User login
Kidney Failure in the 21st Century
The Initiating Dialysis Early and Late (IDEAL) study in Australia and New Zealand1 examined the optimal time to initiate dialysis. This well-designed, randomized, controlled trial gave the nephrology community guidelines for treating patients as they progress through chronic kidney disease to stage 5 (CKD 5). The IDEAL investigators demonstrated that planned early initiation of dialysis did not enhance survival—and in some cases, it hastened death.1
Although most patients have a nephrology provider by the time they reach CKD 5 (ie, kidney failure), primary care providers can be instrumental in preparing the patient for what lies ahead as kidney failure progresses. Presented here is an overview of diagnosis and management of the patient with CKD 5 in the 21st century.
CASE PRESENTATION
A 70-year-old woman with an extensive history of diabetes and hypertension presents to her primary care clinician complaining of a lack of energy. She has just returned from a trip to Disney World, where she says she was unable to keep up with her grandchildren. She sat in the shade while they enjoyed Space Mountain and other attractions, and because of uncustomary fatigue, she required a nap every afternoon.
Physical examination shows an elderly female in no acute distress. Cardiac exam shows a regular heart rate and rhythm, the patient’s lungs are clear, and she has 1+ pitting leg edema bilaterally. The patient’s blood pressure is slightly elevated at 142/92 mm Hg, and no protein is detected on spot urine testing.
Blood work is ordered, including a complete blood count, a comprehensive metabolic panel, and an A1C. Results include a hemoglobin level of 8.7 g/dL (reference range for women, 12.0 to 16.0 g/dL), a serum creatinine level (SCr) of 3 mg/dL (range, 0.6 to 1.2 mg/dL; estimated glomerular filtration rate [eGFR], 15 mL/min/1.73 m2), and an A1C value of 7.5% (normal, & < 7%).
The patient is told that she is in kidney failure and is referred immediately to a nephrology practice, where she is seen the following day. After her appointment, she returns to the primary care office. When she is asked when she will be starting dialysis, she seems surprised, saying, “They told me I was doing well. I need some shots for my blood, but they didn’t seem worried at all.”
Is this patient being managed correctly by the nephrology practitioner?
EPIDEMIOLOGY
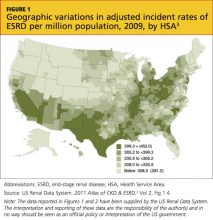
Presently, more than 1 million persons in the United States have CKD 5, and 500,000 are undergoing dialysis. The number of patients with CDK 5 who are on dialysis has doubled since 1994 and is projected to reach 774,000 by 20202,3 (see Figure 13 and Figure 23).
CKD 5 is defined as an eGFR below 15 mL/min/1.73 m2, according to the Modification of Diet in Renal Disease (MDRD) study group formula; or as a creatinine clearance of less than 15 mL/min, using the Cockcroft-Gault formula.4-6 Both formulas have limitations, since they are not fully accurate at extremes of age, with variations in weight, for some racial mixes, or for the very malnourished patient.7,8 However, they do correct for normal age-related loss of kidney function, gender, and SCr, and they are currently the generally accepted formulas.4
A rise in SCr is exponential; for each doubling of the SCr, a reduction in kidney function of approximately 50% occurs. This means that a rise in SCr from 4 to 8 mg/dL is equivalent (in the proportion of loss of kidney function) to a rise of SCr from 0.5 to 1 mg/dL. Commonly, patients are not referred to nephrology until the SCr doubles to 4 or 6 mg/dL—whereas the rise in SCr from 0.5 to 1 mg/dL should be of greatest concern to the primary care provider, prompting a referral.9 Early recognition of reduced renal function allows for identification of potentially reversible etiologies and the slowing of CKD progression.
INDICATIONS FOR RENAL REPLACEMENT THERAPY
Not all patients with an eGFR below 15 mL/min/1.73 m2 are started on dialysis immediately. The decision to initiate dialysis is guided by assessment of a constellation of uremic manifestations—not eGFR alone. Newer data suggest that early initiation of dialysis may be associated with increased mortality.10-15
The IDEAL study,1 a well-designed, randomized, controlled trial of all patients who started dialysis in Australia and New Zealand over a multiyear period, was designed to determine the optimal time to initiate dialysis. Patients were randomized to start dialysis (hemodialysis or peritoneal dialysis) at an eGFR between 10 and 14 mL/min/1.73 m2 or between 5 and 7 mL/min/1.73 m2. While there was some overlap and some patients were started on dialysis outside their randomized eGFR (eg, earlier than the allotted time because symptoms developed), what the IDEAL investigators found surprised the entire nephrology community: Early dialysis starts did not enhance survival and, in some cases, it hastened death.1
The indications for initiation of dialysis often develop long after the patient has progressed within CKD 5, commonly with an eGFR of 10 mL/min/1.73 m2 or less. Medicare has acknowledged this by reimbursing dialysis only in eligible patients whose eGFR is below 10 mL/min/1.73 m2.16 There are also acute indications for initiation of dialysis, such as uremic pericarditis, hyperkalemia, bleeding related to uremic platelet dysfunction, and metabolic encephalopathy related to uremia (and reimbursement for dialysis can be justified), but these are uncommon.
RENAL REPLACEMENT THERAPY CHOICES
The choices of treatment for kidney failure (note: treatment, not cure) are medical management, hemodialysis, peritoneal dialysis, and transplant. Each choice has its advantages and disadvantages, and all patients should receive clear explanations of what they can expect from each modality.
While younger, higher-functioning individuals are likely to benefit from dialysis, patients with extensive comorbid illnesses and/or low functional status tend to respond poorly17; medical management may be the best choice for these patients. In one recent study, the functional status of residents in skilled nursing homes was examined, before and after initiation of dialysis. At one year, 58% of the nursing home residents who underwent dialysis had died, and only 13% had maintained their pre-dialysis functional status.18
Another research team recently compared conservative management of CKD 5 (ie, medical therapy without dialysis) with dialysis in elderly patients. For patients 75 and older with extensive comorbid illness, the researchers found no statistically significant survival benefit to dialysis.19
Dialysis, whether administered as hemodialysis or peritoneal dialysis, is a rigorous, intensive medical therapy. Dialysis does not necessarily prolong life in patients with extensive comorbidities, and it does not always enhance quality of life.18,20-23 The decision to undergo dialysis is personal and individual, and both the patient and family should be actively involved in making it. Primary care providers should be the nephrologist’s ally in the discussion of therapy for renal failure; often, they have cared for the patient for years, and they understand the patient and family dynamics.
An important message the nephrology practitioner must communicate is that choosing medical management without dialysis is not withholding care; sometimes it is a more humane choice.24 Dying of kidney failure can be a peaceful and comfortable death: As the uremic toxins build up (with eGFR ≤ 2 mL/min/1.73 m2, although this can take many years), the patient becomes confused and slowly slips into a sleepiness that leads to death.25 Hospice is usually involved to support the patient and family.
Hemodialysis
Hemodialysis is the most common, best-known treatment modality for CKD in the US, with about 94% of patients choosing it.3 In this process, blood is removed from the body (approximately 500 cc at a time) and filtered through a semipermeable membrane that removes uremic toxins and excess fluid, normalizing the metabolic and electrolyte derangements. The filtered blood is then returned to the patient.
The average dialysis session is four hours long and is conducted three times per week, following recommendations from the 2002 Hemodialysis Study (HEMO).26 Most patients come to a free-standing dialysis center on a Monday/Wednesday/Friday or a Tuesday/Thursday/Saturday schedule.
In theory, there is no such thing as too much dialysis (since the kidneys work 24 hours per day, seven days per week); thus, researchers have recently examined lengthening dialysis in an attempt to extend survival.27-29 According to study results, patients who undergo longer dialysis times generally enjoy better nutrition with a more liberal diet, require fewer medications, have reduced incidence of increased left ventricular mass (a marker for coronary artery disease), and report better quality of life, all in addition to a survival benefit; however, the latter was not considered statistically significant in any of the studies.27-29 Additionally, this survival benefit may not extend to daily hemodialysis; in a recent publication, daily hemodialysis was associated with a 60% higher death rate.30
A number of dialysis units have begun to offer nocturnal dialysis. In this option, patients sleep at the unit for eight hours, three nights per week, for a total of 24 hours of dialysis (vs the typical 12 hours per week). Some patients receive dialysis at home, allowing them to dialyze for six weekly sessions of two to three hours. This strategy attempts to mimic a more “natural” state.
One of the primary challenges associated with hemodialysis is establishing and maintaining a vascular access. An arteriovenous (AV) fistula is the access of choice because its use reduces the likelihood of clotting, improves access survival, and increases clearances during dialysis.31 However, the AV fistula is most effective if it is placed a minimum of six months before use.32
For many patients, an AV fistula is created surgically when the eGFR falls to 15 mL/min/1.73 m2. Fistula placement in preparation for the initiation of hemodialysis is important to reduce the need for hemodialysis catheters, which are associated with higher risk for infection and poorer outcomes.33
Peritoneal Dialysis
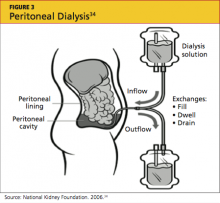
Peritoneal dialysis (PD) filters out uremic toxins and normalizes the metabolic and electrolyte derangements by using the patient’s peritoneal membrane as a filter. Dialysate is instilled through a catheter into the peritoneal cavity where it is allowed to dwell, often for four to six hours (see Figure 334). Exchanges can be performed three to four times per day (allowing six to eight hours of dwell time per exchange for the dialysate), or by way of a cycler at night.35,36
Performing exchanges by way of a cycler requires the patient to be connected via the PD catheter for eight hours; thus the advantage of performing the exchange during sleep. The cycler has a soft, whooshing sound that many patients describe as “white noise” that does not disturb their sleep.
The principal advantage of peritoneal dialysis is extended survival of the patient.37-39 PD also allows the patient a more liberal diet. Although PD must be performed every day, exchanges can be adjusted to the patient’s timing preference. PD reduces cost and time, since the patient need not travel to a dialysis center. PD also preserves residual renal function,39 which is associated with a survival benefit and contributes to the patient’s overall health and well-being. The use of PD requires a committed, competent patient in a clean home environment.
This intervention may not be suitable for patients with a history of abdominal surgeries. However, as a therapy considered gentler than hemodialysis, PD is an excellent choice for the patient with congestive heart failure.40 The PD catheter is not placed until two to four weeks before it will be needed.41
Kidney Transplantation
Last, but certainly not least, is transplant. As entire books have been written on this modality, only the highlights will be addressed here.42 Survival rates following transplantation are reported to exceed those associated with any other treatment modality, even when controlling for comorbidities and patient selection bias.43 A patient can receive a kidney from either a living or a deceased donor. Apart from the perioperative risks associated with transplantation surgery, long-term surgical or medical problems are not common for the living kidney donor.44-46
Since 2002, as a result of a policy change from the United Network for Organ Sharing (UNOS),47 the donor pool has been expanded by including kidneys from what are commonly referred to as extended criteria donors: those who are older than 60, or who are 50 to 59 and have two of the following factors: cerebrovascular accident as the cause of death; preexisting hypertension; or an SCr level exceeding 1.5 mg/dL. These kidneys may be given to any recipients but are primarily used for those age 50 or older.47
Graft survival is statistically shorter in a cadaver kidney than in a living donor kidney.48 However, the larger problem is the long wait time for a cadaver transplant—seven to eight years (or possibly longer) for some centers and some blood types.49 A patient can be referred to a transplant center when the eGFR falls below 25 mL/min/1.73 m2; he or she will then be actively listed for transplant when eGFR reaches 20 mL/min/1.73 m2 or lower.
Early referral for transplant listing buys the patient time before dialysis becomes essential. Patients can receive preemptive transplants (ie, transplantation before dialysis is initiated); these are becoming increasingly popular because they generally extend survival for the recipient.50,51
UNOS (www.unos.org) has set few limits on transplant recipients: patients are required to undergo an extensive medical work-up, but there are no upper age limits (although some centers do set their own) and usually no limits on patients infected with HIV, hepatitis B, or hepatitis C (although there may be separate listing requirements). Patients who are not US citizens are not denied.
Because more than 100,000 patients are currently on the wait list, domino kidney transplants are now being offered at many centers.52-54 These organ exchanges involve altruistic donors who do not match their targeted kidney recipients. Since publication of the first article describing this procedure at Johns Hopkins Medical Center,53 there have been two-way, three-way, and up to 32-way domino transplants. However, patients wishing to engage in this type of trade require a donor; not every patient has access to an altruistic donor.
CKD PATIENT EDUCATION
When Congress passed the Medicare Improvements for Patients and Providers Act of 2008,55 a component little noted outside the nephrology community was the offer of kidney disease education (KDE) classes to Medicare-eligible patients with CKD 4.56-58 Medicare patients with an eGFR of 15 to 30 mL/min/1.73 m2 are eligible to attend KDE classes presented by a physician, an NP, a PA, or a clinical nurse specialist. Medicare will reimburse for six hours of education.
This groundbreaking educational benefit, the first such program paid for by Medicare, was championed by the National Kidney Foundation and other nephrology groups. Many nephrology practices now offer these classes to all their CKD patients, regardless of health insurance status. Instructors educate patients on the choices of renal replacement therapy and help patients select their best option.
KDE classes can be conducted in the office setting or at the patient’s home, and they can be billed on the same day as an evaluation and management (E/M) visit.56,57 This may help explain why, in 2010, gerontologists billed for more home KDE classes than did nephrologists.58
FUTURE TRENDS AND ONGOING TRIALS
The overarching goal of therapy for CKD patients is to diagnose accurately and treat effectively in order to slow or prevent progression to end-stage renal disease (ESRD). Following is a brief review of a selection of new diagnostic tools and therapeutic interventions that may impact the management of CKD in the coming years.
The QxMD Kidney Failure Risk Equation is a newly developed, well-validated predictive model that offers relatively accurate prediction of a patient’s likelihood of progression to ESRD, based on age, sex, eGFR, and levels of albuminuria, SCr, serum phosphorus, serum bicarbonate, and serum albumin59 (see table59). This tool can be accessed online at www.qxmd.com/calculate-online/nephrology/kidney-failure-risk-equation.
Increased awareness of the role that phosphorus plays in the development of vascular calcifications has led to an emphasis on earlier, more aggressive control of dietary phosphorus. Historically, dietary phosphorus control and phosphorus binders were recommended only when the patient’s serum phosphorus exceeded normal limits. Dietary phosphorus control may now be advised as early as CKD 3, based on the understanding that phosphorus is a key component in driving the development of vascular calcifications—which in turn contribute to the high incidence of cardiovascular disease in patients with CKD.60
Bardoxolone is a new medication developed for treatment of diabetic nephropathy.61,62 It works by inhibiting proinflammatory mediators and moderating the effects of oxidative stress, thus interrupting the cascade of inflammation and cellular injury that result in diabetic nephropathy.62 Early clinical trials examining this agent have shown promise in terms of raising eGFR and reducing serum creatinine, but tolerability and toxicity profiles have been an issue.61 Additionally, some reduction in serum creatinine may have been attributable to weight loss as opposed to true improvement of renal function.62
Data have recently been released regarding the use of the herb silymarin for treatment of patients with macroalbuminuria related to diabetic nephropathy. Results from a small (n = 60), randomized, nonblinded clinical trial by Fallahzadeh et al63 indicate that silymarin decreases proteinuria when used in combination with an ACE inhibitor or an angiotensin receptor blocker (ARB). Silymarin, an extract from milk thistle, has been used medicinally for centuries for its antioxidant, anti-inflammatory, and antiviral properties in those with liver “ailments.” In this clinical trial, silymarin was well tolerated and associated with a reduction in pro-inflammatory markers.63
AST-120 is another agent intended to slow progression of CKD. It promotes intestinal adsorption and fecal excretion of the uremic toxin indoxyl sulfate.64 A recently published study demonstrated an association between use of AST-120 and a delay in required initiation of hemodialysis, but no survival benefit.65 This was a nonrandomized trial, and previous research showed no effect of AST-120 on progression of CKD.66 However, patients close to requiring dialysis are likely to welcome a means to delay it.
A growing body of evidence suggests that reversal of CKD-associated acidemia by administering sodium bicarbonate may actually forestall progression of CKD.67 However, treatment with sodium bicarbonate can lead to complications of hypertension and fluid volume overload. In one study, patients at risk for these complications derived equivalent benefit by increasing dietary fruit and vegetable intake to reduce renal acid load.68 However, providers must be mindful of patients’ serum potassium levels, especially patients who are taking an ACE inhibitor or an ARB.
The Provider’s Current Role
Despite the hope offered by new and novel therapies, the mainstay of treatment for CKD continues to be aggressive management of diabetes, hypertension, and the cardiovascular risk profile. As a result of vigorous preventive strategies to address the cardiovascular risks inherent in this patient population, the patient with type 2 diabetes is now more likely to progress to ESRD than to die of a cardiovascular event.69
The well-informed clinician can be instrumental in providing evidence-based medical therapy and excellent patient education from the time of initial CKD diagnosis through CKD 5.
PATIENT OUTCOME
The case patient was started on injections of epoietin alfa for her anemia. She attended KDE classes led by an advanced practitioner in her nephrology group and decided to undergo peritoneal dialysis, since it would allow her to maintain her travel schedule. When last heard from, the patient was preparing for a trip to see the Great Barrier Reef in Australia.
CONCLUSION
The decision to begin renal replacement therapy—whether a form of dialysis, or another management option—depends not on SCr or eGFR alone. Rather, a number of uremic manifestations, comorbidities, lifestyle factors, and other variables must be carefully weighed before the patient, the family, and the clinicians involved can decide on the management plan most likely to enhance the patient’s quality of life and extend survival.
1. Cooper BA, Branley P, Bulfone L, et al; IDEAL Study. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010; 363(7):609-619.
2. Olan G. Policy Update: ASN to CDC: data collection of creatinine levels will advance research. ASN Kidney News. 2012;4(6):16. www.asn-online.org/publications/kidneynews/archives/2012/KN_jun2012.pdf. Accessed August 27, 2012.
3. US Renal Data System, National Institute of Diabetes and Digestive and Kidney Diseases, NIH. 2011 Atlas of CKD & ESRD. Vol 2. Chap 1: Incidence, prevalence, patient characteristics, and modalities. www.usrds.org/2011/pdf/v2_ch01_11.pdf. Accessed September 26, 2012.
4. National Kidney Foundation. Kidney Disease Outcomes Quality Initiative (KDOQI). Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. ?Part 4. Definition and classification of the stages of chronic kidney disease. 2002:43-80. www.kidney.org/professionals/kdoqi/pdf/ckd_evalua tion_classification_stratification.pdf. Accessed August 27, 2012.
5. Levey AS, Bosch JP, Lewis JB, et al; Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Internal Med. 1999;130 (6):461-470.
6. Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the Modification of Diet in Renal Disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16(3):763-773.
7. Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16(5):1413-1419.
8. Zuo L, Ma YC, Zhou YH, et al. Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis. 2005;45(3):463-472.
9. Stevens LA, Stoycheff N, Levey AS. Staging and management of chronic kidney disease. In: Greenberg A, ed. Primer of Kidney Diseases: Expert Consult. 5th ed. Philadelphia, PA: WB Saunders; 2009:436-445.
10. Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ. 2011;183(1):47-53.
11. Beddhu S, Samore MH, Roberts MS, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003;14(9):2305-2312.
12. Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46(5):887-896.
13. Lassalle M, Labeeuw M, Frimat L, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77(8):700-707.
14. Stel VS, Dekker FW, Ansell D, et al. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009;24 (10):3175-3182.
15. Traynor JP, Simpson K, Geddes CC, et al. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002;13(8):2125-2132.
16. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. CMS Form 2728. End-Stage Renal Disease Medical Evidence Report: Medicare Entitlement and/or Patient Registration. www.usrds.org/2008/rg/forms/02_2728_1965.pdf. Accessed September 25, 2012.
17. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis: Clinical Practice Guideline. 2nd ed. Rockville, MD: Renal Physicians Association; Oct 2010:1-12.
18. Kurella Tamura M, Covinsky KE, Chertow GM, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547.
19. Chandna SM, Da Silva-Gane M, Marshall C, et al. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608-1614.
20. Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95:c40-c46.
21. Lamping DL, Constantinovici N, Roderick P, et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: a prospective cohort study. Lancet. 2000;356:1543-1550.
22. O’Connor NR, Kumar P. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med. 2012; 15(2):228-235.
23. Jassal SV, Trpeski L, Zhu N, et al. Changes in survival among elderly patients initiating dialysis from 1990 to 1999. CMAJ. 2007;177(9):?1033-1038.
24. Schmidt RJ. Informing our elders about dialysis: is an age-attuned approach warranted? Clin J Am Soc Nephrol. 2012;7(1):185-191.
25. Holley JL. Providing optimal care before and after discontinuation. Semin Dial. 2012;25(1):?33-34.
26. Eknoyan G, Beck GJ, Cheung AK, et al; Hemodialysis (HEMO) Study Group. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):?2010-2019.
27. Lacson E, Lazarus M. Dialysis time: does it matter? A reappraisal of existing literature. Curr Opin Nephrol Hypertens. 2011;20(2):189-194.
28. Chertow GM, Levin NW, Beck GJ, et al; FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):22878-2300.
29. Lacson E Jr, Xu J, Suri RS, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol. 2012;23(4):687-695.
30. Suri RS, Lindsay RM, Bieber BA, et al. A multinational cohort study of in-center daily hemodialysis and patient survival. Kidney Int. 2012 Sep 12. [Epub ahead of print]
31. Sidawy AN, Spergel LM, Besarab A, et al; Society for Vascular Surgery. Clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. ?J Vasc Surg. 2008;48(5 Suppl):2S-25S.
32. Heaf JG. Algorithm for optimal dialysis access timing. Clin Nephrol. 2007;67(2):96-104.
33. Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int. 2001;60(1):1-13.
34. National Kidney Foundation. Peritoneal dialysis: what you need to know (2006;11-50-0215). www.kidney.org/atoz/pdf/PeritonealDialysis.pdf. Accessed September 24, 2012.
35. Davison SN, Ghangri GS, Jindal K, Pannu N. Comparison of volume overload with cycler-assisted versus continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol. 2009;4(6):1044-1050.
36. Juergensen P, Eras J, McClure B, et al. The impact of various cycling regimens on phosphorus removal in chronic peritoneal dialysis patients. Int J Artif Organs. 2005;28(12):1219-1223.
37. Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl. 2006 Nov;(103):S3-S11.
38. Heaf J, Løkkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant. 2002;17(1):112-117.
39. Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69(10):1726-1732.
40. Cnossen N, Kooman JP, Konings CJ, et al. Peritoneal dialysis in patients with congestive heart failure. Nephrol Dial Transplant. 2006;21 suppl 2: ii63-ii66.
The Initiating Dialysis Early and Late (IDEAL) study in Australia and New Zealand1 examined the optimal time to initiate dialysis. This well-designed, randomized, controlled trial gave the nephrology community guidelines for treating patients as they progress through chronic kidney disease to stage 5 (CKD 5). The IDEAL investigators demonstrated that planned early initiation of dialysis did not enhance survival—and in some cases, it hastened death.1
Although most patients have a nephrology provider by the time they reach CKD 5 (ie, kidney failure), primary care providers can be instrumental in preparing the patient for what lies ahead as kidney failure progresses. Presented here is an overview of diagnosis and management of the patient with CKD 5 in the 21st century.
CASE PRESENTATION
A 70-year-old woman with an extensive history of diabetes and hypertension presents to her primary care clinician complaining of a lack of energy. She has just returned from a trip to Disney World, where she says she was unable to keep up with her grandchildren. She sat in the shade while they enjoyed Space Mountain and other attractions, and because of uncustomary fatigue, she required a nap every afternoon.
Physical examination shows an elderly female in no acute distress. Cardiac exam shows a regular heart rate and rhythm, the patient’s lungs are clear, and she has 1+ pitting leg edema bilaterally. The patient’s blood pressure is slightly elevated at 142/92 mm Hg, and no protein is detected on spot urine testing.
Blood work is ordered, including a complete blood count, a comprehensive metabolic panel, and an A1C. Results include a hemoglobin level of 8.7 g/dL (reference range for women, 12.0 to 16.0 g/dL), a serum creatinine level (SCr) of 3 mg/dL (range, 0.6 to 1.2 mg/dL; estimated glomerular filtration rate [eGFR], 15 mL/min/1.73 m2), and an A1C value of 7.5% (normal, & < 7%).
The patient is told that she is in kidney failure and is referred immediately to a nephrology practice, where she is seen the following day. After her appointment, she returns to the primary care office. When she is asked when she will be starting dialysis, she seems surprised, saying, “They told me I was doing well. I need some shots for my blood, but they didn’t seem worried at all.”
Is this patient being managed correctly by the nephrology practitioner?
EPIDEMIOLOGY
Presently, more than 1 million persons in the United States have CKD 5, and 500,000 are undergoing dialysis. The number of patients with CDK 5 who are on dialysis has doubled since 1994 and is projected to reach 774,000 by 20202,3 (see Figure 13 and Figure 23).
CKD 5 is defined as an eGFR below 15 mL/min/1.73 m2, according to the Modification of Diet in Renal Disease (MDRD) study group formula; or as a creatinine clearance of less than 15 mL/min, using the Cockcroft-Gault formula.4-6 Both formulas have limitations, since they are not fully accurate at extremes of age, with variations in weight, for some racial mixes, or for the very malnourished patient.7,8 However, they do correct for normal age-related loss of kidney function, gender, and SCr, and they are currently the generally accepted formulas.4
A rise in SCr is exponential; for each doubling of the SCr, a reduction in kidney function of approximately 50% occurs. This means that a rise in SCr from 4 to 8 mg/dL is equivalent (in the proportion of loss of kidney function) to a rise of SCr from 0.5 to 1 mg/dL. Commonly, patients are not referred to nephrology until the SCr doubles to 4 or 6 mg/dL—whereas the rise in SCr from 0.5 to 1 mg/dL should be of greatest concern to the primary care provider, prompting a referral.9 Early recognition of reduced renal function allows for identification of potentially reversible etiologies and the slowing of CKD progression.
INDICATIONS FOR RENAL REPLACEMENT THERAPY
Not all patients with an eGFR below 15 mL/min/1.73 m2 are started on dialysis immediately. The decision to initiate dialysis is guided by assessment of a constellation of uremic manifestations—not eGFR alone. Newer data suggest that early initiation of dialysis may be associated with increased mortality.10-15
The IDEAL study,1 a well-designed, randomized, controlled trial of all patients who started dialysis in Australia and New Zealand over a multiyear period, was designed to determine the optimal time to initiate dialysis. Patients were randomized to start dialysis (hemodialysis or peritoneal dialysis) at an eGFR between 10 and 14 mL/min/1.73 m2 or between 5 and 7 mL/min/1.73 m2. While there was some overlap and some patients were started on dialysis outside their randomized eGFR (eg, earlier than the allotted time because symptoms developed), what the IDEAL investigators found surprised the entire nephrology community: Early dialysis starts did not enhance survival and, in some cases, it hastened death.1
The indications for initiation of dialysis often develop long after the patient has progressed within CKD 5, commonly with an eGFR of 10 mL/min/1.73 m2 or less. Medicare has acknowledged this by reimbursing dialysis only in eligible patients whose eGFR is below 10 mL/min/1.73 m2.16 There are also acute indications for initiation of dialysis, such as uremic pericarditis, hyperkalemia, bleeding related to uremic platelet dysfunction, and metabolic encephalopathy related to uremia (and reimbursement for dialysis can be justified), but these are uncommon.
RENAL REPLACEMENT THERAPY CHOICES
The choices of treatment for kidney failure (note: treatment, not cure) are medical management, hemodialysis, peritoneal dialysis, and transplant. Each choice has its advantages and disadvantages, and all patients should receive clear explanations of what they can expect from each modality.
While younger, higher-functioning individuals are likely to benefit from dialysis, patients with extensive comorbid illnesses and/or low functional status tend to respond poorly17; medical management may be the best choice for these patients. In one recent study, the functional status of residents in skilled nursing homes was examined, before and after initiation of dialysis. At one year, 58% of the nursing home residents who underwent dialysis had died, and only 13% had maintained their pre-dialysis functional status.18
Another research team recently compared conservative management of CKD 5 (ie, medical therapy without dialysis) with dialysis in elderly patients. For patients 75 and older with extensive comorbid illness, the researchers found no statistically significant survival benefit to dialysis.19
Dialysis, whether administered as hemodialysis or peritoneal dialysis, is a rigorous, intensive medical therapy. Dialysis does not necessarily prolong life in patients with extensive comorbidities, and it does not always enhance quality of life.18,20-23 The decision to undergo dialysis is personal and individual, and both the patient and family should be actively involved in making it. Primary care providers should be the nephrologist’s ally in the discussion of therapy for renal failure; often, they have cared for the patient for years, and they understand the patient and family dynamics.
An important message the nephrology practitioner must communicate is that choosing medical management without dialysis is not withholding care; sometimes it is a more humane choice.24 Dying of kidney failure can be a peaceful and comfortable death: As the uremic toxins build up (with eGFR ≤ 2 mL/min/1.73 m2, although this can take many years), the patient becomes confused and slowly slips into a sleepiness that leads to death.25 Hospice is usually involved to support the patient and family.
Hemodialysis
Hemodialysis is the most common, best-known treatment modality for CKD in the US, with about 94% of patients choosing it.3 In this process, blood is removed from the body (approximately 500 cc at a time) and filtered through a semipermeable membrane that removes uremic toxins and excess fluid, normalizing the metabolic and electrolyte derangements. The filtered blood is then returned to the patient.
The average dialysis session is four hours long and is conducted three times per week, following recommendations from the 2002 Hemodialysis Study (HEMO).26 Most patients come to a free-standing dialysis center on a Monday/Wednesday/Friday or a Tuesday/Thursday/Saturday schedule.
In theory, there is no such thing as too much dialysis (since the kidneys work 24 hours per day, seven days per week); thus, researchers have recently examined lengthening dialysis in an attempt to extend survival.27-29 According to study results, patients who undergo longer dialysis times generally enjoy better nutrition with a more liberal diet, require fewer medications, have reduced incidence of increased left ventricular mass (a marker for coronary artery disease), and report better quality of life, all in addition to a survival benefit; however, the latter was not considered statistically significant in any of the studies.27-29 Additionally, this survival benefit may not extend to daily hemodialysis; in a recent publication, daily hemodialysis was associated with a 60% higher death rate.30
A number of dialysis units have begun to offer nocturnal dialysis. In this option, patients sleep at the unit for eight hours, three nights per week, for a total of 24 hours of dialysis (vs the typical 12 hours per week). Some patients receive dialysis at home, allowing them to dialyze for six weekly sessions of two to three hours. This strategy attempts to mimic a more “natural” state.
One of the primary challenges associated with hemodialysis is establishing and maintaining a vascular access. An arteriovenous (AV) fistula is the access of choice because its use reduces the likelihood of clotting, improves access survival, and increases clearances during dialysis.31 However, the AV fistula is most effective if it is placed a minimum of six months before use.32
For many patients, an AV fistula is created surgically when the eGFR falls to 15 mL/min/1.73 m2. Fistula placement in preparation for the initiation of hemodialysis is important to reduce the need for hemodialysis catheters, which are associated with higher risk for infection and poorer outcomes.33
Peritoneal Dialysis
Peritoneal dialysis (PD) filters out uremic toxins and normalizes the metabolic and electrolyte derangements by using the patient’s peritoneal membrane as a filter. Dialysate is instilled through a catheter into the peritoneal cavity where it is allowed to dwell, often for four to six hours (see Figure 334). Exchanges can be performed three to four times per day (allowing six to eight hours of dwell time per exchange for the dialysate), or by way of a cycler at night.35,36
Performing exchanges by way of a cycler requires the patient to be connected via the PD catheter for eight hours; thus the advantage of performing the exchange during sleep. The cycler has a soft, whooshing sound that many patients describe as “white noise” that does not disturb their sleep.
The principal advantage of peritoneal dialysis is extended survival of the patient.37-39 PD also allows the patient a more liberal diet. Although PD must be performed every day, exchanges can be adjusted to the patient’s timing preference. PD reduces cost and time, since the patient need not travel to a dialysis center. PD also preserves residual renal function,39 which is associated with a survival benefit and contributes to the patient’s overall health and well-being. The use of PD requires a committed, competent patient in a clean home environment.
This intervention may not be suitable for patients with a history of abdominal surgeries. However, as a therapy considered gentler than hemodialysis, PD is an excellent choice for the patient with congestive heart failure.40 The PD catheter is not placed until two to four weeks before it will be needed.41
Kidney Transplantation
Last, but certainly not least, is transplant. As entire books have been written on this modality, only the highlights will be addressed here.42 Survival rates following transplantation are reported to exceed those associated with any other treatment modality, even when controlling for comorbidities and patient selection bias.43 A patient can receive a kidney from either a living or a deceased donor. Apart from the perioperative risks associated with transplantation surgery, long-term surgical or medical problems are not common for the living kidney donor.44-46
Since 2002, as a result of a policy change from the United Network for Organ Sharing (UNOS),47 the donor pool has been expanded by including kidneys from what are commonly referred to as extended criteria donors: those who are older than 60, or who are 50 to 59 and have two of the following factors: cerebrovascular accident as the cause of death; preexisting hypertension; or an SCr level exceeding 1.5 mg/dL. These kidneys may be given to any recipients but are primarily used for those age 50 or older.47
Graft survival is statistically shorter in a cadaver kidney than in a living donor kidney.48 However, the larger problem is the long wait time for a cadaver transplant—seven to eight years (or possibly longer) for some centers and some blood types.49 A patient can be referred to a transplant center when the eGFR falls below 25 mL/min/1.73 m2; he or she will then be actively listed for transplant when eGFR reaches 20 mL/min/1.73 m2 or lower.
Early referral for transplant listing buys the patient time before dialysis becomes essential. Patients can receive preemptive transplants (ie, transplantation before dialysis is initiated); these are becoming increasingly popular because they generally extend survival for the recipient.50,51
UNOS (www.unos.org) has set few limits on transplant recipients: patients are required to undergo an extensive medical work-up, but there are no upper age limits (although some centers do set their own) and usually no limits on patients infected with HIV, hepatitis B, or hepatitis C (although there may be separate listing requirements). Patients who are not US citizens are not denied.
Because more than 100,000 patients are currently on the wait list, domino kidney transplants are now being offered at many centers.52-54 These organ exchanges involve altruistic donors who do not match their targeted kidney recipients. Since publication of the first article describing this procedure at Johns Hopkins Medical Center,53 there have been two-way, three-way, and up to 32-way domino transplants. However, patients wishing to engage in this type of trade require a donor; not every patient has access to an altruistic donor.
CKD PATIENT EDUCATION
When Congress passed the Medicare Improvements for Patients and Providers Act of 2008,55 a component little noted outside the nephrology community was the offer of kidney disease education (KDE) classes to Medicare-eligible patients with CKD 4.56-58 Medicare patients with an eGFR of 15 to 30 mL/min/1.73 m2 are eligible to attend KDE classes presented by a physician, an NP, a PA, or a clinical nurse specialist. Medicare will reimburse for six hours of education.
This groundbreaking educational benefit, the first such program paid for by Medicare, was championed by the National Kidney Foundation and other nephrology groups. Many nephrology practices now offer these classes to all their CKD patients, regardless of health insurance status. Instructors educate patients on the choices of renal replacement therapy and help patients select their best option.
KDE classes can be conducted in the office setting or at the patient’s home, and they can be billed on the same day as an evaluation and management (E/M) visit.56,57 This may help explain why, in 2010, gerontologists billed for more home KDE classes than did nephrologists.58
FUTURE TRENDS AND ONGOING TRIALS
The overarching goal of therapy for CKD patients is to diagnose accurately and treat effectively in order to slow or prevent progression to end-stage renal disease (ESRD). Following is a brief review of a selection of new diagnostic tools and therapeutic interventions that may impact the management of CKD in the coming years.
The QxMD Kidney Failure Risk Equation is a newly developed, well-validated predictive model that offers relatively accurate prediction of a patient’s likelihood of progression to ESRD, based on age, sex, eGFR, and levels of albuminuria, SCr, serum phosphorus, serum bicarbonate, and serum albumin59 (see table59). This tool can be accessed online at www.qxmd.com/calculate-online/nephrology/kidney-failure-risk-equation.
Increased awareness of the role that phosphorus plays in the development of vascular calcifications has led to an emphasis on earlier, more aggressive control of dietary phosphorus. Historically, dietary phosphorus control and phosphorus binders were recommended only when the patient’s serum phosphorus exceeded normal limits. Dietary phosphorus control may now be advised as early as CKD 3, based on the understanding that phosphorus is a key component in driving the development of vascular calcifications—which in turn contribute to the high incidence of cardiovascular disease in patients with CKD.60
Bardoxolone is a new medication developed for treatment of diabetic nephropathy.61,62 It works by inhibiting proinflammatory mediators and moderating the effects of oxidative stress, thus interrupting the cascade of inflammation and cellular injury that result in diabetic nephropathy.62 Early clinical trials examining this agent have shown promise in terms of raising eGFR and reducing serum creatinine, but tolerability and toxicity profiles have been an issue.61 Additionally, some reduction in serum creatinine may have been attributable to weight loss as opposed to true improvement of renal function.62
Data have recently been released regarding the use of the herb silymarin for treatment of patients with macroalbuminuria related to diabetic nephropathy. Results from a small (n = 60), randomized, nonblinded clinical trial by Fallahzadeh et al63 indicate that silymarin decreases proteinuria when used in combination with an ACE inhibitor or an angiotensin receptor blocker (ARB). Silymarin, an extract from milk thistle, has been used medicinally for centuries for its antioxidant, anti-inflammatory, and antiviral properties in those with liver “ailments.” In this clinical trial, silymarin was well tolerated and associated with a reduction in pro-inflammatory markers.63
AST-120 is another agent intended to slow progression of CKD. It promotes intestinal adsorption and fecal excretion of the uremic toxin indoxyl sulfate.64 A recently published study demonstrated an association between use of AST-120 and a delay in required initiation of hemodialysis, but no survival benefit.65 This was a nonrandomized trial, and previous research showed no effect of AST-120 on progression of CKD.66 However, patients close to requiring dialysis are likely to welcome a means to delay it.
A growing body of evidence suggests that reversal of CKD-associated acidemia by administering sodium bicarbonate may actually forestall progression of CKD.67 However, treatment with sodium bicarbonate can lead to complications of hypertension and fluid volume overload. In one study, patients at risk for these complications derived equivalent benefit by increasing dietary fruit and vegetable intake to reduce renal acid load.68 However, providers must be mindful of patients’ serum potassium levels, especially patients who are taking an ACE inhibitor or an ARB.
The Provider’s Current Role
Despite the hope offered by new and novel therapies, the mainstay of treatment for CKD continues to be aggressive management of diabetes, hypertension, and the cardiovascular risk profile. As a result of vigorous preventive strategies to address the cardiovascular risks inherent in this patient population, the patient with type 2 diabetes is now more likely to progress to ESRD than to die of a cardiovascular event.69
The well-informed clinician can be instrumental in providing evidence-based medical therapy and excellent patient education from the time of initial CKD diagnosis through CKD 5.
PATIENT OUTCOME
The case patient was started on injections of epoietin alfa for her anemia. She attended KDE classes led by an advanced practitioner in her nephrology group and decided to undergo peritoneal dialysis, since it would allow her to maintain her travel schedule. When last heard from, the patient was preparing for a trip to see the Great Barrier Reef in Australia.
CONCLUSION
The decision to begin renal replacement therapy—whether a form of dialysis, or another management option—depends not on SCr or eGFR alone. Rather, a number of uremic manifestations, comorbidities, lifestyle factors, and other variables must be carefully weighed before the patient, the family, and the clinicians involved can decide on the management plan most likely to enhance the patient’s quality of life and extend survival.
The Initiating Dialysis Early and Late (IDEAL) study in Australia and New Zealand1 examined the optimal time to initiate dialysis. This well-designed, randomized, controlled trial gave the nephrology community guidelines for treating patients as they progress through chronic kidney disease to stage 5 (CKD 5). The IDEAL investigators demonstrated that planned early initiation of dialysis did not enhance survival—and in some cases, it hastened death.1
Although most patients have a nephrology provider by the time they reach CKD 5 (ie, kidney failure), primary care providers can be instrumental in preparing the patient for what lies ahead as kidney failure progresses. Presented here is an overview of diagnosis and management of the patient with CKD 5 in the 21st century.
CASE PRESENTATION
A 70-year-old woman with an extensive history of diabetes and hypertension presents to her primary care clinician complaining of a lack of energy. She has just returned from a trip to Disney World, where she says she was unable to keep up with her grandchildren. She sat in the shade while they enjoyed Space Mountain and other attractions, and because of uncustomary fatigue, she required a nap every afternoon.
Physical examination shows an elderly female in no acute distress. Cardiac exam shows a regular heart rate and rhythm, the patient’s lungs are clear, and she has 1+ pitting leg edema bilaterally. The patient’s blood pressure is slightly elevated at 142/92 mm Hg, and no protein is detected on spot urine testing.
Blood work is ordered, including a complete blood count, a comprehensive metabolic panel, and an A1C. Results include a hemoglobin level of 8.7 g/dL (reference range for women, 12.0 to 16.0 g/dL), a serum creatinine level (SCr) of 3 mg/dL (range, 0.6 to 1.2 mg/dL; estimated glomerular filtration rate [eGFR], 15 mL/min/1.73 m2), and an A1C value of 7.5% (normal, & < 7%).
The patient is told that she is in kidney failure and is referred immediately to a nephrology practice, where she is seen the following day. After her appointment, she returns to the primary care office. When she is asked when she will be starting dialysis, she seems surprised, saying, “They told me I was doing well. I need some shots for my blood, but they didn’t seem worried at all.”
Is this patient being managed correctly by the nephrology practitioner?
EPIDEMIOLOGY
Presently, more than 1 million persons in the United States have CKD 5, and 500,000 are undergoing dialysis. The number of patients with CDK 5 who are on dialysis has doubled since 1994 and is projected to reach 774,000 by 20202,3 (see Figure 13 and Figure 23).
CKD 5 is defined as an eGFR below 15 mL/min/1.73 m2, according to the Modification of Diet in Renal Disease (MDRD) study group formula; or as a creatinine clearance of less than 15 mL/min, using the Cockcroft-Gault formula.4-6 Both formulas have limitations, since they are not fully accurate at extremes of age, with variations in weight, for some racial mixes, or for the very malnourished patient.7,8 However, they do correct for normal age-related loss of kidney function, gender, and SCr, and they are currently the generally accepted formulas.4
A rise in SCr is exponential; for each doubling of the SCr, a reduction in kidney function of approximately 50% occurs. This means that a rise in SCr from 4 to 8 mg/dL is equivalent (in the proportion of loss of kidney function) to a rise of SCr from 0.5 to 1 mg/dL. Commonly, patients are not referred to nephrology until the SCr doubles to 4 or 6 mg/dL—whereas the rise in SCr from 0.5 to 1 mg/dL should be of greatest concern to the primary care provider, prompting a referral.9 Early recognition of reduced renal function allows for identification of potentially reversible etiologies and the slowing of CKD progression.
INDICATIONS FOR RENAL REPLACEMENT THERAPY
Not all patients with an eGFR below 15 mL/min/1.73 m2 are started on dialysis immediately. The decision to initiate dialysis is guided by assessment of a constellation of uremic manifestations—not eGFR alone. Newer data suggest that early initiation of dialysis may be associated with increased mortality.10-15
The IDEAL study,1 a well-designed, randomized, controlled trial of all patients who started dialysis in Australia and New Zealand over a multiyear period, was designed to determine the optimal time to initiate dialysis. Patients were randomized to start dialysis (hemodialysis or peritoneal dialysis) at an eGFR between 10 and 14 mL/min/1.73 m2 or between 5 and 7 mL/min/1.73 m2. While there was some overlap and some patients were started on dialysis outside their randomized eGFR (eg, earlier than the allotted time because symptoms developed), what the IDEAL investigators found surprised the entire nephrology community: Early dialysis starts did not enhance survival and, in some cases, it hastened death.1
The indications for initiation of dialysis often develop long after the patient has progressed within CKD 5, commonly with an eGFR of 10 mL/min/1.73 m2 or less. Medicare has acknowledged this by reimbursing dialysis only in eligible patients whose eGFR is below 10 mL/min/1.73 m2.16 There are also acute indications for initiation of dialysis, such as uremic pericarditis, hyperkalemia, bleeding related to uremic platelet dysfunction, and metabolic encephalopathy related to uremia (and reimbursement for dialysis can be justified), but these are uncommon.
RENAL REPLACEMENT THERAPY CHOICES
The choices of treatment for kidney failure (note: treatment, not cure) are medical management, hemodialysis, peritoneal dialysis, and transplant. Each choice has its advantages and disadvantages, and all patients should receive clear explanations of what they can expect from each modality.
While younger, higher-functioning individuals are likely to benefit from dialysis, patients with extensive comorbid illnesses and/or low functional status tend to respond poorly17; medical management may be the best choice for these patients. In one recent study, the functional status of residents in skilled nursing homes was examined, before and after initiation of dialysis. At one year, 58% of the nursing home residents who underwent dialysis had died, and only 13% had maintained their pre-dialysis functional status.18
Another research team recently compared conservative management of CKD 5 (ie, medical therapy without dialysis) with dialysis in elderly patients. For patients 75 and older with extensive comorbid illness, the researchers found no statistically significant survival benefit to dialysis.19
Dialysis, whether administered as hemodialysis or peritoneal dialysis, is a rigorous, intensive medical therapy. Dialysis does not necessarily prolong life in patients with extensive comorbidities, and it does not always enhance quality of life.18,20-23 The decision to undergo dialysis is personal and individual, and both the patient and family should be actively involved in making it. Primary care providers should be the nephrologist’s ally in the discussion of therapy for renal failure; often, they have cared for the patient for years, and they understand the patient and family dynamics.
An important message the nephrology practitioner must communicate is that choosing medical management without dialysis is not withholding care; sometimes it is a more humane choice.24 Dying of kidney failure can be a peaceful and comfortable death: As the uremic toxins build up (with eGFR ≤ 2 mL/min/1.73 m2, although this can take many years), the patient becomes confused and slowly slips into a sleepiness that leads to death.25 Hospice is usually involved to support the patient and family.
Hemodialysis
Hemodialysis is the most common, best-known treatment modality for CKD in the US, with about 94% of patients choosing it.3 In this process, blood is removed from the body (approximately 500 cc at a time) and filtered through a semipermeable membrane that removes uremic toxins and excess fluid, normalizing the metabolic and electrolyte derangements. The filtered blood is then returned to the patient.
The average dialysis session is four hours long and is conducted three times per week, following recommendations from the 2002 Hemodialysis Study (HEMO).26 Most patients come to a free-standing dialysis center on a Monday/Wednesday/Friday or a Tuesday/Thursday/Saturday schedule.
In theory, there is no such thing as too much dialysis (since the kidneys work 24 hours per day, seven days per week); thus, researchers have recently examined lengthening dialysis in an attempt to extend survival.27-29 According to study results, patients who undergo longer dialysis times generally enjoy better nutrition with a more liberal diet, require fewer medications, have reduced incidence of increased left ventricular mass (a marker for coronary artery disease), and report better quality of life, all in addition to a survival benefit; however, the latter was not considered statistically significant in any of the studies.27-29 Additionally, this survival benefit may not extend to daily hemodialysis; in a recent publication, daily hemodialysis was associated with a 60% higher death rate.30
A number of dialysis units have begun to offer nocturnal dialysis. In this option, patients sleep at the unit for eight hours, three nights per week, for a total of 24 hours of dialysis (vs the typical 12 hours per week). Some patients receive dialysis at home, allowing them to dialyze for six weekly sessions of two to three hours. This strategy attempts to mimic a more “natural” state.
One of the primary challenges associated with hemodialysis is establishing and maintaining a vascular access. An arteriovenous (AV) fistula is the access of choice because its use reduces the likelihood of clotting, improves access survival, and increases clearances during dialysis.31 However, the AV fistula is most effective if it is placed a minimum of six months before use.32
For many patients, an AV fistula is created surgically when the eGFR falls to 15 mL/min/1.73 m2. Fistula placement in preparation for the initiation of hemodialysis is important to reduce the need for hemodialysis catheters, which are associated with higher risk for infection and poorer outcomes.33
Peritoneal Dialysis
Peritoneal dialysis (PD) filters out uremic toxins and normalizes the metabolic and electrolyte derangements by using the patient’s peritoneal membrane as a filter. Dialysate is instilled through a catheter into the peritoneal cavity where it is allowed to dwell, often for four to six hours (see Figure 334). Exchanges can be performed three to four times per day (allowing six to eight hours of dwell time per exchange for the dialysate), or by way of a cycler at night.35,36
Performing exchanges by way of a cycler requires the patient to be connected via the PD catheter for eight hours; thus the advantage of performing the exchange during sleep. The cycler has a soft, whooshing sound that many patients describe as “white noise” that does not disturb their sleep.
The principal advantage of peritoneal dialysis is extended survival of the patient.37-39 PD also allows the patient a more liberal diet. Although PD must be performed every day, exchanges can be adjusted to the patient’s timing preference. PD reduces cost and time, since the patient need not travel to a dialysis center. PD also preserves residual renal function,39 which is associated with a survival benefit and contributes to the patient’s overall health and well-being. The use of PD requires a committed, competent patient in a clean home environment.
This intervention may not be suitable for patients with a history of abdominal surgeries. However, as a therapy considered gentler than hemodialysis, PD is an excellent choice for the patient with congestive heart failure.40 The PD catheter is not placed until two to four weeks before it will be needed.41
Kidney Transplantation
Last, but certainly not least, is transplant. As entire books have been written on this modality, only the highlights will be addressed here.42 Survival rates following transplantation are reported to exceed those associated with any other treatment modality, even when controlling for comorbidities and patient selection bias.43 A patient can receive a kidney from either a living or a deceased donor. Apart from the perioperative risks associated with transplantation surgery, long-term surgical or medical problems are not common for the living kidney donor.44-46
Since 2002, as a result of a policy change from the United Network for Organ Sharing (UNOS),47 the donor pool has been expanded by including kidneys from what are commonly referred to as extended criteria donors: those who are older than 60, or who are 50 to 59 and have two of the following factors: cerebrovascular accident as the cause of death; preexisting hypertension; or an SCr level exceeding 1.5 mg/dL. These kidneys may be given to any recipients but are primarily used for those age 50 or older.47
Graft survival is statistically shorter in a cadaver kidney than in a living donor kidney.48 However, the larger problem is the long wait time for a cadaver transplant—seven to eight years (or possibly longer) for some centers and some blood types.49 A patient can be referred to a transplant center when the eGFR falls below 25 mL/min/1.73 m2; he or she will then be actively listed for transplant when eGFR reaches 20 mL/min/1.73 m2 or lower.
Early referral for transplant listing buys the patient time before dialysis becomes essential. Patients can receive preemptive transplants (ie, transplantation before dialysis is initiated); these are becoming increasingly popular because they generally extend survival for the recipient.50,51
UNOS (www.unos.org) has set few limits on transplant recipients: patients are required to undergo an extensive medical work-up, but there are no upper age limits (although some centers do set their own) and usually no limits on patients infected with HIV, hepatitis B, or hepatitis C (although there may be separate listing requirements). Patients who are not US citizens are not denied.
Because more than 100,000 patients are currently on the wait list, domino kidney transplants are now being offered at many centers.52-54 These organ exchanges involve altruistic donors who do not match their targeted kidney recipients. Since publication of the first article describing this procedure at Johns Hopkins Medical Center,53 there have been two-way, three-way, and up to 32-way domino transplants. However, patients wishing to engage in this type of trade require a donor; not every patient has access to an altruistic donor.
CKD PATIENT EDUCATION
When Congress passed the Medicare Improvements for Patients and Providers Act of 2008,55 a component little noted outside the nephrology community was the offer of kidney disease education (KDE) classes to Medicare-eligible patients with CKD 4.56-58 Medicare patients with an eGFR of 15 to 30 mL/min/1.73 m2 are eligible to attend KDE classes presented by a physician, an NP, a PA, or a clinical nurse specialist. Medicare will reimburse for six hours of education.
This groundbreaking educational benefit, the first such program paid for by Medicare, was championed by the National Kidney Foundation and other nephrology groups. Many nephrology practices now offer these classes to all their CKD patients, regardless of health insurance status. Instructors educate patients on the choices of renal replacement therapy and help patients select their best option.
KDE classes can be conducted in the office setting or at the patient’s home, and they can be billed on the same day as an evaluation and management (E/M) visit.56,57 This may help explain why, in 2010, gerontologists billed for more home KDE classes than did nephrologists.58
FUTURE TRENDS AND ONGOING TRIALS
The overarching goal of therapy for CKD patients is to diagnose accurately and treat effectively in order to slow or prevent progression to end-stage renal disease (ESRD). Following is a brief review of a selection of new diagnostic tools and therapeutic interventions that may impact the management of CKD in the coming years.
The QxMD Kidney Failure Risk Equation is a newly developed, well-validated predictive model that offers relatively accurate prediction of a patient’s likelihood of progression to ESRD, based on age, sex, eGFR, and levels of albuminuria, SCr, serum phosphorus, serum bicarbonate, and serum albumin59 (see table59). This tool can be accessed online at www.qxmd.com/calculate-online/nephrology/kidney-failure-risk-equation.
Increased awareness of the role that phosphorus plays in the development of vascular calcifications has led to an emphasis on earlier, more aggressive control of dietary phosphorus. Historically, dietary phosphorus control and phosphorus binders were recommended only when the patient’s serum phosphorus exceeded normal limits. Dietary phosphorus control may now be advised as early as CKD 3, based on the understanding that phosphorus is a key component in driving the development of vascular calcifications—which in turn contribute to the high incidence of cardiovascular disease in patients with CKD.60
Bardoxolone is a new medication developed for treatment of diabetic nephropathy.61,62 It works by inhibiting proinflammatory mediators and moderating the effects of oxidative stress, thus interrupting the cascade of inflammation and cellular injury that result in diabetic nephropathy.62 Early clinical trials examining this agent have shown promise in terms of raising eGFR and reducing serum creatinine, but tolerability and toxicity profiles have been an issue.61 Additionally, some reduction in serum creatinine may have been attributable to weight loss as opposed to true improvement of renal function.62
Data have recently been released regarding the use of the herb silymarin for treatment of patients with macroalbuminuria related to diabetic nephropathy. Results from a small (n = 60), randomized, nonblinded clinical trial by Fallahzadeh et al63 indicate that silymarin decreases proteinuria when used in combination with an ACE inhibitor or an angiotensin receptor blocker (ARB). Silymarin, an extract from milk thistle, has been used medicinally for centuries for its antioxidant, anti-inflammatory, and antiviral properties in those with liver “ailments.” In this clinical trial, silymarin was well tolerated and associated with a reduction in pro-inflammatory markers.63
AST-120 is another agent intended to slow progression of CKD. It promotes intestinal adsorption and fecal excretion of the uremic toxin indoxyl sulfate.64 A recently published study demonstrated an association between use of AST-120 and a delay in required initiation of hemodialysis, but no survival benefit.65 This was a nonrandomized trial, and previous research showed no effect of AST-120 on progression of CKD.66 However, patients close to requiring dialysis are likely to welcome a means to delay it.
A growing body of evidence suggests that reversal of CKD-associated acidemia by administering sodium bicarbonate may actually forestall progression of CKD.67 However, treatment with sodium bicarbonate can lead to complications of hypertension and fluid volume overload. In one study, patients at risk for these complications derived equivalent benefit by increasing dietary fruit and vegetable intake to reduce renal acid load.68 However, providers must be mindful of patients’ serum potassium levels, especially patients who are taking an ACE inhibitor or an ARB.
The Provider’s Current Role
Despite the hope offered by new and novel therapies, the mainstay of treatment for CKD continues to be aggressive management of diabetes, hypertension, and the cardiovascular risk profile. As a result of vigorous preventive strategies to address the cardiovascular risks inherent in this patient population, the patient with type 2 diabetes is now more likely to progress to ESRD than to die of a cardiovascular event.69
The well-informed clinician can be instrumental in providing evidence-based medical therapy and excellent patient education from the time of initial CKD diagnosis through CKD 5.
PATIENT OUTCOME
The case patient was started on injections of epoietin alfa for her anemia. She attended KDE classes led by an advanced practitioner in her nephrology group and decided to undergo peritoneal dialysis, since it would allow her to maintain her travel schedule. When last heard from, the patient was preparing for a trip to see the Great Barrier Reef in Australia.
CONCLUSION
The decision to begin renal replacement therapy—whether a form of dialysis, or another management option—depends not on SCr or eGFR alone. Rather, a number of uremic manifestations, comorbidities, lifestyle factors, and other variables must be carefully weighed before the patient, the family, and the clinicians involved can decide on the management plan most likely to enhance the patient’s quality of life and extend survival.
1. Cooper BA, Branley P, Bulfone L, et al; IDEAL Study. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010; 363(7):609-619.
2. Olan G. Policy Update: ASN to CDC: data collection of creatinine levels will advance research. ASN Kidney News. 2012;4(6):16. www.asn-online.org/publications/kidneynews/archives/2012/KN_jun2012.pdf. Accessed August 27, 2012.
3. US Renal Data System, National Institute of Diabetes and Digestive and Kidney Diseases, NIH. 2011 Atlas of CKD & ESRD. Vol 2. Chap 1: Incidence, prevalence, patient characteristics, and modalities. www.usrds.org/2011/pdf/v2_ch01_11.pdf. Accessed September 26, 2012.
4. National Kidney Foundation. Kidney Disease Outcomes Quality Initiative (KDOQI). Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. ?Part 4. Definition and classification of the stages of chronic kidney disease. 2002:43-80. www.kidney.org/professionals/kdoqi/pdf/ckd_evalua tion_classification_stratification.pdf. Accessed August 27, 2012.
5. Levey AS, Bosch JP, Lewis JB, et al; Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Internal Med. 1999;130 (6):461-470.
6. Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the Modification of Diet in Renal Disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16(3):763-773.
7. Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16(5):1413-1419.
8. Zuo L, Ma YC, Zhou YH, et al. Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis. 2005;45(3):463-472.
9. Stevens LA, Stoycheff N, Levey AS. Staging and management of chronic kidney disease. In: Greenberg A, ed. Primer of Kidney Diseases: Expert Consult. 5th ed. Philadelphia, PA: WB Saunders; 2009:436-445.
10. Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ. 2011;183(1):47-53.
11. Beddhu S, Samore MH, Roberts MS, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003;14(9):2305-2312.
12. Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46(5):887-896.
13. Lassalle M, Labeeuw M, Frimat L, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77(8):700-707.
14. Stel VS, Dekker FW, Ansell D, et al. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009;24 (10):3175-3182.
15. Traynor JP, Simpson K, Geddes CC, et al. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002;13(8):2125-2132.
16. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. CMS Form 2728. End-Stage Renal Disease Medical Evidence Report: Medicare Entitlement and/or Patient Registration. www.usrds.org/2008/rg/forms/02_2728_1965.pdf. Accessed September 25, 2012.
17. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis: Clinical Practice Guideline. 2nd ed. Rockville, MD: Renal Physicians Association; Oct 2010:1-12.
18. Kurella Tamura M, Covinsky KE, Chertow GM, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547.
19. Chandna SM, Da Silva-Gane M, Marshall C, et al. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608-1614.
20. Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95:c40-c46.
21. Lamping DL, Constantinovici N, Roderick P, et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: a prospective cohort study. Lancet. 2000;356:1543-1550.
22. O’Connor NR, Kumar P. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med. 2012; 15(2):228-235.
23. Jassal SV, Trpeski L, Zhu N, et al. Changes in survival among elderly patients initiating dialysis from 1990 to 1999. CMAJ. 2007;177(9):?1033-1038.
24. Schmidt RJ. Informing our elders about dialysis: is an age-attuned approach warranted? Clin J Am Soc Nephrol. 2012;7(1):185-191.
25. Holley JL. Providing optimal care before and after discontinuation. Semin Dial. 2012;25(1):?33-34.
26. Eknoyan G, Beck GJ, Cheung AK, et al; Hemodialysis (HEMO) Study Group. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):?2010-2019.
27. Lacson E, Lazarus M. Dialysis time: does it matter? A reappraisal of existing literature. Curr Opin Nephrol Hypertens. 2011;20(2):189-194.
28. Chertow GM, Levin NW, Beck GJ, et al; FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):22878-2300.
29. Lacson E Jr, Xu J, Suri RS, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol. 2012;23(4):687-695.
30. Suri RS, Lindsay RM, Bieber BA, et al. A multinational cohort study of in-center daily hemodialysis and patient survival. Kidney Int. 2012 Sep 12. [Epub ahead of print]
31. Sidawy AN, Spergel LM, Besarab A, et al; Society for Vascular Surgery. Clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. ?J Vasc Surg. 2008;48(5 Suppl):2S-25S.
32. Heaf JG. Algorithm for optimal dialysis access timing. Clin Nephrol. 2007;67(2):96-104.
33. Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int. 2001;60(1):1-13.
34. National Kidney Foundation. Peritoneal dialysis: what you need to know (2006;11-50-0215). www.kidney.org/atoz/pdf/PeritonealDialysis.pdf. Accessed September 24, 2012.
35. Davison SN, Ghangri GS, Jindal K, Pannu N. Comparison of volume overload with cycler-assisted versus continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol. 2009;4(6):1044-1050.
36. Juergensen P, Eras J, McClure B, et al. The impact of various cycling regimens on phosphorus removal in chronic peritoneal dialysis patients. Int J Artif Organs. 2005;28(12):1219-1223.
37. Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl. 2006 Nov;(103):S3-S11.
38. Heaf J, Løkkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant. 2002;17(1):112-117.
39. Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69(10):1726-1732.
40. Cnossen N, Kooman JP, Konings CJ, et al. Peritoneal dialysis in patients with congestive heart failure. Nephrol Dial Transplant. 2006;21 suppl 2: ii63-ii66.
1. Cooper BA, Branley P, Bulfone L, et al; IDEAL Study. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010; 363(7):609-619.
2. Olan G. Policy Update: ASN to CDC: data collection of creatinine levels will advance research. ASN Kidney News. 2012;4(6):16. www.asn-online.org/publications/kidneynews/archives/2012/KN_jun2012.pdf. Accessed August 27, 2012.
3. US Renal Data System, National Institute of Diabetes and Digestive and Kidney Diseases, NIH. 2011 Atlas of CKD & ESRD. Vol 2. Chap 1: Incidence, prevalence, patient characteristics, and modalities. www.usrds.org/2011/pdf/v2_ch01_11.pdf. Accessed September 26, 2012.
4. National Kidney Foundation. Kidney Disease Outcomes Quality Initiative (KDOQI). Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. ?Part 4. Definition and classification of the stages of chronic kidney disease. 2002:43-80. www.kidney.org/professionals/kdoqi/pdf/ckd_evalua tion_classification_stratification.pdf. Accessed August 27, 2012.
5. Levey AS, Bosch JP, Lewis JB, et al; Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Internal Med. 1999;130 (6):461-470.
6. Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the Modification of Diet in Renal Disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16(3):763-773.
7. Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16(5):1413-1419.
8. Zuo L, Ma YC, Zhou YH, et al. Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis. 2005;45(3):463-472.
9. Stevens LA, Stoycheff N, Levey AS. Staging and management of chronic kidney disease. In: Greenberg A, ed. Primer of Kidney Diseases: Expert Consult. 5th ed. Philadelphia, PA: WB Saunders; 2009:436-445.
10. Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ. 2011;183(1):47-53.
11. Beddhu S, Samore MH, Roberts MS, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003;14(9):2305-2312.
12. Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46(5):887-896.
13. Lassalle M, Labeeuw M, Frimat L, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77(8):700-707.
14. Stel VS, Dekker FW, Ansell D, et al. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009;24 (10):3175-3182.
15. Traynor JP, Simpson K, Geddes CC, et al. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002;13(8):2125-2132.
16. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. CMS Form 2728. End-Stage Renal Disease Medical Evidence Report: Medicare Entitlement and/or Patient Registration. www.usrds.org/2008/rg/forms/02_2728_1965.pdf. Accessed September 25, 2012.
17. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis: Clinical Practice Guideline. 2nd ed. Rockville, MD: Renal Physicians Association; Oct 2010:1-12.
18. Kurella Tamura M, Covinsky KE, Chertow GM, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547.
19. Chandna SM, Da Silva-Gane M, Marshall C, et al. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608-1614.
20. Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95:c40-c46.
21. Lamping DL, Constantinovici N, Roderick P, et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: a prospective cohort study. Lancet. 2000;356:1543-1550.
22. O’Connor NR, Kumar P. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med. 2012; 15(2):228-235.
23. Jassal SV, Trpeski L, Zhu N, et al. Changes in survival among elderly patients initiating dialysis from 1990 to 1999. CMAJ. 2007;177(9):?1033-1038.
24. Schmidt RJ. Informing our elders about dialysis: is an age-attuned approach warranted? Clin J Am Soc Nephrol. 2012;7(1):185-191.
25. Holley JL. Providing optimal care before and after discontinuation. Semin Dial. 2012;25(1):?33-34.
26. Eknoyan G, Beck GJ, Cheung AK, et al; Hemodialysis (HEMO) Study Group. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):?2010-2019.
27. Lacson E, Lazarus M. Dialysis time: does it matter? A reappraisal of existing literature. Curr Opin Nephrol Hypertens. 2011;20(2):189-194.
28. Chertow GM, Levin NW, Beck GJ, et al; FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):22878-2300.
29. Lacson E Jr, Xu J, Suri RS, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol. 2012;23(4):687-695.
30. Suri RS, Lindsay RM, Bieber BA, et al. A multinational cohort study of in-center daily hemodialysis and patient survival. Kidney Int. 2012 Sep 12. [Epub ahead of print]
31. Sidawy AN, Spergel LM, Besarab A, et al; Society for Vascular Surgery. Clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. ?J Vasc Surg. 2008;48(5 Suppl):2S-25S.
32. Heaf JG. Algorithm for optimal dialysis access timing. Clin Nephrol. 2007;67(2):96-104.
33. Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int. 2001;60(1):1-13.
34. National Kidney Foundation. Peritoneal dialysis: what you need to know (2006;11-50-0215). www.kidney.org/atoz/pdf/PeritonealDialysis.pdf. Accessed September 24, 2012.
35. Davison SN, Ghangri GS, Jindal K, Pannu N. Comparison of volume overload with cycler-assisted versus continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol. 2009;4(6):1044-1050.
36. Juergensen P, Eras J, McClure B, et al. The impact of various cycling regimens on phosphorus removal in chronic peritoneal dialysis patients. Int J Artif Organs. 2005;28(12):1219-1223.
37. Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl. 2006 Nov;(103):S3-S11.
38. Heaf J, Løkkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant. 2002;17(1):112-117.
39. Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69(10):1726-1732.
40. Cnossen N, Kooman JP, Konings CJ, et al. Peritoneal dialysis in patients with congestive heart failure. Nephrol Dial Transplant. 2006;21 suppl 2: ii63-ii66.