User login
Technology offers tools for ensuring adherence to medical therapy
Nonadherence to medical therapy is a widespread and complex problem that is a significant variable in the treatment of psychiatric illness and in patients’ prognosis. More than 50% of people who have a chronic illness struggle to comply with their medication regimen—for many reasons.1
Many variables predict poor adherence, so it cannot be expected that a single solution will solve the problem entirely.2 Novel adherence technologies are available, as we discuss in this article, and more are in development.
What is nonadherence to medical therapy?
Nonadherence can be defined primarily as not taking prescribed medication in the recommended dosage or frequency, or not taking prescribed medication at all.3 Nonadherence can result in an increased risk of relapse, hospitalization, poor therapeutic response, and delayed remission and recovery.
Secondarily, non-attendance or irregular attendance at appointments with providers is a form of nonadherence that can have a negative impact on treatment outcomes.4
Why is medical adherence important in psychiatry?
Medication nonadherence has major consequences for psychiatric patients5 and for the greater health care system; it is estimated that, in the United States, the cost of nonadherence is as high as $300 billion a year.6 In psychiatry, the rate of nonadherence to medical therapy has been reported to be 11% to 80% of patients with schizophrenia; 12% to 64% with bipolar disorders; and 30% to 60% with depression.7-9 These surprising statistics make it imperative to design treatment strategies that include an effective patient-centric medication adherence plan, based on diagnosis, patient need, education, and support.
Why are patients nonadherent?
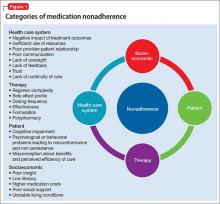
Many variables lead to patient nonadherence (Figure 1). The most common reason is that patients simply forget to take their medication.10 Among psychiatric patients, other reasons are:
- lack of insight
- negative emotional reaction to taking medication11
- feeling better and no longer believing that the medication is needed12,13
- distress associated with side effects14,15
- high cost of medication15
- patient’s perception that medication won’t be effective16,17
- concern about substance abuse18
- fear of dependency19
- complicated dosing regimen20
- general lack of motivation.21
Emotional barriers to medication nonadherence are an underestimated area that can benefit greatly from the expertise and understanding of psychiatrists. These barriers include a sense of losing control, self-stigmatization, denial, poor insight, and beliefs about illness and medications.
Additional patient variables that contribute to nonadherence include:
- suboptimal health literacy
- stigma and shame about the need for psychiatric treatment
- lack of patient involvement in treatment decision-making.
Who is responsible for adherence?
Adherence to medical therapy is not the patient’s responsibility, exclusively. Rather, it is a collection of complex components that generally includes physicians and the health care system. Because barriers to medication adherence are complex and varied, solutions to improve adherence must be multifaceted.
Providers. Patients’ care often is managed by multiple physicians, which can lead to communication lapses about complicated drug regimens and potential adverse effects. To assist patients in adhering to their medication regimen, physicians should recognize, and acknowledge to the patient, that many psychiatric patients have difficulty taking their medications and provide advice and information in how to address this problem.
Families. Likewise, it is important to educate patients and their family about the need for medication—helping the patient see that it is his (her) choice and, indeed, his direct responsibility to take his medication and improve his health. The risk–benefit balance of treatment should be explained to the patient and his family, as well as the nature of the psychiatric diagnosis and how effective patient–physician collaboration can help him function and adhere to his medication regimen in a consistent, reliable manner.
The larger system. Health care systems can contribute to medication adherence by reducing time constraints on visits to providers, to allow time to discuss all aspects of medication adherence. Limited visits in the clinic means physicians are not able to (1) spend adequate time discussing the medication regimen to ensure full patient comprehension and (2) conduct an assessment of medication-taking behaviors. Team-based approaches could improve efficiency, patient understanding, adherence, and early detection of adherence issues.22,23
Strategies such as additional clinic visits and reminder calls to discuss adherence carry a cost, but their long-term advantage is that, if patients understand how to better adhere to their medication regimens, their actions will have a positive impact on their health care costs and outcomes and on the wider health economy—as a result of reduced hospital admissions and reduced need to care for patients whose condition deteriorates because of nonadherence. It is imperative that we build strong relationships with other providers to show that we are committed to building supportive, effective adherence support programs that focus on the individual patient’s needs.
What is the available technology?
There is no standard way to measure nonadherence. The most common, and simplest, measure—asking the patient—is unreliable and severely overestimates adherence.
Direct measures of adherence include observing the patient taking his medications and testing for the concentration of those medications in blood or urine. Indirect adherence assessment methods, such as pill counts, a medication diary, self-report, clinician ratings, pharmacy chart review, and electronic devices that monitor the opening of a lid or tablet strip, have all been used; yet reviews of those methods have shown less than favorable results.6
Pre-packaged pill packs have helped some patients with a simple method for medication management.
Electronic monitoring, using a medication vial cap device (Figure 2) that electronically records the date and time of bottle opening, has become common in general medicine and among patients with schizophrenia.6,13,24-26 Diaz et al24 reported that electronic monitoring detected a greater nonadherence rate (57%) than what prescribers reported (7%) or patients self-reported (5%)—demonstrating that prescribers and patients grossly overestimate adherence. In another study that looked at electronic monitoring, researchers reported that adherence was much higher in depressed youth (87%)27 than what had been seen in adults (67%) in a similar study.13
The downside to pill packs and electronic monitoring? There is no guarantee the patient has actually taken the medication despite the data reported by the system.
Event marker-signaling devices. Novel technologies have been developed to measure adherence:
Proteus Digital Health feedback system (www.proteus.com) requires that patients ingest a tablet containing a tiny, dietary mineral-based “ingestible event marker.” Upon contact with gastric fluid electrolytes, the event marker emits a unique signal that is transmitted through bodily tissue to a small receiver in a patch worn on the torso. The receiver then transmits a signal to a cellular phone, indicating the time and date when the medication was ingested (Figure 3).
A 4-week pilot study28 found that the ingestible event marker is feasible and acceptable to patients: 27 of 28 participants (96%) completed the study, with a mean adherence rate of 74%. Although the system identifies ingestible sensors with high accuracy and is easily tolerated by patients, the pilot study was brief; a longer duration of adherence while wearing the patch needs to be studied.
Breath analysis, facial recognition. Even directly observing ingestion of a medication can be problematic: Some patients don’t swallow the medication and spit it out later. One way around that subterfuge is to consider using other advanced medication adherence solutions that are breath-based or use facial recognition technology and confirm ingestion.
Xhale SMART (www.xhale.com/smart) is a handheld device that generates a reminder to the patient to take his medication; afterward, he (she) must blow into the device so that ingestion of the medication is detected (Figure 4). The medication has breath-detectable adherence markers already incorporated. The adherence marker then is released into the stomach and small intestine, where the adherence marker metabolite is transported through the bloodstream into the lungs and exhaled. The patient must breathe into a breath analysis device, which measures medication ingestion compared with a baseline breath print.
Several articles in the literature have reported the accuracy of this device in detecting the ingested metabolite in every participant, without adverse effects.29,30 Clinical data on the use of the breath-based detector is not available to the public at this time.
AiCure (www.aicure.com) is a facial recognition-based technology platform that can work through any smartphone. The device is powered by artificial intelligence software and motion-sensing technology that can detect, in real time, whether the patient is taking the medication as prescribed. Patients who take an incorrect dose, or who do not use the software, are automatically flagged for immediate follow-up. This technology enables real-time intervention by a provider with the nonadherent patient.
An important note: These innovative technological advances are tools that can help clinicians manage an important aspect of treatment, but they do not show the entire picture: The physician−patient relationship and the therapeutic alliance are key to optimal treatment adherence.
Engage and empower the patient
Novel adherence technologies are, as we’ve described, available, and more are being developed. Incorporating these technologies into clinical care requires continued input and support from clinicians and patients. Digital and mobile health applications are multi-beneficial: They can empower patients to self-manage medication regimens and appointments while they also receive social and psychological information and support as needed. Understanding one’s own illness can, ultimately, improve outcomes and significantly reduce health care costs.
Patient empowerment is key. The physician is an important influencer in this regard.
The role of the physician must not be undervalued in maintaining adherence to therapy; she (he) plays a vital role in continued patient engagement and behavioral training. Integrating physician-led oversight, patient education, and commitment, and novel digital mobile adherence technologies will help deliver better outcomes.
The push to engage. A “one size fits all” approach to maintaining adherence won’t be effective. We need to better understand the individual patient’s underlying cause(s) for nonadherence, then to tailor a solution to influence and change that behavior. One way to do this is by interacting and engaging more directly (and in a digital manner) with patients to monitor adherence.
A recent example of the move toward direct patient engagement is the agreement entered by Otsuka Pharmaceuticals and Proteus Digital Health to develop novel digital health products. The FDA has accepted for review the combination product of Otsuka’s brand of aripiprazole and Proteus’s ingestible sensor. If the product is approved by the FDA, physicians will be able to prescribe aripiprazole with the ingestible sensor embedded in the tablet and then measure medication adherence and other patient physiologic metrics (eg, activity, rest) through the wearable sensor patch and medical software application designed specifically for patient and physician use.
This technology could have huge potential in mental health care, where patients struggle with both adhering to their medication regimen and communicating with the health care team. Physicians could measure adherence when treating adults with schizophrenia, bipolar disorders, and major depressive disorder; flag those who are not adhering as having higher risk of disease progression and poorer outcome; and allow decisions to be made more quickly based on treatment need.
Developing and enhancing these collaborative and patient-centric approaches will increase self-monitoring and patient responsibility, and encourage behavior change.
‘All-in’ strategy. By continuing to use the latest technologies and connecting them to the range of stakeholders—physicians, nurses, pharmacists, payers—we will develop an all-inclusive adherence intervention strategy. All patients will be integrated, and all of them, and their family, will be provided with positive psychoeducational care and motivational counseling (Figure 5). In addition, such a support-based patient experience must be aligned with the work of clinical care providers. Compliance therapy and behavioral training, together with active patient engagement, can help improve insight, acceptance of treatment, and, over the long term, adherence.31,32
1. World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003.
2. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
3. Crowe M, Wilson L, Inder M. Patients’ reports of the factors influencing medication adherence in bipolar disorder – an integrative review of the literature. Int J Nurs Stud. 2011;48(7):894-903.
4. Mert D, Turgut NH, Kelleci M, et al. Perspectives on reasons of medication nonadherence in psychiatric patients. Patient Prefer Adherence. 2015;9:87-93.
5. Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry. 2013;26(5):446-452.
6. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-497.
7. Thompson L, McCabe R. The effect of clinician-patient alliance and communication on treatment adherence in mental health care: a systematic review. BMC Psychiatry. 2012;12:87.
8. Yilmaz S, Buzlu S. Antipsikotik kullanan hastalarda ilaç yan etkileri ve ilaç uyumu. Florence Nightingale Hem˘girelik Dergisi. 2012;20(2):93-103.
9. Kelleci M, Ata EE. Psikiyatri Klini˘ginde yatan hastaların ilaç uyumları ve sosyal destekle iliskisi. [Drug compliance of patients hospitalized in the psychiatry clinic and the relationship with social support]. Psikiyatri Hemsireli˘gi Dergisi. 2011;2(suppl 3):105-110.
10. Bulloch AG, Patten SB. Non-adherence with psychotropic medications in the general population. Soc Psychiatry Psychiatr Epidemiol. 2010;45(1):47-56.
11. Rosenbaum L. Beyond belief—how people feel about taking medications for heart disease. N Engl J Med. 2015;372(2):183-187.
12. Cramer J, Rosenheck R, Kirk G, et al. Medication compliance feedback and monitoring in a clinical trial: predictions and outcomes. Value Health. 2003;6(5):566-573.
13. Nakonezny PA, Byerly MJ, Rush AJ. Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry Res. 2008;157(1-3):259-263.
14. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
15. Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14(6):553-560.
16. Hoencamp E, Stevens A, Haffmans J. Patients’ attitudes toward antidepressants. Psychiatr Serv. 2002;53(9):1180-1181.
17. Keller MB, Hirschfeld RM, Demyttenaere K, et al. Optimizing outcomes in depression: focus on antidepressant compliance. Int Clin Psychopharmacol. 2002;17(6):265-271.
18. Akerblad AC, Bengtsson F, Holgersson M, et al. Identification of primary care patients at risk of nonadherence to antidepressant treatment. Patient Prefer Adherence. 2008;2:376-386.
19. Brown C, Battista DR, Bruehlman R, et al. Beliefs about antidepressant medications in primary care patients: relationship to self-reported adherence. Med Care. 2005;43(12):1203-1207.
20. Demyttenaere K, Adelin A, Patrick M, et al. Six-month compliance with antidepressant medication in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2008;23(1):36-42.
21. Massand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25(8):2289-2304.
22. Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Pub L No. 108-173, 117 Stat 2066.
23. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304-314.
24. Diaz E, Neuse E, Sullivan MC, et al. Adherence to conventional and atypical antipsychotics after hospital discharge. J Clin Psychiatry. 2004;65(3):354-360.
25. Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2-3):129-133.
26. Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30(3):437-452.
27. Nakonezny PA, Hughes CW, Mayes TL, et al. A comparison of various methods of measuring antidepressant medication adherence among children and adolescents with major depressive disorder in a 12-week open trial of fluoxetine. J Child Adolesc Psychopharmacol. 2010;20(5):431-439.
28. Kane JM, Perlis RH, DiCarlo LA, et al. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74(6):e533-e540. doi: 10.4088/JCP.12m08222.
29. Morey TE, Booth MM, Prather RA, et al. Measurement of ethanol in gaseous breath using a miniature gas chromatograph. J Anal Toxicol. 2011;35(3):134-142.
30. Morey TE, Booth M, Wasdo S, et al. Oral adherence monitoring using a breath test to supplement highly active antiretroviral therapy. AIDS Behav. 2013;17(1):298-306.
31. Torem MS. Participatory pharmacotherapy: 10 strategies for enhancing adherence. Current Psychiatry. 2013;12(7):21-25.
32. Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159(10):1653-1664.
Nonadherence to medical therapy is a widespread and complex problem that is a significant variable in the treatment of psychiatric illness and in patients’ prognosis. More than 50% of people who have a chronic illness struggle to comply with their medication regimen—for many reasons.1
Many variables predict poor adherence, so it cannot be expected that a single solution will solve the problem entirely.2 Novel adherence technologies are available, as we discuss in this article, and more are in development.
What is nonadherence to medical therapy?
Nonadherence can be defined primarily as not taking prescribed medication in the recommended dosage or frequency, or not taking prescribed medication at all.3 Nonadherence can result in an increased risk of relapse, hospitalization, poor therapeutic response, and delayed remission and recovery.
Secondarily, non-attendance or irregular attendance at appointments with providers is a form of nonadherence that can have a negative impact on treatment outcomes.4
Why is medical adherence important in psychiatry?
Medication nonadherence has major consequences for psychiatric patients5 and for the greater health care system; it is estimated that, in the United States, the cost of nonadherence is as high as $300 billion a year.6 In psychiatry, the rate of nonadherence to medical therapy has been reported to be 11% to 80% of patients with schizophrenia; 12% to 64% with bipolar disorders; and 30% to 60% with depression.7-9 These surprising statistics make it imperative to design treatment strategies that include an effective patient-centric medication adherence plan, based on diagnosis, patient need, education, and support.
Why are patients nonadherent?
Many variables lead to patient nonadherence (Figure 1). The most common reason is that patients simply forget to take their medication.10 Among psychiatric patients, other reasons are:
- lack of insight
- negative emotional reaction to taking medication11
- feeling better and no longer believing that the medication is needed12,13
- distress associated with side effects14,15
- high cost of medication15
- patient’s perception that medication won’t be effective16,17
- concern about substance abuse18
- fear of dependency19
- complicated dosing regimen20
- general lack of motivation.21
Emotional barriers to medication nonadherence are an underestimated area that can benefit greatly from the expertise and understanding of psychiatrists. These barriers include a sense of losing control, self-stigmatization, denial, poor insight, and beliefs about illness and medications.
Additional patient variables that contribute to nonadherence include:
- suboptimal health literacy
- stigma and shame about the need for psychiatric treatment
- lack of patient involvement in treatment decision-making.
Who is responsible for adherence?
Adherence to medical therapy is not the patient’s responsibility, exclusively. Rather, it is a collection of complex components that generally includes physicians and the health care system. Because barriers to medication adherence are complex and varied, solutions to improve adherence must be multifaceted.
Providers. Patients’ care often is managed by multiple physicians, which can lead to communication lapses about complicated drug regimens and potential adverse effects. To assist patients in adhering to their medication regimen, physicians should recognize, and acknowledge to the patient, that many psychiatric patients have difficulty taking their medications and provide advice and information in how to address this problem.
Families. Likewise, it is important to educate patients and their family about the need for medication—helping the patient see that it is his (her) choice and, indeed, his direct responsibility to take his medication and improve his health. The risk–benefit balance of treatment should be explained to the patient and his family, as well as the nature of the psychiatric diagnosis and how effective patient–physician collaboration can help him function and adhere to his medication regimen in a consistent, reliable manner.
The larger system. Health care systems can contribute to medication adherence by reducing time constraints on visits to providers, to allow time to discuss all aspects of medication adherence. Limited visits in the clinic means physicians are not able to (1) spend adequate time discussing the medication regimen to ensure full patient comprehension and (2) conduct an assessment of medication-taking behaviors. Team-based approaches could improve efficiency, patient understanding, adherence, and early detection of adherence issues.22,23
Strategies such as additional clinic visits and reminder calls to discuss adherence carry a cost, but their long-term advantage is that, if patients understand how to better adhere to their medication regimens, their actions will have a positive impact on their health care costs and outcomes and on the wider health economy—as a result of reduced hospital admissions and reduced need to care for patients whose condition deteriorates because of nonadherence. It is imperative that we build strong relationships with other providers to show that we are committed to building supportive, effective adherence support programs that focus on the individual patient’s needs.
What is the available technology?
There is no standard way to measure nonadherence. The most common, and simplest, measure—asking the patient—is unreliable and severely overestimates adherence.
Direct measures of adherence include observing the patient taking his medications and testing for the concentration of those medications in blood or urine. Indirect adherence assessment methods, such as pill counts, a medication diary, self-report, clinician ratings, pharmacy chart review, and electronic devices that monitor the opening of a lid or tablet strip, have all been used; yet reviews of those methods have shown less than favorable results.6
Pre-packaged pill packs have helped some patients with a simple method for medication management.
Electronic monitoring, using a medication vial cap device (Figure 2) that electronically records the date and time of bottle opening, has become common in general medicine and among patients with schizophrenia.6,13,24-26 Diaz et al24 reported that electronic monitoring detected a greater nonadherence rate (57%) than what prescribers reported (7%) or patients self-reported (5%)—demonstrating that prescribers and patients grossly overestimate adherence. In another study that looked at electronic monitoring, researchers reported that adherence was much higher in depressed youth (87%)27 than what had been seen in adults (67%) in a similar study.13
The downside to pill packs and electronic monitoring? There is no guarantee the patient has actually taken the medication despite the data reported by the system.
Event marker-signaling devices. Novel technologies have been developed to measure adherence:
Proteus Digital Health feedback system (www.proteus.com) requires that patients ingest a tablet containing a tiny, dietary mineral-based “ingestible event marker.” Upon contact with gastric fluid electrolytes, the event marker emits a unique signal that is transmitted through bodily tissue to a small receiver in a patch worn on the torso. The receiver then transmits a signal to a cellular phone, indicating the time and date when the medication was ingested (Figure 3).
A 4-week pilot study28 found that the ingestible event marker is feasible and acceptable to patients: 27 of 28 participants (96%) completed the study, with a mean adherence rate of 74%. Although the system identifies ingestible sensors with high accuracy and is easily tolerated by patients, the pilot study was brief; a longer duration of adherence while wearing the patch needs to be studied.
Breath analysis, facial recognition. Even directly observing ingestion of a medication can be problematic: Some patients don’t swallow the medication and spit it out later. One way around that subterfuge is to consider using other advanced medication adherence solutions that are breath-based or use facial recognition technology and confirm ingestion.
Xhale SMART (www.xhale.com/smart) is a handheld device that generates a reminder to the patient to take his medication; afterward, he (she) must blow into the device so that ingestion of the medication is detected (Figure 4). The medication has breath-detectable adherence markers already incorporated. The adherence marker then is released into the stomach and small intestine, where the adherence marker metabolite is transported through the bloodstream into the lungs and exhaled. The patient must breathe into a breath analysis device, which measures medication ingestion compared with a baseline breath print.
Several articles in the literature have reported the accuracy of this device in detecting the ingested metabolite in every participant, without adverse effects.29,30 Clinical data on the use of the breath-based detector is not available to the public at this time.
AiCure (www.aicure.com) is a facial recognition-based technology platform that can work through any smartphone. The device is powered by artificial intelligence software and motion-sensing technology that can detect, in real time, whether the patient is taking the medication as prescribed. Patients who take an incorrect dose, or who do not use the software, are automatically flagged for immediate follow-up. This technology enables real-time intervention by a provider with the nonadherent patient.
An important note: These innovative technological advances are tools that can help clinicians manage an important aspect of treatment, but they do not show the entire picture: The physician−patient relationship and the therapeutic alliance are key to optimal treatment adherence.
Engage and empower the patient
Novel adherence technologies are, as we’ve described, available, and more are being developed. Incorporating these technologies into clinical care requires continued input and support from clinicians and patients. Digital and mobile health applications are multi-beneficial: They can empower patients to self-manage medication regimens and appointments while they also receive social and psychological information and support as needed. Understanding one’s own illness can, ultimately, improve outcomes and significantly reduce health care costs.
Patient empowerment is key. The physician is an important influencer in this regard.
The role of the physician must not be undervalued in maintaining adherence to therapy; she (he) plays a vital role in continued patient engagement and behavioral training. Integrating physician-led oversight, patient education, and commitment, and novel digital mobile adherence technologies will help deliver better outcomes.
The push to engage. A “one size fits all” approach to maintaining adherence won’t be effective. We need to better understand the individual patient’s underlying cause(s) for nonadherence, then to tailor a solution to influence and change that behavior. One way to do this is by interacting and engaging more directly (and in a digital manner) with patients to monitor adherence.
A recent example of the move toward direct patient engagement is the agreement entered by Otsuka Pharmaceuticals and Proteus Digital Health to develop novel digital health products. The FDA has accepted for review the combination product of Otsuka’s brand of aripiprazole and Proteus’s ingestible sensor. If the product is approved by the FDA, physicians will be able to prescribe aripiprazole with the ingestible sensor embedded in the tablet and then measure medication adherence and other patient physiologic metrics (eg, activity, rest) through the wearable sensor patch and medical software application designed specifically for patient and physician use.
This technology could have huge potential in mental health care, where patients struggle with both adhering to their medication regimen and communicating with the health care team. Physicians could measure adherence when treating adults with schizophrenia, bipolar disorders, and major depressive disorder; flag those who are not adhering as having higher risk of disease progression and poorer outcome; and allow decisions to be made more quickly based on treatment need.
Developing and enhancing these collaborative and patient-centric approaches will increase self-monitoring and patient responsibility, and encourage behavior change.
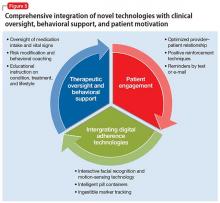
‘All-in’ strategy. By continuing to use the latest technologies and connecting them to the range of stakeholders—physicians, nurses, pharmacists, payers—we will develop an all-inclusive adherence intervention strategy. All patients will be integrated, and all of them, and their family, will be provided with positive psychoeducational care and motivational counseling (Figure 5). In addition, such a support-based patient experience must be aligned with the work of clinical care providers. Compliance therapy and behavioral training, together with active patient engagement, can help improve insight, acceptance of treatment, and, over the long term, adherence.31,32
Nonadherence to medical therapy is a widespread and complex problem that is a significant variable in the treatment of psychiatric illness and in patients’ prognosis. More than 50% of people who have a chronic illness struggle to comply with their medication regimen—for many reasons.1
Many variables predict poor adherence, so it cannot be expected that a single solution will solve the problem entirely.2 Novel adherence technologies are available, as we discuss in this article, and more are in development.
What is nonadherence to medical therapy?
Nonadherence can be defined primarily as not taking prescribed medication in the recommended dosage or frequency, or not taking prescribed medication at all.3 Nonadherence can result in an increased risk of relapse, hospitalization, poor therapeutic response, and delayed remission and recovery.
Secondarily, non-attendance or irregular attendance at appointments with providers is a form of nonadherence that can have a negative impact on treatment outcomes.4
Why is medical adherence important in psychiatry?
Medication nonadherence has major consequences for psychiatric patients5 and for the greater health care system; it is estimated that, in the United States, the cost of nonadherence is as high as $300 billion a year.6 In psychiatry, the rate of nonadherence to medical therapy has been reported to be 11% to 80% of patients with schizophrenia; 12% to 64% with bipolar disorders; and 30% to 60% with depression.7-9 These surprising statistics make it imperative to design treatment strategies that include an effective patient-centric medication adherence plan, based on diagnosis, patient need, education, and support.
Why are patients nonadherent?
Many variables lead to patient nonadherence (Figure 1). The most common reason is that patients simply forget to take their medication.10 Among psychiatric patients, other reasons are:
- lack of insight
- negative emotional reaction to taking medication11
- feeling better and no longer believing that the medication is needed12,13
- distress associated with side effects14,15
- high cost of medication15
- patient’s perception that medication won’t be effective16,17
- concern about substance abuse18
- fear of dependency19
- complicated dosing regimen20
- general lack of motivation.21
Emotional barriers to medication nonadherence are an underestimated area that can benefit greatly from the expertise and understanding of psychiatrists. These barriers include a sense of losing control, self-stigmatization, denial, poor insight, and beliefs about illness and medications.
Additional patient variables that contribute to nonadherence include:
- suboptimal health literacy
- stigma and shame about the need for psychiatric treatment
- lack of patient involvement in treatment decision-making.
Who is responsible for adherence?
Adherence to medical therapy is not the patient’s responsibility, exclusively. Rather, it is a collection of complex components that generally includes physicians and the health care system. Because barriers to medication adherence are complex and varied, solutions to improve adherence must be multifaceted.
Providers. Patients’ care often is managed by multiple physicians, which can lead to communication lapses about complicated drug regimens and potential adverse effects. To assist patients in adhering to their medication regimen, physicians should recognize, and acknowledge to the patient, that many psychiatric patients have difficulty taking their medications and provide advice and information in how to address this problem.
Families. Likewise, it is important to educate patients and their family about the need for medication—helping the patient see that it is his (her) choice and, indeed, his direct responsibility to take his medication and improve his health. The risk–benefit balance of treatment should be explained to the patient and his family, as well as the nature of the psychiatric diagnosis and how effective patient–physician collaboration can help him function and adhere to his medication regimen in a consistent, reliable manner.
The larger system. Health care systems can contribute to medication adherence by reducing time constraints on visits to providers, to allow time to discuss all aspects of medication adherence. Limited visits in the clinic means physicians are not able to (1) spend adequate time discussing the medication regimen to ensure full patient comprehension and (2) conduct an assessment of medication-taking behaviors. Team-based approaches could improve efficiency, patient understanding, adherence, and early detection of adherence issues.22,23
Strategies such as additional clinic visits and reminder calls to discuss adherence carry a cost, but their long-term advantage is that, if patients understand how to better adhere to their medication regimens, their actions will have a positive impact on their health care costs and outcomes and on the wider health economy—as a result of reduced hospital admissions and reduced need to care for patients whose condition deteriorates because of nonadherence. It is imperative that we build strong relationships with other providers to show that we are committed to building supportive, effective adherence support programs that focus on the individual patient’s needs.
What is the available technology?
There is no standard way to measure nonadherence. The most common, and simplest, measure—asking the patient—is unreliable and severely overestimates adherence.
Direct measures of adherence include observing the patient taking his medications and testing for the concentration of those medications in blood or urine. Indirect adherence assessment methods, such as pill counts, a medication diary, self-report, clinician ratings, pharmacy chart review, and electronic devices that monitor the opening of a lid or tablet strip, have all been used; yet reviews of those methods have shown less than favorable results.6
Pre-packaged pill packs have helped some patients with a simple method for medication management.
Electronic monitoring, using a medication vial cap device (Figure 2) that electronically records the date and time of bottle opening, has become common in general medicine and among patients with schizophrenia.6,13,24-26 Diaz et al24 reported that electronic monitoring detected a greater nonadherence rate (57%) than what prescribers reported (7%) or patients self-reported (5%)—demonstrating that prescribers and patients grossly overestimate adherence. In another study that looked at electronic monitoring, researchers reported that adherence was much higher in depressed youth (87%)27 than what had been seen in adults (67%) in a similar study.13
The downside to pill packs and electronic monitoring? There is no guarantee the patient has actually taken the medication despite the data reported by the system.
Event marker-signaling devices. Novel technologies have been developed to measure adherence:
Proteus Digital Health feedback system (www.proteus.com) requires that patients ingest a tablet containing a tiny, dietary mineral-based “ingestible event marker.” Upon contact with gastric fluid electrolytes, the event marker emits a unique signal that is transmitted through bodily tissue to a small receiver in a patch worn on the torso. The receiver then transmits a signal to a cellular phone, indicating the time and date when the medication was ingested (Figure 3).
A 4-week pilot study28 found that the ingestible event marker is feasible and acceptable to patients: 27 of 28 participants (96%) completed the study, with a mean adherence rate of 74%. Although the system identifies ingestible sensors with high accuracy and is easily tolerated by patients, the pilot study was brief; a longer duration of adherence while wearing the patch needs to be studied.
Breath analysis, facial recognition. Even directly observing ingestion of a medication can be problematic: Some patients don’t swallow the medication and spit it out later. One way around that subterfuge is to consider using other advanced medication adherence solutions that are breath-based or use facial recognition technology and confirm ingestion.
Xhale SMART (www.xhale.com/smart) is a handheld device that generates a reminder to the patient to take his medication; afterward, he (she) must blow into the device so that ingestion of the medication is detected (Figure 4). The medication has breath-detectable adherence markers already incorporated. The adherence marker then is released into the stomach and small intestine, where the adherence marker metabolite is transported through the bloodstream into the lungs and exhaled. The patient must breathe into a breath analysis device, which measures medication ingestion compared with a baseline breath print.
Several articles in the literature have reported the accuracy of this device in detecting the ingested metabolite in every participant, without adverse effects.29,30 Clinical data on the use of the breath-based detector is not available to the public at this time.
AiCure (www.aicure.com) is a facial recognition-based technology platform that can work through any smartphone. The device is powered by artificial intelligence software and motion-sensing technology that can detect, in real time, whether the patient is taking the medication as prescribed. Patients who take an incorrect dose, or who do not use the software, are automatically flagged for immediate follow-up. This technology enables real-time intervention by a provider with the nonadherent patient.
An important note: These innovative technological advances are tools that can help clinicians manage an important aspect of treatment, but they do not show the entire picture: The physician−patient relationship and the therapeutic alliance are key to optimal treatment adherence.
Engage and empower the patient
Novel adherence technologies are, as we’ve described, available, and more are being developed. Incorporating these technologies into clinical care requires continued input and support from clinicians and patients. Digital and mobile health applications are multi-beneficial: They can empower patients to self-manage medication regimens and appointments while they also receive social and psychological information and support as needed. Understanding one’s own illness can, ultimately, improve outcomes and significantly reduce health care costs.
Patient empowerment is key. The physician is an important influencer in this regard.
The role of the physician must not be undervalued in maintaining adherence to therapy; she (he) plays a vital role in continued patient engagement and behavioral training. Integrating physician-led oversight, patient education, and commitment, and novel digital mobile adherence technologies will help deliver better outcomes.
The push to engage. A “one size fits all” approach to maintaining adherence won’t be effective. We need to better understand the individual patient’s underlying cause(s) for nonadherence, then to tailor a solution to influence and change that behavior. One way to do this is by interacting and engaging more directly (and in a digital manner) with patients to monitor adherence.
A recent example of the move toward direct patient engagement is the agreement entered by Otsuka Pharmaceuticals and Proteus Digital Health to develop novel digital health products. The FDA has accepted for review the combination product of Otsuka’s brand of aripiprazole and Proteus’s ingestible sensor. If the product is approved by the FDA, physicians will be able to prescribe aripiprazole with the ingestible sensor embedded in the tablet and then measure medication adherence and other patient physiologic metrics (eg, activity, rest) through the wearable sensor patch and medical software application designed specifically for patient and physician use.
This technology could have huge potential in mental health care, where patients struggle with both adhering to their medication regimen and communicating with the health care team. Physicians could measure adherence when treating adults with schizophrenia, bipolar disorders, and major depressive disorder; flag those who are not adhering as having higher risk of disease progression and poorer outcome; and allow decisions to be made more quickly based on treatment need.
Developing and enhancing these collaborative and patient-centric approaches will increase self-monitoring and patient responsibility, and encourage behavior change.
‘All-in’ strategy. By continuing to use the latest technologies and connecting them to the range of stakeholders—physicians, nurses, pharmacists, payers—we will develop an all-inclusive adherence intervention strategy. All patients will be integrated, and all of them, and their family, will be provided with positive psychoeducational care and motivational counseling (Figure 5). In addition, such a support-based patient experience must be aligned with the work of clinical care providers. Compliance therapy and behavioral training, together with active patient engagement, can help improve insight, acceptance of treatment, and, over the long term, adherence.31,32
1. World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003.
2. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
3. Crowe M, Wilson L, Inder M. Patients’ reports of the factors influencing medication adherence in bipolar disorder – an integrative review of the literature. Int J Nurs Stud. 2011;48(7):894-903.
4. Mert D, Turgut NH, Kelleci M, et al. Perspectives on reasons of medication nonadherence in psychiatric patients. Patient Prefer Adherence. 2015;9:87-93.
5. Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry. 2013;26(5):446-452.
6. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-497.
7. Thompson L, McCabe R. The effect of clinician-patient alliance and communication on treatment adherence in mental health care: a systematic review. BMC Psychiatry. 2012;12:87.
8. Yilmaz S, Buzlu S. Antipsikotik kullanan hastalarda ilaç yan etkileri ve ilaç uyumu. Florence Nightingale Hem˘girelik Dergisi. 2012;20(2):93-103.
9. Kelleci M, Ata EE. Psikiyatri Klini˘ginde yatan hastaların ilaç uyumları ve sosyal destekle iliskisi. [Drug compliance of patients hospitalized in the psychiatry clinic and the relationship with social support]. Psikiyatri Hemsireli˘gi Dergisi. 2011;2(suppl 3):105-110.
10. Bulloch AG, Patten SB. Non-adherence with psychotropic medications in the general population. Soc Psychiatry Psychiatr Epidemiol. 2010;45(1):47-56.
11. Rosenbaum L. Beyond belief—how people feel about taking medications for heart disease. N Engl J Med. 2015;372(2):183-187.
12. Cramer J, Rosenheck R, Kirk G, et al. Medication compliance feedback and monitoring in a clinical trial: predictions and outcomes. Value Health. 2003;6(5):566-573.
13. Nakonezny PA, Byerly MJ, Rush AJ. Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry Res. 2008;157(1-3):259-263.
14. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
15. Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14(6):553-560.
16. Hoencamp E, Stevens A, Haffmans J. Patients’ attitudes toward antidepressants. Psychiatr Serv. 2002;53(9):1180-1181.
17. Keller MB, Hirschfeld RM, Demyttenaere K, et al. Optimizing outcomes in depression: focus on antidepressant compliance. Int Clin Psychopharmacol. 2002;17(6):265-271.
18. Akerblad AC, Bengtsson F, Holgersson M, et al. Identification of primary care patients at risk of nonadherence to antidepressant treatment. Patient Prefer Adherence. 2008;2:376-386.
19. Brown C, Battista DR, Bruehlman R, et al. Beliefs about antidepressant medications in primary care patients: relationship to self-reported adherence. Med Care. 2005;43(12):1203-1207.
20. Demyttenaere K, Adelin A, Patrick M, et al. Six-month compliance with antidepressant medication in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2008;23(1):36-42.
21. Massand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25(8):2289-2304.
22. Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Pub L No. 108-173, 117 Stat 2066.
23. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304-314.
24. Diaz E, Neuse E, Sullivan MC, et al. Adherence to conventional and atypical antipsychotics after hospital discharge. J Clin Psychiatry. 2004;65(3):354-360.
25. Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2-3):129-133.
26. Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30(3):437-452.
27. Nakonezny PA, Hughes CW, Mayes TL, et al. A comparison of various methods of measuring antidepressant medication adherence among children and adolescents with major depressive disorder in a 12-week open trial of fluoxetine. J Child Adolesc Psychopharmacol. 2010;20(5):431-439.
28. Kane JM, Perlis RH, DiCarlo LA, et al. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74(6):e533-e540. doi: 10.4088/JCP.12m08222.
29. Morey TE, Booth MM, Prather RA, et al. Measurement of ethanol in gaseous breath using a miniature gas chromatograph. J Anal Toxicol. 2011;35(3):134-142.
30. Morey TE, Booth M, Wasdo S, et al. Oral adherence monitoring using a breath test to supplement highly active antiretroviral therapy. AIDS Behav. 2013;17(1):298-306.
31. Torem MS. Participatory pharmacotherapy: 10 strategies for enhancing adherence. Current Psychiatry. 2013;12(7):21-25.
32. Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159(10):1653-1664.
1. World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003.
2. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
3. Crowe M, Wilson L, Inder M. Patients’ reports of the factors influencing medication adherence in bipolar disorder – an integrative review of the literature. Int J Nurs Stud. 2011;48(7):894-903.
4. Mert D, Turgut NH, Kelleci M, et al. Perspectives on reasons of medication nonadherence in psychiatric patients. Patient Prefer Adherence. 2015;9:87-93.
5. Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry. 2013;26(5):446-452.
6. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-497.
7. Thompson L, McCabe R. The effect of clinician-patient alliance and communication on treatment adherence in mental health care: a systematic review. BMC Psychiatry. 2012;12:87.
8. Yilmaz S, Buzlu S. Antipsikotik kullanan hastalarda ilaç yan etkileri ve ilaç uyumu. Florence Nightingale Hem˘girelik Dergisi. 2012;20(2):93-103.
9. Kelleci M, Ata EE. Psikiyatri Klini˘ginde yatan hastaların ilaç uyumları ve sosyal destekle iliskisi. [Drug compliance of patients hospitalized in the psychiatry clinic and the relationship with social support]. Psikiyatri Hemsireli˘gi Dergisi. 2011;2(suppl 3):105-110.
10. Bulloch AG, Patten SB. Non-adherence with psychotropic medications in the general population. Soc Psychiatry Psychiatr Epidemiol. 2010;45(1):47-56.
11. Rosenbaum L. Beyond belief—how people feel about taking medications for heart disease. N Engl J Med. 2015;372(2):183-187.
12. Cramer J, Rosenheck R, Kirk G, et al. Medication compliance feedback and monitoring in a clinical trial: predictions and outcomes. Value Health. 2003;6(5):566-573.
13. Nakonezny PA, Byerly MJ, Rush AJ. Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry Res. 2008;157(1-3):259-263.
14. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
15. Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14(6):553-560.
16. Hoencamp E, Stevens A, Haffmans J. Patients’ attitudes toward antidepressants. Psychiatr Serv. 2002;53(9):1180-1181.
17. Keller MB, Hirschfeld RM, Demyttenaere K, et al. Optimizing outcomes in depression: focus on antidepressant compliance. Int Clin Psychopharmacol. 2002;17(6):265-271.
18. Akerblad AC, Bengtsson F, Holgersson M, et al. Identification of primary care patients at risk of nonadherence to antidepressant treatment. Patient Prefer Adherence. 2008;2:376-386.
19. Brown C, Battista DR, Bruehlman R, et al. Beliefs about antidepressant medications in primary care patients: relationship to self-reported adherence. Med Care. 2005;43(12):1203-1207.
20. Demyttenaere K, Adelin A, Patrick M, et al. Six-month compliance with antidepressant medication in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2008;23(1):36-42.
21. Massand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25(8):2289-2304.
22. Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Pub L No. 108-173, 117 Stat 2066.
23. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304-314.
24. Diaz E, Neuse E, Sullivan MC, et al. Adherence to conventional and atypical antipsychotics after hospital discharge. J Clin Psychiatry. 2004;65(3):354-360.
25. Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2-3):129-133.
26. Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30(3):437-452.
27. Nakonezny PA, Hughes CW, Mayes TL, et al. A comparison of various methods of measuring antidepressant medication adherence among children and adolescents with major depressive disorder in a 12-week open trial of fluoxetine. J Child Adolesc Psychopharmacol. 2010;20(5):431-439.
28. Kane JM, Perlis RH, DiCarlo LA, et al. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74(6):e533-e540. doi: 10.4088/JCP.12m08222.
29. Morey TE, Booth MM, Prather RA, et al. Measurement of ethanol in gaseous breath using a miniature gas chromatograph. J Anal Toxicol. 2011;35(3):134-142.
30. Morey TE, Booth M, Wasdo S, et al. Oral adherence monitoring using a breath test to supplement highly active antiretroviral therapy. AIDS Behav. 2013;17(1):298-306.
31. Torem MS. Participatory pharmacotherapy: 10 strategies for enhancing adherence. Current Psychiatry. 2013;12(7):21-25.
32. Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159(10):1653-1664.