User login
AstraZeneca recalls lot of Brilinta in US
AstraZeneca has announced a voluntary recall of 1 lot of professional (physician) sample bottles containing 8 tablets of Brilinta® (ticagrelor).
This recall follows a report that a professional sample bottle containing 8 tablets of Brilinta 90 mg also contained Zurampic® (lesinurad) 200 mg tablets, which is also manufactured by AstraZeneca.
The company said this precautionary measure is limited to 1 lot of Brilinta (Brilinta lot #JB5047) distributed to physicians in the US between March and April of 2017.
Other forms and dosage strengths of Brilinta, including medicine obtained via US retail or mail order pharmacies, are not affected by this recall. And this recall does not affect Zurampic.
Potential risks
Unintentional dosing with Zurampic has the potential to lead to adverse renal effects, including acute renal failure, which is more common when Zurampic is given alone, as it should be used in combination with a xanthine oxidase inhibitor.
Missed doses of Brilinta increase the risk of heart attack and stroke. People with a stent who miss doses of Brilinta have a higher risk of stent thrombosis, heart attack, and death. Patients should not stop taking Brilinta without talking to their prescribing doctor.
To date, AstraZeneca has not received any reports of adverse events related to this recall.
Next steps
AstraZeneca is notifying physicians by recall letter and is arranging for the return of all recalled products. Consumers who have medicine that is being recalled should contact their physician.
Consumers with questions regarding this recall can contact the AstraZeneca Information Center at 1-800-236-9933 between the hours of 8 am and 6 pm (Eastern time) Monday to Friday, excluding holidays.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using Brilinta.
Adverse reactions or quality problems related to Brilinta may also be reported to the FDA’s MedWatch Adverse Event Reporting Program.
About Brilinta and Zurampic
Brilinta is intended for use to reduce the rate of cardiovascular death, heart attack, and stroke in patients with acute coronary syndrome or a history of heart attack.
Brilinta is also intended to reduce the rate of stent thrombosis in patients who have been stented for treatment of acute coronary syndrome.
Zurampic is used together with a xanthine oxidase inhibitor, such as allopurinol or Uloric, in adults with gout who still have a high uric acid level.
Brilinta 90 mg tablets are supplied as a round, biconvex, yellow, film-coated tablet, and imprinted with a “90” above a “T” on one side of the pill.
Zurampic tablets 200 mg are blue in color and elliptical/oval in shape. They are imprinted with “LES200” on one side of the pill. ![]()
AstraZeneca has announced a voluntary recall of 1 lot of professional (physician) sample bottles containing 8 tablets of Brilinta® (ticagrelor).
This recall follows a report that a professional sample bottle containing 8 tablets of Brilinta 90 mg also contained Zurampic® (lesinurad) 200 mg tablets, which is also manufactured by AstraZeneca.
The company said this precautionary measure is limited to 1 lot of Brilinta (Brilinta lot #JB5047) distributed to physicians in the US between March and April of 2017.
Other forms and dosage strengths of Brilinta, including medicine obtained via US retail or mail order pharmacies, are not affected by this recall. And this recall does not affect Zurampic.
Potential risks
Unintentional dosing with Zurampic has the potential to lead to adverse renal effects, including acute renal failure, which is more common when Zurampic is given alone, as it should be used in combination with a xanthine oxidase inhibitor.
Missed doses of Brilinta increase the risk of heart attack and stroke. People with a stent who miss doses of Brilinta have a higher risk of stent thrombosis, heart attack, and death. Patients should not stop taking Brilinta without talking to their prescribing doctor.
To date, AstraZeneca has not received any reports of adverse events related to this recall.
Next steps
AstraZeneca is notifying physicians by recall letter and is arranging for the return of all recalled products. Consumers who have medicine that is being recalled should contact their physician.
Consumers with questions regarding this recall can contact the AstraZeneca Information Center at 1-800-236-9933 between the hours of 8 am and 6 pm (Eastern time) Monday to Friday, excluding holidays.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using Brilinta.
Adverse reactions or quality problems related to Brilinta may also be reported to the FDA’s MedWatch Adverse Event Reporting Program.
About Brilinta and Zurampic
Brilinta is intended for use to reduce the rate of cardiovascular death, heart attack, and stroke in patients with acute coronary syndrome or a history of heart attack.
Brilinta is also intended to reduce the rate of stent thrombosis in patients who have been stented for treatment of acute coronary syndrome.
Zurampic is used together with a xanthine oxidase inhibitor, such as allopurinol or Uloric, in adults with gout who still have a high uric acid level.
Brilinta 90 mg tablets are supplied as a round, biconvex, yellow, film-coated tablet, and imprinted with a “90” above a “T” on one side of the pill.
Zurampic tablets 200 mg are blue in color and elliptical/oval in shape. They are imprinted with “LES200” on one side of the pill. ![]()
AstraZeneca has announced a voluntary recall of 1 lot of professional (physician) sample bottles containing 8 tablets of Brilinta® (ticagrelor).
This recall follows a report that a professional sample bottle containing 8 tablets of Brilinta 90 mg also contained Zurampic® (lesinurad) 200 mg tablets, which is also manufactured by AstraZeneca.
The company said this precautionary measure is limited to 1 lot of Brilinta (Brilinta lot #JB5047) distributed to physicians in the US between March and April of 2017.
Other forms and dosage strengths of Brilinta, including medicine obtained via US retail or mail order pharmacies, are not affected by this recall. And this recall does not affect Zurampic.
Potential risks
Unintentional dosing with Zurampic has the potential to lead to adverse renal effects, including acute renal failure, which is more common when Zurampic is given alone, as it should be used in combination with a xanthine oxidase inhibitor.
Missed doses of Brilinta increase the risk of heart attack and stroke. People with a stent who miss doses of Brilinta have a higher risk of stent thrombosis, heart attack, and death. Patients should not stop taking Brilinta without talking to their prescribing doctor.
To date, AstraZeneca has not received any reports of adverse events related to this recall.
Next steps
AstraZeneca is notifying physicians by recall letter and is arranging for the return of all recalled products. Consumers who have medicine that is being recalled should contact their physician.
Consumers with questions regarding this recall can contact the AstraZeneca Information Center at 1-800-236-9933 between the hours of 8 am and 6 pm (Eastern time) Monday to Friday, excluding holidays.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using Brilinta.
Adverse reactions or quality problems related to Brilinta may also be reported to the FDA’s MedWatch Adverse Event Reporting Program.
About Brilinta and Zurampic
Brilinta is intended for use to reduce the rate of cardiovascular death, heart attack, and stroke in patients with acute coronary syndrome or a history of heart attack.
Brilinta is also intended to reduce the rate of stent thrombosis in patients who have been stented for treatment of acute coronary syndrome.
Zurampic is used together with a xanthine oxidase inhibitor, such as allopurinol or Uloric, in adults with gout who still have a high uric acid level.
Brilinta 90 mg tablets are supplied as a round, biconvex, yellow, film-coated tablet, and imprinted with a “90” above a “T” on one side of the pill.
Zurampic tablets 200 mg are blue in color and elliptical/oval in shape. They are imprinted with “LES200” on one side of the pill. ![]()
EC grants drug orphan designation for AML
The European Commission (EC) has granted orphan designation to GMI-1271 for the treatment of acute myeloid leukemia (AML).
GMI-1271 is an E-selectin antagonist being developed by GlycoMimetics, Inc.
The product also has orphan designation, fast track designation, and breakthrough therapy designation in the US.
GMI-1271 is currently being evaluated in a phase 1/2 trial of patients with relapsed or refractory AML and patients age 60 and older with newly diagnosed AML.
The patients are receiving GM-1271 in combination with chemotherapy. The relapsed/refractory group is receiving mitoxantrone, etoposide, and cytarabine. The newly diagnosed patients are receiving cytarabine and idarubicin (7+3).
GlycoMimetics plans to present data from this trial at the 2017 American Society of Clinical Oncology (ASCO) Annual Meeting as abstracts 2520 and 2560.
The company also plans to present the research at the 22nd Congress of the European Hematology Association (EHA) as abstracts P547 and P203.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure.
The European Medicines Agency adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the EC for a final decision. The EC typically makes a decision within 30 days of that submission. ![]()
The European Commission (EC) has granted orphan designation to GMI-1271 for the treatment of acute myeloid leukemia (AML).
GMI-1271 is an E-selectin antagonist being developed by GlycoMimetics, Inc.
The product also has orphan designation, fast track designation, and breakthrough therapy designation in the US.
GMI-1271 is currently being evaluated in a phase 1/2 trial of patients with relapsed or refractory AML and patients age 60 and older with newly diagnosed AML.
The patients are receiving GM-1271 in combination with chemotherapy. The relapsed/refractory group is receiving mitoxantrone, etoposide, and cytarabine. The newly diagnosed patients are receiving cytarabine and idarubicin (7+3).
GlycoMimetics plans to present data from this trial at the 2017 American Society of Clinical Oncology (ASCO) Annual Meeting as abstracts 2520 and 2560.
The company also plans to present the research at the 22nd Congress of the European Hematology Association (EHA) as abstracts P547 and P203.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure.
The European Medicines Agency adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the EC for a final decision. The EC typically makes a decision within 30 days of that submission. ![]()
The European Commission (EC) has granted orphan designation to GMI-1271 for the treatment of acute myeloid leukemia (AML).
GMI-1271 is an E-selectin antagonist being developed by GlycoMimetics, Inc.
The product also has orphan designation, fast track designation, and breakthrough therapy designation in the US.
GMI-1271 is currently being evaluated in a phase 1/2 trial of patients with relapsed or refractory AML and patients age 60 and older with newly diagnosed AML.
The patients are receiving GM-1271 in combination with chemotherapy. The relapsed/refractory group is receiving mitoxantrone, etoposide, and cytarabine. The newly diagnosed patients are receiving cytarabine and idarubicin (7+3).
GlycoMimetics plans to present data from this trial at the 2017 American Society of Clinical Oncology (ASCO) Annual Meeting as abstracts 2520 and 2560.
The company also plans to present the research at the 22nd Congress of the European Hematology Association (EHA) as abstracts P547 and P203.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure.
The European Medicines Agency adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the EC for a final decision. The EC typically makes a decision within 30 days of that submission. ![]()
EC grants drug orphan designation for CTCL
The European Commission (EC) has granted orphan designation to MRG-106 for the treatment of cutaneous T-cell lymphoma (CTCL).
MRG-106 is a locked nucleic acid-modified oligonucleotide inhibitor of miR-155-5p.
miRagen Therapeutics, Inc., the company developing MRG-106, is currently testing the drug in a phase 1 trial of CTCL patients.
Early results from this trial were presented at the 2016 ASH Annual Meeting.
Researchers presented results in 6 patients with stage I-III mycosis fungoides.
The patients received 4 or 5 intratumoral injections of MRG-106 (at 75 mg) over 2 weeks. Four patients received saline injections in a second lesion on the same schedule.
There were 3 adverse events related to MRG-106—pain during injection, burning sensation during injection, and tingling at the injection site.
Adverse events considered possibly related to MRG-106 were pruritus, erythema, skin inflammation, sore on hand, nausea, decrease in white blood cells, neutropenia, and prolonged partial thromboplastin time.
One patient was taken off the trial due to rapid disease progression. The other 5 patients completed the dosing period.
All 5 patients had a reduction in the baseline Composite Assessment of Index Lesion Severity score in MRG-106-treated and saline-treated lesions.
The average maximal reduction was 55% (range, 33% to 77%) in MRG-106-treated lesions and 39% (range, 13% to 75%) in saline-treated lesions.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure.
The European Medicines Agency adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the EC for a final decision. The EC typically makes a decision within 30 days of that submission. ![]()
The European Commission (EC) has granted orphan designation to MRG-106 for the treatment of cutaneous T-cell lymphoma (CTCL).
MRG-106 is a locked nucleic acid-modified oligonucleotide inhibitor of miR-155-5p.
miRagen Therapeutics, Inc., the company developing MRG-106, is currently testing the drug in a phase 1 trial of CTCL patients.
Early results from this trial were presented at the 2016 ASH Annual Meeting.
Researchers presented results in 6 patients with stage I-III mycosis fungoides.
The patients received 4 or 5 intratumoral injections of MRG-106 (at 75 mg) over 2 weeks. Four patients received saline injections in a second lesion on the same schedule.
There were 3 adverse events related to MRG-106—pain during injection, burning sensation during injection, and tingling at the injection site.
Adverse events considered possibly related to MRG-106 were pruritus, erythema, skin inflammation, sore on hand, nausea, decrease in white blood cells, neutropenia, and prolonged partial thromboplastin time.
One patient was taken off the trial due to rapid disease progression. The other 5 patients completed the dosing period.
All 5 patients had a reduction in the baseline Composite Assessment of Index Lesion Severity score in MRG-106-treated and saline-treated lesions.
The average maximal reduction was 55% (range, 33% to 77%) in MRG-106-treated lesions and 39% (range, 13% to 75%) in saline-treated lesions.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure.
The European Medicines Agency adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the EC for a final decision. The EC typically makes a decision within 30 days of that submission. ![]()
The European Commission (EC) has granted orphan designation to MRG-106 for the treatment of cutaneous T-cell lymphoma (CTCL).
MRG-106 is a locked nucleic acid-modified oligonucleotide inhibitor of miR-155-5p.
miRagen Therapeutics, Inc., the company developing MRG-106, is currently testing the drug in a phase 1 trial of CTCL patients.
Early results from this trial were presented at the 2016 ASH Annual Meeting.
Researchers presented results in 6 patients with stage I-III mycosis fungoides.
The patients received 4 or 5 intratumoral injections of MRG-106 (at 75 mg) over 2 weeks. Four patients received saline injections in a second lesion on the same schedule.
There were 3 adverse events related to MRG-106—pain during injection, burning sensation during injection, and tingling at the injection site.
Adverse events considered possibly related to MRG-106 were pruritus, erythema, skin inflammation, sore on hand, nausea, decrease in white blood cells, neutropenia, and prolonged partial thromboplastin time.
One patient was taken off the trial due to rapid disease progression. The other 5 patients completed the dosing period.
All 5 patients had a reduction in the baseline Composite Assessment of Index Lesion Severity score in MRG-106-treated and saline-treated lesions.
The average maximal reduction was 55% (range, 33% to 77%) in MRG-106-treated lesions and 39% (range, 13% to 75%) in saline-treated lesions.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure.
The European Medicines Agency adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the EC for a final decision. The EC typically makes a decision within 30 days of that submission. ![]()
EMA recommends orphan designation for AML drug
The European Medicines Agency (EMA) has recommended orphan designation for Actimab-A, a product intended to treat patients with newly diagnosed acute myeloid leukemia (AML) who are over the age of 60 and are ineligible for standard induction therapy.
Actimab-A targets CD33, a protein expressed on the surface of AML cells, via the monoclonal antibody, HuM195, which carries the cytotoxic radioisotope actinium-225 to the AML cells.
Actinium Pharmaceuticals, Inc., the company developing Actimab-A, is testing the drug in a phase 2 trial.
Results from a phase 1 trial of the drug were presented at the 2016 ASH Annual Meeting.
At that time, researchers reported results in 18 patients who had been newly diagnosed with AML and were age 60 and older. Their median age was 77 (range, 68-87).
The patients received Actimab-A in combination with low-dose cytarabine. Actimab-A was given at 0.5 μCi/kg/fraction (n=3), 1 μCi/kg/fraction (n=6), 1.5 μCi/kg/fraction (n=3), or 2 μCi/kg/fraction (n=6).
Two patients experienced dose-limiting toxicities. Both had grade 4 thrombocytopenia with marrow aplasia for more than 6 weeks after therapy. One patient was in the 1 µCi/kg/fraction cohort, and the other was in the 2 µCi/kg/fraction cohort.
The maximum-tolerated dose was not reached, but 2 µCi/kg/fraction was chosen as the phase 2 dose.
Grade 3/4 toxicities included neutropenia (n=5), thrombocytopenia (n=9), febrile neutropenia (n=6), pneumonia (n=5), other infections (n=3), atrial fibrillation/syncope (n=1), transient creatinine increase (n=1), generalized fatigue (n=1), hypokalemia (n=1), mucositis (n=1), and rectal hemorrhage (n=1).
Twenty-eight percent of patients (5/18) had objective responses to treatment. Two patients achieved a complete response (CR), 1 had a CR with incomplete platelet recovery, and 2 had a CR with incomplete marrow recovery.
The median duration of response was 9.1 months (range, 4.1-16.9).
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
The EMA adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The European Commission typically makes a decision within 30 days. ![]()
The European Medicines Agency (EMA) has recommended orphan designation for Actimab-A, a product intended to treat patients with newly diagnosed acute myeloid leukemia (AML) who are over the age of 60 and are ineligible for standard induction therapy.
Actimab-A targets CD33, a protein expressed on the surface of AML cells, via the monoclonal antibody, HuM195, which carries the cytotoxic radioisotope actinium-225 to the AML cells.
Actinium Pharmaceuticals, Inc., the company developing Actimab-A, is testing the drug in a phase 2 trial.
Results from a phase 1 trial of the drug were presented at the 2016 ASH Annual Meeting.
At that time, researchers reported results in 18 patients who had been newly diagnosed with AML and were age 60 and older. Their median age was 77 (range, 68-87).
The patients received Actimab-A in combination with low-dose cytarabine. Actimab-A was given at 0.5 μCi/kg/fraction (n=3), 1 μCi/kg/fraction (n=6), 1.5 μCi/kg/fraction (n=3), or 2 μCi/kg/fraction (n=6).
Two patients experienced dose-limiting toxicities. Both had grade 4 thrombocytopenia with marrow aplasia for more than 6 weeks after therapy. One patient was in the 1 µCi/kg/fraction cohort, and the other was in the 2 µCi/kg/fraction cohort.
The maximum-tolerated dose was not reached, but 2 µCi/kg/fraction was chosen as the phase 2 dose.
Grade 3/4 toxicities included neutropenia (n=5), thrombocytopenia (n=9), febrile neutropenia (n=6), pneumonia (n=5), other infections (n=3), atrial fibrillation/syncope (n=1), transient creatinine increase (n=1), generalized fatigue (n=1), hypokalemia (n=1), mucositis (n=1), and rectal hemorrhage (n=1).
Twenty-eight percent of patients (5/18) had objective responses to treatment. Two patients achieved a complete response (CR), 1 had a CR with incomplete platelet recovery, and 2 had a CR with incomplete marrow recovery.
The median duration of response was 9.1 months (range, 4.1-16.9).
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
The EMA adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The European Commission typically makes a decision within 30 days. ![]()
The European Medicines Agency (EMA) has recommended orphan designation for Actimab-A, a product intended to treat patients with newly diagnosed acute myeloid leukemia (AML) who are over the age of 60 and are ineligible for standard induction therapy.
Actimab-A targets CD33, a protein expressed on the surface of AML cells, via the monoclonal antibody, HuM195, which carries the cytotoxic radioisotope actinium-225 to the AML cells.
Actinium Pharmaceuticals, Inc., the company developing Actimab-A, is testing the drug in a phase 2 trial.
Results from a phase 1 trial of the drug were presented at the 2016 ASH Annual Meeting.
At that time, researchers reported results in 18 patients who had been newly diagnosed with AML and were age 60 and older. Their median age was 77 (range, 68-87).
The patients received Actimab-A in combination with low-dose cytarabine. Actimab-A was given at 0.5 μCi/kg/fraction (n=3), 1 μCi/kg/fraction (n=6), 1.5 μCi/kg/fraction (n=3), or 2 μCi/kg/fraction (n=6).
Two patients experienced dose-limiting toxicities. Both had grade 4 thrombocytopenia with marrow aplasia for more than 6 weeks after therapy. One patient was in the 1 µCi/kg/fraction cohort, and the other was in the 2 µCi/kg/fraction cohort.
The maximum-tolerated dose was not reached, but 2 µCi/kg/fraction was chosen as the phase 2 dose.
Grade 3/4 toxicities included neutropenia (n=5), thrombocytopenia (n=9), febrile neutropenia (n=6), pneumonia (n=5), other infections (n=3), atrial fibrillation/syncope (n=1), transient creatinine increase (n=1), generalized fatigue (n=1), hypokalemia (n=1), mucositis (n=1), and rectal hemorrhage (n=1).
Twenty-eight percent of patients (5/18) had objective responses to treatment. Two patients achieved a complete response (CR), 1 had a CR with incomplete platelet recovery, and 2 had a CR with incomplete marrow recovery.
The median duration of response was 9.1 months (range, 4.1-16.9).
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
The EMA adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The European Commission typically makes a decision within 30 days. ![]()
FDA approves new formulation of deferasirox
The US Food and Drug Administration (FDA) has approved a new formulation of deferasirox known as Jadenu Sprinkle granules.
The granules are approved for use in the same population as Jadenu film-coated tablets.
Both formulations of Jadenu have accelerated approval from the FDA for the treatment of chronic iron overload due to blood transfusions in patients age 2 and older.
The formulations also have accelerated FDA approval for the treatment of chronic iron overload in patients age 10 and older with non-transfusion-dependent-thalassemia and a liver iron concentration of at least 5 mg Fe per gram of dry weight and a serum ferritin greater than 300 mcg/L.
Continued FDA approval for Jadenu in these indications may be contingent upon verification and description of clinical benefit in confirmatory trials.
Jadenu Sprinkle granules are intended for patients who have difficulty swallowing whole tablets. The granules can be sprinkled over soft foods (eg, yogurt or applesauce) prior to consumption.
Like Jadenu film-coated tablets, Jadenu Sprinkle granules are available in 3 strengths—90 mg, 180 mg, and 360 mg.
Both formulations of Jadenu are products of Novartis. For more details on Jadenu, see the prescribing information. ![]()
The US Food and Drug Administration (FDA) has approved a new formulation of deferasirox known as Jadenu Sprinkle granules.
The granules are approved for use in the same population as Jadenu film-coated tablets.
Both formulations of Jadenu have accelerated approval from the FDA for the treatment of chronic iron overload due to blood transfusions in patients age 2 and older.
The formulations also have accelerated FDA approval for the treatment of chronic iron overload in patients age 10 and older with non-transfusion-dependent-thalassemia and a liver iron concentration of at least 5 mg Fe per gram of dry weight and a serum ferritin greater than 300 mcg/L.
Continued FDA approval for Jadenu in these indications may be contingent upon verification and description of clinical benefit in confirmatory trials.
Jadenu Sprinkle granules are intended for patients who have difficulty swallowing whole tablets. The granules can be sprinkled over soft foods (eg, yogurt or applesauce) prior to consumption.
Like Jadenu film-coated tablets, Jadenu Sprinkle granules are available in 3 strengths—90 mg, 180 mg, and 360 mg.
Both formulations of Jadenu are products of Novartis. For more details on Jadenu, see the prescribing information. ![]()
The US Food and Drug Administration (FDA) has approved a new formulation of deferasirox known as Jadenu Sprinkle granules.
The granules are approved for use in the same population as Jadenu film-coated tablets.
Both formulations of Jadenu have accelerated approval from the FDA for the treatment of chronic iron overload due to blood transfusions in patients age 2 and older.
The formulations also have accelerated FDA approval for the treatment of chronic iron overload in patients age 10 and older with non-transfusion-dependent-thalassemia and a liver iron concentration of at least 5 mg Fe per gram of dry weight and a serum ferritin greater than 300 mcg/L.
Continued FDA approval for Jadenu in these indications may be contingent upon verification and description of clinical benefit in confirmatory trials.
Jadenu Sprinkle granules are intended for patients who have difficulty swallowing whole tablets. The granules can be sprinkled over soft foods (eg, yogurt or applesauce) prior to consumption.
Like Jadenu film-coated tablets, Jadenu Sprinkle granules are available in 3 strengths—90 mg, 180 mg, and 360 mg.
Both formulations of Jadenu are products of Novartis. For more details on Jadenu, see the prescribing information. ![]()
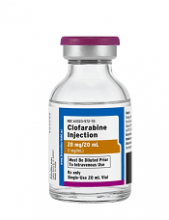
First generic version of clofarabine available in US
Clofarabine Injection, the first-to-market generic version of Sanofi Genzyme’s Clolar, is now available in the US.
The generic, a product of Fresenius Kabi, is available as a single dose vial containing 20 mg per 20 mL clofarabine.
Clofarabine is a purine nucleoside metabolic inhibitor indicated for the treatment of patients ages 1 to 21 with relapsed or refractory acute lymphoblastic leukemia (ALL) who received at least 2 prior treatment regimens.
Clolar was granted accelerated approval for this indication in the US in 2004.
The approval was based on response rates observed in ALL patients. There are no trials verifying that clofarabine confers improvement in survival or disease-related symptoms in ALL patients.
Clofarabine was assessed in a single-arm, phase 2 trial of 61 pediatric patients with relapsed/refractory ALL.
The patients’ median age was 12 (range, 1 to 20 years), and their median number of prior treatment regimens was 3 (range, 2 to 6).
The patients received clofarabine at 52 mg/m2 intravenously over 2 hours daily for 5 days, every 2 to 6 weeks.
The overall response rate was 30%. Seven patient achieved a complete response (CR), 5 had a CR without platelet recovery, and 6 patients had a partial response.
The median duration of CR in patients who did not go on to hematopoietic stem cell transplant was 6 weeks.
The most common grade 3 or higher adverse events were febrile neutropenia, anorexia, hypotension, and nausea.
These results were published in the Journal of Clinical Oncology in 2006. ![]()
Clofarabine Injection, the first-to-market generic version of Sanofi Genzyme’s Clolar, is now available in the US.
The generic, a product of Fresenius Kabi, is available as a single dose vial containing 20 mg per 20 mL clofarabine.
Clofarabine is a purine nucleoside metabolic inhibitor indicated for the treatment of patients ages 1 to 21 with relapsed or refractory acute lymphoblastic leukemia (ALL) who received at least 2 prior treatment regimens.
Clolar was granted accelerated approval for this indication in the US in 2004.
The approval was based on response rates observed in ALL patients. There are no trials verifying that clofarabine confers improvement in survival or disease-related symptoms in ALL patients.
Clofarabine was assessed in a single-arm, phase 2 trial of 61 pediatric patients with relapsed/refractory ALL.
The patients’ median age was 12 (range, 1 to 20 years), and their median number of prior treatment regimens was 3 (range, 2 to 6).
The patients received clofarabine at 52 mg/m2 intravenously over 2 hours daily for 5 days, every 2 to 6 weeks.
The overall response rate was 30%. Seven patient achieved a complete response (CR), 5 had a CR without platelet recovery, and 6 patients had a partial response.
The median duration of CR in patients who did not go on to hematopoietic stem cell transplant was 6 weeks.
The most common grade 3 or higher adverse events were febrile neutropenia, anorexia, hypotension, and nausea.
These results were published in the Journal of Clinical Oncology in 2006. ![]()
Clofarabine Injection, the first-to-market generic version of Sanofi Genzyme’s Clolar, is now available in the US.
The generic, a product of Fresenius Kabi, is available as a single dose vial containing 20 mg per 20 mL clofarabine.
Clofarabine is a purine nucleoside metabolic inhibitor indicated for the treatment of patients ages 1 to 21 with relapsed or refractory acute lymphoblastic leukemia (ALL) who received at least 2 prior treatment regimens.
Clolar was granted accelerated approval for this indication in the US in 2004.
The approval was based on response rates observed in ALL patients. There are no trials verifying that clofarabine confers improvement in survival or disease-related symptoms in ALL patients.
Clofarabine was assessed in a single-arm, phase 2 trial of 61 pediatric patients with relapsed/refractory ALL.
The patients’ median age was 12 (range, 1 to 20 years), and their median number of prior treatment regimens was 3 (range, 2 to 6).
The patients received clofarabine at 52 mg/m2 intravenously over 2 hours daily for 5 days, every 2 to 6 weeks.
The overall response rate was 30%. Seven patient achieved a complete response (CR), 5 had a CR without platelet recovery, and 6 patients had a partial response.
The median duration of CR in patients who did not go on to hematopoietic stem cell transplant was 6 weeks.
The most common grade 3 or higher adverse events were febrile neutropenia, anorexia, hypotension, and nausea.
These results were published in the Journal of Clinical Oncology in 2006. ![]()
FDA grants priority review to NDA for copanlisib
The US Food and Drug Administration (FDA) has granted priority review to the new drug application (NDA) for copanlisib, an intravenous PI3K inhibitor.
The NDA is for copanlisib as a treatment for patients with relapsed or refractory follicular lymphoma (FL) who have received at least 2 prior therapies.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The application for copanlisib is supported by data from the CHRONOS-1 trial. This phase 2 trial enrolled 141 patients with relapsed/refractory, indolent non-Hodgkin lymphoma. Most of these patients had FL (n=104).
In all patients, copanlisib produced an objective response rate of 59.2%, with a complete response rate of 12%. The median duration of response exceeded 98 weeks.
In the FL subset, copanlisib produced an overall response rate of 58.7%, with a complete response rate of 14.4%. The median duration of response exceeded 52 weeks.
In the entire cohort, there were 3 deaths considered related to copanlisib.
The most common treatment-related adverse events were transient hyperglycemia (all grades: 49%/grade 3-4: 40%) and hypertension (all grades: 29%/grade 3: 23%).
“Patients with relapsed or refractory follicular lymphoma have a poor prognosis, and new treatment options which are well tolerated and effective are needed to prolong progression-free survival and improve quality of life for these patients,” said Martin Dreyling, MD, a professor at the University of Munich Hospital (Grosshadern) in Germany and lead investigator of the CHRONOS-1 study.
“Based on the CHRONOS-1 results, where copanlisib showed durable efficacy with a manageable and distinct safety profile, the compound may have the potential to address this unmet medical need.”
Data from CHRONOS-1 were presented at the AACR Annual Meeting 2017.
Data from the FL subset of the trial are scheduled to be presented at the 2017 ASCO Annual Meeting in June.
Copanlisib is being developed by Bayer. The compound has fast track and orphan drug designations from the FDA.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the NDA or biologic license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA. ![]()
The US Food and Drug Administration (FDA) has granted priority review to the new drug application (NDA) for copanlisib, an intravenous PI3K inhibitor.
The NDA is for copanlisib as a treatment for patients with relapsed or refractory follicular lymphoma (FL) who have received at least 2 prior therapies.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The application for copanlisib is supported by data from the CHRONOS-1 trial. This phase 2 trial enrolled 141 patients with relapsed/refractory, indolent non-Hodgkin lymphoma. Most of these patients had FL (n=104).
In all patients, copanlisib produced an objective response rate of 59.2%, with a complete response rate of 12%. The median duration of response exceeded 98 weeks.
In the FL subset, copanlisib produced an overall response rate of 58.7%, with a complete response rate of 14.4%. The median duration of response exceeded 52 weeks.
In the entire cohort, there were 3 deaths considered related to copanlisib.
The most common treatment-related adverse events were transient hyperglycemia (all grades: 49%/grade 3-4: 40%) and hypertension (all grades: 29%/grade 3: 23%).
“Patients with relapsed or refractory follicular lymphoma have a poor prognosis, and new treatment options which are well tolerated and effective are needed to prolong progression-free survival and improve quality of life for these patients,” said Martin Dreyling, MD, a professor at the University of Munich Hospital (Grosshadern) in Germany and lead investigator of the CHRONOS-1 study.
“Based on the CHRONOS-1 results, where copanlisib showed durable efficacy with a manageable and distinct safety profile, the compound may have the potential to address this unmet medical need.”
Data from CHRONOS-1 were presented at the AACR Annual Meeting 2017.
Data from the FL subset of the trial are scheduled to be presented at the 2017 ASCO Annual Meeting in June.
Copanlisib is being developed by Bayer. The compound has fast track and orphan drug designations from the FDA.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the NDA or biologic license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA. ![]()
The US Food and Drug Administration (FDA) has granted priority review to the new drug application (NDA) for copanlisib, an intravenous PI3K inhibitor.
The NDA is for copanlisib as a treatment for patients with relapsed or refractory follicular lymphoma (FL) who have received at least 2 prior therapies.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The application for copanlisib is supported by data from the CHRONOS-1 trial. This phase 2 trial enrolled 141 patients with relapsed/refractory, indolent non-Hodgkin lymphoma. Most of these patients had FL (n=104).
In all patients, copanlisib produced an objective response rate of 59.2%, with a complete response rate of 12%. The median duration of response exceeded 98 weeks.
In the FL subset, copanlisib produced an overall response rate of 58.7%, with a complete response rate of 14.4%. The median duration of response exceeded 52 weeks.
In the entire cohort, there were 3 deaths considered related to copanlisib.
The most common treatment-related adverse events were transient hyperglycemia (all grades: 49%/grade 3-4: 40%) and hypertension (all grades: 29%/grade 3: 23%).
“Patients with relapsed or refractory follicular lymphoma have a poor prognosis, and new treatment options which are well tolerated and effective are needed to prolong progression-free survival and improve quality of life for these patients,” said Martin Dreyling, MD, a professor at the University of Munich Hospital (Grosshadern) in Germany and lead investigator of the CHRONOS-1 study.
“Based on the CHRONOS-1 results, where copanlisib showed durable efficacy with a manageable and distinct safety profile, the compound may have the potential to address this unmet medical need.”
Data from CHRONOS-1 were presented at the AACR Annual Meeting 2017.
Data from the FL subset of the trial are scheduled to be presented at the 2017 ASCO Annual Meeting in June.
Copanlisib is being developed by Bayer. The compound has fast track and orphan drug designations from the FDA.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the NDA or biologic license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
System monitors and maintains drug levels in the body
New technology could make it easier to ensure patients receive the correct dose of chemotherapy and other drugs, according to research published in Nature Biomedical Engineering.
Researchers developed a closed-loop system that was able to continuously regulate drug levels in rabbits and rats.
“This is the first time anyone has been able to continuously control the drug levels in the body in real time,” said study author H. Tom Soh, PhD, of Stanford University in California.
“This is a novel concept with big implications because we believe we can adapt our technology to control the levels of a wide range of drugs.”
The researchers’ system has 3 basic components: a real-time biosensor to continuously monitor drug levels in the bloodstream, a control system to calculate the right dose, and a programmable pump that delivers just enough medicine to maintain a desired dose.
The sensor contains aptamers that are specially designed to bind a drug of interest. When the drug is present in the bloodstream, the aptamer changes shape, which an electric sensor detects. The more drug, the more aptamers change shape.
That information, captured every few seconds, is routed through software that controls the pump to deliver additional drugs as needed.
Researchers tested the technology by administering the chemotherapy drug doxorubicin to rabbits and rats.
Despite physiological and metabolic differences among individual animals, the team was able to keep a constant dosage in all the animals, something not possible with current drug delivery methods.
The researchers also tested for acute drug-drug interactions and found the system was able to stabilize drug levels to moderate what might otherwise be a dangerous spike or dip.
Dr Soh and his colleagues believe this technology could be particularly useful in treating pediatric cancer patients, who are notoriously difficult to dose because a child’s metabolism is usually different from an adult’s.
The team plans to miniaturize the system so it can be implanted or worn by the patient.
At present, the technology is an external apparatus, like a smart IV drip. The biosensor is a device about the size of a microscope slide.
The current setup might be suitable for a chemotherapy drug but not for continual use.
The researchers are also adapting the system with different aptamers so it can sense and regulate the levels of other biomolecules in the body.
New technology could make it easier to ensure patients receive the correct dose of chemotherapy and other drugs, according to research published in Nature Biomedical Engineering.
Researchers developed a closed-loop system that was able to continuously regulate drug levels in rabbits and rats.
“This is the first time anyone has been able to continuously control the drug levels in the body in real time,” said study author H. Tom Soh, PhD, of Stanford University in California.
“This is a novel concept with big implications because we believe we can adapt our technology to control the levels of a wide range of drugs.”
The researchers’ system has 3 basic components: a real-time biosensor to continuously monitor drug levels in the bloodstream, a control system to calculate the right dose, and a programmable pump that delivers just enough medicine to maintain a desired dose.
The sensor contains aptamers that are specially designed to bind a drug of interest. When the drug is present in the bloodstream, the aptamer changes shape, which an electric sensor detects. The more drug, the more aptamers change shape.
That information, captured every few seconds, is routed through software that controls the pump to deliver additional drugs as needed.
Researchers tested the technology by administering the chemotherapy drug doxorubicin to rabbits and rats.
Despite physiological and metabolic differences among individual animals, the team was able to keep a constant dosage in all the animals, something not possible with current drug delivery methods.
The researchers also tested for acute drug-drug interactions and found the system was able to stabilize drug levels to moderate what might otherwise be a dangerous spike or dip.
Dr Soh and his colleagues believe this technology could be particularly useful in treating pediatric cancer patients, who are notoriously difficult to dose because a child’s metabolism is usually different from an adult’s.
The team plans to miniaturize the system so it can be implanted or worn by the patient.
At present, the technology is an external apparatus, like a smart IV drip. The biosensor is a device about the size of a microscope slide.
The current setup might be suitable for a chemotherapy drug but not for continual use.
The researchers are also adapting the system with different aptamers so it can sense and regulate the levels of other biomolecules in the body.
New technology could make it easier to ensure patients receive the correct dose of chemotherapy and other drugs, according to research published in Nature Biomedical Engineering.
Researchers developed a closed-loop system that was able to continuously regulate drug levels in rabbits and rats.
“This is the first time anyone has been able to continuously control the drug levels in the body in real time,” said study author H. Tom Soh, PhD, of Stanford University in California.
“This is a novel concept with big implications because we believe we can adapt our technology to control the levels of a wide range of drugs.”
The researchers’ system has 3 basic components: a real-time biosensor to continuously monitor drug levels in the bloodstream, a control system to calculate the right dose, and a programmable pump that delivers just enough medicine to maintain a desired dose.
The sensor contains aptamers that are specially designed to bind a drug of interest. When the drug is present in the bloodstream, the aptamer changes shape, which an electric sensor detects. The more drug, the more aptamers change shape.
That information, captured every few seconds, is routed through software that controls the pump to deliver additional drugs as needed.
Researchers tested the technology by administering the chemotherapy drug doxorubicin to rabbits and rats.
Despite physiological and metabolic differences among individual animals, the team was able to keep a constant dosage in all the animals, something not possible with current drug delivery methods.
The researchers also tested for acute drug-drug interactions and found the system was able to stabilize drug levels to moderate what might otherwise be a dangerous spike or dip.
Dr Soh and his colleagues believe this technology could be particularly useful in treating pediatric cancer patients, who are notoriously difficult to dose because a child’s metabolism is usually different from an adult’s.
The team plans to miniaturize the system so it can be implanted or worn by the patient.
At present, the technology is an external apparatus, like a smart IV drip. The biosensor is a device about the size of a microscope slide.
The current setup might be suitable for a chemotherapy drug but not for continual use.
The researchers are also adapting the system with different aptamers so it can sense and regulate the levels of other biomolecules in the body.
EMA recommends drug receive orphan designation for PNH
The European Medicines Agency (EMA) has recommended orphan drug designation for the complement C3 inhibitor APL-2 as a treatment for paroxysmal nocturnal hemoglobinuria (PNH).
APL-2 is a synthetic cyclic peptide conjugated to a polyethylene glycol polymer that binds specifically to C3 and C3b, blocking all 3 pathways of complement activation (classical, lectin, and alternative).
This comprehensive inhibition of complement-mediated pathology may have the potential to control symptoms and modify underlying disease in patients with PNH, according to Apellis Pharmaceuticals, Inc., the company developing APL-2.
APL-2 has been evaluated in a pair of phase 1 studies of healthy volunteers. Results from these studies were presented at the 2016 ASH Annual Meeting (abstract 1251).
Now, Apellis is evaluating APL-2 in PNH patients in a pair of phase 1b trials.
In PADDOCK (NCT02588833), researchers are assessing the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary efficacy of multiple doses of APL-2 administered by daily subcutaneous injection in patients with PNH who have not received the standard of care in the past.
In PHAROAH (NCT02264639), researchers are assessing the safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple doses of APL-2 administered by subcutaneous injection as an add-on to the standard of care in patients with PNH.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
The EMA adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The European Commission typically makes a decision within 30 days.
The European Medicines Agency (EMA) has recommended orphan drug designation for the complement C3 inhibitor APL-2 as a treatment for paroxysmal nocturnal hemoglobinuria (PNH).
APL-2 is a synthetic cyclic peptide conjugated to a polyethylene glycol polymer that binds specifically to C3 and C3b, blocking all 3 pathways of complement activation (classical, lectin, and alternative).
This comprehensive inhibition of complement-mediated pathology may have the potential to control symptoms and modify underlying disease in patients with PNH, according to Apellis Pharmaceuticals, Inc., the company developing APL-2.
APL-2 has been evaluated in a pair of phase 1 studies of healthy volunteers. Results from these studies were presented at the 2016 ASH Annual Meeting (abstract 1251).
Now, Apellis is evaluating APL-2 in PNH patients in a pair of phase 1b trials.
In PADDOCK (NCT02588833), researchers are assessing the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary efficacy of multiple doses of APL-2 administered by daily subcutaneous injection in patients with PNH who have not received the standard of care in the past.
In PHAROAH (NCT02264639), researchers are assessing the safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple doses of APL-2 administered by subcutaneous injection as an add-on to the standard of care in patients with PNH.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
The EMA adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The European Commission typically makes a decision within 30 days.
The European Medicines Agency (EMA) has recommended orphan drug designation for the complement C3 inhibitor APL-2 as a treatment for paroxysmal nocturnal hemoglobinuria (PNH).
APL-2 is a synthetic cyclic peptide conjugated to a polyethylene glycol polymer that binds specifically to C3 and C3b, blocking all 3 pathways of complement activation (classical, lectin, and alternative).
This comprehensive inhibition of complement-mediated pathology may have the potential to control symptoms and modify underlying disease in patients with PNH, according to Apellis Pharmaceuticals, Inc., the company developing APL-2.
APL-2 has been evaluated in a pair of phase 1 studies of healthy volunteers. Results from these studies were presented at the 2016 ASH Annual Meeting (abstract 1251).
Now, Apellis is evaluating APL-2 in PNH patients in a pair of phase 1b trials.
In PADDOCK (NCT02588833), researchers are assessing the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary efficacy of multiple doses of APL-2 administered by daily subcutaneous injection in patients with PNH who have not received the standard of care in the past.
In PHAROAH (NCT02264639), researchers are assessing the safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple doses of APL-2 administered by subcutaneous injection as an add-on to the standard of care in patients with PNH.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
The EMA adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The European Commission typically makes a decision within 30 days.
Postmarket safety events common in FDA-approved drugs
New research suggests postmarket safety events are common for therapeutics approved by the US Food and Drug Administration (FDA).
Researchers evaluated more than 200 pharmaceuticals and biologics approved by the FDA from 2001 through 2010 and found that nearly a third of these products were affected by a postmarket safety event.
Most of the events were boxed warnings or safety communications, but there were a few products withdrawn from the market due to safety issues.
Joseph S. Ross, MD, of the Yale University School of Medicine in New Haven, Connecticut, and his colleagues reported these findings in JAMA.
The researchers noted that most pivotal trials that form the basis for FDA approval enroll fewer than 1000 patients and have follow-up of 6 months or less.
Therefore, uncommon or long-term serious safety risks may only become evident after approval, when new therapeutics are used in larger patient populations and for longer periods of time.
With this in mind, Dr Ross and his colleagues examined postmarket safety events for all novel therapeutics approved by the FDA between January 2001 and December 2010 (followed-up through February 2017).
Safety events included withdrawals due to safety concerns, FDA issuance of incremental boxed warnings added in the postmarket period, and FDA issuance of safety communications.
From 2001 through 2010, the FDA approved 222 novel therapeutics—183 pharmaceuticals and 39 biologics.
During a median follow-up of 11.7 years, there were 123 postmarket safety events—3 withdrawals, 61 boxed warnings, and 59 safety communications.
“The fact that the FDA is issuing safety communications means it is doing a good job of following newly approved drugs and evaluating their safety up in the postmarket period,” Dr Ross said.
The 123 safety events identified affected 71 (32%) of the 222 therapeutics.
The median time from FDA approval to the first postmarket safety event was 4.2 years. And 31% of the therapeutics were still affected by a postmarket safety event at 10 years.
The researchers found that postmarket safety events were significantly more frequent in biologics (P=0.03), drugs used to treat psychiatric disease (P<0.001), products approved near their regulatory deadline (P=0.008), and therapeutics granted accelerated approval (P=0.02).
“[The accelerated approval finding] shows that there is the potential for compromising patient safety when drug evaluation is persistently sped up,” Dr Ross said.
On the other hand, the researchers also found that postmarket safety events were significantly less frequent in therapeutics the FDA reviewed in less than 200 days (P=0.02).
The researchers said these findings should be interpreted cautiously, but they can be used to inform ongoing surveillance efforts.
New research suggests postmarket safety events are common for therapeutics approved by the US Food and Drug Administration (FDA).
Researchers evaluated more than 200 pharmaceuticals and biologics approved by the FDA from 2001 through 2010 and found that nearly a third of these products were affected by a postmarket safety event.
Most of the events were boxed warnings or safety communications, but there were a few products withdrawn from the market due to safety issues.
Joseph S. Ross, MD, of the Yale University School of Medicine in New Haven, Connecticut, and his colleagues reported these findings in JAMA.
The researchers noted that most pivotal trials that form the basis for FDA approval enroll fewer than 1000 patients and have follow-up of 6 months or less.
Therefore, uncommon or long-term serious safety risks may only become evident after approval, when new therapeutics are used in larger patient populations and for longer periods of time.
With this in mind, Dr Ross and his colleagues examined postmarket safety events for all novel therapeutics approved by the FDA between January 2001 and December 2010 (followed-up through February 2017).
Safety events included withdrawals due to safety concerns, FDA issuance of incremental boxed warnings added in the postmarket period, and FDA issuance of safety communications.
From 2001 through 2010, the FDA approved 222 novel therapeutics—183 pharmaceuticals and 39 biologics.
During a median follow-up of 11.7 years, there were 123 postmarket safety events—3 withdrawals, 61 boxed warnings, and 59 safety communications.
“The fact that the FDA is issuing safety communications means it is doing a good job of following newly approved drugs and evaluating their safety up in the postmarket period,” Dr Ross said.
The 123 safety events identified affected 71 (32%) of the 222 therapeutics.
The median time from FDA approval to the first postmarket safety event was 4.2 years. And 31% of the therapeutics were still affected by a postmarket safety event at 10 years.
The researchers found that postmarket safety events were significantly more frequent in biologics (P=0.03), drugs used to treat psychiatric disease (P<0.001), products approved near their regulatory deadline (P=0.008), and therapeutics granted accelerated approval (P=0.02).
“[The accelerated approval finding] shows that there is the potential for compromising patient safety when drug evaluation is persistently sped up,” Dr Ross said.
On the other hand, the researchers also found that postmarket safety events were significantly less frequent in therapeutics the FDA reviewed in less than 200 days (P=0.02).
The researchers said these findings should be interpreted cautiously, but they can be used to inform ongoing surveillance efforts.
New research suggests postmarket safety events are common for therapeutics approved by the US Food and Drug Administration (FDA).
Researchers evaluated more than 200 pharmaceuticals and biologics approved by the FDA from 2001 through 2010 and found that nearly a third of these products were affected by a postmarket safety event.
Most of the events were boxed warnings or safety communications, but there were a few products withdrawn from the market due to safety issues.
Joseph S. Ross, MD, of the Yale University School of Medicine in New Haven, Connecticut, and his colleagues reported these findings in JAMA.
The researchers noted that most pivotal trials that form the basis for FDA approval enroll fewer than 1000 patients and have follow-up of 6 months or less.
Therefore, uncommon or long-term serious safety risks may only become evident after approval, when new therapeutics are used in larger patient populations and for longer periods of time.
With this in mind, Dr Ross and his colleagues examined postmarket safety events for all novel therapeutics approved by the FDA between January 2001 and December 2010 (followed-up through February 2017).
Safety events included withdrawals due to safety concerns, FDA issuance of incremental boxed warnings added in the postmarket period, and FDA issuance of safety communications.
From 2001 through 2010, the FDA approved 222 novel therapeutics—183 pharmaceuticals and 39 biologics.
During a median follow-up of 11.7 years, there were 123 postmarket safety events—3 withdrawals, 61 boxed warnings, and 59 safety communications.
“The fact that the FDA is issuing safety communications means it is doing a good job of following newly approved drugs and evaluating their safety up in the postmarket period,” Dr Ross said.
The 123 safety events identified affected 71 (32%) of the 222 therapeutics.
The median time from FDA approval to the first postmarket safety event was 4.2 years. And 31% of the therapeutics were still affected by a postmarket safety event at 10 years.
The researchers found that postmarket safety events were significantly more frequent in biologics (P=0.03), drugs used to treat psychiatric disease (P<0.001), products approved near their regulatory deadline (P=0.008), and therapeutics granted accelerated approval (P=0.02).
“[The accelerated approval finding] shows that there is the potential for compromising patient safety when drug evaluation is persistently sped up,” Dr Ross said.
On the other hand, the researchers also found that postmarket safety events were significantly less frequent in therapeutics the FDA reviewed in less than 200 days (P=0.02).
The researchers said these findings should be interpreted cautiously, but they can be used to inform ongoing surveillance efforts.