User login
Chronic lymphocytic leukemia and apparent hyperkalemia
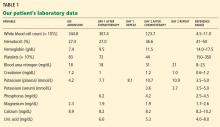
A 64-year-old man with chronic lymphocytic leukemia (CLL), Rai stage IV, was admitted to the hospital to undergo his first cycle of chemotherapy with fludarabine, cyclophosphamide, and rituximab. The physical examination at this time was normal except for splenomegaly and painless bilateral inguinal lymphadenopathy. His laboratory results on admission are shown in Table 1.
On the first day after chemotherapy, laboratory testing revealed an elevated plasma potassium level of 7.7 mmol/L. The specimen was drawn into a BD Vacutainer plasma separator tube with lithium-heparin additive (Becton, Dickinson, and Company, Franklin Lake, NJ) and analyzed on a Unicel DXC 800 chemistry analyzer (Beckman Coulter, Inc, Brea, CA).
1. Which electrocardiographic feature is not associated with hyperkalemia?
- Peaked P waves
- Prolonged PR interval
- Shortened QT interval
- Widened QRS
- Asystole
EVALUATING CARDIAC TOXICITY FROM HYPERKALEMIA
Hyperkalemia is not associated with peaked P waves, but rather with a reduction in the size of the P waves.
Hyperkalemia, defined as a plasma potassium concentration above 5.5 mmol/L, occurs as a result either of a release or a shift of intracellular potassium into the intravascular space or of decreased renal excretion. The earliest changes noted on electrocardiography are peaking and narrowing of T waves, followed by shortening of the QT interval. As hyperkalemia progresses, electrocardiography may show bradycardia, absent P waves, and PR interval prolongation, including second- or third-degree atrioventricular block.
At a plasma potassium concentration greater than 7 mmol/L, there may be a junctional escape rhythm, a sine wave pattern with widening of the QRS complex merging with T waves, ventricular fibrillation, or asystole. However, even with high potassium levels, electrocardiographic changes may be absent.1
The patient denied fatigue, muscle weakness, or palpitations. Electrocardiography did not show peaked T waves, shortened QT intervals, decreased P waves, prolonged PR interval, or widening of the QRS interval.
2. Which is an appropriate intervention for hyperkalemia with cardiac toxicity?
- Membrane stabilization with calcium gluconate
- Shifting potassium into the cells with a beta-adrenergic agonist given by nebulization
- Shifting potassium into the cells with insulin and dextrose
- Removal of potassium with sodium polystyrene sulfonate (Kayexalate)
- Removal of potassium with dialysis
In the setting of cardiac toxicity, the management of hyperkalemia involves stabilizing the cardiac muscle membrane, shifting potassium into the cells, and removing potassium from the body. It is important to do all three interventions when cardiac toxicity is present to provide sustained therapeutic benefit.2
MANAGING HYPERKALEMIA
General principles
When potassium levels are above 6 mmol/L, electrocardiography should always be done. Immediately stop potassium supplementation and any drugs that can cause hyperkalemia, such as potassium-sparing diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, nonsteroidal anti-inflammatory drugs, and trimethoprim-sulfamethoxazole (Bactrim). If the potassium level is greater than 6.5 mmol/L, the patient should be placed on telemetric monitoring, and the potassium level should be measured often.
Hyperkalemia with cardiac toxicity
Membrane stabilization involves intravenous infusion of 10 mL of 10% calcium gluconate. The onset of action is 1 to 3 minutes, and the duration of action is 30 to 60 minutes.
Shifting of potassium is done either with insulin or with a beta-adrenergic agonist nebulizer. With insulin, 10 U of regular insulin is given intravenously along with 50 mL of 50% dextrose; the onset of action is 20 minutes, and the duration is 4 to 6 hours. The dose of beta-adrenergic agonist depends on the type used; the onset of action is 20 minutes, with a duration of 2 to 4 hours.
Removal of potassium is achieved either by drug therapy or by dialysis. Sodium or calcium polystyrene sulfonate is given by mouth, 15 g every 6 hours, or 30 to 60 g by retention enema; the onset of action is 1 to 2 hours, with a duration of 4 to 6 hours. If dialysis is used, 2 to 3 hours is recommended; the onset of action is immediate and lasts for the duration of the dialysis session.
CASE CONTINUED
The patient was moved to a telemetry unit. A repeat plasma potassium measurement after 30 minutes confirmed hyperkalemia (Table 1). The specimen was transported to the laboratory via a pneumatic tube system, centrifuged at 3,300 rpm for 10 minutes, and analyzed on a Beckman Unicel DXC 800 chemistry analyzer. The time from specimen collection to analysis was approximately 60 minutes.
He was treated with oral sodium polystyrene sulfonate because no electrocardiographic changes were observed. Subsequent plasma potassium levels drawn, collected, and analyzed with the same technique described above were persistently high. Repeated electrocardiography continued to show no changes related to hyperkalemia.
DIAGNOSING THE CAUSE OF THE APPARENT HYPERKALEMIA
3. Which is the most likely cause of hyperkalemia in this patient?
- Acute renal failure
- Tumor lysis syndrome
- Hemolysis
- Reverse pseudohyperkalemia
- Pseudohyperkalemia
DIFFERENTIAL DIAGNOSIS
The patient had normal levels of blood urea nitrogen and creatinine and adequate urine output, thus ruling out acute renal failure. Hyperuricemia, hyperphosphatemia, and hypocalcemia were not found, thus ruling out tumor lysis syndrome.
In vitro hemolysis is assessed by visual inspection showing a pink or red hue to serum or plasma, or by hemolysis index calculation using spectrophotometric measurements. Factors associated with in vitro hemolysis include vein fragility, the phlebotomist’s skill and technique, and transportation of the specimen, including duration, mode, and temperature. The plasma potassium level was repeatedly measured from a lithium-heparin tube, thus minimizing the possibility of laboratory error. No evidence of hemolysis was observed during the phlebotomy, transportation, or specimen analysis.
Serum and plasma potassium levels were simultaneously measured to test for pseudohyperkalemia, a falsely elevated serum potassium concentration caused by the release of platelet potassium during clot formation or after venipuncture. Contributing factors include the prolonged use of a tourniquet, hemolysis, and marked leukocytosis or thrombocytosis. A serum-to-plasma potassium gradient greater than 0.4 mmol/L is diagnostic of pseudohyperkalemia.
Reverse pseudohyperkalemia, a falsely high potassium level in plasma samples, is defined as a serum-to-plasma potassium gradient less than 0.4 mmol/L (Table 2). This was the most likely cause of the hyperkalemia in our patient.
On the second day after chemotherapy, two blood samples were collected simultaneously—one into a lithium heparin BD Vacutainer plasma separator tube, and the other into a plain, red-top BD Vacutainer serum collection tube without heparin. The specimens were transported to the laboratory by pneumatic tube and were centrifuged at 3,300 rpm for 10 minutes. The specimens were analyzed simultaneously 20 minutes after collection on a Unicel DXC 800 chemistry analyzer. The analysis revealed a serum-to-plasma potassium gradient of −7.1 mmol/L (serum potassium 3.6 mmol/L and plasma potassium 10.7 mmol/L).3 Repeated potassium measurements drawn in similar fashion after 1 hour continued to show a markedly elevated potassium concentration compared with the serum concentration (Table 1).
Serum and plasma samples were again measured simultaneously 24 hours after detecting hyperkalemia to evaluate for pseudohyperkalemia in this patient. In another published report, serum and plasma measurements were obtained 1 week after observing hyperkalemia.4 Blood gas analysis was done the same day in two other reported cases.4,5
Of note, further review of our patient’s medical history noted hyperkalemia at the time he was diagnosed with CLL. At that time, his plasma potassium level was 7.5 mmol/L, a repeated plasma potassium level was 6.9 mmol/L, and a subsequent blood gas analysis—done 30 minutes after the repeated plasma potassium measurement using a Rapidlab analyzer (Siemens Healthcare Diagnostics, Washington, DC)—showed a potassium level of 3.4 mmol/L. The phenomenon of reverse pseudohyperkalemia was not recognized at that time.
The true mechanism of reverse pseudohyperkalemia has not yet been established. Even minor leakage of intracellular potassium from leukemic cells can have a major effect on the extracellular potassium level. Mechanical stressors in the form of pneumatic tube transport and specimen sampling into vacuum tubes have been implicated as causes of this artifact.5,6 Another possible mechanism is heparin-induced lysis of leukocytes in the setting of hematologic malignancy during laboratory processing.4,7,8
LESSONS LEARNED
In patients with hematologic proliferative disorders who develop hyperkalemia in the absence of electrocardiographic changes and an obvious cause of increased potassium levels (eg, acute renal failure, tumor lysis syndrome), we should entertain the possibility of hemolysis, laboratory error, pseudohyperkalemia, and reverse pseudohyperkalemia. The potassium level should be remeasured to rule out laboratory error and hemolysis. In patients with marked leukocytosis or thrombocytosis, simultaneous measurement of serum and plasma potassium levels helps diagnose pseudohyperkalemia and reverse pseudohyperkalemia. Also, prompt blood gas analysis can help identify spurious hyperkalemia.
- Mirvis DM, Goldberger AL. Electrocardiography. In:Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald's Heart Disease—A Textbook of Cardiovascular Medicine. 9th ed. Boston, MA: Elsevier Saunders; 2011:126–167.
- Rastergar A, Soleimani M. Hypokalaemia and hyperkalaemia. Postgrad Med J 2001; 77:759–764.
- Sevastos N, Theodossiades G, Archimandritis AJ. Pseudohyperkalemia in serum: a new insight into an old phenomenon. Clin Med Res 2008; 6:30–32.
- Meng QH, Krahn J. Reverse pseudohyperkalemia in heparin plasma samples from a patient with chronic lymphocytic leukemia. Clin Biochem 2011; 44:728–730.
- Garwicz D, Karlman M, Øra I. Reverse pseudohyperkalemia in heparin plasma samples from a child with T cell acute lymphoblastic leukemia with hyperleukocytosis [Letter]. Clin Chim Acta 2011; 412:396–397.
- Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis 2005; 46:746–748.
- Abraham B, Fakhar I, Tikaria A, et al. Reverse pseudohyperkalemia in a leukemic patient. Clin Chem 2008; 54:449–551.
- Singh PJ, Zawada ET, Santella RN. A case of ‘reverse’ pseudohyperkalemia. Miner Electrolyte Metab 1997; 23:58–61.
A 64-year-old man with chronic lymphocytic leukemia (CLL), Rai stage IV, was admitted to the hospital to undergo his first cycle of chemotherapy with fludarabine, cyclophosphamide, and rituximab. The physical examination at this time was normal except for splenomegaly and painless bilateral inguinal lymphadenopathy. His laboratory results on admission are shown in Table 1.
On the first day after chemotherapy, laboratory testing revealed an elevated plasma potassium level of 7.7 mmol/L. The specimen was drawn into a BD Vacutainer plasma separator tube with lithium-heparin additive (Becton, Dickinson, and Company, Franklin Lake, NJ) and analyzed on a Unicel DXC 800 chemistry analyzer (Beckman Coulter, Inc, Brea, CA).
1. Which electrocardiographic feature is not associated with hyperkalemia?
- Peaked P waves
- Prolonged PR interval
- Shortened QT interval
- Widened QRS
- Asystole
EVALUATING CARDIAC TOXICITY FROM HYPERKALEMIA
Hyperkalemia is not associated with peaked P waves, but rather with a reduction in the size of the P waves.
Hyperkalemia, defined as a plasma potassium concentration above 5.5 mmol/L, occurs as a result either of a release or a shift of intracellular potassium into the intravascular space or of decreased renal excretion. The earliest changes noted on electrocardiography are peaking and narrowing of T waves, followed by shortening of the QT interval. As hyperkalemia progresses, electrocardiography may show bradycardia, absent P waves, and PR interval prolongation, including second- or third-degree atrioventricular block.
At a plasma potassium concentration greater than 7 mmol/L, there may be a junctional escape rhythm, a sine wave pattern with widening of the QRS complex merging with T waves, ventricular fibrillation, or asystole. However, even with high potassium levels, electrocardiographic changes may be absent.1
The patient denied fatigue, muscle weakness, or palpitations. Electrocardiography did not show peaked T waves, shortened QT intervals, decreased P waves, prolonged PR interval, or widening of the QRS interval.
2. Which is an appropriate intervention for hyperkalemia with cardiac toxicity?
- Membrane stabilization with calcium gluconate
- Shifting potassium into the cells with a beta-adrenergic agonist given by nebulization
- Shifting potassium into the cells with insulin and dextrose
- Removal of potassium with sodium polystyrene sulfonate (Kayexalate)
- Removal of potassium with dialysis
In the setting of cardiac toxicity, the management of hyperkalemia involves stabilizing the cardiac muscle membrane, shifting potassium into the cells, and removing potassium from the body. It is important to do all three interventions when cardiac toxicity is present to provide sustained therapeutic benefit.2
MANAGING HYPERKALEMIA
General principles
When potassium levels are above 6 mmol/L, electrocardiography should always be done. Immediately stop potassium supplementation and any drugs that can cause hyperkalemia, such as potassium-sparing diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, nonsteroidal anti-inflammatory drugs, and trimethoprim-sulfamethoxazole (Bactrim). If the potassium level is greater than 6.5 mmol/L, the patient should be placed on telemetric monitoring, and the potassium level should be measured often.
Hyperkalemia with cardiac toxicity
Membrane stabilization involves intravenous infusion of 10 mL of 10% calcium gluconate. The onset of action is 1 to 3 minutes, and the duration of action is 30 to 60 minutes.
Shifting of potassium is done either with insulin or with a beta-adrenergic agonist nebulizer. With insulin, 10 U of regular insulin is given intravenously along with 50 mL of 50% dextrose; the onset of action is 20 minutes, and the duration is 4 to 6 hours. The dose of beta-adrenergic agonist depends on the type used; the onset of action is 20 minutes, with a duration of 2 to 4 hours.
Removal of potassium is achieved either by drug therapy or by dialysis. Sodium or calcium polystyrene sulfonate is given by mouth, 15 g every 6 hours, or 30 to 60 g by retention enema; the onset of action is 1 to 2 hours, with a duration of 4 to 6 hours. If dialysis is used, 2 to 3 hours is recommended; the onset of action is immediate and lasts for the duration of the dialysis session.
CASE CONTINUED
The patient was moved to a telemetry unit. A repeat plasma potassium measurement after 30 minutes confirmed hyperkalemia (Table 1). The specimen was transported to the laboratory via a pneumatic tube system, centrifuged at 3,300 rpm for 10 minutes, and analyzed on a Beckman Unicel DXC 800 chemistry analyzer. The time from specimen collection to analysis was approximately 60 minutes.
He was treated with oral sodium polystyrene sulfonate because no electrocardiographic changes were observed. Subsequent plasma potassium levels drawn, collected, and analyzed with the same technique described above were persistently high. Repeated electrocardiography continued to show no changes related to hyperkalemia.
DIAGNOSING THE CAUSE OF THE APPARENT HYPERKALEMIA
3. Which is the most likely cause of hyperkalemia in this patient?
- Acute renal failure
- Tumor lysis syndrome
- Hemolysis
- Reverse pseudohyperkalemia
- Pseudohyperkalemia
DIFFERENTIAL DIAGNOSIS
The patient had normal levels of blood urea nitrogen and creatinine and adequate urine output, thus ruling out acute renal failure. Hyperuricemia, hyperphosphatemia, and hypocalcemia were not found, thus ruling out tumor lysis syndrome.
In vitro hemolysis is assessed by visual inspection showing a pink or red hue to serum or plasma, or by hemolysis index calculation using spectrophotometric measurements. Factors associated with in vitro hemolysis include vein fragility, the phlebotomist’s skill and technique, and transportation of the specimen, including duration, mode, and temperature. The plasma potassium level was repeatedly measured from a lithium-heparin tube, thus minimizing the possibility of laboratory error. No evidence of hemolysis was observed during the phlebotomy, transportation, or specimen analysis.
Serum and plasma potassium levels were simultaneously measured to test for pseudohyperkalemia, a falsely elevated serum potassium concentration caused by the release of platelet potassium during clot formation or after venipuncture. Contributing factors include the prolonged use of a tourniquet, hemolysis, and marked leukocytosis or thrombocytosis. A serum-to-plasma potassium gradient greater than 0.4 mmol/L is diagnostic of pseudohyperkalemia.
Reverse pseudohyperkalemia, a falsely high potassium level in plasma samples, is defined as a serum-to-plasma potassium gradient less than 0.4 mmol/L (Table 2). This was the most likely cause of the hyperkalemia in our patient.
On the second day after chemotherapy, two blood samples were collected simultaneously—one into a lithium heparin BD Vacutainer plasma separator tube, and the other into a plain, red-top BD Vacutainer serum collection tube without heparin. The specimens were transported to the laboratory by pneumatic tube and were centrifuged at 3,300 rpm for 10 minutes. The specimens were analyzed simultaneously 20 minutes after collection on a Unicel DXC 800 chemistry analyzer. The analysis revealed a serum-to-plasma potassium gradient of −7.1 mmol/L (serum potassium 3.6 mmol/L and plasma potassium 10.7 mmol/L).3 Repeated potassium measurements drawn in similar fashion after 1 hour continued to show a markedly elevated potassium concentration compared with the serum concentration (Table 1).
Serum and plasma samples were again measured simultaneously 24 hours after detecting hyperkalemia to evaluate for pseudohyperkalemia in this patient. In another published report, serum and plasma measurements were obtained 1 week after observing hyperkalemia.4 Blood gas analysis was done the same day in two other reported cases.4,5
Of note, further review of our patient’s medical history noted hyperkalemia at the time he was diagnosed with CLL. At that time, his plasma potassium level was 7.5 mmol/L, a repeated plasma potassium level was 6.9 mmol/L, and a subsequent blood gas analysis—done 30 minutes after the repeated plasma potassium measurement using a Rapidlab analyzer (Siemens Healthcare Diagnostics, Washington, DC)—showed a potassium level of 3.4 mmol/L. The phenomenon of reverse pseudohyperkalemia was not recognized at that time.
The true mechanism of reverse pseudohyperkalemia has not yet been established. Even minor leakage of intracellular potassium from leukemic cells can have a major effect on the extracellular potassium level. Mechanical stressors in the form of pneumatic tube transport and specimen sampling into vacuum tubes have been implicated as causes of this artifact.5,6 Another possible mechanism is heparin-induced lysis of leukocytes in the setting of hematologic malignancy during laboratory processing.4,7,8
LESSONS LEARNED
In patients with hematologic proliferative disorders who develop hyperkalemia in the absence of electrocardiographic changes and an obvious cause of increased potassium levels (eg, acute renal failure, tumor lysis syndrome), we should entertain the possibility of hemolysis, laboratory error, pseudohyperkalemia, and reverse pseudohyperkalemia. The potassium level should be remeasured to rule out laboratory error and hemolysis. In patients with marked leukocytosis or thrombocytosis, simultaneous measurement of serum and plasma potassium levels helps diagnose pseudohyperkalemia and reverse pseudohyperkalemia. Also, prompt blood gas analysis can help identify spurious hyperkalemia.
A 64-year-old man with chronic lymphocytic leukemia (CLL), Rai stage IV, was admitted to the hospital to undergo his first cycle of chemotherapy with fludarabine, cyclophosphamide, and rituximab. The physical examination at this time was normal except for splenomegaly and painless bilateral inguinal lymphadenopathy. His laboratory results on admission are shown in Table 1.
On the first day after chemotherapy, laboratory testing revealed an elevated plasma potassium level of 7.7 mmol/L. The specimen was drawn into a BD Vacutainer plasma separator tube with lithium-heparin additive (Becton, Dickinson, and Company, Franklin Lake, NJ) and analyzed on a Unicel DXC 800 chemistry analyzer (Beckman Coulter, Inc, Brea, CA).
1. Which electrocardiographic feature is not associated with hyperkalemia?
- Peaked P waves
- Prolonged PR interval
- Shortened QT interval
- Widened QRS
- Asystole
EVALUATING CARDIAC TOXICITY FROM HYPERKALEMIA
Hyperkalemia is not associated with peaked P waves, but rather with a reduction in the size of the P waves.
Hyperkalemia, defined as a plasma potassium concentration above 5.5 mmol/L, occurs as a result either of a release or a shift of intracellular potassium into the intravascular space or of decreased renal excretion. The earliest changes noted on electrocardiography are peaking and narrowing of T waves, followed by shortening of the QT interval. As hyperkalemia progresses, electrocardiography may show bradycardia, absent P waves, and PR interval prolongation, including second- or third-degree atrioventricular block.
At a plasma potassium concentration greater than 7 mmol/L, there may be a junctional escape rhythm, a sine wave pattern with widening of the QRS complex merging with T waves, ventricular fibrillation, or asystole. However, even with high potassium levels, electrocardiographic changes may be absent.1
The patient denied fatigue, muscle weakness, or palpitations. Electrocardiography did not show peaked T waves, shortened QT intervals, decreased P waves, prolonged PR interval, or widening of the QRS interval.
2. Which is an appropriate intervention for hyperkalemia with cardiac toxicity?
- Membrane stabilization with calcium gluconate
- Shifting potassium into the cells with a beta-adrenergic agonist given by nebulization
- Shifting potassium into the cells with insulin and dextrose
- Removal of potassium with sodium polystyrene sulfonate (Kayexalate)
- Removal of potassium with dialysis
In the setting of cardiac toxicity, the management of hyperkalemia involves stabilizing the cardiac muscle membrane, shifting potassium into the cells, and removing potassium from the body. It is important to do all three interventions when cardiac toxicity is present to provide sustained therapeutic benefit.2
MANAGING HYPERKALEMIA
General principles
When potassium levels are above 6 mmol/L, electrocardiography should always be done. Immediately stop potassium supplementation and any drugs that can cause hyperkalemia, such as potassium-sparing diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, nonsteroidal anti-inflammatory drugs, and trimethoprim-sulfamethoxazole (Bactrim). If the potassium level is greater than 6.5 mmol/L, the patient should be placed on telemetric monitoring, and the potassium level should be measured often.
Hyperkalemia with cardiac toxicity
Membrane stabilization involves intravenous infusion of 10 mL of 10% calcium gluconate. The onset of action is 1 to 3 minutes, and the duration of action is 30 to 60 minutes.
Shifting of potassium is done either with insulin or with a beta-adrenergic agonist nebulizer. With insulin, 10 U of regular insulin is given intravenously along with 50 mL of 50% dextrose; the onset of action is 20 minutes, and the duration is 4 to 6 hours. The dose of beta-adrenergic agonist depends on the type used; the onset of action is 20 minutes, with a duration of 2 to 4 hours.
Removal of potassium is achieved either by drug therapy or by dialysis. Sodium or calcium polystyrene sulfonate is given by mouth, 15 g every 6 hours, or 30 to 60 g by retention enema; the onset of action is 1 to 2 hours, with a duration of 4 to 6 hours. If dialysis is used, 2 to 3 hours is recommended; the onset of action is immediate and lasts for the duration of the dialysis session.
CASE CONTINUED
The patient was moved to a telemetry unit. A repeat plasma potassium measurement after 30 minutes confirmed hyperkalemia (Table 1). The specimen was transported to the laboratory via a pneumatic tube system, centrifuged at 3,300 rpm for 10 minutes, and analyzed on a Beckman Unicel DXC 800 chemistry analyzer. The time from specimen collection to analysis was approximately 60 minutes.
He was treated with oral sodium polystyrene sulfonate because no electrocardiographic changes were observed. Subsequent plasma potassium levels drawn, collected, and analyzed with the same technique described above were persistently high. Repeated electrocardiography continued to show no changes related to hyperkalemia.
DIAGNOSING THE CAUSE OF THE APPARENT HYPERKALEMIA
3. Which is the most likely cause of hyperkalemia in this patient?
- Acute renal failure
- Tumor lysis syndrome
- Hemolysis
- Reverse pseudohyperkalemia
- Pseudohyperkalemia
DIFFERENTIAL DIAGNOSIS
The patient had normal levels of blood urea nitrogen and creatinine and adequate urine output, thus ruling out acute renal failure. Hyperuricemia, hyperphosphatemia, and hypocalcemia were not found, thus ruling out tumor lysis syndrome.
In vitro hemolysis is assessed by visual inspection showing a pink or red hue to serum or plasma, or by hemolysis index calculation using spectrophotometric measurements. Factors associated with in vitro hemolysis include vein fragility, the phlebotomist’s skill and technique, and transportation of the specimen, including duration, mode, and temperature. The plasma potassium level was repeatedly measured from a lithium-heparin tube, thus minimizing the possibility of laboratory error. No evidence of hemolysis was observed during the phlebotomy, transportation, or specimen analysis.
Serum and plasma potassium levels were simultaneously measured to test for pseudohyperkalemia, a falsely elevated serum potassium concentration caused by the release of platelet potassium during clot formation or after venipuncture. Contributing factors include the prolonged use of a tourniquet, hemolysis, and marked leukocytosis or thrombocytosis. A serum-to-plasma potassium gradient greater than 0.4 mmol/L is diagnostic of pseudohyperkalemia.
Reverse pseudohyperkalemia, a falsely high potassium level in plasma samples, is defined as a serum-to-plasma potassium gradient less than 0.4 mmol/L (Table 2). This was the most likely cause of the hyperkalemia in our patient.
On the second day after chemotherapy, two blood samples were collected simultaneously—one into a lithium heparin BD Vacutainer plasma separator tube, and the other into a plain, red-top BD Vacutainer serum collection tube without heparin. The specimens were transported to the laboratory by pneumatic tube and were centrifuged at 3,300 rpm for 10 minutes. The specimens were analyzed simultaneously 20 minutes after collection on a Unicel DXC 800 chemistry analyzer. The analysis revealed a serum-to-plasma potassium gradient of −7.1 mmol/L (serum potassium 3.6 mmol/L and plasma potassium 10.7 mmol/L).3 Repeated potassium measurements drawn in similar fashion after 1 hour continued to show a markedly elevated potassium concentration compared with the serum concentration (Table 1).
Serum and plasma samples were again measured simultaneously 24 hours after detecting hyperkalemia to evaluate for pseudohyperkalemia in this patient. In another published report, serum and plasma measurements were obtained 1 week after observing hyperkalemia.4 Blood gas analysis was done the same day in two other reported cases.4,5
Of note, further review of our patient’s medical history noted hyperkalemia at the time he was diagnosed with CLL. At that time, his plasma potassium level was 7.5 mmol/L, a repeated plasma potassium level was 6.9 mmol/L, and a subsequent blood gas analysis—done 30 minutes after the repeated plasma potassium measurement using a Rapidlab analyzer (Siemens Healthcare Diagnostics, Washington, DC)—showed a potassium level of 3.4 mmol/L. The phenomenon of reverse pseudohyperkalemia was not recognized at that time.
The true mechanism of reverse pseudohyperkalemia has not yet been established. Even minor leakage of intracellular potassium from leukemic cells can have a major effect on the extracellular potassium level. Mechanical stressors in the form of pneumatic tube transport and specimen sampling into vacuum tubes have been implicated as causes of this artifact.5,6 Another possible mechanism is heparin-induced lysis of leukocytes in the setting of hematologic malignancy during laboratory processing.4,7,8
LESSONS LEARNED
In patients with hematologic proliferative disorders who develop hyperkalemia in the absence of electrocardiographic changes and an obvious cause of increased potassium levels (eg, acute renal failure, tumor lysis syndrome), we should entertain the possibility of hemolysis, laboratory error, pseudohyperkalemia, and reverse pseudohyperkalemia. The potassium level should be remeasured to rule out laboratory error and hemolysis. In patients with marked leukocytosis or thrombocytosis, simultaneous measurement of serum and plasma potassium levels helps diagnose pseudohyperkalemia and reverse pseudohyperkalemia. Also, prompt blood gas analysis can help identify spurious hyperkalemia.
- Mirvis DM, Goldberger AL. Electrocardiography. In:Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald's Heart Disease—A Textbook of Cardiovascular Medicine. 9th ed. Boston, MA: Elsevier Saunders; 2011:126–167.
- Rastergar A, Soleimani M. Hypokalaemia and hyperkalaemia. Postgrad Med J 2001; 77:759–764.
- Sevastos N, Theodossiades G, Archimandritis AJ. Pseudohyperkalemia in serum: a new insight into an old phenomenon. Clin Med Res 2008; 6:30–32.
- Meng QH, Krahn J. Reverse pseudohyperkalemia in heparin plasma samples from a patient with chronic lymphocytic leukemia. Clin Biochem 2011; 44:728–730.
- Garwicz D, Karlman M, Øra I. Reverse pseudohyperkalemia in heparin plasma samples from a child with T cell acute lymphoblastic leukemia with hyperleukocytosis [Letter]. Clin Chim Acta 2011; 412:396–397.
- Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis 2005; 46:746–748.
- Abraham B, Fakhar I, Tikaria A, et al. Reverse pseudohyperkalemia in a leukemic patient. Clin Chem 2008; 54:449–551.
- Singh PJ, Zawada ET, Santella RN. A case of ‘reverse’ pseudohyperkalemia. Miner Electrolyte Metab 1997; 23:58–61.
- Mirvis DM, Goldberger AL. Electrocardiography. In:Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald's Heart Disease—A Textbook of Cardiovascular Medicine. 9th ed. Boston, MA: Elsevier Saunders; 2011:126–167.
- Rastergar A, Soleimani M. Hypokalaemia and hyperkalaemia. Postgrad Med J 2001; 77:759–764.
- Sevastos N, Theodossiades G, Archimandritis AJ. Pseudohyperkalemia in serum: a new insight into an old phenomenon. Clin Med Res 2008; 6:30–32.
- Meng QH, Krahn J. Reverse pseudohyperkalemia in heparin plasma samples from a patient with chronic lymphocytic leukemia. Clin Biochem 2011; 44:728–730.
- Garwicz D, Karlman M, Øra I. Reverse pseudohyperkalemia in heparin plasma samples from a child with T cell acute lymphoblastic leukemia with hyperleukocytosis [Letter]. Clin Chim Acta 2011; 412:396–397.
- Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis 2005; 46:746–748.
- Abraham B, Fakhar I, Tikaria A, et al. Reverse pseudohyperkalemia in a leukemic patient. Clin Chem 2008; 54:449–551.
- Singh PJ, Zawada ET, Santella RN. A case of ‘reverse’ pseudohyperkalemia. Miner Electrolyte Metab 1997; 23:58–61.