User login
FDA approves biosimilar filgrastim
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Nivestym™ (filgrastim-aafi), a biosimilar to Neupogen (filgrastim).
Nivestym is approved to treat patients with nonmyeloid malignancies who are receiving myelosuppressive chemotherapy or undergoing bone marrow transplant, acute myeloid leukemia patients receiving induction or consolidation chemotherapy, patients undergoing autologous peripheral blood progenitor cell collection, and patients with severe chronic neutropenia.
The FDA’s approval of Nivestym was based on a review of evidence suggesting the drug is highly similar to Neupogen, according to Pfizer, the company developing Nivestym.
The full approved indication for Nivestym is as follows:
- To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever
- To reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy in patients with acute myeloid leukemia
- To reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg, febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant
- For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis
- For chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (eg, fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
For more details on Nivestym, see the full prescribing information.
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Nivestym™ (filgrastim-aafi), a biosimilar to Neupogen (filgrastim).
Nivestym is approved to treat patients with nonmyeloid malignancies who are receiving myelosuppressive chemotherapy or undergoing bone marrow transplant, acute myeloid leukemia patients receiving induction or consolidation chemotherapy, patients undergoing autologous peripheral blood progenitor cell collection, and patients with severe chronic neutropenia.
The FDA’s approval of Nivestym was based on a review of evidence suggesting the drug is highly similar to Neupogen, according to Pfizer, the company developing Nivestym.
The full approved indication for Nivestym is as follows:
- To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever
- To reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy in patients with acute myeloid leukemia
- To reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg, febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant
- For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis
- For chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (eg, fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
For more details on Nivestym, see the full prescribing information.
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Nivestym™ (filgrastim-aafi), a biosimilar to Neupogen (filgrastim).
Nivestym is approved to treat patients with nonmyeloid malignancies who are receiving myelosuppressive chemotherapy or undergoing bone marrow transplant, acute myeloid leukemia patients receiving induction or consolidation chemotherapy, patients undergoing autologous peripheral blood progenitor cell collection, and patients with severe chronic neutropenia.
The FDA’s approval of Nivestym was based on a review of evidence suggesting the drug is highly similar to Neupogen, according to Pfizer, the company developing Nivestym.
The full approved indication for Nivestym is as follows:
- To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever
- To reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy in patients with acute myeloid leukemia
- To reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg, febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant
- For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis
- For chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (eg, fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
For more details on Nivestym, see the full prescribing information.
Diabetics have higher risk of hematologic, other cancers
A review of data from more than 19 million people indicates that diabetes significantly raises a person’s risk of developing cancer.
When researchers compared patients with diabetes and without, both male and female diabetics had an increased risk of leukemias and lymphomas as well as certain solid tumors.
Researchers also found that diabetes conferred a higher cancer risk for women than men, both for all cancers combined and for some specific cancers, including leukemia.
“The link between diabetes and the risk of developing cancer is now firmly established,” said Toshiaki Ohkuma, PhD, of The George Institute for Global Health at the University of New South Wales in Australia.
“We have also demonstrated, for the first time, that women with diabetes are more likely to develop any form of cancer and have a significantly higher chance of developing kidney, oral, and stomach cancers and leukemia.”
Dr Ohkuma and his colleagues reported these findings in Diabetologia.
The researchers conducted a systematic search in PubMed MEDLINE to identify reports on the links between diabetes and cancer. Additional reports were identified from the reference lists of the relevant studies.
Only those cohort studies providing relative risks (RRs) for the association between diabetes and cancer for both women and men were included. In total, 107 relevant articles were identified, along with 36 cohorts of individual participant data.
RRs for cancer were obtained for patients with diabetes (types 1 and 2 combined) versus those without diabetes, for both men and women. The women-to-men ratios of these relative risk ratios (RRRs) were then calculated to determine the excess risk in women if present.
Data on all-site cancer was available from 47 studies, involving 121 cohorts and 19,239,302 individuals.
Diabetics vs non-diabetics
Women with diabetes had a 27% higher risk of all-site cancer compared to women without diabetes (RR=1.27; 95% CI 1.21, 1.32; P<0.001).
For men, the risk of all-site cancer was 19% higher among those with diabetes than those without (RR=1.19; 95% CI 1.13, 1.25; P<0.001).
There were several hematologic malignancies for which diabetics had an increased risk, as shown in the following table.
| Cancer type | RR for women (99% CI) | RR for men (99% CI) |
| Lymphatic and hematopoietic tissue | 1.24 (1.05, 1.46)* | 1.21 (0.98, 1.48) |
| Leukemia | 1.53 (1.00, 2.33) | 1.22 (0.80, 1.85) |
| Myeloid leukemia | 0.83 (0.39, 1.76) | 1.12 (0.77, 1.62) |
| Acute myeloid leukemia | 1.33 (1.12, 1.57)* | 1.14 (0.56, 2.33) |
| Chronic myeloid leukemia | 1.67 (1.27, 2.20)* | 1.62 (1.32, 1.98)* |
| Lymphoid leukemia | 1.74 (0.31, 9.79) | 1.20 (0.86, 1.68) |
| Lymphoma | 2.31 (0.57, 9.30) | 1.80 (0.68, 4.75) |
| Non-Hodgkin lymphoma | 1.16 (1.02, 1.32)* | 1.20 (1.08, 1.34)* |
| Hodgkin lymphoma | 1.20 (0.61, 2.38) | 1.36 (1.05, 1.77)* |
| Multiple myeloma | 1.19 (0.97, 1.47) | 1.12 (0.90, 1.41) |
| *denotes statistical significance with a P value < 0.01 | ||
Sex comparison
Calculation of the women-to-men ratio revealed that women with diabetes had a 6% greater excess risk of all-site cancer compared to men with diabetes (RRR=1.06; 95% CI 1.03, 1.09; P<0.001).
The women-to-men ratios also showed significantly higher risks for female diabetics for:
- Kidney cancer—RRR=1.11 (99% CI 1.04, 1.18; P<0.001)
- Oral cancer—RRR=1.13 (99% CI 1.00, 1.28; P=0.009)
- Stomach cancer—RRR=1.14 (99% CI 1.07, 1.22; P<0.001)
- Leukemia—RRR=1.15 (99% CI 1.02, 1.28; P=0.002).
However, women had a significantly lower risk of liver cancer (RRR=0.88; 99% CI 0.79, 0.99; P=0.005).
There are several possible reasons for the excess cancer risk observed in women, according to study author Sanne Peters, PhD, of The George Institute for Global Health at the University of Oxford in the UK.
For example, on average, women are in the pre-diabetic state of impaired glucose tolerance 2 years longer than men.
“Historically, we know that women are often under-treated when they first present with symptoms of diabetes, are less likely to receive intensive care, and are not taking the same levels of medications as men,” Dr Peters said. “All of these could go some way into explaining why women are at greater risk of developing cancer, but, without more research, we can’t be certain.”
A review of data from more than 19 million people indicates that diabetes significantly raises a person’s risk of developing cancer.
When researchers compared patients with diabetes and without, both male and female diabetics had an increased risk of leukemias and lymphomas as well as certain solid tumors.
Researchers also found that diabetes conferred a higher cancer risk for women than men, both for all cancers combined and for some specific cancers, including leukemia.
“The link between diabetes and the risk of developing cancer is now firmly established,” said Toshiaki Ohkuma, PhD, of The George Institute for Global Health at the University of New South Wales in Australia.
“We have also demonstrated, for the first time, that women with diabetes are more likely to develop any form of cancer and have a significantly higher chance of developing kidney, oral, and stomach cancers and leukemia.”
Dr Ohkuma and his colleagues reported these findings in Diabetologia.
The researchers conducted a systematic search in PubMed MEDLINE to identify reports on the links between diabetes and cancer. Additional reports were identified from the reference lists of the relevant studies.
Only those cohort studies providing relative risks (RRs) for the association between diabetes and cancer for both women and men were included. In total, 107 relevant articles were identified, along with 36 cohorts of individual participant data.
RRs for cancer were obtained for patients with diabetes (types 1 and 2 combined) versus those without diabetes, for both men and women. The women-to-men ratios of these relative risk ratios (RRRs) were then calculated to determine the excess risk in women if present.
Data on all-site cancer was available from 47 studies, involving 121 cohorts and 19,239,302 individuals.
Diabetics vs non-diabetics
Women with diabetes had a 27% higher risk of all-site cancer compared to women without diabetes (RR=1.27; 95% CI 1.21, 1.32; P<0.001).
For men, the risk of all-site cancer was 19% higher among those with diabetes than those without (RR=1.19; 95% CI 1.13, 1.25; P<0.001).
There were several hematologic malignancies for which diabetics had an increased risk, as shown in the following table.
| Cancer type | RR for women (99% CI) | RR for men (99% CI) |
| Lymphatic and hematopoietic tissue | 1.24 (1.05, 1.46)* | 1.21 (0.98, 1.48) |
| Leukemia | 1.53 (1.00, 2.33) | 1.22 (0.80, 1.85) |
| Myeloid leukemia | 0.83 (0.39, 1.76) | 1.12 (0.77, 1.62) |
| Acute myeloid leukemia | 1.33 (1.12, 1.57)* | 1.14 (0.56, 2.33) |
| Chronic myeloid leukemia | 1.67 (1.27, 2.20)* | 1.62 (1.32, 1.98)* |
| Lymphoid leukemia | 1.74 (0.31, 9.79) | 1.20 (0.86, 1.68) |
| Lymphoma | 2.31 (0.57, 9.30) | 1.80 (0.68, 4.75) |
| Non-Hodgkin lymphoma | 1.16 (1.02, 1.32)* | 1.20 (1.08, 1.34)* |
| Hodgkin lymphoma | 1.20 (0.61, 2.38) | 1.36 (1.05, 1.77)* |
| Multiple myeloma | 1.19 (0.97, 1.47) | 1.12 (0.90, 1.41) |
| *denotes statistical significance with a P value < 0.01 | ||
Sex comparison
Calculation of the women-to-men ratio revealed that women with diabetes had a 6% greater excess risk of all-site cancer compared to men with diabetes (RRR=1.06; 95% CI 1.03, 1.09; P<0.001).
The women-to-men ratios also showed significantly higher risks for female diabetics for:
- Kidney cancer—RRR=1.11 (99% CI 1.04, 1.18; P<0.001)
- Oral cancer—RRR=1.13 (99% CI 1.00, 1.28; P=0.009)
- Stomach cancer—RRR=1.14 (99% CI 1.07, 1.22; P<0.001)
- Leukemia—RRR=1.15 (99% CI 1.02, 1.28; P=0.002).
However, women had a significantly lower risk of liver cancer (RRR=0.88; 99% CI 0.79, 0.99; P=0.005).
There are several possible reasons for the excess cancer risk observed in women, according to study author Sanne Peters, PhD, of The George Institute for Global Health at the University of Oxford in the UK.
For example, on average, women are in the pre-diabetic state of impaired glucose tolerance 2 years longer than men.
“Historically, we know that women are often under-treated when they first present with symptoms of diabetes, are less likely to receive intensive care, and are not taking the same levels of medications as men,” Dr Peters said. “All of these could go some way into explaining why women are at greater risk of developing cancer, but, without more research, we can’t be certain.”
A review of data from more than 19 million people indicates that diabetes significantly raises a person’s risk of developing cancer.
When researchers compared patients with diabetes and without, both male and female diabetics had an increased risk of leukemias and lymphomas as well as certain solid tumors.
Researchers also found that diabetes conferred a higher cancer risk for women than men, both for all cancers combined and for some specific cancers, including leukemia.
“The link between diabetes and the risk of developing cancer is now firmly established,” said Toshiaki Ohkuma, PhD, of The George Institute for Global Health at the University of New South Wales in Australia.
“We have also demonstrated, for the first time, that women with diabetes are more likely to develop any form of cancer and have a significantly higher chance of developing kidney, oral, and stomach cancers and leukemia.”
Dr Ohkuma and his colleagues reported these findings in Diabetologia.
The researchers conducted a systematic search in PubMed MEDLINE to identify reports on the links between diabetes and cancer. Additional reports were identified from the reference lists of the relevant studies.
Only those cohort studies providing relative risks (RRs) for the association between diabetes and cancer for both women and men were included. In total, 107 relevant articles were identified, along with 36 cohorts of individual participant data.
RRs for cancer were obtained for patients with diabetes (types 1 and 2 combined) versus those without diabetes, for both men and women. The women-to-men ratios of these relative risk ratios (RRRs) were then calculated to determine the excess risk in women if present.
Data on all-site cancer was available from 47 studies, involving 121 cohorts and 19,239,302 individuals.
Diabetics vs non-diabetics
Women with diabetes had a 27% higher risk of all-site cancer compared to women without diabetes (RR=1.27; 95% CI 1.21, 1.32; P<0.001).
For men, the risk of all-site cancer was 19% higher among those with diabetes than those without (RR=1.19; 95% CI 1.13, 1.25; P<0.001).
There were several hematologic malignancies for which diabetics had an increased risk, as shown in the following table.
| Cancer type | RR for women (99% CI) | RR for men (99% CI) |
| Lymphatic and hematopoietic tissue | 1.24 (1.05, 1.46)* | 1.21 (0.98, 1.48) |
| Leukemia | 1.53 (1.00, 2.33) | 1.22 (0.80, 1.85) |
| Myeloid leukemia | 0.83 (0.39, 1.76) | 1.12 (0.77, 1.62) |
| Acute myeloid leukemia | 1.33 (1.12, 1.57)* | 1.14 (0.56, 2.33) |
| Chronic myeloid leukemia | 1.67 (1.27, 2.20)* | 1.62 (1.32, 1.98)* |
| Lymphoid leukemia | 1.74 (0.31, 9.79) | 1.20 (0.86, 1.68) |
| Lymphoma | 2.31 (0.57, 9.30) | 1.80 (0.68, 4.75) |
| Non-Hodgkin lymphoma | 1.16 (1.02, 1.32)* | 1.20 (1.08, 1.34)* |
| Hodgkin lymphoma | 1.20 (0.61, 2.38) | 1.36 (1.05, 1.77)* |
| Multiple myeloma | 1.19 (0.97, 1.47) | 1.12 (0.90, 1.41) |
| *denotes statistical significance with a P value < 0.01 | ||
Sex comparison
Calculation of the women-to-men ratio revealed that women with diabetes had a 6% greater excess risk of all-site cancer compared to men with diabetes (RRR=1.06; 95% CI 1.03, 1.09; P<0.001).
The women-to-men ratios also showed significantly higher risks for female diabetics for:
- Kidney cancer—RRR=1.11 (99% CI 1.04, 1.18; P<0.001)
- Oral cancer—RRR=1.13 (99% CI 1.00, 1.28; P=0.009)
- Stomach cancer—RRR=1.14 (99% CI 1.07, 1.22; P<0.001)
- Leukemia—RRR=1.15 (99% CI 1.02, 1.28; P=0.002).
However, women had a significantly lower risk of liver cancer (RRR=0.88; 99% CI 0.79, 0.99; P=0.005).
There are several possible reasons for the excess cancer risk observed in women, according to study author Sanne Peters, PhD, of The George Institute for Global Health at the University of Oxford in the UK.
For example, on average, women are in the pre-diabetic state of impaired glucose tolerance 2 years longer than men.
“Historically, we know that women are often under-treated when they first present with symptoms of diabetes, are less likely to receive intensive care, and are not taking the same levels of medications as men,” Dr Peters said. “All of these could go some way into explaining why women are at greater risk of developing cancer, but, without more research, we can’t be certain.”
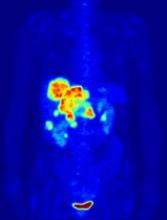
PET-guided treatment didn’t improve outcomes
In the PETAL trial, treatment intensification based on results of an interim positron emission tomography (PET) scan did not improve survival outcomes for patients with aggressive lymphomas.
PET-positive patients did not benefit by switching from R-CHOP to a more intensive chemotherapy regimen.
PET-negative patients did not benefit from 2 additional cycles of rituximab after R-CHOP.
These results were published in the Journal of Clinical Oncology.
PETAL was a randomized trial of patients with newly diagnosed T- or B-cell lymphomas.
Patients received 2 cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone)—plus rituximab (R-CHOP) in CD20-positive lymphomas—followed by a PET scan.
PET-positive patients were randomized to receive 6 additional cycles of R-CHOP or 6 blocks of an intensive protocol used to treat Burkitt lymphoma. This protocol consisted of high-dose methotrexate, cytarabine, hyperfractionated cyclophosphamide and ifosfamide, split-dose doxorubicin and etoposide, vincristine, vindesine, and dexamethasone.
PET-negative patients with CD20-positive lymphomas were randomized to receive 4 additional cycles of R-CHOP or 4 additional cycles of R-CHOP followed by 2 more doses of rituximab.
Among patients with T-cell lymphomas, only PET-positive individuals underwent randomization. PET-negative patients received CHOP. Patients with CD20-positive T-cell lymphomas also received rituximab.
PET-positive results
Of the PET-positive patients (108/862), 52 were randomized to receive 6 additional cycles of R-CHOP, and 56 were randomized to 6 cycles of the Burkitt protocol.
In general, survival rates were similar regardless of treatment. The 2-year overall survival (OS) rate was 63.6% for patients who received R-CHOP and 55.4% for those who received the more intensive protocol.
Two-year progression-free survival (PFS) rates were 49.4% and 43.1%, respectively. Two-year event-free survival (EFS) rates were 42.0% and 31.6%, respectively.
Among patients with diffuse large B-cell lymphoma (DLBCL), the OS rate was 64.8% for patients who received R-CHOP and 47.1% for those on the Burkitt protocol. PFS rates were 55.5% and 41.4%, respectively.
There was a significant difference in EFS rates among the DLBCL patients—52.4% in the R-CHOP arm and 28.3% in the intensive arm (P=0.0186).
Among T-cell lymphoma patients, the OS rate was 22.2% in the R-CHOP arm and 30.0% in the intensive arm. The PFS rates were 12.7% and 30%, respectively. The EFS rates were the same as the PFS rates.
Overall, patients who received the Burkitt protocol had significantly higher rates of grade 3/4 hematologic toxicities, infection, and mucositis.
PET-negative results
Of 754 PET-negative patients, 697 had CD20-positive lymphomas, and 255 of those patients (all with B-cell lymphomas) underwent randomization.
There were 129 patients who were randomized to receive 6 cycles of R-CHOP (2 before and 4 after randomization) and 126 who were randomized to receive 6 cycles of R-CHOP plus 2 additional cycles of rituximab.
Again, survival rates were similar regardless of treatment.
The 2-year OS was 88.2% for patients who received only R-CHOP and 87.2% for those with additional rituximab exposure. PFS rates were 82.0% and 77.5%, respectively. EFS rates were 76.4% and 73.5%, respectively.
In the DLBCL patients, the OS rate was 88.5% in the R-CHOP arm and 85.8% in the intensive arm. PFS rates were 82.3% and 77.7%, respectively. EFS rates were 72.6% and 78.9%, respectively.
As increasing the dose of rituximab did not improve outcomes, the investigators concluded that 6 cycles of R-CHOP should be the standard of care for these patients.
The team also said interim PET scanning is “a powerful tool” for identifying chemotherapy-resistant lymphomas, and PET-positive patients may be candidates for immunologic treatment approaches.
In the PETAL trial, treatment intensification based on results of an interim positron emission tomography (PET) scan did not improve survival outcomes for patients with aggressive lymphomas.
PET-positive patients did not benefit by switching from R-CHOP to a more intensive chemotherapy regimen.
PET-negative patients did not benefit from 2 additional cycles of rituximab after R-CHOP.
These results were published in the Journal of Clinical Oncology.
PETAL was a randomized trial of patients with newly diagnosed T- or B-cell lymphomas.
Patients received 2 cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone)—plus rituximab (R-CHOP) in CD20-positive lymphomas—followed by a PET scan.
PET-positive patients were randomized to receive 6 additional cycles of R-CHOP or 6 blocks of an intensive protocol used to treat Burkitt lymphoma. This protocol consisted of high-dose methotrexate, cytarabine, hyperfractionated cyclophosphamide and ifosfamide, split-dose doxorubicin and etoposide, vincristine, vindesine, and dexamethasone.
PET-negative patients with CD20-positive lymphomas were randomized to receive 4 additional cycles of R-CHOP or 4 additional cycles of R-CHOP followed by 2 more doses of rituximab.
Among patients with T-cell lymphomas, only PET-positive individuals underwent randomization. PET-negative patients received CHOP. Patients with CD20-positive T-cell lymphomas also received rituximab.
PET-positive results
Of the PET-positive patients (108/862), 52 were randomized to receive 6 additional cycles of R-CHOP, and 56 were randomized to 6 cycles of the Burkitt protocol.
In general, survival rates were similar regardless of treatment. The 2-year overall survival (OS) rate was 63.6% for patients who received R-CHOP and 55.4% for those who received the more intensive protocol.
Two-year progression-free survival (PFS) rates were 49.4% and 43.1%, respectively. Two-year event-free survival (EFS) rates were 42.0% and 31.6%, respectively.
Among patients with diffuse large B-cell lymphoma (DLBCL), the OS rate was 64.8% for patients who received R-CHOP and 47.1% for those on the Burkitt protocol. PFS rates were 55.5% and 41.4%, respectively.
There was a significant difference in EFS rates among the DLBCL patients—52.4% in the R-CHOP arm and 28.3% in the intensive arm (P=0.0186).
Among T-cell lymphoma patients, the OS rate was 22.2% in the R-CHOP arm and 30.0% in the intensive arm. The PFS rates were 12.7% and 30%, respectively. The EFS rates were the same as the PFS rates.
Overall, patients who received the Burkitt protocol had significantly higher rates of grade 3/4 hematologic toxicities, infection, and mucositis.
PET-negative results
Of 754 PET-negative patients, 697 had CD20-positive lymphomas, and 255 of those patients (all with B-cell lymphomas) underwent randomization.
There were 129 patients who were randomized to receive 6 cycles of R-CHOP (2 before and 4 after randomization) and 126 who were randomized to receive 6 cycles of R-CHOP plus 2 additional cycles of rituximab.
Again, survival rates were similar regardless of treatment.
The 2-year OS was 88.2% for patients who received only R-CHOP and 87.2% for those with additional rituximab exposure. PFS rates were 82.0% and 77.5%, respectively. EFS rates were 76.4% and 73.5%, respectively.
In the DLBCL patients, the OS rate was 88.5% in the R-CHOP arm and 85.8% in the intensive arm. PFS rates were 82.3% and 77.7%, respectively. EFS rates were 72.6% and 78.9%, respectively.
As increasing the dose of rituximab did not improve outcomes, the investigators concluded that 6 cycles of R-CHOP should be the standard of care for these patients.
The team also said interim PET scanning is “a powerful tool” for identifying chemotherapy-resistant lymphomas, and PET-positive patients may be candidates for immunologic treatment approaches.
In the PETAL trial, treatment intensification based on results of an interim positron emission tomography (PET) scan did not improve survival outcomes for patients with aggressive lymphomas.
PET-positive patients did not benefit by switching from R-CHOP to a more intensive chemotherapy regimen.
PET-negative patients did not benefit from 2 additional cycles of rituximab after R-CHOP.
These results were published in the Journal of Clinical Oncology.
PETAL was a randomized trial of patients with newly diagnosed T- or B-cell lymphomas.
Patients received 2 cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone)—plus rituximab (R-CHOP) in CD20-positive lymphomas—followed by a PET scan.
PET-positive patients were randomized to receive 6 additional cycles of R-CHOP or 6 blocks of an intensive protocol used to treat Burkitt lymphoma. This protocol consisted of high-dose methotrexate, cytarabine, hyperfractionated cyclophosphamide and ifosfamide, split-dose doxorubicin and etoposide, vincristine, vindesine, and dexamethasone.
PET-negative patients with CD20-positive lymphomas were randomized to receive 4 additional cycles of R-CHOP or 4 additional cycles of R-CHOP followed by 2 more doses of rituximab.
Among patients with T-cell lymphomas, only PET-positive individuals underwent randomization. PET-negative patients received CHOP. Patients with CD20-positive T-cell lymphomas also received rituximab.
PET-positive results
Of the PET-positive patients (108/862), 52 were randomized to receive 6 additional cycles of R-CHOP, and 56 were randomized to 6 cycles of the Burkitt protocol.
In general, survival rates were similar regardless of treatment. The 2-year overall survival (OS) rate was 63.6% for patients who received R-CHOP and 55.4% for those who received the more intensive protocol.
Two-year progression-free survival (PFS) rates were 49.4% and 43.1%, respectively. Two-year event-free survival (EFS) rates were 42.0% and 31.6%, respectively.
Among patients with diffuse large B-cell lymphoma (DLBCL), the OS rate was 64.8% for patients who received R-CHOP and 47.1% for those on the Burkitt protocol. PFS rates were 55.5% and 41.4%, respectively.
There was a significant difference in EFS rates among the DLBCL patients—52.4% in the R-CHOP arm and 28.3% in the intensive arm (P=0.0186).
Among T-cell lymphoma patients, the OS rate was 22.2% in the R-CHOP arm and 30.0% in the intensive arm. The PFS rates were 12.7% and 30%, respectively. The EFS rates were the same as the PFS rates.
Overall, patients who received the Burkitt protocol had significantly higher rates of grade 3/4 hematologic toxicities, infection, and mucositis.
PET-negative results
Of 754 PET-negative patients, 697 had CD20-positive lymphomas, and 255 of those patients (all with B-cell lymphomas) underwent randomization.
There were 129 patients who were randomized to receive 6 cycles of R-CHOP (2 before and 4 after randomization) and 126 who were randomized to receive 6 cycles of R-CHOP plus 2 additional cycles of rituximab.
Again, survival rates were similar regardless of treatment.
The 2-year OS was 88.2% for patients who received only R-CHOP and 87.2% for those with additional rituximab exposure. PFS rates were 82.0% and 77.5%, respectively. EFS rates were 76.4% and 73.5%, respectively.
In the DLBCL patients, the OS rate was 88.5% in the R-CHOP arm and 85.8% in the intensive arm. PFS rates were 82.3% and 77.7%, respectively. EFS rates were 72.6% and 78.9%, respectively.
As increasing the dose of rituximab did not improve outcomes, the investigators concluded that 6 cycles of R-CHOP should be the standard of care for these patients.
The team also said interim PET scanning is “a powerful tool” for identifying chemotherapy-resistant lymphomas, and PET-positive patients may be candidates for immunologic treatment approaches.
Health Canada expands approval of obinutuzumab
Health Canada has expanded the approved use of obinutuzumab (Gazyva®).
The anti-CD20 monoclonal antibody is now approved for use in combination with chemotherapy to treat patients with previously untreated follicular lymphoma (FL) that is advanced (stage II bulky, stage III, or stage IV).
In patients who respond to this treatment, obinutuzumab monotherapy can be given as maintenance.
Health Canada previously approved obinutuzumab for the following indications:
- In combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia
- First in combination with bendamustine, then as monotherapy, in FL patients who relapsed after or are refractory to a rituximab-containing regimen.
Phase 3 results
Health Canada’s latest approval of obinutuzumab is based on results from the phase 3 GALLIUM study, which were published in NEJM in October 2017. The following are updated data from the product monograph.
GALLIUM included 1385 patients with previously untreated non-Hodgkin lymphoma, and 1202 of these patients had previously untreated, advanced FL.
Half of the FL patients (n=601) were randomized to receive obinutuzumab plus chemotherapy (followed by obinutuzumab maintenance for up to 2 years), and half were randomized to rituximab plus chemotherapy (followed by rituximab maintenance for up to 2 years).
The different chemotherapies used were CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (cyclophosphamide, vincristine, and prednisone), and bendamustine.
At a median observation time of 41.1 months, the overall response rate was 91% in the obinutuzumab arm and 88% in the rituximab arm. The complete response rates were 28% and 27%, respectively.
The median progression-free survival was not reached in either arm. The hazard ratio, for obinutuzumab compared to rituximab, was 0.72 (95% CI, 0.56-0.93, P=0.0118).
The estimated 3-year progression-free survival was 78.9% in the rituximab arm and 83.4% in the obinutuzumab arm.
Safety was evaluated based on all 1385 patients in the study, 86% of whom had FL and 14% of whom had marginal zone lymphoma.
Serious adverse events (AEs) occurred in 50% of patients in the obinutuzumab arm and 43% in the rituximab arm. Fatal AEs occurred in 5% and 4%, respectively. Infections and second malignancies were the leading causes of these deaths.
During the monotherapy period, the most common AEs (≥ 5%) in patients treated with obinutuzumab were cough (21%), neutropenia (19%), upper respiratory tract infection (15%), viral upper respiratory tract infection (15%), diarrhea (13%), arthralgia (10%), fatigue (9%), sinusitis (9%), infusion reactions (8%), pneumonia (8%), herpes zoster (8%), lower respiratory tract infection (7%), pyrexia (7%), back pain (6%), headache (6%), urinary tract infection (6%), nausea (6%), bronchitis (5%), and vomiting (5%).
Grade 3-4 AEs (≥1%) in patients treated with obinutuzumab included neutropenia (17%), pneumonia (3%), and febrile neutropenia (2%). There were 2 deaths due to pneumonia in the obinutuzumab arm.
Health Canada has expanded the approved use of obinutuzumab (Gazyva®).
The anti-CD20 monoclonal antibody is now approved for use in combination with chemotherapy to treat patients with previously untreated follicular lymphoma (FL) that is advanced (stage II bulky, stage III, or stage IV).
In patients who respond to this treatment, obinutuzumab monotherapy can be given as maintenance.
Health Canada previously approved obinutuzumab for the following indications:
- In combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia
- First in combination with bendamustine, then as monotherapy, in FL patients who relapsed after or are refractory to a rituximab-containing regimen.
Phase 3 results
Health Canada’s latest approval of obinutuzumab is based on results from the phase 3 GALLIUM study, which were published in NEJM in October 2017. The following are updated data from the product monograph.
GALLIUM included 1385 patients with previously untreated non-Hodgkin lymphoma, and 1202 of these patients had previously untreated, advanced FL.
Half of the FL patients (n=601) were randomized to receive obinutuzumab plus chemotherapy (followed by obinutuzumab maintenance for up to 2 years), and half were randomized to rituximab plus chemotherapy (followed by rituximab maintenance for up to 2 years).
The different chemotherapies used were CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (cyclophosphamide, vincristine, and prednisone), and bendamustine.
At a median observation time of 41.1 months, the overall response rate was 91% in the obinutuzumab arm and 88% in the rituximab arm. The complete response rates were 28% and 27%, respectively.
The median progression-free survival was not reached in either arm. The hazard ratio, for obinutuzumab compared to rituximab, was 0.72 (95% CI, 0.56-0.93, P=0.0118).
The estimated 3-year progression-free survival was 78.9% in the rituximab arm and 83.4% in the obinutuzumab arm.
Safety was evaluated based on all 1385 patients in the study, 86% of whom had FL and 14% of whom had marginal zone lymphoma.
Serious adverse events (AEs) occurred in 50% of patients in the obinutuzumab arm and 43% in the rituximab arm. Fatal AEs occurred in 5% and 4%, respectively. Infections and second malignancies were the leading causes of these deaths.
During the monotherapy period, the most common AEs (≥ 5%) in patients treated with obinutuzumab were cough (21%), neutropenia (19%), upper respiratory tract infection (15%), viral upper respiratory tract infection (15%), diarrhea (13%), arthralgia (10%), fatigue (9%), sinusitis (9%), infusion reactions (8%), pneumonia (8%), herpes zoster (8%), lower respiratory tract infection (7%), pyrexia (7%), back pain (6%), headache (6%), urinary tract infection (6%), nausea (6%), bronchitis (5%), and vomiting (5%).
Grade 3-4 AEs (≥1%) in patients treated with obinutuzumab included neutropenia (17%), pneumonia (3%), and febrile neutropenia (2%). There were 2 deaths due to pneumonia in the obinutuzumab arm.
Health Canada has expanded the approved use of obinutuzumab (Gazyva®).
The anti-CD20 monoclonal antibody is now approved for use in combination with chemotherapy to treat patients with previously untreated follicular lymphoma (FL) that is advanced (stage II bulky, stage III, or stage IV).
In patients who respond to this treatment, obinutuzumab monotherapy can be given as maintenance.
Health Canada previously approved obinutuzumab for the following indications:
- In combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia
- First in combination with bendamustine, then as monotherapy, in FL patients who relapsed after or are refractory to a rituximab-containing regimen.
Phase 3 results
Health Canada’s latest approval of obinutuzumab is based on results from the phase 3 GALLIUM study, which were published in NEJM in October 2017. The following are updated data from the product monograph.
GALLIUM included 1385 patients with previously untreated non-Hodgkin lymphoma, and 1202 of these patients had previously untreated, advanced FL.
Half of the FL patients (n=601) were randomized to receive obinutuzumab plus chemotherapy (followed by obinutuzumab maintenance for up to 2 years), and half were randomized to rituximab plus chemotherapy (followed by rituximab maintenance for up to 2 years).
The different chemotherapies used were CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (cyclophosphamide, vincristine, and prednisone), and bendamustine.
At a median observation time of 41.1 months, the overall response rate was 91% in the obinutuzumab arm and 88% in the rituximab arm. The complete response rates were 28% and 27%, respectively.
The median progression-free survival was not reached in either arm. The hazard ratio, for obinutuzumab compared to rituximab, was 0.72 (95% CI, 0.56-0.93, P=0.0118).
The estimated 3-year progression-free survival was 78.9% in the rituximab arm and 83.4% in the obinutuzumab arm.
Safety was evaluated based on all 1385 patients in the study, 86% of whom had FL and 14% of whom had marginal zone lymphoma.
Serious adverse events (AEs) occurred in 50% of patients in the obinutuzumab arm and 43% in the rituximab arm. Fatal AEs occurred in 5% and 4%, respectively. Infections and second malignancies were the leading causes of these deaths.
During the monotherapy period, the most common AEs (≥ 5%) in patients treated with obinutuzumab were cough (21%), neutropenia (19%), upper respiratory tract infection (15%), viral upper respiratory tract infection (15%), diarrhea (13%), arthralgia (10%), fatigue (9%), sinusitis (9%), infusion reactions (8%), pneumonia (8%), herpes zoster (8%), lower respiratory tract infection (7%), pyrexia (7%), back pain (6%), headache (6%), urinary tract infection (6%), nausea (6%), bronchitis (5%), and vomiting (5%).
Grade 3-4 AEs (≥1%) in patients treated with obinutuzumab included neutropenia (17%), pneumonia (3%), and febrile neutropenia (2%). There were 2 deaths due to pneumonia in the obinutuzumab arm.
Transplant strategy not viable for aggressive B-NHL
Transplant with radioimmunotherapy (RIT)-based conditioning is a viable treatment option for patients with indolent—but not aggressive—B-cell non-Hodgkin lymphomas (NHLs), according to researchers.
Long-term follow-up data showed “excellent” outcomes in patients with indolent B-NHL who received conditioning with 90Y-ibritumomab tiuxetan plus fludarabine and low-dose total body irradiation (TBI) prior to HLA-matched hematopoietic stem cell transplant (HSCT).
However, long-term outcomes were inferior in patients with diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL).
Camille E. Puronen, MD, of the University of Washington in Seattle, and her colleagues reported these results in Biology of Blood and Marrow Transplantation.
The study enrolled 40 patients with high-risk B-NHL. This included DLBCL (n=14), chronic lymphocytic leukemia (CLL; n=10), MCL (n=8), follicular lymphoma (FL; n=6); hairy cell leukemia (HCL; n=1), and marginal zone lymphoma (MZL; n=1).
Patients were treated with 0.4 mCi/kg 90Y-ibritumomab tiuxetan, given 2 weeks prior to HSCT, to a maximum dose of 32 mCi.
Patients also received fludarabine at 30 mg/m2 on day 5, 6, and 7 prior to HSCT and 2 Gy TBI given on the day of transplant.
In an earlier report, the objective response rate (ORR) was 60%, and 35% of patients had a complete response (CR) or unconfirmed CR.
The researchers said early responses were not associated with disease bulk or chemoresistance, as the ORR was 59% in patients with bulky or chemoresistant disease.
However, responses were associated with histology, as the ORR was 38% in patients with DLBCL, 50% in those with MCL, 83% in those with FL, and 90% in those with CLL.
Long-term survival
In the current report, 11 of 40 patients were still alive at a median follow up of 9 years (range, 5.3 to 10.2). Fourteen patients died of disease progression, and 14 died from complications of HSCT.
The 5-year overall survival (OS) was 40%, and the 5-year progression-free survival (PFS) was 28%.
The best survival rates were in patients with indolent histology. The 5-year PFS was 44% in these patients, and the 5-year OS was 67%.
The researchers said early CR was not associated with long-term survival. However, patients who had at least stable disease (SD) at earlier time points did have the opportunity to achieve long-term survival. All patients who progressed before day 84 were dead by the 1-year mark.
Of the 11 patients who were still alive at a median follow up of 9 years, 4 had a CR or unconfirmed CR at day 84 (FL: 1; CLL: 2; MCL: 1); 6 were in partial response (CLL: 3; FL: 1; MCL: 1; MZL: 1); and 1 patient with FL had SD.
Among the 18 patients with indolent NHL, long-term PFS was observed in 5 of the 7 patients who achieved early CR and 8 of the 11 patients who did not achieve early CR.
Two of the 4 MCL patients who achieved an early CR had long-term PFS, but none of the MCL patients without an early CR had long-term PFS.
Among DLBCL patients, 1 of the 4 who achieved early CR had long-term PFS, but none of the patients without an early CR had long-term PFS. Only 1 DLBCL patient survived beyond 5 years. None survived beyond 8 years.
The researchers said the favorable outcomes in patients with indolent B-NHL are consistent with the known efficacy of RIT and the graft-versus-leukemia effect in these patients.
The team also noted that, since this trial began, several novel agents have been approved for the treatment of indolent B-NHL, which means allogeneic HSCT is often moved to later in the disease course.
The researchers concluded that 90Y-ibritumomab tiuxetan-based conditioning could “continue to play an important role in these settings,” but “improved strategies are needed” for patients with MCL and DLBCL.
Transplant with radioimmunotherapy (RIT)-based conditioning is a viable treatment option for patients with indolent—but not aggressive—B-cell non-Hodgkin lymphomas (NHLs), according to researchers.
Long-term follow-up data showed “excellent” outcomes in patients with indolent B-NHL who received conditioning with 90Y-ibritumomab tiuxetan plus fludarabine and low-dose total body irradiation (TBI) prior to HLA-matched hematopoietic stem cell transplant (HSCT).
However, long-term outcomes were inferior in patients with diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL).
Camille E. Puronen, MD, of the University of Washington in Seattle, and her colleagues reported these results in Biology of Blood and Marrow Transplantation.
The study enrolled 40 patients with high-risk B-NHL. This included DLBCL (n=14), chronic lymphocytic leukemia (CLL; n=10), MCL (n=8), follicular lymphoma (FL; n=6); hairy cell leukemia (HCL; n=1), and marginal zone lymphoma (MZL; n=1).
Patients were treated with 0.4 mCi/kg 90Y-ibritumomab tiuxetan, given 2 weeks prior to HSCT, to a maximum dose of 32 mCi.
Patients also received fludarabine at 30 mg/m2 on day 5, 6, and 7 prior to HSCT and 2 Gy TBI given on the day of transplant.
In an earlier report, the objective response rate (ORR) was 60%, and 35% of patients had a complete response (CR) or unconfirmed CR.
The researchers said early responses were not associated with disease bulk or chemoresistance, as the ORR was 59% in patients with bulky or chemoresistant disease.
However, responses were associated with histology, as the ORR was 38% in patients with DLBCL, 50% in those with MCL, 83% in those with FL, and 90% in those with CLL.
Long-term survival
In the current report, 11 of 40 patients were still alive at a median follow up of 9 years (range, 5.3 to 10.2). Fourteen patients died of disease progression, and 14 died from complications of HSCT.
The 5-year overall survival (OS) was 40%, and the 5-year progression-free survival (PFS) was 28%.
The best survival rates were in patients with indolent histology. The 5-year PFS was 44% in these patients, and the 5-year OS was 67%.
The researchers said early CR was not associated with long-term survival. However, patients who had at least stable disease (SD) at earlier time points did have the opportunity to achieve long-term survival. All patients who progressed before day 84 were dead by the 1-year mark.
Of the 11 patients who were still alive at a median follow up of 9 years, 4 had a CR or unconfirmed CR at day 84 (FL: 1; CLL: 2; MCL: 1); 6 were in partial response (CLL: 3; FL: 1; MCL: 1; MZL: 1); and 1 patient with FL had SD.
Among the 18 patients with indolent NHL, long-term PFS was observed in 5 of the 7 patients who achieved early CR and 8 of the 11 patients who did not achieve early CR.
Two of the 4 MCL patients who achieved an early CR had long-term PFS, but none of the MCL patients without an early CR had long-term PFS.
Among DLBCL patients, 1 of the 4 who achieved early CR had long-term PFS, but none of the patients without an early CR had long-term PFS. Only 1 DLBCL patient survived beyond 5 years. None survived beyond 8 years.
The researchers said the favorable outcomes in patients with indolent B-NHL are consistent with the known efficacy of RIT and the graft-versus-leukemia effect in these patients.
The team also noted that, since this trial began, several novel agents have been approved for the treatment of indolent B-NHL, which means allogeneic HSCT is often moved to later in the disease course.
The researchers concluded that 90Y-ibritumomab tiuxetan-based conditioning could “continue to play an important role in these settings,” but “improved strategies are needed” for patients with MCL and DLBCL.
Transplant with radioimmunotherapy (RIT)-based conditioning is a viable treatment option for patients with indolent—but not aggressive—B-cell non-Hodgkin lymphomas (NHLs), according to researchers.
Long-term follow-up data showed “excellent” outcomes in patients with indolent B-NHL who received conditioning with 90Y-ibritumomab tiuxetan plus fludarabine and low-dose total body irradiation (TBI) prior to HLA-matched hematopoietic stem cell transplant (HSCT).
However, long-term outcomes were inferior in patients with diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL).
Camille E. Puronen, MD, of the University of Washington in Seattle, and her colleagues reported these results in Biology of Blood and Marrow Transplantation.
The study enrolled 40 patients with high-risk B-NHL. This included DLBCL (n=14), chronic lymphocytic leukemia (CLL; n=10), MCL (n=8), follicular lymphoma (FL; n=6); hairy cell leukemia (HCL; n=1), and marginal zone lymphoma (MZL; n=1).
Patients were treated with 0.4 mCi/kg 90Y-ibritumomab tiuxetan, given 2 weeks prior to HSCT, to a maximum dose of 32 mCi.
Patients also received fludarabine at 30 mg/m2 on day 5, 6, and 7 prior to HSCT and 2 Gy TBI given on the day of transplant.
In an earlier report, the objective response rate (ORR) was 60%, and 35% of patients had a complete response (CR) or unconfirmed CR.
The researchers said early responses were not associated with disease bulk or chemoresistance, as the ORR was 59% in patients with bulky or chemoresistant disease.
However, responses were associated with histology, as the ORR was 38% in patients with DLBCL, 50% in those with MCL, 83% in those with FL, and 90% in those with CLL.
Long-term survival
In the current report, 11 of 40 patients were still alive at a median follow up of 9 years (range, 5.3 to 10.2). Fourteen patients died of disease progression, and 14 died from complications of HSCT.
The 5-year overall survival (OS) was 40%, and the 5-year progression-free survival (PFS) was 28%.
The best survival rates were in patients with indolent histology. The 5-year PFS was 44% in these patients, and the 5-year OS was 67%.
The researchers said early CR was not associated with long-term survival. However, patients who had at least stable disease (SD) at earlier time points did have the opportunity to achieve long-term survival. All patients who progressed before day 84 were dead by the 1-year mark.
Of the 11 patients who were still alive at a median follow up of 9 years, 4 had a CR or unconfirmed CR at day 84 (FL: 1; CLL: 2; MCL: 1); 6 were in partial response (CLL: 3; FL: 1; MCL: 1; MZL: 1); and 1 patient with FL had SD.
Among the 18 patients with indolent NHL, long-term PFS was observed in 5 of the 7 patients who achieved early CR and 8 of the 11 patients who did not achieve early CR.
Two of the 4 MCL patients who achieved an early CR had long-term PFS, but none of the MCL patients without an early CR had long-term PFS.
Among DLBCL patients, 1 of the 4 who achieved early CR had long-term PFS, but none of the patients without an early CR had long-term PFS. Only 1 DLBCL patient survived beyond 5 years. None survived beyond 8 years.
The researchers said the favorable outcomes in patients with indolent B-NHL are consistent with the known efficacy of RIT and the graft-versus-leukemia effect in these patients.
The team also noted that, since this trial began, several novel agents have been approved for the treatment of indolent B-NHL, which means allogeneic HSCT is often moved to later in the disease course.
The researchers concluded that 90Y-ibritumomab tiuxetan-based conditioning could “continue to play an important role in these settings,” but “improved strategies are needed” for patients with MCL and DLBCL.
CHMP recommends CAR T for ALL, DLBCL
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended the approval of tisagenlecleucel (Kymriah®, formerly CTL019) for 2 indications.
According to the CHMP, the chimeric antigen receptor (CAR) T-cell therapy should be approved to treat adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who have received 2 or more lines of systemic therapy and patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation is based on results from a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
Updated results from JULIET were presented at the recent 23rd Annual Congress of the European Hematology Association (EHA) as abstract S799.
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. Of the patients in CR at month 3, 83% remained in CR at month 12. The median duration of response was not reached.
At the time of data cutoff, none of the responders had proceeded to stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended the approval of tisagenlecleucel (Kymriah®, formerly CTL019) for 2 indications.
According to the CHMP, the chimeric antigen receptor (CAR) T-cell therapy should be approved to treat adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who have received 2 or more lines of systemic therapy and patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation is based on results from a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
Updated results from JULIET were presented at the recent 23rd Annual Congress of the European Hematology Association (EHA) as abstract S799.
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. Of the patients in CR at month 3, 83% remained in CR at month 12. The median duration of response was not reached.
At the time of data cutoff, none of the responders had proceeded to stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended the approval of tisagenlecleucel (Kymriah®, formerly CTL019) for 2 indications.
According to the CHMP, the chimeric antigen receptor (CAR) T-cell therapy should be approved to treat adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who have received 2 or more lines of systemic therapy and patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation is based on results from a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
Updated results from JULIET were presented at the recent 23rd Annual Congress of the European Hematology Association (EHA) as abstract S799.
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. Of the patients in CR at month 3, 83% remained in CR at month 12. The median duration of response was not reached.
At the time of data cutoff, none of the responders had proceeded to stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
CHMP recommends CAR T for DLBCL, PMBCL
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for the chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (Yescarta®, formerly KTE-C19).
The recommendation pertains to axicabtagene ciloleucel as a treatment for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) or primary mediastinal large B-cell lymphoma (PMBCL) who have received 2 or more lines of systemic therapy.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The marketing authorization application for axicabtagene ciloleucel is supported by data from the ZUMA-1 trial.
Results from this phase 2 trial were presented at the 2017 ASH Annual Meeting and published simultaneously in NEJM.
The trial enrolled 111 patients with relapsed/refractory B-cell lymphomas. There were 101 patients who received axicabtagene ciloleucel—77 with DLBCL, 8 with PMBCL, and 16 with transformed follicular lymphoma (TFL).
Patients received conditioning with low-dose cyclophosphamide and fludarabine, followed by axicabtagene ciloleucel.
The objective response rate (ORR) was 82% (n=83), and the complete response (CR) rate was 54% (n=55).
Among the DLBCL patients, the ORR was 82% (63/77), and the CR rate was 49% (38/77). In the patients with PMBCL or TFL, the ORR was 83% (20/24), and the CR rate was 71% (17/24).
With a median follow-up of 15.4 months, 42% of patients retained their response, and 40% retained a CR.
At 18 months, the overall survival was 52%. Most deaths were due to disease progression.
However, 2 patients died of adverse events related to axicabtagene ciloleucel, both cytokine release syndrome (CRS).
The most common grade 3 or higher adverse events were neutropenia (78%), anemia (43%), thrombocytopenia (38%), and febrile neutropenia (31%).
Grade 3 or higher CRS occurred in 13% of patients, and grade 3 or higher neurologic events occurred in 28%.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for the chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (Yescarta®, formerly KTE-C19).
The recommendation pertains to axicabtagene ciloleucel as a treatment for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) or primary mediastinal large B-cell lymphoma (PMBCL) who have received 2 or more lines of systemic therapy.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The marketing authorization application for axicabtagene ciloleucel is supported by data from the ZUMA-1 trial.
Results from this phase 2 trial were presented at the 2017 ASH Annual Meeting and published simultaneously in NEJM.
The trial enrolled 111 patients with relapsed/refractory B-cell lymphomas. There were 101 patients who received axicabtagene ciloleucel—77 with DLBCL, 8 with PMBCL, and 16 with transformed follicular lymphoma (TFL).
Patients received conditioning with low-dose cyclophosphamide and fludarabine, followed by axicabtagene ciloleucel.
The objective response rate (ORR) was 82% (n=83), and the complete response (CR) rate was 54% (n=55).
Among the DLBCL patients, the ORR was 82% (63/77), and the CR rate was 49% (38/77). In the patients with PMBCL or TFL, the ORR was 83% (20/24), and the CR rate was 71% (17/24).
With a median follow-up of 15.4 months, 42% of patients retained their response, and 40% retained a CR.
At 18 months, the overall survival was 52%. Most deaths were due to disease progression.
However, 2 patients died of adverse events related to axicabtagene ciloleucel, both cytokine release syndrome (CRS).
The most common grade 3 or higher adverse events were neutropenia (78%), anemia (43%), thrombocytopenia (38%), and febrile neutropenia (31%).
Grade 3 or higher CRS occurred in 13% of patients, and grade 3 or higher neurologic events occurred in 28%.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for the chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (Yescarta®, formerly KTE-C19).
The recommendation pertains to axicabtagene ciloleucel as a treatment for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) or primary mediastinal large B-cell lymphoma (PMBCL) who have received 2 or more lines of systemic therapy.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The marketing authorization application for axicabtagene ciloleucel is supported by data from the ZUMA-1 trial.
Results from this phase 2 trial were presented at the 2017 ASH Annual Meeting and published simultaneously in NEJM.
The trial enrolled 111 patients with relapsed/refractory B-cell lymphomas. There were 101 patients who received axicabtagene ciloleucel—77 with DLBCL, 8 with PMBCL, and 16 with transformed follicular lymphoma (TFL).
Patients received conditioning with low-dose cyclophosphamide and fludarabine, followed by axicabtagene ciloleucel.
The objective response rate (ORR) was 82% (n=83), and the complete response (CR) rate was 54% (n=55).
Among the DLBCL patients, the ORR was 82% (63/77), and the CR rate was 49% (38/77). In the patients with PMBCL or TFL, the ORR was 83% (20/24), and the CR rate was 71% (17/24).
With a median follow-up of 15.4 months, 42% of patients retained their response, and 40% retained a CR.
At 18 months, the overall survival was 52%. Most deaths were due to disease progression.
However, 2 patients died of adverse events related to axicabtagene ciloleucel, both cytokine release syndrome (CRS).
The most common grade 3 or higher adverse events were neutropenia (78%), anemia (43%), thrombocytopenia (38%), and febrile neutropenia (31%).
Grade 3 or higher CRS occurred in 13% of patients, and grade 3 or higher neurologic events occurred in 28%.
Doc reports favorable results from trial on hold
STOCKHOLM—Interim trial results suggest the EZH2 inhibitor tazemetostat can produce durable responses in patients with relapsed or refractory follicular lymphoma (FL).
In patients with EZH2 mutations, the overall response rate (ORR) was 71%, and the median duration of response (DOR) was 32 weeks.
For patients with wild-type (WT) EZH2, the ORR was 33%, and the median DOR was 76 weeks.
Tazemetostat was considered generally well tolerated in this phase 2 trial, which is currently on partial clinical hold.
Gilles Salles, MD, PhD, of the University Hospital of Lyon France, presented results from the trial at the 23rd Congress of the European Hematology Association (EHA) as abstract S100.
The trial is sponsored by Epizyme, Inc.
In April, Epizyme announced that all US-based trials of tazemetostat had been placed on partial hold after a pediatric patient on a phase 1 trial developed secondary T-cell lymphoma.
Enrollment was stopped in all the trials, but patients could continue receiving tazemetostat if they had not progressed on the drug.
The phase 2 trial of tazemetostat in non-Hodgkin lymphoma has enrolled 89 adults with relapsed/refractory FL.
At EHA, Dr Salles presented results in 82 of these patients. There were 28 patients with EZH2-mutated FL and 54 with EZH2-WT FL.
The median age was 61 in both cohorts. Forty-three percent of EZH2-mutated and 63% of WT patients were male.
EZH2-mutated patients had a median of 3 prior therapies, and WT patients had a median of 4. Thirty-eight percent and 42%, respectively, were refractory to their last therapy. Eleven percent and 39%, respectively, had received prior transplant.
The median time from diagnosis was 5.1 years for EZH2-mutated patients and 6.4 years for WT patients. The median time from last prior therapy was 18.4 weeks and 28.1 weeks, respectively.
The patients received tazemetostat at 800 mg twice daily until disease progression or withdrawal.
Safety
In all 82 patients, the rate of treatment-emergent adverse events (AEs) was 95%, and the rate of treatment-related AEs was 78%. The rate of grade 3 or higher treatment-related AEs was 17%, and the rate of serious treatment-related AEs was 4%.
Six percent of patients discontinued treatment due to a related AE, 18% had a dose interruption, and 5% had a dose reduction due to a related AE.
Treatment-related AEs included nausea (20%), fatigue (13%), anemia (13%), diarrhea (11%), alopecia (11%), asthenia (10%), thrombocytopenia (10%), muscle spasms (6%), bronchitis (5%), vomiting (5%), headache (5%), abdominal pain (2%), pyrexia (1%), and cough (1%).
Grade 3 or higher treatment-related AEs included thrombocytopenia (4%), anemia (4%), fatigue (1%), and asthenia (1%).
Efficacy
In the EZH2-mutated cohort, the ORR was 71% (n=20). Eleven percent of patients (n=3) achieved a complete response, and 61% (n=17) had a partial response.
Twenty-nine percent (n=8) had stable disease as their best response. And 21% (n=6) of patients are still on study with stable disease.
All patients in this cohort experienced a reduction in tumor burden. None of the patients had progressive disease as their best response.
At the time of analysis (May 1, 2018), the median DOR was 32.3 weeks, and 55% of responders (n=11) had an ongoing response.
The median progression-free survival was 48.6 weeks.
In patients with WT EZH2 (n=54), the ORR was 33% (n=18). Six percent of patients (n=3) achieved a complete response, and 28% (n=15) had a partial response.
Thirty-one percent of patients (n=17) had stable disease as their best response, including 1 patient who is still receiving treatment.
Thirty-one percent of patients (n=17) progressed. For 4% (n=2), their response status was unknown.
At the time of analysis, the median DOR was 76 weeks, and 56% of responders (n=10) had an ongoing response.
The median progression-free survival was 29.9 weeks.
“I am impressed by the sustained clinical activity and the good tolerability of tazemetostat in this heavily pretreated patient population,” Dr Salles said. “This is important for patients with relapsed or refractory follicular lymphoma, as both the response rates and durations of response usually tend to decrease with each successive line of treatment.”
“I believe tazemetostat has the potential to fill a significant unmet need for these patients, and continued investigation of tazemetostat as a single agent or in combination with other agents is warranted.”
Epizyme’s president and chief executive officer, Robert Bazemore, said the company is still working to resolve the partial clinical hold on tazemetostat trials and is “making good progress.”
STOCKHOLM—Interim trial results suggest the EZH2 inhibitor tazemetostat can produce durable responses in patients with relapsed or refractory follicular lymphoma (FL).
In patients with EZH2 mutations, the overall response rate (ORR) was 71%, and the median duration of response (DOR) was 32 weeks.
For patients with wild-type (WT) EZH2, the ORR was 33%, and the median DOR was 76 weeks.
Tazemetostat was considered generally well tolerated in this phase 2 trial, which is currently on partial clinical hold.
Gilles Salles, MD, PhD, of the University Hospital of Lyon France, presented results from the trial at the 23rd Congress of the European Hematology Association (EHA) as abstract S100.
The trial is sponsored by Epizyme, Inc.
In April, Epizyme announced that all US-based trials of tazemetostat had been placed on partial hold after a pediatric patient on a phase 1 trial developed secondary T-cell lymphoma.
Enrollment was stopped in all the trials, but patients could continue receiving tazemetostat if they had not progressed on the drug.
The phase 2 trial of tazemetostat in non-Hodgkin lymphoma has enrolled 89 adults with relapsed/refractory FL.
At EHA, Dr Salles presented results in 82 of these patients. There were 28 patients with EZH2-mutated FL and 54 with EZH2-WT FL.
The median age was 61 in both cohorts. Forty-three percent of EZH2-mutated and 63% of WT patients were male.
EZH2-mutated patients had a median of 3 prior therapies, and WT patients had a median of 4. Thirty-eight percent and 42%, respectively, were refractory to their last therapy. Eleven percent and 39%, respectively, had received prior transplant.
The median time from diagnosis was 5.1 years for EZH2-mutated patients and 6.4 years for WT patients. The median time from last prior therapy was 18.4 weeks and 28.1 weeks, respectively.
The patients received tazemetostat at 800 mg twice daily until disease progression or withdrawal.
Safety
In all 82 patients, the rate of treatment-emergent adverse events (AEs) was 95%, and the rate of treatment-related AEs was 78%. The rate of grade 3 or higher treatment-related AEs was 17%, and the rate of serious treatment-related AEs was 4%.
Six percent of patients discontinued treatment due to a related AE, 18% had a dose interruption, and 5% had a dose reduction due to a related AE.
Treatment-related AEs included nausea (20%), fatigue (13%), anemia (13%), diarrhea (11%), alopecia (11%), asthenia (10%), thrombocytopenia (10%), muscle spasms (6%), bronchitis (5%), vomiting (5%), headache (5%), abdominal pain (2%), pyrexia (1%), and cough (1%).
Grade 3 or higher treatment-related AEs included thrombocytopenia (4%), anemia (4%), fatigue (1%), and asthenia (1%).
Efficacy
In the EZH2-mutated cohort, the ORR was 71% (n=20). Eleven percent of patients (n=3) achieved a complete response, and 61% (n=17) had a partial response.
Twenty-nine percent (n=8) had stable disease as their best response. And 21% (n=6) of patients are still on study with stable disease.
All patients in this cohort experienced a reduction in tumor burden. None of the patients had progressive disease as their best response.
At the time of analysis (May 1, 2018), the median DOR was 32.3 weeks, and 55% of responders (n=11) had an ongoing response.
The median progression-free survival was 48.6 weeks.
In patients with WT EZH2 (n=54), the ORR was 33% (n=18). Six percent of patients (n=3) achieved a complete response, and 28% (n=15) had a partial response.
Thirty-one percent of patients (n=17) had stable disease as their best response, including 1 patient who is still receiving treatment.
Thirty-one percent of patients (n=17) progressed. For 4% (n=2), their response status was unknown.
At the time of analysis, the median DOR was 76 weeks, and 56% of responders (n=10) had an ongoing response.
The median progression-free survival was 29.9 weeks.
“I am impressed by the sustained clinical activity and the good tolerability of tazemetostat in this heavily pretreated patient population,” Dr Salles said. “This is important for patients with relapsed or refractory follicular lymphoma, as both the response rates and durations of response usually tend to decrease with each successive line of treatment.”
“I believe tazemetostat has the potential to fill a significant unmet need for these patients, and continued investigation of tazemetostat as a single agent or in combination with other agents is warranted.”
Epizyme’s president and chief executive officer, Robert Bazemore, said the company is still working to resolve the partial clinical hold on tazemetostat trials and is “making good progress.”
STOCKHOLM—Interim trial results suggest the EZH2 inhibitor tazemetostat can produce durable responses in patients with relapsed or refractory follicular lymphoma (FL).
In patients with EZH2 mutations, the overall response rate (ORR) was 71%, and the median duration of response (DOR) was 32 weeks.
For patients with wild-type (WT) EZH2, the ORR was 33%, and the median DOR was 76 weeks.
Tazemetostat was considered generally well tolerated in this phase 2 trial, which is currently on partial clinical hold.
Gilles Salles, MD, PhD, of the University Hospital of Lyon France, presented results from the trial at the 23rd Congress of the European Hematology Association (EHA) as abstract S100.
The trial is sponsored by Epizyme, Inc.
In April, Epizyme announced that all US-based trials of tazemetostat had been placed on partial hold after a pediatric patient on a phase 1 trial developed secondary T-cell lymphoma.
Enrollment was stopped in all the trials, but patients could continue receiving tazemetostat if they had not progressed on the drug.
The phase 2 trial of tazemetostat in non-Hodgkin lymphoma has enrolled 89 adults with relapsed/refractory FL.
At EHA, Dr Salles presented results in 82 of these patients. There were 28 patients with EZH2-mutated FL and 54 with EZH2-WT FL.
The median age was 61 in both cohorts. Forty-three percent of EZH2-mutated and 63% of WT patients were male.
EZH2-mutated patients had a median of 3 prior therapies, and WT patients had a median of 4. Thirty-eight percent and 42%, respectively, were refractory to their last therapy. Eleven percent and 39%, respectively, had received prior transplant.
The median time from diagnosis was 5.1 years for EZH2-mutated patients and 6.4 years for WT patients. The median time from last prior therapy was 18.4 weeks and 28.1 weeks, respectively.
The patients received tazemetostat at 800 mg twice daily until disease progression or withdrawal.
Safety
In all 82 patients, the rate of treatment-emergent adverse events (AEs) was 95%, and the rate of treatment-related AEs was 78%. The rate of grade 3 or higher treatment-related AEs was 17%, and the rate of serious treatment-related AEs was 4%.
Six percent of patients discontinued treatment due to a related AE, 18% had a dose interruption, and 5% had a dose reduction due to a related AE.
Treatment-related AEs included nausea (20%), fatigue (13%), anemia (13%), diarrhea (11%), alopecia (11%), asthenia (10%), thrombocytopenia (10%), muscle spasms (6%), bronchitis (5%), vomiting (5%), headache (5%), abdominal pain (2%), pyrexia (1%), and cough (1%).
Grade 3 or higher treatment-related AEs included thrombocytopenia (4%), anemia (4%), fatigue (1%), and asthenia (1%).
Efficacy
In the EZH2-mutated cohort, the ORR was 71% (n=20). Eleven percent of patients (n=3) achieved a complete response, and 61% (n=17) had a partial response.
Twenty-nine percent (n=8) had stable disease as their best response. And 21% (n=6) of patients are still on study with stable disease.
All patients in this cohort experienced a reduction in tumor burden. None of the patients had progressive disease as their best response.
At the time of analysis (May 1, 2018), the median DOR was 32.3 weeks, and 55% of responders (n=11) had an ongoing response.
The median progression-free survival was 48.6 weeks.
In patients with WT EZH2 (n=54), the ORR was 33% (n=18). Six percent of patients (n=3) achieved a complete response, and 28% (n=15) had a partial response.
Thirty-one percent of patients (n=17) had stable disease as their best response, including 1 patient who is still receiving treatment.
Thirty-one percent of patients (n=17) progressed. For 4% (n=2), their response status was unknown.
At the time of analysis, the median DOR was 76 weeks, and 56% of responders (n=10) had an ongoing response.
The median progression-free survival was 29.9 weeks.
“I am impressed by the sustained clinical activity and the good tolerability of tazemetostat in this heavily pretreated patient population,” Dr Salles said. “This is important for patients with relapsed or refractory follicular lymphoma, as both the response rates and durations of response usually tend to decrease with each successive line of treatment.”
“I believe tazemetostat has the potential to fill a significant unmet need for these patients, and continued investigation of tazemetostat as a single agent or in combination with other agents is warranted.”
Epizyme’s president and chief executive officer, Robert Bazemore, said the company is still working to resolve the partial clinical hold on tazemetostat trials and is “making good progress.”
Group updates guidelines on CLL
Recent advances in chronic lymphocytic leukemia (CLL) have prompted an update to the 2008 International Workshop in Chronic Lymphocytic Leukemia (iwCLL) consensus guidelines.
The updated iwCLL guidelines include new information on genomic alterations, the use of clinical staging and prognostic markers/scores, response assessment, minimal residual disease (MRD), and viral diseases in CLL patients.
The update was recently published in Blood.
Diagnosis, prognosis, and staging
To verify CLL diagnosis, the iwCLL guidelines recommend obtaining complete blood counts and differential counts as well as immunophenotyping of peripheral blood lymphocytes. A panel of CD19, CD5, CD20, CD23, κ, and λ typically suffices to establish a diagnosis.
Other tests that may help in prognosis or assessing tumor burden include molecular cytogenetics for del(13q), del(11q), del(17p), and add(12) in peripheral blood lymphocytes and determining TP53 and IGHV mutational status.
These tests can help identify poor-prognosis patients who are not likely to benefit from standard chemotherapy but are likely to benefit from small-molecule inhibitors of BTK, PI3K, or BCL2.
In addition, serum markers such as β2-microglogulin provide insight into overall survival and progression-free survival.
With regard to clinical staging, the guidelines highlight the Binet and Rai systems, which are routinely used in clinical practice and clinical trials.
However, the guidelines also note that “there are a large number of biomarkers that can provide additional prognostic information,” and, recently, “several prognostic scores and stratification systems have been proposed based on multivariate analyses.”
For example, the CLL international prognostic index (CLL-IPI) provides a weighted score that uses clinical stage, age, IGHV mutational status, β2-microglogulin, and the presence of del(17p) and/or TP53 mutations.
Indications for treatment
The guidelines note that active disease must be documented to initiate therapy. At least 1 of the following criteria must be met:
- Evidence of progressive marrow failure
- Massive, progressive, or symptomatic splenomegaly
- Progressive/symptomatic lymphadenopathy or massive nodes
- Progressive lymphocytosis
- Autoimmune complications
- Extranodal involvement
- Disease-related symptoms (unintentional weight loss, significant fatigue, fevers, night sweats for over a month without evidence of infection).
Following relapse, subsequent lines of treatment should follow the same principles as those used for initial treatment decisions.
Response, MRD, and more
The guidelines say 2 groups of parameters must be assessed to determine response to therapy:
- Group A: lymphoid tumor load and constitutional symptoms, including liver and/or spleen size, lymph node evaluation, and circulating lymphocyte count
- Group B: the hematopoietic system (platelet count, hemoglobin, and marrow).
For therapies with a defined treatment duration, response should be assessed at least 2 months after treatment is completed. For continued therapies or maintenance, response should be assessed at a predefined time point or at least 2 months after patients achieve their maximum response.
The guidelines also say MRD should be assessed in clinical trials aimed at maximizing the depth of remission. Furthermore, it “may be important” to confirm MRD negativity in the blood and marrow, as there are therapies that preferentially clear the blood but not the marrow (such as monoclonal antibodies).
In addition to the aforementioned recommendations, the updated iwCLL guidelines also include information on patient eligibility for clinical trials, guidance regarding treatment-related toxicities, and recommendations for supportive care and managing complications.
Recent advances in chronic lymphocytic leukemia (CLL) have prompted an update to the 2008 International Workshop in Chronic Lymphocytic Leukemia (iwCLL) consensus guidelines.
The updated iwCLL guidelines include new information on genomic alterations, the use of clinical staging and prognostic markers/scores, response assessment, minimal residual disease (MRD), and viral diseases in CLL patients.
The update was recently published in Blood.
Diagnosis, prognosis, and staging
To verify CLL diagnosis, the iwCLL guidelines recommend obtaining complete blood counts and differential counts as well as immunophenotyping of peripheral blood lymphocytes. A panel of CD19, CD5, CD20, CD23, κ, and λ typically suffices to establish a diagnosis.
Other tests that may help in prognosis or assessing tumor burden include molecular cytogenetics for del(13q), del(11q), del(17p), and add(12) in peripheral blood lymphocytes and determining TP53 and IGHV mutational status.
These tests can help identify poor-prognosis patients who are not likely to benefit from standard chemotherapy but are likely to benefit from small-molecule inhibitors of BTK, PI3K, or BCL2.
In addition, serum markers such as β2-microglogulin provide insight into overall survival and progression-free survival.
With regard to clinical staging, the guidelines highlight the Binet and Rai systems, which are routinely used in clinical practice and clinical trials.
However, the guidelines also note that “there are a large number of biomarkers that can provide additional prognostic information,” and, recently, “several prognostic scores and stratification systems have been proposed based on multivariate analyses.”
For example, the CLL international prognostic index (CLL-IPI) provides a weighted score that uses clinical stage, age, IGHV mutational status, β2-microglogulin, and the presence of del(17p) and/or TP53 mutations.
Indications for treatment
The guidelines note that active disease must be documented to initiate therapy. At least 1 of the following criteria must be met:
- Evidence of progressive marrow failure
- Massive, progressive, or symptomatic splenomegaly
- Progressive/symptomatic lymphadenopathy or massive nodes
- Progressive lymphocytosis
- Autoimmune complications
- Extranodal involvement
- Disease-related symptoms (unintentional weight loss, significant fatigue, fevers, night sweats for over a month without evidence of infection).
Following relapse, subsequent lines of treatment should follow the same principles as those used for initial treatment decisions.
Response, MRD, and more
The guidelines say 2 groups of parameters must be assessed to determine response to therapy:
- Group A: lymphoid tumor load and constitutional symptoms, including liver and/or spleen size, lymph node evaluation, and circulating lymphocyte count
- Group B: the hematopoietic system (platelet count, hemoglobin, and marrow).
For therapies with a defined treatment duration, response should be assessed at least 2 months after treatment is completed. For continued therapies or maintenance, response should be assessed at a predefined time point or at least 2 months after patients achieve their maximum response.
The guidelines also say MRD should be assessed in clinical trials aimed at maximizing the depth of remission. Furthermore, it “may be important” to confirm MRD negativity in the blood and marrow, as there are therapies that preferentially clear the blood but not the marrow (such as monoclonal antibodies).
In addition to the aforementioned recommendations, the updated iwCLL guidelines also include information on patient eligibility for clinical trials, guidance regarding treatment-related toxicities, and recommendations for supportive care and managing complications.
Recent advances in chronic lymphocytic leukemia (CLL) have prompted an update to the 2008 International Workshop in Chronic Lymphocytic Leukemia (iwCLL) consensus guidelines.
The updated iwCLL guidelines include new information on genomic alterations, the use of clinical staging and prognostic markers/scores, response assessment, minimal residual disease (MRD), and viral diseases in CLL patients.
The update was recently published in Blood.
Diagnosis, prognosis, and staging
To verify CLL diagnosis, the iwCLL guidelines recommend obtaining complete blood counts and differential counts as well as immunophenotyping of peripheral blood lymphocytes. A panel of CD19, CD5, CD20, CD23, κ, and λ typically suffices to establish a diagnosis.
Other tests that may help in prognosis or assessing tumor burden include molecular cytogenetics for del(13q), del(11q), del(17p), and add(12) in peripheral blood lymphocytes and determining TP53 and IGHV mutational status.
These tests can help identify poor-prognosis patients who are not likely to benefit from standard chemotherapy but are likely to benefit from small-molecule inhibitors of BTK, PI3K, or BCL2.
In addition, serum markers such as β2-microglogulin provide insight into overall survival and progression-free survival.
With regard to clinical staging, the guidelines highlight the Binet and Rai systems, which are routinely used in clinical practice and clinical trials.
However, the guidelines also note that “there are a large number of biomarkers that can provide additional prognostic information,” and, recently, “several prognostic scores and stratification systems have been proposed based on multivariate analyses.”
For example, the CLL international prognostic index (CLL-IPI) provides a weighted score that uses clinical stage, age, IGHV mutational status, β2-microglogulin, and the presence of del(17p) and/or TP53 mutations.
Indications for treatment
The guidelines note that active disease must be documented to initiate therapy. At least 1 of the following criteria must be met:
- Evidence of progressive marrow failure
- Massive, progressive, or symptomatic splenomegaly
- Progressive/symptomatic lymphadenopathy or massive nodes
- Progressive lymphocytosis
- Autoimmune complications
- Extranodal involvement
- Disease-related symptoms (unintentional weight loss, significant fatigue, fevers, night sweats for over a month without evidence of infection).
Following relapse, subsequent lines of treatment should follow the same principles as those used for initial treatment decisions.
Response, MRD, and more
The guidelines say 2 groups of parameters must be assessed to determine response to therapy:
- Group A: lymphoid tumor load and constitutional symptoms, including liver and/or spleen size, lymph node evaluation, and circulating lymphocyte count
- Group B: the hematopoietic system (platelet count, hemoglobin, and marrow).
For therapies with a defined treatment duration, response should be assessed at least 2 months after treatment is completed. For continued therapies or maintenance, response should be assessed at a predefined time point or at least 2 months after patients achieve their maximum response.
The guidelines also say MRD should be assessed in clinical trials aimed at maximizing the depth of remission. Furthermore, it “may be important” to confirm MRD negativity in the blood and marrow, as there are therapies that preferentially clear the blood but not the marrow (such as monoclonal antibodies).
In addition to the aforementioned recommendations, the updated iwCLL guidelines also include information on patient eligibility for clinical trials, guidance regarding treatment-related toxicities, and recommendations for supportive care and managing complications.
CPI-613 receives orphan designation for BL
The US Food and Drug Administration (FDA) has granted orphan drug designation to CPI-613 for the treatment of Burkitt lymphoma (BL).
CPI-613 is a novel lipoic acid analogue that inhibits multiple enzyme targets within the tricarboxylic acid cycle.
The drug is in development as a treatment for hematologic malignancies and solid tumors.
In a phase 1 trial of patients with advanced hematologic malignancies, CPI-613 produced a response in a patient with relapsed BL.
Now, Rafael Pharmaceuticals, Inc., the company developing CPI-613, is launching a phase 2 trial of the drug in patients with relapsed or refractory BL and high-grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6.
In the phase 1 trial, the patient with relapsed BL achieved a partial response to CPI-613 monotherapy and was ultimately cleared of disease after surgery.
The patient, a 19-year-old female, began taking CPI-613 (2940 mg/m2) after her second relapse. She achieved a radiographic partial response after the third cycle of CPI-613.
The patient completed 17 cycles of CPI-613 over 51 weeks. She decided to stop treatment after the 17th cycle to pursue a surgical resection of residual tumor. The pathology of the surgical specimen revealed BL with extensive necrosis.
Clinical follow-up on the patient showed no evidence of disease more than 36 months later. And CPI-613 was considered well tolerated in this patient.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to CPI-613 for the treatment of Burkitt lymphoma (BL).
CPI-613 is a novel lipoic acid analogue that inhibits multiple enzyme targets within the tricarboxylic acid cycle.
The drug is in development as a treatment for hematologic malignancies and solid tumors.
In a phase 1 trial of patients with advanced hematologic malignancies, CPI-613 produced a response in a patient with relapsed BL.
Now, Rafael Pharmaceuticals, Inc., the company developing CPI-613, is launching a phase 2 trial of the drug in patients with relapsed or refractory BL and high-grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6.
In the phase 1 trial, the patient with relapsed BL achieved a partial response to CPI-613 monotherapy and was ultimately cleared of disease after surgery.
The patient, a 19-year-old female, began taking CPI-613 (2940 mg/m2) after her second relapse. She achieved a radiographic partial response after the third cycle of CPI-613.
The patient completed 17 cycles of CPI-613 over 51 weeks. She decided to stop treatment after the 17th cycle to pursue a surgical resection of residual tumor. The pathology of the surgical specimen revealed BL with extensive necrosis.
Clinical follow-up on the patient showed no evidence of disease more than 36 months later. And CPI-613 was considered well tolerated in this patient.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to CPI-613 for the treatment of Burkitt lymphoma (BL).
CPI-613 is a novel lipoic acid analogue that inhibits multiple enzyme targets within the tricarboxylic acid cycle.
The drug is in development as a treatment for hematologic malignancies and solid tumors.
In a phase 1 trial of patients with advanced hematologic malignancies, CPI-613 produced a response in a patient with relapsed BL.
Now, Rafael Pharmaceuticals, Inc., the company developing CPI-613, is launching a phase 2 trial of the drug in patients with relapsed or refractory BL and high-grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6.
In the phase 1 trial, the patient with relapsed BL achieved a partial response to CPI-613 monotherapy and was ultimately cleared of disease after surgery.
The patient, a 19-year-old female, began taking CPI-613 (2940 mg/m2) after her second relapse. She achieved a radiographic partial response after the third cycle of CPI-613.
The patient completed 17 cycles of CPI-613 over 51 weeks. She decided to stop treatment after the 17th cycle to pursue a surgical resection of residual tumor. The pathology of the surgical specimen revealed BL with extensive necrosis.
Clinical follow-up on the patient showed no evidence of disease more than 36 months later. And CPI-613 was considered well tolerated in this patient.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.