User login
Screening for Thrombophilia In Pregnancy Called Futile
LISBON — There is absolutely no reason today to universally screen pregnant women for inherited thrombophilias, Dr. Ian A. Greer said at the 15th World Congress of the International Society for the Study of Hypertension in Pregnancy.
Although easy and accurate tests for inherited thrombophilias are available, the best management of women who have these disorders remains unclear. A systematic review of the literature turned up results from just one randomized, controlled trial showing that pregnant women with a thrombophilia—in this case, antiphospholipid syndrome—had a modest benefit from treatment with aspirin and heparin, said Dr. Greer, professor of obstetrics and gynecology at the University of Glasgow, Scotland. But antiphospholipid syndrome is an acquired, not inherited, thrombophilia and no other results from randomized, controlled trials in women with a thrombophilia have been reported, he said.
The top priority today is to run more controlled studies to test various antithrombotic treatments in women with thrombophilia rather than starting widespread screening, Dr. Greer said. Although aspirin, unfractionated heparin, and low-molecular-weight heparin are all treatment options, alone or in combination, not enough evidence currently exists to recommend any specific regimen over the others.
Dr. Greer and his associates have run a cost-effectiveness analysis of thrombophilia screening and treatment, using a hypothetical, representative population of 10,000 pregnant women. They assumed that treatment with low-molecular-weight heparin would have an 80% efficacy for preventing adverse maternal and fetal outcomes, including intrauterine growth restriction, miscarriage, and preeclampsia.
In this analysis, the cost for preventing a single adverse event through universal screening would be about $90,000. The cost to prevent a single adverse event would be about $80,000 using selective screening of women with a personal or family history of thrombophilia or venous thromboembolism, Dr. Greer said.
LISBON — There is absolutely no reason today to universally screen pregnant women for inherited thrombophilias, Dr. Ian A. Greer said at the 15th World Congress of the International Society for the Study of Hypertension in Pregnancy.
Although easy and accurate tests for inherited thrombophilias are available, the best management of women who have these disorders remains unclear. A systematic review of the literature turned up results from just one randomized, controlled trial showing that pregnant women with a thrombophilia—in this case, antiphospholipid syndrome—had a modest benefit from treatment with aspirin and heparin, said Dr. Greer, professor of obstetrics and gynecology at the University of Glasgow, Scotland. But antiphospholipid syndrome is an acquired, not inherited, thrombophilia and no other results from randomized, controlled trials in women with a thrombophilia have been reported, he said.
The top priority today is to run more controlled studies to test various antithrombotic treatments in women with thrombophilia rather than starting widespread screening, Dr. Greer said. Although aspirin, unfractionated heparin, and low-molecular-weight heparin are all treatment options, alone or in combination, not enough evidence currently exists to recommend any specific regimen over the others.
Dr. Greer and his associates have run a cost-effectiveness analysis of thrombophilia screening and treatment, using a hypothetical, representative population of 10,000 pregnant women. They assumed that treatment with low-molecular-weight heparin would have an 80% efficacy for preventing adverse maternal and fetal outcomes, including intrauterine growth restriction, miscarriage, and preeclampsia.
In this analysis, the cost for preventing a single adverse event through universal screening would be about $90,000. The cost to prevent a single adverse event would be about $80,000 using selective screening of women with a personal or family history of thrombophilia or venous thromboembolism, Dr. Greer said.
LISBON — There is absolutely no reason today to universally screen pregnant women for inherited thrombophilias, Dr. Ian A. Greer said at the 15th World Congress of the International Society for the Study of Hypertension in Pregnancy.
Although easy and accurate tests for inherited thrombophilias are available, the best management of women who have these disorders remains unclear. A systematic review of the literature turned up results from just one randomized, controlled trial showing that pregnant women with a thrombophilia—in this case, antiphospholipid syndrome—had a modest benefit from treatment with aspirin and heparin, said Dr. Greer, professor of obstetrics and gynecology at the University of Glasgow, Scotland. But antiphospholipid syndrome is an acquired, not inherited, thrombophilia and no other results from randomized, controlled trials in women with a thrombophilia have been reported, he said.
The top priority today is to run more controlled studies to test various antithrombotic treatments in women with thrombophilia rather than starting widespread screening, Dr. Greer said. Although aspirin, unfractionated heparin, and low-molecular-weight heparin are all treatment options, alone or in combination, not enough evidence currently exists to recommend any specific regimen over the others.
Dr. Greer and his associates have run a cost-effectiveness analysis of thrombophilia screening and treatment, using a hypothetical, representative population of 10,000 pregnant women. They assumed that treatment with low-molecular-weight heparin would have an 80% efficacy for preventing adverse maternal and fetal outcomes, including intrauterine growth restriction, miscarriage, and preeclampsia.
In this analysis, the cost for preventing a single adverse event through universal screening would be about $90,000. The cost to prevent a single adverse event would be about $80,000 using selective screening of women with a personal or family history of thrombophilia or venous thromboembolism, Dr. Greer said.
LMWH During Pregnancy Preserves BMD
LISBON — Long-term treatment with low-molecular-weight heparin during pregnancy did not cause a drop in spinal bone mineral density in a study with 62 women.
Extended administration of low-molecular-weight heparin (LMWH) during pregnancy, as prophylaxis for thrombophilia, also did not produce a clinically important fracture risk, Dr. Marc A. Roger said at the third World Congress of the International Society of Obstetric Medicine.
In contrast, long-term treatment with unfractionated heparin during pregnancy often causes a drop in bone mineral density—frequently a clinically significant drop, said Dr. Roger, head of the thrombosis and hemostasis program at Ottawa Hospital. According to prior study results, up to 2.2% of women who have had prolonged exposure to unfractionated heparin during pregnancy develop osteoporotic fractures.
The new findings came from a prespecified subgroup analysis of data collected in the Thrombophilia in Pregnancy Prophylaxis Study (TIPPS), an ongoing, multicenter trial that was designed to compare prophylaxis using LMWH with placebo for pregnancy outcomes in women with a thrombophilia. The subanalysis was designed to assess the effect of LMWH on bone mineral density.
Both TIPPS and the bone mineral density subanalysis were sponsored by Pfizer, which markets dalteparin (Fragmin), the LMWH used in the studies. Dr. Roger has received research support from and is on a scientific advisory board for Pfizer.
TIPPS enrolled women with confirmed thrombophilia at less than 20 weeks' gestation who were at risk for thromboembolism or had a history of pregnancy complications. They were randomized to placebo or to 5,000 U dalteparin daily through week 20, followed by a regimen of 5,000 U b.i.d. through delivery. All women in the study received dalteparin post partum for 6 weeks.
In the substudy, which involved 62 women, the primary end point was the absolute lumbar-spine bone mineral density measured at 6 weeks post partum. Because of crossovers, 33 women received dalteparin and 29 women received placebo.
The average bone mineral density was 1.15 g/cm2 in the LMWH group and 1.20 g/cm2 in the control group, a difference that was not statistically significant. In addition, the 95% confidence interval for bone mineral density in the dalteparin group did not enter the range that defines osteopenia (less than one standard deviation below the mean).
Hence, “we can say with confidence that if low-molecular-weight heparin causes a difference in bone density, it's a small difference,” Dr. Roger said.
LISBON — Long-term treatment with low-molecular-weight heparin during pregnancy did not cause a drop in spinal bone mineral density in a study with 62 women.
Extended administration of low-molecular-weight heparin (LMWH) during pregnancy, as prophylaxis for thrombophilia, also did not produce a clinically important fracture risk, Dr. Marc A. Roger said at the third World Congress of the International Society of Obstetric Medicine.
In contrast, long-term treatment with unfractionated heparin during pregnancy often causes a drop in bone mineral density—frequently a clinically significant drop, said Dr. Roger, head of the thrombosis and hemostasis program at Ottawa Hospital. According to prior study results, up to 2.2% of women who have had prolonged exposure to unfractionated heparin during pregnancy develop osteoporotic fractures.
The new findings came from a prespecified subgroup analysis of data collected in the Thrombophilia in Pregnancy Prophylaxis Study (TIPPS), an ongoing, multicenter trial that was designed to compare prophylaxis using LMWH with placebo for pregnancy outcomes in women with a thrombophilia. The subanalysis was designed to assess the effect of LMWH on bone mineral density.
Both TIPPS and the bone mineral density subanalysis were sponsored by Pfizer, which markets dalteparin (Fragmin), the LMWH used in the studies. Dr. Roger has received research support from and is on a scientific advisory board for Pfizer.
TIPPS enrolled women with confirmed thrombophilia at less than 20 weeks' gestation who were at risk for thromboembolism or had a history of pregnancy complications. They were randomized to placebo or to 5,000 U dalteparin daily through week 20, followed by a regimen of 5,000 U b.i.d. through delivery. All women in the study received dalteparin post partum for 6 weeks.
In the substudy, which involved 62 women, the primary end point was the absolute lumbar-spine bone mineral density measured at 6 weeks post partum. Because of crossovers, 33 women received dalteparin and 29 women received placebo.
The average bone mineral density was 1.15 g/cm2 in the LMWH group and 1.20 g/cm2 in the control group, a difference that was not statistically significant. In addition, the 95% confidence interval for bone mineral density in the dalteparin group did not enter the range that defines osteopenia (less than one standard deviation below the mean).
Hence, “we can say with confidence that if low-molecular-weight heparin causes a difference in bone density, it's a small difference,” Dr. Roger said.
LISBON — Long-term treatment with low-molecular-weight heparin during pregnancy did not cause a drop in spinal bone mineral density in a study with 62 women.
Extended administration of low-molecular-weight heparin (LMWH) during pregnancy, as prophylaxis for thrombophilia, also did not produce a clinically important fracture risk, Dr. Marc A. Roger said at the third World Congress of the International Society of Obstetric Medicine.
In contrast, long-term treatment with unfractionated heparin during pregnancy often causes a drop in bone mineral density—frequently a clinically significant drop, said Dr. Roger, head of the thrombosis and hemostasis program at Ottawa Hospital. According to prior study results, up to 2.2% of women who have had prolonged exposure to unfractionated heparin during pregnancy develop osteoporotic fractures.
The new findings came from a prespecified subgroup analysis of data collected in the Thrombophilia in Pregnancy Prophylaxis Study (TIPPS), an ongoing, multicenter trial that was designed to compare prophylaxis using LMWH with placebo for pregnancy outcomes in women with a thrombophilia. The subanalysis was designed to assess the effect of LMWH on bone mineral density.
Both TIPPS and the bone mineral density subanalysis were sponsored by Pfizer, which markets dalteparin (Fragmin), the LMWH used in the studies. Dr. Roger has received research support from and is on a scientific advisory board for Pfizer.
TIPPS enrolled women with confirmed thrombophilia at less than 20 weeks' gestation who were at risk for thromboembolism or had a history of pregnancy complications. They were randomized to placebo or to 5,000 U dalteparin daily through week 20, followed by a regimen of 5,000 U b.i.d. through delivery. All women in the study received dalteparin post partum for 6 weeks.
In the substudy, which involved 62 women, the primary end point was the absolute lumbar-spine bone mineral density measured at 6 weeks post partum. Because of crossovers, 33 women received dalteparin and 29 women received placebo.
The average bone mineral density was 1.15 g/cm2 in the LMWH group and 1.20 g/cm2 in the control group, a difference that was not statistically significant. In addition, the 95% confidence interval for bone mineral density in the dalteparin group did not enter the range that defines osteopenia (less than one standard deviation below the mean).
Hence, “we can say with confidence that if low-molecular-weight heparin causes a difference in bone density, it's a small difference,” Dr. Roger said.
Cefazolin Found Still Effective For Antepartum Pyelonephritis
MONTEREY, CALIF. — Cefazolin remained an effective empiric therapy for antepartum pyelonephritis over the last 14 years, Dr. Soldrea Roberts said at the annual meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
A retrospective study compared data on 136 women with antepartum pyelonephritis who were treated at one institution in two time periods, 1992–1993 and 2004–2006. Records revealed positive cultures in 76%, and 89% of these were caused by gram-negative isolates, found in 47 women in the earlier period and 46 in the later period.
Rates of multidrug resistant organisms causing antepartum pyelonephritis were not significantly different between periods but trended upward, from 32% of isolates in 1992–1993 to 43% in 2004–2006. Multidrug resistance was defined as resistance to at least 3 of an average of 10 antimicrobials tested per isolate.
E. coli caused more than 70% of cases. High rates of ampicillin-resistant E. coli were seen in both time periods—51% of cases in 1992–1993 and 54% of cases in 2004–2006—which confirms the inadequacy of ampicillin for empiric therapy of antepartum pyelonephritis, according to Dr. Roberts of Case Western Reserve University, Cleveland, Ohio, and her associates.
E. coli resistance to trimethoprim-sulfamethoxazole increased significantly from 5% of isolates in the earlier years to 23% in the later years, consistent with trends toward greater trimethoprim-sulfamethoxazole resistance in lower urinary tract infections over this time period. Only 5% of E. coli isolates were resistant to cefazolin in 1992–1993 and all isolates in 2004–2006 were susceptible to cefazolin despite concerns about the emergence of multidrug-resistant gram-negative rods over the past two decades, Dr. Roberts said.
The study was 80% powered to detect a 30% increase in multidrug-resistant isolates between the two time periods.
The likelihood of multidrug resistance was not affected by having a history of antepartum pyelonephritis.
Clinical outcomes did not differ significantly between the two time periods. The average length of hospitalization was 3 days in both periods and did not differ between women with or without multidrug-resistant organisms. Antibiotic regimens were changed during hospitalization in 13% in the earlier period and 11% in the later period. In 1992–1993, 96% of the women delivered at term, compared with 65% in 2004–2006.
MONTEREY, CALIF. — Cefazolin remained an effective empiric therapy for antepartum pyelonephritis over the last 14 years, Dr. Soldrea Roberts said at the annual meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
A retrospective study compared data on 136 women with antepartum pyelonephritis who were treated at one institution in two time periods, 1992–1993 and 2004–2006. Records revealed positive cultures in 76%, and 89% of these were caused by gram-negative isolates, found in 47 women in the earlier period and 46 in the later period.
Rates of multidrug resistant organisms causing antepartum pyelonephritis were not significantly different between periods but trended upward, from 32% of isolates in 1992–1993 to 43% in 2004–2006. Multidrug resistance was defined as resistance to at least 3 of an average of 10 antimicrobials tested per isolate.
E. coli caused more than 70% of cases. High rates of ampicillin-resistant E. coli were seen in both time periods—51% of cases in 1992–1993 and 54% of cases in 2004–2006—which confirms the inadequacy of ampicillin for empiric therapy of antepartum pyelonephritis, according to Dr. Roberts of Case Western Reserve University, Cleveland, Ohio, and her associates.
E. coli resistance to trimethoprim-sulfamethoxazole increased significantly from 5% of isolates in the earlier years to 23% in the later years, consistent with trends toward greater trimethoprim-sulfamethoxazole resistance in lower urinary tract infections over this time period. Only 5% of E. coli isolates were resistant to cefazolin in 1992–1993 and all isolates in 2004–2006 were susceptible to cefazolin despite concerns about the emergence of multidrug-resistant gram-negative rods over the past two decades, Dr. Roberts said.
The study was 80% powered to detect a 30% increase in multidrug-resistant isolates between the two time periods.
The likelihood of multidrug resistance was not affected by having a history of antepartum pyelonephritis.
Clinical outcomes did not differ significantly between the two time periods. The average length of hospitalization was 3 days in both periods and did not differ between women with or without multidrug-resistant organisms. Antibiotic regimens were changed during hospitalization in 13% in the earlier period and 11% in the later period. In 1992–1993, 96% of the women delivered at term, compared with 65% in 2004–2006.
MONTEREY, CALIF. — Cefazolin remained an effective empiric therapy for antepartum pyelonephritis over the last 14 years, Dr. Soldrea Roberts said at the annual meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
A retrospective study compared data on 136 women with antepartum pyelonephritis who were treated at one institution in two time periods, 1992–1993 and 2004–2006. Records revealed positive cultures in 76%, and 89% of these were caused by gram-negative isolates, found in 47 women in the earlier period and 46 in the later period.
Rates of multidrug resistant organisms causing antepartum pyelonephritis were not significantly different between periods but trended upward, from 32% of isolates in 1992–1993 to 43% in 2004–2006. Multidrug resistance was defined as resistance to at least 3 of an average of 10 antimicrobials tested per isolate.
E. coli caused more than 70% of cases. High rates of ampicillin-resistant E. coli were seen in both time periods—51% of cases in 1992–1993 and 54% of cases in 2004–2006—which confirms the inadequacy of ampicillin for empiric therapy of antepartum pyelonephritis, according to Dr. Roberts of Case Western Reserve University, Cleveland, Ohio, and her associates.
E. coli resistance to trimethoprim-sulfamethoxazole increased significantly from 5% of isolates in the earlier years to 23% in the later years, consistent with trends toward greater trimethoprim-sulfamethoxazole resistance in lower urinary tract infections over this time period. Only 5% of E. coli isolates were resistant to cefazolin in 1992–1993 and all isolates in 2004–2006 were susceptible to cefazolin despite concerns about the emergence of multidrug-resistant gram-negative rods over the past two decades, Dr. Roberts said.
The study was 80% powered to detect a 30% increase in multidrug-resistant isolates between the two time periods.
The likelihood of multidrug resistance was not affected by having a history of antepartum pyelonephritis.
Clinical outcomes did not differ significantly between the two time periods. The average length of hospitalization was 3 days in both periods and did not differ between women with or without multidrug-resistant organisms. Antibiotic regimens were changed during hospitalization in 13% in the earlier period and 11% in the later period. In 1992–1993, 96% of the women delivered at term, compared with 65% in 2004–2006.
Diagnosis and safe management of placenta previa
CASE Diagnosis precedes sentinel episode of bleeding
“G.A.” is a 39-year-old gravida 6, para 1041 who was diagnosed with complete placenta previa during a target ultrasound exam performed at 18 weeks for advanced maternal age. She had a sentinel episode of vaginal bleeding at 29 weeks and was hospitalized for close monitoring.
Management strategy
One course of steroid was given, vaginal bleeding subsided, and she was discharged for outpatient conservative management, including iron and folic acid supplementation.
The outcome
The patient progressed to 36 weeks’ gestation, when she underwent amniocentesis to assess fetal lung maturity. When the results were reassuring, a cesarean section was scheduled. Intraoperative blood loss was diminished using pelvic vessel embolization. Surgery was uncomplicated, and a healthy infant was delivered.
Placenta previa is a leading and potentially life-threatening cause of third-trimester bleeding.1 Although the overall incidence is about 0.4% in pregnancies exceeding 20 weeks’ gestation,2 that figure rises with the number of cesarean sections and may reach 10% among women who have undergone 4 or more cesarean deliveries.3 Since more women are requesting elective and repeat cesarean deliveries, we are increasingly likely to encounter this condition.
Fortunately, technological advances have improved maternal and neonatal outcomes after placenta previa:
Nevertheless, the condition necessitates cesarean delivery and can cause serious maternal and perinatal morbidity, including:
It can also occur in association with vasa previa, which, though rare, carries a very high perinatal mortality rate.5
Risk factors
An enlarged placenta or endometrial disruption or scarring in the upper uterine segment due to 1 or more of the factors listed below may increase the likelihood of abnormal placental implantation in the unscarred lower uterine segment:3,6,7
Previa often begins with painless vaginal bleeding
The condition often presents as painless, bright red, vaginal bleeding in the third trimester. It is usually distinguished from abruptio placenta by the absence of abdominal pain and uterine contractions.5 However, approximately 20% of women have uterine activity associated with the first episode of vaginal bleeding.13,14 Moreover, in some cases, painful contractions and labor may precipitate vaginal bleeding from placenta previa.5 Therefore, ultrasound examination is strongly recommended for all women with vaginal bleeding during pregnancy.
Ultrasound for other reasons uncovers many cases
With greater routine use of ultrasonography in obstetrics, a large percentage of women with placenta previa are diagnosed prior to the onset of the characteristic painless vaginal bleeding. In a 2003 study by Dola and colleagues,15 approximately 43% of placenta previa cases were diagnosed by ultrasonography performed for other obstetrical indications prior to the onset of vaginal bleeding.
Look for “warning hemorrhage”
The first episode of vaginal bleeding is rarely profuse or life-threatening to the mother or fetus. The bleeding usually subsides spontaneously, although it could recur and become more severe with subsequent episodes. Pregnancy typically continues after the initial bleeding episode.
The mean gestational age at the time of the first bleeding is 29 to 32 weeks.13,14 However, a third of cases have vaginal bleeding before the 30th week of gestation, a third between 30 and 36 weeks, and a third after 36 weeks’ gestation.13-15 Ten percent of women with the condition may be completely asymptomatic and progress to 38 weeks’ gestation without vaginal bleeding.13,14
Which form of ultrasound is most accurate?
With the advanced technology available today, ultrasound has become the standard means of diagnosing placenta previa.16,17
Transabdominal ultrasound has accuracy as high as 95% and a false-negative rate of 7% in the diagnosis of placenta previa.13,19 However, its accuracy may be adversely affected by maternal obesity, acoustic shadowing of the fetal head in a cephalic presentation, inability to locate the internal cervical os (which is critical for correct diagnosis), and difficulty imaging a posterior placenta and the lateral uterine walls. In addition, a full maternal bladder—usually helpful in transabdominal ultrasound imaging—may cause a false-positive diagnosis if the bladder is overly distended. In this situation, the cervix would appear artificially elongated and give a normally implanted placenta the appearance of encroachment into the internal cervical os.
Transvaginal ultrasound is superior for diagnosis of previa
Leerentveld et al20 reported false-positive and false-negative rates of 1% and 2%, respectively—a striking improvement over transabdominal ultrasound, which has rates of 2% to 6% and 7%, respectively.
Transvaginal sonography has several advantages over transabdominal imaging in localization of the placenta. The shorter distance from the vaginal probe transducer to the cervix and lower uterine segment allows the use of higher-frequency ultrasound waves, with improved resolution; therefore, the relationship between the placental edge and the internal os can be determined more accurately.
Some clinicians may worry that the probe used in transvaginal sonography will disrupt the placenta and provoke significant maternal hemorrhage, but this concern is unfounded. Multiple studies have attested to the safety of transvaginal sonography in localization of the placenta.5,20-22 The probe is introduced and positioned under direct ultrasound guidance at all times, and inadvertent insertion of the endovaginal probe into the internal cervical os is virtually impossible due to the anatomical relationship of the vagina and cervix.21
Transperineal ultrasound is another option. Several investigators have found it to be superior to transabdominal and similarly advantageous to transvaginal sonography in the diagnosis and exclusion of placenta previa.18
Start with transabdominal imaging
In current practice, transabdominal ultrasound is usually performed first to localize the placenta. If there is reason to suspect placenta previa, transvaginal or transperineal sonography is then used to confirm the location of the placenta.
Contractions may hinder imaging
Accurate diagnosis or exclusion of placenta previa may be difficult if uterine contractions are present during ultrasound evaluation. Myometrial contractions shorten the distance between the internal cervical os and the placental edge, altering measurement of this distance. In addition, the ultrasound appearance of a contraction may simulate placental tissue, making it difficult to exclude placenta previa.
The trouble with tradition
The 4 types of placenta previa in the traditional classification system—complete, partial, marginal, and low-lying—predate the era of ultrasound diagnosis and are based on digital palpation of the placenta through a partly dilated cervical os during labor.
A new system of 3 types
Along with other authors,5,15-18 we propose a new system with 3 categories—complete, incomplete, and low-lying—because ultrasound may not distinguish a placenta partially covering the internal os (a discrete point) from one that is merely encroaching on it.
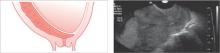
Complete previa
The placenta completely covers the internal cervical os
Incomplete previa
The placental edge is within 2 cm of the internal cervical os, but does not cover the os
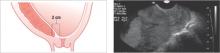
Low-lying previa
The distance from the internal cervical os to the placental edge is between 2 and 3.5 cm
Look for placenta accreta
When placenta previa is diagnosed by ultrasound examination, further diagnostic measures are needed to determine whether placenta accreta is present.5,23 In placenta accreta, neither the normal plane of separation between the placental villi and uterine wall, nor the intervening fibrinoid layer of Nitabuch, is present.5,23
Degrees of abnormal placental implantation
Risk of accreta can reach 67%
There are varying reports on the incidence of placenta accreta, but women with placenta previa and previous cesarean deliveries appear to have the highest incidence.3,23,24 In women with placenta previa and 1 previous cesarean section, the risk of placenta accreta has been estimated at 24%, but it increases to 67% for women with placenta previa and 4 previous cesarean sections.3
Sonographic appearance of placenta accreta
Certain characteristics are suggestive of placenta accreta25,26:
Unfortunately, diagnosis of placenta accreta is difficult prior to delivery, although transvaginal sonography and adjunctive color flow/power Doppler imaging with 2- and 3-dimensional techniques offer improved resolution and have yielded promising results in prenatal diagnosis.27-30
Magnetic resonance imaging (MRI) may also prove useful in detecting placental tissue invasion and evaluating the degree of invasion, especially in a posterior or lateral placenta previa or when there is invasion into the bladder.31-33
Gestational age, symptoms determine management
The management of women with placenta previa in the third trimester depends on the extent of maternal hemorrhage and the fetal gestational age. Clinical categories include:
Some asymptomatic cases resolve
Outpatient management is possible for women who have never bled after diagnosis in the second trimester. These women should abstain from intercourse, avoid digital examination after 20 weeks’ gestation, and immediately present to the hospital if there is any evidence of vaginal bleeding.34
Monthly ultrasound evaluations are necessary to determine whether placenta previa has resolved,34-37 since 90% of cases detected in the second trimester resolve by the third trimester.34 However, if placenta previa persists beyond 24 weeks’ gestation, there is a 50% risk that delivery will be complicated by it.35 If placenta previa persists after 32 weeks, that risk approaches 75%.35
2-fold risk of congenital malformations
Most investigators report a 2-fold increased risk of fetal congenital malformations in cases of placenta previa.5 These malformations include anomalies of the central nervous system, cardiovascular system, respiratory tract, and gastrointestinal tract. Therefore, a target ultrasound examination for fetal anatomy is recommended at the initial ultrasound diagnosis of placenta previa.
Risk of fetal growth restriction warrants heightened surveillance
Some controversy surrounds the incidence of fetal growth restriction in pregnancies complicated by placenta previa. Varma38 reported that fetal growth restriction occurs in 16% of women with placenta previa and is correlated with the number of antepartum bleeding episodes. Other investigators have reported normal fetal growth in women with placenta previa.39 Given this uncertainty, serial follow-up ultrasound evaluations are usually advised for fetal growth assessment.
When patient remains asymptomatic, perform amniocentesis at 36 weeks
Some women progress to the late third trimester without any vaginal bleeding. In these women, amniocentesis is recommended at approximately 36 weeks’ gestation to assess fetal lung maturity.34,40 Elective cesarean delivery can then be planned if pulmonary maturity is documented.
The benefits of elective delivery include a stable patient and an optimally prepared surgical team, as well as the avoidance of emergent surgery, which increases the risk for maternal complications. Emergent surgery also places the fetus at greater risk for anemia, compared with elective procedures(27.7% vs 2.9%, respectively).13
Vaginal bleeding requires inpatient evaluation
Any woman with placenta previa who presents with vaginal bleeding should be admitted to the labor and delivery unit for immediate evaluation of maternal and fetal status, including an estimation of gestational age.
Initial acute care and assessment necessitate34:
If hemorrhage is life-threatening, deliver immediately
During initial evaluation, if the hemorrhage is judged to be massive and life-threatening, resuscitative measures and immediate delivery are necessary to avoid serious maternal morbidity. Recommended measures include constant monitoring of maternal status, aggressive IV fluid resuscitation, transfusion of blood and blood products, assessment of fetal status, and immediate delivery without regard to the maturity of the fetus.
A woman at term or near term (with documented fetal lung maturity) who presents with mild or moderate vaginal bleeding should be delivered via cesarean section.
Conservative management may be appropriate for mild preterm bleeding
If vaginal bleeding is not threatening to the life of the mother, and the fetus is preterm, a conservative approach with aggressive expectant management is appropriate, since most first episodes of vaginal bleeding are self-limited and rarely life-threatening to mother or fetus. Expectant management allows fetal maturation in utero without jeopardizing maternal health. If maternal and fetal health remain stable, the expectant approach allows a safe delay of delivery until the fetus matures.
Hospitalization is recommended. Candidates for expectant management should be hospitalized after the initial episode of vaginal bleeding. Once maternal and fetal conditions stabilize, the woman should be transferred to the antepartum ward for hospital bed rest with bathroom privileges. For expectant management:
Delivery is warranted for life-threatening hemorrhage, fetal lung maturity, and/or the usual maternal and fetal indications.
The question of tocolysis
Third-trimester tocolytic therapy in a woman with vaginal bleeding is controversial. In placenta previa, vaginal bleeding appears to arise from disruption of the placental implantation site as the lower uterine segment develops.41,42 It is unclear whether uterine contractions play a role, as only 20% of women with placenta previa have uterine activity at the time of vaginal bleeding.13,14,42 It is difficult to determine whether these women have true preterm labor, because digital examination of the cervix to document cervical dilatation is impossible.
Does uterine activity precipitate bleeding?
Some investigators believe uterine activity is a predisposing factor for the vaginal bleeding associated with placenta previa, and would consider tocolytic therapy in a stable patient at a premature gestational age. However, further evidence of its safety is needed.
In particular, beta-mimetics should be avoided in hemorrhaging women because their vasodilatory effects can precipitate maternal hypotension. Another side effect of beta-mimetics: maternal tachycardia,43 which may mask the hypovolemic state in women with significant hemorrhage.
Magnesium sulfate has less effect on the maternal cardiovascular system and could be a better choice in symptomatic placenta previa.41 Also consider indomethacin, which appears to have fewer adverse maternal effects.
Inpatient vs outpatient management
Because 2 to 3 weeks of maternal hospitalization can pass between the initial warning hemorrhage and delivery of the fetus, outpatient care has become an option. Several retrospective studies have demonstrated the cost-effectiveness and safety of outpatient management of symptomatic placenta previa.44,45 These studies emphasized careful patient section.
Wing and associates46 conducted a prospective, randomized, controlled trial that reinforces the need for judicious use of outpatient management. In their study, fewer than half the patients diagnosed with placenta previa prior to 37 weeks were candidates. The authors point out the small number of patients in their study, and the fact that vaginal bleeding recurred in approximately 60% of patients. Because of the difficulty of predicting which patients will have recurrent bleeding and when, outpatient management should be reserved for those judged to be compliant with home bed rest who can rapidly return to the hospital, if necessary.
Women with recurrent vaginal bleeding during outpatient management should be rehospitalized.
In the event of massive hemorrhage, immediate compression of the aorta below the level of the renal arteries will reduce the bleeding enough to allow time to evaluate the situation.56 At the same time, aggressive IV fluid resuscitation and blood transfusion should begin. Reevaluate coagulation status after every 5 to 10 U of blood.57
Focused repair may be effective. In some situations, the hemorrhage may be controlled by oversewing and repairing the focal placental site defects.40
Bracketing the bleeding area. Another measure is a circular suture technique in which interrupted sutures are placed on the serosal surface of the anterior and posterior aspects of the uterus and as deeply as possible into the endometrium in a circumferential manner, bracketing the bleeding area.58
The argon beam coagulator can be used to achieve hemostasis; it is more effective than traditional bipolar cautery at ensuring hemostasis in extensive areas.56,57
Stepwise devascularization was effective in 100% of 103 women with postpartum hemorrhage who did not respond to traditional management.59 It involves 5 procedures to be performed in sequence until hemostasis is achieved: unilateral uterine vessel ligation, bilateral uterine vessel ligation, low uterine vessel ligation, unilateral ovarian vessel ligation, and bilateral ovarian vessel ligation.
Hypogastric artery ligation is another option, but it is technically challenging and successful in less than 50% of cases.57 In fact, the time spent on this technique may actually lead to increased blood loss.
Components of safe delivery
A detailed plan is necessary when major hemorrhage is anticipated at the time of elective cesarean delivery for placenta previa, including consultation with experts in different disciplines such as radiology, anesthesiology, urology, pathology, blood bank, neonatology, and gynecologic oncology.
Also pay attention to the maternal red blood cell reserve. Iron and folic acid should be administered to prevent and treat anemia, and antepartum erythropoietin should be considered as a way of increasing the hemoglobin level in women with placenta previa. Autologous blood transfusion, including acute normovolemic hemodilution, is another option.
Pelvic vessel embolization
Elective embolization or occlusion of the hypogastric or uterine arteries has proved to be safe and effective for postpartum hemorrhage, with a success rate of more than 90% in women with normal coagulation.47
In addition, elective catheterization with a balloon-tipped catheter can be used prophylactically to reduce blood flow to the placenta. Prophylactic catheterization of the anterior division of the internal iliac arteries can be performed right before the scheduled cesarean section. An axillary approach is technically easier for fluoroscopically guided catheterization of the internal iliac.48 The actual fluoroscopy time is minutes, so the risk of fetal exposure to radiation and irreversible ovarian damage is minimal.
The fetus is monitored during the procedure, and the balloons are left in the deflated state until after delivery, reducing the risk of uteroplacental insufficiency. Balloon inflation after delivery occludes the hypogastric arteries and diminishes uterine arterial blood flow during surgery. In some cases, the temporary occlusive effect of the balloons may control intraoperative bleeding completely. If substantial bleeding persists, subsequent embolization of the uterine arteries is advised, using absorbable Gelfoam particles, which are temporary and do not damage pelvic organs. Menstruation is not impaired, and normal pregnancies have been reported after this procedure.49,50
In women who undergo cesarean delivery under regional anesthesia, placement of a dry epidural catheter for later dosing of anesthetic agents should be considered prior to balloon catheterization, since the patient’s mobility is restricted after placement of the balloon-tipped catheter.
This therapy is especially useful when there is a high index of suspicion for placenta accreta.
Recommendations at the time of delivery
Hysterectomy for placenta previa, placenta accreta
This procedure is technically challenging when there is a markedly enlarged uterus with engorged collateral vessels. One useful method, delayed ligation technique, was originally described by Dyer et al on the Tulane obstetrics service at Charity Hospital of New Orleans.53 This technique facilitates quick control of all uterine vasculature with rapid hemostasis. Later modification of this method involves successive clamping and severing of all vascular pedicles supplying the uterus, prior to their suture ligation, for quick control of bleeding.
Close follow-up continues even after surgery
Immediately after surgery, close monitoring of hemodynamic status is required, ideally in a critical care setting. Because women with placenta previa/placenta accreta have often received massive transfusions of blood and blood products along with large volumes of crystalloid fluids, pulmonary edema may develop. Conversely, hypovolemia can result from inadequate replacement of blood or persistent intra-abdominal bleeding. Thus, close attention to urinary output allows early detection of pulmonary edema, acute respiratory distress syndrome, hypovolemia, or persistent intra-abdominal bleeding. Patients who undergo peripartum hysterectomy should also be monitored closely for possible ureteral injury.
Thromboprophylaxis should continue until the patient is ambulatory.
Recommended laboratory tests
Get a complete blood count with platelets and fibrinogen immediately after surgery and at frequent intervals as needed. A chemistry panel with calcium, albumin, electrolytes, and creatinine also is helpful.
Serious morbidity in 3% to 5% of women after emergent hysterectomy
Conditions such as acute respiratory distress syndrome from massive blood transfusion and pulmonary capillary leakage, acute tubular necrosis from renal failure, and pulmonary embolism may complicate 3% to 5% of cases.54
Reoperation for persistent intra-abdominal bleeding may be necessary, and 9% of women will have urologic injury. Unfortunately, the maternal mortality rate associated with this procedure is 0.8%, so meticulous postoperative care is mandated.55
The authors report no financial relationships with any company whose products are mentioned in this article.
1. Ananth CV, Savitz DA, Luther ER. Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. Am J Epidemiol. 1996;144:881-889.
2. Faiz AS, Ananth CV. Etiology and risk factors for placenta previa: an overview and meta-analysis of observational studies. J Matern Fetal Neonatal Med. 2003;13:175.-
3. Clark SL, Koonings PP, et al. Placenta previa/accreta and prior cesarean section. Obstet Gynecol. 1985;66:89-92.
4. Neri A, Manor Y, Matityahu A, Blieden L. Placenta previa and congenital cardiac anomalies. Fetal Ther. 1989;4:138-140.
5. Clark SL. Placenta previa and abruptio placenta. In: Creasy RK, Resnik R. Maternal-Fetal Medicine, Principles and Practice. 4th ed. Philadelphia: W.B. Saunders; 1999:616–631.
6. Miller DA, Chollet J, Goodwin TM. Clinical risks factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210-214.
7. Ananth CV, Demissie K, Smulian JC, Vintzileos AM. Placenta previa in singleton and twin births in the United States, 1989 through 1998: a comparison of risk factor profiles and associated conditions. Am J Obstet Gynecol. 2003;188:275-281.
8. Gilbert WM, Nesbitt TS, Danielsen B. Childbearing beyond age 40: pregnancy outcome in 24,032 cases. Obstet Gynecol. 1999;93:9-14.
9. Lavery JP. Placenta previa. Clin Obstet Gynecol. 1990;33:414-421.
10. Taylor VM, Kramer MD, Vaughan TL, Peacock S. Placenta previa in relation to induced and spontaneous abortion: a population- based study. Obstet Gynecol. 1993;82:88-91.
11. Williams MA, Mittendorf R, Lieberman E, Monson RR, Schoenbaum SC, Genest DR. Cigarette smoking during pregnancy in relation to placenta previa. Am J Obstet Gynecol. 1991;165:28-32.
12. Handler AS, Mason ED, Rosenberg DL, Davis FG. The relationship between exposure during pregnancy to cigarette smoking and cocaine use and placenta previa. Am J Obstet Gynecol. 1994;170:884-889.
13. Cotton DB, Read JA, Paul RH, Quilligan EJ. The conservative aggressive management of placenta previa. Am J Obstet Gynecol. 1980;137:687-695.
14. Silver R, Depp R, Sabbagha RE, Dooley SL. Placenta previa: aggressive expectant management. Am J Obstet Gynecol. 1984;150:15-22.
15. Dola CP, Garite TJ, Dowling DD, Friend D, Ahdoot D, Asrat T. Placenta previa: does its type affect pregnancy outcome? Am J Perinatol. 2003;20:353-360.
16. Oppenheimer LW, Farine D, Ritchie JWK, Lewinsky RM, Telford J, Fairbanks LA. What is low-lying placenta? Am J Obstet Gynecol. 1991;165:1036-1038.
17. Bhide A, Thilaganathan B. Recent advances in the management of placenta previa. Curr Opin Obstet Gynecol. 2004;16:447-451.
18. Dawson WB, Dumas MD, Romano WM, Gagnon R, Gratton RJ, Mowbray RD. Translabial ultrasonography and placenta previa: does measurement of the os-placenta distance predict outcome? J Ultrasound Med. 1996;15:44-46.
19. Egley CC. Abruptio placentae and placenta previa. In: Winn HN, Hobbins JC. Clinical Maternal-Fetal Medicine.1st ed. Pearl River, NY: Parthenon Publishing; 2000:47–53.
20. Leerentveld RA, Gilberts E, Arnold M, Wladimiroff JW. Accuracy and safety of transvaginal sonographic placental localization. Obstet Gynecol. 1990;76:759-762.
21. Timor-Tritsch I, Yunis R. Confirming the safety of transvaginal sonography in patients suspected of placenta previa. Obstet Gynecol. 1993;81:742-744.
22. Tan NH, Abu M, Woo JLS, Tahir H. The role of transvaginal sonography in the diagnosis of placenta previa. Aust NZ J Obstet Gynaecol. 1995;35:42-45.
23. Miller DA, Cholleet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210-214.
24. Chattopadhyay SK, Kharif H, Sherbeeni MM. Placenta previa and accreta after previous cesarean section. Eur J Obstet Gynecol Reprod Biol. 1993;52:151.-
25. Comstock CH, Love JJ, Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135-1140.
26. Comstock CH, Lee W, Vettraino IM, Bronsteen RA. The early sonographic appearance of placenta accreta. J Ultrasound Med. 2003;22:19-23.
27. Chou MM, Ho ES. Prenatal diagnosis of placenta previa accreta with power amplitude ultrasonic agiography. Am J Obstet Gynecol. 1997;177:1523-1525.
28. Levine D, Hulka CA, Ludmir J, Li W, Edelman RR. Placenta accreta: evaluation with color Doppler US, power Doppler US, and MR imaging. Radiology. 1997;205:773-776.
29. Taipale P, Orden MR, Berg M, Manninen H, Alafuzoff I. Prenatal diagnosis of placenta accreta and percreta with ultrasonography, color Doppler, and magnetic resonance imaging. Obstet Gynecol. 2004;104:537-540.
30. Chou MM, Tseng JJ, Ho ES, Hwang JI. Three-dimensional color power Doppler imaging in the assessment of uteroplacental neovascularization in placenta previa increta/percreta. Am J Obstet Gynecol. 2001;185:1257-1260.
31. Maldjian C, Adam R, Pelosi M, II, Pelosi M III, Rudelli RD, Maldjian J. MRI appearance of placenta percreta and placenta accreta. Magnetic Resonance Imaging. 1999;17:965-971.
32. Thorp JM, Councell RB, Sandridge DA, Wiest HH. Antepartum diagnosis of placenta previa percreta by magnetic resonance imaging. Obstet Gynecol. 1992;80:506-508.
33. Kay HH, Spritzer CE. Preliminary experience with magnetic resonance imaging in patients with third-trimester bleeding. Obstet Gynecol. 1991;78:424-429.
34. Russo-Stieglitz K, Lockwood CJ. Placenta previa and vasa previa. Up To Date. 2005. Available at: www.uptodate.com.
35. Dashe JS, McIntire DD, Ramus RM, Santos-Ramos R, Twickler DM. Persistence of placenta previa according to gestational age at ultrasound detection. Obstet Gynecol. 2002;99:692-697.
36. Taipale P, Hiilesmaa V, Ylostalo P. Transvaginal ultrasonography at 18-23 weeks in predicting placenta previa at delivery. Ultrasound Obstet Gynecol. 1998;12:422.-
37. Rosati P, Guariglia L. Clinical significance of placenta previa detected at early routine transvaginal scan. J Ultrasound Med. 2000;19:581-585.
38. Varma TR. Fetal growth and placental function in patients with placenta previa. J Obstet Gynaecol Br Commonw. 1973;80:311-315.
39. Crane JM, Van Den Hof MC, et al. Neonatal outcomes with placenta previa. Obstet Gynecol. 1999;93:541-544.
40. Benedetti TJ. Obstetric hemorrhage. In: Gabbe SG, Niebyl, JR, Simpson JL. Obstetrics: Normal and Problem Pregnancies. 4th ed. Philadelphia: Churchill Livingstone; 2002:503–538.
41. Besinger RE, Moniak CW, Paskiewicz LS, et al. The effects of tocolytic use in the management of symptomatic placenta previa. Am J Obstet Gynecol. 1995;172:1770-1778.
42. Magann EF, Johnson CA, Gookin KS, Roberts WE, Martin RW, Morrison JC. Placenta praevia: does uterine activity cause bleeding? Aust NZ J Obstet Gynaecol. 1993;33:22-24
43. Benedetti TJ. Maternal complication of parenteral B-sympathomimetic therapy for premature labor. Am J Obstet Gynecol. 1983;145:1-6.
44. Mouer JR. Placenta previa: Antepartum conservative management, inpatient versus outpatient. Am J Obstet Gynecol. 1994;170:1683-1686.
45. Droste S, Keil K. Expectant management of placenta previa: cost-benefit analysis of outpatient treatment. Am J Obstet Gynecol. 1994;170:1254-1257.
46. Wing D, Paul RH, Millar LK. Management of the symptomatic placenta previa: a randomized, controlled trial of inpatient versus outpatient expectant management. Am J Obstet Gynecol. 1996;175:806-811.
47. Hansch E, Chitkara U, McAlpine J, El-Sayed Y, Dake MD, Razavi MK. Pelvic arterial embolization for control of obstetric hemorrhage: a five-year experience. Am J Obstet Gynecol. 1999;180:1454-1460.
48. Dubois J, Garel L, Grignon A, Lemay M, Leduc L. Placenta percreta: balloon occlusion and embolization of the internal iliac arteries to reduce intraoperative blood losses. Am J Obstet Gynecol. 1997;176:723-726.
49. Suresh V, Goodwin SC, McLucas B, Mohr G. Uterine artery embolization: an underused method of controlling pelvic hemorrhage. Am J Obstet Gynecol. 1997;176:938-948.
50. Salomon LJ, deTayrac R, Castaigne-Meary V, et al. Fertility and pregnancy outcome following pelvic arterial embolization for severe postpartum haemorrhage. A cohort study. Hum Reprod. 2003;18:849-852.
51. Frederiksen MC, Glassenberg R, Stika CS. Placenta previa: a 22-year analysis. Am J Obstet Gynecol. 1999;180:1432-1437.
52. Lockwood CJ, Artal R. Placenta accreta. Obstet Gynecol. 2002;99:1133-1134.
53. Dyer I, Nix GF, Weed JC. Total hysterectomy at cesarean section and the immediate puerperal period. Am J Obstet Gynecol. 1953;65:517-527.
54. Catanzarite VA, Stanco L, Schrimmer D, Conroy C. Managing placenta previa/accreta. Contemporary OB/GYN. 1996;4(5):66-95.
55. Stanco L, Schrimmer D, Paul D, Mishell D. Emergency peripartum hysterectomy and associated risk factors. Am J Obstet Gynecol. 1993;168:879-883.
56. Hudon L, Belfort MA, Broome DR. Diagnosis and management of placenta percreta: a review. Obstet Gynecol Surv. 1998;53:509-517.
57. Shevell T, Malone FD. Management of obstetric hemorrhage. Sem Perinatol. 2003;27:86-104.
58. Cho J, Kim S, Cha K, et al. Interrupted circular suture: bleeding control during cesarean delivery in placenta previa accreta. Obstet Gynecol. 1991;78:876-879.
59. AbdRabbo SA. Stepwise uterine devascularization: a novel technique for management of uncontrollable postpartum hemorrhage with preservation of the uterus. Am J Obstet Gynecol. 1994;171:694-700.
CASE Diagnosis precedes sentinel episode of bleeding
“G.A.” is a 39-year-old gravida 6, para 1041 who was diagnosed with complete placenta previa during a target ultrasound exam performed at 18 weeks for advanced maternal age. She had a sentinel episode of vaginal bleeding at 29 weeks and was hospitalized for close monitoring.
Management strategy
One course of steroid was given, vaginal bleeding subsided, and she was discharged for outpatient conservative management, including iron and folic acid supplementation.
The outcome
The patient progressed to 36 weeks’ gestation, when she underwent amniocentesis to assess fetal lung maturity. When the results were reassuring, a cesarean section was scheduled. Intraoperative blood loss was diminished using pelvic vessel embolization. Surgery was uncomplicated, and a healthy infant was delivered.
Placenta previa is a leading and potentially life-threatening cause of third-trimester bleeding.1 Although the overall incidence is about 0.4% in pregnancies exceeding 20 weeks’ gestation,2 that figure rises with the number of cesarean sections and may reach 10% among women who have undergone 4 or more cesarean deliveries.3 Since more women are requesting elective and repeat cesarean deliveries, we are increasingly likely to encounter this condition.
Fortunately, technological advances have improved maternal and neonatal outcomes after placenta previa:
Nevertheless, the condition necessitates cesarean delivery and can cause serious maternal and perinatal morbidity, including:
It can also occur in association with vasa previa, which, though rare, carries a very high perinatal mortality rate.5
Risk factors
An enlarged placenta or endometrial disruption or scarring in the upper uterine segment due to 1 or more of the factors listed below may increase the likelihood of abnormal placental implantation in the unscarred lower uterine segment:3,6,7
Previa often begins with painless vaginal bleeding
The condition often presents as painless, bright red, vaginal bleeding in the third trimester. It is usually distinguished from abruptio placenta by the absence of abdominal pain and uterine contractions.5 However, approximately 20% of women have uterine activity associated with the first episode of vaginal bleeding.13,14 Moreover, in some cases, painful contractions and labor may precipitate vaginal bleeding from placenta previa.5 Therefore, ultrasound examination is strongly recommended for all women with vaginal bleeding during pregnancy.
Ultrasound for other reasons uncovers many cases
With greater routine use of ultrasonography in obstetrics, a large percentage of women with placenta previa are diagnosed prior to the onset of the characteristic painless vaginal bleeding. In a 2003 study by Dola and colleagues,15 approximately 43% of placenta previa cases were diagnosed by ultrasonography performed for other obstetrical indications prior to the onset of vaginal bleeding.
Look for “warning hemorrhage”
The first episode of vaginal bleeding is rarely profuse or life-threatening to the mother or fetus. The bleeding usually subsides spontaneously, although it could recur and become more severe with subsequent episodes. Pregnancy typically continues after the initial bleeding episode.
The mean gestational age at the time of the first bleeding is 29 to 32 weeks.13,14 However, a third of cases have vaginal bleeding before the 30th week of gestation, a third between 30 and 36 weeks, and a third after 36 weeks’ gestation.13-15 Ten percent of women with the condition may be completely asymptomatic and progress to 38 weeks’ gestation without vaginal bleeding.13,14
Which form of ultrasound is most accurate?
With the advanced technology available today, ultrasound has become the standard means of diagnosing placenta previa.16,17
Transabdominal ultrasound has accuracy as high as 95% and a false-negative rate of 7% in the diagnosis of placenta previa.13,19 However, its accuracy may be adversely affected by maternal obesity, acoustic shadowing of the fetal head in a cephalic presentation, inability to locate the internal cervical os (which is critical for correct diagnosis), and difficulty imaging a posterior placenta and the lateral uterine walls. In addition, a full maternal bladder—usually helpful in transabdominal ultrasound imaging—may cause a false-positive diagnosis if the bladder is overly distended. In this situation, the cervix would appear artificially elongated and give a normally implanted placenta the appearance of encroachment into the internal cervical os.
Transvaginal ultrasound is superior for diagnosis of previa
Leerentveld et al20 reported false-positive and false-negative rates of 1% and 2%, respectively—a striking improvement over transabdominal ultrasound, which has rates of 2% to 6% and 7%, respectively.
Transvaginal sonography has several advantages over transabdominal imaging in localization of the placenta. The shorter distance from the vaginal probe transducer to the cervix and lower uterine segment allows the use of higher-frequency ultrasound waves, with improved resolution; therefore, the relationship between the placental edge and the internal os can be determined more accurately.
Some clinicians may worry that the probe used in transvaginal sonography will disrupt the placenta and provoke significant maternal hemorrhage, but this concern is unfounded. Multiple studies have attested to the safety of transvaginal sonography in localization of the placenta.5,20-22 The probe is introduced and positioned under direct ultrasound guidance at all times, and inadvertent insertion of the endovaginal probe into the internal cervical os is virtually impossible due to the anatomical relationship of the vagina and cervix.21
Transperineal ultrasound is another option. Several investigators have found it to be superior to transabdominal and similarly advantageous to transvaginal sonography in the diagnosis and exclusion of placenta previa.18
Start with transabdominal imaging
In current practice, transabdominal ultrasound is usually performed first to localize the placenta. If there is reason to suspect placenta previa, transvaginal or transperineal sonography is then used to confirm the location of the placenta.
Contractions may hinder imaging
Accurate diagnosis or exclusion of placenta previa may be difficult if uterine contractions are present during ultrasound evaluation. Myometrial contractions shorten the distance between the internal cervical os and the placental edge, altering measurement of this distance. In addition, the ultrasound appearance of a contraction may simulate placental tissue, making it difficult to exclude placenta previa.
The trouble with tradition
The 4 types of placenta previa in the traditional classification system—complete, partial, marginal, and low-lying—predate the era of ultrasound diagnosis and are based on digital palpation of the placenta through a partly dilated cervical os during labor.
A new system of 3 types
Along with other authors,5,15-18 we propose a new system with 3 categories—complete, incomplete, and low-lying—because ultrasound may not distinguish a placenta partially covering the internal os (a discrete point) from one that is merely encroaching on it.
Complete previa
The placenta completely covers the internal cervical os
Incomplete previa
The placental edge is within 2 cm of the internal cervical os, but does not cover the os
Low-lying previa
The distance from the internal cervical os to the placental edge is between 2 and 3.5 cm
Look for placenta accreta
When placenta previa is diagnosed by ultrasound examination, further diagnostic measures are needed to determine whether placenta accreta is present.5,23 In placenta accreta, neither the normal plane of separation between the placental villi and uterine wall, nor the intervening fibrinoid layer of Nitabuch, is present.5,23
Degrees of abnormal placental implantation
Risk of accreta can reach 67%
There are varying reports on the incidence of placenta accreta, but women with placenta previa and previous cesarean deliveries appear to have the highest incidence.3,23,24 In women with placenta previa and 1 previous cesarean section, the risk of placenta accreta has been estimated at 24%, but it increases to 67% for women with placenta previa and 4 previous cesarean sections.3
Sonographic appearance of placenta accreta
Certain characteristics are suggestive of placenta accreta25,26:
Unfortunately, diagnosis of placenta accreta is difficult prior to delivery, although transvaginal sonography and adjunctive color flow/power Doppler imaging with 2- and 3-dimensional techniques offer improved resolution and have yielded promising results in prenatal diagnosis.27-30
Magnetic resonance imaging (MRI) may also prove useful in detecting placental tissue invasion and evaluating the degree of invasion, especially in a posterior or lateral placenta previa or when there is invasion into the bladder.31-33
Gestational age, symptoms determine management
The management of women with placenta previa in the third trimester depends on the extent of maternal hemorrhage and the fetal gestational age. Clinical categories include:
Some asymptomatic cases resolve
Outpatient management is possible for women who have never bled after diagnosis in the second trimester. These women should abstain from intercourse, avoid digital examination after 20 weeks’ gestation, and immediately present to the hospital if there is any evidence of vaginal bleeding.34
Monthly ultrasound evaluations are necessary to determine whether placenta previa has resolved,34-37 since 90% of cases detected in the second trimester resolve by the third trimester.34 However, if placenta previa persists beyond 24 weeks’ gestation, there is a 50% risk that delivery will be complicated by it.35 If placenta previa persists after 32 weeks, that risk approaches 75%.35
2-fold risk of congenital malformations
Most investigators report a 2-fold increased risk of fetal congenital malformations in cases of placenta previa.5 These malformations include anomalies of the central nervous system, cardiovascular system, respiratory tract, and gastrointestinal tract. Therefore, a target ultrasound examination for fetal anatomy is recommended at the initial ultrasound diagnosis of placenta previa.
Risk of fetal growth restriction warrants heightened surveillance
Some controversy surrounds the incidence of fetal growth restriction in pregnancies complicated by placenta previa. Varma38 reported that fetal growth restriction occurs in 16% of women with placenta previa and is correlated with the number of antepartum bleeding episodes. Other investigators have reported normal fetal growth in women with placenta previa.39 Given this uncertainty, serial follow-up ultrasound evaluations are usually advised for fetal growth assessment.
When patient remains asymptomatic, perform amniocentesis at 36 weeks
Some women progress to the late third trimester without any vaginal bleeding. In these women, amniocentesis is recommended at approximately 36 weeks’ gestation to assess fetal lung maturity.34,40 Elective cesarean delivery can then be planned if pulmonary maturity is documented.
The benefits of elective delivery include a stable patient and an optimally prepared surgical team, as well as the avoidance of emergent surgery, which increases the risk for maternal complications. Emergent surgery also places the fetus at greater risk for anemia, compared with elective procedures(27.7% vs 2.9%, respectively).13
Vaginal bleeding requires inpatient evaluation
Any woman with placenta previa who presents with vaginal bleeding should be admitted to the labor and delivery unit for immediate evaluation of maternal and fetal status, including an estimation of gestational age.
Initial acute care and assessment necessitate34:
If hemorrhage is life-threatening, deliver immediately
During initial evaluation, if the hemorrhage is judged to be massive and life-threatening, resuscitative measures and immediate delivery are necessary to avoid serious maternal morbidity. Recommended measures include constant monitoring of maternal status, aggressive IV fluid resuscitation, transfusion of blood and blood products, assessment of fetal status, and immediate delivery without regard to the maturity of the fetus.
A woman at term or near term (with documented fetal lung maturity) who presents with mild or moderate vaginal bleeding should be delivered via cesarean section.
Conservative management may be appropriate for mild preterm bleeding
If vaginal bleeding is not threatening to the life of the mother, and the fetus is preterm, a conservative approach with aggressive expectant management is appropriate, since most first episodes of vaginal bleeding are self-limited and rarely life-threatening to mother or fetus. Expectant management allows fetal maturation in utero without jeopardizing maternal health. If maternal and fetal health remain stable, the expectant approach allows a safe delay of delivery until the fetus matures.
Hospitalization is recommended. Candidates for expectant management should be hospitalized after the initial episode of vaginal bleeding. Once maternal and fetal conditions stabilize, the woman should be transferred to the antepartum ward for hospital bed rest with bathroom privileges. For expectant management:
Delivery is warranted for life-threatening hemorrhage, fetal lung maturity, and/or the usual maternal and fetal indications.
The question of tocolysis
Third-trimester tocolytic therapy in a woman with vaginal bleeding is controversial. In placenta previa, vaginal bleeding appears to arise from disruption of the placental implantation site as the lower uterine segment develops.41,42 It is unclear whether uterine contractions play a role, as only 20% of women with placenta previa have uterine activity at the time of vaginal bleeding.13,14,42 It is difficult to determine whether these women have true preterm labor, because digital examination of the cervix to document cervical dilatation is impossible.
Does uterine activity precipitate bleeding?
Some investigators believe uterine activity is a predisposing factor for the vaginal bleeding associated with placenta previa, and would consider tocolytic therapy in a stable patient at a premature gestational age. However, further evidence of its safety is needed.
In particular, beta-mimetics should be avoided in hemorrhaging women because their vasodilatory effects can precipitate maternal hypotension. Another side effect of beta-mimetics: maternal tachycardia,43 which may mask the hypovolemic state in women with significant hemorrhage.
Magnesium sulfate has less effect on the maternal cardiovascular system and could be a better choice in symptomatic placenta previa.41 Also consider indomethacin, which appears to have fewer adverse maternal effects.
Inpatient vs outpatient management
Because 2 to 3 weeks of maternal hospitalization can pass between the initial warning hemorrhage and delivery of the fetus, outpatient care has become an option. Several retrospective studies have demonstrated the cost-effectiveness and safety of outpatient management of symptomatic placenta previa.44,45 These studies emphasized careful patient section.
Wing and associates46 conducted a prospective, randomized, controlled trial that reinforces the need for judicious use of outpatient management. In their study, fewer than half the patients diagnosed with placenta previa prior to 37 weeks were candidates. The authors point out the small number of patients in their study, and the fact that vaginal bleeding recurred in approximately 60% of patients. Because of the difficulty of predicting which patients will have recurrent bleeding and when, outpatient management should be reserved for those judged to be compliant with home bed rest who can rapidly return to the hospital, if necessary.
Women with recurrent vaginal bleeding during outpatient management should be rehospitalized.
In the event of massive hemorrhage, immediate compression of the aorta below the level of the renal arteries will reduce the bleeding enough to allow time to evaluate the situation.56 At the same time, aggressive IV fluid resuscitation and blood transfusion should begin. Reevaluate coagulation status after every 5 to 10 U of blood.57
Focused repair may be effective. In some situations, the hemorrhage may be controlled by oversewing and repairing the focal placental site defects.40
Bracketing the bleeding area. Another measure is a circular suture technique in which interrupted sutures are placed on the serosal surface of the anterior and posterior aspects of the uterus and as deeply as possible into the endometrium in a circumferential manner, bracketing the bleeding area.58
The argon beam coagulator can be used to achieve hemostasis; it is more effective than traditional bipolar cautery at ensuring hemostasis in extensive areas.56,57
Stepwise devascularization was effective in 100% of 103 women with postpartum hemorrhage who did not respond to traditional management.59 It involves 5 procedures to be performed in sequence until hemostasis is achieved: unilateral uterine vessel ligation, bilateral uterine vessel ligation, low uterine vessel ligation, unilateral ovarian vessel ligation, and bilateral ovarian vessel ligation.
Hypogastric artery ligation is another option, but it is technically challenging and successful in less than 50% of cases.57 In fact, the time spent on this technique may actually lead to increased blood loss.
Components of safe delivery
A detailed plan is necessary when major hemorrhage is anticipated at the time of elective cesarean delivery for placenta previa, including consultation with experts in different disciplines such as radiology, anesthesiology, urology, pathology, blood bank, neonatology, and gynecologic oncology.
Also pay attention to the maternal red blood cell reserve. Iron and folic acid should be administered to prevent and treat anemia, and antepartum erythropoietin should be considered as a way of increasing the hemoglobin level in women with placenta previa. Autologous blood transfusion, including acute normovolemic hemodilution, is another option.
Pelvic vessel embolization
Elective embolization or occlusion of the hypogastric or uterine arteries has proved to be safe and effective for postpartum hemorrhage, with a success rate of more than 90% in women with normal coagulation.47
In addition, elective catheterization with a balloon-tipped catheter can be used prophylactically to reduce blood flow to the placenta. Prophylactic catheterization of the anterior division of the internal iliac arteries can be performed right before the scheduled cesarean section. An axillary approach is technically easier for fluoroscopically guided catheterization of the internal iliac.48 The actual fluoroscopy time is minutes, so the risk of fetal exposure to radiation and irreversible ovarian damage is minimal.
The fetus is monitored during the procedure, and the balloons are left in the deflated state until after delivery, reducing the risk of uteroplacental insufficiency. Balloon inflation after delivery occludes the hypogastric arteries and diminishes uterine arterial blood flow during surgery. In some cases, the temporary occlusive effect of the balloons may control intraoperative bleeding completely. If substantial bleeding persists, subsequent embolization of the uterine arteries is advised, using absorbable Gelfoam particles, which are temporary and do not damage pelvic organs. Menstruation is not impaired, and normal pregnancies have been reported after this procedure.49,50
In women who undergo cesarean delivery under regional anesthesia, placement of a dry epidural catheter for later dosing of anesthetic agents should be considered prior to balloon catheterization, since the patient’s mobility is restricted after placement of the balloon-tipped catheter.
This therapy is especially useful when there is a high index of suspicion for placenta accreta.
Recommendations at the time of delivery
Hysterectomy for placenta previa, placenta accreta
This procedure is technically challenging when there is a markedly enlarged uterus with engorged collateral vessels. One useful method, delayed ligation technique, was originally described by Dyer et al on the Tulane obstetrics service at Charity Hospital of New Orleans.53 This technique facilitates quick control of all uterine vasculature with rapid hemostasis. Later modification of this method involves successive clamping and severing of all vascular pedicles supplying the uterus, prior to their suture ligation, for quick control of bleeding.
Close follow-up continues even after surgery
Immediately after surgery, close monitoring of hemodynamic status is required, ideally in a critical care setting. Because women with placenta previa/placenta accreta have often received massive transfusions of blood and blood products along with large volumes of crystalloid fluids, pulmonary edema may develop. Conversely, hypovolemia can result from inadequate replacement of blood or persistent intra-abdominal bleeding. Thus, close attention to urinary output allows early detection of pulmonary edema, acute respiratory distress syndrome, hypovolemia, or persistent intra-abdominal bleeding. Patients who undergo peripartum hysterectomy should also be monitored closely for possible ureteral injury.
Thromboprophylaxis should continue until the patient is ambulatory.
Recommended laboratory tests
Get a complete blood count with platelets and fibrinogen immediately after surgery and at frequent intervals as needed. A chemistry panel with calcium, albumin, electrolytes, and creatinine also is helpful.
Serious morbidity in 3% to 5% of women after emergent hysterectomy
Conditions such as acute respiratory distress syndrome from massive blood transfusion and pulmonary capillary leakage, acute tubular necrosis from renal failure, and pulmonary embolism may complicate 3% to 5% of cases.54
Reoperation for persistent intra-abdominal bleeding may be necessary, and 9% of women will have urologic injury. Unfortunately, the maternal mortality rate associated with this procedure is 0.8%, so meticulous postoperative care is mandated.55
The authors report no financial relationships with any company whose products are mentioned in this article.
CASE Diagnosis precedes sentinel episode of bleeding
“G.A.” is a 39-year-old gravida 6, para 1041 who was diagnosed with complete placenta previa during a target ultrasound exam performed at 18 weeks for advanced maternal age. She had a sentinel episode of vaginal bleeding at 29 weeks and was hospitalized for close monitoring.
Management strategy
One course of steroid was given, vaginal bleeding subsided, and she was discharged for outpatient conservative management, including iron and folic acid supplementation.
The outcome
The patient progressed to 36 weeks’ gestation, when she underwent amniocentesis to assess fetal lung maturity. When the results were reassuring, a cesarean section was scheduled. Intraoperative blood loss was diminished using pelvic vessel embolization. Surgery was uncomplicated, and a healthy infant was delivered.
Placenta previa is a leading and potentially life-threatening cause of third-trimester bleeding.1 Although the overall incidence is about 0.4% in pregnancies exceeding 20 weeks’ gestation,2 that figure rises with the number of cesarean sections and may reach 10% among women who have undergone 4 or more cesarean deliveries.3 Since more women are requesting elective and repeat cesarean deliveries, we are increasingly likely to encounter this condition.
Fortunately, technological advances have improved maternal and neonatal outcomes after placenta previa:
Nevertheless, the condition necessitates cesarean delivery and can cause serious maternal and perinatal morbidity, including:
It can also occur in association with vasa previa, which, though rare, carries a very high perinatal mortality rate.5
Risk factors
An enlarged placenta or endometrial disruption or scarring in the upper uterine segment due to 1 or more of the factors listed below may increase the likelihood of abnormal placental implantation in the unscarred lower uterine segment:3,6,7
Previa often begins with painless vaginal bleeding
The condition often presents as painless, bright red, vaginal bleeding in the third trimester. It is usually distinguished from abruptio placenta by the absence of abdominal pain and uterine contractions.5 However, approximately 20% of women have uterine activity associated with the first episode of vaginal bleeding.13,14 Moreover, in some cases, painful contractions and labor may precipitate vaginal bleeding from placenta previa.5 Therefore, ultrasound examination is strongly recommended for all women with vaginal bleeding during pregnancy.
Ultrasound for other reasons uncovers many cases
With greater routine use of ultrasonography in obstetrics, a large percentage of women with placenta previa are diagnosed prior to the onset of the characteristic painless vaginal bleeding. In a 2003 study by Dola and colleagues,15 approximately 43% of placenta previa cases were diagnosed by ultrasonography performed for other obstetrical indications prior to the onset of vaginal bleeding.
Look for “warning hemorrhage”
The first episode of vaginal bleeding is rarely profuse or life-threatening to the mother or fetus. The bleeding usually subsides spontaneously, although it could recur and become more severe with subsequent episodes. Pregnancy typically continues after the initial bleeding episode.
The mean gestational age at the time of the first bleeding is 29 to 32 weeks.13,14 However, a third of cases have vaginal bleeding before the 30th week of gestation, a third between 30 and 36 weeks, and a third after 36 weeks’ gestation.13-15 Ten percent of women with the condition may be completely asymptomatic and progress to 38 weeks’ gestation without vaginal bleeding.13,14
Which form of ultrasound is most accurate?
With the advanced technology available today, ultrasound has become the standard means of diagnosing placenta previa.16,17
Transabdominal ultrasound has accuracy as high as 95% and a false-negative rate of 7% in the diagnosis of placenta previa.13,19 However, its accuracy may be adversely affected by maternal obesity, acoustic shadowing of the fetal head in a cephalic presentation, inability to locate the internal cervical os (which is critical for correct diagnosis), and difficulty imaging a posterior placenta and the lateral uterine walls. In addition, a full maternal bladder—usually helpful in transabdominal ultrasound imaging—may cause a false-positive diagnosis if the bladder is overly distended. In this situation, the cervix would appear artificially elongated and give a normally implanted placenta the appearance of encroachment into the internal cervical os.
Transvaginal ultrasound is superior for diagnosis of previa
Leerentveld et al20 reported false-positive and false-negative rates of 1% and 2%, respectively—a striking improvement over transabdominal ultrasound, which has rates of 2% to 6% and 7%, respectively.
Transvaginal sonography has several advantages over transabdominal imaging in localization of the placenta. The shorter distance from the vaginal probe transducer to the cervix and lower uterine segment allows the use of higher-frequency ultrasound waves, with improved resolution; therefore, the relationship between the placental edge and the internal os can be determined more accurately.
Some clinicians may worry that the probe used in transvaginal sonography will disrupt the placenta and provoke significant maternal hemorrhage, but this concern is unfounded. Multiple studies have attested to the safety of transvaginal sonography in localization of the placenta.5,20-22 The probe is introduced and positioned under direct ultrasound guidance at all times, and inadvertent insertion of the endovaginal probe into the internal cervical os is virtually impossible due to the anatomical relationship of the vagina and cervix.21
Transperineal ultrasound is another option. Several investigators have found it to be superior to transabdominal and similarly advantageous to transvaginal sonography in the diagnosis and exclusion of placenta previa.18
Start with transabdominal imaging
In current practice, transabdominal ultrasound is usually performed first to localize the placenta. If there is reason to suspect placenta previa, transvaginal or transperineal sonography is then used to confirm the location of the placenta.
Contractions may hinder imaging
Accurate diagnosis or exclusion of placenta previa may be difficult if uterine contractions are present during ultrasound evaluation. Myometrial contractions shorten the distance between the internal cervical os and the placental edge, altering measurement of this distance. In addition, the ultrasound appearance of a contraction may simulate placental tissue, making it difficult to exclude placenta previa.
The trouble with tradition
The 4 types of placenta previa in the traditional classification system—complete, partial, marginal, and low-lying—predate the era of ultrasound diagnosis and are based on digital palpation of the placenta through a partly dilated cervical os during labor.
A new system of 3 types
Along with other authors,5,15-18 we propose a new system with 3 categories—complete, incomplete, and low-lying—because ultrasound may not distinguish a placenta partially covering the internal os (a discrete point) from one that is merely encroaching on it.
Complete previa
The placenta completely covers the internal cervical os
Incomplete previa
The placental edge is within 2 cm of the internal cervical os, but does not cover the os
Low-lying previa
The distance from the internal cervical os to the placental edge is between 2 and 3.5 cm
Look for placenta accreta
When placenta previa is diagnosed by ultrasound examination, further diagnostic measures are needed to determine whether placenta accreta is present.5,23 In placenta accreta, neither the normal plane of separation between the placental villi and uterine wall, nor the intervening fibrinoid layer of Nitabuch, is present.5,23
Degrees of abnormal placental implantation
Risk of accreta can reach 67%
There are varying reports on the incidence of placenta accreta, but women with placenta previa and previous cesarean deliveries appear to have the highest incidence.3,23,24 In women with placenta previa and 1 previous cesarean section, the risk of placenta accreta has been estimated at 24%, but it increases to 67% for women with placenta previa and 4 previous cesarean sections.3
Sonographic appearance of placenta accreta
Certain characteristics are suggestive of placenta accreta25,26:
Unfortunately, diagnosis of placenta accreta is difficult prior to delivery, although transvaginal sonography and adjunctive color flow/power Doppler imaging with 2- and 3-dimensional techniques offer improved resolution and have yielded promising results in prenatal diagnosis.27-30
Magnetic resonance imaging (MRI) may also prove useful in detecting placental tissue invasion and evaluating the degree of invasion, especially in a posterior or lateral placenta previa or when there is invasion into the bladder.31-33
Gestational age, symptoms determine management
The management of women with placenta previa in the third trimester depends on the extent of maternal hemorrhage and the fetal gestational age. Clinical categories include:
Some asymptomatic cases resolve
Outpatient management is possible for women who have never bled after diagnosis in the second trimester. These women should abstain from intercourse, avoid digital examination after 20 weeks’ gestation, and immediately present to the hospital if there is any evidence of vaginal bleeding.34
Monthly ultrasound evaluations are necessary to determine whether placenta previa has resolved,34-37 since 90% of cases detected in the second trimester resolve by the third trimester.34 However, if placenta previa persists beyond 24 weeks’ gestation, there is a 50% risk that delivery will be complicated by it.35 If placenta previa persists after 32 weeks, that risk approaches 75%.35
2-fold risk of congenital malformations
Most investigators report a 2-fold increased risk of fetal congenital malformations in cases of placenta previa.5 These malformations include anomalies of the central nervous system, cardiovascular system, respiratory tract, and gastrointestinal tract. Therefore, a target ultrasound examination for fetal anatomy is recommended at the initial ultrasound diagnosis of placenta previa.
Risk of fetal growth restriction warrants heightened surveillance
Some controversy surrounds the incidence of fetal growth restriction in pregnancies complicated by placenta previa. Varma38 reported that fetal growth restriction occurs in 16% of women with placenta previa and is correlated with the number of antepartum bleeding episodes. Other investigators have reported normal fetal growth in women with placenta previa.39 Given this uncertainty, serial follow-up ultrasound evaluations are usually advised for fetal growth assessment.
When patient remains asymptomatic, perform amniocentesis at 36 weeks
Some women progress to the late third trimester without any vaginal bleeding. In these women, amniocentesis is recommended at approximately 36 weeks’ gestation to assess fetal lung maturity.34,40 Elective cesarean delivery can then be planned if pulmonary maturity is documented.
The benefits of elective delivery include a stable patient and an optimally prepared surgical team, as well as the avoidance of emergent surgery, which increases the risk for maternal complications. Emergent surgery also places the fetus at greater risk for anemia, compared with elective procedures(27.7% vs 2.9%, respectively).13
Vaginal bleeding requires inpatient evaluation
Any woman with placenta previa who presents with vaginal bleeding should be admitted to the labor and delivery unit for immediate evaluation of maternal and fetal status, including an estimation of gestational age.
Initial acute care and assessment necessitate34:
If hemorrhage is life-threatening, deliver immediately
During initial evaluation, if the hemorrhage is judged to be massive and life-threatening, resuscitative measures and immediate delivery are necessary to avoid serious maternal morbidity. Recommended measures include constant monitoring of maternal status, aggressive IV fluid resuscitation, transfusion of blood and blood products, assessment of fetal status, and immediate delivery without regard to the maturity of the fetus.
A woman at term or near term (with documented fetal lung maturity) who presents with mild or moderate vaginal bleeding should be delivered via cesarean section.
Conservative management may be appropriate for mild preterm bleeding
If vaginal bleeding is not threatening to the life of the mother, and the fetus is preterm, a conservative approach with aggressive expectant management is appropriate, since most first episodes of vaginal bleeding are self-limited and rarely life-threatening to mother or fetus. Expectant management allows fetal maturation in utero without jeopardizing maternal health. If maternal and fetal health remain stable, the expectant approach allows a safe delay of delivery until the fetus matures.
Hospitalization is recommended. Candidates for expectant management should be hospitalized after the initial episode of vaginal bleeding. Once maternal and fetal conditions stabilize, the woman should be transferred to the antepartum ward for hospital bed rest with bathroom privileges. For expectant management:
Delivery is warranted for life-threatening hemorrhage, fetal lung maturity, and/or the usual maternal and fetal indications.
The question of tocolysis
Third-trimester tocolytic therapy in a woman with vaginal bleeding is controversial. In placenta previa, vaginal bleeding appears to arise from disruption of the placental implantation site as the lower uterine segment develops.41,42 It is unclear whether uterine contractions play a role, as only 20% of women with placenta previa have uterine activity at the time of vaginal bleeding.13,14,42 It is difficult to determine whether these women have true preterm labor, because digital examination of the cervix to document cervical dilatation is impossible.
Does uterine activity precipitate bleeding?
Some investigators believe uterine activity is a predisposing factor for the vaginal bleeding associated with placenta previa, and would consider tocolytic therapy in a stable patient at a premature gestational age. However, further evidence of its safety is needed.
In particular, beta-mimetics should be avoided in hemorrhaging women because their vasodilatory effects can precipitate maternal hypotension. Another side effect of beta-mimetics: maternal tachycardia,43 which may mask the hypovolemic state in women with significant hemorrhage.
Magnesium sulfate has less effect on the maternal cardiovascular system and could be a better choice in symptomatic placenta previa.41 Also consider indomethacin, which appears to have fewer adverse maternal effects.
Inpatient vs outpatient management
Because 2 to 3 weeks of maternal hospitalization can pass between the initial warning hemorrhage and delivery of the fetus, outpatient care has become an option. Several retrospective studies have demonstrated the cost-effectiveness and safety of outpatient management of symptomatic placenta previa.44,45 These studies emphasized careful patient section.
Wing and associates46 conducted a prospective, randomized, controlled trial that reinforces the need for judicious use of outpatient management. In their study, fewer than half the patients diagnosed with placenta previa prior to 37 weeks were candidates. The authors point out the small number of patients in their study, and the fact that vaginal bleeding recurred in approximately 60% of patients. Because of the difficulty of predicting which patients will have recurrent bleeding and when, outpatient management should be reserved for those judged to be compliant with home bed rest who can rapidly return to the hospital, if necessary.
Women with recurrent vaginal bleeding during outpatient management should be rehospitalized.
In the event of massive hemorrhage, immediate compression of the aorta below the level of the renal arteries will reduce the bleeding enough to allow time to evaluate the situation.56 At the same time, aggressive IV fluid resuscitation and blood transfusion should begin. Reevaluate coagulation status after every 5 to 10 U of blood.57
Focused repair may be effective. In some situations, the hemorrhage may be controlled by oversewing and repairing the focal placental site defects.40
Bracketing the bleeding area. Another measure is a circular suture technique in which interrupted sutures are placed on the serosal surface of the anterior and posterior aspects of the uterus and as deeply as possible into the endometrium in a circumferential manner, bracketing the bleeding area.58
The argon beam coagulator can be used to achieve hemostasis; it is more effective than traditional bipolar cautery at ensuring hemostasis in extensive areas.56,57
Stepwise devascularization was effective in 100% of 103 women with postpartum hemorrhage who did not respond to traditional management.59 It involves 5 procedures to be performed in sequence until hemostasis is achieved: unilateral uterine vessel ligation, bilateral uterine vessel ligation, low uterine vessel ligation, unilateral ovarian vessel ligation, and bilateral ovarian vessel ligation.
Hypogastric artery ligation is another option, but it is technically challenging and successful in less than 50% of cases.57 In fact, the time spent on this technique may actually lead to increased blood loss.
Components of safe delivery
A detailed plan is necessary when major hemorrhage is anticipated at the time of elective cesarean delivery for placenta previa, including consultation with experts in different disciplines such as radiology, anesthesiology, urology, pathology, blood bank, neonatology, and gynecologic oncology.
Also pay attention to the maternal red blood cell reserve. Iron and folic acid should be administered to prevent and treat anemia, and antepartum erythropoietin should be considered as a way of increasing the hemoglobin level in women with placenta previa. Autologous blood transfusion, including acute normovolemic hemodilution, is another option.
Pelvic vessel embolization
Elective embolization or occlusion of the hypogastric or uterine arteries has proved to be safe and effective for postpartum hemorrhage, with a success rate of more than 90% in women with normal coagulation.47
In addition, elective catheterization with a balloon-tipped catheter can be used prophylactically to reduce blood flow to the placenta. Prophylactic catheterization of the anterior division of the internal iliac arteries can be performed right before the scheduled cesarean section. An axillary approach is technically easier for fluoroscopically guided catheterization of the internal iliac.48 The actual fluoroscopy time is minutes, so the risk of fetal exposure to radiation and irreversible ovarian damage is minimal.
The fetus is monitored during the procedure, and the balloons are left in the deflated state until after delivery, reducing the risk of uteroplacental insufficiency. Balloon inflation after delivery occludes the hypogastric arteries and diminishes uterine arterial blood flow during surgery. In some cases, the temporary occlusive effect of the balloons may control intraoperative bleeding completely. If substantial bleeding persists, subsequent embolization of the uterine arteries is advised, using absorbable Gelfoam particles, which are temporary and do not damage pelvic organs. Menstruation is not impaired, and normal pregnancies have been reported after this procedure.49,50
In women who undergo cesarean delivery under regional anesthesia, placement of a dry epidural catheter for later dosing of anesthetic agents should be considered prior to balloon catheterization, since the patient’s mobility is restricted after placement of the balloon-tipped catheter.
This therapy is especially useful when there is a high index of suspicion for placenta accreta.
Recommendations at the time of delivery
Hysterectomy for placenta previa, placenta accreta
This procedure is technically challenging when there is a markedly enlarged uterus with engorged collateral vessels. One useful method, delayed ligation technique, was originally described by Dyer et al on the Tulane obstetrics service at Charity Hospital of New Orleans.53 This technique facilitates quick control of all uterine vasculature with rapid hemostasis. Later modification of this method involves successive clamping and severing of all vascular pedicles supplying the uterus, prior to their suture ligation, for quick control of bleeding.
Close follow-up continues even after surgery
Immediately after surgery, close monitoring of hemodynamic status is required, ideally in a critical care setting. Because women with placenta previa/placenta accreta have often received massive transfusions of blood and blood products along with large volumes of crystalloid fluids, pulmonary edema may develop. Conversely, hypovolemia can result from inadequate replacement of blood or persistent intra-abdominal bleeding. Thus, close attention to urinary output allows early detection of pulmonary edema, acute respiratory distress syndrome, hypovolemia, or persistent intra-abdominal bleeding. Patients who undergo peripartum hysterectomy should also be monitored closely for possible ureteral injury.
Thromboprophylaxis should continue until the patient is ambulatory.
Recommended laboratory tests
Get a complete blood count with platelets and fibrinogen immediately after surgery and at frequent intervals as needed. A chemistry panel with calcium, albumin, electrolytes, and creatinine also is helpful.
Serious morbidity in 3% to 5% of women after emergent hysterectomy
Conditions such as acute respiratory distress syndrome from massive blood transfusion and pulmonary capillary leakage, acute tubular necrosis from renal failure, and pulmonary embolism may complicate 3% to 5% of cases.54
Reoperation for persistent intra-abdominal bleeding may be necessary, and 9% of women will have urologic injury. Unfortunately, the maternal mortality rate associated with this procedure is 0.8%, so meticulous postoperative care is mandated.55
The authors report no financial relationships with any company whose products are mentioned in this article.
1. Ananth CV, Savitz DA, Luther ER. Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. Am J Epidemiol. 1996;144:881-889.
2. Faiz AS, Ananth CV. Etiology and risk factors for placenta previa: an overview and meta-analysis of observational studies. J Matern Fetal Neonatal Med. 2003;13:175.-
3. Clark SL, Koonings PP, et al. Placenta previa/accreta and prior cesarean section. Obstet Gynecol. 1985;66:89-92.
4. Neri A, Manor Y, Matityahu A, Blieden L. Placenta previa and congenital cardiac anomalies. Fetal Ther. 1989;4:138-140.
5. Clark SL. Placenta previa and abruptio placenta. In: Creasy RK, Resnik R. Maternal-Fetal Medicine, Principles and Practice. 4th ed. Philadelphia: W.B. Saunders; 1999:616–631.
6. Miller DA, Chollet J, Goodwin TM. Clinical risks factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210-214.
7. Ananth CV, Demissie K, Smulian JC, Vintzileos AM. Placenta previa in singleton and twin births in the United States, 1989 through 1998: a comparison of risk factor profiles and associated conditions. Am J Obstet Gynecol. 2003;188:275-281.
8. Gilbert WM, Nesbitt TS, Danielsen B. Childbearing beyond age 40: pregnancy outcome in 24,032 cases. Obstet Gynecol. 1999;93:9-14.
9. Lavery JP. Placenta previa. Clin Obstet Gynecol. 1990;33:414-421.
10. Taylor VM, Kramer MD, Vaughan TL, Peacock S. Placenta previa in relation to induced and spontaneous abortion: a population- based study. Obstet Gynecol. 1993;82:88-91.
11. Williams MA, Mittendorf R, Lieberman E, Monson RR, Schoenbaum SC, Genest DR. Cigarette smoking during pregnancy in relation to placenta previa. Am J Obstet Gynecol. 1991;165:28-32.
12. Handler AS, Mason ED, Rosenberg DL, Davis FG. The relationship between exposure during pregnancy to cigarette smoking and cocaine use and placenta previa. Am J Obstet Gynecol. 1994;170:884-889.
13. Cotton DB, Read JA, Paul RH, Quilligan EJ. The conservative aggressive management of placenta previa. Am J Obstet Gynecol. 1980;137:687-695.
14. Silver R, Depp R, Sabbagha RE, Dooley SL. Placenta previa: aggressive expectant management. Am J Obstet Gynecol. 1984;150:15-22.
15. Dola CP, Garite TJ, Dowling DD, Friend D, Ahdoot D, Asrat T. Placenta previa: does its type affect pregnancy outcome? Am J Perinatol. 2003;20:353-360.
16. Oppenheimer LW, Farine D, Ritchie JWK, Lewinsky RM, Telford J, Fairbanks LA. What is low-lying placenta? Am J Obstet Gynecol. 1991;165:1036-1038.
17. Bhide A, Thilaganathan B. Recent advances in the management of placenta previa. Curr Opin Obstet Gynecol. 2004;16:447-451.
18. Dawson WB, Dumas MD, Romano WM, Gagnon R, Gratton RJ, Mowbray RD. Translabial ultrasonography and placenta previa: does measurement of the os-placenta distance predict outcome? J Ultrasound Med. 1996;15:44-46.
19. Egley CC. Abruptio placentae and placenta previa. In: Winn HN, Hobbins JC. Clinical Maternal-Fetal Medicine.1st ed. Pearl River, NY: Parthenon Publishing; 2000:47–53.
20. Leerentveld RA, Gilberts E, Arnold M, Wladimiroff JW. Accuracy and safety of transvaginal sonographic placental localization. Obstet Gynecol. 1990;76:759-762.
21. Timor-Tritsch I, Yunis R. Confirming the safety of transvaginal sonography in patients suspected of placenta previa. Obstet Gynecol. 1993;81:742-744.
22. Tan NH, Abu M, Woo JLS, Tahir H. The role of transvaginal sonography in the diagnosis of placenta previa. Aust NZ J Obstet Gynaecol. 1995;35:42-45.
23. Miller DA, Cholleet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210-214.
24. Chattopadhyay SK, Kharif H, Sherbeeni MM. Placenta previa and accreta after previous cesarean section. Eur J Obstet Gynecol Reprod Biol. 1993;52:151.-
25. Comstock CH, Love JJ, Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135-1140.
26. Comstock CH, Lee W, Vettraino IM, Bronsteen RA. The early sonographic appearance of placenta accreta. J Ultrasound Med. 2003;22:19-23.
27. Chou MM, Ho ES. Prenatal diagnosis of placenta previa accreta with power amplitude ultrasonic agiography. Am J Obstet Gynecol. 1997;177:1523-1525.
28. Levine D, Hulka CA, Ludmir J, Li W, Edelman RR. Placenta accreta: evaluation with color Doppler US, power Doppler US, and MR imaging. Radiology. 1997;205:773-776.
29. Taipale P, Orden MR, Berg M, Manninen H, Alafuzoff I. Prenatal diagnosis of placenta accreta and percreta with ultrasonography, color Doppler, and magnetic resonance imaging. Obstet Gynecol. 2004;104:537-540.
30. Chou MM, Tseng JJ, Ho ES, Hwang JI. Three-dimensional color power Doppler imaging in the assessment of uteroplacental neovascularization in placenta previa increta/percreta. Am J Obstet Gynecol. 2001;185:1257-1260.
31. Maldjian C, Adam R, Pelosi M, II, Pelosi M III, Rudelli RD, Maldjian J. MRI appearance of placenta percreta and placenta accreta. Magnetic Resonance Imaging. 1999;17:965-971.
32. Thorp JM, Councell RB, Sandridge DA, Wiest HH. Antepartum diagnosis of placenta previa percreta by magnetic resonance imaging. Obstet Gynecol. 1992;80:506-508.
33. Kay HH, Spritzer CE. Preliminary experience with magnetic resonance imaging in patients with third-trimester bleeding. Obstet Gynecol. 1991;78:424-429.
34. Russo-Stieglitz K, Lockwood CJ. Placenta previa and vasa previa. Up To Date. 2005. Available at: www.uptodate.com.
35. Dashe JS, McIntire DD, Ramus RM, Santos-Ramos R, Twickler DM. Persistence of placenta previa according to gestational age at ultrasound detection. Obstet Gynecol. 2002;99:692-697.
36. Taipale P, Hiilesmaa V, Ylostalo P. Transvaginal ultrasonography at 18-23 weeks in predicting placenta previa at delivery. Ultrasound Obstet Gynecol. 1998;12:422.-
37. Rosati P, Guariglia L. Clinical significance of placenta previa detected at early routine transvaginal scan. J Ultrasound Med. 2000;19:581-585.
38. Varma TR. Fetal growth and placental function in patients with placenta previa. J Obstet Gynaecol Br Commonw. 1973;80:311-315.
39. Crane JM, Van Den Hof MC, et al. Neonatal outcomes with placenta previa. Obstet Gynecol. 1999;93:541-544.
40. Benedetti TJ. Obstetric hemorrhage. In: Gabbe SG, Niebyl, JR, Simpson JL. Obstetrics: Normal and Problem Pregnancies. 4th ed. Philadelphia: Churchill Livingstone; 2002:503–538.
41. Besinger RE, Moniak CW, Paskiewicz LS, et al. The effects of tocolytic use in the management of symptomatic placenta previa. Am J Obstet Gynecol. 1995;172:1770-1778.
42. Magann EF, Johnson CA, Gookin KS, Roberts WE, Martin RW, Morrison JC. Placenta praevia: does uterine activity cause bleeding? Aust NZ J Obstet Gynaecol. 1993;33:22-24
43. Benedetti TJ. Maternal complication of parenteral B-sympathomimetic therapy for premature labor. Am J Obstet Gynecol. 1983;145:1-6.
44. Mouer JR. Placenta previa: Antepartum conservative management, inpatient versus outpatient. Am J Obstet Gynecol. 1994;170:1683-1686.
45. Droste S, Keil K. Expectant management of placenta previa: cost-benefit analysis of outpatient treatment. Am J Obstet Gynecol. 1994;170:1254-1257.
46. Wing D, Paul RH, Millar LK. Management of the symptomatic placenta previa: a randomized, controlled trial of inpatient versus outpatient expectant management. Am J Obstet Gynecol. 1996;175:806-811.
47. Hansch E, Chitkara U, McAlpine J, El-Sayed Y, Dake MD, Razavi MK. Pelvic arterial embolization for control of obstetric hemorrhage: a five-year experience. Am J Obstet Gynecol. 1999;180:1454-1460.
48. Dubois J, Garel L, Grignon A, Lemay M, Leduc L. Placenta percreta: balloon occlusion and embolization of the internal iliac arteries to reduce intraoperative blood losses. Am J Obstet Gynecol. 1997;176:723-726.
49. Suresh V, Goodwin SC, McLucas B, Mohr G. Uterine artery embolization: an underused method of controlling pelvic hemorrhage. Am J Obstet Gynecol. 1997;176:938-948.
50. Salomon LJ, deTayrac R, Castaigne-Meary V, et al. Fertility and pregnancy outcome following pelvic arterial embolization for severe postpartum haemorrhage. A cohort study. Hum Reprod. 2003;18:849-852.
51. Frederiksen MC, Glassenberg R, Stika CS. Placenta previa: a 22-year analysis. Am J Obstet Gynecol. 1999;180:1432-1437.
52. Lockwood CJ, Artal R. Placenta accreta. Obstet Gynecol. 2002;99:1133-1134.
53. Dyer I, Nix GF, Weed JC. Total hysterectomy at cesarean section and the immediate puerperal period. Am J Obstet Gynecol. 1953;65:517-527.
54. Catanzarite VA, Stanco L, Schrimmer D, Conroy C. Managing placenta previa/accreta. Contemporary OB/GYN. 1996;4(5):66-95.
55. Stanco L, Schrimmer D, Paul D, Mishell D. Emergency peripartum hysterectomy and associated risk factors. Am J Obstet Gynecol. 1993;168:879-883.
56. Hudon L, Belfort MA, Broome DR. Diagnosis and management of placenta percreta: a review. Obstet Gynecol Surv. 1998;53:509-517.
57. Shevell T, Malone FD. Management of obstetric hemorrhage. Sem Perinatol. 2003;27:86-104.
58. Cho J, Kim S, Cha K, et al. Interrupted circular suture: bleeding control during cesarean delivery in placenta previa accreta. Obstet Gynecol. 1991;78:876-879.
59. AbdRabbo SA. Stepwise uterine devascularization: a novel technique for management of uncontrollable postpartum hemorrhage with preservation of the uterus. Am J Obstet Gynecol. 1994;171:694-700.
1. Ananth CV, Savitz DA, Luther ER. Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. Am J Epidemiol. 1996;144:881-889.
2. Faiz AS, Ananth CV. Etiology and risk factors for placenta previa: an overview and meta-analysis of observational studies. J Matern Fetal Neonatal Med. 2003;13:175.-
3. Clark SL, Koonings PP, et al. Placenta previa/accreta and prior cesarean section. Obstet Gynecol. 1985;66:89-92.
4. Neri A, Manor Y, Matityahu A, Blieden L. Placenta previa and congenital cardiac anomalies. Fetal Ther. 1989;4:138-140.
5. Clark SL. Placenta previa and abruptio placenta. In: Creasy RK, Resnik R. Maternal-Fetal Medicine, Principles and Practice. 4th ed. Philadelphia: W.B. Saunders; 1999:616–631.
6. Miller DA, Chollet J, Goodwin TM. Clinical risks factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210-214.
7. Ananth CV, Demissie K, Smulian JC, Vintzileos AM. Placenta previa in singleton and twin births in the United States, 1989 through 1998: a comparison of risk factor profiles and associated conditions. Am J Obstet Gynecol. 2003;188:275-281.
8. Gilbert WM, Nesbitt TS, Danielsen B. Childbearing beyond age 40: pregnancy outcome in 24,032 cases. Obstet Gynecol. 1999;93:9-14.
9. Lavery JP. Placenta previa. Clin Obstet Gynecol. 1990;33:414-421.
10. Taylor VM, Kramer MD, Vaughan TL, Peacock S. Placenta previa in relation to induced and spontaneous abortion: a population- based study. Obstet Gynecol. 1993;82:88-91.
11. Williams MA, Mittendorf R, Lieberman E, Monson RR, Schoenbaum SC, Genest DR. Cigarette smoking during pregnancy in relation to placenta previa. Am J Obstet Gynecol. 1991;165:28-32.
12. Handler AS, Mason ED, Rosenberg DL, Davis FG. The relationship between exposure during pregnancy to cigarette smoking and cocaine use and placenta previa. Am J Obstet Gynecol. 1994;170:884-889.
13. Cotton DB, Read JA, Paul RH, Quilligan EJ. The conservative aggressive management of placenta previa. Am J Obstet Gynecol. 1980;137:687-695.
14. Silver R, Depp R, Sabbagha RE, Dooley SL. Placenta previa: aggressive expectant management. Am J Obstet Gynecol. 1984;150:15-22.
15. Dola CP, Garite TJ, Dowling DD, Friend D, Ahdoot D, Asrat T. Placenta previa: does its type affect pregnancy outcome? Am J Perinatol. 2003;20:353-360.
16. Oppenheimer LW, Farine D, Ritchie JWK, Lewinsky RM, Telford J, Fairbanks LA. What is low-lying placenta? Am J Obstet Gynecol. 1991;165:1036-1038.
17. Bhide A, Thilaganathan B. Recent advances in the management of placenta previa. Curr Opin Obstet Gynecol. 2004;16:447-451.
18. Dawson WB, Dumas MD, Romano WM, Gagnon R, Gratton RJ, Mowbray RD. Translabial ultrasonography and placenta previa: does measurement of the os-placenta distance predict outcome? J Ultrasound Med. 1996;15:44-46.
19. Egley CC. Abruptio placentae and placenta previa. In: Winn HN, Hobbins JC. Clinical Maternal-Fetal Medicine.1st ed. Pearl River, NY: Parthenon Publishing; 2000:47–53.
20. Leerentveld RA, Gilberts E, Arnold M, Wladimiroff JW. Accuracy and safety of transvaginal sonographic placental localization. Obstet Gynecol. 1990;76:759-762.
21. Timor-Tritsch I, Yunis R. Confirming the safety of transvaginal sonography in patients suspected of placenta previa. Obstet Gynecol. 1993;81:742-744.
22. Tan NH, Abu M, Woo JLS, Tahir H. The role of transvaginal sonography in the diagnosis of placenta previa. Aust NZ J Obstet Gynaecol. 1995;35:42-45.
23. Miller DA, Cholleet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210-214.
24. Chattopadhyay SK, Kharif H, Sherbeeni MM. Placenta previa and accreta after previous cesarean section. Eur J Obstet Gynecol Reprod Biol. 1993;52:151.-
25. Comstock CH, Love JJ, Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135-1140.
26. Comstock CH, Lee W, Vettraino IM, Bronsteen RA. The early sonographic appearance of placenta accreta. J Ultrasound Med. 2003;22:19-23.
27. Chou MM, Ho ES. Prenatal diagnosis of placenta previa accreta with power amplitude ultrasonic agiography. Am J Obstet Gynecol. 1997;177:1523-1525.
28. Levine D, Hulka CA, Ludmir J, Li W, Edelman RR. Placenta accreta: evaluation with color Doppler US, power Doppler US, and MR imaging. Radiology. 1997;205:773-776.
29. Taipale P, Orden MR, Berg M, Manninen H, Alafuzoff I. Prenatal diagnosis of placenta accreta and percreta with ultrasonography, color Doppler, and magnetic resonance imaging. Obstet Gynecol. 2004;104:537-540.
30. Chou MM, Tseng JJ, Ho ES, Hwang JI. Three-dimensional color power Doppler imaging in the assessment of uteroplacental neovascularization in placenta previa increta/percreta. Am J Obstet Gynecol. 2001;185:1257-1260.
31. Maldjian C, Adam R, Pelosi M, II, Pelosi M III, Rudelli RD, Maldjian J. MRI appearance of placenta percreta and placenta accreta. Magnetic Resonance Imaging. 1999;17:965-971.
32. Thorp JM, Councell RB, Sandridge DA, Wiest HH. Antepartum diagnosis of placenta previa percreta by magnetic resonance imaging. Obstet Gynecol. 1992;80:506-508.
33. Kay HH, Spritzer CE. Preliminary experience with magnetic resonance imaging in patients with third-trimester bleeding. Obstet Gynecol. 1991;78:424-429.
34. Russo-Stieglitz K, Lockwood CJ. Placenta previa and vasa previa. Up To Date. 2005. Available at: www.uptodate.com.
35. Dashe JS, McIntire DD, Ramus RM, Santos-Ramos R, Twickler DM. Persistence of placenta previa according to gestational age at ultrasound detection. Obstet Gynecol. 2002;99:692-697.
36. Taipale P, Hiilesmaa V, Ylostalo P. Transvaginal ultrasonography at 18-23 weeks in predicting placenta previa at delivery. Ultrasound Obstet Gynecol. 1998;12:422.-
37. Rosati P, Guariglia L. Clinical significance of placenta previa detected at early routine transvaginal scan. J Ultrasound Med. 2000;19:581-585.
38. Varma TR. Fetal growth and placental function in patients with placenta previa. J Obstet Gynaecol Br Commonw. 1973;80:311-315.
39. Crane JM, Van Den Hof MC, et al. Neonatal outcomes with placenta previa. Obstet Gynecol. 1999;93:541-544.
40. Benedetti TJ. Obstetric hemorrhage. In: Gabbe SG, Niebyl, JR, Simpson JL. Obstetrics: Normal and Problem Pregnancies. 4th ed. Philadelphia: Churchill Livingstone; 2002:503–538.
41. Besinger RE, Moniak CW, Paskiewicz LS, et al. The effects of tocolytic use in the management of symptomatic placenta previa. Am J Obstet Gynecol. 1995;172:1770-1778.
42. Magann EF, Johnson CA, Gookin KS, Roberts WE, Martin RW, Morrison JC. Placenta praevia: does uterine activity cause bleeding? Aust NZ J Obstet Gynaecol. 1993;33:22-24
43. Benedetti TJ. Maternal complication of parenteral B-sympathomimetic therapy for premature labor. Am J Obstet Gynecol. 1983;145:1-6.
44. Mouer JR. Placenta previa: Antepartum conservative management, inpatient versus outpatient. Am J Obstet Gynecol. 1994;170:1683-1686.
45. Droste S, Keil K. Expectant management of placenta previa: cost-benefit analysis of outpatient treatment. Am J Obstet Gynecol. 1994;170:1254-1257.
46. Wing D, Paul RH, Millar LK. Management of the symptomatic placenta previa: a randomized, controlled trial of inpatient versus outpatient expectant management. Am J Obstet Gynecol. 1996;175:806-811.
47. Hansch E, Chitkara U, McAlpine J, El-Sayed Y, Dake MD, Razavi MK. Pelvic arterial embolization for control of obstetric hemorrhage: a five-year experience. Am J Obstet Gynecol. 1999;180:1454-1460.
48. Dubois J, Garel L, Grignon A, Lemay M, Leduc L. Placenta percreta: balloon occlusion and embolization of the internal iliac arteries to reduce intraoperative blood losses. Am J Obstet Gynecol. 1997;176:723-726.
49. Suresh V, Goodwin SC, McLucas B, Mohr G. Uterine artery embolization: an underused method of controlling pelvic hemorrhage. Am J Obstet Gynecol. 1997;176:938-948.
50. Salomon LJ, deTayrac R, Castaigne-Meary V, et al. Fertility and pregnancy outcome following pelvic arterial embolization for severe postpartum haemorrhage. A cohort study. Hum Reprod. 2003;18:849-852.
51. Frederiksen MC, Glassenberg R, Stika CS. Placenta previa: a 22-year analysis. Am J Obstet Gynecol. 1999;180:1432-1437.
52. Lockwood CJ, Artal R. Placenta accreta. Obstet Gynecol. 2002;99:1133-1134.
53. Dyer I, Nix GF, Weed JC. Total hysterectomy at cesarean section and the immediate puerperal period. Am J Obstet Gynecol. 1953;65:517-527.
54. Catanzarite VA, Stanco L, Schrimmer D, Conroy C. Managing placenta previa/accreta. Contemporary OB/GYN. 1996;4(5):66-95.
55. Stanco L, Schrimmer D, Paul D, Mishell D. Emergency peripartum hysterectomy and associated risk factors. Am J Obstet Gynecol. 1993;168:879-883.
56. Hudon L, Belfort MA, Broome DR. Diagnosis and management of placenta percreta: a review. Obstet Gynecol Surv. 1998;53:509-517.
57. Shevell T, Malone FD. Management of obstetric hemorrhage. Sem Perinatol. 2003;27:86-104.
58. Cho J, Kim S, Cha K, et al. Interrupted circular suture: bleeding control during cesarean delivery in placenta previa accreta. Obstet Gynecol. 1991;78:876-879.
59. AbdRabbo SA. Stepwise uterine devascularization: a novel technique for management of uncontrollable postpartum hemorrhage with preservation of the uterus. Am J Obstet Gynecol. 1994;171:694-700.
New Test 'Aces' Preeclampsia Diagnosis
LISBON — A triple test for diagnosing preeclampsia during the third trimester was correct about 90% of the time in a pilot study with 46 women.
“The generalizability of this test needs to be confirmed by using it on more women and on varied populations,” Dr. Brendan J. Smyth said at 15th World Congress of the International Society for the Study of Hypertension in Pregnancy.
A diagnostic test for preeclampsia during the third trimester would aid the management of these women by both making diagnosis during pregnancy more reliable and by paving the way for studies aimed at developing new interventions, said Dr. Smyth, a researcher at Abbott Laboratories in Abbott Park, Ill.
Currently, the only reliable way to distinguish preeclampsia from other blood pressure disorders of pregnancy is retrospectively. The appearance of new-onset hypertension during the final 20 weeks of pregnancy may be preeclampsia, but only if it resolves 12–24 weeks post partum. If it doesn't resolve, then it's chronic hypertension. Preeclampsia can also be hard to distinguish from gestational hypertension because proteinuria might lag behind the rise in blood pressure.
The three-tiered test developed by Dr. Smyth and his associates used serum tests for two biomarkers, body mass index (BMI), and supine systolic blood pressure.
The serum tests measured levels of soluble fms-like tyrosine kinase 1(sFlt-1) and placental growth factor (PlGF). Both factors are produced by the vascular endothelium during the growth of new blood vessels. The assays were run by Abbott Diagnostics, which is developing the tests; the tests are not yet commercially available. The specimens were collected in a study directed by Dr. Jason Umans, associate director of the General Clinical Research Center at Georgetown University in Washington, supported in part by a grant from the National Institutes of Health.
The diagnostic criterion was the ratio of these two analytes. If the ratio of sFlt-1/PlGF was less than 1.9, women were diagnosed as not having preeclampsia. If the ratio was 1.9 or greater, preeclampsia was diagnosed.
BMI was used to diagnose chronic hypertension. Women with a BMI of at least 34 kg/m
These diagnostic criteria were tested on 46 women and 85 of their serum specimens that were collected in the labor and delivery suites of two hospitals in Washington. The subjects included women with preeclampsia, chronic hypertension, superimposed preeclampsia on chronic hypertension, and healthy controls. All of the preeclampsia diagnoses were confirmed by following the patients postpartum.
At least one specimen was collected from each woman in the study. Second, and in some cases third, specimens were collected at weekly intervals from women who remained in the labor and delivery suites for longer periods.
The serum tests correctly classified 83 of the 85 specimens (98%) based on whether they came from preeclamptic or nonpreeclamptic patients. BMI then correctly classified 37 of 43 patients (86%) for the diagnosis of chronic hypertension. Among 42 women without preeclampsia and a BMI of less than 34 kg/m
LISBON — A triple test for diagnosing preeclampsia during the third trimester was correct about 90% of the time in a pilot study with 46 women.
“The generalizability of this test needs to be confirmed by using it on more women and on varied populations,” Dr. Brendan J. Smyth said at 15th World Congress of the International Society for the Study of Hypertension in Pregnancy.
A diagnostic test for preeclampsia during the third trimester would aid the management of these women by both making diagnosis during pregnancy more reliable and by paving the way for studies aimed at developing new interventions, said Dr. Smyth, a researcher at Abbott Laboratories in Abbott Park, Ill.
Currently, the only reliable way to distinguish preeclampsia from other blood pressure disorders of pregnancy is retrospectively. The appearance of new-onset hypertension during the final 20 weeks of pregnancy may be preeclampsia, but only if it resolves 12–24 weeks post partum. If it doesn't resolve, then it's chronic hypertension. Preeclampsia can also be hard to distinguish from gestational hypertension because proteinuria might lag behind the rise in blood pressure.
The three-tiered test developed by Dr. Smyth and his associates used serum tests for two biomarkers, body mass index (BMI), and supine systolic blood pressure.
The serum tests measured levels of soluble fms-like tyrosine kinase 1(sFlt-1) and placental growth factor (PlGF). Both factors are produced by the vascular endothelium during the growth of new blood vessels. The assays were run by Abbott Diagnostics, which is developing the tests; the tests are not yet commercially available. The specimens were collected in a study directed by Dr. Jason Umans, associate director of the General Clinical Research Center at Georgetown University in Washington, supported in part by a grant from the National Institutes of Health.
The diagnostic criterion was the ratio of these two analytes. If the ratio of sFlt-1/PlGF was less than 1.9, women were diagnosed as not having preeclampsia. If the ratio was 1.9 or greater, preeclampsia was diagnosed.
BMI was used to diagnose chronic hypertension. Women with a BMI of at least 34 kg/m
These diagnostic criteria were tested on 46 women and 85 of their serum specimens that were collected in the labor and delivery suites of two hospitals in Washington. The subjects included women with preeclampsia, chronic hypertension, superimposed preeclampsia on chronic hypertension, and healthy controls. All of the preeclampsia diagnoses were confirmed by following the patients postpartum.
At least one specimen was collected from each woman in the study. Second, and in some cases third, specimens were collected at weekly intervals from women who remained in the labor and delivery suites for longer periods.
The serum tests correctly classified 83 of the 85 specimens (98%) based on whether they came from preeclamptic or nonpreeclamptic patients. BMI then correctly classified 37 of 43 patients (86%) for the diagnosis of chronic hypertension. Among 42 women without preeclampsia and a BMI of less than 34 kg/m
LISBON — A triple test for diagnosing preeclampsia during the third trimester was correct about 90% of the time in a pilot study with 46 women.
“The generalizability of this test needs to be confirmed by using it on more women and on varied populations,” Dr. Brendan J. Smyth said at 15th World Congress of the International Society for the Study of Hypertension in Pregnancy.
A diagnostic test for preeclampsia during the third trimester would aid the management of these women by both making diagnosis during pregnancy more reliable and by paving the way for studies aimed at developing new interventions, said Dr. Smyth, a researcher at Abbott Laboratories in Abbott Park, Ill.
Currently, the only reliable way to distinguish preeclampsia from other blood pressure disorders of pregnancy is retrospectively. The appearance of new-onset hypertension during the final 20 weeks of pregnancy may be preeclampsia, but only if it resolves 12–24 weeks post partum. If it doesn't resolve, then it's chronic hypertension. Preeclampsia can also be hard to distinguish from gestational hypertension because proteinuria might lag behind the rise in blood pressure.
The three-tiered test developed by Dr. Smyth and his associates used serum tests for two biomarkers, body mass index (BMI), and supine systolic blood pressure.
The serum tests measured levels of soluble fms-like tyrosine kinase 1(sFlt-1) and placental growth factor (PlGF). Both factors are produced by the vascular endothelium during the growth of new blood vessels. The assays were run by Abbott Diagnostics, which is developing the tests; the tests are not yet commercially available. The specimens were collected in a study directed by Dr. Jason Umans, associate director of the General Clinical Research Center at Georgetown University in Washington, supported in part by a grant from the National Institutes of Health.
The diagnostic criterion was the ratio of these two analytes. If the ratio of sFlt-1/PlGF was less than 1.9, women were diagnosed as not having preeclampsia. If the ratio was 1.9 or greater, preeclampsia was diagnosed.
BMI was used to diagnose chronic hypertension. Women with a BMI of at least 34 kg/m
These diagnostic criteria were tested on 46 women and 85 of their serum specimens that were collected in the labor and delivery suites of two hospitals in Washington. The subjects included women with preeclampsia, chronic hypertension, superimposed preeclampsia on chronic hypertension, and healthy controls. All of the preeclampsia diagnoses were confirmed by following the patients postpartum.
At least one specimen was collected from each woman in the study. Second, and in some cases third, specimens were collected at weekly intervals from women who remained in the labor and delivery suites for longer periods.
The serum tests correctly classified 83 of the 85 specimens (98%) based on whether they came from preeclamptic or nonpreeclamptic patients. BMI then correctly classified 37 of 43 patients (86%) for the diagnosis of chronic hypertension. Among 42 women without preeclampsia and a BMI of less than 34 kg/m
CPAP May Benefit Women At Risk for Preeclampsia
SALT LAKE CITY — Use of continuous positive airway pressure may help prevent preeclampsia in pregnant women at risk for the condition, a study suggests.
In 9 of 12 women with risk factors for preeclampsia who used continuous positive airway pressure (CPAP) and medical therapy beginning before 9 weeks' gestation, blood pressure remained stable and pregnancy was normal, Dr. Christian Guilleminault reported in a poster at the annual meeting of the Associated Professional Sleep Societies.
Sleep-disordered breathing has been suggested by several studies as a possible contributor to preeclampsia. In one study, snoring was linked with preeclampsia, with the disorder occurring in 10% of snorers compared with 4% of nonsnorers. In another study, snoring was shown to be a significant predictor of hypertension and fetal growth retardation, even after controlling for maternal weight, age, and smoking status.
Based on such findings, some researchers have recommended polysomnography and/or CPAP use in pregnant women with risk factors—including snoring—for preeclampsia. In the current study, all 12 participants had risk factors for preeclampsia: All were snorers; seven had hypertension; two had prior preeclampsia; and three were obese, with a body mass index (kg/m
The women, who had a mean age of 29 years, underwent polysomnography at a mean of 7.5 weeks' gestation, and all had flow limitations at the nasal cannula without apnea or hypopnea. Nasal CPAP was used in all participants at an initial pressure of 5–6 cm H2O, and in eight women the pressure was increased to 6–9 cm H2O at between 5 and 6 months' gestation.
Of the seven women with hypertension at baseline, diastolic blood pressure below 90 mm Hg was maintained without a change in medication. All seven delivered healthy, full-term infants, as did one of the women with a history of preeclampsia, reported Dr. Guilleminault, of the department of psychiatry at Stanford (Calif.) University. One of the obese women miscarried at near 14 weeks' gestation and the other delivered at 34 weeks, but did not develop preeclampsia.
The third obese patient and the second woman with prior preeclampsia developed clinical features of preeclampsia, and both underwent cesarean section at 7.5 months' gestation.
Systematic treatment with nasal CPAP initiated prior to 9 weeks' gestation was associated with stable blood pressure and normal pregnancy in most women with risk factors for preeclampsia, Dr. Guilleminault concluded.
SALT LAKE CITY — Use of continuous positive airway pressure may help prevent preeclampsia in pregnant women at risk for the condition, a study suggests.
In 9 of 12 women with risk factors for preeclampsia who used continuous positive airway pressure (CPAP) and medical therapy beginning before 9 weeks' gestation, blood pressure remained stable and pregnancy was normal, Dr. Christian Guilleminault reported in a poster at the annual meeting of the Associated Professional Sleep Societies.
Sleep-disordered breathing has been suggested by several studies as a possible contributor to preeclampsia. In one study, snoring was linked with preeclampsia, with the disorder occurring in 10% of snorers compared with 4% of nonsnorers. In another study, snoring was shown to be a significant predictor of hypertension and fetal growth retardation, even after controlling for maternal weight, age, and smoking status.
Based on such findings, some researchers have recommended polysomnography and/or CPAP use in pregnant women with risk factors—including snoring—for preeclampsia. In the current study, all 12 participants had risk factors for preeclampsia: All were snorers; seven had hypertension; two had prior preeclampsia; and three were obese, with a body mass index (kg/m
The women, who had a mean age of 29 years, underwent polysomnography at a mean of 7.5 weeks' gestation, and all had flow limitations at the nasal cannula without apnea or hypopnea. Nasal CPAP was used in all participants at an initial pressure of 5–6 cm H2O, and in eight women the pressure was increased to 6–9 cm H2O at between 5 and 6 months' gestation.
Of the seven women with hypertension at baseline, diastolic blood pressure below 90 mm Hg was maintained without a change in medication. All seven delivered healthy, full-term infants, as did one of the women with a history of preeclampsia, reported Dr. Guilleminault, of the department of psychiatry at Stanford (Calif.) University. One of the obese women miscarried at near 14 weeks' gestation and the other delivered at 34 weeks, but did not develop preeclampsia.
The third obese patient and the second woman with prior preeclampsia developed clinical features of preeclampsia, and both underwent cesarean section at 7.5 months' gestation.
Systematic treatment with nasal CPAP initiated prior to 9 weeks' gestation was associated with stable blood pressure and normal pregnancy in most women with risk factors for preeclampsia, Dr. Guilleminault concluded.
SALT LAKE CITY — Use of continuous positive airway pressure may help prevent preeclampsia in pregnant women at risk for the condition, a study suggests.
In 9 of 12 women with risk factors for preeclampsia who used continuous positive airway pressure (CPAP) and medical therapy beginning before 9 weeks' gestation, blood pressure remained stable and pregnancy was normal, Dr. Christian Guilleminault reported in a poster at the annual meeting of the Associated Professional Sleep Societies.
Sleep-disordered breathing has been suggested by several studies as a possible contributor to preeclampsia. In one study, snoring was linked with preeclampsia, with the disorder occurring in 10% of snorers compared with 4% of nonsnorers. In another study, snoring was shown to be a significant predictor of hypertension and fetal growth retardation, even after controlling for maternal weight, age, and smoking status.
Based on such findings, some researchers have recommended polysomnography and/or CPAP use in pregnant women with risk factors—including snoring—for preeclampsia. In the current study, all 12 participants had risk factors for preeclampsia: All were snorers; seven had hypertension; two had prior preeclampsia; and three were obese, with a body mass index (kg/m
The women, who had a mean age of 29 years, underwent polysomnography at a mean of 7.5 weeks' gestation, and all had flow limitations at the nasal cannula without apnea or hypopnea. Nasal CPAP was used in all participants at an initial pressure of 5–6 cm H2O, and in eight women the pressure was increased to 6–9 cm H2O at between 5 and 6 months' gestation.
Of the seven women with hypertension at baseline, diastolic blood pressure below 90 mm Hg was maintained without a change in medication. All seven delivered healthy, full-term infants, as did one of the women with a history of preeclampsia, reported Dr. Guilleminault, of the department of psychiatry at Stanford (Calif.) University. One of the obese women miscarried at near 14 weeks' gestation and the other delivered at 34 weeks, but did not develop preeclampsia.
The third obese patient and the second woman with prior preeclampsia developed clinical features of preeclampsia, and both underwent cesarean section at 7.5 months' gestation.
Systematic treatment with nasal CPAP initiated prior to 9 weeks' gestation was associated with stable blood pressure and normal pregnancy in most women with risk factors for preeclampsia, Dr. Guilleminault concluded.
Risks Called Manageable for the Mature Gravida : Consider treating every visit as a preconception visit because many later pregnancies are unintended.
ASHEVILLE, N.C. — The potential pitfalls around conception and childbirth can be daunting—and more so in women over age 35—but the physician can help ensure better outcomes by addressing concerns head-on and being supportive, said Dr. James E. Ferguson II at the Southern Obstetric and Gynecologic Seminar.
Patients would be better served if physicians approached the situation with optimism rather than pessimism, despite increasing odds against successful conception and complication-free childbirth as women age, he said.
It's no secret that pregnancies are being delayed, said Dr. Ferguson, chairman of the obstetrics and gynecology department at the University of Kentucky.
Data on older mothers generally show that postpartum and intrapartum complications—such as obstructed labor, prolonged labor, preeclampsia, and prematurity—are much higher in women over age 40, compared with those aged 20–29, he said.
But there are some contradictions. For instance, the First and Second Trimester Evaluation of Risk of Aneuploidy (FASTER) study, conducted from 1999 to 2002, found no difference in gestational hypertension or preeclampsia between the under- and over-35 groups (Obstet. Gynecol. 2005;105:983–90). One caveat: The study population was mostly healthy, wealthy, and white, said Dr. Ferguson. The risk of spontaneous abortion was higher in the over-40 group, as were chromosomal abnormalities, low birth weight, and perinatal loss.
The likelihood of both maternal and fetal death also increases with age, Dr. Ferguson noted. But it doesn't have to be all bad news, he said. For instance, a mid-1990s study found that there were 4 fetal deaths per 1,000 live births in 34-year-old mothers, compared with 6–9 per 1,000 in 39-year-old mothers (N. Engl. J. Med. 1995;333:1,002–4). Viewed from an individual's perspective, those aren't such bad odds, said Dr. Ferguson. “I personally find this very reassuring rather than very frightening,” he said.
Similarly, though maternal deaths increase significantly after age 39, the rate per 100,000 live births overall is still fairly low, said Dr. Ferguson. Compared with 25- to 29-year-olds, the relative risk of death is 2.3 for the 35- to 39-year-olds and 5.0 for women over 40, according to one study (Obstet. Gynecol. 2003;101:1,015–21). “It's much less significant than it is in the patient's mind,” he said, adding, “You can reassure her.”
Causes of death include pulmonary embolism, pregnancy-induced hypertension, and hemorrhage, either related or not related to ectopic pregnancy. Women over age 40 are at risk because of increased incidence of underlying medical conditions, more postpartum complications, and a higher likelihood of cesarean section. There have been few studies in women over 45, but the few conducted have shown that about half the pregnancies involved complications, and that the risk was much greater for first-time mothers.
Because of the common tendency to put off childbearing—and because almost half of pregnancies are unintended—gynecologists might consider treating every patient office visit as a preconception visit, said Dr. Ferguson.
Patients should be quizzed about medical and immunization histories, and counseled on risks and genetic testing. Physicians should also offer help with reducing risky behavior like smoking and alcohol use.
Women should also be made aware of the latest research on birth spacing—a child born less than 18 months or more than 50 months after the previous birth has a greater risk of bad perinatal outcome, he noted (JAMA 2006;295:1809–23).
For the older mother, “there are some special risks, but I think they are manageable,” Dr. Ferguson said.
ASHEVILLE, N.C. — The potential pitfalls around conception and childbirth can be daunting—and more so in women over age 35—but the physician can help ensure better outcomes by addressing concerns head-on and being supportive, said Dr. James E. Ferguson II at the Southern Obstetric and Gynecologic Seminar.
Patients would be better served if physicians approached the situation with optimism rather than pessimism, despite increasing odds against successful conception and complication-free childbirth as women age, he said.
It's no secret that pregnancies are being delayed, said Dr. Ferguson, chairman of the obstetrics and gynecology department at the University of Kentucky.
Data on older mothers generally show that postpartum and intrapartum complications—such as obstructed labor, prolonged labor, preeclampsia, and prematurity—are much higher in women over age 40, compared with those aged 20–29, he said.
But there are some contradictions. For instance, the First and Second Trimester Evaluation of Risk of Aneuploidy (FASTER) study, conducted from 1999 to 2002, found no difference in gestational hypertension or preeclampsia between the under- and over-35 groups (Obstet. Gynecol. 2005;105:983–90). One caveat: The study population was mostly healthy, wealthy, and white, said Dr. Ferguson. The risk of spontaneous abortion was higher in the over-40 group, as were chromosomal abnormalities, low birth weight, and perinatal loss.
The likelihood of both maternal and fetal death also increases with age, Dr. Ferguson noted. But it doesn't have to be all bad news, he said. For instance, a mid-1990s study found that there were 4 fetal deaths per 1,000 live births in 34-year-old mothers, compared with 6–9 per 1,000 in 39-year-old mothers (N. Engl. J. Med. 1995;333:1,002–4). Viewed from an individual's perspective, those aren't such bad odds, said Dr. Ferguson. “I personally find this very reassuring rather than very frightening,” he said.
Similarly, though maternal deaths increase significantly after age 39, the rate per 100,000 live births overall is still fairly low, said Dr. Ferguson. Compared with 25- to 29-year-olds, the relative risk of death is 2.3 for the 35- to 39-year-olds and 5.0 for women over 40, according to one study (Obstet. Gynecol. 2003;101:1,015–21). “It's much less significant than it is in the patient's mind,” he said, adding, “You can reassure her.”
Causes of death include pulmonary embolism, pregnancy-induced hypertension, and hemorrhage, either related or not related to ectopic pregnancy. Women over age 40 are at risk because of increased incidence of underlying medical conditions, more postpartum complications, and a higher likelihood of cesarean section. There have been few studies in women over 45, but the few conducted have shown that about half the pregnancies involved complications, and that the risk was much greater for first-time mothers.
Because of the common tendency to put off childbearing—and because almost half of pregnancies are unintended—gynecologists might consider treating every patient office visit as a preconception visit, said Dr. Ferguson.
Patients should be quizzed about medical and immunization histories, and counseled on risks and genetic testing. Physicians should also offer help with reducing risky behavior like smoking and alcohol use.
Women should also be made aware of the latest research on birth spacing—a child born less than 18 months or more than 50 months after the previous birth has a greater risk of bad perinatal outcome, he noted (JAMA 2006;295:1809–23).
For the older mother, “there are some special risks, but I think they are manageable,” Dr. Ferguson said.
ASHEVILLE, N.C. — The potential pitfalls around conception and childbirth can be daunting—and more so in women over age 35—but the physician can help ensure better outcomes by addressing concerns head-on and being supportive, said Dr. James E. Ferguson II at the Southern Obstetric and Gynecologic Seminar.
Patients would be better served if physicians approached the situation with optimism rather than pessimism, despite increasing odds against successful conception and complication-free childbirth as women age, he said.
It's no secret that pregnancies are being delayed, said Dr. Ferguson, chairman of the obstetrics and gynecology department at the University of Kentucky.
Data on older mothers generally show that postpartum and intrapartum complications—such as obstructed labor, prolonged labor, preeclampsia, and prematurity—are much higher in women over age 40, compared with those aged 20–29, he said.
But there are some contradictions. For instance, the First and Second Trimester Evaluation of Risk of Aneuploidy (FASTER) study, conducted from 1999 to 2002, found no difference in gestational hypertension or preeclampsia between the under- and over-35 groups (Obstet. Gynecol. 2005;105:983–90). One caveat: The study population was mostly healthy, wealthy, and white, said Dr. Ferguson. The risk of spontaneous abortion was higher in the over-40 group, as were chromosomal abnormalities, low birth weight, and perinatal loss.
The likelihood of both maternal and fetal death also increases with age, Dr. Ferguson noted. But it doesn't have to be all bad news, he said. For instance, a mid-1990s study found that there were 4 fetal deaths per 1,000 live births in 34-year-old mothers, compared with 6–9 per 1,000 in 39-year-old mothers (N. Engl. J. Med. 1995;333:1,002–4). Viewed from an individual's perspective, those aren't such bad odds, said Dr. Ferguson. “I personally find this very reassuring rather than very frightening,” he said.
Similarly, though maternal deaths increase significantly after age 39, the rate per 100,000 live births overall is still fairly low, said Dr. Ferguson. Compared with 25- to 29-year-olds, the relative risk of death is 2.3 for the 35- to 39-year-olds and 5.0 for women over 40, according to one study (Obstet. Gynecol. 2003;101:1,015–21). “It's much less significant than it is in the patient's mind,” he said, adding, “You can reassure her.”
Causes of death include pulmonary embolism, pregnancy-induced hypertension, and hemorrhage, either related or not related to ectopic pregnancy. Women over age 40 are at risk because of increased incidence of underlying medical conditions, more postpartum complications, and a higher likelihood of cesarean section. There have been few studies in women over 45, but the few conducted have shown that about half the pregnancies involved complications, and that the risk was much greater for first-time mothers.
Because of the common tendency to put off childbearing—and because almost half of pregnancies are unintended—gynecologists might consider treating every patient office visit as a preconception visit, said Dr. Ferguson.
Patients should be quizzed about medical and immunization histories, and counseled on risks and genetic testing. Physicians should also offer help with reducing risky behavior like smoking and alcohol use.
Women should also be made aware of the latest research on birth spacing—a child born less than 18 months or more than 50 months after the previous birth has a greater risk of bad perinatal outcome, he noted (JAMA 2006;295:1809–23).
For the older mother, “there are some special risks, but I think they are manageable,” Dr. Ferguson said.
Vancomycin Crosses Placental Barrier, Study Finds
MONTEREY, CALIF. — The first in vivo study of the pharmacokinetics of IV vancomycin in pregnant women found that it crosses the placental barrier, and that the dose recommended for prophylaxis against neonatal group B streptococcus infection may be too high, Dr. Joann Laiprasert said.
Penicillin is the first-line choice for prophylactic treatment of pregnant women colonized with group B streptococcus (GBS) at 35–37 weeks' gestation. For women with penicillin allergy who have a high risk for anaphylaxis, clindamycin is the preferred drug, but 15%–30% of GBS isolates are resistant to clindamycin. Erythromycin should not be used in this situation because it has a similar resistance pattern and its passage through the placenta is incomplete.
Vancomycin is recommended for pregnant women at high risk of anaphylaxis from penicillin who have clindamycin-resistant GBS, she explained at the annual meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
In the study, 13 healthy pregnant women with no indications for antibiotics volunteered to receive 1 g of intravenous vancomycin. Seven women (54%) did not get the full dose because they developed symptoms of Red Man's syndrome, and the drug was stopped. Despite not getting the full dose, all women and fetuses had serum levels of vancomycin above the 1 mcg/mL breakpoint for effective prophylaxis against neonatal GBS, reported Dr. Laiprasert of the University of Michigan, Ann Arbor, and her associates.
The investigators extrapolated the data to model the effects if all women had received 1-g doses and calculated that this would produce supertherapeutic serum levels of the drug in the mother and the fetus. A second model in which all women received only 500 mg of vancomycin still would have resulted in therapeutic levels in maternal and fetal compartments.
The first four women in the study received the drug in 60-minute infusions, and an interim analysis found that three of them (75%) developed symptoms of Red Man's syndrome: pruritus, shortness of breath, rash, or hypotension. For the following nine patients, the infusion duration was lengthened to 90 minutes, and four of them (44%) developed symptoms of Red Man's syndrome.
One woman required treatment with saturated oxygen after developing a moderately severe reaction with symptoms of hypotension, shortness of breath, and oxygen desaturation. Investigators stopped the study after that, with enough data to draw conclusions about vancomycin's pharmacokinetics. Therapeutic drug levels were seen in fetal serum within 30 minutes after completing the 90-minute infusion (120 minutes after starting the infusion) and persisted for 8 hours.
Because less than the recommended 1-g dose of vancomycin produced therapeutic serum levels and because Red Man's syndrome was so common, a 500-mg dose should be considered adequate, Dr. Laiprasert suggested. A slower infusion rate also may be important for safe administration of vancomycin during pregnancy. Redosing vancomycin at 12-hour intervals seems reasonable if needed, she added.
The investigators collected amniotic fluid samples and plan to study them to improve understanding of the metabolism of vancomycin in the fetal circulation.
Dr. Mark D. Pearlman, one of Dr. Laiprasert's associates in the study, commented after her talk that using a lower dose of vancomycin in these patients makes sense. Other treatment options also need to be studied for women with clindamycin-resistant GBS who are at high risk for anaphylaxis from penicillin, added Dr. Pearlman of the university.
“We really need to pressure the FDA and others to start to look at other non-β-lactams that are active against GBS, to have alternatives to vancomycin,” he said. “There are a whole host of newer, extended-spectrum, gram-positive drugs that have great activity against clindamycin-resistant GBS.”
MONTEREY, CALIF. — The first in vivo study of the pharmacokinetics of IV vancomycin in pregnant women found that it crosses the placental barrier, and that the dose recommended for prophylaxis against neonatal group B streptococcus infection may be too high, Dr. Joann Laiprasert said.
Penicillin is the first-line choice for prophylactic treatment of pregnant women colonized with group B streptococcus (GBS) at 35–37 weeks' gestation. For women with penicillin allergy who have a high risk for anaphylaxis, clindamycin is the preferred drug, but 15%–30% of GBS isolates are resistant to clindamycin. Erythromycin should not be used in this situation because it has a similar resistance pattern and its passage through the placenta is incomplete.
Vancomycin is recommended for pregnant women at high risk of anaphylaxis from penicillin who have clindamycin-resistant GBS, she explained at the annual meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
In the study, 13 healthy pregnant women with no indications for antibiotics volunteered to receive 1 g of intravenous vancomycin. Seven women (54%) did not get the full dose because they developed symptoms of Red Man's syndrome, and the drug was stopped. Despite not getting the full dose, all women and fetuses had serum levels of vancomycin above the 1 mcg/mL breakpoint for effective prophylaxis against neonatal GBS, reported Dr. Laiprasert of the University of Michigan, Ann Arbor, and her associates.
The investigators extrapolated the data to model the effects if all women had received 1-g doses and calculated that this would produce supertherapeutic serum levels of the drug in the mother and the fetus. A second model in which all women received only 500 mg of vancomycin still would have resulted in therapeutic levels in maternal and fetal compartments.
The first four women in the study received the drug in 60-minute infusions, and an interim analysis found that three of them (75%) developed symptoms of Red Man's syndrome: pruritus, shortness of breath, rash, or hypotension. For the following nine patients, the infusion duration was lengthened to 90 minutes, and four of them (44%) developed symptoms of Red Man's syndrome.
One woman required treatment with saturated oxygen after developing a moderately severe reaction with symptoms of hypotension, shortness of breath, and oxygen desaturation. Investigators stopped the study after that, with enough data to draw conclusions about vancomycin's pharmacokinetics. Therapeutic drug levels were seen in fetal serum within 30 minutes after completing the 90-minute infusion (120 minutes after starting the infusion) and persisted for 8 hours.
Because less than the recommended 1-g dose of vancomycin produced therapeutic serum levels and because Red Man's syndrome was so common, a 500-mg dose should be considered adequate, Dr. Laiprasert suggested. A slower infusion rate also may be important for safe administration of vancomycin during pregnancy. Redosing vancomycin at 12-hour intervals seems reasonable if needed, she added.
The investigators collected amniotic fluid samples and plan to study them to improve understanding of the metabolism of vancomycin in the fetal circulation.
Dr. Mark D. Pearlman, one of Dr. Laiprasert's associates in the study, commented after her talk that using a lower dose of vancomycin in these patients makes sense. Other treatment options also need to be studied for women with clindamycin-resistant GBS who are at high risk for anaphylaxis from penicillin, added Dr. Pearlman of the university.
“We really need to pressure the FDA and others to start to look at other non-β-lactams that are active against GBS, to have alternatives to vancomycin,” he said. “There are a whole host of newer, extended-spectrum, gram-positive drugs that have great activity against clindamycin-resistant GBS.”
MONTEREY, CALIF. — The first in vivo study of the pharmacokinetics of IV vancomycin in pregnant women found that it crosses the placental barrier, and that the dose recommended for prophylaxis against neonatal group B streptococcus infection may be too high, Dr. Joann Laiprasert said.
Penicillin is the first-line choice for prophylactic treatment of pregnant women colonized with group B streptococcus (GBS) at 35–37 weeks' gestation. For women with penicillin allergy who have a high risk for anaphylaxis, clindamycin is the preferred drug, but 15%–30% of GBS isolates are resistant to clindamycin. Erythromycin should not be used in this situation because it has a similar resistance pattern and its passage through the placenta is incomplete.
Vancomycin is recommended for pregnant women at high risk of anaphylaxis from penicillin who have clindamycin-resistant GBS, she explained at the annual meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
In the study, 13 healthy pregnant women with no indications for antibiotics volunteered to receive 1 g of intravenous vancomycin. Seven women (54%) did not get the full dose because they developed symptoms of Red Man's syndrome, and the drug was stopped. Despite not getting the full dose, all women and fetuses had serum levels of vancomycin above the 1 mcg/mL breakpoint for effective prophylaxis against neonatal GBS, reported Dr. Laiprasert of the University of Michigan, Ann Arbor, and her associates.
The investigators extrapolated the data to model the effects if all women had received 1-g doses and calculated that this would produce supertherapeutic serum levels of the drug in the mother and the fetus. A second model in which all women received only 500 mg of vancomycin still would have resulted in therapeutic levels in maternal and fetal compartments.
The first four women in the study received the drug in 60-minute infusions, and an interim analysis found that three of them (75%) developed symptoms of Red Man's syndrome: pruritus, shortness of breath, rash, or hypotension. For the following nine patients, the infusion duration was lengthened to 90 minutes, and four of them (44%) developed symptoms of Red Man's syndrome.
One woman required treatment with saturated oxygen after developing a moderately severe reaction with symptoms of hypotension, shortness of breath, and oxygen desaturation. Investigators stopped the study after that, with enough data to draw conclusions about vancomycin's pharmacokinetics. Therapeutic drug levels were seen in fetal serum within 30 minutes after completing the 90-minute infusion (120 minutes after starting the infusion) and persisted for 8 hours.
Because less than the recommended 1-g dose of vancomycin produced therapeutic serum levels and because Red Man's syndrome was so common, a 500-mg dose should be considered adequate, Dr. Laiprasert suggested. A slower infusion rate also may be important for safe administration of vancomycin during pregnancy. Redosing vancomycin at 12-hour intervals seems reasonable if needed, she added.
The investigators collected amniotic fluid samples and plan to study them to improve understanding of the metabolism of vancomycin in the fetal circulation.
Dr. Mark D. Pearlman, one of Dr. Laiprasert's associates in the study, commented after her talk that using a lower dose of vancomycin in these patients makes sense. Other treatment options also need to be studied for women with clindamycin-resistant GBS who are at high risk for anaphylaxis from penicillin, added Dr. Pearlman of the university.
“We really need to pressure the FDA and others to start to look at other non-β-lactams that are active against GBS, to have alternatives to vancomycin,” he said. “There are a whole host of newer, extended-spectrum, gram-positive drugs that have great activity against clindamycin-resistant GBS.”
Circulating Soluble Endoglin Predicts Preeclampsia
An early and steep rise in circulating levels of soluble endoglin appears to predict preeclampsia, reported Dr. Richard J. Levine of the National Institute of Child Health and Human Development, Bethesda, Md., and his associates.
Pregnant women's circulating levels of soluble endoglin, an antiangiogenic protein, appears to rise markedly 2–3 months before the onset of preeclampsia. This rise is accompanied by a similar rise in the ratio of another antiangiogenic protein—soluble fms-like tyrosine kinase 1—to placental growth factor (the sFlt-1:PlGF ratio). “Taken together with experimental evidence in rodents, these data suggest that circulating soluble endoglin and sFlt-1, each of which causes endothelial dysfunction by a different mechanism, may both contribute to the syndrome of preeclampsia,” Dr. Levine and his associates said.
“Prospective longitudinal studies are needed to assess whether these biomarkers can predict the imminent onset of clinical disease.”
In an accompanying editorial comment, Dr. Marshall D. Lindheimer and Dr. Jason G. Umans said these findings puts the measurement of antiangiogenic proteins “at the forefront of tests that are potentially useful for predicting preeclampsia.” The findings also point the way to both preventing and treating the disorder, they said.
To examine gestational patterns in levels of these proteins, Dr. Levine and his associates performed a nested case-control study using data and frozen serum samples from a subset of 3,630 women who had participated in the Calcium for Preeclampsia Prevention (CPEP) trial. The CPEP study, conducted in 1992–1995, was a randomized trial that assessed the disorder in healthy nulliparous women with singleton pregnancies.
Of these subjects, 2,469 were normotensive throughout pregnancy and delivered normal-sized infants; these women served as controls in the current study. Another 225 subjects were normotensive but gave birth to small-for-gestational-age (SGA) infants, 651 had gestational hypertension, 213 had preeclampsia at or after 37 weeks (term preeclampsia), and 72 had preeclampsia before 37 weeks' gestation (preterm preeclampsia). For the case-control study, serum specimens that had been obtained during pregnancy were evaluated for 120 subjects randomly selected from the first four categories and from all 72 subjects in the last category.
Serum levels of soluble endoglin rose during the last 2 months of normal pregnancies, but rose earlier and much more steeply in women who later developed preeclampsia, “reaching a peak at the onset of clinical disease,” the researchers said (N. Engl. J. Med. 2006;355:992–1005).
Beginning at 17–20 weeks' gestation, mean serum soluble endoglin levels were significantly higher in women who later developed preterm preeclampsia (10.2 ng/mL) than in controls (5.8 ng/mL). They then showed a steep rise at 33–36 weeks. Similarly, starting at 25–28 weeks' gestation, levels were significantly higher in women who later developed term preeclampsia (8.5 ng/mL) than in controls (5.9 ng/mL). Levels also rose steeply at 33–36 weeks.
After the onset of clinical disease, mean serum levels of soluble endoglin were markedly higher in women with preterm preeclampsia (46.4 ng/mL) than in matched controls (9.8 ng/mL) and were similarly higher in women with term preeclampsia (31.0 ng/mL) than in matched controls (13.3 ng/mL).
“Elevations in soluble endoglin were particularly pronounced—therefore, potentially most useful for prediction—among women in whom preterm preeclampsia developed or women in whom preeclampsia developed who had an SGA infant,” they said.
Increased levels of soluble endoglin were usually accompanied by increased sFlt-1:PlGF ratios. In addition, women with both biomarkers in the highest quartile at 21–32 weeks' gestation had an increased risk of preterm preeclampsia, and those with levels in the highest quartile at 33–42 weeks had an increased risk of term preeclampsia.
“The data can be interpreted to imply that soluble endoglin levels and the sFlt-1:PlGF ratio both contribute to the pathogenesis of preeclampsia. Indeed, a composite measure incorporating all three molecules—the ratio of (sFlt-1 plus soluble endoglin):PlGF—was more strongly predictive of preeclampsia than were individual biomarkers,” the authors said.
An early and steep rise in circulating levels of soluble endoglin appears to predict preeclampsia, reported Dr. Richard J. Levine of the National Institute of Child Health and Human Development, Bethesda, Md., and his associates.
Pregnant women's circulating levels of soluble endoglin, an antiangiogenic protein, appears to rise markedly 2–3 months before the onset of preeclampsia. This rise is accompanied by a similar rise in the ratio of another antiangiogenic protein—soluble fms-like tyrosine kinase 1—to placental growth factor (the sFlt-1:PlGF ratio). “Taken together with experimental evidence in rodents, these data suggest that circulating soluble endoglin and sFlt-1, each of which causes endothelial dysfunction by a different mechanism, may both contribute to the syndrome of preeclampsia,” Dr. Levine and his associates said.
“Prospective longitudinal studies are needed to assess whether these biomarkers can predict the imminent onset of clinical disease.”
In an accompanying editorial comment, Dr. Marshall D. Lindheimer and Dr. Jason G. Umans said these findings puts the measurement of antiangiogenic proteins “at the forefront of tests that are potentially useful for predicting preeclampsia.” The findings also point the way to both preventing and treating the disorder, they said.
To examine gestational patterns in levels of these proteins, Dr. Levine and his associates performed a nested case-control study using data and frozen serum samples from a subset of 3,630 women who had participated in the Calcium for Preeclampsia Prevention (CPEP) trial. The CPEP study, conducted in 1992–1995, was a randomized trial that assessed the disorder in healthy nulliparous women with singleton pregnancies.
Of these subjects, 2,469 were normotensive throughout pregnancy and delivered normal-sized infants; these women served as controls in the current study. Another 225 subjects were normotensive but gave birth to small-for-gestational-age (SGA) infants, 651 had gestational hypertension, 213 had preeclampsia at or after 37 weeks (term preeclampsia), and 72 had preeclampsia before 37 weeks' gestation (preterm preeclampsia). For the case-control study, serum specimens that had been obtained during pregnancy were evaluated for 120 subjects randomly selected from the first four categories and from all 72 subjects in the last category.
Serum levels of soluble endoglin rose during the last 2 months of normal pregnancies, but rose earlier and much more steeply in women who later developed preeclampsia, “reaching a peak at the onset of clinical disease,” the researchers said (N. Engl. J. Med. 2006;355:992–1005).
Beginning at 17–20 weeks' gestation, mean serum soluble endoglin levels were significantly higher in women who later developed preterm preeclampsia (10.2 ng/mL) than in controls (5.8 ng/mL). They then showed a steep rise at 33–36 weeks. Similarly, starting at 25–28 weeks' gestation, levels were significantly higher in women who later developed term preeclampsia (8.5 ng/mL) than in controls (5.9 ng/mL). Levels also rose steeply at 33–36 weeks.
After the onset of clinical disease, mean serum levels of soluble endoglin were markedly higher in women with preterm preeclampsia (46.4 ng/mL) than in matched controls (9.8 ng/mL) and were similarly higher in women with term preeclampsia (31.0 ng/mL) than in matched controls (13.3 ng/mL).
“Elevations in soluble endoglin were particularly pronounced—therefore, potentially most useful for prediction—among women in whom preterm preeclampsia developed or women in whom preeclampsia developed who had an SGA infant,” they said.
Increased levels of soluble endoglin were usually accompanied by increased sFlt-1:PlGF ratios. In addition, women with both biomarkers in the highest quartile at 21–32 weeks' gestation had an increased risk of preterm preeclampsia, and those with levels in the highest quartile at 33–42 weeks had an increased risk of term preeclampsia.
“The data can be interpreted to imply that soluble endoglin levels and the sFlt-1:PlGF ratio both contribute to the pathogenesis of preeclampsia. Indeed, a composite measure incorporating all three molecules—the ratio of (sFlt-1 plus soluble endoglin):PlGF—was more strongly predictive of preeclampsia than were individual biomarkers,” the authors said.
An early and steep rise in circulating levels of soluble endoglin appears to predict preeclampsia, reported Dr. Richard J. Levine of the National Institute of Child Health and Human Development, Bethesda, Md., and his associates.
Pregnant women's circulating levels of soluble endoglin, an antiangiogenic protein, appears to rise markedly 2–3 months before the onset of preeclampsia. This rise is accompanied by a similar rise in the ratio of another antiangiogenic protein—soluble fms-like tyrosine kinase 1—to placental growth factor (the sFlt-1:PlGF ratio). “Taken together with experimental evidence in rodents, these data suggest that circulating soluble endoglin and sFlt-1, each of which causes endothelial dysfunction by a different mechanism, may both contribute to the syndrome of preeclampsia,” Dr. Levine and his associates said.
“Prospective longitudinal studies are needed to assess whether these biomarkers can predict the imminent onset of clinical disease.”
In an accompanying editorial comment, Dr. Marshall D. Lindheimer and Dr. Jason G. Umans said these findings puts the measurement of antiangiogenic proteins “at the forefront of tests that are potentially useful for predicting preeclampsia.” The findings also point the way to both preventing and treating the disorder, they said.
To examine gestational patterns in levels of these proteins, Dr. Levine and his associates performed a nested case-control study using data and frozen serum samples from a subset of 3,630 women who had participated in the Calcium for Preeclampsia Prevention (CPEP) trial. The CPEP study, conducted in 1992–1995, was a randomized trial that assessed the disorder in healthy nulliparous women with singleton pregnancies.
Of these subjects, 2,469 were normotensive throughout pregnancy and delivered normal-sized infants; these women served as controls in the current study. Another 225 subjects were normotensive but gave birth to small-for-gestational-age (SGA) infants, 651 had gestational hypertension, 213 had preeclampsia at or after 37 weeks (term preeclampsia), and 72 had preeclampsia before 37 weeks' gestation (preterm preeclampsia). For the case-control study, serum specimens that had been obtained during pregnancy were evaluated for 120 subjects randomly selected from the first four categories and from all 72 subjects in the last category.
Serum levels of soluble endoglin rose during the last 2 months of normal pregnancies, but rose earlier and much more steeply in women who later developed preeclampsia, “reaching a peak at the onset of clinical disease,” the researchers said (N. Engl. J. Med. 2006;355:992–1005).
Beginning at 17–20 weeks' gestation, mean serum soluble endoglin levels were significantly higher in women who later developed preterm preeclampsia (10.2 ng/mL) than in controls (5.8 ng/mL). They then showed a steep rise at 33–36 weeks. Similarly, starting at 25–28 weeks' gestation, levels were significantly higher in women who later developed term preeclampsia (8.5 ng/mL) than in controls (5.9 ng/mL). Levels also rose steeply at 33–36 weeks.
After the onset of clinical disease, mean serum levels of soluble endoglin were markedly higher in women with preterm preeclampsia (46.4 ng/mL) than in matched controls (9.8 ng/mL) and were similarly higher in women with term preeclampsia (31.0 ng/mL) than in matched controls (13.3 ng/mL).
“Elevations in soluble endoglin were particularly pronounced—therefore, potentially most useful for prediction—among women in whom preterm preeclampsia developed or women in whom preeclampsia developed who had an SGA infant,” they said.
Increased levels of soluble endoglin were usually accompanied by increased sFlt-1:PlGF ratios. In addition, women with both biomarkers in the highest quartile at 21–32 weeks' gestation had an increased risk of preterm preeclampsia, and those with levels in the highest quartile at 33–42 weeks had an increased risk of term preeclampsia.
“The data can be interpreted to imply that soluble endoglin levels and the sFlt-1:PlGF ratio both contribute to the pathogenesis of preeclampsia. Indeed, a composite measure incorporating all three molecules—the ratio of (sFlt-1 plus soluble endoglin):PlGF—was more strongly predictive of preeclampsia than were individual biomarkers,” the authors said.
Summer Viruses May Play Role in Low Birth Weight
MONTEREY, CALIF. — July and August are the peak months for delivering babies weighing less than 1,500 g, but the summer months are not associated with preterm birth, according to a retrospective review from the University of Southern California's Women and Children's Hospital.
The study of 4,108 singleton live births in the 3 years from 2002 through 2004 found no seasonality to preterm deliveries, defined as births before 38 weeks' gestational age. The increase in low-birth-weight babies in summer may correlate with seasonal “summer virus” infections including enteroviruses, adenoviruses, and others, Dr. Ozlem Equils and her associates reported in a poster presentation at the annual meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
In 2002, approximately 13% of babies born in August were low birth weight. About 7% were low birth weight in July 2003, and 6% in July 2004, said Dr. Equils of Cedars-Sinai Medical Center, Los Angeles. Those proportions were higher than in other months of the same years.
Bacterial and viral infections have been associated with preterm birth in other epidemiologic studies, and animal studies have shown an association between infection with enterovirus and preterm birth.
“We know that enterovirus has caused nursing outbreaks—the mother and the baby get sick. We think that it may play a role with these small babies,” Dr. Equils suggested in an interview at the poster.
Obstetricians may want to follow the viral outbreaks in their communities and emphasize infection prevention with patients, she added. If enterovirus is going around, for example, diligent hand washing and perhaps limiting intercourse in the later part of pregnancy may be advisable.
The investigators hope to study placenta samples to see if viruses are present and associated with seasonality and low birth weight. If further research supports these very preliminary associations, it may be possible to identify the presence of viruses from maternal cervical secretions and offer treatment, potentially reducing low-birth-weight deliveries, she speculated.
Previous studies of seasonality and preterm delivery come mainly from developing countries where factors such as droughts and starvation confound the data. It's also difficult to extrapolate observations on seasonality and preterm birth from developed countries in the 1960s through 1980s because of changing environmental conditions and other factors, such as more women entering the labor force, she said.
MONTEREY, CALIF. — July and August are the peak months for delivering babies weighing less than 1,500 g, but the summer months are not associated with preterm birth, according to a retrospective review from the University of Southern California's Women and Children's Hospital.
The study of 4,108 singleton live births in the 3 years from 2002 through 2004 found no seasonality to preterm deliveries, defined as births before 38 weeks' gestational age. The increase in low-birth-weight babies in summer may correlate with seasonal “summer virus” infections including enteroviruses, adenoviruses, and others, Dr. Ozlem Equils and her associates reported in a poster presentation at the annual meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
In 2002, approximately 13% of babies born in August were low birth weight. About 7% were low birth weight in July 2003, and 6% in July 2004, said Dr. Equils of Cedars-Sinai Medical Center, Los Angeles. Those proportions were higher than in other months of the same years.
Bacterial and viral infections have been associated with preterm birth in other epidemiologic studies, and animal studies have shown an association between infection with enterovirus and preterm birth.
“We know that enterovirus has caused nursing outbreaks—the mother and the baby get sick. We think that it may play a role with these small babies,” Dr. Equils suggested in an interview at the poster.
Obstetricians may want to follow the viral outbreaks in their communities and emphasize infection prevention with patients, she added. If enterovirus is going around, for example, diligent hand washing and perhaps limiting intercourse in the later part of pregnancy may be advisable.
The investigators hope to study placenta samples to see if viruses are present and associated with seasonality and low birth weight. If further research supports these very preliminary associations, it may be possible to identify the presence of viruses from maternal cervical secretions and offer treatment, potentially reducing low-birth-weight deliveries, she speculated.
Previous studies of seasonality and preterm delivery come mainly from developing countries where factors such as droughts and starvation confound the data. It's also difficult to extrapolate observations on seasonality and preterm birth from developed countries in the 1960s through 1980s because of changing environmental conditions and other factors, such as more women entering the labor force, she said.
MONTEREY, CALIF. — July and August are the peak months for delivering babies weighing less than 1,500 g, but the summer months are not associated with preterm birth, according to a retrospective review from the University of Southern California's Women and Children's Hospital.
The study of 4,108 singleton live births in the 3 years from 2002 through 2004 found no seasonality to preterm deliveries, defined as births before 38 weeks' gestational age. The increase in low-birth-weight babies in summer may correlate with seasonal “summer virus” infections including enteroviruses, adenoviruses, and others, Dr. Ozlem Equils and her associates reported in a poster presentation at the annual meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
In 2002, approximately 13% of babies born in August were low birth weight. About 7% were low birth weight in July 2003, and 6% in July 2004, said Dr. Equils of Cedars-Sinai Medical Center, Los Angeles. Those proportions were higher than in other months of the same years.
Bacterial and viral infections have been associated with preterm birth in other epidemiologic studies, and animal studies have shown an association between infection with enterovirus and preterm birth.
“We know that enterovirus has caused nursing outbreaks—the mother and the baby get sick. We think that it may play a role with these small babies,” Dr. Equils suggested in an interview at the poster.
Obstetricians may want to follow the viral outbreaks in their communities and emphasize infection prevention with patients, she added. If enterovirus is going around, for example, diligent hand washing and perhaps limiting intercourse in the later part of pregnancy may be advisable.
The investigators hope to study placenta samples to see if viruses are present and associated with seasonality and low birth weight. If further research supports these very preliminary associations, it may be possible to identify the presence of viruses from maternal cervical secretions and offer treatment, potentially reducing low-birth-weight deliveries, she speculated.
Previous studies of seasonality and preterm delivery come mainly from developing countries where factors such as droughts and starvation confound the data. It's also difficult to extrapolate observations on seasonality and preterm birth from developed countries in the 1960s through 1980s because of changing environmental conditions and other factors, such as more women entering the labor force, she said.