User login
When is VBAC appropriate?
At first glance, the issue of vaginal birth after cesarean delivery (VBAC) appears to boil down to a simple question: Should I attempt it, or shouldn’t I?
On deeper inspection, the decision becomes extremely complex, and the evidence can be confusing.
Both planned elective repeat cesarean and planned VBAC are associated with harms as well as benefits. Most experts would agree than an uncomplicated vaginal delivery poses little risk to mother and baby, and that a planned repeat cesarean delivery at term carries some risk to the mother.
The greatest risks for both mother and baby arise when a trial of labor fails and cesarean delivery becomes necessary for maternal or fetal indications. Risks to the mother are largely operative in nature, and the primary risk to the fetus is uterine rupture. However, maternal and fetal risks cannot be truly separated. Uterine rupture not only compromises the fetus in utero but has a severe impact on maternal hemodynamic stability, just as a fetal hypoxic ischemic insult secondary to uterine rupture can have lifelong psychological and social consequences for the mother and family.
We are fortunate that serious adverse outcomes of VBAC are rare. Nevertheless, the only predictable delivery method is planned elective repeat cesarean. Uncertainty over the likelihood of success of VBAC arises when relative risk is confused with absolute risk.
In this article, I examine the literature on the route of delivery after cesarean to assess the overall safety of a trial of labor in various settings and populations.
Data on VBAC are limited
We lack randomized, controlled trials and valid animal studies that assess fetal and maternal outcomes of elective repeat cesarean versus planned vaginal delivery. The vast majority of studies of VBAC are retrospective or cohort studies, which have inherent potential for bias. Many studies lack a standardized definition of adverse outcomes or lack direct evidence that adverse outcomes are wholly attributable to the trial of labor. No studies compare women who are similar in all characteristics except their mode of delivery.
Nor do we fully understand how women choose a course of action after cesarean delivery—except that the decision is almost always multifactorial. Competing voices—health care provider, family members, friends, media, and a woman’s own memory of her previous delivery—and her emotional state—all contribute to the decision.
Clearly, a trial of labor after cesarean delivery can be safe for many women. Successful vaginal delivery is associated with a very low risk of adverse outcomes and may be associated with a lower risk of minor morbidity than is elective repeat cesarean. In fact, the overall success rate for a trial of labor after cesarean is not that different from the success rate for nulliparous women undergoing induction of labor.19 Even so, patients should understand that operative delivery may be necessary, and the physician and hospital must be prepared for this eventuality in accordance with ACOG guidelines.
As I interpret the data, if a woman has undergone one low transverse cesarean delivery for a nonrecurring condition and a nonmacrosomic fetus, a trial of labor after the spontaneous onset of labor should be strongly encouraged. If she has already delivered vaginally in the past, or had a successful VBAC, she is an even better candidate for a trial of labor. In such a case, labor induction with mechanical cervical ripening or appropriate use of oxytocin, or both, may still be appropriate, but the likelihood of success is lower.
If a woman has a history of more than one cesarean delivery without a vaginal birth, she may be better served by scheduled repeat cesarean delivery. The same holds true for women who have a history of preterm cesarean delivery, a short interpregnancy interval, suspected macrosomia, or an unengaged fetal vertex.
Decision-making about delivery should be shared between the provider and patient, after thorough counseling about the risks and benefits in language the patient can easily comprehend.
It would be best to avoid having to make a decision about VBAC by preventing the initial cesarean delivery.
How risky is repeat cesarean?
We are all acutely aware of the skyrocketing rate of cesarean delivery, which reaches 35% to 41% in some areas. Most studies indicate that approximately 50% of all cesarean deliveries are repeat cesarean deliveries. Besides the risks associated with the operation itself, planned repeat cesarean has significant downstream implications for the mother and baby—and for society. For example, multiple cesarean deliveries pose an ever greater risk of abnormal placentation and maternal hemorrhage. Cesarean delivery without labor can also heighten the risk of neonatal respiratory compromise, temperature instability, and slow feeding.1 Cesarean delivery and its longer attendant hospitalization markedly increase costs throughout an already strapped health care system.
On balance, any cesarean delivery imparts an increased risk of maternal morbidity and mortality, compared with vaginal delivery, as well as an increased risk of complications, such as placenta previa and placenta accreta, in subsequent pregnancies.
What are the risks of a trial of labor?
A prospective, 4-year observational study conducted at 19 academic medical centers under the auspices of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network compared the outcomes of 17,898 women undergoing a trial of labor after cesarean delivery with those of 15,801 women having elective repeat cesarean.2 Symptomatic uterine rupture occurred in 0.7% of the women attempting a trial of labor, with no occurrences in the elective cesarean group. Blood transfusion and endomyometritis were more common in the group undergoing a trial of labor, and this difference was statistically significant. These findings are in concordance with those of earlier studies.
The two groups in this study were not exactly the same; more women undergoing a trial of labor had had a previous vaginal delivery. Significant adverse maternal outcomes, such as endomyometritis, uterine rupture, hysterectomy, and the need for transfusion, were much more likely in a failed trial of labor than in a successful one.
The same study found a 0.46% risk of hypoxic-ischemic encephalopathy, which was most likely to occur after symptomatic uterine rupture (7 of 12 cases). No cases of hypoxic-ischemic encephalopathy occurred among women undergoing planned cesarean delivery. Multivariate logistic regression analysis determined that the risks of stillbirth, neonatal death, and hypoxic-ischemic encephalopathy in term infants were increased in the group undergoing a trial of labor, compared with elective repeat cesarean (odds ratio [OR], 2.72; 95% confidence interval [CI], 1.49–4.97).
Can we predict the success of a trial of labor?
Combined success rates from a large number of prospective cohort studies suggest an overall rate of 75.9%. Many clinical characteristics may increase the likelihood of success of a trial of labor after cesarean. In this section, I describe these characteristics and sift the data we have about them.
A history of vaginal delivery ups the odds of success
Women who have delivered vaginally have a much lower risk of rupture during a trial of labor after cesarean than women who have not. Women who have delivered vaginally are also four times more likely to have a successful VBAC. A multicenter, prospective study found a VBAC success rate of 86% among women who had already delivered vaginally, and a success rate of 90% among women who had a history of successful VBAC.3
Many aspects of the cesarean delivery have continuing impact
The type of labor that occurred in the cesarean delivery may help predict subsequent complications and the ultimate success of a trial of labor. For example, induced labor or no labor prior to cesarean delivery is associated with a 2.25-fold risk of uterine rupture in a subsequent trial of labor, compared with a history of spontaneous labor.4
In addition, several studies have demonstrated that the indication for the first cesarean delivery has a bearing on the success of a subsequent trial of labor. For example, an indication of shoulder dystocia reduces the success rate of a subsequent trial of labor by one third.2
Even a brief trial of labor before the cesarean may increase the success of a subsequent trial of labor. One study found that cervical dilation to 8 cm or greater was independently predictive of successful VBAC among women who had a nonrecurring indication for the initial cesarean delivery.5
When the cesarean delivery involves a preterm infant, the risk of uterine rupture during a subsequent trial of labor may increase if the infant is at term. Conversely, the risk of uterine rupture is lower when a term cesarean is followed by a preterm trial of labor.6
A vertical hysterotomy may preclude VBAC
A previous classical hysterotomy is generally an absolute contraindication for a trial of labor because rupture may occur in as many as 14% of women who have this type of scar.
Low transverse hysterotomy does not appear to confer excess risk during a subsequent trial of labor. Less clear is whether a low vertical hysterotomy poses a risk of rupture. In a 2004 prospective cohort study, the rate of uterine rupture among women who had a transverse hysterotomy scar was 0.7%, compared with 2.0% for a low vertical scar. Any difference in the rate of uterine rupture in retrospective studies may be attributable, at least in part, to the subjective nature of the definition of “low vertical” because there is no precise or objective way to ensure that the vertical hysterotomy did not breach the contractile portion of the uterus (FIGURE).
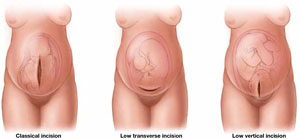
Type of prior hysterotomy influences the VBAC decision
A classical uterine incision is an absolute contraindication to vaginal birth after cesarean (VBAC). A trial of labor is thought to be safe in women who have had a low transverse hysterotomy. The jury is still out on the safety of VBAC in a woman who has had a low vertical incision, however, because of uncertainty over whether the contractile portion of the uterus is involved.
Are multiple cesareans a contraindication to VBAC?
Experts disagree as to whether more than one previous cesarean delivery before a trial of labor increases the risk of uterine rupture. One retrospective study showed no difference in the rate of rupture between women who had a single previous cesarean and those who had more than one.7 A larger prospective study showed a modest increase in the risk of rupture (OR, 1.16) among women who had undergone more than one cesarean—but no decrease in the chance of success.8
Most large retrospective and prospective studies include patients who have had more than one previous cesarean delivery, but their numbers remain low; therefore, statistical significance cannot be determined.
Induction or augmentation of labor may lower odds of success
The likelihood of successful VBAC may be reduced when labor is augmented or induced. The picture is unclear because most studies that have focused on cervical ripening and induction of labor in VBAC are small.
Bujold compared pregnancy outcomes of three groups of women:
- those who underwent cervical ripening via Foley catheter
- those who had amniotomy and oxytocin administration
- those who entered labor spontaneously.
No difference in the rate of uterine rupture was found among the groups. However, the group that underwent cervical ripening had a significantly lower rate of success.9
A large case-control study found no increase in the rate of rupture when oxytocin or prostaglandins were administered, but the rate tripled when both were used together.10
A small, nested, case-control study found an increased risk of uterine rupture only when oxytocin was administered at a rate exceeding 20 mU/mL.11
More than 90% of hysterotomies are transverse
When the obstetric history is incomplete, the clinician may not know what type of hysterotomy was used in the previous cesarean delivery. Most experts believe that VBAC is acceptable when the previous cesarean involved a low transverse hysterotomy. The risk may be much higher with other types of incisions. Today, however, with modern techniques in place, we can assume that more than 90% of hysterotomies are of the low transverse type.
At least one study suggests that the risk of uterine rupture during vaginal birth after cesarean is acceptably low when the type of hysterotomy is unknown. That study explored the effect of augmentation of labor with oxytocin among women who had an unknown scar and found an increased risk of rupture, compared with women who were managed expectantly. However, the overall rate of uterine rupture did not differ from the rate expected when the hysterotomy is known to be of the low transverse type.12
VBAC for twins is rare
Because few women carrying twins attempt VBAC, we have little data to guide counseling on success and complication rates. A multicenter, retrospective, cohort study explored delivery outcomes of 25,005 women who had undergone at least one previous cesarean. Of these women, 24,307 had a singleton pregnancy, and 535 were carrying twins. Women who had a twin gestation were 40% less likely to attempt a trial of labor, but those who did had a chance of success and risk of uterine rupture similar to those of women with a singleton gestation. Women carrying twins who underwent a trial of labor had an elevated risk of requiring transfusion, compared with those carrying singletons, but this risk was similar to that of women delivering twins by elective repeat cesarean. In fact, women who delivered twins by repeat cesarean tended to have more maternal morbidity overall than those who had a trial of labor.13
A short interpregnancy interval precludes VBAC
Data indicate that a trial of labor after cesarean should be avoided in women who have a brief interpregnancy interval. Several retrospective studies had found an increased risk of uterine rupture, as well as a host of other adverse outcomes, among these women. Using 12 months as a reference point, women who had an interpregnancy interval shorter than 6 months had triple the risk of uterine rupture.14 Although the mechanism is unknown, rupture is presumably the result of incomplete healing of the hysterotomy.
Macrosomia may not increase the risk of rupture
Women who are thought to have a macrosomic fetus may be encouraged to attempt VBAC, if they so desire. Macrosomia is a minor risk factor for failure of a trial of labor, but it does not necessarily increase the risk of uterine rupture.15
Elkousy examined VBAC success rates by birth weight, indication for the previous cesarean delivery, and pregnancy history. Not surprisingly, increased birth weight or a history of cephalopelvic disproportion reduced the rate of success, but a history of vaginal delivery negated that risk of failure. A history of successful VBAC improved the chance of success to more than 90%—even when the birth weight exceeded 4,000 g—and the success rate reached 82% when the birth weight exceeded 4,500 g.16
Physician and hospital attitudes toward vaginal birth after cesarean delivery (VBAC) may be a major determinant of its frequency and success. Many forces oppose women who desire a trial of labor after cesarean. Hospitals and insurers make it increasingly difficult to offer a trial of labor, and strict interpretation of ACOG’s guidelines requiring personnel to be “immediately available” during a trial of labor has caused many smaller and isolated hospitals to stop offering this option. The number of women who attempt VBAC has plummeted.20
Two recent surveys by ACOG indicate that an alarming number of providers have stopped offering VBAC because of a lack of insurance and fear of legal liability. As providers offer a trial of labor less and less, skills decline, and so does mentorship of younger physicians.
The NIH weighs in
In March 2010, the National Institutes of Health (NIH) convened a consensus development conference on the topic of VBAC. A panel of health professionals and public representatives reviewed the medical literature and produced a consensus statement. Their conclusion:
- Given the available evidence, [a trial of labor] is a reasonable option for many pregnant women with a prior low transverse uterine incision. The data reviewed in this report show that both [a trial of labor] and elective repeat cesarean for a pregnant woman with a prior transverse uterine incision have important risks and benefits and that these risks and benefits differ for the woman and her fetus.
The panel’s goal was to help women who have a history of cesarean delivery make an informed, evidence-based decision about the subsequent mode of delivery. The panel also acknowledged the general lack of high-quality evidence to confidently quantify the risks and benefits of a trial of labor versus planned repeat cesarean delivery.21
For another point of view on vaginal birth after cesarean, see the Editorial, “Does vaginal birth after cesarean have a future?” by John T. Repke, MD, of the OBG Management Board of Editors.
Repeat cesarean is probably best for obese gravidas
Obesity increases the likelihood of cesarean delivery in all circumstances, so it is not surprising that it is a risk factor for a failed trial of labor after cesarean. Obesity also increases the risks of anesthesia and surgery. Because of these risks, most clinicians opt to deliver obese patients by scheduled elective cesarean rather than risk having to perform emergent cesarean delivery in the case of acute fetal compromise or uterine rupture.
Race is not a risk factor for rupture
Race is probably not a significant independent risk factor for failure of VBAC. A secondary analysis of a multicenter, retrospective, cohort study found that black women were somewhat more likely to fail a trial of labor than white women (OR, 1.50; 95% CI, 1.29–1.74), after adjustment for confounding variables. However, black women undergoing a trial of labor were 40% less likely to suffer a uterine rupture than white women were.17
When comorbidities are well managed, VBAC remains an option
In general, a trial of labor in women who have well managed chronic medical disease does not pose undue risk to mother or baby.
In a population-based, retrospective cohort study using discharge data from California, Gregory and coworkers attempted to delineate clinical variables that might be associated with VBAC success and complications. They examined a wide range of maternal conditions, from diabetes to chorioamnionitis, as well as fetal conditions, such as oligohydramnios and unengaged vertex. Mothers were stratified into low- and high-risk groups, and multivariate logistic regression was performed. Low-risk patients had a 73.7% success rate, whereas high-risk patients had a 50% success rate. Not surprisingly, women who had a fetus with an unengaged vertex had a 9.8% chance of success and an eightfold increase in the risk of uterine rupture.18
1. Tita AT, Landon MB, Spong CY, et al. For Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111-1120.
2. Landon MB, Hauth JC, Leveno KJ, et al. For NICHD MFMU Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351(25):2581-2589.
3. Landon MB, Leindecker S, Spong CY, et al. For NICHD MFMU Network. The MFMU Cesarean Registry: factors affecting the success of trial of labor after previous cesarean delivery. Am J Obst Gynecol. 2005;193(3 Pt 2):1016-1023.
4. Algert CS, Morris JM, Simpson JM, Ford JB, Roberts CL. Labor before a primary cesarean delivery: reduced risk of uterine rupture in a subsequent trial of labor for vaginal birth after cesarean. Obstet Gynecol. 2008;112(5):1061-1066.
5. Kwon JY, Jo YS, Lee GS, Kim SJ, Shin JC, Lee Y. Cervical dilation at the time of cesarean section may affect the success of subsequent vaginal delivery. J Matern Fetal Neonatal Med. 2009;22(11):1057-1062.
6. Sciscione AC, Landon MB, Leveno KJ, et al. For NICHD MFMU Network. Previous preterm delivery and risk of subsequent uterine rupture. Obstet Gynecol. 2008;111(3):648-653.
7. Landon MB, Spong CY, Thom E, et al. For NICHD MFMU Network. Risk of uterine rupture with a trial of labor in women with multiple and single prior cesarean delivery. Obstet Gynecol. 2006;108(1):12-20.
8. Macones GA, Cahill A, Pare E, et al. Obstetric outcomes in women with two prior cesarean deliveries: is vaginal birth after cesarean delivery a viable option? Am J Obstet Gynecol. 2005;192(4):1223-1229.
9. Bujold E, Blackwell SC, Gauthier RJ. Cervical ripening with transcervical foley catheter and the risk of uterine rupture. Obstet Gynecol. 2004;103(1):18-23.
10. Macones G, Peipert J, Nelson D, et al. Maternal complications with vaginal birth after cesarean delivery: a multicenter study. Am J Obstet Gynecol. 2005;193(5):1656-1662.
11. Cahill A, Stamilio D, Odibo A, Peipert J, Stevens E, Macones G. Does a maximum dose of oxytocin affect risk for uterine rupture in candidates for vaginal birth after cesarean delivery? Am J Obstet Gynecol. 2007;197(5):495.e1-e5.
12. Grubb DK, Kjos SL, Paul RH. Latent labor with an unknown uterine scar. Obstet Gynecol. 1996;88(3):351-355.
13. Cahill A, Stamilio DM, Paré E, et al. Vaginal birth after cesarean (VBAC) attempt in twin pregnancies: is it safe? Am J Obstet Gynecol. 2005;193(3 Pt 2):1050-1055.
14. Stamilio DM, DeFranco E, Paré E, et al. Short interpregnancy interval: risk of uterine rupture and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;110(5):1075.-
15. Zelop CM, Shipp TD, Repke JT, Cohen A, Lieberman E. Outcomes of trial of labor following previous cesarean delivery among women with fetuses weighing >4000 g. Am J Obstet Gynecol. 2001;185(4):903-905.
16. Elkousy M, Sammel M, Stevens E, Peipert J, Macones G. The effect of birth weight on vaginal birth after cesarean delivery success rates. Am J Obstet Gynecol. 2003;188(3):824-830.
17. Cahill AG, Stamilio DM, Odibo AO, Peipert J, Stevens E, Macones GA. Racial disparity in the success and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2008;111(3):654-658.
18. Gregory KD, Korst LM, Fridman M, et al. Vaginal birth after cesarean: clinical risk factors associated with adverse outcome. Am J Obstet Gynecol. 2008;198(4):452.e1-e12.
19. Smith GCS, Pell JP, Cameron AD, Dobbie R. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA. 2002;287(20):2684-2690.
20. Hamilton BE, Martin JA, Sutton PD. For US Department of Health and Human Services. Births: preliminary data for 2002. Natl Vital Stat Rep. 2003;51(11):1-20.
21. National Institutes of Health Consensus Development Conference Statement. Vaginal Birth after Cesarean: New Insights. Bethesda, Md: NIH; 2010. http://consensus.nih.gov/2010/images/vbac/vbac_statement.pdf. Accessed June 16, 2010.
At first glance, the issue of vaginal birth after cesarean delivery (VBAC) appears to boil down to a simple question: Should I attempt it, or shouldn’t I?
On deeper inspection, the decision becomes extremely complex, and the evidence can be confusing.
Both planned elective repeat cesarean and planned VBAC are associated with harms as well as benefits. Most experts would agree than an uncomplicated vaginal delivery poses little risk to mother and baby, and that a planned repeat cesarean delivery at term carries some risk to the mother.
The greatest risks for both mother and baby arise when a trial of labor fails and cesarean delivery becomes necessary for maternal or fetal indications. Risks to the mother are largely operative in nature, and the primary risk to the fetus is uterine rupture. However, maternal and fetal risks cannot be truly separated. Uterine rupture not only compromises the fetus in utero but has a severe impact on maternal hemodynamic stability, just as a fetal hypoxic ischemic insult secondary to uterine rupture can have lifelong psychological and social consequences for the mother and family.
We are fortunate that serious adverse outcomes of VBAC are rare. Nevertheless, the only predictable delivery method is planned elective repeat cesarean. Uncertainty over the likelihood of success of VBAC arises when relative risk is confused with absolute risk.
In this article, I examine the literature on the route of delivery after cesarean to assess the overall safety of a trial of labor in various settings and populations.
Data on VBAC are limited
We lack randomized, controlled trials and valid animal studies that assess fetal and maternal outcomes of elective repeat cesarean versus planned vaginal delivery. The vast majority of studies of VBAC are retrospective or cohort studies, which have inherent potential for bias. Many studies lack a standardized definition of adverse outcomes or lack direct evidence that adverse outcomes are wholly attributable to the trial of labor. No studies compare women who are similar in all characteristics except their mode of delivery.
Nor do we fully understand how women choose a course of action after cesarean delivery—except that the decision is almost always multifactorial. Competing voices—health care provider, family members, friends, media, and a woman’s own memory of her previous delivery—and her emotional state—all contribute to the decision.
Clearly, a trial of labor after cesarean delivery can be safe for many women. Successful vaginal delivery is associated with a very low risk of adverse outcomes and may be associated with a lower risk of minor morbidity than is elective repeat cesarean. In fact, the overall success rate for a trial of labor after cesarean is not that different from the success rate for nulliparous women undergoing induction of labor.19 Even so, patients should understand that operative delivery may be necessary, and the physician and hospital must be prepared for this eventuality in accordance with ACOG guidelines.
As I interpret the data, if a woman has undergone one low transverse cesarean delivery for a nonrecurring condition and a nonmacrosomic fetus, a trial of labor after the spontaneous onset of labor should be strongly encouraged. If she has already delivered vaginally in the past, or had a successful VBAC, she is an even better candidate for a trial of labor. In such a case, labor induction with mechanical cervical ripening or appropriate use of oxytocin, or both, may still be appropriate, but the likelihood of success is lower.
If a woman has a history of more than one cesarean delivery without a vaginal birth, she may be better served by scheduled repeat cesarean delivery. The same holds true for women who have a history of preterm cesarean delivery, a short interpregnancy interval, suspected macrosomia, or an unengaged fetal vertex.
Decision-making about delivery should be shared between the provider and patient, after thorough counseling about the risks and benefits in language the patient can easily comprehend.
It would be best to avoid having to make a decision about VBAC by preventing the initial cesarean delivery.
How risky is repeat cesarean?
We are all acutely aware of the skyrocketing rate of cesarean delivery, which reaches 35% to 41% in some areas. Most studies indicate that approximately 50% of all cesarean deliveries are repeat cesarean deliveries. Besides the risks associated with the operation itself, planned repeat cesarean has significant downstream implications for the mother and baby—and for society. For example, multiple cesarean deliveries pose an ever greater risk of abnormal placentation and maternal hemorrhage. Cesarean delivery without labor can also heighten the risk of neonatal respiratory compromise, temperature instability, and slow feeding.1 Cesarean delivery and its longer attendant hospitalization markedly increase costs throughout an already strapped health care system.
On balance, any cesarean delivery imparts an increased risk of maternal morbidity and mortality, compared with vaginal delivery, as well as an increased risk of complications, such as placenta previa and placenta accreta, in subsequent pregnancies.
What are the risks of a trial of labor?
A prospective, 4-year observational study conducted at 19 academic medical centers under the auspices of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network compared the outcomes of 17,898 women undergoing a trial of labor after cesarean delivery with those of 15,801 women having elective repeat cesarean.2 Symptomatic uterine rupture occurred in 0.7% of the women attempting a trial of labor, with no occurrences in the elective cesarean group. Blood transfusion and endomyometritis were more common in the group undergoing a trial of labor, and this difference was statistically significant. These findings are in concordance with those of earlier studies.
The two groups in this study were not exactly the same; more women undergoing a trial of labor had had a previous vaginal delivery. Significant adverse maternal outcomes, such as endomyometritis, uterine rupture, hysterectomy, and the need for transfusion, were much more likely in a failed trial of labor than in a successful one.
The same study found a 0.46% risk of hypoxic-ischemic encephalopathy, which was most likely to occur after symptomatic uterine rupture (7 of 12 cases). No cases of hypoxic-ischemic encephalopathy occurred among women undergoing planned cesarean delivery. Multivariate logistic regression analysis determined that the risks of stillbirth, neonatal death, and hypoxic-ischemic encephalopathy in term infants were increased in the group undergoing a trial of labor, compared with elective repeat cesarean (odds ratio [OR], 2.72; 95% confidence interval [CI], 1.49–4.97).
Can we predict the success of a trial of labor?
Combined success rates from a large number of prospective cohort studies suggest an overall rate of 75.9%. Many clinical characteristics may increase the likelihood of success of a trial of labor after cesarean. In this section, I describe these characteristics and sift the data we have about them.
A history of vaginal delivery ups the odds of success
Women who have delivered vaginally have a much lower risk of rupture during a trial of labor after cesarean than women who have not. Women who have delivered vaginally are also four times more likely to have a successful VBAC. A multicenter, prospective study found a VBAC success rate of 86% among women who had already delivered vaginally, and a success rate of 90% among women who had a history of successful VBAC.3
Many aspects of the cesarean delivery have continuing impact
The type of labor that occurred in the cesarean delivery may help predict subsequent complications and the ultimate success of a trial of labor. For example, induced labor or no labor prior to cesarean delivery is associated with a 2.25-fold risk of uterine rupture in a subsequent trial of labor, compared with a history of spontaneous labor.4
In addition, several studies have demonstrated that the indication for the first cesarean delivery has a bearing on the success of a subsequent trial of labor. For example, an indication of shoulder dystocia reduces the success rate of a subsequent trial of labor by one third.2
Even a brief trial of labor before the cesarean may increase the success of a subsequent trial of labor. One study found that cervical dilation to 8 cm or greater was independently predictive of successful VBAC among women who had a nonrecurring indication for the initial cesarean delivery.5
When the cesarean delivery involves a preterm infant, the risk of uterine rupture during a subsequent trial of labor may increase if the infant is at term. Conversely, the risk of uterine rupture is lower when a term cesarean is followed by a preterm trial of labor.6
A vertical hysterotomy may preclude VBAC
A previous classical hysterotomy is generally an absolute contraindication for a trial of labor because rupture may occur in as many as 14% of women who have this type of scar.
Low transverse hysterotomy does not appear to confer excess risk during a subsequent trial of labor. Less clear is whether a low vertical hysterotomy poses a risk of rupture. In a 2004 prospective cohort study, the rate of uterine rupture among women who had a transverse hysterotomy scar was 0.7%, compared with 2.0% for a low vertical scar. Any difference in the rate of uterine rupture in retrospective studies may be attributable, at least in part, to the subjective nature of the definition of “low vertical” because there is no precise or objective way to ensure that the vertical hysterotomy did not breach the contractile portion of the uterus (FIGURE).

Type of prior hysterotomy influences the VBAC decision
A classical uterine incision is an absolute contraindication to vaginal birth after cesarean (VBAC). A trial of labor is thought to be safe in women who have had a low transverse hysterotomy. The jury is still out on the safety of VBAC in a woman who has had a low vertical incision, however, because of uncertainty over whether the contractile portion of the uterus is involved.
Are multiple cesareans a contraindication to VBAC?
Experts disagree as to whether more than one previous cesarean delivery before a trial of labor increases the risk of uterine rupture. One retrospective study showed no difference in the rate of rupture between women who had a single previous cesarean and those who had more than one.7 A larger prospective study showed a modest increase in the risk of rupture (OR, 1.16) among women who had undergone more than one cesarean—but no decrease in the chance of success.8
Most large retrospective and prospective studies include patients who have had more than one previous cesarean delivery, but their numbers remain low; therefore, statistical significance cannot be determined.
Induction or augmentation of labor may lower odds of success
The likelihood of successful VBAC may be reduced when labor is augmented or induced. The picture is unclear because most studies that have focused on cervical ripening and induction of labor in VBAC are small.
Bujold compared pregnancy outcomes of three groups of women:
- those who underwent cervical ripening via Foley catheter
- those who had amniotomy and oxytocin administration
- those who entered labor spontaneously.
No difference in the rate of uterine rupture was found among the groups. However, the group that underwent cervical ripening had a significantly lower rate of success.9
A large case-control study found no increase in the rate of rupture when oxytocin or prostaglandins were administered, but the rate tripled when both were used together.10
A small, nested, case-control study found an increased risk of uterine rupture only when oxytocin was administered at a rate exceeding 20 mU/mL.11
More than 90% of hysterotomies are transverse
When the obstetric history is incomplete, the clinician may not know what type of hysterotomy was used in the previous cesarean delivery. Most experts believe that VBAC is acceptable when the previous cesarean involved a low transverse hysterotomy. The risk may be much higher with other types of incisions. Today, however, with modern techniques in place, we can assume that more than 90% of hysterotomies are of the low transverse type.
At least one study suggests that the risk of uterine rupture during vaginal birth after cesarean is acceptably low when the type of hysterotomy is unknown. That study explored the effect of augmentation of labor with oxytocin among women who had an unknown scar and found an increased risk of rupture, compared with women who were managed expectantly. However, the overall rate of uterine rupture did not differ from the rate expected when the hysterotomy is known to be of the low transverse type.12
VBAC for twins is rare
Because few women carrying twins attempt VBAC, we have little data to guide counseling on success and complication rates. A multicenter, retrospective, cohort study explored delivery outcomes of 25,005 women who had undergone at least one previous cesarean. Of these women, 24,307 had a singleton pregnancy, and 535 were carrying twins. Women who had a twin gestation were 40% less likely to attempt a trial of labor, but those who did had a chance of success and risk of uterine rupture similar to those of women with a singleton gestation. Women carrying twins who underwent a trial of labor had an elevated risk of requiring transfusion, compared with those carrying singletons, but this risk was similar to that of women delivering twins by elective repeat cesarean. In fact, women who delivered twins by repeat cesarean tended to have more maternal morbidity overall than those who had a trial of labor.13
A short interpregnancy interval precludes VBAC
Data indicate that a trial of labor after cesarean should be avoided in women who have a brief interpregnancy interval. Several retrospective studies had found an increased risk of uterine rupture, as well as a host of other adverse outcomes, among these women. Using 12 months as a reference point, women who had an interpregnancy interval shorter than 6 months had triple the risk of uterine rupture.14 Although the mechanism is unknown, rupture is presumably the result of incomplete healing of the hysterotomy.
Macrosomia may not increase the risk of rupture
Women who are thought to have a macrosomic fetus may be encouraged to attempt VBAC, if they so desire. Macrosomia is a minor risk factor for failure of a trial of labor, but it does not necessarily increase the risk of uterine rupture.15
Elkousy examined VBAC success rates by birth weight, indication for the previous cesarean delivery, and pregnancy history. Not surprisingly, increased birth weight or a history of cephalopelvic disproportion reduced the rate of success, but a history of vaginal delivery negated that risk of failure. A history of successful VBAC improved the chance of success to more than 90%—even when the birth weight exceeded 4,000 g—and the success rate reached 82% when the birth weight exceeded 4,500 g.16
Physician and hospital attitudes toward vaginal birth after cesarean delivery (VBAC) may be a major determinant of its frequency and success. Many forces oppose women who desire a trial of labor after cesarean. Hospitals and insurers make it increasingly difficult to offer a trial of labor, and strict interpretation of ACOG’s guidelines requiring personnel to be “immediately available” during a trial of labor has caused many smaller and isolated hospitals to stop offering this option. The number of women who attempt VBAC has plummeted.20
Two recent surveys by ACOG indicate that an alarming number of providers have stopped offering VBAC because of a lack of insurance and fear of legal liability. As providers offer a trial of labor less and less, skills decline, and so does mentorship of younger physicians.
The NIH weighs in
In March 2010, the National Institutes of Health (NIH) convened a consensus development conference on the topic of VBAC. A panel of health professionals and public representatives reviewed the medical literature and produced a consensus statement. Their conclusion:
- Given the available evidence, [a trial of labor] is a reasonable option for many pregnant women with a prior low transverse uterine incision. The data reviewed in this report show that both [a trial of labor] and elective repeat cesarean for a pregnant woman with a prior transverse uterine incision have important risks and benefits and that these risks and benefits differ for the woman and her fetus.
The panel’s goal was to help women who have a history of cesarean delivery make an informed, evidence-based decision about the subsequent mode of delivery. The panel also acknowledged the general lack of high-quality evidence to confidently quantify the risks and benefits of a trial of labor versus planned repeat cesarean delivery.21
For another point of view on vaginal birth after cesarean, see the Editorial, “Does vaginal birth after cesarean have a future?” by John T. Repke, MD, of the OBG Management Board of Editors.
Repeat cesarean is probably best for obese gravidas
Obesity increases the likelihood of cesarean delivery in all circumstances, so it is not surprising that it is a risk factor for a failed trial of labor after cesarean. Obesity also increases the risks of anesthesia and surgery. Because of these risks, most clinicians opt to deliver obese patients by scheduled elective cesarean rather than risk having to perform emergent cesarean delivery in the case of acute fetal compromise or uterine rupture.
Race is not a risk factor for rupture
Race is probably not a significant independent risk factor for failure of VBAC. A secondary analysis of a multicenter, retrospective, cohort study found that black women were somewhat more likely to fail a trial of labor than white women (OR, 1.50; 95% CI, 1.29–1.74), after adjustment for confounding variables. However, black women undergoing a trial of labor were 40% less likely to suffer a uterine rupture than white women were.17
When comorbidities are well managed, VBAC remains an option
In general, a trial of labor in women who have well managed chronic medical disease does not pose undue risk to mother or baby.
In a population-based, retrospective cohort study using discharge data from California, Gregory and coworkers attempted to delineate clinical variables that might be associated with VBAC success and complications. They examined a wide range of maternal conditions, from diabetes to chorioamnionitis, as well as fetal conditions, such as oligohydramnios and unengaged vertex. Mothers were stratified into low- and high-risk groups, and multivariate logistic regression was performed. Low-risk patients had a 73.7% success rate, whereas high-risk patients had a 50% success rate. Not surprisingly, women who had a fetus with an unengaged vertex had a 9.8% chance of success and an eightfold increase in the risk of uterine rupture.18
At first glance, the issue of vaginal birth after cesarean delivery (VBAC) appears to boil down to a simple question: Should I attempt it, or shouldn’t I?
On deeper inspection, the decision becomes extremely complex, and the evidence can be confusing.
Both planned elective repeat cesarean and planned VBAC are associated with harms as well as benefits. Most experts would agree than an uncomplicated vaginal delivery poses little risk to mother and baby, and that a planned repeat cesarean delivery at term carries some risk to the mother.
The greatest risks for both mother and baby arise when a trial of labor fails and cesarean delivery becomes necessary for maternal or fetal indications. Risks to the mother are largely operative in nature, and the primary risk to the fetus is uterine rupture. However, maternal and fetal risks cannot be truly separated. Uterine rupture not only compromises the fetus in utero but has a severe impact on maternal hemodynamic stability, just as a fetal hypoxic ischemic insult secondary to uterine rupture can have lifelong psychological and social consequences for the mother and family.
We are fortunate that serious adverse outcomes of VBAC are rare. Nevertheless, the only predictable delivery method is planned elective repeat cesarean. Uncertainty over the likelihood of success of VBAC arises when relative risk is confused with absolute risk.
In this article, I examine the literature on the route of delivery after cesarean to assess the overall safety of a trial of labor in various settings and populations.
Data on VBAC are limited
We lack randomized, controlled trials and valid animal studies that assess fetal and maternal outcomes of elective repeat cesarean versus planned vaginal delivery. The vast majority of studies of VBAC are retrospective or cohort studies, which have inherent potential for bias. Many studies lack a standardized definition of adverse outcomes or lack direct evidence that adverse outcomes are wholly attributable to the trial of labor. No studies compare women who are similar in all characteristics except their mode of delivery.
Nor do we fully understand how women choose a course of action after cesarean delivery—except that the decision is almost always multifactorial. Competing voices—health care provider, family members, friends, media, and a woman’s own memory of her previous delivery—and her emotional state—all contribute to the decision.
Clearly, a trial of labor after cesarean delivery can be safe for many women. Successful vaginal delivery is associated with a very low risk of adverse outcomes and may be associated with a lower risk of minor morbidity than is elective repeat cesarean. In fact, the overall success rate for a trial of labor after cesarean is not that different from the success rate for nulliparous women undergoing induction of labor.19 Even so, patients should understand that operative delivery may be necessary, and the physician and hospital must be prepared for this eventuality in accordance with ACOG guidelines.
As I interpret the data, if a woman has undergone one low transverse cesarean delivery for a nonrecurring condition and a nonmacrosomic fetus, a trial of labor after the spontaneous onset of labor should be strongly encouraged. If she has already delivered vaginally in the past, or had a successful VBAC, she is an even better candidate for a trial of labor. In such a case, labor induction with mechanical cervical ripening or appropriate use of oxytocin, or both, may still be appropriate, but the likelihood of success is lower.
If a woman has a history of more than one cesarean delivery without a vaginal birth, she may be better served by scheduled repeat cesarean delivery. The same holds true for women who have a history of preterm cesarean delivery, a short interpregnancy interval, suspected macrosomia, or an unengaged fetal vertex.
Decision-making about delivery should be shared between the provider and patient, after thorough counseling about the risks and benefits in language the patient can easily comprehend.
It would be best to avoid having to make a decision about VBAC by preventing the initial cesarean delivery.
How risky is repeat cesarean?
We are all acutely aware of the skyrocketing rate of cesarean delivery, which reaches 35% to 41% in some areas. Most studies indicate that approximately 50% of all cesarean deliveries are repeat cesarean deliveries. Besides the risks associated with the operation itself, planned repeat cesarean has significant downstream implications for the mother and baby—and for society. For example, multiple cesarean deliveries pose an ever greater risk of abnormal placentation and maternal hemorrhage. Cesarean delivery without labor can also heighten the risk of neonatal respiratory compromise, temperature instability, and slow feeding.1 Cesarean delivery and its longer attendant hospitalization markedly increase costs throughout an already strapped health care system.
On balance, any cesarean delivery imparts an increased risk of maternal morbidity and mortality, compared with vaginal delivery, as well as an increased risk of complications, such as placenta previa and placenta accreta, in subsequent pregnancies.
What are the risks of a trial of labor?
A prospective, 4-year observational study conducted at 19 academic medical centers under the auspices of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network compared the outcomes of 17,898 women undergoing a trial of labor after cesarean delivery with those of 15,801 women having elective repeat cesarean.2 Symptomatic uterine rupture occurred in 0.7% of the women attempting a trial of labor, with no occurrences in the elective cesarean group. Blood transfusion and endomyometritis were more common in the group undergoing a trial of labor, and this difference was statistically significant. These findings are in concordance with those of earlier studies.
The two groups in this study were not exactly the same; more women undergoing a trial of labor had had a previous vaginal delivery. Significant adverse maternal outcomes, such as endomyometritis, uterine rupture, hysterectomy, and the need for transfusion, were much more likely in a failed trial of labor than in a successful one.
The same study found a 0.46% risk of hypoxic-ischemic encephalopathy, which was most likely to occur after symptomatic uterine rupture (7 of 12 cases). No cases of hypoxic-ischemic encephalopathy occurred among women undergoing planned cesarean delivery. Multivariate logistic regression analysis determined that the risks of stillbirth, neonatal death, and hypoxic-ischemic encephalopathy in term infants were increased in the group undergoing a trial of labor, compared with elective repeat cesarean (odds ratio [OR], 2.72; 95% confidence interval [CI], 1.49–4.97).
Can we predict the success of a trial of labor?
Combined success rates from a large number of prospective cohort studies suggest an overall rate of 75.9%. Many clinical characteristics may increase the likelihood of success of a trial of labor after cesarean. In this section, I describe these characteristics and sift the data we have about them.
A history of vaginal delivery ups the odds of success
Women who have delivered vaginally have a much lower risk of rupture during a trial of labor after cesarean than women who have not. Women who have delivered vaginally are also four times more likely to have a successful VBAC. A multicenter, prospective study found a VBAC success rate of 86% among women who had already delivered vaginally, and a success rate of 90% among women who had a history of successful VBAC.3
Many aspects of the cesarean delivery have continuing impact
The type of labor that occurred in the cesarean delivery may help predict subsequent complications and the ultimate success of a trial of labor. For example, induced labor or no labor prior to cesarean delivery is associated with a 2.25-fold risk of uterine rupture in a subsequent trial of labor, compared with a history of spontaneous labor.4
In addition, several studies have demonstrated that the indication for the first cesarean delivery has a bearing on the success of a subsequent trial of labor. For example, an indication of shoulder dystocia reduces the success rate of a subsequent trial of labor by one third.2
Even a brief trial of labor before the cesarean may increase the success of a subsequent trial of labor. One study found that cervical dilation to 8 cm or greater was independently predictive of successful VBAC among women who had a nonrecurring indication for the initial cesarean delivery.5
When the cesarean delivery involves a preterm infant, the risk of uterine rupture during a subsequent trial of labor may increase if the infant is at term. Conversely, the risk of uterine rupture is lower when a term cesarean is followed by a preterm trial of labor.6
A vertical hysterotomy may preclude VBAC
A previous classical hysterotomy is generally an absolute contraindication for a trial of labor because rupture may occur in as many as 14% of women who have this type of scar.
Low transverse hysterotomy does not appear to confer excess risk during a subsequent trial of labor. Less clear is whether a low vertical hysterotomy poses a risk of rupture. In a 2004 prospective cohort study, the rate of uterine rupture among women who had a transverse hysterotomy scar was 0.7%, compared with 2.0% for a low vertical scar. Any difference in the rate of uterine rupture in retrospective studies may be attributable, at least in part, to the subjective nature of the definition of “low vertical” because there is no precise or objective way to ensure that the vertical hysterotomy did not breach the contractile portion of the uterus (FIGURE).

Type of prior hysterotomy influences the VBAC decision
A classical uterine incision is an absolute contraindication to vaginal birth after cesarean (VBAC). A trial of labor is thought to be safe in women who have had a low transverse hysterotomy. The jury is still out on the safety of VBAC in a woman who has had a low vertical incision, however, because of uncertainty over whether the contractile portion of the uterus is involved.
Are multiple cesareans a contraindication to VBAC?
Experts disagree as to whether more than one previous cesarean delivery before a trial of labor increases the risk of uterine rupture. One retrospective study showed no difference in the rate of rupture between women who had a single previous cesarean and those who had more than one.7 A larger prospective study showed a modest increase in the risk of rupture (OR, 1.16) among women who had undergone more than one cesarean—but no decrease in the chance of success.8
Most large retrospective and prospective studies include patients who have had more than one previous cesarean delivery, but their numbers remain low; therefore, statistical significance cannot be determined.
Induction or augmentation of labor may lower odds of success
The likelihood of successful VBAC may be reduced when labor is augmented or induced. The picture is unclear because most studies that have focused on cervical ripening and induction of labor in VBAC are small.
Bujold compared pregnancy outcomes of three groups of women:
- those who underwent cervical ripening via Foley catheter
- those who had amniotomy and oxytocin administration
- those who entered labor spontaneously.
No difference in the rate of uterine rupture was found among the groups. However, the group that underwent cervical ripening had a significantly lower rate of success.9
A large case-control study found no increase in the rate of rupture when oxytocin or prostaglandins were administered, but the rate tripled when both were used together.10
A small, nested, case-control study found an increased risk of uterine rupture only when oxytocin was administered at a rate exceeding 20 mU/mL.11
More than 90% of hysterotomies are transverse
When the obstetric history is incomplete, the clinician may not know what type of hysterotomy was used in the previous cesarean delivery. Most experts believe that VBAC is acceptable when the previous cesarean involved a low transverse hysterotomy. The risk may be much higher with other types of incisions. Today, however, with modern techniques in place, we can assume that more than 90% of hysterotomies are of the low transverse type.
At least one study suggests that the risk of uterine rupture during vaginal birth after cesarean is acceptably low when the type of hysterotomy is unknown. That study explored the effect of augmentation of labor with oxytocin among women who had an unknown scar and found an increased risk of rupture, compared with women who were managed expectantly. However, the overall rate of uterine rupture did not differ from the rate expected when the hysterotomy is known to be of the low transverse type.12
VBAC for twins is rare
Because few women carrying twins attempt VBAC, we have little data to guide counseling on success and complication rates. A multicenter, retrospective, cohort study explored delivery outcomes of 25,005 women who had undergone at least one previous cesarean. Of these women, 24,307 had a singleton pregnancy, and 535 were carrying twins. Women who had a twin gestation were 40% less likely to attempt a trial of labor, but those who did had a chance of success and risk of uterine rupture similar to those of women with a singleton gestation. Women carrying twins who underwent a trial of labor had an elevated risk of requiring transfusion, compared with those carrying singletons, but this risk was similar to that of women delivering twins by elective repeat cesarean. In fact, women who delivered twins by repeat cesarean tended to have more maternal morbidity overall than those who had a trial of labor.13
A short interpregnancy interval precludes VBAC
Data indicate that a trial of labor after cesarean should be avoided in women who have a brief interpregnancy interval. Several retrospective studies had found an increased risk of uterine rupture, as well as a host of other adverse outcomes, among these women. Using 12 months as a reference point, women who had an interpregnancy interval shorter than 6 months had triple the risk of uterine rupture.14 Although the mechanism is unknown, rupture is presumably the result of incomplete healing of the hysterotomy.
Macrosomia may not increase the risk of rupture
Women who are thought to have a macrosomic fetus may be encouraged to attempt VBAC, if they so desire. Macrosomia is a minor risk factor for failure of a trial of labor, but it does not necessarily increase the risk of uterine rupture.15
Elkousy examined VBAC success rates by birth weight, indication for the previous cesarean delivery, and pregnancy history. Not surprisingly, increased birth weight or a history of cephalopelvic disproportion reduced the rate of success, but a history of vaginal delivery negated that risk of failure. A history of successful VBAC improved the chance of success to more than 90%—even when the birth weight exceeded 4,000 g—and the success rate reached 82% when the birth weight exceeded 4,500 g.16
Physician and hospital attitudes toward vaginal birth after cesarean delivery (VBAC) may be a major determinant of its frequency and success. Many forces oppose women who desire a trial of labor after cesarean. Hospitals and insurers make it increasingly difficult to offer a trial of labor, and strict interpretation of ACOG’s guidelines requiring personnel to be “immediately available” during a trial of labor has caused many smaller and isolated hospitals to stop offering this option. The number of women who attempt VBAC has plummeted.20
Two recent surveys by ACOG indicate that an alarming number of providers have stopped offering VBAC because of a lack of insurance and fear of legal liability. As providers offer a trial of labor less and less, skills decline, and so does mentorship of younger physicians.
The NIH weighs in
In March 2010, the National Institutes of Health (NIH) convened a consensus development conference on the topic of VBAC. A panel of health professionals and public representatives reviewed the medical literature and produced a consensus statement. Their conclusion:
- Given the available evidence, [a trial of labor] is a reasonable option for many pregnant women with a prior low transverse uterine incision. The data reviewed in this report show that both [a trial of labor] and elective repeat cesarean for a pregnant woman with a prior transverse uterine incision have important risks and benefits and that these risks and benefits differ for the woman and her fetus.
The panel’s goal was to help women who have a history of cesarean delivery make an informed, evidence-based decision about the subsequent mode of delivery. The panel also acknowledged the general lack of high-quality evidence to confidently quantify the risks and benefits of a trial of labor versus planned repeat cesarean delivery.21
For another point of view on vaginal birth after cesarean, see the Editorial, “Does vaginal birth after cesarean have a future?” by John T. Repke, MD, of the OBG Management Board of Editors.
Repeat cesarean is probably best for obese gravidas
Obesity increases the likelihood of cesarean delivery in all circumstances, so it is not surprising that it is a risk factor for a failed trial of labor after cesarean. Obesity also increases the risks of anesthesia and surgery. Because of these risks, most clinicians opt to deliver obese patients by scheduled elective cesarean rather than risk having to perform emergent cesarean delivery in the case of acute fetal compromise or uterine rupture.
Race is not a risk factor for rupture
Race is probably not a significant independent risk factor for failure of VBAC. A secondary analysis of a multicenter, retrospective, cohort study found that black women were somewhat more likely to fail a trial of labor than white women (OR, 1.50; 95% CI, 1.29–1.74), after adjustment for confounding variables. However, black women undergoing a trial of labor were 40% less likely to suffer a uterine rupture than white women were.17
When comorbidities are well managed, VBAC remains an option
In general, a trial of labor in women who have well managed chronic medical disease does not pose undue risk to mother or baby.
In a population-based, retrospective cohort study using discharge data from California, Gregory and coworkers attempted to delineate clinical variables that might be associated with VBAC success and complications. They examined a wide range of maternal conditions, from diabetes to chorioamnionitis, as well as fetal conditions, such as oligohydramnios and unengaged vertex. Mothers were stratified into low- and high-risk groups, and multivariate logistic regression was performed. Low-risk patients had a 73.7% success rate, whereas high-risk patients had a 50% success rate. Not surprisingly, women who had a fetus with an unengaged vertex had a 9.8% chance of success and an eightfold increase in the risk of uterine rupture.18
1. Tita AT, Landon MB, Spong CY, et al. For Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111-1120.
2. Landon MB, Hauth JC, Leveno KJ, et al. For NICHD MFMU Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351(25):2581-2589.
3. Landon MB, Leindecker S, Spong CY, et al. For NICHD MFMU Network. The MFMU Cesarean Registry: factors affecting the success of trial of labor after previous cesarean delivery. Am J Obst Gynecol. 2005;193(3 Pt 2):1016-1023.
4. Algert CS, Morris JM, Simpson JM, Ford JB, Roberts CL. Labor before a primary cesarean delivery: reduced risk of uterine rupture in a subsequent trial of labor for vaginal birth after cesarean. Obstet Gynecol. 2008;112(5):1061-1066.
5. Kwon JY, Jo YS, Lee GS, Kim SJ, Shin JC, Lee Y. Cervical dilation at the time of cesarean section may affect the success of subsequent vaginal delivery. J Matern Fetal Neonatal Med. 2009;22(11):1057-1062.
6. Sciscione AC, Landon MB, Leveno KJ, et al. For NICHD MFMU Network. Previous preterm delivery and risk of subsequent uterine rupture. Obstet Gynecol. 2008;111(3):648-653.
7. Landon MB, Spong CY, Thom E, et al. For NICHD MFMU Network. Risk of uterine rupture with a trial of labor in women with multiple and single prior cesarean delivery. Obstet Gynecol. 2006;108(1):12-20.
8. Macones GA, Cahill A, Pare E, et al. Obstetric outcomes in women with two prior cesarean deliveries: is vaginal birth after cesarean delivery a viable option? Am J Obstet Gynecol. 2005;192(4):1223-1229.
9. Bujold E, Blackwell SC, Gauthier RJ. Cervical ripening with transcervical foley catheter and the risk of uterine rupture. Obstet Gynecol. 2004;103(1):18-23.
10. Macones G, Peipert J, Nelson D, et al. Maternal complications with vaginal birth after cesarean delivery: a multicenter study. Am J Obstet Gynecol. 2005;193(5):1656-1662.
11. Cahill A, Stamilio D, Odibo A, Peipert J, Stevens E, Macones G. Does a maximum dose of oxytocin affect risk for uterine rupture in candidates for vaginal birth after cesarean delivery? Am J Obstet Gynecol. 2007;197(5):495.e1-e5.
12. Grubb DK, Kjos SL, Paul RH. Latent labor with an unknown uterine scar. Obstet Gynecol. 1996;88(3):351-355.
13. Cahill A, Stamilio DM, Paré E, et al. Vaginal birth after cesarean (VBAC) attempt in twin pregnancies: is it safe? Am J Obstet Gynecol. 2005;193(3 Pt 2):1050-1055.
14. Stamilio DM, DeFranco E, Paré E, et al. Short interpregnancy interval: risk of uterine rupture and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;110(5):1075.-
15. Zelop CM, Shipp TD, Repke JT, Cohen A, Lieberman E. Outcomes of trial of labor following previous cesarean delivery among women with fetuses weighing >4000 g. Am J Obstet Gynecol. 2001;185(4):903-905.
16. Elkousy M, Sammel M, Stevens E, Peipert J, Macones G. The effect of birth weight on vaginal birth after cesarean delivery success rates. Am J Obstet Gynecol. 2003;188(3):824-830.
17. Cahill AG, Stamilio DM, Odibo AO, Peipert J, Stevens E, Macones GA. Racial disparity in the success and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2008;111(3):654-658.
18. Gregory KD, Korst LM, Fridman M, et al. Vaginal birth after cesarean: clinical risk factors associated with adverse outcome. Am J Obstet Gynecol. 2008;198(4):452.e1-e12.
19. Smith GCS, Pell JP, Cameron AD, Dobbie R. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA. 2002;287(20):2684-2690.
20. Hamilton BE, Martin JA, Sutton PD. For US Department of Health and Human Services. Births: preliminary data for 2002. Natl Vital Stat Rep. 2003;51(11):1-20.
21. National Institutes of Health Consensus Development Conference Statement. Vaginal Birth after Cesarean: New Insights. Bethesda, Md: NIH; 2010. http://consensus.nih.gov/2010/images/vbac/vbac_statement.pdf. Accessed June 16, 2010.
1. Tita AT, Landon MB, Spong CY, et al. For Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111-1120.
2. Landon MB, Hauth JC, Leveno KJ, et al. For NICHD MFMU Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351(25):2581-2589.
3. Landon MB, Leindecker S, Spong CY, et al. For NICHD MFMU Network. The MFMU Cesarean Registry: factors affecting the success of trial of labor after previous cesarean delivery. Am J Obst Gynecol. 2005;193(3 Pt 2):1016-1023.
4. Algert CS, Morris JM, Simpson JM, Ford JB, Roberts CL. Labor before a primary cesarean delivery: reduced risk of uterine rupture in a subsequent trial of labor for vaginal birth after cesarean. Obstet Gynecol. 2008;112(5):1061-1066.
5. Kwon JY, Jo YS, Lee GS, Kim SJ, Shin JC, Lee Y. Cervical dilation at the time of cesarean section may affect the success of subsequent vaginal delivery. J Matern Fetal Neonatal Med. 2009;22(11):1057-1062.
6. Sciscione AC, Landon MB, Leveno KJ, et al. For NICHD MFMU Network. Previous preterm delivery and risk of subsequent uterine rupture. Obstet Gynecol. 2008;111(3):648-653.
7. Landon MB, Spong CY, Thom E, et al. For NICHD MFMU Network. Risk of uterine rupture with a trial of labor in women with multiple and single prior cesarean delivery. Obstet Gynecol. 2006;108(1):12-20.
8. Macones GA, Cahill A, Pare E, et al. Obstetric outcomes in women with two prior cesarean deliveries: is vaginal birth after cesarean delivery a viable option? Am J Obstet Gynecol. 2005;192(4):1223-1229.
9. Bujold E, Blackwell SC, Gauthier RJ. Cervical ripening with transcervical foley catheter and the risk of uterine rupture. Obstet Gynecol. 2004;103(1):18-23.
10. Macones G, Peipert J, Nelson D, et al. Maternal complications with vaginal birth after cesarean delivery: a multicenter study. Am J Obstet Gynecol. 2005;193(5):1656-1662.
11. Cahill A, Stamilio D, Odibo A, Peipert J, Stevens E, Macones G. Does a maximum dose of oxytocin affect risk for uterine rupture in candidates for vaginal birth after cesarean delivery? Am J Obstet Gynecol. 2007;197(5):495.e1-e5.
12. Grubb DK, Kjos SL, Paul RH. Latent labor with an unknown uterine scar. Obstet Gynecol. 1996;88(3):351-355.
13. Cahill A, Stamilio DM, Paré E, et al. Vaginal birth after cesarean (VBAC) attempt in twin pregnancies: is it safe? Am J Obstet Gynecol. 2005;193(3 Pt 2):1050-1055.
14. Stamilio DM, DeFranco E, Paré E, et al. Short interpregnancy interval: risk of uterine rupture and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;110(5):1075.-
15. Zelop CM, Shipp TD, Repke JT, Cohen A, Lieberman E. Outcomes of trial of labor following previous cesarean delivery among women with fetuses weighing >4000 g. Am J Obstet Gynecol. 2001;185(4):903-905.
16. Elkousy M, Sammel M, Stevens E, Peipert J, Macones G. The effect of birth weight on vaginal birth after cesarean delivery success rates. Am J Obstet Gynecol. 2003;188(3):824-830.
17. Cahill AG, Stamilio DM, Odibo AO, Peipert J, Stevens E, Macones GA. Racial disparity in the success and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2008;111(3):654-658.
18. Gregory KD, Korst LM, Fridman M, et al. Vaginal birth after cesarean: clinical risk factors associated with adverse outcome. Am J Obstet Gynecol. 2008;198(4):452.e1-e12.
19. Smith GCS, Pell JP, Cameron AD, Dobbie R. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA. 2002;287(20):2684-2690.
20. Hamilton BE, Martin JA, Sutton PD. For US Department of Health and Human Services. Births: preliminary data for 2002. Natl Vital Stat Rep. 2003;51(11):1-20.
21. National Institutes of Health Consensus Development Conference Statement. Vaginal Birth after Cesarean: New Insights. Bethesda, Md: NIH; 2010. http://consensus.nih.gov/2010/images/vbac/vbac_statement.pdf. Accessed June 16, 2010.
Timing of antibiotic prophylaxis for C-section: Better before incision?
There is already a solid body of literature to support preoperative antibiotic prophylaxis for other clean or clean-contaminated surgery.1 There is also substantive evidence that both elective and intrapartum C-sections benefit from antibiotic prophylaxis, with a statistically and clinically significant reduction in endometritis, wound infection, and composite morbidities, compared with no treatment.2 There has been resistance to pre-operative antibiotics for C-section because of a theoretical risk of masking neonatal sepsis.
Strengths of the study
- It was powered sufficiently to answer the fundamental question: Is there a benefit to the mother or risk to the newborn from preoperative antibiotics?
- The studies included were homogenous
- The analysis had biological plausibility
- The findings are congruent with other studies of antibiotic prophylaxis
- The same antibiotic was used in all studies, and both labored and elective cesarean deliveries were included.
Still a question about precise timing
More studies are needed to determine whether the dose of antibiotics should be weight-based and repeated for prolonged cases, as has been suggested for other surgical procedures. The optimal window for preoperative antibiotics also needs to be delineated. For scheduled procedures, including cesarean delivery, there is the luxury of timing the antibiotics fairly precisely 1 or 2 hours before skin incision. Intrapartum cesarean delivery is often unpredictable, is sometimes done in an urgent manner, and arguably carries an even higher risk of infectious morbidity.
This meta-analysis provides good evidence that we should change our practice to administer prophylactic antibiotics 1) for all cesarean deliveries and 2) before the skin incision whenever possible.—AVIVA LEE-PARRITZ, MD
1. Classen DC, Evans RS, Pestotnick SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic antibiotics and the risk of surgical wound infection. N Engl J Med. 1992;326:281-286.
2. Smaill F, Hofmeyr GJ. Antibiotic prophylaxis for cesarean section. Cochrane Database Syst Rev. 2002;(3):CD000933.-
There is already a solid body of literature to support preoperative antibiotic prophylaxis for other clean or clean-contaminated surgery.1 There is also substantive evidence that both elective and intrapartum C-sections benefit from antibiotic prophylaxis, with a statistically and clinically significant reduction in endometritis, wound infection, and composite morbidities, compared with no treatment.2 There has been resistance to pre-operative antibiotics for C-section because of a theoretical risk of masking neonatal sepsis.
Strengths of the study
- It was powered sufficiently to answer the fundamental question: Is there a benefit to the mother or risk to the newborn from preoperative antibiotics?
- The studies included were homogenous
- The analysis had biological plausibility
- The findings are congruent with other studies of antibiotic prophylaxis
- The same antibiotic was used in all studies, and both labored and elective cesarean deliveries were included.
Still a question about precise timing
More studies are needed to determine whether the dose of antibiotics should be weight-based and repeated for prolonged cases, as has been suggested for other surgical procedures. The optimal window for preoperative antibiotics also needs to be delineated. For scheduled procedures, including cesarean delivery, there is the luxury of timing the antibiotics fairly precisely 1 or 2 hours before skin incision. Intrapartum cesarean delivery is often unpredictable, is sometimes done in an urgent manner, and arguably carries an even higher risk of infectious morbidity.
This meta-analysis provides good evidence that we should change our practice to administer prophylactic antibiotics 1) for all cesarean deliveries and 2) before the skin incision whenever possible.—AVIVA LEE-PARRITZ, MD
There is already a solid body of literature to support preoperative antibiotic prophylaxis for other clean or clean-contaminated surgery.1 There is also substantive evidence that both elective and intrapartum C-sections benefit from antibiotic prophylaxis, with a statistically and clinically significant reduction in endometritis, wound infection, and composite morbidities, compared with no treatment.2 There has been resistance to pre-operative antibiotics for C-section because of a theoretical risk of masking neonatal sepsis.
Strengths of the study
- It was powered sufficiently to answer the fundamental question: Is there a benefit to the mother or risk to the newborn from preoperative antibiotics?
- The studies included were homogenous
- The analysis had biological plausibility
- The findings are congruent with other studies of antibiotic prophylaxis
- The same antibiotic was used in all studies, and both labored and elective cesarean deliveries were included.
Still a question about precise timing
More studies are needed to determine whether the dose of antibiotics should be weight-based and repeated for prolonged cases, as has been suggested for other surgical procedures. The optimal window for preoperative antibiotics also needs to be delineated. For scheduled procedures, including cesarean delivery, there is the luxury of timing the antibiotics fairly precisely 1 or 2 hours before skin incision. Intrapartum cesarean delivery is often unpredictable, is sometimes done in an urgent manner, and arguably carries an even higher risk of infectious morbidity.
This meta-analysis provides good evidence that we should change our practice to administer prophylactic antibiotics 1) for all cesarean deliveries and 2) before the skin incision whenever possible.—AVIVA LEE-PARRITZ, MD
1. Classen DC, Evans RS, Pestotnick SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic antibiotics and the risk of surgical wound infection. N Engl J Med. 1992;326:281-286.
2. Smaill F, Hofmeyr GJ. Antibiotic prophylaxis for cesarean section. Cochrane Database Syst Rev. 2002;(3):CD000933.-
1. Classen DC, Evans RS, Pestotnick SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic antibiotics and the risk of surgical wound infection. N Engl J Med. 1992;326:281-286.
2. Smaill F, Hofmeyr GJ. Antibiotic prophylaxis for cesarean section. Cochrane Database Syst Rev. 2002;(3):CD000933.-
Managing placenta accreta
- Placenta accreta occurs in approximately 1 in 2,500 deliveries.
- Risk factors include placenta previa, Asherman’s syndrome, the existence of a prior hysterotomy scar, and advanced maternal age or parity.
- Almost 50% of all cases of placenta accreta are diagnosed antepartum.
- MRI combined with ultrasound has a sensitivity of 100% in identifying placenta accreta.
- Medical management should be considered only when the patient wishes to preserve her fertility and when no active uterine bleeding is present.
- Gravid hysterectomy has been associated with a mortality rate of 7.4%, with a 90% incidence of transfusion, a 28% incidence of postoperative infection, and a 5% incidence of ureteral injuries or fistula formation.
Placenta accreta is an uncommon but potentially lethal complication of pregnancy. It occurs when the placenta is abnormally adherent to the uterine myometrium as a result of partial or complete absence of the decidua basalis and Nitabuch’s layer. The depth of invasion determines the histologic classification: Placenta accreta indicates direct attachment of the placenta to the myometrium; placenta increta describes placental invasion into the myometrium; and placenta percreta indicates full-thickness compromise of the myometrial layer. Deeper invasion is associated with more serious complications.
Incidence and pathophysiology
The incidence of placenta accreta has increased threefold over the past 20 years. Breen and colleagues reported a rate of 1 in 7,000 deliveries in 1977,1 while a later review suggests an incidence closer to 1 in 2,500 deliveries for the period from January 1985 through December 1994.2
Placenta accreta can develop in any setting in which there is an abnormally thin or denuded decidual layer, allowing easy access to the underlying myometrium by the invading trophoblastic tissue. Risk factors include placenta previa, Asherman’s syndrome, the existence of a prior hysterotomy scar, and advanced maternal age or parity. The major contributor to the rise in the incidence of placenta accreta appears to be a concurrent increase in the rate of cesarean section, which is associated with an increased risk for placenta previa.3,4
When placenta accreta occurs in the setting of a prior hysterotomy, the placenta is implanted over the uterine scar, where the decidual layer is already thinned. Clark et al reported the association between placenta accreta and prior cesarean section in a retrospective review of over 97,000 deliveries. They discovered a 5% risk of clinically diagnosed placenta accreta with placenta previa alone, but found this risk increased to 24% with a single prior hysterotomy, to 47% with 2 prior hysterotomies, and to 67% with 3 or more (TABLE 1).3 Miller and colleagues recently demonstrated that women with placenta previa have a 9.3% incidence of placenta accreta, compared with a 0.005% incidence in women with normally located placentae.2
TABLE 1
Incidence of placenta accreta in women with placenta previa and prior hysterotomy
| NUMBER OF HYSTEROTOMIES | INCIDENCE |
|---|---|
| 0 | 5% |
| 1 | 24% |
| 2 | 47% |
| 3 or more | 67% |
Diagnosis
In the past, diagnosis was typically made clinically, suggested by significant postpartum hemorrhage or a placenta that did not separate easily from its uterine attachment. The result was treatment in an emergent setting at the time of delivery. Today, thanks to a better understanding of risk factors and improved diagnostic testing, nearly half of all cases of placenta accreta are diagnosed antepartum.5 Earlier diagnosis makes it possible for the clinician to prepare in advance for delivery and its potential complications, thus improving the ultimate outcome.
Prenatal diagnosis. The assessment of placental morphology and location is a standard part of the obstetric ultrasound examination, allowing many cases of abnormal placentation to be diagnosed antenatally. Ultrasonographic diagnostic criteria (TABLE 2) for placenta accreta include the following:
- thinning or loss of the hypoechoic retroplacental myometrial zone to less than 2 mm6,7;
- absence of the hypoechoic myometrium in the lower uterine segment between the placenta and bladder6;
- thinning or disruption of the hyperechoic uterine serosa-to-bladder interface6;
- focal exophytic masses or extension of the placenta beyond the myometrial boundaries6,7;and
- • lacunar flow within the placenta with prominent venous lakes.8
While these findings are not definitive, they are highly suggestive of the diagnosis. Most authors agree that ultrasound has a sensitivity and specificity exceeding 85% in the detection of this condition.6,9 Transvaginal studies may be preferable to transabdominal ultrasound for improved resolution. In addition, Doppler velocimetry may allow for better identification of venous lakes and areas of increased vascularity within the myometrium. The sonographic detection rate is reduced when the placenta is located posteriorly.
In cases where ultrasound is equivocal, magnetic resonance imaging (MRI) is a useful adjunct. MRI provides better delineation of tissue planes, including the placenta, myometrium, and vasculature. Kay reported 3 cases where MRI was used to identify placenta previa when ultrasonic findings were equivocal.10 Similarly, Levine et al demonstrated a sensitivity of 100% for the identification of placenta accreta using MRI with ultrasound,9 and Thorp and colleagues demonstrated the efficacy of MRI in delineating bladder involvement in a case of placenta percreta.11 As would be expected, MRI has proved most useful when the placenta is located posteriorly. Besides being safe for both mother and fetus, MRI requires little in the way of preparation. Unfortunately, it lacks portability and is more expensive to perform than ultrasound.
Consider medical management only when no active uterine bleeding is present.
Some established biochemical markers have been applied in novel ways in diagnosing placenta accreta. For example, Zelop et al retrospectively reviewed the cases of 11 women who had undergone cesarean hysterectomy for placenta previa with accreta and compared them to 14 women with placenta previa alone. In 5 of 11 cases, women with accreta had alpha-fetoprotein (AFP) levels greater than 2 multiples of the median (MOM), compared to none in the previa-only group.12 This suggests that abnormal placental attachment results in myometrial invasion with increased diffusion of fetal AFP into the maternal circulation.
Hung and colleagues reviewed over 9,000 deliveries in the Taiwan Down Syndrome Screening Group.13 After other causes of elevated maternal AFP were excluded, regression analysis showed a relative risk of 8.3 for the presence of accreta when AFP levels exceeded 2.5 MOM in the second trimester. Ophir et al reported 2 cases of women with elevated creatine kinase levels as early as 22 weeks’ gestation who subsequently were diagnosed with placenta accreta.14 The investigators theorized that trophoblastic invasion of the myometrium results in muscular damage and elevated serum creatine kinase levels. While more studies are needed, serum markers may exist for the presence of accreta, providing another asset for earlier diagnosis and preparation.
TABLE 2
Ultrasound criteria for diagnosis of placenta accreta
| Thinning of the hypoechoic retroplacental myometrium to <2 mm |
| Absence of the hypoechoic myometrium in the lower uterine segment between placenta and bladder |
| Disruption of the hyperechoic uterine serosa-to-bladder interface |
| Extension of the placenta beyond the myometrial boundary |
| Lacunar flow and venous lakes within the placenta |
Medical management
In recent years, reports of select patients undergoing medical management for placenta accreta have begun to appear. Although the number of these patients has been small, with some women ultimately requiring surgical intervention, the vast majority have done well. Even so, medical management should be considered only when the patient wishes to preserve her fertility and when no active uterine bleeding is present. Adequate discussion of the potential risks and benefits also is crucial.
Methotrexate (MTX) is the cornerstone of medical management, although case reports also have described the use of antibiotics, uterotonics, surveillance with ultrasound, and the monitoring of human chorionic gonadotropin (hCG) levels. There is no agreed-upon regimen for the use of MTX or adjunctive therapies such as antibiotics and oxytocin. However, after reviewing the relevant literature, we can suggest some general guidelines.
At the time of delivery, the cord and membranes should be ligated as high as possible. Broad-spectrum antibiotics, for prophylaxis, and oxytocin should be administered during the initial 72 hours. In addition, ultrasound should be performed daily to monitor involution and placental vascularity, which should decrease over time.
If hCG levels plateau, placental vascularity persists, or placental involution stalls after this initial 72-hour period, MTX should be administered (1 mg/kg) on alternate days for a total of 4 to 6 doses. Medical management should be stopped if liver function tests are 2 or more times the normal value or there is evidence of thrombocytopenia (platelet levels below 100,000), neutropenia (white blood cell count below 2,000), or renal dysfunction (creatinine levels greater than 1.5 mg/dL). If the patient becomes clinically unstable or placental tissue fails to resolve following MTX therapy, hysterectomy should be considered.
Expectant management is another valid approach in select cases (TABLE 3). It is more likely to be successful when vascularity is no longer present on ultrasound examination of the placenta. Panoskaltsis and colleagues reported 2 cases of expectant management.15 In 1 case, the placental mass and vascularity regressed spontaneously with time following vaginal delivery, and normal menses resumed at 9 months postpartum. In the second case, MTX was given when the placental mass maintained vascularity on ultrasound exam at postpartum day 12. Ultimately, this mass involuted to a 5-cm mass without vascularity at 1 year. Normal menses resumed, and hCG levels returned to zero. Follow-up in these patients has been short. Fertility has yet to be documented in either patient, although the resumption of menses is an encouraging sign.
When it is successful, medical management has many potential benefits. A woman retains her future fertility and avoids the morbidity and mortality of gravid hysterectomy. Even with antenatal diagnosis of placenta accreta, gravid hysterectomy can result in high-volume blood loss and coagulopathy due to the difficult nature of the procedure.5 Proponents of medical management would further argue that there are few disadvantages to attempting medical management in clinically stable patients, provided follow-up is close. Even when a placental mass fails to resolve or vascularity or vaginal bleeding occurs, an interval of even a few days after delivery may simplify hysterectomy due to uterine involution and a concurrent decrease in vascularity.
Opponents of medical management suggest that it increases the risk of sudden hemorrhage, infection, and/or emergent surgery. While there have been reports of infection, all cases were confined to endometritis and were well controlled with an oral antibiotic regimen. One case report describes a patient given MTX for 6 weeks (50 mg per week). Human chorionic gonadotropin levels decreased, the placental mass was resolving, and there was no evidence of vascularity on ultrasound. However, when a suction dilatation and curettage (D&C) was performed for mild bleeding at 8 weeks postpartum, a massive hemorrhage occurred. Ultimately, the patient required a transfusion of 18 units of packed red blood cells and emergent hysterectomy.16
Opponents of medical management suggest it increases the risk of sudden hemorrhage.
Surgical management
Surgical options for the management of placenta accreta are dictated by the patient’s clinical status, comorbidities, age, and parity, as well as the desire to preserve future fertility. Practitioners should be prepared to manage placenta accreta when suspicious radiologic findings or significant risk factors are present. However, radiologic studies are subject to interpretive errors and definitive diagnosis can be made only at the time of delivery. The physician should lay the groundwork for surgery by counseling the patient extensively regarding possible complications and outcomes.
If hemorrhage occurs, follow a stepwise approach to ensure hemostasis.
Preoperative considerations. The best way to decrease surgical complications is through adequate preparation. To that end, the following steps should be considered when planning an operative delivery for a patient with suspected placenta accreta17:
- Notify anesthesia staff of the potential for a prolonged procedure with significant blood loss.
- Assemble an adequate surgical team, including backup by an experienced gynecologist, gynecologic oncologist, general surgeon, or urologist.
- Notify the blood bank of the potential need for significant blood products in the form of packed cells, clotting factors, and platelets. (Blood should be present in the room at the start of the procedure.)
- Ensure that items such as compression boots, a warming blanket, and a 3-way Foley are available. (The 3-way catheter allows the bladder to be back-filled to check for incidental cystotomy.)
- Consider ureteral stent placement to aid in the identification and protection of ureters if significant dissection is indicated.5
- Consider preoperative placement of angiocatheters for intraoperative embolization of the hypogastric arteries to control operative bleeding.18,19
- If bladder involvement is suspected, preoperative cystoscopy can confirm the diagnosis, allowing mobilization of the urology team.
Intraoperative considerations. Thought also should be given to the actual surgical approach prior to beginning the procedure. Attention to seemingly mundane details can significantly reduce operative complications. Suggestions include the following:
- Make a vertical skin incision to provide optimal exposure to the surgical field.20
- Carefully examine the pelvis to identify any abnormal collateral blood supply and involvement of the sidewall by the placenta.
- Take the time to create a bladder flap, unless there are significant adhesions or clear involvement of the bladder by the invading placenta. The flap will make gravid hysterectomy easier to perform and reduce the possibility of incidental cystotomy.
- Carefully consider the type of uterine incision to be made. If at all possible, incisions should be made away from the placenta.5
- Attempt to develop a cleavage plane between the placenta and uterus.21 If this fails, as much of the placental mass as possible should be manually extracted. Areas of defect or bleeding should be oversewn with chromic suture in an attempt to gain hemostasis. This technique is most useful when there is partial separation of the placenta with only a focal accreta. If the area of the accreta is large but not deep, localized repair of any myometrial defects should be attempted. A sharp curettage of the area in question also may aid in removal of the placental mass, but likely will require oversewing the uterus in order to obtain hemostasis.
The obvious imperative in delivering a gravida with a known abnormal placentation is the safety of both the mother and fetus. The secondary goal is to minimize morbidity, which is tantamount to minimizing blood loss and avoiding disseminated intravascular coagulation (DIC). When faced with excessive hemorrhage, a stepwise approach to securing hemostasis should be pursued.
First, the physician should be aggressive with the administration of blood products to avoid cardiogenic shock and coagulopathy. Second, the uterus should be packed for persistent oozing and reassessed in 12 to 24 hours. The uterine blood supply should be sequentially ligated, beginning with the uterine arteries and proceeding to the lower uterine and ovarian vessels.22 While ligation of the hypogastric arteries may reduce blood flow to the uterus, both Clark and Evans reported that such ligation was associated with a failure rate (for controlling hemorrhage) exceeding 50% because of extensive collateral pelvic circulation.23,24
In cases of balloon occlusion or embolization of the internal iliac arteries for pelvic hemorrhage, a reduction in blood loss and improved visualization of the operative field have been reported, although use in the specific setting of placenta accreta is limited.18,19,25-27 The common approach is axillary, with the catheter tip placed in the bilateral anterior hypogastric arteries prior to beginning the surgery.19 Balloon inflation occurs after delivery of the fetus.
Hysterectomy may become necessary if uterine bleeding cannot be controlled. While attempts may be made to salvage the uterus, immediate hysterectomy is indicated should the patient become unstable.16 Given the significant vascular supply of the cervical branch of the uterine artery and the abnormal placentation in the noncontractile portion of the uterus, the cervix will likely need to be removed at the time of hysterectomy.
Surgical management carries the potential for significant morbidity and mortality. O’Brien and colleagues found a mortality rate of 7.4% with a 90% incidence of transfusion, a 28% incidence of postoperative infection, and a 5% incidence of ureteral injuries or fistula formation in 109 cases of gravid hysterectomy for placenta accreta.5 All maternal deaths were directly related to excessive blood loss, and the median transfusion quantity was 7 units of packed red blood cells for patients managed surgically. This compares to a rate of 5% for infection28 and 0.1% for ureteral injuries29 in simple cesarean sections.
Conclusion
Although hysterectomy traditionally has been the definitive treatment for placenta accreta, clinicians should consider medical management for patients who are clinically stable and wish to preserve fertility. Adequate transfusion facilities; sensitive ultrasound examination and hCG assays; and rapidly responding, highly skilled surgical and anesthesia teams should be available nonetheless. When surgical management is indicated, proper preparation is crucial. If hemorrhage occurs, surgeons should follow a stepwise approach to ensure hemostasis. Further research should focus on preventing hemorrhage, better understanding the mechanism of abnormal placentation, and optimizing medical management regimens.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Breen JL, Neubecker R, Gregori CA, et al. Placenta accreta, increta, and percreta: a survey of 40 cases. Obstet Gynecol. 1977;49:43-47.
2. Miller DA, et al. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210-214.
3. Clark SL, Koonings PP, Phelan JP. Placenta previa/accreta and prior cesarean section. Obstet Gynecol. 1985;66:89-92.
4. To WW, Leung WC. Placenta previa and previous cesarean section. Int J Gynaecol Obstet. 1995;51:25-31.
5. O’Brien JM, et al. The management of placenta percreta: conservative and operative strategies. Am J Obstet Gynecol. 1996;175:1632-1638.
6. Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11:333-343.
7. Rosemond RL, et al. Transvaginal color Doppler sonography in the prenatal diagnosis of placenta accreta. Obstet Gynecol. 1992;80:508-510.
8. Chou MM, Ho ES. Prenatal diagnosis of placenta accreta with power amplitude ultrasonographic angiography. Am J Obstet Gynecol. 1997;177:1523-1525.
9. Levine D, et al. Placenta accreta: evaluation with color Doppler US, power Doppler US, and MRI imaging. Radiology. 1997;205:773-776.
10. Kay HH. Preliminary experience with magnetic resonance imaging in patients with third trimester bleeding. Obstet Gynecol. 1991;78(3 Pt 1):424-429.
11. Thorp JM, Councell RB, Sandridge DA, Weist HH. Antepartum diagnosis of placenta previa percreta by magnetic resonance imaging. Obstet Gynecol. 1992;80:506-508.
12. Zelop C, Nadel A, Frigoletto FD, Pauker S, MacMillan M, Benacerraf BR. Placenta accreta/percreta/increta: a cause of elevated maternal serum alpha-fetoprotein. Obstet Gynecol. 1992;80:693-694.
13. Hung TH, Shau WY, Hsieh CC, Chiu TH, Hsu JJ, Hsieh TT. Risk factors for placenta accreta. Obstet Gynecol. 1999;93:545-550.
14. Ophir E, Tendler R, Odeh M, Khouri S, Oettinger M. Creatine kinase as a biochemical marker for diagnosis of placenta increta and percreta. Am J Obstet Gynecol. 1999;180:1039-1040.
15. Panoskaltsis TA, et al. Placenta increta: evaluation of radiological investigations and therapeutic options of conservative management. Br J Obstet Gynaecol. 2000;107:802-806.
16. Jaffe R, et al. Failure of methotrexate treatment for term placenta percreta. Am J Obstet Gynecol. 1994;171:558-559.
17. Gabbe SG, ed. Obstetrics: Normal and Problem Pregnancies. 3rd ed. Philadelphia: Churchill Livingstone; 1996.
18. Dubois J, Garel L, Grignon A, et al. Placenta percreta: balloon occlusion and embolization of the internal iliac arteries to reduce intraoperative blood loss. Am J Obstet Gynecol. 1997;176:723-726.
19. Mitty HA, Sterling KM, Alvarez M, Gendler R. Obstetric hemorrhage: prophylactic and emergency arterial catheterization and embolotherapy. Radiology. 1993;188:183-187.
20. Rock JA, Thompson JD, eds. Operative Gynecology. 8th ed. Philadelphia: Lippincott-Raven; 1997.
21. Fox H. Placenta accreta 1945-1969. Obstet Gynecol Surv. 1972;27:475-490.
22. Cartwright PS, Pittaway DE, Jones HE, et al. The use of prophylactic antibiotics in obstetrics and gynecology: a review. Obstet Gynecol Surv. 1984;39:537.-
23. Clark SL, Phelan JP, Yeh SY, et al. Hypogastric artery ligation for obstetric hemorrhage. Obstet Gynecol. 1985;66:353-356.
24. Evans S, McShane P. The efficacy of internal iliac artery ligation in obstetric hemorrhage. Surg Gynecol Obstet. 1985;160:250-253.
25. Bakri YN, Linjawi T. Angiographic embolization for control of pelvic genital tract hemorrhage: report of 14 cases. Acta Obstet Gynecol Scand. 1992;71:17-21.
26. Gilbert WM, Moore TR, Resnik R, et al. Angiographic embolization in the management of hemorrhagic complications of pregnancy. Am J Obstet Gynecol. 1992;166:493-497.
27. Greenwood LH, et al. Obstetric and nonmalignant bleeding: treatment with angiographic embolization. Radiology. 1987;164:155-159.
28. Cartwright PS, Pittaway DE, Jones HE, et al. The use of prophylactic antibiotics in obstetrics and gynecology: a review. Obstet Gynecol Surv. 1984;39:537-554.
29. Eisenkop SM, Richman R, Platt LD, et al. Urinary tract injury during cesarean section. Obstet Gynecol. 1982;60:591-596.
30. Buckshee K, Dadhwal V. Medical management of placenta accreta. Int J Gynecol Obstet. 1997;59:47-48.
31. Komulainen MH, Vayrynen MA, Kauko ML, Saarikoski S. Two cases of placenta accreta managed conservatively. Eur J Obstet Gynecol Reprod Biol. 1995;62:135-137.
32. Legro RS, et al. Nonsurgical management of placenta percreta: a case report. Obstet Gynecol. 1994;83:847-849.
33. Raziel A, et al. Repeated ultrasonography and intramuscular methotrexate in the conservative management of residual adherent placenta. J Clin Ultrasound. 1992;20:288-290.
34. Hollander DI, et al. Conservative management of placenta accreta: a case report. J Reprod Med. 1988;33:74-78.
35. Arulkumaran S, et al. Medical treatment of placenta accreta with methotrexate. Acta Obstet Gynecol Scand. 1986;65:285-286.
36. Gorodeski IG, et al. Placenta previa with focal accretion. Isr J Med Sci. 1982;18:277-280.
37. Oumachigui A, et al. Placenta accreta and percreta: a review of 5 cases. Int J Gynaecol Obstet. 1981;19:337-340.
- Placenta accreta occurs in approximately 1 in 2,500 deliveries.
- Risk factors include placenta previa, Asherman’s syndrome, the existence of a prior hysterotomy scar, and advanced maternal age or parity.
- Almost 50% of all cases of placenta accreta are diagnosed antepartum.
- MRI combined with ultrasound has a sensitivity of 100% in identifying placenta accreta.
- Medical management should be considered only when the patient wishes to preserve her fertility and when no active uterine bleeding is present.
- Gravid hysterectomy has been associated with a mortality rate of 7.4%, with a 90% incidence of transfusion, a 28% incidence of postoperative infection, and a 5% incidence of ureteral injuries or fistula formation.
Placenta accreta is an uncommon but potentially lethal complication of pregnancy. It occurs when the placenta is abnormally adherent to the uterine myometrium as a result of partial or complete absence of the decidua basalis and Nitabuch’s layer. The depth of invasion determines the histologic classification: Placenta accreta indicates direct attachment of the placenta to the myometrium; placenta increta describes placental invasion into the myometrium; and placenta percreta indicates full-thickness compromise of the myometrial layer. Deeper invasion is associated with more serious complications.
Incidence and pathophysiology
The incidence of placenta accreta has increased threefold over the past 20 years. Breen and colleagues reported a rate of 1 in 7,000 deliveries in 1977,1 while a later review suggests an incidence closer to 1 in 2,500 deliveries for the period from January 1985 through December 1994.2
Placenta accreta can develop in any setting in which there is an abnormally thin or denuded decidual layer, allowing easy access to the underlying myometrium by the invading trophoblastic tissue. Risk factors include placenta previa, Asherman’s syndrome, the existence of a prior hysterotomy scar, and advanced maternal age or parity. The major contributor to the rise in the incidence of placenta accreta appears to be a concurrent increase in the rate of cesarean section, which is associated with an increased risk for placenta previa.3,4
When placenta accreta occurs in the setting of a prior hysterotomy, the placenta is implanted over the uterine scar, where the decidual layer is already thinned. Clark et al reported the association between placenta accreta and prior cesarean section in a retrospective review of over 97,000 deliveries. They discovered a 5% risk of clinically diagnosed placenta accreta with placenta previa alone, but found this risk increased to 24% with a single prior hysterotomy, to 47% with 2 prior hysterotomies, and to 67% with 3 or more (TABLE 1).3 Miller and colleagues recently demonstrated that women with placenta previa have a 9.3% incidence of placenta accreta, compared with a 0.005% incidence in women with normally located placentae.2
TABLE 1
Incidence of placenta accreta in women with placenta previa and prior hysterotomy
| NUMBER OF HYSTEROTOMIES | INCIDENCE |
|---|---|
| 0 | 5% |
| 1 | 24% |
| 2 | 47% |
| 3 or more | 67% |
Diagnosis
In the past, diagnosis was typically made clinically, suggested by significant postpartum hemorrhage or a placenta that did not separate easily from its uterine attachment. The result was treatment in an emergent setting at the time of delivery. Today, thanks to a better understanding of risk factors and improved diagnostic testing, nearly half of all cases of placenta accreta are diagnosed antepartum.5 Earlier diagnosis makes it possible for the clinician to prepare in advance for delivery and its potential complications, thus improving the ultimate outcome.
Prenatal diagnosis. The assessment of placental morphology and location is a standard part of the obstetric ultrasound examination, allowing many cases of abnormal placentation to be diagnosed antenatally. Ultrasonographic diagnostic criteria (TABLE 2) for placenta accreta include the following:
- thinning or loss of the hypoechoic retroplacental myometrial zone to less than 2 mm6,7;
- absence of the hypoechoic myometrium in the lower uterine segment between the placenta and bladder6;
- thinning or disruption of the hyperechoic uterine serosa-to-bladder interface6;
- focal exophytic masses or extension of the placenta beyond the myometrial boundaries6,7;and
- • lacunar flow within the placenta with prominent venous lakes.8
While these findings are not definitive, they are highly suggestive of the diagnosis. Most authors agree that ultrasound has a sensitivity and specificity exceeding 85% in the detection of this condition.6,9 Transvaginal studies may be preferable to transabdominal ultrasound for improved resolution. In addition, Doppler velocimetry may allow for better identification of venous lakes and areas of increased vascularity within the myometrium. The sonographic detection rate is reduced when the placenta is located posteriorly.
In cases where ultrasound is equivocal, magnetic resonance imaging (MRI) is a useful adjunct. MRI provides better delineation of tissue planes, including the placenta, myometrium, and vasculature. Kay reported 3 cases where MRI was used to identify placenta previa when ultrasonic findings were equivocal.10 Similarly, Levine et al demonstrated a sensitivity of 100% for the identification of placenta accreta using MRI with ultrasound,9 and Thorp and colleagues demonstrated the efficacy of MRI in delineating bladder involvement in a case of placenta percreta.11 As would be expected, MRI has proved most useful when the placenta is located posteriorly. Besides being safe for both mother and fetus, MRI requires little in the way of preparation. Unfortunately, it lacks portability and is more expensive to perform than ultrasound.
Consider medical management only when no active uterine bleeding is present.
Some established biochemical markers have been applied in novel ways in diagnosing placenta accreta. For example, Zelop et al retrospectively reviewed the cases of 11 women who had undergone cesarean hysterectomy for placenta previa with accreta and compared them to 14 women with placenta previa alone. In 5 of 11 cases, women with accreta had alpha-fetoprotein (AFP) levels greater than 2 multiples of the median (MOM), compared to none in the previa-only group.12 This suggests that abnormal placental attachment results in myometrial invasion with increased diffusion of fetal AFP into the maternal circulation.
Hung and colleagues reviewed over 9,000 deliveries in the Taiwan Down Syndrome Screening Group.13 After other causes of elevated maternal AFP were excluded, regression analysis showed a relative risk of 8.3 for the presence of accreta when AFP levels exceeded 2.5 MOM in the second trimester. Ophir et al reported 2 cases of women with elevated creatine kinase levels as early as 22 weeks’ gestation who subsequently were diagnosed with placenta accreta.14 The investigators theorized that trophoblastic invasion of the myometrium results in muscular damage and elevated serum creatine kinase levels. While more studies are needed, serum markers may exist for the presence of accreta, providing another asset for earlier diagnosis and preparation.
TABLE 2
Ultrasound criteria for diagnosis of placenta accreta
| Thinning of the hypoechoic retroplacental myometrium to <2 mm |
| Absence of the hypoechoic myometrium in the lower uterine segment between placenta and bladder |
| Disruption of the hyperechoic uterine serosa-to-bladder interface |
| Extension of the placenta beyond the myometrial boundary |
| Lacunar flow and venous lakes within the placenta |
Medical management
In recent years, reports of select patients undergoing medical management for placenta accreta have begun to appear. Although the number of these patients has been small, with some women ultimately requiring surgical intervention, the vast majority have done well. Even so, medical management should be considered only when the patient wishes to preserve her fertility and when no active uterine bleeding is present. Adequate discussion of the potential risks and benefits also is crucial.
Methotrexate (MTX) is the cornerstone of medical management, although case reports also have described the use of antibiotics, uterotonics, surveillance with ultrasound, and the monitoring of human chorionic gonadotropin (hCG) levels. There is no agreed-upon regimen for the use of MTX or adjunctive therapies such as antibiotics and oxytocin. However, after reviewing the relevant literature, we can suggest some general guidelines.
At the time of delivery, the cord and membranes should be ligated as high as possible. Broad-spectrum antibiotics, for prophylaxis, and oxytocin should be administered during the initial 72 hours. In addition, ultrasound should be performed daily to monitor involution and placental vascularity, which should decrease over time.
If hCG levels plateau, placental vascularity persists, or placental involution stalls after this initial 72-hour period, MTX should be administered (1 mg/kg) on alternate days for a total of 4 to 6 doses. Medical management should be stopped if liver function tests are 2 or more times the normal value or there is evidence of thrombocytopenia (platelet levels below 100,000), neutropenia (white blood cell count below 2,000), or renal dysfunction (creatinine levels greater than 1.5 mg/dL). If the patient becomes clinically unstable or placental tissue fails to resolve following MTX therapy, hysterectomy should be considered.
Expectant management is another valid approach in select cases (TABLE 3). It is more likely to be successful when vascularity is no longer present on ultrasound examination of the placenta. Panoskaltsis and colleagues reported 2 cases of expectant management.15 In 1 case, the placental mass and vascularity regressed spontaneously with time following vaginal delivery, and normal menses resumed at 9 months postpartum. In the second case, MTX was given when the placental mass maintained vascularity on ultrasound exam at postpartum day 12. Ultimately, this mass involuted to a 5-cm mass without vascularity at 1 year. Normal menses resumed, and hCG levels returned to zero. Follow-up in these patients has been short. Fertility has yet to be documented in either patient, although the resumption of menses is an encouraging sign.
When it is successful, medical management has many potential benefits. A woman retains her future fertility and avoids the morbidity and mortality of gravid hysterectomy. Even with antenatal diagnosis of placenta accreta, gravid hysterectomy can result in high-volume blood loss and coagulopathy due to the difficult nature of the procedure.5 Proponents of medical management would further argue that there are few disadvantages to attempting medical management in clinically stable patients, provided follow-up is close. Even when a placental mass fails to resolve or vascularity or vaginal bleeding occurs, an interval of even a few days after delivery may simplify hysterectomy due to uterine involution and a concurrent decrease in vascularity.
Opponents of medical management suggest that it increases the risk of sudden hemorrhage, infection, and/or emergent surgery. While there have been reports of infection, all cases were confined to endometritis and were well controlled with an oral antibiotic regimen. One case report describes a patient given MTX for 6 weeks (50 mg per week). Human chorionic gonadotropin levels decreased, the placental mass was resolving, and there was no evidence of vascularity on ultrasound. However, when a suction dilatation and curettage (D&C) was performed for mild bleeding at 8 weeks postpartum, a massive hemorrhage occurred. Ultimately, the patient required a transfusion of 18 units of packed red blood cells and emergent hysterectomy.16
Opponents of medical management suggest it increases the risk of sudden hemorrhage.
Surgical management
Surgical options for the management of placenta accreta are dictated by the patient’s clinical status, comorbidities, age, and parity, as well as the desire to preserve future fertility. Practitioners should be prepared to manage placenta accreta when suspicious radiologic findings or significant risk factors are present. However, radiologic studies are subject to interpretive errors and definitive diagnosis can be made only at the time of delivery. The physician should lay the groundwork for surgery by counseling the patient extensively regarding possible complications and outcomes.
If hemorrhage occurs, follow a stepwise approach to ensure hemostasis.
Preoperative considerations. The best way to decrease surgical complications is through adequate preparation. To that end, the following steps should be considered when planning an operative delivery for a patient with suspected placenta accreta17:
- Notify anesthesia staff of the potential for a prolonged procedure with significant blood loss.
- Assemble an adequate surgical team, including backup by an experienced gynecologist, gynecologic oncologist, general surgeon, or urologist.
- Notify the blood bank of the potential need for significant blood products in the form of packed cells, clotting factors, and platelets. (Blood should be present in the room at the start of the procedure.)
- Ensure that items such as compression boots, a warming blanket, and a 3-way Foley are available. (The 3-way catheter allows the bladder to be back-filled to check for incidental cystotomy.)
- Consider ureteral stent placement to aid in the identification and protection of ureters if significant dissection is indicated.5
- Consider preoperative placement of angiocatheters for intraoperative embolization of the hypogastric arteries to control operative bleeding.18,19
- If bladder involvement is suspected, preoperative cystoscopy can confirm the diagnosis, allowing mobilization of the urology team.
Intraoperative considerations. Thought also should be given to the actual surgical approach prior to beginning the procedure. Attention to seemingly mundane details can significantly reduce operative complications. Suggestions include the following:
- Make a vertical skin incision to provide optimal exposure to the surgical field.20
- Carefully examine the pelvis to identify any abnormal collateral blood supply and involvement of the sidewall by the placenta.
- Take the time to create a bladder flap, unless there are significant adhesions or clear involvement of the bladder by the invading placenta. The flap will make gravid hysterectomy easier to perform and reduce the possibility of incidental cystotomy.
- Carefully consider the type of uterine incision to be made. If at all possible, incisions should be made away from the placenta.5
- Attempt to develop a cleavage plane between the placenta and uterus.21 If this fails, as much of the placental mass as possible should be manually extracted. Areas of defect or bleeding should be oversewn with chromic suture in an attempt to gain hemostasis. This technique is most useful when there is partial separation of the placenta with only a focal accreta. If the area of the accreta is large but not deep, localized repair of any myometrial defects should be attempted. A sharp curettage of the area in question also may aid in removal of the placental mass, but likely will require oversewing the uterus in order to obtain hemostasis.
The obvious imperative in delivering a gravida with a known abnormal placentation is the safety of both the mother and fetus. The secondary goal is to minimize morbidity, which is tantamount to minimizing blood loss and avoiding disseminated intravascular coagulation (DIC). When faced with excessive hemorrhage, a stepwise approach to securing hemostasis should be pursued.
First, the physician should be aggressive with the administration of blood products to avoid cardiogenic shock and coagulopathy. Second, the uterus should be packed for persistent oozing and reassessed in 12 to 24 hours. The uterine blood supply should be sequentially ligated, beginning with the uterine arteries and proceeding to the lower uterine and ovarian vessels.22 While ligation of the hypogastric arteries may reduce blood flow to the uterus, both Clark and Evans reported that such ligation was associated with a failure rate (for controlling hemorrhage) exceeding 50% because of extensive collateral pelvic circulation.23,24
In cases of balloon occlusion or embolization of the internal iliac arteries for pelvic hemorrhage, a reduction in blood loss and improved visualization of the operative field have been reported, although use in the specific setting of placenta accreta is limited.18,19,25-27 The common approach is axillary, with the catheter tip placed in the bilateral anterior hypogastric arteries prior to beginning the surgery.19 Balloon inflation occurs after delivery of the fetus.
Hysterectomy may become necessary if uterine bleeding cannot be controlled. While attempts may be made to salvage the uterus, immediate hysterectomy is indicated should the patient become unstable.16 Given the significant vascular supply of the cervical branch of the uterine artery and the abnormal placentation in the noncontractile portion of the uterus, the cervix will likely need to be removed at the time of hysterectomy.
Surgical management carries the potential for significant morbidity and mortality. O’Brien and colleagues found a mortality rate of 7.4% with a 90% incidence of transfusion, a 28% incidence of postoperative infection, and a 5% incidence of ureteral injuries or fistula formation in 109 cases of gravid hysterectomy for placenta accreta.5 All maternal deaths were directly related to excessive blood loss, and the median transfusion quantity was 7 units of packed red blood cells for patients managed surgically. This compares to a rate of 5% for infection28 and 0.1% for ureteral injuries29 in simple cesarean sections.
Conclusion
Although hysterectomy traditionally has been the definitive treatment for placenta accreta, clinicians should consider medical management for patients who are clinically stable and wish to preserve fertility. Adequate transfusion facilities; sensitive ultrasound examination and hCG assays; and rapidly responding, highly skilled surgical and anesthesia teams should be available nonetheless. When surgical management is indicated, proper preparation is crucial. If hemorrhage occurs, surgeons should follow a stepwise approach to ensure hemostasis. Further research should focus on preventing hemorrhage, better understanding the mechanism of abnormal placentation, and optimizing medical management regimens.
The authors report no financial relationship with any companies whose products are mentioned in this article.
- Placenta accreta occurs in approximately 1 in 2,500 deliveries.
- Risk factors include placenta previa, Asherman’s syndrome, the existence of a prior hysterotomy scar, and advanced maternal age or parity.
- Almost 50% of all cases of placenta accreta are diagnosed antepartum.
- MRI combined with ultrasound has a sensitivity of 100% in identifying placenta accreta.
- Medical management should be considered only when the patient wishes to preserve her fertility and when no active uterine bleeding is present.
- Gravid hysterectomy has been associated with a mortality rate of 7.4%, with a 90% incidence of transfusion, a 28% incidence of postoperative infection, and a 5% incidence of ureteral injuries or fistula formation.
Placenta accreta is an uncommon but potentially lethal complication of pregnancy. It occurs when the placenta is abnormally adherent to the uterine myometrium as a result of partial or complete absence of the decidua basalis and Nitabuch’s layer. The depth of invasion determines the histologic classification: Placenta accreta indicates direct attachment of the placenta to the myometrium; placenta increta describes placental invasion into the myometrium; and placenta percreta indicates full-thickness compromise of the myometrial layer. Deeper invasion is associated with more serious complications.
Incidence and pathophysiology
The incidence of placenta accreta has increased threefold over the past 20 years. Breen and colleagues reported a rate of 1 in 7,000 deliveries in 1977,1 while a later review suggests an incidence closer to 1 in 2,500 deliveries for the period from January 1985 through December 1994.2
Placenta accreta can develop in any setting in which there is an abnormally thin or denuded decidual layer, allowing easy access to the underlying myometrium by the invading trophoblastic tissue. Risk factors include placenta previa, Asherman’s syndrome, the existence of a prior hysterotomy scar, and advanced maternal age or parity. The major contributor to the rise in the incidence of placenta accreta appears to be a concurrent increase in the rate of cesarean section, which is associated with an increased risk for placenta previa.3,4
When placenta accreta occurs in the setting of a prior hysterotomy, the placenta is implanted over the uterine scar, where the decidual layer is already thinned. Clark et al reported the association between placenta accreta and prior cesarean section in a retrospective review of over 97,000 deliveries. They discovered a 5% risk of clinically diagnosed placenta accreta with placenta previa alone, but found this risk increased to 24% with a single prior hysterotomy, to 47% with 2 prior hysterotomies, and to 67% with 3 or more (TABLE 1).3 Miller and colleagues recently demonstrated that women with placenta previa have a 9.3% incidence of placenta accreta, compared with a 0.005% incidence in women with normally located placentae.2
TABLE 1
Incidence of placenta accreta in women with placenta previa and prior hysterotomy
| NUMBER OF HYSTEROTOMIES | INCIDENCE |
|---|---|
| 0 | 5% |
| 1 | 24% |
| 2 | 47% |
| 3 or more | 67% |
Diagnosis
In the past, diagnosis was typically made clinically, suggested by significant postpartum hemorrhage or a placenta that did not separate easily from its uterine attachment. The result was treatment in an emergent setting at the time of delivery. Today, thanks to a better understanding of risk factors and improved diagnostic testing, nearly half of all cases of placenta accreta are diagnosed antepartum.5 Earlier diagnosis makes it possible for the clinician to prepare in advance for delivery and its potential complications, thus improving the ultimate outcome.
Prenatal diagnosis. The assessment of placental morphology and location is a standard part of the obstetric ultrasound examination, allowing many cases of abnormal placentation to be diagnosed antenatally. Ultrasonographic diagnostic criteria (TABLE 2) for placenta accreta include the following:
- thinning or loss of the hypoechoic retroplacental myometrial zone to less than 2 mm6,7;
- absence of the hypoechoic myometrium in the lower uterine segment between the placenta and bladder6;
- thinning or disruption of the hyperechoic uterine serosa-to-bladder interface6;
- focal exophytic masses or extension of the placenta beyond the myometrial boundaries6,7;and
- • lacunar flow within the placenta with prominent venous lakes.8
While these findings are not definitive, they are highly suggestive of the diagnosis. Most authors agree that ultrasound has a sensitivity and specificity exceeding 85% in the detection of this condition.6,9 Transvaginal studies may be preferable to transabdominal ultrasound for improved resolution. In addition, Doppler velocimetry may allow for better identification of venous lakes and areas of increased vascularity within the myometrium. The sonographic detection rate is reduced when the placenta is located posteriorly.
In cases where ultrasound is equivocal, magnetic resonance imaging (MRI) is a useful adjunct. MRI provides better delineation of tissue planes, including the placenta, myometrium, and vasculature. Kay reported 3 cases where MRI was used to identify placenta previa when ultrasonic findings were equivocal.10 Similarly, Levine et al demonstrated a sensitivity of 100% for the identification of placenta accreta using MRI with ultrasound,9 and Thorp and colleagues demonstrated the efficacy of MRI in delineating bladder involvement in a case of placenta percreta.11 As would be expected, MRI has proved most useful when the placenta is located posteriorly. Besides being safe for both mother and fetus, MRI requires little in the way of preparation. Unfortunately, it lacks portability and is more expensive to perform than ultrasound.
Consider medical management only when no active uterine bleeding is present.
Some established biochemical markers have been applied in novel ways in diagnosing placenta accreta. For example, Zelop et al retrospectively reviewed the cases of 11 women who had undergone cesarean hysterectomy for placenta previa with accreta and compared them to 14 women with placenta previa alone. In 5 of 11 cases, women with accreta had alpha-fetoprotein (AFP) levels greater than 2 multiples of the median (MOM), compared to none in the previa-only group.12 This suggests that abnormal placental attachment results in myometrial invasion with increased diffusion of fetal AFP into the maternal circulation.
Hung and colleagues reviewed over 9,000 deliveries in the Taiwan Down Syndrome Screening Group.13 After other causes of elevated maternal AFP were excluded, regression analysis showed a relative risk of 8.3 for the presence of accreta when AFP levels exceeded 2.5 MOM in the second trimester. Ophir et al reported 2 cases of women with elevated creatine kinase levels as early as 22 weeks’ gestation who subsequently were diagnosed with placenta accreta.14 The investigators theorized that trophoblastic invasion of the myometrium results in muscular damage and elevated serum creatine kinase levels. While more studies are needed, serum markers may exist for the presence of accreta, providing another asset for earlier diagnosis and preparation.
TABLE 2
Ultrasound criteria for diagnosis of placenta accreta
| Thinning of the hypoechoic retroplacental myometrium to <2 mm |
| Absence of the hypoechoic myometrium in the lower uterine segment between placenta and bladder |
| Disruption of the hyperechoic uterine serosa-to-bladder interface |
| Extension of the placenta beyond the myometrial boundary |
| Lacunar flow and venous lakes within the placenta |
Medical management
In recent years, reports of select patients undergoing medical management for placenta accreta have begun to appear. Although the number of these patients has been small, with some women ultimately requiring surgical intervention, the vast majority have done well. Even so, medical management should be considered only when the patient wishes to preserve her fertility and when no active uterine bleeding is present. Adequate discussion of the potential risks and benefits also is crucial.
Methotrexate (MTX) is the cornerstone of medical management, although case reports also have described the use of antibiotics, uterotonics, surveillance with ultrasound, and the monitoring of human chorionic gonadotropin (hCG) levels. There is no agreed-upon regimen for the use of MTX or adjunctive therapies such as antibiotics and oxytocin. However, after reviewing the relevant literature, we can suggest some general guidelines.
At the time of delivery, the cord and membranes should be ligated as high as possible. Broad-spectrum antibiotics, for prophylaxis, and oxytocin should be administered during the initial 72 hours. In addition, ultrasound should be performed daily to monitor involution and placental vascularity, which should decrease over time.
If hCG levels plateau, placental vascularity persists, or placental involution stalls after this initial 72-hour period, MTX should be administered (1 mg/kg) on alternate days for a total of 4 to 6 doses. Medical management should be stopped if liver function tests are 2 or more times the normal value or there is evidence of thrombocytopenia (platelet levels below 100,000), neutropenia (white blood cell count below 2,000), or renal dysfunction (creatinine levels greater than 1.5 mg/dL). If the patient becomes clinically unstable or placental tissue fails to resolve following MTX therapy, hysterectomy should be considered.
Expectant management is another valid approach in select cases (TABLE 3). It is more likely to be successful when vascularity is no longer present on ultrasound examination of the placenta. Panoskaltsis and colleagues reported 2 cases of expectant management.15 In 1 case, the placental mass and vascularity regressed spontaneously with time following vaginal delivery, and normal menses resumed at 9 months postpartum. In the second case, MTX was given when the placental mass maintained vascularity on ultrasound exam at postpartum day 12. Ultimately, this mass involuted to a 5-cm mass without vascularity at 1 year. Normal menses resumed, and hCG levels returned to zero. Follow-up in these patients has been short. Fertility has yet to be documented in either patient, although the resumption of menses is an encouraging sign.
When it is successful, medical management has many potential benefits. A woman retains her future fertility and avoids the morbidity and mortality of gravid hysterectomy. Even with antenatal diagnosis of placenta accreta, gravid hysterectomy can result in high-volume blood loss and coagulopathy due to the difficult nature of the procedure.5 Proponents of medical management would further argue that there are few disadvantages to attempting medical management in clinically stable patients, provided follow-up is close. Even when a placental mass fails to resolve or vascularity or vaginal bleeding occurs, an interval of even a few days after delivery may simplify hysterectomy due to uterine involution and a concurrent decrease in vascularity.
Opponents of medical management suggest that it increases the risk of sudden hemorrhage, infection, and/or emergent surgery. While there have been reports of infection, all cases were confined to endometritis and were well controlled with an oral antibiotic regimen. One case report describes a patient given MTX for 6 weeks (50 mg per week). Human chorionic gonadotropin levels decreased, the placental mass was resolving, and there was no evidence of vascularity on ultrasound. However, when a suction dilatation and curettage (D&C) was performed for mild bleeding at 8 weeks postpartum, a massive hemorrhage occurred. Ultimately, the patient required a transfusion of 18 units of packed red blood cells and emergent hysterectomy.16
Opponents of medical management suggest it increases the risk of sudden hemorrhage.
Surgical management
Surgical options for the management of placenta accreta are dictated by the patient’s clinical status, comorbidities, age, and parity, as well as the desire to preserve future fertility. Practitioners should be prepared to manage placenta accreta when suspicious radiologic findings or significant risk factors are present. However, radiologic studies are subject to interpretive errors and definitive diagnosis can be made only at the time of delivery. The physician should lay the groundwork for surgery by counseling the patient extensively regarding possible complications and outcomes.
If hemorrhage occurs, follow a stepwise approach to ensure hemostasis.
Preoperative considerations. The best way to decrease surgical complications is through adequate preparation. To that end, the following steps should be considered when planning an operative delivery for a patient with suspected placenta accreta17:
- Notify anesthesia staff of the potential for a prolonged procedure with significant blood loss.
- Assemble an adequate surgical team, including backup by an experienced gynecologist, gynecologic oncologist, general surgeon, or urologist.
- Notify the blood bank of the potential need for significant blood products in the form of packed cells, clotting factors, and platelets. (Blood should be present in the room at the start of the procedure.)
- Ensure that items such as compression boots, a warming blanket, and a 3-way Foley are available. (The 3-way catheter allows the bladder to be back-filled to check for incidental cystotomy.)
- Consider ureteral stent placement to aid in the identification and protection of ureters if significant dissection is indicated.5
- Consider preoperative placement of angiocatheters for intraoperative embolization of the hypogastric arteries to control operative bleeding.18,19
- If bladder involvement is suspected, preoperative cystoscopy can confirm the diagnosis, allowing mobilization of the urology team.
Intraoperative considerations. Thought also should be given to the actual surgical approach prior to beginning the procedure. Attention to seemingly mundane details can significantly reduce operative complications. Suggestions include the following:
- Make a vertical skin incision to provide optimal exposure to the surgical field.20
- Carefully examine the pelvis to identify any abnormal collateral blood supply and involvement of the sidewall by the placenta.
- Take the time to create a bladder flap, unless there are significant adhesions or clear involvement of the bladder by the invading placenta. The flap will make gravid hysterectomy easier to perform and reduce the possibility of incidental cystotomy.
- Carefully consider the type of uterine incision to be made. If at all possible, incisions should be made away from the placenta.5
- Attempt to develop a cleavage plane between the placenta and uterus.21 If this fails, as much of the placental mass as possible should be manually extracted. Areas of defect or bleeding should be oversewn with chromic suture in an attempt to gain hemostasis. This technique is most useful when there is partial separation of the placenta with only a focal accreta. If the area of the accreta is large but not deep, localized repair of any myometrial defects should be attempted. A sharp curettage of the area in question also may aid in removal of the placental mass, but likely will require oversewing the uterus in order to obtain hemostasis.
The obvious imperative in delivering a gravida with a known abnormal placentation is the safety of both the mother and fetus. The secondary goal is to minimize morbidity, which is tantamount to minimizing blood loss and avoiding disseminated intravascular coagulation (DIC). When faced with excessive hemorrhage, a stepwise approach to securing hemostasis should be pursued.
First, the physician should be aggressive with the administration of blood products to avoid cardiogenic shock and coagulopathy. Second, the uterus should be packed for persistent oozing and reassessed in 12 to 24 hours. The uterine blood supply should be sequentially ligated, beginning with the uterine arteries and proceeding to the lower uterine and ovarian vessels.22 While ligation of the hypogastric arteries may reduce blood flow to the uterus, both Clark and Evans reported that such ligation was associated with a failure rate (for controlling hemorrhage) exceeding 50% because of extensive collateral pelvic circulation.23,24
In cases of balloon occlusion or embolization of the internal iliac arteries for pelvic hemorrhage, a reduction in blood loss and improved visualization of the operative field have been reported, although use in the specific setting of placenta accreta is limited.18,19,25-27 The common approach is axillary, with the catheter tip placed in the bilateral anterior hypogastric arteries prior to beginning the surgery.19 Balloon inflation occurs after delivery of the fetus.
Hysterectomy may become necessary if uterine bleeding cannot be controlled. While attempts may be made to salvage the uterus, immediate hysterectomy is indicated should the patient become unstable.16 Given the significant vascular supply of the cervical branch of the uterine artery and the abnormal placentation in the noncontractile portion of the uterus, the cervix will likely need to be removed at the time of hysterectomy.
Surgical management carries the potential for significant morbidity and mortality. O’Brien and colleagues found a mortality rate of 7.4% with a 90% incidence of transfusion, a 28% incidence of postoperative infection, and a 5% incidence of ureteral injuries or fistula formation in 109 cases of gravid hysterectomy for placenta accreta.5 All maternal deaths were directly related to excessive blood loss, and the median transfusion quantity was 7 units of packed red blood cells for patients managed surgically. This compares to a rate of 5% for infection28 and 0.1% for ureteral injuries29 in simple cesarean sections.
Conclusion
Although hysterectomy traditionally has been the definitive treatment for placenta accreta, clinicians should consider medical management for patients who are clinically stable and wish to preserve fertility. Adequate transfusion facilities; sensitive ultrasound examination and hCG assays; and rapidly responding, highly skilled surgical and anesthesia teams should be available nonetheless. When surgical management is indicated, proper preparation is crucial. If hemorrhage occurs, surgeons should follow a stepwise approach to ensure hemostasis. Further research should focus on preventing hemorrhage, better understanding the mechanism of abnormal placentation, and optimizing medical management regimens.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Breen JL, Neubecker R, Gregori CA, et al. Placenta accreta, increta, and percreta: a survey of 40 cases. Obstet Gynecol. 1977;49:43-47.
2. Miller DA, et al. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210-214.
3. Clark SL, Koonings PP, Phelan JP. Placenta previa/accreta and prior cesarean section. Obstet Gynecol. 1985;66:89-92.
4. To WW, Leung WC. Placenta previa and previous cesarean section. Int J Gynaecol Obstet. 1995;51:25-31.
5. O’Brien JM, et al. The management of placenta percreta: conservative and operative strategies. Am J Obstet Gynecol. 1996;175:1632-1638.
6. Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11:333-343.
7. Rosemond RL, et al. Transvaginal color Doppler sonography in the prenatal diagnosis of placenta accreta. Obstet Gynecol. 1992;80:508-510.
8. Chou MM, Ho ES. Prenatal diagnosis of placenta accreta with power amplitude ultrasonographic angiography. Am J Obstet Gynecol. 1997;177:1523-1525.
9. Levine D, et al. Placenta accreta: evaluation with color Doppler US, power Doppler US, and MRI imaging. Radiology. 1997;205:773-776.
10. Kay HH. Preliminary experience with magnetic resonance imaging in patients with third trimester bleeding. Obstet Gynecol. 1991;78(3 Pt 1):424-429.
11. Thorp JM, Councell RB, Sandridge DA, Weist HH. Antepartum diagnosis of placenta previa percreta by magnetic resonance imaging. Obstet Gynecol. 1992;80:506-508.
12. Zelop C, Nadel A, Frigoletto FD, Pauker S, MacMillan M, Benacerraf BR. Placenta accreta/percreta/increta: a cause of elevated maternal serum alpha-fetoprotein. Obstet Gynecol. 1992;80:693-694.
13. Hung TH, Shau WY, Hsieh CC, Chiu TH, Hsu JJ, Hsieh TT. Risk factors for placenta accreta. Obstet Gynecol. 1999;93:545-550.
14. Ophir E, Tendler R, Odeh M, Khouri S, Oettinger M. Creatine kinase as a biochemical marker for diagnosis of placenta increta and percreta. Am J Obstet Gynecol. 1999;180:1039-1040.
15. Panoskaltsis TA, et al. Placenta increta: evaluation of radiological investigations and therapeutic options of conservative management. Br J Obstet Gynaecol. 2000;107:802-806.
16. Jaffe R, et al. Failure of methotrexate treatment for term placenta percreta. Am J Obstet Gynecol. 1994;171:558-559.
17. Gabbe SG, ed. Obstetrics: Normal and Problem Pregnancies. 3rd ed. Philadelphia: Churchill Livingstone; 1996.
18. Dubois J, Garel L, Grignon A, et al. Placenta percreta: balloon occlusion and embolization of the internal iliac arteries to reduce intraoperative blood loss. Am J Obstet Gynecol. 1997;176:723-726.
19. Mitty HA, Sterling KM, Alvarez M, Gendler R. Obstetric hemorrhage: prophylactic and emergency arterial catheterization and embolotherapy. Radiology. 1993;188:183-187.
20. Rock JA, Thompson JD, eds. Operative Gynecology. 8th ed. Philadelphia: Lippincott-Raven; 1997.
21. Fox H. Placenta accreta 1945-1969. Obstet Gynecol Surv. 1972;27:475-490.
22. Cartwright PS, Pittaway DE, Jones HE, et al. The use of prophylactic antibiotics in obstetrics and gynecology: a review. Obstet Gynecol Surv. 1984;39:537.-
23. Clark SL, Phelan JP, Yeh SY, et al. Hypogastric artery ligation for obstetric hemorrhage. Obstet Gynecol. 1985;66:353-356.
24. Evans S, McShane P. The efficacy of internal iliac artery ligation in obstetric hemorrhage. Surg Gynecol Obstet. 1985;160:250-253.
25. Bakri YN, Linjawi T. Angiographic embolization for control of pelvic genital tract hemorrhage: report of 14 cases. Acta Obstet Gynecol Scand. 1992;71:17-21.
26. Gilbert WM, Moore TR, Resnik R, et al. Angiographic embolization in the management of hemorrhagic complications of pregnancy. Am J Obstet Gynecol. 1992;166:493-497.
27. Greenwood LH, et al. Obstetric and nonmalignant bleeding: treatment with angiographic embolization. Radiology. 1987;164:155-159.
28. Cartwright PS, Pittaway DE, Jones HE, et al. The use of prophylactic antibiotics in obstetrics and gynecology: a review. Obstet Gynecol Surv. 1984;39:537-554.
29. Eisenkop SM, Richman R, Platt LD, et al. Urinary tract injury during cesarean section. Obstet Gynecol. 1982;60:591-596.
30. Buckshee K, Dadhwal V. Medical management of placenta accreta. Int J Gynecol Obstet. 1997;59:47-48.
31. Komulainen MH, Vayrynen MA, Kauko ML, Saarikoski S. Two cases of placenta accreta managed conservatively. Eur J Obstet Gynecol Reprod Biol. 1995;62:135-137.
32. Legro RS, et al. Nonsurgical management of placenta percreta: a case report. Obstet Gynecol. 1994;83:847-849.
33. Raziel A, et al. Repeated ultrasonography and intramuscular methotrexate in the conservative management of residual adherent placenta. J Clin Ultrasound. 1992;20:288-290.
34. Hollander DI, et al. Conservative management of placenta accreta: a case report. J Reprod Med. 1988;33:74-78.
35. Arulkumaran S, et al. Medical treatment of placenta accreta with methotrexate. Acta Obstet Gynecol Scand. 1986;65:285-286.
36. Gorodeski IG, et al. Placenta previa with focal accretion. Isr J Med Sci. 1982;18:277-280.
37. Oumachigui A, et al. Placenta accreta and percreta: a review of 5 cases. Int J Gynaecol Obstet. 1981;19:337-340.
1. Breen JL, Neubecker R, Gregori CA, et al. Placenta accreta, increta, and percreta: a survey of 40 cases. Obstet Gynecol. 1977;49:43-47.
2. Miller DA, et al. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210-214.
3. Clark SL, Koonings PP, Phelan JP. Placenta previa/accreta and prior cesarean section. Obstet Gynecol. 1985;66:89-92.
4. To WW, Leung WC. Placenta previa and previous cesarean section. Int J Gynaecol Obstet. 1995;51:25-31.
5. O’Brien JM, et al. The management of placenta percreta: conservative and operative strategies. Am J Obstet Gynecol. 1996;175:1632-1638.
6. Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11:333-343.
7. Rosemond RL, et al. Transvaginal color Doppler sonography in the prenatal diagnosis of placenta accreta. Obstet Gynecol. 1992;80:508-510.
8. Chou MM, Ho ES. Prenatal diagnosis of placenta accreta with power amplitude ultrasonographic angiography. Am J Obstet Gynecol. 1997;177:1523-1525.
9. Levine D, et al. Placenta accreta: evaluation with color Doppler US, power Doppler US, and MRI imaging. Radiology. 1997;205:773-776.
10. Kay HH. Preliminary experience with magnetic resonance imaging in patients with third trimester bleeding. Obstet Gynecol. 1991;78(3 Pt 1):424-429.
11. Thorp JM, Councell RB, Sandridge DA, Weist HH. Antepartum diagnosis of placenta previa percreta by magnetic resonance imaging. Obstet Gynecol. 1992;80:506-508.
12. Zelop C, Nadel A, Frigoletto FD, Pauker S, MacMillan M, Benacerraf BR. Placenta accreta/percreta/increta: a cause of elevated maternal serum alpha-fetoprotein. Obstet Gynecol. 1992;80:693-694.
13. Hung TH, Shau WY, Hsieh CC, Chiu TH, Hsu JJ, Hsieh TT. Risk factors for placenta accreta. Obstet Gynecol. 1999;93:545-550.
14. Ophir E, Tendler R, Odeh M, Khouri S, Oettinger M. Creatine kinase as a biochemical marker for diagnosis of placenta increta and percreta. Am J Obstet Gynecol. 1999;180:1039-1040.
15. Panoskaltsis TA, et al. Placenta increta: evaluation of radiological investigations and therapeutic options of conservative management. Br J Obstet Gynaecol. 2000;107:802-806.
16. Jaffe R, et al. Failure of methotrexate treatment for term placenta percreta. Am J Obstet Gynecol. 1994;171:558-559.
17. Gabbe SG, ed. Obstetrics: Normal and Problem Pregnancies. 3rd ed. Philadelphia: Churchill Livingstone; 1996.
18. Dubois J, Garel L, Grignon A, et al. Placenta percreta: balloon occlusion and embolization of the internal iliac arteries to reduce intraoperative blood loss. Am J Obstet Gynecol. 1997;176:723-726.
19. Mitty HA, Sterling KM, Alvarez M, Gendler R. Obstetric hemorrhage: prophylactic and emergency arterial catheterization and embolotherapy. Radiology. 1993;188:183-187.
20. Rock JA, Thompson JD, eds. Operative Gynecology. 8th ed. Philadelphia: Lippincott-Raven; 1997.
21. Fox H. Placenta accreta 1945-1969. Obstet Gynecol Surv. 1972;27:475-490.
22. Cartwright PS, Pittaway DE, Jones HE, et al. The use of prophylactic antibiotics in obstetrics and gynecology: a review. Obstet Gynecol Surv. 1984;39:537.-
23. Clark SL, Phelan JP, Yeh SY, et al. Hypogastric artery ligation for obstetric hemorrhage. Obstet Gynecol. 1985;66:353-356.
24. Evans S, McShane P. The efficacy of internal iliac artery ligation in obstetric hemorrhage. Surg Gynecol Obstet. 1985;160:250-253.
25. Bakri YN, Linjawi T. Angiographic embolization for control of pelvic genital tract hemorrhage: report of 14 cases. Acta Obstet Gynecol Scand. 1992;71:17-21.
26. Gilbert WM, Moore TR, Resnik R, et al. Angiographic embolization in the management of hemorrhagic complications of pregnancy. Am J Obstet Gynecol. 1992;166:493-497.
27. Greenwood LH, et al. Obstetric and nonmalignant bleeding: treatment with angiographic embolization. Radiology. 1987;164:155-159.
28. Cartwright PS, Pittaway DE, Jones HE, et al. The use of prophylactic antibiotics in obstetrics and gynecology: a review. Obstet Gynecol Surv. 1984;39:537-554.
29. Eisenkop SM, Richman R, Platt LD, et al. Urinary tract injury during cesarean section. Obstet Gynecol. 1982;60:591-596.
30. Buckshee K, Dadhwal V. Medical management of placenta accreta. Int J Gynecol Obstet. 1997;59:47-48.
31. Komulainen MH, Vayrynen MA, Kauko ML, Saarikoski S. Two cases of placenta accreta managed conservatively. Eur J Obstet Gynecol Reprod Biol. 1995;62:135-137.
32. Legro RS, et al. Nonsurgical management of placenta percreta: a case report. Obstet Gynecol. 1994;83:847-849.
33. Raziel A, et al. Repeated ultrasonography and intramuscular methotrexate in the conservative management of residual adherent placenta. J Clin Ultrasound. 1992;20:288-290.
34. Hollander DI, et al. Conservative management of placenta accreta: a case report. J Reprod Med. 1988;33:74-78.
35. Arulkumaran S, et al. Medical treatment of placenta accreta with methotrexate. Acta Obstet Gynecol Scand. 1986;65:285-286.
36. Gorodeski IG, et al. Placenta previa with focal accretion. Isr J Med Sci. 1982;18:277-280.
37. Oumachigui A, et al. Placenta accreta and percreta: a review of 5 cases. Int J Gynaecol Obstet. 1981;19:337-340.

