance of all palpable disease on physical exam. Richter’s syndrome was suspected.
"It’s very promising efficacy in this highly pretreated population," Dr. Salles said.
Patients with relapsed/refractory mantle cell lymphoma and diffuse large B-cell lymphoma, especially the ABC subtype, also appear sensitive to ONO-4059. Overall response rates were 43% and 75%, respectively, including three complete responses reported from the phase I study in a separate poster presentation at the meeting.
ONO-4059 had a favorable safety profile with a single dose-limiting toxicity observed in a patient who had Waldenstrom’s macroglobulinemia, was on the 320-mg dose, and was intolerant to all prior therapies. The maximum tolerated dose has not yet been reached.
The majority of adverse events in the CLL patients were grades 1 and 2. There were no clinically significant bleeding events or bruising, and there was a low incidence of diarrhea and rash, Dr. Salles said.
ONO-4059–related grade 3-4 events were independent of dose and included one grade 3 neutropenia at 20 mg and two grade 4 events at 20 mg and 320 mg. Four serious adverse events (febrile neutropenia, pyrexia, rash, and neutropenia) occurred in three patients, all of whom are still in the study and showing good clinical response, Dr. Salles said. Of the 30 patients dosed to date, 22 remain in the study.
No other trials are firmly planned, and pharmacokinetics/pharmacodynamics data continue to be explored in order to assess a phase II dosage, he said in an interview.
Dr. Salles reported consulting for and receiving honoraria from Roche. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
NEW ORLEANS – Early data suggest that the second-generation oral BTK inhibitor ONO-4059 may give ibrutinib a run for its money in chronic lymphocytic leukemia.
The response rate to ONO-4059 monotherapy was 89% overall and 71% in those with the deleterious 17p deletion among 18 heavily pretreated patients with relapsed/refractory or high-risk CLL in a phase I, dose-escalation study.
Patients had already received a median of three prior therapies, including rituximab (84%) and fludarabine (95%), and had no higher priority therapy available to them, said Dr. Gilles Salles of Hospices Civils de Lyon (France), Universite Claude Bernard Lyon.
All patients had improved hemoglobin and platelet counts after 3 months on treatment and rapid reductions in lymph node size within the first 28-day cycle. Tumor burden was reduced by 50% for most patients, and all but one patient experienced a response that was detectable on a CT scan.
"This was true whatever their FISH status or 17p or 11q deletion status," Dr. Salles said at the annual meeting of the American Society of Hematology.
ONO-4059 is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor with antitumor activity in several preclinical models.
No patients had received prior treatment with a P13 kinase or a BTK inhibitor, including ibrutinib (Imbruvica), which recently gained accelerated approval for previously treated mantle cell lymphoma.
ONO-4059 was given at daily doses ranging from 20 mg to 320 mg for up to 6 months, with the option of additional dosing up to 2 years. Sustained BTK inhibition was established at doses of 40 mg and higher.
Overall, the best response was a partial response in 14 patients, as well as two partial responses with lymphocytosis and one stable disease, he said. No complete responses occurred.
One patient progressed roughly 1 month after showing an initial response and complete disappearance of all palpable disease on physical exam. Richter’s syndrome was suspected.
"It’s very promising efficacy in this highly pretreated population," Dr. Salles said.
Patients with relapsed/refractory mantle cell lymphoma and diffuse large B-cell lymphoma, especially the ABC subtype, also appear sensitive to ONO-4059. Overall response rates were 43% and 75%, respectively, including three complete responses reported from the phase I study in a separate poster presentation at the meeting.
ONO-4059 had a favorable safety profile with a single dose-limiting toxicity observed in a patient who had Waldenstrom’s macroglobulinemia, was on the 320-mg dose, and was intolerant to all prior therapies. The maximum tolerated dose has not yet been reached.
The majority of adverse events in the CLL patients were grades 1 and 2. There were no clinically significant bleeding events or bruising, and there was a low incidence of diarrhea and rash, Dr. Salles said.
ONO-4059–related grade 3-4 events were independent of dose and included one grade 3 neutropenia at 20 mg and two grade 4 events at 20 mg and 320 mg. Four serious adverse events (febrile neutropenia, pyrexia, rash, and neutropenia) occurred in three patients, all of whom are still in the study and showing good clinical response, Dr. Salles said. Of the 30 patients dosed to date, 22 remain in the study.
No other trials are firmly planned, and pharmacokinetics/pharmacodynamics data continue to be explored in order to assess a phase II dosage, he said in an interview.
Dr. Salles reported consulting for and receiving honoraria from Roche. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
NEW ORLEANS – Early data suggest that the second-generation oral BTK inhibitor ONO-4059 may give ibrutinib a run for its money in chronic lymphocytic leukemia.
The response rate to ONO-4059 monotherapy was 89% overall and 71% in those with the deleterious 17p deletion among 18 heavily pretreated patients with relapsed/refractory or high-risk CLL in a phase I, dose-escalation study.
Patients had already received a median of three prior therapies, including rituximab (84%) and fludarabine (95%), and had no higher priority therapy available to them, said Dr. Gilles Salles of Hospices Civils de Lyon (France), Universite Claude Bernard Lyon.
All patients had improved hemoglobin and platelet counts after 3 months on treatment and rapid reductions in lymph node size within the first 28-day cycle. Tumor burden was reduced by 50% for most patients, and all but one patient experienced a response that was detectable on a CT scan.
"This was true whatever their FISH status or 17p or 11q deletion status," Dr. Salles said at the annual meeting of the American Society of Hematology.
ONO-4059 is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor with antitumor activity in several preclinical models.
No patients had received prior treatment with a P13 kinase or a BTK inhibitor, including ibrutinib (Imbruvica), which recently gained accelerated approval for previously treated mantle cell lymphoma.
ONO-4059 was given at daily doses ranging from 20 mg to 320 mg for up to 6 months, with the option of additional dosing up to 2 years. Sustained BTK inhibition was established at doses of 40 mg and higher.
Overall, the best response was a partial response in 14 patients, as well as two partial responses with lymphocytosis and one stable disease, he said. No complete responses occurred.
One patient progressed roughly 1 month after showing an initial response and complete disappearance of all palpable disease on physical exam. Richter’s syndrome was suspected.
"It’s very promising efficacy in this highly pretreated population," Dr. Salles said.
Patients with relapsed/refractory mantle cell lymphoma and diffuse large B-cell lymphoma, especially the ABC subtype, also appear sensitive to ONO-4059. Overall response rates were 43% and 75%, respectively, including three complete responses reported from the phase I study in a separate poster presentation at the meeting.
ONO-4059 had a favorable safety profile with a single dose-limiting toxicity observed in a patient who had Waldenstrom’s macroglobulinemia, was on the 320-mg dose, and was intolerant to all prior therapies. The maximum tolerated dose has not yet been reached.
The majority of adverse events in the CLL patients were grades 1 and 2. There were no clinically significant bleeding events or bruising, and there was a low incidence of diarrhea and rash, Dr. Salles said.
ONO-4059–related grade 3-4 events were independent of dose and included one grade 3 neutropenia at 20 mg and two grade 4 events at 20 mg and 320 mg. Four serious adverse events (febrile neutropenia, pyrexia, rash, and neutropenia) occurred in three patients, all of whom are still in the study and showing good clinical response, Dr. Salles said. Of the 30 patients dosed to date, 22 remain in the study.
No other trials are firmly planned, and pharmacokinetics/pharmacodynamics data continue to be explored in order to assess a phase II dosage, he said in an interview.
Dr. Salles reported consulting for and receiving honoraria from Roche. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
AT ASH 2013
Major finding: The response rate was 89% overall and 71% for patients with 17p deletion.
Data source: A prospective, phase I dose-escalation study in 18 patients with relapsed/refractory or high-risk CLL.
Disclosures: Dr. Salles reported honoraria from Janssen, Gilead, and Celgene. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
Ibrutinib approved for mantle cell lymphoma
Ibrutinib is now approved for the treatment of patients with mantle cell lymphoma who have received at least one prior therapy, the Food and Drug Administration announced Nov. 13.
The once-daily, oral therapy, marketed as Imbruvica, is the second drug to receive FDA approval under the breakthrough therapy designation established to speed the development and review of treatments for serious or life-threatening diseases.
"Imbruvica’s approval demonstrates the FDA’s commitment to making treatments available to patients with rare diseases," Dr. Richard Pazdur, director of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in a statement.
Mantle cell lymphoma is an orphan disease, with only about 2,900 new cases of MCL diagnosed each year. MCL comprises only about 6% of all non-Hodgkin’s lymphoma cases in the United States.
Ibrutinib’s approval comes a little more than 4 months after the new drug application was filed in June 2013 and is based on a phase II study reporting an investigator-assessed overall response rate of 66% at a daily dose of 560 mg ibrutinib in 111 patients with relapsed or refractory MCL after a median of three prior therapies. The median duration of response was 17.5 months. An improvement in survival and disease-related symptoms has not been established.
Ibrutinib works by blocking Bruton’s tyrosine kinase, a mediator of the B-cell receptor signaling pathway that has been shown in nonclinical studies to inhibit malignant B-cell survival.
The FDA also granted ibrutinib priority review and orphan-product designation, because the drug demonstrated "the potential to be a significant improvement in safety or effectiveness in the treatment of a serious condition and is intended to treat a rare disease," according to the FDA statement.
Ibrutinib is the third drug approved to treat MCL. In June 2013, the FDA approved the oral thalidomide analogue lenalidomide (Revlimid) for the treatment of MCL that had relapsed or progressed after two prior therapies including bortezomib (Velcade), a subcutaneous therapy that has been available for MCL since 2006.
"It is gratifying to see an early example of the new breakthrough therapy designation pathway meeting its intention – getting promising treatments to patients who are waiting for new options," Dr. Ellen V. Sigal, chairperson and founder of the Washington-based Friends of Cancer Research advocacy organization, said in a statement issued by Janssen Biotech, which is comarketing the drug with Pharmacyclics.
The two companies are expected to continue with phase III studies of ibrutinib and have also submitted the drug to the FDA for the treatment of previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma.
In the pivotal MCL trial, the most common treatment-related adverse events with single-agent ibrutinib were mild or moderate diarrhea, fatigue, and nausea (N. Engl. J. Med. 2013;369:507-16). Grade 3 or higher hematologic events were neutropenia (16%), thrombocytopenia (11%), and anemia (10%).
Ibrutinib is now approved for the treatment of patients with mantle cell lymphoma who have received at least one prior therapy, the Food and Drug Administration announced Nov. 13.
The once-daily, oral therapy, marketed as Imbruvica, is the second drug to receive FDA approval under the breakthrough therapy designation established to speed the development and review of treatments for serious or life-threatening diseases.
"Imbruvica’s approval demonstrates the FDA’s commitment to making treatments available to patients with rare diseases," Dr. Richard Pazdur, director of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in a statement.
Mantle cell lymphoma is an orphan disease, with only about 2,900 new cases of MCL diagnosed each year. MCL comprises only about 6% of all non-Hodgkin’s lymphoma cases in the United States.
Ibrutinib’s approval comes a little more than 4 months after the new drug application was filed in June 2013 and is based on a phase II study reporting an investigator-assessed overall response rate of 66% at a daily dose of 560 mg ibrutinib in 111 patients with relapsed or refractory MCL after a median of three prior therapies. The median duration of response was 17.5 months. An improvement in survival and disease-related symptoms has not been established.
Ibrutinib works by blocking Bruton’s tyrosine kinase, a mediator of the B-cell receptor signaling pathway that has been shown in nonclinical studies to inhibit malignant B-cell survival.
The FDA also granted ibrutinib priority review and orphan-product designation, because the drug demonstrated "the potential to be a significant improvement in safety or effectiveness in the treatment of a serious condition and is intended to treat a rare disease," according to the FDA statement.
Ibrutinib is the third drug approved to treat MCL. In June 2013, the FDA approved the oral thalidomide analogue lenalidomide (Revlimid) for the treatment of MCL that had relapsed or progressed after two prior therapies including bortezomib (Velcade), a subcutaneous therapy that has been available for MCL since 2006.
"It is gratifying to see an early example of the new breakthrough therapy designation pathway meeting its intention – getting promising treatments to patients who are waiting for new options," Dr. Ellen V. Sigal, chairperson and founder of the Washington-based Friends of Cancer Research advocacy organization, said in a statement issued by Janssen Biotech, which is comarketing the drug with Pharmacyclics.
The two companies are expected to continue with phase III studies of ibrutinib and have also submitted the drug to the FDA for the treatment of previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma.
In the pivotal MCL trial, the most common treatment-related adverse events with single-agent ibrutinib were mild or moderate diarrhea, fatigue, and nausea (N. Engl. J. Med. 2013;369:507-16). Grade 3 or higher hematologic events were neutropenia (16%), thrombocytopenia (11%), and anemia (10%).
Ibrutinib is now approved for the treatment of patients with mantle cell lymphoma who have received at least one prior therapy, the Food and Drug Administration announced Nov. 13.
The once-daily, oral therapy, marketed as Imbruvica, is the second drug to receive FDA approval under the breakthrough therapy designation established to speed the development and review of treatments for serious or life-threatening diseases.
"Imbruvica’s approval demonstrates the FDA’s commitment to making treatments available to patients with rare diseases," Dr. Richard Pazdur, director of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in a statement.
Mantle cell lymphoma is an orphan disease, with only about 2,900 new cases of MCL diagnosed each year. MCL comprises only about 6% of all non-Hodgkin’s lymphoma cases in the United States.
Ibrutinib’s approval comes a little more than 4 months after the new drug application was filed in June 2013 and is based on a phase II study reporting an investigator-assessed overall response rate of 66% at a daily dose of 560 mg ibrutinib in 111 patients with relapsed or refractory MCL after a median of three prior therapies. The median duration of response was 17.5 months. An improvement in survival and disease-related symptoms has not been established.
Ibrutinib works by blocking Bruton’s tyrosine kinase, a mediator of the B-cell receptor signaling pathway that has been shown in nonclinical studies to inhibit malignant B-cell survival.
The FDA also granted ibrutinib priority review and orphan-product designation, because the drug demonstrated "the potential to be a significant improvement in safety or effectiveness in the treatment of a serious condition and is intended to treat a rare disease," according to the FDA statement.
Ibrutinib is the third drug approved to treat MCL. In June 2013, the FDA approved the oral thalidomide analogue lenalidomide (Revlimid) for the treatment of MCL that had relapsed or progressed after two prior therapies including bortezomib (Velcade), a subcutaneous therapy that has been available for MCL since 2006.
"It is gratifying to see an early example of the new breakthrough therapy designation pathway meeting its intention – getting promising treatments to patients who are waiting for new options," Dr. Ellen V. Sigal, chairperson and founder of the Washington-based Friends of Cancer Research advocacy organization, said in a statement issued by Janssen Biotech, which is comarketing the drug with Pharmacyclics.
The two companies are expected to continue with phase III studies of ibrutinib and have also submitted the drug to the FDA for the treatment of previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma.
In the pivotal MCL trial, the most common treatment-related adverse events with single-agent ibrutinib were mild or moderate diarrhea, fatigue, and nausea (N. Engl. J. Med. 2013;369:507-16). Grade 3 or higher hematologic events were neutropenia (16%), thrombocytopenia (11%), and anemia (10%).
FDA approves lenalidomide for mantle cell lymphoma
The Food and Drug Administration has approved lenalidomide for the treatment of patients whose mantle cell lymphoma has relapsed or progressed after two prior therapies, one of which included bortezomib.
Lenalidomide, a thalidomide analogue, is already approved for use in combination with dexamethasone for multiple myeloma in patients who have received at least one prior therapy. Lenalidomide also is approved for transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
"There remains a tremendous unmet need for [therapies for] patients with previously treated mantle cell lymphoma," said Dr. Andre Goy, chairman and director, and chief of the division of lymphoma at the John Theurer Cancer Center at Hackensack (N.J.) University Medical Center, in a statement issued by lenalidomide maker Celgene. "The approval of lenalidomide delivers a new option and the first oral therapy in this area of lymphoma."
Mantle cell lymphoma is fairly rare, accounting for about 6% of the 66,360 new cases of non-Hodgkin’s lymphoma diagnosed in the United States each year, according to the Leukemia and Lymphoma Society.
The Food and Drug Administration (FDA) said it based its approval on a single-arm, multicenter study with 134 patients who had relapsed after or were refractory to bortezomib or a bortezomib-containing regimen. In the 133 patients who were evaluable for efficacy, the overall lenalidomide response rate was 26%. Nine patients (7%) had a complete response or unconfirmed complete response, and 25 (19%) had a partial response. In the 34 responders, the median duration of response was 16.6 months.
Due to adverse events, a little more than half of the patients had to interrupt therapy; 38% had a dose reduction and 19% discontinued therapy. The most common reactions included neutropenia, thrombocytopenia, fatigue, anemia, diarrhea, nausea, cough, pyrexia, rash, dyspnea, pruritus, constipation, peripheral edema, and leukopenia, according to the FDA.
In May 2012, the agency also determined that patients taking the drug for newly diagnosed multiple myeloma are at increased risk for secondary cancers.
Lenalidomide was approved at a recommended dose and schedule of 25 mg orally once daily on days 1-21 of repeated 28-day cycles. Celgene also received approval for a new 20-mg strength of lenalidomide.
On Twitter @aliciaault
The Food and Drug Administration has approved lenalidomide for the treatment of patients whose mantle cell lymphoma has relapsed or progressed after two prior therapies, one of which included bortezomib.
Lenalidomide, a thalidomide analogue, is already approved for use in combination with dexamethasone for multiple myeloma in patients who have received at least one prior therapy. Lenalidomide also is approved for transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
"There remains a tremendous unmet need for [therapies for] patients with previously treated mantle cell lymphoma," said Dr. Andre Goy, chairman and director, and chief of the division of lymphoma at the John Theurer Cancer Center at Hackensack (N.J.) University Medical Center, in a statement issued by lenalidomide maker Celgene. "The approval of lenalidomide delivers a new option and the first oral therapy in this area of lymphoma."
Mantle cell lymphoma is fairly rare, accounting for about 6% of the 66,360 new cases of non-Hodgkin’s lymphoma diagnosed in the United States each year, according to the Leukemia and Lymphoma Society.
The Food and Drug Administration (FDA) said it based its approval on a single-arm, multicenter study with 134 patients who had relapsed after or were refractory to bortezomib or a bortezomib-containing regimen. In the 133 patients who were evaluable for efficacy, the overall lenalidomide response rate was 26%. Nine patients (7%) had a complete response or unconfirmed complete response, and 25 (19%) had a partial response. In the 34 responders, the median duration of response was 16.6 months.
Due to adverse events, a little more than half of the patients had to interrupt therapy; 38% had a dose reduction and 19% discontinued therapy. The most common reactions included neutropenia, thrombocytopenia, fatigue, anemia, diarrhea, nausea, cough, pyrexia, rash, dyspnea, pruritus, constipation, peripheral edema, and leukopenia, according to the FDA.
In May 2012, the agency also determined that patients taking the drug for newly diagnosed multiple myeloma are at increased risk for secondary cancers.
Lenalidomide was approved at a recommended dose and schedule of 25 mg orally once daily on days 1-21 of repeated 28-day cycles. Celgene also received approval for a new 20-mg strength of lenalidomide.
On Twitter @aliciaault
The Food and Drug Administration has approved lenalidomide for the treatment of patients whose mantle cell lymphoma has relapsed or progressed after two prior therapies, one of which included bortezomib.
Lenalidomide, a thalidomide analogue, is already approved for use in combination with dexamethasone for multiple myeloma in patients who have received at least one prior therapy. Lenalidomide also is approved for transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
"There remains a tremendous unmet need for [therapies for] patients with previously treated mantle cell lymphoma," said Dr. Andre Goy, chairman and director, and chief of the division of lymphoma at the John Theurer Cancer Center at Hackensack (N.J.) University Medical Center, in a statement issued by lenalidomide maker Celgene. "The approval of lenalidomide delivers a new option and the first oral therapy in this area of lymphoma."
Mantle cell lymphoma is fairly rare, accounting for about 6% of the 66,360 new cases of non-Hodgkin’s lymphoma diagnosed in the United States each year, according to the Leukemia and Lymphoma Society.
The Food and Drug Administration (FDA) said it based its approval on a single-arm, multicenter study with 134 patients who had relapsed after or were refractory to bortezomib or a bortezomib-containing regimen. In the 133 patients who were evaluable for efficacy, the overall lenalidomide response rate was 26%. Nine patients (7%) had a complete response or unconfirmed complete response, and 25 (19%) had a partial response. In the 34 responders, the median duration of response was 16.6 months.
Due to adverse events, a little more than half of the patients had to interrupt therapy; 38% had a dose reduction and 19% discontinued therapy. The most common reactions included neutropenia, thrombocytopenia, fatigue, anemia, diarrhea, nausea, cough, pyrexia, rash, dyspnea, pruritus, constipation, peripheral edema, and leukopenia, according to the FDA.
In May 2012, the agency also determined that patients taking the drug for newly diagnosed multiple myeloma are at increased risk for secondary cancers.
Lenalidomide was approved at a recommended dose and schedule of 25 mg orally once daily on days 1-21 of repeated 28-day cycles. Celgene also received approval for a new 20-mg strength of lenalidomide.
On Twitter @aliciaault
'Highest response rate ever reported' in relapsed mantle cell lymphoma
ATLANTA – More than one-fifth of patients with relapsed or refractory mantle cell lymphoma had a complete response to single-agent therapy with experimental ibrutinib, and another two-fifths had a partial response, investigators reported.
"Colleagues, this is the highest response rate ever reported, ever achieved by one single drug in the history of relapsed mantle cell lymphoma," Dr. Michael Wang told attendees at the annual meeting of the American Society of Hematology.
Dr. Wang’s evident excitement about the data came exactly 1 year to the day after he announced preliminary results of the phase II trial at ASH 2011. At that time, the drug was known only as PCI-32765.
Those early data showed that ibrutinib, an oral inhibitor of Bruton’s tyrosine kinase (BTK) expressed in several hematologic malignancies, induced complete response in 16% and partial response in 53% of patients evaluated at that time for a combined overall response rate of 69%.
The drug demonstrated efficacy against bulky disease, and its effects in early studies appeared to be independent of the MCL [Mantle Cell Lymphoma] International Prognostic Index (MIPI) score.
At this year’s meeting, Dr. Wang of the University of Texas M.D. Anderson Cancer Center, Houston, reported that in an efficacy cohort of 110 patients, 22% had a complete response and 46% a partial response. Among patients who had previously been treated with bortezomib (Velcade), 23% had a complete response, and 49% had a partial response. In bortezomib-naive patients the response rates were 21% and 44%, respectively.
At 9.2 months of follow-up (data cutoff Sept. 21, 2012), the median duration of response had not been reached. Median progression-free survival was 13.9 months.
Ibrutinib was well tolerated
BTK, an essential element of the B-cell antigen receptor–signaling pathway, is expressed in several hematologic malignancies, including lymphoma and chronic lymphocytic leukemia, for which positive clinical trial data were also presented at the meeting. Ibrutinib blocks receptor signaling and induces apoptosis, as well as mantle cell migration and adhesion. It has been shown in in vitro studies to block pERK, pJNK, and NF-KappaB pathways in MCL cell lines.
Dr. Wang and colleagues at 18 U.S. and European centers enrolled patients with confirmed overexpression of cyclin D1 or the 11;14 translocation and measurable disease. The patients had not been able to achieve at least a partial response to prior therapy, or had disease progression following their most recent treatment regimen. All had at least one, but not more than five prior lines of therapy, and adequate end-organ function, and Eastern Cooperative Oncology Group performance status of 2 or lower.
The drug was generally well tolerated, he said, with neutropenia, thrombocytopenia, and anemia being the most frequent hematologic toxicities, and diarrhea, fatigue, nausea, and respiratory tract infections being the most common nonhematologic adverse events.
Dr. Wang noted that longer follow-up of data on 51 patients in the cohort that he presented at ASH 2011 show improvement in complete response rates. The initial rates among bortezomib-naive, bortezomib experienced, and all patients were 16%, 15%, and 16%, respectively, at a median of 3.7 months. In the current follow-up of these patients, however (median 14.7 months), the complete response rates had improved to 40%, 38%, and 39%, respectively, with respective overall response rates of 77%, 71%, and 75%. He called the gradual increase in complete response rates the "phenomenon of incremental response."
Phenomenon of incremental response
Dr. Andrew D. Zelenetz of Memorial Sloan-Kettering Cancer Center, New York, said after Dr. Wang’s presentation that the "phenomenon of incremental response" Dr. Wang described is not really a phenomenon at all.
"The reason you’re giving the drug continuously is that you expect to see a better response," he said. "It’s not unique to this drug, but in rituximab it’s seen when you stop the drug, in lenalidomide it’s seen when you stop the drug, and in radioimmunotherapy it’s seen when you stop the drug. So incremental response is a well described phenomenon in lymphomas," Dr. Zelenetz said.
"This has been just an interim analysis our data. We look forward to updating you with the final results of this clinical trial with excitement, caution, and confidence," Dr.Wang said.
The study was supported by Pharmacyclics. Dr. Wang is on the scientific advisory board of the company. Dr. Zelenetz had no relevant disclosures.
ATLANTA – More than one-fifth of patients with relapsed or refractory mantle cell lymphoma had a complete response to single-agent therapy with experimental ibrutinib, and another two-fifths had a partial response, investigators reported.
"Colleagues, this is the highest response rate ever reported, ever achieved by one single drug in the history of relapsed mantle cell lymphoma," Dr. Michael Wang told attendees at the annual meeting of the American Society of Hematology.
Dr. Wang’s evident excitement about the data came exactly 1 year to the day after he announced preliminary results of the phase II trial at ASH 2011. At that time, the drug was known only as PCI-32765.
Those early data showed that ibrutinib, an oral inhibitor of Bruton’s tyrosine kinase (BTK) expressed in several hematologic malignancies, induced complete response in 16% and partial response in 53% of patients evaluated at that time for a combined overall response rate of 69%.
The drug demonstrated efficacy against bulky disease, and its effects in early studies appeared to be independent of the MCL [Mantle Cell Lymphoma] International Prognostic Index (MIPI) score.
At this year’s meeting, Dr. Wang of the University of Texas M.D. Anderson Cancer Center, Houston, reported that in an efficacy cohort of 110 patients, 22% had a complete response and 46% a partial response. Among patients who had previously been treated with bortezomib (Velcade), 23% had a complete response, and 49% had a partial response. In bortezomib-naive patients the response rates were 21% and 44%, respectively.
At 9.2 months of follow-up (data cutoff Sept. 21, 2012), the median duration of response had not been reached. Median progression-free survival was 13.9 months.
Ibrutinib was well tolerated
BTK, an essential element of the B-cell antigen receptor–signaling pathway, is expressed in several hematologic malignancies, including lymphoma and chronic lymphocytic leukemia, for which positive clinical trial data were also presented at the meeting. Ibrutinib blocks receptor signaling and induces apoptosis, as well as mantle cell migration and adhesion. It has been shown in in vitro studies to block pERK, pJNK, and NF-KappaB pathways in MCL cell lines.
Dr. Wang and colleagues at 18 U.S. and European centers enrolled patients with confirmed overexpression of cyclin D1 or the 11;14 translocation and measurable disease. The patients had not been able to achieve at least a partial response to prior therapy, or had disease progression following their most recent treatment regimen. All had at least one, but not more than five prior lines of therapy, and adequate end-organ function, and Eastern Cooperative Oncology Group performance status of 2 or lower.
The drug was generally well tolerated, he said, with neutropenia, thrombocytopenia, and anemia being the most frequent hematologic toxicities, and diarrhea, fatigue, nausea, and respiratory tract infections being the most common nonhematologic adverse events.
Dr. Wang noted that longer follow-up of data on 51 patients in the cohort that he presented at ASH 2011 show improvement in complete response rates. The initial rates among bortezomib-naive, bortezomib experienced, and all patients were 16%, 15%, and 16%, respectively, at a median of 3.7 months. In the current follow-up of these patients, however (median 14.7 months), the complete response rates had improved to 40%, 38%, and 39%, respectively, with respective overall response rates of 77%, 71%, and 75%. He called the gradual increase in complete response rates the "phenomenon of incremental response."
Phenomenon of incremental response
Dr. Andrew D. Zelenetz of Memorial Sloan-Kettering Cancer Center, New York, said after Dr. Wang’s presentation that the "phenomenon of incremental response" Dr. Wang described is not really a phenomenon at all.
"The reason you’re giving the drug continuously is that you expect to see a better response," he said. "It’s not unique to this drug, but in rituximab it’s seen when you stop the drug, in lenalidomide it’s seen when you stop the drug, and in radioimmunotherapy it’s seen when you stop the drug. So incremental response is a well described phenomenon in lymphomas," Dr. Zelenetz said.
"This has been just an interim analysis our data. We look forward to updating you with the final results of this clinical trial with excitement, caution, and confidence," Dr.Wang said.
The study was supported by Pharmacyclics. Dr. Wang is on the scientific advisory board of the company. Dr. Zelenetz had no relevant disclosures.
ATLANTA – More than one-fifth of patients with relapsed or refractory mantle cell lymphoma had a complete response to single-agent therapy with experimental ibrutinib, and another two-fifths had a partial response, investigators reported.
"Colleagues, this is the highest response rate ever reported, ever achieved by one single drug in the history of relapsed mantle cell lymphoma," Dr. Michael Wang told attendees at the annual meeting of the American Society of Hematology.
Dr. Wang’s evident excitement about the data came exactly 1 year to the day after he announced preliminary results of the phase II trial at ASH 2011. At that time, the drug was known only as PCI-32765.
Those early data showed that ibrutinib, an oral inhibitor of Bruton’s tyrosine kinase (BTK) expressed in several hematologic malignancies, induced complete response in 16% and partial response in 53% of patients evaluated at that time for a combined overall response rate of 69%.
The drug demonstrated efficacy against bulky disease, and its effects in early studies appeared to be independent of the MCL [Mantle Cell Lymphoma] International Prognostic Index (MIPI) score.
At this year’s meeting, Dr. Wang of the University of Texas M.D. Anderson Cancer Center, Houston, reported that in an efficacy cohort of 110 patients, 22% had a complete response and 46% a partial response. Among patients who had previously been treated with bortezomib (Velcade), 23% had a complete response, and 49% had a partial response. In bortezomib-naive patients the response rates were 21% and 44%, respectively.
At 9.2 months of follow-up (data cutoff Sept. 21, 2012), the median duration of response had not been reached. Median progression-free survival was 13.9 months.
Ibrutinib was well tolerated
BTK, an essential element of the B-cell antigen receptor–signaling pathway, is expressed in several hematologic malignancies, including lymphoma and chronic lymphocytic leukemia, for which positive clinical trial data were also presented at the meeting. Ibrutinib blocks receptor signaling and induces apoptosis, as well as mantle cell migration and adhesion. It has been shown in in vitro studies to block pERK, pJNK, and NF-KappaB pathways in MCL cell lines.
Dr. Wang and colleagues at 18 U.S. and European centers enrolled patients with confirmed overexpression of cyclin D1 or the 11;14 translocation and measurable disease. The patients had not been able to achieve at least a partial response to prior therapy, or had disease progression following their most recent treatment regimen. All had at least one, but not more than five prior lines of therapy, and adequate end-organ function, and Eastern Cooperative Oncology Group performance status of 2 or lower.
The drug was generally well tolerated, he said, with neutropenia, thrombocytopenia, and anemia being the most frequent hematologic toxicities, and diarrhea, fatigue, nausea, and respiratory tract infections being the most common nonhematologic adverse events.
Dr. Wang noted that longer follow-up of data on 51 patients in the cohort that he presented at ASH 2011 show improvement in complete response rates. The initial rates among bortezomib-naive, bortezomib experienced, and all patients were 16%, 15%, and 16%, respectively, at a median of 3.7 months. In the current follow-up of these patients, however (median 14.7 months), the complete response rates had improved to 40%, 38%, and 39%, respectively, with respective overall response rates of 77%, 71%, and 75%. He called the gradual increase in complete response rates the "phenomenon of incremental response."
Phenomenon of incremental response
Dr. Andrew D. Zelenetz of Memorial Sloan-Kettering Cancer Center, New York, said after Dr. Wang’s presentation that the "phenomenon of incremental response" Dr. Wang described is not really a phenomenon at all.
"The reason you’re giving the drug continuously is that you expect to see a better response," he said. "It’s not unique to this drug, but in rituximab it’s seen when you stop the drug, in lenalidomide it’s seen when you stop the drug, and in radioimmunotherapy it’s seen when you stop the drug. So incremental response is a well described phenomenon in lymphomas," Dr. Zelenetz said.
"This has been just an interim analysis our data. We look forward to updating you with the final results of this clinical trial with excitement, caution, and confidence," Dr.Wang said.
The study was supported by Pharmacyclics. Dr. Wang is on the scientific advisory board of the company. Dr. Zelenetz had no relevant disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: In an efficacy cohort of 110 patients with relapsed or refractory mantle cell lymphoma, 22% had a complete response and 46% a partial response to ibrutinib.
Data Source: A phase II efficacy and safety trial.
Disclosures: The study was supported by Pharmacyclics. Dr. Wang is on the scientific advisory board of the company. Dr. Zelenetz had no relevant disclosures.
CNS Events Not Immediately Fatal in Mantle Cell Lymphomas
AMSTERDAM – Although it is rare and the prognosis for patients is often poor, the presence of central nervous system involvement at the time of diagnosis of mantle cell lymphoma is not always immediately fatal, according to findings from an international study.
According to the European Mantle Cell Lymphoma Network (EMCLN) findings, the crude prevalence of CNS events was 0.9% at the time of diagnosis and 4.1% at any time. The multicenter, retrospective study found that the median time to a CNS event’s occurring was 15.2 months, with an overall survival of 3.9 months after the event was identified, but some patients were still alive 2 years later.
"We now have some better descriptors about what the expectations and outcome of patients with central nervous system involvement are," Dr. John Seymour said in an interview at the annual congress of the European Hematology Association.
Dr. Seymour, professor and chair of the hematology service at Australia’s Peter MacCallum Cancer Centre in East Melbourne, Victoria, added that even though the overall prognosis of patients who develop CNS involvement is very poor, there are some patients who do better than others.
There is "a subgroup [of patients] who are able to receive high-dose ara-c [cytarabine] or high-dose methotrexate treatment, who are young and fit enough, who do somewhat better," Dr. Seymour said. "A proportion will be alive at 2 years, so it’s not an inevitably, rapidly fatal, and ... futile situation."
Mantle cell lymphomas are a rare type of non-Hodgkin’s lymphoma (NHL), accounting for just less than 3% of all NHL cases in the United States and affecting primarily more elderly patients (Cancer 2008;113:791-8).CNS involvement is also a rare and often devastating event, but it has not previously been very well characterized. As a result, it’s not known whether CNS prophylaxis is of benefit to patients.
The aim of the EMCLN study, therefore, was to look at the problem in more detail, to determine the prevalence of CNS involvement, and to look for any clinically defining features, effect of treatment, and patient outcomes.
A retrospective database review by EMCLN members in 12 centers identified 1,396 patients with mantle cell lymphoma, of whom 1,339 had no CNS involvement. Of the 57 patients with CNS involvement, most (44) developed it at some point during the course of their follow-up.
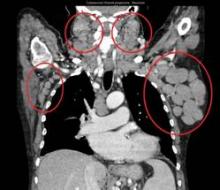
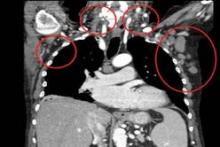
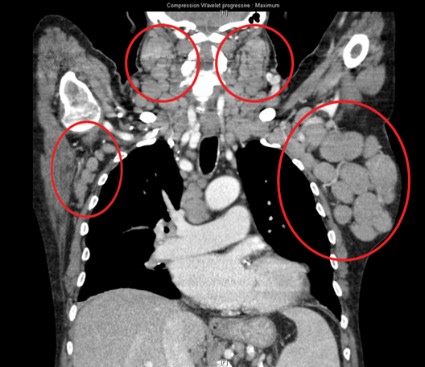
At diagnosis of mantle cell lymphoma, the patients who developed CNS involvement had a median age of 61 years, but this ranged from 38 years to 82 years; patients were predominantly men (70%), with stage IV (91%) disease, and 28% had blastoid histology. Isolated CNS involvement occurred in 15 cases.
Prominent features were a high MIPI (Mantle Cell Lymphoma International Prognostic Index) score (61% of cases), a Ki-67 greater than 30% in 69% of patients, and increased beta2-microglobulin and lactate dehydrogenase in 77% and 75% of cases, respectively. The bone marrow and the peripheral blood were the most common extranodal sites involved, affecting two or more sites in 61% of patients.
At diagnosis of CNS involvement, patients’ neurologic symptoms included weakness, altered mental state, headache, and ocular problems such as double vision. Other symptoms – such as sensory disturbances, pain, sciatica, dizziness, vertigo, ataxia, seizure, and dysphagia – occurred but were less frequent.
CSF cytology and flow cytometry showed a high sensitivity for identifying CNS involvement, with 85% having positive cytology and 91% a positive flow cytometry result.
"Patients had a range of chemotherapies prior to developing central nervous system involvement, but receipt of these regimens was not totally protective," Dr. Seymour said. Data were not collected to enable the relative risk of CNS development with the regimens received.
Chemotherapy was the most frequent treatment strategy to allay CNS disease (67%), and some patients did appear to achieve a complete remission of the CNS disease as a result. In an exploratory analysis, these patients also tended to have improved overall survival, as did those with lower white cell counts (less than 10.9 x 109/L), and who received treatment with high-dose antimetabolites.
"In the longer term, these data will provide a foundation for us to identify predictive factors, to identify – ahead of the event – those people at increased risk," Dr. Seymour said, adding his hope that this will allow preventive steps to be taken.
Dr. Seymour had no conflicts of interest.
AMSTERDAM – Although it is rare and the prognosis for patients is often poor, the presence of central nervous system involvement at the time of diagnosis of mantle cell lymphoma is not always immediately fatal, according to findings from an international study.
According to the European Mantle Cell Lymphoma Network (EMCLN) findings, the crude prevalence of CNS events was 0.9% at the time of diagnosis and 4.1% at any time. The multicenter, retrospective study found that the median time to a CNS event’s occurring was 15.2 months, with an overall survival of 3.9 months after the event was identified, but some patients were still alive 2 years later.
"We now have some better descriptors about what the expectations and outcome of patients with central nervous system involvement are," Dr. John Seymour said in an interview at the annual congress of the European Hematology Association.
Dr. Seymour, professor and chair of the hematology service at Australia’s Peter MacCallum Cancer Centre in East Melbourne, Victoria, added that even though the overall prognosis of patients who develop CNS involvement is very poor, there are some patients who do better than others.
There is "a subgroup [of patients] who are able to receive high-dose ara-c [cytarabine] or high-dose methotrexate treatment, who are young and fit enough, who do somewhat better," Dr. Seymour said. "A proportion will be alive at 2 years, so it’s not an inevitably, rapidly fatal, and ... futile situation."
Mantle cell lymphomas are a rare type of non-Hodgkin’s lymphoma (NHL), accounting for just less than 3% of all NHL cases in the United States and affecting primarily more elderly patients (Cancer 2008;113:791-8).CNS involvement is also a rare and often devastating event, but it has not previously been very well characterized. As a result, it’s not known whether CNS prophylaxis is of benefit to patients.
The aim of the EMCLN study, therefore, was to look at the problem in more detail, to determine the prevalence of CNS involvement, and to look for any clinically defining features, effect of treatment, and patient outcomes.
A retrospective database review by EMCLN members in 12 centers identified 1,396 patients with mantle cell lymphoma, of whom 1,339 had no CNS involvement. Of the 57 patients with CNS involvement, most (44) developed it at some point during the course of their follow-up.
At diagnosis of mantle cell lymphoma, the patients who developed CNS involvement had a median age of 61 years, but this ranged from 38 years to 82 years; patients were predominantly men (70%), with stage IV (91%) disease, and 28% had blastoid histology. Isolated CNS involvement occurred in 15 cases.
Prominent features were a high MIPI (Mantle Cell Lymphoma International Prognostic Index) score (61% of cases), a Ki-67 greater than 30% in 69% of patients, and increased beta2-microglobulin and lactate dehydrogenase in 77% and 75% of cases, respectively. The bone marrow and the peripheral blood were the most common extranodal sites involved, affecting two or more sites in 61% of patients.
At diagnosis of CNS involvement, patients’ neurologic symptoms included weakness, altered mental state, headache, and ocular problems such as double vision. Other symptoms – such as sensory disturbances, pain, sciatica, dizziness, vertigo, ataxia, seizure, and dysphagia – occurred but were less frequent.
CSF cytology and flow cytometry showed a high sensitivity for identifying CNS involvement, with 85% having positive cytology and 91% a positive flow cytometry result.
"Patients had a range of chemotherapies prior to developing central nervous system involvement, but receipt of these regimens was not totally protective," Dr. Seymour said. Data were not collected to enable the relative risk of CNS development with the regimens received.
Chemotherapy was the most frequent treatment strategy to allay CNS disease (67%), and some patients did appear to achieve a complete remission of the CNS disease as a result. In an exploratory analysis, these patients also tended to have improved overall survival, as did those with lower white cell counts (less than 10.9 x 109/L), and who received treatment with high-dose antimetabolites.
"In the longer term, these data will provide a foundation for us to identify predictive factors, to identify – ahead of the event – those people at increased risk," Dr. Seymour said, adding his hope that this will allow preventive steps to be taken.
Dr. Seymour had no conflicts of interest.
AMSTERDAM – Although it is rare and the prognosis for patients is often poor, the presence of central nervous system involvement at the time of diagnosis of mantle cell lymphoma is not always immediately fatal, according to findings from an international study.
According to the European Mantle Cell Lymphoma Network (EMCLN) findings, the crude prevalence of CNS events was 0.9% at the time of diagnosis and 4.1% at any time. The multicenter, retrospective study found that the median time to a CNS event’s occurring was 15.2 months, with an overall survival of 3.9 months after the event was identified, but some patients were still alive 2 years later.
"We now have some better descriptors about what the expectations and outcome of patients with central nervous system involvement are," Dr. John Seymour said in an interview at the annual congress of the European Hematology Association.
Dr. Seymour, professor and chair of the hematology service at Australia’s Peter MacCallum Cancer Centre in East Melbourne, Victoria, added that even though the overall prognosis of patients who develop CNS involvement is very poor, there are some patients who do better than others.
There is "a subgroup [of patients] who are able to receive high-dose ara-c [cytarabine] or high-dose methotrexate treatment, who are young and fit enough, who do somewhat better," Dr. Seymour said. "A proportion will be alive at 2 years, so it’s not an inevitably, rapidly fatal, and ... futile situation."
Mantle cell lymphomas are a rare type of non-Hodgkin’s lymphoma (NHL), accounting for just less than 3% of all NHL cases in the United States and affecting primarily more elderly patients (Cancer 2008;113:791-8).CNS involvement is also a rare and often devastating event, but it has not previously been very well characterized. As a result, it’s not known whether CNS prophylaxis is of benefit to patients.
The aim of the EMCLN study, therefore, was to look at the problem in more detail, to determine the prevalence of CNS involvement, and to look for any clinically defining features, effect of treatment, and patient outcomes.
A retrospective database review by EMCLN members in 12 centers identified 1,396 patients with mantle cell lymphoma, of whom 1,339 had no CNS involvement. Of the 57 patients with CNS involvement, most (44) developed it at some point during the course of their follow-up.
At diagnosis of mantle cell lymphoma, the patients who developed CNS involvement had a median age of 61 years, but this ranged from 38 years to 82 years; patients were predominantly men (70%), with stage IV (91%) disease, and 28% had blastoid histology. Isolated CNS involvement occurred in 15 cases.
Prominent features were a high MIPI (Mantle Cell Lymphoma International Prognostic Index) score (61% of cases), a Ki-67 greater than 30% in 69% of patients, and increased beta2-microglobulin and lactate dehydrogenase in 77% and 75% of cases, respectively. The bone marrow and the peripheral blood were the most common extranodal sites involved, affecting two or more sites in 61% of patients.
At diagnosis of CNS involvement, patients’ neurologic symptoms included weakness, altered mental state, headache, and ocular problems such as double vision. Other symptoms – such as sensory disturbances, pain, sciatica, dizziness, vertigo, ataxia, seizure, and dysphagia – occurred but were less frequent.
CSF cytology and flow cytometry showed a high sensitivity for identifying CNS involvement, with 85% having positive cytology and 91% a positive flow cytometry result.
"Patients had a range of chemotherapies prior to developing central nervous system involvement, but receipt of these regimens was not totally protective," Dr. Seymour said. Data were not collected to enable the relative risk of CNS development with the regimens received.
Chemotherapy was the most frequent treatment strategy to allay CNS disease (67%), and some patients did appear to achieve a complete remission of the CNS disease as a result. In an exploratory analysis, these patients also tended to have improved overall survival, as did those with lower white cell counts (less than 10.9 x 109/L), and who received treatment with high-dose antimetabolites.
"In the longer term, these data will provide a foundation for us to identify predictive factors, to identify – ahead of the event – those people at increased risk," Dr. Seymour said, adding his hope that this will allow preventive steps to be taken.
Dr. Seymour had no conflicts of interest.
AT THE ANNUAL CONGRESS OF THE EUROPEAN HEMATOLOGY ASSOCIATION
Major Finding: Crude prevalences of CNS involvement at diagnosis and overall were 0.9% and 4.1%, respectively, with a median time to an event of 15.2 months and overall survival thereafter of 3.9 months.
Data Source: The EMCLN conducted a retrospective database review of 1,396 patients with mantle cell lymphoma in 12 centers.
Disclosures: Dr. Seymour had no conflicts of interest.