User login
Things We Do for No Reason™: Ova and Parasite Testing in Patients With Acute Diarrhea Arising During Hospitalization
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 54-year-old immunocompetent man admitted to the hospital for non–ST-segment elevation myocardial infarction develops profuse watery diarrhea after his third day of admission. He denies prior episodes of diarrhea. He does not have any fevers, blood in the stool, recent travel, or antibiotic use. Vital signs include a blood pressure of 128/82 mm Hg, heart rate of 120 beats per minute, respiratory rate of 16 breaths per min, oxygen saturation of 100% on room air, and temperature of 36.9 °C. His physical examination is normal, without signs of abdominal tenderness, rebound, or guarding. Complete blood count is normal, without eosinophilia. The comprehensive metabolic panel shows mild hypokalemia of 3.3 mmol/L. The hospitalist resuscitates him with normal saline, provides oral potassium repletion, and orders a stool culture, Clostridioides difficile test, and an ova and parasite (O&P) test. Loperamide and time resolve his symptoms in 2 days. Results of his stool culture, C difficile, and O&P tests return negative in 3 days.
BACKGROUND
Acute diarrhea is a common complaint in both inpatient and outpatient settings. It is defined as the passage of three or more liquid or poorly formed stools in a 24-hour period lasting less than 14 days. Persistent diarrhea lasts from 14 to 29 days, while chronic diarrhea lasts longer than 30 days. There are 47.8 million cases of acute diarrhea per year in the United States, costing $150 million in US health expenditures.1 Viral pathogens remain the most common cause of acute diarrhea in the United States.1,2 Standard O&P testing consists of applying a stool sample to a slide with either saline or iodine (wet mount) and evaluating the specimen with a microscope.
WHY YOU MIGHT THINK O&P TESTING IS HELPFUL
Giardia and Cryptosporidium remain the most commonly implicated parasitic pathogens in acute diarrheal episodes in the United States.3 Cryptosporidium cases in the United States range from 2.2 to 3.9 per 100,000 persons,4 and Giardia cases in the United States range from 5.8 to 6.4 per 100,000 persons.5 To avoid missing potentially treatable causes, providers often order O&P tests reflexively as part of a standard workup for acute diarrhea. From 2001 to 2007, Associated Regional and University Pathologists Laboratories experienced a 379% increase in O&P testing.6 Many providers ordering these tests assume that standard O&P testing covers most, if not all, parasites and that a negative test will rule out a parasitic cause of disease. Furthermore, providers are unaware that more sensitive tests to detect certain parasites have replaced standard O&P microscopy.3
WHY O&P TESTING IS USUALLY UNNECESSARY
Most hospitalized patients do not have a parasitic infection
In a review of 5,681 completed O&P tests from a tertiary care medical center in Canada over a 5-year period, only 1.4% of tests were positive.7 In a 3-year retrospective analysis of stool samples obtained after 3 days of hospitalization, positive results were found in only 1 of 191 stool cultures and in 0 of 90 O&P samples.8 Current practice guidelines suggest not testing patients with stool studies in cases of acute diarrhea lasting less than 7 days in the absence of significant risk factors for parasitic disease because it has been shown to be a rare event and most cases will self-resolve with supportive care only.1,9
The stool O&P test has low sensitivity
Classically ordered stool O&P tests have low sensitivity for the detection of Giardia and Cryptosporidium, the two most common parasites in the United States.6,10,11 O&P studies detect Giardia in only 66% to 79% of specimen samples and Cryptosporidium in less than 5% of specimens. Diagnostic yields can be improved with the use of special stains such as modified acid-fast stain (MAF).6 Despite use of MAF staining, though, sensitivity for Cryptosporidium detection has remained at only 55%.12 Additionally, several studies have shown that physicians are generally unaware of the test characteristics of stool O&P tests and they do not know to order the newer more sensitive enzyme immunoassays (EIA) or direct fluorescent antibody (DFA) tests even in situations when testing for a parasitic infection is appropriate.10,11,13,14 As stated earlier, a parasitic infection without significant risk factors is a rare event. A negative test with low or moderate sensitivity is not additive to such a low clinical suspicion because it does not significantly change posttest probability.
Testing can have adverse consequences
In addition to the low yield, O&P testing is technically complex, is time intensive, and requires an experienced technician’s interpretation. Inappropriate testing increases the cost of care and staff workload without much benefit.6 As such, some institutions have opted to send the O&P tests to labs with experienced technicians. Other institutions have adopted a restrictive stool O&P testing approach that reduces healthcare time and costs and improves the rate of positive tests.13,15 A study at a single tertiary care medical center demonstrated an estimated cost savings of $21,931 annually by implementing a computer-based algorithm to restrict testing for stool cultures and O&P tests to patients with higher probabilities of infection.15 The algorithm directed clinicians to provide further information when attempting to order stool culture, O&P, or other specific stool tests. For patients hospitalized for more than 3 days, the system did not allow certain testing. For patients with worrisome features like severe symptoms or an immunocompromised state, the algorithm directed the clinician to place an infectious disease or gastroenterology consult rather than order stool tests. Decreased laboratory costs of all stool studies (including O&P) in adult inpatient locations led to the cost savings. Additionally, the study authors felt that they likely underestimated the cost savings because they did not account for other expenses in the analysis, such as nursing workload and supplies.15
WHAT YOU SHOULD DO INSTEAD
Clinicians should evaluate patients on a case-by-case basis and determine the need to test based on the presence of high-risk features (Table). 
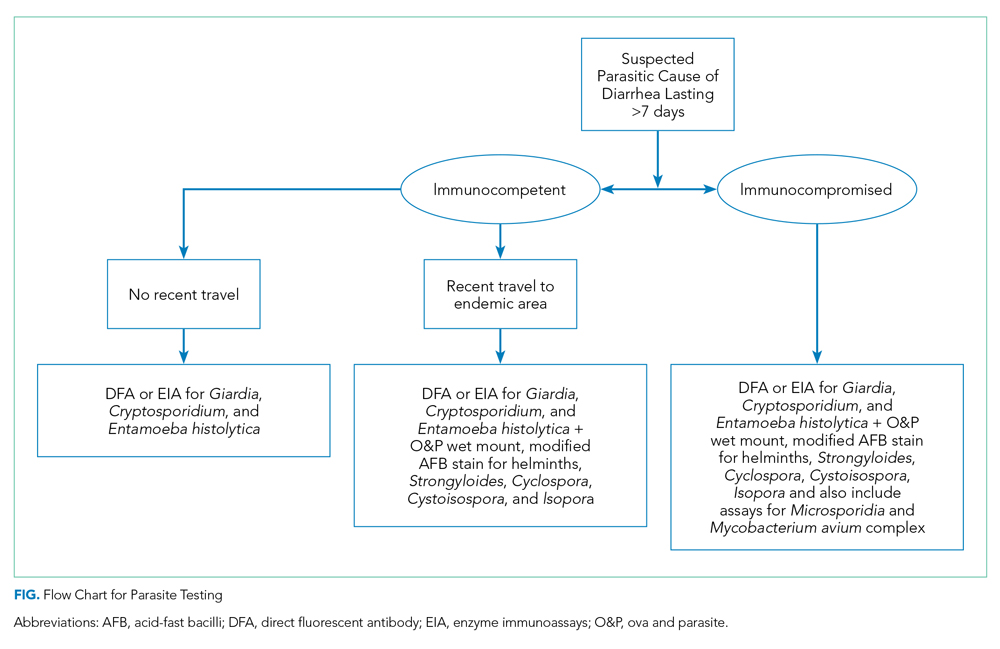
When performing parasitic testing in patients without a recent travel history but with other high-risk features, test for the most prevalent parasites in the United States (ie, Giardia, Cryptosporidium, and Entamoeba histolytica) with DFA or EIA tests.3 DFA testing for Giardia is 99% sensitive.12 In patients with symptoms lasting more than 7 days and recent travel, in addition to the above DFA/EIA tests, perform O&P testing with wet mount, modified acid-fast bacilli stain to detect rare parasites such as helminths, Strongyloides, Cyclospora, and Cystoisospora.3 In patients who live or travel to endemic areas (about 10% of traveler’s diarrhea is caused by parasitic infections), have unexplained eosinophilia, or are part of a community outbreak (eg, childcare institutions or drinking water/food outbreaks), test for Giardia, Cryptosporidium, Cyclospora, Cystoisospora, Entamoeba histolytica, and Isospora belli.9 In addition, among patients with AIDS or immunosuppression, testing should include assays for Microsporidia, Strongyloides, and Mycobacterium avium complex (Figure).9,16 Newer tests, such as the multiplex real-time polymerase chain reaction assay, can also simultaneously detect Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum. For more information on parasitic testing, we suggest reading the review article “Beyond O&P times three.”3 It is important to familiarize yourself with the parasitic tests available at your respective clinics/hospital so the optimal test can be used.
RECOMMENDATIONS
- Prescribe a trial of “wait and see” for patients without high-risk features for parasitic disease.
- Test patients with high-risk features for parasitic disease by utilizing targeted testing.
- For patients with high-risk features but no travel history, first perform DFA, EIA, or multiplex real-time polymerase chain reaction testing to evaluate for Giardia, Cryptosporidium, and Entamoeba histolytica.
- If DFA/EIA testing is negative, obtain O&P tests with and without stains, such as acid-fast bacilli, for detection of other rare parasites.
CONCLUSION
Hospitalists should risk-stratify patients to determine when O&P testing is appropriate. Employ more targeted testing, especially use of DFA/EIA tests when evaluating for parasites. Avoid parasitic testing if symptoms have lasted less than 7 days and the patient has no other high-risk features. Become familiar with the tests available at your institution and their sensitivities. As in our clinical scenario, most acute cases of diarrhea resolve without intervention and should be managed and treated conservatively.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing [email protected] .
Acknowledgments
The authors thank Dr Lenny Feldman for his assistance with editing the manuscript.
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. https://doi.org/10.1038/ajg.2016.126
2. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. https://doi.org/10.1056/nejmra1301069
3. Mohapatra S, Singh DP, Alcid D, Pitchumoni CS. Beyond O&P times three. Am J Gastroenterol. 2018;113(6):805-818. https://doi.org/10.1038/s41395-018-0083-y
4. Painter JE, Hlavsa MC, Collier SA, Xiao L, Yoder JS. Cryptosporidiosis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):1-14.
5. Painter JE, Gargano JW, Collier SA, Yoder JS. Giardiasis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):15-25.
6. Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011;49(2):591-596. https://doi.org/10.1128/jcm.01806-10
7. Mosli M, Gregor J, Chande N, Lannigan R. Nonutility of routine testing of stool for ova and parasites in a tertiary care Canadian centre. Can J Microbiol. 2012;58(5):653-659. https://doi.org/10.1139/w2012-039
8. Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979-982.
9. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. https://doi.org/10.1093/cid/cix669
10. Hennessy TW, Marcus R, Deneen V, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38 (Suppl 3):S203-S211. https://doi.org/10.1086/381588
11. Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? a survey of Connecticut physicians. Arch Intern Med. 1997;157(9):1017-1022.
12. McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712-720. https://doi.org/10.1128/jcm.02877-13
13. Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120(2):206-211.
14. Jones JL, Lopez A, Wahlquist SP, Nadle J, Wilson M; Emerging Infections Program FoodNet Working Group. Survey of clinical laboratory practices for parasitic diseases. Clin Infect Dis. 2004;38(Suppl 3):S198-S202. https://doi.org/10.1086/381587
15. Tewell CE, Talbot TR, Nelson GE, et al. Reducing inappropriate testing for the evaluation of diarrhea among hospitalized patients. Am J Med. 2018;131(2):193-199.e1. https://doi.org/10.1016/j.amjmed.2017.10.006
16. Thielman NM, Guerrant RL. Clinical practice. acute infectious diarrhea. N Engl J Med. 2004;350(1):38-47. https://doi.org/10.1056/nejmcp031534
17. Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31(11):3044-3045. https://doi.org/10.1128/jcm.31.11.3044-3045.1993
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 54-year-old immunocompetent man admitted to the hospital for non–ST-segment elevation myocardial infarction develops profuse watery diarrhea after his third day of admission. He denies prior episodes of diarrhea. He does not have any fevers, blood in the stool, recent travel, or antibiotic use. Vital signs include a blood pressure of 128/82 mm Hg, heart rate of 120 beats per minute, respiratory rate of 16 breaths per min, oxygen saturation of 100% on room air, and temperature of 36.9 °C. His physical examination is normal, without signs of abdominal tenderness, rebound, or guarding. Complete blood count is normal, without eosinophilia. The comprehensive metabolic panel shows mild hypokalemia of 3.3 mmol/L. The hospitalist resuscitates him with normal saline, provides oral potassium repletion, and orders a stool culture, Clostridioides difficile test, and an ova and parasite (O&P) test. Loperamide and time resolve his symptoms in 2 days. Results of his stool culture, C difficile, and O&P tests return negative in 3 days.
BACKGROUND
Acute diarrhea is a common complaint in both inpatient and outpatient settings. It is defined as the passage of three or more liquid or poorly formed stools in a 24-hour period lasting less than 14 days. Persistent diarrhea lasts from 14 to 29 days, while chronic diarrhea lasts longer than 30 days. There are 47.8 million cases of acute diarrhea per year in the United States, costing $150 million in US health expenditures.1 Viral pathogens remain the most common cause of acute diarrhea in the United States.1,2 Standard O&P testing consists of applying a stool sample to a slide with either saline or iodine (wet mount) and evaluating the specimen with a microscope.
WHY YOU MIGHT THINK O&P TESTING IS HELPFUL
Giardia and Cryptosporidium remain the most commonly implicated parasitic pathogens in acute diarrheal episodes in the United States.3 Cryptosporidium cases in the United States range from 2.2 to 3.9 per 100,000 persons,4 and Giardia cases in the United States range from 5.8 to 6.4 per 100,000 persons.5 To avoid missing potentially treatable causes, providers often order O&P tests reflexively as part of a standard workup for acute diarrhea. From 2001 to 2007, Associated Regional and University Pathologists Laboratories experienced a 379% increase in O&P testing.6 Many providers ordering these tests assume that standard O&P testing covers most, if not all, parasites and that a negative test will rule out a parasitic cause of disease. Furthermore, providers are unaware that more sensitive tests to detect certain parasites have replaced standard O&P microscopy.3
WHY O&P TESTING IS USUALLY UNNECESSARY
Most hospitalized patients do not have a parasitic infection
In a review of 5,681 completed O&P tests from a tertiary care medical center in Canada over a 5-year period, only 1.4% of tests were positive.7 In a 3-year retrospective analysis of stool samples obtained after 3 days of hospitalization, positive results were found in only 1 of 191 stool cultures and in 0 of 90 O&P samples.8 Current practice guidelines suggest not testing patients with stool studies in cases of acute diarrhea lasting less than 7 days in the absence of significant risk factors for parasitic disease because it has been shown to be a rare event and most cases will self-resolve with supportive care only.1,9
The stool O&P test has low sensitivity
Classically ordered stool O&P tests have low sensitivity for the detection of Giardia and Cryptosporidium, the two most common parasites in the United States.6,10,11 O&P studies detect Giardia in only 66% to 79% of specimen samples and Cryptosporidium in less than 5% of specimens. Diagnostic yields can be improved with the use of special stains such as modified acid-fast stain (MAF).6 Despite use of MAF staining, though, sensitivity for Cryptosporidium detection has remained at only 55%.12 Additionally, several studies have shown that physicians are generally unaware of the test characteristics of stool O&P tests and they do not know to order the newer more sensitive enzyme immunoassays (EIA) or direct fluorescent antibody (DFA) tests even in situations when testing for a parasitic infection is appropriate.10,11,13,14 As stated earlier, a parasitic infection without significant risk factors is a rare event. A negative test with low or moderate sensitivity is not additive to such a low clinical suspicion because it does not significantly change posttest probability.
Testing can have adverse consequences
In addition to the low yield, O&P testing is technically complex, is time intensive, and requires an experienced technician’s interpretation. Inappropriate testing increases the cost of care and staff workload without much benefit.6 As such, some institutions have opted to send the O&P tests to labs with experienced technicians. Other institutions have adopted a restrictive stool O&P testing approach that reduces healthcare time and costs and improves the rate of positive tests.13,15 A study at a single tertiary care medical center demonstrated an estimated cost savings of $21,931 annually by implementing a computer-based algorithm to restrict testing for stool cultures and O&P tests to patients with higher probabilities of infection.15 The algorithm directed clinicians to provide further information when attempting to order stool culture, O&P, or other specific stool tests. For patients hospitalized for more than 3 days, the system did not allow certain testing. For patients with worrisome features like severe symptoms or an immunocompromised state, the algorithm directed the clinician to place an infectious disease or gastroenterology consult rather than order stool tests. Decreased laboratory costs of all stool studies (including O&P) in adult inpatient locations led to the cost savings. Additionally, the study authors felt that they likely underestimated the cost savings because they did not account for other expenses in the analysis, such as nursing workload and supplies.15
WHAT YOU SHOULD DO INSTEAD
Clinicians should evaluate patients on a case-by-case basis and determine the need to test based on the presence of high-risk features (Table). 
When performing parasitic testing in patients without a recent travel history but with other high-risk features, test for the most prevalent parasites in the United States (ie, Giardia, Cryptosporidium, and Entamoeba histolytica) with DFA or EIA tests.3 DFA testing for Giardia is 99% sensitive.12 In patients with symptoms lasting more than 7 days and recent travel, in addition to the above DFA/EIA tests, perform O&P testing with wet mount, modified acid-fast bacilli stain to detect rare parasites such as helminths, Strongyloides, Cyclospora, and Cystoisospora.3 In patients who live or travel to endemic areas (about 10% of traveler’s diarrhea is caused by parasitic infections), have unexplained eosinophilia, or are part of a community outbreak (eg, childcare institutions or drinking water/food outbreaks), test for Giardia, Cryptosporidium, Cyclospora, Cystoisospora, Entamoeba histolytica, and Isospora belli.9 In addition, among patients with AIDS or immunosuppression, testing should include assays for Microsporidia, Strongyloides, and Mycobacterium avium complex (Figure).9,16 Newer tests, such as the multiplex real-time polymerase chain reaction assay, can also simultaneously detect Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum. For more information on parasitic testing, we suggest reading the review article “Beyond O&P times three.”3 It is important to familiarize yourself with the parasitic tests available at your respective clinics/hospital so the optimal test can be used.

RECOMMENDATIONS
- Prescribe a trial of “wait and see” for patients without high-risk features for parasitic disease.
- Test patients with high-risk features for parasitic disease by utilizing targeted testing.
- For patients with high-risk features but no travel history, first perform DFA, EIA, or multiplex real-time polymerase chain reaction testing to evaluate for Giardia, Cryptosporidium, and Entamoeba histolytica.
- If DFA/EIA testing is negative, obtain O&P tests with and without stains, such as acid-fast bacilli, for detection of other rare parasites.
CONCLUSION
Hospitalists should risk-stratify patients to determine when O&P testing is appropriate. Employ more targeted testing, especially use of DFA/EIA tests when evaluating for parasites. Avoid parasitic testing if symptoms have lasted less than 7 days and the patient has no other high-risk features. Become familiar with the tests available at your institution and their sensitivities. As in our clinical scenario, most acute cases of diarrhea resolve without intervention and should be managed and treated conservatively.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing [email protected] .
Acknowledgments
The authors thank Dr Lenny Feldman for his assistance with editing the manuscript.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 54-year-old immunocompetent man admitted to the hospital for non–ST-segment elevation myocardial infarction develops profuse watery diarrhea after his third day of admission. He denies prior episodes of diarrhea. He does not have any fevers, blood in the stool, recent travel, or antibiotic use. Vital signs include a blood pressure of 128/82 mm Hg, heart rate of 120 beats per minute, respiratory rate of 16 breaths per min, oxygen saturation of 100% on room air, and temperature of 36.9 °C. His physical examination is normal, without signs of abdominal tenderness, rebound, or guarding. Complete blood count is normal, without eosinophilia. The comprehensive metabolic panel shows mild hypokalemia of 3.3 mmol/L. The hospitalist resuscitates him with normal saline, provides oral potassium repletion, and orders a stool culture, Clostridioides difficile test, and an ova and parasite (O&P) test. Loperamide and time resolve his symptoms in 2 days. Results of his stool culture, C difficile, and O&P tests return negative in 3 days.
BACKGROUND
Acute diarrhea is a common complaint in both inpatient and outpatient settings. It is defined as the passage of three or more liquid or poorly formed stools in a 24-hour period lasting less than 14 days. Persistent diarrhea lasts from 14 to 29 days, while chronic diarrhea lasts longer than 30 days. There are 47.8 million cases of acute diarrhea per year in the United States, costing $150 million in US health expenditures.1 Viral pathogens remain the most common cause of acute diarrhea in the United States.1,2 Standard O&P testing consists of applying a stool sample to a slide with either saline or iodine (wet mount) and evaluating the specimen with a microscope.
WHY YOU MIGHT THINK O&P TESTING IS HELPFUL
Giardia and Cryptosporidium remain the most commonly implicated parasitic pathogens in acute diarrheal episodes in the United States.3 Cryptosporidium cases in the United States range from 2.2 to 3.9 per 100,000 persons,4 and Giardia cases in the United States range from 5.8 to 6.4 per 100,000 persons.5 To avoid missing potentially treatable causes, providers often order O&P tests reflexively as part of a standard workup for acute diarrhea. From 2001 to 2007, Associated Regional and University Pathologists Laboratories experienced a 379% increase in O&P testing.6 Many providers ordering these tests assume that standard O&P testing covers most, if not all, parasites and that a negative test will rule out a parasitic cause of disease. Furthermore, providers are unaware that more sensitive tests to detect certain parasites have replaced standard O&P microscopy.3
WHY O&P TESTING IS USUALLY UNNECESSARY
Most hospitalized patients do not have a parasitic infection
In a review of 5,681 completed O&P tests from a tertiary care medical center in Canada over a 5-year period, only 1.4% of tests were positive.7 In a 3-year retrospective analysis of stool samples obtained after 3 days of hospitalization, positive results were found in only 1 of 191 stool cultures and in 0 of 90 O&P samples.8 Current practice guidelines suggest not testing patients with stool studies in cases of acute diarrhea lasting less than 7 days in the absence of significant risk factors for parasitic disease because it has been shown to be a rare event and most cases will self-resolve with supportive care only.1,9
The stool O&P test has low sensitivity
Classically ordered stool O&P tests have low sensitivity for the detection of Giardia and Cryptosporidium, the two most common parasites in the United States.6,10,11 O&P studies detect Giardia in only 66% to 79% of specimen samples and Cryptosporidium in less than 5% of specimens. Diagnostic yields can be improved with the use of special stains such as modified acid-fast stain (MAF).6 Despite use of MAF staining, though, sensitivity for Cryptosporidium detection has remained at only 55%.12 Additionally, several studies have shown that physicians are generally unaware of the test characteristics of stool O&P tests and they do not know to order the newer more sensitive enzyme immunoassays (EIA) or direct fluorescent antibody (DFA) tests even in situations when testing for a parasitic infection is appropriate.10,11,13,14 As stated earlier, a parasitic infection without significant risk factors is a rare event. A negative test with low or moderate sensitivity is not additive to such a low clinical suspicion because it does not significantly change posttest probability.
Testing can have adverse consequences
In addition to the low yield, O&P testing is technically complex, is time intensive, and requires an experienced technician’s interpretation. Inappropriate testing increases the cost of care and staff workload without much benefit.6 As such, some institutions have opted to send the O&P tests to labs with experienced technicians. Other institutions have adopted a restrictive stool O&P testing approach that reduces healthcare time and costs and improves the rate of positive tests.13,15 A study at a single tertiary care medical center demonstrated an estimated cost savings of $21,931 annually by implementing a computer-based algorithm to restrict testing for stool cultures and O&P tests to patients with higher probabilities of infection.15 The algorithm directed clinicians to provide further information when attempting to order stool culture, O&P, or other specific stool tests. For patients hospitalized for more than 3 days, the system did not allow certain testing. For patients with worrisome features like severe symptoms or an immunocompromised state, the algorithm directed the clinician to place an infectious disease or gastroenterology consult rather than order stool tests. Decreased laboratory costs of all stool studies (including O&P) in adult inpatient locations led to the cost savings. Additionally, the study authors felt that they likely underestimated the cost savings because they did not account for other expenses in the analysis, such as nursing workload and supplies.15
WHAT YOU SHOULD DO INSTEAD
Clinicians should evaluate patients on a case-by-case basis and determine the need to test based on the presence of high-risk features (Table). 
When performing parasitic testing in patients without a recent travel history but with other high-risk features, test for the most prevalent parasites in the United States (ie, Giardia, Cryptosporidium, and Entamoeba histolytica) with DFA or EIA tests.3 DFA testing for Giardia is 99% sensitive.12 In patients with symptoms lasting more than 7 days and recent travel, in addition to the above DFA/EIA tests, perform O&P testing with wet mount, modified acid-fast bacilli stain to detect rare parasites such as helminths, Strongyloides, Cyclospora, and Cystoisospora.3 In patients who live or travel to endemic areas (about 10% of traveler’s diarrhea is caused by parasitic infections), have unexplained eosinophilia, or are part of a community outbreak (eg, childcare institutions or drinking water/food outbreaks), test for Giardia, Cryptosporidium, Cyclospora, Cystoisospora, Entamoeba histolytica, and Isospora belli.9 In addition, among patients with AIDS or immunosuppression, testing should include assays for Microsporidia, Strongyloides, and Mycobacterium avium complex (Figure).9,16 Newer tests, such as the multiplex real-time polymerase chain reaction assay, can also simultaneously detect Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum. For more information on parasitic testing, we suggest reading the review article “Beyond O&P times three.”3 It is important to familiarize yourself with the parasitic tests available at your respective clinics/hospital so the optimal test can be used.

RECOMMENDATIONS
- Prescribe a trial of “wait and see” for patients without high-risk features for parasitic disease.
- Test patients with high-risk features for parasitic disease by utilizing targeted testing.
- For patients with high-risk features but no travel history, first perform DFA, EIA, or multiplex real-time polymerase chain reaction testing to evaluate for Giardia, Cryptosporidium, and Entamoeba histolytica.
- If DFA/EIA testing is negative, obtain O&P tests with and without stains, such as acid-fast bacilli, for detection of other rare parasites.
CONCLUSION
Hospitalists should risk-stratify patients to determine when O&P testing is appropriate. Employ more targeted testing, especially use of DFA/EIA tests when evaluating for parasites. Avoid parasitic testing if symptoms have lasted less than 7 days and the patient has no other high-risk features. Become familiar with the tests available at your institution and their sensitivities. As in our clinical scenario, most acute cases of diarrhea resolve without intervention and should be managed and treated conservatively.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing [email protected] .
Acknowledgments
The authors thank Dr Lenny Feldman for his assistance with editing the manuscript.
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. https://doi.org/10.1038/ajg.2016.126
2. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. https://doi.org/10.1056/nejmra1301069
3. Mohapatra S, Singh DP, Alcid D, Pitchumoni CS. Beyond O&P times three. Am J Gastroenterol. 2018;113(6):805-818. https://doi.org/10.1038/s41395-018-0083-y
4. Painter JE, Hlavsa MC, Collier SA, Xiao L, Yoder JS. Cryptosporidiosis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):1-14.
5. Painter JE, Gargano JW, Collier SA, Yoder JS. Giardiasis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):15-25.
6. Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011;49(2):591-596. https://doi.org/10.1128/jcm.01806-10
7. Mosli M, Gregor J, Chande N, Lannigan R. Nonutility of routine testing of stool for ova and parasites in a tertiary care Canadian centre. Can J Microbiol. 2012;58(5):653-659. https://doi.org/10.1139/w2012-039
8. Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979-982.
9. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. https://doi.org/10.1093/cid/cix669
10. Hennessy TW, Marcus R, Deneen V, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38 (Suppl 3):S203-S211. https://doi.org/10.1086/381588
11. Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? a survey of Connecticut physicians. Arch Intern Med. 1997;157(9):1017-1022.
12. McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712-720. https://doi.org/10.1128/jcm.02877-13
13. Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120(2):206-211.
14. Jones JL, Lopez A, Wahlquist SP, Nadle J, Wilson M; Emerging Infections Program FoodNet Working Group. Survey of clinical laboratory practices for parasitic diseases. Clin Infect Dis. 2004;38(Suppl 3):S198-S202. https://doi.org/10.1086/381587
15. Tewell CE, Talbot TR, Nelson GE, et al. Reducing inappropriate testing for the evaluation of diarrhea among hospitalized patients. Am J Med. 2018;131(2):193-199.e1. https://doi.org/10.1016/j.amjmed.2017.10.006
16. Thielman NM, Guerrant RL. Clinical practice. acute infectious diarrhea. N Engl J Med. 2004;350(1):38-47. https://doi.org/10.1056/nejmcp031534
17. Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31(11):3044-3045. https://doi.org/10.1128/jcm.31.11.3044-3045.1993
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. https://doi.org/10.1038/ajg.2016.126
2. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. https://doi.org/10.1056/nejmra1301069
3. Mohapatra S, Singh DP, Alcid D, Pitchumoni CS. Beyond O&P times three. Am J Gastroenterol. 2018;113(6):805-818. https://doi.org/10.1038/s41395-018-0083-y
4. Painter JE, Hlavsa MC, Collier SA, Xiao L, Yoder JS. Cryptosporidiosis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):1-14.
5. Painter JE, Gargano JW, Collier SA, Yoder JS. Giardiasis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):15-25.
6. Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011;49(2):591-596. https://doi.org/10.1128/jcm.01806-10
7. Mosli M, Gregor J, Chande N, Lannigan R. Nonutility of routine testing of stool for ova and parasites in a tertiary care Canadian centre. Can J Microbiol. 2012;58(5):653-659. https://doi.org/10.1139/w2012-039
8. Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979-982.
9. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. https://doi.org/10.1093/cid/cix669
10. Hennessy TW, Marcus R, Deneen V, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38 (Suppl 3):S203-S211. https://doi.org/10.1086/381588
11. Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? a survey of Connecticut physicians. Arch Intern Med. 1997;157(9):1017-1022.
12. McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712-720. https://doi.org/10.1128/jcm.02877-13
13. Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120(2):206-211.
14. Jones JL, Lopez A, Wahlquist SP, Nadle J, Wilson M; Emerging Infections Program FoodNet Working Group. Survey of clinical laboratory practices for parasitic diseases. Clin Infect Dis. 2004;38(Suppl 3):S198-S202. https://doi.org/10.1086/381587
15. Tewell CE, Talbot TR, Nelson GE, et al. Reducing inappropriate testing for the evaluation of diarrhea among hospitalized patients. Am J Med. 2018;131(2):193-199.e1. https://doi.org/10.1016/j.amjmed.2017.10.006
16. Thielman NM, Guerrant RL. Clinical practice. acute infectious diarrhea. N Engl J Med. 2004;350(1):38-47. https://doi.org/10.1056/nejmcp031534
17. Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31(11):3044-3045. https://doi.org/10.1128/jcm.31.11.3044-3045.1993
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Secondary Fracture Prevention for Hospitalized Patients
Osteoporosis is the most prevalent bone disease and a leading cause of morbidity and mortality in older people. According to the National Health and Nutrition Examination Survey, from 2005-2010, there were an estimated 10.2 million adults 50 years and older with osteoporosis and 43.4 million more with low bone mass in the United States.1 Osteoporotic fracture is a leading cause of hospitalization in the United States for women 55 years or older, ahead of heart attacks, stroke, and breast cancer.2 Despite elucidation of the pathogenesis of osteoporosis and the advent of effective and widely available therapies, a “treatment gap” separates the many patients who warrant therapy from the few who receive it. Systematic improvement strategies, such as coordinator-based fracture liaison services, have had a positive impact on addressing this treatment gap.3 There is an opportunity for hospitalists to further narrow this treatment gap.
The American Society of Bone and Mineral Research, in conjunction with the Center for Medical Technology Policy, developed consensus clinical recommendations to address secondary fracture prevention for people 65 years or older who have experienced a hip or vertebral fracture.4 We address six of the fundamental and two of the supplemental recommendations as they apply to the practice of hospital medicine.
KEY RECOMMENDATIONS FOR HOSPITALISTs
Recommendations 1 and 2
Communicate key information to the patient and their usual healthcare provider. Patients 65 years or older with a hip or vertebral fracture likely have osteoporosis and are at high risk for subsequent fractures, which can lead to a decline in function and an increase in mortality. Patients must be counseled regarding their diagnosis, their risks, and the actions they can take to manage their disease. Primary care providers must be notified of the occurrence of the fracture, the diagnosis of osteoporosis, and the plans for management.
We recommend hospitalists act as leading advocates for at-risk patients to ensure that this communication occurs during hospitalization. We encourage hospitals and institutions to adopt systematic interventions to facilitate postdischarge care for these patients. These may include implementing a fracture liaison service, with multidisciplinary secondary fracture–prevention strategies using physicians, pharmacists, nurses, social workers, and case managers for care coordination and treatment initiation.
Elderly patients with osteoporotic fragility fractures are at risk for further morbidity and mortality. Coordination of care between the inpatient care team and the primary care provider is necessary to reduce this risk. In addition to verbal communication and especially when verbal communication is not feasible, discharge documents provided to patients and outpatient providers should clearly identify the occurrence of a hip or vertebral fracture and a discharge diagnosis of osteoporosis if not previously documented, regardless of bone mineral density (BMD) results or lack of testing.
Recommendation 3
Regularly assess fall risk. Patients 65 years or older with a current or prior hip or vertebral fracture must be regularly assessed for risk of falls. Hospitalists can assess patients’ ongoing risk for falls at time of admission or during hospitalization. Risk factors include prior falls; advanced age; visual, auditory, or cognitive impairment; decreased muscle strength; gait and balance impairment; diabetes mellitus; use of multiple medications, and others.5 Specialist evaluation by a physical therapist or a physiatrist should be considered. Active medications should be reviewed for adverse effects and interactions. The use of diuretics, antipsychotics, antidepressants, benzodiazepines, antiepileptics, and opioids should be minimized.
Recommendations 4, 5, 6, and 11
Offer pharmacologic therapy and initiate calcium and vitamin D supplementation. Recommendations 4 through 6 and 11 advocate pharmacologic interventions including bisphosphonates, denosumab, vitamin D, and/or calcium to reduce the risk of future fractures. Bisphosphonates are the cornerstone of pharmacologic therapy for secondary fracture prevention. The efficacy of these agents for prevention of subsequent fractures outweighs the potential for interference in healing of surgically repaired bones.6 Oral bisphosphonate therapy should be initiated in the hospital or at discharge. Parenteral bisphosphonates and denosumab may be utilized in patients unable to tolerate or absorb oral bisphosphonates due to esophageal or other gastrointestinal disease. Initiation of these agents should be delayed until after vitamin D and calcium supplementation have been administered for 2 weeks after the fracture to reduce the risk of precipitating hypocalcemia, and they should not be used in patients with confirmed hypocalcemia until that is resolved. BMD measurement is not necessary prior to pharmacologic therapy initiation because the risk of fracture is elevated for these patients regardless of BMD. Patients without significant dental disease or planned oral or maxillofacial procedures may begin bisphosphonate therapy prior to a full dental assessment because risk of osteonecrosis of the jaw is low.
The guidelines recommend people 65 years or older with a hip or vertebral fracture receive daily supplementation of at least 800 IU vitamin D. Patients unable to achieve an intake of 1,200 mg/day of calcium from food sources should receive daily calcium supplementation. The effect of vitamin D monotherapy on fracture risk is not clear; however, strong evidence suggests that fracture risk is reduced when individuals at high risk of deficiency receive supplementation with vitamin D and calcium. Calcium supplementation alone has not demonstrated reduction in fracture risk. Total daily calcium intake above 1,500 mg has not been shown to provide additional benefit and is potentially harmful.
Recommendation 9
Counsel patients on lifestyle modifications and consider physical therapy. Tobacco has a deleterious effect on bone density and increases risk for osteoporotic fragility fracture.7 Hospitalists should obtain tobacco use history from all patients with an osteoporotic fracture and provide tobacco cessation counseling when appropriate. Excessive alcohol consumption increases the risk of fall injuries.8 Hospitalists should counsel patients to limit alcohol intake to a maximum of two drinks a day for men and one drink a day for women.
Weight-bearing and strength-training exercises, particularly those involving balance and trunk muscle strength, are associated with reduction in fall-risk. Exercise must be tailored to the patient’s physical capacity. Hospitalists may partner with physical therapists or physiatrists to facilitate development of an exercise plan to maximize benefit and minimize risk of injury.
CRITIQUE
We found this document to be highly informative and well cited, with ample evidence to support the recommendations.
Methods in Preparing Guidelines
The multistakeholder coalition did not employ a rigorous and standardized methodology for the guideline, such as GRADE (Grading of Recommendations Assessment, Development, and Evaluation); hence, no assessment of evidence quality, benefits and harms of an intervention, or resource use was provided.
Potential Conflicts for Guideline Authors
Eight guideline authors have pharmaceutical relationships with the manufacturer of one of the medications listed on the guidelines (Amgen-denosumab, Novartis-zoledronic acid). There are no disclosures reported from the multistakeholder coalition members who are not listed as guideline authors.
AREAS IN NEED OF FUTURE STUDY
We anticipate future studies may report outcomes focused on secondary prevention of fractures. Additionally, we would like to see new studies investigating patient-centered outcomes such as improvement in functional status and ambulatory independence based on improved postfracture medical therapies. We see an opportunity for studies assessing real-world outcomes to inform future recommendations, particularly after widespread implementation of secondary fracture prevention therapy either initiated during hospitalization or purposefully planned for after discharge.
We would like to see more trial data comparing the safety and cost-effectiveness of first-line therapy, namely oral bisphosphonates, to alternative treatments, particularly parenteral agents, which may improve treatment compliance because of the convenience in dosing frequency.
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520-2526. https://doi.org/10.1002/jbmr.2269
2. Singer A, Exuzides A, Spangler L, et al. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin Proc. 2015;90(1):53-62. https://doi.org/10.1016/j.mayocp.2014.09.011
3. McLellan AR, Gallacher SJ, Fraser M, McQuillian C. The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int. 2003;14(12):1028-1034. https://doi.org/10.1007/s00198-003-1507-z
4. Conley RB, Adib G, Adler RA, et al. Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res. 2020;35(1):36-52. https://doi.org/10.1002/jbmr.3877
5. Bueno-Cavanillas A, Padilla-Ruiz F, Jiménez-Moleón JJ, Peinado-Alonso CA, Gálvez-Vargas R. Risk factors in falls among the elderly according to extrinsic and intrinsic precipitating causes. Eur J Epidemiol. 2000;16(9):849-859. https://doi.org/10.1023/a:1007636531965
6. Vannucci L, Brandi ML. Healing of the bone with anti-fracture drugs. Expert Opin Pharmacother. 2016;17(17):2267-2272. https://doi.org/10.1080/14656566.2016.1241765
7. Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315(7112):841-846. https://doi.org/10.1136/bmj.315.7112.841
8. Chen CM, Yoon YH. Usual alcohol consumption and risks for nonfatal fall injuries in the United States: results from the 2004-2013 National Health Interview Survey. Subst Use Misuse. 2017;52(9):1120-1132. https://doi.org/10.1080/10826084.2017.1293101
Osteoporosis is the most prevalent bone disease and a leading cause of morbidity and mortality in older people. According to the National Health and Nutrition Examination Survey, from 2005-2010, there were an estimated 10.2 million adults 50 years and older with osteoporosis and 43.4 million more with low bone mass in the United States.1 Osteoporotic fracture is a leading cause of hospitalization in the United States for women 55 years or older, ahead of heart attacks, stroke, and breast cancer.2 Despite elucidation of the pathogenesis of osteoporosis and the advent of effective and widely available therapies, a “treatment gap” separates the many patients who warrant therapy from the few who receive it. Systematic improvement strategies, such as coordinator-based fracture liaison services, have had a positive impact on addressing this treatment gap.3 There is an opportunity for hospitalists to further narrow this treatment gap.
The American Society of Bone and Mineral Research, in conjunction with the Center for Medical Technology Policy, developed consensus clinical recommendations to address secondary fracture prevention for people 65 years or older who have experienced a hip or vertebral fracture.4 We address six of the fundamental and two of the supplemental recommendations as they apply to the practice of hospital medicine.
KEY RECOMMENDATIONS FOR HOSPITALISTs
Recommendations 1 and 2
Communicate key information to the patient and their usual healthcare provider. Patients 65 years or older with a hip or vertebral fracture likely have osteoporosis and are at high risk for subsequent fractures, which can lead to a decline in function and an increase in mortality. Patients must be counseled regarding their diagnosis, their risks, and the actions they can take to manage their disease. Primary care providers must be notified of the occurrence of the fracture, the diagnosis of osteoporosis, and the plans for management.
We recommend hospitalists act as leading advocates for at-risk patients to ensure that this communication occurs during hospitalization. We encourage hospitals and institutions to adopt systematic interventions to facilitate postdischarge care for these patients. These may include implementing a fracture liaison service, with multidisciplinary secondary fracture–prevention strategies using physicians, pharmacists, nurses, social workers, and case managers for care coordination and treatment initiation.
Elderly patients with osteoporotic fragility fractures are at risk for further morbidity and mortality. Coordination of care between the inpatient care team and the primary care provider is necessary to reduce this risk. In addition to verbal communication and especially when verbal communication is not feasible, discharge documents provided to patients and outpatient providers should clearly identify the occurrence of a hip or vertebral fracture and a discharge diagnosis of osteoporosis if not previously documented, regardless of bone mineral density (BMD) results or lack of testing.
Recommendation 3
Regularly assess fall risk. Patients 65 years or older with a current or prior hip or vertebral fracture must be regularly assessed for risk of falls. Hospitalists can assess patients’ ongoing risk for falls at time of admission or during hospitalization. Risk factors include prior falls; advanced age; visual, auditory, or cognitive impairment; decreased muscle strength; gait and balance impairment; diabetes mellitus; use of multiple medications, and others.5 Specialist evaluation by a physical therapist or a physiatrist should be considered. Active medications should be reviewed for adverse effects and interactions. The use of diuretics, antipsychotics, antidepressants, benzodiazepines, antiepileptics, and opioids should be minimized.
Recommendations 4, 5, 6, and 11
Offer pharmacologic therapy and initiate calcium and vitamin D supplementation. Recommendations 4 through 6 and 11 advocate pharmacologic interventions including bisphosphonates, denosumab, vitamin D, and/or calcium to reduce the risk of future fractures. Bisphosphonates are the cornerstone of pharmacologic therapy for secondary fracture prevention. The efficacy of these agents for prevention of subsequent fractures outweighs the potential for interference in healing of surgically repaired bones.6 Oral bisphosphonate therapy should be initiated in the hospital or at discharge. Parenteral bisphosphonates and denosumab may be utilized in patients unable to tolerate or absorb oral bisphosphonates due to esophageal or other gastrointestinal disease. Initiation of these agents should be delayed until after vitamin D and calcium supplementation have been administered for 2 weeks after the fracture to reduce the risk of precipitating hypocalcemia, and they should not be used in patients with confirmed hypocalcemia until that is resolved. BMD measurement is not necessary prior to pharmacologic therapy initiation because the risk of fracture is elevated for these patients regardless of BMD. Patients without significant dental disease or planned oral or maxillofacial procedures may begin bisphosphonate therapy prior to a full dental assessment because risk of osteonecrosis of the jaw is low.
The guidelines recommend people 65 years or older with a hip or vertebral fracture receive daily supplementation of at least 800 IU vitamin D. Patients unable to achieve an intake of 1,200 mg/day of calcium from food sources should receive daily calcium supplementation. The effect of vitamin D monotherapy on fracture risk is not clear; however, strong evidence suggests that fracture risk is reduced when individuals at high risk of deficiency receive supplementation with vitamin D and calcium. Calcium supplementation alone has not demonstrated reduction in fracture risk. Total daily calcium intake above 1,500 mg has not been shown to provide additional benefit and is potentially harmful.
Recommendation 9
Counsel patients on lifestyle modifications and consider physical therapy. Tobacco has a deleterious effect on bone density and increases risk for osteoporotic fragility fracture.7 Hospitalists should obtain tobacco use history from all patients with an osteoporotic fracture and provide tobacco cessation counseling when appropriate. Excessive alcohol consumption increases the risk of fall injuries.8 Hospitalists should counsel patients to limit alcohol intake to a maximum of two drinks a day for men and one drink a day for women.
Weight-bearing and strength-training exercises, particularly those involving balance and trunk muscle strength, are associated with reduction in fall-risk. Exercise must be tailored to the patient’s physical capacity. Hospitalists may partner with physical therapists or physiatrists to facilitate development of an exercise plan to maximize benefit and minimize risk of injury.
CRITIQUE
We found this document to be highly informative and well cited, with ample evidence to support the recommendations.
Methods in Preparing Guidelines
The multistakeholder coalition did not employ a rigorous and standardized methodology for the guideline, such as GRADE (Grading of Recommendations Assessment, Development, and Evaluation); hence, no assessment of evidence quality, benefits and harms of an intervention, or resource use was provided.
Potential Conflicts for Guideline Authors
Eight guideline authors have pharmaceutical relationships with the manufacturer of one of the medications listed on the guidelines (Amgen-denosumab, Novartis-zoledronic acid). There are no disclosures reported from the multistakeholder coalition members who are not listed as guideline authors.
AREAS IN NEED OF FUTURE STUDY
We anticipate future studies may report outcomes focused on secondary prevention of fractures. Additionally, we would like to see new studies investigating patient-centered outcomes such as improvement in functional status and ambulatory independence based on improved postfracture medical therapies. We see an opportunity for studies assessing real-world outcomes to inform future recommendations, particularly after widespread implementation of secondary fracture prevention therapy either initiated during hospitalization or purposefully planned for after discharge.
We would like to see more trial data comparing the safety and cost-effectiveness of first-line therapy, namely oral bisphosphonates, to alternative treatments, particularly parenteral agents, which may improve treatment compliance because of the convenience in dosing frequency.
Osteoporosis is the most prevalent bone disease and a leading cause of morbidity and mortality in older people. According to the National Health and Nutrition Examination Survey, from 2005-2010, there were an estimated 10.2 million adults 50 years and older with osteoporosis and 43.4 million more with low bone mass in the United States.1 Osteoporotic fracture is a leading cause of hospitalization in the United States for women 55 years or older, ahead of heart attacks, stroke, and breast cancer.2 Despite elucidation of the pathogenesis of osteoporosis and the advent of effective and widely available therapies, a “treatment gap” separates the many patients who warrant therapy from the few who receive it. Systematic improvement strategies, such as coordinator-based fracture liaison services, have had a positive impact on addressing this treatment gap.3 There is an opportunity for hospitalists to further narrow this treatment gap.
The American Society of Bone and Mineral Research, in conjunction with the Center for Medical Technology Policy, developed consensus clinical recommendations to address secondary fracture prevention for people 65 years or older who have experienced a hip or vertebral fracture.4 We address six of the fundamental and two of the supplemental recommendations as they apply to the practice of hospital medicine.
KEY RECOMMENDATIONS FOR HOSPITALISTs
Recommendations 1 and 2
Communicate key information to the patient and their usual healthcare provider. Patients 65 years or older with a hip or vertebral fracture likely have osteoporosis and are at high risk for subsequent fractures, which can lead to a decline in function and an increase in mortality. Patients must be counseled regarding their diagnosis, their risks, and the actions they can take to manage their disease. Primary care providers must be notified of the occurrence of the fracture, the diagnosis of osteoporosis, and the plans for management.
We recommend hospitalists act as leading advocates for at-risk patients to ensure that this communication occurs during hospitalization. We encourage hospitals and institutions to adopt systematic interventions to facilitate postdischarge care for these patients. These may include implementing a fracture liaison service, with multidisciplinary secondary fracture–prevention strategies using physicians, pharmacists, nurses, social workers, and case managers for care coordination and treatment initiation.
Elderly patients with osteoporotic fragility fractures are at risk for further morbidity and mortality. Coordination of care between the inpatient care team and the primary care provider is necessary to reduce this risk. In addition to verbal communication and especially when verbal communication is not feasible, discharge documents provided to patients and outpatient providers should clearly identify the occurrence of a hip or vertebral fracture and a discharge diagnosis of osteoporosis if not previously documented, regardless of bone mineral density (BMD) results or lack of testing.
Recommendation 3
Regularly assess fall risk. Patients 65 years or older with a current or prior hip or vertebral fracture must be regularly assessed for risk of falls. Hospitalists can assess patients’ ongoing risk for falls at time of admission or during hospitalization. Risk factors include prior falls; advanced age; visual, auditory, or cognitive impairment; decreased muscle strength; gait and balance impairment; diabetes mellitus; use of multiple medications, and others.5 Specialist evaluation by a physical therapist or a physiatrist should be considered. Active medications should be reviewed for adverse effects and interactions. The use of diuretics, antipsychotics, antidepressants, benzodiazepines, antiepileptics, and opioids should be minimized.
Recommendations 4, 5, 6, and 11
Offer pharmacologic therapy and initiate calcium and vitamin D supplementation. Recommendations 4 through 6 and 11 advocate pharmacologic interventions including bisphosphonates, denosumab, vitamin D, and/or calcium to reduce the risk of future fractures. Bisphosphonates are the cornerstone of pharmacologic therapy for secondary fracture prevention. The efficacy of these agents for prevention of subsequent fractures outweighs the potential for interference in healing of surgically repaired bones.6 Oral bisphosphonate therapy should be initiated in the hospital or at discharge. Parenteral bisphosphonates and denosumab may be utilized in patients unable to tolerate or absorb oral bisphosphonates due to esophageal or other gastrointestinal disease. Initiation of these agents should be delayed until after vitamin D and calcium supplementation have been administered for 2 weeks after the fracture to reduce the risk of precipitating hypocalcemia, and they should not be used in patients with confirmed hypocalcemia until that is resolved. BMD measurement is not necessary prior to pharmacologic therapy initiation because the risk of fracture is elevated for these patients regardless of BMD. Patients without significant dental disease or planned oral or maxillofacial procedures may begin bisphosphonate therapy prior to a full dental assessment because risk of osteonecrosis of the jaw is low.
The guidelines recommend people 65 years or older with a hip or vertebral fracture receive daily supplementation of at least 800 IU vitamin D. Patients unable to achieve an intake of 1,200 mg/day of calcium from food sources should receive daily calcium supplementation. The effect of vitamin D monotherapy on fracture risk is not clear; however, strong evidence suggests that fracture risk is reduced when individuals at high risk of deficiency receive supplementation with vitamin D and calcium. Calcium supplementation alone has not demonstrated reduction in fracture risk. Total daily calcium intake above 1,500 mg has not been shown to provide additional benefit and is potentially harmful.
Recommendation 9
Counsel patients on lifestyle modifications and consider physical therapy. Tobacco has a deleterious effect on bone density and increases risk for osteoporotic fragility fracture.7 Hospitalists should obtain tobacco use history from all patients with an osteoporotic fracture and provide tobacco cessation counseling when appropriate. Excessive alcohol consumption increases the risk of fall injuries.8 Hospitalists should counsel patients to limit alcohol intake to a maximum of two drinks a day for men and one drink a day for women.
Weight-bearing and strength-training exercises, particularly those involving balance and trunk muscle strength, are associated with reduction in fall-risk. Exercise must be tailored to the patient’s physical capacity. Hospitalists may partner with physical therapists or physiatrists to facilitate development of an exercise plan to maximize benefit and minimize risk of injury.
CRITIQUE
We found this document to be highly informative and well cited, with ample evidence to support the recommendations.
Methods in Preparing Guidelines
The multistakeholder coalition did not employ a rigorous and standardized methodology for the guideline, such as GRADE (Grading of Recommendations Assessment, Development, and Evaluation); hence, no assessment of evidence quality, benefits and harms of an intervention, or resource use was provided.
Potential Conflicts for Guideline Authors
Eight guideline authors have pharmaceutical relationships with the manufacturer of one of the medications listed on the guidelines (Amgen-denosumab, Novartis-zoledronic acid). There are no disclosures reported from the multistakeholder coalition members who are not listed as guideline authors.
AREAS IN NEED OF FUTURE STUDY
We anticipate future studies may report outcomes focused on secondary prevention of fractures. Additionally, we would like to see new studies investigating patient-centered outcomes such as improvement in functional status and ambulatory independence based on improved postfracture medical therapies. We see an opportunity for studies assessing real-world outcomes to inform future recommendations, particularly after widespread implementation of secondary fracture prevention therapy either initiated during hospitalization or purposefully planned for after discharge.
We would like to see more trial data comparing the safety and cost-effectiveness of first-line therapy, namely oral bisphosphonates, to alternative treatments, particularly parenteral agents, which may improve treatment compliance because of the convenience in dosing frequency.
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520-2526. https://doi.org/10.1002/jbmr.2269
2. Singer A, Exuzides A, Spangler L, et al. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin Proc. 2015;90(1):53-62. https://doi.org/10.1016/j.mayocp.2014.09.011
3. McLellan AR, Gallacher SJ, Fraser M, McQuillian C. The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int. 2003;14(12):1028-1034. https://doi.org/10.1007/s00198-003-1507-z
4. Conley RB, Adib G, Adler RA, et al. Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res. 2020;35(1):36-52. https://doi.org/10.1002/jbmr.3877
5. Bueno-Cavanillas A, Padilla-Ruiz F, Jiménez-Moleón JJ, Peinado-Alonso CA, Gálvez-Vargas R. Risk factors in falls among the elderly according to extrinsic and intrinsic precipitating causes. Eur J Epidemiol. 2000;16(9):849-859. https://doi.org/10.1023/a:1007636531965
6. Vannucci L, Brandi ML. Healing of the bone with anti-fracture drugs. Expert Opin Pharmacother. 2016;17(17):2267-2272. https://doi.org/10.1080/14656566.2016.1241765
7. Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315(7112):841-846. https://doi.org/10.1136/bmj.315.7112.841
8. Chen CM, Yoon YH. Usual alcohol consumption and risks for nonfatal fall injuries in the United States: results from the 2004-2013 National Health Interview Survey. Subst Use Misuse. 2017;52(9):1120-1132. https://doi.org/10.1080/10826084.2017.1293101
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520-2526. https://doi.org/10.1002/jbmr.2269
2. Singer A, Exuzides A, Spangler L, et al. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin Proc. 2015;90(1):53-62. https://doi.org/10.1016/j.mayocp.2014.09.011
3. McLellan AR, Gallacher SJ, Fraser M, McQuillian C. The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int. 2003;14(12):1028-1034. https://doi.org/10.1007/s00198-003-1507-z
4. Conley RB, Adib G, Adler RA, et al. Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res. 2020;35(1):36-52. https://doi.org/10.1002/jbmr.3877
5. Bueno-Cavanillas A, Padilla-Ruiz F, Jiménez-Moleón JJ, Peinado-Alonso CA, Gálvez-Vargas R. Risk factors in falls among the elderly according to extrinsic and intrinsic precipitating causes. Eur J Epidemiol. 2000;16(9):849-859. https://doi.org/10.1023/a:1007636531965
6. Vannucci L, Brandi ML. Healing of the bone with anti-fracture drugs. Expert Opin Pharmacother. 2016;17(17):2267-2272. https://doi.org/10.1080/14656566.2016.1241765
7. Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315(7112):841-846. https://doi.org/10.1136/bmj.315.7112.841
8. Chen CM, Yoon YH. Usual alcohol consumption and risks for nonfatal fall injuries in the United States: results from the 2004-2013 National Health Interview Survey. Subst Use Misuse. 2017;52(9):1120-1132. https://doi.org/10.1080/10826084.2017.1293101
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Diagnosis and Management of Clostridium difficile in Adults
Clostridium difficile, now referred to as Clostridioides difficile (C. difficile), is the most commonly identified cause of healthcare-associated infection among adults in the United States.1 Because C. difficile infection results in significant mortality and inpatient costs, its persistence threatens to undermine patient safety and the value of healthcare delivery.1 A standardized, evidence-based approach to diagnosis and management is crucial. However, inconsistencies remain with regard to the appropriate threshold for testing, the type of diagnostic tests used, and treatment. Knowledge of these areas has progressed since the publication of the previous C. difficile guidelines in 2010. These guidelines contain 53 recommendations across 35 sections based on a systematic weighting of the strength of recommendation and quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation system. Herein, we have chosen to highlight five of these recommendations most relevant to hospitalists.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Patients with unexplained and new-onset ≥3 unformed stools within 24 hours are the preferred target population for testing for C. difficile infection (weak recommendation, very low quality of evidence). Do not perform repeat testing (within seven days) during the same episode of diarrhea and do not test stool from asymptomatic patients (strong recommendation, moderate quality of evidence).
In the recent past, healthcare facilities employed C. difficile tests with limited sensitivity, leading to frequent and repeat testing of hospitalized patients. Excess testing puts patients at risk for false positive results and unnecessary or prolonged treatment courses. Proper testing requires consideration of pretest probability, including analysis of the alternative causes of diarrhea. Duration of hospitalization and antibiotic exposure are the most significant modifiable risk factors for C. difficile infection in adult inpatients.2 Laxative use within the previous 48 hours, enteral tube feeding, and underlying medical conditions, such as inflammatory bowel disease (IBD), are common causes of improper testing.3 This decision may be difficult, as some underlying causes of diarrhea, such as IBD and enteral tube feeding, also increase the risk of C. difficile infection.3 Laboratories can help by rejecting specimens that are not liquid or soft and employing a multistep algorithm using a combination of nucleic acid testing, antigen testing, and toxin detection to maximize sensitivity and specificity. Because recurrent C. difficile infection is relatively common, repeat testing is appropriate only for recurrence of symptoms following successful treatment and should focus on detection of C. difficile toxin because the persistence of the organism itself can occur after successful treatment.4
Recommendation 2. Either vancomycin (125 mg orally four times per day for 10 days) or fidaxomicin (200 mg twice daily for 10 days) is recommended over metronidazole for an initial episode of nonsevere or severe C. difficile infection (strong recommendation, high quality of evidence). For fulminant C. difficile infection, the regimen of choice is a vancomycin dosage of 500 mg orally four times per day (per rectum every six hours if with ileus) in addition to intravenous metronidazole (strong recommendation, moderate quality of evidence).
For several decades now, metronidazole has been the primary antibiotic agent for initial treatment of nonsevere C. difficile infection. Two recent randomized, placebo-controlled trials, however, have found oral vancomycin to be superior to metronidazole for producing a clinical cure and resolution of diarrhea without recurrence.5,6 Oral vancomycin remains the treatment of choice for severe C. difficile infection. Fidaxomicin, a recently FDA-approved antibiotic, can also be used as initial treatment in place of oral vancomycin. One study found fidaxomicin to be superior to oral vancomycin for producing a sustained clinical response, that is, resolution of diarrhea at the end of treatment without recurrence 25 days later.7 Fulminant disease, which is characterized by hypotension or shock, ileus, or megacolon, requires a higher dose of oral vancomycin (or vancomycin enema if with ileus) in addition to intravenous metronidazole.
Recommendation 3. Treat a first recurrence of C. difficile infection with oral vancomycin as a tapered and pulsed regimen rather than a second standard 10-day course of vancomycin or metronidazole (weak recommendation, low quality of evidence).
Despite the improved treatment response with oral vancomycin, one in four patients will experience recurrence. For a first recurrence of C. difficile infection after a 10-day course of oral vancomycin, an extended taper or pulsed course of vancomycin should be attempted. Various regimens have been tried and found to be effective. For a second recurrence, providers can consider addition of rifaximin following oral vancomycin. Fecal microbiota transplantation is recommended for patients with multiple recurrences of C. difficile infection who have failed these antibiotic treatments.
Recommendation 4. Minimize the frequency and duration of high-risk antibiotic therapy (based on local epidemiology) and the number of antibiotic agents prescribed to reduce C. difficile infection risk (strong recommendation, moderate quality of evidence).
Antibiotic stewardship is a necessary component of any successful effort to reduce C. difficile infections. Antibiotic stewardship programs, which are now commonplace in US hospitals, largely rely on educational initiatives or committee-based order review. Hospitalists should take a structured approach emphasizing the four critical questions of antibiotic prescribing: Does this infection require antibiotics? Have I ordered appropriate cultures and the correct empiric therapy? Can I stop, narrow, or switch to oral agents? Finally, what duration of therapy is needed at discharge?8 Initial efforts should focus on the restriction of fluoroquinolones, clindamycin, and cephalosporins (except for surgical antibiotic prophylaxis) given their known risk to cause C. difficile infection.
Recommendation 5. Contact precautions should be maintained for at least 48 hours after diarrhea has resolved (weak recommendation, low quality of evidence).
Although C. difficile is undetectable in stool samples from most patients by the time diarrhea has resolved, skin and environmental contaminations remain high. No studies demonstrating a benefit to further extending contact precautions beyond 48 hours after resolution of diarrhea are yet available.
CRITIQUE
Methods in Preparing Guidelines
The guideline committee consisted of an interdisciplinary team of healthcare providers with extensive experience in the diagnosis, infection control, treatment, and management of C. difficile. The literature search accessed five different databases (Medline, Embase, Cochrane, Health Technology Assessment, and Database of Abstracts of Reviews and Effects), relevant journals, conference proceedings, and regulatory websites published over the search period of 2009-2016.
A major strength of these guidelines is the extensive work that went into their preparation. The committee reviewed over 14,000 pieces of literature and performed a detailed analysis of each one to determine the quality of evidence in support of each recommendation.
Sources of Potential Conflict of Interest or Bias
To reduce bias, the committee’s work was funded by Infectious Disease Society of America and Society for Healthcare Epidemiology of America. Some authors received funding for work outside of this guideline by companies that manufacture diagnostic assays, vancomycin, and fidaxomicin. These potential conflicts were listed at the end of the article.
Generalizability of the Guideline
Not all studies included in the guideline contain exclusively hospitalized patients, but much of the guideline content is applicable to hospitalized patients. Because C. difficile infection is such a widespread public health problem and these guidelines represent a significant update in knowledge since 2010, the specific recommendations highlighted in this review will impact numerous hospitalists, regardless of the practice setting.
Areas in Need of Future Study
Based on the current literature, as well as statements in the guideline, we expect future guidance around potential screening for and isolation of asymptomatic carriers, including closer guidance on stool transplantation focusing on timing and route, as further data emerge in these areas.
Other Resources
- Grading of Recommendations Assessment, Development, and Evaluation system (http://www.gradeworkinggroup.org)
- Universal Screening for C. difficile in a Tertiary Hospital: risk factors for carriage and clinical disease (Color/Blackhttps://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(19)30048-5/fulltext)
- Effectiveness of Isolating Clostridium Difficile Asymptomatic Carriers on the Incidence of Infections (Color/Blackhttps://clinicaltrials.gov/ct2/show/NCT03223415)
- Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections (Color/Blackhttps://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2516765)
- Clinical Trial Testing Fecal Microbiota Transplant for Recurrent Diarrheal Disease Begins (Color/Blackhttps://www.nih.gov/news-events/news-releases/clinical-trial-testing-fecal-microbiota-transplant-recurrent-diarrheal-disease-begins)
1. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(2):S88-S92. https://doi.org/10.1093/cid/cis335.
2. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693-703. https://doi.org/10.1056/NEJMoa1012413.
3. O’Keefe SJ. Tube feeding, the microbiota, and Clostridium difficile infection. World J Gastroenterol. 2010;16(2):139-142. https://doi.org/10.3748/wjg.v16.i2.139
4. Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381-90; quiz 391. https://doi.org/10.1038/ajg.2015.22.
5. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354. https://doi.org/10.1093/cid/ciu313.
6. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307. https://doi.org/10.1086/519265.
7. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(2):S93-S103. https://doi.org/10.1093/cid/cis499.
8. Tamma, PD, Miller MA, Cosgrove SE. Rethinking how antibiotics are prescribed: incorporating the 4 moments of antibiotic decision making into clinical practice. JAMA. 2018;321(2):139-140. https://doi.org/10.1001/jama.2018.19509.
Clostridium difficile, now referred to as Clostridioides difficile (C. difficile), is the most commonly identified cause of healthcare-associated infection among adults in the United States.1 Because C. difficile infection results in significant mortality and inpatient costs, its persistence threatens to undermine patient safety and the value of healthcare delivery.1 A standardized, evidence-based approach to diagnosis and management is crucial. However, inconsistencies remain with regard to the appropriate threshold for testing, the type of diagnostic tests used, and treatment. Knowledge of these areas has progressed since the publication of the previous C. difficile guidelines in 2010. These guidelines contain 53 recommendations across 35 sections based on a systematic weighting of the strength of recommendation and quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation system. Herein, we have chosen to highlight five of these recommendations most relevant to hospitalists.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Patients with unexplained and new-onset ≥3 unformed stools within 24 hours are the preferred target population for testing for C. difficile infection (weak recommendation, very low quality of evidence). Do not perform repeat testing (within seven days) during the same episode of diarrhea and do not test stool from asymptomatic patients (strong recommendation, moderate quality of evidence).
In the recent past, healthcare facilities employed C. difficile tests with limited sensitivity, leading to frequent and repeat testing of hospitalized patients. Excess testing puts patients at risk for false positive results and unnecessary or prolonged treatment courses. Proper testing requires consideration of pretest probability, including analysis of the alternative causes of diarrhea. Duration of hospitalization and antibiotic exposure are the most significant modifiable risk factors for C. difficile infection in adult inpatients.2 Laxative use within the previous 48 hours, enteral tube feeding, and underlying medical conditions, such as inflammatory bowel disease (IBD), are common causes of improper testing.3 This decision may be difficult, as some underlying causes of diarrhea, such as IBD and enteral tube feeding, also increase the risk of C. difficile infection.3 Laboratories can help by rejecting specimens that are not liquid or soft and employing a multistep algorithm using a combination of nucleic acid testing, antigen testing, and toxin detection to maximize sensitivity and specificity. Because recurrent C. difficile infection is relatively common, repeat testing is appropriate only for recurrence of symptoms following successful treatment and should focus on detection of C. difficile toxin because the persistence of the organism itself can occur after successful treatment.4
Recommendation 2. Either vancomycin (125 mg orally four times per day for 10 days) or fidaxomicin (200 mg twice daily for 10 days) is recommended over metronidazole for an initial episode of nonsevere or severe C. difficile infection (strong recommendation, high quality of evidence). For fulminant C. difficile infection, the regimen of choice is a vancomycin dosage of 500 mg orally four times per day (per rectum every six hours if with ileus) in addition to intravenous metronidazole (strong recommendation, moderate quality of evidence).
For several decades now, metronidazole has been the primary antibiotic agent for initial treatment of nonsevere C. difficile infection. Two recent randomized, placebo-controlled trials, however, have found oral vancomycin to be superior to metronidazole for producing a clinical cure and resolution of diarrhea without recurrence.5,6 Oral vancomycin remains the treatment of choice for severe C. difficile infection. Fidaxomicin, a recently FDA-approved antibiotic, can also be used as initial treatment in place of oral vancomycin. One study found fidaxomicin to be superior to oral vancomycin for producing a sustained clinical response, that is, resolution of diarrhea at the end of treatment without recurrence 25 days later.7 Fulminant disease, which is characterized by hypotension or shock, ileus, or megacolon, requires a higher dose of oral vancomycin (or vancomycin enema if with ileus) in addition to intravenous metronidazole.
Recommendation 3. Treat a first recurrence of C. difficile infection with oral vancomycin as a tapered and pulsed regimen rather than a second standard 10-day course of vancomycin or metronidazole (weak recommendation, low quality of evidence).
Despite the improved treatment response with oral vancomycin, one in four patients will experience recurrence. For a first recurrence of C. difficile infection after a 10-day course of oral vancomycin, an extended taper or pulsed course of vancomycin should be attempted. Various regimens have been tried and found to be effective. For a second recurrence, providers can consider addition of rifaximin following oral vancomycin. Fecal microbiota transplantation is recommended for patients with multiple recurrences of C. difficile infection who have failed these antibiotic treatments.
Recommendation 4. Minimize the frequency and duration of high-risk antibiotic therapy (based on local epidemiology) and the number of antibiotic agents prescribed to reduce C. difficile infection risk (strong recommendation, moderate quality of evidence).
Antibiotic stewardship is a necessary component of any successful effort to reduce C. difficile infections. Antibiotic stewardship programs, which are now commonplace in US hospitals, largely rely on educational initiatives or committee-based order review. Hospitalists should take a structured approach emphasizing the four critical questions of antibiotic prescribing: Does this infection require antibiotics? Have I ordered appropriate cultures and the correct empiric therapy? Can I stop, narrow, or switch to oral agents? Finally, what duration of therapy is needed at discharge?8 Initial efforts should focus on the restriction of fluoroquinolones, clindamycin, and cephalosporins (except for surgical antibiotic prophylaxis) given their known risk to cause C. difficile infection.
Recommendation 5. Contact precautions should be maintained for at least 48 hours after diarrhea has resolved (weak recommendation, low quality of evidence).
Although C. difficile is undetectable in stool samples from most patients by the time diarrhea has resolved, skin and environmental contaminations remain high. No studies demonstrating a benefit to further extending contact precautions beyond 48 hours after resolution of diarrhea are yet available.
CRITIQUE
Methods in Preparing Guidelines
The guideline committee consisted of an interdisciplinary team of healthcare providers with extensive experience in the diagnosis, infection control, treatment, and management of C. difficile. The literature search accessed five different databases (Medline, Embase, Cochrane, Health Technology Assessment, and Database of Abstracts of Reviews and Effects), relevant journals, conference proceedings, and regulatory websites published over the search period of 2009-2016.
A major strength of these guidelines is the extensive work that went into their preparation. The committee reviewed over 14,000 pieces of literature and performed a detailed analysis of each one to determine the quality of evidence in support of each recommendation.
Sources of Potential Conflict of Interest or Bias
To reduce bias, the committee’s work was funded by Infectious Disease Society of America and Society for Healthcare Epidemiology of America. Some authors received funding for work outside of this guideline by companies that manufacture diagnostic assays, vancomycin, and fidaxomicin. These potential conflicts were listed at the end of the article.
Generalizability of the Guideline
Not all studies included in the guideline contain exclusively hospitalized patients, but much of the guideline content is applicable to hospitalized patients. Because C. difficile infection is such a widespread public health problem and these guidelines represent a significant update in knowledge since 2010, the specific recommendations highlighted in this review will impact numerous hospitalists, regardless of the practice setting.
Areas in Need of Future Study
Based on the current literature, as well as statements in the guideline, we expect future guidance around potential screening for and isolation of asymptomatic carriers, including closer guidance on stool transplantation focusing on timing and route, as further data emerge in these areas.
Other Resources
- Grading of Recommendations Assessment, Development, and Evaluation system (http://www.gradeworkinggroup.org)
- Universal Screening for C. difficile in a Tertiary Hospital: risk factors for carriage and clinical disease (Color/Blackhttps://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(19)30048-5/fulltext)
- Effectiveness of Isolating Clostridium Difficile Asymptomatic Carriers on the Incidence of Infections (Color/Blackhttps://clinicaltrials.gov/ct2/show/NCT03223415)
- Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections (Color/Blackhttps://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2516765)
- Clinical Trial Testing Fecal Microbiota Transplant for Recurrent Diarrheal Disease Begins (Color/Blackhttps://www.nih.gov/news-events/news-releases/clinical-trial-testing-fecal-microbiota-transplant-recurrent-diarrheal-disease-begins)
Clostridium difficile, now referred to as Clostridioides difficile (C. difficile), is the most commonly identified cause of healthcare-associated infection among adults in the United States.1 Because C. difficile infection results in significant mortality and inpatient costs, its persistence threatens to undermine patient safety and the value of healthcare delivery.1 A standardized, evidence-based approach to diagnosis and management is crucial. However, inconsistencies remain with regard to the appropriate threshold for testing, the type of diagnostic tests used, and treatment. Knowledge of these areas has progressed since the publication of the previous C. difficile guidelines in 2010. These guidelines contain 53 recommendations across 35 sections based on a systematic weighting of the strength of recommendation and quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation system. Herein, we have chosen to highlight five of these recommendations most relevant to hospitalists.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Patients with unexplained and new-onset ≥3 unformed stools within 24 hours are the preferred target population for testing for C. difficile infection (weak recommendation, very low quality of evidence). Do not perform repeat testing (within seven days) during the same episode of diarrhea and do not test stool from asymptomatic patients (strong recommendation, moderate quality of evidence).
In the recent past, healthcare facilities employed C. difficile tests with limited sensitivity, leading to frequent and repeat testing of hospitalized patients. Excess testing puts patients at risk for false positive results and unnecessary or prolonged treatment courses. Proper testing requires consideration of pretest probability, including analysis of the alternative causes of diarrhea. Duration of hospitalization and antibiotic exposure are the most significant modifiable risk factors for C. difficile infection in adult inpatients.2 Laxative use within the previous 48 hours, enteral tube feeding, and underlying medical conditions, such as inflammatory bowel disease (IBD), are common causes of improper testing.3 This decision may be difficult, as some underlying causes of diarrhea, such as IBD and enteral tube feeding, also increase the risk of C. difficile infection.3 Laboratories can help by rejecting specimens that are not liquid or soft and employing a multistep algorithm using a combination of nucleic acid testing, antigen testing, and toxin detection to maximize sensitivity and specificity. Because recurrent C. difficile infection is relatively common, repeat testing is appropriate only for recurrence of symptoms following successful treatment and should focus on detection of C. difficile toxin because the persistence of the organism itself can occur after successful treatment.4
Recommendation 2. Either vancomycin (125 mg orally four times per day for 10 days) or fidaxomicin (200 mg twice daily for 10 days) is recommended over metronidazole for an initial episode of nonsevere or severe C. difficile infection (strong recommendation, high quality of evidence). For fulminant C. difficile infection, the regimen of choice is a vancomycin dosage of 500 mg orally four times per day (per rectum every six hours if with ileus) in addition to intravenous metronidazole (strong recommendation, moderate quality of evidence).
For several decades now, metronidazole has been the primary antibiotic agent for initial treatment of nonsevere C. difficile infection. Two recent randomized, placebo-controlled trials, however, have found oral vancomycin to be superior to metronidazole for producing a clinical cure and resolution of diarrhea without recurrence.5,6 Oral vancomycin remains the treatment of choice for severe C. difficile infection. Fidaxomicin, a recently FDA-approved antibiotic, can also be used as initial treatment in place of oral vancomycin. One study found fidaxomicin to be superior to oral vancomycin for producing a sustained clinical response, that is, resolution of diarrhea at the end of treatment without recurrence 25 days later.7 Fulminant disease, which is characterized by hypotension or shock, ileus, or megacolon, requires a higher dose of oral vancomycin (or vancomycin enema if with ileus) in addition to intravenous metronidazole.
Recommendation 3. Treat a first recurrence of C. difficile infection with oral vancomycin as a tapered and pulsed regimen rather than a second standard 10-day course of vancomycin or metronidazole (weak recommendation, low quality of evidence).
Despite the improved treatment response with oral vancomycin, one in four patients will experience recurrence. For a first recurrence of C. difficile infection after a 10-day course of oral vancomycin, an extended taper or pulsed course of vancomycin should be attempted. Various regimens have been tried and found to be effective. For a second recurrence, providers can consider addition of rifaximin following oral vancomycin. Fecal microbiota transplantation is recommended for patients with multiple recurrences of C. difficile infection who have failed these antibiotic treatments.
Recommendation 4. Minimize the frequency and duration of high-risk antibiotic therapy (based on local epidemiology) and the number of antibiotic agents prescribed to reduce C. difficile infection risk (strong recommendation, moderate quality of evidence).
Antibiotic stewardship is a necessary component of any successful effort to reduce C. difficile infections. Antibiotic stewardship programs, which are now commonplace in US hospitals, largely rely on educational initiatives or committee-based order review. Hospitalists should take a structured approach emphasizing the four critical questions of antibiotic prescribing: Does this infection require antibiotics? Have I ordered appropriate cultures and the correct empiric therapy? Can I stop, narrow, or switch to oral agents? Finally, what duration of therapy is needed at discharge?8 Initial efforts should focus on the restriction of fluoroquinolones, clindamycin, and cephalosporins (except for surgical antibiotic prophylaxis) given their known risk to cause C. difficile infection.
Recommendation 5. Contact precautions should be maintained for at least 48 hours after diarrhea has resolved (weak recommendation, low quality of evidence).
Although C. difficile is undetectable in stool samples from most patients by the time diarrhea has resolved, skin and environmental contaminations remain high. No studies demonstrating a benefit to further extending contact precautions beyond 48 hours after resolution of diarrhea are yet available.
CRITIQUE
Methods in Preparing Guidelines
The guideline committee consisted of an interdisciplinary team of healthcare providers with extensive experience in the diagnosis, infection control, treatment, and management of C. difficile. The literature search accessed five different databases (Medline, Embase, Cochrane, Health Technology Assessment, and Database of Abstracts of Reviews and Effects), relevant journals, conference proceedings, and regulatory websites published over the search period of 2009-2016.
A major strength of these guidelines is the extensive work that went into their preparation. The committee reviewed over 14,000 pieces of literature and performed a detailed analysis of each one to determine the quality of evidence in support of each recommendation.
Sources of Potential Conflict of Interest or Bias
To reduce bias, the committee’s work was funded by Infectious Disease Society of America and Society for Healthcare Epidemiology of America. Some authors received funding for work outside of this guideline by companies that manufacture diagnostic assays, vancomycin, and fidaxomicin. These potential conflicts were listed at the end of the article.
Generalizability of the Guideline
Not all studies included in the guideline contain exclusively hospitalized patients, but much of the guideline content is applicable to hospitalized patients. Because C. difficile infection is such a widespread public health problem and these guidelines represent a significant update in knowledge since 2010, the specific recommendations highlighted in this review will impact numerous hospitalists, regardless of the practice setting.
Areas in Need of Future Study
Based on the current literature, as well as statements in the guideline, we expect future guidance around potential screening for and isolation of asymptomatic carriers, including closer guidance on stool transplantation focusing on timing and route, as further data emerge in these areas.
Other Resources
- Grading of Recommendations Assessment, Development, and Evaluation system (http://www.gradeworkinggroup.org)
- Universal Screening for C. difficile in a Tertiary Hospital: risk factors for carriage and clinical disease (Color/Blackhttps://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(19)30048-5/fulltext)
- Effectiveness of Isolating Clostridium Difficile Asymptomatic Carriers on the Incidence of Infections (Color/Blackhttps://clinicaltrials.gov/ct2/show/NCT03223415)
- Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections (Color/Blackhttps://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2516765)
- Clinical Trial Testing Fecal Microbiota Transplant for Recurrent Diarrheal Disease Begins (Color/Blackhttps://www.nih.gov/news-events/news-releases/clinical-trial-testing-fecal-microbiota-transplant-recurrent-diarrheal-disease-begins)
1. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(2):S88-S92. https://doi.org/10.1093/cid/cis335.
2. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693-703. https://doi.org/10.1056/NEJMoa1012413.
3. O’Keefe SJ. Tube feeding, the microbiota, and Clostridium difficile infection. World J Gastroenterol. 2010;16(2):139-142. https://doi.org/10.3748/wjg.v16.i2.139
4. Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381-90; quiz 391. https://doi.org/10.1038/ajg.2015.22.
5. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354. https://doi.org/10.1093/cid/ciu313.
6. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307. https://doi.org/10.1086/519265.
7. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(2):S93-S103. https://doi.org/10.1093/cid/cis499.
8. Tamma, PD, Miller MA, Cosgrove SE. Rethinking how antibiotics are prescribed: incorporating the 4 moments of antibiotic decision making into clinical practice. JAMA. 2018;321(2):139-140. https://doi.org/10.1001/jama.2018.19509.
1. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(2):S88-S92. https://doi.org/10.1093/cid/cis335.
2. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693-703. https://doi.org/10.1056/NEJMoa1012413.
3. O’Keefe SJ. Tube feeding, the microbiota, and Clostridium difficile infection. World J Gastroenterol. 2010;16(2):139-142. https://doi.org/10.3748/wjg.v16.i2.139
4. Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381-90; quiz 391. https://doi.org/10.1038/ajg.2015.22.
5. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354. https://doi.org/10.1093/cid/ciu313.
6. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307. https://doi.org/10.1086/519265.
7. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(2):S93-S103. https://doi.org/10.1093/cid/cis499.
8. Tamma, PD, Miller MA, Cosgrove SE. Rethinking how antibiotics are prescribed: incorporating the 4 moments of antibiotic decision making into clinical practice. JAMA. 2018;321(2):139-140. https://doi.org/10.1001/jama.2018.19509.
© 2019 Society of Hospital Medicine