User login
TZD Use Is Linked to Increased Risk of Bone Fracture
NEW YORK — Treatment with a thiazolidinedione, either pioglitazone or rosiglitazone, was linked to an increased rate of bone fractures, particularly in women, in several recently published reports.
Although a definitive link between these drugs and an increased fracture risk has not yet been proved, the evidence amassed so far is suggestive enough to prompt caution in the treatment of patients with a thiazolidinedione (TZD), Dr. Robert G. Josse said at a meeting sponsored by the American Diabetes Association.
“In those with a higher fracture risk, consider other hypoglycemic therapy,” advised Dr. Josse, professor of medicine and nutritional sciences at the University of Toronto and medical director of the department of medicine at the osteoporosis center at St. Michael's Hospital in Toronto. In addition, “if using a TZD, consider therapy to prevent TZD-induced osteoporosis.” Standard therapies for osteoporosis are effective in patients with diabetes—including those with diabetes who develop steroid-induced osteoporosis—but no data now exist on the efficacy of antiosteoporosis treatments for countering the possible effects of TZDs, he noted.
Reasonable steps to reduce the fracture risk in patients who must take a TZD include optimizing calcium intake and the supply of vitamin D, encouraging adequate exercise, and taking precautions to prevent falls. Administration of antiresorptive drugs, such as raloxifene and the bisphosphonates, seems to be effective in patients with diabetes, but the effects of bone anabolic drugs such as teriparatide in these patients isn't known.
The idea that treatment with pioglitazone (Actos) or rosiglitazone (Avandia) may cause osteoporosis and produce an increased rate of bone fractures is biologically plausible, and has been suggested in the results from some studies.
Perhaps the most persuasive evidence so far is a meta-analysis published in January that compiled adverse-event data from 10 randomized, controlled studies with a total of more than 13,000 patients, and also reviewed two observational studies with a total of more than 31,000 patients (Can. Med. Ass. J. 2009;180:32–9). In the 10 randomized trials, patients treated with a TZD had a statistically significant 45% increased risk for bone fracture, compared with patients in the control groups. When the analysis broke the study population down by sex, a statistically significant 2.2-fold increased fracture risk was seen in women treated with a TZD, but absolutely no increased risk was seen in men. Additional analysis by sex showed that, in women, TZD treatment was linked with significant reductions of bone mineral density in the lumbar spine and hip. The two observational studies also showed a significant link between TZD use and fracture risk in women, but not in men.
The two short-term, randomized studies included a study with 50 healthy postmenopausal women without osteoporosis or diabetes who were randomized to treatment with 8 mg rosiglitazone daily or placebo for 14 weeks. Despite the brief period of treatment, the women in the rosiglitazone-treated group had a statistically significant reduction in their total hip bone mineral density, compared with the placebo group (J. Clin. Endocrinol. Metab. 2007;92:1305–10). A second study, published last May, randomized 30 postmenopausal women with polycystic ovary syndrome but without diabetes to treatment with either 30 mg pioglitazone daily or placebo. After 16 weeks, the women treated with pioglitazone had significantly lower lumbar spine and femoral neck density, compared with the controls (J. Clin. Endocrinol. Metab. 2008;93:1696–701). The TZD-treated women also showed significantly decreased blood levels of bone-turnover hormones and enzymes.
Dr. Josse reported receiving research support from, and serving on the speakers bureau and advisory panel for, several companies including Amgen Inc., Eli Lilly & Co., Procter & Gamble Co., and Sanofi-Aventis.
The evidence so far is suggestive enough to prompt caution in the treatment of patients with a thiazolidinedione. DR. JOSSE
NEW YORK — Treatment with a thiazolidinedione, either pioglitazone or rosiglitazone, was linked to an increased rate of bone fractures, particularly in women, in several recently published reports.
Although a definitive link between these drugs and an increased fracture risk has not yet been proved, the evidence amassed so far is suggestive enough to prompt caution in the treatment of patients with a thiazolidinedione (TZD), Dr. Robert G. Josse said at a meeting sponsored by the American Diabetes Association.
“In those with a higher fracture risk, consider other hypoglycemic therapy,” advised Dr. Josse, professor of medicine and nutritional sciences at the University of Toronto and medical director of the department of medicine at the osteoporosis center at St. Michael's Hospital in Toronto. In addition, “if using a TZD, consider therapy to prevent TZD-induced osteoporosis.” Standard therapies for osteoporosis are effective in patients with diabetes—including those with diabetes who develop steroid-induced osteoporosis—but no data now exist on the efficacy of antiosteoporosis treatments for countering the possible effects of TZDs, he noted.
Reasonable steps to reduce the fracture risk in patients who must take a TZD include optimizing calcium intake and the supply of vitamin D, encouraging adequate exercise, and taking precautions to prevent falls. Administration of antiresorptive drugs, such as raloxifene and the bisphosphonates, seems to be effective in patients with diabetes, but the effects of bone anabolic drugs such as teriparatide in these patients isn't known.
The idea that treatment with pioglitazone (Actos) or rosiglitazone (Avandia) may cause osteoporosis and produce an increased rate of bone fractures is biologically plausible, and has been suggested in the results from some studies.
Perhaps the most persuasive evidence so far is a meta-analysis published in January that compiled adverse-event data from 10 randomized, controlled studies with a total of more than 13,000 patients, and also reviewed two observational studies with a total of more than 31,000 patients (Can. Med. Ass. J. 2009;180:32–9). In the 10 randomized trials, patients treated with a TZD had a statistically significant 45% increased risk for bone fracture, compared with patients in the control groups. When the analysis broke the study population down by sex, a statistically significant 2.2-fold increased fracture risk was seen in women treated with a TZD, but absolutely no increased risk was seen in men. Additional analysis by sex showed that, in women, TZD treatment was linked with significant reductions of bone mineral density in the lumbar spine and hip. The two observational studies also showed a significant link between TZD use and fracture risk in women, but not in men.
The two short-term, randomized studies included a study with 50 healthy postmenopausal women without osteoporosis or diabetes who were randomized to treatment with 8 mg rosiglitazone daily or placebo for 14 weeks. Despite the brief period of treatment, the women in the rosiglitazone-treated group had a statistically significant reduction in their total hip bone mineral density, compared with the placebo group (J. Clin. Endocrinol. Metab. 2007;92:1305–10). A second study, published last May, randomized 30 postmenopausal women with polycystic ovary syndrome but without diabetes to treatment with either 30 mg pioglitazone daily or placebo. After 16 weeks, the women treated with pioglitazone had significantly lower lumbar spine and femoral neck density, compared with the controls (J. Clin. Endocrinol. Metab. 2008;93:1696–701). The TZD-treated women also showed significantly decreased blood levels of bone-turnover hormones and enzymes.
Dr. Josse reported receiving research support from, and serving on the speakers bureau and advisory panel for, several companies including Amgen Inc., Eli Lilly & Co., Procter & Gamble Co., and Sanofi-Aventis.
The evidence so far is suggestive enough to prompt caution in the treatment of patients with a thiazolidinedione. DR. JOSSE
NEW YORK — Treatment with a thiazolidinedione, either pioglitazone or rosiglitazone, was linked to an increased rate of bone fractures, particularly in women, in several recently published reports.
Although a definitive link between these drugs and an increased fracture risk has not yet been proved, the evidence amassed so far is suggestive enough to prompt caution in the treatment of patients with a thiazolidinedione (TZD), Dr. Robert G. Josse said at a meeting sponsored by the American Diabetes Association.
“In those with a higher fracture risk, consider other hypoglycemic therapy,” advised Dr. Josse, professor of medicine and nutritional sciences at the University of Toronto and medical director of the department of medicine at the osteoporosis center at St. Michael's Hospital in Toronto. In addition, “if using a TZD, consider therapy to prevent TZD-induced osteoporosis.” Standard therapies for osteoporosis are effective in patients with diabetes—including those with diabetes who develop steroid-induced osteoporosis—but no data now exist on the efficacy of antiosteoporosis treatments for countering the possible effects of TZDs, he noted.
Reasonable steps to reduce the fracture risk in patients who must take a TZD include optimizing calcium intake and the supply of vitamin D, encouraging adequate exercise, and taking precautions to prevent falls. Administration of antiresorptive drugs, such as raloxifene and the bisphosphonates, seems to be effective in patients with diabetes, but the effects of bone anabolic drugs such as teriparatide in these patients isn't known.
The idea that treatment with pioglitazone (Actos) or rosiglitazone (Avandia) may cause osteoporosis and produce an increased rate of bone fractures is biologically plausible, and has been suggested in the results from some studies.
Perhaps the most persuasive evidence so far is a meta-analysis published in January that compiled adverse-event data from 10 randomized, controlled studies with a total of more than 13,000 patients, and also reviewed two observational studies with a total of more than 31,000 patients (Can. Med. Ass. J. 2009;180:32–9). In the 10 randomized trials, patients treated with a TZD had a statistically significant 45% increased risk for bone fracture, compared with patients in the control groups. When the analysis broke the study population down by sex, a statistically significant 2.2-fold increased fracture risk was seen in women treated with a TZD, but absolutely no increased risk was seen in men. Additional analysis by sex showed that, in women, TZD treatment was linked with significant reductions of bone mineral density in the lumbar spine and hip. The two observational studies also showed a significant link between TZD use and fracture risk in women, but not in men.
The two short-term, randomized studies included a study with 50 healthy postmenopausal women without osteoporosis or diabetes who were randomized to treatment with 8 mg rosiglitazone daily or placebo for 14 weeks. Despite the brief period of treatment, the women in the rosiglitazone-treated group had a statistically significant reduction in their total hip bone mineral density, compared with the placebo group (J. Clin. Endocrinol. Metab. 2007;92:1305–10). A second study, published last May, randomized 30 postmenopausal women with polycystic ovary syndrome but without diabetes to treatment with either 30 mg pioglitazone daily or placebo. After 16 weeks, the women treated with pioglitazone had significantly lower lumbar spine and femoral neck density, compared with the controls (J. Clin. Endocrinol. Metab. 2008;93:1696–701). The TZD-treated women also showed significantly decreased blood levels of bone-turnover hormones and enzymes.
Dr. Josse reported receiving research support from, and serving on the speakers bureau and advisory panel for, several companies including Amgen Inc., Eli Lilly & Co., Procter & Gamble Co., and Sanofi-Aventis.
The evidence so far is suggestive enough to prompt caution in the treatment of patients with a thiazolidinedione. DR. JOSSE
MRI, CT Compete to Assess Bone Quality
SAN FRANCISCO — Advances in imaging techniques are providing new insights into trabecular and cortical bone structure and may help assess bone quality, a key component of bone strength identified by a 2001 National Institutes of Health consensus panel.
Recent studies suggest that high-resolution MRI (hrMRI), multidetector CT, and high-resolution peripheral quantitative CT (hr-pQCT) each may be useful in assessing bone quality. But each brings different advantages and disadvantages, and it's unclear which imaging modality will be best for identifying osteoporotic fractures and monitoring treatment-related changes in bone structure.
The three imaging modalities can produce significantly different absolute numbers compared with each other when assessing trabecular or cortical bone structure, yet all correlate reasonably well with micro-CT as a standard of reference, Dr. Thomas M. Link said at a conference sponsored by the International Society for Magnetic Resonance in Medicine. Because of differences in acquisition and analysis of the images, bone structure data from the three imaging modalities are not directly comparable.
Trabecular and cortical bone structure are key components of bone quality, which was deemed to be an important component of bone strength, according to NIH (JAMA 2001;285:785–95).
In one randomized, double-blind study, for example, 51 postmenopausal women with osteopenia were treated with alendronate or placebo and followed over a 2-year period by 3T MRI of the radius, tibia and femur, hr-pQCT of the radius and tibia, and dual x-ray absorptiometry measures of bone mineral density. Both 3T MRI and hr-pQCT results for trabecular bone showed moderate but significant correlation with bone density as a reference, even though there was a twofold to fourfold difference between 3T MRI and hr-pQCT in parameter values such as trabecular number, thickness, or separation (J. Bone Miner. Res. 2008;23:463–74).
In earlier studies, hrMRI showed that salmon-calcitonin nasal spray helped maintain trabecular microarchitecture, compared with placebo (J. Bone Miner. Res. 2005;20:1548–61) and that testosterone replacement may improve trabecular architecture in hypogonadal men (J. Clin. Endocrinol. Metab. 2003;88:1497–502).
Another study of 106 postmenopausal women found no difference in conventional bone mineral density measurements between the 35 women with a history of fractures and the fracture-free women in the rest of the cohort, but hr-pQCT imaging showed significant differences in trabecular structure (J. Clin. Endocrinol. Metab. 2005;90:6508–15).
Multidetector CT was used in a separate study showing significant increases in trabecular microstructure in 65 postmenopausal women who were treated for 12 months with teriparatide for osteoporosis (J. Bone Miner. Res. 2007;22:1426–33).
For corticol bone imaging, a newer area of research, two 2008 studies using hr-pQCT showed substantial differences between postmenopausal women with hip or wrist fractures, compared with fracture-free women, said Dr. Link, professor of radiology at the University of California, San Francisco.
Both hrMRI and hr-pQCT are being used experimentally to assess cortical bone porosity, which affects bone stability. One recent study using hr-pQCT found significant differences between normal premenopausal women, normal postmenopausal women, and postmenopausal women with renal osteodystrophy. “This is quite exciting to see these changes in cortical bone porosity. We don't really know what they mean yet, but they're clearly associated with fracture risk,” said Dr. Link, who has has received research funding and support from Merck, which markets medication to treat osteoporosis.
MRI or hr-pQCT provide high spatial resolution and produce no or relatively little radiation, compared with high-radiation exposure from multidetector CT. Multidector CT has the advantage of allowing imaging of more central skeletal sites such as the spine or proximal femur, he said. The hr-pQCT scanners image only peripheral sites such as small areas of the radius and tibia and possibly the calcaneus, while hrMRI covers larger areas of the radius, tibia, and possibly the femur.
The CT techniques provide measures of bone densitometry. Although hrMRI gives no densitometric data, some studies suggest it may be used to analyze bone marrow composition through spectroscopy in order to assess bone stability. The three techniques appear to have similar rates of reproducibility.
MRI is expensive, and the time needed for imaging results in motion artifacts. In comparison, hr-pQCT requires a dedicated scanner. Although the exam time is shorter with hr-pQCT, motion artifacts remain a problem. Multidetector CT is widely available and requires less time for a scan. Postimage processing requires very sophisticated techniques with MRI, but also is technically challenging with hr-pQCT.
Trabecular and cortical bone architecture of the distal tibia is seen here with hr-pQCT.
High-resolution 3T MRI of the distal tibia shows trabecular and cortical bone architecture. IMAGES COURTESY DR. THOMAS M. LINK
SAN FRANCISCO — Advances in imaging techniques are providing new insights into trabecular and cortical bone structure and may help assess bone quality, a key component of bone strength identified by a 2001 National Institutes of Health consensus panel.
Recent studies suggest that high-resolution MRI (hrMRI), multidetector CT, and high-resolution peripheral quantitative CT (hr-pQCT) each may be useful in assessing bone quality. But each brings different advantages and disadvantages, and it's unclear which imaging modality will be best for identifying osteoporotic fractures and monitoring treatment-related changes in bone structure.
The three imaging modalities can produce significantly different absolute numbers compared with each other when assessing trabecular or cortical bone structure, yet all correlate reasonably well with micro-CT as a standard of reference, Dr. Thomas M. Link said at a conference sponsored by the International Society for Magnetic Resonance in Medicine. Because of differences in acquisition and analysis of the images, bone structure data from the three imaging modalities are not directly comparable.
Trabecular and cortical bone structure are key components of bone quality, which was deemed to be an important component of bone strength, according to NIH (JAMA 2001;285:785–95).
In one randomized, double-blind study, for example, 51 postmenopausal women with osteopenia were treated with alendronate or placebo and followed over a 2-year period by 3T MRI of the radius, tibia and femur, hr-pQCT of the radius and tibia, and dual x-ray absorptiometry measures of bone mineral density. Both 3T MRI and hr-pQCT results for trabecular bone showed moderate but significant correlation with bone density as a reference, even though there was a twofold to fourfold difference between 3T MRI and hr-pQCT in parameter values such as trabecular number, thickness, or separation (J. Bone Miner. Res. 2008;23:463–74).
In earlier studies, hrMRI showed that salmon-calcitonin nasal spray helped maintain trabecular microarchitecture, compared with placebo (J. Bone Miner. Res. 2005;20:1548–61) and that testosterone replacement may improve trabecular architecture in hypogonadal men (J. Clin. Endocrinol. Metab. 2003;88:1497–502).
Another study of 106 postmenopausal women found no difference in conventional bone mineral density measurements between the 35 women with a history of fractures and the fracture-free women in the rest of the cohort, but hr-pQCT imaging showed significant differences in trabecular structure (J. Clin. Endocrinol. Metab. 2005;90:6508–15).
Multidetector CT was used in a separate study showing significant increases in trabecular microstructure in 65 postmenopausal women who were treated for 12 months with teriparatide for osteoporosis (J. Bone Miner. Res. 2007;22:1426–33).
For corticol bone imaging, a newer area of research, two 2008 studies using hr-pQCT showed substantial differences between postmenopausal women with hip or wrist fractures, compared with fracture-free women, said Dr. Link, professor of radiology at the University of California, San Francisco.
Both hrMRI and hr-pQCT are being used experimentally to assess cortical bone porosity, which affects bone stability. One recent study using hr-pQCT found significant differences between normal premenopausal women, normal postmenopausal women, and postmenopausal women with renal osteodystrophy. “This is quite exciting to see these changes in cortical bone porosity. We don't really know what they mean yet, but they're clearly associated with fracture risk,” said Dr. Link, who has has received research funding and support from Merck, which markets medication to treat osteoporosis.
MRI or hr-pQCT provide high spatial resolution and produce no or relatively little radiation, compared with high-radiation exposure from multidetector CT. Multidector CT has the advantage of allowing imaging of more central skeletal sites such as the spine or proximal femur, he said. The hr-pQCT scanners image only peripheral sites such as small areas of the radius and tibia and possibly the calcaneus, while hrMRI covers larger areas of the radius, tibia, and possibly the femur.
The CT techniques provide measures of bone densitometry. Although hrMRI gives no densitometric data, some studies suggest it may be used to analyze bone marrow composition through spectroscopy in order to assess bone stability. The three techniques appear to have similar rates of reproducibility.
MRI is expensive, and the time needed for imaging results in motion artifacts. In comparison, hr-pQCT requires a dedicated scanner. Although the exam time is shorter with hr-pQCT, motion artifacts remain a problem. Multidetector CT is widely available and requires less time for a scan. Postimage processing requires very sophisticated techniques with MRI, but also is technically challenging with hr-pQCT.
Trabecular and cortical bone architecture of the distal tibia is seen here with hr-pQCT.
High-resolution 3T MRI of the distal tibia shows trabecular and cortical bone architecture. IMAGES COURTESY DR. THOMAS M. LINK
SAN FRANCISCO — Advances in imaging techniques are providing new insights into trabecular and cortical bone structure and may help assess bone quality, a key component of bone strength identified by a 2001 National Institutes of Health consensus panel.
Recent studies suggest that high-resolution MRI (hrMRI), multidetector CT, and high-resolution peripheral quantitative CT (hr-pQCT) each may be useful in assessing bone quality. But each brings different advantages and disadvantages, and it's unclear which imaging modality will be best for identifying osteoporotic fractures and monitoring treatment-related changes in bone structure.
The three imaging modalities can produce significantly different absolute numbers compared with each other when assessing trabecular or cortical bone structure, yet all correlate reasonably well with micro-CT as a standard of reference, Dr. Thomas M. Link said at a conference sponsored by the International Society for Magnetic Resonance in Medicine. Because of differences in acquisition and analysis of the images, bone structure data from the three imaging modalities are not directly comparable.
Trabecular and cortical bone structure are key components of bone quality, which was deemed to be an important component of bone strength, according to NIH (JAMA 2001;285:785–95).
In one randomized, double-blind study, for example, 51 postmenopausal women with osteopenia were treated with alendronate or placebo and followed over a 2-year period by 3T MRI of the radius, tibia and femur, hr-pQCT of the radius and tibia, and dual x-ray absorptiometry measures of bone mineral density. Both 3T MRI and hr-pQCT results for trabecular bone showed moderate but significant correlation with bone density as a reference, even though there was a twofold to fourfold difference between 3T MRI and hr-pQCT in parameter values such as trabecular number, thickness, or separation (J. Bone Miner. Res. 2008;23:463–74).
In earlier studies, hrMRI showed that salmon-calcitonin nasal spray helped maintain trabecular microarchitecture, compared with placebo (J. Bone Miner. Res. 2005;20:1548–61) and that testosterone replacement may improve trabecular architecture in hypogonadal men (J. Clin. Endocrinol. Metab. 2003;88:1497–502).
Another study of 106 postmenopausal women found no difference in conventional bone mineral density measurements between the 35 women with a history of fractures and the fracture-free women in the rest of the cohort, but hr-pQCT imaging showed significant differences in trabecular structure (J. Clin. Endocrinol. Metab. 2005;90:6508–15).
Multidetector CT was used in a separate study showing significant increases in trabecular microstructure in 65 postmenopausal women who were treated for 12 months with teriparatide for osteoporosis (J. Bone Miner. Res. 2007;22:1426–33).
For corticol bone imaging, a newer area of research, two 2008 studies using hr-pQCT showed substantial differences between postmenopausal women with hip or wrist fractures, compared with fracture-free women, said Dr. Link, professor of radiology at the University of California, San Francisco.
Both hrMRI and hr-pQCT are being used experimentally to assess cortical bone porosity, which affects bone stability. One recent study using hr-pQCT found significant differences between normal premenopausal women, normal postmenopausal women, and postmenopausal women with renal osteodystrophy. “This is quite exciting to see these changes in cortical bone porosity. We don't really know what they mean yet, but they're clearly associated with fracture risk,” said Dr. Link, who has has received research funding and support from Merck, which markets medication to treat osteoporosis.
MRI or hr-pQCT provide high spatial resolution and produce no or relatively little radiation, compared with high-radiation exposure from multidetector CT. Multidector CT has the advantage of allowing imaging of more central skeletal sites such as the spine or proximal femur, he said. The hr-pQCT scanners image only peripheral sites such as small areas of the radius and tibia and possibly the calcaneus, while hrMRI covers larger areas of the radius, tibia, and possibly the femur.
The CT techniques provide measures of bone densitometry. Although hrMRI gives no densitometric data, some studies suggest it may be used to analyze bone marrow composition through spectroscopy in order to assess bone stability. The three techniques appear to have similar rates of reproducibility.
MRI is expensive, and the time needed for imaging results in motion artifacts. In comparison, hr-pQCT requires a dedicated scanner. Although the exam time is shorter with hr-pQCT, motion artifacts remain a problem. Multidetector CT is widely available and requires less time for a scan. Postimage processing requires very sophisticated techniques with MRI, but also is technically challenging with hr-pQCT.
Trabecular and cortical bone architecture of the distal tibia is seen here with hr-pQCT.
High-resolution 3T MRI of the distal tibia shows trabecular and cortical bone architecture. IMAGES COURTESY DR. THOMAS M. LINK
Postmenopausal dyspareunia— a problem for the 21st century
The author reports that he serves on the speaker’s bureau for Novogyne, TherRx, Warner-Chilcott, and Solvay, and on the advisory board for Upsher-Smith, Novogyne, QuatRx, and Wyeth.
CASE: History of dyspareunia
At her latest visit, a 56-year-old woman who is 7 years postmenopausal relates that she has been experiencing worsening pain with intercourse to the point that she now has very little sex drive at all. This problem began approximately 1 year after she discontinued hormone therapy in the wake of reports that it causes cancer and heart attack. She has been offered both local vaginal and systemic hormone therapy, but is too frightened to use any hormones at all. Sexual lubricants no longer seem to work.
How do you counsel her about these symptoms? And what therapy do you offer?
Physicians and other health-care practitioners are seeing a large and growing number of genitourinary and sexual-related complaints among menopausal women—so much so that it has reached epidemic proportions. Yet dyspareunia is underreported and undertreated, and quality of life suffers for these women.
In this article, I focus on two interrelated causes of this epidemic:
- vaginal dryness and vulvovaginal atrophy (VVA) and the impact of these conditions on women’s sexual function and psychosocial well-being
- barriers to optimal treatment.
I also explore how ObGyns’ role in this area of care is evolving—as a way to understand how you can better serve this expanding segment of our patient population.
Dyspareunia can have many causes, including endometriosis, interstitial cystitis, surgical scarring, injury that occurs during childbirth, and psychosocial origin (such as a history of sexual abuse). Our focus here is on dyspareunia due to VVA.
during sex. What should you do?
- Sexual pain as a category of female sexual dysfunction is relevant at any age; for postmenopausal women dealing with vaginal dryness as a result of estrogen deficiency, it may well be the dominant issue. When determining the cause of a sexual problem in a postmenopausal woman, put dyspareunia caused by vaginal dryness (as well as its psychosocial consequences) at the top of the list of possibilities.
- Bring up the topic of vaginal dryness and sexual pain with postmenopausal patients as part of the routine yearly exam, and explain the therapeutic capabilities of all available options.
- Estrogen therapy, either local or systemic, remains the standard when lubricants are inadequate. Make every effort to counsel the patient about the real risk:benefit ratio of estrogen use.
- If the patient is reluctant to use estrogen therapy, discuss with her the option of short-term local estrogen use, with the understanding that more acceptable options may become available in the near future. This may facilitate acceptance of short-term hormonal treatment and allow the patient to maintain her vaginal health and much of her vaginal sexual function.
- Keep abreast of both present and future options for therapy.
Just how sizable is the postmenopausal population?
About 32% of the female population is older than 50 years.1 That means that around 48 million women are currently menopausal, or will become so over the next few years.
Because average life expectancy approaches 80 years in the United States and other countries of the industrialized world,2 many women will live approximately 40 years beyond menopause or their final menstrual period. Their quality of life during the second half of their life is dependent on both physical and psychosocial health.
Postmenopausal dyspareunia isn’t new
Sexual issues arising from physical causes—dyspareunia among them—have long accounted for a large share of medical concerns reported by postmenopausal women. In a 1985 survey, for example, dyspareunia accounted for 42.5% of their complaints.3
But epidemiologic studies to determine the prevalence of female sexual dysfunction in postmenopausal women are difficult to carry out. Why? Because researchers would need to 1) address changes over time and 2) distinguish problems of sexual function from those brought on by aging.4
The techniques and methodology for researching female sexual dysfunction continue to evolve, creating new definitions of the stages of menopause and new diagnostic approaches to female sexual dysfunction.
However, based on available studies, Dennerstein and Hayes concluded that:
- postmenopausal women report a high rate of sexual dysfunction (higher than men)
- psychosocial factors can ameliorate a decline in sexual function
- “vaginal dryness and dyspareunia seem to be driven primarily by declining estradiol.”4
The WHI and its domino effect
Millions of postmenopausal women stopped taking estrogen-based therapy in the wake of widespread media coverage after 2002 publication of data from the estrogen–progestin arm of the Women’s Health Initiative (WHI), which purported to show, among other things, an increased risk of breast cancer.5
For decades, many postmenopausal women achieved medical management of VVA through long-term use of systemic hormone replacement therapy (HRT), which they used primarily to control other chronic symptoms of menopause, such as hot flashes.
After the WHI data were published (and misrepresented), reduced usage of estrogen-based HRT “unmasked” vaginal symptoms, including sexual pain, due to the effects of estrogen deficiency on the vaginal epithelium and vaginal blood flow. Since then, we have been forced to examine anew the natural history of menopause.
Within days or weeks of discontinuing HRT, women may reexperience the acute vasomotor symptoms that accompany estrogen withdrawal—most commonly hot flashes, night sweats, sleeplessness, palpitations, and headaches. Over time—anywhere from 6 months to several years—the body adjusts to the loss or withdrawal of estrogen, and these vasomotor symptoms eventually diminish or resolve. Not so for the longer-term physical effects of chronic low serum levels of estrogen, which worsen over time.
Approximately 6 months after discontinuing estrogen therapy, postmenopausal women may begin to experience vaginal dryness and VVA. As the years pass, other side effects of estrogen deficiency arise: bone loss, joint pain, mood alteration (including depression), change in skin tone, hair loss, and cardiac and central nervous system changes. These side effects do not resolve spontaneously; in fact, they grow worse as a woman ages. They may have deleterious psychosocial as well as physical impacts on her life—especially on the quality of her intimate relationship.
Clarify the report (adjust appropriately for same-sex partner)
- Where does it hurt? Describe the pain.
- When does it hurt? Does the pain occur 1) with penile contact at the opening of the vagina, 2) once the penis is partially in, 3) with full entry, 4) after some thrusting, 5) after deep thrusting, 6) with the partner’s ejaculation, 7) after withdrawal, or 8) with subsequent micturition?
- Does your body tense when your partner is attempting, or you are attempting, to insert his penis? What are your thoughts and feelings at this time?
- How long does the pain last?
- Does touching cause pain? Does it hurt when you ride a bicycle or wear tight clothes? Does penetration by tampons or fingers hurt?
Assess the pelvic floor
- Do you recognize the feeling of pelvic floor muscle tension during sexual contact?
- Do you recognize the feeling of pelvic floor muscle tension in other (nonsexual) situations?
Evaluate arousal
- Do you feel subjectively excited when you attempt intercourse?
- Does your vagina become sufficiently moist? Do you recognize the feeling of drying up?
Determine the consequences of the complaint
- What do you do when you experience pain during sexual contact? Do you continue? Or do you stop whatever is causing the pain?
- Do you continue to include intercourse or attempts at intercourse in your lovemaking, or do you use other methods of achieving sexual fulfillment? If you use other ways to make love, do you and your partner clearly understand that intercourse will not be attempted?
- What other effect does the pain have on your sexual relationship?
Explore biomedical antecedents
- When and how did the pain start?
- What tests have you undergone?
- What treatment have you received?
Source: Adapted from Basson R, et al.12
Is 60 the new 40?
Many women and men in the large cohort known as the Baby Boomer generation continue to be sexually active into their 60s, 70s, and 80s, as demonstrated by a 2007 study of sexuality and health in older adults.6 In the 57- to 64-year-old age group, 61.6% of women and 83.7% of men were sexually active (defined as sexual activity with a partner within the past 12 months). In the 65- to 74-year-old group, 39.5% of women and 67% of men were sexually active; and in the 75- to 85-year-old group, 16.7% of women and 38.5% of men were sexually active (TABLE).
These findings indicate that fewer women than men remain sexually active during their later years. One reason may be the epidemic of sexual-related symptoms among postmenopausal women. In the same survey, 34.3% of women 57 to 64 years old reported avoiding sex because of:
- pain during intercourse (17.8%)
- difficulty with lubrication (35.9%).
Across all groups, the most prevalent sexual problem was low desire (43%).6 Around 40% of postmenopausal women reported no sexual activity in the past 12 months, as well as lack of interest in sex. This number may include women who have ceased to have sex because of vaginal dryness and dyspareunia, thereby reducing the percentage reporting these symptoms (TABLE).
TABLE
Older adults are having sex—and experiencing sexual problems
| Activity or problem by gender | Number of respondents | Report, by age group (95% confidence interval*) | ||
|---|---|---|---|---|
| 57–64 yr (%) | 65–74 yr (%) | 75–85 yr (%) | ||
| Sexually active in previous 12 months† | ||||
| Men | 1,385 | 83.7 (77.6–89.8) | 67.0 (62.1–72.0) | 38.5 (33.6–43.5) |
| Women | 1,501 | 61.6 (56.7–66.4) | 39.5 (34.6–44.4) | 16.7 (12.5–21.0) |
| Difficulty with lubrication | ||||
| Women | 495 | 35.9 (29.6–42.2) | 43.2 (34.8–51.5) | 43.6 (27.0–60.2) |
| Pain during intercourse | ||||
| Men | 878 | 3.0 (1.1–4.8) | 3.2 (1.2–5.3) | 1.0 (0–2.5) |
| Women | 506 | 17.8 (13.3–22.2) | 18.6 (10.8–26.3) | 11.8 (4.3–19.4) |
| Avoidance of sex due to sexual problems** | ||||
| Men | 533 | 22.1 (17.3–26.9) | 30.1 (23.2–37.0) | 25.7 (14.9–36.4) |
| Women | 357 | 34.3 (25.0–43.7) | 30.5 (21.5–39.4) | 22.7 (9.4–35.9) |
| Source: Adapted from Lindau ST, et al.6 | ||||
| Adjusted odds ratios are based on a logistic regression including the age group and self-rated health status as covariates, estimated separately for men and women. The confidence interval is based on the inversion of the Wald tests constructed with the use of design-based standard errors. | ||||
| † These data exclude 107 respondents who reported at least one sexual problem. | ||||
| ** This question was asked only of respondents who reported at least one sexual problem. | ||||
Assessing menopause-related sexual function is a challenge
Although the transition phases of menopause have been well studied and reported for decades, few of these studies have included questions about the impact of menopause on sexual function.7 When longitudinal studies that included the classification of female sexual dysfunction began to appear, they provided evidence of the important role that VVA and psychosocial factors play in female sexual dysfunction.8
In the fourth year of the Melbourne Women’s Midlife Health Project longitudinal study, six variables related to sexual function were identified. Three were determinate of sexual function:
- feelings for the partner
- problems related to the partner
- vaginal dryness/dyspareunia.
The other three variables—sexual responsiveness, frequency of sexual activity, and libido—were dependent or outcome variables.
By the sixth year of this study, two variables had increased in significance: vaginal dryness/dyspareunia and partner problems.7
Sexual pain and relationship problems can create a vicious cycle
The interrelationship of vaginal dryness, sexual pain, flagging desire, and psychosocial parameters can produce a vicious cycle. A woman experiencing or anticipating pain may have diminished sexual desire or avoid sex altogether. During intercourse, the brain’s awareness of vaginal pain may trigger a physiologic response that can cause the muscles of the vagina to tighten and lubrication to decrease. The result? Greater vaginal pain.
This vicious cycle can contribute to relationship issues with the sexual partner and harm a woman’s psychosocial well-being. Resentment, anger, and misunderstanding may arise when a couple is dealing with problems of sexual function, and these stressors can damage many aspects of the relationship, further exacerbating sexual difficulties.
An additional and very important dimension of these issues is their potential impact on the family unit.
VVA can diminish overall well-being
In a 2007 survey reported at the North American Menopause Society (NAMS), one third to one half of 506 respondents said that VVA had a bad effect on their sexual interest, mood, self-esteem, and the intimate relationship (FIGURE 1).9 Reports from in-depth interviews were consistent with survey results and offered further insight into a woman’s emotional response to the condition of vaginal dryness and its impact on her life. Women found the condition “embarrassing,” something they had to endure but didn’t talk about, and felt that it had a major impact on their self-esteem and intimate relationship.
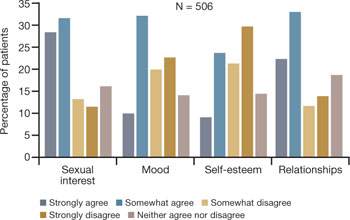
FIGURE 1 Dyspareunia affects more than interest in sex—relationships, mood, and self-esteem suffer
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Clinicians often don’t ask about VVA, and patients are reluctant to talk
Among women of all ages, dyspareunia is underreported and undertreated. In the survey reported at NAMS, 40% of respondents said that their physician had never asked them about the problem of VVA (FIGURE 2).9
Women themselves may be reluctant to discuss the problem with physicians, nurse practitioners, or other health-care providers out of embarrassment or the assumption that there is nothing to be done about the problem. Nevertheless, more than 40% of respondents said they would be highly likely to seek treatment for VVA if they had a concern about urogenital complications of the condition (FIGURE 3).9
Another barrier may be the sense that asking the health-care provider about sex may embarrass him or her. As a result, sufferers do not anticipate help from their physician and other members of the health-care profession and fail to seek treatment or counseling for this chronic medical condition.10,11
In a 1999 telephone survey of 500 adults 25 years of age or older, 71% said they thought that their doctor would dismiss concerns about sexual problems, but 85% said they would talk to their physician anyway if they had a problem, even though they might not get treatment.11 In that survey, 91% of married men and 84% of married women rated a satisfying sex life as important to quality of life.11
Another important and often overlooked limitation on this type of discussion is the time constraints that busy clinicians face, especially with the low reimbursement offered by managed care. Sexual problems can hardly be adequately discussed in 7 to 10 minutes.
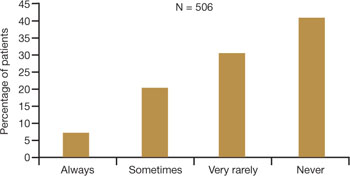
FIGURE 2 Do physicians ask about dyspareunia? Most women surveyed said “rarely” or “never”
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
FIGURE 3 Are these women likely to seek treatment?
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Women have performance anxiety, too
It is well known that men with even a mild degree of erectile dysfunction can suffer from performance anxiety, but the fact that women can also suffer from this phenomenon is not given as much attention. Such anxiety can be a factor in relationship difficulties. With both partners perhaps feeling anxious about sexual performance, a couple may avoid even simple acts of affection, such as holding hands, to avoid raising the other’s expectations.
Exacerbating the situation is the fact that many men use widely prescribed phosphodiesterase type 5 (PDE5) inhibitors, whereas women are contending with barriers to continued sexual activity as they age. It does not take a psychologist to understand that this imbalance often adds to emotional strain and tension between partners.
Popular media address the issue
Look beyond what our postmenopausal patients tell us directly—to the popular media and online forums—to appreciate the scope of sexual pain as a major issue among postmenopausal women. Evidence of psychosocial effects is found on numerous Web sites—some from organizations, others designed by women seeking help from each other.
Red Hot Mamas
This organization aims to empower women through menopause education. Highlighted in the Winter 2007/2008 Red Hot Mamas Report is a survey done in conjunction with Harris Interactive exploring the impact of menopausal symptoms on a woman’s sex life, which found that 47% of women who have VVA have avoided or stopped sex completely because it was uncomfortable, compared with 23% of normal women.
Power Surge
This Web site offers a list of strategies for dealing with sexual pain, including an overview of hormone-based prescription and nonprescription products, along with a variety of over-the-counter, natural, holistic, and herbal therapies for treating dyspareunia.
What is the physician’s role?
Given the epidemic of sexual pain, it is crucial that physicians and others who care for postmenopausal women increase their awareness of this issue and pay special attention to its psychosocial parameters.
Ask patients about sexual function in general and dyspareunia in particular as part of the routine annual visit. A simple opening “Yes/No” question, such as “Are you sexually active?” can lead to further questions appropriate to the patient. For example, if the answer is “No,” the follow-up question might be, “Does that bother you or your partner?” Further discussion may uncover whether the lack of sexual activity is a cause of distress and identify which variables are involved.
If, instead, the answer is “Yes,” follow-up questions can identify the presence of common postmenopausal physical issues, such as vaginal dryness and difficulty with lubrication. The visit then can turn to strategies to ameliorate those conditions.
When a patient reports dyspareunia, further diagnostic information such as precise location, degree of arousal, and reaction to pain can help determine the appropriate course of treatment. For an approach to this aspect of ascertaining patient history, see the list of sample questions above.12
During the physical, pay particular attention to any physical abnormalities or organic causes of sexual pain. Questions designed to characterize the location and nature of the pain can pinpoint the cause. Sexual pain arising from VVA is likely to 1) be localized at the introitus and 2) occur with penile entry.
Since the mid-1990s, the availability of validated scales to measure female sexual function has increased rapidly and enabled researchers to better identify, quantify, and evaluate treatments for female sexual dysfunction.7 Over time, we have moved away from the somewhat mechanical sequence inherent in the linear progression of desire leading to genital stimulation followed by arousal and orgasm toward an appreciation of the multiple physical, emotional, and subjective factors that are at play in women’s sexual function.
By 1998, a classification scheme was developed to further the means to study and discuss disorders of desire, arousal, orgasm, and sexual pain.8 Further contextual definitions of sexual dysfunction are under consideration.13
Basson proposed one new model of female sexual function (see the diagram), and observed that
"…women identify many reasons they are sexual over and beyond inherent sexual drive or “hunger.” Women tell of wanting to increase emotional closeness, commitment, sharing, tenderness, and tolerance, and to show the partner that he or she has been missed (emotionally or physically). Such intimacy-based reasons motivate the woman to find a way to become sexually aroused. This arousal is not spontaneous but triggered by deliberately sought sexual stimuli."13
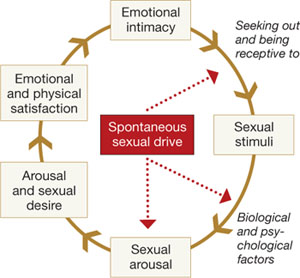
Intimacy-based model of female sexual response cycle
In this flow of physical and emotional variables involved in female sexual function, categories interact. For example, low desire can be and is frequently secondary to the anticipation of pain during sexual intercourse. Arousal can be hampered by lack of vaginal lubrication—perhaps inhibited by the anticipation of pain. Secondary orgasmic disorders can result from low desire, difficulty of arousal, and sexual pain.14 Sexual pain can affect sexual function at any point on this continuum.
Treatments in the pipeline
For decades, hormone-based treatments have been the predominant therapeutic option for vaginal dryness. Often they are a secondary benefit of hormone therapy for vasomotor symptoms and osteoporosis. Estrogen can be delivered in the form of oral tablet, transdermal patch, gel, spray, or vaginal ring for systemic use, or as vaginal cream, ring, or tablet for local use.
However, despite data to the contrary and our reassurances to the patient about overall safety, a large number of women, and many primary care providers, are no longer inclined to use short- or long-term HRT in any presentation.
Other women may have risk factors that contraindicate exogenous hormones.
Nonhormonal options for vaginal dryness and dyspareunia are limited, and there are no approved systemic or oral nonestrogen options. Over-the-counter topical lubricants can ease some of the symptoms of VVA temporarily and allow successful vaginal penetration in many cases. Some may cause vaginal warming and pleasant sensations, but overall they treat the symptom rather than the source of pain. Moreover, many patients consider local lubricants messy and inconvenient and claim they “ruin the mood.”
The use of vaginal dilators along with estrogen or lubricant therapy is an often-forgotten adjunct to therapy for dyspareunia caused by VVA (FIGURE 4).
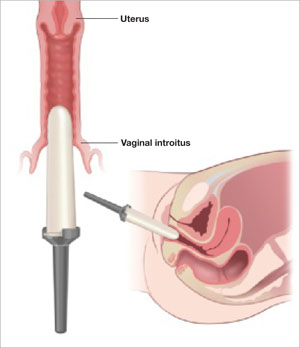
FIGURE 4 Mechanical dilation of the vagina is a useful adjunct
Mechanical dilation is often needed to restore penetration capability in the vagina, even after hormonal treatment. The focus should be on the vaginal introitus, with the top 25% to 35% of the dilator inserted into the opening once a day for 15 minutes, increasing the dilator diameter over time.
New SERMs are in development
Preclinical and clinical research into the diverse class of selective estrogen receptor modulators (SERMs) to treat estrogen-mediated disease produced tamoxifen for breast cancer prevention and raloxifene for both vertebral osteoporosis and breast cancer prevention. Each SERM seems to have unique tissue selectivity. The antiestrogenic activity of tamoxifen and raloxifene extends to the vagina and can exacerbate vaginal dryness.
A new generation of orally active SERMs is under investigation specifically for the treatment of chronic vaginal symptoms. These new agents target the nonvaginal treatment of VVA and associated symptoms. The first oral SERM for long-term treatment of these symptoms, ospemifene (Ophena), may become available in the near future. It is a novel SERM that has both anti-estrogenic and estrogenic actions, depending on the tissue. It was shown to significantly improve both vaginal dryness and dyspareunia in a large placebo-controlled trial.15
1. US Census Bureau. 2006 American community survey. S0101. Age and sex. Available at: http://fact-finder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenuId=&_lang=en&_ts.
2. National Center for Health Statistics. Health, United States, 2007, with Chartbook on Trends in the Health of Americans. Hyattsville, Md: NCHS; 2007. Available at: http://www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed February 2, 2009.
3. Sarrel PM, Whitehead MI. Sex and menopause: defining the issues. Maturitas. 1985;7:217-224.
4. Dennerstein L, Hayes RD. Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med. 2005;2(suppl 3):118-132.
5. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
6. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
7. Dennerstein L, Alexander JL, Kotz K. The menopause and sexual functioning: a review of the population-based studies. Annu Rev Sex Res. 2003;14:64-82.
8. Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888-993.
9. Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
10. Heim LJ. Evaluation and differential diagnosis of dyspareunia. Am Fam Physician. 2001;63:1535-1552.
11. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173-2174.
12. Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med. 2004;1:24-34.
13. Basson R. Female sexual response: the role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-353.
14. Walsh KE, Berman JR. Sexual dysfunction in the older woman: an overview of the current understanding and management. Drugs Aging. 2004;21:655-675.
15. Bachmann GA, Komi J, Hanley R. A new SERM, Ophena (ospemifene), effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Presented at the Endocrine Society annual meeting, San Francisco, Calif, June 2008.
The author reports that he serves on the speaker’s bureau for Novogyne, TherRx, Warner-Chilcott, and Solvay, and on the advisory board for Upsher-Smith, Novogyne, QuatRx, and Wyeth.
CASE: History of dyspareunia
At her latest visit, a 56-year-old woman who is 7 years postmenopausal relates that she has been experiencing worsening pain with intercourse to the point that she now has very little sex drive at all. This problem began approximately 1 year after she discontinued hormone therapy in the wake of reports that it causes cancer and heart attack. She has been offered both local vaginal and systemic hormone therapy, but is too frightened to use any hormones at all. Sexual lubricants no longer seem to work.
How do you counsel her about these symptoms? And what therapy do you offer?
Physicians and other health-care practitioners are seeing a large and growing number of genitourinary and sexual-related complaints among menopausal women—so much so that it has reached epidemic proportions. Yet dyspareunia is underreported and undertreated, and quality of life suffers for these women.
In this article, I focus on two interrelated causes of this epidemic:
- vaginal dryness and vulvovaginal atrophy (VVA) and the impact of these conditions on women’s sexual function and psychosocial well-being
- barriers to optimal treatment.
I also explore how ObGyns’ role in this area of care is evolving—as a way to understand how you can better serve this expanding segment of our patient population.
Dyspareunia can have many causes, including endometriosis, interstitial cystitis, surgical scarring, injury that occurs during childbirth, and psychosocial origin (such as a history of sexual abuse). Our focus here is on dyspareunia due to VVA.
during sex. What should you do?
- Sexual pain as a category of female sexual dysfunction is relevant at any age; for postmenopausal women dealing with vaginal dryness as a result of estrogen deficiency, it may well be the dominant issue. When determining the cause of a sexual problem in a postmenopausal woman, put dyspareunia caused by vaginal dryness (as well as its psychosocial consequences) at the top of the list of possibilities.
- Bring up the topic of vaginal dryness and sexual pain with postmenopausal patients as part of the routine yearly exam, and explain the therapeutic capabilities of all available options.
- Estrogen therapy, either local or systemic, remains the standard when lubricants are inadequate. Make every effort to counsel the patient about the real risk:benefit ratio of estrogen use.
- If the patient is reluctant to use estrogen therapy, discuss with her the option of short-term local estrogen use, with the understanding that more acceptable options may become available in the near future. This may facilitate acceptance of short-term hormonal treatment and allow the patient to maintain her vaginal health and much of her vaginal sexual function.
- Keep abreast of both present and future options for therapy.
Just how sizable is the postmenopausal population?
About 32% of the female population is older than 50 years.1 That means that around 48 million women are currently menopausal, or will become so over the next few years.
Because average life expectancy approaches 80 years in the United States and other countries of the industrialized world,2 many women will live approximately 40 years beyond menopause or their final menstrual period. Their quality of life during the second half of their life is dependent on both physical and psychosocial health.
Postmenopausal dyspareunia isn’t new
Sexual issues arising from physical causes—dyspareunia among them—have long accounted for a large share of medical concerns reported by postmenopausal women. In a 1985 survey, for example, dyspareunia accounted for 42.5% of their complaints.3
But epidemiologic studies to determine the prevalence of female sexual dysfunction in postmenopausal women are difficult to carry out. Why? Because researchers would need to 1) address changes over time and 2) distinguish problems of sexual function from those brought on by aging.4
The techniques and methodology for researching female sexual dysfunction continue to evolve, creating new definitions of the stages of menopause and new diagnostic approaches to female sexual dysfunction.
However, based on available studies, Dennerstein and Hayes concluded that:
- postmenopausal women report a high rate of sexual dysfunction (higher than men)
- psychosocial factors can ameliorate a decline in sexual function
- “vaginal dryness and dyspareunia seem to be driven primarily by declining estradiol.”4
The WHI and its domino effect
Millions of postmenopausal women stopped taking estrogen-based therapy in the wake of widespread media coverage after 2002 publication of data from the estrogen–progestin arm of the Women’s Health Initiative (WHI), which purported to show, among other things, an increased risk of breast cancer.5
For decades, many postmenopausal women achieved medical management of VVA through long-term use of systemic hormone replacement therapy (HRT), which they used primarily to control other chronic symptoms of menopause, such as hot flashes.
After the WHI data were published (and misrepresented), reduced usage of estrogen-based HRT “unmasked” vaginal symptoms, including sexual pain, due to the effects of estrogen deficiency on the vaginal epithelium and vaginal blood flow. Since then, we have been forced to examine anew the natural history of menopause.
Within days or weeks of discontinuing HRT, women may reexperience the acute vasomotor symptoms that accompany estrogen withdrawal—most commonly hot flashes, night sweats, sleeplessness, palpitations, and headaches. Over time—anywhere from 6 months to several years—the body adjusts to the loss or withdrawal of estrogen, and these vasomotor symptoms eventually diminish or resolve. Not so for the longer-term physical effects of chronic low serum levels of estrogen, which worsen over time.
Approximately 6 months after discontinuing estrogen therapy, postmenopausal women may begin to experience vaginal dryness and VVA. As the years pass, other side effects of estrogen deficiency arise: bone loss, joint pain, mood alteration (including depression), change in skin tone, hair loss, and cardiac and central nervous system changes. These side effects do not resolve spontaneously; in fact, they grow worse as a woman ages. They may have deleterious psychosocial as well as physical impacts on her life—especially on the quality of her intimate relationship.
Clarify the report (adjust appropriately for same-sex partner)
- Where does it hurt? Describe the pain.
- When does it hurt? Does the pain occur 1) with penile contact at the opening of the vagina, 2) once the penis is partially in, 3) with full entry, 4) after some thrusting, 5) after deep thrusting, 6) with the partner’s ejaculation, 7) after withdrawal, or 8) with subsequent micturition?
- Does your body tense when your partner is attempting, or you are attempting, to insert his penis? What are your thoughts and feelings at this time?
- How long does the pain last?
- Does touching cause pain? Does it hurt when you ride a bicycle or wear tight clothes? Does penetration by tampons or fingers hurt?
Assess the pelvic floor
- Do you recognize the feeling of pelvic floor muscle tension during sexual contact?
- Do you recognize the feeling of pelvic floor muscle tension in other (nonsexual) situations?
Evaluate arousal
- Do you feel subjectively excited when you attempt intercourse?
- Does your vagina become sufficiently moist? Do you recognize the feeling of drying up?
Determine the consequences of the complaint
- What do you do when you experience pain during sexual contact? Do you continue? Or do you stop whatever is causing the pain?
- Do you continue to include intercourse or attempts at intercourse in your lovemaking, or do you use other methods of achieving sexual fulfillment? If you use other ways to make love, do you and your partner clearly understand that intercourse will not be attempted?
- What other effect does the pain have on your sexual relationship?
Explore biomedical antecedents
- When and how did the pain start?
- What tests have you undergone?
- What treatment have you received?
Source: Adapted from Basson R, et al.12
Is 60 the new 40?
Many women and men in the large cohort known as the Baby Boomer generation continue to be sexually active into their 60s, 70s, and 80s, as demonstrated by a 2007 study of sexuality and health in older adults.6 In the 57- to 64-year-old age group, 61.6% of women and 83.7% of men were sexually active (defined as sexual activity with a partner within the past 12 months). In the 65- to 74-year-old group, 39.5% of women and 67% of men were sexually active; and in the 75- to 85-year-old group, 16.7% of women and 38.5% of men were sexually active (TABLE).
These findings indicate that fewer women than men remain sexually active during their later years. One reason may be the epidemic of sexual-related symptoms among postmenopausal women. In the same survey, 34.3% of women 57 to 64 years old reported avoiding sex because of:
- pain during intercourse (17.8%)
- difficulty with lubrication (35.9%).
Across all groups, the most prevalent sexual problem was low desire (43%).6 Around 40% of postmenopausal women reported no sexual activity in the past 12 months, as well as lack of interest in sex. This number may include women who have ceased to have sex because of vaginal dryness and dyspareunia, thereby reducing the percentage reporting these symptoms (TABLE).
TABLE
Older adults are having sex—and experiencing sexual problems
| Activity or problem by gender | Number of respondents | Report, by age group (95% confidence interval*) | ||
|---|---|---|---|---|
| 57–64 yr (%) | 65–74 yr (%) | 75–85 yr (%) | ||
| Sexually active in previous 12 months† | ||||
| Men | 1,385 | 83.7 (77.6–89.8) | 67.0 (62.1–72.0) | 38.5 (33.6–43.5) |
| Women | 1,501 | 61.6 (56.7–66.4) | 39.5 (34.6–44.4) | 16.7 (12.5–21.0) |
| Difficulty with lubrication | ||||
| Women | 495 | 35.9 (29.6–42.2) | 43.2 (34.8–51.5) | 43.6 (27.0–60.2) |
| Pain during intercourse | ||||
| Men | 878 | 3.0 (1.1–4.8) | 3.2 (1.2–5.3) | 1.0 (0–2.5) |
| Women | 506 | 17.8 (13.3–22.2) | 18.6 (10.8–26.3) | 11.8 (4.3–19.4) |
| Avoidance of sex due to sexual problems** | ||||
| Men | 533 | 22.1 (17.3–26.9) | 30.1 (23.2–37.0) | 25.7 (14.9–36.4) |
| Women | 357 | 34.3 (25.0–43.7) | 30.5 (21.5–39.4) | 22.7 (9.4–35.9) |
| Source: Adapted from Lindau ST, et al.6 | ||||
| Adjusted odds ratios are based on a logistic regression including the age group and self-rated health status as covariates, estimated separately for men and women. The confidence interval is based on the inversion of the Wald tests constructed with the use of design-based standard errors. | ||||
| † These data exclude 107 respondents who reported at least one sexual problem. | ||||
| ** This question was asked only of respondents who reported at least one sexual problem. | ||||
Assessing menopause-related sexual function is a challenge
Although the transition phases of menopause have been well studied and reported for decades, few of these studies have included questions about the impact of menopause on sexual function.7 When longitudinal studies that included the classification of female sexual dysfunction began to appear, they provided evidence of the important role that VVA and psychosocial factors play in female sexual dysfunction.8
In the fourth year of the Melbourne Women’s Midlife Health Project longitudinal study, six variables related to sexual function were identified. Three were determinate of sexual function:
- feelings for the partner
- problems related to the partner
- vaginal dryness/dyspareunia.
The other three variables—sexual responsiveness, frequency of sexual activity, and libido—were dependent or outcome variables.
By the sixth year of this study, two variables had increased in significance: vaginal dryness/dyspareunia and partner problems.7
Sexual pain and relationship problems can create a vicious cycle
The interrelationship of vaginal dryness, sexual pain, flagging desire, and psychosocial parameters can produce a vicious cycle. A woman experiencing or anticipating pain may have diminished sexual desire or avoid sex altogether. During intercourse, the brain’s awareness of vaginal pain may trigger a physiologic response that can cause the muscles of the vagina to tighten and lubrication to decrease. The result? Greater vaginal pain.
This vicious cycle can contribute to relationship issues with the sexual partner and harm a woman’s psychosocial well-being. Resentment, anger, and misunderstanding may arise when a couple is dealing with problems of sexual function, and these stressors can damage many aspects of the relationship, further exacerbating sexual difficulties.
An additional and very important dimension of these issues is their potential impact on the family unit.
VVA can diminish overall well-being
In a 2007 survey reported at the North American Menopause Society (NAMS), one third to one half of 506 respondents said that VVA had a bad effect on their sexual interest, mood, self-esteem, and the intimate relationship (FIGURE 1).9 Reports from in-depth interviews were consistent with survey results and offered further insight into a woman’s emotional response to the condition of vaginal dryness and its impact on her life. Women found the condition “embarrassing,” something they had to endure but didn’t talk about, and felt that it had a major impact on their self-esteem and intimate relationship.

FIGURE 1 Dyspareunia affects more than interest in sex—relationships, mood, and self-esteem suffer
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Clinicians often don’t ask about VVA, and patients are reluctant to talk
Among women of all ages, dyspareunia is underreported and undertreated. In the survey reported at NAMS, 40% of respondents said that their physician had never asked them about the problem of VVA (FIGURE 2).9
Women themselves may be reluctant to discuss the problem with physicians, nurse practitioners, or other health-care providers out of embarrassment or the assumption that there is nothing to be done about the problem. Nevertheless, more than 40% of respondents said they would be highly likely to seek treatment for VVA if they had a concern about urogenital complications of the condition (FIGURE 3).9
Another barrier may be the sense that asking the health-care provider about sex may embarrass him or her. As a result, sufferers do not anticipate help from their physician and other members of the health-care profession and fail to seek treatment or counseling for this chronic medical condition.10,11
In a 1999 telephone survey of 500 adults 25 years of age or older, 71% said they thought that their doctor would dismiss concerns about sexual problems, but 85% said they would talk to their physician anyway if they had a problem, even though they might not get treatment.11 In that survey, 91% of married men and 84% of married women rated a satisfying sex life as important to quality of life.11
Another important and often overlooked limitation on this type of discussion is the time constraints that busy clinicians face, especially with the low reimbursement offered by managed care. Sexual problems can hardly be adequately discussed in 7 to 10 minutes.

FIGURE 2 Do physicians ask about dyspareunia? Most women surveyed said “rarely” or “never”
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
FIGURE 3 Are these women likely to seek treatment?
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Women have performance anxiety, too
It is well known that men with even a mild degree of erectile dysfunction can suffer from performance anxiety, but the fact that women can also suffer from this phenomenon is not given as much attention. Such anxiety can be a factor in relationship difficulties. With both partners perhaps feeling anxious about sexual performance, a couple may avoid even simple acts of affection, such as holding hands, to avoid raising the other’s expectations.
Exacerbating the situation is the fact that many men use widely prescribed phosphodiesterase type 5 (PDE5) inhibitors, whereas women are contending with barriers to continued sexual activity as they age. It does not take a psychologist to understand that this imbalance often adds to emotional strain and tension between partners.
Popular media address the issue
Look beyond what our postmenopausal patients tell us directly—to the popular media and online forums—to appreciate the scope of sexual pain as a major issue among postmenopausal women. Evidence of psychosocial effects is found on numerous Web sites—some from organizations, others designed by women seeking help from each other.
Red Hot Mamas
This organization aims to empower women through menopause education. Highlighted in the Winter 2007/2008 Red Hot Mamas Report is a survey done in conjunction with Harris Interactive exploring the impact of menopausal symptoms on a woman’s sex life, which found that 47% of women who have VVA have avoided or stopped sex completely because it was uncomfortable, compared with 23% of normal women.
Power Surge
This Web site offers a list of strategies for dealing with sexual pain, including an overview of hormone-based prescription and nonprescription products, along with a variety of over-the-counter, natural, holistic, and herbal therapies for treating dyspareunia.
What is the physician’s role?
Given the epidemic of sexual pain, it is crucial that physicians and others who care for postmenopausal women increase their awareness of this issue and pay special attention to its psychosocial parameters.
Ask patients about sexual function in general and dyspareunia in particular as part of the routine annual visit. A simple opening “Yes/No” question, such as “Are you sexually active?” can lead to further questions appropriate to the patient. For example, if the answer is “No,” the follow-up question might be, “Does that bother you or your partner?” Further discussion may uncover whether the lack of sexual activity is a cause of distress and identify which variables are involved.
If, instead, the answer is “Yes,” follow-up questions can identify the presence of common postmenopausal physical issues, such as vaginal dryness and difficulty with lubrication. The visit then can turn to strategies to ameliorate those conditions.
When a patient reports dyspareunia, further diagnostic information such as precise location, degree of arousal, and reaction to pain can help determine the appropriate course of treatment. For an approach to this aspect of ascertaining patient history, see the list of sample questions above.12
During the physical, pay particular attention to any physical abnormalities or organic causes of sexual pain. Questions designed to characterize the location and nature of the pain can pinpoint the cause. Sexual pain arising from VVA is likely to 1) be localized at the introitus and 2) occur with penile entry.
Since the mid-1990s, the availability of validated scales to measure female sexual function has increased rapidly and enabled researchers to better identify, quantify, and evaluate treatments for female sexual dysfunction.7 Over time, we have moved away from the somewhat mechanical sequence inherent in the linear progression of desire leading to genital stimulation followed by arousal and orgasm toward an appreciation of the multiple physical, emotional, and subjective factors that are at play in women’s sexual function.
By 1998, a classification scheme was developed to further the means to study and discuss disorders of desire, arousal, orgasm, and sexual pain.8 Further contextual definitions of sexual dysfunction are under consideration.13
Basson proposed one new model of female sexual function (see the diagram), and observed that
"…women identify many reasons they are sexual over and beyond inherent sexual drive or “hunger.” Women tell of wanting to increase emotional closeness, commitment, sharing, tenderness, and tolerance, and to show the partner that he or she has been missed (emotionally or physically). Such intimacy-based reasons motivate the woman to find a way to become sexually aroused. This arousal is not spontaneous but triggered by deliberately sought sexual stimuli."13

Intimacy-based model of female sexual response cycle
In this flow of physical and emotional variables involved in female sexual function, categories interact. For example, low desire can be and is frequently secondary to the anticipation of pain during sexual intercourse. Arousal can be hampered by lack of vaginal lubrication—perhaps inhibited by the anticipation of pain. Secondary orgasmic disorders can result from low desire, difficulty of arousal, and sexual pain.14 Sexual pain can affect sexual function at any point on this continuum.
Treatments in the pipeline
For decades, hormone-based treatments have been the predominant therapeutic option for vaginal dryness. Often they are a secondary benefit of hormone therapy for vasomotor symptoms and osteoporosis. Estrogen can be delivered in the form of oral tablet, transdermal patch, gel, spray, or vaginal ring for systemic use, or as vaginal cream, ring, or tablet for local use.
However, despite data to the contrary and our reassurances to the patient about overall safety, a large number of women, and many primary care providers, are no longer inclined to use short- or long-term HRT in any presentation.
Other women may have risk factors that contraindicate exogenous hormones.
Nonhormonal options for vaginal dryness and dyspareunia are limited, and there are no approved systemic or oral nonestrogen options. Over-the-counter topical lubricants can ease some of the symptoms of VVA temporarily and allow successful vaginal penetration in many cases. Some may cause vaginal warming and pleasant sensations, but overall they treat the symptom rather than the source of pain. Moreover, many patients consider local lubricants messy and inconvenient and claim they “ruin the mood.”
The use of vaginal dilators along with estrogen or lubricant therapy is an often-forgotten adjunct to therapy for dyspareunia caused by VVA (FIGURE 4).

FIGURE 4 Mechanical dilation of the vagina is a useful adjunct
Mechanical dilation is often needed to restore penetration capability in the vagina, even after hormonal treatment. The focus should be on the vaginal introitus, with the top 25% to 35% of the dilator inserted into the opening once a day for 15 minutes, increasing the dilator diameter over time.
New SERMs are in development
Preclinical and clinical research into the diverse class of selective estrogen receptor modulators (SERMs) to treat estrogen-mediated disease produced tamoxifen for breast cancer prevention and raloxifene for both vertebral osteoporosis and breast cancer prevention. Each SERM seems to have unique tissue selectivity. The antiestrogenic activity of tamoxifen and raloxifene extends to the vagina and can exacerbate vaginal dryness.
A new generation of orally active SERMs is under investigation specifically for the treatment of chronic vaginal symptoms. These new agents target the nonvaginal treatment of VVA and associated symptoms. The first oral SERM for long-term treatment of these symptoms, ospemifene (Ophena), may become available in the near future. It is a novel SERM that has both anti-estrogenic and estrogenic actions, depending on the tissue. It was shown to significantly improve both vaginal dryness and dyspareunia in a large placebo-controlled trial.15
The author reports that he serves on the speaker’s bureau for Novogyne, TherRx, Warner-Chilcott, and Solvay, and on the advisory board for Upsher-Smith, Novogyne, QuatRx, and Wyeth.
CASE: History of dyspareunia
At her latest visit, a 56-year-old woman who is 7 years postmenopausal relates that she has been experiencing worsening pain with intercourse to the point that she now has very little sex drive at all. This problem began approximately 1 year after she discontinued hormone therapy in the wake of reports that it causes cancer and heart attack. She has been offered both local vaginal and systemic hormone therapy, but is too frightened to use any hormones at all. Sexual lubricants no longer seem to work.
How do you counsel her about these symptoms? And what therapy do you offer?
Physicians and other health-care practitioners are seeing a large and growing number of genitourinary and sexual-related complaints among menopausal women—so much so that it has reached epidemic proportions. Yet dyspareunia is underreported and undertreated, and quality of life suffers for these women.
In this article, I focus on two interrelated causes of this epidemic:
- vaginal dryness and vulvovaginal atrophy (VVA) and the impact of these conditions on women’s sexual function and psychosocial well-being
- barriers to optimal treatment.
I also explore how ObGyns’ role in this area of care is evolving—as a way to understand how you can better serve this expanding segment of our patient population.
Dyspareunia can have many causes, including endometriosis, interstitial cystitis, surgical scarring, injury that occurs during childbirth, and psychosocial origin (such as a history of sexual abuse). Our focus here is on dyspareunia due to VVA.
during sex. What should you do?
- Sexual pain as a category of female sexual dysfunction is relevant at any age; for postmenopausal women dealing with vaginal dryness as a result of estrogen deficiency, it may well be the dominant issue. When determining the cause of a sexual problem in a postmenopausal woman, put dyspareunia caused by vaginal dryness (as well as its psychosocial consequences) at the top of the list of possibilities.
- Bring up the topic of vaginal dryness and sexual pain with postmenopausal patients as part of the routine yearly exam, and explain the therapeutic capabilities of all available options.
- Estrogen therapy, either local or systemic, remains the standard when lubricants are inadequate. Make every effort to counsel the patient about the real risk:benefit ratio of estrogen use.
- If the patient is reluctant to use estrogen therapy, discuss with her the option of short-term local estrogen use, with the understanding that more acceptable options may become available in the near future. This may facilitate acceptance of short-term hormonal treatment and allow the patient to maintain her vaginal health and much of her vaginal sexual function.
- Keep abreast of both present and future options for therapy.
Just how sizable is the postmenopausal population?
About 32% of the female population is older than 50 years.1 That means that around 48 million women are currently menopausal, or will become so over the next few years.
Because average life expectancy approaches 80 years in the United States and other countries of the industrialized world,2 many women will live approximately 40 years beyond menopause or their final menstrual period. Their quality of life during the second half of their life is dependent on both physical and psychosocial health.
Postmenopausal dyspareunia isn’t new
Sexual issues arising from physical causes—dyspareunia among them—have long accounted for a large share of medical concerns reported by postmenopausal women. In a 1985 survey, for example, dyspareunia accounted for 42.5% of their complaints.3
But epidemiologic studies to determine the prevalence of female sexual dysfunction in postmenopausal women are difficult to carry out. Why? Because researchers would need to 1) address changes over time and 2) distinguish problems of sexual function from those brought on by aging.4
The techniques and methodology for researching female sexual dysfunction continue to evolve, creating new definitions of the stages of menopause and new diagnostic approaches to female sexual dysfunction.
However, based on available studies, Dennerstein and Hayes concluded that:
- postmenopausal women report a high rate of sexual dysfunction (higher than men)
- psychosocial factors can ameliorate a decline in sexual function
- “vaginal dryness and dyspareunia seem to be driven primarily by declining estradiol.”4
The WHI and its domino effect
Millions of postmenopausal women stopped taking estrogen-based therapy in the wake of widespread media coverage after 2002 publication of data from the estrogen–progestin arm of the Women’s Health Initiative (WHI), which purported to show, among other things, an increased risk of breast cancer.5
For decades, many postmenopausal women achieved medical management of VVA through long-term use of systemic hormone replacement therapy (HRT), which they used primarily to control other chronic symptoms of menopause, such as hot flashes.
After the WHI data were published (and misrepresented), reduced usage of estrogen-based HRT “unmasked” vaginal symptoms, including sexual pain, due to the effects of estrogen deficiency on the vaginal epithelium and vaginal blood flow. Since then, we have been forced to examine anew the natural history of menopause.
Within days or weeks of discontinuing HRT, women may reexperience the acute vasomotor symptoms that accompany estrogen withdrawal—most commonly hot flashes, night sweats, sleeplessness, palpitations, and headaches. Over time—anywhere from 6 months to several years—the body adjusts to the loss or withdrawal of estrogen, and these vasomotor symptoms eventually diminish or resolve. Not so for the longer-term physical effects of chronic low serum levels of estrogen, which worsen over time.
Approximately 6 months after discontinuing estrogen therapy, postmenopausal women may begin to experience vaginal dryness and VVA. As the years pass, other side effects of estrogen deficiency arise: bone loss, joint pain, mood alteration (including depression), change in skin tone, hair loss, and cardiac and central nervous system changes. These side effects do not resolve spontaneously; in fact, they grow worse as a woman ages. They may have deleterious psychosocial as well as physical impacts on her life—especially on the quality of her intimate relationship.
Clarify the report (adjust appropriately for same-sex partner)
- Where does it hurt? Describe the pain.
- When does it hurt? Does the pain occur 1) with penile contact at the opening of the vagina, 2) once the penis is partially in, 3) with full entry, 4) after some thrusting, 5) after deep thrusting, 6) with the partner’s ejaculation, 7) after withdrawal, or 8) with subsequent micturition?
- Does your body tense when your partner is attempting, or you are attempting, to insert his penis? What are your thoughts and feelings at this time?
- How long does the pain last?
- Does touching cause pain? Does it hurt when you ride a bicycle or wear tight clothes? Does penetration by tampons or fingers hurt?
Assess the pelvic floor
- Do you recognize the feeling of pelvic floor muscle tension during sexual contact?
- Do you recognize the feeling of pelvic floor muscle tension in other (nonsexual) situations?
Evaluate arousal
- Do you feel subjectively excited when you attempt intercourse?
- Does your vagina become sufficiently moist? Do you recognize the feeling of drying up?
Determine the consequences of the complaint
- What do you do when you experience pain during sexual contact? Do you continue? Or do you stop whatever is causing the pain?
- Do you continue to include intercourse or attempts at intercourse in your lovemaking, or do you use other methods of achieving sexual fulfillment? If you use other ways to make love, do you and your partner clearly understand that intercourse will not be attempted?
- What other effect does the pain have on your sexual relationship?
Explore biomedical antecedents
- When and how did the pain start?
- What tests have you undergone?
- What treatment have you received?
Source: Adapted from Basson R, et al.12
Is 60 the new 40?
Many women and men in the large cohort known as the Baby Boomer generation continue to be sexually active into their 60s, 70s, and 80s, as demonstrated by a 2007 study of sexuality and health in older adults.6 In the 57- to 64-year-old age group, 61.6% of women and 83.7% of men were sexually active (defined as sexual activity with a partner within the past 12 months). In the 65- to 74-year-old group, 39.5% of women and 67% of men were sexually active; and in the 75- to 85-year-old group, 16.7% of women and 38.5% of men were sexually active (TABLE).
These findings indicate that fewer women than men remain sexually active during their later years. One reason may be the epidemic of sexual-related symptoms among postmenopausal women. In the same survey, 34.3% of women 57 to 64 years old reported avoiding sex because of:
- pain during intercourse (17.8%)
- difficulty with lubrication (35.9%).
Across all groups, the most prevalent sexual problem was low desire (43%).6 Around 40% of postmenopausal women reported no sexual activity in the past 12 months, as well as lack of interest in sex. This number may include women who have ceased to have sex because of vaginal dryness and dyspareunia, thereby reducing the percentage reporting these symptoms (TABLE).
TABLE
Older adults are having sex—and experiencing sexual problems
| Activity or problem by gender | Number of respondents | Report, by age group (95% confidence interval*) | ||
|---|---|---|---|---|
| 57–64 yr (%) | 65–74 yr (%) | 75–85 yr (%) | ||
| Sexually active in previous 12 months† | ||||
| Men | 1,385 | 83.7 (77.6–89.8) | 67.0 (62.1–72.0) | 38.5 (33.6–43.5) |
| Women | 1,501 | 61.6 (56.7–66.4) | 39.5 (34.6–44.4) | 16.7 (12.5–21.0) |
| Difficulty with lubrication | ||||
| Women | 495 | 35.9 (29.6–42.2) | 43.2 (34.8–51.5) | 43.6 (27.0–60.2) |
| Pain during intercourse | ||||
| Men | 878 | 3.0 (1.1–4.8) | 3.2 (1.2–5.3) | 1.0 (0–2.5) |
| Women | 506 | 17.8 (13.3–22.2) | 18.6 (10.8–26.3) | 11.8 (4.3–19.4) |
| Avoidance of sex due to sexual problems** | ||||
| Men | 533 | 22.1 (17.3–26.9) | 30.1 (23.2–37.0) | 25.7 (14.9–36.4) |
| Women | 357 | 34.3 (25.0–43.7) | 30.5 (21.5–39.4) | 22.7 (9.4–35.9) |
| Source: Adapted from Lindau ST, et al.6 | ||||
| Adjusted odds ratios are based on a logistic regression including the age group and self-rated health status as covariates, estimated separately for men and women. The confidence interval is based on the inversion of the Wald tests constructed with the use of design-based standard errors. | ||||
| † These data exclude 107 respondents who reported at least one sexual problem. | ||||
| ** This question was asked only of respondents who reported at least one sexual problem. | ||||
Assessing menopause-related sexual function is a challenge
Although the transition phases of menopause have been well studied and reported for decades, few of these studies have included questions about the impact of menopause on sexual function.7 When longitudinal studies that included the classification of female sexual dysfunction began to appear, they provided evidence of the important role that VVA and psychosocial factors play in female sexual dysfunction.8
In the fourth year of the Melbourne Women’s Midlife Health Project longitudinal study, six variables related to sexual function were identified. Three were determinate of sexual function:
- feelings for the partner
- problems related to the partner
- vaginal dryness/dyspareunia.
The other three variables—sexual responsiveness, frequency of sexual activity, and libido—were dependent or outcome variables.
By the sixth year of this study, two variables had increased in significance: vaginal dryness/dyspareunia and partner problems.7
Sexual pain and relationship problems can create a vicious cycle
The interrelationship of vaginal dryness, sexual pain, flagging desire, and psychosocial parameters can produce a vicious cycle. A woman experiencing or anticipating pain may have diminished sexual desire or avoid sex altogether. During intercourse, the brain’s awareness of vaginal pain may trigger a physiologic response that can cause the muscles of the vagina to tighten and lubrication to decrease. The result? Greater vaginal pain.
This vicious cycle can contribute to relationship issues with the sexual partner and harm a woman’s psychosocial well-being. Resentment, anger, and misunderstanding may arise when a couple is dealing with problems of sexual function, and these stressors can damage many aspects of the relationship, further exacerbating sexual difficulties.
An additional and very important dimension of these issues is their potential impact on the family unit.
VVA can diminish overall well-being
In a 2007 survey reported at the North American Menopause Society (NAMS), one third to one half of 506 respondents said that VVA had a bad effect on their sexual interest, mood, self-esteem, and the intimate relationship (FIGURE 1).9 Reports from in-depth interviews were consistent with survey results and offered further insight into a woman’s emotional response to the condition of vaginal dryness and its impact on her life. Women found the condition “embarrassing,” something they had to endure but didn’t talk about, and felt that it had a major impact on their self-esteem and intimate relationship.

FIGURE 1 Dyspareunia affects more than interest in sex—relationships, mood, and self-esteem suffer
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Clinicians often don’t ask about VVA, and patients are reluctant to talk
Among women of all ages, dyspareunia is underreported and undertreated. In the survey reported at NAMS, 40% of respondents said that their physician had never asked them about the problem of VVA (FIGURE 2).9
Women themselves may be reluctant to discuss the problem with physicians, nurse practitioners, or other health-care providers out of embarrassment or the assumption that there is nothing to be done about the problem. Nevertheless, more than 40% of respondents said they would be highly likely to seek treatment for VVA if they had a concern about urogenital complications of the condition (FIGURE 3).9
Another barrier may be the sense that asking the health-care provider about sex may embarrass him or her. As a result, sufferers do not anticipate help from their physician and other members of the health-care profession and fail to seek treatment or counseling for this chronic medical condition.10,11
In a 1999 telephone survey of 500 adults 25 years of age or older, 71% said they thought that their doctor would dismiss concerns about sexual problems, but 85% said they would talk to their physician anyway if they had a problem, even though they might not get treatment.11 In that survey, 91% of married men and 84% of married women rated a satisfying sex life as important to quality of life.11
Another important and often overlooked limitation on this type of discussion is the time constraints that busy clinicians face, especially with the low reimbursement offered by managed care. Sexual problems can hardly be adequately discussed in 7 to 10 minutes.

FIGURE 2 Do physicians ask about dyspareunia? Most women surveyed said “rarely” or “never”
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
FIGURE 3 Are these women likely to seek treatment?
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Women have performance anxiety, too
It is well known that men with even a mild degree of erectile dysfunction can suffer from performance anxiety, but the fact that women can also suffer from this phenomenon is not given as much attention. Such anxiety can be a factor in relationship difficulties. With both partners perhaps feeling anxious about sexual performance, a couple may avoid even simple acts of affection, such as holding hands, to avoid raising the other’s expectations.
Exacerbating the situation is the fact that many men use widely prescribed phosphodiesterase type 5 (PDE5) inhibitors, whereas women are contending with barriers to continued sexual activity as they age. It does not take a psychologist to understand that this imbalance often adds to emotional strain and tension between partners.
Popular media address the issue
Look beyond what our postmenopausal patients tell us directly—to the popular media and online forums—to appreciate the scope of sexual pain as a major issue among postmenopausal women. Evidence of psychosocial effects is found on numerous Web sites—some from organizations, others designed by women seeking help from each other.
Red Hot Mamas
This organization aims to empower women through menopause education. Highlighted in the Winter 2007/2008 Red Hot Mamas Report is a survey done in conjunction with Harris Interactive exploring the impact of menopausal symptoms on a woman’s sex life, which found that 47% of women who have VVA have avoided or stopped sex completely because it was uncomfortable, compared with 23% of normal women.
Power Surge
This Web site offers a list of strategies for dealing with sexual pain, including an overview of hormone-based prescription and nonprescription products, along with a variety of over-the-counter, natural, holistic, and herbal therapies for treating dyspareunia.
What is the physician’s role?
Given the epidemic of sexual pain, it is crucial that physicians and others who care for postmenopausal women increase their awareness of this issue and pay special attention to its psychosocial parameters.
Ask patients about sexual function in general and dyspareunia in particular as part of the routine annual visit. A simple opening “Yes/No” question, such as “Are you sexually active?” can lead to further questions appropriate to the patient. For example, if the answer is “No,” the follow-up question might be, “Does that bother you or your partner?” Further discussion may uncover whether the lack of sexual activity is a cause of distress and identify which variables are involved.
If, instead, the answer is “Yes,” follow-up questions can identify the presence of common postmenopausal physical issues, such as vaginal dryness and difficulty with lubrication. The visit then can turn to strategies to ameliorate those conditions.
When a patient reports dyspareunia, further diagnostic information such as precise location, degree of arousal, and reaction to pain can help determine the appropriate course of treatment. For an approach to this aspect of ascertaining patient history, see the list of sample questions above.12
During the physical, pay particular attention to any physical abnormalities or organic causes of sexual pain. Questions designed to characterize the location and nature of the pain can pinpoint the cause. Sexual pain arising from VVA is likely to 1) be localized at the introitus and 2) occur with penile entry.
Since the mid-1990s, the availability of validated scales to measure female sexual function has increased rapidly and enabled researchers to better identify, quantify, and evaluate treatments for female sexual dysfunction.7 Over time, we have moved away from the somewhat mechanical sequence inherent in the linear progression of desire leading to genital stimulation followed by arousal and orgasm toward an appreciation of the multiple physical, emotional, and subjective factors that are at play in women’s sexual function.
By 1998, a classification scheme was developed to further the means to study and discuss disorders of desire, arousal, orgasm, and sexual pain.8 Further contextual definitions of sexual dysfunction are under consideration.13
Basson proposed one new model of female sexual function (see the diagram), and observed that
"…women identify many reasons they are sexual over and beyond inherent sexual drive or “hunger.” Women tell of wanting to increase emotional closeness, commitment, sharing, tenderness, and tolerance, and to show the partner that he or she has been missed (emotionally or physically). Such intimacy-based reasons motivate the woman to find a way to become sexually aroused. This arousal is not spontaneous but triggered by deliberately sought sexual stimuli."13

Intimacy-based model of female sexual response cycle
In this flow of physical and emotional variables involved in female sexual function, categories interact. For example, low desire can be and is frequently secondary to the anticipation of pain during sexual intercourse. Arousal can be hampered by lack of vaginal lubrication—perhaps inhibited by the anticipation of pain. Secondary orgasmic disorders can result from low desire, difficulty of arousal, and sexual pain.14 Sexual pain can affect sexual function at any point on this continuum.
Treatments in the pipeline
For decades, hormone-based treatments have been the predominant therapeutic option for vaginal dryness. Often they are a secondary benefit of hormone therapy for vasomotor symptoms and osteoporosis. Estrogen can be delivered in the form of oral tablet, transdermal patch, gel, spray, or vaginal ring for systemic use, or as vaginal cream, ring, or tablet for local use.
However, despite data to the contrary and our reassurances to the patient about overall safety, a large number of women, and many primary care providers, are no longer inclined to use short- or long-term HRT in any presentation.
Other women may have risk factors that contraindicate exogenous hormones.
Nonhormonal options for vaginal dryness and dyspareunia are limited, and there are no approved systemic or oral nonestrogen options. Over-the-counter topical lubricants can ease some of the symptoms of VVA temporarily and allow successful vaginal penetration in many cases. Some may cause vaginal warming and pleasant sensations, but overall they treat the symptom rather than the source of pain. Moreover, many patients consider local lubricants messy and inconvenient and claim they “ruin the mood.”
The use of vaginal dilators along with estrogen or lubricant therapy is an often-forgotten adjunct to therapy for dyspareunia caused by VVA (FIGURE 4).

FIGURE 4 Mechanical dilation of the vagina is a useful adjunct
Mechanical dilation is often needed to restore penetration capability in the vagina, even after hormonal treatment. The focus should be on the vaginal introitus, with the top 25% to 35% of the dilator inserted into the opening once a day for 15 minutes, increasing the dilator diameter over time.
New SERMs are in development
Preclinical and clinical research into the diverse class of selective estrogen receptor modulators (SERMs) to treat estrogen-mediated disease produced tamoxifen for breast cancer prevention and raloxifene for both vertebral osteoporosis and breast cancer prevention. Each SERM seems to have unique tissue selectivity. The antiestrogenic activity of tamoxifen and raloxifene extends to the vagina and can exacerbate vaginal dryness.
A new generation of orally active SERMs is under investigation specifically for the treatment of chronic vaginal symptoms. These new agents target the nonvaginal treatment of VVA and associated symptoms. The first oral SERM for long-term treatment of these symptoms, ospemifene (Ophena), may become available in the near future. It is a novel SERM that has both anti-estrogenic and estrogenic actions, depending on the tissue. It was shown to significantly improve both vaginal dryness and dyspareunia in a large placebo-controlled trial.15
1. US Census Bureau. 2006 American community survey. S0101. Age and sex. Available at: http://fact-finder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenuId=&_lang=en&_ts.
2. National Center for Health Statistics. Health, United States, 2007, with Chartbook on Trends in the Health of Americans. Hyattsville, Md: NCHS; 2007. Available at: http://www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed February 2, 2009.
3. Sarrel PM, Whitehead MI. Sex and menopause: defining the issues. Maturitas. 1985;7:217-224.
4. Dennerstein L, Hayes RD. Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med. 2005;2(suppl 3):118-132.
5. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
6. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
7. Dennerstein L, Alexander JL, Kotz K. The menopause and sexual functioning: a review of the population-based studies. Annu Rev Sex Res. 2003;14:64-82.
8. Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888-993.
9. Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
10. Heim LJ. Evaluation and differential diagnosis of dyspareunia. Am Fam Physician. 2001;63:1535-1552.
11. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173-2174.
12. Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med. 2004;1:24-34.
13. Basson R. Female sexual response: the role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-353.
14. Walsh KE, Berman JR. Sexual dysfunction in the older woman: an overview of the current understanding and management. Drugs Aging. 2004;21:655-675.
15. Bachmann GA, Komi J, Hanley R. A new SERM, Ophena (ospemifene), effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Presented at the Endocrine Society annual meeting, San Francisco, Calif, June 2008.
1. US Census Bureau. 2006 American community survey. S0101. Age and sex. Available at: http://fact-finder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenuId=&_lang=en&_ts.
2. National Center for Health Statistics. Health, United States, 2007, with Chartbook on Trends in the Health of Americans. Hyattsville, Md: NCHS; 2007. Available at: http://www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed February 2, 2009.
3. Sarrel PM, Whitehead MI. Sex and menopause: defining the issues. Maturitas. 1985;7:217-224.
4. Dennerstein L, Hayes RD. Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med. 2005;2(suppl 3):118-132.
5. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
6. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
7. Dennerstein L, Alexander JL, Kotz K. The menopause and sexual functioning: a review of the population-based studies. Annu Rev Sex Res. 2003;14:64-82.
8. Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888-993.
9. Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
10. Heim LJ. Evaluation and differential diagnosis of dyspareunia. Am Fam Physician. 2001;63:1535-1552.
11. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173-2174.
12. Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med. 2004;1:24-34.
13. Basson R. Female sexual response: the role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-353.
14. Walsh KE, Berman JR. Sexual dysfunction in the older woman: an overview of the current understanding and management. Drugs Aging. 2004;21:655-675.
15. Bachmann GA, Komi J, Hanley R. A new SERM, Ophena (ospemifene), effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Presented at the Endocrine Society annual meeting, San Francisco, Calif, June 2008.
Older Black Women May Have Osteoporosis
RIO GRANDE, P.R. — Approximately one in four elderly black women have osteoporosis, findings from a small study suggest.
Physicians should not ignore the possibility of osteoporosis in their older black female patients, although these women are not usually considered at high risk, compared with other demographic groups, said Dr. Sally P. Weaver, research director of the McLennan County Medical Education and Research Foundation, Waco, Texas.
Previous studies of osteoporosis in women have focused mainly on white women because of evidence of an elevated risk for osteoporosis in that population. Yet older women of any ethnicity are prone to age-related fractures if their bone mineral density (BMD) is low, she said in an interview.
Dr. Weaver and her colleagues measured BMD scans from the electronic health records of 44 black women aged 70 years and older. Patients with conditions that could affect bone turnover, vitamin D absorption, or calcium absorption were excluded from the study.
About 50% of the study participants met the criteria for osteopenia and 10% met the criteria for osteoporosis at the left femoral neck. Approximately 25% met criteria for osteopenia or osteoporosis at the lumbar spine. Overall, the left femoral neck had the lowest regional BMD, with an average T score of −1.23. Dr. Weaver presented the results in a poster at the annual meeting of the North American Primary Care Research Group.
Dr. Weaver had no financial conflicts to disclose.
RIO GRANDE, P.R. — Approximately one in four elderly black women have osteoporosis, findings from a small study suggest.
Physicians should not ignore the possibility of osteoporosis in their older black female patients, although these women are not usually considered at high risk, compared with other demographic groups, said Dr. Sally P. Weaver, research director of the McLennan County Medical Education and Research Foundation, Waco, Texas.
Previous studies of osteoporosis in women have focused mainly on white women because of evidence of an elevated risk for osteoporosis in that population. Yet older women of any ethnicity are prone to age-related fractures if their bone mineral density (BMD) is low, she said in an interview.
Dr. Weaver and her colleagues measured BMD scans from the electronic health records of 44 black women aged 70 years and older. Patients with conditions that could affect bone turnover, vitamin D absorption, or calcium absorption were excluded from the study.
About 50% of the study participants met the criteria for osteopenia and 10% met the criteria for osteoporosis at the left femoral neck. Approximately 25% met criteria for osteopenia or osteoporosis at the lumbar spine. Overall, the left femoral neck had the lowest regional BMD, with an average T score of −1.23. Dr. Weaver presented the results in a poster at the annual meeting of the North American Primary Care Research Group.
Dr. Weaver had no financial conflicts to disclose.
RIO GRANDE, P.R. — Approximately one in four elderly black women have osteoporosis, findings from a small study suggest.
Physicians should not ignore the possibility of osteoporosis in their older black female patients, although these women are not usually considered at high risk, compared with other demographic groups, said Dr. Sally P. Weaver, research director of the McLennan County Medical Education and Research Foundation, Waco, Texas.
Previous studies of osteoporosis in women have focused mainly on white women because of evidence of an elevated risk for osteoporosis in that population. Yet older women of any ethnicity are prone to age-related fractures if their bone mineral density (BMD) is low, she said in an interview.
Dr. Weaver and her colleagues measured BMD scans from the electronic health records of 44 black women aged 70 years and older. Patients with conditions that could affect bone turnover, vitamin D absorption, or calcium absorption were excluded from the study.
About 50% of the study participants met the criteria for osteopenia and 10% met the criteria for osteoporosis at the left femoral neck. Approximately 25% met criteria for osteopenia or osteoporosis at the lumbar spine. Overall, the left femoral neck had the lowest regional BMD, with an average T score of −1.23. Dr. Weaver presented the results in a poster at the annual meeting of the North American Primary Care Research Group.
Dr. Weaver had no financial conflicts to disclose.
Topical Tamoxifen Benefits Cyclic Mastalgia
SAN ANTONIO — Afimoxifene, a novel tamoxifen gel applied directly to the breasts, performed favorably as topical therapy for moderate to severe cyclic mastalgia in premenopausal women in a phase II clinical trial.
Although the topical antiestrogen's developer, ASCEND Therapeutics Inc., plans to seek an initial indication for cyclic mastalgia, there also is strong interest in developing afimoxifene as a treatment for male gynecomastia as well as for breast cancer chemoprevention, Dr. Amit Goyal said at the San Antonio Breast Cancer Symposium.
Afimoxifene is 4-hydroxytamoxifen, a highly potent metabolite of tamoxifen, in a proprietary hydroalcoholic gel. Its binding affinity for the alpha and beta estrogen receptors is two- to threefold greater than that of estradiol, explained Dr. Goyal, a surgical oncologist at Cardiff (Wales) University.
Oral tamoxifen, bromocriptine, danazol, and progestins have demonstrated efficacy in treating cyclic mastalgia; however, their systemic side effects render them poorly suited for long-term treatment of a chronic problem.
In contrast, transdermal afimoxifene is highly effective within the breast yet has very low systemic levels, thus reducing the risk of systemic toxicities, he continued.
In a pharmacokinetic study, 16 healthy premenopausal women applied 4 mg of afimoxifene to their breasts daily for 21 days.
At steady state, achieved after 2 weeks of therapy, mean plasma 4-hydroxytamoxifen levels were just 1/18 of those measured in 19 healthy controls taking 20 mg/day of oral tamoxifen.
Based upon those encouraging findings, Dr. Goyal and his coinvestigators next carried out the phase II trial involving 127 premenopausal women with moderate to severe cyclic mastalgia. They were randomized to placebo or either 2 mg or 4 mg of afimoxifene daily for four menstrual cycles.
Significant differences in efficacy between the 4-mg dose and placebo were documented after two cycles.
After four cycles, mean pain intensity measured on a visual analog scale for the 7 worst days per cycle was 64% lower in women on 4 mg/day of afimoxifene than in the placebo group.
Physician global assessments of breast nodularity and tenderness showed reductions of 70% and 67%, respectively, relative to placebo. The 2-mg dose showed less robust albeit favorable trends on all end points, he continued.
In an interview, Dr. Goyal said he has had some success in using oral tamoxifen in men with gynecomastia, a condition for which the only established therapy at present is surgery.
Dr. Goyal said he plans to study topical afimoxifene for this condition in a placebo-controlled trial.
Chemoprevention of breast cancer is a particularly exciting potential application for the topical selective estrogen-receptor modulator.
“The main reason why some women and some physicians are reluctant to use oral tamoxifen, even though we know from the National Surgical Adjuvant Breast and Bowel Project study that it works, is because of side effects. If we can show afimoxifene works to prevent breast cancer as well as oral tamoxifen, I think that would be an important advance,” Dr. Goyal said.
That will require a large, lengthy, and costly phase III clinical trial, he noted.
The mastalgia trial was supported by ASCEND Therapeutics.
Dr. Goyal indicated that he has received research funds from the company but has no other financial involvement with ASCEND.
Transdermal afimoxifene is highly effective within the breast yet has very low systemic levels. DR. GOYAL
SAN ANTONIO — Afimoxifene, a novel tamoxifen gel applied directly to the breasts, performed favorably as topical therapy for moderate to severe cyclic mastalgia in premenopausal women in a phase II clinical trial.
Although the topical antiestrogen's developer, ASCEND Therapeutics Inc., plans to seek an initial indication for cyclic mastalgia, there also is strong interest in developing afimoxifene as a treatment for male gynecomastia as well as for breast cancer chemoprevention, Dr. Amit Goyal said at the San Antonio Breast Cancer Symposium.
Afimoxifene is 4-hydroxytamoxifen, a highly potent metabolite of tamoxifen, in a proprietary hydroalcoholic gel. Its binding affinity for the alpha and beta estrogen receptors is two- to threefold greater than that of estradiol, explained Dr. Goyal, a surgical oncologist at Cardiff (Wales) University.
Oral tamoxifen, bromocriptine, danazol, and progestins have demonstrated efficacy in treating cyclic mastalgia; however, their systemic side effects render them poorly suited for long-term treatment of a chronic problem.
In contrast, transdermal afimoxifene is highly effective within the breast yet has very low systemic levels, thus reducing the risk of systemic toxicities, he continued.
In a pharmacokinetic study, 16 healthy premenopausal women applied 4 mg of afimoxifene to their breasts daily for 21 days.
At steady state, achieved after 2 weeks of therapy, mean plasma 4-hydroxytamoxifen levels were just 1/18 of those measured in 19 healthy controls taking 20 mg/day of oral tamoxifen.
Based upon those encouraging findings, Dr. Goyal and his coinvestigators next carried out the phase II trial involving 127 premenopausal women with moderate to severe cyclic mastalgia. They were randomized to placebo or either 2 mg or 4 mg of afimoxifene daily for four menstrual cycles.
Significant differences in efficacy between the 4-mg dose and placebo were documented after two cycles.
After four cycles, mean pain intensity measured on a visual analog scale for the 7 worst days per cycle was 64% lower in women on 4 mg/day of afimoxifene than in the placebo group.
Physician global assessments of breast nodularity and tenderness showed reductions of 70% and 67%, respectively, relative to placebo. The 2-mg dose showed less robust albeit favorable trends on all end points, he continued.
In an interview, Dr. Goyal said he has had some success in using oral tamoxifen in men with gynecomastia, a condition for which the only established therapy at present is surgery.
Dr. Goyal said he plans to study topical afimoxifene for this condition in a placebo-controlled trial.
Chemoprevention of breast cancer is a particularly exciting potential application for the topical selective estrogen-receptor modulator.
“The main reason why some women and some physicians are reluctant to use oral tamoxifen, even though we know from the National Surgical Adjuvant Breast and Bowel Project study that it works, is because of side effects. If we can show afimoxifene works to prevent breast cancer as well as oral tamoxifen, I think that would be an important advance,” Dr. Goyal said.
That will require a large, lengthy, and costly phase III clinical trial, he noted.
The mastalgia trial was supported by ASCEND Therapeutics.
Dr. Goyal indicated that he has received research funds from the company but has no other financial involvement with ASCEND.
Transdermal afimoxifene is highly effective within the breast yet has very low systemic levels. DR. GOYAL
SAN ANTONIO — Afimoxifene, a novel tamoxifen gel applied directly to the breasts, performed favorably as topical therapy for moderate to severe cyclic mastalgia in premenopausal women in a phase II clinical trial.
Although the topical antiestrogen's developer, ASCEND Therapeutics Inc., plans to seek an initial indication for cyclic mastalgia, there also is strong interest in developing afimoxifene as a treatment for male gynecomastia as well as for breast cancer chemoprevention, Dr. Amit Goyal said at the San Antonio Breast Cancer Symposium.
Afimoxifene is 4-hydroxytamoxifen, a highly potent metabolite of tamoxifen, in a proprietary hydroalcoholic gel. Its binding affinity for the alpha and beta estrogen receptors is two- to threefold greater than that of estradiol, explained Dr. Goyal, a surgical oncologist at Cardiff (Wales) University.
Oral tamoxifen, bromocriptine, danazol, and progestins have demonstrated efficacy in treating cyclic mastalgia; however, their systemic side effects render them poorly suited for long-term treatment of a chronic problem.
In contrast, transdermal afimoxifene is highly effective within the breast yet has very low systemic levels, thus reducing the risk of systemic toxicities, he continued.
In a pharmacokinetic study, 16 healthy premenopausal women applied 4 mg of afimoxifene to their breasts daily for 21 days.
At steady state, achieved after 2 weeks of therapy, mean plasma 4-hydroxytamoxifen levels were just 1/18 of those measured in 19 healthy controls taking 20 mg/day of oral tamoxifen.
Based upon those encouraging findings, Dr. Goyal and his coinvestigators next carried out the phase II trial involving 127 premenopausal women with moderate to severe cyclic mastalgia. They were randomized to placebo or either 2 mg or 4 mg of afimoxifene daily for four menstrual cycles.
Significant differences in efficacy between the 4-mg dose and placebo were documented after two cycles.
After four cycles, mean pain intensity measured on a visual analog scale for the 7 worst days per cycle was 64% lower in women on 4 mg/day of afimoxifene than in the placebo group.
Physician global assessments of breast nodularity and tenderness showed reductions of 70% and 67%, respectively, relative to placebo. The 2-mg dose showed less robust albeit favorable trends on all end points, he continued.
In an interview, Dr. Goyal said he has had some success in using oral tamoxifen in men with gynecomastia, a condition for which the only established therapy at present is surgery.
Dr. Goyal said he plans to study topical afimoxifene for this condition in a placebo-controlled trial.
Chemoprevention of breast cancer is a particularly exciting potential application for the topical selective estrogen-receptor modulator.
“The main reason why some women and some physicians are reluctant to use oral tamoxifen, even though we know from the National Surgical Adjuvant Breast and Bowel Project study that it works, is because of side effects. If we can show afimoxifene works to prevent breast cancer as well as oral tamoxifen, I think that would be an important advance,” Dr. Goyal said.
That will require a large, lengthy, and costly phase III clinical trial, he noted.
The mastalgia trial was supported by ASCEND Therapeutics.
Dr. Goyal indicated that he has received research funds from the company but has no other financial involvement with ASCEND.
Transdermal afimoxifene is highly effective within the breast yet has very low systemic levels. DR. GOYAL
Acupuncture Tied to Long-Term Hot Flash Relief
SAN ANTONIO — A course of acupuncture reduced hot flashes in women with a history of breast cancer by more than half while improving sleep and quality-of-life measures to a similar extent as hormone therapy in a Swedish randomized trial.
Particularly noteworthy was the durability of acupuncture's benefits. Nine months following conclusion of the 3-month course of acupuncture sessions, most patients continued to have a significant reduction in hot flashes and improved measures of well-being, Dr. Jessica Frisk reported at the San Antonio Breast Cancer Symposium.
She added that in her clinical practice, acupuncture has become her first-line treatment for hot flashes. Hormone therapy (HT) is more effective; indeed, it essentially eliminated hot flashes in the women randomized to the HT study arm. But Scandinavian breast cancer patients now reject HT as an option because of reports of an associated increased risk of breast cancer recurrence.
“They want other things—and acupuncture is quite a safe treatment,” said Dr. Frisk, a general surgeon at Linköping (Sweden) University.
She reported on 45 women with hot flashes who had been diagnosed with breast cancer a mean of more than 4 years earlier. In all, 27 women were randomized to 12 weeks of electrostimulated acupuncture, and 18 women to 24 months of HT. The acupuncture program consisted of two 30-minute sessions per week for the first 2 weeks, followed by once-weekly sessions for the next 10.
The median number of hot flashes dropped from 9.6 per 24 hours at baseline to 4.3 per 24 hours at week 12 in 19 women who completed the 12-week course of acupuncture. The median hot flash frequency at 1 year was 4.9 per 24 hours in 14 women who had no additional acupuncture sessions beyond the initial 12 weeks. At 2 years' follow-up, seven women had a median hot flash rate of 2.1 per 24 hours without ever having had an additional acupuncture treatment. The others had similar results with occasional acupuncture booster sessions.
Median scores on the Kupperman Index of 11 menopausal symptoms improved from a baseline of 24 to 12 after 3 months of acupuncture therapy and to 13 at 1 year.
The median score on the Psychological and General Well-Being Index in the acupuncture group improved from 78 at baseline to 79 at 12 weeks and 85 at 1 year. Patient ratings of distress because of night sweats went from a median of 5.1 on a 10-point scale at baseline to 1.3 after 12 weeks of treatment. The patients treated with acupuncture reported waking a median of 3.2 times per night at baseline, 2.2 times per night after 12 weeks, and 1.6 times per night at 1 year.
In the HT group, all patients completed treatment. The median number of hot flashes per 24 hours went from 6.6 at baseline to 0 at 12 weeks. Scores on the Kupperman Index improved from 23 at baseline to 6 at both 12 weeks and 1 year. Median scores on the Psychological and General Well-Being Index went from 75 at baseline to 90 at 12 weeks and 93 at 1 year.
Although there was no placebo arm in the randomized trial, Dr. Frisk considers it highly unlikely that the observed benefits in the acupuncture group were due to the placebo effect.
“These women had menopausal symptoms for a mean of 6–7 years, some for more than 20 years. Then you give them acupuncture, and 4 weeks later their vasomotor symptoms have at least halved,” she noted in an interview.
To view a video interview of Dr. Frisk, go to www.youtube.com/watch?v=yiNwsd5b30E
The median number of hot flashes dropped from 9.6 per 24 hours at baseline to 4.3 per 24 hours at week 12. DR. FRISK
SAN ANTONIO — A course of acupuncture reduced hot flashes in women with a history of breast cancer by more than half while improving sleep and quality-of-life measures to a similar extent as hormone therapy in a Swedish randomized trial.
Particularly noteworthy was the durability of acupuncture's benefits. Nine months following conclusion of the 3-month course of acupuncture sessions, most patients continued to have a significant reduction in hot flashes and improved measures of well-being, Dr. Jessica Frisk reported at the San Antonio Breast Cancer Symposium.
She added that in her clinical practice, acupuncture has become her first-line treatment for hot flashes. Hormone therapy (HT) is more effective; indeed, it essentially eliminated hot flashes in the women randomized to the HT study arm. But Scandinavian breast cancer patients now reject HT as an option because of reports of an associated increased risk of breast cancer recurrence.
“They want other things—and acupuncture is quite a safe treatment,” said Dr. Frisk, a general surgeon at Linköping (Sweden) University.
She reported on 45 women with hot flashes who had been diagnosed with breast cancer a mean of more than 4 years earlier. In all, 27 women were randomized to 12 weeks of electrostimulated acupuncture, and 18 women to 24 months of HT. The acupuncture program consisted of two 30-minute sessions per week for the first 2 weeks, followed by once-weekly sessions for the next 10.
The median number of hot flashes dropped from 9.6 per 24 hours at baseline to 4.3 per 24 hours at week 12 in 19 women who completed the 12-week course of acupuncture. The median hot flash frequency at 1 year was 4.9 per 24 hours in 14 women who had no additional acupuncture sessions beyond the initial 12 weeks. At 2 years' follow-up, seven women had a median hot flash rate of 2.1 per 24 hours without ever having had an additional acupuncture treatment. The others had similar results with occasional acupuncture booster sessions.
Median scores on the Kupperman Index of 11 menopausal symptoms improved from a baseline of 24 to 12 after 3 months of acupuncture therapy and to 13 at 1 year.
The median score on the Psychological and General Well-Being Index in the acupuncture group improved from 78 at baseline to 79 at 12 weeks and 85 at 1 year. Patient ratings of distress because of night sweats went from a median of 5.1 on a 10-point scale at baseline to 1.3 after 12 weeks of treatment. The patients treated with acupuncture reported waking a median of 3.2 times per night at baseline, 2.2 times per night after 12 weeks, and 1.6 times per night at 1 year.
In the HT group, all patients completed treatment. The median number of hot flashes per 24 hours went from 6.6 at baseline to 0 at 12 weeks. Scores on the Kupperman Index improved from 23 at baseline to 6 at both 12 weeks and 1 year. Median scores on the Psychological and General Well-Being Index went from 75 at baseline to 90 at 12 weeks and 93 at 1 year.
Although there was no placebo arm in the randomized trial, Dr. Frisk considers it highly unlikely that the observed benefits in the acupuncture group were due to the placebo effect.
“These women had menopausal symptoms for a mean of 6–7 years, some for more than 20 years. Then you give them acupuncture, and 4 weeks later their vasomotor symptoms have at least halved,” she noted in an interview.
To view a video interview of Dr. Frisk, go to www.youtube.com/watch?v=yiNwsd5b30E
The median number of hot flashes dropped from 9.6 per 24 hours at baseline to 4.3 per 24 hours at week 12. DR. FRISK
SAN ANTONIO — A course of acupuncture reduced hot flashes in women with a history of breast cancer by more than half while improving sleep and quality-of-life measures to a similar extent as hormone therapy in a Swedish randomized trial.
Particularly noteworthy was the durability of acupuncture's benefits. Nine months following conclusion of the 3-month course of acupuncture sessions, most patients continued to have a significant reduction in hot flashes and improved measures of well-being, Dr. Jessica Frisk reported at the San Antonio Breast Cancer Symposium.
She added that in her clinical practice, acupuncture has become her first-line treatment for hot flashes. Hormone therapy (HT) is more effective; indeed, it essentially eliminated hot flashes in the women randomized to the HT study arm. But Scandinavian breast cancer patients now reject HT as an option because of reports of an associated increased risk of breast cancer recurrence.
“They want other things—and acupuncture is quite a safe treatment,” said Dr. Frisk, a general surgeon at Linköping (Sweden) University.
She reported on 45 women with hot flashes who had been diagnosed with breast cancer a mean of more than 4 years earlier. In all, 27 women were randomized to 12 weeks of electrostimulated acupuncture, and 18 women to 24 months of HT. The acupuncture program consisted of two 30-minute sessions per week for the first 2 weeks, followed by once-weekly sessions for the next 10.
The median number of hot flashes dropped from 9.6 per 24 hours at baseline to 4.3 per 24 hours at week 12 in 19 women who completed the 12-week course of acupuncture. The median hot flash frequency at 1 year was 4.9 per 24 hours in 14 women who had no additional acupuncture sessions beyond the initial 12 weeks. At 2 years' follow-up, seven women had a median hot flash rate of 2.1 per 24 hours without ever having had an additional acupuncture treatment. The others had similar results with occasional acupuncture booster sessions.
Median scores on the Kupperman Index of 11 menopausal symptoms improved from a baseline of 24 to 12 after 3 months of acupuncture therapy and to 13 at 1 year.
The median score on the Psychological and General Well-Being Index in the acupuncture group improved from 78 at baseline to 79 at 12 weeks and 85 at 1 year. Patient ratings of distress because of night sweats went from a median of 5.1 on a 10-point scale at baseline to 1.3 after 12 weeks of treatment. The patients treated with acupuncture reported waking a median of 3.2 times per night at baseline, 2.2 times per night after 12 weeks, and 1.6 times per night at 1 year.
In the HT group, all patients completed treatment. The median number of hot flashes per 24 hours went from 6.6 at baseline to 0 at 12 weeks. Scores on the Kupperman Index improved from 23 at baseline to 6 at both 12 weeks and 1 year. Median scores on the Psychological and General Well-Being Index went from 75 at baseline to 90 at 12 weeks and 93 at 1 year.
Although there was no placebo arm in the randomized trial, Dr. Frisk considers it highly unlikely that the observed benefits in the acupuncture group were due to the placebo effect.
“These women had menopausal symptoms for a mean of 6–7 years, some for more than 20 years. Then you give them acupuncture, and 4 weeks later their vasomotor symptoms have at least halved,” she noted in an interview.
To view a video interview of Dr. Frisk, go to www.youtube.com/watch?v=yiNwsd5b30E
The median number of hot flashes dropped from 9.6 per 24 hours at baseline to 4.3 per 24 hours at week 12. DR. FRISK
Menopause Experience Differs by Ethnic Group
LAKE BUENA VISTA, FLA. — Menopause symptoms vary significantly by ethnic group, based on data emerging from a longitudinal study.
The acculturation of women immigrants to the United States, as well as their socioeconomic status, are two factors that might account for these differences, said Dr. Nanette F. Santoro, an endocrinologist who has coauthored multiple studies based on data from the Study of Women's Health Across the Nation (SWAN).
The study included women from seven sites: Boston; Newark, N.J.; Pittsburgh; Detroit; Chicago; Oakland, Calif.; and Los Angeles. Each site recruited white women and women from one ethnic minority group: black, Hispanic, Chinese, or Japanese. More than 10 years later, about 85% of the participants remain in the study.
“We found differences by ethnicity—very intriguing differences,” Dr. Santoro said.
For example, in one study of 11,652 women from SWAN, Dr. Santoro and her colleagues found that 126 participants (1.1%) reported onset of menopause before age 40 years, a condition known as premature ovarian failure (Human Reprod. 2003;18:199–206). This occurred in 1.4% of both black and Hispanic women, 1.0% of white women, 0.5% of Chinese women, and 0.1% of Japanese women. (See bar chart.)
These differences were deemed statistically significant.
Acculturation of immigrants is “a double-edged sword,” Dr. Santoro said at the annual meeting of the North American Menopause Society. It can improve socioeconomic status, access to health care, and attainment of higher education, but at the same time can worsen health through a less-nutritious diet.
In contrast to other minorities, Hispanic women in SWAN and similar studies tend to improve little or even to worsen in terms of health once they are assimilated, she said. Watch for the “Hispanic paradox”: Health outcomes are worse among this population with increased acculturation, despite better socioeconomic status, because of factors such as higher rates of teen pregnancy and cigarette smoking, said Dr. Santoro, director of the division of reproductive endocrinology and infertility at Albert Einstein College of Medicine, New York.
She cautioned, however, that the Hispanic population is heterogeneous and cannot be addressed as a single entity.
The Hispanic SWAN participants came from many different countries and cultures and displayed some internal differences. For example, women from Puerto Rico were more vulnerable to acculturation and reported more menopause-related sleep problems and depressive symptoms than did other Hispanics.
Meanwhile, the acculturation of Japanese women was associated with fewer menopausal symptoms than were seen in Hispanics. Similarly, Chinese participants reported fewer symptoms compared with white, black, and Hispanic women in SWAN. “There are clear-cut differences in symptom reporting by ethnicity,” Dr. Santoro said.
Hispanic and black women were more likely to report depressive symptoms, and Chinese and Japanese women were less likely to do so, the study found.
“This is confounded, possibly, by lower socioeconomic status in the African American and Hispanic groups, and a higher socioeconomic status in Chinese and especially Japanese women,” she said.
Black women in SWAN reported the most hot flashes. Dr. Santoro proposed that increased adiposity among these women might provide more insulation and make them less heat tolerant.
Black women, however, were less bothered by hot flashes than were Hispanic women, who reported more embarrassment with vasomotor symptoms.
SWAN is supported by grants from the Department of Health and Human Services.
ELSEVIER GLOBAL MEDICAL NEWS
LAKE BUENA VISTA, FLA. — Menopause symptoms vary significantly by ethnic group, based on data emerging from a longitudinal study.
The acculturation of women immigrants to the United States, as well as their socioeconomic status, are two factors that might account for these differences, said Dr. Nanette F. Santoro, an endocrinologist who has coauthored multiple studies based on data from the Study of Women's Health Across the Nation (SWAN).
The study included women from seven sites: Boston; Newark, N.J.; Pittsburgh; Detroit; Chicago; Oakland, Calif.; and Los Angeles. Each site recruited white women and women from one ethnic minority group: black, Hispanic, Chinese, or Japanese. More than 10 years later, about 85% of the participants remain in the study.
“We found differences by ethnicity—very intriguing differences,” Dr. Santoro said.
For example, in one study of 11,652 women from SWAN, Dr. Santoro and her colleagues found that 126 participants (1.1%) reported onset of menopause before age 40 years, a condition known as premature ovarian failure (Human Reprod. 2003;18:199–206). This occurred in 1.4% of both black and Hispanic women, 1.0% of white women, 0.5% of Chinese women, and 0.1% of Japanese women. (See bar chart.)
These differences were deemed statistically significant.
Acculturation of immigrants is “a double-edged sword,” Dr. Santoro said at the annual meeting of the North American Menopause Society. It can improve socioeconomic status, access to health care, and attainment of higher education, but at the same time can worsen health through a less-nutritious diet.
In contrast to other minorities, Hispanic women in SWAN and similar studies tend to improve little or even to worsen in terms of health once they are assimilated, she said. Watch for the “Hispanic paradox”: Health outcomes are worse among this population with increased acculturation, despite better socioeconomic status, because of factors such as higher rates of teen pregnancy and cigarette smoking, said Dr. Santoro, director of the division of reproductive endocrinology and infertility at Albert Einstein College of Medicine, New York.
She cautioned, however, that the Hispanic population is heterogeneous and cannot be addressed as a single entity.
The Hispanic SWAN participants came from many different countries and cultures and displayed some internal differences. For example, women from Puerto Rico were more vulnerable to acculturation and reported more menopause-related sleep problems and depressive symptoms than did other Hispanics.
Meanwhile, the acculturation of Japanese women was associated with fewer menopausal symptoms than were seen in Hispanics. Similarly, Chinese participants reported fewer symptoms compared with white, black, and Hispanic women in SWAN. “There are clear-cut differences in symptom reporting by ethnicity,” Dr. Santoro said.
Hispanic and black women were more likely to report depressive symptoms, and Chinese and Japanese women were less likely to do so, the study found.
“This is confounded, possibly, by lower socioeconomic status in the African American and Hispanic groups, and a higher socioeconomic status in Chinese and especially Japanese women,” she said.
Black women in SWAN reported the most hot flashes. Dr. Santoro proposed that increased adiposity among these women might provide more insulation and make them less heat tolerant.
Black women, however, were less bothered by hot flashes than were Hispanic women, who reported more embarrassment with vasomotor symptoms.
SWAN is supported by grants from the Department of Health and Human Services.
ELSEVIER GLOBAL MEDICAL NEWS
LAKE BUENA VISTA, FLA. — Menopause symptoms vary significantly by ethnic group, based on data emerging from a longitudinal study.
The acculturation of women immigrants to the United States, as well as their socioeconomic status, are two factors that might account for these differences, said Dr. Nanette F. Santoro, an endocrinologist who has coauthored multiple studies based on data from the Study of Women's Health Across the Nation (SWAN).
The study included women from seven sites: Boston; Newark, N.J.; Pittsburgh; Detroit; Chicago; Oakland, Calif.; and Los Angeles. Each site recruited white women and women from one ethnic minority group: black, Hispanic, Chinese, or Japanese. More than 10 years later, about 85% of the participants remain in the study.
“We found differences by ethnicity—very intriguing differences,” Dr. Santoro said.
For example, in one study of 11,652 women from SWAN, Dr. Santoro and her colleagues found that 126 participants (1.1%) reported onset of menopause before age 40 years, a condition known as premature ovarian failure (Human Reprod. 2003;18:199–206). This occurred in 1.4% of both black and Hispanic women, 1.0% of white women, 0.5% of Chinese women, and 0.1% of Japanese women. (See bar chart.)
These differences were deemed statistically significant.
Acculturation of immigrants is “a double-edged sword,” Dr. Santoro said at the annual meeting of the North American Menopause Society. It can improve socioeconomic status, access to health care, and attainment of higher education, but at the same time can worsen health through a less-nutritious diet.
In contrast to other minorities, Hispanic women in SWAN and similar studies tend to improve little or even to worsen in terms of health once they are assimilated, she said. Watch for the “Hispanic paradox”: Health outcomes are worse among this population with increased acculturation, despite better socioeconomic status, because of factors such as higher rates of teen pregnancy and cigarette smoking, said Dr. Santoro, director of the division of reproductive endocrinology and infertility at Albert Einstein College of Medicine, New York.
She cautioned, however, that the Hispanic population is heterogeneous and cannot be addressed as a single entity.
The Hispanic SWAN participants came from many different countries and cultures and displayed some internal differences. For example, women from Puerto Rico were more vulnerable to acculturation and reported more menopause-related sleep problems and depressive symptoms than did other Hispanics.
Meanwhile, the acculturation of Japanese women was associated with fewer menopausal symptoms than were seen in Hispanics. Similarly, Chinese participants reported fewer symptoms compared with white, black, and Hispanic women in SWAN. “There are clear-cut differences in symptom reporting by ethnicity,” Dr. Santoro said.
Hispanic and black women were more likely to report depressive symptoms, and Chinese and Japanese women were less likely to do so, the study found.
“This is confounded, possibly, by lower socioeconomic status in the African American and Hispanic groups, and a higher socioeconomic status in Chinese and especially Japanese women,” she said.
Black women in SWAN reported the most hot flashes. Dr. Santoro proposed that increased adiposity among these women might provide more insulation and make them less heat tolerant.
Black women, however, were less bothered by hot flashes than were Hispanic women, who reported more embarrassment with vasomotor symptoms.
SWAN is supported by grants from the Department of Health and Human Services.
ELSEVIER GLOBAL MEDICAL NEWS
Soy Matches HT on Menopause Symptoms, but Not on Lipids
LAKE BUENA VISTA, FLA. — Soy supplements improved somatic and urogenital symptoms of menopause to the same degree as did low-dose combination hormone therapy in a small, randomized, double-blind controlled trial.
A total of 60 women who were 1–13 years past menopause were randomized to one of three groups: soy supplements containing about 90 mg of isoflavones; estradiol 1 mg/norethindrone 0.5 mg; or placebo daily.
After 16 weeks, women in the two treatment groups had significant somatic and urogenital symptom improvements, compared with baseline on the Menopause Rating Scale (MRS).
The changes were also significant, compared with scores among women taking placebo.
The findings suggest a role for dietary soy supplementation for improving hot flashes, joint and muscle pain, and vaginal dryness, with results equivalent to hormone therapy, said gynecologist Adriana O. Pedro.
“I thought that hormone replacement would be better than soy—so I was surprised,” said Dr. Pedro, of the State University of Campinas (Brazil), during a poster session at the annual meeting of the North American Menopause Society.
Women taking hormone therapy fared better, however, in terms of cardiovascular health markers. Women on the low-dose combination hormone therapy showed improvement in total cholesterol and low-density lipoprotein levels; these levels were unchanged in those who got soy supplements.
In addition, total cholesterol decreased 12%, compared with baseline, in the hormone treatment group but remained unchanged in the soy supplement and placebo groups.
The LDL cholesterol level decreased 18% in the hormone therapy group and, again, did not change in the other groups.
“There was no change with soy—probably because they had normal lipid profiles at baseline,” Dr. Pedro said.
Levels of triglycerides, HDL cholesterol, and glucose; body mass index; blood pressure; and abdominal/hip ratio did not change significantly, compared with baseline, in any group.
Total MRS scores were reduced in all groups by 16 weeks. In addition, follicle-stimulating hormone decreased and 17β-estradiol increased, compared with baseline, but only in the hormone replacement group. Psychological symptoms did not change over the treatment period in the soy, hormone replacement, or placebo groups.
Data analysis is ongoing. “We just analyzed symptoms and lipid profiles so far,” Dr. Pedro said. In the future, they plan to publish additional findings for these postmenopausal women regarding any changes in quality of life, vaginal pH, vaginal cytology, or bladder symptoms.
The study was funded by the São Paulo (Brazil) Foundation for the Support of Research.
LAKE BUENA VISTA, FLA. — Soy supplements improved somatic and urogenital symptoms of menopause to the same degree as did low-dose combination hormone therapy in a small, randomized, double-blind controlled trial.
A total of 60 women who were 1–13 years past menopause were randomized to one of three groups: soy supplements containing about 90 mg of isoflavones; estradiol 1 mg/norethindrone 0.5 mg; or placebo daily.
After 16 weeks, women in the two treatment groups had significant somatic and urogenital symptom improvements, compared with baseline on the Menopause Rating Scale (MRS).
The changes were also significant, compared with scores among women taking placebo.
The findings suggest a role for dietary soy supplementation for improving hot flashes, joint and muscle pain, and vaginal dryness, with results equivalent to hormone therapy, said gynecologist Adriana O. Pedro.
“I thought that hormone replacement would be better than soy—so I was surprised,” said Dr. Pedro, of the State University of Campinas (Brazil), during a poster session at the annual meeting of the North American Menopause Society.
Women taking hormone therapy fared better, however, in terms of cardiovascular health markers. Women on the low-dose combination hormone therapy showed improvement in total cholesterol and low-density lipoprotein levels; these levels were unchanged in those who got soy supplements.
In addition, total cholesterol decreased 12%, compared with baseline, in the hormone treatment group but remained unchanged in the soy supplement and placebo groups.
The LDL cholesterol level decreased 18% in the hormone therapy group and, again, did not change in the other groups.
“There was no change with soy—probably because they had normal lipid profiles at baseline,” Dr. Pedro said.
Levels of triglycerides, HDL cholesterol, and glucose; body mass index; blood pressure; and abdominal/hip ratio did not change significantly, compared with baseline, in any group.
Total MRS scores were reduced in all groups by 16 weeks. In addition, follicle-stimulating hormone decreased and 17β-estradiol increased, compared with baseline, but only in the hormone replacement group. Psychological symptoms did not change over the treatment period in the soy, hormone replacement, or placebo groups.
Data analysis is ongoing. “We just analyzed symptoms and lipid profiles so far,” Dr. Pedro said. In the future, they plan to publish additional findings for these postmenopausal women regarding any changes in quality of life, vaginal pH, vaginal cytology, or bladder symptoms.
The study was funded by the São Paulo (Brazil) Foundation for the Support of Research.
LAKE BUENA VISTA, FLA. — Soy supplements improved somatic and urogenital symptoms of menopause to the same degree as did low-dose combination hormone therapy in a small, randomized, double-blind controlled trial.
A total of 60 women who were 1–13 years past menopause were randomized to one of three groups: soy supplements containing about 90 mg of isoflavones; estradiol 1 mg/norethindrone 0.5 mg; or placebo daily.
After 16 weeks, women in the two treatment groups had significant somatic and urogenital symptom improvements, compared with baseline on the Menopause Rating Scale (MRS).
The changes were also significant, compared with scores among women taking placebo.
The findings suggest a role for dietary soy supplementation for improving hot flashes, joint and muscle pain, and vaginal dryness, with results equivalent to hormone therapy, said gynecologist Adriana O. Pedro.
“I thought that hormone replacement would be better than soy—so I was surprised,” said Dr. Pedro, of the State University of Campinas (Brazil), during a poster session at the annual meeting of the North American Menopause Society.
Women taking hormone therapy fared better, however, in terms of cardiovascular health markers. Women on the low-dose combination hormone therapy showed improvement in total cholesterol and low-density lipoprotein levels; these levels were unchanged in those who got soy supplements.
In addition, total cholesterol decreased 12%, compared with baseline, in the hormone treatment group but remained unchanged in the soy supplement and placebo groups.
The LDL cholesterol level decreased 18% in the hormone therapy group and, again, did not change in the other groups.
“There was no change with soy—probably because they had normal lipid profiles at baseline,” Dr. Pedro said.
Levels of triglycerides, HDL cholesterol, and glucose; body mass index; blood pressure; and abdominal/hip ratio did not change significantly, compared with baseline, in any group.
Total MRS scores were reduced in all groups by 16 weeks. In addition, follicle-stimulating hormone decreased and 17β-estradiol increased, compared with baseline, but only in the hormone replacement group. Psychological symptoms did not change over the treatment period in the soy, hormone replacement, or placebo groups.
Data analysis is ongoing. “We just analyzed symptoms and lipid profiles so far,” Dr. Pedro said. In the future, they plan to publish additional findings for these postmenopausal women regarding any changes in quality of life, vaginal pH, vaginal cytology, or bladder symptoms.
The study was funded by the São Paulo (Brazil) Foundation for the Support of Research.
Early Data on Compounded Transdermal HT
NEW ORLEANS — Compounded transdermal hormone therapy relieves menopausal symptoms while improving cardiovascular risk factors and inflammatory and thrombotic biomarkers, according to a preliminary study.
“By replacing the hormone that's deficient via transdermal dosing it may be possible to more closely mimic normal physiology and favorably impact cardiometabolic clinical biomarkers.
“Despite FDA expressed concerns of dangers of compounded hormone use, our data suggest that transdermal compounded hormones may offer a safe and effective treatment for hormone-related symptoms when utilizing dosages targeting physiologic reference ranges and compounds, which meet USP standards for potency,” Dr. Kenna Stephenson said at the annual scientific sessions of the American Heart Association.
“Our study would suggest this is a superior way to treat women. Sure, Premarin [conjugated estrogens] gets rid of hot flashes, but it also increases C-reactive protein and increases thrombotic risk,” added Dr. Stephenson, a family physician active in clinical research in women's health at the University of Texas Health Science Center at Tyler.
Her group's ongoing study involves 150 women, mean age 51.9 years, with menopausal symptoms, who were randomized to usual care or individualized transdermal plant-derived estrogen, progesterone, testosterone, and dehydroandrostenidione therapy prepared by a compounding pharmacist.
After 12 months of follow-up, the women on transdermal therapy showed significant reductions in triglycerides, blood pressure, fasting blood glucose, C-reactive protein, plasma fibrinogen, insulin-like growth factor-I, and factor VII along with significant symptomatic and quality of life improvements (see chart). The study will continue through 3 years of follow-up.
Ever since analysis of data from the Women's Health Initiative linked oral hormone replacement therapy to increased risks of breast cancer and cardiovascular events, women with menopausal symptoms have expressed growing interest in alternative forms of hormonal therapy.
“In my clinical practice I would say every week I see a patient who's already had an MI or a stroke, she's in her 50s or maybe her 40s, and she's been told she can never have hormones again,” Dr. Stephenson observed in an interview.
As in the ongoing study, her clinical practice is to take a history of hormone-related symptoms such as hot flashes, night sweats, mood changes, sleep deprivation, and unexplained fatigue, measure the patient's sex hormone levels, and then prescribe a low-dose transdermal hormone compounded specifically for her. Transdermal therapy avoids first-pass hepatic metabolism, thereby preventing buildup of atherogenic sex hormone metabolites, Dr. Stephenson explained.
“What I see in clinical practice as well as in my research studies is their biomarkers improve. They have adequate symptom relief, which is what's most important to the patients. And once their symptoms are relieved they're more likely to make positive nutritional and lifestyle changes: They feel like exercising; they feel like eating the way they're supposed to,” the family physician continued.
She uses the university medical center's compounding pharmacy. There are a growing number of such pharmacies around the country as a result of increasing applications for compounded transdermal therapy in pain medicine, oncology, dermatology, and sports medicine, as well as hormone therapy. She noted that interested physicians can locate a compounding pharmacist through the member registry maintained by the International Academy of Compounding Pharmacists (www.iacprx.org
A home salivary specimen shipped to a CLIA-certified laboratory provides the most accurate way to assess a woman's hormone status. “Traditional blood tests are not helpful, in my clinical experience. The reference ranges in serum testing for sex hormones are too broad,” Dr. Stephenson said.
She is the author of “Awakening Athena: Resilience, Restoration, and Rejuvenation for Women” (Hallsville, Texas: Health, Heart, and Mind Institute, 2004) a book that goes into the details of individualized transdermal compounded hormone therapy.
In January 2008, the Food and Drug Administration announced a controversial new policy of restricted access to medications containing estriol that could have a negative impact on compounded transdermal hormone therapy for women, since prescribing physicians are required to fill out an Investigational New Drug application. Resolutions have been introduced in both the Senate (S.Con.Res. 88) and House of Representatives (H.Con.Res. 342) calling on the FDA to reverse this policy.
A video interview of Dr. Stephenson discussing her study is available at www.youtube.com/watch?v=IXDCo4nw86Q
'What I see in clinical practice as well as in my research studies is their biomarkers improve.' DR. STEPHENSON
ELSEVIER GLOBAL MEDICAL NEWS
NEW ORLEANS — Compounded transdermal hormone therapy relieves menopausal symptoms while improving cardiovascular risk factors and inflammatory and thrombotic biomarkers, according to a preliminary study.
“By replacing the hormone that's deficient via transdermal dosing it may be possible to more closely mimic normal physiology and favorably impact cardiometabolic clinical biomarkers.
“Despite FDA expressed concerns of dangers of compounded hormone use, our data suggest that transdermal compounded hormones may offer a safe and effective treatment for hormone-related symptoms when utilizing dosages targeting physiologic reference ranges and compounds, which meet USP standards for potency,” Dr. Kenna Stephenson said at the annual scientific sessions of the American Heart Association.
“Our study would suggest this is a superior way to treat women. Sure, Premarin [conjugated estrogens] gets rid of hot flashes, but it also increases C-reactive protein and increases thrombotic risk,” added Dr. Stephenson, a family physician active in clinical research in women's health at the University of Texas Health Science Center at Tyler.
Her group's ongoing study involves 150 women, mean age 51.9 years, with menopausal symptoms, who were randomized to usual care or individualized transdermal plant-derived estrogen, progesterone, testosterone, and dehydroandrostenidione therapy prepared by a compounding pharmacist.
After 12 months of follow-up, the women on transdermal therapy showed significant reductions in triglycerides, blood pressure, fasting blood glucose, C-reactive protein, plasma fibrinogen, insulin-like growth factor-I, and factor VII along with significant symptomatic and quality of life improvements (see chart). The study will continue through 3 years of follow-up.
Ever since analysis of data from the Women's Health Initiative linked oral hormone replacement therapy to increased risks of breast cancer and cardiovascular events, women with menopausal symptoms have expressed growing interest in alternative forms of hormonal therapy.
“In my clinical practice I would say every week I see a patient who's already had an MI or a stroke, she's in her 50s or maybe her 40s, and she's been told she can never have hormones again,” Dr. Stephenson observed in an interview.
As in the ongoing study, her clinical practice is to take a history of hormone-related symptoms such as hot flashes, night sweats, mood changes, sleep deprivation, and unexplained fatigue, measure the patient's sex hormone levels, and then prescribe a low-dose transdermal hormone compounded specifically for her. Transdermal therapy avoids first-pass hepatic metabolism, thereby preventing buildup of atherogenic sex hormone metabolites, Dr. Stephenson explained.
“What I see in clinical practice as well as in my research studies is their biomarkers improve. They have adequate symptom relief, which is what's most important to the patients. And once their symptoms are relieved they're more likely to make positive nutritional and lifestyle changes: They feel like exercising; they feel like eating the way they're supposed to,” the family physician continued.
She uses the university medical center's compounding pharmacy. There are a growing number of such pharmacies around the country as a result of increasing applications for compounded transdermal therapy in pain medicine, oncology, dermatology, and sports medicine, as well as hormone therapy. She noted that interested physicians can locate a compounding pharmacist through the member registry maintained by the International Academy of Compounding Pharmacists (www.iacprx.org
A home salivary specimen shipped to a CLIA-certified laboratory provides the most accurate way to assess a woman's hormone status. “Traditional blood tests are not helpful, in my clinical experience. The reference ranges in serum testing for sex hormones are too broad,” Dr. Stephenson said.
She is the author of “Awakening Athena: Resilience, Restoration, and Rejuvenation for Women” (Hallsville, Texas: Health, Heart, and Mind Institute, 2004) a book that goes into the details of individualized transdermal compounded hormone therapy.
In January 2008, the Food and Drug Administration announced a controversial new policy of restricted access to medications containing estriol that could have a negative impact on compounded transdermal hormone therapy for women, since prescribing physicians are required to fill out an Investigational New Drug application. Resolutions have been introduced in both the Senate (S.Con.Res. 88) and House of Representatives (H.Con.Res. 342) calling on the FDA to reverse this policy.
A video interview of Dr. Stephenson discussing her study is available at www.youtube.com/watch?v=IXDCo4nw86Q
'What I see in clinical practice as well as in my research studies is their biomarkers improve.' DR. STEPHENSON
ELSEVIER GLOBAL MEDICAL NEWS
NEW ORLEANS — Compounded transdermal hormone therapy relieves menopausal symptoms while improving cardiovascular risk factors and inflammatory and thrombotic biomarkers, according to a preliminary study.
“By replacing the hormone that's deficient via transdermal dosing it may be possible to more closely mimic normal physiology and favorably impact cardiometabolic clinical biomarkers.
“Despite FDA expressed concerns of dangers of compounded hormone use, our data suggest that transdermal compounded hormones may offer a safe and effective treatment for hormone-related symptoms when utilizing dosages targeting physiologic reference ranges and compounds, which meet USP standards for potency,” Dr. Kenna Stephenson said at the annual scientific sessions of the American Heart Association.
“Our study would suggest this is a superior way to treat women. Sure, Premarin [conjugated estrogens] gets rid of hot flashes, but it also increases C-reactive protein and increases thrombotic risk,” added Dr. Stephenson, a family physician active in clinical research in women's health at the University of Texas Health Science Center at Tyler.
Her group's ongoing study involves 150 women, mean age 51.9 years, with menopausal symptoms, who were randomized to usual care or individualized transdermal plant-derived estrogen, progesterone, testosterone, and dehydroandrostenidione therapy prepared by a compounding pharmacist.
After 12 months of follow-up, the women on transdermal therapy showed significant reductions in triglycerides, blood pressure, fasting blood glucose, C-reactive protein, plasma fibrinogen, insulin-like growth factor-I, and factor VII along with significant symptomatic and quality of life improvements (see chart). The study will continue through 3 years of follow-up.
Ever since analysis of data from the Women's Health Initiative linked oral hormone replacement therapy to increased risks of breast cancer and cardiovascular events, women with menopausal symptoms have expressed growing interest in alternative forms of hormonal therapy.
“In my clinical practice I would say every week I see a patient who's already had an MI or a stroke, she's in her 50s or maybe her 40s, and she's been told she can never have hormones again,” Dr. Stephenson observed in an interview.
As in the ongoing study, her clinical practice is to take a history of hormone-related symptoms such as hot flashes, night sweats, mood changes, sleep deprivation, and unexplained fatigue, measure the patient's sex hormone levels, and then prescribe a low-dose transdermal hormone compounded specifically for her. Transdermal therapy avoids first-pass hepatic metabolism, thereby preventing buildup of atherogenic sex hormone metabolites, Dr. Stephenson explained.
“What I see in clinical practice as well as in my research studies is their biomarkers improve. They have adequate symptom relief, which is what's most important to the patients. And once their symptoms are relieved they're more likely to make positive nutritional and lifestyle changes: They feel like exercising; they feel like eating the way they're supposed to,” the family physician continued.
She uses the university medical center's compounding pharmacy. There are a growing number of such pharmacies around the country as a result of increasing applications for compounded transdermal therapy in pain medicine, oncology, dermatology, and sports medicine, as well as hormone therapy. She noted that interested physicians can locate a compounding pharmacist through the member registry maintained by the International Academy of Compounding Pharmacists (www.iacprx.org
A home salivary specimen shipped to a CLIA-certified laboratory provides the most accurate way to assess a woman's hormone status. “Traditional blood tests are not helpful, in my clinical experience. The reference ranges in serum testing for sex hormones are too broad,” Dr. Stephenson said.
She is the author of “Awakening Athena: Resilience, Restoration, and Rejuvenation for Women” (Hallsville, Texas: Health, Heart, and Mind Institute, 2004) a book that goes into the details of individualized transdermal compounded hormone therapy.
In January 2008, the Food and Drug Administration announced a controversial new policy of restricted access to medications containing estriol that could have a negative impact on compounded transdermal hormone therapy for women, since prescribing physicians are required to fill out an Investigational New Drug application. Resolutions have been introduced in both the Senate (S.Con.Res. 88) and House of Representatives (H.Con.Res. 342) calling on the FDA to reverse this policy.
A video interview of Dr. Stephenson discussing her study is available at www.youtube.com/watch?v=IXDCo4nw86Q
'What I see in clinical practice as well as in my research studies is their biomarkers improve.' DR. STEPHENSON
ELSEVIER GLOBAL MEDICAL NEWS
“Bioidentical” hormones: What you (and your patient) need to know
OBG Management Senior Editor Janelle Yates contributed to this article.
Hear Dr. Pinkerton discuss this article
The Women’s Health Initiative (WHI) caused a sea change in women’s attitudes toward menopausal hormone therapy and aroused many fears—not always rational—that remain almost palpable today. One study of the aftermath of the WHI found that 70% of women who were taking hormone therapy discontinued it, and 26% of women lost confidence in medical recommendations in general.1
Into the chaos stepped Suzanne Somers, Michael Platt, and other celebrities, touting the benefits of a new kind of hormone: bioidentical. You don’t have to read Somers’ bestseller, The Sexy Years, to encounter the claims it makes on behalf of bioidenticals; the cover itself makes them clear: Discover the Hormone Connection—The Secret to Fabulous Sex, Great Health and Vitality, for Women and Men. Since publication of the book, the demand for bioidentical hormones has only increased, as women remain fearful about conventional hormone therapy.
Many ObGyns regularly field requests from patients for specially compounded bioidentical regimens. In most cases, the women who ask for these drugs are poorly informed about their risks and willing to pay out of pocket to acquire them. JoAnn V. Pinkerton, MD, sees many of these patients at The Women’s Place Midlife Health Center in Charlottesville, Virginia. OBG Management recently sat down with Dr. Pinkerton to discuss her concerns about the growing ubiquity of compounded bioidentical hormones. In the Q&A that follows, we talk about what “bioidentical” actually means, whether these hormones are ever justified, common misconceptions about them, and other issues.
In a special accompanying commentary, former Food and Drug Administration (FDA) Senior Medical Officer Bruce Patsner, MD, JD, also weighs in on the issue.
- “They’re identical to the hormones in my body”
- “They occur naturally”
- “They are safer and more effective than conventional hormone therapy”
- “They’re risk-free”
- “They are monitored by the FDA”
- “They are the fountain of youth”
- “They prevent breast cancer”
- “Celebrities know more about them than physicians and menopause and hormone experts do”
- “Doctors oppose bioidentical hormone therapy because they are in the pocket of Big Pharma”
- “Bioidentical hormones are not a huge money-making enterprise”
What is “bioidentical”?
OBG MANAGEMENT: Let’s start with the basics. What does the word “bioidentical” mean? Is it a legitimate medical term?
DR. PINKERTON: Bioidentical hormones are exogenous hormones that are biochemically similar to those produced endogenously by the body or ovaries. These include estrone, estradiol, estriol, progesterone, testosterone, dehydroepiandrosterone (DHEA), and cortisol. The FDA has approved many prescription products that contain bioidentical hormones. However, the term “bioidentical” is often used to refer to custom-compounded hormones. The major difference between the FDA-approved prescription bioidentical hormone products and custom-compounded products is that the former are regulated by the FDA and tested for purity, potency, efficacy, and safety.
Bioidenticals are not “natural” hormones, although many consumers think they are. In reality, compounded bioidentical hormones and FDA-approved bioidentical hormones all come from the same precursors. They begin as soy products or wild yam and then get converted to the different hormones in a laboratory in Germany before finding their way to the various world markets.
The claim that all bioidentical hormones are bioengineered to contain the same chemical structure as natural female sex hormones is false. As one expert noted, “the term ‘bioidentical’ has become inappropriately synonymous with ‘natural’ or ‘not synthetic’ and should be redefined to correct patient misconceptions.”2
Common misconceptions
OBG MANAGEMENT: What are some of the other false impressions you encounter among patients who ask for bioidenticals?
DR. PINKERTON: That the hormones are safer or more effective than hormone therapy, that they carry no risks, and that they are as well-monitored as FDA-approved products, to name a few. (For more, see “10 erroneous beliefs patients have about compounded hormones”).
OBG MANAGEMENT: Where do these ideas originate?
DR. PINKERTON: They are propagated by self-proclaimed experts and celebrities or by laypersons and physicians who devote the bulk of their time to promoting these hormones, usually at considerable cost to the patient.
OBG MANAGEMENT: What are the risks of compounded bioidentical hormones?
DR. PINKERTON: According to FDA guidance for industry, in the absence of data about these hormones, the risks and benefits should be assumed to be identical to those of FDA-approved hormone therapies, with the caveat that we don’t know from batch to batch what a woman is receiving. However, they are not regulated or monitored by the FDA, so we are lacking testing for purity, potency, efficacy, and safety. When the FDA did analyze compounded bioidentical hormones, a significant percentage (34%) failed one or more standard quality tests.3 In comparison, FDA-approved drugs fail analytical testing at a rate of less than 2%.3

BRUCE PATSNER, MD, JD
Dr. Patsner is Research Professor of Law at the University of Houston Law Center in Houston, Tex. He served as Senior Medical Officer at the US Food and Drug Administration (FDA), where he was one of the agency’s experts on pharmacy compounding of prescription hormone drug therapy for the treatment of menopausal conditions.
The FDA has nothing against compounding pharmacies per se. Individualized preparation of a customized medication for a patient, based on a valid prescription, is an essential part of the practice of pharmacy. However, some actors in the pharmacy compounding business have taken the practice to a different level, not just in terms of the volume of business they do, but in the way compounded hormones are advertised and promoted. The courts aren’t necessarily interested in intervening in cases involving high volume alone. And when it comes to unsubstantiated claims of benefit, the FDA has found it difficult to assert jurisdiction over pharmacy compounding in general, making it hard to assert control over the advertising claims these pharmacies make on behalf of compounded drugs.
The result? The FDA has been unable to rein in claims that compounded prescription drugs are safer or better than commercially prepared medications. These drugs are probably as safe and effective as their manufactured counterparts, but there are no data to confirm this assumption.
What’s in a name?
“Bioidentical” isn’t a bona fide term. There is no definition of it in any medical dictionary; it’s just a name the industry cooked up, a catchy one at that. And when bioidenticals are advertised and promoted, the term “natural” is usually in close proximity. Most patients equate the word natural with plant-derived substances that have not been chemically altered. The fact is, many compounded prescription drugs are derived from plants—but they are also chemically altered.
Some applications are legitimate
A number of women use compounded medications because they make it possible to obtain hormone combinations that are not readily available in cream form. For example, if a patient wants testosterone as part of a cream of estrogen and progesterone, a compounded product is the only option.
Show me the data
No studies have compared compounded drugs with commercial drugs—and such studies are exceedingly unlikely. Compounding pharmacies have no incentive to conduct or participate in such studies. The pharmaceutical compounding industry is a multibillion-dollar enterprise in this country, and compounded prescription drugs for menopausal conditions are probably the biggest product outside of the oncology arena. Proponents of compounded hormones have a captive audience, so to speak, made up of women who don’t like commercial drug manufacturers or who prefer products that appear to be natural, or both.
The problem is that these women receive no package insert or prescription drug label with their hormones. Warning labels are not required because compounded drugs are not regulated by the FDA. Consumers are basically at the mercy of whatever claims they read on the Internet or in the lay literature, which tends to be written by people who have a financial interest in affiliating with the compounding industry. It’s a very frustrating situation for a lot of people.
Unintended consequences of the WHI
The Women’s Health Initiative (WHI) stirred demand for bioidentical hormones by casting the safety claims for some commercial hormone therapy products in a less than favorable light. That wasn’t the investigators’ intent, of course, and some of the findings of the WHI have since been questioned.
The goal of the WHI was to critically evaluate some of the touted health benefits of commercial hormone therapy prescription drugs, but, by questioning some of these claims, it inadvertently pushed a significant percentage of patients toward compounded prescription drugs—and we have no safety data on them.
No one knows exactly how many women were swayed, but the consensus is that they were, and no one’s been happy about that.
The main problem with the compounded hormones, as I see it, is that women who use them do not receive any written information from the compounding pharmacist about risks and benefits. Nor do they receive the black box warnings on FDA-approved estrogen therapy. I believe women need to be adequately educated about the potential risks and benefits, as well as the lack of efficacy data and quality control, if compounded products are requested. That means it’s up to the prescriber to educate the patient about the potential risks and benefits.
Rosenthal states that symptomatic menopausal women or those who fear breast cancer or heart disease can be considered a vulnerable population: “Patients do not have the background to decipher credible sources from noncredible sources.” False claims present convincing arguments for laypersons. A woman may be vulnerable to unsubstantiated claims by virtue of her symptoms and the anxiety and even depression that they can produce. Without comprehensive education, these women cannot be assumed to be adequately informed.
Let me put it in perspective. If a patient with a history of breast cancer complains about severe vaginal dryness that interferes with her sex life, I might decide to give her the smallest amount of topical estrogen that I can—for example, a dime-sized amount of estrogen to apply to her vulvar area twice a week. This amount of estrogen can’t be detected in her system with current assays. I know that some of it will be systemically absorbed, but it cannot be detected. When the patient buys that commercially prepared cream from the pharmacist, she will receive the same black box warning that comes with all systemic hormones since the WHI. However, if she goes to a compounding pharmacist with a prescription for bioidentical hormone therapy, she will not get the warning, regardless of the ingredients or dosage.
The American College of Obstetricians and Gynecologists (ACOG), North American Menopause Society (NAMS), and The Endocrine Society have all issued statements noting the lack of safety data on compounded bioidentical hormones. Here’s what they say:
ACOG
“Most compounded products have not undergone rigorous clinical testing for safety or efficacy, and issues regarding purity, potency and quality are a concern. Compounded hormone products have the same safety issues as those associated with hormone therapy agents that are approved by the US Food and Drug Administration and may have additional risks intrinsic to compounding. There is no scientific evidence to support claims of increased efficacy or safety for individualized estrogen or progesterone regimens.”
NAMS
“NAMS does not recommend custom-compounded products over well-tested, government-approved products for the majority of women—and does not recommend saliva testing to determine hormone levels” (www.menopause.org/bioidentical_NAMS.aspx).
The Endocrine Society
“‘Bioidentical hormones,’ particularly estrogen and progesterone, have been promoted as safer and more effective alternatives to more traditional hormone therapies, often by people outside of the medical community. In fact, little or no scientific and medical evidence exists to support such claims about ‘bioidentical hormones.’ Additionally, many ‘bioidentical hormone’ formulations are not subject to FDA oversight and can be inconsistent in dose and purity….”
Are compounded bioidenticals ever justified?
OBG MANAGEMENT: According to the FDA, compounding of drug products is justified only when a practitioner finds that an FDA-approved drug does not meet the patient’s needs. Do you think this is ever really the case, given the availability of FDA-approved bioidentical hormone preparations?
DR. PINKERTON: In rare cases, compounding of bioidentical hormones is justified, such as when a patient cannot tolerate an FDA-approved product. The problem is that women have been especially concerned about the safety of hormone therapy since the WHI, and bioidentical hormones have been promoted as being safer than FDA-regulated preparations, despite the lack of evidence of their safety or efficacy in peer-reviewed literature. So many women request them.
In a recent commentary, Boothby and Doering call bioidentical hormone therapy “a panacea that lacks supportive evidence.” They say, “It’s our belief that pharmacists are compounding these with the best intentions, but they are ill informed regarding the lack of scientific underpinning associated with efficacy and safety.”3
OBG MANAGEMENT: Do you ever prescribe bioidentical hormones?
DR. PINKERTON: Yes, but rarely, and primarily for women who can’t tolerate FDA-approved hormones or who, after adequate information and education, refuse FDA-approved hormone therapy.
Is salivary hormone testing informative?
OBG MANAGEMENT: Many clinicians who prescribe bioidentical hormones base the dosage on salivary hormone testing. They claim that this allows them to offer individualized formulations. Is this a reliable claim?
DR. PINKERTON: No, it isn’t. Although compounded bioidentical hormone therapy is often prescribed on the basis of salivary hormone testing, there is no scientific evidence that a correlation exists between a patient’s symptoms and salivary hormones, or that salivary hormone testing reflects what is happening at the tissue level. As Fugh-Berman and Bythrow have observed, this type of testing is often used to convince asymptomatic consumers to use hormones—or symptomatic women to take higher dosages. That practice is likely to lead to adverse events.5 The practice also directly contradicts evidence-based guidelines, which recommend that hormone therapy be individualized on the basis of symptoms, not hormone levels.6
There are no published studies in the peer-reviewed literature that show that salivary testing is a reliable measure on which to safely and effectively base dosing decisions. Indeed, The Endocrine Society issued a position statement that notes, among other issues, that salivary hormone tests are “inaccurate and should not be considered reliable measures of hormones in the body.”7 The American College of Obstetricians and Gynecologists also advises against salivary testing, observing that:
- 1) there is no biologically meaningful relationship between salivary sex steroidal hormone concentrations and free serum hormone concentrations
- 2) there is large within-patient variability in salivary hormone concentrations. Salivary hormone levels vary depending on diet, time of day of testing, the specific hormone being tested, and other variables.3
Do bioidenticals protect against cancer?
OBG MANAGEMENT: Some reports mention the fact that many women believe that bioidentical hormones—specifically, estriol—can reduce their risk of breast and endometrial cancer. Is there any truth behind this belief?
DR. PINKERTON: Estriol is a weak estrogen. There is no evidence that, if it is given at a dosage high enough to relieve symptoms, it is any safer than estradiol.
In regard to endometrial cancer, if the exogenous estrogen—bioidentical or otherwise—is unopposed or inadequately opposed, the risk of endometrial cancer is elevated. The problem is that it is hard to determine whether estrogen is being adequately opposed, particularly when transdermal compounded progesterone is given, because the progesterone molecule is too large to be well-absorbed systemically.9
In regard to breast cancer, estriol is a less potent estrogen than estradiol, but it is believed to carry the same risks if it is dosed at effective levels. There is nothing about estriol per se in the peer-reviewed literature that shows that it protects against breast cancer.
The data on risk of breast cancer with estrogen therapy is confusing, with potentially higher risks if estrogen is combined with progestogen. Most of the data we have on estriol come from animals, but a study from 1980 in humans showed that, when older women with breast cancer were treated with estriol, 25% had increased growth of metastases.8
How do you monitor use of bioidentical hormones?
OBG MANAGEMENT: When you do prescribe a compounded bioidentical hormone, how do you monitor the patient?
DR. PINKERTON: First, I want to reiterate that I prescribe these hormones after considerable patient education about FDA-approved options and their potential risks. Second, when a patient needs or requests hormone therapy, I recommend conventional therapy. Only when she cannot tolerate or refuses FDA-approved drugs do I consider prescribing compounded bioidentical hormones—which, as I said earlier, are assumed to carry risks identical to those of FDA-approved hormones.
In some cases, I provide gynecologic care for patients who obtain compounded bioidentical hormones from other sources. What I will sometimes do, just to give myself some idea of how much estrogen they are getting, is to measure the peak and trough estradiol and estrone levels. That is, I measure the hormone level within 4 hours of the patient taking the drug to see how high it goes, and again about 12 hours later to see how low it goes. I measure both because estradiol may be peripherally converted to estrone.
Regrettably, we don’t know what to do about the various hormone levels. It isn’t like treating thyroid disorders; we normally dose estrogen therapy based on symptoms.
Who pays?
OBG MANAGEMENT: Who pays for salivary testing and compounded bioidentical hormones? Does health insurance cover them?
DR. PINKERTON: Like other “natural” products, compounded bioidenticals may cost more than their commercially prepared counterparts and often are not covered by insurance. In addition, prescribers may charge more for a “consultation” than do practitioners who accept insurance; they also may recommend salivary testing, which is expensive. Patients can end up paying large sums out of pocket.
As Rosenthal noted, many women do not appear to be concerned about the added costs.2 That may be because compounded bioidentical hormone therapy is usually offered to economically advantaged patients.2
Ethical considerations
OBG MANAGEMENT: That raises an important question: What ethical considerations are inherent in the prescribing of compounded bioidenticals?
DR. PINKERTON: The fact that women who are able to pay out of pocket are the primary users of these drugs is one important point. In her analysis of the ethics surrounding bioidentical hormones, Rosenthal noted that the drugs remain “an unequal alternative, and any data collected would not be representative of the overall menopausal community.”2
A critical issue pointed out by Rosenthal is that perimenopausal and menopausal women may be particularly vulnerable to the unsubstantiated claims of purveyors of bio identical hormones. “A substantial number of women seek out bioidentical hormone replacement therapy to restore sexual well-being and functioning, in particular, who may be psychologically more vulnerable,” she writes.2
Another concern arises when the practitioner who prescribes bioidentical hormones also happens to sell them. This poses a potential conflict of interest and “violates professional ethical conduct.”2
OBG MANAGEMENT: Do physicians aggravate the problem when they accede to a patient’s request for compounded hormones?
DR. PINKERTON: Physicians and health-care providers need to stop and educate the patient about the lack of safety and efficacy data, the risks and benefits, and recognize the possibility that she has been influenced by unsubstantiated claims.
1. Schonberg MA, Davis RB, Wee CC. After the Women’s Health Initiative: decision making and trust of women taking hormone therapy. Womens Health Issues. 2005;15:187-195.
2. Rosenthal MS. Ethical problems with bioidentical hormone therapy. Int J Impot Res. 2008;20:45-52.
3. ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 322. Compounded bioidentical hormones. Obstet Gynecol. 2005;106(5 Pt 1):1139-1140.
4. Boothby LA, Doering PL. Bioidentical hormone therapy: a panacea that lacks supportive evidence. Curr Opin Obstet Gynecol. 2008;20:400-407.
5. Fugh-Berman A, Bythrow J. Bioidentical hormones for menopausal hormone therapy: variation on a theme. J Gen Intern Med. 2007;22:1030-1034.
6. Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health. 2007;16:600-631.
7. Bioidentical hormones lack evidence for safety and effectivness, according to new statement by The Endocrine Society [press release]. Chevy Chase, Md: The Endocrine Society; June 14, 2008.
8. Lemon HM. Pathophysiologic considerations in the treatment of menopausal patients with oestrogens: mammary carcinoma. Acta Endocrinol Suppl [Copenhagen]. 1980;233:17-27.
9. Wren BG, Champion SM, Willets K, et al. Transdermal progesterone and its effects on vasomotor symptoms, blood lipid levels, bone metabolic markers, mood, and quality of life for postmenopausal women. Menopause. 2003;10:13-18.
OBG Management Senior Editor Janelle Yates contributed to this article.
Hear Dr. Pinkerton discuss this article
The Women’s Health Initiative (WHI) caused a sea change in women’s attitudes toward menopausal hormone therapy and aroused many fears—not always rational—that remain almost palpable today. One study of the aftermath of the WHI found that 70% of women who were taking hormone therapy discontinued it, and 26% of women lost confidence in medical recommendations in general.1
Into the chaos stepped Suzanne Somers, Michael Platt, and other celebrities, touting the benefits of a new kind of hormone: bioidentical. You don’t have to read Somers’ bestseller, The Sexy Years, to encounter the claims it makes on behalf of bioidenticals; the cover itself makes them clear: Discover the Hormone Connection—The Secret to Fabulous Sex, Great Health and Vitality, for Women and Men. Since publication of the book, the demand for bioidentical hormones has only increased, as women remain fearful about conventional hormone therapy.
Many ObGyns regularly field requests from patients for specially compounded bioidentical regimens. In most cases, the women who ask for these drugs are poorly informed about their risks and willing to pay out of pocket to acquire them. JoAnn V. Pinkerton, MD, sees many of these patients at The Women’s Place Midlife Health Center in Charlottesville, Virginia. OBG Management recently sat down with Dr. Pinkerton to discuss her concerns about the growing ubiquity of compounded bioidentical hormones. In the Q&A that follows, we talk about what “bioidentical” actually means, whether these hormones are ever justified, common misconceptions about them, and other issues.
In a special accompanying commentary, former Food and Drug Administration (FDA) Senior Medical Officer Bruce Patsner, MD, JD, also weighs in on the issue.
- “They’re identical to the hormones in my body”
- “They occur naturally”
- “They are safer and more effective than conventional hormone therapy”
- “They’re risk-free”
- “They are monitored by the FDA”
- “They are the fountain of youth”
- “They prevent breast cancer”
- “Celebrities know more about them than physicians and menopause and hormone experts do”
- “Doctors oppose bioidentical hormone therapy because they are in the pocket of Big Pharma”
- “Bioidentical hormones are not a huge money-making enterprise”
What is “bioidentical”?
OBG MANAGEMENT: Let’s start with the basics. What does the word “bioidentical” mean? Is it a legitimate medical term?
DR. PINKERTON: Bioidentical hormones are exogenous hormones that are biochemically similar to those produced endogenously by the body or ovaries. These include estrone, estradiol, estriol, progesterone, testosterone, dehydroepiandrosterone (DHEA), and cortisol. The FDA has approved many prescription products that contain bioidentical hormones. However, the term “bioidentical” is often used to refer to custom-compounded hormones. The major difference between the FDA-approved prescription bioidentical hormone products and custom-compounded products is that the former are regulated by the FDA and tested for purity, potency, efficacy, and safety.
Bioidenticals are not “natural” hormones, although many consumers think they are. In reality, compounded bioidentical hormones and FDA-approved bioidentical hormones all come from the same precursors. They begin as soy products or wild yam and then get converted to the different hormones in a laboratory in Germany before finding their way to the various world markets.
The claim that all bioidentical hormones are bioengineered to contain the same chemical structure as natural female sex hormones is false. As one expert noted, “the term ‘bioidentical’ has become inappropriately synonymous with ‘natural’ or ‘not synthetic’ and should be redefined to correct patient misconceptions.”2
Common misconceptions
OBG MANAGEMENT: What are some of the other false impressions you encounter among patients who ask for bioidenticals?
DR. PINKERTON: That the hormones are safer or more effective than hormone therapy, that they carry no risks, and that they are as well-monitored as FDA-approved products, to name a few. (For more, see “10 erroneous beliefs patients have about compounded hormones”).
OBG MANAGEMENT: Where do these ideas originate?
DR. PINKERTON: They are propagated by self-proclaimed experts and celebrities or by laypersons and physicians who devote the bulk of their time to promoting these hormones, usually at considerable cost to the patient.
OBG MANAGEMENT: What are the risks of compounded bioidentical hormones?
DR. PINKERTON: According to FDA guidance for industry, in the absence of data about these hormones, the risks and benefits should be assumed to be identical to those of FDA-approved hormone therapies, with the caveat that we don’t know from batch to batch what a woman is receiving. However, they are not regulated or monitored by the FDA, so we are lacking testing for purity, potency, efficacy, and safety. When the FDA did analyze compounded bioidentical hormones, a significant percentage (34%) failed one or more standard quality tests.3 In comparison, FDA-approved drugs fail analytical testing at a rate of less than 2%.3

BRUCE PATSNER, MD, JD
Dr. Patsner is Research Professor of Law at the University of Houston Law Center in Houston, Tex. He served as Senior Medical Officer at the US Food and Drug Administration (FDA), where he was one of the agency’s experts on pharmacy compounding of prescription hormone drug therapy for the treatment of menopausal conditions.
The FDA has nothing against compounding pharmacies per se. Individualized preparation of a customized medication for a patient, based on a valid prescription, is an essential part of the practice of pharmacy. However, some actors in the pharmacy compounding business have taken the practice to a different level, not just in terms of the volume of business they do, but in the way compounded hormones are advertised and promoted. The courts aren’t necessarily interested in intervening in cases involving high volume alone. And when it comes to unsubstantiated claims of benefit, the FDA has found it difficult to assert jurisdiction over pharmacy compounding in general, making it hard to assert control over the advertising claims these pharmacies make on behalf of compounded drugs.
The result? The FDA has been unable to rein in claims that compounded prescription drugs are safer or better than commercially prepared medications. These drugs are probably as safe and effective as their manufactured counterparts, but there are no data to confirm this assumption.
What’s in a name?
“Bioidentical” isn’t a bona fide term. There is no definition of it in any medical dictionary; it’s just a name the industry cooked up, a catchy one at that. And when bioidenticals are advertised and promoted, the term “natural” is usually in close proximity. Most patients equate the word natural with plant-derived substances that have not been chemically altered. The fact is, many compounded prescription drugs are derived from plants—but they are also chemically altered.
Some applications are legitimate
A number of women use compounded medications because they make it possible to obtain hormone combinations that are not readily available in cream form. For example, if a patient wants testosterone as part of a cream of estrogen and progesterone, a compounded product is the only option.
Show me the data
No studies have compared compounded drugs with commercial drugs—and such studies are exceedingly unlikely. Compounding pharmacies have no incentive to conduct or participate in such studies. The pharmaceutical compounding industry is a multibillion-dollar enterprise in this country, and compounded prescription drugs for menopausal conditions are probably the biggest product outside of the oncology arena. Proponents of compounded hormones have a captive audience, so to speak, made up of women who don’t like commercial drug manufacturers or who prefer products that appear to be natural, or both.
The problem is that these women receive no package insert or prescription drug label with their hormones. Warning labels are not required because compounded drugs are not regulated by the FDA. Consumers are basically at the mercy of whatever claims they read on the Internet or in the lay literature, which tends to be written by people who have a financial interest in affiliating with the compounding industry. It’s a very frustrating situation for a lot of people.
Unintended consequences of the WHI
The Women’s Health Initiative (WHI) stirred demand for bioidentical hormones by casting the safety claims for some commercial hormone therapy products in a less than favorable light. That wasn’t the investigators’ intent, of course, and some of the findings of the WHI have since been questioned.
The goal of the WHI was to critically evaluate some of the touted health benefits of commercial hormone therapy prescription drugs, but, by questioning some of these claims, it inadvertently pushed a significant percentage of patients toward compounded prescription drugs—and we have no safety data on them.
No one knows exactly how many women were swayed, but the consensus is that they were, and no one’s been happy about that.
The main problem with the compounded hormones, as I see it, is that women who use them do not receive any written information from the compounding pharmacist about risks and benefits. Nor do they receive the black box warnings on FDA-approved estrogen therapy. I believe women need to be adequately educated about the potential risks and benefits, as well as the lack of efficacy data and quality control, if compounded products are requested. That means it’s up to the prescriber to educate the patient about the potential risks and benefits.
Rosenthal states that symptomatic menopausal women or those who fear breast cancer or heart disease can be considered a vulnerable population: “Patients do not have the background to decipher credible sources from noncredible sources.” False claims present convincing arguments for laypersons. A woman may be vulnerable to unsubstantiated claims by virtue of her symptoms and the anxiety and even depression that they can produce. Without comprehensive education, these women cannot be assumed to be adequately informed.
Let me put it in perspective. If a patient with a history of breast cancer complains about severe vaginal dryness that interferes with her sex life, I might decide to give her the smallest amount of topical estrogen that I can—for example, a dime-sized amount of estrogen to apply to her vulvar area twice a week. This amount of estrogen can’t be detected in her system with current assays. I know that some of it will be systemically absorbed, but it cannot be detected. When the patient buys that commercially prepared cream from the pharmacist, she will receive the same black box warning that comes with all systemic hormones since the WHI. However, if she goes to a compounding pharmacist with a prescription for bioidentical hormone therapy, she will not get the warning, regardless of the ingredients or dosage.
The American College of Obstetricians and Gynecologists (ACOG), North American Menopause Society (NAMS), and The Endocrine Society have all issued statements noting the lack of safety data on compounded bioidentical hormones. Here’s what they say:
ACOG
“Most compounded products have not undergone rigorous clinical testing for safety or efficacy, and issues regarding purity, potency and quality are a concern. Compounded hormone products have the same safety issues as those associated with hormone therapy agents that are approved by the US Food and Drug Administration and may have additional risks intrinsic to compounding. There is no scientific evidence to support claims of increased efficacy or safety for individualized estrogen or progesterone regimens.”
NAMS
“NAMS does not recommend custom-compounded products over well-tested, government-approved products for the majority of women—and does not recommend saliva testing to determine hormone levels” (www.menopause.org/bioidentical_NAMS.aspx).
The Endocrine Society
“‘Bioidentical hormones,’ particularly estrogen and progesterone, have been promoted as safer and more effective alternatives to more traditional hormone therapies, often by people outside of the medical community. In fact, little or no scientific and medical evidence exists to support such claims about ‘bioidentical hormones.’ Additionally, many ‘bioidentical hormone’ formulations are not subject to FDA oversight and can be inconsistent in dose and purity….”
Are compounded bioidenticals ever justified?
OBG MANAGEMENT: According to the FDA, compounding of drug products is justified only when a practitioner finds that an FDA-approved drug does not meet the patient’s needs. Do you think this is ever really the case, given the availability of FDA-approved bioidentical hormone preparations?
DR. PINKERTON: In rare cases, compounding of bioidentical hormones is justified, such as when a patient cannot tolerate an FDA-approved product. The problem is that women have been especially concerned about the safety of hormone therapy since the WHI, and bioidentical hormones have been promoted as being safer than FDA-regulated preparations, despite the lack of evidence of their safety or efficacy in peer-reviewed literature. So many women request them.
In a recent commentary, Boothby and Doering call bioidentical hormone therapy “a panacea that lacks supportive evidence.” They say, “It’s our belief that pharmacists are compounding these with the best intentions, but they are ill informed regarding the lack of scientific underpinning associated with efficacy and safety.”3
OBG MANAGEMENT: Do you ever prescribe bioidentical hormones?
DR. PINKERTON: Yes, but rarely, and primarily for women who can’t tolerate FDA-approved hormones or who, after adequate information and education, refuse FDA-approved hormone therapy.
Is salivary hormone testing informative?
OBG MANAGEMENT: Many clinicians who prescribe bioidentical hormones base the dosage on salivary hormone testing. They claim that this allows them to offer individualized formulations. Is this a reliable claim?
DR. PINKERTON: No, it isn’t. Although compounded bioidentical hormone therapy is often prescribed on the basis of salivary hormone testing, there is no scientific evidence that a correlation exists between a patient’s symptoms and salivary hormones, or that salivary hormone testing reflects what is happening at the tissue level. As Fugh-Berman and Bythrow have observed, this type of testing is often used to convince asymptomatic consumers to use hormones—or symptomatic women to take higher dosages. That practice is likely to lead to adverse events.5 The practice also directly contradicts evidence-based guidelines, which recommend that hormone therapy be individualized on the basis of symptoms, not hormone levels.6
There are no published studies in the peer-reviewed literature that show that salivary testing is a reliable measure on which to safely and effectively base dosing decisions. Indeed, The Endocrine Society issued a position statement that notes, among other issues, that salivary hormone tests are “inaccurate and should not be considered reliable measures of hormones in the body.”7 The American College of Obstetricians and Gynecologists also advises against salivary testing, observing that:
- 1) there is no biologically meaningful relationship between salivary sex steroidal hormone concentrations and free serum hormone concentrations
- 2) there is large within-patient variability in salivary hormone concentrations. Salivary hormone levels vary depending on diet, time of day of testing, the specific hormone being tested, and other variables.3
Do bioidenticals protect against cancer?
OBG MANAGEMENT: Some reports mention the fact that many women believe that bioidentical hormones—specifically, estriol—can reduce their risk of breast and endometrial cancer. Is there any truth behind this belief?
DR. PINKERTON: Estriol is a weak estrogen. There is no evidence that, if it is given at a dosage high enough to relieve symptoms, it is any safer than estradiol.
In regard to endometrial cancer, if the exogenous estrogen—bioidentical or otherwise—is unopposed or inadequately opposed, the risk of endometrial cancer is elevated. The problem is that it is hard to determine whether estrogen is being adequately opposed, particularly when transdermal compounded progesterone is given, because the progesterone molecule is too large to be well-absorbed systemically.9
In regard to breast cancer, estriol is a less potent estrogen than estradiol, but it is believed to carry the same risks if it is dosed at effective levels. There is nothing about estriol per se in the peer-reviewed literature that shows that it protects against breast cancer.
The data on risk of breast cancer with estrogen therapy is confusing, with potentially higher risks if estrogen is combined with progestogen. Most of the data we have on estriol come from animals, but a study from 1980 in humans showed that, when older women with breast cancer were treated with estriol, 25% had increased growth of metastases.8
How do you monitor use of bioidentical hormones?
OBG MANAGEMENT: When you do prescribe a compounded bioidentical hormone, how do you monitor the patient?
DR. PINKERTON: First, I want to reiterate that I prescribe these hormones after considerable patient education about FDA-approved options and their potential risks. Second, when a patient needs or requests hormone therapy, I recommend conventional therapy. Only when she cannot tolerate or refuses FDA-approved drugs do I consider prescribing compounded bioidentical hormones—which, as I said earlier, are assumed to carry risks identical to those of FDA-approved hormones.
In some cases, I provide gynecologic care for patients who obtain compounded bioidentical hormones from other sources. What I will sometimes do, just to give myself some idea of how much estrogen they are getting, is to measure the peak and trough estradiol and estrone levels. That is, I measure the hormone level within 4 hours of the patient taking the drug to see how high it goes, and again about 12 hours later to see how low it goes. I measure both because estradiol may be peripherally converted to estrone.
Regrettably, we don’t know what to do about the various hormone levels. It isn’t like treating thyroid disorders; we normally dose estrogen therapy based on symptoms.
Who pays?
OBG MANAGEMENT: Who pays for salivary testing and compounded bioidentical hormones? Does health insurance cover them?
DR. PINKERTON: Like other “natural” products, compounded bioidenticals may cost more than their commercially prepared counterparts and often are not covered by insurance. In addition, prescribers may charge more for a “consultation” than do practitioners who accept insurance; they also may recommend salivary testing, which is expensive. Patients can end up paying large sums out of pocket.
As Rosenthal noted, many women do not appear to be concerned about the added costs.2 That may be because compounded bioidentical hormone therapy is usually offered to economically advantaged patients.2
Ethical considerations
OBG MANAGEMENT: That raises an important question: What ethical considerations are inherent in the prescribing of compounded bioidenticals?
DR. PINKERTON: The fact that women who are able to pay out of pocket are the primary users of these drugs is one important point. In her analysis of the ethics surrounding bioidentical hormones, Rosenthal noted that the drugs remain “an unequal alternative, and any data collected would not be representative of the overall menopausal community.”2
A critical issue pointed out by Rosenthal is that perimenopausal and menopausal women may be particularly vulnerable to the unsubstantiated claims of purveyors of bio identical hormones. “A substantial number of women seek out bioidentical hormone replacement therapy to restore sexual well-being and functioning, in particular, who may be psychologically more vulnerable,” she writes.2
Another concern arises when the practitioner who prescribes bioidentical hormones also happens to sell them. This poses a potential conflict of interest and “violates professional ethical conduct.”2
OBG MANAGEMENT: Do physicians aggravate the problem when they accede to a patient’s request for compounded hormones?
DR. PINKERTON: Physicians and health-care providers need to stop and educate the patient about the lack of safety and efficacy data, the risks and benefits, and recognize the possibility that she has been influenced by unsubstantiated claims.
OBG Management Senior Editor Janelle Yates contributed to this article.
Hear Dr. Pinkerton discuss this article
The Women’s Health Initiative (WHI) caused a sea change in women’s attitudes toward menopausal hormone therapy and aroused many fears—not always rational—that remain almost palpable today. One study of the aftermath of the WHI found that 70% of women who were taking hormone therapy discontinued it, and 26% of women lost confidence in medical recommendations in general.1
Into the chaos stepped Suzanne Somers, Michael Platt, and other celebrities, touting the benefits of a new kind of hormone: bioidentical. You don’t have to read Somers’ bestseller, The Sexy Years, to encounter the claims it makes on behalf of bioidenticals; the cover itself makes them clear: Discover the Hormone Connection—The Secret to Fabulous Sex, Great Health and Vitality, for Women and Men. Since publication of the book, the demand for bioidentical hormones has only increased, as women remain fearful about conventional hormone therapy.
Many ObGyns regularly field requests from patients for specially compounded bioidentical regimens. In most cases, the women who ask for these drugs are poorly informed about their risks and willing to pay out of pocket to acquire them. JoAnn V. Pinkerton, MD, sees many of these patients at The Women’s Place Midlife Health Center in Charlottesville, Virginia. OBG Management recently sat down with Dr. Pinkerton to discuss her concerns about the growing ubiquity of compounded bioidentical hormones. In the Q&A that follows, we talk about what “bioidentical” actually means, whether these hormones are ever justified, common misconceptions about them, and other issues.
In a special accompanying commentary, former Food and Drug Administration (FDA) Senior Medical Officer Bruce Patsner, MD, JD, also weighs in on the issue.
- “They’re identical to the hormones in my body”
- “They occur naturally”
- “They are safer and more effective than conventional hormone therapy”
- “They’re risk-free”
- “They are monitored by the FDA”
- “They are the fountain of youth”
- “They prevent breast cancer”
- “Celebrities know more about them than physicians and menopause and hormone experts do”
- “Doctors oppose bioidentical hormone therapy because they are in the pocket of Big Pharma”
- “Bioidentical hormones are not a huge money-making enterprise”
What is “bioidentical”?
OBG MANAGEMENT: Let’s start with the basics. What does the word “bioidentical” mean? Is it a legitimate medical term?
DR. PINKERTON: Bioidentical hormones are exogenous hormones that are biochemically similar to those produced endogenously by the body or ovaries. These include estrone, estradiol, estriol, progesterone, testosterone, dehydroepiandrosterone (DHEA), and cortisol. The FDA has approved many prescription products that contain bioidentical hormones. However, the term “bioidentical” is often used to refer to custom-compounded hormones. The major difference between the FDA-approved prescription bioidentical hormone products and custom-compounded products is that the former are regulated by the FDA and tested for purity, potency, efficacy, and safety.
Bioidenticals are not “natural” hormones, although many consumers think they are. In reality, compounded bioidentical hormones and FDA-approved bioidentical hormones all come from the same precursors. They begin as soy products or wild yam and then get converted to the different hormones in a laboratory in Germany before finding their way to the various world markets.
The claim that all bioidentical hormones are bioengineered to contain the same chemical structure as natural female sex hormones is false. As one expert noted, “the term ‘bioidentical’ has become inappropriately synonymous with ‘natural’ or ‘not synthetic’ and should be redefined to correct patient misconceptions.”2
Common misconceptions
OBG MANAGEMENT: What are some of the other false impressions you encounter among patients who ask for bioidenticals?
DR. PINKERTON: That the hormones are safer or more effective than hormone therapy, that they carry no risks, and that they are as well-monitored as FDA-approved products, to name a few. (For more, see “10 erroneous beliefs patients have about compounded hormones”).
OBG MANAGEMENT: Where do these ideas originate?
DR. PINKERTON: They are propagated by self-proclaimed experts and celebrities or by laypersons and physicians who devote the bulk of their time to promoting these hormones, usually at considerable cost to the patient.
OBG MANAGEMENT: What are the risks of compounded bioidentical hormones?
DR. PINKERTON: According to FDA guidance for industry, in the absence of data about these hormones, the risks and benefits should be assumed to be identical to those of FDA-approved hormone therapies, with the caveat that we don’t know from batch to batch what a woman is receiving. However, they are not regulated or monitored by the FDA, so we are lacking testing for purity, potency, efficacy, and safety. When the FDA did analyze compounded bioidentical hormones, a significant percentage (34%) failed one or more standard quality tests.3 In comparison, FDA-approved drugs fail analytical testing at a rate of less than 2%.3

BRUCE PATSNER, MD, JD
Dr. Patsner is Research Professor of Law at the University of Houston Law Center in Houston, Tex. He served as Senior Medical Officer at the US Food and Drug Administration (FDA), where he was one of the agency’s experts on pharmacy compounding of prescription hormone drug therapy for the treatment of menopausal conditions.
The FDA has nothing against compounding pharmacies per se. Individualized preparation of a customized medication for a patient, based on a valid prescription, is an essential part of the practice of pharmacy. However, some actors in the pharmacy compounding business have taken the practice to a different level, not just in terms of the volume of business they do, but in the way compounded hormones are advertised and promoted. The courts aren’t necessarily interested in intervening in cases involving high volume alone. And when it comes to unsubstantiated claims of benefit, the FDA has found it difficult to assert jurisdiction over pharmacy compounding in general, making it hard to assert control over the advertising claims these pharmacies make on behalf of compounded drugs.
The result? The FDA has been unable to rein in claims that compounded prescription drugs are safer or better than commercially prepared medications. These drugs are probably as safe and effective as their manufactured counterparts, but there are no data to confirm this assumption.
What’s in a name?
“Bioidentical” isn’t a bona fide term. There is no definition of it in any medical dictionary; it’s just a name the industry cooked up, a catchy one at that. And when bioidenticals are advertised and promoted, the term “natural” is usually in close proximity. Most patients equate the word natural with plant-derived substances that have not been chemically altered. The fact is, many compounded prescription drugs are derived from plants—but they are also chemically altered.
Some applications are legitimate
A number of women use compounded medications because they make it possible to obtain hormone combinations that are not readily available in cream form. For example, if a patient wants testosterone as part of a cream of estrogen and progesterone, a compounded product is the only option.
Show me the data
No studies have compared compounded drugs with commercial drugs—and such studies are exceedingly unlikely. Compounding pharmacies have no incentive to conduct or participate in such studies. The pharmaceutical compounding industry is a multibillion-dollar enterprise in this country, and compounded prescription drugs for menopausal conditions are probably the biggest product outside of the oncology arena. Proponents of compounded hormones have a captive audience, so to speak, made up of women who don’t like commercial drug manufacturers or who prefer products that appear to be natural, or both.
The problem is that these women receive no package insert or prescription drug label with their hormones. Warning labels are not required because compounded drugs are not regulated by the FDA. Consumers are basically at the mercy of whatever claims they read on the Internet or in the lay literature, which tends to be written by people who have a financial interest in affiliating with the compounding industry. It’s a very frustrating situation for a lot of people.
Unintended consequences of the WHI
The Women’s Health Initiative (WHI) stirred demand for bioidentical hormones by casting the safety claims for some commercial hormone therapy products in a less than favorable light. That wasn’t the investigators’ intent, of course, and some of the findings of the WHI have since been questioned.
The goal of the WHI was to critically evaluate some of the touted health benefits of commercial hormone therapy prescription drugs, but, by questioning some of these claims, it inadvertently pushed a significant percentage of patients toward compounded prescription drugs—and we have no safety data on them.
No one knows exactly how many women were swayed, but the consensus is that they were, and no one’s been happy about that.
The main problem with the compounded hormones, as I see it, is that women who use them do not receive any written information from the compounding pharmacist about risks and benefits. Nor do they receive the black box warnings on FDA-approved estrogen therapy. I believe women need to be adequately educated about the potential risks and benefits, as well as the lack of efficacy data and quality control, if compounded products are requested. That means it’s up to the prescriber to educate the patient about the potential risks and benefits.
Rosenthal states that symptomatic menopausal women or those who fear breast cancer or heart disease can be considered a vulnerable population: “Patients do not have the background to decipher credible sources from noncredible sources.” False claims present convincing arguments for laypersons. A woman may be vulnerable to unsubstantiated claims by virtue of her symptoms and the anxiety and even depression that they can produce. Without comprehensive education, these women cannot be assumed to be adequately informed.
Let me put it in perspective. If a patient with a history of breast cancer complains about severe vaginal dryness that interferes with her sex life, I might decide to give her the smallest amount of topical estrogen that I can—for example, a dime-sized amount of estrogen to apply to her vulvar area twice a week. This amount of estrogen can’t be detected in her system with current assays. I know that some of it will be systemically absorbed, but it cannot be detected. When the patient buys that commercially prepared cream from the pharmacist, she will receive the same black box warning that comes with all systemic hormones since the WHI. However, if she goes to a compounding pharmacist with a prescription for bioidentical hormone therapy, she will not get the warning, regardless of the ingredients or dosage.
The American College of Obstetricians and Gynecologists (ACOG), North American Menopause Society (NAMS), and The Endocrine Society have all issued statements noting the lack of safety data on compounded bioidentical hormones. Here’s what they say:
ACOG
“Most compounded products have not undergone rigorous clinical testing for safety or efficacy, and issues regarding purity, potency and quality are a concern. Compounded hormone products have the same safety issues as those associated with hormone therapy agents that are approved by the US Food and Drug Administration and may have additional risks intrinsic to compounding. There is no scientific evidence to support claims of increased efficacy or safety for individualized estrogen or progesterone regimens.”
NAMS
“NAMS does not recommend custom-compounded products over well-tested, government-approved products for the majority of women—and does not recommend saliva testing to determine hormone levels” (www.menopause.org/bioidentical_NAMS.aspx).
The Endocrine Society
“‘Bioidentical hormones,’ particularly estrogen and progesterone, have been promoted as safer and more effective alternatives to more traditional hormone therapies, often by people outside of the medical community. In fact, little or no scientific and medical evidence exists to support such claims about ‘bioidentical hormones.’ Additionally, many ‘bioidentical hormone’ formulations are not subject to FDA oversight and can be inconsistent in dose and purity….”
Are compounded bioidenticals ever justified?
OBG MANAGEMENT: According to the FDA, compounding of drug products is justified only when a practitioner finds that an FDA-approved drug does not meet the patient’s needs. Do you think this is ever really the case, given the availability of FDA-approved bioidentical hormone preparations?
DR. PINKERTON: In rare cases, compounding of bioidentical hormones is justified, such as when a patient cannot tolerate an FDA-approved product. The problem is that women have been especially concerned about the safety of hormone therapy since the WHI, and bioidentical hormones have been promoted as being safer than FDA-regulated preparations, despite the lack of evidence of their safety or efficacy in peer-reviewed literature. So many women request them.
In a recent commentary, Boothby and Doering call bioidentical hormone therapy “a panacea that lacks supportive evidence.” They say, “It’s our belief that pharmacists are compounding these with the best intentions, but they are ill informed regarding the lack of scientific underpinning associated with efficacy and safety.”3
OBG MANAGEMENT: Do you ever prescribe bioidentical hormones?
DR. PINKERTON: Yes, but rarely, and primarily for women who can’t tolerate FDA-approved hormones or who, after adequate information and education, refuse FDA-approved hormone therapy.
Is salivary hormone testing informative?
OBG MANAGEMENT: Many clinicians who prescribe bioidentical hormones base the dosage on salivary hormone testing. They claim that this allows them to offer individualized formulations. Is this a reliable claim?
DR. PINKERTON: No, it isn’t. Although compounded bioidentical hormone therapy is often prescribed on the basis of salivary hormone testing, there is no scientific evidence that a correlation exists between a patient’s symptoms and salivary hormones, or that salivary hormone testing reflects what is happening at the tissue level. As Fugh-Berman and Bythrow have observed, this type of testing is often used to convince asymptomatic consumers to use hormones—or symptomatic women to take higher dosages. That practice is likely to lead to adverse events.5 The practice also directly contradicts evidence-based guidelines, which recommend that hormone therapy be individualized on the basis of symptoms, not hormone levels.6
There are no published studies in the peer-reviewed literature that show that salivary testing is a reliable measure on which to safely and effectively base dosing decisions. Indeed, The Endocrine Society issued a position statement that notes, among other issues, that salivary hormone tests are “inaccurate and should not be considered reliable measures of hormones in the body.”7 The American College of Obstetricians and Gynecologists also advises against salivary testing, observing that:
- 1) there is no biologically meaningful relationship between salivary sex steroidal hormone concentrations and free serum hormone concentrations
- 2) there is large within-patient variability in salivary hormone concentrations. Salivary hormone levels vary depending on diet, time of day of testing, the specific hormone being tested, and other variables.3
Do bioidenticals protect against cancer?
OBG MANAGEMENT: Some reports mention the fact that many women believe that bioidentical hormones—specifically, estriol—can reduce their risk of breast and endometrial cancer. Is there any truth behind this belief?
DR. PINKERTON: Estriol is a weak estrogen. There is no evidence that, if it is given at a dosage high enough to relieve symptoms, it is any safer than estradiol.
In regard to endometrial cancer, if the exogenous estrogen—bioidentical or otherwise—is unopposed or inadequately opposed, the risk of endometrial cancer is elevated. The problem is that it is hard to determine whether estrogen is being adequately opposed, particularly when transdermal compounded progesterone is given, because the progesterone molecule is too large to be well-absorbed systemically.9
In regard to breast cancer, estriol is a less potent estrogen than estradiol, but it is believed to carry the same risks if it is dosed at effective levels. There is nothing about estriol per se in the peer-reviewed literature that shows that it protects against breast cancer.
The data on risk of breast cancer with estrogen therapy is confusing, with potentially higher risks if estrogen is combined with progestogen. Most of the data we have on estriol come from animals, but a study from 1980 in humans showed that, when older women with breast cancer were treated with estriol, 25% had increased growth of metastases.8
How do you monitor use of bioidentical hormones?
OBG MANAGEMENT: When you do prescribe a compounded bioidentical hormone, how do you monitor the patient?
DR. PINKERTON: First, I want to reiterate that I prescribe these hormones after considerable patient education about FDA-approved options and their potential risks. Second, when a patient needs or requests hormone therapy, I recommend conventional therapy. Only when she cannot tolerate or refuses FDA-approved drugs do I consider prescribing compounded bioidentical hormones—which, as I said earlier, are assumed to carry risks identical to those of FDA-approved hormones.
In some cases, I provide gynecologic care for patients who obtain compounded bioidentical hormones from other sources. What I will sometimes do, just to give myself some idea of how much estrogen they are getting, is to measure the peak and trough estradiol and estrone levels. That is, I measure the hormone level within 4 hours of the patient taking the drug to see how high it goes, and again about 12 hours later to see how low it goes. I measure both because estradiol may be peripherally converted to estrone.
Regrettably, we don’t know what to do about the various hormone levels. It isn’t like treating thyroid disorders; we normally dose estrogen therapy based on symptoms.
Who pays?
OBG MANAGEMENT: Who pays for salivary testing and compounded bioidentical hormones? Does health insurance cover them?
DR. PINKERTON: Like other “natural” products, compounded bioidenticals may cost more than their commercially prepared counterparts and often are not covered by insurance. In addition, prescribers may charge more for a “consultation” than do practitioners who accept insurance; they also may recommend salivary testing, which is expensive. Patients can end up paying large sums out of pocket.
As Rosenthal noted, many women do not appear to be concerned about the added costs.2 That may be because compounded bioidentical hormone therapy is usually offered to economically advantaged patients.2
Ethical considerations
OBG MANAGEMENT: That raises an important question: What ethical considerations are inherent in the prescribing of compounded bioidenticals?
DR. PINKERTON: The fact that women who are able to pay out of pocket are the primary users of these drugs is one important point. In her analysis of the ethics surrounding bioidentical hormones, Rosenthal noted that the drugs remain “an unequal alternative, and any data collected would not be representative of the overall menopausal community.”2
A critical issue pointed out by Rosenthal is that perimenopausal and menopausal women may be particularly vulnerable to the unsubstantiated claims of purveyors of bio identical hormones. “A substantial number of women seek out bioidentical hormone replacement therapy to restore sexual well-being and functioning, in particular, who may be psychologically more vulnerable,” she writes.2
Another concern arises when the practitioner who prescribes bioidentical hormones also happens to sell them. This poses a potential conflict of interest and “violates professional ethical conduct.”2
OBG MANAGEMENT: Do physicians aggravate the problem when they accede to a patient’s request for compounded hormones?
DR. PINKERTON: Physicians and health-care providers need to stop and educate the patient about the lack of safety and efficacy data, the risks and benefits, and recognize the possibility that she has been influenced by unsubstantiated claims.
1. Schonberg MA, Davis RB, Wee CC. After the Women’s Health Initiative: decision making and trust of women taking hormone therapy. Womens Health Issues. 2005;15:187-195.
2. Rosenthal MS. Ethical problems with bioidentical hormone therapy. Int J Impot Res. 2008;20:45-52.
3. ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 322. Compounded bioidentical hormones. Obstet Gynecol. 2005;106(5 Pt 1):1139-1140.
4. Boothby LA, Doering PL. Bioidentical hormone therapy: a panacea that lacks supportive evidence. Curr Opin Obstet Gynecol. 2008;20:400-407.
5. Fugh-Berman A, Bythrow J. Bioidentical hormones for menopausal hormone therapy: variation on a theme. J Gen Intern Med. 2007;22:1030-1034.
6. Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health. 2007;16:600-631.
7. Bioidentical hormones lack evidence for safety and effectivness, according to new statement by The Endocrine Society [press release]. Chevy Chase, Md: The Endocrine Society; June 14, 2008.
8. Lemon HM. Pathophysiologic considerations in the treatment of menopausal patients with oestrogens: mammary carcinoma. Acta Endocrinol Suppl [Copenhagen]. 1980;233:17-27.
9. Wren BG, Champion SM, Willets K, et al. Transdermal progesterone and its effects on vasomotor symptoms, blood lipid levels, bone metabolic markers, mood, and quality of life for postmenopausal women. Menopause. 2003;10:13-18.
1. Schonberg MA, Davis RB, Wee CC. After the Women’s Health Initiative: decision making and trust of women taking hormone therapy. Womens Health Issues. 2005;15:187-195.
2. Rosenthal MS. Ethical problems with bioidentical hormone therapy. Int J Impot Res. 2008;20:45-52.
3. ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 322. Compounded bioidentical hormones. Obstet Gynecol. 2005;106(5 Pt 1):1139-1140.
4. Boothby LA, Doering PL. Bioidentical hormone therapy: a panacea that lacks supportive evidence. Curr Opin Obstet Gynecol. 2008;20:400-407.
5. Fugh-Berman A, Bythrow J. Bioidentical hormones for menopausal hormone therapy: variation on a theme. J Gen Intern Med. 2007;22:1030-1034.
6. Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health. 2007;16:600-631.
7. Bioidentical hormones lack evidence for safety and effectivness, according to new statement by The Endocrine Society [press release]. Chevy Chase, Md: The Endocrine Society; June 14, 2008.
8. Lemon HM. Pathophysiologic considerations in the treatment of menopausal patients with oestrogens: mammary carcinoma. Acta Endocrinol Suppl [Copenhagen]. 1980;233:17-27.
9. Wren BG, Champion SM, Willets K, et al. Transdermal progesterone and its effects on vasomotor symptoms, blood lipid levels, bone metabolic markers, mood, and quality of life for postmenopausal women. Menopause. 2003;10:13-18.