User login
Methicillin‐resistant Staphylococcus aureus bacteremia due to prostatic abscess
Community‐associated methicillin‐resistant Staphylococcus aureus (MRSA) infection is an evolving disease that is changing medical practice. MRSA has become the most frequent cause of skin and soft‐tissue infections presenting to most emergency departments in the United States.1 In comparison with methicillin‐sensitive S. aureus, community‐associated MRSA is more likely to present as soft‐tissue abscesses or necrotizing pneumonia.2 In 2005 alone, 94,360 invasive MRSA infections were estimated to have occurred in the United States, most of which were associated with MRSA bacteremia.3 In the hospital, MRSA infections are associated with greater lengths of stay, higher mortality, and increased costs.3
We report a patient with persistent MRSA bacteremia due to a prostatic abscess. Prostatitis or prostatic abscess with MRSA has rarely been reported. Resolution of the bacteremia was achieved only after drainage of the abscess. This case highlights the importance of recognizing this clinical condition and draining any MRSA‐associated abscesses. In addition, the abscess‐forming characteristics of MRSA may suggest that the incidence of prostatic abscess due to this microbe is on the rise.
CASE REPORT
A 40‐year‐old human immunodeficiency viruspositive man presented with a 10‐day history of intermittent fever, urinary hesitancy, weak urinary stream, and intermittent abdominal pain relieved following urination. He denied dysuria, hematuria, chest pain, dyspnea, nausea, weight loss, diarrhea, or decreased functional status. His last CD4 count was 528/L on highly active antiretroviral therapy 1 year prior to presentation; however, he had run out of medications several months prior to presentation. His medical history was also significant for incision and drainage of skin abscesses with unknown microbiology several months prior to presentation. He currently used tobacco but denied illicit drug use. He was sexually abstinent for over a year prior to presentation but had a history of having unprotected sex with men.
On physical examination, his vital signs were as follows: temperature, 37.8C; blood pressure, 133/84 mm Hg; heart rate, 107 beats/minute; respiration rate, 16 breaths/minute; and oxygen saturation, 100% on room air. He was under no distress. His heart sound was tachycardic and regular with no murmur noted. Lung sounds were clear. Abdominal examination revealed no tenderness or organomegaly. His prostate was boggy, minimally tender, and slightly enlarged. The rest of his physical examination was normal. The white blood cell count was 7.5 109/L with 79% neutrophils. The serum chemistry was normal. The prostate‐specific antigen level was 2.9 ng/mL. A repeat CD4 count was 140/L. Urinalysis revealed large leukocyte esterase, no nitrites, and 60 white blood cells per high‐power field. He was diagnosed with prostatitis and discharged on levofloxacin.
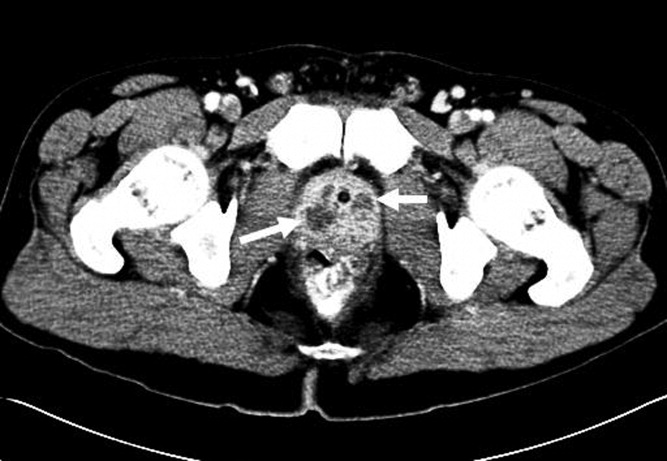
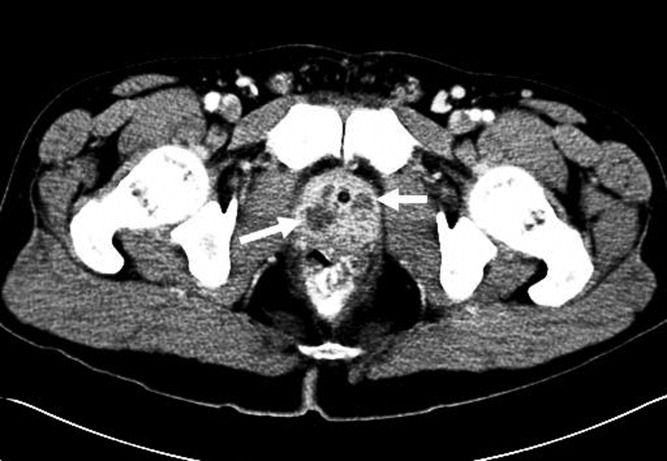
The subsequent day, he returned to the emergency department with an inability to void. A Foley catheter was placed with resolution of his symptoms. Later that day, blood cultures from his initial admission grew MRSA in 2 out of 4 bottles, and he was admitted to the hospital. A review of the prior urine culture showed no growth. Computed tomography (CT) of the abdomen and pelvis showed an enlarged prostate with multiple noncommunicating peripherally enhancing hypodensities consistent with prostatic abscesses (arrows in Figure 1). These abscesses were associated with periprostatic fat stranding, edematous seminal vesicles, diffuse urinary bladder wall thickening, and mild inguinal adenopathy. Therapy with intravenous vancomycin was initiated.
Over the next 6 days, the patient continued to have intermittent fevers. Blood cultures continued to grow MRSA. Gentamicin was added for possible synergy on hospital day 3 without improvement. A transesophageal echocardiogram was normal. On hospital day 6, repeat CT showed no change in the size of the prostatic abscesses but an increased capsular bulge along the right mid gland. The urology service was consulted. They performed bedside transperineal drainage of the largest abscess with transrectal ultrasound guidance. No indwelling drain was placed. A culture of the purulent drainage grew MRSA. Three days after the surgical drainage, the patient was afebrile, urinary symptoms had resolved, and serial blood cultures remained negative. He was discharged on hospital day 13 to complete a 4‐week course of intravenous vancomycin. The patient missed an appointment for follow‐up imaging.
DISCUSSION
We report a case of community‐associated MRSA bacteremia secondary to a prostatic abscess, as confirmed by cultures from serum and of the abscess. S. aureus bacteremia often stems from infections of the respiratory tract, skin, and soft tissues, endocarditis, or infections of indwelling devices.4S. aureus can then create embolic foci, including in the prostate, which can serve as sources of persistent bacteremia. However, new evidence suggests community‐associated MRSA might be sexually transmitted among men who have sex with men.5 An MRSA prostatic abscess could then potentially be related to an ascending urinary tract infection or translocation from the gastrointestinal tract.
The vast majority of prostatitis cases prostatic abscesses are caused by Escherichia coli and other gram‐negative bacilli.6 Staphylococcus species are less common, and MRSA isolates have been described as a rare etiologic agent. Only four other case reports describing MRSA bacteremia associated with prostatitis or prostatic abscesses have been published.710 Both our patient and the three other case reports in the literature with prostatic abscesses and MRSA bacteremia required drainage of the abscess as well as antibiotics for resolution. The other reported cases involved drainage by transurethral resection of the prostate,79 whereas our case used transperineal drainage of the prostatic abscess via transrectal ultrasound guidance. In addition to intravenous antibiotics, drainage appears to be a key therapeutic measure for resolution of MRSA prostatic abscesses.
With the increasing incidence of community‐associated and healthcare‐associated MRSA infections, the incidence of prostatitis or prostatic abscesses secondary to MRSA may be increasing. MRSA seems to have a specific predilection for abscess formation in skin and soft‐tissue infections and pneumonia.2 Clinicians must be alert to a potentially higher frequency of MRSA as a cause of prostatic abscesses and prostatitis.
In the evaluation of a patient with persistent MRSA bacteremia, the potential for the prostate as a source of infection should be considered. Urinary symptoms may be subtle. Clinicians should have a low threshold for imaging studies such as CT to evaluate for a possible source of MRSA bacteremia. If a prostatic abscess is found, prompt surgical drainage or debridement is necessary for cure.
- ,,, et al.Methicillin‐resistant S. aureus infections among patients in the emergency department.N Engl J Med.2006;355:666–674.
- ,,, et al.Comparison of both clinical features and mortality risk associated with bacteremia due to community‐acquired methicillin‐resistant Staphylococcus aureus and methicillin‐susceptible S. aureus.Clin Infect Dis.2008;46:799–806.
- ,,, et al.Invasive methicillin‐resistant Staphylococcus aureus infections in the United States.JAMA.2007;298:1763–1771.
- ,,,,,.The current spectrum of Staphylococcus aureus infection in a tertiary care hospital.Medicine (Baltimore).1994;73:186–208.
- ,,, et al.Emergence of multidrug‐resistant, community‐associated, methicillin‐resistant Staphylococcus aureus clone USA300 in men who have sex with men.Ann Intern Med.2008;148:249–257.
- ,,, et al.Prostatic abscess in the antibiotic era.Rev Infect Dis.1988;10:239–249.
- ,,.Community‐acquired methicillin‐resistant Staphylococcus aureus prostatic abscess.Urology.2004;64:808–810.
- ,,.Persistent methicillin‐resistant Staphylococcus aureus bacteremia due to a prostatic abscess.Scand J Infect Dis.2003;35:273–274.
- ,.Dual perinephric and prostatic abscesses from methicillin resistant Staphylococcus aureus.South Med J.2007;100:515–516.
- ,.Methicillin‐resistant Staphylococcus aureus prostatitis.Urology.2007;69:779.e1–779.e3.
Community‐associated methicillin‐resistant Staphylococcus aureus (MRSA) infection is an evolving disease that is changing medical practice. MRSA has become the most frequent cause of skin and soft‐tissue infections presenting to most emergency departments in the United States.1 In comparison with methicillin‐sensitive S. aureus, community‐associated MRSA is more likely to present as soft‐tissue abscesses or necrotizing pneumonia.2 In 2005 alone, 94,360 invasive MRSA infections were estimated to have occurred in the United States, most of which were associated with MRSA bacteremia.3 In the hospital, MRSA infections are associated with greater lengths of stay, higher mortality, and increased costs.3
We report a patient with persistent MRSA bacteremia due to a prostatic abscess. Prostatitis or prostatic abscess with MRSA has rarely been reported. Resolution of the bacteremia was achieved only after drainage of the abscess. This case highlights the importance of recognizing this clinical condition and draining any MRSA‐associated abscesses. In addition, the abscess‐forming characteristics of MRSA may suggest that the incidence of prostatic abscess due to this microbe is on the rise.
CASE REPORT
A 40‐year‐old human immunodeficiency viruspositive man presented with a 10‐day history of intermittent fever, urinary hesitancy, weak urinary stream, and intermittent abdominal pain relieved following urination. He denied dysuria, hematuria, chest pain, dyspnea, nausea, weight loss, diarrhea, or decreased functional status. His last CD4 count was 528/L on highly active antiretroviral therapy 1 year prior to presentation; however, he had run out of medications several months prior to presentation. His medical history was also significant for incision and drainage of skin abscesses with unknown microbiology several months prior to presentation. He currently used tobacco but denied illicit drug use. He was sexually abstinent for over a year prior to presentation but had a history of having unprotected sex with men.
On physical examination, his vital signs were as follows: temperature, 37.8C; blood pressure, 133/84 mm Hg; heart rate, 107 beats/minute; respiration rate, 16 breaths/minute; and oxygen saturation, 100% on room air. He was under no distress. His heart sound was tachycardic and regular with no murmur noted. Lung sounds were clear. Abdominal examination revealed no tenderness or organomegaly. His prostate was boggy, minimally tender, and slightly enlarged. The rest of his physical examination was normal. The white blood cell count was 7.5 109/L with 79% neutrophils. The serum chemistry was normal. The prostate‐specific antigen level was 2.9 ng/mL. A repeat CD4 count was 140/L. Urinalysis revealed large leukocyte esterase, no nitrites, and 60 white blood cells per high‐power field. He was diagnosed with prostatitis and discharged on levofloxacin.
The subsequent day, he returned to the emergency department with an inability to void. A Foley catheter was placed with resolution of his symptoms. Later that day, blood cultures from his initial admission grew MRSA in 2 out of 4 bottles, and he was admitted to the hospital. A review of the prior urine culture showed no growth. Computed tomography (CT) of the abdomen and pelvis showed an enlarged prostate with multiple noncommunicating peripherally enhancing hypodensities consistent with prostatic abscesses (arrows in Figure 1). These abscesses were associated with periprostatic fat stranding, edematous seminal vesicles, diffuse urinary bladder wall thickening, and mild inguinal adenopathy. Therapy with intravenous vancomycin was initiated.

Over the next 6 days, the patient continued to have intermittent fevers. Blood cultures continued to grow MRSA. Gentamicin was added for possible synergy on hospital day 3 without improvement. A transesophageal echocardiogram was normal. On hospital day 6, repeat CT showed no change in the size of the prostatic abscesses but an increased capsular bulge along the right mid gland. The urology service was consulted. They performed bedside transperineal drainage of the largest abscess with transrectal ultrasound guidance. No indwelling drain was placed. A culture of the purulent drainage grew MRSA. Three days after the surgical drainage, the patient was afebrile, urinary symptoms had resolved, and serial blood cultures remained negative. He was discharged on hospital day 13 to complete a 4‐week course of intravenous vancomycin. The patient missed an appointment for follow‐up imaging.
DISCUSSION
We report a case of community‐associated MRSA bacteremia secondary to a prostatic abscess, as confirmed by cultures from serum and of the abscess. S. aureus bacteremia often stems from infections of the respiratory tract, skin, and soft tissues, endocarditis, or infections of indwelling devices.4S. aureus can then create embolic foci, including in the prostate, which can serve as sources of persistent bacteremia. However, new evidence suggests community‐associated MRSA might be sexually transmitted among men who have sex with men.5 An MRSA prostatic abscess could then potentially be related to an ascending urinary tract infection or translocation from the gastrointestinal tract.
The vast majority of prostatitis cases prostatic abscesses are caused by Escherichia coli and other gram‐negative bacilli.6 Staphylococcus species are less common, and MRSA isolates have been described as a rare etiologic agent. Only four other case reports describing MRSA bacteremia associated with prostatitis or prostatic abscesses have been published.710 Both our patient and the three other case reports in the literature with prostatic abscesses and MRSA bacteremia required drainage of the abscess as well as antibiotics for resolution. The other reported cases involved drainage by transurethral resection of the prostate,79 whereas our case used transperineal drainage of the prostatic abscess via transrectal ultrasound guidance. In addition to intravenous antibiotics, drainage appears to be a key therapeutic measure for resolution of MRSA prostatic abscesses.
With the increasing incidence of community‐associated and healthcare‐associated MRSA infections, the incidence of prostatitis or prostatic abscesses secondary to MRSA may be increasing. MRSA seems to have a specific predilection for abscess formation in skin and soft‐tissue infections and pneumonia.2 Clinicians must be alert to a potentially higher frequency of MRSA as a cause of prostatic abscesses and prostatitis.
In the evaluation of a patient with persistent MRSA bacteremia, the potential for the prostate as a source of infection should be considered. Urinary symptoms may be subtle. Clinicians should have a low threshold for imaging studies such as CT to evaluate for a possible source of MRSA bacteremia. If a prostatic abscess is found, prompt surgical drainage or debridement is necessary for cure.
Community‐associated methicillin‐resistant Staphylococcus aureus (MRSA) infection is an evolving disease that is changing medical practice. MRSA has become the most frequent cause of skin and soft‐tissue infections presenting to most emergency departments in the United States.1 In comparison with methicillin‐sensitive S. aureus, community‐associated MRSA is more likely to present as soft‐tissue abscesses or necrotizing pneumonia.2 In 2005 alone, 94,360 invasive MRSA infections were estimated to have occurred in the United States, most of which were associated with MRSA bacteremia.3 In the hospital, MRSA infections are associated with greater lengths of stay, higher mortality, and increased costs.3
We report a patient with persistent MRSA bacteremia due to a prostatic abscess. Prostatitis or prostatic abscess with MRSA has rarely been reported. Resolution of the bacteremia was achieved only after drainage of the abscess. This case highlights the importance of recognizing this clinical condition and draining any MRSA‐associated abscesses. In addition, the abscess‐forming characteristics of MRSA may suggest that the incidence of prostatic abscess due to this microbe is on the rise.
CASE REPORT
A 40‐year‐old human immunodeficiency viruspositive man presented with a 10‐day history of intermittent fever, urinary hesitancy, weak urinary stream, and intermittent abdominal pain relieved following urination. He denied dysuria, hematuria, chest pain, dyspnea, nausea, weight loss, diarrhea, or decreased functional status. His last CD4 count was 528/L on highly active antiretroviral therapy 1 year prior to presentation; however, he had run out of medications several months prior to presentation. His medical history was also significant for incision and drainage of skin abscesses with unknown microbiology several months prior to presentation. He currently used tobacco but denied illicit drug use. He was sexually abstinent for over a year prior to presentation but had a history of having unprotected sex with men.
On physical examination, his vital signs were as follows: temperature, 37.8C; blood pressure, 133/84 mm Hg; heart rate, 107 beats/minute; respiration rate, 16 breaths/minute; and oxygen saturation, 100% on room air. He was under no distress. His heart sound was tachycardic and regular with no murmur noted. Lung sounds were clear. Abdominal examination revealed no tenderness or organomegaly. His prostate was boggy, minimally tender, and slightly enlarged. The rest of his physical examination was normal. The white blood cell count was 7.5 109/L with 79% neutrophils. The serum chemistry was normal. The prostate‐specific antigen level was 2.9 ng/mL. A repeat CD4 count was 140/L. Urinalysis revealed large leukocyte esterase, no nitrites, and 60 white blood cells per high‐power field. He was diagnosed with prostatitis and discharged on levofloxacin.
The subsequent day, he returned to the emergency department with an inability to void. A Foley catheter was placed with resolution of his symptoms. Later that day, blood cultures from his initial admission grew MRSA in 2 out of 4 bottles, and he was admitted to the hospital. A review of the prior urine culture showed no growth. Computed tomography (CT) of the abdomen and pelvis showed an enlarged prostate with multiple noncommunicating peripherally enhancing hypodensities consistent with prostatic abscesses (arrows in Figure 1). These abscesses were associated with periprostatic fat stranding, edematous seminal vesicles, diffuse urinary bladder wall thickening, and mild inguinal adenopathy. Therapy with intravenous vancomycin was initiated.

Over the next 6 days, the patient continued to have intermittent fevers. Blood cultures continued to grow MRSA. Gentamicin was added for possible synergy on hospital day 3 without improvement. A transesophageal echocardiogram was normal. On hospital day 6, repeat CT showed no change in the size of the prostatic abscesses but an increased capsular bulge along the right mid gland. The urology service was consulted. They performed bedside transperineal drainage of the largest abscess with transrectal ultrasound guidance. No indwelling drain was placed. A culture of the purulent drainage grew MRSA. Three days after the surgical drainage, the patient was afebrile, urinary symptoms had resolved, and serial blood cultures remained negative. He was discharged on hospital day 13 to complete a 4‐week course of intravenous vancomycin. The patient missed an appointment for follow‐up imaging.
DISCUSSION
We report a case of community‐associated MRSA bacteremia secondary to a prostatic abscess, as confirmed by cultures from serum and of the abscess. S. aureus bacteremia often stems from infections of the respiratory tract, skin, and soft tissues, endocarditis, or infections of indwelling devices.4S. aureus can then create embolic foci, including in the prostate, which can serve as sources of persistent bacteremia. However, new evidence suggests community‐associated MRSA might be sexually transmitted among men who have sex with men.5 An MRSA prostatic abscess could then potentially be related to an ascending urinary tract infection or translocation from the gastrointestinal tract.
The vast majority of prostatitis cases prostatic abscesses are caused by Escherichia coli and other gram‐negative bacilli.6 Staphylococcus species are less common, and MRSA isolates have been described as a rare etiologic agent. Only four other case reports describing MRSA bacteremia associated with prostatitis or prostatic abscesses have been published.710 Both our patient and the three other case reports in the literature with prostatic abscesses and MRSA bacteremia required drainage of the abscess as well as antibiotics for resolution. The other reported cases involved drainage by transurethral resection of the prostate,79 whereas our case used transperineal drainage of the prostatic abscess via transrectal ultrasound guidance. In addition to intravenous antibiotics, drainage appears to be a key therapeutic measure for resolution of MRSA prostatic abscesses.
With the increasing incidence of community‐associated and healthcare‐associated MRSA infections, the incidence of prostatitis or prostatic abscesses secondary to MRSA may be increasing. MRSA seems to have a specific predilection for abscess formation in skin and soft‐tissue infections and pneumonia.2 Clinicians must be alert to a potentially higher frequency of MRSA as a cause of prostatic abscesses and prostatitis.
In the evaluation of a patient with persistent MRSA bacteremia, the potential for the prostate as a source of infection should be considered. Urinary symptoms may be subtle. Clinicians should have a low threshold for imaging studies such as CT to evaluate for a possible source of MRSA bacteremia. If a prostatic abscess is found, prompt surgical drainage or debridement is necessary for cure.
- ,,, et al.Methicillin‐resistant S. aureus infections among patients in the emergency department.N Engl J Med.2006;355:666–674.
- ,,, et al.Comparison of both clinical features and mortality risk associated with bacteremia due to community‐acquired methicillin‐resistant Staphylococcus aureus and methicillin‐susceptible S. aureus.Clin Infect Dis.2008;46:799–806.
- ,,, et al.Invasive methicillin‐resistant Staphylococcus aureus infections in the United States.JAMA.2007;298:1763–1771.
- ,,,,,.The current spectrum of Staphylococcus aureus infection in a tertiary care hospital.Medicine (Baltimore).1994;73:186–208.
- ,,, et al.Emergence of multidrug‐resistant, community‐associated, methicillin‐resistant Staphylococcus aureus clone USA300 in men who have sex with men.Ann Intern Med.2008;148:249–257.
- ,,, et al.Prostatic abscess in the antibiotic era.Rev Infect Dis.1988;10:239–249.
- ,,.Community‐acquired methicillin‐resistant Staphylococcus aureus prostatic abscess.Urology.2004;64:808–810.
- ,,.Persistent methicillin‐resistant Staphylococcus aureus bacteremia due to a prostatic abscess.Scand J Infect Dis.2003;35:273–274.
- ,.Dual perinephric and prostatic abscesses from methicillin resistant Staphylococcus aureus.South Med J.2007;100:515–516.
- ,.Methicillin‐resistant Staphylococcus aureus prostatitis.Urology.2007;69:779.e1–779.e3.
- ,,, et al.Methicillin‐resistant S. aureus infections among patients in the emergency department.N Engl J Med.2006;355:666–674.
- ,,, et al.Comparison of both clinical features and mortality risk associated with bacteremia due to community‐acquired methicillin‐resistant Staphylococcus aureus and methicillin‐susceptible S. aureus.Clin Infect Dis.2008;46:799–806.
- ,,, et al.Invasive methicillin‐resistant Staphylococcus aureus infections in the United States.JAMA.2007;298:1763–1771.
- ,,,,,.The current spectrum of Staphylococcus aureus infection in a tertiary care hospital.Medicine (Baltimore).1994;73:186–208.
- ,,, et al.Emergence of multidrug‐resistant, community‐associated, methicillin‐resistant Staphylococcus aureus clone USA300 in men who have sex with men.Ann Intern Med.2008;148:249–257.
- ,,, et al.Prostatic abscess in the antibiotic era.Rev Infect Dis.1988;10:239–249.
- ,,.Community‐acquired methicillin‐resistant Staphylococcus aureus prostatic abscess.Urology.2004;64:808–810.
- ,,.Persistent methicillin‐resistant Staphylococcus aureus bacteremia due to a prostatic abscess.Scand J Infect Dis.2003;35:273–274.
- ,.Dual perinephric and prostatic abscesses from methicillin resistant Staphylococcus aureus.South Med J.2007;100:515–516.
- ,.Methicillin‐resistant Staphylococcus aureus prostatitis.Urology.2007;69:779.e1–779.e3.