User login
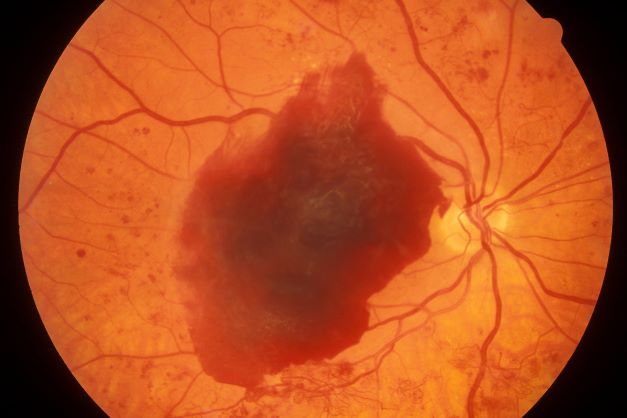
Pruritus and swelling
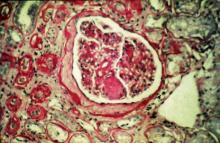
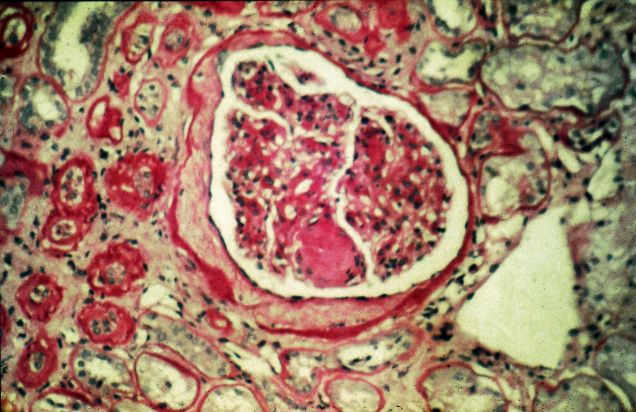
The history and findings in this case are suggestive of chronic kidney disease (CKD).
CKD affects between 8% and 16% of the population worldwide. Risk factors for CKD are numerous and include T2D, hypertension, and prediabetes. Diabetes is the leading cause of CKD. Up to 40% of patients with diabetes develop diabetic kidney disease, which can progress to end-stage renal disease (ESRD) requiring dialysis or kidney transplantation. In fact, diabetic kidney disease is the top cause of ESRD in the United States.
Diagnostic criteria for CKD include elevated urinary albumin excretion (albuminuria) and/or eGFR < 60 mL/1.73 m2 that persists for more than 3 months. The normal presentation of diabetic kidney disease includes long-standing diabetes, retinopathy, albuminuria without gross hematuria, and gradually progressive decline of eGFR. However, signs of diabetic kidney disease may be present in patients at diagnosis or without retinopathy in T2D. Reduced eGFR without albuminuria has been frequently reported in both type 1 diabetes (T1D) and T2D and is becoming increasingly common as the prevalence of diabetes rises in the United States.
Chronic kidney disease is usually identified through routine screening with serum chemistry profile and urine studies or as an incidental finding. Less often, patients may present with symptoms, such as gross hematuria, "foamy urine" (a sign of albuminuria), nocturia, flank pain, or decreased urine output. In advanced cases, patients may report fatigue, poor appetite, nausea, vomiting, a metallic taste, unintentional weight loss, pruritus, changes in mental status, dyspnea, and/or peripheral edema.
The American Diabetes Association (ADA) 2023 Standards of Care in Diabetes describes five stages of CKD. Stages 1-2 are defined by evidence of high albuminuria with eGFR ≥ 60 mL/min/1.73 m2, while stages 3-5 are defined by progressively lower ranges of eGFR. Of note, at any eGFR, the degree of albuminuria is associated with risk for cardiovascular disease, CKD progression, and mortality. Thus, as noted by the ADA Standards, both eGFR and albuminuria should be used to guide treatment decisions; additionally, eGFR levels are essential for modifying drug dosages or restrictions of use, and the degree of albuminuria should influence selection of antihypertensive agents and glucose-lowering medications.
According to the ADA 2023 Standards of Care in Diabetes, for people with non–dialysis-dependent CKD, dietary protein intake should be ∼0.8 g/kg body weight per day (the recommended daily allowance), as this level has been shown to slow GFR decline compared with higher levels of dietary protein intake, with evidence of a greater effect over time. Conversely, higher levels of dietary protein intake (> 20% of daily calories from protein or > 1.3 g/kg/d) have been associated with increased albuminuria, more rapid kidney function loss, and cardiovascular disease mortality. For patients on dialysis, higher levels of dietary protein intake should be considered, because malnutrition is a significant problem in some of these patients.
Urinary excretion of sodium and potassium may be impaired in patients with reduced eGFR. Thus, restriction of dietary sodium to < 2300 mg/d may help to control blood pressure and reduce cardiovascular risk, and restriction of dietary potassium may be necessary to control serum potassium concentration.
Intensive glycemic control with the goal of achieving near-normoglycemia has been shown to delay the onset and progression of albuminuria and reduced eGFR in patients with diabetes. Insulin alone was used to lower blood glucose in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study of T1D while a variety of agents were used in clinical trials of T2D, supporting the conclusion that glycemic control itself helps prevent CKD and its progression. However, the presence of CKD affects the risks and benefits of intensive glycemic control and several glucose-lowering medications. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial of T2D, increased adverse effects of intensive glycemic control (hypoglycemia and mortality) were seen among patients with kidney disease at baseline. Moreover, it may take at least 2 years to see improved eGFR outcomes as an effect of intensive glycemic control. Therefore, in some patients with prevalent CKD and substantial comorbidity, target A1c levels may be less intensive.
According to guidance from the US Food and Drug Administration, eGFR should be monitored while taking metformin and metformin is contraindicated in patients with an eGFR < 30 mL/min/1.73 m2. Clinicians should assess the benefits and risks of continuing treatment when eGFR falls to < 45 mL/min/1.73 m2.
The ADA recommends that sodium–glucose cotransporter 2 inhibitors be given to all patients with stage 3 CKD or higher and T2D, regardless of glycemic control, as they have been shown to delay CKD progression and reduce heart failure risk independent of glycemic control. Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) also have direct effects on the kidney and have been reported to improve renal outcomes compared with placebo. In patients for whom cardiovascular risk is a predominant problem, the ADA suggests using GLP-1 RAs for cardiovascular risk reduction.
Comprehensive guidance on the management of CKD in patients with T2D is available in the ADA 2023 Standards of Care in Diabetes.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of chronic kidney disease (CKD).
CKD affects between 8% and 16% of the population worldwide. Risk factors for CKD are numerous and include T2D, hypertension, and prediabetes. Diabetes is the leading cause of CKD. Up to 40% of patients with diabetes develop diabetic kidney disease, which can progress to end-stage renal disease (ESRD) requiring dialysis or kidney transplantation. In fact, diabetic kidney disease is the top cause of ESRD in the United States.
Diagnostic criteria for CKD include elevated urinary albumin excretion (albuminuria) and/or eGFR < 60 mL/1.73 m2 that persists for more than 3 months. The normal presentation of diabetic kidney disease includes long-standing diabetes, retinopathy, albuminuria without gross hematuria, and gradually progressive decline of eGFR. However, signs of diabetic kidney disease may be present in patients at diagnosis or without retinopathy in T2D. Reduced eGFR without albuminuria has been frequently reported in both type 1 diabetes (T1D) and T2D and is becoming increasingly common as the prevalence of diabetes rises in the United States.
Chronic kidney disease is usually identified through routine screening with serum chemistry profile and urine studies or as an incidental finding. Less often, patients may present with symptoms, such as gross hematuria, "foamy urine" (a sign of albuminuria), nocturia, flank pain, or decreased urine output. In advanced cases, patients may report fatigue, poor appetite, nausea, vomiting, a metallic taste, unintentional weight loss, pruritus, changes in mental status, dyspnea, and/or peripheral edema.
The American Diabetes Association (ADA) 2023 Standards of Care in Diabetes describes five stages of CKD. Stages 1-2 are defined by evidence of high albuminuria with eGFR ≥ 60 mL/min/1.73 m2, while stages 3-5 are defined by progressively lower ranges of eGFR. Of note, at any eGFR, the degree of albuminuria is associated with risk for cardiovascular disease, CKD progression, and mortality. Thus, as noted by the ADA Standards, both eGFR and albuminuria should be used to guide treatment decisions; additionally, eGFR levels are essential for modifying drug dosages or restrictions of use, and the degree of albuminuria should influence selection of antihypertensive agents and glucose-lowering medications.
According to the ADA 2023 Standards of Care in Diabetes, for people with non–dialysis-dependent CKD, dietary protein intake should be ∼0.8 g/kg body weight per day (the recommended daily allowance), as this level has been shown to slow GFR decline compared with higher levels of dietary protein intake, with evidence of a greater effect over time. Conversely, higher levels of dietary protein intake (> 20% of daily calories from protein or > 1.3 g/kg/d) have been associated with increased albuminuria, more rapid kidney function loss, and cardiovascular disease mortality. For patients on dialysis, higher levels of dietary protein intake should be considered, because malnutrition is a significant problem in some of these patients.
Urinary excretion of sodium and potassium may be impaired in patients with reduced eGFR. Thus, restriction of dietary sodium to < 2300 mg/d may help to control blood pressure and reduce cardiovascular risk, and restriction of dietary potassium may be necessary to control serum potassium concentration.
Intensive glycemic control with the goal of achieving near-normoglycemia has been shown to delay the onset and progression of albuminuria and reduced eGFR in patients with diabetes. Insulin alone was used to lower blood glucose in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study of T1D while a variety of agents were used in clinical trials of T2D, supporting the conclusion that glycemic control itself helps prevent CKD and its progression. However, the presence of CKD affects the risks and benefits of intensive glycemic control and several glucose-lowering medications. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial of T2D, increased adverse effects of intensive glycemic control (hypoglycemia and mortality) were seen among patients with kidney disease at baseline. Moreover, it may take at least 2 years to see improved eGFR outcomes as an effect of intensive glycemic control. Therefore, in some patients with prevalent CKD and substantial comorbidity, target A1c levels may be less intensive.
According to guidance from the US Food and Drug Administration, eGFR should be monitored while taking metformin and metformin is contraindicated in patients with an eGFR < 30 mL/min/1.73 m2. Clinicians should assess the benefits and risks of continuing treatment when eGFR falls to < 45 mL/min/1.73 m2.
The ADA recommends that sodium–glucose cotransporter 2 inhibitors be given to all patients with stage 3 CKD or higher and T2D, regardless of glycemic control, as they have been shown to delay CKD progression and reduce heart failure risk independent of glycemic control. Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) also have direct effects on the kidney and have been reported to improve renal outcomes compared with placebo. In patients for whom cardiovascular risk is a predominant problem, the ADA suggests using GLP-1 RAs for cardiovascular risk reduction.
Comprehensive guidance on the management of CKD in patients with T2D is available in the ADA 2023 Standards of Care in Diabetes.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of chronic kidney disease (CKD).
CKD affects between 8% and 16% of the population worldwide. Risk factors for CKD are numerous and include T2D, hypertension, and prediabetes. Diabetes is the leading cause of CKD. Up to 40% of patients with diabetes develop diabetic kidney disease, which can progress to end-stage renal disease (ESRD) requiring dialysis or kidney transplantation. In fact, diabetic kidney disease is the top cause of ESRD in the United States.
Diagnostic criteria for CKD include elevated urinary albumin excretion (albuminuria) and/or eGFR < 60 mL/1.73 m2 that persists for more than 3 months. The normal presentation of diabetic kidney disease includes long-standing diabetes, retinopathy, albuminuria without gross hematuria, and gradually progressive decline of eGFR. However, signs of diabetic kidney disease may be present in patients at diagnosis or without retinopathy in T2D. Reduced eGFR without albuminuria has been frequently reported in both type 1 diabetes (T1D) and T2D and is becoming increasingly common as the prevalence of diabetes rises in the United States.
Chronic kidney disease is usually identified through routine screening with serum chemistry profile and urine studies or as an incidental finding. Less often, patients may present with symptoms, such as gross hematuria, "foamy urine" (a sign of albuminuria), nocturia, flank pain, or decreased urine output. In advanced cases, patients may report fatigue, poor appetite, nausea, vomiting, a metallic taste, unintentional weight loss, pruritus, changes in mental status, dyspnea, and/or peripheral edema.
The American Diabetes Association (ADA) 2023 Standards of Care in Diabetes describes five stages of CKD. Stages 1-2 are defined by evidence of high albuminuria with eGFR ≥ 60 mL/min/1.73 m2, while stages 3-5 are defined by progressively lower ranges of eGFR. Of note, at any eGFR, the degree of albuminuria is associated with risk for cardiovascular disease, CKD progression, and mortality. Thus, as noted by the ADA Standards, both eGFR and albuminuria should be used to guide treatment decisions; additionally, eGFR levels are essential for modifying drug dosages or restrictions of use, and the degree of albuminuria should influence selection of antihypertensive agents and glucose-lowering medications.
According to the ADA 2023 Standards of Care in Diabetes, for people with non–dialysis-dependent CKD, dietary protein intake should be ∼0.8 g/kg body weight per day (the recommended daily allowance), as this level has been shown to slow GFR decline compared with higher levels of dietary protein intake, with evidence of a greater effect over time. Conversely, higher levels of dietary protein intake (> 20% of daily calories from protein or > 1.3 g/kg/d) have been associated with increased albuminuria, more rapid kidney function loss, and cardiovascular disease mortality. For patients on dialysis, higher levels of dietary protein intake should be considered, because malnutrition is a significant problem in some of these patients.
Urinary excretion of sodium and potassium may be impaired in patients with reduced eGFR. Thus, restriction of dietary sodium to < 2300 mg/d may help to control blood pressure and reduce cardiovascular risk, and restriction of dietary potassium may be necessary to control serum potassium concentration.
Intensive glycemic control with the goal of achieving near-normoglycemia has been shown to delay the onset and progression of albuminuria and reduced eGFR in patients with diabetes. Insulin alone was used to lower blood glucose in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study of T1D while a variety of agents were used in clinical trials of T2D, supporting the conclusion that glycemic control itself helps prevent CKD and its progression. However, the presence of CKD affects the risks and benefits of intensive glycemic control and several glucose-lowering medications. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial of T2D, increased adverse effects of intensive glycemic control (hypoglycemia and mortality) were seen among patients with kidney disease at baseline. Moreover, it may take at least 2 years to see improved eGFR outcomes as an effect of intensive glycemic control. Therefore, in some patients with prevalent CKD and substantial comorbidity, target A1c levels may be less intensive.
According to guidance from the US Food and Drug Administration, eGFR should be monitored while taking metformin and metformin is contraindicated in patients with an eGFR < 30 mL/min/1.73 m2. Clinicians should assess the benefits and risks of continuing treatment when eGFR falls to < 45 mL/min/1.73 m2.
The ADA recommends that sodium–glucose cotransporter 2 inhibitors be given to all patients with stage 3 CKD or higher and T2D, regardless of glycemic control, as they have been shown to delay CKD progression and reduce heart failure risk independent of glycemic control. Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) also have direct effects on the kidney and have been reported to improve renal outcomes compared with placebo. In patients for whom cardiovascular risk is a predominant problem, the ADA suggests using GLP-1 RAs for cardiovascular risk reduction.
Comprehensive guidance on the management of CKD in patients with T2D is available in the ADA 2023 Standards of Care in Diabetes.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 56-year-old Hispanic man presents and reports a 2-month history of fatigue, loss of appetite, pruritus, and swelling of the legs, ankles, and feet. The patient was diagnosed with type 2 diabetes (T2D), hypertension, and hyperlipidemia 7 years ago after an ophthalmologist diagnosed him with diabetic retinopathy and referred him for medical care. Since then, he has been inconsistent with attending regular follow-up visits. He is a current smoker (40-pack/year history).
At today's visit, the patient's blood pressure is 150/95 mm Hg, heart rate is 97 beats/min, and respiration rate is 29 breaths/min. He is 5 ft 9 in and weighs 210 lb (BMI 31). Current medications include metformin ER 1000 mg/d, atorvastatin 40 mg/d, amlodipine 10 mg/d, and hydrochlorothiazide 25 mg/d. At a routine visit 4 months ago, the patient's estimated glomerular filtration rate (eGFR) was 59 mL/min/1.73 m2; at a subsequent follow-up visit, his eGFR was 57 mL/min/1.73 m2.
Pertinent laboratory findings today include eGFR 56 mL/min/1.73 m2, serum creatinine 2.7 g/dL, serum albumin 3.3 g/dL, A1c 8.8%, glucose 189 mg/dL, and an albumin-creatinine ratio of 225 mg/g. All other findings are within normal ranges.
T2D Medications II
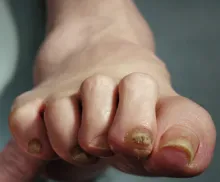
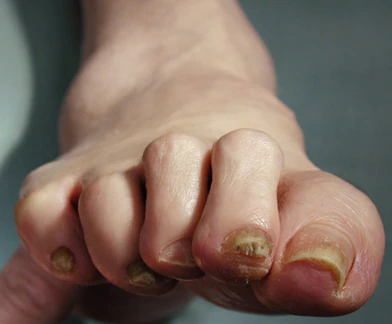
Complaints of foot pain
This patient's physical findings are consistent with a diagnosis of claw toe, which can be caused by diabetes-related peripheral neuropathy.
According to the International Diabetes Federation, diabetes currently affects approximately 537 million adults worldwide. The number of individuals living with diabetes is expected to exceed 640 million by 2030 and 780 million by 2045. In the United States, more than 37 million people are living with diabetes.
Foot complications related to diabetes represent a significant economic and social burden and can profoundly affect a patient's quality of life and medical outcomes. Common diabetes-related foot complications include foot deformity and peripheral neuropathy, both of which increase the risk for ulceration and amputation. The most common deformity is at the metatarsophalangeal joint (MTPJ). As many as 85% of patients with a history of ulcers and amputation have an MTPJ deformity such as claw toe or hammertoe.
Although they are often grouped together, claw toe and hammertoe have distinct features. Extended MTPJ, flexed proximal interphalangeal joint (PIPJ), and flexed distal interphalangeal joint (DIPJ) are characteristic of claw toe. While hammertoe also has extended MTPJ and flexed PIPJ, the DIPJ is extended rather than flexed. In both cases, the area of high pressure at risk for skin breakdown and ulceration is at the metatarsal head as a result of MTPJ hyperextension deformity.
Prompt detection and care of diabetes-related foot complications can minimize progression and negative consequences on patients' health and quality of life. According to the American Diabetes Association, all patients with diabetes should undergo a comprehensive foot evaluation at least annually to identify risk factors for ulceration and amputation, which include foot deformities, poor glycemic control, peripheral neuropathy, cigarette smoking, preulcerative callus or corn, peripheral artery disease, chronic kidney disease, visual impairment, and a history of ulceration or amputation. When patients present with a history of ulceration or amputation, a foot inspection should be conducted at each visit.
A comprehensive foot evaluation should include inspection of the skin, evaluation of any foot deformities, a neurologic assessment (10-g monofilament testing with at least one other assessment: pinprick, temperature, vibration), and a vascular assessment, including pulses in the legs and feet.
Patients should be educated on risk factors and appropriate management of foot-related complications, including the importance of effective glycemic control and daily monitoring of feet. Treatment may be medical, surgical, or both, as indicated by the individual patient's presentation. Conservative treatment approaches include footwear that is extra wide or deep, avoiding high-heeled and narrow-toed shoes, use of a metatarsal bar or pad, cushioning sleeves or stocking caps with silicon linings, and a longitudinal pad beneath the toes.
Complete recommendations on achieving glycemic control in T2D can be found in the 2022 American Diabetes Association Standards of Medical Care. Guidelines on foot care are also available.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's physical findings are consistent with a diagnosis of claw toe, which can be caused by diabetes-related peripheral neuropathy.
According to the International Diabetes Federation, diabetes currently affects approximately 537 million adults worldwide. The number of individuals living with diabetes is expected to exceed 640 million by 2030 and 780 million by 2045. In the United States, more than 37 million people are living with diabetes.
Foot complications related to diabetes represent a significant economic and social burden and can profoundly affect a patient's quality of life and medical outcomes. Common diabetes-related foot complications include foot deformity and peripheral neuropathy, both of which increase the risk for ulceration and amputation. The most common deformity is at the metatarsophalangeal joint (MTPJ). As many as 85% of patients with a history of ulcers and amputation have an MTPJ deformity such as claw toe or hammertoe.
Although they are often grouped together, claw toe and hammertoe have distinct features. Extended MTPJ, flexed proximal interphalangeal joint (PIPJ), and flexed distal interphalangeal joint (DIPJ) are characteristic of claw toe. While hammertoe also has extended MTPJ and flexed PIPJ, the DIPJ is extended rather than flexed. In both cases, the area of high pressure at risk for skin breakdown and ulceration is at the metatarsal head as a result of MTPJ hyperextension deformity.
Prompt detection and care of diabetes-related foot complications can minimize progression and negative consequences on patients' health and quality of life. According to the American Diabetes Association, all patients with diabetes should undergo a comprehensive foot evaluation at least annually to identify risk factors for ulceration and amputation, which include foot deformities, poor glycemic control, peripheral neuropathy, cigarette smoking, preulcerative callus or corn, peripheral artery disease, chronic kidney disease, visual impairment, and a history of ulceration or amputation. When patients present with a history of ulceration or amputation, a foot inspection should be conducted at each visit.
A comprehensive foot evaluation should include inspection of the skin, evaluation of any foot deformities, a neurologic assessment (10-g monofilament testing with at least one other assessment: pinprick, temperature, vibration), and a vascular assessment, including pulses in the legs and feet.
Patients should be educated on risk factors and appropriate management of foot-related complications, including the importance of effective glycemic control and daily monitoring of feet. Treatment may be medical, surgical, or both, as indicated by the individual patient's presentation. Conservative treatment approaches include footwear that is extra wide or deep, avoiding high-heeled and narrow-toed shoes, use of a metatarsal bar or pad, cushioning sleeves or stocking caps with silicon linings, and a longitudinal pad beneath the toes.
Complete recommendations on achieving glycemic control in T2D can be found in the 2022 American Diabetes Association Standards of Medical Care. Guidelines on foot care are also available.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's physical findings are consistent with a diagnosis of claw toe, which can be caused by diabetes-related peripheral neuropathy.
According to the International Diabetes Federation, diabetes currently affects approximately 537 million adults worldwide. The number of individuals living with diabetes is expected to exceed 640 million by 2030 and 780 million by 2045. In the United States, more than 37 million people are living with diabetes.
Foot complications related to diabetes represent a significant economic and social burden and can profoundly affect a patient's quality of life and medical outcomes. Common diabetes-related foot complications include foot deformity and peripheral neuropathy, both of which increase the risk for ulceration and amputation. The most common deformity is at the metatarsophalangeal joint (MTPJ). As many as 85% of patients with a history of ulcers and amputation have an MTPJ deformity such as claw toe or hammertoe.
Although they are often grouped together, claw toe and hammertoe have distinct features. Extended MTPJ, flexed proximal interphalangeal joint (PIPJ), and flexed distal interphalangeal joint (DIPJ) are characteristic of claw toe. While hammertoe also has extended MTPJ and flexed PIPJ, the DIPJ is extended rather than flexed. In both cases, the area of high pressure at risk for skin breakdown and ulceration is at the metatarsal head as a result of MTPJ hyperextension deformity.
Prompt detection and care of diabetes-related foot complications can minimize progression and negative consequences on patients' health and quality of life. According to the American Diabetes Association, all patients with diabetes should undergo a comprehensive foot evaluation at least annually to identify risk factors for ulceration and amputation, which include foot deformities, poor glycemic control, peripheral neuropathy, cigarette smoking, preulcerative callus or corn, peripheral artery disease, chronic kidney disease, visual impairment, and a history of ulceration or amputation. When patients present with a history of ulceration or amputation, a foot inspection should be conducted at each visit.
A comprehensive foot evaluation should include inspection of the skin, evaluation of any foot deformities, a neurologic assessment (10-g monofilament testing with at least one other assessment: pinprick, temperature, vibration), and a vascular assessment, including pulses in the legs and feet.
Patients should be educated on risk factors and appropriate management of foot-related complications, including the importance of effective glycemic control and daily monitoring of feet. Treatment may be medical, surgical, or both, as indicated by the individual patient's presentation. Conservative treatment approaches include footwear that is extra wide or deep, avoiding high-heeled and narrow-toed shoes, use of a metatarsal bar or pad, cushioning sleeves or stocking caps with silicon linings, and a longitudinal pad beneath the toes.
Complete recommendations on achieving glycemic control in T2D can be found in the 2022 American Diabetes Association Standards of Medical Care. Guidelines on foot care are also available.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 59-year-old woman newly diagnosed with type 2 diabetes (T2D) and hypercholesterolemia presents with complaints of foot pain, particularly while wearing shoes. Physical examination reveals an extended metatarsophalangeal joint, a flexed proximal interphalangeal joint, and flexed distal interphalangeal joint. Her toenails are discolored with a yellowish hue, and callus formation is noted over the metatarsal area. The patient reports pain at the tip of the toe from pressure against the point of the distal phalanx. She states that she has not experienced any numbness, tingling, or muscle weakness. Before her recent diabetes diagnosis, the patient had not been receiving regular medical care. The patient's current medications include metformin 500 mg/d, empagliflozin 10 mg/d, and rosuvastatin 10 mg/d.
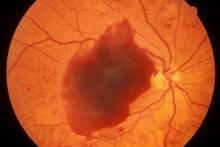
Flickering sensation in eyes
The American Diabetes Association (ADA) position statement on diabetic retinopathy states that hyperglycemia has been the most consistently associated risk factor for retinopathy. A large and consistent set of observational studies and clinical trials confirms the association of poor glucose control and retinopathy.
The Diabetes Control and Complications Trial (DCCT), a randomized controlled clinical trial of intensive glycemic control vs conventional glycemic control in people with type 1 diabetes (T1D), demonstrated that intensive therapy reduced the development or progression of diabetic retinopathy by 34%-76%. The DCCT also demonstrated a definitive relationship between hyperglycemia and diabetic microvascular complications, including retinopathy. Early treatment with intensive therapy was effective.
The UK Prospective Diabetes Study (UKPDS) of patients with newly diagnosed T2D conclusively demonstrated that improved blood glucose control reduced the risk for retinopathy and nephropathy and, possibly, neuropathy. The overall microvascular complication rate was decreased by 25% in patients receiving intensive therapy vs conventional therapy. Epidemiologic analysis of the UKPDS data showed a continuous relationship between the risk for microvascular complications and glycemia, such that every percentage-point decrease in A1c (eg, 9% to 8%) was associated with a 35% reduction in the risk for microvascular complications.
More recently, the ACCORD trial of medical therapies demonstrated that intensive glycemic control reduced the risk for progression of diabetic retinopathy in people with T2D of 10 years' duration. This study included 2856 ACCORD participants enrolled in the ACCORD Eye Study and followed for 4 years.
The ADA recommends screening by an ophthalmologist for diabetic retinopathy within 5 years of the diagnosis of T1D and at the time of diagnosis of T2D. Women with preexisting diabetes who are planning pregnancy or who have become pregnant should be screened before pregnancy or in the first trimester.
While optimization of blood glucose, blood pressure, and serum lipid levels in conjunction with appropriately scheduled dilated eye examinations can substantially decrease the risk for vision loss from diabetic retinopathy, a significant proportion of those affected with diabetes develop diabetic macular edema or proliferative changes that require intervention. ADA treatment recommendations are:
• Refer patients with any level of macular edema, severe nonproliferative diabetic retinopathy (a precursor of proliferative diabetic retinopathy), or proliferative diabetic retinopathy to an ophthalmologist knowledgeable and experienced in the management and treatment of diabetic retinopathy.
• Laser photocoagulation therapy reduces the risk for vision loss in patients with high-risk proliferative diabetic retinopathy and, in some cases, severe nonproliferative diabetic retinopathy.
• Intravitreous injections of anti–vascular endothelial growth factor are indicated for central-involved diabetic macular edema, which occurs beneath the foveal center and may threaten reading vision.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The American Diabetes Association (ADA) position statement on diabetic retinopathy states that hyperglycemia has been the most consistently associated risk factor for retinopathy. A large and consistent set of observational studies and clinical trials confirms the association of poor glucose control and retinopathy.
The Diabetes Control and Complications Trial (DCCT), a randomized controlled clinical trial of intensive glycemic control vs conventional glycemic control in people with type 1 diabetes (T1D), demonstrated that intensive therapy reduced the development or progression of diabetic retinopathy by 34%-76%. The DCCT also demonstrated a definitive relationship between hyperglycemia and diabetic microvascular complications, including retinopathy. Early treatment with intensive therapy was effective.
The UK Prospective Diabetes Study (UKPDS) of patients with newly diagnosed T2D conclusively demonstrated that improved blood glucose control reduced the risk for retinopathy and nephropathy and, possibly, neuropathy. The overall microvascular complication rate was decreased by 25% in patients receiving intensive therapy vs conventional therapy. Epidemiologic analysis of the UKPDS data showed a continuous relationship between the risk for microvascular complications and glycemia, such that every percentage-point decrease in A1c (eg, 9% to 8%) was associated with a 35% reduction in the risk for microvascular complications.
More recently, the ACCORD trial of medical therapies demonstrated that intensive glycemic control reduced the risk for progression of diabetic retinopathy in people with T2D of 10 years' duration. This study included 2856 ACCORD participants enrolled in the ACCORD Eye Study and followed for 4 years.
The ADA recommends screening by an ophthalmologist for diabetic retinopathy within 5 years of the diagnosis of T1D and at the time of diagnosis of T2D. Women with preexisting diabetes who are planning pregnancy or who have become pregnant should be screened before pregnancy or in the first trimester.
While optimization of blood glucose, blood pressure, and serum lipid levels in conjunction with appropriately scheduled dilated eye examinations can substantially decrease the risk for vision loss from diabetic retinopathy, a significant proportion of those affected with diabetes develop diabetic macular edema or proliferative changes that require intervention. ADA treatment recommendations are:
• Refer patients with any level of macular edema, severe nonproliferative diabetic retinopathy (a precursor of proliferative diabetic retinopathy), or proliferative diabetic retinopathy to an ophthalmologist knowledgeable and experienced in the management and treatment of diabetic retinopathy.
• Laser photocoagulation therapy reduces the risk for vision loss in patients with high-risk proliferative diabetic retinopathy and, in some cases, severe nonproliferative diabetic retinopathy.
• Intravitreous injections of anti–vascular endothelial growth factor are indicated for central-involved diabetic macular edema, which occurs beneath the foveal center and may threaten reading vision.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The American Diabetes Association (ADA) position statement on diabetic retinopathy states that hyperglycemia has been the most consistently associated risk factor for retinopathy. A large and consistent set of observational studies and clinical trials confirms the association of poor glucose control and retinopathy.
The Diabetes Control and Complications Trial (DCCT), a randomized controlled clinical trial of intensive glycemic control vs conventional glycemic control in people with type 1 diabetes (T1D), demonstrated that intensive therapy reduced the development or progression of diabetic retinopathy by 34%-76%. The DCCT also demonstrated a definitive relationship between hyperglycemia and diabetic microvascular complications, including retinopathy. Early treatment with intensive therapy was effective.
The UK Prospective Diabetes Study (UKPDS) of patients with newly diagnosed T2D conclusively demonstrated that improved blood glucose control reduced the risk for retinopathy and nephropathy and, possibly, neuropathy. The overall microvascular complication rate was decreased by 25% in patients receiving intensive therapy vs conventional therapy. Epidemiologic analysis of the UKPDS data showed a continuous relationship between the risk for microvascular complications and glycemia, such that every percentage-point decrease in A1c (eg, 9% to 8%) was associated with a 35% reduction in the risk for microvascular complications.
More recently, the ACCORD trial of medical therapies demonstrated that intensive glycemic control reduced the risk for progression of diabetic retinopathy in people with T2D of 10 years' duration. This study included 2856 ACCORD participants enrolled in the ACCORD Eye Study and followed for 4 years.
The ADA recommends screening by an ophthalmologist for diabetic retinopathy within 5 years of the diagnosis of T1D and at the time of diagnosis of T2D. Women with preexisting diabetes who are planning pregnancy or who have become pregnant should be screened before pregnancy or in the first trimester.
While optimization of blood glucose, blood pressure, and serum lipid levels in conjunction with appropriately scheduled dilated eye examinations can substantially decrease the risk for vision loss from diabetic retinopathy, a significant proportion of those affected with diabetes develop diabetic macular edema or proliferative changes that require intervention. ADA treatment recommendations are:
• Refer patients with any level of macular edema, severe nonproliferative diabetic retinopathy (a precursor of proliferative diabetic retinopathy), or proliferative diabetic retinopathy to an ophthalmologist knowledgeable and experienced in the management and treatment of diabetic retinopathy.
• Laser photocoagulation therapy reduces the risk for vision loss in patients with high-risk proliferative diabetic retinopathy and, in some cases, severe nonproliferative diabetic retinopathy.
• Intravitreous injections of anti–vascular endothelial growth factor are indicated for central-involved diabetic macular edema, which occurs beneath the foveal center and may threaten reading vision.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 48-year-old Black man with type 2 diabetes (T2D) presented with complaints of a "flickering" sensation and a decrease in brightness of colors in both eyes as well as floaters in his left eye for several weeks. He reported that his symptoms fluctuate with changes in his blood glucose levels. His last eye examination was 2 years ago and his ocular history was unremarkable. His medical history was significant with a history of hypertension and T2D requiring insulin. His most recent glycated hemoglobin (A1c), 2 months ago, was 8.4%. His BMI was 31.2. The patient's medications were dulaglutide 0.75 mg injection pen, glargine insulin 42 units, losartan 100 mg, and amlodipine 10 mg.
On examination, his best-corrected visual acuity was 20/20 in the right eye and 20/30 in the left eye. Confrontation fields were intact, extraocular movements were full and extensive, and both pupils were equal, round, and reactive to light without afferent pupillary defects. Anterior segment examination was unremarkable in both eyes, without iris neovascularization. Intraocular pressures were 17 mm Hg in the right eye and 16 mm Hg in the left eye. On dilated fundus examination, the cup-to-disc ratio was 0.45 horizontally and vertically, with the presence of 1/4 disc diameters of neovascularization of the disc in the right eye and 2/3 disc diameters of neovascularization of the disc in the left eye.
Posterior segment findings were significant for scattered microaneurysms and dot/blot hemorrhages in the maculae. In the periphery of both eyes, there were tortuous vessels, scatter microaneurysms with dot/blot hemorrhages, and multiple areas of neovascularization elsewhere, with several foci of vitreous traction. There was no vitreous hemorrhage or tractional retinal detachment of either eye.
Spectral domain optical coherence tomography revealed an epiretinal membrane in the right eye and a blunted foveal contour with parafoveal cystic spaces, probably secondary to vitreomacular contraction. The left eye also had an epiretinal membrane and blunted foveal contour secondary to vitreomacular adhesion. The patient was diagnosed with bilateral high-risk proliferative diabetic retinopathy.
Type 2 Diabetes Treatment
Pruritus and pitting edema
The 2020 Kidney Disease Improving Global Outcomes (KDIGO) diabetes management in CKD guideline states that most patients with diabetic nephropathy and an eGFR ≥ 30 mL/min/1.73 m2 benefit from treatment with both metformin and a sodium-glucose cotransporter 2 (SGLT2) inhibitor, which have been demonstrated to offer substantial benefits in reducing the risks for diabetic nephropathy and cardiovascular disease.
In patients who do not reach individualized targets with metformin and an SGLT2 inhibitor, or who are unable to use these medications, a long-acting glucagon-like peptide 1 (GLP-1) receptor antagonist may be used.
Metformin should be administered with caution to patients with CKD because it may increase the risk for lactic acidosis. It is contraindicated in patients with an eGFR < 30, but this patient's eGFR is adequate. Many clinicians might use a lower metformin dosage (1500 mg) as a precaution. Given how high his A1c is, adding a GLP-1 receptor antagonist is probably going to be needed because an SGLT2 inhibitor is only intermediate in terms of glucose reduction.
For control of his hypertension, the American Diabetes Association recommends either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) as first-line treatment. However, one agent alone is unlikely to control this patient's hypertension. At his level of eGFR, a thiazide diuretic is unlikely to be very effective. Therefore, a loop diuretic should be initiated with the ACE inhibitor or ARB, especially because he has edema.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The 2020 Kidney Disease Improving Global Outcomes (KDIGO) diabetes management in CKD guideline states that most patients with diabetic nephropathy and an eGFR ≥ 30 mL/min/1.73 m2 benefit from treatment with both metformin and a sodium-glucose cotransporter 2 (SGLT2) inhibitor, which have been demonstrated to offer substantial benefits in reducing the risks for diabetic nephropathy and cardiovascular disease.
In patients who do not reach individualized targets with metformin and an SGLT2 inhibitor, or who are unable to use these medications, a long-acting glucagon-like peptide 1 (GLP-1) receptor antagonist may be used.
Metformin should be administered with caution to patients with CKD because it may increase the risk for lactic acidosis. It is contraindicated in patients with an eGFR < 30, but this patient's eGFR is adequate. Many clinicians might use a lower metformin dosage (1500 mg) as a precaution. Given how high his A1c is, adding a GLP-1 receptor antagonist is probably going to be needed because an SGLT2 inhibitor is only intermediate in terms of glucose reduction.
For control of his hypertension, the American Diabetes Association recommends either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) as first-line treatment. However, one agent alone is unlikely to control this patient's hypertension. At his level of eGFR, a thiazide diuretic is unlikely to be very effective. Therefore, a loop diuretic should be initiated with the ACE inhibitor or ARB, especially because he has edema.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The 2020 Kidney Disease Improving Global Outcomes (KDIGO) diabetes management in CKD guideline states that most patients with diabetic nephropathy and an eGFR ≥ 30 mL/min/1.73 m2 benefit from treatment with both metformin and a sodium-glucose cotransporter 2 (SGLT2) inhibitor, which have been demonstrated to offer substantial benefits in reducing the risks for diabetic nephropathy and cardiovascular disease.
In patients who do not reach individualized targets with metformin and an SGLT2 inhibitor, or who are unable to use these medications, a long-acting glucagon-like peptide 1 (GLP-1) receptor antagonist may be used.
Metformin should be administered with caution to patients with CKD because it may increase the risk for lactic acidosis. It is contraindicated in patients with an eGFR < 30, but this patient's eGFR is adequate. Many clinicians might use a lower metformin dosage (1500 mg) as a precaution. Given how high his A1c is, adding a GLP-1 receptor antagonist is probably going to be needed because an SGLT2 inhibitor is only intermediate in terms of glucose reduction.
For control of his hypertension, the American Diabetes Association recommends either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) as first-line treatment. However, one agent alone is unlikely to control this patient's hypertension. At his level of eGFR, a thiazide diuretic is unlikely to be very effective. Therefore, a loop diuretic should be initiated with the ACE inhibitor or ARB, especially because he has edema.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 47-year-old Black man presents with shortness of breath, pruritus, and pitting edema of the bilateral extremities, which have been present for 6 weeks. He has a 7-year history of type 2 diabetes, hypertension, and hyperlipidemia, as well as a 30–pack-year history of smoking. His blood pressure is 160/95 mm Hg, heart rate is 97 beats/min (regular rate and rhythm), and respiration is 26 breaths/min. He also has proliferative retinopathy. He is 5 ft 10 in and weighs 220 lb (BMI 31.6). He is taking metformin 2550 mg/d. Other medications include simvastatin 20 mg, amlodipine 10 mg, and hydrochlorothiazide 25 mg. He admits to being nonadherent to his medication regimen. A year ago, his estimated glomerular filtration rate (eGFR) was 66 mL/min/1.73 m2 and he had 1+ proteinuria.
Laboratory tests reveal hemoglobin of 8.7 g/dL, creatinine of 3.4 g/dL, eGFR of 32 mL/min/1.73 m2, serum albumin of 3.3 g/dL, A1c of 8.8%, low-density lipoprotein of 143 mg/dL, high-density lipoprotein of 43 mg/dL, random glucose of 186 mg/dL, albumin-creatinine ratio of 3250 mg/g, calcium of 8.7 mg/dL, phosphorus of 4.2 mg/dL, plasma parathyroid hormone of 77 pg/mL, and C-reactive protein of 12.
In summary, this patient has normal albumin levels and increased proteinuria with decreased eGFR. His glucose level and A1c are not controlled. In addition, he has anemia, a low serum albumin level, and edema.
This patient has diabetic nephropathy and is at risk for a cardiovascular event because of his eGFR and long history of diabetes, hypertension, tobacco use, and hyperlipidemia. Intervention to control these risk factors should start immediately to prevent progression to chronic kidney disease (CKD).