User login
Postsurgical pain: Optimizing relief while minimizing use of opioids
CASE Managing pain associated with prolapse and SUI surgery
A 46-year-old woman (G4P4) described 3 years of worsening symptoms related to recurrent stage-3 palpable uterine prolapse. She had associated symptomatic stress urinary incontinence. She had been treated for uterine prolapse 5 years ago with vaginal hysterectomy, bilateral salpingectomy, and high uterosacral-ligament suspension.
After consultation, the patient elected to undergo laparoscopic sacral colpopexy, a mid-urethral sling, and possible anterior and posterior colporrhaphy. Appropriate discussion about the risks and benefits of mesh was provided preoperatively. The surgical team judged her to be highly motivated; she wanted same-day outpatient surgery so that she could go home and then return to work. She had excellent support at home.
How would you counsel this patient about expected postoperative pain? Which medications would you administer to her preoperatively and perioperatively? Which ones would you prescribe for her to manage pain postoperatively?
Adverse impact of prescription opioids in the United States
Although fewer than 5% of the world’s population live in the United States, nearly 80% of the world’s opioids are written for them.1 In 2012, 259 million prescriptions were written for opioids in the United States—more than enough to give every American adult their own bottle of pills.2 Sadly, drug overdose is now a leading cause of accidental death in the United States, with 52,404 lethal drug overdoses in 2015. A startling statistic is that prescription opioid abuse is driving this epidemic, with 20,101 overdose deaths related to prescription pain relievers and 12,990 overdose deaths related to heroin in 2015.3
It is likely that there are multiple reasons prescribing of opioids is epidemic. Surgical pain is a common indication for opioid prescriptions; fewer than half of patients who undergo surgery report adequate postoperative pain relief.4 Recognition of these deficits in pain management has inspired national campaigns to improve patients’ experience with pain and aggressively address pain with drugs such as opioids.5
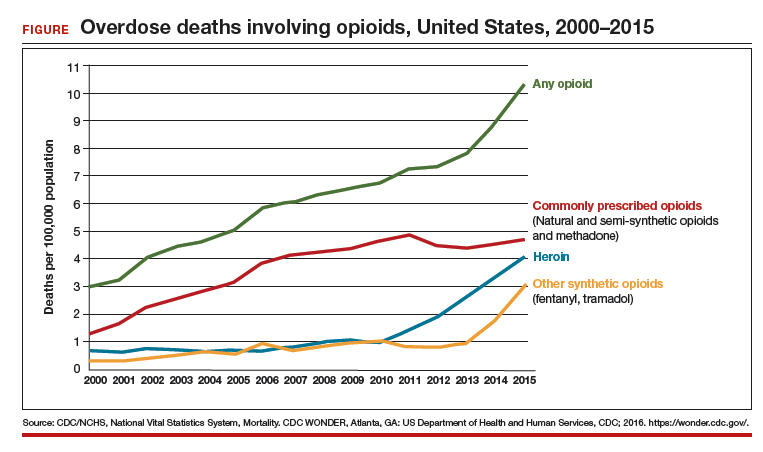
At the same time, marketing efforts by the pharmaceutical industry sought to reassure the medical community that patients would not become addicted to prescription opioid pain relievers if physical pain was the indication for such prescriptions. In response, health care providers began to prescribe opioids at a greater rate. As providers were encouraged to increase prescriptions, opioid medications began to be misused—and only then did it become clear that these medications are, in fact, highly addictive.6 Opioid abuse and overdose rates began to increase; in 2015, more than 33,000 Americans died because of an opioid overdose, including prescription opioids and heroin7 (FIGURE). In fact, although most people recognize the threat posed by illegal heroin, most of the 2 million who abused opioids in 2015 in the United States suffered from prescription abuse; only about a quarter, or about 600,000, abused heroin.8 In addition, more than 80% of people who abuse heroin initially abused prescription opioids.9
Read about medications and strategies for multimodal pain management.
Multimodal approach to pain management
The goals of postsurgical pain treatment are to relieve suffering, optimize bodily functioning after surgery, limit length of the stay, and optimize patient satisfaction. Pain-control regimens should consider the specific surgical procedure and the patient’s medical, psychological, and physical conditions; age; level of fear or anxiety; personal preference; and response to previous treatments.10
Optimally, postsurgical pain management starts well before the day of surgery. Employing such strategies as Enhanced Recovery after Surgery (ERAS) protocols does not necessarily mean providing the same care for every patient, every time. Rather, ERAS serves as a checklist to ensure that all applicable categories of pain medication and pain-control strategies are considered, selected, and dosed according to individual needs.11 (See “Preoperative management of pain expectations.”)
Ideally, before surgery, provide the patient with an opportunity to learn that:
- Her expectations about postsurgical pain should be realistic, and that freedom from pain is not realistic.
- Pain-reduction options should optimize her bodily function and mobility, reduce the degree to which pain interferes with activities, and relieve associated psychological stressors.
- Inherent in the pain management plan should be a goal of minimizing the risks of opioid misuse, abuse, and addiction—for the patient and for her family members and friends.
Opioids
Opioids have been employed to treat pain for 700 years.12 They are powerful pain relievers because they target central mechanisms involved in the perception of pain. Regrettably, because of their central action, opioids have many adverse effects in addition to being highly addictive.
Nonopioid alternatives
Expert consensus, including recommendations of the World Health Organization,11 favors using nonopioids as first-line medications to address surgical pain. Nonopioid analgesic options are acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and adjuvant medications. In addition, nonanalgesic medications such as sedatives, sleep aids, and muscle relaxants can relieve postsurgical pain. Optimal use of these nonopioid medications can significantly reduce or eliminate the need for opioid medications to treat pain. Goals are to 1) reserve opioids for the most severe pain and 2) minimize the number of doses/pills of opioids required to control postsurgical pain.
Acetaminophen. At dosages of 325 to 1,000 mg orally every 4 to 6 hours, to a maximum dosage of 4,000 mg/d, acetaminophen can be used to treat mild pain and, in combination with other medications, moderate-to-severe pain. The drug also can be administered intravenously (IV), although use of the IV route is limited in many hospitals because of its significantly higher expense compared to the oral form.
The mechanism of action of acetaminophen is unique among pain relievers; it can therefore be used in combination with other pain relievers to more effectively treat pain with fewer concerns about medication-induced adverse effects or opioid overdose. However, keep in mind when considering combining analgesics, that acetaminophen is an active ingredient in hundreds of over-the-counter (OTC) and prescription formulations, and that a combination of more than one acetaminophen-containing product can create the risk of overdose.
Acetaminophen should be used with caution in patients with liver disease. That being said, multiple trials have documented safe use in normal body weight adults who do not have hepatic disease, at dosages as high as 4,000 mg over a 24-hour period.13
NSAIDs. A combination of an NSAID and acetaminophen has been documented to reduce the amount of opioid medications required to treat postsurgical pain. In most circumstances, especially for minor surgery, acetaminophen and NSAIDS can be administered just before surgery starts. This preoperative treatment, called “preventive analgesia” or “preemptive analgesia,” has been demonstrated in multiple clinical trials to reduce postoperative pain.14
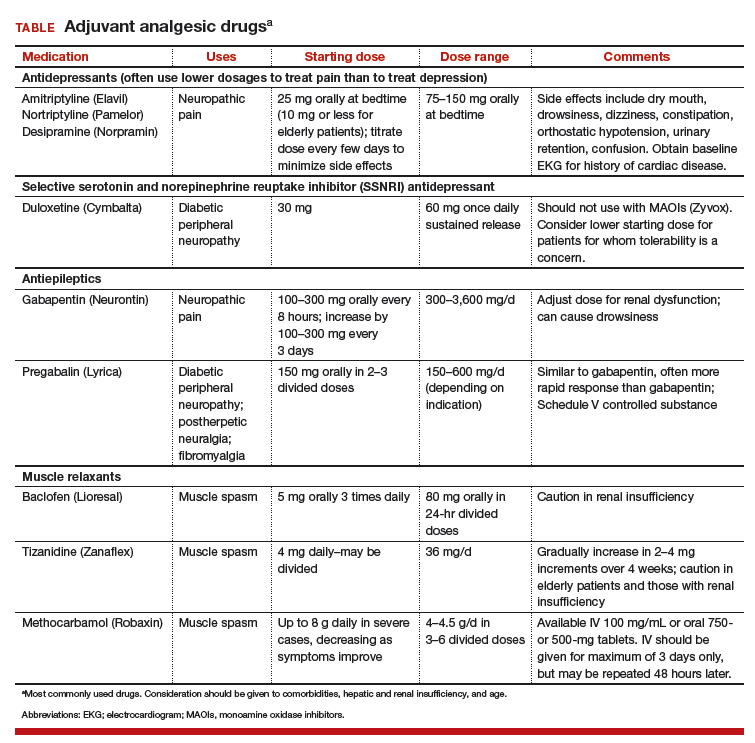
Adjuvant pain medications. Antidepressants, antiepileptic agents, and muscle relaxants—agents that have a primary indication for a condition (or conditions) other than pain and do not directly provide analgesia—have been used as adjuvant pain medications. When employed with traditional analgesics, they have been demonstrated to reduce postsurgical pain scores and the amount of opioids required. These medications need to be used cautiously because some are associated with serious sedation and vertigo (TABLE). Take caution when using adjuvant pain medications in patients older than 65 years; guidance on their use in older patients has been outlined by the American Geriatrics Society and other professional organizations.15
Case Continued
The patient was given the expectation that the 11-mm left lower-quadrant port site would likely be the most bothersome site of pain—a rating of 4 or 5 on a visual analogue scale of 1 to 10, on postoperative day 1, while standing. The other 3 (5-mm) laparoscopic ports, she was told, would, typically, be less bothersome. The patient was educated regarding the role of analgesics and adjuvant medications and cautioned not to exceed 4,000 mg of acetaminophen in any 24-hour period. She was told that gabapentin may make her feel sedated or dizzy, or both; she was encouraged to hold this medication if she found these adverse effects bothersome or limiting.
The following multimodal pain management was established.
Preoperatively, the patient was given:
- Acetaminophen 1.5 g orally (as a liquid, 45 mL of a suspension of 500 mg/15 mL liquid), 2 to 3 hours preoperatively; the surgical suite did not stock IV acetaminophen.
- Gabapentin 600 mg orally, with a sip of water, the morning of surgery.
- Celecoxib 100 mg orally, with a sip of water, the morning of surgery.
Prescriptions for home postoperative pain management were provided preoperatively:
- OTC acetaminophen 1,000 mg (as 2 500-mgtablets) taken as a scheduled dose every 8 hours for the first 48 hours postoperatively.
- Meloxicam 15 mg daily as the NSAID, taken as a scheduled dose once per day for the first 48 hours postoperatively, then as needed.
- Gabapentin 300 mg (in addition to the preoperative dose, above), taken as a scheduled dose every 8 hours for the first 48 hours postoperatively, then as needed.
- Oxycodone 5 mg (without acetaminophen) for breakthrough pain.
Intraoperatively:
- Meticulous attention was paid to patient positioning, to reduce the possibility of back and upper- and lower-extremity injury postoperatively.
- A corticosteroid (dexamethasone 8 mg IV) was administered to minimize postoperative nausea and vomiting and as an adjuvant medication for postoperative pain control.
- Careful attention was paid to limit residual CO2 gas and intraoperative intra-abdominal pressures.
- All laparoscopic port sites were injected with 30 mL of 0.25% bupivacaine with epinephrine, extending to subcutaneous, fascial, and peritoneal layers.
Read about why a multimodal approach is best for postsurgical pain.
Why a multimodal plan to treat pain?
Pain following laparoscopy has been associated with many variables, including patient positioning, port size and placement, amount of port manipulation, and gas retention. After a laparoscopic surgical procedure, patients report pain in the abdomen, back, and shoulders.
Postsurgical pain has 3 components:
- Shoulder pain, thought to result from phrenic nerve irritation caused by lingering CO2 in the abdominal cavity.
- Visceral pain, occurring secondary to stretching of the abdominal cavity.
- Somatic pain, caused by the surgical incision; of the 3 components to pain, somatic pain can have the least impact because laparoscopic incisions are small.
For our patient, prior to the incisions being made, she received local anesthesia intraoperatively to the laparoscopic port sites to include the subcutaneous, fascial, and peritoneal layers. Involving these layers allows for more of a block. An ultrasonography-guided transversus abdominis plane (TAP) block, if available, is highly effective at decreasing postoperative pain, but its efficacy is dependent on the anatomy and the skill of the physician (whether anesthesiologist, gynecologist, or surgeon) who is placing it.16
We used dexamethasone 8 mg IV, intraoperatively because this single dose has been shown to decrease the perception of pain postoperatively. Dexamethasone also has been shown to decrease consumption of oxycodone during the 24 hours after laparoscopic gynecologic surgery.17
CO2 used to insufflate the patient’s abdomen can take as long as 2 days to fully resorb, resulting in increased pain. This discomfort has been described as delayed; the patient might not notice it until she goes home. In a study, 70% of patients had shoulder discomfort following laparoscopy 24 hours after their procedure.18 For this reason, we employed several techniques to reduce this effect:
- We reduced the intra-abdominal pressure limit to 10 mm Hg (from 15 mm Hg) once dissection was complete.
- At the end of the procedure, careful attention was paid to removing as much intra-abdominal gas as possible, including placing the patient in the Trendelenburg position and having the anesthesiologist induce a Valsalva maneuver. This action has been shown to significantly improve pain control compared to placebo intervention.19
- We used humidified CO2, which has been demonstrated to reduce pain in laparoscopic surgery.20
Preemptively, we provided this patient with acetaminophen, celecoxib, and gabapentin, which have been demonstrated to be effective in gynecologic patients to decrease the need for postoperative opioids.21 Also, our patient received counseling, with specific expectations for what to expect following the surgical procedure.
CASE Resolved
Our patient did exceptionally well following surgery. She used only one of the oxycodone pills and did not require unplanned interventions. She took gabapentin, acetaminophen, and meloxicam at their scheduled doses for 2 days. She continued to use meloxicam for 4 more days for mild abdominal pain, then discontinued all medications.She flushed her 9 unused oxycodone pills down the toilet. (See “A word about disposal of ‘excess’ opioids”22) The patient returned to her administrative duties at work 2 weeks after the procedure and reported that she was “very satisfied” with her surgical experience.
The US Food and Drug Administration (FDA) recommends disposing of certain drugs through a take-back program or, if such a program is not readily available, by flushing them down a toilet or sink. In a recent study, investigators concluded that opioids on the FDA's so-called flush list include most opioids in clinical use--even if the entire supply prescribed is to be flushed down the drain. Conservative estimates of environmental degradation were employed in the study; the investigators' conclusion was that these drugs pose a "negligible" eco-toxicologic risk.1
Reference
- Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration "flush list". Sci Total Environ. 2017;609:1023-1040.
In conclusion
Postoperative pain is a complex entity that must be considered to require individualized strategies and, possibly, multiple interventions. Optimally, thorough education, including pain management options, is provided to the patient prior to surgery. Given the current state of opioid abuse in the United States, all gynecologic surgeons should be familiar with multimodal pain therapy and how to employ nonmedical techniques to reduce postsurgical pain without relying solely on opioids. (See “Online resources for pain management”.)
- Drug Disposal Information
(US Department of Justice Drug Enforcement Administration)
https://www.deadiversion.usdoj.gov/drug_disposal/index.html - Surgical Pain Consortium
http://surgicalpainconsortium.org/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- United Nations International Narcotics Control Board. Narcotic drugs: Report 2016: Estimated world requirements for 2017-statistics for 2015. New York, NY. https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2016/Narcotic_Drugs_Publication_2016.pdf. Published 2017. Accessed January 7, 2018.
- Centers for Disease Control and Prevention. Opioid painkiller prescribing: Where you live makes a difference. Atlanta, GA. https://www.cdc.gov/vitalsigns/pdf/2014-07-vitalsigns.pdf. Published July 2014. Accessed January 5, 2018.
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- Gan TJ, Habib AS, Miller TE, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–160.
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625.
- Van Zee A. The promotion and marketing of OxyContin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221–227.
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Results from the 2015 national survey on drug use and health: Detailed tables. Rockville, MD. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm. Published 2016. Accessed January 5, 2018.
- Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. http://www.samhsa.gov/data/sites/default/files/DR006/DR006/nonmedical-pain-reliever-use-2013.htm. Published August 2013. Accessed January 5, 2018.
- Joshi GP. Multimodal analgesia techniques and postoperative rehabilitation. Anesthesiol Clin North America. 2005;23(1):185–202.
- Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 suppl):6S–11S.
- Brownstein, MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci USA. 1993;90(12):5391–5393.
- US Food and Drug Administration. Acetaminophen. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm. Published November 2017. Accessed January 7, 2018.
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal anti-inflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170–1179.
- Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications included in the use of high‐risk medications in the elderly and potentially harmful drug–disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63(12):e8–e18.
- Joshi GP, Janis JE, Haas EM, et al. Surgical site infiltration for abdominal surgery: A novel neuroanatomical-based approach. Plast Reconstr Surg Glob Open. 2016;4(12):e1181. https://insights.ovid.com/crossref?an=01720096-201612000-00021. Accessed January 5, 2018.
- Jokela RM, Ahonen JV, Tallgren MK, et al. The effective analgesic dose of dexamethasone after laparoscopic hysterectomy. Anesth Analg. 2009;109(2):607–615.
- Hohlrieder M, Brimacombe J, Eschertzhuber S, et al. A study of airway management using the ProSeal LMA laryngeal mask airway compared with the tracheal tube on postoperative analgesia requirements following gynaecological laparoscopic surgery. Anaesthesia. 2007;62(9):913–918.
- Phelps P, Cakmakkaya OS, Apfel CC, Radke OC. A simple clinical maneuver to reduce laparoscopy-induced shoulder pain: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1155–1160.
- Sammour T, Kahokehr A, Hill AG. Meta‐analysis of the effect of warm humidified insufflation on pain after laparoscopy. Br J Surg. 2008;95(8):950–956.
- Reagan KM, O’Sullivan DM, Gannon R, Steinberg AC. Decreasing postoperative narcotics in reconstructive pelvic surgery; A randomized controlled trial. Am J Obstet Gynecol. 2017;217(3):325.e1–e10.
- Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration “flush list”. Sci Total Environ. 2017;609:1023–1040.
CASE Managing pain associated with prolapse and SUI surgery
A 46-year-old woman (G4P4) described 3 years of worsening symptoms related to recurrent stage-3 palpable uterine prolapse. She had associated symptomatic stress urinary incontinence. She had been treated for uterine prolapse 5 years ago with vaginal hysterectomy, bilateral salpingectomy, and high uterosacral-ligament suspension.
After consultation, the patient elected to undergo laparoscopic sacral colpopexy, a mid-urethral sling, and possible anterior and posterior colporrhaphy. Appropriate discussion about the risks and benefits of mesh was provided preoperatively. The surgical team judged her to be highly motivated; she wanted same-day outpatient surgery so that she could go home and then return to work. She had excellent support at home.
How would you counsel this patient about expected postoperative pain? Which medications would you administer to her preoperatively and perioperatively? Which ones would you prescribe for her to manage pain postoperatively?

Adverse impact of prescription opioids in the United States
Although fewer than 5% of the world’s population live in the United States, nearly 80% of the world’s opioids are written for them.1 In 2012, 259 million prescriptions were written for opioids in the United States—more than enough to give every American adult their own bottle of pills.2 Sadly, drug overdose is now a leading cause of accidental death in the United States, with 52,404 lethal drug overdoses in 2015. A startling statistic is that prescription opioid abuse is driving this epidemic, with 20,101 overdose deaths related to prescription pain relievers and 12,990 overdose deaths related to heroin in 2015.3
It is likely that there are multiple reasons prescribing of opioids is epidemic. Surgical pain is a common indication for opioid prescriptions; fewer than half of patients who undergo surgery report adequate postoperative pain relief.4 Recognition of these deficits in pain management has inspired national campaigns to improve patients’ experience with pain and aggressively address pain with drugs such as opioids.5
At the same time, marketing efforts by the pharmaceutical industry sought to reassure the medical community that patients would not become addicted to prescription opioid pain relievers if physical pain was the indication for such prescriptions. In response, health care providers began to prescribe opioids at a greater rate. As providers were encouraged to increase prescriptions, opioid medications began to be misused—and only then did it become clear that these medications are, in fact, highly addictive.6 Opioid abuse and overdose rates began to increase; in 2015, more than 33,000 Americans died because of an opioid overdose, including prescription opioids and heroin7 (FIGURE). In fact, although most people recognize the threat posed by illegal heroin, most of the 2 million who abused opioids in 2015 in the United States suffered from prescription abuse; only about a quarter, or about 600,000, abused heroin.8 In addition, more than 80% of people who abuse heroin initially abused prescription opioids.9

Read about medications and strategies for multimodal pain management.
Multimodal approach to pain management
The goals of postsurgical pain treatment are to relieve suffering, optimize bodily functioning after surgery, limit length of the stay, and optimize patient satisfaction. Pain-control regimens should consider the specific surgical procedure and the patient’s medical, psychological, and physical conditions; age; level of fear or anxiety; personal preference; and response to previous treatments.10
Optimally, postsurgical pain management starts well before the day of surgery. Employing such strategies as Enhanced Recovery after Surgery (ERAS) protocols does not necessarily mean providing the same care for every patient, every time. Rather, ERAS serves as a checklist to ensure that all applicable categories of pain medication and pain-control strategies are considered, selected, and dosed according to individual needs.11 (See “Preoperative management of pain expectations.”)
Ideally, before surgery, provide the patient with an opportunity to learn that:
- Her expectations about postsurgical pain should be realistic, and that freedom from pain is not realistic.
- Pain-reduction options should optimize her bodily function and mobility, reduce the degree to which pain interferes with activities, and relieve associated psychological stressors.
- Inherent in the pain management plan should be a goal of minimizing the risks of opioid misuse, abuse, and addiction—for the patient and for her family members and friends.
Opioids
Opioids have been employed to treat pain for 700 years.12 They are powerful pain relievers because they target central mechanisms involved in the perception of pain. Regrettably, because of their central action, opioids have many adverse effects in addition to being highly addictive.
Nonopioid alternatives
Expert consensus, including recommendations of the World Health Organization,11 favors using nonopioids as first-line medications to address surgical pain. Nonopioid analgesic options are acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and adjuvant medications. In addition, nonanalgesic medications such as sedatives, sleep aids, and muscle relaxants can relieve postsurgical pain. Optimal use of these nonopioid medications can significantly reduce or eliminate the need for opioid medications to treat pain. Goals are to 1) reserve opioids for the most severe pain and 2) minimize the number of doses/pills of opioids required to control postsurgical pain.
Acetaminophen. At dosages of 325 to 1,000 mg orally every 4 to 6 hours, to a maximum dosage of 4,000 mg/d, acetaminophen can be used to treat mild pain and, in combination with other medications, moderate-to-severe pain. The drug also can be administered intravenously (IV), although use of the IV route is limited in many hospitals because of its significantly higher expense compared to the oral form.
The mechanism of action of acetaminophen is unique among pain relievers; it can therefore be used in combination with other pain relievers to more effectively treat pain with fewer concerns about medication-induced adverse effects or opioid overdose. However, keep in mind when considering combining analgesics, that acetaminophen is an active ingredient in hundreds of over-the-counter (OTC) and prescription formulations, and that a combination of more than one acetaminophen-containing product can create the risk of overdose.
Acetaminophen should be used with caution in patients with liver disease. That being said, multiple trials have documented safe use in normal body weight adults who do not have hepatic disease, at dosages as high as 4,000 mg over a 24-hour period.13
NSAIDs. A combination of an NSAID and acetaminophen has been documented to reduce the amount of opioid medications required to treat postsurgical pain. In most circumstances, especially for minor surgery, acetaminophen and NSAIDS can be administered just before surgery starts. This preoperative treatment, called “preventive analgesia” or “preemptive analgesia,” has been demonstrated in multiple clinical trials to reduce postoperative pain.14
Adjuvant pain medications. Antidepressants, antiepileptic agents, and muscle relaxants—agents that have a primary indication for a condition (or conditions) other than pain and do not directly provide analgesia—have been used as adjuvant pain medications. When employed with traditional analgesics, they have been demonstrated to reduce postsurgical pain scores and the amount of opioids required. These medications need to be used cautiously because some are associated with serious sedation and vertigo (TABLE). Take caution when using adjuvant pain medications in patients older than 65 years; guidance on their use in older patients has been outlined by the American Geriatrics Society and other professional organizations.15

Case Continued
The patient was given the expectation that the 11-mm left lower-quadrant port site would likely be the most bothersome site of pain—a rating of 4 or 5 on a visual analogue scale of 1 to 10, on postoperative day 1, while standing. The other 3 (5-mm) laparoscopic ports, she was told, would, typically, be less bothersome. The patient was educated regarding the role of analgesics and adjuvant medications and cautioned not to exceed 4,000 mg of acetaminophen in any 24-hour period. She was told that gabapentin may make her feel sedated or dizzy, or both; she was encouraged to hold this medication if she found these adverse effects bothersome or limiting.
The following multimodal pain management was established.
Preoperatively, the patient was given:
- Acetaminophen 1.5 g orally (as a liquid, 45 mL of a suspension of 500 mg/15 mL liquid), 2 to 3 hours preoperatively; the surgical suite did not stock IV acetaminophen.
- Gabapentin 600 mg orally, with a sip of water, the morning of surgery.
- Celecoxib 100 mg orally, with a sip of water, the morning of surgery.
Prescriptions for home postoperative pain management were provided preoperatively:
- OTC acetaminophen 1,000 mg (as 2 500-mgtablets) taken as a scheduled dose every 8 hours for the first 48 hours postoperatively.
- Meloxicam 15 mg daily as the NSAID, taken as a scheduled dose once per day for the first 48 hours postoperatively, then as needed.
- Gabapentin 300 mg (in addition to the preoperative dose, above), taken as a scheduled dose every 8 hours for the first 48 hours postoperatively, then as needed.
- Oxycodone 5 mg (without acetaminophen) for breakthrough pain.
Intraoperatively:
- Meticulous attention was paid to patient positioning, to reduce the possibility of back and upper- and lower-extremity injury postoperatively.
- A corticosteroid (dexamethasone 8 mg IV) was administered to minimize postoperative nausea and vomiting and as an adjuvant medication for postoperative pain control.
- Careful attention was paid to limit residual CO2 gas and intraoperative intra-abdominal pressures.
- All laparoscopic port sites were injected with 30 mL of 0.25% bupivacaine with epinephrine, extending to subcutaneous, fascial, and peritoneal layers.
Read about why a multimodal approach is best for postsurgical pain.
Why a multimodal plan to treat pain?
Pain following laparoscopy has been associated with many variables, including patient positioning, port size and placement, amount of port manipulation, and gas retention. After a laparoscopic surgical procedure, patients report pain in the abdomen, back, and shoulders.
Postsurgical pain has 3 components:
- Shoulder pain, thought to result from phrenic nerve irritation caused by lingering CO2 in the abdominal cavity.
- Visceral pain, occurring secondary to stretching of the abdominal cavity.
- Somatic pain, caused by the surgical incision; of the 3 components to pain, somatic pain can have the least impact because laparoscopic incisions are small.
For our patient, prior to the incisions being made, she received local anesthesia intraoperatively to the laparoscopic port sites to include the subcutaneous, fascial, and peritoneal layers. Involving these layers allows for more of a block. An ultrasonography-guided transversus abdominis plane (TAP) block, if available, is highly effective at decreasing postoperative pain, but its efficacy is dependent on the anatomy and the skill of the physician (whether anesthesiologist, gynecologist, or surgeon) who is placing it.16
We used dexamethasone 8 mg IV, intraoperatively because this single dose has been shown to decrease the perception of pain postoperatively. Dexamethasone also has been shown to decrease consumption of oxycodone during the 24 hours after laparoscopic gynecologic surgery.17
CO2 used to insufflate the patient’s abdomen can take as long as 2 days to fully resorb, resulting in increased pain. This discomfort has been described as delayed; the patient might not notice it until she goes home. In a study, 70% of patients had shoulder discomfort following laparoscopy 24 hours after their procedure.18 For this reason, we employed several techniques to reduce this effect:
- We reduced the intra-abdominal pressure limit to 10 mm Hg (from 15 mm Hg) once dissection was complete.
- At the end of the procedure, careful attention was paid to removing as much intra-abdominal gas as possible, including placing the patient in the Trendelenburg position and having the anesthesiologist induce a Valsalva maneuver. This action has been shown to significantly improve pain control compared to placebo intervention.19
- We used humidified CO2, which has been demonstrated to reduce pain in laparoscopic surgery.20
Preemptively, we provided this patient with acetaminophen, celecoxib, and gabapentin, which have been demonstrated to be effective in gynecologic patients to decrease the need for postoperative opioids.21 Also, our patient received counseling, with specific expectations for what to expect following the surgical procedure.
CASE Resolved
Our patient did exceptionally well following surgery. She used only one of the oxycodone pills and did not require unplanned interventions. She took gabapentin, acetaminophen, and meloxicam at their scheduled doses for 2 days. She continued to use meloxicam for 4 more days for mild abdominal pain, then discontinued all medications.She flushed her 9 unused oxycodone pills down the toilet. (See “A word about disposal of ‘excess’ opioids”22) The patient returned to her administrative duties at work 2 weeks after the procedure and reported that she was “very satisfied” with her surgical experience.
The US Food and Drug Administration (FDA) recommends disposing of certain drugs through a take-back program or, if such a program is not readily available, by flushing them down a toilet or sink. In a recent study, investigators concluded that opioids on the FDA's so-called flush list include most opioids in clinical use--even if the entire supply prescribed is to be flushed down the drain. Conservative estimates of environmental degradation were employed in the study; the investigators' conclusion was that these drugs pose a "negligible" eco-toxicologic risk.1
Reference
- Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration "flush list". Sci Total Environ. 2017;609:1023-1040.
In conclusion
Postoperative pain is a complex entity that must be considered to require individualized strategies and, possibly, multiple interventions. Optimally, thorough education, including pain management options, is provided to the patient prior to surgery. Given the current state of opioid abuse in the United States, all gynecologic surgeons should be familiar with multimodal pain therapy and how to employ nonmedical techniques to reduce postsurgical pain without relying solely on opioids. (See “Online resources for pain management”.)
- Drug Disposal Information
(US Department of Justice Drug Enforcement Administration)
https://www.deadiversion.usdoj.gov/drug_disposal/index.html - Surgical Pain Consortium
http://surgicalpainconsortium.org/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Managing pain associated with prolapse and SUI surgery
A 46-year-old woman (G4P4) described 3 years of worsening symptoms related to recurrent stage-3 palpable uterine prolapse. She had associated symptomatic stress urinary incontinence. She had been treated for uterine prolapse 5 years ago with vaginal hysterectomy, bilateral salpingectomy, and high uterosacral-ligament suspension.
After consultation, the patient elected to undergo laparoscopic sacral colpopexy, a mid-urethral sling, and possible anterior and posterior colporrhaphy. Appropriate discussion about the risks and benefits of mesh was provided preoperatively. The surgical team judged her to be highly motivated; she wanted same-day outpatient surgery so that she could go home and then return to work. She had excellent support at home.
How would you counsel this patient about expected postoperative pain? Which medications would you administer to her preoperatively and perioperatively? Which ones would you prescribe for her to manage pain postoperatively?

Adverse impact of prescription opioids in the United States
Although fewer than 5% of the world’s population live in the United States, nearly 80% of the world’s opioids are written for them.1 In 2012, 259 million prescriptions were written for opioids in the United States—more than enough to give every American adult their own bottle of pills.2 Sadly, drug overdose is now a leading cause of accidental death in the United States, with 52,404 lethal drug overdoses in 2015. A startling statistic is that prescription opioid abuse is driving this epidemic, with 20,101 overdose deaths related to prescription pain relievers and 12,990 overdose deaths related to heroin in 2015.3
It is likely that there are multiple reasons prescribing of opioids is epidemic. Surgical pain is a common indication for opioid prescriptions; fewer than half of patients who undergo surgery report adequate postoperative pain relief.4 Recognition of these deficits in pain management has inspired national campaigns to improve patients’ experience with pain and aggressively address pain with drugs such as opioids.5
At the same time, marketing efforts by the pharmaceutical industry sought to reassure the medical community that patients would not become addicted to prescription opioid pain relievers if physical pain was the indication for such prescriptions. In response, health care providers began to prescribe opioids at a greater rate. As providers were encouraged to increase prescriptions, opioid medications began to be misused—and only then did it become clear that these medications are, in fact, highly addictive.6 Opioid abuse and overdose rates began to increase; in 2015, more than 33,000 Americans died because of an opioid overdose, including prescription opioids and heroin7 (FIGURE). In fact, although most people recognize the threat posed by illegal heroin, most of the 2 million who abused opioids in 2015 in the United States suffered from prescription abuse; only about a quarter, or about 600,000, abused heroin.8 In addition, more than 80% of people who abuse heroin initially abused prescription opioids.9

Read about medications and strategies for multimodal pain management.
Multimodal approach to pain management
The goals of postsurgical pain treatment are to relieve suffering, optimize bodily functioning after surgery, limit length of the stay, and optimize patient satisfaction. Pain-control regimens should consider the specific surgical procedure and the patient’s medical, psychological, and physical conditions; age; level of fear or anxiety; personal preference; and response to previous treatments.10
Optimally, postsurgical pain management starts well before the day of surgery. Employing such strategies as Enhanced Recovery after Surgery (ERAS) protocols does not necessarily mean providing the same care for every patient, every time. Rather, ERAS serves as a checklist to ensure that all applicable categories of pain medication and pain-control strategies are considered, selected, and dosed according to individual needs.11 (See “Preoperative management of pain expectations.”)
Ideally, before surgery, provide the patient with an opportunity to learn that:
- Her expectations about postsurgical pain should be realistic, and that freedom from pain is not realistic.
- Pain-reduction options should optimize her bodily function and mobility, reduce the degree to which pain interferes with activities, and relieve associated psychological stressors.
- Inherent in the pain management plan should be a goal of minimizing the risks of opioid misuse, abuse, and addiction—for the patient and for her family members and friends.
Opioids
Opioids have been employed to treat pain for 700 years.12 They are powerful pain relievers because they target central mechanisms involved in the perception of pain. Regrettably, because of their central action, opioids have many adverse effects in addition to being highly addictive.
Nonopioid alternatives
Expert consensus, including recommendations of the World Health Organization,11 favors using nonopioids as first-line medications to address surgical pain. Nonopioid analgesic options are acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and adjuvant medications. In addition, nonanalgesic medications such as sedatives, sleep aids, and muscle relaxants can relieve postsurgical pain. Optimal use of these nonopioid medications can significantly reduce or eliminate the need for opioid medications to treat pain. Goals are to 1) reserve opioids for the most severe pain and 2) minimize the number of doses/pills of opioids required to control postsurgical pain.
Acetaminophen. At dosages of 325 to 1,000 mg orally every 4 to 6 hours, to a maximum dosage of 4,000 mg/d, acetaminophen can be used to treat mild pain and, in combination with other medications, moderate-to-severe pain. The drug also can be administered intravenously (IV), although use of the IV route is limited in many hospitals because of its significantly higher expense compared to the oral form.
The mechanism of action of acetaminophen is unique among pain relievers; it can therefore be used in combination with other pain relievers to more effectively treat pain with fewer concerns about medication-induced adverse effects or opioid overdose. However, keep in mind when considering combining analgesics, that acetaminophen is an active ingredient in hundreds of over-the-counter (OTC) and prescription formulations, and that a combination of more than one acetaminophen-containing product can create the risk of overdose.
Acetaminophen should be used with caution in patients with liver disease. That being said, multiple trials have documented safe use in normal body weight adults who do not have hepatic disease, at dosages as high as 4,000 mg over a 24-hour period.13
NSAIDs. A combination of an NSAID and acetaminophen has been documented to reduce the amount of opioid medications required to treat postsurgical pain. In most circumstances, especially for minor surgery, acetaminophen and NSAIDS can be administered just before surgery starts. This preoperative treatment, called “preventive analgesia” or “preemptive analgesia,” has been demonstrated in multiple clinical trials to reduce postoperative pain.14
Adjuvant pain medications. Antidepressants, antiepileptic agents, and muscle relaxants—agents that have a primary indication for a condition (or conditions) other than pain and do not directly provide analgesia—have been used as adjuvant pain medications. When employed with traditional analgesics, they have been demonstrated to reduce postsurgical pain scores and the amount of opioids required. These medications need to be used cautiously because some are associated with serious sedation and vertigo (TABLE). Take caution when using adjuvant pain medications in patients older than 65 years; guidance on their use in older patients has been outlined by the American Geriatrics Society and other professional organizations.15

Case Continued
The patient was given the expectation that the 11-mm left lower-quadrant port site would likely be the most bothersome site of pain—a rating of 4 or 5 on a visual analogue scale of 1 to 10, on postoperative day 1, while standing. The other 3 (5-mm) laparoscopic ports, she was told, would, typically, be less bothersome. The patient was educated regarding the role of analgesics and adjuvant medications and cautioned not to exceed 4,000 mg of acetaminophen in any 24-hour period. She was told that gabapentin may make her feel sedated or dizzy, or both; she was encouraged to hold this medication if she found these adverse effects bothersome or limiting.
The following multimodal pain management was established.
Preoperatively, the patient was given:
- Acetaminophen 1.5 g orally (as a liquid, 45 mL of a suspension of 500 mg/15 mL liquid), 2 to 3 hours preoperatively; the surgical suite did not stock IV acetaminophen.
- Gabapentin 600 mg orally, with a sip of water, the morning of surgery.
- Celecoxib 100 mg orally, with a sip of water, the morning of surgery.
Prescriptions for home postoperative pain management were provided preoperatively:
- OTC acetaminophen 1,000 mg (as 2 500-mgtablets) taken as a scheduled dose every 8 hours for the first 48 hours postoperatively.
- Meloxicam 15 mg daily as the NSAID, taken as a scheduled dose once per day for the first 48 hours postoperatively, then as needed.
- Gabapentin 300 mg (in addition to the preoperative dose, above), taken as a scheduled dose every 8 hours for the first 48 hours postoperatively, then as needed.
- Oxycodone 5 mg (without acetaminophen) for breakthrough pain.
Intraoperatively:
- Meticulous attention was paid to patient positioning, to reduce the possibility of back and upper- and lower-extremity injury postoperatively.
- A corticosteroid (dexamethasone 8 mg IV) was administered to minimize postoperative nausea and vomiting and as an adjuvant medication for postoperative pain control.
- Careful attention was paid to limit residual CO2 gas and intraoperative intra-abdominal pressures.
- All laparoscopic port sites were injected with 30 mL of 0.25% bupivacaine with epinephrine, extending to subcutaneous, fascial, and peritoneal layers.
Read about why a multimodal approach is best for postsurgical pain.
Why a multimodal plan to treat pain?
Pain following laparoscopy has been associated with many variables, including patient positioning, port size and placement, amount of port manipulation, and gas retention. After a laparoscopic surgical procedure, patients report pain in the abdomen, back, and shoulders.
Postsurgical pain has 3 components:
- Shoulder pain, thought to result from phrenic nerve irritation caused by lingering CO2 in the abdominal cavity.
- Visceral pain, occurring secondary to stretching of the abdominal cavity.
- Somatic pain, caused by the surgical incision; of the 3 components to pain, somatic pain can have the least impact because laparoscopic incisions are small.
For our patient, prior to the incisions being made, she received local anesthesia intraoperatively to the laparoscopic port sites to include the subcutaneous, fascial, and peritoneal layers. Involving these layers allows for more of a block. An ultrasonography-guided transversus abdominis plane (TAP) block, if available, is highly effective at decreasing postoperative pain, but its efficacy is dependent on the anatomy and the skill of the physician (whether anesthesiologist, gynecologist, or surgeon) who is placing it.16
We used dexamethasone 8 mg IV, intraoperatively because this single dose has been shown to decrease the perception of pain postoperatively. Dexamethasone also has been shown to decrease consumption of oxycodone during the 24 hours after laparoscopic gynecologic surgery.17
CO2 used to insufflate the patient’s abdomen can take as long as 2 days to fully resorb, resulting in increased pain. This discomfort has been described as delayed; the patient might not notice it until she goes home. In a study, 70% of patients had shoulder discomfort following laparoscopy 24 hours after their procedure.18 For this reason, we employed several techniques to reduce this effect:
- We reduced the intra-abdominal pressure limit to 10 mm Hg (from 15 mm Hg) once dissection was complete.
- At the end of the procedure, careful attention was paid to removing as much intra-abdominal gas as possible, including placing the patient in the Trendelenburg position and having the anesthesiologist induce a Valsalva maneuver. This action has been shown to significantly improve pain control compared to placebo intervention.19
- We used humidified CO2, which has been demonstrated to reduce pain in laparoscopic surgery.20
Preemptively, we provided this patient with acetaminophen, celecoxib, and gabapentin, which have been demonstrated to be effective in gynecologic patients to decrease the need for postoperative opioids.21 Also, our patient received counseling, with specific expectations for what to expect following the surgical procedure.
CASE Resolved
Our patient did exceptionally well following surgery. She used only one of the oxycodone pills and did not require unplanned interventions. She took gabapentin, acetaminophen, and meloxicam at their scheduled doses for 2 days. She continued to use meloxicam for 4 more days for mild abdominal pain, then discontinued all medications.She flushed her 9 unused oxycodone pills down the toilet. (See “A word about disposal of ‘excess’ opioids”22) The patient returned to her administrative duties at work 2 weeks after the procedure and reported that she was “very satisfied” with her surgical experience.
The US Food and Drug Administration (FDA) recommends disposing of certain drugs through a take-back program or, if such a program is not readily available, by flushing them down a toilet or sink. In a recent study, investigators concluded that opioids on the FDA's so-called flush list include most opioids in clinical use--even if the entire supply prescribed is to be flushed down the drain. Conservative estimates of environmental degradation were employed in the study; the investigators' conclusion was that these drugs pose a "negligible" eco-toxicologic risk.1
Reference
- Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration "flush list". Sci Total Environ. 2017;609:1023-1040.
In conclusion
Postoperative pain is a complex entity that must be considered to require individualized strategies and, possibly, multiple interventions. Optimally, thorough education, including pain management options, is provided to the patient prior to surgery. Given the current state of opioid abuse in the United States, all gynecologic surgeons should be familiar with multimodal pain therapy and how to employ nonmedical techniques to reduce postsurgical pain without relying solely on opioids. (See “Online resources for pain management”.)
- Drug Disposal Information
(US Department of Justice Drug Enforcement Administration)
https://www.deadiversion.usdoj.gov/drug_disposal/index.html - Surgical Pain Consortium
http://surgicalpainconsortium.org/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- United Nations International Narcotics Control Board. Narcotic drugs: Report 2016: Estimated world requirements for 2017-statistics for 2015. New York, NY. https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2016/Narcotic_Drugs_Publication_2016.pdf. Published 2017. Accessed January 7, 2018.
- Centers for Disease Control and Prevention. Opioid painkiller prescribing: Where you live makes a difference. Atlanta, GA. https://www.cdc.gov/vitalsigns/pdf/2014-07-vitalsigns.pdf. Published July 2014. Accessed January 5, 2018.
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- Gan TJ, Habib AS, Miller TE, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–160.
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625.
- Van Zee A. The promotion and marketing of OxyContin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221–227.
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Results from the 2015 national survey on drug use and health: Detailed tables. Rockville, MD. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm. Published 2016. Accessed January 5, 2018.
- Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. http://www.samhsa.gov/data/sites/default/files/DR006/DR006/nonmedical-pain-reliever-use-2013.htm. Published August 2013. Accessed January 5, 2018.
- Joshi GP. Multimodal analgesia techniques and postoperative rehabilitation. Anesthesiol Clin North America. 2005;23(1):185–202.
- Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 suppl):6S–11S.
- Brownstein, MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci USA. 1993;90(12):5391–5393.
- US Food and Drug Administration. Acetaminophen. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm. Published November 2017. Accessed January 7, 2018.
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal anti-inflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170–1179.
- Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications included in the use of high‐risk medications in the elderly and potentially harmful drug–disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63(12):e8–e18.
- Joshi GP, Janis JE, Haas EM, et al. Surgical site infiltration for abdominal surgery: A novel neuroanatomical-based approach. Plast Reconstr Surg Glob Open. 2016;4(12):e1181. https://insights.ovid.com/crossref?an=01720096-201612000-00021. Accessed January 5, 2018.
- Jokela RM, Ahonen JV, Tallgren MK, et al. The effective analgesic dose of dexamethasone after laparoscopic hysterectomy. Anesth Analg. 2009;109(2):607–615.
- Hohlrieder M, Brimacombe J, Eschertzhuber S, et al. A study of airway management using the ProSeal LMA laryngeal mask airway compared with the tracheal tube on postoperative analgesia requirements following gynaecological laparoscopic surgery. Anaesthesia. 2007;62(9):913–918.
- Phelps P, Cakmakkaya OS, Apfel CC, Radke OC. A simple clinical maneuver to reduce laparoscopy-induced shoulder pain: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1155–1160.
- Sammour T, Kahokehr A, Hill AG. Meta‐analysis of the effect of warm humidified insufflation on pain after laparoscopy. Br J Surg. 2008;95(8):950–956.
- Reagan KM, O’Sullivan DM, Gannon R, Steinberg AC. Decreasing postoperative narcotics in reconstructive pelvic surgery; A randomized controlled trial. Am J Obstet Gynecol. 2017;217(3):325.e1–e10.
- Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration “flush list”. Sci Total Environ. 2017;609:1023–1040.
- United Nations International Narcotics Control Board. Narcotic drugs: Report 2016: Estimated world requirements for 2017-statistics for 2015. New York, NY. https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2016/Narcotic_Drugs_Publication_2016.pdf. Published 2017. Accessed January 7, 2018.
- Centers for Disease Control and Prevention. Opioid painkiller prescribing: Where you live makes a difference. Atlanta, GA. https://www.cdc.gov/vitalsigns/pdf/2014-07-vitalsigns.pdf. Published July 2014. Accessed January 5, 2018.
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- Gan TJ, Habib AS, Miller TE, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–160.
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625.
- Van Zee A. The promotion and marketing of OxyContin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221–227.
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Results from the 2015 national survey on drug use and health: Detailed tables. Rockville, MD. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm. Published 2016. Accessed January 5, 2018.
- Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. http://www.samhsa.gov/data/sites/default/files/DR006/DR006/nonmedical-pain-reliever-use-2013.htm. Published August 2013. Accessed January 5, 2018.
- Joshi GP. Multimodal analgesia techniques and postoperative rehabilitation. Anesthesiol Clin North America. 2005;23(1):185–202.
- Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 suppl):6S–11S.
- Brownstein, MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci USA. 1993;90(12):5391–5393.
- US Food and Drug Administration. Acetaminophen. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm. Published November 2017. Accessed January 7, 2018.
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal anti-inflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170–1179.
- Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications included in the use of high‐risk medications in the elderly and potentially harmful drug–disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63(12):e8–e18.
- Joshi GP, Janis JE, Haas EM, et al. Surgical site infiltration for abdominal surgery: A novel neuroanatomical-based approach. Plast Reconstr Surg Glob Open. 2016;4(12):e1181. https://insights.ovid.com/crossref?an=01720096-201612000-00021. Accessed January 5, 2018.
- Jokela RM, Ahonen JV, Tallgren MK, et al. The effective analgesic dose of dexamethasone after laparoscopic hysterectomy. Anesth Analg. 2009;109(2):607–615.
- Hohlrieder M, Brimacombe J, Eschertzhuber S, et al. A study of airway management using the ProSeal LMA laryngeal mask airway compared with the tracheal tube on postoperative analgesia requirements following gynaecological laparoscopic surgery. Anaesthesia. 2007;62(9):913–918.
- Phelps P, Cakmakkaya OS, Apfel CC, Radke OC. A simple clinical maneuver to reduce laparoscopy-induced shoulder pain: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1155–1160.
- Sammour T, Kahokehr A, Hill AG. Meta‐analysis of the effect of warm humidified insufflation on pain after laparoscopy. Br J Surg. 2008;95(8):950–956.
- Reagan KM, O’Sullivan DM, Gannon R, Steinberg AC. Decreasing postoperative narcotics in reconstructive pelvic surgery; A randomized controlled trial. Am J Obstet Gynecol. 2017;217(3):325.e1–e10.
- Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration “flush list”. Sci Total Environ. 2017;609:1023–1040.