User login
A new nonhormonal option for menopausal hot flashes: What prescribers should know
This transcript has been edited for clarity.
Hello. I am Dr. JoAnn Pinkerton, professor of obstetrics and gynecology at the University of Virginia and a North American Menopause Society–credentialed menopause specialist.
I am excited to tell you about a brand-new, just-approved non-estrogen therapy for treatment of menopausal symptoms. . The manufacturer, Astellas, is expected to make fezolinetant available at pharmacies before the end of this year. This medication binds to and blocks the neurokinin 3 (NK3) receptor, which plays a role in regulating body temperature, leading to a reduction in hot flashes.
For women suffering from frequent moderate to severe hot flashes, fezolinetant is an exciting breakthrough in women’s health as it is a highly effective nonhormonal treatment that reduces hot flashes and improves quality of life.
In two phase 3 clinical trials (Johnson et al. and Lederman et al.), fezolinetant 45 mg reduced the frequency of vasomotor symptoms by about 65%, significantly more than placebo, and similar to the 75% reduction seen with hormone therapy. Fezolinetant’s efficacy becomes evident within 1 week, reducing both frequency and severity of hot flashes.
With respect to side effects, 1%-2% of the menopausal women participating in clinical trials reported adverse events, including headaches, abdominal pain, diarrhea, insomnia, back pain, hot flushes, and reversible elevated hepatic transaminases. Serious adverse events were infrequent.
Subgroup analysis of data presented at ACOG’s 2023 annual meeting noted fezolinetant’s effectiveness among diverse populations, including White or Black race, body mass index of 30 or higher, those younger or older than age 55, smokers, former smokers, and never smokers, in U.S. as well as in European trial participants.
With respect to safety, a 52-week placebo-controlled safety trial confirmed safety for this time period. Adverse effects on the endometrium were neither seen nor expected, as fezolinetant is a centrally acting non–estrogen-containing medication. In addition, no loss of bone density was seen.
Prior trials of neurokinin receptor antagonists suggested the potential for hepatotoxicity. Increases in ALT or AST noted in one of the phase 3 trials of fezolinetant were described as asymptomatic, isolated, intermittent, or transient and returned to baseline during treatment or after discontinuation. However, the FDA placed a warning about liver injury potential. Package labeling recommends baseline liver function tests before starting fezolinetant and at 3, 6, and 9 months. In addition, concomitant use of moderate CYP1A2 inhibitors, including many antidepressants and cimetidine, should be avoided.
As with other recently approved medications, I am concerned that high cost could prevent appropriate candidates from having access.
Until now, the FDA had approved only one nonhormone therapy for vasomotor symptoms, 7.5 mg paroxetine salt. However, neither this formulation nor off-label use of other SSRIs, SNRIs, gabapentinoids, oxybutynin, or clonidine are as effective as hormone therapy or fezolinetant for moderate to severe vasomotor symptoms.
For women with bothersome menopausal hot flashes who can’t or choose not to use hormone therapy, including those with estrogen-sensitive breast or uterine cancers, fezolinetant offers a much-needed, highly effective, safe, nonhormone/non-estrogen option to treat their hot flashes.
The FDA approved it for treating vasomotor symptoms of menopause (hot flashes and night sweats) but it also appears to improve sleep disruption, mood, and quality of life.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I am Dr. JoAnn Pinkerton, professor of obstetrics and gynecology at the University of Virginia and a North American Menopause Society–credentialed menopause specialist.
I am excited to tell you about a brand-new, just-approved non-estrogen therapy for treatment of menopausal symptoms. . The manufacturer, Astellas, is expected to make fezolinetant available at pharmacies before the end of this year. This medication binds to and blocks the neurokinin 3 (NK3) receptor, which plays a role in regulating body temperature, leading to a reduction in hot flashes.
For women suffering from frequent moderate to severe hot flashes, fezolinetant is an exciting breakthrough in women’s health as it is a highly effective nonhormonal treatment that reduces hot flashes and improves quality of life.
In two phase 3 clinical trials (Johnson et al. and Lederman et al.), fezolinetant 45 mg reduced the frequency of vasomotor symptoms by about 65%, significantly more than placebo, and similar to the 75% reduction seen with hormone therapy. Fezolinetant’s efficacy becomes evident within 1 week, reducing both frequency and severity of hot flashes.
With respect to side effects, 1%-2% of the menopausal women participating in clinical trials reported adverse events, including headaches, abdominal pain, diarrhea, insomnia, back pain, hot flushes, and reversible elevated hepatic transaminases. Serious adverse events were infrequent.
Subgroup analysis of data presented at ACOG’s 2023 annual meeting noted fezolinetant’s effectiveness among diverse populations, including White or Black race, body mass index of 30 or higher, those younger or older than age 55, smokers, former smokers, and never smokers, in U.S. as well as in European trial participants.
With respect to safety, a 52-week placebo-controlled safety trial confirmed safety for this time period. Adverse effects on the endometrium were neither seen nor expected, as fezolinetant is a centrally acting non–estrogen-containing medication. In addition, no loss of bone density was seen.
Prior trials of neurokinin receptor antagonists suggested the potential for hepatotoxicity. Increases in ALT or AST noted in one of the phase 3 trials of fezolinetant were described as asymptomatic, isolated, intermittent, or transient and returned to baseline during treatment or after discontinuation. However, the FDA placed a warning about liver injury potential. Package labeling recommends baseline liver function tests before starting fezolinetant and at 3, 6, and 9 months. In addition, concomitant use of moderate CYP1A2 inhibitors, including many antidepressants and cimetidine, should be avoided.
As with other recently approved medications, I am concerned that high cost could prevent appropriate candidates from having access.
Until now, the FDA had approved only one nonhormone therapy for vasomotor symptoms, 7.5 mg paroxetine salt. However, neither this formulation nor off-label use of other SSRIs, SNRIs, gabapentinoids, oxybutynin, or clonidine are as effective as hormone therapy or fezolinetant for moderate to severe vasomotor symptoms.
For women with bothersome menopausal hot flashes who can’t or choose not to use hormone therapy, including those with estrogen-sensitive breast or uterine cancers, fezolinetant offers a much-needed, highly effective, safe, nonhormone/non-estrogen option to treat their hot flashes.
The FDA approved it for treating vasomotor symptoms of menopause (hot flashes and night sweats) but it also appears to improve sleep disruption, mood, and quality of life.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I am Dr. JoAnn Pinkerton, professor of obstetrics and gynecology at the University of Virginia and a North American Menopause Society–credentialed menopause specialist.
I am excited to tell you about a brand-new, just-approved non-estrogen therapy for treatment of menopausal symptoms. . The manufacturer, Astellas, is expected to make fezolinetant available at pharmacies before the end of this year. This medication binds to and blocks the neurokinin 3 (NK3) receptor, which plays a role in regulating body temperature, leading to a reduction in hot flashes.
For women suffering from frequent moderate to severe hot flashes, fezolinetant is an exciting breakthrough in women’s health as it is a highly effective nonhormonal treatment that reduces hot flashes and improves quality of life.
In two phase 3 clinical trials (Johnson et al. and Lederman et al.), fezolinetant 45 mg reduced the frequency of vasomotor symptoms by about 65%, significantly more than placebo, and similar to the 75% reduction seen with hormone therapy. Fezolinetant’s efficacy becomes evident within 1 week, reducing both frequency and severity of hot flashes.
With respect to side effects, 1%-2% of the menopausal women participating in clinical trials reported adverse events, including headaches, abdominal pain, diarrhea, insomnia, back pain, hot flushes, and reversible elevated hepatic transaminases. Serious adverse events were infrequent.
Subgroup analysis of data presented at ACOG’s 2023 annual meeting noted fezolinetant’s effectiveness among diverse populations, including White or Black race, body mass index of 30 or higher, those younger or older than age 55, smokers, former smokers, and never smokers, in U.S. as well as in European trial participants.
With respect to safety, a 52-week placebo-controlled safety trial confirmed safety for this time period. Adverse effects on the endometrium were neither seen nor expected, as fezolinetant is a centrally acting non–estrogen-containing medication. In addition, no loss of bone density was seen.
Prior trials of neurokinin receptor antagonists suggested the potential for hepatotoxicity. Increases in ALT or AST noted in one of the phase 3 trials of fezolinetant were described as asymptomatic, isolated, intermittent, or transient and returned to baseline during treatment or after discontinuation. However, the FDA placed a warning about liver injury potential. Package labeling recommends baseline liver function tests before starting fezolinetant and at 3, 6, and 9 months. In addition, concomitant use of moderate CYP1A2 inhibitors, including many antidepressants and cimetidine, should be avoided.
As with other recently approved medications, I am concerned that high cost could prevent appropriate candidates from having access.
Until now, the FDA had approved only one nonhormone therapy for vasomotor symptoms, 7.5 mg paroxetine salt. However, neither this formulation nor off-label use of other SSRIs, SNRIs, gabapentinoids, oxybutynin, or clonidine are as effective as hormone therapy or fezolinetant for moderate to severe vasomotor symptoms.
For women with bothersome menopausal hot flashes who can’t or choose not to use hormone therapy, including those with estrogen-sensitive breast or uterine cancers, fezolinetant offers a much-needed, highly effective, safe, nonhormone/non-estrogen option to treat their hot flashes.
The FDA approved it for treating vasomotor symptoms of menopause (hot flashes and night sweats) but it also appears to improve sleep disruption, mood, and quality of life.
A version of this article first appeared on Medscape.com.
Is vaginal estrogen used for GSM associated with a higher risk of CVD or cancer?
Expert Commentary
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. December 17, 2018. doi: 10.1097/GME.0000000000001284.
GSM, a chronic and often progressive condition, occurs in almost 50% of postmenopausal women and has been shown to impair sexual function and quality of life.1 Symptoms include vaginal dryness, vulvar or vaginal itching, dyspareunia, urinary urgency or frequency, and increased urinary tract infections. Although lubricants or vaginal moisturizers may be sufficient to treat GSM, targeted hormonal therapy may be needed to improve the symptoms and resolve the underlying cause, due to vaginal hormone loss.
Despite lack of any observational or clinical trial evidence for chronic health disease risks related to low-dose vaginal estrogen use, there remains an US Food and Drug Administration boxed warning on the package label for low-dose vaginal estrogen related to risks of heart disease, stroke, venous thromboembolism, pdementia, and breast cancer. The objective of the investigation by Bhupathiraju and colleagues was to evaluate associations between vaginal estrogen use and health outcomes, including CVD (myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal), and hip fracture.
Details of the study
The prospective analysis included 896 postmenopausal current users of vaginal estrogen in the Nurses’ Health Study (NHS; 1982–2012), compared with 52,901 nonusers. Eighteen years of follow-up was evaluated. Users of systemic hormone therapy were excluded from the analysis. For the NHS, self-reported data were collected every 2 years on questionnaires for vaginal estrogen use and health outcomes. Investigators used medical records to confirm health outcomes.
After adjusting for covariates, no significant differences in risks were found for CVD, cancer, and hip fracture between users and nonusers of vaginal estrogen, regardless of hysterectomy status.
Key findings
After adjusting for multiple variables (including age, race, physical activity, age at menopause, hysterectomy, aspirin use, parental history of cancer, etc), health outcomes for CVDs, all cancers, and hip fracture were:
- myocardial infarction: hazard ratio (HR), 0.73 (95% confidence interval [CI], 0.47–1.13)
- stroke: HR, 0.85 (95% CI, 0.56–1.29)
- pulmonary embolism/deep vein thrombosis: HR, 1.06 (95% CI, 0.58–1.93)
- hip fracture: HR, 0.91 (95% CI, 0.60–1.38)
- all cancers: HR, 1.05 (95% CI, 0.89–1.25).
Continue to: Health outcomes for specific invasive cancers
Health outcomes for specific invasive cancers (risk for endometrial cancer included only women with an intact uterus) were:
- invasive breast cancer: HR, 1.07 (95% CI, 0.78–1.47)
- ovarian cancer: HR, 1.17 (95% CI, 0.52–2.65)
- endometrial cancer: HR, 1.62 (95% CI, 0.88–2.97)
- colorectal cancer: HR, 0.77 (95% CI, 0.45–1.34).
Study strengths and weaknesses
A causal relationship cannot be proven as the study was observational. However, a strength included the 18 years of follow-up. Women used vaginal estrogen for an average of 3 years, which provided longer-term safety data than available 12-month clinical trial data. Data were collected through self-report on questionnaires every 2 years, which is a drawback; however, participants were registered nurses, who have been shown to provide reliable health-related information. Comparisons between therapies were not possible as data were not collected about type or dosage of vaginal estrogen. Available therapies during the NHS included vaginal estrogen tablets, creams, and an estradiol ring, with higher doses available during earlier parts of the study than the lower doses commonly prescribed in current day.
Overall
The findings from this long-term follow-up of the NHS provide support for the safety of vaginal estrogen for treatment of GSM. No statistically significant increased health risks were found for users of vaginal estrogen, similar to earlier reported findings from the large Women’s Health Initiative.2 Low-dose vaginal estrogen is recommended for treatment of GSM by The North American Menopause Society, the American College of Obstetricians and Gynecologists, and the Endocrine Society.
Absorption of low-dose vaginal estrogen preparations appears minimal, and they are effective and generally safe for the treatment of GSM for women at any age. Progesterone is not recommended with low-dose vaginal estrogen therapies, based primarily on randomized clinical trial safety data of 12 months.3 Postmenopausal bleeding, however, needs to be thoroughly evaluated. For women with breast cancer, include the oncologist in decision making about the use of low-dose vaginal estrogen.
Despite the boxed warning on vaginal estrogen, the findings from this study support the safety of vaginal estrogen use for effective relief of GSM in women with and without a uterus.
JOANN V. PINKERTON, MD, NCMP
- Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;251:704-711.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018;25:11-20.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
Expert Commentary
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. December 17, 2018. doi: 10.1097/GME.0000000000001284.
GSM, a chronic and often progressive condition, occurs in almost 50% of postmenopausal women and has been shown to impair sexual function and quality of life.1 Symptoms include vaginal dryness, vulvar or vaginal itching, dyspareunia, urinary urgency or frequency, and increased urinary tract infections. Although lubricants or vaginal moisturizers may be sufficient to treat GSM, targeted hormonal therapy may be needed to improve the symptoms and resolve the underlying cause, due to vaginal hormone loss.
Despite lack of any observational or clinical trial evidence for chronic health disease risks related to low-dose vaginal estrogen use, there remains an US Food and Drug Administration boxed warning on the package label for low-dose vaginal estrogen related to risks of heart disease, stroke, venous thromboembolism, pdementia, and breast cancer. The objective of the investigation by Bhupathiraju and colleagues was to evaluate associations between vaginal estrogen use and health outcomes, including CVD (myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal), and hip fracture.
Details of the study
The prospective analysis included 896 postmenopausal current users of vaginal estrogen in the Nurses’ Health Study (NHS; 1982–2012), compared with 52,901 nonusers. Eighteen years of follow-up was evaluated. Users of systemic hormone therapy were excluded from the analysis. For the NHS, self-reported data were collected every 2 years on questionnaires for vaginal estrogen use and health outcomes. Investigators used medical records to confirm health outcomes.
After adjusting for covariates, no significant differences in risks were found for CVD, cancer, and hip fracture between users and nonusers of vaginal estrogen, regardless of hysterectomy status.
Key findings
After adjusting for multiple variables (including age, race, physical activity, age at menopause, hysterectomy, aspirin use, parental history of cancer, etc), health outcomes for CVDs, all cancers, and hip fracture were:
- myocardial infarction: hazard ratio (HR), 0.73 (95% confidence interval [CI], 0.47–1.13)
- stroke: HR, 0.85 (95% CI, 0.56–1.29)
- pulmonary embolism/deep vein thrombosis: HR, 1.06 (95% CI, 0.58–1.93)
- hip fracture: HR, 0.91 (95% CI, 0.60–1.38)
- all cancers: HR, 1.05 (95% CI, 0.89–1.25).
Continue to: Health outcomes for specific invasive cancers
Health outcomes for specific invasive cancers (risk for endometrial cancer included only women with an intact uterus) were:
- invasive breast cancer: HR, 1.07 (95% CI, 0.78–1.47)
- ovarian cancer: HR, 1.17 (95% CI, 0.52–2.65)
- endometrial cancer: HR, 1.62 (95% CI, 0.88–2.97)
- colorectal cancer: HR, 0.77 (95% CI, 0.45–1.34).
Study strengths and weaknesses
A causal relationship cannot be proven as the study was observational. However, a strength included the 18 years of follow-up. Women used vaginal estrogen for an average of 3 years, which provided longer-term safety data than available 12-month clinical trial data. Data were collected through self-report on questionnaires every 2 years, which is a drawback; however, participants were registered nurses, who have been shown to provide reliable health-related information. Comparisons between therapies were not possible as data were not collected about type or dosage of vaginal estrogen. Available therapies during the NHS included vaginal estrogen tablets, creams, and an estradiol ring, with higher doses available during earlier parts of the study than the lower doses commonly prescribed in current day.
Overall
The findings from this long-term follow-up of the NHS provide support for the safety of vaginal estrogen for treatment of GSM. No statistically significant increased health risks were found for users of vaginal estrogen, similar to earlier reported findings from the large Women’s Health Initiative.2 Low-dose vaginal estrogen is recommended for treatment of GSM by The North American Menopause Society, the American College of Obstetricians and Gynecologists, and the Endocrine Society.
Absorption of low-dose vaginal estrogen preparations appears minimal, and they are effective and generally safe for the treatment of GSM for women at any age. Progesterone is not recommended with low-dose vaginal estrogen therapies, based primarily on randomized clinical trial safety data of 12 months.3 Postmenopausal bleeding, however, needs to be thoroughly evaluated. For women with breast cancer, include the oncologist in decision making about the use of low-dose vaginal estrogen.
Despite the boxed warning on vaginal estrogen, the findings from this study support the safety of vaginal estrogen use for effective relief of GSM in women with and without a uterus.
JOANN V. PINKERTON, MD, NCMP
Expert Commentary
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. December 17, 2018. doi: 10.1097/GME.0000000000001284.
GSM, a chronic and often progressive condition, occurs in almost 50% of postmenopausal women and has been shown to impair sexual function and quality of life.1 Symptoms include vaginal dryness, vulvar or vaginal itching, dyspareunia, urinary urgency or frequency, and increased urinary tract infections. Although lubricants or vaginal moisturizers may be sufficient to treat GSM, targeted hormonal therapy may be needed to improve the symptoms and resolve the underlying cause, due to vaginal hormone loss.
Despite lack of any observational or clinical trial evidence for chronic health disease risks related to low-dose vaginal estrogen use, there remains an US Food and Drug Administration boxed warning on the package label for low-dose vaginal estrogen related to risks of heart disease, stroke, venous thromboembolism, pdementia, and breast cancer. The objective of the investigation by Bhupathiraju and colleagues was to evaluate associations between vaginal estrogen use and health outcomes, including CVD (myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal), and hip fracture.
Details of the study
The prospective analysis included 896 postmenopausal current users of vaginal estrogen in the Nurses’ Health Study (NHS; 1982–2012), compared with 52,901 nonusers. Eighteen years of follow-up was evaluated. Users of systemic hormone therapy were excluded from the analysis. For the NHS, self-reported data were collected every 2 years on questionnaires for vaginal estrogen use and health outcomes. Investigators used medical records to confirm health outcomes.
After adjusting for covariates, no significant differences in risks were found for CVD, cancer, and hip fracture between users and nonusers of vaginal estrogen, regardless of hysterectomy status.
Key findings
After adjusting for multiple variables (including age, race, physical activity, age at menopause, hysterectomy, aspirin use, parental history of cancer, etc), health outcomes for CVDs, all cancers, and hip fracture were:
- myocardial infarction: hazard ratio (HR), 0.73 (95% confidence interval [CI], 0.47–1.13)
- stroke: HR, 0.85 (95% CI, 0.56–1.29)
- pulmonary embolism/deep vein thrombosis: HR, 1.06 (95% CI, 0.58–1.93)
- hip fracture: HR, 0.91 (95% CI, 0.60–1.38)
- all cancers: HR, 1.05 (95% CI, 0.89–1.25).
Continue to: Health outcomes for specific invasive cancers
Health outcomes for specific invasive cancers (risk for endometrial cancer included only women with an intact uterus) were:
- invasive breast cancer: HR, 1.07 (95% CI, 0.78–1.47)
- ovarian cancer: HR, 1.17 (95% CI, 0.52–2.65)
- endometrial cancer: HR, 1.62 (95% CI, 0.88–2.97)
- colorectal cancer: HR, 0.77 (95% CI, 0.45–1.34).
Study strengths and weaknesses
A causal relationship cannot be proven as the study was observational. However, a strength included the 18 years of follow-up. Women used vaginal estrogen for an average of 3 years, which provided longer-term safety data than available 12-month clinical trial data. Data were collected through self-report on questionnaires every 2 years, which is a drawback; however, participants were registered nurses, who have been shown to provide reliable health-related information. Comparisons between therapies were not possible as data were not collected about type or dosage of vaginal estrogen. Available therapies during the NHS included vaginal estrogen tablets, creams, and an estradiol ring, with higher doses available during earlier parts of the study than the lower doses commonly prescribed in current day.
Overall
The findings from this long-term follow-up of the NHS provide support for the safety of vaginal estrogen for treatment of GSM. No statistically significant increased health risks were found for users of vaginal estrogen, similar to earlier reported findings from the large Women’s Health Initiative.2 Low-dose vaginal estrogen is recommended for treatment of GSM by The North American Menopause Society, the American College of Obstetricians and Gynecologists, and the Endocrine Society.
Absorption of low-dose vaginal estrogen preparations appears minimal, and they are effective and generally safe for the treatment of GSM for women at any age. Progesterone is not recommended with low-dose vaginal estrogen therapies, based primarily on randomized clinical trial safety data of 12 months.3 Postmenopausal bleeding, however, needs to be thoroughly evaluated. For women with breast cancer, include the oncologist in decision making about the use of low-dose vaginal estrogen.
Despite the boxed warning on vaginal estrogen, the findings from this study support the safety of vaginal estrogen use for effective relief of GSM in women with and without a uterus.
JOANN V. PINKERTON, MD, NCMP
- Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;251:704-711.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018;25:11-20.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;251:704-711.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018;25:11-20.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
Managing menopausal vasomotor and genitourinary symptoms after breast cancer
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.
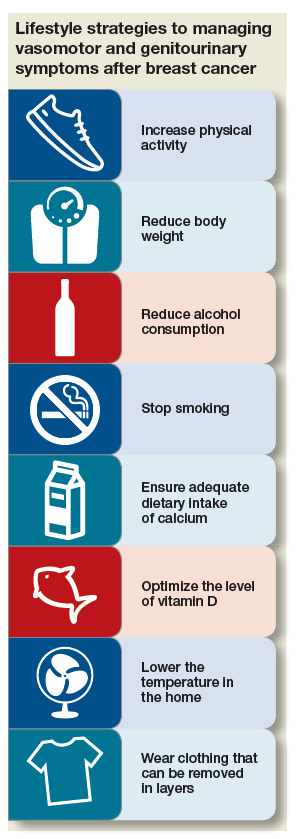
The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.

The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.

The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
Managing menopausal symptoms in breast cancer survivors
Conjugated estrogen plus bazedoxifene—a new approach to estrogen therapy
In this special installment of Cases in Menopause, I interview series contributor and menopause expert JoAnn V. Pinkerton, MD. We discuss a fairly new therapy: the combination conjugated estrogen and bazedoxifene (CE/BZA; Duavee) for the treatment of moderate to severe hot flashes due to menopause and the prevention of menopausal osteoporosis.
Much of my practice has focused on the treatment of menopausal women, but which of my patients can benefit from this particular combination of CE 0.45 mg plus BZA 20 mg? I asked Dr. Pinkerton this question, and more.
Which patients can benefit most?
Dr. Pinkerton CE/BZA was tested in healthy postmenopausal women with a uterus at risk for bone loss who were reporting 50 or more moderate to severe hot flashes per week. The combination of CE and BZA is a good choice for women who have bothersome menopausal symptoms: hot flashes, night sweats, and sleep disruption or symptomatic vulvovaginal atrophy (VVA)—although it’s not approved for VVA.
Efficacy and safety data show that compared with placebo:
- CE/BZA decreases the frequency and severity of hot flashes at 12 weeks, and those decreases are maintained at 12 months.1,2
- Women taking CE/BZA have greater improvements in sleep, with both decreased sleep disturbance and time to fall asleep.3
- CE/BZA maintained or prevented lumbar spine and hip bone loss in postmenopausal women at risk for osteoporosis. 1,4,5
Although fracture data were not captured and the drug was not tested in osteoporotic women, study results showed bone loss prevention at 12 months, which was sustained at 24 months. The improvement in bone mineral density from baseline was about 1% to 1.5%. This was compared with a bone loss of 1.8% in women taking placebo (P<.01).
In clinical studies, women taking CE/BZA versus placebo also have reported a lower incidence of painful intercourse,6 and some improvement in health-related quality of life and treatment satisfaction.7,8
In short, CE/BZA is a good option for symptomatic menopausal women with a uterus who have bothersome hot flashes, night sweats, and sleep disruptions and want to prevent bone loss.
What about adverse effects?
Dr. Pinkerton In general, CE/BZA has a favorable safety and tolerability profile, with an overall incidence of adverse events similar to placebo. The rates of cardiovascular and cerebrovascular events, cancers (breast, endometrial, and ovarian), and mortality are comparable to placebo in 2-year trials. These data are limited; studies have been conducted in healthy postmenopausal women. Future studies need to define the full risk profile, particularly among overweight or obese women and different ethnic groups and for longer-term use.
Is there a role among women with breast cancer?
Dr. Pinkerton CE/BZA has not been tested in women at risk for or with prior breast cancer. In preclinical trials of up to 2 years, involving healthy postmenopausal women, the rates for breast cancer with CE/BZA were similar to placebo. There are no long-term data, however, and there are no data in women at risk for breast cancer. I recommend that women who have or are at high risk for breast cancer consider nonhormonal treatment options.9–11
Has there been an associated increase in breast density with CE/BZA?
Dr. Pinkerton No. Data from two randomized clinical trials showed that the breast density changes with 12-month CE/BZA treatment was similar to placebo—which is markedly different from comparisons of placebo and combination estrogen-progestin therapy (EPT), where EPT increased breast density. If indeed this lack of an association translates into fewer breast cancers, it would be wonderful, but we do not have long-term data. We can tell our patients that using CE/BZA has not been shown to increase the risk of breast cancer, at least up to 2 years.
What makes CE/BZA different from traditional EPT?
Dr. Pinkerton There are two exciting differences:
- The incidences of breast pain and tenderness were found to be similar to placebo, and were significantly less than those with the comparator EPT (conjugated estrogens 0.45 mg plus medroxyprogesterone acetate [CE/MPA] 1.5 mg).9,10,12
- Bleeding and spotting rates were significantly less than those found with CE/MPA.13
In addition, high rates of amenorrhea have been found—comparable to placebo.13
CE/BZA is similar to traditional EPT in several ways. For instance, compared with placebo, at 2 years, CE/BZA was not found to increase the incidence of endometrial hyperplasia, endometrial thickness (increase from baseline was <1 mm and comparable to placebo), or endometrial cancers.14 Lastly, similar to EPT, there is probably a twofold risk of venous thromboembolism (VTE) with BZA 20 mg alone.15 Importantly, there has been no additive effect on VTE risk when combining CE with BZA; however, we will need longer studies, in older women, to fully evaluate this risk.1
Overall, in symptomatic postmenopausal women with a uterus, randomized controlled data show the same improvement with CE/BZA as that seen with traditional oral EPTs, with improvements in hot flashes; night sweats, with fewer sleep disruptions; and prevention of bone loss. In addition, the changes in cholesterol (an increase in triglyceride levels) and effect on the vagina are the same. Yet, CE/BZA appears to have a neutral effect on the breast and protects against endometrial hyperplasia and endometrial cancer without causing bleeding.9,10 CE/BZA’s VTE and stroke risks are expected to be similar to traditional oral EPT.
Therefore, the major benefit of CE/BZA for women who have a uterus is the lack of significant breast tenderness, lack of changes in breast density, and lack of vaginal bleeding that is often seen with traditional EPT.12
Then, is progestogen the harmful agent in traditional HT options?
Dr. Pinkerton There is evidence that estrogen plus progestogen therapy has more risk for breast cancer than estrogen alone. But in women who have a uterus, you need to protect against uterine cancer so, up until now, the only option was to add progestogen. Some studies suggest the risk of breast cancer may differ depending on the type of progestogen. So it’s a laudable goal to try to protect the endometrium without using a progestogen.
Given its safety profile, do you see CE/BZA being indicated for women without a uterus?
Dr. Pinkerton CE/BZA has been tested only in women with a uterus; there is no indication for using it in hysterectomized women. In the future, unless trial data show a benefit to hysterectomized women—by a reduction in breast cancer compared with estrogen alone—there would be no reason to add BZA to the CE for these women. You would just use CE or another type of estrogen alone.
Do you anticipate BZA being used alone?
Dr. Pinkerton For treating osteoporosis in postmenopausal women at increased fracture risk, BZA alone has greater benefits than risks. It is approved in other countries to prevent or treat osteoporosis. In 2008, Wyeth received an approval letter from the US Food and Drug Administration for BZA alone but, for whatever reason, the drug was not brought to market. BZA reduces the number of new lumbar spine fractures by 4% (vs 2% for placebo), with efficacy better in those with a higher risk of fractures. Like raloxifene, it has not been shown effective at reducing nonvertebral fractures, although it maintains spinal bone density.16
BZA available as monotherapy could tempt clinicians to pair it with other estrogens. We must recognize that the combination of the specific estrogen and BZA dose and type need to be balanced to provide endometrial hyperplasia protection. It would not be safe or effective to take BZA as a selective estrogen-receptor modulator and pair it with any other untested systemic estrogen. I do not anticipate, in this country, that BZA will become available as monotherapy.
New options are welcome
Dr. Moore Novel strategies for clinicians to optimally treat menopausal symptoms are always welcome. I look forward to more data from the SMART trials on CE/BZA and to moving forward as we gain experience with using this new treatment option.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic bone parameters and overall safety profile. Fertil Steril. 2009;92(3):1025–1038.
2. Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens. Menopause. 2009;16:(6)1116–1124.
3. Pinkerton JV, Pan K, Abraham L, et al. Sleep parameters and health-related quality of life with bazedoxifene/conjugated estrogens. Menopause. 2014;21(3):252–259.
4. Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex (TSEC) of bazedoxifene/conjugated estrogens (BZA/CE) for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92(3):1045–1052.
5. Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone. J Clin Endocrinol Metab. 2014;99(2):E189–E198.
6. Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens (BZA/CE) for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause. 2010;17(2):281–289.
7. Utian W, Yu H, Bobula J, Mirkin S, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens and quality of life in postmenopausal women. Maturitas. 2009;63:(4)329–335.
8. Abraham L, Pinkerton JV, Messig M, Ryan KA, Komm BS, Mirkin S. Menopause-specific quality of life across varying menopausal populations with conjugated estrogens/bazedoxifene. Maturitas. 2014;78(3):212–218.
9. Harvey JA, Pinkerton JV, Baracat EC, Shi H, Chines AA, Mirkin S. Breast density changes in a randomized controlled trial evaluating bazedoxifene/conjugated estrogens. Menopause. 2013;20:(2)138–145.
10. Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens. Obstet Gynecol. 2013;121(5):959–968.
11. Kaunitz AM. When should a menopausal woman discontinue hormone therapy? OBG Manag. 2014;26(2):59–65.
12. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15:(5)411–418.
13. Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril. 2009;92:1039–1044.
14. Pickar JH, Yeh I-T, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex (TSEC) containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril. 2009; 92(3):1018–1024.
15. Mirkin S, Komm BS. Tissue-selective estrogen complexes for postmenopausal women. Maturitas. 2013;76(3):213–220.
16. Ellis AG, Reginster JY, Luo X, et al. Bazedoxifene versus oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women with osteoporosis at higher risk of fracture. Value Health. 2014;17(4):424–432.
In this special installment of Cases in Menopause, I interview series contributor and menopause expert JoAnn V. Pinkerton, MD. We discuss a fairly new therapy: the combination conjugated estrogen and bazedoxifene (CE/BZA; Duavee) for the treatment of moderate to severe hot flashes due to menopause and the prevention of menopausal osteoporosis.
Much of my practice has focused on the treatment of menopausal women, but which of my patients can benefit from this particular combination of CE 0.45 mg plus BZA 20 mg? I asked Dr. Pinkerton this question, and more.
Which patients can benefit most?
Dr. Pinkerton CE/BZA was tested in healthy postmenopausal women with a uterus at risk for bone loss who were reporting 50 or more moderate to severe hot flashes per week. The combination of CE and BZA is a good choice for women who have bothersome menopausal symptoms: hot flashes, night sweats, and sleep disruption or symptomatic vulvovaginal atrophy (VVA)—although it’s not approved for VVA.
Efficacy and safety data show that compared with placebo:
- CE/BZA decreases the frequency and severity of hot flashes at 12 weeks, and those decreases are maintained at 12 months.1,2
- Women taking CE/BZA have greater improvements in sleep, with both decreased sleep disturbance and time to fall asleep.3
- CE/BZA maintained or prevented lumbar spine and hip bone loss in postmenopausal women at risk for osteoporosis. 1,4,5
Although fracture data were not captured and the drug was not tested in osteoporotic women, study results showed bone loss prevention at 12 months, which was sustained at 24 months. The improvement in bone mineral density from baseline was about 1% to 1.5%. This was compared with a bone loss of 1.8% in women taking placebo (P<.01).
In clinical studies, women taking CE/BZA versus placebo also have reported a lower incidence of painful intercourse,6 and some improvement in health-related quality of life and treatment satisfaction.7,8
In short, CE/BZA is a good option for symptomatic menopausal women with a uterus who have bothersome hot flashes, night sweats, and sleep disruptions and want to prevent bone loss.
What about adverse effects?
Dr. Pinkerton In general, CE/BZA has a favorable safety and tolerability profile, with an overall incidence of adverse events similar to placebo. The rates of cardiovascular and cerebrovascular events, cancers (breast, endometrial, and ovarian), and mortality are comparable to placebo in 2-year trials. These data are limited; studies have been conducted in healthy postmenopausal women. Future studies need to define the full risk profile, particularly among overweight or obese women and different ethnic groups and for longer-term use.
Is there a role among women with breast cancer?
Dr. Pinkerton CE/BZA has not been tested in women at risk for or with prior breast cancer. In preclinical trials of up to 2 years, involving healthy postmenopausal women, the rates for breast cancer with CE/BZA were similar to placebo. There are no long-term data, however, and there are no data in women at risk for breast cancer. I recommend that women who have or are at high risk for breast cancer consider nonhormonal treatment options.9–11
Has there been an associated increase in breast density with CE/BZA?
Dr. Pinkerton No. Data from two randomized clinical trials showed that the breast density changes with 12-month CE/BZA treatment was similar to placebo—which is markedly different from comparisons of placebo and combination estrogen-progestin therapy (EPT), where EPT increased breast density. If indeed this lack of an association translates into fewer breast cancers, it would be wonderful, but we do not have long-term data. We can tell our patients that using CE/BZA has not been shown to increase the risk of breast cancer, at least up to 2 years.
What makes CE/BZA different from traditional EPT?
Dr. Pinkerton There are two exciting differences:
- The incidences of breast pain and tenderness were found to be similar to placebo, and were significantly less than those with the comparator EPT (conjugated estrogens 0.45 mg plus medroxyprogesterone acetate [CE/MPA] 1.5 mg).9,10,12
- Bleeding and spotting rates were significantly less than those found with CE/MPA.13
In addition, high rates of amenorrhea have been found—comparable to placebo.13
CE/BZA is similar to traditional EPT in several ways. For instance, compared with placebo, at 2 years, CE/BZA was not found to increase the incidence of endometrial hyperplasia, endometrial thickness (increase from baseline was <1 mm and comparable to placebo), or endometrial cancers.14 Lastly, similar to EPT, there is probably a twofold risk of venous thromboembolism (VTE) with BZA 20 mg alone.15 Importantly, there has been no additive effect on VTE risk when combining CE with BZA; however, we will need longer studies, in older women, to fully evaluate this risk.1
Overall, in symptomatic postmenopausal women with a uterus, randomized controlled data show the same improvement with CE/BZA as that seen with traditional oral EPTs, with improvements in hot flashes; night sweats, with fewer sleep disruptions; and prevention of bone loss. In addition, the changes in cholesterol (an increase in triglyceride levels) and effect on the vagina are the same. Yet, CE/BZA appears to have a neutral effect on the breast and protects against endometrial hyperplasia and endometrial cancer without causing bleeding.9,10 CE/BZA’s VTE and stroke risks are expected to be similar to traditional oral EPT.
Therefore, the major benefit of CE/BZA for women who have a uterus is the lack of significant breast tenderness, lack of changes in breast density, and lack of vaginal bleeding that is often seen with traditional EPT.12
Then, is progestogen the harmful agent in traditional HT options?
Dr. Pinkerton There is evidence that estrogen plus progestogen therapy has more risk for breast cancer than estrogen alone. But in women who have a uterus, you need to protect against uterine cancer so, up until now, the only option was to add progestogen. Some studies suggest the risk of breast cancer may differ depending on the type of progestogen. So it’s a laudable goal to try to protect the endometrium without using a progestogen.
Given its safety profile, do you see CE/BZA being indicated for women without a uterus?
Dr. Pinkerton CE/BZA has been tested only in women with a uterus; there is no indication for using it in hysterectomized women. In the future, unless trial data show a benefit to hysterectomized women—by a reduction in breast cancer compared with estrogen alone—there would be no reason to add BZA to the CE for these women. You would just use CE or another type of estrogen alone.
Do you anticipate BZA being used alone?
Dr. Pinkerton For treating osteoporosis in postmenopausal women at increased fracture risk, BZA alone has greater benefits than risks. It is approved in other countries to prevent or treat osteoporosis. In 2008, Wyeth received an approval letter from the US Food and Drug Administration for BZA alone but, for whatever reason, the drug was not brought to market. BZA reduces the number of new lumbar spine fractures by 4% (vs 2% for placebo), with efficacy better in those with a higher risk of fractures. Like raloxifene, it has not been shown effective at reducing nonvertebral fractures, although it maintains spinal bone density.16
BZA available as monotherapy could tempt clinicians to pair it with other estrogens. We must recognize that the combination of the specific estrogen and BZA dose and type need to be balanced to provide endometrial hyperplasia protection. It would not be safe or effective to take BZA as a selective estrogen-receptor modulator and pair it with any other untested systemic estrogen. I do not anticipate, in this country, that BZA will become available as monotherapy.
New options are welcome
Dr. Moore Novel strategies for clinicians to optimally treat menopausal symptoms are always welcome. I look forward to more data from the SMART trials on CE/BZA and to moving forward as we gain experience with using this new treatment option.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this special installment of Cases in Menopause, I interview series contributor and menopause expert JoAnn V. Pinkerton, MD. We discuss a fairly new therapy: the combination conjugated estrogen and bazedoxifene (CE/BZA; Duavee) for the treatment of moderate to severe hot flashes due to menopause and the prevention of menopausal osteoporosis.
Much of my practice has focused on the treatment of menopausal women, but which of my patients can benefit from this particular combination of CE 0.45 mg plus BZA 20 mg? I asked Dr. Pinkerton this question, and more.
Which patients can benefit most?
Dr. Pinkerton CE/BZA was tested in healthy postmenopausal women with a uterus at risk for bone loss who were reporting 50 or more moderate to severe hot flashes per week. The combination of CE and BZA is a good choice for women who have bothersome menopausal symptoms: hot flashes, night sweats, and sleep disruption or symptomatic vulvovaginal atrophy (VVA)—although it’s not approved for VVA.
Efficacy and safety data show that compared with placebo:
- CE/BZA decreases the frequency and severity of hot flashes at 12 weeks, and those decreases are maintained at 12 months.1,2
- Women taking CE/BZA have greater improvements in sleep, with both decreased sleep disturbance and time to fall asleep.3
- CE/BZA maintained or prevented lumbar spine and hip bone loss in postmenopausal women at risk for osteoporosis. 1,4,5
Although fracture data were not captured and the drug was not tested in osteoporotic women, study results showed bone loss prevention at 12 months, which was sustained at 24 months. The improvement in bone mineral density from baseline was about 1% to 1.5%. This was compared with a bone loss of 1.8% in women taking placebo (P<.01).
In clinical studies, women taking CE/BZA versus placebo also have reported a lower incidence of painful intercourse,6 and some improvement in health-related quality of life and treatment satisfaction.7,8
In short, CE/BZA is a good option for symptomatic menopausal women with a uterus who have bothersome hot flashes, night sweats, and sleep disruptions and want to prevent bone loss.
What about adverse effects?
Dr. Pinkerton In general, CE/BZA has a favorable safety and tolerability profile, with an overall incidence of adverse events similar to placebo. The rates of cardiovascular and cerebrovascular events, cancers (breast, endometrial, and ovarian), and mortality are comparable to placebo in 2-year trials. These data are limited; studies have been conducted in healthy postmenopausal women. Future studies need to define the full risk profile, particularly among overweight or obese women and different ethnic groups and for longer-term use.
Is there a role among women with breast cancer?
Dr. Pinkerton CE/BZA has not been tested in women at risk for or with prior breast cancer. In preclinical trials of up to 2 years, involving healthy postmenopausal women, the rates for breast cancer with CE/BZA were similar to placebo. There are no long-term data, however, and there are no data in women at risk for breast cancer. I recommend that women who have or are at high risk for breast cancer consider nonhormonal treatment options.9–11
Has there been an associated increase in breast density with CE/BZA?
Dr. Pinkerton No. Data from two randomized clinical trials showed that the breast density changes with 12-month CE/BZA treatment was similar to placebo—which is markedly different from comparisons of placebo and combination estrogen-progestin therapy (EPT), where EPT increased breast density. If indeed this lack of an association translates into fewer breast cancers, it would be wonderful, but we do not have long-term data. We can tell our patients that using CE/BZA has not been shown to increase the risk of breast cancer, at least up to 2 years.
What makes CE/BZA different from traditional EPT?
Dr. Pinkerton There are two exciting differences:
- The incidences of breast pain and tenderness were found to be similar to placebo, and were significantly less than those with the comparator EPT (conjugated estrogens 0.45 mg plus medroxyprogesterone acetate [CE/MPA] 1.5 mg).9,10,12
- Bleeding and spotting rates were significantly less than those found with CE/MPA.13
In addition, high rates of amenorrhea have been found—comparable to placebo.13
CE/BZA is similar to traditional EPT in several ways. For instance, compared with placebo, at 2 years, CE/BZA was not found to increase the incidence of endometrial hyperplasia, endometrial thickness (increase from baseline was <1 mm and comparable to placebo), or endometrial cancers.14 Lastly, similar to EPT, there is probably a twofold risk of venous thromboembolism (VTE) with BZA 20 mg alone.15 Importantly, there has been no additive effect on VTE risk when combining CE with BZA; however, we will need longer studies, in older women, to fully evaluate this risk.1
Overall, in symptomatic postmenopausal women with a uterus, randomized controlled data show the same improvement with CE/BZA as that seen with traditional oral EPTs, with improvements in hot flashes; night sweats, with fewer sleep disruptions; and prevention of bone loss. In addition, the changes in cholesterol (an increase in triglyceride levels) and effect on the vagina are the same. Yet, CE/BZA appears to have a neutral effect on the breast and protects against endometrial hyperplasia and endometrial cancer without causing bleeding.9,10 CE/BZA’s VTE and stroke risks are expected to be similar to traditional oral EPT.
Therefore, the major benefit of CE/BZA for women who have a uterus is the lack of significant breast tenderness, lack of changes in breast density, and lack of vaginal bleeding that is often seen with traditional EPT.12
Then, is progestogen the harmful agent in traditional HT options?
Dr. Pinkerton There is evidence that estrogen plus progestogen therapy has more risk for breast cancer than estrogen alone. But in women who have a uterus, you need to protect against uterine cancer so, up until now, the only option was to add progestogen. Some studies suggest the risk of breast cancer may differ depending on the type of progestogen. So it’s a laudable goal to try to protect the endometrium without using a progestogen.
Given its safety profile, do you see CE/BZA being indicated for women without a uterus?
Dr. Pinkerton CE/BZA has been tested only in women with a uterus; there is no indication for using it in hysterectomized women. In the future, unless trial data show a benefit to hysterectomized women—by a reduction in breast cancer compared with estrogen alone—there would be no reason to add BZA to the CE for these women. You would just use CE or another type of estrogen alone.
Do you anticipate BZA being used alone?
Dr. Pinkerton For treating osteoporosis in postmenopausal women at increased fracture risk, BZA alone has greater benefits than risks. It is approved in other countries to prevent or treat osteoporosis. In 2008, Wyeth received an approval letter from the US Food and Drug Administration for BZA alone but, for whatever reason, the drug was not brought to market. BZA reduces the number of new lumbar spine fractures by 4% (vs 2% for placebo), with efficacy better in those with a higher risk of fractures. Like raloxifene, it has not been shown effective at reducing nonvertebral fractures, although it maintains spinal bone density.16
BZA available as monotherapy could tempt clinicians to pair it with other estrogens. We must recognize that the combination of the specific estrogen and BZA dose and type need to be balanced to provide endometrial hyperplasia protection. It would not be safe or effective to take BZA as a selective estrogen-receptor modulator and pair it with any other untested systemic estrogen. I do not anticipate, in this country, that BZA will become available as monotherapy.
New options are welcome
Dr. Moore Novel strategies for clinicians to optimally treat menopausal symptoms are always welcome. I look forward to more data from the SMART trials on CE/BZA and to moving forward as we gain experience with using this new treatment option.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic bone parameters and overall safety profile. Fertil Steril. 2009;92(3):1025–1038.
2. Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens. Menopause. 2009;16:(6)1116–1124.
3. Pinkerton JV, Pan K, Abraham L, et al. Sleep parameters and health-related quality of life with bazedoxifene/conjugated estrogens. Menopause. 2014;21(3):252–259.
4. Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex (TSEC) of bazedoxifene/conjugated estrogens (BZA/CE) for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92(3):1045–1052.
5. Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone. J Clin Endocrinol Metab. 2014;99(2):E189–E198.
6. Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens (BZA/CE) for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause. 2010;17(2):281–289.
7. Utian W, Yu H, Bobula J, Mirkin S, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens and quality of life in postmenopausal women. Maturitas. 2009;63:(4)329–335.
8. Abraham L, Pinkerton JV, Messig M, Ryan KA, Komm BS, Mirkin S. Menopause-specific quality of life across varying menopausal populations with conjugated estrogens/bazedoxifene. Maturitas. 2014;78(3):212–218.
9. Harvey JA, Pinkerton JV, Baracat EC, Shi H, Chines AA, Mirkin S. Breast density changes in a randomized controlled trial evaluating bazedoxifene/conjugated estrogens. Menopause. 2013;20:(2)138–145.
10. Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens. Obstet Gynecol. 2013;121(5):959–968.
11. Kaunitz AM. When should a menopausal woman discontinue hormone therapy? OBG Manag. 2014;26(2):59–65.
12. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15:(5)411–418.
13. Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril. 2009;92:1039–1044.
14. Pickar JH, Yeh I-T, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex (TSEC) containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril. 2009; 92(3):1018–1024.
15. Mirkin S, Komm BS. Tissue-selective estrogen complexes for postmenopausal women. Maturitas. 2013;76(3):213–220.
16. Ellis AG, Reginster JY, Luo X, et al. Bazedoxifene versus oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women with osteoporosis at higher risk of fracture. Value Health. 2014;17(4):424–432.
1. Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic bone parameters and overall safety profile. Fertil Steril. 2009;92(3):1025–1038.
2. Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens. Menopause. 2009;16:(6)1116–1124.
3. Pinkerton JV, Pan K, Abraham L, et al. Sleep parameters and health-related quality of life with bazedoxifene/conjugated estrogens. Menopause. 2014;21(3):252–259.
4. Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex (TSEC) of bazedoxifene/conjugated estrogens (BZA/CE) for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92(3):1045–1052.
5. Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone. J Clin Endocrinol Metab. 2014;99(2):E189–E198.
6. Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens (BZA/CE) for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause. 2010;17(2):281–289.
7. Utian W, Yu H, Bobula J, Mirkin S, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens and quality of life in postmenopausal women. Maturitas. 2009;63:(4)329–335.
8. Abraham L, Pinkerton JV, Messig M, Ryan KA, Komm BS, Mirkin S. Menopause-specific quality of life across varying menopausal populations with conjugated estrogens/bazedoxifene. Maturitas. 2014;78(3):212–218.
9. Harvey JA, Pinkerton JV, Baracat EC, Shi H, Chines AA, Mirkin S. Breast density changes in a randomized controlled trial evaluating bazedoxifene/conjugated estrogens. Menopause. 2013;20:(2)138–145.
10. Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens. Obstet Gynecol. 2013;121(5):959–968.
11. Kaunitz AM. When should a menopausal woman discontinue hormone therapy? OBG Manag. 2014;26(2):59–65.
12. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15:(5)411–418.
13. Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril. 2009;92:1039–1044.
14. Pickar JH, Yeh I-T, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex (TSEC) containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril. 2009; 92(3):1018–1024.
15. Mirkin S, Komm BS. Tissue-selective estrogen complexes for postmenopausal women. Maturitas. 2013;76(3):213–220.
16. Ellis AG, Reginster JY, Luo X, et al. Bazedoxifene versus oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women with osteoporosis at higher risk of fracture. Value Health. 2014;17(4):424–432.
How I screen patients at increased risk for breast cancer
Related article: Your menopausal patient’s breast biopsy reveals atypical hyperplasia JoAnn V. Pinkerton, MD; Andrew M. Kaunitz, MD, James A. Simon, MD (Cases in Menopause, May 2013)
Related article: Your menopausal patient’s breast biopsy reveals atypical hyperplasia JoAnn V. Pinkerton, MD; Andrew M. Kaunitz, MD, James A. Simon, MD (Cases in Menopause, May 2013)
Related article: Your menopausal patient’s breast biopsy reveals atypical hyperplasia JoAnn V. Pinkerton, MD; Andrew M. Kaunitz, MD, James A. Simon, MD (Cases in Menopause, May 2013)
Your menopausal patient’s breast biopsy reveals atypical hyperplasia
A new series brought to you by the menopause experts

| Andrew M. Kaunitz, MD Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a NAMS Board member and certified menopause practitioner. He also serves on the OBG Management Board of Editors. |

| JoAnn V. Pinkerton, MD Professor, Department of Obstetrics and Gynecology, and Director, Division of Midlife Women’s Health, University of Virginia. Dr. Pinkerton is a North American Menopause Society (NAMS) past president and certified menopause practitioner. She also serves on the OBG Management Board of Editors. |

| James A. Simon, MD Clinical Professor, Department of Obstetrics and Gynecology, George Washington University, and Medical Director, Women’s Health & Research Consultants, Washington, DC. Dr. Simon is a NAMS past president, a certified menopause practitioner, and a certified clinical densitometrist. He also serves on the OBG Management Board of Editors. |
Disclosures
Dr. Kaunitz reports that his institution receives grant or research support from Agile, Bayer, Endoceutics, Teva, Medical Diagnostic Laboratories, and Noven, and that he is a consultant to Bayer, Merck, and Teva.
Dr. Pinkerton reports that her institution receives consulting fees from Pfizer, DepoMed, Shionogi, and Noven and multicenter research fees from DepoMed, Endoceutics, and Bionova.
Dr. Simon reports being a consultant to or on the advisory boards of Abbott Laboratories, Agile Therapeutics, Amgen, Ascend Therapeutics, BioSante, Depomed, Lelo, MD Therapeutics, Meda Pharmaceuticals, Merck, Noven, Novo Nordisk, Novogyne, Pfizer, Shionogi, Shippan Point Advisors LLC, Slate Pharmaceuticals, Sprout Pharmaceuticals, Teva, Warner Chilcott, and Watson. He also reports receiving (currently or in the past year) grant/research support from BioSante, EndoCeutics, Novo Nordisk, Novogyne, Palatin Technologies, Teva, and Warner Chilcott. He reports serving on the speakers bureaus of Amgen, Merck, Novartis, Noven, Novo Nordisk, Novogyne, Teva, and Warner Chilcott. Dr. Simon is currently the Chief Medical Officer for Sprout Pharmaceuticals.
CASE: Atypical ductal hyperplasia
Your 56-year-old, married, white patient has been on hormone therapy (HT) since age 52 for the treatment of vasomotor symptoms. She is taking a low-dose oral estrogen and micronized progesterone combination as she has an intact uterus. Her family history is positive for breast cancer, as her mother was diagnosed at age 68.
Her most recent annual screening mammogram shows linear calcifications. Because fine, linear, branching or casting calcifications are worrisome for atypical ductal hyperplasia (ADH) or ductal carcinoma in situ, a biopsy is recommended.
She elects to wean off and discontinue HT during the evaluation of her abnormal mammogram. The mammographic-guided stereotactic biopsy reveals ADH. She undergoes an open excisional biopsy, the results of which reveal extensive ADH with negative margins.
Six weeks after a lumpectomy she returns to your office reporting moderate to severe hot flashes that occur seven to 10 times per day and impair her sleep, leading to fatigue and “brain fog.” In addition, she is noticing vaginal dryness and dyspareunia despite use of lubricants. She requests treatment for her symptoms and wonders if she can restart HT systemically or vaginally.
How do you manage her hot flashes?
What are the alternatives to HT for hot flashes?
Certain lifestyle changes have been reported to provide relief for hot flushes.1 These include:
- use of layered clothing
- maintenance of cool ambient temperature (particularly during sleep)
- consumption of cool foods or beverages
- relaxation techniques (such as deep breathing, or paced respirations, for 20 min three times per day).
Despite sparse data, avoiding triggers such as spicy or hot foods or alcohol may be helpful.
Therapies such as evening primrose oil, dong quai, ginseng, wild yam, magnet therapy, reflexology, and homeopathy have not been found more effective in treating hot flashes than placebo.2
Phytoestrogens (such as equol), acupuncture, yoga, and hypnosis continue to be tested in randomized trials with mixed results.
Off-label drug options offer modest help. There are currently no FDA-approved nonhormonal pharmaceutical options for relief of hot flashes; the gold standard for treatment remains estrogen therapy. For moderate to severe bothersome hot flashes, potentially effective drug therapies used off label include clonidine, selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs), and gabapentin (TABLE 1).2,4 In large, randomized, controlled trials, the following agents were modestly more effective than placebo: desvenlafaxine,5 low-dose paroxetine salt,6 escitalopram,7 and gastroretentive gabapentin.8 Participants in these trials included women with both spontaneous and surgically induced menopause.
Although sponsors have applied for approval for three of these agents, the FDA so far has declined to approve these agents for vasomotor treatment due to concerns about risks versus benefits. Benefits of these nonhormonal prescription therapies need to be weighed carefully against side effects, because the reduction in absolute hot flushes is modest.
Many small trials have assessed other medications and complementary and alternative therapies regarding management of menopausal symptoms. Most, however, are limited by small numbers of enrolled participants and shorter study duration (≤12 weeks). In addition, enrolled participants have variable numbers of hot flashes, often less than 14 per week.2,4
TABLE 1
Nonhormonal treatment of vasomotor symptoms
| Treatment | Study Design* | Findings |
|---|---|---|
| Complementary/alternative medicines (black cohosh, St. John’s Wort, red clover, acupuncture, exercise) | Duration: 4–52 wk; OL and RPL trials; entry criteria for most trials: >14 hot flashes/wk | Mixed results, mostly with no sustained improvement |
| SSRIs** (paroxetine, fluoxetine, sertraline, citalopram, escitalopram) | Duration: 4–36 wk; RPL trials with all agents; N = 20–90 in active arms; entry criteria for most trials: >14 hot flashes/wk | Reduction in vasomotor symptoms (frequency, composite scores): 28%–55% |
| SNRIs** (venlafaxine, desvenlafaxine) | Duration, 12–52 wk; RPL trials with all agents; N = 20–65 in VEN; N = 120–200 in DVS; Entry criteria >14 hot flashes/wk for VEN; >50/wk for DVS | Reduction in VMS (frequency, composite scores): 35%–58% for VEN, 55%–68% for DVS |
| Gabapentin** | Duration: 4–12 wk; RPL trials; N = 20–100; entry criteria for most trials: >14–50 hot flashes/wk | Reduction in vasomotor symptoms (frequency, composite scores): 50%–70% |
| *All studies of menopausal, nondepressed women. **Treatment is off label. Hall E, et al. Drugs. 2011;71:287-304. Reprinted with permission. Pinkerton JV, Shapiro M. The North American Menopause Society. Overview of available treatment options for VMS and VVA. Medscape Education Web site. http://www.medscape.org/viewarticle/763413. Published May 24, 2012. Accessed February 23, 2013.19 | ||
Can your patient restart HT? If so, should HT be offered vaginally or systemically?
Non-HT may be enough for vaginal dryness. Benefit has been shown with the use of vaginal moisturizers twice weekly and lubricants as needed for sexual activity.9 Therefore, the local application of daily lubricants, such as olive oil, along with the use of moisturizers with regular sexual intercourse may be enough to maintain vaginal health and function.
In randomized trials, phytoestrogens lack benefit data for vaginal atrophy. Small pilot studies of the effect of oral/ vaginal phytoestrogens on vaginal atrophy do not show any benefit on vaginal pH or vaginal maturation index and mixed improvement in vaginal dryness. In addition, no clear effect of these agents has been seen compared with placebo, except that there may be less progression of vaginal atrophy over time with phytoestrogens.10 It is possible that the benefits of phytoestrogens may take longer to take effect than the 12 weeks required to see an effect with HT.
Vaginal estrogen: limited safety data. No published clinical trials have assessed the impact of topical vaginal estrogen on risk of recurrence in breast cancer survivors, and concern exists because detectable estradiol levels have been reported in women who take aromatase inhibitors and have very atrophic vaginal mucosa.11 NAMS recommends that the discussion about vaginal estrogen be individualized between the patient, her provider, and her oncologist.12
Vaginal estrogen creams and tablets (Vagifem 10 μg per tablet) are often started daily for 2 weeks for a “priming dose” then dosed twice per week. To minimize systemic absorption, creams may be used externally or with smaller doses vaginally. The higher the dose or more frequent the use, the greater the risk of significant systemic absorption, particularly when the vagina is atrophic.13
Another option is the vaginal estradiol ring, which delivers a low dose (7.5 μg per day) for 90 days.14,15
Progestogen therapy is generally not needed when low-dose estrogen is administered locally to treat vaginal atrophy.12
A new oral option. In February 2013, ospemifene, a selective estrogen receptor modulator (SERM), was approved for pain with intercourse and vaginal dryness.
Related article: “New treatment option for vulvar and vaginal atrophy,” by Andrew M. Kaunitz, MD (May 2013)
There is concern with systemic estrogen use
If her hot flashes remain persistent and bothersome, low-dose estrogen could be considered. However, data from the Breast Cancer Surveillance Consortium9 showed that as postmenopausal HT use decreased (from 35% to 11% between 1999 and 2005), rates of ADH decreased from a peak of 5.5 per 10,000 mammograms in 1999 to 2.4 per 10,000 mammograms in 2005. Similarly, rates of invasive cancer with ADH decreased from a peak of 4.3 per 10,000 mammograms in 2003 to 3.3 per 10,000 mammograms in 2005. This finding—that rates of ADH and invasive breast cancer were significantly linked to postmenopausal use of HT—raises concern about using HT in women with prior ADH. Of note, the cancers linked with ADH were of lower grade and stage and more estrogen receptor–positive than cancers not linked with ADH.17
How do you manage your patient’s increased risk of breast cancer?
In examining the answer to this management point, we need to first ask and answer, “How does ADH develop?” and “What is her risk of developing breast cancer?”
The development of invasive breast cancer is believed to involve a complex, multistep process. Initially, there is disruption of normal cell development and growth, with overproduction of normal-looking cells (hyperplasia). These excess cells stack up and/or become abnormal. Then, there is continued change in appearance and multiplication, becoming ductal carcinoma in situ (noninvasive). If left untreated, the cells may develop into invasive cancer.18 See TABLE 2 for the relative risk of a patient with ADH developing breast cancer.10
Now, we can address, “How do you manage this patient’s increased risk of breast cancer?”
TABLE 2. Relative risk of developing breast cancer
| Risk | Relative Risk |
|---|---|
| Atypical ductal hyperplasia | 4-5 |
| Atypical ductal hyperplasia and positive family history | 6-8 |
More frequent breast screening!
- Clinical breast exams twice per year
- Screening mammograms annually
- Screening tomosynthesis (These are additional digital screening views which provide almost a 3D view.)
- Screening breast ultrasound
- Screening breast MRI — if she has a 20% lifetime risk of breast cancer (family history or genetic predisposition) (TABLE 3).
TABLE 3. ACS guidelines for screening breast MRI
| Risk | Recommendation |
|---|---|
| <15% lifetime risk | MRI not recommended |
| 15% to 20% lifetime risk | Talk about benefits and limitations of MRI screening |
| >20% lifetime risk | Annual mammogram and annual MRI alternating every 6 months |
Careful consideration of medications
Since it is possible that estrogen may fuel the growth of some breast cancers, avoiding systemic menopausal HT may be safest.
Counsel her about strategies to reduce breast cancer risk
These include:
- Lifestyle changes, including weight loss, exercise, and avoiding excess alcohol intake.
- Preventive medications. Tamoxifen or raloxifene (Evista) can be used for 5 years. These medications block estrogen from binding to the breast estrogen receptors. Another option is an aromatase inhibitor, which decreases estrogen production.
- Risk-reducing (prophylactic) mastectomy.
Management approach for this patient
This patient has had her ADH surgically excised. She will remain at higher risk for breast cancer and should consider strategies to decrease her risk, including lifestyle changes and the possible initiation of medications such as tamoxifen or raloxifene. New screening modalities, such as tomosynthesis or breast ultrasound, may be used to screen for breast cancer, and she may be a candidate for alternating mammograms and MRIs at 6-month intervals.
For her vaginal dryness, over-the-counter lubricants and moisturizers may be helpful. If not, topical or vaginal estrogen is available (as creams, tablets, or a ring) and provides primarily local benefit with limited systemic absorption.
For her bothersome hot flashes, if lifestyle changes don’t work, nonhormonal therapies can be offered off label, such as effexor, desvenlafaxine, gabapentin, or any of the SSRIs—including those tested in large, randomized, controlled trials, such as escitalopram and low-dose paroxetine salt, at low doses.
If she is taking tamoxifen, however, SSRIs such as paroxetine should be avoided due to P450 interaction.
If her hot flashes remain persistent and bothersome, low-dose estrogen could be considered, with education about the potential risks, as she is already at higher risk for breast cancer.
Acknowledgment
The author would like to thank Andrew M. Kaunitz, MD, for his forward thinking in helping to establish this new series on menopause.
Suggest it to our expert panel. They may address your management dilemma in a future issue!
Email us at [email protected] or send a Letter to the Editor to [email protected]
1. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause. 2004;11(1):11-33.
2. Pinkerton JV, Stovall DW, Kightlinger RS. Advances in the treatment of menopausal symptoms. Womens Health (Lond Engl). 2009;5(4):361-384.
3. Ghaleiha A, Jahangard L, Sherafat Z, et al. Oxybutynin reduces sweating in depressed patients treated with sertraline: a double-blink, placebo-controlled, clinical study. Neuropsychiatr Dis Treat. 2012;8:407-412.
4. Hall E, Frey BN, Soares CN. Non-hormonal treatment strategies for vasomotor symptoms: a critical review. Drugs. 2011;71(3):287-304.
5. Pinkerton JV, Archer DF, Guico-Pabia CJ, Hwang E, Cheng RF. Maintenance of the efficacy of desvenlafaxine in menopausal vasomotor symptoms: a 1-year randomized controlled trial. Menopause. 2013;20(1):38-46.
6. Simon JA, Sanacora G, Bhaskar S, Lippman J. Safety and efficacy of low-dose mesylate salt of paroxetine (LDMP) for the treatment of vasomotor symptoms (VMS) associated with menopause: a 24-week randomized, placebo-controlled phase 3 study. Menopause. 2012;19(12):1371.-
7. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267-274.
8. Pinkerton JV, Kagan R, Portman D, Sathyanarayan RK, Sweeney M. Efficacy of gabapentin extended release in the treatment of menopausal hot flashes: results of the Breeze 3 study. Menopause. 2012;19(12):1377.-
9. MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85(1):87-94.
10. Bedell S, Nachtigall M, Naftolin F. The pros and cons of plant estrogens for menopause [published online ahead of print December 25 2012]. J Steroid Biochem Mol Biol. 2012. doi: 10.1016/j.jsbmb.2012.12.004.
11. Moegele M, Buchholz S, Seitz S, Ortmann O. Vaginal estrogen therapy in postmenopausal breast cancer patients treated with aromatase inhibitors. Arch Gynecol Obstet. 2012;285(5):1397-402.
12. North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14(3 Pt 1):355-371.
13. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2010;17(1):194-203.
14. Simon JA. Vulvovaginal atrophy: new and upcoming approaches. Menopause. 2009;16(1):5-7.
15. Kaunitz AM. Transdermal and vaginal estradiol for the treatment of menopausal symptoms: the nuts and bolts. Menopause. 2012;19(6):602-603.
16. Pruthi S, Simon JA, Early AP. Current overview of the management of urogenital atrophy in women with breast cancer. Breast J. 2011;17(4):403-408.
17. Menes TS, Kerlikowske K, Jaffer S, Seger D, Miglioretti D. Rates of atypical ductal hyperplasia declined with less use of postmenopausal hormone treatment: findings from the Breast Cancer Surveillance Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2822-2828.
18. Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353(3):275-285.
19. Pinkerton JV, Shapiro M. The North American Menopause Society. Overview of available treatment options for VMS and VVA. Medscape Education Web site. http://www.medscape.org/viewarticle/763413. Published May 24 2012. Accessed February 23, 2013.
A new series brought to you by the menopause experts

| Andrew M. Kaunitz, MD Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a NAMS Board member and certified menopause practitioner. He also serves on the OBG Management Board of Editors. |

| JoAnn V. Pinkerton, MD Professor, Department of Obstetrics and Gynecology, and Director, Division of Midlife Women’s Health, University of Virginia. Dr. Pinkerton is a North American Menopause Society (NAMS) past president and certified menopause practitioner. She also serves on the OBG Management Board of Editors. |

| James A. Simon, MD Clinical Professor, Department of Obstetrics and Gynecology, George Washington University, and Medical Director, Women’s Health & Research Consultants, Washington, DC. Dr. Simon is a NAMS past president, a certified menopause practitioner, and a certified clinical densitometrist. He also serves on the OBG Management Board of Editors. |
Disclosures
Dr. Kaunitz reports that his institution receives grant or research support from Agile, Bayer, Endoceutics, Teva, Medical Diagnostic Laboratories, and Noven, and that he is a consultant to Bayer, Merck, and Teva.
Dr. Pinkerton reports that her institution receives consulting fees from Pfizer, DepoMed, Shionogi, and Noven and multicenter research fees from DepoMed, Endoceutics, and Bionova.
Dr. Simon reports being a consultant to or on the advisory boards of Abbott Laboratories, Agile Therapeutics, Amgen, Ascend Therapeutics, BioSante, Depomed, Lelo, MD Therapeutics, Meda Pharmaceuticals, Merck, Noven, Novo Nordisk, Novogyne, Pfizer, Shionogi, Shippan Point Advisors LLC, Slate Pharmaceuticals, Sprout Pharmaceuticals, Teva, Warner Chilcott, and Watson. He also reports receiving (currently or in the past year) grant/research support from BioSante, EndoCeutics, Novo Nordisk, Novogyne, Palatin Technologies, Teva, and Warner Chilcott. He reports serving on the speakers bureaus of Amgen, Merck, Novartis, Noven, Novo Nordisk, Novogyne, Teva, and Warner Chilcott. Dr. Simon is currently the Chief Medical Officer for Sprout Pharmaceuticals.
CASE: Atypical ductal hyperplasia
Your 56-year-old, married, white patient has been on hormone therapy (HT) since age 52 for the treatment of vasomotor symptoms. She is taking a low-dose oral estrogen and micronized progesterone combination as she has an intact uterus. Her family history is positive for breast cancer, as her mother was diagnosed at age 68.
Her most recent annual screening mammogram shows linear calcifications. Because fine, linear, branching or casting calcifications are worrisome for atypical ductal hyperplasia (ADH) or ductal carcinoma in situ, a biopsy is recommended.
She elects to wean off and discontinue HT during the evaluation of her abnormal mammogram. The mammographic-guided stereotactic biopsy reveals ADH. She undergoes an open excisional biopsy, the results of which reveal extensive ADH with negative margins.
Six weeks after a lumpectomy she returns to your office reporting moderate to severe hot flashes that occur seven to 10 times per day and impair her sleep, leading to fatigue and “brain fog.” In addition, she is noticing vaginal dryness and dyspareunia despite use of lubricants. She requests treatment for her symptoms and wonders if she can restart HT systemically or vaginally.
How do you manage her hot flashes?
What are the alternatives to HT for hot flashes?
Certain lifestyle changes have been reported to provide relief for hot flushes.1 These include:
- use of layered clothing
- maintenance of cool ambient temperature (particularly during sleep)
- consumption of cool foods or beverages
- relaxation techniques (such as deep breathing, or paced respirations, for 20 min three times per day).
Despite sparse data, avoiding triggers such as spicy or hot foods or alcohol may be helpful.
Therapies such as evening primrose oil, dong quai, ginseng, wild yam, magnet therapy, reflexology, and homeopathy have not been found more effective in treating hot flashes than placebo.2
Phytoestrogens (such as equol), acupuncture, yoga, and hypnosis continue to be tested in randomized trials with mixed results.
Off-label drug options offer modest help. There are currently no FDA-approved nonhormonal pharmaceutical options for relief of hot flashes; the gold standard for treatment remains estrogen therapy. For moderate to severe bothersome hot flashes, potentially effective drug therapies used off label include clonidine, selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs), and gabapentin (TABLE 1).2,4 In large, randomized, controlled trials, the following agents were modestly more effective than placebo: desvenlafaxine,5 low-dose paroxetine salt,6 escitalopram,7 and gastroretentive gabapentin.8 Participants in these trials included women with both spontaneous and surgically induced menopause.
Although sponsors have applied for approval for three of these agents, the FDA so far has declined to approve these agents for vasomotor treatment due to concerns about risks versus benefits. Benefits of these nonhormonal prescription therapies need to be weighed carefully against side effects, because the reduction in absolute hot flushes is modest.
Many small trials have assessed other medications and complementary and alternative therapies regarding management of menopausal symptoms. Most, however, are limited by small numbers of enrolled participants and shorter study duration (≤12 weeks). In addition, enrolled participants have variable numbers of hot flashes, often less than 14 per week.2,4
TABLE 1
Nonhormonal treatment of vasomotor symptoms
| Treatment | Study Design* | Findings |
|---|---|---|
| Complementary/alternative medicines (black cohosh, St. John’s Wort, red clover, acupuncture, exercise) | Duration: 4–52 wk; OL and RPL trials; entry criteria for most trials: >14 hot flashes/wk | Mixed results, mostly with no sustained improvement |
| SSRIs** (paroxetine, fluoxetine, sertraline, citalopram, escitalopram) | Duration: 4–36 wk; RPL trials with all agents; N = 20–90 in active arms; entry criteria for most trials: >14 hot flashes/wk | Reduction in vasomotor symptoms (frequency, composite scores): 28%–55% |
| SNRIs** (venlafaxine, desvenlafaxine) | Duration, 12–52 wk; RPL trials with all agents; N = 20–65 in VEN; N = 120–200 in DVS; Entry criteria >14 hot flashes/wk for VEN; >50/wk for DVS | Reduction in VMS (frequency, composite scores): 35%–58% for VEN, 55%–68% for DVS |
| Gabapentin** | Duration: 4–12 wk; RPL trials; N = 20–100; entry criteria for most trials: >14–50 hot flashes/wk | Reduction in vasomotor symptoms (frequency, composite scores): 50%–70% |
| *All studies of menopausal, nondepressed women. **Treatment is off label. Hall E, et al. Drugs. 2011;71:287-304. Reprinted with permission. Pinkerton JV, Shapiro M. The North American Menopause Society. Overview of available treatment options for VMS and VVA. Medscape Education Web site. http://www.medscape.org/viewarticle/763413. Published May 24, 2012. Accessed February 23, 2013.19 | ||
Can your patient restart HT? If so, should HT be offered vaginally or systemically?
Non-HT may be enough for vaginal dryness. Benefit has been shown with the use of vaginal moisturizers twice weekly and lubricants as needed for sexual activity.9 Therefore, the local application of daily lubricants, such as olive oil, along with the use of moisturizers with regular sexual intercourse may be enough to maintain vaginal health and function.
In randomized trials, phytoestrogens lack benefit data for vaginal atrophy. Small pilot studies of the effect of oral/ vaginal phytoestrogens on vaginal atrophy do not show any benefit on vaginal pH or vaginal maturation index and mixed improvement in vaginal dryness. In addition, no clear effect of these agents has been seen compared with placebo, except that there may be less progression of vaginal atrophy over time with phytoestrogens.10 It is possible that the benefits of phytoestrogens may take longer to take effect than the 12 weeks required to see an effect with HT.
Vaginal estrogen: limited safety data. No published clinical trials have assessed the impact of topical vaginal estrogen on risk of recurrence in breast cancer survivors, and concern exists because detectable estradiol levels have been reported in women who take aromatase inhibitors and have very atrophic vaginal mucosa.11 NAMS recommends that the discussion about vaginal estrogen be individualized between the patient, her provider, and her oncologist.12
Vaginal estrogen creams and tablets (Vagifem 10 μg per tablet) are often started daily for 2 weeks for a “priming dose” then dosed twice per week. To minimize systemic absorption, creams may be used externally or with smaller doses vaginally. The higher the dose or more frequent the use, the greater the risk of significant systemic absorption, particularly when the vagina is atrophic.13
Another option is the vaginal estradiol ring, which delivers a low dose (7.5 μg per day) for 90 days.14,15
Progestogen therapy is generally not needed when low-dose estrogen is administered locally to treat vaginal atrophy.12
A new oral option. In February 2013, ospemifene, a selective estrogen receptor modulator (SERM), was approved for pain with intercourse and vaginal dryness.
Related article: “New treatment option for vulvar and vaginal atrophy,” by Andrew M. Kaunitz, MD (May 2013)
There is concern with systemic estrogen use
If her hot flashes remain persistent and bothersome, low-dose estrogen could be considered. However, data from the Breast Cancer Surveillance Consortium9 showed that as postmenopausal HT use decreased (from 35% to 11% between 1999 and 2005), rates of ADH decreased from a peak of 5.5 per 10,000 mammograms in 1999 to 2.4 per 10,000 mammograms in 2005. Similarly, rates of invasive cancer with ADH decreased from a peak of 4.3 per 10,000 mammograms in 2003 to 3.3 per 10,000 mammograms in 2005. This finding—that rates of ADH and invasive breast cancer were significantly linked to postmenopausal use of HT—raises concern about using HT in women with prior ADH. Of note, the cancers linked with ADH were of lower grade and stage and more estrogen receptor–positive than cancers not linked with ADH.17
How do you manage your patient’s increased risk of breast cancer?
In examining the answer to this management point, we need to first ask and answer, “How does ADH develop?” and “What is her risk of developing breast cancer?”
The development of invasive breast cancer is believed to involve a complex, multistep process. Initially, there is disruption of normal cell development and growth, with overproduction of normal-looking cells (hyperplasia). These excess cells stack up and/or become abnormal. Then, there is continued change in appearance and multiplication, becoming ductal carcinoma in situ (noninvasive). If left untreated, the cells may develop into invasive cancer.18 See TABLE 2 for the relative risk of a patient with ADH developing breast cancer.10
Now, we can address, “How do you manage this patient’s increased risk of breast cancer?”
TABLE 2. Relative risk of developing breast cancer
| Risk | Relative Risk |
|---|---|
| Atypical ductal hyperplasia | 4-5 |
| Atypical ductal hyperplasia and positive family history | 6-8 |
More frequent breast screening!
- Clinical breast exams twice per year
- Screening mammograms annually
- Screening tomosynthesis (These are additional digital screening views which provide almost a 3D view.)
- Screening breast ultrasound
- Screening breast MRI — if she has a 20% lifetime risk of breast cancer (family history or genetic predisposition) (TABLE 3).
TABLE 3. ACS guidelines for screening breast MRI
| Risk | Recommendation |
|---|---|
| <15% lifetime risk | MRI not recommended |
| 15% to 20% lifetime risk | Talk about benefits and limitations of MRI screening |
| >20% lifetime risk | Annual mammogram and annual MRI alternating every 6 months |
Careful consideration of medications
Since it is possible that estrogen may fuel the growth of some breast cancers, avoiding systemic menopausal HT may be safest.
Counsel her about strategies to reduce breast cancer risk
These include:
- Lifestyle changes, including weight loss, exercise, and avoiding excess alcohol intake.
- Preventive medications. Tamoxifen or raloxifene (Evista) can be used for 5 years. These medications block estrogen from binding to the breast estrogen receptors. Another option is an aromatase inhibitor, which decreases estrogen production.
- Risk-reducing (prophylactic) mastectomy.
Management approach for this patient
This patient has had her ADH surgically excised. She will remain at higher risk for breast cancer and should consider strategies to decrease her risk, including lifestyle changes and the possible initiation of medications such as tamoxifen or raloxifene. New screening modalities, such as tomosynthesis or breast ultrasound, may be used to screen for breast cancer, and she may be a candidate for alternating mammograms and MRIs at 6-month intervals.
For her vaginal dryness, over-the-counter lubricants and moisturizers may be helpful. If not, topical or vaginal estrogen is available (as creams, tablets, or a ring) and provides primarily local benefit with limited systemic absorption.
For her bothersome hot flashes, if lifestyle changes don’t work, nonhormonal therapies can be offered off label, such as effexor, desvenlafaxine, gabapentin, or any of the SSRIs—including those tested in large, randomized, controlled trials, such as escitalopram and low-dose paroxetine salt, at low doses.
If she is taking tamoxifen, however, SSRIs such as paroxetine should be avoided due to P450 interaction.
If her hot flashes remain persistent and bothersome, low-dose estrogen could be considered, with education about the potential risks, as she is already at higher risk for breast cancer.
Acknowledgment
The author would like to thank Andrew M. Kaunitz, MD, for his forward thinking in helping to establish this new series on menopause.
Suggest it to our expert panel. They may address your management dilemma in a future issue!
Email us at [email protected] or send a Letter to the Editor to [email protected]
A new series brought to you by the menopause experts

| Andrew M. Kaunitz, MD Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a NAMS Board member and certified menopause practitioner. He also serves on the OBG Management Board of Editors. |

| JoAnn V. Pinkerton, MD Professor, Department of Obstetrics and Gynecology, and Director, Division of Midlife Women’s Health, University of Virginia. Dr. Pinkerton is a North American Menopause Society (NAMS) past president and certified menopause practitioner. She also serves on the OBG Management Board of Editors. |

| James A. Simon, MD Clinical Professor, Department of Obstetrics and Gynecology, George Washington University, and Medical Director, Women’s Health & Research Consultants, Washington, DC. Dr. Simon is a NAMS past president, a certified menopause practitioner, and a certified clinical densitometrist. He also serves on the OBG Management Board of Editors. |
Disclosures
Dr. Kaunitz reports that his institution receives grant or research support from Agile, Bayer, Endoceutics, Teva, Medical Diagnostic Laboratories, and Noven, and that he is a consultant to Bayer, Merck, and Teva.
Dr. Pinkerton reports that her institution receives consulting fees from Pfizer, DepoMed, Shionogi, and Noven and multicenter research fees from DepoMed, Endoceutics, and Bionova.
Dr. Simon reports being a consultant to or on the advisory boards of Abbott Laboratories, Agile Therapeutics, Amgen, Ascend Therapeutics, BioSante, Depomed, Lelo, MD Therapeutics, Meda Pharmaceuticals, Merck, Noven, Novo Nordisk, Novogyne, Pfizer, Shionogi, Shippan Point Advisors LLC, Slate Pharmaceuticals, Sprout Pharmaceuticals, Teva, Warner Chilcott, and Watson. He also reports receiving (currently or in the past year) grant/research support from BioSante, EndoCeutics, Novo Nordisk, Novogyne, Palatin Technologies, Teva, and Warner Chilcott. He reports serving on the speakers bureaus of Amgen, Merck, Novartis, Noven, Novo Nordisk, Novogyne, Teva, and Warner Chilcott. Dr. Simon is currently the Chief Medical Officer for Sprout Pharmaceuticals.
CASE: Atypical ductal hyperplasia
Your 56-year-old, married, white patient has been on hormone therapy (HT) since age 52 for the treatment of vasomotor symptoms. She is taking a low-dose oral estrogen and micronized progesterone combination as she has an intact uterus. Her family history is positive for breast cancer, as her mother was diagnosed at age 68.
Her most recent annual screening mammogram shows linear calcifications. Because fine, linear, branching or casting calcifications are worrisome for atypical ductal hyperplasia (ADH) or ductal carcinoma in situ, a biopsy is recommended.
She elects to wean off and discontinue HT during the evaluation of her abnormal mammogram. The mammographic-guided stereotactic biopsy reveals ADH. She undergoes an open excisional biopsy, the results of which reveal extensive ADH with negative margins.
Six weeks after a lumpectomy she returns to your office reporting moderate to severe hot flashes that occur seven to 10 times per day and impair her sleep, leading to fatigue and “brain fog.” In addition, she is noticing vaginal dryness and dyspareunia despite use of lubricants. She requests treatment for her symptoms and wonders if she can restart HT systemically or vaginally.
How do you manage her hot flashes?
What are the alternatives to HT for hot flashes?
Certain lifestyle changes have been reported to provide relief for hot flushes.1 These include:
- use of layered clothing
- maintenance of cool ambient temperature (particularly during sleep)
- consumption of cool foods or beverages
- relaxation techniques (such as deep breathing, or paced respirations, for 20 min three times per day).
Despite sparse data, avoiding triggers such as spicy or hot foods or alcohol may be helpful.
Therapies such as evening primrose oil, dong quai, ginseng, wild yam, magnet therapy, reflexology, and homeopathy have not been found more effective in treating hot flashes than placebo.2
Phytoestrogens (such as equol), acupuncture, yoga, and hypnosis continue to be tested in randomized trials with mixed results.
Off-label drug options offer modest help. There are currently no FDA-approved nonhormonal pharmaceutical options for relief of hot flashes; the gold standard for treatment remains estrogen therapy. For moderate to severe bothersome hot flashes, potentially effective drug therapies used off label include clonidine, selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs), and gabapentin (TABLE 1).2,4 In large, randomized, controlled trials, the following agents were modestly more effective than placebo: desvenlafaxine,5 low-dose paroxetine salt,6 escitalopram,7 and gastroretentive gabapentin.8 Participants in these trials included women with both spontaneous and surgically induced menopause.
Although sponsors have applied for approval for three of these agents, the FDA so far has declined to approve these agents for vasomotor treatment due to concerns about risks versus benefits. Benefits of these nonhormonal prescription therapies need to be weighed carefully against side effects, because the reduction in absolute hot flushes is modest.
Many small trials have assessed other medications and complementary and alternative therapies regarding management of menopausal symptoms. Most, however, are limited by small numbers of enrolled participants and shorter study duration (≤12 weeks). In addition, enrolled participants have variable numbers of hot flashes, often less than 14 per week.2,4
TABLE 1
Nonhormonal treatment of vasomotor symptoms
| Treatment | Study Design* | Findings |
|---|---|---|
| Complementary/alternative medicines (black cohosh, St. John’s Wort, red clover, acupuncture, exercise) | Duration: 4–52 wk; OL and RPL trials; entry criteria for most trials: >14 hot flashes/wk | Mixed results, mostly with no sustained improvement |
| SSRIs** (paroxetine, fluoxetine, sertraline, citalopram, escitalopram) | Duration: 4–36 wk; RPL trials with all agents; N = 20–90 in active arms; entry criteria for most trials: >14 hot flashes/wk | Reduction in vasomotor symptoms (frequency, composite scores): 28%–55% |
| SNRIs** (venlafaxine, desvenlafaxine) | Duration, 12–52 wk; RPL trials with all agents; N = 20–65 in VEN; N = 120–200 in DVS; Entry criteria >14 hot flashes/wk for VEN; >50/wk for DVS | Reduction in VMS (frequency, composite scores): 35%–58% for VEN, 55%–68% for DVS |
| Gabapentin** | Duration: 4–12 wk; RPL trials; N = 20–100; entry criteria for most trials: >14–50 hot flashes/wk | Reduction in vasomotor symptoms (frequency, composite scores): 50%–70% |
| *All studies of menopausal, nondepressed women. **Treatment is off label. Hall E, et al. Drugs. 2011;71:287-304. Reprinted with permission. Pinkerton JV, Shapiro M. The North American Menopause Society. Overview of available treatment options for VMS and VVA. Medscape Education Web site. http://www.medscape.org/viewarticle/763413. Published May 24, 2012. Accessed February 23, 2013.19 | ||
Can your patient restart HT? If so, should HT be offered vaginally or systemically?
Non-HT may be enough for vaginal dryness. Benefit has been shown with the use of vaginal moisturizers twice weekly and lubricants as needed for sexual activity.9 Therefore, the local application of daily lubricants, such as olive oil, along with the use of moisturizers with regular sexual intercourse may be enough to maintain vaginal health and function.
In randomized trials, phytoestrogens lack benefit data for vaginal atrophy. Small pilot studies of the effect of oral/ vaginal phytoestrogens on vaginal atrophy do not show any benefit on vaginal pH or vaginal maturation index and mixed improvement in vaginal dryness. In addition, no clear effect of these agents has been seen compared with placebo, except that there may be less progression of vaginal atrophy over time with phytoestrogens.10 It is possible that the benefits of phytoestrogens may take longer to take effect than the 12 weeks required to see an effect with HT.
Vaginal estrogen: limited safety data. No published clinical trials have assessed the impact of topical vaginal estrogen on risk of recurrence in breast cancer survivors, and concern exists because detectable estradiol levels have been reported in women who take aromatase inhibitors and have very atrophic vaginal mucosa.11 NAMS recommends that the discussion about vaginal estrogen be individualized between the patient, her provider, and her oncologist.12
Vaginal estrogen creams and tablets (Vagifem 10 μg per tablet) are often started daily for 2 weeks for a “priming dose” then dosed twice per week. To minimize systemic absorption, creams may be used externally or with smaller doses vaginally. The higher the dose or more frequent the use, the greater the risk of significant systemic absorption, particularly when the vagina is atrophic.13
Another option is the vaginal estradiol ring, which delivers a low dose (7.5 μg per day) for 90 days.14,15
Progestogen therapy is generally not needed when low-dose estrogen is administered locally to treat vaginal atrophy.12
A new oral option. In February 2013, ospemifene, a selective estrogen receptor modulator (SERM), was approved for pain with intercourse and vaginal dryness.
Related article: “New treatment option for vulvar and vaginal atrophy,” by Andrew M. Kaunitz, MD (May 2013)
There is concern with systemic estrogen use
If her hot flashes remain persistent and bothersome, low-dose estrogen could be considered. However, data from the Breast Cancer Surveillance Consortium9 showed that as postmenopausal HT use decreased (from 35% to 11% between 1999 and 2005), rates of ADH decreased from a peak of 5.5 per 10,000 mammograms in 1999 to 2.4 per 10,000 mammograms in 2005. Similarly, rates of invasive cancer with ADH decreased from a peak of 4.3 per 10,000 mammograms in 2003 to 3.3 per 10,000 mammograms in 2005. This finding—that rates of ADH and invasive breast cancer were significantly linked to postmenopausal use of HT—raises concern about using HT in women with prior ADH. Of note, the cancers linked with ADH were of lower grade and stage and more estrogen receptor–positive than cancers not linked with ADH.17
How do you manage your patient’s increased risk of breast cancer?
In examining the answer to this management point, we need to first ask and answer, “How does ADH develop?” and “What is her risk of developing breast cancer?”
The development of invasive breast cancer is believed to involve a complex, multistep process. Initially, there is disruption of normal cell development and growth, with overproduction of normal-looking cells (hyperplasia). These excess cells stack up and/or become abnormal. Then, there is continued change in appearance and multiplication, becoming ductal carcinoma in situ (noninvasive). If left untreated, the cells may develop into invasive cancer.18 See TABLE 2 for the relative risk of a patient with ADH developing breast cancer.10
Now, we can address, “How do you manage this patient’s increased risk of breast cancer?”
TABLE 2. Relative risk of developing breast cancer
| Risk | Relative Risk |
|---|---|
| Atypical ductal hyperplasia | 4-5 |
| Atypical ductal hyperplasia and positive family history | 6-8 |
More frequent breast screening!
- Clinical breast exams twice per year
- Screening mammograms annually
- Screening tomosynthesis (These are additional digital screening views which provide almost a 3D view.)
- Screening breast ultrasound
- Screening breast MRI — if she has a 20% lifetime risk of breast cancer (family history or genetic predisposition) (TABLE 3).
TABLE 3. ACS guidelines for screening breast MRI
| Risk | Recommendation |
|---|---|
| <15% lifetime risk | MRI not recommended |
| 15% to 20% lifetime risk | Talk about benefits and limitations of MRI screening |
| >20% lifetime risk | Annual mammogram and annual MRI alternating every 6 months |
Careful consideration of medications
Since it is possible that estrogen may fuel the growth of some breast cancers, avoiding systemic menopausal HT may be safest.
Counsel her about strategies to reduce breast cancer risk
These include:
- Lifestyle changes, including weight loss, exercise, and avoiding excess alcohol intake.
- Preventive medications. Tamoxifen or raloxifene (Evista) can be used for 5 years. These medications block estrogen from binding to the breast estrogen receptors. Another option is an aromatase inhibitor, which decreases estrogen production.
- Risk-reducing (prophylactic) mastectomy.
Management approach for this patient
This patient has had her ADH surgically excised. She will remain at higher risk for breast cancer and should consider strategies to decrease her risk, including lifestyle changes and the possible initiation of medications such as tamoxifen or raloxifene. New screening modalities, such as tomosynthesis or breast ultrasound, may be used to screen for breast cancer, and she may be a candidate for alternating mammograms and MRIs at 6-month intervals.
For her vaginal dryness, over-the-counter lubricants and moisturizers may be helpful. If not, topical or vaginal estrogen is available (as creams, tablets, or a ring) and provides primarily local benefit with limited systemic absorption.
For her bothersome hot flashes, if lifestyle changes don’t work, nonhormonal therapies can be offered off label, such as effexor, desvenlafaxine, gabapentin, or any of the SSRIs—including those tested in large, randomized, controlled trials, such as escitalopram and low-dose paroxetine salt, at low doses.
If she is taking tamoxifen, however, SSRIs such as paroxetine should be avoided due to P450 interaction.
If her hot flashes remain persistent and bothersome, low-dose estrogen could be considered, with education about the potential risks, as she is already at higher risk for breast cancer.
Acknowledgment
The author would like to thank Andrew M. Kaunitz, MD, for his forward thinking in helping to establish this new series on menopause.
Suggest it to our expert panel. They may address your management dilemma in a future issue!
Email us at [email protected] or send a Letter to the Editor to [email protected]
1. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause. 2004;11(1):11-33.
2. Pinkerton JV, Stovall DW, Kightlinger RS. Advances in the treatment of menopausal symptoms. Womens Health (Lond Engl). 2009;5(4):361-384.
3. Ghaleiha A, Jahangard L, Sherafat Z, et al. Oxybutynin reduces sweating in depressed patients treated with sertraline: a double-blink, placebo-controlled, clinical study. Neuropsychiatr Dis Treat. 2012;8:407-412.
4. Hall E, Frey BN, Soares CN. Non-hormonal treatment strategies for vasomotor symptoms: a critical review. Drugs. 2011;71(3):287-304.
5. Pinkerton JV, Archer DF, Guico-Pabia CJ, Hwang E, Cheng RF. Maintenance of the efficacy of desvenlafaxine in menopausal vasomotor symptoms: a 1-year randomized controlled trial. Menopause. 2013;20(1):38-46.
6. Simon JA, Sanacora G, Bhaskar S, Lippman J. Safety and efficacy of low-dose mesylate salt of paroxetine (LDMP) for the treatment of vasomotor symptoms (VMS) associated with menopause: a 24-week randomized, placebo-controlled phase 3 study. Menopause. 2012;19(12):1371.-
7. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267-274.
8. Pinkerton JV, Kagan R, Portman D, Sathyanarayan RK, Sweeney M. Efficacy of gabapentin extended release in the treatment of menopausal hot flashes: results of the Breeze 3 study. Menopause. 2012;19(12):1377.-
9. MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85(1):87-94.
10. Bedell S, Nachtigall M, Naftolin F. The pros and cons of plant estrogens for menopause [published online ahead of print December 25 2012]. J Steroid Biochem Mol Biol. 2012. doi: 10.1016/j.jsbmb.2012.12.004.
11. Moegele M, Buchholz S, Seitz S, Ortmann O. Vaginal estrogen therapy in postmenopausal breast cancer patients treated with aromatase inhibitors. Arch Gynecol Obstet. 2012;285(5):1397-402.
12. North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14(3 Pt 1):355-371.
13. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2010;17(1):194-203.
14. Simon JA. Vulvovaginal atrophy: new and upcoming approaches. Menopause. 2009;16(1):5-7.
15. Kaunitz AM. Transdermal and vaginal estradiol for the treatment of menopausal symptoms: the nuts and bolts. Menopause. 2012;19(6):602-603.
16. Pruthi S, Simon JA, Early AP. Current overview of the management of urogenital atrophy in women with breast cancer. Breast J. 2011;17(4):403-408.
17. Menes TS, Kerlikowske K, Jaffer S, Seger D, Miglioretti D. Rates of atypical ductal hyperplasia declined with less use of postmenopausal hormone treatment: findings from the Breast Cancer Surveillance Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2822-2828.
18. Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353(3):275-285.
19. Pinkerton JV, Shapiro M. The North American Menopause Society. Overview of available treatment options for VMS and VVA. Medscape Education Web site. http://www.medscape.org/viewarticle/763413. Published May 24 2012. Accessed February 23, 2013.
1. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause. 2004;11(1):11-33.
2. Pinkerton JV, Stovall DW, Kightlinger RS. Advances in the treatment of menopausal symptoms. Womens Health (Lond Engl). 2009;5(4):361-384.
3. Ghaleiha A, Jahangard L, Sherafat Z, et al. Oxybutynin reduces sweating in depressed patients treated with sertraline: a double-blink, placebo-controlled, clinical study. Neuropsychiatr Dis Treat. 2012;8:407-412.
4. Hall E, Frey BN, Soares CN. Non-hormonal treatment strategies for vasomotor symptoms: a critical review. Drugs. 2011;71(3):287-304.
5. Pinkerton JV, Archer DF, Guico-Pabia CJ, Hwang E, Cheng RF. Maintenance of the efficacy of desvenlafaxine in menopausal vasomotor symptoms: a 1-year randomized controlled trial. Menopause. 2013;20(1):38-46.
6. Simon JA, Sanacora G, Bhaskar S, Lippman J. Safety and efficacy of low-dose mesylate salt of paroxetine (LDMP) for the treatment of vasomotor symptoms (VMS) associated with menopause: a 24-week randomized, placebo-controlled phase 3 study. Menopause. 2012;19(12):1371.-
7. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267-274.
8. Pinkerton JV, Kagan R, Portman D, Sathyanarayan RK, Sweeney M. Efficacy of gabapentin extended release in the treatment of menopausal hot flashes: results of the Breeze 3 study. Menopause. 2012;19(12):1377.-
9. MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85(1):87-94.
10. Bedell S, Nachtigall M, Naftolin F. The pros and cons of plant estrogens for menopause [published online ahead of print December 25 2012]. J Steroid Biochem Mol Biol. 2012. doi: 10.1016/j.jsbmb.2012.12.004.
11. Moegele M, Buchholz S, Seitz S, Ortmann O. Vaginal estrogen therapy in postmenopausal breast cancer patients treated with aromatase inhibitors. Arch Gynecol Obstet. 2012;285(5):1397-402.
12. North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14(3 Pt 1):355-371.
13. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2010;17(1):194-203.
14. Simon JA. Vulvovaginal atrophy: new and upcoming approaches. Menopause. 2009;16(1):5-7.
15. Kaunitz AM. Transdermal and vaginal estradiol for the treatment of menopausal symptoms: the nuts and bolts. Menopause. 2012;19(6):602-603.
16. Pruthi S, Simon JA, Early AP. Current overview of the management of urogenital atrophy in women with breast cancer. Breast J. 2011;17(4):403-408.
17. Menes TS, Kerlikowske K, Jaffer S, Seger D, Miglioretti D. Rates of atypical ductal hyperplasia declined with less use of postmenopausal hormone treatment: findings from the Breast Cancer Surveillance Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2822-2828.
18. Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353(3):275-285.
19. Pinkerton JV, Shapiro M. The North American Menopause Society. Overview of available treatment options for VMS and VVA. Medscape Education Web site. http://www.medscape.org/viewarticle/763413. Published May 24 2012. Accessed February 23, 2013.
Can nonhormonal treatments relieve hot flushes in breast Ca survivors?
A frequent challenge facing clinicians who manage breast cancer survivors is identifying treatment options to attenuate hot flushes and night sweats without resorting to estrogen, which is contraindicated because it can induce cancer growth.
Variables associated with a high prevalence of hot flushes in this population:
- age at diagnosis (>50 years)
- abrupt discontinuation of estrogen therapy at diagnosis
- induction of premature menopause by therapy (i.e., chemotherapy and surgical or medical ovarian ablation)
- induction of estrogen deficiency symptoms by chemotherapy (e.g., tamoxifen or an aromatase inhibitor).
There are no FDA-approved nonhormonal pharmaceutical options to alleviate bothersome hot flushes that accompany breast cancer treatment and the menopausal transition, whether spontaneous or induced. Moreover, meaningful guidance from published trials is limited by the small number of enrolled subjects and short duration of study (i.e., ≤12 weeks).
How hot flushes happen
According to Freedman, body temperature is regulated over a range called the thermo-neutral zone.1,2 Reduced or fluctuating ovarian hormones are believed to narrow the thermoregulatory zone such that variations in core body temperature trigger heat loss mechanisms. A narrowed thermoregulatory zone has been associated with increased norepinephrine.
Clonidine, an α-2 adrenergic agonist, decreases norepinephrine and has been found to reduce hot flushes. Other nonhormonal agents that have been found to be at least partially effective in reducing hot flushes include SSRI and SNRI antidepressants, which enhance central serotonin and norepinephrine activity. Gabapentin has also proved to be effective.
Potential adverse events or negative side effects, such as insomnia, weight gain, drowsiness, and sedation, need to be taken into account when evaluating the benefits of pharmaceutical options.
There are no FDA-approved nonhormonal therapies for hot-flush reduction in breast cancer survivors.
Potentially effective drug therapies include clonidine, SSRIs, SNRIs, and gabapentin. Benefits need to be weighed carefully against side effects, because the reduction in absolute hot flushes is only mild to moderate.
Nonpharmaceutical therapies that are not beneficial include homeopathy, magnet therapy, and acupuncture. However, more recent RCT data suggest that, in breast cancer patients, traditional acupuncture (and, in some studies, sham acupuncture) may, in fact, significantly reduce frequency of hot flushes, with a prolonged reduction at 3 to 6 months.3,4 Relaxation therapy has a modest benefit.
When a patient reports bothersome hot flushes, I recommend that she avoid overheating, use cooling techniques, and try relaxation therapy or acupuncture.
For medical therapy, I usually recommend venlafaxine or gabapentin, both of which have an effect on hot flushes within 2 weeks and both of which are associated with side effects. I start with 37.5 mg of venlafaxine, increasing to 75 mg after 2 weeks, if needed. If using gabapentin, I start with 300 mg, increasing to 600 mg at night and adding 300 mg in the morning or afternoon, if needed, aiming for 900 to 1,800 mg per day.
For vaginal dryness, I recommend vaginal moisturizers (used twice weekly) and lubricants (as needed) for sexual activity. The use of vaginal dilators or topical estrogen therapy is individualized.—JOANN V. PINKERTON, MD
We want to hear from you! Tell us what you think.
1. Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181(1):66-70.
2. Freedman RR. Core body temperature variation in symptomatic and asymptomatic postmenopausal women: brief report. Menopause. 2002;9(6):399-401.
3. Hervik J, Mialand O. Acupuncture for the treatment of hot flashes in breast cancer patients: a randomized, controlled trial. Breast Cancer Res Treat. 2009;116(2):311-316.
4. de Valois BA, Young TE, Robinson N, McCourt C, Maher EJ. Using traditional acupuncture for breast cancer-related hot flashes and night sweats. J Altern Complement Med. 2010;16(10):1047-1057.
A frequent challenge facing clinicians who manage breast cancer survivors is identifying treatment options to attenuate hot flushes and night sweats without resorting to estrogen, which is contraindicated because it can induce cancer growth.
Variables associated with a high prevalence of hot flushes in this population:
- age at diagnosis (>50 years)
- abrupt discontinuation of estrogen therapy at diagnosis
- induction of premature menopause by therapy (i.e., chemotherapy and surgical or medical ovarian ablation)
- induction of estrogen deficiency symptoms by chemotherapy (e.g., tamoxifen or an aromatase inhibitor).
There are no FDA-approved nonhormonal pharmaceutical options to alleviate bothersome hot flushes that accompany breast cancer treatment and the menopausal transition, whether spontaneous or induced. Moreover, meaningful guidance from published trials is limited by the small number of enrolled subjects and short duration of study (i.e., ≤12 weeks).
How hot flushes happen
According to Freedman, body temperature is regulated over a range called the thermo-neutral zone.1,2 Reduced or fluctuating ovarian hormones are believed to narrow the thermoregulatory zone such that variations in core body temperature trigger heat loss mechanisms. A narrowed thermoregulatory zone has been associated with increased norepinephrine.
Clonidine, an α-2 adrenergic agonist, decreases norepinephrine and has been found to reduce hot flushes. Other nonhormonal agents that have been found to be at least partially effective in reducing hot flushes include SSRI and SNRI antidepressants, which enhance central serotonin and norepinephrine activity. Gabapentin has also proved to be effective.
Potential adverse events or negative side effects, such as insomnia, weight gain, drowsiness, and sedation, need to be taken into account when evaluating the benefits of pharmaceutical options.
There are no FDA-approved nonhormonal therapies for hot-flush reduction in breast cancer survivors.
Potentially effective drug therapies include clonidine, SSRIs, SNRIs, and gabapentin. Benefits need to be weighed carefully against side effects, because the reduction in absolute hot flushes is only mild to moderate.
Nonpharmaceutical therapies that are not beneficial include homeopathy, magnet therapy, and acupuncture. However, more recent RCT data suggest that, in breast cancer patients, traditional acupuncture (and, in some studies, sham acupuncture) may, in fact, significantly reduce frequency of hot flushes, with a prolonged reduction at 3 to 6 months.3,4 Relaxation therapy has a modest benefit.
When a patient reports bothersome hot flushes, I recommend that she avoid overheating, use cooling techniques, and try relaxation therapy or acupuncture.
For medical therapy, I usually recommend venlafaxine or gabapentin, both of which have an effect on hot flushes within 2 weeks and both of which are associated with side effects. I start with 37.5 mg of venlafaxine, increasing to 75 mg after 2 weeks, if needed. If using gabapentin, I start with 300 mg, increasing to 600 mg at night and adding 300 mg in the morning or afternoon, if needed, aiming for 900 to 1,800 mg per day.
For vaginal dryness, I recommend vaginal moisturizers (used twice weekly) and lubricants (as needed) for sexual activity. The use of vaginal dilators or topical estrogen therapy is individualized.—JOANN V. PINKERTON, MD
We want to hear from you! Tell us what you think.
A frequent challenge facing clinicians who manage breast cancer survivors is identifying treatment options to attenuate hot flushes and night sweats without resorting to estrogen, which is contraindicated because it can induce cancer growth.
Variables associated with a high prevalence of hot flushes in this population:
- age at diagnosis (>50 years)
- abrupt discontinuation of estrogen therapy at diagnosis
- induction of premature menopause by therapy (i.e., chemotherapy and surgical or medical ovarian ablation)
- induction of estrogen deficiency symptoms by chemotherapy (e.g., tamoxifen or an aromatase inhibitor).
There are no FDA-approved nonhormonal pharmaceutical options to alleviate bothersome hot flushes that accompany breast cancer treatment and the menopausal transition, whether spontaneous or induced. Moreover, meaningful guidance from published trials is limited by the small number of enrolled subjects and short duration of study (i.e., ≤12 weeks).
How hot flushes happen
According to Freedman, body temperature is regulated over a range called the thermo-neutral zone.1,2 Reduced or fluctuating ovarian hormones are believed to narrow the thermoregulatory zone such that variations in core body temperature trigger heat loss mechanisms. A narrowed thermoregulatory zone has been associated with increased norepinephrine.
Clonidine, an α-2 adrenergic agonist, decreases norepinephrine and has been found to reduce hot flushes. Other nonhormonal agents that have been found to be at least partially effective in reducing hot flushes include SSRI and SNRI antidepressants, which enhance central serotonin and norepinephrine activity. Gabapentin has also proved to be effective.
Potential adverse events or negative side effects, such as insomnia, weight gain, drowsiness, and sedation, need to be taken into account when evaluating the benefits of pharmaceutical options.
There are no FDA-approved nonhormonal therapies for hot-flush reduction in breast cancer survivors.
Potentially effective drug therapies include clonidine, SSRIs, SNRIs, and gabapentin. Benefits need to be weighed carefully against side effects, because the reduction in absolute hot flushes is only mild to moderate.
Nonpharmaceutical therapies that are not beneficial include homeopathy, magnet therapy, and acupuncture. However, more recent RCT data suggest that, in breast cancer patients, traditional acupuncture (and, in some studies, sham acupuncture) may, in fact, significantly reduce frequency of hot flushes, with a prolonged reduction at 3 to 6 months.3,4 Relaxation therapy has a modest benefit.
When a patient reports bothersome hot flushes, I recommend that she avoid overheating, use cooling techniques, and try relaxation therapy or acupuncture.
For medical therapy, I usually recommend venlafaxine or gabapentin, both of which have an effect on hot flushes within 2 weeks and both of which are associated with side effects. I start with 37.5 mg of venlafaxine, increasing to 75 mg after 2 weeks, if needed. If using gabapentin, I start with 300 mg, increasing to 600 mg at night and adding 300 mg in the morning or afternoon, if needed, aiming for 900 to 1,800 mg per day.
For vaginal dryness, I recommend vaginal moisturizers (used twice weekly) and lubricants (as needed) for sexual activity. The use of vaginal dilators or topical estrogen therapy is individualized.—JOANN V. PINKERTON, MD
We want to hear from you! Tell us what you think.
1. Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181(1):66-70.
2. Freedman RR. Core body temperature variation in symptomatic and asymptomatic postmenopausal women: brief report. Menopause. 2002;9(6):399-401.
3. Hervik J, Mialand O. Acupuncture for the treatment of hot flashes in breast cancer patients: a randomized, controlled trial. Breast Cancer Res Treat. 2009;116(2):311-316.
4. de Valois BA, Young TE, Robinson N, McCourt C, Maher EJ. Using traditional acupuncture for breast cancer-related hot flashes and night sweats. J Altern Complement Med. 2010;16(10):1047-1057.
1. Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181(1):66-70.
2. Freedman RR. Core body temperature variation in symptomatic and asymptomatic postmenopausal women: brief report. Menopause. 2002;9(6):399-401.
3. Hervik J, Mialand O. Acupuncture for the treatment of hot flashes in breast cancer patients: a randomized, controlled trial. Breast Cancer Res Treat. 2009;116(2):311-316.
4. de Valois BA, Young TE, Robinson N, McCourt C, Maher EJ. Using traditional acupuncture for breast cancer-related hot flashes and night sweats. J Altern Complement Med. 2010;16(10):1047-1057.
“Bioidentical” hormones: What you (and your patient) need to know
OBG Management Senior Editor Janelle Yates contributed to this article.
Hear Dr. Pinkerton discuss this article
The Women’s Health Initiative (WHI) caused a sea change in women’s attitudes toward menopausal hormone therapy and aroused many fears—not always rational—that remain almost palpable today. One study of the aftermath of the WHI found that 70% of women who were taking hormone therapy discontinued it, and 26% of women lost confidence in medical recommendations in general.1
Into the chaos stepped Suzanne Somers, Michael Platt, and other celebrities, touting the benefits of a new kind of hormone: bioidentical. You don’t have to read Somers’ bestseller, The Sexy Years, to encounter the claims it makes on behalf of bioidenticals; the cover itself makes them clear: Discover the Hormone Connection—The Secret to Fabulous Sex, Great Health and Vitality, for Women and Men. Since publication of the book, the demand for bioidentical hormones has only increased, as women remain fearful about conventional hormone therapy.
Many ObGyns regularly field requests from patients for specially compounded bioidentical regimens. In most cases, the women who ask for these drugs are poorly informed about their risks and willing to pay out of pocket to acquire them. JoAnn V. Pinkerton, MD, sees many of these patients at The Women’s Place Midlife Health Center in Charlottesville, Virginia. OBG Management recently sat down with Dr. Pinkerton to discuss her concerns about the growing ubiquity of compounded bioidentical hormones. In the Q&A that follows, we talk about what “bioidentical” actually means, whether these hormones are ever justified, common misconceptions about them, and other issues.
In a special accompanying commentary, former Food and Drug Administration (FDA) Senior Medical Officer Bruce Patsner, MD, JD, also weighs in on the issue.
- “They’re identical to the hormones in my body”
- “They occur naturally”
- “They are safer and more effective than conventional hormone therapy”
- “They’re risk-free”
- “They are monitored by the FDA”
- “They are the fountain of youth”
- “They prevent breast cancer”
- “Celebrities know more about them than physicians and menopause and hormone experts do”
- “Doctors oppose bioidentical hormone therapy because they are in the pocket of Big Pharma”
- “Bioidentical hormones are not a huge money-making enterprise”
What is “bioidentical”?
OBG MANAGEMENT: Let’s start with the basics. What does the word “bioidentical” mean? Is it a legitimate medical term?
DR. PINKERTON: Bioidentical hormones are exogenous hormones that are biochemically similar to those produced endogenously by the body or ovaries. These include estrone, estradiol, estriol, progesterone, testosterone, dehydroepiandrosterone (DHEA), and cortisol. The FDA has approved many prescription products that contain bioidentical hormones. However, the term “bioidentical” is often used to refer to custom-compounded hormones. The major difference between the FDA-approved prescription bioidentical hormone products and custom-compounded products is that the former are regulated by the FDA and tested for purity, potency, efficacy, and safety.
Bioidenticals are not “natural” hormones, although many consumers think they are. In reality, compounded bioidentical hormones and FDA-approved bioidentical hormones all come from the same precursors. They begin as soy products or wild yam and then get converted to the different hormones in a laboratory in Germany before finding their way to the various world markets.
The claim that all bioidentical hormones are bioengineered to contain the same chemical structure as natural female sex hormones is false. As one expert noted, “the term ‘bioidentical’ has become inappropriately synonymous with ‘natural’ or ‘not synthetic’ and should be redefined to correct patient misconceptions.”2
Common misconceptions
OBG MANAGEMENT: What are some of the other false impressions you encounter among patients who ask for bioidenticals?
DR. PINKERTON: That the hormones are safer or more effective than hormone therapy, that they carry no risks, and that they are as well-monitored as FDA-approved products, to name a few. (For more, see “10 erroneous beliefs patients have about compounded hormones”).
OBG MANAGEMENT: Where do these ideas originate?
DR. PINKERTON: They are propagated by self-proclaimed experts and celebrities or by laypersons and physicians who devote the bulk of their time to promoting these hormones, usually at considerable cost to the patient.
OBG MANAGEMENT: What are the risks of compounded bioidentical hormones?
DR. PINKERTON: According to FDA guidance for industry, in the absence of data about these hormones, the risks and benefits should be assumed to be identical to those of FDA-approved hormone therapies, with the caveat that we don’t know from batch to batch what a woman is receiving. However, they are not regulated or monitored by the FDA, so we are lacking testing for purity, potency, efficacy, and safety. When the FDA did analyze compounded bioidentical hormones, a significant percentage (34%) failed one or more standard quality tests.3 In comparison, FDA-approved drugs fail analytical testing at a rate of less than 2%.3

BRUCE PATSNER, MD, JD
Dr. Patsner is Research Professor of Law at the University of Houston Law Center in Houston, Tex. He served as Senior Medical Officer at the US Food and Drug Administration (FDA), where he was one of the agency’s experts on pharmacy compounding of prescription hormone drug therapy for the treatment of menopausal conditions.
The FDA has nothing against compounding pharmacies per se. Individualized preparation of a customized medication for a patient, based on a valid prescription, is an essential part of the practice of pharmacy. However, some actors in the pharmacy compounding business have taken the practice to a different level, not just in terms of the volume of business they do, but in the way compounded hormones are advertised and promoted. The courts aren’t necessarily interested in intervening in cases involving high volume alone. And when it comes to unsubstantiated claims of benefit, the FDA has found it difficult to assert jurisdiction over pharmacy compounding in general, making it hard to assert control over the advertising claims these pharmacies make on behalf of compounded drugs.
The result? The FDA has been unable to rein in claims that compounded prescription drugs are safer or better than commercially prepared medications. These drugs are probably as safe and effective as their manufactured counterparts, but there are no data to confirm this assumption.
What’s in a name?
“Bioidentical” isn’t a bona fide term. There is no definition of it in any medical dictionary; it’s just a name the industry cooked up, a catchy one at that. And when bioidenticals are advertised and promoted, the term “natural” is usually in close proximity. Most patients equate the word natural with plant-derived substances that have not been chemically altered. The fact is, many compounded prescription drugs are derived from plants—but they are also chemically altered.
Some applications are legitimate
A number of women use compounded medications because they make it possible to obtain hormone combinations that are not readily available in cream form. For example, if a patient wants testosterone as part of a cream of estrogen and progesterone, a compounded product is the only option.
Show me the data
No studies have compared compounded drugs with commercial drugs—and such studies are exceedingly unlikely. Compounding pharmacies have no incentive to conduct or participate in such studies. The pharmaceutical compounding industry is a multibillion-dollar enterprise in this country, and compounded prescription drugs for menopausal conditions are probably the biggest product outside of the oncology arena. Proponents of compounded hormones have a captive audience, so to speak, made up of women who don’t like commercial drug manufacturers or who prefer products that appear to be natural, or both.
The problem is that these women receive no package insert or prescription drug label with their hormones. Warning labels are not required because compounded drugs are not regulated by the FDA. Consumers are basically at the mercy of whatever claims they read on the Internet or in the lay literature, which tends to be written by people who have a financial interest in affiliating with the compounding industry. It’s a very frustrating situation for a lot of people.
Unintended consequences of the WHI
The Women’s Health Initiative (WHI) stirred demand for bioidentical hormones by casting the safety claims for some commercial hormone therapy products in a less than favorable light. That wasn’t the investigators’ intent, of course, and some of the findings of the WHI have since been questioned.
The goal of the WHI was to critically evaluate some of the touted health benefits of commercial hormone therapy prescription drugs, but, by questioning some of these claims, it inadvertently pushed a significant percentage of patients toward compounded prescription drugs—and we have no safety data on them.
No one knows exactly how many women were swayed, but the consensus is that they were, and no one’s been happy about that.
The main problem with the compounded hormones, as I see it, is that women who use them do not receive any written information from the compounding pharmacist about risks and benefits. Nor do they receive the black box warnings on FDA-approved estrogen therapy. I believe women need to be adequately educated about the potential risks and benefits, as well as the lack of efficacy data and quality control, if compounded products are requested. That means it’s up to the prescriber to educate the patient about the potential risks and benefits.
Rosenthal states that symptomatic menopausal women or those who fear breast cancer or heart disease can be considered a vulnerable population: “Patients do not have the background to decipher credible sources from noncredible sources.” False claims present convincing arguments for laypersons. A woman may be vulnerable to unsubstantiated claims by virtue of her symptoms and the anxiety and even depression that they can produce. Without comprehensive education, these women cannot be assumed to be adequately informed.
Let me put it in perspective. If a patient with a history of breast cancer complains about severe vaginal dryness that interferes with her sex life, I might decide to give her the smallest amount of topical estrogen that I can—for example, a dime-sized amount of estrogen to apply to her vulvar area twice a week. This amount of estrogen can’t be detected in her system with current assays. I know that some of it will be systemically absorbed, but it cannot be detected. When the patient buys that commercially prepared cream from the pharmacist, she will receive the same black box warning that comes with all systemic hormones since the WHI. However, if she goes to a compounding pharmacist with a prescription for bioidentical hormone therapy, she will not get the warning, regardless of the ingredients or dosage.
The American College of Obstetricians and Gynecologists (ACOG), North American Menopause Society (NAMS), and The Endocrine Society have all issued statements noting the lack of safety data on compounded bioidentical hormones. Here’s what they say:
ACOG
“Most compounded products have not undergone rigorous clinical testing for safety or efficacy, and issues regarding purity, potency and quality are a concern. Compounded hormone products have the same safety issues as those associated with hormone therapy agents that are approved by the US Food and Drug Administration and may have additional risks intrinsic to compounding. There is no scientific evidence to support claims of increased efficacy or safety for individualized estrogen or progesterone regimens.”
NAMS
“NAMS does not recommend custom-compounded products over well-tested, government-approved products for the majority of women—and does not recommend saliva testing to determine hormone levels” (www.menopause.org/bioidentical_NAMS.aspx).
The Endocrine Society
“‘Bioidentical hormones,’ particularly estrogen and progesterone, have been promoted as safer and more effective alternatives to more traditional hormone therapies, often by people outside of the medical community. In fact, little or no scientific and medical evidence exists to support such claims about ‘bioidentical hormones.’ Additionally, many ‘bioidentical hormone’ formulations are not subject to FDA oversight and can be inconsistent in dose and purity….”
Are compounded bioidenticals ever justified?
OBG MANAGEMENT: According to the FDA, compounding of drug products is justified only when a practitioner finds that an FDA-approved drug does not meet the patient’s needs. Do you think this is ever really the case, given the availability of FDA-approved bioidentical hormone preparations?
DR. PINKERTON: In rare cases, compounding of bioidentical hormones is justified, such as when a patient cannot tolerate an FDA-approved product. The problem is that women have been especially concerned about the safety of hormone therapy since the WHI, and bioidentical hormones have been promoted as being safer than FDA-regulated preparations, despite the lack of evidence of their safety or efficacy in peer-reviewed literature. So many women request them.
In a recent commentary, Boothby and Doering call bioidentical hormone therapy “a panacea that lacks supportive evidence.” They say, “It’s our belief that pharmacists are compounding these with the best intentions, but they are ill informed regarding the lack of scientific underpinning associated with efficacy and safety.”3
OBG MANAGEMENT: Do you ever prescribe bioidentical hormones?
DR. PINKERTON: Yes, but rarely, and primarily for women who can’t tolerate FDA-approved hormones or who, after adequate information and education, refuse FDA-approved hormone therapy.
Is salivary hormone testing informative?
OBG MANAGEMENT: Many clinicians who prescribe bioidentical hormones base the dosage on salivary hormone testing. They claim that this allows them to offer individualized formulations. Is this a reliable claim?
DR. PINKERTON: No, it isn’t. Although compounded bioidentical hormone therapy is often prescribed on the basis of salivary hormone testing, there is no scientific evidence that a correlation exists between a patient’s symptoms and salivary hormones, or that salivary hormone testing reflects what is happening at the tissue level. As Fugh-Berman and Bythrow have observed, this type of testing is often used to convince asymptomatic consumers to use hormones—or symptomatic women to take higher dosages. That practice is likely to lead to adverse events.5 The practice also directly contradicts evidence-based guidelines, which recommend that hormone therapy be individualized on the basis of symptoms, not hormone levels.6
There are no published studies in the peer-reviewed literature that show that salivary testing is a reliable measure on which to safely and effectively base dosing decisions. Indeed, The Endocrine Society issued a position statement that notes, among other issues, that salivary hormone tests are “inaccurate and should not be considered reliable measures of hormones in the body.”7 The American College of Obstetricians and Gynecologists also advises against salivary testing, observing that:
- 1) there is no biologically meaningful relationship between salivary sex steroidal hormone concentrations and free serum hormone concentrations
- 2) there is large within-patient variability in salivary hormone concentrations. Salivary hormone levels vary depending on diet, time of day of testing, the specific hormone being tested, and other variables.3
Do bioidenticals protect against cancer?
OBG MANAGEMENT: Some reports mention the fact that many women believe that bioidentical hormones—specifically, estriol—can reduce their risk of breast and endometrial cancer. Is there any truth behind this belief?
DR. PINKERTON: Estriol is a weak estrogen. There is no evidence that, if it is given at a dosage high enough to relieve symptoms, it is any safer than estradiol.
In regard to endometrial cancer, if the exogenous estrogen—bioidentical or otherwise—is unopposed or inadequately opposed, the risk of endometrial cancer is elevated. The problem is that it is hard to determine whether estrogen is being adequately opposed, particularly when transdermal compounded progesterone is given, because the progesterone molecule is too large to be well-absorbed systemically.9
In regard to breast cancer, estriol is a less potent estrogen than estradiol, but it is believed to carry the same risks if it is dosed at effective levels. There is nothing about estriol per se in the peer-reviewed literature that shows that it protects against breast cancer.
The data on risk of breast cancer with estrogen therapy is confusing, with potentially higher risks if estrogen is combined with progestogen. Most of the data we have on estriol come from animals, but a study from 1980 in humans showed that, when older women with breast cancer were treated with estriol, 25% had increased growth of metastases.8
How do you monitor use of bioidentical hormones?
OBG MANAGEMENT: When you do prescribe a compounded bioidentical hormone, how do you monitor the patient?
DR. PINKERTON: First, I want to reiterate that I prescribe these hormones after considerable patient education about FDA-approved options and their potential risks. Second, when a patient needs or requests hormone therapy, I recommend conventional therapy. Only when she cannot tolerate or refuses FDA-approved drugs do I consider prescribing compounded bioidentical hormones—which, as I said earlier, are assumed to carry risks identical to those of FDA-approved hormones.
In some cases, I provide gynecologic care for patients who obtain compounded bioidentical hormones from other sources. What I will sometimes do, just to give myself some idea of how much estrogen they are getting, is to measure the peak and trough estradiol and estrone levels. That is, I measure the hormone level within 4 hours of the patient taking the drug to see how high it goes, and again about 12 hours later to see how low it goes. I measure both because estradiol may be peripherally converted to estrone.
Regrettably, we don’t know what to do about the various hormone levels. It isn’t like treating thyroid disorders; we normally dose estrogen therapy based on symptoms.
Who pays?
OBG MANAGEMENT: Who pays for salivary testing and compounded bioidentical hormones? Does health insurance cover them?
DR. PINKERTON: Like other “natural” products, compounded bioidenticals may cost more than their commercially prepared counterparts and often are not covered by insurance. In addition, prescribers may charge more for a “consultation” than do practitioners who accept insurance; they also may recommend salivary testing, which is expensive. Patients can end up paying large sums out of pocket.
As Rosenthal noted, many women do not appear to be concerned about the added costs.2 That may be because compounded bioidentical hormone therapy is usually offered to economically advantaged patients.2
Ethical considerations
OBG MANAGEMENT: That raises an important question: What ethical considerations are inherent in the prescribing of compounded bioidenticals?
DR. PINKERTON: The fact that women who are able to pay out of pocket are the primary users of these drugs is one important point. In her analysis of the ethics surrounding bioidentical hormones, Rosenthal noted that the drugs remain “an unequal alternative, and any data collected would not be representative of the overall menopausal community.”2
A critical issue pointed out by Rosenthal is that perimenopausal and menopausal women may be particularly vulnerable to the unsubstantiated claims of purveyors of bio identical hormones. “A substantial number of women seek out bioidentical hormone replacement therapy to restore sexual well-being and functioning, in particular, who may be psychologically more vulnerable,” she writes.2
Another concern arises when the practitioner who prescribes bioidentical hormones also happens to sell them. This poses a potential conflict of interest and “violates professional ethical conduct.”2
OBG MANAGEMENT: Do physicians aggravate the problem when they accede to a patient’s request for compounded hormones?
DR. PINKERTON: Physicians and health-care providers need to stop and educate the patient about the lack of safety and efficacy data, the risks and benefits, and recognize the possibility that she has been influenced by unsubstantiated claims.
1. Schonberg MA, Davis RB, Wee CC. After the Women’s Health Initiative: decision making and trust of women taking hormone therapy. Womens Health Issues. 2005;15:187-195.
2. Rosenthal MS. Ethical problems with bioidentical hormone therapy. Int J Impot Res. 2008;20:45-52.
3. ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 322. Compounded bioidentical hormones. Obstet Gynecol. 2005;106(5 Pt 1):1139-1140.
4. Boothby LA, Doering PL. Bioidentical hormone therapy: a panacea that lacks supportive evidence. Curr Opin Obstet Gynecol. 2008;20:400-407.
5. Fugh-Berman A, Bythrow J. Bioidentical hormones for menopausal hormone therapy: variation on a theme. J Gen Intern Med. 2007;22:1030-1034.
6. Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health. 2007;16:600-631.
7. Bioidentical hormones lack evidence for safety and effectivness, according to new statement by The Endocrine Society [press release]. Chevy Chase, Md: The Endocrine Society; June 14, 2008.
8. Lemon HM. Pathophysiologic considerations in the treatment of menopausal patients with oestrogens: mammary carcinoma. Acta Endocrinol Suppl [Copenhagen]. 1980;233:17-27.
9. Wren BG, Champion SM, Willets K, et al. Transdermal progesterone and its effects on vasomotor symptoms, blood lipid levels, bone metabolic markers, mood, and quality of life for postmenopausal women. Menopause. 2003;10:13-18.
OBG Management Senior Editor Janelle Yates contributed to this article.
Hear Dr. Pinkerton discuss this article
The Women’s Health Initiative (WHI) caused a sea change in women’s attitudes toward menopausal hormone therapy and aroused many fears—not always rational—that remain almost palpable today. One study of the aftermath of the WHI found that 70% of women who were taking hormone therapy discontinued it, and 26% of women lost confidence in medical recommendations in general.1
Into the chaos stepped Suzanne Somers, Michael Platt, and other celebrities, touting the benefits of a new kind of hormone: bioidentical. You don’t have to read Somers’ bestseller, The Sexy Years, to encounter the claims it makes on behalf of bioidenticals; the cover itself makes them clear: Discover the Hormone Connection—The Secret to Fabulous Sex, Great Health and Vitality, for Women and Men. Since publication of the book, the demand for bioidentical hormones has only increased, as women remain fearful about conventional hormone therapy.
Many ObGyns regularly field requests from patients for specially compounded bioidentical regimens. In most cases, the women who ask for these drugs are poorly informed about their risks and willing to pay out of pocket to acquire them. JoAnn V. Pinkerton, MD, sees many of these patients at The Women’s Place Midlife Health Center in Charlottesville, Virginia. OBG Management recently sat down with Dr. Pinkerton to discuss her concerns about the growing ubiquity of compounded bioidentical hormones. In the Q&A that follows, we talk about what “bioidentical” actually means, whether these hormones are ever justified, common misconceptions about them, and other issues.
In a special accompanying commentary, former Food and Drug Administration (FDA) Senior Medical Officer Bruce Patsner, MD, JD, also weighs in on the issue.
- “They’re identical to the hormones in my body”
- “They occur naturally”
- “They are safer and more effective than conventional hormone therapy”
- “They’re risk-free”
- “They are monitored by the FDA”
- “They are the fountain of youth”
- “They prevent breast cancer”
- “Celebrities know more about them than physicians and menopause and hormone experts do”
- “Doctors oppose bioidentical hormone therapy because they are in the pocket of Big Pharma”
- “Bioidentical hormones are not a huge money-making enterprise”
What is “bioidentical”?
OBG MANAGEMENT: Let’s start with the basics. What does the word “bioidentical” mean? Is it a legitimate medical term?
DR. PINKERTON: Bioidentical hormones are exogenous hormones that are biochemically similar to those produced endogenously by the body or ovaries. These include estrone, estradiol, estriol, progesterone, testosterone, dehydroepiandrosterone (DHEA), and cortisol. The FDA has approved many prescription products that contain bioidentical hormones. However, the term “bioidentical” is often used to refer to custom-compounded hormones. The major difference between the FDA-approved prescription bioidentical hormone products and custom-compounded products is that the former are regulated by the FDA and tested for purity, potency, efficacy, and safety.
Bioidenticals are not “natural” hormones, although many consumers think they are. In reality, compounded bioidentical hormones and FDA-approved bioidentical hormones all come from the same precursors. They begin as soy products or wild yam and then get converted to the different hormones in a laboratory in Germany before finding their way to the various world markets.
The claim that all bioidentical hormones are bioengineered to contain the same chemical structure as natural female sex hormones is false. As one expert noted, “the term ‘bioidentical’ has become inappropriately synonymous with ‘natural’ or ‘not synthetic’ and should be redefined to correct patient misconceptions.”2
Common misconceptions
OBG MANAGEMENT: What are some of the other false impressions you encounter among patients who ask for bioidenticals?
DR. PINKERTON: That the hormones are safer or more effective than hormone therapy, that they carry no risks, and that they are as well-monitored as FDA-approved products, to name a few. (For more, see “10 erroneous beliefs patients have about compounded hormones”).
OBG MANAGEMENT: Where do these ideas originate?
DR. PINKERTON: They are propagated by self-proclaimed experts and celebrities or by laypersons and physicians who devote the bulk of their time to promoting these hormones, usually at considerable cost to the patient.
OBG MANAGEMENT: What are the risks of compounded bioidentical hormones?
DR. PINKERTON: According to FDA guidance for industry, in the absence of data about these hormones, the risks and benefits should be assumed to be identical to those of FDA-approved hormone therapies, with the caveat that we don’t know from batch to batch what a woman is receiving. However, they are not regulated or monitored by the FDA, so we are lacking testing for purity, potency, efficacy, and safety. When the FDA did analyze compounded bioidentical hormones, a significant percentage (34%) failed one or more standard quality tests.3 In comparison, FDA-approved drugs fail analytical testing at a rate of less than 2%.3

BRUCE PATSNER, MD, JD
Dr. Patsner is Research Professor of Law at the University of Houston Law Center in Houston, Tex. He served as Senior Medical Officer at the US Food and Drug Administration (FDA), where he was one of the agency’s experts on pharmacy compounding of prescription hormone drug therapy for the treatment of menopausal conditions.
The FDA has nothing against compounding pharmacies per se. Individualized preparation of a customized medication for a patient, based on a valid prescription, is an essential part of the practice of pharmacy. However, some actors in the pharmacy compounding business have taken the practice to a different level, not just in terms of the volume of business they do, but in the way compounded hormones are advertised and promoted. The courts aren’t necessarily interested in intervening in cases involving high volume alone. And when it comes to unsubstantiated claims of benefit, the FDA has found it difficult to assert jurisdiction over pharmacy compounding in general, making it hard to assert control over the advertising claims these pharmacies make on behalf of compounded drugs.
The result? The FDA has been unable to rein in claims that compounded prescription drugs are safer or better than commercially prepared medications. These drugs are probably as safe and effective as their manufactured counterparts, but there are no data to confirm this assumption.
What’s in a name?
“Bioidentical” isn’t a bona fide term. There is no definition of it in any medical dictionary; it’s just a name the industry cooked up, a catchy one at that. And when bioidenticals are advertised and promoted, the term “natural” is usually in close proximity. Most patients equate the word natural with plant-derived substances that have not been chemically altered. The fact is, many compounded prescription drugs are derived from plants—but they are also chemically altered.
Some applications are legitimate
A number of women use compounded medications because they make it possible to obtain hormone combinations that are not readily available in cream form. For example, if a patient wants testosterone as part of a cream of estrogen and progesterone, a compounded product is the only option.
Show me the data
No studies have compared compounded drugs with commercial drugs—and such studies are exceedingly unlikely. Compounding pharmacies have no incentive to conduct or participate in such studies. The pharmaceutical compounding industry is a multibillion-dollar enterprise in this country, and compounded prescription drugs for menopausal conditions are probably the biggest product outside of the oncology arena. Proponents of compounded hormones have a captive audience, so to speak, made up of women who don’t like commercial drug manufacturers or who prefer products that appear to be natural, or both.
The problem is that these women receive no package insert or prescription drug label with their hormones. Warning labels are not required because compounded drugs are not regulated by the FDA. Consumers are basically at the mercy of whatever claims they read on the Internet or in the lay literature, which tends to be written by people who have a financial interest in affiliating with the compounding industry. It’s a very frustrating situation for a lot of people.
Unintended consequences of the WHI
The Women’s Health Initiative (WHI) stirred demand for bioidentical hormones by casting the safety claims for some commercial hormone therapy products in a less than favorable light. That wasn’t the investigators’ intent, of course, and some of the findings of the WHI have since been questioned.
The goal of the WHI was to critically evaluate some of the touted health benefits of commercial hormone therapy prescription drugs, but, by questioning some of these claims, it inadvertently pushed a significant percentage of patients toward compounded prescription drugs—and we have no safety data on them.
No one knows exactly how many women were swayed, but the consensus is that they were, and no one’s been happy about that.
The main problem with the compounded hormones, as I see it, is that women who use them do not receive any written information from the compounding pharmacist about risks and benefits. Nor do they receive the black box warnings on FDA-approved estrogen therapy. I believe women need to be adequately educated about the potential risks and benefits, as well as the lack of efficacy data and quality control, if compounded products are requested. That means it’s up to the prescriber to educate the patient about the potential risks and benefits.
Rosenthal states that symptomatic menopausal women or those who fear breast cancer or heart disease can be considered a vulnerable population: “Patients do not have the background to decipher credible sources from noncredible sources.” False claims present convincing arguments for laypersons. A woman may be vulnerable to unsubstantiated claims by virtue of her symptoms and the anxiety and even depression that they can produce. Without comprehensive education, these women cannot be assumed to be adequately informed.
Let me put it in perspective. If a patient with a history of breast cancer complains about severe vaginal dryness that interferes with her sex life, I might decide to give her the smallest amount of topical estrogen that I can—for example, a dime-sized amount of estrogen to apply to her vulvar area twice a week. This amount of estrogen can’t be detected in her system with current assays. I know that some of it will be systemically absorbed, but it cannot be detected. When the patient buys that commercially prepared cream from the pharmacist, she will receive the same black box warning that comes with all systemic hormones since the WHI. However, if she goes to a compounding pharmacist with a prescription for bioidentical hormone therapy, she will not get the warning, regardless of the ingredients or dosage.
The American College of Obstetricians and Gynecologists (ACOG), North American Menopause Society (NAMS), and The Endocrine Society have all issued statements noting the lack of safety data on compounded bioidentical hormones. Here’s what they say:
ACOG
“Most compounded products have not undergone rigorous clinical testing for safety or efficacy, and issues regarding purity, potency and quality are a concern. Compounded hormone products have the same safety issues as those associated with hormone therapy agents that are approved by the US Food and Drug Administration and may have additional risks intrinsic to compounding. There is no scientific evidence to support claims of increased efficacy or safety for individualized estrogen or progesterone regimens.”
NAMS
“NAMS does not recommend custom-compounded products over well-tested, government-approved products for the majority of women—and does not recommend saliva testing to determine hormone levels” (www.menopause.org/bioidentical_NAMS.aspx).
The Endocrine Society
“‘Bioidentical hormones,’ particularly estrogen and progesterone, have been promoted as safer and more effective alternatives to more traditional hormone therapies, often by people outside of the medical community. In fact, little or no scientific and medical evidence exists to support such claims about ‘bioidentical hormones.’ Additionally, many ‘bioidentical hormone’ formulations are not subject to FDA oversight and can be inconsistent in dose and purity….”
Are compounded bioidenticals ever justified?
OBG MANAGEMENT: According to the FDA, compounding of drug products is justified only when a practitioner finds that an FDA-approved drug does not meet the patient’s needs. Do you think this is ever really the case, given the availability of FDA-approved bioidentical hormone preparations?
DR. PINKERTON: In rare cases, compounding of bioidentical hormones is justified, such as when a patient cannot tolerate an FDA-approved product. The problem is that women have been especially concerned about the safety of hormone therapy since the WHI, and bioidentical hormones have been promoted as being safer than FDA-regulated preparations, despite the lack of evidence of their safety or efficacy in peer-reviewed literature. So many women request them.
In a recent commentary, Boothby and Doering call bioidentical hormone therapy “a panacea that lacks supportive evidence.” They say, “It’s our belief that pharmacists are compounding these with the best intentions, but they are ill informed regarding the lack of scientific underpinning associated with efficacy and safety.”3
OBG MANAGEMENT: Do you ever prescribe bioidentical hormones?
DR. PINKERTON: Yes, but rarely, and primarily for women who can’t tolerate FDA-approved hormones or who, after adequate information and education, refuse FDA-approved hormone therapy.
Is salivary hormone testing informative?
OBG MANAGEMENT: Many clinicians who prescribe bioidentical hormones base the dosage on salivary hormone testing. They claim that this allows them to offer individualized formulations. Is this a reliable claim?
DR. PINKERTON: No, it isn’t. Although compounded bioidentical hormone therapy is often prescribed on the basis of salivary hormone testing, there is no scientific evidence that a correlation exists between a patient’s symptoms and salivary hormones, or that salivary hormone testing reflects what is happening at the tissue level. As Fugh-Berman and Bythrow have observed, this type of testing is often used to convince asymptomatic consumers to use hormones—or symptomatic women to take higher dosages. That practice is likely to lead to adverse events.5 The practice also directly contradicts evidence-based guidelines, which recommend that hormone therapy be individualized on the basis of symptoms, not hormone levels.6
There are no published studies in the peer-reviewed literature that show that salivary testing is a reliable measure on which to safely and effectively base dosing decisions. Indeed, The Endocrine Society issued a position statement that notes, among other issues, that salivary hormone tests are “inaccurate and should not be considered reliable measures of hormones in the body.”7 The American College of Obstetricians and Gynecologists also advises against salivary testing, observing that:
- 1) there is no biologically meaningful relationship between salivary sex steroidal hormone concentrations and free serum hormone concentrations
- 2) there is large within-patient variability in salivary hormone concentrations. Salivary hormone levels vary depending on diet, time of day of testing, the specific hormone being tested, and other variables.3
Do bioidenticals protect against cancer?
OBG MANAGEMENT: Some reports mention the fact that many women believe that bioidentical hormones—specifically, estriol—can reduce their risk of breast and endometrial cancer. Is there any truth behind this belief?
DR. PINKERTON: Estriol is a weak estrogen. There is no evidence that, if it is given at a dosage high enough to relieve symptoms, it is any safer than estradiol.
In regard to endometrial cancer, if the exogenous estrogen—bioidentical or otherwise—is unopposed or inadequately opposed, the risk of endometrial cancer is elevated. The problem is that it is hard to determine whether estrogen is being adequately opposed, particularly when transdermal compounded progesterone is given, because the progesterone molecule is too large to be well-absorbed systemically.9
In regard to breast cancer, estriol is a less potent estrogen than estradiol, but it is believed to carry the same risks if it is dosed at effective levels. There is nothing about estriol per se in the peer-reviewed literature that shows that it protects against breast cancer.
The data on risk of breast cancer with estrogen therapy is confusing, with potentially higher risks if estrogen is combined with progestogen. Most of the data we have on estriol come from animals, but a study from 1980 in humans showed that, when older women with breast cancer were treated with estriol, 25% had increased growth of metastases.8
How do you monitor use of bioidentical hormones?
OBG MANAGEMENT: When you do prescribe a compounded bioidentical hormone, how do you monitor the patient?
DR. PINKERTON: First, I want to reiterate that I prescribe these hormones after considerable patient education about FDA-approved options and their potential risks. Second, when a patient needs or requests hormone therapy, I recommend conventional therapy. Only when she cannot tolerate or refuses FDA-approved drugs do I consider prescribing compounded bioidentical hormones—which, as I said earlier, are assumed to carry risks identical to those of FDA-approved hormones.
In some cases, I provide gynecologic care for patients who obtain compounded bioidentical hormones from other sources. What I will sometimes do, just to give myself some idea of how much estrogen they are getting, is to measure the peak and trough estradiol and estrone levels. That is, I measure the hormone level within 4 hours of the patient taking the drug to see how high it goes, and again about 12 hours later to see how low it goes. I measure both because estradiol may be peripherally converted to estrone.
Regrettably, we don’t know what to do about the various hormone levels. It isn’t like treating thyroid disorders; we normally dose estrogen therapy based on symptoms.
Who pays?
OBG MANAGEMENT: Who pays for salivary testing and compounded bioidentical hormones? Does health insurance cover them?
DR. PINKERTON: Like other “natural” products, compounded bioidenticals may cost more than their commercially prepared counterparts and often are not covered by insurance. In addition, prescribers may charge more for a “consultation” than do practitioners who accept insurance; they also may recommend salivary testing, which is expensive. Patients can end up paying large sums out of pocket.
As Rosenthal noted, many women do not appear to be concerned about the added costs.2 That may be because compounded bioidentical hormone therapy is usually offered to economically advantaged patients.2
Ethical considerations
OBG MANAGEMENT: That raises an important question: What ethical considerations are inherent in the prescribing of compounded bioidenticals?
DR. PINKERTON: The fact that women who are able to pay out of pocket are the primary users of these drugs is one important point. In her analysis of the ethics surrounding bioidentical hormones, Rosenthal noted that the drugs remain “an unequal alternative, and any data collected would not be representative of the overall menopausal community.”2
A critical issue pointed out by Rosenthal is that perimenopausal and menopausal women may be particularly vulnerable to the unsubstantiated claims of purveyors of bio identical hormones. “A substantial number of women seek out bioidentical hormone replacement therapy to restore sexual well-being and functioning, in particular, who may be psychologically more vulnerable,” she writes.2
Another concern arises when the practitioner who prescribes bioidentical hormones also happens to sell them. This poses a potential conflict of interest and “violates professional ethical conduct.”2
OBG MANAGEMENT: Do physicians aggravate the problem when they accede to a patient’s request for compounded hormones?
DR. PINKERTON: Physicians and health-care providers need to stop and educate the patient about the lack of safety and efficacy data, the risks and benefits, and recognize the possibility that she has been influenced by unsubstantiated claims.
OBG Management Senior Editor Janelle Yates contributed to this article.
Hear Dr. Pinkerton discuss this article
The Women’s Health Initiative (WHI) caused a sea change in women’s attitudes toward menopausal hormone therapy and aroused many fears—not always rational—that remain almost palpable today. One study of the aftermath of the WHI found that 70% of women who were taking hormone therapy discontinued it, and 26% of women lost confidence in medical recommendations in general.1
Into the chaos stepped Suzanne Somers, Michael Platt, and other celebrities, touting the benefits of a new kind of hormone: bioidentical. You don’t have to read Somers’ bestseller, The Sexy Years, to encounter the claims it makes on behalf of bioidenticals; the cover itself makes them clear: Discover the Hormone Connection—The Secret to Fabulous Sex, Great Health and Vitality, for Women and Men. Since publication of the book, the demand for bioidentical hormones has only increased, as women remain fearful about conventional hormone therapy.
Many ObGyns regularly field requests from patients for specially compounded bioidentical regimens. In most cases, the women who ask for these drugs are poorly informed about their risks and willing to pay out of pocket to acquire them. JoAnn V. Pinkerton, MD, sees many of these patients at The Women’s Place Midlife Health Center in Charlottesville, Virginia. OBG Management recently sat down with Dr. Pinkerton to discuss her concerns about the growing ubiquity of compounded bioidentical hormones. In the Q&A that follows, we talk about what “bioidentical” actually means, whether these hormones are ever justified, common misconceptions about them, and other issues.
In a special accompanying commentary, former Food and Drug Administration (FDA) Senior Medical Officer Bruce Patsner, MD, JD, also weighs in on the issue.
- “They’re identical to the hormones in my body”
- “They occur naturally”
- “They are safer and more effective than conventional hormone therapy”
- “They’re risk-free”
- “They are monitored by the FDA”
- “They are the fountain of youth”
- “They prevent breast cancer”
- “Celebrities know more about them than physicians and menopause and hormone experts do”
- “Doctors oppose bioidentical hormone therapy because they are in the pocket of Big Pharma”
- “Bioidentical hormones are not a huge money-making enterprise”
What is “bioidentical”?
OBG MANAGEMENT: Let’s start with the basics. What does the word “bioidentical” mean? Is it a legitimate medical term?
DR. PINKERTON: Bioidentical hormones are exogenous hormones that are biochemically similar to those produced endogenously by the body or ovaries. These include estrone, estradiol, estriol, progesterone, testosterone, dehydroepiandrosterone (DHEA), and cortisol. The FDA has approved many prescription products that contain bioidentical hormones. However, the term “bioidentical” is often used to refer to custom-compounded hormones. The major difference between the FDA-approved prescription bioidentical hormone products and custom-compounded products is that the former are regulated by the FDA and tested for purity, potency, efficacy, and safety.
Bioidenticals are not “natural” hormones, although many consumers think they are. In reality, compounded bioidentical hormones and FDA-approved bioidentical hormones all come from the same precursors. They begin as soy products or wild yam and then get converted to the different hormones in a laboratory in Germany before finding their way to the various world markets.
The claim that all bioidentical hormones are bioengineered to contain the same chemical structure as natural female sex hormones is false. As one expert noted, “the term ‘bioidentical’ has become inappropriately synonymous with ‘natural’ or ‘not synthetic’ and should be redefined to correct patient misconceptions.”2
Common misconceptions
OBG MANAGEMENT: What are some of the other false impressions you encounter among patients who ask for bioidenticals?
DR. PINKERTON: That the hormones are safer or more effective than hormone therapy, that they carry no risks, and that they are as well-monitored as FDA-approved products, to name a few. (For more, see “10 erroneous beliefs patients have about compounded hormones”).
OBG MANAGEMENT: Where do these ideas originate?
DR. PINKERTON: They are propagated by self-proclaimed experts and celebrities or by laypersons and physicians who devote the bulk of their time to promoting these hormones, usually at considerable cost to the patient.
OBG MANAGEMENT: What are the risks of compounded bioidentical hormones?
DR. PINKERTON: According to FDA guidance for industry, in the absence of data about these hormones, the risks and benefits should be assumed to be identical to those of FDA-approved hormone therapies, with the caveat that we don’t know from batch to batch what a woman is receiving. However, they are not regulated or monitored by the FDA, so we are lacking testing for purity, potency, efficacy, and safety. When the FDA did analyze compounded bioidentical hormones, a significant percentage (34%) failed one or more standard quality tests.3 In comparison, FDA-approved drugs fail analytical testing at a rate of less than 2%.3

BRUCE PATSNER, MD, JD
Dr. Patsner is Research Professor of Law at the University of Houston Law Center in Houston, Tex. He served as Senior Medical Officer at the US Food and Drug Administration (FDA), where he was one of the agency’s experts on pharmacy compounding of prescription hormone drug therapy for the treatment of menopausal conditions.
The FDA has nothing against compounding pharmacies per se. Individualized preparation of a customized medication for a patient, based on a valid prescription, is an essential part of the practice of pharmacy. However, some actors in the pharmacy compounding business have taken the practice to a different level, not just in terms of the volume of business they do, but in the way compounded hormones are advertised and promoted. The courts aren’t necessarily interested in intervening in cases involving high volume alone. And when it comes to unsubstantiated claims of benefit, the FDA has found it difficult to assert jurisdiction over pharmacy compounding in general, making it hard to assert control over the advertising claims these pharmacies make on behalf of compounded drugs.
The result? The FDA has been unable to rein in claims that compounded prescription drugs are safer or better than commercially prepared medications. These drugs are probably as safe and effective as their manufactured counterparts, but there are no data to confirm this assumption.
What’s in a name?
“Bioidentical” isn’t a bona fide term. There is no definition of it in any medical dictionary; it’s just a name the industry cooked up, a catchy one at that. And when bioidenticals are advertised and promoted, the term “natural” is usually in close proximity. Most patients equate the word natural with plant-derived substances that have not been chemically altered. The fact is, many compounded prescription drugs are derived from plants—but they are also chemically altered.
Some applications are legitimate
A number of women use compounded medications because they make it possible to obtain hormone combinations that are not readily available in cream form. For example, if a patient wants testosterone as part of a cream of estrogen and progesterone, a compounded product is the only option.
Show me the data
No studies have compared compounded drugs with commercial drugs—and such studies are exceedingly unlikely. Compounding pharmacies have no incentive to conduct or participate in such studies. The pharmaceutical compounding industry is a multibillion-dollar enterprise in this country, and compounded prescription drugs for menopausal conditions are probably the biggest product outside of the oncology arena. Proponents of compounded hormones have a captive audience, so to speak, made up of women who don’t like commercial drug manufacturers or who prefer products that appear to be natural, or both.
The problem is that these women receive no package insert or prescription drug label with their hormones. Warning labels are not required because compounded drugs are not regulated by the FDA. Consumers are basically at the mercy of whatever claims they read on the Internet or in the lay literature, which tends to be written by people who have a financial interest in affiliating with the compounding industry. It’s a very frustrating situation for a lot of people.
Unintended consequences of the WHI
The Women’s Health Initiative (WHI) stirred demand for bioidentical hormones by casting the safety claims for some commercial hormone therapy products in a less than favorable light. That wasn’t the investigators’ intent, of course, and some of the findings of the WHI have since been questioned.
The goal of the WHI was to critically evaluate some of the touted health benefits of commercial hormone therapy prescription drugs, but, by questioning some of these claims, it inadvertently pushed a significant percentage of patients toward compounded prescription drugs—and we have no safety data on them.
No one knows exactly how many women were swayed, but the consensus is that they were, and no one’s been happy about that.
The main problem with the compounded hormones, as I see it, is that women who use them do not receive any written information from the compounding pharmacist about risks and benefits. Nor do they receive the black box warnings on FDA-approved estrogen therapy. I believe women need to be adequately educated about the potential risks and benefits, as well as the lack of efficacy data and quality control, if compounded products are requested. That means it’s up to the prescriber to educate the patient about the potential risks and benefits.
Rosenthal states that symptomatic menopausal women or those who fear breast cancer or heart disease can be considered a vulnerable population: “Patients do not have the background to decipher credible sources from noncredible sources.” False claims present convincing arguments for laypersons. A woman may be vulnerable to unsubstantiated claims by virtue of her symptoms and the anxiety and even depression that they can produce. Without comprehensive education, these women cannot be assumed to be adequately informed.
Let me put it in perspective. If a patient with a history of breast cancer complains about severe vaginal dryness that interferes with her sex life, I might decide to give her the smallest amount of topical estrogen that I can—for example, a dime-sized amount of estrogen to apply to her vulvar area twice a week. This amount of estrogen can’t be detected in her system with current assays. I know that some of it will be systemically absorbed, but it cannot be detected. When the patient buys that commercially prepared cream from the pharmacist, she will receive the same black box warning that comes with all systemic hormones since the WHI. However, if she goes to a compounding pharmacist with a prescription for bioidentical hormone therapy, she will not get the warning, regardless of the ingredients or dosage.
The American College of Obstetricians and Gynecologists (ACOG), North American Menopause Society (NAMS), and The Endocrine Society have all issued statements noting the lack of safety data on compounded bioidentical hormones. Here’s what they say:
ACOG
“Most compounded products have not undergone rigorous clinical testing for safety or efficacy, and issues regarding purity, potency and quality are a concern. Compounded hormone products have the same safety issues as those associated with hormone therapy agents that are approved by the US Food and Drug Administration and may have additional risks intrinsic to compounding. There is no scientific evidence to support claims of increased efficacy or safety for individualized estrogen or progesterone regimens.”
NAMS
“NAMS does not recommend custom-compounded products over well-tested, government-approved products for the majority of women—and does not recommend saliva testing to determine hormone levels” (www.menopause.org/bioidentical_NAMS.aspx).
The Endocrine Society
“‘Bioidentical hormones,’ particularly estrogen and progesterone, have been promoted as safer and more effective alternatives to more traditional hormone therapies, often by people outside of the medical community. In fact, little or no scientific and medical evidence exists to support such claims about ‘bioidentical hormones.’ Additionally, many ‘bioidentical hormone’ formulations are not subject to FDA oversight and can be inconsistent in dose and purity….”
Are compounded bioidenticals ever justified?
OBG MANAGEMENT: According to the FDA, compounding of drug products is justified only when a practitioner finds that an FDA-approved drug does not meet the patient’s needs. Do you think this is ever really the case, given the availability of FDA-approved bioidentical hormone preparations?
DR. PINKERTON: In rare cases, compounding of bioidentical hormones is justified, such as when a patient cannot tolerate an FDA-approved product. The problem is that women have been especially concerned about the safety of hormone therapy since the WHI, and bioidentical hormones have been promoted as being safer than FDA-regulated preparations, despite the lack of evidence of their safety or efficacy in peer-reviewed literature. So many women request them.
In a recent commentary, Boothby and Doering call bioidentical hormone therapy “a panacea that lacks supportive evidence.” They say, “It’s our belief that pharmacists are compounding these with the best intentions, but they are ill informed regarding the lack of scientific underpinning associated with efficacy and safety.”3
OBG MANAGEMENT: Do you ever prescribe bioidentical hormones?
DR. PINKERTON: Yes, but rarely, and primarily for women who can’t tolerate FDA-approved hormones or who, after adequate information and education, refuse FDA-approved hormone therapy.
Is salivary hormone testing informative?
OBG MANAGEMENT: Many clinicians who prescribe bioidentical hormones base the dosage on salivary hormone testing. They claim that this allows them to offer individualized formulations. Is this a reliable claim?
DR. PINKERTON: No, it isn’t. Although compounded bioidentical hormone therapy is often prescribed on the basis of salivary hormone testing, there is no scientific evidence that a correlation exists between a patient’s symptoms and salivary hormones, or that salivary hormone testing reflects what is happening at the tissue level. As Fugh-Berman and Bythrow have observed, this type of testing is often used to convince asymptomatic consumers to use hormones—or symptomatic women to take higher dosages. That practice is likely to lead to adverse events.5 The practice also directly contradicts evidence-based guidelines, which recommend that hormone therapy be individualized on the basis of symptoms, not hormone levels.6
There are no published studies in the peer-reviewed literature that show that salivary testing is a reliable measure on which to safely and effectively base dosing decisions. Indeed, The Endocrine Society issued a position statement that notes, among other issues, that salivary hormone tests are “inaccurate and should not be considered reliable measures of hormones in the body.”7 The American College of Obstetricians and Gynecologists also advises against salivary testing, observing that:
- 1) there is no biologically meaningful relationship between salivary sex steroidal hormone concentrations and free serum hormone concentrations
- 2) there is large within-patient variability in salivary hormone concentrations. Salivary hormone levels vary depending on diet, time of day of testing, the specific hormone being tested, and other variables.3
Do bioidenticals protect against cancer?
OBG MANAGEMENT: Some reports mention the fact that many women believe that bioidentical hormones—specifically, estriol—can reduce their risk of breast and endometrial cancer. Is there any truth behind this belief?
DR. PINKERTON: Estriol is a weak estrogen. There is no evidence that, if it is given at a dosage high enough to relieve symptoms, it is any safer than estradiol.
In regard to endometrial cancer, if the exogenous estrogen—bioidentical or otherwise—is unopposed or inadequately opposed, the risk of endometrial cancer is elevated. The problem is that it is hard to determine whether estrogen is being adequately opposed, particularly when transdermal compounded progesterone is given, because the progesterone molecule is too large to be well-absorbed systemically.9
In regard to breast cancer, estriol is a less potent estrogen than estradiol, but it is believed to carry the same risks if it is dosed at effective levels. There is nothing about estriol per se in the peer-reviewed literature that shows that it protects against breast cancer.
The data on risk of breast cancer with estrogen therapy is confusing, with potentially higher risks if estrogen is combined with progestogen. Most of the data we have on estriol come from animals, but a study from 1980 in humans showed that, when older women with breast cancer were treated with estriol, 25% had increased growth of metastases.8
How do you monitor use of bioidentical hormones?
OBG MANAGEMENT: When you do prescribe a compounded bioidentical hormone, how do you monitor the patient?
DR. PINKERTON: First, I want to reiterate that I prescribe these hormones after considerable patient education about FDA-approved options and their potential risks. Second, when a patient needs or requests hormone therapy, I recommend conventional therapy. Only when she cannot tolerate or refuses FDA-approved drugs do I consider prescribing compounded bioidentical hormones—which, as I said earlier, are assumed to carry risks identical to those of FDA-approved hormones.
In some cases, I provide gynecologic care for patients who obtain compounded bioidentical hormones from other sources. What I will sometimes do, just to give myself some idea of how much estrogen they are getting, is to measure the peak and trough estradiol and estrone levels. That is, I measure the hormone level within 4 hours of the patient taking the drug to see how high it goes, and again about 12 hours later to see how low it goes. I measure both because estradiol may be peripherally converted to estrone.
Regrettably, we don’t know what to do about the various hormone levels. It isn’t like treating thyroid disorders; we normally dose estrogen therapy based on symptoms.
Who pays?
OBG MANAGEMENT: Who pays for salivary testing and compounded bioidentical hormones? Does health insurance cover them?
DR. PINKERTON: Like other “natural” products, compounded bioidenticals may cost more than their commercially prepared counterparts and often are not covered by insurance. In addition, prescribers may charge more for a “consultation” than do practitioners who accept insurance; they also may recommend salivary testing, which is expensive. Patients can end up paying large sums out of pocket.
As Rosenthal noted, many women do not appear to be concerned about the added costs.2 That may be because compounded bioidentical hormone therapy is usually offered to economically advantaged patients.2
Ethical considerations
OBG MANAGEMENT: That raises an important question: What ethical considerations are inherent in the prescribing of compounded bioidenticals?
DR. PINKERTON: The fact that women who are able to pay out of pocket are the primary users of these drugs is one important point. In her analysis of the ethics surrounding bioidentical hormones, Rosenthal noted that the drugs remain “an unequal alternative, and any data collected would not be representative of the overall menopausal community.”2
A critical issue pointed out by Rosenthal is that perimenopausal and menopausal women may be particularly vulnerable to the unsubstantiated claims of purveyors of bio identical hormones. “A substantial number of women seek out bioidentical hormone replacement therapy to restore sexual well-being and functioning, in particular, who may be psychologically more vulnerable,” she writes.2
Another concern arises when the practitioner who prescribes bioidentical hormones also happens to sell them. This poses a potential conflict of interest and “violates professional ethical conduct.”2
OBG MANAGEMENT: Do physicians aggravate the problem when they accede to a patient’s request for compounded hormones?
DR. PINKERTON: Physicians and health-care providers need to stop and educate the patient about the lack of safety and efficacy data, the risks and benefits, and recognize the possibility that she has been influenced by unsubstantiated claims.
1. Schonberg MA, Davis RB, Wee CC. After the Women’s Health Initiative: decision making and trust of women taking hormone therapy. Womens Health Issues. 2005;15:187-195.
2. Rosenthal MS. Ethical problems with bioidentical hormone therapy. Int J Impot Res. 2008;20:45-52.
3. ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 322. Compounded bioidentical hormones. Obstet Gynecol. 2005;106(5 Pt 1):1139-1140.
4. Boothby LA, Doering PL. Bioidentical hormone therapy: a panacea that lacks supportive evidence. Curr Opin Obstet Gynecol. 2008;20:400-407.
5. Fugh-Berman A, Bythrow J. Bioidentical hormones for menopausal hormone therapy: variation on a theme. J Gen Intern Med. 2007;22:1030-1034.
6. Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health. 2007;16:600-631.
7. Bioidentical hormones lack evidence for safety and effectivness, according to new statement by The Endocrine Society [press release]. Chevy Chase, Md: The Endocrine Society; June 14, 2008.
8. Lemon HM. Pathophysiologic considerations in the treatment of menopausal patients with oestrogens: mammary carcinoma. Acta Endocrinol Suppl [Copenhagen]. 1980;233:17-27.
9. Wren BG, Champion SM, Willets K, et al. Transdermal progesterone and its effects on vasomotor symptoms, blood lipid levels, bone metabolic markers, mood, and quality of life for postmenopausal women. Menopause. 2003;10:13-18.
1. Schonberg MA, Davis RB, Wee CC. After the Women’s Health Initiative: decision making and trust of women taking hormone therapy. Womens Health Issues. 2005;15:187-195.
2. Rosenthal MS. Ethical problems with bioidentical hormone therapy. Int J Impot Res. 2008;20:45-52.
3. ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 322. Compounded bioidentical hormones. Obstet Gynecol. 2005;106(5 Pt 1):1139-1140.
4. Boothby LA, Doering PL. Bioidentical hormone therapy: a panacea that lacks supportive evidence. Curr Opin Obstet Gynecol. 2008;20:400-407.
5. Fugh-Berman A, Bythrow J. Bioidentical hormones for menopausal hormone therapy: variation on a theme. J Gen Intern Med. 2007;22:1030-1034.
6. Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health. 2007;16:600-631.
7. Bioidentical hormones lack evidence for safety and effectivness, according to new statement by The Endocrine Society [press release]. Chevy Chase, Md: The Endocrine Society; June 14, 2008.
8. Lemon HM. Pathophysiologic considerations in the treatment of menopausal patients with oestrogens: mammary carcinoma. Acta Endocrinol Suppl [Copenhagen]. 1980;233:17-27.
9. Wren BG, Champion SM, Willets K, et al. Transdermal progesterone and its effects on vasomotor symptoms, blood lipid levels, bone metabolic markers, mood, and quality of life for postmenopausal women. Menopause. 2003;10:13-18.
Does the HRT-associated risk of breast cancer vary by histologic type and hormone regimen?
Using data from their large population-based, frequency-matched control study from Germany (3,464 breast cancer cases, 6,657 matched controls), they found a higher risk of lobular cancer with use of norethisterone- and levonorgestrel-derived progestogens than with progestogens derived from progesterone.
Other studies have also suggested that women who use estrogen–progestogen therapy (EPT) are more likely to be given a diagnosis of lobular, rather than ductal, cancer, and that various dosages, routes of administration, frequency of use, and type of estrogen or progestogen differentially affect the type of breast cancer—although the reason for this intriguing observation is unknown.
Fournier and associates, for example, found that EPT regimens containing progesterone or dydrogesterone were associated with a lower risk of breast cancer than other types of progestogen.1 The Women’s Health Initiative (WHI) evaluated only one type of oral EPT and found too few lobular cancers to answer the question of whether breast cancers found in women in the EPT group differed by histologic type.2
Li and colleagues3 and Flesch-Janys and associates found no statistically significant risk of breast cancer 5 years after EPT was discontinued. Heiss and colleagues reported that, 2.4 years after cessation of EPT, the risk of breast cancer remained stable for prior users in the WHI, compared with women taking placebo (5-year data are not yet available).4
Estrogen-only therapy in the WHI was not associated with an increased risk after 6.7 years of use.5
In the study by Flesch-Janys and associates, 67.9% of cases and 59.5% of controls had ever used HRT. Cases had a higher age at menopause and lower parity, and were less likely to have breastfed, more likely to have a family history of breast cancer, and more likely to have had benign breast disease.
Study design may invite bias
Case-control methodology is appropriate to study rare cases, examine conditions that develop over time, and generate preliminary hypotheses. Bias can occur, however, depending on the quality of existing records or self-recall and whether it is possible to collect information from all eligible controls.
Recall bias can occur because cases tend to provide more accurate information than controls. In the study by Flesch-Janys and colleagues, trained interviewers conducted face-to-face interviews to limit recall bias, but the low response rate, particularly among controls, is of concern, as is the variety of HRT preparations used by participants. The lack of verification of histologic subtype or re-review of histology by blinded pathologists limits the generalizability of the findings.
Lobular carcinoma, found more often in this study, accounts for only about 15% of invasive breast cancers. It is usually hormonally sensitive and considered more treatable than ductal cancer. However, it is more difficult to detect by mammography and physical examination.
Encourage your patients to establish their risk of breast cancer using the Gail model or another tool; to perform breast examination; to undergo mammography; and, in a setting of high risk, to consider magnetic resonance imaging of the breasts. Counsel patients that the risk of breast cancer may be diminished by regular exercise, weight loss, smoking cessation, limitation of alcohol, and adequate intake of vitamin D.
If hormone therapy is indicated in a given patient, using a lower dosage or, potentially, different types of estrogen–progestogen regimens could minimize the risk of breast cancer, although more research on these measures is needed before recommendations can be made.
For women who are at high risk of breast cancer, you can offer Food and Drug Administration-approved chemoprevention with tamoxifen or raloxifene.—JOANN V. PINKERTON, MD
1. Fournier A, Fabre A, Mesrine S, Boutron-Rualt M-C, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol. 2008;26:1260-1268.
2. Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas. 2006;55:103-115.
3. Li CI, Malone KE, Porter PL, et al. Relationship between menopausal hormone therapy and risk of ductal, lobular, and ductal–lobular breast carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:43-50.
4. Heiss G, Wallace R, Anderson GL, et al. For the WHI Investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036-1045.
5. Prentice RL, Chlebowski RT, Stefanick ML, et al. Conjugated equine estrogens and breast cancer risk in the Women’s Health Initiative clinical trial and observational study. Am J Epidemiol. 2008;167:1407-1415.
Using data from their large population-based, frequency-matched control study from Germany (3,464 breast cancer cases, 6,657 matched controls), they found a higher risk of lobular cancer with use of norethisterone- and levonorgestrel-derived progestogens than with progestogens derived from progesterone.
Other studies have also suggested that women who use estrogen–progestogen therapy (EPT) are more likely to be given a diagnosis of lobular, rather than ductal, cancer, and that various dosages, routes of administration, frequency of use, and type of estrogen or progestogen differentially affect the type of breast cancer—although the reason for this intriguing observation is unknown.
Fournier and associates, for example, found that EPT regimens containing progesterone or dydrogesterone were associated with a lower risk of breast cancer than other types of progestogen.1 The Women’s Health Initiative (WHI) evaluated only one type of oral EPT and found too few lobular cancers to answer the question of whether breast cancers found in women in the EPT group differed by histologic type.2
Li and colleagues3 and Flesch-Janys and associates found no statistically significant risk of breast cancer 5 years after EPT was discontinued. Heiss and colleagues reported that, 2.4 years after cessation of EPT, the risk of breast cancer remained stable for prior users in the WHI, compared with women taking placebo (5-year data are not yet available).4
Estrogen-only therapy in the WHI was not associated with an increased risk after 6.7 years of use.5
In the study by Flesch-Janys and associates, 67.9% of cases and 59.5% of controls had ever used HRT. Cases had a higher age at menopause and lower parity, and were less likely to have breastfed, more likely to have a family history of breast cancer, and more likely to have had benign breast disease.
Study design may invite bias
Case-control methodology is appropriate to study rare cases, examine conditions that develop over time, and generate preliminary hypotheses. Bias can occur, however, depending on the quality of existing records or self-recall and whether it is possible to collect information from all eligible controls.
Recall bias can occur because cases tend to provide more accurate information than controls. In the study by Flesch-Janys and colleagues, trained interviewers conducted face-to-face interviews to limit recall bias, but the low response rate, particularly among controls, is of concern, as is the variety of HRT preparations used by participants. The lack of verification of histologic subtype or re-review of histology by blinded pathologists limits the generalizability of the findings.
Lobular carcinoma, found more often in this study, accounts for only about 15% of invasive breast cancers. It is usually hormonally sensitive and considered more treatable than ductal cancer. However, it is more difficult to detect by mammography and physical examination.
Encourage your patients to establish their risk of breast cancer using the Gail model or another tool; to perform breast examination; to undergo mammography; and, in a setting of high risk, to consider magnetic resonance imaging of the breasts. Counsel patients that the risk of breast cancer may be diminished by regular exercise, weight loss, smoking cessation, limitation of alcohol, and adequate intake of vitamin D.
If hormone therapy is indicated in a given patient, using a lower dosage or, potentially, different types of estrogen–progestogen regimens could minimize the risk of breast cancer, although more research on these measures is needed before recommendations can be made.
For women who are at high risk of breast cancer, you can offer Food and Drug Administration-approved chemoprevention with tamoxifen or raloxifene.—JOANN V. PINKERTON, MD
Using data from their large population-based, frequency-matched control study from Germany (3,464 breast cancer cases, 6,657 matched controls), they found a higher risk of lobular cancer with use of norethisterone- and levonorgestrel-derived progestogens than with progestogens derived from progesterone.
Other studies have also suggested that women who use estrogen–progestogen therapy (EPT) are more likely to be given a diagnosis of lobular, rather than ductal, cancer, and that various dosages, routes of administration, frequency of use, and type of estrogen or progestogen differentially affect the type of breast cancer—although the reason for this intriguing observation is unknown.
Fournier and associates, for example, found that EPT regimens containing progesterone or dydrogesterone were associated with a lower risk of breast cancer than other types of progestogen.1 The Women’s Health Initiative (WHI) evaluated only one type of oral EPT and found too few lobular cancers to answer the question of whether breast cancers found in women in the EPT group differed by histologic type.2
Li and colleagues3 and Flesch-Janys and associates found no statistically significant risk of breast cancer 5 years after EPT was discontinued. Heiss and colleagues reported that, 2.4 years after cessation of EPT, the risk of breast cancer remained stable for prior users in the WHI, compared with women taking placebo (5-year data are not yet available).4
Estrogen-only therapy in the WHI was not associated with an increased risk after 6.7 years of use.5
In the study by Flesch-Janys and associates, 67.9% of cases and 59.5% of controls had ever used HRT. Cases had a higher age at menopause and lower parity, and were less likely to have breastfed, more likely to have a family history of breast cancer, and more likely to have had benign breast disease.
Study design may invite bias
Case-control methodology is appropriate to study rare cases, examine conditions that develop over time, and generate preliminary hypotheses. Bias can occur, however, depending on the quality of existing records or self-recall and whether it is possible to collect information from all eligible controls.
Recall bias can occur because cases tend to provide more accurate information than controls. In the study by Flesch-Janys and colleagues, trained interviewers conducted face-to-face interviews to limit recall bias, but the low response rate, particularly among controls, is of concern, as is the variety of HRT preparations used by participants. The lack of verification of histologic subtype or re-review of histology by blinded pathologists limits the generalizability of the findings.
Lobular carcinoma, found more often in this study, accounts for only about 15% of invasive breast cancers. It is usually hormonally sensitive and considered more treatable than ductal cancer. However, it is more difficult to detect by mammography and physical examination.
Encourage your patients to establish their risk of breast cancer using the Gail model or another tool; to perform breast examination; to undergo mammography; and, in a setting of high risk, to consider magnetic resonance imaging of the breasts. Counsel patients that the risk of breast cancer may be diminished by regular exercise, weight loss, smoking cessation, limitation of alcohol, and adequate intake of vitamin D.
If hormone therapy is indicated in a given patient, using a lower dosage or, potentially, different types of estrogen–progestogen regimens could minimize the risk of breast cancer, although more research on these measures is needed before recommendations can be made.
For women who are at high risk of breast cancer, you can offer Food and Drug Administration-approved chemoprevention with tamoxifen or raloxifene.—JOANN V. PINKERTON, MD
1. Fournier A, Fabre A, Mesrine S, Boutron-Rualt M-C, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol. 2008;26:1260-1268.
2. Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas. 2006;55:103-115.
3. Li CI, Malone KE, Porter PL, et al. Relationship between menopausal hormone therapy and risk of ductal, lobular, and ductal–lobular breast carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:43-50.
4. Heiss G, Wallace R, Anderson GL, et al. For the WHI Investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036-1045.
5. Prentice RL, Chlebowski RT, Stefanick ML, et al. Conjugated equine estrogens and breast cancer risk in the Women’s Health Initiative clinical trial and observational study. Am J Epidemiol. 2008;167:1407-1415.
1. Fournier A, Fabre A, Mesrine S, Boutron-Rualt M-C, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol. 2008;26:1260-1268.
2. Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas. 2006;55:103-115.
3. Li CI, Malone KE, Porter PL, et al. Relationship between menopausal hormone therapy and risk of ductal, lobular, and ductal–lobular breast carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:43-50.
4. Heiss G, Wallace R, Anderson GL, et al. For the WHI Investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036-1045.
5. Prentice RL, Chlebowski RT, Stefanick ML, et al. Conjugated equine estrogens and breast cancer risk in the Women’s Health Initiative clinical trial and observational study. Am J Epidemiol. 2008;167:1407-1415.